Note: Descriptions are shown in the official language in which they were submitted.
CA 02375210 2001-11-26
WO 00/73797 PCT/US00/14827
HOMOGENEOUS TESTS FOR SEQUENTIALLY DETERMINING
LIPOPROTEIN FRACTIONS
CROSS-REFERENCES TO RELATED APPLICATIONS
This application is a continuation-in-part of U.S. provisional patent
application 60/136,709, filed May 28, 1999, the contents of which are hereby
incorporated by reference.
STATEMENT AS TO RIGHTS TO INVENTIONS MADE UNDER
FEDERALLY SPONSORED RESEARCH AND DEVELOPMENT
This invention is owned by the United States Government.
BACKGROUND OF THE INVENTION
Serum total cholesterol (total-C) is an important risk factor for coronary
artery
disease (Wilson, P. et al., Circulation 97:1837-47 (1998)). The measurement of
the
cholesterol content of the lipoprotein fractions, however, is more valuable in
establishing
the risk for coronary artery disease, because the various lipoprotein
fractions do not have
the same effect on the process of atherosclerosis. Low density lipoprotein
("LDL,"
cholesterol associated with LDL is known as low density lipoprotein-
cholesterol, or
"LDL-C") is a pro-atherogenic lipoprotein fraction and more closely correlates
with the
risk for coronary artery disease than does total-C. In contrast, high density
lipoprotein
("HDL," cholesterol associated with HDL is known as high density lipoprotein-
cholesterol, or "HDL-C") is a negative risk factor for atherosclerosis (Gordon
T, et al.,
Am JMed 62:707-14. (1977)). HDL is believed to be beneficial in reversing the
process
of atherosclerosis because of its ability to increase reverse cholesterol
transport, a
pathway by which excess cholesterol is transported from peripheral cells to
the liver for
excretion (Badimon J.J. et al., Circulation 86:86-94 (1992)). Recent
recommendations
for screening of cholesterol as a risk factor for coronary artery disease
include the
measurement of both LDL-C and HDL-C (The Expert Panel. JAMA 269:3015-23
(1993)).
Until recently, the most common procedure for determining LDL-C and
HDL-C involved performing three tests, namely a measurement of total-C, a
measurement of total triglyceride, and a measurement of HDL-C. HDL-C has often
been
determined after the precipitation and the physical removal of the apoB-
containing
WO 00/73797 CA 02375210 2001-11-26 PCT/US00/14827
lipoproteins (the apoB containing lipoproteins are considered to be
chylomicrons, LDL,
and very low density lipoproteins, or "VLDL") by centrifugation. For example,
Polymedco (Cortland Manor, NY), provides a test based on magnetic bead
precipitation
method. LDL-C can be calculated from the total-C, HDL-C and triglyceride
values by
the Friedewald equation (Friedewald W.T. et al., Clin Chem 18:499-502 (1972)).
The
above procedure, however, has several limitations (Schectman G., et al., Clin
Chem
42:732-737 (1996)) and is relatively costly when used as a screening test
because
multiple tests are required and because of the complexity of the tests, in
particular of the
HDL-C determination.
Various additional approaches to HDL measurement have been developed,
such as those described in Kerscher, U.S. Patent No. 4,892,815 ("Kerscher I"),
and
Kerscher, U.S. Patent No. 4,851,335 ("Kerscher II"). One recent improvement in
lipoprotein cholesterol fraction measurement is the use of homogenous assays
for HDL-
C, that is, assays which do not require the physical separation of HDL or of
the apoB-
containing lipoproteins to make the measurements. Since homogenous HDL-C
assays
can be performed in one tube, they are easier to perform, easier to automate,
less costly,
and offer superior analytic performance (Lin M. et al., Clin Chem 44:1050-52
(1998);
Rifai N. et al., Clin Chem 44:1452-58 (1998); Nauck M. et al., Clin Chem
44:144-51
(1998)). See also, Ziegenhorn, U.S. Patent No. 4,544,630, and Miki, European
Patent
Application EP 0 754 948 A1.
One of the approaches taken to determining HDL-C homogeneously has
been to add an anti-apoB antibody to serum to form an antibody-antigen
complex, after
which HDL-C is measured. For example, Sigma Diagnostics (St. Louis, MO)
markets the
EZ-HDLT"" cholesterol reagent kit. This kit permits measurement of HDL-C by
using an
anti-apoB antibody to render the complexed apoB-containing fractions
inaccessible to
subsequently added cholesterol-measuring enzymes, followed by a standard
enzymatic
measurement of the level of HDL-C present, for which they provide premixed
reagents.
Typically, the HDL-C is measured by reacting the HDL-C with cholesterol
esterase to
liberate cholesterol ester bound to the HDL, and then reacting the now free
cholesterol
with cholesterol oxidase and oxygen to form hydrogen peroxide. The hydrogen
peroxide
can then be measured by a variety of means.
In Japanese Patent Kokai No. Hei 6-242110, lipoprotein fractions one does
not want to measure are complexed with an appropriate antibody. Enzymes for
2
WO 00/73797 CA 02375210 2001-11-26 PCT/US00/14827
measuring cholesterol content are then added, and the cholesterol release and
oxidation
are permitted to proceed, and reagents are added to permit measurement of the
hydrogen
peroxide by colorimetric changes. To facilitate reading the colorimetric
changes, which
are impeded by turbidity caused by the presence of the antibody-antigen
complex, a
detergent is added to dissolve the complex. To prevent the measurement of the
lipoprotein fraction being measured from being affected by the presence of the
lipoprotein
fraction being freed from the antibody-antigen complex, a heavy metal is added
at the
same time to poison the enzymes and terminate the enzymatic reaction.
Reagents other than antibodies have also been used to render non-HDL
lipoproteins inaccessible to enzymatic reaction so that the HDL fraction can
be measured.
Roche Diagnostics (Basel, Switzerland), for example, provides a polyethylene
glycol
("PEG") based system in which sulfated a-cyclodextrin, dextran sulfate and
MgCl2 form
water soluble complexes with the non-HDL lipoproteins present in a sample,
after which
pegylated cholesterol esterase and cholesterol oxidase are introduced. The non-
HDL
1 S complexes are not accessible to the PEG-modified enzymes, permitting
measurement of
the HDL fraction.
Another homogeneous assay is provided by Genzyme Diagnostics (San
Carlos, CA and Cambridge MA), under the name Liquid N-geneous~ HDL. In this
method, synthetic polyanions adsorb to the surface of the non-HDL lipoproteins
and
render them unavailable to cholesterol esterase, while the HDL fraction is
solubilized and
undergoes conventional enzymatic reactions. The various homogenous assays for
HDL
are discussed and compared in, for example, Nauck et al., Clin Chem 44:1143-
1451
(1998) and Harris et al., Clin Chem 43:816-823 (1997). The N-geneous~ system
is
compared to phosphotungstic acid precipitation in, for example, Halloran et
al., Arch Path
Lab Med 123:317-326 (1999) and Hubbard et al., Am J Clin Path 110:495-502
(1998).
The use of other polyanions to complex lipoprotein fractions is also known.
See, e.g.,
Burstein et al., J. Lipid Res 11:583-595 (1970).
The reference method for determining LDL-C is the (3-quantification
method. This method arnves at an LDL-C measurement by a chemical precipitation
using heparin and a divalent cation, such as manganese, magnesium or calcium
and
ultracentrifugation, which leaves the HDL fraction in the supernatant. The
supernatant
can then be removed and the LDL solubilized and reacted to determine the
amount
3
WO 00/73797 CA 02375210 2001-11-26 PCT/US00/14827
present. LDL-C values have been also been obtained by the Friedewald
calculation, once
total-C, triglycerides, and HDL-C have been measured.
More recently, two homogeneous assays for LDL-C have been developed.
One, introduced by Roche Diagnostics (Basel, Switzerland), employs reagents --
MgCl2,
sulfated a,-cyclodextrin, and dextran sulfate -- to render the non-LDL
fractions
unavailable to the enzymes used in the enzymatic determinations. A non-ionic
detergent
is then introduced to selectively solubilize the LDL-C, which is then measured
by
conventional enzymatic reaction. In the second, commercially available from
Genzyme
(Cambridge, MA), a detergent selectively solubilizes the non-LDL fractions,
which are
then reacted with enzymes in the absence of a color label. After the non-LDL-C
has been
reacted, a second detergent solubilizes the LDL-C in the presence of a color
substrate to
permit conventional enzymatic measurement of the LDL-C. The relative merits
and
problems of these methods are reviewed and compared, for example, in Nauck and
Rifai,
Clinica Chimica Acta 294:77-92 (2000).
SUMMARY OF THE INVENTION
The invention relates to a method for performing a sequential
homogeneous assay for cholesterol associated with lipoprotein fractions
present in a
sample. The method comprises, in the following order: contacting a first
lipoprotein
fraction in the sample with a complex-forming agent which selectively forms a
complex
with the first lipoprotein fraction to form an agent-first lipoprotein
fraction complex, with
the proviso that the complex is not a substrate for cholesterol esterase;
measuring the
amount of any cholesterol associated with a second lipoprotein fraction
present in the
sample by using cholesterol esterase and cholesterol oxidase to obtain a first
cholesterol
value; dissociating the first lipoprotein fraction from the first lipoprotein
fraction/agent
complex; and determining the total amount of cholesterol present in the
sample. In some
embodiments, the complex is also not a substrate for cholesterol oxidase and
in others, it
is not a substrate for cholesterol dehydroxgenase.
Further, by subtracting the value obtained for the first lipoprotein fraction
from the total-C, the amount of the second lipoprotein fraction can also be
determined.
Thus, the methods of the invention can be used to determine HDL and total-C,
allowing
4
WO 00/73797 CA 02375210 2001-11-26 PCT/US00/14827
calculation of the non-HDL-C in the sample, or to determine LDL-C and total-C,
which
allows calculation of the non-LDL-C in the sample.
The complex-forming agent can be, for example, an antibody which binds
selectively to the first lipoprotein fraction, a polyanion, or a sulfated
cyclodextrin.
Suitable polyanions include heparin, dextran sulfate, phosplotungstic acid,
polyvinyl
sulfate, heparin sulfate, chondroitin sulfate, hyaluronic acid, and sulfated
oligosaccharides.
In some embodiments, a non-denaturing detergent is used to dissociate the
first lipoprotein fraction from the complex-forming agent. In a preferred
embodiment,
the detergent is deoxycholate.
The methods of the invention include measuring the amount of cholesterol
present in the first and second lipoprotein fractions by reacting cholesterol
ester in the
fractions with cholesterol esterase, so that all the cholesterol in the
lipoprotein fraction
being measured is in the form of free cholesterol. Depending on the system
chosen by the
1 S practitioner, the free cholesterol is then typically reacted with
cholesterol oxidase or with
cholesterol dehydrogenase. If desired, the value obtained for the first
lipoprotein fraction
is subtracted from the value obtained for the total cholesterol.
In some embodiments, the amount of the cholesterol present in the sample
is determined by an optical means. In preferred embodiments, the optical means
is a
change in absorption or emission spectra of an indicator molecule. In some
preferred
embodiments, the indicator molecule is a dye. In some other preferred
embodiments, the
indicator molecule is NAD or NADP.
In yet another aspect of the invention, the method further comprises
determining the amount of any triglycerides present in the sample.
In other aspects, the invention relates to kits for determining amounts of
cholesterol present in a sample, comprising a complex forming agent, a non-
denaturing
detergent, and instructions for performing a method of the invention. The
complex-
forming agent can be, for example, an anti-apoB antibody or an anti-apoAI or
anti-apoAII
antibody. The complex-forming agent can alternatively be a synthetic polyanion
or a
sulfated cyclodextrin. The non-denaturing detergent can be, for example,
deoxycholate.
The kit can further comprise one or more enzymes useful in cholesterol or
triglyceride
measurements, such as lipase, glycerol kinase, glykinase, glycerol phosphate
WO 00/73797 CA 02375210 2001-11-26 pC'T/US00/14827
dehydrogenase, glycerol phosphate oxidase, peroxidase, pyruvate kinase, and
lactate
dehydrogenase.
BRIEF DESCRIPTION OF THE DRAWINGS
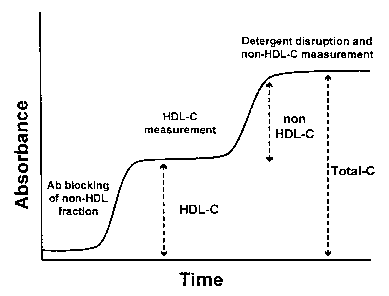
Figure 1. Diagram of DHT reaction profile.
Figure Z. Reaction profile of DHT assay. Panel A: HDL (50 mg/dL); Panel B:
LDL (150 mg/dL); Panel C: HDL (50 mg/dL) plus LDL (150 mg/dL); Panel D:
serum. Arrows indicate the point of adding the reagents for step 2 and step 3
for the
DHT assay.
Figure 3. Lipoprotein cross reactivity of DHT assay. Panel A: HDL-C was fixed
at
50 mg/dL and LDL-C was added at the indicated concentration shown on the X-
axis. Measured values for HDL-C (O) in step 2 and LDL-C (0) in step 4 and
total-
C (0) are shown on the Y-axis. Panel B: LDL-C was fixed at 100 mg/dL and
HDL-C was added at the indicated concentration shown on the X-axis. Measured
values for HDL-C (O) in step 2 and LDL-C (0) in step 4 and total-C (~) are
shown
on the Y-axis. Results are shown as the mean plus and minus 1 S.D.
Figure 4. Linearity of HDL-C and Total-C for the DHT assay. Panel A: Linearity
was determined by dilution of a concentrated sample of HDL and is shown as the
measured HDL-C on the Y-axis versus the assigned concentrations of HDL-C on
the X-axis. Panel B: Linearity was determined by dilution of a concentrated
sample
of LDL and is shown as the measured Total-C on the Y-axis versus the assigned
concentrations of LDL-C on the X-axis. Results are shown as the mean plus and
minus 1 S.D.
Figure 5. Precision of the DHT assay. Results for HDL-C and total-C are shown
for within-run (n=20) and between-run (n=10) precision. Results are shown as
the
coefficient of variation (CV).
Figure 6. Comparison of DHT assay to standard assays. Panel A: HDL-C as
determined by the DHT method (Y-axis) was compared to the results obtained by
PolyMedco method (X-axis). Panel B: Total cholesterol as determined by the DHT
method (Y-axis) was compared to the results obtained by Roche method (X-axis).
6
WO 00/73797 CA 02375210 2001-11-26 PCT/US00/14827
Panel C: Non-HDL-C as determined by the DHT method (Y-axis) was compared to
the results obtained by subtraction of total cholesterol (Roche method) from
HDL-C
(PolyMedco method). (X-axis). Panel D: LDL-C calculated by the Friedewald
equation using HDL-C and total cholesterol as determined by the DHT method (Y-
axis) was compared to the calculated LDL-C using the standard assay for HDL-C
(PolyMedco) and total cholesterol (Roche) (X-axis). Results are shown as the
mean
plus and minus 1 S.D.
DETAILED DESCRIPTION
I. Introduction
The invention provides new methods for determining the amounts of
cholesterol associated with the various lipoprotein fractions present in a
sample, such as a
patient serum sample, in a series of simple steps. Although each of the steps
is simple, in
combination, they permit the determination of HDL-C and total-C, or of LDL-C
and
1 S total-C, to be made from a single sample in a single tube in a single
liquid phase.
Optionally, the level of triglycerides present can also be determined. We
initially the
assay method to determine HDL and total cholesterol levels, and therefore
dubbed the
assay the dual HDL/total cholesterol, or "DHT" assay. For convenience, we
continue to
refer to our method by this name, even though it can also be used to determine
LDL-C
and total-C, and, with an extra step, triglycerides.
The standard DHT assay is performed in four steps, performed in order. In
the first step, an agent is added which can complex selectively with a chosen
lipoprotein
fractions (the lipoprotein fraction which will be complexed with the complex-
forming
agent will be referred to hereafter as the "first lipoprotein fraction") which
might be
present in a sample. For example, an anti-apoB antibody can be added to
complex with
apoB-containing lipoproteins (LDL, VLDL, and chylomicrons) present in the
sample,
leaving the HDL-C lipoprotein uncomplexed. Or, an anti-apoAI or anti-apoAII
antibody
may be used to complex with apoAI- or apoAII-containing lipoproteins, such as
HDL.
The complex-forming agent renders the lipoprotein fraction with which it
reacts
unavailable to enzymatic reaction with cholesterol esterase and preferably at
least one of
the enzymes cholesterol oxidase or cholesterol dehydrogenase, which are used
in
common assays of cholesterol content.
In the second step, a measurement is made of the lipoprotein fraction
which remains uncomplexed (the "second lipoprotein fraction"). Thus, in the
example
7
WO 00/73797 CA 02375210 2001-11-26 PCT/US00/14827
just cited, if the complex-forming agent is an anti-apoB antibody, the HDL-C
in the
sample will remain uncomplexed and available for measurement. Typically, the
measurement is performed by conventional enzymatic reactions. In one form of
these
conventional assays, the lipoprotein is first contacted with cholesterol
esterase. The
enzyme converts all the cholesterol in the lipoprotein, which is typically
present as free
cholesterol and as cholesterol ester, into free cholesterol. The free
cholesterol is then
reacted with cholesterol oxidase, in the presence of a reporter enzyme
(typically
peroxidase) which induces a colorimetric change of the indicator molecule in
the presence
of the product of the reaction with cholesterol oxidase. In a second
conventional method,
called the "NAD" method, following the reaction with cholesterol esterase, the
cholesterol is reacted with cholesterol dehydroxgenase. This reduces NAD to
NADH and
results in a change in the absorption profile of the solution, which can be
correlated to the
cholesterol content. See, e.g., Kayamoriy et al., Clin. Chem. 45:2158-63
(1999). In a
variation of this method, the assay is performed utilizing NADP rather than
NAD.
Cholesterol associated with the lipoproteins bound in the complex is
sterically blocked from reacting with the enzymes of the method chosen and is
not
available as a substrate for the enzymes. The enzymes thus react with only the
cholesterol associated with any HDL-C fraction present in the sample (if the
non-HDL-C
was complexed) or with any LDL-C present in the sample (if the HDL-C was
complexed
with the complex-forming agent).
Once the enzymes have effectively reacted fizlly with the cholesterol
present in the second lipoprotein fraction (the fraction which was not
complexed with the
complex-forming agent) the third step is performed. In the third step, a
detergent which
can disrupt the agent-lipoprotein complex without denaturing the enzymes is
added to the
sample, enabling the enzymatic measurement of cholesterol associated with the
lipoprotein fractions which were originally complexed with the agent (the
first lipoprotein
fraction). Since the enzymes have not been denatured, they are available to
react with the
cholesterol in the first lipoprotein fraction. Further, since all or
substantially all of the
cholesterol in the second lipoprotein fraction was reacted before addition of
the detergent,
any additional cholesterol which reacts with the enzymes after the addition of
the
detergent is considered to have come from the first lipoprotein fraction.
In the fourth step, the change in absorbance from the baseline in step 1 to
the end of step 3 is measured. This change is proportional to total-C. The
cholesterol in
the first lipoprotein fractions (those which were originally complexed with
the complex-
8
WO 00/73797 CA 02375210 2001-11-26 pCT/US00/14827
forming agent) can then be calculated by subtracting the results from the
total cholesterol
measurement (step 4) from the measurement of the second lipoprotein fraction
(step 2).
A diagram of the theoretical reaction profile of the DHT assay is shown in
Fig. 1. It
should be noted that, while the method has been described in four steps for
convenience
and clarity of presentation, in practice some of the steps can be combined and
performed
together. For example, steps l and 2, or steps 3 and 4 can be combined
By subtracting the value obtained for the first lipoprotein fraction from the
total-C, the amount of the second lipoprotein fraction can also be determined.
Thus, the
methods of the invention can be used, for example, to determine HDL and total-
C,
allowing calculation of the LDL-C and VLDL-C in the sample, or to determine
LDL-C
and total-C, which allows calculation of the non-LDL-C in the sample. It
should be noted
that a calculation of non-LDL-C in the sample will combine the HDL-C in the
sample
(which is usually the next lipoprotein fraction of interest) with VLDL-C and
other minor
lipoprotein fractions. Similarly, when determining HDL-C by the methods of the
invention, thus complexing the non-HDL-C, VLDL-C will generally be complexed
with
the LDL-C (which in this assay is the lipoprotein fraction of greatest
interest next to the
HDL-C).
Optionally, in yet another aspect, triglyceride levels can be measured.
This is usually performed using lipase, glycerol phosphate dehydrogenase,
glycerol
phosphate oxidase, and peroxidase (which, like the cholesterol content assay
using
peroxidase, provides a colorimetric change) or, alternatively, by the "NAD"
method,
using lipase, glycerol kinase, pyruvate kinase, and lactate dehydrogenase
(which, like the
NAD method for determining cholesterol content, provides a change in the
absorption
profile of the solution, permitting correlation to the triglycerides present).
Various
conventional assays for measuring triglycerides are known in the art. For
example, some
nine commercially available assays are compared in Sampson et al., Clin. Chem.
40:221-
226 (1994).
Measurement of the triglycerides present further extends the value of the
assay. For example, if the method is used to measure HDL-C and then total-C
and then
triglycerides, LDL-C can be calculated by the Friedewald equation. Conversely,
the
method can be used to measure LDL-C and then total-C and, optionally,
triglycerides,
permitting non-LDL-C to be calculated by the Freidewald equation. Thus, the
method is
flexible and permits all the commonly measured lipoprotein fractions to be
measured
homogeneously in a single tube.
9
WO 00/73797 CA 02375210 2001-11-26
PCT/US00/14827
Based on the results obtained in numerous assays and comparisons to
standard assays (such as those shown in Figures 2-6), the DHT assay has
acceptable
analytic performance and produces results similar to standard assays. But the
DHT assay
simplifies the approach for measuring the cholesterol content of lipoprotein
fractions.
Besides the DHT test, only an assay for total triglyceride is needed for
determining the
cholesterol content of lipoprotein fractions. Because the DHT test is a
homogenous test,
the assay can be performed in a single tube and no preprocessing steps, such
as
precipitation and centrifugation, are necessary. The DHT test is also cost
effective,
because no additional reporter enzymes are needed to measure total cholesterol
once the
HDL or LDL measurement has been made. Reducing the overall complexity and cost
of
performing lipoprotein fraction analysis is important because such analysis is
widely used
both for screening the population at risk for coronary artery disease and for
guiding
therapy. Moreover, the same test procedure can be used to assay either LDL-C
or HDL-
C. If the choice is to directly assay HDL, a complex-forming agent that
complexes with
LDL, such as an anti-apoB antibody, is used so that the LDL becomes the first
lipoprotein
fraction. If the choice is to directly assay LDL, a complex-forming agent that
complexes
with HDL, such as an anti-apoAI- or anti-apoAII antibody is used, so that the
HDL in the
sample becomes the first lipoprotein fraction.
In sum, the DHT test is a relatively simple method for the dual
measurement of HDL-C and total cholesterol or of LDL-C and total cholesterol,
and, if
desired, of triglycerides. It provides a cost effective alternative for
performing cholesterol
lipoprotein fraction analysis. The DHT test is also flexible, allowing the use
of different
types of complex-forming agents. Examples 1 and 2, below, show the use of the
invention using antibodies as the complex-forming agents. Examples 3 and 4
show that
satisfactory results have been obtained using a synthetic polyanion and
Example 5 shows
that satisfactory results were obtained using a sulfated cyclodextrin as the
complex
forming agent.
The discussion below provides further information regarding definitions of
the terms used herein. It then proceeds to discuss complex-forming agents
appropriate for
use in the invention, detergents which can be used to disrupt the complex-
forming agent-
first lipoprotein fraction complex formed in step 1, systems for measuring the
levels of
cholesterol present in each lipoprotein fraction, and assays for measuring
triglycerides as
an optional series of steps in employing the inventive method.
WO 00/73797 CA 02375210 2001-11-26
PCT/US00/14827
II. Definitions
Unless defined otherwise, all scientific and technical terms used herein
have the same meaning as commonly understood by one of ordinary skill in the
art to
which this invention belongs. Although any materials and methods similar to or
equivalent to those described herein can be used in the practice of the
invention, preferred
methods and materials are described. For purposes of the present invention,
the following
terms are described below.
According to Stedman's Medical Dictionary (Hensyl, ed., 25th Ed.,
Williams & Wilkins, Baltimore MD, 1990) ("Stedman's"), a "lipoprotein" refers
to a
complex or compound containing lipid and protein. Stedman's notes that almost
all the
lipids in plasma are in the form of lipoproteins. See, Stedman's, at page 886,
definition of
"lipoprotein."
Stedman's classifies lipoproteins by their flotation constants, or densities
("d"), as follows: chylomicra, < 1.006; very low density lipoproteins
("VLDL"), 1.006-
1.019; low density lipoproteins ("LDL"), 1.019-1.063; high density
lipoproteins, 1.063-
1.121, and very high density lipoproteins ("VHDL"), > 1.21. Id. Although
interest has
grown in the effect of the VHDL fraction in preventing arthlerosclerosis, VHDL
is
usually measured together with I-IDL. Unless otherwise specified or made clear
in
context, therefore, references herein to "HDL" also include any VHDL which may
be
present.
As used herein, "HDL" and "HDL-C" are generally used synonymously
unless otherwise indicated or required by context.
As used herein, "LDL" and "LDL-C" are generally used synonymously
unless otherwise indicated or required by context.
The term "associated with," means, with respect to the cholesterol content
of a particular lipoprotein fraction, that the cholesterol is complexed with
protein in
particles classified, by their flotation constant, as belonging to the
particular lipoprotein
fraction under discussion.
The "cholesterol" in lipoproteins is present both as free cholesterol and as
cholesterol ester. Typically, a lipoprotein is reacted with cholesterol
esterase to convert
the cholesterol ester in the lipoprotein to free cholesterol. All of the
cholesterol in the
lipoprotein is then available for further reactions. Unless otherwise
indicated or required
in context, references herein to "cholesterol" in a lipoprotein fraction refer
to both free
cholesterol and that present as cholesterol ester.
11
WO 00/73797 CA 02375210 2001-11-26 PCT/US00/14827
The term "complex-forming agent" (sometimes referred to simply as
"agent") refers to a compound or molecule capable of selectively binding to a
particular
lipoprotein fraction, for example, to HDL or to LDL, to non-apoB containing
lipoproteins, or to non-apoAI or non-apoAII containing lipoproteins.
Frequently, the
complex-forming agent is an antibody, such as an anti-apoB antibody.
Polyanions and
sulfated cyclodextrins can also be used to bind to a selected lipoprotein
fraction.
Preferably, the binding of the complex-forming agent does not alter or destroy
the
lipoprotein fraction to which it binds or complexes. Even more preferably, the
agent
sterically blocks the interaction of the lipoprotein to which it is complexed
with
cholesterol esterase, cholesterol oxidase, or both.
As used herein, a "non-denaturing detergent" is a detergent that (1) is able
to dissolve the complex formed by a complex-forming agent, such as an
antibody, and a
lipoprotein fraction, and (2) does not inactivate cholesterol esterase,
cholesterol oxidase,
or significantly affect colorimetric measurement of the amount of cholesterol
with
standard reagents.
An "indicator molecule" refers to a molecule that can be used to monitor a
reaction. Typically, this is done using a molecule such as a dye which changes
its
absorbance or emission spectra in the presence of the product of an enzymatic
reaction of
interest. In the case of a peroxidase-coupled reaction, the oxidation of the
dye changes its
absorption spectrum. In the case of the NAD and NADP reaction systems, the
indicator
molecule is the NAD or NADP, respectively, which change absorbance when
reduced to
NADH or NADPH, respectively.
As used herein, "determining" includes measuring, including by adding to
or subtracting from a value to obtain a second value.
"Dissociating" means, with regard to a complex formed by a complex-
forming agent and a lipoprotein fraction, to separate the complex, typically
by disrupting
or dissolving it.
An "anti-apoAI antibody" or "anti-apoAII antibody" means an antibody or
antibody fragment which specifically recognizes and complexes with
apolipoprotein AI
or AII, respectively.
As used herein, an "anti-apoB antibody" means an antibody or antibody
fragment which specifically recognizes and complexes with antigenic
determinants on
chylomicrons, VLDL, and LDL. Specifically, the term relates to antibodies
which
recognize apolipoprotein B.
12
WU 00/73797 CA 02375210 2001-11-26 pCT/[JS00/14827
An "anti-apoC antibody" is an antibody or antibody fragment which
specifically recognizes apolipoprotein C. Similarly, an "anti-apoE antibody"
is an
antibody or antibody fragment which specifically recognizes apolipoprotein E.
"Specifically bind" means, with reference to a complex-forming agent
such as an antibody, that the agent preferentially associates with a
lipoprotein of the
designated fraction and not at all, or in much smaller amounts, to other
lipoprotein
fractions. With respect to antibodies in particular, it is recognized that a
certain degree of
non-specific interaction may occur between a molecule and a non-target
lipoprotein
fraction. Nevertheless, specific binding may be distinguished as mediated
through
specific recognition of the antigen.
As used herein, an "antibody" refers to a protein functionally defined as a
binding protein and structurally defined as comprising an amino acid sequence
that is
recognized by one of skill as being derived from the framework region of an
immunoglobulin encoding gene of an animal producing antibodies. An antibody
can
consist of one or more polypeptides substantially encoded by immunoglobulin
genes or
fragments of immunoglobulin genes. The recognized immunoglobulin genes include
the
kappa, lambda, alpha, gamma, delta, epsilon and mu constant region genes, as
well as
myriad immunoglobulin variable region genes. Light chains are classified as
either kappa
or lambda. Heavy chains are classified as gamma, mu, alpha, delta, or epsilon,
which in
turn define the immunoglobulin classes, IgG, IgM, IgA, IgD and IgE,
respectively.
A typical immunoglobulin (antibody) structural unit is known to comprise
a tetramer. Each tetramer is composed of two identical pairs of polypeptide
chains, each
pair having one "light" (about 25 kD) and one "heavy" chain (about SO-70 kD).
The N-
terminus of each chain defines a variable region of about 100 to 110 or more
amino acids
primarily responsible for antigen recognition. The terms variable light chain
(VL) and
variable heavy chain (VH) refer to these light and heavy chains respectively.
Antibodies exist as intact immunoglobulins or as a number of well
characterized fragments produced by digestion with various peptidases. Thus,
for
example, pepsin digests an antibody below the disulfide linkages in the hinge
region to
produce F(ab)'2, a dimer of Fab which itself is a light chain joined to VH-CH1
by a
disulfide bond. The F(ab)'2 may be reduced under mild conditions to break the
disulfide
linkage in the hinge region thereby converting the (Fab')2 dimer into an Fab'
monomer.
The Fab' monomer is essentially an Fab with part of the hinge region (see,
Fundamental
Immunology, W.E. Paul, ed., Raven Press, N.Y. (1993), for a more detailed
description of
13
WO 00/73797 CA 02375210 2001-11-26 PCT/US00/14827
other antibody fragments). While various antibody fragments are defined in
terms of the
digestion of an intact antibody, one of skill will appreciate that such Fab'
fragments may
be synthesized de novo either chemically or by utilizing recombinant DNA
methodology.
Thus, the term antibody also includes antibody fragments either produced by
the
modification of whole antibodies or synthesized de novo using recombinant DNA
methodologies, and also includes single chain antibodies (antibodies that
exist as a single
polypeptide chain). The term also encompasses single chain Fv antibodies (sFv
or scFv)
in which a variable heavy and a variable light chain are joined together
(directly or
through a peptide linker) to form a continuous polypeptide. The single chain
Fv antibody
is a covalently linked VH-VL heterodimer which may be expressed from a nucleic
acid
including VH- and VL- encoding sequences either joined directly or joined by a
peptide-
encoding linker. Huston, et al. (1988) Proc. Nat. Acad. Sci. USA, 85: 5879-
5883. While
the VH and VL are connected to each as a single polypeptide chain, the VH and
VL
domains associate non-covalently. Preferred antibodies include scFv, Fv, Fab
and
disulfide linked Fv (Reiter et al. (1995) Protein Eng. 8: 1323-1331).
Antibodies can also
include diantibodies and miniantibodies.
Methods of producing polyclonal and monoclonal antibodies are known to
those of skill in the art. See, e.g., Coligan (1991), CURRENT PRO'rocoLS IN
IMMUNOLOGY,
Wiley/Greene, NY; and Harlow and Lane; Stites et al. (eds.) BASIC AND CLINICAL
IMMUNOLOGY (4th ed.) Lange Medical Publications, Los Altos, CA, and references
cited
therein; Goding (1986), MONOCLONAL ANTIBODIES: PRINCIPLES AND PRACTICE (2d
ed.)
Academic Press, New York, NY; and Kohler and Milstein (1975), Nature, 256:495-
497.
Such techniques include antibody preparation by selection of antibodies from
libraries of
recombinant antibodies in phage or similar vectors. See, Huse et al. (1989),
Science,
246:1275-1281; and Ward et al. (1989), Nature, 341:544-546.
III. Complex-Forming Agents
Complex-forming agents suitable for use in the present invention are those
which specifically recognize structural determinants on lipoprotein fractions
other than
the one to be measured in a particular assay and sterically interfere with
access by
cholesterol esterase and typically of cholesterol oxidase to the complex.
Further, the
complex formed between the complex-forming agent and the lipoprotein fraction
with
which it complexes should be capable of disruption and the agent should not
destroy or
cleave the lipoprotein or otherwise act on the lipoprotein in a manner which
prevents the
14
WO 00/73797 CA 02375210 2001-11-26
PCT/US00/14827
lipoprotein from reacting with cholesterol esterase or cholesterol oxidase
once the
complex between the agent and the lipoprotein is disrupted.
In one important set of preferred embodiments, the complex-forming
agents are antibodies. Anti-apoB antibodies constitute one important set of
antibodies
useful in the present invention. Apolipoprotein B, or "apoB," is associated
with
chylomicrons, VLDL, and LDL; thus, an anti-apoB antibody added to a sample
complexes with all the non-HDL lipoprotein fractions. Low density lipoproteins
are now
considered by some in the art to comprise a group of related lipoproteins
which can be
differentiated by their associated proteins.
Anti-apoAI and anti-apoAII antibodies constitute a second important set of
antibodies useful in the present invention. ApoAI constitutes about 75-80% of
HDL
lipoprotein and is found on all HDL species. Accordingly, anti-apoAI
antibodies can be
used alone to obtain a good approximation of LDL and HDL levels. For more
accurate
readings, anti-apoAII antibodies can be used. Apolipoprotein All constitutes
about 20%
of total HDL protein. It is also present on a small percentage of LDL species;
it is
anticipated however, that complexing with this small percentage of the overall
LDL
present in a sample would not significantly affect the overall accuracy of the
test and
would not, for example, bring its overall accuracy of measuring HDL below the
level set
as acceptable by the National Cholesterol Education Program (see, e.g.,
Warnick and
Wood, Clin. Chem. 41:1427-33 (1995). Thus, anti-apoAI and anti-apoAII
antibodies
complex with the HDL fraction and are used when the desire is to measure LDL-C
and
total-C using the methods herein.
While the invention will be most often be used to determine HDL, LDL,
and total-C levels present in a sample, it is occasionally desirable to
determine the amount
of particular subfractions of lipoproteins present. Such subfractions can be
determined
using the method of the invention, using a complex-forming agent that
complexes with
the lipoprotein fraction of interest. For example, antibodies which recognize
apoE, apoC,
or apolipoprotein J can be used to determine the amounts of lipoprotein
present bearing
those determinants.
Use of the antibodies results in the formation of an antibody/lipoprotein
complex which sterically blocks interaction of the complexed lipoproteins with
the
enzymes used to measure cholesterol, typically cholesterol esterase and
cholesterol
oxidase. The cholesterol in the lipoprotein fraction which has not been
complexed is then
measured, until all the cholesterol available in the non-complexed fraction
has been
WO 00/73797 CA 02375210 2001-11-26 pCT/US00/14827
reacted. The complex is then dissolved, freeing the previously-complexed
lipoproteins to
be available as substrates for the enzymes and the amount of cholesterol freed
can then be
measured.
In addition to antibodies, synthetic polymers, in particular, polyanions, can
be used as complex-forming agents. In a preferred embodiment, the synthetic
polyanion
supplied by Genzyme in its N-geneous~ HDL test kit has proven satisfactory in
the
methods of the invention. A number of polyanions are known in the art, such as
heparin,
dextran sulfate, phosphotungstic acid, and polyvinyl sulfate, as well as
divalent cations,
such as calcium, magnesium and manganese chloride. See, e.g., Kerscher, U.S.
Patent
No. 4,746,605 (which for example sets forth molecular weights and preferred
concentration ranges); Karl, U.S. Patent No. 5,804,450 (which for example sets
forth
additional polyanions); and Hino, U.S. Patent No. 5,773,304 (which for example
sets
forth use of polyanions or divalent cations in the presence of surfactants).
Miki, U.S.
Patent No. 5,925,534, also lists sulfated cyclodextrin, heparan sulfate,
chondroitin sulfate,
hyaluronic acid, sulfated oligosaccharides, sulfated polyacrilymides,
carboxymethylated
polyacrylamides and salts of these as polyanions, although it states that the
better known
polyanions heparin, phosphotungstic acid and dextran sulfate and salts thereof
are
preferred. It indicates that the concentrations of the polyanion in a reagent
to be mixed
with a patient sample is usually 0.0001 % to 10 % (w/v) and is preferably
0.001 % to 1
(w/v). It further states the polyanions can be used singly or in combination.
The '534 patent lists sulfated cyclodextrin as a polyanion, presumably
because of the charge conferred by the sulfate groups. Unsulfated
cyclodextrins complex
with lipoproteins, however, sulfated cyclodextrins complex more selectively
due to their
charge. As discussed further in Example 5, below, DHT assays performed using a-
cyclodextrin and the other reagents in the Roche LDL-C measuring system
successfully
determined both LDL-C and total-C, permitting the calculation of non-LDL-C,
and
compared well to standard assays measuring just LDL-C or just total-C.
The suitability of any particular complex-forming agent, such as a
cyclodextrin, a polyanion, or an anti-apoB antibody, to block the reactivity
of cholesterol
esterase and cholesterol oxidase with the complexed lipoprotein fraction and
the amount
to use can be determined by performing "ranging" assays using a known amount
of a
purified lipoprotein fraction of the type to which the particular complex-
forming agent
binds. For this purpose, a cholesterol assay can be performed, as described
below,
without first adding a complex-forming agent normally added in step 1. The
cholesterol
16
W~ 00/73797 CA 02375210 2001-11-26
PCT/US00/14827
measurement resulting from the ranging assay should represent the total
cholesterol
content of LDL. Thereafter, a series of further assays can be performed, in
which each
successive assay contains an amount of the complex-forming agent which is
greater than
that used in the preceding assay. If the complex-forming agent is forming a
complex and
hindering access of the agent to the This should result in a successively
decreasing
amount of cholesterol measured.
The amount of complex-forming agent to be used should be sufficient to
completely block the detection of cholesterol from the lipoprotein fraction to
which it is
intended to complex. The series of assays described above can provide this
value. To
ensure that the amount of antibody used is sufficient, the concentration of
the lipoprotein
used in the ranging assays described above should be in the upper range of the
concentrations of that lipoprotein likely to be encountered. The normal ranges
for each
lipoprotein fraction, and the abnormal values reached in individuals with
pathological
conditions or unusual genetic conditions is known in the art. For example,
with respect to
LDL-C, a value of approximately 500 mg/dL is sufficient.
IV. Enzymes
Enzymes used in the DHT assay include cholesterol esterase, cholesterol
oxidase, peroxidase; enzymes used in the triglyceride assay include lipase,
glycerol
kinase, glycerol phosphate dehydrogenase, and glycerol phosphate oxidase.
These
enzymes are commercially available from a number of suppliers, including Sigma
Diagnostics (St. Louis, MO), Roche Molecular Biochemicals (Indianapolis, Il~,
Wako
Chemicals (Richmond, VA), and ICN Biomedicals (Costa Mesa, CA). Most or all of
the
commercially sold enzymes are of bacterial source. Cholesterol esterase
("CHE") and
lipase from Pseudomonas, cholesterol oxidase ("CO") from Nocardia, and
glycerol
kinase from Candida in the assays have proven satisfactory. The glycerol
phosphate
oxidase is, in standard reference, E.C. 1.1.3.21.
While a problem in conducting the assays with enzymes from any
particular source has not been noted, if desired, an enzyme can be tested for
its suitability
for use in the assays set forth herein, such as the DHT assay, or the DHT and
triglyceride
assay, by running the assays on a known concentration of cholesterol, of
triglycerides, or
of both, and determining whether the results of the assay are within an
acceptable range
of the known value. Reagents containing known amounts of cholesterol and of
17
CA 02375210 2001-11-26
WO 00/73797 PCT/US00/14827
triglycerides are available from a number of sources, including Roche and
Sigma
Diagnostics.
In Examples 1 and 2, below, the reagents used in steps 1 and 2 of the assay
are Reagents 1 and 2 of the EZ-HDLT"" cholesterol measurement kit from Sigma
Diagnostics. If desired, however, the reagents contained in this kit (anti-
human apoB
antibody and the reporter compounds peroxidase (POD) and 4-aminoantipyrine
("4AA")
in the case of Reagent 1 and CHE and CO and FDAOS (N-ethyl-N-(2-hydroxy-3-
sulfopropyl)-3,5-dimethoxy-4-fluoroanaline, sodium salt) in the case of
Reagent 2) can be
added separately. Determination of the amount of antibody to be used can be
determined
by the assays described in the preceding section. Appropriate amounts of the
reporter
compounds and enzymes can be determined by performing a series of test assays
on
commercially available reagents containing known concentrations of lipoprotein
fractions. Examples 3, 4, and 5, report the results of assays using different
complex-
forming agents to measure HDL-C and total-C or LDL-C and total-C. The enzymes
used
in these assays were from the same sources as those noted above.
Triglyceride measurements are most commonly made using glycerol
kinase, glycerol phosphodehydrogenase, glycerol phosphate oxidase, and
peroxidase.
Other enzymatic assays for measuring triglycerides can, however, be
incorporated into
the methods taught herein. For example the "NAD" method uses glycerol kinase,
pyruvate kinase and lactate dehydrogenase to measure triglycerides. All of
these
enzymes are also commercially available from Sigma and other suppliers.
V. Detergents
Following measurement of the uncomplexed lipoprotein component of the
sample, the complex between the complex-forming agent and the complexed
lipoprotein
fraction is treated to separate the two. For purposes of the methods taught
herein, the
complex is preferably separated by dissolving the complex with a non-
denaturing
detergent. The detergent should be strong enough to dissolve the complex, but
gentle
enough not to denature the enzymes used during the course of the lipoprotein
or
triglyceride assays. A non-denaturing detergent suitable for use in the
invention is
therefore one which (1) dissolves the complex formed between the complex-
forming
agent and the complexed lipoprotein fraction and (2) does not inactivate
cholesterol
esterase, cholesterol oxidase, or peroxidase and does not interfere with the
measurement
18
WO 00/73797 CA 02375210 2001-11-26 PCT/US00/14827
of the amount of cholesterol present. The strength of a detergent can be
measured and
characterized by, for example, its hydrophilic lipophilic balance, as taught
in U.S. Patent
No. 5,766,629.
Detergents preferred for use in the invention are deoxycholate, NP-40, and
octyl glucoside, with deoxycholate being the most preferred. Any non-
denaturing
detergent meeting the test above, however, can be used. Any particular
detergent can be
readily tested to determine whether it is satisfactory for use in the
invention. For
example, a sample containing known concentrations of HDL and LDL can be tested
by
complexing the LDL component with anti-apoB antibodies, reacting the HDL with
CHE
and CO, and then using the detergent under consideration. If the resulting
measurement
of the LDL component is within an acceptable range of the known concentration,
then the
detergent has successfully dissolved the complex-forming agent/lipoprotein
complex and
has not impaired the measurement. A reading well below the known amount of LDL
present would indicate that the detergent has failed one of the prongs of the
test and is not
1 S satisfactory for use. Conveniently, the detergent is tested on a portion
of a sample
assayed in parallel with a second portion of the sample tested with
deoxycholate as the
detergent to verify that any the problem is with the detergent under
consideration and not
with the equipment or with an experimental error. As previously noted,
reagents
containing known concentrations of cholesterol (sometimes known in the art as
cholesterol calibrators) are commercially available from several sources,
including Roche
and Sigma Diagnostics.
VI. Optical measuring systems
In preferred embodiments, the amount of cholesterol present is determined
by optical means. In colorimetric assays, reagents are used to permit a
measurement of
the amount of light absorbed by a dye which undergoes a color change
calibrated to the
amount of substrate (ultimately, cholesterol) present in the sample. In
fluorescent assays,
a reagent is added which is excited by a light and which emits light of a
different color,
the amount of which is calibrated to the amount of substrate, and ultimately,
the amount
of cholesterol present. In chemiluminescent assays, light is spontaneously
emitted by the
reagents in response to the amount of substrate, permitting a measure of
cholesterol
present.
In a particularly preferred embodiment, chromogenic systems are used in
which the amount of cholesterol present is determined by changes in the
absorbance of
19
WO 00/73797 CA 02375210 2001-11-26 pCT/Ug00/14827
the sample solution as calibrated to the amount of light reaching a detector
at 600 nm.
Typically, cholesterol esterase and cholesterol oxidase react with cholesterol
in the
presence of H20 and 02 to form cholestenone, fatty acid and H202. Chromogenic
systems which can then be used to detect the presence of H202, such as 4-
aminoantipyrine ("4AA") and 3-methyl-2-benzothiazolinone hydrazone (MBTH) or
its
sulphonated derivative, are well known. See, e.g., U.S. Patent No. 4,851,335.
Additional
reagents, such as FDAOS (N-ethyl-N-(2-hydroxy-3-sulfopropyl)-3,5-dimethoxy-4-
fluoroanaline, sodium salt), are often included in commercial reagent kits to
speed the
reaction.
In NAD and NADP systems, the reduction of NAD or NADP molecules,
respectively, present in a solution containing a sample causes a change in the
absorbance
profile of the solution (typically measured at 340 nm). This absorbance change
can be
correlated to the amount of cholesterol or of triglycerides present in the
sample, and does
not require the presence of a dye.
VII. Triglyceride measurement
Triglycerides are comprised of fatty acid molecules bound to a glycerol
backbone. See, e.g., Stryer, Biochemistry, W. H. Freeman and Co. New York (3rd
Ed.
1988), Chapter 20. In the classic enzymatic reaction for measuring
triglyceride levels,
lipase is used to cleave the fatty acids from their glycerol backbones. ATP is
added, and
glycerol kinase is used to phosphorylate the glycerol. Glycerol phosphate
oxidase then
generates glycerol and HZO2. An alternative assay format uses lipase, glycerol
kinase,
pyruvate kinase, and lactate dehydrogenase. See, e.g., Sampson et al., Clin.
Chem.
40:221-226 (1994). Triglyceride measurements can be made following step 4 of
the
method of the invention. When using the classic reaction with glycerol
phosphate
oxidase, the H202 formed by the reaction can be detected by the chromogenic
systems
already in the tube in connection with the lipoprotein fraction measurements,
as discussed
in the previous section.
Reagent kits containing enzymes for triglyceride measurements, along
with reporter compounds, are commercially available from a number of
suppliers, such as
Sigma and Wako.
WO 00/73797 CA 02375210 2001-11-26 PCT/US00/14827
VIII. Modifications to Automated Assay Formats
Some automated assay devices, such as the Genzyme N-geneous~ HDL
system, are designed for the addition of only two reagents. Reagent 1 contains
the
polyanion, and reagent 2 contains the cholesterol esterase, cholesterol
oxidase, and other
enzymatic reagents used for the colorimetric measurement of cholesterol
content in the
HDL fraction. The methods of the invention can be practiced in such systems by
combining the polyanion and the enzymes into a single reagent so that the
detergent can
be added as the second reagent. An exemplary assay as performed in our
laboratory is set
forth in the Examples.
Automated systems which permit the addition of more than two reagents
should be usable in the methods of the invention without modification. If the
system
permits only two reagents to be added, and uses a complex-forming agent other
than a
polyanion, a test should be performed to see if the complex-forming agent and
the
enzymes used for the release and measurement of cholesterol can be combined
without
significantly affecting the results. This can be easily accomplished by taking
a sample
containing of a known amount of LDL-C or of HDL-C and dividing it into two
portions.
One portion is assayed in the system following the standard methodology for
that system,
and the second portion is tested in the system with the reagents provided in
the system
combined into a single reagent and the detergent added as a second reagent. A
difference
in the results of the two assays which is greater than the difference
permitted by the
standard deviation of the system as determined by the manufacturer indicates
that the
reagents supplied by the system cannot be combined into single reagent for use
in the
assays taught herein.
IX. Kits
The reagents necessary or useful for practicing the methods of the
invention can be conveniently provided as kits. Typically, the kits will
provide a
container containing one or more complex-forming agents, such as an anti-apoB
or an
anti-apoAI antibody, a cyclodextrin, or a polyanion. The kit may further
provide a
container holding a detergent, such as deoxycholate. The kit may further
provide
containers holding one or more enzymes for enzymatic determination of
cholesterol, such
as cholesterol esterase, cholesterol oxidase, and may also provide reporter
dyes to detect
the formation of hydrogen peroxide generated by the action of cholesterol
oxidase on
cholesterol released from a lipoprotein fraction by cholesterol esterase.
21
WU 00/73797 CA 02375210 2001-11-26 PCT/US00/14827
In addition, the kit may provide containers holding one or enzymes for the
measurement of triglycerides. For the standard assay, these enzymes may
include lipase,
glycerol kinase, glycerol phosphate dehydrogenase, glycerol phosphate oxidase,
and
peroxidase. Alternatively, or in addition, the kit may provide enzymes useful
in the NAD
method of measuring triglycerides, specifically, lipase, glycerol kinase,
pyruvate kinase,
and lactate dehydrogenase.
EXAMPLES
Example 1
This example demonstrates use of a method of the invention to determine
the HDL-C and total cholesterol content of a sample.
Materials and Methods
HDL (d=1.063-1.21 g/dL) and LDL (d=1.009-1.063 g/dL) were obtained
by density gradient ultracentrifugation, as previously described (Schuxnaker,
V.N. et al.,
Academic Press Inc., London 128:155-169 (1986)). Deoxycholate and a homogenous
HDL-cholesterol kit (EZ-HDLT"") were obtained from Sigma Diagnostics (St.
Louis,
MO). Cholesterol calibrators and reagents for total cholesterol were obtained
from Roche
(Indianapolis, IN) and performed on a Hitachi 917 analyzer (Roche). The HDL
precipitation method was performed with reagents from PolyMedco (Cortland
Manor,
NY) and cholesterol was measured on a Cobas Fara analyzer using reagents from
Roche.
The DHT test was performed on a Cobas Fara II analyzer (Roche) by
modifying the Sigma homogenous HDL cholesterol assay kit, using the parameters
shown
in Table 1. HDL-C was calibrated using Preciset cholesterol calibrators
(Roche) at 3
levels (50, 200, and 400 mgldL). Total-C was calibrated by taking the factor
(slope)
generated by the Cobas step 2 and applying it to the raw data generated during
step 3.
Table 1. DHT Assay Parameters
STEP VOLUME 'I'~
Step 1
Add sample 2.5 ~L
Add antibody 225 ~,L 4 min
22
WO 00/73797 CA 02375210 2001-11-26 pCTNS00/14827
Step 2
Add CE, CO (enzymatic reagents 75 ~L 5 min
for cholesterol determination)
Read absorbance.
Step 3
Add 100 mmol/L DOC 15 wL 5 min
Step 4
Note: enzymatic reagents for Read absorbance after
cholesterol measurement are akeady incubation with
present from Step 2 detergent
Legend: CE = Cholesterol esterase, CO = Cholesterol oxidase, DOC =
Deoxycholate
(Note: this legend is also followed in the Tables below.)
Results
The reaction profile of the DHT assay for purified fractions of HDL, LDL,
and serum is shown in Fig. 2. In each panel of Figure 2, the arrow for "Step
2" represents
a time point at which enzymes have been added to a mixture of sample and Anti-
apoB
antibody. In each panel, "Step 3" represents a time point at which detergent
has been
added and further colorimetric measurements made. As shown in Figure 2A, HDL-C
is
detected in only the second step, whereas Fig. 2B shows LDL-C was detected
only after
the addition of detergent in the third step. When a mixture of HDL and LDL was
analyzed, cholesterol was detected in both steps 2 and 3 (Fig. 2C). Likewise,
a positive
reaction for cholesterol was detected in both steps 2 and 3 when serum was
analyzed (Fig.
2D). The enzymatic reactions for detecting cholesterol in each step at
37°C were
complete after approximately 5 minutes.
The cross reactivity of HDL-C and LDL-C during each step of the DHT
reaction was demonstrated using purified HDL and LDL fractions (see Fig. 3).
In Fig 3A,
a fixed concentration of HDL-C was measured in the presence of an increasing
amount of
LDL-C (shown on the X-axis). In Fig 3B, a fixed concentration of LDL-C was
measured
in the presence of an increasing amount of HDL-C (shown on the X-axis). As can
be
23
WD 00/73797 CA 02375210 2001-11-26 PCT/US00/14827
seen in Fig. 3A, the concentration of HDL-C when measured in step 2 did not
significantly change as the amount of LDL-C in the sample was increased. The
increasing amount of LDL-C was not detected during step 2 but was measured in
step 4.
Similarly, increasing amounts of HDL-C did not significantly affect the
measurement of
LDL-C in step 3 (Fig. 3B). The sum of HDL-C and non-HDL-C was equal to the
total-C
measurement as determined by the overall change in absorbance from step 1 to
step 4.
The linearity of HDL-C and total cholesterol for the DHT assay is shown
in Fig. 4. Both the HDL-C and total cholesterol portions of the assay were
linear
throughout the typical range of serum HDL-C and total cholesterol
concentration. The
within-run and between-run precision for the DHT assay is shown in Fig. 5 for
a frozen
serum pool. Both the HDL-C and total cholesterol portions of the assay showed
relatively good precision compared to other HDL-C and total cholesterol
assays.
The measurement of HDL-C and total cholesterol as determined by the
DHT assay was compared to standard assays on sera from patients with a broad
range of
lipoprotein values (Fig. 6). Both the HDL-C and total cholesterol portions of
the assay
compared favorably to the standard assays. In addition, the calculated non-HDL
cholesterol and the calculated LDL-C by the DHT assay also showed a close
correlation
with the standard assays.
Discussion
Total cholesterol in serum is typically measured enzymatically using
cholesterol esterase and cholesterol oxidase. Cholesterol esterase ("CE" or
"CHE")
converts cholesterol ester to free cholesterol. The free cholesterol is then
oxidized by
cholesterol oxidase ("CO"), which generates a molecule of hydrogen peroxide.
The
generation of hydrogen peroxide is then detected by the oxidation of various
dyes by
peroxidase. The oxidation of the reporter dye results in a change of its
absorption
spectrum, which is used to determine the concentration of total cholesterol.
Assays for
total cholesterol typically contain a detergent, which does not adversely
affect the activity
of cholesterol esterase and cholesterol oxidase but accelerates the rate of
the reaction by
increasing the substrate availability of cholesterol esterase and cholesterol
oxidase. The
detergent disrupts and solubilizes the lipoproteins into smaller micelles,
thus increasing
the surface area of the lipid interface and increasing the rate of reaction of
cholesterol
24
WO 00/73797 CA 02375210 2001-11-26
PCT/US00/14827
esterase and cholesterol oxidase. Addition of detergent at the beginning of
the present
procedure, however, would impede the ability to measure HDL-C in the first
measurement. Accordingly, in the present methods, the detergent is not added
until after
the HDL-C reading is taken.
Homogenous assays for HDL-C use various strategies for blocking the
reactivity of cholesterol esterase and cholesterol oxidase to the apoB
containing
lipoproteins (Nauck M. et al., Clin Chem 44:144-51 (1998)). The original
homogenous
HDL-C assay that was modified in creating the DHT assay uses an antibody
directed
against apoB to sterically block the reactivity of the apoB containing
lipoproteins to
cholesterol esterase and cholesterol oxidase. The assay is performed in the
absence of
detergent in order to prevent any disruption in the interaction of the
antibody to the apoB
containing lipoproteins. In the DHT assay of the invention, deoxycholate, a
non-
denaturing detergent is added after the measurement of HDL-C is complete in
step 2. As
can be seen in Fig. 2, after approximately 5 minutes, the reaction of
cholesterol esterase
and cholesterol oxidase with HDL is complete. This is not because the enzymes
are no
longer active or because of the consumption of the unoxidized dye but because
of the
enzymes have depleted the cholesterol on the HDL present in the sample. The
addition of
deoxycholate disrupts the antibody-apoB complex, which results in an
additional increase
in absorbance from the enzymatic reaction of cholesterol on the apoB
containing
lipoproteins.
Example 2
This Example describes coupling the DHT assay to an assay for
triglycerides. It sets forth the standard protocol we employ for the DHT
assay, as well as
the modifications employed to accommodate the triglyceride assay.
The DHT assay and the triglyceride assay can be performed in sequence
on a Cobas Fara II analyzer. Due to limitations on the sample volume that can
be handled
by the automatic pipetter of the analyzer, and to constraints on the ability
of the device to
read the absorbance of the dye above certain concentration levels, when a
triglyceride
assay will be performed following the DHT assay, we have found it useful to
make a 10
fold dilution of the sample (25 ~.L serum + 225 ~L of saline). The analyzer is
then set to
pipette the sample at 12.5 pL (which is 5 times the volume -- 2.5 p,L -- used
when only
WO 00/73797 CA 02375210 2001-11-26 PCT/US00/14827
the DHT assay is being performed), so that the final dilution of the sample is
half the
concentration used for the DHT assay, along with 225 p.L of Reagent I of the
EZ-HDLT""
cholesterol measurement kit (Sigma Diagnostics), which is a premixed solution
of the
appropriate compounds.
After a four minute incubation and the first reading, the analyzer adds 75
pL of Reagent 2 (Sigma Diagnostics EZ-HDLT"" kit), which contains CHE and CO
and
FDAOS. After a five minute incubation, reading 2 is taken, and then 1 S p,L of
100
mmol/L deoxycholate is added. After a five minute incubation, reading 3 is
taken, and 50
pL of a mixture of lipase (2500 U/L), glycerol kinase (1250 U/L), glycerol
phosphate
dehydroxgenase (2000 U/L), and glycerol phosphate oxidase (2500 U/L), is added
which
contains the enzymes needed for the triglyceride measurement with the GPO
Trinder
reaction. It should be noted that the 4AA, POD, and FDAOS added for the first
reading
are still in the sample mixture and act to permit the colorimetric
determination of the level
of triglycerides present, expressed in mg/dL.
For the readings set forth in the Figures, the amounts of the various
lipoprotein fractions were determined by readings of absorbance taken at 600
nm on a
device calibrated by the absorbance of known amounts of cholesterol and
triglycerides.
Example 3
In this Example, compatibility of the invention with the Genzyme N-
geneous~ HDL measurement kit was determined. The N-geneous~ kit was used
according to the manufacturing directions to perform steps 1 and 2 of the
invention; that
is, to complex a lipoprotein fraction (in the case of the N-geneous~ kit, the
LDL fraction)
and to obtain a HDL reading. The remaining steps of the method were then
performed.
The procedure is set forth in the following Table:
Table 2.
STEP VOLUME TIME RESULT
Step 1
Add patient or other sample 2.5 pL
Add Genzyme Reagent 1 (polyanion) 250 ~L 5 min
26
WO 00/73797 CA 02375210 2001-11-26
PCT/US00/14827
Step 2
Add Genzyme Reagent 2 (CE, CO, 85~L 5 min HDL-C
enzymatic reagents
for cholesterol determination)
Read absorbance
Step 3
Add 100 mmol/L DOC 15 wL 5 min
Step 4
Read Total-C absorbance following incubation with detergent. Total-C
(Enzymatic reagents are already present from Step 2.)
1 S Results.
The Genzyme N-geneous~ system was completely compatible with the
methods of the invention. The synthetic polyanion used to complex with the LDL
was
readily solubilized with the detergent, deoxycholate, which permitted the
reading of total-
C. Non-HDL-C can then be calculated, if desired, by subtracting the HDL-C
value from
the total-C reading obtained in step 4.
Example 4
In this Example, the Genzyme N-geneous~ HDL-C measuring system was
modified. The system is designed for the addition of two reagents. Since the
method of
the invention employs a detergent as an additional reagent, the reagents used
in the
Genzyme system were combined so that the detergent could be added as the
second
reagent. The modified system was tested, as shown in the following table:
27
WO 00/73797 CA 02375210 2001-11-26 PCT/US00/14827
Table 3.
STEP VOLUME TIME RESULT
Steps 1 and 2
Add sample 2.4 wL
(Optional: read baseline absorbance)
Add combination of Genzyme 320 ~L 5 min HDL -C
Reagent 1 (polyanion) and Genzyme
Reagent 2 (CE, CO, enzymatic reagents
for cholesterol determination)
Read absorbance
Step 3
Add 100 mmol/L DOC 15 wL 5 min
Step 4
Read absorbance following Total-C
incubation with detergent.
(Enzymatic reagents are already present from Step 2.)
Results.
The modified Genzyme N-geneous~ system was completely compatible
with the methods of the invention. The combined reagents (synthetic polyanion
used to
complex with the LDL and the enzymes for determining ILL-C) could be added
together
and the LDL-polyanion complex was readily solubilized with the detergent,
deoxycholate, which permitted the reading of total-C. Non-HDL-C can then be
calculated, if desired, by subtracting the HDL-C value obtained from the total-
C reading
obtained in step 4.
Example 5
This Example shows the use of the assays of the invention to determine
LDL-C. The Roche LDL-C assay uses sulfated a-cyclodextrin to complex apoB-
28
WO 00/73797 CA 02375210 2001-11-26 PCT/[JS00/14827
containing lipoproteins. The assay was performed using the Roche reagents in a
Cobas
Fara II analyzer.
The assay procedure is set forth in the following Table:
Table 4. Using Roche LDL-C reagents to determine LDL + TC in the DHT assay.
STEP VOLUME TIME RESULT
Step 1
Add sample 3.0 ~L
Add Roche Reagent 1 (a-Cyclodextrin, 225 ~.L 5 min
buffer)
Read baseline absorbance
Step 2
Add CE, CO (enzymatic reagents 75 ~L 5 min LDL -C
for cholesterol determination)
Read absorbance
Step 3
Add 100 mmol/L DOC 1 S ~tL 10 min
Step 4
Read absorbance Total-C
(Enzymatic necessary for reading are present from
Step 2).
Tests on a number of patient samples showed satisfactory correspondence
between the
LDL and Total-C measurements obtained by the DHT assay and the measurements
obtained using standard tests for LDL-C and for Total-C.
29
WO 00/73797 CA 02375210 2001-11-26 PCT/US00/14827
All publications and patent applications cited in this specification are
herein incorporated by reference as if each individual publication or patent
application
were specifically and individually indicated to be incorporated by reference.
Although the foregoing invention has been described in some detail by
way of illustration and example for purposes of clarity of understanding, it
will be readily
apparent to those of ordinary skill in the art in light of the teachings of
this invention that
certain changes and modifications may be made thereto without departing from
the spirit
or scope of the appended claims.