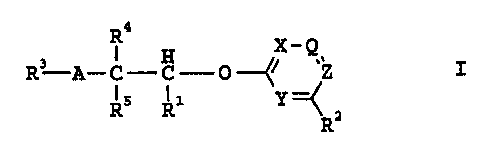Note: Descriptions are shown in the official language in which they were submitted.
CA 02375666 2001-11-30
1
NOVEL CARBOXYLIC ACID DERIVATIVES COMPRISING ARYL
SUBSTITUTED NITROGEN HETEROCYCLES, THEIR PRODUCTION AND
THEIR USE AS ENDOTHELIN RECEPTOR ANTAGONISTS
The present invention relates to novel carboxylic acid
derivatives, their preparation and use.
Endothelia is a peptide composed of 21 amino acids, which is
synthesized and released by vascular endothelium. Endothelia
exists in three isoforms, ET-1, ET-2 and ET-3. In the following,
"endothelia" or "ET" designates one or all isoforms of
endothelia. Endothelia is a potent vasoconstrictor and has a
strong effect on vessel tone. It is known that this
vasoconstriction is caused by the binding of endothelia to its
receptor (Nature, 3~. 411-415, 1988 FEBS Letters, ?~~,, 440-444,
1988 and Hiochem. Biophys. Res. Commun., 15.4., 868-875, 1988).
Increased or abnormal release of endothelia causes persistent
vasoconstriction in peripheral, renal and cerebral blood vessels,
which may lead to diseases: As reported in the literature,
endothelia is involved in a number of diseases. These include:
hypertension, acute mycocardial infarct, pulmonary hypertension,
Raynaud's syndrome, cerebral vasospasms, stroke, benign prostate
hypertrophy, atherosclerosis, asthma and prostate cancer (J.
Vascular Med. Biology 2., 207 (1990), J. Am. Med. Association 2~4,
2868 (1990), Nature , 114 (1990), N. Engl. J. Med: ~,. 205
(1989), N. Engl. J. Med. 3~$, 1732 (1993), Nephron ~~, 373
(1994), Stroke ~., 904 (1994), Nature ~, 759 (1993), J. Mol.
Cell. Cardiol. 22, A234 (1995) Cancer Research .~,~, 663 (1996),
Nature Medicine ~., 944,(1995)).
At least two endothelia receptor subtypes, ETA and ETB receptors,
have to date been described in the literature (Nature ~,$.. 730
(1990), Nature ~.$~ 732 (1990)). Accordingly, substances which
inhibit the binding of endothelia to one or both receptors should
antagonize the physiological effects of endothelia and therefore
represent valuable drugs.
The preparation and use of endothelia receptor antagonists has
already been described in WO 95/26716, WO 96/11914, WO 97/38980,
WO 97/38982, W098/09953, w098/27070, WO 98/58916, WO 99/11629,
DE 19748238.4, DE 19806438.1, DE 19809144.3 and DE 19836044.4.
These are compounds which each comprise a heteroaromatic system
with at least one nitrogen but no phenyl substitution.
0050/50031 CA 02375666 2001-11-30
2
It is an object of the present invention to provide further
endothelin receptor antagonists with valuable pharmacological
properties.
The invention relates to carboxylic acid derivatives of the
formula I
R4
X-Q
R3 A-C-C-0-~~ ~Z
R5 Ri Y ~ z
R
in which R1 is tetrazolyl or a group
I I
C-R
in which R has the following meaning:
a) a radical OR6 in which R6 is:
hydrogen, the cation of an alkali metal, the cation of an
alkaline earth metal, a physiologically tolerated~organic
ammonium ion such as tertiary C1-CQ-alkylammonium or the
ammonium ion;
C3-Ca-cycloalkyl, Cl-Ce-alkyl, CHZ-phenyl which may be
substituted by one or more of the following radicals:
halogen, vitro, cyano, Cl-C4-alkyl, C1-C4-haloalkyl,
hydroxyl, C1-C4-alkoxy, mercapto, Cl-C4-alkylthio, amino,
,.... ~(C1-C4_alkyl). N(C1-C4-alkyl)2:
A CZ-C6-alkenyl or C3-C6-alkynyl group. it being possible
for these groups in turn to carry one to five halogen
atoms;
R6 can also be a phenyl radical which may carry one to
five halogen atoms and/or one to three of the following
radicals: vitro, cyano, C1-C4-alkyl, C1-C4-haloalkyl,
hydroxyl, C1-C4-alkoxy, mercapto, C1-C4-alkylthio, amino,
NH(C1-C4-alkyl), N(C1-C4-alkyl)2;
b) a 5-membered heteroaromatic system which is linked via a
nitrogen atom, such as pyrrolyl, pyrazolyl, imidazolyl
and triazolyl, which may_carry one to two halogen atoms
0050/50031 CA 02375666 2001-11-30
or one to two Cl-C4-alkyl or one to two Cl-CQ-alkoxy
groups;
c) a group
~I'~x
-O- ~ ,~l,p_S,_R7
in which k assumes the values 0, 1 and 2, p assumes the
values 1, 2, 3 and 4, and R~ is
C1-C4-alkyl, C3-CB-cycloalkyl, Cz-C6-alkenyl, Cz-C6-alkynyl
or phenyl which may be substituted by one or more, for
example one to three, of the following radicals:
halogen, vitro, cyano, C1-C4-alkyl, C1-C4-haloalkyl,
hydroxyl, C1-C4-alkoxy, Ci-C4-alkylthio. mercapto, amino,
._.~
NH(Cl-C4-alkyl). N(C1-C4-alkyl)z;
d) a radical
O
Ii
-N S-Re
I l
0
in which R8 is:
Cl-C4-alkyl, Cz-C6-alkenyl, Cz-C6-alkynyl, C3-CB-cycloalkyl,.
it being possible for these radicals to carry a
Cl-C4-alkoxy, Cl-C4-alkylthio and/or a phenyl radical as
mentioned under c);
phenyl which may be substituted by one to three of the
following radicals: halogen, vitro, cyano. Cl-CQ-alkyl,
Cl-CQ-haloalkyl, hydroxyl, Cl-C4-alkoxy, Cl-C4-alkylthio,
mercapto, amino, NH(Cl-CQ-alkyl), N(C1-C4-alkyl)z.
The other substituents have the following meanings:
Rz phenyl or phenoxy, benzyl, benzyloxy, it being possible for
all the aryl radicals to carry one to five halogen atoms
and/or one to three of the following radicals: hydroxyl,
mercapto, carboxyl, vitro, cyano, C1-C4-alkyl,
C1-C~-haloalkyl, C1-CQ-alkoxy, C3-C6-alkenyloxy,
C3-C6-alkynyloxy, C1-C4-alkylthio, C1-C4-haloalkoxy,
C1-C4-alkylcarbonyl, R9, C1-C4-alkoxycarbonyl,
(C1-C4-alkyl)NHcarbonyl, (C1-CQ-alkyl)zNcarbonyl,
~~5~/50031 CA 02375666 2001-11-30
4
C3-C8-alkylcarbonylalkyl, amino, NH(Cl-C4-alkyl),
N(C1-C4-alkyl)2, phenoxy or phenyl, it being possible for said
aryl radicals in turn to be substituted once to three times
by halogen, C1-C4-alkyl, C~,-C4-haloalkyl, C1-C4-alkoxy,
C1-C4-haloalkoxy, methylenedioxy, ethylenedioxy,
C1-C4-alkylthio:
a five- or six-membered heteroaromatic system which contains
one to three nitrogen atoms and/or one sulfur or oxygen atom
and which may carry one to four halogen atoms and/or one to
two of the following radicals: Cl-C4-alkyl, C1-C4-haloalkyl,
C1-C4-alkoxy, C1-C4-haloalkoxy, C1-C4-alkylthio, phenyl,
phenoxy or phenylcarbonyl, it being possible for the phenyl
radicals in turn to carry one to five halogen atoms and/or
--. 15 one to three of the following radicals: C1-C4-alkyl,
Cl-C4-haloalkyl, C1-C4-alkoxy, C1-C4-haloalkoxy and/or
C1-C4-alkylthio;
C3-Ce-cycloalkyl which may carry one to three of the following
radicals: C1-C4-alkyl, C1-C4-haloalkyl, C1-C4-alkoxy,
C1-C4-haloalkoxy, C1-C4-alkylthio.
R3 hydrogen;
C1-C8-alkyl, C3-C6-alkenyl, C3-C6-alkynyl or C3-CB-cycloalkyl,
it being possible for each of these radicals to be
substituted one or more times by: hydroxyl, mercapto,
carboxyl, halogen, vitro, cyano, C1-C4-alkoxy,
C3-C6-alkenyloxy, C3-C6-alkynyloxy, C1-C4-alkylthio,
Cl-C4-haloalkoxy, C1-C4-alkylcarbonyl, C1-C4-alkoxycarbonyl,
(C1-C4-alkyl)NHCarbonyl, (C1-C4-alkyl)2Ncarbonyl,
C3-Ce-alkylcarbonylalkyl, amino, NH(Cl-Ca-alkyl),
N(C1-C4-alkyl)2, phenoxy, phenyl or hetaryl, five- or
six-membered, containing one to three nitrogen atoms and/or
one sulfur or oxygen atom, it being possible for all said
aryl and hetaryl radicals to be substituted once to three
times by: halogen, vitro, cyano, C1-C4-alkyl, C1-C4-haloalkyl,
C1-C4-alkoxy, C1-C4-haloalkoxy, mercapto, carboxyl, hydroxyl,
amino, R9, C1-C4-alkoxycarbonyl, NH(Cl-C4-alkyl),
N(C1-C4-alkyl)2, methylenedioxy, ethylenedioxy,
Cl-C4-alkylthio, phenyl or phenoxy;
phenyl or naphthyl, each of which may be substituted by one
or more of the following radicals: halogen, vitro, cyano,
hydroxyl, amino, C1-C4-alkyl,,Cl-CQ-haloalkyl, C1-C4-alkoxy,
CA 02375666 2001-11-30
C1-C4-haloalkoxy, phenoxy, C.l-CQ-alkylthio, NH(Cl-C4-alkyl),
N(C1-C4-alkyl)2 or methylenedioxy or ethylenedioxy;
a five- or six-membered heteroaromatic system which contains
5 one to three nitrogen atoms and/or one sulfur or oxygen atom
and which may carry one to four halogen atoms and/or one to
two of the following radicals: C1-C4-alkyl, C1-C4-haloalkyl,
C1-C4-alkoxy, C1-CQ-haloalkoxy, C1-C4-alkylthio, phenyl,
phenoxy or phenylcarbonyl, it being possible for the phenyl
radicals in turn to carry one to five halogen atoms and/or
one to three of the following radicals: Cl-C4-alkyl,
C1-CQ-haloalkyl, Cl-C4-alkoxy, C1-C4-haloalkoxy and/or
C1-C4-alkylthio.
R4 and R$ (which may be identical or different):
phenyl or naphthyl, each of which may be substituted by one
or more of the following radicals: halogen, vitro, cyano,
hydroxyl, C1-C4-alkyl, C1-C4-haloalkyl, Cl-C4-alkoxy,
C1-C4-haloalkoxy, phenoxy, C1-C4-alkylthio, amino,
NH (C1-C4-alkyl ) , N (C1-CQ-alkyl ) 2; or
phenyl or naphthyl which are connected together in ortho
positions by a direct linkage, a methylene, ethylene or
ethenylene group, an oxygen or sulfur atom or an SOZ, NH or
N-alkyl group:
or C3-C~-cycloalkyl.
X and Y (which may be identical or different):
nitrogen or methine; with the proviso that if X = Y = methine
then Z = nitrogen.
Z nitrogen or CRlo.
Q nitrogen or CR11; with the proviso that if X = Y = Z =
nitrogen then Q = CRli,
R9 C1-C4-alkyl, C1-C4-alkylthio, C1-C4-alkoxy, each of which
carry one of the following radicals: hydroxyl, carboxyl,
. amino, NH(C1-C4-alkyl), N(C1-C4-alkyl)2, carbamoyl or
CON(C1-C4-alkyl)2.
R1° hydrogen, halogen, hydroxyl, NH2, NH(C1-C4-alkyl),
N(C1-C4-alkyl)2, Cl-CQ-alkyl,, C2-C4-alkenyl, CZ-C4-alkynyl,
C;-C4-hydroxyalkyl, C1-C4-haloalkyl, C1-C4-alkoxy,
C1-C4-haloalkoxy or C1-C4-alkylthio, or CRlo.forms together
OU'rJ~/50031 CA 02375666 2001-11-30
6
with CRll a 5- or 6-membered alkylene or alkenylene ring which
may be substituted by one or two C1-C4-alkyl groups and in
which in each case one or more methylene groups can be
replaced by oxygen, sulfur, -NH or -N(C1-C4-alkyl);
phenyl which may carry one to three of the following
radicals: C1-C4-alkyl, C1-C4-haloalkyl, C1-C4-alkoxy,
C1-CQ-haloalkoxy, Cl-C4-alkylthio.
R11 hydrogen, hydroxyl, NHZ, NH(Cl-C4-alkyl), N(C1-C4-alkyl)Z,
halogen, C1-C4-alkyl, C2-CQ-alkenyl, C2-C4-alkynyl,
C3-Cs-alkenyloxy, C1-C4-alkylcarbonyl, C1-C4-alkoxycarbonyl,
C1-C4-hydroxyalkyl, C1-CQ-haloalkyl, Cl-C4-alkoxy,
C1-CQ-haloalkoxy, -NH-0-C1-C4-alkyl, Cl-C4-alkylthio,
C3-Ce-cycloalkyl or CR11 forms together with CRl~ and as
~.__, indicated for Rl~ a 5- or 6-membered ring;
phenyl or phenoxy, each of which may carry one to five
halogen atoms and/or one to three of the following radicals:
hydroxyl, mercapto, carboxyl, nitro, cyano, C1-CQ-alkyl,
Cl-C4-haloalkyl, C1-C4-alkoxy, C3-C6-alkenyloxy,
C3-C6-alkynyloxy, C1-C4-alkylthio, C1-C4-haloalkoxy,
C1-C4-alkylcarbonyl, R9, C1-C4-alkoxycarbonyl,
(C1-C4-alkyl)NHcarbonyl, (C1-CQ-alkyl)2Ncarbonyl,
C3-C8-alkylcarbonylalkyl, amino. NH(C1-C4-alkyl),
N(C1-C4-alkyl)a, phenoxy or phenyl, it being possible for said.
aryl radicals in turn to be substituted once to three times
by halogen, C1-C4-alkyl, Cl-C4-haloalkyl, C1-C4-alkoxy,
C1-C4-haloalkoxy, methylenedioxy, ethylenedioxy,
Cl-C4-alkylthio:
.;
a five- or six-membered heteroaromatic system which contains
one to three nitrogen atoms and/or one sulfur or oxygen atom
and which may carry one to four halogen atoms and/or one to
two of the following radicals: C1-C4-alkyl, C1-C4-haloalkyl,
C1-C4-alkoxy, C1-C4-haloalkoxy, C1-C4-alkylthio, phenyl,
phenoxy or phenylcarbonyl, it being possible f or the phenyl
radicals in turn to carry one to five halogen atoms and/or
one to three of the following radicals: C1-CQ-alkyl,
C1-C4-haloalkyl, Cl-C4-alkoxy, C1-CQ-haloalkoxy and/or
C1-CQ-alkylthio.
A sulfur or oxygen.
The following definitions apply in this connection and
hereinaf ter
0050/50031 CA 02375666 2001-11-30
7
an alkali metal is, for example, lithium, sodium, potassium;
an alkaline earth metal is, for example, calcium, magnesium,
barium;
organic ammonium ions are protonated amines such as. for example,
ethanolamine, diethanolamine, ethylenediamine, diethylamine or
piperazine;
C3-CB-cycloalkyl is, for example, cyclopropyl, cyclobutyl,
cyclopentyl, cyclohexyl or cycloheptyl;
C1-C4-haloalkyl can be linear or branched, such as, for example,
fluoromethyl, difluoromethyl, trifluoromethyl,
chlorodifluoromethyl, dichlorofluoromethyl, trichloromethyl,
_. 1-fluoroethyl, 2-fluoroethyl, 2,2-difluoroethyl,
2,2,2-trifluoroethyl, 2-chloro-2,2-difluoroethyl,
2,2-dichloro-2-fluoroethyl, 2,2,2-trichloroethyl or
pentafluoroethyl;
C1-C4-haloalkoxy can be linear or branched, such as, for example,
difluoromethoxy, trifluoromethoxy, chlorodifluoromethoxy,
1-fluoroethoxy, 2,2-difluoroethoxy, 1,1,2,2-tetraf luoroethoxy,
2,2,2-trifluoroethoxy, 2-chloro-1,1,2-trifluoroethoxy,
2-fluoroethoxy or pentafluoroethoxy;
C1-C4-alkyl can be linear or branched, such as, for example,
methyl, ethyl, 1-propyl, 2-propyl, 2-methyl-2-propyl,
2-methyl-1-propyl, 1-butyl or 2-butyl;
C2-C4-alkenyl can be linear or branched, such as, for example,
ethenyl, 1-proper-3-yl, 1-proper-2-yl, 1-proper-1-yl,
2-methyl-1-propenyl, 1-butenyl or 2-butenyl;
C2-C4-alkynyl can be linear or branched, such as, for example,
ethynyl, 1-propyn-1-yl, 1-propyn-3-yl, 1-butyn-4-yl or
2-butyn-4-yl;
C1-C4-alkoxy can be linear or branched, such as, for example,
methoxy, ethoxy, propoxy, 1-methylethoxy, butoxy,
1-methylpropoxy, 2-methylpropoxy or 1,1-dimethylethoxy;
C3-C6-alkenyloxy can be linear or branched, such as. for example,
allyloxy, 2-buten-1-yloxy or 3-buten-2-yloxy;
CA 02375666 2001-11-30
8
C3-C6-alkynyloxy can be linear or branched, such as, for example,
2-propyn-1-yloxy, 2-butyn-1-yloxy or 3-butyn-2-yloxy;
C1-C4-alkylthio can be linear or branched, such as, for example,
methylthio, ethylthio, propylthio, 1-methylethylthio, butylthio,
1-methylpropylthio, 2-methylpropylthio or 1,1-dimethylethylthio;
Cl-C4-alkylcarbonyl can be linear or branched, such as, for
example, acetyl, ethylcarbonyl or 2-propylcarbonyl;
C1-C4-alkoxycarbonyl can be linear or branched, such as. for
example, methoxycarbonyl, ethoxycarbonyl, n-propoxycarbonyl,
i-propoxycarbonyl or n-butoxycarbonyl;
C3-Ce-alkylcarbonylalkyl can be linear or branched, for example
-- 2-oxoprop-1-yl, 3-oxobut-1-yl or 3-oxobut-2-yl;
C1-CB-alkyl can be linear or branched, such as, for example,
C1-C4-alkyl, pentyl, hexyl, heptyl or octyl;
ao
halogen is, for example, fluorine, chlorine, bromine. iodine.
The invention further relates to compounds from which the
compounds of the formula I can be liberated (called prodrugs),
for example amides of the acids embraced by formula I.
Preferred prodrugs are those with which the liberation takes
place under conditions like those prevailing in particular
compartments of the body, for example in the stomach, intestine,
bloodstream, liver.
The compounds, and.the intermediates for preparing them, such as,
for example, II and IV, may have one or more asymmetric
substituted carbon atoms. Compounds of this type may be in the
form of pure enantiomers or pure diastereomers or a mixture
thereof. The use of an enantiomerically pure compound as active
ingredient is preferred.
The invention further relates to the use of the abovementioned
carboxylic acid derivatives for producing drugs, in particular
for producing inhibitors for endothelin receptors.
Compounds of the general formula IV in which Z is sulfur or
oxygen (IV) can be prepared as described in WO 96/11914.
0050/50031 CA 02375666 2001-11-30
9
R4 0 Ri R4 H
+ R3-Z-H ~ R3-A~C OH
R5 R5 Ri
II III IV
Compounds of the general formula III either are known or can be
synthesized, for example, by reducing the corresponding
carboxylic acids or esters thereof, or by other generally known
methods.
Compounds of the formula IV can be obtained in enantiomerically
pure form by acid-catalyzed transesterification as described in
WO 98/09953.
It is also possible to obtain enantiomerically pure compounds of
the formula IV by carrying out a conventional racemate resolution
of racemic or diastereomeric compounds of the formula IV using
suitable enantiomerically pure bases. Examples of suitable bases
of this type are 4-chlorophenylethylamine and the bases mentioned
in WO 96/11914.
The compounds according to the invention in which the
substituents have the meaning indicated for the general formula I
can be prepared, for example, by reacting the carboxylic acid
derivatives of the general formula IV in which the substituents
have the stated meaning with compounds of the general formula V.
X-Q
w. . IV + Ria ~/ 'Z ~ I
Y =-~
R~
V
R12 in formula V is halogen or R13-SOz where R13 Can be
C1-C4-alkyl, C1-C4-haloalkyl or phenyl. The reaction preferably
takes place in an inert solvent or diluent with the addition of a
suitable base, i.e. a base which deprotonates the intermediate
IV, at a temperature in the range from room temperature to the
boiling point of the solvent.
If R1 is an ester, the compounds with R1 = COON can be prepared by
acidic, basic or catalytic cleavage of the ester group.
0050/50031 CA 02375666 2001-11-30
' Compounds of type I with R1 = COOH can also be obtained directly
by deprotonating the intermediate IV in which Rl is COOH with two
equivalents of a suitable base, and reacting with compounds of
the general formula V. This reaction also takes place in an inert
5 solvent and at a temperature in the range from room temperature
to the boiling point of the solvent.
Examples of such solvents or diluents are aliphatic, alicyclic
and aromatic hydrocarbons, each of which may optionally by
10 chlorinated, such as, for example, hexane, cyclohexane, petroleum
ether, naphtha, benzene, toluene, xylene, methylene chloride,
chloroform, carbon tetrachloride, ethyl chloride and
trichloroethylene, ethers such as, for example, diisopropyl
ether, dibutyl ether, methyl tert-butyl ether, propylene oxide,
dioxane and tetrahydrofuran, nitriles such as, for example,
f,.. acetonitrile and propionitrile, amides such as, for example,
dimethylformamide, dimethylacetamide and N-methylpyrrolidone,
sulfoxides and sulfones such as, for example, dimethyl sulfoxide
and sulfolane.
Compounds of the formula v are known, some of them can be bought,
or they can be prepared in a generally known manner.
It is possible to use as base an alkali metal or alkaline earth
metal hydride such as sodium hydride, potassium hydride or
calcium hydride, a carbonate such as alkali metal carbonate, for
example sodium or potassium carbonate, an alkali metal or
alkaline earth metal hydroxide such as sodium or potassium
hydroxide, an organometallic compound such as butyllithium or an
alkali metal amide such as lithium diisopropylamide.
i
Compounds of the formula I can also be prepared by starting from
the corresponding carboxylic acids, i.e. compounds of the formula
I in which R1 is COON, and initially converting these in a
conventional way into an activated form such as an acid halide,
anhydride or imidazolide, and then reacting this with an
appropriate hydroxyl compound HORS. This reaction can be carried
out in the conventional solvents and of ten requires addition of a
base such as; for example, triethylamine, pyridine, imidazole or
diazabicycloundecene. These two steps can also be simplified, for
example, by allowing the carboxylic acid to act in the presence
of a dehydrating agent such as a carbodiimide on the hydroxyl
compound.
It is also possible to prepare compounds of the formula I by
starting from salts of the corresponding carboxylic acids, i.e.
from compounds of the formula I in which R1 is a COOM group, where
0050/50031 CA 02375666 2001-11-30
11
M can be an alkali metal cation or the equivalent of an alkaline
earth metal cation. These salts can be reacted with many
compounds of the formula R1-D where D is a conventional
nucleofugic leaving group, for example halogen such as chlorine,
bromine, iodine or optionally halogen-, alkyl- or
haloalkyl-substituted aryl- or alkylsulfonyl such as, for
example, toluenesulfonyl and methylsulfonyl or another equivalent
leaving group. Compounds of the formula R1-D with a reactive
substituent D are known or can easily be obtained with general
expert knowledge. This reaction can be carried out in
conventional solvents and is advantageously effected with
addition of a base, in which case those mentioned above are
suitable.
In a few cases it is necessary to use generally known protective
--- group techniques to prepare the compounds I according to the
invention. If, for example, R6 = 4-hydroxyphenyl, the hydroxyl
group can initially be protected as the benzyl ether, which is
then cleaved at a suitable stage in the reaction sequence.
ao
Compounds of the formula I in which Rl is tetrazolyl can be
prepared as described in WO 96/11914. Further possibilities are
mentioned, for example, in: Synthesis, 767 (1993); J. Org. chem.
~, 2395 (1991) .
a5
With a view to the biological effect, preferred carboxylic acid
derivatives of the general formula I - both as pure enantiomers
and pure diastereomers or as mixtures thereof - are those in
which the substituents have the following meanings:
Rz phenyl or phenoxy, each of which 'may carry one to three of
the following radicals: halogen, C1-C4-alkyl, C1-C4-haloalkyl,
C1-C4-alkoxy, C1-C4-alkylthio, Cl-C4-haloalkoxy,
C1-C4-alkylcarbonyl, Cy-C4-alkoxycarbonyl,
(C1-C4-alkyl)-NHcarbonyl, (Cl-C4-alkyl)2Ncarbonyl,
NH(C1-C4-alkyl), N(C1-C4-alkyl)2;
a five- or six-membered heteroaromatic system which contains
one nitrogen atom and%or one sulfur or oxygen atom and which
may carry one tv two of the following radicals: halogen,
C1-C4-alkyl, C1-Ca-haloalkyl, C1-C4-alkoxy, C1-C4-haloalkoxy,
C1-Ca-alkylthio, phenyl which in turn may carry one to three
of the following radicals: halogen, C1-C4-alkyl,
C1-C4-haloalkyl, C1-C4-alkoxy, C1-C4-haloalkoxy,
C1-C4-alkylthio;
005~~50031 CA 02375666 2001-11-30
12
CS-C6-cycloalkyl.
R3 hydrogen;
C1-C8-alkyl, C3-C6-alkenyl, C3-C6-alkynyl or C3-Ce-cycloalkyl,
it being possible for each of these radicals to be
substituted one or more times by: hydroxyl, halogen,
C1-C4-alkoxy, C1-CQ-alkylthio, C1-C4-haloalkoxy,
C1-CQ-alkylcarbonyl, C1-C4-alkoxycarbonyl,
(C1-C4-alkyl)NHcarbonyl, (C1-C4-alkyl)ZNcarbonyl, amino,
NH(Cl-CQ-alkyl), N(Cl-CQ-alkyl)2, phenoxy or phenyl, hetaryl,
five- or six-membered, containing one to three nitrogen atoms
and/or one sulfur or oxygen atom, it being possible for said
aryl and hetaryl radicals to be substituted once to three
(--. 15 times by: halogen, C1-C4-alkyl, C1-C4-haloalkyl, Cl-C4-alkoxy,
._ C1-C4-haloalkoxy, R9, Cl-C4-alkoxycarbonyl, NH(Cl-C4-alkyl),
N(C1-C4-alkyl)2, methylenedioxy, ethylenedioxy,
C1-C4-alkylthio;
phenyl or naphthyl, each of which can be substituted by one
or more of the following radicals: halogen, C1-C4-alkyl,
C1-C4-haloalkyl, C1-C4-alkoxy, Cl-C4-haloalkoxy,
C1-CQ-alkylthio, NH(C1-C4-alkyl), N(Cy-Ca-alkyl)2,
methylenedioxy or ethylenedioxy;
a five- or six-membered heteroaromatic system which contains
one to three nitrogen atoms and/or one sulfur or oxygen atom
and which may carry one to two of the following radicals:
. C1-C4-alkyl, C1-C4-haloalkyl, C1-C4-alkoxy, C1-C4-haloalkoxy,
C1-C4-alkylthio, phenyl which may in turn carry one to five
_ ' halogen atoms and/or one to three of the following radicals:
Cz-Ca-alkyl, C1-C4-haloalkyl, Cl-C4-alkoxy, C1-CQ-haloalkoxy,
C1-C4-alkylthio.
R4 and R5 (which may be identical or different):
phenyl or naphthyl, each of which can be substituted by one
or more of the following radicals: halogen, C1-C4-alkyl,
C1-CQ-haloalkyl, C1-C4-alkoxy, C1-C4-haloalkoxy,
C1-C4-alkylthio; or
phenyl or naphthyl which are linked together in ortho
positions by a direct linkage, a methylene, ethylene or
ethenylene group; or
cyclohexyl.
005/50031 CA 02375666 2001-11-30
13
X and Y (which may be identical or different):
nitrogen or methine; with the proviso that if X = Y = methine
then Z = nitrogen.
Z nitrogen or CRla; with the proviso that if Z = nitrogen then
Q = CR11.
Q nitrogen or CRli,
R9 C1-C4-alkyl, C1-C4-alkylthio, C1-C4-alkoxy, which carry one of
the following radicals: hydroxyl, carbamoyl or
CON(C1-C4-alkyl)2.
Rl~ hydrogen, halogen, C1-C4-alkyl, C1-C4-haloalkyl, C1-CQ-alkoxy,
C1-CQ-haloalkoxy or C1-C4-alkylthio, or CRla forms together
..__. with CR11 a 5- or 6-membered alkylene or alkenylene ring which
may be substituted by one or two methyl groups and in which
in each case one or more methylene groups can be replaced by
oxygen or sulfur.
R11 hydrogen, NFi(C1-C4-alkyl), N(C1-C4-alkyl)2, C1-C4-alkyl,
C1-C4-haloalkyl, C1-C4-alkoxy, C1-C4-haloalkoxy,
C1-C4-alkylthio, C5-C6-cycloalkyl or CR11 forms together with
CRlo as indicated under Rlo a 5- or 6-membered ring;
.
phenyl or phenoxy, each of which may carry one to three of
the following radicals: halogen, CI-CQ-alkoxy,
' C1-C4-alkylthio, C1-C4-haloalkoxy, C1-CQ-alkylcarbonyl,
C1-C4-alkoxycarbonyl, (C1-C4-alkyl)NHcarbonyl,
(C1-C4-alkyl)2Ncarbonyl, NH(C1-C4-alkyl), N(C1-C4-alkyl)Z;
a five- or six-membered heteroaromatic system which contains
one nitrogen atom and/or one sulfur or oxygen atom and which
may carry one to two of the following radicals: halogen,
C1-C4-alkyl, C1-CQ-haloalkyl, C1-C4-alkoxy, C1-C4-haloalkoxy,
C1-CQ-alkylthio, phenyl which may in turn carry one to three
of the following radicals: halogen, Cl-C4-alkyl,
C1-CQ-haloalkyl, C1-C4-alkoxy, Cl-C4-haloalkoxy,
C1-C4-alkylthio.
A sulfur or oxygen.
Particularly preferred compounds of the formula I - both as pure
enantiomers and pure diastereomers or as mixtures thereof - are
those in which the substituents have the following meanings:
X050/50031 CA 02375666 2001-11-30
14
R2 phenyl or phenoxy, each of which may carry one to three of
the following radicals: halogen, C1-C4-alkyl, C1-C4-haloalkyl,
C1-CQ-alkoxy, C1-C4-alkylthio, C1-C4-haloalkoxy;
- a five- or six-membered heteroaromatic system which contains
one nitrogen atom and/or one sulfur or~oxygen atom and which
may carry one to two of the following radicals: halogen,
C1-CQ-alkyl, C1-C4-haloalkyl, C1-C4-alkoxy, C1-Ca-haloalkoxy,
C1-C4-alkylthio;
CS-C6-cycloalkyl.
R3 hydrogen;
C1-Ce-alkyl or C3-C6-cycloalkyl, it being possible for each of
~- these radicals to be substituted once to three times by:
hydroxyl, halogen, C1-C4-alkoxy, C1-C4-alkylthio,
C1-C4-haloalkoxy, phenoxy, phenyl, hetaryl, five- or
six-membered, containing one nitrogen atom and/or one sulfur
. or oxygen atom, it being possible for said aryl and hetaryl
radicals to be substituted once to three times by: halogen,
C1-C4-alkyl, Cl-C4-haloalkyl, C1-C4-alkoxy, C1-C4-haloalkoxy,
R9, methylenedioxy, ethylenedioxy, Cl-CQ-alkylthio;
phenyl or naphthyl, each of which may be substituted by one
or more of the following radicals: halogen, Cl-C4-alkyl,
C1-C4-haloalkyl, C1-C4-alkoxy, C1-C4-haloalkoxy,
C1-C4-alkylthio, methylenedioxy or ethylenedioxy;
a five-membered heteroaromatic system which contains one
sulfur or oxygen atom and which may carry one to two of the
following radicals: C1-C4-alkyl, C1-CQ-haloalkyl,
C1-C4-alkoxy, C1-C4-haloalkoxy, C1-C4-alkylthio.
R4 and R5 (which may be identical or different):
phenyl or naphthyl, each of which may be substituted by one
or more of the following radicals: halogen, C1-C4-alkyl,
C1-C4-haloalkyl, C1-C4-alkoxy, C1-C4-haloalkoxy,
C1-CQ-alkylthio; or
phenyl or naphthyl which are connected in ortho positions by
a direct linkage or a methylene group.
0050/50031 CA 02375666 2001-11-30
X and Y (which may be identical or different):
nitrogen or methine; with the proviso that if X = Y = methine
then Z = nitrogen;
5 Z nitrogen or CRlo
Q CR11.
R9 Cl-C4-alkyl, C1-C4-alkylthio, Cl-C4-alkoxy, each of which may
10 carry a hydroxyl group.
Rlo hydrogen, halogen, methyl, trifluoromethyl, methoxy, or CRlo
forms together with CR11 a 5- or 6-membered alkylene ring
which may be substituted by one or two methyl groups and in
15 which in each case one or more methylene groups may be
-. replaced by oxygen or sulfur.
R11 hydrogen, Cl-CQ-alkyl, C1-C4-haloalkyl, C1-C4-alkoxy,
C1-CQ-haloalkoxy, C1-C4-alkylthio, C5-C6-cycloalkyl or CRli
forms together with CRlo as indicated under Rlo a 5- or
6-membered ring.
A sulfur or oxygen.
The compounds of the present invention offer a novel potential
treatment of hypertension, pulmonary hypertension, myocardial
infarct, angina pectoris, arrhythmia, acute/chronic kidney
failure, chronic heart failure, renal insufficiency, cerebral
vasospasms, cerebral ischemia, subarachnoid hemorrhages,
migraine, asthma, atherosclerosis, endotoxic shock,
endotoxin-induced organ failure, intravascular coagulation,
restenosis of ter angioplasty and bypass operations, benign
prostate hyperplasia, erectile dysfunction, glaucoma, ischemic
and intoxication-induced kidney failure or hypertension,
metastasis and growth of mesenchymal tumors, contrast
agent-induced kidney failure, pancreatitis, gastrointestinal
ulcers.
The invention further relates to combinations of endothelin
receptor antagonists of the formula I and inhibitors of the
renin-angiotensin system. Inhibitors of the renin-angiotensin
system are renin inhibitors, angiotensin II antagonists and
angiotensin converting enzyme (ACE) inhibitors. Combinations of
endothelin receptor antagonists of the formula I and ACE
inhibitors are preferred.
0050/50031 CA 02375666 2001-11-30
16
The invention further relates to combinations of endothelia
receptor antagonists of the formula I and beta blockers.
The invention further relates to combinations of endothelia
receptor antagonists of the formula I and diuretics.
The invention further relates to combinations of endothelia
receptor antagonists of the formula I and substances which block
the action of VEGF (vascular endothelial growth f actor). Examples
of such substances are antibodies directed against VEGF or
specific binding proteins or else low molecular weight substances
capable of specific inhibition of VEGF release or receptor
binding.
The aforementioned combinations can be administered concurrently
or sequentially. They can be employed either in a single
pharmaceutical formulation or else in separate formulations. The
administration form may also differ; for example, the endothelia
receptor antagonists can be administered orally, and VEGF
inhibitors can be administered parenterally.
These combination products are particularly suitable for treating
and preventing hypertension and its. sequelae, and for treating
heart failure.
The good effect of the compounds can be shown in the following
tests:
Receptor-binding studies
'., ~ Cloned human ETA or ETB receptor-expressing CHO cells were
employed for binding studies.
Membrane preparation
The ETA or ETB receptor-expressing CHO cells were grown in DMEM
NUT MIX Fla medium (Gibco, No. 21331-020) with 10% fetal calf
serum (PAA Laboratories GmbH, Linz, No. A15-022), 1 mM glutamine
(Gibco No. 25030-024), 100 U/ml penicillin and 100 ~,g/ml
streptomycin (Sigma No. P-0781). After 48 hours, the cells were
washed with PBS and incubated with 0.05% trypsin-containing PBS
at 37~C for 5 minutes. This was followed by neutralization with
medium, and the cells were collected by centrifugation at
300 x g.
' UUS~/50031 CA 02375666 2001-11-30
17
For membrane preparation, the cells were adjusted to a
concentration of 10a cells/ml of buffer (50 mM Tris~HCl buffer,.
pH 7.4) and then disintegrated with ultrasound (Branson Sonifier
250, 40-70 seconds/constant output 20).
Binding assays
For the ETA and ETB receptor-binding assay, the membranes were
suspended in incubation buffer (50 mM Tris-HC1, pH 7.4 with 5 mM
MnClz, 40 mg/ml bacitracin and 0.2% BSA) in a concentration of
50 ~,g of protein per assay mixture and incubated with 25 pM
[lzsI~ -ET1 (ETA receptor assay) or 25 pM [l2sl~ _ET3 (ETB receptor
assay) in the presence and absence of test substance at 25°C. The
nonspecific binding was determined using 10-~ M ETl. After 30 min,
filtration through GF/B glass fiber filters (Whatman, England) in
a Skatron cell harvester (Skatron, Lier, Norway) separated free
and bound radioligand, and the filters were washed with ice-cold
Tris-HC1 buffer, pH 7.4 with 0.2% BSA. The radioactivity
collected on the filters was quantified using a Packard 2200 CA
liquid scintillation counter.
Functional test on vessels for endothelia receptor antagonists
Segments of rabbit aorta are, after an initial tension of 2 g and
a relaxation time of 1 h in Krebs-Henseleit solution at 37°C and
pH 7.3-7.4, first induced to contract with K+. After washing out,
an endothelia dose-effect plot up to the maximum is constructed.
Potential endothelia antagonists are administered to other
preparations of the same vessel 15 min before starting the
endothelia dose-effect plot. The effects of the endothelia are
calculated as percent of the K+-induced contraction. Effective
endothelia antagonists result in a shift to the right in the
endothelia dose-effect plot.
Testing of ET antagonists in vivo:
Male SD rats weighing 250 - 300 g were anesthetized with
amobarbital, artificially ventilated, vagotomized and pithed. The
carotid artery and jugular vein were catheterized.
In control animals, intravenous administration of 1 ~tg/kg ET1
results in a marked rise in blood pressure which persists for a
lengthy period.
X050/50031 CA 02375666 2001-11-30
18
The test animals received i.v. injection (1 ml/kg) of the test
compounds 30 min before administration of ET1. To determine the
ET-antagonistic properties, the changes in blood pressure in the
test animals were compared with those in the control animals.
Oral testing of mixed ETp, and ETB antagonists:
Male normotensive rats (Sprague Dawley, Janvier) weighing
250-350 g are pretreated with the test substances orally. 80
minutes later, the animals are anesthetized with urethane, and
the carotid artery (for measuring the blood pressure) and the
jugular vein (administration of big endothelin/endothelin 1) are
catheterized.
After a stabilization period, big endothelia (20 ~,g/kg, admin.
vol. 0.5 ml/kg) or ET1 (0.3 ~g/kg, adman. vol. 0.5 ml/kg) is
given intravenously. Blood pressure and heart rate are recorded
continuously for 30 minutes. The marked and long-lasting changes
in blood pressure are calculated as the area under the curve
(AUC). To determine the antagonistic effect of the test
substances, the AUC for the animals treated with substance is
compared with the AUC for the control animals.
The novel compounds can be administered orally or parenterally
(subcutaneously, intravenously, intramuscularly,
intraperitoneally) in a conventional way. Administration can also
take place with vapors or sprays through the nasopharyngeal
space.
The dosage depends on the age, condition and weight of the
patient and on the mode of administration. As a rule, the daily
dose of active ingredient is from about 0.5 to 50 mg/kg of body
weight on oral administration and from about 0.1 to 10 mg/kg of
body weight on parenteral administration.
The novel compounds can be administered in conventional solid or
liquid pharmaceutical forms, e.g. as uncoated or (film-)coated
tablets, capsules, powders, granules, suppositories, solutions,
ointments, creams or sprays. These are produced in a conventional
way. The active ingredients can for this purpose be processed
with conventional pharmaceutical aids such as tablet binders,
bulking agents, preservatives, tablet disintegrants, flow
regulators, plasticizers, wetting agents, dispersants,
emulsifiers, solvents, release-slowing agents, antioxidants
and/or propellant gases (cf. H. Sucker et al.: Pharmazeutische
Technologie, Thieme-Verlag, Stuttgart, 1991). The administration
0050/50031 CA 02375666 2001-11-30
19
forms obtained in this way normally contain from 0.1 to 90% by
weight of active ingredient.
Synthesis examples:
Example 1:
4-Methyl-2-methylsulfanyl-6-phenylpyrimidine
1 molar sodium hydroxide solution (4.10 ml; 4.10 mmol) and
iodomethane (86 ~,1; 194 mg: 1.37 mmol) were successively added to
a solution of 4-methyl-6-phenylpyrimidine-2-thiol (300 mg;
1.37 mmol with 92% purity; synthesized by the method of D.J.
Brown et al., Aust. J. Chem. 1984, 37, 155) in methanol (15 ml).
The mixture was stirred at room temperature for 16 hours; it was
then diluted with water (50 ml) and acidified with hydrochloric
acid. The mixture was extracted with ethyl acetate (3x), the
combined organic phases were dried over magnesium sulfate, and
the solvent was removed in vacuo. 290 mg (1.24 mmol, 91% yield,
HPLC purity 93%) of the required pyrimidine were obtained.
Example 2:
2-Methanesulfonyl-4-methyl-6-phenylpyrimidine
Oxone~ (Aldrich; 973 mg; 1.58 mmol) and 4 molar sodium hydroxide
solution (0.85 ml; 3.33 mmol) were simultaneously added to a
solution of 4-methyl-2-methylsulfanyl-6-phenylpyrimidine (278 mg,
1.19 mmol with 93% purity) in methanol (10 ml) and water (10 ml)
while cooling in ice in such a way that the pH was always pH 2-3.
The mixture was then stirred at room temperature for 16 h, and
the reaction was then stopped by dilution with water (75 ml). The
mixture was extracted with ether (2x) and then with ethyl acetate
(lx); the combined organic phases were dried over magnesium
sulfate, and the solvent was removed in vacuo. 290 mg (1.11 mmol,
93% yield, HPLC purity 95%) of the~required sulfonylpyrimidine
were obtained; it was used further without further purification.
Example 3:
3-Ethoxy-2-(4-methyl-6-phenylpyrimidin-2-yloxy)-3,3-diphenyl-
propionic acid (I-180)
50% sodium hydride (72 mg, 1.51 mmol) and then a solution of
3-ethoxy-2-hydroxy-3,3-diphenylpropionic acid (150 mg, 0.50 mmol;
synthesis described in WO 96/11914) in dimethyl formamide were
added to a solution of 2-methanesulfonyl-4-methyl-6-phenyl-
" CA 02375666 2001-11-30
0050/50031
pyrimidine (145 mg; 0.55 mmol with 95% purity) in anhydrous
dimethylformamide (10 ml), and the mixture was stirred at room
temperature. After 3 hours, tire sulfonylpyrimidine was completely
consumed but the hydroxy acid had not yet reacted completely, so
5 that a further 30 mg of the sulfonylpyrimidine were added. After
stirring at room temperature for a further hour, the reaction was
virtually complete and it was stopped by adding water.
Acidification with hydrochloric acid was followed by extraction
with ether (3x). The ethereal extracts were extracted with 1 molar
10 potassium hydroxide solution; the aqueous alkaline extracts were
combined and again acidified and extracted three times with
ether. The ethereal extracts resulting from this were dried over
magnesium sulfate and evaporated. The remaining crude product was
purified by flash chromatography on silica gel and isolated by
15 freeze drying. 47 mg of the target compound (0.09 mmol; 19%
yield) were obtained.
1H-NMR (200 MHz, CDC13): 7.9-8.1 (m, 2 H); 7.1-7.7 (m, 14 H); 6.5
(s, 1 H) ; 3.3-3 .7 (m, 2 H) ; 2.5 (s, 3 H) ; 1.2 (t, 3 H) .
ESI-MS: M+ = 454.
Example 4:
3-Methoxy-2-(4-methyl-6-phenylpyrimidin-2-yloxy)-3,3-diphenyl-
propionic acid (I-58)
1H-NMR (200 MHz, CDC13): 7.9-8.1 (m, 2 H); 7.2-7.7 (m, 14 H); 6.4
(s, 1 H) ; 3.3 (s, 3 H) ; 2 .5 (s, 3 H) .
ESI-MS: M+ = 440.
Example 5:
2-Methylsulfanyl-6-phenylpyrimidin-4-of
1 molar sodium hydroxide solution (15.0 ml; 15.0 mmol) and
iodomethane (0.33 ml; 735 mg; 5.18 mmol) Were successively added
to a solution of 2-mercapto-6-phenylpyrimidin-4-of (1.06 g;
5.18 mmol; synthesized by the method of H.I. Skulnick et al., J.
Med. Chem. 1986, 29 (8), 1499) in methanol (10 ml). The mixture
was stirred at room temperature for 30 minutes; it was then
diluted with water (100 ml) and acidified with hydrochloric acid.
The mixture was extracted with ethyl acetate (3x), the combined
organic phases were dried over magnesium sulfate, and the solvent
0050/50031
CA 02375666 2001-11-30
21
was removed in vacuo. 750 mg (3.44 mmol, 66% yield) of the.
required product were obtained.
Example 6:
4-Chloro-2-methylsulfanyl-6-phenylpyrimidine
A solution of 2-methylsulfanyl-6-phenylpyrimidin-4-of (854 mg;
3.91 mmol) in phosphorus oxychloride (10.0 ml) was heated with
stirring to 80°C and stirred at this temperature for 2 hours.
After cooling, the phosphorus oxychloride was removed in vacuo;
the residue was taken up in ethyl acetate and washed with water
(3x). The organic phase was dried over magnesium sulfate, and the
solvent was evaporated off in vacuo. Evaporation once again with
toluene afforded the required chloropyrimidine in 95% purity
(950 mg; 3.81 mmol; 97% yield).
Example 7:
4-Methoxy-2-methylsulfanyl-6-phenylpyrimidine
30% strength methanolic sodium methanolate solution (4.50 ml) was
added to a mixture of 4-chloro-2-methylsulfanyl-6-phenyl-
pyrimidine (950 mg, 3.81 mmol with 95% purity) in anhydrous
methanol (15 ml) under a nitrogen atmosphere. The resulting
mixture was heated to boiling and refluxed at this temperature
for 90 minutes. It was then stirred at room temperature for 16
hours, and the solvent was evaporated off. The residue was mixed
with water and extracted with ethyl acetate (3x), The organic
phase was dried over magnesium~sulfate and evaporated. The
required compound was obtained in the form of yellow crystals
(854 mg; 3.60 mmol; 94%) .
Example 8:
2-Methanesulfonyl-4-methoxy-6-phenylpyrimidine
Oxone~ (Aldrich; 3.32 g; 5.40 mmol) and 4 molar sodium hydroxide
solution (2.50 ml: 10.0 mmol) were simultaneously added to a
solution of 4-methoxy-2-methylsulfanyl-6-phenylpyrimidine
(854 mg, 3.60 mmol) in methanol (15 ml) and water (15 ml) while
cooling in ice in such a way that the pH was always at pH 2-3.
The mixture was then stirred at room temperature for 16 h, and
the reaction was then stopped by dilution with water (75 ml). The
mixture was extracted with ethyl acetate (2x); the combined
organic phases were dried over magnesium sulfate, and the solvent
was removed in vacuo. 928 mg (3.17 mmol, 88% yield, HPLC purity
CA 02375666 2001-11-30
22
90%) of the required sulfonylpyrimidine were obtained; it was
used further without further purification.
Example 9:
3-Methoxy-2-(4-methoxy-6-phenylpyrimidin-2-yloxy)-3,3-diphenyl-
propionic acid (I-64)
50% sodium hydride (106 mg; 2.20 mmol) was added to a stirred
solution of 3-methoxy-2-hydroxy-3,3-diphenylpropionic acid
(200 mg; 0.73 mmol; synthesis described in WO 96/11914) in
anhydrous dimethylformamide (10 ml) while cooling in ice under a
nitrogen atmosphere. After 10 minutes,
2-methanesulfonyl-4-methoxy-6-phenylpyrimidine (320 mg; 1.09 mmol
with 90% purity) dissolved in a little dimethylformamide was
.._ added. The cooling bath was removed, and the mixture was stirred
at room temperature for 16 hours. The reaction was then stopped
by cautious addition of water, followed by acidification with
hydrochloric acid and extraction with ether (3x). The ethereal
extracts were extracted with 1 molar potassium hydroxide
solution; the aqueous alkaline extracts were combined and again
acidified and extracted three times with ether. The ethereal
extracts obtained thereby were dried over magnesium sulfate and,
after addition of some hexane, evaporated at low temperature.
317 mg (0.67 mmol; 92% yield) of the target compound were
obtained.
1H-NMR (200 MHz, CDC13): 7.9-8.1 (m, 2 H); 7.2-7.6 (m, 13 H); 6.8
(s, 1 H); 6.3 (s, 1 H); 3.9 (s, 3 H); 3.3 (s, 3 H).
Melting point: 110-115°C.
Example 10:
3-Ethoxy-2-(4-methoxy-6-phenylpyrimidin-2-yloxy)-3,3-diphenyl-
propionic acid (I-95)
Example 11:
3-Methoxy-2-[4-methoxy-6-(4-trifluoromethylphenyl)pyrimidin-2-
yloxy]-3,3-diphenylpropionic acid (I-6)
1H-NMR (200 MHz, ds-DMSO): 8.3 (d, 2 H); 7.8 (d, 2 H); 7.4 (m app
t, 4 H) ; 7, 1 (s, 1 H) ; 7. 0-7. 3 (m, 6 H) ; 6 .3 (s, 1 H) ; 3. 9 (s,
3 H) ; 3 .4 (s, 3 H) .
CA 02375666 2001-11-30
23
ESI-MS: M+ = 524.
Example 12:
3-Ethoxy-2-[4-methoxy-6-(4-trifluoromethylphenyl)pyrimidin-2-
yloxy]-3,3-diphenylpropionic acid (I-159)
1H-l~tR (200 MHz, CDC13) : 8. 0 (d, 2 H) ; 7.7 (d, 2 H) ; 7.6 (m, 2 H) ;
7.2-7.5 (m, 8 H); 6.8 (s, 1 H); 6.4 (s, 1 H); 4.0 (s, 3 H); 3.5
(mc, 2 H) ; 1.3 (t, 3 H) .
ESI-MS: M'* = 538.
Example 13:
2-[4-(4-Isopropylphenyl)-6-methoxypyrimidin-2-yloxy]-3-methoxy-
3,3-diphenylpropionic acid (I-87)
1H-NMR (200 MHz, CDC13): 7.9 (d, 2 H); 7.6 (m app d, 2 H); 7.2-7.4
(m, 10 H); 6.8 (s, 1 H); 6.4 (s, 1 H); 3.9 (s, 3 H); 3.3 (s, 3
H); 2.9 (sept, 1 H); 1.3 (d, 6 H).
ESI-MS: M+ = 498.
Example 14:
2,4-Dichloro-6-ethyl-[1,3,5]-triazine
A 2 molar solution of ethylmagnesium chloride in tetrahydrofuran
(100 ml; 200 mmol) was added dropwise over the course of 20
minutes to a solution of cyanuric chloride (23.1 g; 184 mmol) in
anhydrous toluene (200 ml) while cooling in ice under a nitrogen
atmosphere. The temperature gradually rose to 15°C during this;
after completion of the addition, the mixture was stirred at room
temperature for 2 hours. To stop the reaction, water (40 ml) was
very cautiously (NB) added to the mixture and, of ter addition of
solid magnesium sulfate (40 g), it was filtered. The filtrate was
evaporated and the residue was extracted with hexane. Evaporation
of the hexane was followed by purification by flash
chromatography on silica gel; 8.80 g (49.4 mmol; 40% yield) of an
oil were obtained.
Example 15:
2-Chloro-4-ethyl-6-phenyl-[1,3,5]-triazine
~~5~/50031 CA 02375666 2001-11-30
24
A solution of 2,4-dichloro-6-ethyl-[1,3,5]-triazine (1.78 g;
10.0 mmol) in anhydrous dichloromethane (50 ml) was cooled to -10
to -20°C under a nitrogen atmosphere and a 2 molar solution of
phenylmagnesium chloride in tetrahydrofuran (5.50 ml; 11.0 mmol)
was added over the course of 5 minutes. The mixture was allowed
to warm to room temperature and was then stirred at room
temperature for 1 hour. The reaction was stopped by cautious
addition of water (3 ml); this was followed by addition of 3 g of
solid magnesium sulfate. After removal of insolubles by
filtration, the solvent was evaporated off and the remaining oil
was purified by chromatography. 1.35 g of an oil were obtained;
despite chromatography, its HPLC purity was only 66% (4.07 mmol;
41% yield).
Example 16:
Benzyl 3-ethoxy-2-hydroxy-3,3-diphenylpropionate
Absolute ethanol (10.0 ml) and boron trifluoride etherate (3-4
drops) were successively added to a solution of benzyl
3,3-diphenyloxirane-2-carboxylate (10.0 g; 30.3 mmol with 92%
purity) in anhydrous ether (100 ml) while cooling in ice under a
nitrogen atmosphere. The cooling bath was removed, and the
mixture was stirred at room temperature for 2 hours. Washing with
saturated sodium bicarbonate solution and water was followed by
drying over magnesium sulfate and evaporation. The residue was
purified by crystallization from ether/n-hexane; 6.60 g
(17.5 mmol; 58% yield) of the pure hydroxy ester were obtained.
~30 1H-NMR (270 MHz, CDC13): 7.2-7.5 (m, 15 H); 5.2 (d, 1 H); 5.0 (s,
a _ 2 H) ; 3.4 (m, 1 H) ; 3. 2 (m, 1 H) ; 3. 0 (d, 1 H) ; 1.1 (t, 3 H) .
Example 17:
Henzyl 3-ethoxy-2-(4-ethyl-6-phenyl-[1,3,5]-triazin-2-yloxy)-3,3-
diphenylpropionate (I-17)
A solution of benzyl 3-ethoxy-2-hydroxy-3,3-diphenylpropionate
(376 mg; 1.00 mmol) and 2-chloro-4-ethyl-6-phenyl-[1,3,5]-
triazine (497 mg; 1.50 mmol with 66% purity) in anhydrous
dimethylformamide (30 ml) was mixed with potassium carbonate
(276 mg; 2.00 mmol) and stirred at room temperature for 16 hours.
The mixture was diluted with water (150 ml), acidified with
citric acid and extracted with ether (2x). The combined ethereal
extracts were dried over magnesium sulfate, and the solvent was
removed in vacuo; the oily residue was purified by flash
CA 02375666 2001-11-30
0050/50031
chromatography. 431 mg (0.77 mmol, 77% yield) of the pure target
compound were obtained.
Example 18:
5
3-Ethoxy-2-(4-ethyl-6-phenyl-[1,3,5]-triazin-2-yloxy)-3,3-
diphenylpropionic acid (I-102)
A solution of benzyl 3-ethoxy-2-(4-ethyl-6-phenyl-[1,3,5]-
10 triazin-2-yloxy)-3,3-diphenylpropionate (430 mg; 0.77 mmol) in
ethyl acetate (60 ml) was mixed under protective gas with a
palladium/carbon hydrogenation catalyst and then stirred under a
hydrogen atmosphere at room temperature for 3 days. The mixture
was then filtered to remove catalyst and was evaporated.
15 Crystallization of the oily residue from n-hexane afforded 187 mg
(0.40 mmol; 52% yield) of the pure carboxylic acid.
1H-NMR (200 MHz, CDC13): 8.4 (d, 2 H); 7.2-7.7 (m, 13 H); 6.5
(s, 1 H); 3.5-3.7 (m, 1 H); 3.25-3.45 (m, 1 H); 2.9 (q. 2 H);
20 1.2-1.4 (m, 6 H).
ESI-MS: M+ = 469.
The following were prepared analogously:
Example 19:
2-(4-Ethyl-6-phenyl-[1,3,5]-triazin-2-yloxy)-3-methoxy-3,3-
diphenylpropionic acid (I-109)
1H-1~IR (200 MHz, CDC13): 8.5 (d, 2 H); 7.2-7.7 (m, 13 H); 6.5
(s, 1 H); 3.3 (s, 3 H); 2.9 (q, 2 H); 1.3 (t, 3 H).
BSI-MS: M+ = 455.
Example 20:
2-[4-Ethyl-6-(4-methoxyphenyl)-[1,3,5]-triazin-2-yloxy]-3-
methoxy-3,3-diphenylpropionic acid (I-23)
1H-NMR (200 MHz, CDC13): 8.4 (d, 2 H); 7.5-7.6 (m, 2 H); 7.2-7.5
(m, 8 H) ; 7. 0 (d, 2 H) ; 6.4 (s, 1 H) ; 3 .9 (s, 3 H) ; 3. 3 (s, 3 H) ;
2 . 9 (q, 2 H) ; 1. 3 ( t, 3 H) .
ESI-MS: M+ = 485.
' CA 02375666 2001-11-30
0050/50031
26
Example 21:
3-Ethoxy-2-[4-ethyl-6-(4-methoxyphenyl)-[1,3,5]-triazin-2-yloxy]-
3,3-diphenylpropionic acid (I-147)
1H-NMR (200 MHz, CDC13): 8.4 (d, 2 H)-; 7.5-7.6 (m, 2 H); 7.2-7.5
(m, 8 H); 7.0 (d, 2 H); 6.S (s, 1 H); 3.9 (s, 3 H); 3.3-3.7
(m, 2 H); 2.8 (q, 2 H); 1.35 (t, 3 H); 1.25 (t, 3 H).
ESI-MS: M'' = 499.
Example 22:
2-[4,6-biphenyl)-[1,3,5]-triazin-2-yloxy]-3-methoxy-3,3-diphenyl-
propionic acid (I-29)
1H-NMR (200 MHz, CDC13): 8.6 (d, 4 H); 7.2-7.7 (m, 16 H); 6.5
(s. 1 H) ; 3.3 (s, 3 H) .
ESI-MS: M+ = 503.
Example 23:
2-[4,6-biphenyl)-[1,3,5]-triazin-2-yloxy]-3-ethoxy-3,3-diphenyl-
propionic acid (I-41)
1H-NMR (200 MHz, CDC13): 8.6 (d, 4 H); 7.2-7.7 (m, 16 H); 6.6
(s, 1 H) ; 3.3-3.7 (m, 2 H) ; 1.3 (t, 3 H) .
ESI-MS: M+ = 517.
The compounds listed in Table I can be prepared analogously or as
described in the general section.
40
CA 02375666 2001-11-30
0050/50031
27
d O O O O O O O O O O O O O O O v~
w~~ O ~ .~ O
N U ~ ~ ~ ~ ~ z ~ U ~ ~ U U U U U
~' z z z z z z z z z z z z z z z z
x z z z z z z z z z z z z z z z z
a
~. ~ .c ~ a.
p>' "~.i, ~~, ~ b ~ fs' ~ 3
.ti .s~
A. ~ Q
°'~~ ~~~ N
0
xv -x
aG x
I x~ '~ x ~ ~ x ~~
'~ r ~, v x ~, " x
'~~ ~~' ;, ~ N ~''
x
x ~ W ~ U ~ ~ U ~ ~ x U t.Ot.'' U ~ U
>, ..1, .L
'~'a~ .1, c~ -!~
..L a ~, ~.
.f'.~,r~. NC~'~C~bAb
M
~ x x x x x z x x x z x x x ~ x
0 0 0 0 0 0 0 0 0 0 0 0
O O O O O O O ~ O ~ ~ O O ~ O O
U U U U U U U U U U U U U U U U
~, ca mn r- o, °
CA 02375666 2001-11-30
0050/50031
28
d O O O O O O O O O O O O O v~ O O O O O O O O
a~ a~ a ~", ~ ~ a a o a~ as a~
~ W
x x x x x x x x x x x x x x
z U U U U z z z U U U U z U U U U U z z ~ U
~' z z z z z z z z z z z z z z z z z z v v z z
x z z z z z z z z z z z z z z z z z z z z z x
~ ,~
_ _ _
~~., ~ ~' ~1 '1 ~1 Q
tY" ~ ~ T ~ ~ ~ N >, ~, ~ .b ~
"'L' O O N N g .d ~ .G1
~~~~~~~~~~~~a~~~q~~~~~~
x~'
~, ~ x~'
x x ~,x x~ x ~x ~x x '~ x
x ~ ~, x ~, ~, ~ x x
x x
x ~ Y ~
a .a .rs
~~ ~~~ ~~q~ w
_. ~UUU".~N~~~~~a'a,cv~ c~
a
~ a
.a
>, >, >, ~ ~. P" 'ti 'sa ~ i, >, >, >, >; a, >, >, >, >, a,
..~ a a a a a a a a a
~ a'~, a. ~ a'~, ~ ~ ~ ~ ~ ~ ate, c'~, a'~, a~. a''~, a'~. a'°.
x
U
O O O O ~ ~ ~ O ~ O O ~ O O O ~ O ~ O ~ O O
O O O ~ U U U OU U OU OU U OU OU OU U ~ U O O O O
C ~ ~ ~ O .-~ N e~ dW 1 ~O l'~ 00 O~ O .., N e'~ d' ~1 ~O t~ 00
CA 02375666 2001-11-30
0050/50031
29
O O O O O O O O O O O O O v~ O O O O O O O O
a
N N ~. N N w~ ~ ~ 1. O ~O ~
~~c~c~ooxxx'~c~c~Sc~c~~v c~ c~z~~c~
N x x x x ~ ~ ~ x x ~ x x x x x x x x
U U z U U Z U U z z U U U U U U U U
z z Z z z z z z z z z z U z z x z z z z z z
x z z z z z z z z z z z z z z z z z z z z z z
''
J, ~ o ,J; ~, '~ ~ T p pa, ~ ~'' ~''
i.> >, ~ ~ ,~ .b .CI .C b
~~'~~r~~_~ ~ .~
xN x~ ~, x
N ~ ~ x ~ c~
x x ~ x
x a
~ x ~ ~ x~
~, x ~ '~ b
i, a, ~ o a, a, ~" b ~, a, ,, ~,
j ~ w ~ w ~ N x N a
>, ,~ >,
.c
a .~ .°c~ .a a a .~ ~ .°~ .b ,~ .°r
C~ C4 W C.4 fs~ iS~ W ~ W i~r ~ GL N W W i~r i~r ~ P4 i~r W
x >,
. x U x x ~ x x x x x x x x x x x x x x x x x
0 0 0 0 ~ 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0
O O O O ~ O O O O V D O O O O O O O O V O O
on c .., N ~~~, ~n .o c~ 00 0. o ,~ h n ,ii n ° h
:~~~~~~~~~~~~~~~a
CA 02375666 2001-11-30
0050/50031
~d Orn000000000000000 rn 0000
ax
~ .~ ~~ ~ ~ ~ w ~ ~ ~ ~ ~ ~~ ~. ~ ~-~
c~ ~ ~ ~ ~ ~ ~ ~ ~ ~ z z
x x x x x x x x x x x x x x x x x x x
z U U U U ~ U U U U U U U z U U U U U U U U
~' z z z z z z z z z z z z z z z z U U z z z z
x z z z z z z z z z z z z z z z z z z z z z z
T T
C~
>, ~ ~ ~ ~T
N
~ N
>, al" ~ .C ~ r~.. °~
"c7 ~ 'tt ~?~ ~ T ~ T
M
a ~
C~ p °~ p '~ J, o
-a ~L ~ .a 'r~ °,~ a. '~ o
~L i, >, ~ ~' ~ ~ ~ ~ ~ '$ 'tta, xM
>, _~
x
as w~I ~ ~ ~ ~ x a, a, ~ ~ ~ ~T N a, ~,
wN
x U ~ oa
s
c~T3~ ~ ~~'~T
°~ a
Is, G~" .s~ ~ ,-. r.. .... .-. .-. ... .., ... .... ... .-, ...
>, >, >, T >, >, T ~r, T _O .~, ?, ~s ?, >,
A, N N fig C4 Q, W W ~ P~ is, C~ PH fir P~ i~
h
x x
x x x x x x x x x v x x x x x x x x x x x x
0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0
0 0 0 ~ 0 0 0 ~ ~ 0 0 0 0 0 0 0 0 0 0 0 0 0
O ~~ N t~1 h ~O l~ 00 Q~ O ~ N en et' v1 ~O C'~ 00 Ov O ~ N
CA 02375666 2001-11-30
0050/50031
31
Q~ O O O O O O O O O O O O O O O O O O O O rn O
a
° w "~ ~ ~ ~ a~ a a ~ .~ ..~ ~ w
z z 5
N x x x x x x x x x~ x x x x x x x x x
U U U U U U U U U z U U U U U U z z z U U
~' z z z z z z z z z U U z z z z z z v z z z z
x z z z z z z z z z z v z z z z z z U U z z z
y
a, a, .~ ~ -La,
1 .~ ~ ~ y a, >, ~ a, ~ o
W ~' ~ ~ ~ 1 y ~' ~ ya, ~ ~ .a ~ ~ ~ ~ ~ ~ '' a
x~ ~ ~ xyxl~
xN x ~ ~, x~ x~
x y o x ~' '~ ~ '~ '~
U ~ ~ a, ~ ~ x ~ x
x
x a, a. ~ a, x ~ ~ ~ ~, a, ~ w ~ ~, a,
a, ~ ~ J
V ~ ~ V ~ U ~ ~ ~ ~ ~ ~ W U 'O.L~' ~ ~ ~ ~ W U U
,~ >, ,.-, >, ~»
a a '~ a ~ ~ °x'
ct .b as
a a w ~ a' a a ~
a". ~ a'~, ax, o~'.~ a~. o'~, ~
M
x
U
Qi
x x x ~ x x x x x x x x x x x x x x x~ x ~ x
0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0
0 0 0 0 ~ 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0
O .-~ N M ~t
p e~1 V1 ~O l~ 00 O~ O ~ N M et V1 ~O C'~ CO Q~ O O O O O
CA 02375666 2001-11-30
0050/50031
32
~d O 0 0 0 0 0 0 0 0 0. 0 0 0 0 rr~ 0 0 0 0 0 v~ 0
a
a~ a> >' a~ w as a~ a~ ~ ~ M
w o W .~ ~ W ~ ~ ~ ~ ~ ~ W ~ ,~ w w
U ~ ~ ~ U
x x x x x x x x x x x x x x x x x x x x x
U U U U z U U U U U U U U U U U U U U U U U
~' z z z z z z z z z z z z z z z z z z z z z z
x z z z z z-z x z z z z z z z z z z z z z z z
~ .1. ~ ~ .l. -~~ '1'a, .1. a,
tx ~ ~ ~l. '~ a a ~ >' a a, a a, ~ ~ ~ a a.
w .r~ ~ ~ .c '" ~ a
w ~, ,.., a ~ ~ w ~, ~ ~ M
x x~, x
x 1~ '~, '~ x~" ~ ~ x
x ~ ~' ~ x ~ ~, x x
x c~ ~ x ~ x x w
~ z ~ ~ ~ ~ ~ ~ ~ ~ ~ .a
° ~
b
~ ~ x z '~
w w U x ~ ~ ~ ~ ~ w
~~,
~ ~, ~s
a ~, ~ a v .~ ~ aa.
~ v x x x x x x ~ x x x x x x x ~ x v x x x x
0 0 0 0 0 0 0 0 0 0 0 0 0 ~' 0 0 0 0 0
0 0 0 0 0 0 0 0 0 ~ a ~ 0 0 0 °o~ 0 0 0 0 0 0
~W D r- 00 Cv O .-~ N en d' ~W O t~~ 00 Ov O ~ N t~'1 et ~1 ~O
p O O O O O .--~ .--i ~ .-~ -r ~~ ~~ ~1 ~~ .-~ N N N N N N N
~ ~ v-.~ ~ ~ ..r ....i .-H -r ~ ~ ..-wr ~.r rr ~ ~ ~ ~ n-i ~
CA 02375666 2001-11-30
0050/50031
33
d O 0.1 O O O O O O O O O O O O vi rr~ O O O O O O
a
N x x x x x x x x x x x x x x x x x x x x x
U U U U U U U U U U U U U U U U U U U U z U
~' z z z z z z z z z z z z z z z z z z z z z z
x z z z z z z z z z z z z z z z z z z z z z z
1
a ~~ -1. ...I. uT ~ ~, a a a ~ ~ a ~ ~ xN
A,r~,, ~, ~ ~ .cs .mss .~ .~ ~ .'~ ,~ ~ ~ .a
b ~ .~ b ~, ~, .
~' x ~ .~ o ~ x
~~~~~~~N~~~~~~;~~~~~~~'~
x~ x
x~ ~ Y
. x~ ~, N ~ ~' x ~ x
c~ x
x x
o b
'~ w
w ~ W.; ~ ~ ~ ~ ~, ~ ~ ~ ~ ~ ~ ~ w
a~ a ~ a~
7, ~ ~ >, >, ?, >, >, T ~ ~ >, T >, ~ ?, >, >, >, ~ >,
~t ~ .~ .C x ,~ .c3 .~ .C ~'1 ~ .r1 .C
Ar f~1~ C~, Q, OH W w ~ O.r G4 G~ i~ W p, W P.i i~
C
~ v x x x x x x x x x x v x x x x x ~ v x x x
0 0 0 0 0 0 0 0 0 o z o 0 0 0 0 0 ~Z o 0 0 0
0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0
U U U U U U U U U U U U U U U U U U U U U U
(~ CO O~ O ~ N M d' V'f ~O h~ 00 O~ O ~ N M d' ~f b n 00
Q N N N M M M M M M M M M M ~' <Y'
.-q v--~ ,.y .-~ H ...a rr .H H ..r N ~.-yr .r ..-I ~ ~ r.1 r.q ~.q ..1 ~
z
CA 02375666 2001-11-30
0050/50031
34
d O O rr~ ~n O O O O O O O O v~ O O O.O O O O O v~
d w"'
a~ a~ a ° a a~ ° ~v d a~ ° d a
~ ... ~ ~ ~ ~ ~ ~ ~ ~ ~ ~ w~
z ~ ~ ~ ~ ~ ~ c~ c~
N x x x x x x x x x x x x x x x x x x
U U U U U U U U U U U U z z U U U U z z. U U
~' z z z z z z z z z z z z v z z z z z U U z z
~ z z z z z z z z z z z z z z z z z z z z z z
g .!, -"~, ~ ~. ~ .~, >,
H ~, .c x r~ ~ ~ ,, ° ~ y y ,°, .° ~, y
N~ N 'r~ .C i~ ~ .cS
N ~ ~ ~ ~ N
x~
y >,
,.1. ~, d "!, .~ 1
~, a .c .1, y s' c ,w1.
x~ ~ ~ -.
>, >, ~ ~ ~ ~ a, ~, ~~ ~" ~ w ~ ~ ~ a
_>
& ~ a
°' ~ ... .~ ~.. ...~ r. .~
a w a a a a ~ a a a a ~ ~ a ° a ~ ~ ~ c
a'°. a~.~ ~ a~, ~ a'~. a'~, a'~. a"', a, a, a'~, a~, U~' a'~, a'~, ~
a~,
~ x x x x x x x x x x x x x x x x x ~ x x ~' x
0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0
O O O V O O O V O ~ O O O O O O O O D ~ O O
G1 O ~ N M ~ W O I~~ 00 Cv ~ N ch Y1 ~D l~ 00 C~ O
p ~t v1 V1 w'1 Y1 vn v1 H ~n . v1 TWO ~O ~O ~D v0 ~O vo vD l~
z ~ ~ ~ ~ ~ ~ ~ ~ ~ ~ ~ ~ ~ ~ ~ ~ ~ a a
CA 02375666 2001-11-30
0050/50031
d' O O r~ O O v~ O O O O
a
N x x x x x x x x x x
U U U U U U U U U U
~' z z z z z z z z z z
~ z z z z z x z z z z
1
a .1.
.~ ~ ~ >,
a
.J. a, '~"
.a~~3 ..L a ~ ~ ~ °' 1
~' ~' a,
0
~ ~ N ~ w w w
h
x ~ ~~,
~'
~ v v x x x x x x x x
0 0 0 0 0 0 ~ 0 0 0
0
CA 02375666 2001-11-30
0050/50031
36
O O O O O O O O O O O O O O
a
w'~ ° ~ _
U ~ ~ U
N
H
N
U U U U U U z U U U U U U U
~' z z z z z z z z z z z z z z
~ z z z z z z z z z z z z z z
h
~w'
~ i v
x ~ xN
~ ~ 1 x
~xx
j >, a, _, a ~ >, >, >, a .!,
~ ~ N ~ x ~ ~ ~ a,
U ~ V :~ ~ ~ ~ U W ''°.~
A
N
~ P4
~' ~, >, >, a, >, 9, ~, >. ?, >, ~,
a v a ~ w w ~ a
'" x x x x x x z x x x x x x x
a o 0 0 0 0 0 0 0 0 0 0 0 0 0
H o 0 0 0 0 0 0 0 0 0 0 0 0 0
U U U U U U U U U U U U U U
cv rmn t~
i
' CA 02375666 2001-11-30
0050/50031
37
Example 24:
The binding assay described above was used to measure receptor
binding data far the compounds listed below.
The results are shown in Table 2.
Table 2
Receptor binding data (K1 values)
Compound ETp, [nM]
I-180 83
I-102 130
I-109 133
I-23 5
I-147 14
30
40