Note: Descriptions are shown in the official language in which they were submitted.
CA 02375119 2001-11-26
WO 00/73480 PCT/US00/14481
COMPOSITIONS AND METHODS FOR PRODUCTION OF RECOMBINANT VIRUS USING A CARRIER
VECTOR DERIVED FROM A NONMAMMALIAN VIRUS
TECHNICAL FIELD OF THE INVENTION
This invention relates to novel nonmammalian carrier vectors and
viruses useful in the production of high titers of recombinant viruses which
may
contain foreign DNA inserts or which may be point-mutated or deleted viruses,
and
methods of producing those viruses. The nonmammalian carrier vector ("carrier
vector") is a chimeric vector which includes those portions of a nonmammalian
virus backbone which allow replication in a nonmammalian host cell. The
carrier
vector includes various nucleic acid cassettes, which may include an embedded
recombinant viral genome containing a desired transgene, components necessary
for
production of a replication-defective recombinant virus containing the
transgene,
and domains that permit the carrier vector to bind to mammalian cells. The
invention also provides methods of producing high concentrations of
recombinant
virus as a substantially homogeneous preparation, compositions to produce the
recombinant virus, and novel recombinant viruses.
CA 02375119 2001-11-26
WO 00/73480 PCT/US00/14481
-2-
BACKGROUND OF THE INVENTION
A recombinant virus carrying a foreign DNA insert may be used to
deliver genes to cells, where the gene may be expressed, if desired, to permit
production of recombinant proteins in vitro or in vivo, vaccination of human
and
non-human mammals, or treatment or amelioration of diseases or genetic defects
in
humans or non-human mammals. One may treat or ameliorate diseases or genetic
defects by providing normal gene products, increased levels of gene products
or by
blocking endogenous production of a gene, whose expression would be
deleterious
to the cell or organism.
Methods for delivering an exogenous gene to a mammalian cell
include the use of mammalian viral vectors, such as those which are derived
from
retroviruses, adenoviruses, herpes viruses, vaccinia viruses, polio viruses,
adeno-
associated viruses, hybrid viruses (e.g., hybrid adenovirus-AAV, see U.S. Pat.
No.
S,8S6,1 S2) and the like. Other methods include direct injection of DNA,
biolistic
administration of DNA, electroporation, calcium phosphate precipitation, as
well as
methods of administration which utilize ligand-DNA conjugates, liposome
conjugates of DNA, polycation-DNA complexes or adenovirus-ligand-DNA
conjugates.
A transgene is a nucleic acid encoding a protein of interest; it may be
2 0 a ~~ene to allow for genetic or drug selection, e.g., a gene conferring
resistance to
antibiotics, or a reporter gene allowing detection, e.g., by color in the case
of the
use of green fluorescent protein. Alternatively, the transgene may be one that
is
useful for corrective applications. For instance, a transgene may be a normal
gene
CA 02375119 2001-11-26
WO 00/73480 PCT/US00/14481
_3_
that replaces or augments the function of a patient's defective gene. The
transgene
may be one that counteracts the effects of a disease, such as introduction and
expression of a gene that is distinct from the one that it replaces or
augments, but
which has the same function or compensates for the defective gene's function.
The
transgene may be a gene which blocks or represses the expression of a
malfunctioning, mutated, or viral gene in the patient, thereby giving rise to
a
corrective effect. A transgene may also be used for immunization against
various
agents, by provoking an immunogenic response in an animal. Delivery of
therapeutic transgenes to a patient thus effects a correction of a defect or
prevention of disease. The transgene also may be one which is useful for
production of proteins in vitro, such as for large-scale production of
therapeutic
proteins.
Appropriate genes for expression in the cell include, without
limitation, those genes which are normally expressed in cells but whose
products are
produced in insufficient amounts. Alternatively, the appropriate gene for
expression
is one which expresses a normal gene product which replaces a defective gene
product, encodes ribozymes or antisense molecules which repair or destroy
mutant
cellular RNAs expressed from mutated genes, or modifies or destroys viral
RNAs.
Transgenes used for production of proteins in vitro include proteins such as
2 0 secreted factors, including hormones, growth factors and enzymes.
Many gene therapy methods involve supplying an exogenous gene to
overcome a deficiency in the expression of a gene in a patient. Some of these
deficiencies are congenital and are due to a mutation in a particular gene in
all the
CA 02375119 2001-11-26
WO 00/73480 PCT/US00/14481
-4-
cells of the patient. For instance, in cystic fibrosis, there are one or more
mutations
in the gene encoding the cystic fibrosis transmembrane conductance regulator
(CFTR) which prevents the CFTR protein from functioning properly. In other
cases, a deficiency in gene expression is due to an accident or disease that
occurs
during the patient's life. For instance, in Type I diabetes mellitus, the ~3
pancreatic
islet cells, which produce insulin, are destroyed, such that patients with
this disease
can no longer synthesize insulin. In other cases, the endogenous gene may be
structurally normal but is not produced in high enough quantities due to
disease,
medical treatment or other environmental conditions, or mutations in the
regulatory
elements of the endogenous gene. For example, there are a number of blood
disorders, such as anemia, in which there is insufFicient production of red
blood
cells, which may be treated with erythropoietin (EPO) or with a transgene
encoding
EPO. Transgenes may also be used for genetic immunization, i.e., to elicit an
immune response to a pathogen in an animal, including humans. For instance, a
transgene may include a sequence from a viral, bacterial or fungal pathogen,
such as
influenza virus, human immunodeficiency virus (HIV), or mycobacterium
tuberculosis.
Certain methods are amenable to targeted delivery of the exogenous
gene to specific tissues, such as liver tissue. One method of delivering genes
to
2 0 specific cells relies upon the function of a cell-specific receptor. The
asialoglycoprotein receptor (ASGP-R), which is present on the surface of
hepatocytes (Spiess et al., 1990, Biochem. 29:10009-10018), is a lectin which
has
affinity for the terminal galactose residues of glycoproteins, and has been
used to
CA 02375119 2001-11-26
WO 00/73480 PCT/US00/14481
-5-
target gene delivery to liver hepatocytes. For example, a DNA complex is bound
to
a ASGP-R on the cell surface, allowing subsequent endoyctosis by the liver
hepatocyte.
Viruses that are commonly used in gene delivery applications are
modified by replacing viral nucleic acid with a desired transgene. Frequently,
DNA
removed from the virus encodes proteins necessary for viral replication or
encapsidation, in which case the recombinant virus containing a transgene is
replication-deficient and will not replicate or encapsidate in the host. To
permit
replication and encapsidation, current methods recognize that those portions
of
DNA which have been deleted must be supplied by wild-type or modified viruses
or
by plasmids containing DNA encoding the required gene products. Supplying wild-
type or modified virus may result in recombinant virus stocks contaminated
with
wild-type or modified virus. Supplying plasmids encoding the required gene
products through cotransfection results in low efficiency of recombinant virus
production, as well as recombination events which yield wild-type virus
contaminants.
A number of dii~erent viruses have been used to deliver a transgene
to mammalian cells. These viruses include retrovirus, hepatitis B virus (HBV),
adenovirus, adeno-associated virus (AAV) and herpesvirus. AAV possesses unique
2 0 features that make it attractive as a vector for delivering foreign DNA
(i.e., a
transgene) to cells, and various groups have studied the potential use of AAV
in the
treatment of disease states.
CA 02375119 2001-11-26
WO 00/73480 PCT/US00/14481
-6-
AAV is a parvovirus, the genome of which is about 4.7 kb in length,
including 145 nucleotide inverted terminal repeats (ITRs). The AAV genome
encodes two genes, rep and cap, each of which expresses a family of related
proteins from separate open reading frames and produced as a result of
alternative
mRNA splicing. Rep polypeptides (rep78, rep68, rep52, and rep40) are involved
in
replication, rescue and integration of the AAV genome. Cap proteins (VP 1,
VP2,
and VP3) form the virion capsid. Flanking the rep and cap open reading frames
at
the 5' and 3' ends of the AAV genome are the 145 by ITRs, the first 125 by of
which are capable of forming Y- or T- shaped duplex structures. The entire
nucleic
acid encoding rep and cap can be excised and replaced with a transgene [B. J.
Carter, in "Handbook of Parvoviruses", ed., P. Tijsser, CRC Press, pp.155-168
( 1990)] . The ITRs represent the minimal sequence required for replication,
rescue,
packaging, and integration of the AAV genome.
When this nonpathogenic human virus infects a human cell, the viral
genome integrates into chromosome 19 resulting in latent infection of the
cell.
Production of infectious virus and replication of the virus does not occur
unless the
cell is coinfected with a lytic helper virus, such as adenovirus (Ad) or
herpesvirus.
Upon infection with a helper virus, the AAV provirus is rescued and amplified,
and
both AAV and helper virus are produced. The infecting parental ssDNA is
2 0 converted to duplex replicating form (RF) DNAs in a rep dependent manner.
The
rescued AAV genomes are packaged into preformed protein capsids (icosahedral
symmetry approximately 20 nm in diameter) and released as infectious virions
that
have packaged either + or - ss DNA genomes following cell lysis. However,
CA 02375119 2001-11-26
WO 00/73480 PCT/US00/14481
_7_
progress towards establishing AAV as a transducing vector for the delivery of
DNA
in the form of a desired transgene has been slow for a variety of reasons.
Replacing the rep and cap sequences with a desired transgene yields
a recombinant virus capable of delivering the transgene to target host cells.
However, because AAV requires a particular genome packaging size, addition of
a
transgene results in deletion of necessary gene functions for rep and cap. In
current
methods, necessary gene functions replaced by the transgene are supplied by
viruses
or additional plasmids. Furthermore, the requirement by AAV for helper virus
functions also requires the use of helper viruses (either wildtype or crippled
viruses)
or plasmids containing the helper virus functions.
One method that has been used to produce recombinant AAV
(rAAV) vectors comprises co-transfecting eukaryotic cells with a plasmid
containing rAAV (the cis plasmid) and a plasmid containing rep and cap (the
traps
plasmid), and infecting the cells with a helper virus (e.g., adenovirus or
herpes
virus). See U.S. Pat. No. 5,753,500. The disadvantage ofthis method is that
the
rAAV vector stock is contaminated with helper virus, which is labor-intensive
and
difficult to separate from the helper virus, and co-transfection of two
plasmids along
with infection by a helper virus is inefficient and cannot be easily scaled up
for
industrial production of rAAV.
2 0 A second method that has been used to produced rAAV involves a
triple plasmid transfection of eukaryotic cells. In this method, one plasmid
carries
the transgene and ITRs (the cis plasmid), a second plasmid encodes the rep and
cap
genes (the traps plasmid), and the third plasmid encodes the helper virus
functions,
CA 02375119 2001-11-26
WO 00/73480 PCTNS00/14481
_g_
i.e. adenoviral genes such as Ela, Elb, E2a and E4 (the helper plasmid). The
disadvantage of this method is that a triple transfection is also inefficient
and is
difficult to scale up.
A third method involves the use of a packaging cell line such as one
including AAV functions rep and cap. See U.S. Pat. No. 5,658,785 and U.S.
Serial
No. PCT US98/19463. The packaging cell line may be transfected with a cis
plasmid comprising the transgene and ITRs, and infected by wild-type
adenovirus
(Ad) helper. See U.S. Pat. No. 5,658,785. Alternatively, the packaging cell
line
may be co-infected by a hybrid Ad/AAV, in which a hybrid Ad vector caries the
cis
plasmid in the El locus (see U.S. Pat. No. 5,856,152), and by a wild-type or
mutant
Ad that supplies E1. The disadvantage of this method is that wild-type Ad may
be
produced, which must be separated from the rAAV vector before use in a
patient.
Thus, current methods of producing recombinant AAV are incapable
of yielding the high amounts of essentially homogeneous virus for
pharmaceutical
compositions needed for the treatment of a large number of patients in a
easily
scaled industrial production.
Nonmammalian viruses have been used to transiently express
particular individual exogenous proteins in either mammalian or non-mammalian
cells. For example, viruses of the family Baculoviridae, or "baculoviruses",
which
2 0 normally infect members of the order Lepidoptera, have been used to
express
exogenous genes in insect cells. Baculoviruses have also been reported to
enter
mammalian cells, and baculoviral DNA has been detected in nuclear extracts of
mammalian cells (Volkman et al., 1983, Appl. Environ. Microbiol. 45:1085-
1093).
CA 02375119 2001-11-26
WO 00/73480 PCT/US00/14481
_9_
While one report of baculovirus-mediated gene expression in mammalian cells
has
appeared, the authors later attributed the apparent reporter gene activity to
the
reporter gene product being carried into the cell after a prolonged incubation
of the
cell with the virus (Carbonell et al., 1987, Appl. Environ. Microbiol. 53:1412-
1417). These authors reported that, when the exogenous gene gains access to
the
cell as part of the baculovirus genome, the exogenous gene is not expressed de
r~~mo. Subsequent studies have demonstrated baculovirus-mediated gene
expression
of particular proteins in mammalian cells (Boyce et al., 1996, Proc. Natl.
Acad. Sci.
USA, 93:2348-2352).
While baculovirus has been used for expressing particular proteins in
a mammalian cell, see U.S. Pat. No. 5,731,182, baculovirus has not been used
to
produce pharmaceutical compositions of replication-deficient recombinant virus
using an easily scaled industrial process. As disclosed in U.S. Pat. No.
5,731,182,
the genome of the baculovirus may be modified by insertion of ligand DNA,
which
comprises a gene encoding a mammalian receptor specific protein that allows
the
baculovirus to bind and enter mammalian cells. The nonmammalian virus
infecting
the mammalian cells allows only for transient expression of the transgene
within the
mammalian cell. In addition, the methods disclosed in U.S. Pat. No. 5,731,182
do
not result in production of an altogether distinct, essentially homogeneous
2 0 recombinant virus, at high titers.
The problem of generating recombinant replication-deficient virus
that is produced in the absence of helper viruses and by an efficient method
that is
applicable to large-scale industrial production has not been solved until the
present
CA 02375119 2001-11-26
WO 00/73480 PCT/US00/14481
-10-
invention. Current viral production methods include costly and time consuming
purification and concentration steps, and are incapable of producing
sufficient
recombinant virus for pharmaceutic applications. In the case of AAV, for
example,
current methods produce at most on the order of 104-105 genomic copies (gc) of
recombinant virus per producer cell. Similarly, current methods are suitable
for
producing recombinant adenovirus in amounts on the order of 104 particles per
producer cell, and retroviruses in amounts on the order of 102-104 colony
forming
units (cfu) per producer cell. Current production methods result in
contaminating
helper virus which must be inactivated and/or removed from the final products
prior
to pharmaceutical application. Thus, there exists an unfulfilled need for a
method of
manufacturing recombinant mammalian virus at high titers free of other
contaminating virus in order to produce recombinant viruses capable of
delivering a
desired transgene to mammalian cells, or immunizing cells against viral or
bacterial
infection by the use of such recombinant viruses, in a stable fashion.
SUMMARY OF THE INVENTION
The invention exploits the properties of nonmammalian and
mammalian viruses to create novel chimeric vectors and viruses for the
manufacture
of an essentially homogeneous recombinant virus preparation in the absence of
contaminating helper virus using a process that may be easily scaled for
industrial
2 0 production. The essentially homogeneous recombinant virus may be used for
various purposes, including delivering a desired transgene to mammalian cells
for
pharmaceutic applications including immunization and correction of genetic
defects;
CA 02375119 2001-11-26
WO 00/73480 PCT/US00/14481
-11-
transient and stable gene transfer in vivo, in vitro and ex vivo; production
of
proteins in oivo or in vitro; and other methods in which high levels of gene
transduction into a cell are required, e.g., in the production of expression
libraries
for screening compounds or for introducing genes into cells that are not
easily
transfected.
The carrier vector of the invention is a chimeric vector backbone
derived from the nucleic acid of a nonmammalian virus, and includes one or
more of
the following elements: 1) an embedded recombinant viral genome; 2) nucleic
acid
sequences which encode proteins required for replication and encapsidation of
the
recombinant virus genome; 3) nucleic acid sequences encoding helper functions
(if
the recombinant virus to be produced is helper-dependent, e.g., AAV); 4)
nucleic
acid sequences encoding a ligand that can interact with a mammalian cell; and
S)
re~~ulatory control sequences that regulate nucleic acid sequences in the
nonmammalian virus backbone or in a replication-deficient portion or
modification
thereof. The carrier vector may also include any other nucleic acid sequences
that
are required to produce a replication-deficient recombinant virus.
In one embodiment of the invention, one or more carrier vectors may
comprise all of the elements required to produce a replication-deficient
recombinant
vector in a particular host cell or cell line. The number and type of elements
that
2 0 are required will depend upon the particular host cell used and the type
of
recombinant vector produced. For instance, if a recombinant AAV vector is
desired
and the host cell line is one which has rep and cap stably integrated in its
genome,
the carrier vector or vectors would comprise 1 ) an embedded recombinant viral
CA 02375119 2001-11-26
WO 00/73480 PCT/US00/14481
-12-
genome comprising the AAV ITRs and the transgene and 2) separate helper
functions, which may include any nucleic acid sequence required for
replication and
encapsidation of the rAAV. For instance, these helper functions may include
any
one or a combination of E1, E2a, E40RF6 and VAI from adenovirus (Ad). If a
recombinant AAV vector is to be produced in a host cell line that does not
express
rc.~p and cap, then the carrier vector or vectors may also include the DNA
sequences
encoding nep and cap.
Alternatively, if a recombinant retrovirus is desired, the carrier
vector or vectors would comprise 1 ) an embedded recombinant viral genome
comprising the retroviral LTRs and the transgene of interest driven from the
retroviral LTRs or from a heterologous promoter, and 2) DNA sequences encoding
anv one or a combination of gag, pol and env for the functions of replication
and
encapsidation of the retrovirus not supplied in the host cell. In a preferred
embodiment, all of the required elements to produce a recombinant virus in a
particular host cell are contained on a single carrier vector because the use
of a
single carrier vector having all functions not supplied by a host cell
increases the
efficiency of transduction, and can be more easily scaled for industrial
production of
the embedded recombinant virus.
In an alternative embodiment, the carrier vector comprises an
2 0 embedded recombinant viral genome, and any required replication,
encapsidation
and/or helper functions are provided by a helper virus or a plasmid.
The embedded recombinant viral genome may comprise a transgene
and DNA elements required for replication of a mammalian virus. The transgene
CA 02375119 2001-11-26
WO 00/73480 PCT/US00/14481
-13-
comprises the gene of interest, regulatory elements to regulate its
expression, and
an optional DNA spacer. The transgene is flanked by the DNA elements required
for replication of a mammalian virus, such as the ITRs of AAV, the LTRs of
retrovirus, or the ITRs of adenovirus. The recombinant viral genome is
embedded
within the nonmammalian virus backbone, optionally along with one or more of
the
other DNA sequences listed above, resulting in a chimeric carrier vector of
the
presentmvention.
In an alternative embodiment, the embedded recombinant viral
genome does not contain a transgene but the recombinant viral genome itself
contains point mutations or deletions. In this embodiment, the point mutations
or
deletions function to attenuate the replication of the subsequently-produced
recombinant virus. The attenuated recombinant virus may be any virus which
could
be useful for vaccination, including, without limitation, picornaviruses such
as
poliovirus; hepatitis viruses such as hepatitis B and hepatitis C; cold-
adapted
respiratory syncytial virus (RSV); cold-adapted influenza virus; parainfluenza
virus
types I, 2 and 3; and rotavirus.
The carrier vector is replication-proficient in its native host cells.
For example, employing a baculovirus backbone results in a chimeric carrier
vector
that is replication-proficient in insect cells. In contrast, the embedded
recombinant
2 0 viral genome, optionally containing a transgene, is unable to excise,
replicate, and
package into virions because its promoters are inactive in insect cells.
However,
once the chimeric carrier vector infects a mammalian cell, the essential gene
products required for replication and packaging of the carrier vector in its
CA 02375119 2001-11-26
WO 00/73480 PCT/US00/14481
- 14-
permissive native cell are no longer expressed. Thus, the carrier vector does
not
replicate in mammalian cells, and instead exists transiently within the
mammalian
cell.
In contrast, once the carrier vector has infected a mammalian cell,
the mammalian regulatory sequences within the carrier vector controlling the
embedded recombinant viral genome and other mammalian DNA sequences are
activated, such that the recombinant viral genome is capable of being excised
from
the carrier vector and replicated. The capsid proteins which form the capsid
of the
recombinant virus are expressed such that the recombinant viral genome is
encapsidated, which yields an infectious recombinant virus. The recombinant
virus
is essentially free of carrier vector because the carrier vector is not
replicated in
mammalian cells.
In a preferred embodiment, the recombinant virus is replication-
deficient because there are no replication or helper functions present in the
newly
formed virions; i.e., the recombinant virus lacks part or all of the coding
regions of
the native virus genome. In embodiments of the invention in which the
recombinant
virus is helper-dependent, such as rAAV, the recombinant virus lacks both
functional replication and encapsidation functions. In embodiments of the
invention
in which the recombinant virus is not helper-dependent, the recombinant virus
lacks
2 0 functional replication coding regions or other essential genes.
In cases where helper functions are required for recombinant virus
production, recombinant virus may be produced without the need for coinfection
and subsequent production of helper virus if a carrier vector includes the
necessary
CA 02375119 2001-11-26
WO 00/73480 PCT/US00/14481
-15-
helper functions. Thus, the invention yields lysates of substantially pure and
essentially homogeneous preparations of the particular recombinant virus of
interest
in the absence of helper virus.
This invention thus has many advantages over current methods for
manufacturing recombinant viruses. These advantages include: ( 1 ) the
nonmammalian virus backbone permits insertion of large DNA sequences without
compromising the efficiency of recombinant virus production; (2) sequences
normally toxic to mammalian cells (e.g., AAV rep, VSV-G, retroviral envelope
proteins, eukaryotic regulatory proteins, etc.) are not expressed in
substantial
amounts from their mammalian regulatory sequences in the nonmammalian host
cell
of the nonmammalian carrier vector and thus can be tolerated by the
nonmammalian
carrier vector during the course of its replication in the nonmammalian host
cell; (3)
nonmammalian viruses do not replicate in mammalian cells, precluding
contamination of the final eukaryotic vector stocks with the nonmammalian
carrier
vector; (4) in some embodiments no helper viruses are necessary, with the
result
that the final recombinant virus preparation is essentially free of helper
virus; (5)
frequency of wildtype virus production due to homologous or non-homologous
recombination is minimized; and (6) the methods of the present invention are
particularly suitable to large scale production of recombinant viruses which
are
2 0 themselves replication-deficient. Additionally, nonmammalian viruses are
not
normally pathogenic to mammalian cells, may be propagated in serum free media,
and may be grown to a high titer. Other features and advantages of the
invention
CA 02375119 2001-11-26
WO 00/73480 PCT/US00/14481
-16-
will be apparent from the following drawings, the description of the invention
and
its preferred embodiments, and the examples described herein.
In one embodiment, the present invention includes nonmammalian
carrier vectors containing elements that are required to produce replication-
deficient
recombinant viral vectors. In a preferred embodiment, the nonmammalian carrier
vector contains all the elements required to produce a replication-deficient
recombinant viral vector. In an even more preferred embodiment, a single
nonmammalian carrier vector contains all the required elements to produce a
replication-deficient recombinant viral vector. In another preferred
embodiment,
the nonmammalian carrier vector is a baculovirus.
In another embodiment, the invention includes a method of
producing replication-deficient recombinant viral vector lysates and stocks
that are
free of helper or other contaminating virus. In a preferred embodiment, the
method
is one which is easily scaled for industrial production of recombinant viral
vectors.
In another preferred embodiment, the method is one in which a high titer of
recombinant viral vector lysates and stocks is achieved.
In another embodiment, the invention includes attenuated,
replication-competent recombinant viruses and a method of producing such
viruses
free of helper or other contaminating virus. In a preferred embodiment, these
2 0 attenuated, replication-competent viruses may be used for immunization.
CA 02375119 2001-11-26
WO 00/73480 PCT/US00/14481
-17-
BRIEF DESCRIPTION OF THE DRAWINGS
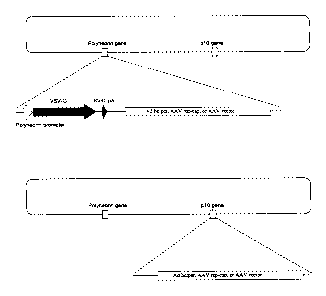
Figure 1 is a schematic diagram of recombinant baculoviruses with
target genes inserted into the loci of either polyhedrin or p 10 genes.
Figures 2A and 2B represent a genetic map of AAV type 2. Figure
2A is a schematic representation of the viral genome. rep encodes replication
proteins (Rep78, Rep68, Rep52, and Rep40) and cap encodes encapsidation
functions (VP1, VP2, and VP3). Right-angled arrows: the p5, p19, and p40 viral
promoters;. downward vertical arrow: common polyadenylation signal upstream of
the 3'-ITR Figure 2B represents the transcripts derived from each of the three
promoters. A": polyadenylation.
Figure 3 is a schematic diagram of constructed plasmids used in this
invention.
Figure 4 shows the steps involved in rAAV production by traditional
adenovirus infection/plasmid co-transfection method (Shenk et al., US patent
#5,436,146).
Figure 5 shows the steps required for rAAV production through the
use of two recombinant baculoviruses (BV-EiOV-RC and BV-cisEFGFP)
Figure 6 shows the steps required for rAAV production through the
use of stable cell line 293-CG3 together with one recombinant baculovirus (BV-
2 0 EiOV-RC).
Figure 7 shows the steps required for rAAV production through the
use of stable cell line expressing AAV rep and cap genes together with
recombinant
baculovirus (BV-EiOV-cisEFGFP-E1).
CA 02375119 2001-11-26
WO 00/73480 PCT/US00/14481
-18-
DETAILED DESCRIPTION OF THE INVENTION
Definitions and General Techniques
Unless otherwise defined, all technical and scientific terms used
herein have the meaning as commonly understood by one of ordinary skill in the
art
to which this invention belongs. The practice of the present invention
employs,
unless otherwise indicated, conventional techniques of chemistry, molecular
biology, microbiology, recombinant DNA, genetics, virology and immunology.
See, e.g., Sambrook et al., 1989, Ausubel et al., 1992, Harlow et al. 1989
(which
are incorporated herein by reference).
A "recombinant viral genome" comprises all or a part of a viral
genome, wherein the viral genome may be wild type or may contain point
mutations
or deletions, and optionally comprises a transgene operably linked to
expression
control sequences. In one embodiment, the transgene is flanked by flanking
elements. The recombinant viral genome of the invention is embedded in the
genome of the carrier vector, and is ultimately packaged into a recombinant
virus.
A "recombinant virus" is a virus derived from the recombinant viral
genome described above. The recombinant virus may comprise a transgene, may be
an attenuated, replication-competent virus without a transgene, may be a
replication-competent virus with one or more point mutation(s), or may be a
2 0 replication-deficient virus with one or more point mutations or genomic
deletions,
or combinations thereof. The recombinant virus comprising a transgene is
capable
of transducing mammalian cells and delivering the transgene thereto.
A "flanking element" or "flanking nucleic acid" is a nucleic acid
CA 02375119 2001-11-26
WO 00/73480 PCT/US00/14481
-19-
sequence generally derived from a mammalian virus which, when located in
positions flanking a transgene, permits the packaging of the transgene into a
recombinant virus. Flanking elements may be the naturally-occurring flanking
elements from a mammalian virus which permit the packaging of the recombinant
virus, or may be artificial nucleic acid elements, e.g. mutated sequences of
flanking
elements, that have the same or similar packaging function. Flanking elements
include, without limitation, the inverted terminal repeats (ITRs) of AAV or
Ad, the
long terminal repeats (LTRs) of retrovirus, the "a" or packaging sequence of
herpes
simplex virus (HSV), as well as any other sequences that are required for
packaging
from other viruses known in the art.
A "transgene" is a nucleic acid sequence that is to be delivered or
transferred to a mammalian cell. A transgene may encode a protein, peptide or
polypeptide that is useful as a marker, reporter or therapeutic molecule. A
transgene may also encode a protein, polypeptide or peptide that is useful for
protein production, diagnostic assays or for any transient or stable gene
transfer in
vitro or in vivo. Alternatively, a transgene may not encode a protein but
rather be
used as an antisense molecule, ribozyme or other regulatory nucleic acid to
inhibit
replication, transcription or translation of a nucleic acid to which it is
complementary or to target a complementary mRNA for degradation.
2 0 "Expression control sequences" are nucleic acid sequences that
regulate the expression of a gene by being operably linked to the gene of
interest.
"Operably linked" sequences include both expression control sequences that are
contiguous with the gene of interest and expression control sequences that act
in
CA 02375119 2001-11-26
WO 00/73480 PCT/US00/14481
-20-
tran.s or at a distance to control the gene of interest. Expression control
sequences
include appropriate transcription initiation, termination, promoter and
enhancer
sequences; efficient RNA processing signals such as splicing and
polyadenylation
signals; sequences that stabilize cytoplasmic mRNA; sequences that enhance
translation efficiency (i.e., Kozak consensus sequence); sequences that
enhance
protein stability; and when desired, sequences that enhance protein secretion.
As used herein, a "carrier vector" means a nucleic acid molecule
comprising a nonmammalian viral nucleic acid backbone and nucleic acid
sequences
derived from mammalian sources, mammalian viral sources, nonmammalian sources,
and nonmammalian viral sources. The nonmammalian viral nucleic acid backbone
may be selected from a wide variety of sources, see, for example Table 1 of U.
S.
Pat. No. 5,731,182, herein incorporated by reference. The nonmammalian viral
nucleic acid backbone, upon transfection of the carrier vector nucleic acid
into non-
mammalian cells, is sufficient to produce packaged carrier virus comprising
the
nucleic acid sequences inserted into the carrier vector.
A "carrier virus" is an encapsidated carrier vector capable of binding
to a mammalian cell and delivering the earner vector's genome to the cell's
nucleus.
As used herein, "ligand nucleic acid" means a nucleic acid which
encodes a protein which allows the carrier virus of the invention to bind to
and
2 0 enter a mammalian cell. The nucleic acid encoding the protein may be
operably
linked to expression control sequences that regulate the expression of the
nucleic
acid encoding the ligand.
"Helper function nucleic acid" is one or more nucleic acid sequences
CA 02375119 2001-11-26
WO 00/73480 PCT/US00/14481
-21 -
that encode one or more proteins, peptides or polypeptides, or that is
transcribed to
an RNA, wherein the one or more proteins, peptides, polypeptides or RNAs are
required by certain viruses for production of recombinant viruses. The
sequences
may be naturally-occurring helper functions or may be sequences that have been
mutated or altered but which retain their respective helper functions. The
sequences
may be derived from helper viruses or may be naturally-occurring or artificial
nucleic acid sequences that encode non-viral proteins that act as helper
functions for
production of recombinant viruses. The nucleic acid sequences that are
transcribed
to RNA or which encode the proteins, polypeptides or peptides may be operably
linked to expression control sequences that regulate the expression of the
nucleic
acid encoding the helper functions.
"Replication and/or encapsidation nucleic acid" is a nucleic acid
sequence or sequences which encode proteins or polypeptides that are required
for
replication and encapsidation of the recombinant virus. The sequences may be
naturally-occurring replication or encapsidation sequences or may be sequences
that
have been mutated or altered but which retain their respective functions of
replication or encapsidation. The nucleic acid sequences encoding the proteins
may
be operably linked to expression control sequences that regulate the
expression of
the nucleic acid encoding the replication and encapsidation sequences.
2 0 A "replicon" is an episomal replication origin and those necessary
proteins (or DNA encoding these proteins) to initiate nucleic acid
replication.
CA 02375119 2001-11-26
WO 00/73480 PCT/US00/14481
-22-
The Carrier Vector
The carrier vector of the invention is a chimeric vector backbone
derived from the nucleic acid of a nonmammalian virus. The carrier vector
comprises suf~'icient vector sequences to be able to replicate and encapsidate
within
the appropriate nonmammalian host cell. The carrier vector also includes one
or
more of the following inserts: an embedded recombinant viral genome; a ligand
nucleic acid providing for expression of a protein which can interact with a
mammalian cell; replication and/or encapsidation nucleic acid required to
replicate
and encapsidate a recombinant virus; and helper virus functions nucleic acids.
In a preferred embodiment, the carrier vector comprises an
embedded recombinant viral genome within its nonmammalian virus genomic
backbone. The recombinant viral genome may comprise a transgene with
associated expression regulatory sequences, wherein the transgene and
regulatory
sequences are bordered by flanking elements of a mammalian virus.
Alternatively,
the recombinant viral genome does not contain a transgene but rather contains
deletions or point mutations in its sequence such that it produces an
attenuated,
replication-proficient recombinant virus, or other deletions or point
mutations that
produce a replication-deficient recombinant virus.
In a more preferred embodiment, the carrier vector comprises the
2 0 embedded recombinant viral genome and either or both of 1) nucleic acid
sequences
encoding replication and/or encapsidation and 2) nucleic acid sequences
encoding
helper functions. In an even more preferred embodiment of this invention, the
carrier vector additionally comprises a ligand nucleic acid providing for
expression
CA 02375119 2001-11-26
WO 00/73480 PCT/US00/14481
- 23 -
of a protein which can interact with a mammalian cell. In another preferred
embodiment, the ligand nucleic acid encodes a protein which can bind to a
specific
mammalian cell receptor.
In the most preferred embodiment, the carrier vector comprises the
embedded recombinant viral genome and all of those nucleic acid inserts
required
for production of a recombinant virus in a mammalian cell. For instance, if
the
carrier virus comprising the carrier vector is to be used to infect a cell
line which
expresses replication and encapsidation proteins for a recombinant AAV virus
(e.g.,
the A64 cell line described in U.S. Pat. No. 5,658,785 and the B50 cell line
described in PCT US98/19463), then the carrier vector would comprise the
embedded recombinant viral genome and the helper functions, and optionally the
ligand nucleic acid. Alternatively, if carrier virus is to be used to infect a
cell line
which expresses a helper function for a recombinant AAV virus (e.g., the 293
cell
line which expresses EI), then the earner vector would comprise the embedded
recombinant viral genome, the replication and encapsidation nucleic acids for
AAV
(rc.~p and cap), and the helper functions required in addition to El (e.g.,
E2a,
E40RF6 and VAI RNA), and optionally the ligand nucleic acid.
If the carrier virus is to be used to produce a recombinant retrovirus,
which does not require helper functions, the carrier vector would comprise the
2 0 embedded recombinant retroviral viral genome and the nucleic acids
required for its
replication and encapsidation (e.g., gag, pol and env) and optionally, in
cases where
the retrovirus is a lentivirus, one or more of the nucleic acids encoding
regulatory or
auxilliary proteins (e.g., tat, rev, nef, vpr, vpu). If the earner virus is to
be used to
CA 02375119 2001-11-26
WO 00/73480 PCT/US00/14481
-24-
produce a recombinant retrovirus in a cell line that expresses gag, pol and
env or
the other functions described above in the case of lentiviruses, then the
carrier virus
would need only comprise the embedded recombinant retroviral genome and
optionally the ligand nucleic acid. Similarly, if the carrier virus is to be
used to
produce a recombinant adenovirus, the carrier vector would comprise the
embedded
recombinant adenoviral genome and the nucleic acid sequences required for its
replication and encapsidation. The type of nucleic acid sequences required for
replication and encapsidation of the recombinant adenoviral genome depends
upon
which adenoviral genes are deleted from the recombinant adenoviral genome and
whether the mammalian cell line that the carrier virus infects expresses any
adenoviral genes (e.g., 293 cells express E1). Any carrier vector genome may
optionally comprise a ligand nucleic acid to increase infection by the carrier
virus of
a mammalian cell.
The embedded recombinant viral genome and other nucleic acid
inserts may be carried on separate carrier vectors, but in the most preferred
embodiment, the embedded recombinant viral genome and all other desired
nucleic
acid inserts are carried on a single earner vector. The advantage of a single
carrier
vector is that only a single infection by the carrier virus of the mammalian
host cell
is required in order to produce a recombinant virus.
2 0 In another embodiment of the invention, the inability of the carrier
vector to replicate in mammalian cells is overcome by supplying a mammalian
replicon to the carrier vector. The provision of a replicon assures that
mammalian
cells infected by the carrier vector maintain a sufficient copy number of the
carrier
CA 02375119 2001-11-26
WO 00/73480 PCT/US00/14481
- 25 -
vector extrachromosomally throughout a population of proliferating and
dividing
mammalian cells.
Based on this description, other embodiments of the carrier vector
will be readily apparent to those of ordinary skill in the art.
Nonmammalian Virus Backbone
The chimeric carrier vector is constructed from a backbone of a
nonmammalian virus. The backbone need not be the entire genome of the
nonmammalian virus, but may be only that portion of the genome necessary for
replication in a nonmammalian host. Preferably, the vector backbone is derived
from an invertebrate virus. Table 1 of U. S. Pat. No. 5,731,182 lists several
examples of viruses that may be used to form the backbone of the chimeric
vector,
the sequences of which are available from various sources, such as Genbank. In
a
preferred embodiment, the invertebrate DNA virus is a baculovirus. In a more
preferred embodiment, the bacuolovirus is a Granulovirus or
Nucleopolyhedrovirus.
In an even more preferred embodiment, the nonmammalian viral backbone is
derived from the baculovirus Autographa californica nuclear polyhedrosis virus
(AcNPV). See, e.g., GenBank Accession No. L22858.
In a preferred embodiment, the nonmammalian virus backbone must
be capable of replication in its ordinary host cell, but incapable of
replication in a
2 0 mammalian cell. For example, the baculovirus virus backbone exemplified
herein
replicates only in insect cells.
CA 02375119 2001-11-26
WO 00/73480 PCT/US00/14481
-26-
The Embedded Recombinant Viral Genome
The methods of the present invention allow for large scale
production of high titers of recombinant virus, i.e., one that has a transgene
inserted
therein to be delivered to target mammalian cells, or one that does not have a
transgene but rather has a mutation or deletion in a viral gene and is to be
used as a
vaccine, e.g., an attenuated and replication-proficient recombinant virus or a
replication-deficient mutant virus. The recombinant virus may be any virus of
interest for use to deliver transgenes to mammalian cells or for use as a
vaccine.
Preferred recombinant viruses for delivery of a transgene include
adenoviruses,
retroviruses, adeno-associated viruses, herpesvirus amplicons and hepatitis B
mruses.
In order to manufacture a recombinant virus containing a transgene,
the method of the present invention begins with a desired transgene, then
associates
the transgene with appropriate expression regulatory sequences (ERS), e.g.,
promoter, enhancer, polyadenylation site, then inserts this ERS-transgene
construct
between the packaging elements of the virus to be manufactured, in place of
the
genes normally found therein. Where the length of the replacement is shorter
than
that being replaced, and that shorter length would pose an obstacle to proper
packaging, an optional spacer or "stuffer" sequence may be inserted in order
to
2 0 maintain the proper length for packaging. The entire construct of the ERS-
transgene constructed bordered by the flanking elements is the genome of the
recombinant virus of the present invention, which is then embedded in the
carrier
vector's genome, at which point it subsists as an embedded recombinant viral
CA 02375119 2001-11-26
WO 00/73480 PCT/US00/14481
-27-
genome. Each of these elements is described in detail below:
T he %i-ansgene
The composition of the transgene sequence depends upon the
intended use for the resulting recombinant virus. For example, one type of
transgene sequence comprises a reporter or marker sequence, which upon
expression produces a detectable signal. Such reporter or marker sequences
include, without limitation, DNA sequences encoding E. coli (3-lactamase, (3-
galactosidase (LacZ), alkaline phosphatase, HSV thymidine kinase, green
fluorescent protein (GFP), bacterial chloramphenicol acetyltransferase (CAT),
firefly luciferase, eukaryotic membrane bound proteins including, for example,
CD2,
CD4, CDB, the influenza hemagglutinin protein, and others well known in the
art, to
which high affinity antibodies directed to them exist or can be made
routinely, and
fusion proteins comprising a membrane bound protein appropriately fused to an
antigen tag domain from, among others, hemagglutinin or myc.
These sequences, when associated with regulatory elements which
drive their expression, provide signals detectable by conventional means,
including
enzymatic, radiographic, colorimetric, fluorescence or other spectroscopic
assays,
fluorescent activated cell sorting assay and immunological assays, including
ELISA,
RIA and immunohistochemistry. For example, where the transgene is the LacZ
2 0 gene, the presence of a recombinant virus is detected by assays for (3-
galactosidase
activity. Similarly, where the transgene is luciferase, the recombinant virus
gene
expression may be measured by light production in a luminometer.
CA 02375119 2001-11-26
WO 00/73480 PCT/US00/14481
-28-
However, desirably, the transgene is a non-marker gene which can
be delivered to a cell or an animal via the recombinant virus produced by this
method. The transgene may be selected from a wide variety of gene products
useful
in biology and medicine, such as proteins, antisense nucleic acids (e.g.,
RNAs), or
catalytic RNAs. The invention may be used to correct or ameliorate gene
deficiencies, wherein normal genes are expressed but at less than normal
levels, and
may also be used to correct or ameliorate genetic defects wherein a functional
gene
product is not expressed. A preferred type of transgene sequence is a
therapeutic
gene which expresses a desired corrective gene product in a host cell. These
therapeutic nucleic acid sequences typically encode products which, upon
expression, are able to correct, complement or compensate an inherited or non-
inherited genetic defect, or treat an epigenetic disorder or disease. However,
the
selected transgene may encode any product desirable for study. The selection
of the
transgene sequence is not a limitation of this invention. Choice of a
transgene
sequence is within the skill of the artisan in accordance with the teachings
of this
application.
The invention also includes methods of producing recombinant virus
and compositions thereof which can be used to correct or ameliorate a gene
defect
caused by a mufti-subunit protein. In certain situations, a different
transgene may
2 0 be used to encode each subunit of the protein. This may be desirable when
the size
of the DNA encoding the protein subunit is large, e.g., for an immunoglobulin
or
the platelet-derived growth factor receptor. In order for the cell to produce
the
mufti-subunit protein, a cell would be infected with recombinant virus
expressing
CA 02375119 2001-11-26
WO 00/73480 PCT/US00/14481
-29-
each of the different subunits.
Alternatively and more preferably, different subunits of a protein
may be encoded by the same transgene. In this case, a single transgene would
include the DNA encoding each of the subunits, with the DNA for each subunit
separated by an internal ribosome entry site (IRES). The use of IRES permits
the
creation of multigene or polycistronic mRNAs. IRES elements are able to bypass
the ribosome scanning model of 5' methylated cap-dependent translation and
begin
translation at internal sites (Pelletier and Sonenberg, 1988). IRES elements
from
two members of the picornavirus family (polio and encephalomyocarditis) have
been described (Pelletier and Sonenberg, 1988), as well an IRES from a
mammalian
mRNA (Macejak and Sarnow, 1991). IRES elements can be linked to heterologous
open reading frames. By virtue of the IRES element, each open reading frame is
accessible to ribosomes for efficient translation. Thus, multiple genes can be
efficiently expressed using a single promoter/enhancer to transcribe a single
message. This is preferred when the size of the DNA encoding each of the
subunits
is sufficiently small that the total of the DNA encoding the subunits and the
IRES is
no greater than the maximum size of the DNA insert that the virus can
encompass.
For instance, for rAAV, the insert size can be no greater than approximately
4.8
kilobases; however, for an adenovirus which lacks all of its helper functions,
the
2 0 insert size is approximately 28 kilobases.
Useful gene products include hormones and growth and
differentiation factors including, without limitation, insulin, glucagon,
growth
hormone (GH), parathyroid hormone (PTH), calcitonin, growth hormone releasing
CA 02375119 2001-11-26
WO 00/73480 PCT/US00/14481
-30-
factor (GRF), thyroid stimulating hormone (TSH), adrenocorticotropic hormone
(ACTH), prolactin, melatonin, vasopressin, ~3-endorphin, met-enkephalin, leu-
enkephalin, prolactin-releasing factor, prolactin-inhibiting factor,
corticotropin-
releasing hormone, thyrotropin-releasing hormone (TRH), follicle stimulating
hormone (FSH), luteinizing hormone (LH), chorionic gonadotropin (CG), vascular
endothelial growth factor (VEGF), angiopoietins, angiostatin, endostatin,
granulocyte colony stimulating factor (GCSF), erythropoietin (EPO), connective
tissue growth factor (CTGF), basic fibroblast growth factor (bFGF), bFGF2,
acidic
fibroblast growth factor (aFGF), epidermal growth factor (EGF), transforming
growth factor a (TGFa), platelet-derived growth factor (PDGF), insulin-like
growth factors I and II (IGF-I and IGF-II), any one of the transforming growth
factor ~3 (TGF~3) superfamily comprising TGF~3, activins, inhibins, or any of
the
bone morphogenic proteins (BMP) BMPs 1-15, any one of the
heregulin/neuregulin/ARIA/neu differentiation factor (NDF) family of growth
factors, nerve growth factor (NGF), brain-derived neurotrophic factor (BDNF),
neurotrophins NT-3, NT-4/5 and NT-6, ciliary neurotrophic factor (CNTF), glial
cell line derived neurotrophic factor (GDNF), neurtuin, persephin, agrin, any
one of
the family of semaphorins/collapsins, netrin-1 and netrin-2, hepatocyte growth
factor (HGF), ephrins, noggin, sonic hedgehog and tyrosine hydroxylase.
2 0 Other useful gene products include proteins that regulate the
immune system including, without limitation, cytokines and lymphokines such as
thrombopoietin (TPO), interleukins (IL) IL-1 a, IL-1 ~3, IL-2, IL,-3, IL-4, IL-
5, IL,-6,
IL-7, IL-8, IL-9, IL-10, IL-11, IL,-12, IL-13, IL,-14, II,-15, IL,-16, and IL,-
17,
CA 02375119 2001-11-26
WO 00/73480 PCT/US00/14481
-31 -
monocyte chemoattractant protein (MCP-1), leukemia inhibitory factor (LIF),
granulocyte-macrophage colony stimulating factor (GM-CSF), granulocyte colony
stimulating factor (G-CSF), monocyte colony stimulating factor (M-CSF), Fas
ligand, tumor necrosis factors a and ~3 (TNFa and TNF~3), interferons (IFN)
IFN-a,
IFN-(3 and IFN-y, stem cell factor, flk-2/flt3 ligand. Gene products produced
by
the immune system are also encompassed by this invention. These include,
without
limitations, immunglobulins IgG, IgM, IgA, IgD and IgE, chimeric
immunoglobulins, humanized antibodies, single chain antibodies, T cell
receptors,
chimeric T cell receptors, single chain T cell receptors, class I and class II
MHC
molecules, as well as engineered MHC molecules including single chain MHC
molecules. Useful gene products also include complement regulatory proteins
such
as membrane cofactor protein (MCP), decay accelerating factor (DAF), CR1, CRZ
and CD59.
Still other useful gene products include any one of the receptors for
the hormones, growth factors, cytokines, lymphokines, regulatory proteins and
immune system proteins. Examples of such receptors include fZt-1, ,fZk-l, TIE-
2;
the trk family of receptors such as TrkA, MUSK, Eph, PDGF receptor, EGF
receptor, HER2, insulin receptor, IGF-1 receptor, the FGF family of receptors,
the
TGF~3 receptors, the interleukin receptors, the interferon receptors,
serotonin
2 0 receptors, a-adrenergic receptors, ~3-adrenergic receptors, the GDNF
receptor, p75
neurotrophin receptor, among others. The invention encompasses receptors for
extracellular matrix proteins, such as integrins, counter-receptors for
transmembrane-bound proteins, such as intercellular adhesion molecules (ICAM-
1,
CA 02375119 2001-11-26
WO 00/73480 PCTNS00/14481
-32-
ICAM-2, ICAM-3 and ICAM-4), vascular cell adhesion molecules (VCAM), and
selectins E-selectin, P-selectin and L-selectin. The invention encompasses
receptors
for cholesterol regulation, including the LDL receptor, HDL receptor, VLDL
receptor, and the scavenger receptor. The inventions encompasses the
apolipoprotein ligands for these receptors, including ApoAI, ApoAIV and ApoE.
The invention also encompasses gene products such as steroid hormone receptor
superfamily including glucocorticoid receptors and estrogen receptors, Vitamin
D
receptors and other nuclear receptors. In addition, useful gene products
include
antimicrobial peptides such as defensins and maginins, transcription factors
such as
,jur~,.fos, max, mad, serum response factor (SRF), AP-1, AP-2, myb, MRG1,
CREM, Alx4, FREACl, NF-oB, members of the leucine zipper family, C2H4 zinc
finger proteins, including Zif268, EGRl, EGR2, C6 zinc finger proteins,
including
the glucocorticoid and estrogen receptors, POU domain proteins, exemplified by
Pitl, homeodomain proteins, including HOX-1, basic helix-loop-helix proteins,
including myc, MyoD and myogenin, ETS-box containing proteins, TFE3, E2F,
ATFl, ATF2, ATF3, ATF4, ZFS, NEAT, CREB, HNF-4, C/EBP, SP1, CCAAT-
box binding proteins, interferon regulation factor 1 (IRF-I), Wilms tumor
protein,
ETS-binding protein, STAT, GATA-box binding proteins, e.g., GATA-3, and the
forkhead family of winged helix proteins.
2 0 Other usefizl gene products include carbamoyl synthetase I, ornithine
transcarbamylase, arginosuccinate synthetase, arginosuccinate lyase, arginase,
fumarylacetoacetate hydrolase, phenylalanine hydroxylase, alpha-1 antitrypsin,
glucose-6-phosphatase, porphobilinogen deaminase, factor VII, factor VIII,
factor
CA 02375119 2001-11-26
WO 00/73480 PCT/US00/14481
- 33 -
IX, factor II, factor V, factor X, factor XII, factor XI, von Willebrand
factor,
superoxide dismutase, glutathione peroxidase and reductase, heme oxygenase,
angiotensin converting enzyme, endothelin-1, atrial natriuetic peptide, pro-
urokinase, urokinase, plasminogen activator, heparin cofactor II, activated
protein
C (Factor V Leiden), Protein C, antithrombin, cystathione beta-synthase,
branched
chain ketoacid decarboxylase, albumin, isovaleryl-CoA dehydrogenase, propionyl
CoA carboxylase, methyl malonyl CoA mutase, glutaryl CoA dehydrogenase,
insulin, beta-glucosidase, pyruvate carboxylase, hepatic phosphorylase,
phosphorylase kinase, glycine decarboxylase (also referred to as P-protein), H-
protein, T-protein, Menkes disease protein, tumor suppressors (e.g., p53),
cystic
fibrosis transmembrane regulator (CFTR), the product of Wilson's disease gene
PWD, Cu/Zn superoxide dismutase, aromatic aminoacid decarboxylase, tyrosine
hydroxylase, acetylcholine synthetase, prohormone convertases, protease
inhibitors,
lactase, lipase, trypsin, gastrointestinal enzymes including chyromotrypsin,
and
pepsin, adenosine deaminase, al anti-trypsin, tissue inhibitor of
metalloproteinases
(TIMP), GLUT-1, GLUT-2, trehalose phosphate synthase, hexokinases I, II and
III, glucokinase, any one or more of the individual chains or types of
collagen,
elastin, fibronectin, thrombospondin, vitronectin and tenascin, and suicide
genes
such as thymidine kinase and cytosine deaminase.
2 0 Other useful transgenes include non-naturally occurring
polypeptides, such as chimeric or hybrid polypeptides or polypeptides having a
non-
naturally occurring amino acid sequence containing insertions, deletions or
amino
acid substitutions. For example, single-chain engineered immunoglobulins could
be
CA 02375119 2001-11-26
WO 00/73480 PCT/US00/14481
-34-
useful in certain immunocompromised patients. Other useful proteins include
truncated receptors which lack their transmembrane and cytoplasmic domain.
These truncated receptors can be used to antagonize the function of their
respective
ligands by binding to them without concomitant signaling by the receptor.
Other
types of non-naturally occurring gene sequences include antisense molecules
and
catalytic nucleic acids, such as ribozymes, which could be used to reduce
overexpression of a gene.
Other useful transgenes include those that encode antigenic peptides
capable of generating an immune response. Recombinant vectors comprising these
transgenes can be used for genetic immunization. Useful transgenes include
those
that encode peptides specific for Epstein Barr virus; HIV; simian
immunodeficiency
virus (SIV); human T-cell leukemia viruses I and II (HTLV-I and HTLV-II);
hepatitis A, B, C, D and E; pseudorabies virus; rabies virus; cytomegalovirus;
respiratory syncytial virus; parainfluenza virus types I-4; mumps virus;
rubella virus;
polio virus; rubeola virus; influenza virus types A, B and C; rotavirus;
herpes
simplex viruses types 1 and 2; varicella-zoster virus; human herpes virus type
6;
hantavirus; adenoviruses; chlamydia pneumoniae; chlamydia trachomatis;
mycoplasma pneumoniae; mycobacterium tuberculosis; atypical mycobacteria;
feline
leukemia virus; feline immunodeficiency virus; bovine immunodeficiency virus;
2 0 equine infectious anemia virus; caprine arthritis encephalitis virus;
visna virus;
Staphlococcus species and Streptococcus species. The transgenes may also be
directed against peptides from tumor antigens to provide immunization for
tumors
and cancers.
CA 02375119 2001-11-26
WO 00/73480 PCT/US00/14481
-35-
Txpressiorr Control Sequences
A great number of expression control sequences -- native,
constitutive, inducible and/or tissue-specific -- are known in the art and may
be
utilized to drive expression of the transgene and the nucleic acid sequences
encoding the replication and encapsidation functions of the recombinant virus,
the
helper functions and the ligand. The choice of expression control sequence
depends
upon the type of expression desired. For eukaryotic cells, expression control
sequences typically include a promoter, an enhancer, such as one derived from
an
immunoglobulin gene, SV40, cytomegalovirus, etc., and a polyadenylation
sequence
which may include splice donor and acceptor sites. The polyadenylation
sequence
generally is inserted following the transgene sequences and before the 3'
flanking
sequence of the transgene. A transgene-carrying molecule useful in the present
invention may also contain an intron, desirably located between the
promoter/enhancer sequence and the transgene. One possible intron sequence is
also derived from SV-40, and is referred to as the SV-40 T intron sequence.
Another vector element that may be used is an internal ribosome entry site
(IRES),
as described above. An IRES sequence is used to produce more than one
polypeptide from a single gene transcript. An IRES sequence can be used for
the
transgene or for any of the other nucleic acid sequences encoding the
replication
2 0 and encapsidation polypeptides, the helper functions or the ligand.
Selection of
these and other common vector elements are conventional and many such
sequences are available [see, e.g., Sambrook et al, and references cited
therein at,
for example, pages 3.18-3.26 and 16.17-16.27 and Ausubel et al., Current
CA 02375119 2001-11-26
WO 00/73480 PCT/US00/14481
-36-
Protocols in Molecular Biolo~y, John Wiley & Sons, New York, 1989].
In one embodiment, high-level constitutive expression will be
desired. Examples of such promoters include, without limitation, the
retroviral
Rous sarcoma virus (RSV) LTR promoter/enhancer, the cytomegalovirus (CMV)
immediate early promoter/enhancer [see, e.g., Boshart et al, Cell, 41:521-530
(1985)], the SV40 promoter, the dihydrofolate reductase promoter, the
cytoplasmic
(3-actin promoter and the phosphoglycerol kinase (PGK) promoter.
In another embodiment, inducible promoters may be desired.
Inducible promoters are those which are regulated by exogenously supplied
compounds, either in cis or in traps, including without limitation, the zinc-
inducible
sheep metallothionine (MT) promoter; the dexamethasone (Dex)-inducible mouse
mammary tumor virus (MMTV) promoter; the T7 polymerase promoter system
[WO 98/10088]; the ecdysone insect promoter [No et al, Proc. Natl. Acad. Sci.
USA, 93:3346-3351 (1996)]; the tetracycline-repressible system [Gossen et al,
Proc. Natl. Acad. Sci. USA, 89:5547-5551 (1992)]; the tetracycline-inducible
system [Gossen et al., Science, 268:1766-1769 (1995); see also Harvey et al.,
Curr.
Olin. Chem. Biol., 2:512-518 (1998)]; the RU486-inducible system [Wang et al.,
Nat. Biotech., 15:239-243 (1997) and Wang et al., Gene Ther., 4:432-441
(1997)];
and the rapamycin-inducible system [Magari et al., J. Clip. Invest., 100:2865-
2872
(1997); Rivera et al., Nat. Medicine. 2:1028-1032 (1996)]. Other types of
inducible
promoters which may be useful in this context are those which are regulated by
a
specific physiological state, e.g., temperature, acute phase, or in
replicating cells
only. In a preferred embodiment, the transgene is under the control of the
native p5
CA 02375119 2001-11-26
WO 00/73480 PCT/US00/14481
-37-
promoter of AAV.
In another embodiment, the native promoter for the transgene or
nucleic acid sequence of interest will be used. The native promoter may be
preferred when it is desired that expression of the transgene or the nucleic
acid
sequence should mimic the native expression. The native promoter may be used
when expression of the transgene or other nucleic acid sequence must be
regulated
temporally or developmentally, or in a tissue-specific manner, or in response
to
specific transcriptional stimuli. In a further embodiment, other native
expression
control elements, such as enhancer elements, polyadenylation sites or Kozak
consensus sequences may also be used to mimic the native expression.
In one embodiment, the recombinant viral genome comprises a
transgene operably linked to a tissue-specific promoter. For instance, if
expression
in skeletal muscle is desired, a promoter active in muscle may be used. These
include the promoters from genes encoding skeletal a-actin, myosin light chain
2A,
dystrophin, muscle creatine kinase, as well as synthetic muscle promoters with
activities higher than naturally-occurring promoters [see Li et al., Nat.
Biotech.,
17:241-245 (1999)]. Examples of promoters that are tissue-specific are known
for
liver [albumin, Miyatake et al. J. Virol., 71:5124-32 (1997); hepatitis B
virus core
promoter, Sandig et al., Gene Ther., 3:1002-9 (1996); alpha-fetoprotein (AFP),
2 0 Arbuthnot et al., Hum. Gene Ther.. 7:1503-14 (1996)], bone [osteocalcin,
Stein et
al., Mol. Biol. Ren., 24:185-96 (1997); bone sialoprotein, Chen et al., J.
Bone
Miner. Res., 11:654-64 (1996)], lymphocytes [CD2, Hansal et al., J. Immunol.,
161:1063-8 (1998); immunoglobulin heavy chain; T cell receptor a chain],
neuronal
CA 02375119 2001-11-26
WO 00/73480 PCT/US00/14481
-38-
[neuron-specific enolase (NSE) promoter, Andersen et al. Cell. Mol.
Neurobiol..
13:503-15 (1993); neurofilament light-chain gene, Piccioli et al., Proc. Natl.
Acad.
Sci. USA, 88:5611-5 (1991); the neuron-specific vgf gene, Piccioli et al.,
Neuron.
15:373-84 (1995)]; among others.
Of course, not all vectors and expression control sequences will
function equally well to express all of the transgenes or other nucleic acid
sequences
of this invention. However, one of skill in the art may make a selection among
these expression control sequences without departing from the scope of this
invention. Suitable promoter/enhancer sequences which function in the
appropriate
host cell of choice may be selected by one of skill in the art using the
guidance
provided by this application. Such selection is a routine matter and is not a
limitation of the molecule or construct.
In one method of identifying a suitable expression control sequence
for a desired nucleic acid sequence, one may select one or more expression
control
sequences and operably link the expression control sequence to the nucleic
acid
sequence to be regulated. Then, one may insert these operably linked sequences
comprising the expression control sequence and regulated sequence into the
genome of the carrier vector. In one embodiment, one may insert a recombinant
viral genome comprising the expression control sequence and the transgene into
a
2 0 nonmammalian vector of the instant invention. After following one of the
methods
for producing and packaging the recombinant vector as taught in this
specification
one may infect suitable cells in vitro or in vivo. The number of copies of the
transgene in the cell may be monitored by Southern blotting or quantitative
PCR;
CA 02375119 2001-11-26
WO 00/73480 PCT/US00/14481
-39-
the level of RNA expression may be monitored by Northern blotting or
quantitative
RT-PCR; and the level of protein expression may be monitored by Western
blotting,
immunohistochemistry, ELISA, RIA, tests of the transgene's gene product's
biological activity, either in vitro or in vivo, or tests for correction or
amelioration
of a genetic defect.
In a similar fashion, one may select one or more expression control
sequences and operably link it to a nucleic acid sequence encoding replication
and
encapsidation proteins, helper functions or a ligand, and insert the resultant
desired
nucleic acid molecule into a vector of the instant invention. One may also
select
one or more vector replication sequences and insert them into a vector of the
instant
invention. After packaging and infecting nonmammalian cells, one may measure
the
particular effects, e.g., on expression of the ligand or on replication of the
vector,
by one of the methods described above. One may also use a functional test to
determine if one or more particular expression control sequences operably
linked to
a nucleic acid sequence encoding a ligand produces a carrier virus which is
able to
infect mammalian cells efficiently. One may assay a number of different
expression
control sequences to determine which one is most effective for mammalian cell
infection. The same may be done using a variety of vector replication
sequences.
Furthermore, after infecting mammalian host cells and obtaining
2 0 recombinant virus, one may infect mammalian cells with the recombinant
virus, then
measure the expression of the replication and encapsidation proteins and/or
helper
functions by one of the methods described above. One may also use a functional
test to determine if one or more particular expression control sequences
operably
CA 02375119 2001-11-26
WO 00/73480 PCT/US00/14481
-40-
linked to one or more helper functions or replication or encapsidation
functions is
capable of supporting production of a infectious recombinant virus. One may
determine which of many expression control sequences are most effective in
producing a high titer of infectious recombinant virus.
I % lcruking Elements
Flanking elements are required for replication, excision and
packaging of many viruses, and each type of virus has its own type of flanking
elements. In a wild-type virus, these elements flank the viral genes when the
viral
DNA integrates into a host cell chromosome. In the case of integrating
viruses,
when the wild-type virus is rescued from the host chromosome, the flanking
elements excise along with the viral DNA and remain in flanking positions
surrounding the rescued viral DNA, in a form suitable for packaging into
virions.
For non-integrating, extrachromosomal viruses (e.g. HSV), flanking sequences
serve functions in DNA replication and packaging. In recombinant viruses, much
or
all of the viral nucleic acid sequences between the flanking elements are
removed
from the virus and are replaced with a transgene and its associated expression
regulatory sequences.
In one embodiment of the invention, the recombinant virus is a
recombinant adenovirus, and comprises a selected transgene operably linked to
2 0 expression regulatory sequences and the adenoviral flanking elements.
Adenoviral
flanking elements are ITRs and are 100-200 by in length. A large number of
adenoviral flanking elements are known, such as those from human adenoviruses
CA 02375119 2001-11-26
WO 00/73480 PCT/US00/14481
-41 -
types 1-46, chimpanzee adenoviruses, canine adenoviruses, bovine adenoviruses
[all
available from the American Type Culture Collection (ATCC), 10801 University
Boulevard, Manassas, VA 20110-2209].
In another embodiment, the recombinant virus is a recombinant
retrovirus, and comprises a selected transgene operably linked to expression
regulatory sequences and retroviral flanking elements. The flanking elements
are
long terminal repeat (LTR) sequences that are present at the 5' and 3' ends of
the
retroviral genome. These LTRs contain strong promoter and enhancer sequences
and are also required for integration in the host cell genome (Coffin, 1990).
A large
number of retroviral LTRs are known. See, for instance, U.S. Pat. No.
5,672,510.
In yet another embodiment of the invention, the recombinant virus is
a recombinant AAV, and comprises a selected transgene operably linked to
expression regulatory sequences and AAV flanking elements. The naturally-
occurring AAV ITRs consist of approximately 145 by at the 5' and 3' ends of
the
AAV genome. The AAV ITRs are required for replication, excision and
encapsidation of both wild type and recombinant AAV virions.
In another embodiment, the recombinant virus is either a herpesvirus
derivative containing one or more mutations or deletions of viral genes, or is
a
herpesvirus amplicon. In either case, the flanking elements would be the viral
2 0 terminal repeats (e.g., the "a" sequence if the virus is HSV). HSV
amplicons are
defective HSV genomes containing the packaging sequence (a), viral origin of
DNA
replication (ori) and the transgene cassette of interest operably linked to
the desired
expression regulatory sequences. In the presence of helper herpesvirus or
substitute
CA 02375119 2001-11-26
WO 00/73480 PCT/US00/14481
-42-
helper functions, the amplicon is replicated and packaged as head-to-tail
concatemers to form wild-type size genomes.
In another embodiment, the recombinant virus is a recombinant
defective hepatitis B virus (HBV) and comprises a selected transgene operably
linked to expression regulatory sequences and inserted into the HBV genome. In
oilro studies have shown that the recombinant hepatitis B virus retains the
ability for
helper-dependent packaging and reverse transcription despite the deletion of
up to
80% of its genome (Horwich et al., 1990). This suggests that large portions of
the
genome may be replaced with foreign genetic material. The hepatotropism and
persistence (integration) were particularly attractive properties for liver-
directed
gene transfer.
Li~and DNA
Most nonmammalian viruses are not infectious to mammalian cells;
however, it has been reported that in some cases, nonmammalian viruses will
infect
certain particularly infection-susceptible mammalian cell lines. [Barsoum et
al.,
Human Gene Therapy 8:2011-2018 (Nov. 20, 1997)]. Where the host cell to be
used for manufacturing the recombinant virus is not susceptible to infection
by the
nonmammalian virus, the nonmammalian virus may be modified by incorporating
ligand DNA in the nonmammalian backbone. The nonmammalian backbone may
2 0 also be modified by incorporation of ligand DNA to increase infection of
mammalian host cell by the nonmammalian virus. The expression of the ligand
DNA by the subsequently produced nonmammalian virus will permit infection or
CA 02375119 2001-11-26
WO 00/73480 PCT/US00/14481
- 43 -
increase infection of mammalian cells. The backbone of the carrier vector of
the
present invention is modified by addition of DNA encoding components needed to
produce a ligand which is recognized by a desired mammalian cell.
The ligand DNA is selected from genes which, when expressed, yield
a gene product that will be present on the surface of the encapsidated carrier
vector,
thus presenting the ligand to the mammalian cell target receptors and allowing
the
carrier vector to bind to and enter mammalian cells. [Barsoum et al., Human
Gene
Therapy 8:2011-2018 (Nov. 20, 1997).] The ligand DNA is placed under the
regulatory control of expression regulatory sequences that insure that the
ligand
gene product is expressed coordinately with the replicated DNA of the carrier
vector to allow efficient chimeric carrier vector production in nonmammalian
host
cells. The nonmammalian expression regulatory sequence may be identical,
similar,
or distinct from the backbone's native regulatory sequences, so long as it is
capable
of regulatory functions in the nonmammalian host cell. In a preferred
embodiment,
the expression regulatory sequences comprise a promoter derived from the
native
nonmammalian virus from which the nonmammalian vector backbone is derived. In
a more preferred embodiment, the promoter is polyhedrin early promoter (poles)
and the nonmammalian vector backbone is derived from baculovirus.
In general, if a ligand DNA is regulated by nonmammalian
2 0 expression regulatory sequences, the ligand DNA will not be expressed when
the
chimeric vector infects the mammalian host cell. The absence of expression of
the
ligand encoded by the DNA may be useful to prevent incorporation of ligand
into
the recombinant virus coat encoded from the carrier nonmammalian vector, which
CA 02375119 2001-11-26
WO 00/73480 PCT/US00/14481
-44-
could disrupt its structural integrity or cause adverse immunogenic reactions
in an
animal. However, if ligand expression is desired in the mammalian host cell,
then
alternative or additional expression regulatory sequences may be operably
linked to
the sequences encoding the ligand to permits its expression in mammalian and
nonmammalian host cells. Alternatively, there may be some instances in which
the
ligand DNA is expressed because the nonmammalian expression regulatory
sequences are also activated in the mammalian cells.
The ligand DNA can be essentially any nucleic acid that encodes a
protein, polypeptide or peptide that modifies the mature nonmammalian virus to
enable it to bind to and enter mammalian cells. The ligand can be naturally-
occurring protein, a fragment of a naturally-occurring protein that has a
desired
binding capability, or an artificial or mutated polypeptide or peptide that
has a
desired binding capability. The ligand can be one of general specificity,
which
would allow binding to a wide variety of mammalian cells (e.g., vesicular
stomatitis
virus glycoprotein G (VSV-G) gene, bovine syncytial virus (BSV) envelope
glycoprotein gene, or amphotropic envelope gene as illustrated below), or it
may be
more specific, allowing binding to targeted specific cell types. For instance,
the
ligand may cause the virus to bind via electrostatic interactions or other
general
mechanism of interacting the mammalian cell, or it may be a specific ligand-
receptor
2 0 interaction.
Usefizl ligand nucleic acids may be any nucleic acid which encodes a
ligand that permits the nonmammalian virus to interact with the mammalian
cell.
For instance, the ligand may be one which increases the electrostatic
interaction
CA 02375119 2001-11-26
WO 00/73480 PCT/US00/14481
- 45 -
between the virus and the mammalian cell for a receptor found on the mammalian
host cells that are to be infected by the carrier virus. Other useful ligand
nucleic
acids include, without limitation, nucleic acids encoding peptide hormones,
growth
factors, or other normally secreted factors for which the mammalian host cell
of
interest expresses a receptor. The nucleic acids useful as a ligand include
all those
secreted factors, peptide hormones and growth factors which have a normal
cellular
receptor and which are disclosed above for transgenes. For instance, the
ligand
nucleic acid may encode PDGF, EGF, bFGF, aFGF, insulin, IGF-I, IGF-II, apoE,
apoAl, apoA4, EPO, PTH, GH or GRF. The ligand nucleic acid may encode a
native or genetically engineered immunoglobulin (e.g., ScFv, chimeric
immunoglobulin, humanized immunoglobulin, etc.) or MHC molecule that
specifically binds to a particular cell surface protein on the mammalian cell.
Other
ligand nucleic acids of interest encode a member of the extracellular matrix
such as
a collagen, elastin, thrombospondin, tenascin or vitronectin, which bind to
integrins
and other cellular transmembrane receptors. The nucleic acid sequence encoding
a
ligand which is normally secreted may be modified by incorporating a nucleic
acid
sequence encoding an "anchoring domain" at either the 5' or 3' end of the
coding
sequence for the ligand. The anchoring domain is a region that secures the
ligand in
the viral coat. In a preferred embodiment, the anchoring domain is at the 3'
end of
2 0 the coding sequence for the ligand. In a further preferred embodiment, the
anchoring domain is derived from a viral coat protein, such as HIV gp41 (which
anchors gp 120 coat protein to the viral envelope). Other examples include E
protein of dengue virus or the 14 kDa protein of vaccinia virus.
CA 02375119 2001-11-26
WO 00/73480 PCTNS00/14481
-46-
The ligand nucleic acid also may encode a protein that is normally
anchored in the cell membrane of a mammalian cell which binds to a particular
cell
surface protein or counter-receptor on a mammalian host cell. Examples of this
type of ligand nucleic acid include a number of the CD antigens, such as the T
cell
receptor (TCR), CTLA-4 receptor and B-7, integrins such as Mac-1, LFA-1, and
p 150,95, intercellular adhesion molecules such as ICAM-1, ICAM-2, ICAM-3 and
ICAM-4, and selectins, such as E-selectin, P-selectin and L-selectin. The
ligand
may also be an artificial or mutated counter-receptor, such as a cell-surface
anchored or hybrid immunoglobulin or TCR.
In one embodiment, the ligand is one that is normally present on a
virus and which mediates binding to a mammalian cell, for example, gp 120 of
HIV
or HA from influenza. In another embodiment, the ligand is one that is
normally
present on a bacterial cell and which mediates binding to a mammalian cell,
for
example, Protein A from Staphylococcus aureus is known to bind to
immunoglobulins.
In another embodiment, the mammalian host cell is genetically
engineered to express a receptor which specifically binds to a ligand. Thus,
one can
design mammalian host cell-carrier virus systems that promote highly specific
binding of the carrier virus to the mammalian host cell. For example, one may
2 0 engineer a mammalian host cell line to express a growth factor receptor,
such as the
EPO receptor, and design the carrier vector to comprise a ligand nucleic acid
comprising the EPO gene. One of skill in the art, in light of the instant
specification, would be able to identify a large number of mammalian host cell-
CA 02375119 2001-11-26
WO 00/73480 PCT/US00/14481
-47-
carrier virus interactive receptor-ligand systems.
In one embodiment the ligand DNA is the VSV-G gene. This gene
may be placed under the control of the baculovirus polyhedrin (pPH) early
promoter. The VSV-G protein, when expressed, modifies the mature carrier virus
such that it may bind to mammalian host cells and thereby infect them.
[Barsoum,
snprcr]. In another embodiment of the present invention, the ligand DNA is the
BSV env gene, which functions in the context of the invention in a similar
manner.
In another preferred embodiment, the present invention exploits the
fact that nonmammalian viruses normally do not terminate glycoproteins with
sialic
acid. Thus, the ligand DNA is a gene which expresses an asialoglycoprotein,
which
binds to mammalian lectins (e.g., the hepatic asialoglycoprotein receptor),
which
would then facilitate entry into the mammalian cell.
Replication and Encapsidation Nucleic Acids
The replication and encapsidation functions are required for
replication, excision and encapsidation of the recombinant viral genome into
an
infectious recombinant virion or virus. Each type of recombinant virus will
require
a different type of replication and encapsidation function. For instance, if
the
recombinant virus is a retrovirus, then the replication and encapsidation
functions
include the retroviral gag, pol and env genes (and in the case of lentiviruses
will also
2 0 include regulatory or accessory genes such as HIV tat, rev, nef, vpu or
vpr), while
if the recombinant virus is an AAV, then the replication and encapsidation
functions
include the rep and cap genes from an AAV.
CA 02375119 2001-11-26
WO 00/73480 PCT/US00/14481
-48-
As discussed above, either the carrier vector or the mammalian host
cell may comprise nucleic acids encoding those replication and encapsidation
functions required for a particular recombinant virus. Mammalian host cells
such as
A64 cell line described in U.S. Pat. No. 5,658,785 and the B50 cell line
described in
PCT US98/19463) express AAV rep and cap genes for replication and packaging
of recombinant AAV. Similarly, mammalian host cells expressing adenoviral
genes
required for replication and packaging of recombinant adenovirus are known
[see,
e.g., U.S. Pat. No. 5,851,806 and Amaltifano et al., Proc. Natl. Acad. .Sci.
I~SA
9 3:3352-6 (1996)] or may be constructed, and a number of mammalian host cells
expressing retroviral genes required for replication and packaging of
recombinant
retroviruses have been constructed [see, e.g., Cone et al., Proc. Natl. Acad.
Sci.
USA 81:6349-6353 (1984); Miller et al., Mol. Cell. Biol. 6:2895-2902 (1986);
Miller et al., Mol. Cell. Biol. 5:431-437 (1985); and Sorge et al., Mol. Cell.
Biol.
4:1730-1737 (1984)]. Cell lines comprising genes required for packaging of
herpesviruses (see, e.g., U.S. Pat. No. 5,851,826) are also known.
If a cell line comprises all the necessary replication and encapsidation
functions to replicate, excise and package a particular recombinant viral
genome,
then the carrier vector need not comprise any replication and/or encapsidation
nucleic acid sequences. The cell line may comprise the necessary replication
and
2 0 encapsidation functions either by being transiently or stably transduced
with the
nucleic acid encoding the appropriate proteins. In a preferred embodiment, the
cell
line stably comprises the replication and encapsidation functions.
Furthermore, the
cell line may express the replication and encapsidation functions
constitutively or
CA 02375119 2001-11-26
WO 00/73480 PCT/US00/14481
-49-
inducibly. Constitutive or inducible expression may be controlled by using any
of
the expression regulatory sequences known in the art or as discussed above
under
"Expression Regulatory Sequences." In a preferred embodiment, the expression
of
the replication and encapsidation functions is inducible. In a more preferred
embodiment, the replication and encapsidation functions are stably transfected
or
infected and are inducibly expressed. In an even more preferred embodiment,
the
expression of the replication and encapsidation functions is regulated by
their native
promoters.
A mammalian cell line used in the instant invention may comprise
none of the functions required for replication or encapsidation, or may
comprise
only a part of the functions required for replication or encapsidation. If a
mammalian cell line comprises none of the functions required for replication
or
encapsidation, these functions must be introduced into the cell by a vector
for
production of the recombinant virus. In a preferred embodiment, one or more
carrier viruses of the instant invention are used to transduce the mammalian
cell line
with the nucleic acids encoding the replication and encapsidation functions.
In a
more preferred embodiment, a single Garner virus comprising the replication
and
encapsidation functions are used to transduce the mammalian cell line. In an
even
more preferred embodiment, a single Garner virus comprising the replication
and
2 0 encapsidation functions, the embedded recombinant viral genome, and any
other
nucleic acid sequences required for recombinant virus production are used to
transduce the mammalian cell line.
If the mammalian cell line comprises some of the replication or
CA 02375119 2001-11-26
WO 00/73480 PCTNS00/14481
-50-
encapsidation functions, these functions must be introduced into the cell by a
vector
for production of the recombinant virus. In a preferred embodiment, one or
more
carrier viruses are used to transduce the mammalian cell line with the nucleic
acids
encoding the missing replication and encapsidation functions. In a more
preferred
embodiment, a single carrier virus comprising the missing replication and
encapsidation functions are used to transduce the mammalian cell line. In an
even
more preferred embodiment, a single carrier virus comprising the missing
replication
and encapsidation functions, the embedded recombinant viral genome, and any
other nucleic acid sequences required for recombinant virus production are
used to
transduce the mammalian cell line.
The replication and encapsidation functions required for a
recombinant virus differ depending upon the type of recombinant virus. In
general,
the required replication and encapsidation functions are known in the art for
the
various recombinant viruses. In preferred embodiment of recombinant vectors,
recombinant AAV requires rep and cap for replication and encapsidation,
recombinant retroviruses require gag, pol and env (and tat, rev and nef for
lentiviruses), recombinant adenoviruses require all of part of the functions
encoded
by E1, E2, E4, L1-L5, pIX and IVa2 genes, alone or in combination, and
recombinant herpesviruses require a large number of genes, which may be
provided
2 0 by a helper herpesvirus or by a carrier vector comprising the required
herpesvirus
genes. Together the host mammalian cell and the carrier virus must contribute
the
necessary replication and encapsidation functions for the particular
recombinant
virus in order to obtain infectious recombinant virus from the mammalian host
cells.
CA 02375119 2001-11-26
WO 00/73480 PCT/US00/14481
- Sl -
In one embodiment, the replication and encapsidation functions are
encoded by nucleic acids encoding the naturally-occurring proteins having the
replication and encapsidation functions. In another embodiment, the
replication and
encapsidation functions are encoded by nucleic acids encoding fragments or
muteins
of the naturally-occurring proteins but which retain their respective
replication and
encapsidation functions. In another embodiment of the invention, other
recombinant viruses may be produced using nucleic acids encoding the
appropriate
replication and encapsidation functions for the particular recombinant virus
desired.
Other types of recombinant viruses and the replication and encapsidation
functions
they require are known in the art.
In a preferred embodiment, when production of a recombinant AAV
is desired, the rep and cap sequences are regulated by a native AAV p5
promoter.
In another preferred embodiment, when production of a recombinant adenovirus
is
desired, the nucleic acid sequences encoding the replication and encapsidation
functions for adenovirus are regulated by their native adenovirus promoters.
Native
promoters may also be used for regulating the expression of replication and
encapsidation functions of other recombinant viruses, including, without
limitation,
herpesvirus and HBV.
In a more preferred embodiment, the replication and encapsidation
2 0 functions are encoded by nucleic acid sequences inserted in the carrier
vector. The
advantage of having these sequences on the carrier vector is that no cell line
has to
be constructed before infection by the carrier virus. It is often difficult to
create and
maintain cell lines expressing replication and encapsidation functions because
many
CA 02375119 2001-11-26
WO 00/73480 PCT/US00/14481
-52-
of the proteins that provide these functions are toxic to mammalian cells.
Thus,
another advantage of inserting the replication and encapsidation sequences on
the
carrier vector is that the replication and encapsidation functions are only
expressed
in the mammalian cells when the cells are infected with the carrier virus when
the
production of a recombinant virus is desired. In a more preferred embodiment,
the
carrier virus has an embedded recombinant viral genome comprising a transgene
and the ITRs from AAV and further has rep and cap gene sequences for
replication
and encapsidation of the embedded recombinant AAV genome. In an even more
preferred embodiment, the expression of the rep and cap genes is regulated by
their
native promoters or replcap is separated from the promoter to decrease or
eliminate
homologous or non-homologous recombination to form wt AAV. Similarly, in a
preferred embodiment of carrier viruses that produce recombinant retrovirus,
adenovirus, herpesvirus and HBV, the carrier viruses contain nucleic acid
sequences
that encode replication and encapsidation functions. In a more preferred
embodiment, the nucleic acid sequences encoding the replication and
encapsidation
functions are regulated by their native promoters.
Helper Functions
A number of viruses are unable to replicate, excise and package on
their own, and require helper functions to do so. Helper functions may also be
2 0 required for the production of recombinant viruses which have had a large
amount
of their genome deleted for insertion of the transgene. The nature of the
helper
function may differ depending upon the type of recombinant virus and/or the
CA 02375119 2001-11-26
WO 00/73480 PCT/US00/14481
-53-
amount of genome that has been deleted. Helper functions include viral
proteins,
non-viral proteins, as well as physical and/or chemical agents. One may
identify
which helper functions are required from what is known in the art. For
instance, it
is known that AAV requires helper functions from adenovirus or herpesvirus or
from different chemical or physical agents. Alternatively, one of skill in the
art may
determine what helper functions are required by producing recombinant viruses
using the composition and methods disclosed in the instant specification.
To identify which helper functions are required for high levels of
recombinant virus production, one may infect mammalian host cells with the
carrier
virus in the absence of helper functions and measure the titer of infectious
recombinant virus. One may then transduce the mammalian host cells with
various
nucleic acids encoding potential helper functions. Such helper functions may
be any
nucleic acid that is known or thought to encode a helper function. In a
preferred
embodiment, the helper function is one or more viral proteins. In a more
preferred
embodiment, the helper virus proteins are insufficient to produce a mature
helper
virus. After transducing the mammalian host cell with the nucleic acid
encoding the
potential helper function, one may then measure the titer of the recombinant
virus.
If the carrier virus comprises a recombinant AAV genome, helper
functions are required for production of infectious recombinant AAV. In a
2 0 preferred embodiment, the helper functions are nucleic acids derived from
a virus.
In a more preferred embodiment, the helper functions are derived from
adenovirus,
herpes simplex virus (HSV) HSV-1, HSV-2, cytomegalovirus (CMV) or
pseudorabies virus (PRV). In an even more preferred embodiment, the helper
CA 02375119 2001-11-26
WO 00/73480 PCT/US00/14481
-54-
functions are at least Ela, Elb and E2a from adenovirus, and may also include
E40RF6 and VAI. In another preferred embodiment, the nucleic acid encodes the
helper functions from the helicase-primase complex of HSV (ULS, LJL8 and UL52)
and the major single-stranded DNA binding protein of HSV (IJL29). The helper
functions may also include all 7 HSV DNA replication genes (ULS, 8, 52, 29,
30, 9
and 42). Alternatively, helper functions for recombinant AAV may be provided
by
chemical or physical agents, including ultraviolet light, cycloheximide,
hydroxyurea
and various carcinogens.
The required helper functions for production of a recombinant virus
may be delivered to the mammalian host cell by any method known in art. The
helper functions may be delivered by transfection with a vector, such as a
plasmid,
by infection with a viral vector comprising the helper functions, or by any
other
method known in the art, including those discussed above (e.g., biolistic
injection of
DNA, use of DNA conjugates, etc.). The transfection or infection may be stable
or
transient. Alternatively, the mammalian cell line may stably express (either
on an
extrachromosomal episome or through integration in the cell's genome) the
helper
functions. In addition, some of the helper functions may be expressed by the
mammalian cell line while other helper functions are introduced by a vector.
For
example, 293 cells (ATCC CRL-1573) constitutively produce adenoviral Ela and
2 0 Elb proteins. Thus, for production of recombinant AAV, the helper
functions
required for the production of infectious recombinant AAV, such as E2A, E40RF6
and VAI, are introduced into the host cell by transfection or infection of a
vector.
In a preferred embodiment, the helper functions are transduced into
CA 02375119 2001-11-26
WO 00/73480 PCT/US00/14481
-55-
the mammalian cells by a carrier virus. In a more preferred embodiment, some
or
all of the helper functions are transduced into the mammalian cell by a
carrier virus
comprising the embedded recombinant viral genome. In an even more preferred
embodiment, all of the helper functions are transduced into the mammalian cell
by a
carrier virus comprising the embedded recombinant viral genome, any required
replication and encapsidation functions, and, optionally, a ligand DNA. In the
most
preferred embodiment, the carrier vector has a baculovirus backbone. An
internal
ribosome entry site (IRES) sequence may be placed between E2A and E4orf6 if
only a single promoter is to be used for these two proteins. Alternatively,
each
helper function gene may be supplied with its own promoter. These genes may be
under the regulatory control of a variety of promoters, constitutive or
inducible,
such as the CMV immediate-early promoter/enhancer or the MMTV LTR,
respectively. Whether the helper functions are provided on the carrier vector
itself
or are provided by the host cells, the promoters regulating those genes may be
constitutive or inducible.
The expression of the helper functions may be regulated by any of
the expression regulatory sequences known in the art or as described above,
including cis or traps regulation. The expression regulatory sequences may
provide
for constitutive expression, inducible expression, tissue-, cell type- or
differentiation
2 0 state-specific expression, or expression from the helper function
protein's native
promoter. In a preferred embodiment, the native promoter of the helper
function
protein is used. In another preferred embodiment, an inducible promoter of a
helper
function protein is used. In another preferred embodiment, a constitutive
promoter
CA 02375119 2001-11-26
WO 00/73480 PCT/US00/14481
-56-
of a helper function protein is used. In a further preferred embodiment, the
constitutive promoter is the CMV promoter. In another preferred embodiment,
one
or more constitutive promoters are used for certain helper function proteins,
and
one or more native promoters are used for other helper function proteins.
In one embodiment, each protein or polypeptide required for helper
function is encoded by a nucleic acid whose expression is regulated by its own
promoter and polyadenylation signal, as well as optional sequences such as
enhancers. In another embodiment, a nucleic acid is transcribed to a single
transcript that encodes more than one protein or polypeptide required for
helper
function. In this case, an IRES may be placed between the coding sequences of
each of the individual proteins or polypeptide to permit subsequent
translation of
the polycistronic mRNA. If only a single polycistronic transcript is produced,
only
a single promoter, optional enhancer, and polyadenylation signal are required
for
regulation of the transcription of the nucleic acid encoding the helper
function. One
may also encode the helper function by using both monocistronic mRNAs that
encode single proteins and polycistronic mRNAs encoding multiple proteins.
In a preferred embodiment of the instant invention, the carrier vector
comprises a embedded recombinant AAV genome and helper functions. In a
further preferred embodiment, the helper functions comprise adenovirus Ela,
Elb
2 0 and E2a, and more preferably include E40RF6 and VAI. In an even more
preferred
embodiment, the helper functions are encoded by a single polycistronic
transcript,
and the promoter for the helper functions is a constitutive promoter,
preferably the
CMV promoter.
CA 02375119 2001-11-26
WO 00/73480 PCT/US00/14481
-57-
Other recombinant viruses would require different helper functions
or none at all, but in all cases those helper functions may be provided on the
carrier
vector that carries the embedded recombinant viral genome, on a separate
carrier
virus, on a different type of vector capable of transducing a mammalian host
cell, or
is endogenously expressed in the mammalian host cell itself.
Mammalian Host Cells
Any type of mammalian host cell which can be adapted to cell
culture may be used to produce the recombinant viral genome. In general, a
mammalian host cell used in this invention is one that may be infected by a
nonmammalian carrier virus. The mammalian host cell may be one that may be
infected by a nonmammalian carrier virus that does not express a ligand
encoded by
a ligand nucleic acid, may be one that may be infected by a nonmammalian
carrier
virus that expresses a ligand encoded by a ligand nucleic acid, or may be a
cell that
is infected by a carrier vector that either expresses or does not express a
ligand
nucleic acid. Alternatively, the mammalian host cell may be one that is not
usually
infected by a carrier virus, but which can be transduced with a cellular
receptor such
that it may bind to a nonmammalian host cell. For instance, a mammalian host
cell
may be transduced with a growth factor receptor such that it can be infected
by a
carrier virus that expresses the particular growth factor as its ligand.
2 0 In addition to the ability to be infected by the carrier virus, another
preferred characteristic of the mammalian host cell is that it is able to
uncoat the
nonmammalian carrier virus. A third preferred characteristic of the mammalian
host
CA 02375119 2001-11-26
WO 00/73480 PCT/US00/14481
-58-
cell is its ability to replicate the recombinant virus at high levels. In a
preferred
embodiment, the mammalian host cell is one that takes up the nonmammalian
carrier virus at high levels, uncoats the carrier virus efficiently, and
replicates the
recombinant virus at high levels.
Appropriate mammalian host cells include, without limitation, CHO,
BHK, MDCK and various murine cells, e.g., 1 OT 1 /2 and WEHI cells, African
green
monkey cells such as COS 1, COS 7, BSC 1, BSC 40, and BMT 10, and human
cells such as VERO, WI38, MRCS, A549, and HT1080 cells. In a preferred
embodiment, appropriate mammalian cell include 293 cells (human embryonic
kidney cells which express adenoviral Ela and Elb proteins), B-50 cells (HeLa
cells
which express AAV rep and cap, see PCT US98/19463), 3T3 cells (mouse
embryonic fibroblast cell line), NIH3T3 cells (subline of 3T3 cells), HepG2
cells
(human liver carcinoma cell line), Saos-2 cells (human osteogenic sarcoma cell
line),
HuH7 cells or HeLa cells (human carcinoma cell line).
In addition to the mammalian host cells listed above, other
mammalian host cells may be used. One may determine whether a cell line would
be suited for use as a mammalian host cell by infecting the cell line with a
carrier
virus containing all the required components to produce a recombinant virus,
culturing the cells under conditions in which recombinant virus is produced,
and
2 0 then measuring the titer of infectious recombinant virus that is produced.
One may
then compare the titer of infectious virus produced in the potential host cell
with the
titers produced by other host cells to determine whether the cell line is good
for
recombinant virus production.
CA 02375119 2001-11-26
WO 00/73480 PCT/US00/14481
-59-
Although the receptors) for nonmammalian carrier virus such as
baculovirus on both insect and mammalian cell is/are unknown, it is thought
that the
baculovirus may bind to the cell, at least in part, via heparan sulfate
expressed on
the cell surface. Without wishing to be bound by any theory, cells which
express
high levels of heparan sulfate on their cell surface may be more easily
infected by
carrier viruses, especially baculovirus, than cells which express low levels
of
heparan sulfate on their cell surface. Thus, one method of identifying whether
a
particular cell line is a potential mammalian host cell is to measure the
level of
heparan sulfate on the cell surface.
Method of Making and Producing Carrier Viruses
The present invention includes methods of constructing the novel
carrier vectors described above and producing large quantities of the carrier
vector.
This method comprises the steps of:
1. Modifying a nonmammalian virus backbone DNA, or a
replication-proficient portion thereof, by inserting one or more nucleic acid
inserts
comprising 1 ) a recombinant viral genome comprising a transgene operably
linked
to expression regulatory sequences and flanked by flanking elements; 2)
nucleic acid
sequences encoding helper functions operably linked to expression regulatory
2 0 sequences; 3) nucleic acid sequences encoding replication and/or
encapsidation
functions for the recombinant virus; 4) a ligand DNA operably linked to
expression
regulatory sequences that are active in nonmammalian cells; and S) regulatory
control sequences that regulate sequences in the nonmammalian virus backbone,
a
CA 02375119 2001-11-26
WO 00/73480 PCT/US00/14481
-60-
modified nonmammalian virus backbone or a replication-proficient portion of
the
backbone or modified backbone;
2. transducing the resulting carrier vector into nonmammalian host
cells;
3. growing the nonmammalian host cells under conditions in which
carrier virus is produced; and
4. collecting the carrier virus from the nonmammalian host cells.
In a preferred embodiment, the carrier vector will be modified such
that it comprises the recombinant viral genome. In a more preferred
embodiment,
the carrier vector will be modified such that it comprises the recombinant
viral
genome and either or both of the nucleic acid inserts encoding the replication
and/or
encapsidation functions and the helper functions required for production of a
recombinant virus. In an even more preferred embodiment, the carrier vector
will
be modified such that it comprises the recombinant viral genome, all of the
nucleic
acid inserts encoding the replication and/or encapsidation functions and the
helper
functions required for production of a recombinant virus, and the nucleic acid
insert
encoding the ligand.
The nonmammalian host cell may be any host cell known in the art
or described in Table 1 of U. S. Pat. No. 5,731,182. The nonmammalian virus
2 0 backbone DNA may be derived from any virus that infects nonmammalian
species,
including those known in the art or described in Table 1 of U. S. Pat. No.
5,731,182.
In a preferred embodiment, the nonmammalian backbone of the carrier vector is
derived from a baculovirus and the nonmammalian host cells are insect cells.
In a
CA 02375119 2001-11-26
WO 00/73480 PCT/US00/14481
-61 -
more preferred embodiment, the carrier virus produced by the baculoviral
carrier
virus in the insect cells is produced at a high titer. Preferably, when
baculovirus is
used, the titers of the carrier baculovirus produced in any embodiment are
greater
than 10~ pfu/ml in insect cells or 109 pfu/ml; more preferably, the titers are
greater
than 10"' pfu/ml or 10'1 pfu/ml; and even more preferably, the titers are
greater than
I 0''- pfu/ml. The instant invention also encompasses lysates and supernatants
of
nonmammalian host cells comprising baculoviral carrier viruses having similar
titers.
The nonmammalian host cells comprising the carrier vector may be
grown by any method known in the art or as described herein. Methods for
producing large amounts of nonmammalian viruses are well known in the art and
are described in U.S. Pat. No. 5,871,986. The nonmammalian carrier virus may
be
purified from the supernatant produced by the nonmammalian host cells or from
lysed cells by any method known in the art or as described herein. Methods for
collecting and purifying nonmammalian viruses are well known in the art and
are
described in U.S. Pat. No. 5,871,986. A method of collecting and purifying the
nonmammalian viruses is described in Example 6.
The carrier virus produced when the carrier vector is encapsidated
has the normal wild-type capsid optionally modified by addition of the ligand.
In
general, the expression of the ligand nucleic acid is regulated by expression
2 0 regulatory sequences which promote transcription and translation in the
nonmammalian host cells. Such expression regulatory sequences may include a
nonmammalian promoter active in the nonmammalian host cells of interest, and
may
optionally include enhancer sequences, polyadenylation signals, or any other
CA 02375119 2001-11-26
WO 00/73480 PCT/US00/14481
-62-
expression regulatory sequences known in the art or described above. In a
preferred embodiment, the other nucleic acid inserts in the nonmammalian
backbone
may not be expressed or may be expressed at lower levels because their
promoters
are inactive or less active in nonmammalian cells. Because potentially toxic
viral
components, such as helper functions or replication/encapsidation functions,
are
either not expressed or expressed at lower levels, a high titer of carrier
virus may be
produced in nonmammalian cells.
Methods of Producing Recombinant Virus from the Carrier Vector
Another aspect of the instant invention is a method of producing
recombinant virus by using a carrier virus, produced by the method described
above, to infect mammalian cells and subsequently collecting and purifying the
recombinant virus from the mammalian cells. The method comprises the steps of:
1. Infecting mammalian host cells with a carrier virus, wherein he
carrier virus optionally expresses a ligand on the surface of the carrier
virus;
2. growing the infected mammalian host cells under conditions in
which the embedded recombinant viral genome is replicated, excised and
encapsidated; and
3. collecting the recombinant virus from the mammalian host cells.
The mammalian host cells may be any mammalian host cell known in
2 0 the art, described in the specification above under "Mammalian Host
Cells," or
identified by the method described under "Mammalian Host Cells" as an
appropriate
host cell. The mammalian host cell, prior to infection, may be one that
expresses
CA 02375119 2001-11-26
WO 00/73480 PCT/US00/14481
-63-
one or more of the following: 1 ) replication and/or encapsidation functions
(e.g., B-
50 cells or one of the retroviral cell lines described previously); 2) some or
all
necessary helper functions (e.g., 293 cells); and/or 3) an embedded
recombinant
viral genome stably integrated in the mammalian host cell genome.
Alternatively,
the mammalian host cell comprises none of these other elements before
infection by
the carrier virus.
The mammalian host cells may be infected and grown by any method
known in the art or as described herein. Methods for infecting mammalian host
cells with nonmammalian viruses are described herein and in Barsoum et al.,
supra.
Once the mammalian host cell has been infected, the expression regulatory
sequences that are operably linked to any required replication and/or
encapsidation
functions and helper functions are activated. Expression of the replication
and/or
encapsidation functions and helper functions, along with the mammalian host
cell's
native transcriptional and translational components, permits replication,
excision
and encapsidation of the embedded recombinant viral genome, thereby causing
the
manufacture of the recombinant virus.
In general, the nonmammalian carrier virus is capable of infecting a
mammalian cell, but the carrier virus will not replicate in the mammalian cell
because the components required for replication of the nonmammalian carrier
virus
2 0 are not present in the mammalian cell. If the carrier virus comprises a
ligand nucleic
acid, the expression regulatory sequences controlling ligand expression
generally
will not function in mammalian cells, such that ligand expression does not
occur in
the mammalian host cell. However, if replication of the carrier virus or if
CA 02375119 2001-11-26
WO 00/73480 PCTNS00/14481
-64-
expression of the ligand is desired in the mammalian host cell, then
additional
expression regulatory sequences may be operably linked to the sequences
required
for replication, excision and/or packaging of the nonmammalian carrier virus
and/or
expression of the ligand.
The recombinant virus may be purified from the supernatant
produced by the mammalian host cells or from lysed cells by any method known
in
the art or as described herein. Methods for collecting and purifying various
types of
recombinant viruses from mammalian host cells are well known in the art and
are
described in PCT US97/15716. A method of collecting and purifying the
recombinant viruses is also described in Example 6.
As discussed above, in a preferred embodiment, one or more carrier
viruses comprises all those nucleic acid inserts required for production of
recombinant virus in a particular mammalian host cell. Thus, if the host cell
comprises replication and encapsidation functions, then the carrier viruses
comprise
the embedded recombinant viral genome and any necessary helper functions.
Similarly, if the host cell comprises the embedded recombinant viral genome,
then
one or more carrier viruses will comprise the required replication and
encapsidation
functions and any necessary helper functions, while if the host cell comprises
the
necessary helper functions, then one or more carrier viruses will comprise the
2 0 required replication and encapsidation functions and the embedded
recombinant
viral genome.
In a preferred embodiment, a single Garner virus comprises all of the
nucleic acid inserts required for production of a recombinant virus in a
particular
CA 02375119 2001-11-26
WO 00/73480 PCT/US00/14481
-65-
mammalian host cell. For example, if a recombinant AAV is to be produced in B-
50 cells, the carrier virus will comprise the embedded recombinant viral
genome and
those helper functions required for AAV production. Similarly, if a
recombinant
AAV is to be produced in a mammalian host cell that does not express any of
the
required functions for AAV production, the carrier virus will comprise the
embedded recombinant viral genome, the replication and encapsidation functions
and the helper functions required for AAV production.
The method of producing recombinant virus described is useful
because it produces an essentially homogeneous recombinant virus that is free
from
helper virus and wild-type virus without purification. The recombinant virus
is free
from helper virus because there are insufficient helper virus genes to produce
a
mature helper virus. The recombinant virus is free of wild-type virus because
homologous recombination is avoided by a variety of techniques. For instance,
wild-type AAV produced through homologous recombination may be avoided using
several strategies. The replcap sequences and the embedded recombinant viral
genome may be positioned at separate loci on the carrier vector, minimizing
the
likelihood of a recombination event. The replcap sequences and the embedded
recombinant viral genome also may be designed so that they have no regions of
homology. Additionally, because AAV is intolerant of packaging greater than
5.0
2 0 kb, one may incorporate a "stuffer" nucleic acid sequence to be inserted
between a
required sequence, such as rep or cap and its promoter. Thus, even if
recombination took place, the resulting AAV genome would be too large to
package and no wtAAV would be produced. In order to maintain the integrity of
CA 02375119 2001-11-26
WO 00/73480 PCT/US00/14481
-66-
translation of rep, the stuffier sequence may be constructed using splice
donor and
acceptor sites, such that the resulting mRNA and ultimately the rep protein
produced would be unaffected. Similar methods may be employed for other types
of recombinant viruses to avoid recombination and production of wild-type
virus.
The method is also easily scaled to industrial production because it
may require only a single infection of mammalian host cells by a carrier virus
that
can be produced in large amounts at high titers. In a preferred embodiment,
the
recombinant virus produced by the instantly described method is produced at a
high
titer. For recombinant AAV, the titer is preferably greater than 104 particles
per
producing cell, more preferably, greater than 105 to 106 particles per
producing cell,
and even more preferably, greater than 10' particles per producing cell. For
recombinant adenovirus, the titer is preferably greater than 104 particles per
producing cell, more preferably, greater than 105 particles per producing
cell, and
even more preferably, greater than 106 particles per producing cell. For
recombinant herpesvirus, the titer preferably is greater than 101° pfu
per ml, more
preferably, greater than 101' pfu per ml and even more preferably, greater
than 10'3
pfu per ml. For retroviruses, the titer preferably is greater than 106 to 10'
colony
forming units (cfu) per ml, more preferably, 108 cfu per ml, and even more
preferably, 109 cfu per ml. The instant invention also encompasses lysates and
2 0 supernatants of mammalian host cells comprising recombinant viruses. These
lysates and supernatants direr from those produced by prior art methods
because
they do not contain wild-type virus or helper virus.
In a preferred embodiment, the method is used to manufacture
CA 02375119 2001-11-26
WO 00/73480 PCT/US00/14481
-67-
recombinant AAV at high titers and in the absence of helper virus or wild-type
AAV. The desired transgene, with appropriate expression regulatory sequences
operably linked thereto, is placed between the AAV ITRs, by means known in the
art. In order to maintain the length of the insert at a length compatible with
eventual packaging, spacer DNA may optionally be inserted therein. This
recombinant viral genome is then embedded in a baculovirus, in a non-essential
locus, by means known in the art. The required helper functions, replication
and
encapsidation functions, and/or embedded recombinant viral genome may be
placed
in the polyhedrin gene site, the p10 gene site, or one could be placed at the
polyhedrin gene site and the other may be placed at the p 10 gene site (see
Figure 1 ).
The baculovirus backbone also may be modified to comprise a ligand nucleic
acid,
such as the VSV-G gene. The baculovirus may also be modified to comprise rep
and cap sequences and helper functions from adenovirus, comprising E 1 a, E
1b,
E2a, E40RF6 and VAI, or HSV genes ULS, UL8, UL52 and UL29. The carrier
1 S vector is transduced into insect cells, such as Sf~ cells, the cells are
grown under
conditions in which baculovirus is produced, and the baculovirus is collected
and
purified. The baculovirus is then used to infect mammalian cells, the
mammalian
cells are grown under conditions in which the recombinant virus is replicated,
excised and encapsidated, and the recombinant AAV is collected and,
optionally,
2 0 purified.
In another preferred embodiment, the method is used to manufacture
recombinant "gutless" adenovirus deleted of all adenoviral genes at high
titers and
in the absence of helper virus or wild-type adenovirus. Previously, one would
make
CA 02375119 2001-11-26
WO 00/73480 PCT/US00/14481
-68-
a "gutted" adenovirus plasmid from the adenovirus genome in which all of the
adenovirus genes were removed except for the ITRs and the cis-acting packaging
signal. Foreign DNA containing the transgene of interest, transcriptional
regulatory
sequences, and, optionally, stufl'er DNA would be added to obtain an insert of
approximately 36 kb. The plasmid is designed such that a DNA cassette contains
the packaging signal upstream of the transgene and stuffer DNA, and the Ad
ITRs
flank such DNA cassette. The plasmid was transfected into cells, such as 293
cells.
A helper adenovirus lacking the adenoviral E1 and E3 genes, as well as
sequences
within the adenoviral packaging signal was used to infect the 293 cells
transfected
with the gutted Ad plasmid to provide replication and encapsidation functions
in
trans. Using this method, low levels of homologous recombination would rescue
the deletion in the helper Ad's packaging signal, thus both helper and
"gutless"
adenovirus would be produced. CsCI gradients would have to be performed to
separate the helper adenovirus from the recombinant "gutless" adenovirus
vector.
In addition, other disadvantages included high levels of contaminating helper
virus
and low yields of the gutless Ad vector.
Using the method of the instant invention, homologous
recombination resulting in generation of contaminating helper Ad can be
avoided.
Rather than using helper adenovirus, one may construct a carrier vector
comprising
2 0 the adenoviral functions necessary for replication and packaging of the
gutted Ad
genome. In one embodiment, the carrier vector contains the complete genome of
adenovirus without the ITR's, El, the packaging signal, and, optionally,
without
E3. Then, one may infect mammalian cells with the carrier virus and transfect
the
CA 02375119 2001-11-26
WO 00/73480 PCT/US00/14481
-69-
cells with the plasmid described above containing the transgene, ITRs and
packaging signal. In another embodiment, one may construct two separate
carrier
vectors, one comprising helper adenoviral functions described above and the
other
comprising the gutted Ad construct containing the Ad ITR's, packaging signal
and
the transgene cassette. Alternatively, one may construct a single carrier
vector
comprising both the helper adenoviral functions and the transgene cassette
comprising the ITRs, packaging signal and transgene/stuffer DNA. The
adenoviral
functions and transgene cassette may be placed in the polyhedrin gene locus,
the
p 10 gene locus, or one could be placed at the polyhedrin gene locus and the
other
may be placed at the p 10 gene locus (see Figure 1 ).
In another preferred embodiment, the method is used to manufacture
recombinant herpesvirus amplicon vectors. As discussed above, herpesvirus
amplicons require the "a" sequence for packaging, and the HSV origin of
replication. Either the oris or oriL origin of replication may be used, but
the oris
origin is preferred. One may construct a carrier vector comprising a
herpesvirus
amplicon. In one embodiment, the cassette would contain, in the 5' to 3'
direction,
the "a" sequence, followed by the transgene of interest, followed by the HSV
origin
of replication, followed by an optional spacer, and followed by another "a"
sequence. This may be inserted into either the polyhedrin or the p I O gene
loci in
2 0 baculovirus, for instance. The carrier virus that is subsequently produced
may be
used to infect mammalian cells that have been coinfected with helper
herpesvirus.
Alternatively, the helper herpesvirus functions may be placed on the same or a
separate carrier vector and used to infect the mammalian cells. Recombinant
CA 02375119 2001-11-26
WO 00/73480 PCT/US00/14481
-70-
herpesvirus amplicon vectors may then be isolated and purified from the
mammalian
cells.
Recombinant Virus Compositions
Another embodiment of the present invention is the recombinant
virus produced by the methods of the invention. Unlike other preparations of
recombinant virus, the preparations produced by the methods of this invention
yield
high titers of essentially homogeneous recombinant virus which is helper-free
and
wild-type virus free. The recombinant virus may be formulated as a
pharmacological composition for use for any form of transient and stable gene
transfer in vivo and in vitro. The recombinant virus may be used for in vivo
and ex
vioo gene therapy, genetic immunization, in vitro protein production and
diagnostic
assays.
For gene therapy, the recombinant virus may be introduced into cells
ex vivo or in vivo. Where the virus is introduced into a cell ex vivo, the
recombinant virus may be used to infect a cell in vitro, and then the cell may
subsequently be introduced into a mammal (e.g., into the portal vein or into
the
spleen), if desired. Alternatively, the recombinant virus may be administered
to a
mammal directly, e.g., intravenously or intraperitoneally. A slow-release
device,
2 0 such as an implantable pump, may be used to facilitate delivery of the
virus to a cell.
Where the virus is administered to a mammal, the specific cells to be infected
may
be targeted by controlling the method of delivery. For example, intravascular
administration of the recombinant virus to the portal vein or to the hepatic
artery
CA 02375119 2001-11-26
WO 00/73480 PCT/US00/14481
-71-
may be used to facilitate targeting the recombinant virus to a liver cell.
The recombinant virus produced by the above-described method
may be administered to a patient, preferably suspended in a biologically
compatible
solution or pharmaceutically acceptable delivery vehicle. A suitable vehicle
includes
sterile saline. Other aqueous and non-aqueous sterile suspensions known to be
pharmaceutically acceptable carrier and well known to those of skill in the
art may
be employed for this purpose.
The recombinant virus is administered in sufficient amounts to infect
the desired cells and provide sufficient levels of transduction and expression
of the
selected transgene (or viral gene products in the case of a vaccine) to
provide a
corrective effect without undue adverse or with medically acceptable
physiological
effects, which can be determined by those skilled in the medical arts.
Conventional
and pharmaceutically acceptable routes of administration include direct
administration to the target organ, tissue or site; intranasal; intravenous;
intramuscular; subcutaneous; intradermal; oral and other parenteral routes of
administration. Routes of administration may be combined, if desired.
Dosages of the recombinant virus will depend primarily on factors
such as the type of recombinant virus (i.e., whether the virus is AAV,
adenovirus,
retrovirus, etc.), the condition being treated and the selected gene. The
dosage may
2 0 also vary depending upon the age, weight and health of the patient. For
example,
an effective human dosage of a recombinant adenovirus is generally in the
range of
from about 0.5 ml to 50 ml of saline solution containing adenovirus at
concentrations of 1 x 10' or 1 x 10g or 1 x 109 or 1 x 101° or 1 x 1011
or 1 x 1012 or
CA 02375119 2001-11-26
WO 00/73480 PCT/US00/14481
_72_
1 x 10'3 or 1 x 10'4 or 1 x 10'5 particles per dose administered. The dosage
will be
adjusted to balance the corrective benefits against any adverse side eiTects.
The
levels of expression of the selected gene may be monitored to determine the
type
and frequency of dosage administration.
The following examples of the present inventions are illustrative
only, and are not intended to limit the scope of the invention.
EXAMPLE 1
Cell Line Maintenance and Virus Propa_ ation
The human embryonic kidney cell line 293 (ATCC CRL 1573) was
maintained in Dulbecco's Modification of Eagle's Medium (DMEM; GIBCO BRL)
supplemented with 10% FBS (Hyclone) and 50 pg of penicillin, 50 p.g of
streptomycin, and 10 ~.g of neomycin/ml (GIBCO BRL). Insect cell line IPLB-
Sfzl
(CLONTECH Laboratories, Inc.) was maintained in SF900-II medium (GIBCO
BRL) supplemented with 10% FBS and SO p.g of penicillin, 50 p.g of
streptomycin,
and 10 pg of neomycin/ml. Human adenovirus type 5 (ATCC VR-5) was
propagated on 293 cells and purified through CsCI gradient centrifugation
(Jones
and Shenk, 1978).
EXAMPLE 2
Recombinant Plasmid Construction
2 0 Standard DNA recombinant techniques were employed to create
recombinant plasmids (Sambrook et al, 1989). The Rep and Cap sequence of pAV2
CA 02375119 2001-11-26
WO 00/73480 PCT/US00/14481
- 73 -
(ATCC 37216) between the DraIII site upstream ofthe p5 promoter and the NcoI
site downstream of the polyadenylation signal was removed. The Rep and Cap
sequence was replaced through multiple cloning steps with a cassette
containing
GFP under the control of elongation factor 1 alpha (EFla) promoter to create
pAV2cisEFGFP (Fig. 2). The entire cassette containing both AAV ITRs and the
GFP gene was then cloned into the SpeI and BgIII sites of BV-CZPG (baculovirus
shuttle plasmid with VSV-G gene under control of polyhedrin promoter; kindly
provided by Dr. Jim Barsoum of Biogen, Inc.) through multiple cloning steps to
obtain pBV-cisEFGFP (Fig. 3).
Adenovirus helper genes E2A, E40RF6, and VAI were subcloned
from Ad5 DNA. Briefly, E40RF6 was first inserted into the Smal and XbaI sites
of
pIRESlneo (CLONTECH Laboratories, Inc.) to obtain pIRESORF6. Next, a
Sau3AI-BsrGI fragment containing E2A coding sequences was inserted into the
BamHIBstXI sites of the plasmid pIRESORF6 through multiple cloning steps to
obtain the plasmid pE2AiORF6. In this construct, E2A and E40RF6 genes are
separated by an encephalomyocarditis virus (ECMV)-derived IRES, and both genes
are under the transcriptional control of a single human cytomegalovirus (CMV)
promoter upstream of the E2A gene. Next, a NcoI-BamHI fragment of Ad5 DNA
containing the VAI gene was inserted into the XhoI site of pE2AiORF6 through
2 0 blunt-end cloning to obtain pE2AiORF6-VAI. The entire cassette containing
CMV-E2AiORF6-VAI was cloned into the HpaI and SpeI sites of the baculovirus
shuttle plasmid BV-CZPG to obtain pBV-EiOV (Fig. 2).
AAV-2 rep and cap genes located between a Dra III site, which is
CA 02375119 2001-11-26
WO 00/73480 PCT/US00/14481
-74-
upstream of the AAV-2 p5 promoter and a BsaI site, which is downstream of the
polyadenylation signal (Fig. 2), were cloned into the SpeI and PacI sites of
pBV-
EiOV through multiple cloning steps to obtain pBV-EiOV-RC (Fig. 3). Ad5 El
and cisEFGFP were cloned into pBV-EiOV through multiple steps to obtain the
pBV-EiOV-cisEFGFP-El plasmid (Fig. 3). Plasmid pAc-cisEFGFP was
constructed by inserting a cassette containing the GFP gene flanked by AAV-2
ITRs into pAcUWI (Pharmingen) through several cloning steps. The Xbal-SspI
fragment of EBVOri from pEBVHisA (Invitrogen) was inserted into pBV-
cisEFGFP and pBV-EiOV-RC to create pBV-cisEFGFP-EBVOri (Fig. 3) and
pBV-EiOV-RC-EBVOri (Fig. 3). AAV-2 rep and cap genes were cloned into
pAdOF6 (plasmid carrying Ad helper genes E2A, the entire E4, and VAI; kindly
provided by Dr. Guangping Gao of the University of Pennsylvania) to obtain
pAd~F6-RC (Fig. 3).
Reference to a construct preceded by a "p" refers to a plasmid, while
reference to a construct without a "p" preceding it refers to a virus. For
example,
pBV-EiOV-RC refers to a plasmid, while BV-EiOV-RC refers to a modified
baculovirus.
EXAMPLE 3
Transfection of 293 Cells and Selection for 293-CG3 Stable Cell Line
293 cells were grown to ~70% confluency in 6-cm dia. tissue culture
dishes and co-transfected overnight with 1 p.g pIRESlneo and 10 p.g
pAV2cisEFGFP by the calcium phosphate transfection method (Sambrook et al.,
CA 02375119 2001-11-26
WO 00/73480 PCT/US00/14481
-75-
1989). Cells were fed with fresh medium containing 10% FBS and cultured for 24
hours. Following trypsinization, cells were seeded at a 1:20 dilution in fresh
medium containing 10% FBS. After incubation for another 24 hours, fresh medium
containing 1,250 ~g/ml of 6418 (GIBCO BRL) was added to the cell monolayer to
select for 6418-resistant cells. The medium containing 6418 was replaced every
3-
4 days until most of the original 6418-resistant cell colonies had formed. A
total of
fifty colonies were picked, six of which demonstrated constitutive GFP
expression.
These six clones were expanded in the presence of 6418 and tested for their
ability
to rescue functional rAAV by transfection with plasmid pBV-EiOV-RC. Normally,
when a clone was established, it was maintained in 6418-containing medium for
3
to 5 passages to ensure that all nonresistant cells had been killed. Then, the
cells
were maintained in 6418-free medium. One cell clone, 293-CG3, showed high
efficiency of rAAV rescue and was expanded and used for further experiments.
EXAMPLE 4
Functional Test of 293-CG3 for rAAV Production
Several plasmids were used to test the efficiency of 293-CG3 cells
for rAAV production. The cells were first seeded on 6-well plates at a density
of 1
X 10'' cells/well at 2 to 4 hours before transfection. The cells in each well
received
5 pg of plasmid DNA in a final volume of 167 ~,1 of CaP04 (Sambrook et al.,
1989).
2 0 After incubation for 16 hours, cells were fed with fresh medium. Three
days later,
cells were harvested and rAAV titers (transducing units) were determined. The
results are presented in Table 1 as an average of values determined from two
CA 02375119 2001-11-26
WO 00/73480 PCT/US00/14481
-76-
separate experiments. They indicate that the 293-CG3 cell line is very
efficient for
rAAV production, producing approximately 30 to 170 transducing units of rAAV
per cell depending on the configuration of helper plasmid used.
Table 1.
Transfection Plasmid 5 Cell rAAV titer (TU/106
line cells)
1. BV-EiOV-RC 293-CG3 3.1 X 10'
2. AdOF6-RC 293-CG3 1.7 X 10g
3. BV-EiOV 293-CG3 0
4 BV-RC 293-CG3 0
EXAMPLE 5
Generation of Recombinant Baculoviruses
To create VSV-G pseudotyped recombinant baculoviruses, the
BacPAK baculovirus expression system (CLONTECH Laboratories, Inc.) was
used. Plasmid DNA, one of pBV-EiOV-RC, pBV-cisEFGFP, or pBV-EiOV-
cisEFGFP-E1, was cotransfected with Bsu36I-digested BacPAK6 DNA into Sf21
cells according to the manufacturer's protocol. The medium was harvested 3
days
after transfection and recombinant baculoviruses were screened on 96-well
plates by
limited dilution assay. Briefly, the medium harvested from transfection was
diluted
to 10-2, 10-~, 10-4, and 10-5 each in 10 ml of insect medium containing Sf21
cells at 2
2 0 X I OS cells/ml. The mixture was then plated on 96-well plates at 100
p.l/well.
After infection for 5-7 days, cells were examined for signs of viral
infection (cell fusion mediated by VSV-G expression; see Eidelman et al.,
1984).
The week that showed viral infection in the lowest dilution were marked and
the
virus harvested (Chen et al., 1994). The cells were lysed and used for DNA
CA 02375119 2001-11-26
WO 00/73480 PCT/US00/14481
_77_
hybridization to verify the presence of recombinant DNA. Positive clones were
amplified into 10 ml, and 1 ml of each clone was used to transduce 293 cells
grown
in 6-well plates. The 293 cells were transfected with a plasmid carrying the
elements not provided by the recombinant baculovirus for rAAV rescue. After
transduction for 3 days, cells were lysed and the lysates were used to
transduce 84-
31 cells (E1/E4 double complementing cell lines derived from 293 cells; see
Fischer
et al., J. Virol., 70:8934-8943, 1996). The expression of a marker gene
indicated
the rescuing of rAAV. The cloned that could best support rAAV rescue was
screened for 3 to 4 more rounds in order to obtain pure recombinant
baculovirus.
and tested for their support of rAAV rescue Functional clones were further
screened on 96-well plates for 3 to 4 rounds to obtain pure recombinant
baculoviruses.
To create non-VSV-G pseudotyped recombinant baculoviruses, a
Baculovirus Expression Vector System (Pharmingen) was used. Plasmid DNA was
co-transfected with baculoviral DNA into Sf21 cells according to
manufacturer's
protocol. Recombinant baculovirus was screened on 96-well plates the same as
described for the VSV-G pseudotyped baculoviruses above except that X-gal
staining was used to distinguish recombinant baculovirus from wild type
baculovirus.
CA 02375119 2001-11-26
WO 00/73480 PCT/US00/14481
_78_
EXAMPLE 6
Production of rAAV by Using the Methods of this Invention
Method to Transduce Cells
For transduction, recombinant baculoviruses were first pelleted by
centrifugation at 4"C at 20,000 rpm for 30 minutes. The pellets were then
resuspended in serum-free DMEM. The medium was removed from the cell
monolayer and baculovirus was added to the cells. After incubating for 8-16
hours,
cells were fed with fresh medium containing 10% FBS. Cells were harvested 72
hours after transduction. Baculovirus-transduced cells were harvested and
lysed in
DOC lysis buffer (50 mM Tris-HCI, pH 7.4, 1 mM MgCIZ, 0.5% sodium
deoxycholate) by sonication on ice water (three sonication pulses for 1 minute
each). Cell debris was removed by centrifugation at 13,000 rpm for 5 minutes
at
4 ° C and the supernatant was collected. The supernatant was used for
rAAV
titration as described in Example 7.
7i°crfzsduction of 2~3 cells by Baculoviruses BhEiOV RC and BV cisEFGFP
BV-EiOV-RC provides Ad helper genes E2A, E40RF6, and VAI as
well as AAV rep and cap genes. BV-cisEFGFP provides the AAV vector sequence
with both AAV ITRs flanking the marker gene GFP. 293 cells express Ela and
Elb. Thus, transduction of 293 cells with both BV-EiOV-RC and BV-cisEFGFP
2 0 provides to the cells the embedded AAV viral genome comprising the GFP
transgene operably linked to the EFla promoter and flanked by the AAV ITRs; a
VSV-G ligand; helper functions comprising Ela, Elb, E2a, E40RF6, and VAI; and
CA 02375119 2001-11-26
WO 00/73480 PCT/US00/14481
-79-
the replication and encapsidation functions, rep and cap. These functions
allow the
cells to produce recombinant AAV.
%i~crrr.sdrrction of'293-CG3 Cells by Baculovirus BhEiOhRC
Because the AAV vector was stably integrated in the 293-CG3 cells,
only BV-EiOV-RC was needed to provide a ligand nucleic acid, helper functions
and replication and encapsidation functions to produce recombinant AAV.
%ioro.sduction of Rep-Cap Expressing Cells by
Baculovirus BhEiOT~ cisEFGFP-El
The baculovirus BV-EiOV-cisEFGFP-E1 provides the Ad helper
genes E1, E2A, E40RF6, and VAI, as well as the AAV vector with both AAV-
ITRS flanking the marker gene GFP. The AAV rep-cap genes are provided by
stable rep-cap cell lines such as B50 (Gao et al. 1998).
EXAMPLE 7
Titration of rAAV Produced by Baculovirus Transduction
An rAAV lysate from baculovirus-transduced cells prepared as
described in Example 6, was diluted at 10-2, 10-3 and 10-4 with DMEM
containing
10% FBS and used for the titration assay. 24-well plates were first coated
with
0.1 % gelatin for 30 minutes and then plated with 2 X 105 cells/well of 293-
based
2 0 84-3 I cells (Fischer, et al., 1996). After 3 to 4 hours of incubation,
the cells were
infected with adenovirus at 100 particles/cell for 30 minutes (adenovirus
helps the
conversion of single stranded AAV into double stranded AAV and is widely used
CA 02375119 2001-11-26
WO 00/73480 PCT/US00/14481
-80-
for AAV titration), and then the diluted rAAV lysate was added. After
transduction
by the rAAV for 24 hours, the cells were fixed with 4% paraformaldehyde in PBS
for 30 minutes. The paraformaldehyde was replaced with PBS, and GFP-
expressing cells were counted under by fluorescent microscopy.
EXAMPLE 8
Production of rAAV Using VSV-G Pseudotvped Baculovirus
Recombinant baculovirus BV-EiOV-RC was used to transduce 293-
CG3 cells for rAAV production. The cells were first plated on 6-well plates at
a
density of 1 X 106 cells/well at 2 to 4 hours prior to transduction.
Baculovirus BV-
EiOV-RC was concentrated by centrifugation at 20,000 rpm at 4°C for 30
minutes,
resuspended in serum-containing DMEM, and then added to the cells at the
indicated amounts as shown in Table 2. After incubation for 16 hours, cells
were
fed with fresh medium. Following transduction for a total of 3 days, cells
were
harvested and rAAV titers (transducing units) determined. The results are
presented in Table 2 as an average of values determined from two separate
experiments. They results indicate that recombinant baculovirus carrying Ad
helper
and AAV rep-cap genes can successfully transduce 293-CG3 cells and produce
rAAV. By increasing the multiplicity of infection (moi) of input baculovirus,
higher
titers of rAAV were produced.
CA 02375119 2001-11-26
WO 00/73480 PCT/US00/14481
_81 _
Table 2
TransductionBV-EiOV-RC-H6 Cell line rAAV titers
fu/cell TU/106 cells
1. 0 293-CG3 0
2. 6.25 293-CG3 1 X 10z
3 I 2. S 293-CG3 4.9 X 103
4. 25 293-CG3 2.6 X 104
5 50 293-CG3 2.7 X 105
6. 100 293-CG3 1.8 X 106
EXAMPLE 9
Transduction Efficiency of Different Mammalian Cell Lines
by VSV-G Pseudotyped and Non-Pseudotyped Baculoviruses
In order to identify a suitable cell line that is efficiently transduced by
baculovirus, a number of cell lines were tested. Cells were seeded on 6-well
plates
and grown to ~80% confluency. Baculoviruses were added to the cells at the
indicated moi's in serum-free DMEM, incubated with the cells overnight, and
replaced with fresh medium 12-15 hours later. GFP-expressing cells were scored
as
a percentage of all cells in the monolayer at 48 hours post-transduction by
the
recombinant baculovirus.
The results presented in Table 3 indicate that, in general, VSV-G
2 0 pseudotyped baculovirus (BV-cisEFGFP) transduce mammalian cells much more
efficiently than the non-pseudotyped baculovirus (Ac-cisEFGFP). However, HepG2
and Saos-2 cell lines were found to be more transducible than HeLa and 293
cell
lines by baculovirus. Insertion of Ad El genes into the chromosome of these
cell,
similar to the scenario in 293 cells, should further facilitate production of
rAAV
2 5 from these cells using the recombinant baculoviruses cited in previous
examples. It
is noteworthy that Saos-2 cells are highly permissive for infection by
baculovirus
CA 02375119 2001-11-26
WO 00/73480 PCT/US00/14481
-82-
irrespective of the presence or absence of the VSV-G glycoprotein on the
baculoviral coat. The use of this cell line could provide an advantage for
rAAV
production using non-pseudotyped baculovirus.
All documents cited above are incorporated by reference herein.
Numerous modifications and variations of the present invention are included in
the above-identified specification and are expected to be obvious to one of
skill
in the art. Such modifications and alterations to the processes of the present
invention are believed to be encompassed in the scope of the claims appended
hereto.
CA 02375119 2001-11-26
WO 00/73480 PCT/US00/14481
- 83 -
Table 3
TransductionCell Baculovirus Results
line
BV-cisEFGFPAc-cisEFGFP% reen cells
1. He G2 10 fu/cell 60
2. He G2 50 fu/cell 90
3. He G2 100 fu/cell 90
4. He G2 10 fu/cell 1020
S. He G2 50 fu/cell 60
6. He G2 100 fu/cell70
7. Saos-2 10 fu/cell 70
8. Saos-2 50 fu/cell 100
9. Saos-2 100 fu/cell 100
10. Saos-2 10 fu/cell 70
11. Saos-2 50 fu/cell 100
12. Saos-2 100 fu/cell100
13. Hela 10 fu/cell ~5
14. Hela 50 fu/cell ~15
I5. Hela 100 fu/cell ~15
16. Hela 10 fu/cell
17. Hela 50 fu/cell
2 18. Hela 100 fu/cell~5
0
19. 293 10 fu/cell 10
20. 293 50 fu/cell 50
21. 293 100 fu/cell 50
22. 293 10 fu/cell ~5
2 23 . 293 50 fu/cell 20
5
24. ~ 293 ~ 100 pfu/cell20
CA 02375119 2001-11-26
WO 00/73480 PCT/US00/14481
-84-
References
1. Jones N, Shenk T. Isolation of deletion and substitution mutants of
adenovirus type 5. Cell 1978 Jan;l3(1):181-8
2. Sambrook, J., Fritsch, E.F., and Maniatis, T. "Molecular Cloning-A
Laboratory Manual". Cold Spring Harbor Laboratory Press. 1989.
3. Gao GP, Qu G, Faust LZ, Engdahl RK, Xiao W, Hughes JV, Zoltick PW,
Wilson JM. High-titer adeno-associated viral vectors from a Rep/Cap cell line
and
hybrid shuttle virus. Hum Gene Ther 1998 Nov 1;9(16):2353-62.
4. Fisher KJ, Gao GP, Weitzman MD, DeMatteo R, Burda JF, Wilson JM.
Transduction with recombinant adeno-associated virus for gene therapy is
limited
by leading-strand synthesis. J Virol 1996 Jan;70(1):520-32.
5. Eidelman, O., Schlegel, R., Tralka, T.S., and Blumenthal, R. J. Biol. Chem.
1996, 259:4622-4628.
6. Chen, H. and Padmanabhan, R. Biotechniques 1994, 17:40-42.