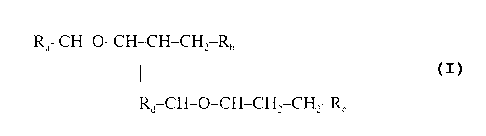Note: Descriptions are shown in the official language in which they were submitted.
CA 02373630 2001-11-13
WO 00/67726 PCT/US00/12743
HOST-GUEST PROCESSES AND FORMULATIONS FOR
DELIVERING BIO-AFFECTING COMPOUNDS
FIELD OF THE INVENTION
The present invention relates to processes and formulations capable of
protecting,
stabilizing, and/or delivering one or more bio-affecting compounds. More
particularly,
the invention relates to processes of making a composition having a host
compound
capable of accepting one or more bio-affecting guest compounds and new
compositions
formed by the processes. The processes are particularly useful in formulating
compositions for the topical delivery of the bio-affecting compounds.
BACKGROUND OF THE INVENTION
Biological systems depend on a balance between water-soluble compounds and
fat-soluble compounds. Frequently, natural enzymatic reaction will convert a
compound
to help maintain the balance between water-soluble compounds and lipid-soluble
compounds in the biological system, for example, ascorbyl palmitate, which is
lipid
soluble, can be converted by enzymatic action to ascorbic acid, which is water
soluble.
The flow of both water-soluble and lipid-soluble compounds into and out of
biological systems are controlled by cell membranes. To penetrate a membrane,
a
compound needs to have an appropriate structure. In addition, the transfer of
the
compound across a cell membrane is governed by enzymes, pH, and salt balance.
Thus,
the cell membranes also help maintain the balance between water-soluble
compounds and
lipid-soluble compounds in the biological system.
The formation of compositions capable of delivering a compound to a cell
membrane and in a structural form or environment that will encourage the
transfer of the
compound across the cell membrane into the biological system has been the
subject of
considerable research using many different approaches.
For example, it is known that ascorbic acid (Vitamin C) can be beneficial for
healing the skin. In a composition for topical application to the skin, a
relatively high
concentration of ascorbic acid, preferably at least 8% by weight of the
composition, is
desirable, and perhaps necessary, to be effective in penetrating the dermal
layer and
-1-
CA 02373630 2001-11-13
WO 00/67726 PCT/US00/12743
activating collagen in the skin. When the ascorbic acid composition is exposed
to air,
however, and particularly at such high concentrations, the ascorbic acid tends
to rapidly
oxidize. A stabilizing environment for the ascorbic acid is necessary to
protect it from
oxidation or the composition will lose its effectiveness.
It is also known that alpha tocopherol (Vitamin E) can be beneficial for
healing
and/or preventing damage to the skin by scavenging free radicals in the
biological system.
In a composition for topical application to the skin, a relatively high
concentration of
alpha tocopherol, preferably at least 5% by weight of the composition, is
desirable, and
perhaps necessary, to be effective in penetrating the dermal layer and
reducing free
radicals. When the alpha tocopherol composition is exposed to air, however,
the alpha
tocopherol tends to oxidize, that is, become rancid. A stabilizing environment
for the
alpha tocopherol is necessary to protect it from becoming rancid or the
composition will
lose its effectiveness.
Considerable research has been conducted on stabilizing ascorbic acid, which
is
water soluble, and on stabilizing alpha tocopherol, which is lipid soluble.
Because of their
widely-different solubility characteristics, however, obtaining high
concentrations of both
in the same composition continues to be a particular challenge. Many
compositions for
topical application contain low concentrations of both ascorbic acid (as a
preservative)
and alpha tocopherol (as an antioxidant) at levels below 0.5% by weight. At
these low
concentrations, however, the ascorbic acid and alpha tocopherol are much less
effective
in repairing skin damage.
Furthermore, compositions having high concentrations of certain bio-affecting
compounds, such as ascorbic acid, have been particularly difficult to
stabilize. In this
regard, standard stability test procedures that are used to determine the
shelf life of a
product do not tell the whole story. A standard stability test is conducted at
elevated
temperatures and humidity is commonly used for determining the shelf life of a
composition. Because the rates of chemical reactions and the growth of
bacteria tend to
double with each 10°C (18°F) increase in temperature, testing
the stability of a product
at elevated temperatures can be used to calculate its expected shelf life at
ordinary
3o temperatures with a reasonable degree of confidence. The standard test
requires the
compound to be placed in the sealed container in which it is to be sold or
stored, for 30,
60, and 90 days at 31°C (87°F) and at 45°C (113°F)
in a chamber at 80% relative
-2-
CA 02373630 2001-11-13
WO 00/67726 PCT/US00/12743
humidity. This test does not, however, determine the stability of the product
after the
sealed container has been opened. Unfortunately, many products that pass the
shelf-life
stability test become unstable in a much shorter period of time once the
container has been
opened, and quickly lose effectiveness, and worse, may allow the growth of
pathogenic
bacteria.
Another type of problem encountered in designing topical delivery systems for
bio-
affecting compounds is encountered when the bio-affecting compound is
virtually or
totally insoluble in either water or lipids. For example, bio-affecting
ingredients for ultra-
violet sun block protection include zinc oxide (Zn0) and/or titanium dioxide
(TiO~),
to which can be used for blocking UV-A and/or UV-B radiation, respectively.
Unfortunately, it is difficult to find acarrierforevenly dispersing these
insoluble inorganic
compounds in a sufficient concentration to form a protective layer over the
skin without
also imparting a whitening color, which most people find aesthetically
undesirable.
There has been a long-felt need for a process of formulating a composition
that
would be capable of stabilizing at least two different bio-affecting compounds
having
diverse solubility characteristics. Such a process can be used to stabilize
only one bio-
affecting compound, but it is expected to have particularly beneficial use
when it is
desirable to prepare a composition having at least two different bio-affecting
compounds
of diverse solubility characteristics. There has also been a long-felt need
for a process of
formulating a composition that is capable of protecting and stabilizing high
concentrations
of certain bio-affecting compounds that have been particularly difficult to
stabilize at such
high concentrations. In addition, there has been a particular long-felt need
for a process
of formulating a composition that is capable of remaining stable for long
periods of time
even after the container has been opened causing the composition to be exposed
to the
ambient air environment. It would also be desirable to produce a composition
that
discourages the growth of bacteria. These problems have been particularly
acute with
respect to compositions and products that are expected to be used over an
extended
period of time after the sealed container has been opened. By way of further
example,
there has been a long-felt need for a topical delivery system capable of
evenly dispersing
3o a bio-affecting compound that is insoluble.
-3-
CA 02373630 2001-11-13
WO 00/67726 PCT/US00/12743
SUMMARY OF THE INVENTION
The formulation processes and compositions according to the invention depend
on the initial formation of a host composition having a host capable of
accepting a guest
in host-guest coordination. One or more bio-active compounds can then be mixed
with
the host composition for creation of a stable molecular environment, that is,
according to
a process of molecular stacking. For compositions including water, the
formulation
processes preferably include establishing a desired pH range to help maintain
the stability
of pH-sensitive compounds. A wide range of bio-affecting compositions can be
made
according to the general approach of the invention. In addition, specific
formulation
processes and compositions are provided.
According to a general aspect of the invention, a process is provided for
making
a host composition having a host for at least one guest, the process
comprising mixing,
in any order:
(i) a non-ionic surfactant selected from the group consisting of compounds
having a chemical structure:
' R~ CH-O-CH-CH-CH~ Rn
Rd CH-O-CH-CH2-CH2-R
where "-CH-O-CH-" represents an epoxide group,
where R~ and Rh are hydrocarbons that can be the same or different,
where at least one of the R~ and Rb hydrocarbons includes an epoxide
group within 3 carbons of the hydrocarbon attachment to contribute to the
desired hydro-lipid balance of 7 - 9,
where R~ is hydrogen or a methyl group, and
where Rd is a methylene group (-CH~ ), an ethyl group (-CHI CHI ), or
a structurally equivalent link with a bond length range about the same as
or shorter than that provided by an ethyl group, and
having a hydro-lipid balance in the range of 7 - 9,
or any combination of two or more thereof;
-4-
CA 02373630 2001-11-13
WO 00/67726 PCT/US00/12743
(ii) in a stoichiometric proportion of at least 1:6 relative to the non-ionic
surfactant, an amphoteric surfactant selected from the group consisting of
organic compounds having the chemical formula NH3-R-COOH, where
R is a straight, branched, or aromatic hydrocarbon structure having 6 - 24
carbons, or any combination of two or more thereof;
(iii) at least a sufficient amount of a solvent to dissolve the amphoteric
surfactant, the solvent comprising one or more compounds selected from
the group consisting of water, alcohols having straight or branched
hydrocarbon structure having up to 6 carbons, glycosamionoglucans, or
any combination of two or more of the foregoing;
(iv) in a stoichiometric proportion of at least 1:240 relative to the non-
ionic
surfactant, an aromatic selected from the group consisting of compounds
having at least one aromatic five or six-member ring structure, or any
combination of two or more thereof;
(v) in a stoichiometric proportion of at least 1:240 relative to the non-ionic
surfactant, of an aluminum canon;
(vi) in a stoichiometric proportion of at least 1:1200 relative to the non-
ionic
surfactant of at least one Lewis acid that is not a Bronsted-Lowry acid;
(vii) at least 0.003 molar concentration of at least one Bronsted-Lowry acid.
Without being limited by suggesting a theoretical explanation at the molecular
level for how these ingredients react to create a host composition, it is
believed that a
process according to this general approach is capable of producing a
composition having
one or more host molecular complexes such as crown ethers,
crystahemispherands,
calixerands, calixarenes, carcerands, rotoxanes or other host molecular
configurations
capable of forming a host-guest relationship with guest molecular structures,
which is
accomplished without the use of a guest transitional metal. Instead of a
transition metal,
it is believed that a five- or six-member aromatic ring structure is of the
appropriate
molecular size and provides the appropriate electron orbitals to coordinate in
the
formation of the host complex. After the initial formation of a host
composition having
a host complex, a bio-affecting compound, either organic or inorganic, can be
mixed with
the host composition according to the formulation processes for creation of a
stable
molecular environment, that is, according to a process of molecular stacking.
-5-
CA 02373630 2001-11-13
WO 00/67726 PCT/US00/12743
The particular compounds used can be varied according to the principles of the
invention dependent on the desired molecular stacking parameters for
stabilizing one or
more bio-affecting ingredients. For example, nonoxyl-9, which includes a
phosphate
group that can be considered to be a Lewis acid but not a Bronsted-Lowry acid,
can be
used for providing both the aromatic and the Lewis acid. Nonoxyl-9 has the
further
advantage of being a well-known germicide. By way of another example, in a
composition including water, aluminum sulfate, which in water forms a small
amount of
sulfuric acid, can be used for providing both the aluminum canon and the
Bronsted-Lowry
acid.
1o As will hereinafter be described in more detail, and illustrated by way of
representative examples, the relative proportions of these compounds can vary
considerably without departing from the scope of the invention.
According to a further aspect of the invention, one or more compounds are
selected to be sequentially mixed with the host composition to form a stable
molecular
environment, which is sometimes referred to herein as a process of molecular
stacking.
The sequence of mixing the additional bio-affecting compounds is based on the
following
factors:
(i) the one or more desired bio-affecting compounds to be added to create a
desired composition for a specific application; and
(ii) the desired point of attachment to the host complexes.
The systematic addition, i.e., molecular stacking, of the bio-affecting and
other desirable
ingredients into the host composition is also based on a consideration of the
following
factors:
(i) the molecular structure of each ingredient;
(ii) the solubility of each ingredient compound and the hydro-lipid balance of
the composition, and the possibility of changing the solubility
characteristics by changing the form of the ingredient, e.g., by using a salt
form of the ingredient;
(iii) in an aqueous composition, the effect of each ingredient on the pH, and
the sensitivity of each ingredient to pH; and
(iv) the temperature required for "setting" or "stacking" the ingredient into
the
host composition.
-6-
CA 02373630 2001-11-13
WO 00/67726 PCT/US00/12743
In formulation processes including water, the process preferably includes
establishing a desired pH range to help maintain the stability of pH-sensitive
compounds.
Establishing the desired pH range often has a substantial influence on the
selection of one
or more additional compounds to be mixed with the host composition and the
mixing
sequence.
Those skilled in the art will appreciate that a wide range of bio-affecting
compositions can be made according to the general approach of the invention.
In
addition, specific formulation processes and compositions are provided for
various bio-
affecting compositions, which compositions are highly effective for certain
biological
purposes, such as skin exfoliation, collagen activation in the skin, the
topical delivery of
salicylic acid, and other pain relievers to local areas of pain and/or
inflammation, to
promote skin healing processes, and other purposes, such as the delivery of
plant growth
hormones, such as diterpenes, or even the topical dispersion of UV radiation
blocking
compounds. Thus, the formulation processes according to the invention are
expected to
be useful in the production of a wide array of compositions having bio-
affecting purposes.
As used herein, water soluble means that a compound or mixture of compounds
has a solubility characteristic of at least 0.2 g/IOOg of distilled water at
standard
temperature and pressure. To the extent the compound or mixture of compounds
does
not meet this solubility criteria, it would be expected to be lipid soluble.
It is to be
understood that this bright-line criteria between water solubility and lipid
solubility is
arbitrarily assigned as a matter of clarity of definition, and that the
solubility characteristics
in relation to complex mixtures can be blurred by factors such as temperature,
pressure,
pH, chemical reaction, complex coordination, and mutual solubility. Of course,
some
compounds, particularly inorganic compounds, can be nearly or completely
insoluble in
both water and lipids.
For example, a process according to the invention can be used to formulate
specific compositions including one or more compounds that can be considered
to be
water soluble selected from the group consisting of: ascorbic acid, ascorbyl
salts, 7-
dehydroxy cholesterol, alpha-hydroxy acids, beta-hydroxy acids, glycolic
acids,
isoprenoids, bioflavinoids, fatty acids, glycosaminoglucans, flavin mono
nucleotides, flavin
mono nucleotide derivatives, diterpenes, glycerophospholipids, beta-carotene,
trans
CA 02373630 2001-11-13
WO 00/67726 PCT/US00/12743
retinol, traps retinoic acid, allontoin, nonoxyl-9, betaine, and any
combination of two or
more of the foregoing.
A process according to the invention can be used to formulate specific
compositions including one or more compounds that can be considered to be
lipid-soluble
selected from the group consisting of: alpha tocopherol, alpha tocopherol
ester, co
enzymes, ubiquinones, menaquinones, phylloquinones, 7-dehydroxy cholesterol,
steroids,
bioflavinoids, terpenes, saponified fatty acids, unsaponifiedfats,
glycerophospholipids, and
any combination of two or more of the foregoing.
Similarly, the host complexes can be used for a variety of formulations that
do not
require direct activation of ingredients in response to the system in which
they are
introduced. Rather, the stabilization of crown complexes can provide an
alternative at the
other end of the spectrum of products. By way of further example, a process
according
to the invention can be used to formulate specific compositions including one
or more
compounds that can be considered to be nearly or completely insoluble in
either water and
lipids, such as inorganic compounds, and more particularly, titanium dioxide
and/or zinc
oxide. The processes according to the invention allow for the inclusion of non-
hydrocarbon chemicals that are bio-affecting as not only resistant to external
forces (like
UV radiation) within mammalian systems, but also provide the consistent
ability to release
other organic products as a resultant of the interacting system.
It is important to note, however, that solubility characteristics of chemical
compounds are typically reported based on studies of a purified form of the
particular
chemical. Many naturally-occurring chemicals are found and extracted in
conjunction
with derivatives that substantially affect solubility of the naturally-
occurring mixtures.
_g_
CA 02373630 2001-11-13
WO 00/67726 PCT/US00/12743
General Objects of the Invention
The invention has one or more of the following illustrative objects, which are
not
intended to limit the invention to being able to accomplish all of the
following objects:
(a) to provide a host composition having a host capable of accepting at least
one bio-affecting guest for use in topical delivery of the bio-affecting guest
to the
mammalian skin/dermal layer or plant membrane of a target biological system;
(b) to provide a host composition capable of stabilizing a sufficiently high
concentration of one or more bio-affecting compounds to activate the natural
biochemical
pathways after delivery to and penetration through the mammalian skin/dermal
layer or
plant membrane of a target biological system;
(c) to create a molecular environment that is capable of protecting and
stabilizing at least two bio-affecting compounds that have widely-different
solubility
characteristics;
(d) to create a molecular environment and coordinated complex that will
release one or more bio-affecting compounds to the membrane of a target
biological
system; andlor
(e) to provide specific formulations and compositions for the topical delivery
of the bio-affecting compounds.
DETAILED DESCRIPTION OF PRESENTLY PREFERRED EMBODIMENTS
The formulation processes and compositions according to the invention depend
on the initial formation of a host composition capable of accepting a guest in
host-guest
coordination. Thereafter, one or more bio-active compounds can be mixed with
the host
composition according to the formulation process for creation of a stable
molecular
environment for the bio-active compounds, that is, according to a process of
molecular
stacking.
_9_
CA 02373630 2001-11-13
WO 00/67726 PCT/US00/12743
A. Formulation of a Host Composition
1. Crown Ethers and Other Host Compounds
Crown ethers were first discovered by Charles Pederson in 1967. Crown ethers
act as ionophores trapping guest atoms or molecules that would otherwise not
be able to
transfer across membranes because the trapped molecule is not soluble in the
fatty
compounds found in the membrane. Traditionally, the guest has been a
transition metal.
Crown ethers tend to mimic enzymes in function because they act as carriers of
other
compounds across membranes. The most commonly occurring crown ether structure
in
biological systems is ferrechrome which has hemoglobin at the center of a C-N-
O ether
ring. Other forms of host molecules include, for example, crystahemispherands,
calixarenes, carcerands, and rotoxanes. It is important to recognize that all
these host
compounds are not necessarily cyclic in nature, but many are actually open
crowns that
are generally horseshoe shaped.
With the presence of transitional metals, crown ethers are more rigid and
interlocking similar to amino acid building blocks in DNA. Without the
presence of a
transitional metal, the crown ethers tend to be flat and soft, more like a
rubber band, and
without the presence of a well-defined cavity. To act as a host, a guest
molecule must be
present to give the crown ether or other host compound its shape and function.
Crown ethers are typically formed and stabilized with transitional metals and
acyl
or acetyl groups by establishing a catalytic reaction which allows for
synthesis of a crown
ether including repeating (-C-O-C-)" groups and stabilized by electrophilic
attraction.
The larger the transitional metal, the larger the crown ether formed with the
transition
metal.
After being formed, crown ethers and other forms of host complexes can attach
to cyclic and aromatic compounds. These host forms allow for attachment or
coordination of molecules having widely-different solubility characteristics.
Of course,
these host complexes can also allow for attachment or coordination with
inorganic
compounds, such as transition metal compounds, which may be virtually or
completely
insoluble in either water or lipids.
-10-
CA 02373630 2001-11-13
WO 00/67726 PCT/US00/12743
2. Surfactants
Surfactants in water can create lamellar configurations, vesicles, micelles,
and
reverse micelles which will incorporate compounds that have the opposite
solubility of the
chemical system, water in oil, oil in water. These configurations align the
polar end of a
molecule in one direction and the non-polar end in the opposite direction. The
specific
configuration will vary dependent upon the type of surfactant, the
concentration of the
surfactant, the compounds) involved, temperature, and mix order of the
compounds.
Generally, surfactants will create an emulsion or a micro-emulsion.
Surfactants also alter
the interfacial tension of the chemical system. Low temperatures and/or
elevated
pressures will cause separation of the oil and water phases in a surfactant
system.
Examples of surfactant systems are found naturally in biological systems, the
most
prominent is the lung surfactant containing 50% to 60% dipalmitoylphosphatidyl
choline.
The lung surfactant maintains high surface tension preventing collapse of the
alveoli upon
air expulsion from the lungs. The phophatidyl choline is a phopholipid which
will also
create electron orbitals that will share with both lipid and water-soluble
compounds. In
combination with certain bio-affecting compounds, the stability of that
compound is
lessened.
3. New Method of Making Host Compounds With Surfactants
According to the presently preferred embodiment of the invention, a
transitional
crown ether "host" can be formed in a what is referred to herein as a "host
composition."
The tdiversely-solublensitional crown ether allows for electrophilic
attachment of
diversely-soluble compounds.
A host complex is created by using a combination of non-ionic and amphoteric
surfactant in an aqueous environment with aluminum sulfate. The non-ionic
surfactant is
selected from the group consisting of compounds having the following chemical
structure:
R~ CH-O-CH-CH-CH~ Rb
Rd CH-O-CH-CHI CH, R
where "-CH-O-CH-" represents an epoxide group,
-11-
CA 02373630 2001-11-13
WO 00/67726 PCT/US00/12743
where Ra and Rb are hydrocarbons that can be the same or different,
where at least one of the R~ and Rb hydrocarbons includes an epoxide group
within 3 carbons of the hydrocarbon attachment to contribute to the desired
hydrolypid balance of 7 - 9,
where R~ is hydrogen or a methyl group, and
where Rd is a methylene group (-CHZ ), an ethyl group(-CHI-CHI ), or a
structudiversely-solublellyequivalentlink with abond length diversely-
solublenge
about the same as or shorter than that provided by an ethyl group, and
having a hydro-lipid balance in the diversely-solublenge of 7 - 9,
or any combination of two or more thereof.
It is believed that the epoxide group included with R~ or Rb is necessary to
contribute to
the desired hydrolypid balance of 7 - 9 of the non-ionic surfactant. More
prefediversely-
solublebly, R~ includes the required epoxide group. Most preferably, R~ is a
hydrogen.
Without being limited by this theoretical explanation, it is believed, and as
further
supported by conformational analysis and preliminary GC mass spectral analysis
data, that
the -CH-O-CH-CH, CHI-R~ group of this type of structure is susceptible to
being
cleaved from Rd, which cleaved group further reacts to provide the chemical
structural
building blocks for the creation of a host complex, probably a crown ether,
and probably
an 18-crown-6 ether. To form a perfect crown ether structure, R~ is most
preferably
hydrogen.
As used herein, "hydrocarbon" generally refers to a chemical structure made up
of hydrogen and carbon atoms. Unless the context clearly requires otherwise,
however,
it is to be understood that the term does not exclude hydrocarbon structures
having other
atoms or chemical functionalities, so long as such variations do not interfere
with the
chemistry of the formulation processes and compositions.
The presently most preferred components for the formation of the host
composition are:
(i) a branched chain non-ionic surfactant having the chemical structure R-
(CHZ),-2,3-epoxide-(CHZ)4-8,9-epoxide-CHZ)3-13(-(CH~)~-3,4-epoxide-
R')-14-R", where R is a hydrocarbon structure having 3 to 6 carbons,
where R' is hydrogen or a methyl group, and R" is a hydrocarbon
-12-
CA 02373630 2001-11-13
WO 00/67726 PCT/US00/12743
structure having 1 to 12 carbons, and where n = 1 or 2 (carbon numbering
on branch assuming n = 2);
(ii) an amphoteric surfactant having the chemical formula NH3-R-COON,
where R is a straight, branched, or aromatic hydrocarbon structure having
6 to 24 carbons;
(iii) ethanol solvent for dissolving the amphoteric surfactant;
(iv) water;
(v) nonoxyl-9, which is an aromatic compound with a single phosphate group,
and where the phosphate group provides a Lewis acid, and
(vi) aluminum sulfate, which in the presence of water pi°ovides both
the
aluminum canon and a small quantity of sulfuric acid, which is a Bronsted-
Lowry acid.
The non-ionic surfactant is made from naturally-occurring compositions and is
sometimes commercially referred to as a "dodecatriethoxylate" or "E03" type
composition, which is presently commercially available from Hoechst under the
trade
name "Genapol UD079."
The attachment process includes various intermediate steps which can lead to
one
of several resultant chemical structures including a pure crown ether without
a transitional
metal, a crystahemispherand, a calixarene, a carcerand, a rotoxane, or another
form of a
2o host-guest complex. The presence of the chemical unit -C-O-C- can be
immediately
attached to an aromatic or cyclic compound as long as the end resultant
complex
completes a cyclic configuration. This reaction stages into repetitive
additions of that
unit. The larger the (-C-O-C-)n Host molecule, the larger the guest molecule
that
coordinates with the host.
Non-ionic surfactants with 12 to 16 carbons and three epoxides having a hydro-
lipid balance factor of 7 - 9 will yield an -C-O-C- ether unit. Non-ionic
surfactants are
formed from alcohols. By treating the alcohol with potassium hydroxide, 96% of
the
alcohol bonds will form into epoxide groups. By final treatment with aluminum
hydroxide, the remaining 4% alcohols will also yield epoxide bonds.
Conversely,
aluminum sulfate (providing the Bronsted-Lowry acid) will cleave the branched
chain,
opening the -C-C- bond in the epoxide group that has the oxygen attached. In
the
-13-
CA 02373630 2001-11-13
WO 00/67726 PCT/US00/12743
presence of zwitterions and at temperatures between 18°C - 46°C
(65°F - 115°F), the
reaction yields relatively large percentages of the ether link.
In this presently most preferred embodiment of the invention, the water is
preferably in a stoichiometric proportion of at least 1:1.2 relative to the
non-ionic
sunactant.
More particularly, it is believed that the basic chemistry is as follows. The
host
complex is formed by cleaving the branched chain of the non-ionic surfactant.
The
branched chain contains an epoxide group. According to the presently most
preferred
embodiment, the key components for cleaving the branched chain and forming the
host
complex are the presence of an amphoteric surfactant (zwitterion) dissolved in
a solvent
such as a short chain alcohol, a phosphate group (Lewis acid), an aromatic
aryl
hydrocarbon (e.g., attached to phosphate group), aluminum sulfate, and water.
The aluminum sulfate has two roles in this reaction. First, aluminum sulfate
in the
presence of water and a phosphate group will generate sufficient sulfuric acid
to cleave
the epoxide branch chain of the non-ionic surfactant. Second, the aluminum
sulfate acts
as a temporary binding site for the formation of the host complex unit ( -C-O-
C-)n,
where n is at least 3. In the presence of alcohol, the epoxide group breaks
between the
C-C bond.
In the process, the more polar aromatic group of the phosphated aromatic aryl
hydrocarbon replaces the aluminum sulfate creating one of the various host
complexes.
The alcohol participates in the formation of the host complex unit by sharing
electrons
while the temperature is ramped up to about 49°C (120°F). The
host complex unit at
lower temperatures of 21 °C - 32°C (70°F - 90°F)
can join together by producing an 18-
crown-6 ether with a single aromatic ring in the middle to provide rigidity.
The internal
diameter of 18-crown-6 ether is 7.86A, whereas the external diameter of the
benzene ring
is 4.56 A.
The less polar end of the aromatic aryl hydrocarbon is at a 75 degree to 90
degree
angle from the plane of the host complex. As the temperature increases, the
shape of the
host complex is that of calixarenes and carcerands without a transition metal
in the middle
of the structure. Due to the presence of ringed structures in calixarenes and
carcerands,
several compounds with an aromatic group can provide the rigidity for the host
complex.
-14-
CA 02373630 2001-11-13
WO 00/67726 PCT/US00/12743
The zwitterion adds stability to the reaction by partial sharing of electrons
during
the formation of the host complex. The addition of aromatic compounds with
repeating
isoprenoid units become the guest in the host complex. The repeating units,
whether
isoprenoids or polymeric units, provide for subsequent molecular stacking of
additional
ingredients into the host composition.
At least three molecules of the branched chain non-ionic surfactant is
required for
the formation of a host complex molecule. Preferably, at least about one
weight percent
of the host complex in the total composition is formed for the purpose of
stabilizing
diversely-soluble compounds. Proportionately, one host complex provides inter-
molecular attachment sites for three diversely-soluble molecules. This, in
turn, provides
three or more stacking sites for other like molecules. The stacked molecules
are then
sandwiched between non-ionic surfactant layers.
Without being unnecessarily limited by the theoretical explanation, it is
believed
that the important factors that drive the host-formation reaction are
temperature,
solubility, electron transfer, anion balance, and hydro-lipid balance. The
anion balance
(e.g., phosphate-sulfate balance) is the balance of electrons available from
different
orbitals due to the molecular configuration of the anions. The crown ethers
that are
formed are extremel y unstable until they become attached within the system
based on both
nucleophilic and electrophilic reaction. The key to the reaction is the
cleavage of the
branched chain non-ionic surfactant, i.e., the separation of the -(CH,)"-3,4-
epoxide-R"
group. Calixerands, crystahemispherands, calixarenes, carcerands, rotoxanes,
or other
host molecules can be formed in the process of producing a crown by
introducing
aromatic compounds prior to full cyc~ization of the crown ether. Because of
greater
electron affinity to the paired electrons found in the oxygen molecule and,
similarly, in
double-bonded shared electrons in cyclic and aromatic compounds, other host
complexes
can be formed. Refer to Figure 1.
The subsequent sequence of mixing additional compounds with the host
composition allows for stabilization of the one or more host compounds, which
then
allows for molecular stacking of diversely-soluble compounds. Host
compositions begin
to stabilize the first addition of the aromatic to the center of the host
compound. The host
compositions become more stable as more guest compounds are added because the
host
-15-
CA 02373630 2001-11-13
WO 00/67726 PCT/US00/12743
becomes entrapped rather than entrapping as in the first step of host-guest
complex
formation.
The remaining system is balanced with assorted surfactants (lamellas layering)
allowing for other compounds to be added in a hydro-lipid balanced
environment. In the
presence of a non-ionic surfactant and an amphoteric surfactant, the apolar
ends of the
guest molecules will first be directed toward the aqueous phase with the polar
ends
directed to the crown ether type structure, which also is polar. As the
isoprenoid unit
extends into the aqueous phase, there will be lamellas layer of similar
compounds,
especially those that have a ubiqionone structure attached to the hydrocarbon
chain. The
primary stabilizing component of this compounding method is the introduction
of selected
compounds during the formation of either the pure 18-crown-6 ether or any
resultant
compound where the introduced substrate binds to the (- C-O-C)~ complex.
The resultant host-guest complex creates an electronic environment where
hydrogen bonding, Van Der Waal forces, and inter-molecular adhesion occurs.
And, by
partial electron polarity and sharing, the various molecules will align
through a process
of molecular stacking and layering. It is important to note that this is not
the same as
forming a 'liposorrie' system, which requires the presence of what can be
various
phosphatidyl cholines. Nor is this an emulsion, which is generally created in
cold
compounding procedures by utilizing high revolutions-per-minute (RPM) mixing,
rather
than the relatively low RPM mixing preferred according to the inventive
formulation
processes.
B. Molecular Stacking into Host Composition One or More Bio-active
Compounds
A process of molecular stacking of one or more bio-active compounds is used in
the further formulation of stabilized compositions. The bio-active compounds
are selected
based on the desired function of the final product and the desired activity
level of each of
the selected ingredients. The identification and desired bio-active compounds
to be
3o stabilized and delivered by the formulation can be determined from the
known uses and
effectiveness of the bio-effective compound. Numerous classes of compounds are
-16-
CA 02373630 2001-11-13
WO 00/67726 PCTNS00/12743
available, including synthetic and natural compounds, as well as natural
essential
compounds.
Major factors governing the addition of compounds to the host composition
environment include:
(i) the one or more desired bio-affecting compounds desired to be added to
create a desired composition for a specific application; and
(ii) the desired point of attachment to the host complexes.
The systematic addition, i.e., molecular stacking, of the bio-affecting and
other
desirable ingredients into the host composition is also based on a
consideration of the
1o following factors:
(i) the molecular structure of each ingredient;
(ii) the solubility of each ingredient compound and the hydro-lipid balance of
the composition, and the possibility of changing the solubility
characteristics by changing the form of the ingredient, e.g., by using a salt
form of the ingredient;
(iii) in an aqueous composition, the effect of each ingredient on the pH, and
the sensitivity of each ingredient to pH; and
(iv) the temperature required for "setting" or "stacking" the ingredient into
the
host composition.
1. Molecular Structure
The initial molecular structure required for maintaining stability for
diversely-
soluble compounds is based on partial polarization of long chain hydrocarbons
and the
electron protection of aromatic, cyclomatic, and suspected non-metal-
containing crown-
like ether compounds in the host composition. The initial molecular structure
is
established through mixing non-ionic surfactants (epoxide groups configured
inward
protecting the oxygen molecules), establishing divalent charges with the
addition of an
amphoteric (zwitterion) surfactant, an aromatic group (such as provided by the
germicide
nonoxyl-9) for initially coordinating with the molecular structure of the
host.
The molecular infrastructure, although similar to a liposome, is not reliant
on
phosphate compounds such as found in phosphatydal choline. The molecular
infrastructure is reliant on the structure of quinones, ubiqinones,
menaquinones, and
-17-
CA 02373630 2001-11-13
WO 00/67726 PCT/US00/12743
hydroquinones. Although a broad classification of compounds, the molecular
structure
of quinones and similar structures allow for a molecular stacking in multiple
layers due to
the diversity of configurations containing cyclics, aromatics, aromatic
branched chains,
and aromatic cyclic branched chains. Refer to Figures 2, 3, and 4.
There are many classes and examples of compounds that can be added to the host-
guest composition with the essential factor being the presence of a polar
compound
having an aromatic ring and a repeating apolar hydrocarbon unit. The variation
of the
selected compound for stabilization is determined by its stability at a
specified temperature
and the desired point of attachment in the complex. By selecting a bio-
affecting
compound that is an active ingredient, the number of compounds that are useful
is
reduced. The key is the repeating hydrocarbon branch that is found in
compounds like
alpha-tocopherol, phylioquinones, ubiquinones, menaquinones, and
querciquinones. It is
also possible to attach polyisoprenoid groups as found in Beta-carotene
because the length
of the repeating unit between end cyclics.
The selected order is totally dependent on the desired outcome of the bio-
affecting
product. This is driven by bond angle, electron energy, bond length, and
attachment
temperature. Once the stabilizing compound, for example, alpha-tocopherol, is
selected,
the next compound that is desired for the end product is selected. The
resulting
environment is capable of accepting both water- and lipid-soluble compounds.
The key
is to select compounds that in later steps are compatible in structure with at
least one
structural component of the compounds to be later added. This is similar to
solubility
standards in batch mixing. The difference is that the form of the compound
must be able
to stack into the host-guest environment of the host composition.
The mix order depends on the molecular stacking concept. The long hydrocarbon
chain will easily affiliate or coordinate with the hydrocarbon component (less
polar
component) of the croon ether host-guest complex or the short branch from the
cyclic
component of the selected compound will electronically associate with the
oxygen
component of the crown ether. Further, to stabilize the molecular environment,
many
natural products can be added prior to a specific compound. For instance,
carrot seed oil
which is high in Beta-carotene needs to be added prior to highly purified Beta-
carotene.
The other components of carrot seed oil do not attach to the crown ether
environment,
but allow for later components (chemicals) to be added. Carrot seed oil, for
instance, has
-18-
CA 02373630 2001-11-13
WO 00/67726 PCT/US00/12743
flavinoids and fatty acids which allow for later addition through mutual
solubility of a
wide variety of both natural products like St. John's Wort and other highly-
purified
chemicals like vitamin B's.
The molecular stacking process is continued on the basis of both attachment to
the
crown ether and maintaining the hydro-lipid balance as well as future
chemicals necessary
for an effective bio-affecting compound. This system is based on quinone
structures with
repeating hydrocarbon units. There is an alternating requirement for the
attachment to
the crown ether system; if the first compound attached is water soluble, then
the next
compound must be lipid soluble to maintain the hydro-lipid balance as well as
the
electrophilic and nucleophilic balance. Nowhere in this process should a
compound or
series of compounds be added that would substantially diminish that balance.
For
instance, compounds high in redox potential added in significantly high
quantity can
disrupt that balance. By way of a more particular example, co-enzymes with a
quinone
structure can be added, but high concentration of flavinoids cannot be added
without pre
treatment that would create a reduced compound.
2. Solubility and Hydro-lipid l;alance
In setting the order of successive or "stacking" additions, if is essential
that the
solubility factor and the temperature factor be considered. At the point that
a compound
like alpha-tocopherol is added, the system has become more hydrophobic.
Therefore, it
is essential that the next addition be lipid soluble with a water-soluble
component to
maintain the hydro-lipid balance. It is also essential that this compound be
able to
electronically associate with the oxygen component of the crown ether.
According to the molecular stacking process, the inter-molecular polarity of
the
host composition is preferably controlled to be between about 1.8 debye to
about 20
debye at 21 °C (70°F).
According to one approach to controlling the hydro-lipid balance during the
molecular stacking, the process includes the steps of:
(a) controlling the inter-molecular polarity of the host composition to be
between about 1.8 debye to about 8 debye at 21 °C (70°F); and
(b) mixing at least one lipid-soluble compound with the host composition to
obtain a lipi-guest composition.
-19-
CA 02373630 2001-11-13
WO 00/67726 PCT/US00/12743
Subsequently, the process more preferably further includes the steps of:
(a) controlling the inter-molecular polarity of the lipi-guest composition to
be
between about 8 debye to about 20 debye at 21 °C (70°F); and
(b) mixing at least one water-soluble compound with the second composition
to obtain a lipi- and hydro-guest composition.
The inter-molecular polarity of the host composition can be easily controlled
by adding
water to the composition.
According to another approach to controlling the hydro-lipid balance during
the
molecular stacking, the process includes the steps of:
(a) controlling the inter-molecular polarity of the host composition to be
between about 8 debye to about 20 debye at 21 °C (70°F); and
(b) mixing at least one water-soluble compound with the second composition
to obtain a hydro-Guest composition.
Subsequently, this process more preferably further includes the steps of:
(a) controlling the inter-molecular polarity of the hydro-guest composition to
be between about 1.8 debye to about 8 debye at 21 °C (70°F); and
(b) mixing at least one lipid-soluble compound with the host composition to
obtain a lipi- and hydro-guest composition.
3. If in an Aqueous Composition, the Effect on pH
In an aqueous composition, a buffer compound such as betaine is preferably
added
at some point during the formulation process to help control the pH within a
desired
range.
In the initial formulation of the host composition having the crown ether and
a
hydro-lipid balanced environment, an amphoteric surfactant NH3-R-COOH is
included
because of its ability to allow for creation of an electron sharing neutral
environment
where electrophilic ornucleophilic compounds can be added without
substantially altering
the established pH. This amphoteric compound, being a zwitterion, allows for
prolonged
additions of the above-mentioned types of compounds.
In cases where it is desirable or necessary to control the pH within certain
limits,
for example, in the case of the need to stabilize a pH-sensitive compound, the
pH
stabilizing (i.e., buffering) effects of the amphoteric surfactant may need to
be overcome.
-20-
CA 02373630 2001-11-13
WO 00/67726 PCT/US00/12743
To control the pH, one or more other zwitterions can be added during the
formulation
process. The choice of the other zwitterions is based on the pH range required
to stabilize
a pH-sensitive bio-affecting compound.
The desired pH range is preferably maintained by buffering. For example, the
pH
must be maintained below the pKa of any additional compound or the pKa of any
potential bond reaction within the compound to maintain stability within the
molecular
system. This pKa factor becomes very evident when L-ascorbic acid is one of
the
compounds to reside in the stable molecular environment. Compounds are added
in ratio
with their salt form such that the pH is below the pKa.
For example, in the case of a composition for the topical delivery of
concentrated
Vitamin C, which should be stabilized in a composition having a pH of less
than 4.5, a
small concentration of a short-length zwitterion, like a betaine, helps adjust
and maintain
the pH in an acidic range below 4.5. A zwitterion such as allantoin can be
preferably
added for its additional benefit of having desirable bio-effecting properties,
such as
softening of the skin. Allantoin has both cyclic and ring structures included,
which
structures can be taken advantage of for the purposes of selecting the
molecular stacking
sequence, as well as the zwitterion effect.
Prior to adding the L-ascorbic acid, the pH has to be lowered to below 4.
Several
choices can be made, all of which are either alpha hydroxy acids or beta-
hydroxy acids.
Both will work in stabilizing the L-ascorbic acid. The difference is that the
selection of
a beta-hydroxy acid is more compatible with the bio-affecting application of
the product.
The amount of the beta-hydroxy acid, in this case salicylic acid, is based on
the desired
pH. The pH has to be below the C=O bond's pKa of the L-ascorbic acid, 4.5. The
salicylic acid, as with most beta-hydroxy acid, is unstable without the
combination with
its own salt form to allow for there to be pH balance in conjunction with the
zwitterion
complex previously added. According to a presently most preferred embodiment
of the
invention for a vitamin C composition, there are several ratios that are
preferably
followed: Ascorbic acid:ascorbyl palmitate:calcium ascorbate = 3:2:1; and
Salicylic
acid: sodium salicylate = 2:1.
3o To finalize such a Vitamin C product, there are still some chemical lose
ends.
There is the presence of natural bioflavinoids because of the previous
addition of carrot
seed oil. To align these compounds into a stable state, another natural
product with
-21-
CA 02373630 2001-11-13
WO 00/67726 PCT/US00/12743
nearly opposite ratios of compounds needs to be added. It is important to note
that,
within this process, other compounds can be substituted as long as they meet
the crown
ether host-guest, hydro-lipid balance in the system, and the molecular
stacking
requirements. The selection of compounds is entirely based on the bio-
affecting system
for which it is designed.
4. Temperature Required for Stacking
The temperature must be increased to allow for the complete attachment of the
selected first compound. In general, it is preferable to begin stacking the
ingredients into
to the composition in the sequence of highest melting point to lowest. The
temperature of
the host composition is preferably adjusted (typically heated) to be at least
as high as the
melting point of the first bio-affecting compound to be added to the mixture
to create a
molecular environment in which the first bio-affecting compound to be added
can be set
into the host molecule. By raising the temperature, the resultant reaction
allows for the
addition of a lipid-soluble compound such as alpha tocopherol or a water-
soluble
compound such as 7-dehydroxy cholesterol.
For example, in the case of adding alpha tocopherol (Vitamin E), the
temperature
of the host composition is increased to at least about 49°C
(120°F). Once the alpha
tocopherol has been mixed with the host composition and sets in the lameller
layers, the
temperature is reduced to below the melting point of the next compound to be
added.
The speed of mixing and the required amount of time for mixing varies
according to
volume, but once determined is consistent for that volume.
The resultant mix sets the diverse compounds in a configuration that allows
for
the first addition of compound containing electrophilic and neuclophilic
compound, e.g.,
ascorbyl palmitate. Upon this addition (with temperature control) there exists
molecular
chains by which either electrophilic or neuclophilic compounds can be added as
long as
an appropriate pH range is maintained by buffering. The pH must be maintained
below
the pKa of any additional compound or the pKa of any potential bond reaction
within the
compound to maintain stability within the molecular system. This pKa factor
becomes
very evident when L-ascorbic acid is one of the compounds to reside in the
stable
molecular environment. Compounds are added in ratio with their salt form such
that the
pH is below the pKa.
-22-
CA 02373630 2001-11-13
WO 00/67726 PCT/US00/12743
Following the completion of the attachment of the alpha tocopheral, other
compounds can be added. The next compound to be added is preferably a lipid-
soluble
derivative of one of the active ingredients in order to begin the basis for
molecular
stacking of like molecules and reduction of pH, especially in the case of
stabilizing the
molecular environment for L-ascorbic acid. In this case, because of the future
requirement of L-ascorbic acid, ascorbyl palmitate is selected because the
palmitate group
is lipid soluble and the tail of the ascorbic acid group can electronically
attach to the
oxygen group of the crown ether system. Note that the system has to be reduced
in
temperature to accept the ascorbyl palmitate, as it would for other compounds
that are
bio-affecting, but not necessarily for stabilization of the system.
A pH buffering compound such as betaine should be added soon after the
temperature decreases to about 46°C (115°F). The temperature is
maintained at about
45 °C (112 °F) until all the remaining pH stabilizing
ingredients have been mixed together.
C. Resulting Compositions
Following the formulation processes according to the present invention
produces
a stable product containing one or more bio-affecting compounds. As will be
appreciated
by those skilled in the art, a wide variety of bio-affecting compositions can
be made
according to the formulation process. In order to accomplish the additions of
these
various other compounds, certain process steps have to be completed. The
compound has
various partially-charged components that can be used to add other essential
compounds
and maintain stability of the complex. Each addition requires adjustment of
temperature
of either the added component or the complex itself. For example, in the case
of lecithin,
a phosphatidyl choline used for oily skin, the addition of the phosphatidyl
choline
(depending on the precise structure of the phosphatidyl choline) temperature
must be
adjusted to allow for the compound to inter-molecularly bind with the complex.
In the
case of aloe, used for dry skin, because of its mucopolysaccharide components
must be
combined with a beta-hydroxy acid to attach with the complex. In the case of
Shea
Butter, the temperature of the Shea Butter has to be adjusted to become
soluble with the
complex.
-23-
CA 02373630 2001-11-13
WO 00/67726 PCT/US00/12743
Most additional ingredients are present for effectiveness and bio-affecting
applications. Not all ingredients can be added without considering solubility,
pH, and
temperature.
The resulting composition is highly stable, and certain formulations have been
tested to be capable of remaining stable until the air-to-composition ratio in
the container
is 6:1. At that time there is a 14-day period before one or more of the
composition's
active ingredients begin to degrade to the point the product composition may
become
ineffective for one or more of its intended purposes.
D. Process and Formulation Examples
The formulations are based on host-guest formation and chemical structure
addition. It is necessary to have similar lipid- or water-soluble components
to the
compounds so that the chemicals can either attach to the crown ether type
structure or
layer by molecular stacking between the compounds. In the case of a pH-
sensitive bio-
affecting compound such as vitamin C, the pH must below 4.5 before adding the
ascorbic
acid or, otherwise, the -C=O external to the ring will be subject to
oxidation. There are
several ratios that are preferably followed: Ascorbic acid:ascorbyl
palmitate:calcium
ascorbate = 3:2:1; and Salicylic acid:sodium salicylate = 2:1.
The molecular configuration for quinones requires balancing the attached chain
groups to the available electron orbitals from other compounds with similar
chain groups.
The principle involved is the stacking of compounds with similar aromatic and
cyclic
structures through the lamellar layer created in the process. The ring
structures will tend
to stack one upon the other with the apolar ends acting as tails. The
isoprenoid units
become the apolar end and are aligned in the opposite direction from the polar
cyclics.
Some cyclics will exhibit a partial polarity based on hydroxylation to the
ring.
Each of the following formulations are examples of mixture formulations and
steps
that can be used to produce a stable composition for the delivery of one or
more bio-
affecting compounds to a target biological system. The first 10 of the
following
formulation examples are from actual mixes. The temperatures can vary with
reasonable
ranges of the precise temperatures used in the examples without departing from
the scope
and spirit of the examples. The initial phase of the mix preferably includes
the step of
-24-
CA 02373630 2001-11-13
WO 00/67726 PCT/US00/12743
increasing the temperature to help drive the formation of the host complex.
Although the
maximum mixing temperature can exceed 49°C (120°F), there is a
point where the
complex will not accept the initial substrate for setting the rigidity of the
host-guest
complex. This temperature point will vary depending of the guest chemical, but
typically
is expected to be a maximum of about 54°C (130°F).
The RPM of the mix motor can vary depending of the equipment and the type of
propeller used in the procedure. However, it is important that the mixing not
be
unnecessarily fast to minimize the introduction of air (i.e., oxygen) into the
composition.
Excessive mixing forces may also tend to destabilize the molecular stacking
within the
mixtures.
Each compound is selected for its bio-affecting capacity within the system and
for
the stated purposes of the composition. Upon considering the general
description of the
invention and the followingprocess and formulation examples, those skilled in
the art will
be able to make numerous modifications and substitutions in the formulations
within the
scope and spirit of the invention.
Formulation #1 - Skin Slou~hing/Exfoliation
This formulation is designed to promote skin slouching. The presence of trans
retinoic acid, a form of retinol (Vitamin A) at the 1.0 % or greater level
will increase the
rate of exfoliation. The first step sets the molecular environment as well as
establishes the
crown ether host complex. It is important to note that the 6% amino
dodecacarboxylic
acid is in an ethanol solution. The ethanol provides a solubility factor
allowing for the
formation of the host complex and the stage for lamellar layering. As the
temperature is
increased during the mixing steps of the process, the ethanol gradually
evaporates.
The addition, in this case the alpha tocopherol at 41 °C
(106°F), is for the purpose
of establishing an attachment to the host-guest complex. The host-guest
complex does
not go to completion in the procedure until 49°C (120°F). For
other processes and
mixtures, this temperature can vary dependent on the desired guest chemical
and the
primary decision on which is the first compound to be added to the host
composition. As
previously mentioned, the first solubility decision drives the mix order and
the following
decisions as to molecular stacking of additional compounds.
-25-
CA 02373630 2001-11-13
WO 00/67726 PCT/US00/12743
The salicylic acid and sodium salicylate are present to reduce the pH so that
ascorbic acid can be added below a pH of 4.5, which is the pKa of the C=O
external to
the ring structure of the ascorbic acid. It also acts as an exfoliation
chemical due to being
a beta-hydroxy acid.
The other natural extracts in this formulation example contain natural
solublizing
compounds allowing for protection from other factors like pH and salt content.
These
natural products are preferably mixed into the composition before the desired
specific
compound for biochemical function, in this case, the ascorbic acid.
Formulation #1
- Skin Slou hina/Exfoliation
Substance WeiahtPercentTem erature Mixing RPM
C
Purified water 9.0 16-32 15 to 20
67o Amino dodecc 5.6
carboxylic acid Increase
to 41
Nonoxyl 9 3.1
Dodecotriethoxylate15.0
Aluminum Sulfate 0.003
Purified water 22.0
Alpha tocopherol 5.0 Increase 20 for 15 minutes
to 49
Lower to
47
Ascorbyl palmitate3.4 30-45 for 10 minutes
Purified water 17.0 Lower to 30 for 5 minutes
43
Trans-retinoic 1.0 30 for 5 minutes
acid
Carrot seed oil 0.38 Lower to 30 for 3 minutes
42
Beta-Carotene 0.007 30 for 10 minutes
Cholecalciferol 0.007 35 for 10 minutes
Calcium ascorbate1.2 Lower to 45 for 10 minutes
41
Purified water 9.0 45 for 3 minutes
Co-enzyme Q10 0.01 45 for 5 minutes
Betaine 0.75 35 for 5 minutes
Salicylic acid 0.41 35 for 10 minutes
Sodium salycilate0.205 45 for 5 minutes
Ascorbic acid 6.8 45 for 10 minutes
St John's Wort 0.43 35 for 5 minutes
FMN ' 0.04 35 for 5 minutes
Formulation #2 - Collagen I Activating Complex
This formulation is designed to maximize the production of collagen I in skin.
Therefore, the percentages of ascorbic acid and ascorbic acid derivatives are
increased so
as to produce a composition having above 8% by weight of water-soluble
ascorbic acid.
Likewise, the balance needed in the molecular complexing for alpha tocopherol
is
-26-
CA 02373630 2001-11-13
WO 00/67726 PCT/US00/12743
increased to be above 5% to act in balance to the ascorbic acid and for free
radical
reduction within the body. With the co-enzyme Q 10 being part of the free
radical
reduction system, the complex requires a very small, but active, percentage to
accelerate
the free radical reduction.
The same concept as in Formulation 1 is required for the molecular stacking.
In
Formulation 1 only betaine is used for creating a pH buffering balance.
Allantoin, which
is a longer chained compound and has better molecular stacking properties, is
added due
to the increased amounts of active ingredients and additional compounds like
orange oil
which contains limonene. Allantoin also has properties that increase the
integrity of the
lipid membrane in the dermal layer. Limonene, found in orange oil, is very
penetrating in
the dermal layer and aids in carrying other compounds across the dermal layer.
These
additions alone do not count for the complete penetration of the dermal layer
and the
hyperdermal layer. Lavender is added in the complex because its natural
components
contain bioflavinoids that are compatible in ring structure and has been
reported to
promote healing in the skin as does cholecalciferal, a form of Vitamin D. The
percent of
carrot seed oil is increased relative to the amount in Formulation 1 to
increase the
concentration of natural beta-carotene and to increase the protection of the
beta-carotene
by providing a natural screen for 234 nm UV light, which causes beta-carotene
to convert
to traps-retinol, Vitamin A, which, in turn, would then upset the pH balance
of the
complex by oxygenating the C=O in the ascorbic acid.
-27-
CA 02373630 2001-11-13
WO 00/67726 PCT/US00/12743
Formul ation #2
- Colla
en I Activating
Com lex
Substance WeiahtPercentTem erature Mixing RPM
C
Purified water 9.0 16-32 15 to 20
6% Amino dodeca 5.6
carboxylic acid Increase
to 41
Nonoxyl 9 3.1
Dodecotriethoxylate15.0
Aluminum Sulfate 0.003
Purified water 19.0
Alpha tocopherol 5.3 Increase 20 for 15 minutes
to 49
Lower to
47
Ascorbyl palmitate6.0 30-45 for 10 minutes
Purified water 13.0 Lower to 31 for 5 minutes
43
Carrot seed oil 1.0 Lower to 30 for 3 minutes
42
Beta-Carotene 0.025 30 for 10 minutes
Cholecalciferol 0.025 35 for 10 minutes
Calcium ascorbate3.0 Lower to 45 for 10 minutes
41
Purified water 6.2 45 for 3 minutes
Co-enzyme Q10 0.02 45 for 5 minutes
Betaine 0.25 35 for 5 minutes
Allantoin 0.75 35 for 5 minutes
Salicylic acid 0.8 35 for 10 minutes
Sodium salycilate0.4 45 for 5 minutes
Ascorbic acid 9.0 45 for 10 minutes
St John's Wort 0.5 35 for 5 minutes
~
Orange oil 0.5 35 for 5 minutes
Lavender 0.5 35 for 5 minutes
Formulation #3 - Collagen I Activating Formulation for Dry Skin
The primary difference between this formulation and formulation #2, is the
presence of Aloe, which contains mucopolysaccharides, amino poly~lycans,
glycolic, and
hyaluronic acids. By changing the mix order to eliminate the addition of
purified water
in step 2, prior to the addition of alpha tocopherol, the molecular stacking
results in the
reduction of the number the apolar ends creating areas within the complex that
will accept
the amino and polysaccharide groups without disrupting the solubility balance.
Likewise,
this formulation can be adjusted for oily skin by changing the aloe to
lecithin which is a
phosphatidyl choline. The phosphatidyl choline can be added at the end of the
order
without disrupting the balance within the complex because the phosphate ions
will not
-28-
CA 02373630 2001-11-13
WO 00/67726 PCT/US00/12743
form liposomes which adsorb oxygen rapidly. The oxygen adsorption will change
the pH
affecting the C=O of the ascorbic acid, destabilizing the water-soluble
ascorbic acid.
Formulation #3
- Colla en I
Activatin Formulation
for Dr Skin
Substance Wei ht PercentTem erature Mixin RPM
C
Purified water 9.0 16-32 15 to 20
6% Amino dodeca 5.6
carboxylic acid Increase
to 41
Nonoxyl 9 3.1
Dodecotriethoxylate15.0
Aluminum Sulfate 0.003
Alpha tocopherol 5.3 Increase 20 for 15 minutes
to 49
Lower to
47
Ascorbyl palmitate6.0 30-55 for 10 minutes
Purified water 13.0 Lower to 32 for 5 minutes
43
Carrot seed oil 1.0 Lower to 30 for 3 minutes
42
Beta-Carotene 0.025 30 for 10 minutes
Cholecalciferol 0.025 35 for 10 minutes
Calcium ascorbate3.0 Lower to 45 for 10 minutes
41
Purified water 6.2 45 for 3 minutes
Co-enzyme Q10 0.02 45 for 5 minutes
Betaine 0.25 35 for 5 minutes
Allantoin 0.75 35 for 5 minutes
Salicylic acid 0.8 35 for 10 minutes
Sodium salycilate0.4 45 for 5 minutes
Ascorbic acid 9.0 45 for 10 minutes
St John's Wort 0.5 35 for 5 minutes
Orange oil 0.5 35 for 5 minutes
Lavender 0.5 35 for 5 minutes
Aloe 19.0 35 for 5 minutes
Formulation #4 - Relief Creme for Arthritis, Rheumatism, Swelling and
Inflammation
This formulation is designed to be able to penetrate the dermal and
hyperdermal
layers to deliver a series of compounds to fibroblast and osteoblast found in
joints,
tendons, and muscles.
The ascorbic acid activates the production of Collagen I, bringing collagen
complex back into balance.
Important to this formulation is Shea Butter which contains a relatively high
4o amount of unsaponifiable fats as compared to other natural oil extracts.
The presence of
the ratio of unsaponifiable fats to saponifiable fats allows for the stability
of the complex
-29-
CA 02373630 2001-11-13
WO 00/67726 PCT/US00/12743
without cleaving the Ca bond in the calcium ascorbate due to the reducing
capability of
unsaponifiable fats. Also, as an active agent, the Shea Butter tends to hold
the complex
in place on the dermis for a longer period of time allowing for the active
components to
penetrate the hyperdermal layer and reach the fibroblast, osteoblast, and
other tissues.
Note that the Shea Butter needs to be heated to be able change from a granular
form to
a liquid so that the unsaponifiable fats can molecularly stack within the
formulation.
Note that the amount of salicylic acid has been increased to 2%. Salicylic
acid
derivatives, acetyl salicylic acid and methyl salicylate, have been shown to
decrease
inflammation when taken internally. The delivery system in this complex allows
for the
salicylic acid and the sodium salicylate to reach the inflamed area, thereby
reducing
inflammation and swelling.
-30-
CA 02373630 2001-11-13
WO 00/67726 PCT/US00/12743
Formulation #4
- Relief Creme
Substance Weight PercentTem erature Mixin RPM
F
Purified water 9.0 16-32 15 to 20
6% Amino dodeca 5.6
carboxylic acid Increase
to 41
Nonoxyl 9 3.1
Dodecotriethoxylate15.0
Aluminum Sulfate 0.003
Purified water 10.0
7-dehydroxycholesterol0.5 Increase 20 for 15 minutes
to 49
Lower to
47
Ascorbyl palmitate6.0 30-45 for 10 minutes
Purified water 13.0 Lower to 33 for 5 minutes
43
Carrot seed oil 1.0 Lower to 30 for 3 minutes
42
Beta-Carotene 0.025 30 for 10 minutes
A1 ha toco herol 0.025 35 for 10 minutes
Calcium ascorbate 3.0 Lower to 45 for 10 minutes
41
Purified water 6.2 45 for 3 minutes
Co-enzyme Q10 0.02 45 for 5 minutes
Betaine 0.25 ~ 35 for 5 minutes
Allantoin 0.75 35 for 5 minutes
Salicylic acid 2.0 35 for 10 minutes
Sodium salycilate 1.0 45 for 5 minutes
Ascorbic acid 9.0 45 for 10 minutes
St John's Wort 0.5 35 for 5 minutes
Orange oil 0.5 35 for 5 minutes
Lavender 0.5 35 for 5 minutes
Shea Butter 8.0 Shea Butter 35 for 5 minutes
separatel
y
heated to
47
before adding
3o Formulation #5- After treatment preparation for intrusive skin care
treatments like
microdermabrasion and carbon dioxide laserin~.
This formulation is designed to provide relief from procedures that remove
layers
of skin from the face. The combination of the Shea Butter and the Aloe provide
this
relief. In order for this formulation to remain stable, the amino polyglycans
in the Aloe
are added first to provide additional buffering before adding the
unsaponifiable fats since
the amount of the salicylic acid has been reduced to a level similar to
previous
formulations. The molecular configuration within the lamellar layering is
subject to partial
reversal of the micelles that are created if added in a different order.
-31-
CA 02373630 2001-11-13
WO 00/67726 PCT/US00/12743
Formulation #5-
After treatment
re aration
Substance Wei ht PercentTem erature Mixin RPM
C
Purified water 9.0 16-32 15 to 20
6Io Amino dodeca 5.6
carboxylic acid Increase
to 41
Nonoxyl 9 3.1
Dodecotriethoxylate15.0
Aluminum Sulfate 0.003
Purified water 10.0
Alpha tocopherol 5.3 Increase 20 for 15 minutes
to 49
Lower to
47
Ascorbyl palmitate6.0 30-45 for 10 minutes
Purified water 13.0 Lower to 34 for 5 minutes
43
Carrot seed oil 1.0 Lower to 30 for 3 minutes
42
Beta-Carotene 0.025 30 for 10 minutes
Cholecalciferol 0.025
Calcium ascorbate3.0 Lower to 45 for 10 minutes
41
Purified water 6.2 45 for 3 minutes
Co-enzyme Q10 0.02 45 for 5 minutes
Betaine 0.25 35 for 5 minutes
Allantoin 0.75 35 for 5 minutes
Salicylic acid 0.8 35 for 10 minutes
Sodium salycilate0.4 45 for 5 minutes
Ascorbic acid 9.0 45 for 10 minutes
St John's Wort 0.5 35 for 5 minutes
Orange oil 0.5 35 for 5 minutes
Lavender 0.5 35 for 5 minutes
Aloe 9.0 35 for 5 minutes
Shea Butter 9.0 Shea Butter 45 for 10 minutes
separately
heated to
47
before addin
Formulation #6 - Oily Skin Treatment Complex
This formulation is altered from Formulation #5 only by the substitution of
lecithin
for Aloe and requires a change in percentage due to the properties of
phosphatidylcholine.
-32-
CA 02373630 2001-11-13
WO 00/67726 PCT/US00/12743
Formulation #6
- Oil Skin Treatment
Com lex
Substance Wei ht PercentTem erature Mixing RPM
F
Purified water 9.0 16-32 15 to 20
6% Amino dodeca 5.6 _
carboxylic acid Increase
to 41
Nonoxyl 9 3.1
Dodecotriethoxylate15.0
Aluminum Sulfate 0.003
Purified water 2.0
Alpha tocopherol 5.3 Increase 20 for 15 minutes
to 49
Lower to
47
Ascorbyl palmitate6.0 30-45 for 10 minutes
Purified water 17.0 Lower to 36 for 5 minutes
43
Carrot seed oil 1.0 Lower to 30 for 3 minutes
42
Beta-Carotene .025 30 for 10 minutes
Cholecalciferol .025
Calcium ascorbate3.0 Lower to 45 for 10 minutes
41
Purified water 6.2 45 for 3 minutes
Co-enzyme Q10 0.02 45 for 5 minutes
Betaine 0.25 ~ 35 for 5 minutes
Allantoin 0.75 35 for 5 minutes
Salicylic acid 0.8 35 for 10 minutes
Sodium salycilate0.4 45 for 5 minutes
Ascorbic acid 9.0 45 for 10 minutes
St John's Wort 0.5 35 for 5 minutes
Orange oil 0.5 35 for 5 minutes
Lavender 0.5 35 for 5 minutes
Lecithin 6.33 37 for 5 minutes
Shea Butter 7.5 Shea Butter 45 for 10 minutes
separately
heated to
47
before addin
Formulation #7 - Non-greasy Moisturizing Complex
This formulation contains a reducing agent, FMNHz, which, in combination with
the bioflavinoids found in St. John's Wort, will attach to the saponifiable
fats in Shea
Butter. There is an overall decrease in the amount of purified water in the
complex. This
reduces the chance of destabilizing the ascorbic acid with the reduction in
lamellar layering
created by having both Aloe and Shea Butter present without the increased
amounts of
salicylic acid which keeps the pH below 4.0, the C=O pKa of 4.5.
-33-
CA 02373630 2001-11-13
WO 00/67726 PCTNS00/12743
Formula tion #7
- Non-areas
Moisturizin
Com lex
Substance WeiahtPercentTem erature Mixin RPM
C
Purified water 9.0 16-32 15 to 20
6% Amino dodeca 5.6
carboxylic acid Increase
to 41
Nonoxyl 9 3.1
Dodecotriethoxylate15.0
Aluminum Sulfate 0.003
Purified water 8.0
1o Alpha tocopherol 5.3 Increase 20 for 15 minutes
to 49
Lower to
47
Ascorbyl palmitate6.0 30 for 10 minutes
Purified water 17.0 Lower to 30 for 5 minutes
43
Can-ot seed oil 1.0 Lower to 30 for 3 minutes
42
Beta-Carotene .025 30 for 10 minutes
Cholecalciferol .025 35 for 10 minutes
Ph llo uinone 2.0 35 for 10 minutes
Calcium ascorbate3.0 Lower to 45 for 10 minutes
41
Purified water 6.2 _ 45 for 3 minutes
Co-enzyme Q10 0.02 45 for 5 minutes
Betaine 0.25 35 for 5 minutes
Allantoin 0.75 35 for 5 minutes
Salicylic acid 0.8 35 for 10 minutes
Sodium salycilate0.4 45 for 5 minutes
Ascorbic acid 9.0 45 for 10 minutes
St John's Wort 0.5 35 for 5 minutes
.
FMNH~ 0.04 35 for 3 minutes
Orange oil 0.5 35 for 5 minutes
Lavender 0.5 35 for 5 minutes
Aloe 6.0 31 for 5 minutes
Shea Butter 3.0 Shea Butter 45 for 10 minutes
separately
heated to
47
before addin
Formulation #8 - Dermal Peeling Complex
This formulation is designed as an acid peel. The pH is lower than the regular
pH
of 3.5 to 3Ø This formulation illustrates that the use to the host
composition for the
stabilization and delivery of a single active ingredient, in that this
formulation need not
contain alpha tocopherol as the host-guest setting compound. Instead, a
mucopolysaccaharide complex containing amino polyglycans and glycolic acid can
be
substituted and the mix order changed based on solubility. The different
components like
-34-
CA 02373630 2001-11-13
WO 00/67726 PCT/US00/12743
glycine are present to stabilize the polar ends of the complex so that the
ascorbic acid
stability is maintained.
Formulation #8 lex
- Dermal Peelin
Com
Substance Wei ht PercentTem erature Mixing RPM
C
Purified water 9.0 16-32 15 to 20
6% Amino dodecc 5.6
carboxylic acid Increase
to 41
Nonoxyl 9 3.1
Dodecotriethoxylate15.0
Aluminum Sulfate 0.003
Purified water 24.0
Mucopolysaccaharide9.0 Increase 20 for 15 minutes
to 49
Lower to
47
Ascorbyl palmitate3.4 30 to 45 for 10
minutes
Purified water 21.0 Lower to 32 for 5 minutes
43
Beta-Carotene 0.25 30 for 10 minutes
Cholecalciferol 0.25 Lower to 35 for 10 minutes
42
Calcium ascorbate3.4 Lower to 45 for 10 minutes
41
Purified water 9.1 45 for 3 minutes
Glycine 5.0 45 for 3 minutes
Co-enzyme Q10 0.01 45 for 5 minutes
Betaine 0.75 35 for 5 minutes
Salicylic acid 2.0 35 for 10 minutes
_
Sodium salycilate1.0 45 for 5 minutes
GI colic acid 3.0 45 for 10 minutes
Shea Butter 10% Shea Butter 35 for 5 minutes
separately
heated to
47
before addin
Formulation #9 - Plant Growth Hormone Delivery System
This formulation is designed as a delivery system for plant growth hormones,
diterpenes. This formulation also illustrates that the use to the host
composition for the
stabilization and delivery of a single active ingredient, in that this
formulation need not
contain alpha tocopherol as the host-guest setting compound. Instead, a
mucopolysaccaharide complex containing amino polyglycans and glycolic acid can
be
substituted and the mix order changed based on solubility. The host complex is
first
attached to the polar end of the amino polyglycans. The nitrogen complex
within the amino
group creates a molecular attachment point for the diterpenes. In turn, these
isoprenoid
units are directed 90 degrees from the crown ether complex providing for
lamellar layer of
-35-
CA 02373630 2001-11-13
WO 00/67726 PCT/US00/12743
the Co-enzyme Q 10 and the betaine. The chlorophyll, a porphorin compound,
attaches
parallel to the crown ether complex. Upon penetration of the plant membrane,
the diterpene
is released into the plant cell.
Formulation #9
- Plant Growth
Hormone Deliver
S stem
Substance Wei ht PercentTem erature Mixing RPM
C
Purified water 9.0 16-32 15 to 20
67o Amino dodecc 5.6
carboxylic acid Increase
to 41
to Nonoxyl9 3.1
Dodecotriethoxylate15.0
Aluminum Sulfate 0.003
Purified water 24.0
Mucopolysaccaharide15.0 Increase 20 for 15 minutes
to 49
Lower to
47
Diterpene
Co-enzyme Q 10 25.0 Lower to
42
0.5 .
Purified water 11.0 Lower to 35 for 5 minutes
41
Betaine 1.0 45 for 5 minutes
Chlorophyll 10.0 35 for 5 minutes
Formulation #10 - Antioxidant Treatment for Rough Dry Skin
This formulation is designed to penetrate the dermal layer when it has become
'sequacious', which is an overlayering condition of the skin. This formulation
illustrates the
ability of the formulation process to provide a stable complex with primarily
lipid-soluble
components.
-36-
CA 02373630 2001-11-13
WO 00/67726 PCT/US00/12743
Formulation #10
- Antioxidant
Treatment for
Rou h Dr Skin
Substance Wei ht PercentTem erature Mixin RPM
F
Purified water 9.0 16-32 15 to 20
6% Amino dodeca 5.6
carboxylic acid Increase
to 41
Nonoxyl 9 3.1
Dodecotriethoxylate15.0
Aluminum Sulfate 0.003
Purified water 24.0
Mucopolysaccaharide15.0 Increase 20 for 15 minutes
to 49
Lower to
43
Alpha tocopherol 10.0
Co-enz me Q 10 0.25 Lower to
42
Purified water 15.0 Lower to 35 for 5 minutes
41
Betaine 0.5 45 for 5 minutes
Ascorb 1 almitate1.0
St John's Wort 1.0 35 for 5 minutes
2o Formulation #11 - Partial Antioxidant and Chemical Peel Treatment for Dry
This formulation is designed to penetrate the dermal layer acting as a
chemical peel.
This formulation illustrates the ability of the formulation process to provide
a stable
composition by first mixing a water-soluble bio-affecting compound with the
host
composition.
Formulation #11
- Partial Antioxidant
and Chemical
Peel Treatment
for Dr Skin
Substance WeiahtPercentTem erature Mixing RPM
C
Purified water 9.0 16-32 15 to 20
6% Amino dodeca 5.6
carboxylic acid Increase
to 41
Nonoxyl 9 3.1
Dodecotriethoxylate15.0
Aluminum Sulfate 0.003
Salicylic acid 1.0 Increase 20 for 40 minutes
to 49
Ascorbic acid 11.0 Lower to 20 for 15 minutes
44
Al ha toco herol 6.0 Lower to 35 for 20 minutes
41
Purified water 47.3 Lower to 35 for 5 minutes
41
Sodium salicylate0.5 45 for 5 minutes
Betaine 0.5 45 for 5 minutes
St John's Wort 1.0 35 for 5 minutes
-37-
CA 02373630 2001-11-13
WO 00/67726 PCT/US00/12743
Formulation #12 - Metallo Complexes for Anti-oxidant, Moisturizing Sun
Protectors
This formulation is designed to provide sun protection from UV-A/UV-B wave
lenghts by including metallo complexes known to provide in vivo and in vitro
coverage, i.e.,
zinc oxide and titanium dioxide. Additional ingredients are preferably added
for anti-
oxidant benefits, occlusion, and moisturizing capabilities.
Formulation #12
- Metallo Com
lexes for Sun
Protectors
Substance Wei ht PercentTem erature Mixing RPM
C
Purified water 9.0 16-32 15 to 20
6% Amino dodeca 5.6
carboxylic acid Increase
to 49
Nonoxyl 9 3.1
Dodecotriethoxylate15.0
Aluminum Sulfate 0.003
Titanium Dioxide 2.0 Lower to 30 to 45 minutes
46
Carrot Seed Oil 0.1
Melatonin 0.1
Zinc Oxide 12.5
Purified water 12.0
2o Pentasilicone 1.0 Maintain 45 to 60 minutes
at 46
Vegetable Glycerine8.05
Shea Butter 5.4
Alpha tocopherol 2.1
Ascorbyl Palmitate8.5
Aloe 4.3
Grapeseed Oil 1.3 Maintain 60 to 90 minutes
at 46
Purified water 19.3
Gamma Oryzanol 1.0
C clomethisilicone5.0
Conclusion
These and other aspects of the invention will be apparent to those skilled in
the art.
The illustrative examples discussed herein are not for the purpose of pointing
out what an
infringement would be, but are only for the purpose of illustrating various
aspects of the
invention. Those skilled in the art will recognize that numerous variations in
the examples
according to the formulation processes and compositions are possible, and that
numerous
substitutions of compounds can be made without departing from the scope and
spirit of the
invention.
-38-