Note: Descriptions are shown in the official language in which they were submitted.
CA 02370964 2001-10-22
WO 00/65149 PCT/US00/10777
SULFUR RECOVERY FROM SPENT LIQUOR
GASIFICATION PROCESS
CROSS REFERENCE TO RELATED APPLICATIONS
Reference is made to the corresponding U.S. patent application of William
Downs,
titled ULTRA HIGH PARTICULATE COLLECTION OF SUB-MICRON AEROSOLS, U.S.
Serial No. ~/~filed April 23, 1999 and the U.S. patent application of Jerry D.
Blue,
William Downs, Timothy A. Fuller, Christopher L. Verrill, Paul S. Weitzel, and
Phung H. M.
Chan, titled GASIFICATION PROCESS FOR SPENT LIQUOR AT HIGH TEMPERATURE
AND HIGH PRESSURE, U.S. Serial No.D~/~?~filed April 23, 1999, the text of
which
are hereby incorporated by reference ~s though fully set forth herein. Unless
otherwise stated,
definitions of terms in those applications are valid for this disclosure also.
FIELD AIVD BACgGROUND OF THE INVENTION
The present invention relates in general to sulfur recovery and in particular
to a new
and useful method and apparatus for recovering sulfur and other useful
products from spent
liquor gasification systems.
There is a large body of prior art relating to the removal and/or recovery of
HZS from
petroleum and natural gas processes and from pulp and paper spent liquor
chemical recovery
processes. The motivation for removing H2S from petroleum and natural gas
processes is
CA 02370964 2001-10-22
WO 00/65149 PCT/US00/10777
-2
singularly to improve the quality of the product. Usually, these processes
convert the H2S to
solid elemental sulfur because it facilitates storage and transportation. Most
sulfur thus
produced is ultimately converted to sulfuric acid at the point of use. In a
few instances, HZS
is converted to sulfuric acid directly. Prior art for H2S recovery in the pulp
and paper industry
varies according to the specifics of the process. U.S. Patent No. 3,323,858
deals with the
absorption of H2S with carbonate liquor. The carbonate liquor is then
causticized to caustic
liquor. U.S. Patent No. 4,297,330 uses hot potassium carbonate to produce an
acid gas stream
containing H2S, COZ and H20. The selectivity of that process for HZS recovery
over COZ
recovery is only about 12 to 1. By comparison, as set forth in the DESCRIPTION
OF THE
PAD EMBODIIViENTS of the present invention, the selectivity of H2S recovery
over
C02 recovery according to the present invention must be typically better than
100 to 1. The
process described in U.S Patent No. 4,297,330 is not capable of achieving that
degree of
selectivity. U.S. Patent No. 4,609,388 describes a process that separates all
of the components
of a fuel gas into separate pure component streams. This process requires the
complete
dehydration of the fuel gas. This fact alone makes this process inappropriate
for a spent liquor
gasification process. U.S. Patent No. 5,205,908 deals directly with the issue
of absorbing HZS
from a fuel gas generated by gasification of spent liquor. It is quite
specific in stating that the
absorption of HZS is done with an alkaline wash solution that is not green
liquor and has a
composition where the mole ratio OH'/HS' is greater than 8. This patent does
not deal at all
with the issue of the co-absorption of COZ and therefore is missing -primary
elements for its
practical application. U.S. Patent No. 5,556,605 uses carbonate liquor to
absorb both H2S and
C02. followed by steam stripping out the H2S and using it outside the kraft
pulping process in
a process such as the Neutral Sulfite Semi-Chemical (NSSC) pulping process.
Finally, U.S.
Patent No. 5,660,685 deals with spent liquor gasification in such a way that
H2S is removed
from the fuel gas and then returned to the gasifier so that the carbonate
liquor produced by
dissolving the molten salts from the gasifier has a very high sulfidity, and
little carbonate. In
the extreme, this approach has the possible advantage of eliminating the
causticizing step.
Although this-idea has certain appeal, it has some significantly difficult
steps; e.g., a Claus
Reactor, H2S compression and re-injection, and would be very difficult to
implement.
CA 02370964 2001-10-22
WO 00/65149 - 3 - PCT/US00/10777
SUMMARY OF THE INVENTION
The approach of the present invention to H2S recovery and reuse differs
significantly
from the prior art.
An object of the present invention is to provide a method and apparatus for
processing
a waste stream from digestion of lignocellulosic material to form useful
products, comprising:
partially oxidizing the waste stream to form hot gases and molten salts;
cooling the hot gases
and molten salts using a quench liquor to form quenched gas and carbonate
liquor; removing
particles from the quenched gas to form a raw fuel gas; removing H2S from the
raw fuel gas
using an HZS removal process which is more selective for HZS than it is for
C02, the removing
step forming usable fuel gas as one useful product, and acid gases; and
further processing the
acid gases to form additional useful products.
The various features of novelty which characterize the invention are pointed
out with
particularity in the claims annexed to and forming a part of this disclosure.
For a better
understanding of the invention, its operating advantages and specific objects
attained by its
uses, reference is made to the accompanying drawings anti descriptive matter
in which a
preferred embodiment of the invention is illustrated.
BRIEF DESCRIPTION OF THE DRAWINGS
In the drawings:
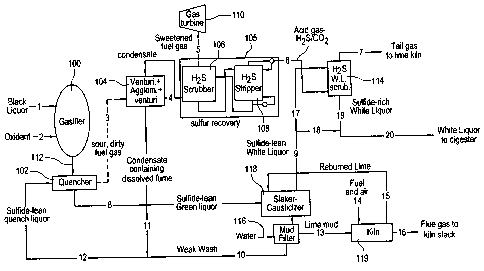
Fig. 1 is a flow chart showing the apparatus and method of the present
invention;
Fig. 2 is a graph plotting carbonate content against the hydrogen
sulfide~arbon
dioxide ratio;
Fig. 3 is a flow chart showing a typical proprietary SELEXOL process used in
accordance with the present invention;
Fig. 4 is a flow chart similar to Fig. -i, but showing a conventional process;
and
Fig. 5 is a flow chart showing an alternative embodiment employing a plurality
of
absorption-stripping units connected in series.
CA 02370964 2001-10-22
WO 00/65149 - 4 - PcTmsoono~~~
DESCRIPTION OF THE PREFERRED EMBODIMENT
Referring to the drawings generally, wherein like reference numerals designate
the
same or functionally similar elements throughout the several drawings, and to
Fig. 1 in
particular, in its broadest form the process of the present invention begins
with the
atomization, partial combustion and gasification of a mixed organic/inorganic
waste stream
(Stream 1) resulting from the digestion of wood or other lignocellulosic
materials. An oxidant
(Stream 2) such as air or oxygen is used for, the partial combustion. Of
course, while the
method anti apparatus of the present invention will likely find first
commercial application to
the processing of black liquor produced in the well known kraft pulping and
recovery process,
the present invention is not limited to that particular type of pulping
process. For example,
the present invention can also be applied to process alkaline, acidic, or
neutral sulfite spent
liquors, as well as polysulfide spent liquors: As is known to those skilled in
the art, the terms
"black liquor" or "smelt" are commonly used in connection with the kraft
pulping process,
while sulfite spent liquors are commonly called "red" liquors and not "black",
and polysulfite
pulping liquor is commonly called "orange" liquor and not "white" liquor.
Accordingly, it
will be understood that while the terms black liquor, smelt, green liquor,
white liquor, lime
mud, and weak wash have been employed in the Figures and in the following
description of
the preferred embodiment of the invention, persons skilled in the art will
appreciate that the
invention is not limited merely to the kraft pulping process. Corresponding
broader
terminology such as spent liquor, molten salts, carbonate liquor, caustic
liquor, and calcium
carbonate solids may be substituted, respectively, for those terms as
applicable, together with
the same term weak wash depending upon the particular type of pulping process
that is
involved. Such broader terminology has been employed in the claims append to
and forming
a part of this specification. Similarly, the present invention employs the
term "lignocellulosic"
to encompass all of the various types of feed stocks which one might want to
employ in a
pulping process, to broadly include woody and non-woody plants, whether or not
the kraft
type pulping process or other types of pulping processes are employed. For
further details of
the various aspects of pulping processes used in the paper industry, the
reader is referred to
STEAM Its Generation and Use, 40~' Ed., Stultz and Kitto, Eds., ~ 1992 The
Babcock &
CA 02370964 2001-10-22
WO 00/65149 - 5 - PCT/US00/10777
Wilcox Company, particularly to Chapter 26 - Chemical and Heat Recovery in the
Paper
Industry, the text of which is hereby incorporated by reference as though
fully set forth herein.
This process takes place in suspension in a gasifler vessel 100 that is
operated at above
atmospheric pressure, typically up to 800 psia, preferably between 300 and 600
psia. The hot
fuel gases produced proceed to a quench zone 102 where a spray comprising
process water aad
condensate (Stream 12), preferably a sulfide-lean quench liquor, rapidly cools
the fuel gases.
These quenched, sour, dirty fuel gases (Stream 3) will have sufficient heating
value for use in
a gas turbine, schematically indicated at 110. However, they will also contain
alkali fume,
carbonaceous aerosols, and reduced sulfur compounds that must be removed
before the fuel
gas can proceed to the gas turbine.
The particulate in the fuel gases will be predominantly sub-micron aerosol.
The fuel
gas first proceeds to a particulate removal stage 104 where up to 99.9999%
(six nines control)
of the alkali fume and carbonaceous aerosol are removed. Although this level
of particulate
removal is extreme, it is necessary to meet the very tight specification for
alkali contamination
of fuel gases entering the gas turbine 110. This particulate cleanup stage 104
will comprise
a combination of one or more inertial-type dust collectors and may include an
electrostatic dust
collector/agglomerator to meet the most severe particulate requirements. For
details of one
such type of particulate removal equipment, reference is made to the
aforementioned U.S.
Patent application of William Downs, tided ULTRA-HIGH PARTICULATE COLLECTION
OF SUB-MICRON AEROSOLS. Upon exiting from the particulate removal stage 104,
the
fuel gases (Stream 4) will then proceed to a system generally designated 105
for removal of
H2S from the fuel gas and which is designed for high selectivity of HZS over
C02. System 105
includes a process unit 106 designed to remove H2S from the fuel gas, and
preferably
comprises an absorption step or HZS scrubber and one or more stripping steps
at 108. The fuel
gases, after passing through the H2S absorption step (Stream 5), will proceed
into the gas
turbine 110 or other suitable power generation equipment such as a steam
generator. In the
power generation equipment, any residual HZS in the fuel gas will be oxidized
to SOz. S02
emissions resulting from the power generation step will be held below
environmental emission
limits by controlling the efficiency of the upstream HZS removal system 105.
CA 02370964 2001-10-22
WO 00/65149 - 6 - PCT/US00/10777
In the gasifier 100, where the organic portion of the waste was gasified by
partial
combustion and the water shift reaction, the inorganic alkali portion of this
stream 112 will be
liberated as a stream of molten salts. In the context of a kraft recovery
process, the molten
salt stream at 112 is referred to as smelt. This stream 112 consists
principally of sodium
carbonate and sodium sulfide. Much of the molten salts will impinge on the
walls of the
gasifler 100 and flow by gravity towards the quench zone 102. Some relatively
coarse droplets
of molten salts will remain suspended in the fuel gas, but both of these
streams will be
effectively captured in the quench zone 102. The fume and carbonaceous aerosol
will not be
efficiently captured in the quench zone 102 but will instead proceed along
with the fuel gas and
be collected by the particulate removal stage 104 described above. The molten
salts produced
by this high temperature, high-pressure gasification process will be lean in
sodium sulfide
when compared with those produced in a conventional Tomlinson boiler. The
aqueous fluid
stream 12 used for quenching the fuel gas will consist of condensate
containing dissolved fume
(Stream 11) and a weak alkaline process water stream commonly referred to in
the industry
as weak wash (Stream 10). This stream 10, in turn, comes from the washing with
fresh water
at 116 of the calcium carbonate precipitate (a.k.a. lime mud) that is created
from a causticizing
operation 118 to be described. The fluid used in the quencher 102 is thus a
sulfide-lean
quench liquor.
The sulfide-lean quench liquor 12, when combined in the quencher 102 with the
molten
salts at 112 from the gasifier 100, will form a solution of principally sodium
carbonate, sodium
disulfide and either sodium bicarbonate or sodium hydroxide. This solution is
known in the
kraft pulp and paper industry as green liquor or, more broadly, as carbonate
liquor. Since the
molten salts from which the carbonate liquor is formed are lean is sodium
sulfide, so is the
carbonate liquor (Stream 8), especially when compared to the carbonate liquor
formed in the
conventional kraft recovery process. This sulfide-lean carbonate liquor
(Stream 8) is next
taken to the causticizing plant 118 where the carbonate liquor first contacts
powdered lime
(Stream 15) in a conventional slaker-causticizer. The purpose of the slaker-
causticizer 118 is
to react slaked lime (calcium hydroxide) with aqueous sodium carbonate to form
solid calcium
carbonate and aqueous sodium hydroxide. A competing and undesirable reaction
is between
CA 02370964 2001-10-22
WO 00/65149 _ ~ _ PCT/US00/10777
solid calcium hydroxide and aqueous sodium sulfide to form solid calcium
sulfide and aqueous
sodium hydroxide. Since the carbonate liquor (Stream 8) of the invention is
lean in sodium
sulfide, the causticizing is therefore more efficient when compared to a
conventional kraft
recovery process. Therefore, the amount of undesirable carbonate that stays
with the caustic
liquor (a.k.a. white liquor) (Stream 9) following the causticizer 118 will be
less here than in
a conventional process.
The caustic liquor (Stream 9) produced in this causticizer 118 is deficient in
sulfide
(i.e., sulfide-lean) when compared to conventional kraft recovery processes.
For some pulping
processes this would be a desirable trait. However, for the conventional kraft
recovery
processes, high sulfidity caustic liquor is preferred. Sulfidity is an
industrial term, and is
commonly defined as the molar ratio of HS- to (HS- + OH-). To recover this
sulfur value to
the caustic liquor, it will be necessary to contact a portion of this caustic
liquor (Stream 9) with
the acid gases from the HZS stripper 108 (Stream 6). In order to do this
without overly
carbonating the caustic liquor (Stream 9), it is necessary that the molar
ratio of HZS to COZ in
Stream 6 coming from the HZS stripper 108 be greater than about 2. The
influence of the H2S
over COZ ratio on the caustic liquor (Stream 9) composition can best be
illustrated with an
example. If a tray type absorption column is used to scrub the H2S and if the
selectivity of H2S
over COZ is say 10, then an absorption column that is designed to remove 99 ~
of the HZS will
remove approximately 37% of the COZ in that gas. In this example, it is
assumed that the
sulfide-lean caustic liquor (Stream 9) has a sulfidity of 12.3 % and a
carbonation extent of
13.7 ~ . If that caustic liquor in Stream 17 contacts an acid gas (Stream 6)
containing an H2S
to COZ ratio of 2.0 in an H2S caustic liquor scrubber 114, then the sulfide-
rich caustic liquor
(Stream 19) leaving the H2S contactor or scrubber 114 in this example would
have a sulfidity
of about 32.5 % and a carbonation level of about 17.3 % . The influence of the
HZS to COZ ratio
entering the caustic liquor scrubber 114 on the caustic liquor composition
leaving the scrubber
is illustrated in Fig. 2. The amount of carbonation of the caustic liquor
(Stream 19) will
depend therefore on the ratio of HZS to COZ in the acid gas (Stream 6)
entering the caustic
liquor scrubber 114. It also depends on the selectivity of that scrubber 114
to absorb H2S in
preference to C02. Any number of commercially available absorption columns can
be used
CA 02370964 2001-10-22
WO 00/65149 PCT/IJS00/10777
_g_
for the selective absorption of HzS over COZ. The caustic liquor, (sulfide-
rich white liquor -
Stream 19) upon leaving the scrubber 114, is suitable for use as pulping
liquor without any
further treatment.
A typical fuel gas (Stream 4) composition entering the HZS removal system 105
is
depicted in Table 1.
Table 1
COMPONENT MOLE %
H20 0.69
HZ 34.48
NZ 0.63
pr 1.41
CO 31.65
COZ 26.79
CH4 2.03
HZS 2.32
The ratio of HZS to C02 in this example is 0.0866. Recall from above that the
HZS to
COZ ratio needs to be about 2.0 or higher before contacting the caustic liquor
(Stream 17).
The absorption-stripping operation therefore has two distinct functions. The
first is to reduce
the HzS concentration of the fuel gas 110 sufficiently so that when combusted
in the gas turbine
110 the S02 concentrations in the turbine exhaust will be environmentally
acceptable. The
second function is to produce an acid gas stream with an H2S to C02 ratio of
at least 2Ø This
means that the H2S selectivity over COZ must be very high. Selectivity in this
context is
defined as the ratio of mass transfer coefficients, e.g. Kgax=S I Ksacol .
Using a to represent
this selectivity, it can be shown that the selectivity can be expressed in
terms of transfer units,
NTU where NTU can be approximated by:
NTUH=S = -ln(1- s)H=s
CA 02370964 2001-10-22
WO 00/65149 - 9 - PCT/US00/10777
Then,
NTUH=s
NTUcoI
If in this example the H2S concentration leaving the scrubber 106 must be
lowered from
2.32% to 100 ppm, that will require a removal efficiency of a = 1-.0001/.0232
= .9957 or
99.57 % . Conversely, the C02 removal efficiency must be exceedingly low. If
the acid gas
is to have a ratio of HZS to COZ of 2, then no more than 2.32 x .9957/2 moles
of C02 can be
absorbed (about 1.16 moles CO~. Therefore, the C02 removal efficiency must not
exceed
1.16/26.79, or 4.33 % . Then the required selectivity must be:
-ln(1-.9957)
a = = 123.1
-ln(1-:0433)
This selectivity of 123.1 is beyond the capability of conventional absorption-
stripping
processes known to the inventors. A conventional absorption-stripping process
or system is
meant to imply a single absorpdoa tower coupled with a single stripper tower.
Even sterically
hindered tertiary amines are capable of HZS to C02 selectivities of no better
than about 30.
A system that is capable of achieving, adequate selectivity is the SELEXOL
process.
SEL.EXOL is a trademark of UOP Canada Inc., Toronto, Canada, for its process
of scrubbing
H2S. This commercially available process incorporates the use of a physical
solvent and
therefore absorbs various acid gas compounds in Proportion to their partial
pressure. The
SELEXOL solvent itself is proprietary. Solvent regeneration is by pressure
letdown of rich
solvent. The solvent can be regenerated without heat. However, to reduce
treated gas
contaminants to low concentration, the solvent can be regenerated by a
stripping medium such
as an inert gas, or regeneration can be enhanced by the application of heat.
Additional
information concerning the publicly available SELEXOL process can also be
found in
HYDROGEN PROCESSING, April 1998, page 123.
A generalized SELEXOL process flow diagram is depicted in Fig. 3. Feed gas
enters
an absorber 201 where contaminants are absorbed by the SELEXOL solvent. Rich
solvent
CA 02370964 2001-10-22
WO 00/65149 - to - PCT/US00/10777
from the bottom then flows to a recycle flash drum 202 to separate and
compress 203 any co-
absorbed product gas back to the absorber. Further pressure reduction on the
drum 204
releases off gases. In some applications, the solvent is regenerated in a
stripper column 205.
The regenerated solvent is then pumped through a cooler 206 and recycled back
to the
absorber 201.
The gases leaving the HZS removal process such as the SELEXOL process are
passed
to a tower (114 in Fig. 1) where they are contacted with a portion of the
sulfide lean caustic
liquor (Stream 17) in Fig. 1. By proper design of this caustic liquor
absorption tower 114, a
selectivity of HZS over COZ of about 10 to_ 15 is achievable. By designing
this absorption
tower to remove 99+ % of the H2S, the tail gases leaving this tower (Stream 7)
can be taken
directly to the lime kiln 119 for incineration or they can be delivered to the
pulp mill's non-
condensible odor control system.
The SELEXOL solvent and process can be obtained from UOP Canada Inc. of
Toronto
Canada. There are SELEXOL processes which are available and which can be
tailored to
specific applications to enhance process performance. In the particular case
of spent liquor
gasification, the feed gas typically has a H2S/COZ ratio which is less than
1:20. The
requirement is for selective HZS removal to less than 100 ppmv in the product
gas while
minimizing C02 co-absorption, such that the resulting acid gas to sulfur
recovery has a
HzS/C02 ratio of at least about 1:1. See Fig. 2. In order to accomplish these
goals effectively,
the basic SELEXOL process is modified to a more specialized process
illustrated in Fig. 3 that
involves both selective absorption and selective desorption/regeneration.
The person having ordinary skill in this art can therefore practice the
SELEXOL
process, H2S removal. aspects of the present invention based on publicly
available information.
An alternate to the SELEXOL process which removes more H2S than C02 is to
subject
the gases to a plurality of absorption-stripping units connected in series.
For example, suppose
that a conventional absorption-stripping system based on methyldiethanolamine
(MDEA) were
designed to contact the HZS bearing fuel gas to achieve the desired level of
HZS control. If the
fuel gas contained 1 part H2S per 23 parts CO2, the acid gas evolved from the
stripper portion
of the absorption-stripping unit could achieve a HZS to C02 ratio of about 1
part HZS to 1.8
CA 02370964 2001-10-22
WO 00/65149 PCT/US00/10777
-11
parts CO2. If this acid gas is now taken as the feed gas to a second
absorption-stripping set,
then the HZS to COZ ratio achievable could be about 1.9:1. If a still higher
ratio of HZS to COZ
is desired before contacting the acid gas with caustic liquor, as in tower 114
of Fig. 1, then
a third absorption-stripping unit could be used. Fig. 5 illustrates one form
of such a plurality
of absorption-stripping units connected in series. As shown, stream 22 is the
acid gas product
from the first absorber-stripper set. Stream 22 becomes the feed to the second
absorber-
stripper set, whose output is the acid-gas stream 6 provided to the H2S
caustic liquor scrubber
114.
The principal improvement of the process of the invention is the ability of
this
gasification system to recover the H2S from the fuel gas generated by
gasifying spent liquor
without increasing the burden on the causdcizing system. The principal
advantage has to do
with savings in energy, i.e. fuel oil, that is required to calcine calcium
carbonate that is
produced in the causticizer. This advantage is derived by adding an
intermediate step in the
HZS recovery system, i.e. the SELEXOL process or equivalent, that first
creates an acid gas
stream with a high H2S to C02 concentration before contacting the acid gas
with caustic liquor.
A more conventional approach to H2S recovery is depicted in Fig. 4. Here the
fuel gas stream
400 is contacted directly with a mixture of weak wash 402 and caustic liquor
406 in a
multistage tower 404. Because the COZ concentration is so much higher than the
H2S
concentration, most of the caustic that was produced in the causticizer 118 is
consumed by the
absorption of C02. Therefore, that extra COZ must be recycled to the
causticizer through the
quencher via streams 412 and 414 and that COZ is therefore discharged through
the lime kiln
stack. Calcining of calcium carbonate is a highly energy intensive process and
therefore
creates a significant burden on the energy efficiency of this process.
Moreover, many kraft
pulp and paper mills have limited lime processing capacity in their rotary
kih~s 410. The
additional amount of calcium carbonate that must be handled may require
additional capital
investment.
A second advantage of the present invention concerns the ability to produce
both high
sulfidity and low sulfidity caustic liquor. The portion of sulfide-lean
caustic liquor used to
recover sulfur from the acid gas becomes saturated with HS- ion. This sulfide
rich caustic
CA 02370964 2001-10-22
WO 00/65149 _ 12 - PCT/iJS00/10777
liquor can be used advantageously to improve pulp properties by application to
wood early in
the kraft digestion process. It can alternately be blended with lean caustic
liquor (Stream 18
in Fig. 1) to produce a conventional caustic liquor (Stream 20) of typical
sulfidity and
carbonate content.
While a specific embodiment of the invention has been shown and described in
detail
to illustrate the application of the principles of the invention, it will be
understood that the
invention may be embodied otherwise without departing from such principles.