Note: Descriptions are shown in the official language in which they were submitted.
CA 02382461 2005-10-20
APPARATUS AND METHOD FOR
WELDING DUPLEX STAINLESS STEEL
Technical Field of the Invention
The present invention relates to welding processes for
duplex stainless steels. More particularly, the invention
relates to the use of a flux, and optionally a weld ring, to
weld duplex stainless steel using, for example, an orbital
welder.
Background of the Invention
Duplex stainless steel is becoming more widely used in
applications that require high strength and corrosion
resistance. A typical example is deep sea applications in the
oil and gas industry. Particularly for high pressure
operations, thick walled or heavy duplex tubing may be
required. By "thick walled" or "heavy" tubing, which are used
interchangeably herein, is meant duplex tubing greater than 2
mm wall thickness. For most applications, duplex tubing is
welded either to additional sections of duplex tubing or to
fittings, valves and so forth all of which maybe made of duplex
steel.
Duplex steel is characterized by a phase balance between
austenite and ferrite in the steel crystalline structure. In
general, duplex stainless steels contain about 30 to 70 vol.°
ferrite, more typically about 35 to 60 vol.o ferrite, even more
typically about 40 to 45 vol.o ferrite, with the balance being
1
CA 02382461 2002-02-20
WO 01/14094 PCT/US00/17414
austenite. Maintaining the austenite/fernte phase balance is very important in
that the austenitic phase contributes to the pitting corrosion resistance of
the
steel while the fernte phase contributes to greater strength and resistance to
chloride stress corrosion cracking.
Welding of duplex steels presents special challenges, sine a proper
phase balance and nitrogen content must be maintained in the weld metal as
well as in the surrounding heat affect zone (HAZ). This is because welding
subjects the material forming the weld bead to additional high heat, melting,
cooling and solidification. Many factors associated with the welding process
can affect the phase balance in the weld metal. These factors include the
welding temperature, cooling rate, type of purge gas used during the welding
operation and the chemistry of the weld pool. If the final weld solidifies
with
too much austenite, the strength of the weld can be compromised. If the weld
solidifies with too much fernte, the weld and HAZ may exhibit lower corrosion
resistance.
Welding of steel tubing is done both manually and by machine. In both
operations, sagging or drop through of the weld pool should be avoided so that
the weld bead is uniform in profile along its entire length. In addition, the
weld
bead should not be too wide in profile, since a weld bead which solidifies too
slowly may exhibit improper metallurgy in terms of both chemistry and phase
structure. Incomplete penetration through the tube wall should also be
avoided.
Machine welding of steel tubing is typically done commercially using
orbital welding equipment in which heat for welding is derived from an
electric
arc generated by a pulsed electric current. The arc emanates from an electrode
2
WO 01/14094 CA 02382461 2002-02-20 pCT~S00/17414
positioned outside the tubing adjacent the weld junction to be formed, with
the
electrode being moved by the machine orbitally (circumferentially) around the
tubing along its entire circumference. Preferably, orbital welding is
accomplished in a single pass (plus an additional 30° to 120° in
some instances
to complete the weld smoothly), since this approach minimizes problems
occurnng when a previously formed weld bead is remelted.
Thick walled duplex tubing is particularly difficult to weld, since the
factors causing poor weld profile and improper phase balance magnify as tube
wall thickness increases. Therefore, it has not been possible to achieve
acceptable weld quality when machine welding duplex steel tubing of heavy
wall thickness. Although manual welding can achieve acceptable weld quality,
a highly skilled welder is required. Moreover, multiple weld passes are also
required, which only exacerbates the complexity and expense of the welding
process.
It is, therefore, an object of the present invention to provide welding
apparatus and methods that significantly improve the weldability of duplex
stainless steel by producing acceptable weld profiles and weld beads
exhibiting
a proper duplex phase balance and nitrogen retention.
It is a further object of the present invention to provide a welding
process and apparatus that facilitate machine based welding, especially single
pass orbital welding, of duplex stainless steel tubing, especially thick
walled
duplex stainless steel tubing.
Summary of the Invention
3
CA 02382461 2002-02-20
WO 01/14094 PCT/US00/17414
In accordance with the present invention, it has been found that duplex
steel tubing and other parts can be easily joined by machine based arc welding
provided that a high-refractory flux is present in the HAZ (heat affected
zone)
and further that the electric arc generated for welding is non-pulsed. In
particular, it has been found that the weld bead produced when welding duplex
steel parts together will reliably and consistently achieve the desired duplex
phase balance, nitrogen levels and bead profile if a high-refractory flux is
present in the HAZ, provided that the arc supplying the weld heat is generated
by a non-pulsed electric current.
Accordingly, the present invention in its broader aspects provides a new
process for welding duplex steel parts in which formation of a weld bead
having
a duplex stainless steel phase structure is facilitated by carrying out the
welding
operation in the presence of a high refractory flux. In addition, the present
invention also provides a new process for arc welding duplex steel tubing in
which a weld bead having a duplex stainless steel phase structure, a desired
nitrogen level and a uniform profile is achieved by carrying out welding in
the
presence of a high refractory flux with the heat for welding being derived
from
an arc generated by a non-pulsed electric current.
Brief Description of the Drawing
The invention may take physical form in certain parts and arrangements
of parts, preferred embodiments and a method of which will be described in
detail in this specification and illustrated in the accompanying drawing which
form a part hereof, and wherein is illustrated an apparatus for welding duplex
steel thick walled tubing.
4
WO 01/14094 CA 02382461 2002-02-20 pCT~S00/17414
Detailed Description of the Preferred Embodiments
The present invention can be used for welding a wide variety of parts
together that are made of duplex stainless steel, especially but not
necessarily
thick walled or heavy duplex tubing and tube ends. In particular, the present
invention is directed to welding together two or more parts, at Ieast one of
which is formed from a duplex steel, and further in which the weld bead formed
by the welding operation also has a duplex structure.
As mentioned above, duplex stainless steels contain about 30 to 70
vol.% ferrite, more typically about 35 to 60 vol.% fernte, even more typically
about 40 to 45 vol.% ferrite, with the balance being austenite. The weld beads
produced by the inventive process also have a duplex stainless steel phase
structure, meaning they also contain these amounts of austenite and fernte,
since this balance of phases is needed to achieve the high strength and
corrosion
resistance characteristic of duplex steels.
In accordance with the present invention, it has been found that a weld
bead having the above desired phase balance and nitrogen levels, as well as an
appropriate profile, can be formed when welding duplex steel, provided that a
high-refractory flux is present in the HAZ and the electric arc used for
supplying the welding heat is non-pulsed.
Weld fluxes are well known products of commerce extensively used in
the welding industry. Basically, they serve as surface active agents causing
the
molten weld pool to flow in a desired manner, i.e. to amalgamate or
consolidate
along the surface being heated into a compact mass. The effectiveness of a
weld flux in promoting consolidation of a weld pool is measured in terms of
CA 02382461 2005-10-20
penetration characteristic of the weld, which is the ratio of
the weld depth to its width at its widest point. In accordance
with the present invention, welds can be produced with
penetration coefficients of 0.33 or greater, preferably 0.5 or
greater, more preferably 1.0 or greater.
Many different materials have bee n used for weld fluxes.
Most typical are chlorides and fluorides such as magnesium
chloride, ferric chloride, tin chloride and various sulfur
containing compounds. In accordance with the present invention,
we have discovered that these typical weld fluxes are
ineffective in achieving a duplex weld bead with the desired
phase balance and profile. In particular, we have determined
that such fluxes contaminate the weld pool with extraneous
materials such as chloride, fluoride and/or sulfur atoms,
thereby adversely affecting the phase balance and chemistry of
the weld bead ultimately produced. In the present invention,
therefore, a different type of weld flux is used, referred to
herein as ~~high refractory" fluxes.
A high refractory flux in accordance with the present
invention is any material which will impart surface active
properties to the weld pool which it contacts in the manner of
conventional welding fluxes, but which also does not
contaminate the molten weld pool with extraneous atoms as a
result of the welding operation. Examples of materials which
are useful for this purpose are the refractory oxides such as
silica, titania, magnesia, chromia, TiO, and the like. An
especially preferred weld flux is composed of a mixture of
Cr203, Si02 and an oxide of titanium, particularly a mixture of
about 30 to 70 wt.'o of a titanium oxide (Ti0 and/or Ti02) 20 to
76 wt~ Cr203 and 5 to 27 wt.o Si02, as described in US Patent No.
-6-
CA 02382461 2005-10-20
5,804,792. A flux comprising about 50% oxide of titanium, about
40o Cr203 and about 10% SiOz is especially preferred. Generally,
such fluxes are supplied in admixture with a liquid carrier
such as water or an organic material such as acetone or methyl
ethyl ketone. An exemplary flux of this type is LFX-SS7 (trade-
mark) flux available from Liburdi Dimetrics Company of Dundas,
Ontario, Canada.
These fluxes are used in accordance with the present
invention in the same way as conventional welding fluxes. Thus
they may be applied in the same amounts, to the same locations,
and at the same time, as conventional fluxes. Where a weld ring
is used, in accordance with a preferred embodiment of the
invention as describe below, the weld flux may be applied to
the weld ring only, before or after the weld ring is joined to
the tubes being welded, or it may be applied to the tubes
themselves, or to both the weld ring and the tubes.
Once the weld flux is applied, the duplex parts to be
joined are welded in a conventional manner. Where welding is
accomplished by arc welding, it is preferable in accordance
with the present invention to use non-pulsed welding - i.e.,
arc welding in which the electric current generating the arc is
non-pulsed, preferably continuous. In a typical orbital
welding operation, pulsed electrode currents are used because
they are easier to regulate and to use to control heat at
the weld zone. However, we have found that weld beads
produced with pulsed arcs are unacceptably porous and non-
uniform if a high refractory weld flux, as described above, is
present during welding. Although not wishing to be bound to any
theory, we believe unacceptable welds are produced when pulsed
arcs are used together with high refractory fluxes
CA 02382461 2002-02-20
WO 01/14094 PCT/US00/17414
because of excessive turbulence created in the weld pool. Non-pulsed arcs
smooth out the rate heat is applied and thereby reduce or eliminate excessive
heat generation and attendant turbulence during peaks in the electrical cycle.
In standard arc welding, current pulsing occurs at 2 to 20 Hertz,
typically, with amplitudes generally varying between 100% and 30% of peak.
"Non-pulsed" as used herein means that the period of the pulse is lengthened
and/or the variation between maximum and minimum amplitude is reduced so
that violent turbulence and it attendant adverse effect on weld quality is
substantially eliminated. Preferably, direct (continuous) current is used, as
this
completely eliminates the adverse effects of pulsing. Using non-pulsed arcs in
accordance with the present invention has also be found to reduce the total
amount of electrical power required for welding.
A particular advantage of the present invention is that high quality welds
having the desired austenite/ferrite balance, nitrogen levels and bead profile
can
be produced in a single electrode pass, even if the wall thickness of the
tubing
being welded exceeds 2 mm. Orbital welding of duplex steel tubing using
conventional technology is difficult at best and impossible, as a practical
matter,
when the wall thickness of the tubing exceeds 2 mm. Welding duplex tubing of
this thickness manually is possible, but very difficult, and in any event
multiple
passes are required. In accordance with the present invention, however, tubing
of this thickness can be readily welded together with conventional orbital
welders even when the welders are operated in a single pass mode. In this
connection, it should be understood that single pass operation as contemplated
herein includes extending the pass of the electrode by an additional 30, 45,
90,
8
CA 02382461 2002-02-20
WO 01/14094 PCT/LTS00/17414
120 or even 180 degrees beyond a single complete revolution in order to smooth
out completion of the weld. Good results, however, can still be accomplished
with a single pass of 360 degrees.
In accordance with another embodiment of the invention, the weld pool
is formed using additional alloy elements supplied by a weld filler material.
Using weld fillers to supply additional alloying elements to a weld is a
common
welding practice. Since the metallurgy of a weld can often be different from
that of the underlying base metal, additional alloying elements supplied by a
weld filler can be used to alter the chemistry of the weld so as to achieve a
more
desirable chemistry and metallurgy. This effect can be used in welding duplex
steels in accordance with the present invention to tailor the austenite/fernte
balance of the weld bead produced closer to a desired value.
In this connection, welds produced form duplex steels tend to have a
lower proportion of austenite than the base metals from which they are melted.
Therefore, this effect can be offset, and the desired phase balance
maintained, at
least approximately, by using a weld filler whose alloying elements tend to
promote austenite formation. Nickel helps stabilize or enhance austenite
formation during solidification, while chromium fosters fernte formation.
Therefore, using a weld filler that is over alloyed with nickel compared to
the
base metal being welded is a desirable approach in accordance with the present
invention. For example, a weld filler made of 25.10.4.L filler material
available
from Sandvik Corporation can be used advantageously for welding 2507 duplex
stainless steel also available from Sandvik.
9
CA 02382461 2002-02-20
WO 01/14094 PCT/US00/17414
Weld filler materials in accordance with this aspect of the invention can
be supplied in any conventional manner. For example, they can be supplied in
the form of wire, for use in manual as well as machine based welding, or they
can be supplied in the form of weld rings for insertion between and attachment
to the ends of the tubes being welded. Preferably, the weld filler is supplied
in
the form of a weld ring T-shaped in profile since this allows the tube ends to
be
physically secured together before welding. Also, if desired, the high
refractory
flux to be used in the inventive process can be applied to the weld ring
separately from the tube ends to be welded before or after the weld ring is
attached to the tubing to be welded. Indeed, the ingredients of the high
refractory flux can even be incorporated into the weld ring when it is made,
if
desired.
Orbital welding can be carned out using an open system, that is
apparatus in which the gap between the weld electrode and tubing being welded
is open to the atmosphere, or a closed system in which this gap is enclosed.
In
either case, it is customary to flush the gap with a shielding gas for
substantially
eliminating oxygen from the vicinity of the weld and for carrying off any
gases
produced by the welding operation.
A variety of different gases have been used as shielding gases in
conventional welding processes. Examples are the noble gases, especially
argon, nitrogen and other gases. Nitrogen when used as a shielding gas in
concentrations as low as 2% is known to enhance austenite formation in many
different steels and so is a preferred choice in many applications. Hydrogen
has also been used. In accordance with the present invention, however, it has
CA 02382461 2005-10-20
been found that nitrogen and hydrogen, in concentrations as low
as 20, cause an "explosion" of the weld pool created in the
inventive process when a high refractory flux is present.
Accordingly, hydrogen, nitrogen and alI other gases having a
similar effect are preferably avoided in carrying out the
present invention. Thus, the shield gas used in this embodiment
of the invention can include inert gases such as the noble
gases (helium, argon, neon and xenon) as well as any other gas
which does not react with the weld pool or the high refractory
flux under the conditions encountered during welding.
The present invention will now be exemplified by a
particular embodiment which is illustrated in the drawing:
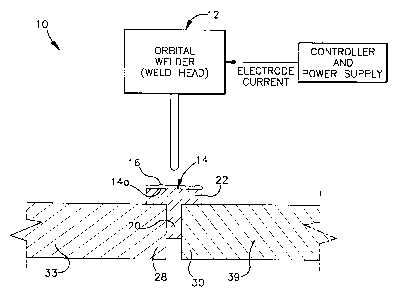
Apparatus 10 for welding together tube ends 28 and 30 of
duplex steel tubes 33 and 39 includes in a preferred embodiment
an orbital welder device 12, a weld ring 14 and a flux material
16. The orbital welder may be, for example, an orbital welding
system and power supply such as model M-100* available from
Swagelok Company of Solon, Ohio. Other welding techniques well
known to those of ordinary skill in the art can be used
however, including but not limited to manual welding systems.
In the exemplary embodiment, duplex steel tubes 33 and 39 are
formed from SAF 2507 steel available from Sandvik Corporation
of Sandviken, Sweden.
A weld filler material, such as for example, Alloy
25.10.4.L also available from Sandvik, is formed into
a consumable insert or weld ring 14. Weld ring 14 is
completely consumed in the weld puddle during the
welding operation. A significant characteristic of Alloy
25.10.4.L is that it is over alloyed with nickel
compared to the base metal being welded. The additional
*Trade-mark
-11-
CA 02382461 2002-02-20
WO 01/14094 PCT/US00/17414
nickel helps stabilize or enhance the austenite formation in the weld during
solidification.
Alloy 25.10.4.L is commercially available in wire form from Sandvik
Corporation. In accordance with one aspect of the invention, the filler wire
is
formed into a weld ring having a radial inner ring 20 and a circumferential
axially extending ring 22 integral therewith. The weld ring 14 is
appropriately
dimensioned to slip onto each end of the tube ends being welded together. The
weld ring 14 shape also aids in joint alignment which is particularly useful
with
orbital welding apparatus. Weld ring 14 can be formed by any convenient
process such as by sintering, stamping and so forth.
Flux 16 is LFX-SS7 flux available from Liburdi Dimetrics. Other fluxes
may be used as mentioned above. Preferably, flux 16 is applied to an outer
surface 14a of the weld ring and on adjacent tube surfaces. Surface
application
facilitates the penetration enhancing characteristic of the flux. The flux 16
is
typically available in powder form, but in this case is mixed with a liquid
carrier
to form a paste that is manually brushed on weld ring 14. The liquid carrier
evaporates and the flux remains loosely adhered to the weld ring 14. The flux
is
preferably entirely consumed during the welding operation, however, flux
residue can be easily cleaned from the final weldment as required. Preferably,
the flux is kept near the outer surface of the weld ring 14, as shown in the
figure.
Flux 16 improves heat penetration in the weld, thus reducing the weld
width which reduces the potential for sagging and other weld profile problems
that commonly occur during attempts to do a single pass welding operation with
12
CA 02382461 2002-02-20
WO 01/14094 PCT/US00/17414
thick walled components. By more efficiently directing the heat inward to
reduce weld pool spread, the welding operation uses lower currents for full
penetration. Reduced current allows for welding with smaller lower power
weld heads and power supplies and also further aids in maintaining austenite
in
the weld.
In operation, the tube ends 28 and 30 of tube sections 33 and 39 are
abutted together with weld ring 14 therebetween. Orbital welder 12 is used to
perform a single pass welding operation using argon gas as the shield gas. For
a
typical duplex tube having a wall thickness of 0.095 inch and a tube diameter
of
0.5 inch, acceptable welds are achieved using a welding current/voltage of 50
amps and 9 Volts at an electrode travel speed of 2.1 inches per minute. The
weld bead produced in this manner has an austenite/ferrite ratio of 58/42 and
a
uniform profile along its entire width with a penetration characteristic
(ratio of
weld depth to maximum weld width) of 0.5.
The invention has been described with reference to the preferred
embodiment. Obviously, modifications and alterations will occur to others
upon a reading and understanding of this specification. It is intended to
include
all such modifications and alterations insofar as they come within the scope
of
the appended claims or the equivalents thereof.
13