Note: Descriptions are shown in the official language in which they were submitted.
CA 02370241 2001-10-22
WO 00/64886 PCT/US00/10647
-1-
TETRAHYDROISOQUINOLINYL-INDOLE DERIVATIVES FOR THE
TREATMENT OF DEPRESSION
FIELD OF INVENTION
This invention relates to compounds which are useful for the treatment of
diseases affected by disorders of the serotonin-affected neurological systems,
such as
depression and anxiety. More specifically, the present invention is directed
to
dihydroisoquinolinyl-indole derivatives useful in the treatment of such
disorders.
BACKGROUND OF INVENTION
Pharmaceuticals which enhance neurotransmission of serotonin (5-HT) are
useful for the treatment of many psychiatric disorders, including depression
and
anxiety. The first generation of non-selective serotonin-affecting drugs
operated
through a variety of physiological functions which caused them to possess
numerous
undesired side-effects. The more recently prescribed drugs, the selective
serotonin
reuptake inhibitors (SSRIs), act predominately by inhibiting 5-HT, which is
released
at the synapses, from being actively removed from the synaptic cleft via a
presynaptic
serotonin transport carrier. Since SSRIs require several weeks before they
exert their
full therapeutic effect, this 5-HT blockade mechanism cannot fully account for
their
therapeutic activity. It is speculated that this two week induction, which
occurs
before a full antidepressant effect is observed, is due to the involvement of
the 5-
HT1A autoreceptors which suppress the firing activity of 5-HT neurons, causing
a
dampening of the therapeutic effect. Studies suggest that after several weeks
of SSRI
administration, a desensitization of the 5-HT autoreceptors occurs allowing a
full
antidepressant effect in most patients (see, e.g., LePaul et al., Arch.
Pharmacol.,
352:141 (1995)). Hence, it is believed that overriding this negative feedback
by
using SHT1A antagonists would potentially increase and accelerate the clinical
antidepressant response. Recent studies by Artigas et al., Trends Neurosci.,
19:378-
383 (1996) suggested a combination of 5-HT1A activity and inhibition of 5-HT
uptake within a single molecular entity can achieve a more robust and fast-
acting
antidepressant effect.
CA 02370241 2001-10-22
WO 00/64886 PCT/L1S00/10647
-2-
The present invention relates to a new class of molecules which have the
ability to act at the 5-HT1A autoreceptors and concommitantly with the 5-HT
transporter. Such compounds are therefore potentially useful for the treatment
of
depression as well as other serotonin disorders.
U.S. Patent No. 5,468,767 discloses a series of substituted indoles of the
following formula useful for the treatment of disorders associated with
dysfunction in
serotonergic neurotransmission, including depression.
wherein:
R, is hydrogen or C,~, alkyl; and
R.: is C,~ alkyl or (CHZ)PAr.
WO 9415928 discloses reports a series of piperazine derivatives of the
following formula for the treatment of CNS disorders, including depression.
R
n
R1-~ R2
R3
"'
wherein:
R is hydrogen or alkyl;
R, and RZ are each mono or bicyclic aryl or heteroaryl radicals;
R3 is hydrogen, alkyl, or a spirocycloalkyl group; and
n is 1 or 2; and m is 1 or 3.
CA 02370241 2001-10-22
WO 00/64886 PCT/US00/10647
-3-
WO 93/10092 discloses a series of substituted cyclohexenes of the following
formula for the treatment of dopaminergic disorders.
R2-( CH2) n / C H2)m-R1
wherein:
R' is aryl; 2-, 3- or 4 pyridinyl; 2-, 4- or 5- pyrimidinyl; 2-pyrazinyl; 2-or
3-thienyl;
2-or 3-furanyl; or 2-, 4- or 5-thiazolyl;
m is zero or an integer from 1 to 2;
Rz is
-N~R1
R1 or
~J
~\~OH
and
R1
n is zero or an integer of 1 to 4.
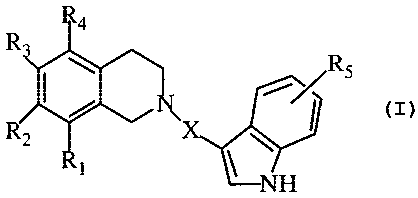
According to the present invention there is provided dihydroisoquinolino-
indole derivatives represented by Formula I:
I
wherein:
R,, R" R3 and RQ are, independently, hydrogen, halogen, alkoxy, or
carboxamide;
RS is hydrogen, halogen, CF3, CN, carbamide, or alkoxy; and
X is (CH2)~ or a 4-6-membered carbocyclic ring, wherein n is an integer of 2
to 4;
or pharmaceutically acceptable salts thereof.
CA 02370241 2001-10-22
WO 00/64886 PCT/US00/10647
-4-
Preferably, the compounds of the present invention are those of Formula I,
wherein:
R,, Rz, R3, and R4 are, independently, hydrogen or alkoxy;
RS is halogen or CN; and
X is (CHz)~ or a 6-membered carbocyclic ring, wherein n is an integer of 2 to
3;
or pharmaceutically acceptable salts thereof.
Most preferably, the compounds of the present invention are selected from:
3-[( 1,4-cis)-4-(7-Methoxy-3,4-dihydro-1 H-isoquinolin-2-yl)-cyclohexyl]-1 H-
indole-
5-carbonitrile;
3-[( 1,4-traps)-4-(7-Methoxy-3,4-dihydro-1H-isoquinolin-2-yl)-cyclohexyl]-1H-
indole-5-carbonitrile;
3-[( 1,4-cis)-4-(8-Methoxy-3,4-dihydro-1 H-isoquinolin-2-yl)-cyclohexyl]-1 H-
indole-
5-carbonitrile;
3-[(1,4-traps)-4-(8-Methoxy-3,4-dihydro-1H-isoquinolin-2-yl)-cyclohexyl]-1H-
indole-5-carbonitrile;
3-[( 1,4-cis)-4-(6-Methoxy-3,4-dihydro-1 H-isoquinolin-2-yl)-cyclohexyl]-1 H-
indole-
5-carbonitrile;
3-[( 1,4-traps)-4-(6-Methoxy-3,4-dihydro-1 H-isoquinolin-2-yl)cyclohexyl]-1 H-
indole-5-carbonitrile;
3-[( 1,4-cis)-4-(5-Methoxy-3,4-dihydro-1 H-isoquinolin-2-yl)-cyclohexyl]-1 H-
indole-
5-carbonitrile;
3-[( 1,4-traps)-4-(5-Methoxy-3,4-dihydro-1 H-isoquinolin-2-yl)-cyclohexyl]-1 H-
indole-5-carbonitrile;
3-[(1,4-cis)-4-(3,4-Dihydro-1H-isoquinolin-2-yl)-cyclohexyl]-1H-indole-
carbonitrile;
3-[( 1,4-traps)-4-(3,4-Dihydro-1 H-isoquinolin-2-yl)-cyclohexyl]-1 H-indole-
carbonitrile;
2-[3-(5-Fluoro-1H-indol-3-yl)-propyl]-5-methoxy-1,2,3,4-
tetrahydroisoquinoline;
2-[3-(5-Fluoro-1H-indol-3-yl)-propyl]-6-methoxy-1,2,3,4-
tetrahydroisoquinoline;
and
2-[3-(5-Fluoro-1H-indol-3-yl)-propyl]-1,2,3,4-tetrahydroisoquinoline.
CA 02370241 2001-10-22
WO 00/64886 PCT/US00/10647
-5-
As used herein, the term "alkoxy" includes both straight and branched alkyl
chains and preferably contains 1 to 6 carbon atoms, more preferably 1 to 4
carbon
atoms. Most preferably it is methoxy. The term "halogen" includes fluorine,
chlorine, bromine and iodine. The term "carbocyclic ring" means saturated or
unsaturated carbon rings such as aryl or cycloalkyl. Examples are phenyl and
cycloalkyl of 4 to 6 carbon atoms. Preferably, the carbocyclic ring is a
cycloalkyl of
4 to 6 carbon atoms. Most preferably, it is cyclohexyl.
The most preferred value of n is 3.
The compounds of Formula I also may be used in the form of a
pharmaceutically acceptable acid addition salt having the utility of the free
base.
Such salts, prepared by methods well known to the art are formed with both
inorganic
or organic acids, for example: fumaric, malefic, benzoic, ascorbic, pamoic,
succinic,
bismethylenesalicylic, methanesulfonic, ethanedisulfonic, acetic, oxalic,
propionic,
tartaric, salicyclic, citric, gluconic, lactic, malic, mandelic, cinnamic,
citraconic,
aspartic, stearic, palmitic, itaconic, glycolic, p-aminobenzoic, glutamic,
benzene-
sulfonic, hydrochloric hydrobromic, sulfuric, cyclohexylsulfamic, phosphoric
and
nitric acids.
The compounds of the present invention may be prepared by any suitable
method which will be recognized by those skilled in the art. However, the
present
compounds may be advantageously prepared according to Scheme I below.
CA 02370241 2001-10-22
WO 00/64886 PCT/US00/10647
-6-
Scheme 1
H R3
I ~ NHz ' \ N~CH3~ / I
\ NH
/ I / O Ri
R' R~ Rz O
Rz R
z
(1) (2)
Ra
a N Rs \ N
R3 / I / N
R, \ I NH ..~ \ I ~ R~ Rz
Rz '--N/H ~ H
Ex 1 R~=OMe Rz=R3=Ra=H
Ex 2 Rz=OMe R3=R~=Ra=H
Ex 3 R3=OMe R~=Rz=Ra=H
Ex 4 Ra=OMe R~=Rz=R3=H
Ex 5 Ri=Rz=R3=Ra=H
H C H3 C H3
y ~ I~ 1 p 1
iN / iN / NH
(5) (6)
R~ H
R~ Rz \
R \ ~- ~ ~ Br ~ I / N
I v
/ NH N I ~
H Ex 6 R~=OMe Rz=H
Ex 7 R~=H Rz=OMe
Ex 8 R~=Rz=H
Processes according to the above Scheme form a further aspect of the
invention. Specific exemplification of the preparation of representative
compounds
of this invention is provided in the following procedures.
INTERMEDIATE la
N-(Methoxycarbonyl)-2-(4-methoxyphenyl)ethylamine
To a solution of 4-methoxy-phenethylamine (5.81 mL, 39.7 mmol) in
anhydrous THF (200 mL) was added Et3N (6.6 mL, 48 mmol). The solution was
cooled to 0°C and methyl chloroformate (15.38 mL, 199 mmol) was added
slowly via
CA 02370241 2001-10-22
WO 00/64886 PCT/US00/10647
_7_
syringe. The reaction was stirred at 0°C for 2 hours and then allowed
to warm to
room temperature and stirred overnight. To the reaction mixture was added H20
(50
mL) and the resulting solution was extracted into Et20 ( 1 x 100 mL) and then
into
EtOAc (2 x 75 mL). The combined organic fractions were washed with brine (200
mL) and 1 M HCl (200 mL), dried over NazS04, filtered and concentrated under
vacuum, affording 7.98 g (96%) of the title compound as a pale yellow solid.
INTERMEDIATE lb
N-(Methoxycarbonyl)-2-(3-methoxyphenyl)ethylamine
This compound was prepared in the manner described for Intermediate la,
replacing 4-methoxy-phenethylamine with 3-methoxy-phenethylamine ( 10 g, 66
mmol) affording 13.6 g (98°l0) of the title compound as a gold oil.
INTERMEDIATE 2a
7-Methoxy-3,4-dihydro-2H-isoquinolin-1-one
To a stirred solution of polyphosphoric acid (SOg) at 145°C was
added N-
(methoxycarbonyl)-2-(4-methoxyphenyl)ethylamine (4.5 g, 21.5 mmol). The
resulting brown solution was stirred at 140°C for 40 min. The hot
reaction mixture
was poured onto ice, and extracted in CH2Cl2 (3 X 200 mL). The organic
fractions
were combined, dried over Na2S04, filtered, and concentrated. The crude oil
was
purified by column chromatography with 10 % MeOH/ CH2C12 as the eluent,
affording 1.11 g (30°Io) of the title compound as a pale yellow solid:
mp 90-93°C.
Elemental Analysis for C,oH"NOz:
Calc'd: C, 67.78; H, 6.26; N, 7.90
Found: C, 67.88; H, 6.54; N, 7.90
INTERMEDIATE 2b
6-Methoxy-3,4-dihydro-2H-isoquinolin-1-one
This compound was prepared in the same manner as Intermediate 2a, replacing
N-(methoxycarbonyl)-2-(4-methoxyphenyl)ethylamine with N-(methoxycarbonyl)-2-
(3-
CA 02370241 2001-10-22
WO 00/64886 PCT/US00/10647
_g_
methoxyphenyl)ethylamine (6 g, 28.7 mmol) affording 2.42 g (48%) of the title
compound as a white solid: mp 131-133 °C.
Elemental Analysis for C,oH"NOz
Calc'd: C, 67.78; H, 6.26; N, 7.90
Found: C, 67.55; H, 6.36; N, 7.86
INTERMEDIATE 2c
8-Methoxy-3,4-dihydro-2H-isoquinolin-1-one
This compound was isolated at the same time as Intermediate 2b, affording
0.67 g (13%) as a white solid: mp 140-142°C.
Elemental Analysis for C,oH"NO~~O.1H20
Calc'd: C, 67.10; H, 6.31; N, 7.82
Found: C, 66.92; H, 6.49; N, 7.70
INTERMEDIATE 3a
7-Methoxy-1,2,3,4-tetrahydro-isoquinoline
A solution of Intermediate 2a ( 1 g, 5.6 mmol) dissolved in anhydrous THF
(30 mL) was dripped slowly into a LAH solution (7.35 mL, 1 M in THF) at
0°C under
a N2 atmosphere. The resulting solution was heated at reflux for 2 hours. The
reaction mixture was cooled to 0°C and H20 was added dropwise to
quench. The
resulting solution was basified with 1 M NaOH ( 100 mL), filtered through a
bed of
celite, and extracted into EtOAc (3 X 150 mL). The organic fractions were
combined, dried over Na2S04, filtered and concentrated yielding 0.88 g (96%)
of a
pale yellow oil. The HCl salt was made in EtOAc affording a white solid: mp
224-
227°C.
Elemental Analysis for C,oH,3N~HCl
Calc'd: C, 60.15; H, 7.07; N, 7.01-
Found: C, 60.11; H, 7.12; N, 6.87
CA 02370241 2001-10-22
WO 00/64886 PCT/US00/10647
-9-
INTERMEDIATE 3b
6-Methoxy-1,2,3,4-tetrahydro-isoquinoline
This compound was prepared in the same manner as Intermediate 3a by
replacing Intermediate 2a with Intermediate 2b ( 1 g, 5.6 mmol) affording 0.87
g
(95%) of the title compound as a pale yellow oil. The HCl salt was made in
EtOAc
affording a white solid: mp 236-238 °C.
Elemental Analysis for C,oH,3N~HCl
Calc'd: C, 60.15; H, 7.07; N, 7.01
Found: C, 60.12; H, 6.99; N, 6.82
INTERMEDIATE 3c
8-Methoxy-1,2,3,4-tetrahydro-isoquinoline
This compound was prepared in the same manner as Intermediate 3a by
replacing Intermediate 2a with Intermediate 2c (0.600 g, 3.4 mmol) affording
0.41 g
(74%) of the title compound as a pale yellow oil. The HCl salt was made in
EtOAc
affording a white solid: mp dec >210°C.
Elemental Analysis for C,oH,3N~HCl
Calc' d: C, 60.15; H, 7.07; N, 7.01
Found: C, 59.98; H, 6.97; N, 6.83
INTERMEDIATE 5
5-Methoxy-isoquinoline
To an oven dry three neck flask was added 5-hydroxy-quinoline (5 g, 34.5
mmol), and triphenylphosphine. The solids were dissolved in THF ( 100 mL) and
DEAD (8.19 mL, 51.7 mmol) was added slowly. The maroon reaction mixture was
stirred at room temperature overnight and then poured into H20 (100 mL) and
extracted into EtOAc (3 x 150 mL). The combined organic fractions were dried
over
Na2S04, filtered, concentrated, and purified by column chromatography using 20
%
EtOAc/hexanes as the eluent. The title compound was isolated as 3.5 g (64%) of
a
white solid.
CA 02370241 2001-10-22
WO 00/64886 PCT/US00/10647
- 10-
INTERMEDIATE 6
5-Methoxy-1,2,3,4-tetrahydro-isoquinoline
To a Parr hydrogenation flask was added PtOZ ( 165 mg) and the solid was
purged with N2 for 10 minutes. A solution of 5-methoxy-isoquinoline in HOAc
(40
mL) was added to the flask and hydrogenated at 40 psi overnight. The resulting
solution was filtered through celite, concentrated and basified with 1 M NaOH
(100
mL) and extracted into EtOAc (3 x 150 mL). The organic fractions were
combined,
dried over Na2S04, filtered, concentrated and purified by column
chromatography
with 10%MeOH/CH2CH2/NH40H as the eluent. The title compound was obtained
as 2.49 g (70%) of a white solid: mp 124-126; MS EI m/e 163 M+.
EXAMPLE la
3-[(1,4-cis)-4-(7-Methoxy-3,4-dihydro-1H-isoquinolin-2-yl)-cyclohexyl)-1H
indole-5-carbonitrile
To a solution of 7-methoxy-1,2,3,4-tetrahydro-isoquinoline (500 mg,
3.lmmol),4-(5-cyano-1H-3-indolyl)-cyclohexanone (730 mg, 3.1 mmol), and sodium
triacetoxyborohydride (975 mg, 4.6 mmol) in dichloroethane (50 mL) was added
acetic acid (0.35 mL, 6.1 mmol) and the mixture was stirred overnight at room
temperature. The reaction was quenched with 1 M NaOH ( 100 mL) and extracted
in
CH2Cl2 (3 x 100 mL) and EtOAc (2 x 100 mL). The organic fractions were
combined, dried over NazS04, concentrated, filtered and chromatographed (5%
MeOH/EtOAc) yielding 480 mg (40%) of the cis isomer as a gold oil. The HCl
salt
was generated from EtOAc yielding a pale yellow solid: mp dec > 145 °C.
Elemental Analysis for CZSHz,N30~HCl~0.25H20
Calc'd: C, 70.41; H, 6.74; N, 9.85
Found: C, 69.91; H, 6.69; N, 9.75
CA 02370241 2001-10-22
WO 00/64886 PCT/US00/10647
-11-
EXAMPLE lb
3-[(1,4-trans)-4-(7-Methoxy-3,4-dihydro-1H-isoquinolin-2-yl)-cyclohexyl]-1H
indole-5-carbonitrile
The trans isomer was isolated at the same time as Example la, affording 320
mg (27%) as a pale yellow solid: mp dec > 140°C.
Elemental Analysis for CZSH2,N30~0.25Hz0
Calc'd: C, 76.99; H, 7.11; N, 10.77
Found: C, 76.79; H, 7.09; N, 10.50
EXAMPLE 2a
3-[(1,4-cis)-4-(8-Methoxy-3,4-dihydro-1H-isoquinolin-2-yl)-cyclohexyl]-1H
indole-5-carbonitrile
This compound was prepared in the same manner as Example la replacing
7-methoxy-1,2,3,4-tetrahydro-isoquinoline with 8-methoxy-1,2,3,4-tetrahydro-
iso-
quinoline (300 mg, 1.85 mmol) to afford 300 mg (44%) of the title compound as
a
yellow oil. The HCl salt was prepared from EtOAc yielding a white solid: mp
dec
>155°C.
Elemental Analysis for CZSHZ,N30~HCl~0.50H20
Calc'd: C, 69.67; H, 6.78; N, 9.75
Found: C, 69.91; H, 6.77; N, 9.89
EXAMPLE 2b
3-[(1,4-trans)-4-(8-Methoxy-3,4-dihydro-1H-isoquinolin-2-yl)-cyclohexyl]-1H
indole-5-carbonitrile
The trans isomer was isolated at the same time as the cis isomer of Example
2a, affording 200 mg (28%) as a pale yellow solid. The HCl salt was generated
from
EtOAc affording a white solid: mp dec > 250°C.
Elemental Analysis for CZSHz,N30~HCI~0.25H20
Calc' d: C, 70.41; H, 6.74; N, 9.85
Found: C, 70.48; H, 6.65; N, 9.69
CA 02370241 2001-10-22
WO 00/64886 PCT/US00/10647
-12-
EXAMPLE 3a
3-[(1,4-cis)-4-(6-Methoxy-3,4-dihydro-1H-isoquinolin-2-yl)-cyclohexyl]-1H
indole-5-carbonitrile
This compound was prepared in the same manner as Example 1 a, replacing
7-methoxy-1,2,3,4-tetrahydro-isoquinoline with 6-methoxy-1,2,3,4-tetrahydro-
isoquinoline (500 mg, 3.1 mmol) to afford 520 mg (44%) of the title compound
as a
yellow oil. The HCl salt was prepared from EtOAc yielding a white solid: mp
dec
> 180 °C.
Elemental Analysis for CZSHZ,N30~HCl~0.25H20
Calc'd: C, 70.41; H, 6.74; N, 9.85
Found: C, 70.21; H, 6.80; N, 9.63
EXAMPLE 3b
3-[(1,4-trans)-4-(6-Methoxy-3,4-dihydro-1H-isoquinolin-2-yl)-cyclohexyl]-1H-
indole-5-carbonitrile
The trans isomer was isolated at the same time as the cis isomer of Example
3a, affording 250 mg (21%) as a pale yellow solid: mp dec > 200°C.
Elemental Analysis for C2SHz,N30~0.25H20
Calc'd: C, 76.99; H, 7.11; N, 10.77
Found: C, 76.81; H, 7.08; N, 10.56
EXAMPLE 4a
3-[(1,4-cis)-4-(5-Methoxy-3,4-dihydro-1H-isoquinolin-2-yl)-cyclohexyl]-1H
indole-5-carbonitrile
This compound was prepared in the same manner as Example la, replacing
7-methoxy-1,2,3,4-tetrahydro-isoquinoline with 5-methoxy-1,2,3,4-tetrahydroiso-
quinoline (500 mg, 3.1 mmol) to afford 400 mg (34%) of the title compound as a
pale
yellow solid: mp 223-226 °C.
Elemental Analysis for CZSHz,N30~0.85H20
Calc'd: C, 74.91; H, 7.22; N, 10.48
Found: C, 75.30; H, 7.15; N, 10.08
CA 02370241 2001-10-22
WO 00/64886 PCT/US00/10647
-13-
EXAMPLE 4b
3-[(1,4-trans)-4-(5-Methoxy-3,4-dihydro-1H-isoquinolin-2-yl)-cyclohexyl]-1H
indole-S-carbonitrile
The trans isomer was isolated at the same time as the cis isomer of Example
4a affording 200 mg (17%) as a white solid. The HCl salt was generated in
EtOAc
yielding a white solid: mp dec > 181°C.
Elemental Analysis for CZSHZ,N30~HCl~1H20
Calc'd: C, 68.25; H, 6.87; N, 9.55
Found: C, 68.23; H, 6.64; N, 9.33
EXAMPLE 5a
3-[(1,4-cis)-4-(3,4-Dihydro-1H-isoquinolin-2-yl)-cyclohexyl]-1H-indole-
carbonitrile
This compound was prepared in the same manner as Example la replacing
7-methoxy-1,2,3,4-tetrahydro-isoquinoline with commercially available 1,2,3,4-
tetra-
hydro-isoquinoline (250 mg, 2.1 mmol) to afford 330 mg (44%) of the title
compound as a yellow solid. The HCl salt was generated from EtOAc yielding an
off
white solid: mp 188-191 °C.
Elemental Analysis for CZQHz5N3~HCl~0.75Hz0
Calc'd: C, 71.10; H, 6.84; N, 10.36
Found: C, 71.37; H, 6.82; N, 9.89
EXAMPLE Sb
3-[(1,4-trans)-4-(3,4-Dihydro-1H-isoquinolin-2-yl)-cyclohexyl]-1H-indole-
carbonitrile
The trans isomer was isolated at the same time as the cis isomer of Example
Sa affording 250 mg (21 %) as a pale yellow solid: mp 196-200 °C.
Elemental Analysis for Cz,H25N3~0.25Hz0
Calc'd: C, 80.08; H, 7.14; N, 11.67
Found: C, 80.01; H, 7.14; N, 11.37
CA 02370241 2001-10-22
WO 00/64886 PCT/US00/10647
- 14-
EXAMPLE 6
2-[3-(S-Fluoro-1H-indol-3-yl)-propyl]-5-methoxy-1,2,3,4-tetrahydroisoquinoline
A solution of 5-methoxy-1,2,3,4-tetrahydro-isoquinoline (500 mg, 3.1 mmol),
3-(5-fluoro-indolyl)-propylbromide (604 mg, 2.36 mmol) and Et3N (0.86 mL, 6.2
mmol) was dissolved in DMSO (20 mL) and heated at 100°C for 5 hours
then at room
temperature overnight. The reaction was poured into H20 ( 150 mL) and
extracted
into EtOAc (2 x 100 mL). The organic fractions were combined and washed with
NaHC03 (150 mL), dried over Na2S04, filtered, and concentrated. The resulting
oil
was purified by column chromatography using 5°lo MeOH/EtOAc as the
eluent
yielding 570 mg (71 %) of a gold oil. The HCl salt was generated from EtOAc
affording a white solid: mp 183-186°C.
Elemental Analysis for CZ,H23FNz0~HCl
Calc'd: C, 67.28; H, 6.45; N, 7.47
Found: C, 67.00; H, 6.52; N, 7.30
EXAMPLE 7
2-[3-(5-Fluoro-1H-indol-3-yl)-propyl]-6-methoxy-1,2,3,4-tetrahydroisoquinoline
This compound was prepared in the same manner as Example 6, replacing
5-methoxy-1,2,3,4-tetrahydro-isoquinoline with 6-methoxy-1,2,3,4-tetrahydroiso-
quinoline ( 190 mg, 1.16 mmol) affording the title compound as a brown oil.
The HCl
salt was generated from EtOAc yielding a hygroscopic tan solid: mp dec > 90
°C.
Elemental Analysis for CZ,H23FNZ0~HCl~HZO
Calc' d: C, 64.20; H, 6.67; N, 7.13
Found: C, 64.18; H, 6.51; N, 6.90
EXAMPLE-8
2-[3-(5-Fluoro-1H-indol-3-yl)-propyl]-1,2,3,4-tertrahydroisoquinoline
This compound was prepared in the same manner as Example 6, replacing
5-methoxy-1,2,3,4-tetrahydro-isoquinoline with commercial 1,2,3,4-tetrahydro-
CA 02370241 2001-10-22
WO 00/64886 PCT/US00/10647
-15-
isoquinoline (500 mg, 4.2 mmol) affording 730 mg as a waxy orange solid. The
HCl
salt was generated from EtOAc yielding a pale yellow solid: mp 235-
238°C.
Elemental Analysis for CZOH2,FN2~HCl
Calc'd: C, 69.66; H, 6.43; N, 8.12
Found: C, 69.55; H, 6.34; N, 7.84
The activity of the present compounds is demonstrated by the following
standard pharmacological test procedures.
The PCR cloning of the human 5-HT,A receptor subtype from a human
genomic library has been described previously by Chanda et al., Mol.
Pharmacol.,
43:516 (1993). A stable Chinese hamster ovary cell line expressing the human 5-
HT,A
receptor subtype (5-HT,A.CHO cells) was employed throughout this study. Cells
were
maintained in DMEM supplemented with 10% fetal calf serum, non-essential amino
acids and penicillin/ streptomycin.
Cells were grown to 95-100% confluency as a monolayer before membranes
were harvested for binding studies. Cells were gently scraped from the culture
plates,
transferred to centrifuge tubes, and washed twice by centrifugation (2000 rpm
for 10
min., 4°C) in buffer (50 mM Tris; pH 7.5). The resulting pellets were
aliquoted and
maintained at -80°C. On the day of assay, the cells were thawed on ice,
and
resuspended in buffer. Studies were conducted using [3HJ8-OH-DPAT as the
radioligand. The binding assay was performed in 96 well microtiter plates in a
final
total volume of 250 ~,L of buffer. Comparison experiments were performed by
using
7 concentrations of unlabelled drug and a final ligand concentration of 1.5 nM
. Non-
specific binding was determined in the presence of 10 ~.M SHT. Saturation
analysis
was conducted by using [3H]8-OH-DPAT at concentrations ranging from 0.3-30 nM.
Following a 30 minute incubation at room temperature, the reaction was
terminated
by the addition of ice cold buffer and rapid filtration using a M-96 Brandel
Cell
Harvester (Gaithersburg, MD) through a GFB filter presoaked for 30 minutes in
0.5% polyethyleneimine.
CA 02370241 2001-10-22
WO 00/64886 PCT/US00/10647
- 16-
A protocol similar to that used by Cheetham et al., Neuropharmacol. , 32:737
(1993) was used to determine the affinity of compounds for the serotonin
transporter.
Briefly, frontal cortical membranes prepared from male Sprague-Dawley rats
were
incubated with'H-paroxetine (0.1 nM) for 60 min at 25°C. All tubes also
contained
either vehicle, test compound (one to eight concentrations), or a saturating
concentration of fluoxetine (10 p,M) to define specific binding. All reactions
were
terminated by the addition of ice cold Tris buffer followed by rapid
filtration using a
Tom Tech filtration device to separate bound from free 3H-paroxetine. Bound
radioactivity was quantitated using a Wallac 1205 Beta Plate~ counter.
Nonlinear
regression analysis was used to determine ICSO values which were converted to
Ki
values using the method set forth in Cheng and Prusoff, Biochem. Pharmacol.,
22:3099 (1973) (Ki = IC50/((Radioligand conc.)/(1 + KD)).
The [35S]-GTFyS binding assay was similar to that used by Lazareno and
Birdsall, Br. J. Pharmacol. 109:1120 (1993). Briefly, 5-HT,A cloned receptor
membrane fragments (as used for 5-HT,A receptor binding assays) were stored at
-70°C. When needed, membranes were rapidly thawed, centrifuged at
40,000 x g for
10 minutes and resuspended at 4°C for 10 minutes in assay buffer (25 mM
HEPES,
3 mM MgCl2, 100 mM NaCI, 1 mM EDTA, 10 uM GDP, 500 mM DTT, pH 8.0).
These membranes were then incubated for 30 min at 30 °C with [35S]GTPgS
(1 nM) in
the presence of vehicle, test compound (one to eight concentrations), or
excess 8-OH-
DPAT to define maximum agonist response. All reactions were terminated by the
addition of ice cold Tris buffer followed by rapid filtration using a Tom
Tech~
filtration device to separate bound from free [35S]GTPgS. Agonists produced an
increase in the amount of [35S]GTPgS bound whereas antagonists produced no
increase in binding. Bound radioactivity was counted and analyzed as above.
The following assays were performed by incubating the cells with DMEM
containing 25 mM HEPES, 5 mM theophylline and 10 ~,M pargyline for a period of
20 minutes at 37°C. Functional activity was assessed by treating the
cells with
CA 02370241 2001-10-22
WO 00/64886 PCT/US00/10647
-17-
forskolin (1 uM final concentration) followed immediately by test compound (6
concentrations) for an additional 10 min at 37°C. In separate
experiments, 6
concentrations of antagonist were preincubated for 20 min prior to the
addition of
nM 8-OH-DPAT and forskolin. The reaction was terminated by removal of the
5 media and addition of 0.5 ml ice cold assay buffer. Plates were stored at -
20°C prior
to assessment of cAMP formation by a cAMP SPA assay (Amersham).
TABLE A
Example No. 5-HT,A ST GTPgS ED50
(K;, nM,) (%EMax)
[Ki, nM: (% inh @
1 uM)]
la (26 %) 0.8
lb (48 %) 12
2a (14 %) 1.0
2b (46 %) 7.5
3a (21 %) 0.1
3b 300 8.0 (0 %)
4a (7 %) 0.61 (12 %)
4b (47 %) 12.0 350 (33 %)
Sa (0 %) 1.3
Sb (10 %) 10.0
6 243 1.9 1346 (23 %)
7 288 1.4 (100 %)
8 (48 %) 4.85 (13 %)
As demonstrated by the results set forth above, the compounds of the present
invention are active towards SHT,A receptors and generally elevate serotonin
levels
by inhibiting 5-HT transport. Accordingly, the present compounds should be
useful
in treating disorders related to defects in serotonin concentration.
The compounds of formula I for use in methods of treatment or therapy form
further aspects of the present invention.
CA 02370241 2001-10-22
WO 00/64886 PCT/US00/10647
- 18-
The compounds of this invention may be administered orally or parenterally,
neat or in combination with conventional pharmaceutical carriers. Applicable
solid
Garners can include one or more substances which may also act as flavoring
agents,
lubricants, solubilizers, suspending agents, fillers, glidants, compression
aids, binders
or tablet-disintegrating agents or an encapsulating material. In powders, the
Garner is
a finely divided solid which is in admixture with the finely divided active
ingredient.
In tablets, the active ingredient is mixed with a carrier having the necessary
compression properties in suitable proportions and compacted in the shape and
size
desired. The powders and tablets preferably contain up to 99% of the active
ingredient. Any of the solid Garners known to those skilled in the art may be
used
with the compounds of this invention. Particularly suitable solid Garners
include, for
example, calcium phosphate, magnesium stearate, talc, sugars, lactose,
dextrin,
starch, gelatin, cellulose, methyl cellulose, sodium carboxymethyl cellulose,
polyvinylpyrrolidone, low melting waxes and ion exchange resins.
Liquid Garners may be used in preparing solutions, suspensions, emulsions,
syrups and elixirs of the compounds of this invention. The compounds of this
invention can be dissolved or suspended in a pharmaceutically acceptable
liquid
carrier such as water, an organic solvent, a mixture of both or
pharmaceutically
acceptable oils or fat. The liquid Garner can contain other suitable
pharmaceutical
additives such as solubilizers, emulsifiers, buffers, preservatives,
sweeteners,
flavoring agents, suspending agents, thickening agents, colors, viscosity
regulators,
stabilizers or osmo-regulators. Suitable examples of liquid Garners for oral
and
parenteral administration include water (particularly containing additives as
above,
e.g., cellulose derivatives, preferably sodium carboxymethyl cellulose
solution),
alcohols (including monohydric alcohols and polyhydric alcohols, e.g.,
glycols) and
their derivatives and oils (e.g., fractionated coconut oil and arachis oil).
For
parenteral administration, the carrier can also be an oily ester such as ethyl
oleate and
isopropyl myristate. Sterile liquid carriers are used in compositions for
parenteral
administration.
Liquid pharmaceutical compositions which are sterile solutions or
suspensions can be utilized by, for example, intramuscular, intraperitoneal or
subcutaneous injection. Sterile solutions can also be administered
intravenously.
Compositions for oral administration may be either liquid or solid composition
form.
CA 02370241 2001-10-22
WO 00/64886 PCT/US00/10647
- 19-
Preferably, the pharmaceutical compositions containing the compounds of this
invention are in unit dosage form, e.g., tablets or capsules. In such form,
the
compositions may be sub-divided in unit doses containing appropriate
quantities of
the present compounds. The unit dosage forms can be packaged compositions, for
example, packeted powders, vials, ampoules, prefilled syringes or sachets
containing
liquids. Alternatively, the unit dosage form can be, for example, a capsule or
tablet
itself, or it can be the appropriate number of any such compositions in
package form.
Pharmaceutical compositions comprising compounds of formula I and a
pharmaceutically acceptable carrier form a further aspect of the present
invention.
The therapeutically effective amount of the compounds of this invention that
is administered and the dosage regimen depends on a variety of factors,
including the
weight, age, sex, and medical condition of the subject, the severity of the
disease, the
route and frequency of administration, and the specific compound employed, and
thus
may vary widely. However, it is believed that the pharmaceutical compositions
may
contain the compounds of this invention in the range of about 0.1 to about
2000 mg,
preferably in the range of about 0.5 to about 500 mg and more preferably
between
about 1 and about 100 mg. Projected daily dosages of active compound are about
0.01 to about 100 mg/kg body weight. The daily dose can be conveniently
administered two to four times per day.
The present invention may be embodied in other specific forms without
departing from the spirit and essential attributes thereof and accordingly,
reference
should be made to the appended claims, rather than to the foregoing
specification, as
indicating the scope of the invention.