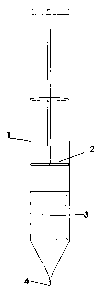Note: Descriptions are shown in the official language in which they were submitted.
WO 00/75623 cA 02375449 2001-11-3o pCT/GB00/02211
1 -
Sample Processing Device
The present invention relates to a sample processing device and in particular
to a
sample processing device that can purify biomolecules from crude starting
materials
such as blood, tissue, plants, microbes, agricultural, food etc. The system
can also be
used to purify or manipulate any biomolecule or compound from aqueous or non-
aqueous samples in a fully automated or manual mode.
Conventional chromatography columns are not suitable for direct extraction of
biomolecules from crude starting materials containing particulate matter,
viscous
material, or cellular debris. This is due to the type of solid phase employed
and the
design of the column or cartridge that are prone to blocking or clogging. This
also
applies to mini-chromatography columns processed using a vacuum or
centrifugation
to pass the liquid over the solid supports. This clogging problem is
exacerbated
when attempting to extract high molecular weight DNA without shearing it into
small fragments. Clogging of columns or cartridges may be due to frits (a
thick,
rigid, porous disc/membrane or plug) with small pore sizes or the small size
of
standard solid phase particles (usually less than 200 microns in diameter)
and/or
close packing of stationary solid phase material (dependant on size and shape
and
compressibility).
We have now devised equipment and a method which reduces these problems.
According to the invention there is provided equipment for extracting a
material from
a liquid mixture containing the material which equipment comprises a container
containing a solid phase able to adsorb the material to be extracted and a
reversible
suction means adapted to apply suction to the solid phase to draw up the
liquid
mixture over the solid phase and which is able to be reversed so as to pass
the liquid
back over the solid phase.
CA 02375449 2001-11-30
WO 00/75623 PCT/GB00/02211
- 2 -
The reversible suction means can be in the form of a syringe and the solid
phase is
contained within the syringe below the piston so that when the nozzle of the
syringe
is placed in the liquid mixture and the piston is withdrawn liquid is drawn up
over
the solid phase and when the piston is depressed the liquid is passed back
over the
solid phase.
Alternatively the container containing the solid phase can be attached to the
nozzle of
a syringe.
In another embodiment of the invention the reversible suction means comprises
a
pipette and there is a plug of the solid phase contained within the pipette
tip.
It is a feature of the invention that the novel design allows almost any
starting
material to be used without clogging the automated extraction system and in a
closed
environment reducing the risks of contamination to the operator, instrument or
to
adjacent sample tubes.
The design allows the use of existing solid phase extraction methods used in
chromatography as well as novel reagents and materials described below.
The invention is especially suitable for extracting large macromolecules such
as
nucleic acids (DNA and RNA) that tend to block or clog existing devices.
The syringe or pumping device with sucking and blowing action can be used in
conjunction with a specially modified chromatography cartridge that resists
clogging.
For example, a biological sample e.g. animal or plant tissue, blood, cells,
hair, faeces,
agricultural, water, food etc. is homogenised to release the nucleic acids and
then
passed through a solid phase material to capture the nucleic acids, nuclei or
nucleated
cells. The cellular debris or contaminants pass up and down to waste leaving
the
nucleic acids immobilised on the solid phase support. Alternatively, the solid-
phase
CA 02375449 2001-11-30
WO 00/75623 PCT/GB00/02211
- 3 -
material can be used to remove unwanted cellular debris leaving the DNA or
biomolecule in solution.
The material to be extracted can be passed up and down the solid phase using
the
pumping action of the syringe or peristaltic pump. Any type of solid phase can
be
used since the cartridges are designed to be interchangeable for a wide
variety of
solid phase extractions.
Additionally, the system will allow homogenisation of samples by introducing a
shredding device in the primary cartridge.
This allows difficult samples, such as plant extracts, animal tissue, faeces,
food or
other samples requiring maceration to release cellular contents such as
proteins or
nucleic acids. The target molecules can then be captured on the solid-phase
and the
homogenisation and purification process is thus completely automated.
An enrichment step is often used to remove initial debris and this can be
performed
using flocculating agents such as cellulose, diatomaceous earth, silica gel,
dextrans,
PEG or any substance that promotes rapid flocculation and sedimentation of
debris or
contaminants without requiring centrifugation.
The instrument of the invention can be designed to handle single or multiple
rows of
standard syringes (disposable or non-disposable) of different sizes e.g up to
100m1 .
A single disposable unit with 4, 8, 12, 24 or 96 channels can also be used in
conjunction with a pumping system. Thus it is able to dispense large or small
volumes in an 8 by 12 tube array.
A feature of the instrument of the invention is that the final purified
product can be
presented in a microtitre plate format of 8 x 12 tubes or as single tubes
regardless of
the starting volumes of samples. Therefore a great many large samples of blood
e.g.
CA 02375449 2001-11-30
WO 00/75623 PCT/GB00/02211
- 4 -
Sml, can be processed for the extraction of DNA simultaneously and then
concentrated down to small 1 ml tubes.
By using sucking and blowing rather than flow-through of reagents and samples,
the
disposable cartridge retains the sample completely, preventing contamination
of any
interfaces with the instrument such as tubes, valves and ports of the
disposable
cartridge. This also minimises the risk to the operator as the disposable item
can be
automatically discarded.
Alternatively, a whole microtitre plate can be processed containing small
samples
e.g. I ml buccal cell scrapes, using smaller syringes or pumping devices.
A syringe or mufti-channel disposable cartridge system is able to operate in
X, Y and
Z dimensions and can accommodate any pitch changes necessary to handle
different
sample volumes. The purified analyte may be transferred automatically to a
UV/visible spectrophotometer or fluorescent photometer to estimate analyte
concentration and purity.
Most conventional UV spectrophotometers require a relatively large sample to
analyse in a silica quartz cuvette. Unfortunately, in biological samples the
amount of
analyte is often in tiny volumes or low concentration. This results in either
sacrificing the whole sample or diluting into a bigger volumes which then may
make
detection very difficult. By having a disposable probe that dips into the
sample the
solution can be measured at full strength and without wastage.
The instrument design allows incorporation of electrodes or metal meshes or
conductive plastic meshes that can be made positively charged to bind nucleic
acids
from crude extracts. The positive charge can then be turned off or reversed to
release
the purified nucleic acids. The pumping effect of the instrument allows rapid
mixing
increasing the contact of the target molecules. In one format there could be a
mesh,
WO 00/75623 CA 02375449 2001-11-30 PCT/GB00/02211
- 5 -
bead or tip incorporated as a plastic disposable that can carry a positive
charge by
applying a potential difference or induction. As the sample passes across the
mesh or
membrane the nuclei acids bind and can be released as the charge is reversed
or
switched off.
Large highly porous or non-porous solid phase beads may be used to avoid
clogging
and maintain high flow rates. For example; porous plastic beads with a
diameter of
150microns or greater with very large pores e.g. 1 to 20 microns, made from
polypropylene, polyethylene or any polymer with a natural affinity for
specific
biomolecules. Other materials can also be used; cellulose, agarose, glass,
silica or
any suitable material that may be derivatised to extract a target analyte.
The solid-phase may be derivatised with imidazole groups, amine, carboxy or
any
group with an affinity for nucleic acids or the target molecule/compound. The
beads
may be used with a frit or membrane or a single hole or mufti hole mesh
depending
on the flow rates required.
The beads may be composed of material that has an inherent affinity of
biomolecules
such as poly vinyl pyridine that is positively charged at pH 4 and will bind
DNA and
elute it at pH 8. Any polymeric compound can be converted into beads or
particles or
surfaces for binding and may include groups such as pyrazole, pyrole,
pyrroldine,
indole, pyrimidine, nucleic acid bases, imidazole, imines, amines, lysines or
any
groups that have a pKa in the range of 3 to 12. Preferably a pKa of 5 to 8 is
employed
to maintain physiological conditions if biological samples are being processed
and
can thus be manipulated by pH to turn a positive charge on or off.
The beads may also be converted for chelation of biomolecules such as
iminodiacetic
acid beads bound with ferric ions at pH 3 to bind nucleic acids. Raising the
pH
removes the purified nucleic acids. Calcium, Magnesium or Ammonium ions can
also be used to chelate biomolecules.
CA 02375449 2001-11-30
WO 00/75623 PCT/GB00/02211
- 6 -
The beads can be held stationary using frits or membranes as long as the pore
diameters are large enough to allow easy passage of crude matter. In most
cases the
frits or membranes require relatively large holes (pin holes) not found in
conventional materials. Alternatively the beads may be held in place by
narrowing
the inlet and outlet of the cartridge removing the need for frits or they may
be held in
place by a mesh incorporated into the design of the cartridge.
The larger beads can be allowed to move inside the column by having space
inside
the cartridge. This helps with mixing and reduces clogging. Alternatively, the
beads
can be added to the crude mixture as a suspension and then trapped in a
cartridge
when the target molecules are bound thus achieving separation.
Large diameter beads are also useful for preparing magnetic solid phases which
are
not prone to aggregation during isolation of large macromolecules such as
nucleic
acids. Most non-porous magnetic (paramagnetic) beads are less than SOmicrons
but
are difficult to handle when extracting genomic or microbial DNA. Large porous
magnetic beads may lead to internal entrapment of contaminants as the
macromolecule such as DNA bind to the outer surface of the beads, these
contaminants are only released when the DNA is eluted contaminating the final
2 0 preparation.
Porous membranes or frits can be modified by adding larger holes and used in
spaced
stacks or individually to bind biomolecules from crude extracts, e.g. blood.
Existing
membranes or frits block instantly when encountering crude extracts or high
molecular weight DNA. The invention describes the use of a unique pore size
modification that allows the treatment of crude materials in large or small
volumes
that is also amenable to automation.
For DNA extraction from blood, a small or large device can be constructed
based on
the same design. The small device relies on a standard plastic pipette tip
that
WO 00/75623 CA 02375449 2001-11-30 pCT/GB00/02211
incorporates a single porous plug, wadding, or frit with a pore size that
prevents
blocking. This can be used with a conventional pipette or syringe in a manual
mode
or in a fully automated pipetting station. Alternatively, they can be
incorporated into
Deep Well plates, Microtitre plates or PCR tubes and used with centrifugation
or
vacuum manifolds. The frits may also be incorporated directly inside a syringe
1 ml
to 60m1 instead of an extra cartridge.
The plug or frit may be derivatised to bind DNA or any biomolecule. For larger
extractions additional plugs or frits can be added in stacks separated by a
small air
barrier to avoid blocking and maintain exposed surfaces for binding the target
compound.
The material for the membrane or frit may be porous polyethylene with a
primary
pore size of 1 to 200 microns or preferably 20 microns , or any porous
plastic, porous
glass, cellulose with pores large enough to allow passage of crude matter.
E.g. 20
microns or larger. The material may have small pore sizes that will enable
binding of
target molecule, but they must also possess larger holes e.g. 0.1 mm or
greater to
avoid blocking. This larger hole may be at the edges or in the middle or part
of a cut
away section.
A variety of frits can be incorporated in a single cartridge to perform
sequential or
discrete separations, e.g. one frit separates the nuclei and another
derivatised with
silica or imidazole groups purifies the DNA further up the cartridge or in a
separate
device. Alternatively, the initial capture of DNA, RNA, or other biomolecules
can be
performed, then the analyte is washed off and carried up the syringe and
precipitated
with a compound such as PEG, alcohol, ammonium or sodium sulphate. The
precipitated compound may then be re-captured allowing the soluble
contaminants to
pass to waste.
CA 02375449 2001-11-30
WO 00/75623 PCT/GB00/02211
_ g _
A syringe based system can be used to filter a crude sample by incorporating a
filter
membrane or plug at the tip of the cartridge or pipette tip. The debris is
left behind
as the liquid is sucked up through the plug. After rinsing the plug free of
debris the
clarified solution is dispensed into a new tube.
This technique can be assisted by using a filter-aid such as silica gel,
titanium oxide,
fibrous cellulose etc.
Following homogenisation, a filter-aid is added to flocculate and compact the
debris
at the bottom of the tube leaving the target molecule in the supernatant ready
for
processing or purification.
The filter-aid may be soluble, possess temperature dependant solubility or be
insoluble.
If smaller solid-phase beads are employed for conventional extractions. For
example,
less than 100 micron glass, then a by-pass channel can be introduced that
allows
larger particles or debris to pass up and down without clogging the cartridge.
A by-
pass channel may be created as small tube that by passes the solid phase or a
porous
material with large pores e.g. 20 microns or greater, that surrounds the solid
phase.
For very pure DNA further purifications can be performed with another
cartridge in-
line to allow the use of the salting out technique. This salting out technique
can be
used in conjunction with alcoholic precipitation and capture of the insoluble
nucleic
acids.
If smaller solid-phase beads are employed for conventional extractions. For
example,
less than 100 micron glass, then the solid -phase can be allowed to move
internally so
clogging is avoided. The introduction of ridges, spirals or obstructions
inside the
cartridge helps prevent the solid-phase moving in bulk maintaining good mixing
and
WO 00/75623 CA 02375449 2001-11-30 pCT/GB00/02211
_ g _
separation of solid-phase. If the solid-phase particles are large enough of
sufficiently
dense, e.g. 200 micron glass, a mini-fluidised bed can be generated.
Clogging of cartridges may also be avoided by the pre-addition of a mobile
solid-
s phase to capture the target molecule. The loose solid-phase or paramagnetic
beads
are added as a suspension and the contaminants are washed away leaving the
immobilised target compound ready for further purification in a cartridge or
analysis.
Any type of solid phase can be added with a preference for material that will
sediment quickly or be flocculated by filter aids to avoid centrifugation or
are
paramagnetic.
The instrument and disposable cartridge or tip system has a variety of
applications
including molecular biology such as affinity purification of cell antibodies,
enzymes
and other proteins, purging of mixtures to remove unwanted compounds,
combinatorial chemistry, ion exchange purification, hydrophobic
chromatography,
enzyme assays using immobilised antibodies, nucleic acids or antigens, enzyme
catalysis on solid phase supports, food screening for pathogens, genomic DNA,
toxins, allergens, etc., clinical sample processing for pathogenic organisms,
mixing
adjuvants for immunisation and making stable emulsions, pipetting larger
volumes,
removal of lipoproteins for cholesterol assays, detecting or concentrating
pathogens
in milk, food or water.
A device of the invention is described in the accompanying drawings in which:-
Fig. 1 illustrates with the solid phase inside a syringe
Fig.2 shows the use of a cartridge
Fig. 3 shows a pipette
Figs. 4, 5 and 6 show alternative cartridges and
Figs. 7, 8 and 9 show different disc arrangements.
WO 00/75623 CA 02375449 2001-11-30 pCT/GB00/02211
- 10 -
Referring to fig. 1 a syringe ( 1 ) having a moveable piston (2) has an
adsorbent solid
phase (3) held within it. In use the nozzle (4) is placed within the liquid
from which
material is to be separated and the piston withdrawn to suck up the liquid
through (3).
When the piston is depressed the liquid is forced back over (3) and this
process can
be repeated if desired so that there is better adsorption of material from the
liquid.
Referring to fig. 2 the syringe (5) with a piston (6) has nozzle (7) placed in
cartridge
(8) containing a solid adsorbent and the cartridge (8) has its inlet (9)
placed in the
liquid from which material is to be separated. When the piston (6) is
withdrawn the
liquid is drawn up through the cartridge and material is adsorbed, when the
piston is
depressed the liquid is forced back over the adsorbent in the cartridge so
that there is
better adsorption of material from the liquid.
Referring to fig. 4 the adsorbent material (10) can be in the form of frits or
beads and
can fill the cartridge.
Referring to fig. 5 there can be a by-pass channel ( 11 ) round the outside of
the solid
adsorbent so that larger particles can pass up and down without clogging.
Referring to fig. 6 there are discs (12) positioned within the cartridge and
each disc
consists of an adsorbent membrane, the discs can have large pores as
illustrated in
fig. 7 and can have cut away sections as shown in fig. 8 to prevent blocking.
The
discs can be stacked on top the other and can have a raised lip (14) as shown
in fig. 9
so that the discs are only in contact through this lip.
Referring to fig. 3 a pipette ( 15) has an aerosol plug ( 16) to prevent
contamination
and contains a plug (16) of adsorbent material such as a porous plastic
material as
shown. In us the tip of the pipette (17) is placed in the liquid and liquid is
sucked up
over the plug (16), by blowing down the pipette the liquid is forced back over
the
plug (16) so that there is better adsorption of material from the liquid.
CA 02375449 2001-11-30
WO 00/75623 PCT/GB00/02211
- 11 -
The adsorbed material can be removed from the solid phase by conventional
elution
methods.
The invention is described in the following examples in which the isolated or
eluted
products were identified using conventional laboratory analysis methods.
Example 1
Using the equipment of fig. 2 polystyrene porous carboxylated beads (200 - 500
microns or 16 - 50 mesh size) were loaded into a chromatography cartridge and
held
in place with plastic mesh with pore sizes of about 100 microns.
Whole blood was diluted 10 times with IOmM Ammonium Bicarbonate, lOmM
Ammonium Carbonate and 0.1 % Tween 20 pH9 and sucked up and down the
cartridge with a syringe and passed back through the cartridge. The dilution
buffer
can be any hypotonic solution that causes lysis of the red blood cell
fraction, but
maintains the integrity of nuclei, white blood cells or chromatin. The nuclei
became
immobilised on the beads and the lysed blood was taken to waste. Direct
elution of
the nuclear DNA was achieved using hot water. To obtain greater purity DNA,
the
eluate from the first cartridge was then further processed using another
cartridge
containing a solid-phase with poly imidazole groups.
To collect the white blood cell fraction, the same solution is made isotonic
with
saline and the cells were captured in a similar manner.
Example 2
Using the equipment of fig. 3 polystyrene porous carboxylated beads (200 - 500
microns or 16 - 50 mesh size) were loaded into a 1 ml pipette tip.
Whole blood was diluted 10 times with 1 OmM Ammonium Bicarbonate and 0.01
Tween 20 pH9 and sucked up and down the tip of the pipette. The nuclei became
immobilised on the beads and the lysed blood was removed to waste. Direct
elution
of the nuclear DNA was achieved using alkaline detergent solutions and by
boiling
3 0 water.
CA 02375449 2001-11-30
WO 00/75623 PCT/GB00/02211
- 12 -
Example 3
Using the equipment of fig. 2 agarose was treated with carbonyldiimidazole in
anhydrous organic solvent and then left in water at pH 3 to maintain the
imidazole
groups. The derivatised agarose was placed in a cartridge and the supernatant
from a
plasmid alkaline lysis preparation was sucked up and down immobilising the
plasmid
DNA on the beads at pH 5. After washing, the plasmid DNA was eluted with l OmM
Tris HC1, pH 9.
The above was repeated with carboxylated polystyrene and dextrans of various
sizes
and DNA obtained by elution as above.
Example 4
Extraction of nuclei or DNA from whole blood
Using the equipment of fig. 2 with the packing of fig. 6 whole blood was lysed
with
5 volumes of IOmM Ammonium Bicarbonate containing 0.1 %Tween 20 pH9. The
lysed blood was passed through several 20micron porous polyethylene frits
modified
with larger pores of 1 mm in diameter, housed in a plastic cartridge attached
to a 2m1
syringe and plunger. Each frit was spaced 3mm apart to allow free flow of
liquid.
The nuclei or white blood cell fraction bound to the frit allowing all the
contaminating proteins and lipids to pass through to waste in a single pass or
several
strokes of the plunger. The frit and nuclei was then washed to remove residual
proteins using deionised water or chaotropes or alcohols or detergents such
SDS or
Tween 20 or combinations or lactic and salicylic acids or their salts, or poly
phosphates or per chlorates and either eluted off using hot water or alkaline
solutions
of detergents or further purified inside the cartridge using chaotropic agents
or
proteases.
Example 5
Purification of buccal cell DNA
CA 02375449 2001-11-30
WO 00/75623 PCT/GB00/02211
- 13 -
Using the equipment of fig. 3 a plug of porous polyethylene was derivatised
with
imidazole groups and inserted into the tip of a standard 1 ml pipette tip. A
further
non derivatised plug was inserted at the top to act as an aerosol and liquid
barrier to
prevent contamination of the pipette.
A buccal scrape was taken and mixed with 0.2M guanidine isothiocyanate, 3%
Tween 20, Proteinase K and SOmM MES pH5 at 30°C for 15 minutes. The
mixture
was then sucked up and down the tip several times allowing the DNA to bind to
the
derivatised plug. The plug was washed with 1mM MES pH5 and then the DNA
eluted with 1 OmM Tris. HCI pH9. The same protocol was repeated using 0.01 %
to
10% SDS with or without salts and buffers. Fast degradation of the buccal
cells can
also be achieved using salicylic acid, lactic acid, or MgCl2 at concentrations
of 0.05
to SM. Combinations of the above salts and reagents can also be used.
Example 6
1 gram of carboxylated polystyrene beads with a diameter of about 60 microns
or 200
to 400 mesh was suspended in a hypotonic solution of ammonium bicarbonate l
OmM
with 0.1 % Tween 20 pH9. A five fold excess of this suspension was added to a
Sml
blood sample and mixed once. The beads captured the nuclei and sedimented.
After
several washes with water, the DNA was eluted with hot water. To concentrate
the
2 0 DNA the equipment of fig. 2 was used with the packing of fig. 6 and the
DNA was
captured on a porous disc in the cartridge and subsequently eluted off in a
small
volume and analysed using PCR or Restriction Digestion
Example 7 Removal and purification of human I~G from serum
An agarose gel coupled to Protein A was placed in the cartridge of fig. 2 and
washed
with phosphate buffered saline. A solution containing human IgG in serum was
sucked up and down the solid phase until all the IgG was bound. After washing
the
solid phase with PBS, the IgG was eluted with 0.1 M glycine, 0.1 SM NaCI,
pH2.8
and immediately neutralised with Tris. HCI.
CA 02375449 2001-11-30
WO 00/75623 PCT/GB00/02211
- 14 -
Example 8 Purification and isolation of specific white blood cell tykes from
whole
blood
Non-porous glass particles of 175 microns were coupled to CD4 monoclonal
antibodies and the solid phase placed in a cartridge as in Example 4 with
80micron
frits. A diluted solution of Buffy Coat was sucked slowly up and down through
the
glass beads immobilising the T cell sub-population which could be released.
Example 9 Recombinant~rotein purification
An agarose gel containing Iminodiacetic acid-Nickel ion groups was packed into
a
cartridge as in Example 4.
A bacterial lysate containing a recombinant protein possessing a 6 histidine
tail was
sucked up and down the cartridge and the protein was bound to the co-ordinated
nickel. Release of the protein was effected by eluting with O.SM Imidazole pH
6.
Example 10 Extraction of HIV RNA from serum
A cartridge as in fig. 2 was packed with 60 micron silica and a sample of
serum
diluted 5 times with 6M guanidine isothiocyanate, 0.1 % Tween 20, 20mM EDTA,
100mM Tris. HCl pH6 was sucked up and down through the solid phase. After
washing the solid phase with isopropanol and drying the RNA was eluted using
water
at 60C.
Example 11 Purification of PCR reactions
A cartridge as in fig. 2 was packed with 60 micron silica and a sample of a
PCR
reaction diluted 5 times with 6M guanidine isothiocyanate, 0. 1 % Tween 20,
20mM
EDTA, 100mM Tris-HCl pH6 was sucked up through the solid phase. After washing
the solid phase with isopropanol and drying the DNA was eluted using water.
Example 12 Extraction of RNA from Liver
Fresh liver was homogenised in a mixture of 50% Phenol containing 6M Guanidine
isothiocyanate, lOmM DTT, 0.1 M Sodium Acetate pH 4. Chloroform was added to
CA 02375449 2001-11-30
WO 00/75623 PCT/GB00/02211
- 15 -
separate the phases and the top layer containing the RNA was sucked up and
down
through a cartridge of fig. 2 containing 60micron silica. The silica was
washed with
alcohol, air dried and the RNA eluted with hot water ready for processing.
Example 13 Isolation of mRNA
A cartridge was packed with COOH polystyrene beads coupled to oligo dT 30 5'
NH2.
A sample of white blood cells prepared earlier in a cartridge were treated
with an
excess of 1% SDS, O.SM LiCI, lOmM DTT, lOmM Tris. HCl pH 7.5 and sucked up
and down through the affinity resin several times to shear the DNA and bind
the
mRNA. The resin was then washed in 0.1 M LiCI and air dried. Elution of mRNA
was performed by hot water.
The above experiment was repeated with a carboxylated plastic porous frit as
in
Example 4 that was coupled to oligo dT30 and used for binding less than 5
micrograms of mRNA.
Example 14 Streptavidin immobilised on solid-phase
Streptavidin was immobilised onto porous frits by mixing the protein in 0.1 M
sodium phosphate buffer with 0.01 % glutaldehyde pH7 as in example 4.
Biotinylated primers used to generate a PCR product were then isolated on the
immobilised streptavidin. The PCR product was then made single stranded using
heat
or 0.1 M NaOH and used for sequencing or probe analysis.
Example 15
Use of electrodes, static charge, induction, electrophoresis to isolate DNA or
RNA
Whole blood was diluted down 10 times in l OmM ammonium carbonate/bicarbonate,
SOmM Tris. HC1 with 1 % Tween 20, 1001g/ml proteinase K pH9. Electrodes were
surrounded by dialysis tubing containing the same buffer and dipped into the
solution. A 12 volt direct current from a battery was connected and the nuclei
or
CA 02375449 2001-11-30
WO 00/75623 PCT/GB00/02211
- 16 -
DNA was captured on the outside of the dialysis tubing at the positive
electrode after
a 1 hour incubation. The DNA could be removed by elution with water.