Note: Descriptions are shown in the official language in which they were submitted.
CA 02378191 2002-O1-03
WO 01/05901 PCT/EP00/06423
Curable composition
The invention relates to a curable composition based on acidic polyesters and
on certain
polygiycidyf compounds and to a powder coating composition based thereon.
JP-A-Sho 57-108166 discloses a curable composition and the use thereof as a
powder
coating composition, the composition comprising
(A) from 60 to 96 parts by weight of a polyester having an acid number of from
20 to
200 mg of KOH per gram, a softening point of from 80 to 150 °C and a
molecular
weight (number average) of from 1000 to 10 000,
(B) from 3 to 40 parts by weight of an acrylic polymer containing giycidyl
groups and
having a molecular weight (number average) of from 300 to 5000,
(C) from 1 to 20 parts by weight of a further epoxy resin, which may be, inter
alia, diglycidyl
te~ephthaiate or trigfycidyl isocyanurate, and
(D) an accelerator for the reaction of epoxy groups with carboxyl groups.
There are used as component (B) polymers or copolymers that contain identical
or different
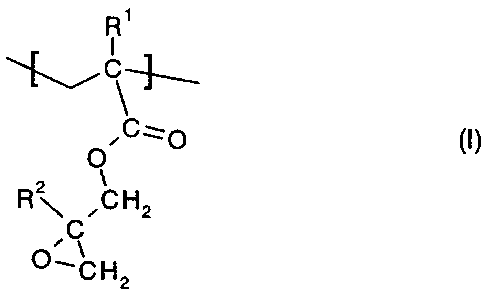
structural repeating units of formula (I):
R'
~C~
O
O
z 1
R~ ,CH2
,C
~~CH2
wherein the radicals R' and R2 are each independently of the other a hydrogen
atom or a
methyl group.
As can be seen from the above quantitative data, the said compositions contain
at least
approximately 2.5 % by weight of component (B), based on the total amount of
components
(A), (B) and (C). The flow behaviour of the compositions is generally stilt
not satisfactory,
however, and accordingly when such compositions are used in the production of
powder
CA 02378191 2002-O1-03
WO 01/05901 PCT/EP00/06423
-2-
resin coatings there is, in particular, a risk that the known orange-peel
effect will occur, that
is to say, that a coating having a very uneven surface will be formed.
The present invention is based on the surprising discovery that the flow
behaviour of the
above-mentioned curable compositions is substantially improved but,
nevertheless, cured
material having excellent physical properties, especially excellent mechanical
properties, is
obtainable by using as component (B) of the compositions a maximum of 2
percent by
weight, based on the total amount of components (A), (B) and (C), of one or
more polymers
or copolymers that each have an epoxy value of at least 3 equivalents per
kilogram and a
molecular weight (number average Mn) of from 800 to 20 000 and that contain
identical or
different structural repeating units of the formula (I) already mentioned
above.
The present invention accordingly relates to a curable composition comprising:
(A) from 80 to 97.9 percent by weight, based on the total amount of components
(A), (B)
and (C), of a polyester component having an acid number in total of from 10 to
40 mg
of KOH per gram of the component and consisting of one or more polyesters
containing free carboxyl groups;
(B) from 0.2 to 2 percent by weight, based on the total amount of components
(A), (B) and
(C), of a component consisting of one or more polymers or copolymers that each
have
an epoxy value of at least 3 equivalents per kilogram and a molecular weight
(number
average Mn) of from 800 to 20 000 and that contain identical or different
structural
repeating units of formula (I):
R'
C-
2 \
R ~ ~CH2
,C
~'CH2
wherein R' and R2 are each independently of the other a hydrogen atom or a
methyl
group; and
(C) a component consisting of one or more monomeric polyglycidyl compounds
having an
epoxy value of at least 3.5 equivalents per kilogram and a maximum molecular
weight
of 600, the amount of component (C) in percent by weight, based on the total
amount
CA 02378191 2002-O1-03
WO 01/05901 PCT/EP00/06423
-3-
of components (A), (B) and (C), corresponding to the difference between 100
and the
sum of the percentage amounts of components (A) and (B) based on the total
amount
of components (A), (B) and (C); and
(D) an accelerator for the reaction of epoxy groups with carboxyl groups,
wherein, furthermore, component (B) constitutes from 5 to 95 percent of the
total weight of
components (B) and (C) and the molar ratio of glycidyl groups to carboxyl
groups in the
composition is from 1.3:1 to 1:1.3.
Curable compositions according to the present invention are distinguished,
inter alia, by a
very good flow behaviour and yield a cured material that has a high
crosslinking density and
excellent mechanical properties.
The curable compositions according to the invention are suitable, for example,
as adhesives,
casting resins or compression-moulding compositions, it being possible for
further
components that are conventional in the field of use in question to be admixed
therewith, as
desired.
Special preference is given to powder coating compositions that contain as
curable
component system the compositions according to the invention. The invention
accordingly
relates also to powder coating compositions based on the curable compositions
according to
the invention.
The polyester component (A) constitutes preferably from 85 to 96 percent by
weight,
especially from 88 to 95 percent by weight, of the composition according to
the invention
based on the total amount of components (A), (B) and (C).
The acid number of from 10 to 40 mg of KOH per gram applies to the polyester
component
as a whole, and therefore corresponds, when several different polyesters are
used, to the
average acid number of the totality of those polyesters. Preference is given
to compositions
according to the invention in which the polyester component (A) has an acid
number in total
of from 20 to 40 mg, preferably from 22 to 38 mg, of KOH per kilogram of the
component.
The polyesters of component (A) are advantageously solid at room temperature
(from 15 to
35°C) and have, for example, a molecular weight (number average Mn) of
from 1000 to
CA 02378191 2002-O1-03
WO 01/05901 PCT/EP00/06423
-4-
000. The ratio of Mw (weight average of the molecular weight) to Mn of those
polyesters
is generally from 2 to 10. There are especially suitable for the present
invention, for example,
free-carboxyl-group-containing polyesters having a molecular weight (weight
average Mw
from GPC measurement using polystyrene calibration) of from 4000 to 15 000,
especially
from 6500 to 11 000, and a glass transition temperature (Tg) of from 35 to
120°C, preferably
from 50 to 90°C.
Polyesters such as those mentioned are described, for example, in US-A-3 397
254 and EP-
A-0 600 546. Polyesters suitable for the present invention are condensation
products of
difunctional, trifunctional and/or polyfunctional alcohols (polyols) with
dicarboxylic acids and,
optionally, trifunctional and/or polyfunctional carboxylic acids, or with
corresponding
carboxylic acid anhydrides. The polyols used include, for example, ethylene
glycol,
diethylene glycol, the propylene glycols, butylene glycol, 1,3-butanediol, 1,4-
butanediol,
neopentanediol, isopentyl glycol, 1,6-hexanediol, glycerol, hexanetriol,
trimethylolethane,
trimethylolpropane, erythritol, pentaerythritol, cyclohexanediol and 1,4-
dimethylolcyclo-
hexane. Suitable dicarboxylic acids include, for example, isophthalic acid,
terephthalic acid,
phthalic acid, methyl-substituted derivatives of the said acids,
tetrahydrophthalic acid, methyl-
tetrahydrophthalic acids, for example 4-methyltetrahydrophthalic acid,
cyclohexane-
dicarboxylic acids, succinic acid, glutaric acid, adipic acid, pimelic acid,
suberic acid, azelaic
acid, sebacic acid, dodecanedicarboxylic acid, fumaric acid, malefic acid and
4,4'-diphenyl-
dicarboxylic acid etc.. Suitable tricarboxylic acids include, for example,
aliphatic tricarboxylic
acids, such as 1,2,3-propanetricarboxylic acid, aromatic tricarboxylic acids,
such as trimesic
acid, trimellitic acid and hemimellitic acid, and cycloaliphatic tricarboxylic
acids, such as
6-methylcyclohex-4-ene-1,2,3-tricarboxylic acid. Suitable tetracarboxylic
acids include, for
example, pyromellitic acid and benzophenone-3,3',4,4'-tetracarboxylic acid.
Commercially
available polyesters especially are very commonly based on neopentyl glycol
and/or
trimethylolpropane as the main alcoholic monomer constituents) and on adipic
acid and/or
terephthalic acid and/or isophthalic acid and/or trimellitic acid as the main
acidic monomer
component(s).
Component (B) constitutes preferably from 0.7 to 2 percent by weight,
especially from 0.7 to
1.8 percent by weight, of the composition according to the invention based on
the total
weight of components (A), (B) and (C). It is furthermore preferred for
component (B) to
CA 02378191 2002-O1-03
WO 01/05901 PCT/EP00/06423
-5-
constitute from 5 to 50 percent, especially from 7.5 to 25 percent, of the
total weight of the
polyglycidyl components (B) and (C).
The polymers and copolymers constituting component (B) of the compositions
according to
the invention preferably have a molecular weight (number average Mn) of from
1000 to
000. The polymers and copolymers can be prepared, for example, by a
conventional free-
radical polymerisation of a monomer selected from glycidyl acrylate, glycidyl
methacrylate, ~3-
methylglycidyl acrylate and ~3-methylglycidyl methacrylate, optionally in the
presence of a
suitable amount of free-radically polymerisable comonomer(s). The
polymerisation can be
carried out, for example, by dissolving the monomers in a suitable organic
solvent, such as
toluene, 1-methoxy-2-propanol, 1-methoxy-2-propyl acetate or methyl isobutyl
ketone, or in a
mixture of the said solvents, and heating the resulting solution in the
presence of a suitable
initiator, such as, for example, a,a'-azoisobutyronitrile or dicumyl peroxide,
optionally in the
presence of a chain terminator, such as, for example, allyl glycidyl ether.
Suitable
comonomers include, for example, (meth)acrylic acid esters, such as ethyl
acrylate, butyl
acrylate, 2-ethyl hexylacrylate, and also, especially, C,-C4alkyl
methacrylates, such as
methyl methacrylate, ethyl methacrylate and butyl methacrylate, and
furthermore, for
example, acrylo- and methacrylo-nitrites, (meth)acrylamides and also vinyl
compounds, for
example aromatic vinyl compounds, especially styrene and styrene derivatives.
It is also possible, however, to glycidylise a poly(meth)acrylic acid polymer
or a poly(meth)-
acrylic acid copolymer according to one of the conventional methods, for
example by
reacting the polymer or copolymer with an excess of epichlorohydrin in the
presence of a
suitable catalyst, after which the resulting polyhalohydrin esters are
dehydrochlorinated
using a hydrogen-chloride-binding agent.
Component (B) consists preferably of polymers or copolymers each having an
epoxy value
of at least 5 equivalents per kilogram.
It is more especially preferred for component (B) to be constituted by
polymers that consist
substantially of identical or different structural repeating units of formula
(I):
CA 02378191 2002-O1-03
WO 01/05901 PCT/EP00/06423
-6-
R'
~C~
~C~O
O (I),
R~ ,CH2
,C
~~CH2
wherein R' and RZ are each independently of the other a hydrogen atom or a
methyl group,
especially when R2 in formula (I) is a hydrogen atom. Finally, the
polyglycidyl component (C)
constitutes preferably from 0.1 to 38 percent by weight, especially from 5 to
15 percent by
weight, of the composition according to the invention based on the total
amount of
components (A), (B) and (C).
Component (C) consists of one or more polyglycidyl or poly(~i-methyl)glycidyl
compounds
each having an epoxy value of at least 3.5 equivalents per kilogram. Those
compounds, in
contrast to the polyglycidyl compounds constituting component (B) of the
compositions
according to the invention, are monomeric compounds having a maximum molecular
weight
of 600 (molecular weight being understood in this case to mean the sum,
calculated
theoretically for each of the compounds, of the weights of the atoms of the
molecule), for
example corresponding diglycidyl or triglycidyl compounds that are preferably
solid at room
temperature, or corresponding polyglycidyl compounds having an even larger
number of
glycidyl groups per molecule.
Preferably, component (C) of the compositions consists of one or more glycidyl
compounds
having an epoxy value of at least 4 equivalents, especially at least 5
equivalents, per
kilogram.
Examples of glycidyl compounds that are suitable as component (C) of the
compositions
according to the invention include, inter alia, trimellitic acid triglycidyl
ester, triglycidyl
isocyanurate, hexahydroterephthalic acid diglycidyl ester, especially traps-
hexahydro-
terephthalic acid diglycidyl ester, hexahydroisophthalic acid diglycidyl
ester, hexahydro-
trimellitic acid triglycidyl ester, terephthalic acid diglycidyl ester,
isophthalic acid diglycidyl
ester and cyclohexanedimethanol diglycidyl ether.
CA 02378191 2002-O1-03
WO 01/05901 PCT/EP00/06423
_7_
For the composition according to the invention it is furthermore preferred for
the epoxy value
of components (B) and (C) together likewise to be at least 3.5 equivalents,
preferably at least
equivalents, per kilogram of the mixture of the two components.
The compositions according to the invention preferably have a slight molar
excess of epoxy
groups. The molar ratio of glycidyl groups to carboxyl groups in the
compositions is thus
preferably from 1.3:1 to 1:1, for example approximately from 1.2:1 to 1.1:1.
In order that the curing reaction can proceed sufficiently rapidly, the
compositions according
to the invention also contain, as component (D), a catalyst for the reaction
of epoxy groups
with carboxyl groups. Such a catalyst is frequently an organic amine or a
derivative of an
amine, especially a tertiary amine or a nitrogen-containing heterocyclic
compound. Preferred
catalysts for the reaction of epoxy groups with carboxyl groups are
phenylimidazole, N-
benzyldimethylamine and 1,8-diazabicyclo[5.4.0]-7-undecene, optionally on a
silicate support
or triphenylphosphine, Actiron~ NXJ-60 (2-propylimidazole), Actiron~ NXJ-60 P
(60 % by
weight of 2-propylimidazole on 40 % by weight of solid support),
Beschleuniger~ DT 3126
([C,6H33N(CH3)3]+Br ). The catalyst or a catalyst mixture is preferably added
in such an
amount that the gel time of the mixture at 180°C (determined according
to DIN 55990) is
approximately from 70 to 400 seconds, preferably from 90 to 300 seconds.
Generally,
approximately from 0.1 to 10 percent by weight, especially from 0.5 to 5
percent by weight,
of catalyst will be required for that purpose. Of course some commercially
available
polyesters that are used as a constituent of component (A) of the compositions
according to
the invention will already contain a certain amount of one of the above-
mentioned catalysts
or of a comparable catalyst, and that amount should be taken into account in
the above
percentage by weight figure for catalyst; the mentioned preferred gel times
can be used to
provide an indication of how much catalyst still needs to be added.
In addition to the components already mentioned, the compositions according to
the
invention may, of course, contain further components, which may vary according
to the field
of application of the compositions and which will be known to the person
skilled in the art in
question.
CA 02378191 2002-O1-03
WO 01/05901 PCT/EP00/06423
_g_
Powder coating compositions based on the compositions according to the
invention may, for
example, also contain additives customary in the surface-coating industry in
the amounts
conventionally used therefor, such as, inter alia, light stabilizers, curing
accelerators, dyes,
pigments, for example titanium dioxide pigment, degassing agents, for example
benzoin,
and also additional flow agents. Suitable flow agents include, for example,
polyvinyl acetals,
such as polyvinyl butyral, polyethylene glycol, polyvinylpyrrolidone, glycerol
and acrylic mixed
polymers, such as, for example, those available under the names
Modaflow° and Acrylron°.
The powder coating compositions according to the invention can be prepared
simply by
mixing the constituents together, for example in a ball mill. Another, more
preferred
possibility comprises melting together, blending and homogenising the
constituents,
preferably using an extrusion machine, such as a Buss co-kneader, and cooling
and
comminuting the resulting mass. In that procedure, the fact that either
immediately after
extrusion, or at least after they have been left to stand for a few hours, for
example from 24
to 48 hours, the powder coating compositions according to the invention become
so hard and
brittle that they can readily be ground, has proved especially advantageous.
The powder
coating mixtures preferably have a particle size in the range from 0.015 to
500 pm, especially
from 10 to 75 Vim. In some cases it may also be advantageous first of all to
prepare a
masterbatch from portions of the binder, the epoxy resins and, optionally,
further
components, the masterbatch then being mixed and homogenised in a second step
with the
remainder of the binder and the remaining constituents to yield the finished
powder coating
composition.
After application to the article to be coated, the powder coating compositions
are cured at a
temperature of at least approximately 100°C, preferably at from 150 to
220°C. Curing
generally takes approximately from 5 to 60 minutes. All materials that are
stable at the
temperatures required for the curing, especially ceramics and metals, are
suitable for
coating. The substrate may already have one or more base surface-coatings that
are
compatible with the powder coating composition.
The powder coating compositions exhibit a good flow behaviour combined with
good
mechanical properties, good weather resistance and good resistance to
chemicals.
CA 02378191 2002-O1-03
WO 01/05901 PCT/EP00/06423
_g_
Example 1: Preparation of a glycidyl methacrylate (= GMA) homopolymer
0
O~O AIBN; ~o~l
Solvent
O O
GMA O
O
The free-radical polymerisation is carried out using AIBN (a,a'-
azoisobutyronitrile) as
initiator. The desired low molecular weight (MW or M~) is achieved by
selecting the amount of
initiator, the amount of chain terminator (allyl glycidyl ether in the present
case) and the
polymerisation temperature. In the polymerisation procedure used in this
instance, the major
portion of the monomer mixture (90 %), the full amount of initiator and the
major portion of
the solvent are metered within a period of two hours into an initial charge in
the reactor, the
initial charge consisting of the remainder of the monomer mixture (10 %) and
the remainder
of the solvent. This allows good control of the polymerisation exotherm and
helps to achieve
a molecular weight that is reproducible. Many variants of the reaction
procedure are, of
course, possible; the person skilled in the art will find that it is simple to
determine suitable
conditions.
The polymerisation apparatus consists of a heatable 3000 ml double-walled
jacket reactor
with stirrer, reflux condenser, thermometer, nitrogen purging means, feed
receptacle and
feed pump ("Masterflex" peristaltic pump). A monomer mixture consisting of
1300.50 g of
methacrylic acid glycidyl ester and 144.50 g of allyl glycidyl ether is
prepared. The reactor is
charged with 144.50 g of the monomer mixture and 867.00 g of methoxypropyl
acetate
(MPA, solvent). The feed receptacle is filled with the remainder of the
monomer mixture
(1300.50 g), 96.50 g of MPA and 72.25 g of AIBN. The AIBN should be
homogeneously
dissolved. The entire apparatus (including the feed receptacle) is rendered
inert with
nitrogen, the reactor is heated to an internal temperature of 100°C
(jacket temperature of
105°C) and the feed is then started. The rate of feed is so selected
that the feed takes about
2 hours. The stirring speed is 100 revs/min. When the feed is complete,
polymerisation is
continued for a further 2 hours at 100°C under nitrogen. The viscous
solution is precipitated
in hexane and the solvent is decanted off. The greasy residue is dissolved in
acetone and
precipitated from water. The precipitation residue is reprecipitated once more
from
CA 02378191 2002-O1-03
WO 01/05901 PCT/EP00/06423
- 10-
acetone/water. The solid polymer is filtered off and dried in vacuo. 1200.00 g
of colourless
polymer in powder form, having the following analytical data, remain:
T9 , measured by DSC : 31 °C
GPC (polystyrene calibration): MW = 7147; M~ = 2986
Epoxy value, titrimetric: 6.44 equivalents/kg
Example 2: Preparation of a further GMA homopolymer
The following are polymerised in accordance with the procedure indicated in
Example 1:
Monomer mixture: 144.50 g of glycidyl methacrylate + 25.50 g of allyl glycidyl
ether
Initial reactor charge: 17.00 g of monomer mixture + 102.00 g of MPA
Feed: 153.00 g of monomer mixture + 11.30 g of MPA + 8.50 g of AIBN
158.30 g of colourless polymer in powder form, having the following analytical
data, are
obtained:
T9: 42°C
GPC (polystyrene calibration): Mw = 6099; M~ = 1304
Epoxy value, titrimetric: 6.39 equivalents/kg
Example 3: Preparation of a GMA copolymer (Rt 2549/1 )
The following are polymerised in accordance with the procedure indicated in
Example 1:
Monomer mixture: 25.50 g of butyl methacrylate + 127.50 g of glycidyl
methacrylate +
17.00 g of allyl glycidyl ether
Initial reactor charge: 17.00 g of monomer mixture and 102.00 g of MPA
Feed: 153.00 g of monomer mixture + 11.30 g of MPA + 8.50 g of AIBN
The isolation of the solid polymer is slightly modified: the polymer solution
is concentrated to
dryness using a rotary evaporator, the residue is dissolved in acetone and
then precipitated
from ice-water. 157.00 g of colourless polymer in powder form, having the
following
analytical data, are obtained:
GPC (polystyrene calibration): MW = 7182; M~ = 1926
Epoxy value, titrimetric: 5.47 equivalents/kg
CA 02378191 2002-O1-03
WO 01/05901 PCT/EP00/06423
_11_
Example 4: Free-radical polymerisation of GMA in diglycidyl terephthalate
(DGT)
O O
DGT +
O
O
O
O O
O O
200 g of diglycidyl terephthalate (DGT) are introduced into a 1 litre flat
ground flask. 40 g of
glycidyl methacrylate (GMA) and 4 g of 1,1-bis(tert-butylperoxy)-3,3,5-
trimethylcyclohexane
(50% solution = Trigonox 29, C50 from AKZO) are introduced into a 250 ml taper-
necked
flask. The two flasks are connected to each other. The flat ground flask is
heated by means
of an oil bath until the DGT has melted. When all of the DGT has melted, the 1
litre flat
ground flask, together with the 250 ml supply flask connected to it, is
evacuated and filled
with nitrogen a total of five times. The mixture of GMA and 1,1-bis(tert-
butylperoxy)-3,3,5-
trimethylcyclohexane is then fed from the 250 ml supply flask into the molten
DGT within a
period of 20 minutes, with stirring, and the batch is left to react for 3.5
hours with further
stirring. On subsequent cooling, the liquid mass solidifies.
Yield: 236 g (98%)
GPC (polystyrene calibration): MW = 15 700; M~ = 8921
Epoxy value, titrimetric: 6.86 equivalents/kg
Example 5:
Using an extruder (laboratory extruder from PRISM, The Old Stables, England),
the powder
coating compositions indicated in the following Tables 1 a and 1 b are
homogenised at a
temperature of from 60 to 80°C. The total amount of powder coating
composition in each
case is approximately from 100 to 200 grams. The cooled extrudates are ground
to give the
finished powder coating composition having a particle size of approximately 40
Vim.
CA 02378191 2002-O1-03
WO 01/05901 PCT/EP00/06423
-12-
Table 1 a: Powder coating composition formulations A to C according to the
invention and
comparison coating compositions E to G
Formulation/ Invention Com
arison
amounts % b wt. A B C E F G
Uralac P 3485 58.98 59.96 90.77 59.08 59.81 59.95
DGT 4.69 3.97 6.01 4.89 --- ---
GMA of mer of Ex. --- 1.03 --- --- 5.16 ---
1
GMA of mer of Ex. --- --- 1.52 --- --- 5.02
2
GMA of mer 6.7 a 0.80
/k
DT 3126-1 0.50 --- --- 1.00 --- ---
Benzoin 0.20 0.20 0.20 0.20 0.20 0.20
Ac Iron 1.50 1.50 1.50 1.50 1.50 1.50
Ti02 i ment 33.33 33.33 --- 33.33 33.33 33.33
rmyester ~asea on terepnthalic acid, isophthalic acid and neopentyl glycol
having an acid number of
28 and a glass transition temperature Tg of 71 °C;
2~ Alkylammonium salt in polyester;
3~ Acrylic mixed polymer as flow agent.
Table 1 b: Powder coating composition formulations H to K and P according to
the invention
Formulation Invention
[% b wt. H I J K P
Polyester"' Uralac CrylcoatCrylcoatAlftalatUralac
P-3485 801 D-6210 9952 P-3485
58.71 57_.61 59.66 58.90 59.09
DGT/GMA
mixed of mer 5.765 6.865 4.815 5.575 5.386
Benzoin 0.20 0.20 0.20 0.20 0.50
Ac Iron 1.50 1.50 1.50 1.50 0.20
XB 3126 0.50 0.50 0.50 0.50 1.50
Ti02 i ment 33.33 33.33 33.33 33.33 33.33
Polyester based on terephthalic acid, isophthalic acid and neopentyl glycol
having an acid number
and a glass transition temperature according to the following Table:
Pol ester C Icoat C !coat D-6210Alftalat
801 9952
Acid -
number 34 23 27
T C 73 72 64
'First DSC run.
5~ Mixture of approximately 86 % by weight DGT and 14 % by weight GMA
homopolymer having an
epoxy value of 6.37 equivalents per kilogram of the mixture.
6~ Mixture of DGT and GMA homopolymer according to Example 4.
Using an electrostatic spray pistol, the powder coating compositions are
applied to Q panels
as substrate. The coated panels are then placed in an oven in order to melt
and fully cure
the powder coating composition. The gel time, the curing temperature and the
curing time,
CA 02378191 2002-O1-03
WO 01/05901 PCT/EP00/06423
-13-
and also the thickness of the resulting powder resin coating, are indicated in
the following
Tables 2a and 2b together with properties of the resulting coatings that are
important from
the standpoint of surface-coating technology.
Table 2a: Properties, important from the standpoint of surface-coating
technology, of the
coatings obtained using compositions A, B, C and E, F, G
Invention Com arison
A B C E F G
Gel time'' at 220 170 90 205 25 20
180C s
Curing conditions15 min/15 min./15 min./15 min./15 min./15 min./
180C 180C 180C 180C 180C 180C
Layer thickness
m 60 53 59 60 59 60
60 loss 94 96 124 87 18 21
20 loss 85 77 117 95 60 65
Ye~~owness value0.2 -0.8 --- -1.2 -1 -0
1 8
. .
Flow ~~ [rating]10 10 12 12 orange-orange-
eel eel
Impact
deformation,
reverse 9~ 160 160 160 160 <5 <5
k cm
Impact
deformation,
front
k cm 160 160 140 160 80 80
Acetone test, 5
"''
1 min. [rating]3 3 3 not 3 3
cured
Appearance satis- satis- satis- satis- mat mat
facto facto facto facto
tmpmcai scale from 0 (very good) to 18 (orange-peel)
9~ The impact deformation is determined by dropping onto the coated face a
punch weighing 2 kg,
having a 20 mm diameter ball on its underside, with the underside leading,
from a specific height from
behind (reverse side) or from the front. The value indicated is the product of
the weight of the punch in
kg and the test height in cm at which there is still no detectable damage to
the coating.
'°~ According to DIN 53320. The specimen is kept in acetone for 1
minute. The result is evaluated in
accordance with the following scale of five ratings: 0 = unchanged; 1 =
resistant, cannot be scratched
with a finger nail; 2 = difficult to scratch, may stain cottonwool pad; 3 =
softened, easily scratchable;
4 = beginning to separate or dissolve; 5 = complete dissolution.
CA 02378191 2002-O1-03
WO 01/05901 PCT/EP00/06423
-14-
Table 2b: Surface-coating technology properties of the coatings using
compositions
HtoK,P
Invention
Fi I J K P
Gel time at 225 275 180 170 260
180C
s
Curing conditions15 min. 15 min. 15 min. 15 min. 15 min.
/ / / / /
200C 200C 200C 200C 180C
Layer thickness
m 60 55 50 47 46
60 loss 89 95 94 96 95
20 loss 76 88 84 83 85
Yellowness value-0.4 0.0 -1.0 -1.8 -1.5
Yi
Flow [ratin 8 8 10 10 10
Impact deform-
ation, reverse
k cm] >160 120 >160 >160 >160
Impact deform-
ation, direct
k cm >160 >160 >160 >160 >160
Acetone test,
1 min
[ratin
3 3 3 3 3
Appearance satis- satis- satis- satis- satis-
facto facto facto facto facto