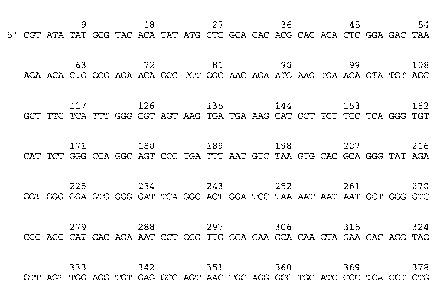Note: Descriptions are shown in the official language in which they were submitted.
CA 02378989 2002-O1-03
WO 01/04308 PCT/US00/19014
HUMAN LIM DOMAIN PROTEINS
TECHNICAL FIELD
This invention relates to nucleic acid and amino acid sequences of human LIM
domain proteins
and to the use of these sequences in the diagnosis, treatment, and prevention
of cell proliferative,
developmental, and cell motility disorders.
BACKGROUND OF THE INVENTION
The LIM domain is a cysteine-rich motif of about 60 amino acid residues that
forms two loop
structures, each of which binds a zinc ion. (Reviewed in Sanchez-Garcia, I.
and T. H. Rabbitts (1994)
Trends Genet. 10:315-320; Dawid, I. B. et al. (1998) Trends Genet. 14:156-
162). Although the LIM
domain double zinc finger structure resembles the GATA zinc finger
transcription factors, no direct
evidence has been found that LIM domains bind DNA. Instead, LIM domains appear
to function as-'
protein interaction modules, similar to SH2, SH3, PDZ, and other domains which
mediate the
assembly of multiprotein signaling complexes (Pawson, T. and J. D. Scott
(1997) Science 278:2075-
2080). LIM domains have been identified in a variety of proteins, including
transcription factors,
cytoskeletal proteins, and signaling molecules. LIM proteins are involved in
cell fate determination,
growth regulation, and oncogenesis. More than sixty members of the LIM family
have been
described, and LIM proteins have been found in a variety of species.
The LIM proteins have been classified into three groups based upon homology of
their LIM
domains and other structural features (Dawid, supra). The group 1 LIM domain
proteins are
classified into three protein families: the LMO proteins, which contain two
tandem LIM domains
only; the LHX proteins, containing two tandem LIM domains followed by a
homeodomain; and the
LIMK proteins, containing two tandem LIM domains followed by a protein kinase
domain. Proteins
in group 2 consist mostly of LIM domains, while those in group 3 contain from
one to five tandem
LIM domains at the C-terminus, and may include other protein binding motifs in
the N-terminal
regions. Group 1 LIM domain proteins are primarily found in the nucleus, while
group 2 and 3 LIM
domain proteins are found primarily in the cytoplasm.
LHX proteins are involved in the control of differentiation of specific cell
types. The LHX
family includes the three proteins for which LIM was named, Lin-11 from C.
ele~ans, ISL1 from rat,
and Mec-3 from C. eleg_ans, all of which are transcription factors that play a
role in cell fate
determination during development. Lin-11 protein controls asymmetric cell
divisions in C. ele~ans
during vulval development, while Mec-3 is required for the differentiation of
mechanosensory
neurons. ISLl, which is expressed in islet cells, binds to the enhancer of the
insulin gene and
probably regulates the cell-specific expression of insulin. Liml/Lhxl, a
mammalian LHX family
CA 02378989 2002-O1-03
WO 01/04308 PCT/US00/19014
protein, has a fundamental early role in vertebrate embryogenesis, as
disruption of this gene in mice
results in headless embryos. Other mammalian LHX genes exhibit specific
functions in varied
tissues. Disruption of Lhx2 in mice leads to massive brain defects, including
a lack of eyes, while
disruption of Lhx3 results in specific ablation of the anterior and
intermediate lobes of the pituitary.
The LHX proteins bind DNA through their homeodomains. The LIM domains appear
to exert a
negative regulatory effect on DNA binding, with binding of protein cofactors
to the LIM domains
being required to activate LHX function (Dawid, supra).
The LMO genes were originally identified as a result of their activation by
chromosomal
translocations found in human tumors. LMO1 and LM02 are believed to be
involved in certain
human T-cell acute leukemias, as these genes are associated with recurring
chromosomal
translocations. In addition, transgenic expression of LMO1 or LM02 produces
leukemia and
lymphoma in mice (McGuire, E.A. et al. (1992) Mol. Cell. Biol. 12:4186-4196;
Fisch, P. et al. (1992)
Oncogene 7:238 -2397). The normal function of LM02 is in the development of
hematopoietic
lineages, as knockout mice carrying LM02 null mutations are defective in
erythroid differentiation.
The LM02 gene product does not seem to directly bind DNA, but is found in a
multiprotein complex
that regulates transcription, in which it appears to bridge two DNA-binding
arms of the complex.
Improper expression of LM02 in T cells is believed to result in the formation
of an abnormal DNA
transcription complex which leads to leukemia (Rabbitts, T. H. et al. (1999)
Cancer Res. 59 (7
suppl.):1794s-1798s).
Group 2 LIM domain proteins include the cysteine-rich protein (CRP) family,
which display
two LIM domains and are implicated in muscle development. A recently
characterized family in this
group are the FHL (four and a half LIM domain) proteins which consist of tour
LIM domains plus an
additional N-terminal single zinc finger that resembles the C-terminal half of
the LIM domain. FHL
proteins are expressed in skeletal and cardiac muscle in a manner which
appears to be coordinated with
critical events in myogenesis (Morgan, M. J. and A. J. A. Magwick (1999)
Biochem. Biophys. Res.
Commun. 255:245-250).
Many group 3 LIM domain proteins are localized primarily in focal adhesions,
macromolecular
complexes which mediate the contact between extracellular matrix receptors and
the cytoskeleton.
These proteins include zyxin and paxillin. Paxillin is a 68 kilodalton protein
containing four tandem
LIM domains, as well as binding sites for the SH3 and SH2 domains of the c-Src
and v-Crk oncogene
products, respectively. Paxillin is a substrate for various tyrosine kinases
including focal adhesion
tyrosine kinase and the hematopoietic c-Abl oncogene product. Tyrosine
phosphorylation of paxillin
is induced in response to growth factor stimulation and oncogenic
transformation. Such
phosphorylation events are correlated with changes in the actin cytoskeleton
(Salgia, R. et al. (1995)
J. Biol. Chem. 270:5039-5047). Zyxin plays a role in the spatial control of
actin assembly. This
CA 02378989 2002-O1-03
WO 01/04308 PCT/US00/19014
protein contains three tandem LIM domains, through which it binds to proteins
of the CRP family.
Zyxin also interacts with a-actinin, a cross linker of actin filaments,
through its proline rich N-
terminus (Beckerle, M. C. (1997) BioEssays 19:949-957).
Other LIM domain proteins include reversion-induced LIM (RIL) protein from
rat, which has
a single C-terminal LIM domain. RIL is highly expressed in fibroblasts and is
down-regulated in H-
ras transformed cells. Expression of RIL is restored in phenotypic revenants
derived from H-ray
transformed cells. RIL is thus proposed to be involved in the maintenance of
normal cell growth
(Kiess, M. et al. (1995) Oncogene 10:61-68).
The discovery of new human LIM domain proteins and the polynucleotides
encoding them
satisfies a need in the art by providing new compositions which are useful in
the diagnosis, prevention,
and treatment of cell proliferative, developmental, and cell motility
disorders.
SUMMARY OF THE INVENTION
The invention features purified polypeptides, human LIM domain proteins,
referred to
collectively as "LIMD" and individually as "LIMD-1" and "LIMD-2." In one
aspect, the invention
provides an isolated polypeptide comprising an amino acid sequence selected
from the group consisting
of a) an amino acid sequence selected from the group consisting of SEQ ID N0:1-
2, b) a naturally
occurring amino acid sequence having at least 95 % sequence identity to an
amino acid sequence
selected from the group consisting of SEQ ID NO:1-2, c) a biologically active
fragment of an amino
acid sequence selected from the group consisting of SEQ ID NO:1-2, and d) an
immunogenic fragment
of an amino acid sequence selected from the group consisting of SEQ ID NO:1-2.
In one alternative,
the invention provides an isolated polypepdde comprising the amino acid
sequence of SEQ ID NO:1-2.
The invention funkier provides an isolated polynucleotide encoding a
polypeptide comprising an
amino acid sequence selected from the group consisting of a) an amino acid
sequence selected from the
group consisting of SEQ ID NO:1-2, b) a naturally occurring amino acid
sequence having at least 95%
sequence identity to an amino acid sequence selected from the group consisting
of SEQ ID N0:1-2, c) a
biologically active fragment of an amino acid sequence selected from the group
consisting of SEQ ID
NO:1-2, and d) an immunogenic fiagment of an amino acid sequence selected from
the group consisting
of SEQ ID NO:1-2. In one alternative, the polynucleotide encodes a polypeptide
selected from the
group consisting of SEQ ID NO:1-2. In another alternative, the polynucleotide
is selected from the
group consisting of SEQ ID N0:3-4.
Additionally, the invention provides a recombinant polynucleotide comprising a
promoter
sequence operably linked to a polynucleotide encoding a polypeptide comprising
an amino acid
sequence selected from the group consisting of a) an amino acid sequence
selected from the group
CA 02378989 2002-O1-03
WO 01/04308 PCT/US00/19014
consisting of SEQ ID NO:1-2, b) a naturally occurring amino acid sequence
having at least 95%
sequence identity to an amino acid sequence selected from the group consisting
of SEQ ID NO:1-2, c) a
biologically active fragment of an amino acid sequence selected from the group
consisting of SEQ ID
NO:1-2, and d) an immunogenic fragment of an amino acid sequence selected from
the group consisting
of SEQ ID NO:1-2. In one alternative, the invention provides a cell
transformed with the recombinant
polynucleotide. In another alternative, the invention provides a transgenic
organism comprising the
recombinant polynucleotide.
The invention also provides a method for producing a polypeptide comprising an
amino acid
sequence selected from the group consisting of a) an amino acid sequence
selected from the group
consisting of SEQ ID N0:1-2, b) a naturally occurring amino acid sequence
having at least 95%
sequence identity to an amino acid sequence selected from the group consisting
of SEQ ID N0:1-2, c) a
biologically active fragment of an amino acid sequence selected from the group
consisting of SEQ ID
NO:1-2, and d) an immunogenic fragment of an amino acid sequence selected from
the group consisting
of SEQ ID NO:I-2. The method comprises a) culturing a cell under conditions
suitable for expression
of the polypeptide, wherein said cell is transformed with a recombinant
polynucleotide comprising a
promoter sequence operably linked to a polynucleotide encoding the
polypeptide, and b) recovering the
polypeptide so expressed.
Additionally, the invention provides an isolated antibody which specifically
binds to a
polypeptide comprising an amino acid sequence selected from the group
consisting of a) an amino acid
sequence selected from the group consisting of SEQ ID N0:1-2, b) a naturally
occurring amino acid
sequence having at least 95 % sequence identity to an amino acid sequence
selected from the group
consisting of SEQ ID NO:1-2, c) a biologically active fragment of an amino
acid sequence selected
from the group consisting of SEQ ID NO:1-2, and d) an immunogenic fragment of
an amino acid
sequence selected from the group consisting of SEQ 1D NO:1-2.
The invention further provides an isolated polynucleotide comprising a
polynucleotide sequence
selected from the group consisting of a) a polynucleotide sequence selected
from the group consisting of
SEQ ID N0:3-4, b) a naturally occurring polynucleotide sequence having at
least 90% sequence
identity to a polynucleotide sequence selected from the group consisting of
SEQ ID N0:3-4, c) a
polynucleotide sequence complementary to a), d) a polynucleotide sequence
complementary to b), and e)
an RNA equivalent of a)-d). In one alternative, the polynucleotide comprises
at least 60 contiguous
nucleotides.
Additionally, the invention provides a method for detecting a target
polynucleotide in a sample,
said target polynucleodde having a sequence of a polynucleotide comprising a
polynucleotide sequence
selected ITOm the group consisting of a) a polynucleotide sequence selected
from the group consisting of
CA 02378989 2002-O1-03
WO 01/04308 PCT/US00/19014
SEQ ID N0:3-4, b) a naturally occurring polynucleotide sequence having at
least 90% sequence
identity to a polynucleotide sequence selected from the group consisting of
SEQ ID N0:3-4, c) a
polynucleotide sequence complementary to a), d) a polynucleotide sequence
complementary to b), and e)
an RNA equivalent of a)-d). The method comprises a) hybridizing the sample
with a probe comprising
at least 20 contiguous nucleotides comprising a sequence complementary to said
target polynucleotide
in the sample, and which probe specifically hybridizes to said target
polynucleotide, under conditions
whereby a hybridization complex is formed between said probe and said target
polynucleotide or
fragments thereof, and b) detecting the presence or absence of said
hybridization complex, and
optionally, if present, the amount thereof. In one alternative, the probe
comprises at least 60 contiguous
nucleotides.
The invention further provides a method for detecting a target polynucleotide
in a sample, said
target polynucleotide having a sequence of a polynucleotide comprising a
polynucleotide sequence
selected from the group consisting of a) a polynucleotide sequence selected
from the group consisting of
SEQ ID N0:3-4, b) a naturally occurring polynucleotide sequence having at
least 90% sequence
identity to a polynucleotide sequence selected from the group consisting of
SEQ ID N0:3-4, c) a
polynucleotide sequence complementary to a), d) a polynucleotide sequence
complementary to b), and e)
an RNA equivalent of a)-d). The method comprises a) amplifying said target
polynucleotide or
fragment thereof using polymerase chain reaction amplification, and b)
detecting the presence or
absence of said amplified target polynucleotide or fragment thereof, and,
optionally, if present, the
amount thereof.
The invention further provides a pharmaceutical composition comprising an
effective amount
of a polypeptide comprising an amino acid sequence selected from the group
consisting of a) an amino
acid sequence selected from the group consisting of SEQ ID NO:1-2, b) a
naturally occurring amino
acid sequence having at least 95 % sequence identity to an amino acid sequence
selected from the group
consisting of SEQ ID N0:1-2, c) a biologically active fragment of an amino
acid sequence selected
from the group consisting of SEQ ID N0:1-2, and d) an immunogenic fragment of
an amino acid
sequence selected from the group consisting of SEQ ID N0:1-2, and a
pharmaceutically acceptable
excipient. In one embodiment, the pharmaceutical composition comprises an
amino acid sequence
selected from the group consisting of SEQ ID NO:1-2. The invention
additionally provides a method of
treating a disease or condition associated with decreased expression of
functional LIMD, comprising
administering to a patient in need of such treatment the pharmaceutical
composition.
The invention also provides a method for screening a compound for
effectiveness as an
agonist of a polypeptide comprising an amino acid sequence selected from the
group consisting of a) an
amino acid sequence selected from the group consisting of SEQ 1D NO:1-2, b) a
naturally cx;curring
CA 02378989 2002-O1-03
WO 01/04308 PCT/US00/19014
amino acid sequence having at least 95 % sequence identity to an amino acid
sequence selected from the
group consisting of SEQ ID NO:1-2, c) a biologically active fragment of an
amino acid sequence
selected from the group consisting of SEQ ID NO:l-2, and d) an immunogenic
fragment of an amino
acid sequence selected from the group consisting of SEQ ID N0:1-2. The method
comprises a)
exposing a sample comprising the polypeptide to a compound, and b) detecting
agonist activity in the
sample. In one alternative, the invention provides a pharmaceutical
composition comprising an agonist
compound identified by the method and a pharmaceutically acceptable excipient.
In another
alternative, the invention provides a method of treating a disease or
condition associated with
decreased expression of functional LIMD, comprising administering to a patient
in need of such
treatment the pharmaceutical composition.
Additionally, the invention provides a method for screening a compound for
effectiveness as
an antagonist of a polypeptide comprising an amino acid sequence selected from
the group consisting
of a) an amino acid sequence selected from the group consisting of SEQ ID N0:1-
2, b) a naturally
occurring amino acid sequence having at least 95 % sequence identity to an
amino acid sequence
selected from the group consisting of SEQ ID NO:1-2, c) a biologically active
fragment of an amino
acid sequence selected from the group consisting of SEQ ID NO:1-2, and d) an
immunogenic fragment
of an amino acid sequence selected from the group consisting of SEQ ID NO:1-2.
The method
comprises a) exposing a sample comprising the polypeptide to a compound, and
b) detecting
antagonist activity in the sample. In one alternative, the invention provides
a pharmaceutical
composition comprising an antagonist compound identified by the method and a
pharmaceutically
acceptable excipient. In another alternative, the invention provides a method
of treating a disease or
condition associated with overexpression of functional LIMD, comprising
administering to a patient
in need of such treatment the pharmaceutical composition.
The invention further provides a method of screening for a compound that
specifically binds
to a polypeptide comprising an amino acid sequence selected from the group
consisting of a) an amino
acid sequence selected from the group consisting of SEQ ID NO:1-2, b) a
naturally occurring amino
acid sequence having at least 95 % sequence identity to an amino acid sequence
selected from the group
consisting of SEQ ID NO:1-2, c) a biologically active fragment of an amino
acid sequence selected
from the group consisting of SEQ ID NO:1-2, and d) an immunogenic fragment of
an amino acid
sequence selected from the group consisting of SEQ ID NO:1-2. The method
comprises a) combining
the polypeptide with at least one test compound under suitable conditions, and
b) detecting binding
of the polypeptide to the test compound, thereby identifying a compound that
specifically binds to the
polypeptide.
The invention further provides a method of screening for a compound that
modulates the
activity of a polypeptide comprising an amino acid sequence selected from the
group consisting of a)
CA 02378989 2002-O1-03
WO 01/04308 PCT/US00/19014
an amino acid sequence selected from the group consisting of SEQ ID NO:1-2, b)
a naturally
occurring amino acid sequence having at least 95% sequence identity to an
amino acid sequence
selected from the group consisting of SEQ 1D NO:1-2, c) a biologically active
fragment of an amino
acid sequence selected from the group consisting of SEQ ID NO:l-2, and d) an
immunogenic
fragment of an amino acid sequence selected from the group consisting of SEQ
ID NO:1-2. The
method comprises a) combining the polypeptide with at least one test compound
under conditions
permissive for the activity of the polypeptide, b) assessing the activity of
the polypeptide in the
presence of the test compound, and c) comparing the activity of the
polypeptide in the presence of the
test compound with the activity of the polypeptide in the absence of the test
compound, wherein a
change in the activity of the polypeptide in the presence of the test compound
is indicative of a
compound that modulates the activity of the polypeptide.
The invention further provides a method for screening a compound for
effectiveness in
altering expression of a target polynucleotide, wherein said target
polynucleotide comprises a
sequence selected from the group consisting of SEQ ID N0:3-4, the method
comprising a) exposing a
sample comprising the target polynucleotide to a compound, and b) detecting
altered expression of
the target polynucleotide.
The invention further provides a method for assessing toxicity of a test
compound, said
method comprising a) treating a biological sample containing nucleic acids
with the test compound;
b) hybridizing the nucleic acids of the treated biological sample with a probe
comprising at least 20
contiguous nucleotides of a polynucleotide comprising a polynucleotide
sequence selected from the
group consisting of i) a polynucleotide sequence selected from the group
consisting of SEQ ID N0:3-
4, ii) a naturally occurring polynucleotide sequence having at least 90%
sequence identity to a
polynucleotide sequence selected from the group consisting of SEQ ID N0:3-4,
iii) a polynucleotide
sequence complementary to i), iv) a polynucleotide sequence complementary to
ii), and v) an RNA
equivalent of i)-iv). Hybridization occurs under conditions whereby a specific
hybridization complex
is formed between said probe and a target polynucleotide in the biological
sample, said target
polynucleotide comprising a polynucleotide sequence selected from the group
consisting of SEQ ID
N0:3-4, ii) a naturally occurring polynucleotide sequence having at least 90%
sequence identity to a
polynucleotide sequence selected from the group consisting of SEQ ID N0:3-4,
iii) a polynucleotide
sequence complementary to i), iv) a polynucleotide sequence complementary to
ii), and v) an RNA
equivalent of i)-iv). Alternatively, the target polynucleotide comprises a
fragment of the above
polynucleotide sequence; c) quantifying the amount of hybridization complex;
and d) comparing the
amount of hybridization complex in the treated biological sample with the
amount of hybridization
complex in an untreated biological sample, wherein a difference in the amount
of hybridization
complex in the treated biological sample is indicative of toxicity of the test
compound.
CA 02378989 2002-O1-03
WO 01/04308 PCT/US00/19014
BRIEF DESCRIPTION OF THE FIGURES AND TABLES
Figures lA, 1B, 1C, and 1D show the amino acid sequence (SEQ ID NO:1) and
nucleic acid
sequence (SEQ ID N0:3) of LIMD-1. The alignments were produced using MACDNASIS
PRO
software (Hitachi Software Engineering Co. Ltd., San Bruno, CA).
Figures 2A, 2B, 2C, 2D, 2E, 2F, and 2G show the amino acid sequence (SEQ ID
N0:2) and
nucleic acid sequence (SEQ ID N0:4) of LIMD-2.
Figures 3A and 3B show the amino acid sequence alignment between LIMD-1
(Incyte Clone
number 4084014; SEQ ID NO:1) and human FHL-1 (GI 2853224; SEQ ID NO:S). The
alignments
were produced using the multisequence alignment program of LASERGENE software
(DNASTAR
Inc., Madison WI).
Figures 4A, 4B, and 4C show the amino acid sequence alignment between LIMD-2
(Incyte
Clone number 5640004; SEQ ID N0:2) and human LIM-type zinc finger protein (GI
2624922; SEQ
ID N0:6).
Table 1 shows polypeptide and nucleotide sequence identification numbers (SEQ
ID NOs),
clone identification numbers (clone IDs), cDNA libraries, and cDNA fragments
used to assemble full-
length sequences encoding LIMD.
Table 2 shows features of each polypeptide sequence, including potential
motifs, homologous
sequences, and methods, algorithms, and searchable databases used for analysis
of LIMD.
Table 3 shows selected fragments of each nucleic acid sequence; the tissue-
specific expression
patterns of each nucleic acid sequence as determined by northern analysis;
diseases, disorders, or
conditions associated with these tissues; and the vector into which each cDNA
was cloned.
Table 4 describes the tissues used to construct the cDNA libraries from which
cDNA clones
encoding LIMD were isolated.
Table 5 shows the tools, programs, and algorithms used to analyze the
polynudeotides and
polypeptides of the invention, along with applicable descriptions, references,
and threshold parameters.
DESCRIPTION OF THE INVENTION
Before the present proteins, nucleotide sequences, and methods are described,
it is understood
that this invention is not limited to the particular machines, materials and
methods described, as these
may vary. It is also to be understood that the terminology used herein is for
the purpose of describing
particular embodiments only, and is not intended to limit the scope of the
present invention which will
be limited only by the appended claims.
It must be noted that as used herein and in the appended claims, the singular
forms "a," "an,"
CA 02378989 2002-O1-03
WO 01/04308 PCT/US00/19014
and "the" include plural reference unless the context clearly dictates
otherwise. Thus, for example, a
reference to "a host cell" includes a plurality of such host cells, and a
reference to "an antibody" is a
reference to one or more antibodies and equivalents thereof known to those
skilled in the art, and so
forth.
Unless defined otherwise, all technical and scientific terms used herein have
the same meanings
as commonly understood by one of ordinary skill in the art to which this
invention belongs. Although
any machines, materials, and methods similar or equivalent to those described
herein can be used to
practice or test the present invention, the preferred machines, materials and
methods are now described.
All publications mentioned herein are cited for the purpose of describing and
disclosing the cell lines,
protocols, reagents and vectors which are reported in the publications and
which might be used in
connection with the invention. Nothing herein is to be construed as an
admission that the invention is
not entitled to antedate such disclosure by virtue of prior invention.
DEFINITIONS
"LIMD" refers to the amino acid sequences of substantially purified LIMD
obtained from any
species, particularly a mammalian species, including bovine, ovine, porcine,
murine, equine, and
human, and from any source, whether natural, synthetic, semi-synthetic, or
recombinant.
The term "agonist" refers to a molecule which intensifies or mimics the
biological activity of
LIMD. Agonists may include proteins, nucleic acids, carbohydrates, small
molecules, or any other
compound or composition which modulates the activity of LIMD either by
directly interacting with
LIMD or by acting on components of the biological pathway in which LIMD
participates.
An "allelic variant" is an alternative form of the gene encoding LIMD. Allelic
variants may
result from at least one mutation in the nucleic acid sequence and may result
in altered mRNAs or in
polypeptides whose structure or function may or may not be altered. A gene may
have none, one, or
many allelic variants of its naturally occurring form. Common mutational
changes which give rise to
allelic variants are generally ascribed to natural deletions, additions, or
substitutions of nucleotides.
Each of these types of changes may occur alone, or in combination with the
others, one or more times in
a given sequence.
"Altered" nucleic acid sequences encoding LIMD include those sequences with
deletions,
insertions, or substitutions of different nucleotides, resulting in a
polypeptide the same as LIMD or a
polypeptide with at least one functional characteristic of LIMD. Included
within this definition are
polymorphisms which may or may not be readily detectable using a particular
oligonucleotide probe of
the polynucleotide encoding LIMD, and improper or unexpected hybridization to
allelic variants, with a
locus other than the normal chromosomal locus for the polynucleotide sequence
encoding LIMD. The
encoded protein may also be "altered," and may contain deletions, insertions,
or substitutions of amino
CA 02378989 2002-O1-03
WO 01/04308 PCT/US00/19014
acid residues which produce a silent change and result in a functionally
equivalent LIMD. Deliberate
amino acid substitutions may be made on the basis of similarity in polarity,
charge, solubility,
hydrophobicity, hydrophilicity, and/or the amphipathic nature of the residues,
as long as the biological
or immunological activity of LIMD is retained. For example, negatively charged
amino acids may
include aspartic acid and glutamic acid, and positively charged amino acids
may include lysine and
arginine. Amino acids with uncharged polar side chains having similar
hydrophilicity values may
include: asparagine and glutamine; and serine and threonine. Amino acids with
uncharged side chains
having similar hydrophilicity values may include: leucine, isoleucine, and
valine; glycine and alanine;
and phenylalanine and tyrosine.
The terms "amino acid" and "amino acid sequence" refer to an oligopeptide,
peptide,
polypeptide, or protein sequence, or a fragment of any of these, and to
naturally occurring or synthetic
molecules. Where "amino acid sequence" is recited to refer to a sequence of a
naturally occurring
protein molecule, "amino acid sequence" and like terms are not meant to limit
the amino acid sequence
to the complete native amino acid sequence associated with the recited protein
molecule.
"Amplification" relates to the production of additional copies of a nucleic
acid sequence.
Amplification is generally carried out using polymerise chain reaction (PCR)
technologies well known
in the art.
The term "antagonist" refers to a molecule which inhibits or attenuates the
biological activity of
LIMD. Antagonists may include proteins such as antibodies, nucleic acids,
carbohydrates, small
molecules, or any other compound or composition which modulates the activity
of LIMD either by
directly interacting with LIMD or by acting on components of the biological
pathway in which LIMD
participates.
The term "antibody" refers to intact immunoglobulin molecules as well as to
fragments thereof,
such as Fab, F(ab')2, and Fv fragments, which are capable of binding an
epitopic determinant.
Antibodies that bind LIMD polypeptides can be prepared using intact
polypeptides or using fragments
containing small peptides of interest as the immunizing antigen. The
polypeptide or oligopeptide used
to immunize an animal (e.g., a mouse, a rat, or a rabbit) can be derived from
the translation of RNA, or
synthesized chemically, and can be conjugated to a carrier protein if desired.
Commonly used carriers
that are chemically coupled to peptides include bovine serum albumin,
thyroglobulin, and keyhole
limpet hemocyanin (KL,H). The coupled peptide is then used to immunize the
animal.
The term "antigenic determinant" refers to that region of a molecule (i.e., an
epitope) that
makes contact with a particular antibody. When a protein or a fragment of a
protein is used to
immunize a host animal, numerous regions of the protein may induce the
production of antibodies which
bind specifically to antigenic determinants (particular regions or three-
dimensional structures on the
protein). An antigenic determinant may compete with the intact antigen (i.e.,
the immunogen used to
CA 02378989 2002-O1-03
WO 01/04308 PCT/US00/19014
elicit the immune response) for binding to an antibody.
The term "antisense" refers to any composition capable of base-pairing with
the "sense"
(coding) strand of a specific nucleic acid sequence. Antisense compositions
may include DNA; RNA;
peptide nucleic acid (PNA); oligonucleotides having modified backbone linkages
such as
phosphorothioates, methylphosphonates, or benzylphosphonates; oligonucleotides
having modified
sugar groups such as 2'-methoxyethyl sugars or 2'-methoxyethoxy sugars; or
oligonucleotides having
modified bases such as 5-methyl cytosine, 2'-deoxyuracil, or 7-deaza-2'-
deoxyguanosine. Antisense
molecules may be produced by any method including chemical synthesis or
transcription. Once
introduced into a cell, the complementary antisense molecule base-pairs with a
naturally occurring
nucleic acid sequence produced by the cell to form duplexes which block either
transcription or
translation. The designation "negative" or "minus" can refer to the antisense
strand, and the
designation "positive" or "plus" can refer to the sense strand of a reference
DNA molecule.
The term "biologically active" refers to a protein having structural,
regulatory, or biochemical
functions of a naturally occurring molecule. Likewise, "immunologically
active" or "immunogenic"
refers to the capability of the natural, recombinant, or synthetic LIMD, or of
any oligopeptide thereof,
to induce a specific immune response in appropriate animals or cells and to
bind with specific
antibodies.
"Complementary" describes the relationship between two single-stranded nucleic
acid
sequences that anneal by base-pairing. For example, 5'-AGT-3' pairs with its
complement,
3'-TCA-5'.
A "composition comprising a given polynucleodde sequence" and a "composition
comprising a
given amino acid sequence" refer broadly to any composition containing the
given polynucleotide or
amino acid sequence. The composition may comprise a dry formulation or an
aqueous solution.
Compositions comprising polynucleotide sequences encoding LIMD or fragments of
LIMD may be
employed as hybridization probes. The probes may be stored in freeze-dried
form and may be
associated with a stabilizing agent such as a carbohydrate. In hybridizations,
the probe may be
deployed in an aqueous solution containing salts (e.g., NaCl), detergents
(e.g., sodium dodecyl sulfate;
SDS), and other components (e.g., Denhardt's solution, dry milk, salmon sperm
DNA, etc.).
"Consensus sequence" refers to a nucleic acid sequence which has been
subjected to repeated
DNA sequence analysis to resolve uncalled bases, extended using the XL-PCR kit
(PE Biosystems,
Foster City CA) in the 5' and/or the 3' direction, and resequenced, or which
has been assembled from
one or more overlapping cDNA, EST, or genomic DNA fragments using a computer
program for
fragment assembly, such as the GELVIEW fragment assembly system (GCG, Madison
WI) or Phrap
(University of Washington, Seattle WA). Some sequences have been both extended
and assembled to
11
CA 02378989 2002-O1-03
WO 01/04308 PCT/US00/19014
produce the consensus sequence.
"Conservative amino acid substitutions" are those substitutions that are
predicted to least
interfere with the properties of the original protein, i.e., the structure and
especially the function of the
protein is conserved and not significantly changed by such substitutions. The
table below shows amino
acids which may be substituted for an original amino acid in a protein and
which are regarded as
conservative amino acid substitutions.
Original Residue Conservative Substitution
Ala Gly, Ser
Arg His, Lys
Asn Asp, Gln, His
Asp Asn, Glu
Cys Ala, Ser
Gln Asn, Glu, His
Glu Asp, Gln, His
Gly Ala
His Asn, Arg, Gln, Glu
Ile Leu, Val
Leu Ile, Val
Lys Arg, Gln, Glu
Met Leu, Ile
Phe His, Met, Leu, Trp, Tyr
Ser Cys, Thr
Thr Ser, Val
Trp Phe, Tyr
Tyr His, Phe, Trp
Val Ile, Leu, Thr
Conservative amino acid substitutions generally maintain (a) the structure of
the polypeptide
backbone in the area of the substitution, for example, as a beta sheet or
alpha helical conformation,
(b) the charge or hydrophobicity of the molecule at the site of the
substitution, and/or (c) the bulk of the
side chain.
A "deletion" refers to a change in the amino acid or nucleotide sequence that
results in the
absence of one or more amino acid residues or nucleotides.
The term "derivative" refers to a chemically modified polynucleotide or
polypeptide. Chemical
modifications of a polynucleotide sequence can include, for example,
replacement of hydrogen by an
alkyl, acyl, hydroxyl, or amino group. A derivative polynucleotide encodes a
polypeptide which retains
at least one biological or immunological function of the natural molecule. A
derivative polypeptide is
one modified by glycosylation, pegylation, or any similar process that retains
at least one biological or
immunological function of the polypeptide from which it was derived.
A "detectable label" refers to a reporter molecule or enzyme that is capable
of generating a
measurable signal and is covalently or noncovalently joined to a
polynucleotide or polypeptide.
12
CA 02378989 2002-O1-03
WO 01/04308 PCT/US00/19014
A "fragment" is a unique portion of LIMD or the polynucleotide encoding LIMD
which is
identical in sequence to but shorter in length than the parent sequence. A
fragment may comprise up
to the entire length of the defined sequence, minus one nucleotide/amino acid
residue. For example, a
fragment may comprise from 5 to 1000 contiguous nucleotides or amino acid
residues. A fragment
used as a probe, primer, antigen, therapeutic molecule, or for other purposes,
may be at least 5, 10,
15, 16, 20, 25, 30, 40, 50, 60, 75, 100, 150, 250 or at least 500 contiguous
nucleotides or amino acid
residues in length. Fragments may be preferentially selected from certain
regions of a molecule. For
example, a polypeptide fragment may comprise a certain length of contiguous
amino acids selected
from the first 250 or 500 amino acids (or first 25% or 50% of a polypeptide)
as shown in a certain
defined sequence. Clearly these lengths are exemplary, and any length that is
supported by the
specification, including the Sequence Listing, tables, and figures, may be
encompassed by the present
embodiments.
A fragment of SEQ ID N0:3-4 comprises a region of unique polynucleotide
sequence that
specifically identifies SEQ ID N0:3-4, for example, as distinct liom any other
sequence in the
genome from which the fragment was obtained. A fragment of SEQ ID N0:3-4 is
useful, for
example, in hybridization and amplification technologies and in analogous
methods that distinguish
SEQ ID N0:3-4 from related polynucleotide sequences. The precise length of a
fragment of SEQ ID
N0:3-4 and the region of SEQ ID N0:3-4 to which the fragment corresponds are
routinely
determinable by one of ordinary skill in the art based on the intended purpose
for the fragment.
A fragment of SEQ ID NO:1-2 is encoded by a fragment of SEQ ID N0:3-4. A
fragment of
SEQ ID NO:1-2 comprises a region of unique amino acid sequence that
specifically identifies SEQ ID
NO:1-2. For example, a fiagment of SEQ ID NO:1-2 is useful as an immunogenic
peptide for the
development of antibodies that specifically recognize SEQ ID NO:1-2. The
precise length of a
liagment of SEQ ID NO:1-2 and the region of SEQ ID NO:l-2 to which the
fragment corresponds are
routinely determinable by one of ordinary skill in the art based on the
intended purpose for the
fragment.
A "full-length" polynucleotide sequence is one containing at least a
translation initiation colon
(e.g., methionine) followed by an open reading frame and a translation
termination colon. A "full-
length" polynucleotide sequence encodes a "full-length" polypeptide sequence.
"Homology" refers to sequence similarity or, interchangeably, sequence
identity, between two
or more polynucleotide sequences or two or more polypeptide sequences.
The terms ''percent identity" and "% identity," as applied to polynucleotide
sequences, refer to
the percentage of residue matches between at least two polynucleotide
sequences aligned using a
standardized algorithm. Such an algorithm may insert, in a standardized and
reproducible way, gaps in
the sequences being compared in order to optimize alignment between two
sequences, and therefore
13
CA 02378989 2002-O1-03
WO 01/04308 PCT/US00/19014
achieve a more meaningful comparison of the two sequences.
Percent identity between polynucleotide sequences may be determined using the
default
parameters of the CLUSTAL V algorithm as incorporated into the MEGALIGN
version 3.12e sequence
alignment program. This program is part of the LASERGENE software package, a
suite of molecular
biological analysis programs (DNASTAR, Madison WI). CLUSTAL V is described in
Higgins, D.G.
and P.M. Sharp (1989) CABIOS 5:151-153 and in Higgins, D.G. et al. (1992)
CABIOS 8:189-191.
For pairwise alignments of polynucleotide sequences, the default parameters
are set as follows:
Ktuple=2, gap penalty=5, window=4, and "diagonals saved"=4. The "weighted"
residue weight table is
selected as the default. Percent identity is reported by CLUSTAL V as the
"percent similarity" between
aligned polynucleotide sequences.
Alternatively, a suite of commonly used and freely available sequence
comparison algorithms is
provided by the National Center for Biotechnology Information (NCBI) Basic
Local Alignment Search
Tool (BLAST) (Altschul, S.F. et al. (1990) J. Mol. Biol. 215:403-410), which
is available from several
sources, including the NCBI, Bethesda, MD, and on the Internet at
http://www.ncbi.nlm.nih.gov/BLAST/. The BLAST software suite includes various
sequence analysis
programs including "blastn," that is used to align a known polynucleotide
sequence with other
polynucleotide sequences from a variety of databases. Also available is a tool
called "BLAST 2
Sequences" that is used for direct pairwise comparison of two nucleotide
sequences. "BLAST 2
Sequences" can be accessed and used interactively at
http://www.ncbi.nlm.nih.gov/gorf/bl2.html. The
"BLAST 2 Sequences" tool can be used for both blastn and blastp (discussed
below). BLAST
programs are commonly used with gap and other parameters set to default
settings. For example, to
compare two nucleotide sequences, one may use blastn with the "BLAST 2
Sequences" tool Version
2Ø12 (April-21-2000) set at default parameters. Such default parameters may
be, for example:
Matrix: BLOSUM62
Reward for match: 1
Penalty for mismatch: -2
Open Gap: 5 and Extension Gap: 2 penalties
Gap x drop-off: SD
Expect: 10
Word Size: 11
Filter: on
Percent identity may be measured over the length of an entire defined
sequence, for example, as
defined by a particular SEQ ID number, or may be measured over a shorter
length, for example, over
the length of a fragment taken from a larger, defined sequence, for instance,
a liagment of at least 20, at
14
CA 02378989 2002-O1-03
WO 01/04308 PCT/US00/19014
least 30, at least 40, at least 50, at least 70, at least 100, or at least 200
contiguous nucleotides. Such
lengths are exemplary only, and it is understood that any fragment length
supported by the sequences
shown herein, in the tables, figures, or Sequence Listing, may be used to
describe a length over which
percentage identity may be measured.
Nucleic acid sequences that do not show a high degree of identity may
nevertheless encode
similar amino acid sequences due to the degeneracy of the genetic code. It is
understood that changes in
a nucleic acid sequence can be made using this degeneracy to produce multiple
nucleic acid sequences
that all encode substantially the same protein.
The phrases "percent identity" and "% identity," as applied to polypeptide
sequences, refer to
the percentage of residue matches between at least two polypeptide sequences
aligned using a
standardized algorithm. Methods of polypeptide sequence alignment are well-
known. Some alignment
methods take into account conservative amino acid substitutions. Such
conservative substitutions,
explained in more detail above, generally preserve the charge and
hydrophobicity at the site of
substitution, thus preserving the structure (and therefore function) of the
polypeptide.
Percent identity between polypeptide sequences may be determined using the
default parameters
of the CLUSTAL V algorithm as incorporated into the MEGALIGN version 3.12e
sequence alignment
program (described and referenced above). For pairwise alignments of
polypeptide sequences using
CLUSTAL V, the default parameters are set as follows: Ktuple=1, gap penalty=3,
window=5, and
"diagonals saved"=5. The PAM250 matrix is selected as the default residue
weight table. As with
polynucleotide alignments, the percent identity is reported by CLUSTAL V as
the "percent similarity"
between aligned polypeptide sequence pairs.
Alternatively the NCBI BLAST software suite may be used. For example, for a
pairwise
comparison of two polypeptide sequences, one may use the "BLAST 2 Sequences"
tool Version 2Ø12
(Apr-21-2000) with blastp set at default parameters. Such default parameters
may be, for example:
Matrix: BLOSUM62
Open Gap: 1l and Extension Gap: I penalties
Gap x drop-off:' S0
Expect: 10
Word Size: 3
Filter: on
Percent identity may be measured over the length of an entire defined
polypeptide sequence, for
example, as defined by a particular SEQ ID number, or may be measured over a
shorter length, for
example, over the length of a fragment taken from a larger, defined
polypeptide sequence, for instance,
a fragment of at least 15, at least 20, at least 30, at least 40, at least 50,
at least 70 or at least 150
CA 02378989 2002-O1-03
WO 01/04308 PCT/US00/19014
contiguous residues. Such lengths are exemplary only, and it is understood
that any fragment length
supported by the sequences shown herein, in the tables, figures or Sequence
Listing, may be used to
describe a length over which percentage identity may be measured.
"Human artificial chromosomes" (HACs) are linear microchromosomes which may
contain
DNA sequences of about 6 kb to 10 Mb in size, and which contain all of the
elements required for
chromosome replication, segregation and maintenance.
The term "humanized antibody" refers to an antibody molecule in which the
amino acid
sequence in the non-antigen binding regions has been altered so that the
antibody more closely
resembles a human antibody, and still retains its original binding ability.
"Hybridization" refers to the process by which a polynucleotide strand anneals
with a
complementary strand through base pairing under defined hybridization
conditions. Specific
hybridization is an indication that two nucleic acid sequences share a high
degree of complementarity.
Specific hybridization complexes form under permissive annealing conditions
and remain hybridized
after the "washing" step(s). The washing steps) is particularly important in
determining the stringency
of the hybridization process, with more stringent conditions allowing less non-
specific binding, i.e.,
binding between pairs of nucleic acid strands that are not perfectly matched.
Permissive conditions for
annealing of nucleic acid sequences are routinely determinable by one of
ordinary skill in the art and
may be consistent among hybridization experiments, whereas wash conditions may
be varied among
experiments to achieve the desired stringency, and therefore hybridization
specificity. Permissive
annealing conditions occur, for example, at 68°C in the presence of
about 6 x SSC, about 1 % (w/v)
SDS, and about 100 ~g/ml sheared, denatured salmon sperm DNA.
Generally, stringency of hybridization is expressed, in part, with reference
to the temperature
under which the wash step is carried out. Such wash temperatures are typically
selected to be about
5°C to 20°C lower than the thermal melting point (T~ for the
specific sequence at a defined ionic
strength and pH. The Tm is the temperature (under defined ionic strength and
pH) at which 50% of the
target sequence hybridizes to a perfectly matched probe. An equation for
calculating Tm and conditions
for nucleic acid hybridization are well known and can be found in Sambrook, J.
et al., 1989, Molecular
Cloning: A Laboratory Manual, 2nd ed., vol. 1-3, Cold Spring Harbor Press,
Plainview NY; specifically
see volume 2, chapter 9.
High stringency conditions for hybridization between polynucleotides of the
present invention
include wash conditions of 68°C in the presence of about 0.2 x SSC and
about 0.1% SDS, for 1 hour.
Alternatively, temperatures of about 65°C, 60°C, 55°C, or
42°C may be used. SSC concentration may
be varied from about 0.1 to 2 x SSC, with SDS being present at about 0.1 %.
Typically, blocking
reagents are used to block non-specific hybridization. Such blocking reagents
include, for instance,
16
CA 02378989 2002-O1-03
WO 01/04308 PCT/US00/19014
sheared and denatured salmon sperm DNA at about 100-200 ~g/ml. Organic
solvent, such as
formamide at a concentration of about 35-50% v/v, may also be used under
particular circumstances,
such as for RNA:DNA hybridizations. Useful variations on these wash conditions
will be readily
apparent to those of ordinary skill in the art. Hybridization, particularly
under high stringency
conditions, may be suggestive of evolutionary similarity between the
nucleotides. Such similarity is
strongly indicative of a similar role for the nucleotides and their encoded
polypeptides.
The term "hybridization complex" refers to a complex formed between two
nucleic acid
sequences by virtue of the formation of hydrogen bonds between complementary
bases. A hybridization
complex may be formed in solution (e.g., Cot or Rot analysis) or formed
between one nucleic acid
sequence present in solution and another nucleic acid sequence immobilized on
a solid support (e.g.,
paper, membranes, filters, chips, pins or glass slides, or any other
appropriate substrate to which cells
or their nucleic acids have been fixed).
The words "insertion" and "addition" refer to changes in an amino acid or
nucleotide sequence
resulting in the addition of one or more amino acid residues or nucleotides,
respectively.
"immune response" can refer to conditions associated with inflammation,
trauma, immune
disorders, or infectious or genetic disease, etc. These conditions can be
characterized by expression of
various factors, e.g., cytokines, chemokines, and other signaling molecules,
which may affect cellular
and systemic defense systems.
An "immunogenic fragment" is a polypeptide or oligopeptide fragment of LIMD
which is
capable of eliciting an immune response when introduced into a living
organism, for example, a
mammal. The term "immunogenic fragment" also includes any polypeptide or
oligopeptide fragment of
LIMD which is useful in any of the antibody production methods disclosed
herein or known in the art.
The term "microarray" refers to an arrangement of a plurality of
polynucleotides, polypeptides,
or other chemical compounds on a substrate.
The terms "element" and "array element" refer to a polynucleotide,
polypeptide, or other
chemical compound having a unique and defined position on a microarray.
The term "modulate" refers to a change in the activity of LIMD. For example,
modulation may
cause an increase or a decrease in protein activity, binding characteristics,
or any other biological,
functional, or immunological properties of LIMD.
The phrases "nucleic acid" and "nucleic acid sequence" refer to a nucleotide,
oligonucleotide,
polynucleotide, or any fragment thereof. These phrases also refer to DNA or
RNA of genomic or
synthetic origin which may be single-stranded or double-stranded and may
represent the sense or the
antisense strand, to peptide nucleic acid (PNA), or to any DNA-like or RNA-
like material.
"Operably linked" refers to the situation in which a first nucleic acid
sequence is placed in a
17
CA 02378989 2002-O1-03
WO 01/04308 PCT/US00/19014
functional relationship with a second nucleic acid sequence. For instance, a
promoter is operably
linked to a coding sequence if the promoter affects the transcription or
expression of the coding
sequence. Operably linked DNA sequences may be in,close proximity or
contiguous and, where
necessary to join two protein coding regions, in the same reading frame.
"Peptide nucleic acid" (PNA) refers to an antisense molecule or anti-gene
agent which
comprises an oligonucleotide of at least about 5 nucleotides in length linked
to a peptide backbone of
amino acid residues ending in lysine. The terminal lysine confers solubility
to the composition. PNAs
preferentially bind complementary single stranded DNA or RNA and stop
transcript elongation, and
may be pegylated to extend their lifespan in the cell.
"Post-translational modification" of an LIMD may involve lipidation,
glycosylation,
phosphorylation, acetylation, racemizadon, proteolytic cleavage, and other
modifications known in the
art. These processes may occur synthetically or biochemically. Biochemical
modifications will vary by
cell type depending on the enzymatic milieu of LIMD.
"Probe" refers to nucleic acid sequences encoding LIMD, their complements, or
fragments
thereof, which are used to detect identical, allelic or related nucleic acid
sequences. Probes are
isolated oligonucleotides or polynucleotides attached to a detectable label or
reporter molecule. Typical
labels include radioactive isotopes, ligands, ~hemiluminescent agents, and
enzymes. "Primers" are
short nucleic acids, usually DNA oligonucleotides, which may be annealed to a
target polynucleotide by
complementary base-pairing. The primer may then be extended along the target
DNA strand by a DNA
polymerase enzyme. Primer pairs can be used for amplification (and
identification) of a nucleic acid
sequence, e.g., by the polymerase chain reaction (PCR).
Probes and primers as used in the present invention typically comprise at
least 15 contiguous
nucleotides of a known sequence. In order to enhance specificity, longer
probes and primers may also
be employed, such as probes and primers that comprise at least 20, 25, 30, 40,
50, 60, 70, 80, 90, 100,
or at least 150 consecutive nucleotides of the disclosed nucleic acid
sequences. Probes and primers may
be considerably longer than these examples, and it is understood that any
length supported by the
specification, including the tables, figures, and Sequence Listing, may be
used.
Methods for preparing and using probes and primers are described in the
references, for
example Sambrook, J. et al., 1989, Molecular Cloning: A Laboratory Manual,
2°d ed., vol. 1-3, Cold
Spring Harbor Press, Plainview NY; Ausubel, F.M. et a1.,1987, Current
Protocols in Molecular
Biolo~v, Greene Publ. Asscx. & Wiley-Intersciences, New York NY; Innis, M. et
al., 1990, PCR
Protocols, A Guide to Methods and Applications, Academic Press, San Diego CA.
PCR primer pairs
can be derived from a known sequence, for example, by using computer programs
intended for that
purpose such as Primer (Version 0.5, 1991, Whitehead Institute for Biomedical
Research, Cambridge
MA).
18
CA 02378989 2002-O1-03
WO 01/04308 PCT/US00/19014
Oligonucleotides for use as primers are selected using software known in the
art for such
purpose. For example, OLIGO 4.06 software is useful for the selection of PCR
primer pairs of up to
100 nucleotides each, and for the analysis of oligonucleotides and larger
polynucleotides of up to 5,000
nucleotides from an input polynucleotide sequence of up to 32 kilobases.
Sinular primer selection
programs have incorporated additional features for expanded capabilities. For
example, the PrimOU
primer selection program (available to the public from the Genome Center at
University of Texas South
West Medical Center, Dallas TX) is capable of choosing specific primers from
megabase sequences
and is thus useful for designing primers on a genome-wide scope. The Primer3
primer selection
program (available to the public from the Whitehead InstitutelMIT Center for
Genome Research,
Cambridge MA) allows the user to input a "mispriming library," in which
sequences to avoid as primer
binding sites are user-specified. Primer3 is useful, in particular, for the
selection of oligonucleotides for
microarrays. (The source code for the latter two primer selection programs may
also be obtained from
their respective sources and modified to meet the user's specific needs.) The
PrimeGen program
(available to the public from the UK Human Genome Mapping Project Resource
Centre, Cambridge
UK) designs primers based on multiple sequence alignments, thereby allowing
selection of primers that
hybridize to either the most conserved or least conserved regions of aligned
nucleic acid sequences.
Hence, this program is useful for identification of both unique and conserved
oligonucleotides and
polynucleotide fragments. The oligonucleotides and polynucleotide fragments
identified by any of the
above selection methods are useful in hybridization technologies, for example,
as PCR or sequencing
primers, microarray elements, or specific probes to identify fully or
partially complementary
polynucleotides in a sample of nucleic acids. Methods of oligonucleotide
selection are not limited to
those described above.
A "recombinant nucleic acid" is a sequence that is not naturally occurring or
has a sequence
that is made by an artificial combination of two or more otherwise separated
segments of sequence.
This artificial combination is often. accomplished by chemical synthesis or,
more commonly, by the
artificial manipulation of isolated segments of nucleic acids, e.g., by
genetic engineering techniques
such as those described in Sambrook, su ra. The term recombinant includes
nucleic acids that have
been altered solely by addition, substitution, or deletion of a portion of the
nucleic acid. Frequently, a
recombinant nucleic acid may include a nucleic acid sequence operably linked
to a promoter sequence.
Such a recombinant nucleic acid may be part of a vector that is used, for
example, to transform a cell.
Alternatively, such recombinant nucleic acids may be part of a viral vector,
e.g., based on a
vaccinia virus, that could be use to vaccinate a mammal wherein the
recombinant nucleic acid is
expressed, inducing a protective immunological response in the mammal.
A "regulatory element" refers to a nucleic acid sequence usually derivett liom
untranslated
19
CA 02378989 2002-O1-03
WO 01/04308 PCT/US00/19014
regions of a gene and includes enhancers, promoters, introns, and 5' and 3'
untranslated regions (UTRs).
Regulatory elements interact with host or viral proteins which control
transcription, translation, or RNA
stability.
"Reporter molecules" are chemical or biochemical moieties used for labeling a
nucleic acid,
amino acid, or antibody. Reporter molecules include radionuclides; enzymes;
fluorescent,
chemilunlinescent, or chromogenic agents; substrates; cofactors; inhibitors;
magnetic particles; and
other moieties known in the art.
An "RNA equivalent," in reference to a DNA sequence, is composed of the same
linear
sequence of nucleotides as the reference DNA sequence with the exception that
all occurrences of the
nitrogenous base thymine are replaced with uracil, and the sugar backbone is
composed of ribose
instead of deoxyribose.
The term "sample" is used in its broadest sense. A sample suspected of
containing nucleic
acids encoding LIMD, or fragments thereof, or LIMD itself, may comprise a
bodily fluid; an extract
from a cell, chromosome, organelle, or membrane isolated from a cell; a cell;
genomic DNA, RNA, or
cDNA, in solution or bound to a substrate; a tissue; a tissue print; etc.
The terms "specific binding" and "specifically binding" refer to that
interaction between a
protein or peptide and an agonist, an antibody, an antagonist, a small
molecule, or any natural or
synthetic binding composition. The interaction is dependent upon the presence
of a particular structure
of the protein, e.g., the antigenic determinant or epitope, recognized by the
binding molecule. For
example, if an antibody is specific for epitope "A," the presence of a
polypeptide comprising the epitope
A, or the presence of free unlabeled A, in a reaction containing free labeled
A and the antibody will
reduce the amount of labeled A that binds to the antibody.
The term "substantially purified" refers to nucleic acid or amino acid
sequences that are
removed from their natural environment and are isolated or separated, and are
at least 60% free,
preferably at least 75% free, and most preferably at least 90% free fiom other
components with which
they are naturally associated.
A "substitution" refers to the replacement of one or more amino acid residues
or nucleotides by
different amino acid residues or nucleotides, respectively.
"Substrate" refers to any suitable rigid or semi-rigid support including
membranes, filters,
chips, slides, wafers, fibers, magnetic or nonmagnetic beads, gels, tubing,
plates, polymers,
microparticles and capillaries. The substrate can have a variety of surface
forms, such as wells,
trenches, pins, channels and pores, to which polynucleotides or polypeptides
are bound.
A "transcript image" refers to the collective pattern of gene expression by a
particular cell type
or tissue under given conditions at a given time.
CA 02378989 2002-O1-03
WO 01/04308 PCT/US00/19014
"Transformation" describes a process by which exogenous DNA is introduced into
a recipient
cell. Transformation may occur under natural or artificial conditions
according to various methods well
known in the art, and may rely on any known method for the insertion of
foreign nucleic acid sequences
into a prokaryotic or eukaryotic host cell. The method for transformation is
selected based on the type
of host cell being transformed and may include, but is not limited to,
bacteriophage or viral infection,
electroporation, heat shock, lipofection, and particle bombardment. The term
"transformed" cells
includes stably transformed cells in which the inserted DNA is capable of
replication either as an
autonomously replicating plasmid or as part of the host chromosome, as well as
transiently transformed
cells which express the inserted DNA or RNA for limited periods of time.
A "transgenic organism," as used herein, is any organism, including but not
limited to
animals and plants, in which one or more of the cells of the organism contains
heterologous nucleic
acid introduced by way of human intervention, such as by transgenic techniques
well known in the
art. The nucleic acid is introduced into the cell, directly or indirectly by
introduction into a precursor
of the cell, by way of deliberate genetic manipulation, such as by
microinjection or by infection with
a recombinant virus. The term genetic manipulation does not include classical
cross-breeding, or in
vitro fertilization, but rather is directed to the introduction of a
recombinant DNA molecule. The
transgenic organisms contemplated in accordance with the present invention
include bacteria,
cyanobacteria, fungi, plants, and animals. The isolated DNA of the present
invention can be
introduced into the host by methods known in the art, for example infection,
transfection,
transformation or transconjugation. Techniques for transferring the DNA of the
present invention
into such organisms are widely known and provided in references such as
Sambrook et al. (1989),
supra.
A "variant" of a particular nucleic acid sequence is defined as a nucleic acid
sequence having at
least 40% sequence identity to the particular nucleic acid sequence over a
certain length of one of the
nucleic acid sequences using blastn with the "BLAST 2 Sequences" tool Version
2Ø9 (May-07-1999)
set at default parameters. Such a pair of nucleic acids may show, for example,
at least 50%, at least
60%, at least 70%, at least 80%, at least 85%, at least 90%, at least 95% or
at least 98% or greater
sequence identity over a certain defined length. A variant may be described
as, for example, an "allelic"
(as defined above), "splice," "species," or "polymorphic" variant. A splice
variant may have significant
identity to a reference molecule, but will generally have a greater or lesser
number of polynucleotides
due to alternative splicing of exons during mRNA processing. The corresponding
polypeptide may
possess additional functional domains or lack domains that are present in the
reference molecule.
Species variants are polynucleotide sequences that vary from one species to
another. The resulting
polypeptides generally will have significant amino acid identity relative to
each other. A polymorphic
variant is a variation in the polynucleotide sequence of a particular gene
between individuals of a given
21
CA 02378989 2002-O1-03
WO 01/04308 PCT/US00/19014
species. Polymorphic variants also may encompass "single nucleotide
polymorphisms" (SNPs) in
which the polynucleotide sequence varies by one nucleotide base. The presence
of SNPs may be
indicative of, for example, a certain population, a disease state, or a
propensity for a disease state.
A "variant" of a particular polypeptide sequence is defined as a polypeptide
sequence having at
least 40% sequence identity to the particular polypeptide sequence over a
certain length of one of the
polypeptide sequences using blastp with the "BLAST 2 Sequences" tool Version
2Ø9 (May-07-1999)
set at default parameters. Such a pair of polypeptides may show, for example,
at least 50%, at least
60%, at least 70%, at least 80%, at least 90%, at least 95%, or at least 98%
or greater sequence
identity over a certain defined length of one of the polypeptides.
THE INVENTION
The invention is based on the discovery of new human human LIM domain proteins
(LIMD),
the polynucleotides encoding LIMD, and the use of these compositions for the
diagnosis, treatment, or
prevention of cell proliferative, developmental, and cell motility disorders.
Table 1 lists the Incyte clones used to assemble full length nucleotide
sequences encoding
LIMD. Columns 1 and 2 show the sequence identification numbers (SEQ ID NOs) of
the polypeptide
and nucleotide sequences, respectively. Column 3 shows the clone IDs of the
Incyte clones in which
nucleic acids encoding each LIMD were identified, and column 4 shows the cDNA
libraries from which
these clones were isolated. Column 5 shows Incyte clones and their
corresponding cDNA libraries.
Clones for which cDNA libraries are not indicated were derived from pooled
cDNA libraries. The
Incyte clones in column 5 were used to assemble the consensus nucleotide
sequence of each LIMD and
are useful as fragments in hybridization technologies.
The columns of Table 2 show various properties of each of the polypeptides of
the invention:
column 1 references the SEQ ID NO; column 2 shows the number of amino acid
residues in each
polypeptide; column 3 shows potential phosphorylation sites; column 4 shows
potential glycosylation
sites; column 5 shows the amino acid residues comprising signature sequences
and motifs; column 6
shows homologous sequences as identified by BLAST analysis; and column 7 shows
analytical methods
and in some cases, searchable databases to which the analytical methods were
applied. The methods of
column 7 were used to characterize each polypeptide through sequence homology
and protein motifs.
As shown in Figures 3A and 3B, LIMD-1 has chemical and structural homology
with human FHL-1
(GI 2853224; SEQ ID NO:S). In particular, LIMD-1 and human FHI,-1 share 93%
identity overall,
although LIMD-1 is shorter and therefore lacks homology to the first 90
residues of SEQ ID NO:S.
As shown in Figures 4A, 4B, and 4C, LIMD-2 has chemical and structural
homology with human
LIM-type zinc finger protein (GI 2624922; SEQ 1D N0:6). In particular, LIMD-2
and human LIM-
type zinc finger protein share 18% identity overall, and 58% identity in the C-
terminal LIM domain,
from C501 through S564 in SEQ ID N0:2 and from C272 through S335 in SEQ ID
N0:6.
22
CA 02378989 2002-O1-03
WO 01/04308 PCT/US00/19014
The columns of Table 3 show the tissue-specificity and diseases, disorders, or
conditions
associated with nucleotide sequences encoding LIMD. The first column of Table
3 lists the nucleotide
SEQ ID NOs. Column 2 lists Iiagments of the nucleotide sequences of column 1.
These fragments are
useful, for example, in hybridization or amplification technologies to
identify SEQ ID N0:3-4 and to
distinguish between SEQ ID N0:3-4 and related polynucleotide sequences. The
polypeptides encoded
by these fragments are useful, for example, as immunogenic peptides. Column 3
lists tissue categories
which express LIMD as a fraction of total tissues expressing LIMD. Column 4
lists diseases,
disorders, or conditions associated with those tissues expressing LIMD as a
fraction of total tissues
expressing LIMD. Column 5 lists the vectors used to subclone each cDNA
library.
The columns of Table 4 show descriptions of the tissues used to construct the
cDNA libraries
from which cDNA clones encoding LIMD were isolated. Column 1 references the
nucleotide SEQ ID
NOs, column 2 shows the cDNA libraries from which these clones were isolated,
and column 3 shows
the tissue origins and other descriptive information relevant to the cDNA
libraries in column 2.
SEQ ID N0:3 maps to chromosome X within the interval from 173.6 to 179.8
centiMorgans.
This interval also contains genes associated with Lowe oculocerebrorenal
syndrome, Simpson
dysmorphia syndrome, Lesch-Nyhan syndrome, and fragile X mental retardation.
SEQ ID N0:4 maps to chromosome 4 within the interval from 56.7 to 60.5
centiMorgans.
This interval also contains a gene associated with familial
hypercholesterolemia.
The invention also encompasses LIMD variants. A preferred LIMD variant is one
which has at
least about 85%, or alternatively at least about 90%, or even at least about
95% amino acid sequence
identity to the LIMD amino acid sequence, and which contains at least one
functional or structural
characteristic of LIMD.
The invention also encompasses polynucleotides which encode LIMD. In a
particular
embodiment, the invention encompasses a polynucleolide sequence comprising the
sequence of SEQ ID
N0:3, as shown in Figures 1 A, 1B, 1C, and 1 D, which encodes a LIMD. In a
further embodiment, the
invention encompasses the polynucleotide sequence comprising the sequence of
SEQ ID N0:4, as
shown in Figures 2A, 2B, 2C, 2D, 2E, 2F, and 2G, which encodes a LIMD. The
polynucleotide
sequences of SEQ ID N0:3-4, as presented in the Sequence Listing, embrace the
equivalent RNA
sequences, wherein occurrences of the nitrogenous base thymine are replaced
with uracil, and the sugar
backbone is composed of ribose instead of deoxyribose.
The invention also encompasses a variant of a polynucleotide sequence encoding
LIMD. 1n
particular, such a variant polynucleotide sequence will have at least about
85%, or alternatively at least
about 90%, or even at least about 95% polynucleotide sequence identity to the
polynucleotide sequence
encoding LIMD. A particular aspect of the invention encompasses a variant of a
polynucleotide
sequence comprising a sequence selected from the group consisting of SEQ ID
N0:3-4 which has at
23
CA 02378989 2002-O1-03
WO 01/04308 PCT/US00/19014
least about 85%, or alternatively at least about 90%, or even at least about
95% polynucleotide
sequence identity to a nucleic acid sequence selected from the group
consisting of SEQ ID N0:3-4.
Any one of the polynucleodde variants described above can encode an amino acid
sequence which
contains at least one functional or structural characteristic of L1MD.
S It will be appreciated by those skilled in the art that as a result of the
degeneracy of the genetic
code, a multitude of polynucleotide sequences encoding LIMD, some bearing
minimal similarity to the
polynucleotide sequences of any known and naturally occurring gene, may be
produced. Thus, the
invention contemplates each and every possible variation of polynucleotide
sequence that could be made
by selecting combinations based on possible codon choices. These combinations
are made in
accordance with the standard triplet genetic code as applied to the
polynucleotide sequence of naturally
occurring LIMD, and all such variations are to be considered as being
specifically disclosed.
Although nucleotide sequences which encode LIMD and its variants are generally
capable of
hybridizing to the nucleotide sequence of the naturally occurring LIMD under
appropriately selected
conditions of stringency, it may be advantageous to produce nucleotide
sequences encoding LIMD or its
derivatives possessing a substantially different codon usage, e.g., inclusion
of non-naturally occurring
codons. Codons may be selected to increase the rate at which expression of the
peptide occurs in a
particular prokaryotic or eukaryotic host in accordance with the frequency
with which particular codons
are utilized by the host. Other reasons for substantially altering the
nucleotide sequence encoding
LIMD and its derivatives without altering the encoded amino acid sequences
include the production of
RNA transcripts having more desirable properties, such as a greater half-life,
than transcripts produced
from the naturally occurring sequence.
The invention also encompasses production of DNA sequences which encode LIMD
and LIMD
derivatives, or fragments thereof, entirely by synthetic chemistry. After
production, the synthetic
sequence may be inserted into any of the many available expression vectors and
cell systems using
reagents well known in the art. Moreover, synthetic chemistry may be used to
introduce mutations into
a sequence encoding LIMD or any fragment thereof.
Also encompassed by the invention are polynucleotide sequences that are
capable of
hybridizing to the claimed polynucleotide sequences, and, in particular, to
those shown in SEQ ID
N0:3-4 and fragments thereof under various conditions of stringency. (See,
e.g., Wahl, G.M. and S.L.
Berger (1987) Methods Enzymol. 152:399-407; Kimmel, A.R. (1987) Methods
Enzymol. 152:507-
511.) Hybridization conditions, including annealing and wash conditions, are
described in
"Definitions."
Methods for DNA sequencing are well known in the art and may be used to
practice any of the
embodiments of the invention. The methods may employ such enzymes as the
HIenow fragment of
24
CA 02378989 2002-O1-03
WO 01/04308 PCT/US00/19014
DNA polymerase I, SEQUENASE (US Biochemical, Cleveland OH), Taq polymerase (PE
Biosystems,
Foster City CA), thermostable T7 polymerase (Amersham Pharmacia Biotech,
Piscataway NJ), or
combinations of polymerases and proofreading exonucleases such as those found
in the ELONGASE
amplification system (Life Technologies, Gaithersburg MD). Preferably,
sequence preparation is
automated with machines such as the MICROLAB 2200 liquid transfer system
(Hamilton, Reno NV),
PTC200 thermal cycler (MJ Research, Watertown MA) and ABI CATALYST 800 thermal
cycler (PE
Biosystems). Sequencing is then carried out using either the ABI 373 or 377
DNA sequencing system
(PE Biosystems), the MEGABACE 1000 DNA sequencing system (Molecular Dynamics,
Sunnyvale
CA), or other systems known in the art. The resulting sequences are analyzed
using a variety of
algorithms which are well known in the art. (See, e.g., Ausubel, F.M. (1997)
Short Protocols in
Molecular Biolo~v, John Wiley & Sons, New York NY, unit 7.7; Meyers, R.A.
(1995) Molecular
Biolo~y and Biotechnolo~y, Wiley VCH, New York NY, pp. 856-853.)
The nucleic acid sequences encoding LIMD may be extended utilizing a partial
nucleotide
sequence and employing various PCR-based methods known in the art to detect
upstream sequences,
such as promoters and regulatory elements. For example, one method which may
be employed,
restriction-site PCR, uses universal and nested primers to amplify unknown
sequence from genomic
DNA within a cloning vector. (See, e.g., Sarkar, G. (1993) PCR Methods Applic.
2:318-322.)
Another method, inverse PCR, uses primers that extend in divergent directions
to amplify unknown
sequence from a circularized template. The template is derived from
restriction fragments comprising a
known genomic locus and surrounding sequences. (See, e.g., Triglia, T. et al.
(1988) Nucleic Acids
Res. 16:8186.) A third method, capture PCR, involves PCR amplification of DNA
fragments adjacent
to known sequences in human and yeast artificial chromosome DNA. (See, e.g.,
Lagerstrom, M. et al.
(1991) PCR Methods Applic. 1:111-119.) In this method, multiple restriction
enzyme digestions and
ligations may be used to insert an engineered double-stranded sequence into a
region of unknown
sequence before performing PCR. Other methods which may be used to retrieve
unknown sequences
are known in the art. (See, e.g., Parker, J.D. et al. (1991) Nucleic Acids
Res. 19:3055-3060).
Additionally, one may use PCR, nested primers, and PROMOTERFINDER libraries
(Clontech, Palo
Alto CA) to walk genomic DNA. This procedure avoids the need to screen
libraries and is useful in
finding intron/exon junctions. For all PCR-based methods, primers may be
designed using
commercially available software, such as OLIGO 4.06 Primer Analysis software
(National Biosciences,
Plymouth MN) or another appropriate program, to be about 22 to 30 nucleotides
in length, to have a
GC content of about 50% or more, and to anneal to the template at temperatures
of about 68°C to
72°C.
When screening for full-length cDNAs, it is preferable to use libraries that
have been
CA 02378989 2002-O1-03
WO 01/04308 PCT/US00/19014
size-selected to include larger cDNAs. In addition, random-primed libraries,
which often include
sequences containing the 5' regions of genes, are preferable for situations in
which an oligo d(T) library
does not yield a full-length cDNA. Genomic libraries may be useful for
extension of sequence into 5'
non-transcribed regulatory regions.
Capillary electrophoresis systems which are commercially available may be used
to analyze the
size or confirm the nucleotide sequence of sequencing or PCR products. In
particular, capillary
sequencing may employ flowable polymers for electrophoretic separation, four
different nucleotide-
specific, laser-stimulated fluorescent dyes, and a charge coupled device
camera for detection of the
emitted wavelengths. Outputllight intensity may be converted to electrical
signal using appropriate
software (e.g., GENOTYPER and SEQUENCE NAVIGATOR, PE Biosystems), and the
entire
process from loading of samples to computer analysis and electronic data
display may be computer
controlled. Capillary electrophoresis is especially preferable for sequencing
small DNA fragments
which may be present in limited amounts in a particular sample.
In another embodiment of the invention, polynucleotide sequences or fragments
thereof which
encode LIMD may be cloned in recombinant DNA molecules that direct expression
of LIMD, or
fragments or functional equivalents thereof, in appropriate host cells. Due to
the inherent degeneracy of
the genetic code, other DNA sequences which encode substantially the same or a
functionally equivalent
amino acid sequence may be produced and used to express LIMD.
The nucleotide sequences of the present invention can be engineered using
methods generally
known in the art in order to alter LIMD-encoding sequences for a variety of
purposes including, but not
limited to, modification of the cloning, processing, and/or expression of the
gene product. DNA
shuffling by random fragmentation and PCR reassembly of gene fragments and
synthetic
oligonucleotides may be used to engineer the nucleotide sequences. For
example, oligonucleotide-
mediated site-directed mutagenesis may be used to introduce mutations that
create new restriction sites,
alter glycosylation patterns, change codon preference, produce splice
variants, and so forth.
'The nucleotides of the present invention may be subjected to DNA shuffling
techniques such
as MOLECULARBREEDING (Maxygen Inc., Santa Clara CA; described in U.S. Patent
Number
5,837,458; Chang, C.-C. et al. (1999) Nat. Biotechnol. 17:793-797; Christians,
F.C. et al. (1999) Nat.
Biotechnol. 17:259-264; and Crameri, A. et al. (1996) Nat. Biotechnol. 14:315-
319) to alter or
improve the biological properties of LIMD, such as its biological or enzymatic
activity or its ability to
bind to other molecules or compounds. DNA shuffling is a process by which a
library of gene
variants is produced using PCR-mediated recombination of gene fragments. The
library is then
subjected to selection or screening procedures that identify those gene
variants with the desired
properties. These preferred variants may then be pooled and further subjected
to recursive rounds of
DNA shuffling and selection/screening. Thus, genetic diversity is created
through "artificial"
26
CA 02378989 2002-O1-03
WO 01/04308 PCT/US00/19014
breeding and rapid molecular evolution. For example, fragments of a single
gene containing random
point mutations may be recombined, screened, and then reshuffled until the
desired properties are
optimized. Alternatively, fragments of a given gene may be recombined with
fragments of
homologous genes in the same gene family, either from the same or different
species, thereby
maximizing the genetic diversity of multiple naturally occurring genes in a
directed and controllable
manner.
In another embodiment, sequences encoding LIMD may be synthesized, in whole or
in part,
using chemical methods well known in the art. (See, e.g., Caruthers, M.H. et
al. (1980) Nucleic Acids
Symp. Ser. 7:215-223; and Horn, T. et al. (1980) Nucleic Acids Symp. Ser.
7:225-232.) Alternatively,
LIMD itself or a fragment thereof may be synthesized using chemical methods.
For example, peptide
synthesis can be performed using various solution-phase or solid-phase
techniques. (See, e.g.,
Creighton, T. (1984) Proteins, Structures and Molecular Properties, WH
Freeman, New York NY, pp.
55-60; and Roberge, J.Y. et al. (1995) Science 269:202-204.) Automated
synthesis may be achieved
using the ABI 431A peptide synthesizer (PE Biosystems). Additionally, the
amino acid sequence of
LIMD, or any part thereof, may be altered during direct synthesis and/or
combined with sequences from
other proteins, or any part thereof, to produce a variant polypeptide or a
polypeptide having a sequence
of a naturally occurring polypeptide.
The peptide may be substantially purified by preparative high performance
liquid
chromatography. (See, e.g., Chiez, R.M. and F.Z. Regnier (1990) Methods
Enzymol. 182:392-421.)
The composition of the synthetic peptides may be confirmed by amino acid
analysis or by sequencing.
(See, e.g., Creighton, supra, pp. 28-53.)
In order to express a biologically active LIMD, the nucleotide sequences
encoding LIMD or
derivatives thereof may be inserted into an appropriate expression vector,
i.e., a vector which contains
the necessary elements for transcriptional and translational control of the
inserted coding sequence in a
suitable host. These elements include regulatory sequences, such as enhancers,
constitutive and
inducible promoters, and 5' and 3' untranslated regions in the vector and in
polynucleotide sequences
encoding LIMD. Such elements may vary in their strength and specificity.
Specific initiation signals
may also be used to achieve more efficient translation of sequences encoding
LIMD. Such signals
include the ATG initiation codon and adjacent sequences, e.g. the Kozak
sequence. In cases where
sequences encoding LIMD and its initiation codon and upstream regulatory
sequences are inserted into
the appropriate expression vector, no additional transcriptional or
translational control signals may be
needed. However, in cases where only coding sequence, or a fragment thereof,
is inserted, exogenous
translational control signals including an in-frame ATG initiation codon
should be provided by the
vector. Exogenous translational elements and initiation codons may be of
various origins, both natural
and synthetic. The efficiency of expression may be enhanced by the inclusion
of enhancers appropriate
27
CA 02378989 2002-O1-03
WO 01/04308 PCT/US00/19014
for the particular host cell system used. (See, e.g., Scharf, D. et al. (1994)
Results Probl. Cell Differ.
20:125-162.)
Methods which are well known to those skilled in the art may be used to
construct expression
vectors containing sequences encoding LIMD and appropriate transcriptional and
translational control
elements. These methods include in vitro recombinant DNA techniques, synthetic
techniques, and in
vivo genetic recombination. (See, e.g., Sambrook, J. et al. (1989) Molecular
Cloning, A Laboratory
Manual, Cold Spring Harbor Press, Plainview NY, ch. 4, 8, and 16-17; Ausubel,
F.M. et al. (1995)
Current Protocols in Molecular Bioloav, John Wiley & Sons, New York NY, ch. 9,
13, and 16.)
A variety of expression vector/host systems may be utilized to contain and
express sequences
encoding LIMD. These include, but are not limited to, microorganisms such as
bacteria transformed
with recombinant bacteriophage, plasmid, or cosmid DNA expression vectors;
yeast transformed with
yeast expression vectors; insect cell systems infected with viral expression
vectors (e.g., baculovirus);
plant cell systems transformed with viral expression vectors (e.g.,
cauliflower mosaic virus, CaMV, or
tobacco mosaic virus, TMV) or with bacterial expression vectors (e.g., Ti or
pBR322 plasmids); or
animal cell systems. (See, e.g., Sambrook, supra; Ausubel, su ra; Van Heeke,
G. and S.M. Schuster
(1989) J. Biol. Chem. 264:5503-5509; Bitter, G.A. et al. (1987) Methods
Enzymol. 153:516-544;
Scorer, C.A. et al. (1994) Bio/Technology 12:181-184; Engelhard, E.K. et al.
(1994) Proc. Nati.
Acad. Sci. USA 91:3224-3227; Sandig, V. et al. (1996) Hum. Gene Ther. 7:1937-
1945; Takamatsu,
N. (1987) EMBO J. 6:307-311; Coruzzi, G. et al. (1984) EMBO J. 3:1671-1680;
Brogue, R. et al.
(1984) Science 224:838-843; Winter, J. et al. (1991) Results Probl. Cell
Differ. 17:85-105; The
McGraw Hill Yearbook of Science and Technology (1992) McGraw Hill, New York
NY, pp.
191-196; Logan, J. and T. Shenk (1984) Proc. Nati. Acad. Sci. USA 81:3655-
3659; and Harrington,
J.J. et al. (1997) Nat. Genet. 15:345-355.) Expression vectors derived from
retroviruses,
adenoviruses, or herpes or vaccinia viruses, or from various bacterial
plasmids, may be used for
delivery of nucleotide sequences to the targeted organ, tissue, or cell
population. (See, e.g., Di
Nicola, M. et al. (1998) Cancer Gen. Ther. 5(6):350-356; Yu, M. et al., (1993)
Proc. Natl. Acad. Sci.
USA 90(13):6340-6344; Buller, R.M. et al. (1985) Nature 317(6040):813-815;
McGregor, D.P. et al.
(1994) Mol. Immunol. 31(3):219-226; and Verma, LM. and N. Somia (1997) Nature
389:239-242.)
The invention is not limited by the host cell employed.
In bacterial systems, a number of cloning and expression vectors may be
selected depending
upon the use intended for polynucleotide sequences encoding LIMD. For example,
routine cloning,
subcloning, and propagation of polynucleotide sequences encoding LIMD can be
achieved using a
multifunctional E. coli vector such as PBLUESCRIPT (Stratagene, La Jolla CA)
or PSPORT1 plasmid
(Life Technologies). Ligation of sequences encoding LIMD into the vector's
multiple cloning site
disrupts the lacZ gene, allowing a calorimetric screening procedure for
identification of transformed
28
CA 02378989 2002-O1-03
WO 01/04308 PCT/US00/19014
bacteria containing recombinant molecules. In addition, these vectors may be
useful for in vitro
transcription, dideoxy sequencing, single strand rescue with helper phage, and
creation of nested
deletions in the cloned sequence. (See, e.g., Van Heeke, G. and S.M. Schuster
(1989) J. Biol. Chem.
264:5503-5509.) When large quantities of LIMD are needed, e.g. for the
production of antibodies,
vectors which direct high level expression of LIMD may be used. For example,
vectors containing the
strong, inducible T5 or T7 bacteriophage promoter may be used.
Yeast expression systems may be used for production of LIMD. A number of
vectors
containing constitutive or inducible promoters, such as alpha factor, alcohol
oxidase, and PGH
promoters, may be used in the yeast Saccharomyces cerevisiae or
Pichia~astoris. In addition, such
vectors direct either the secretion or intracellular retention of expressed
proteins and enable integration
of foreign sequences into the host genome for stable propagation. (See, e.g.,
Ausubel, 1995, supra;
Bitter, supra; and Scorer, supra.)
Plant systems may also be used for expression of LIMD. Transcription of
sequences encoding
LIMD may be driven viral promoters, e.g., the 35S and 19S promoters of CaMV
used alone or in
combination with the omega leader sequence from TMV (Takamatsu, N. (1987) EMBO
J. 6:307-311).
Alternatively, plant promoters such as the small subunit of RUBISCO or heat
shock promoters may be
used. (See, e.g., Coruzzi, supra; Broglie, su ra; and Winter, supra.)
These.constructs can be
introduced into plant cells by direct DNA transformation or pathogen-mediated
transfection. (See, e.g.,
The McGraw Hill Yearbook of Science and Technoloey (1992) McGraw Hill, New
York NY, pp.
191-196.)
In mammalian cells, a number of viral-based expression systems may be
utilized. In cases
where an adenovirus is used as an expression vector, sequences encoding LIMD
may be ligated into an
adenovirus transcription/translation complex consisting of the late promoter
and tripartite leader
sequence. Insertion in a non-essential E1 or E3 region of the viral genome may
be used to obtain
infective virus which expresses LIMD in host cells. (See, e.g., Logan, J. and
T. Shenk (1984) Proc.
Natl. Acad. Sci. USA 81:3655-3659.) In addition, transcription enhancers, such
as the Rous sarcoma
virus (RSV) enhancer, may be used to increase expression in mammalian host
cells. SV40 or EBV-
based vectors may also be used for high-level protein expression.
Human artificial chromosomes (HACs) may also be employed to deliver larger
fragments of
DNA than can be contained in and expressed from a plasmid. HACs of about 6 kb
to 10 Mb are
constructed and delivered via conventional delivery methods (liposomes,
polycationic amino polymers,
or vesicles) for therapeutic purposes. (See, e.g., Harrington, J.J. et al.
(1997) Nat. Genet. 15:345-355.)
For long term production of recombinant proteins in mammalian systems, stable
expression of
LIMD in cell lines is preferred. For example, sequences encoding LIMD can be
transformed into cell
29
CA 02378989 2002-O1-03
WO 01/04308 PCT/US00/19014
lines using expression vectors which may contain viral origins of replication
and/or endogenous
expression elements and a selectable marker gene on the same or on a separate
vector. Following the
introduction of the vector, cells may be allowed to grow for about 1 to 2 days
in enriched media before
being switched to selective media. The purpose of the selectable marker is to
confer resistance to a
selective agent, and its presence allows growth and recovery of cells which
successfully express the
introduced sequences. Resistant clones of stably transformed cells may be
propagated using tissue
culture techniques appropriate to the cell type.
Any number of selection systems may be used to recover transformed cell lines.
These include,
but are not limited to, the herpes simplex virus thymidine kinase and adenine
phosphoribosyltransferase
genes, for use in tk- and apr cells, respectively. (See, e.g., Wigler, M. et
al. (1977) Cell 11:223-232;
Lowy, I. et al. (1980) Cell 22:817-823.) Also, antimetabolite, antibiotic, or
herbicide resistance can be
used as the basis for selection. For example, dhfr confers resistance to
methotrexate; neo confers
resistance to the aminoglycosides neomycin and G-418; and als and pat confer
resistance to
chlorsulli.~ron and phosphinotricin acetyltransferase, respectively. (See,
e.g., Wigler, M. et al. (1980)
Proc. Natl. Acad. Sci. USA 77:3567-3570; Colbere-Garapin, F. et al. (1981) J.
Mol. Biol. 150:1-14.)
Additional selectable genes have been described, e.g., trpB and hisD, which
alter cellular requirements
for metabolites. (See, e.g., Hartman, S.C. and R.C. Mulligan (1988) Prcx;.
Natl. Acad. Sci. USA
85:8047-8051.) Visible markers, e.g., anthcxyanins, green fluorescent proteins
(GFP; Clontech),13
glucuronidase and its substrate 13-glucuronide, or luciferase and its
substrate luciferin may be used.
These markers can be used not only to identify transformants, but also to
quantify the amount of
transient or stable protein expression attributable to a specific vector
system. (See, e.g., Rhodes, C.A.
(1995) Methods Mol. Biol. 55:121-131.)
Although the presence~absence of masker gene expression suggests that the gene
of interest is
also present, the presence and expression of the gene may need to be
confirmed. For example, if the
sequence encoding LIMD is inserted within a marker gene sequence, transformed
cells containing
sequences encoding LIMD can be identified by the absence of marker gene
function. Alternatively, a
marker gene can be placed in tandem with a sequence encoding LIMD under the
control of a single
promoter. Expression of the marker gene in response to induction or selection
usually indicates
expression of the tandem gene as well.
In general, host cells that contain the nucleic acid sequence encoding LIMD
and that express
LIMD may be identified by a variety of prcx;edures known to those of skill in
the art. These procedures
include, but are not limited to, DNA-DNA or DNA-RNA hybridizations, PCR
amplification, and
protein bioassay or immunoassay techniques which include membrane, solution,
or chip based
technologies for the detection and/or quantification of nucleic acid or
protein sequences.
CA 02378989 2002-O1-03
WO 01/04308 PCT/US00/19014
Inununological methods for detecting and measuring the expression of L1MD
using either
specific polyclonal or monoclonal antibodies are known in the art. Examples of
such techniques include
enzyme-linked immunosorbent assays (ELISAs), radioimmunoassays (RIAs), and
fluorescence
activated cell sorting (FACS). A two-site, monoclonal-based inununoassay
utilizing monoclonal
antibodies reactive to two non-interfering epitopes on LIMD is preferred, but
a competitive binding
assay may be employed. These and other assays are well known in the art. (See,
e.g., Hampton, R. et
al. (1990) Serological Methods, a Laboratory Manual, APS Press, St. Paul MN,
Sect. IV; Coligan, J.E.
et al. (1997) Current Protocols in Immunology, Greene Pub. Associates and
Wiley-lnterscience, New
York NY; and Pound, J.D. (1998) lmmunochemical Protocols, Humana Press, Totowa
NJ.)
A wide variety of labels and conjugation techniques are known by those skilled
in the art and
may be used in various nucleic acid and amino acid assays. Means for producing
labeled hybridization
or PCR probes for detecting sequences related to polynucleotides encoding LIMD
include oligolabeling,
nick translation, end-labeling, or PCR amplification using a labeled
nucleotide. Alternatively, the
sequences encoding LIMD, or any fragments thereof, may be cloned into a vector
for the production of
an mRNA probe. Such vectors are known in the art, are commercially available,
and may be used to
synthesize RNA probes in vitro by addition of an appropriate RNA polymerise
such as T7, T3, or SP6
and labeled nucleotides. These procedures may be conducted using a variety of
commercially available
kits, such as those provided by Amersham Pharmacia Biotech, Promega (Madison
WI), and US
Biochemical. Suitable reporter molecules or labels which may be used for ease
of detection include
radionuclides, enzymes, fluorescent, chemiluminescent, or chromogenic agents,
as well as substrates,
cofactors, inhibitors, magnetic particles, and the like.
Host cells transformed with nucleotide sequences encoding LIMD may be cultured
under
conditions suitable for the expression and recovery of the protein from cell
culture. The protein
produced by a transformed cell may be secreted or retained intracellularly
depending on the sequence
and/or the vector used. As will be understood by those of skill in the art,
expression vectors containing
polynucleotides which encode LIMD may be designed to contain signal sequences
which direct
secretion of LIMD through a prokaryotic or eukaryotic cell membrane.
In addition, a host cell strain may be chosen for its ability to modulate
expression of the
inserted sequences or to process the expressed protein in the desired fashion.
Such modifications of the
polypeptide include, but are not limited to, acetylation, carboxylation,
glycosylation, phosphorylation,
lipidation, and acylation. Post-translational processing which cleaves a
"prepro" or "pro" form of the
protein may also be used to specify protein targeting, folding, and/or
activity. Different host cells
which have specific cellular machinery and characteristic mechanisms for post-
translational activities
(e.g., CHO, HeLa, MDCK, HEK293, and WI38) are available from the~American Type
Culture
31
CA 02378989 2002-O1-03
WO 01/04308 PCT/US00/19014
Collection (ATCC, Manassas VA) and may be chosen to ensure the correct
modification and processing
of the foreign protein.
In another embodiment of the invention, natural, modified, or recombinant
nucleic acid
sequences encoding LIMD may be ligated to a heterologous sequence resulting in
translation of a fusion
protein in any of the aforementioned host systems. For example, a chimeric
LIMD protein containing a
heterologous moiety that can be recognized by a commercially available
antibody may facilitate the
screening of peptide libraries for inhibitors of LIMD activity. Heterologous
protein and peptide
moieties may also facilitate purification of fusion proteins using
commercially available affinity
matrices. Such moieties include, but are not limited to, glutathione S-
transferase (GST), maltose
binding protein (MBP), thioredoxin (Trx), calmodulin binding peptide (CBP), 6-
His, FLAG, c-myc, and
hemagglutinin (HA). GST, MBP, Trx, CBP, and 6-His enable purification of their
cognate fusion
proteins on immobilized glutathione, maltose, phenylarsine oxide, calmodulin,
and metal-chelate resins,
respectively. FLAG, c-myc, and hemagglutinin (HA) enable immunoaffinity
purification of fusion
proteins using commercially available monoclonal and polyclonal antibodies
that specifically recognize
these epitope tags. A fusion protein may also be engineered to contain a
proteolytic cleavage site
located between the LIMD encoding sequence and the heterologous protein
sequence, so that LIMD
may be cleaved away from the heterologous moiety following purification.
Methods for fusion protein
expression and purification are discussed in Ausubel (1995, supra, eh. 10). A
variety of commercially
available kits may also be used to facilitate expression and purif canon of
fusion proteins.
In a further embodiment of the invention, synthesis of radiolabeled LIMD may
be achieved in
vitro using the TNT rabbit reticulocyte lysate or wheat germ extract system
(Promega). These systems
couple transcription and translation of protein-coding sequences operably
associated with the T7, T3, or
SP6 promoters. Translation takes place in the presence of a radiolabeled amino
acid precursor, for
example, 35S-methionine.
LIMD of the present invention or fragments thereof may be used to screen for
compounds
that specifically bind to LIMD. At least one and up to a plurality of test
compounds may be screened
for specific binding to LIMD. Examples of test compounds include antibodies,
oligonucleotides,
proteins (e.g., receptors), or small molecules.
In one embodiment, the compound thus identified is closely related to the
natural ligand of
LIMD, e.g., a ligand or fragment thereof, a natural substrate, a structural or
functional mimetic, or a
natural binding partner. (See, Coligan, J.E. et al. (1991) Current Protocols
in Immunology 1(2):
Chapter 5.) Similarly, the compound can be closely related to the natural
receptor to which LIMD
binds, or to at least a fragment of the receptor, e.g., the ligand binding
site. In either case, the
compound can be rationally designed using known techniques. In one embodiment,
screening for
these compounds involves producing appropriate cells which express LIMD,
either as a secreted
32
CA 02378989 2002-O1-03
WO 01/04308 PCT/US00/19014
protein or on the cell membrane. Preferred cells include cells from mammals,
yeast, Drosophila, or E.
colt. Cells expressing LIMD or cell membrane fractions which contain LIMD are
then contacted with
a test compound and binding, stimulation, or inhibition of activity of either
LIMD or the compound is
analyzed.
An assay may simply test binding of a test compound to the polypeptide,
wherein binding is
detected by a fluorophore, radioisotope, enzyme conjugate, or other detectable
label. For example,
the assay may comprise the steps of combining at least one test compound with
LIMD, either in
solution or affixed to a solid support, and detecting the binding of LIMD to
the compound.
Alternatively, the assay may detect or measure binding of a test compound in
the presence of a
labeled competitor. Additionally, the assay may be carried out using cell-free
preparations, chemical
libraries, or natural product mixtures, and the test compounds) may be tree in
solution or affixed to a
solid support.
LIMD of the present invention or fragments thereof may be used to screen for
compounds
that modulate the activity of LIMD. Such compounds may include agonists,
antagonists, or partial or
inverse agonists. In one embodiment, an assay is performed under conditions
permissive for LIMD
activity, wherein LIMD is combined with at least one test compound, and the
activity of LIMD in the
presence of a test compound is compared with the activity of LIMD in the
absence of the test
compound. A change in the activity of LIMD in the presence of the test
compound is indicative of a
compound that modulates the activity of LIMD. Alternatively, a test compound
is combined with an
in vitro or cell-free system comprising LIMD under conditions suitable for
LIMD activity, and the
assay is performed. In either of these assays,.a test compound which modulates
the activity of LIMD
may do so indirectly and need not come in direct contact with the test
compound. At least one and up
to a plurality of test compounds may be screened.
In another embodiment, polynucleotides encoding LIMD or their mammalian
homologs may
be "knocked out" in an animal model system using homologous recombination in
embryonic stem
(ES) cells. Such techniques are well known in the art and are useful for the
generation of animal
models of human disease. (See, e.g., U.S. Patent No. 5,175,383 and U.S. Patent
No. 5,767,337.) For
example, mouse ES cells, such as the mouse 129/SvJ cell line, are derived from
the early mouse
embryo and grown in culture. The ES cells are transformed with a vector
containing the gene of
interest disrupted by a marker gene, e.g., the neomycin phosphotransferase
gene (neo; Capecchi, M.R.
(1989) Science 244:1288-1292). The vector integrates into the corresponding
region of the host
genome by homologous recombination. Alternatively, homologous recombination
takes place using
the Cre-loxP system to knockout a gene of interest in a tissue- or
developmental stage-specific
manner (Marth, J.D. (1996) Clin. Invest. 97:1999-2002; Wagner, K.U. et al.
(1997) Nucleic Acids
Res. 25:4323-4330). Transformed ES cells are identified and microinjected into
mouse cell
33
CA 02378989 2002-O1-03
WO 01/04308 PCT/US00/19014
blastocysts such as those from the C57BL/6 mouse strain. The blastocysts are
surgically transferred
to pseudopregnant dams, and the resulting chimeric progeny are genotyped and
bred to produce
heterozygous or homozygous strains. Transgenic animals thus generated may be
tested with potential
therapeutic or toxic agents.
Polynucleotides encoding LIMD may also be manipulated in vitro in ES cells
derived from
human blastocysts. Human ES cells have the potential to differentiate into at
least eight separate cell
lineages including endoderm, mesoderm, and ectodermal cell types. These cell
lineages differentiate
into, for example, neural cells, hematopoietic lineages, and cardiomyocytes
(Thomson, J.A. et al.
(1998) Science 282:1145-1147).
Polynucleotides encoding LIMD can also be used to create "knockin" humanized
animals
(pigs) or transgenic animals (mice or rats) to model human disease. With
knockin technology, a
region of a polynucleotide encoding LIMD is injected into animal ES cells, and
the injected sequence
integrates into the animal cell genome. Transformed cells are injected into
blastulae, and the
blastulae are implanted as described above. Transgenic progeny or inbred lines
are studied and
treated with potential pharmaceutical agents to obtain information on
treatment of a human disease.
Alternatively, a mammal inbred to overexpress LIMD, e.g., by secreting LIMD in
its milk, may also
serve as a convenient source of that protein (Janne, J. et al. (1998)
Biotechnol. Annu. Rev. 4:55-74).
THERAPEUTICS
Chemical and structural similarity, e.g., in the context of sequences and
motifs, exists
between regions of LIMD and human LIM domain proteins. In addition, the
expression of LIMD is
closely associated with cell proliferation. Therefore, LIMD appears to play a
role in cell proliferative,
developmental, and cell motility disorders. In the treatment of disorders
associated with increased
LIMD expression or activity, it is desirable to decrease the expression or
activity of LIMD. In the
treatment of disorders associated with decreased LIMD expression or activity,
it is desirable to
increase the expression or activity of LIMD.
Therefore, in one embodiment, LIMD or a fragment or derivative thereof may be
administered to a subject to treat or prevent a disorder associated with
decreased expression or
activity of LIMD. Examples of such disorders include, but are not limited to,
a cell proliferative
disorder, such as actinic keratosis, arteriosclerosis, atherosclerosis,
bursitis, cirrhosis, hepatitis, mixed
connective tissue disease (MCTD), myelofibrosis, paroxysmal nocturnal
hemoglobinuria,
polycythemia vera, psoriasis, primary thrombocythemia, and cancers including
adenocarcinoma,
leukemia, lymphoma, melanoma, myeloma, sarcoma, teratocarcinoma, and, in
particular, cancers of
the adrenal gland, bladder, bone, bone marrow, brain, breast, cervix, gall
bladder, ganglia,
gastrointestinal tract, heart, kidney, liver, lung, muscle, ovary, pancreas,
parathyroid, penis, prostate,
salivary glands, skin, spleen, testis, thymus, thyroid, and uterus; a
developmental disorder, such as
34
CA 02378989 2002-O1-03
WO 01/04308 PCT/US00/19014
renal tubular acidosis, anemia, Cushing's syndrome, achondroplastic dwarfism,
Duchenne and Becker
muscular dystrophy, epilepsy, gonadal dysgenesis, WAGR syndrome (Wilms' tumor,
aniridia,
genitourinary abnormalities, and mental retardation), Smith-Magenis syndrome,
myelodysplastic
syndrome, hereditary mucoepithelial dysplasia, hereditary keratodermas,
hereditary neuropathies such
as Charcot-Marie-Tooth disease and neurofibromatosis, hypothyroidism,
hydrocephalus, seizure
disorders such as Syndenham's chorea and cerebral palsy, spina bifida,
anencephaly,
craniorachischisis, congenital glaucoma, cataract, and sensorineural hearing
loss; and a disorder of
cell motility, such as ankylosing spondylitis, Chediak-Higashi syndrome,
Duchenne and Becker
muscular dystrophy, intrahepatic cholestasis, myocardial hyperplasia,
cardiomyopathy, early onset
peridondtis, cancers such as adenocarcinoma, ovarian carcinoma, and chronic
myelogenous leukemia,
and bacterial and helminthic infections.
In another embodiment, a vector capable of expressing LIMD or a fragment or
derivative
thereof may be administered to a subject to treat or prevent a disorder
associated with decreased
expression or activity of LIMD including, but not limited to, those described
above.
In a further embodiment, a pharmaceutical composition comprising a
substantially purified
LIMD in conjunction with a suitable pharmaceutical carrier may be administered
to a subject to treat or
prevent a disorder associated with decreased expression or activity of LIMD
including, but not limited
to, those provided above.
In still another embodiment, an agonist which modulates the activity of LIMD
may be
administered to a subject to treat or prevent a disorder associated with
decreased expression or activity
of LIMD including, but not limited to, those listed above.
In a further embodiment, an antagonist of LIMD may be administered to a
subject to treat or
prevent a disorder associated with increased expression or activity of LIMD.
Examples of such
disorders include, but are not linuted to, those cell proliferative,
developmental, and cell motility
disorders described above. In one aspect, an antibody which specifically binds
LIMD may be used
directly as an antagonist or indirectly as a targeting or delivery mechanism
for bringing a
pharmaceutical agent to cells or tissues which express LIMD.
In an additional embodiment, a vector expressing the complement of the
polynucleotide
encoding LIMD may be administered to a subject to treat or prevent a disorder
associated with
increased expression or activity of LIMD including, but not limited to, those
described above.
In other embodiments, any of the proteins, antagonists, antibodies, agonists,
complementary
sequences, or vectors of the invention may be administered in combination with
other appropriate
therapeutic agents. Selection of the appropriate agents for use in combination
therapy may be made by
one of ordinary skill in the art, according to conventional pharmaceutical
principles. The combination
of therapeutic agents may act synergistically to effect the treatment or
prevention of the various
CA 02378989 2002-O1-03
WO 01/04308 PCT/US00/19014
disorders described above. Using this approach, one may be able to achieve
therapeutic efficacy with
lower dosages of each agent, thus reducing the potential for adverse side
effects.
An antagonist of LIMD may be produced using methods which are generally known
in the art.
In particular, purified LIMD may be used to produce antibodies or to screen
libraries of pharmaceutical
agents to identify those which specifically bind LIMD. Antibodies to LIMD may
also be generated
using methods that are well known in the art. Such antibodies may include, but
are not limited to,
polyclonal, monoclonal, chimeric, and single chain antibodies, Fab fragments,
and fragments produced
by a Fab expression library. Neutralizing antibodies (i.e., those which
inhibit dimer formation) are
generally preferred for therapeutic use.
For the production of antibodies, various hosts including goats, rabbits,
rats, mice, humans,
and others may be immunized by injection with LIMD or with any fragment or
oligopeptide thereof
which has immunogenic properties. Depending on the host species, various
adjuvants may be used to
increase immunological response. Such adjuvants include, but are not limited
to, Freund's, mineral gels
such as aluminum hydroxide, and surface active substances such as
lysolecithin, pluronic polyols,
polyanions, peptides, oil emulsions, KLH, and dinitrophenol. Among adjuvants
used in humans, BCG
(bacilli Calmette-Guerin) and Corynebacterium parvum are especially
preferable.
It is preferred that the oligopeptides, peptides, or fragments used to induce
antibodies to LIMD
have an amino acid sequence consisting of at least about 5 amino acids, and
generally will consist of at
least about 10 amino acids. It is also preferable that these oligopeptides,
peptides, or fragments are
identical to a portion of the amino acid sequence of the natural protein.
Short stretches of LIMD amino
acids may be fused with those of another protein, such as KLH, and antibodies
to the chimeric molecule
may be produced.
Monoclonal antibodies to LIMD may be prepared using any technique which
provides for the
production of antibody molecules by continuous cell lines in culture. These
include, but are not limited
to, the hybridoma technique, the human B-cell hybridoma technique, and the EBV-
hybridoma
technique. (See, e.g., Kohler, G. et al. (1975) Nature 256:495-497; Kozbor, D.
et al. (1985) J.
Immunol. Methods 81:31-42; Cote, R.J. et al. (1983) Proc. Natl. Acad. Sci. USA
80:2026-2030; and
Cole, S.P. et al. (1984) Mol. Cell Biol. 62:109-120.)
In addition, techniques developed for the production of "chimeric antibodies,"
such as the
splicing of mouse antibody genes to human antibody genes to obtain a molecule
with appropriate
antigen specificity and biological activity, can be used. (See, e.g.,
Morrison, S.L. et al. (1984) Proc.
Natl. Acad. Sci. USA 81:6851-6855; Neuberger, M.S. et al. (1984) Nature
312:604-608; and Takeda,
S. et al. (1985) Nature 314:452-454.) Alternatively, techniques described for
the production of single
chain antibodies may be adapted, using methods known in the art, to produce
LIMD-specific single
36
CA 02378989 2002-O1-03
WO 01/04308 PCT/US00/19014
chain antibodies. Antibodies with related specificity, but of distinct
idiotypic composition, may be
generated by chain shuffling from random combinatorial immunoglobulin
libraries. (See, e.g., Burton,
D.R. (1991) Proc. Natl. Acad. Sci. USA 88:10134-10137.)
Antibodies may also be produced by inducing in vivo production in the
lymphocyte population
or by screening immunoglobulin libraries or panels of highly specific binding
reagents as disclosed in
the literature. (See, e.g., Orlandi, R. et al. (1989) Proc. Natl. Acad. Sci.
USA 86:3833-3837; Winter,
G. et al. (1991) Nature 349:293-299.)
Antibody fragments which contain specific binding sites for LIMD may also be
generated. For
example, such fragments include, but are not limited to, F(ab~2 fragments
produced by pepsin digestion
of the antibody molecule and Fab fragments generated by reducing the disulfide
bridges of the F(ab~2
fragments. Alternatively, Fab expression libraries may be constructed to allow
rapid and easy
identification of monoclonal Fab fragments with the desired specificity. (See,
e.g., Huse, W.D. et al.
(1989) Science 246:1275-1281.)
Various immunoassays may be used for screening to identify antibodies having
the desired
specificity. Numerous protocols for competitive binding or immunoradiometric
assays using either
polyclonal or monoclonal antibodies with established specificities are well
known in the art. Such
immunoassays typically involve the measurement of complex formation between
LIMD and its specific
antibody. A two-site, monoclonal-based immunoassay utilizing monoclonal
antibodies reactive to two
non-interfering LIMD epitopes is generally used, but a competitive binding
assay may also be employed
(Pound, su ra).
Various methods such as Scatchard analysis in conjunction with
radioimmunoassay techniques
may be used to assess the affinity of antibodies for LIMD. Affinity is
expressed as an association
constant, Ka, which is defined as the molar concentration of LIMD-antibody
complex divided by the
molar concentrations of free antigen and free antibody under equilibrium
conditions. The Ka determined
for a preparation of polyclonal antibodies, which are heterogeneous in their
affinities for multiple LIMD
epilopes, represents the average affinity, or avidity, of the antibodies for
LIMD. The Ka deternuned for
a preparation of monoclonal antibodies, which are monospecific for a
particular LIMD epitope,
represents a true measure of affinity. High-affinity antibody preparations
with K.a ranging from about
109 to 10'2 L/mole are preferred for use in immunoassays in which the LIMD-
antibody complex must
withstand rigorous manipulations. Low-affinity antibody preparations with Ka
ranging from about 106
to 10' L/mole are preferred for use in immunopurification and sinular
procedures which ultimately
require dissociation of LIMD, preferably in active form, from the antibody
(Catty, D. (1988)
Antibodies, Volume I: A Practical Approach, IRL Press, Washington DC; Liddell,
J.E. and A. Cryer
(1991) A Practical Guide to Monoclonal Antibodies, John Wiley & Sons, New York
NY).
37
CA 02378989 2002-O1-03
WO 01/04308 PCT/US00/19014
The titer and avidity of polyclonal antibody preparations may be further
evaluated to determine
the quality and suitability of such preparations for certain downstream
applications. For example, a
polyclonal antibody preparation containing at least 1-2 mg specific
antibody/n~l, preferably 5-10 mg
specific antibody/ml, is generally employed in procedures requiring
precipitation of LIMD-antibody
complexes. Procedures for evaluating antibody specificity, titer, and avidity,
and guidelines for
antibody quality and usage in various applications, are generally available.
(See, e.g., Catty, supra, and
Coligan et al., supra.)
In another embodiment of the invention, the polynucleotides encoding LIMD, or
any fragment
or complement thereof, may be used for therapeutic purposes. In one aspect,
modifications of gene
expression can be achieved by designing complementary sequences or antisense
molecules (DNA, RNA,
PNA, or modified oligonucleotides) to the coding or regulatory regions of the
gene encoding LIMD.
Such technology is well known in the art, and antisense oligonucleotides or
larger fragments can be
designed from various locations along the coding or control regions of
sequences encoding LIMD.
(See, e.g., Agrawal, S., ed. (1996) Antisense Therapeutics, Humana Press Inc.,
Totawa NJ.)
In therapeutic use, any gene delivery system suitable for introduction of the
antisense
sequences into appropriate target cells can be used. Antisense sequences can
be delivered
intracellularly in the form of an expression plasmid which, upon
transcription, produces a sequence
complementary to at least a portion of the cellular sequence encoding the
target protein. (See, e.g.,
Slater, J.E. et al. (1998) J. Allergy Clin. Immunol. 102(3):469-475; and
Scanlon, K.J. et al. (1995)
9(13):1288-1296.) Antisense sequences can also be introduced intracellularly
through the use of viral
vectors, such as retrovirus and adeno-associated virus vectors. (See, e.g.,
Miller, A.D. (1990) Blood
76:271; Ausubel, su ra; Uckert, W. and W. Walther (1994) Pharmacol. Ther.
63(3):323-347.) Other
gene delivery mechanisms include liposome-derived systems, artificial viral
envelopes, and other
systems known in the art. (See, e.g., Rossi, J.J. (1995) Br. Med. Bull.
51(1):217-225; Boado, R.J. et
al. (1998) J. Pharm. Sci. 87(11):1308-1315; and Moms, M.C. et al. (1997)
Nucleic Acids Res.
25(14):2730-2736.)
In another embodiment of the invention, polynucleotides encoding L1MD may be
used for
somatic or germline gene therapy. Gene therapy may be performed to (i) correct
a genetic deficiency
(e.g., in the cases of severe combined immunodeficiency (SCID)-Xl disease
characterized by X-linked
inheritance (Cavazzana-Calvo, M. et al. (2000) Science 288:669-672), severe
combined
immunodeficiency syndrome associated with an inherited adenosine deaminase
(ADA) deficiency
(Blaese, R.M. et al. (1995) Science 270:475-480; Bordignon, C. et al. (1995)
Science 270:470-475),
cystic fibrosis (Zabner, J. et al. (1993) Cell 75:207-216; Crystal, R.G. et
al. (1995) Hum. Gene
Therapy 6:643-666; Crystal, R.G. et al. (1995) Hum. Gene Therapy 6:667-703),
thalassamias, familial
hypercholesterolemia, and hemophilia resulting from Factor VIII or Factor IX
deficiencies (Crystal,
38
CA 02378989 2002-O1-03
WO 01/04308 PCT/US00/19014
R.G. (1995) Science 270:404-410; Verma, LM. and Somia, N. (1997) Nature
389:239-242)), (ii)
express a conditionally lethal gene product (e.g., in the case of cancers
which result from unregulated
cell proliferation), or (iii) express a protein which affords protection
against intracellular parasites (e.g.,
against human retroviruses, such as human immunodeliciency virus (HIV)
(Baltimore, D. (1988)
Nature 335:395-396; Poeschla, E. et al. (1996) Proc. Natl. Acad. Sci. USA.
93:11395-11399),
hepatitis B or C virus (HBV, HCV); fungal parasites, such as Candida albicans
and Paracoccidioides
brasiliensis; and protozoan parasites such as Plasmodium falciparum and
Trvaanosoma cruzi). In the
case where a genetic deficiency in LIMD expression or regulation causes
disease, the expression of
LIMD from an appropriate population of transduced cells may alleviate the
clinical manifestations
caused by the genetic deficiency.
In a further embodiment of the invention, diseases or disorders caused by
deficiencies in LIMD
are treated by constructing mammalian expression vectors encoding LIMD and
introducing these
vectors by mechanical means into LIMD-deficient cells. Mechanical transfer
technologies for use with
cells in vivo or ex vitro include (i) direct DNA microinjection into
individual cells, (ii) ballistic gold
particle delivery, (iii) liposome-mediated transfection, (iv) receptor-
mediated gene transfer, and (v) the
use of DNA transposons (Morgan, R.A. and W.F. Anderson (1993) Annu. Rev.
Biochem. 62:191-217;
Ivics, Z. (1997) Cell 91:501-510; Boulay, J-L. and H. Recipon (1998) Curr.
Opin. Biotechnol. 9:445-
450).
Expression vectors that may be effective for the expression of L1MD include,
but are not
limited to, the PCDNA 3.1, EPITAG, PRCCMV2, PREP, PVAX vectors (Invitrogen,
Carlsbad CA),
PCMV-SCRIPT, PCMV-TAG, PEGSH/PERV (Stratagene, La Jolla CA), and PTET-OFF,
PTET-ON, PTRE2, PTRE2-LUC, PTK-HYG (Clontech, Palo Alto CA). LIMD may be
expressed
using (i) a constitutively active promoter, (e.g., from cytomegalovirus (CMV),
Rous sarcoma virus
(RSV), SV40 virus, thymidine kinase (TK), or (3-actin genes), (ii) an
inducible promoter (e.g., the
tetracycline-regulated promoter (Gossen, M. and H. Bujard (1992) Proc. Natl.
Acad. Sci. USA
89:5547-5551; Gossen, M. et al. (1995) Science 268:1766-1769; Rossi, F.M.V.
and H.M. Blau (1998)
Curr. Opin. Biotechnol. 9:451-456), commercially available in the T-REX
plasmid (Invitrogen)); the
ec:dysone-inducible promoter (available in the plasmids PVGRXR and PIND;
Invitrogen); the
FK506/rapamycin inducible promoter; or the RU486/mifepristone inducible
promoter (Rossi, F.M.V.
and H.M. Blau, supra)), or (iii) a tissue-specific promoter or the native
promoter of the endogenous
gene encoding LIMD from a normal individual.
Conunercially available liposome transformation kits (e.g., the PERFECT LIPID
TRANSFECT10N KIT, available from Invitrogen) allow one with ordinary skill in
the art to deliver
polynucleotides to target cells in culture and require minimal effort to
optimize experimental
39
CA 02378989 2002-O1-03
WO 01/04308 PCT/US00/19014
parameters. In the alternative, transformation is performed using the calcium
phosphate method
(Graham, F.L. and A.J. Eb (1973) Virology 52:456-467), or by electroporation
(Neumann, E. et al.
(1982) EMBO J. 1:841-845). The introduction of DNA to primary cells requires
modification of these
standardized mammalian transfection protocols.
In another embodiment of the invention, diseases or disorders caused by
genetic defects with
respect to LIMD expression are treated by constructing a retrovirus vector
consisting of (i) the
polynucleotide encoding LIMD under the control of an independent promoter or
the retrovirus long
terminal repeat (LTR) promoter, (ii) appropriate RNA packaging signals, and
(iii) a Rev-responsive
element (RRE) along with additional retrovirus cis-acting RNA sequences and
coding sequences
required for efficient vector propagation. Retrovirus vectors (e.g., PFB and
PFBNEO) are
commercially available (Stratagene) and are based on published data (Riviere,
I. et al. (1995) Proc.
Natl. Acad. Sci. USA 92:6733-6737), incorporated by reference herein. The
vector is propagated in an
appropriate vector producing cell line (VPCL) that expresses an envelope gene
with a tropism for
receptors on the target cells or a promiscuous envelope protein such as VSVg
(Armentano, D. et al.
(1987) J. Virol. 61:1647-1650; Bender, M.A. et al. (1987) J. Virol. 61:1639-
1646; Adam, M.A. and
A.D. Miller (1988) J. Virol. 62:3802-3806; Dull, T. et al. (1998) J. Virol.
72:8463-8471; Zufferey, R.
et al. (1998) J. Virol. 72:9873-9880). U.S. Patent Number 5,910,434 to Rigg
("Method for obtaining
retrovirus packaging cell lines producing high transducing efficiency
retroviral supernatant") discloses a
method for obtaining retrovirus packaging cell lines and is hereby
incorporated by reference.
Propagation of retrovirus vectors, transduction of a population of cells
(e.g., CD4+ T-cells), and the
return of transduced cells to a patient are procedures well known to persons
skilled in the art of gene
therapy and have been well documented (Ranga, U. et al. (1997) J. Virol.
71:7020-7029; Bauer, G. et
al. (1997) Blood 89:2259-2267; Bonyhadi, M.L. (1997) J. Virol. 71:4707-4716;
Ranga, U. et al.
(1998) Proc. Natl. Acad. Sci. USA 95:1201-1206; Su, L. (1997) Blood 89:2283-
2290).
In the alternative, an adenovirus-based gene therapy delivery system is used
to deliver
polynucleotides encoding LIMD to cells which have one or more genetic
abnormalities with respect to
the expression of LIMD. The construction and packaging of adenovirus-based
vectors are well known
to those with ordinary skill in the art. Replication defective adenovirus
vectors have proven to be
versatile for importing genes encoding immunoregulatory proteins into intact
islets in the pancreas
(Csete, M.E. et al. (1995) Transplantation 27:263-268). Potentially useful
adenoviral vectors are
described in U.S. Patent Number 5,707,618 to Armentano ("Adenovirus vectors
for gene therapy"),
hereby incorporated by reference. For adenoviral vectors, see also Antinozzi,
P.A. et al. (1999) Annu.
Rev. Nutr. 19:511-544; and Verma, LM. and N. Somia (1997) Nature 18:389:239-
242, both
incorporated by reference herein.
CA 02378989 2002-O1-03
WO 01/04308 PCT/US00/19014
In another alternative, a herpes-based, gene therapy delivery system is used
to deliver
polynucleotides encoding LIMD to target cells which have one or more genetic
abnormalities with
respect to the expression of LIMD. The use of herpes simplex virus (HSV)-based
vectors may be
especially valuable for introducing LIMD to cells of the central nervous
system, for which HSV has a
tropism. The construction and packaging of herpes-based vectors are well known
to those with
ordinary skill in the art. A replication-competent herpes simplex virus (HSV)
type I-based vector has
been used to deliver a reporter gene to the eyes of primates (Liu, X. et al.
(1999) Exp. Eye
Res.169:385-395). The construction of a HSV-1 virus vector has also been
disclosed in detail in U.S.
Patent Number 5,804,413 to DeLuca ("Herpes simplex virus strains for gene
transfer"), which is
hereby incorporated by reference. U.S. Patent Number 5,804,413 teaches the use
of recombinant HSV
d92 which consists of a genome containing at least one exogenous gene to be
transferred to a cell under
the control of the appropriate promoter for purposes including human gene
therapy. Also taught by this
patent are the construction and use of recombinant HS V strains deleted for
ICP4, ICP27 and ICP22.
For HSV vectors, see also Goins, W.F. et al. (1999) J. Virol. 73:519-532 and
Xu, H. et al. (1994) Dev.
Biol. 163:152-161, hereby incorporated by reference. The manipulation of
cloned herpesvirus
sequences, the generation of recombinant virus following the transfection of
multiple plasmids
containing different segments of the large herpesvirus genomes, the growth and
propagation of
herpesvirus, and the infection of cells with herpesvirus are techniques well
known to those of ordinary
skill in the art.
In another alternative, an alphavirus (positive, single-stranded RNA virus)
vector is used to
deliver polynucleotides encoding LIMD to target cells. The biology of the
prototypic alphavirus,
Semliki Forest Virus (SFV), has been studied extensively and gene transfer
vectors have been based on
the SFV genome (Garoff, H. and K.-J. Li (1998) Curr. Opin. Biotech. 9:464-
469). During alphavirus
RNA replication, a subgenomic RNA is generated that normally encodes the viral
capsid proteins. This
subgenomic RNA replicates to higher levels than the full-length genomic RNA,
resulting in the
overproduction of capsid proteins relative to the viral proteins with
enzymatic activity (e.g., protease
and polymerase). Similarly, inserting the coding sequence for LIMD into the
alphavirus genome in
place of the capsid-coding region results in the production of a large number
of LIMD-coding RNAs
and the synthesis of high levels of LIMD in vector transduced cells. While
alphavirus infection is
typically associated with cell lysis within a few days, the ability to
establish a persistent infection in
hamster normal kidney cells (BHK-21) with a variant of Sindbis virus (SIN)
indicates that the lytic
replication of alphaviruses can be altered to suit the needs of the gene
therapy application (Dryga, S.A.
et al. (1997) Virology 228:74-83). The wide host range of alphaviruses will
allow the introduction of
LIMD into a variety of cell types. The specific transduction of a subset of
cells in a population may
41
CA 02378989 2002-O1-03
WO 01/04308 PCT/US00/19014
require the sorting of cells prior to transduction. The methods of
manipulating infectious cDNA clones
of alphaviruses, performing alphavirus cDNA and RNA transfections, and
performing alphavirus
infections, are well known to those with ordinary skill in the art.
Oligonucleoddes derived from the transcription initiation site, e.g., between
about positions -10
and +10 from the start site, may also be employed to inhibit gene expression.
Similarly, inhibition can
be achieved using triple helix base-pairing methodology. Triple helix pairing
is useful because it causes
inhibition of the ability of the double helix to open sufficiently for the
binding of polymerases,
transcription factors, or regulatory molecules. Recent therapeutic advances
using triplex DNA have
been described in the literature. (See, e.g., Gee, J.E. et al. (1994) in
Huber, B.E. and B.I. Carr,
Molecular and Immunolo~ic Annroaches, Futura Publishing, Mt. Kisco NY, pp. 163-
177.) A
complementary sequence or antisense molecule may also be designed to block
translation of mRNA by
preventing the transcript from binding to ribosomes.
Ribozymes, enzymatic RNA molecules, may also be used to catalyze the specific
cleavage of
RNA. The mechanism of ribozyme action involves sequence-specific hybridization
of the ribozyme
molecule to complementary target RNA, followed by endonucleolytic cleavage.
For example,
engineered hammerhead motif ribozyme molecules may specifically and
efficiently catalyze
endonucleolytic cleavage of sequences encoding L1MD.
Specific ribozyme cleavage sites within any potential RNA target are initially
identified by
scanning the target molecule for ribozyme cleavage sites, including the
following sequences: GUA,
GUU, and GUC. Once identified, short RNA sequences of between 15 and 20
ribonucleotides,
corresponding to the region of the target gene containing the cleavage site,
may be evaluated for
secondary structural features which may render the oligonucleotide inoperable.
The suitability of
candidate targets may also be evaluated by testing accessibility to
hybridization with complementary
oligonucleoddes using ribonuclease protection assays.
Complementary ribonucleic acid molecules and ribozymes of the invention may be
prepared by
any method known in the art for the synthesis of nucleic acid molecules. These
include techniques for
chemically synthesizing oligonucleotides such as solid phase phosphoramidite
chemical synthesis.
Alternatively, RNA molecules may be generated by in vitro and in vivo
transcription of DNA sequences
encoding LIMD. Such DNA sequences may be incorporated into a wide variety of
vectors with suitable
RNA polymerase promoters such as T7 or SP6. Alternatively, these cDNA
constructs that synthesize
complementary RNA, constitutively or inducibly, can be introduced into cell
lines, cells, or tissues.
RNA molecules may be modified to increase intracellular stability and half-
life. Possible
modifications include, but are not limited to, the addition of flanking
sequences at the 5' and/or 3' ends
of the molecule, or the use of phosphorothioate or 2' O-methyl rather than
phosphodiesterase linkages
42
CA 02378989 2002-O1-03
WO 01/04308 PCT/US00/19014
within the backbone of the molecule. This concept is inherent in the
production of PNAs and can be
extended in all of these molecules by the inclusion of nontraditional bases
such as inosine, queosine, and
wybutosine, as well as acetyl-, methyl-, thio-, and similarly modified forms
of adenine, cytidine,
guanine, thymine, and uridine which are not as easily recognized by endogenous
endonucleases.
An additional embodiment of the invention encompasses a method for screening
for a
compound which is effective in altering expression of a polynucleotide
encoding LIMD. Compounds
which may be effective in altering expression of a specific polynucleotide may
include, but are not
limited to, oligonucleotides, antisense oligonucleotides, triple helix-forming
oligonucleotides,
transcription factors and other polypeptide transcriptional regulators, and
non-macromolecular
chemical entities which are capable of interacting with specific
polynucleotide sequences. Effective
compounds may alter polynucleotide expression by acting as either inhibitors
or promoters of
polynucleotide expression. Thus, in the treatment of disorders associated with
increased LIMD
expression or activity, a compound which specifically inhibits expression of
the polynucleodde
encoding LIMD may be therapeutically useful, and in the treament of disorders
associated with
decreased LIMD expression or activity, a compound which specifically promotes
expression of the
polynucleotide encoding LIMD may be therapeutically useful.
At least one, and up to a plurality, of test compounds may be screened for
effectiveness in
altering expression of a specific polynucleotide. A test compound may be
obtained by any method
commonly known in the art, including chemical modification of a compound known
to be effective in
altering polynucleotide expression; selection from an existing, commercially-
available or proprietary
library of naturally-occurring or non-natural chemical compounds; rational
design of a compound
based on chemical and/or structural properties of the target polynucleotide;
and selection from a
library of chemical compounds created combinatorially or randomly. A sample
comprising a
polynucleotide encoding LIMD is exposed to at least one test compound thus
obtained. The sample
may comprise, for example, an intact or permeabilized cell, or an in vitro
cell-free or reconstituted
biochemical system. Alterations in the expression of a polynucleotide encoding
LIMD are assayed by
any method commonly known in the art. Typically, the expression of a specific
nucleotide is detected
by hybridization with a probe having a nucleotide sequence complementary to
the sequence of the
polynucleotide encoding LIMD. The amount of hybridization may be quantified,
thus forming the
basis for a comparison of the expression of the polynucleotide both with and
without exposure to one
or more test compounds. Detection of a change in the expression of a
polynucleotide exposed to a
test compound indicates that the test compound is effective in altering the
expression of the
polynucleotide. A screen for a compound effective in altering expression of a
specific polynucleotide
can be carried out, for example, using a Schizosaccharomyces pombe gene
expression system (Atkins,
D. et al. (1999) U.S. Patent No. 5.932,435; Arndt, G.M. et al. (2000) Nucleic
Acids Res. 28:E15) or a
43
CA 02378989 2002-O1-03
WO 01/04308 PCT/US00/19014
human cell line such as HeLa cell (Clarke, M.L. et al. (2000) Biochem.
Biophys. Res. Commun.
268:8-13). A particular embodiment of the present invention involves screening
a combinatorial
library of oligonucleotides (such as deoxyribonucleotides, ribonucleotides,
peptide nucleic acids, and
modified oligonucleotides) for antisense activity against a specific
polynucleotide sequence (Bruice,
T.W. et al. (1997) U.S. Patent No. 5,686,242; Bruice, T.W. et al. (2000) U.S.
Patent No. 6,022,691).
Many methods for introducing vectors into cells or tissues are available and
equally suitable for
use in vivo, in vitro, and ex vivo. For ex vivo therapy, vectors may be
introduced into stem cells taken
from the patient and clonally propagated for autologous transplant back into
that same patient.
Delivery by transfection, by liposome injections, or by polycationic amino
polymers may be achieved
using methods which are well known in the art. (See, e.g., Goldman, C.K. et
al. (1997) Nat.
Biotechnol. 15 :462-466. )
Any of the therapeutic methods described above may be applied to any subject
in need of such
therapy, including, for example, mammals such as humans, dogs, cats, cows,
horses, rabbits, and
monkeys.
An additional embodiment of the invention relates to the administration of a
pharmaceutical
composition which generally comprises an active ingredient formulated with a
pharmaceutically
acceptable excipient. Excipients may include, for example, sugars, starches,
celluloses, gums, and
proteins. Various formulations are commonly known and are thoroughly discussed
in the latest edition
of Remington's Pharmaceutical Sciences (Maack Publishing, Easton PA). Such
pharmaceutical
compositions may consist of LIMD, antibodies to LIMD, and mimetics, agonists,
antagonists, or
inhibitors of LIMD.
The pharmaceutical compositions utilized in this invention may be administered
by any number
of routes including, but not limited to, oral, intravenous, intramuscular,
infra-arterial, intramedullary,
intrathecal, intraventricular, pulmonary, transdermal, subcutaneous,
intraperitoneal, intranasal, enteral,
topical, sublingual, or rectal means.
Pharmaceutical compositions for pulmonary administration may be prepared in
liquid or dry
powder form. These compositions are generally aerosolized immediately prior to
inhalation by the
patient. In the case of small molecules (e.g. traditional low molecular weight
organic drugs), aerosol
delivery of fast-acting formulations is well-known in the art. In the case of
macromolecules (e.g. larger
peptides and proteins), recent developments in the field of pulmonary delivery
via the alveolar region of
the lung have enabled the practical delivery of drugs such as insulin to blood
circulation (see, e.g.,
Patton, J.S. et al., U.S. Patent No. 5,997,848). Pulmonary delivery has the
advantage of administration
without needle injection, and obviates the need for potentially toxic
penetration enhancers.
Pharmaceutical compositions suitable for use in the invention include
compositions wherein the
active ingredients are contained in an effective amount to achieve the
intended purpose. The
44
CA 02378989 2002-O1-03
WO 01/04308 PCT/US00/19014
determination of an effective dose is well within the capability of those
skilled in the art.
Specialized forms of pharmaceutical compositions may be prepared for direct
intracellular
delivery of macromolecules comprising LIMD or fragments thereof. For example,
liposome
preparations containing a cell-impermeable macromolecule may promote cell
fusion and intracellular
delivery of the macromolecule. Alternatively, LIMD or a fragment thereof may
be joined to a short
cationic N-terminal portion from the HIV Tat-1 protein. Fusion proteins thus
generated have been
found to transduce into the cells of all tissues, including the brain, in a
mouse model system (Schwarze,
S.R. et al. (1999) Science 285:1569-1572).
For any compound, the therapeutically effective dose can be estimated
initially either in cell
culture assays, e.g., of neoplastic cells, or in animal models such as mice,
rats, rabbits, dogs, monkeys,
or pigs. An animal model may also be used to determine the appropriate
concentration range and route
of administration. Such information can then be used to determine useful doses
and routes for
administration in humans.
A therapeutically effective dose refers to that amount of active ingredient,
for example LIMD
or fragments thereof, antibodies of LIMD, and agonists, antagonists or
inhibitors of LIMD, which
ameliorates the symptoms or condition. Therapeutic efficacy and toxicity may
be determined by
standard pharmaceutical procedures in cell cultures or with experimental
animals, such as by
calculating the EDso (the dose therapeutically effective in 50% of the
population) or LDSO (the dose
lethal to 50% of the population) statistics. The dose ratio of toxic to
therapeutic effects is the
therapeutic index, which can be expressed as the LDso/EDSO ratio.
Pharmaceutical compositions which
exhibit large therapeutic indices are preferred. The data obtained fiom cell
culture assays and animal
studies are used to formulate a range of dosage for human use. The dosage
contained in such
compositions is preferably within a range of circulating concentrations that
includes the EDSO with little
or no toxicity. The dosage varies within this range depending upon the dosage
form employed, the
sensitivity of the patient, and the route of adnunistration.
The exact dosage will be determined by the practitioner, in light of factors
related to the subject
requiring treatment. Dosage and administration are adjusted to provide
sufficient levels of the active
moiety or to maintain the desired effect. Factors which may be taken into
account include the severity
of the disease state, the general health of the subject, the age, weight, and
gender of the subject, time
and frequency of administration, drug combination(s), reaction sensitivities,
and response to therapy.
Long-acting pharmaceutical compositions may be administered every 3 to 4 days,
every week, or
biweekly depending on the half life and clearance rate of the particular
formulation.
Normal dosage amounts may vary from about 0.1 ~cg to 100,000 fig, up to a
total dose of
about 1 gram, depending upon the route of administration. Guidance as to
particular dosages and
CA 02378989 2002-O1-03
WO 01/04308 PCT/US00/19014
methods of delivery is provided in the literature and generally available to
practitioners in the art.
Those skilled in the art will employ different formulations for nucleotides
than for proteins or their
inhibitors. Similarly, delivery of polynucleotides or polypeptides will be
spec;itic to particular cells,
conditions, locations, etc.
DIAGNOSTICS
In another embodiment, antibodies which specifically bind LIMD may be used for
the diagnosis
of disorders characterized by expression of LIMD, or in assays to monitor
patients being treated with
LIMD or agonists, antagonists, or inhibitors of LIMD. Antibodies useful for
diagnostic purposes may
be prepared in the same manner as described above for therapeutics. Diagnostic
assays for LIMD
include methods which utilize the antibody and a label to detect LIMD in human
body tluids or in
extracts of cells or tissues. The antibodies may be used with or without
modification, and may be
labeled by covalent or non-covalent attachment of a reporter molecule. A wide
variety of reporter
molecules, several of which are described above, are known in the art and may
be used.
A variety of protocols for measuring LIMD, including ELISAs, RIAs, and FACS,
are known in
the art and provide a basis for diagnosing altered or abnormal levels of LIMD
expression. Normal or
standard values for LIMD expression are established by combining body fluids
or cell extracts taken
from normal mammalian subjects, for example, human subjects, with antibody to
LIMD under
conditions suitable for complex formation. The amount of standard complex
formation may be
quantitated by various methods, such as photometric means. Quantities of LIMD
expressed in subject,
control, and disease samples from biopsied tissues are compared with the
standard values. Deviation
between standard and subject values establishes the parameters for diagnosing
disease.
In another embodiment of the invention, the polynucleotides encoding LIMD may
be used for
diagnostic purposes. The polynucleotides which may be used include
oligonucleotide sequences,
complementary RNA and DNA molecules, and PNAs. The polynucleotides may be used
to detect and
quantify gene expression in biopsied tissues in which expression of LIMD may
be correlated with
disease. The diagnostic assay may be used to determine absence, presence, and
excess expression of
LIMD, and to monitor regulation of LIMD levels during therapeutic
intervention.
Tn one aspect, hybridization with PCR probes which are capable of detecting
polynucleotide
sequences, including genomic sequences, encoding LIMD or closely related
molecules may be used to
identify nucleic acid sequences which encode LIMD. The specificity of the
probe, whether it is made
from a highly specific region, e.g., the 5'regulatory region, or from a less
specific region, e.g., a
conserved motif, and the stringency of the hybridization or amplification will
determine whether the
probe identifies only naturally occurring sequences encoding LIMD, allelic
variants, or related
sequences.
46
CA 02378989 2002-O1-03
WO 01/04308 PCT/US00/19014
Probes may also be used for the detection of related sequences, and may have
at least 50%
sequence identity to any of the LIMD encoding sequences. The hybridization
probes of the subject
invention may be DNA or RNA and may be derived from the sequence of SEQ ID
N0:3-4 or from
genomic sequences including promoters, enhancers, and introns of the LIMD
gene.
Means for producing specific hybridization probes for DNAs encoding LIMD
include the
cloning of polynucleotide sequences encoding LIMD or LIMD derivatives into
vectors for the
production of mRNA probes. Such vectors are known in the art, are commercially
available, and may
be used to synthesize RNA probes in vitro by means of the addition of the
appropriate RNA
polymerases and the appropriate labeled nucleotides. Hybridization probes may
be labeled by a variety
of reporter groups, for example, by radionuclides such as 32P or 35S, or by
enzymatic labels, such as
alkaline phosphatase coupled to the probe via avidin/biotin coupling systems,
and the like.
Polynucleotide sequences encoding LIMD may be used for the diagnosis of
disorders associated
with expression of LIMD. Examples of such disorders include, but are not
limited to, a cell
proliferative disorder, such as actinic keratosis, arteriosclerosis,
atherosclerosis, bursitis, cirrhosis,
hepatitis, mixed connective tissue disease (MCTD), myelofibrosis, paroxysmal
nocturnal
hemoglobinuria, polycythemia vera, psoriasis, primary thrombocythemia, and
cancers including
adenocarcinoma, leukemia, lymphoma, melanoma, myeloma, sarcoma,
teratocarcinoma, and, in
particular, cancers of the adrenal gland, bladder, bone, bone marrow, brain,
breast, cervix, gall
bladder, ganglia, gastrointestinal tract, heart, kidney, liver, lung, muscle,
ovary, pancreas, parathyroid,
penis, prostate, salivary glands, skin, spleen, testis, thymus, thyroid, and
uterus; a developmental
disorder, such as renal tubular acidosis, anemia, Cushing's syndrome,
achondroplastic dwarfism,
Duchenne and Becker muscular dystrophy, epilepsy, gonadal dysgenesis, WAGR
syndrome (Wilms'
tumor, aniridia, genitourinary abnormalities, and mental retardation), Smith-
Magenis syndrome,
myelodysplastic syndrome, hereditary mucoepithelial dysplasia, hereditary
keratodermas, hereditary
neuropathies such as Charcot-Marie-Tooth disease and neurofibromatosis,
hypothyroidism,
hydrocephalus, seizure disorders such as Syndenham's chorea and cerebral
palsy, spina bifida,
anencephaly, craniorachischisis, congenital glaucoma, cataract, and
sensorineural hearing loss; and a
disorder of cell motility, such as ankylosing spondylitis, Chediak-Higashi
syndrome, Duchenne and
Becker muscular dystrophy, intrahepatic cholestasis, myocardial hyperplasia,
cardiomyopathy, early
onset peridontitis, cancers such as adenocarcinoma, ovarian carcinoma, and
chronic myelogenous
leukemia, and bacterial and helminthic infections. The polynucleotide
sequences encoding LIMD may
be used in Southern or northern analysis, dot blot, or other membrane-based
technologies; in PCR
technologies; in dipstick, pin, and multiformat ELISA-like assays; and in
microarrays utilizing fluids or
tissues from patients to detect altered LIMD expression. Such qualitative or
quantitative methods are
well known in the art.
47
CA 02378989 2002-O1-03
WO 01/04308 PCT/US00/19014
In a particular aspect, the nucleotide sequences encoding LIMD may be useful
in assays that
detect the presence of associated disorders, particularly those mentioned
above. The nucleotide
sequences encoding LIMD may be labeled by standard methods and added to a
fluid or tissue sample
from a patient under conditions suitable for the formation of hybridization
complexes. After a suitable
incubation period, the sample is washed and the signal is quantified and
compared with a standard
value. If the amount of signal in the patient sample is significantly altered
in comparison to a control
sample then the presence of altered levels of nucleotide sequences encoding
LIMD in the sample
indicates the presence of the associated disorder. Such assays may also be
used to evaluate the efficacy
of a particular therapeutic treatment regimen in animal studies, in clinical
trials, or to monitor the
treatment of an individual patient.
In order to provide a basis for the diagnosis of a disorder associated with
expression of LIMD,
a normal or standard profile for expression is established. This may be
accomplished by combining
body fluids or cell extracts taken from normal subjects, either animal or
human, with a sequence, or a
fragment thereof, encoding LIMD, under conditions suitable for hybridization
or amplification.
Standard hybridization may be quantified by comparing the values obtained from
normal subjects with
values from an experiment in which a known amount of a substantially purified
polynucleotide is used.
Standard values obtained in this manner may be compared with values obtained
from samples from
patients who are symptomatic for a disorder. Deviation from standard values is
used to establish the
presence of a disorder.
Once the presence of a disorder is established and a treatment protocol is
initiated,
hybridization assays may be repeated on a regular basis to determine if the
level of expression in the
patient begins to approximate that which is observed in the normal subject.
The results obtained from
successive assays may be used to show the efficacy of treatment over a period
ranging from several
days to months.
With respect to cancer, the presence of an abnormal amount of transcript
(either under- or
overexpressed) in biopsied tissue from an individual may indicate a
predisposition for the development
of the disease, or may provide a means for detecting the disease prior to the
appearance of actual
clinical symptoms. A more definitive diagnosis of this type may allow health
professionals to employ
preventative measures or aggressive treatment earlier thereby preventing the
development or further
progression of the cancer.
Additional diagnostic uses for oligonucleotides designed from the sequences
encoding LIMD
may involve the use of PCR. These oligomers may be chemically synthesized,
generated enzymatically,
or produced in vitro. Oligomers will preferably contain a fragment of a
polynucleotide encoding LIMD,
or a fragment of a polynucleotide complementary to the polynucleotide encoding
LIMD, and will be
48
CA 02378989 2002-O1-03
WO 01/04308 PCT/US00/19014
employed under optimized conditions for identification of a specific gene or
condition. Oligomers may
also be employed under less stringent conditions for detection or
quantification of closely related DNA
or RNA sequences.
In a particular aspect, oligonucleotide primers derived tiom the
polynucleotide sequences
encoding LIMD may be used to detect single nucleotide polymorphisms (SNPs).
SNPs are
substitutions, insertions and deletions that are a frequent cause of inherited
or acquired genetic disease
in humans. Methods of SNP detection include, but are not limited to, single-
stranded conformation
polymorphism (SSCP) and fluorescent SSCP (fSSCP) methods. In SSCP,
oligonucleotide primers
derived from the polynucleotide sequences encoding LIMD are used to amplify
DNA using the
polymerase chain reaction (PCR). The DNA may be derived, for example, from
diseased or normal
tissue, biopsy samples, bodily fluids, and the like. SNPs in the DNA cause
differences in the secondary
and tertiary structures of PCR products in single-stranded form, and these
differences are detectable
using gel electrophoresis in non-denaturing gels. In fSCCP, the
oligonucleotide primers are
tluorescently labeled, which allows detection of the amplimers in high-
throughput equipment such as
DNA sequencing machines. Additionally, sequence database analysis methods,
termed in silico SNP
(isSNP), are capable of identifying polymorphisms by comparing the sequence of
individual
overlapping DNA fragments which assemble into a common consensus sequence.
These computer-
based methods filter out sequence variations due to laboratory preparation of
DNA and sequencing
errors using statistical models and automated analyses of DNA sequence
chromatograms. In the
alternative, SNPs may be detected and characterized by mass spectrometry
using, for example, the high
throughput MASSARRAY system (Sequenom, Inc., San Diego CA).
Methods which may also be used to quantify the expression of LIMD include
radiolabeling or
biotinylating nucleotides, coamplification of a control nucleic acid, and
interpolating results from
standard curves. (See, e.g., Melby, P.C. et al. (1993) J. Immunol. Methods
159:235-244; Duplaa, C. et
al. (1993) Anal. Biochem. 212:229-236.) The speed of quantitation of multiple
samples may be
accelerated by running the assay in a high-throughput format where the
oligomer or polynucleotide of
interest is presented in various dilutions and a spectrophotometric or
calorimetric response gives rapid
quantitation.
In further embodiments, oligonucleotides or longer fragments derived tiom any
of the
polynucleotide sequences described herein may be used as elements on a
microarray. The microarray
can be used in transcript imaging techniques which monitor the relative
expression levels of large
numbers of genes simultaneously as described in Seilhamer, J.J. et al.,
"Comparative Gene Transcript
Analysis," U.S. Patent No. 5,840,484, incorporated herein by reference. The
microarray may also be
used to identify genetic variants, mutations, and polymorphisms. This
information may be used to
49
CA 02378989 2002-O1-03
WO 01/04308 PCT/US00/19014
determine gene function, to understand the genetic basis of a disorder, to
diagnose a disorder, to monitor
progression/regression of disease as a function of gene expression, and to
develop and monitor the
activities of therapeutic agents in the treatment of disease. In particular,
this information may be used
to develop a pharmacogenomic profile of a patient in order to select the most
appropriate and effective
treatment regimen for that patient. For example, therapeutic agents which are
highly effective and
display the fewest side effects may be selected for a patient based on his/her
pharmacogenomic profile.
In another embodiment, antibodies specific for LIMD, or LIMD or fragments
thereof may be
used as elements on a microarray. The microarray may be used to monitor or
measure protein-protein
interactions, drug-target interactions, and gene expression profiles, as
described above.
A particular embodiment relates to the use of the polynucleotides of the
present invention to
generate a transcript image of a tissue or cell type. A transcript image
represents the global pattern of
gene expression by a particular tissue or cell type. Global gene expression
patterns are analyzed by
quantifying the number of expressed genes and their relative abundance under
given conditions and at a
given time. (See Seilhamer et al., "Comparative Gene Transcript Analysis,"
U.S. Patent Number
5,840,484, expressly incorporated by reference herein.) Thus a transcript
image may be generated by
hybridizing the polynucleotides of the present invention or their complements
to the totality of
transcripts or reverse transcripts of a particular tissue or cell type. In one
embodiment, the
hybridization takes place in high-throughput format, wherein the
polynucleotides of the present
invention or their complements comprise a subset of a plurality of elements on
a microarray. The
resultant transcript image would provide a profile of gene activity.
Transcript images may be generated using transcripts isolated from tissues,
cell lines, biopsies,
or other biological samples. The transcript image may thus reflect gene
expression in vivo, as in the
case of a tissue or biopsy sample, or in vitro, as in the case of a cell line.
Transcript images which profile the expression of the polynucleotides of the
present invention
may also be used in conjunction with in vitro model systems and preclinical
evaluation of
pharmaceuticals, as well as toxicological testing of industrial and naturally-
occurring environmental
compounds. All compounds induce characteristic gene expression patterns,
frequently termed
molecular fingerprints or toxicant signatures, which are indicative of
mechanisms of action and toxicity
(Nuwaysir, E.F. et al. (1999) Mol. Carcinog. 24:153-159; Steiner, S. and N.L.
Anderson (2000)
Toxicol. Lett. 112-113:467-471, expressly incorporated by reference herein).
If a test compound has a
signature similar to that of a compound with known toxicity, it is likely to
share those toxic properties.
These fingerprints or signatures are most useful and refined when they contain
expression information
from a large number of genes and gene families. Ideally, a genome-wide
measurement of expression
provides the highest quality signature. Even genes whose expression is not
altered by any tested
CA 02378989 2002-O1-03
WO 01/04308 PCT/US00/19014
compounds are important as well, as the levels of expression of these genes
are used to normalize the
rest of the expression data. The normalization procedure is useful for
comparison of expression data
after treatment with different compounds. While the assignment of gene
function to elements of a
toxicant signature aids in interpretation of toxicity mechanisms, knowledge of
gene function is not
necessary for the statistical matching of signatures which leads to prediction
of toxicity. (See, for
example, Press Release 00-02 from the National Institute of Environmental
Health Sciences, released
February 29, 2000, available at http://www.niehs.nih.gov/oc/news/toxchip.htm.)
Therefore, it is
important and desirable in toxicological screening using toxicant signatures
to include all expressed
gene sequences.
In one embodiment, the toxicity of a test compound is assessed by treating a
biological sample
containing nucleic acids with the test compound. Nucleic acids that are
expressed in the treated
biological sample are hybridized with one or more probes specific to the
polynucleotides of the
present invention, so that transcript levels corresponding to the
polynucleotides of the present
invention may be quantified. The transcript levels in the treated biological
sample are compared with
levels in an untreated biological sample. Differences in the transcript levels
between the two samples
are indicative of a toxic response caused by the test compound in the treated
sample.
Microarrays may be prepared, used, and analyzed using methods known in the
art. (See, e.g.,
Brennan, T.M. et al. (1995) U.S. Patent No. 5,474,796; Schena, M. et al.
(1996) Proc. Natl. Acad. Sci.
USA 93:10614-10619; Baldeschweiler et al. (1995) PCT application W095/251116;
Shalom D. et al.
(1995) PCT application W095/35505; Heller, R.A. et al. (1997) Proc. Natl.
Acad. Sci. USA 94:2150-
2155; and Heller, M.J. et al. (1997) U.S. Patent No. 5,605,662.) Various types
of microarrays are well
known and thoroughly described in DNA Microarrays: A Practical Approach, M.
Schena, ed. (1999)
Oxford University Press, London, hereby expressly incorporated by reference.
In another embodiment of the invention, nucleic acid sequences encoding LIMD
may be used to
generate hybridization probes useful in mapping the naturally occurring
genomic sequence. Either
coding or noncoding sequences may be used, and in some instances, noncoding
sequences may be
preferable over coding sequences. For example, conservation of a coding
sequence among members
of a multi-gene family may potentially cause undesired cross hybridization
during chromosomal
mapping. The sequences may be mapped to a particular chromosome, to a specific
region of a
chromosome, or to artificial chromosome constructions, e.g., human artificial
chromosomes (HACs),
yeast artificial chromosomes (YACs), bacterial artificial chromosomes (BACs),
bacterial P1
constructions, or single chromosome cDNA libraries. (See, e.g., Harrington,
J.J. et al. (1997) Nat.
Genet. 15:345-355; Price, C.M. (1993) Blood Rev. 7:127-134; and Trask, B.J.
(1991) Trends Genet.
7:149-154.) Once mapped, the nucleic acid sequences of the invention may be
used to develop genetic
linkage maps, for example, which correlate the inheritance of a disease state
with the inheritance of a
51
CA 02378989 2002-O1-03
WO 01/04308 PCT/US00/19014
particular chromosome region or restriction fragment length polymorphism
(RFLP). (See, e.g.,
Lander, E.S. and D. Botstein (1986) Proc. Natl. Acad. Sci. USA 83:7353-7357.)
Fluorescent in situ hybridization (FISH) may be correlated with other physical
and genetic map
data. (See, e.g., Heinz-Ulrich, et al. (1995) in Meyers, su ra, pp. 965-968.)
Examples of genetic map
data can be found in various scientific journals or at the Online Mendelian
Inheritance in Man (OMIM)
World Wide Web site. Correlation between the location of the gene encoding
LIMD on a physical map
and a specific disorder, or a predisposition to a specific disorder, may help
define the region of DNA
associated with that disorder and thus may further positional cloning efforts.
In situ hybridization of chromosomal preparations and physical mapping
techniques, such as
linkage analysis using established chromosomal markers, may be used for
extending genetic maps.
Often the placement of a gene on the chromosome of another mammalian species,
such as mouse, may
reveal associated markers even if the exact chromosomal locus is not known.
This information is
valuable to investigators searching for disease genes using positional cloning
or other gene discovery
techniques. Once the gene or genes responsible for a disease or syndrome have
been crudely localized
by genetic linkage to a particular genomic region, e.g., ataxia-telangiectasia
to l 1q22-23, any sequences
mapping to that area may represent associated or regulatory genes for further
investigation. (See, e.g.,
Gatti, R.A. et al. (1988) Nature 336:577-580.) The nucleotide sequence of the
instant invention may
also be used to detect differences in the chromosomal location due to
translocation, inversion, etc.,
among normal, carrier, or affected individuals.
In another embodiment of the invention, LIMD, its catalytic or immunogenic
fragments, or
oligopeptides thereof can be used for screening libraries of compounds in any
of a variety of drug
screening techniques. The fragment employed in such screening may be free in
solution, affixed to a
solid support, borne on a cell surface, or located intracellularly. The
formation of binding complexes
between LIMD and the agent being tested may be measured.
Another technique for drug screening provides for high throughput screening of
compounds
having suitable binding affinity to the protein of interest. (See, e.g.,
Geysen, et al. (1984) PCT
application W084/03564.) In this method, large numbers of different small test
compounds are
synthesized on a solid substrate. The test compounds are reacted with LIMD, or
fragments thereof, and
washed. Bound LIMD is then detected by methods well known in the art. Purified
LIMD can also be
coated directly onto plates for use in the aforementioned drug screening
techniques. Alternatively,
non-neutralizing antibodies can be used to capture the peptide and immobilize
it on a solid support.
In another embodiment, one may use competitive drug screening assays in which
neutralizing
antibodies capable of binding LIMD specifically compete with a test compound
for binding LIMD. In
this manner, antibodies can be used to detect the presence of any peptide
which shares one or more
52
CA 02378989 2002-O1-03
WO 01/04308 PCT/US00/19014
antigenic determinants with LIMD.
In additional embodiments, the nucleotide sequences which encode LIMD may be
used in any
molecular biology techniques that have yet to be developed, provided the new
techniques rely on
properties of nucleotide sequences that are currently known, including, but
not limited to, such
properties as the triplet genetic axle and specific base pair interactions.
Without further elaboration, it is believed that one skilled in the art can,
using the preceding
description, utilize the present invention to its fullest extent. The
following preferred specific
embodiments are, therefore, to be construed as merely illustrative, and not
limitative of the remainder
of the disclosure in any way whatsoever.
The disclosures of all patents, applications and publications, mentioned above
and below, in
particular U.S. Ser. No. 60/143,426 are hereby expressly incorporated by
reference.
EXAMPLES
I. Construction of cDNA Libraries
RNA was isolated from tissues described in Table 4. For construction of the
CONFNOT02
cDNA library, the frozen tissue was homogenized and lysed in TRIZOL reagent
(lgm tissue/10 ml
TRIZOL reagent; Life Technologies), a monophasic solution of phenol and
guanidine isothiocyanate,
using a Polytron PT-3000 homogenizer (Brinkman Instruments, Westbury NY).
Alter a brief
incubation on ice, chloroform was added (1:5 v/v), and the mixture was
centrifuged to separate the
phases. The upper aqueous phase was removed to a fresh tube, and isopropanol
was added to
precipitate RNA. The RNA was resuspended in RNase-free water and treated with
DNase. The RNA
was re-extracted as necessary with acid phenol-chloroform to increase purity,
and the RNA was
reprecipitated with sodium acetate and ethanol. Construction of the UTRSTMRO1
cDNA library was
carried out in the same way except that the frozen tissue was homogenized and
lysed in TRIZOL
reagent (0.8 gm tissue/12 ml Trizol), and the RNA was not treated with DNase.
For each library, poly(A+) RNA was isolated using oligo d(T)-coupled
paramagnetic particles
(Promega), OLIGOTEX latex particles (QIAGEN, Chatsworth CA), or an OLIGOTEX
mRNA
purification kit (QIAGEN). Alternatively, RNA was isolated directly from
tissue lysates using other
RNA isolation kits, e.g., the POLY(A)PURE mRNA purification kit (Ambion,
Austin TX).
In some cases, Stratagene was provided with RNA and constructed the
corresponding cDNA
libraries. Otherwise, cDNA was synthesized and cDNA libraries were constructed
with the UNIZAP
vector system (Stratagene) or SUPERSCRIPT plasmid system (Life Technologies),
using the
recommended procedures or similar methods known in the art. (See, e.g.,
Ausubel, 1997, supra, units
5.1-6.6.) Reverse transcription was initiated using oligo d(T) or random
primers. Synthetic
oligonucleotide adapters were ligated to double stranded cDNA, and the cDNA
was digested with the
53
CA 02378989 2002-O1-03
WO 01/04308 PCT/US00/19014
appropriate restriction enzyme or enzymes. For most libraries, the cDNA was
size-selected (300-1000
bp) using SEPHACRYL S 1000, SEPHAROSE CL2B, or SEPHAROSE CL4B column
chromatography (Amersham Pharmacia Biotech) or preparative agarose gel
electrophoresis. cDNAs
were ligated into compatible restriction enzyme sites of the polylinker of a
suitable plasmid, e.g.,
PBLUESCRIPT plasmid (Stratagene), PSPORT1 plasmid (Life Technologies),
pcDNA2.1 plasmid
(Invitrogen, Carlsbad CA), or pINCY plasmid (Incyte Genomics, Palo Alto CA).
Recombinant
plasmids were transformed into competent E. coli cells including XL1-Blue, XL1-
BlueMRF, or SOLR
from Stratagene or DHSa, DH10B, or ElectroMAX DH10B from Life Technologies.
II. Isolation of cDNA Clones
Plasmids obtained as described in Example I were recovered from host cells by
in vivo excision
using the UNIZAP vector system (Stratagene) or by cell lysis. Plasmids were
purified using at least
one of the following: a Magic or WIZARD Minipreps DNA purification system
(Promega); an AGTC
Miniprep purification kit (Edge Biosystems, Gaithersburg MD); and QIAWELL 8
Plasmid, QIAWELL
8 Plus Plasmid, QIAWELL 8 Ultra Plasmid purification systems or the R.E.A.L.
PREP 96 plasmid
purification kit from QIAGEN. Following precipitation, plasmids were
resuspended in 0.1 ml of
distilled water and stored, with or without lyophilization, at 4°C.
Alternatively, plasmid DNA was amplified from host cell lysates using direct
link PCR in a
high-throughput format (Rao, V.B. (1994) Anal. Biochem. 216:1-14). Host cell
lysis and thermal
cycling steps were carried out in a single reaction mixture.- Samples were
processed and stored in 384-
well plates, and the concentration of amplified plasmid DNA was quantified
fluorometrically using
P1COGREEN dye (Molecular Probes, Eugene OR) and a FLUOROSKAN II fluorescence
scanner
(Labsystems Oy, Helsinki, Finland).
I1I. Sequencing and Analysis
Incyte cDNA recovered in plasmids as described in Example II were sequenced as
follows.
Sequencing reactions were processed using standard methods or high-throughput
instrumentation
such as the ABI CATALYST 800 (PE Biosystems) thermal cycler or the PTC-200
thermal cycler (MJ
Research) in conjunction with the HYDRA microdispenser (Robbins Scientific) or
the MICROLAB
2200 (Hamilton) liquid transfer system. cDNA sequencing reactions were
prepared using reagents
provided by Amersham Pharmacia Biotech or supplied in ABI sequencing kits such
as the ABI
PRISM BIGDYE Terminator cycle sequencing ready reaction kit (PE Biosystems).
Electrophoretic
separation of cDNA sequencing reactions and detection of labeled
polynucleotides were carried out
using the MEGABACE 1000 DNA sequencing system (Molecular Dynamics); the ABI
PRISM 373 or
377 sequencing system (PE Biosystems) in conjunction with standard ABI
protocols and base calling
software; or other sequence analysis systems known in the art. Reading frames
within the cDNA
54
CA 02378989 2002-O1-03
WO 01/04308 PCT/US00/19014
sequences were identified using standard methods (reviewed in Ausubel, 1997,
supra, unit 7.7). Some
of the cDNA sequences were selected for extension using the techniques
disclosed in Example VI.
The polynucleotide sequences derived.from cDNA sequencing were assembled and
analyzed
using a combination of software programs which utilize algorithms well known
to those skilled in the
art. Table 5 summarizes the tools, programs, and algorithms used and provides
applicable descriptions,
references, and threshold parameters. The first column of Table 5 shows the
tools, programs, and
algorithms used, the second column provides brief descriptions thereof, the
third column presents
appropriate references, all of which are incorporated by reference herein in
their entirety, and the fourth
column presents, where applicable, the scores, probability values, and other
parameters used to evaluate
the strength of a match between two sequences (the higher the score, the
greater the homology between
two sequences). Sequences were analyzed using MACDNASIS PRO software (Hitachi
Software
Engineering, South San Francisco CA) and LASERGENE software (DNASTAR).
Polynucleotide and
polypeptide sequence alignments were generated using the default parameters
specified by the clustal
algorithm as incorporated into the MEGALIGN multisequence alignment program
(DNASTAR), which
also calculates the percent identity between aligned sequences.
The polynucleodde sequences were validated by removing vector, linker, and
polyA sequences
and by masking ambiguous bases, using algorithms and programs based on BLAST,
dynamic
programing, and dinucleotide nearest neighbor analysis. The sequences were
then queried against a
selection of public databases such as the GenBank primate, rodent, mammalian,
vertebrate, and
eukaryote databases, and BLOCKS, PRINTS, DOMO, PRODOM, and PFAM to acquire
annotation
using programs based on BLAST, FASTA, and BLIMPS. The sequences were assembled
into full
length polynucleotide sequences using programs based on Phred, Phrap, and
Conned, and were screened
for open reading frames using programs based on GeneMark, BLAST, and FASTA.
The full length
polynucleotide sequences were translated to derive the corresponding full
length amino acid sequences,
and these full length sequences were subsequently analyzed by querying against
databases such as the
GenBank databases (described above), SwissProt, BLOCKS, PRINTS, DOMO, PRODOM,
Prosite,
and Hidden Markov Model (HMM)-based protein family databases such as PFAM. HMM
is a
probabilistic approach which analyzes consensus primary structures of gene
families. (See, e.g.,
Eddy, S.R. (1996) Curr. Opin. Struct. Biol. 6:361-365.)
The programs described above for the assembly and analysis of full length
polynucleotide and
amino acid sequences were also used to identify polynucleotide sequence
fragments from SEQ ID
N0:3-4. Fragments from about 20 to about 4000 nucleotides which are useful in
hybridization and
amplification technologies were described in The Invention section above.
IV. Analysis of Polynucleotide Expression
CA 02378989 2002-O1-03
WO 01/04308 PCT/US00/19014
Northern analysis is a laboratory technique used to detect the presence of a
transcript of a gene
and involves the hybridization of a labeled nucleotide sequence to a membrane
on which RNAs from a
particular cell type or tissue have been bound. (See, e.g., Sambrook, supra,
eh. 7; Ausubel, 1995,
su ra, eh. 4 and 16.)
Analogous computer techniques applying BLAST were used to search for identical
or related
molecules in cDNA databases such as GenBank or LIFESEQ (Incyte Genomics). This
analysis is
much faster than multiple membrane-based hybridizations. In addition, the
sensitivity of the computer
search can be modified to determine whether any particular match is
categorized as exact or similar.
The basis of the search is the product score, which is defined as:
BLAST Score x Percent Identity
5 x minimum {length(Seq. 1), length(Seq. 2)}
The product score takes into account both the degree of similarity between two
sequences and the length
of the sequence match. The product score is a normalized value between 0 and
100, and is calculated
as follows: the BLAST score is multiplied by the percent nucleotide identity
and the product is divided
by (5 times the length of the shorter of the two sequences). The BLAST score
is calculated by
assigning a score of +5 for every base that matches in a high-scoring segment
pair (HSP), and -4 for
every mismatch. Two sequences may share more than one HSP (separated by gaps).
If there is more
than one HSP, then the pair with the highest BLAST score is used to calculate
the product score. The
product score represents a balance between fractional overlap and quality in a
BLAST alignment. For
example, a product score of 100 is produced only for 100% identity over the
entire length of the shorter
of the two sequences being compared. A product score of 70 is produced either
by 100% identity and
70% overlap at one end, or by 88% identity and 100% overlap at the other. A
product score of 50 is
produced either by 100% identity and 50% overlap at one end, or 79% identity
and 100% overlap.
The results of northern analyses are reported as a percentage distribution of
libraries in which
the transcript encoding LIMD occurred. Analysis involved the categorization of
cDNA libraries by
organ/tissue and disease. The organ/tissue categories included cardiovascular,
dermatologic,
developmental, endocrine, gastrointestinal, hematopoietic/immune,
musculoskeletal, nervous,
reproductive, and urologic. The disease/condition categories included cancer,
inflammation, trauma,
cell proliferation, neurological, and pooled. For each category, the number of
libraries expressing the
sequence of interest was counted and divided by the total number of libraries
across all categories.
Percentage values of tissue-specific and disease- or condition-specific
expression are reported in Table
3.
V. Chromosomal Mapping of LIMD Encoding Polynucleotides
56
CA 02378989 2002-O1-03
WO 01/04308 PCT/US00/19014
The cDNA sequences which were used to assemble SEQ ID N0:3 and SEQ ID N0:4
were
compared with sequences from the Incyte L1FESEQ database and public domain
databases using
BLAST and other implementations of the Snuth-Waterman algorithm. Sequences
from these databases
that matched SEQ ID N0:3 and SEQ ID N0:4 were assembled into clusters of
contiguous and
overlapping sequences using assembly algorithms such as Phrap (Table 5).
Radiation hybrid and
genetic mapping data available from public resources such as the Stanford
Human Genome Center
(SHGC), Whitehead Institute for Genome Research (WIGR), and Genethon were used
to determine if
any of the clustered sequences had been previously mapped. Inclusion of a
mapped sequence in a
cluster resulted in the assignment of all sequences of that cluster, including
its particular SEQ ID NO:,
to that map location.
The genetic map locations of SEQ ID N0:3 and SEQ ID N0:4 are described in The
Invention
as ranges, or intervals, of human chromosomes. The map position of an
interval, in centiMorgans, is
measured relative to the terminus of the chromosome's p-arm. (The centiMorgan
(cM) is a unit of
measurement based on recombination frequencies between chromosomal markers. On
average, 1 cM is
roughly equivalent to 1 megabase (Mb) of DNA in humans, although this can vary
widely due to hot
and cold spots of recombination.) The cM distances are based on genetic
markers mapped by Genethon
which provide boundaries for radiation hybrid markers whose sequences were
included in each of the
clusters. Diseases associated with the public and Incyte sequences located
within the indicated intervals
are also reported in the Invention where applicable.
Vl. Extension of LIMD Encoding Polynucleotides
The full length nucleic acid sequences of SEQ ID N0:3-4 were produced by
extension of an
appropriate fragment of the full length molecule using oligonucleotide primers
designed from this
fragment. One primer was synthesized to initiate 5' extension of the known
fragment, and the other
primer, to initiate 3' extension of the known fiagment. The initial primers
were designed using OL1G0
4.06 software (National Biosciences), or another appropriate program, to be
about 22 to 30 nucleotides
in length, to have a GC content of about 50% or more, and to anneal to the
target sequence at
temperatures of about 68°C to about 72°C. Any stretch of
nucleotides which would result in hairpin
structures and primer-primer dimerizations was avoided.
Selected human cDNA libraries were used to extend the sequence. If more than
one extension
was necessary or desired, additional or nested sets of primers were designed.
High fidelity amplification was obtained by PCR using methods well known in
the art. PCR
was performed in 96-well plates using the PTC-200 thermal cycler (MJ Research,
Ine.). The reaction
mix contained DNA template, 200 nmol of each primer, reaction buffer
containing Mg2+, (NH4)ZS04,
and (3-mercaptoethanol, Taq DNA polymerase (Amersham Pharmacia Biotech),
ELONGASE enzyme
57
CA 02378989 2002-O1-03
WO 01/04308 PCT/US00/19014
(Life Technologies), and Pfu DNA polymerase (Stratagene), with the following
parameters for primer
pair PCI A and PCI B: Step 1: 94°C, 3 min; Step 2: 94°C, 15 sec;
Step 3: 60°C, 1 min; Step 4: 68°C,
2 min; Step 5: Steps 2, 3, and 4 repeated 20 times; Step 6: 68 °C, 5
min; Step 7: storage at 4°C. In the
alternative, the parameters for primer pair T7 and SK+ were as follows: Step
1: 94°C, 3 min; Step 2:
94°C, 15 sec; Step 3: 57°C, 1 min; Step 4: 68°C, 2 min;
Step 5: Steps 2, 3, and 4 repeated 20 times;
Step 6: 68 °C, 5 min; Step 7: storage at 4°C.
The concentration of DNA in each well was determined by dispensing 100 ~1
PICOGREEN
quantitation reagent (0.25% (v/v) PICOGREEN; Molecular Probes, Eugene OR)
dissolved in 1X TE
and 0.5 ~1 of undiluted PCR product into each well of an opaque fluorimeter
plate (Corning Costar,
Acton MA), allowing the DNA to bind to the reagent. The plate was scanned in a
Fluoroskan 1I
(I,absystems Oy, Helsinki, Finland) to measure the fluorescence of the sample
and to quantify the
concentration of DNA. A 5 ~1 to 10 ~1 aliquot of the reaction mixture was
analyzed by electrophoresis
on a 1 % agarose mini-gel to determine which reactions were successful in
extending the sequence.
The extended nucleotides were desalted and concentrated, transferred to 384-
well plates,
digested with CviJI cholera virus endonuclease (Molecular Biology Research,
Madison WI), and
sonicated or sheared prior to religation into pUC 18 vector (Amersham
Pharmacia Biotec;h). For
shotgun sequencing, the digested nucleotides were separated on low
concentration (0.6 to 0.8%) agarose
gels, fragments were excised, and agar digested with Agar ACE (Promega).
Extended clones were
religated using T4 ligase (New England Biolabs, Beverly MA) into pUC 18 vector
(Amersham
Pharmacia Biotech), treated with Pfu DNA polymerase (Stratagene) to fill-in
restriction site overhangs,
and transfected into competent E. coli cells. Transformed cells were selected
on antibiotic-containing
media, and individual colonies were picked and cultured overnight at
37°C in 384-well plates in LB/2x
carb liquid media.
The cells were lysed, and DNA was amplified by PCR using Taq DNA polymerase
(Amersham
Pharmacia Biotech) and Pfu DNA polymerase (Stratagene) with the following
parameters: Step 1:
94°C, 3 min; Step 2: 94°C, 15 sec; Step 3: 60°C, 1 min;
Step 4: 72°C, 2 min; Step 5: steps 2, 3, and 4
repeated 29 times; Step 6: 72°C, 5 min; Step 7: storage at 4°C.
DNA was quantified by PICOGREEN
reagent (Molecular Probes) as described above. Samples with low DNA recoveries
were reamplified
using the same conditions as described above. Samples were diluted with 20%
dimethysulfoxide (1:2,
v/v), and sequenced using DYENAMIC energy transfer sequencing primers and the
DYENAMIC
DIRECT kit (Amersham Pharmacia Biotech) or the ABI PRISM BIGDYE Terminator
cycle
sequencing ready reaction kit (PE Biosystems).
In like manner, the polynucleotide sequences of SEQ ID N0:3-4 are used to
obtain 5'
regulatory sequences using the procedure above, along with oligonucleotides
designed for such
58
CA 02378989 2002-O1-03
WO 01/04308 PCT/US00/19014
extension, and an appropriate genomic library.
VII. Labeling and Use of Individual Hybridization Probes
Hybridization probes derived from SEQ ID N0:3-4 are employed to screen cDNAs,
genomic
DNAs, or mRNAs. Although the labeling of oligonucleotides, consisting of about
20 base pairs, is
specifically described, essentially the same procedure is used with larger
nucleotide fragments.
Oligonucleotides are designed using state-of-the-art software such as OLIGO
4.06 software (National
Biosciences) and labeled by combining 50 pmol of each oligomer, 250 ~Ci of [y-
32P] adenosine
triphosphate (Amersham Pharmacia Biotech), and T4 polynucleotide kinase
(DuPont NEN, Boston
MA). The labeled oligonucleotides are substantially purified using a SEPHADEX
G-25 superfine size
exclusion dextran bead column (Amersham Pharmacia Biotech). An aliquot
containing 10' counts per
minute of the labeled probe is used in a typical membrane-based hybridization
analysis of human
genomic DNA digested with one of the following endonucleases: Ase I, Bgl II,
Eco RI, Pst I, Xba I, or
Pvu II (DuPont NEN).
The DNA from each digest is fractionated on a 0.7% agarose gel and transferred
to nylon
membranes (Nytran Plus, Schleicher & Schuell, Durham NH). Hybridization is
carried out for 16
hours at 40°C. To remove nonspecific signals, blots are sequentially
washed at room temperature
under conditions of up to, for example, 0.1 x saline sodium citrate and 0.5%
sodium dodecyl sulfate.
Hybridization patterns are visualized using autoradiography or an alternative
imaging means and
compared.
VIII. Microarrays
The linkage or synthesis of array elements upon a microarray can be achieved
utilizing
photolithography, piezoelectric printing (ink jet printing, See, e.g.,
Baldeschweiler, supra), mechanical
microspotting technologies, and derivatives thereof. The substrate in each of
the aforementioned
technologies should be uniform and solid with a non-porous surface (Schena
(1999), supra). Suggested
substrates include silicon, silica, glass slides, glass chips, and silicon
wafers. Alternatively, a procedure
analogous to a dot or slot blot may also be used to arrange and link elements
to the surface of a
substrate using thermal, UV, chemical, or mechanical bonding procedures. A
typical array may be
produced using available methods and machines well known to those of ordinary
skill in the art and may
contain any appropriate number of elements. (See, e.g., Schena, M. et al.
(1995) Science 270:467-470;
Shalom D. et al. (1996) Genome Res. 6:639-645; Marshall, A. and J. Hodgson
(1998) Nat. Biotechnol.
16:27-31.)
Full length cDNAs, Expressed Sequence Tags (SSTs), or fragments or oligomers
thereof may
comprise the elements of the microarray. Fragments or oligomers suitable for
hybridization can be
selected using software well known in the art such as LASERGENE software
(DNASTAR). The array
59
CA 02378989 2002-O1-03
WO 01/04308 PCT/US00/19014
elements are hybridized with polynucleotides in a biological sample. The
polynucleotides in the
biological sample are conjugated to a fluorescent label or other molecular tag
for ease of detection.
After hybridization, nonhybridized nucleotides from the biological sample are
removed, and a
fluorescence scanner is used to detect hybridization at each array element.
Alternatively, laser
desorbtion and mass spectrometry may be used for detection of hybridization.
The degree of
complementarity and the relative abundance of each polynucleotide which
hybridizes to an element on
the microarray may be assessed. In one embodiment, microarray preparation and
usage is described in
detail below.
Tissue or Cell Sample Preparation
Total RNA is isolated from tissue samples using the guanidinium thiocyanate
method and
poly(A)+ RNA is purified using the oligo-(dT) cellulose method. Each poly(A)+
RNA sample is
reverse transcribed using MMLV reverse-transcriptase, 0.05 pg/E.~l oligo-(dT)
primer (2lmer), 1X first
strand buffer, 0.03 units/lil RNase inhibitor, 500 pM dATP, 500 plVl dGTP, 500
NM dTTP, 40 l~M
dCTP, 40 NIVI dCTP-Cy3 (BDS) or dCTP-Cy5 (Amersham Pharmacia Biotech). The
reverse
transcription reaction is performed in a 25 ml volume containing 200 ng
poly(A)+ RNA with
GEMBRIGHT kits (Incyte). Specific control poly(A)+ RNAs are synthesized by in
vitro transcription
from non-coding,yeast genomic DNA. After incubation at 37 °C for 2 hr,
each reaction sample (one
with Cy3 and another with Cy5 labeling) is treated with 2.5 ml of O.SM sodium
hydroxide and
incubated for 20 minutes at 85 °C to the stop the reaction and degrade
the RNA. Samples are purified
using two successive CHROMA SPIN 30 gel filtration spin columns (CLONTECH
Laboratories, Ine.
(CLONTECH), Palo Alto CA) and after combining, both reaction samples are
ethanol precipitated
using 1 ml of glycogen (1 mg/ml), 60 ml sodium acetate, and 300 ml of 100%
ethanol. The sample is
then dried to completion using a SpeedVAC (Savant Instruments Inc., Holbrook
NY) and
resuspended in 14 l~l SX SSC/0.2% SDS.
Microarray Preparation
Sequences of the present invention are used to generate array elements. Each
array element is
amplified from bacterial cells containing vectors with cloned cDNA inserts.
PCR amplification uses
primers complementary to the vector sequences flanking the cDNA insert. Array
elements are
amplified in thirty cycles of PCR from an initial quantity of 1-2 ng to a
final quantity greater than 5
Ng. Amplified array elements are then purified using SEPHACRYL-400 (Amersham
Pharmacia
Biotech).
Purified array elements are immobilized on polymer-coated glass slides. Glass
microscope
slides (Corning) are cleaned by ultrasound in 0.1 % SDS and acetone, with
extensive distilled water
washes between and after treatments. Glass slides are etched in 4%
hydrofluoric acid (VWR
Scientific Products Corporation (VWR), West Chester PA), washed extensively in
distilled water, and
CA 02378989 2002-O1-03
WO 01/04308 PCT/US00/19014
coated with 0.05% aminopropyl silane (Sigma) in 95% ethanol. Coated slides are
cured in a 110°C
oven.
Array elements are applied to the coated glass substrate using a procedure
described in US
Patent No. 5,807,522, incorporated herein by reference. 1 irl of the array
element DNA, at an average
concentration of 100 ng/~.il, is loaded into the open capillary printing
element by a high-speed robotic
apparatus. The apparatus then deposits about 5 n1 of array element sample per
slide.
Microarrays are UV-crosslinked using a STRATALINKER UV-crosslinker
(Stratagene).
Microarrays are washed at room temperature once in 0.2% SDS and three times in
distilled water.
Non-specific binding sites are blocked by incubation of microarrays in 0.2%
casein in phosphate
buffered saline (PBS) (Tropix, Inc., Bedford MA) for 30 minutes at 60
°C followed by washes in
0.2% SDS and distilled water as before.
Hybridization
Hybridization reactions contain 9 Ed of sample mixture consisting of 0.2 pg
each of Cy3 and
Cy5 labeled cDNA synthesis products in SX SSC, 0.2% SDS hybridization buffer.
The sample
mixture is heated to 65 °C for 5 minutes and is aliquoted onto the
microarray surface and covered with
an 1.8 cm2 coverslip. The arrays are transferred to a waterproof chamber
having a cavity just slightly
larger than a microscope slide. The chamber is kept at 100% humidity
internally by the addition of
140 ~.xl of SX SSC in a corner of the chamber. The chamber containing the
arrays is incubated for
about 6.5 hours at 60°C. The arrays are washed for 10 min at 45
°C in a first wash buffer (1X SSC,
0.1% SDS), three times for 10 minutes each at 45 °C in a second wash
buffer (0.1X SSC), and dried.
Detection
Reporter-labeled hybridization complexes are detected with a microscope
equipped with an
Innova 70 mixed gas 10 W laser (Coherent, Inc., Santa Clara CA) capable of
generating spectral lines
at 488 nm for excitation of Cy3 and at 632 nm for excitation of CyS. The
excitation laser light is
focused on the array using a 20X microscope objective (Nikon, Inc., Melville
NY). The slide
containing the array is placed on a computer-controlled X-Y stage on the
microscope and raster-
scanned past the objective. The 1.8 cm x 1.8 cm array used in the present
example is scanned with a
resolution of 20 micrometers.
In two separate scans, a mixed gas multiline laser excites the two
fluorophores sequentially.
Emitted light is split, based on wavelength, into two photomultiplier tube
detectors (PMT 81477,
Hamamatsu Photonics Systems, Bridgewater NJ) corresponding to the two
fluorophores. Appropriate
filters positioned between the array and the photomultiplier tubes are used to
filter the signals. The
emission maxima of the lluorophores used are 565 nm for Cy3 and 650 nm for
CyS. Each array is
typically scanned twice, one scan per fluorophore using the appropriate
filters at the laser source,
although the apparatus is capable of recording the spectra from both
fluorophores simultaneously.
61
CA 02378989 2002-O1-03
WO 01/04308 PCT/US00/19014
The sensitivity of the scans is typically calibrated using the signal
intensity generated by a
cDNA control species added to the sample mixture at a known concentration. A
specific location on
the array contains a complementary DNA sequence, allowing the intensity of the
signal at that
location to be correlated with a weight ratio of hybridizing species of
1:100,000. When two samples
from different sources (e.g., representing test and control cells), each
labeled with a different
fluorophore, are hybridized to a single array for the purpose of identifying
genes that are differentially
expressed, the calibration is done by labeling samples of the calibrating cDNA
with the two
fluorophores and adding identical amounts of each to the hybridization
mixture.
The output of the photomultiplier tube is digitized using a 12-bit RTI-835H
analog-to-digital
(A/D) conversion board (Analog Devices, Inc., Norwood MA) installed in an IBM-
compatible PC
computer. The digitized data are displayed as an image where the signal
intensity is mapped using a
linear 20-color transformation to a pseudocolor scale ranging from blue (low
signal) to red (high
signal). The data is also analyzed quantitatively. Where two different
fluorophores are excited and
measured simultaneously, the data are first corrected for optical crosstalk
(due to overlapping
emission spectra) between the fluorophores using each fluorophore's emission
spectrum.
A grid is superimposed over the fluorescence signal image such that the signal
from each spot
is centered in each element of the grid. The fluorescence signal within each
element is then integrated
to obtain a numerical value corresponding to the average intensity of the
signal. The software used
for signal analysis is the GEMTOOLS gene expression analysis program (Incyte).
1X. Complementary Polynucleotides
Sequences complementary to the LIMD-encoding sequences, or any parts thereof,
are used to
detect, decrease, or inhibit expression of naturally occurring LIMD. Although
use of oligonucleotides
comprising from about 15 to 30 base pairs is described, essentially the same
procedure is used with
smaller or with larger sequence fragments. Appropriate oligonucleotides are
designed using OLIGO
4.06 software (National Biosciences) and the coding sequence of LIMD. To
inhibit transcription, a
complementary oligonucleotide is designed from the most unique 5' sequence and
used to prevent
promoter binding to the coding sequence. To inhibit translation, a
complementary oligonucleotide is
designed to prevent ribosomal binding to the LIMD-encoding transcript.
X. Expression of LIMD
Expression and purification of LIMD is achieved using bacterial or virus-based
expression
systems. For expression of LIMD in bacteria, cDNA is subcloned into an
appropriate vector containing
an antibiotic resistance gene and an inducible promoter that directs high
levels of cDNA transcription.
Examples of such promoters include, but are not limited to, the trp-lac (tae)
hybrid promoter and the
TS or T7 bacteriophage promoter in conjunction with the lac operator
regulatory element.
Recombinant vectors are transformed into suitable bacterial hosts, e.g.,
BL21(DE3). Antibiotic
62
CA 02378989 2002-O1-03
WO 01/04308 PCT/US00/19014
resistant bacteria express LIMD upon induction with isopropyl beta-D-
thiogalactopyranoside (IPTG).
Expression of LIMD in eukaryotic cells is achieved by infecting insect or
mammalian cell lines with
recombinant Auto~raphica californica nuclear polyhedrosis virus (AcMNPV),
conmioNy known as
baculovirus. The nonessential polyhedrin gene of baculovirus is replaced with
cDNA encoding LIMD
by either homologous recombination or bacterial-mediated transposition
involving transfer plasmid
intermediates. Viral infectivity is maintained and the strong polyhedrin
promoter drives high levels of
cDNA transcription. Recombinant baculovirus is used to infect Spodoptera
fru~iperda (Sf9) insect
cells in most cases, or human hepatocytes, in some cases. Infection of the
latter requires additional
genetic modifications to baculovirus. (See Engelhard, E.K. et al. (1994) Proc.
Natl. Acad. Sci. USA
91:3224-3227; Sandig, V. et al. (1996) Hum. Gene Ther. 7:1937-1945.)
In most expression systems, LIMD is synthesized as a fusion protein with,
e.g., glutathione S-
transferase (GST) or a peptide epitope tag, such as FLAG or 6-His, permitting
rapid, single-step,
affinity-based purification of recombinant fusion protein from crude cell
lysates. GST, a 26-kilodalton
enzyme from Schistosoma laponicum, enables the purification of fusion proteins
on immobilized
glutathione under conditions that maintain protein activity and antigenicity
(Amersham Pharmacia
Biotech). Following purification, the GST moiety can be proteolytically
cleaved from LIMD at
specifically engineered sites. FLAG, an 8-amino acid peptide, enables
immunoaffinity purification
using commercially available moncxlonal and polyclonal anti-FLAG antibodies
(Eastman Kodak). 6-
His, a stretch of six consecutive histidine residues, enables purification on
metal-chelate resins
(QIAGEN). Methods for protein expression and purification are discussed in
Ausubel (1995, supra,
ch. 10 and 16). Purified LIMD obtained by these methods can be used directly
in the assays shown in
Examples XI and XV.
XI. Demonstration of LIMD Activity
The binding of Znz+ to LIMD is assayed by monitoring the resulting changes in
enthalpy (heat
production or absorption) in an isothermal titration microcalorimeter (Micro-
Cal Inc., Northampton,
MA). Titration microcalorimetry measurements do not require labeling of the
ligand or receptor
molecules; detection is based solely on the intrinsic change in the heat of
enthalpy upon binding.
Multiple computer-controlled injections of a known volume of ZnCl2 solution
are directed into a
thermally-controlled chamber containing LIMD. The change in enthalpy after
each injection is plotted
against the number of injections, producing a binding isotherm. The volumes
and concentrations of the
injected ZnCl2 solution and of the LIMD solution are used along with the
binding isotherm to calculate
values for the number, affinity, and asscx;iation constant of L1MD with the
Zn2+ ligand.
XII. Functional Assays
LIMD function is assessed by expressing the sequences encoding LIMD at
physiologically
63
CA 02378989 2002-O1-03
WO 01/04308 PCT/US00/19014
elevated levels in mammalian cell culture systems. cDNA is subcloned into a
mammalian expression
vector containing a strong promoter that drives high levels of cDNA
expression. Vectors of choice
include pCMV SPORT plasmid (Life Technologies) and pCR3.1 plasmid
(Invitrogen), both of which
contain the cytomegalovirus promoter. 5-10 ~cg of recombinant vector are
transiently transfected into a
human cell line, for example, an endothelial or hematopoietic cell line, using
either liposome
formulations or electroporation. 1-2 ~cg of an additional plasmid containing
sequences encoding a
marker protein are co-transfected. Expression of a marker protein provides a
means to distinguish
transfected cells from nontransfected cells and is a reliable predictor of
cDNA expression from the
recombinant vector. Marker proteins of choice include, e.g., Green Fluorescent
Protein (GFP;
Clontech), CD64, or a CD64-GFP fusion protein. Flow cytometry (FCM), an
automated, laser optics-
based technique, is used to identify transfected cells expressing GFP or CD64-
GFP and to evaluate the
apoptotic state of the cells and other cellular properties. FCM detects and
quantifies the uptake of
fluorescent molecules that diagnose events preceding or coincident with cell
death. These events include
changes in nuclear DNA content as measured by staining of DNA with propidium
iodide; changes in
cell size and granularity as measured by forward light scatter and 90 degree
side light scatter; down-
reguladon of DNA synthesis as measured by decrease in bromodeoxyuridine
uptake; alterations in
expression of cell surface and intracellular proteins as measured by
reactivity with specific antibodies:
and alterations in plasma membrane composition as measured by the binding of
fluorescein-conjugated
Annexin V protein to the cell surface. Methods in flow cytometry are discussed
in Ormerod, M.G.
(1994) Flow C ometry, Oxford, New York NY.
The influence of LIMD on gene expression can be assessed using highly purified
populations of
cells transfected with sequences encoding LIMD and either CD64 or CD64-GFP.
CD64 and CD64-
GFP are expressed on the surface of transfected cells and bind to conserved
regions of human
immunoglobulin G (IgG). Transfected cells are efficiently separated from
nontransfected cells using
magnetic beads coated with either human IgG or antibody against CD64 (DYNAL,
Lake Success NY).
mRNA can be purified from the cells using methods well known by those of skill
in the art. Expression
of mRNA encoding LIMD and other genes of interest can be analyzed by northern
analysis or
microarray techniques.
XIII. Production of L1MD Specific Antibodies
LIMD substantially purified using polyacrylamide gel electrophoresis (PAGE;
see, e.g.,
Harrington, M.G. (1990) Methods Enzymol. 182:488-495), or other purification
techniques, is used to
immunize rabbits and to produce antibodies using standard protocols.
Alternatively, the LIMD amino acid sequence is analyzed using LASERGENE
software
(DNASTAR) to deternune regions of high immunogenicity, and a corresponding
oligopeptide is
64
CA 02378989 2002-O1-03
WO 01/04308 PCT/US00/19014
synthesized and used to raise antibodies by means known to those of skill in
the art. Methods for
selection of appropriate epitopes, such as those near the C-terminus or in
hydrophilic regions are well
described in the art. (See, e.g., Ausubel, 1995, su ra, ch. 11.)
Typically, oligopeptides of about 15 residues in length are synthesized using
an ABI 431A
peptide synthesizer (PE Biosystems) using FMOC chemistry and coupled to KLH
(Sigma-Aldrich, St.
Louis MO) by reaction with N-maleimidobenzoyl-N-hydroxysuccinimide ester (MBS)
to increase
immunogenicity. (See, e.g., Ausubel, 1995, supra.) Rabbits are immunized with
the oligopeptide-KLH
complex in complete Freund's adjuvant. Resulting antisera are tested for
antipeptide and anti-LIMD
activity by, for example, binding the peptide or LIMD to a substrate, blocking
with 1 % BSA, reacting
with rabbit antisera, washing, and reacting with radio-iodinated goat anti-
rabbit IgG.
XIV. Purification of Naturally Occurring LIMD Using Specific Antibodies
Naturally occurring or recombinant LIMD is substantially purified by
immunoaffinity
chromatography using antibodies specific for LIMD. An immunoaflinity column is
constructed by
covalently coupling anti-LIMD antibody to an activated chromatographic resin,
such as
CNBr-activated SEPHAROSE (Amersham Pharmacia Biotech). After the coupling, the
resin is
blocked and washed according to the manufacturer's instructions.
Media containing LIMD are passed over the immunoaffinity column, and the
column is washed
under conditions that allow the preferential absorbance of LIMD (e.g., high
ionic strength buffers in the
presence of detergent). The column is eluted under conditions that disrupt
antibody/LIMD binding
(e.g., a buffer of pH 2 to pH 3, or a high concentration of a chaotrope, such
as urea or thiocyanate ion),
and LIMD is collected.
XV. Identification of Molecules Which Interact with LIMD
LIMD, or biologically active fragments thereof, are labeled with'zsI Bolton-
Hunter reagent.
(See, e.g., Bolton A.E. and W.M. Hunter (1973) Biochem. J. 133:529-539.)
Candidate molecules
previously arrayed in the wells of a mufti-well plate are incubated with the
labeled LIMD, washed, and
any wells with labeled LIMD complex are assayed. Data obtained using different
concentrations of
LIMD are used to calculate values for the number, aftinity, and association of
LIMD with the candidate
molecules.
Alternatively, molecules interacting with LIMD are analyzed using the yeast
two-hybrid
system as described in Fields, S. and O. Song (1989, Nature 340:245-246), or
using commercially
available kits based on the two-hybrid system, such as the MATCHMAKER system
(Clontech).
LIMD may also be used in the PATHCALLING process (CuraGen Corp., New Haven CT)
which employs the yeast two-hybrid system in a high-throughput manner to
determine all interactions
between the proteins encoded by two large libraries of genes (Nandabalan, K.
et al. (2000) U.S. Patent
CA 02378989 2002-O1-03
WO 01/04308 PCT/US00/19014
No. 6,057,101 ).
Various modifications and variations of the described methods and systems of
the invention will
be apparent to those skilled in the art without departing from the scope and
spirit of the invention.
Although the invention has been described in connection with certain
embodiments, it should be
understood that the invention as claimed should not be unduly limited to such
specific embodiments.
Indeed, various modifications of the described modes for carrying out the
invention which are obvious
to those skilled in molecular biology or related fields are intended to be
within the scope of the following
claims.
66
CA 02378989 2002-O1-03
WO 01/04308 PCT/US00/19014
N
O
H
O fx
z
w
z
0 0
U o0
O -- o~
x x ~ w w x
~ ~r ._ ~ o
~
H r1 U1 M lD
H
N O r-i O1
O lfl 00
O M E-~ M CO
d~ O
E-~ O O N M
d'
00
O O z O~ N
O O
M V~ Ul N M
~
O
W . p.,' .
W .-. .-.
a V' 'r M M
N r1 H
O O r1 O O
O
H H
H O fs-~ O
O O W
x z ~ z z w
z ~
00 r-i Ei
E-~ U H U~
Cia
~
o p,' ~ P: W
O E-~
m P4 oo CO W
U PO
M
U1 r-I
r-I <-i r-1
10 lO r1
x w . x x H
x
N d~ ~ O r-1
OO d~
r-I M N l0
l0 00 O
.-I
O M O 01 O
O l~ O
00 E-Wn O
V' V' O
Q l0 ,~ 00 O
~ M ~'
01 [-i L~
O N M l0
r-i H N M M
~ Lf7
'J
a -. w -.
w-W W -I .-I
-i e-I M
U7 O O O O O N
1.W -i r1 H
[~
[~
fx P: v~ O
cn W O
O
H
N ~ L~ U' U
~ fk
~ ~
d; f0 N ~I7
W R: O
a x .o a a
a~ oa
Cia M 00
N c-I
~-I H P~,
O
z H
w
z x
a o H
U
W d~ d~
H r1 O
O O O
O
O
O lD
U
'd
O
~~
z
q M d~
r~
va
~w
z~
~o
.,~
z
l~ r1 N
q
O
~'
a
~''
w
67
CA 02378989 2002-O1-03
WO 01/04308 PCT/US00/19014
x cn x m
x >~ x
'Lf c0 ~
U ~ U
' ~ O
r0 ~ ~
c~ G ~ U U
cn
U N N C7-~ P~1 N C=.~ W U1
u~
-r-i C7 L4 I C7 W I W
U1 W
U7
~ ~S I v1 l u1 I u1 I cn a
a
O ,~ E-~ Cr-. E-~ LT-n R: CL
R: W H H
~
r-1 U2 H W ~i U) H W ,~," Cin
.~ G4 U
~
c0 ry' E-~ FC E-~ H O
J-1 H O U1
J-~
a~ a o ~ a a o ~ a x
~a x w
~ ca oa z x w w z x on w
w u~
z a
v H -r-I
a ro
U U1 ~ U7 U G U7
U7 ~-i >~ s~ ~ O ~ s~ -rl
~ T5
~ -
U
x - ~ ~
.
N ~ -
N fs~ C~ +~ >~ DC CL O f.~,
O N r-I r6 Wit'O -rl f~ f~ 00
N 'Li N N
b1 rtf ~ U1 S-W', LL N l0
U N i~ N N
O ~ J-1 -.-i ~
N C~ --i N S-I -r1
01
rl N U O M b rt1 O O~ J ~
N ~ N O d~
r~ 1-1 .~.,W l~ .~., N I C71
tf1 J-1 ~,, N
N ~'., O ,~', >~ ''C O Ol
O 00 ~ ~ O O ~O
O N x H ~-I O O >~ x d~ H -rl
x N ~-1 x N
x ~n a:n a a. a U ~ a ~ a ~I
~ ~ a~ ~ ~
ui
w
.r., ..
v
o Zs
z " ~w
U7 N
1-1
~ O ~
-i ~ Ln
~ N
N U7 ~ .,-
-r-I -ri -~
i-1 N ~ ~ U ~
N R~ N
rtf
~ O p I I o~ -I
~1 p
,~ ,
'~ N O ~ '~
~
1
r
~ C7 U U ~ U
~ ~
-r-I H -.-I H
N
~
v~ a ~n a
u~
~a
N
0
-,
o
c~
.~,
s~
o
m
N U
N
J-1 N
'y
1~
O r-I .-I
-ri
w cWn z
N
N 01 N
O
J~ r1 N 01
'.-I E-~ N .--i d' l~ 00
U1 ~C1 d~ d~ .-I ~i'
M ~-I U1 H N U7
'~', 00 CO Ul
O E-a ri N d'
-r1 l0 10 V' N O
11 O r1 M Lf1 l0 ~
c(f l0 r-i U1 W .-i V7
r-1 [~ U7 U1
r-1 O u-i L~ OO
(a L~ O 1D II7 00 i.!1
10
-ri v-i r1 N M ~ V~ N
O
A-1 U1 W Ul U1 Ul r-I CI1
,s;
H
N UI d~ N d~ ri ~D t!7 l0
L1 l0 LI7 01 l0 l0 OD N 01
O
O ~ r1 r1 d' N dW0 N d'
W W v1 U1 UW U1 E~ U1 U1
N
U N
00 r-1
O -~ o0
.-I u1
-r1
N
Ca
H
N
r1 N
W
1~
68
CA 02378989 2002-O1-03
WO 01/04308 PCT/US00/19014
~'
O U U
z z
U H H
f.~ CA
Ln M
N
O O
v v
O ~ N
--
-ri ~ N >~
r-I N
rtf O M O M
O J-1 l~
O O
H (~
'-'
O S-1 ~-I
U O G N ~
4-I
I O 4--t
O
~.I -ri -r1
-rl -r-I
O r-i -1
f~ i i-~
m
O O ~0 O N
N ~.I ~-I
-ri
U7 W W
t~
to t0
U
N r1 ~--I
~ r~ .-I
U7 r1 r-I
~-I 4~ W
-ri N ~ N >~
fs~
f~ U H U H
~
.-.
M
O r1 d~
.-~ Lf~
l0 r.
O .-I N M
O
M ~ o, o
.
v
(IS N O O N
O . ~ .-.-
~ ,H
~1 -ri O fij O
O
~1 co
C.' ~.I
,-
N N r~ r1
W l~ N
~I ~ U v
O
. o U
N
>~ 1~ v (R
U7 +~
1~
W U c~ to
O G U
-ri ~ ,~ U7
-r-I ,7
N CS ~ O
1~ O ''O
O
U O -ri O -.-t
~I O
~
N ~ ~ f~
~ N
wl N cti N cti
C~ c~ N
H x U z U
-- c7 x
O
U
s~
to
N
1~
b'
N M
O
U1 O d'
r1
tn N N
c0 d~ ~ <i
''O
~-I I I I
-r-I
fs.~~O o a~
U
Q,' O o0
l0
d~ M ri
N rt
U
1~
-ri
U
N
N
ri
r1
U
N
~n
z
ro
~
o
o
z
q M d~
U
H
as
~,
w
0
w
69
CA 02378989 2002-O1-03
WO 01/04308 PCT/US00/19014
a~
v
f~
~' o ~
~
-
~a
~ ,
N ~r cn E-~ >~
o ~ 3
~
~
N N -~ .y,
t
U ,~ ~ O
U7 1--i r1 O U1 r-1
f~ ca
U1 N ctf 1-I O
~t v -r-I
-.-i -r1 U ~ ~
U r-1 U7 >~
1> >~ S.I N ~-i
-rl a N S-a
~ S-i O ~0
S-i N
' W
f~ 'LS ~ 1~
W
W ~i O (1l r-I
J.1 r1
~ x
U7 ~ ,~ v -.
.11 ~ S-I
~., -ri ~1 ,7 -.~
1-)
O 1~ N rl 1-I S-W
-r-I G rtS N N
-r-1 IO N
-r-I
1~ r-i -rl G ~ U
>~, 'J
O U ~ ?-i -.-I O
O
N U N b~ '~ ''O
N '~
U7 -r-1 J-! r0 ~ ~
.~ N
N 1~ ~ 5 N N Ei
'L5
~ o ~
S-i - N b~
w N '~ w G U1 O -rl
~
'-d -ri ''C ~ ~ -
''d ~ 'r
N ~ O ~ N O ?-i
a S~ a ro ~ '~
'~ -~I O
cd cti N O N
~
r-I r-I r-I N ((S
-r-I r-I
U1
O C~ O rl ~ w ..-i
U
U7 G U7 c~ w -r1
~ N ,.i~''
S~
S-I N 'LS ~
~
't3 S. s~ '~ N N
a
O ~'-, -r-1
-rl 1J -rl
!U .L~ !!j f~ ~ 1J
b~ r1 C3~ -~ +~
u~ N
~
O
-~I ~ -.
,~ -~ cd
N N
U7 QJ U1 U U 3
4a 1-~ ~ ~ c<5 Sa
'~
U
N ~ -~ ~ O ''C
N
~ v
~ 0.i ~ O ?-I ~
~ N
~ ~
r-I ~
t~ ~
U7 (d U7 ~ N cff
~-1 U7 r~ ca
~
U -~I ~ N Sa U U7
N rti r1
O rtS O ?, U -rl
'O -.-I ~ G~,
'~ U r
-
~ l U1 1J N
fir
W O S-I U7 V~ N S-I
-r-I N
.1J (~ I N UI U
r1
3 3
~ -~
~
N f~ ~, ~ U 1~
-rl
O
N ,~ ~ N N
N ~
U ~I N S-I w .r1
.~ 'd o -r1
Ln ''t~ ..Q ~-1 ~,'
1.-1 4-I
O
-r-I -r-I ''~ 1W
r-I 0 Qi -ri
N
?-t r-I c~ r-I N N -rl
(<j U G
'ZS
~ 0
~
U7 ~ UI O O O O
1~ r C
-I .2~ -
,f~ -~I O -~ ~ fo Ti
s~ U u7 m G
-.-1.C ~I ,~ N >~ ~
N S~' U7 ~., N
a H ~ ~ H ~ a~ a~
.~I
N r-1
O O
S-i H fx
c0 O
z H
w
z x
a o H
U
N
'C5
O
O
z
q M d~
U
H
a
~
w
0
w
CA 02378989 2002-O1-03
WO 01/04308 PCT/US00/19014
y L
II as ° v ~ C
Yin
L
° ~ C L. ~n O O
II P. ~ 'b ° ~ a~ II ~ ~''~ d O.
H _ ~
'~f' S V7 O Y ~ ° ~f ~ it ~ .~ O W '
O c0 V ~ II ~ ,a ~ U OD ~ C U --~
.G > 3; N 4. 'D W ~ 4. y ~ . cue, >_
0
y _Y O iG 5 ~ O ~ S ° dA C_l7 d U
E, ,°~ ~ ~ W ~ y N o ° ~..' w 'b '° c
L V .~ ,~.. W y "C3 N bU O .~.. N p ~ .b Y ~ °
U Gs~-i O w.pa0y>~O Or+vl,~~. O~Gw
e~~0 ~ ~~~7 Er°~' ovXay
A, ~ ~.~tl o ~Y > W ~ ~ ~ w ~i w r~ ~ ~ P; v~ ' 7
U
U G ,_~ N '~ n
~ 00
A"' '~' OW' Z C'7 p~ ca
0'10 Gr ~ ~ ~ ,~ ~ ~ ~ N
p _ N
~_~o~o ~ ~w ~ ~N
N " ~ ~, ~ .~ N N ~, ~ "'.a p
d Nd d ~ ~ C N ~ N c,.., ' G G ~ f~ W M
U U U ~ M ~ ~ ~ ~s ~ o ~ W x ~ o ~ 'o
y ~Q y ~w~ ~.~ N3~ xxb~,~ N
U o v~ c~~ ;.~ c'! c v~ ~ ~ N s_° b 'Y' ~ i
°~ ~ ~i ~''. ~ W ~ ~ x' ~ °' 3 v? ~ G,'
v~ ~ N in 'r U N ~ d b U' ~ ~ .~ . 0v G' b
w° w ~ w ~Qrx ~~_ ~ '~ ~ '~~~-d °" ~'~!'-d
~~ U
Pr U ~! ~ cC °~ ~~ ccf O cC M .
(~ O .b ~'7 V7 ~ E-r
:~ :~ ~j a~.n ,-, U ~ O . Cn . c~'~, ~n U
U rn v~ C v~ VJ ~f' Q c~C O~ y G1 w O O 4-, .~ O
C ~ cn ~ ~ ~ th U ~ U ~ CY w ~ ;~C , ~ d O z
O_ O U O ~p~~ °Q~~d .Wn ~~ ~.-~-X00
w ~ ~ ~ ~ ~ v-, v 'c~ Y ~ .b > c b x ~ ~ o v-i ~
a~ ~., ~ ~c b a~ U
x ~ a a~, a aNz wz3 ~d xd~NU
N . . . w
o c, ~ 'n ~ y '~ o d
~n U c~ 4. G ~ U bA v~
U N U ~ ~ ~ y cC ~ O ~ U w
O p' ~ cC 4." O U ~ >?. w y ~ O b
U bIJ ~ v~ O 'U ~ ~ N C O .J _U ~ ~ ~ O cd U
~b C w .d c~ ~ L ~ ~ U c3 Gw >, ~ ~ cOn
V O O U ~ W n _~ ~ 'n N v~
O' ~ U ~ O G ~ ~ ~ (%~ '+" ~ ~r b ~ i-y~0 U
° ~ " U V7 iC ~y O Q y y.., U ~ ~ ~~ ~ N
~s ~ o-
o T y.., w
7., _N ~ U~~ ~ N _~~ ~~Cb U O
C v~
j N ~ ,.O ~° ~ ~ '~ v~ O a' N ~ ~ U ~ ~ U v cOn
O~ OU ~ ~mV_c~ ~~~,w ,bO ~ cwt'.
N ~ ~ ~~ ~ ~ ~ ~ O ~ ~ > i
c°c '° d~~a-L'y a3y~° rs:cA._~?.~ who
b.a ~~0~ ~.bD~~~ ~~>,
r-a .N yn p T N a7 ,~G N p..' w p
o. cn ~ ,~ y b7~ .~ c U o ~ '~ c > .= o c O ~ .~. a
.v O .x ~ O o c~a ~ .,~ .v a> a w c~ a ~ ~ 0~0 a~ ~ ~O .~?
o. ~ w G a, a7 a~ U ~ w y ~. ~ ~ w Q. p L on
L1 Q ~ d ~ d d ' ~ w d '~ °~' u? ~ °' ° .~ '"
Q ~n A w w Q ~ a.
w
a
Q
U c~
ud, Q Q ~ d
E
c~ Q ~ ~ rya w ~ x
71
CA 02378989 2002-O1-03
WO 01/04308 PCT/US00/19014
C7 ~a
U ~
L
n1 w
cC
b OU G L by
N
~
>
T
Vi i ~ V _
a-.
~r '~ ~
(W
~
O
E~ ' 1-1 O s'.. L
O
V
x L ~ Y O
~ O
y b cc N M
_~'
II II
CC ~'. V LU.i U i.V.
U N
,
w
-
~
z ~
~ '
~
~ '~~~ G
~
_ w.,
d ~ R./,
~
.
v0 Gv1 p G c
a
y
W~ . pp ~ ~ ~ ~
pp b_ ~
G
G1 ~ G Ov h C G ~
V
w V OL
~,, ~ ~ y w
'-i V C. (~ ""' V V
N ~
V y, c~ C ~i
, o yd ~= ~~3
U~ G ~
. 'n
~~ ca ~0.'7 0 ~ ~
,D ~? ' a
c~
y . C
'
L a\ d, a1 ~
U ~ ,~
CC
CC
N Ov FG ~ ~ V O~ m O~ G
~ > ~ O~ ,
. ~
4 N Ow -
. , O
c~ i~ G ~., pp ~ G _ ~ _ ~
y xN ' ~ '~ ~
~ ~' ~ i '~
a
a
~ ~ ~ ~ i~
~r 3 ~
~ '''
w o ~a; ~~ ~ >
~
> >~Q ~~ ~ u: ~ c ~1~ x
4 c3
c _ ~ ~; ~ ' .~
a
O ~ ~v, ~ ~ ~ y ~ ~ 'U 00 O v0 U "~~
~ N 00 Y ,O ~ G CL
: o~ . D a o N
~ ~ .~
~ o
a , ~ ~ o ~ d ,'
o r',
3
Ix C7 C7 W oo v~ d 3 C7 Z ~ <x1
Z ... ~ v~ R: U c~
~ U
c
b
'
.D .n ~ ~G o o w
' ~ i
i
a
aS ~ ~ V ~ r3 b-0 ~ T C C~
' V
y C m U G
s.. C y i~ 0 ~ C
' c~
. > ~ ~ ~ sN. b
C
~ D'
~(/ ~ cVCC
N~
N .-. ~ ~
~
L p~ ~ C ~ ~ ,~ ~ ~ w C
p,r ~ G
G c0 ~ ~ ~ w ~ dA ~ O b
N
C ~p '~'' ~, G. O G O. N
N ~ U C
. S V N i N
p y ~ N ~
G ~ ~ > n
4~ . '~'
1 ~ d
-, ~ N V 'O
0
G 3
~ ~
Y w ~ ~a ~ 'b o ~ k
~.~., o ~
on .
o c ~ ~ .c o ~ o
~' U p
.
o ~ 8 0 ~ , ' ~ ~
~ ~
~
. ~ ~ . ~ ~ s
:r ~ ~ .
Ow O V V V '-' ~ ~ ~ ~ _. ~ ~ yn
~ ~ ~ N
6
~ .
N N ~ N ~. cd 'i~ O
~ ~ a . ~ N .b
~- ~- ~ ~- O
o
G .
G1 d ~ ~ d ~' d v~ ~ d ~ d ~ d n.
n. ~ ~ a.
c
U
8
W ~ c~ ~ U
0.~ c~: G~..~ ~ U
72
CA 02378989 2002-O1-03
WO 01/04308 PCT/US00/19014
SEQUENCE LISTING
<110> INCYTE GENOMICS, INC.
TANG, Y. Tom
YUE, Henry
AZIMZAI, Yalda
<120> HUMAN LIM DOMAIN PROTEINS
<130> PF-0717 PCT
<140> To Be Assigned
<141> Herewith
<150> 60/143,426
<151> 1999-07-13
<160> 6
<170> PERL Program
<210> 1
<211> 188
<212> PRT
<213> Homo sapiens
<220>
<221> misc_feature
<223> Incyte Clone No: 4084014CD1
<400> 1
Met Gln Pro Pro Ala Glu Pro Val Ser Gly Ala Ile Gln Leu Leu
1 5 10 15
Pro Ser Ala Gly Asp Gln Asn Val Glu Tyr Lys Gly Thr Val Trp
20 25 30
His Lys Asp Cys Phe Thr Cys Ser Asn Cys Lys Gln Val Ile Gly
35 40 45
Thr Gly Ser Phe Phe Pro Lys Gly Glu Asp Phe Tyr Cys Val Thr
50 55 60
Cys His Glu Thr Lys Phe Ala Lys His Cys Val Lys Cys Asn Lys
65 70 75
Ala Ile Thr Ser Gly Gly Ile Thr Tyr Gln Asp Gln Pro Trp His
80 85 90
Ala Asp Cys Phe Val Cys Val Thr Cys Ser Lys Lys Leu Ala Gly
95 100 105
Gln Arg Phe Thr Ala Val Glu Asp Gln Tyr Tyr Cys Val Asp Cys
110 115 120
Tyr Lys Asn Phe Val Ala Lys Lys Cys Ala Gly Cys Lys Asn Pro
125 130 135
Ile Thr Gly Phe Gly Lys Gly Ser Ser Val Val Ala Tyr Glu Gly
140 145 150
Gln Ser Trp His Asp Tyr Cys Phe His Cys Lys Lys Cys Ser Val
155 160 165
Asn Leu Ala Asn Lys Arg Phe Val Phe His Gln Glu Gln Val Tyr
170 175 180
Cys Pro Asp Cys Ala Lys Lys Leu
185
1/6
CA 02378989 2002-O1-03
WO 01/04308 PCT/US00/19014
<210> 2
<211> 571
<212> PRT
<213> Homo Sapiens
<220>
<221> misc_feature
<223> Incyte Clone No: 5640004CD1
<400> 2
Met Lys Tyr Leu Arg Gln Gln Ser Leu Pro Pro Pro Lys Phe Thr
1 5 10 15
Ala Thr Val Glu Thr Thr Ile Ala Arg Ala Ser Val Leu Asp Thr
20 25 30
Ser Met Ser Ala Gly Ser Gly Ser Pro Ser Lys Thr Val Thr Pro
35 40 45
Lys Ala Val Pro Met Leu Thr Pro Lys Pro Tyr Ser Gln Pro Lys
50 55 60
Asn Ser Gln Asp Val Leu Lys Thr Phe Lys Val Asp Gly Lys Val
65 70 75
Ser Val Asn Gly Glu Thr Val His Arg Glu Glu Glu Lys Glu Arg
80 85 90
Glu Cys Pro Thr Val Ala Pro Ala His Ser Leu Thr Lys Ser Gln
95 100 105
Met Phe Glu Gly Val Ala Arg Val His Gly Ser Pro Leu Glu Leu
110 115 120
Lys Gln Asp Asn Gly Ser Ile Glu Ile Asn Ile Lys Lys Pro Asn
125 130 135
Ser Val Pro Gln Glu Leu Ala Ala Thr Thr Glu Lys Thr Glu Pro
140 145 150
Asn Ser Gln Glu Asp Lys Asn Asp Gly Gly Lys Ser Arg Lys Gly
155 160 165
Asn Ile Glu Leu Ala Ser Ser Glu Pro Gln His Phe Thr Thr Thr
170 175 180
Val Thr Arg Cys Ser Pro Thr Val Ala Phe Val Glu Phe Pro Ser
185 190 195
Ser Pro Gln Leu Lys Asn Asp Val Ser Glu Glu Lys Asp Gln Lys
200 205 210
Lys Pro Glu Asn Glu Met Ser Gly Lys Val Glu Leu Val Leu Ser
215 220 225
Gln Lys Val Val Lys Pro Lys Ser Pro Glu Pro Glu Ala Thr Leu
230 235 240
Thr Phe Pro Phe Leu Asp Lys Met Pro Glu Ala Asn Gln Leu His
245 250 255
Leu Pro Asn Leu Asn Ser Gln Val Asp Ser Pro Ser Ser Glu Lys
260 265 270
Ser Pro Val Thr Thr Pro Phe Lys Phe Trp Ala Trp Asp Pro Glu
275 280 285
Glu Glu Arg Arg Arg Gln Glu Lys Trp Gln Gln Glu Gln Glu Arg
290 295 300
Leu Leu Gln Glu Arg Tyr Gln Lys Glu Gln Asp Lys Leu Lys Glu
305 310 315
Glu Trp Glu Lys Ala Gln Lys Glu Val Glu Glu Glu Glu Arg Arg
320 325 330
Tyr Tyr Glu Glu Glu Arg Lys Ile Ile Glu Asp Thr Val Val Pro
335 340 345
Phe Thr Val Ser Ser Ser Ser Ala Asp Gln Leu Ser Thr Ser Ser
350 355 360
Ser Met Thr Glu Gly Ser Gly Thr Met Asn.Lys Ile Asp Leu Gly
365 370 375
2/6
CA 02378989 2002-O1-03
WO 01/04308 PCT/US00/19014
Asn Cys Gln Asp Glu Lys Gln Asp Arg Arg Trp Lys Lys Ser Phe
380 385 390
Gln Gly Asp Asp Ser Asp Leu Leu Leu Lys Thr Arg Glu Ser Asp
395 400 405
Arg Leu Glu Glu Lys Gly Ser Leu Thr Glu Gly Ala Leu Ala His
410 415 420
Ser Gly Asn Pro Val Ser Lys Gly Val His Glu Asp His Gln Leu
425 430 435
Asp Thr Glu Ala Gly Ala Pro His Cys Gly Thr Asn Pro Gln Leu
440 445 450
Ala Gln Asp Pro Ser Gln Asn Gln Gln Thr Ser Asn Pro Thr His
455 460 465
Ser Ser Glu Asp Val Lys Pro Lys Thr Leu Pro Leu Asp Lys Ser
470 475 480
Ile Asn His Gln Ile Glu Ser Pro Ser Glu Arg Arg Lys Ser Ile
485 490 495
Ser Gly Lys Lys Leu Cys Ser Ser Cys Gly Leu Pro Leu Gly Lys
500 505 510
Gly Ala Ala Met Ile Ile Glu Thr Leu Asn Leu Tyr Phe His Ile
515 520 525
Gln Cys Phe Arg Cys Gly Ile Cys Lys Gly Gln Leu Gly Asp Ala
530 535 540
Val Ser Gly Thr Asp Val Arg Ile Arg Asn Gly Leu Leu Asn Cys
545 550 555
Asn Asp Cys Tyr Met Arg Ser Arg Ser Ala Gly Gln Pro Thr Thr
560 565 570
Leu
<210> 3
<211> 1284
<212> DNA
<213> Homo Sapiens
<220>
<221> misc_feature
<223> Incyte Clone No: 4084014CB1
<400> 3
cgtatatatg cgtacacata tatgctcgca cacacgcaca cactcggaga ctaaagaaca 60
ctggcgagaa cagcctgtgg caacagaatg aagtgaacag tatgtagcgc tttctcattt 120
gggcgtagta agtgatgaaa gcatgcttct tcctcagggt gtcattctgg gccaggcagt 180
ccctgattta atgtctaagt gcacgcaggg tatagaggtg ggggagtggg ggattcaggc 240
actggatcct aaaataataa tgctggggtc cccacccatg acagaaatcc tgggttggca 300
caagcacaag tagaacacag gtaggttagt tggaggtgtg aggccagtaa ctgcagggcc 360
tgcatcccct cacctctgga gggcctgggg aggggagctg agtggatgca gccccctgca 420
gagcctgtca gtggggctat ccaattgctt ccctctgcag gagatcaaaa cgtggagtac 480
aaggggaccg tctggcacaa agactgcttc acctgtagta actgcaagca agtcatcggg 540
actggaagct tcttccctaa aggggaggac ttctactgcg tgacttgcca tgagaccaag 600
tttgccaagc attgcgtgaa gtgcaacaag gccatcacat ctggaggaat cacttaccag 660
gatcagccct ggcatgccga ttgctttgtg tgtgttacct gctctaagaa gctggctggg 720
cagcgtttca ccgctgtgga ggaccagtat tactgcgtgg attgctacaa gaactttgtg 780
gccaagaagt gtgctggatg caagaacccc atcactgggt ttggtaaagg ctccagtgtg 840
gtggcctatg aaggacaatc ctggcacgac tactgcttcc actgcaaaaa atgctccgtg 900
aatctggcca acaagcgctt tgttttccac caggagcaag tgtattgtcc cgactgtgcc 960
aaaaagctgt aaactgacag gggctcctgt cctgtaaaat ggcatttgaa tctcgttctt 1020
tgtgtcctta ctttctgccc tataccatca ataggggaag agtggtcctt cccttcttta 1080
aagttctcct tccgtctttt ctcccatttt acagtattac tcaaataagg gcacacagtg 1140
atcatattag catttagcaa aaagcaaccc tgcagcaaag tgaatttctg tccggctgca 1200
3/6
CA 02378989 2002-O1-03
WO 01/04308 PCT/US00/19014
atttaaaaat gaaaacttag gtagattgac tcttctgcat gtttctcata gagcagaaaa 1260
gtgctaatca tttagccact tagg 1284
<210> 4
<211> 2621
<212> DNA
<213> Homo Sapiens
<220>
<221> misc_feature
<223> Incyte Clone No: 5640004CB1
<400> 4
gccaaaaatt ctggaaagaa gccattcaac agagccaaat ttatcctcct tcctgaatga 60
ccccaatccc atgaaatacc tgcggcaaca gtcactgcct ccacccaaat tcactgccac 120
tgttgaaacc accattgctc gtgccagtgt tctggatacc agcatgtcag caggcagtgg 180
gtctccaagc aaaactgtca ctcccaaagc agtgcctatg ctgacaccca agccttactc 240
ccagcccaaa aattctcaag atgttctgaa gacctttaag gtagacggga aagtcagtgt 300
gaatggagag acggttcata gagaggagga gaaggaaaga gagtgtccca cggtggcacc 360
tgcccactcc ttaaccaaat cccagatgtt tgaaggtgtg gccagagtgc acgggtctcc 420
actggagctg aaacaagaca acggtagcat cgagatcaac ataaagaagc caaactctgt 480
tccccaagag ctcgcagcaa ccactgagaa aacggaaccg aatagtcaag aggacaagaa 540
tgatggtgga aaatcaagaa aagggaatat agaacttgcc tcatcagaac cacagcattt 600
tacaacaact gtgactcgat gcagcccgac cgtggccttt gtggaatttc cctccagccc 660
ccagctgaag aatgatgtgt cggaagaaaa agaccagaag aaaccagaaa atgaaatgag 720
tggaaaggtg gagttggtgc tgtcacaaaa ggtggtaaag ccaaaatctc cagaacccga 780
agcaacgctg acatttccat ttctggacaa aatgcctgaa gccaaccaac tacatttgcc 840
aaatctcaat tctcaagtgg attctccaag cagtgagaag tcacctgtta cgacaccttt 900
taagttctgg gcatgggacc cagaagagga gcgcaggcga caggaaaaat ggcaacagga 960
acaggaacgt ttgctccagg agagatacca gaaggagcag gacaagctga aagaagagtg 1020
ggaaaaggcc caaaaggagg tggaagagga agaacgcaga tactatgagg aggagcgtaa 1080
gataattgaa gacactgtgg ttccatttac tgtttcttca agttccgctg accagctgtc 1140
tacctcttcc tccatgactg aaggcagtgg gacaatgaat aagatagacc tgggaaactg 1200
tcaagatgaa aaacaagaca gaagatggaa gaaatcattc cagggagatg acagtgactt 1260
attgctgaag actagggaaa gtgatcgact ggaggagaag ggcagcctaa ctgaaggggc 1320
cttggctcat tctgggaacc ctgtatcaaa aggagtccat gaagaccatc agctggatac 1380
cgaggctggg gccccacact gtggaacaaa cccacagctt gctcaggatc catcccagaa 1440
tcagcagaca tcaaatccaa cgcacagttc agaagatgtg aagccaaaaa ccctcccgct 1500
ggataaaagc attaaccatc agatcgagtc tcccagtgaa aggcggaagt ctataagtgg 1560
aaagaagctg tgctcttcct gtgggcttcc tttgggtaaa ggagctgcaa tgatcatcga 1620
gaccctcaat ctctattttc acatccagtg tttcaggtgt ggaatttgta aaggccagct 1680
tggagatgca gtgagtggga cggatgttag gattcgaaat ggtctcctga actgtaatga 1740
ttgctacatg cgatccagaa gtgccgggca gcctacaaca ttgtgacacg gctttcaagc 1800
ttccggatca ctcaccattt ctttactgag agtgtcccct ggcaactgct taacaaaatc 1860
ccaagctcag gggcttctca gcatttacct aatttctgaa aggctcttct gaaaggtggt 1920
atctgttctt tcgtagcaca gtgtttatgt ttttcctgtt tattgttttg gttttttttt 1980
tttttttgca tttgcacagt atacacaaaa gaatatgggg ttgtaatgat cctgaatagc 2040
tcaaaaaagg ttttagcatg gtcaaacagg cttatggttt aaaatgtgtt attctcttct 2100
ttgggaatta gctaaatgat gcaataaacc tgttttgttt tagaatgtct aggaattaaa 2160
cactttatgt ttacagaatt gagctgcaga aagtgcaaga catgccaatt tgagacacac 2220
ggtcttctaa gactgaagga taaatttaat gcatttcaga aactaaacat cacagcaagc 2280
tctatctctg agctataatt tgtttttaat gcaaagacac tagtttgata atatatactg 2340
taatcctgaa acatttgtgt tacttacctt tggaggtaga aattatacca ataaattatt 2400
gcaccgttag tattagattc tgtgtacctt ggaagttatg tcattaatat aggctggttc 2460
atcaaataaa gcaaaacctt gcaatatcag ctagatttac actccgggac gttgcccaaa 2520
ggtaggaaga aagcagaggg aaatatttca gtcatcattt ccaaagtcat tatcaaaatc 2580
tgtgaggaag tttaatcttc caagagtcca tgtcagacat c 2621
<210> 5
4/6
CA 02378989 2002-O1-03
WO 01/04308 PCT/US00/19014
<211> 280
<212> PRT
<213> Homo Sapiens
<220>
<221> misc_feature
<223> GenBank ID No: 82853224
<400> 5
Met Ala Glu Lys Phe Asp Cys His Tyr Cys Arg Asp Pro Leu Gln
1 5 10 15
Gly Lys Lys Tyr Val Gln Lys Asp Gly His His Cys Cys Leu Lys
20 25 30
Cys Phe Asp Lys Phe Cys Ala Asn Thr Cys Val Glu Cys Arg Lys
35 40 45
Pro Ile Gly Ala Asp Ser Lys Glu Val His Tyr Lys Asn Arg Phe
50 55 60
Trp His Asp Thr Cys Phe Arg Cys Ala Lys Cys Leu Gln Pro Leu
65 70 75
Ala Asn Glu Thr Phe Val Ala Lys Asp Asn Lys Ile Leu Cys Asn
80 85 90
Lys Cys Thr Thr Arg Glu Asp Phe Pro Lys Cys Lys Gly Cys Phe
95 100 105
Lys Ala Ile Val Ala Gly Asp Gln Asn Val Glu Tyr Lys Gly Thr
110 115 120
Val Trp His Lys Asp Cys Phe Thr Cys Ser Asn Cys Lys Gln Val
125 130 135
Ile Gly Thr Gly Ser Phe Phe Pro Lys Gly Glu Asp Phe Tyr Cys
140 145 150
Val Thr Cys His Glu Thr Lys Leu Ala Lys His Cys Val Lys Cys
155 160 165
Asn Lys Ala Ile Thr Ser Gly Gly Ile Thr Tyr Gln Asp Gln Pro
170 175 180
Trp His Ala Asp Cys Phe Val Cys Val Thr Cys Ser Lys Lys Leu
185 190 195
Ala Gly Gln Arg Phe Thr Ala Val Glu Asp Gln Tyr Tyr Cys Val
200 205 210
Asp Cys Tyr Lys Asn Phe Val Ala Lys Lys Cys Ala Gly Cys Lys
215 220 225
Asn Pro Ile Thr Gly Phe Gly Lys Gly Ser Ser Val Val Ala Tyr
230 235 240
Glu Gly Gln Ser Trp His Asp Tyr Cys Phe His Cys Lys Lys Cys
245 250 255
Ser Val Asn Leu Ala Asn Lys Arg Phe Val Phe His Gln Glu Gln
260 265 270
Val Tyr Cys Pro Asp Cys Ala Lys Lys Leu
275 280
<210> 6
<211> 341
<212> PRT
<213> Homo Sapiens
<220>
<221> misc_feature
<223> GenBank ID No: 82624922
<400> 6
5/6
CA 02378989 2002-O1-03
WO 01/04308 PCT/US00/19014
Val Ile Glu Arg Glu Arg Lys Trp Glu Gln Gln Leu Gln Glu Glu
1 5 10 15
Gln Glu Gln Lys Arg Leu Gln Ala Glu Ala Glu Glu Gln Lys Arg
20 25 30
Pro Ala Glu Glu Gln Lys Arg Gln Ala Glu Ile Glu Arg Glu Thr
35 40 45
Ser Val Arg Ile Tyr Gln Tyr Arg Arg Pro Val Asp Ser Tyr Asp
50 55 60
Ile Pro Lys Thr Glu Glu Ala Ser Ser Gly Phe Leu Pro Gly Asp
65 70 75
Arg Asn Lys Ser Arg Ser Thr Thr Glu Leu Asp Asp Tyr Ser Thr
80 85 90
Asn Lys Asn Gly Asn Asn Lys Tyr Leu Asp Gln Ile Gly Asn Thr
95 100 105
Thr Ser Ser Gln Arg Arg Ser Lys Lys Glu Gln Val Pro Ser Gly
110 115 120
Ala Glu Leu Glu Arg Gln Gln Ile Leu Gln Glu Met Arg Lys Arg
125 130 135
Thr Pro Leu His Asn Asp Asn Ser Trp Ile Arg Gln Arg Ser Ala
140 145 150
Ser Val Asn Lys Glu Pro Val Ser Leu Pro Gly Ile Met Arg Arg
155 160 165
Gly Glu Ser Leu Asp Asn Leu Asp Ser Pro Arg Ser Asn Ser Trp
170 175 180
Arg Gln Pro Pro Trp Leu Asn Gln Pro Thr Gly Phe Tyr Ala Ser
185 190 195
Ser Ser Val Gln Asp Phe Ser Arg Pro Pro Pro Gln Leu Val Ser
200 205 210
Thr Ser Asn Arg Ala Tyr Met Arg Asn Pro Ser Ser Ser Val Pro
215 220 225
Pro Pro Ser Ala Gly Ser Val Lys Thr Ser Thr Thr Gly Val Ala
230 235 240
Thr Thr Gln Ser Pro Thr Pro Arg Ser His Ser Pro Ser Ala Ser
245 250 255
Gln Ser Gly Ser Gln Leu Arg Asn Arg Ser Val Ser Gly Lys Arg
260 265 270
Ile Cys Ser Tyr Cys Asn Asn Ile Leu Gly Lys Gly Ala Ala Met
275 280 285
Ile Ile Glu Ser Leu Gly Leu Cys Tyr His Leu His Cys Phe Lys
290 295 300
Cys Val Ala Cys Glu Cys Asp Leu Gly Gly Ser Ser Ser Gly Ala
305 310 315
Glu Val Arg Ile Arg Asn His Gln Leu Tyr Cys Asn Asp Cys Tyr
320 325 330
Leu Arg Phe Lys Ser Gly Arg Pro Thr Ala Met
335 340
6/6