Note: Descriptions are shown in the official language in which they were submitted.
CA 02372026 2002-02-28
Poly-l3-1~4 -N-Acetylcxlucosamine
1. INTRODUCTION
The present invention relates, first; to a
purified, easily produced poly-~-1-~4-N-
acetylglucosamine (p-GlcNAc) polysaccharide species.
The p-GlcNAc of the invention is a polymer of high
molecular weight whose constituent monosaccharide
sugars are attached in a ,8-1-~4 conformation, and which
is free of proteins, and substantially free of single
amino acids, and other organic and inorganic
contaminants. In addition, derivatives and
reformulations of p-GlcNAc are described. The present
invention further relates to methods for the
purification of the p-GlcNAc of the invention from
microalgae, preferably diatom, starting sources.
Still furthez, the invention relates to methods for
the derivatization and reformulation of the p-GlcNAc.
Additionally, the present invention relates to the
uses of pure p-GlcNAc, its derivatives, and/or its
reformulations.
2. BACKGROUND OF THE INVENTION
There exists today an extensive literature on the
properties, activities, and uses of polysaccharides
that consist, in part, of p-GlcNAc. A class of such
materials has been generically referred to as
"chitin", while deacetylated chitin derivatives have
been referred to as "chitosan". When these terms were
first used, around 1823, it was believed that chitin
and chitosan always occurred in nature as distinct,
well-defined, unique, and invariant chemical species,
with chitin being fully acetylated and chitosan being
fully deacetylated compositions. It was approximately
a century later, however, before it was discovered
CA 02372026 2002-02-28
- 2 -
that the terms "chitin" and "chitosan" are, in fact,
very ambiguous. Rather than referring to well-defined
compounds, these terms actually refer to a family of
compounds that exhibit widely differing physical and
chemical properties. These differences are due,to the
products' varying molecular weights, varying degrees
of acetylation, and the presence of contaminants such
as covalently bound, species-specific proteins, single
amino acid and inorganic contaminants. Even today,
the terms "chitin" and "chitosan". are used
ambiguously, and actually refer to poorly defined
mixtures of many different compounds.
For example, the properties of -"chitins" isolated
from conventional sources such as crustacean outer
shells and fungal mycelial mats are unpredictably
variable. Such variations are due not only to species
differences. but are also due to varying environmental
and seasonal effects that determine some of the
biochemical characteristics of the "chitin"-producing
species. In fact, the unpredictable variability of
raw material is largely responsible for the slow
growth of chitin-based industries.
No reports exist today in the scientific
literature describing the isolation and production,
from material sources, of pure, fully acetylated p-
GlcNAc, i.e., a product or products uncontaminated by
organic or inorganic impurities. While McLachlan et
al. (McLachlan, A.G. et al., 1965, Can. J. Botany
43:707-713) reported the isolation of chitin,
subsequent studies have shown that the "pure"
substance obtained, in fact contained proteins and
other contaminants.
Deacetylated and partially deacetylated chitin
preparations exhibit potentially beneficial chemical
properties, such as high reactivity, dense cationic
CA 02372026 2002-02-28
- 3 -
charges, powerful metal chelating capacity, the
ability to covalently attach proteins, and solubility
in many aqueous solvents. The unpredictable
variability of these preparations, as described above,
however, severely limits the utility of these
heterogenous compounds. For example, the currently
available "chitins" and "chitosans°' give rise to
irreproducible data and to unacceptably wide
variations in experimental results. Additionally, the
available preparations are not sufficiently homogenous
or pure, and the preparation constituents are not
sufficiently reproducible for these preparations to be
acceptable for use in applications, especially in
medical ones. Thus, although extremely desirable, a
true, purified preparations of chitin and chitosan,
whose properties are highly reproducible and which are
easily manufactured, do not currently exist.
3. SUMMARY OF THE INVENTION
The present invention relates, first, to an
isolated, easily produced, pure p-GlcNAc species.. The
p-GlcNAc of the invention is a polymer of high
molecular weight whose constituent monosaccharides are
attached in a (3-1-~4 conformation, and which is free of
proteins, substantially free of other organic
contaminants, and substantially free of inorganic
contaminants.
The importance of the present invention resides
in the fact that the problem of unpredictable raw
material variability has been overcome. It is, for
the first time, possible to produce, by simple means,
and on a commercial scale, biomedically pure, p-GlcNAc
of high molecular weight and consistent properties.
The material produced in the present invention is
highly crystalline and is produced from carefully
CA 02372026 2002-02-28
- 4 -
controlled, aseptic cultures of one of a number of
marine microalgae, preferably diatoms, which have been
grown in a defined medium.
The present invention further describes w
derivatives and reformulations of p-GlcNAc as well as
methods for the production of such derivatives and
reformulations. Such derivatizations may include, but
are not limited to polyglucosamine and its
derivatives, and such reformulations may include, but
are not limited to membranes, filaments, non-woven
textiles, sponges, and three dimensional matrices.
Still further, the present invention relates to .
methods for the purification of the p-GlcNAc of the
invention from microalgae, preferably diatom, sources.
Additionally, the present invention relates to the
uses of~the purified p-GlcNAc, its derivatives, and/or
its reformulations. Among these uses are novel
commercial applications relating to such industries as
the biomedical;.pharmaceutical, and cosmetic
industries, all of which require starting materials of
the highest degree.of purity. For example, the p-
GlcNAc materials of the invention may be formulated to
exhibit controllable biodegradation properties, and,
further, may be used as part of slow drug delivery
systems, as cell encapsulation systems, and as
treatments for the prevention of post-surgical
adhesions.
4. BRIEF DESCRIPTION OF~THE FIGURES
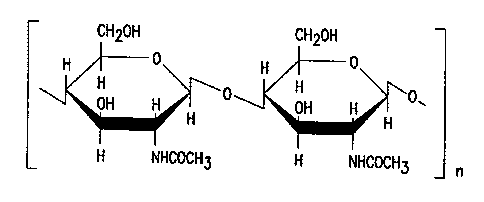
FIG. 1. Chemical structure of 100% p-GIcNAc.
"n" refers to an integer ranging from about 4,000 to
about 150,000, with about 4,000 to about 15,000 being
preferred.
CA 02372026 2002-02-28
_ 5 _
FIG. 2. Carbohydrate analysis of p-GlcNAc, Gas
Chromatography-Mass Spectroscopy data. Solid squares
represent p-GlcNAc purified using the acid
treatment/neutralization variation of the
Chemical/Biological method, as described in Section
5.3.2, below. '
FIG. 3A: Circular dichroism spectra of solid
membranes of pure p-GlcNAc.
FIG. 3B. Circular dichroism spectra of solid
membranes of Deacetylated p-GlcNAc. The disappearance
of the 211 nm minimum and 195 nm maximum observed in
pure p-GlcNAc (FIG. 3A) indicates complete
Deacetylation under the conditions used, as described
in Section 5.4 below.
FIG. 4A. Infra-red spectra analyses of thin
membranes of pure diatom p-GlcNAc prepared by the
mechanical force purification method, top, and the
. chemical/biological purification method, bottom.
FIG. 4B. Infra-red spectra analyses of two
preparations of commercial "chitin" cast into
membranes according to the methods detailed in Section
5.5, below. .
FIG. 4C. Infra-red spectra analyses of pure p-
GlcNAc which was modified by heat denaturation (top)
and by chemical deacetylation (bottom), according to
the methods detailed in Section 5.4, below.
FIG. 4D. Infra-red spectrum analysis of a p-
GlcNAc membrane derived from the diatom Thallasiosira
fluviatilis, using the chemical/biological
CA 02372026 2002-02-28
- 6 -
purification method, as detailed in Section 5.3.2,
below.
FIG. 4E. Infra-red'spectrum analysis of a p-
GlcNAc membrane prepared by the mechanical force
purification method, as described in Section 5.3.1,
below, following autoclaving.
FIG. 5A. NMR analysis of p-GlcNAc purified using
to the chemical/biological purification method as
described in Section 5.3.2, below. Chart depicting
peak amplitudes, areas, and ratios relative to
reference controls. Ratio of total areas of peaks.
i5 FIG. 5B. NMR analysis of p-GlcNAc purified using
the chemical/biological purification method as
described in Section 5.3.2. The graph depicts the
ratios of total areas of peaks.
20 FIG. 6A-68. Transmission electron micrographs
(TEM) of a p-GlcNAc membrane prepared by the
mechanical force purification method as described in
Section 5.3.1, below. Magnification: 6A: 4190x; 6B:
16,250x.
FIG. ?A-?B. Transmission electron micrographs
(TEM) of a p-GlcNAc membrane by HF treatment as
described in the discussion of the chemical/biological
purification method in Section 5.3.2, below.
Magnification: ?A: 52?Ox; 78: 8150x.
FIG. 8A-88. Transmission electron micrographs
(TEM) of a p-GlcNAc membrane prepared by the acid
treatment/neutralization variation of the
chemical/biological purification method, as described
CA 02372026 2002-02-28
-
in Section 5.3.2, below. Magnification: 8A: 5270x;
88: 16,700x.
FIG. 9A. Scanning electron micrograph depicting
a p-GlcNAc membrane prepared by the acid
treatment/neutralization variation of the
chemical/biological purification method as described
in Section 5.3.2, below.
Magnification: 200x.
i0
FIG. 9B. Scanning electron micrograph depicting
a p-GlcNAc membrane prepared by the acid
treatment/neutralization variation of the
chemical/biological purification method as described
in Section 5.3.2, below:
Magnification: 1000x.
FIG. 9C. Scanning electron micrograph depicting
a.p-GlcNAc membrane prepared by the acid
treatment/neutralization variation of the
chemical/biological purification method as described
in Section 5.3.2, below:
Magnification: 5000x.
FIG. 9D. Scanning electron micrograph depicting
a p-GlcNAc membrane prepared by the acid
treatment/neutralization variation of the
chemical/biological purification method as described
in Section 5.3.2, below.
Magnification: 10;000x.
FIG. 9E. Scanning electron micrograph depicting
a p-GlcNAc membrane prepared by the acid
treatment/neutralization variation of the
CA 02372026 2002-02-28
-
chemical/biological purification method as described
in Section 5.3.2, below. Magnification: 20,000x.
FIG. l0A-10B. Scanning electron micrographs of a
pure p-GlcNAc membrane made from material which was
initially produced using the cell
dissolution/neutralisation purification method
described in Section 5.3, below, dissolved in
dimethylacetamide/lithium chloride, and
reprecipitated in Ii20 into a mat, as described below in
Section 5.5. Magnification: 10A: 1000x; 108: 10,000x.
FIG. 11A-118. Scanning electron micrographs of a
deacetylated p-GlcNAc mat. Magnification: 11A:
1000x; 11B: 10,000x.
FIG. 12A-12B. Photographs .of diatoms. Note the
p-GlcNAc.fibers extending from the diatom cell bodies.
FIG. 13. Diagram depicting some of the possible
p-GlcNAc and deacetylated p-GlcNAc derivatives of the
invention. (Adapted.from S. Hirano, "Production and
Application of Chitin and Chitosan in Japan", in
"Chitin and Chitosan", 1989, Skjak-Braek, Anthonsen,
and Sanford, eds. Elsevier Science Publishing Co.,
PP~ 37-43.)
FIG. 14. Cell viability study of cells grown in
the presence or absence of p-GlcNAc membranes. Closed
circle (~): cells grown on p-GlcNAc matrix; open
circles (o): cells grown in absence of matrix.
FIG. 15A-15B. SEM micrographs of transformed
mouse fibroblast cells grown on p-GlcNAc membranes.
Magnification: 15A: 1000x; 15B: 3000x.
CA 02372026 2002-02-28
- g -
FIG:. 16A. Scanning electron micrograph (SEM) of
a collagen-only control material prepared according to
the method described, below, in Section 13.1.
Magnification 100x.
FIG. 168. Scanning electron micrograph (~SEM) of
a collagen/p-GlcNAc hybrid material prepared according
to the method described, below, in Section 13.1.
Ratio collagen suspension:p-GlcNAc suspension equals
3:1, with final concentrations~of 7.5 mg/ml collagen
and 0:07 mg/ml p-GlcNAc. Magnification 100x.
FIG. 16C. Scanning electron micrograph (SEM) of
a collagen/p-GlcNAc hybrid material prepared according
to the method described, below, in Section 13:1.
Ratio collagen suspension:p-GlcNAc suspension equals
1:1, with final concentrations of 5.0 mg/ml collagen
and 0.12 mg/m1 p-GlcNAc. Magnification 100x.
FIG. 16D. Scanning electron micrograph (SEM) of
~a collagen/p-GlcNAc hybrid material prepared according
to the method described, below, in Section 13.1.
Ratio collagen suspension:p-GlcNAc suspension equals
2:2, with final concentrations of 10.0 mg/ml collagen
and 0.25 mg/ml p-GlcNAc. Magnification 100x.
FIG. 16E. Scanning electron micrograph (SEM) of
a collagen/p-GlcNAc hybrid material prepared according
to the method described, below, in Section 13.1.
Ratio collagen suspension:p-GlcNAc suspension equals
CA 02372026 2002-02-28
- 1~ -
1:3, with final concentrations of 2.5 mg/ml collagen
and 0.25 mg/ml p-GlcNAc. Magnification 100x
FIG. 17A. SEM of mouse 3T3 fibroblast cells
cultured on.the collagen-only control material of FIG.
16A, above. Magnification 100x.
FIG. 17B. SEM of mouse 3T3 fibroblast cells
cultured on the collagen/p-GlcNAc material of FIG.
16B, above. Magnification 100x.
FIG. 17C. SEM of mouse 3T3 fibroblast cells
cultured on the collagen/p-GlcNAc material of FIG.
16C, above. Magnification 100x.
FIG. 17D. SEM of mouse 3T3 fibroblast cells
cultured on the collagen/p-GlcNAc material of FIG.
16D, above. Magnification 100x.
FIG. 18: Transformed NMR data curves, used to ,
obtain areas for each carbon atom and to then
calculate the CH3(area) to c-atom(area) ratios. -
FIG. 19. Typical p-GlcNAc Cl'-NMR spectrum. The
individual peaks represent the contribution to the
spectrum of each unique carbon atom in the molecule.
FIG. 20. Transformed NMR spectrum data
representing values calculated for CH3(area) to C
atom(area) ratios. Top: Graphic.depiction of data;
bottom: numerical depiction of data.
FIG. 21A-G. Three dimensional p-GlcNAc matrices
produced in various solvents. Specifically, the p-
GlcNAc matrices were produced in distilled water (FIG.
CA 02372026 2002-02-28
- 11 -
21A, FIG. 21D), 10% methanol in distilled water (FIG.
21B), 25% methanol in distilled water (FIG. 21C), 10%
ethanol in distilled water (FIG. 21E), 25% ethanol in
distilled water (FIG. 21F) and 40% ethanol in
distilled water (FIG. 21G). Magnification: 200x. A
scale marking of 200 microns is indicated on each.of
these figures.
FIG. 22A-G. Fibroblast cells grown on three
dimensional p-GlcNAc matrices prepared by lyophilizing
p-GlcNAc in distilled water.. Magnification: lOOx
(FIGS. 22A, 22E), 500x (FIG. 22B), 100Ox (FIGS. 22C,
22F), 5000x (FIGS. 22D, 22G). Scales marking 5, 20,
50, or 200 microns, as indicated; are included in each
of the figures.
FIG. 23. A typical standard curve obtained using
the procedure described, below, in Section 18.1. A
standard curve such as this one was used in the
lysozyme-chitinase assay also described, below, in
Section 18.1.
FIG. 24: p-GlcNAc lysozyme digestion data. The
graph presented here depicts the accumulation of N-
acetylglucosamine over time, as p-GlcNAc membranes are
digested with lysozyme. The graph compares the
degradation rate of fully acetylated p-GlcNAc to
partially (50%) deacetylated p-GlcNAc, and
demonstrates that the degradation rate for the
partially deacetylated p-GlcNAc was substantially
higher than that of the fully acetylated p-GlcNAc
material.
FIG. 25. p-GlcNAc lysozyme digestion data. The
graph presented here depicts the accumulation of N-
CA 02372026 2002-02-28
- 12 -
acetylglucosamine over time, as p-GlcNAc membranes are
digested with lysozyme. The graph compares the
degradation rate of two partially deacetylated p-
GlcNAc membranes (specifically a 25% and a 50%
deacetylated p-GlcNAc membrane). The data demonstrate
that the degradation rate increases as the percent of
deacetylation increases, with the degradation rate for
the 50% deacetylated p-GlcNAc membrane being
substantially higher than that of the 25% deacetylated
p-GlcNAc membrane.
FIG. 26A-26E. p-GlcNAc in vivo biodegradability
data. FIG. 26A-26C depict rats which have had
prototype 1 (fully acetylated p-GlcNAc) membrane
abdominally implanted, as described, below, in Section
18.1. FIG. 26A shows a rat at day 0 of the
implantation; FIG. 26B shows a rat at day 14 post-
implantation; FIG 26C shows a rat at day 2l post-
implantation. FIG: 26D-26E depict rats which have had
prototype 3A (lyophilized and partially deacetylated
p-GlcNAc membrane) abdominally implanted, as
described, below, in Section 18.1. FIG. 26D shows a
rat at day O of the implantation; FIG. 26E shows a rat
at day 14 post-implantation.
FIG. 27. The graph depicted here illustrates
data concerning the percent increase in tumor size of
' animals which either received no treatment (1) or
received p-GlcNAc-lactate/5'Flurouracil (FU) (O), as
described, below, in Section 20.1.
FIG. 28. The graph depicted here illustrates
data concerning the percent increase in tumor size of
animals which either received p-GlcNAc-lactate alone
CA 02372026 2002-02-28
- 13 -
(~) or received p-GlcNAc-lactate/5°Flurouracil (FU)
(O), as described; below, in Sectian 20.1.
FIG. 29. The graph depicted here illustrates
data concerning the percent increase in tumor size. of
animals which either received no treatment (~) br
received p-GlcNAc-lactate/mitomycin (mito) (O), as
described, below, in Section 20.1.
FIG. 30. The graph depicted here illustrates
data concerning the percent increase in tumor size of
animals which either received p-GlcNAc-lactate alone
(~) or received p-GlcNAc-lactate/5' mitomycin (mito)
(O), as described, below, in Sectian 20.1.
FIG. 31. The bar graph depicted here illustrates
the average percent change in tumor size per animal of
animals treated with p-GlcNAc/5'FU high dose (bar 1),
p-GlcNAc/5'FU low dose (bar 2), p-GlcNAc membrane
alone (bar 3), and untreated (bar 4). N=4 for bars 1
and 2, n=2 for bars 3 and 4.
5. DETAILED DESCRIPTION OF THE INVENTION
Presented below, is, first, a description of
physical characteristics of the purified p-GlcNAc
species of the invention,. of the p-GlcNAc derivatives,
and of their reformulations. Next, methods are
described for the purification of the p-GlcNAc species
of the invention from microalgae, preferably diatom,
starting sources. Third, derivatives and
reformulations of the p-GlcNAc, and methods for the
production of such derivatives and reformulations are
presented. Finally, uses are presented for the p-
GlcNAc, p-GlcNAc derivatives and/or p-GlcNAc
reformulations of the invention.
CA 02372026 2002-02-28
- 14 -
5.1 p-GlcNAc
The p-GlcNAc polysaccharide species of the
invention is a polymer of high molecular weight.
ranging from a weight average of about 800,000 daltons
to about 30 million daltons, based upon gel permeation
chromatography measurements. Such a molecular weight
range represents a p-GlcNAc species having about 4,000
to about 150,000 N-acetylglucosamine monosaccharides
attached in a ~i-1-~4 configuration, with about 4, 000 to
about 15,000 N-acetylglucosamine monosaccharides being
preferred (FIG. 1) .
The variability of the p-GlcNAc of the invention
is very low, and its purity.is very high, both of
which are evidenced by chemical and physical criteria.
Among these-are chemical composition and non-
polysaccharide contaminants. First, chemical
composition data for the p-GlcNAc produced using two
different purification methods, both of which are
described in Section 5.3, below, is shown in Table I
below. As can be seen, the chemical composition of
the p-GlcNAc produced by both methods is, within the
bounds of experimental error, the same as the formula
compositions of p-GlcNAc. Second, as is also shown in
Table I, the p-GlcNAc produced is free of detectable
protein contaminants, is substantially free of other
organic contaminants such as free amino acids, and is
substantially free of inorganic contaminants such as
ash and metal ions (the p-GlcNAc of the invention may
contain up to about 0.05% trace metals). Further, the
p-GlcNAc of the invention exhibits a very low
percentage of bound water.
CA 02372026 2002-02-28
- 15 -
TABLE I
CHEMICAL ANALYSIS DATA (% by weir
Theoretical Values for Pure b-GlcNAc:
Carbon - 47.29
Hydrogen - 6.40
Nitrogen - 6.89
Oxygen - 39.41
Protein - 0.00
,
Experimental Data one-GlcNAc Mats:
(Number of experimental batches for each membrane type
being greater than 30 for each membrane type)
MECHANICAL FORCE CHEMICAL~BIOLOGICAL
METHOD METHOD
Normalized 1 % Dev. Normalized 1 % Dev.
Carbon 47.21 0:08 -0.17 47.31 0.11 +0.04
Hydrogen 6.45 0.08 +0.78 6.34 0.08 -0.94
Nitrogen 6.97 0.18 +0:87 6.94 0.16 +0.73
Oxygen 39.55 0.36 +0.36 39.41 0.10 0.00
Average Values Average Values
Protein 0:00 0.00
' Ash 1.30 0.98
Moisture 2.0 1.2
1 Raw analytical normalized account
data have been to
for ash and moisture content of the samples.
The pure p-GlcNAc of the invention exhibits a
carbohydrate analysis profile substantially similar to
that shown in FIG. 2. The primary monosaccharide of
the pure p-GlcNAc of the invention is N-
acetylglucosamine. Further, the pure p-GlcNAc of the
invention does not contain the monosaccharide
glucosamine.
CA 02372026 2002-02-28
- 16 -
The.circular dichroism (CD) and sharp infra-red
spectra (IR) of the p-GlcNAc of the invention are
shown 'in FIGS. 3A, and FIGS. 4A and 4D, respectively,
which present analyses of material produced using the
methods described in Section 5.3, below. Such
physical data corroborates that the~p-GlcNAc of the
invention is of high purity and crystallinity. The
methods used to obtain the CD and IR data are
described, below, in the Working Example in Section 6:
i0 NMR analysis of the pure p-GlcNAc of the
invention exhibits a pattern substantially similar to
that~seen in FIGS. 5A, 5B, 18A and 18B. Such an NNgt
pattern indicates not only data which is consistent
with the~p-GlcNAc of the invention being a fully
acetylated polymer, but also demonstrates the lack of
contaminating organic matter within the p-GlcNAc
species.
The electron micrographic structure of~the p
GlcNAc of the invention, as produced using the methods
described in Section 5.3, below and demonstrated in
the Working Examples presented, below, in Section 8
and 9, is depicted in FIGS. 6A through FIG. 9E.
The p-GlcNAc of the invention exhibits a high
degree of biocompatability. Biocompatability may be
determined by a variety of technicyues, including, but
not limited to such procedures as the elution test,
intramuscular implantation, or intracutaneous or
systemic injection into animal subjects. Briefly, an
elution test (U. S. Pharmacopeia XXII, 1990, pp. 1415-
1497; U.S. Pharmacopeia XXII, 1991, Supplement 5, pp.
2702-2703) is designed to evaluate the
biocompatability of test article extracts, and assays ..
the biological reactivity of a mammalian cell culture
line which is sensitive to extractable cytotoxic
articles (such as, for example, the L929 cell line) in
CA 02372026 2002-02-28
- l.~ -
response;to the test article. The Working Example
presented in Section 10, below, demonstrates the high
biocompatability of the p-GlcNAc of the invention.
5.2 METHODS OF PRODUCING MICROALGAL
SOURCES OF D-G1CNA~
5.2.1 MICROALGAL SOURCES OF p-GIcNAC
The p-GlcNAc of the invention is produced by, and
may be purified from, microalgae, preferably diatoms.
The diatoms of several genuses and numerous species
within such genuses may be utilized as p-GlcNAc
starting sources. Each of these diatoms produce
fibers composed of p-GlcNAc which extend from their
cell bodies. See FIG. 12A-12B for photographs of such "
diatoms. The diatoms which may be used as starting
sources for the production of the p-GlcNAc of the
invention include, but are not limited to members of
the Coscinodiscus genus, the Cyclotella genus, and the
Thalassiosira genus, with the Thalassiosira genus
being preferred.
Among the Coscinodiscus genus, the species of
diatom that may be used to produce the p-GlcNAc of the
invention include, but are not limited to the
concinnus and radiatus species. The diatoms among the
Cyclotella genus which may be used include, but are
not limited to the caspia, cryptica, and meneghiniana
species. The Thalassiosira diatoms that may be
utilized to produce the starting material for the p-
GlcNAc of the invention include, but are not limited
to the nitzschoides, aestivalis, antarctica,
deciphens, eccentrica, floridana, fluviatilis,
gravida, guillardii, hyalina, minima, nordenskioldii,_
oceanica, polychorda, pseudonana; rotula, tubifera,
tumida, and weissflogii species, with the fluviatilis
and weissflogii species being preferred.
CA 02372026 2002-02-28
_ 18
Diatoms such as those described above may be
obtained, for example, from the culture collection of
the Bigelow Laboratory for Ocean Sciences, Center for
Collection of Marine Phytoplankton (McKown Point, West
Boothbay Harbor, Maine, 04575).
5.2.2 METHODS FOR GROWING DIATOMS
Any of the diatoms described in Section 5.2.1,
above, may be grown by utilizing, for example, the
methods described in this section. New diatom
cultures are initiated by inoculating, under sterile
conditions, Nutrient Medium with an aliquot of a
mature diatom culture. The Nutrient Medium must be
free of all other microorganisms, therefore all
materials, including water, organic components, and
inorganic components used in the preparation of the
Nutrient Medium must be sterile. In addition, it is
mandatory that all procedures involved in this
operation be conducted under strictly sterile
2-0 conditions, i.e., all containers, all transfers of
substances from one vessel to another, etc. must be
performed in a sterile environment. The quantity of
Nutrient Medium to be prepared at one time should not
exceed what is necessary to start a new culture. For
example, Fernbach flasks which occupy approximately
one square foot of surface may be used as vessels for
the diatom cultures, and such vessels require one
liter of Nutrient Medium for optimum growth of the
diatom organism.
Preparation of the nutrient medium involves the
following operations:
a) Acquisition and processing of seawater
b) Preparation of distilled and deionized
water.
c) Preparation of primary nutrient stocks
CA 02372026 2002-02-28
- 19 -
d) Preparation of nutrient working stocks
e) Preparation of the final nutrient medium
Filtered seawater may be obtained, for example,
from the Marine Biology Laboratory (Woods Hole,
Massachusetts). Seawater containers should be stored
at 5° C. When required, the necessary volume of water
may be filtered through a Buchner filtration unit,
using a nitrocellulose filter membrane with 0.45
micron pore size (Millipore, Inc.). The seawater is
then sterilized by autoclaving at, for example, 121° C.
for 15 minutes per liter. On completion of the
sterilization process, the capped are immediately
cooled, preferably by transfer to a cold room capable
of allowing the solutions to reach a temperature of
approximately 5° C. When it is to be used, solutions
are allowed to reach room temperature.
Tap water is distilled and deionized using
standard equipment and procedures, and collected and
stored in sterile, securely capped, preferably glass, _
containers.
Listed below are formulas which may be follawed
in preparing the stock solutions necessary for the
preparation of the Nutrient Medium. It is to be
understood that while such formulas are to be used as
guides, it is intended that routine variations of such
formulas which contribute to the preparation of a
Nutrient Medium capable of sustaining microalgal
diatom growth sufficient~for the p-OlcNAc preparative
processes described here also be within the scope of
the present invention.
I. Trace Metal Primar~r Stocks (TMPS)
a. 39 mM CuS04~ 5H20 (copper [II] sulfate
pentahydrate) (9.8g copper [II] sulfate/L)
CA 02372026 2002-02-28
- 20 -
b . 7 . 5 mM ZnS04 ~ 7Hz0 ( Zinc sul f ate
heptahydrate) (22g zinc sulfate/L)
c. 42 mM CoCl2~ 6H20 (Cobalt [II] chloride
hexahydrate) (lOg cobalt [II] chloride/L)
d. 91 mM MnClz~ 4HZ0 (Manganese [II]
chloride tetrahydrate) 18g manganese [II] chloride/L)
e. 26 mM NaMo04.2H20 (Sodium molybdate
dehydrate) 6.3g sodium molybdate/L)
f. 153.5 mM HZSe03 (Selenious acid) (12.9g
selenious acid/L).
Sterile filter each nutrient with a filter of no
greater than 0.2mm pore size.
II. Vitamin Primary_Stocks (VPS~
a. l mg/ml Vitamin Bl2
b. 0.1 mg/ml Biotin
Sterile filter both stocks with a filter of no greater
than 0.2mm pore size. .
III. Sodium Salts Working Stocks (SSWS)
a. Sodium nitrate working stock: 0.88 M
(75 g NaN03/L)
b. Sodium phosphate monobasic monohydrate
working stock: 36.2 mM NaH2P04~ H20 (5 g NaHzP04~ H20/L)
c. Sodium metasilicate nonahydrate working
stock: 0.11 M NaZSi03~ 9H20 (30 g NaZSi03~ 9H20/L)
Sterile filter each of the SSWS with a filter of no
greater than 0.2mm pore size.
IV. Trace Metal Working Stocks (TMWS)
11.7 mM Na2EDTA (Ethylenediamine Tetraacetic
acid, disodium salt dehydrate) (4.36 g/L)
11. 7 mM FeCl3 ~ 6H20 ( I ron [ I I I ] chloride
hexahydrate) (3.15 g/L)
CA 02372026 2002-02-28
- 21 -
1 ml/L of each of the six primary trace
metal stocks listed above.
Sterile filter with a filter of no greater than 0.2mm
pore size. Note that the trace metal working stock
must be prepared fresh each time a new Nutrient Medium
is assembled. '
V. Vitamin Workincr Stock (VWS)
1.0 ~.g/ml Biotin (1.0 ml primary Biotin
Stock/100 ml)
1.0 ~Cg/ml Vitamin B12 (0.1 ml Vitamin B12
primary stock/100 ml)
mg of Thiamine HC1 (Thiamine
hydrochloride/100 ml).
15 Sterile filter with a filter of no greater than 0.2mm
pore size. Note that a new Vitamin Working Stock
should be prepared fresh every time a new nutrient
medium is being assembled.
20 Described below are techniques which may be
followed for the preparation of Nutrient Medium and
for diatom culturing. It is to be understood that, in
addition to these techniques, any routine variation in
the formulas and/or procedures described herein which
result in a Nutrient Medium and in procedures capable
of sustaining diatom growth sufficient for the
preparative processes described herein is intended to
be within the scope of the present invention. '
Nutrient Medium may be prepared, for example, as
follows: To each liter of filtered and sterilized
seawater may be added 1 ml of the NaN03 working stock,
1 ml of the NaH2P04~H20 working stock, 1 ml of the
Trace Metal working stock, and 1 ml of the Na2Si03~ 9H20
working stock. Simultaneously with the addition of
Na2Si0,~ 9H20, 2 mls of 1 N HC1 may be added and the
CA 02372026 2002-02-28
- 22 -
solution may be shaken to mix. Next, 1.5 mls 1 N NaOH
may be added and the solution may again be shaken to
mix. Finally, 0.5 ml of the Vitamin working stock may
be added.
In order to grow a new diatom culture, 7 ml of a
mature culture, (having a cell density of '
approximately 1 x 105 cells/ml); may be transferred to
a sterile container containing 100 ml of sterile
Nutrient Medium, which may be prepared according to
the methods described above. The inoculated culture
may then be incubated for 8 days under the following
conditions:
Temperature: 20 degrees Centigrade
Constant illumination.
Agitation: Gentle swirling of flasks once
for two or three seconds every morning and every
evening.
After 8 days of incubation, 80 ml of this
incubated culture may be transferred, under sterile
conditions, to 1000 ml of Nutrient Medium, which may,
for example, be contained in a 2.8 L Fernbach flask,
protected by a cotton wool plug covered by
cheesecloth. Such a culture may be allowed to
incubate and grow to the desired cell density, or
alternatively, may be used to inoculate new diatom
cultures. Once a culture. reaches a desired cell
density, the culture's p-GlcNAc fibers may be
harvested, and the p-GlcNAc of the invention may be
purified, using methods such as those described below
in Section 5.3, below.
COs may be dissolved in the culture solution in
order to maintain a culture pH of approximately 7 to .,
8, with approximately 7.4 being preferred. The
maintenance of such a neutral pH environment, greatly
CA 02372026 2002-02-28
- 23 -
increases the p-GlcNAc yield that may be obtained from
each diatom culture.
5.3 METHODS FOR ISOLATION, PURIFICATION, AND
CONCENTRATION OF t~-GlcNAc -FIBERS
Presented in this Section are methods which may
be utilized for the preparation of p-GlcNAc fibers
from diatom cultures such as those described, above,
in Section 5.2..
While each of the methods described below for the
purification of p-GlcNAc from microalgae, preferably
diatom, starting sources produces very pure,
unadulterated, crystalline p-GlcNAc, each of the
methods yields p-GlcNAc having specific
characteristics and advantageous features. For
example, the p-GlcNAc of the invention purified via
the Mechanical Force method presented in Section
5.3.1, below, produces a p-GlcNAc membrane that
provides a superior substrate for the attachment of
cells to the p-GlcNAc. The second method, described
below in Section 5.3.2, the Chemical/Biological
method, produces a much higher average yield than the
average p-GlcNAc yield produced by the Mechanical
Force method. Additionally, the acid treatment/
neutralization variation described as part of the
Chemical/Biological method of Section 5.3.2, below,
produces extremely long p-GlcNAc fibers, with some
fibers being in excess of 100 Vim, and of very high
molecular weight, as high as 20-30 million daltons.
5.3.1 MECHANICAL FORCE METHOD FOR PREPARATION
OF PURE n-GlcNAc
The p-GlcNAc fibers may be separated from diatom
cell bodies by subjecting the contents of the culture
to an appropriate mechanical force. Such a mechanical
force may include, but is not limited to, a shear
CA 02372026 2002-02-28
- 24 -
force generated by, for example, a colloid mill, an
ultrasound device, or a bubble generator, or a cutting
force generated by, for example, a blaring blender.
The resulting suspension of diatom cell bodies
and p-GlcNAc fibers are then segregated. For example,
the suspension may be subjected to a series of '
centrifugation steps which segregate the p-GlcNAc
fibers from the cell bodies, yielding a clear
supernatant exhibiting little, if any, visible
flocculent material. A fixed angle rotor, and a
temperature of about l0° C. are preferred for the
centrifugation steps. The speed, duration, and total
number of centrifugation steps required may vary
depending on, for example, the specific centrifugation
rotor being used, but the determination of the values
for such parameters will be apparent to one of
ordinary skill in the art.
The p-GlcNAc fibers: in the supernatant may then
be concentrated using techniques well known to those
of skill in the art. Such techniques may include, but
are not limited to suction and filtration devices.
Finally, the concentrated p-GlcNAc fibers are
washed with, for example, distilled-deionized water,
HC1 and ethanol, or other appropriate solvents,
preferably solvents, such as alcohols, in which both
organic and inorganic materials dissolve.
The Working Example presented in Section 7,
below, demonstrates the use of this method for the
purification of p-GlcNAc.
5.3.2. CHEMICAL/BIOLOGICAL METHOD FOR
PURIFICATION OF p-GlcNAc
In this method, p-GlcNAc fibers are separated
from diatom cell bodies by subjecting them to chemical
and/or biological agents as described in more detail
below.
CA 02372026 2002-02-28
- 25 -
Diatom cultures may be treated with a chemical
capable of weakening diatom cell walls, which leads to
a release of the p-GlcNAc fibers without altering
their structure. Such a chemical may include, but is
not limited to, hydrofluoric acid (HF).
Alternatively, a mature diatom culture may be treated
with a biological agent capable of altering a
biological process may be used to inhibit p-GlcNAc
fiber synthesis, thus releasing the fibers already
IO present. For example, such an agent may include; but
is not limited to, polyoxin-D, an inhibitor of the
enzyme N-acetylglucosaminyl-P-transferase.
The cell bodies and p-GlcNAc-containing fibers of
diatom cultures treated with a member of the above
described chemical or biological agents are then
segregated. For example, the contents of treated
diatom cultures may be allowed to settle such that the
contents of the cultures are allowed to form two
distinct layers: The upper layer will contain
primarily the p-GlcNAc fibers, while the bottom layer
will contain the cell bodies. The upper p-GlcNAc
fiber-containing layer may be siphoned off, leaving
behind the settled cellular material of the bottom
layer.
The siphoned off p-GlcNAc fiber-containing layer
may then be further purified to remove protein and
other unwanted matter by treatment with a detergent
that will not damage the p-GlcNAc fibers. Such a
detergent may include; but is not limited to, sodium
dodecyl sulfate (SDS) .
When acid treatment, such as HF treatment; is
used to separate p-GlcNAc fibers from diatom cell
bodies, a step may be included for the dispersal of
the fibers. Such a step may include, but is not
limited to, the use of mechanical force for fiber
CA 02372026 2002-02-28
- 26 -
dispersal,,such as a step in which the fibers are
subjected to a blaring blender dispersal.
Alternatively, the acid-treated suspension may,
in an optional step, be neutralized prior to further
purification by detergent treatment. Such
neutralization will, in general, change the pH of the
suspension from approximately 1.8 to approximately
7.0, and may be accomplished.by, for example, the
addition of an appropriate volume of 1M Tris (pH 8.0)
or the addition of an appropriate. volume of sodium
hydroxide (NaOH). Neutralization, in general; yields
pure p-GlcNAc fibers of a substantially greater length
than the other purification methods discussed herein.
The purified p-GlcNAc fibers may then be
concentrated using techniques well known to those of
skill in the art, such as by utilizing a suction and
filtration device. Finally, the p-GlcNAc fibers are
washed, in a series of steps with distilled-deionized
water, HCl and ethanol, or other appropriate solvents,
preferably solvents, such as alcohols, in which both
organic and inorganic materials dissolve.
The Working Example presented, below, in Section
8 demonstrates the successful utilization of such a
purification method.
5.4 DERIVATIZATION OF n-GlcNAc
The pure, fully acetylated p-GIcNAc of the
invention may be derivatized, by utilizing a variety
of controlled conditions and procedures, into a large
range of different compounds. See FIG. 13 for a
diagram depicting some of these compounds. Such
derivatized compounds may include, but are not limited ,
to, partially or completely deacetylated p-GlcNAc,
which has been modified via chemical and/or enzymatic
means, as described in further detail, below:
CA 02372026 2002-02-28
- 27 -
Additionally, p-GlcNAc, or its deacetylated
derivative, may be derivatized by being sulfated,
phosphorylated, and/or nitrated. Further, as detailed
below, O-sulfonyl, N-acyl, O-alkyl, N-alkyl,
deoxyhalogen, and N-alkylidene and N-arylidene and
other derivatives may be prepared from the p-GlcNAc or
deacetylated p-GlcNAc of the invention. The
deacetylated p-GlcNAc of the invent~.on may also be
used to prepare a variety of organic salts and/or
metal chelates. Further, the p-GlcNAc, or a
derivative thereof, of the invention may have attached
to it, either covalently or non-covalently, any of a
variety of molecules. Still further, the p-GlcNAc of
the invention, or a derivative thereof, may be
subjected to controlled hydrolysis conditions which
yield groups of molecules having uniform and discrete
molecular weight characteristics.
One or more of the monosaccharide units of the p-
GlcNAc of the-invention may be deacetylated to form a
poly-,B-1->4-N-glucosamine species. A poly-Q-1-~4-N-
glucosamine species of the invention in which each of
the monosaccharide units of the poly-(3-1~4-N-
acetylglucosamine species of the invention has been
deacetylated wil have a molecular weight of about
640,000 daltons to about 24 million daltons, with
about 640,000 daltons to about 2.4 million daltons
being preferred. A species with such a molecular
weight range represents a species having about 4000 to
about 150,000 glucosamine monosaccharides covalently
attached in a ~i-1-.4 configuration, with about 4, 000 to
about 15,000 glucosamine monosaccharides being
preferred. At least one of the monosaccharide units
of the poly-(3-1-~4-N-glucosamine species may remain
acetylated, with about 25% to about 75% acetylation
CA 02372026 2002-02-28
- 28 -
being preferred, and about 30% acetylation being most
preferred.
The p-GlcNAc of the invention may be deacetylated
by treatment with a base to yield glucosamines with
free amino groups. This hydrolysis process may be
carried out with solutions of concentrated sodium
hydroxide or potassium hydroxide at elevated
temperatures. To precisely control the extent of
deacetylation and to avoid degradation of the main
carbohydrate chain of the polysaccharide molecule,
however, it is preferable that an enzymatic procedure
utilizing a chitin deacetylase enzyme be used for p-
GlcNAc deacylation. Such a deacetylase enzymatic
procedure is well known to those of skill in the art
and may be performed as in (U:S. Patent No.
5,219,749), which is incorporated herein, by
reference, i,n its entirety.
One or more of the monosaccharide units of the p-
GlcNAc of the invention may be-derivatized to contain
at least one sulfate group, or, alternatively, may be
phosphorylated or nitrated, as depicted below:
30
CA 02372026 2002-02-28
- 29 -
CH20R
O
H NHCOCH3
or
NHR2
where, R and/or R1, in place of a hydrogen, and/or R2,
in place of -COCH3, may be a sulfate (-SH03) , a
phosphate (-P(OH)2) , or a nitrate (--N02) group.
Described below are methods by which such p-
GlcNAc derivatives may be prepared. Before performing
methods such as those described in this Section, it
may be advantageous to first lyophilize, freeze in
liquid nitrogen, and pulverize the ;p-GlcNAc starting
material.
Sulphated p-GlcNAc derivatives may be generated,
by, for example, a two step process. In the first
step, 0-carboxymethyl p-GlcNAc may be prepared from
the p-GlcNAc and/or p-GlcNAc derivatives of the
invention by, for example, utilizing techniques such
as those described by Tokura et al. (Tokura, S. et
al., 1983, Polym. J. 15:485). Second, the sulfation
step may be carried out with, for example, N, N-
dimethyl-formamide-sulfur trioxide, according to
techniques well known to those of skill in the art,
such as are described by Schweiger (Schweiger, R.G.,
192, Carbohydrate Res. 21:219). The resulting
product may be isolated as a sodium salt.
Phosphorylated p-GlcNAc derivatives of the
invention may be prepared, for example, by utilizing
techniques well known to those of skill in the art,
such as those described by Nishi et al. (Nishi, N. et
al., 1986, in "Chitin in Nature and Technology,
CA 02372026 2002-02-28
- 30 -
Muzzarelli,et al., eds. Plenum Press, New York, pp.
297-299). Briefly, p-GlcNAc/methanesulfonic acid
mixture may be treated with phosphorus pentoxide (in
an approximately 0.5 to 4.0 molar equivalent) with
stirring, at a temperature of about 0° C. to about 5°
C. Treatment may be for about 2 hours. The resulting
product may then be precipitated and washed using
standard techniques well known to those of skill in
the art. For example, the sample may be precipitated
with a solvent such as ether, centrifuged, washed with
a solvent such as ether, acetone, or methanol; and
dried.
Nitrated p-GlcNAc derivatives may be prepared by
utilizing techniques well known to those of skill in
the art, such as those described by Schorigin and Halt
(Schorigin, R. and Halt, E., 1934, Chem. Ber.
67:1712). Briefly, p-GlcNAc and/or a p-GlcNAc
derivative may be treated with concentrated nitric
acid to, form a stable nitrated product.
One or more of the monosaccharide units of the p-
GlcNAc of the invention may contain a sulfonyl group,
as depicted below:
CH20S02R3
H ~/H o,\
~~.o
OH H H
i
H NHCOCH3
where R3 may be an alkyl, an aryl, an alkenyl, or an ;
alkynyl moiety: Such a derivative may be generated by
well known methods such as the method described in
Kurita et al. (Kurita, K. et al., 1990, Polym. Prep
[Am. Chem. Soc., Div. Polym. Chem.] 31:624-625).
Briefly, an aqueous alkali p-GlcNAc solution may be
CA 02372026 2002-02-28
- 31 -
reacted with a chloroform solution of tosyl chloride,
and the reaction may then be allowed to proceed
smoothly at low temperatures.
One or more of the monosaccharides of the p-
GIcNAc of the invention or its deacetylated derivative
may contain one or more O-acyl groups, as depicted
below:
CH20COR4
~O
H ,~ H
' O '
' ~O
OCR5 H H
H NH2
o~
NH~Rg
O
where R9 and/or R5, in place of hydrogen, may be an
alkyl., an alkenyl, or an alkynyl moiety, and R6 may be
an alkyl, an alkenyl, or an alkynyl moiety. An
example of such a derivative may be generated by well
known methods such as those described by Komai (Komai,
T. et al., 1986, in "Chitin in Nature and Technology",
Muzzarelli et al., eds., Plenum Press, New York, pp.
49_506). Briefly, p-GlcNAc may be reacted with any
of a number of suitable acyl chlorides in
methanesulfonic acid to yield p-GlcNAc derivatives
which include, but are not limited to, caproyl,
capryl, lanroyl, or benzoyl derivatives.
One or more of the monosaccharides of the
deaceylated p-GlcNAc of the invention may contain an
N-acyl group, as depicted below:
CA 02372026 2002-02-28
- 32 -
CH20H
O
where R7 may be an alkyl, an alkenyl, or an alkynyl
moiety. Such a derivatization maybe obtained by
utilizing techniques well known to those of skill in
the art, such as the technique described in Hirano et
al. tHirano, S. et al., 1976, Carbohydrate Research
4~315-320).
Deacetylated p-GlcNAc is soluble in a number of
aqueous solutions of organic acids. The addition of
selected carboxylic anhydrides to such p-GlcNAc-
containing solutions, in aqueous methanolic acetic
acid, results in the formation of N-acyl p-GlcNAc
derivatives.
One or more of the monosaccharides of the
deacetylated p-GIcNAc of the invention or of its
deacetylated derivative, may contain an O-alkyl group,
as depicted below:
O
or
NNCOCH3
H NHCRT
O
CH20R$
CA 02372026 2002-02-28
- 33
where Ra may be an alkyl, and alkenyl; or a alkynyl
moiety. Such a derivatization may be obtained by
using techniques well known to those of skill in the
art. For example, the procedure described by Maresh
et al. (Maresh, G. et al., in ~~Chitin and Chitosan,."
Skjak-Braek, G. et al.; eds., 1989, Elsevier
Publishing Co., pp. 389-395). Briefly, deacetylated
p-GlcNAc may be dispersed in dimethoxyethane (DME) and
reacted with an excess of propylene oxide. The period
of the reaction may be 24 hours,~and the reaction
takes place in an autoclave at 40 to 90° C. The
mixture may then be diluted with water and filtered.
The DME may be removed by distillation. Finally, the
end-product may be isolated via lyophilization.
One or more of the monosaccharide units of the p-
GlcNAc of the invention may be an alkali derivative,
as depicted below:
CH20Na
25 Such a derivative may be obtained by using techniques
well known to those of skill in the art. For example,
a method such as that described,by Noguchi et al.
(Noguchi, J. et al., 1969, Kogyo Kagaku Zasshi 72:796-
799) may be utilized. Briefly, p-GIcNAc may be
steeped, under vacuo, in NaOH (43%, preferably) for a
period of approximately two hours at about 0°C.
Excess.NaOH may then be removed by, for example,
centrifugation in a basket centrifuge and by
mechanical pressing.
H NHCOCH3
CA 02372026 2002-02-28
- 34 -
One or more of the monosaccharide units of the
deacetylated derivative of the p-GlcNAc of the
invention may contain an N-alkyl group, as depicted
below:
CH20H
O
H CH3 itCH~
where R9 may be an alkyl, an alkenyl, or an alkynyl
moiety. Such a derivatization may be obtained by
utilizing, for example, a procedure such as that of
Maresh et al. (Maresh, G. et al., in "Chitin and
Chitosan," Skjak-Brack, G. et al., eds. 1989, Elsevier
Publishing Co., pp. 389-395), as described, above, for
the production of 0-alkyl p-GTcNAc derivatives.
One or more of the moriosaccharide units of the
deacetylated derivative of the p-GlcNAc of the
invention may~contain at least one deoxyhalogen
derivative, as depicted below:
CH2R'o
0
where Rlo may be F, C1, Br, or I, with I being
preferred. Such a derivative may be obtained by using
techniques well known to those of skill in the art.
For example, a procedure such as that described by
Kurita et al. (Kurita, K. et al., 1990, Polym. Prep.
H NH2
CA 02372026 2002-02-28
- 35 -
[Am. Chem. Soc. Div. Polym. Chem.] 31:624-625) may be
utilized. Briefly, a tosylated p-GlcNAc is made to
react with a sodium halide in dimethylsulfoxide,
yielding a deoxyhalogen derivative. p-GlcNAc
tosylation may be performed by reacting an aqueous
alkali p-GlcNAc solution with a chloroform solution of
tosyl chloride. Such a reaction may proceed smoothly
at low temperatures.
One or more of the monosaccharide units of the
deacetylated derivative of the p-GlcNAc of the
invention may form a salt, as depicted below:
CHyOH
. __ .. . O
H H
O
~ OH H H
H ~H3H'~~tt
where Rll may be an alkyl, an alkenyl, or an.alkynyl
moiety. Such a derivatization maybe obtained by
using techniques well known to those of skill in the
art. For example, a procedure such as that described
by Austin and Sennett (Austin, P.R. and Sennett, S.,
in "Chitin in Nature and Technology," 1986 ,
Muzzarelli, R.A.A. et al., eds. Plenum Press, pp: 279-
286) may be utilized. Briefly, deacetylated p-GlcNAc
may be suspended in an organic medium such as, for
example, ethyl acetate or isopropanoi, to which may be
added an appropriate organic acid such as, for
example, formic, acetic, glycolic, ~r lactic acid.
The mixture may be allowed to stand for a period of
time (1 to 3 hours, for example). The temperature of
reaction and drying may vary from about 12° to about
35° C., with 20° to 25°C being preferred. The salts
CA 02372026 2002-02-28
- 36 -
may then be separated by filtration, washed with fresh
medium; and the residual medium evaporated.
One or more of the monosaccharide units of the
deacetylated derivative of the p-GIcNAc of the ' '
invention may form a metal chelate, as depicted below:
CHZOH ~ '
~O
H / H I~
"' O
' OH H H
H HNH
X-Rt2 X
I
X
where Rlz may be a metal ion, particularly one of the
transition metals, and X is the dative bond
established by the nitrogen electrons present in the
amino and substituted amino groups present in the
deacetylated p-GlcNAc. ~ .
One or more of the monosaccharide units of the
~ deacetylated derivative of the p-GlcNAc of the
invention may contain an N-alkylidene or an N-
arylidene group, as depicted below:
CH20H
..i o
H ,.H
\O
OH H H
3 0 H NHCR~g
where Rl, may be an alkyl, an alkenyl, an alkynyl, or
an aryl moiety. Such a derivatization maybe obtained
by using techniques well known to those of skill in
the art. For example, a procedure such as that
described by Hirano et al. (Hirano, S. et al., 1981,
CA 02372026 2002-02-28
- 37 -
J. Biomed. Mat. Res. 15:903-911) may be utilized.
Briefly, an N-substitution reaction of deacetylated p-
GlcNAc may be performed with carboxylic anhydrides
and/or arylaldehydes to yield acyl- and/or arylidene
derivatives.
Further; the p-GlcNAc of the invention, om its
deacetylated derivative, may be subjected to
controlled hydrolysis conditions, which yield groups
of molecules having uniform, discrete molecular weight
and other physical characteristics. Such hydrolysis
conditions may include, for example, treatment with
the enzyme, lysozyme. p-GlcNAc may be exposed to
lysozyme for varying periods of time, in order to
control the extent of hydrolysis. In addition, the
rate of hydrolysis may be controlled as a function of
the extent to which the p-GlcNAc that is being
lysozyme treated has been deacetylated. Deacetylation
conditions may be as described earlier in this
Section. The more fully a p-GlcNAc molecule has been
deacetylated, the more fully the molecule will be
hydrolyzed. Changes in physical characteristics; in
addition to the lowering of molecular weight, may be
elicited by hydrolysis and/or deacetylation
treatments. Extensive hydrolysis causes liquefication
of the p-GlcNAc. The results of a
hydrolysis/deacetylation procedure are presented below
in the Working Example of Section 9, below.
Further, heat denaturation may function to modify
the crystalline structure of the p-GlcNAc. Such a
modification of the p-GlcNAc product crystalline
structure may advantageously affect, for example, the
reactivity of the p-GlcNAc.
Further, a variety of molecules may be covalently
yr non-covalently functionally attached to the
deacetylated derivatives of the p-GIcNAc of the
CA 02372026 2002-02-28
- 38 -
invention., Such molecules may include, but are not
limited to such polypeptides as growth factors, such
as nerve growth factor, proteases, such as pepsin,
hormones, or peptide recognition sequences such as RGD
sequences, fibronectin recognition sequences, laminin,
integrins, cell adhesion molecules, and the like.
_:Covalent attachment of molecules to the exposed
primary amines of deacetylated p-GlcNAc may be
accomplished by, for example, chemical attachment
utilizing bi-functional cross-linking reagents that
act as specific length chemical. spacers . Such
techniques are well known to those of skill in the
art, and may resemble, for example, the methods of
Davis and Preston (Davis, M. and Preston, J.F. 1981,
Anal. Biochem. 116:404-407) and Staros et al. (Staros,
J. V. et al., 1986, Anal. Biochem. 156:220-222).
Briefly, carboxylic residues on the peptide to be
attached to the deacetylated or partially deacetylated
p-GlcNAc of the invention may be activated and then
crosslinked to the p-GlcNAc. Activation may be
accomplished, for example, by the addition of a
solution such as carbodiimide EDC (1-ethyl-3-(3-
dimethylaminopropyl)carbodiimide) to a peptide
solution in a phosphate buffer. Preferably, this
solution would additionally contain a reagent such as
sulpho-NHS (N-hydroxysulphosuccinimide) to enhance
coupling. The activated peptide may be crosslinked to
the deacetylated p-GlcNAc by mixing in a high pH
buffer, such as carbonate buffer (pH 9.0-9.2).
Alternatively, such molecules such as those
described above may be non-covalently attached to
deacetylated p-GlcNAc using techniques well known to
those of skill in the art. For example, a molecule or
molecules of choice may be mixed with a deacetylated
p-GlcNAc solution prior to lyophilization.
CA 02372026 2002-02-28
- 39 -
Alternatively, hybrids comprising p-GlcNAc and/or
p-GIcNAc derivatives may be formed. Such hybrids may
contain any of a number of natural and/or synthetic
materials, in addition to p-GlcNAc and/or p-GlcNac
derivatives. For example, hybrids may be formed of
p-GlcNaC and/or p-GlcNac derivatives plus one or more
extracellular matrix (ECM) components. Such ECM
components may include, but are not limited to,
collagen, fibronectin, glycosaminoglycans, and/or
peptidoglycans. Hybrids may also be formed of p-
GlcNAc and/or p-GlcNAc derivatives plus one or more
synthetic materials such as, for example,
polyethylene: Such a p-GlcNac/polyethylene or p-
GlcNac derivative/polyethylene hybrid may be made by
thermally linking the hybrid components via, for
example, autoclaving.
Additionally, an iodo-p-GlcNAc derivative may be
copolymerized with, for example, styrene, for the
manufacture of novel plastic materials. Iodo-p-GlcNAc
can be prepared by a process similar to that described
by Kurita and Inoue (Kurita, K. and Inoue, S., 1989,
in "Chitin and Chitosan", Skjak-Braek et al., eds.,
Elsevier Science Publishing Co., Inc., p. 365), via
tosylation and iodination of p-GlcNAc. The iodo
derivative of p-GlcNAc can then be dispersed in
nitrobenzene and reacted with styrene, with tin (IV)
chloride being used as a catalyst.
In the case of a collagen/p-GlcNAc hybrid,
briefly, a p-GlcNAc suspension and a collagen
suspension may be mixed and lyophilized, and
crosslinked, preferably dehydrothermally crosslinked.
The collagen species of such hybrids may be native or
synthetic, and may be of human or non-human, such as
bovine, for example, origin. p-GlcNAc/collagen and/or
p-GlcNAc derivative/collagen hybrid materials exhibit
CA 02372026 2002-02-28
40 -
uniform properties, and form a porous matrix that may
act, for example, as an efficient three-dimensional
matrix for the attachment and growth of cells. The
Working Example presented in Section 13, below '
demonstrates the formation, properties and usefulness
of such a p-GlcNAc/collagen hybrid.
Hybrids comprising combinations of deacetylated
p-GlcNAc and such compounds as, for example, heparin,
sodium alginate, and carboxymethyl p-GlcNAc may be
formulated using techniques such as those described
herein. Such combinations may be formed or reformed
into, for example, membranes and fibers.
Complexes of deacetylated p-GIcNAc with
polyanions such as, for example; polyacrylic acid or
pectin, possessing both positive and negative charges,
may be formulated. The formation of such complexes
may be accomplished according to a method similar to
that described by Mireles et a1. -(Mireles, C. et al.,
1992, in "Advances in Chitin and Chitosan", Brine,
C.J. et al., eds., Elsevier Publishers, Ltd.).
Deacetylated p-GlcNAc and polyacrylic acid,
carrageenan or pectin, for example, are dissolved in
HC1 and NaCl, respectively, and the reactant
solutions, with equal pH, are mixed. This operation
produces effective flocculating molecules possessing
both positive and negative characteristics, useful,
for example, in the processing of waste waters.
5.5 REFORMLTLATIONS
The p-GlcNAc of the invention, as well as its
deacetylated derivatives and/or their derivatizations,
such as those described, above, in Section 5.4, may be __
dissolved and subsequently reformulated into a variety
of shapes and configurations.
CA 02372026 2002-02-28
- 41 -
Solution of the p-GlcNAc of the invention can be
achieved by treatment with dimethyl acetamide
(DMA)/lithium chloride. p-GlcNAc may be readily
dissolved by stirring in a DMA solution containing 5%
LiCl (by weight of the DMA). Water. soluble p-GlcNAc
derivatives; such as p-GlcNAc salts, may be dissolved
in water. P-GlcNAc which has been at least about 75%
deacetylated may be gut into solution in, for example,
a mild acidic solution, such as 1% acetic acid.
p-GlcNAc derivatives that are water-insoluble may be
put into solution in organic solvents.
Derivativization of p-GlcNAc in DMA:LiCl with
phenyl isocyanates may be used to produce
carbanilates. Further, derivatization of p-GlcNAc in
DMA:LiCl with toluene-p-sulphonylchloride may be used
to produce toluene-p-sulfonate.
The p-GlcNAc of the invention, its deacetylated
derivatives, and/or.their derivatizations in solution
.. may then be precipitated and reformulated into shapes
which include, but are not limited to, mats, strings,
ropes, microsgheres, microbeads, membranes, fibers,
powders, and sponges. Further, ultrathi.n (i-e., less
than about 1 micron thick) uniform membranes may be
formulated.
Such reformulati:ons may be achieved, by, for
example, taking advantage. of the fact that pure p-
GlcNAc is insoluble in-solutions such as water and
alcohol, preferably ethanol. Introduction, by
conventional means, such as by injection, for example,
of the p-GlcNAc-containing DMA/LiCl mixture into such
a water or alcohol, preferably ethanol, solution will
bring about the reprecipitation, and therefore
reformulation, of the dissolved p-GlcNAc. Such a pure
p-GlcNAc reformulation is demonstrated in the Working
Example presented, below, in Section 11. In the case
CA 02372026 2002-02-28
- 42 -
of water soluble p-GlcNAc derivatives, reformulations
may be achieved by reprecipitating in such organic
solvents as, for example, ethyl acetate or
isopropanol. Reformulations of p-GlcNAc which has '
been at least about ?5% deacetylated may be achieved
by reprecipitating in an alkaline solution. Water- '
insoluble p-GlcNAc derivatives may be reformulated by
reprecipitation is aqueous solutions, such as, for
example, water.
Deacetylated p-GlcNAc, in donjunction with
oxidized cellulose, may be formulated to produce p-
GlcNAc/cellulose hybrid materials improving the wet-
strength of paper products. An oxidized cotton
substrate can be approached closely by the
deacetylated p-GlcNAc chain which has a flat ribbon-
like shape, similar to that of cotton. Such proximity
maximizes the contribution of the ver der Waals forces
to the forces promoting adsorption, thus enhancing the
wet-strength properties of the hybrid p-GlcNAc-
cellulose materials.
p-GlcNAc membranes and three dimensional p-GlcNAc
matrices may be produced via methods which provide for
the formation of controlled average pore sizes within
either the membranes or the matrices. Pore size can
be controlled in membranes and matrices by varying the
amount of p-GlcNAc material used, and by the addition
of certain solvents such as methanol or ethanol, with
ethanol being preferred; in specific amounts, ranging
from about 5% to about 40%, prior to the formation of
membranes and/or matrices. In general, the greater
the percentage of solvent, the smaller the average
pore size formed will be. The Example presented; .,
below, in Section 15, demonstrates the synthesis and
characterization of~such porous p-GlcNAc structures.
CA 02372026 2002-02-28
- 43 -
5.6 USES
The p-GlcNAc of the invention, as well as
its deacetylated derivatives and their
derivatizations, such as those described, above, in
Section 5.4, and reformulations, such as those
described above, in Section 5.5, have a variety'of
uses. For example, the non-toxic, non-pyrogenic,
biodegradable, and biocompatible properties of the
molecules of the invention, in addition to the
advantageous properties of the p-GlcNAc and its
derivatives, as described herein, lend themselves to
applications in such diverse fields as agriculture,
cosmetics, the biomedical industry, animal nutrition
and health, and the food, chemical, photographic, and
pharmaceutical industries.
5.6.1 BIOMEDICAL USES OF p-GIcNAc MATERIALS
5.6.1.1 DRUG IMMOBILIZATION/DELIVERY.USES
Biomedical uses of p-GlcNAc material may include,
for.example, enzyme and/or drug
immobilization/delivery methods. For example, the p-
GlcNAc of the invention or its derivatives, may have
peptides of interest (growth factors, for example)
covalently attached to them, as described, above, in
Section 5.4. Peptide-containing p-GlcNAc may be
administered to a patient. using standard procedures
well known to those of skill in the art, which
include, but are not limited to injection;
implantation, arthroscopic, laparoscopic or similar
means. Upon introduction of the peptide-containing p-
GlcNAc into a patient, the p-GlcNAc of the invention
biodegrades, such that the attached peptides are
gradually released into the bloodstream of the
patient, thus providing a method for controlled drug
delivery.
4
CA 02372026 2002-02-28
- 44
Deacetylated or partially deacetylated p-GlcNAc
species may be produced having a predictable rate of
biodegradability. For example, the percentage of
deacetylation affects the rate at which the p-GlcNAc '
species degrades. Generally, the higher the
percentage of deacetylation, the faster the~rate of
biodegradability and resorption will be. Thus, the
degree of p-GlcNAc biodegradability and the in vivo
rate of resorption may be controlled during the p-
GlcNAc's production. Examples of the production and
characterization of such p-GlcNAc materials are
presented in Section 18 , below. p-GlcNAc materials
having such controllable biodegradability rates may be
formulated into membranes, gels, sponges,
microspheres, fibers, and the like. These p-GlcNAc
products adhere and mold to tissues, both soft and
hard tissues, in the human body with no need for
suturing. The p-GlcNAc.materials may, for example, be
applied during general or minimally invasive surgery;
such as laparoscopic surgery..
p-GlcNAc materials having a controllable rate,of
biodegradation may be useful, for example, to promote
hemostasis in bleeding tissues, organs and blood
vessels, to provide periodontal barriers for the
separation of soft and hard tissue during the repair
process following periodontal surgery, to provide
surgical space fillers, to promote soft tissue
augmentation, particularly in the skin for the purpose
of reducing skin wrinkles, and as urinary sphincter
augmentation; for the purpose of controlling ,:
incontinence. The Example presented in Section 19,
below, demonstrates the use of such p-GlcNAc materials ,
in one such application, namely, to promote
hemostasis.
CA 02372026 2002-02-28
- 45 -
In addition, the molecules of the invention may
serve as slow release drug delivery vehicles wherein
the drug of interest has been encapsulated by the p-
GlcNAc, or a derivative thereof. A drug/p-GlcNAc
encapsulation may be produced, for example, by ,
following a modification of the acid
treatment/neutralization variation of the
chemical/biological purification method presented,
above, in Section 5.3.2. Rather than raising the pH
of the p-GlcNAc solution to approximately neutral pH
range (i.e., approximately 7.4), one may create a
basic pH environment, by raising the pH to
approximately 9.0 after the purification of the
p-GlcNAc is completed. At a more basic pH, the
structure of the p-GlcNAc of the invention, or a
derivative thereof, assumes a more three dimensional
or "open" configuration. As the pH is lowered, the
molecule's configuration reverts to a more compact,
"closed" configuration. Thus, a drug of interest may
be added to a p-GlcNAc at a high pH, then the pH of
the p-GlcNAc/drug suspension may be lowered, thereby
"trapping" or encapsulating the drug~of interest
within a p-GlcNAc matrix.
Such p-GlcNAc encapsulations may be administered
to a patient using standard techniques well known to
those of skill in the art., so that, upon
administration, the encapsulated drug is slowly
released into the system of the patient as the p-,
GlcNAc of the encapsulation degrades.
p-GlcNAc-based gels and membranes have a variety
of applications as therapeutic drug delivery systems.
Such applications include, for example, anti-tumor
drug delivery systems. The drug delivery systems
described herein are feasible for use with any anti-
tumor drug. Such drugs are well known to those of
CA 02372026 2002-02-28
- 46 -
skill in the art, and may be formulated into p-GlcNAc
gels or membranes, for example, so as to provide site-
specific slow-release delivery directly to the tumor
or to the region vacated by the tumor following
surgery. Such an immobilized slow-release p-GlcNAc
drug product can act as an important initial defensive
procedure after surgery. Such p-GlcNAc anti-tumor
drug delivery systems are particularly useful in
treating tumors which are totally or partially
inaccessible through surgery, such as, for example, is
the case with certain brain tumors.
Additional targets for p-GlcNAc anti-tumor
systems include, but are not limited to, skin, GI
tract, pancreatic, lung, breast,,urinary tract and
uterine tumors, and HIV-related Kaposi's sarcomas.
Antitumor drugs that are radiation enhancers are
preferred for instances in which radiation therapy
treatment is to be prescribed, either in lieu of, or
following surgery. Examples of such drugs include,
for example, 5'-fluorouracil, mitomycin, cis-platin
and its derivatives, taxol, adriamycin, actinomycin,
bleomycins, daunomycins, and methamycins.
Dose ranges for anti-tumor drugs may be lower
than, equal to or greater than the typical daily doses
prescribed for systemic treatment of patients. Higher
doses may be tolerated in. that the drugs are delivered
locally at the site of a tumor. Other tissues,
therefore, including blood cells, are not as readily
exposed to the drugs. Doses of such drugs are well
known to those of skill in the art, and may,.
alternatively, routinely be determined using standard
techniques well known to those of skill in the art,
such as, for example, are described, below, at the end
of this Section.
CA 02372026 2002-02-28
- 47 -
The p-GlcNAc/drug delivery systems of the
invention may, additionally, be used for the treatment
of infections. For such an application, antibiotics,
either water soluble or water insoluble, may be
immobilized/formulated in p-GlcNAc based materials,
such as, for example, gels and membranes. Antibiotics
are well known to those of skill in the art, and
include, for example, penicillins, cephalosporins,
tetracyclines, ampicillin, aureothicin, bacitracin,
chloramphenicol, cycloserine,'erythromycin,
gentamicin, gramacidins, kanamycins, neomycins,
streptomycins, tobramycin, and vancomycin. Doses of
such drugs are well known to those of skill in the
art, and may, alternatively, routinely be determined
using standard techniques well known to those of skill
in the art, such as, for example, are described,
below, at the end of this Section.
Such p-GlcNAc antibiotic products may be used to
treat bacterial infections that occur either
externally, e-cr., on skin, scalp, dermal ulcers or
eyes, or internally, e-a, localized infections of the
brain, muscles, abdomen. A prominent application is
for treatment of. HIV-related opportunistic infections.
The p-GlcNAc/drug delivery systems of the
invention may be formulated with anti-inflammatory
drugs to control dysfunctional activity of the
inflammatory and immune processes. For example, p-
GlcNAc may be formulated with non-steroidal anti-
inflammatory drugs (NSAIDs) and used to the reduction
of local pain nd inflammation induced by diseases such
as Rheumatoid arthritis, osteoarthritis and systemic
lupus, to name a few. The localized delivery of such
NSAIDs using the p-GlcNAc gel or membrane/drug
delivery systems of the invention may serve to reduce
NSAID side effects, which may include gastric
CA 02372026 2002-02-28
- 48 -
irritation, azotemia; platelet disfunction and liver
function abnormalities. NSAIDs are well known to
those of skill in the art and include inhibitors of
cycloxygenase, such as aspirin, etodolac, fenoprofen
and naproxen. Other anti-inflammatory drugs may be
utilized as part of the p-GlcNAc/drug delivery systems '
of the invention, such as, for example, inhibitors of
lipid inflammatory mediators; such as leucotrienes.
Doses for such drugs are well known to those of skill
in the art, and may, alternatively, routinely be
determined using standard techniques well known to
those of skill in the art, such as, for example, are
described, below, at the end of this Section.
The p-GlcNAc/drug delivery systems of the
invention may additionally be formulated with
antifungal agents, using techniques described above,
for the treatment of specific fungal diseases.
Antifungal agents are well known to those of skill in
the art, and may include, for example, amphotericin,
anisomycin, antifungone, blastomycin, griseofulvins,
and nystatin. Doses of such drugs are well known to
those of skill in the art, and may, alternatively,
routinely be determined using standard techniques well
known to those of skill in the art, such as, for
example, are described, below, at the end of this
Section.
The p-GlcNAc/drug delivery systems of the
invention may also be formulated with antiprotozoal
agents, using techniques described above, for the
treatment of specific protozoal infections.
Antiprotozoal agents are well known to those of skill
in the art, and may include, for example, antiamoebin,
antiprotozin, monomyci:n, paromomycin and trichomycin.
Doses of such drugs are well known to those of skill
in the art, and may, alternatively , routinely be
CA 02372026 2002-02-28
- 49 -
determined using standard techniques well known to
those of skill in the art, such as, for example, are
described, below, at the end of this Section.
The p-GlcNAc drug delivery systems of the
invention may be formulated with spermicidal
compounds, using techniques such as those described,
above, to produce effective contraceptives.
Appropriate spermicides are well known to those of
skill in the art. Doses of such spermicides are well
known to those of skill in the art, and may,
alternatively, routinely be determined using standard
techniques well known to those of skill in the art,
such as, for example, are described, below, at the end
of this Section.
The p-GlcNAc drug delivery systems of the
invention may, still further, be formulated using
therapeutic protein agents. Such formulations may be
produced using, for example, techniques such as those
described above. By utilizing such p-GlcNAc
therapeutic protein systems, it is possible to deliver
specific proteins directly to desired target sites and
to effect slow release of the proteins at such sites,
Examples of possible proteins include, but are not
limited to insulin, monoclonal antibodies, breast
cancer immunotoxin, tumor necrosis factor,
interferons, human growth. hormone, lymphokines, colony
stimulating factor, interleukins and human serum
albumin. Doses of such therapeutic protein agents are
well known to those of skill in the art, and may,
alteratively, routinely be determined using standard
techniques well known to those of skill in the art;
such as, for example, are described, below, at the end
of this Section.
Because the p-GlcNAc materials of the invention
are themselves immunoneutral, in that they do not
CA 02372026 2002-02-28
-
elicit an immune response in humans, such p-GlcNAc
devices, as described above, comprising p-GlcNAc
membranes, 3D porous matrices and/or gels that harbor ,
immobilized drugs, may deliver such drugs in a manner
that there is no immune response. Certain additional
materials, such as natural alginates and synthetic
polymers, may be used in some cases to construct such
devices in combination with the p-GIcNAc material.
The therapeutically effective doses of any of the
drugs or agents described above, in conjunction with
the p-GlcNAc-based systems described herein, may
routinely be determined using techniques well known to
those of skill in the art. A "therapeutically
effective " dose refers to that amount of the compound
sufficient to result in amelioration of symptoms of
the processes and/or diseases described herein.
Toxicity and therapeutic efficacy of the drugs
can be determined by standard pharmaceutical
procedures in cell cultures or experimental animals,
e.cx., for determining the LDso (the dose lethal to 50%
of the population) and the EDso (the dose
therapeutically effective in 50% of the population).
The dose ratio between toxic and therapeutic effects
is the therapeutic index and it can be expressed as
the ratio LDso/EDso. Compounds which exhibit large
therapeutic indices are preferred. While compounds
that exhibit toxic side effects may be used, care
should be taken to design a delivery system that
targets such compounds to the site of affected tissue
'30 in order to minimize potential damage to uninfected
cells and, thereby, reduce side effects.
The data obtained from the cell culture assays
and animal studies can be used in formulating a range
of dosage for use in humans. The dosage of such
compounds lies preferably within a range of
CA 02372026 2002-02-28
- 51 -
circulating concentrations that include the ED50 with
little or no toxicity. The dosage may vary.within
this range depending upon the dosage form employed and
the route of administration utilized. For any
compound used in the method of the invention, the
therapeutically effective dose can be estimated
initially from cell culture assays. A dose may be
formulated in animal models to achieve a circulating
plasma concentration range that includes the IC50
(i.e., the concentration of the test compound which
achieves a half-maximal inhibition of symptoms) as
determined in cell culture. Such information can be
used to more accurately determine useful doses in
humans. Levels in plasma may be measured, for
example, by high performance liquid chromatography.
5.6.1.2. p-GlcNAc CELL ENCAPSULATION USES
p-GlcNAc encapsulated cells may be formulated,
and such p-GlcNAc encapsulated cells may be
administered to a patient, via standard techniques
well known to those of skill in the art. See, for
example, the administration techniques described,
above, in Section 5.6.1.1. Alternatively, see, for
example, Aebisher et al. (Aebisher, P. et al., in
"Fundamentals of Animal Cell Encapsulation and
Immobilization", 1993, CRC Press, pp. 197-224), which
is incorporated herein by reference in its entirety.
Cells may be encapsulated by, on, or within p-GlcNAc
or partially deacetylated p-GlcNAc membranes, three
dimensional p-GlcNAc porous matrices, or p-GlcNAc
gels.
Three dimensional matrices can be seeded with
cells and used in certain applications without further
encapsulation. Alternatively, cells can be
encapsulated into microspheres or droplets of p-
CA 02372026 2002-02-28
- 52 -
GlcNAc-based polymer gels such as, for example, a p-
GlcNAc-lactate polyelectrolyte polymer (a polycationic
polymer). Gels, droplets or microspheres into which
cells have been encapsulated may then be coated with a '
second polyelectrolyte of opposite charge (e. a., with
a polyanion, such as an alginate) to form an outer
capsule which provides immuno-isolation for the
encapsulated cells, thus reducing the risk of immune
rejection by the host organism.
Additionally, cells entrapped in p-GlcNAc gels,
three dimensional p-GlcNAc matrices, or both, can be
loaded into thermoplastic capsules in yet another
method of formulation. Thermoplastic-based capsules
can also be utilized to provide immuno-protection for
implanted cells in a host organism. Such
thermoplastic capsules are made of materials such as
hydroxyethyl~methylacrylate-methylmethacrylate
copolymer (HEMA-MMA). Thermoplastic-derived
microcapsules are formed, for example, by the
coextrusion of a solution of HEMA-N~MA in polyethylene
glycol and the cell-containing p-GlcNAc matrix and/or
gel'medium, into an appropriate organic solvent such
as hexadecane. See, for example, the method described
by Aebisher et al. (Aebisher, P. et al., in
"Fundamentals of Animal Cell Encapsulation and
Immobilization", 1993, CRC Press, pp. 197-224).
The p-GlcNAc cell encapsulations have a variety
of applications. First, they may be utilized for the
delivery of therapeutic compounds, synthesized and
secreted by cells attached to and encapsulated in the
membranes, matrices or gels. For example and not by
way of limitation, the p-GlcNAc/cell encapsulations
may be used for delivery of insulin in the treatment
of diabetes, nerve growth factor for the treatment of
Alzheimer's disease, factor VIII and other clotting
CA 02372026 2002-02-28
- 53 -
factors for the treatment of hemophilia, dopamine for
the treatment of Parkinson's disease, enkephalins via
adrenal chromaffin cells for the treatment of chronic
pain, dystrophin for the treatment of muscular
dystrophy, and human growth hormone for the treatment
of abnormal growth.
Further, because the p-GlcNAc materials of the
invention are themselves immunoneutral, as they do not
elicit an immune response in humans, it is possible to
engineer and construct devices consisting of p-GIcNAc
membranes, three-dimensional porous p-GlcNAc matrices
and/or p-GlcNAc gels that harbor attached cells which
can deliver cell-based therapeutics in a manner such
that the cells are immuno-isolated, i.e., no anti-cell
host immune response is elicited. Certain additional
materials, such as, for example, natural alginates and
synthetic polymers, may be used to construct such
devices in addition to the p-GlcNAc material itself.
p-GlcNAc/cell encapsulation compositions may
additionally be utilized for the delivery of cells to
seed, tissue regeneration. Applications of specific
cell types encapsulated for the seeding of cell growth
leading to tissue regeneration at the site of an
injury may include, but~are not limited to
regeneration of skin, cartilage, nerves, bone, liver,
and blood vessels. The t~.ssue regeneration
applications of cells encapsulated in p-GlcNAc
materials are advantageous, in part, because of the
ability of the p-GlcNAc material to adhere to injured
tissue, to provide a substrate for mammalian cell
growth, and to undergo bioresorbtion coincident with
the growth of new healthy tissue during the tissue
regeneration process at the site of injury. Examples
include, but are not limited to the regeneration of
CA 02372026 2002-02-28
- 54 -
skin, bone, cartilage, liver, tendon, and ligament
tissues.
5.6.1.3 UTILIZING p-GlcNAc MATERIALS FOR THE
PREVENTION OF POST SURGICAL ADHESIONS
Additionally, p-GlcNAc membranes may be used to .
provide a biodegradable, biocompatible mechanical
barrier to prevent post-surgical adhesions. The
Example presented in Section 17, below, demonstrate
such a p-GlcNAc application. Solid p-GlcNAc or p-
GlcNAc derivatives formulated into membranes or
sponges may be utilized for such an application.
Preferred membranes are thin, generally less than
about 1 mm in thickness. Preferable p-GlcNAc
derivatives are p-GlcNAc derivatives which have been
about 50-80% deacetylated. Such p-GlcNAc derivatives
will generally be resorbed approximately 7-21 days
post implantation:
Liquid p-GlcNAc derivatives are also suitable for
use in the prevention of post surgical adhesions.
Preferable liquid p-GlcNAc derivatives for such an
application are deacetylated p-GlcNAc~salt derivatives
and carboxymethyl p-GlcNAc derivatives. A p-GlcNAc
derivative which is particularly preferred for the
Prevention of post surgical adhesions is a p-GlcNAc-
lactate derivative, especially a p-GlcNAc-lactate gel
derivative. Such p-GlcNAc-lactate derivatives may be
formulated using propylene glycol and water, as, for
example, described in Section 17.1. p-GlcNAc-lactate
derivatives may be produced having high and low
viscosities, which allows for the ability to tailor
the-p-GicNAc used to the specific indication of
a
interest. For example, it may be useful to use a p-
GlcNAc product having a lower viscosity for delivery
through a syringe or via a spray, while it may be
desirable to use a p-GlcNAc product having a higher
CA 02372026 2002-02-28
- 55 -
viscosity, and therefore greater lubrication
properties, when the indication is an orthopedic one.
For the prevention of post surgical adhesions,
solid p-GlcNAc formulations are suitable for clearly
circumscribed wound sites. Such p-GlcNAc formulations
should be applied following the surgical procedure and
the material should completely cover the traumatized
tissue. It can be applied either in conjunction with
either general or minimally invasive (e. g.,
laparoscopic) surgical procedures. The solid p-GlcNAc
formulations can be cut and applied using standard
surgical procedures and instrumentation well known to
those of skill in the art.
The liquid p-GlcNAc formulations can be applied,
for the prevention of post surgical adhesions, in
larger areas prone to form such postoperative
adhesions. The p-GlcNAc-lactate gel, for example, can
be applied before the surgical procedure to provide
additional lubrication and thus reduce the amount of
traumatized tissue. Alternatively; the liquid p-
GlcNAc formulation, such as p-GlcNAc-lactate, can be
applied following the-surgical procedure to form a
physical barrier to prevent postoperative adhesion
formation.
The p-GlcNAc material can be painted, sprayed or
dropped from a syringe device onto the wounded site.
In laparoscopic procedures, low viscosity materials
can, for example, be delivered with standard suction
irrigation devices. Higher viscosity materials will
require pressure to reach its target. The pressure
can be provided by a compressed gas powered piston or
a syringe type device.
The amount of liquid p-GlcNAc formulation, such
as the p-GlcNAc-lactate gel formulation, required for
prevention of post surgical adhesions is proportional
CA 02372026 2002-02-28
- 56 -
to the extent of the traumatized tissue. The p-GlcNAc
material administered should be applied in the range
of 0.1 ml to l.5 ml per sq. cm of surface area. ,
5.6.1.4 OTHER BIOMEDICAL USES OF ~-GlcNAc MATERIALS
Other biomedical uses of p-GlcNAc materials
include, for example, the use of such materials as
cell culture substrates. For example; as shown in the
Working Example presented in Section 12, below, the p-
GlcNAc of the invention acts as~a very efficient
substrate for mammalian cells grown in culture.
Further, three dimensional configurations of p-GlcNAc
may be used as a medium components which will allow
three dimensional cell culture growth.
The cell substrate capabilities of the p-GlcNAc
of the invention may also be utilized in vivo. Here,
the p-GlcNAc of the invention, or a derivative
thereof, as described herein, may act to facilitate
tissue regeneration (e-, regeneration of connective
tissue covering teeth near the gum line, vascular
grafts, ligament, tendon; cartilage, bone, skin, nerve
tissues): The p-GlcNAc molecules of the invention
may, therefore, for example, have extensive plastic
surgery applications.
Deacetylated p-GlcNAc is preferred for use as a
sealant of vascular grafts. Deacetylated p-GlcNAc
derivatives such as N-carboxymethyl and N-carboxybutyl
deacetylated p-GlcNAc are preferred as tissue
regeneration reagents. N-carboxymethyl deacetylated
p-GlcNAc may, for example, be inoculated into the
cornea to induce neovascularization.
Further biomedical applications of the p-GlcNAc
of the invention or of its derivatives, as described
herein, may involve the molecules' use in wound
CA 02372026 2002-02-28
- 57 -
dressing, wound healing ointments, and surgical
sutures, sponges, and the like.
Still further, such molecules may be used, for
example, in the treatment of osteoarthritis, in the
reduction of blood serum cholesterol levels, as anti
viral agents; as anti-bacterial agents, as
immunomodulators, as anticoagulants, as dialysis and
ultrafiltration membranes, as anti-.tumor agents, as
contact lens material, and as oral adsorbents for
iremic toxins when administered to kidney failure
patients. Microcrystalline p-GlcNAc suspensions or
water soluble p-GlcNAc derivatives are preferred for
the treatment of arthritis, by, for example, injection
directly into arthritic joints.
p-GlcNAc has additional applications as a
component of artificial or donor skin. For example,
p-GlcNAc, preferably as non-woven p-GlcNAc films, may
be applied to split thickness skin donor sites, over,
for example, donor dermis. .
Deacetylated p-GlcNAc to which a protease, such
as pepsin, has bgen attached may be used for the
' controlled digestion of~proteins in contact with such
p-GlcNAc/protease compounds.
Certain derivatizations of the p-GlcNAc of the
invention; or of its derivatives, maybe preferred for
specific applications. (Derivatizations are described
in Section 5.4, above.) For example, sulfated,
phosphorylated, and/or nitrated p-GlcNAc derivatives
may be preferred as anticoagulants or as lipoprotein
lipase activators. N-acyl p-GlcNAc derivatives may
also be preferred for anticoagulants, in addition to
being preferred for, for example, use in production of
artificial blood vessels, anti-viral compounds, anti-
tumor (specifically, cancer cell aggregating
compounds), dialysis and ultrafiltration membranes,
CA 02372026 2002-02-28
_ 5g _
and in the. production of controlled release drug
delivery systems. O-alkyl p-GlcNAc and its
deacetylated derivatives may also be preferred in the
production of controlled release drug delivery
systems. N-alkyl p-GlcNAc derivatives may be
preferred as anti-bacterial agents. Oxido deaminated
derivatives may be preferred as anti-cancer agents,
specifically their use in conjunction with
immunotherapy for cancer cells. Deacetylated p-GlcNAc
derivatives may be preferred as wound healing agents.
N-alkylidene and N-arylidene p-GlcNAc derivatives may
be preferred for the enzyme immobilization
applications.
5.6.2 AGRICULTURAL USES OF p-GlcNAc MATERIALS
The p-GlcNAc of the invention or its derivatives
may be used in various agricultural applications, as
well. Such applications include, but are not limited
to insecticide, fungicide, bactericide,~and nematocide
applications. N-carboxymethyl. deacetylated p-GlcNAc
derivatives are. preferred for use as effective
bacteriostatic reagents. N-alkyl p-GlcNAc derivatives
may be preferred for fungicide applications.
Additionally, the molecules of the invention may be
used in various soil treatment applications,
including, but not limited to; fertilizer
compositions. Further, controlled release of
agrochemicals may be achieved by entrapping such
chemicals via the immobilization, encapsulation, and
other methods described, above, in this Section.
,Additionally, analogs of, for example, Rhizobium
modulation factors and/or nitrogen fixation inducers
may be immobilized onto, and administered via, the p-
GlcNAc and/or p-GlcNAc derivatives of the invention.
CA 02372026 2002-02-28
- 59 -
5.6.3 NUTRITION/FOOD INDUSTRY USES OF p-GlcNAc
MATERIALS
The p-GlcNAc of the invention and its derivatives
as described herein additionally have applications in
the fields of animal and.human nutrition. For
example, the molecules of the invention may be used as
feed ingredients Techniques such as those described,
above, in this Section, may be used in the production.
of controlled release products in animal systems.
Additionally, the biomedical applications described
. above may be utilized in animal systems by
incorporating routine modifications well known to
those of ordinary skill in the art.
Food industry applications of the p-GlcNAc of the
invention and of its derivatives, as described herein,
may include, but are not limited to anticholesterol
(i.e., hypocholesterol.emic compounds), fat-binding
compounds, emulsifiers, carriers, preservatives,
seasonings, and food texturizers, in addition to fruit
coatings, and food packaging products.
5.6.4 COSMETIC USES OF ~-GlcNAc MATERIALS
Cosmetic applications of the p-GlcNAc of the
invention may include, but are not limited to, the
Production of products for hair and skin care. Skin
care products may include, for example, cosmetics
utilizing deacetylated p-GlcNAc salts, carboxymethyl
p-GlcNAc-containing products, and cosmetic packs
containing deacetylated p-GIcNAc and such derivatives
as hydroxypropyl-, N-succinyl-, and quaternary p-
GlcNAc derivatives. Hair products may include, for
example, carboxymethyl p-GlcNAc-containing products,
and film-forming p-GlcNAc derivatives.
CA 02372026 2002-02-28
60 -
5.6.5 CHEMICAL ENGINEERING APPLICATIONS OF p-GlcNAc
MATERIALS
The p-GlcNAc of the invention and its derivatives
have a variety of applications that are useful in the .
chemical engineering industry. For example, p-GlcNAc
may be used as a coupling agent for~adhesion of. metals
to polymers, membranes formed by glycol p-GlcNAc may
be used in desalination applications, and membranes
formed by other p-GlcNAc derivatives may be used for
transport of halogen ions. Other applications may
include the production of flame retardants; and the
manufacture of metal chelating compounds and compounds
capable of removing trace and heavy metals from
liquids as well as water-soluble industrial
Pollutants, such as PCBs, for example. p-GlcNAc
and/or p-GlcNAc derivatives may be used in
photographic applications. For example, the ability
of p-GlcNAc and/or p-GlcNAc derivatives to chelate
metals, such as silver halides, may be utilized by
~ contacting photographic solutions to recast mats, such
as thin membranes, of p-GlcNAc and/or p-GlcNAc
derivatives.
6. EXAMPLE: PHYSICAL CHARACTERIZATION OF PURE
PREPARATIONS OF p-GIcNAC
Presented in this Example; are circular dichroism
(CD) and infra-red spectra (IR) analyses of p-GIcNAC
and deacetylated p-GIcNAC membranes.
6.1 MATERIALS AND METHODS
p-GIcNAC and commercial "chitin° preparations:
The p-GlcNAc used in the.CD studies was prepared
using the Mechanical Force purification method
described, above, in Section 5.3.1.
CA 02372026 2002-02-28
- 61 -
Commercial "chitin" was purchased from NovaChem,
Ltd., PO Box 1030 Armdale, Halifax, Nova Scotia,
Canada, B3L 4K9.
The p-GIcNAC membranes used in the IR studies
were prepared by either the Mechanical Force
purification method as described, above, in Section
5.3.1, or by the Chemical/Biological purification
method, as described; above, in Section 5.3.2, as
indicated.
The commercial "p-GleNAc" preparations were cast
into membranes by dissolving in a dimethylacetamide
solution containing 5% lithium chloride, and layering
onto distilled, deionized water until membranes
precipitated.
p-GIcNAC derivatives and treatments: The
Deacetylated p-GIcNAC used in both the CD and IR
studies was prepared by treatment of the p-GIcNAC with
50% NaOH at 60° C. for 2 hours. The heat-denatured p-
GIcNAC membranes used in the IR studies were modified
by boiling in 0.2mM EDTA for 3 minutes. Autoclaved p-
GlcNAc was autoclaved or 30 minutes at 122° C.
CD'techniques: Solid state CD techniques were
carried out essentially according to Domard (Domard,
A., 1986, Int. J. Macromol. 8:243-246).
6.2 . RESULTS
6.2.1 CD ANALYSIS
In the CD spectra obtained from untreated p-
GlcNAc (FIG. 3A) , the .expected n-~r' and ~-r*' optically
active electronic transitions (220-185nM) were
observed due to the presence of the carbonyl group in
the acetyl moiety of p-GlcNAc are present. Such peaks
are completely absent in the CD spectrum obtained from
the deacetylated p-GlcNAc product, as shown in FIG.
3B.
CA 02372026 2002-02-28
- s2 -
6.2.2 IR SPECTRA ANALYSIS
The IR spectra obtained in this study are
consistent with the chemical structure of p-GlcNAc. ,
Additionally, the sharp definition of each IR peak is
indicative of the presence of an ordered and regular
(i.e., pseudocrystalline) structure in the p-GlcNAc
fibers. See FIG. 4A for the IR spectrum of p-GlcNAc
purified via the Mechanical Force purification method,
and FIG. 4D for the IR spectrum of g-GlcNAc purified
via the Chemical/Biological method. For comparison,
see FIG. 4B, which demonstrates the IR spectrum of a
commercial "chitin" preparation.
The IR spectrum obtained from the autoclaved p-
GlcNAc material (FIG. 4E) does not differ visibly from
the IR spectrum observed in FIG. 4A. This data
indicates that the p-GlcNAc material may be sterilized
by autoclaving with no loss of polymer structure.
7. EXAMPLE: PURIFICATION OF p-GIcNAC USING THE
MECHANICAL FORCE PURIFICATION METHOD
In this section, p-GIcNAC was purified using the
Mechanical Force technique described above, in Section
5.3.1.
~~1 MATERIALS AND METHODS/RESULTS
Diatom culture conditions: The diatom species
Thallasiosira fluviatilis~was grown in culture
according the procedures described, above, in Sections
5.1 and 5.2.
SEM procedures: The SEM techniques used here are
as those described, below, in Section 12.1.
p-GlcNAc Purification nrocedure:p-GIcNAC was
purified from the diatom culture by utilizing the -'
Mechanical Force technique described above, in Section
5-3~1. Specifically, the p-GlcNAc fibers were
separated from the diatom cell bodies by subjecting
CA 02372026 2002-02-28
- 63 -
the contents of the culture to three short bursts of
top speed mixing motion in a blaring blender. Total
time of the three bursts was about one second. The
resulting suspension was centrifuged at 3500 rpm in a
Sorvall GS-4 fixed angle rotor, for 20 minutes at
about 10°C. The supernatant was decanted, and
centrifuged again, this time at, 4000 rpm in a Sorvall
GS-4 fixed angle rotor for 20 minutes at about 10°C.
Once again, the supernatant was decanted and
centrifuged at 4000 rpm at 10° C. The final
supernatant of the third centrifugation was clear,
with little, if any, visible flocs floating in the
liquid. The clear supernatant was decanted into a
Buchner filtration unit equipped with nitrocellulose
with 0.8~m pore size, suction was then applied and the
liquid was filtered from the fiber suspension,
allowing the fibers to be collected onto the membrane.
The collected fibers were washed with 1 liter of
distilled, deionized H20 at 70° C: When almost all of
the water had been drained, fibers were washed, with
suction, with 1 liter of 1 N HC1 at 70°C. When most
of'the acid solution had been drained, the fibers were
washed with 1 liter of distilled, deionized H20 at
70°C, using suction. When most of the wash water had
been drained, the fibers were washed with 1 liter of
95% ethanol at room temperature, and vacuum was
applied. The filter membrane on which the white fiber
membrane had been collected was then removed from the
filtration unit and the membrane and its membrane
support was dried in a drying oven at 58°C for 20
minutes, after which the membrane and its support was
placed in a desiccator for 15 hours.
Following this purification procedure, the yield
of p-GlcNAc from a 1000 m1 culture was 6.85 milligrams
per liter of diatom culture. SEM photographs of the
CA 02372026 2002-02-28
- 64 -
membrane formed by the collection of the p-GIcNAC
fibers via this technique is shown in FIG. 6A-6B.
8. EXAMPLE: PURIFICATION OF p-GIcNAC USING THE
BIOLOGICAL/CHEMICAL PURIFICATION
METHOD
In this section, p-GIcNAC was purified using two
of the Chemical/Biological techniques described above,
in Section 5.3.2. Briefly, p-GIcNAC was purified via
HF treatment, in one case, and via acid
treatment/neutralization in the second case.
8.1 MATERIALS AND METHODS/RESULTS
diatom culture conditions: The diatom species
i5 Thall~siosira fluviatilis was grown in culture
according the procedures described, above, in Sections
5.1 and 5.2.
SEM procedures: The techniques utilized in this
study were as described, below, in Section 12.1.
i'urificat~on procedure: First, p-GIcNAC was
purified by HF treatment, the results of which are
shown in FIG. 7A-7B. Specifically, under a fume hood,
2.42 ml of a 49% (29N) HF solution was added to the
diatom contents of the culture, at room temperature,
for each 1000 ml of the volume of the original cell
culture, resulting in a 0.07 M HF solution. The
mixture was then shaken vigorously for about 30
seconds, causing persistent foam to appear over the
liquid. The container: was allowed to stand
undisturbed for 5-6 hours to allow heavy particulates
to settle.' At the end of this time, a layer of foam
had formed, while the liquid itself was divided into
two strata: first, a narrow, very dark green layer
resting on the bottom of the container below a second,
much lighter colored grayish-green and murky phase
which represented perhaps 85-90% of the total volume
CA 02372026 2002-02-28
- ss -
of liquid. The foam layer was carefully siphoned off,
using a capillary glass tube and vacuum suction. The
grayish cloudy supernatant was then siphoned off, with
care being taken to not disturb the dark bottom layer,
which consisted mainly of settled cell bodies, and was
transferred to a separate plastic container. The
grayish cloudy supernatant was allowed to stand
undisturbed for an additional 16 hours. The liquid
was initially almost colorless, light grey, but not
transparent. After 16 hours settling time, a small
amount of foam remained on top of the main body of
liquid and a small amount of green matter had settled
on the bottom of the container. The liquid was
lighter in color; but still not transparent. The foam
on top of the liquid was siphoned off as before. The
main body of liquid was then carefully siphoned off,
leaving behind the small amount of -settled green
material at the bottom of the container. The liquid
which had thus been isolated, contained the majority
of the p-GlcNAc fibers and some impurities.
To remove proteins and other unwanted matter
liberated by the diatoms during the preceding steps in w
the procedure from the fiber-containing liquid, the
suspension of fibers and cell remnants was washed with
sodium dodecyl sulfate (SDS). Specifically, the
necessary volume of a 20% SDS solution was added to
make the final concentration of the liquid 0.5% SDS by
volume. The container holding the liquid was sealed,
secured in a horizontal position on a shaking machine,
and agitated for 24 hours at about 100 shakes a
minute. Soon after shaking began, large flocs of
white p-GlcNAc fibers appeared in the suspension, and
a considerable amount of foam accumulated in the head
space of the containers. At the end of the SDS
washing, the contents of the containers were
CA 02372026 2002-02-28
- 66 -
transferred to Buchner ffiltration equipment equipped
with a 0.8~~cm (Supor Filter, Gelman) filter membrane.
The liquid was filtered with suction, and the p-GlcNAc
fibers in the liquid were collected on the filter
membrane.
The p-GlcNAc fibers collected on the filter
membrane were then washed further. First, the fibers
were washed with hot (70° C.) distilled, deionized H20,
using three times the volume of the original
f
suspension. With a water jet, using distilled,
deionized HZO, the white fiber clumps collected on the
filter membrane of the Buchner filter were transferred
to a blaring blender, and the fiber clumps were
disintegrated with about 10 short mixing bursts. The
suspension of disintegrated fibers was transferred to
a Buchner filter funnel equipped with a nitrocellulose
filter membrane as described above, and the liquid was
removed under suction. The collected fibers were
washed with 1000 ml of hot (70°C).1N' HCl solution, and
subsequently further washed with 1000 ml hot (70°C)
distilled, deionized H20. Finally, the fibers were
washed with 1000 ml 95% ethanol at room temperature,
and filtered to dryness: The fiber membrane and the
filter membrane supporting the fiber membrane were
then dried in a drying oven at 58°C for 20 minutes.
The membrane and membrane support was then placed in a
desiccator for 16 hours. The membrane was then
carefully detached from the filter membrane.
Second, p-GlcNAc was purified by using the acid
treatment/neutralization method described, above, in
Section 5.3:2. Specifically, the p-GlcNAc was
processed as described earlier in this Section, until
prior to the SDS wash step, at which point the
solution was neutralized to a pH of approximately 7.0
by the addition of a 2.9M Tris solution. The p-GlcNAc
CA 02372026 2002-02-28
- 67 -
yield from this purification procedure was 20.20
milligrams per liter of diatom culture. On average,
approximately 60 milligrams per liter diatom culture
are obtained. SEM micrographs of membranes formed
during the purification procedure are shown in FIGS.
8A-8B and 9A-9E.
9. EXAMPLE: p-GlcNAc DEACETYLATION
A p-GlcNAc membrane was suspended in a solution
i0 containing 50% NaOH. The suspension was heated at 80°C
for 2 hours. The resulting deacetylated membrane was
dried and studied by scanning electron microscopy, as
shown in FIG. 11A-11B.
i5 10. EXAMPLE: p-GlcNAc BIOCOMPATIBILITY
In this Example, it is demonstrated that the p-
GlcNAc of the invention exhibits no detectable
biological reactivity, as assayed by elution tests,
intramuscular implantation in rabbits, intracutaneous
20 injection in rabbits, and systemic injections in mice.
10.1. MATERIALS AND METHODS
10.1.1. ELUTION TEST
Conditions for the elution test conformed to the
25 Specifications set forth in the U.S. Pharmacopeia
XXII, 1990, pp. 1415-1497 and to U.S. Pharmacopeia
XXII, Supplement 5 , 1991, pp. 2702-2703.
Cell culture: Mouse fibroblast L929 cell line
(American Type Culture: Collection Rockville, MD; ATCC
30 No. CCL1; NCTC clone 929) was utilized. A 24 hour
confluent monolayer of L929 cells was propagated in
complete Minimum Essential Medium (MEM).
p-GlcNAc: a solid membrane of p-GlcNAc which had
been prepared according to the Mechanical Force method
35 of purification described, above, in Section 5.3.1,
CA 02372026 2002-02-28
- 68 -
was extracted in 20 ml serum-supplemented MEM as per
U.S. Pharmacopeia XXII (1990) requirements.
Controls: Natural rubber was used as a positive
control, and silicone was used as a negative control. '
Controls were tested in the same manner as the test
article, p-GlcNAc. '
Extracts: Extracts were prepared at 3?°C, in a
humidified atmosphere containing 5% carbon dioxide,
for 24 hours. Extracts were evaluated for a change in
pH, and adjustments were made to bring the pH to
within +/- 0.2 pH units of the original medium.
Adjustments were made with HC1 lower extract pH on
with NaHC03 to raise the extract pH. Extracts were
sterile filtered by passage through a 0.22 micron
filter, prior to being applied to the cell monolayer.
Dosinct: 3 mls of p-GlcNAc or control extracts
were used to replace the maintenance medium of cell
cultures. All extracts were tested in duplicate.
Evaluation Criteria: Response of the cell
monolayer was evaluated either visually or under a
microscope. The biological reactivity, i.e., cellular
degeneration and/or malformation, was rated on a scale
of 0 to 4, as shown below.. The test system is
suitable if no signs of cellular reactivity (Grade 0)
are noted for the negative control article, and the
positive control article shows a greater than mild
reactivity (Grade 2). The test article (i.e., p-
GlcNAc) meets the biocompatibility test if none of the
cultures treated with the test article show a greater
than mild reactivity.
Grade Reactivity Description of Reactivity Zone
0 None Discrete intracytoplasmic
granules; No cell Lysis
CA 02372026 2002-02-28
- 69 -
1 Slightly Not more than 20% of the cells
are round, loosely attached, and
without intra-cytoplasmic
granules; occasional lysed cells
are present
2 Mild Not more than 50% of the cells
are round and devoid of
intracytoplasmic granules;
extensive cell lysis and empty
areas between cells
3 Moderate Not more than 70% of the cell
layers contain rounded cells
and/or are lysed
4 Severe Nearly complete destruction of
the cell layers
10.1.2. INTRAMUSCULAR IMPLANTATIONS
Animals: Healthy, New Zealand White Rabbits,
male and female, (Eastern Rabbit Breeding Laboratory,
Taunton, MA) were used. Rabbits,were individually
housed using suspended stainless steel cages. Upon
receipt, animals were placed in quarantine for 8 days,
under the same conditions, as for the actual test.
Hardwood chips (Sani-chipsTM, J.P. Murphy Forest
Products, Montvale, NJ) were used as non-contact
bedding under cages. The animal facility was
maintained at a temperature of 68° ~-/- 3°F, with a
relative humidity at 30-70%, a minimum of 10-13
complete air exchanges per hour, and a 12-hour
light/dark cycle using full spectrum fluorescent
lights. Animals were supplied with commercial feed
(Agway ProLab, Waverly, NY) under cantrolled
conditions and municipal tap water ad libitum. No
known'contaminants were present in the feed, bedding,
or water which would be expected to interfere with the
test results. Animals selected for the study were
Chosen from a larger pool of animals. Rabbits were
CA 02372026 2002-02-28
weighted to nearest lOg and individually identified by
ear tattoo.
p,-GlcNAc: The p-GlcNAc used was as described,
above, in Section 10.1.1. '
5 Implantation Test: Two rabbits were used for each
implantation test. On the day of the test, the'animal
skin on both sides of the spinal column was clipped
free of fur. Each animal was anesthetized to prevent
muscular movement. Using sterile hypodermic needles
10 and stylets, four strips of the test p-GlcNAc (lmm x
lmm x lOmm) were implanted into the paravertebral
muscle on one side of the spine of each of two rabbits
(2.5 to 5cm from the midline, parallel to the spinal
column, and about 2.5 cm from each other). In a
15 similar fashion, two strips of the USP negative
control plastic RS (lmm x lmm x l0mm) were implanted
in the apposite muscle of each animal. Animals were
maintained for.a period of 7 days. At the end of the
observation period, the animals were weighed and
20 euthanized by ~n injectable barbituate, Euthanasia-5
(Veterinary Laboratories, Inc., Lenexa, KS).
Sufficient time was allowed to elapse for the tissue
to be cut without bleeding. The area of the tissue
surrounding the center portion of each implant strip
25 was examined macroscopically using a magnifying lens.
Hemorrhaging, necrosis, discolorations and infections
were scored using the following scale: 0=Normal,
1=Mild, 2=Moderate, and 3=Severe. Encapsulation, if
present, was scored by first measuring the width of
30 the capsule (i.e., the distance from the periphery of
the implant to the periphery of the capsule) rounded
to the nearest O.lmm. The encapsulation was scored as
follows
CA 02372026 2002-02-28
- 71 -
Capsule Width Score
None 0
up to 0.5 mm 1
0.6 - 1.0 mm 2
1.1 - 2.0 mm 3
Greater than 2.0 mm 4
The differences between the average scores for
the p-GlcNAc and the positive control article were
calculated. The test is considered negative if, in
each rabbit, the difference between the average scores
for each category of biological reaction for the p-
GlcNAc and the positive control plastic implant sites
does not exceed 1.0; or, if the difference between the
mean scores for all categories of biological reaction
for each p-GlcNAc article and the average score for
all categories for all the positive control plastic
implant' sites does not exceed 1.0; for not more than
one of four p-GlcNAc strips.
10.1.3. INTRACUTANEOUS INJECTIONS
Animals: New Zealand white rabbits were used and
maintained as described; above, in Section 10.1.2
p-GlcNAc: A solid membrane of p-GlcNAc which had
been prepared according to the mechanical force method
of purification described; above, in Section 5.3.1,
was placed in an extraction flask, to which 20 ml of
the appropriate medium were added. Extractions were
performed by heating to 70° for 24 hours. Following
this procedure, extracts were cooled to room
temperature. Each extraction bottle was shaken
vigorously prior to administration.
Intracutaneous Test: On the day of the test,
animals were clipped free of fur on the dorsal side.
A volume of 0.2 ml of each p-GlcNAc extract was
CA 02372026 2002-02-28
_ 72 _ . ,
injected intracutaneously at five sites on one side of
each of two rabbits. More than one p-GlcNAc extract
was used per rabbit. At five sites on the other side
of each rabbit, 0.2 ml of the corresponding control
was injected. Injection sites were observed for signs
of erythema, edema, and necrosis at 24, 48, and~72
hours after injection. Observations were scored
according to the Draize Scale for the Scoring Skin
Reaction (USP Pharmacopeia XXII, 1990, 1497-1500; USP
Pharmacopeia XXII, Supplement 5,F, 1991, 2703-2705) as
shown in Table II, below:
TABLE II
Draize Scale for Scoring Skin Reactions
Value
Erythema and Eschar Formation
No erythema . . . . . : . . . . . . . . . . . . . . 0
Very slight erythema (barely perceptible) . . . . . 1
well defined erytheina . . . . . . . . . . . . . . 2
Moderate to severe erythema . . . . . . . . . . . 3
Severe erythema (beet redness) to slight eschar
formation (injuries in depth) . . . . . . . . . , . 4 '
Total possible erythema score = 4
Edema Formation
No edema . . . . . . . . . . . . . . . . . . . . . 0
Very slight edema (barely perceptible) . . . . . . 1
Slight edema (edges are well defined by definite
raising) . . . . . . : . . . . . . . . . . . . . . 2
Moderate edema (raised approximately lmm and
extending beyond area of exposure) . . . . . . , . 3
Severe edema (raised more than lmm and extending
beyond area of exposure) . . . . . . . . . . . . , 4
Total possible edema scare = 4
CA 02372026 2002-02-28
- 73 -
All erythema and edema scores at 24, 48, and 72 hours
were totaled separately and divided by 12 (i.e., 2
animals x 3 scoring periods x 2 scoring categories) to
determine the overall mean score for the p-GIcNAc
versus the corresponding control. Animals were
weighed at the end of the observation period and
euthanized by injection of a barbituate, Euthanasia-5
(Veterinary Laboratories, Inc., Lenexa, KS). The
results of the test are met if the difference between
the p-GlcNAc and the control means reaction scores
(erythema/edema) is 1.0 or less).
10.1.4. SYSTEMIC INJECTIONS
Animals: Albino Swiss mice (Mus musculus),
female, (Charles River Breeding Laboratories,
Wilmington, MA) were used. Groups of 5 mice were
housed in polypropylene cages fitted with stainless
steel lids. Hardwood chips (Sani-chips'"', J.P. Murphy
Forest Products, Montvale, NJ) were used as contact
bedding in the cages. The animal facility was
maintained as a limited access area. The animal rooms
were kept at a temperature of 68 +/- 3°F, with a
relative humidity of 30-70%, a minimum of 10-13
complete air exchanges per hour, and a 12 hour
light/dark cycle using full spectrum fluorescent
lights. Mice were supplied with commercial feed and
municipal tap water ad libitum. There were no known
contaminants present in the feed, bedding, or water
which would be expected to interfere with the test
results. Animals selected for the study were chosen
from a larger pool of animals. Mice were weighed to
the nearest O.lg and individually identified by ear
punch.
p-GlcNAc: The samples used were as described,
above, in Section 10.1.1. Extracts Were prepared
CA 02372026 2002-02-28
- 74 -
according to the procedures described in Section
10.1.3, above.
Systemic Injection Test: Groups of 5 mice were
injected with either p-GlcNAc extract or a
corresponding control article, in the same amounts and
by the same routes as set forth below:
Test Article Dosing Route Dose/Kg Injection
or Control Rate
Article
Extracts
0.9% Sodium Intravenous 50 m1 0.1 ml/sec
Chloride
Injection, USP
(0.9% NaCl)
1 in 20 Intravenous 50 ml 0.1 ml/sec
Alcohol in
0.9% Sodium
Chloride
Injection USP
(EtOH:NaCl)
Polyethylene Intraperitoneal .10 g r
_
Glycol 400
(PEG 400
Cottonseed Oil Intraperitoneal 50 ml .
(CSO)
Extracts of the p-GlcNAc prepared with PEG 400, and
the corresponding
control, were diluted
with 0.9%
NaCl, to obtain 200 mg;of PEG 400 per ml. For the
Test, PEG 400 was diluted
Intracutaneous with 0.9%
NaCl to obtain 120 mg of PEG 400 per ml.
The animals were observed immediately after
injection, at 24 hours, 48 hours, and 72 hours after
injection. Animals were weighed at the end of the
observation period and euthanized by exposure to
carbon dioxide gas. The requirements of the test are
met if none of the animals treated with the p-GlcNAc
shows a significantly greater biological reactivity
than the animals treated with the control article.
CA 02372026 2002-02-28
- 75 -
10.2 RESULTS
10.2.1. ELUTION TEST
The response of the cell monolayer to the p-
GlcNAc test article was evaluated visually and under a
microscope. No cytochemical stains were used in the
evaluation. No signs of cellular biological
reactivity (Grade 0) were observed by 48 hours post-
exposure to the negative control article or to the p-
GlcNAc. Severe reactivity (Grade 4) was noted for the
positive control article, as shown below in Table III:
TABLE III
REACTIVITY GRADES
Control Articles
Time p-GlcNAe Negative Positive
A B A B A B
0 Hours 0 0 0 0 0 0
24 Hours 0 0 0 0 4 4
4g Hours 0 0 0 0 4 4
The p-GlcNAc of the invention, therefore, passes
requirements of the elution test for biocompatibility,
and, thus, is non-cytotoxic.
10.2.2 INTRAMUSCULAR IMPLANTATIONS
. Both rabbits (A and B) tested increased in body
weight and exhibited no signs of toxicity. See Table
IV for data. In addition, there were no overt signs
of toxicity noted in either animal. Macroscopic
evaluation of the test and control article implant
sites showed no inflammation, encapsulation,
hemorrhage, necrosis, or discoloration. See Table IV
for results. The test, therefore, demonstrates that
CA 02372026 2002-02-28
- 76 -
the p-GlcNAc assayed exhibits no biological
reactivities, in that, in each rabbit, the difference
between the average scores for all of the categories
of biological reaction for all of the p-GlcNAc implant
sites and the average score for all categories for all
the control implant sites did not e~cceed 1Ø
15
25
35
CA 02372026 2002-02-28
77 _
aw
~
o 0 0 0 0
zw
U
N O 0 0 0 0 0[ O)
U '
N O O O O O C7[O[
U
W
O O O O O
W W
H ~
~' o 0 0 0 0 0[ o[
. .
E-~
W
a
oa
H
''~ 0 0 0 0 0 0[ o[
E.,
N O O O O O O[ O[
E~
O[
O U
~
~ z ~ ,-a o c c c o o y ~
H
H a~~ x
s~
~ a o o
~ ~ W Z
O - ~ U -~ ~ .~ ~ ~ W
[
i3~U O t- W ~ tT cd G4 O
..n -..
Ew O -~ !~. V~ rt r-irt GO l.t O V
~n
U ~t Cl~~t
a ~~
m ~ E c ~ ~ ~ W
FC ~ ~ ~ n . ~
0 rt t~,i~ O O rtf
~ to m r-trt O sr U b
a ~ E E ~
U v~ w U E U ~ ~ o
w c~m ~ -~ N ~ ~ a~ a~ -:~~ w w
.~
~ H ~ ~ N H w x z A H ~
,.
CA 02372026 2002-02-28
aw
00
0 0 0 0 0
w
U
U
O O O O O O' O~
'~ 0 0 0 0 0 0~ 0~
U
W
o o 0 o O
W W
O
V d,
-- H_ o 0 0 0 0 0~ of
H
W
a
H~ E o 0 0 0 0 0~ o~
N O O O O O O) O
O
'~ O O O O O O~ O
H
a
~ O
t",O O ~
H 1~f~S~1 f~ Eli O
~
W ~ U U
E ~ ~ O v
~
W ~ ~ ~
1 c~ O S U rtf~
- -i
E W E
~ ~ a ~ ~ O
7
E-~ H W x '~..L..1Ei ~
'.'
CA 02372026 2002-02-28
_ ~g _
10.2.3. INTRACUTANEOUS TEST
All of the animals increased in weight. See
Table V for data. There were no signs of erythema or
edema observed at any of the p-GlcNAc or control
article sites. Overt signs of toxicity were not
observed in any animal. Because the difference
between the p-GlcNAc and control article mean reaction
scores (erythema/edema) was less than 1.0, the p-
GlcNAc meets the requirements of the intracutaneous
test. See Table VI for results. Therefore; as
assayed by this test, the p-GlcNAc demonstrates no
biological reactivity.
20
30
CA 02372026 2002-02-28
W
~
O
~
~
~
~
' O O O O O O '
O
~nHz z z z z z
M
A
x
a~
tT1d'. M M ~ tD apO
O
.-1O O O O O O 5..1
tlS
N
.~
~ O O O O O O
U
3
O
M ~ '~
tf1 d' Ln tD CO t'
~
v (a N N N N Ca N N y~
~ ~
r-1
E
a -~ 3
0 0
H ~ b i ~ ~ ~ ~ ~
? m ?, >
.
IY1 A N N N N A N N
N
d'1
O .
. U O
O U ~
rl r~l r-1
~C ~ E ~ ~
E ~
H .~~ a ~ w ~ w ~ w
o c
n
~ ~s
0
a
~a ~a
~
z
U b M w tn to rti
~ ~ ~ ~ ~
.
c~ Z3t~ ,-
i
., M M f~'1M U ~
U (a
N N N N ~ ~qU
O O
N 1~r1 U
~
-I U U O
o o
z
z z
s~ o o .c~ o ~ ~ ~
~ eW 01~ ',L'.~. e-1 E i1
U7 Ul W W
O o~U rnU OW OA. C1, E
C7 O 0 W W H U) H
.~! ~ ~ vtJ
CA 02372026 2002-02-28
$1
O O O O O O
O O
\\\ \ \\\ \
~ a o00 0 00o a
a
O O O O O O
O O
\\\ \ \\\ \
O O O O O O
O O
N i-I ~-I
i.~ ~
~1
E ,~ ~ .~
.~ .O
.~
-ri d' df GO
00 N
N
[-f N e~ N d~
~ I~
tIt O O O O O O
. O O I
I \\\ \ \\\ \
U oo0 0 000 0,
Ca N O O O O O O
O O I
W t \\\ '\ \\\ \
\ H O O O O O O
A O
W
i
'-'d~ OOO O OOO O',
f \\\ \ \\\ \
!
U O O O O O O
O O
H
(~ df O O O O O O
O O !
1 \.\\ \ . \\\ \
'.
H O O O O O O
O O I
H M O O O O O O
O O
f \\\ \ \\\ \
j
U aoo 0 000 0
W
pa M 0 0 O 0 0 O
0 0
f \\\ \ \\\ \
H ~ E-f o00 0 000 0
~ N O O O O O O
O O
f \\a \ \w, \,
p; W U o00 0 000 0
pq
N O O O O O O
~ O O
cn ~ f \\\ \ \\\ \
W -~-i E-r O O O O O O
O O
W
U
Q~ H e-1 0 0 O 0 0 O
0 0
Cla (!J W f \\\ \ \\\ \
U o00 0 000 0
W ~
N ~
H ~ ' r-1 G1 O O O O
O O
O
f \\\ \ \\\ \
(-a O O O O O O
O O
U
H r-1 N '
cf) ri O .-t
W C9 1~.1 U .~-i r-i
t~ ~ ~ U .i~U .i~
v~ O ~ c6 O rtf O
v a z H z H
o v a
W r-i U U
~
~ r-~
-1
(-i 1J ~i iJ fI9 M
f.~ m SC E .-a
~
U ~cC N W -~
~ A M M
~ N N
t.,
H m En V
Z ~ " of
CA 02372026 2002-02-28
- 82 -
m ~ o00 0 000 0
\\\ W \\\ \
U o00 0 000 0
000 0 000 0
\\\ w \\\ \
EI o00 0 000 0
~
-r- dl CD d'
i N 40
N
H N et~ N d~
l~ t~
In O O O O O O
O O
1 \\\ \ \\\ \
U o00 0 000 o
Gt I,n oo0 0 000 o
W 1 \\\ \ \\\
Ea o00 0 000 o
W
d' O O O O O O
O O
1 \\\ \ \\\ \
U o00 0 000 0
H
tY. O O O O O O
d~ O O
~ O 1 \\\ \ \\\ \
E-~ O O O O O O
O O
C
M O O O O O O
O O
U 1 \\\ \ \\\ \
U oo0 0 000 0
H
n''1 O O 0 O O O
O O
1 \\\ \ .\\\ \
N o00 0 000 0
N N W
~ v W v
W U o00 0 000 0
~\\ \ \\\
EI O O O O O O
O O
W
-a o00 0 00o 0
H
1 \\\ \ \\\ \
U o00 0' o00 0
r-t o00 0 000 0
1 \\\ \ \\\ \
N o00 0 000 0
a
w ro
U
E, U
U
~~ ri
W A
M
O H N N
U
CA 02372026 2002-02-28
- 83 -
000 0 000 0
\\\ \ \\\ \
U o o O o o o
o 0
000 o 000 0
\\\ '~.\\\ \
[-I oo0 o 00o o
rl dr d~ CO
CO N
N
[-I N ~ N d~
~ C
. II1 O O O O O O
O O
i \\\ ~ \\\ \
U o00 0 000 0
L1 In o00 0 000 0
W I \\\ \ \\\
\ E-~ 000 0 000 0
w
~r o00 0 000 0
I \\\ \ \\\ \
t7 U o0o a o00 0
z
H
p.,' O O O O O O
d~ O O
.-. Q 1 \\\ \ \\\ \
U H o00 0 000 0
O M O O O O O O
O O
U I \\\ a \\\ \
'-' U G~ 0 O O O
O O
O
H
r~1 o O O O O O
O O
I \\\ \ \\\ \
W E-~ 0 0 O 0 0 O
0 0
a
N O O O O O O
Ei I O \ O \
\\\ \\\
~
w U o00 0 000 0
C4
N O O . O O O
O O O
t \\\ \ \\\ \
E-t O O O O O O
O O
W
0 0 O 0 0 O
0 0
I . \\\ \ \\\ \
U 00o 0 000 0
rl o00 0 000 0
\\\ ~ \\\ \
E-I o00 0 000 0
~.1 U r-) .-I
U .~, ~ x ~a ,~ x
it .~ U O r.~U O .u
S~ GY c~ O c~f O
.t~ r.~
zw H zw H
x
o ~a ~n ~o
w .~
\ ra M
rl b N N
H
U
z
CA 02372026 2002-02-28
- 84 -
000 0 000 0
\\\ \ \\\ \
U o00 0 000 0
O o 0 0 0 O
0 o
\\ \ \\\ \
o0o 0 o00 o
N S-t ~.I
i-W.t i-~
i~i
E ~ ~ ~ ~
~ ~
rl cr d~ 00
ao N
N
E1 N d~ N d~
t'~ I~
t11 O O O O O p
O O
\\''~.\ \\\ \
U o 0 0 o 0 .o
0 0
W n o00 0 000 0
W W ,\\ \ \\\ \
\ H O O O O O O
O O
W
O O O O O O
O O
i \\\ \ \\\
C7 U o00 0 o00 o
2
H
rx d~ ooo o 000 o
.. p ~ \\\ \ \~.\ \
U wE-~ 000 0 000 o
G
O cn oao 0 000 0
U ~ \\\ \ \\\
U o00 0' Ooo O
H
r1 O O O O O O
O O
\\\ \ \\\ \
W E-~ 0 0 0 0 0 0
0 0
a
~ N O O O O O O
E1 1 ' O O
\\\ \\\
~, \ \
W U o00 0 000 0
~ _
y N O O O O O O
O O
1 \\\ \ \\\ \
(-a O O O O O O
O O
W
,~a o00 o 000 0
H
~ \\\ \ \\\ \
U O O O O O O
O O
r-I 0 0 O 0 0 O
0 0
1 \\\ \ \\\ \
H o00 0 000 0
U r-1 ,-I
x ~
c~ C~
U
ro
E ~ .-i .-t
x
W ~ fa M M
H N N
W
f.1
CA 02372026 2002-02-28
- 85 -
10.2.4. SYSTEMIC TEST
All of the mice treated with the p-GlcNAc extract
or the control article increased in weight. See Table
VII for data. In addition, there were no overt signs
of toxicity observed in any.p-GlcNAc or control
animal. See Table VI for results. It is concluded,
therefore, that none of the p-GlcNAc test animals
showed a significantly greater biological reactivity
than the animals treated with the control article.
15
25
35
CA 02372026 2002-02-28
- ss -
TABLE va=
ANIMAL WEIGHTS AND CLINICAL OBSERVATIONS
Body Weight
(g)
Group Sex Dose Animal Day Day Weight Signs
0 3 of
(ml) # Change Toxicity
t
NaCl: Female 1.03 I. 20.6 22.8 2.2 None
EtOH Female 1.06 II. 21.1 23.4 2.3. None
1 0 Test Female 1.02 III. 20.4 22.6 2.2 None
50m1/kg Female 1.11 IV. 22.2 24.5 2.3 None
Female 1.05 V. 21.0 23.2 2.2 None
Mean 21.1 23.3
SD +/- 0.7 0.7
NaCl: Female 1.04 VI. 20,7 23.2 2.5 None
EtOH Female 1.04 VII. 20.8 23.5 2.7 None
Control Female 1.02 VIII. 20.3 22.3 2.0 None
50m1/kg Female 0:91 IX. 18.2 20.6 2.4 None
Female 0.94 X. 18.7 20.9 2.2 None
Mean 19.7 22.1
SD +/- 1.2 1.3
2
0
pEG Female 1.02 XI. 20.3 22.? 2.4 None
Test Female 0.96 XII. 19.2 21.4 2.2 None
l0ml/kg Female 0.95 XIII. 18.9 21.6 2.7 None
Female 1.05 XIV. 20.9 22.7 1.8 None
Female 0.94 XV. 18.7 21.2 2.5 None
Mean 19.6 21.9
SD +/- 1.0 0.7
PEG Female 1.01 XVI. 20.1 22.3 2.2 None
Control Female 0.99 XVII. 19.8 22.0 2.2 None
lOg/kg Female 1.10 XVIII. 22.0 24.3 2.3 None
Female 1.07 XIX. 21.4 23.6 2.2 None
Female 1.03 XX. 20.6 22.4 1.8 None
Mean 20.8 22.9
3 sn +/- 0.9 1.0
0
*Summary of observations - 0, 4, 24, 48, and 72 h
after injection
CA 02372026 2002-02-28
- 87 -
11. EXAMPLE: n-GlcNAc REFORMULATION
In the Working Example presented in this Section,
a p-GlcNAc membrane(16.2 mg) was dissolved in i ml of
a dimethylacetamide solution containing 5% LiCl. The
p-GlcNAc-containing solution was placed in a syringe
and extruded into 50 m1 of pure water to precipitate a
fiber. The resulting fiber was studied with scanning
electron microscopy, as shown in FIG. 10A-lOB.
12. EXAMPLE: CELL ATTACHMENT TO b-G~.cNAc
In this working example, it is demonstrated that
p-GlcNAc represents an efficient substrate for cell
attachment and growth in culture.
12.1 MATERIALS AND METHODS
Ce s:The transformed mouse 3T3 fibroblast cell
line was used, and was grown in DMEM supplemented with
10 fetal bovine serum (FBS).
p-GlcNAc membranes:p-GlcNAc was prepared w
according to the methods described, above, in Sections
5.3.1 and 8.
p-GlcNAc membranes were initially stuck to a ~i
(i8mm) Corning cover glass using one drop of water,
and were attached by autoclaving at 121° C. for 30
minutes. Membranes prepared in this manner were then
placed in culture wells of 6 well culture plates.
Cell counts: Cell numbers were determined in media
by direct counting with a hemocytometer, and on matrix
by first rinsing membranes with fresh medium DMEM +
10% FBS) followed by treatment with trypsin (10%, at
37° C. for 5 minutes) prior to counting.
SEM onerating conditions: A Zeiss 962 instrument
was utilized with an accelerating voltage of lOkv, and
a working distance of l5mm. Polaroid type 55 p/n (u4)
CA 02372026 2002-02-28
was utilized at various magnifications, as indicated.
Sample coat: carbon coat (1000 & 100 aupd.
Specimen preparation: For primary fixation, the
culture growth medium was replaced with 2%
glutaraldehyde in Eagle's DMEM without serum. Several
changes were performed to ensure a complete transition
from growth media to Fixative. Fixation proceeded for
0.5 hours at room temperature. Cover slips were
transferred to fresh vials containing 2%
Glutaraldehyde in O.lM Na Cacodylate pH 7.2 with O.1M
Sucrose and fixed for a further 1.5 hours at room
temperature.
Dehvdration. CPD, Mount and Sputter Coating:
Samples were rinsed in O.1M Na Cacodylate pH 7.2,
and cover slips were transferred to a CPD holder.
Dehydration was performed in ethanol series (300, 50%,
75%, 85%, 95o and 3 x 100%, 5 mins each), and samples
were critical point dried. Cover slips were then
mounted on A1 stubs, carbon coated, using vacuum
Evaporator (Q 100A) and Sputter Coated with 100 A
AuPd.
12.2 RESULTS
p-GlcNAc membranes were tested for an ability to
form a substrate on which cells may be grown in
culture. Mouse fibroblast cells were grown in wells
in the presence or absence of p-GlcNAc membranes and
cell counts were taken daily to assay the viability of
cultures. The results of one such series of cell
counts in shown in FIG. 14. As indicated, by day 5
after plating, only the wells containing p-GlcNAc
membranes were able to continue to sustain viable
cells, demonstrating that p-GlcNAc membranes are
capable of acting as efficient substrates for the
continued growth of cells in culture.
CA 02372026 2002-02-28
- 89 -
Further, the SEM micrographs depicted in FIG.
15A-15B show healthy cells strongly attached to p-
GlcNAc membranes.
13. EXAMPLE: p-GlcNAc/COLLAGEN HYBRIDS
Presented in this Working Example is the
formation and characterization of a p-GlcNAc/collagen
hybrid material.
13.1 MATERIALS AND METHODS
Materials: Bovine Type I collagen was used in
preparation of the hybrids described in this study.
p-GlcNAc was prepared according to the mechanical
force method described, above, in Section 5:3.2:
i5 Hybrid preparation: Collagen (10 milligrams/ml)
and p-GlcNAc (0.25 milligrams/ml) suspensions were
mixed, in different ratios, frozen in liquid N2 (-
80°C.), thermal soaked at -9° C. for 4 hours, and
lyophilized. Material Was dehydrothermally cross-
linked under vacuum (approximately 0.030 Torr) at 60°C.
for 3 days.
Cell Culture: Mouse 3T3 fibroblast cells were
grown on the collagen/p-GlcNAc hybrids produced.
Standard culturing procedures were followed, and SEM
micrographs were taken after 8 days in culture.
13.2 RESULTS
Collagen and p-GlcNAc suspensions were mixed in
differing ratios (namely, 3:1, 1:1, 2:2, and 1:3
collagen:p-GlcNAc suspension ratios), frozen,
lyophilized, and crosslinked. Such a procedure
yielded collagen/p-GlcNAc slabs. SEM micrographs of
the resulting materials are shown in FIGS. 16 B-E.
Fig. 16A represents a collagen-only control material.
Note the porous structure of the hybrid material.
CA 02372026 2002-02-28
- 90 -
The collagen/p-GlcNAc hybrids of the invention
provide an efficient three-dimensional structure for
the attachment and growth of cells, as shown in the .
SEM micrographs in FIGS. 17A-D.
14.EXAMPLE: NMR CHARACTERIZATION OF PURE PREPARATIONS
OF p-GlcNAc
Presented in this Example is an NMR (nuclear
magnetic resonance) analysis of pure p-GlcNAc
Preparations.
14.1 MATERIALS AND METHODS
g-GlcNAc preparations: The p-GlcNAc used in the
NMR studies described here was prepared using the
chemical purification method described, above, in
Section -5.3.2, with hydrofluoric acid utilized as the
chemical reagent.
NMR techniques: Solid state NMR data was
obtained using a Bruker 500MFi NMR spectrometer.
Computer image analysis was used to transform the raw
NMR spectrum data so as to eliminate background and to
normalize baselines. An example of such transformed
data are shown in FIG. 18. Transformed NMR curves
such as that in Figure 18 were used to obtain areas
for every carbon atom type, and to then calculate the
CH3(area) to C-atom(area) ratios. Such values,
obtained as described are provided in FIG. 20.
14:2 RESULTS
Solid state NMR data was obtained by measuring
the C13-NMR spectrum of a 500mg sample of p-GlcNAc. A
typical NMR spectrum is shown in FIG. 19. The'
individual peaks represent the contribution to the
spectrum of each unique carbon atom in the molecule.
The relative percentage of each type of carbon atom in
the molecule was determined dividing the area of the
CA 02372026 2002-02-28
- 91 -
peak generated by that carbon species by the total sum
of the areas under all of the NMR peaks obtained in
the spectrum. Thus, it was possible to calculate the
ratio of each of the atoms of the molecule measured by
a reference atom. All p-GlcNAc molecules consist of
N-acetylated glucosamine residues having C1, C2; C3,
C4, C5 and C6 atoms, by definition. The ratio, then,
of the area of the N-acetyl CH3 carbon atom peak to
the areas of any of the glucosamine residue carbon
atom peaks, above, should be 1.0 if all of the
glucosamine residues in the polymer are N-acetylated.
Data such as those in FIG. 20 were used to obtain
values for the CH3(area) ratios.
The calculated ratios in Fig. 20 are in many
cases equal to or nearly equal to 1.0, within
experimental error, e.g. CH3/C2=1.097, CH3/C6=0.984,
CH3/C5=1.007, CH3/C1=0.886. These results are
consistent with the conclusion that the p-GlcNAc -
material of the invention is free of contaminants and
is fully acetylated (i.e. that essentially 100% of the
glucosamine residues are N-acetylated).
15. EXAMPLE: SYNTHESIS AND BIOLOGICAL
CHARACTERIZATLON OF CONTROLLED PORE
SIZE THREE-DIMENSIONAL p-GlcNAc
MATRICES
Described below, are. methods for the production
of three-dimensional p-GlcNAc based porous matrices
having controlled average pore sizes. Such matrices
have a variety of important applications,
particularly, for example, as means for the
encapsulation of cells. Such cell encapsulation
compositions are useful as transplantable cell-based
therapeutics, and in other cell & tissue engineering
applications such as in cartilage regeneration. The
capability to manipulate the morphology and
CA 02372026 2002-02-28
_ 92 _
dimensionality of p-GlcNAc materials, as demonstrated
here, provides a powerful tool in expanding the
potential applications of the p-GlcNAc material of the
invention.
15.1 MATERIALS AND METIiODS '
p-GlcNAc starting material: p-GlcNAc was
prepared using the chemical purification method
described, above, in Section 5.3.2, with hydrofluoric
utilized as the chemical reagent:
Matrix formation: Suspensions (5mls) containing
mg p-GlcNAc samples were made in the solvents
listed below in Section 15.2, prior to lyophilization.
Samples were then poured into wells of.tissue culture
15 dishes and frozen at -20°C. The frozen samples were
then lyophilized to dryness, and the resulting three
dimensional matrices were removed.
Scannincr electron microscogv techniques: The
procedures utilized here Were performed as described,
20 above, in Section 12.1: The images shown in FIGS.
21A-G. are 200X magnifications of the matrix material,
and a scale marking of 200 microns is indicated on
each of these figures.
15.2 RESULTS
p-GlcNAc samples were obtained from each of the
following solvents, as described, above, in Section
15.1:
A. Distilled water
B. lo% methanol in distilled water
C. 25% methanol in distilled water
Distilled water only
E. 10% ethanol in distilled water
F. 25% ethanol in distilled water v
G. 40% ethanol in distilled water
CA 02372026 2002-02-28
- 93 -
Samples of matrix formed using each of the
solvents were subjected to scanning, electron
microscopic (SEM) analysis, as shown in FIGS. 21A-G.
These figures reveal that the average matrix pore size
decreases as the percentage of either methanol or
ethanol increases in each suspension.
Specifically, pore diameter in the two water
suspensions (FIGS. 21A and 21D) approach 200 microns
on average. Pore size in the samples depicted in
FIGS. 21C and 21F (25% methanol and ethanol,
respectively) are between 30 and 50 microns on
average.
The results shown here suggest that while both
ethanol and methanol may successfully used to control
p-GlcNAc.pore size, ethanol may be more efficient than
methanol in enabling the control of the p-GlcNAc
matrix pore size.
16. EXAMPLE: CELL GROWTH ON THREE DIMENSIONAL
POROUS p-GlcNAc MATRICES
Described in this Section are results
demonstrating the successful use of three dimensional
p-GlcNAc porous matrices as substrates for the
culturing of cells.
16.1 MATERIALS AND METHODS
p-GlcNAc starting material: p-GlcNAc was
prepared using the chemical purification method
described, above, in Section 5.3.2, with hydrofluoric
acid utilized as the chemical reagent.
Matrix formation: Three-dimensional p-GlcNAc
matrices were prepared by the lyophilization of
suspensions of p-GlcNAc'in water, water-ethanol, or
water-methanol mixtures.
CA 02372026 2002-02-28
- 94 -
Suspensions (5 mls) containing 20 mgs p-GlcNAc
were prepared in the following solvents prior to
lyophilization:
~l. Distilled water only
2. 10% methanol in distilled water
3. 25°s methanol in distilled water
4. Distilled water only
5. 10% ethanol in distilled water
6. 25% ethanol in distilled water
7. 40% ethanol in distilled water
Samples were poured into circular wells of
plastic tissue culture dishes and were frozen at -20°C.
The frozen samples were then lyophilized to dryness,
and the resulting three dimensional matrices were
removed. Samples of each matrix were subjected to
scanning electron microscopic (SEM) analysis.
Cells: Mouse embryo BALBC/3T3 fibroblast cell
line (clone A31), obtained from the ATCC, were used
for culturing on the three dimensional porous p-GlcNAc
matrices.
Culturinct°conditions: One cma samples of porous
matrices were placed in tissue culture wells and were
covered with a standard tissue-culture growth medium.
Each-well was seeded and cells were cultured for
days at 3 7 ° C in a C02 incubator ( 5 % C02 ) .
SEM procedures: Matrix samples were fixed and
subjected to SEM analysis as described, above, in
Section 12.1. The matrices were prepared by
lyophilizing p-GlcNAc in distilled water. Images vary
in magnification from 100X to 5000X, as indicated in
figure legends (FIGS. 22A-G).
16:2 RESULTS
SEM photographs of p-GlcNAc matrices containing
attached mouse fibroblast cells attached are shown in
FIGS 22A-G. These photographs show that the three
dimensional p-GlcNAc matrices contain attached mouse
CA 02372026 2002-02-28
- 95 -
fibroblast cells. Further, the photographs reveal
that there is a close interaction and connection
between the cells and the p-GlcNAc matrix material. It
is also notable that the cells have a rounded three-
s dimensional morphology which is different from the.
flat, spread shape of the cells when cultured directly
onto plastic culture dishes. Cell viabilities were
determined to be greater than 95%.
17. EXAMPLE: p-GlcNAc SUCCESSFULLY PREVENTS
POST SURGICAL ADHESIONS
The Example presented herein demonstrates the
successful use of p-GlcNAc materials,, specifically a
p-GlcNAc membrane and gel formulation, to prevent the
formation of post surgical adhesions in a series of
animal models for such adhesions.
17.1 MATERIALS AND METHODS
Synthesis p-GlcNAc-lactate: p-GlcNAc membrane
starting material was produced by the chemical method,
as described, above, in Section 5.3.2, with
hydrofluoric acid utilized as the chemical reagent.
The p-GlcNAc was converted to deacetylated p-
GlcNAc by the following method. (It should be noted
that approximately 1.4 g of p-GlcNAc are needed to -
produce each 1 g of p-GlcNAc lactate, given the
expected loss in mass of approximately 15°s which
occurs upon deacetylation.) Approximately 200mg of p-
GlcNAc membrane material were mixed vigorously with
approximately 2OO m1 60% NaOH. The vigorous shaking
served to separate the p-GlcNAc membrane material to
the extent possible. The NaOH solution used was made
at least 12 hours before using. Samples were placed
in an 80°C water bath for 6 hrs, with periodic shaking
to separate and wet p-GlcNAc material. After 6 hrs,
the samples were taken from water bath and the NaOH
CA 02372026 2002-02-28
- 96 -
solution was immediately removed. The membrane
materials were washed with ddH20, at room temperature,
until a pH of 7 was reached. The membranes were
removed from the water and dried on a Teflon sheet.
At this point a 2 ,mg sample was collected for C,
H, N analysis in order to determine extent of '
deacetylation. Further, solubility in 1% acetic acid
was checked, with a solubility of 1 mg/ml indicating
that the p-GlcNAc material was appropriately
deacetylated.
The partially deacetylated pGlcNAc was then
converted to pGlcNAc-lactate using the following
method: Sufficient 2-propanol (containing 10% water)
to wet all of the partially deacetylated pG7:cNAc
material and to allow for stirring was added to lg of
the partially deacetylated p-GlcNAc in a 250 ml
Erlenmeyer flask. (Approximately 30 mls 2-propanol
necessary.) 2-propanol must be reagent grade, and
fresh prior to each synthesis. With stirring, 1.1 mL
of a 50% aqueous lactic acid solution. Lactic acid
should be reagent grade; and must be analyzed to
determine exact concentration of available (i.e., non- -
esterified) lactic acid present: This was generally
accomplished by titration with O.1N NaOH to the
phenopthalein end-point (pH 7.0). The concentration
of lactic acid used must be constant, i.e., must be
+/- 1 percent, for each,p-GlcNAc synthesis. The
mixture was allowed to stir for at least two hours.
It is possible to add low heat in order to elevate the
reaction rate. Reaction time may be extended, or~the
amount,of 50% aqueous lactic acid maybe increased so
that the reaction goes to completion. After stirring,
the contents of the flask were poured through a
Buchner funnel using quantitative ashless filter
paper. The material was washed with 15 ml of
CA 02372026 2002-02-28
_ 97 _
anhydrous 2-propanol. The material was allowed to air
dry in a fume hood for 2 hours and then placed in an
oven at 40°C overnight: For every gram of partially
deacetylated p-GlcNAc starting material, a final p-
GlcNAc-lactate weight of approximately 1.4 g, (i.e.,
an increase of 40% in mass) should be obtained.'
Formulation of p-GlcNAc-lactate as a gel: The
p-GlcNAc-lactate material was formulated into a gel as
follows: p-GlcNAc-lactate starting was dissolved in
dd-deionized water to a concentration of between 0.1-
4.0% p-GlcNAc-lactate, by weight. Reagent grade
propylene glycol (2-propandi.ol) Was then added to a
final propylene glycol concentration of between 1-10%.
In some cases, a preservative was added to prevent
bacterial and/or fungal'contamination of the product.
Typically, concentrations of p-GlcNAc-lactate of
between 0.1%-4.0% were prepared. The viscosity of
. these preparations increases dramatically as the p-
GlcNAc.-lactate percentage increases, such.that
formulations having 0.5% or more of the p-GlcNAc-
lactate behave as gels.
Animal models:
Spractue-Dawley rats: Adhesions are produced in
this model by abrading or irritating the serosal
surface of the cecum and apposing it to an area of
parietal peritoneum. The.success rate for inducing
adhesions in control animals with this method is
reported at an average 80%.
Specifically, the surgical procedure for inducing
post surgical adhesions in these rats involved the
following. Animals were placed in dorsal recumbency
and prepared and draped accordingly for aseptic
surgery. Abdominal cavities were exposed through a
midline incision. An area, approximately 0.5 cm x 1.0
cm, of parietal peritoneum on the left abdominal wall
CA 02372026 2002-02-28
_ 98 _
was carefully excised, removing a thin layer of
muscle, along with the peritoneum.
The cecum was then elevated and isolated. An .
area, approximately 0.5 cm x 1.0 cm, on the lateral
surface of the proximal end of the cecum was abraded
by rubbing ten times with a dry gauze. The cecum was '
then scraped with a scalpel blade to cause minute
petechial hemorrhages. The cecal abrasion and the
peritoneal incision were left exposed for 15 minutes.
After 15 minutes, the test article (i.e., the p-
GlcNAc material) or control article was applied to the
cecal wound. The cecal abrasion and the peritoneal
wound were then opposed and held in contact with Allis
tissue forceps for an additional 15 minutes.
The cecum was then replaced into the abdomen such
that the abraded area of the cecum was adjacent to the
peritoneal site. The abdominal incision was closed
and the animal was allowed to recover from the
anesthesia. . . ..
Fourteen days after surgery, animals were
euthanized and the abraded area was examined for the
formation of post surgical adhesions. If adhesions
were present, the entire area involved in the adhesion
(i.e., body wall, test or control article, and
internal organs) were dissected free of the animal.
The extent of involvement and tenacity of
adhesions was evaluated according to the following
scales:
Extent of involvement scores:
0 no adhesion
1 adhesion<= 25% of the area
2 adhesion <= 50% of the area
3 adhesion <= 75% of the area
4 adhesion > 25% of the area
CA 02372026 2002-02-28
_ 99 _
Tenacity Scores:
o no adhesion
1 adhesion freed with blunt dissection
2 adhesion freed with aggressive dissection
3 adhesion requiring sharp dissection
Additionalan_im_al models: Pig and horse large
animal bowel models were used to assess the prevention
of peritoneal adhesions:'
Surgical procedure: The animals were placed in
dorsal recumbency and prepared and draped accordingly
for aseptic surgery. The abdominal cavity was exposed
through a midline incision. The small intestine was.
elevated and a 2 cm X 2 cm section was identified,
extensively abraded (approximately 200 strikes using a
scalpel), and allowed to dry for 10 minutes. The test
article (i.e., p-GlcNAc material) or control article
was then applied to the abraded wound, and the wounded
section of the small intestine was replaced into the
abdomen. In such a large bowel type of animal model,
six wounds, each separated by 4 inches of bowel from
the adjacent wound provides an environment highly
prone to form adhesions. Following the last of the
wounds, the abdominal incision is closed and the
animal is allowed to recover from the anesthesia.
Analysis of peritoneal adhesions: Twenty one
days after surgery, animals were euthanized and the
abraded area was examined; with adhesion formation
being evaluated following a procedure similar to that
of the Sprague-Dawley rat cecum model.
1?.2 RESULTS
when injury occurs, the body sets in motion a
complex set of responses designed to restore the
injured area. In the final stages of healing,
connective tissue forms at the wound site to
CA 02372026 2002-02-28
- 1~0 -
regenerate the body structure and protect the affected
area from further damage. In some instances this
cascade of events does not work properly and can lead
to life threatening conditions.
For example, as a visceral organ heals following
surgery, a fibrin clot generated during the surgical
procedure may invade the surface of adjoining organs
forming a link which allows for fibroblast migration.
This migration leads to collagen deposition and tissue
growth, which in turn causes t-he organs involved to
adhere to one another.
Such adhesions, referred to as, post surgical
adhesions, may produce pain, obstruction and
malfunction by distorting the organ-or organs
involved. Immobilized joints, intestinal obstruction
and infertility are often linked to the formation of
post-surgical adhesions. Furthermore, post surgical
adhesion will complicate and extend the length of
future surgical procedures in the surrounding region.
This. last issue is of particular relevance to open
heart surgeries and cesarean section obstetrical
procedures where additional surgeries may be required.
The formation of adhesions is very Gammon following
abdominal, cardiovascular and orthopedic surgical
procedures.
When adhesions become pathological and seriously
interfere with organ function, surgical adhesiolysis
(sharp or blunt dissection of the adhesion in
conjunction with meticulous surgical techniques) is
the treatment that is currently used to eliminate
adhesions. In 1991, approximately 500,000
adhesiolysis procedures were performed. This
procedure is, however, notoriously ineffective, with
the frequency of recurrence of adhesion formation
reported to be as high as 90%. Further, no other
CA 02372026 2002-02-28
- l~l -
technique or composition has proven effective in the
prevention of such post surgical adhesions:
The results presented herein, therefore, are
significant in that they demonstrate the effectiveness
of the p-GlcNAc materials of the invention for the
prevention of post surgical adhesions. Specifically,
the results presented here demonstrate the efficacy of
p-GlcNAc based solid and liquid formulations as
barriers to the formation of abdominal post surgical
adhesions. in accepted rat and pig animal model
systems.
One of the accepted animal models used to study
adhesion formation employs visceral-parietal
peritoneal adhesions in Sprague-Dawley rats. Both
partially deacetylated p-GlcNAc membranes and p-
GlcNAc-lactate gel formulations prevented and/or
considerably reduced the incidence of adhesion
formation as compared with either non-treated controls
treated with InterCEED~ (Johnson & Johnson), the only
commercially available product for this indication.
Specifically, a total of l8 rats were used to
test p-GlcNAc-lactate gel formulations. 12 animals
were used as controls, with 6 receiving no treatment
and 6 receiving InterCeedTM. 6 animals received 0.25%
p-GlcNAc-lactate gel, TC% propylene glycol, water.
Animals receiving the p-GlcNAc-lactate gel treatment
showed a significantly reduced incidence of
postoperative adhesion formation, compared to either
of the controls, as shown, below, in Table VIII.
35
CA 02372026 2002-02-28
- 102 -
TABLE VIII
Extent of Tenacity
Involvement '
Control (No treatment) 1 +/- 2:1 1 +/- 1.5
InterCEED~" 1 +/- 1.8 1 +/- 1.5
p-GlcNAc-lactate gel 0 +/- 0~8 1 +/- 1.2
Partially deacetylated p-GlcNAc membranes were
also tested for their ability to prevent to occurrence
of post surgical adhesions in the rat animal model. A
A total of 22 rats were used in the study. 12 animals
were used as controls, with 6 receiving no treatment
and 6 receiving InterCEED'~. Ten animals each received
a lcm x lcm membrane 4f an approximately 60%
deacetylated p-glcNAc formulation. The animals which
received the partially deacetylated p-GlcNAc membrane
showed a significant reduction in the incidence of
formation of postoperative adhesions, as compared with
the non-treated controls and InterCEED'", as shown,
below, in Table IX.
TABLE IX
Extent of Tenacity
Involvement
Control (No treatment) 3 +/- 1.8 1 +/- 0.6
InterCEED'~ 3 +/- 1.6 1 +/- 0:4
p-GlcNAc-membrane 1 +/- 0.8 1 +/- 0.3
CA 02372026 2002-02-28
- 103 -
Large animal bowel models for the prevention of
peritoneal adhesions were also used to test p-GlcNAc
compositions. Specifically, six pigs and one horse
were used to study both the partially deacetylated p-
GlcNAc membrane and the p-GlcNAc-lactate gel. The
partially deacetylated p-GI.cNAc membrane consisted of
a 2 cm X 2 cm piece of 60% deacetylated p-GlcNAc
membrane, while the p-GlcNAc-lactate gel consisted of
0.25% p-GIcNAc lactate formulated with 10% propylene
glycol and water. Control animals received no
treatment to the wounded site.
The results of these large animal studies
revealed that, while the control sites formed multiple
adhesions and scare tissue in the surrounding site,
both the p-GIcNAc membrane and gel formulations
effectively prevented the formation of adhesions.
Samples from control and treated sites were
additionally examined using SEM, which showed an
increased amount of fibrosis~in the control sites as
compared to the treated tissues.
18. EXAMPLE: BIODEGRADABILITY OF p-GlcNAc
MATERIALS
The Example presented in this Section
demonstrates that p-GlcNAc materials of the invention
may be prepared which exhibit controllable in vitro
and in vivo biodegradability and rates of resorption.
18.1 MATERIALS AND METHODS
P-GlcNAc materials: Prototype I was made by the
method described, above; in Section 5.3.2, via the
chemical method, with hydrofluoric acid being utilized
as the chemical reagent. Prototype I represented 100%
acetylated p-GlcNAc.
The p-GlcNAc starting material of prototype 3A
was made by the method described, above, in Section
CA 02372026 2002-02-28
- 104 -
5.3.2, via the chemical method, with hydrofluoric acid
being utilized as the chemical reagent. The p-GlcNAc
material was then deac.etylated by the method
described, above, in Section 5.4. Specifically, the
p-GlcNAc material was treated with a 40% NaOH solution'
at 60°C. for 30 minutes. The resulting prototype 3A
was determined to be 30% deacetylated.
The p-GlcNAc starting material of prototype 4 was
made by the method described, above, in Section 5.3.2,
via the chemical method; with hydrofluoric acid being
utilized as the chemical reagent. The p-GlcNAc
material was then deacetylated by treatment with a 40%
NaOH solution at 60°C. for 30 minutes. Next, the
fibers were suspended in distilled water, frozen at -
20°C., and lyophilized to dryness. Prototype 4 was
also determined to be 30% deacetylated.
Abdominal implantation model: Sprague Dawley
albino rats were utilized for the abdominal
implantation model studies. Animals were anesthetized
and prepared for surgery, and an incision was made in
the skin and abdominal muscles. The cecum was located
and lifted out. A 1 cm x 1 cm membrane of p-GlcNAc
material was placed onto the cecum, and the incision
was closed with nylon. Control animals were those in
which no material was placed onto the cecum.
Animals were ogened:at 14 and 21 days post
implantation. Photographs were taken during the
implant and explant procedures (FIGS. 23A-El. Samples
of cecum~were prepared for histopathology after the
explant procedure.
p-GlcNAc in vitro ~ecrradation lysozyme-chitinase
assa The assay is a colorimetric assay for N-acetyl
glucosamine, and was performed as follows: 150~C1 of a
reaction sample was pipetted into 13x100mm glass
disposable test tubes, in duplicate. 25~e1 of 0.25M
CA 02372026 2002-02-28
- 105 -
potassium phosphate buffer (pH 7.1) was added to each
test tube, followed by the addition of 35,1 of 0.8M
potassium borate solution (pH 9.8). Tubes were
immediately immersed into an ice-bath for a minimum of
2 minutes. Samples were then removed from the ice-
bath, lml of freshly prepared DMAB reagent was added,
and the samples. were vortexed. DMAB (Dimethyl
aminobenzaldehyde) reagent was made by adding 70m1s of
glacial acetic acid and lOmls of 11.6N (concentrated)
HCl to 8 grams of p-dimethyl aminobenzaldehyde.
Samples were then incubated at 37°C for 20 minutes.
To prepare a standard curve, the following
procedure was utilized. A GlcNAc stock'solution was
diluted to O.lmg/ml with O.OlOM sodium acetate buffer
(pH 4.5) , and 0~,1, 20.1, 301, 90.1 or 120.1 of the
diluted GlcNAc solution was added to a set of test
tubes. This was followed by the addition of 150.1,
130.1, 60,1 or 30.1, respectively, of O.OlOM sodium
acetate buffer (pH 4.5) to the test tubes. Next, 25~c1
of 0.25M potassium phosphate buffer (pH 7.1) and 35.1
of 0.8M potassium borate buffer (pH 9.8) were added to
each test tube. A duplicate set of test tubes is
prepared by the same~procedure.
The test tubes are capped and boiled at 100°C. for
for exactly 3 minutes. The tubes are then immersed in
an ice bath. The tubes are removed from the ice bath
and lml of DMAB reagent , freshly prepared according to
the method described above in the Section, is added to
each tube. The tubes are incubated at 37°C for 20
minutes. The absorbance of the contents of each tube
is read at 585nM. Absorbance should be read as
quickly as possible. The standard curve is plotted on
graph paper and used to determine the concentration of
N-acetyl glucosamine in the reaction samples. A
typical standard curve is shown in FIG. 23.
CA 02372026 2002-02-28
- 106 -
18.2 RESULTS
The in-vitro biodegradability of p-GlcNAc
materials was studied in experiments which assayed the
relative susceptibility 'of p-GlcNAc membrane materials
to degradation by lysozyme. p-GlcNAc membranes were
exposed to an excess of lysozyme in a lOmM acetate
buffer, and the subsequent release of N-acetyl
glucosamine was determined using the assay described,
above, in Section 18.1.
The results of these experiments indicated that
partially deacetylated membranes are more susceptible
to digestion by lysozyme (see FIG. 24) and, further,
that the rate of lysozyme degradation is directly
related to the extent of deacetylation (see FIG. 25,
which compares the degradation rates of a 50% to a 25%
deacetylated p-GlcNAc membrane).
Additionally, experiments were performed which
addressed the in-vivo biodegradability of p-GlcNAc
materials.. Such experiments utilized an abdominal
implantation model. Three p-GlcNAc materials, as
listed below, were tested.
p-GlcNAc materials tested:
1) p-GlcNAc, fully acetylated (designated
prototype 1);
2) partially deacetylated p-GlcNAc membrane
(designated prototype 3A); and
3) lyophilized and partially deacetylated p-
GlcNAc membrane(designated.prototype 4).
The fully acetylated p-GlcNAc (prototype 1)
was resorbed within 2l days, as shown in FIGS. 26A-
26C. The partially deacetylated p-GlcNAc membrane
(prototype 3A) was completely resorbed within l4 days,
as shown in FIGS. 26D-26E. The lyophilized and
CA 02372026 2002-02-28
'- 107 -
partially deacetylated p-GlcNAc membrane(prototype 4)
had not yet been completely resorbed after 21 days
post-implantation.
Histopathology analyses showed that once the
p-GlcNAc material has been resorbed there were no
histolQgical differences detectable between tissue
samples obtained from the treated and from the control
animals.
19. EXAMPLE: p-GlcNAc HEMOSTASIS STUDIES
The experiments described herein study the
efficacy of the p-GlcNAc materials of the invention
for the control of bleeding. The success of the p-
GlcNAc materials in controlling bleeding is, further,
compared against commercially available hemostatic
products.
19.1 MATERIALS AND M$THODS
p-GlcNAc and control materials: partially
deacetylated (approximately 70%) p-GleNAc membranes
were made using the chemical separation technique
described, above, in Section 5.3.2, with hydrofluoric
acid being utilized as the chemical reagent, and the
techniques described, above, in Section 5.4. 2 cm x 1
cm pieces were used. p-GIcNAc-lactate gel (4% p-
GlcNAc-lactate, formulated in propylene glycol and
water) was produced following the methods described,
above, in Section .17.1. The control material utilized
for the study of bleeding in the spleen and liver was
GelfoamTM (Upjohn Company) . GelfoamT'" and Avitene'"'
(Medchem Products, Inc.) were the control materials
used in the study of small blood vessel bleeding.
Test animals: New Zealand White rabbits were
used. 3 animals received two wounds in the spleen and
one wound in the liver. 4 animals received five
CA 02372026 2002-02-28
- 108 -
surgical wounds to blood vessels of similar size in
the caudal mesenteric artery system.
Surgical preparation: The animals were
anesthetized with ketamine HC1 and Xylazine. The .'
animals were placed in dorsal recumbency, and all the
hair from the abdomen was removed. The abdomen was
then scrubbed with povidone-iodine and 70~ isopropyl
alcohol and draped for aseptic surgery:
Liver/spleen surgical procedures: A midline
incision was made and either the~spleen or liver was
exteriorized and packed with moist lap sponges. A 3-4
mm diameter cork bore wa used to make a circular
wound of about 2 mm depth at one end of the organ.
Once the splenic tissue was removed, a pre-weighed
4" X 4" gauze sponge was used to absorb all the blood
lost from the splenic wound for a period of one
minute. The sponge was re-weighed to quantify the
amount of blood lost from that particular wound. The
test animal was then treated by application to the
wound of one of the treatment materials. The time
until hemo.stasis and the amount of blood lost prior to
hemostasis was recorded.
After hemostasis in the first wound was achieved,
a second wound in the spleen and one wound in the
liver were made following the same procedure.
Small blood vessel surclr'cal_procedure: A midline
incision was made and the small bowel was exteriorized
exposing the caudal mesenteric artery system. The
bowel was packed with moist lap sponges and five blood
vessels of about the same size were identified. A
scalpel was used to make a wound of about 1 mm depth '
at one of the vessels. A pre-weighed 4" x 4" gauze
sponge was used to absorb all the blood lost from the
vessel wound for a period of one minute. The sponge
was re-weighed to quantify the amount of blood lost
CA 02372026 2002-02-28
- 109 -
from that particular wound. The animal was then
treated by application to the wound of one of the
treatment materials. The time until hemostasis and
the amount of blood lost prior to hemostasis was
recorded. .
After hemostasis in the first wound was achieved,
four more wounds were made following the same
procedure.
19.2 RESULTS
p-GlcNAc materials were tested for their ability
to control bleeding in the spleen and liver of rat
animal models. The p-GlcNAc material's tested were:
1) partially deacetylated (approximately 70%) p-
GIcNAc; and 2) p-GlcNAc-lactate gel (4% p-GlcNAc-
lactate, formulated in propylene glycol and water).
The effectiveness of these p-GlcNAc materials was
compared to Gelfoam"" (Upjohn Company) .
Each material was.tested three times (twice in
20~ the spleen and once in the liver). Both of the p-
GlcNAc based materials exhibited an effectiveness in
controlling bleeding within the first minute after
application which was comparable to that of Gelfoam~".
The p-GlcNAc based materials have additional
advantages. Specifically, the p-GlcNAc materials do
not need to be held in place during the procedure, may
be left in the body, where they will be resorbed
within two to three weeks (GelfoamTM is not indicated
for this purpose), are compatible with both general
and minimally invasive surgical procedures.
Next, the efficacy of p-GlcNAc based materials in
the control of bleeding in small blood vessels was
studied, and compared against commercially available
hemostatic products.
CA 02372026 2002-02-28
11~
Each material was tested five times (twice in one
of the animals and once in the other three anima~.s).
The p-GlcNAc membrane and gel formulations were easily
applied to the site and controlled the bleeding within
2 minutes. GelfoamTM, which had to be held in place in ,
order to perform its function achieved hemostasis
within the same 2 minute range as the p-GlcNAc
materials. Avitene~", a fibrous material made of
collagen, was difficult to handle and required more
ZO than five minutes to control the bleeding.
Thus, the results described herein demonstrate
that the p-GlcNAc materials tested here represent
effective, convenient hemostatic agents.
20. EXAMPLE: p-GlcNAc DRUG DELIVERY SYSTEMS
Described herein are studies demonstrating the
successful use of p-GlcNAc materials to deliver anti-
tumor drugs to the site of malignant skin cancer and
colon cancer tumors such that the delivered anti tumor
drugs exhibit a therapeutic impact upon the tumors.
20.1 MATERIALS AND METHODS
p-GlcNAc-lactate drucr delivery compositions:
Mixtures of 5'-fluorouracil (5'-FU) and p-GlcNAc-
lactate were formulated as follows; 0.5mL of 5'-FU
(50mg/mL) was mixed with 0.5mL of propylene glycol,
and 2.OmL of 4% p-GlcNAc-lactate was added and mixed.
The p-GlcNAc-lactate was produced using the techniques
described, above, in Section - . Even after
extensive mixing, the 5'FU did not completely dissolve
into the p-GlcNAc-lactate gel. Assuming complete
mixing, the final concentration of 5'-FU would be
6.25mg/mL.
Mixtures of mitomycin (Mito) and p-GlcNAc-lactate
were formulated as follows; 0.5mg of Mito (lyophilized
CA 02372026 2002-02-28
- 111 -
powder) were dissolved in 5m1 of propylene glycol, and
0.5m1 of the Mito solution was mixed with 0.5mL of
MPT's 4% p-GIcN-lactate preparation to give a final
Mito concentration of 0.2mg/ml and a final p-GlcNAc-
lactate concentration of 2%. The materials were
compatible, with the Mito dissolving easily into~the
p-GlcNAc-lactate gel.
g-GlcNAc membrane 5'FU deliverv compositions:
Samples of 5'-fluorouracil (5'-FU) were immobilized
into discs of pure p-GlcNAc membrane material produced
using the chemical separation method described, above,
in Section 5.3.2, with hydrofluoric acid being
utilized as the chemical reagent. Each disc described
here had a diameter of l.Scm, as described here.
For the preparation of high dose (HD) discs,
0.64mL of a SOmg/mL solution of 5'-FU was mixed,with
suspensions containing approximately 8 mg of pure
p-GlcNAc. The mixtures were allowed to stand for
several hours to promote the absorption of 5'-FU into
20. the p-GlcNAc, and were then dried at 55°C for 3.5
hours. The resulting HD discs contained a total of
32mg 5'-FU, which is equivalent to approximately twice
the normal total 14 day dose of 5'-FU typically given
to a cancer patient,
Low dose (LD) 5'-FU containing p-GlcNAc discs
were prepared in the same manner, except that the LD
discs contained l7mg of 5'-FU, an amount equivalent to
equal the normal total human dose for a 14 day period,
normalized to the weight of the experimental mice
based on the typical dose of 5'-FU per Kg body weight
for humans.
Test Animals: For the 5'FU study, SLID (severe
combined immunodeficiency) mice were inoculated with
subcutaneous flank injections of HT-29 colon cancer
cells (ATCC; 1X105 cells per inoculum) obtained by
CA 02372026 2002-02-28
- 112 -
standard tissue culture methods, in order to produce
HT-29 colon cancer tumors. These injections led to
palpable tumors which were harvested in 14-21 days.
Tumors were dissected and necrotic tissue was cut
away. The HT-29 colon cancer tumors were sliced into
3x3x3mm pieces.
The experimental SCID mice were anesthetized via
intra-peritoneal injections with a standard dose of
avetin, and a slice of HT-29 colon caper tumor was
implanted onto the cecum of each mouse. Specifically,
each mouse was surgically opened to expose its abdomen
and the cecum was located, which was nicked with a
scalpel to make a small incision. A 3x3x3mm tumor
slice was sutured over the incision onto the cecum
using 5.0 silk sutures. The abdomen was then closed
using Clay Adams staples.
All mice were caged singly and fed for two weeks.
All mice were healthy and none had obstructed colons
at the end of the two week period.-w
=On day 14, each mouse was anesthetized, and its
abdomen was reopened. The growing tumors were
measured (Length. and horizontal dimensions). Tumors
were then treated with the p-GlcNAc/anti-tumor drug or
were used as controls.
Six mice were used for the p-GlcNAc-lactate 5'FU
study, and 15 mice were used for the p-GlcNAc membrane
5'FU study.
For the mitomycin study, nine SCID mice were
inoculated with sub-cutaneous injections of A431
squamous cell skin cancer cells (ATCC; 1X105 cells per
inoculum). Tumors resulted in all mice within 14
days.
Treatments: For the p-GlcNAc-lactate 5'FU study,
animals were treated once daily by "painting' the 5'-
fluorouracil (5'-FU)-containing p-GlcNAc gel mixture
CA 02372026 2002-02-28
- 113 -
onto the skin area over the tumor mass. Measurements
of the tumor size were obtained daily. Control
animals included animals treated with p-GlcNAc alone,
without 5'-FU, and animals which received no
treatment.
For the p-GlcNAc membrane 5'FU study, the HT29
colon tumors in the SLID mice were~treated by
surgically implanting discs of the drug-containing p-
GlcNAc membrane material directly onto their surface,
after having allowed the tumor to grow on the colon
for 14 days. Mice were sacrificed 14 days following
the implant procedure . Measurements of tumor volumes
were made immediately prior to implanting the drug-
containing p-GlcNAc membranes on day 0 and at the
termination of the experiment on day 14. Control
animals included ones treated with the p-GlcNAc
membrane without 5'-FU, and controls which received no
treatment: Additionally, two animals received daily
systemic injections of 5'-FU in doses equivalent Go
the HD and LD regimen.
For the p-GlcNAc-lactate Mito study, animals were
treated daily as in the p-GlcNAc-lactate 5'-FU study,
with 3 animals being treated with the Mito containing
mixture. In addition, 3 animals were treated with p-
GlcNAc minus Mito, 2 animals received no treatment,
and 1 animal received propylene glycol.
20.2 RESULTS
20.2.1 p-GlcNAc-LACTATE 5'FU
Experiments designed to study the effect of p-
GlcNAc-lactate 5'FU drug delivery systems on tumor
size were conducted, as described, above, in Section
20.1.
The largest length and width dimension were
measured for each tumor and the cross-sectional area
CA 02372026 2002-02-28
- 114 -
10
20
30
using these dimensions was calculated. The cross-
sectional area values are shown in Table X, below.
CA 02372026 2002-02-28
- 115 -
Table X
Animal # Treatment Tumor Size (em2)
Day O Day Day 11 Day 15
4
1 CL + 5FU 63 90 168 156
2 CL + 5FU 48 56 70 88
3 CL 21 36 88 108
Control
4 CL 58 110 150 195,30
Control
5 Nothing 40 64 132 234
6 Nothing 28 42 100 132
% Increase
in
Size
Day 0 Day Day 11 Day 15
4
1 CL + SFU 0 43 167 147
2 CL + 5FU 0 17 47 84
3 CL 0 71 319 414
Control
4 CL 0 90 160 289
Control
5 Nothing ~ 0 61 232 488
6 Nothing 0 48 253 366
The data comparing p-GlcNAc-lactate 5'FU
treated animals with controls are shown in FIGS. 27-
28. The data summarized in Table X and~FIGS. 27-28
clearly suggest that the HT-29 subcutaneous tumors in
the rats treated with the 5'-FU containing p-Gl.cNAc-
CA 02372026 2002-02-28
- 116 -
lactate gels have a significantly retarded rate of
growth compared to controls. Their growth has been
slowed 2.5-fold in comparison to the p-GlcNAc-lactate
gel controls and 4-fold compared to the no treatment
controls.
20.2.2 p-GlcNAc-LACTATE MITO
Experiments designed to study the effect of p
GlcNAc-lactate 5~FU drug delivery systems on tumor
size were also conducted, as described, above, in
Section 20.1.
The largest length and width dimensions were
measured for each tumor and the cross sectional area
using these dimensions was calculated. The cross-
sectional area values Were as shown in Table XI,
below.
25
'
CA 02372026 2002-02-28
- 117 -
Table XI
Animal # Treatment Tumor Size (cm~)
'Day 0 Day 3 Day 5 Day 8
1 pGlcN-L + 23 23 42 49
Mito
2 pGlcN-L + 23 16 54 63
Mito
3 pGlcN-L + 72 99 Term Term
Mito
4 pGlcN-L 27 54 140 203
control
5 pGlcN-L 30 54 96 140
control
6 pGlcN-L 30 58 200 221
control
7 Nothing 48. 75 126 300
8 Nothing . 44 80 20? Dead
9 Propyl 4 9 8 6 18 0 21:
ene 6
glycol
% Increase in Size
Day 0 Day Day 5 Day
3 8
1 pGlcN-L + 0 0 83 . 135
Mito
2 pGlcN-L + 0 -30 135 174
Mito
3 pGlcN-L + 0 38 Term Term
Mito
CA 02372026 2002-02-28
- 118 -
4 pGlcN-L 0 100 419 652
control
pGlcN-L 0 80 220 367 -
5 control
6 pGlcN-L 0 93 567 ~ 637
control
7 Nothing 0 56 163 525
8 Nothing 0 82 370 Dead
9 Propylene 0 76 267 341
glycol
'the data comparing p-GIcNAc-lactate Mito treated
animals with controls are shown in FIGS. 29-30. The
data summarized in Table XI and FIGS. 29-30 clearly
suggest that the tumors growing in the rats treated
with the Mitomycin-containing p-GlcNAc-lactate gels
animals have a significantly retarded rate of. growth.
Their growth was been slowed by 4-fold in comparison
to the p-GlcNAc-lactate gel controls and.4-fold
compared to the no treatment controls:
20.2.3 p-GIcNAe MEMBRANE 5'FU
Next, experiments designed to study the effect of
p-GlcNAc membrane 5'FU drug delivery systems on skin
cancer tumor size were conducted, as described, above,
in Section 20.1.
The tumor volume data obtained during the study,
including percent change in volume caused by the
different treatments, is summarized in Table XII,
below. Tumors were assumed to be cylindrical in '
shape: Their volumes were determined by measuring
their width and length, and using the following
CA 02372026 2002-02-28
- 119 -
equation: V=~rr2l, where the radius r is 0.5 times the
width and 1 is the length.
10
20
30
CA 02372026 2002-02-28
- 120 -
m
N
-.r
. b ,
. 'C3
y_
41 t~ n O
O
ro . ~ O o0 O O M O~ O O M '~; O N
' ' '
' M
~, 00 O ~O O .-~ N 'O M . p
U N ~O M cn ~'o N M N
p ; M
. ,
.
r--i . .
ro .
-rlGI : :
~
, '
o ro~ ~ ' . '
-r1~ ~ o o ~ ~n: o N oo ~c'
~ ~
ro ~i N M h . .. ~ ~ N
i~ ..
v
~1 O i . .
~
H i0
1
H O W ' . '
a~
~
E ~ ~~
+ ~o ~ . .
ro" M tn Ov O~
M l~ ~ l'~ ~ tODN ~ , ~ _
O .
~' . . .
V
U ~i . O O '
U U
'
x x x x .' A A A D : Q ~ .
E w w w w ~ ~ w w : ~ ,,~
; u u .
. . ..
,
o ~ ~n v~ ~n~ N ~n in ~n v~ o a, o.
G7. o .r ~a
~~ N M et~ A h vD I~ 00~ O Cv
.~ y 3 ,~ U
a ,
~ x o ~ ~ a
~. z
ro
~ c~;, ~ w ~ ~ ~ ..
Q a ai ' >
Q v
CA 02372026 2002-02-28
- 121 -
M M
N it ~..y.r
O O
O O b 'fl
V V
~
~
_
_
0 . ~ ~O
'
N'
h . ~ ~ ..M..n
lr"
O
U
..
'
N
Q
Ew v N
, v v v
O
w,
O
O
U
0
a a
::.
a .
e~ N
a . ,5 0 0
~. ~ A A ~
o E~ E~ . N
~ 3 3 .c
.
~ ~
o z z ~ ~ x
U i ~
..rN ~ e'~M
~
O ~ ~ ~ y .-~~ ~-
r
O _
.O
Q
'
>
H
w
a Q w
CA 02372026 2002-02-28
- 122 -
FIG. 31 summarizes a portion of the data
presented, above, in Table XII. as shown in FIG. 31,
the data strongly suggest that tumors treated with the
high dose (HD) 5'-FU-containing p-GlcNAc membranes
have stopped growing and have, in all cases, actually
gotten significantly smaller. The low dose (LD)~
polymer materials resulted in disease stability and
slight decrease in tumor size. In contrast, the
tumors in the control animals continued to rapidly
increase in size. It is interesting to note that two
of the three control animals which were treated via IV
died during the study, indicating that systemic
delivery of the equivalent amount of 5'-FU is lethal,
whereas site-specific delivery via the p-GlcNAc
polymer is efficacious in ridding the animal of the
disease.
20.3 CONCLUSION
The data presented in-this Section strongly
suggest that the site-specific delivery of anti-tumor
drugs has a positive effect, in_retarding and reversing
tumor growth. Successful results were obtained using
p-GlcNAc drug delivery compositions produced having
two different formulations, namely p-GlcNAc-lactate
and p-GlcNAc membrane formulations. Further,
successful results were obtained using two different
anti-tumor drugs, 5'-FU and Mito. Thus, the p-GlcNAc
drug delivery systems of the invention exhibit anti-
tumor activity, useful, for example, in the delivery
of drugs specifically to the site of the tumor cells
of interest.
It is apparent that many modifications and
variations of this invention as set forth here may be
made without departing from the spirit and scope
CA 02372026 2002-02-28
- 123
thereof. The specific embodiments described above are
given by way of example only, and the invention is
limited only by the terms of the appended claims.
10
20
30