Note: Descriptions are shown in the official language in which they were submitted.
CA 02369892 2001-10-04
WO 00/62062 PCT/KR00/00329
1
METHOD FOR QUANTIFYING TRANSFORMING GROWTH FACTOR-~31 AND
METHOD FOR DETECTING CANCER BY USING SAME
FIELD OF THE INVENTION
The present invention relates to a method for
quantifying the concentration of transforming growth
factor-(31 (TGF-X31) in a body fluid, a method for
detecting cancer by using same, a composition for
detecting cancer, and a TGF-(31-specific monoclonal
antibody.
BACKGROUND OF THE INVENTION
Transforming growth factor-(3 (TGF-(3) regulates the
growth and differentiation of several cells, its mode of
action depending on the cell configuration and the
presence of other growth factors(Sporn et al., Science,
233, 532-534 (1986); and Roberts and Sporn, Adv. Cancer
Res., ~l_, 107-145 (1988)).
Three forms of TGF-(3 factor, TGF-(31, -(32 and -~i3,
occur in mammals, and, among these, TGF-(31 is believed
to play a key role in the physiological mechanism and
disease progression. It has been reported that it acts
abnormally in an invasion process, e.g., carcinogenesis.
This suggests that TGF-(31 is useful as a tumor marker in
cancer diagnosis, and that a method for quantifying TGF-
(31 in a body fluid with a high precision can be critical
in cancer diagnosis.
EP Publication No. 0 722 773 A1 discloses a method
for detecting cancer by contacting a blood sample
containing TGF-(31 with an absorbent, OH-carbonated
hydroxyapatite, to adsorb TGF-(31 thereto, eluting the
absorbed TGF-(31 with a buffer, and determining the
amount of TGF-(31 eluted with UV spectrometry. However,
this method suffers from the problems of limited
CA 02369892 2001-10-04
WO 00/62062 PCT/KR00/00329
2
sensitivity and imprecision manifested by large
fluctuations in measured values.
Therefore, there has existed a need to develop an
improved method for quantifying the amount of TGF-(31 in
plasma.
S~TMMARY OF THE INVENTION
Accordingly, it is an object of the present
invention to provide a method for quantifying the amount
of TGF-(31 in a sample with high precision and
sensitivity.
Another object of the present invention is to
provide a method for detecting cancer by using said
method .
A further object of the present invention is to
provide a composition for detecting cancer.
A still further object of the present invention is
to provide a TGF-(31-specific monoclonal antibody and a
hybridoma cell line producing the monoclonal antibody.
In accordance with one aspect of the present
invention, there is provided a method for quantifying
the amount of TGF-ail in a sample which comprises
treating the sample with a TGF-(31-specific receptor to
form a complex between TGF-(31 and the receptor and
measuring the amount of the complex.
BRIEF DESCRIPTION OF THE DRAWINGS
The above objects and features of the present
invention will become apparent from the following
description of preferred embodiments taken in
conjunction with the accompanying drawings, in which:
Fig. 1 shows the optical density-TGF-ail
concentration correlations obtained in Example 1 for
TGF-(31 type III and type II receptors, respectively;
CA 02369892 2001-10-04
WO 00/62062 PCT/KR00/00329
3
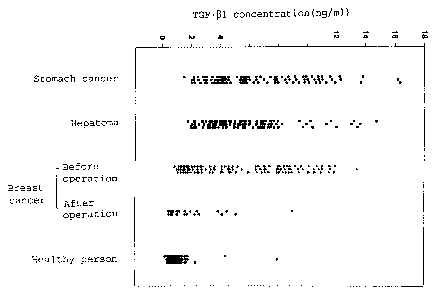
Fig. 2 depicts the respective distributions of
TGF-(31 concentrations in plasma samples taken from
healthy persons, stomach cancer patients, hepatoma
patients and breast cancer patients; and
Fig. 3 provides the respective distributions of
TGF-(31 concentrations in plasma samples taken from
healthy persons, lung cancer patients, rectal-colic
cancer patients, prostate cancer patients and uterine
cervical cancer patients.
DETAILED DESCRIPTION OF THE INVENTION
The TGF-(31-specific receptors which may be used in
the present invention include TGF-(31 type I, II and III
receptors (RI , RII and RIII ) , and preferred is TGF-X31
type III receptor, RIII. The TGF-(31 receptors may be
obtained by expressing a TGF-(31 receptor gene in a
mammal or insect cell line in accordance with a
conventional method(Burand, J.P. et al., Virology 101,
286-290 (1980)). For example, the TGF-(31 receptor may
be obtained by infecting an insect cell line, e.g.,
Sf21(Invitrogen, Netherlands), with a recombinant
baculovirus containing a TGF-(31 receptor gene;
extracting a water-insoluble receptor protein expressed
in the insect cell; solubilizing the water-insoluble
receptor protein with guanidine HC1 or urea; refolding
the solubilized receptor protein by removing the
guanidine or urea to restore the affinity for TGF-(31.
The TGF-(31-specific antibody which may be used in
the present invention may be prepared by immunizing a
mammal with TGF-ail or a part thereof . The TGF-(31
specific antibody may be a monoclonal antibody or a
polyclonal antibody having a specificity only for TGF-(31.
A preferred method for quantifying the amount of
TGF-(31 in a body fluid, e.g., plasma or urine, in
accordance with the present invention comprises
CA 02369892 2001-10-04
WO 00/62062 PCT/KR00/00329
4
(a) attaching a TGF-(31-specific receptor to a solid
support;
(b) adding a body fluid sample to the supported
receptor to form a TGF-(31-receptor complex;
(c) binding a TGF-(31-specific antibody conjugated
with a label to the complex; and
(d) measuring the amount of TGF-(31 using the label
as a detection marker.
Representative labels which may be employed in the
present invention include horseradish peroxidase, biotin
and fluorescence.
A first preferred embodiment of the present
invention comprises attaching a TGF-(31 receptor to a
solid support, e.g., the well of a microtiter plate;
adding an appropriately diluted sample containing TGF-(31
to the TGF-(31 receptor to allow the formation of a
complex between TGF-(31 and the TGF-(31 receptor; washing
the support with a phosphate buffered saline(PBS);
adding thereto a chromogenic enzyme-conjugated anti-TGF-
(31 antibody and developing the chromogenic enzyme; and
measuring the optical density of the resulting solution
to quantify the content of TGF-X31 in the sample.
In a second preferred embodiment of the present
invention, a liquid containing a TGF-(31-specific
receptor may be used in place of the supported TGF-(31
receptor. In this method, the amount of TGF-(31 in a
sample may be quantified by adding the sample to the
liquid containing a TGF-(31-specific receptor; adding a
TGF-(31 specific antibody conjugated with a label
thereto; precipitating an antibody-TGF-(31-receptor
complex; and measuring the optical density thereof.
The inventive method is capable of detecting TGF-
(31 at a very low concentration range of 30 pg/ml or
below.
The above method is particularly useful in cancer
diagnosis, since the TGF-(31 concentration in a cancer
CA 02369892 2001-10-04
WO 00/62062 PCT/KR00/00329
patient's body fluid is distinctly different from that
of a healthy person. Accordingly, a cancer may be
detected by repeating the above method to quantify the
TGF-(31 level in a patient's body fluid sample, e.g.,
5 plasma or urine; and comparing the TGF-(31 concentration
with that of a healthy person.
A preferred embodiment of the inventive method for
detecting a cancer comprises
(a) attaching a TGF-(31-specific receptor to a solid
support;
(b) adding a body fluid sample to .the supported
receptor to form the TGF-X31-receptor complex;
(c) binding a TGF-ail-specific antibody conjugated
with a label to the complex;
(d) measuring the amount of TGF-ail using the label
as a detection marker; and
(e) comparing the TGF-(31 amount with that of a
healthy person.
The above method is particularly effective in
detecting stomach cancer, hepatoma, breast cancer, lung
cancer, rectal-colic cancer, prostate cancer and uterine
cervical cancer.
A composition which may be used in the method for
detecting a cancer comprises a TGF-(31 receptor,
preferably RIII, and a TGF-(31 specific antibody.
In order to improve the sensitivity, the
monoclonal antibody may be obtained by preparing a
hybridoma cell line which produces TGF-(31-specific
monoclonal antibody using TGF-(31 or an antigenic
determinant part thereof as an immunogen according to a
conventional cell fusion method; and isolating the
monoclonal antibody from the hybridoma cell line. For
example, such a hybridoma cell line may be prepared by
immunizing a mouse with human TGF-(31; fusing the mouse
spleen cell with myeloma cell according to the cell
fusion method described by Kohler and Milstein(Eur. J.
CA 02369892 2001-10-04
WO 00/62062 PCT/KR00/00329
6
Immunol., ~., 511-519 (1976)); selecting by way of using
ELISA a hybridoma cell line having a specificity only
for human TGF-(31; determining the subclass of the
monoclonal antibody produced by the hybridoma cell line
using an immunodiffusion method; and selecting a
hybridoma cell line secreting IgGl subclass with the
highest antibody titer. The hybridoma cell line thus
obtained was designated hTGF-46 and deposited with
Korean Collection for Type Culture(Address: #52, Oun-
dong, Yusong-ku, Taejon 305-600, Republic of Korea) on
April 20, 1998 under the accession number of KCTC 0460BP,
in accordance with the terms of the Budapest Treaty on
the International Recognition of the Deposit of
Microorganism for the Purpose of Patent Procedure.
Hybridoma cell line hTGF-46 originates from (3-
limphoma, and continuously divides while producing human
TGF-(31-specific, IgGl subclass antibody. The hybridoma
cell line may be cultured in RPMI 1640 medium(Gibco-BRL,
USA) containing loo bovine fetal serum at 37 °C under an
atmosphere of 5% COz and 100% humidity. The cell number
doubles in 12 to 14 hours. This hybridoma cell line
floats on the medium without attaching itself to the
bottom of the culture flask and has a round shape having
a diameter of 15 to 20 ~,m.
To produce a large amount of the TGF-ail-specific
monoclonal antibody from the hybridoma cell line, the
hybridoma cell line is injected to a mouse and when its
abdominal cavity swells the ascites containing a high
concentration of hybridoma cells is taken to isolate the
monoclonal antibody therefrom.
When TGF-(31, -X32 and -(33 are subjected to
electrophoresis followed by western blotting, the
monoclonal antibody of the present invention recognizes
only TGF-X31, but not TGF-(32 or -(33. This suggests that
the present monoclonal antibody has a unique specificity
for TGF-ail. The monoclonal antibody of the present
CA 02369892 2001-10-04
WO 00/62062 PCT/KR00/00329
7
invention also shows a high affinity toward human TGF-(31,
and binds to the epitope region corresponding to the 5th
to 80th amino acid residues of TGF-(31.
The following Examples are intended to further
illustrate the present invention without limiting its
scope.
Further, percentages given below for solid in
solid mixture, liquid in liquid, and solid in liquid are
on a wt/wt, vol/vol and wt/vol basis, respectively,
unless specifically indicated otherwise.
Exam lp a 1: Sensitivity of TGF-(31 for Receptor
(Step 1) Preparation of TGF-(31 type III receptor
Plasmid pCEP4(Invitrogen, Netherlands) containing
a full length cDNA of Human TGF-(31 type III receptor
was subjected to polymerase chain reaction(PCR) using
primers RIII1 and RIII2(SEQ ID NOs: 1 and 2) to obtain
a DNA fragment encoding an extracellular domain of the
receptor which is composed of 400 amino acid
residues(1 to 400). The DNA fragment thus obtained was
inserted into baculovirus vector pCRBac(Invitrogen,
Netherlands) to obtain recombinant plasmid pCRBac-TGFR.
E. coli cells were transformed with the recombinant
plasmid pCRBac-TGFR and the transformed E. coli cells
were selected on a selective medium, LB medium
containing ampicillin.
Vector pCRBac-TGFR and Bac-N-Blue DNA(Invitrogen,
Netherlands) were cointroduced to insect cell line Sf
21(Invitrogen, Netherlands) using the liposome
transfection method(Burand, J.P., Virology, 101, 286
290 (1980)) and cultured for 3 days to obtain a virus
product. After 3 days, the virus thus obtained was
subjected to plaque analysis using lacZ gene as a
selective marker to select the recombinant virus. The
CA 02369892 2001-10-04
WO 00/62062 PCT/KR00/00329
8
recombinant virus thus obtained was subjected to PCR
using forward primer(SEQ ID NO: 3) and reverse
primer(SEQ ID NO: 4) to confirm the presence of the
TGF-(31 receptor gene. The wild vaculovirus showed a
839 by PCR product whereas the recombinant virus gave
a 1.5 kbp PCR product.
Insect cell line SF21 was infected with the
recombinant virus and then cultured for 5 days. The
culture was centrifuged to remove cell debris and the
supernatant containing virus was collected.
Insect cell line Sf21 was inoculated with the
supernatant and then cultured at 27 °C for 72 days in
Grace Insect medium(Invitrogen, Netherlands)
containing 10% fetal bovine serum(FBS), 7.3% TC
yeastolate, and 73% lactoalbumin hydrolysate. The
culture was centrifuged to collect cells and the cells
were washed with PBS. Protein lysis solution(50 mM
Tris-HC1 (pH 7. 5) , 50 mM NaCl, 10 mM (3-mercaptoethanol,
1% Triton X-100 and 2 mM BMSF) was added thereto and
then the resulting solution was heated at 100 °C for 10
minutes to prepare a sample.
The sample was subjected to SDS-PAGE in 12.5%
SDS-polyacrylamide gel and the resulting gel was
stained with coomassie brilliant blue. The gel was
subjected to western blotting which was conducted by
electrically transferring the proteins separated on
the gel to a filter, binding the antibody for TGF-(31
receptor obtained from R&D Systems Inc., USA to the
proteins of the filter and then analyzing the TGF-X31
using horseradish peroxidase(HRP)-conjugated anti-IgG
secondary antibody(Chemicon, USA) to confirm the
expression of the TGF-(31 III receptor.
Since the TGF-(31 receptor is water-insoluble, 8M
guanidine HCl (pH 8 . 0 ) was added to the sample and the
resulting solution was stirred for 1 hour. The
resulting solution was centrifuged at 7,000 rpm for 40
CA 02369892 2001-10-04
WO 00/62062 PCT/KR00/00329
9
minutes and then the supernatant was adjusted to a
protein concentration of 2 mg/ml. To restore the
binding activity of the TGF-(31 receptor to TGF-X31, the
resulting solution was added to a refolding buffer(100
mM Tris, 0.5 M arginin, 0.2 M EDTA, pH 8.0) to a
protein concentration of 150 ~g/ml and kept at 10 °C
for 40 hours. The resulting solution was dialyzed
with 20 mM Tris solution (pH 8 . 0) , successively in the
order of twice every 4 hours, once after overnight,
and twice every 2 hours thereafter, to effectively
refold the TGF-(31 receptor.
(Step 2) Sensitivity of TGF-X31 type III receptor for
TGF- (31
Each 2 ~g of the TGF-X31 type III receptor
obtained in Step 1 was placed in the wells of a
microtiter plate and the resulting plate was held at
an ambient temperature for 24 hours to attach the
receptor on the plate. 2 ng of purified TGF-(31(R&D
systems Inc., USA) was dissolved in PBS and diluted
serially. Each dilution solution was added in an
amount of 100 ~l to the well and then held at an
ambient temperature for 3 hours to allow the TGF-(31
bind the receptor. Each well was washed with PBS
containing 0.050 of Tween 20(PBST) and then HRP-
conjugated anti-TGF-(31 antibody (R&D systems Inc . , USA)
was added thereto. The resulting plate was left at
room temperature for 1.5 hours. Each well was washed
with PBST. 100 ~l of TMB-ELISA(Gibco-BRL, USA), a
substrate of HRP, was added thereto and the resulting
plate was left at an ambient temperature for 20
minutes to develop. The development reaction was
terminated by adding 25 ~l of 2N sulfuric acid. The
optical density of the reaction mixture was determined
at a measuring wavelength of 450 nm and a correction
CA 02369892 2001-10-04
WO 00/62062 PCT/KR00/00329
wavelength of 570 nm, and the result were plotted to
obtain a standard optical density-concentration
correlation.
Fig. 1 shows that the correlation thus obtained
5 is a straight line with a correlation coefficient of
0.999 and a slope of 0.28. The slope represents the
sensitivity of the receptor used in the measurements
and the TGF-(31 type III receptor is deemed to have an
excellent sensitivity toward TGF-(31 binding. The
10 correlation in Fig. 1 also shows that an extremely low
concentration of TGF-(31, down to 10 pg/ml, can be
detected by the present method.
The above procedure was repeated using TGF-(31
type II receptor(R&D systems Inc., USA) to determine
the sensitivity of the TGF-(31 type II receptor. The
results which are also plotted in Fig. 1 show that the
correlation obtained using TGF-(31 type II receptor is
also a straight line with a correlation coefficient of
0.999 and a slope of 0.57. Accordingly, the type II
receptor may also be used in quantifying the
concentration of TGF-(31 but its sensitivity is
considerably lower than that of type III receptor.
Example 2: Specificity of TGF-(31 Type III Receptor for
TGF- (31
The procedure of step 2 of Example 1 was repeated
except that a mixture containing 2,000 pg/ml TGF-(31,
2, 000 pg/ml TGF-(32 and 2, 000 pg/ml TGF-(33 (R&D Systems
Inc., USA) was used in place of TGF-(31. The procedure
of step 2 of Example 1 was repeated using 2, 000 pg/ml
TGF-(31 as a control. Results are shown in Table I.
CA 02369892 2001-10-04
WO 00/62062 PCT/KR00/00329
11
Table I
TGF-(31 TGF-(31 + TGF-(32 + TGF-(33
TGF-ail III receptor 100% 91.50
As can be seen from Table I, the TGF-X31 type III
receptor binds only with TGF-(31 without the occurrence
a crossreaction with TGF-(32 or TGF-(33.
Example 3: Measurement of Plasam TGF-(31 Concentration
in Cancer Patients Using Monoclonal Antibody
Blood samples were taken from 101 healthy persons,
111 stomach cancer patients, 100 hepatoma patients and
151 breast cancer patients. Blood samples were
collected with vacuumtainer containing 0.081 ml of 15%
ethylene diamine tetraacetic acid(EDTA) as an
anticoagulant, and then the resulting mixture was
centrifuged at 3,000 rpm for 20 minutes to obtain a
plasma sample. 0.1 ml of the plasma sample was added
to 0.1 ml of 2.5 N acetic acid/10 M urea solution. The
resulting mixture was kept at room temperature for 10
minutes, and neutralized with 0.1 ml of 2.7 N NaOH
containing 1M hydroxyethyl piperazine ethanesulfonic
acid(HEPES). Activated plasma thus obtained was
diluted 4-fold with PBST to obtain a plasma sample
solution which was subj ected to the following process
for measuring its TGF-X31 concentration.
0.1 ml of the plasma sample solution thus
obtained, as well as 0.1 ml portions of TGF-ail
standard solutions(0, 100, 1,000 and 2,000 pg/ml),
were respectively added to the wells of a 96-well
plate containing TGF-(31 type III receptor, kept at
room temperature for 3 hours, and then, washed three
times with PBST. Purified TGF-(31 monoclonal antibody-
HRP complex(Sigma) was added to each well and then the
CA 02369892 2001-10-04
WO 00/62062 PCT/KR00/00329
12
plate was kept at room temperature for 1.5 hours,
followed by washing the wells three times with PBST.
100 ~l of TMB ELISA(Gibco-BRL, USA), a substrate of
HRP, was added to each well and the plate was kept at
room temperature for 20 minutes to develop. The
development reaction was terminated by adding 25 ~l of
2N sulfuric acid. The optical density of the reaction
mixture was determined at a measuring wavelength of
450 nm, and a correction wavelength of 570 nm. The
TGF-X31 concentration of the plasma sample was
determined based on the calibration curve obtained
using standard solutions, and the results are shown in
Table II and Fig. 2.
Table II
Plasma Sample Group Mean Standard Range
Error (ng/ml) (ng/ml)
Stomach Cancer(n=111) 6.53 0.31 1.5 - 16.35
Hepatoma(n=100) 5,gg 0,3 1.77 - 14.76
Breast Before 5.49 0.32 0.87 - 13.44
Cancer Operation
(n=117)
After 2.15 0.42 0.46 - 9
Operation
(n=34)
[Healthy Person(n=101) I 1.03 0.08 0.27 - 8
As can be seen in Fig. 2 which depicts
distribution patterns of plasma TGF-(31 concentrations
of respective patient groups, the plasma samples of
cancer patients, display TGF-(31 concentration patterns
which are distinctly different from that of the
healthy group. This suggests that the above-mentioned
cancers can be detected by measuring plasma TGF-(31
concentration in accordance with the above procedure.
CA 02369892 2001-10-04
WO 00/62062 PCT/KR00/00329
13
Example 4: Measurement of Plasma TGF-(31 Concentration
of Cancer Patient Using Monoclonal Antibody
The procedure of Example 3 was repeated using
blood samples taken from 288 healthy persons, 29 lung
cancer patients, 48 rectal-colic cancer patents, 50
prostate cancer patients and 88 uterine cervical cancer
patients to measure respective plasma TGF-X31
concentrations and the results are shown in Table III
and Fig. 3.
Table III
Plasma Sample Mean Standard Standard
Error(ng/ml) Deviation
Lung Cancer(n=29) g,4g 1,2~ 4.16
Rectal-colic Cancer(n=48) 5,1g p,g~ 3.69
Prostate Cancer(n=50) 4.12 0.53 2.30
Uterine Cervical Cancer g.55 0.92 5.25
(n=88)
Healthy Person(n=288) 1,17 0.05 0.55
P<0.01
As can be seen in Fig. 3 which shows distribution
patterns of plasma TGF-(31 concentrations of respective
patient groups, the plasma samples of cancer patients,
display TGF-ail concentration patterns which are
distinctly different from that of the healthy group.
This suggests that the above-mentioned cancers can be
detected by measuring plasma TGF-(31 concentration in
accordance with the above procedure.
Exam 1~: Measurement of Plasma TGF-ail Concentration
of Cancer Patient Using Polyclonal Antibody
The procedure of Example 3 was repeated using
blood samples taken from 50 healthy persons, 50 hepatoma
CA 02369892 2001-10-04
WO 00/62062 PCT/KR00/00329
14
patients and 50 breast cancer patients, except that a
polyclonal antibody was used in place of the monoclonal
antibody, to measure respective plasma TGF-(31
concentrations and the results are shown in Table IV.
Table IV
Plasma Sample Mean Standard Standard Range
Error(ng/ml) Deviation (ng/ml)
Hepatoma 5.14 0.57 2.92 1.44-16.96
(n=50)
Breast Cancer 5.31 0.46 1.67 2.07-10.27
(n=50)
Healthy Person 1.19 0.08 0.29 0.70-1.9
(n=50)
P<0.05
As can be seen in Table IV, the plasma samples of
cancer patients display TGF-ail concentration patterns
which are distinctly different from that of the healthy
group. This suggests that the above-mentioned cancers
can be detected by measuring plasma TGF-(31 concentration
in accordance with the above procedure.
Example 6: Preparation of Hybridoma Cell Line
Producing a Monoclonal Antibody for TGF-(31
(Step 1) Immunization of Mouse
TGF-(31 was mixed with an equal volume of Complete
Freund Adjuvant until the mixture became fluid and the
resulting mixture was injected, in an amount of 100
~1/mouse, to the caudal vein of a 7 weeks-old Balb/c
mouse. After 2 weeks, the same amount of TGF-(31 as in
the first injection, which was mixed with Freund's
incomplete adjuvant, was injected to the caudal vein of
the mouse . Af ter 4 to 5 days , a small amount of blood
was taken from the tail and the presence of an antibody
CA 02369892 2001-10-04
WO 00/62062 PCT/KR00/00329
for TGF-(31 was confirmed by ELISA. 30 ~g of human TGF-
(31 dissolved in 0.85% PBS was then intravenously
injected 3 to 4 days before the following cell fusion
procedure.
5
(Step 2) Cell Fusion
Myeloma cell SP2/O~Agl4 (ATCC CRL 1581) was used as
a mother cell in the cell fusion procedure. The mother
10 cell was cultured in RPMI medium containing 10% FBS
while maintaining a maximum cell density of 5x105/ml.
The immunized mouse obtained in Step 1 was
anesthetized using ether and its spleen was removed to
be homogenized with a tissue homogenizer. The resulting
15 homogenate was suspended in HBSS(Gibco-BRL, USA) and the
resulting suspension was placed in a 15 ml centrifugal
tube and centrifuged. This procedure was repeated twice
to wash the spleen cells thoroughly. The mother cells,
SP2/O~Agl4, were suspended in HBSS and centrifuged.
This procedure was repeated twice . The spleen cells and
the SP2/O~Agl4 cells were respectively resuspended in 10
ml of HBSS to count the cell number in each suspension.
108 spleen cells and 10' SP2/O~Agl4 cells taken from
respective suspensions were mixed in a centrifugal tube
and then centrifuged to precipitate the cells. The
centrifugal tube was tapped with fingers to disperse the
precipitated cells and, then, kept at 37 °C for 1 minute.
1 ml of HBSS containing 45% PEG(w/v) and 5o DMSO were
added thereto over a period of 1 minute, followed by
shaking the tube for 1 minute. 9 ml of RPMI medium was
added thereto over a period of 3 minute and then RPMI
medium was added thereto until the total volume of the
cell suspension became 50 ml while shaking the tube.
The resulting suspension was centrifuged and the cell
pellet thus obtained was resuspended at a concentration
of 1 to 2 x 105/ml in HAT medium(Gibco-BRL, USA). 0.2 ml
CA 02369892 2001-10-04
WO 00/62062 PCT/KR00/00329
16
portions of the resulting resuspension were placed in
the wells of a 96-well microtiter plate and then
cultured for several days in an incubator, maintaining
the condition of 37 °C, 5% C02 and 100 % humidity.
(Step 3) Selection of Hybridoma cell Producing
Monoclonal antibody
The hybridoma cells obtained in Step 2 were
subjected to ELISA using human TGF-(31 antigen to obtain
cells which specifically react with TGF-(31, as described
below.
Human TGF-(31 antigen was added to the wells of a
microtiter plate in an amount of 50 x,1(2 ~g/ml)/well and
kept at room temperature for 12 hours to attach the
antigen to the well surface. The wells were washed with
PBST to remove unattached antigen.
The hybridoma cell culture obtained in Step 2 was
added in an amount of 50 ~1/cell to each well and kept
at 37 °C for 1 hour. The wells were washed with PBST to
remove the culture. Goat anti-mouse IgG-HRP(sigma, USA)
was added thereto, held at room temperature for 1 hour
and washed with PBST. 100 ~l of Substrate solution(OPD,
Sigma) was added thereto, held at room temperature for
20 minutes, and the optical density of the resulting
reaction mixture was measured at 492 nm.
Hybridoma cell lines secreting antibodies having
high specificity for human TGF-(31 antigen were first
selected, and each of these hybridoma cell lines was
subjected to ELISA using human TGF-(31, -(32 and -X33 to
screen hybridoma cells which have specificity only for
human TGF-(31 antigen. Each of the hybridoma cells thus
obtained was subjected to limiting dilution to obtain 7
hybridoma cell line clones producing a monoclonal
antibody, hTGF-7, -8, -31, -46, -70, -119 and -207.
Each clone was freeze-dried.
CA 02369892 2001-10-04
WO 00/62062 PCT/KR00/00329
17
The hybridoma cell culture was centrifuged and the
supernatant was subjected to ELISA to determine the
antibody titer and then subjected to immunotype
kit(Sigma, USA) to determine the subclass type of the
antibody. The results are shown in Table V.
Table V
Clone No. Optical Density(492 nm) Subclass Type
hTGF-46 2.125 IgGl
HTGF-7 1.644 IgGl
HTGF-70 2.590 IgGl
HTGF-8 2.395 IgGl
HTGF-207 1.735 IgGl
HTGF-119 2.462 IgGl
HTGF-31 2.282 IgGl
As can be seen from Table V, all of the 7 clones
were IgGl.
Among the 7 clones, the clone having the highest
titer, hTGF-46, was selected and injected
intraperitoneally to a mouse. Then its ascites was
collected and subjected to western blotting. The
results showed that the hybridoma cell line clone hTGF-
46 secrets a monoclonal antibody having a high
specificity for human TGF-(31. The hybridoma cell line
hTGF-46 was deposited with Korean Collection for Type
Culture(Address: #52, Oun-dong, Yusong-ku, Taejon 305-
600, Republic of Korea) on April 20, 1998 under
accession number of KCTC 0460BP, in accordance with the
terms of the Budapest Treaty on the International
Recognition of the Deposit of Microorganism for the
Purpose of Patent Procedure.
Examx~le 7: Production of Monoclonal Antibody for TGF-(31
To produce monoclonal antibody for TGF-(31 using
the hybridoma cell line obtained in Example 6, 0.5 ml of
CA 02369892 2001-10-04
WO 00/62062 PCT/KR00/00329
18
pristane was injected intraperitoneally to Balb/c mice,
and after 1 week, 5x106 hybridoma cells were injected to
each mouse. From the mice having swollen abdominal
cavity, ascites containing a high concentration of
hybridoma cells was taken and centrifuged at 10,000 rpm
to remove the hybridoma cells. The supernatant was
stored at -2 0 °C .
Column was filled with protein G beads and then
washed four times with lxPBS. 2 ml of the supernatant
was applied dropwise at a rate of 5 drops/minute to the
column, and 0.1 M glycine-HC1 solution was introduced at
a rate of 1 drop/10 minutes to the column to elute IgG.
HRP was activated in 0.1 M sodium phosphate
buffer(pH 6.5) containing 1.25 % glutaraldehyde and the
activated HRP was dialyzed against carbonate buffer(pH
9.2). The dialyzed HRP was reacted with the IgG to
obtain a HRP-conjugated IgG. After completion of the
reaction, the RZ value (A4oa/Azeo) was determined by
measuring the optical density of the reaction mixture at
280nm and 403 nm. In order to determine the activity of
the enzyme-conjugated antibody, each well of a
microtiter plate was coated with 1 ~.g of TGF-(31 and then
reacted with the HRP-conjugated IgG to determine the
activity. Futher, the HRP-conjugated IgG was subjected
to Western blotting to confirm the activity thereof.
Example 8: Western Blotting
To examine whether the TGF-(31-specific monoclonal
antibody obtained in Example 7 reacts with TGF-~i2 and
TFG-X33, the monoclonal antibody was subjected to SDS-
PAGE and western blotting as follows.
Human TGF-(31, -(32 and -(33 proteins were subjected
to SDS-PAGE on a 10% SDS-polyacrylamide gel and then
transferred electrically to a nitrocellulose filter
membrane. The membrane was reacted with the monoclonal
CA 02369892 2001-10-04
WO 00/62062 PCT/KR00/00329
19
hours. The resulting membrane was treated with 3%
bovine serum albumin at room temperature for 12 to 14
hours to block nonspecific reactions of the proteins.
The membrane was washed three times with PBS containing
0.5% Tween 20 and then reacted with HRP-conjugated anti-
mouse IgG(Sigma, USA) at room temperature. The membrane
was washed with PBS containing 0.5% Tween 20, and then,
a substrate solution(TMB, Gibco-BRL, USA) was added to
the membrane to develop.
The result showed that the monoclonal antibody of
the present invention reacts only with human TGF-(31 and
did not reacted with human TGF-~i2 and -(33. Therefore,
the present monoclonal antibody has specificity only for
human TGF-X31.
While the invention has been described with
respect to the above specific embodiments, it should be
recognized that various modifications and changes may be
made to the invention by those skilled in the art which
also fall within the scope of the invention as defined
by the appended claims.
CA 02369892 2001-10-04
WO 00/62062 PCT/KR00/00329
~ ~~oPtw t o ~t
I~NAT1~NAL P~tM
~~c~~~r rrr ~ ~~.s~ a~ err o~r~u~r~, r~~posrr
tta~~e~.~
To: Choc, Yox~-Iipung
3hindongA .Apt 1-2G3, i~ roan t Yot~oa~-dons, Doug l~u. Tacwu $00=200,
Ftepubltc of I~osen
z zbcA~ort of ~ ~oo~~arusM
_ .~. .-
Identltlcation refs given by ttm Atllutiber given by the
bT'I'c~R INTERNATIONAL DEPt7SITARY
AZTTI~O~RTi'Y:
hT(~ln - 4s
~CZC O~a~P
II. SCIENTIFTC DESCLtI~ON ANb/dRP.~p0~8D TA~CONC~C DfiSTGNATION
'Ihe m3nism ideatlfl0d rmdaar I abovo arcs mecompa~od bar:
I x 1 a ~tcientifyc dosCtiption
I 1 a ~oposed tsxaasoomic doai,Baa~oa
(Mm3c wlth a at~ss arhere a~tpliC3We)
1IL REGh>~ AND ACC~YTANC6
This Int~c~onsl bepasiraiy Anthaaity aooepts the mitsnargantsm Idaatisied
m~dat I above,
which waR receivad by it oa I~pel1 3Q 1098.
IV. RFC»pT' aFRBQtJ~STPQk SIGN
'11~e mi~a~gaaism iclenttfiad ~mder I ebonra vas roceiv~od by this
Intomational Dapoeitary
Acthacity on and a zto convert tha original dapoQit to a depp~it
artder tha B~apest ~enty ~aaa iecelvod tr~ritvn
V. INTERNATiI~NAt.. DEP(!S!"L"ARY' AZI7:~IOR1TY
Name: K area Bessarch Instltutti of 5igdt0at~c(a)uEDc~cacx~(s) having thepower
to
B ins ci et1 C ti o t1d B ~ tee b aOln g d ~temtit the IntornntioaeI
Dcpositary
Korean Coltecfmn tofTgpe Cuttan~ Aut6cxityorofautha~fzcdotfrcia6(s);
Adcfiroas: KC'PC. KR~SB
X52 4W~.-dung, ~s6i~ ku.
1'ae~bn g05-FOCI, Xyurrg Sooic Bata, Ctuxtor
Rcpubtlc of Korea. Dao~: qty ~ 1888
CA 02369892 2001-10-04
WO 00/62062 PCT/KR00/00329
1
Sequence Listing
<110> Hanmi Pllann. Co., Ltd
<120> METHOD FOR QUANTIFYING TRANSFORMING GROWTH FACTOR-
(31 AND METHOD FOR DETECTING CANCER BY USING SAME
<130> PCA00418/HMY
<150> KR 1999-12568
< 151 > 1999-04-09
<150> KR 1999-43935
<151> 1999-10-12
<160> 4
<170> KOPATIN 1.5
<210> 1
<211> 18
<212> DNA
<213> Artificial Sequence
<220>
<223> Primer RIII1
<400> 1
atggcagtga catcccac 1 g
<210> 2
<211> 12
<212> DNA
<213> Artificial Sequence
CA 02369892 2001-10-04
WO 00/62062 PCT/KR00/00329
2
<220>
<223> Primer RIII2
<400> 2
atttgggctt cc 12
<210>3
<211>24
<212>DNA
<213>Artificial Sequence
<220>
<223> Forward primer
<400> 3
tttactgttt tcgtaacagt tttg 24
<210>4
<211>21
<212>DNA
<213>Artificial Sequence
<220>
<223> Reverse primer
<400> 4
caacaacgca cagaatctag c 21