Note: Descriptions are shown in the official language in which they were submitted.
CA 02372920 2001-11-06
WO 00/70124 PCT/FI00/00440
1
Method of providing electrochemical prevention
of corrosion in changing conditions
The invention relates to a method of providing electrochemical prevention of
corrosion in changing conditions.
The method may be used in providing both cathodic and anodic corrosion
prevention
systems.
Electrochemical corrosion prevention refers to a method where the
electrochemical
surface potential of a metal surface being protected is changed, i.e.
polarized in an
advantageous direction by passing electrical current to the surface. The
electrical
current may be derived either from less noble i.e. sacrificial electrodes
galvanically
connected to the object being protected or alternatively from an external
source of
direct current supplied through a separate electrode.
Electrochemical surface potential is measured by a reference electrode which
is
2 0 placed close to the surface to be protected and galvanically insulated
from the
surface. Many types of reference electrodes are known and their usefulness
depends
on the chemical and physical properties of the prevailing electrolyte. A
characteristic
of a usable reference electrode is a specific potential that remains
relatively constant
in operating conditions.
The direction of polarization which is advantageous with respect to
electrochemical
protection may be sought either in the potential range of an optimally dense
oxide
layer or alternatively in the immune range of a metal that is being protected,
i.e. in
the potential range in which the metal atom is thermodynamically stable and
thus
3 0 does not corrode.
CA 02372920 2001-11-06
WO 00/70124 PCT/FI00/00440
2
In the most commonly known situations, such as, for example, the protection of
carbon steel in soil or in sea water, protection potentials are well know and
constant.
In relatively stable corrosion conditions, optimal protection potential can be
determined using known corrosion examination methods, such as, determination
of
a polarization curve, potentiostatic weight loss tests or resistance
measurements of
an oxide layer.
By using sacrificial anodes, the potential of the protected object can be
affected only
by varying the number and location of the anodes. In potentiostatic protective
systems accomplished by means of an external current source, the potential of
the
object is measured and it is sought to be maintained as close as possible to a
preselected target potential by automatically changing the current supplied by
the
current source.
The potentiostatic corrosion prevention method is well-known and various ways
of
its application are described, for instance, in US patents 4, 528, 460 and 4,
713,158. In
the corrosion prevention method in accordance with US patent 4,713,158,
potential
is allowed to move within predetermined limits.
2 0 In processes where conditions clearly change as a function of time, the
potentiostatic
method cannot be applied without risk. If certain chemical or physical
variables
affecting corrosion reactions, such as, for example, pH, the proportion of
aggressive
ions, or temperature change considerably, it may lead to a situation where
there exists
no optimal protection potential covering the entire process period.
Moreover, the potentiostatic method does not operate in the best possible way
in an
environment where protection is based not on an absolute potential value but,
for
example, on realized polarization.
3 0 In connection with those metals in particular whose resistance to
corrosion is based
on formation of a protective oxide layer, protection potential is greatly
affected by
environmental factors. Moreover, the optimum range of the protection potential
of
CA 02372920 2001-11-06
WO 00/70124 PCT/FI00/00440
3
a metal that has reached the limits of its resistance to corrosion is
generally very
small, and thus there is hardly any margin of error.
In all advanced potentiostatic corrosion prevention systems it is possible to
determine
protection potential freely, but it always requires that the operator takes an
active part
in determining and accomplishing a change. If the changes are rapid, the
control of
protection takes too much working time and is therefore almost impossible. If
the
changes occur irregularly and infrequently, the risk of failing to detect them
increases. Moreover, the operating personnel of factories and plants do not
usually
have sufficient skill to perform the necessary measurements and draw the
necessary
conclusions of the need for change.
Conditions which are difficult to control or which cannot be controlled by
means of
conventional potentiostatic corrosion inhibition include almost all batch
processes of
the chemical industry. In batch processes, the changes in conditions may be
dependent on time or alternatively the change is triggered by some process
quantity,
such as, temperature or the start of chemical supply. In continuous processes,
the
discontinuities causing problems include, for example, changes in the type of
product
that is being produced. In them, the change is triggered by a change in the
source of
2 0 raw material. By the potentiostatic method it is also difficult to control
the cathodic
protection of reinforcement steels, in which, instead of an absolute potential
value,
the magnitude of a change caused in potential is generally used as the
criterion for
protection.
2 5 It is true that corrosion examination methods offer a possibility of
monitoring the
development of corrosion aggressiveness also such that optimal protection
potential
can be determined at short time intervals. However, the examinations have
typically
been momentary tasks which relate to the determination of potentiostatic
protection
and which have been performed in the laboratory on a sample taken from a
process.
3 0 Continuous corrosion examination of the monitoring type directly carried
out in the
process environment is noticeably uncommon and until now there has not even
existed any need for it because in a stable environment a limited sampling
rate
CA 02372920 2001-11-06
WO 00/70124 PCT/FI00/00440
4
suffices to get an idea of the environment. It is, however, already possible
to measure
in real time a number of process variables affecting corrosion reactions.
In other words, regarding the current state of the art, it must be noted that
although
the far advanced potentiostatic corrosion inhibition system operates very well
in
stable conditions, for changing conditions there exist no method and apparatus
which
automatically adapt to changes.
An object of the invention is to provide a method in which the prevention of
corrosion adapts its operation to changing corrosion conditions automatically
without
delay. A more specific object of the invention is to provide a method that can
utilize
both the measurement data of process variables and corrosion measurement data
generated by the method itself, and change the current or voltage supply to
the
corrosion prevention provided by an external current source in such a way that
optimal protection potential for conditions prevailing at any given time is
achieved.
A broader object of the invention is also to be able to utilize data measured
from the
process and processed on the bases of corrosion directly or indirectly for
control of
the process such that the corrosion aggressiveness of the process diminishes.
2 0 The objective of the invention is achieved by a method which is
characterized in that
in the method:
(a) at least one detector device is placed in contact with the electrolyte
present in
an object that is being protected such that the detector device is
electrically
2 5 insulated from the object that is being protected;
(b) the electrochemical properties of said electrolyte or the properties of
said
electrolyte affecting the rate of corrosion reactions are measured by said
detector device at time intervals shorter than the time it takes for the
electrolyte
3 0 to change significantly in terms of corrosion;
CA 02372920 2001-11-06
WO 00/70124 PCT/FI00/00440
(c) the measurement results of said detector device are passed to a measuring
and
data processing unit, and a new optimum potential is determined on the basis
of said measurement results; and
(d) the current or voltage supplied by a current source for corrosion
prevention
5 is changed such that said new optimum potential is achieved.
In the method in accordance with the invention, the inventors have realized
the
possibility to apply commonly known electrochemical and other methods of
measuring quantities that affect the rate of corrosion reactions to the
determination
of an optimum potential for corrosion prevention accomplished by means of an
external current source in such a way that the optimum potential is
continuously
redetermined during operation, thereby making it possible to automatically
adapt to
changing corrosion conditions. In that case, we may speak of potentiodynamic
corrosion inhibition. In the case where process sequences are repeated
unchanged in
their corrosion conditions, the data from measurements performed previously
may
be utilized in determining a new optimum potential.
In the method in accordance with the invention, a corrosion prevention control
system redetermines and changes the optimum potential for corrosion prevention
2 0 when the corrosion conditions change. The optimum potentials are
preferably
determined by electrochemical corrosion examination methods, such as, for
example,
a polarization curve run, linear polarization resistance or CER (Contact
Electric
Resistance). In protection of reinforcement steels, in redetermining optimum
potential, a depolarization test is preferably used in which the realized
polarization
2 5 of the steels is measured by switching off the protection current for a
given period of
time and measuring the depolarization from which the voltage drop (IR drop)
caused
by electric field and the specific resistance of the electrolyte has been
eliminated. The
determination of optimum potential in accordance with this invention is not
limited
to the above-mentioned methods, but all electrochemical corrosion rate
3 0 measurements can be made use of for the purpose of the invention. In the
cases where
corrosion conditions have a clear correlation with a given process variable or
variables that can be unambiguously measured, such as, for example, pH,
temperature
CA 02372920 2001-11-06
WO 00/70124 PCT/FI00/00440
6
or the concentration of some electrolyte component, the measurement of said
process
variable may be used for controlling the change of optimum potential. Thus,
the
automatic change of the optimum potential in corrosion prevention during
operation
in accordance with the invention does not necessarily require electrochemical
corrosion measurements during operation.
For the purpose of determining optimum potentials during operation, at least
one
detector, preferably several detectors, is/are fitted in the object to be
protected such
that the detectors will be in contact with the electrolyte present in the
object that is
being protected. The detectors are electrically insulated from the object that
is being
protected and they are so designed that they withstand the chemical and
physical
conditions of the electrolyte. Measurement electronics and a data processing
unit may
be situated in connection with the detector device or, in the case of several
detector
devices, centrally such that one data processing unit operates several
detector devices.
The determination of optimum potential may take place at given preset
intervals that
can be selected or the determination can be triggered when a given process
variable
affecting corrosion conditions in a known fashion changes. In the case where
process
sequences are repeated unchanged in their corrosion conditions, the optimum
2 o potential values determined previously may be utilized. Said optimum
potential
values may also be determined by a periodic corrosion examination in the
process or
at the laboratory.
The method in accordance with the invention is suitable for controlling all
corrosion
2 5 prevention systems accomplished by means of an external current source,
most
advantageously when the corrosion conditions in the environment of the object
to be
protected vary considerably.
The invention will be described in detail with reference to an advantageous
embodi-
3 0 ment of the invention shown in the figures of the accompanying drawings,
to which
embodiment the invention is, however, not intended to be exclusively confined.
CA 02372920 2001-11-06
WO 00/70124 PCT/FI00/00440
7
Figure 1 graphically shows examples of polarization curves in changing
conditions
when variable process quantities are pH and CI' content.
Figure 2 is a schematic view of an advantageous embodiment of a measurement
system used in the method in accordance with the invention.
In Fig. 1 it is seen how polarization curves change with changing process
conditions.
At the point of time 0, pH and C1G content are on the level shown in Fig. 1.
At the
point of time t, the C1G content drops, at the point of time t, the pH drops,
at the
point of time t3 the C1G content rises, and at the point of time t~ the C1G
content drops
again. Beginning from the point of time t,, the pH remains constant.
It is seen in Fig. 1 that in the time period 0 - t, the value of optimum
potential B is on
the level shown on the left-hand side of Fig. 1. In a corresponding way, the
point A
of the polarization curve indicates the point having a maximum corrosion rate,
i.e.
current I~o,r is at the maximum. When the potential exceeds a point C on the
polariz-
ation curve, metal becomes susceptible to pit corrosion. It is seen in Fig. 1
how the
polarization curves change such that the optimum potential B, the point A
repre-
senting the maximum corrosion rate and the point C representing the onset of
pit
2 0 corrosion change with changing process conditions pH, C1G. It is also seen
in Fig.
1 that, for example, the optimum potential usable at the time interval t,-t3
causes pit
corrosion at the time interval t3-t4 when the conditions have changed.
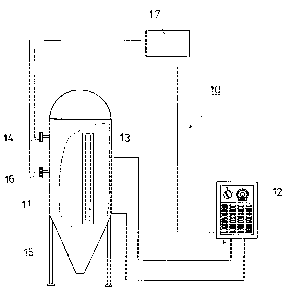
In Fig. 2, the measurement system in accordance with the invention is
generally
2 5 denoted with the reference numeral 10. The measurement system 10 comprises
a
direct current source 12, a current supply electrode 13, a measuring detector
14, and
a measuring and data processing unit 17. The object to be protected, which in
this
embodiment is a container containing a process liquid 15, is denoted with the
reference numeral 11. In the embodiment shown in Fig. 2, the measuring system
10
3 0 additionally comprises a measuring detector 16, which may measure any
process
variable or process variables, such as, for example, pH, temperature T,
concentration
CA 02372920 2001-11-06
WO 00/70124 PCT/FI00/00440
8
C, etc. Thus, the measuring detector 16 measures the chemical and physical
properties of the process liquid 15.
In the method in accordance with the invention, the detector device 14 is
placed in
contact with the electrolyte 1~ present in the object 11 that is being
protected in such
a way that the detector device 14 is electrically insulated from the process
equipment.
The detector device 14 serves to measure the electrochemical properties of the
electrolyte or its properties affecting the rate of corrosion reactions at
time intervals
shorter than the time it takes for the electrolyte to change significantly in
terms of
corrosion. The detector device 14 also measures the electrochemical potential
of the
protected object 11. The measurement results of the detector device 14 are
passed to
the measuring and data processing unit 17, and a new optimum potential B is
determined based on the measurement results. The measuring and data processing
unit 17 delivers a control signal to the current source 12, and the current or
voltage
supplied by the current source is changed such that the new optimum potential
B is
reached. In addition, the measurement results of the measuring detector 16 are
passed, if needed, to the measuring and data processing unit 17.
The invention allows the target potential of electrochemical corrosion
prevention to
2 0 be automatically changed when changing corrosion conditions or the
fulfilling of the
criteria for protection so require. The method in accordance with the
invention may
be employed in providing both cathodic and anodic corrosion prevention
systems.
The invention also makes it possible that the data measured in the process and
processed on the bases of corrosion can be utilized directly or indirectly for
control
2 5 of the process such that the corrosion aggressiveness of the process
diminishes.
The embodiment shown in Fig. 2 illustrates cathodic protection. If the +
terminal and
the - terminal in the embodiment shown in Fig. 2 are exchanged with each
other, the
protection in question is anodic protection.
CA 02372920 2001-11-06
WO 00/70124 PCT/FI00/00440
9
Above, only one advantageous embodiment of the invention has been described,
and
it is obvious to a person skilled in the art that numerous modifications may
be made
thereto within the inventive idea stated in the accompanying claims.