Note: Descriptions are shown in the official language in which they were submitted.
CA 02371141 2001-10-22
WO 01/07834 PCT/GB00/01595
COMBUSTION OF PYROLYSIS OIL
The present invention relates to a method of combusting pyrolysis oil in a
standard
high-speed compression ignition engine, and using the heat and power produced
by the
engine to generate electricity. Pyrolysis oil is manufactured by the
controlled combustion of
biomass matter in an inert atmosphere. Pyrolysis oils have low calorific
values and poor
ignition qualities.
High-speed compression ignition engines are fuel specific and only tend to
operate
efficiently on the petrochemical-based oils that have been designed for this
type of engine.
The formulation of these mineral fuels is carefully controlled to ensure that
they combust
reliably in high-speed compression ignition engines.
l0 Carbon dioxide is emitted into the atmosphere when fossil-based fuels are
combusted.
Carbon dioxide is a greenhouse gas and it is now widely accepted that the
build up of this
gas in the atmosphere could be a major cause of global warming.
An alternative to fossil fuels would be to use oils derived from renewable
biomass
sources. One such alternative is pyrolysis oil, which is manufactured by the
controlled
combustion of plant material, such as wood chips, straw or grasses, in an
inert atmosphere.
"Pyrolysis oil" as referred to herein is not truly an "oil" as such and is
sometimes referred to
by those skilled in the art as "pyrolysis fuel" or "pyrolysis biofuel" as
well.
The carbon dioxide that is emitted during the combustion of pyrolysis oil was
recently sequestered from the atmosphere by the plants used as the raw
material to produce
the oil. These plants can be regrown after cropping and they will reabsorb
carbon dioxide
from the atmosphere. The carbon dioxide released during the combustion of
pyrolysis oil is
therefore not a net contributor towards the greenhouse effect.
A major limitation to the use of pyrolysis oil, as a replacement for
petrochemical-
based fuels, is that the oil is of relatively poor quality and difficult to
ignite.
The composition of pyrolysis oil is very different from mineral oils, and it
even
differs considerably from other potential renewable non-fossil liquid fuels
such as vegetable
oils and animal fats.
CA 02371141 2001-10-22
WO 01/07834 PCT/GB00/01595
2
Diesel fuel oil consists of a combustible mixture of alkanes and aromatic
compounds.
Pyrolysis oil, however, is a much more diverse and complex mixture of
chemicals, typically
comprising of lignin, aldehydes, carboxylic acids, carbohydrates, ketones,
phenols, alcohols,
water and char material. Pyrolysis oil typically has a high and acidic
moisture content, a low
calorific value and poor ignition qualities. The high moisture content in
particular means that
pyrolysis oil is difficult to ignite and combust. In addition to its low
calorific value and poor
quality as a fuel, pyrolysis oil may typically have a water content of from
about 15 to 30%,
and normally the water content is about 25%. The properties of diesel fuel oil
and typical
pyrolysis oil are compared in Table 1.
to
Table 1
Tynical Properties of Diesel Oil and Pwsis Oil
Property Diesel Oil Pyrolysis Oil
Calorific Value 43 17
MJ/kg
Density g/cm 0.83 1.20
Acidity pH Neutral 2.5
Moisture Content Nil 25
%
Composition Mass C=86; H=14 C=42; H=8;
% O=50 ~
2o The design and method of operation of compression ignition engines has been
established for many years. Liquid fuel is injected into air that has been
compressed by a
piston travelling up a cylinder, and the fuel and air mixture is further
compressed until the
temperature is high enough to ignite the fuel. This leads to a rapid increase
in temperature
and pressure inside the combustion chamber, which forces the piston back down
the cylinder
on its power stroke.
The engine manufacturer will normally supply a specification rating for the
engine
including a recommended power output and optimum speed setting for continuous
operation
of the engine. This is based on a specific type of fuel i.e. diesel oil for a
compression
ignition engine. The engine is not designed to run on other types of fuel.
Often a maximum
3o power output (at the optimum speed) is also specified, and beyond this
level it is expected
that inefficient combustion and undesirable black smoke production would
occur.
CA 02371141 2001-10-22
WO 01/07834 PCT/GB00/01595
The fuel for this type of engine needs the appropriate ignition properties to
be able
to ignite by means of compression only, and the formulation of conventional
diesel mineral
oil is carefully controlled to achieve these qualities.
For fuels that are more difficult to ignite, the alternative to compression
ignition is
to use a more complicated internal combustion engine design that incorporates
either a pilot
or a spark ignition system in the engine to initiate ignition of the fuel.
A combustion trial was carried out in the laboratory using a Lister-Petter
high-speed,
twin cylinder, four-stroke diesel engine, with direct fuel injection and a
nominal capacity of
one litre, and using typical pyrolysis oil as fuel.
l0 The pyrolysis oil had been manufactured from a pine wood chip feedstock,
and had
a moisture content of 24.6 %, with a pH of 2.3, and a calorific value of 17
MJ/kg. Char and
ash particles suspended in the oil that were above a certain size, for example
greater than 15
microns, would block the fuel injectors of the engine. These were removed by
centrifuging
the oil at 2500 rpm, and then utilising only the clear top layer from the
centrifuged liquor as
15 the fuel for the engine. Alternative techniques, such as filtration or
pulverisation, could also
be used to remove or reduce the larger sized particulates present in the oil,
prior to injection
into the engine.
The combustion trial, under normal operating conditions, confirmed that the
pyrolysis
oil would not reliably ignite by means of compression only in the Lister-
Petter high-speed
20 test engine.
Attempts have been made to burn pyrolysis oil in compression ignition engines
by
using a pre-ignition means eg. pre-ignition with diesel oil. Spark ignition
engines have also
been tried.
'The potential for using pyrolysis oil as a fuel would be greatly improved if
the crude
25 oil could be combusted effectively in a standard, high-speed diesel engine,
without the need
for either a special means of ignition or expensive modifications to the
composition of the
oil to improve its ignition qualities.
The present invention seeks to provide an improved method of combusting
pyrolysis
oil using a standard compression ignition engine. It has surprisingly been
found that this can
CA 02371141 2001-10-22
WO 01/07834 PCT/GB00/01595
4
be achieved by providing an enriched oxygen atmosphere in the combustion
chamber of the
engine and then running the engine at its optimum speed. From a first broad
aspect,
therefore, the invention provides a method of combusting pyrolysis oil in a
compression
ignition engine wherein an enriched oxygen atmosphere is provided in the
combustion
chamber of the engine.
Operating the engine under these conditions enables the pyrolysis oil to self
ignite
under the heat of compression only and then combust effectively and cleanly.
Using an enriched oxygen atmosphere inside the combustion chamber of internal
combustion engines has been studied before. However, past research has been
limited in
1o scope and has concentrated on conventional petrochemical-based fuels, in
combination with
oxygen enrichment, as a means of reducing the environmental pollution
associated with
engines that are used for transport applications.
There is no evidence that oxygen enrichment has been considered to aid the
combustion in compression ignition engines of low calorific, biomass based
fuels, such as
pyrolysis oil, which consist of a complex mixture of chemicals having poor
ignition qualities.
It will be appreciated that the invention also extends to a combustion system
for
operation in accordance with the invention. Viewed from a further aspect
therefore, the
invention provides a combustion system comprising of a compression ignition
engine, a
means for supplying an enriched oxygen atmosphere to the combustion chamber of
said
2o engine, and a means to supply the liquid pyrolysis fuel to said combustion
chamber.
The composition of different pyrolysis oils varies and is dependent on the
biomass
feedstock from which the oil is made and the manufacturing technique used to
produce the
oil. However one factor which is peculiar to pyrolysis oil is the high water
content, as
mentioned above. The method of the invention can surprisingly be employed to
combust
pyrolysis oil having a high water content e.g. above 10%, especially 15 to 30%
water. The
level of oxygen enrichment would be dependent on the specific composition of
the fuel.
Combustion tests with the trial pyrolysis oil suggested that generally, the
level of oxygen
enrichment would preferably be between 4% and 6% above normally aspirated
conditions
(25% oxygen, 75% nitrogen and 27% oxygen, 73% nitrogen respectively). However,
CA 02371141 2001-10-22
WO 01/07834 PCT/GB00/01595
pyrolysis oils that have very poor ignition qualities may require an oxygen
concentration
greater than 6% above normal in order to initiate ignition of the fuel. The
present invention
may thus also provide for the efficient combustion of pyrolysis oils of
different compositions
by controlling the level of oxygen enrichment in the combustion chamber of the
engine to
5 suit individual oil specifications and provide optimum combustion
conditions.
In general, the engine operating conditions, including the level of oxygen in
the air,
will be set at an optimum level determined by the quality of fuel. All the
engine operating
parameters are monitored and small adjustments in any one of them may be
desirable to
maintain efficient and smooth running of the engine.
The level of carbon monoxide (CO), oxides of nitrogen (NOx) and the exhaust
temperature in the exhaust gas stream may also be measured.
In particular, the carbon monoxide level in the exhaust gas stream is a good
indicator
of efficient combustion. Thus if carbon monoxide levels increase, the oxygen
concentration
of the inlet air stream may be adjusted to compensate so that carbon monoxide
is returned
to the desired level. Any such variation in exhaust gas emission may be caused
for example
by differences in the composition of the fuel during engine operation.
In the case of pyrolysis oil, it may be particularly desirable to control the
oxygen
concentration of the inlet gas and other operating conditions, in dependence
upon an analysis
of the CO level in the exhaust gas stream. This is because the composition and
quality of the
pyrolysis oil may vary considerably from one batch to the next and even within
the same
batch. It is therefore generally desirable to predetermine an optimum or
desired level of CO
in the exhaust gas stream which corresponds to an optimum or desired
efficiency of
combustion, and to adjust other engine operating parameters such as the oxygen
inlet
concentration in order to maintain the desired CO concentration in the exhaust
gas stream.
The oxygen level can be adjusted manually or electronically, and may
conveniently
be controlled in dependence on the analysis of carbon monoxide levels in the
exhaust gas
stream.
Using an enriched oxygen atmosphere in the combustion chamber, to aid the
combustion of the pyrolysis oil, results in an increased emission level of
nitrogen oxides,
CA 02371141 2001-10-22
WO 01/07834 PCT/GB00/01595
6
when compared to the combustion of diesel oil under naturally aspirated
conditions. The
nitrogen oxides in the exhaust gas can be abated down to an acceptable
environmental level
by means, for example, of catalytic reduction with ammonia.
The oxygen or oxygen rich air can be supplied by a number of commercially
available means, including gas separation membranes, pressure swing
adsorption, vacuum
swing adsorption and cryogenic systems. A particularly surprising benefit of
the invention
is that the gas separation system employed will also produce a supply of
nitrogen or nitrogen
rich air, which can be used to provide the inert atmosphere in the pyrolysis
oil manufacturing
process. A complete system including both the air separation module and the
pyrolysis oil
to combustion unit on the same site as the pyrolysis oil manufacturing plant
is therefore
especially advantageous. The ability to have a complementary use for the
separated gases
eliminates the need for two different gas separation systems and results in a
cost effective
gas supply to both the pyrolysis and the combustion processes.
Hence a preferred aspect of the invention is a method of combusting pyrolysis
oil
in a compression ignition engine wherein an enriched oxygen atmosphere is
provided in the
combustion chamber of the engine, wherein a gas separator provides oxygen or
oxygen
enriched air for the combustion atmosphere and also residual nitrogen or
nitrogen rich air,
the nitrogen or nitrogen rich air being used to provide an inert atmosphere in
a pyrolysis oil
manufacturing process. From a further aspect, the invention also provides a
system
2o comprising a compression ignition engine; a gas separation system for
producing oxygen or
oxygen enriched air and nitrogen or nitrogen enriched air; and a pyrolysis oil
production
plant; the oxygen or oxygen enriched air produced by the separator being
supplied to the
engine and the nitrogen or nitrogen enriched air being supplied to the
pyrolysis oil
production plant.
Heat taken from the engine cooling system and from the hot exhaust gas, as
well as
steam from the steam boiler, can also be utilised within the pyrolysis oil
manufacturing
process.
Also provided is a method of producing energy from biomass, comprising the
combustion of plant material in an inert atmosphere to produce pyrolysis oil
and the
CA 02371141 2001-10-22
WO 01/07834 PCT/GB00/01595
7
combustion of said pyrolysis oil in a compression ignition engine in an
enriched oxygen
atmosphere.
Preferably, the inert atmosphere is nitrogen or a nitrogen-containing mixture
of gases
e.g. nitrogen-rich air and even more preferably the nitrogen is provided by an
air separation
unit which also supplies oxygen for the oxygen-enriched combustion atmosphere.
The present invention will have particular application in the commercial
generation
of electricity. Accordingly, a preferred aspect of the invention includes a
method of
generating power wherein an engine operating in accordance with the invention
is coupled
mechanically to an electrical power generating device. Furthermore, the heat
from the
l0 engine can be used for localised heating purposes and/or to produce steam
to drive a steam
turbine, which in turn drives a further electrical generating device. In a
system comprising
a pyrolysis oil production plant, the heat may be used in the pyrolysis
process.
Viewed from yet a further aspect, the invention provides an electrical power
generating system comprising a generator coupled to an engine, said engine
being able to
combust poor quality pyrolysis liquid fuel by means of an enriched oxygen
atmosphere in
the combustion chamber of the engine.
The invention will now be described by way of example with reference to the
following example and to the accompanying drawings, in which:
Figure 1 shows a schematic illustration of a cylinder of a compression
ignition
2o engine.
Figure 2 shows a schematic illustration of a system to generate electricity
that utilises
pyrolysis oil as the fuel in a compression ignition engine.
Figure 3 shows a schematic illustration of a process to manufacture the
pyrolysis oil
that utilises the product streams available from the combustion system.
Figure 4 shows the difference in smoke density of the exhaust gases when
diesel oil
is burnt at 9 kWe power in 21 % oxygen (air) as compared against combustion of
pyrolysis
oil in 26% oxygen-enriched air at 8 kWe power.
CA 02371141 2001-10-22
WO 01/07834 PCT/GB00/01595
8
Example
To confirm that pyrolysis oil could be effectively combusted in a compression
ignition engine,-with the aid of an enriched oxygen atmosphere in the
combustion chamber,
practical trials were carried out in the laboratory using the Lister-Petter
test engine.
The engine was run at its point of maximum thermal efficiency that is when the
maximum Brake Mean Effective Pressure was achieved throughout the engine
revolution
range. The best operating BMEP was found to occur at a speed of 2300 rpm. The
engine was
operated in a special test rig, where the mechanical load consisted of a high
power direct
current motor with a variable field voltage.
l0 The engine manufacturer recommended that the most favourable power output,
when
running continuously at 2300 rpm and using diesel oil as fuel, was between 8
and 9-kWe.
To establish the normal engine operating parameters, the engine was first run
naturally
aspirated (21% oxygen, 79% nitrogen) at a power output of 8.5 kWe using
regular diesel oil
as fuel. The exhaust temperature was measured and the exhaust gas was analysed
for carbon
monoxide, oxides of nitrogen and smoke opacity.
The engine was then run at a power output of 8.5 kWe using the pyrolysis oil
as fuel
and an enriched oxygen atmosphere in the combustion chamber that was 5% above
normal
(26% oxygen, 74% nitrogen).
Again the exhaust emissions of carbon monoxide and oxides of nitrogen, the
exhaust
2o temperature and the opacity of the smoke from the engine were measured.
Under this enriched oxygen condition of 5% above normal it was established
that the
pyrolysis oil would self ignite by means of compression only.
The results of the trial runs with diesel oil and pyrolysis oil are compared
in Table
2. For ease of comparison most of the results are given relative to naturally
aspirated diesel
oil at 8.5 kWe power output.
CA 02371141 2001-10-22
WO 01/07834 PCT/GB00/01595
9
Table 2
Engine Trials Using_P~Ysis Oil and Diesel Oil
Property Diesel Oil Pyrolysis
Oil
21% Oxygen 26% Oxygen
Actual Power 8.5 8.5
Output kWe
Power Output 1.0 1.0
Relative
Carbon Monoxide1.0 0.46
to Emission Relative
Nitrogen Oxides1.0 2.91
Emission Relative
Actual Exhaust428 409
Temperature
C
The presence of carbon monoxide in the exhaust gas is a sign of incomplete
combustion and the level of carbon monoxide provides a good indication of the
efficiency
of the combustion process. Because of the constituents in pyrolysis oil
(aldehydes, carboxylic
acids, ketones and phenols), it is important that the pyrolysis oil is
effectively combusted so
2o that the exhaust gas emitted from the engine is as unpolluted as possible.
The results in Table 2 show that the method of the invention provided very
effective
combustion of the pyrolysis oil. The carbon monoxide emitted during the oxygen-
enriched
combustion of the pyrolysis oil was about 50% less than the level produced
with naturally
aspirated diesel oil.
The carbon monoxide concentration was in fact so low that the engine could
probably
have been operated at a power output much higher than 8.5 kWe without
producing an
unacceptable level of carbon monoxide in the exhaust gas stream.
The smoke coming from the engine when combusting the pyrolysis oil under
enriched oxygen conditions was clear, and the level of particulate matter in
the smoke was
3o significantly less than in the smoke emitted when running naturally
aspirated diesel oil. This
provided further confirmation of the effective combustion of the pyrolysis
oil. This is
illustrated in Figure 4 from which it can be seen that the exhaust gas from
pyrolysis oil
combustion was almost completely colourless, in contrast to the exhaust smoke
from diesel
CA 02371141 2004-08-23
WO 01/07834 PGT/GB00/01595
oil combustion which was visibly black and dirty.
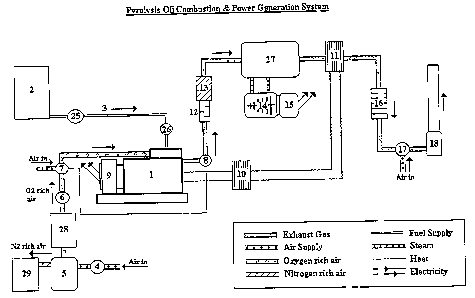
With reference to Figures I and 2, fuel 3 from storage tank 2 is pumped by
pump 25
via a control valve 26 to the fuel injector 22 of a compression ignition
engine 1. The control
valve 26 regulates the rate of fuel injection dependent on an analysis by
sensors that monitor
the engine performance.
Air is pumped by pump 4 into a gas separation unit 5. Oxygen or oxygen rich
air
from the gas separation unit 5 is transferred to a storage tank 28. The oxygen
or oxygen rich
air is pumped by pump 6 from tank 28 to control valve 7, where it is mixed
with normal
atmospheric air to the required enriched oxygen composition to provide optimum
engine
10 operating conditions. The outlet of the control valve 7 is connected to the
air intake manifold
of the engine 1.
The nitrogen or nitrogen rich residual air from thegasseparation unit 5 is
transferred
to a storage tank 29.
The oxygen rich air is introduced to the combustion chamber 19 of the engine
cylinder 20 via the air inlet valve 21 in the cylinder head of the engine and
the air is
compressed by piston 23 travelling up the cylinder.
Pyrolysis oil is injected into the combustion chamber 19 by injector valve 22.
As the
piston 23 continues on its compression stroke, the fuel ignites, the
temperature and pressure
in the combustion chamber 19 rise rapidly and the piston 23 is forced back
down the cylinder
20 on its power stroke.
The exhaust gas valve 24 opens to allow the exhaust gases lefr from the
combustion
process to be expelled when the piston 23 returns up the cylinder 20 on its
exhaust stroke.
Sensor 8, which is linked to control valve 7, continually analyses the
composition of the
exhaust gas stc~eam for example the CO concentration leaving the engine, so
that valve 7 can
adjust the level of oxygen concentration in the engine air supply to provide
optimum
combustion conditions if necessary.
The engine 1 powers a generator 9 to produce electricity. The engine cooling
system
is connected to a heat exchange unit 10 so that heat from the engine can be
used for heating
purposes, in conjunction with heat taken from the hot exhaust gas by heat
exchanger 11.
CA 02371141 2001-10-22
WO 01/07834 PCT/GB00/01595
The exhaust gas stream passes through a silencer 12 and is then treated in an
abatement unit 13 where excessive levels of oxides of nitrogen are reduced by
catalytic
reduction with ammonia. The hot exhaust gas is used to raise steam in boiler
27. Steam from
boiler 27 is supplied to a steam turbine 14, which drives a further generator
15 to produce
more electricity. Excess steam from boiler 27 can be used for other purposes.
After leaving the heat exchanger 11 the exhaust gas is cleaned in a bag filter
16 to
remove particulates that may be present in the exhaust gas stream. The exhaust
gas is then
diluted with air by an induced draft fan 17 and vented to the atmosphere
through flue stack
18.
The pyrolysis oil manufacturing process can utilise product streams from the
combustion system as illustrated with reference to Figure 3.
Organic matter 39, such as wood chips, is fed to a processing plant 40 where
it is
chopped to size and dried by heat 34 supplied from the heat exchangers 10 and
11 in the
combustion system. The dried material 41 is fed to a hopper 42.
Nitrogen or nitrogen rich air from the gas separation unit 5 that is stored in
tank 29
is fed by pump 52 into the dried material 41 and they are blown together onto
the bed of the
pyrolysis reactor 43. More nitrogen from tank 29 is pre-heated in a chamber
44, by heat 34
from heat exchangers 10 and 11, and is used to fluidise the bed of the
reactor.
Further heat 34 for the pyrolysis reaction is supplied from heat exchangers 10
and 11,
along with hot steam from the steam boiler 27, to the reactor 43. Char product
from the
reaction process is removed at a cyclone unit 45. The char is dried in a
drying unit 46 using
heat 34 from heat exchangers 10 and 11 and hot steam. The dry char is
collected for use as
a soil conditioner.
The hot pyrolysis gas, which includes water vapour, is condensed at a water-
cooled
condenser 47 and the liquid oil residues are pumped by pump 48 to an oil
separation /
filtration unit 49. The separated filtered oil is stored in tank 2 for use as
fuel in the
compression ignition engine 1.
Residual pyrolysis gas from the condenser 47, which mainly consists of
nitrogen,
carbon dioxide and a small amount of carbon monoxide, is filtered at bag
filter 50. The gas
CA 02371141 2001-10-22
WO 01/07834 PCT/GB00/01595
12
is vented to the atmosphere through flue 18 after being mixed with the exhaust
gas from the
engine and diluted with air by the induced draft fan 17.
From the above, it will be seen that the present invention enables pyrolysis
oil, a low
calorific fuel comprising of a complex mixture of chemicals with poor ignition
qualities, to
be effectively and cleanly combusted in a standard design of high-speed
compression
ignition engine. This is achieved by introducing an enriched oxygen atmosphere
to the
combustion chamber of the engine, igniting the pyrolysis oil by means of
compression only
and running the engine at its optimum speed. Pyrolysis oil is a renewable, non-
fossil fuel
manufactured from biomass material. The heat and power produced by the engine
can
l0 therefore be used to generate electricity without emitting exhaust gases to
the atmosphere
that significantly add to the greenhouse effect.
Although the research was carried out using a high-speed engine, the method of
the
invention would also be applicable to low and medium-speed compression
ignition engines.
These types of engine usually have larger cylinder bores than high-speed
engines, however,
their method of providing compression ignition of fuels is similar.