Note: Descriptions are shown in the official language in which they were submitted.
CA 02373757 2001-11-13
WO 01/03694 PCT/DK00/00377
TREATMENT OF NEUROTIC DISORDERS
Field of invention
The present invention relates to the use of the compound escitalopram (INN-
name), which is
the S-enantiomer of the well-known antidepressant drug citalopram, i.e. (S)-1-
[3-(dimethyl-
amino)propyl]-1-(4-fluorophenyl)-1,3-dihydro-5-isobenzofurancarbonitrile, or a
pharma-
ceutically acceptable salt thereof for the preparation of medicaments for the
treatment of
neurotic disorders, including anxiety states and panic attacks.
Background of the Invention
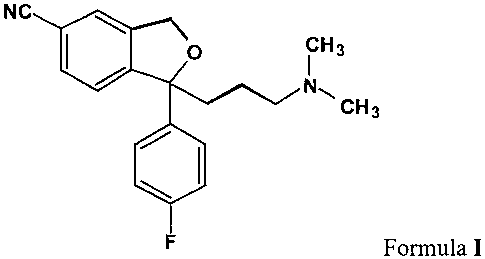
Citalopram is a well-known antidepressant drug that has now been on the market
for some
years and has the following structure:
NC
p i H3
N,
CH3
F Formula I
It is a selective, centrally acting serotonin (5-hydroxytryptamine; 5-HT)
reuptake inhibitor,
accordingly having antidepressant activities. The antidepressant activity of
the compound
has been reported in several publications, eg. J. Hyttel, Prog. Neuro-
Psychopharmacol. &
Biol. Psychiat., 1982, 6, 277-295 and A. Gravem, Acta Psychiatr. Scand., 1987,
75, 478-486,
and it is now marketed for the treatment of depression and panic disorders.
The compound
has further been disclosed to show effects in the treatment of dementia and
cerebrovascular
disorders, EP-A 474580.
Escitolopram and a method for its preparation are disclosed in US Patent No
4,943,590. The
stereo selectivity of citalopram, i.e. the 5-HT-reuptake inhibition in the S-
enantiomer, and
accordingly, the antidepressant effect of said enantiomer is also disclosed. S-
citalopram is
now in development as an antidepressant.
CONFIRMATION COPY
CA 02373757 2004-06-09
WO 01/03694 PCT/DKU0J00377
2
Studies have shown that patients suffering from neurotic disorders including
anxiety
disorders, especially generalised anxiety, and panic attacks, in particular in
association with
agoraphobia, have a quality of life impairment comparable with or greater than
the disability
found in patients with alcoholism, schizophrenia or personality disorders.
Furthermore, 5 current treatments are not always effective or cause
unacceptable side effects.
Consequently, there is a need for alternative therapies useful in the
treatment of neurotic
disorders.
1o Escitalopram has now been found to show potent effects in models of
neurotic disorders such
as anxiolytic effect and prominent effect in the treatment of panic attacks
and obsessive
compulsive disorder.
15 Description of the Invention
According to the present invention, a novel use of escitalopram, namely for
the preparation
of a medicament useful in the treatment of neurotic disorders is provided.
20 Throughout this specification and claims the term neurotic disorders is
used to designate a
group of mental disorders, including anxiety states, in particular generalised
anxiety disorder
and social anxiety disorder, post traumatic stress disorder, obsessive
compulsive disorder anu
panic attacks.
The terms generalised anxiety disorder, social anxiety disorder, post
traumatic stress disorder and
25 obsessive compulsive disorder are as defined in the fourth edition of
"Diagnostic and Statistical
Manual of Mental Disorders (DSM-IV-TRTM, American Psychiatric Association,
American
Psychiatric Press, 2000).
The phrase "panic attacks" contemplates treatment of any disease, which is
associated with
panic attacks including panic disorder, specific phobias, social phobia and
agoraphobia in
30 which panic attacks occur. These disorders are further defined in the DSM
IV. A panic
attack is a discrete period in which there is a sudden onset of intense
apprehension,
fearfulness or terror, often associated with feelings of impending doom.
During the attack,
symptoms such as palpitations, sweating, trembling, sensations of shortness of
breath,
CA 02373757 2001-11-13
WO 01/03694 PCT/DK00/00377
3
feeling of choking, chest pain or discomfort, nausea, feeling dizzy, feelings
of unreality, fear
of losing control or going crazy, fear of dying, paresthesias and chills or
hot flushes are
present.
Panic disorders are characterised by recurrent unexpected panic attacks about
which there is
a persistent concern. Agoraphobia is anxiety about, or avoidance of, places or
situations
from which escape might be difficult or in which help may not be available in
the event of a
panic attack. Specific phobia and social phobia (together formerly simple
phobia) are
characterised by marked and persistent fear that is excessive or unreasonable,
cued by the
presence or anticipation of a specific object or situation (flying, heights,
animals, seeing
blood etc.) or social performance situations.
The disorders in which panic attacks occur are differentiated from each other
by the
predictability of the occurrence of the attacks, for example, in panic
disorder the attacks are
unpredictable and not associated with any particular event, whereas in
specific phobia the
attacks are triggered by specific stimuli.
The phrase "treatment of panic disorder" means a reduction in the number or
prevention of
attacks and/or relief of the severity of the attacks. Similarly, the treatment
of generalised
anxiety disorder, social anxiety disorder, post traumatic stress disorder and
obsessive
compulsive disorder include the treatment or prevention of these diseases, or
the relief of the
symptoms thereof.
According to the invention, escitalopram may be used as the base of the
compound or as a
pharmaceutically acceptable acid addition salt thereof or as an anhydrate or
hydrate of such
salt. The salts of the compound used in the invention are salts formed with
non-toxic organic
or inorganic acids, in particular the oxalate.
Escitalopram has been found to show prominent effects different from the
effects of the
racemate in the "Inhibition of footshock-induced ultrasonic vocalisation in
adult rats" - test,
the "Mice Black and White Test" setup, and in the polydipsia test. These
models are standard
CA 02373757 2001-11-13
WO 01/03694 PCT/DK00/00377
4
animal models for anxiolytic effect and effect on panic attacks and for
obsessive compulsive
disorder, respectively.
According to the invention, escitalopram or a pharmaceutically acceptable salt
thereof may
be administered in any suitable way e.g. orally or parenterally, and it may be
presented in
any suitable form for such administration, e.g. in the form of tablets,
capsules, powders,
syrups or solutions or dispersions for injection. Preferably, and in
accordance with the
purpose of the present invention, the compound of the invention is
administered in the form
of a solid pharmaceutical entity, suitably as a tablet or a capsule or in the
form of a
suspension, solution or dispersion for injection.
Methods for the preparation of solid pharmaceutical preparations are well
known in the art.
Tablets may thus be prepared by mixing the active ingredients with ordinary
adjuvants
and/or diluents and subsequently compressing the mixture in a convenient
tabletting
machine. Examples of adjuvants or diluents comprise: corn starch, lactose,
talcum,
magnesium stearate, gelatine, lactose, gums, and the like. Any other adjuvant
or additive
such as colourings, flavourings, preservatives, etc. may also be used provided
that they are
compatible with the active ingredients.
The compound of the invention is most conveniently administered orally in unit
dosage
forms such as tablets or capsules, containing the active ingredient in a dose
from about 1.0
mg to 50 mg, preferably 5 mg/day to 40 mg/day, most preferably 10 mg/day to 20
mg/day.
The oxalate of escitalopram may be prepared as described in US Patent No
4,943,590 and
the base and other pharmaceutically acceptable salts may be obtained therefrom
by standard
procedures.
Thus the acid addition salts used according to the invention may be obtained
by treatment of
escitalopram with the acid in an inert solvent followed by precipitation,
isolation and
optionally re-crystallisation by known methods and if desired micronisation of
the crystalline
product by wet or dry milling or another convenient process, or preparation of
particles from
a solvent-emulsification process.
CA 02373757 2004-06-09
WO 01/03694 PCT/DK00/00377
Pharmacological Tests
Escitalopram was tested in well recognised and reliable test models of effects
on neurotic
disorders. Citalopram-racemate was included for comparison purposes.
5
The footshock- induced vocalisation test in adult rats.
The footshock- induced vocalisation test in adult rats (described in detail in
Sanchez C.,
Effect of serotonergic drugs on footshock-induced ultrasonic vocalization in
adult male rats.
Behav. Pharmacol. 1993; 4:267-277) is a test for anxiolytic and anti-panic
effects.
Experimental Procedure
Male rats (Wistar WU, Charles River, Germany), weighing 150-175 g at the
beginning of the
study were used.
TM
Briefly, test cages (22 cm x 22 cm x 22 cm) made of grey Perspex and equipped
with a metal
grid floor were used. Footshocks were delivered from a two pole shocker and a
microphone
sensitive to ultrasounds in the range of 20-30 kHz was placed in the centre of
the lid of the
test cage. The ultrasounds were sent from the microphone to a preamplifier and
converted
from AC signals to DC signals in a signal rectifier. The accumulated time, in
which the
voltage of the rectified signal was larger than the voltage of a previously
determined treshold
level, was recorded.
Twenty-four hours before the first test session the animals were primed. A rat
was placed in
each test cage and received, immediately thereafter, four 1.0 mA inescapable
footshocks
each of a duration of 10 sec and with an intershock interval of 5 sec. The
animals were left
in the test cage for 6 min after the last shock. On test days, drug or saline
was given 30 min
before test. The rats received four 1.0 mA inescapable footshocks each of a
duration of 10
sec. The intershock interval was 5 sec. Recording of ultrasonic vocalisation
started 1 min
after the last shock and lasted for 5 min. The total time spent on
vocalisation was recorded.
After a wash-out period of one week the rats were used in a new test session.
The rats were
used for a total of 7-8 weeks. At each test session, the animal groups were
randonily
allocated to treatment with saline or test drug. Each treatment group
consisted of 8 animals,
CA 02373757 2004-06-09
WO 01/03694 PCT/DKOO/00377
6
one saline and 2-4 drug treated groups were included at each session. Each
drug was tested
at least in two separate experiments with overlapping doses.
Results
The experiments showed that the maximum effect was 60-70% inhibition for
citalopram-
racemate whereas escitalopram was able to inhibit vocalisation completely.
Black and White Box Test
This is a test for anxiolytic effects. The test model is further described in
Sanchez, C. (1995)
1o Phan nacol. Toxicol. 77, 71-78.
Test procedure
Male mice (Lundbeck strain, Charles River, Germany) weighing 30-35 g were
housed in
groups of 4 in macrolon cages type 11 under a reversed 12 h day /night cycle
(lights off 7
p.m.). The mice were adapted to the reversed light/dark cycle for at least 3
weeks prior to
testing. The room temperature (21 t 2 C), relative humidity (55 5%), and air
exchange (16
times per h) were automatically controlled. The animals had free access to
commercii.al food
pellets and water.
The test box used was designed as described by Sanchez (1995) (supra).
Briefly, the test box
(45 cm x 27 cm x 27 cm) was open-topped and divided into two compartments
(ratio 2:3) by
a partition which was black on the side facing the black compartment and white
on the sid
facing the white compartment. The smaller chamber was made of black perspex.
The larger
chamber was made of white perspex except for the lowest 7.5 cm. This part was
made of
tra¾sparent perspex (outer walls) and black perspex (partition). The white
compartment was
connected to the black compartment by a 7.5 em x 7.5 cm opening in the
partition. The floor
of the white compartment was divided into 9 fields, and the floor of the black
was divided
TM
into 6 fields. The wliite compartment was illuminated by means of a Schott KL
1500
electronic lamp emitting cold light corresponding to a light intensity of 560
Lux. The mouse
test-system was fully automated by 2 rows of 11 infrared light sources and
photocells in the
transverse direction and 1 row of 16 in the longitudinal direction (lower
row). The lower row
of photocells (2 cm above cage floor) detected horizontal locomotor activity
(crossing,
entries, and time in each compartment), whereas the upper row of photocells (5
cm above
CA 02373757 2004-06-09
WO 01/03694 7 PCT/DK00/00377
cage floor) detected rearing activity. The accumulated data for 1 min
intervals were recorded
TM
from 4 test boxed simultaneously and stored in a Paradox data base.
The test boxes were placed in a dark and quiet room. The mice were transported
to the test
room in a darkened container about 2 h before test. The test room was
separated into two
parts by a black curtain. The drug treatment took place in one part of the
room using a
TM
minimum of red light_ Affter dosing, the mice were placed individually in
macrolon type II
cages until test. The pretreatment time was 30 min. The test boxes were placed
in the other
part of the room. The test was started by placing the mouse in the centre of
the brightly-lit
white compartment facing the opening to the black compartment. The test
duration was 5
lo min and the number of rears and line crossings between squares in both the
black and the
white compartment, number of entries into the black compartment and time spent
in the
white compartment were assessed.
Results
Escitalopram showed prominent effects in this model.
Schedule-induced Polydipsfa
Food deprived rats exposed to a procedure in which food is delivered
intermittently will
drink large amounts of water if given the opportunity to do so. This
behavioural phenomenon
is called schedule-induced polydipsia and can be considered as an excessive
expression of a
normal behaviour. Schedule-induced polydipsia is regarded as a model of
obsessive-
compulsive disorder (Woods et al. 1993).
Test Procedure:
Male wistar rats (Mrallegard) housed in pairs and kept on a food-restricted
diet (80% of
normal body weight) for 2 weeks before the start of testing and throughout the
duration of
testing. To induce polydipsia rats were placed in test chambers where a pellet
dispenser
automatically dispensed one 60 mg food pellet every 60 seconds. Water was
available at all
times in the test chamber. Rats were tested 4-5 times per week, after 3-4
weeks training 70%
of the rats were drinking >l Oml per 30 min test session.
Once the rats had attained a steady drinking level compounds could be tested.
Citalopram
(40 mg/kg) or Lu 26-054 (20 mg/kg) were administered orally 60 min prior to
testing and at
CA 02373757 2001-11-13
WO 01/03694 PCT/DK00/00377
8
10:00 on the non-test days. The water intake was presented as a percentage of
the pre-dosing
(baseline) level.
Results:
Escitalopram produced a significant reduction in water intake, whereas
citalopram was
without effect.
All these studies show that escitalopram has potent anti neurotic diseases
effects, in
particular anxiolytic effects and effects on panic attacks and obsessive
compulsive disorder.