Note: Descriptions are shown in the official language in which they were submitted.
CA 02378868 2002-O1-09
WO 01/05815 PCT/US00/15018
PROCESS FOR DEACYLATION OF LIPODEPSIPEPTIDES
FIELD OF THE INVENTION
The present invention relates to lipodepsipeptides, in
particular, deacylation of the N-acyl side-chain of
pseudomycin and syringomycin natural products and the
compounds produced therefrom.
BACKGROUND OF THE INVENTION
Pseudomycins and syringomycins are natural products
isolated from liquid cultures of Pseudomonas syringae
(plant-associated bacterium) and have been shown to have
antifungal activities. (see i.e., Harrison, L., et al.,
"Pseudomycins, a family of novel peptides from Pseudomonas
syringae possessing broad-spectrum antifungal activity," J.
Gen. Microbiology, 137(12), 2857-65 (1991) and US Patent
Nos. 5,576,298 and 5,837,685) Unlike the previously
described antimycotics from P. syringae (e. g.,
syringomycins, syringotoxins and syringostatins),
pseudomycins A-C contain hydroxyaspartic acid, aspartic
acid, serine, dehydroaminobutyric acid, lysine and
diaminobutyric acid.
The peptide moiety for pseudomycins A, A', B, B', C, C'
corresponds to L-Ser-D-Dab-L-Asp-L-Lys-L-Dab-L-aThr-Z-Dhb-L-
Asp(3-OH)-L-Thr(4-Cl) with the terminal carboxyl group
CA 02378868 2002-O1-09
WO 01/05815 PCT/US00/15018
closing a macrocyclic ring on the OH group of the N-terminal
Ser. The analogs are distinguished by the N-acyl side
chain, i.e., pseudomycin A is N-acylated by
3,4-dihydroxytetradeconoyl, pseudomycin A' by
3,4-dihydroxypentadecanoyl, pseudomycin B by
3-hydroxytetradecanoyl, pseudomycin B' by
3-hydroxydodecanoyl, pseudomycin C by
3,4-dihydroxyhexadecanoyl and pseudomycin C' by
3-hydroxyhexadecanoyl. (see i.e., Ballio, A., et al.,
"Novel bioactive lipodepsipeptides from Pseudomonas
syringae: the pseudomycins," FEBS Letters, 355(1), 96-100,
(1994) and Coiro, V.M., et al., "Solution conformation of
the Pseudomonas syringae MSU 16H phytotoxic lipodepsipeptide
Pseudomycin A determined by computer simulations using
distance geometry and molecular dynamics from NMR data,"
Eur. J. Biochem., 257(2), 449-456 (1998).)
Pseudomycins and syringomycins are known to have
certain adverse biological effects. For example,
destruction of the endothelium of the vein, destruction of
tissue, inflammation, and local toxicity to host tissues
have been observed when pseudomycin is administered
intraveneously. Therefore, there is a need to identify
compounds within this class that are useful for treating
fungal infections without the currently observed adverse
side effects.
2
CA 02378868 2002-O1-09
WO 01/05815 PCT/US00/15018
BRIEF SUMMARY OF THE INVENTION
The present invention provides a process for
deacylating the N-acyl side-chain of a lipodepsipeptide
natural product to produce the corresponding nucleus. The
deacylation of pseudomycin compounds produces the
pseudomycin amino nucleus represented by the following
structure I.
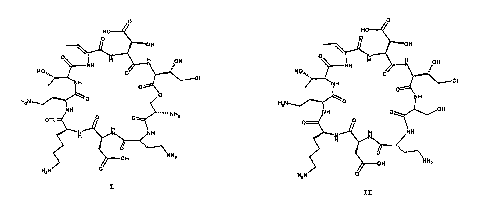
0
HO
O
OOH
N
H
~NH
O
HzN
I
The nucleus is useful as a starting material for producing
semi-synthetic derivatives of the corresponding natural
product.
The process includes reacting a pseudomycin natural
product with a deacylase enzyme selected from the group
consisting of ECB deacylase and polymyxin acylase to produce
3
CA 02378868 2002-O1-09
WO 01/05815 PCT/US00/15018
the corresponding nucleus represented by structure I. The
free amine may rearrange to produce a cyclic peptide nucleus
having a free hydroxy group represented by structure II
below (also referred to as pseudomycin hydroxy nucleus).
0
HO
OOH
H2N
HZN v
II
Compound II may then serve as starting material to generate
novel derivatives which may be pharmaceutically active.
In another embodiment of the present invention, the
process described above is used to deacylate syringomycin
compounds to provide a syringomycin amino nucleus. For
example, the amino nucleus of Syringomycin E has the
following structure III.
4
-OH NH2
CA 02378868 2002-O1-09
WO 01/05815 PCT/US00/15018
0
HO
O
OH
O ~ \N
H OH
NH N
O ~CI
O
HzN
'~~
N "' N HZ
-OH
III
Like the pseudomycin amino nucleus, the syringomycin
amino nucleus may rearrange to form the following Compound
IV (also referred to as syringomycin hydroxy nucleus).
O
HO
O
OH
N
H H OH
N
O ~CI
O~
NH
5
CA 02378868 2002-O1-09
WO 01/05815 PCT/US00/15018
IV
Even though specific chiral forms are depicted above for
Compounds I, II, III and IV, other chiral forms are within
the spirit of the present invention. Each of the compounds
may also exist as pharmaceutically acceptable salts,
hydrates or solvates thereof.
Definitions
As used herein, the term "pseudomycin" refers to
compounds having the following formula:
O
HO
O
OH
O~N H
NH H N OH
HO, O
NH ~CI
O
O
H2N
NH
p O O 'NHR
N NH
O
OH NH2
HzN O
where R is a lipophilic moiety. The lipophilic moiety
includes C9-C15 alkyl, Cg-C15 hydroxyalkyl, C9-C1s
dihydroxyalkyl , C9-C15 alkenyl , C9-C15 hydroxyalkenyl , or C9-
C15 dihydroxyalkenyl. The pseudomycin compounds A, A', B,
6
CA 02378868 2002-O1-09
WO 01/05815 PCT/LTS00/15018
B', C, C' are represented by the formula I above where R is
as defined below.
Pseudomycin A R = 3,4-dihydroxytetradecanoyl
Pseudomycin A' R = 3,4-dihydroxypentadecanoyl
Pseudomycin B R = 3-hydroxytetradecanoyl
Pseudomycin B' R = 3-hydroxydodecanoyl
Pseudomycin C R = 3,4-dihydroxyhexadecanoyl
Pseudomycin C' R = 3-hydroxyhexadecanoyl
DETAILED DESCRIPTION OF THE INVENTION
Applicants have discovered a process for enzymatically
deacylating the N-acyl side-chain of a broad spectrum of
lipodepsipeptide natural products to produce the
corresponding nucleus. Surprisingly, the free amine nucleus
rearranges to produce the free hydroxy derivative such as
the compounds shown above as structures II and IV.
Compounds I and III can be converted to Compounds II and IV,
respectively, by exposing Compound I or III to a pH >_ 6. If
the desired product is Compound I or III, then one could
reduce the rate at which the rearranged product forms from
the deacylated pseudomycin or deacylated syringomycin with
the addition of an acid, such as trifluoroacetic acid.
However, the addition of an acid could result in lower
yields of the amine nucleus. At lower pHs, the enzyme may
precipitate out of the reaction mixture thus stopping the
7
CA 02378868 2002-O1-09
WO 01/05815 PCT/US00/15018
conversion. Therefore, the pH of the reaction mixture is
preferably not lowered less than about 5.5. One could
prevent enzyme precipitation by separating the enzyme from
the reaction through a molecular weight membrane (i.e.,
10,000 to 50,000 molecular weight cutoff). The effluent
through the membrane would contain compounds having a
molecular weight less than 10,000 to 5,000 (e. g., Compounds
I-IV) and would exclude the higher molecular weight enzyme.
The effluent could then be pH adjusted down to stabilize the
product.
Unlike acid deacylation processes (e. g.,
trifluoroacetic acid in an aqueous solvent at room
temperature), the inventive enzymatic process may be used to
deacylate pseudomycin or syringomycin analogs with or
without gamma or delta hydroxy side chains. Therefore, the
spectrum of starting natural products is expanded
significantly. For example, one may deacylate pseudomycin
A, A', B, B', C or C' using the inventive process. Whereas,
the acid deacylation process is useful only with pseudomycin
A, A' and C.
Suitable enzymes include ECB deacylase and Polymyxin
acylase (available in both a crude & pure form as 161-16081
Fatty Acylase, Pure and 164-16081 Fatty Acylase, Crude, from
Wako Pure Chemical Industries, Ltd.) ECB deacylase can be
obtained from Actinoplanes utahensis (see e.g., LaVerne, D,
8
CA 02378868 2002-O1-09
WO 01/05815 PCT/US00/15018
et al, "Deacylation of Echinocandin B by Actinoplanes
utahensis," J. of Antibiotics, 42(3), 382-388 (1989).) The
Actinoplanes utahensis ECB deacylase enzyme may be purified
by the process described in U.S. Patent No. 5,573,936,
incorporated herein by reference. One may also use an
enzyme that has been cloned and expressed in Streptomyces
Iividans. Attempts to deacylate pseudomycin A with Pen G
Amidase and Phthalyl Amidase were not successful.
The enzymatic deacylation may be accomplished using
standard deacylation procedures well known to those skilled
in the art. For example, general procedures for using
Polymyxin acylase may be found in Yasuda, N., et al, Agric.
Biol. Chem., 53, 3245 (1989) and Kimura, Y., et al., Agric.
Biol. Chem., 53, 497 (1989).
The deacylation process is generally ran at
temperatures between about 20°C and about 60°C, preferably
between about room temperature (25°C) and about 40°C.
Higher temperatures may promote the formation of the
rearranged product (Compound II). The enzyme is optimally
active at pH 8.0 and at a temperature between about 50°C and
60°C. Although the reaction is faster at the higher pH and
higher temperature, more rearranged product may be observed
at the higher pH. Therefore, the pH of the reaction is
9
CA 02378868 2002-O1-09
WO 01/05815 PCT/L1S00/15018
generally kept between about 5.5 and about 8Ø The
reaction time will vary depending upon the pH and the
temperature. However, with limiting enzyme concentration
and saturated substrate concentration at high temperatures
and pH, the reaction is linear through 10 minutes. Since
Pseudomcyin A is unstable at higher pHs, deacylation of
Pseudomycin A is generally ran at a lower pH (between about
5.0 and 6.0) and temperature (about 25°C). For example,
deacylation of Pseudomycin A can be ran in a buffered
solution containing 0.05 M KP04 and 0.8 M KCl. A saturated
level of substrate is generally between about 0.5 mg and
about 1 mg per ml of reaction.
As discussed earlier, pseudomycins are natural products
isolated from the bacterium Pseudomonas syringae that have
been characterized as lipodepsinonapetpides containing a
cyclic peptide portion closed by a lactone bond and
including the unusual amino acids 4-chlorothreonine (ClThr),
3-hydroxyaspartic acid (HOAsp), 2,3-dehydro-2-aminobutyric
acid (Dhb), and 2,4-diaminobutyric acid (Dab). Methods for
growth of various strains of P. syringae to produce the
different pseudomycin analogs (A, A', B, B', C, and C') are
generally described below and also described in more detail
in PCT Patent Application Serial No. PCT/US00/08728 filed by
Hilton, et al. on April 14, 2000 entitled "Pseudomycin
Production by Pseudomonas Syringae," incorporated herein by
CA 02378868 2002-O1-09
WO 01/05815 PCT/LTS00/15018
reference, PCT Patent Application Serial No. PCT/US00/08727
filed by Kulanthaivel, et al. on April 14, 2000 entitled
"Pseudomycin Natural Products," incorporated herein by
reference, and U.S. Patent Nos. 5,576,298 and 5,837,685,
each of which are incorporated herein by reference.
Isolated strains of P. syringae that produce one or
more pseudomycins are known in the art. Wild type strain
MSU 174 and a mutant of this strain generated by transposon
mutagenesis, MSU 16H are described in U.S. Patent Nos.
5,576,298 and 5,837,685; Harrison, et al., "Pseudomycins, a
family of novel peptides from Pseudomonas syringae
possessing broad-spectrum antifungal activity," J. Gen.
Microbiology, 137, 2857-2865 (1991); and Lamb et al.,
"Transposon mutagenesis and tagging of fluorescent
pseudomonas: Antimycotic production is necessary for control
of Dutch elm disease," Proc. Natl. Acad. Sci. USA, 84, 6447-
6451 (1987).
A strain of P. syringae that is suitable for production
of one or more pseudomycins can be isolated from
environmental sources including plants (e. g., barley plants,
citrus plants, and lilac plants) as well as, sources such as
soil, water, air, and dust. A preferred stain is isolated
from plants. Strains of P. syringae that are isolated from
environmental sources can be referred to as wild type. As
11
CA 02378868 2002-O1-09
WO 01/05815 PCT/LTS00/15018
used herein, "wild type" refers to a dominant genotype which
naturally occurs in the normal population of P. syringae
(e.g., strains or isolates of P. syringae that are found in
nature and not produced by laboratory manipulation). Like
most organisms, the characteristics of the pseudomycin-
producing cultures employed (P. syringae strains such as MSU
174, MSU 16H, MSU 206, 25-B1, 7H9-1) are subject to
variation. Hence, progeny of these strains (e..g.,
recombinants, mutants and variants) may be obtained by
methods known in the art.
Mutant strains of P. syringae are also suitable for
production of one or more pseudomycins. As used herein,
"mutant" refers to a sudden heritable change in the
phenotype of a strain, which can be spontaneous or induced
by known mutagenic agents, such as radiation (e. g.,
ultraviolet radiation or x-rays), chemical mutagens (e. g.,
ethyl methanesulfonate (EMS), diepoxyoctane, N-methyl-N-
nitro-N'-nitrosoguanine (NTG), and nitrous acid), site-
specific mutagenesis, and transposon mediated mutagenesis.
Pseudomycin-producing mutants of P. syringae can be produced
by treating the bacteria with an amount of a mutagenic agent
effective to produce mutants that overproduce one or more
pseudomycins, that produce one pseudomycin (e. g.,
pseudomycin B) in excess over other pseudomycins, or that
produce one or more pseudomycins under advantageous growth
12
CA 02378868 2002-O1-09
WO 01/05815 PCT/US00/15018
conditions. While the type and amount of mutagenic agent to
be used can vary, a preferred method is to serially dilute
NTG to levels ranging from 1 to 100 ~,g/ml. Preferred
mutants are those that overproduce pseudomycin B and grow in
minimal defined media.
Environmental isolates, mutant strains, and other
desirable strains of P. syringae can be subjected to
selection for desirable traits of growth habit, growth
medium nutrient source, carbon source, growth conditions,
amino acid requirements, and the like. Preferably, a
pseudomycin producing strain of P. syringae is selected for
growth on minimal defined medium such as N21 medium and/or
for production of one or more pseudomycins at levels greater
than about 10 ~,g/ml. Preferred strains exhibit the
characteristic of producing one or more pseudomycins when
grown on a medium including three or fewer amino acids and
optionally, either a lipid, a potato product or combination
thereof .
Recombinant strains can be developed by transforming
the P. syringae strains, using procedures known in the art.
Through the use of recombinant DNA technology, the P.
syringae strains can be transformed to express a variety of
gene products in addition to the antibiotics these strains
produce. For example, one can modify the strains to
13
CA 02378868 2002-O1-09
WO 01/05815 PCT/US00/15018
introduce multiple copies of the endogenous pseudomycin-
biosynthesis genes to achieve greater pseudomycin yield.
To produce one or more pseudomycins from a wild type or
mutant strain of P. syringae, the organism is cultured with
agitation in an aqueous nutrient medium including an
effective amount of three or fewer amino acids, preferably
glutamic acid, glycine, histidine, or a combination thereof.
Alternatively, glycine is combined with one or more of a
potato product and a lipid. Culturing is conducted under
conditions effective for growth of P. syringae and
production of the desired pseudomycin or pseudomycins.
Effective conditions include temperatures from about 22°-C to
about 27°-C, and a duration of about 36 hours to about 96
hours. Controlling the concentration of oxygen in the
medium during culturing of P. syringae is advantageous for
production of a pseudomycin. Preferably, oxygen levels are
maintained at about 5 to 50% saturation, more preferably
about 30o saturation. Sparging with air, pure oxygen, or
gas mixtures including oxygen can regulate the concentration
of oxygen in the medium.
Controlling the pH of the medium during culturing of P.
syringae is also advantageous. Pseudomycins are labile at
basic pH, and significant degradation can occur if the pH of
the culture medium is above about 6 for more than about 12
hours. Preferably, the pH of the culture medium is
14
CA 02378868 2002-O1-09
WO 01/05815 PCT/US00/15018
maintained between 6 and 4. P. syringae can produce one or
more pseudomycins when grown in batch culture. However,
fed-bath or semi-continuous feed of glucose and optionally,
an acid or base (e.g., ammonium hydroxide) to control pH,
enhances production. Pseudomycin production can be further
enhanced by using continuous culture methods in which
glucose and ammonium hydroxide are fed automatically.
Choice of P. syringae strain can affect the amount and
distribution of pseudomycin or pseudomycins produced. For
example, strains MSU 16H and 67 H1 each produce
predominantly pseudomycin A, but also produce pseudomycin B
and C, typically in ratios of 4:2:1. Strain 67 H1 typically
produces levels of pseudomycins about three to five fold
larger than are produced by strain MSU 16H. Compared to
strains MSU 16H and 67 H1, strain 25-B1 produces more
pseudomycin B and less pseudomycin C. Strain 7H9-1 are
distinctive in producing predominantly pseudomycin B and
larger amount of pseudomycin B than other strains. For
example, this strain can produce pseudomycin B in at least a
ten fold excess over either pseudomycin A or C.
As discussed earlier, the process described herein is
also useful for deacylating syringomycin compounds.
Syringomycin E, syringotoxin B, and syringostatin A may be
produced from cultures of Pseudomonas syringae pv. syringae
strains B301D, PS268, and SY12, respectively. Syringomycin
CA 02378868 2002-O1-09
WO 01/05815 PCT/US00/15018
A1 and G may be isolated from Pseudomonas syringae pv.
syringae as well. Strains B301D and PS268 are grown in
potato dextrose broth as described by Zhang, L., and J. Y.
Takemoto, "Effects of Pseudomonas syringae phytotoxin,
syringomycin, on plasma membrane functions of Rhodotorula
pilimanae," Phytopathol. 77(2):297-303 (1987). Strain SY12
was grown in syringomycin minimal medium supplemented with
100M arbutin (Sigma Chemical Co., A 4256; St. Louis, Mo.)
and 0.1o fructose (SRMAF) (19, 23). SR-E, ST-B, and SS-A
are purified by high performance liquid chromatography as
described previously by Bidwai, A. P., and J. Y. Takemoto,
"Bacterial phytotoxin, syringomycin, induces a protein
kinase-mediatedphosphorylation of red beet plasma membrane
polypeptides," Proc. Natl. Acad. Sci. USA, 84:6755-6759
(1987). Solubilized AmB containing 35% sodium deoxycholate
(Sigma Chemical Co., A 9528; St. Louis, Mo.) and
ketoconazole (Sigma Chemical Co., K-1003; St. Louis, Mo.)
are used as test standards. A detailed description for the
production and isolation of three cyclic
lipodepsinonapeptides syringomycin E, syringotoxin B, and
syringostatin A may be found in U.S. Patent No. 5,830,855,
incorporated herein by reference.
The pseudomycin or syringomycin nucleus or
corresponding rearranged compounds (Compounds II and IV) may
be isolated and used per se or in the form of its
16
CA 02378868 2002-O1-09
WO 01/05815 PCT/US00/15018
pharmaceutically acceptable salt or solvate. The term
"pharmaceutically acceptable salt" refers to non-toxic acid
addition salts derived from inorganic and organic acids.
Suitable salt derivatives include halides, thiocyanates,
sulfates, bisulfates, sulfites, bisulfites, arylsulfonates,
alkylsulfates, phosphonates, monohydrogen-phosphates,
dihydrogenphosphates, metaphosphates, pyrophosphonates,
alkanoates, cycloalkylalkanoates, arylalkonates, adipates,
alginates, aspartates, benzoates, fumarates,
glucoheptanoates, glycerophosphates, lactates, maleates,
nicotinates, oxalates, palmitates, pectinates, picrates,
pivalates, succinates, tartarates, citrates, camphorates,
camphorsulfonates, digluconates, trifluoroacetates, and the
like.
The term "solvate" refers to an aggregate that
comprises one or more molecules of the solute (i.e.,
pseudomycin and syringomycin compound) with one or more
molecules of a pharmaceutical solvent, such as water,
ethanol, and the like. When the solvent is water, then the
aggregate is referred to as a hydrate. Solvates are
generally formed by dissolving the nucleus or rearranged
compound (Compounds II or IV) in the appropriate solvent
with heat and slowing cooling to generate an amorphous or
crystalline solvate form.
17
CA 02378868 2002-O1-09
WO 01/05815 PCT/US00/15018
EXAMPLES
Biological Samples
P. syringae MSU 16H is publicly available from the
American Type Culture Collection, Parklawn Drive, Rockville,
MD, USA as Accession No. ATCC 67028. P. syringae strains
25-B1, 7H9-1, and 67 H1 were deposited with the American
Type Culture Collection on March 23, 2000 and were assigned
the following Accession Nos.:
25-B1 Accession No. PTA- 1622
7H9-1 Accession No. PTA- 1623
67 H1 Accession No. PTA- 1621
Chemical Abbreviations
The following abbreviations are used through out the
examples to represent the respective listed materials:
ACN - acetonitrile
TFA - trifluoroacetic acid
DMF - dimethylformamide
Example 1
Example illustrates the deacylation of Pseudomycin A
using ECB Deacylase enzyme.
Pseudomycin A (50 fig) and purified ECB Deacylase (50
~l) in 900 ~,1 of an aqueous buffer solution containing 0.05
M potassium phosphate and 0.8 M potassium chloride. The pH
remained between 6.0 and 8Ø The temperature was increased
18
CA 02378868 2002-O1-09
WO 01/05815 PCT/US00/15018
from 25°C to 40°C. The reaction was monitored by HPLC
(Waters C18 ~,Bondapak 3.9 X 300 mm column, 235 nm, 1%
acetonitrile/0.2o trifluoroacetic acid (4 minutes) to 60%
acetonitrile/0.2o trifluoroacetic acid (16 minutes)). Both
the pseudomycin amine nucleus (Compound I) and the
rearranged pseudomycin hydroxy nucleus (Compound II) were
observed.
Both Compounds I and II showed identical N!+H ion (m/z
981.3) in the electrospray ionization mass spectroscopy
(ESIMS) corresponding to a molecular formula of
C37H61C1N1z017- (See Table I below) Detailed analysis of 1H
and TOCSY (total correlation spectroscopy) NMR spectra
enabled the assignment of all protons for the hydrolysis
products which supports structures I and II. The 1H NMR
chemical shifts of the (3-protons (4.83 and 4.46 ppm) of the
serine residue of I were consistent with those found in
pseudomycins A, B and C, indicating that the peptide
macrocycle was intact. Furthermore, as expected, the TOCSY
spectrum did not show the typical amide proton as part of
the serine spin system. On the other hand, in II the serine
(3-protons underwent considerable upfield shifts (3.78 and
3.74 ppm) suggesting that these protons were not bearing the
lactone functionality. This and the fact that the (3-
protons, in addition to the a proton, correlated to an amide
19
CA 02378868 2002-O1-09
WO 01/05815 PCT/US00/15018
proton at 8.04 ppm in the TOCSY spectrum indicated that the
lactone of the macrocycle rearranged to a peptide core as
depicted in II.
Table I
1H NMR dataa of I and II in H20+CD3CN
Amino Acid Position I II
Ser NH - 8.04
a, 4.30 4.30
4.83 3.78
2 4.46 3.74
Dab-1 NH 9.19 7.99
4.06 4.19
2.03 2.15
2.01
3.03 2.92
2.96
Asp NH 8.51 8.20
a, 4.61 4.56
2.89 2.84
~j2 2.83 2.75
Lys NH 7.90 8.11
4.23 4.06
1.79 1.76
1.71 1.68
1.27 1.30
1.25
g 1.54 1.54
2.84 2.84
NH2 7.34 7.34
Dab-2 NH 8.35 8.31
4.29 4.34
2.14 2.09
1.98 1.91
2.90 2.92
NH2 7.53 7.49
Thr NH 7.73 7.74
4.24 4.21
3.98 3.98
1.18 1.16
CA 02378868 2002-O1-09
WO 01/05815 PCT/US00/15018
Table I (continued)
Amino Acid Position I II
Dhb NH 9.65 9.26
6.69 6.62
1.69 1.66
OHAsp NH 7.82 7.83
a, 4.95 4.99
4.72 4.75
ClThr NH 7.92 7.95
4.90 4.62
4.27 4.25
3.48 3.57
3.42 3.51
Chemical shifts reported are relative to solvent signal
(1.94 ppm).
Assignments may be interchanged.
Other pseudomycin or syringomycin compounds having an
N-acyl group may be deacylated using the same general
procedures described above.
21