Note: Descriptions are shown in the official language in which they were submitted.
CA 02381714 2002-02-11
WO 01/13102 PCT/GB00/03054
- 1 -
SENSOR DEVICES AND ANALYTICAL METHODS FOR THEIR USE.
This invention relates to improved sensor devices and
methods for their analytical use, and more particularly to
improved forms of enzyme electrodes.
There is an increasing need for procedures and devices
which can enable the presence and amount of particular
components (analytes) in biological media to be measured
without having to rely on taking samples periodically and
taking them away to be analysed in a laboratory . Such old
"sampling" procedures - though usually accurate - are too
slow and involve a significant delay in obtaining the result
of the measurement, and in many circumstances this delay can
be inconvenient and even dangerous to a subject.
Instead, it is very desirable to have a continuing
monitoring procedure can be carried and used to detect
changes as near as possible to the moment at which they
occur, especially if the changes are abrupt or fluctuating.
This is important because conditions in some biological
environments, and especially conditions in vivo, can change
unexpectedly and quickly -- and some measurements may be
useful as indications of progress or even critically
important as vital indication or warning of a need for
speedy remedial action.
Especial interest exists in measuring glucose levels in
body tissue or body fluids, as glucose is vital for life and
its level is greatly affected by some conditions, for
example diabetes mellitus. Other analytes, e.g. drugs and
metabolic products, are also of comparable interest.
For a continuous monitoring system, it is very
desirable to implant a sensor device in the biological
environment or medium, and especially an in vivo
environment, so that parameters of a living environment or
process can be made. Indeed, the only routinely usable
monitoring system for an ambulatory diabetic would be either
a portable or an in vivo device.
CA 02381714 2002-02-11
WO 01/13102 PCT/GB00/03054
- 2 -
A variety of such implant devices have been proposed,
but their success and applicability vary considerably. The
devices or approaches suggested hitherto could, in
principle, eventually allow for reliable monitoring but they
suffer from various deficiencies and are not entirely
satisfactory. For example, many can lack sensitivity or
specificity and, as results are often plotted as graphs,
show difficulty in making true measurements independently of
base-line or response slope variability in such graphs.
Further, in some cases it can be impossible to arrive at
meaningful in vivo data without in vivo calibration,
particularly in a tissue matrix, and the implant may even
provoke tissue rejection.
It has been proposed to meet some of these needs by
inserting into living tissue a sensor device substantially
in the form of a needle incorporating a sensor electrode.
Such an electrode may be used in conjunction with a
reference electrode - which may be combined with the sensing
electrode in the sensor device or may be used separately
from it, for example on the skin of the subject.
Such sensors commonly use an active sensor electrode in
conjunction with an enzyme, so that the sensor device can be
made to respond to selected analyte species which would in
themselves be inactive at the electrode and so would not be
detected by it, e.g. glucose oxidase for measuring glucose
content. Likewise, various forms of coating materials or
membranes (permeable or permselective) are commonly proposed
for regulating the access of analyte to the active electrode
or reducing interference from other compounds which could
interfere with the effectiveness of the measurements of the
desired analyte if they reach the electrode surface.
It has been proposed to use fine wires of an
appropriate electrode material, bare or appropriately
coated, compatible with the environment in which it is to be
inserted. Even the smallest sensor devices proposed so far
CA 02381714 2002-02-11
WO 01/13102 PCT/GB00/03054
- 3 -
have been found to have several difficulties in use, which
it would be very desirable to overcome, for example:-
(1) Present devices can be too large for easy use (e. g. they
may cause undue discomfort to the subject) so greater
miniaturisation is desirable to reduce the effect the
implanted sensor may have on the subject's behaviour and
tolerance of the implant. Also it is desirable to achieve a
size of device which allows for an adequate balance between
rigidity and flexibility, so that the device is more durable
and more easily implanted.
(2) Many known devices employing any coating on the
electrode surface - enzyme, membrane, etc. - suffer from
difficulties in the stage of insertion into the subject
tissue. The enzyme and/or membrane material can be
destroyed or displaced using the insertion, to the detriment
of the sensor device's effectiveness in subsequent use.
This is almost unavoidable, as the friction between the
tissue and the coating on the sensor device can be
considerable --especially as the coating material is usually
of a very delicate nature.
(3) It is very difficult to make sensor devices which are
both small and effective. The fabrication of very small
devices can be difficult, and it is also difficult to
achieve the formation of reliable and stable coatings on
them. For example, some devices proposed so far are little
more than wires having an exposed tip which is the
enzyme/membrane system -- and it is especially difficult to
coat the tip of a thin wire.
For use, it has been proposed to implant electrodes in
tissue through a cannula to avoid undesirable damage during
the insertion stage, but this still involves some friction
within the cannula. Attempts to enclose the whole device in
a sheath of protective material which may contribute to the
electrical function of the sensor electrode (e.g. a
reference electrode) or as a strengthening or stiffening aid
CA 02381714 2002-02-11
WO 01/13102 PCT/GB00/03054
- 4 -
have been proposed, but these tend to increase the size of
the device and complicate provision of the necessary degree
of exposure of enzyme-coated electrode for effective
measuring activity.
The aim of our invention is to reduce the size of
sensor implants to the practicable minimum compatible with
robust mechanical integrity, and also to provide a compliant
insert that reduces patient discomfort beyond that achieved
to date.
We have now found that miniaturised devices which can
overcome such disadvantages can be achieved by modifying the
shape of the sensor device (especially one incorporating a
wire electrode) by forming cavities in the otherwise smooth
material of the sensor device and using these cavities to
retain the enzyme. In this way, the enzyme can be retained
within the profile of the sensor device and, as it no longer
needs to protrude beyond the surface of the sensor device,
it is thereby rendered less susceptible to being removed by
friction or abrasion during insertion into tissue.
Thus according to our invention we provide new sensor
devices comprising an enzyme electrode sensor in which
active electrode material carries an enzyme, characterised
in that the enzyme is retained within one or more cavities
formed in the said electrode sensor.
By placing the cavity (or cavities) along the length of
the electrode core, the enzyme therein can face laterally
instead of being on a mechanically vulnerable wire tip.
The core of active electrode material may be made of
any of the conventional conducting materials known for use
in the art of sensor electrodes. Preferably it is a noble
metal, for example gold or platinum, or an alloy of these
with each other or one or more other elements . Preferably
the material is platinum itself, but as platinum itself is
relatively soft it can be hardened by alloying with a
proportion of iridium.
CA 02381714 2002-02-11
WO 01/13102 PCT/GB00/03054
- 5 -
The shape of the sensor device is most conveniently of
a substantially circular cross-section, as is customarily
the case when the active electrode material is a wire core,
e.g. conventional drawn metal wire as available
commercially, but may be be of any other cross-section if
desired. The size of the core material is preferably in the
range 50 to 150 Jum. though larger or smaller sizes may be
used if desired. Our aim is to use as thin a core as can be
found to be practical, consistent with the requirement that
its strength and integrity are not impaired by the size of
the cavities.
As the purpose of the construction is for the sensor to
present an enzyme-covered surface to its environment, it
will normally be found that if any bare active electrode
material is also exposed to the environment it will
interfere with the measurements made at the surface beneath
the enzyme. A bare surface of active electrode material can
be tolerated if such interference does not occur, but the
preferred form is that in which a core of the active
electrode material is covered with a coating of insulating
material to prevent bare active electrode material coming
into contact with the environment media and the analyte to
be detected and measured. Such an insulating material should
be suitably durable, stable and resistant to the environment
media, effectively sealed over the core of active electrode
material, and - when intended for use in vivo - be suitably
bio-compatible and harmless in use. Such materials are well
known in the art.
Consequently, when the sensor to be used has such a
coating of insulating material, the cavities required for
our invention may be made in several ways . One way is for
the insulation to be stripped off to expose a bare core of
active electrode material and form the cavity into which the
enzyme can then be placed. Alternatively, both the
insulation and some of the core of active electrode material
CA 02381714 2002-02-11
WO 01/13102 PCT/GB00/03054
- 6 -
can be removed by using an appropriate micro-machining
technique, so that the insulation is removed and a cavity is
also formed in the core of active electrode material itself.
This latter procedure has the advantage that the fabrication
is simplified and also that the cavities in the active
electrode material allow for a greater surface area of the
active electrode material to be exposed to enzyme and used
to generate stronger signal outputs.
The cavities may be formed by conventional procedures,
for example drilling, punching, grinding, boring, cutting,
or any combination of these techniques, and the size and
shape of the cavities may take any form which is considered
most convenient and capable of retaining the desired enzyme
in sufficient quantity. Likewise, the number of cavities
may may be as large or small as desired, and will be
determined to some degree by the size, shape and position of
the cavities used in any particular instance. Our
preference is for the cavities to be of a size up to about
half of the overall thickness of the sensor material, so
that the strength of the sensor is not unduly reduced. The
optimum in any particular case may be readily determined by
simple trial. The method used for forming the cavities may
be a mechanical one, though that can be difficult on the
micro-scale required; therefore we prefer to use an ion beam
or laser method (commonly referred to a "micro-machining")
as this is more easily used on the scale of size involved
here. Thus a laser or ion beam can be used to etch, cut or
bore into the material of the sensor to form the required
cavities.
Examples of suitable shapes for the cavities include
circular, oval, square, polygonal, cruciform, star-shaped
and combinations of these. The cavities may regular or
irregular in their, size, shape, number and distribution,
though it is generally preferred (as being more convenient)
to make all the cavities of substantially the same shape and
CA 02381714 2002-02-11
WO 01/13102 PCT/GB00/03054
size. It is preferred that the form of the cavities should
be chosen so that they can readily retain the enzyme; thus
dish-shaped cavities may be less efficient if the enzyme is
not strongly held, and cavities which are more like "pits"
are usually to be preferred as they achieve a stronger hold
on their enzyme contents.
Another useful cavity shape is a slot cut into the
sensor in a substantially lengthways direction (i.e. in the
direction of the axis of a wire electrode), as this can
minimise the number of cavities to be made. The size of the
slot (length and breadth) may be varied to suit particular
needs and usually are not critical.
An especially useful form of cavity is one which passes
completely through the core of electrode material, in effect
forming a tunnel, open at both ends, running transversely to
the general direction of the inner core. This allows the
enzyme to be packed into this tunnel and the enzyme contents
to be exposed to analyte and (in the case of an oxidase)
also oxygen, as needed for reaction - thus giving very
effective enzymatic action and consequent measurement
efficiency. If desired the cavity may contain more than one
enzyme, e.g. as laminate layers, so that a succession of
reactions can be catalysed --- one enzyme acting on an
analyte substrate to form a product which, in turn, is acted
upon by the second enzyme to generate a further product
which can then be satisfactorily detected and measured at
the active electrode surface.
The enzyme may be used in conventional formulations and
compositions, and placed in the cavities and retained
therein by conventional coating methods and fixing methods.
For example the enzyme may be applied as a composition which
coats and fills the cavities (e.g. by dipping) followed by
wiping or passing through a collar to remove the surplus and
especially any on the main surface where it is not required.
The enzyme can then be fixed in place by cross-linking, e.g.
CA 02381714 2002-02-11
WO 01/13102 PCT/GB00/03054
_ g _
by treatment with glutaraldehyde.
The enzyme may be any of the conventional enzymes used
in sensor enzyme electrodes for electrochemical analysis,
but we find oxidase or dehydrogenase enzymes are most
useful. An especially useful example is glucose oxidase,
which allows the device to be used for detection and
measurement of glucose concentrations in tissues. Though we
describe our invention with particular reference to glucose
and glucose oxidase, however, it is not limited to this
specific system and it is applicable to other
substrate/enzyme systems, of which several are well known in
the art for analytical purposes.
The sensor devices of our invention preferably also
comprise coatings over the enzymes held within the cavities.
Thus, additional layers of material may be deposited over
the enzyme after that has been put into the cavities. Such
over-coating layers may be composed of materials of
appropriate permeability (simple or selective) to regulate
the passage of components from a sample under examination to
the enzyme and active electrode surface, or excluding or
limiting access by materials which could interfere with the
measurements. These materials are well known in the art and
are usually in a thin form which serves as a permselective
membrane, and may be applied by conventional means also well
known in the art.
Examples of such materials include various polymers and
polymer compositions, e.g. polyaryl ether sulphones and
modified polyurethanes.
The electrodes of this invention may be used by any of
the conventional procedures well known in the art, but of
all the electrochemical procedures available we prefer to
use an amperometric procedure with the active electrode
material as the anode.
The sensor devices of our invention can be used in vivo
or in vitro, and the mode of insertion into the sites for
CA 02381714 2002-02-11
WO 01/13102 PCT/GB00/03054
- 9 -
making measurements are conventional. For in vivo sites,
they may be inserted directly (transcutaneously into tissue)
or through a cannula or even fine tubing. e.g. of nylon.
The advantages of the invention are especially in the
way it provides new sensors which are small, light in
weight, and potentially much more robust, flexible and
suitable for implantation without attendant discomfort and
other problems. Other advantages stem from the way in which
the new sensors provide a different orientation and profile
for the sensing surfaces - side-oriented micro-machined
sensing surfaces - to the samples under examination,
The invention is illustrated but not limited by the
accompanying drawings, which illustrate some forms which the
improved electrodes can take.
These drawings are schematic and not drawn to scale,
but are intended to show the principal features - in some
cases emphasised by being out of scale.
In the drawings Figures 1 to 4 represent, in
perspective view, various forms of sensor electrode devices
of according to this invention and the type and disposition
of cavities, and Figures 5 to 10 illustrate cross-sectional
views through such sensor devices at the position at which
the cavity is made.
In detail, all of Figures 1 to 4 show a thin platinum
wire covered with insulation (1), with the said insulation
covering the end (2) as well as the main body (1). The end
(3) is adapted for continuation on and connection to the
electrical measuring system (not shown).
In Figure 1, which shows the simplest form of the
invention, the insulated wire (1) is pierced by a hole (4)
to form a cavity (5) which is filled with an immobilised
enzyme composition.
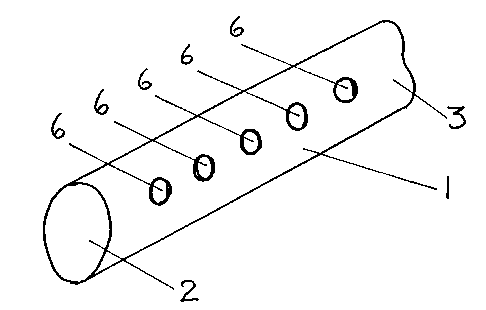
In Figure 2, there is shown a form in which the
insulated wire (1) is pierced by a series of holes (6), each
forming cavities filled with immobilised enzyme as in Figure
CA 02381714 2002-02-11
WO 01/13102 PCT/GB00/03054
- 10 -
1. In Figure 3, there is shown a form in which the
insulated wire (1) is pierced by a series of holes (7), each
forming cavities filled with immobilised enzyme as in Figure
1 but of a shape different from those in Figure 2, i.e.
cruciform instead of round.
In Figure 4, there is shown a form in which the
insulated wire (1) is stripped of its insulation to form a
slot (8) in the direction of the axis of the insulated wire
(1), and the resulting slot is filled with immobilised
enzyme; in this variant, the slot (8) has been made by
cutting out the cover layer of insulation but without
cutting into the core of platinum wire itself, but a further
alternative (not shown) is that of cutting the slot into the
core of platinum metal in addition to cutting away the outer
layer of insulation.
All of Figures 5 to 10 illustrate cross-sectional views
of alternative forms of the cavity through a thin platinum
wire covered with insulation where the cavity is made.
In Figure 5, which shows the simplest form of the
invention, the insulated wire comprises a core of thin
platinum wire (1) covered by an outer layer of insulation
(2) with a part of the insulation cut away to leave a cavity
(3) filled with an immobilised enzyme composition.
In Figure 6, a cavity (3) has been bored through the
inner platinum wire core, passing through it completely from
one side to the other, and is filled with an immobilised
enzyme composition. The enzyme-filled cavity (3) is coated
at each end with a layer of a membrane coating (4) which
acts to protect the enzyme and provide a chosen degree of
selectivity or regulation of access of components of the
surrounding medium to the enzyme.
In Figure 7 , the cavity ( 3 ) has been bored through the
inner platinum wire core, passing through it completely from
one side to the other, and is filled with an immobilised
enzyme composition as in Figure 6, except that two different
CA 02381714 2002-02-11
WO 01/13102 PCT/GB00/03054
- 11 -
enzyme compositions (3A) and (3B) are used to fill the
cavity (3). The enzyme contents are coated at each end with
a layer of a membrane coating (4) as in Figure 6.
In Figure 8, the arrangement is essentially the same as
for Figure 5 except that the cavity (3) is bored through the
outer insulation layer (2) and also into the inner platinum
wire core to form an inner pit in the platinum. This form
has the advantage of having a larger surface area of the
platinum exposed to enzyme and this produces greater
response signals for measurement.
In Figure 9, the arrangement is essentially the same as
for Figure 6 except that the cavity (3) bored through the
outer insulation layer (2) and completely through the inner
platinum wire core has not been given any coating (4), so
that the enzyme composition contents of the cavity (3) are
exposed directly to the surrounding media.
In Figure 10, the construction is that of Figure 6 but
shows - with added arrows (9) - indication of flows of fluid
past the membrane-covered apertures in the insulation.
These flows may be fast or slow, large or small, and may be
the same or different. One variant which is practicable is
for the two flows (9A) arid (9B) to be different -- with one
(9B) being the sample medium, from which analyte components
can diffuse in to the enzyme through the membrane, while the
other flow (9B) can provide similar access by diffusion for
necessary substrates for the enzyme system. For example,
(9A) may provide the main source of glucose to a glucose
oxidase system while (9B) may provide more of the oxygen
necessary for the enzyme to function.
To fabricate the devices illustrated, the insulated
platinum wire may be etched to remove the insulation layer
and expose the platinum within, and subsequent
polymerisation or other conventional techniques may be used
to deposit the enzyme on the platinum surface. Covering the
enzyme in the cavity can be achieved by conventional
CA 02381714 2002-02-11
WO 01/13102 PCT/GB00/03054
- 12 -
techniques, for example application from solution by
dipping. The thickness of the coating may vary, but should
be thin enough to allow adequate diffusion of a desired
analyte without unduly impeding the ability of the material
contacting the enzyme to change frequently enough to give a
satisfactory overall view of the rate of changes there may
be in the medium surrounding the sensor device.
The etching and boring operations indicated above were
carried out using a copper laser.
The thin platinum wire used is a commercially available
one, as commonly used for making wire electrodes, since it
is not necessary to use a special wire. If the coating of
insulation on commercially available wire is not suitable
for use in any particular intended environment, the coating
as supplied can if necessary be removed and replaced by re-
coating with a more suitable insulation or a coating of the
preferred insulation material may be deposited over the one
already on the commercial wire.
The method of fabrication according to this invention,
and illustrated herein, enables insulated monopolar platinum
wire electrodes having an outer diameter of 50 to 150~um and
a series of laser-drilled transaxial cavities (which we
refer to as fenestrations) each approximately 30 hum in
diameter for wire-shaft enzyme loading to be made without
undue difficulty.
Using laser-etching of an insulated platinum wire of 25
to 50~um outer diameter to remove insulation locally allows
further miniaturisation and can create an ultra-fine working
electrode surface on which thin layers of enzyme and barrier
films can be deposited.
These provide embodiments provide protection for the
active enzyme and have unique device geometry;
configurations as illustrated provide side-oriented enzyme
surfaces in micro-machined sensing surfaces designed to
retain and protect them.
CA 02381714 2002-02-11
WO 01/13102 PCT/GB00/03054
- 13 -
Construction as in Figure 6 in particular internalises
the enzyme in the working electrode, giving a unique twin-
surface enzyme with radial product diffusion into the
surrounding platinum surface. The greater area of active
platinum surface (i.e. surface exposed to the enzyme) can
improve the efficiency with which the enzyme functions and
so improve the measurements, for example in the linearity of
response.
In use, the device can be inserted into tissue and,
though the tip naturally must enter first, this entry does
not damage the enzyme-containing areas of the surface. When
the wire is pure platinum it may be too soft for direct
insertion insertion to be easy, so subcutaneous implantation
of such "soft" wires will be through narrow bore nylon
tubes. Alternatively, direct insertion can be made easier
by using a platinum/iridium alloy wire, which is more rigid.
For determination of analytes, the procedures used were
conventional ones and not described in detail here as they
are well known in the art and do not involve any departures
from the usual ones.
_____ p _____