Note: Descriptions are shown in the official language in which they were submitted.
CA 02371651 2001-10-31
WO 00/66560 PCT/US00/11835
QUINAZOLINONE AND BENZOXAZINE DERIVATIVES AS
PROGESTERONE RECEPTOR MODULATORS
Field of the Invention
This invention relates to compounds which are agonists and antagonists of the
progesterone receptor, their preparation and utility.
Background of the Invention
Intracellular receptors (IR) form a class of structurally related gene
regulators
known as "ligand dependent transcription factors" (R. M. Evans, Science, 240,
889,
1988). The steroid receptor family is a subset of the IR family, including
progesterone
receptor (PR), estrogen receptor (ER), androgen receptor (AR), glucocorticoid
receptor (GR), and mineralocorticoid receptor (MR).
The natural hormone, or ligand, for the PR is the steroid progesterone, but
synthetic compounds, such as medroxyprogesterone acetate or levonorgestrel,
have
been made which also serve as ligands. Once a ligand is present in the fluid
surrounding a cell, it passes through the membrane via passive diffusion, and
binds to
the IR to create a receptor/ligand complex. This complex binds to specific
gene
promoters present in the cell's DNA. Once bound to the DNA the complex
modulates
the production of mRNA and protein encoded by that gene.
A compound that binds to an IR and mimics the action of the natural hormone
is termed an agonist, whilst a compound which inhibits the effect of the
hormone is an
antagonist.
PR agonists (natural and synthetic) are known to play an important role in the
health of women. PR agonists are used in birth control formulations, typically
in the
presence of an ER agonist. ER agonists are used to treat the symptoms of
menopause,
but have been associated with a proliferative effect on the uterus that can
lead to an
CA 02371651 2001-10-31
WO 00/66560 PCT/US00/11835
-2-
increased risk of uterine cancers. Co-administration of a PR agonist reduces
or ablates
that risk.
PR antagonists may also be used in contraception. In this context they may be
administered alone (Ulmann, et al, Ann. N. Y. Acad. Sci., 261, 248, 1995), in
combination with a PR agonist (Kekkonen, et al, Fertility and Sterility, 60,
610, 1993)
or in combination with a partial ER antagonist such as tamoxifen (WO 96/19997
A1
July 4, 1996).
PR antagonists may also be useful for the treatment of hormone dependent
breast cancers (Horwitz, et al, Horm Cancer, 283, pub: Birkhaeuser, Boston,
Mass.,
ed. Vedeckis) as well as uterine and ovarian cancers. PR antagonists may also
be
usefizl for the treatment of non-malignant chronic conditions such as fibroids
(Murphy,
et al, J. Clin. Endo. Metab. , 76, 513, 1993) and endometriosis (Kettel, et
al, Fertility
and Sterility, 56, 402, 1991).
PR antagonists may also be useful in hormone replacement therapy for post
menopausal patients in combination with a partial ER antagonist such as
tamoxifen
(US 5719136).
PR antagonists, such as mifepristone and onapristone, have been shown to be
effective in a model of hormone dependent prostate cancer, which may indicate
their
utility in the treatment of this condition in men (Michna, et al, Ann. N. Y.
Acad. Sci. ,
761, 224, 1995).
Jones, et al, (U.5. Patent No. 5,688,810) describe the PR antagonist
dihydroquinofine 1.
CA 02371651 2001-10-31
WO 00/66560 PCT/US00/11835
-3-
N
1
Me
Jones, et al, described the enol ether 2 (U.S. Patent No. 5,693,646) as a PR
ligand.
F
Me
H Me
2
Jones, et al, described compound 3 (U. S. Patent No. 5,696,127) as a PR
ligand.
F
3
Me
CA 02371651 2001-10-31
WO 00/66560 PCT/US00/11835
-4-
Zhi, et al, described lactones 4, 5 and 6 as PR antagonists (J. Med. Chem.,
41,
291, 1998).
\ 0 O Me I \ Me
\ ~ / \
Me Ae ~ Me
\ ~ O O \ N
H Me H Me
4 5 6
Zhi, et al, described the ether 7 as a PR antagonist (J. Med. Chem., 41, 291,
1998).
Me
7
Combs, et al., disclosed the amide 8 as a ligand for the PR (J. Med. Chem.,
38,
4880, 1995).
F
Br
O
8
Perlman, et. al., described the vitamin D analog 9 as a PR ligand (Tet.
Letters,
35, 2295, 1994).
Me
CA 02371651 2001-10-31
WO 00/66560 PCT/US00/11835
-5-
9
Hamann, et al, described the PR antagonist 10 (Ann. N. Y. Acad. Sci., 761,
383,
1995).
10
Chen, et al, described the PR antagonist 11 (Chen, et al, POI-37, 16't' Int.
Cong. Het. Chem., Montana, 1997).
11
Kurihari, et. al., described the PR ligand 12 (J. Antibiotics, 50, 360, 1997).
CA 02371651 2001-10-31
WO 00/66560 PCT/US00/11835
-6-
12
A number of publications reported the synthesis and utilities of
benzodiazinones and benzoxazines. However, none of examples in this literature
contained substituents necessary for the compounds to be active as
progesterone
receptor modulators. Included in this literature is the patent by Kubla et al.
(US
4666913) which claimed that the compound such as A and B could be used as
cardiotonic agents. Ning et al. reported the synthesis of quinazolinones such
as C.
Hz N
H
N ~ ~ \ NH
H O
A H
/cH2onn~
., ~o
C H
CA 02371651 2001-10-31
WO 00/66560 PCT/US00/11835
-7_
Other prior art close to this invention is the literature which disclosed the
benzoxazines. Among these publications, Gromachevskaya et al. CChem
Heterocycl.
Compd. (N.Y.), 33(10), 1209-1214 (1998)) studied the bromination process of
certain
benzoxazines such as compound D. Kobzina et al. (U.S. Patent No. 3,917,592)
claimed that compounds such as E can be used as a herbicidal agent.
HsG ,CHs
'O
\ \ CHs
CH3 CI
NOZ E
Pffegel et al. (Pharmazie, 37(10), 714-717(1982)) disclosed quinazolin-2-
thiones such as compound F in their study of polarography of heterocyclics. No
activity of the compound F was mentioned.
F
CA 02371651 2001-10-31
WO 00/66560 PCT/US00/11835
_g_
Description of the invention
The compounds of this invention have been shown to act as competitive
inhibitors of progesterone binding to the PR and act as agonists and/or
antagonists in
functional models, either/or in-vitro and in-vivo. These compounds may be used
for
contraception, in the treatment of fibroids, endometriosis, breast, uterine,
ovarian and
prostate cancer, and post menopausal hormone replacement therapy.
The compounds in the present invention contain a pendent aromatic
substituent. The aromatic substituents proved to be critical for the resultant
compounds being active as progesterone receptor modulators and have broad
structural diversity which may consists of aryl, substituted aryl, heteroaryl
or
substituted heteroaryl group.
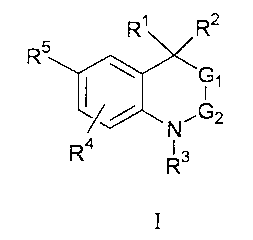
This invention provides compounds of Formula I having the structure:
R~ R2
R5
I
~/ N~G2
Ra R3
I
wherein:
R', Rz are independent substituents selected from the group which includes H,
C, to C6 alkyl, substituted C, to C6 alkyl, Cz to C6 alkenyl, substituted Cz
to C6 alkenyl,
Cz to C6 alkynyl, substituted Cz to C6 alkynyl, C3 to C8 cycloallcyl,
substituted C3 to C8
cycloalkyl, aryl, substituted aryl, heterocyclic, substituted heterocyclic,
CORA, or
NRBCORA;
or R' and Rz are fused to form:
a) an optionally substituted 3 to 8 membered spirocyclic alkyl ring;
b) an optionally substituted 3 to 8 membered spirocyclic alkenyl; or
CA 02371651 2001-10-31
WO 00/66560 PCT/US00/11835
-9-
c) an optionally substituted 3 to 8 membered heterocyclic ring
containing one to three heteroatoms from the group including O, S and N; the
spirocyclic rings of a), b) and c) being optionally substituted by from 1 to 4
groups
selected from fluorine, C, to C6 alkyl, C, to C6 alkoxy, C, to C6 thioalkyl, -
CF3, -OH, -
CN, NHz, -NH(C, to C6 alkyl), or -N(C, to C6 alkyl)z;
RA is H, C, to C3 alkyl, substituted C, to C3 alkyl, aryl, substituted aryl,
C, to
C3 alkoxy, substituted C, to C3 alkoxy, C, to C3 aminoalkyl, substituted C, to
C3
aminoalkyl,
RB is H, C, to C3 alkyl, substituted C, to C3 alkyl,
R3 is H, OH, NHz, C, to C6 alkyl, substituted C, to C6 alkyl, C3 to C6
alkenyl,
substituted C, to C6 alkenyl, alkynyl, or substituted alkynyl, CORD,
R~ is H, C, to C3 alkyl, substituted C, to C3 alkyl, aryl, substituted aryl,
C, to
C3 alkoxy, substituted C, to C3 alkoxy, C, to C3 aminoallcyl, substituted C,
to C3
aminoalkyl,
R4 is H, halogen, CN, NOz, C, to C6 alkyl, substituted C, to C6 alkyl,
alkynyl,
or substituted alkynyl, C, to C6 alkoxy, substituted C, to C6 alkoxy, amino,
C, to C6
aminoalkyl, substituted C, to C6 aminoallcyl,
RS is a trisubstituted benzene ring containing the substituents X, Y and Z as
shown below,
Y' Z
\\/~
X-
X is taken from the group including halogen, CN, C, to C3 alkyl, substituted
C,
to C3 alkyl, alkynyl, or substituted alkynyl, C, to C3 alkoxy, substituted C,
to C3
alkoxy, C, to C3 thioalkoxy, substituted C, to C3 thioalkoxy, amino, C, to C3
aminoalkyl, substituted C, to C3 aminoallcyl, NOz, C, to C3 perfluoroalkyl, 5
or 6
CA 02371651 2001-10-31
WO 00/66560 PCT/US00/11835
- 10-
membered heterocyclic ring containing 1 to 3 heteroatoms, CORD, OCORD, or
NRECORD;
RD is H, C, to C3 alkyl, substituted C, to C3 alkyl, aryl, substituted aryl,
C, to
C3 alkoxy, substituted C, to C3 alkoxy, C, to C3 aminoalkyl, or substituted C,
to C3
aminoalkyl;
RE is H, C, to C3 alkyl, substituted C, to C3 alkyl;
Y and Z are independent substituents taken from the group including H,
halogen, CN, NOz, amino, aminoalkyl, C, to C3 alkoxy, C, to C3 alkyl, or C, to
C3
thioalkoxy;
or
RS is a five or six membered ring with 1, 2, or 3 heteroatoms from the group
including O, S, SO, SOz or NR6 and containing one or two independent
substituents
from the group including H, halogen, CN, NOz, amino, and C, to C3 alkyl, C, to
C3
alkoxy, C, to C3 aminoalkyl, CORE, or NR~CORF;
RF is H, C, to C3 alkyl, substituted C, to C3 alkyl, aryl, substituted aryl,
C, to
C3 alkoxy, substituted C, to C3 alkoxy, C, to C3 aminoalkyl, or substituted C,
to C3
aminoalkyl;
RG is H, C, to C3 alkyl, or substituted C, to C3 alkyl;
R6 is H or C, to C3 alkyl;
G, is O, NR~, or CR~Rs;
Gz is CO, CS, or CR~RB;
provided that when G, is O, Gz is CR,RB, and G, and Gz cannot both be
CR~Rs;
R~ and R8 are independent substituents selected from H or an optionally
substituted alkyl, aryl, or heterocyclic moiety;
or pharmaceutically acceptable salt thereof.
Preferred compounds are those of Formula I
CA 02371651 2001-10-31
WO 00/66560 PCT/US00/11835
-11-
R~ R2
R5
I
~/ N~G2
Ra Rs
I
wherein:
R' is H, C, to C6 alkyl, substituted C, to C6 alkyl, C3 to Cg cycloalkyl,
substituted C3 to Cs cycloalkyl, aryl, substituted aryl, heterocyclic,
substituted
heterocyclic, CORA, or NRBCOR";
Rz is H, C, to C6 alkyl, substituted C, to C6 alkyl, Cz to C6 alkenyl,
substituted
Cz to C6 alkenyl, C3 to C8 cycloalkyl, substituted C3 to C8 cycloalkyl, aryl,
substituted
aryl, heterocyclic, substituted heterocyclic, CORA, or NRBCORA;
or
R' and Rz are fused to form an optionally substituted 3 to 8 membered
spirocyclic alkyl, alkenyl or heterocyclic ring containing one to three
heteroatoms from
the group including O, S and N, as described above;
RA is H, C, to C3 alkyl, substituted C, to C3 alkyl, aryl, substituted aryl,
C, to
C3 alkoxy, substituted C, to Cs alkoxy, C, to C3 aminoalkyl, or substituted C,
to C3
aminoalkyl;
RB is H, C, to C3 alkyl, or substituted C, to C3 alkyl,
R3 is H, OH, NHz, C, to C6 alkyl, substituted C, to C6 alkyl, C3 to C6
alkenyl,
substituted C, to C6 alkenyl, alkynyl, or substituted alkynyl, CORD;
R~ is H, C, to C4 alkyl, substituted C, to Ca alkyl, aryl, substituted aryl,
C, to
Ca alkoxy, substituted C, to C4 alkoxy, C, to Ca aminoalkyl, or substituted C,
to C4
aminoalkyl;
R4 is H, halogen, CN, NOz, C, to C6 alkyl, substituted C, to C6 alkyl, C, to
C6
alkoxy, substituted C, to C6 alkoxy, amino, C, to C6 aminoalkyl, substituted
C, to C6
aminoalkyl,
CA 02371651 2001-10-31
WO 00/66560 PCT/US00/11835
- 12-
RS is a trisubstituted benzene ring containing the substituents X, Y and Z as
shown below:
Y
/~
X-
X is selected from halogen, CN, C, to C3 alkyl, substituted C, to C3 alkyl, C,
to
C3 alkoxy, substituted C, to C3 alkoxy, C, to C3 thioalkoxy, substituted C, to
C3
thioalkoxy, amino, C, to C3 aminoallcyl, substituted C, to C3 aminoalkyl, NOz,
C, to C3
perfluoroalkyl, 5 membered heterocyclic ring containing 1 to 3 heteroatoms,
CORD,
OCORD, or NRECORD;
RD is H, C, to C3 alkyl, substituted C, to C3 alkyl, aryl, substituted aryl,
C, to
C3 alkoxy, substituted C, to C3 alkoxy, C, to C3 aminoalkyl, or substituted C,
to C3
aminoalkyl;
RE is H, C, to C3 alkyl, or substituted C, to C3 alkyl;
Y and Z are independent substituents taken from the group including H,
halogen, CN, NOz, C, to C3 alkoxy, C, to C3 alkyl, or C, to C3 thioalkoxy;
or
RS is a five or six membered ring with 1, 2, or 3 heteroatoms from the group
including O, S, SO, SOz or NR6 and containing one or two independent
substituents
from the group including H, halogen, CN, NOz, amino, and C, to C3 alkyl, C, to
C3
alkoxy.
R6 is H, or C, to C3 alkyl.
G, is O, NR,, or CR,RB
Gz is CO, CS, or CR,Re , with the proviso that when G, is O, Gz is CR,Ra, and
G, and Gz cannot both be CR,RB;
wherein R, and Rg are independent substituent selected from H, alkyl,
substituted alkyl, aryl, substituted aryl, heterocyclic, or substituted
heterocyclic
CA 02371651 2001-10-31
WO 00/66560 PCT/US00/11835
-13-
or a pharmaceutically acceptable salt thereof.
Still, more preferred compounds are those of Formula I
R~ R2
R5
\ ~G~
N.G2
R4 Rs
I
wherein:
R' = Rz and are selected from C, to C3 alkyl, substituted C, to C3 alkyl, or
spirocyclic alkyl constructed by fusing R' and Rz to form a 3 to 6 membered
spirocyclic ring;
R3 is H, OH, NHz, C, to C6 alkyl, substituted C, to C6 alkyl, -COH, -CO(C, to
C4 alkyl) or-CO(C, to C4 alkoxy);
R4 is H, halogen, NOz, C, to C3 alkyl, substituted C, to C3 alkyl,
RS is a disubstituted benzene ring containing the substituents X, and Y as
shown below
x
3'
4'
V
5'
X is taken from the group including halogen, CN, C, to C3 alkoxy, C, to C3
alkyl, NOz, C, to C3 perfluoroalkyl, 5 membered heterocyclic ring containing 1
to 3
heteroatoms, C, to C3 thioalkoxy,
Y is a substituent on the 4' or 5' position from the group including H,
halogen,
CN, NOz, C1 t0 C3 alkoxy, Cl t0 C4 alkyl, C1 t0 C3 tlnoalkoxy
or
RS is a five membered ring with the structure shown below
CA 02371651 2001-10-31
WO 00/66560 PCT/US00/11835
-14-
x'
v,
a
U is O, S, or NR6,
R6 is H, or C, to C3 alkyl, C, to C4 COzalkyl,
X' is from the group including halogen, CN, NOz, C, to C3 alkyl and C, to C3
alkoxy;
Y' is from the group including H and C, to Ca aryl
or
RS is a six membered ring with the structure shown
X1
N~
X' is N or CXz,
Xz is halogen, CN, alkoxy, or NOz,
G, is O, NR~, or CR~Rg
Gz is CO, CS, or CR,RB
provided that when G, is O, Gz is CR,RB, and G, and Gz cannot both be
CR,RB;
wherein R~ and R8 are independent substituents selected from H, alkyl,
substituted alkyl, aryl, substituted aryl, heterocyclic, or substituted
heterocyclic;
or pharmaceutically acceptable salt thereof
Still. even more preferred compounds are those of Formula I
CA 02371651 2001-10-31
WO 00/66560 PCT/US00/11835
-15-
R~ R2
R5
I
~/ N~G2
R4 R3
I
wherein:
R' = Rz and are selected from the group which includes CH3 and spirocyclic
alkyl constructed by fusing R' and RZ to form a 6 membered spirocyclic ring,
R3 is H, OH, NHz, CH3> substituted methyl, CORD,
R~ is H, C, to C3 alkyl, C, to C4 alkoxy,
R4 is H, halogen, C, to C3 alkyl,
RS is a disubstituted benzene ring containing the substituents X, and Y as
shown below
x
3'
4'
Y-
5'
X is taken from the group including halogen, CN, methoxy, NOz, 2-thiazole,
Y is a substituent on the 4' or 5' position from the group including H and F,
or
RS is a five membered ring with the structure shown below
x'
Y'
U
U is O, S, or NH,
X' is from the group including halogen, CN, NO2,
Y' is from the group including H and C, to C4 allcyl
G, is O, NR~, or CR~Rs
CA 02371651 2001-10-31
WO 00/66560 PCT/US00/11835
- 16-
Gz is CO, CS, or CR,RB
provided that when G, is O, Gz is CR,Rs, and G, and Gz cannot both be
CR,RB;
R, and R8 are independent substituent selected from H, alkyl, substituted
alkyl,
aryl, substituted aryl, heterocyclic, or substituted heterocychc;
and pharmaceutically acceptable salts thereof.
The compounds of this invention may contain an asymmetric carbon atom and
some of the compounds of this invention may contain one or more asymmetric
centers
and may thus give rise to optical isomers and diastereomers. While shown
without
respect to stereochemistry in Formula I, the present invention includes such
optical
isomers and diastereomers; as well as the racemic and resolved,
enantiomerically pure
R and S stereoisomers; as well as other mixtures of the R and S stereoisomers
and
pharn~aceutically acceptable salts thereof. It will be understood by one
skilled in the
art that the number of substituents listed for spirocyclic or
heterospirocyclic rings
formed by fusing R, and Rz will be determined by the size of the spirocyclic
ring.
The term "alkyl" is used herein to refer to both straight- and branched-chain
saturated aliphatic hydrocarbon groups having one to eight carbon atoms;
"alkenyl" is
intended to include both straight- and branched-chain alkyl group with at
least one
carbon-carbon double bond and two to eight carbon atoms; "alkynyl" group is
intended to cover both straight- and branched-chain alkyl group with at least
one
carbon-carbon triple bond and two to eight carbon atoms.
The terms "substituted alkyl", "substituted alkenyl", and "substituted
alkynyl"
refer to alkyl, alkenyl, and alkynyl as just described having one or more
substituents
from the group including halogen, CN, OH, NOz, amino, aryl, heterocyclic,
substituted
aryl, substituted heterocyclic, alkoxy, aryloxy, substituted alkyloxy,
alkylcarbonyl,
alkylcarboxy, alkylamino, arylthio. These substituents may be attached to any
carbon
of alkyl, alkenyl, or alkynyl group provided that the attachment constitutes a
stable
chemical moiety.
CA 02371651 2001-10-31
WO 00/66560 PCT/US00/11835
-17-
The term "aryl" is used herein to refer to an aromatic system which may be a
single ring or multiple aromatic rings fused or linked together as such that
at least one
part of the fused or linked rings forms the conjugated aromatic system The
aryl
groups include but not limited to phenyl, naphthyl, biphenyl, anthryl,
tetrahydronaphthyl, phenanthryl.
The term "substituted aryl" refers to aryl as just defined having one to four
substituents from the group including halogen, CN, OH, NOZ, amino, alkyl,
cycloalkyl,
alkenyl, alkynyl, alkoxy, aryloxy, substituted alkyloxy, alkylcarbonyl,
alkylcarboxy,
alkylamino, or arylthio.
The term "heterocyclic" is used herein to describe a stable 4- to 7-membered
monocyclic or a stable multicyclic heterocyclic ring which is saturated,
partially
unsaturated, or unsaturated, and which consists of carbon atoms and from one
to four
heteroatoms selected from the group including N, O, and S atoms. The N and S
atoms
may be oxidized. The heterocyclic ring also includes any multicyclic ring in
which any
of above defined heterocyclic rings is fused to an aryl ring. The heterocyclic
ring may
be attached at any heteroatom or carbon atom provided the resultant structure
is
chemically stable. Such heterocyclic groups include, for example,
tetrahydrofuran,
piperidinyl, piperazinyl, 2-oxopiperidinyl, azepinyl, pyrrolidinyl,
imidazolyl, pyridyl,
pyrazinyl, pyrimidinyl, pyridazinyl, oxazolyl, isoxazolyl, morpholinyl,
indolyl,
quinolinyl, thienyl, furyl, benzofuranyl, benzothienyl, thiamorpholinyl,
thiamorpholinyl
sulfoxide, and isoquinolinyl.
The term "substituted heterocyclic" is used herein to describe the
heterocyclic
just defined having one to four substituents selected from the group which
includes
halogen, CN, OH, NO2, amino, alkyl, substituted alkyl, cycloalkyl, alkenyl,
substituted
alkenyl, alkynyl, alkoxy, aryloxy, substituted alkyloxy, alkylcarbonyl,
alkylcarboxy,
allcylamino, or arylthio. The term "alkoxy" refers to the OR group, where R is
alkyl or
substituted alkyl. The term "aryloxy" is used herein to indicate the OR group,
where R
is aryl or substituted aryl. The term "alkylcarbonyl" refers to the RCO group,
where R
CA 02371651 2001-10-31
WO 00/66560 PCT/US00/11835
-18-
is alkyl or substituted alkyl. The term "alkylcarboxy" refers to the COOR
group,
where R is alkyl or substituted alkyl. The term "aminoallcyl" refers to both
secondary
and tertiary amines wherein the alkyl or substituted alkyl groups, containing
one to
eight carbon atoms, which may be either same or different and the point of
attachment
is on the nitrogen atom The term "halogen" refers to Cl, Br, F, and I element.
The compounds of this invention can be prepared following the Schemes
illustrated below:
S cheme I
:00R'
F:MgBr, THF, rt, Nx '
Rz
1
NHz
F
A~
OH
ArB(OH)z, Pd(Ph3P)a, NaxC03 OH
DME/HzO, Nz, 85 degrees C
R3
Rz 3
A
R6COR~, p-TSA, toulene
Rs
R
Rz 4
As demonstrated in Scheme I, the compounds of this invention are generally
prepared by employing the suitable coupling reaction as a key step. An
appropriately
substituted ortho-amino benzoic acid or its derivatives such as ethyl ester (X
= Br, I,
CA 02371651 2001-10-31
WO 00/66560 PCT/US00/11835
- 19-
Cl, or a latent coupling precursor such as alkoxy group which can be converted
into
OTf group suitable in the coupling reaction) was treated with a suitable
organo
metallic reagent, e.g. Grignard reagent, in appropriate nonprotic solvents
which
include but are not limited to THF or ether to give ortho-amino carbinol 2
under an
inert atmosphere such as argon or nitrogen at -78 °C to room
temperature. The
arylation of amino carbinol 2 to yield 3 can be effected by various coupling
reactions
including Suzuki, Stille reactions. These reactions are commonly performed in
the
presence of a transition metallic catalyst, e.g., palladium or nickel complex
often with
phosphino ligands, e.g., Ph3P, dppf, dppe or a catalyst such as palladium
acetate.
Under this catalytic condition, an appropriately substituted nucleophilic
reagent, e.g.,
aryl boronic acid, arylstannane, or aryl zinc compound, is coupled with amino
carbinol
2 to give 3. If a base is needed in the reaction, the commonly used bases
include but
are not limited to sodium bicarbonate, sodium carbonate, potassium phosphate,
barium
carbonate, cesium fluoride, or potassium acetate. The most commonly used
solvents
in these reactions include benzene, DMF, isopropanol, ethanol, DME, ether,
acetone,
or a mixture of above solvent and water. The coupling reaction is generally
executed
under an inert atmosphere such as nitrogen or argon at temperatures ranging
from
room temperature to 95 °C. The compounds of this invention 4 can be
effected by
treatment of amino carbinol 3 with an appropriate ketone in the presence of an
suitable
acid catalyst such as p-toluenesulfonic acid in a suitable solvent such as
toluene,
benzene under an inert atmosphere such as argon or nitrogen at room
temperature to
reflux.
Scheme II describes the procedure to prepare benzoxazines bearing two
different substituents at position-4. The Weinreb amide 6 can be prepared from
an
appropriately substituted isatoic anhydride 5 when treated with N , O-
dimethylhydroxyl-amine hydrochloride salt in a protic solvent such as ethanol,
or
isopropanol at reffux under an inert atmosphere such as argon or nitrogen.
Coupling
of amide 6 with an aryl electrophile such as aryl boronic acid or arylstannane
to give 7
CA 02371651 2001-10-31
WO 00/66560 PCT/US00/11835
-20-
can be effected by employing a typical coupling reaction such as Suzuki,
Stille
coupling procedure in a similar fashion as described for the preparation of
compound
3. Treatment of Weinreb amide 7 with organo metallic compounds, e.g.,
alkyllithium,
alkynyllithium, aryllithium, or their Grignard counterpart in a nonprotic
solvent such as
THF or ether under an inert atmosphere such as argon or nitrogen at -78
° to room
temperature affords amino ketone 8. Conversion of ketone 8 to carbinol 9 can
be
effected by treatment of 8 with an organo metallic reagent such as alkyl,
alkynyl, or
aryl Grignard reagent in a nonprotic solvent such as THF or ether under an
inert
atmosphere such as argon or nitrogen at -78 °C to room temperature.
Conversion of
ketone 8 to carbinol 9 can also be effected by reduction of ketone group of 8
to the
carbinol moiety of 9 using an appropriate reducing reagent such as lithium
aluminum
hydride, sodium borohydride in a suitable solvent such as THF, ether, or
anhydrous
alcohol under an inert atmosphere in the temperature ranging from 0 °C
to the boiling
point of the solvent. Further conversion of 9 to the compounds of this
invention can
be effected as described in scheme I for the preparation of compound 4.
CA 02371651 2001-10-31
WO 00/66560 PCT/US00/11835
-21-
Scheme II
EtOH, NHOMeMe, HCI, reflex
ArB(OH)z, Pd(Pkt~P)e, Ne=CO~
DME/HzO, 85 degrees C, Nz
R6L.i or R6MgX, T'f~', -78 degrees C to rt'
R~MgX, -78 degrees C to rt, Nz
or reducing agent
Alternatively, ortho-amino ketone 8 can be prepared by treatment of ortho-
amino benzonitrile 11 with an organo metallic compound such as organo lithium
reagent or Grignard reagent in a suitable solvent such as THF or ether under
an inert
atmosphere such as argon or nitrogen at temperatures ranging from -78
°C to room
temperature as illustrated in Scheme III. Benzonitrile 11 can be readily
prepared from
an appropriately substituted benzonitrile such as bromobenzonitrile 10 using a
suitable
coupling reaction such as Stille or Suzuki protocol carried out in a similar
fashion as
described for the preparation of the Weinreb amide 7.
CA 02371651 2001-10-31
WO 00/66560 PCT/US00/11835
-22-
Scheme III
CN q CN
ArB(OH)z, NazC03
Pd(0), DME/I-IzO, N
NH2 R NI-IZ
Rz 11
R6L,i or R6MgX,0 degrees C
R2
8
Scheme IV illustrates the synthesis of 3,4-dihydroquinazolin-2-ones. The
S substituted 2-aminobenzonitrile 11 is treated with an organo metallic
compound such
as an organo lithium or Grignard reagent in a nonprotic solvent such as THF or
ether
under an inert atmosphere such argon or nitrogen at -78 °C to room
temperature to
produce an imino intermediate which is reacted with a suitable carbonate such
as
diethyl carbonate or dimethyl carbonate in situ at 0 °C to 60 °C
to give quinazolin-2-
10 ones 12. Protection of quinazolin-2-ones 12 with a suitable protective
group such as a
para-methoxybenzyl moiety can be effected by treating 12 with a suitable base
such as
potassium hydride, potassium t-butoxide, or sodium hydride followed by
addition of a
protective reagent such as para-methoxybenzyl chloride in an appropriate
solvent such
as DMF, or a mixture of solvents such as THF and DMF under an inert atmosphere
such as nitrogen or argon at 0 °C to room temperature. The Michael
addition of a
suitable organo metallic compound such as organo lithium or Grignard reagent
to the
CA 02371651 2001-10-31
WO 00/66560 PCT/US00/11835
-23-
protected quinazolin-2-ones 12 to give 13 can be accomplished in the presence
of a
suitable Lewis acid such as magnesium triflate in a nonprotic solvent such as
THF or
ether under an inert atmosphere such as argon or nitrogen at 0 °C to
room
temperature.
Scheme IV
CN
~z
R6IvlgBr or R 6I,i, ether
MezC03, rt to 60 degrees C
Rz 11
Rz 12
(1) PMBC1, NaH, DMF, 0 degrees C to rt
(2) Mg(OTf)z, R yLi or R ~MgBr
THF, 0 degrees C to r~ Nx
Rz
13
TFA, CHxCIx, or Can, 0 degrees to rt
or 1 R
( ) 8l, DMF, NaH, 0 degrees C to rt
(2) TFA, CHaCIz> or Can, 0 degrees C to rt
1
Rz
14
Lawesson's reagent, toluene
w
reflux, Nx
Rz
CA 02371651 2001-10-31
WO 00/66560 PCT/US00/11835
-24-
Removal of the protective group can be effected by treating 13 with a suitable
deprotecting reagent, e.g. for the para-methoxybenzyl protective group it can
be
removed by treatment of 13 with protic acid such as TFA or with Ceric ammonium
nitrate in a suitable solvent such as methylene chloride at 0 °C to
room temperature
under an inert atmosphere such as argon or nitrogen. Prior to the removal of
protective group, the alkylation of 3-nitrogen can be achieved by treating 13
with an
appropriate base such as sodium hydride, potassium hydride, or potassium t-
butoxide
in a suitable solvent such as DMF followed by quenching the reaction solution
with an
organo iodide or an organo triflate such as iodomethane tinder an inert
atmosphere
such as argon or nitrogen at 0 °C to room temperature. The compounds of
the present
invention 14 can be prepared when the protective group is removed with a
stutable
reagent, e.g. for the para-methoxybenzyl protective group it can be removed by
treatment of 13 with protic acid such as TFA or with Ceric ammonium nitrate in
a
suitable solvent such as methylene chloride at 0 °C to room temperature
under an inert
atmosphere such as argon or nitrogen.
The conversion of compounds 14 to 3,4-dihydroquinazolin-2-thiones 15 can be
accomplished by treatment of 14 with a stutable sulfur reagent such as
Lawesson's
reagent in a nonprotic solvent such as o-xylene, chlorobenzene, or toluene
under an
inert atmosphere such as argon or nitrogen at reffux.
As illustrated in scheme V, the compounds 14 or 15 can be further derivatized
at position-1 via numerous approaches leading to a variety of the novel
derivatives
including 1-alkyl, substituted 1-alkyl, 1-carbonyl, substituted 1-carbonyl, 1-
carboxy,
substituted 1-carboxy derivatives. For example, alkyl or substituted alkyl
derivatives
16 or 17 can be formed by treatment of carba.mate 14 or 15 with a suitable
base such
as soditun hydride in a suitable solvent such as DMF under an inert atmosphere
such as
argon or nitrogen followed by addition of an appropriate electrophile such as
alkyl or
substituted alkyl bromide, iodide, or triffate. Such transformation of 14 or
15 to 16 or
17 at position-1 can also be effected using biphasic condition as indicated in
scheme V
CA 02371651 2001-10-31
WO 00/66560 PCT/US00/11835
-25-
in which allcylation is executed using a biphasic catalyst such as
tributylammonium
bromide in a suitable solvent such as acetonitrile. A further example of such
modification in position-1 includes but not limited to the one depicted in
scheme V in
that heating of 14 or 15 with triethyl orthoformate affords 1-substituted
derivatives of
compound 14 or 15.
Scheme V
X, NaH, DMF,
r RX, KxC03, CH3CN,
3wNBr
or CH(OEt)3, heat
R R2
2
14(X=0) 16(X=0)
(X=S) 17 (X=S)
RCOX, CH,CN, DMAP
18 (X=O)
19 (X=S)
C1N2,NaH, THF, EtrO
R2
(X=O)
21 (X=S)
CA 02371651 2001-10-31
WO 00/66560 PCT/US00/11835
-26-
The acylation or carboxylation of the compound 14 or 15 at position-1 to give
compound 18 or 19 can be readily effected by treatment of 14 or 15 with a
suitable
acylating or carboxylating reagent such as di-t-butyl dicarbonate in the
presence of a
suitable basic catalyst such as DMAP in a suitable solvent such as
acetonitrile under an
inert atmosphere such as argon or nitrogen. The amination of position-1 of
compound
14 or 15 to give compounds 20 and 21 can be furnished using a suitable
aminating
reagent such as chloroamine in the presence of a suitable base such as sodium
hydride
in a suitable solvent such as THF or diethyl ether following the literature
procedure
(Metlesics et al. J. Org. Chem. 30, 1311(1965).).
According to scheme VI an appropriate aniline such as 4-bromoaniline 22, is
reacted in the presence of a base in a suitable nonprotic solvent with an
acryloyl
chloride 23 to form the amide 24. The base is preferably a strong base such as
sodium
hydride or sodium or potassium hexamethyldisilylamide, utilizing THF as the
solvent
under an inert atmosphere (nitrogen or argon) from 0°C up to the reflux
temperature
of the solvent.
CA 02371651 2001-10-31
WO 00/66560 PCT/US00/11835
-27-
Scheme VI
Br Br
23 O R~ ~ O Ri
~~NH CI'~~Rz R~ ~ ~ Rz
R 2 H
24
22
R~ R2
Br
A1C13, heat
R/ ~ N
H
Ri R2
Ar
Pd(0), base,
ArB(OH)z
N
H
26
Ri Rz
Ar
Lawesson's agent
R~ ~ H S
27
Reaction of the amide 24 under strongly acidic conditions, sulfuric acid,
borontriffuoride etherate, or preferably aluminum chloride either as a melt,
or in an
inert solvent (dichlorobenzenes) under an inert atmosphere (nitrogen or argon)
from 0
5 °C up to the reflex temperature, of the solvent then provides the
cyclic amide 25.
Subsequent reaction of compound 25 with an aryl or heteroaryl boronic acid,
boronic
acid anhydride or trialkyl stannane then provides access to the desired biaryl
compound
26. The reaction can be carried out in a solvent such as acetone, ethanol,
benzene,
toluene or THF, under an inert atmosphere (nitrogen or argon) from 0 °C
up to the
10 reflex temperature of the solvent, in the presence of a palladium catalyst
such as
CA 02371651 2001-10-31
WO 00/66560 PCT/US00/11835
-28-
tetrakis(triphenylphosphine) palladium (0) or palladium acetate and may
require an
additive such as sodium carbonate, cesium fluoride or potassium phosphate.
The conversion of compounds 26 to thioamide 27 can be accomplished by
treatment of 26 with a suitable sulfur reagent such as Lawesson's reagent in a
nonprotic solvent such as o-xylene, chlorobenzene, or toluene under an inert
atmosphere such as argon or nitrogen at reflux.
Scheme VII
R~ RZ
R~ R2 NaH gr \
Br I \
_ ~ N 0
O ~ \ /
25 I ~ Oi
28
R~ R2 R~ Rz
Pd(0), Base, Ar I \ 1.~ Ar \
N O
2.NH4C1 N
ArB(OH)z \
o~
29
R~ R2
Pd/C, HZ Ar \
N
H
31
10 According to scheme VII, an appropriate cyclic amide such as 25, is allowed
to
react with NaH in THF to form the anion species and then a benzyl halide is
added to
convert the starting material to the N-protected amide product, 28. Reaction
of 28
with an aryl boronic acid in the presence of a palladium catalyst such as
tetrakis(triphenylphosphine)palladium(0) or palladium acetate permits a
coupling of the
15 two aromatic species to yield 29. The reaction is normally carried out
under biphasic
CA 02371651 2001-10-31
WO 00/66560 PCT/US00/11835
-29-
conditions. That is, water is often employed along with an appropriate organic
solvent, such as toluene or DMF. The palladium catalyst is typically added
last and the
reaction mixture is refluxed in the presence of an inert gas such as nitrogen.
The
product is treated with a Grignard Reagent, an alkyl magnesium halide, in THF
followed by the addition of ammonium chloride solution to afford the enamine
derivative 30. The reduction of the double bond in 30 and removal of the
protecting
group can be accomplished in a single step by catalytic reduction in a Parr
Hydrogenation Apparatus using palladium on charcoal to form the target
compound
31.
Scheme VIII
I.RMgX Br \
28 2.NH4C1 ~ ~ ,
N
32 I / O/
Br \
Nay 3 ( / Triflic acid Br \
N ~ ~ N
H
/ O/
33 34
CN CN
H
F \
Pd(0) , N
H
10
CA 02371651 2001-10-31
WO 00/66560 PCT/US00/11835
-30-
3-Fluoro-5-(2,4,4-trimethyl-1,2,3,4-hydro-qinolinyl)-benzonitrile (compound
35) can be prepared by Scheme VIII ,a process similar to Scheme VII .
According to
scheme VIII, compound 28 is allowed to react with a Grignard reagent, an alkyl
magnesium halide, in THF followed by the addition of ammonium chloride
solution to
afford the enamine derivative 32. Reduction of the double bond with sodium
cyanoborohydride affords the reduced derivative 33. Removal of the protecting
group
with a strong acid such as triflic or sulfuric acid affords the deprotected
compound 34
which can then be coupled with a suitably substituted phenylboronic acid in
the
presence of a palladium catalyst such as
tetrakis(triphenylphosphine)palladium(0) or
palladium acetate permits a coupling of the two aromatic species to yield 35.
The
reaction is normally carried out under biphasic conditions. That is, water is
often
employed along with an appropriate organic solvent, such as toluene or DMF.
The compounds of the present invention can be used in the form of salts
derived from pharmaceutically or physiologically acceptable acids or bases.
These
salts include, but are not limited to, the following salts with inorganic
acids such as
hydrochloric acid, sulfuric acid, nitric acid, phosphoric acid and, as the
case may be,
such organic acids as acetic acid, oxalic acid, succinic acid, and malefic
acid. Other
salts include salts with alkali metals or alkaline earth metals, such as
sodium,
potassium, calcium or magnesium in the form of esters, carbamates and other
conventional "pro-drug" forms, which, when administered in such form, convert
to the
active moiety in vivo.
This invention includes pharmaceutical compositions and treatments which
comprise administering to a mammal a pharmaceutically effective amount of one
or
more compounds as described above wherein GZ is C=O as antagonists of the
progesterone receptor. The invention further provides comparable methods and
compositions which utilize one or more compounds herein wherein Gz is C=S as
agonists of the progesterone receptor. Moreover the invention further provides
comparable methods and compositions which utilize one or more compounds herein
CA 02371651 2001-10-31
WO 00/66560 PCT/US00/11835
-31-
wherein G,=O and Gz =CR~CRB are agonists of the progesterone receptor and when
G,=CR~CRB and Gz=CR,CRB are agonists of the progesterone receptor.
The progesterone receptor antagonists of this invention, used alone or in
combination, can be utilized in methods of contraception and the treatment
and/or
prevention of benign and malignant neoplastic disease. Specific uses of the
compounds and pharmaceutical compositions of invention include the treatment
and/or
prevention of uterine myometrial fibroids, endometriosis, benign prostatic
hypertrophy;
carcinomas and adenocarcinomas of the endometrium, ovary, breast, colon,
prostate,
pituitary, meningioma and other hormone-dependent tumors. Additional uses of
the
present progesterone receptor antagonists include the synchronization of the
estrus in
livestock. When used in contraception the progesterone receptor antagonists of
the
current invention may be used either alone in a continuous administration of
between 1
and S00 mg per day, or alternatively used in a different regimen which would
entail 2-4
days of treatment with the progesterone receptor antagonist after 21 days of a
progestin, in this regimen between 0.1 and 500 mg daily doses of the progestin
(e.g.
levonorgestrel, trimegestone, gestodene, norethistrone acetate, norgestimate
or
cyproterone acetate) would be followed by between 0.1 and 500 mg daily doses
of the
progesterone receptor antagonists of the current invention.
The progesterone receptor antagonists of this invention, used alone or in
combination, can also be utilized in methods of treatment and/or prevention of
benign
and malignant neoplastic disease. Specific uses of the compounds and
pharmaceutical
compositions of invention include the treatment and/or prevention of uterine
myometrial fibroids, endometriosis, benign prostatic hypertrophy; carcinomas
and
adenocarcinomas of the endometrium, ovary, breast, colon, prostate, pituitary,
meningioma and other hormone-dependent tumors. Additional uses of the present
progesterone receptor antagonists include the synchronization of the estrus in
livestock.
CA 02371651 2001-10-31
WO 00/66560 PCT/US00/11835
-32-
The progesterone receptor agonists of this invention, used alone or in
combination, can be utilized in methods of contraception and the treatment
and/or
prevention of dysfunctional bleeding, uterine leiomyomata, endometriosis;
polycystic
ovary syndrome, carcinomas and adenocarcinomas of the endometrium, ovary,
breast,
colon, prostate. Additional uses of the invention include stimulation of food
intake.
When used in contraception the progesterone receptor agonists of the current
invention are preferably used in combination or sequentially with an estrogen
agonist
(e.g. ethinyl estradiol). The preferred dose of the progesterone receptor
agonist is
between 0.01 and 500 mg per day.
When the compounds are employed for the above utilities, they may be
combined with one or more pharmaceutically acceptable carriers or excipients,
for
example, solvents, diluents and the like, and may be administered orally in
such forms
as tablets, capsules, dispersible powders, granules, or suspensions
containing, for
example, from about 0.05 to 5% of suspending agent, syrups containing, for
example,
from about 10 to 50% of sugar, and elixirs containing, for example, from about
20 to
50% ethanol, and the like, or parenterally in the form of sterile injectable
solutions or
suspensions containing from about 0.05 to 5% suspending agent in an isotonic
medium. Such pharmaceutical preparations may contain, for example, from about
25
to about 90% of the active ingredient in combination with the Garner, more
usually
between about 5% and 60% by weight.
The effective dosage of active ingredient employed may vary depending on the
particular compound employed, the mode of administration and the severity of
the
condition being treated. However, in general, satisfactory results are
obtained when
the compounds of the invention are administered at a daily dosage of from
about 0.5 to
about 500 mg/kg of animal body weight, preferably given in divided doses two
to four
times a day, or in a sustained release form For most large mammals, the total
daily
dosage is from about 1 to 100 mg, preferably from about 2 to 80 mg. Dosage
forms
suitable for internal use comprise from about 0.5 to 500 mg of the active
compound in
CA 02371651 2001-10-31
WO 00166560 PCT/US00/11835
-33-
intimate admixture with a solid or liquid pharmaceutically acceptable carrier.
This
dosage regimen may be adjusted to provide the optimal therapeutic response.
For
example, several divided doses may be administered daily or the dose may be
proportionally reduced as indicated by the exigencies of the therapeutic
situation.
These active compounds may be administered orally as well as by intravenous,
intramuscular, or subcutaneous routes. Solid carriers include starch, lactose,
dicalcium
phosphate, microcrystalline cellulose, sucrose and kaolin, while liquid
carriers include
sterile water, polyethylene glycols, non-ionic surfactants and edible oils
such as corn,
peanut and sesame oils, as are appropriate to the nature of the active
ingredient and the
particular form of administration desired. Adjuvants customarily employed in
the
preparation of pharmaceutical compositions may be advantageously included,
such as
flavoring agents, coloring agents, preserving agents, and antioxidants, for
example,
vitamin E, ascorbic acid, BHT and BHA.
The preferred pharmaceutical compositions from the standpoint of ease of
preparation and administration are solid compositions, particularly tablets
and hard
filled or liquid-filled capsules. Oral administration of the compounds is
preferred.
These active compounds may also be administered parenterally or
intraperitoneally. Solutions or suspensions of these active compounds as a
free base or
pharmacologically acceptable salt can be prepared in water suitably mixed with
a
surfactant such as hydroxypropylcellulose. Dispersions can also be prepared in
glycerol, liquid, polyethylene glycols and mixtures thereof in oils. Under
ordinary
conditions of storage and use, these preparations contain a preservative to
prevent the
growth of microorganisms.
'The pharmaceutical forms suitable for injectable use include sterile aqueous
solutions or dispersions and sterile powders for the extemporaneous
preparation of
sterile injectable solutions or dispersions. In all cases, the form must be
sterile and
must be fluid to the extent that easy syringe ability exits. It must be stable
under
conditions of manufacture and storage and must be preserved against the
CA 02371651 2001-10-31
WO 00/66560 PCT/US00/11835
-34-
contaminating action of microorganisms such as bacterial and fungi. The
carrier can
be a solvent or dispersion medium containing, for example, water, ethanol
(e.g.,
glycerol, propylene glycol and liquid polyethylene glycol), suitable mixtwes
thereof,
and vegetable oil.
The following non-limiting examples illustrate preparation of compounds of the
invention.
EXAMPLE 1
1-(4-Amino-3'-chloro-biphenyl-3-yl)-ethanone
A mixtwe of 2-amino-5-bromo-N methoxy-N methylbenzamide (7.78g, 30
mmol), 3-chlorophenyl boronic acid (5.63g, 36 mmol),
tetrakis(triphenylphosphine)palladium (0) (1.73g, 1.5 mmol), and sodiwn
carbonate
(7.63g, 72 mmol) in a mixture of DME and water (150 mL/30 mL) was degassed to
remove the oxygen and was then heated at 85 °C under nitrogen for 3
bows. The
reaction mixtwe was cooled to room temperatwe and treated with brine (30 mL)
and
ethyl acetate (100 mL). The organic layer was separated and aqueous layer was
extracted with ethyl acetate (3x40 mL). The combined organic layers were
washed
with brine and dried with MgS04. After removal of solvent, the residue was
purified
by a flash chromatography (silica gel, hexane:ethyl acetate/1:1) to give 5-(3-
chlorophenyl)-N methoxy-N-methylbenzamide as a brown oil (5g, 57%). To a
solution of this benzamide (5g, 17.2 mmol) in anhydrous THF was added in a
dropwise fashion a solution of methyllithium in ether (1.4M, 28.6 mL, 40 mL)
at -78
°C under nitrogen. After stirring for 30 minutes, the reaction mixtwe
was treated with
a satwated aqueous ammonium chloride solution (50 mL) at -78 °C. Ethyl
acetate
(100 mL) was added, the organic layer was separated, and the aqueous layer was
extracted with ethyl acetate (3x20 mL). The combined organic layers were
washed
(brine) and dried (MgSOa). After removal of solvent, the residue was purified
by a
CA 02371651 2001-10-31
WO 00/66560 PCT/US00/11835
-35-
flash chromatography (silica gel, hexane: ethyl acetate/2:1) to afford 1-(4-
amino-3'-
chloro-biphenyl-3-yl)-ethanone as yellow solid (2g, 47%): mp 89-90 °C;
'H-NMR
(CDCl3) 8 7.89 (d, 1H, J= 2.0 Hz), 7.51 (m, 2H), 7.25-7.40 (m, 3H), 6.73 (d,
1H, J=
8.6 Hz), 6.38 (br, 2H), 2.65 (s, 3H); MS (EI) m/z 268([M+Na]+, 60%); Anal.
Calc.
For C~4H~zC1N0: C, 68.44, H, 4.92, N, 5.70. Found: C, 68.40, H, 4.89, N, 5.61.
EXAMPLE 2
1-(4-Amino-3'-chloro-biphenyl-3-yl)-dimethyl-methanol
To a solution of 1-(4-amino-3'-chloro-biphenyl-3-yl)-ethanone (.55g, 2.2
mmol) in anhydrous THF under nitrogen was added a solution of methyl magnesium
bromide (3.0 M in diethyl ether, 1 mL, 3 mmol) at 0 °C. The mixture was
slowly
warmed to room temperature and kept stirring under nitrogen for 18 hours. The
mixture was treated with 10 mL of saturated ammonium chloride aqueous solution
and
ethyl acetate (SO mL) was added. The organic layer was separated and the
aqueous
layer was extracted with ethyl acetate (3x15 mL). The combined organic layers
were
washed with brine (20 mL) and dried (MgSOa). After removal of the solvent, the
residue was purified by a flash chromatography (silica gel, hexane:ethyl
acetate/2:1) to
afford the title compound as an off white solid: 186-188 °C (HCl salt).
Anal. Calc.
For C,sH»ClzNO: C, 60.42, H, 5.75, N, 4.7. Found: C, 60.51, H, 5.62, N, 4.56.
EXAMPLE 3
6-l3-Chloro-nhenvll-2.4,4-trimethvl-2-trifluoromethvl-1.4-dihvdro-2H-
benzo f dl ( 1,31 oxazine
A mixture of (4-amino-3'-chloro-biphenyl-3-yl)-dimethyl-methanol (0.25 g,
0.95 mmol), trifiuoromethylacetone (0.16 g, 1.43 mmol), andp-toluenesulfonic
acid
(0.01 g, 0.05 mmol) in dry toluene (5 mL) was stirred under a blanket of
nitrogen for
48 hours. Upon completion of the reaction, the toluene was removed and the
residue
purified via flash chromatography (silica gel, 10% ethyl acetate/hexane) to
give 6-(3-
CA 02371651 2001-10-31
WO 00/66560 PCT/US00/11835
-36-
chloro-phenyl)-2,4,4-trimethyl-2-trifluoromethyl-1,4-dihydro-2H-benzo [d] [
1,3]-
oxazine (0.22 g, 65%) as a clear oil. The oil was dissolved in ether at -78
°C and then
treated with a solution of 1N HCl in ether to give the hydrochloride salt of
the title
compound as a white solid: 'H-NMR (DMSO-d6) 8 7.67 (bs, 1H), 7.58 (d, 1H, J=
7.88 Hz), 7.42 (m, 3H), 7.32 (d, 1H, J= 7.96 Hz), 6.84 (d, 1H, J= 8.03 Hz),
1.52 (s,
3H), 1.5 (s, 6H); MS (APCI) m/z 354 ([M-H]-, 100%); Anal. Calc. For
CIaH»C1F3N0: C, 55.12; H, 4.62; N, 3.57. Found: C, 54.97; H, 4.59; N, 3.41.
EXAMPLE 4
6-(3-Chloro-phenyl)-2,2,4,4-tetramethyl-1,4-dihydro-2H-benzofd111,31oxazine
A mixture of (4-amino-3~-chloro-biphenyl-3-yl)-dimethyl-methanol (0.46 g, 1.8
mmol), acetone (0.16 g, 2.7 mmol), andp-toluenesulfonic acid (0.017 g, 0.09
mmol) in
dry toluene (6 mL) was heated at 33 °C under a blanket of nitrogen
overnight. Upon
completion of the reaction, the toluene was removed and the compound purified
via a
flash chromatography (silica gel, 15% ethyl acetate/hexane) to give 6-(3-
chloro-
phenyl)-2,2,4,4-tetramethyl-1,4-dihydro-2H-benzo[d][1,3]oxazine (0.36 g, 68%)
as a
yellow oil. The oil was dissolved in ether at -78 °C and then treated
with a solution of
1N HCl in ether to give the hydrochloride salt of the title compound as a
yellow solid:
'H-NMR (DMSO-d6) 8 7.70 (bs, 1H), 7.61 (d, 1H, J= 7.82 Hz), 7.52 (bs, 1H),
7.44
(m, 2H), 7.33 (d, 1H, J= 8.21 Hz), 6.87 (d, 1H, J= 7.85 Hz), 1.5 (s, 6H), 1.4
(s, 6H);
MS (ESI) m/z 302 ([M+H]+, 100%); Anal. Calc. For C,sHzoClNO: C, 63.91; H,
6.26; N, 4.14. Found: C, 64.08; H, 6.43; N, 4.14.
EXAMPLE 5
6-(3-Nitro-nhenvl)-2,2,4; trimethyl-1,4-dihydro-2H-benzold111,31oxazine
Prepared from 1-(4-amino-3'-nitro-biphenyl-3-yl)-ethanol and acetone in the
same fashion as that of Example 4. Yellow solid: mp 188-189 °C; Anal.
Calc. For
C"H,8Nz03~0.35 H20: C, 67.02; H, 6.19; N, 9.20. Found: C, 66.7; H, 5.89; N,
9.03.
CA 02371651 2001-10-31
WO 00/66560 PCT/US00/11835
-37-
EXAMPLE 6
4-Amino-3'-chloro-binhenyl-3-carbonitrile
A mixture of 2-amino-5-bromobenzonitrile (1 Og, 50 mmol), 3-chlorophenyl
boronic acid (9.5g, 60 mmol), tetrakis(triphenylphosphine)-palladium (0)
(3.5g, 3
mmol), and sodium carbonate (13g, 120 mmol) in a mixture of DME and water (100
mL/25 mL) was degassed to remove the oxygen and then heated to 85 °C
under a
blanket of nitrogen for 5 hours. The reaction mixture was cooled to ambient
temperature and quenched with a saturated aqueous ammonium chloride solution
(80
mL). Ethyl acetate (200 mL) was added, the organic layer was separated, and
the
aqueous layer was extracted with ethyl acetate (3x40 mL). The combined organic
layers were washed with brine and dried with MgS04. The solvent was removed in
vacuo and the residue was purified by a silica gel flash chromatography
(hexane: ethyl
acetate/4:1) to afford 4-amino-3'-chloro-biphenyl-3-carbonitrile as an
of~white solid
(8g, 87%): mp 118-119 °C; 'H-NMR (DMSO-d6) 8 7.80 (d, 1H, J= 2.3 Hz),
7.65-
7.72 (m, 2H), 7.57 (d, 1H, J= 8.0 Hz), 7.42 (t, 1H, J= 7.9 Hz), 7.31 (m, 1H),
6.87
(d, 1H, J= 8.7 Hz), 6.29 (br, 2H); Anal. Calc. For C,3H9C1Nz: C, 68.28, H,
3.97, N,
12.25. Found: C, 67.68, H, 4.06, N, 11.89.
EXAMPLE 7
6-(3-Chlorophenyl)-4-cyclonrouyl-1H-guinazolin-2-one
Prepared using a similar procedure as described in the literature (Tucker et
al.
J. Med. Chem., 1994, 37, 2437-2444). To a solution of cyclopropylinagnesium
bromide prepared from magnesium (0.9g, 37 mmol) and cyclopropyl bromide (3.2
mL,
40 mmol) in anhydrous THF was added at 50 °C under nitrogen a solution
of 4-amino-
3'-chloro-biphenyl-3-carbonitrile (2.3g, 10 mmol) in anhydrous THF. After
addition,
the reaction mixture was kept at 50 °C for 30 minutes under nitrogen
and treated with
dimethyl carbonate in a dropwise manner. The reaction solution was stirred at
50 °C
CA 02371651 2001-10-31
WO 00/66560 PCT/US00/11835
-38-
under nitrogen for 30 minutes and cooled to ambient temperature. A saturated
aqueous ammonium chloride solution (30 mL) was added followed by addition of
ethyl
acetate (80 mL). The organic layer was separated and the aqueous layer was
extracted
with ethyl acetate (3x40 mL). The combined organic layers were washed with
brine
and dried with MgSOa. After removal of the solvent, the residue was purified
by a
flash chromatography (silica gel, methylene chloride:methanol/25:1) to give 6-
(3-
chlorophenyl)-4-cyclopropyl-1H-quinazolin-2-one as a yellowish solid (0.55g,
18%):
mp 189-190 °C; 'H-NMR (DMSO-d6) 8 11.71 (s, 1H, Dz0 exchangeable), 8.56
(d,
1 H, J = 1.3 Hz), 8. 09 (dd, 1 H, J = 8. 6, 1. 6 Hz), 7. 92 (s, 1 H), 7. 77
(d, 1 H, J = 7. 7 Hz),
7.52 (t, 1H, J= 7.9 Hz), 7.45 (d, 1H, J= 8.1 Hz), 7.36 (d, 1H, J= 8.6 Hz),
3.15 (m,
1H), 1.20 (m, 4H); MS (CI) m/z 297 ([M+H~+, 100%); Anal. Calc. For C"H13C1N20:
C, 69.98, H, 4.49, N, 9.23. Found: C, 67.98, H, 4.46, N, 9.10.
EXAMPLE 8
6-(3-Chlorophenyl)-4-cyclopropyl-1-(4-methoxybenzyl)-1H-QUinazolin-2-one
To a suspension of 6-(3-chlorophenyl)-4-cyclopropyl-1H-quinazolin-2-one
(0.5g, 1.68 mmol) in anhydrous DMF was added potassium hexamethylsilyl amide
(0.45g, 2.1 mmol) at ambient temperature under nitrogen. The reaction mixture
was
stirred at ambient temperature for 30 minutes, treated withp-methoxy benzyl
chloride
(0.35 mL, 2.5 mmol), and heated at 55 °C for 5 hours. The mixture was
then cooled
to room temperature and quenched with a saturated aqueous ammonium chloride
solution (10 mL). Methylene chloride (50 mL) was added and organic layer was
separated. The aqueous layer was extracted with methylene chloride (2x20 mL)
and
the combined organic layers were washed with brine and dried (MgS04). After
removal of solvent, the residue was separated on a flash chromatography
(silica gel,
hexane:ethyl acetate/l:l) to afford 6-(3-chlorophenyl)-4-cyclopropyl-1-(4-
methoxybenzyl)-1H-quinazolin-2-one as an off white solid: mp 173-174
°C; 'H-NMR
(DMSO-d6) 8 8.65 (d, 1H, J= 1.8 Hz), 8.10 (dd, 1H, J= 8.9, 1.8 Hz), 7.93 (d,
1H, J
CA 02371651 2001-10-31
WO 00/66560 PCT/US00/11835
-39-
= 1.6 Hz), 7. 79 (d, 1H, J = 7.6 Hz), 7.53 (d, 2H, J = 8.4 Hz), 7.46 (t, 1H, J
= 8.1 Hz),
7.21 (d, 2H, J = 8.6 Hz), 6. 88 (d, 2H, J = 8.6 Hz), 5.41 (s, 2H), 3. 73 (s,
3H), 3.18 (m,
1H), 1.18-1.27 (m, 4H); MS (CI) m/z 417([M+H]+, 100%); Anal. Calc. For
Cz5Hz1C1NzOz: C, 72.02, H, 5.08, N, 6.72. Found: C, 71.88, H, 4.91, N, 6.70.
6-(3-Chlorophenyl)-4-cyclopropyl-2-(4-methoxybenzyloxy)quinazoline was
obtained as a side product, off white solid: mp 158-159 °C;'H-NMR (DMSO-
d6) 8
8.75 (d, 1H, J= 1.7 Hz), 8.26 (dd, 1H, J= 8.8, 1.8 Hz), 8.01 (s, 1H), 7.84 (m,
2H),
7.56 (t, 1H, J= 7.9 Hz), 7.45 (m, 3H), 6.96 (d, 2H, J= 8.6 Hz), 5.38 (s, 2H),
3.75 (s,
3H), 3.24 (m, 1H), 1.25 (m, 4H); MS (CI) m/z 417([M+H]+, 100%); Anal. Calc.
For
CzsHz~ CINzOz: C, 72.02, H, 5.08, N, 6.72. Found: C, 72.19, H, 4.91, N, 6.65.
EXAMPLE 9
6-(3-Chlorophenvl)-4-cyclopronvl-4-methyl-3,4-dihyd ro-1H-
puinazolin-2-one
To a solution of 6-(3-chlorophenyl)-4-cyclopropyl-1-(4-methoxybenzyl)-1H-
quinazolin-2-one (0.25g, 0.6 mmol) in anhydrous ether was added, at ambient
temperature under nitrogen, magnesium trifiate (0.78g, 2.4 mmol). The mixture
was
stirred for 30 minutes and treated with a solution of methylinagnesium bromide
in
ether (3.0 M, 1.0 mL, 3.0 mmol). The reaction mixture was kept at room
temperature
under nitrogen for 3 hours and quenched with a mixture of a saturated aqueous
ammonium chloride solution (10 mL) and 1N aqueous HCl solution (5 mL). The
mixture was stirred at room temperature for 20 minutes and ethyl acetate (40
mL) was
added. The organic layer was separated and the aqueous layer was extracted
with
ethyl acetate (3x10 mL). The combined organic layers were washed with brine
and
dried (MgS04). The solvent was removed and a half portion of the residue was
dissolved in TFA (3 mL) and was stirred under nitrogen at room temperature for
72
hours. The solution was poured onto ice-water and the white precipitate
obtained was
collected on a filter. The solid was washed with water and then purified by a
flash
CA 02371651 2001-10-31
WO 00/66560 PCT/US00/11835
-40-
chromatography (methylene chloride:methanol/25:1, silica gel) to give 6-(3-
chlorophenyl)-4-cyclopropyl-4-methyl-3,4-dihydro-1H-quinazolin-2-one as off
white
solid(40 mg, 43%): mp 125-127 °C; 'H-NMR (DMSO-d6) 8 9.21 (s, 1H), 7.71
(s,
1H), 7.61 (d, 1H, J= 7.8 Hz), 7.55 (d, 1H, J= 1.6 Hz), 7.47 (dd, 1H, J= 8.3,
1.8
Hz), 7.45 (t, 1H, J= 7.8 Hz), 7.36 (d, 1H, J= 8.1 Hz), 6.84 (d, 1H, J= 8.2
Hz), 6.79
(s, 1H), 1.54 (s, 3H), 1.11 (m, 1H), 0.42 (m, 1H), 0.15-0.20 (m, 3H); MS (ESI)
m/z
313((M+H]+, 100%).
EXAMPLE 10
6-(3-Chlorophenyl)-4-cyclopropyl-3,4-dimethyl-3,4-dihydro-1H-QUinazolin-2-one
To a solution of half of the crude addition product from Example 9 in
anhydrous DMF (5 mL) was added under nitrogen at room temperature sodium
hydride (25 mg, 0.63 mmol). The mixture was stirred at ambient temperature for
30
minutes and treated with methyl iodide (0.5 mL, excess). After stirring for
3.5 hours,
a mixture of saturated aqueous ammonium chloride and 1N HCl aqueous solution
(10
mL/5 mL) was added to the reaction mixture. Ethyl acetate (30 mL) was added
and
organic layer was separated. The aqueous layer was extracted with ethyl
acetate (3x10
mL) and the combined organic layers were washed with brine and dried (MgS04).
The
solvent was removed and residue was dissolved in a mixture of methylene
chloride and
TFA (2 mL/2 mL). After stirring for 3 hours, the solution was poured onto ice-
water
and neutralized by addition of a saturated aqueous sodium bicarbonate
solution. The
ethyl acetate (30 mL) was added and organic layer was separated. The aqueous
layer
was extracted with ethyl acetate (3x10 mL). The combined organic layers were
washed with brine and dried (MgS04). The solvent was removed in vacuo and the
residue was purified by a flash chromatography (silica gel, hexane:ethyl
acetate/1:1) to
afford 6-(3-chlorophenyl)-4-cyclopropyl-3,4-dimethyl-3,4-dihydro-1H-quinazolin-
2-
one as a white solid (11 mg, 11% for three steps): mp 193-194 °C; 'H-
NMR (DMSO-
d6) 8 9.51 (s, 1H), 7.68 (s, 1H), 7.59 (d, 1H, J= 8.0 Hz), 7.57 (d, 1H, J= 1.6
Hz),
CA 02371651 2001-10-31
WO 00/66560 PCT/US00/11835
-41 -
7.51 (dd, 1H, J= 8.3, 1.7 Hz), 7.46 (t, 1H, J= 7.8 Hz), 7.35 (d, 1H, J= 8.1
Hz), 6.87
(d, 1H, J= 8.3 Hz), 3.02 (s, 3H), 1.51 (s, 3H), 1.25 (m, 1H), 0.32-0.51 (m,
3H), 0.25
(m, 1H); MS (CI) m/z 327 ([M+H]', 100%). Anal. Calc. For C,9H,gC1N2O'O.3 HZO:
C, 68.69, H, 5.95, N, 8.43. Found: C, 68.69, H, 5.70, N, 8.18.
EXAMPLE 11
6-(3-Chloro-phenyl)-4,4-dimethyl-3,4-dihydro-1H-puinolin-2-one
Sodium hydride (1.16 g, ca 29 mmol, 60% in oil) was washed with hexane (x3)
then placed in anhydrous THF (50 mL) under nitrogen. To this slurry was then
added
dropwise a solution of 4-bromoaniline (5.0 g, 29 mmol) in dry THF (100 ml).
After
0.5 h, the mixture was cooled to 0 °C and treated with a solution of
3,3-
dimethylacryloyl chloride in anhydrous THF (30 ml). After 4 h, the mixture was
quenched with saturated aqueous ammonium chloride solution, and extracted with
diethyl ether. The organic layer was washed with water, brine, dried (NaZSOa)
and
evaporated. The residue was then crystallized from EtOAc/hexane to afford N
(3,3'-
dimethylacryoloyl)-4-bromoaniline (3.36 g, 13.22 mmol, 46%) as a white solid:
1H
NMR (CDC13) 8 7.42 (s, 4H), 7.06 (s, 1H), 5.68 (s, 1H), 2.21 (s, 3H), 1.09 (s,
3H);
MS (EI) m/z 253 [M+].
N (3,3'-Dimethylacryoloyl)-4-bromoaniline (4.0 g, 15.38 mmol) was heated
under nitrogen to ca. 130 - 140 °C causing the solid to melt. Aluminum
chloride (3.07
g, 23 mmol) was added and heating continued. After 1 h, the mixture was cooled
and
quenched carefully with water and then extracted with dichloromethane (3x60
mL).
The combined organic layers were washed with water, dried (MgSOa) and
evaporated.
The residue was then subjected to column chromatography (Si02, EtOAc/hexane
gradient elution) to afford 6-bromo-4,4-dimethyl-3,4-dihydro-1H-quinolin-2-one
(1.69g, 6.6 mmol, 47%) as a white solid: 'H-NMR (CDC13) 8 8.82 (s, 1H), 7.39
(d,
1H, J= 2 Hz), 7.29 (dd, 1H, J= 8.3, 2.0 Hz), 6.71 (d, 1H, J= 8.0 Hz), 2.47 (s,
2H),
1.32 (s, 6H).
CA 02371651 2001-10-31
WO 00/66560 PCT/US00/11835
-42-
To a solution of the last cited compound (0.5 g, 1.96 mmol) in
dimethoxyethane (15 mL) was added tetrakis(triphenylphosphine)palladium (0)
(0.11
g, 0.09 mmol) under nitrogen. After 15 min., 3-chlorophenylboronic acid (0.6
g, 3.9
mmol) was added followed by potassium carbonate (1.62 g, 11.7 mmol) in water
(7.5
mL). Afler 1.5 h at reflux, the mixture was cooled, filtered, and extracted
with
EtOAc. 'The organic layer was washed with water, dried (MgS04) and evaporated.
'The residue was then subjected to column chromatography (SiOz,
EtOAc:hexane/3:1)
and crystallized from dichloromethane/hexane to afford 6-(3-chloro-phenyl)-4,4-
dimethyl-3,4-dihydro-1H-quinolin-2-one (0.21 g, 0.73 mmol, 37%) as a white
solid;
mp. 211 - 215 °C;'H-NMR (CDC13) 8 8.48 (s, 1H), 7.53 (s, 1H), 7.47 (d,
1H, J= 2.0
Hz), 7.44 - 7.29 (m, 4H), 6.87 (1H, d, J= 2.0 Hz), 2.54 (s, 2H), 1.39 (s, 6H);
MS ((-
)ES) m/z 284 [M-H]-.
EXAMPLE 12 - Pharmacolo~y
The compounds of this invention were tested in the relevant assay as described
below and their potency are in the range of 0.01 nM to 5 pM in the in vitro
assays and
0.001 to 300 mg/kg in the in vivo assays. The selected examples are listed
below
CA 02371651 2001-10-31
WO 00/66560 PCTNS00/11835
-43-
Table 1
R3
Compound R1 RZ R3 hPR CV-1
1C'~o ~~nM~1
1 Me Me Cl 10.0
2 CF3 Me Cl 50.0
3 Me H NOz 1675
R = H, hPR CV-1, ICso = 1075 nM hPR CV-1, ICso = 1075 nM
R = Me, hPR CV-1, ICso = 580 nM
CA 02371651 2001-10-31
WO 00/66560 PCT/US00/11835
-44-
A. In-vitro biology
The in-vitro biology is determined by (1) competitive
Radioligand Binding: using the A-form of the human progesterone receptor with
progesterone as the radioligand; (2) co-transfection assay, which provides
functional
activity expressed as agonist EC50 and Antagonist IC50 values; (3) a T47D cell
proliferation, which is a further functional assay which also provides agonist
and
antagonist data; and (4) T47D cell alkaline phosphatase assay, which is a
further
functional assay which also provides agonist and antagonist data.
1. hPR Binding assay - This assay is carried out in
accordance with: Pathirana, C.; Stein, R.B.; Berger, T.S.; Fenical, W.;
Ianiro, T.; Mais,
D.E.; Tones, A.; Glodman, M.E., Nonsteroidal human progesterone receptor
modulators from the marine alga cymoplia barbata, J. Steroid Biochem. Mol.
Biol.,
1992, 41, 733-738.
2. PRE-luciferase assay in CV-1 cells
The object of this assay is to determine a compound's
progestational or antiprogestational potency based on its effect on PRE-
luciferase
reporter activity in CV-1 cells co-transfected with human PR and PRE-
luciferase
plasmids. The materials methods used in the assay are as follows.
a. Medium: The growth medium was as follows:
DMEM (BioWhittaker) containing 10% (v/v) fetal bovine serum (heat
inactivated), 0.1
mM MEM non-essential amino acids, 100U/ml penicillin, 100mg/ml streptomycin,
and
2 mM GlutaMax (GIBCO, BRL). The experimental medium was as follows:
DMEM (BioWhittaker), phenol red-free, containing 10% (v/v) charcoal-stripped
fetal
bovine serum (heat-inactivated), 0.1 mM MEM non-essential amino acids, 100U/ml
penicillin, 100mg/ml streptomycin, and 2 mM GlutaMax (GIBCO, BRL).
CA 02371651 2001-10-31
WO 00/66560 PCT/US00/11835
-45-
b. Cell culture, transfection, treatment, and luciferase
assay
Stock CV-1 cells are maintained in growth medium
Co-transfection is done using 1.2x10' cells, 5 mg pLEM plasmid with hPR-B
inserted
at Sphl and BamHl sites, 10 mg pGL3 plasmid with two PREs upstream of the
luciferase sequence, and 50 mg sonicated calf thymus DNA as carrier DNA in 250
ml.
Electroporation is carried out at 260 V and 1,000 mF in a Biorad Gene Pulser
II.
After electroporation, cells are resuspended in growth medium and plated in 96-
well
plate at 40,000 cells/well in 200 p1. Following overnight incubation, the
medium is
changed to experimental medium Cells are then treated with reference or test
compounds in experimental medium Compounds are tested for antiprogestational
activity in the presence of 3 nM progesterone. Twenty-four hr. after
treatment, the
medium is discarded, cells are washed three times with D-PBS (GIBCO, BRL).
Fifty
p1 of cell lysis buffer (Promega, Madison, WI) is added to each well and the
plates are
shaken for 15 min in a Titer Plate Shaker (Lab Line Instrument, Inc.).
Luciferase
activity is measured using luciferase reagents from Promega.
c. Analysis of Results:
Each treatment consists of at least 4 replicates. Log
transformed data are used for analysis of variance and nonlinear dose response
curve
fitting for both agonist and antagonist modes. Huber weighting is used to
downweight
the effects of outliers. ECso or ICso values are calculated from the
retransformed
values. JMP software (SAS Institute, Inc.) is used for both one-way analysis
of
variance and non-linear response analyses.
d. Reference Compounds:
Progesterone and trimegestone are reference progestins and
RU486 is the reference antiprogestin. All reference compounds are run in full
dose-
response curves and the ECSO or ICSO values are calculated.
CA 02371651 2001-10-31
WO 00/66560 PCT/US00/11835
-46-
Table 2. Estimated ECSp, standard error (SE), and 95% confidence intervals
(CI) for reference progestins from three individual studies
EC50 95% CI
Compound Exp. (nM) SE lower upper
Progesterone 1 0.616 0.026 0.509 0.746
2 0.402 0.019 0.323 0.501
3 0.486 0.028 0.371 0.637
Trimegestone 1 0.0075 0.0002 0.0066 0.0085
2 0.0081 0.0003 0.0070 0.0094
3 0.0067 0.0003 0.0055 0.0082
Table 3. Estimated ICso, standard error (SE), and 95% confident interval (CI)
for the antiprogestin, RU486 from three individual studies
IC 50 95%
CI
Compound Exp. (nNi) SE lower upper
RU486 1 0.028 0.002 0.019 0.042
2 0.037 0.002 0.029 0.048
3 0.019 0.001 0.013 0.027
Progestational activity: Compounds that increase PRE-luciferase activity
significantly (p<0.05) compared to vehicle control are considered active.
Antiprogestational activity: Compounds that decrease 3 nM progesterone
induced PRE-luciferase activity significantly (p<0.05)
ECSO: Concentration of a compound that gives half maximal increase PRE-
luciferase activity (default-nM) with SE.
ICSO: Concentration of a compound that gives half maximal decrease in 3 nM
progesterone induced PRE-luciferase activity (default-nM) with SE.
CA 02371651 2001-10-31
WO 00/66560 PCT/US00/11835
-47-
3. T47D cell proliferation assaX
The objective of this assay is the determination of
progestational and antiprogestational potency by using a cell proliferation
assay in
T47D cells. A compound's effect on DNA synthesis in T47D cells is measured.
The
materials and methods used in this assay are as follows.
a. Growth medium: DMEM: F 12 ( 1:1 )
(GIBCO, BRL) supplemented with 10% (v/v) fetal bovine serum (not heat-
inactivated), 100U/ml penicillin, 100mg/ml streptomycin, and 2 mM GlutaMax
(GIBCO, BRL).
b. Treatment medium Minimum Essential
Medium (MEM) (#51200-038GIBC0, BRL) phenol red-free supplemented with 0.5%
charcoal stripped fetal bovine serum, 100U/ml penicillin, 200 mg/ml
streptomycin, and
2 mM GlutaMax (GIBCO, BRL).
c. Cell culture
Stock T47 D cells are maintained in growth medium For BrdU incorporation
assay, cells are plated in 96-well plates (Falcon, Becton Dickinson Labware)
at 10,000
cells/well in growth medium. After overnight incubation, the medium is changed
to
treatment medium and cells are cultured for an additional 24 hr before
treatment.
Stock compounds are dissolved in appropriate vehicle (100% ethanol or 50%
ethanol/50% DMSO), subsequently diluted in treatment medium and added to the
cells. Progestin and antiprogestin reference compounds are run in full dose-
response
curves. The final concentration of vehicle is 0.1 %. In control wells, cells
receive
vehicle only. Antiprogestins are tested in the presence of 0.03 nM
trimegestone, the
reference progestin agonist. Twenty-four hours after treatment, the medium is
discarded and cells are labeled with 10 mM BrdU (Amersham Life Science,
Arlington
Heights, IL) in treatment medium for 4 hr.
CA 02371651 2001-10-31
WO 00/66560 PCT/U~00/11835
-48-
d. Cell Proliferation Assay
At the end of BrdU labeling, the medium is removed and BrdU
incorporation is measured using a cell proliferation ELISA kit (#RPN 250,
Amersham
Life Science) according to manufacturer's instructions. Briefly, cells are
fixed in an
ethanol containing fixative for 30 min, followed by incubation in a blocking
buffer for
30 min to reduce background. Peroxidase-labeled anti-BrdU antibody is added to
the
wells and incubated for 60 min. The cells are rinsed three times with PBS and
incubated with 3,3'5,5'-tetramethylbenzidine (TMB) substrate for 10-20 min
depending upon the potency of tested compounds. Then 25 p1 of 1 M sulfuric
acid is
added to each well to stop color reaction and optical density is read in a
plate reader at
450 nm within 5 min.
e. Analysis of Results:
Square root-transformed data are used for analysis of variance and nonlinear
dose response curve fitting for both agonist and antagonist modes. Huber
weighting is
used to downweight the effects of outliers. ECSO or ICSO values are calculated
from the
retransformed values. JMP software (5A5 Institute, Inc.) is used for both one-
way
analysis of variance and non-linear dose response analyses in both single dose
and dose
response studies.
f. Reference Com op ands:
Trimegestone and medroxyprogesterone acetate (MPA) are reference
progestins and RU486 is the reference antiprogestin. All reference compounds
are run
in full dose-response curves and the ECSO or ICSO values are calculated.
CA 02371651 2001-10-31
WO 00/66560 PCT/US00/118~5
-49-
Table 4. Estimated ECSp, standard error (SE), and 95% confidence intervals
(CI) for individual studies
ECso 95% CI
Compound Exp (nM) SE lower upper
Trimegestone 1 0.017 0.003 0.007 0.040
2 0.014 0.001 0.011 0.017
3 0.019 0.001 0.016 0.024
MPA 1 0.019 0.001 0.013 0.027
2 0.017 0.001 0.011 0.024
Table 5. Estimated ICso, standard error, and 95% confident interval for the
antiprogestin, RU486
ICso 95% CI
Compound Exp (nM) SE lower upper
RU486 1 0.011 0.001 0.008 0.014
2 0.016 0.001 0.014 0.020
3 0.018 0.001 0.014 0.022
ECSO: Concentration of a compound that gives half maximal increase in BrdU
incorporation with SE; ICso: Concentration of a compound that gives half
maximal
decrease in 0.1 trimegestone induced BrdU incorporation with SE
4. T47D cell alkaline phosphatase assay
The purpose of this assay is to identify progestins or antiprogestins by
determining a compound's effect on alkaline phosphatase activity in T47D
cells. The
materials and methods used in this assay are as follows.
CA 02371651 2001-10-31
WO 00/66560 PCT/US00/11835
-50-
a. Culture medium: DMEM:F12 (1:1) (GIBCO, BRL)
supplemented with 5% (v/v) charcoal stripped fetal bovine serum (not heat-
inactivated).
100U/ml penicillin, 100 p,g/ml streptomycin, and 2 mM GlutaMax (GIBCO, BRL).
b. Alkaline phosphatase assay buffer:
I. 0.1 M Tris-HCI, pH 9.8, containing 0.2% Triton X-100
II. 0.1 M Tris-HCI, pH 9.8 containing 4 mM p-nitrophenyl phosphate
(Sigma).
c. Cell Culture and Treatment:
Frozen T47D cells were thawed in a 37°C water
bath and diluted to 280,000 cells/ml in culture medium. To each well in a 96-
well plate
(Falcon, Becton Dickinson Labware), 180 ~1 of diluted cell suspension was
added.
Twenty p1 of reference or test compounds diluted in the culture medium was
then added
to each well. When testing for progestin antagonist activity, reference
antiprogestins or
test compounds were added in the presence of 1 nM progesterone. The cells were
incubated at 37°C in a 5% COz/humidified atmosphere for 24 hr.
d. Alkaline Phosphatase Enzyme Assay:
At the end of treatment, the medium was removed
from the plate and fifty ~.1 of assay buffer I was added to each well. The
plates were
shaken in a titer plate shaker for 15 min. Then 150 p1 of assay buffer II was
added to
each well. Optical density measurements were taken at 5 min intervals for 30
min at a
test wavelength of 405 nM.
e. Analysis of Results: Analysis of dose-response data
For reference and test compounds, a dose response curve
is generated for dose (X-axis) vs. the rate of enzyme reaction (slope) (Y-
axis). Square
root-transformed data are used for analysis of variance and nonlinear dose
response
curve fitting for both agonist and antagonist modes. Huber weighting is used
to
downweight the effects of outliers. ECS° or ICS° values are
calculated from the
retransformed values. JMP software (SAS Institute, Inc.) is used for both one-
way
CA 02371651 2001-10-31
WO 00/66560 PCT/US00/11835
-51-
analysis of variance and non-linear dose response analyses in both single dose
and dose
response studies.
f. Reference Compounds:
Progesterone and trimegestone are reference progestins and RU486 is the
reference antiprogestin. All reference compounds are run in full dose response
curves
and the ECSO or ICSO values are calculated.
Table 6. Estimated ECSp, standard error (SE), and 95% confidence intervals
(CI) for reference progestins from three independent experiments
EC50 95% CI
Compound Exp. (nM~l SE lower upper
Progesterone 1 0.839 0.030 0.706 0.996
2 0.639 0.006 0.611 0.669
3 1.286 0.029 1.158 1.429
Trimegestone 1 0.084 0.002 0.076 0.091
2 0.076 0.001 0.072 0.080
3 0.160 0.004 0.141 0.181
Table 7. Estimated ICSp, standard error, and 95% confident interval for the
reference antiprogestin RU486 from three independent experiments
IC 50 95% CI
Compound Exp (nM) SE lower upper
RU486 1 0.103 0.002 0.092 0.115
2 0.120 0.001 0.115 0.126
3 0.094 0.007 0.066 0.134
CA 02371651 2001-10-31
WO 00/66560 PCT/US00/11835
-52-
B. In-vivo Biology
The primary in-vivo assay is the rat decidualization model which may be used
to determine progestational effects of both agonists and antagonists. The
secondary in-
vivo assay is the rat ovulation inhibition model which is under development
and hence
the protocol is un-available.
1. Rat decidualization assay The objective of this procedure is used to
evaluate the effect of progestins and antiprogestins on rat uterine
decidualization and
compare the relative potencies of various test compounds. The materials and
methods
used in this assay are as follows.
a. Methods: Test compounds are dissolved in 100% ethanol
and mixed with corn oil (vehicle). Stock solutions of the test compounds in
oil
(MazolaTM) are then prepared by heating (~80 °C) the mixture to
evaporate ethanol.
Test compounds are subsequently diluted with 100% corn oil or 10% ethanol in
corn
oil prior to the treatment of animals. No difference in decidual response was
found
1 S when these two vehicles were compared.
b. Animals (RACUC protocol #5002)
Ovariectomized mature female Sprague-Dawley rats (~60-day old and 230g)
are obtained from Taconic (Taconic Farms, NY) following surgery. Ovariectomy
is
performed at least 10 days prior to treatment to reduce circulating sex
steroids.
Animals are housed under 12 hr light/dark cycle and given standard rat chow
and
water ad libitum
c. Treatment
Rats are weighed and randomly assigned to groups of 4 or 5
before treatment. Test compounds in 0.2 ml vehicle are administered by
subcutaneous
injection in the nape of the neck or by lavage using 0.5 ml. The animals are
treated
once daily for seven days. For testing antiprogestins, animals are given the
test
compounds and a ECgO dose of progesterone (5.6 mg/kg) during the first three
days
CA 02371651 2001-10-31
WO 00/66560 PCT/US00/11835
-53-
of treatment. Following decidual stimulation, animals continue to receive
progesterone until necropsy four days later.
d. Do_ sina
Doses are prepared based upon mg/kg mean group body
weight. In all studies, a control group receiving vehicle is included.
Determination of
dose-response curves is carried out using doses with half log increases (e.g.
0.1, 0.3,
1.0, 3.0 mg/kg).
e. Decidual induction
Approximately 24 hr after the third injection, decidualization
is induced in one of the uterine horns by scratching the antimesometrial
luminal
epithelium with a blunt 21 G needle. The contralateral horn is not scratched
and serves
as an unstimulated control. Approximately 24 hr following the final treatment,
rats are
sacrificed by C02 asphyxiation and body weight measured. Uteri are removed and
trimmed of fat. Decidualized (D-horn) and control (C-horn) uterine horns are
weighed
separately.
f. Analysis of Results:
The increase in weight of the decidualized uterine horn is
calculated by D-hom/C-horn and logarithmic transformation is used to maximize
normality and homogeneity of variance. The Huber M-estimator is used to down
weight the outlying transformed observations for both dose-response curve
fitting and
one-way analysis of variance. JMP software (SAS Institute, Inc.) is used for
both one-
way ANOVA and non-linear dose-response analyses.
g. Reference Compounds:
All progestin reference compounds were run in full dose-
response curves and the ECSO for uterine wet weight were calculated.
CA 02371651 2001-10-31
WO 00/66560 PCT/US00/11835
-54-
Table 8. Estimated ECso, standard error (SE), and 95% confidence intervals for
individual studies
ECso 95% CI
Compound Exp (m~/kg. s.c.)SE lower upper
Progesterone 1 5.50 0.77 4.21 7.20
2 6.21 1.12 4.41 8.76
3-Ketodesogestrel1 0.11 0.02 0.07 0.16
2 0.10 0.05 0.11 0.25
3 0.06 0.03 0.03 0.14
Levonorgestrel1 0.08 0.03 0.04 0.16
2 0.12 0.02 0.09 0.17
3 0.09 0.02 0.06 0.13
4 0.09 0.02 0.06 0.14
MPA 1 0.42 0.03 0.29 0.60
2 0.39 0.05 0.22 0.67
3 0.39 0.04 0.25 0.61
Table 9. Estimated average
ECso, standard error, and
95% confidence
intervals for dose-response
curves of 3 reference compounds
EC50 95% CI
Compound (m~/kg, s.c, SE lower upper
Progesterone 5.62 0.62 4.55 7.00
3-Ketodesogestrel 0.10 0.02 0.07 0.14
Levonorgestrel 0.10 0.01 0.08 0.12
CA 02371651 2001-10-31
WO 00/66560 PCT/US00/11835
-55-
Table 10. Estimated ICso, standard error, and 95% confident interval for the
antiprogestin, RU 486
ICSO 95% CI
Compound Exp. (m~/k~. p.o.) SE lower upper
RU 486 1 0.21 0.07 0.05 0.96
2 0.14 0.02 0.08 0.27
Concentration: Compound concentration in assay(default-mg/kg body weight)
Route of administration: Route the compound is administered to the animals
Body weight: Mean total animal body weight (default-kg)
D-horn: Wet weight of decidualized uterine horn (default-mg)
C-horn: Wet weight of control uterine horn (default-mg)
Decidual response: [(D-C)/C]x100%
Progestational activity: Compounds that induce decidualization significantly
(p<0.05) compared to vehicle control are considered active
Antiprogestational activity: Compounds that decrease ECso progesterone
induced decidualization significantly (p<0.05)
ECSO for uterine weight: Concentration of compound that gives half maximal
increase in decidual response (default-mg/kg)
ICSO for uterine weight: Concentration of compound that gives half maximal
decrease in ECSO progesterone induced decidual response (default-mg/kg)
EXAMPLE 13
5-(2,4,4-Trimethyl-1,4-dihydro-2H-3,1-benzoxazin-6-yl)thiophene-2-carbonitrile
A solution of 2-amino-5-bromobenzoic acid (1 Og, 46 mmol) in dry THF (200
mL) was treated at -78 °C under nitrogen with a solution of
methylmagnesium
bromide in ether (3.0 M, 90 mL, 270 mmol). The reaction mixture was slowly
CA 02371651 2001-10-31
WO 00/66560 PCT/US00/11835
-56-
warmed to ambient temperature, kept stirring for 48 hours under nitrogen and
then
poured into a cold 0.5 N aqueous hydrochloride solution (300 mL). The mixture
was
neutralized with aqueous 1 N sodium hydroxide solution and ethyl acetate (300
mL)
was added. 'The organic layer was separated and aqueous layer was extracted
with
ethyl acetate (3x100 mL). The combined organic layers were washed with brine
and
dried (MgS04). A$er removal of solvent in vacuo, the residue was purified by a
silica
gel flash column chromatography(hexane:ethyl acetate/3:2) to give 2-(2-amino-5-
bromophenyl)propan-2-of as off white solid (6g, 57%): mp 62-63 °C; 'H-
NMR
(CDC13) 8 7.19 (d, 1H, J= 2.3 Hz), 7.12 (dd, 1H, J= 8.4, 2.3 Hz), 6.51 (d, 1H,
J=
8.4 Hz), 4.70 (s, 2H), 1.82 (s, 1H), 1.65 (s, 6H).
To a solution of 2-(2-amino-5-bromophenyl)propan-2-of (27g, 125 mmol) in
anhydrous toluene was added at ambient temperature under a blanket of nitrogen
acetylaldehyde (10.5 mL, 187 mmol). After 10 minutes, the mixture was passed
through a pad of silica gel and filtrate was concentrated to yield 6-bromo-
2,4,4-
trimethyl-1,4-dihydro-2H-3,1-benzoxazine as off white solid (25g, 78%): 'H-NMR
(DMS O-d6) 8 7.22 (d, 1 H, J = 2.2 Hz), 7. 08 (dd, 1 H, J = 8. 6, 2.3 Hz),
6.51 (d, 1 H, J
= 8.6 Hz), 6.36 (s, 1H), 4.72 (m, 1H), 1.45 (s, 3H), 1.40 (s, 3H), 1.25 (d,
3H, J= 5.5
Hz).
A mixture of 6-bromo-2,4,4-trimethyl-1,4-dihydro-2H-3,1-benzoxazine (3.6g,
14 mmol), bis(pinacolato)diboron (5 g, 19.7 mmol), potassium acetate (4g, 41
mmol),
and [1,1'-bis(diphenylphosphino)ferrocene]palladium (II) chloride (1:1 complex
with
methylene chloride, 0.4g, 0.5 mmol) in DMF (80 mL) was subject to a positive
nitrogen flow to remove oxygen and then heated at 85 °C under a blanket
of nitrogen
for 18 hours. The mixture was allowed to cool to ambient temperature, treated
with
S-bromo-2-thiophenecarbonitrile (4g, 21 mmol), [1,1'
bis(diphenylphosphino)ferrocene]palladium (II) chloride (1:1 complex with
methylene
chloride, 0.4g, 0.5 mmol), and aqueous sodium carbonate solution (2M, 35 mL,
70
mmol), and then heated at 85 °C under nitrogen for 3 hours. The
reaction mixture was
CA 02371651 2001-10-31
WO 00/66560 PCT/US00/11835
-57-
allowed to cool to ambient temperature, brine (100 mL) and ethyl acetate (150
mL)
were added. The organic layer was separated and aqueous layer was extracted
with
ethyl acetate (3x50 mL). The combined organic layers were dried (MgS04) and
concentrated. The residue was purified by a flash silica gel column
chromatography(THF:hexane/1:4) to a.$'ord the title compound as an off white
solid
(1g, 25%): mp 172-173 °C;'H-NMR (DMSO-d6) 8 7.88 (d, 1H, J= 4.0 Hz),
7.47 (d,
1 H, J = 4. 0 Hz), 7.43 (d, 1 H, J = 2. 0 Hz), 7. 32 (dd, 1 H, J = 8. 36, 2.4
Hz), 6. 77 (s,
1H), 6.60 (d, 1H, J= 8.4 Hz), 4.83 (m, 1H), 1.51 (s, 3H), 1.48 (s, 3H), 1.28
(d, 3H, J
= 5.6 Hz); MS (ESI) m/z 283 [M-H]-.
EXAMPLE 14
3-Fluoro-5-(2,4,4-trimethyl-1,4-dihydro-2H-3,l-benzoxazin-6-vl)benzonitrile
Prepared according to the procedure for Example 13 from 6-bromo-2,4,4-
trimethyl-1,4-dihydro-2H-3,1-benzoxazine and 3-bomo-5-fluorobenzonitrile. A
white
solid: mp 163-164 °C;'H-NMR (DMSO-d6) 8 8.02 (t, 1H, J= 1.5 Hz), 7.87
(dt, 1H, J
= 10.6, 2.2 Hz), 7.65 (m, 1H), 7.55 (d, 1H, J= 2.2 Hz), 7.44 (dd, 1H, J= 8.4,
2.2
Hz), 6.63 (d, 1H, J = 8.4 Hz), 6.58 (s, 1H), 4.82 (m, 1H), 1.52 (s, 3H), 1.50
(s, 3H),
1.28 (d, 3H, J= 5.1 Hz); MS (ESI) mlz 295 [M-H]-.
EXAMPLE 15
4-(2,4,4-Trimethyl-1,4-dihydro-2H-3,1-benzoxazin-6-yl)thiophene-2-carbonitrile
Prepared according to the procedure for Example 13 from 6-bromo-2,4,4-
trimethyl-1,4-dihydro-2H-3,1-benzoxazine and 4-bromo-2-thiophenecarbonitrile.
An
off white solid: mp 175-176 °C;'H-NMR (DMSO-d6) 8 8.39 (d, 1H, J= 1.5
Hz), 8.13
(d, 1H, J= 1.5 Hz), 7.47 (d, 1H, J= 1.9 Hz), 7.36 (dd, 1H, J= 8.4, 1.9 Hz),
6.59 (d,
1H, J= 8.4 Hz), 6.41 (s, 1H), 4.78 (m, 1H), 1.51 (s, 3H), 1.47 (s, 3H), 1.28
(d, 3H, J
= 5.4 Hz); MS (ESI) m/z 285 [M+H]+.
CA 02371651 2001-10-31
WO 00/66560 PCT/US00/11835
-58-
EXAMPLE 16
4-Methyl-5-(2,4,4-trimethvl-1,4-dihydro-2H-3,1-benzoxazin-6-yl)thiophene-2-
carbonitrile
Prepared according to the procedure for Example 13 from 6-bromo-2,4,4-
trimethyl-1,4-dihydro-2H-3,1-benzoxazine and 5-bromo-4-methyl-2-
thiophenecarbonitrile. A yellowish solid: mp 145-146 °C;'H-NMR (DMSO-
d6) 8 7.79
(s, 1H), 7.18 (d, 1H, J= 2.0 Hz), 7.13 (dd, 1H, J= 8.4, 2.0 Hz), 6.68 (s, 1H),
6.54 (d,
1H, J= 8.3 Hz), 4.83 (m, 1H), 2.26 (s, 3H), 1.49 (s, 3H), 1.46 (s, 3H), 1.28
(d, 3H, J
= 5.5 Hz); MS (ESI) m/z 299 [M+H]+.
EXAMPLE 17
3- f (2R,4S)-2,4-Dimethyl-4-phenyl-1,4-dihyd ro-2H-3,1-benzoxazin-6-yll-5-
fluorobenzonitrile
To a solution of 2-amino-4-bomobenzonitrile (5g, 25 mmol) in anhydrous
THF, was added at 0 °C under nitrogen, phenylinagnesium bromide (3 M in
ether, 25
mL, 75 mmol). The mixture was allowed to warm to room temperature, stirred
under
nitrogen for 15 hours, and treated with 2N aqueous hydrogen chloride solution
(100
mL). The aqueous solution was heated to 50 °C for 3 hours, cooled to
room
temperature, and neutralized with a cold saturated sodium bicarbonate
solution. Ethyl
acetate (100 mL) was added and the organic layer was separated and aqueous
layer
was extracted with ethyl acetate (3x40 mL). The combined organic layers were
dried
(MgS04) and evaporated. The residue was purified by a flash column
chromatography(silica gel, hexane: ethyl acetate/4:1) to yield (2-amino-5-
bromophenyl)(phenyl)methanone as a yellow crystal (2.13g, 31%): MS (ESI) m/z
276/278 [M+H]+.
To a solution of (2-amino-5-bromophenyl)(phenyl)methanone (1g, 3.6 mmol)
in anhydrous THF (15 mL) was added at room temperature under nitrogen
methylmagnesium bromide (3M in ether, 3 mL, 9 mmol). After 3 hours, the
mixture
CA 02371651 2001-10-31
WO 00/66560 PCT/US00/11835
-59-
was treated with a saturated aqueous ammonium sulfate solution (30 mL) and
ethyl
acetate (50 mL). The organic layer was separated, dried (MgSOa), and
evaporated.
The residue was then dissolved in anhydrous toluene and treated at ambient
temperature under nitrogen with acetylaldehyde (2 mL). After 2 minutes, the
solvent
was removed and residue was purified by a column chromatography(silica gel,
hexane:ethyl acetate/4:1) to afford 6-bromo-2,4-dimethyl-4-phenyl-1,4-dihydro-
ZH-
3,1-benzoxazine as a yellowish solid (0. 8g, 70%): MS (ESI) m/z 318/320
[M+H)+.
A mixture of 6-bromo-2,4-dimethyl-4-phenyl-1,4-dihydro-2H-3,1-benzoxazine
(0.6g, 1.9 mmol), 3-cyano-S-fluorobenzene boronic acid (0.45g, 2.7 mmol),
tetrakis(triphenylphosphine)palladium (0) (0.2g, 0.17 mmol), sodium carbonate
(0.6g,
5.7 mmol) in a mixture of DME and water (20/5 mL) was subject to a positive
nitrogen flow to remove oxygen and then heated to 85 °C under a blanket
of nitrogen
for 2 hours. The mixture was allowed to cool to ambient temperature. Brine (30
mL)
and ethyl acetate (100 mL) were added. The organic layer was separated and
aqueous
layer was extracted with ethyl acetate (3x30 mL). The combined organic layers
were
dried (MgS04), and evaporated. The residue was purified by a silica gel column
chromatography(hexane:ethyl acetate/4:1) to afford the title compound as off
white
solid (0.09g, 13%): mp128-129 °C; 'H-NMR (DMSO-d6) 8 8.08 (s, 1H), 7.91
(dt,
1H, J= 10.7, 1.9 Hz), 7.71 (d, 1H, J= 2.0 Hz), 7.65 (m, 1H), 7.57 (dd, 1H, J=
8.8,
2.4 Hz), 7.32-7.36 (m, 2H), 7.24-7.29 (m, 3H), 6.71 (d, 1H, J= 1.6 Hz), 6.69
(d, 1H,
J= 8.3 Hz), 4.33 (m, 1H), 1.84 (s, 3H), 1.24 (d, 3H, J = 5.5 Hz); MS (ESI) mlz
357
[M-H]-.
EXAMPLE 18
ten Butvl 2-cyano-5-(2,4,4-trimethvl-1,4-dihydro-2H-3,1-benzogazin-6-yl)-1H-
nyrrole-1-carboxylate
tert-Butyl-5-(2,4,4-trimethyl-1,4-dihydro-2H-3,1-benzoxazin-6-yl)-1 H-pyrrole-
1-carboxylate was prepared according to the coupling procedure for Example 17
from
CA 02371651 2001-10-31
WO 00/66560 PCT/CTS00/11835
-60-
6-bromo-2,4,4-trimethyl-1,4-dihydro-2H-3,1-benzoxazine and 1-t-
butoxycarbonylpyrrol-2-yl boronic acid. To a solution of tert-butyl-5-(2,4,4-
trimethyl-
1,4-dihydro-2H-3,1-benzoxazin-6-yl)-1H-pyrrole-1-carboxylate (1g, 2.9 mmol) in
anhydrous THF (20 mL) was added at -78 °C under nitrogen chlorosulfonyl
isocyanate (0.35 mL, 4.0 mmol). The mixture was kept stirring at -78 °C
under
nitrogen for 2 hours, treated with anhydrous DMF (5 mL), and allowed to warm
to
room temperature. Aqueous ammonium sulfate solution (50 mL) and ethyl acetate
(100 mL) was added and organic layer was separated, dried (MgS04), and
evaporated.
The residue was purified by a silica gel column chromatography(hexane:ethyl
acetate/4:1) to afford the title compound as a white solid (0.019g, 1.78%): 'H-
NMR
(DMSO-d6) 8 7.48 (d, 1H, J = 2.0 Hz), 7.36 (dd, 1H, J= 8.3, 2.0 Hz), 7.32 (d,
1H, J
= 3.6 Hz), 7.14 (d, 1H, J= 8.3 Hz), 6.46 (d, 1H, J= 4.0 Hz), 5.35 (q, 1H, J=
5.2
Hz), 1.58 (d, 3H, J= 5.6 Hz), 1.56 (s, 3H), 1.51 (s, 3H), 1.38 (s, 9H); MS
(ESI) m/z
366 [M-H]~.
EXAMPLE 19
9H-Fluoren-9-ylmethyl-6-f 1-(tert-butoxvcarbonyl)-5-cyano-1H-pyrrol-2-vll-
2,4,4-trimethyl-2H-3,1-benzozazine-1 (4H)-carbogylate
A mixture of tert-butyl-5-(2,4,4-trimethyl-1,4-dihydro-2H-3,1-benzoxazin-6-
yl)-1H-pyrrole-1-carboxylate (1.7g, 4.96 mmol), 9-ffuorenylinethyl
chloroformate
(1.92g, 7.5 mL), sodium carbonate (4g, 37 mmol) in dioxane (50 mL) and water
(50
mL) was stirred at room temperature under a blanket of nitrogen for 6 hours.
Ethyl
acetate (100 mL) was added and organic layer was separated, dried (MgS04), and
evaporated. The residue was purified by a silica gel column
chromatography(hexane:ethyl acetate/6:1) to afford 9H-fiuoren-9-ylmethyl-6-[1-
(tert-
butoxycarbonyl)-1H-pyrrol-2-yl]-2,4,4-trimethyl-2H-3,1-benzoxazine-1 (4H)-
carboxylate as a clean oil.
CA 02371651 2001-10-31
WO 00/66560 PCT/US00/11835
-61-
Using the procedure for Example 18, the title compound (0.8g, 65%) was
prepared from 9H-ffuoren-9-ylmethyl-6-[1-(tert-butoxycarbonyl)-1H-pyrrol-2-yl]-
2,4,4-trimethyl-2H-3,1-benzoxazine-1(4H)-carboxylate (1.2g, 2.1 mmol) and
chlorosulfonyl isocyanate (0.28 mL, 3.1 mmol). A white solid: mp 135-136
°C; 'H-
NMR (DMSO-d6) 8 7.90 (t, 2H, J = 6.7 Hz), 7.64 (d, 1H, J= 7.5 Hz), 7.59 (d,
1H, J
= 7.1 Hz), 7.40 (td, 2H, J= 7.2, 2.0 Hz), 7.31-7.34 (m, 3H), 7.29 (d, 1H, J =
1.2 Hz),
7.04-7.09 (m, 2H), 6.44 (d, 1H, J = 3.57 Hz), 5.30 (q, 1H, J= 5.6 Hz), 4.86
(dd, 1H,
J= 10.7, 5.2 Hz), 4.64 (dd, 1H, J= 10.8, 5.2 Hz), 4.33 (t, 1H, J= 4.7 Hz).
1.50 (s,
3H), 1.30 (s, 9H), 1.20 (s, 3H), 1.03 (d, 3H, J= 5.6 Hz); MS (ESI) mlz 590
[M+H]+.
EXAMPLE 20
5-(2,4,4-Trimethyl-1,4-dihydro-2H-3,1-benzogazin-6-yp-1H-nyrrole-2-
carbonitrile
9H-Fluoren-9-ylmethyl-6-[ 1-(tert-butoxycarbonyl)-5-cyano-1 H-pyrrol-2-yl]-
2,4,4-trimethyl-2H-3,1-benzoxazine-1 (4H)-carboxylate (0.5g, 0.84 mmol) was
heated
under a blanket of nitrogen at 160 °C until gas evolution ceased. After
cooling to
room temperature, 9H-ffuoren-9-ylmethyl-6-[5-cyano-1H-pyrrol-2-yl]-2,4,4-
trimethyl-
2H-3,1-benzoxazine-1 (4H)-carboxylate was obtained as a white solid (0.4g,
97%).
A solution of 9H-ffuoren-9-ylmethyl-6-[5-cyano-1H-pyrrol-2-yl]-2,4,4-
trimethyl-2H-3,1-benzoxazine-1 (4H)-carboxylate (0.1 g, 0.2 mmol) in 20%
piperidine
in DMF (5 mL) was stirred at room temperature under nitrogen for 10 minutes.
The
mixture was poured into a saturated ammonium sulfate solution (30 mL) and
extracted
with diethyl ether (3x30 mL). The combined organic layers were dried (MgS04)
and
evaporated. The residue was purified by a silica gel column
chromatography(hexane:ethyl acetate/3:1) to afford the title compound as a
white solid
(0.03g, 56%): mp 201-202 °C;'H-NMR (DMSO-d6) 8 12.27 (s, 1H), 7.44 (d,
1H, J=
2.1 Hz), 7.32 (dd, 1H, J= 8.3, 1.1 Hz), 6.92 (dd, 1H, J= 4.1, 2.6 Hz), 6.57
(d, 1H, J
CA 02371651 2001-10-31
WO 00/66560 PCT/US00/11835
-62-
= 8.3 Hz), 6.50 (dd, 1H, J = 4.2, 2.6 Hz), 6.40 (s, 1H), 4.77-4.80 (m, 1H),
1.51 (s,
3H), 1.46 (s, 3H), 1.27 (d, 3H, J= 5.7 Hz); MS (ESI) mlz 266 [M-H]-.
EXAMPLE 21
9H-Fluoren-9-vlmethvl6-(5-cyano-1-methvl-1H-nvrrol-2-yl)-2,4,4-trimethvl-2H-
'3,1-benzoxazine-1(4H)-carbogvlate
A mixture of 9H-ffuoren-9-ylmethyl-6-[5-cyano-1H-pyrrol-2-yl]-2,4,4-
trimethyl-2H-3,1-benzoxazine-1 (4H)-carboxylate (0.4g, 0.8 mmol) and potassium
carbonate (1.5g) in anhydrous DMF was treated at ambient temperature under a
blanket of nitrogen with iodomethane (1.5 mL, excess). The mixture was stirred
for
30 minutes. A saturated ammonium sulfate solution (50 mL) and ethyl acetate
(50
mL) was added. The organic layer was separated and aqueous layer was extracted
with ethyl acetate (3x15 mL). The combined organic layers were dried (MgS04),
evaporated to yield the title compound as a white solid (0.35g, 87%): mp 63-64
°C;
'H-NMR (DMSO-d6) 8 7.90 (m, 2H), 7.62 (d, 1H, J= 7.7 Hz), 7.58 (d, 1H, J= 7.7
Hz), 7.40 (m, 2H), 7.29-7.32 (m, 3H), 7.14 (dd, 1H, J = 8.1, 1.9 Hz), 7.01-
7.04 (m,
2H), 6.33 (d, 1H, J = 4.3 Hz), 5.31 (q, 1H, J= 5.8 Hz), 4.88 (dd, 1H, J= 10.8,
5.0
Hz), 4.65 (dd, 1H, J= 10.8, 4.6 Hz), 4.34 (t, 1H, J= 4.6 Hz), 3.71 (s, 3H),
1.52 (s,
3H), 1.21 (s, 3H), 1.06 (d, 3H, J= 5.4 Hz); MS (ESI) mlz 504 [M+H]+.
EXAMPLE 22
1-Methvl-5-(2,4,4-trimethvl-1,4-dihvdro-2H-3,1-benzozazin-6-vll-1H-ovrrole-2-
carbonitrile
Prepared according to the procedure for Example 20 from 9H-fluoren-9-
ylmethyl6-(5-cyano-1-methyl-1H-pyrrol-2-yl)-2,4,4-trimethyl-2H-'3,1-
benzoxazine-
1(4H)-carboxylate (0.3g, 0.6 mmol) and 20% piperidine in DMF. A white solid
(0.07g, 42%): mp 195-196 °C; 'H-NMR (DMSO-d6) 8 7.16 (d, 1H, J= 2.8
Hz), 7.08
(dd, 1 H, J = 8.1, 1. 9 Hz), 6. 98 (d, 1 H, J = 4.1 Hz), 6. 63 (d, 1H, J = 8.
5 Hz), 6. 61 (s,
CA 02371651 2001-10-31
WO 00/66560 PCT/US00/11835
-63-
1H), 6.20 (d, 1H, J = 4.lHz), 4.79-4.81 (m, 1H), 3.67 (s, 3H), 1.49 (s, 3H),
1.45 (s,
3H), 1.27 (d, 3H, J= 5.5 Hz); MS (ESI) m/z 282 [M+H]+.
EXAMPLE 23
S 5~2-Methylspirof4H-3,1-benzoxazine-4,1'-cyclopentanel-6-yl)-4 methyl-2-
thiophenecarbonitrile
2-Methyl-6-bromospiro[4H-3,1-benzoxazine-4,1'-cyclopentane] was prepared
using the same procedure as for 6-bromo-2,4,4-trimethyl-1,4-dihydro-2H-3,1-
benzoxazine in Example 13.
The title compound was prepared according to the procedure for Example 13
from2-methyl-6-bromospiro[4H-3,1-benzoxazine-4,1'-cyclopentane] ands-bromo-4-
methyl-2-thiophenecarbonitrile. A yellowish solid: mp 58-60 °C;'H-NMR
(DMSO-
d6) 8 7. 79 (s, 1 H), 7.16 (d, 1 H, J = 1.9 Hz), 7.12 (dd, 1 H, J = 8.4, 2.2
Hz), 6. 66 (s,
1H), 6.63 (d, 1H, J= 8.4 Hz), 4.75 (m, 1H), 2.26 (s, 3H), 2.14 (m, 1H), 1.87
(m, 1H),
1.4-1.7 (m, 6H), 1.32 (d, 3H, J= 5.5 Hz).
EXAMPLE 24
4-(2-Methylspiro f 2H-3,1-benzoxazine-4,1'-cyclopentanel-6-yl)-2-
thiophenecarbonitrile
Prepared according to the procedure for Example 13 from 2-methyl-6-
bromospiro[4H-3,1-benzoxazine-4,1'-cyclopentane] and 4-bromo-2-
thiophenecarbonitrile. An off white solid: mp 103-104 °C.
CA 02371651 2001-10-31
WO 00/66560 PCT/US00/11835
-64-
EXAMPLE 25
5-(2-methyls pi ro l2H-3,1-benzoxazi ne-4,1'-cyclohexanel-6-yl)-4-methyl-2-
thiophenecarbonitrile
2-Methyl-6-bromospiro[2H-3,1-benzoxazine-4,1'-cyclohexane] was prepared
using the same procedure as for 6-bromo-2,4,4-trimethyl-1,4-dihydro-2H-3,1-
benzoxazine in Example 13.
The title compound was prepared according to the procedure for Example 13
from2-methyl-6-bromospiro[4H-3,1-benzoxazine-4,1'-cyclohexane] ands-bromo-4-
methyl-2-thiophenecarbonitrile. A brown solid: 'H-NMR (DMSO-d6) 8 7.78 (s,
1H),
7.17 (d, 1H, J = 1. 8 Hz), 7.14 (dd, 1 H, J = 8.4, 2.2 Hz), 6. 64 (s, 1 H),
6.63 (d, 1 H, J =
8.2 Hz), 4.74 (m, 1H), 2.26 (s, 3H), 2.14 (m, 1H), 1.87 (m, 1H), 1.4-1.7 (m,
8H), 1.31
(d, 3H, J= 5.3 Hz); MS (ESI) mlz 337 [M-H]-.
EXAMPLE 26
4-(2-Methylspirol2H-3,1-benzoxazine-4,1'-cyclohexanel-6-yp2-
thiophenecarbonitrile
Prepared according to the procedure for Example 13 from 2-methyl-6-
bromospiro[4H-3,1-benzoxazine-4,1'-cyclohexane] and 4-bromo-2-
thiophenecarbonitrile. A brown solid: mp 111-112 °C; 'H-NMR (DMSO-d6) 8
8.42
(s, 1H), 8.14 (s, 1H), 7.47 (s, 1H), 7.35 (dd, 1H, J= 8.3, 1.1 Hz), 6.58 (d,
1H, J = 8.4
Hz), 6.38 (s, 1H), 4.72 (m, 1H), 1.92-2.16 (m, 2H), 1.35-1.75 (m, 8H), 1.31
(d, 3H, J
= 5.3 Hz); MS (ESI) m/z 325 [M+H]+.
EXAMPLE 27
6-(3-Fluorophenyl)-2,4,4-trimethyl-1,4-dihydro-2H-3,1-benzoxazine
Prepared according to the coupling procedure for Example 17 from 3-
fluorophenyl boronic acid and 6-bromo-2,4,4-trimethyl-1,4-dihydro-2H-3,1-
benzoxazine. Ayellow solid: mp 139-140°C;'H-NMR (CDCl3) 8 7.40-7.19 (m,
6H),
CA 02371651 2001-10-31
WO 00/66560 PCT/US00/11835
-65-
7.01-6.94 (m, 1H), 6.72 (d, 1H, J= 8.24 Hz), 4.90 (q, 1H, J= 5.48 Hz), 1.62
(s, 3H),
1.59 (s, 3H), 1.46 (d, 3H, J= 5.5 Hz); MS (ES) mlz 272 ([M+Hj+)
EXAMPLE 28
6-(3-Chlorophenyl)-2,4,4-trimethyl-1,4-dihydro-2H-3,1-benzoxazine
Prepared according to the coupling procedure for Example 17 from 3-
chlorophenyl boronic acid and 6-bromo-2,4,4-trimethyl-1,4-dihydro-2H-3,1-
benzoxazine. A orange solid: mp 144-146 °C; 'H-NMR (CDCl3) 8 7.50 (t,
1H, J=
1.78 Hz), 7.40 (dt, 1H, J= 7.61, 1.45 Hz), 7.33 (t, 1H, J= 7.76 Hz), 7.29-7.22
(m,
4H) 6.72 (d, 1H, J= 8.24 Hz), 4.90 (q, 1H, J= 5.45 Hz), 1.62 (s, 3H), 1.59 (s,
3H),
1.4 6 (d, 3H, J= 5.5 Hz); MS (ES) mlz 288/290 ([M + H]+)
EXAMPLE 29
6-(3-Chloro-4-flu orophenyl)-2,4,4-trimethyl-1,4-dihyd ro-2H-3,1-benzoxazine
A mixture of 6-bromo-2,4,4-trimethyl-1,4-dihydro-2H-3,1-benzoxazine (3.0 g,
11.7 mmol), 3-chloro-4-fluorobenzeneboronic acid (3.1 g, 17.6 mmol),
tetrakis(triphenylphosphine)palladium (0) (0.67 g, 0.59 mmol), and sodium
carbonate
(3.72 g, 35.1 mmol) in DME (80 mL) and water (40 mL) was subject to a blanket
of
nitrogen flow for 15 minutes at 50°C and then was heated at 85°C
under nitrogen for 1
hour. The reaction was cooled to room temperature and ethyl acetate (200 mL)
was
added. The organic layer was washed twice with aqueous ammonium chloride (50
mL) and once with brine (50 mL), dried over sodium sulfate and concentrated to
a
yellow solid. The solid was triturated with ether (25 mL) and hexane (25 mL),
collected on a filter, and dried to give 6-(3-chloro-4-fluorophenyl)-2,4,4-
trimethyl-1,4-
dihydro-2H-3,1-benzoxazine (1.87 g, 35%) as a yellow solid: mp 173-
175°C;'H-
NMR (DMSO-d6) 8 7.79 (dd, 1H, J= 6.84, 2.14 Hz), 7.60-7.57 (m, 1H), 7.43-7.39
(m, 2H), 7.29 (dd, 1H, J= 8.12, 2.14 Hz), 6.62 (d, 1H, J= 8.54 Hz), 6.40 (s,
1H),
CA 02371651 2001-10-31
WO 00/66560 PCT/US00/11835
-66-
4.79 (q, 1H, J= 5.55 Hz), 1.53 (s, 3H), 1.48 (s, 3H), 1.28 (d, 3H, J= 5.55
Hz); MS
(ES) m/z 306/308([M + H]+).
EXAMPLE 30
2-Fluoro-5-(2,4,4-trimethvl-1,4-dihydro-2H-3,1-benzogazin-6-yl)benzonitrile
Prepared according to the coupling procedure for Example 13 from 6-bromo-
2,4,4-trimethyl-1,4-dihydro-2H-3,1-benzoxazine and 5-bromo-2-
ffuorobenzonitrile. A
white solid: mp 184-186°C;'H-NMR (DMSO-d6) 8 8.17 (dd, 1H, J= 5.86,
3.42 Hz),
8.00-7.98 (m, 1H), 7.52 (t, 1H, J= 9.28 Hz), 7.45 (d, 1H, J= 1.95 Hz), 7.34
(dd, 1H,
J= 8.30, 1.95 Hz), 6.63 (d, 1H, J= 8.3 Hz), 6.45 (d, 1H), 4.8 (q, 1H, J= 5.37
Hz),
1.53 (s, 3H), 1.49 (s, 3H), 1.29 (d, 3H, J= 5.37 Hz); MS (ES) mlz 297 ([M +
H]+).
EXAMPLE 31
4-(2,4,4-Trimethvl-1,4-dihyd ro-2H-3,1-benzozazin-6-yl)-2-furonitrile
Prepared using the coupling procedure for Example 13 from 6-bromo-2,4,4-
trimethyl-1,4-dihydro-2H-3,1-benzoxazine and 4-bromo-2-cyanofuran. A light
brown
solid: mp 116-118 °C;'H-NMR (DMSO-d6) 8 8.43 (s, 1H), 8.06 (s, 1H),
7.38 (d, 1H,
J= 1.98 Hz), 7.25 (dd, 1H, J= 7.93, 1.98 Hz) 6.58 (d, 1H, J= 7.93 Hz), 6.37
(s, 1H),
4.77 (q, 1H, J= 5.55 Hz), 1.50 (s, 3H), 1.46 (s, 3H), 1.27 (d, 3H, J= 5.55
Hz); MS
(ES) m/z 269 ([M+H]+); Anal. Calc. For C~6H16N2O2~ C, 71.62; H, 6.01, N,
10.44.
Found: C, 71.55; H, 6.26, N, 10.17.
EXAMPLE 32
3-f 4,4-Dimethyl-2-(trifluoromethyl)-1,4-dihydro-2H-3,1-benzozazin-6-yll-5-
fluorobenzonitrile
A mixture of 2-(2-amino-5-bromo-phenyl)-propan-2-of (5 g, 21.7 mmol),
triffuoroacetaldehyde methyl hemiacetal (5 mL), and magnesium sulfate (10 g)
in
toluene (75 mL) was heated at 80°C under nitrogen. After disappearance
of the
CA 02371651 2001-10-31
WO 00/66560 PCT/US00/11835
-67-
starting material, the reaction was filtered through a pad of silica gel and
the filtrate
dried over sodium sulfate and concentrated to give 6-bromo-4,4-dimethyl-2-
(trifluoromethyl)-1,4-dihydro-2H-3,1-benzoxazine as an amorphous solid: 'H-NMR
(DMSO-d6) 8 7.31 (d, 1H, J= 1.99 Hz), 7.19 (dd, 1H, J= 8.73, 2.38 Hz), 6.88 (s
1H), 6.74 (d, 1H, J= 8.33 Hz), 5.32 (m, 1H), 1.52 (s, 6H).
A mixture of 6-bromo-4,4-dimethyl-2-(triffuoromethyl)-1,4-dihydro-2H-3,1-
benzoxazine (0.5 g, 1.61 mmol), 3-cyano-5-ffuorobenzeneboronic acid (0.39 g,
1.8
mmol), tetrakis(triphenylphosphine)-palladium (0) (0.2 g, 0.161 mmol), and
sodium
carbonate (0.5 g, 4. 83 mmol) in DME (50 mL) and water (25 mL) was subject to
a
blanket of nitrogen flow for 15 minutes at 50°C and then was heated at
85°C under
nitrogen for 1 hour. The reaction mixture was cooled to room temperature and
ethyl
acetate (200 mL) was added. The organic layer was washed twice with aqueous
ammonium chloride (20 mL) and once with brine (20 mL), dried over sodium
sulfate
and concentrated to a yellow solid. Purification via flash column
chromatography
(silica gel, 5% ethyl acetate/hexane) gave 3-[4,4-dimethyl-2-(triffuoromethyl)-
1,4-
dihydro-2H-3,1-benzoxazin-6-yl]-5-ffuorobenzonitrile (0.396 g, 73%) as a white
solid:
mp 102-103°C;'H-NMR (DMSO-d6) 8 8.07 (s, 1H), 7.91 (d, 1H, J= 10.7 Hz),
7.7 (d,
1H, J= 10.7 Hz) 7.62 (d, 1H, J= 1.98 Hz), 7.53 (dd, 1H, J= 8.33, 1.98 Hz),
7.05 (s,
1H), 6.87 (d, 1H, J= 8.33 Hz), 5.39-5.37 (m, 1H), 1.61 (s, 3H), 1.59 (s, 3H);
MS
(ES) m/z 349 ([M-H]~).
EXAMPLE 33
4-14.4-Dimethvl-2-(trifluoromethvl)-1,4-dihvdro-2H-3,1-benzoxazin-6-
yllthiophene-2-carbonitrile
Prepared using the coupling procedure for Example 13 starting with 6-bromo-
4,4-dimethyl-2-(trifluoromethyl)-1,4-dihydro-2H-3,1-benzoxazine and 4-bromo-2-
thiophenecarbonitrile. A yellow solid: 'H-NMR (DMSO-d6) 8 8.43 (s, 1H), 8.19
(s,
1H), 7.54 (d, 1H, J= 1.98 Hz), 7.46 (dd,lH, J= 8.33, 1.98 Hz), 6.90 (s, 1H),
6.83 (d,
CA 02371651 2001-10-31
WO 00/66560 PCT/US00/11835
-68-
1H, J= 8.33 Hz), 5.35 (d, 1H, J= 3.57 Hz), 1.59 (s, 3H), 1.57 (s, 3H); MS (ES)
mlz
337 ([M-H]-).
EXAMPLE 34
4-f 1-Acetyl-4,4-dimethyl-2-(trifluoromethyl)-1,4-dihydro-2H-3,1-benzoxazin-6-
yllthiophene-2-carbonitrile
4-[4,4-dimethyl-2-(triffuoromethyl)-1,4-dihydro-2H-3,1-benzoxazin-6-
yl]thiophene-2-carbonitrile (0.25 g, 0.74 mmol) was dissolved in DMF (10 mL),
NaH
(0.09 g, 2.22 mmol) was added and the mixture was stirred for 30 minutes prior
to the
addition of acetyl chloride (0.079 mL, 1.11 mmol). Upon disappearance of the
starting
material the reaction mixture was poured into brine (100 mL) and the product
extracted with ether (150 mL), dried over sodium sulfate and concentrated. The
residue was purified by a flash column chromatography (silica gel, 25% ethyl
acetate/hexane) to yield 4-[1-acetyl-4,4-dimethyl-2-(trifluoromethyl)-1,4-
dihydro-2H-
3,1-benzoxazin-6-yl]thiophene-2-carbonitrile (0.1 g, 36%) as an amorphous
solid: 'H-
NMR (DMSO-d6) 8 8.58 (s, 1H), 8.5 (s, 1H), 7.83-7.80 (m, 2H), 7.64 (d, 1H, J=
8.74 Hz), 6.42 (q, 1H, J= 10 Hz), 2.24 (s, 3H), 1.73 (s, 3H), 1.42 (s, 3H).
EXAMPLE 35
(6(5-Cyanothien-3-yl)-4,4-dimethyl-2-(trifluoromethyl)-2H-3,1-benzoxazin-
1(4H)-vl)methvl pivalate
4-[4,4-dimethyl-2-(trifiuoromethyl)-1,4-dihydro-2H-3,1-benzoxazin-6-
yl]thiophene-2-carbonitrile (0.30 g, 0.89 mmol) was dissolved in DMF (10 mL),
NaH
(0.065 g, 2.7 mmol) was added and the was mixture stirred for 30 minutes prior
to the
addition of tert-butyl chloroacetate (0.19 mL, 1.3 mmol). Upon disappearance
of the
starting material the reaction mixture was poured into brine (100 mL) and the
product
extracted with ether (150 mL), dried over sodium sulfate and concentrated.
Flash
column chromatography (silica gel, 8% ethyl acetate/hexane) gave (6(5-
cyanothien-3-
CA 02371651 2001-10-31
WO 00/66560 PCT/US00/11835
-69-
yl)-4,4-dimethyl-2-(triffuoromethyl)-2H-3,1-benzoxazin-1(4H)-yl)methyl
pivalate
(0.127 g, 32%) as an amorphous solid: 'H-NMR (CDC13) b 7.82 (s, 1H), 7.58 (s,
1H),
7.41 (dd, 1H, J= 8.33, 1.98 Hz), 7.25 (d, 1H, J= 1.98 Hz) 7.10 (d, 1H, J= 8.33
Hz),
6.79 (d, 1H, J= 8.33 Hz), 5.66 (d, 1H, J= 11.9 Hz), 5.38 (d, 1H, J= 11.9 Hz),
1.72
(s, 3H), 1.55 (s, 3H), 1.18 (s, 9H).
EXAMPLE 36
3-Fluoro-5-(2,2,4,4-tetramethyl-1,4-dihydro-2H-3,1-benzozazin-6-
yl)benzonitrile
Prepared using the coupling procedure for Example 13 starting with 6-bromo-
2,2,4,4-tetramethyl-1,4-dihydro-2H-3,1-benzoxazine and 3-bromo-5-
fluorobenzonitrile. A white solid: 'H-NMR (DMSO-d6) 8 8.03 (s, 1H), 7.87 (d,
1H, J
= 10.7 Hz), 7.65 (d, 1H, J= 10.7 Hz), 7.57 (d, 1H, J= 1.98 Hz) 7.45 (dd, 1H,
J=
8.33, 1.98 Hz), 6.65 (d, 1H, J= 8.33 Hz), 6.42 (s, 1H), 1.52 (s, 6H), 1.34 (s,
6H);
MS (ES) m/z 311 ([M+H]+).
EXAMPLE 37
4-(2,2,4,4-Tetramethyl-1,4-dihyd ro-2H-3,1-benzozazin-6-yl)thio phene-2-
carbonitrile
Prepared using the coupling procedure for Example 13 starting with 6-bromo-
2,2,4,4-tetramethyl-1,4-dihydro-2H-3,1-benzoxazine and 4-bromo-2-
cyanothiophene.
An amorphous orange solid: 'H-NMR (DMSO-d6) 8 8.4 (d, 1H, J= 1.69 Hz), 8.14
(d,
1H, J = 1.69 Hz), 7.49 (d, 1H, J= 2.20 Hz), 7.38 (dd, 1H, J= 8.43, 2.02 Hz),
6.61
(d, 1H, J = 8.43 Hz), 6.26 (s, 1H), 1.49 (s, 6H), 1.32 (s, 6H); MS (ES) mlz
299
QM+H]+).
CA 02371651 2001-10-31
WO 00/66560 PCT/US00/11835
-70-
EXAMPLE 38
5-14,4-Dimethyl-2-(trifluoromethyl)-1,4-dihydro-2H-3,1-benzoxazin-6-yll-4-
methylthionhene-2-carbonitrile
Prepared according to the coupling procedure for Example 13 from 6-bromo-
4,4-dimethyl-2-(triffuoromethyl)-1,4-dihydro-2H-3,1-benzoxazine and 5-bromo-4-
methyl-2-thiophene-carbonitrile. A yellowish solid: mp 97-98 °C;'H-NMR
(DMSO-
d6) 8 7.82 (s, 1H), 7.26 (d, 1H, J = 1.7 Hz), 7.21 (dd, 1H, J= 8.1, 2.1 Hz),
7.16 (s,
1H), 6.88 (d, 1H, J = 8.1 Hz), 5.40 (m, 1H), 2.27 (s, 3H), 1.56 (s, 3H), 1.55
(s, 3H);
MS (ESI) m/z 351 [M-H]-.
EXAMPLE 39
6-(3-Chlorophenyl)-4,4-dimethyl-2-(trifluoromethyl)-1,4-dihydro-2H-3,1-
benzoxazine
Prepared according to the coupling procedure for Example 17 from 6-bromo-
4,4-dimethyl-2-(trifluoromethyl)-1,4-dihydro-2H-3,1-benzoxazine and 3-
chlorophenyl
boronic acid. A yellowish solid: mp 108-109 °C; 'H-NMR (DMSO-d6) 8 7.69
(t, 1H,
J = 1.7 Hz), 7.59 (d, 1H, J = 7.8 Hz), 7.35-7.50 (m, 3H), 7.32 (dt, 1H, J=
8.1, 1.1
Hz), 6.91 (s, 1H), 6.87 (d, 1H, J = 8.4 Hz), 5.35 (m, 1H), 1.60 (s, 3H), 1.59
(s, 3H);
MS (ESI) m/z 340 [M-H]-.
EXAMPLE 40
6-(3-Fluorophenyl)-4,4-dimethyl-3,4-dihydroguinolin-2(1H)-one
Prepared by coupling 3-ffuorophenylboronic acid with an equivalent amount of
6-bromo-4,4-dimethyl-3,4-dihydo-1H-quinolin-2-one using a catalytic amount of
tetrakis(triphenylphosphine)palladium(0) with overnight reffuxing in toluene
containing
an equivalent amount of potassium carbonate dissolved in water. Work up in the
usual
manner followed by recrystallization from ethanol gave a gray solid: mp 190-
192° C .
CA 02371651 2001-10-31
WO 00/66560 PCT/US00/11835
_71_
'H-NMR (DMSO-d6) 8 10.27 (s, 1H), 7.57 (s, 1H), 7.50 (m, 4H), 7.15 (m, 1H),
6.95
(d, 1H J= 8.2Hz), 2.39 (s, 2H), 1.29 (s, 6H); MS (APCI (-)) mlz 268 [M-H]-
Anal.
Calc. For C,~H,6FN0 0.25 H20: C, 74.57, H, 6.07, N, 5.12. Found: C, 74.86, H,
5.97,
N 5.06.
EXAMPLE 41
3-(4,4-Dimethyl-2-oxo-1,2,3,4-tetrahydroguinolin-6-yl)-5-fluorobenzonitrile
6-bromo-4,4-quinolin-2-one was allowed to couple with an equivalent of
bis(pinacolato)diboron in refluxing DMF containing an equivalent of sodium
carbonate
dissolved in a minimal amount of water and a catalytic quantity of
tetrakis(triphenylphosphine)palladium(0). After refluxing overnight an
equivalent of 3-
bromo-5-fluorobenzonitrile was added. Another equivalent of sodium carbonate
was
then added, followed by an additional amount of the same catalyst. After
several
hours of reflux, the reaction mixture was filtered and taken to dryness in
vacuo. The
residue was extracted into ethyl acetate and the solution was dried over
magnesium
sulfate, filtered and the filtrate was again roto-evaporated to give a solid
residue.
Recrystallization from ethanol afforded the title compound as a gray solid: mp
249-
250° C. 'H-NMR (DMSO-d6) 8 10.32 (s, 1H), 8.09 (t, 1H, J= l.6Hz), 7.93
(d, 1H, J
= 12.7 Hz) 7.77, (d, 1H, J= 8.lHz), 7.70 (s, 1H), 7.61(d, 1H J= 8.9Hz), 6.95
(d,
1H, J= 8.3Hz),2.40 (s, 2H), 1.30 (s, 6H); MS (APCI (-)) mlz 293 [M-H]- Anal.
Calc.
For C,aH~SFN20 I.SHzO : C, 67.28, H, 5.65, N, 8.72. Found: C, 67.36, H, 4.90,
N
s8.44..
EXAMPLE 42
3-(4,4-Dimethvl-2-oxo-1,2,3,4,-tetrahvdro4uinolin-6-vl)benzonitrile
Prepared by coupling 3-cyanophenylboronic acid with an equivalent amount of
6-bromo-4,4-dimethyl-3,4-dihydo-1H-quinolin-2-one using a catalytic amount of
tetrakis(triphenylphosphine)palladium(0) as a catalyst with overnight
refluxing in
toluene containing an equivalent amount of potassium carbonate dissolved in
water in
CA 02371651 2001-10-31
WO 00/66560 PCT/US00/11835
-72-
the usual manner followed by recrystallization from ethanol gave a gray solid:
mp. 190
- 192 ° C; 'H-NMR (DMSO-d6) 8 10.29 (s,lH), 8.17 (s, 1H), 8.00 (d,lH, J
= 7.9
Hz), 7.77 (d, 1H, J = 6.4Hz), 7.65 (m, 2H), 7.55 (d, 1H, J = 8.2 Hz), 6.97 (d,
1H, J =
8.3 Hz), 2.40 (s, 2H), 1.30 (s, 6H); MS (APCI (-)) m/z 275 [M-H]- Anal. Calc.
For
C~sH,6Nz0 0.25H20: C, 75.77, H, 6.01, N, 9.82. Found: C, 75.45, H, 5.65, N
9.20.
EXAMPLE 43
6-(3-Chlorophenyl)-4,4-dimethyl-3,4-dihydroQuinoline-2(1H)-thione
Prepared by heating under reflux overnight a mixture of 6-(3-chlorophenyl)-
4,4-dimethyl-3,4-dihydroquinoline-2(1H)-one and an equal weight of phosphorus
pentasulfide in pyridine was stirred. Removal of the pyridine in vacuo
followed by
treating the residue with 6N hydrochloric acid and recrystallization of the
residue in
ethanol gave the product as a yellow solid: mp. 197 - 198° C. 'H-NMR
(DMSO-d6) 8
12.34 (s, 1H), 7.75 (m, 1H), 7.64 (m, 2H), 7.57 (dd , 1H J= 9.3 and 2.1 Hz),
7.48 (t,
1 H, J = 7. 7 Hz), 7. 41 (m, 1 H), 7. 20 (d, 1 H, J = 8. 3 Hz), 3. 34 (s, 2H),
1. 26 (s, 6H); MS
(APCI (-)) m/z 300 [M-H]- Anal. Calc. For C»H,6C1NS: C, 67.65, H, 5.34, N,
4.64.
Found: C, 67.77, H, 5.57, N 4.54.
EXAMPLE 44
6-(3-Fluoroohenvll-4.4-dimethvl-3,4-dihvdroauinoline-2(1H)-thione
Prepared by heating under reflux overnight a mixture of 6-(3-fluororophenyl)-
4,4-dimethyl-3,4-dihydroquinoline-2(1H)-one and an equal weight of phosphorus
pentasulfide was stirred in pyridine. Workup as in the previous example gave a
yellow
solid, mp 209-211°C.'H-NMR (DMSO-d6) 8 12.34 (s,lH), 7.65 (d,lHJ--2.2
Hz )
7.57 (dd ,1H J, = 8.24 JZ= 2.2Hz), 7.51(m,3H), 7.18(m,2H), 2.84(s,2H),
1.26(s,6H).
MS m/z 284 [M-H]- Anal. Calc. For C»H,6F1NS: C,71.55, H,5.65, N,4.91. Found:
C,71.18, H,5.59, N4.82.
CA 02371651 2001-10-31
WO 00/66560 PCT/US00/11835
-73-
EXAMPLE 45
3-(4,4-Dimethyl-2-thioxo-1,2,3,4; tetrahvdro4uinolin-6-vl)benzonitrile
Prepared by heating under reffux overnight a stirred mixture of 3-(4,4-
dimethyl-2-oxo-1,2,3,4,-tetrahydroquinolin-6-yl)benzonitrile and an equal
weight of
phosphorus pentasulfide in pyridine and workup as in the previous example gave
a
yellow solid: mp 220-223 ° C dec. 'H-NMR (DMSO-d6) 8 12.35 (s, 1H),
8.21 (s, 1H),
8.10 (d, 1H, J= 6.0 Hz), 7.80 (d, 1H, J= 7.9Hz), 7.72 (s, 1H), 7.65 (m, 2H),
7.21 (d,
1H, J= 8.4 Hz), 2.85 (s, 2H), 1.27 (s, 6H); MS (APCI (-)) mlz 291 [M-H]- Anal.
Calc. For C~sH,6NzS 3H20: C, 62.40, H, 6.40, N, 8.09. Found: C, 62.12, H,
4.88, N
7.77.
EXAMPLE 46
3-(4,4-Dimethyl-2-thioxo-1,2,3,4-tetrahydroa uinolin-6-yl)-5-
tluorobenzonitrile
Prepared by heating under reffux overnight a stirred mixture of 3-(4,4-
dimethyl-2-oxo-1,2,3,4-tetrahydroquinolin-6-yl)-5-ffuorobenzonitrile and an
equal
weight of phosphorus pentasulfide in pyridine and workup as in the previous
example
gave a yellow solid: mp. 240-242° C dec.; 'H-NMR (DMSO-d6) 8 12.37 (s,
1H), 8.13
(s, 1H), 7.98 (dt,lH, J= 10.4 and 5.4 Hz), 7.81 (d, 1H, J = 8.4Hz), 7.7 (d,
1H, J =
1.BHz), 7.68 (dd, 1H, J = 8.3 and 1.9 Hz), 7.22 (d, 1H, J = 8.3Hz), 2.85 (s,
2H), 1.27
(s, 6H); MS m/z 309 [M-H]- Anal. Calc. For CISH~sFNzS 0.10 HzO: C, 69.25, H,
4.91, N, 8.97. Found: C, 69.15, H, 4.74, N 8.75.
EXAMPLE 47
6-(3-Fluoro-phenyl)-2,4,4-trimethyl-1,2,3,4-tetrahydro-nuinoline
6-Bromo-1-(4-methoxy-benz~l)-4,4-dimethyl-3,4-dihvdro-1H-quinolin-2-one.
To a solution of 6-bromo-4,4-dimethyl-3,4-dihydro-1H-quinolin-2-one
CA 02371651 2001-10-31
WO 00/66560 PCT/US00/11835
-74-
(0. 5g, 1.97mmo1) in THF (25mL) was added 60% NaH (0.12g, 2.95mmol) suspended
in mineral oil. The resulting reaction mixture was stirred at room temperature
for 30
min., 4-methoxybenzyl chloride (0.34g, 2.17) added, and heated under reffux
for 20 h.
The reaction was cooled to room temperature then quenched slowly with water.
After
extraction with ethyl acetate, the organic layer was dried (MgSOa), evaporated
and the
residue purified by chromatography (SiOz 3:7 ethyl acetate/hexane). The white
crystalline product was obtained (0.35g, 48%); mp 118-119 °C,'H NMR
(DMSO-d6)
8 1.23 (s, 6H), 2.59 (s, 2H), 3.70 (s, 3H), 3.72 (s, 1H), 4.41 (d, 1H, J=
5.86Hz), 5.09
(s, 1H), 6.87 (m, 2H), 7.01 (d, 1H, J= 8.78Hz), 7.17 (d, 1H, J= 8.98Hz), 7.23
(d,
1H, J= 8.79), 7.34 (dd, 1H, J= 6.59 and 2.2 Hz), 7.43( d, 1H, J= 2.2Hz); MS
(APCI (+)) [M+H]+ =374/376.
6-(3-Fluoro-phenylL4-methoxy-benzyl)-4,4-dimethyl-3,4-dihydro-1H-quinolin-2-
one.
A mixture of 6-Bromo-1-(4-methoxy-benzyl)-4,4-dimethyl-3,4-dihydro-1H
quinolin-2-one (2.9g, 7.75mmo1) in ethylene glycol dimethyl ether (SOmL),
KZC03
1.18 g, 8.53mmo1) in Hz0 (S.OmL), and a catalytic amount of
tetrakistriphenylphosphine palladium was heated under reffux overnight. After
cooling
to room temperature, the mixture was extracted with ethyl acetate and the
organic
phase was washed with NaHC03 solution, brine, dried over MgSOa, concentrated,
and
crystallized from ethanol to obtain the product as a white crystalline
material (1.8 g,
60%): mp 159 - 162 °C, 'H NMR (DMSO-d6) 8 1.32 (s, 6H), 2.63 (s, 2H ),
3.70 (s,
3H), 5.15 (s, 2H), 6. 89 (d, 2H, J = 11.72Hz), 7.14 (m, 2H), 7.22 (d, 2H, J =
8.8Hz),
7.53 (m, 4H), 7.60 (d, 1H, J= 2.2Hz); MS (APCI (+)) [M+H]+ = 390.
6-Bromo-1-(4-methoxy-benzyl)-2,4,4-trimethyl-1,4-dihydro-quinoline.
To a solution of 6-(3-Fluoro-phenyl)-1-(4-methoxy-benzyl)-4,4-dimethyl-3,4-
dihydro-1H-quinolin-2-one (1.16g, 2.99mmo1) in THF (lSmL) at room temperature
was added a solution of 1.4 M MeMgBr (2.6mL, 12.56mmo1) and the resulting
reaction mixture was stirred for 6h. The solution was quenched with HzO,
extracted
CA 02371651 2001-10-31
WO 00/66560 PCT/US00/11835
-75-
with EtOAc, treated with ammonium chloride solution, washed with brine, dried
over
MgSOa, and concentrated. The product was purified by column chromatography
using a 2:8 hexane/ethyl acetate mixture and used below.
To a solution of 6-(3-Fluoro-phenyl)-1-(4-methoxy-benzyl)-2,4,4-trimethyl-
1,2,3,4-tetrahydroquinoline (0.15g, 0.39mmol) in EtOH (35mL) was added 10%Pd/C
and hydrogenated for 10 h at 40 psi. The catalyst was removed by filtration
through
celite and the product purified by chromatography. A reddish liquid was
obtained. 'H
NMR (DMSO-d6) 8 1.15 ( d, 3H, J= 6.73Hz), 1.21( s, 3H ), 1.34(m, 4H), 1.60(
dd,
1H, J, =10.1,J z= 2.69, Hz), 3.40( m, 1H), 5.95( s, 1H), 6.55( d, 1H, J--
8.08Hz),
7.01( m, 1H), 7.21(dd, 1H, J, =5.78 JZ= 2.02 Hz), 7.35(m,4H); MS( FI Pos)
[M+H]+
=270.
Still other compounds, including 5-(2,4,4-Trimethyl-1,2,3,4-tetrahydro-
quinolin-6-yl)-1H-pyrrole-2-carbonitrile, 1-Methyl-5-(2,4,4-trimethyl-1,2,3,4-
tetrahydro-quinolin-6-yl)-1H-pyrrole-2- carbonitrile , and 3-Fluoro-5-(2,4,4-
trimethyl-
1,2,3,4-tetrahydro-quinolin-6-yl)-benzonitrile may be prepared using the
methods
described above.
All publications cited in this specification are incorporated herein by
reference
herein. While the invention has been described with reference to a
particularly
preferred embodiment, it will be appreciated that modifications can be made
without
departing from the spirit of the invention. Such modifications are intended to
fall
within the scope of the appended claims.