Note: Descriptions are shown in the official language in which they were submitted.
CA 02382866 2006-07-07
Method for Bleaching Pulp with Chlorine dioxide
The present invention relates to a method and an apparatus for treatment of
pulp. The method and
the apparatus according to the invention are particularly well applicable to
treatment of chemical
pulp by using chlorine dioxide in a treatment phase in which the use of
chlorine dioxide has been
optimized.
It is a known fact from the prior art that about two decades ago cellulose
fiber suspension was still
bleached mainly with elemental chlorine Cl. This was done by mixing into the
pulp elemental
chlorine which had been absorbed into cold water, max. temperature 20 C. Then
the bleaching
process was allowed to continue at a low LC consistency (typical consistency
about 3 - 4 %) at a
temperature of 20 - 40 C for about 60 min. In the 80's the use of chlorine
started to be reduced
mainly due to environmental reasons because the AOX content of effluents had
become an
important criteria in the protection of environment and the AOX emissions
could be reduced most
efficiently by replacing elemental chlorine with chlorine dioxide in the
bleaching.
It was found out already in the 1940's that chlorine dioxide, C1O2, which in
every-day language is
called chlorine dioxide, is a usable chemical in the bleaching of pulp and in
fact the only chemical
used at that time with which sulfate pulp could be bleached to the full
brightness of over 86 - 90 %
(ISO). Thus it was natural to use these chemicals originating from chlorine,
i.e. both elemental
chlorine and chlorine dioxide, side by side in successive treatment stages and
later on in the same
treatment stage to improve the quality of pulp. From this time comes also the
practice of
performing the bleaching process containing chlorine at a relatively low
temperature. Chlorine had
taught the pulp mills that a suitable bleaching temperature is approximately
the room temperature
because chlorine gas dissolved in water evaporates at higher temperatures and
the process cannot be
controlled. One reason for the difficulty in the control of the process was
the lack of efficient
mixing methods. Until the 1970's, mixing of large chlorine volumes into the
pulp was successful
only in a water solution at a low consistency because the operation of
chemical mixers was
fluctuating and the mixing results were crucially different from each other in
different processes.
CA 02382866 2006-07-07
2
The problem caused by the mixing of chemicals was practically solved when the
high-intensity
fluidizing mixers became available on the market and they were introduced both
into MC and LC
bleaching stages. At the same time it became possible to use chlorine in gas
form instead of
elemental chlorine in water solution, the gas being dissolved directly into
the liquid in the pulp
suspension and reacting there with the pulp without a separate dissolving
process. At its best the
whole chemical dose could be consumed efficiently during the process because
of the efficient
mixing.
Mixing is an important stage in the bleaching of pulp, which ensures that
there is an even chemical
consistency around each fiber. Mixing of chlorine dioxide into the
chlorination stage was
considered advantageous in view of the pulp quality and at the same the
temperature of the
chlorination stage could be raised as, due to the efficient mixing, all the
chemical was consumed
very fast and evenly, and the addition of chlorine dioxide was considered to
protect the pulp from
quality losses at the high temperature.
As the leading trend ever since the 70's has been to close the processes and
to reduce the volume of
effluents, the use of a higher temperature in the C, C/D and Do stage has
provided benefits for the
heat economy of the mill. As mills were provided with modern biological
effluent purification
plants, it became necessary to start performing the bleaching stages at the MC
consistency, as the
operation of the purification plants required an essential reduction in the
volume of water and an
increase in the concentrations. Finally, for environmental reasons the use of
chlorine was given up
and the first typically delignifying electrophilic bleaching stage was
transformed into a mere
chlorine dioxide stage, i.e. the so-called D stage.
In other words, the temperature of the chlorine dioxide bleaching stage used
as the first stage in a
bleaching sequence has been raised very slowly, in pace with the withdrawal of
prejudice so that
today the normal treatment temperature at the MC consistency stages varies
within a wide range of
the order of 45 - 70 C. Not until recently, around the middle of the 90's, the
delignifying chlorine
dioxide bleaching D has been suggested to be performed at a higher
temperature, i.e. at a
temperature close to 100 degrees or even higher, in which case a completely
pressurized process is
CA 02382866 2006-07-07
3
used in order to prevent the water in the fiber suspension from boiling.
However, in all cases the
risk of deterioration of the pulp quality has been experienced. In the process
technology in general,
raising the temperature results in acceleration of chemical reactions and thus
faster consumption of
chemicals, and as a detrimental property when chlorine dioxide is used, an
increase in the volume
of chloride-containing exhaust gases. Further, at the high temperature of over
90 C, the danger of
corrosion in the presence of chloride-containing liquid increases. Also, the
temperature of over
90 C is difficult in view of the heat economy of the mill.
Chlorine dioxide treatments are, because of their chemical character, divided
in the bleaching
sequences into two different stages each having their own purposes and process
conditions. The so-
called D. stage is a delignifying treatment stage, the main purpose of which
is to decrease the
Kappa number of the pulp and the main reaction of which has been described in
the wood chemical
publications as an electrophilic stage. As publications discussing the subject
are mainly more than
ten years old the literature does not deal with a mere D. stage but a combined
CD stage is
discussed, i.e. chlorination utilizing chlorine dioxide combined with
elemental chlorine. The Dl
and D2 etc. stages following later in the bleaching sequence are brightening
stages which aim at
increasing the brightness of the pulp and the chemical reaction of which is
mainly nucleophilic.
This has a different chemical character and totally different control
parameters have influence on it
than on a D. stage. Literature (e.g. Pulp and Paper Manufacture, Volume 5,
Alkaline Pulping,
Tappi 1989) very clearly recommends, in particular for bleaching chlorine
dioxide stages, a
temperature of 50 - 90 C and a treatment time of more than two hours. The
nucleophilic
(brightening) and the electrophilic (delignifying) chlorine dioxide stage are
separated in the
literature based on the pH so that the former has a pH range of 1.8 - 4 and
the latter a pH range of 3
- 6.5. Patent literature, however, mentions pH ranges of up to 8. The pH
ranges mentioned overlap
to some extent as the pH changes when the treatment stage proceeds and as the
pH is adjusted
according to the number, i.e. two or three, of the chlorine dioxide stages in
the bleaching.
Controversial views on the optimal conditions of a Do stage have been
presented in many contexts
and it has not been possible to give flawless grounds for the optimal
conditions.
CA 02382866 2006-07-07
4
In practice, bleaching has mostly been carried out at a pH of 1.8 - 3.5,
preferably 2.2 - 3, at a
temperature of 45 - 60 C and with a retention of about %z - 1 hours but it has
not been possible to
prove that a rise in the temperature even nearly always results directly in
quality losses.
Controversial views on the optimal conditions may be partly due to the
development of mixing
techniques. The D stage may have worked differently even in the same mill due
to different
mixing efficiencies. In our studies we have proved that the chemicals are
consumed very quickly
and the change of temperature within a small range does not as a whole have as
a remarkable effect
on the quality of pulp as has been thought.
The dosing of chlorine dioxide into the treatment stage has been controlled by
state of the art
methods so that sensors have been provided in the feed duct from the mixer to
the reactor, which
both measure the residual chemical and indicate the pulp brightness and the
feed of the chemical
has been controlled based on the information given by the sensors. The amount
of the chemical
needed in the treatment stage has been adjusted with the apparatus but from
time to time
interpretation of the information given by the sensors has been found
problematic due to the
varying process conditions. For technical reasons the measurement has been
performed after a
retention time of less than half a minute after the mixing of the chemicals
itself at which time in the
prior art processes typically 30 - 60 minutes of the retention of the whole
bleaching treatment is still
left. Thus it is clear that the residual chemical measured after a very short
retention after the mixer
is indicative and does not give a comprehensive picture of the proceeding of
the bleaching reaction
or its speed.
The studies we have performed have shown that if the mixing of the pulp is
efficient enough the
chemical can be totally consumed in the chlorine dioxide stage in a short
reaction time and at a
remarkably lower temperature than is suggested by the kinetic model (Chlorine
dioxide
Delignification Kinetics and Eop Extraction of Softwood Kraft Pulp, The
Canadian Journal of
Chemical Engineering, Volume 75, February 1997) which has recently been
accepted in general
discussion. In the tests we have performed, the chlorine dioxide mixed into
the pulp was
completely consumed in a few minutes already at a temperature of 60 C when the
mixing was
adequately efficient. The same kind of results were received from the
treatment of pulp in tests in
both one stage and in several stages. An efficient laboratory reactor was used
in all the tests.
CA 02382866 2006-07-07
Although the temperature could not be proved to have a direct correlation with
the decrease of the
Kappa number, the chemical residue and the brightness of the pulp in this
connection, its
significance as a factor accelerating the reaction is known from prior art
installations and the basic
chemical literature and it has been proved also in other laboratory tests.
Based on the tests, it is
5 clear that the temperature need not be over 85 - 90 C in order to have a
quick reaction. Thus, the
kinetic model mentioned indicates a slower chemical consumption than it really
is if the chemical is
dosed using efficient mixing. The study leading to the kinetic model in
question has been made in
laboratory conditions and probably the test have not been made with modem
laboratory reactors
used now allowing fluidizing mixing. Thus in the kinetic model of the test,
the reaction kinetics is
limited by the mixing because the significance of the mixing has not been
taken into account. Thus
it can be concluded that raising the temperature, increasing the mixing
efficiency and increasing the
retention will compensate each other.
EP-B1-0 496 782 discusses Dl and D2 stages which are brightening, nucleophilic
treatment stages.
According to the publication these stages are performed in two steps from
which the first at a pH of
the order of 6.0 - 10.0 and with a mixing time of 5- 40 minutes and the second
step at a pH of 1.9 -
4.2 and with a treatment time of two hours or longer. The temperature in both
the steps is about 55
- 85 C. The purpose of the two-step treatment described in the publication is
to achieve a higher
brightness with a certain chlorine dioxide dose than before or to achieve the
same brightness with a
smaller chlorine dioxide dose. In the treatment stage according to the
publication chlorine dioxide
need not necessarily be added to the second step at all but according to the
publication also a low
pH achieved by acidifying is adequate for the result desired.
SE-C2-504 210 deals with a multistage bleaching process in which chlorine
dioxide is used in at
least one stage. According to the publication, the chlorine dioxide stage is
performed at a
temperature of 90 - 130 degrees, at a pressure of 0.1 - 10 bar, at a
consistency of 8 - 40 %, and
with a retention time of 1 - 90 minutes. According to the publication, the
pressurized chlorine
dioxide stage mentioned is suitable for both the beginning
CA 02382866 2006-07-07
6
and the end of the sequence. The method described in this patent publication
is based on a study
made by Sunds Defibrator Industries AB, which is also discussed in the article
"Advancing the
Chlorine dioxide Process", Norden & Mellander, The 12th Sunds Defibrator
International
Technical Seminar, May 29. - 31. 1996, Sundsvall. The starting point of both
the article mentioned
and the patent publication is that the advantageous treatment time is 15
minutes or longer. Thus the
reaction kinetics of the method described in the patent publication and the
article mentioned
corresponds to the kinetic model presented in the study mentioned above when
the kinetic model is
calculated for the temperature 90 - 130 C discussed.
WO-A-98/00602 deals with a multistage bleaching sequence which uses as a
starting point the
chlorine dioxide stage described in patent SE-C2-504 210. This stage has been
continued without
an intermediate wash in a way by another treatment step in which the treatment
pressure and the
temperature of the pulp in a down-flow tower have been reduced whereby the
chlorine dioxide
residues in the pulp are separated to the gas phase of the down-flow tower and
can be removed
from there.
The prior art publications mentioned above disclose thus both the hot and the
pressurized chlorine
dioxide stage, as well as a two-step chlorine dioxide stage, in which only a
short retention and
mixing time do not suffice, even if it is intended particularly for a
bleaching stage. The
publications also partly aim at reducing the volume of chlorine dioxide to be
used and at
intensifying the bleaching process. However, these goals are striven for only
by raising the
temperature and by arranging the bleaching stage two-stepped. All the
processes cited above have
the typical feature that either the chlorine dioxide stage is very long, of
the order of more than 30
minutes, or the stage is very hot and pressurized in which case the high
temperature and the
pressurization is considered to allow the use of a little shorter reaction
time. However, none of the
publications suggests performing the first chlorine dioxide treatment
following the brown stock
washing or oxygen bleaching with a treatment time of a few minutes at a known
low temperature
when the pH is between 2 and 4 at the beginning of the treatment and between 2
and 3 at the end of
it.
CA 02382866 2006-07-07
7
The studies we have performed have shown, however, that prior art methods have
several weak
points which may be improved and thus the operation economy of the chlorine
dioxide stage may
be brought to a quite new level. These prior art methods do not for example
pay attention to the
significance of mixing as an accelerator of the reaction, or at least it has
not been systematically
reported, and on the other hand the temperature has not been used as an active
parameter in
controlling the consumption of chemical. Further, in the prior art solutions
the chlorine dioxide
treatment has been carried out based on laboratory tests performed with low-
intensity mixing; thus
the influence of the mixing has not been understood. On the other hand, even
when fluidizing
mixers have been used, the meaning of the mixing efficiency they provide has
not been understood
and made fully use of.
One of the main objectives of the present invention is to minimize the
consumption of chlorine
dioxide without compromising the efficiency and the result of the treatment.
Another objective is to reduce the size of the equipment needed in the D
stage and thus to reduce
the investments of the mill. Prior art D stages have been performed with a
relatively long
treatment time which requires a fairly large treatment tower which in turn
requires space and in
particular increases investment costs as, because of the corroding effect of
chlorine dioxide, the
tower must be constructed of titanium or a corrosion resistant special steel,
provided with brick
lining.
A third objective is to improve the process technical controllability of the
stage, whereby the pulp is
subjected to contact with the chemical only in conditions in which the
bleaching chemical is used in
the reaction, and to raise the temperature only as much as is required to
adjust the bleaching
reaction to last only as long as the pulp needs to flow from the mixer to the
discharge of the
reaction vessel. Thus the reaction can be adjusted actively with the
temperature.
A fourth objective is to optimize the ratio of the mixing needed and the
reactor volume so that, by
ensuring efficient mixing, the bleaching reaction is completed at the
temperature of 50 - 85 C and
at the same time to prevent high temperatures which are unfavorable in view of
the heat economy.
The objective is to reduce the corrosion risk of
CA 02382866 2006-07-07
8
the stage by using a low temperature. The temperature is of essential
importance also because with
the temperature rise the treatment of exhaust gases becomes problematic.
Temperatures below 90
degrees allow using indirect heating methods.
A fifth objective is to minimize, in the recycling of filtrates, the contact
of the pulp with the
reaction products in order to avoid harmful by-reactions. Tests we have
performed show that
chlorine dioxide is consumed in by-reactions for example with the organic
substance in the fiber
suspension coming to the chlorine dioxide stage Do either from the brown stock
washing or the
oxygen stage. In other words, chlorine dioxide does not only react with the
lignin of the fibers but
also with the organic matter in the liquid phase of the suspension.
A further objective is to reduce the amount of toxic chlorinated phenol
compounds which are, as is
known, produced during the chlorine dioxide treatment. It has been found out
that these phenol
compounds are produced when the chlorine dioxide reacts with the organic
matter produced during
the treatment or present in the fiber pulp. In other words, when reacting with
the organic matter
dissolved in the liquid phase of the fiber suspension, chlorine dioxide forms
these chlorinated
phenol compounds. Our invention relates to a treatment method in which the
reactions of the
chlorine dioxide with the organic by-products of the liquid phase, i.e. with
the organic matter
measurable with the COD, are minimized.
In order to reduce the environmental load of a pulp mill, it is specifically
desirable to reduce the
volume of effluent from the bleaching process. Many industrial prior art
installations have ended
up in using the apparatus arrangement illustrated in Figure 1. However,
already when deciding on
the apparatus arrangement it has been known that recycling the reaction
products from the washer
subsequent to the treatment tower with the filtrate back to the pulp
suspension will reduce the
brightness of the pulp at the end of the bleaching stage and increase the
consumption of chemicals
during the bleaching. This has been established also in our test, in which
pulp was treated with
recycled filtrate and clean water. When bleaching with recycled filtrate, the
brightness of the pulp
in the D. stage was reduced by 2 - 5 % (ISO) brightness units compared with
pulp diluted with
clean water.
CA 02382866 2006-07-07
. =
9
Similar results were received also by the authors of the kinetic model cited
above in their
experiment, in which the brightness of the pulp began to drop in the first
chlorination/chlorine
dioxide stage after a certain time. According to the study, the highest
brightness level was reached
exactly at that moment when the chemical had been completely consumed in the
conditions of the
test. As only reactions products of the stage remained in the pulp suspension,
the detrimental
impact of the reaction product of the Do stage on the development of the
brightness was easy to see.
The same applies both to the reactions products originating from the recycling
and to the reaction
products, which remain in the pulp when reactions cease as all the
chemical.has been consumed.
These two studies, i.e. our own and the external study, indicate that both the
time the pulp stays in
contact with the reaction products and the amount of the reaction products are
detrimental to the
development of the brightness. In order to solve this problem, the retention
of the chlorine dioxide
stage, i.e. the time from the chemical feed to the subsequent wash of the
pulp, should be adjusted to
be only as long as is necessary for the reactions of the lignin, i.e. until
all the bleaching chemical
has been consumed, and the bleaching should be stopped when the chemicals have
been consumed.
In this way the time the pulp is in contact with the effluent of the bleaching
is minimized and thus
the bleaching losses are reduced to the minimum. In practice, the detrimental
by-reactions take
place a little after the main reaction as the by-reactions require the
presence of reaction products in
the liquid phase. When the main reactions with the bleaching chemical can be
actively controlled
by adjusting the temperature, also the amount of the detrimental by-reactions,
i.e. the time available
for these reactions can be reduced to a minimum. This is essential always in a
Do stage but it is of
particular importance when the original pulp has been diluted with filtrate
obtained from the same
D. stage. In order to minimize the drawbacks of this recycling, the retention
of the D. stage must
be adjusted short.
The studies we have performed have shown that a Do-E bleaching of pulp, in
which the E is an
alkaline stage, and the use of a short chlorine dioxide stage and intensive
mixing in the presence of
filtrate produces a brightness level which is very close to the level reached
with bleaching processes
using clean water. As the brightness has
CA 02382866 2006-07-07
developed well in the presence of the reaction products of the Do stage,
shortening the treatment
time of pulp also reduced brigbtness losses in a stage where reactions
products of the bleaching
stage are present. This is natural as tests have proved that a prolonged
reaction time drops the
brightness when all the chemicals have been consumed.
5
Tests we have performed have also shown that the organic matter originating
from the oxygen stage
require a certain bleaching chemical content to cause the bleaching chemicals
to be consumed in
the oxidation of the organic matter. In our tests this consumption of
chemicals practically stopped
altogether when the chemical content in the liquid was 2 g/l active chlorine.
Thus, the chlorine
10 dioxide content should be kept relative low, if the consumption of the
bleaching chemical in
reactions with the organic matter of the filtrate is to be reduced. If
necessary, chlorine dioxide may
be dosed into the pulp at several stages. Then the chlorine dioxide content in
the liquid phase
remains relatively low and the chlorine dioxide reacts with the lignin
compound in the pulp, only.
Among other things these goals have been reached in a bleaching stage, in
which the treatments
have been chained so that the chlorine dioxide is dosed into the pulp in
several steps if necessary
and the similar treatment of pulp is repeated successively several times.
Although retention time
must be allowed between the treatments, the retention required to consume the
chemical to an
adequate degree may be only a few seconds depending on the conditions.
It was already stated above that the chlorine dioxide stage retention from the
chemical feed to the
subsequent wash should be only long enough to allow the chlorine dioxide to do
what it used for,
i.e. to react with the lignin in the pulp. This is because it has been found
out that the quality of pulp
deteriorates if the pulp is too long in contact with the reaction products.
However, the state-of-the-
art chlorine dioxide stages have been constructed so that the pulp is
discharged from a chlorine
dioxide tower to an atmospheric space which may be a drop leg, a tank or a
corresponding member
in which the pulp is diluted if required and from which the pulp is pumped
further to a washer.
This retention allows the pulp to continue reacting with the reaction products
and consequently the
quality of the pulp degrades.
CA 02382866 2006-07-07
11
A characteristic feature of a preferred embodiment of the invention is that
it, among other things,
solves the problem described above by arranging the chlorine dioxide stage
washer as close to the
chlorine dioxide reactor as possible. Experiments have shown already that pulp
can be fed with the
chlorine dioxide stage pump through the reactor straight up to the washer as
the consumption of
chemicals is very fast and the reactions can be controlled well.
This procedure, however, involves a new problem. When a washer is brought
close to the chlorine
dioxide reactor it must be ensured that no harmful amounts of chlorine dioxide
end up in the washer
because of the corrosion hazard caused by chlorine dioxide. Depending on the
material of the
washer, some chlorine dioxide may be allowed in the pulp flowing to the
washer; in other words,
the amount of the chlorine dioxide allowed depends on the materials used. It
is possible to
determine both experimentally and based on studies for each material the
allowable residual
chlorine dioxide level, which secures adequate life.
Thus, because of this problem, it is necessary to make sure that either there
is no chlorine dioxide
left in the discharge of the reactor, that the chlorine dioxide can be
eliminated from the pulp leaving
the reactor, or that the chlorine dioxide amount is acceptable in view of the
material of the
subsequent apparatus.
There are several solutions to this problem. In principle these are divided
into two groups: ways to
make sure that there is no chlorine dioxide in the pulp discharged from the
reactor, and ways to
eliminate chlorine dioxide from the pulp discharged from the reactor.
Solutions in the first group
mentioned are for example selecting the reaction temperature, reaction time
and reactor size so that
the chlorine dioxide has time to react well before the pulp is discharged from
the reactor.
Naturally, this is facilitated by mixing the chlorine dioxide with pulp as
efficiently as possible, i.e.
by using a fluidizing, in other words high-intensity mixer.
The second group involves destroying the chlorine dioxide discharged from the
reactor with the
pulp before the pulp is fed to a washer. The destruction of chlorine dioxide
is usually performed by
mixing chemical, in most cases sulphur chlorine dioxide water or sodium
bisulphate into the pulp
which reacts with the chlorine dioxide producing inert compounds which are
easy to remove from
the pulp during the wash. An essential feature in carrying out the process of
CA 02382866 2006-07-07
12
our invention is that the chemical is mixed with the pulp very efficiently.
The purpose is to
distribute the chemical into the pulp so that it can react with the residual
chlorine dioxide as fast as
possible, i.e. before the pulp comes to the washer. Thus, the invention covers
both measures
aiming at fast consumption of chemicals and destroying the residual chemicals
prior to the washer,
which provides for the use of short retention times reliably and without risk.
Other characteristic features of the method and apparatus of the present
invention are disclosed in
the appended patent claims.
The methods and the apparatus according to the invention are described more in
detail below with
reference to the accompanying drawings of which
Fig. 1 illustrates schematically the apparatus arrangement of a process
simulated in our tests;
Fig. 2 illustrates the change in the brightness of the fiber suspension in
different bleaching stages
determined based on our studies, as a function of the number of filtrate
recycles;
Fig. 3 illustrates the COD content of the fiber suspension liquid phase in
different bleaching stages
determined based on our studies, as a function of the number of filtrate
recycles;
Fig. 4 illustrates the change in the brightness of the fiber suspension in
different bleaching stages
determined based on our studies, as a function of the number of filtrate
recycles;
Fig. 5 illustrates an apparatus arrangement for carrying out the chlorine
dioxide treatment according
to a preferred embodiment of the invention; and
Fig. 6 illustrates an apparatus arrangement for carrying out the chlorine
dioxide treatment according
to another preferred embodiment of the invention.
Fig. 1 illustrates schematically the apparatus arrangement of a process
simulated in the tests,
comprising a washing apparatus 10, a press, a washer or a corresponding
member, and illustrating
the practical situation in which an essential portion of the water and the
reaction products
originating from digestion or oxygen bleaching is removed from the
CA 02382866 2006-07-07
13
pulp coming from a preceding treatment stage, i.e. either from screening and
washing following the
digestion or additionally also from oxygen bleaching or ozone treatment, which
all are in the
following called brown stock. After the washing apparatus 10, particularly if
the washer is a press,
the pulp is diluted at point 12 to the MC consistency before it is guided to a
mixer 16. Further it is
advantageous to heat the pulp to a desired temperature before the mixer. The
pulp can be heated
either by using the wash water of the washing apparatus 10 or after the
washing apparatus by
appropriate means. The heating can be carried out for example directly with
the dilution liquid, in a
separate feed tank or with a separate steam heater 14, preferably by means of
direct steam heating
or by heating the wash liquid of a preceding washer. Also chlorine dioxide can
be mixed into the
heated and diluted pulp in the mixer 16. The mixer 16 may also be used for
mixing into the pulp
alkali or acid in order to adjust the pH of the pulp to be appropriate for the
treatment. In addition to
heating the pulp before the mixer 16, the pulp may be heated in some cases
alternatively after the
mixer 16 if desired for example with an indirect heat exchanger. After the
dilution, heating,
preferably steam heating, if they have been desired, and the mixing of
chlorine dioxide the pulp is
guided to a treatment vessel 18, from which the pulp is further taken to a
wash apparatus 20,
preferably at the pressure of the apparatus feed pump (not illustrated).
As was already stated above, it must be ensured that no chlorine dioxide
causing corrosion is
brought to the washer with the pulp. This is ensured for example by providing
the discharge end of
the reactor with a discharger by means of which chemical is mixed into the
pulp to eliminate
chlorine dioxide from the pulp. The discharger must have an adequately high
intensity so as to
reach absolutely even distribution of the chemical into the pulp. Preferably
the apparatus comprises
an apparatus containing a fluidizing means, whereby the mixing of chemical
takes place in a
fluidized state. However, there are also static high-intensity mixers
available with which
fluidization can be reached in a narrow slot. Other mixing devices may be for
example a valve
provided in the discharge of the reactor or in the vicinity of it, which, when
throttled under an
adequate pressure difference (>1.0 -1.5 bar), fluidizes the pulp to a state
which fully corresponds to
the mixing obtained with a prior art fluidizing mixer provided with a rotating
mixing member. Also
a fluidizing discharger may be used as the mixing member, which has been
provided with means, for
CA 02382866 2006-07-07
14
example vanes, for increasing the pressure. In some cases, it is also possible
to use a fluidizing
pump, for example a so-called MC pump, both for mixing the chemicals and for
pumping pulp to a
washer.
In other words, the apparatus of the invention comprises a pump, from which
pulp is pumped to a
reactor, which due to the short reaction time may be a relatively short duct,
a mixer placed
preceding the pump if chlorine dioxide is not mixed in the pump, a mixing
apparatus disposed
substantially at the discharge end of the reactor vessel or in the vicinity of
it for mixing the
chemical which eliminates the chlorine dioxide, and a washer. The essential
feature of the
apparatus described is that pulp travels from the chlorine dioxide mixing to
the washer in a closed
space and thus chlorine-containing compounds are prevented from escaping to
the atmosphere.
When studying the invention the apparatus illustrated in Figure 1 was
simulated in a laboratory by
introducing to dilution 12 continuously a pulp batch the properties of which
correspond to the ones
of pulp obtained conventionally from brown stock washing. Here brown stock
means, as was
stated above, chemical pulp which has been washed and screened, or washed,
screened and
bleached with oxygen, or washed, screened and treated with ozone. As brown
stock washing is
typically performed with a press, the pulp was brought to the dilution at the
discharge consistency
of the press, which is typically about 30 %. After the first dilution with
clean water to the
consistency of about ten percent, chlorine dioxide was added to the pulp under
vigorous mixing
substantially during the whole time the chlorine dioxide was added. Finally,
the pulp was bleached
in laboratory equipment and the filtrate separated while thickening the pulp
was returned from the
wash, which corresponds to the washer 20 in Fig. 1, to be used as dilution
liquid in the new pulp
batch to be bleached, which procedure corresponds to the dilution 12 after the
washing apparatus
10. As particularly the wash filtrate during the continuous recycling was to
be studied, a sample
was taken from the wash filtrate from each cycle, and the amount of organic
solids in it was
determined by an analysis depicting it, i.e. by the so-called COD (mg/1).
Quite a number of these
bleaching processes were performed by recycling the filtrate of the washer in
the way described
above. It was found out that the recycling of the filtrate from the bleaching
decreased the bleaching
result by several
CA 02382866 2006-07-07
brightness units whereby the presence of reaction products in the Da stage
results in decreasing of
the bleaching result at the end of the sequence (Fig. 2). The sequence Do EP-
DI was used in the
experiment.
5 Figures 2 and 4 illustrate the decrease in the final brightness as a result
of the increase in the
amount of reaction products in the bleaching sequence Do-EP-DI. The horizontal
axis in the figure
illustrates how many times the filtrate from the washing stage ending the Do
stage was recycled
back to the dilution preceding the Do stage. The figure illustrates how the
recycling times of the
filtrate, i.e. in this case how many times there are reaction products from
the Do treatment present
10 in the bleaching stage, decrease the brightness of the pulp and thus create
needs to increase the
amount of the chemical required in order to reach the desired brightness.
Since the retention is the
same in each bleaching, the concentration of the reaction products from the
bleaching has a
significant role in the decrease of the brightness in this case. Figure 2
further indicates that the
increase in the volume of reaction products does not affect strongly the
brightness after the Do stage
15 but only the brightness after the EP and Dl stages which at its worst
decrease by more then 10 ISO
units.
Figure 3 illustrates the increase of COD as a function of the number of
recycling times in a three-
stage Do EP-Dl bleaching when all the stages have been simulated as
illustrated in Fig. 1. In other
words there has been no intermediate wash of the pulp but filtrate has been
pressed out from the
pulp and the pulp has been diluted for the following stage with filtrate
obtained from the following
stage mentioned by pressing it out from the previous pulp batch. It should be
noted that the COD
content increases in the Do stage in the liquid phase remarkably but does not
increase much with the
number of the treatment times; thus, chlorine dioxide oxidizes and consumes in
the D. stage the
organic matter which is measurable with the COD analysis. This means that in
the conditions
prevailing in the D. stage, chlorine dioxide oxidizes non-selectively the
organic matter both in the
pulp and in the filtrate. In other bleaching stages the phenomenon is not as
remarkable and the
increase of the COD content continues in the liquid phase for a longer time.
On the other hand, the
increase of impurities in the liquid phase reduces the final brightness
reached.
CA 02382866 2006-07-07
16
Filtrate from an oxygen stage, i.e. brown stock treatment, was treated with
chlorine dioxide
solution under bleaching conditions. The results indicate that when the
concentration of the
bleaching chemicals at the beginning has been very high, the bleaching
chemical has reacted with
the COD of the filtrate so that the COD of the filtrate has not increased
almost at all irrespective of
the number of the filtrate recycling times. On the other hand, the tests
showed that when the
concentration of the bleaching chemical in the filtrate was 2 g/l calculated
as active chlorine the
chlorine-containing chemical is almost not at all active with the filtrate of
the oxygen stage but the
COD content of the liquid phase can rise relatively freely. When the chemical
concentration was
2.5 g/l calculated as active chlorine the COD content already rose to some
extent, i.e. in practice
the activity of the chlorine chemical relative to the filtrate of the oxygen
stage has reduced
remarkably. This speaks for arranging the chlorine dioxide stage so that the
concentration of the
bleaching chemical is not allowed to rise much over 2.5 g/l in the filtrate
during one treatment stage
or step.
Thus, the present invention also relates to different ways of running
bleaching processes according
to a preferred embodiment of the invention so that the chlorine dioxide
concentration in the fiber
suspension to be treated remains less then 2.5 g/l, preferably less than 2.0
g/l calculated as active
chlorine. The tests we have performed have indicated that the treatment time
in the reaction vessel
should be shorter than 10 minutes, preferably shorter than 7.5 minutes, more
preferably shorter than
5 minutes. According to our tests the temperature of the pulp should be over
40 C whereby
naturally the temperature and the treatment time are inversely proportional to
each other, i.e. when
the temperature is high the treatment time can be shorter and vice versa. The
pH in tuYn should be
1.5 - 6.5, preferably 2- 4.
Another test aiming at determining the limit for the chlorine dioxide
concentration is presented in
table 1.
CA 02382866 2006-07-07
17
Table 1
Original solution: OZ stage solution, COD 14800 mg/1
Results from chlorine dioxide treatment of solution
dosed solution at end COD
active Cl, g ml active Cl, at end
g/l
0.3 500 0.24 14800
3 500 1.85 10700
6 500 2.02 7300
9 500 1.93 5600
The test presented in the table was performed by dosing 0.3 - 9 g/l chlorine
dioxide calculated as
active chlorine into filtrate from an oxygen stage the COD content of which
was 14800 mg/l.
Finally, both the COD and the amount of residual chlorine dioxide were
determined. The results
indicate that with a chlorine dioxide dose of 3 g/1 and more, the COD of the
solution has reduced
while the amount of residual chlorine dioxide has remained at 2 g/l. In other
words, also this test
supports the theory presented earlier that the chlorine dioxide concentration
calculated as active
chlorine should be less than 2.5 g/l, preferably less than 2.0 g/1, in order
to prevent the chlorine
dioxide from being consumed in undesirable reactions with the COD.
Example 1
The following table presents the chlorine dioxide bleaching tests performed
with a short bleaching
stage Do EP the results from which have been determined always after a similar
alkaline extraction
stage. A laboratory reactor provided with high intensity mixing and automatic
dosing equipment
has been used in tests 1 -7. Only for the comparison, the results from a
corresponding bleaching
test performed in a plastic bag with low intensity mixing are presented (test
8). After the tests the
residual chemical content was always determined from the pulp in order to make
sure that all the
CA 02382866 2006-07-07
18
chlorine dioxide had been consumed in the bleaching reactions in all the
tests.
Table 2
DO staee
Consistency 10 %, amount of softwood pulp 135 g, Kappa 16.7, Viscosity 1108,
Brightness 35.5
Code Injection Dilution Temp D dose Time Kappa Visco Brightness
C kg/adt min (after E stage)
1 100% water 60 38.41 5 3.2 1027 57.4
2 100% water 70 38.41 5 3.3 1036 57.2
3 100% water 85 38.41 5 3.4 1030 57.7
4 50+50 water 70 38.4 3+3 3.3 1019 57.2
5 50+50 water 85 38.41 3+3 3.4 992 58.1
6 50+50 Do clean 70 38.41 2+2 3.3 1007 55.3
7 50+50 Do clean 85 38.41 3+3 3.5 964 56.4
8 100 water 60 38.41 45 4.0 1042 57.9
CA 02382866 2006-07-07
19
In tests 1 - 3, clean water was used as dilution liquid, 38.41 kg/adt of
chlorine dioxide calculated as
active chlorine was dosed all at the same time under intensive agitation, the
treatment time was 4 -
6 minutes and only the temperature was changed in order to find out the
influence of the
temperature increase on the end result. As can be seen from the results, the
influence of the
temperature on the brightness or the Kappa number of pulp is not significant.
In fact, the
differences both in the Kappa numbers and the brightnesses determined between
the tests remain
within the limits of measurement accuracy.
Tests 4 and 5 were performed otherwise the same way as described above except
that the chlorine
dioxide was dosed at two stages so that the treatment time in both stages was
three minutes. When
the temperature was changed, no essential changes were detected in the Kappa
number and also the
changes in the brightness remained under one ISO unit.
In tests 6 and 7, filtrate from a chlorine dioxide treatment was used in the
dilution, the chemical was
dosed in two stages and the treatment time in both the stages was in test 6
two minutes and in test 7
three minutes. Between the tests, the temperature was raised from 70 degrees
to 85 degrees. Still
the Kappa number and the brightness remained practically unchanged. It is
worth noticing that
neither a treatment time, which was 50 % longer, nor a temperature, which was
15 degrees higher,
did bring a clear improvement in the results in test 7.
Comparative test 8, which was performed in a plastic bag, corresponds
primarily to test 1,
compared with which the only difference in addition to the chlorine dioxide
mixing method is that
the treatment time, 45 minutes, used in the test is the time given by the
kinetic theory for a chlorine
dioxide treatment performed at the temperature of 60 degrees. The results
indicate that the Kappa
number remains a little weaker than in the tests 1 - 7 performed applying the
method of our
invention. On the average the same brightness was reached as in the tests
simulating our invention.
CA 02382866 2006-07-07
It may be concluded from the tests that it is possible to make all the
chemical (chlorine dioxide) to
be consumed by using intensive mixing at a remarkably shorter time than is
suggested by the
kinetic model, and in a test series performed at a temperature below 85 - 90
C, quality losses
measured with viscosity (scan standard method) are not significant and are
mainly due to prolonged
5 mixing occurring in the laboratory equipment.
Example 2
130 g pulp having a consistency of 10 % and a Kappa number of 29.7 was
supplied to the
laboratory reactor used in the tests 1- 7 of example 1. 60 kg/adt chlorine
dioxide calculated as
10 active chlorine was mixed into the pulp, the pulp was mixed well and the
reaction was allowed to
continue for three minutes at a temperature of 60 C. After this the residual
chlorine dioxide was
determined from the pulp; it was 0.2 kg/adt. In other words, more than 50 %
larger dose of chlorine
dioxide than used in tests of the previous example was practically all
consumed in three minutes.
15 Based on the test in example 2, it can be expected that it is possible in
industrial scale to treat
brown stock with chlorine dioxide amounts in the order of 70 -80 kg/adt
(calculated as active
chlorine) at a temperature below 90 C and to bring the delignification process
substantially to its
end in less than ten minutes, preferably in a few minutes. In other words,
compared with the
kinetic model referred to above, only about one tenth of the time given in the
model is needed for
20 the reaction. In practice this can be transferred directly to the size of
the reaction vessel, which may
possibly be only a tube reactor instead of a large tower used today.
According to a preferred embodiment of the invention, a probe measuring the
chemical residue has
been provided following the treatment vessel, which in some cases may be only
a tube. When the
chemical dose has been selected based on the lignin and the brightness
indicator of the pulp, the
temperature of the process is adjusted based on the residual chemical so that
the whole chemical
dose is consumed in the pulp suspension within the treatment time determined
by the size of the
treatment vessel and the production of the line.
CA 02382866 2006-07-07
, =
21
In the adjustment method described above, the temperature is an active
parameter in ensuring the
consumption of chemical and in optimizing the treatment time. This can be done
in the process
according to the present invention because the retention between the mixing of
the chemicals and
the end of the process is short. As the mixing of chemicals is according to
our study an essential
part of the process in order to ensure a quick reaction, the process is
provided with one or several
efficient chemical mixer/s. Thus the chemical may be mixed for example with
two mixers disposed
one after the other in a tube line.
According to a further embodiment of the present invention, an essential part
of the process is to
guide the process with the temperature so that the process is in contact with
the chemicals only as
long as is necessary for the consumption of the bleaching chemical. In the
prior art, i.e. in almost
all the Do stages used in the world, the process conditions have been
determined so carefully
(mainly according to the kinetic model already referred to earlier in several
connections) that all the
chemical is consumed much before the top of the tower and what is left at end
stage is the reaction
products and the pulp treated with the bleaching chemical. This permits
reduction of pulp quality
losses, particularly to decrease of pulp brightness as by-reactions continue
for a long time before
the pulp bleaching stage ends, i.e. before the pulp arrives to a washer/press.
The method according
to our invention allows active adjustment of the bleaching reaction to
continue during the whole
retention of the reactor but prevents it from continuing to a level where the
bleaching chemical has
already been consumed and the quality losses begin to occur without the
positive bleaching effect
provided by the bleaching chemical.
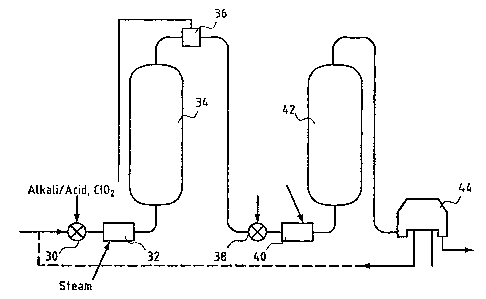
Figure 5 illustrates an apparatus according to a preferred embodiment of the
invention for
performing a chlorine dioxide bleaching stage Do. It comprises a high-
intensity, preferably a so-
called fluidizing mixer 30, by means of which chlorine dioxide and if
necessary either acid or alkali
is mixed into the pulp in order to adjust the pH. Close to the mixer 30 in
connection with the Da
stage, preferably prior to the mixer 30, a heater has been provided, which may
be for example a
direct or an indirect steam heater 32 or a liquid - liquid heater. After the
chemical mixing and the
heating, the pulp is guided to a reaction vessel, i.e. a treatment tower 34.
After the tower 34,
residual chlorine dioxide is determined from the pulp by a residue
measurement, based on which it
is possible, and in
CA 02382866 2006-07-07
22
fact the intention, in this embodiment of the invention to adjust the
temperature of the pulp, if
necessary, preferably before the pulp is fed to the first mixer 30. If
chlorine dioxide remains in the
pulp after the reaction vessel 34, the temperature is raised by the heater 32
and, after the retention
of the treatment stage, it is checked if there still is chlorine dioxide left
in the pulp. On the other
hand, if all the chlorine dioxide has been consumed the temperature may be
decreased in order to
increase the treatment time. By this measure it can be ensured that the whole
treatment time is used
in a beneficial bleaching reaction. At the same time, the time is minimized
during which the pulp is
in contact with the reaction products. From the residue measurement 36 the
pulp flows in this
embodiment to another mixer 38, in which a new dose of chlorine dioxide is
mixed and, if
necessary, chemical needed for the adjustment of the pH. Another heater 40 has
been provided in
connection with the mixer 38, by means of which the temperature of the second
step may be raised,
if necessary. After this the pulp is guided to another treatment vessel or
tower 42 and from there
further to a washer 44.
According to a preferred method, the chlorine dioxide residue is measured by
selecting a small
chlorine dioxide amount in a way as the target value towards which the process
is adjusted.
Because of the corrosion risk the monitoring of the residual volume is of
vital importance in order
to ensure adequate life of the equipment. Then the value guiding the
adjustment is the relative
residue, i.e. the difference between the target value and the measured value.
In addition to the steam heating methods described above of heating the pulp
close to the chemical
mixing with low-pressure steam before the pulp is pumped to the bleaching
stage, the heating may
be performed also by heating the diluting filtrate or by heating the
displacing wash liquid of the
previous washer. All these methods give a similar result if the temperature
measurement has been
arranged and connected to the control circuit. Thus the heating method is not
essential, but the
adjustment of the temperature must be active and the temperature must be
adjustable within a
certain range. Thus the adjustment based on the information from the residue
measurement may be
adjustment of the volume of the steam used as the heat source, adjustment of
the heating of the
filtrate used for dilution or adjustment of the heating of the wash liquid of
the washer.
CA 02382866 2006-07-07
23
Some kind of a processor is preferably connected with the apparatus described
above and in
particular with the adjustment system used in connection with it, although
also manual adjustment
can be used, to guide for example the volume of steam flowing into the heater
32 based on the
residual chlorine dioxide. At least two adjustment methods are thinkable.
According to the first
method, the amount of chlorine dioxide in the treated pulp indicated by the
residue measurement
only guides more efficiency to the heating of the pulp, for example more steam
to the heater. It is
advantageous to add a retention connection with the system so that a chlorine
dioxide finding in the
residue measurement immediately causes a certain raise in the heating
efficiency which is kept
constant at least for the time the bleaching process takes so that the change
caused by the heating
has time to arrive to the residue measurement. After this the result of the
residue measurement is
reassessed and the necessary changes are made in the heating efficiency.
The second method is actually a continuation of the previous one in that sense
that by adding some
logic to the adjustment system, it is possible to "teach" the system to adjust
itself optimally. In
other words, when the system looks for the optimal increases in the heating
efficiency in relation to
the chlorine dioxide residue the way described above, the "correct" pairs of
chlorine dioxide
residues and efficiency increases may be saved in the memory of the processor,
whereby the
adjustment system can in continuous operation adjust the heating quickly
without "searching".
Naturally, in the "teaching phase" the increases in the heating efficiency
should be made in
relatively small steps. Then there is no risk of having raised the temperature
too quickly, which
would result in the bleaching reaction coming to its end too early and the
pulp having too much
time to start reacting with the reaction products of the bleaching reaction.
Further it is possible, if so desired or based on further tests considered
necessary, to take into
consideration in the adjustment logic also the pulp temperature. In other
words, the increase in the
heating efficiency would not be directly dependent on the chlorine residue,
only, but to some extent
also for example on the temperature of the pulp after the temperature
adjustment or the temperature
at the residue measurement.
CA 02382866 2006-07-07
= ~
24
When the process apparatus described above is desired to be used as optimally
as possible it works
so that so much chlorine dioxide is dosed to the first treatment step, i.e. to
the mixer 30, that its
consistency in the fiber suspension becomes less than 2.5 g/1, preferably 2.0
g/1 calculated as active
chlorine. In most cases this dose can be performed by calculating in relation
to the pulp flow as the
travel of chlorine dioxide counter-currently from the filtrates obtained from
wash stages subsequent
to the process is prevented. The bleaching stage tower is used, in the process
sense, to refer to the
retention taking place after the mixing, which retention may also be realized
for example by a tube
or an enlargement of a tube. The bleaching stage and the retention after it
have been dimensioned
in the process for a certain production whereby the residue measurement has
been provided, in view
of the adjustability of the process, advantageously in the discharge of the
pulp; based on the
measurement the heating of the pulp prior to its feeding to the reaction
vessel and the amount of
chlorine dioxide to be fed may be controlled so that the residue remains,
according to one way of
operation, practically at zero. According to another way of operation, the
temperature of the pulp
to be fed to another mixer is adjusted based on the residue measurement at the
discharge of the
tower so that if some unreacted chlorine dioxide has remained in the pulp in
the fust tower, the
residue after the second tower does not remain detrimentally high.
Figure 6 illustrates a bleaching apparatus according to yet another preferred
embodiment of the
invention. The embodiment of Figure 6 differs from the one in Figure 5 in fact
only in that in the
embodiment illustrated in Figure 6 the process apparatus comprises three
subsequent treatment
towers and 30', 34' and 36' correspond with 30, 34 and 36. The apparatus
embodiment depends
mainly on the fact that it may be necessary in the D. stage to reduce the
Kappa number, i.e. the
lignin content of the pulp more than can be achieved in two towers using the
low chlorine dioxide
contents of the invention. Then it is sensible to divide the chlorine dioxide
dose into three
substantially equal portions so that the chlorine dioxide content in each
tower remains under the
limit value given above and on the other hand the chemical consumption is
even.
Naturally it is clear that at its simplest many of the advantages of the
invention are achievable
already with apparatus, which comprises from the apparatus illustrated in
figure 5 only the first
reaction vessel 34 with its pulp dilution and heating apparatus 32,
CA 02382866 2006-07-07
chemical feed apparatus 30, residual chlorine dioxide measurement 36 and wash
apparatus 44.
Even with this apparatus the pulp treatment time with chlorine dioxide can be
optimized so that the
pulp does not stay too long in contact with the reaction products.
5 Thus it is clear that the bleaching process according to the invention may
be adapted to the required
decrease in Kappa number merely by changing the number of treatment towers. It
should be noted
that even if this seems now to be a solution involving expensive apparatus, it
is quite the opposite.
If the initial situation is that prior art chlorine dioxide treatments require
treatment times of 0.5 - 1
hour, they have to be performed in large bricked bleaching towers. A bricked
tower instead of an
10 ordinary metal tower is required because of the strong corrosive influence
of chlorine dioxide.
Now, when treatment times of only in the order of 1- 10 minutes are required,
a substantially
smaller treatment vessel is required, which may be even a glass fiber or
standard tube vessel, which
is small compared with the conventional towers, and the manufacturing costs as
well as
construction, erection, isolation and instrumentation costs of it are only a
fraction of those of the
15 corresponding prior art bricked towers.
When the reactions of the pulp can be arranged to take place practically in
one closed space up to
the wash apparatus, the process eliminates gaseous emissions; thus no gas
emission collecting
devices used in conventional stages are needed. This saves investment costs
and is also
20 advantageous in view of the climate protection.
As can be understood from the above description, a new chlorine dioxide
bleaching method
avoiding the drawbacks and problems of prior art methods and apparatus has
been developed, the
characteristic features of which are disclosed in the appended claims. Thus it
is clear that only a
25 few preferred embodiments of the invention have been described above, which
in no way intend to
limit the scope of protection of the invention from what is defined by the
patent claims.