Note: Descriptions are shown in the official language in which they were submitted.
CA 02377217 2001-12-28
1
DESCRIPTION
COMPOUND WITH TETRAHYDRONAPHTHALENE SKELETON AND LIQUID
CRYSTAL COMPOSITION CONTAINING SAME
TECHNICAL FIELD
The present invention relates to a novel liquid crystal compound comprising a
tetrahydronaphthalene derivative which is useful as an electrooptical liquid
crystal display
material, as well as a liquid crystal composition containing such a compound
and a liquid
crystal display element utilizing the compound.
BACKGROUND ART
Liquid crystal display elements are now used not only in clocks and
calculators, but
also in various types of measuring instruments, automobile instrument panels,
word
processors, personal digital assistants, printers, computers and televisions.
Typical
examples of liquid crystal display methods include TN (twisted nematic) types,
STN (super
twisted nematic) types, DS (dynamic scattering) types, GH (guest host) types,
or methods
which enable high speed response such as FLC (ferroelectric liquid crystal)
types or AFLC
(antiferroelectric liquid crystal) types. In addition, multiplex driving is
replacing the
conventional static driving as the most common type of driving method, and
moreover,
simple matrix systems, and more recently, active matrix systems, have also
come into
practical use.
Extremely large numbers of different liquid crystal compounds have been
synthesized
for use as liquid crystal materials, and these are selected and used depending
on the display
CA 02377217 2001-12-28
2
method, the driving method and the final use of the display. However the
demand for
improved performance (such as improved display quality or increased display
screen size)
from liquid crystal display elements has grown stronger over the years, and
development of
new liquid crystal compounds to satisfy these demands is ongoing.
Liquid crystal compounds generally comprise a central skeleton part known as
the
core, with terminal portions on both sides. Typically, the most common ring
structure that
composes the core portion of the liquid crystal compound is a 1,4-phenylene
group (which
may be substituted with one or two halogen atoms, cyano groups, or methyl
groups or the
like) and a trans-l,4-cyclohexylene group. However, liquid crystal compounds
composed
solely of a 1,4-phenylene group and a trans-l,4-cyclohexylene group are
limited in terms of
the variety of possible compounds and the associated characteristics, and are
currently unable
to adequately accommodate the aforementioned demands.
Ring structures which have been investigated other than the 1,4-phenylene
group and
trans-l,4-cyclohexylene group described above include heterocyclic ring
systems such as a
pyridine-2,5-diyl group, pyrimidine-2,5-diyl group, and a 1,3-dioxane-trans-
2,5-diyl group,
and fused ring systems such as a trans-decahydronaphthalene-2,6-diyl group,
naphthalene-
2,6-diyl group, tetrahydronaphthalene-2,6-diyl group, bicyclo [2.2.2] octane-
1,4-diyl group,
and a spiro[3.3]heptane-2,6-diyl group, although production problems
(including technical
problems and cost) and stability problems mean that only a very small number
are currently
used in practical applications.
Of these fused ring systems, the tetrahydronaphthalene-2,6-diyl group is a
ring
structure that has been known for considerable time, although synthetic
examples are
extremely few, and furthermore, characteristics of the ring structure other
than the liquid
crystallinity (the phase transition temperature), in particular the
characteristics of the structure
CA 02377217 2001-12-28
3
as a nematic liquid crystal, are virtually unknown. (Recently, it was reported
that an
optically active alcohol ester of tetrahydronaphthalene-2-carboxylic acid
showed smectic
liquid crystal characteristics, and in particular highly interesting
characteristics as a
ferroelectric liquid crystal and an antiferroelectric liquid crystal.
Furthermore, a
ferroelectric liquid crystal skeleton comprising a fluorine containing
tetrahydronaphthalene
structure has also been recently reported.)
In a typical liquid crystal compound, at least one of the terminal portions is
a chain
like (side chain) group, and in cases in which the dielectric anisotropy is
positive, so-called p-
type liquid crystals, then the other terminal portion is usually a polar
group.
In TN or STN display methods, in order to reduce the associated driving
voltage, a
compound with a large positive dielectric anisotropy (p-type) is required. In
order to satisfy
this requirement, typically compounds with a cyano group at the molecule
terminal, and
moreover at least one fluorine atom in the same direction on the molecule are
used. The
reported tetrahydronaphthalene derivatives almost all have a dielectric
anisotropy between 0
and a negative value, so-called n-type liquid crystals, and the only examples
of p-type liquid
crystals where a polar group has been introduced onto a tetrahydronaphthalene
skeleton are
limited to compounds with a phenyltetrahydronaphthalene skeleton or a phenyl
tetrahydronaphthalene-2-carboxylate skeleton, and no comments have been made
about the
electrooptical characteristics, nor examples given of potential applications
(Helv. Chim. Acta,
65, 1318-1330 (1982)).
Examples of p-type compounds for use with the aforementioned active matrix
driving
method include only compounds with a fluorine atom, a fluoroalkoxy group or a
fluoroalkyl
group as the polar group, and no reports have been made of a
tetrahydronaphthalene
compound. Furthermore, although many liquid crystal compounds which are
CA 02377217 2001-12-28
4
tetrahydronaphthalene derivatives are not particularly co-soluble with other
liquid crystal
compounds, it is thought that introducing a side substituent (a fluorine atom
is particularly
desirable) into the tetrahydronaphthalene skeleton would be effective in
alleviating this
problem. In terms of application to the aforementioned active matrix system,
it is thought
that substitution with a fluorine group would also be effective in the case in
which a polar
group is introduced directly into the tetrahydronaphthalene ring. There are no
actual
synthetic examples of this type of fluorotetrahydronaphthalene derivative, and
at present it is
impossible to even estimate the properties of this type of compound.
In liquid crystal compounds, it is known that replacing the alkyl group
typically used
as the side chain portion with an alkenyl group, results in superior effects
such as an
improvement in liquid crystallinity, a reduction in viscosity, and
improvements in the
sharpness of the display characteristics. However, these alkenyl groups are
usually
introduced via direct bonding to a cyclohexane ring, and no compounds have
been reported
where such an introduction has occurred on an aromatic ring, or more
particularly a
tetrahydronaphthalene ring.
Similarly, tetrahydronaphthalene derivatives with a side chain such as an
alkoxylalkyl
group, a fluoroalkyl group, a fluoroalkenyl group, or a fluoroalkenyloxy group
or the like,
have also not been reported.
In terms of the ring structure linkage group within the core of a liquid
crystal
compound, in addition to single bonds and ester groups (-COO-, -OCO-), many
bivalent
organic groups such as a 1,2-ethylene group (-CH2CH2-), an ethynylene group (-
C=C-) or a
difluoroethenylene group (-CF=CF-) are also known, although such groups are
unknown
within tetrahydronaphthalene derivatives.
CA 02377217 2001-12-28
DISCLOSURE OF INVENTION
The problem which the present invention attempts to resolve is to provide a
novel
compound with a tetrahydronaphthalene skeleton, and furthermore to provide a
practical
liquid crystal composition using such a compound.
5 As a result of intensive investigations aimed at resolving the above
problem, the
present inventors discovered compounds with a tetrahydronaphthalene skeleton
which could
be produced with ease, which in the main showed liquid crystallinity over a
wide temperature
range, and which regardless of the liquid crystallinity or otherwise of any
single compound,
upon addition to a composition, could be mixed with the composition without
any lowering
in the response speed (in a large number of cases the response speed
improves), without any
marked narrowing of the targeted liquid crystal temperature range (in a large
number of cases
the temperature range widens), and without any layer separation, and thereby
completed the
present invention.
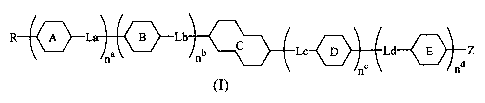
Invention 1: A tetrahydronaphthalene derivative represented by a general
formula (I)
R A La B Lb
na nb Lc Ld Z
nd
(~)
(wherein, R represents a saturated or unsaturated alkyl group or alkoxyl group
of 1 to 20
carbon atoms which may incorporate a branched chain and may be substituted
with 1 to 7
fluorine atoms or alkoxyl groups of 1 to 7 carbon atoms; the linkage groups
La, Lb, Lc and
Ld each represent independently a single bond, -CH2CH2-, -CH=CH-, -CH(CH3)CH2-
,
-CH2CH(CH3)-, -CH(CH3)CH(CH3)-, -CF2CF2-, -CF=CF-, -CH2O-, -OCH2-, -OCH(CH3)-,
-CH(CH3)O-, -C=C-, -CF2O-, -OCF2-, -COO-, -OCO-, -COS- or -SCO-; Z represents
a
fluorine atom, chlorine atom, cyano group, cyanato group, trifluoromethoxy
group or a
CA 02377217 2001-12-28
6
difluoromethoxy group; ring A, ring B and ring D each represent independently
a trans-1,4-
cyclohexylene group, trans-decahydronaphthalene-2,6-diyl group, trans-l,3-
dioxane-2,4-diyl
group, or a 1,4-phenylene group which may be substituted with one or two
fluorine atoms,
pyridine-2,5-diyl group, pyrimidine-2,5-diyl group, pyrazine-2,5-diyl group, a
pyridazine-3,6-
diyl group, and a naphthalene-2,6-diyl group which may be substituted with one
or two
fluorine atoms; ring E represents independently a 1,4-phenylene group which
may be
substituted with one or two fluorine atoms, and a naphthalene-2,6-diyl group
which may be
substituted with one or two fluorine atoms; ring C represents either the
general formula (IIa)
or the general formula (IIb)
x x5 x6
x' x2 (IIa) x4 (IIb)
(wherein, XI, X2, X3, X4, X5 and X6 each represent a hydrogen atom or a
fluorine atom); and
a b c d
n , n , n and n each represent independently either 0 or 1.
However, in the case in which nc = 1, nd = 0, the ring D represents a 1,4-
phenylene
group which may be substituted with one or two fluorine atoms and/or a
naphthalene-2,6-diyl
group which may be substituted with one or two fluorine atoms.
Furthermore, in the case in which Z is a cyano group, R is an unsubstituted
and
saturated alkyl group or alkoxyl group, na = n = nd = 0 and nb = 1, or nb = n
= nd = 0 and n a=
1, ring A and ring B are 1,4-phenylene groups, La and Lb are single bonds, and
ring C is the
general formula (IIa), then at least one of Xl, X2 and X3 represents a
fluorine atom.
Furthermore, in the case in which Z is a cyano group, R is an unsubstituted
and
saturated alkyl group or alkoxyl group, na = nb = n = 0 and nd = 1, or na =
nb = nd = 0 and nc _
1, ring C and ring D are 1,4-phenylene groups, Lc and Ld are single bonds or -
COO- linkages,
and ring C is the general formula (IIa), then at least one of Xl, X2 and X3
represents a fluorine
CA 02377217 2001-12-28
7
atom.
Furthermore, in the case in which Z is a cyano group, R is an unsubstituted
and
saturated alkyl group or alkoxyl group, na = nb = d = 0 and nd = 1, or ne = nb
= nd = 0 and n =
1, ring C and ring D are 1,4-phenylene groups, Lc and Ld are single bonds or -
COO- linkages,
and ring C is the general formula (IIb), then at least one of X4, X5 and X6
represents a fluorine
atom.
Furthermore, in the case in which Z is a fluorine atom, R is an unsubstituted
and
saturated alkyl group or alkoxyl group, na = nb = n= 0 and nd = 1, or na = nb
= nd = 0 and n _
1, ring C and ring D are 1,4-phenylene groups, Lc and Ld are -COO- linkages,
and ring C is
the general formula (IIb), then at least one of X4, X5 and X6 represents a
fluorine atom.
In addition, in the case in which ring C is the general formula (IIb), at
least one of n'
and n is 1.).
Invention 2: A tetrahydronaphthalene derivative according to Invention 1,
wherein in
the general formula (I), the ring C is represented by the formula (11a).
Invention 3: A tetrahydronaphthalene derivative according to Invention 1,
wherein in
the general formula (I), the ring C is represented by the forrnula (IIb).
Invention 4: A tetrahydronaphthalene derivative according to any one of
Inventions 1
through 3, wherein in the general formula (I), either na or nb is 0.
Invention 5: A tetrahydronaphthalene derivative according to any one of
Inventions 1
through 4, wherein in the general formula (I), either n or nd is 0.
Invention 6: A tetrahydronaphthalene derivative according to any one of
Inventions 1
through 5, wherein in the general formula (I), na = nb = 0.
Invention 7: A tetrahydronaphthalene derivative according to any one of
Inventions 1,
2, 4 and 5, wherein in the general formula (I), n = nd = 0.
CA 02377217 2001-12-28
8
Invention 8: A tetrahydronaphthalene derivative according to any one of
Inventions 1
through 7, wherein in the general formula (I), at least one of na, nb, n and
nd is 1.
Invention 9: A tetrahydronaphthalerie derivative according to any one of
Inventions 1
through 8, wherein in the general formula (I), the linkage groups La, Lb, Lc
and Ld are each
selected independently from a group consisting of a single bond, -CH2CH2-, and
-C=C-.
Invention 10: A tetrahydronaphthalene derivative according to any one of
Inventions 1
through 9, wherein in the general formula (I), the linkage groups La, Lb, Lc
and Ld are each
selected independently from a group consisting of a single bond and -CH2CH2-.
Invention 11: A tetrahydronaphthalene derivative according to any one of
Inventions 1
through 10, wherein in the general formula (I), the linkage groups La, Lb, Lc
and Ld are each
a single bond.
Invention 12: A tetrahydronaphthalene derivative according to any one of
Inventions I
through 11, wherein in the general formula (I), the ring A, the ring B and the
ring D are each
independently selected from a group consisting of a trans-1,4-cyclohexylene
group, a trans-
decahydronaphthalene-2,6-diyl group, a trans-1,3-dioxane-2,4-diyl group, or a
1,4-phenylene
group which may be substituted with one or two fluorine atoms, and a
naphthalene-2,6-diyl
group which may be substituted with one or two fluorine atoms.
Invention 13: A tetrahydronaphthalene derivative according to any one of
Inventions 1
through 12, wherein in the general formula (I), Z is a fluorine atom.
Invention 14: A tetrahydronaphthalene derivative according to any one of
Inventions 1
through 12, wherein in the general formula (I), Z is a cyano group.
Invention 15: A tetrahydronaphthalene derivative according to any one of
Inventions 1
through 12, wherein in the general formula (I), Z is a trifluoromethoxy group.
Invention 16: A tetrahydronaphthalene derivative according to any one of
Inventions I
CA 02377217 2001-12-28
9
through 15, wherein in the general formula (I), R is a saturated or
unsaturated alkyl group of I
to 20 carbon atoms which may incorporate a branched chain and may be
substituted with 1 to
7 fluorine atoms or alkoxyl groups of 1 to 7 carbon atoms.
Invention 17: A tetrahydronaphthalene derivative according to any one of
Inventions 1
through 16, wherein in the general formula (I), R is a saturated or
unsaturated straight chain
alkyl group of 1 to 20 carbon atoms.
Invention 18: A tetrahydronaphthalene derivative according to any one of
Inventions I
through 17, wherein in the general formula (I), X3, X4 and X5 in the formula
(IIa) and the
formula (IIb) are hydrogen atoms.
Invention 19: A tetrahydronaphthalene derivative according to any one of
Inventions 1
through 18, wherein in the general formula (I), X2 in the formula (IIa) is a
hydrogen atom and
Xl is a fluorine atom.
Invention 20: A tetrahydronaphthalene derivative according to any one of
Inventions 1
through 18, wherein in the general formula (I), X1 in the formula (IIa) is a
hydrogen atom and
X2 is a fluorine atom.
Invention 21: A tetrahydronaphthalene derivative according to any one of
Inventions I
through 20 which shows liquid crystallinity.
Invention 22: A tetrahydronaphthalene derivative which forms a nematic phase.
Invention 23: A tetrahydronaphthalene derivative according to any one of
Inventions 1
through 22 which upon addition to a nematic liquid crystal composition forms a
nematic
phase.
Invention 24: A liquid crystal composition containing one or more compounds of
the
general formula (I) according to any one of Inventions 1 through 23.
Invention 25: A liquid crystal composition according to Invention 24 which can
be
CA 02377217 2001-12-28
used for active matrix driving.
Invention 26: A liquid crystal element comprising a liquid crystal composition
according to Invention 25 as a structural element.
Invention 27: An active matrix driven liquid crystal display element utilizing
a liquid
5 crystal composition according to Invention 26.
BEST MODE FOR CARRYING OUT THE INVENTION
In the compound of the general formula (I) described above, suitable examples
of the
R, the linkage groups La, Lb, Lc and Ld, the polar group Z, the ring A, the
ring B, the ring C,
10 the ring D and the ring E, as well as na, nb, nc and nd are described
below.
na, nb, n and nd are either 0 or 1, and with the exception of the case in
which ring C is
represented by the formula (lIb), any combinations are possible, although in
cases in which na
+ nb + n + nd = 3 or 4 the melting point and the viscosity increase, and in
the case in which na
+ nb + n + nd = 0 the liquid crystallinity deteriorates, and so cases in
which na + nb + n + nd
= 1 or 2 are preferred.
Examples of the group R include straight chain saturated alkyl groups such as
a
methyl group, ethyl group, propyl group, butyl group, pentyl group, hexyl
group, heptyl
group, octyl group, nonyl group, decyl group, undecyl group, dodecyl group,
tridecyl group,
tetradecyl group, pentadecyl group, hexadecyl group, heptadecyl group,
octadecyl group,
nonadecyl group and an eicosyl group; branched saturated alkyl groups such as
a 1-
methylethyl group, 1-methylpropyl group, 2-methylpropyl group, 1,2-
dimethylpropyl group,
1-methylbutyl group, 2-methylbutyl group, 3-methylbutyl group, 1,2-
dimethylbutyl group,
1,3-dimethylbutyl group, 2,3-dimethylbutyl group, 1-methylpentyl group, 2-
methylpentyl
group, 3-methylpentyl group, 4-methylpentyl group, 1,2-dimethylpentyl group,
1,3-
CA 02377217 2001-12-28
11
dimethylpentyl group, 1-methylhexyl group, 2-methylhexyl group, 3-methylhexyl
group, 4-
methylhexyl group, 5-methylhexyl group, 1,2-dimethylhexyl group, 1,3-
dimethylhexyl group,
1-methylheptyl group, 2-methylheptyl group, 3-methylheptyl group, 4-
methylheptyl group, 5-
methylheptyl group, 6-methylheptyl group, 1,2-dimethylheptyl group, 1,3-
dimethylheptyl
group, 1-methyloctyl group, 2-methyloctyl group, 3-methyloctyl group, 4-
methyloctyl group,
5-methyloctyl group, 6-methyloctyl group, 7-methyloctyl group, 1,2-
dimethyloctyl group,
1,3-dimethyloctyl group, 1-methyinonyl group, 2-methylnonyl group, 3-
methylnonyl group,
4-methylnonyl group, 5-methylnonyl group, 6-methylnonyl group, 7-methylnonyl
group, 8-
methylnonyl group, 1,2-dimethylnonyl group, 1,3-dimethylnonyl group, 1-
methyldecyl group,
2-methyldecyl group, 3-methyldecyl group, 1,2-dimethyldecyl group, 1,3-
dimethyldecyl
group, 1-methylundecyl group, 2-methylundecyl group, 3 -methylundecyl group,
1,2-
dimethylundecyl group, 1,3-dimethylundecyl group, 1-methyldodecyl group, 2-
methyldodecyl
group, 3-methyldodecyl group, 1,2-dimethyldodecyl group, 1,3-dimethyldodecyl
group, 1-
methyltridecyl group, 2-methyltridecyl group, 3-methyltridecyl group, 1,2-
dimethyltridecyl
group and a 1,3-dimethyltridecyl group; unsaturated alkyl groups such as a
vinyl group, trans-
1-propenyl group, 2-propenyl group, trans-l-butenyl group, trans-2-butenyl
group, 3-butenyl
group, trans-1-pentenyl group, trans-2-pentenyl group, trans-3-pentenyl group,
4-pentenyl
group, trans-1-hexenyl group, trans-2-hexenyl group, trans-3-hexenyl group,
trans-4-hexenyl
group, 5-hexenyl group, trans-l-heptenyl group, trans-2-heptenyl group, trans-
3-heptenyl
group, trans-4-heptenyl group, trans-5-heptenyl group, 6-heptenyl group, trans-
l-octenyl
group, trans-2-octenyl group, trans-3-octenyl group, trans-4-octenyl group,
trans-5-octenyl
group, trans-6-octenyl group, 7-octenyl group, trans-l-nonenyl group, 8-
nonenyl group, trans-
1-decenyl group, 9-decenyl group, trans-l-undecenyl group, 10-undecenyl group,
trans-l-
dodecenyl group, 11-dodecenyl group, ethynyl group, 1-propynyl group, 2-
propynyl group, 1-
CA 02377217 2001-12-28
12
butynyl group, 2-butynyl group, 3-butynyl group, 1-pentynyl group, 2-pentynyl
group, 3-
pentynyl group, 4-pentynyl group, 1-hexynyl group, 2-hexynyl group, 3-hexynyl
group, 4-
hexynyl group, 5-hexynyl group, 1-heptynyl group, 2-heptynyl group, 3-heptynyl
group, 4-
heptynyl group, 5-heptynyl group, 6-heptynyl group, 1-octynyl group, 2-octynyl
group, 3-
octynyl group, 4-octynyl group, 5-octynyl group, 6-octynyl group, 7-octynyl
group, 1-nonynyl
group, 8-nonynyl group, 1-decynyl group, 9-decynyl group, 1-undecynyl group,
10-undecynyl
group, 1-dodecynyl group, 11-dodecynyl group, 1-tridecynyl group and a 12-
tridecynyl group;
fluorine substituted alkyl groups such as a fluoromethyl group, difluoromethyl
group,
trifluoromethyl group, 2-fluoromethyl group, 2,2-difluoroethyl group, 2,2,2-
trifluoroethyl
group, 1,1,2,2,2-pentafluoroethyl group, 3-fluoropropyl group, 2-fluoropropyl
group, 1-
fluoropropyl group, 3,3-difluoropropyl group, 3,3,3-trifluoropropyl group,
2,2,3,3-
tetrafluoropropyl group, 2,2,3,3,3-pentafluoropropyl group, 1,1,2,2,3,3,3-
heptafluoropropyl
group, 4-fluorobutyl group, 3-fluorobutyl group, 2-fluorobutyl group, 1-
fluorobutyl group,
4,4-difluorobutyl group, 4,4,4-trifluorobutyl group, 3,3,4,4-tetrafluorobutyl
group, 3,3,4,4,4-
pentafluorobutyl group, 2,2,3,3,4,4,4-heptafluorobutyl group, 5-fluoropentyl
group, 4-
fluoropentyl group, 3-fluoropentyl group, 2-fluoropentyl group, 1-fluoropentyl
group, 5,5-
difluoropentyl group, 5,5,5-trifluoropentyl group, 4,4,5,5-tetrafluoropentyl
group, 4,4,5,5,5-
pentafluoropentyl group, 3,3,4,4,5,5,5-heptafluoropentyl group, 6-fluorohexyl
group, 5-
fluorohexyl group, 4-fluorohexyl group, 3-fluorohexyl group, 2-fluorohexyl
group, 1-
fluorohexyl group, 6,6-difluorohexyl group, 6,6,6-trifluorohexyl group,
5,5,6,6,6-
pentafluorohexyl group, 4,4,5,5,6,6,6-heptafluorohexyl group, 7-fluoroheptyl
group, 6-
fluoroheptyl group, 5-fluoroheptyl group, 4-fluoroheptyl group, 3-fluoroheptyl
group, 2-
fluoroheptyl group, 1-fluoroheptyl group, 7,7-difluoroheptyl group, 7,7,7-
trifluoroheptyl
group, 6,6,7,7-tetrafluoroheptyl group, 6,6,7,7,7-pentafluoroheptyl group,
5,5,6,6,7,7,7-
CA 02377217 2001-12-28
13
heptafluoroheptyl group, 8-fluorooctyl group, 7-fluorooctyl group, 6-
fluorooctyl group, 5-
fluorooctyl group, 4-fluorooctyl group, 3-fluorooctyl group, 2-fluorooctyl
group, 1-
fluorooctyl group, 8,8-difluorooctyl group, 8,8,8-trifluorooctyl group,
7,7,8,8-tetrafluorooctyl
group, 7,7,8,8,8-pentafluorooctyl group and a 6,6,7,7,8,8,8-heptafluorooctyl
group; fluorine
substituted unsaturated alkyl groups such as a 2,2-difluoroethenyl group, (E)-
1,2-
difluoroethenyl group, (Z)-1,2-difluoroethenyl group, 3,3-difluoro-2-propenyl
group, (E)-2,3-
difluoro-2-propenyl group, (Z)-2,3-difluoro-2-propenyl group, 4,4-difluoro-3-
butenyl group,
(E)-3,4-difluoro-3-butenyl group, (Z)-3,4-difluoro-3-butenyl group, 5,5-
difluoro-4-pentenyl
group, (E)-4,5-difluoro-4-pentenyl group, (Z)-4,5-difluoro-4-pentenyl group,
6,6-difluoro-5-
hexenyl group, (E)-5,6-difluoro-5-hexenyl group, (Z)-5,6-difluoro-5-hexenyl
group, (E)-1,2-
difluoro-l-propenyl group, (E)-1,2-difluoro-l-butenyl group, (E)-1,2-difluoro-
l-pentenyl
group, (E)-1,2-difluoro-l-hexenyl group, (Z)-1-fluoro-l-propenyl group, (Z)-1-
fluoro-l-
butenyl group, (Z)-1-fluoro-1-pentenyl group, (Z)-1-fluoro-l-hexenyl group,
(Z)-2-fluoro-l-
propenyl group, (Z)-2-fluoro-l-butenyl group, (Z)-2-fluoro-l-pentenyl group,
(Z)-2-fluoro-l-
hexenyl group, (E)-2,3-difluoro-2-butenyl group, (E)-2,3-difluoro-2-pentenyl
group, (E)-2,3-
difluoro-2-hexenyl group, (Z)-2-fluoro-2-butenyl group, (Z)-2-fluoro-2-
pentenyl group, (Z)-
2-fluoro-2-hexenyl group, (Z)-3-fluoro-2-butenyl group, (Z)-3-fluoro-2-
pentenyl group and a
(Z)-3-fluoro-2-hexenyl group; alkoxyl group substituted alkyl groups such as a
methoxymethyl group, ethoxymethyl group, propoxymethyl group, butoxymethyl
group,
pentyloxymethyl group, hexyloxymethyl group, heptyloxymethyl group, 1-
methoxyethyl
group, 1-ethoxyethyl group, 1-propoxyethyl group, 1-butoxyethyl group, 1-
pentyloxyethyl
group, 1-hexyloxyethyl group, 1-heptyloxyethyl group, 2-methoxyethyl group, 2-
ethoxyethyl
group, 2-propoxyethyl group, 2-butoxyethyl group, 2-pentyloxyethyl group, 2-
hexyloxyethyl
group, 2-heptyloxyethyl group, 1-methoxypropyl group, 1-ethoxypropyl group, 1-
aftis u puu 'pauaja.zd am -D-D- Pue -zHDzHD- 'puoq ai2uts t, 'asaql jo pun '-
ODS- zO -SOD-
'-ODO- '-OOD- '-z3DO- '-Oz3D- '-D=D- '-O(~HD)HD- '-(~HD)HDO- '-zHDO- '-OzHD-
'-3D=3D- '-z3Dz3D- '-(~HD)HD(~HO)HD- '-(~HD)HDzHD- '-zHD(~HD)HD- '-HD=HD-
'-zHDzHD- 'puoq ai2uts n apnjoui pq pun o-I 'q-I'n-I sdnoz2 aBnxt.tij atpjo
saidumxg
-dno.t2 iXualdaq-9 t, pu>? 'dnoB iSuajdaq-S-sun4 'dnoa jXuajdau-{, OZ
-sut?zl 'dnoi2 i.Kuajdaq-~-su>3tj'dnoi2 j,Cuajdaq-Z-sue4 'dnoig j,Cuaidau-j-
sunzl 'dnoj2 iXuaxau
-s 'dno.z2 jXuaxau-{,-suu4 'dno.t2 i/,uaxaq-E-suu4 'dnoB iXuaxaq-Z-
suuij'dno.z2 jXuaxau
-i-suv.tl 'dno.z2 jXuajuad-t, 'dnoa jXuajuad-~-suntj'dnot2 i,iuajuad-Z-suu4
'dno.zS iXualuad
-i-sut,4 'dno.z2 jXuainq-~ 'dno.i2 i,Cuajnq-Z-suezj 'dnozS iAuainq-l-suu4
'dnoz2 ixuado.zd
-Z 'dnoz2 iXuado.td-j-su>?n 'dnoA If.uin 'dnoa ifloo 'dnoB 1/4datj 'dnoiB
jXxaq 'dno.zs IXIuad 91
'dnozg ji4nq 'dno.t2 iXdotd 'dnoa2 iXtpa 'dnoj2 i,Ktpauz u apnjout sdno.t2
aiqvitsap Xi.znjnatwd
pum 'pa.uajaad a.re sdno.r2 iXxiu pairm}usun puu sdnoz2 if.xTe palumivs ureuo
jqi~re4s
'sdno.t2iXxjvqons jO -pauajaid a.re sdnoz2 iXxit, q2notpje 'joaaaqj sdnoa
jxxoxit? aql
pu>? 'dnoi2 iXxaqXxoiXldaq-9 L, puu dno.t2 jXxaufxoiXxaq-q 'dno.i2
iXxauXxoiXluad-9 'dnoi2
iXxaqAxolnq-9 'dnoA jXxaqXxodozd-9 'dnoa iXxauXxotpa-9 'dnoj2 i,ixauAxotpaux-q
'dnoi2 0t
jfquadUxoj,44daq-S 'dnoa2 1,44uadUxoiXxau-S 'dnoB jf4uadAxoi)4uad-S 'dnoj2
I,44uadAxolnq-S
'dnoz2 jf4uadUxodozd-S 'dnoa jA4uadAxocpa-S 'dno.zB if4uaaxo.tpauz-S 'dno.zS
If4nqAxojA4daq
-t,'dnozg jf4nqAxoj,Cxaq-t, 'dnoa2 if4nqAxojAjuad-{, 'dno.tS j,44nqAxojnq-{,
'dnoiB
jf4nq,ixodojd-t,'dnoa if4nqAxotpa-t, 'dnoa jf4nqXxoqlauz-t,'dnoaB
iXdoidUxoif4dau-~ 'dnozB
i,ido.tcUxojXxau-~ 'dnoB ifdo.zdAxoiAjuad-~ 'dno.tB iAdozdUxolnq-~ 'dno.tS
jXdoidAxodozd S
-~ 'dnoj2 iAdo.iaxotpa-E 'dnoi? iAdo.tdAxotpauz-~ 'dnozB iAdo.tduxojf4daq-Z
'dnoiS
iXdozdXxolAxaq-Z 'dnozg jAdoadUxojAjuad-Z 'dno.t2 iAdo.zdAxolnq-Z 'dnozS
lAdo.zduxodoid
-Z 'dno.zS iAdo idUxotpa-Z 'dnoag iXdoidAxoqlauz-Z 'dnoaS iAdo.tdAxoif4daq-
i'dnoaO
i,fdozdAxolAxau- j'dnoa jAdojdUxojf4uad- j'dnoi$ iAdoidAxolnq- i'dno.iS
iAdoadUxodoid
bi
8Z-ZT-TOOZ LTZLL~ZO FIO
CA 02377217 2001-12-28
bond is particularly preferred.
Examples of the group Z include a fluorine atom, chlorine atom, cyano group,
cyanato
group, trifluoromethoxy group or a difluoromethoxy group, although a fluorine
atom, a cyano
group, or a trifluoromethoxy group are preferred, and a fluorine atom or a
trifluoromethoxy
5 group are particularly desirable.
Examples of the ring A, the ring B and the ring D include the types of
structures
shown in the formulas group 1.
0
F F F
F
F F F
N-
~ ~ ~
F F
N N N- N-
~ ~ -C N- ~N}- -~-?~
F
N-N - - -
~ ~ ~ ~ ~ ~ ~ ~ ~
- - - F
F
- F -
F F
F F F F
C ~ ~ ~ ~ ~ ~
F - - F -
F F F F
F AP F F F
formulas group I
CA 02377217 2001-12-28
16
Of these ring structures, the structures shown in the formulas group 2 are
preferred.
F F
F
- - - F -
F F F
F - F -
F F
formulas group 2
Of these ring structures, the structures shown in the formulas group 3 are
particularly
desirable.
F F
F F
~ ~
- F
F
(~ ~
- F
formulas group 3
Examples of the ring E, and the ring D in the case in which n = 0 include the
types of
structures shown in the formulas group 4.
CA 02377217 2001-12-28
17
F F F
F
F F F
b- --0- - )-
F F F
~ ~ ~ ~ - - F
F F F F F F
- F
F F F
F
F F F F
F
F - F
F
formulas group 4
Of these ring structures, the structures shown in the formulas group 5 are
preferred.
F F
F F
~ ~ ~ ~ ~
- F -
F F F
C
F 5 F F
formulas group 5
CA 02377217 2001-12-28
18
Of these ring structures, the structures shown in the formulas group 6 are
particularly
desirable.
F F _
~
F F F
F
formulas group 6
Examples of the formula (Ila) and the formula (lIb) of the ring C include the
types of
structures shown in the formulas group 7.
-CQ- -C
F F
F F F
F F F F F F
F F
cb
F F F F F F
F F
formulas group 7
Of these ring structures, the structures shown in the formulas group 8 are
preferred.
CA 02377217 2001-12-28
19
F
~ ~ ~ ~ ~ $-
F F
F
cb- -cb
formulas group 8
As described above, the compound of the aforementioned general formula (I)
incorporates a very large variety of compounds depending on the selection of
the group R, the
linkage groups La, Lb, Lc and Ld, the polar group Z, the ring A, the ring B,
the ring C, the
ring D and the ring E, and na, nb, nc and nd, and by appropriate selection of
each of these
structural sites, compounds can be prepared which are applicable to a wide
range of uses and
fields. As a result, compounds of the general formula (I) show characteristics
such as a
broad nematic temperature range, good stability against light and heat, and a
high voltage
retention, that are extremely useful for electrooptical elements, and are
particular preferable
for STN-LCD and AM-LCD devices. Specifically, of the compounds represented by
the
general formula (I), particularly preferred compounds include those listed
below.
In the compounds listed, the following abbreviations are used, so that R'
represents
any one of a methyl group, ethyl group, propyl group, butyl group, pentyl
group, hexyl
group, heptyl group, octyl group, vinyl group, trans-l-propenyl group, 2-
propenyl group,
trans-l-butenyl group, trans-2-butenyl group, 3-butenyl group, trans-1-
pentenyl group, trans-
2-pentenyl group, trans-3-pentenyl group, 4-pentenyl group, trans-l-hexenyl
group, trans-2-
hexenyl group, trans-3-hexenyl group, trans-4-hexenyl group, 5-hexenyl group,
trans-l-
heptenyl group, trans-2-heptenyl group, trans-3-heptenyl group, trans-4-
heptenyl group, trans-
5-heptenyl group, and a 6-heptenyl group.
CA 02377217 2001-12-28
F
Cy : Ph 1 : Ph2: F
F
Ph3: \ / Np l : Np2:
F -
F
Np3: Np4:
F
Th l : / \ Th2: Th3: / \ Th4:
F F
F
Te l : \ / Te2: \ /
In the case in which ring C is the formula (IIa),
then in the case in which na = l, nb = 0 or na = 0, nb = 1, and nc= nd = 0,
and Z is a fluorine
atom,
5 R'-Cy-ThI-F, R'-Phl-Thl-F, R'-Ph2-Thl-F, R'-Ph3-Thl-F, R'-Npl-Thl-F,
R'-Np2-Thl-F, R'-Np3-Thl-F, R'-Np4-Thl-F, R'-Cy-Th2-F, R'-Phl-Th2-F,
R'-Ph2-Th2-F, R'-Ph3-Th2-F, R'-Npl-Th2-F, R'-Np2-Th2-F, R'-Np3-Th2-F,
R'-Np4-Th2-F, R'-Cy-Th3-F, R'-Phl-Th3-F, R'-Ph2-Th3-F, R'-Ph3-Th3-F,
R'-Npl-Th3-F, R'-Np2-Th3-F, R'-Np3-Th3-F, R'-Np4-Th3-F, R'-Cy-Th4-F,
10 R' -Ph 1-Th4-F, R' -Ph2-Th4-F, R'-Ph3 -Th4-F, R' -Np 1-Th4-F, R -Np2-Th4-F,
R'-Np3-Th4-F, R'-Np4-Th4-F, R'-Cy-CH2CH2-Thl-F, R'-PhI-CHZCHZ-ThI-F,
R' -Ph2-CHZCH2-Th l-F, R' -Ph3 -CH2CH2-Th 1-F, R' -Np 1-CH2CH2-Th l-F,
R'-Np2-CH2CH2-Thl-F, R'-Np3-CH2CH2-Thl-F, R'-Np4-CH2CH2-Thl-F,
R'-Cy-CH2CH2-Th2-F, R'-Phl-CH2CH2-Th2-F, R'-Ph2-CH2CH2-Th2-F,
CA 02377217 2001-12-28
21
R' -Ph3 -CHZCH2-Th2-F, R' -Np 1-CH2CH2-Th2-F, R' -Np2-CH2CH2-Th2-F,
R'-Np3-CH2CH2-Th2-F, R'-Np4-CH2CH2-Th2-F, R'-Cy-CH2CH2-Th3-F,
R'-Phl-CH2CH2-Th3-F, R'-Ph2-CH2CH2-Th3-F, R'-Ph3-CHZCH2-Th3-F,
R'-Npl-CH2CH2-Th3-F, Rt-Np2-CH2CH2-Th3-F, R'-Np3-CH2CH2-Th3-F,
R'-Np4-CH2CHZ-Th3-F, R'-Cy-CH2CH2-Th4-F, R'-Phl-CH2CH2-Th4-F,
R'-Ph2-CH2CH2-Th4-F, R'-Ph3-CH2CH2-Th4-F, R'-Npl-CH2CH2-Th4-F,
R'-Np2-CH2CH2-Th4-F, R'-Np3-CH2CH2-Th4-F, R'-Np4-CH2CH2-Th4-F,
in the case in which na = 1, nb = 0 or na = 0, nb = 1, and n = nd = 0, and Z
is a cyano group,
R'-Cy-Thl-CN, R'-Ph2-Thl-CN, R'-Ph3-Thl-CN, R'-Npl-Thl-CN, R'-Np2-Thl-CN,
R' -Np3-Th 1-CN, R'-Np4-Th 1-CN, R'-Cy-Th2-CN, R'-Ph 1-Th2-CN, R' -Ph2-Th2-CN,
R'-Ph3-Th2-CN, R'-Npl-Th2-CN, R'-Np2-Th2-CN, R'-Np3-Th2-CN, R'-Np4-Th2-CN,
R'-Cy-Th3-CN, R'-Phl-Th3-CN, R'-Ph2-Th3-CN, R'-Ph3-Th3-CN, R'-Npl-Th3-CN,
R'-Np2-Th3-CN, R'-Np3-Th3-CN, R'-Np4-Th3-CN, R'-Cy-Th4-CN, R'-Phl-Th4-CN,
R'-Ph2-Th4-CN, R'-Ph3-Th4-CN, R'-Npl-Th4-CN, R'-Np2-Th4-CN, R'-Np3-Th4-CN,
R'-Np4-Th4-CN,
R'-Cy-CH2CH2-Th1-CN, R'-Ph1-CH2CH2-Th1-CN, R'-Ph2-CHZCH2-Th1-CN,
R'-Ph3-CH2CH2-Th1-CN, R'-Np1-CH2CH2-Th1-CN, R'-Np2-CH2CH2-Th1-CN,
R'-Np3-CH2CH2-Thl-CN, R'-Np4-CH2CH2-Thl-CN, RI-Cy-CH2CH2-Th2-CN,
R'-Phl-CH2CH2-Th2-CN, R'-Ph2-CH2CH2-Th2-CN, R'-Ph3-CH2CH2-Th2-CN,
R'-Npl-CH2CH2-Th2-CN, R'-Np2-CH2CH2-Th2-CN, R'-Np3-CH2CH2-Th2-CN,
R'-Np4-CH2CH2-Th2-CN, R'-Cy-CH2CH2-Th3-CN, R'-Phl-CH2CH2-Th3-CN,
R'-Ph2-CH2CH2-Th3-CN, R'-Ph3-CHzCH2-Th3-CN, R'-Npl-CH2CH2-Th3-CN,
R'-Np2-CH2CH2-Th3-CN, R'-Np3-CH2CH2-Th3-CN, R'-Np4-CH2CH2-Th3-CN,
R' -Cy-CH2CH2-Th4-CN, R' -Ph 1-CH2CH2-Th4-CN, R' -Ph2-CH2CH2-Th4-CN,
CA 02377217 2001-12-28
22
R'-Ph3-CH2CH2-Th4-CN, R'-Npl-CH2CH2-Th4-CN, R'-Np2-CH2CH2-Th4-CN,
R'-Np3-CH2CH2-Th4-CN, R'-Np4-CH2CH2-Th4-CN,
in the case in which na = 1, nb = 0 or na = 0, nb = 1, and nc = nd = 0, and Z
is a trifluoromethyl
group,
R'-Cy-ThI-OCF3, R'-Phl-ThI-OCF3, R'-Ph2-Thl-OCF3, R'-Ph3-Thl-OCF3,
R'-Npl-ThI-OCF3, R'-Np2-Thl-OCF3, R'-Np3-Thl-OCF3, R'-Np4-Thl-OCF3,
R'-Cy-Th2-OCF3, R'-Phl-Th2-OCF3, R'-Ph2-Th2-OCF3, R'-Ph3-Th2-OCF3,
R'-Npl-Th2-OCF3, R'-Np2-Th2-OCF3, R'-Np3-Th2-OCF3, R'-Np4-Th2-OCF3,
R'-Cy-Th3-OCF3, R'-Phl-Th3-OCF3, R'-Ph2-Th3-OCF3, R'-Ph3-Th3-OCF3,
R'-Npl-Th3-OCF3, R'-Np2-Th3-OCF3, R'-Np3-Th3-OCF3, R'-Np4-Th3-OCF3,
R'-Cy-Th4-OCF3, R'-Phl-Th4-OCF3, R'-Ph2-Th4-OCF3, R'-Ph3-Th4-OCF3,
R'-Npl-Th4-OCF3, R'-Np2-Th4-OCF3, R'-Np3-Th4-OCF3, R'-Np4-Th4-OCF3,
R'-Cy-CH2CH2-Thl-OCF3, R'-Phl-CH2CH2-Thl-OCF3, R'-Ph2-CH2CH2-Thl-OCF3,
R'-Ph3-CH2CH2-Th1-OCF3, R'-Npl-CH2CH2-Thl-OCF3, R'-Np2-CH2CH2-Thl-OCF3,
R'-Np3-CH2CH2-Th1-OCF3, Rl-Np4-CH2CH2-Th1-OCF3, R'-Cy-CH2CH2-Th2-OCF3,
R'-Phl-CH2CH2-Th2-OCF3, R'-Ph2-CH2CH2-Th2-OCF3, R'-Ph3-CH2CH2-Th2-OCF3,
R' -Np 1-CH2CH2-Th2-OCF3, R' -Np2-CH2CH2-Th2-OCF3, R' -Np3 -CH2CH2-Th2-OCF3,
R'-Np4-CH2CH2-Th2-OCF3, R'-Cy-CH2CH2-Th3-OCF3, R'-Phl-CH2CH2-Th3-OCF3,
R'-Ph2-CH2CH2-Th3-OCF3, R'-Ph3-CH2CH2-Th3-OCF3, R'-Np1-CHZCH2-Th3-OCF3,
R'-Np2-CH2CH2-Th3-OCF3, R'-Np3-CH2CH2-Th3-OCF3, R'-Np4-CH2CH2-Th3-OCF3,
R'-Cy-CH2CH2-Th4-OCF3, R' -Ph 1-CH2CH2-Th4-OCF3, R'-Ph2-CH2CH2-Th4-OCF3,
R'-Ph3-CH2CH2-Th4-OCF3, R'-Npl-CH2CH2-Th4-OCF3, R'-Np2-CH2CH2-Th4-OCF3,
R'-Np3-CH2CHZ-Th4-OCF3, R'-Np4-CH2CH2-Th4-OCF3,
in the case in which na = nb = 1, and n = nd = 0, and Z is a fluorine atom,
CA 02377217 2001-12-28
23
R'-Cy-Cy-Thl -F, R' -Phl -Cy-Th 1-F, R'-Ph2-Cy-Th 1-F, R'-Ph3-Cy-Th 1-F,
R'-Np1-Cy-Th1-F, R'-Np2-Cy-Th1-F, R'-Np3-Cy-Th1-F, R'-Np4-Cy-Th1-F,
R'-Cy-Cy-Th2-F, R'-Phl-Cy-Th2-F, R'-Ph2-Cy-Th2-F, R'-Ph3-Cy-Th2-F,
R'-Npl-Cy-Th2-F, R'-Np2-Cy-Th2-F, R'-Np3-Cy-Th2-F, R'-Np4-Cy-Th2-F,
R'-Cy-Cy-Th3-F, R'-Phl-Cy-Th3-F, R'-Ph2-Cy-Th3-F, R'-Ph3-Cy-Th3-F,
R'-Npl-Cy-Th3-F, R'-Np2-Cy-Th3-F, R'-Np3-Cy-Th3-F, R'-Np4-Cy-Th3-F,
R'-Cy-Cy-Th4-F, R'-Phl-Cy-Th4-F, R'-Ph2-Cy-Th4-F, R'-Ph3-Cy-Th4-F,
R'-Npl-Cy-Th4-F, R'-Np2-Cy-Th4-F, R'-Np3-Cy-Th4-F, R'-Np4-Cy-Th4-F,
R' -Cy-CH2CH2-Cy-Th 1-F, R' -Ph 1-CH2CH2-Cy-Th 1-F, R' -Ph2-CH2CHz-Cy-Th 1-F,
R' -Ph3-CH2CH2-Cy-Th 1-F, R' -Np 1-CH2CH2-Cy-Th 1-F, R' -Np2-CH2CH2-Cy-Th 1-F,
R'-Np3-CH2CH2-Cy-ThI-F, R'-Np4-CHZCH2-Cy-Thl-F, R'-Cy-CH2CH2-Cy-Th2-F,
R'-Phl-CH2CH2-Cy-Th2-F, R'-Ph2-CH2CH2-Cy-Th2-F, R'-Ph3-CH2CH2-Cy-Th2-F,
R'-Npl-CH2CH2-Cy-Th2-F, R'-Np2-CHZCH2-Cy-Th2-F, R'-Np3-CH2CH2-Cy-Th2-F,
R'-Np4-CH2CH2-Cy-Th2-F, R'-Cy-CH2CH2-Cy-Th3-F, R'-Phl-CH2CHZ-Cy-Th3-F,
R'-Ph2-CH2CH2-Cy-Th3-F, Rl-Ph3-CH2CH2-Cy-Th3-F, R'-Npl-CH2CH2-Cy-Th3-F,
R'-Np2-CHZCH2-Cy-Th3-F, Rl-Np3-CH2CH2-Cy-Th3-F, Rl-Np4-CH2CH2-Cy-Th3-F,
R'-Cy-CH2CH2-Cy-Th4-F, Rl-Phl-CH2CH2-Cy-Th4-F, R'-Ph2-CH2CH2-Cy-Th4-F,
R1-Ph3-CH2CH2-Cy-Th4-F, R'-NpI-CHZCH2-Cy-Th4-F, R'-Np2-CH2CH2-Cy-Th4-F,
R'-Np3-CH2CH2-Cy-Th4-F, Rl-Np4-CH2CH2-Cy-Th4-F,
R'-Cy-Phl-Thl-F, R'-Phl-Phl-Thl-F, Rl-Ph2-Phl-Thl-F, R'-Ph3-Phl-Thl-F,
R'-Npl-Phl-Thl-F, R'-Np2-Phl-Thl-F, R'-Np3-Phl-Thl-F, R'-Np4-Phl-Thl-F,
R'-Cy-Phl-Th2-F, R'-Phl-Phl-Th2-F, R'-Ph2-Phl-Th2-F, R'-Ph3-Phl-Th2-F,
R'-Npl-Phl-Th2-F, R'-Np2-Phl-Th2-F, R'-Np3-Phl-Th2-F, R'-Np4-Phl-Th2-F,
R'-Cy-Phl-Th3-F, R'-Phl-Phl-Th3-F, R'-Ph2-Phl-Th3-F, R'-Ph3-Phl-Th3-F,
CA 02377217 2001-12-28
24
R'-Np1-Phl-Th3-F, R'-Np2-Ph1-Th3-F, R'-Np3-Ph1-Th3-F, R'-Np4-Ph1-Th3-F,
R'-Cy-Ph1-Th4-F, R'-Ph1-Ph1-Th4-F, R'-Ph2-Ph1-Th4-F, R'-Ph3-Ph1-Th4-F,
R'-Np 1-Ph 1-Th4-F, R'-Np2-Phl -Th4-F, R'-Np3-Ph 1-Th4-F, R'-Np4-Phl -Th4-F,
R' -Cy-CH2CH2-Ph 1-Th 1-F, R' -Ph 1-CHZCHZ-Ph l-Th 1-F, R' -Ph2-CH2CH2-Ph 1-Th
1-F,
R'-Ph3-CH2CH2-Ph1-Th1-F, R'-Np1-CH2CH2-Ph1-Th1-F, R'-Np2-CH2CH2-Ph1-Thl-F,
R'-Np3-CH2CH2-Phl-Thl-F, R'-Np4-CH2CH2-Phl-Thl-F, R'-Cy-CH2CH2-Phl-Th2-F,
R'-Phl-CH2CH2-Phl-Th2-F, R'-Ph2-CH2CH2-Phl-Th2-F, R'-Ph3-CH2CH2-Phl-Th2-F,
R' -Np 1-CH2CH2-Ph 1-Th2-F, R' -Np2-CH2CH2-Ph 1-Th2-F, R' -Np3-CH2CH2-Ph 1-Th2-
F,
R' -Np4-CH2CH2-Ph 1-Th2-F, R' -Cy-CH2CH2-Ph 1-Th3 -F, R' -Ph 1-CH2CH2-Ph 1-Th3
-F,
R'-Ph2-CH2CH2-Phl-Th3-F, R'-Ph3-CHZCH2-Phl-Th3-F, R'-Npl-CH2CH2-Phl-Th3-F,
R'-Np2-CH2CH2-Phl-Th3-F, R'-Np3-CH2CH2-Phl-Th3-F, R'-Np4-CH2CH2-Phl-Th3-F,
R' -Cy-CHZCHZ-Ph l-Th4-F, R' -Ph l-CH2CH2-Ph 1-Th4-F, R' -Ph2-CH2CHZ-Ph 1-Th4-
F,
R' -Ph3 -CHZCH2-Ph 1-Th4-F, R' -Np 1-CH2CH2-Ph 1-Th4-F, R' -Np2-CH2CH2-Ph 1-
Th4-F,
R'-Np3-CH2CH2-Phl-Th4-F, R'-Np4-CH2CH2-Phl-Th4-F,
R'-PhI-C=C-Phl-Thl-F, R'-Ph2-C=C-Phl-Thl-F, R'-Ph3-C=C-Phl-Thl-F,
R'-Phl -C=C-Phl -Th2-F, R'-Ph2-C-C-Phl -Th2-F, R'-Ph3-C=C-Phl -Th2-F,
R'-Phl-C-C-Phl-Th3-F, R'-Ph2-C=C-Phl-Th3-F, R'-Ph3-C=C-Phl-Th3-F,
R' -Ph 1-C=C-Ph 1-Th4-F, R' -Ph2-C=C-Ph 1-Th4-F, R' -Ph3 -C=C-Ph 1-Th4-F,
R'-Cy-Ph2-Th1-F, R'-Ph1-Ph2-Th1-F, R'-Ph2-Ph2-Th1-F, R'-Ph3-Ph2-Th1-F,
R'-Np1-Ph2-Th1-F, R'-Np2-Ph2-Thl-F, R'-Np3-Ph2-Thl-F, R'-Np4-Ph2-Th1-F,
R'-Cy-Ph2-Th2-F, R'-Phl-Ph2-Th2-F, R'-Ph2-Ph2-Th2-F, R'-Ph3-Ph2-Th2-F,
R'-Npl-Ph2-Th2-F, R'-Np2-Ph2-Th2-F, R'-Np3-Ph2-Th2-F, R'-Np4-Ph2-Th2-F,
R'-Cy-Ph2-Th3-F, R'-Phl-Ph2-Th3-F, R'-Ph2-Ph2-Th3-F, R'-Ph3-Ph2-Th3-F,
R'-Npl-Ph2-Th3-F, R'-Np2-Ph2-Th3-F, R'-Np3-Ph2-Th3-F, R'-Np4-Ph2-Th3-F,
CA 02377217 2001-12-28
R'-Cy-Ph2-Th4-F, R'-Phl-Ph2-Th4-F, R'-Ph2-Ph2-Th4-F, R'-Ph3-Ph2-Th4-F,
R'-Np1-Ph2-Th4-F, R'-Np2-Ph2-Th4-F, R'-Np3-Ph2-Th4-F, R'-Np4-Ph2-Th4-F,
R' -Cy-CH2CH2-Ph2-Th 1-F, R' -Ph 1-CHZCH2-Ph2-Th 1-F, R' -Ph2-CHZCH2-Ph2-Th 1-
F,
R' -Ph3-CH2CH2-Ph2-Th 1-F, R' -Np 1-CH2CH2-Ph2-Th 1-F, R' -Np2-CH2CH2-Ph2-Th 1-
F,
5 R'-Np3-CH2CH2-Ph2-Thl-F, Rl-Np4-CH2CH2-Ph2-Thl-F, R'-Cy-CH2CH2-Ph2-Th2-F,
R'-Phl-CH2CH2-Ph2-Th2-F, R'-Ph2-CH2CH2-Ph2-Th2-F, R'-Ph3-CH2CH2-Ph2-Th2-F,
R'-Npl-CH2CH2-Ph2-Th2-F, R'-Np2-CH2CH2-Ph2-Th2-F, R'-Np3-CH2CH2-Ph2-Th2-F,
R'-Np4-CH2CH2-Ph2-Th2-F, RI-Cy-CH2CH2-Ph2-Th3-F, R'-Phl-CHZCH2-Ph2-Th3-F,
R'-Ph2-CHZCH2-Ph2-Th3-F, R'-Ph3-CH2CH2-Ph2-Th3-F, R'-Npl-CH2CH2-Ph2-Th3-F,
10 R'-Np2-CH2CH2-Ph2-Th3-F, R'-Np3-CH2CH2-Ph2-Th3-F, R'-Np4-CH2CH2-Ph2-Th3-F,
R'-Cy-CH2CH2-Ph2-Th4-F, R'-Phl-CH2CH2-Ph2-Th4-F, R'-Ph2-CH2CH2-Ph2-Th4-F,
R' -Ph3 -CH2CHZ-Ph2-Th4-F, R' -Np 1-CHZCH2-Ph2-Th4-F, R' -Np2-CH2CH2-Ph2-Th4-
F,
R'-Np3-CHZCH2-Ph2-Th4-F, R'-Np4-CH2CH2-Ph2-Th4-F,
R' -Ph 1-C=C-Ph2-Th 1-F, R' -Ph2-C=C-Ph2-Th 1-F, R' -Ph3-C=C-Ph2-Th 1-F,
15 R'-PhI-C=C-Ph2-Th2-F, R'-Ph2-C=C-Ph2-Th2-F, R'-Ph3-C=C-Ph2-Th2-F,
R'-Phl-C=C-Ph2-Th3-F, R'-Ph2-C=C-Ph2-Th3-F, R'-Ph3-C=C-Ph2-Th3-F,
R' -Ph 1-C=C-Ph2-Th4-F, R' -Ph2-C=C-Ph2-Th4-F, R' -Ph3-CGC-Ph2-Th4-F,
R'-Cy-Ph3-Thl-F, R'-Phl-Ph3-Thl-F, R'-Ph2-Ph3-Thl-F, R'-Ph3-Ph3-Thl-F,
R'-Npl-Ph3-Thl-F, R'-Np2-Ph3-Thl-F, Rl-Np3-Ph3-Thl-F, R'-Np4-Ph3-Th1-F,
20 R'-Cy-Ph3-Th2-F, R'-Phl-Ph3-Th2-F, R'-Ph2-Ph3-Th2-F, R'-Ph3-Ph3-Th2-F,
R'-Npl-Ph3-Th2-F, R'-Np2-Ph3-Th2-F, R'-Np3-Ph3-Th2-F, R'-Np4-Ph3-Th2-F,
R'-Cy-Ph3-Th3-F, R'-Phl-Ph3-Th3-F, Rl-Ph2-Ph3-Th3-F, R'-Ph3-Ph3-Th3-F,
R'-Npl-Ph3-Th3-F, R'-Np2-Ph3-Th3-F, R'-Np3-Ph3-Th3-F, R'-Np4-Ph3-Th3-F,
R'-Cy-Ph3-Th4-F, R'-Phl-Ph3-Th4-F, Rl-Ph2-Ph3-Th4-F, R'-Ph3-Ph3-Th4-F,
CA 02377217 2001-12-28
26
R'-Npl-Ph3-Th4-F, R'-Np2-Ph3-Th4-F, R'-Np3-Ph3-Th4-F, R'-Np4-Ph3-Th4-F,
R'-Cy-CHZCH2-Ph3-Thl-F, R'-Phl-CH2CH2-Ph3-Thl-F, R'-Ph2-CH2CH2-Ph3-Thl-F,
R'-Ph3-CH2CH2-Ph3-Thl-F, R'-Npl-CH2CH2-Ph3-Thl-F, R'-Np2-CH2CH2-Ph3-Thl-F,
R'-Np3-CHZCH2-Ph3-Thl-F, R'-Np4-CH2CH2-Ph3-Thl-F, R'-Cy-CH2CHZ-Ph3-Th2-F,
R'-Phi-CH2CH2-Ph3-Th2-F, R'-Ph2-CH2CH2-Ph3-Th2-F, R'-Ph3-CH2CH2-Ph3-Th2-F,
R' -Np 1-CH2CH2-Ph3-Th2-F, R'-Np2-CH2CH2-Ph3-Th2-F, R'-Np3-CH2CH2-Ph3-Th2-F,
R'-Np4-CH2CH2-Ph3-Th2-F, R'-Cy-CH2CH2-Ph3-Th3-F, R'-Phl-CH2CH2-Ph3-Th3-F,
R'-Ph2-CHZCH2-Ph3-Th3-F, R'-Ph3-CHZCH2-Ph3-Th3-F, R'-Npl-CH2CH2-Ph3-Th3-F,
R'-Np2-CH2CH2-Ph3-Th3-F, R'-Np3-CH2CH2-Ph3-Th3-F, R'-Np4-CH2CH2-Ph3-Th3-F,
RI-Cy-CH2CH2-Ph3-Th4-F, R'-Phl-CH2CH2-Ph3-Th4-F, R'-Ph2-CH2CH2-Ph3-Th4-F,
R'-Ph3-CH2CH2-Ph3-Th4-F, R'-Npl-CH2CH2-Ph3-Th4-F, R'-Np2-CH2CH2-Ph3-Th4-F,
R'-Np3-CH2CH2-Ph3-Th4-F, R'-Np4-CHZCH2-Ph3-Th4-F,
R'-Phi-C=C-Ph3-Thl-F, Rl-Ph2-C=C-Ph3-Thl-F, R'-Ph3-C=C-Ph3-Thl-F,
R'-PhI-C=C-Ph3-Th2-F, R'-Ph2-C-C-Ph3-Th2-F, R'-Ph3-C=C-Ph3-Th2-F,
R'-Phl-C-C-Ph3-Th3-F, R'-Ph2-C=C-Ph3-Th3-F, R'-Ph3-C=C-Ph3-Th3-F,
R'-PhI-C=C-Ph3-Th4-F, R'-Ph2-C=C-Ph3-Th4-F, R'-Ph3-C=C-Ph3-Th4-F,
R'-Cy-Npl-Thl-F, R'-Phl-Npl-Thl-F, R'-Ph2-Npl-Thl-F, R'-Ph3-Npl-Thl-F,
R'-Cy-Np 1-Th2-F, R'-Ph 1-Np 1-Th2-F, R'-Ph2-Np 1-Th2-F, R' -Cy-Np 1-Th3-F,
R'-Ph1-Npl-Th3-F, R'-Ph2-Np1-Th3-F, R'-Ph3-Np1-Th3-F, R'-Cy-Np1-Th4-F,
R'-Phl-Npl-Th4-F, R'-Ph2-Npl-Th4-F, R'-Ph3-Npl-Th4-F,
R' -Cy-CH2CH2-Np 1-Th 1-F, R' -Ph 1-CH2CH2-Np 1-Th 1-F, R' -Ph2-CH2CH2-Np 1-Th
1-F,
R 1-Ph3 -CH2CH2-Np 1-Th 1-F, R' -Cy-CH2CH2-Np 1-Th2-F, R' -Ph 1-CH2CH2-Np 1-
Th2-F,
R' -Ph2-CH2CH2-Np 1-Th2-F, R' -Ph3-CH2CH2-Np 1-Th2-F, R' -Cy-CH2CH2-Np 1-Th3-
F,
R'-Phl -CH2CH2-Np 1-Th3-F, R'-Ph2-CH2CH2-Np 1-Th3-F, R' -Ph3-CH2CH2-Np 1-Th3-
F,
CA 02377217 2001-12-28
27
R' -Cy-CH2CH2-Np 1-Th4-F, R' -Ph 1-CH2CH2-Np 1-Th4-F, R' -Ph2-CH2CH2-Np 1-Th4-
F,
R' -Ph3-CH2CH2-Np 1-Th4-F,
in the case in which na = nb = 1, and nc = nd = 0, and Z is a cyano group,
R' -Cy-Cy-Th 1-CN, R' -Ph 1-Cy-Th 1-CN, R'-Ph2-Cy-Thl -CN, R' -Ph3-Cy-Th 1-CN,
R'-Np 1-Cy-Thl -CN, R'-Np2-Cy-Thl -CN, R'-Np3-Cy-Th 1-CN, R'-Np4-Cy-Thl -CN,
R'-Cy-Cy-Th2-CN, R'-Phl-Cy-Th2-CN, R'-Ph2-Cy-Th2-CN, R'-Ph3-Cy-Th2-CN,
R'-Npl-Cy-Th2-CN, R'-Np2-Cy-Th2-CN, R'-Np3-Cy-Th2-CN, R'-Np4-Cy-Th2-CN,
R'-Cy-Cy-Th3-CN, R'-Phl-Cy-Th3-CN, R'-Ph2-Cy-Th3-CN, R'-Ph3-Cy-Th3-CN,
R'-Npl-Cy-Th3-CN, R'-Np2-Cy-Th3-CN, R'-Np3-Cy-Th3-CN, R'-Np4-Cy-Th3-CN,
R'-Cy-Cy-Th4-CN, R'-Phl-Cy-Th4-CN, R'-Ph2-Cy-Th4-CN, R'-Ph3-Cy-Th4-CN,
R'-Npl-Cy-Th4-CN, R'-Np2-Cy-Th4-CN, R'-Np3-Cy-Th4-CN, R'-Np4-Cy-Th4-CN,
R' -Cy-CH2CH2-Cy-Th 1-CN, R' -Ph 1-CH2CH2-Cy-Th 1 -CN, R' -Ph2-CH2CH2-Cy-Th 1-
CN,
R'-Ph3-CH2CH2-Cy-ThI-CN, R'-Npl-CH2CH2-Cy-Thl-CN, Rl-Np2-CH2CH2-Cy-Thl-CN,
R'-Np3-CHZCH2-Cy-Thl-CN, R'-Np4-CH2CH2-Cy-Thl-CN, RI-Cy-CH2CH2-Cy-Th2-CN,
R'-Phl-CH2CH2-Cy-Th2-CN, R'-Ph2-CH2CH2-Cy-Th2-CN, R'-Ph3-CH2CH2-Cy-Th2-CN,
R1-Npl-CH2CH2-Cy-Th2-CN, Rl-Np2-CH2CH2-Cy-Th2-CN, Rl-Np3-CH2CH2-Cy-Th2-CN,
R'-Np4-CH2CH2-Cy-Th2-CN, R'-Cy-CH2CH2-Cy-Th3-CN, R'-Phl-CH2CH2-Cy-Th3-CN,
R'-Ph2-CH2CH2-Cy-Th3-CN, R'-Ph3-CH2CH2-Cy-Th3-CN, R'-Npl-CH2CH2-Cy-Th3-CN,
R'-Np2-CH2CH2-Cy-Th3-CN, Rl-Np3-CH2CH2-Cy-Th3-CN, R'-Np4-CH2CH2-Cy-Th3-CN,
R'-Cy-CH2CH2-Cy-Th4-CN, Rl-Phl-CH2CHZ-Cy-Th4-CN, R'-Ph2-CH2CH2-Cy-Th4-CN,
R'-Ph3-CH2CH2-Cy-Th4-CN, Rl-Npl-CH2CH2-Cy-Th4-CN, R'-Np2-CHZCH2-Cy-Th4-CN,
R'-Np3-CH2CH2-Cy-Th4-CN, R'-Np4-CH2CH2-Cy-Th4-CN,
R'-Cy-Ph1-Th1-CN, R'-Ph1-Ph1-Th1-CN, R'-Ph2-Ph1-Th1-CN, R'-Ph3-Ph1-Th1-CN,
R'-Npl-Phl-Thl-CN, R'-Np2-Phl-Thl-CN, R'-Np3-Phl-Thl-CN,
CA 02377217 2001-12-28
28
R'-Np4-Ph1-Th1-CN, R~-Cy-Phi-Th2-CN, R'-Ph1-Ph1-Th2-CN, R'-Ph2-Phl-Th2-CN,
R'-Ph3-Phl-Th2-CN, R'-Npl-Phl-Th2-CN, R'-Np2-Phl-Th2-CN,
R'-Np3-Ph1-Th2-CN, R'-Np4-Ph1-Th2-CN, R'-Cy-Ph1-Th3-CN, R'-Ph1-Ph1-Th3-CN,
R' -Ph2-Ph 1-Th3 -CN, R' -Ph3 -Ph 1-Th3 -CN, R' -Np 1-Ph 1-Th3 -CN,
R'-Np2-Phl-Th3-CN, R'-Np3-Phl-Th3-CN, R'-Np4-Phl-Th3-CN, R'-Cy-Phl-Th4-CN,
R' -Ph 1-Ph 1-Th4-CN, R' -Ph2-Ph 1-Th4-CN, R' -Ph3 -Ph 1-Th4-CN,
R' -Np 1-Ph 1-Th4-CN, R' -Np2-Ph 1-Th4-CN, R' -Np3 -Ph 1-Th4-CN,
R' -Np4-Ph 1-Th4-CN,
R' -Cy-CH2CH2-Ph 1-Th 1-CN, Rl -Ph 1-CHZCH2-Ph 1-Th 1-CN, RI -Ph2-CH2CH2-Ph 1-
Th 1-CN,
R'-Ph3-CH2CH2-Phl-Thl-CN, R'-Npl-CH2CH2-Phl-Thl-CN,
R'-Np2-CHZCH2-Phl-Thl-CN, R'-Np3-CH2CH2-Phl-Thl-CN,
R' -Np4-CH2CH2-Ph 1-Th 1-CN, R' -Cy-CH2CH2-Ph 1-Th2-CN,
R' -Ph 1-CH2CH2-Phl -Th2-CN,
R'-Ph2-CH2CH2-Phl-Th2-CN, R'-Ph3-CH2CH2-Phl-Th2-CN,
R' -Np 1-CH2CH2-Ph 1-Th2-CN, R' -Np2-CH2CH2-Ph i-Th2-CN,
R'-Np3-CH2CH2-Phl-Th2-CN, R'-Np4-CH2CH2-Phl-Th2-CN,
R' -Cy-C H2 C H2-Ph 1-Th3 -CN,
R'-Phl-CH2CH2-Phl-Th3-CN, R'-Ph2-CH2CH2-Phl-Th3-CN,
R' -Ph3 -CH2CH2-Ph 1-Th3 -CN, R' -Np 1-CH2CH2-Ph 1-Th3 -CN,
R'-Np2-CH2CH2-Phl-Th3-CN, R'-Np3-CH2CH2-Phl-Th3-CN,
R' -Np4-CH2CH2-Ph 1-Th3 -CN, R' -Cy-CHZCH2-Ph 1-Th4-CN,
R' -Ph 1-CH2CH2-Ph 1-Th4-CN,
R' -Ph2-CHzCH2-Ph 1-Th4-CN, R' -Ph3-CH2CH2-Ph l -Th4-CN,
R'-NpI-CH2CH2-Phl-Th4-CN, R'-Np2-CH2CH2-Phl-Th4-CN,
CA 02377217 2001-12-28
29
R'-Np3-CH2CH2-Phl-Th4-CN, R'-Np4-CH2CH2-Phl-Th4-CN,
R'-Ph1-C=C-Ph1-Th1-CN, R'-Ph2-C=C-Ph1-Th1-CN, R'-Ph3-C=C-Ph1-Th1-CN,
R' -Ph 1-C=C-Ph 1-Th2-CN, R' -Ph2-C=C-Ph 1-Th2-CN, R' -Ph3-C=C-Ph l-Th2-CN,
R'-Phl-C=C-Phl-Th3-CN, R'-Ph2-C=C-Phl-Th3-CN, R'-Ph3-C=C-Phl-Th3-CN,
R' -Ph 1-C=C-Ph 1-Th4-CN, R' -Ph2-C=C-Ph 1-Th4-CN, R' -Ph3 -C=C-Ph 1-Th4-CN,
R' -Cy-Ph2-Th 1-CN, R' -Ph i-Ph2-Th 1-CN, R' -Ph2-Ph2-Th 1-CN, R' -Ph3-Ph2-Th
l-CN,
R' -Np 1-Ph2-Th 1-CN, R' -Np2-Ph2-Th 1-CN, R' -Np3 -Ph2-Th 1-CN,
R'-Np4-Ph2-Thl-CN, R'-Cy-Ph2-Th2-CN, R'-Phl-Ph2-Th2-CN, R'-Ph2-Ph2-Th2-CN,
R'-Ph3-Ph2-Th2-CN, R'-Np1-Ph2-Th2-CN, R'-Np2-Ph2-Th2-CN,
R'-Np3-Ph2-Th2-CN, R'-Np4-Ph2-Th2-CN, R'-Cy-Ph2-Th3-CN, R'-Phl-Ph2-Th3-CN,
R'-Ph2-Ph2-Th3-CN, R'-Ph3-Ph2-Th3-CN, R'-Npl-Ph2-Th3-CN,
R'-Np2-Ph2-Th3-CN, R'-Np3-Ph2-Th3-CN, R'-Np4-Ph2-Th3-CN, R'-Cy-Ph2-Th4-CN,
R'-Phl-Ph2-Th4-CN, R'-Ph2-Ph2-Th4-CN, R'-Ph3-Ph2-Th4-CN,
R'-Npl-Ph2-Th4-CN, R'-Np2-Ph2-Th4-CN, R'-Np3-Ph2-Th4-CN,
R'-Np4-Ph2-Th4-CN,
R' -Cy-CH2CH2-Ph2-Th 1-CN, R' -Ph l-CH2CH2-Ph2-Th 1-CN, R' -Ph2-CH2CH2-Ph2-Th
1-CN,
R'-Ph3-CHZCH2-Ph2-Th1-CN, R'-Npl-CH2CH2-Ph2-Thl-CN,
R'-Np2-CH2CH2-Ph2-Thl -CN, R'-Np3-CH2CH2-Ph2-Thl -CN,
R'-Np4-CH2CH2-Ph2-Thl-CN, R'-Cy-CH2CH2-Ph2-Th2-CN,
R'-Phl-CH2CH2-Ph2-Th2-CN,
R'-Ph2-CH2CH2-Ph2-Th2-CN, R'-Ph3-CH2CH2-Ph2-Th2-CN,
R'-Npl-CH2CH2-Ph2-Th2-CN, R'-Np2-CHZCH2-Ph2-Th2-CN,
R'-Np3-CH2CH2-Ph2-Th2-CN, R'-Np4-CH2CH2-Ph2-Th2-CN,
R' -Cy-CH2CH2-Ph2-Th3 -CN,
CA 02377217 2001-12-28
R'-Phl-CH2CH2-Ph2-Th3-CN, R'-Ph2-CH2CH2-Ph2-Th3-CN,
R' -Ph3-CHZCH2-Ph2-Th3-CN, R'-Np l -CH2CH2-Ph2-Th3-CN,
R'-Np2-CH2CH2-Ph2-Th3-CN, R'-Np3-CH2CH2-Ph2-Th3-CN,
R'-Np4-CHZCH2-Ph2-Th3-CN, R'-Cy-CH2CH2-Ph2-Th4-CN,
5 R' -Ph 1-CH2CH2-Ph2-Th4-CN,
R'-Ph2-CH2CH2-Ph2-Th4-CN, R'-Ph3-CH2CH2-Ph2-Th4-CN,
R'-Npl-CH2CH2-Ph2-Th4-CN, R'-Np2-CH2CH2-Ph2-Th4-CN,
R'-Np3-CHZCH2-Ph2-Th4-CN, R'-Np4-CH2CH2-Ph2-Th4-CN,
R' -Ph l-C_C-Ph2-Th 1-CN, R' -Ph2-C=C-Ph2-Th l-CN, R'-Ph3-C=C-Ph2-Th 1-CN,
10 R'-PhI-C=C-Ph2-Th2-CN, R'-Ph2-C=C-Ph2-Th2-CN, R'-Ph3-C=C-Ph2-Th2-CN,
R'-PhI-C=C-Ph2-Th3-CN, R'-Ph2-CGC-Ph2-Th3-CN, R'-Ph3-C=C-Ph2-Th3-CN,
R'-Phl-C=C-Ph2-Th4-CN, R'-Ph2-C=C-Ph2-Th4-CN, R'-Ph3-C=C-Ph2-Th4-CN,
R'-Cy-Ph3-Thl-CN, R'-Phl-Ph3-Thl-CN, R'-Ph2-Ph3-Thl-CN, R'-Ph3-Ph3-Thl-CN,
R' -Np l -Ph3 -Th l -CN, R' -Np2-Ph3 -Th l -CN, R' -Np3 -Ph3 -Th 1-CN,
15 R'-Np4-Ph3-Thl-CN, R'-Cy-Ph3-Th2-CN, R'-Phl-Ph3-Th2-CN, R'-Ph2-Ph3-Th2-CN,
R'-Ph3-Ph3-Th2-CN, R'-Npl-Ph3-Th2-CN, R'-Np2-Ph3-Th2-CN,
R'-Np3-Ph3-Th2-CN, R'-Np4-Ph3-Th2-CN, R'-Cy-Ph3-Th3-CN, R'-Phl-Ph3-Th3-CN,
R' -Ph2-Ph3 -Th3 -CN, R' -Ph3 -Ph3 -Th3 -CN, R' -Np 1-Ph3 -Th3 -CN,
R'-Np2-Ph3-Th3-CN, R'-Np3-Ph3-Th3-CN, R'-Np4-Ph3-Th3-CN, R'-Cy-Ph3-Th4-CN,
20 R'-Phl-Ph3-Th4-CN, R'-Ph2-Ph3-Th4-CN, R'-Ph3-Ph3-Th4-CN,
R' -Np 1 -Ph3 -Th4-CN, R'-Np2-Ph3-Th4-CN, R'-Np3 -Ph3 -Th4-CN,
R'-Np4-Ph3-Th4-CN,
R'-Cy-CHZCH2-Ph3-Thl-CN, R'-Phl-CH2CH2-Ph3-Thl-CN, R'-Ph2-CH2CH2-Ph3-Thl-CN,
R'-Ph3-CH2CH2-Ph3-Thl-CN, R'-Npl-CH2CH2-Ph3-Thl-CN,
CA 02377217 2001-12-28
31
R'-Np2-CH2CH2-Ph3-Thl-CN, R'-Np3-CH2CH2-Ph3-Thl-CN,
R' -Np4-CH2CH2-Ph3-Th 1-CN, R' -Cy-CH2CH2-Ph3-Th2-CN,
R' -Ph 1-CHZCH2-Ph3-Th2-CN,
R'-Ph2-CH2CH2-Ph3-Th2-CN, R'-Ph3-CH2CH2-Ph3-Th2-CN,
R' -Np 1-CH2CH2-Ph3 -Th2-CN, R' -Np2-CH2CH2-Ph3 -Th2-CN,
R'-Np3-CH2CH2-Ph3-Th2-CN, R'-Np4-CH2CH2-Ph3-Th2-CN,
R' -Cy-CHZCH2-Ph3-Th3-CN,
R'-Phl-CH2CH2-Ph3-Th3-CN, R'-Ph2-CH2CH2-Ph3-Th3-CN,
R'-Ph3-CH2CH2-Ph3-Th3-CN, R'-Npl-CH2CH2-Ph3-Th3-CN,
R'-Np2-CH2CH2-Ph3-Th3-CN, R'-Np3-CH2CH2-Ph3-Th3-CN,
R'-Np4-CH2CH2-Ph3-Th3-CN, R'-Cy-CH2CH2-Ph3-Th4-CN,
R' -Ph 1-CHZCH2-Ph3-Th4-CN,
R'-Ph2-CHZCH2-Ph3-Th4-CN, R'-Ph3-CH2CH2-Ph3-Th4-CN,
R'-Npl-CH2CH2-Ph3-Th4-CN, R'-Np2-CH2CH2-Ph3-Th4-CN,
R'-Np3-CH2CH2-Ph3-Th4-CN, R'-Np4-CH2CH2-Ph3-Th4-CN,
R'-PhI-C=C-Ph3-Thl-CN, R'-Ph2-C=C-Ph3-Thl-CN, R'-Ph3-C=C-Ph3-Thl-CN,
R'-Phl -C=C-Ph3-Th2-CN, R'-Ph2-C=C-Ph3-Th2-CN, R'-Ph3-C-C-Ph3-Th2-CN,
R'-PhI-C=C-Ph3-Th3-CN, R'-Ph2-C=C-Ph3-Th3-CN, R'-Ph3-C=C-Ph3-Th3-CN,
R' -Ph 1-C=C-Ph3 -Th4-CN, R' -Ph2-C=C-Ph3 -Th4-CN, R' -Ph3 -C=C-Ph3 -Th4-CN,
R'-Cy-Npl-Thl-CN, R'-Phl-Npl-Thl-CN, R'-Ph2-Npl-Thl-CN, R'-Ph3-Npl-Thl-CN,
R'-Cy-Np1-Th2-CN, Rl-Ph1-Np1-Th2-CN, R'-Ph2-Np1-Th2-CN, R'-Ph3-Np1-Th2-CN,
R'-Cy-Npl-Th3-CN, R'-Phl-Npl-Th3-CN, R'-Ph2-Npl-Th3-CN, Rl-Ph3-Npl-Th3-CN,
R' -Cy-Np 1-Th4-CN, R' -Ph 1-Np 1-Th4-CN, R' -Ph2-Np 1-Th4-CN, R' -Ph3 -Np 1-
Th4-CN,
R' -Cy-C H2CH2-Np 1-Th 1-CN, R' -Ph 1-CH2CH2-Np 1-Th 1-CN,
CA 02377217 2001-12-28
32
R' -Ph2-CH2CH2-Np 1-Th 1-CN,
R' -Ph3 -CH2CH2-Np 1-Th l-CN, R' -Cy-CH2CH2-Np 1-Th2-CN,
R' -Ph 1-CH2CH2-Np 1-Th2-CN,
R'-Ph2-CH2CH2-Npl-Th2-CN, R'-Ph3-CH2CH2-Npl-Th2-CN,
R'-Cy-CH2CH2-Npl-Th3-CN,
R'-Phl-CH2CH2-Npl-Th3-CN, Rl-Ph2-CHZCH2-Npl-Th3-CN,
R'-Ph3-CH2CH2-Npl-Th3-CN, R'-Cy-CH2CH2-Npl-Th4-CN,
R'-Ph 1-CH2CH2-Np 1-Th4-CN,
R' -Ph2-CHZCH2-Np 1-Th4-CN, R' -Ph3 -CH2CH2-Np 1-Th4-CN,
in the case in which ne = nb = 1, and n = nd = 0, and Z is a fluorine atom,
R' -Cy-Cy-Th 1-OCF3, Rl -Ph 1-Cy-Th 1-OCF3, R' -Ph2-Cy-Th 1-OCF3,
R'-Ph3-Cy-ThI-OCF3, R'-Npl-Cy-Thl-OCF3, Rl-Np2-Cy-ThI-OCF3,
R'-Np3-Cy-ThI-OCF3, R'-Np4-Cy-ThI-OCF3, RI-Cy-Cy-Th2-OCF3,
R'-Phl-Cy-Th2-OCF3, R'-Ph2-Cy-Th2-OCF3, R'-Ph3-Cy-Th2-OCF3,
R'-Npl-Cy-Th2-OCF3, RI-Np2-Cy-Th2-OCF3, Rl-Np3-Cy-Th2-OCF3,
R'-Np4-Cy-Th2-OCF3, RI-Cy-Cy-Th3-OCF3, R'-Phl-Cy-Th3-OCF3,
R'-Ph2-Cy-Th3-OCF3, R'-Ph3-Cy-Th3-OCF3, R'-Npl-Cy-Th3-OCF3,
R'-Np2-Cy-Th3-OCF3, R'-Np3-Cy-Th3-OCF3, R'-Np4-Cy-Th3-OCF3,
R'-Cy-Cy-Th4-OCF3, R'-Phl-Cy-Th4-OCF3, R'-Ph2-Cy-Th4-OCF3,
R'-Ph3-Cy-Th4-OCF3, Rl-Npl-Cy-Th4-OCF3, Rl-Np2-Cy-Th4-OCF3,
R'-Np3-Cy-Th4-OCF3, Ri-Np4-Cy-Th4-OCF3,
RI-Cy-CH2CH2-Cy-Thl-OCF3, R'-Phl-CH2CH2-Cy-Thl-OCF3,
R' -Ph2-CH2CH2-Cy-Th 1-OCF3, R' -Ph3-CH2CH2-Cy-Th 1-OCF3,
Rl-Npl-CH2CH2-Cy-Thl-OCF3, R'-Np2-CH2CH2-Cy-Thl-OCF3,
CA 02377217 2001-12-28
33
R'-Np3-CH2CH2-Cy-Thl-OCF3, R'-Np4-CH2CH2-Cy-ThI-OCF3,
R' -Cy-CH2CH2-Cy-Th2-OCF3, R' -Ph 1-CH2CH2-Cy-Th2-OCF3,
R'-Ph2-CH2CH2-Cy-Th2-OCF3, R'-Ph3-CH2CH2-Cy-Th2-OCF3,
R'-Npl-CH2CH2-Cy-Th2-OCF3, R'-Np2-CHZCH2-Cy-Th2-OCF3,
R' -Np3-CHZCH2-Cy-Th2-OCF3, R' -Np4-CH2CH2-Cy-Th2-OCF3,
R'-Cy-CH2CH2-Cy-Th3-OCF3, R'-Phl-CH2CH2-Cy-Th3-OCF3,
R'-Ph2-CH2CH2-Cy-Th3-OCF3, R'-Ph3-CH2CH2-Cy-Th3-OCF3,
R'-Npl-CH2CH2-Cy-Th3-OCF3, R'-Np2-CH2CH2-Cy-Th3-OCF3,
R'-Np3-CH2CH2-Cy-Th3-OCF3, R'-Np4-CH2CH2-Cy-Th3-OCF3,
R' -Cy-CH2CH2-Cy-Th4-OCF3, R'-Ph 1-CH2CHZ-Cy-Th4-OCF3,
R'-Ph2-CH2CH2-Cy-Th4-OCF3, R'-Ph3-CH2CH2-Cy-Th4-OCF3,
R' -Np 1-CH2CH2-Cy-Th4-OCF3, R' -Np2-CH2CH2-Cy-Th4-OCF3,
R1-Np3-CH2CH2-Cy-Th4-OCF3, R'-Np4-CH2CH2-Cy-Th4-OCF3,
R'-Cy-Phl-Thl-OCF3, R'-Phl-Phl-ThI-OCF3, R'-Ph2-Phl-ThI-OCF3,
R'-Ph3-Phl-ThI-OCF3, R'-Npl-Phl-ThI-OCF3, R'-Np2-Phl-ThI-OCF3,
R'-Np3-Phl-ThI-OCF3, R'-Np4-Phl-ThI-OCF3, RI-Cy-Phl-Th2-OCF3,
R'-Phl-Phl-Th2-OCF3, R'-Ph2-Phl-Th2-OCF3, R'-Ph3-Phl-Th2-OCF3,
R'-Np1-Ph1-Th2-OCF3, R'-Np2-Ph1-Th2-OCF3, R'-Np3-Ph1-Th2-OCF3,
R'-Np4-Phl-Th2-OCF3, R'-Cy-Phl-Th3-OCF3, R1-Phl-Phl-Th3-OCF3,
R'-Ph2-Phl-Th3-OCF3, R'-Ph3-Phl-Th3-OCF3, R1-Npl-Phl-Th3-OCF3,
R'-Np2-Phl-Th3-OCF3, R'-Np3-Phl-Th3-OCF3, R'-Np4-Phl-Th3-OCF3,
R'-Cy-Ph1-Th4-OCF3, R'-Phl-Phl-Th4-OCF3, R'-Ph2-Ph1-Th4-OCF3,
R'-Ph3-Phl-Th4-OCF3, R'-Npl-Phl-Th4-OCF3, R'-Np2-Phl-Th4-OCF3,
R' -Np3-Ph 1-Th4-OCF3, R'-Np4-Ph 1-Th4-OCF3,
CA 02377217 2001-12-28
34
R'-Cy-CHZCH2-Phl-Thl-OCF3, R'-Phi-CH2CH2-Phl-ThI-OCF3,
R'-Ph2-CH2CH2-Phl-Thl-OCF3, R'-Ph3-CH2CH2-Phl-Thl-OCF3,
R'-Npl-CH2CH2-Phl-ThI-OCF3, R'-Np2-CH2CH2-Phl-ThI-OCF3,
R'-Np3-CH2CH2-Phl-ThI-OCF3, R'-Np4-CH2CH2-Phl-ThI-OCF3,
R' -Cy-CH2CH2-Ph l-Th2-OCF3, R' -Ph 1-CH2CH2-Ph 1-Th2-OCF3,
R' -Ph2-CH2CH2-Ph 1-Th2-OCF3, R' -Ph3 -CH2CH2-Ph 1-Th2-OCF3,
R' -Np 1-CH2CH2-Ph 1-Th2-OCF3, R' -Np2-CH2CH2-Ph 1-Th2-OCF3,
R'-Np3-CH2CH2-Phl-Th2-OCF3, R'-Np4-CH2CH2-Phl-Th2-OCF3,
R'-Cy-CH2CH2-Phl-Th3-OCF3, R'-Phl-CH2CH2-Phl-Th3-OCF3,
R'-Ph2-CH2CH2-Phl-Th3-OCF3, R'-Ph3-CH2CH2-Phl-Th3-OCF3,
R'-NpI-CHZCH2-Phl-Th3-OCF3, R'-Np2-CH2CH2-Phl-Th3-OCF3,
R'-Np3-CH2CH2-Phl-Th3-OCF3, R'-Np4-CH2CH2-Phl-Th3-OCF3,
R' -Cy-CH2CHZ-Ph 1-Th4-OCF3, R' -Ph 1-CH2CH2-Ph 1-Th4-OCF3,
R' -Ph2-CH2CH2-Ph 1-Th4-OCF3, R'-Ph3-CH2CH2-Ph l -Th4-OCF3,
R' -Np 1-CH2CH2-Ph 1-Th4-OCF3, R' -Np2-CH2CH2-Ph 1-Th4-OCF3,
R' -Np3 -CH2CH2-Ph l -Th4-OCF3, R' -Np4-CH2CH2-Ph 1-Th4-OCF3,
R' -Ph 1-C-C-Ph 1-Th 1-OCF3, R' -Ph2-C=C-Ph l-Th 1-OCF3,
R'-Ph3-C=C-Phl-ThI-OCF3, R'-PhI-C=C-Ph1-Th2-OCF3,
R'-Ph2-C=C-Phl-Th2-OCF3, R'-Ph3-C=C-Phl-Th2-OCF3,
R' -Ph 1-C-C-Ph 1-Th3 -OCF3, R' -Ph2-C=C-Ph 1-Th3 -OCF3,
R'-Ph3-C=C-Phl-Th3-OCF3, R'-Phl-C=C-Phl-Th4-OCF3,
R'-Ph2-C-C-Phl-Th4-OCF3, R'-Ph3-C=C-Phl-Th4-OCF3,
R'-Cy-Ph2-Thl-OCF3, R'-Phl-Ph2-Thl-OCF3, R'-Ph2-Ph2-Thl-OCF3,
R'-Ph3-Ph2-Thl-OCF3, R'-Npl-Ph2-Thl-OCF3, R'-Np2-Ph2-Thl-OCF3,
CA 02377217 2001-12-28
R'-Np3-Ph2-Thl-OCF3, R'-Np4-Ph2-Thl-OCF3i R'-Cy-Ph2-Th2-OCF3,
R'-Phl-Ph2-Th2-OCF3, R'-Ph2-Ph2-Th2-OCF3, R'-Ph3-Ph2-Th2-OCF3,
R'-Npl-Ph2-Th2-OCF3, R'-Np2-Ph2-Th2-OCF3, R'-Np3-Ph2-Th2-OCF3,
R' -Np4-Ph2-Th2-OCF3, R' -Cy-Ph2-Th3 -OCF3, R' -Ph 1-Ph2-Th3 -OCF3,
5 R'-Ph2-Ph2-Th3-OCF3, R'-Ph3-Ph2-Th3-OCF3, R'-Npl-Ph2-Th3-OCF3,
R'-Np2-Ph2-Th3-OCF3, R'-Np3-Ph2-Th3-OCF3, R'-Np4-Ph2-Th3-OCF3,
R' -Cy-Ph2-Th4-OCF3, R' -Ph 1-Ph2-Th4-OCF3, R' -Ph2-Ph2-Th4-OCF3,
R' -Ph3 -Ph2-Th4-OCF3, R' -Np 1-Ph2-Th4-OCF3, R' -Np2-Ph2-Th4-OCF3,
R'-Np3-Ph2-Th4-OCF3, R'-Np4-Ph2-Th4-OCF3,
10 R'-Cy-CH2CH2-Ph2-Thl-OCF3, R'-Phl-CH2CH2-Ph2-Thl-OCF3,
R'-Ph2-CH2CH2-Ph2-Thl-OCF3, R'-Ph3-CH2CH2-Ph2-Thl-OCF3,
R'-Npl-CH2CH2-Ph2-Thl-OCF3, R'-Np2-CH2CH2-Ph2-Thl-OCF3,
R'-Np3-CH2CH2-Ph2-Thl-OCF3, R'-Np4-CH2CH2-Ph2-Thl-OCF3,
R'-Cy-CH2CH2-Ph2-Th2-OCF3, R'-Phl-CH2CH2-Ph2-Th2-OCF3,
15 R'-Ph2-CHZCH2-Ph2-Th2-OCF3, R'-Ph3-CH2CH2-Ph2-Th2-OCF3,
R'-Npl-CH2CH2-Ph2-Th2-OCF3, R'-Np2-CH2CH2-Ph2-Th2-OCF3,
R'-Np3-CHZCH2-Ph2-Th2-OCF3, R'-Np4-CH2CH2-Ph2-Th2-OCF3,
R'-Cy-CH2CH2-Ph2-Th3-OCF3, R'-Phl-CH2CH2-Ph2-Th3-OCF3,
R' -Ph2-CHZCH2-Ph2-Th3-OCF3, R'-Ph3-CH2CH2-Ph2-Th3-OCF3,
20 R' -Np 1-CH2CH2-Ph2-Th3-OCF3, R'-Np2-CH2CH2-Ph2-Th3-OCF3,
R' -Np3-CH2CH2-Ph2-Th3-OCF3, R'-Np4-CH2CH2-Ph2-Th3-OCF3,
R'-Cy-CH2CH2-Ph2-Th4-OCF3, R'-Phl-CH2CH2-Ph2-Th4-OCF3,
R' -Ph2-CH2CH2-Ph2-Th4-OCF3, R' -Ph3-CHZCH2-Ph2-Th4-OCF3,
R'-Np 1-CH2CH2-Ph2-Th4-OCF3, R'-Np2-CH2CH2-Ph2-Th4-OCF3,
CA 02377217 2001-12-28
36
R'-Np3-CH2CH2-Ph2-Th4-OCF3, R'-Np4-CH2CH2-Ph2-Th4-OCF3,
R' -Ph 1-C=C-Ph2-Th l-OCF3, R' -Ph2-C=C-Ph2-Th 1-OCF3,
R'-Ph3-C=C-Ph2-Thl-OCF3, R'-PhI-C=C-Ph2-Th2-OCF3,
R'-Ph2-C=C-Ph2-Th2-OCF3, Rl-Ph3-C=C-Ph2-Th2-OCF3,
R'-Phl-C=C-Ph2-Th3-OCF3, R'-Ph2-C=C-Ph2-Th3-OCF3,
R'-Ph3-C=C-Ph2-Th3-OCF3, R'-PhI-C=C-Ph2-Th4-OCF3,
R' -Ph2-C=C-Ph2-Th4-OCF3, R' -Ph3 -C=C-Ph2-Th4-OCF3,
R'-Cy-Ph3-Thl-OCF3, R'-Phl-Ph3-Thl-OCF3, R'-Ph2-Ph3-Thl-OCF3,
R'-Ph3-Ph3-Thl-OCF3, R'-Npl-Ph3-Thl-OCF3, R'-Np2-Ph3-Th1-OCF3,
R'-Np3-Ph3-Thl-OCF3, Rl-Np4-Ph3-Thl-OCF3, RI-Cy-Ph3-Th2-OCF3,
R'-Phl-Ph3-Th2-OCF3, R'-Ph2-Ph3-Th2-OCF3, R'-Ph3-Ph3-Th2-OCF3,
R'-Np 1-Ph3-Th2-OCF3, R'-Np2-Ph3-Th2-OCF3, R'-Np3-Ph3-Th2-OCF3,
R'-Np4-Ph3-Th2-OCF3, R'-Cy-Ph3-Th3-OCF3, R'-Phl -Ph3 -Th3 -OCF3,
R'-Ph2-Ph3-Th3-OCF3, R'-Ph3-Ph3-Th3-OCF3, R'-Npl-Ph3-Th3-OCF3,
R'-Np2-Ph3-Th3-OCF3, R'-Np3-Ph3-Th3-OCF3, R'-Np4-Ph3-Th3-OCF3,
R'-Cy-Ph3-Th4-OCF3, R'-Ph1-Ph3-Th4-OCF3, R'-Ph2-Ph3-Th4-OCF3,
R'-Ph3-Ph3-Th4-OCF3, R'-Npl-Ph3-Th4-OCF3, R'-Np2-Ph3-Th4-OCF3,
R'-Np3-Ph3-Th4-OCF3, R'-Np4-Ph3-Th4-OCF3,
R'-Cy-CH2CH2-Ph3-Thl-OCF3, R'-Phl-CH2CH2-Ph3-Thl-OCF3,
R'-Ph2-CH2CH2-Ph3-Thl-OCF3, R'-Ph3-CH2CH2-Ph3-Thl -OCF3,
R'-Npl-CH2CH2-Ph3-Thl-OCF3, R'-Np2-CH2CH2-Ph3-Thl-OCF3,
R'-Np3-CH2CH2-Ph3-Thl-OCF3, R'-Np4-CH2CH2-Ph3-Thl-OCF3,
R' -Cy-CHZCH2-Ph3-Th2-OCF3, R' -Ph 1-CH2CH2-Ph3-Th2-OCF3,
R'-Ph2-CH2CH2-Ph3-Th2-OCF3, R'-Ph3-CH2CH2-Ph3-Th2-OCF3,
CA 02377217 2001-12-28
37
R' -Np 1-CH2CH2-Ph3-Th2-OCF3, R' -Np2-CH2CH2-Ph3-Th2-OCF3,
R'-Np3-CH2CH2-Ph3-Th2-OCF3, R'-Np4-CH2CH2-Ph3-Th2-OCF3,
R'-Cy-CH2CH2-Ph3-Th3-OCF3, R'-Phl-CH2CH2-Ph3-Th3-OCF3,
R'-Ph2-CH2CH2-Ph3-Th3-OCF3, R'-Ph3-CH2CH2-Ph3-Th3-OCF3,
R'-Npl-CH2CH2-Ph3-Th3-OCF3, R'-Np2-CH2CH2-Ph3-Th3-OCF3,
R' -Np3-CH2CH2-Ph3-Th3-OCF3, R'-Np4-CH2CH2-Ph3-Th3-OCF3,
R' -Cy-CH2CH2-Ph3-Th4-OCF3, R'-Ph 1-CH2CH2-Ph3-Th4-OCF3,
R'-Ph2-CH2CH2-Ph3-Th4-OCF3, R'-Ph3-CH2CH2-Ph3-Th4-OCF3,
R'-Npl-CH2CH2-Ph3-Th4-OCF3, R'-Np2-CH2CH2-Ph3-Th4-OCF3,
R'-Np3-CH2CH2-Ph3-Th4-OCF3, R'-Np4-CH2CH2-Ph3-Th4-OCF3,
R'-Cy-C=C-Ph3-Thl-OCF3, R'-PhI-C=C-Ph3-Thl-OCF3,
R'-Ph2-C=C-Ph3-Thl-OCF3, R'-Ph3-C=C-Ph3-Th1 -OCF3,
R'-Phl-C=C-Ph3-Th2-OCF3, R'-Ph2-C=C-Ph3-Th2-OCF3,
R'-Ph3-C-C-Ph3-Th2-OCF3, R'-Phl-C-C-Ph3-Th3-OCF3,
R'-Ph2-C=C-Ph3-Th3-OCF3, R'-Ph3-C=C-Ph3-Th3-OCF3,
R'-Phl-C=C-Ph3-Th4-OCF3, R'-Ph2-C=C-Ph3-Th4-OCF3,
R' -Ph3-C=C-Ph3-Th4-OCF3,
R' -Cy-Np l-Th l-OCF3, R' -Ph 1-Np 1-Th 1-OCF3, R' -Ph2-Np 1-Th 1-OCF3,
R' -Ph3 -Np 1-Th 1-OCF3, R' -Np 1-Np 1-Th 1-OCF3, R' -Np2-Np 1-Th 1-OCF3,
R'-Np3-Npl-ThI-OCF3, R'-Np4-Npl-ThI-OCF3, R'-Cy-Npl-Th2-OCF3,
R' -Ph l-Np l-Th2-OCF3, R' -Ph2-Np l-Th2-OCF3, R' -Ph3 -Np l-Th2-OCF3,
R'-Cy-Np1-Th3-OCF3, R'-Ph1-Np1-Th3-OCF3, R'-Ph2-Np1-Th3-OCF3,
R'-Ph3-Np1-Th3-OCF3, R'-Cy-Np1-Th4-OCF3, R'-Ph1-Np1-Th4-OCF3,
R' -Ph2-Np 1-Th4-OCF3, R' -Ph3-Np 1-Th4-OCF3,
CA 02377217 2001-12-28
38
R' -Cy-CHZCH2-Np 1-Th 1-OCF3, R' -Ph 1-CH2CH2-Np 1-Th 1-OCF3,
R'-Ph2-CHzCH2-Npl-ThI-OCF3, R'-Ph3-CH2CH2-Npl-ThI-OCF3,
R' -Cy-CH2CH2-Np 1-Th2-OCF3, R' -Ph 1-CH2CH2-Np 1-Th2-OCF3,
R'-Ph2-CH2CH2-Npl-Th2-OCF3, R'-Ph3-CHZCH2-Npl-Th2-OCF3,
R'-Cy-CHZCH2-Npl-Th3-OCF3, R'-Phl-CH2CH2-Npl-Th3-OCF3,
R'-Ph2-CHZCH2-Npl-Th3-OCF3, R'-Ph3-CH2CH2-Npl-Th3-OCF3,
R' -Cy-CHZCH2-Np 1-Th4-OCF3, R' -Ph 1-CH2CH2-Np 1-Th4-OCF3,
R'-Ph2-CH2CH2-Npl-Th4-OCF3, R'-Ph3-CH2CH2-Npl-Th4-OCF3,
in the case in which na = nb = 0, and n = 1, nd = 0 or n' = 0, nd = 1, and Z
is a fluorine atom,
R'-Th1-Ph1-F, R'-Thl-Ph2-F, R'-Th1-Ph3-F, R'-Th1-Np1-F, R'-Th1-Np2-F,
R'-Th1-Np3-F, R'-Th1-Np4-F, R'-Th2-Ph1-F, R'-Th2-Ph2-F, R'-Th2-Ph3-F,
R'-Th2-Npl-F, R'-Th2-Np2-F, R'-Th2-Np3-F, R'-Th2-Np4-F, R'-Th3-Phl-F,
R'-Th3-Ph2-F, R'-Th3-Ph3-F, R'-Th3-Npl-F, R'-Th3-Np2-F, R'-Th3-Np3-F,
R'-Th3-Np4-F, R'-Th4-Phl-F, R'-Th4-Ph2-F, Rl-Th4-Ph3-F, R'-Th4-Npl-F,
R'-Th4-Np2-F, R'-Th4-Np3-F, Rl-Th4-Np4-F,
R'-Th1-CH2CH2-Ph1-F, R'-Th1-CHZCH2-Ph2-F, R'-Th1-CHZCH2-Ph3-F,
R' -Th 1-CH2CH2-Np 1-F, R' -Th 1-CHZCH2-Np2-F, R' -Th 1-CH2CH2-Np3 -F,
R'-Thl-CH2CH2-Np4-F, R'-Th2-CH2CH2-Phl-F, R'-Th2-CH2CH2-Ph2-F,
R'-Th2-CH2CH2-Ph3-F, R'-Th2-CH2CH2-Npl-F, R'-Th2-CH2CH2-Np2-F,
R'-Th2-CHZCH2-Np3-F, R'-Th2-CH2CH2-Np4-F, R'-Th3-CH2CH2-Phl-F,
R'-Th3-CH2CH2-Ph2-F, R'-Th3-CH2CH2-Ph3-F, R'-Th3-CH2CH2-Npl-F,
R'-Th3-CH2CH2-Np2-F, R'-Th3-CHZCH2-Np3-F, R'-Th3-CHZCH2-Np4-F,
R' -Th4-CH2CH2-Ph 1-F, R' -Th4-CHZCH2-Ph2-F, R' -Th4-CH2CH2-Ph3 -F,
R'-Th4-CH2CH2-Npl-F, R'-Th4-CH2CH2-Np2-F, R'-Th4-CH2CH2-Np3-F,
CA 02377217 2001-12-28
39
R' -Th4-CH2CH2-Np4-F,
R'-Thl-C=C-Ph1-F, R'-Th1-C=C-Ph2-F, R'-Th1-C=C-Ph3-F,
R' -Th2-C=C-Ph l -F, R' -Th2-C=C-Ph2-F, R' -Th2-C=C-Ph3 -F,
R'-Th3-C=C-PhI-F, R'-Th3-C=C-Ph2-F, R'-Th3-C-C-Ph3-F,
R'-Th4-C-C-PhI-F, Rl-Th4-C=C-Ph2-F,
in the case in which na = nb = 0, and n= 1, nd = 0 or n = 0, nd = 1, and Z is
a cyano group,
R'-Th1-Ph1-CN, R'-Thl-Ph2-CN, R'-Thl-Ph3-CN, Rl-Thl-Npl-CN, R'-Thl-Np2-CN,
R'-Thl-Np3-CN, R'-Thl-Np4-CN, R'-Th2-Phl-CN, R'-Th2-Ph2-CN, R'-Th2-Ph3-CN,
R'-Th2-Npl-CN, R'-Th2-Np2-CN, R'-Th2-Np3-CN, R'-Th2-Np4-CN, R'-Th3-Phl-CN,
R'-Th3-Ph2-CN, R'-Th3-Ph3-CN, R'-Th3-Npl-CN, R'-Th3-Np2-CN, R'-Th3-Np3-CN,
R'-Th3-Np4-CN, R'-Th4-Phl-CN, R'-Th4-Ph2-CN, R'-Th4-Ph3-CN, R'-Th4-Npl-CN,
R' -Th4-Np2-CN, R' -Th4-Np3 -CN, R' -Th4-Np4-CN,
R'-Thl-CH2CH2-Phl-CN, Rl-Thl-CH2CH2-Ph2-CN, R'-Thl-CH2CH2-Ph3-CN,
R'-Thl-CH2CH2-Npl-CN, R'-Thl-CH2CH2-Np2-CN, R'-ThI-CHZCH2-Np3-CN,
R'-Thl-CH2CH2-Np4-CN, R'-Th2-CHZCH2-Phl-CN, R'-Th2-CH2CH2-Ph2-CN,
R'-Th2-CH2CH2-Ph3-CN, R'-Th2-CH2CH2-Npl-CN, R'-Th2-CH2CH2-Np2-CN,
R'-Th2-CH2CH2-Np3-CN, R'-Th2-CH2CH2-Np4-CN, R'-Th3-CH2CH2-Phl-CN,
R'-Th3-CH2CH2-Ph2-CN, R'-Th3-CH2CHZ-Ph3-CN, R'-Th3-CH2CH2-Npl-CN,
R'-Th3-CH2CH2-Np2-CN, R'-Th3-CHZCH2-Np3-CN, R'-Th3-CHZCH2-Np4-CN,
R'-Th4-CH2CH2-Phl-CN, R'-Th4-CH2CH2-Ph2-CN, R'-Th4-CH2CH2-Ph3-CN,
R'-Th4-CH2CHZ-NpI-CN, R'-Th4-CH2CH2-Np2-CN, R'-Th4-CH2CH2-Np3-CN,
R' -Th4-CH2CH2-Np4-CN,
R'-Th1-C=C-Ph1-CN, R'-Th1-C-C-Ph2-CN, R'-Th1-C=C-Ph3-CN,
R'-Th2-C=C-PhI-CN, R'-Th2-C=C-Ph2-CN, R'-Th2-C-C-Ph3-CN,
CA 02377217 2001-12-28
R'-Th3-C=C-Ph1-CN, R'-Th3-CaC-Ph2-CN, R'-Th3-C=-C-Ph3-CN,
R'-Th4-C=C-Phl-CN, Rl-Th4-C=C-Ph2-CN,
in the case in which na = nb = 0, and n = 1, n = 0 or n = 0, nd = 1, and Z
is a trifluoromethyl
group,
5 R'-Thl-PhI-OCF3, Rl-Thl-Ph2-OCF3, R'-Thl-Ph3-OCF3, R'-Thl-NpI-OCF3,
R'-Thl-Np2-OCF3, R'-Thl-Np3-OCF3, R'-Th1-Np4-OCF3, R'-Th2-Phl-OCF3,
R'-Th2-Ph2-OCF3, R'-Th2-Ph3-OCF3, R'-Th2-Npl-OCF3i R'-Th2-Np2-OCF3,
R'-Th2-Np3-OCF3, R'-Th2-Np4-OCF3, R'-Th3-Phl-OCF3, R'-Th3-Ph2-OCF3,
R'-Th3-Ph3-OCF3, R'-Th3-Np1-OCF3, R'-Th3-Np2-OCF3, R'-Th3-Np3-OCF3,
10 R'-Th3-Np4-OCF3, R'-Th4-Phl-OCF3, R'-Th4-Ph2-OCF3, R'-Th4-Ph3-OCF3,
R'-Th4-Npl-OCF3, R'-Th4-Np2-OCF3, R'-Th4-Np3-OCF3, R'-Th4-Np4-OCF3,
R'-ThI-CHZCH2-Phl-OCF3, R'-Thl-CH2CH2-Ph2-OCF3, R'-Thl-CH2CH2-Ph3-OCF3,
R'-Thl-CH2CH2-Np1-OCF3, R'-Thl-CH2CH2-Np2-OCF3, R'-Thl-CH2CH2-Np3-OCF3,
R' -Th 1-CH2CH2-Np4-OCF3, R' -Th2-CH2CH2-Ph l-OCF3, R' -Th2-CHZCH2-Ph2-OCF3,
15 R'-Th2-CH2CH2-Ph3-OCF3, R'-Th2-CH2CH2-Npl-OCF3, R'-Th2-CH2CH2-Np2-OCF3,
R'-Th2-CH2CH2-Np3-OCF3, R'-Th2-CH2CH2-Np4-OCF3, R'-Th3-CH2CH2-Phl-OCF3,
R'-Th3-CH2CH2-Ph2-OCF3, R'-Th3-CH2CH2-Ph3-OCF3, R'-Th3-CH2CH2-Npl-OCF3,
R'-Th3-CH2CH2-Np2-OCF3, R'-Th3-CH2CH2-Np3-OCF3, R'-Th3-CH2CH2-Np4-OCF3,
R'-Th4-CH2CH2-Phl-OCF3, R'-Th4-CH2CH2-Ph2-OCF3, R'-Th4-CH2CH2-Ph3-OCF3,
20 R'-Th4-CH2CH2-Npl-OCF3, R'-Th4-CH2CH2-Np2-OCF3, R'-Th4-CH2CH2-Np3-OCF3,
R' -Th4-CH2CH2-Np4-OCF3,
R'-Thl-C=C-PhI-OCF3, R'-Thl-C=C-Ph2-OCF3, R'-ThI-C=C-Ph3-OCF3,
R'-Th2-C=C-Phl-OCF3, R'-Th2-C=C-Ph2-OCF3, R'-Th2-C=C-Ph3-OCF3,
R'-Th3-C_C-PhI-OCF3, R'-Th3-C=C-Ph2-OCF3, R'-Th3-C=C-Ph3-OCF3,
CA 02377217 2001-12-28
41
R'-Th4-C=C-Ph1-OCF3, Rl-Th4-C=C-Ph2-OCF3,
in the case in which na = nb = 0, and n = nd = 1, and Z is a fluorine atom,
R'-Th1-Cy-Ph1-F, R'-Th1-Cy-Ph2-F, R'-Th1-Cy-Ph3-F, R'-Th1-Cy-Np1-F,
R'-Th1-Cy-Np2-F, R'-Thl-Cy-Np3-F, R'-Thl-Cy-Np4-F, R'-Th2-Cy-PhI-F,
R'-Th2-Cy-Ph2-F, R'-Th2-Cy-Ph3-F, R'-Th2-Cy-Npl-F, R'-Th2-Cy-Np2-F,
R'-Th2-Cy-Np3-F, R'-Th2-Cy-Np4-F, R'-Th3-Cy-PhI-F, R'-Th3-Cy-Ph2-F,
R'-Th3-Cy-Ph3-F, R'-Th3-Cy-NpI-F, R'-Th3-Cy-Np2-F, R'-Th3-Cy-Np3-F,
R'-Th3-Cy-Np4-F, R'-Th4-Cy-PhI-F, R'-Th4-Cy-Ph2-F, R'-Th4-Cy-Ph3-F,
R'-Th4-Cy-NpI-F, R'-Th4-Cy-Np2-F, R'-Th4-Cy-Np3-F, R'-Th4-Cy-Np4-F,
R'-Thl-Phl-Phl-F, R'-Thl-Phl-Ph2-F, R'-Thl-Phl-Ph3-F, R'-Thl-Phl-Npl-F,
R'-Th1-Phl-Np2-F, R'-Thl-Phl-Np3-F, R'-Th1-Ph1-Np4-F, R'-Th2-Phl-Ph1-F,
R'-Th2-Phl-Ph2-F, R'-Th2-Phl-Ph3-F, R'-Th2-Phl-Npl-F, R'-Th2-Phl-Np2-F,
R'-Th2-Ph1-Np3-F, R'-Th2-Ph1-Np4-F, R'-Th3-Phl-Ph1-F, R'-Th3-Ph1-Ph2-F,
R'-Th3-Phl-Ph3-F, R'-Th3-Phl-Npl-F, R'-Th3-Ph1-Np2-F, R'-Th3-Phl-Np3-F,
R'-Th3-Ph1-Np4-F, R'-Th4-Ph1-Ph1-F, R'-Th4-Ph1-Ph2-F, R'-Th4-Ph1-Ph3-F,
R'-Th4-Ph 1-Np 1-F, R'-Th4-Ph 1-Np2-F, R'-Th4-Ph 1-Np3-F, R'-Th4-Ph 1-Np4-F,
R'-Th1-Ph2-Ph1-F, R'-Thl-Ph2-Ph2-F, R'-Thl-Ph2-Ph3-F, R'-Th1-Ph2-Np1-F,
R'-Thl-Ph2-Np2-F, R'-Thl-Ph2-Np3-F, R'-Thl-Ph2-Np4-F, R'-Th2-Ph2-Phl-F,
R'-Th2-Ph2-Ph2-F, R'-Th2-Ph2-Ph3-F, R'-Th2-Ph2-Npl-F, R'-Th2-Ph2-Np2-F,
R'-Th2-Ph2-Np3-F, R'-Th2-Ph2-Np4-F, R'-Th3-Ph2-Phl-F, R'-Th3-Ph2-Ph2-F,
R'-Th3-Ph2-Ph3-F, R'-Th3-Ph2-Npl-F, R'-Th3-Ph2-Np2-F, R'-Th3-Ph2-Np3-F,
R'-Th3-Ph2-Np4-F, R'-Th4-Ph2-Phl-F, R'-Th4-Ph2-Ph2-F, R'-Th4-Ph2-Ph3-F,
R'-Th4-Ph2-Np1-F, R'-Th4-Ph2-Np2-F, R'-Th4-Ph2-Np3-F, R'-Th4-Ph2-Np4-F,
R'-Thl-Ph3-Phl-F, R'-Thl-Ph3-Ph2-F, R'-Thl-Ph3-Ph3-F, R'-Thl-Ph3-Npl-F,
CA 02377217 2001-12-28
42
R'-Thl-Ph3-Np2-F, R'-Thl-Ph3-Np3-F, R'-Thl-Ph3-Np4-F, R'-Th2-Ph3-Phl-F,
R'-Th2-Ph3-Ph2-F, R'-Th2-Ph3-Ph3-F, R'-Th2-Ph3-Npl-F, R'-Th2-Ph3-Np2-F,
R'-Th2-Ph3-Np3-F, R'-Th2-Ph3-Np4-F, R'-Th3-Ph3-Phl-F, R'-Th3-Ph3-Ph2-F,
R'-Th3-Ph3-Ph3-F, R'-Th3-Ph3-Npl-F, R'-Th3-Ph3-Np2-F, R'-Th3-Ph3-Np3-F,
R'-Th3-Ph3-Np4-F, R'-Th4-Ph3-Phl-F, R'-Th4-Ph3-Ph2-F, R'-Th4-Ph3-Ph3-F,
R'-Th4-Ph3-Npl-F, R'-Th4-Ph3-Np2-F, R'-Th4-Ph3-Np3-F, R'-Th4-Ph3-Np4-F,
R'-Thl-Npl-Phl-F, R'-Thl-Npl-Ph2-F, R'-Thl-Npl-Ph3-F, R'-Th2-Npl-Phl-F,
R'-Th2-Npl-Ph2-F, R'-Th2-Npl-Ph3-F, R'-Th3-Npl-Phl-F, R'-Th3-Npl-Ph2-F,
R'-Th3-Npl-Ph3-F, R'-Th4-Npl-Phl-F, Rl-Th4-Npl-Ph2-F, R'-Th4-Npl-Ph3-F,
R'-Thl-Np2-Phl-F, R'-Thl-Np2-Ph2-F, R'-Thl-Np2-Ph3-F, R'-Th2-Np2-Phl-F,
R'-Th2-Np2-Ph2-F, R'-Th2-Np2-Ph3-F, R'-Th3-Np2-Phl-F, R'-Th3-Np2-Ph2-F,
R'-Th3-Np2-Ph3-F, R'-Th4-Np2-Phl-F, R'-Th4-Np2-Ph2-F, R'-Th4-Np2-Ph3-F,
R'-Thl-Np3-Phl-F, R'-Thl-Np3-Ph2-F, R'-Thl-Np3-Ph3-F, R'-Th2-Np3-Phl-F,
R'-Th2-Np3-Ph2-F, R'-Th2-Np3-Ph3-F, R'-Th3-Np3-Phl-F, R'-Th3-Np3-Ph2-F,
R'-Th3-Np3-Ph3-F, R'-Th4-Np3-Phl-F, R'-Th4-Np3-Ph2-F, R'-Th4-Np3-Ph3-F,
R'-Th1-Np4-Ph1-F, R'-Th1-Np4-Ph2-F, R'-Thl-Np4-Ph3-F, R'-Th2-Np4-Ph1-F,
R'-Th2-Np4-Ph2-F, R'-Th2-Np4-Ph3-F, R'-Th3-Np4-Phl-F, R'-Th3-Np4-Ph2-F,
R'-Th3-Np4-Ph3-F, R'-Th4-Np4-Phl-F, R'-Th4-Np4-Ph2-F, R'-Th4-Np4-Ph3-F,
R'-Thl-Cy-CH2CH2-Phl-F, R"-Thl-Cy-CH2CH2-Ph2-F, R'-Thl-Cy-CH2CH2-Ph3-F,
R'-Thl-Cy-CH2CHZ-NpI-F, R'-Thl-Cy-CH2CH2-Np2-F, R'-Thl-Cy-CH2CH2-Np3-F,
R'-Thl-Cy-CH2CH2-Np4-F, R'-Th2-Cy-CH2CH2-Phl-F, R'-Th2-Cy-CH2CH2-Ph2-F,
R' -Th2-Cy-CH2CH2-Ph3-F, R' -Th2-Cy-CH2CH2-Np 1-F, R' -Th2-Cy-CH2CH2-Np2-F,
R'-Th2-Cy-CH2CH2-Np3-F, R'-Th2-Cy-CH2CH2-Np4-F, R'-Th3-Cy-CH2CH2-Phl-F,
R'-Th3-Cy-CH2CH2-Ph2-F, R'-Th3-Cy-CH2CHZ-Ph3-F, R'-Th3-Cy-CH2CH2-Np 1-F,
CA 02377217 2001-12-28
43
R'-Th3-Cy-CH2CH2-Np2-F, R'-Th3-Cy-CH2CH2-Np3-F, R'-Th3-Cy-CH2CH2-Np4-F,
R'-Th4-Cy-CH2CH2-Phl-F, R'-Th4-Cy-CH2CH2-Ph2-F, R'-Th4-Cy-CH2CH2-Ph3-F,
R'-Th4-Cy-CH2CH2-Npl-F, R'-Th4-Cy-CH2CH2-Np2-F, R'-Th4-Cy-CHZCH2-Np3-F,
R' -Th4-Cy-CH2CH2-Np4-F, R' -Th 1-Ph 1-CH2CH2-Ph 1-F, R' -Th 1-Ph i-CH2CH2-Ph2-
F,
R' -Th l-Ph l-CH2CH2-Ph3-F, R' -Th l-Ph l-CH2CH2-Np l-F, R' -Th l-Ph l-CHZCHZ-
Np2-F,
R'-Thl-Phl-CH2CH2-Np3-F, R'-Thl-Phl-CH2CH2-Np4-F, R'-Th2-Ph1 -CH2CH2-Phl-F,
R'-Th2-Phl-CH2CH2-Ph2-F, R'-Th2-Phl-CH2CH2-Ph3-F, R'-Th2-Phl-CH2CH2-Npl-F,
R'-Th2-Phl-CH2CH2-Np2-F, R'-Th2-Phl-CH2CH2-Np3-F, R'-Th2-Phl-CHZCH2-Np4-F,
R'-Th3-Phl-CH2CH2-Phl-F, R'-Th3-Phl-CH2CH2-Ph2-F, R'-Th3-Phl-CH2CH2-Ph3-F,
R'-Th3-Phl-CH2CH2-Npl-F, R'-Th3-Phl-CH2CH2-Np2-F, R'-Th3-Phl-CH2CH2-Np3-F,
R' -Th3 -Ph 1-CH2CH2-Np4-F, R' -Th4-Ph 1-CH2CH2-Ph 1-F, R' -Th4-Ph 1-CH2CH2-
Ph2-F,
R'-Th4-Ph1-CH2CH2-Ph3-F, R'-Th4-Ph1-CH2CH2-Np1-F, R'-Th4-Ph1-CH2CH2-Np2-F,
R'-Th4-Phl-CH2CH2-Np3-F, R'-Th4-Phl-CH2CH2-Np4-F, R'-Thl-Ph2-CHZCH2-Phl-F,
R'-Thl-Ph2-CH2CHZ-Ph2-F, R'-Thl-Ph2-CHZCH2-Ph3-F, R'-Thl-Ph2-CH2CH2-Npl-F,
R'-Thl-Ph2-CH2CH2-Np2-F, R'-Thl-Ph2-CH2CHZ-Np3-F, R'-Thl-Ph2-CH2CH2-Np4-F,
R'-Th2-Ph2-CH2CH2-Phl-F, R'-Th2-Ph2-CH2CH2-Ph2-F, R'-Th2-Ph2-CH2CH2-Ph3-F,
R'-Th2-Ph2-CH2CH2-Npl-F, R'-Th2-Ph2-CH2CH2-Np2-F, R'-Th2-Ph2-CH2CH2-Np3-F,
R'-Th2-Ph2-CH2CH2-Np4-F, R'-Th3-Ph2-CH2CHZ-Phl -F, R'-Th3-Ph2-CH2CH2-Ph2-F,
R'-Th3-Ph2-CH2CH2-Ph3-F, R'-Th3-Ph2-CH2CH2-Npl-F, R'-Th3-Ph2-CH2CH2-Np2-F,
R'-Th3-Ph2-CHZCH2-Np3-F, R'-Th3-Ph2-CH2CH2-Np4-F, R'-Th4-Ph2-CH2CH2-Phl-F,
R'-Th4-Ph2-CH2CH2-Ph2-F, R'-Th4-Ph2-CH2CH2-Ph3-F, R'-Th4-Ph2-CH2CH2-Npl-F,
R' -Th4-Ph2-CHZCH2-Np2-F, R' -Th4-Ph2-CH2CH2-Np3-F, R' -Th4-Ph2-CH2CH2-Np4-F,
R'-Thl-Ph3-CH2CH2-Phl-F, R'-Thl-Ph3-CH2CHz-Ph2-F, R'-Thl-Ph3-CH2CHZ-Ph3-F,
R'-Thl-Ph3-CH2CH2-Npl-F, R'-Thl-Ph3-CH2CH2-Np2-F, R'-Thl-Ph3-CH2CH2-Np3-F,
CA 02377217 2001-12-28
44
R'-Thl-Ph3-CH2CH2-Np4-F, R'-Th2-Ph3-CH2CH2-Phl-F, R'-Th2-Ph3-CH2CH2-Ph2-F,
R'-Th2-Ph3-CH2CH2-Ph3-F, R'-Th2-Ph3-CH2CH2-Npl-F, R'-Th2-Ph3-CH2CH2-Np2-F,
R'-Th2-Ph3-CH2CH2-Np3-F, R'-Th2-Ph3-CH2CHZ-Np4-F, R'-Th3-Ph3-CHZCHZ-Phl -F,
R'-Th3-Ph3-CH2CH2-Ph2-F, R'-Th3-Ph3-CH2CH2-Ph3-F, R'-Th3-Ph3-CH2CH2-Npl-F,
R'-Th3-Ph3-CH2CH2-Np2-F, R'-Th3-Ph3-CH2CH2-Np3-F, R'-Th3-Ph3-CH2CH2-Np4-F,
R'-Th4-Ph3-CH2CH2-Phl-F, R'-Th4-Ph3-CHZCH2-Ph2-F, R'-Th4-Ph3-CH2CH2-Ph3-F,
R'-Th4-Ph3-CH2CH2-Npl-F, R'-Th4-Ph3-CH2CH2-Np2-F, R'-Th4-Ph3-CHZCH2-Np3-F,
R' -Th4-Ph3 -CH2CH2-Np4-F, R' -Th 1-Np 1-CH2CH2-Ph 1-F, R' -Th 1-Np 1-CH2CH2-
Ph2-F,
R'-Thl-Npl-CH2CHZ-Ph3-F, R'-Th2-Npl-CH2CH2-Phl-F, R'-Th2-Npl-CH2CH2-Ph2-F,
R'-Th2-Npl-CH2CH2-Ph3-F, Rl-Th3-Npl-CH2CH2-Phl-F, R'-Th3-Npl-CH2CHz-Ph2-F,
R' -Th3 -Np 1-CH2CH2-Ph3-F, R' -Th4-Np 1-CH2CH2-Ph 1-F, R' -Th4-Np 1-CH2CH2-
Ph2-F,
R'-Th4-Npl-CHZCH2-Ph3-F, R'-Thl-Np2-CH2CH2-Phl-F, R'-Thl-Np2-CH2CH2-Ph2-F,
R'-Thl-Np2-CH2CH2-Ph3-F, R'-Th2-Np2-CH2CH2-Ph1-F, R'-Th2-Np2-CH2CHZ-Ph2-F,
R'-Th2-Np2-CH2CH2-Ph3-F, R'-Th3-Np2-CH2CH2-Phl-F, R'-Th3-Np2-CH2CH2-Ph2-F,
R'-Th3-Np2-CH2CH2-Ph3-F, R'-Th4-Np2-CH2CH2-Phl-F, R'-Th4-Np2-CH2CH2-Ph2-F,
R'-Th4-Np2-CH2CH2-Ph3-F, R'-Thl-Np3-CH2CH2-Phl-F, R'-Thl-Np3-CH2CH2-Ph2-F,
R'-Thl-Np3-CH2CH2-Ph3-F, R'-Th2-Np3-CH2CH2-Phl-F, R'-Th2-Np3-CH2CH2-Ph2-F,
R'-Th2-Np3-CH2CH2-Ph3-F, Rl-Th3-Np3-CH2CH2-Phl-F, R'-Th3-Np3-CH2CH2-Ph2-F,
R'-Th3-Np3-CH2CH2-Ph3-F, R'-Th4-Np3-CH2CH2-Phl-F, R'-Th4-Np3-CH2CH2-Ph2-F,
R'-Th4-Np3-CHZCH2-Ph3-F, R'-Thl-Np4-CH2CH2-Phl-F, R'-Thl-Np4-CH2CH2-Ph2-F,
R'-Thl-Np4-CH2CH2-Ph3-F, R'-Th2-Np4-CHZCH2-Phl-F, R'-Th2-Np4-CH2CH2-Ph2-F,
R'-Th2-Np4-CH2CH2-Ph3-F, R'-Th3-Np4-CH2CH2-Phl-F, R'-Th3-Np4-CH2CH2-Ph2-F,
R'-Th3-Np4-CH2CH2-Ph3-F, R'-Th4-Np4-CH2CH2-Phl-F, R'-Th4-Np4-CH2CH2-Ph2-F,
R'-Th4-Np4-CH2CH2-Ph3-F, R'-Thl-CH2CH2-Cy-PhI-F, R'-Thl-CH2CHz-Cy-Ph2-F,
CA 02377217 2001-12-28
R' -Th 1-CH2CH2-Cy-Ph3-F, R~-Th 1-CH2CH2-Cy-Np 1-F, R' -Th 1-CHZCH2-Cy-Np2-F,
R'-Th1-CHZCH2-Cy-Np3-F, R'-Thl-CH2CH2-Cy-Np4-F, Rl-Th2-CH2CH2-Cy-Ph1-F,
R'-Th2-CH2CH2-Cy-Ph2-F, R'-Th2-CH2CH2-Cy-Ph3-F, R'-Th2-CH2CH2-Cy-Npl-F,
R'-Th2-CH2CH2-Cy-Np2-F, R~-Th2-CH2CH2-Cy-Np3-F, Rl-Th2-CH2CH2-Cy-Np4-F,
5 R'-Th3-CH2CH2-Cy-Phl-F, R'-Th3-CH2CH2-Cy-Ph2-F, R'-Th3-CH2CH2-Cy-Ph3-F,
R'-Th3-CH2CH2-Cy-NpI-F, R'-Th3-CH2CH2-Cy-Np2-F, R'-Th3-CH2CH2-Cy-Np3-F,
R'-Th3-CH2CH2-Cy-Np4-F, R'-Th4-CH2CH2-Cy-Phl-F, Rl-Th4-CH2CH2-Cy-Ph2-F,
R'-Th4-CH2CH2-Cy-Ph3-F, Rl-Th4-CH2CH2-Cy-Npl-F, Rl-Th4-CH2CH2-Cy-Np2-F,
R'-Th4-CH2CH2-Cy-Np3-F, R'-Th4-CHZCH2-Cy-Np4-F, R'-Thl-CH2CH2-Phl-Phl-F,
10 R'-Th1-CH2CH2-Ph1-Ph2-F, R'-Th1-CHZCH2-Ph1-Ph3-F, R'-Th1-CH2CH2-Ph1-Np1-F,
R'-Thl-CH2CH2-Phl-Np2-F, R'-Th1-CH2CH2-Phl-Np3-F, R'-Thl-CH2CH2-Phl-Np4-F,
R'-Th2-CHZCH2-Phl-Phl-F, R'-Th2-CH2CH2-Phl-Ph2-F, R'-Th2-CH2CH2-Phl-Ph3-F,
R'-Th2-CH2CH2-Phl-Npl-F, Rl-Th2-CH2CH2-Phl-Np2-F, R'-Th2-CH2CH2-Phl-Np3-F,
R'-Th2-CHZCH2-Phl-Np4-F, Rl-Th3-CH2CH2-Phl-Phl-F, R'-Th3-CH2CH2-Ph1-Ph2-F,
15 R'-Th3-CH2CH2-Phl-Ph3-F, R'-Th3-CH2CH2-Phl-Npl-F, Rl-Th3-CH2CH2-Phl-Np2-F,
R'-Th3-CH2CH2-Phl-Np3-F, R~-Th3-CH2CH2-Phl-Np4-F, R'-Th4-CH2CH2-Phl-Phl-F,
R'-Th4-CH2CH2-Phl-Ph2-F, R'-Th4-CH2CH2-Phl-Ph3-F, R'-Th4-CH2CH2-Ph1-Npl-F,
R'-Th4-CH2CH2-Phl-Np2-F, R'-Th4-CH2CH2-Phl-Np3-F, R'-Th4-CH2CH2-Phl-Np4-F,
R'-Th1-CH2CH2-Ph2-Phl-F, R'-Thl-CH2CH2-Ph2-Ph2-F, R'-ThI-CHZCHZ-Ph2-Ph3-F,
20 R'-Th1-CH2CH2-Ph2-Npl-F, R~-Thl-CH2CH2-Ph2-Np2-F, R'-Thl-CH2CH2-Ph2-Np3-F,
R'-ThI-CHzCH2-Ph2-Np4-F, R'-Th2-CHZCH2-Ph2-Phl-F, R'-Th2-CH2CH2-Ph2-Ph2-F,
R' -Th2-CH2CH2-Ph2-Ph3-F, R'-Th2-CH2CH2-Ph2-Np 1-F, R'-Th2-CH2CH2-Ph2-Np2-F,
R'-Th2-CH2CH2-Ph2-Np3-F, Rl-Th2-CH2CH2-Ph2-Np4-F, R'-Th3-CH2CH2-Ph2-Phl -F,
R' -Th3-CH2CH2-Ph2-Ph2-F, R'-Th3-CH2CH2-Ph2-Ph3-F, R'-Th3-CH2CH2-Ph2-Np 1-F,
CA 02377217 2001-12-28
46
R'-Th3-CH2CH2-Ph2-Np2-F, R'-Th3-CH2CH2-Ph2-Np3-F, R'-Th3-CH2CH2-Ph2-Np4-F,
R' -Th4-CH2CH2-Ph2-Ph 1-F, R' -Th4-CH2CH2-Ph2-Ph2-F, R' -Th4-CH2CH2-Ph2-Ph3 -
F,
R'-Th4-CH2CH2-Ph2-Npl-F, R'-Th4-CH2CH2-Ph2-Np2-F, R'-Th4-CH2CH2-Ph2-Np3-F,
R'-Th4-CHZCH2-Ph2-Np4-F, R'-Thl-CH2CH2-Ph3-Phl-F, R'-ThI-CHZCH2-Ph3-Ph2-F,
R'-Thl-CH2CH2-Ph3-Ph3-F, R'-Thl-CH2CH2-Ph3-Npl-F, R'-Thl-CH2CH2-Ph3-Np2-F,
R'-Thl-CH2CH2-Ph3-Np3-F, R'-Thl-CH2CH2-Ph3-Np4-F, R'-Th2-CHZCH2-Ph3-Phl-F,
R'-Th2-CH2CH2-Ph3-Ph2-F, R'-Th2-CH2CH2-Ph3-Ph3-F, R'-Th2-CH2CH2-Ph3-Npl-F,
R'-Th2-CH2CH2-Ph3-Np2-F, R'-Th2-CH2CH2-Ph3-Np3-F, R'-Th2-CH2CH2-Ph3-Np4-F,
R'-Th3-CH2CH2-Ph3-Phl-F, R'-Th3-CH2CH2-Ph3-Ph2-F, R'-Th3-CH2CH2-Ph3-Ph3-F,
R'-Th3-CH2CH2-Ph3-Npl-F, R'-Th3-CH2CH2-Ph3-Np2-F, R'-Th3-CH2CH2-Ph3-Np3-F,
R'-Th3-CH2CH2-Ph3-Np4-F, Rl-Th4-CH2CH2-Ph3-Phl -F, R'-Th4-CHZCHZ-Ph3-Ph2-F,
R'-Th4-CH2CH2-Ph3-Ph3-F, R'-Th4-CHZCH2-Ph3-Np 1-F, R'-Th4-CH2CH2-Ph3-Np2-F,
R'-Th4-CH2CH2-Ph3-Np3-F, R'-Th4-CHZCH2-Ph3-Np4-F, R'-Thl-CH2CHZ-Npl-Phl-F,
R' -Th 1-CHZCH2-Np 1-Ph2-F, R' -Th 1-CH2CH2-Np 1-Ph3 -F, R' -Th2-CH2CH2-Np 1-
Ph 1-F,
R'-Th2-CH2CH2-Npl-Ph2-F, R'-Th2-CH2CH2-Npl-Ph3-F, R'-Th3-CH2CH2-Npl-Phl-F,
R'-Th3-CH2CHZ-Npl-Ph2-F, R'-Th3-CH2CH2-Npl-Ph3-F, R'-Th4-CH2CH2-Npl-Phl-F,
R' -Th4-CH2CH2-Np 1 -Ph2-F, R'-Th4-CH2CH2-Np 1-Ph3-F, R'-Th 1-CH2CH2-Np2-Ph 1-
F,
R'-ThI-CHZCH2-Np2-Ph2-F, R'-Thl-CH2CH2-Np2-Ph3-F, R'-Th2-CH2CH2-Np2-Phl-F,
R'-Th2-CH2CH2-Np2-Ph2-F, R'-Th2-CH2CHZ-Np2-Ph3-F, R'-Th3-CH2CH2-Np2-Phl-F,
R'-Th3-CH2CH2-Np2-Ph2-F, R'-Th3-CHZCH2-Np2-Ph3-F, R'-Th4-CH2CH2-Np2-Phl-F,
R'-Th4-CH2CH2-Np2-Ph2-F, R'-Th4-CH2CH2-Np2-Ph3-F, R'-Thl-CH2CH2-Np3-Phl-F,
R'-Thl-CH2CH2-Np3-Ph2-F, R'-Thl-CH2CH2-Np3-Ph3-F, R'-Th2-CH2CH2-Np3-Phl-F,
R'-Th2-CH2CH2-Np3-Ph2-F, R'-Th2-CH2CH2-Np3-Ph3-F, R'-Th3-CH2CH2-Np3-Ph 1-F,
R'-Th3-CHZCH2-Np3-Ph2-F, R'-Th3-CH2CH2-Np3-Ph3-F, R'-Th4-CH2CH2-Np3-Phl -F,
CA 02377217 2001-12-28
47
R'-Th4-CH2CH2-Np3-Ph2-F, R'-Th4-CH2CH2-Np3-Ph3-F, R'-Thl-CH2CH2-Np4-Phl-F,
R' -Th 1-CH2CH2-Np4-Ph2-F, R' -Th 1-CH2CH2-Np4-Ph3-F, R' -Th2-CH2CH2-Np4-Ph 1-
F,
R'-Th2-CH2CH2-Np4-Ph2-F, R'-Th2-CH2CH2-Np4-Ph3-F, R'-Th3-CHZCH2-Np4-Phl-F,
R'-Th3-CHZCH2-Np4-Ph2-F, R'-Th3-CH2CH2-Np4-Ph3-F, R'-Th4-CH2CH2-Np4-Phl-F,
R'-Th4-CH2CH2-Np4-Ph2-F, R'-Th4-CH2CH2-Np4-Ph3-F,
R'-Th1-Ph1-C=C-Ph1-F, R'-Th1-Ph1-C=C-Ph2-F, R'-Th1-Ph1-C=C-Ph3-F,
R' -Th2-Ph 1-C=C-Ph 1-F, R' -Th2-Ph 1-C=C-Ph2-F, R'-Th2-Ph 1-C-C-Ph3-F,
R' -Th3-Ph l-C=C-Ph l-F, R' -Th3 -Ph l-C=C-Ph2-F, R' -Th3-Ph l-C=C-Ph3-F,
R' -Th4-Ph 1-C=C-Ph 1-F, R' -Th4-Ph 1-C=C-Ph2-F, R' -Th4-Ph 1-C-C-Ph3 -F,
R'-Thl-Ph2-C-C-Phl-F, Rl-Thl-Ph2-C=C-Ph2-F, R'-Thl-Ph2-C=C-Ph3-F,
R'-Th2-Ph2-C=C-Phl-F, R'-Th2-Ph2-C=C-Ph2-F, R'-Th2-Ph2-C=C-Ph3-F,
R'-Th3-Ph2-C-C-Phl-F, R'-Th3-Ph2-C-C-Ph2-F, R'-Th3-Ph2-C=C-Ph3-F,
R'-Th4-Ph2-C=C-PhI-F, R'-Th4-Ph2-C-C-Ph2-F, R'-Th4-Ph2-C=C-Ph3-F,
R'-Thl-Ph3-C=C-Phl-F, R'-Thl-Ph3-C=C-Ph2-F, R'-Thl-Ph3-C-C-Ph3-F,
R'-Th2-Ph3-C=C-Phl-F, R'-Th2-Ph3-C=C-Ph2-F, R'-Th2-Ph3-C=C-Ph3-F,
R'-Th3-Ph3-C-C-Phl-F, R'-Th3-Ph3-C=C-Ph2-F, R'-Th3-Ph3-C=C-Ph3-F,
R' -Th4-Ph3-C=C-Phl -F, R'-Th4-Ph3-C=C-Ph2-F, R'-Th4-Ph3-C=C-Ph3-F,
R' -Th 1-C=C-Ph l-Ph 1-F, Rl -Th 1-C=C-Ph 1-Ph2-F, R' -Th 1-C_C-Ph 1-Ph3 -F,
R'-Th2-C=C-Phl-Phl-F, Rl-Th2-C=C-Phl-Ph2-F, R'-Th2-C=C-Phl-Ph3-F,
R'-Th3-C=C-Phl-Phl-F, R'-Th3-C=C-Phl-Ph2-F, R'-Th3-C=C-Phl-Ph3-F,
R' -Th4-C-C-Ph 1-Ph 1-F, R' -Th4-C=C-Ph 1-Ph2-F, R' -Th4-C=C-Ph 1-Ph3 -F,
R' -Th 1-C=C-Ph2-Ph 1-F, R' -Th l-C=C-Ph2-Ph2-F, R' -Th 1-C=C-Ph2-Ph3 -F,
R'-Th2-C=C-Ph2-Phl-F, R'-Th2-C=C-Ph2-Ph2-F, R'-Th2-C=C-Ph2-Ph3-F,
CA 02377217 2001-12-28
48
R'-Th3-C=C-Ph2-Phl-F, R'-Th3-C=C-Ph2-Ph2-F, R'-Th3-C=C-Ph2-Ph3-F,
R'-Th4-C=C-Ph2-Phl-F, R'-Th4-C=C-Ph2-Ph2-F, R'-Th4-C-C-Ph2-Ph3-F,
R' -Th 1-C=C-Ph3 -Ph 1-F, R' -Th 1-C=C-Ph3 -Ph2-F, R' -Th 1-C-C-Ph3 -Ph3 -F,
R'-Th2-C=C-Ph3-Phl-F, R'-Th2-C=C-Ph3-Ph2-F, R'-Th2-C=C-Ph3-Ph3-F,
R'-Th3-C=C-Ph3-Phl-F, Rt-Th3-C=C-Ph3-Ph2-F, R'-Th3-C=C-Ph3-Ph3-F,
R'-Th4-C=C-Ph3-Phl-F, R'-Th4-C=C-Ph3-Ph2-F, R'-Th4-C=C-Ph3-Ph3-F,
inthecaseinwhichna= 1,nb=0orna=0,nb= 1,andn = 1,nd=0orn=0,nd 1,andZ
is a fluorine atom,
R'-Cy-Th1-Ph1-F, R'-Cy-Th1-Ph2-F, R'-Cy-Th1-Ph3-F, R'-Cy-Th1-Np1-F,
R'-Cy-Thl-Np2-F, R'-Cy-Thl-Np3-F, R'-Cy-Thl-Np4-F,
R'-Cy-CH2CH2-Thl-Phl-F, R'-Cy-CH2CH2-Thl-Ph2-F, R'-Cy-CH2CH2-Thl-Ph3-F,
R'-Cy-CH2CH2-Thl-Npl-F, R'-Cy-CH2CH2-Thl-Np2-F, R'-Cy-CH2CH2-Thl-Np3-F,
R' -Cy-CH2CH2-Th 1-Np4-F, R' -Cy-Th 1-CH2CH2-Ph 1-F, R' -Cy-Th 1-CH2CH2-Ph2-F,
R'-Cy-ThI-CHZCH2-Ph3-F, R'-Cy-Thl-CH2CH2-Npl-F, R'-Cy-Thl-CH2CHZ-Np2-F,
R'-Cy-Thl-CH2CH2-Np3-F, R'-Cy-Thl-CH2CH2-Np4-F,
R'-Cy-Th1-C=C-Ph1-F, R'-Cy-Thl-C=C-Ph2-F, R'-Cy-Th1-C=C-Ph3-F,
R'-Phl-Thl-Phl-F, R'-Phl-Thl-Ph2-F, R'-Phl-Thl-Ph3-F, R'-Phl-Thl-Npl-F,
R'-Phl-Thl-Np2-F, R'-Phl-Thl-Np3-F, R'-Phl-Thl-Np4-F,
R'-Ph1-CH2CH2-Th1-Ph1-F, R'-Ph1-CH2CH2-Th1-Ph2-F, R'-Ph1-CH2CH2-Th1-Ph3-F,
R'-Phl-CH2CH2-Thl-Np1 -F, R'-Phl-CH2CH2-Thl-Np2-F, R'-PhI-CHZCH2-Thl-Np3-F,
R'-Phl-CH2CH2-Thl-Np4-F, R'-Phl-Thl-CH2CH2-Phl-F, R'-Phl-Thl-CH2CH2-Ph2-F,
R'-Phl-Thl-CH2CH2-Ph3-F, R'-Phl-Thl-CH2CH2-Npl-F, R'-Phl-Thl-CH2CH2-Np2-F,
R' -Ph 1-Th 1-CHZCH2-Np3 -F, R' -Ph i-Th 1-CH2CH2-Np4-F,
R' -Ph 1-C=C-Th 1-Ph 1-F, R' -Ph l-C=C-Th 1-Ph2-F, R' -Ph 1-C=C-Th 1-Ph3 -F,
CA 02377217 2001-12-28
49
R'-Phl-Th1-C=C-Ph1-F, R'-Phl-Thl-C=C-Ph2-F, R'-Ph1-Th1-C=C-Ph3-F,
R'-Ph2-Th1-Ph1-F, R'-Ph2-Th1-Ph2-F, R'-Ph2-Th1-Ph3-F, R'-Ph2-Th1-Np1-F,
R'-Ph2-Thl-Np2-F, R'-Ph2-Thl-Np3-F, R'-Ph2-Thl-Np4-F,
R'-Ph2-CHZCH2-Th1-Ph1-F, R'-Ph2-CH2CH2-Th1-Ph2-F, R'-Ph2-CH2CH2-Th1-Ph3-F,
R'-Ph2-CH2CH2-Thl-Npl-F, R'-Ph2-CH2CH2-Thl-Np2-F, R'-Ph2-CHZCH2-Thl-Np3-F,
R' -Ph2-CHZCH2-Th 1-Np4-F, R' -Ph2-Th 1-CH2CH2-Ph 1-F, R' -Ph2-Th 1-CH2CH2-Ph2-
F,
R'-Ph2-Thl-CH2CH2-Ph3-F, R'-Ph2-Thl-CH2CH2-Npl-F, R'-Ph2-Thl-CH2CH2-Np2-F,
R'-Ph2-Thl-CH2CH2-Np3-F, R'-Ph2-Thl-CH2CH2-Np4-F,
R' -Ph2-C_C-Th 1-Ph 1-F, R' -Ph2-C=C-Th 1-Ph2-F, R' -Ph2-C=C-Th 1-Ph3 -F,
R' -Ph2-Th 1-C=C-Ph 1-F, R' -Ph2-Th 1-C=C-Ph2-F, R' -Ph2-Th 1-C=C-Ph3-F,
R'-Ph3-Thl-Phl-F, R'-Ph3-Thl-Ph2-F, R'-Ph3-Thl-Ph3-F, R'-Ph3-Thl-Npl-F,
R'-Ph3-Thl-Np2-F, R'-Ph3-Thl-Np3-F, R'-Ph3-Thl-Np4-F,
R'-Ph3-CH2CH2-Thl-Phl-F, R'-Ph3-CH2CH2-Thl-Ph2-F, R'-Ph3-CH2CH2-Thl-Ph3-F,
R'-Ph3-CH2CH2-Thl-Npl-F, R'-Ph3-CH2CH2-Thl-Np2-F, R'-Ph3-CH2CH2-Thl-Np3-F,
Rl-Ph3-CH2CH2-Thl-Np4-F, R'-Ph3-Thl-CH2CH2-Phl-F, R'-Ph3-Thl-CH2CH2-Ph2-F,
R'-Ph3-Thl-CH2CH2-Ph3-F, R'-Ph3-Thl-CH2CH2-Npl-F, R'-Ph3-Thl-CH2CH2-Np2-F,
R'-Ph3-Thl-CH2CH2-Np3-F, R'-Ph3-Thl-CH2CH2-Np4-F,
R' -Ph3 -C-C-Th 1-Ph 1-F, R' -Ph3 -C-C-Th 1-Ph2-F, R' -Ph3 -C-C-Th 1-Ph3 -F,
R'-Ph3-Thl-C=C-PhI-F, R'-Ph3-Thl-C=C-Ph2-F, R'-Ph3-Thl-C_C-Ph3-F,
R'-Npl-Thl-Phl-F, R'-Npl-Thl-Ph2-F, R'-Npl-Thl-Ph3-F,
R'-Np1-CH2CH2-Thl-Ph1-F, R'-Np1-CH2CH2-Thl-Ph2-F, R'-Np1-CH2CH2-Thl-Ph3-F,
R' -Np 1-Th 1-CH2CH2-Ph 1-F, R' -Np 1-Th 1-CH2CH2-Ph2-F, R' -Np 1-Th 1-CH2CH2-
Ph3 -F,
R' -Np2-Th l-Ph l-F, R' -Np2-Th l-Ph2-F, R' -Np2-Th l-Ph3-F,
R' -Np2-CH2CH2-Th 1-Ph 1-F, Rl -Np2-CH2CHZ-Th 1-Ph2-F, R' -Np2-CH2CH2-Th 1-Ph3
-F,
CA 02377217 2001-12-28
R' -Np2-Th 1-CH2CH2-Ph 1-F, R' -Np2-Th 1-CH2CH2-Ph2-F, R' -Np2-Th 1-CH2CH2-Ph3-
F,
R'-Np3-Thl-Phi-F, R'-Np3-Thl-Ph2-F, R'-Np3-Thl-Ph3-F,
R'-Np3-CHZCHZ-Thl-Phl-F, R'-Np3-CH2CH2-Thl-Ph2-F, R'-Np3-CH2CH2-Thl-Ph3-F,
R'-Np3-Thl-CH2CH2-Phl-F, R'-Np3-Thl-CH2CH2-Ph2-F, R'-Np3-Thl-CH2CH2-Ph3-F,
5 R'-Np1-Th1-Ph1-F, R'-Np1-Thl-Ph2-F, R'-Np1-Th1-Ph3-F,
R'-Np4-CH2CH2-Thl-Phl-F, R'-Np4-CH2CH2-Thl-Ph2-F, R'-Np4-CH2CH2-Thl-Ph3-F,
R'-Np4-Thl-CH2CH2-Phl-F, R'-Np4-Thl-CH2CHZ-Ph2-F, R'-Np4-Thl-CHZCH2-Ph3-F,
R'-Cy-Th2-Phl-F, R'-Cy-Th2-Ph2-F, R'-Cy-Th2-Ph3-F, R'-Cy-Th2-Npl-F,
R'-Cy-Th2-Np2-F, R'-Cy-Th2-Np3-F, R'-Cy-Th2-Np4-F,
10 R'-Cy-CH2CH2-Th2-Phl-F, R'-Cy-CH2CH2-Th2-Ph2-F, R'-Cy-CH2CH2-Th2-Ph3-F,
R'-Cy-CH2CH2-Th2-Npl-F, R'-Cy-CHZCH2-Th2-Np2-F, R'-Cy-CHZCH2-Th2-Np3-F,
R' -Cy-CH2CH2-Th2-Np4-F, R' -Cy-Th2-CH2CH2-Ph l -F, R' -Cy-Th2-CHZCH2-Ph2-F,
R'-Cy-Th2-CH2CH2-Ph3-F, R'-Cy-Th2-CH2CH2-Npl-F, R'-Cy-Th2-CH2CH2-Np2-F,
R'-Cy-Th2-CH2CH2-Np3-F, R'-Cy-Th2-CH2CH2-Np4-F,
15 R'-Cy-Th2-C=C-PhI-F, R'-Cy-Th2-C=C-Ph2-F, R'-Cy-Th2-C=C-Ph3-F,
R'-Phl-Th2-Phl-F, R'-Phl-Th2-Ph2-F, R'-Phl-Th2-Ph3-F, R'-Phl-Th2-Npl-F,
R'-Phl-Th2-Np2-F, R'-Phl-Th2-Np3-F, R'-Phl-Th2-Np4-F,
R'-PhI-CHZCH2-Th2-Phl-F, R'-PhI-CHZCH2-Th2-Ph2-F, R'-Phl-CHZCH2-Th2-Ph3-F,
R'-Phl-CH2CH2-Th2-Npl-F, R'-Phl-CH2CH2-Th2-Np2-F, R'-Phl-CH2CH2-Th2-Np3-F,
20 R'-Phl-CH2CH2-Th2-Np4-F, R'-Phl-Th2-CH2CH2-Phl-F, Rl-Phl-Th2-CH2CH2-Ph2-F,
R' -Ph 1-Th2-CHZCH2-Ph3-F, R' -Ph 1-Th2-CHZCH2-Np 1-F, R' -Ph 1-Th2-CH2CH2-Np2-
F,
R' -Ph 1-Th2-CH2CH2-Np3-F, R' -Ph 1-Th2-CH2CH2-Np4-F,
R' -Ph 1-C=C-Th2-Ph 1-F, R' -Ph 1-C=C-Th2-Ph2-F, R' -Ph 1-C-C-Th2-Ph3 -F,
R' -Ph 1-Th2-C=-C-Ph 1-F, R' -Ph 1-Th2-C=C-Ph2-F, R' -Ph 1-Th2-C=C-Ph3 -F,
CA 02377217 2001-12-28
51
R'-Ph2-Th2-Phl-F, R'-Ph2-Th2-Ph2-F, R'-Ph2-Th2-Ph3-F, R'-Ph2-Th2-Npl-F,
R' -Ph2-Th2-Np2-F, R' -Ph2-Th2-Np3-F, R' -Ph2-Th2-Np4-F,
R' -Ph2-CH2CH2-Th2-Ph l-F, R' -Ph2-CH2CH2-Th2-Ph2-F, R' -Ph2-CH2C HZ-Th2-Ph3 -
F,
R'-Ph2-CH2CH2-Th2-Npl-F, R'-Ph2-CH2CH2-Th2-Np2-F, R'-Ph2-CHZCH2-Th2-Np3-F,
R'-Ph2-CH2CH2-Th2-Np4-F, R'-Ph2-Th2-CH2CH2-Phl-F, R'-Ph2-Th2-CH2CH2-Ph2-F,
R'-Ph2-Th2-CH2CH2-Ph3-F, R'-Ph2-Th2-CH2CH2-Npl-F, R'-Ph2-Th2-CH2CH2-Np2-F,
R'-Ph2-Th2-CH2CH2-Np3-F, R'-Ph2-Th2-CH2CH2-Np4-F,
R'-Ph2-C-C-Th2-Phl-F, R'-Ph2-C=C-Th2-Ph2-F, R'-Ph2-C=C-Th2-Ph3-F,
R'-Ph2-Th2-C-C-Ph1-F, R'-Ph2-Th2-C=C-Ph2-F, R'-Ph2-Th2-C=C-Ph3-F,
R'-Ph3-Th2-Phl-F, R'-Ph3-Th2-Ph2-F, R'-Ph3-Th2-Ph3-F, R'-Ph3-Th2-Npl-F,
R'-Ph3-Th2-Np2-F, R'-Ph3-Th2-Np3-F, R'-Ph3-Th2-Np4-F,
R'-Ph3-CH2CH2-Th2-Phl-F, R'-Ph3-CH2CH2-Th2-Ph2-F, R'-Ph3-CH2CH2-Th2-Ph3-F,
R'-Ph3-CH2CH2-Th2-Npl-F, R'-Ph3-CH2CH2-Th2-Np2-F, R'-Ph3-CH2CH2-Th2-Np3-F,
R'-Ph3-CH2CH2-Th2-Np4-F, R'-Ph3-Th2-CH2CH2-Phl -F, R'-Ph3-Th2-CH2CH2-Ph2-F,
R'-Ph3-Th2-CH2CH2-Ph3-F, R'-Ph3-Th2-CH2CH2-Npl-F, R'-Ph3-Th2-CH2CH2-Np2-F,
R'-Ph3-Th2-CH2CH2-Np3-F, R'-Ph3-Th2-CH2CH2-Np4-F,
R'-Ph3-C=C-Th2-Phl-F, R'-Ph3-C=C-Th2-Ph2-F, R'-Ph3-C=C-Th2-Ph3-F,
R'-Ph3-Th2-C-C-PhI-F, R'-Ph3-Th2-C=C-Ph2-F, R'-Ph3-Th2-C=C-Ph3-F,
R' -Np 1-Th2-Ph 1-F, R' -Np 1-Th2-Ph2-F, R' -Np 1-Th2-Ph3-F,
R' -Np 1-CH2CH2-Th2-Ph 1-F, R' -Np 1-CH2CH2-Th2-Ph2-F, R' -Np 1-CH2CH2-Th2-Ph3
-F,
R' -Np 1-Th2-CH2CH2-Ph 1-F, R' -Np 1-Th2-CH2CH2-Ph2-F, R' -Np 1-Th2-CHZCH2-Ph3
-F,
R' -Np2-Th2-Ph 1-F, R' -Np2-Th2-Ph2-F, R' -Np2-Th2-Ph3 -F,
R '-Np2-CHZCH2-Th2-Ph 1-F, R' -Np2-CH2CH2-Th2-Ph2-F, R' -Np2-CH2CH2-Th2-Ph3 -
F,
R' -Np2-Th2-CH2CH2-Ph 1-F, R' -Np2-Th2-CHZCH2-Ph2-F, R' -Np2-Th2-CH2CH2-Ph3 -
F,
CA 02377217 2001-12-28
52
R' -Np3 -Th2-Ph 1-F, R' -Np3 -Th2-Ph2-F, R' -Np3 -Th2-Ph3 -F,
R'-Np3-CH2CH2-Th2-Phl-F, R'-Np3-CH2CH2-Th2-Ph2-F, R'-Np3-CH2CH2-Th2-Ph3-F,
R'-Np3-Th2-CHZCHZ-Phl-F, R'-Np3-Th2-CH2CH2-Ph2-F, R'-Np3-Th2-CH2CH2-Ph3-F,
R' -Np 1-Th2-Phl -F, R'-Np l-Th2-Ph2-F, R'-Np 1-Th2-Ph3-F,
R'-Np4-CHZCH2-Th2-Phl-F, R'-Np4-CH2CH2-Th2-Ph2-F, R'-Np4-CH2CH2-Th2-Ph3-F,
R' -Np4-Th2-CH2CH2-Ph l -F, R'-Np4-Th2-CH2CH2-Ph2-F, R' -Np4-Th2-CHZCH2-Ph3 -
F,
R'-Cy-Th3-Phl-F, R'-Cy-Th3-Ph2-F, R'-Cy-Th3-Ph3-F, R'-Cy-Th3-Npl-F,
R'-Cy-Th3-Np2-F, R'-Cy-Th3-Np3-F, R'-Cy-Th3-Np4-F,
R'-Cy-CH2CH2-Th3-Phl-F, R'-Cy-CH2CH2-Th3-Ph2-F, R'-Cy-CH2CH2-Th3-Ph3-F,
R'-Cy-CH2CH2-Th3-Npl-F, R'-Cy-CH2CH2-Th3-Np2-F, R'-Cy-CH2CH2-Th3-Np3-F,
R'-Cy-CH2CH2-Th3-Np4-F, R'-Cy-Th3-CHZCH2-Phl-F, R'-Cy-Th3-CH2CH2-Ph2-F,
R'-Cy-Th3-CHZCH2-Ph3-F, R'-Cy-Th3-CH2CH2-Npl-F, R'-Cy-Th3-CH2CH2-Np2-F,
R'-Cy-Th3-CH2CH2-Np3-F, R'-Cy-Th3-CH2CH2-Np4-F,
R' -Cy-Th3 -C=C-Ph 1-F, R' -Cy-Th3 -C-C-Ph2-F, R' -Cy-Th3 -C-C-Ph3 -F,
R'-Phl-Th3-Phl-F, R'-Phl-Th3-Ph2-F, R'-Phl-Th3-Ph3-F, R'-Phl-Th3-Npl-F,
R'-Phl-Th3-Np2-F, R'-Phl-Th3-Np3-F, R'-Phl-Th3-Np4-F,
R'-Phl-CH2CH2-Th3-Ph1 -F, R'-Phl-CH2CH2-Th3-Ph2-F, R'-Phl-CH2CH2-Th3-Ph3-F,
R'-Phl-CH2CH2-Th3-Np1 -F, R'-PhI-CHZCHZ-Th3-Np2-F, R'-PhI-CHzCH2-Th3-Np3-F,
R'-Phl-CH2CH2-Th3-Np4-F, R'-Phl-Th3-CH2CH2-Phl-F, R'-Phl-Th3-CH2CH2-Ph2-F,
R'-Phl-Th3-CHZCH2-Ph3-F, R'-Phl-Th3-CHZCH2-Npl-F, R'-Phl-Th3-CH2CH2-Np2-F,
R'-Phl-Th3-CH2CH2-Np3-F, R'-Phl-Th3-CH2CH2-Np4-F,
R'-PhI-C-C-Th3-Phl-F, R'-PhI-C=C-Th3-Ph2-F, R'-PhI-C=C-Th3-Ph3-F,
R'-Phl-Th3-C=C-Phl-F, R'-Phl-Th3-C=C-Ph2-F, R'-Phl-Th3-C=C-Ph3-F,
R'-Ph2-Th3-Phl-F, R'-Ph2-Th3-Ph2-F, R'-Ph2-Th3-Ph3-F, R'-Ph2-Th3-Npl-F,
CA 02377217 2001-12-28
53
R'-Ph2-Th3-Np2-F, R'-Ph2-Th3-Np3-F, R'-Ph2-Th3-Np4-F,
R'-Ph2-CH2CH2-Th3-Ph1-F, R'-Ph2-CH2CH2-Th3-Ph2-F, R'-Ph2-CH2CH2-Th3-Ph3-F,
R'-Ph2-CH2CH2-Th3-Np 1-F, R'-Ph2-CH2CH2-Th3-Np2-F, R'-Ph2-CH2CH2-Th3-Np3-F,
R'-Ph2-CH2CH2-Th3-Np4-F, R'-Ph2-Th3-CH2CH2-Phl-F, R'-Ph2-Th3-CH2CH2-Ph2-F,
R'-Ph2-Th3-CH2CH2-Ph3-F, R'-Ph2-Th3-CH2CH2-Npl-F, R'-Ph2-Th3-CH2CH2-Np2-F,
R'-Ph2-Th3-CH2CH2-Np3-F, R'-Ph2-Th3-CH2CH2-Np4-F,
R'-Ph2-C=C-Th3-Phl-F, R'-Ph2-C=C-Th3-Ph2-F, R'-Ph2-C=C-Th3-Ph3-F,
R'-Ph2-Th3-C=C-Phl -F, R'-Ph2-Th3-C=C-Ph2-F, R'-Ph2-Th3-C=C-Ph3-F,
R'-Ph3-Th3-Phl-F, R'-Ph3-Th3-Ph2-F, R'-Ph3-Th3-Ph3-F, R'-Ph3-Th3-Np1-F,
R'-Ph3-Th3-Np2-F, R'-Ph3-Th3-Np3-F, R'-Ph3-Th3-Np4-F,
R'-Ph3-CH2CH2-Th3-Phl -F, R'-Ph3-CH2CH2-Th3-Ph2-F, R'-Ph3-CH2CH2-Th3-Ph3-F,
R'-Ph3-CH2CH2-Th3-Np1-F, R'-Ph3-CHZCHZ-Th3-Np2-F, R'-Ph3-CH2CH2-Th3-Np3-F,
R'-Ph3-CH2CH2-Th3-Np4-F, R'-Ph3-Th3-CH2CH2-Phi-F, R'-Ph3-Th3-CHZCH2-Ph2-F,
R'-Ph3-Th3-CH2CH2-Ph3-F, R'-Ph3-Th3-CH2CH2-Npl-F, R'-Ph3-Th3-CH2CH2-Np2-F,
R'-Ph3-Th3-CH2CH2-Np3-F, R'-Ph3-Th3-CH2CH2-Np4-F,
R' -Ph3 -C-C-Th3 -Ph 1-F, R' -Ph3 -C=C-Th3 -Ph2-F, R' -Ph3 -C=C-Th3 -Ph3 -F,
R'-Ph3-Th3-C=C-Ph1-F, R'-Ph3-Th3-C=C-Ph2-F, R'-Ph3-Th3-C=C-Ph3-F,
R' -Np 1-Th3 -Ph 1-F, R' -Np 1-Th3 -Ph2-F, R' -Np 1-Th3 -Ph3 -F,
R'-NpI-CHZCH2-Th3-Phl-F, R'-Npl-CH2CH2-Th3-Ph2-F, R'-Npl-CH2CH2-Th3-Ph3-F,
R'-Npl-Th3-CH2CH2-Phl-F, R'-Np1-Th3-CH2CH2-Ph2-F, R'-Npl-Th3-CH2CH2-Ph3-F,
R'-Np2-Th3-Phl-F, R'-Np2-Th3-Ph2-F, R'-Np2-Th3-Ph3-F,
R'-Np2-CH2CH2-Th3-Phl-F, R'-Np2-CH2CHZ-Th3-Ph2-F, R'-Np2-CH2CH2-Th3-Ph3-F,
R'-Np2-Th3-CH2CH2-Phl-F, R'-Np2-Th3-CHZCH2-Ph2-F, R'-Np2-Th3-CH2CH2-Ph3-F,
R'-Np3-Th3-Ph1-F, R'-Np3-Th3-Ph2-F, R'-Np3-Th3-Ph3-F,
CA 02377217 2001-12-28
54
R'-Np3-CH2CH2-Th3-Phl-F, R'-Np3-CH2CH2-Th3-Ph2-F, R'-Np3-CHZCHZ-Th3-Ph3-F,
R'-Np3-Th3-CH2CH2-Phl-F, R'-Np3-Th3-CH2CH2-Ph2-F, R'-Np3-Th3-CHZCH2-Ph3-F,
R'-Npl-Th3-Phl-F, R'-Npl-Th3-Ph2-F, R'-Npl-Th3-Ph3-F,
R'-Np4-CH2CH2-Th3-Phl-F, R'-Np4-CH2CH2-Th3-Ph2-F, R'-Np4-CH2CH2-Th3-Ph3-F,
R'-Np4-Th3-CH2CH2-Phl-F, R'-Np4-Th3-CH2CH2-Ph2-F, R'-Np4-Th3-CH2CH2-Ph3-F,
R'-Cy-Th3-Phl-F, R'-Cy-Th3-Ph2-F, R'-Cy-Th3-Ph3-F, R'-Cy-Th3-Npl-F,
R'-Cy-Th3-Np2-F, R'-Cy-Th3-Np3-F, R'-Cy-Th3-Np4-F,
R'-Cy-CH2CH2-Th3-Phl-F, RI-Cy-CH2CH2-Th3-Ph2-F, R'-Cy-CH2CH2-Th3-Ph3-F,
R'-Cy-CH2CH2-Th3-Npl-F, R'-Cy-CH2CH2-Th3-Np2-F, RI-Cy-CH2CH2-Th3-Np3-F,
R'-Cy-CHZCH2-Th3-Np4-F, R'-Cy-Th3-CH2CH2-Phl-F, R'-Cy-Th3-CH2CH2-Ph2-F,
R'-Cy-Th3-CH2CH2-Ph3-F, R'-Cy-Th3-CH2CH2-Npl-F, R'-Cy-Th3-CH2CH2-Np2-F,
R'-Cy-Th3-CH2CH2-Np3-F, RI-Cy-Th3-CH2CH2-Np4-F,
R'-Cy-Th3-C=C-PhI-F, R'-Cy-Th3-C=C-Ph2-F, R'-Cy-Th3-C-=C-Ph3-F,
R'-Phl-Th3-Phl-F, R'-Phl-Th3-Ph2-F, R'-Phl-Th3-Ph3-F, R'-Phl-Th3-Npl-F,
R'-Phl-Th3-Np2-F, R'-Phl-Th3-Np3-F, R'-Phl-Th3-Np4-F,
R'-Phi-CH2CH2-Th3-Phl-F, R'-Phl-CH2CH2-Th3-Ph2-F, R'-Phl-CH2CH2-Th3-Ph3-F,
R'-Phl-CH2CH2-Th3-Npl-F, R'-Phl-CH2CH2-Th3-Np2-F, RI-Phl-CH2CH2-Th3-Np3-F,
R'-Phl-CH2CH2-Th3-Np4-F, R'-Phl-Th3-CH2CH2-Phl-F, R'-Phl-Th3-CH2CH2-Ph2-F,
R'-Phl-Th3-CH2CH2-Ph3-F, R'-Phl-Th3-CH2CH2-Npl-F, R'-Phl-Th3-CHZCHZ-Np2-F,
R' -Ph 1-Th3 -CH2CH2-Np3 -F, R' -Ph 1-Th3 -CH2CH2-Np4-F,
R' -Ph 1-C=C-Th3 -Ph 1-F, R' -Ph 1-C=C-Th3 -Ph2-F, R' -Ph 1-C-C-Th3 -Ph3 -F,
R'-Phl-Th3-C=C-Phl-F, R'-Phl-Th3-C=C-Ph2-F, R'-Phl-Th3-C=C-Ph3-F,
R'-Ph2-Th3-Phl-F, R'-Ph2-Th3-Ph2-F, R'-Ph2-Th3-Ph3-F, R'-Ph2-Th3-Npl-F,
R'-Ph2-Th3-Np2-F, R'-Ph2-Th3-Np3-F, R'-Ph2-Th3-Np4-F,
CA 02377217 2001-12-28
R'-Ph2-CH2CH2-Th3-Phl-F, R'-Ph2-CH2CH2-Th3-Ph2-F, R'-Ph2-CH2CH2-Th3-Ph3-F,
R'-Ph2-CH2CH2-Th3-Npl-F, R'-Ph2-CH2CH2-Th3-Np2-F, R'-Ph2-CH2CH2-Th3-Np3-F,
R'-Ph2-CHZCH2-Th3-Np4-F, R'-Ph2-Th3-CH2CH2-Phl -F, R'-Ph2-Th3-CH2CH2-Ph2-F,
R'-Ph2-Th3-CH2CH2-Ph3-F, R'-Ph2-Th3-CH2CH2-Npl-F, R'-Ph2-Th3-CH2CH2-Np2-F,
5 R'-Ph2-Th3-CH2CH2-Np3-F, R'-Ph2-Th3-CH2CH2-Np4-F,
R'-Ph2-C=C-Th3-Phl-F, R'-Ph2-C=C-Th3-Ph2-F, R'-Ph2-C=C-Th3-Ph3-F,
R'-Ph2-Th3-CGC-PhI-F, R'-Ph2-Th3-C=C-Ph2-F, R'-Ph2-Th3-C=C-Ph3-F,
R'-Ph3-Th3-Phl-F, R'-Ph3-Th3-Ph2-F, R'-Ph3-Th3-Ph3-F, R'-Ph3-Th3-Npl-F,
R' -Ph3-Th3-Np2-F, R'-Ph3-Th3-Np3-F, R'-Ph3-Th3-Np4-F,
10 R'-Ph3-CH2CH2-Th3-Phl-F, R'-Ph3-CH2CH2-Th3-Ph2-F, R'-Ph3-CH2CH2-Th3-Ph3-F,
R'-Ph3-CH2CH2-Th3-Npl-F, R'-Ph3-CH2CH2-Th3-Np2-F, R'-Ph3-CH2CH2-Th3-Np3-F,
R'-Ph3-CH2CH2-Th3-Np4-F, R'-Ph3-Th3-CH2CH2-Phl -F, R'-Ph3-Th3-CH2CH2-Ph2-F,
R'-Ph3-Th3-CH2CH2-Ph3-F, R'-Ph3-Th3-CH2CH2-Np 1-F, R'-Ph3-Th3-CH2CH2-Np2-F,
R'-Ph3-Th3-CH2CH2-Np3-F, R'-Ph3-Th3-CH2CH2-Np4-F,
15 R'-Ph3-C=C-Th3-Phl-F, R'-Ph3-C-C-Th3-Ph2-F, R'-Ph3-C-C-Th3-Ph3-F,
R'-Ph3-Th3-C=C-PhI-F, R'-Ph3-Th3-C=C-Ph2-F, R'-Ph3-Th3-C=C-Ph3-F,
R'-Np1-Th3-Ph1-F, R'-Np1-Th3-Ph2-F, R'-Np1-Th3-Ph3-F,
R'-Npl-CH2CH2-Th3-Phl-F, R'-Npl-CH2CH2-Th3-Ph2-F, R'-Npl-CH2CH2-Th3-Ph3-F,
R'-Npl-Th3-CH2CH2-PhI-F, R'-Np1-Th3-CHZCH2-Ph2-F, R'-Npl-Th3-CH2CH2-Ph3-F,
20 R'-Np2-Th3-Phl-F, R'-Np2-Th3-Ph2-F, R'-Np2-Th3-Ph3-F,
R'-Np2-CH2CH2-Th3-Phl-F, R'-Np2-CH2CH2-Th3-Ph2-F, R'-Np2-CH2CH2-Th3-Ph3-F,
R'-Np2-Th3-CH2CH2-Phl-F, R'-Np2-Th3-CH2CH2-Ph2-F, R'-Np2-Th3-CH2CH2-Ph3-F,
R'-Np3-Th3-Phl-F, R'-Np3-Th3-Ph2-F, R'-Np3-Th3-Ph3-F,
R'-Np3-CH2CH2-Th3-Phl-F, R'-Np3-CH2CH2-Th3-Ph2-F, R'-Np3-CH2CH2-Th3-Ph3-F,
CA 02377217 2001-12-28
56
R'-Np3-Th3-CH2CH2-Phl-F, R'-Np3-Th3-CH2CH2-Ph2-F, R'-Np3-Th3-CH2CHZ-Ph3-F,
R'-Np1-Th3-Phl-F, R'-Np1-Th3-Ph2-F, R'-Np1-Th3-Ph3-F,
R'-Np4-CH2CH2-Th3-Phl-F, R' -Np4-CH2CH2-Th3-Ph2-F, R'-Np4-CH2CH2-Th3-Ph3-F,
R'-Np4-Th3-CH2CH2-Phl-F, R'-Np4-Th3-CH2CH2-Ph2-F, R'-Np4-Th3-CH2CH2-Ph3-F,
inthecaseinwhichna=1,nb=0orne=0,nb=1,andn =1,nd=0orn 0,nd=1,andZ
is a cyano group,
R'-Cy-Th1-Ph1-CN, R'-Cy-Th1-Ph2-CN, R'-Cy-Th1-Ph3-CN, R'-Cy-Th1-Np1-CN,
R' -Cy-Th 1-Np2-CN, R'-Cy-Th 1-Np3-CN, R'-Cy-Th 1-Np4-CN,
R' -Cy-CH2CH2-Th 1-Ph 1-CN, R' -Cy-CH2CH2-Th 1-Ph2-CN, R' -Cy-CH2CH2-Th 1-Ph3 -
CN,
R'-Cy-CH2CH2-Thl-Npl-CN, R' -Cy-CH2CH2-Th 1 -Np2-CN, R'-Cy-CH2CH2-Thl-Np3-CN,
R'-Cy-CH2CH2-Thl-Np4-CN, R'-Cy-Thl-CH2CH2-Phl -CN, R'-Cy-Thl-CH2CH2-Ph2-CN,
R'-Cy-Thl-CH2CH2-Ph3-CN, R'-Cy-Thl-CH2CH2-Npl-CN, R'-Cy-Thl-CHZCHZ-Np2-CN,
R'-Cy-Thl-CH2CH2-Np3-CN, R'-Cy-Thl-CH2CH2-Np4-CN,
R'-Cy-Th1-C-C-Ph1-CN, R'-Cy-Th1-C=C-Ph2-CN, R'-Cy-Th1-C=C-Ph3-CN,
R'-Phl-Thl-Phl-CN, R'-Phl-Thl-Ph2-CN, R'-Phl-Thl-Ph3-CN,
R'-Phl-Thl-Npl-CN, R'-Phl-Thl-Np2-CN, R'-Phl-Thl-Np3-CN,
R'-Ph l -Th 1-Np4-CN,
R' -Ph 1-CHZCH2-Th 1-Ph 1-CN, R' -Ph 1-CHZCH2-Th 1-Ph2-CN,
R' -Ph l-CH2CH2-Th l-Ph3-CN, R' -Ph l-CHZCH2-Th l-Np l-CN,
R' -Ph 1-CH2CH2-Th 1-Np2-CN, R' -Ph 1-CH2CH2-Th 1-Np3 -CN,
R'-Phl-CH2CH2-Thl-Np4-CN, R'-Phl-Thl-CH2CH2-Phl-CN,
R' -Ph 1-Th 1-CH2CH2-Ph2-CN, R' -Ph i-Th 1-CH2CH2-Ph3 -CN,
R'-Ph1-Th1-CH2CH2-Np1-CN, R'-Ph1-Th1-CH2CH2-Np2-CN,
R'-Phl-Thl-CH2CH2-Np3-CN, R'-Phl-Thl-CH2CH2-Np4-CN,
CA 02377217 2001-12-28
57
R' -Phl -C=C-Thl -Ph 1-CN, R'-Ph l-C=C-Th 1-Ph2-CN, R'-Phl -C=C-Th l-Ph3-CN,
R'-Ph1-Th1-C-C-PhI-CN, R'-Ph1-Thl-C=C-Ph2-CN, R'-Phl-Th1-C=C-Ph3-CN,
R'-Ph2-Th 1-Ph 1-CN, R' -Ph2-Th 1-Ph2-CN, R' -Ph2-Th l-Ph3 -CN,
R' -Ph2-Th 1-Np 1-CN, R' -Ph2-Th 1-Np2-CN, R' -Ph2-Th 1-Np3 -CN,
R' -Ph2-Th 1-Np4-CN,
R'-Ph2-CHZCH2-Thl-Phl-CN, R'-Ph2-CH2CH2-Thl-Ph2-CN,
R'-Ph2-CHZCH2-Thl-Ph3-CN, R'-Ph2-CH2CH2-Thl-Npl-CN,
R'-Ph2-CH2CH2-Thl-Np2-CN, R'-Ph2-CH2CH2-Thl-Np3-CN,
R' -Ph2-CH2CH2-Th 1-Np4-CN, R'-Ph2-Th 1-CH2CH2-Ph1-CN,
R' -Ph2-Th 1-CH2CH2-Ph2-CN, R' -Ph2-Th l-CH2CH2-Ph3 -CN,
R' -Ph2-Th l-CH2CH2-Np l-CN, R' -Ph2-Th l-CH2CH2-Np2-CN,
R'-Ph2-Thl-CH2CH2-Np3-CN, R'-Ph2-Thl-CH2CH2-Np4-CN,
R' -Ph2-C-C-Th 1-Ph 1-CN, R' -Ph2-C=C-Th 1-Ph2-CN, R' -Ph2-C-C-Th 1-Ph3 -CN,
R' -Ph2-Th l-C=C-Ph 1-CN, R' -Ph2-Th l-C=C-Ph2-CN, R' -Ph2-Th 1-C=C-Ph3 -CN,
R'-Ph3-Thl-Phl-CN, R'-Ph3-Thl-Ph2-CN, R'-Ph3-Thl-Ph3-CN,
R'-Ph3-Thl-Npl-CN, Ri-Ph3-Thl-Np2-CN, R'-Ph3-Thl-Np3-CN,
R' -Ph3-Th 1-Np4-CN,
R'-Ph3-CH2CH2-Thl-Phl-CN, R'-Ph3-CH2CH2-Thl-Ph2-CN,
R'-Ph3-CH2CH2-Thl-Ph3-CN, R'-Ph3-CH2CH2-Thl-Npl-CN,
R'-Ph3-CH2CH2-Thl-Np2-CN, R'-Ph3-CH2CH2-Thl-Np3-CN,
R'-Ph3-CH2CH2-Thl-Np4-CN, R'-Ph3-Thl-CH2CH2-Phl-CN,
R'-Ph3-Thl-CH2CH2-Ph2-CN, R'-Ph3-Thl-CH2CH2-Ph3-CN,
R'-Ph3-Thl-CH2CH2-Np 1 -CN, R'-Ph3-Thl-CH2CH2-Np2-CN,
R' -Ph3-Th 1-CH2CH2-Np3-CN, R' -Ph3-Th 1-CH2CH2-Np4-CN,
CA 02377217 2001-12-28
58
R' -Ph3-C=C-Th 1-Ph 1-CN, R'-Ph3-C=C-Th 1-Ph2-CN, R' -Ph3-C=C-Th 1-Ph3-CN,
R'-Ph3-Thl-C=C-Ph1-CN, R'-Ph3-Th1-C=C-Ph2-CN, R'-Ph3-Thl-C=C-Ph3-CN,
R' -Np 1-Th l-Ph 1-CN, R' -Np 1-Th 1-Ph2-CN, R' -Np 1-Th l-Ph3 -CN,
R' -Np 1-CH2CH2-Th l-Ph l-CN, R' -Np 1-CH2CH2-Th 1-Ph2-CN,
R'-Np1-CH2CH2-Thl-Ph3-CN, R'-Np1-Th1-CH2CH2-Ph1-CN,
R' -Np 1-Th 1-CHZCH2-Ph2-CN, R' -Np 1-Th 1-CH2CH2-Ph3-CN,
R' -Np2-Th 1-Ph 1-CN, R' -Np2-Th 1-Ph2-CN, R' -Np2-Th 1-Ph3 -CN,
R' -Np2-CH2CH2-Th 1-Ph l-CN, R' -Np2-CH2CH2-Th 1-Ph2-CN,
R'-Np2-CH2CH2-Thl-Ph3-CN, R'-Np2-Thl-CHZCH2-Phl-CN,
R'-Np2-Thl-CH2CH2-Ph2-CN, Rl-Np2-Thl-CHZCH2-Ph3-CN,
R'-Np3-Th1-Ph1-CN, R'-Np3-Thl-Ph2-CN, R'-Np3-Th1-Ph3-CN,
R'-Np3-CHZCH2-Thl-Phl-CN, R'-Np3-CH2CHZ-Thl-Ph2-CN,
R'-Np3-CHZCH2-Thl-Ph3-CN, R'-Np3-Thl-CH2CH2-Phl-CN,
R'-Np3-Thl-CH2CH2-Ph2-CN, R'-Np3-Thl-CH2CH2-Ph3-CN,
R'-Npl-Thl-Phl-CN, R'-Npl-Thl-Ph2-CN, R'-Npl-Thl-Ph3-CN,
R' -Np4-CH2CH2-Th l-Ph l-CN, R' -Np4-CH2CH2-Th 1-Ph2-CN,
R'-Np4-CH2CH2-Thl-Ph3-CN, R'-Np4-Thl-CH2CH2-Phl-CN,
R'-Np4-Thl-CH2CH2-Ph2-CN, R'-Np4-Thl-CH2CH2-Ph3-CN,
R'-Cy-Th2-Phl-CN, R'-Cy-Th2-Ph2-CN, R'-Cy-Th2-Ph3-CN, R'-Cy-Th2-Npl-CN,
R'-Cy-Th2-Np2-CN, R'-Cy-Th2-Np3-CN, R'-Cy-Th2-Np4-CN,
R'-Cy-CH2CH2-Th2-Phl-CN, R'-Cy-CHZCH2-Th2-Ph2-CN, R'-Cy-CH2CH2-Th2-Ph3-CN,
R'-Cy-CH2CH2-Th2-Npl-CN, R'-Cy-CH2CH2-Th2-Np2-CN, R'-Cy-CH2CH2-Th2-Np3-CN,
R'-Cy-CH2CH2-Th2-Np4-CN, R'-Cy-Th2-CH2CH2-Phl-CN, R'-Cy-Th2-CH2CHZ-Ph2-CN,
R' -Cy-Th2-CH2CH2-Ph3-CN, R' -Cy-Th2-CH2CH2-Np 1-CN, R' -Cy-Th2-CH2CH2-Np2-CN,
CA 02377217 2001-12-28
59
R' -Cy-Th2-CH2CH2-Np3-CN, R' -Cy-Th2-CH2CH2-Np4-CN,
R'-Cy-Th2-C=C-Phl-CN, R'-Cy-Th2-C=C-Ph2-CN, R'-Cy-Th2-C=C-Ph3-CN,
R' -Ph l-Th2-Ph l-CN, R' -Ph l-Th2-Ph2-CN, R' -Ph l-Th2-Ph3-CN,
R'-Phl-Th2-Npl-CN, R'-Phl-Th2-Np2-CN, R'-Phl-Th2-Np3-CN,
R' -Ph 1-Th2-Np4-CN,
R' -Ph l-CH2CH2-Th2-Ph l-CN, R' -Ph l-CH2CH2-Th2-Ph2-CN,
R'-Phl-CH2CH2-Th2-Ph3-CN, R'-Phl-CH2CH2-Th2-Npl-CN,
R'-Phl-CH2CH2-Th2-Np2-CN, R'-Phl-CH2CH2-Th2-Np3-CN,
R' -Ph l-CH2CH2-Th2-Np4-CN, R' -Ph l-Th2-CHZCH2-Ph 1-CN,
R' -Ph 1-Th2-CH2CH2-Ph2-CN, R' -Ph 1-Th2-CH2CH2-Ph3 -Th2-CH2CH2-Ph3-CN,
R' -Ph 1-Th2-CH2CH2-Np l-CN, R' -Ph 1-Th2-CH2CHZ-Np2-CN,
R' -Ph 1-Th2-CH2CH2-Np3-CN, R' -Ph 1-Th2-CH2CH2-Np4-CN,
R' -Ph 1-C_C-Th2-Ph 1-CN, R' -Ph l-C-C-Th2-Ph2-CN, R' -Ph l-C=C-Th2-Ph3 -CN,
R' -Ph 1-Th2-C=C-Ph 1-CN, R' -Ph 1-Th2-C=C-Ph2-CN, R' -Ph 1-Th2-C=C-Ph3-CN,
R'-Ph2-Th2-Phl-CN, R'-Ph2-Th2-Ph2-CN, R'-Ph2-Th2-Ph3-CN,
R'-Ph2-Th2-Npl-CN, R'-Ph2-Th2-Np2-CN, R'-Ph2-Th2-Np3-CN,
R'-Ph2-Th2-Np4-CN,
R'-Ph2-CH2CH2-Th2-Phl-CN, R'-Ph2-CH2CH2-Th2-Ph2-CN,
R'-Ph2-CH2CH2-Th2-Ph3-CN, R'-Ph2-CHzCH2-Th2-Npl-CN,
R1-Ph2-CH2CH2-Th2-Np2-CN, R'-Ph2-CH2CH2-Th2-Np3-CN,
R'-Ph2-CH2CH2-Th2-Np4-CN, R'-Ph2-Th2-CHZCH2-Phl-CN,
R'-Ph2-Th2-CH2CH2-Ph2-CN, R'-Ph2-Th2-CH2CH2-Ph3-CN,
R'-Ph2-Th2-CH2CH2-Npl-CN, R'-Ph2-Th2-CH2CH2-Np2-CN,
R' -Ph2-Th2-CH2CH2-Np3-CN, R' -Ph2-Th2-CHZCH2-Np4-CN,
CA 02377217 2001-12-28
R'-Ph2-C=C-Th2-Phl-CN, R'-Ph2-C=C-Th2-Ph2-CN, R'-Ph2-C-C-Th2-Ph3-CN,
R'-Ph2-Th2-C=C-PhI-CN, R'-Ph2-Th2-C=C-Ph2-CN, R'-Ph2-Th2-C-C-Ph3-CN,
R' -Ph3 -Th2-Ph 1-CN, R' -Ph3 -Th2-Ph2-CN, R' -Ph3 -Th2-Ph3 -CN,
R'-Ph3-Th2-Npl-CN, Rl-Ph3-Th2-Np2-CN, R'-Ph3-Th2-Np3-CN,
5 R'-Ph3-Th2-Np4-CN,
R'-Ph3-CH2CH2-Th2-Phl-CN, R'-Ph3-CHzCH2-Th2-Ph2-CN,
R'-Ph3-CHZCH2-Th2-Ph3-CN, R'-Ph3-CH2CH2-Th2-Npl-CN,
R'-Ph3-CH2CH2-Th2-Np2-CN, R'-Ph3-CH2CH2-Th2-Np3-CN,
R'-Ph3-CHZCH2-Th2-Np4-CN, R'-Ph3-Th2-CH2CH2-Phl-CN,
10 R'-Ph3-Th2-CH2CH2-Ph2-CN, R'-Ph3-Th2-CH2CH2-Ph3-CN,
R'-Ph3-Th2-CH2CH2-Npl-CN, R'-Ph3-Th2-CH2CH2-Np2-CN,
R'-Ph3-Th2-CH2CH2-Np3-CN, R'-Ph3-Th2-CH2CH2-Np4-CN,
R'-Ph3-C=C-Th2-Phl-CN, R'-Ph3-C=C-Th2-Ph2-CN, R'-Ph3-C=C-Th2-Ph3-CN,
R'-Ph3-Th2-C=C-Phl-CN, R'-Ph3-Th2-C=C-Ph2-CN, R'-Ph3-Th2-C=C-Ph3-CN,
15 R' -Np 1-Th2-Ph1-CN, R'-Np 1-Th2-Ph2-CN, R'-Np 1-Th2-Ph3-CN,
R'-Npl-CH2CH2-Th2-Phl-CN, R'-Npl-CH2CH2-Th2-Ph2-CN,
R'-Npl-CH2CH2-Th2-Ph3-CN, R'-Npl-Th2-CH2CH2-Phl-CN,
R'-Npl-Th2-CH2CH2-Ph2-CN, R'-Npl-Th2-CHZCH2-Ph3-CN,
R'-Np2-Th2-Ph1-CN, R'-Np2-Th2-Ph2-CN, R'-Np2-Th2-Ph3-CN,
20 R' -Np2-CH2CH2-Th2-Ph l -CN, R' -Np2-CH2CH2-Th2-Ph2-CN,
R'-Np2-CH2CH2-Th2-Ph3-CN, R'-Np2-Th2-CHZCH2-Phl-CN,
R'-Np2-Th2-CH2CH2-Ph2-CN, R'-Np2-Th2-CH2CH2-Ph3-CN,
R'-Np3-Th2-Phl -CN, R'-Np3-Th2-Ph2-CN, R'-Np3-Th2-Ph3-CN,
R'-Np3-CH2CHz-Th2-Phl-CN, R'-Np3-CH2CH2-Th2-Ph2-CN,
CA 02377217 2001-12-28
61
R'-Np3-CH2CH2-Th2-Ph3-CN, R'-Np3-Th2-CHZCH2-Phl-CN,
R'-Np3-Th2-CH2CH2-Ph2-CN, R'-Np3-Th2-CH2CH2-Ph3-CN,
R' -Np l-Th2-Ph 1-CN, Rt -Np l-Th2-Ph2-CN, R' -Np 1-Th2-Ph3-CN,
R' -Np4-CH2CH2-Th2-Ph 1-CN, R'-Np4-CH2CH2-Th2-Ph2-CN,
R'-Np4-CH2CH2-Th2-Ph3-CN, R'-Np4-Th2-CH2CH2-Phl-CN,
R'-Np4-Th2-CH2CHZ-Ph2-CN, R'-Np4-Th2-CH2CH2-Ph3-CN,
R'-Cy-Th3-Phl-CN, R'-Cy-Th3-Ph2-CN, R'-Cy-Th3-Ph3-CN, R'-Cy-Th3-Npl-CN,
R' -Cy-Th3-Np2-CN, R'-Cy-Th3-Np3-CN, R'-Cy-Th3-Np4-CN,
R'-Cy-CH2CH2-Th3-Phl-CN, R'-Cy-CH2CH2-Th3-Ph2-CN, R'-Cy-CH2CH2-Th3-Ph3-CN,
R'-Cy-CH2CH2-Th3-Npl-CN, R'-Cy-CHZCH2-Th3-Np2-CN, R'-Cy-CH2CH2-Th3-Np3-CN,
R'-Cy-CH2CH2-Th3-Np4-CN, R'-Cy-Th3-CH2CH2-Phl-CN, R'-Cy-Th3-CH2CH2-Ph2-CN,
R'-Cy-Th3-CHZCH2-Ph3-CN, R'-Cy-Th3-CHZCH2-Npl-CN, R'-Cy-Th3-CHZCH2-Np2-CN,
R' -Cy-Th3-CH2CH2-Np3-CN, R' -Cy-Th3-CH2CH2-Np4-CN,
R'-Cy-Th3-C=C-PhI-CN, R'-Cy-Th3-C=C-Ph2-CN, R'-Cy-Th3-C=C-Ph3-CN,
R'-Ph1-Th3-Ph1-CN, R'-Phi-Th3-Ph2-CN, R'-Phl-Th3-Ph3-CN,
R'-Phl-Th3-Npl-CN, R'-Phl-Th3-Np2-CN, R'-Phl-Th3-Np3-CN,
R' -Ph 1-Th3-Np4-CN,
R'-Phl-CH2CH2-Th3-Phl-CN, R'-Phl-CH2CH2-Th3-Ph2-CN,
R'-PhI-CHzCH2-Th3-Ph3-CN, R'-Phl-CH2CH2-Th3-Npl-CN,
R'-Phl-CH2CH2-Th3-Np2-CN, R'-Phl-CH2CH2-Th3-Np3-CN,
R'-Phl -CH2CH2-Th3-Np4-CN, R'-Phl-Th3-CH2CH2-Phl-CN,
R'-Phl -Th3-CHZCH2-Ph2-CN, R'-Phl -Th3-CH2CH2-Ph3-CN,
R'-Phl-Th3-CH2CH2-Npl-CN, R'-Phl-Th3-CH2CH2-Np2-CN,
R'-Phl-Th3-CH2CH2-Np3-CN, R'-Phl-Th3-CH2CH2-Np4-CN,
CA 02377217 2001-12-28
62
R'-PhI-C=C-Th3-Phl-CN, R'-Phl-C=C-Th3-Ph2-CN, R'-Phi-C=C-Th3-Ph3-CN,
R' -Ph 1-Th3 -C=C-Ph 1-CN, R' -Ph l-Th3 -C=C-Ph2-CN, R' -Ph 1-Th3 -C=C-Ph3 -
CN,
R'-Ph2-Th3-Ph1-CN, R'-Ph2-Th3-Ph2-CN, R'-Ph2-Th3-Ph3-CN,
R'-Ph2-Th3-Npl-CN, Rl-Ph2-Th3-Np2-CN, R'-Ph2-Th3-Np3-CN,
R'-Ph2-Th3-Np4-CN,
R' -Ph2-CH2CH2-Th3-Phl -CN, R'-Ph2-CH2CH2-Th3-Ph2-CN,
R'-Ph2-CHZCH2-Th3-Ph3-CN, Rl-Ph2-CHZCH2-Th3-Npl-CN,
R'-Ph2-CH2CH2-Th3-Np2-CN, R'-Ph2-CH2CH2-Th3-Np3-CN,
R'-Ph2-CH2CH2-Th3-Np4-CN, R'-Ph2-Th3-CH2CH2-Phl-CN,
R'-Ph2-Th3-CH2CH2-Ph2-CN, R'-Ph2-Th3-CH2CH2-Ph3-CN,
R' -Ph2-Th3 -CH2CH2-Np 1-CN, R' -Ph2-Th3 -CH2CH2-Np2-CN,
R'-Ph2-Th3-CH2CH2-Np3-CN, R'-Ph2-Th3-CH2CH2-Np4-CN,
R'-Ph2-C=C-Th3-Phl-CN, R'-Ph2-C=C-Th3-Ph2-CN, R'-Ph2-C=C-Th3-Ph3-CN,
R'-Ph2-Th3-C=C-Phl-CN, R'-Ph2-Th3-C=C-Ph2-CN, R'-Ph2-Th3-C=C-Ph3-CN,
R'-Ph3-Th3-Phl-CN, R'-Ph3-Th3-Ph2-CN, R'-Ph3-Th3-Ph3-CN,
R'-Ph3-Th3-Npl-CN, R'-Ph3-Th3-Np2-CN, R'-Ph3-Th3-Np3-CN,
R'-Ph3-Th3-Np4-CN,
R'-Ph3-CH2CH2-Th3-Phl-CN, R-Ph3-CH2CH2-Th3-Ph2-CN,
R'-Ph3-CH2CH2-Th3-Ph3=CN, R'-Ph3-CH2CH2-Th3-Npl-CN,
R'-Ph3-CH2CH2-Th3-Np2-CN, R'-Ph3-CH2CH2-Th3-Np3-CN,
R'-Ph3-CH2CH2-Th3-Np4-CN, R'-Ph3-Th3-CH2CH2-Phl -CN,
R'-Ph3-Th3-CH2CH2-Ph2-CN, R'-Ph3-Th3-CH2CH2-Ph3-CN,
R'-Ph3-Th3-CH2CH2-Npl-CN, R'-Ph3-Th3-CH2CH2-Np2-CN,
R'-Ph3-Th3-CH2CH2-Np3-CN, R'-Ph3-Th3-CH2CH2-Np4-CN,
CA 02377217 2001-12-28
63
R'-Ph3-C=C-Th3-Phl-CN, R'-Ph3-C=C-Th3-Ph2-CN, R'-Ph3-C=C-Th3-Ph3-CN,
R'-Ph3-Th3-C=C-PhI-CN, R'-Ph3-Th3-C=C-Ph2-CN, R'-Ph3-Th3-C=C-Ph3-CN,
R' -Np 1-Th3 -Ph 1-CN, R' -Np 1-Th3 -Ph2-CN, R' -Np 1-Th3 -Ph3 -CN,
R'-Npl-CH2CH2-Th3-Phl-CN, R'-Npl-CH2CH2-Th3-Ph2-CN,
R' -Np 1-CH2CH2-Th3-Ph3 -CN, R' -Np 1-Th3-CH2CH2-Ph 1-CN,
R'-Npl-Th3-CH2CH2-Ph2-CN, R'-Npl-Th3-CH2CH2-Ph3-CN,
R'-Np2-Th3-Phl-CN, R'-Np2-Th3-Ph2-CN, R'-Np2-Th3-Ph3-CN,
R'-Np2-CH2CH2-Th3-Phl-CN, R'-Np2-CH2CH2-Th3-Ph2-CN,
R'-Np2-CH2CH2-Th3-Ph3-CN, R'-Np2-Th3-CH2CH2-Phl-CN,
R'-Np2-Th3-CH2CH2-Ph2-CN, R'-Np2-Th3-CH2CH2-Ph3-CN,
R' -Np3 -Th3 -Ph 1-CN, R' -Np3 -Th3 -Ph2-CN, R' -Np3 -Th3 -Ph3 -CN,
R'-Np3-CH2CH2-Th3-Phl-CN, R'-Np3-CH2CH2-Th3-Ph2-CN,
R'-Np3-CH2CH2-Th3-Ph3-CN, R'-Np3-Th3-CHZCH2-Phl-CN,
R'-Np3-Th3-CH2CHZ-Ph2-CN, R'-Np3-Th3-CH2CH2-Ph3-CN,
R' -Np 1-Th3-Phl -CN, R'-Np 1-Th3-Ph2-CN, R'-Np 1-Th3-Ph3-CN,
R'-Np4-CH2CH2-Th3-Phl-CN, R'-Np4-CH2CH2-Th3-Ph2-CN,
R' -Np4-CH2CH2-Th3-Ph3-CN, R'-Np4-Th3-CH2CH2-Phl -CN,
R'-Np4-Th3-CH2CH2-Ph2-CN, R'-Np4-Th3-CH2CH2=Ph3-CN,
R'-Cy-Th3-Phl-CN, R'-Cy-Th3-Ph2-CN, R'-Cy-Th3-Ph3-CN, R'-Cy-Th3-Npl-CN,
R'-Cy-Th3-Np2-CN, R'-Cy-Th3-Np3-CN, R'-Cy-Th3-Np4-CN,
R'-Cy-CHZCH2-Th3-Phl-CN, R'-Cy-CH2CH2-Th3-Ph2-CN, R'-Cy-CH2CH2-Th3-Ph3-CN,
R'-Cy-CH2CH2-Th3-Npl-CN, R'-Cy-CH2CH2-Th3-Np2-CN, R'-Cy-CH2CH2-Th3-Np3-CN,
R'-Cy-CH2CH2-Th3-Np4-CN, R'-Cy-Th3-CH2CH2-Ph1-CN, R'-Cy-Th3-CH2CH2-Ph2-CN,
RI-Cy-Th3-CH2CH2-Ph3-CN, R'-Cy-Th3-CH2CH2-Npl-CN, R'-Cy-Th3-CHZCHZ-Np2-CN,
CA 02377217 2001-12-28
64
R'-Cy-Th3-CH2CH2-Np3-CN, R'-Cy-Th3-CH2CH2-Np4-CN,
R'-Cy-Th3-C=C-Phl-CN, R'-Cy-Th3-C=C-Ph2-CN, R'-Cy-Th3-C=C-Ph3-CN,
R' -Ph 1-Th3 -Ph 1-CN, R' -Ph 1-Th3 -Ph2-CN, R' -Ph 1-Th3 -Ph3 -CN,
R'-Phl-Th3-Npl-CN, R'-Phl-Th3-Np2-CN, R'-Phl-Th3-Np3-CN,
R'-Phl-Th3-Np4-CN,
R'-Phl-CH2CH2-Th3-Phl-CN, R'-PhI-CH2CH2-Th3-Ph2-CN,
R'-Phl-CH2CH2-Th3-Ph3-CN, R'-Phl-CH2CH2-Th3-Npl-CN,
R' -Phl -CH2CH2-Th3-Np2-CN, R'-Phl -CHZCH2-Th3-Np3-CN,
R'-Phl-CH2CH2-Th3-Np4-CN, R'-Phl-Th3-CH2CH2-Phl-CN,
R'-Phl-Th3-CH2CH2-Ph2-CN, R'-Phl-Th3-CH2CH2-Ph3-CN,
R'-Phl-Th3-CH2CH2-Npl-CN, R'-Phl-Th3-CH2CH2-Np2-CN,
R'-Phl-Th3-CH2CH2-Np3-CN, R'-Phl-Th3-CH2CH2-Np4-CN,
R'-Phl-C=C-Th3-Phl-CN, R'-Phl-C=C-Th3-Ph2-CN, R'-Phl-C=C-Th3-Ph3-CN,
R'-Ph1-Th3-C=C-Ph1-CN, R'-Ph1-Th3-C=C-Ph2-CN, R'-Ph1-Th3-C=C-Ph3-CN,
R'-Ph2-Th3-Phl-CN, Rl-Ph2-Th3-Ph2-CN, R'-Ph2-Th3-Ph3-CN,
R'-Ph2-Th3-Npl-CN, R'-Ph2-Th3-Np2-CN, R'-Ph2-Th3-Np3-CN,
R' -Ph2-Th3-Np4-CN,
R'-Ph2-CH2CH2-Th3-Phl-CN, R'-Ph2-CHZCH2-Th3-Ph2-CN,
R'-Ph2-CH2CH2-Th3-Ph3-CN, R'-Ph2-CH2CH2-Th3-Npl-CN,
R'-Ph2-CHZCH2-Th3-Np2-CN, R'-Ph2-CH2CH2-Th3-Np3-CN,
R'-Ph2-CH2CH2-Th3-Np4-CN, R'-Ph2-Th3-CH2CH2-Phl-CN,
R'-Ph2-Th3-CH2CH2-Ph2-CN, R'-Ph2-Th3-CH2CH2-Ph3-CN,
R'-Ph2-Th3-CH2CH2-NpI-CN, R'-Ph2-Th3-CH2CH2-Np2-CN,
R'-Ph2-Th3-CH2CH2-Np3-CN, R'-Ph2-Th3-CH2CH2-Np4-CN,
CA 02377217 2001-12-28
R'-Ph2-C-C-Th3-Phl-CN, R'-Ph2-C=C-Th3-Ph2-CN, R'-Ph2-C=C-Th3-Ph3-CN,
R'-Ph2-Th3-C=C-PhI-CN, R'-Ph2-Th3-C=C-Ph2-CN, R'-Ph2-Th3-C-C-Ph3-CN,
R' -Ph3-Th3-Phl -CN, R'-Ph3-Th3-Ph2-CN, R'-Ph3-Th3-Ph3-CN,
R' -Ph3 -Th3 -Np l -CN, R' -Ph3 -Th3 -Np2-CN, R' -Ph3 -Th3 -Np3 -CN,
5 R'-Ph3-Th3-Np4-CN,
R'-Ph3-CH2CH2-Th3-Phl-CN, R'-Ph3-CH2CH2-Th3-Ph2-CN,
R'-Ph3-CH2CH2-Th3-Ph3-CN, R'-Ph3-CHZCH2-Th3-Npl-CN,
R'-Ph3-CH2CH2-Th3-Np2-CN, R'-Ph3-CH2CH2-Th3-Np3-CN,
R'-Ph3-CH2CH2-Th3-Np4-CN, R'-Ph3-Th3-CHZCH2-Phl-CN,
10 R'-Ph3-Th3-CH2CH2-Ph2-CN, R'-Ph3-Th3-CH2CH2-Ph3-CN,
R'-Ph3-Th3-CH2CH2-Npl-CN, R'-Ph3-Th3-CH2CH2-Np2-CN,
R'-Ph3-Th3-CH2CH2-Np3-CN, R'-Ph3-Th3-CHZCH2-Np4-CN,
R'-Ph3-C=C-Th3-Phl-CN, R'-Ph3-C=C-Th3-Ph2-CN, R'-Ph3-C=C-Th3-Ph3-CN,
R'-Ph3-Th3-C=C-PhI-CN, R'-Ph3-Th3-C=C-Ph2-CN, R'-Ph3-Th3-C=C-Ph3-CN,
15 R'-Np1-Th3-Ph1-CN, R'-Np1-Th3-Ph2-CN, R'-Np1-Th3-Ph3-CN,
R'-Np1-CH2CH2-Th3-Ph1-CN, R'-Np1-CH2CH2-Th3-Ph2-CN,
R'-NpI-CHZCH2-Th3-Ph3-CN, R'-Npl-Th3-CH2CH2-Phl-CN,
R' -Np 1-Th3 -CH2CH2-Ph2-CN, R' -Np 1-Th3 -CH2CH2-Ph3 -CN,
R'-Np2-Th3-Phl-CN, R'-Np2-Th3-Ph2-CN, R'-Np2-Th3-Ph3-CN,
20 R'-Np2-CH2CH2-Th3-Phl-CN, R'-Np2-CHZCH2-Th3-Ph2-CN,
R'-Np2-CH2CH2-Th3-Ph3-CN, R'-Np2-Th3-CH2CH2-Phl-CN,
R'-Np2-Th3-CH2CH2-Ph2-CN, R'-Np2-Th3-CH2CH2-Ph3-CN,
R'-Np3-Th3-Phl-CN, R'-Np3-Th3-Ph2-CN, R'-Np3-Th3-Ph3-CN,
R'-Np3-CHZCH2-Th3-Phl-CN, R'-Np3-CHZCH2-Th3-Ph2-CN,
CA 02377217 2001-12-28
66
R'-Np3-CHz2CHz2-Th3-Ph3-CN, R'-Np3-Th3-CHZCH2-Phl-CN,
R'-Np3-Th3-CHZCH2-Ph2-CN, R'-Np3-Th3-CH2CH2-Ph3-CN,
R'-Np1-Th3-Phl-CN, R'-Np1-Th3-Ph2-CN, R'-Np1-Th3-Ph3-CN,
R'-Np4-CH2CH2-Th3-Phl-CN, Rl-Np4-CH2CH2-Th3-Ph2-CN,
R1-Np4-CH2CH2-Th3-Ph3-CN, R'-Np4-Th3-CH2CH2-Phl-CN,
R'-Np4-Th3-CH2CH2-Ph2-CN, R'-Np4-Th3-CH2CH2-Ph3-CN,
inthecaseinwhichne= 1,nb=0orna=0,nb= 1,andn = 1,nd=0orn'=0,nd 1,andZ
is a trifluoromethyl group,
R'-Cy-Thl-Phl-CF3, R'-Cy-Thl-Ph2-CF3, R'-Cy-Thl-Ph3-CF3, RI-Cy-Thl-Npl-CF3,
R'-Cy-Thl-Np2-CF3, RI-Cy-Thl-Np3-CF3, RI-Cy-Thl-Np4-CF3,
R'-Cy-CH2CH2-Thl-Phl-CF3, R'-Cy-CHZCH2-Thl-Ph2-CF3, R'-Cy-CH2CH2-Thl-Ph3-CF3,
R'-Cy-CH2CH2-Thl-Npl-CF3, RI-Cy-CHZCH2-Thl-Np2-CF3,
R' -Cy-CH2CHZ-Th 1-Np3 -CF3,
R'-Cy-CHZCH2-Thl-Np4-CF3, R'-Cy-Th 1 -CH2CH2-Phl-CF3, RI-Cy-Thl-CH2CH2-Ph2-
CF3,
R'-Cy-Thl-CH2CH2-Ph3-CF3, R'-Cy-Thl-CH2CH2-Npl-CF3, R'-Cy-Thl-CH2CH2-Np2-CF3,
R'-Cy-Thl-CH2CH2-Np3-CF3, R'-Cy-Thl-CH2CH2-Np4-CF3,
R'-Cy-ThI-C=C-Phl-CF3, R'-Cy-Thl-C=C-Ph2-CF3, R'-Cy-ThI-C-C-Ph3-CF3,
R' -Ph 1-Th 1-Ph 1-CF3, R' -Ph 1-Th l-Ph2-CF3, R' -Ph 1-Th 1-Ph3 -CF3,
R'-Phl-Thi-Npl-CF3, R'-Phl-Thl-Np2-CF3, R'-Phl-Thl-Np3-CF3,
R'-Phl-Thl-Np4-CF3,
R'-PhI-CHZCH2-Thl-Phl-CF3, Rl-Phl-CH2CH2-Thl-Ph2-CF3,
R'-Ph1-CH2CH2-Th1-Ph3-CF3, R'-Ph1-CH2CH2-Th1-Np1-CF3,
R'-Phl-CH2CH2-Thl-Np2-CF3, R'-Phl-CH2CH2-Thl-Np3-CF3,
R'-Ph1-CH2CH2-Th1-Np4-CF3, R'-Ph1-Th1-CH2CH2-Ph1-CF3,
CA 02377217 2001-12-28
67
R' -Ph 1-Th 1-CH2CH2-Ph2-CF3, R' -Ph 1-Th 1-CHZCH2-Ph3-CF3,
R' -Ph 1-Th 1-CH2CH2-Np 1-CF3, R' -Ph 1-Th 1-CH2CH2-Np2-CF3,
R'-Ph l-Th l-CHZCH2-Np3-CF3, R' -Ph l-Th l-CH2CH2-Np4-CF3,
R' -Ph l-C=C-Th l-Ph l-CF3, R' -Ph l-C=C-Th 1-Ph2-CF3, R' -Ph l-C=C-Th l-Ph3-
CF3,
R' -Ph l-Th l-C=C-Ph l-CF3, R' -Ph l-Th 1-C=C-Ph2-CF3, R' -Ph l-Th l-C=-C-Ph3 -
CF3,
R'-Ph2-Thl-Phl-CF3, R'-Ph2-Thl-Ph2-CF3, R'-Ph2-Thl-Ph3-CF3,
R'-Ph2-Thl-Npl-CF3, Rl-Ph2-Thl-Np2-CF3, R'-Ph2-Thl-Np3-CF3,
R' -Ph2-Th 1-Np4-CF3,
R' -Ph2-CH2CH2-Th 1-Ph 1-CF3, R' -Ph2-CH2CH2-Th 1-Ph2-CF3,
R'-Ph2-CHZCH2-Th1-Ph3-CF3, R'-Ph2-CH2CH2-Thl-Np1-CF3,
R'-Ph2-CH2CH2-Thl-Np2-CF3, R'-Ph2-CH2CH2-Thl-Np3-CF3,
R' -Ph2-CH2CH2-Th 1-Np4-CF3, R' -Ph2-Th 1-CH2CH2-Ph 1-CF3,
R'-Ph2-Thl-CH2CH2-Ph2-CF3, R'-Ph2-Thl-CHZCHZ-Ph3-CF3,
R' -Ph2-Th 1-CH2CH2-Np 1-CF3, R' -Ph2-Th 1-CH2CH2-Np2-CF3,
R'-Ph2-Thl-CH2CH2-Np3-CF3, R'-Ph2-Thl-CH2CH2-Np4-CF3,
R'-Ph2-C=C-Thl-Phl-CF3, R'-Ph2-C=C-Thl-Ph2-CF3, R'-Ph2-C-C-Thl-Ph3-CF3,
R'-Ph2-Thl-C=C-Phl-CF3, R'-Ph2-Thl-C=C-Ph2-CF3, R'-Ph2-Thl-C=C-Ph3-CF3,
R'-Ph3-Thl-Phl-CF3, R'-Ph3-Thl-Ph2-CF3, R'-Ph3-Thl-Ph3-CF3,
R'-Ph3-Thl-Npl-CF3, R'-Ph3-Thl-Np2-CF3, R'-Ph3-Thl-Np3-CF3,
R'-Ph3-Thl-Np4-CF3,
R'-Ph3-CH2CH2-Thl-Phl-CF3, R'-Ph3-CHZCH2-Thl-Ph2-CF3,
R'-Ph3-CH2CH2-Thl-Ph3-CFa, R'-Ph3-CH2CH2-Thl-Npl-CF3,
R'-Ph3-CH2CH2-Thl-Np2-CF3, R'-Ph3-CH2CH2-Thl-Np3-CF3,
R'-Ph3-CH2CH2-Thl-Np4-CF3, R'-Ph3-Thl-CH2CH2-Phl-CF3,
CA 02377217 2001-12-28
68
R'-Ph3-Thl-CH2CH2-Ph2-CF3, R'-Ph3-Thl-CH2CH2-Ph3-CF3,
R'-Ph3-Thl-CH2CH2-Npl-CF3, R'-Ph3-Thl-CH2CH2-Np2-CF3,
R'-Ph3-Thl-CH2CH2-Np3-CF3, R'-Ph3-Thl-CH2CH2-Np4-CF3,
R'-Ph3-C=C-Thl-Phl-CF3, Rl-Ph3-C=C-Thl-Ph2-CF3, R'-Ph3-C=C-Thl-Ph3-CF3,
R'-Ph3-Th1-C=C-Ph1-CF3, R'-Ph3-Th1-C=C-Ph2-CF3, R'-Ph3-Th1-C=C-Ph3-CF3,
R' -Np l-Th l-Ph l-CF3, R'-Np l-Th l-Ph2-CF3, R' -Np l-Th l-Ph3 -CF3,
R' -Np l-CH2CHZ-Th l-Ph l-CF3, R'-Np l-CH2CH2-Th l-Ph2-CF3,
R'-Np1-CH2CH2-Th1-Ph3-CF3, Rl-Np1-Th1-CH2CH2-Ph1-CF3,
R' -Np 1-Th l-CH2CHZ-Ph2-CF3, R' -Np 1-Th l-CH2CH2-Ph3-CF3,
R'-Np2-Thl-Phl-CF3, R'-Np2-Thl-Ph2-CF3, R'-Np2-Thl-Ph3-CF3,
R' -Np2-CH2CHZ-Th 1-Ph 1-CF3, R' -Np2-CH2CH2-Th 1-Ph2-CF3,
R'-Np2-CHZCH2-Thl-Ph3-CF3, R'-Np2-Thl-CH2CH2-Phl-CF3,
R' -Np2-Th 1-CHZCH2-Ph2-CF3, R' -Np2-Th 1-CH2CH2-Ph3 -CF3,
R'-Np3-Thl-Phl-CF3, R'-Np3-Thl-Ph2-CF3, R'-Np3-Thl-Ph3-CF3,
R'-Np3-CH2CH2-Thl-Phi-CF3, R'-Np3-CH2CH2-Thl-Ph2-CF3,
R'-Np3-CHZCH2-Thl-Ph3-CF3, R'-Np3-Thl-CH2CH2-Phl-CF3,
R'-Np3-Thl-CH2CH2-Ph2-CF3, R'-Np3-Thl-CHZCH2-Ph3-CF3,
R'-Npl-Thl-Phl-CF3, R'-Npl-Thl-Ph2-CF3, R'-Npl-Thl-Ph3-CF3,
R'-Np4-CHZCH2-Thl-Phl-CF3, R'-Np4-CH2CH2-Thl-Ph2-CF3,
R'-Np4-CH2CH2-Thl-Ph3-CF3, R'-Np4-Thl-CHZCH2-Phl-CF3,
R' -Np4-Th 1-CH2CH2-Ph2-CF3, R' -Np4-Th 1-CH2CH2-Ph3 -CF3,
R'-Cy-Th2-Phl-CF3, R'-Cy-Th2-Ph2-CF3, R'-Cy-Th2-Ph3-CF3, R'-Cy-Th2-Npl-CF3,
R'-Cy-Th2-Np2-CF3, R'-Cy-Th2-Np3-CF3, R'-Cy-Th2-Np4-CF3,
R'-Cy-CH2CH2-Th2-Phl-CF3, R'-Cy-CH2CHZ-Th2-Ph2-CF3, R'-Cy-CH2CH2-Th2-Ph3-CF3,
CA 02377217 2001-12-28
69
R'-Cy-CH2CHZ-Th2-Npl-CF3, R'-Cy-CHZCH2-Th2-Np2-CF3,
R' -Cy-CH2CH2-Th2-Np3-CF3,
R'-Cy-CH2CH2-Th2-Np4-CF3, R'-Cy-Th2-CH2CH2-Phl-CF3, R'-Cy-Th2-CH2CH2-Ph2-CF3,
R'-Cy-Th2-CH2CH2-Ph3-CF3, R'-Cy-Th2-CH2CH2-Npl-CF3, R'-Cy-Th2-CH2CH2-Np2-CF3,
R'-Cy-Th2-CH2CH2-Np3-CF3, R'-Cy-Th2-CH2CH2-Np4-CF3,
R' -Cy-Th2-C=C-Ph 1-CF3, R' -Cy-Th2-C=C-Ph2-CF3, R' -Cy-Th2-C=C-Ph3 -CF3,
R'-Ph1-Th2-Ph1-CF3, R'-Ph1-Th2-Ph2-CF3, R'-Ph1-Th2-Ph3-CF3,
R'-Ph1-Th2-Np1-CF3, R'-Ph1-Th2-Np2-CF3, R'-Ph1-Th2-Np3-CF3,
R' -Ph 1-Th2-Np4-CF3,
RI-Phl-CH2CH2-Th2-Phl-CF3, R'-Phl-CH2CH2-Th2-Ph2-CF3,
R'-Phl-CH2CH2-Th2-Ph3-CF3, R'-Phl-CH2CH2-Th2-Npl-CF3,
R'-Phl-CH2CH2-Th2-Np2-CF3, R'-Phl-CH2CHz-Th2-Np3-CF3,
R' -Ph 1-CH2CHz-Th2-Np4-CF3, R' -Ph 1-Th2-CH2CH2-Ph 1-CF3,
R' -Ph 1-Th2-CH2CH2-Ph2-CF3, R' -Ph 1-Th2-CH2CH2-Ph3 -CF3,
R'-Phl-Th2-CH2CH2-Npl-CF3i R-Phl-Th2-CH2CH2-Np2-CF3,
R'-Phl-Th2-CH2CH2-Np3-CF3, R'-Phl-Th2-CHZCH2-Np4-CF3,
R'-PhI-C_C-Th2-Phl-CF3, R'-PhI-C=C-Th2-Ph2-CF3, R'-Phl-C=C-Th2-Ph3-CF3,
R'-Phl-Th2-C=C-Phl-CF3, R'-Phl-Th2-C=C-Ph2-CF3, R'-Phl-Th2-C=C-Ph3-CF3,
R'-Ph2-Th2-Phl-CF3, R'-Ph2-Th2-Ph2-CF3, R'-Ph2-Th2-Ph3-CF3,
R'-Ph2-Th2-Npl-CF3, R'-Ph2-Th2-Np2-CF3, R'-Ph2-Th2-Np3-CF3,
R' -Ph2-Th2-Np4-CF3,
R'-Ph2-CHZCH2-Th2-Phl-CF3, R'-Ph2-CH2CH2-Th2-Ph2-CF3,
R'-Ph2-CH2CH2-Th2-Ph3-CF3, R'-Ph2-CH2CH2-Th2-Np 1-CF3,
R'-Ph2-CH2CH2-Th2-Np2-CF3, R'-Ph2-CH2CH2-Th2-Np3-CF3,
CA 02377217 2001-12-28
R'-Ph2-CH2CH2-Th2-Np4-CF3, R'-Ph2-Th2-CH2CH2-Phl-CF3,
R'-Ph2-Th2-CHZCH2-Ph2-CF3, R'-Ph2-Th2-CH2CH2-Ph3-CF3,
R'-Ph2-Th2-CH2CH2-Npl-CF3, R'-Ph2-Th2-CH2CH2-Np2-CF3,
R'-Ph2-Th2-CH2CH2-Np3-CF3, R'-Ph2-Th2-CH2CH2-Np4-CF3,
5 R'-Ph2-C=C-Th2-Phl-CF3, R'-Ph2-C=C-Th2-Ph2-CF3, R'-Ph2-C=C-Th2-Ph3-CF3,
R'-Ph2-Th2-C-C-Phl-CF3, R'-Ph2-Th2-C=C-Ph2-CF3, R'-Ph2-Th2-C=C-Ph3-CF3,
R'-Ph3-Th2-Phl-CF3, R'-Ph3-Th2-Ph2-CF3, R'-Ph3-Th2-Ph3-CF3,
R'-Ph3-Th2-Np1-CF3, R'-Ph3-Th2-Np2-CF3, R1-Ph3-Th2-Np3-CF3,
R'-Ph3-Th2-Np4-CF3,
10 R'-Ph3-CH2CH2-Th2-Phl-CF3, R'-Ph3-CH2CH2-Th2-Ph2-CF3,
R'-Ph3-CH2CH2-Th2-Ph3-CF3, R'-Ph3-CH2CH2-Th2-Npl-CF3,
R'-Ph3-CH2CH2-Th2-Np2-CF3, R'-Ph3-CH2CH2-Th2-Np3-CF3,
R'-Ph3-CH2CH2-Th2-Np4-CF3, R'-Ph3-Th2-CH2CH2-Phl-CF3,
R'-Ph3-Th2-CH2CH2-Ph2-CF3, R'-Ph3-Th2-CH2CH2-Ph3-CF3,
15 R'-Ph3-Th2-CH2CH2-Npl-CF3, R'-Ph3-Th2-CH2CH2-Np2-CF3,
R'-Ph3-Th2-CH2CH2-Np3-CF3, R'-Ph3-Th2-CH2CH2-Np4-CF3,
R'-Ph3-C=C-Th2-Phl-CF3, R'-Ph3-C=C-Th2-Ph2-CF3, R'-Ph3-C=C-Th2-Ph3-CF3,
R'-Ph3-Th2-C=C-Ph1-CF3, R'-Ph3-Th2-C=C-Ph2-CF3, R'-Ph3-Th2-C-C-Ph3-CF3,
R'-Np1-Th2-Ph1-CF3, R'-Np1-Th2-Ph2-CF3, R'-Np1-Th2-Ph3-CF3,
20 R'-Np 1-CH2CH2-Th2-Phl -CF3, R'-Np 1-CH2CH2-Th2-Ph2-CF3,
R' -Np 1-CH2CHZ-Th2-Ph3-CF3, R'-Np 1-Th2-CHZCH2-Ph 1-CF3,
R' -Np 1-Th2-CHZCH2-Ph2-CF3, R'-Np 1-Th2-CH2CH2-Ph3-CF3,
R' -Np2-Th2-Ph 1-CF3, R' -Np2-Th2-Ph2-CF3, R' -Np2-Th2-Ph3-CF3,
R'-Np2-CHZCH2-Th2-Phl-CF3, R'-Np2-CH2CH2-Th2-Ph2-CF3,
CA 02377217 2001-12-28
71
R'-Np2-CH2CH2-Th2-Ph3-CF3, R'-Np2-Th2-CH2CH2-Phl-CF3,
R'-Np2-Th2-CH2CHZ-Ph2-CF3, R'-Np2-Th2-CH2CH2-Ph3-CF3,
R'-Np3-Th2-Phl-CF3, R'-Np3-Th2-Ph2-CF3, R'-Np3-Th2-Ph3-CF3,
R'-Np3-CH2CH2-Th2-Phl-CF3, R'-Np3-CH2CH2-Th2-Ph2-CF3,
R'-Np3-CH2CH2-Th2-Ph3-CF3, R'-Np3-Th2-CH2CH2-Phl-CF3,
R'-Np3-Th2-CH2CHZ-Ph2-CF3, R'-Np3-Th2-CH2CH2-Ph3-CF3,
R'-Np1-Th2-Ph1-CF3, R'-Np1-Th2-Ph2-CF3, R'-Np1-Th2-Ph3-CF3,
R'-Np4-CH2CH2-Th2-Phl-CF3, R'-Np4-CHZCH2-Th2-Ph2-CF3,
R'-Np4-CH2CH2-Th2-Ph3-CF3, R'-Np4-Th2-CH2CH2-Phl-CF3,
R'-Np4-Th2-CH2CH2-Ph2-CF3, R'-Np4-Th2-CH2CH2-Ph3-CF3,
R'-Cy-Th3-Phl-CF3, R'-Cy-Th3-Ph2-CF3, R'-Cy-Th3-Ph3-CF3, R'-Cy-Th3-Npl-CF3,
R'-Cy-Th3-Np2-CF3, R'-Cy-Th3-Np3-CF3, R'-Cy-Th3-Np4-CF3,
R'-Cy-CH2CH2-Th3-Phl-CF3, R'-Cy-CH2CH2-Th3-Ph2-CF3, R'-Cy-CH2CH2-Th3-Ph3-CF3,
R'-Cy-CH2CH2-Th3-Npl-CF3, R'-Cy-CH2CH2-Th3-Np2-CF3,
R'-Cy-CH2CH2-Th3-Np3-CF3,
R'-Cy-CH2CH2-Th3-Np4-CF3, R'-Cy-Th3-CH2CH2-Phl-CF3, R'-Cy-Th3-CH2CH2-Ph2-CF3,
R'-Cy-Th3-CH2CH2-Ph3-CF3, R'-Cy-Th3-CH2CH2-Npl-CF3, R'-Cy-Th3-CHzCH2-Np2-CF3,
R' -Cy-Th3 -CH2CH2-Np3 -CF3, R' -Cy-Th3 -CH2CH2-Np4-CF3,
R'-Cy-Th3-C=C-Phl -CF3, R'-Cy-Th3-C=C-Ph2-CF3, R'-Cy-Th3-C=C-Ph3-CF3,
R'-Phl-Th3-Phl-CF3, R'-Phl-Th3-Ph2-CF3, R'-Phl-Th3-Ph3-CF3,
R'-Phl-Th3-Npl-CF3, R'-Phl-Th3-Np2-CF3, R'-Phl-Th3-Np3-CF3,
R' -Ph 1-Th3-Np4-CF3,
R'-Phl-CH2CH2-Th3-Phl-CF3, R'-Phl-CH2CH2-Th3-Ph2-CF3,
R'-Phl-CH2CH2-Th3-Ph3-CF3, R'-PhI-CHZCH2-Th3-Npl-CF3,
CA 02377217 2001-12-28
72
R' -Ph 1-CHZCHZ-Th3-Np2-CF3, R' -Ph 1-CH2CH2-Th3-Np3-CF3,
R'-Phl-CH2CH2-Th3-Np4-CF3, R'-Phl-Th3-CH2CH2-Phl-CF3,
R'-Phl-Th3-CH2CH2-Ph2-CF3, R'-Phl-Th3-CH2CH2-Ph3-CF3,
R'-Phl-Th3-CH2CH2-Npl-CF3, R'-Phl-Th3-CH2CH2-Np2-CF3,
R' -Ph 1-Th3 -CH2CH2-Np3 -CF3, R' -Ph 1-Th3 -CH2CH2-Np4-CF3,
R'-Phl-C=C-Th3-Phl-CF3, R'-Phl-C=C-Th3-Ph2-CF3, R'-Phl-C=C-Th3-Ph3-CF3,
R'-Phl-Th3-C=C-Phl-CF3, R'-Phl-Th3-C=C-Ph2-CF3, R'-Phl-Th3-C=C-Ph3-CF3,
R'-Ph2-Th3-Phl-CF3, R'-Ph2-Th3-Ph2-CF3, R'-Ph2-Th3-Ph3-CF3,
R'-Ph2-Th3-Npl-CF3, R'-Ph2-Th3-Np2-CF3, R'-Ph2-Th3-Np3-CF3,
R'-Ph2-Th3-Np4-CF3,
R' -Ph2-CH2CH2-Th3-Ph 1-CF3, R' -Ph2-CH2CH2-Th3-Ph2-CF3,
R'-Ph2-CH2CH2-Th3-Ph3-CF3, R'-Ph2-CH2CH2-Th3-Np 1-CF3,
R1-Ph2-CH2CH2-Th3-Np2-CF3, R'-Ph2-CH2CH2-Th3-Np3-CF3,
R'-Ph2-CH2CH2-Th3-Np4-CF3, Rl-Ph2-Th3-CH2CH2-Phl-CF3,
R'-Ph2-Th3-CH2CH2-Ph2-CF3, R'-Ph2-Th3-CH2CH2-Ph3-CF3,
R'-Ph2-Th3-CH2CH2-Npl-CF3, R'-Ph2-Th3-CH2CH2-Np2-CF3,
R'-Ph2-Th3-CH2CH2-Np3-CF3, R'-Ph2-Th3-CH2CH2-Np4-CF3,
R'-Ph2-C=C-Th3-Phl-CF3, R'-Ph2-C=C-Th3-Ph2-CF3, R'-Ph2-C=C-Th3-Ph3-CF3,
R'-Ph2-Th3-C=C-Phl-CF3, R'-Ph2-Th3-C=C-Ph2-CF3, R'-Ph2-Th3-C-C-Ph3-CF3,
R'-Ph3-Th3-Phl-CF3, R'-Ph3-Th3-Ph2-CF3, R'-Ph3-Th3-Ph3-CF3,
R'-Ph3-Th3-Npl-CF3, R'-Ph3-Th3-Np2-CF3, R'-Ph3-Th3-Np3-CF3,
R'-Ph3-Th3-Np4-CF3,
R'-Ph3-CHZCH2-Th3-Phl-CF3, R'-Ph3-CH2CH2-Th3-Ph2-CF3,
R' -Ph3-CH2CH2-Th3-Ph3-CF3, R'-Ph3-CH2CH2-Th3-Np 1-CF3,
CA 02377217 2001-12-28
73
R'-Ph3-CH2CH2-Th3-Np2-CF3, R'-Ph3-CH2CH2-Th3-Np3-CF3,
R'-Ph3-CHZCH2-Th3-Np4-CF3, R'-Ph3-Th3-CH2CH2-Phl-CF3,
R'-Ph3-Th3-CH2CHZ-Ph2-CF3, R'-Ph3-Th3-CH2CH2-Ph3-CF3,
R'-Ph3-Th3-CH2CH2-Npl-CF3, R'-Ph3-Th3-CH2CH2-Np2-CF3,
R'-Ph3-Th3-CH2CH2-Np3-CF3, R'-Ph3-Th3-CH2CH2-Np4-CF3,
R'-Ph3-C=C-Th3-Phl-CF3, R'-Ph3-C=C-Th3-Ph2-CF3, R'-Ph3-C=C-Th3-Ph3-CF3,
R'-Ph3-Th3-C=C-Phl-CF3, R'-Ph3-Th3-C=C-Ph2-CF3, R'-Ph3-Th3-C-C-Ph3-CF3,
R'-Npl-Th3-Phl-CF3, R'-Npl-Th3-Ph2-CF3, R'-Npl-Th3-Ph3-CF3,
R'-Npl-CH2CH2-Th3-Phl-CF3, R'-Npl-CH2CH2-Th3-Ph2-CF3,
R'-Npl-CH2CH2-Th3-Ph3-CF3, R'-Npl-Th3-CH2CH2-Phl-CF3,
R'-Npl-Th3-CH2CH2-Ph2-CF3, R'-Npl-Th3-CH2CH2-Ph3-CF3,
R'-Np2-Th3-Phl-CF3, R'-Np2-Th3-Ph2-CF3, R'-Np2-Th3-Ph3-CF3,
R'-Np2-CH2CH2-Th3-Phl-CF3, R'-Np2-CH2CH2-Th3-Ph2-CF3,
R' -Np2-CHZCH2-Th3-Ph3-CF3, R'-Np2-Th3-CH2CH2-Ph 1-CF3,
R'-Np2-Th3-CH2CH2-Ph2-CF3, R'-Np2-Th3-CHZCH2-Ph3-CF3,
R'-Np3-Th3-Phl -CF3, R'-Np3-Th3-Ph2-CF3, R'-Np3-Th3-Ph3-CF3,
R'-Np3-CH2CH2-Th3-Phl-CF3, R'-Np3-CHZCH2-Th3-Ph2-CF3,
R'-Np3-CH2CH2-Th3-Ph3-CF3, R'-Np3-Th3-CHZCH2-Phi-CF3,
R'-Np3-Th3-CH2CH2-Ph2-CF3, R'-Np3-Th3-CH2CH2-Ph3-CF3,
R'-Npl-Th3-Phl-CF3, R'-Npl-Th3-Ph2-CF3, R'-Npl-Th3-Ph3-CF3,
R'-Np4-CH2CH2-Th3-Phl-CF3, R'-Np4-CH2CH2-Th3-Ph2-CF3,
R'-Np4-CH2CH2-Th3-Ph3-CF3, R'-Np4-Th3-CH2CH2-Phl-CF3,
R'-Np4-Th3-CH2CH2-Ph2-CF3, R'-Np4-Th3-CHZCH2-Ph3-CF3,
R'-Cy-Th3-Phl-CF3, R'-Cy-Th3-Ph2-CF3, R'-Cy-Th3-Ph3-CF3, R'-Cy-Th3-Npl-CF3,
CA 02377217 2001-12-28
74
R'-Cy-Th3-Np2-CF3, RI-Cy-Th3-Np3-CF3, R'-Cy-Th3-Np4-CF3,
R'-Cy-CH2CH2-Th3-Phl-CF3, R'-Cy-CH2CH2-Th3-Ph2-CF3, R'-Cy-CH2CH2-Th3-Ph3-CF3,
R1-Cy-CH2CH2-Th3-Npl-CF3, RI-Cy-CH2CH2-Th3-Np2-CF3,
R'-Cy-CH2CH2-Th3-Np3-CF3,
R'-Cy-CH2CH2-Th3-Np4-CF3, R'-Cy-Th3-CH2CH2-Phl-CF3, RI-Cy-Th3-CH2CH2-Ph2-CF3,
R'-Cy-Th3-CH2CH2-Ph3-CF3, R'-Cy-Th3-CH2CH2-Npl-CF3, R'-Cy-Th3-CH2CHZ-Np2-CF3,
R'-Cy-Th3-CHZCH2-Np3-CF3, R'-Cy-Th3-CH2CH2-Np4-CF3,
R'-Cy-Th3-C=C-Phl-CF3, R'-Cy-Th3-C=C-Ph2-CF3, R'-Cy-Th3-C=C-Ph3-CF3,
R'-Phl-Th3-Phl-CF3, R'-Phl-Th3-Ph2-CF3, R'-Phl-Th3-Ph3-CF3,
R'-Phl-Th3-Npl-CF3, R'-Phl-Th3-Np2-CF3, R'-Phl-Th3-Np3-CF3,
R' -Ph 1-Th3 -Np4-CF3,
R'-Phl-CH2CH2-Th3-Phl-CF3, R'-Phl-CH2CH2-Th3-Ph2-CF3,
R'-Phl-CH2CH2-Th3-Ph3-CF3, Rl-Phl-CH2CH2-Th3-Npl-CF3,
R'-Phl-CH2CH2-Th3-Np2-CF3, R'-Phl-CH2CHz-Th3-Np3-CF3,
R'-Phl-CH2CH2-Th3-Np4-CF3, R'-Phl-Th3-CH2CH2-Phl-CF3,
R'-Phl-Th3-CH2CH2-Ph2-CF3, R'-Phl-Th3-CH2CH2-Ph3-CF3,
R'-Phl-Th3-CH2CH2-Npi-CF3, R'-Phl-Th3-CH2CH2-Np2-CF3,
R'-Phl-Th3-CH2CH2-Np3-CF3, R'-Phl-Th3-CH2CH2-Np4-CF3,
R'-PhI-C=C-Th3-Phl-CF3, R'-PhI-C-C-Th3-Ph2-CF3, R'-PhI-C=C-Th3-Ph3-CF3,
R'-Phl-Th3-C=C-Phl-CF3, R'-Phl-Th3-C=C-Ph2-CF3, R'-Phl-Th3-C_C-Ph3-CF3,
R'-Ph2-Th3-Phl-CF3, R'-Ph2-Th3-Ph2-CF3, R'-Ph2-Th3-Ph3-CF3,
R'-Ph2-Th3-Npl-CF3, R'-Ph2-Th3-Np2-CF3, R'-Ph2-Th3-Np3-CF3,
R'-Ph2-Th3-Np4-CF3,
R'-Ph2-CH2CH2-Th3-Phl-CF3, R'-Ph2-CH2CH2-Th3-Ph2-CF3,
CA 02377217 2001-12-28
R'-Ph2-CH2CH2-Th3-Ph3-CF3, R'-Ph2-CH2CH2-Th3-Npl-CF3,
R'-Ph2-CH2CH2-Th3-Np2-CF3, R'-Ph2-CH2CH2-Th3-Np3-CF3,
R'-Ph2-CH2CH2-Th3-Np4-CF3, R'-Ph2-Th3-CH2CH2-Phl-CF3,
R'-Ph2-Th3-CH2CH2-Ph2-CF3, R'-Ph2-Th3-CH2CH2-Ph3-CF3,
5 R'-Ph2-Th3-CH2CH2-Npl-CF3, R'-Ph2-Th3-CH2CH2-Np2-CF3,
R'-Ph2-Th3-CH2CH2-Np3-CF3, R'-Ph2-Th3-CH2CH2-Np4-CF3,
R'-Ph2-C=C-Th3-Phl-CF3, R'-Ph2-C=C-Th3-Ph2-CF3, R'-Ph2-C=C-Th3-Ph3-CF3,
R'-Ph2-Th3-C=C-Phl-CF3, R'-Ph2-Th3-C=C-Ph2-CF3, R'-Ph2-Th3-C=C-Ph3-CF3,
R'-Ph3-Th3-Phl-CF3, R'-Ph3-Th3-Ph2-CF3, R'-Ph3-Th3-Ph3-CF3,
10 R'-Ph3-Th3-Npl-CF3, R'-Ph3-Th3-Np2-CF3, R'-Ph3-Th3-Np3-CF3,
R'-Ph3-Th3-Np4-CF3,
R'-Ph3-CH2CH2-Th3-Phl-CF3, R'-Ph3-CH2CH2-Th3-Ph2-CF3,
R'-Ph3-CHZCH2-Th3-Ph3-CF3, R'-Ph3-CH2CH2-Th3-Npl-CF3,
R'-Ph3-CHZCH2-Th3-Np2-CF3, R'-Ph3-CH2CH2-Th3-Np3-CF3,
15 R'-Ph3-CH2CH2-Th3-Np4-CF3, R'-Ph3-Th3-CH2CH2-Phl-CF3,
R'-Ph3-Th3-CH2CH2-Ph2-CF3, R'-Ph3-Th3-CH2CHZ-Ph3-CF3,
R'-Ph3-Th3-CH2CH2-Npl-CF3, R'-Ph3-Th3-CH2CH2-Np2-CF3,
R'-Ph3-Th3-CH2CH2-Np3-CF3, R'-Ph3-Th3-CH2CH2-Np4-CF3,
R'-Ph3-C=C-Th3-Phl-CF3, R'-Ph3-C=C-Th3-Ph2-CF3, R'-Ph3-C=C-Th3-Ph3-CF3,
20 R'-Ph3-Th3-C=C-Phl-CF3, R'-Ph3-Th3-C=C-Ph2-CF3, R'-Ph3-Th3-C_C-Ph3-CF3,
R'-Npl-Th3-Phl-CF3, R'-Npl-Th3-Ph2-CF3, R'-Npl-Th3-Ph3-CF3,
R'-Npl-CH2CH2-Th3-Phl-CF3, R'-NpI-CHZCH2-Th3-Ph2-CF3,
R' -Np 1-CH2CH2-Th3-Ph3-CF3, R' -Np 1-Th3-CHZCH2-Ph 1-CF3,
R'-Np 1-Th3-CH2CH2-Ph2-CF3, R'-Np 1-Th3-CH2CH2-Ph3-CF3,
CA 02377217 2001-12-28
76
R'-Np2-Th3-Phl-CF3, R'-Np2-Th3-Ph2-CF3, R'-Np2-Th3-Ph3-CF3,
R'-Np2-CH2CH2-Th3-Phl-CF3, R'-Np2-CH2CH2-Th3-Ph2-CF3,
R'-Np2-CH2CH2-Th3-Ph3-CF3, R'-Np2-Th3-CH2CH2-Phl-CF3,
R'-Np2-Th3-CH2CH2-Ph2-CF3, R'-Np2-Th3-CH2CH2-Ph3-CF3,
R'-Np3-Th3-Phl-CF3, R'-Np3-Th3-Ph2-CF3, R'-Np3-Th3-Ph3-CF3,
R'-Np3-CH2CH2-Th3-Phl-CF3, R'-Np3-CH2CH2-Th3-Ph2-CF3,
R'-Np3-CH2CH2-Th3-Ph3-CF3, R'-Np3-Th3-CH2CH2-Phl-CF3,
R'-Np3-Th3-CH2CH2-Ph2-CF3, R'-Np3-Th3-CH2CH2-Ph3-CF3,
R'-Npl-Th3-Phl-CF3, R'-Np1-Th3-Ph2-CF3, R'-Npl-Th3-Ph3-CF3,
R'-Np4-CH2CH2-Th3-Phl -CF3, R'-Np4-CH2CH2-Th3-Ph2-CF3,
R'-Np4-CHZCH2-Th3-Ph3-CF3, R'-Np4-Th3-CH2CH2-Phl-CF3,
R'-Np4-Th3-CH2CH2-Ph2-CF3, R'-Np4-Th3-CH2CH2-Ph3-CF3,
In the case in which ring C is the formula (IIb),
then in the case in which na = nb = 0, and d = 1, nd = 0 or n = 0, nd = 1,
and Z is a fluorine
atom,
R'-Te1-Phl-F, R'-Te1-Ph2-F, R'-Te1-Ph3-F, R'-Te1-Np1-F, R'-Tel-Np2-F,
R'-Tel-Np3-F, R'-Tel-Np4-F, R'-Te2-Phl-F, R'-Te2-Ph2-F, R'-Te2-Ph3-F,
R'-Te2-Npl-F, R'-Te2-Np2-F, R'-Te2-Np3-F, R'-Te2-Np4-F,
R'-Te1-CH2CH2-Ph2-F, R'-Tel-CH2CH2-Ph3-F, R'-Te1-CH2CH2-Np1-F,
R'-Tel-CH2CH2-Np2-F, R'-Tel-CH2CH2-Np3-F, R'-Tel-CH2CH2-Np4-F,
R'-Te2-CHzCH2-Ph1-F, R'-Te2-CH2CH2-Ph2-F, R'-Te2-CH2CH2-Ph3-F,
R'-Te2-CH2CHZ-Npl -F, R'-Te2-CH2CH2-Np2-F, R'-Te2-CH2CH2-Np3-F,
R' -Te2-CHzCH2-Np4-F,
in the case in which na = nb = 0, and n = 1, nd = 0 or n = 0, nd = 1, and Z
is a cyano group,
CA 02377217 2001-12-28
77
R'-Te1-Ph2-CN, R'-Te1-Ph3-CN, R'-Tel-Npl-CN, R'-Te1-Np2-CN, R'-Te1-Np3-CN,
R'-Tel-Np4-CN, R'-Te2-Phl-CN, R'-Te2-Ph2-CN, R'-Te2-Ph3-CN, R'-Te2-Npl-CN,
R'-Te2-Np2-CN, R'-Te2-Np3-CN, Rl-Te2-Np4-CN,
R' -Te 1-CH2CH2-Ph2-CN, Rl -Te 1-CH2CH2-Ph3-CN, Rl -Te 1-CH2CH2-Np 1-CN,
R'-Tel-CH2CH2-Np2-CN, Rl-Tel-CH2CH2-Np3-CN, R'-Tel-CH2CH2-Np4-CN,
R'-Te2-CHZCH2-Phl-CN, R'-Te2-CH2CH2-Ph2-CN, Rl-Te2-CH2CH2-Ph3-CN,
R'-Te2-CH2CH2-Npl-CN, R'-Te2-CH2CH2-Np2-CN, R'-Te2-CH2CH2-Np3-CN,
R' -Te2-CH2CH2-Np4-CN,
inthecaseinwhichna=nb=0,andn = 1, n d = 0 or n'= 0, n d = 1,andZisa
trifluoromethoxy group,
R'-Tel-PhI-OCF3, R'-Tel-Ph2-OCF3, R'-Tel-Ph3-OCF3, Rl-Tel-NpI-OCF3,
R'-Tel-Np2-OCF3, R'-Tel-Np3-OCF3, R'-Tel-Np4-OCF3, R'-Te2-Phl-OCF3,
R'-Te2-Ph2-OCF3, R'-Te2-Ph3-OCF3, R'-Te2-Npl-OCF3, R'-Te2-Np2-OCF3,
R'-Te2-Np3-OCF3, Rl-Te2-Np4-OCF3,
R' -Te l-CHZCH2-Ph2-OCF3, Rl-Te 1-CHZCH2-Ph3-OCF3, R'-Te 1-CH2CH2-Np 1-OCF3,
R'-Tel-CH2CH2-Np2-OCF3, Rl-TeI-CHZCH2-Np3-OCF3, Rl-Tel-CHzCH2-Np4-OCF3,
R'-Te2-CHZCH2-Phl-OCF3, Rl-Te2-CH2CHZ-Ph2-OCF3, R1-Te2-CH2CH2-Ph3-OCF3,
R'-Te2-CH2CH2-Npl-OCF3, Rl-Te2-CH2CH2-Np2-OCF3, R'-Te2-CH2CH2-Np3-OCF3,
Rl -Te2-CH2CH2-Np4-OCF3,
in the case in which na = 1, nb = 0 or na = 0, nb = l, and n = 1, nd = 0 or n
= 0, nd = 1, and Z
is a fluorine atom,
R' -Cy-Te 1-Ph 1-F, R' -Cy-Te 1-Ph2-F, R' -Cy-Te 1-Ph3-F, R' -Cy-Te 1-Np 1-F,
R'-Cy-Te1-Np2-F, R'-Cy-Te1-Np3-F, R'-Cy-Te1-Np4-F, RI-Cy-Te2-Ph1-F,
R'-Cy-Te2-Ph2-F, R'-Cy-Te2-Ph3-F, R'-Cy-Te2-Npl-F, R'-Cy-Te2-Np2-F,
CA 02377217 2001-12-28
78
R' -Cy-Te2-Np3 -F, R' -Cy-Te2-Np4-F,
R' -Cy-Te 1-CH2CH2-Ph2-F, R' -Cy-Te 1-CH2CH2-Ph3-F, R' -Cy-Te 1-CH2CH2-Np 1-F,
R' -Cy-Te 1-CH2CH2-Np2-F, R' -Cy-Te 1-CH2CH2-Np3 -F, R' -Cy-Te 1-CH2CH2-Np4-F,
R'-Cy-Te2-CH2CH2-Phl-F, R'-Cy-Te2-CH2CH2-Ph2-F, R'-Cy-Te2-CH2CH2-Ph3-F,
R'-Cy-Te2-CH2CH2-Npl-F, RI-Cy-Te2-CH2CH2-Np2-F, R'-Cy-Te2-CH2CH2-Np3-F,
R'-Cy-Te2-CH2CH2-Np4-F,
R'-Phl-Tel-Phl-F, R'-Phl-Tel-Ph2-F, R'-Phl-Tel-Ph3-F, R'-Phl-Tel-Npl-F,
R' -Ph 1-Te l-Np2-F, R'-Phl -Te l-Np3-F, R'-Ph l-Te l-Np4-F, R' -Phl -Te2-Ph l-
F,
R'-Phl-Te2-Ph2-F, R'-Phl-Te2-Ph3-F, R'-Phl-Te2-Npl-F, Rl-Phl-Te2-Np2-F,
R'-Phl-Te2-Np3-F, R'-Phl-Te2-Np4-F,
R'-Phl-Te1-CHZCH2-Ph2-F, Rl-Ph1-Te1-CH2CH2-Ph3-F, R'-Ph1-Tel-CH2CH2-Np1-F,
R' -Ph 1-Te l-CH2CH2-Np2-F, R' -Ph 1-Te 1-CH2CH2-Np3 -F, R' -Ph 1-Te 1-CH2CH2-
Np4-F,
R'-Phl-Te2-CH2CH2-Phl-F, R'-Phl-Te2-CH2CHZ-Ph2-F, R'-Phl-Te2-CH2CH2-Ph3-F,
R'-Phl-Te2-CH2CH2-Npl-F, R'-Phl-Te2-CH2CH2-Np2-F, R'-Phl-Te2-CH2CH2-Np3-F,
R'-Phl-Te2-CH2CH2-Np4-F,
R'-Ph2-Te1-Ph1-F, R'-Ph2-Te1-Ph2-F, R'-Ph2-Te1-Ph3-F, R'-Ph2-Te1-Np1-F,
R' -Ph2-Te 1-Np2-F, R'-Ph2-Te 1-Np3-F, R'-Ph2-Te 1-Np4-F, R' -Ph2-Te2-Ph 1-F,
R'-Ph2-Te2-Ph2-F, R'-Ph2-Te2-Ph3-F, R'-Ph2-Te2-Npl-F, R'-Ph2-Te2-Np2-F,
R'-Ph2-Te2-Np3-F, R'-Ph2-Te2-Np4-F,
R' -Ph2-Te 1-CH2CH2-Ph2-F, R' -Ph2-Te 1-CH2CH2-Ph3-F, R' -Ph2-Te 1-CH2CHz-Np 1-
F,
R'-Ph2-Tel-CH2CH2-Np2-F, Rl-Ph2-Tel-CH2CH2-Np3-F, Rl-Ph2-Tel-CH2CH2-Np4-F,
R'-Ph2-Te2-CH2CH2-Phl -F, R'-Ph2-Te2-CH2CH2-Ph2-F, R'-Ph2-Te2-CH2CH2-Ph3-F,
R'-Ph2-Te2-CH2CH2-Npl-F, R'-Ph2-Te2-CH2CH2-Np2-F, R'-Ph2-Te2-CH2CH2-Np3-F,
R' -Ph2-Te2-CH2CH2-Np4-F,
CA 02377217 2001-12-28
79
R'-Ph3-Tel-Phl-F, R'-Ph3-Tel-Ph2-F, R'-Ph3-Tel-Ph3-F, R'-Ph3-Tel-Npl-F,
R' -Ph3-Te l-Np2-F, R'-Ph3-Te l-Np3-F, R' -Ph3-Te l-Np4-F, R' -Ph3-Te2-Ph l-F,
R'-Ph3-Te2-Ph2-F, R'-Ph3-Te2-Ph3-F, R'-Ph3-Te2-Npl-F, R'-Ph3-Te2-Np2-F,
R' -Ph3 -Te2-Np3 -F, R' -Ph3 -Te2-Np4-F,
R'-Ph3-Tel-CH2CH2-Ph2-F, R'-Ph3-Tel-CH2CH2-Ph3-F, R'-Ph3-Tel-CH2CH2-Npl-F,
R'-Ph3-Tel-CH2CH2-Np2-F, R'-Ph3-Tel-CH2CH2-Np3-F, R'-Ph3-Tel-CHZCH2-Np4-F,
R'-Ph3-Te2-CH2CH2-Phl -F, R'-Ph3-Te2-CH2CH2-Ph2-F, R'-Ph3-Te2-CH2CH2-Ph3-F,
R'-Ph3-Te2-CH2CH2-Npl-F, R'-Ph3-Te2-CH2CH2-Np2-F, R'-Ph3-Te2-CHZCH2-Np3-F,
R' -Ph3 -Te2-CH2CH2-Np4-F,
R'-Npl-Tel-Phl-F, Rl-Npl-Tel-Ph2-F, R'-Npl-Tel-Ph3-F, R'-Npl-Te2-Phl-F,
R' -Np 1-Te2-Ph2-F, R'-Np 1-Te2-Ph3-F,
Rl-Npl-Tel-CHZCH2-Ph2-F, Rl-Npl-Tel-CHZCH2-Ph3-F, Rl-Npl-Te2-CH2CH2-Phl-F,
R'-Npl-Te2-CH2CH2-Ph2-F, R'-Npl-Te2-CH2CH2-Ph3-F,
R'-Np2-Te1-Ph1-F, R'-Np2-Te1-Ph2-F, R'-Np2-Te1-Ph3-F, R1-Np2-Te2-Ph1-F,
R'-Np2-Te2-Ph2-F, R'-Np2-Te2-Ph3-F,
R' -Np2-Te 1-CH2CH2-Ph2-F, R' -Np2-Te 1-CH2CH2-Ph3 -F, R' -Np2-Te2-CH2CH2-Ph 1-
F,
R'-Np2-Te2-CH2CH2-Ph2-F, R'-Np2-Te2-CH2CH2-Ph3-F,
R'-Np3-Tel-Phl-F, R'-Np3-Tel-Ph2-F, R'-Np3-Tel-Ph3-F, R'-Np3-Te2-Phl-F,
R'-Np3-Te2-Ph2-F, R'-Np3-Te2-Ph3-F,
R'-Np3-Tel-CH2CH2-Ph2-F, R'-Np3-Tel-CH2CH2-Ph3-F, R'-Np3-Te2-CH2CH2-Phl-F,
R'-Np3-Te2-CH2CH2-Ph2-F, R'-Np3-Te2-CH2CH2-Ph3-F,
R' -Np4-Te 1-Ph 1-F, R' -Np4-Te 1-Ph2-F, R' -Np4-Te 1-Ph3 -F, R' -Np4-Te2-Ph 1-
F,
R' -Np4-Te2-Ph2-F, R' -Np4-Te2-Ph3 -F,
R' -Np4-Te 1-CHZCH2-Ph2-F, Rl -Np4-Te 1-CH2CH2-Ph3-F, R' -Np4-Te2-CHzCH2-Ph 1-
F,
CA 02377217 2001-12-28
R'-Np4-Te2-CH2CH2-Ph2-F, R'-Np4-Te2-CH2CH2-Ph3-F, R'-Cy-CH2CH2-Tel-Phl-F,
R' -Cy-CH2CH2-Te 1-Ph2-F, R' -Cy-CH2CH2-Te 1-Ph3-F, R' -Cy-CH2CH2-Te 1-Np 1-F,
R'-Cy-CH2CH2-Tel-Np2-F, R'-Cy-CH2CH2-Tel-Np3-F, R'-Cy-CH2CH2-Tel-Np4-F,
R'-Cy-CH2CHZ-Te2-Phl-F, R'-Cy-CH2CH2-Te2-Ph2-F, R1-Cy-CH2CH2-Te2-Ph3-F,
5 R'-Cy-CH2CH2-Te2-Npl-F, R'-Cy-CH2CH2-Te2-Np2-F, R'-Cy-CH2CH2-Te2-Np3-F,
RI-Cy-CH2CH2-Te2-Np4-F, R'-Phl-CH2CH2-Tel-Phl-F, Rl-Phl-CH2CH2-Tel-Ph2-F,
R'-Phl-CH2CH2-Tel-Ph3-F, Rl-Phl-CH2CH2-Tel-Npl-F, R'-Phl-CH2CH2-Tel-Np2-F,
R' -Phl -CH2CH2-Te 1-Np3-F, R' -Ph 1-CH2CH2-Te 1-Np4-F, R' -Phl -CH2CH2-Te2-Ph
1-F,
Rl-Phl-CH2CHZ-Te2-Ph2-F, R'-Phl-CH2CH2-Te2-Ph3-F, R'-Phl-CH2CH2-Te2-Npl-F,
10 R'-PhI-CHZCH2-Te2-Np2-F, R'-Phl-CH2CH2-Te2-Np3-F, Rl-Phl-CH2CH2-Te2-Np4-F,
R' -Ph2-CH2CH2-Te 1-Ph 1-F, R' -Ph2-CH2CH2-Te 1-Ph2-F, R' -Ph2-CH2CH2-Te 1-Ph3-
F,
R' -Ph2-CHZCH2-Te 1-Np 1-F, R' -Ph2-CH2CH2-Te 1-Np2-F, R' -Ph2-CHZCHZ-Te 1-Np3
-F,
R' -Ph2-CH2CH2-Te 1-Np4-F, R' -Ph2-CH2CH2-Te2-Ph 1-F, R' -Ph2-CH2CH2-Te2-Ph2-
F,
R'-Ph2-CHZCH2-Te2-Ph3-F, R'-Ph2-CH2CH2-Te2-Npl-F, R'-Ph2-CH2CH2-Te2-Np2-F,
15 R'-Ph2-CH2CH2-Te2-Np3-F, R'-Ph2-CH2CH2-Te2-Np4-F, Rl-Ph3-CH2CH2-Tel-Phl-F,
R'-Ph3-CH2CH2-Tel-Ph2-F, R'-Ph3-CH2CH2-Tel-Ph3-F, R'-Ph3-CH2CH2-Te2-Phl-F,
R'-Ph3-CH2CH2-Te2-Ph2-F, R'-Ph3-CH2CH2-Te2-Ph3-F, R'-Npl-CH2CH2-Tel-Phl-F,
R1-Npl-CH2CH2-Tel-Ph2-F, R1-Npl-CH2CH2-Tel-Ph3-F, R'-Npl-CH2CH2-Te2-Phl-F,
R'-Npl-CH2CH2-Te2-Ph2-F, R'-Npl-CHZCH2-Te2-Ph3-F, R'-Np2-CH2CH2-Tel-Phl-F,
20 R'-Np2-CH2CH2-Tel-Ph2-F, R'-Np2-CH2CH2-Tel-Ph3-F, R'-Np2-CH2CH2-Te2-Phl-F,
R'-Np2-CH2CH2-Te2-Ph2-F, R'-Np2-CH2CH2-Te2-Ph3-F, Rl-Np3-CH2CH2-Tel-Phl-F,
R'-Np3-CH2CHZ-Tel-Ph2-F, R'-Np3-CH2CH2-Tel-Ph3-F, R'-Np3-CH2CH2-Te2-Phl-F,
R'-Np3-CH2CH2-Te2-Ph2-F, R'-Np3-CH2CH2-Te2-Ph3-F, R'-Np4-CH2CH2-Tel-Phi-F,
R'-Np4-CH2CH2-Tel-Ph2-F, R'-Np4-CH2CH2-Te1-Ph3-F, R'-Np4-CH2CH2-Te2-Phl-F,
CA 02377217 2001-12-28
81
R'-Np4-CHZCH2-Te2-Ph2-F, Rl-Np4-CH2CH2-Te2-Ph3-F,
R' -Ph 1-C=C-Te 1-Phl -F, R'-Ph 1-C=C-Te 1-Ph2-F, R'-Ph 1-C=C-Te 1-Ph3-F,
R'-Ph1-C=C-Te2-Ph1-F, R'-Ph1-C=C-Te2-Ph2-F, R'-Ph1-C=C-Te2-Ph3-F,
R' -Ph2-C-C-Te 1-Ph1-F, R'-Ph2-C-C-Te 1-Ph2-F, R'-Ph2-C-C-Te 1-Ph3-F,
R' -Ph2-C=C-Te2-Ph 1-F, R' -Ph2-C=C-Te2-Ph2-F, R' -Ph2-C=C-Te2-Ph3 -F,
R'-Ph3-C=C-Tel-Phl-F, R'-Ph3-C=C-Tel-Ph2-F, R'-Ph3-C-C-Tel-Ph3-F.,
R'-Ph3-C=C-Te2-Phl-F, R'-Ph3-C=C-Te2-Ph2-F, R'-Ph3-C=C-Te2-Ph3-F,
inthecaseinwhichna=1,nb=0orna=0,nb=1,andn'=1,nd=0orn~=0,nd1,andZ
is a cyano group,
Rl -Cy-Te l-Ph l-CN, R'-Cy-Te l-Ph2-CN, R' -Cy-Te l-Ph3-CN, R' -Cy-Te l-Np l-
CN,
R'-Cy-Te 1-Np2-CN, R'-Cy-Te 1-Np3-CN, R'-Cy-Te 1-Np4-CN, R' -Cy-Te2-Ph 1-CN,
R'-Cy-Te2-Ph2-CN, R'-Cy-Te2-Ph3-CN, R'-Cy-Te2-Npl-CN, R'-Cy-Te2-Np2-CN,
R'-Cy-Te2-Np3-CN, R'-Cy-Te2-Np4-CN,
R'-Cy-Te1-CH2CH2-Ph2-CN, R'-Cy-Te1-CH2CH2-Ph3-CN, R'-Cy-Te1-CH2CH2-Np1-CN,
R'-Cy-Tel-CH2CH2-Np2-CN, R'-Cy-Tel-CH2CH2-Np3-CN, R'-Cy-TeI-CH2CH2-Np4-CN,
R'-Cy-Te2-CH2CH2-Phl-CN, R'-Cy-Te2-CH2CH2-Ph2-CN, R'-Cy-Te2-CH2CH2-Ph3-CN,
R'-Cy-Te2-CH2CH2-Npl-CN, R'-Cy-Te2-CH2CH2-Np2-CN, R'-Cy-Te2-CH2CH2-Np3-CN,
R1-Cy-Te2-CH2CH2-Np4-CN,
R' -Ph 1-Te 1-Ph i-CN, R' -Ph 1-Te 1-Ph2-CN, R' -Ph 1-Te 1-Ph3 -CN,
R'-Phl-Tel-Npl-CN, R'-Phl-Tel-Np2-CN, R'-Phl-Tel-Np3-CN,
R' -Ph 1-Te 1-Np4-CN, R' -Ph 1-Te2-Ph 1-CN, R' -Ph 1-Te2-Ph2-CN,
R'-Ph1-Te2-Ph3-CN, R'-Ph1-Te2-Np1-CN, R'-Ph1-Te2-Np2-CN,
R' -Ph i -Te2-Np3 -CN, R' -Ph 1-Te2-Np4-CN,
R' -Ph 1-Te l-CH2CH2-Ph2-CN, R' -Ph 1-Te 1-CH2CH2-Ph3-CN,
CA 02377217 2001-12-28
82
R' -Ph 1-Te 1-CH2CH2-Np 1-CN, R' -Ph 1-Te l-CH2CH2-Np2-CN,
R' -Ph l-Te l-CH2CH2-Np3-CN, R' -Ph l-Te l-CH2CH2-Np4-CN,
R'-Phl-Te2-CH2CH2-Phl-CN, R'-Phl-Te2-CH2CH2-Ph2-CN,
R'-Phl-Te2-CH2CH2-Ph3-CN, R'-Phl-Te2-CH2CH2-Npl-CN,
R'-Phl -Te2-CH2CH2-Np2-CN, R'-Phl-Te2-CH2CH2-Np3-CN,
R' -Ph 1-Te2-CH2CH2-Np4-CN,
R'-Ph2-Te1-Ph1-CN, R'-Ph2-Te1-Ph2-CN, R'-Ph2-Te1-Ph3-CN,
R' -Ph2-Te l-Np 1-CN, R' -Ph2-Te l-Np2-CN, R'-Ph2-Te l-Np3-CN,
R'-Ph2-Tel-Np4-CN, R'-Ph2-Te2-Phl-CN, R'-Ph2-Te2-Ph2-CN,
R'-Ph2-Te2-Ph3-CN, R'-Ph2-Te2-Npl-CN, R'-Ph2-Te2-Np2-CN,
R'-Ph2-Te2-Np3-CN, R'-Ph2-Te2-Np4-CN,
R' -Ph2-Te 1-CH2CH2-Ph2-CN, R' -Ph2-Te 1-CH2CH2-Ph3-CN,
R' -Ph2-Te 1-CH2CH2-Np 1-CN, R' -Ph2-Te 1-CH2CH2-Np2-CN,
R'-Ph2-Tel-CH2CH2-Np3-CN, R'-Ph2-Tel-CH2CH2-Np4-CN,
R'-Ph2-Te2-CH2CH2-Phl-CN, R'-Ph2-Te2-CH2CH2-Ph2-CN,
R'-Ph2-Te2-CH2CH2-Ph3-CN, R'-Ph2-Te2-CH2CH2-Npl-CN,
R'-Ph2-Te2-CH2CH2-Np2-CN, R'-Ph2-Te2-CH2CH2-Np3-CN,
R' -Ph2-Te2-C H2CH2-Np4-CN,
R' -Ph3 -Te 1-Ph 1-CN, R' -Ph3 -Te 1-Ph2-CN, R' -Ph3 -Te 1-Ph3 -CN,
R'-Ph3-Tel-Np1-CN, R'-Ph3-Tel-Np2-CN, R'-Ph3-Tel-Np3-CN,
R'-Ph3-Tel-Np4-CN, R'-Ph3-Te2-Phl-CN, R'-Ph3-Te2-Ph2-CN,
R'-Ph3-Te2-Ph3-CN, R'-Ph3-Te2-Np 1-CN, R'-Ph3-Te2-Np2-CN,
R'-Ph3-Te2-Np3-CN, R'-Ph3-Te2-Np4-CN,
R'-Ph3-Tel-CH2CH2-Ph2-CN, R'-Ph3-Tel-CH2CH2-Ph3-CN,
CA 02377217 2001-12-28
83
R' -Ph3-Te 1-CH2CH2-Np 1-CN, R' -Ph3-Te 1-CH2CH2-Np2-CN,
R'-Ph3-Tel-CH2CH2-Np3-CN, R'-Ph3-Tel-CH2CH2-Np4-CN,
R'-Ph3-Te2-CHZCH2-Phl-CN, R'-Ph3-Te2-CH2CH2-Ph2-CN,
R'-Ph3-Te2-CH2CH2-Ph3-CN, R'-Ph3-Te2-CH2CH2-Npl-CN,
R'-Ph3-Te2-CH2CH2-Np2-CN, R'-Ph3-Te2-CH2CH2-Np3-CN,
R' -Ph3 -Te2-CH2 CH2-Np4-CN,
R'-Np 1-Te l-Ph 1-CN, R'-Np 1-Te l-Ph2-CN, R'-Np 1-Te l-Ph3-CN,
R' -Np 1-Te2-Ph 1-CN, R' -Np l-Te2-Ph2-CN, R' -Np l-Te2-Ph3 -CN,
Ri-Npl-Te1-CH2CH2-Ph2-CN, R'-Np1-Te1-CH2CH2-Ph3-CN,
R'-Np 1-Te2-CH2CH2-Ph 1-CN, R'-Np 1-Te2-CH2CH2-Ph2-CN,
R'-Np 1-Te2-CH2CH2-Ph3-CN,
R'-Np2-Tel-Phl-CN, R'-Np2-Tel-Ph2-CN, R'-Np2-Tel-Ph3-CN,
R'-Np2-Te2-Phl -CN, R'-Np2-Te2-Ph2-CN, R'-Np2-Te2-Ph3-CN,
Rl-Np2-Tel-CH2CH2-Ph2-CN, R'-Np2-Tel-CH2CH2-Ph3-CN,
Rl-Np2-Te2-CH2CH2-Phl -CN, R'-Np2-Te2-CHZCH2-Ph2-CN,
RI-Np2-Te2-CH2CH2-Ph3-CN, R'-Np2-Te2-CH2CH2-Np4-CN,
R'-Np3-Tel-Phl-CN, R'-Np3-Tel-Ph2-CN, R'-Np3-Tel-Ph3-CN,
R'-Np3-Te2-Phl-CN, R'-Np3-Te2-Ph2-CN, R'-Np3-Te2-Ph3-CN,
R'-Np3-Tel-CH2CHZ-Ph2-CN, R'-Np3-Te1-CH2CH2-Ph3-CN,
R'-Np3-Te2-CH2CH2-Phl -CN, R'-Np3 -Te2-CH2CH2-Ph2-CN,
R' -Np3 -Te2-CHZCH2-Ph3 -CN,
R' -Np4-Te 1-Ph 1-CN, R' -Np4-Te l-Ph2-CN, R'-Np4-Te 1-Ph3-CN,
R' -Np4-Te2-Ph 1-CN, R' -Np4-Te2-Ph2-CN, R' -Np4-Te2-Ph3 -CN,
R' -Np4-Te 1-CH2CH2-Ph2-CN, R'-Np4-Te 1-CH2CH2-Ph3-CN,
CA 02377217 2001-12-28
84
R' -Np4-Te2-CH2CH2-Ph 1-CN, R'-Np4-Te2-CH2CH2-Ph2-CN,
R' -Np4-Te2-CH2CH2-Ph3-CN,
R' -Cy-CH2CH2-Te 1-Ph 1-CN, R' -Cy-CHZCH2-Te 1-Ph2-CN, R' -Cy-CHZCH2-Te 1-Ph3 -
CN,
R'-Cy-CH2CH2-Tel-Npl-CN, R'-Cy-CHZCH2-Tel-Np2-CN, R'-Cy-CHZCH2-Tel-Np3-CN,
R'-Cy-CH2CH2-Tel-Np4-CN, R'-Cy-CH2CH2-Te2-Phl-CN, R'-Cy-CH2CH2-Te2-Ph2-CN,
R'-Cy-CH2CH2-Te2-Ph3-CN, R'-Cy-CH2CH2-Te2-Npl-CN, R'-Cy-CH2CH2-Te2-Np2-CN,
R'-Cy-CH2CH2-Te2-Np3-CN, RI-Cy-CH2CH2-Te2-Np4-CN, R'-Phl-CH2CH2-Tel-Phl-CN,
R'-Phl-CH2CH2-Tel-Ph2-CN, R'-Phl-CH2CH2-Tel-Ph3-CN,
R' -Ph 1-CH2CH2-Te 1-Np 1-CN, R 1-Ph 1-CH2CH2-Te 1-Np2-CN,
R'-Phi-CH2CH2-Tel-Np3-CN, Rl-Phl-CH2CH2-Tel-Np4-CN,
R'-Phl-CH2CH2-Te2-Phl-CN, Rl-Phl-CH2CH2-Te2-Ph2-CN,
R' -Ph l-CHZCH2-Te2-Ph3-CN, R' -Ph 1-CH2CH2-Te2-Np 1-CN,
R'-Phl-CH2CH2-Te2-Np2-CN, R'-Phl-CH2CH2-Te2-Np3-CN,
R' -Ph l-CH2CH2-Te2-Np4-CN, R' -Ph2-CH2CH2-Te 1-Ph 1-CN,
R' -Ph2-CH2CH2-Te 1-Ph2-CN, R' -Ph2-CH2CH2-Te 1-Ph3-CN,
R' -Ph2-CH2CH2-Te 1-Np 1-CN, R'-Ph2-CH2CH2-Te 1-Np2-CN,
R'-Ph2-CH2CH2-Tel-Np3-CN, R'-Ph2-CH2CH2-Tel-Np4-CN,
R'-Ph2-CH2CH2-Te2-Phl-CN, Rl-Ph2-CH2CH2-Te2-Ph2-CN, 11 R' -Ph2-CH2CH2-Te2-Ph3-
CN, R' -Ph2-CH2CH2-Te2-Np 1-CN,
R' -Ph2-CHZCH2-Te2-Np2-CN, R' -Ph2-CH2CH2-Te2-Np3-CN,
R'-Ph2-CH2CH2-Te2-Np4-CN, R'-Ph3-CH2CH2-Tel-Phl-CN,
R'-Ph3-CH2CH2-Tel-Ph2-CN, R'-Ph3-CH2CH2-Tel-Ph3-CN,
R'-Ph3-CHZCH2-Tel-Npl-CN, R'-Ph3-CH2CH2-Tel-Np2-CN,
R'-Ph3-CH2CH2-Tel-Np3-CN, R'-Ph3-CH2CH2-Tel-Np4-CN,
CA 02377217 2001-12-28
R'-Ph3-CH2CH2-Te2-Phl-CN, R'-Ph3-CH2CH2-Te2-Ph2-CN,
R' -Ph3-CH2CH2-Te2-Ph3-CN, R'-Ph3-CH2CH2-Te2-Np I -CN,
R'-Ph3-CH2CH2-Te2-Np2-CN, R'-Ph3-CH2CH2-Te2-Np3-CN,
R' -Ph3-CH2CH2-Te2-Np4-CN, R' -Np 1-CH2CH2-Te 1-Ph 1-CN,
5 R' -Np 1-CH2CH2-Te 1-Ph2-CN, R' -Np 1-CH2CH2-Te 1-Ph3-CN,
R' -Np l-CH2CH2-Te2-Ph l-CN, R' -Np 1-CH2CH2-Te2-Ph2-CN,
R' -Np 1-CH2CH2-Te2-Ph3-CN, R'-Np2-CH2CH2-Te 1-Ph 1-CN,
R'-Np2-CH2CH2-Tel-Ph2-CN, R'-Np2-CH2CH2-Tel-Ph3-CN,
R'-Np2-CH2CH2-Te2-Phl-CN, R'-Np2-CH2CH2-Te2-Ph2-CN,
10 R'-Np2-CH2CH2-Te2-Ph3-CN, R'-Np3-CHZCH2-Tel-Phl-CN,
R'-Np3-CH2CH2-Tel-Ph2-CN, R'-Np3-CH2CH2-Tel-Ph3-CN,
R'-Np3-CH2CH2-Te2-Phl-CN, R'-Np3-CH2CH2-Te2-Ph2-CN,
R'-Np3-CHZCH2-Te2-Ph3-CN, R'-Np4-CH2CH2-Tel-Phl-CN,
Rl-Np4-CHZCH2-Tel-Ph2-CN, R'-Np4-CH2CH2-Tel-Ph3-CN,
15 R'-Np4-CH2CH2-Te2-Phl-CN, R'-Np4-CH2CH2-Te2-Ph2-CN,
R1-Np4-CH2CH2-Te2-Ph3-CN,
inthecaseinwhichna=1,nb=0orna=0,nb=l,andn'=1,nd0orn 0,ndl,andZ
is a trifluoromethoxy group,
R'-Cy-Te1-Ph1-OCF3, R'-Cy-Te1-Ph2-OCF3, R'-Cy-Te1-Ph3-OCF3,
20 R'-Cy-Te1-Np1-OCF3, R'-Cy-Te1-Np2-OCF3, R'-Cy-Te1-Ph3-OCF3,
R' -Cy-Te 1-Np4-OCF3, R' -Cy-Te2-Ph 1-OCF3, R' -Cy-Te2-Ph2-OCF3,
R'-Cy-Te2-Ph3-OCF3, R'-Cy-Te2-Npl-OCF3, R'-Cy-Te2-Np2-OCF3,
R'-Cy-Te2-Ph3-OCF3, R'-Cy-Te2-Np4-OCF3,
R' -Cy-Te 1-CH2CH2-Ph2-OCF3, R' -Cy-Te 1-CH2CH2-Ph3-OCF3,
CA 02377217 2001-12-28
86
R'-Cy-Tel-CH2CH2-Npl-OCF3, R'-Cy-Tel-CH2CH2-Np2-OCF3,
R'-Cy-Tel-CHZCH2-Ph3-OCF3, R'-Cy-Tel-CH2CH2-Np4-OCF3,
R'-Cy-Te2-CH2CH2-Phl-OCF3, R'-Cy-Te2-CH2CH2-Ph2-OCF3,
R'-Cy-Te2-CHZCH2-Ph3-OCF3, R'-Cy-Te2-CH2CH2-Npl-OCF3,
R'-Cy-Te2-CHZCH2-Np2-OCF3, R'-Cy-Te2-CH2CH2-Ph3-OCF3,
R' -Cy-Te2-CH2CH2-Np4-OCF3,
R' -Ph 1-Te l-Ph 1-OCF3, R' -Ph 1-Te 1-Ph2-OCF3, R' -Ph 1-Te 1-Ph3 -OCF3,
R'-Phl-Tel-NpI-OCF3, R'-Phl-Tel-Np2-OCF3, R'-Phl-Tel-Ph3-OCF3,
R'-Ph1-Tel-Np4-OCF3, R'-Ph1-Te2-Ph1-OCF3, R'-Ph1-Te2-Ph2-OCF3,
R' -Ph 1-Te2-Ph3-OCF3, R'-Ph 1 -Te2-Np 1-OCF3, R'-Ph 1-Te2-Np2-OCF3,
R'-Phl-Te2-Ph3-OCF3, R'-Phl-Te2-Np4-OCF3,
R'-Ph1-Te1-CH2CH2-Ph2-OCF3, R'-Ph1-Te1-CH2CH2-Ph3-OCF3,
R' -Ph 1-Te 1-CH2CH2-Np 1-OCF3, R'-Phl -Te 1-CH2CH2-Np2-OCF3,
R'-Ph1-Te1-CH2CH2-Ph3-OCF3, R'-Ph1-Te1-CH2CH2-Np4-OCF3,
R'-Phl-Te2-CH2CHZ-PhI-OCF3, R'-Phl-Te2-CH2CH2-Ph2-OCF3,
R'-Phl-Te2-CH2CH2-Ph3-OCF3, R'-Phl-Te2-CH2CH2-Npl-OCF3,
R'-Phl-Te2-CH2CH2-Np2-OCF3, R'-Phl-Te2-CH2CH2-Ph3-OCF3,
R' -Ph l -Te2-CH2CH2-Np4-OCF3,
R' -Ph2-Te 1-Ph 1-OCF3, R' -Ph2-Te 1-Ph2-OCF3, R' -Ph2-Te 1-Ph3 -OCF3,
R'-Ph2-Te1-Np1-OCF3, R'-Ph2-Te1-Np2-OCF3, R'-Ph2-Te1-Ph3-OCF3,
R' -Ph2-Te 1-Np4-OCF3, R' -Ph2-Te2-Ph 1-OCF3, R' -Ph2-Te2-Ph2-OCF3,
R' -Ph2-Te2-Ph3 -OCF3, R' -Ph2-Te2-Np 1-OCF3, R' -Ph2-Te2-Np2-OCF3,
R' -Ph2-Te2-Ph3-OCF3, R' -Ph2-Te2-Np4-OCF3,
R'-Ph2-Tel-CH2CH2-Ph2-OCF3, R'-Ph2-Tel-CH2CH2-Ph3-OCF3,
CA 02377217 2001-12-28
87
R' -Ph2-Te 1-CHZCH2-Np 1-OCF3, R' -Ph2-Te 1-CH2CH2-Np2-OCF3,
R'-Ph2-Te l -CHZCH2-Ph3-OCF3, R'-Ph2-Te 1-CH2CH2-Np4-OCF3,
R'-Ph2-Te2-CH2CH2-Phl-OCF3, R'-Ph2-Te2-CH2CH2-Ph2-OCF3,
R' -Ph2-Te2-CH2CH2-Ph3 -OCF3, R' -Ph2-Te2-CH2CH2-Np 1-OCF3,
R'-Ph2-Te2-CH2CH2-Np2-OCF3, R'-Ph2-Te2-CH2CH2-Ph3-OCF3,
R' -Ph2-Te2-CH2CH2-Np4-OCF3,
R'-Ph3-Tel-PhI-OCF3, R'-Ph3-Tel-Ph2-OCF3, R'-Ph3-Tel-Ph3-OCF3,
R'-Ph3-Tel-Npl-OCF3, R'-Ph3-Tel-Np2-OCF3, R'-Ph3-Tel-Ph3-OCF3,
R'-Ph3-Tel-Np4-OCF3, R'-Ph3-Te2-Phl-OCF3, R'-Ph3-Te2-Ph2-OCF3,
R'-Ph3-Te2-Ph3-OCF3, R'-Ph3-Te2-Npl-OCF3, R'-Ph3-Te2-Np2-OCF3,
R'-Ph3-Te2-Ph3-OCF3, R'-Ph3-Te2-Np4-OCF3,
R'-Ph3-Tel-CH2CH2-Ph2-OCF3, R'-Ph3-Tel-CH2CH2-Ph3-OCF3,
R' -Ph3 -Te 1-CH2CH2-Np 1-OCF3, R' -Ph3 -Te 1-CH2CH2-Np2-OCF3,
R'-Ph3-Tel-CHZCH2-Ph3-OCF3, R'-Ph3-Tel-CH2CH2-Np4-OCF3,
R'-Ph3-Te2-CH2CH2-Phl-OCF3, R'-Ph3-Te2-CH2CH2-Ph2-OCF3,
R'-Ph3-Te2-CH2CH2-Ph3-OCF3, R'-Ph3-Te2-CH2CH2-Npl-OCF3,
R'-Ph3-Te2-CH2CH2-Np2-OCF3, R'-Ph3-Te2-CH2CH2-Ph3-OCF3,
R' -Ph3 -Te2-CH2CH2-Np4-OCF3,
R'-Npl-Tel-PhI-OCF3, R'-Npl-Tel-Ph2-OCF3, R'-Npl-Tel-Ph3-OCF3,
R'-Np1-Te1-Ph3-OCF3, R'-Np1-Te2-Ph1-OCF3, R'-Np1-Te2-Ph2-OCF3,
R'-Npl-Te2-Ph3-OCF3, R'-Npl-Te2-Ph3-OCF3,
R' -Np 1-Te l-CH2CH2-Ph2-OCF3, R' -Np 1-Te l-CH2CH2-Ph3 -OCF3,
R' -Np 1-Te 1-CH2CH2-Np 1-OCF3, R' -Np 1-Te 1-CH2CH2-Np2-OCF3,
R' -Np 1-Te 1-CH2CH2-Ph3-OCF3, R' -Np 1-Te2-CH2CHz-Ph 1-OCF3,
CA 02377217 2001-12-28
88
R' -Np 1-Te2-CH2CH2-Ph2-OCF3, R'-Np 1-Te2-CHZCH2-Ph3-OCF3,
R' -Np 1-Te2-CH2CH2-Ph3-OCF3,
R'-Np2-Te1-Ph1-OCF3, R'-Np2-Te1-Ph2-OCF3, R'-Np2-Te1-Ph3-OCF3,
R'-Np2-Tel-Ph3-OCF3, R'-Np2-Tel-Np4-OCF3, R'-Np2-Te2-Ph1-OCF3,
R'-Np2-Te2-Ph2-OCF3, R'-Np2-Te2-Ph3-OCF3, R'-Np2-Te2-Ph3-OCF3,
R' -Np2-Te2-Np4-OCF3,
R'-Np2-Te 1-CH2CH2-Ph2-OCF3, R'-Np2-Te 1-CHZCH2-Ph3-OCF3,
R'-Np2-Tel-CH2CH2-Ph3-OCF3, R'-Np2-Tel-CH2CH2-Np4-OCF3,
R'-Np2-Te2-CH2CH2-Phl-OCF3, R'-Np2-Te2-CH2CH2-Ph2-OCF3,
R'-Np2-Te2-CH2CH2-Ph3-OCF3,
R'-Ph3-Tel-PhI-OCF3, R'-Ph3-Tel-Ph2-OCF3, R'-Ph3-Tel-Ph3-OCF3,
R'-Ph3-Tel-NpI-OCF3, R'-Ph3-Tel-Np2-OCF3, R'-Ph3-Te1-Ph3-OCF3,
R'-Ph3-Tel-Np4-OCF3, R'-Ph3-Te2-Phl-OCF3, R'-Ph3-Te2-Ph2-OCF3,
R'-Ph3-Te2-Ph3-OCF3, R'-Ph3-Te2-Npl-OCF3, R'-Ph3-Te2-Np2-OCF3,
R'-Ph3-Te2-Ph3-OCF3, R'-Ph3-Te2-Np4-OCF3,
R'-Ph3-Tel-CH2CHZ-Ph2-OCF3, R'-Ph3-Tel-CH2CH2-Ph3-OCF3,
R' -Ph3-Te 1-CHZCH2-Np 1-OCF3, R'-Ph3-Te 1-CH2CH2-Np2-OCF3,
R'-Ph3-Tel-CHZCH2-Ph3-OCF3, R'-Ph3-Tel-CH2CH2-Np4-OCF3,
R'-Ph3-Te2-CH2CH2-Phl -OCF3, R'-Ph3-Te2-CH2CH2-Ph2-OCF3,
R'-Ph3-Te2-CH2CH2-Ph3-OCF3, R'-Ph3-Te2-CH2CH2-Npl-OCF3,
R'-Ph3-Te2-CH2CH2-Np2-OCF3, R'-Ph3-Te2-CH2CH2-Ph3-OCF3,
R' -Ph3 -Te2-CH2CH2-Np4-OC F3,
R' -Np4-Te 1-Phl -OCF3, R'-Np4-Te 1-Ph2-OCF3, R'-Np4-Te 1-Ph3-OCF3,
R' -Np4-Te 1-Np 1-OCF3, R' -Np4-Te 1-Np2-OCF3, R' -Np4-Te 1-Ph3 -OCF3,
CA 02377217 2001-12-28
89
R' -Np4-Te l-Np4-OCF3, R' -Np4-Te2-Ph 1-OCF3, R' -Np4-Te2-Ph2-OCF3,
R'-Np4-Te2-Ph3-OCF3, R'-Np4-Te2-Npl-OCF3, R'-Np4-Te2-Np2-OCF3,
R'-Np4-Te2-Ph3-OCF3, R'-Np4-Te2-Np4-OCF3,
R' -Np4-Te 1-CH2CH2-Ph2-OCF3, R' -Np4-Te l -CH2CH2-Ph3-OCF3,
R' -Np4-Te2-CH2CH2-Ph 1-OCF3, R' -Np4-Te2-CH2CH2-Ph2-OCF3,
R'-Np4-Te2-CHZCH2-Ph3-OCF3, R'-Cy-CH2CH2-Tel-PhI-OCF3,
R'-Cy-CH2CH2-Tel-Ph2-OCF3, R'-Cy-CH2CH2-Tel-Ph3-OCF3,
R'-Cy-CH2CH2-Tel-Npl-OCF3, R'-Cy-CH2CH2-Tel-Np2-OCF3,
R' -Cy-CH2CH2-Te l -Ph3-OCF3, R'-Cy-CH2CH2-Te 1-Np4-OCF3,
R'-Cy-CH2CH2-Te2-Phl-OCF3, R'-Cy-CH2CH2-Te2-Ph2-OCF3,
R'-Cy-CHZCH2-Te2-Ph3-OCF3, R'-Cy-CH2CH2-Te2-Npl-OCF3,
R'-Cy-CH2CH2-Te2-Np2-OCF3, R'-Cy-CH2CH2-Te2-Ph3-OCF3,
R' -Cy-CH2CH2-Te2-Np4-OCF3, R' -Ph i-CH2CH2-Te 1-Ph 1-OCF3,
R'-Ph1-CH2CH2-Te1-Ph2-OCF3, R'-Ph1-CH2CH2-Te1-Ph3-OCF3,
R'-Ph1-CH2CH2-Te1-Np1-OCF3, R'-Ph1-CH2CH2-Te1-Np2-OCF3,
R' -Ph 1-CH2CH2-Te 1-Ph3-OCF3, R' -Ph 1-CH2CH2-Te 1-Np4-OCF3,
R' -Ph 1-CH2CH2-Te2-Ph 1-OCF3, R' -Ph 1-CH2CH2-Te2-Ph2-OCF3,
R'-Phl-CH2CH2-Te2-Ph3-OCF3, R'-Phl-CH2CH2-Te2-Npl-OCF3,
R'-Phl-CH2CH2-Te2-Np2-OCF3, R'-Phl-CH2CH2-Te2-Ph3-OCF3,
R' -Ph 1-CH2CH2-Te2-Np4-OCF3, R' -Ph2-CH2CH2-Te 1-Ph 1-OCF3,
Rl-Ph2-CH2CH2-Tel-Ph2-OCF3, R'-Ph2-CH2CH2-Tel-Ph3-OCF3,
R' -Ph2-CH2CH2-Te 1-Np 1-OCF3, R' -Ph2-CH2CH2-Te 1-Np2-OCF3,
R' -Ph2-CH2CH2-Te 1-Ph3-OCF3, R' -Ph2-CHZCHZ-Te 1-Np4-OCF3,
R' -Ph2-CH2CH2-Te2-Phl -OCF3, R'-Ph2-CH2CH2-Te2-Ph2-OCF3,
CA 02377217 2001-12-28
R'-Ph2-CH2CH2-Te2-Ph3-OCF3, R'-Ph2-CH2CH2-Te2-Npl-OCF3,
R'-Ph2-CH2CH2-Te2-Np2-OCF3, R'-Ph2-CH2CH2-Te2-Ph3-OCF3,
R'-Ph2-CHZCH2-Te2-Np4-OCF3, R'-Ph3-CH2CH2-Tel-PhI-OCF3,
R'-Ph3-CH2CH2-Tel-Ph2-OCF3, R'-Ph3-CH2CH2-Tel-Ph3-OCF3,
5 R' -Ph3-CH2CH2-Te 1-Np 1-OCF3, Rt-Ph3-CH2CH2-Te 1-Np2-OCF3,
R'-Ph3-CHZCH2-Tel-Ph3-OCF3, R'-Ph3-CH2CH2-Tel-Np4-OCF3,
Rl-Ph3-CH2CH2-Te2-Phl-OCF3, R'-Ph3-CH2CH2-Te2-Ph2-OCF3,
R' -Ph3-CH2CH2-Te2-Ph3-OCF3, R' -Ph3-CH2CH2-Te2-Np 1-OCF3,
Rl-Ph3-CH2CH2-Te2-Np2-OCF3, R'-Ph3-CH2CH2-Te2-Ph3-OCF3,
10 R'-Ph3-CH2CH2-Te2-Np4-OCF3, R'-Npl-CH2CH2-Tel-PhI-OCF3,
R' -Np 1-CH2CH2-Te 1-Ph2-OCF3, R'-Np 1-CH2CH2-Te 1-Ph3-OCF3,
R' -Np 1-CH2CH2-Te 1-Np 1-OCF3, R'-Np 1-CH2CH2-Te 1-Np2-OCF3,
R'-Np1-CH2CH2-Te1-Ph3-OCF3, R'-Np1-CHZCH2-Te1-Np4-OCF3,
R' -Np 1-CH2CH2-Te2-Ph 1-OCF3, R' -Np 1-CH2CH2-Te2-Ph2-OCF3,
15 Ri-Npl-CH2CH2-Te2-Ph3-OCF3, R'-Np2-CH2CH2-Tel-Phl-OCF3,
R' -Np2-CH2CHz-Te 1-Ph2-OCF3, R' -Np2-CH2CH2-Te 1-Ph3-OCF3,
R'-Np2-CH2CH2-Tel-NpI-OCF3, Rl-Np2-CH2CH2-Tel-Np2-OCF3,
R'-Np2-CH2CH2-Tel-Ph3-OCF3, R'-Np2-CH2CH2-Tel-Np4-OCF3,
R'-Np2-CHZCH2-Te2-Phl-OCF3, R'-Np2-CH2CH2-Te2-Ph2-OCF3,
20 R'-Np2-CH2CH2-Te2-Ph3-OCF3, R'-Ph3-CH2CH2-Tel-Phl-OCF3,
R1-Ph3-CH2CH2-Tel-Ph2-OCF3, R'-Ph3-CH2CH2-Tel-Ph3-OCF3,
Rl-Ph3-CH2CH2-Tel-NpI-OCF3, Rl-Ph3-CH2CH2-Tel-Np2-OCF3,
R'-Ph3-CH2CH2-Tel-Ph3-OCF3, R'-Ph3-CH2CH2-Tel-Np4-OCF3,
R'-Ph3-CH2CH2-Te2-Phl-OCF3, R'-Ph3-CH2CH2-Te2-Ph2-OCF3.
CA 02377217 2001-12-28
91
R'-Ph3-CH2CH2-Te2-Ph3-OCF3, Rl-Ph3-CH2CH2-Te2-Npl-OCF3,
R'-Ph3-CH2CH2-Te2-Np2-OCF3, R'-Ph3-CH2CHZ-Te2-Ph3-OCF3,
R'-Ph3-CH2CH2-Te2-Np4-OCF3, R'-Np4-CH2CH2-Te1-Phl-OCF3,
R'-Np4-CH2CH2-Tel-Ph2-OCF3, R'-Np4-CH2CH2-Tel-Ph3-OCF3,
R'-Np4-CH2CH2-Te2-Phl-OCF3, R'-Np4-CH2CH2-Te2-Ph2-OCF3,
R'-Np4-CHZCH2-Te2-Ph3-OCF3, R'-Np4-CH2CH2-Te2-Ph3-OCF3,
R' -Ph 1-C=C-Te 1-Ph l-OCF3i R' -Ph 1-C-C-Te 1-Ph2-OCF3,
R'-PhI-C=C-Tel-Ph3-OCF3, R'-Phl-C-C-Te1-Np1-OCF3,
R'-PhI-C_C-Tel-Np2-OCF3, R'-PhI-C-C-Te1-Ph3-OCF3,
R' -Ph 1-C=C-Te 1-Np4-OCF3, R' -Ph 1-C=C-Te2-Ph 1-OCF3,
R' -Ph 1-C=C-Te2-Ph2-OCF3, R'-Ph 1-C=C-Te2-Ph3-OCF3,
R' -Ph 1-C=C-Te2-Np 1-OCF3, R' -Ph 1-C=C-Te2-Np2-OCF3,
R' -Ph 1-C=C-Te2-Ph3 -OCF3, R' -Ph 1-C-C-Te2-Np4-OCF3,
R' -Ph2-C-C-Te 1-Ph i-OCF3, R'-Ph2-C-C-Te 1-Ph2-OCF3,
R' -Ph2-C=C-Te 1-Ph3 -OCF3, R' -Ph2-C=C-Te 1-Np 1-OCF3,
Rl-Ph2-C-C-Tel-Np2-OCF3, R'-Ph2-C=C-Tel-Ph3-OCF3,
R' -Ph2-C-C-Te 1-Np4-OCF3, R' -Ph2-C=C-Te2-Ph 1-OCF3,
R'-Ph2-C=C-Te2-Ph2-OCF3, R'-Ph2-C=C-Te2-Ph3-OCF3,
R'-Ph2-C=C-Te2-Npl-OCF3, R'-Ph2-C=C-Te2-Np2-OCF3,
R'-Ph2-C-C-Te2-Ph3-OCF3, R'-Ph2-C=C-Te2-Np4-OCF3,
R'-Ph3-C-C-Tel-PhI-OCF3, R'-Ph3-C=C-Tel-Ph2-OCF3,
R'-Ph3-C=C-Te 1-Ph3-OCF3, R'-Ph3-C=C-Te 1-Np 1-OCF3,
R'-Ph3-C=C-Te 1-Np2-OCF3, R'-Ph3-C=C-Te 1-Ph3-OCF3,
CA 02377217 2001-12-28
92
R'-Ph3-C=C-Tel-Np4-OCF3, R'-Ph3-C=-C-Te2-Phl-OCF3,
R'-Ph3-C=C-Te2-Ph2-OCF3, R'-Ph3-C_C-Te2-Ph3-OCF3,
R'-Ph3-C=C-Te2-Npl-OCF3, R'-Ph3-C=C-Te2-Np2-OCF3,
R'-Ph3-C=C-Te2-Ph3-OCF3, R'-Ph3-C=C-Te2-Np4-OCF3,
R'-Ph3-C=C-Tel-PhI-OCF3, R'-Ph3-C-C-Tel-Ph2-OCF3,
R'-Ph3-C=C-Tel-Ph3-OCF3, R'-Ph3-C=C-Tel-NpI-OCF3,
R'-Ph3-C=C-Tel-Np2-OCF3, R'-Ph3-C=C-Tel-Ph3-OCF3,
R'-Ph3-C=C-Tel-Np4-OCF3, R'-Ph3-C-C-Te2-Phl-OCF3,
R'-Ph3-C=C-Te2-Ph2-OCF3, R'-Ph3-C=C-Te2-Ph3-OCF3,
R'-Ph3-C-C-Te2-Np 1=OCF3, R'-Ph3-C-C-Te2-Np2-OCF3,
R'-Ph3-C=C-Te2-Ph3-OCF3, R'-Ph3-C=C-Te2-Np4-OCF3,
in the case in which na = nb = 0, and n'= nd = 1, and Z is a fluorine atom,
R' -Te l-Cy-Phl -F, R' -Te l-Cy-Ph2-F, R'-Te l-Cy-Ph3-F, R' -Te 1-Cy-Np 1-F,
R'-Tel-Cy-Np2-F, R'-Tel-Cy-Np3-F, R'-Tel-Cy-Np4-F, R'-Te2-Cy-Ph1-F,
R'-Te2-Cy-Ph2-F, R'-Te2-Cy-Ph3-F, R'-Te2-Cy-NpI-F, R'-Te2-Cy-Np2-F,
R'-Te2-Cy-Np3-F, R'-Te2-Cy-Np4-F,
R'-Tel-Cy-CHZCH2-Ph2-F, R'-Tel-Cy-CHZCH2-Ph3-F, R'-Tel-Cy-CHZCH2-Npl-F,
R' -Te 1-Cy-CH2CH2-Np2-F, R' -Te l-Cy-CH2CH2-Np3 -F, R' -Te l-Cy-CH2CH2-Np4-F,
R'-Te2-Cy-CHZCH2-Phl-F, R'-Te2-Cy-CH2CH2-Ph2-F, R'-Te2-Cy-CH2CH2-Ph3-F,
R'-Te2-Cy-CHZCHZ-Npl-F, R'-Te2-Cy-CH2CH2-Np2-F, R'-Te2-Cy-CH2CH2-Np3-F,
R 1-Te2-Cy-CH2CH2-Np4-F, R' -Te 1-CH2CH2-Cy-Ph 1-F, R' -Te 1-CH2CH2-Cy-Ph2-F,
R' -Te l-CH2CH2-Cy-Ph3-F, R' -Te l-CH2CH2-Cy-Np 1-F, R' -Te 1-CH2CHZ-Cy-Np2-F,
R' -Te l-CH2CH2-Cy-Np3 -F, R' -Te l-CH2CH2-Cy-Np4-F, R' -Te2-CH2CH2-Cy-Ph 1-F,
R' -Te2-CH2CH2-Cy-Ph2-F, R' -Te2-CH2CH2-Cy-Ph3-F, R' -Te2-CH2CH2-Cy-Np 1-F,
CA 02377217 2001-12-28
93
R' -Te2-CH2CH2-Cy-Np2-F, R'-Te2-CH2CH2-Cy-Np3-F, R' -Te2-CH2CH2-Cy-Np4-F,
R'-Tel-Phl-Phl-F, R'-Tel-Phl-Ph2-F, R'-Tel-Phl-Ph3-F, R'-Tel-Phl-Npl-F,
R'-Tel-Phl-Np2-F, R'-Te1-Ph1-Np3-F, R'-Te1-Ph1-Np4-F, R'-Te2-Ph1-Ph1-F,
R'-Te2-Ph1-Ph2-F, R'-Te2-Ph1-Ph3-F, R'-Te2-Ph1-Np1-F, R'-Te2-Ph1-Np2-F,
R'-Te2-Phl-Np3-F, R'-Te2-Phl-Np4-F,
R'-Te1-Ph1-CH2CH2-Ph2-F, R'-Te1-Ph1-CH2CH2-Ph3-F, R'-Te1-Ph1-CH2CH2-Np1-F,
R'-Tel-Ph1-CH2CH2-Np2-F, R'-Te1-Ph1-CHZCH2-Np3-F, R'-Tel-Ph1-CH2CH2-Np4-F,
R'-Te2-Phl-CH2CH2-Phl-F, R'-Te2-Phl-CH2CH2-Ph2-F, R'-Te2-Phl-CH2CH2-Ph3-F,
R'-Te2-Phl-CH2CH2-Npl-F, R'-Te2-Phl-CH2CH2-Np2-F, Rl-Te2-Phl-CH2CH2-Np3-F,
R'-Te2-Phl-CH2CH2-Np4-F,
R'-Te 1-Ph 1-C=C-Ph2-F, Rl-Te 1-Ph 1-C=C-Ph3-F, Rl-Te 1-Ph 1-C-C-Np 1-F,
R'-Te1-Ph1-C=C-Np2-F, R'-Te1-Ph1-C=C-Np3-F, Rl-Te1-Ph1-C=C-Np4-F,
R'-Te2-Phl-C=C-Phl-F, R'-Te2-Phl-C-C-Ph2-F, R'-Te2-Phl-C=C-Ph3-F,
R'-Te2-Phl-C=C-NpI-F, R'-Te2-Phl-C-C-Np2-F, Rl-Te2-Phl-C=C-Np3-F,
R'-Te2-Phl-C=C-Np4-F,
R'-Te1-Ph2-Ph1-F, R'-Te1-Ph2-Ph2-F, R'-Te1-Ph2-Ph3-F, R'-Tel-Ph2-Np1-F,
R' -Te l-Ph2-Np2-F, R' -Te 1-Ph2-Np3 -F, R' -Te 1-Ph2-Np4-F, R' -Te2-Ph2-Ph 1-
F,
R'-Te2-Ph2-Ph2-F, R'-Te2-Ph2-Ph3-F, R'-Te2-Ph2-Npl-F, R'-Te2-Ph2-Np2-F,
R'-Te2-Ph2-Np3-F, R'-Te2-Ph2-Np4-F,
R'-Tel-Ph2-CH2CH2-Ph2-F, R'-Tel-Ph2-CH2CH2-Ph3-F, Rl-Tel-Ph2-CH2CH2-Npl-F,
R' -Te l-Ph2-CH2CH2-Np2-F, R' -Te 1-Ph2-CH2CH2-Np3-F, R'-Te 1-Ph2-CH2CH2-Np4-
F,
R'-Te2-Ph2-CH2CH2-Phi-F, R'-Te2-Ph2-CH2CH2-Ph2-F, R'-Te2-Ph2-CH2CH2-Ph3-F,
R'-Te2-Ph2-CH2CH2-Npl-F, R'-Te2-Ph2-CH2CH2-Np2-F, R'-Te2-Ph2-CHZCH2-Np3-F,
R' -Te2-Ph2-CH2CH2-Np4-F,
CA 02377217 2001-12-28
94
R' -Te l-Ph2-C=C-Ph2-F, R' -Te 1-Ph2-C=C-Ph3-F, R' -Te2-Ph2-C=C-Ph 1-F,
R'-Te2-Ph2-C=C-Ph2-F, R'-Te2-Ph2-C=C-Ph3-F,
R'-Tel-Ph3-Phl-F, R'-Tel-Ph3-Ph2-F, R'-Tel-Ph3-Ph3-F, R'-Tel-Ph3-Npl-F,
R'-Te l-Ph3-Np2-F, R' -Te l-Ph3-Np3-F, R'-Te 1-Ph3-Np4-F, R'-Te2-Ph3-Ph 1-F,
R'-Te2-Ph3-Ph2-F, R'-Te2-Ph3-Ph3-F, R'-Te2-Ph3-Npl-F, R'-Te2-Ph3-Np2-F,
R'-Te2-Ph3-Np3-F, R'-Te2-Ph3-Np4-F,
R'-Tel-Ph3-CH2CHZ-Ph2-F, R'-Tel-Ph3-CH2CH2-Ph3-F, R'-Tel-Ph3-CH2CH2-Npl-F,
R'-Tel-Ph3-CH2CH2-Np2-F, R'-Tel-Ph3-CH2CH2-Np3-F, R'-Tel-Ph3-CH2CH2-Np4-F,
R'-Te2-Ph3-CH2CH2-Phl -F, R'-Te2-Ph3-CH2CH2-Ph2-F, R'-Te2-Ph3-CH2CH2-Ph3-F,
R'-Te2-Ph3-CH2CH2-Npl-F, R'-Te2-Ph3-CH2CH2-Np2-F, R'-Te2-Ph3-CHZCHZ-Np3-F,
R' -Te2-Ph3 -CH2CH2-Np4-F,
R'-Tel-Ph3-C-C-Ph2-F, R'-Tel-Ph3-C=C-Ph3-F, R'-Te2-Ph3-C=C-Phl-F,
R'-Te2-Ph3-C-C-Ph2-F, R'-Te2-Ph3-C=C-Ph3-F,
R' -Te 1-Np 1-Ph l-F, R' -Te l-Np 1-Ph2-F, R'-Te 1-Np 1-Ph3-F, R'-Te2-Np l-Ph
1-F,
R' -Te2-Np 1-Ph2-F, R' -Te2-Np 1-Ph3 -F,
R'-Te1-Np1-CH2CH2-Ph2-F, R'-Te1-Np1-CH2CH2-Ph3-F, R'-Te2-Np1-CH2CH2-Ph1-F,
R'-Te2-Np1-CHZCHZ-Ph2-F, R'-Te2-Np1-CH2CH2-Ph3-F,
R'-Tel-Np2-Phl-F, Rl-Tel-Np2-Ph2-F, R'-Tel-Np2-Ph3-F, R'-Te2-Np2-Phl-F,
R' -Te2-Np2-Ph2-F, R' -Te2-Np2-Ph3-F,
R'-Tel-Np2-CH2CH2-Ph2-F, R'-Tel-Np2-CH2CH2-Ph3-F, R'-Te2-Np2-CH2CH2-Phl-F,
R'-Te2-Np2-CH2CHZ-Ph2-F, R'-Te2-Np2-CH2CH2-Ph3-F,
R'-Tel-Np3-Phl-F, R'-Tel-Np3-Ph2-F, R'-Tel-Np3-Ph3-F, R'-Te2-Np3-Phl-F,
R'-Te2-Np3-Ph2-F, R'-Te2-Np3-Ph3-F,
R'-Tel-Np3-CH2CH2-Ph2-F, R'-Tel-Np3-CH2CH2-Ph3-F, R'-Te2-Np3-CH2CH2-Phl-F,
CA 02377217 2001-12-28
R'-Te2-Np3-CH2CH2-Ph2-F, R'-Te2-Np3-CH2CH2-Ph3-F,
R'-Te1-Np4-Ph1-F, R'-Tel-Np4-Ph2-F, R'-Te1-Np4-Ph3-F, R'-Te2-Np4-Ph1-F,
R'-Te2-Np4-Ph2-F, R'-Te2-Np4-Ph3-F,
R'-Tel-Np4-CH2CH2-Ph2-F, R'-Tel-Np4-CH2CH2-Ph3-F, R'-Te2-Np4-CH2CH2-Phl-F,
5 R'-Te2-Np4-CH2CH2-Ph2-F, R'-Te2-Np4-CH2CH2-Ph3-F,
R'-Tel-CHZCH2-Phl-Phl-F, R'-Tel-CH2CH2-Phl-Ph2-F, R'-Tel-CH2CH2-Phl-Ph3-F,
R'-Tel-CH2CH2-Phl-Npl-F, R'-Tel-CH2CH2-Ph1-Np2-F, R'-Tel-CH2CH2-Phl-Np3-F,
R'-Tel-CH2CH2-Phl-Np4-F, R'-Te2-CH2CH2-Phl-Phl-F, R'-Te2-CH2CH2-Phl-Ph2-F,
R'-Te2-CHZCH2-Phl-Ph3-F, R'-Te2-CH2CH2-Phl-Npl-F, R'-Te2-CH2CH2-Phl-Np2-F,
10 R'-Te2-CH2CH2-Phl-Np3-F, R'-Te2-CH2CH2-Phl-Np4-F, R'-Tel-CH2CH2-Ph2-Phl-F,
R'-Tel-CH2CH2-Ph2-Ph2-F, R'-Tel-CH2CH2-Ph2-Ph3-F, R'-Tel-CH2CH2-Ph2-Npl-F,
R' -Te l-CHZCH2-Ph2-Np2-F, R' -Te 1-CH2CH2-Ph2-Np3 -F, R' -Te 1-CH2CH2-Ph2-Np4-
F,
R' -Te2-CH2CH2-Ph2-Ph l -F, R' -Te2-CH2CH2-Ph2-Ph2-F, R' -Te2-CH2CH2-Ph2-Ph3 -
F,
R'-Te2-CH2CH2-Ph2-Npl-F, R'-Te2-CH2CH2-Ph2-Np2-F, R'-Te2-CH2CH2-Ph2-Np3-F,
15 R'-Te2-CH2CH2-Ph2-Np4-F, R'-Tel-CH2CH2-Ph3-Phl-F, R'-Tel-CHzCH2-Ph3-Ph2-F,
R'-Tel-CH2CH2-Ph3-Ph3-F, R'-Tel-CH2CH2-Ph3-Npl-F, R'-Tel-CH2CH2-Ph3-Np2-F,
R'-Tel-CH2CH2-Ph3-Np3-F, R'-Tel-CH2CH2-Ph3-Np4-F, R'-Te2-CH2CH2-Ph3-Phl-F,
R'-Te2-CHZCH2-Ph3-Ph2-F, R'-Te2-CH2CH2-Ph3-Ph3-F, R'-Te2-CH2CH2-Ph3-Npl-F,
R'-Te2-CH2CH2-Ph3-Np2-F, R'-Te2-CH2CH2-Ph3-Np3-F, R'-Te2-CHZCHZ-Ph3-Np4-F,
20 R'-Te1-CH2CH2-Np1-Ph1-F, R'-Tel-CH2CH2-Np1-Ph2-F, R'-Te1-CH2CHZ-Np1-Ph3-F,
R'-Te2-CH2CH2-Npl-Phl-F, R'-Te2-CH2CH2-Npl-Ph2-F, R'-Te2-CH2CH2-Npl-Ph3-F,
R'-Tel-CH2CH2-Np2-Phl-F, R'-Tel-CH2CH2-Np2-Ph2-F, R'-Tel-CH2CH2-Np2-Ph3-F,
R'-Te2-CH2CH2-Np2-Phl-F, R'-Te2-CH2CH2-Np2-Ph2-F, R'-Te2-CH2CH2-Np2-Ph3-F,
Rl-Tel-CHZCH2-Np2-CH2CH2-Ph2-F, R'-Tel-CH2CH2-Np2-CHZCH2-Ph3-F,
CA 02377217 2001-12-28
96
R'-Te2-CH2CH2-Np2-CH2CH2-Phl-F, R'-Te2-CH2CH2-Np2-CH2CH2-Ph2-F,
R' -Te2-CH2CH2-Np2-CH2CH2-Ph3 -F,
R'-Tel-CH2CH2-Np3-Phl-F, R'-Tel-CHZCH2-Np3-Ph2-F, R'-Tel-CH2CH2-Np3-Ph3-F,
R1-Te2-CH2CH2-Np3-Phl-F, R'-Te2-CH2CH2-Np3-Ph2-F, R'-Te2-CH2CH2-Np3-Ph3-F,
R' -Te l-CH2CH2-Np4-Ph 1-F, R' -Te 1-CH2CH2-Np4-Ph2-F, R' -Te 1-CHZCH2-Np4-Ph3
-F,
R' -Te 1-C=C-Ph l-Ph 1-F, R' -Te 1-C=C-Ph 1-Ph2-F, R' -Te 1-C-C-Ph l-Ph3 -F,
R' -Te2-C=C-Ph 1-Ph 1-F, R' -Te2-C=C-Ph l-Ph2-F, R' -Te2-C=C-Ph 1-Ph3 -F,
R'-TeI-C=C-Ph2-Phl-F, R'-Tel-C=C-Ph2-Ph2-F, R'-TeI-C=C-Ph2-Ph3-F,
R'-Te2-C=C-Ph2-Phl-F, R'-Te2-C=C-Ph2-Ph2-F, R'-Te2-C-C-Ph2-Ph3-F,
R'-Te1-C=C-Ph3-Ph1-F, R'-Tel-C=C-Ph3-Ph2-F, R'-Tel-C-C-Ph3-Ph3-F,
R'-Te2-C=C-Ph3-Phl-F, R'-Te2-C=C-Ph3-Ph2-F, R'-Te2-C=C-Ph3-Ph3-F,
in the case in which na = nb = 0, and n = nd = 1, and Z is a cyano group,
R'-Tel-Cy-Ph1-CN, R'-Te1-Cy-Ph2-CN, R'-Te1-Cy-Ph3-CN, R'-Te1-Cy-Np1-CN,
R' -Te l-Cy-Np2-CN, R' -Te 1-Cy-Np3-CN, R' -Te l-Cy-Np4-CN, Rl -Te2-Cy-Ph 1-
CN,
R'-Te2-Cy-Ph2-CN, R'-Te2-Cy-Ph3-CN, R'-Te2-Cy-NpI-CN, R'-Te2-Cy-Np2-CN,
R' -Te2-Cy-Np3 -CN, R' -Te2-Cy-Np4-CN,
R' -Te l-Cy-CH2CH2-Ph2-CN, R' -Te 1-Cy-CH2CH2-Ph3-CN, R' -Te 1-Cy-CH2CH2-Np 1-
CN,
R'-Tel-Cy-CH2CH2-Np2-CN, R'-Tel-Cy-CH2CH2-Np3-CN, R'-Tel-Cy-CH2CH2-Np4-CN,
R'-Te2-Cy-CH2CH2-Phl-CN, R'-Te2-Cy-CH2CH2-Ph2-CN, R'-Te2-Cy-CH2CH2-Ph3-CN,
R'-Te2-Cy-CH2CH2-Npl-CN, R'-Te2-Cy-CH2CH2-Np2-CN, R'-Te2-Cy-CH2CH2-Np3-CN,
R' -Te2-Cy-CH2CH2-Np4-CN, R' -Te l-CH2CH2-Cy-Ph 1-CN, R' -Te l-CHZCH2-Cy-Ph2-
CN,
R' -Te l-CH2CH2-Cy-Ph3 -CN, R' -Te 1-CH2CH2-Cy-Np 1-CN, R' -Te 1-CH2CH2-Cy-Np2-
CN,
R1-Tel-CH2CH2-Cy-Np3-CN, R'-Tel-CH2CH2-Cy-Np4-CN, R'-Te2-CH2CH2-Cy-PhI-CN,
R'-Te2-CHZCH2-Cy-Ph2-CN, R'-Te2-CH2CH2-Cy-Ph3-CN, R'-Te2-CH2CH2-Cy-Npl-CN,
CA 02377217 2001-12-28
97
R' -Te2-CH2CH2-Cy-Np2-CN, Rl-Te2-CH2CH2-Cy-Np3-CN, R' -Te2-CH2CH2-Cy-Np4-CN,
R' -Te 1-CH2CH2-Cy-CH2CH2-Ph2-CN,
R'-Tel-Phl-Phl-CN, R'-Tel-Phl-Ph2-CN, R'-Tel-Phl-Ph3-CN,
R'-Te1-Ph1-Np1-CN, R'-Te1-Ph1-Np2-CN, R'-Te1-Ph1-Np3-CN,
R' -Te l-Ph l-Np4-CN, R' -Te2-Ph l-Ph l-CN, R' -Te2-Ph l-Ph2-CN,
R' -Te2-Ph 1-Ph3 -CN, R' -Te2-Ph 1-Np 1-CN, R' -Te2-Ph 1-Np2-CN,
R'-Te2-Phl-Np3-CN, R'-Te2-Ph1-Np4-CN,
R' -Te 1-Ph 1-CH2CH2-Ph2-CN, R' -Te 1-Ph l-CH2CH2-Ph3-CN,
R' -Te 1-Ph 1-CH2CH2-Np 1-CN, R' -Te 1-Ph 1-CH2CH2-Np2-CN,
R'-Tel-Phl-CH2CH2-Np3-CN, R'-Tel-Phl-CHZCH2-Np4-CN,
R' -Te2-Ph l-CH2CH2-Ph 1-CN, R' -Te2-Ph 1-CHZCH2-Ph2-CN,
R'-Te2-Phl-CH2CH2-Ph3-CN, R'-Te2-Phl-CH2CH2-Npl-CN,
R' -Te2-Ph 1-CH2CH2-Np2-CN, R' -Te2-Ph 1-CH2CH2-Np3 -CN,
R' -Te2-Ph 1-CH2CH2-Np4-CN,
R' -Te l-Ph l-C=C-Ph2-CN, R' -Te l-Ph l-C=C-Ph3-CN, R' -Te2-Ph l-C=C-Ph l-CN,
R' -Te2-Ph 1-C=C-Ph2-CN, R'-Te2-Ph 1-C=C-Ph3-CN,
R' -Te 1-Ph2-Ph 1-CN, R' -Te 1-Ph2-Ph2-CN, R' -Te 1-Ph2-Ph3 -CN,
R' -Te 1-Ph2-Np 1-CN, R' -Te 1-Ph2-Np2-CN, R' -Te 1-Ph2-Np3 -CN,
R' -Te l-Ph2-Np4-CN, R' -Te2-Ph2-Ph 1-CN, R'-Te2-Ph2-Ph2-CN,
R'-Te2-Ph2-Ph3-CN, R'-Te2-Ph2-Npl-CN, R'-Te2-Ph2-Np2-CN,
R' -Te2-Ph2-Np3-CN, R' -Te2-Ph2-Np4-CN,
R' -Te 1-Ph2-CHzCH2-Ph2-CN, R' -Te 1-Ph2-CH2CH2-Ph3 -CN,
R' -Te 1-Ph2-CHZCH2-Np 1-CN, R' -Te 1-Ph2-CH2CH2-Np2-CN,
R'-Tel-Ph2-CHZCH2-Np3-CN, R'-Tel-Ph2-CHZCH2-Np4-CN,
CA 02377217 2001-12-28
98
R'-Te2-Ph2-CH2CH2-Phl-CN, R'-Te2-Ph2-CH2CH2-Ph2-CN,
R' -Te2-Ph2-CH2CH2-Ph3-CN, R' -Te2-Ph2-CHZCH2-Np 1-CN,
R'-Te2-Ph2-CH2CH2-Np2-CN, R'-Te2-Ph2-CH2CH2-Np3-CN,
R' -Te2-Ph2-CH2CH2-Np4-CN,
R' -Te 1-Ph2-C=C-Ph2-CN, R' -Te 1-Ph2-C=C-Ph3-CN, R' -Te2-Ph2-C=C-Ph 1-CN,
R'-Te2-Ph2-C=C-Ph2-CN, R'-Te2-Ph2-C=C-Ph3-CN,
R'-Tel-Ph3-Phl-CN, R'-Tel-Ph3-Ph2-CN, R'-Tel-Ph3-Ph3-CN,
R'-Tel-Ph3-Npl-CN, R'-Tel-Ph3-Np2-CN, R'-Tel-Ph3-Np3-CN,
R'-Tel-Ph3-Np4-CN, R'-Te2-Ph3-Phl-CN, R'-Te2-Ph3-Ph2-CN,
R'-Te2-Ph3-Ph3-CN, R'-Te2-Ph3-Npl-CN, R'-Te2-Ph3-Np2-CN,
R'-Te2-Ph3-Np3-CN, R'-Te2-Ph3-Np4-CN,
R'-Tel-Ph3-CH2CH2-Ph2-CN, R'-Tel-Ph3-CH2CH2-Ph3-CN,
R'-Tel-Ph3-CH2CH2-Npl-CN, R'-Tel-Ph3-CH2CH2-Np2-CN,
R'-Te 1-Ph3-CH2CH2-Np3-CN, R'-Te 1-Ph3-CH2CH2-Np4-CN,
R' -Te2-Ph3-CH2CH2-Ph 1-CN, R' -Te2-Ph3-CH2CH2-Ph2-CN,
R'-Te2-Ph3-CH2CH2-Ph3-CN, R'-Te2-Ph3-CH2CH2-Npl-CN,
R'-Te2-Ph3-CH2CH2-Np2-CN, R'-Te2-Ph3-CH2CH2-Np3-CN,
R' -Te2-Ph3-CH2CH2-Np4-CN,
R' -Te 1-Ph3 -C=C-Ph2-CN, R' -Te 1-Ph3 -C=C-Ph3 -CN,
R'-Te1-Np1-Ph1-CN, R'-Te1-Np1-Ph2-CN, R'-Te1-Np1-Ph3-CN,
R' -Te 1-Np 1-CH2CH2-Ph2-CN, R' -Te 1-Np 1-CH2CH2-Ph3-CN,
R' -Te2-Np l-CHZCH2-Ph 1-CN, R' -Te2-Np l-CH2CH2-Ph2-CN,
R' -Te2-Np 1-CH2CH2-Ph3-CN,
R' -Te l-Np2-Ph l-CN, R' -Te l-Np2-Ph2-CN, R' -Te l-Np2-Ph3 -CN,
CA 02377217 2001-12-28
99
R' -Te2-Np2-Ph 1-CN, R' -Te2-Np2-Ph2-CN, R' -Te2-Np2-Ph3-CN,
R' -Te l -Np2-CH2CH2-Ph2-CN, R' -Te 1-Np2-CH2CH2-Ph3-CN,
R' -Te2-Np2-CH2CH2-Ph l -CN, R' -Te2-Np2-CH2CH2-Ph2-CN,
R' -Te2-Np2-CH2CH2-Ph3-CN,
R'-Te1-Np3-Ph1-CN, R'-Te1-Np3-Ph2-CN, R'-Tel-Np3-Ph3-CN,
R'-Te2-Np3-Phl-CN, R'-Te2-Np3-Ph2-CN, R'-Te2-Np3-Ph3-CN,
R'-Tel-Np3-CH2CH2-Ph2-CN, R'-Tel-Np3-CH2CH2-Ph3-CN,
R' -Te2-Np3 -CH2CH2-Ph l -CN, R' -Te2-Np3 -CH2CH2-Ph2-CN,
R' -Te2-Np3 -CH2CH2-Ph3 -CN,
R'-Te 1-Np4-Ph 1-CN, R' -Te l-Np4-Ph2-CN, R'-Te l-Np4-Ph3-CN,
R' -Te2-Np4-Ph 1-CN, R' -Te2-Np4-Ph2-CN, R' -Te2-Np4-Ph3 -CN,
R'-Tel-Np4-CH2CH2-Ph2-CN, R'-Tel-Np4-CH2CH2-Ph3-CN,
R' -Te2-Np4-CHZCH2-Ph 1-CN, R' -Te2-Np4-CH2CH2-Ph2-CN,
R' -Te l-CH2CH2-Ph 1-Ph 1 -CN, R' -Te 1-CH2CH2-Ph l-Ph2-CN,
R'-Te1-CHZCH2-Ph1-Ph3-CN, R'-Te1-CH2CH2-Ph1-Np1-CN,
R'-Tel-CH2CH2-Phl-Np2-CN, R'-Tel-CH2CH2-Phl-Np3-CN,
R'-Tel-CHzCH2-Phl-Np4-CN, R'-Te2-CH2CH2-Ph1-Ph1-CN,
R'-Te2-CH2CH2-Phl-Ph2-CN, R'-Te2-CH2CH2-Phl-Ph3-CN,
R' -Te2-CH2CH2-Ph 1-Np 1-CN, R' -Te2-CH2CH2-Ph 1-Np2-CN,
R' -Te2-CH2CH2-Ph 1-Np3 -CN, R' -Te2-CH2CH2-Ph l-Np4-CN,
R' -Te 1-CH2CHz-Ph2-Ph l-CN, R' -Te l-CH2CH2-Ph2-Ph2-CN,
R' -Te l-CH2CH2-Ph2-Ph3 -CN, R' -Te l-CHZCH2-Ph2-Np 1-CN,
R'-Tel-CH2CH2-Ph2-Np2-CN, R'-Tel-CHZCH2-Ph2-Np3-CN,
R'-Tel-CH2CH2-Ph2-Np4-CN, R'-Te2-CH2CH2-Ph2-Phl-CN,
CA 02377217 2001-12-28
100
R'-Te2-CH2CHZ-Ph2-Ph2-CN, R'-Te2-CH2CH2-Ph2-Ph3-CN,
R' -Te2-CH2CH2-Ph2-Np 1-CN, R'-Te2-CH2CH2-Ph2-Np2-CN,
R'-Te2-CH2CH2-Ph2-Np3-CN, Rl-Te2-CH2CHz-Ph2-Np4-CN,
R' -Te 1-CH2CH2-Ph3-Ph 1-CN, R' -Te l-CH2CH2-Ph3-Ph2-CN,
R'-Tel-CH2CH2-Ph3-Ph3-CN, R'-Tel-CH2CH2-Ph3-Npl-CN,
R'-Te 1-CH2CH2-Ph3-Np2-CN, R'-Te l -CH2CH2-Ph3-Np3-CN,
R'-Tel-CH2CH2-Ph3-Np4-CN, R'-Te2-CH2CH2-Ph3-Phl-CN,
R'-Te2-CH2CH2-Ph3-Ph2-CN, R'-Te2-CH2CH2-Ph3-Ph3-CN,
R'-Te2-CH2CH2-Ph3-Npl-CN, R'-Te2-CH2CH2-Ph3-Np2-CN,
R'-Te2-CH2CHZ-Ph3-Np3-CN, R'-Te2-CHZCH2-Ph3-Np4-CN,
R' -Te 1-CH2CHZ-Np 1-Ph 1-CN, R' -Te 1-CH2CH2-Np 1-Ph2-CN,
R' -Te 1-CH2CH2-Np 1-Ph3 -CN, Rl -Te2-CHZCH2-Np 1-Ph 1-CN,
R'-Te2-CH2CH2-Npl-Ph2-CN, Rl-Te2-CH2CH2-Npl-Ph3-CN,
R'-Te l -CH2CH2-Np2-Ph1-CN, Rl-Te 1-CH2CH2-Np2-Ph2-CN,
R'-Tel-CH2CH2-Np2-Ph3-CN, R'-Te2-CHZCH2-Np2-Phl-CN,
R'-Te2-CH2CH2-Np2-Ph2-CN, R'-Te2-CH2CH2-Np2-Ph3-CN,
R'-Tel-CH2CH2-Np3-Phl-CN, R'-Tel-CH2CH2-Np3-Ph2-CN,
R'-Tel-CH2CH2-Np3-Ph3-CN, R'-Te2-CH2CH2-Np3-Phl-CN,
R'-Te2-CH2CH2-Np3-Ph2-CN, R'-Te2-CH2CH2-Np3-Ph3-CN,
R' -Te 1-CH2CH2-Np4-Ph 1 -CN, R' -Te 1-CH2CH2-Np4-Ph2-CN,
R'-Tel-CH2CH2-Np4-Ph3-CN, R'-Te2-CH2CH2-Np4-Phl-CN,
R' -Te2-CH2CH2-Np4-Ph2-CN, R' -Te2-CH2CH2-Np4-Ph3-CN,
R' -Te l-C=C-Ph l-Ph l-CN, R' -Te l-C=C-Ph l-Ph2-CN, R' -Te l-C=C-Ph l-Ph3-CN,
R'-Te2-C=C-Phl-Phl-CN, R'-Te2-C=C-Phl-Ph2-CN, R'-Te2-C=C-Phl-Ph3-CN,
CA 02377217 2001-12-28
101
R' -Te l-C-C-Ph2-Ph 1-CN, R' -Te l-C=C-Ph2-Ph2-CN, R' -Te 1-C=C-Ph2-Ph3-CN,
R' -Te2-C=C-Ph2-Ph 1-CN, R' -Te2-C=C-Ph2-Ph2-CN, R' -Te2-C=C-Ph2-Ph3 -CN,
R'-Te1-C_C-Ph3-Phl-CN, R'-Tel-C=C-Ph3-Ph2-CN, R'-Tel-C=C-Ph3-Ph3-CN,
R'-Te2-C=C-Ph3-Phl-CN, R'-Te2-C=C-Ph3-Ph2-CN, R'-Te2-C=C-Ph3-Ph3-CN,
in the case in which na = nb = 0, and n = nd = 1, and Z is a trifluoromethoxy
group,
R'-Tel-Cy-Phl-OCF3, R'-Tel-Cy-Ph2-OCF3, R'-Tel-Cy-Ph3-OCF3,
R' -Te 1-Cy-Np 1-OCF3, R'-Te 1-Cy-Np2-OCF3, Rl -Te l-Cy-Np3-OCF3,
R'-Tel-Cy-Np4-OCF3, Rl-Te2-Cy-PhI-OCF3, R'-Te2-Cy-Ph2-OCF3,
R'-Te2-Cy-Ph3-OCF3, R'-Te2-Cy-NpI-OCF3, Rl-Te2-Cy-Np2-OCF3,
R'-Te2-Cy-Np3-OCF3, Rl-Te2-Cy-Np4-OCF3,
R' -Te l -Cy-CHZCH2-Ph2-OCF3, R'-Tel-Cy-CH2CH2-Ph3-OCF3,
R'-Tel-Cy-CH2CH2-Npl-OCF3, R'-Tel-Cy-CHZCH2-Np2-OCF3,
R'-Tel-Cy-CH2CH2-Np3-OCF3, R'-Tel-Cy-CH2CH2-Np4-OCF3,
R'-Te2-Cy-CH2CH2-Phl-OCF3, R'-Te2-Cy-CH2CH2-Ph2-OCF3,
R'-Te2-Cy-CH2CH2-Ph3-OCF3, R'-Te2-Cy-CH2CH2-Np 1-OCF3,
R'-Te2-Cy-CH2CH2-Np2-OCF3, R'-Te2-Cy-CH2CH2-Np3-OCF3,
R' -Te2-Cy-CH2CH2-Np4-OCF3, R' -Te 1-CHZCH2-Cy-Ph 1-OCF3,
R'-Tel-CHZCH2-Cy-Ph2-OCF3, R'-Tel-CH2CH2-Cy-Ph3-OCF3,
R1-Te 1-CH2CH2-Cy-Np 1-OCF3, R'-Te 1-CH2CH2-Cy-Np2-OCF3,
R'-Tel-CH2CH2-Cy-Np3-OCF3, R'-Tel-CH2CH2-Cy-Np4-OCF3,
R'-Te2-CHZCHZ-Cy-Phl-OCF3, R'-Te2-CH2CH2-Cy-Ph2-OCF3,
R'-Te2-CHZCH2-Cy-Ph3-OCF3, R'-Te2-CH2CH2-Cy-Npl-OCF3,
R'-Te2-CH2CH2-Cy-Np2-OCF3, R'-Te2-CH2CH2-Cy-Np3-OCF3,
R1-Te2-CH2CH2-Cy-Np4-OCF3,
CA 02377217 2001-12-28
102
R'-Tel-Phl-PhI-OCF3, R~-Tel-Phl-Ph2-OCF3, R'-Tel-Phl-Ph3-OCF3,
R'-Tel-Phl-Npl-OCF3, R'-Tel-Phl-Np2-OCF3, R'-Tel-Phl-Np3-OCF3,
R' -Te 1-Ph 1-Np4-OCF3, R' -Te2-Ph 1-Ph 1-OCF3, R' -Te2-Ph 1-Ph2-OCF3,
R' -Te2-Ph 1-Ph3-OCF3, R~-Te2-Ph 1-Np 1-OCF3, R' -Te2-Ph 1-Np2-OCF3,
R'-Te2-Phl-Np3-OCF3, R'-Te2-Phl-Np4-OCF3,
R'-Tel-Phl-CH2CH2-Ph2-OCF3, R'-Tel-Phl-CH2CH2-Ph3-OCF3,
R'-Tel-Phl-CH2CH2-Npl-OCF3, Rl-Tel-Phi-CH2CH2-Np2-OCF3,
R'-Tel-Phl-CH2CH2-Np3-OCF3, R'-Tel-Phl-CH2CH2-Np4-OCF3,
R'-Te2-Phl-CH2CH2-Phl-OCF3, R'-Te2-Phl-CH2CH2-Ph2-OCF3,
R' -Te2-Ph 1-CH2CH2-Ph3 -OCF3, R' -Te2-Ph 1-CH2CH2-Np 1-OCF3,
R' -Te2-Ph 1-CH2CH2-Np2-OCF3, R' -Te2-Ph 1-CH2CH2-Np3-OCF3,
R' -Te2-Phi -CH2CH2-Np4-OCF3,
R' -Te 1-Ph 1-C=C-Ph2-OCF3, R' -Te l-Ph 1-C=C-Ph3 -OCF3,
R'-Te2-Phl-C=C-PhI-OCF3, Rl-Te2-Phl-C=C-Ph2-OCF3,
R' -Te2-Ph 1-C=C-Ph3-OCF3,
R' -Te 1-Ph2-Ph l-OCF3, R' -Te 1-Ph2-Ph2-OCF3, R' -Te l-Ph2-Ph3-OCF3,
R'-Tel-Ph2-Npl-OCF3, Rl-Tel-Ph2-Np2-OCF3, R'-Tel-Ph2-Np3-OCF3,
R' -Te 1-Ph2-Np4-OCF3, R' -Te2-Ph2-Ph 1-OCF3, R' -Te2-Ph2-Ph2-OCF3,
R' -Te2-Ph2-Ph3-OCF3, R'-Te2-Ph2-Np 1-OCF3, R'-Te2-Ph2-Np2-OCF3,
R' -Te2-Ph2-Np3-OCF3, R' -Te2-Ph2-Np4-OCF3,
R' -Te 1-Ph2-CH2CH2-Ph2-OCF3, R' -Te 1-Ph2-CH2CH2-Ph3-OCF3,
R'-Tel-Ph2-CHZCH2-Npl-OCF3, R'-Tel-Ph2-CH2CH2-Np2-OCF3,
R' -Te 1-Ph2-CH2CH2-Np3-OCF3, R' -Te 1-Ph2-CH2CH2-Np4-OCF3,
R' -Te2-Ph2-CH2CH2-Ph 1-OCF3, R' -Te2-Ph2-CHZCHZ-Ph2-OCF3,
CA 02377217 2001-12-28
103
Rl-Te2-Ph2-CHZCH2-Ph3-OCF3, Rl-Te2-Ph2-CH2CH2-Npl-OCF3,
R'-Te2-Ph2-CH2CH2-Np2-OCF3, R'-Te2-Ph2-CH2CH2-Np3-OCF3,
R' -Te2-Ph2-CHZCHZ-Np4-OCF3,
R' -Te 1-Ph2-C=C-Ph2-OCF3, R' -Te 1-Ph2-C=C-Ph3 -OCF3,
R'-Te2-Ph2-C=C-Phl-OCF3, Rl-Te2-Ph2-C=C-Ph2-OCF3,
R' -Te2-Ph2-C-C-Ph3-OCF3,
R'-Tel-Ph3-Phl-OCF3, R'-Tel-Ph3-Ph2-OCF3, R'-Tel-Ph3-Ph3-OCF3,
R'-Tel-Ph3-Npl-OCF3, Rl-Tel-Ph3-Np2-OCF3, R'-Tel-Ph3-Np3-OCF3,
R'-Te l -Ph3-Np4-OCF3, R1-Te2-Ph3-Phl -OCF3, Rl-Te2-Ph3-Ph2-OCF3,
R'-Te2-Ph3-Ph3-OCF3, Rl-Te2-Ph3-Npl-OCF3, R'-Te2-Ph3-Np2-OCF3,
R'-Te2-Ph3-Np3-OCF3, R'-Te2-Ph3-Np4-OCF3,
R'-Tel-Ph3-CHZCH2-Ph2-OCF3, Rl-Tel-Ph3-CH2CH2-Ph3-OCF3,
R'-Tel-Ph3-CH2CH2-Npl-OCF3, R'-Tel-Ph3-CH2CH2-Np2-OCF3,
R'-Tel-Ph3-CH2CH2-Np3-OCF3, R~-Tel-Ph3-CH2CH2-Np4-OCF3,
R'-Te2-Ph3-CH2CH2-Phl-OCF3, R'-Te2-Ph3-CH2CH2-Ph2-OCF3,
R'-Te2-Ph3-CHZCH2-Ph3-OCF3, R'-Te2-Ph3-CH2CH2-Np 1-OCF3,
R'-Te2-Ph3-CHZCH2-Np2-OCF3, R'-Te2-Ph3-CHzCH2-Np3-OCF3,
R' -Te2-Ph3 -C H2CH2-Np4-OCF3,
R'-Tel-Ph3-C=C-Ph2-OCF3, R'-Tel-Ph3-C=C-Ph3-OCF3,
R1-Te2-Ph3-C_C-Phl-OCF3, Rl-Te2-Ph3-C=C-Ph2-OCF3,
R' -Te2-Ph3 -C=C-Ph3 -OCF3,
R'-Tel-Npl-Phl-OCF3, R'-Tel-Npl-Ph2-OCF3, R'-Tel-Npl-Ph3-OCF3,
R'-Te2-Npl-PhI-OCF3, Rl-Te2-Npl-Ph2-OCF3, R'-Te2-Npl-Ph3-OCF3,
R' -Te 1-Np 1-CHZCH2-Ph2-OCF3, R' -Te 1-Np 1-CHZCH2-Ph3-OCF3,
CA 02377217 2001-12-28
104
R' -Te2-Np 1-CH2CH2-Ph l-OCF3, R' -Te2-Np l-CH2CH2-Ph2-OCF3,
Rl-Te2-Np 1-CH2CH2-Ph3-OCF3,
R' -Te 1-Np2-Ph 1-OCF3, R' -Te l-Np2-Ph2-OCF3, R' -Te 1-Np2-Ph3 -OCF3,
R'-Te2-Np2-Phl -OCF3, R'-Te2-Np2-Ph2-OCF3, R'-Te2-Np2-Ph3-OCF3,
R' -Te l-Np2-CH2CH2-Ph2-OCF3, R'-Te 1-Np2-CH2CH2-Ph3-OCF3,
R'-Te2-Np2-CHZCH2-Phl-OCF3, R'-Te2-Np2-CHZCH2-Ph2-OCF3,
R1-Te2-Np2-CH2CH2-Ph3-OCF3,
R'-Tel-Np3-Phl-OCF3, R'-Tel-Np3-Ph2-OCF3, R'-Tel-Np3-Ph3-OCF3,
R'-Te2-Np3-Phl-OCF3, R'-Te2-Np3-Ph2-OCF3, R'-Te2-Np3-Ph3-OCF3,
R'-Tel-Np3-CH2CH2-Ph2-OCF3, R'-Tel-Np3-CH2CH2-Ph3-OCF3,
R'-Te2-Np3-CH2CH2-Phl-OCF3, R'-Te2-Np3-CH2CH2-Ph2-OCF3,
R' -Te2-Np3 -CH2CH2-Ph3 -OCF3,
R'-Tel-Np4-Phl-OCF3, R'-Tel-Np4-Ph2-OCF3, R'-Tel-Np4-Ph3-OCF3,
R' -Te2-Np4-Ph 1-OCF3, R' -Te2-Np4-Ph2-OCF3, R' -Te2-Np4-Ph3 -OCF3,
R'-Tel-Np4-CH2CH2-Ph2-OCF3, R'-Tel-Np4-CH2CH2-Ph3-OCF3,
R' -Te2-Np4-CH2CH2-Ph l -OCF3, R' -Te2-Np4-CH2CH2-Ph2-OCF3,
R' -Te2-Np4-CH2CH2-Ph3-OCF3,
R'-Tel-CH2CH2-Phl-Phl-OCF3, R'-Tel-CH2CH2-Phl-Ph2-OCF3,
R'-Tel-CH2CH2-Phl-Ph3-OCF3, R'-Tel-CH2CH2-Phl-NpI-OCF3,
R' -Te 1-CH2CH2-Ph 1-Np2-OCF3, R' -Te l-CHZCH2-Ph 1-Np3-OCF3,
R' -Te 1-CH2CH2-Ph 1-Np4-OCF3, R' -Te2-CHZCH2-Ph 1-Ph l-OCF3,
R'-Te2-CHZCH2-Phl-Ph2-OCF3, R'-Te2-CH2CH2-Phl-Ph3-OCF3,
R'-Te2-CHZCH2-Phl-Npl-OCF3, Rl-Te2-CH2CH2-Phl-Np2-OCF3,
R' -Te2-CH2CH2-Ph l -Np3-OCF3, Rl -Te2-CH2CH2-Ph 1-Np4-OCF3,
CA 02377217 2001-12-28
105
R'-Tel-CH2CH2-Ph2-Phl-OCF3, R'-Tel-CHZCH2-Ph2-Ph2-OCF3,
R' -Te l-CH2CH2-Ph2-Ph3-OCF3, R' -Te l-CH2CH2-Ph2-Np 1-OCF3,
R'-Tel-CH2CH2-Ph2-Np2-OCF3, R'-Tel-CH2CH2-Ph2-Np3-OCF3,
R' -Te l -CH2CH2-Ph2-Np4-OCF3, R' -Te2-CH2CH2-Ph2-Ph 1-OCF3,
R'-Te2-CH2CH2-Ph2-Ph2-OCF3, R'-Te2-CH2CH2-Ph2-Ph3-OCF3,
R'-Te2-CH2CH2-Ph2-Npl-OCF3, R'-Te2-CH2CH2-Ph2-Np2-OCF3,
R'-Te2-CH2CH2-Ph2-Np3-OCF3, R'-Te2-CH2CH2-Ph2-Np4-OCF3,
R'-Tel-CH2CH2-Ph3-Phl-OCF3, R'-Tel-CH2CH2-Ph3-Ph2-OCF3,
R'-Tel-CH2CH2-Ph3-Ph3-OCF3, Rl-Te2-CH2CH2-Ph3-Phl-OCF3,
R'-Te2-CH2CH2-Ph3-Ph2-OCF3, R'-Te2-CH2CH2-Ph3-Ph3-OCF3,
R'-Te2-CHZCH2-Ph3-Npl-OCF3, R'-Te2-CH2CH2-Ph3-Np2-OCF3,
R'-Te2-CH2CH2-Ph3-Np3-OCF3, R'-Te2-CH2CH2-Ph3-Np4-OCF3,
R' -Te 1-CH2CH2-Np 1-Ph l-OCF3, R' -Te 1-CH2CH2-Np 1-Ph2-OCF3,
R' -Te l-CH2CH2-Np 1-Ph3-OCF3, R' -Te2-CH2CH2-Np 1-Ph 1-OCF3,
R' -Te2-CH2CH2-Np 1-Ph2-OCF3, R' -Te2-CH2CH2-Np 1-Ph3-OCF3,
R' -Te 1-CH2CH2-Np2-Phl -OCF3, R' -Te l -CH2CH2-Np2-Ph2-OCF3,
R'-Tel-CH2CH2-Np2-Ph3-OCF3, R'-Te2-CH2CH2-Np2-Phl-OCF3,
R1-Te2-CHZCH2-Np2-Ph2-OCF3, R-Te2-CH2CH2-Np2-Ph3-OCF3,
R' -Te 1-CH2CH2-Np3 -Ph 1-OCF3, R' -Te l-CH2CH2-Np3 -Ph2-OCF3,
R'-Tel-CH2CH2-Np3-Ph3-OCF3, R'-Te2-CH2CH2-Np3-Phl-OCF3,
R' -Te2-CH2CH2-Np3-Ph2-OCF3, R' -Te2-CH2CH2-Np3-Ph3-OCF3,
R'-Tel-CHZCH2-Np4-Phl-OCF3, R'-Tel-CHZCH2-Np4-Ph2-OCF3,
R' -Te 1-CH2CH2-Np4-Ph3-OCF3, R' -Te2-CH2CH2-Np4-Ph 1-OCF3,
R1-Te2-CH2CH2-Np4-Ph2-OCF3, R'-Te2-CH2CH2-Np4-Ph3-OCF3,
CA 02377217 2001-12-28
106
R'-Te 1-C=C-Ph 1-Ph i-OCF3, Rl -Te 1-C=C-Ph 1-Ph2-OCF3i
R'-Te l-C=C-Ph l-Ph3-OCF3, R'-Te2-C=-C-Ph l-Ph l-OCF3,
R'-Te2-C=C-Phl-Ph2-OCF3, Rl-Te2-C=C-Ph1-Ph3-OCF3,
R' -Te 1-C-C-Ph2-Ph 1-OCF3, R1-Te 1-C=C-Ph2-Ph2-OCF3,
Rl-Te l -C=C-Ph2-Ph3-OCF3, Rl-Te2-C=C-Ph2-Phl -OCF3,
R'-Te2-C=C-Ph2-Ph2-OCF3, Rl-Te2-C=C-Ph2-Ph3-OCF3, ,
R'-Tel-C=C-Ph3-Phl-OCF3, Rl-Tel-C=C-Ph3-Ph2-OCF3,
R'-Te l -C=C-Ph3-Ph3-OCF3i R'-Te2-C=C-Ph3-Phl -OCF3,
R'-Te2-C=C-Ph3-Ph2-OCF3, R'-Te2-C=C-Ph3-Ph3-OCF3,
R'-Te 1-C=C-Ph3-CH2CH2-Ph2-OCF3, Rl-Te 1-C=C-Ph3-CHZCHZ-Ph3-OCF3,
The compounds of the general formula (I) can be produced by a variety of
synthetic
methods depending on the selection of the group R, the linkage groups La, Lb,
Lc and Ld, the
polar group Z, the ring A, the ring B, the ring C, the ring D and the ring E,
and na, nb, nc and
nd, and representative examples of these synthetic methods are presented
below.
The case in which ring C is the formula (IIa)
In the case in which nb = 1, nc = nd = 0, the linkage group La is a single
bond,
-CH2CH2-, -CH(CH3)CH2-, -CH2CH(CH3)-, -CH(CH3)CH(CH3)- or -CF2CF2-, and the
linkage group Lb is a single bond, synthesis can be completed in accordance
with the
following schemes.
CA 02377217 2001-12-28
107
R A La
O Z 1) SOCIZ O Z ' a(MtOH X' X' Va-1)
2) AlCi3
x3 xZ CHZ=CH2 3 2
X X
(IIIa-1) (IVa-1)
HO X' 4-CH3-C6H4-SO3H
R A La' na B Z
(VIa-1) x3 xz
x'
R A La' B H2
ne Z cat.
(VIIa-1) X3 Xz
R O-La2 ne B Z
(I-a) x3 x2
(wherein, R, the polar group Z, the ring A, the ring B, na, XI, X2 and X3 have
the same
meaning as described above for the general formula (I), LaI represents a
single bond,
-CH2CH2-, -CH=CH-, -C=C-, -CH(CH3)CH2-, -CH2CH(CH3)-, -CH(CH3)CH(CH3)- or
-CF2CF2-, La2 represents a single bond, -CH2CH2-, -CH(CH3)CH2-, -CH2CH(CH3)-,
-CH(CH3)CH(CH3)- or -CF2CF2-, and Mtl' represents a metal ion such as Li,
BrMg, or IMg.)
That is, by using thionyl chloride or the like to convert the phenylacetic
acid
represented by the general formula (IIIa-1) into an acid chloride, and
subsequently conducting
a reaction with ethylene in the presence of aluminum chloride, a tetralone
derivative
represented by the general formula (IVa-1) can be prepared. By reacting this
derivative with
a lithium or magnesium reagent represented by the general formula (Va-1),
either in or out of
the presence of a metal salt such as cerium chloride or manganese chloride and
a Lewis acid,
an alcohol represented by the general formula (VIa-1) is obtained, and by
heating this alcohol
in the presence of an acid catalyst such as p-toluenesulfonic acid, a
dihydronaphthalene
CA 02377217 2001-12-28
108
compound represented by the general formula (VIIa-1) is obtained. Subsequent
hydrogenation of this dihydronaphthalene compound in the presence of a metal
catalyst such
as Pd-C, Rh-C, Pt-C or Pd(OH)2 or the like, yields the target compound
represented by the
general formula (I-a).
The lithium or magnesium reagent represented by the general formula (VIa-1) is
a
compound frequently used in liquid crystal production, and can be easily
produced from the
corresponding halide or the like.
As shown in the schemes below, by replacing the lithium or magnesium reagent
represented by the general formula (Va-1) with an acetylide represented by the
general
formula (Va-2), a compound represented by the general formula (I-b) can be
produced.
R A La~ B = Mtl l
o XI
ne (Va-2)
X3 X2
(IVa-1)
H X 4-CH3-C6H4-SO3H
R D-Lal B
ne - ~~ Z
(VIa-2) X3 X2
X1 H
R A Lal B = 2
ne Z cat.
(VIIa-2) X 3 Xz
=
R A LaZ B Xi
ne Z
(I-b) 3 XZ
(wherein, R, the polar group Z, the ring A, the ring B, na, X1, X2 and X3 have
the same
meaning as described above for the general formula (I), Lal represents a
single bond,
-CH2CH2-, -CH=CH-, -C-C-, -CH(CH3)CH2-, -CH2CH(CH3)-1 -CH(CH3)CH(CH3)- or
CA 02377217 2001-12-28
109
-CF2CF2-, La2 represents a single bond, -CH2CH2-, -CH(CH3)CH2-, -CH2CH(CH3)-,
-CH(CH3)CH(CH3)- or -CF2CF2-, and Mtll represents a metal ion such as Li,
BrMg, or IMg.)
Furthermore, as shown in the schemes below, the compound represented by the
general formula (I-b) can also be prepared by reacting the tetralone
derivative represented by
the general formula (IVa-1) with an ylide represented by the general formula
(Vb-1), and then
reducing the thus obtained compound (VIIb-2).
R A La B PPh
X na s (Vb-1)
O Z
X3 X2
(IVa-1)
R A La B X ~ H
z
ne - / \
Z cat.
(VIIb-2)
X3 XZ
R A Laz B XI
na Z
(I-b) 3 X2
(wherein, R, the polar group Z, the ring A, the ring B, na, X1, X2 and X3 have
the same
meaning as described above for the general formula (I), Lal represents a
single bond,
-CH2CH2-, -CH=CH-, -C=C-, -CH(CH3)CH2-, -CH2CH(CH3)-, -CH(CH3)CH(CH3)- or
-CF2CF2-, and La2 represents a single bond, -CH2CH2-, -CH(CH3)CH2-, -
CH2CH(CH3)-,
-CH(CH3)CH(CH3)- or -CF2CF2-.)
In addition, the compounds represented by the general formulas (I-b) and (I-c)
can
also be produced by a method shown in the schemes below.
CA 02377217 2001-12-28
110
Xi Me0 X
O ~PPh3
Z Z
X3 XZ X3 XZ
(IVa-1) (VIIIa-1)
R A Lat
na \PPh3 R aLaB X
ne Z
(I-c)
X3 Xz .
H2 R A La2 B Xi
cat. ne Z
(I-b) X3 XZ
(wherein, R, the polar group Z, the ring A, the ring B, na, X1, X2 and X3 have
the same
meaning as described above for the general formula (I), Lal represents a
single bond,
-CH2CH2-, -CH=CH-, -CGC-, -CH(CH3)CH2-, -CH2CH(CH3)-, -CH(CH3)CH(CH3)- or
-CF2CF2-, and La2 represents a single bond, -CH2CH2-, -CH(CH3)CH2-, -
CH2CH(CH3)-,
-CH(CH3)CH(CH3)- or -CF2CF2-.)
In the case in which nb = 1 , n = nd = 0, the ring C is the formula (IIa),
and X', X2 and
X3 are hydrogen atoms, and Z is a cyano group or a trifluoromethoxy group,
synthesis can be
achieved in the manner described below.
CA 02377217 2001-12-28
111
N
R A La B Lb--( =O H
na ~J ~O
(IXa-1) 2)
R @-La B Lb -
O and/or
na
(IXb-1)
R A La B Lb
ne b O
(IXb-2)
CuBrZy R A La aLb-Ca
Li n8 OH
Br
(IXc-1)
R A La ne B Lb b Zi
(I-d)
(wherein, R, La, Lb, the ring A, the ring B and na have the same meaning as
described above
for the general formula (I), and Z' represents a cyano group or a
trifluoromethoxy group.)
That is, by subjecting a cyclohexanone derivative represented by the general
formula
(IXa-1) to a dehydration reaction with pyrrolidine, subsequent reaction with
methyl vinyl
ketone, and then treatment with an acid, a mixture of the octahydronaphthalene
derivatives
represented by the general formulas (IXb-1) and (IXb-2) can be obtained. By
subjecting this
mixture to an oxidation using copper (II) bromide or lithium bromide, a
compound
represented by the general formula (IXc-1) can be obtained. Following
conversion of the
hydroxyl group to a triflate, if the compound is reacted with copper cyanide,
then a compound
of the general formula (I-d) in which the Z' group is a cyano group can be
obtained.
Furthermore, if the phenol derivative represented by the general formula (IXc-
1) is reacted
with carbon tetrachloride, and subsequently reacted with potassium fluoride,
then a
compound of the general formula (I-d) in which the Z' group is a
trifluoromethoxy group can
CA 02377217 2001-12-28
112
be obtained.
In addition, by producing the compound of the general formula (Xa-1) from the
formula (IIIa-2), in the same manner as for the production of the compound of
the general
formula (I-a), and subsequently performing a lithiation using a base such as
butyllithium or
lithium diisopropylamide or the like, and then reacting the product with
carbon dioxide, the
carboxylic acid derivative represented by the general formula (Xb-1) can be
obtained. By
converting this carboxylic acid to an acid chloride, and subsequently to an
amide using
ammonia gas, and then subjecting the product to the action of a dehydration
agent, a
compound represented by the general formula (I-e) can be obtained.
F F
O OH O
(IIIa-2) (IVa-2)
F
R A LaZ B /\ 1) base
na 2) CO2
(Xa-1) F
F
R A LaZ B
ne ~ ~ COOH
(Xb-1) F
F
R A La2 B
e CN
n
(I-e) F
(wherein, R, the ring A, the ring B and na have the same meaning as described
above for the
general formula (I), and La2 represents a single bond, -CH2CH2-, -CH(CH3)CH2-,
-CH2CH(CH3)-, -CH(CH3)CH(CH3)- or -CF2CF2-.)
Furthermore, by lithiating the compound represented by the general formula (Xa-
1),
and carrying out a subsequent reaction with trimethyl borate, and then with
hydrogen
CA 02377217 2001-12-28
113
peroxide, a compound represented by the general formula (IXc-2) can be
prepared. The
compound represented by the general formula (I-f) can be derived from this
compound in the
same manner as was described for the compound represented by the general
formula (IXc-1).
F
R D-Laz B /\ 1) base
ne 2) B(OMe)3
(Xa-1) F 3) H202
F
R A LaZ ---
ne OH
(IXc-2) F
F
R A La2ne B Zl
(I-f) F
(wherein, R, the ring A, the ring B and na have the same meaning as described
above for the
general formula (I), La2 represents a single bond, -CH2CH2-, -CH(CH3)CH2-, -
CH2CH(CH3)-1
-CH(CH3)CH(CH3)- or -CF2CF2-, and Z' represents a cyano group or a
trifluoromethoxy
group.)
In the case in which n d= 1, n = 0, the ring C is the formula (IIa), and the
linkage
group Lb is a single bond, synthesis can be achieved in the manner described
below.
CA 02377217 2001-12-28
114
R A La B Lb
n8 nb OH
x 3 xZ
(IXc-3) ~~
MtIZ-( E r-Z'
X' ~!
R A Lb (XIa-1)
ne y
~ OTf
(IXd-1) x3 xZ
x'
R A La Lb E Z'
nb -
x3 xz (I-g)
(wherein, R, the ring A, the ring B, the ring E, na, nb, Xl, X2 and X3 have
the same meaning
as described above for the general formula (I), Zl represents a fluorine atom
or a
trifluoromethoxy group, Tf represents a trifluoromethanesulfonyl group, and
Mtl2 represents
Li, C1Mg, BrMg, IMg or (HO)2B.)
That is, reacting a compound represented by the general formula (IXc-3), which
can
be prepared in the same manner as the compounds represented by the general
formulas (IXc-
1) and (IXc-2), with trifluoromethanesulfonic anhydride or
trifluoromethanesulfonyl chloride
or the like, in the presence of a base such as pyridine , diethylamine or
triethylamine, and in a
solvent such as dichloromethane or chloroform yields a triflate represented by
the general
formula (IXd-1). By reacting this triflate with a compound represented by the
general
formula (XIa-1) in the presence of a transition metal catalyst such as
tetrakis(triphenylphosphine) palladium(0) or tetrakis(triphenylphosphine)
nickel(0), a
compound represented by the general formula (I-g) can be synthesized.
Furthermore, by reacting the compound represented by the general formula (IXd-
1)
with an acetylene compound represented by the general formula (XIb-1), in the
presence of
cuprous iodide and a transition metal catalyst such as
dichlorobis(triphenylphosphine)
CA 02377217 2001-12-28
115
palladium or tetrakis(triphenylphosphine) palladium(0), a compound represented
by the
general formula (I-h) can be prepared.
= E Z
R A La Lb (
ne nb OTf XIb-1)
(IXd-1) x3 x2
xl
R G)'La Lb
na nb E Z
x3 x2 (I-h)
(wherein, R, the polar group Z, the ring A, the ring B, the ring E, na nb, XI,
X2 and X3 have
the same meaning as described above for the general formula (I), and Tf
represents a
trifluoromethanesulfonyl group.)
Furthermore, reacting a compound represented by the general formula (IXb-3),
which
can be prepared in the same manner as the compounds represented by the general
formulas
(IXb-1) and (IXb-2), with an aryl lithium or magnesium reagent represented by
the general
formula (Xla-2), followed by dehydration, yields a compound represented by the
general
formula (XIIa-1), which upon subsequent oxidation with an oxidizing agent such
as 2,3-
dichloro-5,6-dicyano-1,4-benzoquinone or bromine or the like, yields a
compound
represented by the general formula (I-i).
CA 02377217 2001-12-28
116
MtII--( E )-ZI
R A La OLb - ~(/XIa-2)
na nb O
(IXb-3)
R A La B Lb
na nb Zi
(XIIa-1)
R aLa B Lb ZI
ne nb
(I-i)
(wherein, R, the ring A, the ring B, the ring E, na and nb have the same
meaning as described
above for the general formula (I), Z' represents a fluorine atom or a
trifluoromethoxy group,
and Mtll represents Li, C1Mg, BrMg, or IMg.)
The case in which ring C is the formula (IIb)
In the case in which na = nb = n = 0, nd = 1, and the linkage group Ld is a
single bond,
synthesis can be achieved in the manner described below.
x 5 x 6
R ~ ~ + Mtll -~ E )---ZZ
O ~/
X4
(IVb-1) (XIa-3)
x 5 x 6
R ~ ~ jOH E ZZ
X4
(XIIIa-1)
XS xb
R q ~ E Z2
X(XIVa-1)
x 5 x 6
R ~ ~ E ZZ
X4 (I-J)
CA 02377217 2001-12-28
117
(wherein, R, X4, X5, X6 and the ring E have the same meaning as described
above for the
general formula (I), Z2 represents a fluorine atom, a chlorine atom, a
trifluoromethyl group, a
trifluoromethoxy group or a difluoromethoxy group, and Mtll represents a metal
ion such as
Li, C1Mg, BrMg, or IMg.)
That is, by reacting a ketone represented by the general formula (IVb-1) with
an aryl
lithium or magnesium reagent represented by the general formula (XIa-3) either
in or out of
the presence of a metal salt such as cerium chloride or manganese chloride and
a Lewis acid,
an alcohol represented by the general formula (XIIIa-1) can be obtained, and
subsequent
heating in the presence of an acid catalyst such as p-toluenesulfonic acid,
yields a
dihydronaphthalene compound represented by the general formula (XIVa-1), which
can be
subsequently hydrogenated in the presence of a metal catalyst such as Pd-C, Rh-
C, Pt-C or
Pd(OH)Z or the like to obtain the target compound represented by the general
formula (I j).
The compound (IVb-1) can be prepared by a variety of different methods,
although as
shown by the schemes below, a representative method comprises conversion of a
phenylacetic acid derivative represented by the general formula (IIIb-1) to an
acid chloride,
and a subsequent reaction with ethylene in the presence of aluminum chloride.
x 5 x 6 x 5 x6
1) SOCIZ -b
R / COOH 2) CH2=CHZ R ~ 0
4 AIC3 X4
(IIIb-1) (IVb-1)
(wherein, R, X4, X5 and X6 have the same meaning as described above for the
general
formula (I).)
Furthermore, in the case in which X4, X5 and X6 are all hydrogen atoms, then
as
shown in the schemes below, a compound represented by the general formula (IVb-
2) can be
synthesized by hydrogenating a naphthol derivative represented by the general
formula (XVa-
CA 02377217 2001-12-28
118
1) in the presence of a transition metal catalyst such as palladium, rhodium,
platinum or
ruthenium, followed by subsequent oxidation, where necessary.
R \/ ~ OH --~ R Cb-OH and/or R Cb-0
(XVa-1) (IVb-2)
(wherein, R has the same meaning as described for the general formula (I).)
Furthermore as shown in the schemes below, subjecting
octahydronaphthalenedione
monoacetal, which can be synthesized by reacting 1,4-cyclohexanedione
monoacetal with an
amine such as pyrrolidine followed by reaction with methyl vinyl ketone, to
the action of an
organometallic reagent represented by R'-Mtll, and performing a subsequent
dehydration
reaction, yields an octahydronaphthalenone acetal. By aromatizing this
compound, either
using a metal catalyst such as palladium, rhodium, ruthenium or platinum as a
dehydrogenation catalyst, or using the action of an oxidizing agent such as
2,3-dichloro-5,6-
dicyano-1,4-benzoquinone, or using a material such as sulfur, bromine or
iodine, and
subsequently converting the acetal to a ketone, a compound represented by the
general
formula (IVb-3) can be synthesized.
1)'N/
(03 -\ iH- O O RI-Mtll
O=~/X, - --
O 2) o a
HO O
R~ O~ - ~
O 0
Rl-q O
O Rl \ /
~ O
(IVb-3)
(wherein, R' represents a saturated alkyl group of 1 to 20 carbon atoms which
may
CA 02377217 2001-12-28
119
incorporate a branched chain and which may be substituted with 1 to 7 fluorine
atoms or
alkoxyl groups of 1 to 7 carbon atoms.)
In the case in which na = nb = nc = 0, nd = 1, and the linkage group Ld is -
CH2CH2-,
synthesis can be achieved in the manner described below.
x5 x6
R b + Mtll - E ZZ
O
X4
(IVb-1) (XIa-4)
x5 x6
R \ Zx5 x6 (XIIIa-2)
R ~ ~ 3 _ Zz
X
6 (XIVa-2)
R ~ ~ -( ~ Z2
5 x4 (I-k
(wherein, R, X4, X5, X6 and the ring E have the same meaning as described
above for the
general formula (I), Z2 represents a fluorine atom, a chlorine atom, a
trifluoromethyl group, a
trifluoromethoxy group or a difluoromethoxy group, and Mtll represents a metal
ion such as
Li, C1Mg, BrMg, or IMg.)
That is, by reacting theketone represented by the general formula (IVb-1) with
an aryl
lithium or magnesium reagent represented by the general formula (XIa-4) either
in or out of
the presence of a metal salt such as cerium chloride or manganese chloride and
a Lewis acid,
an alcohol represented by the general formula (XIIIa-2) can be obtained, and
subsequent
heating in the presence of an acid catalyst such as p-toluenesulfonic acid,
yields a
dihydronaphthalene compound represented by the general formula (XIVa-2), which
can be
subsequently hydrogenated in the presence of a metal catalyst such as Pd-C, Rh-
C, Pt-C or
CA 02377217 2001-12-28
120
Pd(OH)2 or the like to obtain the target compound represented by the general
formula (I-k).
Furthermore, if compounds represented by (XIa-5) and (XIa-6) are used instead
of the
compounds (XIa-3) and (XIa-4) shown above, then compounds represented by the
general
formulas (I-1) and (I-m) can be prepared.
Mtl'--D j-Ld'<E rZ2 (XIa-5)
Mtl' = D Ldl<1J rZ2 (XIa-6)
~J
x 5 x 6
R \/ D Ld' <D-Z 2
Xa x 5 x 6
R Xa~ ~ ll Ld >/J~-Z2 (I-m)
~ \~V~
(wherein, R, X4, X5, X6, the ring D and the ring E have the same meaning as
described above
for the general formula (I), Z2 represents a fluorine atom, a chlorine atom, a
trifluoromethyl
group, a trifluoromethoxy group or a difluoromethoxy group, and Ld' represents
a single
bond or a -CH2CH2-.)
In addition, as shown by the schemes below, by reacting the compound
represented by
the general formula (IVb-1) with an ylide compound represented by the general
formula (Vc-
1), and then hydrogenating the thus obtained olefin compound (XVIa- 1), a
compound
represented by the general formula (I-n) can be produced.
CA 02377217 2001-12-28
121
x5 x6
R E Z ~
\ / + Ph,
X4 0 PePh (Vc-1)
(IVb-1)
x5 x6
R \ / E Z
X4
(XVIa-1)
x5 x6
R \ / Z
x (I-n)
(wherein, R, X4, X5, X6, the ring E and Z have the same meaning as described
above for the
general formula (I).)
In addition, as shown by the schemes below, by reacting the
tetrahydronaphthalenone
represented by the general formula (IVb-1) with an ylide compound prepared
from a
methoxymethyl phosphonium salt, and treating the thus obtained product with
acid, an
aldehyde represented by the general formula (VIIIb-1) can be obtained. By
reacting this
aldehyde with an ylide compound represented by the formula (Vc-2), and
hydrogenating the
thus produced olefin (I-o), a compound represented by the general formula (I-
n) can be
prepared.
CA 02377217 2001-12-28
122
x 5 x6
R Ph, _f OCH3
~ ~ O + ph"P
Xa Ph
(IVb-1)
6
X X E~Z
ph_p\
R ph Ph (VC-2)
CHO
Xa
(VIIIb-1)
X5 x6
R Z --
Xa
(I-o)
x5 x6
R ~ ~ Z
xa (I-n)
(wherein, R, X4, X5, X6, the ring E and Z have the same meaning as described
above for the
general formula (I).)
In the method described above, by using the compound (IVb-2) in place of (IVb-
1), a
5 compound represented by the general formula (XVIIa) can be produced.
x5 x6
Br ~ (IVb-2)
x5 x6
Br ~\
Lcl Ldl-E -Z
xa ' ~/(XVIIa-1)
(wherein, X4, X5, X6, the ring D, the ring E and Z have the same meaning as
described above
for the general formula (I), and Lci and Ld' represent a single bond or a-
CHZCHz-.)
By substituting the bromine atom in the compound (XVIIa-1), a large number of
compounds of the general formula (I) can be produced.
CA 02377217 2001-12-28
123
For example, if the acetylene compound represented by the formula (XVIIIa-1)
is
reacted with the compound (XVIIa-1) in the presence of a palladium catalyst
such as
PdCl2(PPh3)2 or Pd(PPh3)4 and triethylamine, then a compound represented by
the general
formula (I-p) can be obtained. In addition, subsequent hydrogenation in the
presence of a
metal catalyst can convert the acetylene diyl group (-C=C-) to an ethylene
group (-CH2CH2-).
x 5 x 6
Br /~\
Lct Ld'-{ E?-Z + R~ -
X4 nc ~/
(XVIIa-1) (XVIIIa-1)
x 5 x 6
Rt - ~\
Lct Ld'-( Z
Xa
(I-P)
(wherein, X4, X5, X6, the ring D, the ring E and Z have the same meaning as
described above
for the general formula (I), R' represents a saturated or an unsaturated alkyl
group of 1 to 18
carbon atoms which may incorporate a branched chain and which may be
substituted with 1
to 7 fluorine atoms or alkoxyl groups of 1 to 7 carbon atoms, and LcI and Ldt
represent a
single bond or a -CH2CH2-.)
In addition, by altering the compound (XVIIIa-1) to (XVIIIa-2), a compound
represented by the general formula (I-q) can be produced. Subsequent
hydrogenation of this
compound in the presence of a metal catalyst can also be used to convert the
acetylene diyl
group (-C=C-) to an ethylene group (-CH2CH2-).
CA 02377217 2001-12-28
124
x 5 x 6
Br 0-z Lci Ld'- + R A -
Xa (XVIIa-1) (XVIIIa-1)
x5 x 6
R-- DA-
Lc D LdlXD-Z
X4 nc (I-q)
(wherein, R, X4, X5, X6, the ring A, the ring D, the ring E and Z have the
same meaning as
described above for the general formula (I), and Lcl and Ld' represent a
single bond or a
-CH2CH2-.)
Furthermore, by reacting the organometallic reagent represented by (XIb- 1)
with the
compound (XVIIa-1) in the presence of a nickel or a palladium catalyst, a
compound
represented by the general formula (I-r) can be produced.
x 5 x 6
Br ~/ Lc~ D Ld~ E Z + R &Mtl2
Xa
(XVIIa-1) (XIb-1)
x 5 x 6
/ Lci Ldi-( Z
Xa
(I-r)
(wherein, R, X4, X5, X6, the ring D, the ring E and Z have the same meaning as
described
above for the general formula (I), the ring A' represents a 1,4-phenylene
group which may be
substituted with one or two fluorine atoms and a naphthalene-2,6-diyl group
which may be
substituted with one or two fluorine atoms, Lcl and Ld' represent a single
bond or a
-CH2CH2-, and Mtl2 represents Li, ClMg, BrMg, IMg or (HO)2B.)
CA 02377217 2001-12-28
125
Representative examples of the compounds represented by the general formula
(I)
produced using the methods described above are shown below.
n-CA n-CA
F F
F
F
n-C3H7 F n-CA -0- F
(1-1-4) F
F
n-C3H~ n~3H~ ~
F / F
F
(1-2-2)
F
n-CA n-CA
/ ~
F CN
-0- -(:
F F
(1-2-3) (1-2-4)
n-CA
- / F n-CA
F F
(1-2-5) F
n-C3H~ ~ / F n-C3H~ ~ /
~ / &OCF3
F
(1-3-2) (1-3-3)
F
n-CA F n-C3H7
(1-3-4) (1-3-5)
The present invention also provides a liquid crystal composition comprising at
least
one compound represented by the general formula (I) as a constituent.
In a liquid crystal composition comprising at least one compound represented
by the
general formula (I) as a constituent, provided the composition shows liquid
crystallinity, any
other compound may be incorporated into the composition in addition to the
compound
CA 02377217 2001-12-28
126
represented by the general formula (I), although as a first constituent the
composition should
comprise at least one compound represented by the general formula (I), and in
addition
should also preferably comprise at least one constituent from the second
through fourth
constituents described below.
Namely, the second constituent is a so-called fluorine system (halogen system)
p-type
liquid crystal compound, and comprises a compound represented by the general
formulas
(A 1) to (A3) shown below.
AlkB Fa La Fz Pa (Al)
Alka Fa La-( Fb )---Lb Fz Pa (A2)
Alka Fa Le_ Fb f-Lb ~@_Lc Fz Pa (A3)
wherein Alka represents an alkyl group of 1 to 12 carbon atoms, which may be
either a
straight chain or contain methyl or ethyl branches, may contain a 3 to 6
membered ring
structure, may have any particular -CH2- structure within the group replaced
by a -0-,
-CH=CH-, -CH=CF-, -CF=CH-, -CF=CF- or -C=C-, and may have any particular
hydrogen
atom within the group substituted with a fluorine atom or a trifluoromethoxy
group, although
straight chain alkyl groups of 2 to 7 carbon atoms, straight chain 1-alkenyl
groups of 2 to 7
carbon atoms, straight chain 3-alkenyl groups of 4 to 7 carbon atoms, and
alkyl groups of 1 to
5 carbon atoms in which the terminal is substituted with an alkoxyl group of 1
to 3 carbon
atoms are preferred. Furthermore, in those cases in which branching leads to
an asymmetric
carbon atom, either optically active compounds or racemic mixtures may be
used.
The ring Fa, the ring Fb and the ring Fc each represent independently
a trans- 1,4-cyclohexylene group, a transdecahydronaphthalene-trans-2,6-diyl
group, a 1,4-
phenylene group which may be substituted with one or more fluorine atoms, a
naphthalene-
CA 02377217 2001-12-28
127
2,6-diyl group which may be substituted with one or more fluorine atoms, a
tetrahydronaphthalene-2,6-diyl group which may be substituted with one or more
fluorine
atoms, a 1,4-cyclohexenylene group which may be substituted with a fluorine
atom, a 1,3-
dioxane-trans-2,5-diyl group, a pyrimidine-2,5-diyl group or a pyridine-2,5-
diyl group,
although a trans-1,4-cyclohexylene group, a transdecahydronaphthalene-trans-
2,6-diyl group,
a naphthalene-2,6-diyl group which may be substituted with a fluorine atom or
a 1,4-
phenylene group which may be substituted with one or two fluorine atoms are
preferred.
Particularly in those cases in which the ring Fb is a trans-1,4-cyclohexylene
group or a
transdecahydronaphthalene-trans-2,6-diyl group, it is preferable that the ring
Fa is a trans-1,4-
cyclohexylene group, and in those cases in which the ring Fc is a trans-l,4-
cyclohexylene
group or a transdecahydronaphthalene-trans-2,6-diyl group, it is preferable
that the ring Fb
and the ring Fa are trans-1,4-cyclohexylene groups. Furthermore in (A3), it is
preferable
that the ring Fa is a trans-1,4-cyclohexylene group.
La, Lb and L are linkage groups, and each represent, independently, a single
bond,
an ethylene group (-CH2CH2-), a 1,2-propylene group (-CH(CH3)CH2- and -
CH2CH(CH3)-),
a 1,4-butylene group, -COO-, -OCO-, -OCF2-, -CF2O-, -CH=CH-, -CH=CF-, -CF=CH-,
-CF=CF-, -C=C- or -CH=N-N=CH-, although a single bond,
an ethylene group, a 1,4-butylene group, -COO-, -OCF2-, -CFzO-, -CF=CF- or -
C=C- are
preferred, and a single bond or an ethylene group are particularly desirable.
Furthermore it
is preferable that at least one of these linkage groups in (A2), and at least
two of the linkage
groups in (A3) are single bonds.
The ring Fz is an aromatic ring, and can be represented by the general
formulas (Ga)
to (Gc) shown below.
CA 02377217 2001-12-28
128
J' Je J8 Jf
a Jh
\ ~ \ / \
Jb e 3~ J~
(Ga) (Gb) (Gc)
wherein, Ja to JJ each represent independently a hydrogen atom or a fluorine
atom, although in
(Ga) it is preferable that at least one of Ja and jb is a fluorine atom, and
in (Gb) it is preferable
that at least one of Jd to Jf is a fluorine atom, with the structure in which
J is a fluorine atom
being particularly desirable.
The terminal group Pa represents a fluorine atom, a chlorine atom, a
trifluoromethoxy
group, a difluoromethoxy group, or an alkoxyl group, alkyl group, alkenyl
group or
alkenyloxy group of 2 or 3 carbon atoms substituted with a trifluoromethyl
group or a
difluoromethyl group or 2 or more fluorine atoms, although a fluorine atom, a
trifluoromethoxy group or a difluoromethoxy group are preferred, and a
fluorine atom is
particularly desirable.
Furthermore, compounds of the general formula (I) of the present invention are
not
included in (A 1) to (A3).
The third constituent is a so-called cyano system p-type liquid crystal
compound, and
comprises a compound represented by the general formulas (B1) to (B3) shown
below.
Alk' __(Fd)_L d Fy Pa (B1)
Alkb Fd Ld Fe Le Fy Pa (B2)
Alkb Fd Ld Fe Le Ff Lf Fy Pa (B3)
wherein Alkb represents an alkyl group of 1 to 12 carbon atoms, which may be
either a
straight chain or contain methyl or ethyl branches, may contain a 3 to 6
membered ring
CA 02377217 2001-12-28
129
structure, may have any particular -CHZ- structure within the group replaced
by a -0-,
-CH=CH-, -CH=CF-, -CF=CH-, -CF=CF- or -C=C-, and may have any particular
hydrogen
atom within the group substituted with a fluorine atom or a trifluoromethoxy
group, although
straight chain alkyl groups of 2 to 7 carbon atoms, straight chain 1-alkenyl
groups of 2 to 7
carbon atoms, straight chain 3-alkenyl groups of 4 to 7 carbon atoms, and
alkyl groups of 1 to
5 carbon atoms in which the terminal is substituted with an alkoxyl group of 1
to 3 carbon
atoms are preferred. Furthermore, in those cases in which branching leads to
an asymmetric
carbon atom, either optically active compounds or racemic mixtures may be
used.
The ring Fd, the ring Fe and the ring Ff each represent independently
a trans- 1,4-cyclohexylene group, a transdecahydronaphthalene-trans-2,6-diyl
group, a 1,4-
phenylene group which may be substituted with one or more fluorine atoms, a
naphthalene-
2,6-diyl group which may be substituted with one or more fluorine atoms, a
tetrahydronaphthalene-2,6-diyl group which may be substituted with one or more
fluorine
atoms, a 1,4-cyclohexenylene group which may be substituted with a fluorine
atom, a 1,3-
dioxane-trans-2,5-diyl group, a pyrimidine-2,5-diyl group or a pyridine-2,5-
diyl group,
although a trans-1,4-cyclohexylene group, a transdecahydronaphthalene-trans-
2,6-diyl group,
a naphthalene-2,6-diyl group which may be substituted with a fluorine atom or
a 1,4-
phenylene group which may be substituted with one or two fluorine atoms are
preferred.
Particularly in those cases in which the ring Fe is a trans-l,4-cyclohexylene
group or a
transdecahydronaphthalene-trans-2,6-diyl group, it is preferable that the ring
Fd is a trans-l,4-
cyclohexylene group, and in those cases in which the ring Ff is a trans-l,4-
cyclohexylene
group or a transdecahydronaphthalene-trans-2,6-diyl group, it is preferable
that the ring Fd
and the ring Fe are trans-1,4-cyclohexylene groups. Furthermore in (B3), it is
preferable
that the ring Fd is a trans-1,4-cyclohexylene group.
CA 02377217 2001-12-28
130
L , Le and Lf are linkage groups, and each represent, independently, a single
bond,
an ethylene group (-CH2CH2-), a 1,2-propylene group (-CH(CH3)CH2- and -
CH2CH(CH3)-),
a 1,4-butylene group, -COO-, -OCO-, -OCFZ-, -CF2O-, -CH=CH-, -CH=CF-, -CF=CH-,
-CF=CF-, -C=C-, -OCH2-, -CHZO- or -CH=N-N=CH-, although a single bond, an
ethylene
group, -COO-, -OCF2-, -CF2O-, -CF=CF- or -C_C- are preferred, and a single
bond, an
ethylene group or a -COO- are particularly desirable. Furthermore it is
preferable that at
least one of these linkage groups in (B2), and at least two of the linkage
groups in (B3) are
single bonds.
The ring Fy is an aromatic ring, and can be represented by the general
formulas (Gd)
to (Gf) shown below.
Jk J Jm JP
JQ
n
(Gd) J (Ge) (Gf)
wherein, Jk to jq each represent independently a hydrogen atom or a fluorine
atom, although
in (Ge) it is preferable that J" and J are hydrogen atoms.
The terminal group Pa represents a cyano group (-CN), a cyanato group (-OCN)
or a
-C=CCN group, although a cyano group is preferred.
Furthermore, compounds of the general formula (I) of the present invention are
not
included in (B 1) to (B3 ).
The fourth constituent is a so-called n-type liquid crystal compound in which
the
dielectric anisotropy is approximately zero, or a value below zero, and
comprises a compound
represented by the general formulas (Cl) to (C3) shown below.
CA 02377217 2001-12-28
131
Alkc Fg Lg Fh Alkd (C1)
Alk Fg Lg Fh Lh Fi Allcd (C2)
Alk Fg Lg-G -Lh Fi Li FJ Alkd (C3)
wherein Alk and Alkd each represent, independently, an alkyl group of 1 to 12
carbon atoms,
which may be either a straight chain or contain methyl or ethyl branches, may
contain a 3 to 6
membered ring structure, may have any particular -CH2- structure within the
group replaced
by a -0-, -CH=CH-, -CH=CF-, -CF=CH-, -CF=CF- or -C=C-, and may have any
particular
hydrogen atom within the group substituted with a fluorine atom or a
trifluoromethoxy group,
although straight chain alkyl groups of 1 to 7 carbon atoms, straight chain 1-
alkenyl groups of
2 to 7 carbon atoms, straight chain 3-alkenyl groups of 4 to 7 carbon atoms,
straight chain
alkoxyl groups of 1 to 3 carbon atoms and straight chain alkyl groups of 1 to
5 carbon atoms
in which the terminal is substituted with an alkoxyl group of 1 to 3 carbon
atoms are
preferred, and moreover, compounds in which at least one of Alkc and Alkd
represent a
straight chain alkyl group of 1 to 7 carbon atoms, a straight chain 1-alkenyl
group of 2 to 7
carbon atoms, or a straight chain 3-alkenyl group of 4 to 7 carbon atoms are
particularly
desirable.
The ring Fg, the ring Fh, the ring Fi and the ring Fj each represent
independently a
trans-1,4-cyclohexylene group, a transdecahydronaphthalene-trans-2,6-diyl
group, a 1,4-
phenylene group which may be substituted with one or two fluorine atoms or
methyl groups,
a naphthalene-2,6-diyl group which may be substituted with one or more
fluorine atoms, a
tetrahydronaphthalene-2,6-diyl group which may be substituted with one or two
fluorine
atoms, a 1,4-cyclohexenylene group which may be substituted with one or two
fluorine
atoms, a 1,3-dioxane-trans-2,5-diyl group, a pyrimidine-2,5-diyl group or a
pyridine-2,5-diyl
CA 02377217 2001-12-28
132
group, although in each compound it is preferable that there be no more than
one
transdecahydronaphthalene-trans-2,6-diyl group, naphthalene-2,6-diyl group
which may be
substituted with one or more fluorine atoms, tetrahydronaphthalene-2,6-diyl
group which may
be substituted with one or two fluorine atoms, 1,4-cyclohexenylene group which
may be
substituted with a fluorine atom, 1,3-dioxane-trans-2,5-diyl group, pyrimidine-
2,5-diyl group
or pyridine-2,5-diyl group, and that the other rings in such cases should
preferably be a trans-
1,4-cyclohexylene group or a 1,4-phenylene group which may be substituted with
one or two
fluorine atoms or methyl groups.
Lg, Lh and L' are linkage groups, and each represent, independently, a single
bond,
an ethylene group (-CH2CH2-), a 1,2-propylene group (-CH(CH3)CH2- and -
CH2CH(CH3)-),
a 1,4-butylene group, -COO-, -OCO-, -OCF2-, -CF2O-, -CH=CH-, -CH=CF-, -CF=CH-,
-CF=CF-, -C=C-, or -CH=N-N=CH-, although a single bond, an ethylene group, a
1,4-
butylene group, -COO-, -OCO-, -OCF2-, -CF2O-, -CF=CF-, -C=C-, or -CH=N-N=CH-
are
preferred, and it is also preferable that at least one of these linkage groups
in (C2), and at least
two of the linkage groups in (C3) are single bonds.
Preferred forms for (C 1) can be represented by the general formulas (C 1 a)
to (C 1 h)
shown below.
CA 02377217 2001-12-28
133
A(k' Fgl Fhl Alkf ~- Alk' F92 Fh2 Alkf
(Cla) (Clb)
Alke Fg2 = Fh2 Alkf O Fh2 Alkf
Alk' FP,2
(Clc)
(Cld)
F
f
Alke Fg2 ~ A~ O Fh2 Alkf
Alk' <94 F
F F
(Cle) (Cif)
Alke Fg2 Fh2 A1kf
(Clg) Alke Fg3 N_N Fh3 Alkf
(Clh)
In each of the formulas above, Alk' and Alkf each represent, independently, a
straight
chain alkyl group of 1 to 7 carbon atoms, a straight chain 1-alkenyl group of
2 to 7 carbon
atoms, a straight chain 3-alkenyl group of 4 to 7 carbon atoms, a straight
chain alkoxyl group
of 1 to 3 carbon atoms or a straight chain alkyl groups of 1 to 5 carbon atoms
in which the
terminal is substituted with an alkoxyl group of 1 to 3 carbon atoms, although
at least one of
Alk' and A1kf represents a straight chain alkyl group of 1 to 7 carbon atoms,
a straight chain
1-alkenyl group of 2 to 7 carbon atoms, or a straight chain 3 -alkenyl group
of 4 to 7 carbon
atoms. In those cases in which the rings Fgl to Fg3 are aromatic, the
corresponding ALke
excludes 1-alkenyl groups and alkoxyl groups, and in those cases in which the
rings Fhl to
Fh3 are aromatic, the corresponding ALkf excludes 1-alkenyl groups and alkoxyl
groups.
The ring Fg 1 and the ring Fh 1 each represent independently a trans-1,4-
cyclohexylene
group, a transdecahydronaphthalene-trans-2,6-diyl group, a 1,4-phenylene group
which may
be substituted with one or two fluorine atoms or methyl groups, a naphthalene-
2,6-diyl group
which may be substituted with one or more fluorine atoms, a
tetrahydronaphthalene-2,6-diyl
group which may be substituted with one or two fluorine atoms, a 1,4-
cyclohexenylene group
CA 02377217 2001-12-28
134
which may be substituted with one or two fluorine atoms, a 1,3-dioxane-trans-
2,5-diyl group,
a pyrimidine-2,5-diyl group or a pyridine-2,5-diyl group, although in each
compound it is
preferable that there be no more than one transdecahydronaphthalene-trans-2,6-
diyl group,
naphthalene-2,6-diyl group which may be substituted with one or more fluorine
atoms,
tetrahydronaphthalene-2,6-diyl group which may be substituted with one or two
fluorine
atoms, 1,4-cyclohexenylene group which may be substituted with a fluorine
atom, 1,3-
dioxane-trans-2,5-diyl group, pyrimidine-2,5-diyl group or pyridine-2,5-diyl
group, and that
the other ring in such cases should preferably be a trans-1,4-cyclohexylene
group or a 1,4-
phenylene group which may be substituted with one or two fluorine atoms or
methyl groups.
The ring Fg2 and the ring Fh2 each represent independently a trans-l,4-
cyclohexylene group,
a transdecahydronaphthalene-trans-2,6-diyl group, a 1,4-phenylene group which
may be
substituted with one or two fluorine atoms or methyl groups, a naphthalene-2,6-
diyl group
which may be substituted with one or more fluorine atoms, or a
tetrahydronaphthalene-2,6-
diyl group which may be substituted with one or two fluorine atoms, although
in each
compound it is preferable that there be no more than one
transdecahydronaphthalene-trans-
2,6-diyl group, naphthalene-2,6-diyl group which may be substituted with one
or more
fluorine atoms, or tetrahydronaphthalene-2,6-diyl group which may be
substituted with one or
two fluorine atoms, and that the other ring in such cases should preferably be
a trans-l,4-
cyclohexylene group or a 1,4-phenylene group which may be substituted with one
or two
fluorine atoms or methyl groups. The ring Fg3 and the ring Fh3 each represent
independently a 1,4-phenylene group which may be substituted with one or two
fluorine
atoms or methyl groups, a naphthalene-2,6-diyl group which may be substituted
with one or
more fluorine atoms, or a tetrahydronaphthalene-2,6-diyl group which may be
substituted
with one or two fluorine atoms, although in each compound it is preferable
that there be no
CA 02377217 2001-12-28
135
more than one naphthalene-2,6-diyl group which may be substituted with one or
more
fluorine atoms or one tetrahydronaphthalene-2,6-diyl group which may be
substituted with
one or two fluorine atoms.
Preferred forms for (C2) can be represented by the general formula (C2a) to
(C2m)
shown below.
Ajke Fgl Fhl Fil Alkf (C2a)
Alke S Fh2 Fi2 Alkf (C2b)
O Fi2Alkf
Alke F92 Fh2 (C2c)
O
A]ke Fg2 Fh3 = Fi3 Alkf (C2d)
Alke Fg2 Fh2 Fi2 Alkf (C2e)
O Fi3 Alkf
Alke Fg3 Fh3 (C2f)
F
FF
Fi3 Alkf
A1ke Fg3 Fh3 O (C2g)
Alke Fg2 O Fi2 Alkf (C2h)
Fh2
O O
Alke Fg2 O Fi2 Alkf (C2i)
O-~C'~~l ''
O
O O
Alke Fg2 Fi2 Alkf (C2j)
O Fh2 O
Alke Fg2 O O Fi2 Alkf (C2k)
~-- Fh2
O O
Alke Fg2
Fh3 = Fi3 Alkf (C21)
O Fi2 Alkf
Alke Fg3 = Fh3 (C2m)
0
CA 02377217 2001-12-28
136
In each of the formulas above, the ring Fg 1, ring Fg2, ring Fg3, ring Fh 1,
ring Fh2 and
the ring Fh3 each represent the same meaning as that described above, and the
ring Fi 1
represents the same meaning as the ring Fgl, the ring Fi2 the same as the ring
Fg2, and the
ring Fi3 the same as the ring Fg3. Furthermore, in each of the compounds
listed above, it is
preferable that there be no more than one transdecahydronaphthalene-trans-2,6-
diyl group,
naphthalene-2,6-diyl group which may be substituted with one or more fluorine
atoms,
tetrahydronaphthalene-2,6-diyl group which may be substituted with one or two
fluorine
atoms, 1,4-cyclohexenylene group which may be substituted with a fluorine
atom, 1,3-
dioxane-trans-2,5-diyl group, pyrimidine-2,5-diyl group or pyridine-2,5-diyl
group, and that
the other rings in such cases should preferably be a trans-1,4-cyclohexylene
group or a 1,4-
phenylene group which may be substituted with one or two fluorine atoms or
methyl groups.
Preferred forms for (C3) can be represented by the general formulas (C3a) to
(C3f)
shown below.
A1ke Fgl Fhl Fil Fjl Alkf (C3a)
p]ke Fgl Fhl Fi2 Fj2 Alkf(C3b)
O
A]ke Fgl Fhl Fi2 f (C3c)
O Fj2 Alk
Alhe Fg] Fhl Fi2 O Fj2 Alkf (C3d)
O O
Alke Fg2 Fh2 ~ f (C3e)
O Fi2 FJ2 Alk
O
Alke Fg2 Fh2 Fj2 Alkf (CM)
O Fi2
In each of the formulas above, the ring Fgl, ring Fg2, ring Fhl, ring Fh2,
ring Fi 1 and
the ring Fi2 each represent the same meaning as that described above, and the
ring Fj I
CA 02377217 2001-12-28
137
represents the same meaning as the ring Fgl, and the ring Fj2 the same as the
ring Fg2.
Furthermore, in each of the compounds listed above, it is preferable that
there be no more
than one transdecahydronaphthalene-trans-2,6-diyl group, naphthalene-2,6-diyl
group which
may be substituted with one or more fluorine atoms, tetrahydronaphthalene-2,6-
diyl group
which may be substituted with one or two fluorine atoms, 1,4-cyclohexenylene
group which
may be substituted with a fluorine atom, 1,3-dioxane-trans-2,5-diyl group,
pyrimidine-2,5-
diyl group or pyridine-2,5-diyl group, and that the other rings in such cases
should preferably
be a trans-l,4-cyclohexylene group or a 1,4-phenylene group which may be
substituted with
one or two fluorine atoms or methyl groups.
As will be shown in the examples described below, when a practical liquid
crystal
composition with a nematic phase upper limit temperature of 117 C is prepared
using the
aforementioned compounds, and 20% by weight of a compound represented by the
general
formula (I) is added, the compound represented by the general formula (I)
displays superior
solubility with respect to the host liquid crystal composition, and it is
clear that the liquid
crystal phase can be exhibited over a very wide temperature range.
In addition, when the voltage holding rate of this composition was measured,
the
results before and after heating and after ultraviolet light irradiation were
quite high and
similar to those of the host liquid crystal composition.
In this manner, a compound of the present invention shows superior liquid
crystallinity, and superior co-solubility with currently used liquid crystal
compounds and
liquid crystal compositions. Furthermore, the temperature range over which
liquid
crystallinity is shown is broad, the threshold voltage is low, and in terms of
preparing a liquid
crystal composition capable of high speed response, the compound displays
superior results
CA 02377217 2001-12-28
138
to conventional compounds.
Consequently, compounds of the general formula (I) can be suitably used in
mixtures
with other nematic liquid crystal compounds, for electric field effect type
display cells of TN
and STN type cells, and are particularly applicable to liquid crystal
materials with a broad
temperature range for which low voltage driving is possible. Furthermore of
the compounds
represented by the general formula (I), those compounds which incorporate no
cyano groups
or ester linkages offer a large specific resistance and a high voltage holding
rate, and so may
also be used as constituents in active matrix driven liquid crystal materials.
In addition, use
of the compounds of the general formula (I) is not limited to nematic liquid
crystals, and
application as a low viscosity material essential for realizing the high speed
response of
ferroelectric liquid crystals and antiferroelectric liquid crystals can also
be expected.
EXAMPLES
As follows is a description of specific examples of the present invention,
with a
detailed description of the advantages offered by a tetrahydronaphthalene
derivative of the
present invention and a production method thereof, and a liquid crystal
composition
comprising a tetrahydronaphthalene derivative of the present invention as a
constituent,
although the purport and range of application of the present invention is of
course not limited
to these examples.
(Example 1) Synthesis of 2-(4-propylphenyl)-6-fluoro-1,2,3,4-
tetrahydronaphthalene (I-1-1)
CA 02377217 2001-12-28
139
OH
O 1) SOC12 n-CA ---~&MgBr
2) CHZ=CH2 O /~ F
A1C13 -
HO
n-C3H~ ~ / 4CH3-C6H4-SO3H n~3H~ ~ / ~
/ ~ F F
HZ n-C3H7 -Q / ~ F
Pd-C _
(1-1) Synthesis of 6-fluoro-3,4-dihydro-2(1 H)-naphthalenone
To a solution of 30 g of 4-fluorophenylacetic acid and 48.9 g of thionyl
chloride in 60
ml of 1,2-dichloroethane was added a catalytic quantity of pyridine, and the
solution was then
refluxed for 5 hours under an atmosphere of nitrogen. Following removal of the
1,2-
dichloroethane, the product was added dropwise to an ice cooled suspension of
48.6 g of
aluminum chloride in 200 ml of dichloromethane. Following stirring for 30
minutes,
ethylene gas was blown into the reaction vessel, and after a further 5 hours
of stirring, dilute
hydrochloric acid was added, and following separation of the organic layer,
the aqueous layer
was extracted with toluene. The toluene extract was combined with the organic
layer and
washed subsequently with water, a saturated aqueous solution of sodium
bicarbonate, water,
and a saturated aqueous solution of sodium chloride, and was then dried over
anhydrous
sodium sulfate and the solvent removed by evaporation, and subsequently
purified by
distillation (75 C, 2 Torr) to obtain 19.4 g of 6-fluoro-3,4-dihydro-2(1H)-
naphthalenone.
IR 1723, 1617m 1596 cm' 1
'H NMR (CDC13) S 7.1-6.9 (m,3H), 3.6 (s, 2H), 3.0-2.5 (m, 4H)
13C NMR (CDC13) S 210, 160, 139, 130, 129, 115, 114,44, 38,28
MS m/z 164, 149, 135, 122, 115, 109, 101, 96, 83, 75, 63, 57
CA 02377217 2001-12-28
140
(1-2) Synthesis of 2-(4-propylphenyl)-6-fluoro- 1,2,3,4-tetrahydronaphthalene
(I-1-1)
3.5 g of magnesium was suspended in 4 ml of tetrahydrofuran (THF), and a
solution
of 25.7 g of 4-propylbromobenzene in 100 ml of THF was then added dropwise to
the
suspension over an approximately 30 minute period at such a rate that the THF
refluxed
gently. The mixture was then stirred for a further 1 hour, and a solution of
9.4 g of 6-fluoro-
3,4-dihydro-2(1 H)-naphthalenone obtained from (1-1) in 80 ml of THF was then
added
dropwise to the mixture over a 30 minute period. Following stirring for a
further 1 hour, 50
ml of 10% hydrochloric acid was added. 100 ml of hexane was then added, the
organic
layer was separated, and the aqueous layer was extracted with a further 100 ml
of hexane
which was combined with the organic layer. The combined organic extract was
then
washed with water, a saturated aqueous solution of sodium bicarbonate, and a
saturated
aqueous solution of sodium chloride, and was then dried over anhydrous sodium
sulfate.
The solvent was then removed by evaporation, 100 ml of toluene and 2.0 g of p-
toluenesulfonic acid monohydrate were added, and the mixture was heated at 110
C with
stirring while evaporated water was separated and removed. When the
evaporation of water
had ceased, the temperature was reduced to room temperature, 50 ml of water
was added, and
the organic layer was separated. The organic layer was washed with a saturated
aqueous
solution of sodium bicarbonate, water, and a saturated aqueous solution of
sodium chloride,
and was then dried over anhydrous sodium sulfate. The solvent was removed by
evaporation, and the whole residue was dissolved in 200 ml of ethyl acetate.
2.0 g of 5%
palladium-carbon (wet) was then added, and the mixture was stirred in an
autoclave under a
hydrogen pressure of 4 Kg/cm2. After stirring for 5 hours at room temperature,
the catalyst
was removed by filtration through celite, the solvent was removed by
evaporation, and the
residue was purified by silica gel column chromatography (hexane), and was
recrystallized
CA 02377217 2001-12-28
141
twice from ethanol to obtain 17.2 g of white crystals of 2-(4-propylphenyl)-6-
fluoro-1,2,3,4-
tetrahydronaphthalene.
(Example 2) Synthesis of 2-(4-propylphenyl)-5,6,7-trifluoro-1,2,3,4-
tetrahydronaphthalene
(1-1-2)
Under the same conditions as those described for the example 1, using 3,4,5-
trifluorophenylacetic acid instead of 4-fluorophenylacetic acid yielded 2-(4-
propylphenyl)-
5,6, 7-trifluoro-1,2,3,4-tetrahydronaphthalene.
(Example 3) Synthesis of 2-(4-propylphenyl)-5,7-difluoro-6-cyano-1,2,3,4-
tetrahydronaphthalene
OH F F
O n-C3H7 ~ ~ ~ ~ 1) BuLi
--
~
F 2) COZ
- F 1) SOZCI
n-CA
COOH 2) NH3
3) POCI3
F
n-CA
CN
--CI
F
Under the same conditions as those described for the example 1, using 3,5-
difluorophenylacetic acid instead of 4-fluorophenylacetic acid yielded 2-(4-
propylphenyl)-
5,7-difluoro-1,2,3,4-tetrahydronaphthalene. 10 g of this 2-(4-propylphenyl)-
5,7-difluoro-
1,2,3,4-tetrahydronaphthalene was lithiated using butyllithium, converted to a
benzoic acid by
blowing carbon dioxide into the reaction vessel, subsequently converted to an
acid chloride
with thionyl chloride, and an amide then synthesized by blowing ammonia gas
into the
reaction mixture was dissolved in 40 ml of DMF, 2.5 ml of phosphorus
oxychloride was
CA 02377217 2001-12-28
142
added, and the reaction was allowed to proceed for 2 hours at 25 C. The
reaction liquid was
poured into water with ice, dilute hydrochloric acid was added, and the water
layer was
extracted with toluene. The toluene extract was combined with the organic
layer, and
following washing with water, a saturated aqueous solution of sodium
bicarbonate, water and
a saturated aqueous solution of sodium chloride, was dried over anhydrous
sodium sulfate.
The crude product was purified using silica gel column chromatography
(hexane/dichloromethane = 6/4), and then recrystallized from ethanol to yield
6.3 g of 2-(4-
propylphenyl)-5,7-difluoro-6-cyano-1,2,3,4-tetrahydronaphthalene.
(Example 4) Synthesis of 2-[2-(trans-4-propylcyclohexyl)ethyl]-6-fluoro-
1,2,3,4-
tetrahydronaphthalene (I-1-3)
1) /=PPh3 /-\ PPh3
Me0 n-C3H7-(~/}
F ~ /
2) H+ O F
n C3H7-0 F ? n-C3H7--( / F
Pd-C
(4-1) Synthesis of 6-fluoro-1,2,3,4-tetrahydronaphthalene-2-carbaldehyde
A solution of 50 g of 6-fluoro-3,4-dihydro-2(1 H)-naphthalenone obtained from
(1-
1) in 250 ml THF was added dropwise, at a temperature of 0 C, to a Wittig
reagent prepared
from 156.6 g of inethoxymethyltriphenylphosphonium chloride and 51.3 g of
potassium t-
butoxide in ice cooled THF. After 1 hour of reaction, the mixture was returned
to room
temperature, water was added, and the organic layer was concentrated. Hexane
was then
added to dissolve the residue, the insoluble triphenylphosphine oxide was
removed by
filtration, and the product was washed in a 1/1 solvent mixture of methanol
and water. The
CA 02377217 2001-12-28
143
crude product obtained by concentrating the hexane layer was dissolved in 250
ml of THF,
250 ml of dilute hydrochloric acid was added, and the mixture was refluxed for
3 hours.
Toluene was then added, and following washing with water, the product was
dried over
anhydrous sodium sulfate and the solvent removed by evaporation to yield 46.7
g of 6-fluoro-
1,2,3,4-tetrahydronaphthalene-2-carbaldehyde.
(4-2) Synthesis of 2-[2-(trans-4-propylcyclohexyl)ethyl]-6-fluoro-1,2,3,4-
tetrahydronaphthalene (I-1-3)
A solution comprising all of the 6-fluoro-1,2,3,4-tetrahydronaphthalene-2-
carbaldehyde obtained from (4-1) dissolved in 250 ml THF was added dropwise,
at a
temperature of 0 C, to a Wittig reagent prepared from 188.7 g of 2-(trans-4-
propylcyclohexyl) methyltriphenyiphosphonium bromide and 44.1 g of potassium t-
butoxide
in ice cooled THF. After 1 hour of reaction, the mixture was returned to room
temperature,
water was added, and the organic layer was concentrated. Hexane was then added
to
dissolve the residue, the insoluble triphenylphosphine oxide was removed by
filtration, and
the product was washed in a 1/1 solvent mixture of methanol and water. The
crude product
obtained by concentrating the hexane layer was dissolved in 200 ml of ethyl
acetate, 10 g of
5% palladium-carbon (wet) was added, and the mixture was stirred in an
autoclave under a
hydrogen pressure of 4 Kg/cm2. After stirring for 5 hours at room temperature,
the catalyst
was removed by filtration through celite, the solvent was removed by
evaporation, and the
residue was purified by silica gel column chromatography (hexane), and two
separate
recrystallizations from ethanol to obtain 50.7 g of white crystals of 2-[2-
(trans-4-
propylcyclohexyl)ethyl]-6-fluoro-1,2,3,4-tetrahydronaphthalene (1-1-3).
(Example 5) Synthesis of 2-[2-(trans-4-propylcyclohexyl)ethyl]-5,6,7-trifluoro-
1,2,3,4-
CA 02377217 2001-12-28
144
tetrahydronaphthalene (I-1-4)
Under the same conditions as those described for the example 4, using the
5,6,7-
trifluoro-3,4-dihydro-2(1 H)-naphthalenone synthesized in the example 2
instead of 6-fluoro-
3,4-dihydro-2(1 H)-naphthalenone yielded 2-[2-(trans-4-propylcyclohexyl)ethyl]-
5,6,7-
trifluoro- 1,2,3,4-tetrahydronaphthalene.
(Example 6) Synthesis of 2-propyl-6-(3,4-difluorophenyl)-1,2,3,4-
tetrahydronaphthalene (I-
2-1)
F
1) CNH 1)BrM O F F F
n-C3H7-~ FO 2 n-C3H7~ 2) p-TsOH n-C3H7 - _
~J ~O O / \ /
Br2 F
am n-CsH7 - -
F
(6-1) Synthesis of 6-propyl-4,4a,5,6,7,8-hexahydro-3H-naphthalen-2-one
212 g of 4-propylcyclohexanone and 200 ml of pyrrolidine was dissolved in 600
ml of
toluene, and the mixture was heated with stirring for 3 hours, and any
azeotropically distilled
water was removed. Excess pyrrolidine was then removed by azeotropic
distillation with
toluene, to obtain 1-(4-propylcyclohexa-l-en-1-yl)-pyrrolidine. The crude
product was
cooled, as is, to room temperature, a further 800 ml of toluene was added, the
mixture was
cooled in a water bath, and 120 ml of methyl vinyl ketone was added dropwise
over a period
of 1 hour at a temperature of 25 C or lower. Following completion of the
addition, the
mixture was immediately heated and refluxed for 20 hours. The solution was
then cooled to
room temperature, a buffer solution of pH 5 prepared from 63 g of sodium
acetate, 120 ml of
acetic acid and 140 ml of water was added, and the reflux was continued for a
further 4 hours.
After the solution had been cooled to room temperature, the organic layer was
separated and
CA 02377217 2001-12-28
145
washed with water and a saturated aqueous solution of sodium chloride. The
organic layer
was then dried over anhydrous sodium sulfate, and the solvent removed by
evaporation to
obtain 320 g of a crude product of 6-propyl-4,4a,5,6,7,8-hexahydro-3H-
naphthalen-2-one.
(6-2) Synthesis of 2-propyl-6-(3,4-difluorophenyl)-1,2,3,4,8,8a-
hexahydronaphthalene
27.1 g of magnesium was suspended in 70 ml of tetrahydrofuran (THF), and a
solution of 195.7 g of 3,4-difluorobromobenzene dissolved in 800 ml of THF was
then added
dropwise with the mixture under conditions of heated reflux. Following
stirring for a
further one hour, a solution of 150 g of the 6-propyl-4,4a,5,6,7,8-hexahydro-
3H-naphthalen-
2-one obtained from (6-1) above in 600 ml of THF was added dropwise, with
continuous
stirring and with the reaction mixture cooled in a water bath. Following
stirring for a further
two hours, the mixture was cooled in an ice bath, and 1000 ml of 10%
hydrochloric acid was
added. The product was then extracted with toluene, washed subsequently with
water, and a
saturated aqueous solution of sodium chloride, and was then dried over
anhydrous
magnesium sulfate. Subsequently, the crude product obtained by removal of the
solvent by
evaporation was redissolved in 1200 ml of toluene, 14.9 g of p-toluenesulfonic
acid was
added, and the mixture was heated with stirring for 3 hours and any
azeotropically distilled
water was removed. After the solution had been cooled to room temperature, the
toluene
layer was washed subsequently with water and a saturated aqueous solution of
sodium
chloride, and then dried over anhydrous magnesium sulfate, before the solvent
was removed
by evaporation to obtain 250 g of a crude product of 2-propyl-6-(3,4-
difluorophenyl)-
1,2,3,4,8,8a-hexahydronaphthalene.
(6-3) Synthesis of 2-propyl-6-(3,4-difluorophenyl)-1,2,3,4-
tetrahydronaphthalene
All of the crude 2-propyl-6-(3,4-difluorophenyl)-1,2,3,4,8,8a-
hexahydronaphthalene
obtained from (6-2) above was dissolved in 1200 ml of methylene chloride, the
solution was
CA 02377217 2001-12-28
146
cooled in an ice bath, and 44.2 ml of bromine was then added dropwise with
stirring, with the
stirring continued for a further 3 hours following completion of the addition.
Subsequently,
an aqueous solution of sodium hydrogen sulfite was added, and following 30
minutes of
vigorous stirring, the methylene chloride layer was washed with a saturated
aqueous solution
of sodium chloride, dried over anhydrous magnesium sulfate, and concentrated
to obtain a
crude product of 2-propyl-6-(3,4-difluorophenyl)-1,2,3,4-
tetrahydronaphthalene. This crude
product was subsequently purified by silica gel column chromatography
(hexane), and then
dissolved in 800 ml of ethyl acetate, and stirred for 6 hours in an autoclave
with 20 g of 5%
palladium-carbon, under a hydrogen atmosphere of 4 Kg/cm2. Following
filtration, the
solvent was removed by evaporation, and from the 230 g of crude product thus
obtained, 20 g
was purified by silica gel column chromatography (hexane), and then
recrystallized 3 times
from ethanol to obtain 6.5 g of white crystals of 2-propyl-6-(3,4-
difluorophenyl)-1,2,3,4-
tetrahydronaphthalene.
(Example 7) Synthesis of 2-propyl-6-(3,4,5-trifluorophenyl)-1,2,3,4-
tetrahydronaphthalene
(1-2-2)
CuBr2, LiBr Tf20
n-C3H7~0 n-C3H7 OH ,: n-C3H7
\ / OTf
F
(HO)2B a F
F n'CsH7
\ / \ / F
Pd(PPh3)4 F
(7-1) Synthesis of 2-propyl- 1,2,3,4-tetrahydro-6-naphthol
5 g of 6-propyl-4,4a,5,6,7,8-hexahydro-3H-naphthalen-2-one obtained from (6-1)
was
dissolved in 10 ml of acetonitrile, and a solution of 11.6 g of copper (II)
bromide and 2.3 g of
lithium bromide in 50 ml of acetonitrile was then added dropwise at room
temperature.
CA 02377217 2001-12-28
147
Following stirring of the reaction mixture for a fiuther two hours, the
solvent was removed by
evaporation, and the residue was redissolved in ethyl acetate and filtered to
remove any
insoluble components. The solution was then washed with water and a saturated
aqueous
solution of sodium chloride. Following drying over anhydrous sodium sulfate,
the solvent
was removed by evaporation to obtain a crude product of 2-propyl-1,2,3,4-
tetrahydro-6-
naphthol.
(7-2) Synthesis of 2-propyl-1,2,3,4-tetrahydronaphthalen-6-yl
trifluoromethanesulfonate
The crude 2-propyl-1,2,3,4-tetrahydro-6-naphthol obtained from (7-1) was
dissolved
in 20 ml of dichloromethane, 4.7 ml of anhydrous trifluoromethanesulfonic acid
was then
added and suspended, and the mixture was cooled to 5 C. 4.6 ml of pyridine was
then
added dropwise with vigorous stirring, and the mixture then stirred for a
further one hour.
ml of water was then added, the reaction was halted, and the organic layer was
separated
off. The aqueous layer was extracted with 20 ml of dichloromethane, which was
combined
with the organic layer, and the combined organic layer was then washed with
dilute
15 hydrochloric acid, a saturated aqueous solution of sodium bicarbonate,
water, and then a
saturated aqueous solution of sodium chloride, and subsequently dried over
anhydrous
sodium sulfate. Following removal of the solvent by evaporation, the crude
product was
purified by silica gel column chromatography (hexane) to obtain 3.8 g of
2-propyl-1,2,3,4-tetrahydronaphthalen-6-yl trifluoromethanesulfonate.
20 (7-3) Synthesis of 2-propyl-6-(3,4,5-trifluorophenyl)- 1,2,3,4-
tetrahydronaphthalene
A mixture of 3.8 g of the produced 2-propyl-1,2,3,4-tetrahydronaphthalen-6-yl
trifluoromethanesulfonate, 3.0 g of 3,4,5-trifluorophenylboric acid (this
compound was
produced by reacting a Grignard reagent prepared from 3,4,5-
trifluorobromobenzene and
magnesium, with trimethoxy borane, and then performing a hydrolysis with
dilute
CA 02377217 2001-12-28
148
hydrochloric acid), 0.13 g of tetrakis(triphenylphosphine) palladium(0), and
3.6 g of
potassium phosphate in 20 ml of dimethyl formamide (DMF) was stirred for 10
hours at
80 C. The mixture was subsequently cooled to room temperature, 20 ml of water
was
added, the mixture was extracted with toluene, and the organic layer was
washed
subsequently with water and then a saturated aqueous solution of sodium
chloride, and
subsequently dried over anhydrous sodium sulfate. The crude product obtained
by removal
of the solvent by evaporation was purified by silica gel column chromatography
(hexane) and
then recrystallized 3 times from ethanol to obtain 0.5 g of 2-propyl-6-(3,4,5-
trifluorophenyl)-
1,2,3,4-tetrahydronaphthalene.
(Example 8) Synthesis of 2-(trans-4-propylcyclohexyl)-6-(3,4,5-
trifluorophenyl)-1,2,3,4-
tetrahydronaphthalene (1-2-3)
F
1) [CNH 1) BrM9 a F
n-CgH~ _ F _
n C3H7 -0--(:)=O 2) 0 2) p-TsOH
0
n_C3H7 ~ F DDQ n_C3H7 F
F F
F F
(8-1) Synthesis of 2-(4-propylcyclohexyl)-6-(3,4,5-trifluorophenyl)-
1,2,3,4,8,8a-
hexahydronaphthalene
A synthesis was performed in the same manner as (6-1) of the example 6, with
the
exception of using 4-(4-propylcyclohexyl)-cyclohexanone instead of 4-
propylcyclohexanone,
and yielded 6-(4-propylcyclohexyl)-4,4a,5,6,7,8-hexahydro-3H-naphthalen-2-one.
Subsequently, the synthesis was continued in the same manner as (6-2), with
the exception of
using 3,4,5-trifluorobromobenzene instead of 3,4-difluorobromobenzene, and
yielded 2-(4-
propylcyclohexyl)-6-(3,4,5-trifluorophenyl)-1,2,3,4,8,8a-hexahydronaphthalene.
CA 02377217 2001-12-28
149
(8-2) Synthesis of 2-(4-propylcyclohexyl)-6-(3,4,5-trifluorophenyl)-1,2,3,4-
tetrahydronaphthalene
A mixture of 20 g of 2-(4-propylcyclohexyl)-6-(3,4,5-trifluorophenyl)-
1,2,3,4,8,8a-
hexahydronaphthalene obtained from (8-1) above and 14 g of 2,3-dichloro-5,6-
dicyano-1,4-
benzoquinone (DDQ) in 80 ml of toluene was stirred for 3 hours at room
temperature.
Water and toluene were then added, the mixture was filtered, and the toluene
layer was then
separated and washed subsequently in water and a saturated aqueous solution of
sodium
chloride, before being dried over anhydrous magnesium sulfate. Following
removal of the
solvent by evaporation, the residue was purified by silica gel column
chromatography
(hexane) to obtain 21.1 g of a crude product. All of this crude product was
subsequently
dissolved in 80 ml of ethyl acetate, and then stirred for 6 hours in an
autoclave with 4 g of 5%
palladium-carbon, under a hydrogen atmosphere of 4 Kg/cm2. Following
filtration, the
solvent was removed by evaporation, and the crude product thus obtained was
purified by
silica gel column chromatography (hexane), and then recrystallized 3 times
from
ethanol/toluene to obtain 6.2 g of 2-(4-propylcyclohexyl)-6-(3,4,5 -
trifluorophenyl)- 1,2,3,4-
tetrahydronaphthalene.
(Example 9) Synthesis of 2-propyl-6-(4-cyano-3,5-difluorophenyl)-1,2,3,4-
tetrahydronaphthalene (1-2-4)
F
1) BrM9 0 F
1) n-BuLi
n_C3H7 _ F n-C3H7 Is
O 2) p-TsOH 2) COZ
3) DDQ F 3) SOCIz
4) NH3
F F
n-CsH7 POC13 n-C3H7
CONH2 CN
F F
CA 02377217 2001-12-28
150
(9-1) Synthesis of 2-propyl-6-(3,5-difluorophenyl)-1,2,3,4-
tetrahydronaphthalene
A synthesis was performed in the same manner as (6-2) of the example 6, with
the
exception of replacing 3,4-difluorobromobenzene with 3,5-difluorobromobenzene,
and
yielded 212.5 g of 2-propyl-6-(3,5-difluorophenyl)-1,2,3,4,8,8a-
hexahydronaphthalene
from 200 g of 6-propyl-4,4a,5,6,7,8-hexahydro-3H-naphthalen-2-one.
Subsequently, the
synthesis was continued in the same manner as (8-2), and an additional vacuum
distillation
then carried out, to obtain 112 g of 2-propyl-6-(3,5-difluorophenyl)-1,2,3,4-
tetrahydronaphthalene.
(9-2) Synthesis of 2-propyl-6-(3,5-difluoro-4-carbamoylphenyl)-1,2,3,4-
tetrahydronaphthalene
27 .1 g of the 2-propyl-6-(3,5-difluorophenyl)-1,2,3,4-tetrahydronaphthalene
obtained
in (9-1) above was dissolved in 120 ml of THF, and with the solution cooled to
-50 C, 68.9
ml of a 1.51 M hexane solution of n-butyllithium was added dropwise. Carbon
dioxide gas
was then blown through the solution, and then water was added and the product
was extracted
with ethyl acetate. The organic layer was then washed subsequently with water
and a
saturated aqueous solution of sodium chloride, and then dried over anhydrous
magnesium
sulfate. Following removal of the solvent by evaporation, the crude product
was redissolved
in 80 ml of 1,2-dichloroethane and 8.2 ml of thionyl chloride was added.
Following the
dropwise addition of a catalytic quantity of pyridine, the solution was
stirred for 2 hours at
50 C and any excess thionyl chloride and the 1,2-dichloroethane were removed
by
evaporation. The residue was dissolved in 100 ml of methylene chloride, and
ammonia gas
was blown into the solution at room temperature, with stirring, and when the
heat generation
had ceased, water was added, the product was extracted with ethyl acetate, and
the organic
layer was washed subsequently with water and a saturated aqueous solution of
sodium
CA 02377217 2001-12-28
151
chloride, before being dried over anhydrous magnesium sulfate. The solvent was
removed
by evaporation and yielded a crude product of 2-propyl-6-(3,5-difluoro-4-
carbamoylphenyl)-
1,2,3,4-tetrahydronaphthalene.
(9-3) Synthesis of 2-propyl-6-(4-cyano-3,5-difluorophenyl)-1,2,3,4-
tetrahydronaphthalene
All of the crude product of 2-propyl-6-(3,5-difluoro-4-carbamoylphenyl)-
1,2,3,4-
tetrahydronaphthalene obtained in (9-2) above was dissolved in 120 ml of DMF,
13 ml of
phosphorus oxychloride was added, and the reaction was allowed to proceed for
2 hours at
25 C. The reaction liquid was then poured into water with ice, dilute
hydrochloric acid was
added, and the water layer was extracted with toluene. The toluene extract was
combined
with the organic layer, and following washing with water, a saturated aqueous
solution of
sodium bicarbonate and a saturated aqueous solution of sodium chloride, was
dried over
anhydrous sodium sulfate. The crude product was purified using silica gel
column
chromatography (toluene) followed by alumina column chromatography (toluene),
further
purified by activated carbon treatment in an acetone solution, and then
recrystallized seven
times from ethanol to obtain 4.0 g of 2-propyl-6-(4-cyano-3,5-difluorophenyl)-
1,2,3,4-
tetrahydronaphthalene.
(Example 10) Synthesis of 2-propyl-5-fluoro-6-(3,4,5-trifluorophenyl)-1,2,3,4-
tetrahydronaphthalene, and 2-propyl-7-fluoro-6-(3,4,5-trifluorophenyl)-1,2,3,4-
tetrahydronaphthalene (1-2-5)
CA 02377217 2001-12-28
152
F
N N I n ~H7
_ F F ~Fa)2 \ / OH TfZO
n-C3H~ - -~
OH
n-C3H7
\ / OH
F
F F
F F n-C3H7
[nC3H7OTf F
(HO)p8 A ~ F
F ! F
Pd(PPh3)4 F
n ~H7 n-~H7
\ / OTf F
F F F
(10-1) Synthesis of 2-propyl-5-fluoro-1,2,3,4-tetrahydro-6-naphthol, and 2-
propyl-7-fluoro-
1,2,3,4-tetrahydro-6-naphthol
200 g of the 2-propyl-1,2,3,4-tetrahydro-6-naphthol obtained in (7-1) was
dissolved in
1000 ml of dichloromethane, 5 g of sodium trifluoromethanesulfonate was added,
and the
mixture was stirred vigorously. 243 g of N,N'-difluoro-2,2'-bipyridinium
bistetrafluoroborate was gradually added to the reaction mixture, and the
resulting mixture
was stirred for a further 5 hours at room temperature. Water, and then a 10%
aqueous
solution of sodium hydroxide were added, any excess fluorinating reagent was
decomposed,
and following restoration to an acidic state by addition of dilute
hydrochloric acid, the organic
layer was separated. The aqueous layer was extracted with dichloromethane, the
extract was
combined with the separated organic layer, and this combined organic extract
was washed
with water and a saturated aqueous solution of sodium chloride, before being
dried over
anhydrous sodium sulfate. The crude product obtained by removal of the solvent
by
evaporation was separated and purified by silica gel column chromatography
(toluene), to
obtain 57.0 g of 2-propyl-5-fluoro-1,2,3,4-tetrahydro-6-naphthol, and 85.5 g
of 2-propyl-7-
fluoro- 1,2,3,4-tetrahydro-6-naphthol.
(10-2) Synthesis of 2-propyl-5-fluoro-6-(3,4,5-trifluorophenyl)-1,2,3,4-
CA 02377217 2001-12-28
153
tetrahydronaphthalene, and 2-propyl-7-fluoro-6-(3,4,5-trifluorophenyl)-1,2,3,4-
tetrahydronaphthalene
Syntheses were performed in the same manner as (7-2) and (7-3), with the
exception
of using the 2-propyl-5-fluoro-1,2,3,4-tetrahydro-6-naphthol and the 2-propyl-
7-fluoro-
1,2,3,4-tetrahydro-6-naphthol obtained in (10-1) above instead of the 2-propyl-
1,2,3,4-
tetrahydro-6-naphthol in (7-2), and yielded 35 g of 2-propyl-5-fluoro-6-(3,4,5-
trifluorophenyl)-1,2,3,4-tetrahydronaphthalene, and 53 g of 2-propyl-7-fluoro-
6-(3,4,5-
trifluorophenyl)-1,2,3,4-tetrahydronaphthalene respectively.
(Example 11) Synthesis of 2-propyl-6-(3,4,5-trifluorophenyl)ethynyl-1,2,3,4-
tetrahydronaphthalene
F
_ =i F F
n-C3H~ - F n-C3H7
10 F
\ / OTf Pd(PPh3)4
A mixture of 50 g of the 2-propyl-1,2,3,4-tetrahydronaphthalen-6-yl
trifluoromethanesulfonate obtained in (7-2), 33.9 g of 2-(3,4,5-
trifluorophenyl)acetylene, 3.6
g of tetrakis(triphenylphosphine) palladium(0), and 51.4 g of potassium
phosphate in 200 ml
of DMF was stirred for 10 hours at 80 C. The mixture was subsequently cooled
to room
temperature, water was added, the mixture was extracted with toluene, and the
organic layer
was washed subsequently with water and a saturated aqueous solution of sodium
chloride,
and subsequently dried over anhydrous sodium sulfate. The crude product
obtained by
removal of the solvent by evaporation was purified by silica gel column
chromatography
(hexane) and then recrystallized 3 times from ethanol to obtain 32.6 g of 2-
propyl-6-(3,4,5-
trifluorophenyl)ethynyl-1,2,3,4-tetrahydronaphthalene.
CA 02377217 2001-12-28
154
(Example 12) Synthesis of 6-propyl-2-(3,4,5-trifluorophenyl)-1,2,3,4-
tetrahydronaphthalene
(1-3-2)
F
BrMg ~ ~ F F
n-C3H~ C OH
n C3H~ cb O F
F
F
n-CA
F F
n-C3H~ Et~ ~ F
F
A Grignard reagent was prepared by the dropwise addition of a solution of 30 g
of
3,4,5-trifluorobromobenzene in 100 ml of tetrahydrofuran to 4.0 g of
magnesium.
Following addition of 150 ml of toluene to this reagent, approximately 100 ml
of solvent was
removed under reduced pressure, at room temperature. The mixture was then
heated to
60 C, and a solution of 25 g of 6-propyl-1,2,3,4-tetrahydronaphthalen-2-one in
50 ml of
toluene was added dropwise over a period of 20 minutes. The resulting mixture
was stirred
for a further 30 minutes, returned to room temperature, and then poured into a
10%
hydrochloric acid solution. The organic layer was washed with a saturated
aqueous solution
of sodium chloride, dried over anhydrous sodium sulfate, and then
concentrated. The thus
obtained residue was purified by silica gel column chromatography
(hexane/ethyl acetate =
6/1) and yielded 29 g of 2-hydroxy-6-propyl-2-(3,4,5-trifluorophenyl)-1,2,3,4-
tetrahydronaphthalene. To this product was added 100 ml of toluene and 3 g of
p-
toluenesulfonic acid, and the resulting mixture was stirred with heating for
one hour. The
reaction solution was then cooled and concentrated, and the residue purified
by silica gel
CA 02377217 2001-12-28
155
column chromatography (hexane) to obtain 26 g of 6-propyl-2-(3,4,5-
trifluorophenyl)-3,4-
dihydronaphthalene. To this product was added 50 ml of ethanol, 50 ml of ethyl
acetate and
3 g of 5% palladium-carbon, and the resulting mixture was stirred under an
atmosphere of
hydrogen for 2 hours at room temperature. The reaction mixture was filtered
through celite,
and the filtrate concentrated by removal of the solvent. The residue was
purified by silica
gel column chromatography (hexane), and yielded 26 g of 6-propyl-2-(3,4,5-
trifluorophenyl)-
1,2,3,4-tetrahydronaphthalene. This compound displayed a purity of 99% when
analyzed by
gas chromatography, and had a molecular weight of 304.
(Example 13) Synthesis of 2-(3,4-difluorophenyl)-6-propyl-1,2,3,4-
tetrahydronaphthalene
(1-3-1)
A synthesis was performed under the same conditions as the example 12, with
the
exception of using 3,4-difluorobromobenzene instead of 3,4,5-
trifluorobromobenzene, and
yielded 2-(3,4-difluorophenyl)-6-propyl-1,2,3,4-tetrahydronaphthalene. Purity
99%,
molecular weight 286.
(Example 14) Synthesis of 6-propyl-2-(4-trifluoromethoxyphenyl)-1,2,3,4-
tetrahydronaphthalene (1-3-3)
A synthesis was performed under the same conditions as the example 12, with
the
exception of using 4-trifluoromethoxybromobenzene instead of 3,4,5-
trifluorobromobenzene,
and yielded 6-propyl-2-(4-trifluoromethoxyphenyl)-1,2,3,4-
tetrahydronaphthalene. Purity
99%, molecular weight 334.
(Example 15) Synthesis of 2-(3,4-difluorophenyl)-6-(trans-4-propylcyclohexyl)-
1,2,3,4-
CA 02377217 2001-12-28
156
tetrahydronaphthalene (1-3-4)
F
F
BrMg ~ ~ F Br ~ / OH -
F
Br ~ ~ O ~ ~
- F
Br Br
~ ~ F ~ ~ \ / F
n-CA -0=0 OH F
n-CA
F
n-CA
F
n-C3H7 ~ ~ -
F
A Grignard reagent was prepared by the dropwise addition of a solution of 25 g
of
3,4-difluorobromobenzene in 100 ml of tetrahydrofuran to 3.5 g of magnesium.
The reagent
mixture was filtered through a glass filter, and following the addition of 150
ml of toluene to
the filtrate, approximately 100 ml of solvent was removed under reduced
pressure, at room
temperature. The mixture was then heated to 60 C, and a solution of 27 g of 6-
bromo-
1,2,3,4-tetrahydronaphthalen-2-one in 50 ml of toluene was added dropwise over
a period of
20 minutes. The resulting mixture was stirred for a further 30 minutes,
returned to room
temperature, and then poured into a 10% hydrochloric acid solution. The
organic layer was
washed with a saturated aqueous solution of sodium chloride, dried over
anhydrous sodium
sulfate, and then concentrated. The thus obtained residue was purified by
silica gel column
chromatography (hexane/ethyl acetate = 6/1) and yielded 29 g of 6-bromo-2-(3,4-
difluorophenyl)-2-hydroxy-1,2,3,4-tetrahydronaphthalene. To this product was
added 100
ml of toluene and 3 g of p-toluenesulfonic acid, and the resulting mixture was
stirred with
heating for one hour. The reaction solution was then cooled and concentrated,
and the
CA 02377217 2001-12-28
157
residue purified by silica gel column chromatography (hexane) to obtain 26 g
of 6-bromo-2-
(3,4-difluorophenyl)-3,4-dihydronaphthalene. To this product was added 50 ml
of ethanol,
50 ml of ethyl acetate and 3 g of 5% rhodium-carbon, and the resulting mixture
was stirred
under an atmosphere of hydrogen for 2 hours at room temperature. The reaction
mixture
was then filtered through celite, and the filtrate concentrated by removal of
the solvent. The
residue was purified by silica gel column chromatography (hexane), and yielded
26 g of 6-
bromo-2-(3,4-difluorophenyl)-1,2,3,4-tetrahydronaphthalene. A Grignard reagent
was then
prepared by the dropwise addition of a solution of this 26 g of 6-bromo-2-(3,4-
difluorophenyl)-1,2,3,4-tetrahydronaphthalene in 70 ml of tetrahydrofuran to
2.3 g of
magnesium. A solution of 13.5 g of 4-propylcyclohexanone in 30 ml of
tetrahydrofuran was
then added dropwise to this reagent. The resulting mixture was stirred for a
further 30
minutes, and then poured into a 10% hydrochloric acid solution. The organic
layer was
washed with a saturated aqueous solution of sodium chloride, dried over
anhydrous sodium
sulfate, and then concentrated. The thus obtained residue was purified by
silica gel column
chromatography (hexane/ethyl acetate = 6/1) and yielded 23 g of 2-(3,4-
difluorophenyl)-2-
hydroxy-6-(trans-4-propylcyclohexyl)-1,2,3,4-tetrahydronaphthalene. To this
product was
added 80 ml of toluene and 2 g of p-toluenesulfonic acid, and the resulting
mixture was
stirred with heating for one hour. The reaction solution was then cooled and
concentrated,
and the residue purified by silica gel column chromatography (hexane) to
obtain 26 g of 2-
(3,4-difluorophenyl)-6-(trans-4-propylcyclohexyl)-3,4-dihydronaphthalene. To
this product
was added 30 ml of ethanol, 50 ml of ethyl acetate and 2 g of 5% palladium-
carbon, and the
resulting mixture was stirred under an atmosphere of hydrogen for 2 hours at
room
temperature. The reaction mixture was then filtered through celite, and the
filtrate
concentrated by removal of the solvent. 50 ml of dimethyl formamide and 5.5 g
of
CA 02377217 2001-12-28
158
potassium t-butoxide were then added to the residue, and the mixture was
stirred for 2 hours
at 70 C. 10% hydrochloric acid was then added to the reaction mixture, which
was then
extracted with toluene, and the extracted organic layer was then washed with a
saturated
aqueous solution of sodium chloride, dried over anhydrous sodium sulfate and
concentrated
by removal of the solvent. The residue was purified by silica gel column
chromatography
(hexane), and then recrystallized from ethanol, and yielded 20 g of 2-(3,4-
difluorophenyl)-6-
(trans-4-propylcyclohexyl)-1,2,3,4-tetrahydronaphthalene. This compound
displayed a
purity of 99.8% when analyzed by gas chromatography, and had a molecular
weight of 368.
(Example 16) Synthesis of 2-(3,4-difluorophenyl)-6-(4-propylphenyl)-1,2,3,4-
tetrahydronaphthalene (1-3-5)
- F n-C3H7 ~ ~ B(OH)Z
Br ~ ~ -
~ ~ F
n-C3H7
F
-0
A solution of 4.5 g of 4-propylphenylboric acid obtained by reaction of a
Grignard
reagent prepared from 4-propylbromobenzene and magnesium with trimethoxy
borane and
subsequent hydrolysis with dilute hydrochloric acid, 5Ø g of 6-bromo-2-(3,4-
difluorophenyl)-
1,2,3,4-tetrahydronaphthalene synthesized in the example 15, 0.3 g of
tetrakis(triphenylphosphine) palladium(0), and 3.0 g of potassium phosphate in
30 ml of
dimethyl formamide was stirred for 10 hours at 80 C. The mixture was
subsequently cooled
to room temperature, water was added, and the mixture was extracted with
toluene. The
organic layer was washed with a saturated aqueous solution of sodium chloride,
dried over
anhydrous sodium sulfate, and then concentrated. The residue was purified by
silica gel
CA 02377217 2001-12-28
159
column chromatography (hexane) and then recrystallized from ethanol, and
yielded 4 g of 2-
(3,4-difluorophenyl)-6-(4-propylphenyl)-1,2,3,4-tetrahydronaphthalene. This
compound
displayed a purity of 99.7% when analyzed by gas chromatography, and had a
molecular
weight of 362.
(Example 17) Preparation 1 of a liquid crystal composition
The versatile host liquid crystal (H), which has a broad temperature range, is
of low
viscosity, and can be used in active matrix driving, was prepared.
50%
F (H)
50%
(wherein, the cyclohexane rings represent trans forms). This host liquid
crystal (H) exhibits
a nematic phase at 116.7 C or lower, and has a melting point of 11 C. The
physical
property values of this composition, and the measured value at 20 C of the
threshold voltage
(Vth) of a TN cell (cell thickness: 4.5 m) prepared using this composition
were as shown
below.
Nematic phase upper limit temperature (TN_,): 116.7 C
Dielectric anisotropy (&): 4.80
Threshold voltage (Vth): 1.88 V
Response time (i): 21.5 ms
Next, when a liquid crystal composition (M- 1) was prepared from 80% of this
host
liquid crystal (H) and 20% of the compound (I-1-2) of the present invention
obtained in the
example 2, the liquid crystal phase upper limit temperature (TN_1) was 72.3 C.
The value of
CA 02377217 2001-12-28
160
TN_1 was also measured after leaving the composition (M- 1) to stand for 20
hours at a
temperature of 150 C, but no variation was observed from the value prior to
heating.
Furthermore, the composition was also irradiated with ultraviolet radiation
for 20 hours, but
no change was observed in the value of TN_I. When the voltage holding ratio of
this
composition was measured it exhibited a high value similar to that of the host
liquid crystal
(H), at the time of preparation, following heating, and following irradiation
with ultraviolet
radiation.
Next, a liquid crystal element was prepared by using the composition (M-1) to
fill a
TN cell with a cell thickness of 4.6 m, and measurement of the electrooptical
characteristics
of the element revealed the values shown below.
Dielectric anisotropy (Os): 5.20
Threshold voltage (Vth): 1.51 V
Response time (i): 29.3 ms
Consequently, addition of 20% of the compound (I-1-2) enabled the fall in the
nematic phase upper limit temperature (TN_I) to be limited to 44 C, while the
threshold
voltage (Vth) could be reduced by 0.37 V. In addition, the increase in the
response time
could be suppressed to 8 ms. Furthermore, even after standing for 1 week at 0
C, crystal
precipitation did not occur. Moreover, when the composition was crystallized
by rapid
cooling and the melting point (TC_N) was then measured, it was found to be 13
C, which is
almost the same as the host liquid crystal (H), indicating that the compound
(1-2) dissolves
readily in the host liquid crystal.
Next, the voltage holding rates of this element were measured at room
temperature
and at 80 C, and both were extremely favorable, indicating that this element
can be
adequately used for active matrix driving.
CA 02377217 2001-12-28
161
(Example 18) Preparation 2 of a liquid crystal composition
A liquid crystal composition (M-2) was prepared in the same manner as the
example
17, using the compound (1-2-3) of the present invention obtained in the
example 8 instead of
the compound (1-1-2), and adding 20% of this compound to the host liquid
crystal (H). This
composition exhibited a nematic phase upper limit temperature (TN_I) of 118.1
C. The value
of TN_i was also measured after leaving the composition (M-2) to stand for 20
hours at a
temperature of 150 C, but no variation was observed from the value prior to
heating.
Furthermore, the composition was also irradiated with ultraviolet radiation
for 20 hours, but
no change was observed in the value of TN.I. When the voltage holding ratio of
this
composition was measured it exhibited a high value similar to that of the host
liquid crystal
(H), at the time of preparation, following heating, and following irradiation
with ultraviolet
radiation.
Next, a liquid crystal element was prepared by using the composition (M-2) to
fill a
TN cell with a cell thickness of 6.0 m, and measurement of the electrooptical
characteristics
of the element revealed the values shown below.
Dielectric anisotropy (DE): 5.50
Threshold voltage (Vth): 1.92 V
Response time (ti): 34.2 ms
Consequently, addition of 20% of the compound (1-2-3) enabled the nematic
phase
temperature range to be widened, while the threshold voltage (Vth) was reduced
by 0.22 V.
In addition, the increase in the response time could be suppressed to 9 ms.
(Example 19) Preparation 3 of a liquid crystal composition
CA 02377217 2001-12-28
162
A liquid crystal composition (M-3) was prepared in the same manner as the
example
17, using 20% of the compound (1-3-2) of the present invention obtained in the
example 12
instead of the compound (I-1-2), and the liquid crystal phase upper limit
temperature (TN_1) of
this composition was 74 C. The value of TN_I was also measured after leaving
the
composition (M-3) to stand for 20 hours at a temperature of 150 C, but no
variation was
observed from the value prior to heating. Furthermore, the composition was
also irradiated
with ultraviolet radiation for 20 hours, but no change was observed in the
value of TN_1.
When the voltage holding ratio of this composition was measured it exhibited a
high value
similar to that of the host liquid crystal (H), at the time of preparation,
following heating, and
following irradiation with ultraviolet radiation.
Next, a liquid crystal element was prepared by using the composition (M- 1) to
fill a
TN cell with a cell thickness of 4.5 m, and measurement of the electrooptical
characteristics
of the element revealed the values shown below.
Dielectric anisotropy (Os): 5.50
Threshold voltage (Vth): 1.55 V
Response time (i): 30.5 ms
Consequently, addition of 20% of the compound (1-3-2) enabled the fall in the
nematic phase upper limit temperature (TN_I) to be limited to 46 C, while the
threshold
voltage (Vth) could be reduced by 0.33 V. In addition, the increase in the
response time
could be suppressed to 9 ms. Furthermore, even after standing for 1 week at 0
C, crystal
precipitation did not occur. Moreover, when the composition was crystallized
by rapid
cooling and the melting point (TC_N) was then measured, it was found to be 12
C, which is
almost the same as the host liquid crystal (H), indicating that the compound
(1-2) dissolves
readily in the host liquid crystal.
CA 02377217 2001-12-28
163
Next, the voltage holding rates of this element were measured at room
temperature
and at 80 C, and both were extremely favorable, indicating that this element
can be
adequately used for active matrix driving.
(Comparative Example 1)
A liquid crystal composition (M-4) was prepared in the same manner as the
example
17, using trans-4-propyl-(3,4,5-trifluorophenyl)cyclohexane instead of the
compound (1-1-2),
and adding 20% of this compound to the host liquid crystal (H). This
composition exhibited
a nematic phase upper limit temperature (TN_1) of 70 C, and the liquid
crystallinity was
considerably low.
The physical property values of this composition, together with the
electrooptical
properties of an element prepared in the same manner as that described above
are as shown
below.
Nematic phase upper limit temperature (TN_,): 70.0 C
Dielectric anisotropy (As): 5.60
Threshold voltage (Vth): 1.58 V
Response time (i): 30.0 ms
Birefringence (An): 0.080
INDUSTRIAL APPLICABILITY
A tetrahydronaphthalene derivative provided in the present invention shows
superior
liquid crystallinity and co-solubility with conventional liquid crystal
compounds and
compositions. Furthermore, addition of such a compound enables the threshold
voltage to
be significantly reduced with almost no deleterious effect on the response. In
addition, a
CA 02377217 2001-12-28
164
compound of the present invention can also be easily produced industrially, is
colorless, and
is chemically stable. Consequently, liquid crystal compositions containing
such a
compound are extremely useful as liquid crystals, and are particularly
suitable for liquid
crystal displays requiring a wide operating temperature range, low voltage
driving and a quick
response.