Note: Descriptions are shown in the official language in which they were submitted.
CA 02375098 2001-11-23
WO 00/75353 PCT/US00/00415
COMPOSITIONS AND METHODS USEFUL FOR PRODUCTION OF
RECOMBINANT VIRUSES WHICH REQUIRE HELPER VIRUSES
Field of the Invention
The present invention relates generally to compositions and methods useful in
producing recombinant viruses, and particularly, those methods which require
the use of a helper virus. More particularly, the invention provides
compositions and
methods useful in obtaining a recombinant viral particle in a form which is
readily
separated from cultures in which helper virus was used in the production.
Background of the Invention
The usefulness of traditional adenovirus vectors is often limited because of
induction of strong cell-mediated immune response [Yang et al., Gene Ther.,
3:137-
144 ( 1996)]. Previous studies with E 1 deleted vector demonstrated that viral
gene
expression contributes to this problem [Yang et al, J. Virol, 70:7209-7212 (
1996);
Yang et al, Immunity, 1:433-442 (1994)]. Newly developed helper dependent
adenovirus vectors, constructed through complete removal of all adenovirus
coding
regions [Mitani, Proc. Natl. Acad. Sci. USA, 92:3854-3858 (1995)], have become
very promising gene therapy vectors since prolongation of transgene expression
and
increased capacity have been tested in a number of animal experiments [Fisher
et al,
Virology, 217:11-22 (1996); Morsy et al, Proc. Natl. Acad. Sci. USA, 95:7866-
7871
(1998); Schiedner et al, Nat. Genet., 18:180-183 (1998); Whittle, Trends
Genet.,
14:136-137 (1998)]. However, such vectors make poor pharmaceutical quality
vectors because helper virus contamination make them difficult to produce.
Uncontrolled helper virus replication competes with the vector replication and
packaging, which also decrease the yield. The commonly used cre-loxP system
has
been the primary system available to generate helper dependent adenovirus
vectors
with diminished helper virus [Parks et al, Proc. Natl. Acad. Sci USA, 93:13565-
13570
(1996); Parks, J. Virol., 71:3293-3298 (1997)].
What are needed are methods of producing recombinant viruses which are
substantially free of contamination with helper virus.
aatctgatca accggaggcg atttcagca
CA 02375098 2001-11-23
WO 00/75353 PCT/US00/00415
Summary of the Invention
The present invention provides compositions and methods which permit
production of recombinant viral vectors which are readily purified from helper
viruses. The method uses a recombinant helper virus which has been designed to
contain at least one site for a rare-cutting restriction enzyme, a viral
vector which is
helper-dependent for packaging into a virus, and a recombinant host cell which
expresses the rare-cutting restriction enzyme. In a preferred embodiment, the
rare-
cutting enzyme is I-SceI. The method involves transfecting or infecting the
host cell
with the helper virus and viral vector and incubating the cell under
conditions which
permit packaging of the viral vector. Thereafter, the rare-cutting restriction
enzyme
expressed by the host cell digests the helper virus, permitting ready
separation of the
digested fragments of the helper virus from the packaged recombinant virus. In
a
preferred embodiment, the restriction enzyme is I-Scel and the viral vector is
an
adenovirus.
I S Thus, in one aspect, the present invention provides a method for producing
helper-dependent virus which involves the following steps. A host cell capable
of
expressing a rare-cutting restriction enzyme (e.g., I-SceI) is provided. The
host cell
is transfected with a recombinant viral vector comprising a minigene
containing a
transgene encoding a selected protein and regulatory sequences which control
expression of said protein. Subsequently, the host cell is infected with a
recombinant
helper virus engineered to contain at least one, and preferably multiple
restriction
sites for the rare-cutting restriction enzyme, e.g., I-SceI. The host cell
with the
recombinant viral vector and helper virus is then cultured under conditions
which
permit packaging of the recombinant viral vector in a viral particle, wherein
the
helper virus and the host cell provide to the recombinant adenovirus vector
the
necessary viral genes for viral packaging.
In another aspect, the present invention provides a recombinant virus
produced by the method of the invention, which is substantially purified from
helper
virus.
In yet another aspect, the invention provides a stable mammalian cell line
which expresses a rare-cutting restriction enzyme, e.g., I-SceI.
2
CA 02375098 2001-11-23
WO 00/75353 PCT/US00/00415
In still another aspect, the invention provides a recombinant helper virus
useful in helper-dependent production of a recombinant virus vector, wherein
said
helper virus is engineered to contain a site for a rare-cutting restriction
enzyme
downstream of the packaging signal.
In still a further aspect, the invention provides a method for the helper-
dependent production of a recombinant virus which involves transfecting a host
cell
with a recombinant viral vector comprising a minigene containing a transgene
encoding a selected protein and regulatory sequences which control expression
of said
protein. The host cell is further infected with a recombinant helper virus
engineered
to contain a site for a rare-cutting enzyme, e.g., I-SceI, and the
corresponding
restriction enzyme, e.g., I-SceI, is delivered to the host cell. The host cell
is then
cultured under conditions which permit packaging of the recombinant virus
vector in
a viral particle. Suitably, the helper virus and the host cell provide to the
recombinant viral vector the necessary viral genes for viral packaging and the
I S restriction enzyme cleaves the recombinant helper virus following
generation of the
recombinant viral vector.
In yet a further aspect, the invention provides a cell lysate comprising a
recombinant virus which is substantially free of helper virus.
Other aspects and advantages of the invention will be readily apparent from
the following detailed description of the invention.
Brief Description of the Drawines
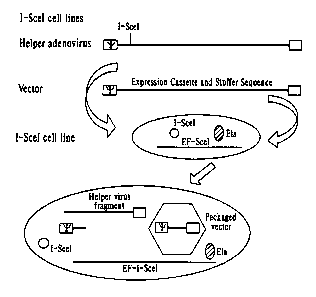
Fig. lA is a schematic illustration of the generation of recombinant
adenoviruses using I-SceI expressed from the human 293 cell line.
Fig. 1B is a schematic illustration of the generation of recombinant
adenoviruses using recombinant AAV vectors to deliver the functional I-SceI
enzymes
Fig. 2 is a detailed restriction map for a recombinant adenovirus helper virus
of the invention, Ad-3I. There are three I-SceI sites, one located before the
CMV
promoter, one located between the CMV promoter and the al-antitrypsin gene and
CA 02375098 2001-11-23
WO 00/75353 PCT/US00/00415
one located after the al-antitrypsin gene, respectively. The CMV-al-
antitrypsin
cassette was located right after the 3' end of adenovirus genome.
Fig. 3 is a line graph providing a time course of gene expression from Ad-3I
in the presence of I-SceI enzyme in human 293 cells. Ad-3I (MOI 10) infected
either
293 cells, 293-I-SceI cell line or 293 cells along with rAAV-I-SceI at MOI 10.
The
amount of human al-antitrypsin in media was measured by ELISA at the various
times indicated in the graph.
Fig. 4 is a bar chart illustrating that 293 I-SceI cell lines enhanced helper
dependent adenovirus production.
Fig. 5 is a bar graph illustrating the performance of an I-SceI cell line of
the
invention, as determined by helper virus at 48 hours post-infection shown as
the al-
antitrypsin secreted into the media. The digested helper virus is not able to
express
al-antitrypsin. The same amount of Ad-0-LacZ vector and Ad-3I mix were used to
infect the same amount of 293, 293-I-Scel(a), 293-I-SceI(b) cells. The viruses
were
harvested 48 hours post-infection. The lacZ-forming unit (LFU) was determined
by
X-Gal staining.
Detailed Description of the Invention
The present invention provides compositions and methods which permit
production of recombinant viral vectors which are readily separated from
helper
viruses. Thus, the invention is particularly suited for production of viral
vectors
which are dependent upon helper viruses for packaging and/or encapsidation
into a
recombinant virus. The invention uses a recombinant helper virus which has
been
designed to contain at least one, and preferably, multiple sites for a
selected rare-
cutting restriction enzyme and a recombinant host cell which is capable of
expressing
the rare-cutting restriction enzyme. As used herein, a helper virus is any
virus which,
in conjunction with the selected host cell, provides the necessary viral
products to
permit packaging and/or encapsidation of the selected viral vector into an
infectious
recombinant virus.
The host cell may be a cell line stably or transiently expressing the rare-
cutting restriction enzyme. The method involves delivering the helper virus
and viral
4
CA 02375098 2001-11-23
WO 00/75353 PCT/US00/00415
vector to a host cell which expresses the restriction enzyme (or is modified
to express
the enzyme) and culturing the cell under conditions which permit encapsidation
of the
viral vector. Thereafter, the restriction enzyme expressed by the host cell
digests the
helper virus, permitting ready separation of the digested fragments of the
helper virus
from the packaged recombinant virus.
As defined herein, a rare-cutting restriction enzyme is a restriction enzyme
which is not naturally present in the selected host cell and/or selected viral
vector
genome or which recognizes a site which is present sufficiently infrequently
in the
genomes of the species of the host or virus, that the site (and/or enzyme) is
unlikely to
occur naturally in the host cell or viral vector. In preferred embodiment, a
suitable
rare-cutting enzyme for use in the present invention is the restriction enzyme
I-SceI.
The I-SceI enzyme is an endonuclease encoded by the group I intron of S.
cerevisiae mitochondria [L. Colleaux et al, Proc. Natl. Acad. Sci. USA,
85:6022-6026
( 1988), which has high specificity for an 18 by nonpalindromic nucleotide
sequence
[I-SceI site: SEQ ID NO:1: 5'-tagggataa/cagggtaat]. In a human genome with
about 3
x 109 nucleotides, a common restriction endonuclease generally recognizes a
short
stretch of nucleotides of 4 to 8 base pairs; thus, there would be about one
million such
sites in one human genome. In contrast, the I-SceI site occurs randomly only
once in
every 20 human genomes. The rarity of I-SceI sites has been partially
confirmed by
the fact that there are no I-SceI sites in the genomes of many organisms,
including
viruses, bacteria and yeast. Based on the information provided herein, one of
skill in
the art can readily substitute other suitable rare-cutting restriction enzymes
for I-SceI
for use in the invention.
Such rare-cutting restriction enzymes may be selected, for example, from
among various restriction enzymes which are native to non-mammalian animals,
plants, yeast, fungi, and/or insects, which restriction enzymes are not native
to
mammalian species. Examples of rare-cutting restriction enzymes, in addition
to I-
SceI include, PspI, I-Ceul, and the like. Other suitable rare-cutting
restriction
enzymes may be selected from among those which recognized sites which are at
least
about 12 to about 40 nucleotides in length, preferably about 14 to about 20
nucleotides in length, and most preferably, at least about 18 nucleotides in
length.
5
CA 02375098 2001-11-23
WO 00/75353 PCT/US00/00415
Such restriction enzymes are available from a variety of commercial sources,
including, e.g., New England BioLabs, Promega, and Boehringer Mannheim.
Alternatively, these enzymes or their coding sequences may be produced
synthetically or using recombinant technology.
For example, the I-SceI enzyme may be purchased from commercial sources
(e.g., Boehringer Mannheim, Germany). Alternatively, the sequence of the
enzyme
may be produced by conventional chemical synthesis. [See, e.g., G. Barony and
R.B.
Merrifield, The Peptides: Analysis, Synthesis & Biology, Academic Press, pp. 3-
285
(1980)]. Preferably, the native coding sequence for this enzyme (or another
selected
rare-cutting restriction enzyme) is altered to optimize expression in
mammalian cells,
which are the preferred host cells. Techniques for optimizing expression,
e.g., by
altering preference codons, are well known to those of skill in the art.
Similarly, the
sequences for the I-SceI site and, other selected rare-cutting restriction
enzyme sites,
may be produced synthetically, recombinantly, or obtained using other suitable
I S techniques. See, e.g., Barony and Merrifield, cited above; Sambrook et al,
Molecular
Cloning: A Laboratory Manual, Cold Spring Harbor Laboratories, Cold Spring
Harbor, New York.
I. Host Cells
The invention provides host cells which are useful in the production of helper-
dependent viruses. In one embodiment, the invention provides cell lines which
stably
express a rare-cutting restriction enzyme. In another embodiment, the
sequences
encoding the rare-cutting restriction enzyme are delivered in traps to a
selected host
cell via a suitable vector or other nucleic acid molecule. For convenience
throughout
this specification, reference will be made to the I-SceI enzyme. However, it
will be
readily understood that another rare-cutting restriction enzyme and/or its
restriction
enzyme site, as defined herein, may be substituted.
A. Stable Cell Line Expressing Rare-Cutting Restriction Enzyme
Functions
A cell line of the invention may be constructed by providing the
selected host cell line with a nucleic acid molecule encoding a rare-cutting
restriction
6
CA 02375098 2001-11-23
WO 00/75353 PCT/US00/00415
enzyme or a functional fragment thereof operably linked to regulatory
sequences
which control expression thereof using conventional techniques. As used
herein,
"operably linked" sequences include both expression control sequences that are
contiguous with the gene of interest and expression control sequences that act
in traps
or at a distance to control the gene of interest.
Expression control sequences include appropriate transcription
initiation, termination, promoter and enhancer sequences; efficient RNA
processing
signals such as splicing and polyadenylation (polyA) signals; sequences that
stabilize
cytoplasmic mRNA; sequences that enhance translation efficiency (i.e., Kozak
consensus sequence); sequences that enhance protein stability; and when
desired,
sequences that enhance secretion of the encoded product. A great number of
expression control sequences, native, constitutive, inducible and/or tissue-
specific, are
known in the art and may be utilized.
Desirably, the nucleic acid molecule encoding the rare-cutting
I S restriction enzyme, e.g., I-Scel, is further provided with a nuclear
localization signal,
which targets the I-SceI sequences to the nucleus. Suitable nuclear
localization
signals are known to those of skill in the art and are not a limitation of the
present
invention.
In one embodiment, the host cell stably expresses the rare-cutting
restriction enzyme, e.g., I-SceI, under the control of a constitutive
promoter.
Examples of such promoters include, without limitation, the retroviral Rous
sarcoma
virus (RSV) LTR promoter (optionally with the RSV enhancer), the
cytomegalovirus
(CMV) promoter (optionally with the CMV enhancer) [see, e.g., Boshart et al,
Cell,
41:521-530 (1985)], the SV40 promoter, the dihydrofolate reductase promoter,
the
(3-actin promoter, the phosphoglycerol kinase (PGK) promoter, and the EF 1 a
promoter [Invitrogen].
In another embodiment, the rare-cutting restriction enzyme, e.g., I-
SceI, is stably expressed by the host cell under the control of an inducible
promoter.
Inducible promoters are regulated by exogenously supplied compounds,
including,
the zinc-inducible sheep metallothionine (MT) promoter, the dexamethasone
(Dex)
inducible mouse mammary tumor virus (MMTV) promoter, the T7 polymerise
7
CA 02375098 2001-11-23
WO 00/75353 PCT/US00/00415
promoter system [WO 98/10088]; the ecdysone insect promoter [No et al, Proc.
Natl.
Acad. Sci. USA, 93:3346-3351 (1996)], the tetracycline-repressible system
[Gossen et
al, Proc. Natl. Acad. Sci. USA, 89:5547-5551 (1992)], the tetracycline-
inducible
system [Gossen et al, Science, 268:1766-1769 (1995), see also Harvey et al,
Curr.
Opin. Chem. Biol., 2:512-518 (1998)], the RU486-inducible system [Wang et al,
Nat.
Biotech., 15:239-243 (1997) and Wang et al, Gene Ther., 4:432-441 (1997)] and
the
rapamycin-inducible system [Magari et al, J. Clip. Invest., 100:2865-2872
(1997)].
Most preferably, the cell line selected for transformation with the
nucleic acid molecule encoding the rare-cutting restriction enzyme, e.g., I-
SceI, and,
optionally, expression control sequences therefor, is a mammalian cell line,
including,
without limitation, cells such as A549, WEHI, 3T3, IOTl/2, BHK, MDCK, COS1,
COS7, BSC 1, BSC 40, BMT 10, VERO, WI38, HeLa, Saos, C2C12, L cells,
HT1080, HepG2 and primary fibroblast, hepatocyte and myoblast cells derived
from
mammals including human, monkey, mouse, rat and hamster. Preferred cells
include
human cells, and most preferably, cells which express adenovirus E 1
functions, e.g.,
293 cells. However, other cell lines may be readily obtained from the American
Type
Culture Collection (ATCC) or a variety of commercial and academic sources.
B. Delivery of Rare-cutting Restriction Enzyme
In another embodiment, the method of the invention is performed by
delivery of sequences encoding the rare-cutting restriction enzyme, e.g., I-
SceI, or a
functional fragment thereof to a selected host cell in traps. The nucleic acid
molecule
carrying the rare-cutting restriction enzyme (e.g, I-SceI) coding sequences
and
expression control sequences may be in any form which transfers these
components
to the host cell and permits expression of the restriction enzyme, preferably
in the cell
nucleus. In this embodiment, the selected host cell is preferably a human cell
line,
e.g., 293. However, other suitable host cells may be readily selected from
among
those known in the art. See discussion of host cells above.
The nucleic acid molecule carrying the sequences encoding the rare-
cutting restriction enzyme (e.g., I-SceI), as well as the sequences which
regulate
expression thereof, are provided to the host cell by any suitable method,
including
transfection, electroporation, liposome delivery, membrane fusion techniques,
high
8
CA 02375098 2001-11-23
WO 00/75353 PCT/US00/00415
velocity DNA-coated pellets, viral infection and protoplast fusion. See, for
instance,
Sambrook et al, Molecular Cloning: A Laboratory Manual, Cold Spring Harbor
Laboratories, Cold Spring Harbor, New York. Most suitably, these sequences are
contained within a vector. A "vector" includes, without limitation, any
genetic
element, such as a plasmid, phage, transposon, cosmid, chromosome, virus,
virion,
etc..
In one desirable embodiment, the restriction enzyme (e.g., I-SceI)
coding sequences are delivered via a viral vector, and most preferably, via a
recombinant, infectious virus. Selection of the enzyme delivery virus is not a
limitation on the present invention. Suitable recombinant enzyme (e.g., I-
SceI)
delivery viruses may be readily engineered utilizing such viruses as adeno-
associated
viruses (AAV), retroviruses, adenoviruses, hybrid adeno-AAV viruses,
lentiviruses,
baculovirus, herpes virus, and pox viruses, among others.
In one currently preferred embodiment, the enzyme delivery virus is a
recombinant AAV (rAAV) containing the enzyme coding sequences operably linked
to suitable expression control sequences, which direct expression of the
enzyme (e.g.,
I-SceI) in the host cell and target the nucleus. This I-SceI-rAAV and other
delivery
virus constructs of the invention are prepared using the rare-cutting
restriction
enzyme sequences, obtained as described herein, and using known methods. For
example, methods for producing rAAV vectors have been described. [See, W. Xiao
et
al, J. Virol., 72:10222-10226 (1998); US Patent No. 5,658,776; US Patent No.
5,622,856, among others].
Generally, an I-SceI-rAAV of the invention employs the cis-acting 5'
and 3' inverted terminal repeat (ITR) sequences [see, e.g, B.J. Carter, in
"Handbook
of Parvoviruses", ed., P. Tijsser, CRC Press, pp. 155-168 (1990)] flanking the
sequences encoding I-SceI and directing expression thereof. The ITR sequences
are
about 145 by in length. Preferably, substantially the entire sequences
encoding the
ITRs are used in the molecule, although some degree of minor modification of
these
sequences is permissible. The ability to modify these ITR sequences is within
the
skill of the art. [See, e.g., texts such as Sambrook et al, "Molecular
Cloning. A
Laboratory Manual.", 2d edit., Cold Spring Harbor Laboratory, New York (1989);
9
CA 02375098 2001-11-23
WO 00/75353 PCT/US00/00415
Carter et al, cited above; and K. Fisher et al., , ZQ:520-532 (1996)]. An
example of such a molecule employed in the present invention is a "cis-acting"
plasmid containing the transgene, in which the selected transgene sequence and
associated regulatory elements are flanked by the 5' and 3' AAV ITR sequences.
The AAV ITR sequences may be obtained from any known AAV,
including presently identified human AAV types. Similarly, AAVs known to
infect
other animals may also provide these ITRs employed in the molecules or
constructs
of this invention. For example, the ITRs may be provided by AAV type 1, AAV
type
2, AAV type 3, AAV type 4, AAV type 5, parvovirus type Hl, MVM, LuIII, or by
any other parvovirus or AAV serotype. A variety of AAV strains are available
from
the American Type Culture Collection or are available by request from a
variety of
commercial and institutional sources. In the following exemplary embodiments
an
AAV-2 is used for convenience. However, the selection of the species and
serotype
of AAV that provides these sequences is within the skill of the artisan
according to
the teachings of this application and does not limit the following invention.
In addition, the enzyme delivery virus contains the restriction enzyme
nucleic acid sequences, a nuclear localization signal, and conventional
regulatory
elements necessary to drive expression of the enzyme in a host cell
transfected
(infected) with this enzyme delivery virus. Such expression control elements
include
promoters, including both constitutive and inducible promoters, as are
described
above, poly A sequences, and the like. Other heterologous nucleic acid
sequences
optionally present in this enzyme delivery virus include sequences providing
signals
required for efficient polyadenylation of the RNA transcript, and introns with
functional splice donor and acceptor sites. A common poly-A sequence which is
employed in the enzyme delivery viruses useful in this invention is that
derived from
the papovavirus SV-40. In the I-SceI-rAAV delivery virus, the poly-A sequence
generally is inserted following the transgene sequences and before the 3' AAV
ITR
sequence. An enzyme delivery virus useful in the present invention may also
contain
an intron, desirably located between the promoter/enhancer sequence and the
transgene. One possible intron sequence is also derived from SV-40, and is
referred
CA 02375098 2001-11-23
WO 00/75353 PCT/US00/00415
to as the SV-40 T intron sequence. Selection of these and other common vector
elements are conventional and many such sequences are available [see, e.g.,
Sambrook et al, and references cited therein at, for example, pages 3.18-3.26
and
16.17-16.27]. Optionally, the enzyme delivery virus may contain a selectable
marker
or reporter sequences, such as sequences encoding hygromycin or purimycin,
among
others. See the discussion of reporter sequences below.
The engineering methods used to construct any embodiment of this
invention are known to those with skill in nucleic acid manipulation and
include
genetic engineering, recombinant engineering, and synthetic techniques. See,
e.g.,
Sambrook et al, cited above; and International Patent Application NO.
W095/13598.
Suitably, the I-SceI-rAAV may be delivered to the selected host cells
at a multiplicity of infection (MOI) of about 5 to about 200 rAAV genome
particles,
and preferably at an MOI of 10 to 100 rAAV genome particles. Suitable MOI for
other selected enzyme delivery viruses may be in this range, or may be
adjusted as
desired by one of skill in the art. Alternatively, where the I-SceI is
delivered by a
vector which lacks the ability to infect host cells, the vector is delivered
to the host
cells in an amount of about 5 pg to about 100 pg DNA by any suitable means
known
to those of skill in the art.
The enzyme delivery vehicle (e.g., I-SceI-rAAV) may be provided to
the host cells at any time prior to cell lysis, including prior to delivery of
the vector
(e.g., by infection or transfection), prior to delivery of the helper virus
sequences
(e.g., infection with the helper virus), or after delivery of either or both
of these
components to the host cell. For example, where the enzyme delivery vehicle
constitutively expresses the restriction enzyme (e.g., I-SceI), it may be
desirable to
provide this enzyme delivery vehicle to the host cells following delivery of
the viral
vector and the helper virus. Alternatively, where the enzyme delivery vehicle
inducibly expresses the restriction enzyme, the timing of delivery of the
enzyme may
not be critical. However, the selection of promoters, and the determination of
timing
of delivery of an enzyme delivery virus (or other nucleic acid molecule) to
the host
cell may be made by one of skill in the art in view of the information
provided herein.
11
CA 02375098 2001-11-23
WO 00/75353 PCT/US00/00415
II. Recombinant Vector
The compositions and methods of the present invention are particularly well
suited for packaging of a recombinant viral vector which lacks sufficient
viral genes
to permit encapsidation in a capsid in the absence of a helper virus and host
cell.
Together, the helper virus and host cell provide the gene functions necessary
to
encapsidate the recombinant virus. This present invention may be utilized for
any
viral vector which is dependent upon a helper virus for packaging. Such viral
vectors
may include, without limitation, recombinant adeno-associated vectors (rAAV),
recombinant adenovirus vectors, hybrid adenovirus/AAV vectors, retroviruses,
and
lentiviruses. Selection of a suitable viral vector is not a limitation of the
present
invention.
In one embodiment, the method of the invention is particularly well suited for
packaging of adenoviral vectors. In a preferred embodiment, the adenoviral
vector
contains only minimal adenovirus sequences. In one example, the recombinant
adenovirus vector, referred to herein as pAdO is described below. Methods of
producing these recombinant adenoviral vectors are known in the art. See,
e.g.,
International Patent Publication No. W096/13597.
A. The Adenovirus Sequences of pAdd
The adenovirus nucleic acid sequences of the pAdO vector provide the
minimum adenovirus sequences which enable a viral particle to be produced with
the
assistance of a helper virus and, optionally, a packaging cell line. These
sequences
assist in delivery of a recombinant transgene genome to a target cell by the
resulting
recombinant virus.
The DNA sequences of a number of adenovirus types are available
from Genbank, including type Ad5 [Genbank Accession No. M73260]. The
adenovirus sequences may be obtained from any known adenovirus serotype, such
as
serotypes 2, 3, 4, 7, 12 and 40, and further including any of the presently
identified
human types [see, e.g., Horwitz, cited above]. Similarly adenoviruses known to
infect other animals may also be employed in the vector constructs of this
invention.
The selection of the adenovirus type is not anticipated to limit the following
invention. A variety of adenovirus strains are available from the American
Type
12
CA 02375098 2001-11-23
WO 00/75353 PCT/US00/00415
Culture Collection, Manassas, Virginia, or available by request from a variety
of
commercial and institutional sources. In the following exemplary embodiment,
an
adenovirus type 5 (Ad5) is used for convenience.
However, it is desirable to obtain a variety of pAdO vectors based on
different human adenovirus serotypes. It is anticipated that a library of such
plasmids
and the resulting Ad0 viruses would be useful in a therapeutic regimen to
evade
cellular, and possibly humoral, immunity, and lengthen the duration of
transgene
expression, as well as improve the success of repeat therapeutic treatments.
Additionally the use of various serotypes is anticipated to produce
recombinant
viruses with different tissue targeting specificities.
Specifically, the pAd~ vector lacks nucleic acid sequences encoding
all functional adenoviral genes. Such functional genes include E1, E2, E3, E4,
the
intermediate genes (IVa and IX) and late genes (L1, L2, L3, L4, L5). More
specifically, the adenovirus sequences employed are the cis-acting 5' and 3'
inverted
terminal repeat (ITR) sequences of an adenovirus (which function as origins of
replication) and the native 5' packaging/enhancer domain that contains
sequences
necessary for packaging linear Ad genomes and enhancer elements for the E1
promoter. These sequences are necessary for replication and virion
encapsidation.
See, e.g., P. Hearing et al, ~, x(8):2555-2558 (1987); M. Grable and P.
Hearing, , 44(5): 2047-2056 (1990); and M. Grable and P. Hearing, ,
x(2):723-731 ( 1992).
The entire adenovirus 5' sequence containing the 5' ITR and
packaging/enhancer region can be employed as the 5' adenovirus sequence in the
pAdO vector. This left terminal (5') sequence of the Ad5 genome useful in this
invention spans by 1 to about 360 of the conventional adenovirus genome, also
referred to as map units 0-1 of the viral genome. This sequence includes the
5' ITR
and the packaging/enhancer domain. Preferably, this adenovirus 5' region is
employed in the vector in its native, unmodified form. However, some
modifications
including deletions, substitutions and additions to this sequence which do not
adversely effect its biological function may be acceptable. See, e.g.,
International
Patent Publication No. WO 93/24641, published December 9, 1993. The ability to
13
CA 02375098 2001-11-23
WO 00/75353 PCT/US00/00415
modify these ITR sequences is within the ability of one of skill in the art.
See, e.g.,
texts such as Sambrook et al, "Molecular Cloning. A Laboratory Manual.", 2d
edit.,
Cold Spring Harbor Laboratory, Cold Spring Harbor, New York ( 1989).
The 3' adenovirus sequences of the vector include the right terminal
(3') ITR sequence of the adenoviral genome spanning about by 35,353 - end of
the
adenovirus genome, or map units 98.4-100. This entire sequence is desirably
employed as the 3' sequence of a pAd~ vector. Preferably, the native
adenovirus 3'
region is employed in the shuttle vector in unmodified form. However, some
modifications to this sequence which do not adversely effect its biological
function
may be acceptable.
An exemplary pAdO vector used in this invention, described below,
lacks adenovirus sequences encoding functional adenoviral genes. The pAdO
vector
contains Ad5 5' and 3' cis-elements, as well as the transgene sequences
described
below. Suitably, these 5' and 3' elements may flank the transgene (e.g., 5'
cis-
elements, transgene, 3' cis-elements). Alternatively, these 5' ITRs and 3'
ITRs may
be oriented in a head-to-tail configuration, located upstream of the
transgene. Such a
vector may be constructed using conventional genetic engineering techniques,
e.g.,
homologous recombination and the like. See, e.g., US Patent No. 6,001,557.
From the foregoing information, it is expected that one of skill in the
art may employ other equivalent adenovirus sequences for use in the pAdO
vectors of
this invention. These sequences may include other adenovirus strains, or the
above
mentioned cis-acting sequences with minor modifications. Further, one of skill
in
the art will readily appreciate that the method of the invention is useful in
overcoming
difficulties in separating helper virus from production cultures for other
viral vectors
lacking gene functions required for packaging. Such other vectors include rAd
vectors lacking one or more adenoviral genes selected from E1, E2, E4, the
intermediate genes and the late genes, as well as non-adenoviral vectors.
Other non-
adenoviral vectors which require helper functions for production include ,
without
limitation, parvoviruses, including adeno-associated viruses and retroviruses,
including, lentiviruses. One of skill in the art can readily select other
suitable systems
for use of the present invention.
14
CA 02375098 2001-11-23
WO 00/75353 PCT/US00/00415
B. The Transgene
The transgene sequence of the recombinant vector and the virus
resulting from the method of the invention is a nucleic acid sequence,
heterologous to
the virus sequence, which encodes a polypeptide, protein, or other product, of
interest.
The transgene is operatively linked to regulatory components in a manner which
permits transgene transcription.
The composition of the transgene sequence will depend upon the use
to which the resulting virus will be put. For example, one type of transgene
sequence
includes a reporter sequence, which upon expression produces a detectable
signal.
Such reporter sequences include without limitation, DNA sequences encoding ~3-
lactamase, (3-galactosidase (LacZ), alkaline phosphatase, thymidine kinase,
green
fluorescent protein (GFP), chloramphenicol acetyltransferase (CAT),
luciferase,
membrane bound proteins including, for example, CD2, CD4, CD8, the influenza
hemagglutinin protein, and others well known in the art, to which high
affinity
antibodies directed thereto exist or can be produced by conventional means,
and
fusion proteins comprising a membrane bound protein appropriately fused to an
antigen tag domain from, among others, hemagglutinin or Myc.
These sequences, when associated with regulatory elements which
drive their expression, provide signals detectable by conventional means,
including
enzymatic, radiographic, colorimetric, fluorescence or other spectrographic
assays,
fluorescent activating cell sorting assays and immunological assays, including
enzyme linked immunosorbent assay (ELISA), radioimmunoassay (RIA) and
immunohistochemistry. For example, where the marker sequence is the LacZ gene,
the presence of helper virus is detected by assays for beta-galactosidase
activity.
Where the transgene is luciferase, the helper virus may be measured by light
production in a luminometer.
However, desirably, the transgene is a non-marker sequence encoding
a product which is useful in biology and medicine, such as proteins, peptides,
anti-
sense nucleic acids (e.g., RNAs), enzymes, or catalytic RNAs. The transgene
may be
used to correct or ameliorate gene deficiencies, which may include
deficiencies in
which normal genes are expressed at less than normal levels or deficiencies in
which
CA 02375098 2001-11-23
WO 00/75353 PCT/US00/00415
the functional gene product is not expressed. A preferred type of transgene
sequence
encodes a therapeutic protein or polypeptide which is expressed in a host
cell. The
invention further includes using multiple transgenes, e.g., to correct or
ameliorate a
gene defect caused by a mufti-subunit protein. In certain situations, a
different
transgene may be used to encode each subunit of a protein, or to encode
different
peptides or proteins. This is desirable when the size of the DNA encoding the
protein
subunit is large, e.g., for an immunoglobulin, the platelet-derived growth
factor, or a
dystrophin protein. In order for the cell to produce the mufti-subunit
protein, a cell is
infected with the recombinant virus containing each of the different subunits.
Alternatively, different subunits of a protein may be encoded by the same
transgene.
In this case, a single transgene includes the DNA encoding each of the
subunits, with
the DNA for each subunit separated by an internal ribozyme entry site (IRES).
This
is desirable when the size of the DNA encoding each of the subunits is small,
e.g., the
total size of the DNA encoding the subunits and the IRES is less than five
kilobases.
However, the selected transgene may encode any product desirable for study.
The
selection of the transgene sequence is not a limitation of this invention.
Useful products encoded by the transgene include hormones and
growth and differentiation factors including, without limitation, insulin,
glucagon,
growth hormone (GH), parathyroid hormone (PTH), growth hormone releasing
factor
(GRF), follicle stimulating hormone (FSH), luteinizing hormone (LH), human
chorionic gonadotropin (hCG), vascular endothelial growth factor (VEGF),
angiopoietins, angiostatin, granulocyte colony stimulating factor (GCSF),
erythropoietin (EPO), connective tissue growth factor (CTGF), basic fibroblast
growth factor (bFGF), acidic fibroblast growth factor (aFGF), epidermal growth
factor (EGF), transforming growth factor a (TGFa), platelet-derived growth
factor
(PDGF), insulin growth factors I and II (IGF-I and IGF-II), any one of the
transforming growth factor (3 superfamily, including TGF (3, activins,
inhibins, or any
of the bone morphogenic proteins (BMP) BMPs 1-15, any one of the
heregluin/neuregulin/ARIA/neu differentiation factor (NDF) family of growth
factors,
nerve growth factor (NGF), brain-derived neurotrophic factor (BDNF),
neurotrophins
NT-3 and NT-4/5, ciliary neurotrophic factor (CNTF), glial cell line derived
16
CA 02375098 2001-11-23
WO 00/75353 PCT/US00/00415
neurotrophic factor (GDNF), neurturin, agrin, any one of the family of
semaphorins/collapsins, netrin-1 and netrin-2, hepatocyte growth factor (HGF),
ephrins, noggin, sonic hedgehog and tyrosine hydroxylase.
Other useful transgene products include proteins that regulate the
immune system including, without limitation, cytokines and lymphokines such as
thrombopoietin (TPO), interleukins (IL) IL-1 through IL-17, monocyte
chemoattractant protein, leukemia inhibitory factor, granulocyte-macrophage
colony
stimulating factor, Fas ligand, tumor necrosis factors a and ~3, interferons
a, (3, and y,
stem cell factor, flk-2/flt3 ligand. Gene products produced by the immune
system are
also useful in the invention. These include, without limitations,
immunoglobulins
IgG, IgM, IgA, IgD and IgE, chimeric immunoglobulins, humanized antibodies,
single chain antibodies, T cell receptors, chimeric T cell receptors, single
chain T cell
receptors, class I and class II MHC molecules, as well as engineered
immunoglobulins and MHC molecules. Useful gene products also include
complement regulatory proteins such as complement regulatory proteins,
membrane
cofactor protein (MCP), decay accelerating factor (DAF), CR1, CF2 and CD59.
Still other useful gene products include any one of the receptors for the
hormones, growth factors, cytokines, lymphokines, regulatory proteins and
immune
system proteins. The invention encompasses receptors for cholesterol
regulation,
including the low density lipoprotein (LDL) receptor, high density lipoprotein
(HDL)
receptor, the very low density lipoprotein (VLDL) receptor, and the scavenger
receptor. The invention also encompasses gene products such as members of the
steroid hormone receptor superfamily including glucocorticoid receptors and
estrogen
receptors, Vitamin D receptors and other nuclear receptors. In addition,
useful gene
products include transcription factors such as jun, fos, max, mad, serum
response
factor (SRF), AP-1, AP2, myb, MyoD and myogenin, ETS-box containing proteins,
TFE3, E2F, ATF1, ATF2, ATF3, ATF4, ZFS, NFAT, CREB, HNF-4, C/EBP, SP1,
CCAAT-box binding proteins, interferon regulation factor (IRF-1), Wilms tumor
protein, ETS-binding protein, STAT, GATA-box binding proteins, e.g., GATA-3,
and
the forkhead family of winged helix proteins.
17
CA 02375098 2001-11-23
WO 00/75353 PCT/US00/00415
Other useful gene products include, carbamoyl synthetase I, ornithine
transcarbamylase, arginosuccinate synthetase, arginosuccinate lyase, arginase,
fumarylacetacetate hydrolase, phenylalanine hydroxylase, alpha-1 antitrypsin,
glucose-6-phosphatase, porphobilinogen deaminase, factor VIII, factor IX,
cystathione beta-synthase, branched chain ketoacid decarboxylase, albumin,
isovaleryl-coA dehydrogenase, propionyl CoA carboxylase, methyl malonyl CoA
mutase, glutaryl CoA dehydrogenase, insulin, beta-glucosidase, pyruvate
carboxylate,
hepatic phosphorylase, phosphorylase kinase, glycine decarboxylase, H-protein,
T-
protein, a cystic fibrosis transmembrane regulator (CFTR) sequence, and a
dystrophin
cDNA sequence.
Other useful gene products include, non-naturally occurring
polypeptides, such as chimeric or hybrid polypeptides having a non-naturally
occurring amino acid sequence containing insertions, deletions or amino acid
substitutions. For example, single-chain engineered immunoglobulins could be
useful in certain immunocompromised patients. Other types of non-naturally
occurring gene sequences include antisense molecules and catalytic nucleic
acids,
such as ribozymes, which could be used to reduce overexpression of a gene.
Other
suitable transgenes may be readily selected by one of skill in the art. The
selection of
the transgene is not considered to be a limitation of this invention.
C. Regulatory Elements
In addition to the major elements identified above for the viral vector,
(e.g, the adenovirus sequences and the transgene), the vector also includes
conventional control elements necessary to drive expression of the transgene
in a cell
transfected with the viral vector. Thus the vector contains a selected
promoter which
is linked to the transgene and located, with the transgene, between the viral
sequences
of the vector. Suitable promoters may be readily selected from among
constitutive
and inducible promoters, such as those discussed herein. Selection of these
and other
common vector elements are conventional and many such sequences are available
(see, e.g., Sambrook et al, and references cited therein].
The combination of the transgene, promoter/enhancer, and the other
regulatory vector elements is referred to as a "minigene" for ease of
reference herein.
18
CA 02375098 2001-11-23
WO 00/75353 PCT/US00/00415
In a preferred embodiment, the minigene is flanked by the 5' and 3' cis-acting
adenovirus sequences described above. Such a minigene may have a size in the
range
of several hundred base pairs up to about 30 kb due to the absence of
adenovirus early
and late gene sequences in the vector. Thus, this viral vector (e.g., pAdO)
system
permits a great deal of latitude in the selection of the various components of
the
minigene, particularly the selected transgene, with regard to size. Provided
with the
teachings of this invention, the design of such a minigene can be made by
resort to
conventional techniques.
D. Delivery of Recombinant Vector to Host Cell
In the performance of the method of the invention, the recombinant
viral vector, e.g., pAdO, is delivered to the host cells using conventional
techniques.
Delivery can be by any suitable method, including transfection,
electroporation,
liposome delivery, membrane fusion techniques, high velocity DNA-coated
pellets,
viral infection and protoplast fusion.
Currently, transfection is a particularly desirable method of delivering
the viral vector (e.g., pAdO) to the host cell. One suitable method is
described in
Fisher et al, Virol., 217:11-22 (1996), which is incorporated by reference
herein.
Particularly, the pAdO is linearized by a suitable restriction enzyme and
added in
transfection cocktail. The transfection is then performed using the calcium-
phosphate
based techniques described in Cullen, in "Methods in Enzymology", ed. S.L.
Berger
and A.R. Kimmel, Vol. 152, pp. 684-704, Academic Press, San Diego ( 1987).
Other
suitable transfection techniques are known and may readily be utilized to
deliver the
recombinant vector to the host cell.
Generally, when delivering the recombinant vector (e.g., pAdO) by
transfection, the vector is delivered in an amount from about 5 pg to about
100 pg
DNA, and preferably about 10 to about 50 pg DNA to about 1 x 104 cells to
about 1 x
10'3 cells, and preferably about 105 cells. However, the relative amounts of
vector
DNA to host cells may be adjusted, taking into consideration such factors as
the
selected vector, the delivery method and the host cells selected.
19
CA 02375098 2001-11-23
WO 00/75353 PCT/US00/00415
III. Helper Virus
The present invention utilizes a recombinant helper virus for production of
the
viral vector, which helper virus has been engineered to contain heterologous
restriction sites for the rare-cutting restriction enzyme, e.g., I-SceI.
Normally, the
production of a recombinant virus which utilizes helper virus containing a
full
complement of required genes results in recombinant virus contaminated by
excess
production of the helper virus. Thus, extensive purification of the
recombinant virus
from the contaminating helper virus is required. However, the present
invention
provides a way to facilitate purification and reduce contamination by
enzymatic
digestion of the helper virus by the rare-cutting restriction enzyme (e.g., I-
SceI)
expressed by the host cell. Thus, the helper viruses of the invention contain
at least
one, and preferably multiple, rare-cutting restriction enzyme sites, which are
located
within the genome of the helper virus such that the digested helper virus is
composed
of fragments which are small enough to be readily distinguishable from the
recombinant virus and any nucleic acid used to deliver the restriction enzyme.
Generally, a helper virus is utilized in the production of a recombinant virus
from a vector which contains insufficient viral genes to package (or
encapsidate) the
vector. Because of the limited amount of viral sequence present in the viral
vector, a
helper virus of this invention must, alone or in concert with a packaging cell
line,
provide the gene sequences necessary for a productive viral infection. A
suitable
helper virus may be readily selected by one of skill in the art, taking into
consideration the viral vector to be packaged. For example, an AAV vector may
be
packaged in either a rAAV or a recombinant adenovirus (Ad) capsid, using a
rAAV
or Ad helper virus of a selected serotype, as appropriate. However, this and
other
vectors may be packaged using other selected helper viruses. Suitably, the
helper
viruses used in the present invention are capable of replication in the
selected host
cell. In one desirable embodiment, the helper viruses are replication-
competent in the
selected host cells and replication-incompetent in other cells, which do not
provide
the necessary gene functions to permit replication.
In one preferred embodiment, a pAdO vector is packaged into an adenoviral
capsid using helper viruses which contain selected adenovirus gene sequences,
and
CA 02375098 2001-11-23
WO 00/75353 PCT/US00/00415
optionally reporter sequences operably linked to expression control sequences.
The
adenovirus sequences forming the helper virus may be obtained from the sources
identified above in the discussion of the pAd vector. Use of different Ad
serotypes as
helper viruses enables production of recombinant viruses containing the pAdO
vector
sequences in a capsid formed by the other serotype adenovirus. Use of these
different
Ad serotype helper viruses may also demonstrate advantages in recombinant
virus
production, stability and packaging.
The helper virus supplies the adenovirus sequences necessary for
encapsidation of pAdO, including early genes E1, E2, E4, or fragments of a
gene
which perform the same or substantially the same function as the intact
complete
gene (i.e., functional fragments), and all remaining late, intermediate,
structural and
non-structural genes of the adenovirus genome, which are not present in the
pAdO
vector or provided by the cell line. Most suitably, the helper virus and cell
line
provide, at a minimum, adenovirus E 1 a, E 1 b, E2a, or functional fragments
thereof.
More preferably, the helper virus and cell line provide, Ela, Elb, E2a, E4,
and VAI,
or functional fragments of these genes (e.g., E4 ORF6) and the helper virus
provides
the adenoviral gene IX function. Preferably, the recombinant virus is an
adenovirus
and, most preferably, an adenovirus which replicates in the selected host
cell.
Alternatively, other suitable viruses, e.g., herpesviruses, may be used as
helpers.
The selected helper virus is engineered to contain at least one, and
preferably
multiple, restriction enzyme sites (e.g., I-SceI). In one embodiment, the
helper virus
useful in the present invention contains three heterologous restriction enzyme
sites.
The presence of multiple restriction enzyme sites permits the helper virus to
be
digested by the rare-cutting restriction enzyme expressed in the cell,
resulting in small
fragments of helper virus which are readily distinguishable from and purified
from
the packaged recombinant virus (e.g., Ad0) and any remaining unpackaged viral
vector (e.g., pAdO).
Most desirably, the helper virus contains a restriction enzyme site (e.g., a I-
Sce-I site) located downstream of the packaging sequences, so that the helper
virus is
disabled for replication and packaging following digestion with the
restriction
enzyme expressed in the host cell. Other restriction enzyme sites may be
readily
21
CA 02375098 2001-11-23
WO 00/75353 PCT/US00/00415
engineered into other suitable sites to disrupt functions which are no longer
required
following packaging of the viral vector and/or to permit monitoring of helper
virus
levels, e.g. by expression of a marker (i.e. reporter) sequence, which upon
expression
produces a detectable signal. Examples of suitable marker sequences are
described
above.
In one suitable embodiment, a rare-cutting restriction enzyme site (e.g., I-
SceI) is placed upstream of the sequences encoding the marker, permitting
monitoring
of changing levels of intact helper virus by monitoring a decrease in the
levels of
detectable marker. In one desirable embodiment, the helper virus is composed
of
adenovirus 5' sequences, including a packaging signal, a first I-SceI site, a
promoter,
a second I-SceI site, a reporter gene, a third I-SceI site, and additional
adenovirus
sequences. Other suitable helper viruses may be readily designed and produced
using the information provided herein.
IV. Method of the Invention
In one embodiment, the present invention provides a method of producing
helper-dependent viruses in a host cell which stably expresses the selected
rare-
cutting restriction enzyme (e.g., I-SceI). Suitably, the host cell is
transfected with the
recombinant viral vector, e.g., pAdO, and infected with the helper virus using
conventional methods. See, generally, Sambrook et al, cited above. See also,
the
methods described in K.J. Fisher et al, Yirol., 217:11-22 (1996). The host
cell is then
cultured under suitable conditions to permit encapsidation of recombinant
viral vector
in a first round of amplification. Suitably, where the rare-cutting
restriction enzyme
expression (e.g., I-SceI) is under the control of an inducible promoter, the
inducing
agent is added 6 to 72 hours, and preferably 24 hours, after infection of the
cells with
the helper virus.
In an alternative embodiment, the host cell is transiently transfected or
infected with the sequences encoding the rare-cutting restriction enzyme
(e.g., I-
SceI). The host cell, provided with the recombinant viral vector and helper
virus as
described above, is then cultured in a similar manner to provide recombinant
virus in
a viral capsid.
22
CA 02375098 2001-11-23
WO 00/75353 PCT/US00/00415
Optionally, recombinant virus produced by this first round of viral
amplification may then be recovered by conventional means. For example, the
host
cells may be harvested and lysed using known methods, e.g., three rounds of
freeze
(e.g., ethanol, dry ice) - thawing (e.g., 37°C). The cell lysate may
then be centrifuged
to remove cell debris. An advantage of the present invention is that, due to
the
difference in size between the recovered virus and the fragments into which
the
helper virus is cleaved by the restriction enzyme (e.g., I-SceI), the
recovered virus is
readily separated from the helper virus fragments. Where desired, the
recovered virus
may be subjected to further purification steps. See, Fisher et al, cited
above.
Alternatively, the recovered lysate may be subjected to a second round of
amplification, e.g., using the steps and host cells described above in the
first round of
amplification. This virus is useful for a variety of purposes known in the
art.
The recombinant viruses produced according to the method of the invention
are substantially free of helper virus contamination and are useful for a
variety of
purposes, which are well known to those of skill in the art.
The following examples are provided to illustrate methods for producing the
compositions useful in the method of the invention and methods for performing
the
invention. Such examples do not limit the scope of the present invention. One
skilled in the art will appreciate that although specific reagents and
conditions are
outlined in the following examples, modifications can be made which are meant
to be
encompassed by the spirit and scope of the invention.
Example 1 - Production of Helper Virus Containing I-SceI Sites
The helper adenovirus described in this example is termed Ad-3I since it
carries three I-SceI sites in its genome.
A. Production of pAd-linker-3I
The I-SceI coding region was synthesized by Anagen Inc. (CA, USA)
and contains modified codons to optimize expression in mammalian cells. This
23
CA 02375098 2001-11-23
WO 00/75353 PCT/US00/00415
complete coding sequence for I-SceI is: SEQ ID N0:2: GGGGCGGCCG
CAGCCGCCAT ATGAAGAACA TCAAGAAGAA CCAGGTGATG
AACCTGGGCC CCAACAGCAA GCTGCTGAAG GAGTACAAGA
GCCAGCTGAT CGAGCTGAAC ATCGAGCAGT TCGAGGCCGG
CATCGGCCTG ATCCTGGGCG ACGCCTACAT CAGGAGCAGG
GACGAGGGCA AGACCTACTG CATGCAGTTC GAGTGGAAGA
ACAAGGCCTA CATGGACCAC GTGTGCCTGC TGTACGACCA
GTGGGTGCTG AGCCCCCCCC ACAAGAAGGA GAGGGTGAAC
CACCTGGGCA ACCTGGTGAT CACCTGGGGC GCCCAGACCT
TCAAGCACCA GGCCTTCAAC AAGCTGGCCA ACCTGTTCAT
CGTGAACAAC AAGAAGACCA TCCCCAACAA CCTGGTGGAG
AACTACCTGA CCCCCATGAG CCTGGCCTAC TGGTTCATGG
ACGACGGCGG CAAGTGGGAC TACAACAAGA ACAGCACCAA
CAAGAGCATC GTGCTGAACA CCCAGAGCTT CACCTTCGAG
GAGGTGGAGT ACCTGGTGAA GGGCCTGAGG AACAAGTTCC
AGCTGAACTG CTACGTGAAG ATCAACAAGA ACAAGCCCAT
CATCTACATC GACAGCATGA GCTACCTGAT CTTCTACAAC
CTGATCAAGC CCTACCTGAT CCCCCAGATG ATGTACAAGC
TGCCCAACAC CATCAGCAGC GAGACCTTCC TGAAGTGATA TCGGG.
B. Production of Adenoviral Helper Virus
The pAd-linker-3I was constructed as follows. The synthetic I-SceI
oligonucleotide described in A above was inserted into pAd-linked at Bgl II
and
Hind III [Fisher et al, cited above). This plasmid contains an intact
adenoviral
genome located on a pBR322 backbone. Another I-SceI site is cloned into pCI-
AAT
([Xiao et al, J. Virol., 72:10222-10226 (1998)] following digestion with XhoI.
The
Hind III-Cla I fragment of pCI-AAT with one I-SceI was then cloned into pAd-
linkerl with two I-SceI sites following digestion with EcoRV and CIaI, to
obtain
pAd-linker-3I.
The helper virus Ad-3I was constructed by homologous recombination
between pAd-linker-3I and adenovirus DNA in 293 cells as described elsewhere
[Gao
24
CA 02375098 2001-11-23
WO 00/75353 PCT/US00/00415
et al, J. Virol., 70:8934-8943 (1996)]. The genome organization of Ad-3I is
shown
in Figure 2.
The resulting helper virus, Ad-3I, contains from 5' to 3': an
adenovirus 5' ITR and packaging signal, a first I-SceI site, a CMV promoter, a
second
I-SceI site between the CMV promoter and the al-antitrypsin cDNA, and a third
I-
SceI site between a 1-antitrypsin and the other adenovirus early and late
genes. The
E 1 a and E 1 b genes are disrupted in this construct, which replicates in the
presence of
the E 1 functions provided by the cell line.
The al-antitrypsin gene driven by CMV is included in this helper
virus to serve as a reporter gene. Digestion of the second I-SceI site
(located between
the CMV promoter and al-antitrypsin cDNA) results in the loss of al-
antitrypsin
gene expression. Additionally, digestion of any of the three I-SceI sites
disables Ad-
3I for replication and packaging since the 5' terminal repeat is thereby
detached from
helper adenovirus genome.
1 S C. Digestion of Ad-3I Helper Virus
The in vitro digestion of DNA by I-SceI was carried out using
commercially available I-SceI (Boehringer Mannheim, Germany) under the
conditions recommended by the vendor. To obtain Ad-3I DNA, the purified virus
was digested with proteinase K and extracted from CsCI gradient purified
adenovirus
with phenol-chloroform before ethanol precipitation. The digested DNA was
electrophoresed in 0.8% agarose gel. The size of the bands was identified. The
complete digestion of Ad-3I results in four fragments of sizes of
approximately 34 kb,
1.7 kb, 1.1 kb and 400 bp.
Example 2 - Production of Human Cell Line Ex rp essirtg Functional I-SceI
Enzvme
The synthesized I-SceI sequence described in Example 1 A was fused at its N-
terminus with a nuclear translocation signal (NLS) to ensure that the enzyme
will
reach the nucleus where adenovirus DNA replication takes place. This fusion
was
performed by inserting the I-SceI sequence downstream of a NLS in a suitable
plasmid backbone. The NLS-I-SceI fragment was then removed by digestion with
BspHI and NotI and the resulting fragment was cloned into plasmid, pEF-myc-nuc
CA 02375098 2001-11-23
WO 00/75353 PCT/US00/00415
(purchased from Invitrogen), to get pEF-SceI. pEF-myc-nuc contains a strong
constitutive promoter derived from the human elongation faction 1 a. The 293-I-
SceI
cell line was constructed by transfecting plasmid pEF-SceI into 293 cells
using
lipofectAmine (Gibco BRL) selected under 200 ~g/ml of 6418.
The 6418 resistant clones (22) were selected and analysed for I-SceI
expression following infection with Ad-3I at MOI 10. The Hirt DNA was
extracted
36 hours post-infection. Southern analysis was performed using a human al-
antitrypsin cDNA probe. Fragments of 1.7 kb and 2.8 kb were obtained. The 1.7
kb
fragment results from the digestion of I-SceI sites before and after al-
antitrypsin gene
in Ad-3I. The results indicated that most 6418 positive clones had the ability
to
digest helper Ad-3I genome in vivo.
These cell lines were maintained in Dulbecco's modified Eagle media
(DMEM) supplemented with 10% fetal calf serum in 5% COZ humidified
environment at 37°C.
The infectious Ad-3I helper virus was used for screening the selected cell
clones. The digestion of Ad-3I genome, which generates multiple small
fragments in
the Southern blot, is a indicator of the active I-SceI in the cell. Nearly all
cell clones
had the functional enzyme to digest the input adenovirus. On the other hand,
in
contrast to what observed in the in vitro digestion, the in vivo digestion
seems not to
be complete. The 2.8 kb fragment can be ready observed, which suggests that
the
second I-SceI site located between CMV promoter and al-antitrypsin is not
digested
to 100%. This is probably due to the fact that cellular environment of
mammalian
cells is not the optimized condition as the in vitro digestion.
Example 3 - Production of Recombinant Adenovirus
A. Generation of Hel en rDependent Vector
A helper-dependent vector, Ad-0-lacZ, was produced essentially as
described in Fisher et al, Virol., 217:11-22 (1996). This vector contains the
sequence
from the left-end of Ad5 encompassing by 1-360 (5' inverted terminal repeat
(ITR), a
lacZ minigene under the transcriptional control of the cytomegalovirus (CMV)
immediate early enhancer/promoter, and sequence from the right end of Ad5
26
CA 02375098 2001-11-23
WO 00/75353 PCT/US00/00415
spanning by 35,353 to the end of the genome (3' ITR). This plasmid was
designed
so that digestion with EcoRI releases the terminal ends of the Ad-O-lacZ
genome
from the plasmid backbone.
B. Production of Recombinant Adenovirus
The plasmid Ad-0-lacZ was linearized by EcoRI and transfected into
293 cells or a 293 I-SceI expression cell line of the invention. Helper virus
Ad-3I
was infected at MOI of 5. The cells were harvested at full cytopathic effect
(CPE).
After freezing and thawing the cells three times, the cell lysates were used
for
determination of virus titer by X-Gal staining, as described in Fisher et al,
cited
above. Cell lysates were also subjected to a second round of amplification to
permit
rescue of helper dependent vector Ad-0-lacZ, which was performed essentially
as
described by Fisher et al, cited above.
C. I-SceI Cell Lines Enhanced Hel ep r Dependent Adenovirus Production
The performance of the 293 I-SceI cell line in production of a
recombinant adenovirus deleted of all adenoviral genes is presented in Fig. 4.
The
deleted Ad vector, EcoRI-digested pAd-0-lacZ ( 10 pg), was transfected in 1 x
105
cells of each of three cell lines: a conventional 293 cell line and two
independent
clones of 293-I-SceI (termed (a) and (b)). After transfection, the cells were
infected
with Ad-3I helper virus at MOI 5. The cell lysates were harvested 96 hours
after
infection. A portion of the cell lysates were used to determine the titer of
Ad-D-lacZ
virus titer for the first round of amplification. The remainder of the cell
lysate was
used for a second round of amplification in the same cell lines. The titer is
shown as
lacZ forming unit (LFU) per field under microscopy under identical conditions.
See
Fig. 4.
Interestingly, the initial rescue of helper dependent viruses was better
in 293 cells while subsequent amplification of helper virus is better in 293-I-
SceI cell
lines. This may reflect the importance in the initial rescue step of adequate
amounts
of helper virus. In the 293-I-SceI cell line, the amount of helper virus is
decreased
because of I-SceI digestion. In the amplification procedure, the balance of
helper
virus and vector is more critical.
27
CA 02375098 2001-11-23
WO 00/75353 PCT/US00/00415
A further characterization of the Ad-3I-293-I-SceI system is shown in
Fig. 5. In this experiment, the same amounts of helper virus and EcoRI-
digested
plasmid Ad-D-lacZ were used to infect the same amounts of 293 cells, 293-I-
SceI(a)
cells and 293-I-SceI(b) cells (two different clones). The amount of helper
virus was
decreased in the 293-I-SceI cell line. However, the helper virus yield is
increased 10-
100 fold in a simple step of amplification.
Example 4 - Production of Hel ep rDependent Recombinant Adenovirus
A. Production of rAAV vector ex rp essing I-SceI enzyme
The plasmid pAAV-SceI was constructed by cloning the blunt ended
EF-nuc-I-SceI cassette from pEF-SceI into pSub201 at the XbaI site. Infectious
AAV-SceI was generated using the techniques described previously [Xiao, J.
Virol,
72:10222-10226 (1998)].
B. Production of Hel en rDependent Recombinant Adenovirus
293 cells ( 1 x 105) are transfected with 10 pg of EcoRI-digested pAd-
O-IacZ and rAAV-SceI (MOI 10). After transfection, the cells are infected with
Ad-
3I at MOI 5. The cell lysates are harvested 96 hours after infection.
Example 5 - Characterization of 293-I-SceI Cell Line and rAAV-SceI Vectors
To further study the helper-dependent rAd virus production methods of the
invention, the levels of reporter gene expression (al-antitrypsin gene
expression)
after Ad-3I infection (MOI from 0.1, 1, 10, 100, 200) in media from either 293
cells,
the 293-I-SceI cell line, or 293 cells with rAAV-EF-I-SceI (MOI 10) co-
infection
were monitored at various time points by ELISA which was performed as
described
by [Xiao et al, J. Virol., 72:10222-10226 (1998)].
A Southern blot of the Hirt DNA was performed using a probe specific for
human al-antitrypsin cDNA. DNA fragments of 1.7 kb, 2.8 kb and 32 kb were
obtained. These fragments are indicative of digestion with the I-SceI. The
Hirt DNA
was extracted 24 hours post infection and electrophoresed in 0.8% agarose gel.
The
results clearly showed that replication of helper virus, Ad-3I, was lowered
dramatically in the presence of I-SceI gene expression in the cell line.
28
CA 02375098 2001-11-23
WO 00/75353 PCT/US00/00415
Ad-3I genome is disabled by I-SceI restriction digestion, which most likely
causes the decrease in the copy number of the al-antitrypsin gene available
for
transcription. The Southern blot also further confirmed that this is the case.
In either
293-I-SceI cell or with rAAV-I-SceI co-infection, the replication of Ad-3I is
also
reduced. This is evident since the large fragment (>30kb) in the presence of I-
SceI
expression is less than that generated in 293 cells. The digested small
fragments are
also evident in the blots. Moreover, this experiment suggested the I-SceI
enzyme
delivered by rAAV vector was more potent than the I-SceI enzyme expressed from
293-I-SceI cell line. This is probably due to the fact that infectious rAAV
transduced
the 293 cells with more copies of I-SceI gene than are present in the
integrated
counterpart (i.e., the 293-I-SceI cell line).
All publications cited in this specification are incorporated herein by
reference
herein. While the invention has been described with reference to a
particularly
preferred embodiment, it will be appreciated that modifications can be made
without
1 S departing from the spirit of the invention. Such modifications are
intended to fall
within the scope of the appended claims.
29