Note: Descriptions are shown in the official language in which they were submitted.
CA 02371782 2001-07-24
WO 00/43750 PCT/US00/01764
IMAGING OF TISSUE USING POLARIZED LIGHT
RELATED APPLICATIONS
This application claims the benefit of U.S. Provisional Application No.
60/117,221, filed on January 25, 1999, the contents of which the are
incorporated
herein by reference.
BACKGROUND OF THE INVENTION
More then 90% of cancer lesions are epithelial in origin. Several of the most
common forms of epithelial cancers such as colorectal, esophageal, bladder,
cervical
and oral cancers have a well defined, detectable pre-cancer stage called
dysplasia.
Dysplasia is characterized by sequential accumulation of mutations in defined
oncogenes and tumor suppresser genes. If detected, the absolute majority of
the
dysplastic lesions are curable. Clinical efforts to detect and treat this pre-
cancerous
stage of epithelial cancer have been shown to reduce the mortality rate.
Diagnosis of epithelial dysplasia remains difficult because it typically does
not form macroscopic structures such as polyps, and is usually only visible
after
cancer has developed. Standard methods of detecting epithelial dysplasia are
based
on random biopsies and pathologic examination of the stained biopsy material.
However, random biopsies have high sampling error. In many cases less than 1 %
of
the epithelial surface at risk for dysplasia can be examined.
All types of epithelial dysplasia have several common characteristics,
namely enlargement of epithelial cell nuclei with an increase in the nuclear
to
CA 02371782 2001-07-24
WO 00/43750 PCT/US00/01764
-2-
cytoplasmic ratio, nuclear hyperchromatism, and increased number and
stratification of epithelial cells. Despite these well-characterized
epithelial changes,
classification has been difficult as demonstrated by high inter-observer
disagreement, even among experienced pathologists.
SUMMARY OF THE INVENTION
Non-invasive, in-vivo methods of detecting epithelial dysplasia provide for
surveillance of epithelial surfaces, and the pathological diagnosis of pre-
cancerous
conditions in humans.
Optical techniques are well suited to be a substitution for random biopsies,
since they are non-invasive, do not require tissue removal, and can be
performed in-
vivo. Moreover, they are fast (can be applied in real time), are relatively
non-
expensive, are able to work on microscopic scale, and thus can find very small
dysplastic sites. The latter are highly likely to be missed by random
biopsies.
The present invention relates to light scattering spectroscopy of polarized
light to provide information about scatterers in surface layers of turbid
media such as
tissue. This process need not utilize fluorescence or absorption spectral
features, but
rather scattering properties of surface tissues such as epithelial layers. It
can
characterize properties of large scatterers (cell nuclei) in human epithelium
and
provide histological information about human tissues and diagnose dysplasia in
real
time in human organs in-vivo.
The idea of light scattering spectroscopy of unpolarized light to determine
features of epithelial tissue has been described in U.S. Serial No. 08/948,734
filed on
October 10, 1997, and in International Application No. PCT/LTS98/21450 filed
on
October 9, 1998, which designated the United States, the entire contents of
these
applications being incorporated herein by reference. The major centers of
light
scattering in epithelium are cellular organelles such as mitochondria and
nuclei with
the refractive index higher than that of the surrounding cytoplasm. Light
backscattered from surface epithelial cell nuclei has an oscillatory
wavelength
dependent component. The periodicity of this component increases with nuclear
CA 02371782 2001-07-24
WO 00/43750 PCT/US00/01764
-3-
size, and its amplitude is related to the density of the nuclei. Thus, by
analyzing the
amplitude and frequency of the oscillatory component, the density and size
distribution of epithelial nuclei can be determined. Normal nuclei have a
characteristic diameter l=4-7 ~,m. In contrast, dysplastic nuclei can be as
large as 20
p,m. Nuclear size and density are important indicators of neoplastic
precancerous
changes in biological tissue. The ability to measure nuclear size distribution
in vivo
and in real time has valuable applications in clinical medicine. This enables
the
diagnosis of precancerous changes in various human organs such as esophagus,
colon, bladder, oral cavity, cervix, etc. non-invasively and in-real-time.
Epithelium covers surfaces of organs in the human body. The thickness of
epithelium ranges from 20 ~,m (one cell layer) to a few hundred microns
(multiple
cell layers). Beneath epithelium there are layers of relatively acellular
connective
and muscular tissues. Since dysplasia is limited to the epithelium, it is
important to
differentiate between the signal associated with the epithelium and underlying
tissues. The backscattered component which carries information about surface
epithelial nuclei is present in light reflected from mucosal tissues. However,
it is
ordinarily very small in amplitude, and easily masked by a background signal
formed by diffuse scattering from the underlying tissue. To analyze that
component
the background signal must be removed. One can remove the diffuse background
by
modeling the general spectral features of the background. However, to make the
approach more useful in practical medicine, and to be able to diagnose
dysplasia in
vivo, in real time, and in different organs, it is necessary to develop more
robust
method of removing or significantly reducing the diffuse component of the
scattered
light.
The present invention provides a method of measuring scattering features of
epithelial cells by using polarized light spectroscopy. Note that initially
polarized
light loses its polarization while traveling through a turbid medium (tissue
is an
example of turbid medium). On the other hand the light scattered backward
after a
single scattering preserves polarization. Thus, by removing the nonpolarized
, component of the scattered light, one is able to distinguish light scattered
by
CA 02371782 2001-07-24
WO 00/43750 PCT/US00/01764
-4-
epithelial cells. The residual spectrum can be further analyzed so that the
size
distribution of the nuclei and their density can be determined.
A preferred embodiment of the inventions includes a fiber optic light
delivery and collection system for diagnoses of tissue. The fiber optic system
can be
S housed in a probe housing proximal and distal ends where the distal end can
be
inserted into the various lumens of the human body for in vivo measurements of
tissue. Polarizers can be used on the distal ends of both delivery and
collection
fibers. With optical fibers that preserve the polarization of light, the
polarizers can
be positioned at the proximal end of the probe. In a three fiber system, the
probe can
use a central delivery fiber and two off center collection fibers that collect
two
different polarization components of light returning from the tissue. The
polarizers
can be birefringent crystalline materials such as quartz, sapphire or calcite.
The
calcite must be sealed from the working environment.
Another preferred embodiment of the invention includes imaging systems
using polarized light to detect and image dysplasia. These systems can be used
to
image tissue samples or perform in vivo imaging of internal organs using
endoscopic systems.
The direct backscatter signal from epithelial tissue, which carnes the desired
information on nuclear size distribution, and the diffuse backscatter signal,
which
must be removed before the size analysis, can be distinguished both by the
polarization of the backscattered light and by its angular distribution. A
preferred
embodiment of a useful light scattering diagnostic takes advantage of both
distinguishing characteristics. Such a diagnostic can be a point measurement,
using
fiberoptic probes, or an imaging diagnostic using lenses and spatial filters
for
angular differentiation as well as polarization-sensitive components for
polarization
differentiation. Preferred embodiments of both fiberoptic, point measurement
systems and video imaging systems are described which are able to highlight
areas
of dysplastic tissue in vivo and in real time.
The wavelength dependence of light scattering from enlarged cell nuclei is
the physical basis for applying light scattering spectroscopy to the detection
of
CA 02371782 2001-07-24
WO 00/43750 PCT/US00/01764
-5-
dysplastic changes. The theory of the scattering of a plane electromagnetic
wave
from a transparent, homogeneous sphere was provided by Mie in 1908 and process
has become known as Mie scattering. The theory shows that the intensity and
the
polarization of the scattered light varies with the angle at which it is
scattered. The
intensity and polarization distribution is determined by five parameters; the
sphere
diameter, the sphere's refractive index, the refractive index of the medium in
which
the sphere is embedded, the wavelength of the incident light in the medium and
the
polarization of the incident light. Generally normal nuclei can be represented
as
spheres with diameters of 5 to 7 ~m and refractive indices of about 1.42, or
generally in the range of 1.40 to 1.45, in a medium with a refractive index
close to
that of water (1.33). Dysplastic nuclei can be considered to be spheres with
diameters of 10 ~m and above.
Much of the diffusion of light through tissue is governed by the scattering of
light from particles and in homogeneities which are smaller than a wavelength.
This
scattered intensity is uniform for all angles in the plane perpendicular to
the plane of
polarization of the light. In the plane of polarization, the scattered
intensity forms
two equal lobes in the forward and backward direction with no light scattered
directly along the axis of polarization. For scattering sites with diameters
of about
one wavelength, the total scattered intensity is strongly peaked in the
forward
direction and the backscattered intensity is very small. The scattering from
these
relatively small sites dominates the scattered light which exits the tissue
surface
in the reverse direction after multiple scattering events deeper in the
tissue. Such
light has a very broad (diffuse) angular distribution and is essentially
depolarized. Light exiting the tissue at a given point within an illuminated
area
of tissue is the sum of light scattered from all entrance points within that
illuminated area so that polarization anisotropies in the individual
scattering
paths are averaged over all angles.
In contrast, scattering sites with diameters relatively large compared to
the wavelength such as dysplastic nuclei, exhibit increasing backscattered
intensity with increasing diameter. This backscattered intensity retains the
CA 02371782 2001-07-24
WO 00143750 PCT/US00/01764
-6-
polarization of the incident light and is also sharply peaked with an angular
distribution which is typically less than five degrees in width. Lobes in
these
backscattered angular distributions also shift direction and intensity with
changes in the illuminating wavelength, giving rise to the spectroscopic
signatures which are used to determine the diameter of the scattering site.
Even
though the absolute backscattered intensity is much smaller than the forward
scattered intensity (it is typically smaller by a factor of 103) its narrow
angle
means that it can be efficiently collected by an optical fiber or imaging
system,
even when the optical aperture subtends a small solid angle. The collection
efficiency of diffusely backscattered scattered light, for the same optical
aperture, is significantly lower. Typically, only about 0.1% of the diffuse
light is
collected by a single optical fiber held a few millimeters from the tissue. In
a
properly designed light scattering spectroscopy probe, the backscatter signal
can
thus be equal to or stronger than the diffuse scatter signal.
The detailed designs of the fiberoptic, point-measurement systems and video
imaging systems described below take advantage of these differences in angular
distribution and polarization between the directly backscattered light
carrying the
desired information and the diffuse backscattered light which dilutes that
signal. By
performing differential measurements, based on polarization or angle or both,
the
desired signal can be extracted from the background, facilitating the analysis
of
nuclear size distribution in the epithelial tissue. The resulting diagnostic
instruments
are able to detect and/or image areas of dysplastic epithelium in vivo and in
real time
with out the need for the human-assisted analysis previously required.
BRIEF DESCRIPTION OF THE DRAWINGS
Figure 1 illustrates a preferred embodiment of a polarization-based light
scattering spectroscopic system.
Figures 2 A and B are reflectance spectra of the two-layered tissue phantom
(polystyrene beads on top of gel containing blood and BaS04) for parallel and
perpendicular polarizations (notice characteristic hemoglobin dips)
respectively.
CA 02371782 2001-07-24
WO 00/43750 PCT/US00/01764
_7_
Figures 3 A-D illustrate differences of two polarizations for (A) 4.56 ~.m
beads in water (relative refractive index h ~ 1.19), (B) 9.5 ~,m beads in
water
(n ~ 1.19), (C) 5.7 p,m beads in glycol (n ~ 1.09), (D) 8.9 ~.m beads in
glycerol
(h ~ 1.07) where the signals (dashed lines) are in good agreement with Mie
calculations (solid lines) and the absorption features of hemoglobin are
completely
removed.
Figure 4 is a spectrum of the polarized (residual) component of back-
scattered light: experimental data vs. fit of the Mie calculations for the
polarized
back-scattering for T84 cancerous colonic cells where best fits provide the
following
sets of parameters: average size 10.2 ~,m, standard deviation 1.5 ~,m,
relative
refractive index 1.045, and the sizes and standard deviations are in agreement
with
those measured using light microscopy.
Figure 5 is a spectrum of the polarized (residual) component of back-
scattered light: experimental data vs. fit of the Mie calculations for the
polarized
back-scattering for normal intestinal cells where best fits provide the
following sets
of parameters: average size 5.0 ~,m, standard deviation 0.5 ~,m, relative
refractive
index 1.035, and the sizes and standard deviations are in agreement with those
measured using light microscopy.
Figure 6 shows the nuclear size distribution for normal intestinal cells and
T84 cancerous colonic cells where in each case, the solid line is the
distribution
extracted from the data, and the dashed line is the distribution measured
using light
microscopy.
Figure 7 schematically illustrates a fiber optic probe system for performing
in vivo optical measurements of tissue in accordance with the invention.
Figures 8A and 8B show the distal end of a probe of a preferred embodiment
of the invention.
Figures 9A-9C illustrate another preferred embodiment of a fiber optic probe
in accordance with the invention.
Figures l0A-l OC illustrate a preferred embodiment of a fiber optic probe
device for delivery and collection of light.
CA 02371782 2001-07-24
WO 00/43750 PCT/US00/01764
_g_
Figures 1 lA-11D illustrate preferred embodiments of an imaging system in
accordance with the invention.
Figure 12 is a cross-sectional view of a rigid probe imaging system in
accordance with the invention.
Figure 13 illustrates a distal end of a probe imaging system in accordance
with the invention.
Figure 14 is a cross-sectional end view of an imaging endoscope.
Figure 15 is a detailed cross-sectional view of a liquid crystal light valve
to
control illumination of the imaging sensor.
Figure 16 illustrates the resulting illumination of the imaging sensor.
Figure 17 illustrates a simple patient measurement probe.
Figure 18 illustrates another preferred embodiment of a probe tip.
Figure 19 illustrates another preferred embodiment of a probe tip in
accordance with the invention.
Figure 20 illustrates a multifiber probe in accordance with the invention.
Figures 21A-21D illustrate features scattering measurements.
Figures 22A-B graphically illustrate results of scattering measurements.
The foregoing and other objects, features and advantages of the invention
will be apparent from the following more particular description of preferred
embodiments of the invention, as illustrated in the accompanying drawings in
which
like reference characters refer to the same parts throughout the different
views. The
drawings are not necessarily to scale, with emphasis being placed upon
illustrating
the principles of the invention.
DETAILED DESCRIPTION OF THE INVENTION
To determine properties of epithelial cells, one can correlate measured
spectrum of the backscattered light with a model or representation. Using Mie
theory, which provides the exact solution for the problem of light scattering
by
spherical objects of arbitrary sizes, the sizes and relative refractive
indexes of the
scatterers can be determined.
CA 02371782 2001-07-24
WO 00/43750 PCT/US00/01764
-9-
For polarized incident light, light scattered by a spherical particle with
diameter d has components which are polarized parallel and perpendicular to
the
plane of scattering. For a plane polarized wave incident in direction so,
light
scattered into direction s will have components which are polarized parallel
(p) and
perpendicular (s) to the plane of scattering. Intensities Ip and IS of these
components
are related to the intensities of the incident light Ip~°> and
ISO°~ as follows:
IS ("s, s ) z
Ins) - 4 Kzdz In~so) (1)
I'Sz ~S~ So ) z o
IS~S) - 4 Kzdz In ~So)
where k is the wavenumber of the incident light, SI and SZ are scattering
amplitudes
which can be calculated numerically using Mie theory, and s, and s2 are unit
vectors
defining propagation of the incident and scattered light. Scattering
amplitudes are
functions of a scattering angle ,~ =cos' (s~so ) and are normalized so that
integral
f ( S, (,~)IZ + SZ (~) Z ) sin ,~d ~ equals the total elastic scattering cross
section.
0
Now consider an experiment in which linearly polarized incident light,
intensity Io, is distributed over solid angle OSZo and scattering is collected
over solid
angle OSZ. The polarization, ~o , of the incident light can be decomposed into
a
component sp , in the scattering plane (i.e. the plane formed by s and so ),
and a
perpendicular component s° . By means of analyzers, we detect two
orthogonal
components of the scattered light intensity, I~~ having polarization sQ and Il
having
CA 02371782 2001-07-24
WO 00/43750 PCT/US00/01764
-10-
perpendicular polarization sa . The scattered intensity components are then
given
by
I ~ _ ~kd, ~ d"s f dsolo (so )ISz ~so ~ S~ cos ~p cos ~po + S, (so, s) sin ~p
sin ~po I z
n~ nn
(3)
I - 2 f ds f ds I (s ) S (s s) cos sin - S (s s) sin cos
~kd2 0 0 0 2 0 ~ ~P ~Po ~ o ~ ~P ~Po
n~ n~
(4)
If the incident light is completely collimated (OSZo =0), light scattered
directly backward will be polarized parallel to the incident light
polarization. In this
case we can orient one of the analyzers parallel to the incident polarization
direction
( so ~ sa ) . If the solid angles of the incident and collected light are
sufficiently
small and approximately equal, both I~~ and Il will be present. However, the
analyzer can still be positioned such that ( so ~ sa ~ . Thus, in this case
the
collected light will still be highly polarized, and I~,, » 11 . For this case
the
expression for the residual intensity, 1~~ - Il can be simplified:
4I a~
I ~ - Ii ~ kd 2 ~ Re(S; (a)Sz (a)) sin 9d ~ ,
0
(5)
with ~ o =
2~
Consider a system of two layers of scattering media such as epithelial tissue
in which a thin layer of large scattered (d»~.) covers a highly turbid layer
of
underlying tissue. Each of these layers gives rise to a different type of
scattering.
This two layer system represents optical properties of many human tissues with
the
first layer correlated with epithelium and second layer correlated with other
tissue
CA 02371782 2001-07-24
WO 00/43750 PCT/US00/01764
-11-
layers beneath epithelium. The upper layer is optically thin so that it does
not allow
multiple scattering. Small portions of incident linearly polarized light is
backscattered by the particles in the upper layer. The rest of the signal
penetrates to
the second layer that is optically thick. Light propagating through the second
layer
S is randomized by means of multiple scattering. This diffusive light, if not
absorbed
in the second layer, returns to the surface. Thus, emerging light has two
contributions: one from light backscattered by the particles of the first
layer, Ib, and
the other being diffusely reflected from the second layer, Id. Ib has high
degree of
linear polarization that is parallel to the polarization of the incident
light: lob»Ilb.
As a result of multiple scatterings in the second layer, diffusely reflected
light is
depolarized and hd=lia. Therefore, the residual intensity of the emerging
light li
-I1 ~I~b-I1b 1S dominated by the contribution from the upper layer and is
substantially free from both absorption and scattering from the tissue below.
Expressions (3)-(5) relate h -li to the scattering amplitudes S~ and S2. The
amplitudes depend on the wavelength of the light being scattered ~,=~/k, the
scatter's
size d and the ratio of its refractive index to that of the surrounding
medium, relative
refractive index h. Therefore, the spectrum of the residual intensity varies
with the
scatterer's size and relative refractive index. Thus, sizes and refractive
indexes of
the scatterers can be found by fitting the representation of the Mie theory
using
equations (3)-(5) to the residual intensity spectrum.
A system 10 that measures excised tissue samples in vitro is illustrated in
Figure 1. This system 10 delivers collimated polarized light on tissue 12 and
separates two orthogonal polarizations of back-scattered light. The difference
of
these two components provides information about light scattered in the
epithelial
layer only. Since linearly polarized light is depolarized faster than
circularly
polarized light while passing through a random medium, linear polarization was
used. The system provides light from a broadband source 14 (250 W tungsten
lamp,
Model 66181, Oriel Instruments, Inc., Stratford, CT) is collimated and then
refocused with a small solid angle onto the sample using a fiber 16, a lens 18
and an
aperture 20. A broadband polarizer 22 linearly polarizes the beam, before it
is
CA 02371782 2001-07-24
WO 00/43750 PCT/US00/01764
-12-
delivered to the surface of a scattering medium through beamsplitter 24. The
light
beam strikes the surface of the sample with an angle ~ 15° relative to
the normal in
order to avoid specular reflectance. The diameter of the beam is 2mm. The
reflected light is collected in a narrow cone (0.015 radian) with apertures 26
and
S mirror 28 and two polarizations, parallel Iii and orthogonal Ii to the
initial
polarization, are separated by a broadband polarizing beam splitter cube 28
which
also acts as our analyzer (Melles Griot, Inc.). The output from this analyzer
is
delivered through lenses 30 and 200~,m optical fibers 32, 34 (Ocean Optics,
Inc.,
Dunedin, FL) into two channels of a multichannel spectroscope 36 (quadruple
spectroscope, Model SQ200, Ocean Optics, Inc., Dunedin, FL). This enables the
spectra of both components to be measured simultaneously in the range from 300
nm to 1200 or optionally in the range from 400 nm to 900 run.
The beams are not perfectly collinear, and when they pass through the
polarizer and analyzer cubes this gives rise to a small amount of distortion.
Furthermore, the beamsplitter has different reflectivities for s andp
polarizations. A
diffusely reflective white surface was used as standard to correct for
wavelength
non-uniformity, and to calibrate the signals in the two channels. I1 ~~,) and
III ~~,) were each normalized to the corresponding background spectra, Ii ~~,)
and
IIIB (~,) were each normalized to the corresponding background spectra, IIiB
(~,) and
Il (~,) taken with the white diffusing surface. This removed spectral non-
uniformities in the light source. Thus, the experiments actually measured the
normalized residual intensity, 0I:
01-III _11
Ia Ia
II
(5)
Measurements on simple single- and two-layer systems were conducted to
determine operational parameters. The single layer system included polystyrene
beads of various sizes ranging from O.S~,m to 10~.m (Polyscience, Inc.)
embedded in
CA 02371782 2001-07-24
WO 00/43750 PCT/US00/01764
-13-
de-ionized water, glycol, or glycerol. The thickness of these layers was
varied so
that the optical thickness i ranged from 0.1 to 5 a photon propagating through
a
medium with i=1, undergoes one scattering event on average). The beads of
large
sizes 4-10 ~,m were used to represent cell nuclei. Since the relative
refractive index
of the polystyrene beads in water is about 1.2 (absolute refractive index is
about
h=1.59) and is substantially higher than that of the cell nuclei relative to
the
cytoplasm which is in the range from 1.03 to l.l, glycol (n4 1.45) and
glycerol
(nQ 1.48) were used instead of water to decrease the relative refractive index
of the
beads and, therefore, better approximate biological conditions.
In the single layer measurements the component of the backscattered light
with the same state of polarization as the incoming light (denoted by I~ was
almost
100 times larger than the component with the polarization orthogonal to the
polarization of the incoming light (denoted by Ii). This establishes that
single
scattering from large spheroidal particles preserves polarization.
In the measurements with two layer models the first layer consisted of
polystyrene beads embedded in water, glycol, or glycerol and was prepared as
in the
single layer measurements. The second layer included a gel containing solution
of
BaS04 powder which provided scattering properties of the second layer and
human
blood. Hemoglobin content of the blood provided absorptive properties to the
model. This physical model simulated epithelium and underlying tissues.
Adjusting
concentrations of the BaS04 powder and blood, the scattering and absorption
properties, can be made similar to those of a biological tissue, since in the
optical
spectral region hemoglobin is known to be the major absorber.
Figures 2A and 2B shows spectra of the parallel IA and orthogonal 11
polarized components of the light reflected from a two layer system. In this
measurement, the first layer contained beads embedded in glycol. The beads had
an
average diameter of 4.56 ~,m. Standard deviation of their sizes was 0.03 ~,m.
Optical thickness of the first layer was z ~ 0.8. The second layer was
optically thick
and its scattering and absorptive properties were comparable to those of a
biological
tissue. The spectrum of I1 is dominated by characteristic hemoglobin
absorption
CA 02371782 2001-07-24
WO 00/43750 PCT/US00/01764
-14-
bands. At the same time, characteristic spectral features of light scattered
by 4.56
~,m beads in the first layer, namely apparent ripple structure, and hemoglobin
absorption in the second layer are seen in the spectrum of Iu.
The residual spectrum dI is shown in Figure 3A. No hemoglobin absorption
features are seen and the diffusive background coming from the second layer
was
completely removed. The ripple structure characteristic of scattering from
spheres is
evident. The comparison with Mie theory representation for scatterers with d =
4.56 ~,m , 0d=0.03~,m and n=1.035 correspond with the ~,m shown in Figure 3B
shows high degree of accuracy. The residual spectra obtained in measurements
with
other bead sizes (5.7~,m, 8.9~,m, and 9.S~,m) embedded in any of the media
used
(water, glycol, and glycerol) had no measurable diffusive background component
and agreed with Mie theory. Figure 3B shows the agreement between the theory
and
the measurements for 9.5 ~m beads.
Similarly, the results of the measurements for 5.7 ~,m and 8.9 ~.m beads in
glycerol and glycol are shown in Figures 3(C) and (D) respectively. Mie theory
corresponds with the measured values in these cases as well. The high
frequency
ripple structure decreases as the relative refractive index gets smaller. The
law
frequency oscillations remain evident. Measurements showed that the instrument
was able to detect signal from the bead solution of as low optical thickness
as 0.05.
Small disagreements seen in the spectrum can result from imperfect calibration
of
the instrument for the wavelength dependence of the optical elements used. The
beams are not perfectly collinear and so there arises some imperfections in
the
polarized signals from the two channels when the beam passes through the
polarizer
and the analyzer elements. Further, the beam sputter used has different
reflectivities
for the s and thep polarized beams. However, using just a white standard,
signals in
the two channels were corrected for any wavelength non-uniformity and further
used
for calibration of signals.
Measurements with cell monolayers were conducted and the results are
described in connection with Figures 4-6. A layer of gel containing solution
of
BaS04 powder and human blood under the monolayers is used to represent
CA 02371782 2001-07-24
WO 00/43750 PCT/US00/01764
-15-
underlying tissue. The concentrations of the BaS04 powder and blood, were
adjusted to match optical properties of the biological tissue. Three types of
cells
were measured: normal intestinal cells, T84 cancer colonic cells and the
fibroblasts.
The measurements were similar to the measurements with beads. Nuclei of cells,
however, had relative refractive indexes smaller then those of beads as well
as larger
size distributions which substantially eliminate the ripple structure. Fitting
of the
observed residual spectrum to Mie theory was performed. Three parameters in
the
fitting procedure were average size of the nuclei, standard deviation in size
(a
Gaussian size distribution was assumed), and relative refractive index.
For normal intestinal cells, the best fit was obtained using
d=S.O~m,Od=O.S~,m, and n=1.045 (Figure 4). For the fibroblast cells, d-7.0
~,m,
0d=1.0 ~,m and n=1.051 were obtained. For the T84 colon cancer cells the
corresponding values were d=9.8 ~,m ~d=1.5 ~,m, and n=1.04 (Figure 5).
In order to check these results, the distribution of the average size of the
cell
nuclei was measured using light microscopy. The sizes and their standard
deviations
were in agreement with the parameters from Mie theory. A histogram showing the
size distributions obtained for the normal T84 cells are shown in Figure 6.
The
accuracy of the average size is estimated to be 0.1 ~,m, and the accuracy in n
as
0.001. Note the larger value of n obtained for cancerous cells, which is in
agreement
with the hyperchromaticity of cancer cell nuclei observed in conventional
histopathology of stained tissue sections.
The backscattered signal can be described by Mie theory if the average size
of the nuclei d, standard deviation in sizes Od, and relative refractive index
n are
varied. Note that in Mie theory, dependence on d and n does not always come as
a
(n-1 )d product. Thus, the residual spectra have enough information to extract
d and
n simultaneously.
The size distributions for monolayers were compared to light microscopy
and were in a good agreement for all three lines of cells. The accuracy of
size and
standard deviation extraction was approximately 0.1 ~,m which makes the method
CA 02371782 2001-07-24
WO 00/43750 PCT/US00/01764
-16-
useful in differentiating nuclei of different cell types, including cancerous
and non-
cancerous cells of the same organ.
Ability to detect cell nuclear enlargement and changes in refractive index of
the nucleus (which can be related to the amount of DNA and protein in the
nucleus)
has valuable applications in clinical medicine.
The method of tissue diagnosis can be implemented either in a diagnostic
device in which light can be delivered to points on the surface of the tissue,
and
collected and analyzed at each of those points on the surface of the tissue,
and
collected and analyzed at each of those points. In an in vivo system optical
fibers are
used to deliver and collect light. The fiber probe can be inserted in the
endoscope
biopsy channel or any similar device (depending on the type of the procedure
and
organ under study). Polarizer and analyzer can be placed at the tip of the
probe in
front of the delivery and collection fibers. Such an instrument can be used
during
routine endoscopic procedures to detect precancerous changes in-vivo in real
time.
Such a probe system 40 is shown generally in Figure 7. This system 40
includes a broadband light source 42 that is optically coupled to a delivery
fiber 44
extending through probe S0. As schematically shown in Figure 7, the probe 50
can
be inserted through a channel in an endoscope 48, however the probe 50 can be
constructed to be used separately. In a preferred embodiment described
hereinafter,
the light from source is directed through a polarizer at the distal end of
probe S0.
However, in another embodiment using polarization preserving optical fibers, a
polarizer 26 can be used at the proximal end of probe fiber 44 to direct
polarized
light 46 through the fiber. Similarly, the proximal ends of collection fibers
65, 66
can employ polarizing elements 65, 66 respectively to transmit selected
polarization
components into the multichannel fiber spectrometer 54. The data can then be
processed by computer 56, stored in computer 56, stored in computer memory and
displayed on display 60 as needed.
The probe system can include a fiber optic probe having a distal end
incorporating polarizers as seen in Figure 8A and 8B.
CA 02371782 2001-07-24
WO 00/43750 PCT/US00/01764
-17-
Figures 8A and 8B show the distal end of a probe 100 for the use of
polarized light for in vivo diagnosis. Figure 8A shows a fiber optic device
that is
divided into three sections, the inner delivery fiber and two sets of
collection fibers
150 and 152 that collect different polarization components. The cross-section
of
Figure 8B shows fibers 156 delivering light onto the tissue 140. They have to
pass
through a polarizer 120 which is also seen in the cross-section view of Figure
8B.
The polarizing element is divided into at least two parts or elements 122,
126.
Optical fibers 152 are arranged to collect the back reflected light from the
tissue
surface.
The backscattered light has two polarization components, corresponding to
the parallel and the perpendicular components to the incident light. The two
are
differentiated by two different birefringent analyzers shown by two sectioned
ring
elements 122, 126. A first element 122 allows the parallel component to pass
through while the second element 126 allows perpendicular component. A portion
of element 122 polarizes light exiting fiber 156. As the fibers have low
numerical
apertures to collect light over very small angles, it is necessary to extend
the distance
136 between the fiber ends and the aperture surface 142 opening to the tissue
surface
140. It can be as long as Smm. To avoid spurious internal reflections a glass
block
130 is shown having refractive index n2 lower than that of the shield 132 with
refractive index nl. The shield 132 can be coated with an absorbing element so
that
light hitting the boundaries is refracted out and then absorbed by the
absorbing
coating on the outer wall of the shield 132. The glass element 130 is beveled
to
avoid specular reflections from the tissue surface as it is described to
increase the
relative signal strength of the back-scattering. The light having the two
orthogonal
polarizations is separated and coupled to two spectrometer channels for
detection
and analysis.
Another preferred embodiment of a fiber optic probe 160 is illustrated in
Figures 9A-9C. In this embodiment, delivery 156 and collection 162 fibers are
housed in flexible tube 164 that is attached to a distal annular housing 166.
Housing
166 includes a fiber retainer 106 and a polarizer 168 which can be a
birefringent
CA 02371782 2001-07-24
WO 00/43750 PCT/US00/01764
-18-
crystal such as calcite, quartz or sapphire. Delivery fiber 156 delivers light
from
source 42 to polarizer 168 which delivers ordinary ray 170 through aperture
175 and
window 178. Light returning through aperture 175 has ordinary 170 and
extraordinary 172 components. The perpendicular component is collected by
fibers
162 and the parallel component is collected by fibers 161. The delivery fiber
156 is
positioned along the optical axis 176 of the crystal 168. Fibers 161, 156 are
aligned
along the aperture 175 of absorbing plate 178.
An improved method for this analysis involves performing a differential
measurement of the backscattered light. Taking advantage of the fact that
backward
Mie scatter is differentiated by both angle and polarization from diffuse
scatter. In
this embodiment, a fiberoptic probe measures the backscattered light at two
angles
with a single polarization filter. By subtracting the two measured spectra the
signal
to noise ratio of the measurement is increased and the need to perform a
parametric
fit is eliminated.
Initially, since the original reflectance measurement technique required
spectra for every point measurement, it seemed that an imaging device would be
impractical. However, the use of polarized light for imaging can be provided
using
a system that generates a plurality of images at discrete wavelengths that
detect
different polarization and angular components.
The features of such an imaging system include an optical system which can
take two images of the tissue, in a narrow wavelength band, that discriminates
between the backscattered angle of the reflected light and the polarization of
the
reflected light. This accomplishes the differential measurement. These
differential
images are then acquired at as many different wavelengths as required to
accomplish
a final image which highlights areas of tissue with enlarged cell nuclei
compared to
normal cell nuclei.
The scattering of light by spheroidal particles as a function of angle and
polarization is well described by Mie theory. Most incident light scattered
from a
cell nucleus continues generally in a forward direction. A small fraction,
however,
is backscattered within a narrow angle cone, with the same polarization as the
CA 02371782 2001-07-24
WO 00/43750 PCT/US00/01764
-19-
incident light. Generally, light scattered from large particles is more
strongly
peaked in the backwards direction than light scattered from smaller nuclei
with a
strength which has an oscillating dependence on the diameter of the cell
nucleus
relative to the wavelength of light. Analysis of this backscattered light, as
a function
of wavelength, provides a distribution of the density and diameter of the
scattering
particles as described above. Other types of scattered light, however, can
dilute this
backscattered light, making the analysis more difficult. Forward scattered
light,
after many repeated scattering events, may also exit the tissue in a generally
backwards direction with a wide (diffuser) angular distribution. When a large
area
of tissue is illuminated with polarized light, as in the described imaging
system, the
diffused light exiting the tissue from any given point within the illuminated
area has
essentially no preferred polarization. This diffused light is the sum of light
scattered
to that point from all of the entrance points of the illuminated area. The
polarization
effects which can be seen on a single ray of light which has propagated
through the
tissue at a specific angle from the input polarization plane and a given
distance from
the entrance point are thus averaged out. This will be the case for all of the
imaged
points on the tissue surface as long as the illumination area extends
sufficiently
beyond the imaged area. The problem to be solved is the enhancement of the
detection of the small quantity of directly backscattered light in the
presence of the
larger quantity of multiply scattered light.
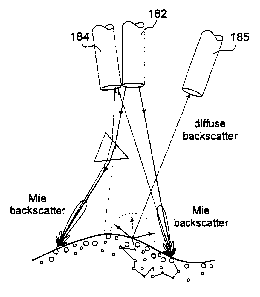
The assembled view of Figures 9A and 9B show how the fibers are held
together to maintain their relative angle. An appropriate sleeve 185 is placed
around
this assembly to protect the three long fibers 182, 184, 186 in the endoscope
channel
and to prevent direct light from entering the assembly from the side. The
exploded
view of the assembly in Figure 9C shows how the tip is assembled. The three
fibers
are surface glued (LJV-curing polymer) onto a semi-cylindrical carrier which
is
molded from plastic with alignment grooves for the fibers. The cap semi-
cylinder is
glued on to hold them rigidly in place. The three fiber tips are then polished
simultaneously so their surfaces are perpendicular to the Garner longitudinal
axis.
The carrier assembly is then optically glued to the end window.
CA 02371782 2001-07-24
WO 00/43750 PCT/US00/01764
-20-
Figure 22A shows that direct Mie backscatter from large particles can be
collected more efficiently than diffuse backscatter from small particles by
properly
choosing the angular direction and collection solid angle of the receiving
fiber. This
improves the signal (direct backscatter) to noise (diffuse scatter) ratio. The
tilt
prevents direct reflections from the tip/tissue interface from getting into
the
receiving fibers.
These drawings assume that the direct backscatter is picked up by the same
fiber that transmits the light to the tissue. Such single fiber designs have
the virtue
of lower costs for materials and assembly. The technical difficulty which must
be
overcome in their design is that surfaces perpendicular to the direction of
the
illumination beam in the optical train reflect some fraction of the light
backwards
into the detectors which are looking for tissue reflection. The design must
thus
avoid such surface reflections by tilting them at a sufficient angle to
prevent such
reflected light from propagating in the optical fiber. For typical fiber
numerical
apertures this requires a tilt of about 14 degrees. These single-fiber devices
are
shown in Figures 17 through 20.
The present embodiment of the invention accomplishes that enhancement by
acquiring two, separate images of the tissue (in pairs, at multiple
wavelengths, for
example). Figure 1 lA shows a preferred embodiment of the imaging system that
can be used for in vitro analysis or for exposed surface tissue. The optical
train
enhances the detection of the directly backscattered light in one image
detected
through lens 218 with image sensor 219 by passing only the light polarized
parallel
to the incident light, polarized light along with a spatial filter 217 at a
focus in the
optical train which passes only light within a narrow cone angle from the axis
of the
incident light. In this first image, some of the undesired light from diffuse,
multiple-
scatter events reaches the image. A second image detected with image sensor
223
receives light from lens 222 and spatial filter 221, also shown in Figure 11C,
that
blocks the passage of the directly backscattered light and passes only the off
axis
light with a polarization perpendicular to the incident light. The images are
recorded
electronically with separate monochrome image sensor 219, 223 which can be
CA 02371782 2001-07-24
WO 00/43750 PCT/US00/01764
-21-
charge-coupled device (CCD) cameras. Electronically subtracting a fraction of
the
second image from the first image leads to a final image of the tissue which
consists
primarily of the directly backscattered light. This process is repeated at a
sufficient
number of wavelengths to allow the size of enlarged cell nuclei in the tissue
to be
differentiated from normal cell nuclei. This selection of wavelengths can be
accomplished with a rotating filter wheel 204 shown in Figure 11B that is
mounted
on a rotating spindle 224 in front of a broadband light source 200, or by an
electronically tuned liquid crystal filter in front of a broadband light
source 200, or
by a series of narrowband light sources combined onto one axis with a grating
or
scanning mirror. A lens 202 couples light from source 200 onto the filters 203
on
wheel 204. A second lens 205 couples light exiting each filter 203 into a
fiber 206,
through aperture 207, prism 208 in optical coupler 209, lens 210, mirror 21 l,
beamsplitter 212 and onto a tissue surface 213. Light returning from the
tissue
passes through beamsplitter 212, lens 214 and into optical coupler 209. The
light
returning from the tissue is either reflected by beamsplitter 215 onto mirror
220 and
into filter 221 or is transmitted by beamsplitter 215 through aperture 216 and
filter
217.
Alternatively, instead of elements 211, 212, another embodiment illustrated
in Figure 11D uses mirror 228 and a nonpolarizing beamsplitter 226. This
emobidment rduces the amount of back reflection occurancy in the embodiment fo
Figure 1 lA. Beamsplitter 226 can also be used to replace element 215, for
example,
in system 209.
Shown in Figure 12 is a probe assembly 250 using a relay lens system in a
distal section 254 that can be dimensioned appropriately for examinations of
the oral
cavity, cervix, or tissue exposed during laparoscopy. The proximal section 252
of
probe 250 can employ the general design described in connection with Figure
11A.
Image sensors 270 and 272 collect images having different polarization
components. The optical housing 280 includes mirror 282, beamsplitter 284,
polarizing prism 285, polarizing beamsplitters 286, 288, spatial filters 266,
268, 290,
and 292, delivery fiber 264, image reducing lens 274 and 276. Lens 260 and 262
CA 02371782 2001-07-24
WO 00/43750 PCT/US00/01764
-22-
can be spaced to form a telecentric optical coupling system. A window 258 can
provide direct coupling to tissue surface 256.
Figure 13 shows a reflectance imaging system positioned in an endoscope
tip that uses both differential polarization and differential angle. The
arrangement
shown in the top drawing only uses both differential polarization and
differential
angle. The arrangement shown in the top drawing only uses polarization as
discussed below. The modified design shown at the bottom to the figure below
the
end-on view uses both polarization and angle to detect the direct Mie
backscatter.
Generally the liquid crystal switches work better in collimated light which
favors the
polarization only design, but they operate as shown in the polarization/angle
design
with some reduction of contrast. A further description regarding the use of
twisted
nematic, liquid crystal spatial light modulators is B.E.A. Saleh and
M.C.Teich,
Fundamentals ofPhotonics, Wley, New York, NY, 1991, pp 724-726, ISBN 0-471-
83965-5, TA1520.524, the contents of which is incorporated herein by
reference.
Figure 13 shows an embodiment of an endoscope-based reflectance imaging
system which enhances the detection of the direct Mie backscatter from cell
nuclei
with a polarization differentiation technique alone. The rigid endoscope tip,
300, is
attached to the flexible section, 302, and capped with an end plug, 304. The
end
plug, shown in Figure 14 carnes an imaging objective lens group, 306, along
with
the usual biopsy channel, 308, suction channel 310, and an auxiliary
fiberoptic
illumination port, 312, for normal, white light illumination of the tissue.
The
objective lens group, 306, images the tissue surface, 314, onto a CCD, video
camera
chip, 316, utilizing a second lens group, 318, which, along with 306, forms a
telecentric imaging system. A non-polarizing, broadband beamsplitter, 320,
couples
illumination light onto the imaging axis. This illumination light comes from a
small
diameter optical fiber, 322, which is polarized by a transmission filter, 324.
The
diameter of the optical fiber, along with the focal length of the main
objective lens
group, 306, sets the angle subtended by the illumination onto the tissue for
the
reflectance measurement. A twisted, nematic, liquid-crystal cell, 328, is
placed in
the collimated beam after the lens group 318. A polarizer, crossed with
respect to
CA 02371782 2001-07-24
WO 00/43750 PCT/IJS00/01764
-23-
the fiber optic polarizer, 324, is placed in front of the CCD camera where it
is
recorded as a digital image. This image is thus composed of the direct, Mie
backscattered reflection (polarized) and half of the diffuse backscattered
reflection
from the tissue (unpolarized). When a longitudinal electric field is placed on
the
liquid crystal cell it passes both polarizations of the diffuse reflected
light from the
tissue and the direct Mie backscatter without rotating their polarizations. A
second
digital image is taken. In this image half of the diffuse reflected light
passes through
the polarizer, 330, and the direct Mie backscatter is blocked. Since the
diffuse
scatter is unpolarized, the two image components from the diffuse backscatter
are
identical. Taking the difference between the first image and the second image
thus
determines the direct Mie backscatter image alone. An iris placed at the focus
of the
telecentric lens system sets the angular extent of the reflected light which
is passed
for analysis.
A second embodiment shown in Figure 15 modifies the liquid crystal cell,
332, and its position in the imaging optical train. This allows the liquid
crystal cell
to block the direct Mie backscattered light both in terms of its polarization
and
angle. In this embodiment the cell is placed at the focus of the telecentric
lens
system so that it is sensitive to the angle of the imaging light rays. Only
the central
portion of the liquid crystal cell, 332, has an applied longitudinal voltage
as shown
in the orthogonal view of the liquid cell, 336 in Figure 16. In this
embodiment, only
the central rays, with the polarization of the illumination light, are blocked
at the
CCD camera. As before, two images are taken and their difference indicates the
portion of the image due to direct Mie backscatter. The liquid crystal cell
loses
some of its ability to affect polarization at steep angles and practice may
show that
removing the lens elements, 338, to increase the focal length of the
telecentric lens
group may improve the image. This increases the overall length of the
endoscope
tip, however, which should be avoided if possible.
The same techniques of angle and polarization control can be used with
single fiber point measuring reflectance probe as shown in Figures 17-20.
CA 02371782 2001-07-24
WO 00/43750 PCT/US00/01764
-24-
The optical system of Figure 17 launches the broadband light from source,
400, through an optical assembly, 402, into the fiber through a 50/50, non-
polarizing
beamsplitter, 404. This allows the return light from the tissue to pass
through to the
lens 406, which delivers it onto a detector or a spectrograph. A plate of
absorbing
glass, 408, is optically glued to the back surface of the beamsplitter
assembly 404 to
absorb the illumination light which is not directed to the fiber. The probe
fiber tip is
placed in a block 410, and the two are polished at the same angle as the
beamsplitter
assembly output face. The block thus keeps the fiber tip properly aligned to
that
face. A small drop of index matching fluid assists in reducing scatter from
the fiber
tip. Note that all of the surfaces through which the beam passes are tilted to
avoid
back reflections.
The single optical fiber, 412, carnes the light to a probe tip, 41 S, which
must
provide, in general, a window 414 which holds the fiber tip at an offset from
the
tissue to allow the beam to expand somewhat and not present a puncture risk to
the
patient. The fiber tip in the probe is also held in a block 413 to facilitate
polishing it
at an angle. The tip of this window must also be tilted in window probe 417
shown
in Figure 18 that provides two specific benefits. First, the negative lens,
416,
shortens the length of the probe tip by making the tip of the transmit/receive
fiber
appear to be farther from the tissue surface. There is an optimal apparent
optical
distance for the received fiber tip from the tissue which maximizes the signal
to
noise ratio for a single fiber reflectance measurement. The negative lens
provides
this optical path in a shorter probe tip, allowing the tip to fit more easily
through
narrow, curved endoscope channels. The directly backscattered light is not
significantly affected by this lens because it retraces its path back through
the lens to
the fiber tip from which the illumination came.
Probe 421 illustrated in Figure 19 shows that the optical element which
accomplishes the redirection of the illumination does not have to be a common,
spherical lens. The refracting surface in optical element 418 in distal end
421 is
cone-shaped with an included angle which is less than 90 degrees to prevent a
corner-cube reflection condition back into the transmit/receive fiber tip.
This lens is
CA 02371782 2001-07-24
WO 00/43750 PCT/US00/01764
-25-
commercially available in broadband-transmitting sapphire at a very low price
because this shape is used for jeweled pivot bearings. Note that there is no
transmitted light which goes directly forward because of element 420 so that
for this
portion of the window, the amount of diffuse reflected light which makes it
back
into the transmit/receive fiber is also reduced.
Probe 425 illustrated in Figure 20 shows a preferred probe tip which also
included the addition of a linear polarizing filter, 422, at the window tip.
The
directly backscattered light from the cell nuclei is polarized in the same
plane as the
illumination. The polarizing filter at the window tip absorbs all but one
polarization
of the illumination light and passes all of the directly backscattered light.
The
diffuse backscatter, however, is not polarized since it has undergone many out-
of
plane scattering events which randomly rotates its polarization. Thus the
diffuse
backscattered light is reduced by a factor of two before it enters the
transmit/receive
fiber.
1 S The additional received fibers, 424, shown in the preferred embodiment of
probe 425 are aligned parallel to the central transmit/receive fiber to
collect off axis
reflected light which is predominately due to the diffuse scattering
processes. These
fibers can be arranged in a circular ring around the central fiber and can be
much
narrower than the central fiber to maintain the flexibility of the total
bundle. A
fraction of the signal from these off axis fibers can be subtracted from the
signal
from the central fiber to provide a differential measurement of the Mie direct
backscattered light which carries the desired information on the tissue cell
nuclei
diameters. The appropriate subtraction factor can be measured by looking at
tissue
phantoms with very small scattering particles which only provide diffuse
backscatter.
The high refractive index of sapphire allows the internal spaces 426 in the
preferred probe between the tilted transmit/receive fiber tip and the sapphire
window
to be filled with a low-refractive-index fluid, such as water. This further
reduces
direct backscatter from the probe tip which might make it back to the
detection
system.
CA 02371782 2001-07-24
WO 00/43750 PCT/US00/01764
-26-
The graph of Figure 22B shows a result of a measurement of backscattered
light from spherical particles in an index matching fluid illustrated
generally in
Figures 21A-D that the signal for either case of particle size is zero at zero
distance
because the overlap of the illumination and reception fiber view fields is
zero. As
the fibers pull back the overlap increases. At some point the '/rZ loss
dominates and
the signals stop increasing. With a further increase in distance the signal to
noise
increase. Eventually, however, the signal to noise drops due to the fixed
noise
inherent in the detection of the signal. The optimal position will be a
distance where
most of the large diameter backward scattering is collected but the '/r2
effect has not
reduced the total signal to the point where it is comparable to thermal noise
in the
detectors. The apparent distance of the receiving fiber tip can be increased
by using
a negative lens at a short distance from the fiber tips, which could replace
the usual
plane window. This achieves the increase in signal to noise without requiring
an 8
mm probe tip length. Figure 22B illustrates the results of differencing the
two
signals in Figure 22A.
While this invention has been particularly shown and described with
references to preferred embodiments thereof, it will be understood by those
skilled
in the art that various changes in form and details may be made therein
without
departing from the spirit and scope of the invention as defined by the
appended
claims.