Note: Descriptions are shown in the official language in which they were submitted.
CA 02370739 2001-10-16
WO 00/65347 PCT/US00/11207
1
MULTIPLE DIAGNOSTIC DEVICE FOR A WOMAN'S HEALTH
FIELD OF THE INVENTION
The present invention relates to a multiple diagnostic device which detects
two or
more types of hormones related to a woman's menstrual cycle, ovulation, pre-
menstrual
syndrome and pregnancy. Also, the multiple diagnostic device may be placed
within or in
a conventional type of feminine protection absorbent article and an
incontinent absorbent
article. The multiple diagnostic device may also be used to detect one or more
types of
bacteria, virus and other known disease indicators.
BACKGROUND OF THE INVENTION
Today, disposable articles, such as absorbent articles, adult incontinence
briefs,
sanitary napkins and tampons, are widely used in infant and toddler care and
in the care of
incontinent or menstruating adults as a means of containing, isolating and
disposing of
bodily wastes. These articles have generally replaced reusable, washable cloth
garments
as the preferred means for these applications because of their convenience and
reliability.
The disposable articles respond to a defecation, urination or discharge event
by absorbing
or containing bodily wastes deposited on the article. Some disposable articles
also signal
a defecation, urination or discharge event after it has occurred (e.g.,
wetness indicators,
temperature change detection). Other disposable absorbent articles known in
the art
comprise a chemically reactive means to detect various substances in the
wearer's
waste(s). However, none of these specifically detect target potentially
pathogenic
microorganisms such as bacteria, viruses, fungi, and parasites (e.g.,
protozoans) and/or
related biomolecules, all of which require a high degree of selectivity (i.e.,
specificity) and
sensitivity versus purely chemical agents. Additionally, the articles do not
predict when a
health-related event is about to occur and signal wearer or caregiver that
prophylactic or
remedial action is required prior to the onset of clinically observable
symptoms.
CA 02370739 2001-10-16
WO 00/65347 PCT/US00/11207
2
SUMMARY OF THE INVENTION
Accordingly, the invention provides a multiple diagnostic device for a woman's
health comprising a biosensor being able to detect multiple target analytes in
a woman's
bodily fluids and on or through the skin, wherein the analytes are germane to
a women's
health. The bodily fluids consist of vaginal secretions, menses, blood, urine
and saliva.
Preferably, the biosensor comprises at least one bio-recognition element for
use in
detecting multiple biological analytes.
As previously stated, biological analytes tested are germane to a woman's
health
indicating at least one of the following including 1) the onset of
menstruation, 2) the
presence of ovulation, 3) the presence of a sexually transmitted disease, 4)
the state of
pregnancy, 5) the presence of infection, 6) the presence of hormone
fluctuations, 7)
ovarian reserve, 8) the presence of menopause, 9) the presence of
osteoporosis, 10) the
presence of an iron deficiency, 11) electrolyte balance, 12) nutritional
status, 13) stress
level and 14) combinations thereof.
Also preferably, an absorbent article may comprise the multiple diagnostic
device
of the present invention. The absorbent article is a conventional one known in
the art
having a topsheet, a backsheet joined to the topsheet, and an absorbent core
positioned
between the topsheet and the backsheet. The topsheet has a top surface facing
a user and
a bottom surface facing away from a user. The multiple diagnostic device may
be
positioned on the top surface of the topsheet. The multiple diagnostic device
may be
positioned between the topsheet and the backsheet of the absorbent article.
Preferably,
the multiple diagnostic device will be positioned adjacent to the bottom
surface of the
topsheet and above the absorbent core. The multiple diagnostic device may also
be
placed within the absorbent core of the absorbent article.
Ideal absorbent articles for the invention herein include one selected from
the
group consisting of a sanitary napkin, an interlabial device, a tampon, a
patch, a liquid
collection device, an incontinent device and combinations thereof.
CA 02370739 2001-10-16
WO 00/65347 PCT/US00/11207
3
BRIEF DESCRIPTIONS OF THE DRAWINGS
While the specification concludes with claims particularly pointing out and
distinctly claiming the subject matter which is regarded as forming the
present invention,
it is believed that the invention will be better understood from the following
descriptions
which are taken in conjunction with the accompanying drawings in which like
designations are used to designate substantially identical elements, and in
which:
DETAILED DESCRIPTION OF THE INVENTION
As used herein, the term "sanitary napkin" or "napkin" refers to an absorbent
article which is worn by females adjacent to the pudendal region, generally
external to the
urogenital region, and which is intended to absorb and contain menstrual
fluids and other
vaginal discharges from the wearer's body (e.g., blood, menses, and urine). As
used
herein, the term "pudendal" refers to the externally visible female genitalia.
It should be
understood, however, that the present invention is also applicable to other
feminine
hygiene or catamenial pads such as pantiliners, or other absorbent articles
such as
incontinence pads, and the like. By the term "zone of menses insult" it is
meant herein
that area on the sanitary napkin most likely to consistently receive a menses
discharge
from a female wearer.
Non-limiting examples of panty liners and sanitary napkins which may be
provided with a diagnostic device include those manufactured by The Procter &
Gamble
Company of Cincinnati, Ohio as: ALWAYS~ Pantiliners with DriWeave~
manufactured according to U.S. Patent Nos. 4,324,246; 4,463,045; and
6,004,893;
ALWAYS~ Ultrathin Slender Maxi with Wings manufactured according to U.S.
Patent
Nos. 4,342,314, 4,463,045, 4,556,146, Bl 4,589,876, 4,687,478, 4,950,264,
5,009,653,
5,267,992, and Re. 32,649; ALWAYS~ Regular Maxi; ALWAYS~ Ultra Maxi with
Wings; ALWAYS~ Maxi with Wings; ALWAYS~ Ultra Long Maxi with Wings;
ALWAYS~ Long Super Maxi with Wings; and ALWAYS~ Overnight Maxi with
CA 02370739 2001-10-16
WO 00/65347 PCT/US00/11207
4
Wings. An example of a panty liner with a diagnostic device is shown in Figs.
1 and lA.
An example of a sanitary napkin with a diagnostic device is shown in Fig. 2.
Non-limiting examples of tampons which may be provided with a diagnostic
device, and applicators therefor, are described in U.S. Patent Nos. 4,726,805;
4,846,802;
4,960,417; 5,087,239; 5,279,541; 5,346,468; 5,348,534; 5,531,674; and
5,566,435. In
addition, the diagnostic device could also be placed on a digitally insertable
tampon. An
example of a tampon with a diagnostic device is shown in Fig. S.
The cyclic nature of the hormones of the menstrual cycle (i.e., the full 28
day
cycle) make them useful in understanding fertility and, in general, the
position of an
individual during her cycle. This goes beyond current uses of hormones to
predict
ovulation and pregnancy. For example, progesterone peaks and then drops just
prior to
menstruation. Estrogen also declines just prior to menstruation. Thus, in
combination,
assay for these two hormones will allow reliable prediction of the onset and
the presence
of menstruation. The timing of the peak of these hormones, along with their
subsequent
drop, may allow an almost daily marking of the time before menstruation.
Similar examples may be developed for other points of interest in the cycle.
Follicle Stimulating Hormone (FSH) exhibits a peak about one week prior to
ovulation,
giving more advance timing for pregnancy planning than assays for the
luteinizing
hormone, which exhibits a sharp peak at the time of ovulation. Two to three
days of
fertility may be missed when relying only on assay of the luteinizing hormone.
Thus, in a
diagnostic for ovulation herein, it is highly preferred to measure for both
the Follicle
Stimulating Hormone and the luteinizing hormone along with estrogen.
A rise in Follicle Stimulating Hormone to a near constant amount signals the
approach of menopause. This may be of use in planning healthy approaches to
menopause, such as Hormone Replacement Therapy, nutritional changes, and
checks for
osteoporosis.
CA 02370739 2001-10-16
WO 00/65347 PCT/US00/11207
Thus, a diagnostic testing for multiple hormones presents a much broader and
more useful information set to a woman than single tests of individual cycle
information
proposed by the prior art. Technologies which allow multiple analyte assays
are thus of
potential use in this invention proposed by the prior art. These include, but
are not limited
to, antibody labeled microbeads, Silas TM surface analysis, or membrane based
biosensors.
SILASTM or SILICON Assay Surface Technology is a proven method for the
detection of specific target molecules. This thin film based technology has
successfully
been used for the development of diagnostic tests to detect bacterial and
viral antigens
from Group A Streptococcus, Group B Streptococcus, Chlamydia, and Influenza A
and B
(Optical Immunoassay (OIA~)).
The wafer consists of a silicon support with an optical coating and attachment
layer. This wafer surface technology enables the direct visual detection of a
physical
change in the optical thickness of molecular thin films. This change in
thickness is due to
the specific capture of an analyte on the surface. When a substrate is added,
this binding
event is amplified and again increases the surface thickness of the molecular
thin film.
This change in thickness alters the reflected light path and is visually
perceived as a color
change. Slight changes in optical thickness produce a distinct visible color
change. A
positive result appears as a purple spot on the predominant gold background.
When a
target is not present in the sample, no binding takes place. Therefore, the
optical
thickness remains unchanged and the surface retains the original gold color
indicating a
negative result.
Thus, in a first aspect, the invention features a device for detecting the
amount or
presence of an analyte of interest. The device includes a substrate which has
an optically
active surface exhibiting a first color in response to light impinging
thereon. This first
color is defined as a spectral distribution of the emanating light. The
substrate also
exhibits a second color which is different from the first color (by having
combination of
wavelengths of light which differ from that combination present in the first
color, or
having a different spectral distribution, or by having an intensity of one or
more of those
CA 02370739 2001-10-16
WO 00/65347 PCT/US00/11207
6
wavelengths different from those present in the first color). The second color
is exhibited
in response to the same light when the analyte is present on the surface.
An "optically active surface" is a surface that participates in the generation
of an
optical effect such that the light impinging upon that surface is in some way
altered. Such
optically active surfaces may be adapted to respond not only to polychromatic
light (e.g.,
white light) but also to mono-chromatic light (e.g., laser light, which may be
inherently
polarized). Devices of this invention preferably produce a color signal that
strongly
contrasts the background interference color of the unreacted test surface and
a reacted
surface.
Specifically, the invention features similar devices in which the substrate
has an
attachment layer formed from a chemical selected from the group consisting of
dendrimers, star polymers, molecular self assembling polymers, polymeric
siloxanes, and
film forming latexes; the substrate itself is formed from a material selected
from the group
consisting of monocrystalline silicon, a amorphous silicon on glass, amorphous
silicon on
plastic, a ceramic, polycrystalline silicon, and composites of these
materials; and the
substrate may have an optical thin film formed from a material selected from
the group
consisting of silicon nitride, silicon/silicon dioxide composites, titanates,
diamond, oxides
of zirconium, and silicon carbide.
The substrate is selected from the group consisting of glass, and plastic,
comprising a layer of amorphous silicon on its surface, whereby an optically
active
surface is produced; the optically active surface includes monocrystalline
silicon or
metal; the substrate in metal further having a layer of amorphous silicon; a
receptor layer
receptive to an analyte is provided with a specific binding partner for the
analyte; the
receptor layer is formed from material selected from the group consisting of
antigens,
antibodies, oligonucleotides, chelators, enzymes, bacteria, bacterial pili,
bacterial flagellar
materials, nucleic acids, polysaccharides, lipids, proteins, carbohydrates,
metals, viruses,
hormones, and receptors for said materials; and the first color is golden in
appearance and
the second color is purple or blue in appearance to the eye.
CA 02370739 2001-10-16
WO 00/65347 PCT/US00/11207
7
In another related aspect, the invention features a method for detecting an
analyte
of interest in a sample, by the steps of providing a thin film optical
immunoassay device
having a substrate, having an upper and a lower surface, and supporting on its
upper
surface, an unlabeled antibody layer bound to the substrate, at least one
layer containing
the analyte from the sample, the analyte containing layer supporting at least
one layer
having an enzyme conjugate complex with the analyte; contacting the enzyme
conjugate
with a precipitating agent; incubating for a time period sufficient to cause
precipitation of
product from interaction of the precipitating agent and the enzyme; and
optically
measuring the mass change of the enzyme conjugate layer and the unlabeled
antibody
layer as an indication of the amount of the analyte in the test sample.
Preferably, the enzyme conjugate has an immobilized peroxidase or an anti-
bacterial anti-body-horseradish peroxidase complex; or the enzyme conjugate is
alkaline
phosphatase and comprises an anti-bacterial -antibody- alkaline phosphatase
complex;
and the precipitating agent is a substrate containing 5-bromo-4chloro-3indolyl
phosphate.
In practice, an array of sensors may be placed on a surface to be brought into
contact with a suitable sample. Samples include, but are not limited to urine,
saliva,
sweat, and vaginal discharge. Alternatively, the sensor may be placed on the
skin. The
individual sensors respond to their respective analytes and produce a visually
detectable
signal. This may be as simple as a color or refractive index change, or may
involve a
change in an electrical signal such as due to current flow through a biosensor
membrane.
The latter type of assay lends itself to a device which may incorporate an
algorithm to detect the changes in hormone levels. This may then be displayed
in an easy
to understand format for the user.
For example, antibodies to the appropriate hormones may be immobilized on the
surface of the Silas optical wafers by methods known in the art. These wafers
may then
be separated and arranged in a known pattern on a detection article, for
example, an
absorbent article. Similarly, these antibodies could be immobilized on
microbeads and
arranged in a lateral flow assay device suitable for urine or saliva matrices.
Again,
multiple reagents are used in an array giving readout of all analytes
simultaneously. Also,
CA 02370739 2001-10-16
WO 00/65347 PCT/US00/11207
8
other analytes may be detected in combination with hormones. These include
biomarkers
for other conditions of interest, such as infections, osteoporosis, etc.
Another immunoassay method comprises applying an aqueous solution containing
the analyte antigen to one end of a mufti-zoned test strip device such that
the solution
moves along the strip by capillary action. The zones are arranged so that the
solution (a)
first contacts and reconstitutes dry, diffusible labeled component comprising
colloidal
gold conjugated to an antibody specific for said analyte antigen and then (b)
contacts and
reconstitutes dry, diffusible biotinylated second antibody specific for the
analyte antigen
such that a diffusible, dispersed sandwich reaction product forms. The
reaction product
diffuses along the strip with the solution and into a zone containing capture
component
consisting of a latex and avidin complex. The avidin collects the reaction
product by
means of reaction with its biotin moiety. Thus, gold particles are collected
and
concentrated in the detection zone for visual detection.
There are also home test for LH (luteinizing hormone) and similar clinical
tests for
Strep A, and Chlamydia. Other sources have laboratory single-analyte tests
using this
chromatographic principle for human corionic gonadotropin (HCG), common
infectious
diseases, and DAU (drugs of abuse in urine).
Carter-Wallace's home pregnancy test, First Response, uses plain microspheres
(~l~m) coated with one antibody to hCG and very small (<SOnm) red gold sol
particles
coated with an antibody to another hCG epitope. On mixing with a sample of
urine, if the
sample contains hCG, the particles are coagglutinated, thus yielding red
clumps. The
mixture is poured through a filter which catches the red clumps to yield a
pink colored
filter. With negative urine, un- coagglutinated red particles pass through the
filter and no
color develops on it.
Optionally, one or more of the sensors can be replaced by a sensor to detect
pregnancy. In 1988 a new over-the-counter pregnancy test (Clearblue EasyTM),
developed
and patented by Unipath, was introduced. The test uses dyed microspheres in a
sandwich
format to give a one step test. To prepare the test, small dark-blue dyed
microspheres (O)
are first coated with antibody (Ab,) to HCG (human chorionic gonadotropin);
the
CA 02370739 2001-10-16
WO 00/65347 PCT/US00/11207
9
microspheres (O-Abl) are dried on one part of a nitrocellulose strip; a second
antibody
(Ab2) to HCG is immobilized on another section of the strip.
In use the strip is wetted at one end with urine. As the urine moves by
capillary
action, it picks up the blue microspheres (O-Ably, and carnes them downstream;
any
HCG in the urine reacts with Abl on the microspheres (O-Abl- HCG). When the
flow
reaches the immobilized Ab2-1, the dyed microspheres with HCG (O-Abl- HCG) are
captured by Ab2-I to form a blue line caused by the HCG sandwich (O-Abl- HCG-
Ab2-
1). The blue line signals a positive pregnancy test. Further downstream there
is another
line of immobilized protein (Ab3-1) which catches unconjugated O-Abl.as (O-Abl-
Ab3-
1) (independent of HCG) to form another blue line which acts as a positive
procedural
control. If the second line does not form, the test results are invalid.
The absorbent article herein (i.e., sanitary napkins, interlabial devices, and
tampons) preferably also includes at least one multiple diagnostic device
having a
biosensor 60. As used herein, the term "biosensor" includes a component
comprising one
or more elements being adapted to detect one or more hormones, target
pathogenic
microorganisms or related biomolecules (e.g., an enzyme sensor, organella
sensor, tissue
sensor, microorganism sensor, immunosensor or electrochemical sensor),
additionally
having the capability to provide a signal of said detection to the wearer,
caretaker, or an
actuator. In certain embodiments, the elements may be biologically reactive,
chemically
reactive, binding, or they may operate by physical entrapment. The term
"biologically
reactive" is defined as having the capability to selectively interact with,
and preferably
bind, target pathogenic microorganisms and/or related biomolecules as
described herein.
The term "biologically reactive" includes, but is not limited to elements that
detect the
presence of enzymes. Generally, biosensors function by providing a means of
specifically
binding, and therefore detecting, a target biologically active analyte. In
this way, the
biosensor is highly selective, even when presented with a mixture of many
chemical and
biological entities, such as feces, menses, sweat, and saliva. Chemical
sensors, on the
other hand, which rely on chemically reactive means, generally do not have
either the high
selectivity or the amplification properties of biosensors and, therefore, are
not as well
suited to detect biologically reactive analytes, especially when they are
present in low
concentrations and/or in a complex media such as bodily fluids, bodily waste,
and other
CA 02370739 2001-10-16
WO 00/65347 PCT/US00/11207
bodily discharges. Often the target biological analyte is a minor component of
a complex
mixture comprising a multiplicity of biological and other components. Thus, in
many
biosensor applications, detection of target analytes to the parts-per-billion,
parts-per-
trillion, or even lower levels is necessary. Accordingly, discrimination
ratios of about
107-108 or greater may be required for the biosensor to recognize the target
biological
analyte in a complex mixture.
The biosensor of the present invention comprises a bio-recognition element, or
molecular recognition element, that provides the highly specific binding or
detection
selectivity for a particular analyte. The bio-recognition element, or system,
may be a
biologically derived material such as an enzyme or sequence of enzymes; an
antibody; a
membrane receptor protein; DNA; an organelle, a natural or synthetic cell
membrane; an
intact or partial viable or nonviable bacterial, plant or animal cell; or a
piece of plant or
mammalian tissues, and generally functions to interact specifically with a
target biological
analyte. The bio-recognition element is responsible for the selective
recognition of the
analyte and the physico-chemical signal that provides the basis for the output
signal.
Biosensors may include biocatalytic biosensors, and bioaffinity biosensors. In
biocatalytic biosensor embodiments, the bio-recognition element is
"biocatalytic" and
may comprise an enzyme, organelle, piece of plant or mammalian tissue, or
whole cells,
the selective binding sites "turn over" (i.e., can be used again during the
detection
process), resulting in a significant amplification of the input signal.
Biocatalytic sensors
such as these are generally useful for real-time, continuous sensing.
Bioaffinity sensors are generally applicable to bacteria, viruses, and toxins
and
include chemoreceptor-based biosensors and/or immunological sensors (i.e.
immunosensors). Chemoreceptors are complex biomolecular macroassemblies
responsible, in part, for a viable organism's ability to sense chemicals in
its environment
with high selectivity. Chemoreceptor-based biosensors comprise one or more
natural or
synthetic chemoreceptors associated with a means to provide a signal (visual,
electrical,
etc.) of the presence or concentration of a target biological analyte. In
certain
embodiments, the chemoreceptor may be associated with an electrode (i.e., an
electrical
transducer) so as to provide a detectable electrical signal. Chemoreceptors
may include
whole or partial nerve bundles (e.g., from antennae or other sensing organs)
and/or whole
CA 02370739 2001-10-16
WO 00/65347 PCT/US00/11207
11
or partial natural or synthetic cell membranes. On the other hand, the bio-
recognition
elements of immunosensors are generally antibodies. Antibodies are highly
specific and
can be made toward bacteria, viruses, fragments of microorganisms (e.g.,
bacterial cell
walls, parasite eggs or portions thereof, etc.), and large biomolecules.
Suitable antibodies
may be monoclonal or polyclonal. In any case, bioaffinity biosensors are
generally
irreversible because the receptor sites of the biosensor become saturated when
exposed to
the target biological analyte.
In certain embodiments, biocatalytic bioaffinity biosensors may be combined,
such
as RNA/DNA probes or other high-affinity binding systems wherein the initial
bio-
recognition event is followed by biological amplification of the signal. For
example, a
specific bacteria may be detected by a biosensor comprising genetic material,
such as
DNA, as a bio-recognition element and PCR (i.e., polymerase chain reaction)
amplification to detect small numbers of organisms, preferably less than or
equal to about
500. Biocatalytic and bioaffinity biosensor systems are described in more
detail in
Journal of Chromatography, 510 (1990) 347-354 and in the Kirk-Othmer Encyclo
edp is of
Chemical Technolo~y, 4th ed. (1992), John Wiley & Sons, NY, the disclosure of
which is
incorporated by reference herein.
The biosensors of the present invention preferably detect biologically active
analytes related to impending (i.e., future presentation of symptoms is
likely) or current
human systemic disease states, including, but not limited to, pathogenic
bacteria, parasites
(e.g., any stage of the life cycle, including eggs or portions thereof, cysts,
or mature
organisms), viruses, fungi such as Candida albicans, antibodies to pathogens,
and/or
microbially produced toxins. Additionally, the biosensor may target
biologically active
analytes related to impending or current localized health issues, such as
stress proteins
(e.g., cytokines) and IL-la (interleukin 1-alpha) that may precede the
clinical presentation
of skin irritation or inflammation. In preferred embodiments, the biosensor
functions as a
proactive sensor, detecting and signaling the wearer or caretaker of the
impending
condition prior to the presentation of clinical symptoms. This allows time to
administer
prophylactic or remedial treatments to the wearer which can significantly
reduce, if not
prevent, the severity and duration of the symptoms. Further, the biosensor, by
detecting
CA 02370739 2001-10-16
WO 00/65347 PCT/US00/11207
12
the presence of a target biological analyte in the wearer's bodily waste
(e.g., menses), may
detect residual contamination on a surface, such as skin, in contact with the
biosensor, and
provide an appropriate signal.
The physico-chemical signal generated by the bio-recognition element or
elements
may be communicated visually to the wearer or caretaker (i.e., via a color
change visible
to the human eye as in a colorimetric sensor). Other embodiments may produce
optical
signals, which may require other instrumentation to enhance the signal. These
include
flourescence, bioluminesence, total internal reflectance resonance, surface
plasmon
resonance, Raman methods and other laser-based methods. Exemplary surface
plasmon
resonance biosensors which may comprise bioconjugate surfaces as bio-
recognition
elements are available as IBIS I and IBIS II from XanTec Analysensysteme of
Muenster,
Germany. Alternatively, the signal may be processed via an associated
transducer which,
for example, may produce an electrical signal (e.g., current, potential,
inductance, or
impedance) that may be displayed (e.g., on a readout such as an LED or LCD
display) or
which triggers an audible or tactile (e.g., vibration) signal or which may
trigger an
actuator, as described herein. The signal may be qualitative (e.g., indicating
the presence
of the target biological analyte) or quantitative (i.e., a measurement of the
amount or
concentration of the target biological analyte). In such embodiments, the
transducer may
optionally produce an optical, thermal or acoustic signal.
In any case, the signal may also be durable (i.e., stable and readable over a
length
of time typically at least of the same magnitude as the usage life of the
article) or transient
(i.e., registering a real-time measurement). Additionally, the signal may be
transmitted to
a remote indicator site (e.g., via a wire, or transmitter, such as an infrared
or rf
transmitter) including other locations within or on the article or remote
devices. Further,
the biosensor, or any of its components, may be adapted to detect and/or
signal only
concentrations of the target biological analyte above a predefined threshold
level (e.g., in
cases wherein the target biological analyte is normally present in the bodily
fluids, bodily
waste, or other bodily discharges, or when the concentration of the analyte is
below a
known "danger" level).
CA 02370739 2001-10-16
WO 00/65347 PCT/US00/11207
13
As described above, the target analytes that the biosensors of the present
invention
are adapted to detect may be pathogenic microorganisms such as the pathogenic
microorganisms implicated in human gastrointestinal diseases, especially those
resulting
in diarrhea. This type of pathogen is particularly important to monitor due to
the number
of children who become seriously ill or die each year from diarrheal diseases.
It has been
found that severe chronic diarrhea may result in weight loss and permanent
physical and
mental developmental retardation. A non-limiting list of pathogenic bacteria
that the
biosensor may detect include any of the various pathogenic strains of
Escherichia coli
(commonly known as E. Coli), including enteropathenogenic E. coli (EPEC),
enterotoxigenic E. coli (ETEC), enterohemorragic E. coli (EHEC),
enteroinvasive E. coli
(EIEC), and enteroadherent E. coli (EAEC) strains; Salmonella strains,
including S. typhi,
S. paratyphi, S. enteriditis, S. typhimuriunz, and S. heidelberg; Shigella
strains such as
Shigella sonnei, Shigella flexneri, Shigella boydii, and Shigella dysenteriae;
Vibrio
cholerae; Mycobacterium tuberculosis; Yersinia enterocolitica; Aeromonas
hydrophila;
Plesiomonas shigelloides; Campylobacter strains such as C. jejuni and C. coli;
Bacteroides fragilis; and Clostridia strains, including C. septicum, C.
perfringens, C.
botulinum, and C. difficile. Non-limiting examples of commercially available
biosensors
adapted to detect E. coli are available from AndCare, Inc. of Durham, NC, as
test kit
#4001 and from Meridian Diagnostics, Inc. of Cincinnati, OH, as
ImmunoCard~STAT!
E. coli 0157 Plus assay. Another non-limiting example of a commercially
available
biosensor adapted to detect rotavirus is available from Meridian Diagnostics,
Inc. of
Cincinnati, OH, as ImmunoCard~STAT! Rotavirus assay. Another non-limiting
example
of a commercially available biosensor adapted to detect Cryptosporidium and
Giardia
lamblia is available from Meridian Diagnostics, Inc. of Cincinnati, OH, as the
Merifluor
Crypto/Giardia assay. Another non-limiting example of a commercially available
biosensor adapted to detect C. difficile toxin is available from Meridian
Diagnostics, Inc.
of Cincinnati, OH, as the Premier C. difficile Toxin A assay. ABTECH,
Scientific, Inc.,
of Yardley, PA offers "bioanalytical biotransducers", available as BB Au-
1050.5-FD-X,
which may be rendered biospecific (for microorganisms or other target
biological analytes
as described herein) by covalently immobilizing polypeptides, enzymes,
antibodies, or
CA 02370739 2001-10-16
WO 00/65347 PCT/US00/11207
14
DNA fragments to their surfaces. Other suitable microbial biosensors, or
sensing
systems, for one or more of the pathogens of interest are described in U.S.
Patent Nos.
5,948,694; 6,001,556; 5,106,965 (adenovirus); 5,869,272 (gram negative
organisms);
5,795,717 (Shigella); 5,830,341; 5,795,453; 5,354,661; 5,783,399; 5,840,488;
5,827,651;
5,723,330; and 5,496,700, all of which are incorporated herein by reference.
The target analytes that the biosensors of the present invention are adapted
to
detect may also be viruses. These may include diarrhea-inducing viruses such
as
rotavirus, adenovirus (a dsDNA virus), astrovirus (an RNA virus), calcivirus
(an RNA
virus), and Norwalk viruses (RNA viruses), or other viruses such as rhinovirus
and human
immunodeficiency virus (HIV). An exemplary biosensor adapted to detect HIV is
described in U.S. Patent Nos. 5,830,341 and 5,795,453, referenced above. The
disclosure
of each of these patents is incorporated by reference herein.
In alternative embodiments, the target analytes that the biosensors of the
present
invention are adapted to detect may also be parasites, especially those which
inhabit the
gastrointestinal tract during some point in their life-cycle (e.g., eggs or
portions thereof,
oocytes, trophozoites, adults). Such parasites may include protozoans, worms,
and other
gastrointestinal parasites. Other examples of parasites which may be detected
include
Entamoeba histolytica (which cause amoebic dysentery), cryptosporidium,
Giardia
lamblia, and Dientomeba fragilis, Trypana cruzi (which causes Chagas disease),
and
Plasmodium falciparum.
In yet other embodiments, the target analytes the biosensors of the present
invention are adapted to detect may be fungi such as Candida albicans. In
addition to
pathogenic bacteria, certain beneficial colonic bacteria may be detected
and/or measured
as a health indicator, such as Bifidobacteria and Lactobacillus strains.
The target analytes that the biosensors of the present invention are adapted
to
detect may also be proteins or antigens related to skin distress. Preferably,
these analytes
are detectable on or at the skin surface, preferably prior to the presentation
of clinically
observable skin irritation. These may include stress proteins such as
cytokines, histamine,
and other immune response factors including interleukins (such as IL-la, IL-2,
IL-3, IL-4,
and IL-8) and interferons (including interferons a and g). Again, these are
preferably
detectable by the biosensor 60 prior to the onset of clinically observable
redness,
CA 02370739 2001-10-16
WO 00/65347 PCT/US00/11207
irntation, or dermatitis. Additionally, the biosensors of the present
invention may be
adapted to detect enzymes, or other biological factors, implicated in skin
irritation (e.g.,
absorbent article dermatitis), including tryspin, chymotrypsin, and lipase.
In certain preferred embodiments of the present invention, the article may
comprise a diagnostic panel. A "diagnostic panel", as used herein, comprises
the
combination of two or more biosensors, or other types of indicators, adapted
to detect the
presence of at least two of a specific group of substances. These substances
can be
indicators of the physical conditions or state of well being of a user, or the
cause of a
particular disease state, such as diarrhea, vaginal infections, sexually
transmitted diseases
("STD's"), and other diseases. The biosensors can, for example, be adapted to
detect the
presence of at least two of a specific group of pathogens for the purpose of
determining
the class of pathogens or specific pathogens) causing a particular disease
state, generally
in order to provide a diagnosis leading to a specific course of remedial
medical treatment.
For example, the article may comprise a diagnostic panel adapted to determine
the
pathogenic cause, or causes, of diarrhea or vaginal infections. Examples of
physical
conditions or the state of well being that the diagnostic panel can be adapted
to detect
include, but are not limited to ovulation and the onset of menstruation.
Examples of
substances that the diagnostic panel can be adapted to detect in order to
determine the
onset of menstruation include, but are not limited to: progesterone, pH, and
red blood
cells (hemoglobin).
Alternatively, the article may comprise a diagnostic panel adapted to detect
any of
the potential bacterial causes of diarrhea or vaginal infections. In certain
preferred
embodiments, the diagnostic panel may comprise biosensors adapted to detect at
least two
of the following group of bacteria: SPEC, ETEC, EHEC, EAEC, EIEC,
Campylobacter
jejuni, Vibrio cholerae, and Shigella strains, including S. sonnei and S.
flexneri.
Preferably, the presence of any of the above bacterial causes of diarrhea or
vaginal
infections are indicated by the diagnostic panel. In any case, the signal to
the user,
caretaker, or health professional from the diagnostic panel preferably
indicates the
specific cause (i.e., bacterial pathogen) of the diarrhea or vaginal
infections, allowing the
early and specific treatment of the health condition.
CA 02370739 2001-10-16
WO 00/65347 PCT/US00/11207
16
Alternatively, the article may comprise a diagnostic panel adapted to detect
any of
the potential viral and bacterial causes of vaginal infections. In certain
preferred
embodiments, the diagnostic panel may comprise one or more biosensors adapted
to
detect at least one virus and one or more biosensors adapted to detect at
least one bacteria.
In any case, the signal to the user, caretaker, or health professional from
the diagnostic
panel preferably indicates the specific cause of vaginal infections, allowing
the early and
specific treatment of the health condition.
A non-limiting embodiment of an exemplary diagnostic panel 10 suitable for
incorporation into a disposable absorbent article is shown in Figures 6B-6E.
The
diagnostic panel 10 includes two biosensors 12, a biosensor 14 adapted to
detect E. coli
H0157 and a biosensor 16 adapted to detect rotavirus. The diagnostic panel 10
may be
made by obtaining biosensors 12 from the hereinbefore mentioned
ImmunoCard~STAT!
E. coli 0157 Plus and ImmunoCard~STAT! Rotavirus kits, available from Meridian
Diagnostics. The biosensors are removed from their respective "cards" and
attached to an
exposed surface of a substrate 18 via any attachment or bonding means as known
in the
art, such as an adhesive. The substrate 18 is preferably a stiff cardboard
material,
although it may comprise any substrate such as paper, cardboard, a
polyolefinic film, etc.
As shown in Figure 6C, a mask 20 having openings corresponding to the
biosensors 12
may be applied to the surface of the substrate 18 to ensure fluid/waste
contact only with
the biosensors 12 themselves and not the remainder of the substrate 18
surface. The
substrate 18, or mask 20, may be made of any material such as plastic,
cardboard, or
paper, and may comprise markings, instructions, or other indicia to aid in
performance of
the test or the interpretation of the results. For example, the substrate 18
may comprise a
color change "key" to assist the user in the correct interpretation of the
results. The
diagnostic panel 10 is attached to the wearing-facing surface of the absorbent
article
topsheet in the crotch region of the absorbent article corresponding to the
location of a
female wearer's pudendal region via any attachment or bonding means as known
in the
art, such as an adhesive. Alternatively, the diagnostic panel may be made by
attaching the
hereinbefore mentioned E. coli 0157 Plus and ImmunoCard~STAT! Rotavirus
biosensors directly to the wearing-facing surface of the absorbent article
topsheet in the
CA 02370739 2001-10-16
WO 00/65347 PCT/US00/11207
17
region of the absorbent article corresponding to the location of a female
wearer's
pudendal region.
In any of the above embodiments, the absorbent article topsheet may comprise
at
least one aperture and the diagnostic panel 15 may be attached to the region
of the
underlying absorbent core corresponding to the topsheet aperture(s). The
fluid/waste
sample may optionally be diluted with a diluent, such as the diluent provided
with the
ImmunoCard~STAT! Kit, upon removal of the absorbent article from the wearer or
application of the fluid/waste sample to the biosensors 12. In any event, the
results from
the biosensors 12 may be read approximately 10 minutes after insult by a
female wearer's
fluids or waste or, if diluent was added, 10 minutes after the sample
dilution.
The biosensors of the present invention may also comprise bio-recognition
systems, including enzymes or binding proteins such as antibodies immobilized
onto the
surface of physico-chemical transducers. For example, a specific strain of
bacteria may
be detected via biosensors employing antibodies raised against that bacterial
strain.
Alternatively, a target bacteria may be detected by a bio-recognition element
(including
antibodies and synthetic or natural molecular receptors) specific to
extracellular products
of the target bacteria, such as toxins produced by that strain (e.g., E.
coli). Exemplary
enzyme electrodes that may be used to detect phenols (e.g. in urine) include
tyrosinase
based electrodes or polyphenol oxidase enzyme electrodes described in U.S.
Patent No.
5,676,820 entitled "Remote Electrochemical Sensor," issued to Joseph Wang et
al. on
October 14, 1997 and U.S. Patent No. 5,091,299 entitled "An Enzyme Electrode
For Use
In Organic Solvents," issued to Anthony P. F. Turner et al. on February 25,
1992,
respectively. Both of these patents are incorporated by reference herein.
In any of the foregoing examples, the specific microorganism may be directly
detected or may be detected by binding a toxin, enzyme, or other protein
produced by the
organism or an antibody, such as a monoclonal antibody, specific to the
organism.
Exemplary biosensors adapted to detect proteolytic enzymes described in U.S.
Patent No.
5,607,567 and toxins in U.S. Patent Nos. 5,496,452; 5,521,101; and 5,567,301.
CA 02370739 2001-10-16
WO 00/65347 PCTNS00/11207
18
In a non-limiting embodiment of an exemplary diagnostic panel for vaginal
infections, the biosensor may be adapted to detect various specific types of
bacteria that
may be the cause of bacterial vaginosis, including Gardnerella vaginalis,
Prevotella
bivia, Bacteroides species, Mycoplasma hominis, Mobiluncus species. The
biosensor may
be adapted to detect non-specific types of bacteria that may be the cause of
bacterial
vaginosis. The biosensor may also be adapted to detect fungi such as Candida
species,
which is the cause of yeast vaginitis (or yeast infections). The biosensor may
also be
adapted to detect protozoa such as Trichomonas vaginalis, which is the cause
of
Trichomoniasis, a non-reportable sexually transmitted disease, Chlamydia, or
other
sexually-transmitted diseases. A non-limiting example of a commercially
available
biosensors adapted to detect G. vaginalis is the FEM EXAM~ G. vaginalis PIP
Activity
TestCard available from Litmus Concepts, Inc. of Santa Clara, CA. The FEM
EXAM~
G. vaginalis TestCard is described in U.S. Patent No. 5,571,684. A non-
limiting example
of a commercially available biosensors adapted to detect non-specific causes
of bacterial
vaginosis is the FEM EXAMO pH and Amines TestCard available from Litmus
Concepts, Inc. The FEM EXAM~ pH and Amines TestCard is described in U.S.
Patent
No. 5,660,790. Other Litmus Concepts patents and patent publications of
interest include:
5,268,146; 5,416,003; 5,585,273; 5,897,834; and PCT Publication WO 94/24306.
Non-
limiting examples of biosensor adapted to detect Candida and Chlamydia are
described in
U.S. Patent Nos. 5,741,662 and 5,773,234, respectively, issued to Quidel
Corporation of
San Diego, CA.
In other preferred embodiments, the diagnostic panel may comprise biosensors
adapted to detect at least two of the following group of bacteria: various
types of bacteria
that may be the cause of bacterial vaginosis, including Gardnerella vaginalis,
Prevotella
bivia, Bacteroides species, Mycoplasma hominis, Mobiluncus species.
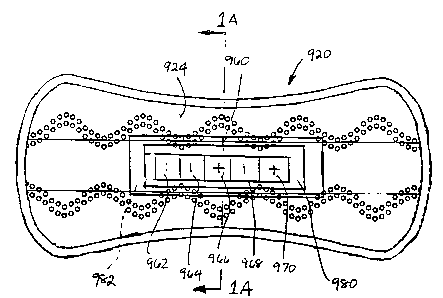
Fig. 1 shows a non-limiting panty liner embodiment 920 containing an exemplary
diagnostic panel 960 for detecting the various causes of vaginitis. The
diagnostic panel
960 shown in Fig. 1 contains five sensor elements, 962, 964, 966, 968, and
970. Each of
these sensor elements is adapted to detect one or more of the causes of
vaginitis alone, or
in combination with one or more of the other sensor elements. Sensor element
962 is
CA 02370739 2001-10-16
WO 00/65347 PCT/US00/11207
19
adapted to detect pH. Sensor element 964 is adapted to detect the presence of
amines.
Sensor element 966 is adapted to detect G. vaginalis. Sensor element 968 is
adapted to
detect Candida species. Sensor element 970 is adapted to detect Tr-ichomonas
vaginalis.
The combination of sensor elements 962 and 964 can be used to detect non-
specific causes of bacterial vaginosis. An early study of bacterial vaginosis
(BV) involved
comparisons of the pH of vaginal fluids of women known to be suffering from BV
with
those known to be free of the disease - Gardner, H.L., et al., Am. J. Obstet.
Gynecol. 69:
962 (1955). All of the BV positive women in the study were determined to have
a vaginal
fluid pH greater than 4.5, and 91 % of these women had a vaginal fluid pH
greater than
5Ø Studies subsequent have now adjusted the pH threshold to 4.7.
The whiff test, which is one of the Amsel criteria, originated in a study by
Pheifer,
et al., N. En~l. J. Med. 298: 1429-1434 (1978), that reported the presence of
a
characteristic fishy amine odor upon the addition of 10% KOH to a vaginal
fluid
specimen from a woman with BV. The odor is caused by the alkaline
volatilization of
amine salts found in the vaginal fluid of women with BV.
An example is a test device for analyzing an aqueous liquid sample (usually a
biological specimen) for a pH equal to or greater than a critical point in the
range of 4.6 to
4.8 (preferably about 4.7) by a detectable transition.
A further example is a test device for detecting salts of volatile amines in
an
aqueous liquid sample (again, usually a biological specimen). This device
contains a dry,
solid gaseous amine-releasing substance in addition to an amine indicator
retained in a
matrix that is impermeable to aqueous liquids.
Preferred pH indicators are bromophenol blue, bromochlorophenol blue,
bromocresol green, bromocresol purple, bromothymol blue, brilliant yellow, and
nitrazine
yellow. A particularly preferred pH indicator is nitrazine yellow which, when
in
combination with quaternary ammonium groups, changes directly from greenish-
yellow to
blue over a narrow pH range of approximately 0.1 pH units as the pH rises, the
transition
centering around pH 4.7.
CA 02370739 2001-10-16
WO 00/65347 PCTNS00/11207
The quaternary ammonium groups can be any groups capable of asserting a
positive charge sufficient to form an ionic attraction with the negatively
charged groups)
in the indicator. Preferred quaternary ammonium groups are lower alkyl
ammonium
groups in which the alkyl groups are C~ - C.~ alkyl groups. Trimethylammonium
groups
are particularly preferred.
The amine test differentiates between amines volatilized by alkali and those
that
are not volatilized by alkali by incorporating solid alkali accessible to the
specimen, an
indicator accessible to a liquid specimen, and an indicator accessible only to
vapors
emitted by the specimen, in the same device. Thus, the specimen is first
contacted with
the solid alkali, then applied to both indicators, one of which sill undergo a
color change
regardless of the presence or absence of volatile amines, and the other a
color change only
in the presence of volatile amines.
The choice of solid alkali for the gas-releasing lamina is not critical and
can vary.
In general, alkali and alkaline earth metal aluminates, carbonates and
hydroxides can be
used. Best results will most often be achieved with the use of either sodium
aluminate,
sodium carbonate, or magnesium hydroxide. Sodium aluminate is particularly
preferred.
Any indicator that changes color upon exposure to amines, and preferably
amines
in a fluid specimen that would otherwise be acidic, may be used. Bromocresol
green is
one example, and may be used here as well as in the pH test. Other examples
are
bromophenol blue, bromocresol purple, bromochlorophenol blue, nitrazine
yellow, and
various other indicators.
Sensor elements 962 and 964 can comprise the hereinbefore mentioned FEM
EXAM~ pH and Amines TestCard sensors available from Litmus Concepts, Inc. to
detect
non-specific causes of bacterial vaginosis.
Sensor element 966 is adapted to detect G. vaginalis. In 1988, a report by
Thomason, et al. (Obstet. Gyneco1.,71 (4): 607 ( 1988)) suggested that
bacterial enzyme
activity, specifically proline iminopeptidase activity, in vaginal fluid may
be a suitable
marker for BV.
CA 02370739 2001-10-16
WO 00/65347 PCT/US00/11207
21
The assay is performed by contacting the sample with a solid-phase conjugate
which is susceptible to cleavage by the hydrolase, and either during or
subsequent thereto,
contacting the sample with an indicator which undergoes a detectable change
upon the
action of a reporter group which is a portion of the conjugate and is
liberated from it
either partly or entirely by the action of the hydrolase.
The term "conjugate" is used herein to refer to a reporter group coupled to a
substrate residue yet capable of cleavage or decoupling therefrom upon contact
with the
catalytically active hydrolase whose presence is being detected. The term
"reporter
group" or (interchangeably) "marker group" is used herein to refer to a moiety
which can
be hydrolytically released from the substrate residue by a hydrolase and
which, in its free
form, can react with an indicator to produce a detectable change. Such
reporter groups
include, but are not limited to, the following: phenols, naphthols, aromatic
amines, amino
acids, their derivatives and analogs. In a particularly preferred embodiment,
naphthylamine, its derivatives or analogs are used as the reporter group.
If the hydrolase of interest hydrolyzes the conjugate at any other point other
than
freeing the reporter group, the hydrolase by itself would be incapable of
releasing the
reporter group in active form. One or more assisting hydrolases which could
only act in
conjunction with the hydrolase of interest could then be incorporated into the
assay to
complete the release of the reporter group in active form. The assisting
hydrolase or
hydrolases must therefore be ones which are incapable of releasing the
reporter group
directly from the intact conjugate, but instead capable of releasing the
reporter group only
from the cleavage product generated by the hydrolase of interest.
First, the hydrolase of interest, unable to release the reporter group
directly,
specifically hydrolyzes one or more bonds in the conjugate, thereby releasing
a molecular
fragment containing the inactive reporter group. Next, the assisting hydrolase
(or
hydrolases) releases the reporter group by hydrolyzing the bond between the
substrate
residue fragment and the reporter group in one or more steps. The net effect
of the
foregoing reaction sequence is the release of the reporter group only when the
hydrolase
of interest is present in the sample.
CA 02370739 2001-10-16
WO 00/65347 PCT/US00/11207
22
To illustrate an implementation of the present invention for detecting proline
iminopeptidase activity, the sample is placed in a device which contains first
and second
solid supports, the first solid support being a Mylar~ polyethylene laminate
on which an
L-prolyl-beta-naphthylamide, L-prolyl-beta-methoxynaphthylamide of hydroxy-L-
prolyl-
beta-naphthylamide conjugate is deposited, the second solid support being a
Mylar~
polyethylene laminate on which Fast Garnet GBC, a chromogenic indicator which
undergoes a detectable change upon action of beta-naphthylamine, is deposited.
The
sample is placed in the device in such a manner that the sample contacts the
first and
second solid supports such that any beta-naphthylamine released by proline
iminopeptidase activity in the sample is permitted to diffuse through the
sample to the
second solid support. The Fast Garnet GBC is then observed for a detectable
change as
an indication of the presence of the enzyme in the sample. The conjugate may
be
incorporated in a matrix of water-soluble polymer such as hydroxypropyl
cellulose. The
Fast Garnet GBC indicator may be incorporated in a water-insoluble matrix of
ethylcellulose which contains a penetrant such as manganese chloride.
Sensor element 966 can comprise the hereinbefore mentioned FEM EXAM~ G.
vaginalis PIP Activity TestCard available from Litmus Concepts, Inc.
Sensor element 968 is adapted to detect Candida species. It has been
discovered
that enzymatically active Candida albicans aspartic protease is present in the
vaginal fluid
of women with vulvovaginal candidiasis. It has further been discovered that
the presence
of enzymatically active aspartic protease in a sample or specimen can serve as
a marker
for the detection and diagnosis of candidiasis. Accordingly, a method has now
been
developed for detecting candidiasis by assaying for the presence of
enzymatically active
aspartic protease in a sample.
In this method, a sample, e.g., vaginal fluid, is contacted with a solid
support. The
solid support with which the sample is contacted has a reporter enzyme (i.e.,
a signal
generating enzyme) immobilized thereon. The reporter enzyme is immobilized on
the
solid support in a manner such that it is released from the solid support upon
action of the
enzymatically active aspartic protease if the enzymatically active aspartic
protease is, in
CA 02370739 2001-10-16
WO 00/65347 PCT/US00/11207
23
fact, present in the sample. The sample after having been contacted with the
solid support
is combined with an indicator. The indicator is any chemical species which is
susceptible
to a visible or detectable change (such as, for example, a change in color)
upon action of
the reporter enzyme. If after contact with the sample the indicator undergoes
a detectable
change, enzymatically active aspartic protease is present in the sample and,
hence, it can
be said that candidiasis is present.
The term "reporter enzyme" or (interchangeably) "marker enzyme" is used herein
to refer to a signal generating enzyme, i.e., an enzyme whose activity brings
about a
detectable change. Such reporter enzymes include, but are not limited to, the
following:
peroxidases, phosphatases, oxidoreductases, dehydrogenases, transferases,
isomerases,
kinases, reductases, deaminases, catalases, urease, and glucuronidase.
Presently preferred reporter enzymes are the peroxidases, such as, for
example,
horseradish peroxidase.
The reporter enzyme is immobilized on a solid support, i.e., an insoluble
polymeric material, inorganic or organic matrix, gel, aggregate, precipitate
or resin, in
such a manner whereby the reporter enzyme is released upon action of the
hydrolase
whose presence is being assayed. Preferred solid supports in accordance with
the present
invention include, but are not limited to, the following: cellulose, agarose,
dextran,
polyacrylate, polyacrylamide, or their derivatives, chitin, sepharose, oxirane
acrylic beads
and polymeric dialdehyde, starch, collagen, keratin, elastin, bovine hide
powder, bacterial
cell wall peptidoglycan or fragments thereof, nylon, polyethylene
terephthalates,
polycarbonates, and controlled pore glass. Immobilization of the reporter
enzyme on the
solid support is carded out using conventional methods and procedures known to
and
understood by those skilled in the art.
The term "indicator" is used herein to refer to any chemical species which
undergoes a detectable change as a result of the reaction or as a result of
the culmination
of reactions occurnng when the enzymatically active hydrolase is present in
the sample or
specimen. The resulting detectable change is an indication that the
enzymatically active
hydrolase is present in the sample or specimen.
Preferred indicators are visual indicators and, in particular, chromogenic
indicators, i.e., those in which the visible change is a change in color,
including the
CA 02370739 2001-10-16
WO 00/65347 PCT/US00/11207
24
formation of color in an otherwise colorless material, upon action of the
reporter or
marker enzyme when it is released from the solid support by the enzymatically
active
hydrolase whose presence is being detected. Alternatively, the reporter enzyme
may be
capable of catalyzing the formation of a fluorescent signal, a phosphorescent
signal, a
bioluminescent signal, a chemiluminescent signal, or an electrochemical signal
upon its
release from the solid support by the action of the hydrolase. Additionally,
the reporter
enzyme may be capable of producing other visible or detectable signals, such
as, for
example, a clot, an agglutination, a precipitation, or a clearing zone.
A wide variety of chromogenic indicators (i.e., chromogens) and other species
having a similar effect may be used as visual indicators with horseradish
peroxidase as the
reporter enzyme. Preferred chromogenic indicators in accordance with the
present
invention comprise a hydroperoxide and a chromogen including, but not limited
to, one of
the following: guaiac, 2-2'-azino-bis(3-ethyl-benthiazoline-6-sulfonic acid),
tetramethylbenzidine, phenol, 4-aminoantipyrine, and 4, 5-
dihydroxynaphthatlene-2, 7-
disulfonic acid. A particularly preferred chromogenic indicator is comprised
of a
hydroperoxide and guaiac, a chromogen which is colorless in its reduced state
and deep
blue in its oxidized state.
Sensor element 970 is adapted to detect Trichomonas vaginalis. In yet another
aspect of the present invention, a method is provided for detecting
Trichomonas vaginalis
by assaying for the presence of enzymatically active thiol protease in a
sample, this
method comprising: (a) contacting the sample with a solid support, the solid
support
having a reporter enzyme immobilized thereon in such a manner whereby the
reporter
enzyme is released upon action of the enzymatically active thiol protease; (b)
combining
the sample after having been contacted with the solid support with an
indicator, the
indicator being one which is susceptible to a detectable change upon action of
the reporter
enzyme; and (c) observing whether the indicator undergoes a detectable change,
the
detectable change being an indication of the presence of enzymatically active
thiol
protease in the sample and thus, Trichomonas vaginalis.
The panty liner 920 shown in Fig. 9 comprises a hybrid topsheet as described
in
U.S. Patent No. 6,004,893, Van Tilburg. The sensor elements can be attached to
the
wearing-facing surface of the panty liner topsheet 924. The sensors can be
attached in the
CA 02370739 2001-10-16
WO 00/65347 PCT/US00/11207
region of the panty liner corresponding to the location of the wearer's
vagina. The
sensors can be attached via any attachment or bonding means as known in the
art, such as
an adhesive. Alternatively, the topsheet may comprise at least one aperture
and the
sensors may be attached to the region of the underlying absorbent core
corresponding to
the topsheet aperture(s).
The sensor elements may be in the nature of a plus or minus sign to indicate
the
presence or absence of the test analytes in a quantity above a certain
threshold as shown in
Figs. 1 and lA. Alternatively, they may be adapted to provide a colorimetric
indication of
the quantity of test analytes as shown in Fig. 3, and the darkness of the
color on the sensor
elements can be compared with a comparison chart, such as that shown in Fig.
4, which
indicates the level of test analytes present. The comparison chart can be
provided in a
number of suitable formats, including, but not limited to, in the form of a
card that is
packaged with the article on which the sensors are located, or on the outside
of the
package.
The sensor elements can be covered by a covering to prevent the test reagents
in
the sensors from coming in contact with the wearer's body, if test reagents
are present.
Preferably, the covering is clear and also flexible, so that it will not
interfere with wearing
the article, if the article is of a type to be worn adjacent to a wearer's
body. The covering
can be made of any suitable material, such as plastic, SARAN~ wrap, MYLAR~, or
the
like. The covering can be apertured to allow body fluids to come into contact
with the
sensors, or it may be unapertured.
If a covering is used, it may be desirable to provide a fluid transport
element, such
as a wicking strip, underneath and/or on the sides of the sensors to bring the
bodily fluids
of interest into contact with the sensors.
Fig. 2 shows a non-limiting sanitary napkin embodiment 1220 containing an
exemplary diagnostic panel 1260.
CA 02370739 2001-10-16
WO 00/65347 PCT/US00/11207
26
Figs. 7-9 show a non-limiting interlabial device embodiment 1320 containing an
exemplary diagnostic panel 1360. Fig. 10 shows how the interlabial device 1320
may be
held by a user for insertion into the space between the wearer's labia. Fig.
11 shows the
interlabial device in place relative to the wearer's body.
Fig. 5 shows a non-limiting tampon embodiment 1620 containing an exemplary
diagnostic panel 1660.
The biosensor used in the present invention may comprise one or more
"proactive
sensors". This is especially useful in embodiments where the detection of the
target
biologically reactive analyte precedes the onset of clinically observable
health symptoms.
As used in this application, the term "proactive sensor" refers to a sensor
that is capable of
detecting changes or signals on the body of the wearer (i.e., skin) or in the
waste, i.e.,
inputs, that directly relate or, at a minimum, correlate to the occurrence of
an impending
or potential health or skin related even. Proactive sensors may respond to one
or more
specific inputs as described above.
A proactive sensor may detect an impending event or detect a parameter that
directly relates, or at a minimum correlates to the occurrence of an impending
event,
particularly a systemic or skin health event or condition (i.e., the
presentation of clinically
observable indications or symptoms). An impending event that may be detected
or
predicted by a proactive sensor of the present invention may include diarrheal
disease,
skin irntation or rash (including candidiasis), and/or other types of illness
or medical
conditions of the wearer such as a parasitic infestation. The detected
biological analyte
may be one or more steps removed from the actual presentation of clinical
symptoms. For
example, the biosensor may detect potential precursors to the above conditions
(e.g., fecal
contamination of the skin that may precede the elicitation of stress proteins
which may, in
turn, precede clinically observable skin irntation. A parameter that
correlates to an event
is any measurable input, signal such as one or more of the potential inputs
listed above,
that correlates with the occurrence of the event within the frame of reference
of the system
(i.e., a signal caused by the waste or the wearer). Proactive sensors in an
article may
measure one or more different inputs in order to predict an event. For
example, the
proactive sensor may monitor for Candida albicans in the feces and residual
colonic
CA 02370739 2001-10-16
WO 00/65347 PCT/US00/11207
27
bacteria on the skin (i.e., detecting residual contamination) both of which
are signals that
may precede skin irritation.
In biosensor embodiments wherein the bio-recognition element does not produce
an easily visible signal (e.g., a color change), the biosensor may include a
transducer in
communication with the bio-recognition element in order to convert the physico
chemical
signal from the bio-recognition element into a usable signal to the wearer,
caretaker, or
component of the article (e.g., and actuator). Exemplary transducers may
include
electrochemical transducers (including potentiometric, amperometric, and
conductimetric
transducers), optical transducers (including flourescence, bioluminesence,
total internal
reflective resonance, and surface plasmon resonance), thermal transducers, and
acoustic
transducers, as known in the art. A power source, such as a miniature 3 volt
watch battery
or printed thin film lithium battery, may be connected with the biosensor 60
to provide
any required power.
The effectiveness of the biosensors of the present invention may be measured
with
the Response Factor Test described in the Test Method section below. The
Response
Factor describes the ratio of the response of the biosensor when exposed to
fluid/waste
test material compared to the response of the biosensor when exposed to
physiological
saline solution and is useful in assessing the sensitivity of the biosensor
for biologically
active analytes expected to be found. The biosensors of the present invention
preferably
have a response factor of at least 2, 3, or 5, more preferably at least 10,
and even more
preferably at least 20.
If microorganisms are incorporated into a biosensor, they may be immobilized
in
the biosensor by techniques known in the art such as entrapment, adsorption,
crosslinking,
encapsulation, covalent attachment, any combination thereof, or the like.
Further, the
immobilization can be carned out on many different substrates such as known
the art. In
certain preferred embodiments, the immobilization substrate may be selected
from the
group of polymer based materials, hydrogels, tissues, nonwoven materials,
woven
materials.
In certain embodiments, the biosensor, including any biosensor embodiments,
may
comprise, be disposed on, or be operatively associated with a microchip, such
as a silicon
chip, MEMs (i.e., micro electromechanical system) device, or an integrated
circuit.
CA 02370739 2001-10-16
WO 00/65347 PCT/US00/11207
28
Microchip-based biosensors may be known as "biochips". Regardless of the type
of
sensor, the microchip may comprise a multiplicity of sensor components having
similar or
different sensitivities, kinetics, and/or target analytes (i.e., markers) in
an array adapted to
detect differing levels or combinations of said analyte(s). Further, each
biosensor in such
an array may provide a different type of signal, including those types
disclosed herein, and
may be associated with different actuators and/or controllers. Also, each
biosensor in an
array may operate independently or in association with (e.g., in parallel,
combination, or
series) any number of other sensors in the array.
The biosensor may be disposed in and/or operatively connected to any portion
of
an absorbent article that will be exposed to the input that the biosensor is
designed to
detect. For the purposes of the present invention, the term "operatively
connected" refers
to a means of communication such that the biosensor may signal some portion of
the
article 10 when the biosensor detects an input. The biosensor may be separate
from and
operatively connected to another portion of the biosensor, another biosensor,
an actuator,
a controller or some other portion or component of the absorbent article 10.
"Operatively
connected" may, for example, include a means of communication such as an
electrical
connection via a conductive wire or member, via a transmitted signal such as
radio
frequency, infrared or another transmitted frequency communication.
Alternatively, the
biosensor may be operatively connected via a mechanical connection such as a
pneumatic
or a hydraulic connection.
The biosensor may be integral with the absorbent article 10, or may be
installed by
the caretaker or the wearer. The biosensor during the course of wearing the
article, may
also become at least partially detached from the article and may be adhered to
the
wearer's skin. The biosensor may be affixed, permanently or detachably (e.g.,
via a
mechanical fastening system like VelcroTM or a water soluble adhesive) to a
support
structure, including adhesive tapes, cellulosic or synthetic webs, nonwoven
highlofts,
films, scrims, foams, and the like. Further, the biosensor may be completely
contained
within the absorbent article 10 or may have a receiving portion located in the
absorbent
article 10 such that it will come into contact with the desired input and
another portion
such as a transmitting portion located either in the article or outside the
article. The
biosensor may be external to the absorbent article 10 yet operatively
connected to some
CA 02370739 2001-10-16
WO 00/65347 PCT/US00/11207
29
portion of the absorbent article 10 such that the biosensor may detect an
input external to
the absorbent article 10 and provide a signal to a controller and/or an
actuator. In some
embodiments, the biosensor may be separate from the article, e.g., separately
applied to
some portion of the wearer via adhesive or other means as known in the art,
and/or may
have one or more components separate from the article.
In some embodiments, a wiping means or element may be provided to allow the
wearer or caretaker to clean sufficient bodily waste from the biosensor to
allow a visual
assessment or reading of the signal (especially for biosensor embodiments that
provide
such a signal). The wiping element may include a web (cellulosic or
synthetic),
nonwoven highloft, film, foam, rigid or semi-rigid squeegee like element, and
the like
disposed in the article and adapted such that the element may be used to clean
the
biosensor display. The wiping element may be at least partially affixed to an
element (e.g.,
topsheet, backsheet, absorbent core) of the article, such as a topsheet, in
proximity to the
biosensor by any known means in the art. The wiping means may optionally
comprise
water or any other known cleaning aid to facilitate cleaning of the wearer or
the biosensor
display.
In certain preferred embodiments, the absorbent article 10 also may comprise
an
actuator. As used in this application, the term "actuator" refers to a device
that comprises
"potential" and a means of transforming that potential to perform or activate
a "responsive
function." The potential of the actuator may comprise either stored or
potential energy or
stored material. The actuator thus may perform or activate a responsive
function by
transforming potential energy to kinetic energy or by releasing or delivering
a stored
material. A "responsive function" is defined for the purposes of the present
invention as a
function performed upon the bodily waste, the wearer, the article, or a
component or
components thereof, or a signal to the wearer or the caretaker. A component of
bodily
waste may include, for example, moisture, electrolytes, enzymes, volatile
gases, bacteria,
blood, etc. A component of the wearer may also include skin, genitalia, the
pudendal
region, the anus, the anal sphincter muscle, etc. A component of the article
may also
include leg cuffs, waist cuffs or other waste barners and/or containment
components, side
panels, ears, a chassis, an absorbent core, an acquisition component, a
fastening system,
the longitudinal or end edges, etc. Potential energy may be stored as
mechanical,
CA 02370739 2001-10-16
WO 00/65347 PCT/US00/11207
electrical, chemical or thermal energy. "Kinetic energy" as used in this
application refers
to the capacity to do work or to perform a responsive function as described
above (e.g.,
expansion of a compressed device, rotation of a twisted device, a gel that
moves as it
changes phases, coating or treatment of skin, inhibition of an enzyme,
adjustment of pH,
etc.).
The absorbent article 10 may also include a controller. A "controller" is
defined
for the purposes of this application as a device that receives an input from a
biosensor and
determines if one or more actions are to be taken. The controller may receive
a signal
from the biosensor and direct the actuator to perform a responsive function
upon the
bodily waste, the wearer, the article or a component thereof. Alternatively,
the actuator
may receive the signal directly from the biosensor and perform a responsive
function upon
the wearer, the waste, the article or a component thereof. The controller may
include
materials that undergo chemical or physical change, may be a chemical,
mechanical or
electrical device that processes information from a biosensor, etc. The
controller may
include a transducer comprising a polylayer Langmuir-Blodgett film, wherein
one or more
layers includes a bio-recognition element. Upon contact with water, Langmuir-
Blodgett
films are known to spontaneously reorganize, resulting in regions with more
layers than
the original film and other regions having fewer layers. This reorganization
may expose
the bio-recognition element to the environment preferentially in the presence
of water,
such as in bodily waste, which may contain the target biological analyte.
Thus, the
number of false positives can be reduced and the shelf life of the biosensor
can be
extended. Alternatively, an electrical controller that receives signals such
as electrical
potential from an electrochemical biosensor may receive and monitor multiple
electrical
signals and may repeatedly trigger the actuator. The controller may be
integral with the
biosensor component, integral with the actuator component, or a separate
component of
the system.
The controller may be disposed in and/or operatively connected to any portion
of a
disposable article that will allow the controller to receive a signal from the
biosensor and
to provide a signal to the actuator. The controller may be integral with the
absorbent
article 10, or may be installed by the caretaker or the wearer. The controller
may be
completely contained within the article such as absorbent article 10, may have
a portion
CA 02370739 2001-10-16
WO 00/65347 PCT/US00/11207
31
located in the article and a portion located outside the article, or may be
located
completely outside the absorbent article 10. The controller or a portion of a
controller
may be operatively connected to one or more biosensors, one or more actuators
90,
another portion of the controller or another portion of the absorbent article
10. The
controller, for example, may receive a signal from the biosensor and provide a
signal to
the actuator, e.g., by a radio frequency (rf) transmission.
Although distinct structural elements may perform the biosensor, actuator and
controller functions, the biosensor, actuator and/or controller functions of
the present
invention need not be performed by distinct structural elements. The biosensor
and
controller functions, for example, may be performed by the same structural
element.
A "responsive system" is defined for the purposes of this application as a
system
that includes a biosensor and an actuator that acts upon the bodily waste, the
wearer, the
article, or a component or components thereof when the biosensor detects the
appropriate
triggering input. Upon sensing a given input parameter, the actuator effects
the release of
stored energy or the release or delivery of stored material to perform a
responsive
function. For example, when a proactive biosensor including a transducer
detects an
impending event, the transducer provides a signal to the actuator effecting
the release of
stored energy. By detecting an input signal prior to the impending event, a
responsive
system in the article may be triggered to prepare for the event or to signal
the caregiver or
the wearer of the impending event. This allows construction of articles in
which the
waste-management or treating technology is initially "hidden" or unobtrusive,
but which
is available at, or just before, the moment of need and/or in which the
article may provide
the caregiver or the wearer the opportunity to prepare for an event in advance
(e.g.,
administer a prohylactic treatment to the wearer in the event of detected
pathogenic
microorganisms). Regardless of the specific input, the biosensor in these
embodiments
may trigger an actuator to perform an action on the article, the wearer or the
environment
to prepare for the occurrence of the event or provide a signal to the
caregiver that the
impending event is about to occur. If the biosensor comprises a sensing
system, one
actuator may be triggered by different biosensors and/or signals, or different
actuators
may be triggered by different biosensors and/or signals. Alternatively, one
biosensor
and/or signal may trigger multiple actuators.
CA 02370739 2001-10-16
WO 00/65347 PCT/US00/11207
32
A responsive system may respond in either a "continuous" or a "discontinuous"
manner. As used in this application, a "continuous responsive system" refers
to a
responsive system in which the output is quantitatively dependent upon the
quantity of the
input, i.e., continuously increasing quantities of the input are required to
effect
continuously increasing quantities of the output, or where the output of the
responsive
system comprises a passive release of a stored material. A super absorbent
polymer
placed in an absorbent core of an article, for example, provides a continuous
response in
which the output is quantitatively dependent upon the quantity of the input,
i.e., as
increasing quantities of liquid waste contact the super absorbent polymer, an
increasing
amount of the polymer contains that liquid until the capacity of the polymer
is exhausted.
A stoichiometric chemical reaction is another example of a system having a
continuous
response to increasing output. In the reaction A + excess B -~ C, for example,
the
amount of excess B converted to C is stoichiometrically and, therefore
"continuously,"
related to the amount of A available in the system.
A "discontinuous responsive system" of the present invention, however, refers
to a
responsive system that has an output function that is essentially independent
of the
quantity of the input beyond a threshold level. For example, when one or more
threshold
levels of a given input are met, the responsive system may release all or a
pre-designated
portion of its stored energy or deliver, i.e., actively transport, all or a
pre-designated
portion of its stored material to perform a specific responsive function. In
an ideal
embodiment of the present invention, the output function, f(x), includes a
"step" function
as shown in Figure 3A. In this embodiment, the rate of change in the output
with
increasing levels of input (d(output)/d(input)), i.e., the slope or first
derivative f (x) of the
output function f(x), is preferably essentially zero when the amount of input
is above or
below the threshold level. At the threshold level, however, the d(output)/
d(input) rate of
change preferably approaches infinity. Thus, in the ideal discontinuous
response, the
limit of the function f(x-E) as s-~0 is not equal to the limit of the function
f(x+E) as s-~0,
i.e., lim f(x-s) ~ lim f(x+s).
s~0 s-~0
CA 02370739 2001-10-16
WO 00/65347 PCT/US00/11207
33
The present invention, however, recognizes that in the physical world an ideal
instantaneous step change at the threshold level is not necessary and may not
even be
possible in many instances. In a preferred embodiment, it is only necessary
that the
output function have a virtual step change with very little change in the
input at or around
the threshold level of the input. Thus, the present invention contemplates a
discontinuous
responsive system of the present invention having an output function that
responds in a
sufficiently discontinuous manner in the transition region such that the
output function
has at least a minimum relative degree of steepness in the transition region.
While not
wishing to be limited to a particular method of describing or modeling a
discontinuous
system, in a preferred method of determining whether a given output function
performs in
a sufficiently discontinuous manner as defined for the purposes of the present
invention,
the slope of the output curve at the inflection point is compared with the
relative slope of
a line between the first and last points of the transition region. For
example, Figure 4A
shows a graph of an exemplary output function, f(x) along with aligned graphs
of the first,
f (x), and second, f '(x), and third, f"(x), derivatives of the exemplary
output function.
The output function f(x) describes the effect of the in put (x or I) on the
output or
response (R(I)). For purposes of the present invention, the transition region
is defined as
the region between the relative maxima, R(h), and the minima, R(Iz), of the
second
derivative, f '(x), of the output function, f(x). The relative maxima, R(I~),
and the relative
minima, R(I2), are points at which the third derivative, f"(x), equals zero.
The inflection
point, Io, is defined as the point in the transition region at which the
second derivative,
f '(x), equals zero, i.e.,
dZR
- ~ - 0.
dI2 ~ I=to
The comparison of the slope of the output function at the inflection point to
the
slope of a line between the first and the last points of the transition region
can be
described by the equation:
CA 02370739 2001-10-16
WO 00/65347 PCT/US00/11207
34
dR ~ ( 0 RT )
_ k
dI ~ I=Io ( DIT )
In this equation dR/dI at the inflection point is the first derivative of the
output
function at that point. The term DIT is the change in the input to the
responsive system
between the first, I,, and last, IZ, points of the transition region, i.e., IZ
- Il, and the term O
RT is the change in the response of the output function between the first and
last points of
the transition region, i.e., R(IZ) - R(I~). The coefficient k is a
proportional constant that
describes the relative steepness of the slope of the output function at the
inflection point,
Io, compared to the slope of a line between the first and last points of the
transition region.
In order that the responsive system have a discontinuous output function, the
proportional
constant k must be at least about 2.0, preferably at least about 3.0, more
preferably at least
about 5.0, even more preferably at least about 10.0, with at least about 100.0
being the
most preferred.
In certain embodiments, the relative degree of steepness in the transition
region of
a discontinuous responsive system may also be modeled by a transfer function
of a control
system having a series of an integer number, n, first order lags with an equal
time
constant. The transfer function of the responsive system is defined for the
purposes of the
present invention as the ratio of the Laplace transforms of the output
(responding
variable) to the input (disturbing variable). See, e.g., Robert H. Perry & Don
Green,
Perry's Chemical Engineers' Handbook, Sixth Ed., Chap. 22 (McGraw Hill, Inc.
1984).
As shown in Figure 12, the relative degree of steepness of an output function
may be
approximated by the formula: KG(s) = K/(Ts + 1)" in which KG(s) is the
transfer
function, K is a proportional element, T is the time constant of the system,
and n is the
integer number of first order time lags. In this model, as the number n
increases, the
steepness of the output function in the transition region increases, and the
model begins to
approximate a discontinuous responsive system. Certain discontinuous
responsive
systems of the present invention preferably may be modeled by the above
formula when n
is greater than or equal to about 25, with n being greater than or equal to
about 50 being
more preferred, and n being greater than or equal to about 100 being the most
preferred.
CA 02370739 2001-10-16
WO 00/65347 PCT/US00/11207
As shown in Figure 13A, a responsive system of the present invention may
include
a single threshold level at which the responsive system may release all of its
stored energy
to perform a specific responsive function or may include multiple threshold
levels at
which the system may release a pre-designated portion of its stored energy to
perform one
or more specific responsive functions at each of the threshold levels. In an
embodiment
having a single threshold level, for example, the responsive system may
release all of its
stored energy to perform the entire responsive function when that threshold
level is met.
In such a single threshold embodiment, In this example, the discontinuous
responsive
system includes a system that has two states such as on or off. When a
threshold quantity
of an input such as a target biological material is present in the absorbent
article, the
responsive system may perform a single responsive function upon the waste, the
wearer,
the article or a component thereof, such as enveloping the waste away from the
skin of the
user or providing an easily detectable visual signal to the wearer or
caregiver. Thus, the
discontinuous responsive system may perform a one-time "switch-like" function
that
changes from one state to another in the presence of a threshold level of an
input.
Alternatively, as shown in Figure 13B, the responsive system may have multiple
threshold levels at which when each threshold level is met the system may
release a given
"quanta" of energy or deliver a given quantity of material to perform a
specific responsive
function. In this embodiment, when each threshold level is met, a portion of
the entire
responsive function may be performed and/or different independent responsive
functions
may be performed in response to different threshold levels being met. For
example, a
responsive system may monitor a target enzyme and when each threshold enzyme
level is
met may deliver an equal or unequal quantity of enzyme inhibitors) or lotion,
or deliver a
pH buffer at the first threshold level and perform another responsive function
such as
delivering a quantity of enzyme inhibitors) at the second threshold level. In
each
transition region, the responsive system responds essentially the same as the
transition
region in the single threshold embodiment described above.
In addition, a responsive system may monitor multiple inputs such as one or
more
pathogenic bacteria and/or one or more target enzymes and perform one or more
responsive functions when the threshold levels of the different inputs are met
or may
perform one responsive function only when two or more of the threshold levels
of the
CA 02370739 2001-10-16
WO 00/65347 PCT/US00/11207
36
different inputs are met. Thus, a controller may monitor multiple different
inputs and
perform a different responsive function when the threshold level of the
different inputs are
met. Alternatively, the controller may perform a logic OR-gate type function
such that a
responsive function may be performed when one or more threshold levels of the
multiple
inputs are met. The controller may also perform a logic AND-gate type function
such that
a responsive function may be performed when each threshold level of two or
more
different inputs is met.
The responsive system may also comprise a "closed loop" or an "open loop"
system. A "closed loop" system, which is also referred to as a "feedback
control loop"
system, includes distinct biosensor and actuator components and performs a
responsive
function upon the input. In some preferred embodiments, the system may also
use a
detection or a measurement of an element or a parameter of the output
condition as at
least one trigger of the responsive function that is performed upon the input.
The output
condition may be the state of the input condition after the actuator has had
the opportunity
to perform a responsive function on the input condition. The responsive
function may be
performed when the output condition reaches a threshold level, or may be
performed only
when the output condition and one or more other conditions are met. Acting
upon the
input may include acting upon the element sensed, e.g., sensing a
microorganism and
acting upon the microorganism, or may include acting upon a composition of
which the
element sensed is an integral component. As described above, a feedback
control loop
system includes at least two distinct components: the biosensor 60 and the
actuator. The
biosensor detects an event, or a parameter associated with that event. The
actuator
receives a signal and performs a responsive function on the input condition
detected by
the biosensor. The feedback control loop may further include a controller. In
this case,
the biosensor may provide a signal to the controller, and the controller may
direct the
actuator to perform a responsive function upon the input condition. The
controller may be
a separate component of the responsive system or the controller function may
be
performed by the biosensor and/or the actuator.
The feedback control loop may be "non-modulating" or "modulating." In a "non-
modulating" feedback control loop responsive system the responsive system acts
as a one-
time switch in which the actuator performs a responsive function on the input
when the
CA 02370739 2001-10-16
WO 00/65347 PCT/US00/11207
37
threshold level of the output condition is met. For example, the biosensor may
detect the
presence of or measure the concentration of a specific pathogenic
microorganism, and the
actuator may signal the caretaker of a potential incipient infection. In this
example, the
actuator acts upon the input detected by the biosensor. A "modulating"
feedback control
loop, however, includes a biosensor, an actuator and a controller. In a
modulating
feedback control loop, the output condition is monitored constantly or
repeatedly, and the
controller directs the actuator to perform a responsive function on the input
in order to
maintain the output condition at a desired set point or within a desired range
or to provide
a continuous measurement of the level or concentration of the target
biological analyte.
An "open loop" system, however, is a system that responds to the input to
perform
a responsive function without using feedback, i.e., the output has no effect
upon the
sensed input entering the system. An open loop system may include a responsive
system
that has a single device that performs the functions of both the biosensor and
the actuator
or may have distinct biosensor and actuator components in which the actuator
acts upon
something other than the input. A super absorbent polymer placed in an
absorbent core of
a disposable absorbent article, for example, provides an open loop response
because the
polymer only includes a single device that performs the functions of the
biosensor and
actuator. Alternatively, an open loop responsive system may include a
biosensor that
detects bodily waste or a component of that bodily waste, and an actuator that
performs a
responsive function in a continuous or a discontinuous manner on something
other than
the input detected by the biosensor.
The present invention includes responsive systems that provide a discontinuous
or
continuous response, whether open loop or closed loop. Other responsive
systems are
described in United States Patent Application Numbers 09/106,424 entitled
"Disposable
Article Having A Discontinuous Responsive System" filed on June 29, 1998 (P&G
Case
Number 7197); 09/107,563 entitled "Disposable Article Having A Responsive
System
Including A Feedback Control Loop" filed on June 29, 1998 (P&G Case Number
7198);
and 09/106,225 entitled "Disposable Article Having A Responsive System
Including A
Mechanical Actuator" filed on June 29, 1998 (P&G Case Number 7199), each of
which is
incorporated herein by reference.
CA 02370739 2001-10-16
WO 00/65347 PCT/US00/11207
38
The disclosures of all patents, as well as any corresponding published foreign
patent applications), and publications mentioned throughout this patent
application are
hereby incorporated by reference herein. It is expressly not admitted,
however, that any of
the documents incorporated by reference herein teach or disclose the present
invention.