Note: Descriptions are shown in the official language in which they were submitted.
CA 02376570 2001-12-19
WO 01/02338 PCT/US99/15056
1
QUATERNARY NITROGEN COMPOUND, FABRIC CARE COMPOSITION
CONTAINING SAME, AND PROCESS FOR FORMING SAME
FIELD OF THE INVENTION
The present invention relates to a quaternary nitrogen compound, a fabric
care composition containing a quaternary nitrogen compound, and a process for
forming a quaternary nitrogen compound.
BACKGROUND OF THE INVENTION
Fabric softening actives are known additives for laundry applications
which provide softer fabrics, reduce or eliminate static, reduce or eliminate
wrinkles and enhance in-wear comfort. Traditionally, such fabric softening
actives were used to provide benefits on fabrics such as clothes. These fabric
softening actives are typically added in either the wash cycle as part of the
detergent, or separately in the rinse and/or drying cycles of a laundering
operation as, for example, liquid or solid fabric care compositions. During
the
wash, rinse, and/or drying cycles, these fabric softening actives transfer
(e.g.,
from solution or a dryer sheet) onto clothes, to provide one or more of the
above
benefits.
Typical fabric softening actives known in the art include amine-containing
compounds, cationic fabric softening compounds such as quaternary nitrogen
compounds, nonionic fabric softening compounds, and mixtures thereof.
Processes for forming fabric softening actives are also known.
As concern over the environment increases, it is becoming increasingly
desirable to provide biodegradable fabric care compositions. While current
fabric
softening actives may be biodegradable, and may soften clothes and other
fabrics, they tend to sufFer from certain drawbacks. For example, they may not
remain stable in an aqueous solution and/or in a fabric care composition; the
CA 02376570 2001-12-19
WO 01/02338 PCT/US99/15056
2
typical fabric softening active is either biodegradable, or stable during
storage,
but rarely both. Thus, a biodegradable fabric softening active's stability
over time
may be limited, which in turn limits its commercial applicability. In addition
to a
loss of fabric softening activity, the degradation of a fabric softening
active may
40 create malodor problems, especially in hot, humid climates or storage
conditions.
Another disadvantage of many fabric softening actives is that they are
often extremely viscous fluids, or solids which are accordingly difficult to
formulate in an aqueous solution, especially for a clear, aqueous
compositions.
Thus, solvents and hydrotropes are often required in a clear fabric care
45 composition. While these solvents and hydrotropes help solubilize the
fabric
softening active, they often are expensive, and/or do not otherwise improve
the
product's performance.
It is thus desirable to improve fabric care composition stability, reduce
formulation obstacles, improve softening performance, and improve anti-static
50 performance, while simultaneously reducing formulation costs. Furthermore,
it is
desirable to increase both the stability and the biodegradability of a fabric
softening active.
Accordingly, there is a need for a fabric softening active which possesses
improved stability, is easier to formulate, is biodegradable, and which has
55 improved softening performance. There also exists a need for a process for
making such a fabric softening active, and for a fabric care composition
containing such a fabric softening active.
SUMMARY OF THE INVENTION
60 In one aspect, the present invention relates to a novel quaternary nitrogen
compound of Formula I:
O
+ W O)n
R2 4
(Formula I),
where each R, is independently selected from the group consisting of ethyl,
propyl, isopropyl, and butyl; each RZ is independently selected from the group
65 consisting of a saturated C,_22 alkyl group, and an unsaturated C,_z2
alkylgroup;
each n is independently from about 1 to about 25; and X- is an anion.
In another aspect, the present invention relates to a fabric care
composition comprising a fabric softening active and a carrier. The fabric
CA 02376570 2001-12-19
WO 01/02338 PCT/US99/15056
3
softening active comprises a mixture of quaternary nitrogen compounds of the
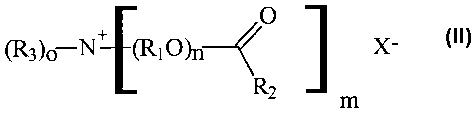
70 general Formula II:
O
~3)o N+ W O)n~ X
RZ m (Formula II),
where each R, is independently selected from the group consisting of ethyl,
propyl, isopropyl, and butyl; each RZ is independently selected from the group
consisting of a saturated C,_22 alkyl group, and an unsaturated C,_22 alkyl
group;
75 each R3 is independently selected form the group consisting of a saturated
C,_22
alkyl group, and an unsaturated C,_zZ alkyl group; each n is independently
from
about 1 to about 25; m is from 0 to 4; o is from 0 to 4; m + o = 4; and X- is
an
anion. The mixture of quaternary nitrogen compounds comprises at least about
5% mono-ester quaternary nitrogen compound with m = 1 and o = 3, at least
80 about 15% di-ester quaternary nitrogen compound with m = 2 and o = 2, and
at
least about 5% tri-ester quaternary nitrogen compound with m = 3 and o = 1.
In yet another aspect, the present invention relates to a process for
forming a quaternary nitrogen compound comprising the steps of providing a
starting material selected from the group consisting of ammonia, monoalkyl
85 amine, dialkyl amines, and mixtures thereof, providing an alkoxylated
reactant,
providing a fatty acid reactant, and providing a quaternizing agent. The
starting
material is alkylated with the alkoxylated reactant to form a tertiary
nitrogen
compound, which is in turn reacted with the fatty acid reactant to form a
tertiary
alkoxylated ester having from 2 to 3 alkoxylated fatty acid moieties. This
tertiary
90 alkoxylated ester is then quaternized with the quaternizing agent to form a
quaternary nitrogen compound.
In another aspect, the present invention comprises a process for forming a
quaternary nitrogen compound comprising the steps of providing a starting
material selected from the group consisting of ammonia, monoalkyl amine,
dialkyl
95 amine, and mixtures thereof, providing an alkoxylated reactant, and
providing a
fatty acid reactant. The starting material is alkylated with the alkoxylated
reactant
to form a quaternized alkoxylated amine, which is in turn reacted with the
fatty
acid reactant to form a quaternary alkoxylated ester having from 3 to 4
alkoxylated fatty acid moieties.
100 It has now been found that a novel quaternary nitrogen compound and
mixtures of quaternary nitrogen compounds may serve as a fabric softening
CA 02376570 2001-12-19
WO 01/02338 PCT/US99/15056
4
active. Furthermore, when included in a fabric care composition, this
quaternary
nitrogen compound may provide one or more benefits such as softer clothes,
reduced static, easier formulation, increased biodegradability, lower
formulation
105 costs, and improved storage stability. This improved stability is
especially
desirable in hot, humid climates and hot, humid storage conditions, which may
promote hydrolysis and increase malodor issues.
Moreover, it has been found that when the mono-, di-, and tri-ester forms
of the quaternary nitrogen compounds described are combined, they provide
110 synergistic benefits, for example, improved softening benefits, easier
formulation,
and/or a significant reduction of hydrotropes. It has also now been found that
when certain starting materials are employed, the quaternary nitrogen
compounds described herein may be formed in an economical manner.
These and other features, aspects, advantages, and variations of the
115 present invention, and the embodiments described herein, will become
evident to
those skilled in the art from a reading of the present disclosure with the
appended claims, and are covered within the scope of these claims.
DETAILED DESCRIPTION OF THE INVENTION
120 All percentages, ratios and proportions herein are by weight, unless
otherwise specified. All temperatures are in degrees Celsius (°C)
unless
otherwise specified. All documents cited are incorporated herein by reference
in
their entireties. Citation of any reference is not an admission regarding any
determination as to its availability as prior art to the claimed invention.
125 As used herein, the term "alkyl" means a hydrocarbyl moiety which is
straight or branched, saturated or unsaturated. Unless otherwise specified,
alkyl
moieties are preferably saturated or unsaturated with double bonds, preferably
with one or two double bonds. Included in the term "alkyl" is the alkyl
portion of
acyl groups.
130 As defined herein, the terms "mono-ester quaternary nitrogen compound"
indicates a compound of Formula II where m = 1 and o = 3, while "di-ester
quaternary nitrogen compound" indicates a compound of Formula II where m = 2
and o = 2, and "tri-ester quaternary nitrogen compound" indicates a compound
of
Formula II where m = 3 and o = 1. As used herein, the term "quaternary-ester
135 quaternary nitrogen compound" indicates a compound of Formula I, which
correlates to a compound of Formula II, where m = 4 and o = 0.
CA 02376570 2001-12-19
WO 01/02338 PCT/US99/15056
The present invention is directed towards a novel quaternary nitrogen
compound, a fabric care composition containing a synergistic mixture of
quaternary nitrogen compounds, and a process for forming a quaternary nitrogen
140 compound. The quaternary nitrogen compounds herein may be included in a
fabric care composition, such as, a fabric softening composition, and/or a
laundering composition. Preferred fabric care compositions include a liquid or
solid rinse-added fabric softening composition, and a fabric softening
composition for use in the drying-cycle. More preferably, the fabric care
145 composition is a rinse-added liquid fabric softening composition. The
liquid fabric
care compositions herein may be either clear, transparent, translucent, or
opaque fabric care compositions.
Quaternary Nitrogen Compound
150 The fabric softening active herein is a quaternary nitrogen compound, or a
mixture of quaternary nitrogen compounds. This quaternary nitrogen compound
may also be easier to formulate, and easy to prepare. Without intending to be
limited by theory, it is believed that these compounds possess improved
resistance to hydrolysis (e.g., alkaline-induced hydrolysis) during storage
while
155 remaining readily biodegradable. It is believed that these quaternary
nitrogen
compounds are less subject to hydrolysis because they possess extended
"oxyalkylene spacers" between the ester carbonyls and the quaternized
nitrogen.
These quaternary nitrogen compounds are especially resistant to hydrolysis if
two or more such oxyalkylene spacers are present. This reduces the esters'
160 susceptibility to hydrolysis by increasing the spatial distance between
the ester
and nitrogen functional groups. Furthermore, the presence of alkoxy, and
especially ethoxy groups, provide significant moisturization benefits for the
fabric,
improving the softening benefits. Without intending to be limited by theory,
it is
further believed that the alkoxy groups are susceptible to hydrolysis under
acidic
165 conditions, and therefore are more easily biodegradable by, for example,
bacteria.
Accordingly, the novel quaternary nitrogen compounds of the present
invention correspond to Formula I:
O
+ (R10~-
RZ 4
(Formula I),
CA 02376570 2001-12-19
WO 01/02338 PCT/US99/15056
6
170 where each R, is independently selected from the group consisting of
ethyl,
propyl, isopropyl, and butyl; preferably ethyl, and propyl; and more
preferably
ethyl. Each Rz is independently selected from the group consisting of a
saturated
C,_zz alkyl group, and an unsaturated C,_zz alkyl group; and is preferably
selected
from the group consisting of a saturated C,z_,$ alkyl group, and an
unsaturated
175 C,z_,$ alkyl group. Such Rz groups may be straight chain, or branched, may
be
aliphatic, cyclic, or aromatic, and may or may not include other functional
groups.
Each n indicates the average degree of alkoxylation, and is independently from
about 1 to about 25; preferably from about 1.1 to about 10; and more
preferably
from about 1.5 to about 5. In Formula I, X- is an anion suitable. for use in a
fabric
180 care composition; preferably X- is selected from the group consisting of a
halide,
and an alkyl sulfate; and more preferably X- is a halide.
In addition to the novel quaternary nitrogen compound of Formula I, the
present invention is also directed to a fabric care composition containing a
fabric
softening active comprising a mixture of quaternary nitrogen compounds of the
185 general Formula II:
O
~3)o N+ ~t O)n
m
R2 (Formula II),
where each R,, Rz, n, and X- are as defined above for Formula I. Each R3 is
independently selected from the group consisting of a saturated C,_zz alkyl
group,
and an unsaturated C,_zz alkyl group; and is preferably selected from the
group
190 consisting of a saturated C,z_,8 alkyl group, and an unsaturated C,z_,$
alkyl group.
Such R3 groups may be straight chain, or branched, may be aliphatic, cyclic,
or
aromatic, and may or may not include other functional groups. Furthermore, m
is
fromOto4;oisfromOto4;andm+o=4.
When a mixture of quaternary nitrogen compounds of Formula II are
195 present in a fabric care composition, it has been found that significant
benefits
are found when the mono-, di-, and tri-ester quaternary nitrogen compounds are
present at certain minimum levels. The mono-ester quaternary nitrogen
compound of Formula II, where m = 1 and o = 3, is thus present at a level of
at
least about 5%, preferably about 20%, and more preferably about 30%, by
200 weight, of the mixture of quaternary nitrogen compounds of Formula II. It
has
also been found that it is desirable to maximize the proportion of di-ester
quaternary nitrogen compound, to provide a balance between desirable fabric
CA 02376570 2001-12-19
WO 01/02338 PCT/US99/15056
7
softening and solubility benefits. Therefore, the di-ester quaternary nitrogen
compound of Formula II, where m = 2 and o = 2, comprises at least about 15%,
205 preferably at least about 30%, and more preferably at least about 40%, by
weight, of the mixture of quaternary nitrogen compounds of Formula II. The tri-
ester quaternary nitrogen compound of Formula II, where m = 3 and o = 1, will
typically be from about 5% to about 33%, preferably from about 7% to about
30%, and more preferably from about 10% to about 25%, by weight, of the
210 mixture of quaternary nitrogen compounds of Formula II. Thus, the mixture
of
quaternary nitrogen compounds comprises at least about 5% mono-ester
quaternary nitrogen compound, at least about 15% di-ester quaternary nitrogen
compound, and at least about 5% tri-ester quaternary nitrogen compound.
Such fabric care compositions may further contain a quaternary-ester
215 quaternary nitrogen compound of Formula I. If present, then the quaternary
ester quaternary nitrogen compound of Formula I typically comprises from about
5% to about 33%, preferably from about 5% to about 30%, and more preferably
from about 5% to about 25%, by weight, of the mixture of quaternary nitrogen
compounds.
220 The compounds of Formula I and Formula II, and their weight percent in a
composition or preparation, may be differentiated by 1 H NMR analysis and/or
high performance liquid chromatography methods, as described below.
If a clear, aqueous, isotropic liquid fabric care composition is desired, then
it is highly preferred that the amount of di-ester quaternary nitrogen
compound of
225 Formula II be at least equal, or greater than the total amount of the
quaternary
ester quaternary nitrogen compound of Formula I plus the tri-ester quaternary
nitrogen compound of Formula II. Without intending to be limited by theory, it
is
believed that while they provide better fabric softening benefits, the tri-
and
quaternary-ester quaternary nitrogen compounds are less soluble in aqueous
230 media than the mono- and di-ester quaternary nitrogen compounds. However,
when present in the synergistic levels described above, it is believed that
the
mono- and di-ester quaternary nitrogen compounds, and especially the di-ester
quaternary nitrogen compounds, serve as hydrotropes to help keep the tri-ester
quaternary nitrogen compounds, and optionally the quaternary-ester quaternary
235 nitrogen compounds, in solution. Therefore, when present in these levels,
a
clear, aqueous, isotropic liquid fabric care composition may be produced. Such
liquid fabric care compositions typically possess a viscosity of less than
about
CA 02376570 2001-12-19
WO 01/02338 PCT/US99/15056
8
200 cps, preferably from about 50 cps to about 100 cps, as measured at 25
°C,
by means of a glass capillary viscometer as set forth in Dow Corning Corporate
240 Test Method CTM0004, dated July 20, 1970.
The fabric softening active comprising a mixture of quaternary nitrogen
compounds of Formula II (and optionally Formula I) is typically present in the
fabric care composition at a level of from about 0.1 % to about 60%,
preferably
from about 0.2% to about 35%, and more preferably from about 20% to about
245 35%, by weight of the fabric care composition.
Carrier
A liquid fabric softening composition typically requires a liquid carrier. The
level of carrier is generally greater than about 50%, preferably greater than
250 about 65%, more preferably greater than about 70% of the fabric care
composition. The carrier employed herein is preferably water due to its low
cost,
availability, safety, and environmental compatibility. The level of water in
the
liquid carrier is generally more than about 50%, preferably more than about
80%,
and more preferably more than about 85%, by weight of the carrier. Mixtures of
255 water and organic solvents having low molecular weight, e.g., less than
about
100 g/mol, are useful as the liquid carrier. Preferred organic solvents
include
low molecular weight alcohols such as ethanol, propanol, isopropanol or
butanol;
propylene carbonate; and/or glycol ethers. Useful low molecular weight
alcohols
also include dihydric alcohols (e.g., glycol), trihydric alcohols (e.g.,
glycerol), and
260 polyhydric (polyols) alcohols, such as C2_g polyhydric alcohols.
Principle Solvent
The fabric care compositions may take the form of clear, transparent, or
translucent liquid compositions. In such instances, the compositions may also
265 include a principle solvent. The principle solvent is one or more organic
solvents
which balance the level of fabric softening active and water, to produce a
clear,
transparent, or translucent liquid composition. Such a principle solvent
possesses a CIogP value, as described below. The principle solvent is also
selected to minimize solvent odor impact in the composition and to provide a
low
270 viscosity to the final composition. It has been found that a principle
solvent may
further reduce turbidity, increase delivery of the fabric softening active to
the
CA 02376570 2001-12-19
WO 01/02338 PCT/US99/15056
9
fabric, increase stability, reduce viscosity for easy in-use dissolution,
reduce
formulation costs, and/or provide a desirable odor.
The preferred principle solvent provides a highly concentrated fabric care
275 composition which has one or more of the benefits described above. The
most
preferred principle solvent may be identified by the appearance of softener
vesicles, as observed via cryogenic electron microscopy of the compositions
that
have been diluted to the concentration used in the rinse. These dilute
compositions appear to have dispersions of fabric softener that exhibit a more
280 uni-lamellar appearance than conventional fabric softener compositions.
The
closer to uni-lamellar the appearance, the better the compositions seem to
perform. These compositions provide surprisingly good fabric softening and
deposition as compared to similar compositions prepared in the conventional
way
with the same fabric softener active. Thus, the principle solvent in the
principle
285 solvent system must be carefully selected. For example, isopropyl alcohol
is not
very effective at forming the desired softener vesicles, and has a strong
odor. n-
Propyl alcohol is more effective at forming the desired vesicles, but also has
a
distinct odor. Several butyl alcohols also have odors, but may be used for
effective clarity/stability, especially when combined with another principle
solvent
290 to minimize their odor. The preferred principle solvent is also selected
for
optimum low temperature stability, and is able to form a composition that is
liquid,
with an acceptable low viscosity and translucent, preferably clear, down to
about
40 °F (about 4.4 °C), and is able to recover after storage down
to about 5 °F
(about -15 °C).
295 Accordingly, the suitability of any principle solvent for the formulation
of
the liquid, concentrated, preferably clear, fabric softener compositions
herein with
the requisite stability is surprisingly selective. A suitable principle
solvent may be
selected based upon its octanol/water partition coefficient (P). The
octanol/water
partition coefficient of a principle solvent is the ratio between its
equilibrium
300 concentration in octanol and in water. The partition coefficients of the
principle
solvent of this invention is conveniently given in the form of their logarithm
to the
base 10, IogP.
The IogP of many ingredients has been reported. For example, the
Pomona92 database, available from Daylight Chemical Information Systems, Inc.
305 (Daylight CIS), Irvine, California, contains many, along with citations to
the
original literature. However, the IogP values are most conveniently calculated
by
CA 02376570 2001-12-19
WO 01/02338 PCT/US99/15056
the "CLOGP" program, also available from Daylight CIS. This program also lists
experimental IogP values when they are available in the Pomona92 database.
The "calculated IogP" (CIogP) is determined by the fragment approach of Hansch
310 and Leo (cf., A. Leo, in Comprehensive Medicinal Chemistry, Vol. 4, C.
Hansch,
P. G. Sammens, J. B. Taylor and C. A. Ramsden, Eds., p. 295, Pergamon Press,
1990, incorporated herein by reference). The fragment approach is based on the
chemical structure of each ingredient, and takes into account the numbers and
types of atoms, the atom connectivity, and chemical bonding. These CIogP
315 values, which are the most reliable and widely used estimates for this
physicochemical property, are preferably used instead of the experimental IogP
values in the selection of the principle solvent useful in the present
invention.
Other methods that may be used to compute CIogP include, e.g., Crippen's
fragmentation method as disclosed in J. Chem. Inf. Comput. Sci., 27, 21
(1987);
320 Viswanadhan's fragmentation method as disclose in J. Chem. Inf. Comput.
Sci.,
29, 163 (1989); and Broto's method as disclosed in Eur. J. Med. Chem. - Chim.
Theor., 19, 71 (1984).
The principle solvent useful herein is selected from those solvents having
a CIogP of from about 0.15 to about 0.64, preferably from about 0.25 to about
325 0.62, and more preferably from about 0.40 to about 0.60. The principle
solvent is
preferably at least somewhat asymmetric, and preferably has a melting, or
solidification, point that allows it to be liquid at, or near room
temperature. A
principle solvent that has a low molecular weight and is biodegradable is also
desirable. The more assymetric solvents appear to be very desirable, whereas
330 the highly symmetrical solvents such as 1,7-heptanediol, or 1,4-
bis(hydroxymethyl) cyclohexane, which have a center of symmetry, appear to be
unable to provide clear compositions when used alone, even though their CIogP
values fall in the preferred range.
Operable principle solvents are disclosed and listed below which have
335 CIogP values which fall within the requisite range. The principle solvent
useful
herein is selected from the group consisting of mono-ols, C6 diols, C, diols,
octanediol isomers, butanediol derivatives, trimethylpentanediol isomers,
ethylmethylpentanediol isomers, propyl pentanediol isomers, dimethylhexanediol
isomers, ethylhexanediol isomers, methylheptanediol isomers, octanediol
340 isomers, nonanediol isomers, alkyl glyceryl ethers, di(hydroxy alkyl)
ethers, and
aryl glyceryl ethers, aromatic glyceryl ethers, alicyclic diols and
derivatives, C3 C,
CA 02376570 2001-12-19
WO 01/02338 PCT/US99/15056
11
diol alkoxylated derivatives, aromatic diols, unsaturated diols, and mixtures
thereof. A particularly preferred principle solvent is selected from the group
consisting of hexanediols, such as 1,2-hexanediol and 2-ethyl-1,3-hexanediol,
345 pentanediols, such as 2,2,4-trimethyl-1,3-pentanediol, and mixtures
thereof.
These principle solvents are disclosed in copending U.S. Patent Application
numbers 08/621,019; 08/620,627; 08/620,767; 08/620,513; 08/621,285;
08/621,299; 08/621,298; 08/620,626; 08/620,625; 08/620,772; 08/621,281;
08/620,514; and 08/620,958, all filed March 22, 1996, and all having the title
350 "CONCENTRATED, STABLE, PREFERABLY CLEAR, FABRIC SOFTENING
COMPOSITION".
When employed in the present invention, the principle solvent preferably
comprises less than about 40%, preferably from about 2% to about 35%, more
preferably from about 3% to about 25%, and even more preferably from about
355 4% to about 14%, by weight of the composition.
Mono-Long-Chain-Alkyl Cationic Surfactant
In a preferred embodiment, the fabric care composition further contains a
mono-long-chain-alkyl (water-soluble) cationic surfactant, in addition to any
360 quaternary nitrogen compounds of Formula II which are included in the tri-
ester
and/or quaternary-ester quaternary nitrogen compound synthesis process.
Without intending to be limited by theory, it is believed that the
mono-long-chain-alkyl cationic surfactant also acts as a hydrotrope for the
tri-
and quaternary-ester quaternary nitrogen compounds, and may further provide a
365 fabric softening, or other benefits. For example, the mono-long-chain
alkyl
cationic surfactants may also be present as dispersability modifiers.
If present, the mono-long-chain-alkyl cationic surfactants in liquid
compositions are typically at a level of up to about 30%, preferably from
about
0.5% to about 10% by weight of the final composition. To form a clear, or
370 transparent liquid fabric care composition, the weight ratio of the
mono-long-chain-alkyl cationic surfactant to the mixture of quaternary
nitrogen
compounds is from about 1:1 to about 1:8, preferably from about 1:1 to about
1:6.
The mono-long-chain-alkyl cationic surfactants useful in the present
375 invention are, preferably, quaternary ammonium salts of the general
formula:
(R2N+R3) X_
CA 02376570 2001-12-19
WO 01/02338 PCT/US99/15056
12
wherein the R2 group is a C10-C22 hydrocarbon group, preferably C12-C18
alkyl group or the corresponding ester linkage interrupted group with a short
alkylene (C1-C4) group between the ester linkage and the N, and having a
380 similar hydrocarbon group, e.g., a fatty acid ester of choline, preferably
C12-C14
(coco) choline ester and/or C16- C1g tallow choline ester; each R is a C1-C4
alkyl or substituted (e.g., hydroxy) alkyl, or hydrogen, preferably methyl,
and the
counterion X- is a softener-compatible anion, for example, chloride, bromide,
methyl sulfate, etc.
385 The ranges above represent the amount of the single-long-chain-alkyl
cationic surfactant which is preferably added to the composition of the
present
invention.
The long chain group R2, of the single-long-chain-alkyl cationic
surfactant, typically contains an alkyl, or alkylene group having from about
10 to
390 about 22 carbon atoms, preferably from about 12 to about 16 carbon atoms
for
solid compositions, and preferably from about 12 to about 18 carbon atoms for
liquid compositions. This R2 group can be attached to the cationic nitrogen
atom through a group containing one, or more, ester, amide, ether, amine,
etc.,
preferably ester, linking groups which can be desirable for increased
395 hydrophilicity, biodegradability, etc. Such linking groups are preferably
within
about three carbon atoms of the nitrogen atom. Suitable biodegradable
single-long-chain alkyl cationic surfactants containing an ester linkage in
the long
chain are described in U.S. Pat. No. 4,840,738 to Hardy and Walley, issued
June 20, 1989.
400 It will be understood that the main function of the water-soluble cationic
surfactant is to lower the composition's viscosity and/or increase the
dispersability of the fabric softening active. Thus, it is not essential that
the
cationic surfactant itself have substantial softening properties, although
this may
be the case. Also, surfactants having only a single long alkyl chain,
presumably
405 because they have greater solubility in water, may protect the fabric
softener
active from interacting with anionic surfactants and/or detergent builders.
Other cationic materials with ring structures such as alkyl imidazoline,
imidazolinium, pyridine, and pyridinium salts having a single C12-C3p alkyl
chain
can also be used. A very low pH is required to stabilize these ring structures
410 (e.g., imidazoline).
CA 02376570 2001-12-19
WO 01/02338 PCT/US99/15056
13
Some alkyl imidazolinium salts useful in the present invention have the
general formula:
C H2-C H2
N\ ,N~ C2H4-Yz-R7 X-
C ~R6
R$
415
wherein Y2 is -C(O)-O-, -O-(O)-C-, -C(O)-N(R5), or -N(R5)-C(O)- in which R5
is hydrogen or a C1-C4 alkyl radical; R6 is a C1-C4 alkyl radical; R7 and R8
are
each independently selected from R and R2 as defined herein before for the
single-long-chain cationic surfactant with only one being R2.
420 Some alkyl pyridinium salts useful in the present invention have the
general formula:
R2-+N~ ~ X-
wherein R2 and X-are as defined above. A typical material of this type is
cetyl
pyridinium chloride.
425 Amine oxides can also be used. Suitable amine oxides include those with
one alkyl, or hydroxyalkyl, moiety of about 8 to about 22 carbon atoms,
preferably from about 10 to about 18 carbon atoms, more preferably from about
12 to about 14 carbon atoms, and two alkyl moieties selected from the group
consisting of alkyl groups and hydroxyalkyl groups containing from one to
about
430 three carbon atoms.
Examples of amine oxides include: dimethyloctylamine oxide;
diethyldecylamine oxide; dimethyldodecylamine oxide; dipropyltetradecylamine
oxide; dimethyl-2-hydroxyoctadecylamine oxide; dimethylcoconutalkylamine
oxide; and bis-(2-hydroxyethyl)dodecylamine oxide.
435
Additional Ingredients
Fully formulated fabric softening compositions may contain, in addition to
the herein before described components, one or more of the following
ingredients.
CA 02376570 2001-12-19
WO 01/02338 PCT/US99/15056
14
440 Concentrated compositions of the present invention may require organic
and/or inorganic concentration aids to go to even higher concentrations and/or
to
meet higher stability standards depending on the other ingredients. Surfactant
concentration aids are typically selected from the group consisting of single
long
chain alkyl cationic surfactants; nonionic surfactants; amine oxides; fatty
acids; or
445 mixtures thereof. If present, such concentration aids are typically
present at up
to about 15% of the composition.
In addition, the compositions of the present invention may include less
than about 1 % by weight of an amphoteric surfactant. Preferably, the
compositions include less than about 0.9% and more preferably less than about
450 0.75% by weight of an amphoteric surfactant.
Viscosity/dispersability modifiers may be added for the purpose of
facilitating the solubilization and/or dispersion, concentration, and/or
improving
phase stability (e.g., viscosity stability). Preferred dispersability
modifiers may
include nonionic surfactants.
455 Suitable nonionic surfactants to serve as the viscosity/dispersability
modifier include addition products of ethylene oxide and, optionally,
propylene
oxide, with fatty alcohols, fatty acids, fatty amines, etc. They are referred
to
herein as, e.g., ethoxylated fatty alcohols, ethoxylated fatty acids, and
ethoxylated fatty amines.
460 Any of the alkoxylated materials of the particular type described
hereinafter can be used as the nonionic surfactant. In general terms, the
nonionic surfactant herein, when used alone, is present at a level of up to
about
5%, preferably from about 0.1 % to about 5%, more preferably from about 0.2%
to about 3%. Suitable compounds are substantially water-soluble surfactants of
465 the general formula:
R2 - Y - (C2H40)Z - C2H40H
wherein R2 is selected from the group consisting of primary, secondary and
branched chain alkyl and/or acyl hydrocarbyl groups; primary, secondary and
branched chain alkenyl hydrocarbyl groups; and primary, secondary and
470 branched chain alkyl- and alkenyl-substituted phenolic hydrocarbyl groups;
said
hydrocarbyl groups having a hydrocarbyl chain length of from about 8 to about
20, preferably from about 10 to about 18 carbon atoms. More preferably the
hydrocarbyl chain length is from about 16 to about 18 carbon atoms. In the
general formula for the ethoxylated nonionic surfactants herein, Y is
typically -O-,
CA 02376570 2001-12-19
WO 01/02338 PCT/US99/15056
475 -C(O)O-, -C(O)N(R)-, or -C(O)N(R)R-, preferably -O-, and in which R2, and
R,
when present, have the meanings given herein before, and/or R can be
hydrogen, and z is at least about 8, preferably at least about 10-11.
Performance and, usually, stability of the softener composition decrease when
fewer ethoxylate groups are present.
480 The nonionic surfactants herein are characterized by an HLB
(hydrophilic-lipophilic balance) of from about 7 to about 20, preferably from
about
8 to about 15. Of course, by defining R2 and the number of ethoxylate groups,
the HLB of the surfactant is, in general, determined. However, it is to be
noted
that the nonionic ethoxylated surfactants useful herein, for concentrated
liquid
485 compositions, contain relatively long chain R2 groups and are relatively
highly
ethoxylated. While shorter alkyl chain surfactants having short ethoxylated
groups may possess the requisite HLB, they are not as effective herein.
Nonionic surfactants are preferred over other viscosity/dispersability
modifiers disclosed herein for compositions with higher levels of perfume.
490 Examples of nonionic surfactants follow. The nonionic surfactants of this
invention are not limited to these examples. In the following examples, the
integer defines the number of ethoxy (E0) groups in the molecule.
The deca-, undeca-, dodecc-, tetradeca-, and pentadecaethoxylates of
n-hexadecanol, and n-octadecanol having an HLB within the range recited
495 herein are useful viscosity/dispersability modifiers in the context of
this invention.
Exemplary ethoxylated primary alcohols useful herein as the
viscosity/dispersability modifiers of the compositions are n-C18E0(10); and
n-C10E0(11 ). The ethoxylates of mixed natural or synthetic alcohols in the
"tallow" chain length range are also useful herein. Specific examples of such
500 materials include tallow alcohol-EO(11 ), tallow alcohol-EO(18), and
tallow
alcohol -EO(25).
The deca-, undeca-, dodecc-, tetradeca-, pentadeca-, octadeca-, and
nonadeca-ethoxylates of 3-hexadecanol, 2-octadecanol, 4-eicosanol, and
5-eicosanol having and HLB within the range recited herein are useful
505 viscosity/dispersability modifiers in the context of this invention.
Exemplary
ethoxylated secondary alcohols useful herein as the viscosity/dispersability
modifiers of the compositions are: 2-C1 gE0(11 ); 2-C20E0(11 ); and 2
-ClgEO(14).
CA 02376570 2001-12-19
WO 01/02338 PCT/US99/15056
16
As in the case of the alcohol alkoxylates, the hexa- through
510 octadeca-ethoxylates of alkylated phenols, particularly monohydric
alkylphenols,
having an HLB within the range recited herein are useful as the
viscosity/dispersability modifiers of the instant compositions. The hexa-
through
octadeca-ethoxylates of p-tridecylphenol, m-pentadecylphenol, and the like,
are
useful herein. Exemplary ethoxylated alkylphenols useful as the
515 viscosity/dispersability modifiers of the mixtures herein are: p-
tridecylphenol
EO(11 ) and p-pentadecylphenol EO(18).
As used herein and as generally recognized in the art, a phenylene group
in the nonionic formula is the equivalent of an alkylene group containing from
2
to 4 carbon atoms. For present purposes, nonionics containing a phenylene
520 group are considered to contain an equivalent number of carbon atoms
calculated as the sum of the carbon atoms in the alkyl group plus about 3.3
carbon atoms for each phenylene group.
The alkenyl alcohols, both primary and secondary, and alkenyl phenols
corresponding to those disclosed immediately hereinabove may be ethoxylated
525 to an HLB within the range recited herein and used as the
viscosity/dispersability
modifiers of the instant compositions.
Branched chain primary and secondary alcohols which are available from
the well-known "0X0" process may be ethoxylated and employed as the
viscosity/dispersability modifiers of compositions herein.
530 The above ethoxylated nonionic surfactants are useful in the present
compositions alone or in combination, and the term "nonionic surfactant"
encompasses mixed nonionic surface active agents.
Mixtures of the above viscosity/dispersability modifiers are highly
desirable. The single long chain cationic surfactant provides improved
535 dispersability and protection for the quaternary nitrogen compounds
against
anionic surfactants and/or detergent builders, such as those that may be
carried
over from the wash solution. If present, the viscosity/dispersability
modifiers are
present at a level of from about 0.1 % to about 30%, preferably from about
0.2%
to about 20%, by weight of the fabric care composition.
540 Stabilizers may be present in the compositions of the present invention.
The term "stabilizer," as used herein, includes antioxidants and reductive
agents
both of which are well-known in the art. These agents are typically present at
a
level of up to about 2%, preferably from about 0.01 % to about 0.2%, more
CA 02376570 2001-12-19
WO 01/02338 PCT/US99/15056
17
preferably from about 0.035% to about 0.1 % by weight of the composition for
545 antioxidants, and more preferably from about 0.01 % to about 0.2% by
weight of
the composition for reductive agents. These assure good odor stability under
long term storage conditions for the compositions and compounds stored in
molten form. The use of antioxidants and reductive agent stabilizers is
especially desirable for low scent products (low perfume).
550 Optionally, the compositions herein contain from 0.01 % to about 10%,
preferably from about 0.1 % to about 5%, more preferably from about 0.1 % to
about 2%, of a soil release agent. Preferably, such a soil release agent is a
polymer. Polymeric soil release agents useful in the present invention include
copolymeric blocks of terephthalate and polyethylene oxide or polypropylene
555 oxide, and the like. U.S. Patent No. 4,956,447 to Gosselink, et al.,
issued
September 11, 1990, discloses specific preferred soil release agents
comprising
cationic functionalities.
A preferred soil release agent is a copolymer having blocks of
terephthalate and polyethylene oxide. More specifically, these polymers are
560 comprised of repeating units of ethylene and/or propylene terephthalate
and
polyethylene oxide terephthalate at a molar ratio of ethylene terephthalate
units
to polyethylene oxide terephthalate units of from about 25:75 to about 35:65,
said
polyethylene oxide terephthalate containing polyethylene oxide blocks having
molecular weights of from about 300 to about 2,000 g/mol. The molecular weight
565 of this polymeric soil release agent is in the range of from about 5,000
to about
55,000 g/mol.
U.S. Patent No. 4,976,879 to Maldonado, et al., issued December 11,
1990, discloses specific preferred soil release agents which may also provide
improved antistatic benefits, said patent being incorporated herein by
reference.
570 Another preferred polymeric soil release agent is a crystallizable
polyester
with repeat units of ethylene terephthalate units containing from about 10% to
about 15% by weight of ethylene terephthalate units together with from about
10% to about 50% by weight of polyoxyethylene terephthalate units, derived
from
a polyoxyethylene glycol of average molecular weight of from about 300 to
about
575 6,000 g/mol, and the molar ratio of ethylene terephthalate units to
polyoxyethylene terephthalate units in the crystallizable polymeric compound
is
between 2:1 and 6:1. Examples of this polymer include the commercially
available materials Zelcon~ 4780 (from DuPont) and Milease~ T (from ICI).
CA 02376570 2001-12-19
WO 01/02338 PCT/US99/15056
18
A more complete disclosure of these highly preferred soil release agents
580 is contained in European Patent Application 185,427 to Gosselink,
published
June 25, 1986.
An optional cellulase may be used in the compositions herein. The
cellulase can be any bacterial or fungal cellulase. Suitable cellulase is
disclosed,
for example, in GB-A-2 075 028, GB-A-2 095 275 and DE-OS-24 47 832.
585 Examples of such cellulase are cellulase produced by a strain of Humicola
insolens (Humicola ra isea var. thermoidea), particularly by the Humicola
strain
DSM 1800, and cellulase 212-producing fungus belonging to the genus
Aeromonas, and cellulase extracted from the hepatopancreas of a marine
mollusk (Dolabella Auricula Solander).
590 The cellulase added to the composition of the invention can be in the form
of a non-dusting granulate, e.g. "marumes" or "prills", or in the form of a
liquid,
e.g., one in which the cellulase is provided as a cellulase concentrate
suspended
in a nonionic surfactant, or dissolved in an aqueous medium.
Preferred cellulase for use herein are characterized in that they provide at
595 least 10% removal of immobilized radioactive labeled carboxymethyl-
cellulose
according to the C,4CMC-method described in European Patent Application 350
098 A at 25x10-6 % by weight of cellulase protein in the laundry test
solution.
A highly preferred cellulase is that described in International Patent
Application WO 91/17243. For example, a cellulase preparation useful in the
600 compositions of the invention can consist essentially of a homogeneous
endoglucanase component, which is immunoreactive with an antibody raised
against a highly purified 43kD cellulase derived from Humicola insolens, DSM
1800, or which is homologous to said 43kD endoglucanase.
The cellulase herein should be used in the liquid fabric-conditioning
605 compositions of the present invention at a level equivalent to an activity
from
about 1 to about 125 CEVU/gram of composition (CEVU = Cellulase Equivalent
Viscosity Unit, as described, for example, in WO 91/13136, and preferably an
activity of from about 5 to about 100.
Other preferred optional ingredients include, but are not limited to, dye
610 transfer inhibiting agents, polymeric dispersing agents, suds suppressors,
optical
brighteners or other brightening or whitening agents, dye fixing agents, light
fading protection agents, oxygen bleach protection agents, traditional fabric
softening agents (e.g., fabric softening clay, anti-static agents, anti-
wrinkle
CA 02376570 2001-12-19
WO 01/02338 PCT/US99/15056
19
agents, and/or other active ingredients), carriers, hydrotropes, processing
aids,
615 dyes or pigments, bactericides, colorants, perfumes, preservatives,
opacifiers,
anti-shrinkage agents, fabric crisping agents, spotting agents, germicides,
fungicides, anti-corrosion agents, and the like.
Synthesis Process
620 The quaternary nitrogen compounds may be formed by first providing a
starting material, an alkoxylated reactant, a fatty acid reactant, and a
quaternizing agent. To form the mono-ester and di-ester quaternary nitrogen
compounds, the starting material may be selected from various aliphatic and
aromatic amines known in the art. However, to form the tri-ester and
quaternary-
625 ester quaternary nitrogen compounds described by Formula II and Formula I,
respectively, the starting material is selected from the group consisting of
ammonia, monoalkyl amine, dialkyl amine, and mixtures thereof, preferably
ammonia, monoalkyl amine, and mixtures thereof, and more preferably
ammonia. Such starting materials are widely available, for example, from Kanto
630 Chemicals, Tokyo, Japan; Wako Chemicals, Osaka, Japan; and Sigma-Aldrich
Chemicals, St. Louis, U.S.A.
The alkoxylated reactant is typically a poly(oxyalkylene) material,
corresponding to Formula III:
X -(R ~ O )n-H (Formula III),
635 where R, and n correspond to R, and n as described in Formula I and
Formula
II, and where X is a halide or alkyl sulfate, preferably a halide or methyl
sulfate,
and more preferably chlorine or bromine. Thus, the alkoxylated reactants are
typically selected from the group consisting of halide and methyl sulfate
derivatives of polyethylene oxides, polypropylene oxides, polyisopropylene
640 oxides, polybutylene oxides, and mixtures thereof; preferably polyethylene
oxide
halides, polyethylene oxide methyl sulfates, polypropylene oxide halides,
polypropylene oxide methyl sulfates, and mixtures thereof; and more preferably
polyethylene oxide chloride, polypropylene oxide chloride, and mixtures
thereof.
These alkoxylated reactants are available, for example, from Tokyo-Kasei
645 Chemicals, Tokyo, Japan; and Sigma-Aldrich Chemicals, St. Louis, U.S.A.
The starting material is then alkylated with the alkoxylated reactant in a
sealed vessel at about 90 °C for about 24 hours, after which time the
vessel is
cooled to room temperature and the reaction worked up. The key parameters of
CA 02376570 2001-12-19
WO 01/02338 PCT/US99/15056
the alkylation reaction are dependent upon the degree of alkylation desired
for
650 the particular starting material. To a single molar equivalent of amine
are added
sufficient alkoxylated reactant to achieve this alkylation and sufficient
alkoxide
base to neutralize the intermediate ammonium salt formed. The alkylation
reaction takes place via a nucleophilic attack of the starting material's
central
nitrogen onto the carbon bearing the leaving group of the alkoxylated
reactant.
655 The alkoxide base neutralizes the intermediate ammonium species to provide
a
free amine which can then undergo additional alkylation reaction to form a
tertiary nitrogen compound. By adjusting the starting material, the ratio of
starting material to alkoxylated reactant, and the ratio of starting material
to
alkoxide base, one can control the level of alkylation, so as to form a non,
singly,
660 doubly, triply, or tertiary-alkylated tertiary nitrogen compound.
Typically a
mixture of these non, singly, doubly, and triply-etherified tertiary nitrogen
compounds will be formed.
In a preferred embodiment, the degree of alkylation is carefully controlled
so as to provide the desired ratio of mono-ether, di-ether, tri-ether, and
optionally
665 quaternary-ether quaternary nitrogen compound. This is especially
desirable, as
it allows much easier formulation and dispersal of the fabric softening active
into
a liquid. This is particularly desirable if the fabric care composition is to
be a
clear, liquid fabric care composition.
The fatty acid reactant corresponds to the R2 group of Formula I and/or
670 Formula II. Thus, the fatty acid reactant typically consists of an RZ
group, and a
reactive moiety joined by a carbonyl group. The fatty acid reactant is
typically of
Formula IV:
,,o
R --~2
(Formula IV),
where RZ corresponds to RZ of Formula I and Formula II, and where X is the
675 reactive moiety. Thus, the R2 group is selected form the group consisting
of a
saturated C,_22 alkyl group, and an unsaturated C,_22 alkyl group; preferably
a
saturated C,2_,8 alkyl group, and an unsaturated C,2_,$ alkyl group. The
reactive
moiety is typically selected form the group consisting of a halide, and an -OH
group; preferably chloride, bromide, and an -OH group; and more preferably
680 chloride, and an -OH group.
CA 02376570 2001-12-19
WO 01/02338 PCT/US99/15056
21
Such fatty acid reactants are available, for example, from Kanto
Chemicals, Tokyo, Japan; Wako Chemicals, Osaka, Japan; and Sigma-Aldrich
Chemicals, St. Louis, U.S.A.
The fatty acid reactant and the tertiary nitrogen compound are then
685 typically reacted in the presence of a solvent, to form a tertiary
alkoxylated ester.
This tertiary alkoxylated ester will typically contain a mixture of compounds
having from zero to three, preferably two to three alkoxylated fatty acid
moieties.
When the fatty acid reactant is a fatty acid halide, then it is preferred that
the
solvent be an inert solvent, such as tetrahydrofuran. The fatty acid halide
690 reactant and the tertiary nitrogen compound are reacted in the presence of
an
organic acid scavenger in an inert solvent for about 3 hours at room
temperature
and then about 6 hours at elevated temperature to form a tertiary amine
alkoxylated ester. The organic acid scavenger is typically a tertiary amine
(pyridine, triethylamine, etc.) which accelerates the reaction and avoids
695 unwanted side reactions. Upon completion of the reaction and cooling to
room
temperature, this acid scavenger is typically converted to an ammonium salt
which is easily removed from the reaction mixture. Following this, the
reaction
mixture may be worked up with ordinary procedures. This tertiary alkoxylated
ester will typically contain a mixture of compounds having from zero to three,
700 preferably two to three alkoxylated ester moieties.
When the fatty acid reactant is a fatty acid, it is preferable that the
solvent
also include a catalyst, preferably an alkaline earth metal hydroxide, in this
reaction. The tertiary nitrogen compound, fatty acid, and alkaline earth metal
hydroxide are then heated to form the tertiary alkoxylated ester. Without
705 intending to be limited by theory, it is believed that this helps increase
yield, and
achieve the final, desired levels of mono-, di-, tri-, and quaternary-ester
quaternary nitrogen compounds.
The quaternizing agent useful herein may be any of the quaternizing
agents known in the art, such as alkyl halides, alkyl sulfates, or
combinations
710 thereof, preferably alkyl halides and more preferably methyl chloride.
However,
if a quaternary-ester quaternary nitrogen compound is desired, then the
quaternizing agent must be an appropriately substituted ester alkoxylate.
These
quaternizing agents are available from, for example, as chloromethane from
Sigma-Aldrich Chemical or Acros Organics, iodomethane from Sigma-Aldrich
715 Chemical or Acros Organics, ethylbromide or benzyl bromide from Sigma-
Aldrich
CA 02376570 2001-12-19
WO 01/02338 PCT/US99/15056
22
Chemical or Acros Organics, and dimethyl sulfate from Sigma-Aldrich Chemical
or Acros Organics.
The tertiary alkoxylated ester is then reacted with the quaternizing agent
in the presence of methanol, for about 24 hours, at about 35 °C, to
form a
720 quaternary nitrogen compound as described herein.
In another embodiment of the present invention, the quaternary nitrogen
compounds may be formed by first providing a starting material, an alkoxylated
reactant, and a fatty acid reactant, as described above. The starting material
is
alkylated with the alkoxylated reactant to form a quaternized alkoxylated
amine,
725 and then reacted with the fatty acid reactant to form a quaternary
nitrogen
compound having from 3 to 4 alkoxylated fatty acid moieties, preferably a
quaternary nitrogen compound of Formula I, having 4 alkoxylated fatty acid
moieties. In this embodiment, the fatty acid reactant is preferably a fatty
acid, as
described above.
730 Typically, the quaternary nitrogen compound will contain a mixture of
mono-ester quaternary nitrogen compounds, di-ester quaternary nitrogen
compounds, tri-ester quaternary nitrogen compounds, and quaternary-ester
quaternary nitrogen compounds. Preferably, the quaternary nitrogen compound
contains a mixture of mono-ester quaternary nitrogen compounds to di-ester
735 quaternary nitrogen compounds to tri-ester quaternary nitrogen compounds,
and
optionally quaternary-ester quaternary nitrogen compounds, as is described
above. This makes it easier to formulate these compounds in a fabric care
composition, because it is less viscous, more easily dispersible, and more
soluble in aqueous media than the pure tri-ester or quaternary-ester
quaternary
740 nitrogen compounds themselves.
The reactant products and/or the finished composition may be analyzed
by, 1 H NMR and high performance liquid chromatography (HPLC) methods
known in the art. The preferred technique for analyzing the presence of mono
ester, di-ester, tri-ester, or quaternary-ester quaternary nitrogen compounds
is
745 by HPLC analysis of the mixture of quaternary nitrogen compounds.
HPLC methods are especially useful, as they allow simultaneous analysis
and fractionation of the mixture of quaternary nitrogen compounds. Using
ordinary HPLC equipment and detection systems, the quaternary nitrogen
compound species may be separated and quantitated with an ion-exchange
750 column (for example, Whatman PARTTISIL 10 SCX) and methanol containing
CA 02376570 2001-12-19
WO 01/02338 PCT/US99/15056
23
ammonium formate as mobile phase. Under these conditions, the more highly
alkoxylated ester quaternary nitrogen compounds will elute more quickly with
the
least alkoxylated ester quaternary nitrogen compound requiring the longest
elution time. From this, the weight percentage of mono-ester, di-ester, tri-
ester,
755 and quaternary-ester quaternary nitrogen compounds may easily be
calculated.
Examples of the invention are set forth hereinafter by way of illustration
and are not intended to be in any way limiting of the invention.
EXAMPLE 1
760 A 4.0 g portion of NaOH (100 mmole) is dissolved in .10 mL water and
allowed to cool to room temperature in a thick walled glass cylinder fitted
with a
locking screw-type seal. To this is added a 36 mL portion (250 mmole) of 2-(2-
(2-chloroethoxy)ethoxy)ethanol (E0=3, Tokyo Kasei Chemical, Tokyo, Japan)
with stirring. A 3.4 mL portion of 28% aqueous ammonia (50 mmole, Wako
765 Chemicals, Osaka, Japan) is also added. The apparatus is sealed tightly,
and
then heated to 90 °C for 24 hours. This corresponds to a molar ratio of
ammonia
to polyethylene oxide chloride of about 1:5 and forms a triply-alkylated
tertiary
nitrogen compound upon extraction and drying. The polyethylene oxide chloride
is provided in excess to insure production of the triply-alkylated tertiary
nitrogen
770 compound.
A 5 gm portion of the tertiary nitrogen compound (12 mmole) is dissolved
in 100 mL THF (tetrahydrofuran) containing 5.3 mL triethylamine (38 mmole).
This is cooled to 0 °C and a 50 mL portion of THF containing 12.8 mL
stearoyl
chloride (38 mmole) is dripped in over a period of about 45 minutes. To assure
775 complete formation of the tertiary ethoxylated ester having three ester
moieties,
the fatty acid chloride is in a molar excess as compared to the alcohol
moieties of
the tertiary nitrogen compound. After stirring at room temperature for 2
hours,
the mixture is heated to 60 °C and stirred for an additional 3 hours.
After cooling
to room temperature, the reaction mixture is further cooled to 0 °C and
allowed to
780 stir overnight. The following day, the solid material is removed by
suction
filtration and the THF solvent of the liquid portion is removed in vacuo. The
resulting syrupy material is dissolved in chloroform, extracted with 1 M NaOH,
and dried with sodium sulfate. The solvent is then removed in vacuo.
A 10 g portion of the tertiary ethoxylated ester (8.2 mmole) is dissolved in
785 150 mL ethanol with stirring. This is treated with 1 mL iodomethane (16.4
mL)
CA 02376570 2001-12-19
WO 01/02338 PCT/US99/15056
24
and the solution is gradually warmed to 35 °C with stirring. After
stirring for 24
hours, the solvent is removed in vacuo and the solid material recovered (the
mixture of quaternary nitrogen compounds) is re-crystallized from hexane. HPLC
analysis confirms presence of about 40% mono-ester quaternary nitrogen
790 compound, about 50% di-ester quaternary nitrogen compound, and about 10%
tri-ester quaternary nitrogen compound. This is then fractionated with a high
performance liquid chromatography column and stored.
EXAMPLE 2
795 A 6.0 g portion of NaOH (15 mmole) is dissolved in .10 mL water and
allowed to cool to room temperature in a thick walled glass cylinder fitted
with a
locking screw-type seal. To this is added a 36 mL portion (250 mmole) of 2-(2-
(2-chloroethoxy)ethoxy)ethanol (E0=3, Tokyo Kasei Chemical, Tokyo, Japan)
with stirring. A 3.4 mL portion of 28% aqueous ammonia (50 mmole, Wako
800 Chemical, Osaka, Japan) is also added. The apparatus is sealed tightly and
heated to 90 °C for 24 hours. This corresponds to a molar ratio of
ammonia to
polyethylene oxide chloride of about 1:5 and forms a quaternized ethoxylated
amine with four ethylene oxide substituents. After extraction to remove excess
polyethyleneoxide derivative, the aqueous layer is dried to remove water and
the
805 quaternized ethoxylated amine is poured away from the sodium chloride. The
polyethylene oxide chloride is provided in excess to insure production of the
quaternized ethoxylated amine.
A 10 gm portion of the quaternized ethoxylated amine (17 mmole) is
heated with 19.5 g stearic acid (68 mmole) to 220 °C in the presence of
0.2 g tin
810 powder. This is stirred mechanically for at least 2 hours until the
removal of
water is complete. The mixture is then cooled to approximately 60 °C
and the
viscous oil is poured away from the tin powder and allowed to cool into a
solid
mass. The mixture contains about 30% mono-ester quaternary nitrogen
compound, about 45% di-ester quaternary nitrogen compound, about 15% tri-
815 ester quaternary nitrogen compound, and about 10% quaternary-ester
quaternary nitrogen compound. This is then fractionated with a high
performance liquid chromatography column and stored.
EXAMPLE 3
820 Liquid fabric softening compositions are prepared as follows:
CA 02376570 2001-12-19
WO 01/02338 PCT/US99/15056
The fabric softening active is melted in a water bath at a temperature of
from about 70 °C to about 75 °C to from a molten organic phase.
The mono-
long-chain-alkyl cationic surfactant is premixed with 1,2 hexane diol and then
added to the molten organic phase. Separately, the silicone anti-foaming agent
825 and hydrochloric acid are added to water, covered and heated to a
temperature
of from about 70 °C to about 75 °C.
The aqueous system is transferred to an insulated baffled mixing vessel
which is fitted with a turbine blade impeller at a temperature of from about
70 °C
to about 75 °C. The molten organic phase is slowly added to the aqueous
phase
830 under high speed agitation. The dispersion becomes highly .viscous. A
small
portion of the total calcium chloride is slowly added to the dispersion as a
2.5%
solution.
The dispersion is milled using a probe rotor-stator high shear device for a
period of time corresponding to batch size. The milled product is chilled in
an ice
835 bath to room temperature over a 3-6 minute period. The other ingredients,
minors, and remaining calcium chloride are added with vigorous mixing. Dye is
then added as desired. The final product is very fluid with a viscosity of
less than
100 cps.
These liquid fabric softening compositions are formulated as follows:
840 A B C D E
In redient Wt.% Wt.% Wt.% Wt.% Wt.%
Fabric Softenin Active 24 - 10 5 12
1
Fabric Softenin Active - 25 10 30 12
2
Iso ro anol 0.3 0.3 0.3 0.3 0.3
Calcium Chloride 0.05 - - 0.1 -
H drochloric acid 0.5 0.5 0.5 0.5 0.5
Soil Release A ent 0.2 - 0.2 0.2 0.2
Silicone Anti-foam 0.01 0.01 0.01 - 0.01
Pol eth lene I co14000 0.6 0.6 0.6 0.6 0.6
1, 2 hexane diol 4.5 6 - 8 4
Mono-long-chain-alkyl - 6 4 10 10
cationic surfactant
3
Water + minors to 100 to 100 to 100 to 100 to 100
(1 ) quaternary nitrogen compound mixture of Example 1
(2) quaternary nitrogen compound mixture of Example 2
CA 02376570 2001-12-19
WO 01/02338 PCT/US99/15056
26
(3) C,2 C,4 dimethyl hydroxyethyl quaternary ammonium chloride
The above formulation examples provide excellent softening benefits, and
845 are surprisingly stable, while remaining readily biodegradable. Examples B-
E,
are clear, transparent, aqueous, isotropic liquids.