Note: Descriptions are shown in the official language in which they were submitted.
CA 02383489 2002-02-12
WO 01/13050 PCT/US99/18344
HEAT TEMPERATURE RAISING SYSTEM
Technical Field
This invention relates to a process and apparatus for raising heat
temperature, i.e.,
transferring heat from a low temperature heat source to a higher temperature
heat sink.
More specifically, in one embodiment, the invention relates to a process and
apparatus for
transforming a heat carrying substance under a first pressure Pi and producing
a vapor of a
second heat carrying substance at a second pressure P2.
Background of the Invention
The process of the invention is based on the principle that the melting point
of a
substance changes as the applied pressure changes. A set of multiple heat
conductive
and pressure rated (i.e. pressurizable) conduits is connected with
pressurizing equipment.
Inside these conduits is a mass of Heat Temperature Raising Medium (HTR
medium)
which is capable of undergoing a phase change upon the application of
pressure. When
the HTR medium is subjected to a pressure variation, it allows the said medium
to absorb
and store heat at low temperature. After the HTR medium has absorbed and
stored the
heat at low temperature, a high pressure is applied to the HTR medium. This
increase in
pressure on the HTR medium causes the melting point of the medium to increase
and
allows the medium to release the heat it has stored to the higher surrounding
temperature.
Thus, the HTR medium can successfully elevate the temperature from a low
temperature
to a higher temperature. Another medium, Heat Carrying Medium (HCM medium), is
used in the present invention to assist and complete the cycle of raising heat
temperature
from low temperature heat source to a higher temperature heat sink.
The use of a melting point inversion for water purification of saline water is
disclosed in U.S. Patent number 3,354,083. This process requires a large sum
of saline
water and mediums to be exposed to high pressure and low pressure. Due to the
difficulty
of such operation, this process was unsuccessful. Hence there remains a need
in the art for
a process and apparatus which can make use of the latent heat of fusion and/or
latent heat of
evaporation of heat transfer materials to transfer heat without subjecting
large quantities of
process fluids to high pressure.
SUBSTITUTE SHEET (RULE 26)
CA 02383489 2002-02-12
WO 01/13050 PCT/US99/18344
2
Disclosure of the Invention
It is accordingly an aspect of the invention to provide a process and
apparatus for
transferring heat from a heat source at one temperature to a heat sink at a
higher
temperature.
It is another aspect of the invention to provide a process and apparatus, as
above,
which accomplishes the heat transfer by effecting a phase change of a heat
transfer
medium using variable pressure to alter the temperature at which the phase
change
occurs.
It is another aspect of the invention to provide a process and apparatus, as
above,
which minimizes the amount of fluid subjected to the variable pressure.
Unlike the above prior process, the present invention maintains the Heat
Temperature Raising Medium (HTR medium) inside conduits. By subjecting the HTR
medium to a pressure fluctuation between low pressure and high pressure, the
invention
allows the HTR medium to absorb heat at low temperature and release heat at a
higher
temperature. Thus, the HTR medium can successfully elevate the heat
temperature, i.e.,
transfer heat from a first temperature to a second, higher temperature without
the need of
exposing large quantities of materials to low pressure and high-pressure
operation.
In the present invention, the high-pressure zone is preferably stationary and
is
secured inside a Heat Temperature Raiser (HTR unit), while the transportation
of the heat
between the low temperature source and high temperature sink is accomplished
by the Heat
Carrying Medium (HCM medium) operating at low pressure. Therefore, large
quantities of
material moving between low pressure and high-pressure operation are not
required.
The present invention provides for a Heat Temperature Raising System, referred
to
as a HTR system, for taking in heat from a low temperature (TL) heat source
and supplying
heat to a high temperature (TH) heat sink. A HTR system comprises a heat
temperature
raising unit, referred as a HTR unit, a mass of heat temperature raising
medium, referred to
as HTR medium, and a mass of a first heat carrying medium, referred to as HCM-
1
medium, and a mass of second heat carrying medium, referred to as HCM-2
medium. The
system is divided into three compartments: a central compartment referred to
as a HTR
compartment, containing the HTR units, a heat source compartment and a heat
sink
compartment. There is a first valuing means separating the heat source
compartment and
SUBSTITUTE SHEET (RULE 26)
CA 02383489 2002-02-12
WO 01/13050 PCT/US99/18344
3
the HTR compartment and a second valuing means separating the heat sink
compartment
and the HTR compartment.
The HTR unit is preferably a stationary unit, i.e., the HTR medium is not
itself
conveyed between the heat source and the heat sink. The HTR unit comprises a
set of heat
conductive and pressure sustaining conduits, a mass of HTR medium contained in
the HTR
unit and a pressurizer for pressurizing and depressurizing the HTR medium
contained in the
HTR unit.
The pressurizer can be, for example, a mechanical compressor such as a plunger
mechanism, a steam source, or other compressive fluid source, or any other
device which
can apply substantially hydrostatic pressure to the HTR medium. The HTR medium
is
subjected to a series of cyclic operations that comprise melting under a first
pressure and a
first temperature, respectively referred to as a first HTR pressure and a
first HTR
temperature and solidifying under a second pressure and a second temperature,
respectively
referred to as a second HTR pressure and a second HTR temperature. A mass of
first heat
carrying medium HCM-1 is vaporized by receiving heat from the heat source to
form a
HCM-1 vapor and the vapor passes through the first valuing means to come in
contact with
the HTR unit and melt the HTR medium. The HCM-1 vapor is thereby condensed to
form
a mass of HCM-1 condensate. Then, a mass of the second heat carrying medium
HCM-2 is
brought in contact with the HTR medium to thereby solidify the HTR medium
under the
second HTR pressure and temperature and thereby form a vapor stream of the
second heat
carrying medium, referred to as HCM-2 vapor. The HCM-2 vapor passes through
the
second valuing means and releases heat to the heat sink and thereby condenses
to form a
condensate of HCM-2 medium. The condensate is recycled to the vaporization
operation
described. It is noted that since the HTR medium is preferably contained
within a
stationary HTR unit, it takes the first and second HCM medium to exchange heat
with the
heat source and the heat sink.
It is also within the scope of the invention to use the same medium for both
HCM-1
and HCM-2. 1n this alternative, the HCM-1 condensate formed in the low
temperature
condensation operation may be used in the high temperature evaporation
operation, and the
HCM-2 condensate formed in the high temperature condensation operation may be
used in
the low temperature vaporization operation.
SUBSTITUTE SHEET (RULE 26)
CA 02383489 2002-02-12
WO 01/13050 PCT/US99/18344
4
A HTR system can be used in providing chill water and air conditioning and
used in
vacuum freezing processes, in ice production processes, ice storage processes,
distillative
freezing processes and mufti-effect evaporation processes.
Brief Description of the Drawing
For a full understanding of the invention, the following detailed description
should be read in conjunction with the drawings, wherein:
Fig. 1 is a schematic illustration of one embodiment of an HTR system of the
invention;
Fig. 2 illustrates one embodiment of an HTR unit of the invention;
Fig. 2A illustrates another embodiment of an HTR unit of the invention;
Fig 2B illustrates the structure of one embodiment of a longitudinal fin unit
of the
invention;
Fig. 2C is a partial cutaway view of an HTR unit containing the fin unit of
Fig.
2B;
Fig. 3A is a cross-sectional view of one embodiment of a multiple connected
conduit unit of the invention;
Fig. 3B is a cross-sectional view of the multiple conduit unit of Fig. 3A with
longitudinal fin units installed;
Fig. 4A is a cross-sectional view of another embodiment of a multiple conduit
unit of the invention;
Fig. 4B is a cross-sectional view of the conduit unit of Fig. 4A with
longitudinal
fin units installed;
Fig. 5A is a cross-sectional view of one embodiment of mufti-void metal block
unit of the invention;
Fig. 5B is a cross-sectional view of the mufti-void metal block unit of Fig.
5A
with longitudinal fin units installed;
Fig. 6A illustrates one embodiment of an HTR system of the invention with heat
transfer from a first Heat Carrying Medium;
Fig. 6B illustrates the HTR system of Fig. 6A with heat transfer to a second
Heat
Carrying Medium;
SUBSTITUTE SHEET (RULE 26)
CA 02383489 2002-02-12
WO 01/13050 PCT/US99/18344
Fig. 7A illustrates another embodiment of an HTR system of the invention with
heat transfer from a first Heat Carrying Medium;
Fig. 7B illustrates the HTR system of Fig. 7A with heat transfer to a second
Heat
Carrying Medium;
5 Fig. 8A illustrates an embodiment of an HTR system of the invention which is
useful in vacuum freezing with heat transfer from a first Heat Carrying
Medium;
Fig. 8B illustrates the HTR system of Fig. 8A with heat transfer to a second
Heat
Carrying Medium;
Fig. 9A illustrates another embodiment of an HTR system of the invention
useful
in vacuum freezing with heat transfer from a first Heat Carrying Medium;
Fig. 9B illustrates the HTR system of Fig. 9B with heat transfer to a second
Heat
Carrying Medium;
Fig. l0A illustrates another embodiment of the HTR system of the invention
useful in vacuum crystallization of an aqueous solution and non-aqueous
mixtures with
heat transfer from a first Heat Carrying Medium;
Fig. lOB illustrates the HTR system of Fig. l0A with heat transfer to a second
Heat Carrying Medium;
Fig. 11A illustrates one embodiment of a multiple effect evaporating system
incorporating HTR units of the invention and operated in a first cycle;
Fig. 11B illustrates the mufti-effect evaporating system of Fig. 11A operated
a
second cycle;
Fig. 12A illustrates a multiple effect evaporator system similar to that of
Fig. 1 lA
and employing corrugated metal walls to form falling film evaporators and
operated in a
first cycle;
Fig. 12B illustrates the multiple effect evaporator of Fig. 12A operated in a
second cycle;
Fig. 13 illustrates an automatic valuing system of the invention; and3
Fig. 13A illustrates a single valve of the invention.
Detailed Description of the Preferred Embodiments
A heat temperature raising system (HTR system) of the present invention
utilizes a
heat temperature raising medium (HTR medium) that undergoes cyclic
solidification and
SUBSTITUTE SHEET (RULE 26)
CA 02383489 2002-02-12
WO 01/13050 PCT/US99/18344
6
melting operations and one or more heat carrying mediums (HCM mediums) that
undergo
vaporization and condensation operations. An HTR system is used to take in
heat at a low
temperature heat source and discharge heat to a higher temperature heat sink.
Figure 1 illustrates the processing steps of an HTR system. In this and other
figures,
like numerals denote the same structure. In the system, a mass of HTR medium
contained
within a multitude of heat conductive and pressure sustaining conduits is
subjected to a
cyclic operations undergoing: (a) a first step of melting most of the HTR
medium under
pressure PHTRI ~d temperature T~1, [state 1 - state2]; (b) a second step of
varying the
medium pressure from PH-,-R, to PHT~ [state 2 - state 3]; (c) a third step of
solidifying most
of the HTR medium under pressure PHT~ and temperature THT~ [state 3 - state
4]; and (d)
a fourth step of varying the medium pressure from PHA to PHTRi [state 4 -
state 1].
A first heat carrying medium [HCM-1 medium] receives heat from a low
temperature heat source and thereby generates a first HCM medium vapor, HCM-1
medium
vapor, which is condensed by releasing heat Q~ to the HTR medium in step 1. A
second
heat carrying medium [HCM-2 medium] receives heat from the HTR medium in step
3 to
form a HCM-2 medium vapor, which is condensed by rejecting heat QH to the heat
sink at
an elevated temperature.
Figure 2 illustrates the construction of a Heat Temperature Raising unit (HTR
unit). It comprises a multitude of heat conductive and pressure sustaining
conduits 2, a
mass of heat temperature raising medium (HTR medium) 3, a header 4, and a
cylinder
and piston 5 for pressurizing and depressurizing the HTR medium. The use of a
cylinder
and piston is of course merely one example of a method for pressurizing the
HTR
medium. Other well-known methods will readily occur to those skilled in the
art.
Figure 2A illustrates another heat temperature raising unit similar to that of
Figure 2, except that there is a fin unit 6 installed in each conduit to
enhance heat
transfer. Figure 2B illustrates the structure of a longitudinal fin unit 6 and
Figure 2C
illustrates a cutaway view of a conduit 2 containing a fin unit 6.
Figure 3A illustrates a cross section of a unit of multiple connected conduits
7 unit
formed by bonding two sheets 8 of corrugated material. The neighboring
conduits are
connected by wings 9. Figure 3B illustrated a cross section of a multiple
conduit 7 unit
similar to that of Figure 3A with a fin unit 10 installed in each conduit 7 to
enhance heat
transfer.
SUBSTITUTE SHEET (RULE 26)
CA 02383489 2002-02-12
WO 01/13050 PCT/US99/18344
7
Figure 4A illustrates a cross section of a multiple conduit unit or a multiple
tube
assembly in which each conduit 7 is isolated from neighboring conduit. Figure
4B
illustrates cross-sections of a multiple conduit unit similar to that of
Figure 4A with a fin
unit 10 installed in each conduit to enhance heat transfer.
Figure SA illustrates a cross-section of multivoid metal blocks that has
multiple
conduits 12. Figure 5B illustrates a cross section of a unit similar to that
of Figure SA with
a fin unit 13 in each of the conduits.
It is noted that the mass of the wall (MW) of a HTR unit, e.g., walls of the
conduits
and header (see Figure 2) and block (see Figures SA, SB) is a major factor
that affects the
efficiency of the HTR operation. It is important to keep the ratio of the mass
of the wall of
the HTR unit (MW) to the mass of HTR medium to a low value. Referring again to
Figure
l, as the HTR unit is heated in step 2, the wall (not shown) is heated from T~
to TZ and
thereby absorbs sensible heat. Therefore a part of the HTR medium is
solidified to meet the
energy balance relation. Thus, the amount of the remaining HTR medium to be
solidified
becomes less and the heat released in step 3 becomes less. Again in Figure 1,
as the HTR
unit is cooled in step 4, the wall is cooled from TZ to T~ and thereby
releases sensible heat.
Thus, a part of the HTR medium is melted to meet the energy balance relation.
Therefore,
the amount of the remaining HTR medium to be melted becomes less and the heat
absorbed
in step 1 becomes less. The problem described is referred to as "Thermal
Inertia Problem".
It is important to note that the higher the ratio of the mass of the wall of a
HTR unit to the
mass of the HTR medium, the more serious the "Thermal Inertia Problem" becomes
and the
lower the productivity of the HTR unit becomes and the lower the efficiency of
the HTR
unit becomes.
Suitable materials of construction of the HTR unit include aluminum, steel,
copper,
brass, and other metal and non-metal materials having sufficient heat transfer
characteristics
to permit acceptable heat transfer from the first Heat Carrying Medium and
from the HTR
unit to the second Heat Carrying Medium including heat transfer through the
walls.
The mass ratio of the wall to the HTR medium is the lowest for a HTR unit with
multiple tube assembly illustrated by Figures 4A and 4B. The mass ratio of the
wall to the
HTR medium is higher in a HTR unit with connected walls illustrated by Figures
3A and
3B. The mass ratio of the wall to HTR medium is the highest in a HTR unit made
of a
multivoid block illustrated by Figures SA and SB.
SUBSTITUTE SHEET (RULE 26)
CA 02383489 2002-02-12
WO 01/13050 PCT/US99/18344
8
Thus, the use of a multivoid block is generally less preferred, because the
mass ratio
of wall to HTR medium is so high that the efficiency of HTR operation is low,
its efficiency
becomes so low that it is not very usefizl when the TZ - Tl, referred to as
temperature lift, is
high.
Figures 6A and 6B show a HTR system that comprises a Heat Temperature Raising
Zone 15, HTR Zone (Zone - 1), a flash cooling Zone 16 (Zone - 2), and a direct
contact
condensation Zone 17 (Zone - 3). Figure 6A shows that a feed liquid 21 is
flash vaporized
to be cooled and generate a heat carrying medium vapor V1 (HCM-1 vapor). The
HCM-1
medium vapor is then passed through an automatic valve 18 (made of, for
example, a
grating and flaps made of thin film), and it is brought in contact with the
outer surface 22 of
the HTR conduits for exchanging heat with the HTR medium in the HTR unit to
thereby
melt the HTR medium at T~RI and P,-i~~. Figure 6B shows that a mass of heat
carrying
medium is brought in contact with the HTR unit under pressure PHA and at
temperature
THE to thereby generate a heat carrying medium vapor VZ (HCM-2 vapor) and
solidify the
HTR medium. The HCM-2 medium vapor is then passed through an automatic valve
19,
(made of, again for example, a grating and flaps made of thin film), and is
brought in direct
contact with a fluid 24 introduced in Zone-3 to contact the HCM-2 vapor and
heat the said
fluid. This system is useful in producing chilled water for air conditioning
and also for
other industrial cooling operations.
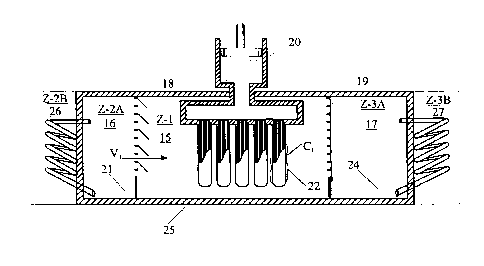
Figure 7A and 7B illustrate another HTR system that comprises a HTR Zone 15
(Zone 1), HCM-1 vapor generation Zone 16 (Zone-2A) and heat source Zone 26
(Zone 2B),
a HCM-2 vapor condensation Zone 17 (Zone-3A) and a heat sink Zone 17 (Zone-
3B).
Figure 7A illustrates that a HCM medium is brought in heat exchange relation
with a heat
source in Zone-2B to generate HCM-1 vapor V1 in Zone 2A. The HCM-1 vapor is
condensed and the HTR medium is melted at T~1 and PHI in Zone 1. Figure 7B
shows
that a mass of HCM-2 is applied on the outer surface 23 of the conduit of the
HTR unit and
is vaporized to form HCM-2 vapor VZ and solidify the HTR medium at THE and P
Hue.
The HCM-2 vapor is then pass through a second valve 19 and condensed in Zone-
3A by
releasing heat to a heat sink 27 in Zone-3B.
Figures 8A and 8B illustrate a HTR system used in a vacuum freezing operation.
This system is useful in seawater desalination, industrial solution
concentration, waste water
concentration and crystallization of aqueous solution and non-aqueous
mixtures. The
SUBSTITUTE SHEET (RULE 26)
CA 02383489 2002-02-12
WO 01/13050 PCT/US99/18344
9
system comprises a HTR Zone 29 (Zone-1), a vacuum-freezing Zone 30 (Zone-2), a
crystal
melting Zone 34 (Zone-3) and a crystal washing Zone 23 (Zone-4).
The processing steps conducted in the system are explained by referring to sea
water
desalination as an example. Referring to Fig 8A, a sea water feed is subjected
to a
deaeration and a heat exchange operation and is flash vaporized in Zone-2 to
form a first
low pressure water vapor that is designated as HCM-1 vapor V~ and a mass of
ice crystals
35. The pressures of the HCM-1 vapor is around 3.5 torr, which is lower than
the triple
point pressure of water (4.58 torr). The mass of ice crystals and the
concentrated mother
liquid form a slurry stream that is subjected to a crystal washing Zone 23
(Zone 4) and the
purified ice is introduced to Zone-3. The low-pressure water vapor V~ (HCM-1
vapor) is
brought in contact with the HTR unit that is at pressure PHTRi and temperature
T ,-,~1. The
water vapor is desublimed to form a mass of desublimate (ice) 36 on the outer
surfaces of
the HTR unit and the HTR medium is melted. Referring to Figure 8B, the HTR
unit is then
subjected to pressure PHA and temperature T HT~ to generated a second water
vapor VZ
(HCM-2 vapor) at a pressure around 5 ton, which is higher than the triple
point pressure of
water. The second water vapor Vz is brought in contact with the ice in Zone 3
to thereby
simultaneously melt the ice and condense the second water vapor VZ as output
stream 39.
Both the condensate of the second vapor VZ and the melt 39 of the ice become
purified
product water.
The system illustrated in Figures 9A and 9B is similar to that of Figures 8A
and 8B
and operations conducted in this system are also similar. In this system the
HCM-2 vapor is
brought into an indirect contact heat exchange with the purified crystals in
Zone 3. Melt
liquid exits in stream 47.
The system illustrated by Figures 10A and lOB is usefizl in vacuum
crystallization
of aqueous solution and non-aqueous mixtures. In this system the HCM-2 vapor
formed is
condensed by a cooling medium in Zone-3, and the crystal formed is not melted
by the
HCM-2 vapor. This system is particularly usefixl in ice block making whereby
the small ice
made in Zone 2 can be compressed to form ice block. This system is also very
useful in
conducting a distillative freezing process as described in U.S. Patent Nos.:
4218893,
4433558, 4451273 and 4578093, which are hereby incorporated by reference in
their
entirety.
SUBSTITUTE SHEET (RULE 26)
CA 02383489 2002-02-12
WO 01/13050 PCT/US99/18344
Figures 11A and 11B illustrates a mufti-effect evaporating system that
comprises a
first multiple effect evaporator, Z-lA, a second multiple effect evaporator, Z-
1B, in the
major processing Zone Z-1, a first HTR unit 61 in Z-2A and a second HTR unit
62 in Z-2B
at the first end of the system, a third HTR unit 63 in Z-3A and a fourth HTR
unit 64 in Z-3B
S at the second end of the system. The HTR units are operated cyclically and
the mufti-effect
evaporators are operated nearly continuously.
The first multiple effect evaporator Z-lA comprises, as an example, nine
evaporators 69-77 (ZE-1 through ZE-9) connected in series, with operating
pressures
decreasing successively in the direction from the left end ZE-1 toward the
right end ZE-9.
10 The second multiple effect evaporator Z-1B, comprises nine evaporators 78-
86 (ZE'-1
through ZE'-9) connected in series, with operating pressures decreasing
successively in the
direction from the right end ZE'-1 toward the left end ZE'-9. It is readily
understood that
more or less than 9 effects may be used, the actual number being selected
based on
perameters such as operating conditions and economics.
1 S Each of the four HTR units is operated cyclically and alternately serves
as an
evaporator and as a condenser. The four HTR units are operated in a
coordinated manner.
While one of the two HTR units at each end serves as an evaporator, the other
unit serves as
a condenser. As illustrated in Figure 11A, the HTR units in Z-2A and Z-3B
serves as two
vapor generators and the HTR units in Z-2B and Z-3A serve as two condensers.
The vapor streams generated are used as steam supplies to the two ZE-1 Zones
and
ZE'-1 Zones and thereby initiate the multiple effect evaporator operations.
The vapor
steams leaving the last effect ZE-9 and ZE'-9 are condensed in the two HTR-
units serving
as condensers. Figure 11B illustrates the same system in the other half of the
cycle, in
which the HTR units in Z-2B and Z-3A become the vapor generators and the HTR
units in
Z-2A and Z-3B become the condensers.
Figures 12A and 12B illustrate a multiple effect evaporator system similar to
those
illustrated by Figures 11A and 11B. In this system, corrugated metal walls 96,
97 are used
to form falling film evaporators 89-95 and 98-104.
Figure 13 illustrates an Automatic valuing system that provides vapor passages
from
one chamber to another without any mechanical actuating device or any
electrical switch.
The automatic valuing system is made of a mesh 105 for structural support as
well as a
grating 107 for the two chambers with flaps 106 (made of thin filin) attached
on to the
SUBSTITUTE SHEET (RULE 26)
CA 02383489 2002-02-12
WO 01/13050 PCT/US99/18344
11
grating 107. The gratings with attached thin films fiznction as dividers
between two
chambers. The thin film flaps 106 are pressure sensitive where when one
chamber's
pressure is higher than the other, the flap will automatically opens allowing
the vapor to
flow from the first chamber with higher pressure to the second chamber with
the low
S pressure. It automatically closes when the pressure of the second chamber
becomes higher
than that in the first chamber.
Figure 13A illustrates a single valve made of thin film flap attached onto a
holder on
the top of flap.
In nature, heat flows from a high temperature heat source to a low temperature
heat
sink. The present invention discloses a process and apparatus with proper
input of energy
to accomplish just the opposite of what nature does. Theoretically the work
input (applied
pressure times the volume change of the HTR medium) to the HTR for a given
amount of
heat raised (the latent heat of HTR medium) per unit temperature rise is
inversely
proportional to the absolute temperature. This relationship may be derived
from the
Clausius-Clapeyron equation (Journal of Chemical Physics, Volume 25, No. 3),
and can
take the form: f POV/~T}=1/T, representing the law of heat temperature raiser.
This
invention is based on the principle that as the applied pressure changes the
melting point
changes.
There are generally two types of compounds suitable for an HTR medium: a Type
A substance is the most common, for which, as the pressure applied on the
medium
increases, its melting point increases. Thus, the said medium will absorb heat
at low
temperature and low pressure; and as the applied pressure increases, the
melting point of the
medium increases, allowing it to release heat and solidify at a higher
temperature. For a
Type B substance, such as water, as the applied pressure increases, the
melting temperature
decreases. As the pressure applied upon ice increases, the melting point
decreases whereby
allowing ice to absorb heat at temperature below 0°C to melt and again
solidify at 0°C as the
pressure is released. Nonetheless, both types of substances can be used to
absorb heat at a
lower temperature and to release heat at a higher temperature by subjecting
the medium to a
pressure variation. Therefore, any medium with proper variation in melting
point can be
used as a Heat Temperature Raising Medium. Suitable Heat Temperature Raising
Medium
include compounds having melting points ranging from between -30°C and
100°C as
described, for example, in the Handbook of Chemistry and Physics, which is
incorporated
SUBSTITUTE SHEET (RULE 26)
CA 02383489 2002-02-12
WO 01/13050 PCT/US99/18344
12
herein by reference. Any resulting mixture should have a eutectic point range
of between -
30°C and 100°C. A mixture can also be used as a HTR medium. The
figures will now
again be discussed in fiuther detail.
In the system of Figure l, a mass of HTR medium contained within a multitude
of
heat conductive and pressure sustaining conduits is subjected to a cyclic
operations
undergoing: (a) a first step of melting most of the HTR medium under pressure
PHTRI ~d
temperature THAI, [state 1 - state2]. (b) a second step of varying the medium
pressure from
PHTRI to PH~z [state 2 - state 3]. (c) a third step of solidifying most of the
HTR medium
under pressure PHT~ and temperature TE,T~ [state 3 - state 4], and (d) a
fourth step of
varying the medium pressure from PHA to P,~RI [state 4 - state 1]. A first
heat carrying
medium [HCM-1 medium] receives heat from a low temperature heat source and
thereby
generate a first HCM medium vapor, HCM-1 medium vapor, which is condensed by
releasing heat QL to the HTR medium in step 1. A second heat carrying medium
[HCM-2
medium] receives heat from the HTR medium in step 3 to form a HCM-2 medium
vapor,
which is condensed by rejecting heat QH to the heat sink at an elevated
temperature.
Still referring to Figure 1, the masses of HTR medium in the solid and liquid
states
in state 1 are respectively represented by (ms)~~,1 and (mL)~,1; the masses of
HTR
medium in the solid and liquid states in state 2 are respectively represented
by (ms)~-,T~ and
(m~)HT~; the mass of HTR medium in the solid and liquid states in state 3 are
respectively
represented by (ms)~,3 and (m~)~,3; the mass of HTR medium in the solid and
liquid
states in state 4 are represented by (ms),-ITRa and (mL),-,~,a. Then, the heat
taken in at the
low temperature heat source QL is given by: QL={(mL)HTR,z - (mL)HT~,1 } x ~.m
where 7,,", is
the latent heat of melting of the HTR medium. It also shows that the heat
given to the high
temperature heat sink minus QH is given by: - QH ={(ms)HTa,a - (ms)H~ra,3} x
~,,~". As the
HTR unit changes its temperatures from TH~,4 to THTa,I, the HTR unit releases
sensible
heat. Therefore, a portion of the HTR medium melts to satisfy the energy
balance relation.
Therefore, (mL)HTR,i is greater than (m~)~~4. This makes the heat removable
from the low
temperature heat source smaller. Similarly, as the HTR unit changes its
temperature from
T~ to THE the HTR unit absorbs sensible heat. Therefore, a portion of the HTR
medium solidifies to satisfy the energy balance relation. Therefore, (ms)H~,3
is greater than
(ms)HTR,2. This makes the heat available to the high temperature heat sink
smaller. The loss
in the amount of heat transferable is referred to as "Thermal Inertia
Problem." It can be
SUBSTITUTE SHEET (RULE 26)
CA 02383489 2002-02-12
WO 01/13050 PCT/US99/18344
13
shown that as the mass of the HTR conduits increases, the more serious the
Thermal Inertia
Problem becomes, and the greater the temperature lift, {TH~,Z - THra,i } is
the more serious
the Thermal Inertial Problem becomes.
The figure also shows that one may use the same substance for both HCM-l and
HCM-2 mediums. In this case, one may use as the HCM-1 a condensate obtained in
the
low temperature condensation operation as the HCM-2 medium and subject it to a
high
temperature vaporization operation and one may also used the HCM-2 condensate
formed
in the high temperature condensation operation as the HCM-1 medium and subject
it to a
low temperature vaporization operation.
Figure 2 illustrates the construction of a heat temperature raising unit (HTR
unit) 1.
It comprises a multitude of heat conductive and pressure sustaining tubes 2, a
mass of heat
temperature raising medium 3, a header 4, and a cylinder and a piston 5 for
pressurizing and
depressurizing the HTR medium. This pressure device can be just a piston or
any other
type of pressurizing device.
By changing the pressure applied to the Heat Temperature Raiser unit, the Heat
Temperature Raising Medium (HTR medium) 3 absorbs heat at a low temperature
and
releases heat at a higher temperature. The present invention will be
illustrated by use of a
type A substance as a Heat Temperature Raising Medium. The HTR at a low
pressure
melts and store the heat in the form of latent heat of the HTR medium. As the
applied
pressure is increased, the melting point of the HTR medium increases. Under
the higher
pressure, the latent heat of the medium HTR medium will be released at a
higher
temperature, and the HTR medium will solidify again. Therefore by changing the
applied
pressure, HTR will allow the HTR medium to perform a batch processing of the
elevating
heat temperature from a lower temperature to a higher temperature. The faster
the rate of
the heat transfer through the conduits of the Heat Temperature Raiser, the
faster the HTR
medium can absorb and release its latent heat. Therefore, the pressure changes
have to take
place faster. Therefore the amount of the heat temperature raising per unit
length of the
conduits of the HTR per unit time will be greater. A set of fins can be
installed inside of the
conduits to increase the rate of the heat transfer within the HTR medium.
Figure 2A shows
a HTR unit similar to that of Figure 2, except that there are a set of
longitudinal fins 6
installed in the conduits. Figure 2B shows the construction of a longitudinal
fin. Figure 2C
shows a partial cut away view of a conduit with a longitudinal fin installed
therein.
SUBSTITUTE SHEET (RULE 26)
CA 02383489 2002-02-12
WO 01/13050 PCT/US99/18344
14
The conduit of the Heat Temperature Raiser is made of a heat conductive and
pressure sustaining material. There are different ways of constructing
conduits used in
constructing a HTR unit:
Figure 3A illustrates a set of conduits 7 formed by bonding together
corrugated
plates 8. The neighboring conduits are connected by wings 9. Figure 3B
illustrates a set of
conduits with connecting wings similar to that shown in Figure 3A, except that
longitudinal
fins are installed within the conduits.
Figure 4A illustrates a set of conduits 7 that have substantially uniform wall
thickness 8 and that the conduits are individually separated without having
any connecting
walls between two neighboring conduits. Figure 4B shows a similar unit with
fins 10 in the
conduits.
Figure SA illustrates a multivoid metal block having conduits that do not have
substantial uniform thicknesses. Figure SB illustrates a similar structure
with fins in the
conduits.
During the high-pressure operation of the HTR, the melting point of HTR medium
will increase as the pressure is increased, thereby allowing the HTR medium to
release heat
at the higher temperature. At the same time, the outer wall of the conduits of
the HTR will
also increase its temperature by absorbing heat released by HTR medium. After
pressure is
released from HTR, the pressure of HTR will decrease and the melting point of
the HTR
medium will decrease which will allow the HTR medium to absorb heat at the
lower
temperature while the outer walls of the conduit of the HTR will reduce the
temperature by
releasing heat. The effectiveness of HTR in upgrading heat temperature of the
HCM
medium from a low temperature heat source to a higher temperature heat sink is
dependent
on the amount of latent heat of HTR medium elevated by the batch process of
HTR minus
the amount of sensible heat used by the outer walls of the conduits.
Therefore, the less
sensible heat used by the outer wall of the conduits, the more effective the
HTR in
upgrading heat temperature will be. Thus, materials with less sensible heat
retention used in
constructing the HTR, the less sensible heat will be lost, and the
effectiveness of the HTR
will increase.
Therefore, in the above mentioned types of conduits, a set of conduits with
relatively uniform wall thickness is preferred over the multivoid block
conduits for
constructing HTR because a set of conduits with relatively uniform wall
thickness will
SUBSTITUTE SHEET (RULE 26)
CA 02383489 2002-02-12
WO 01/13050 PCT/US99/18344
minimize the loss of sensible heat per unit volume of conduit. The amount of
the material
used by the multivoid block conduits is too large causing too much sensible
heat loss per
unit volume of conduit; thus it is not preferred and may be suitable for use
in the HTR to
elevate heat temperature in the HTR. On the other hand, a set of the conduits
with
5 relatively uniform wall thickness has a relatively small mass of material
used in the walls of
the conduits which can reduce the loss in effectiveness due to the sensible
heat per unit
volume of the conduit.
By alternating between high-pressure and low-pressure in the HTR, the
temperature
of HCM medium vapor will increase; whereby HCM medium vapor will condense onto
the
10 HTR allowing its latent heat to transfer through the walls of the conduits
into the HTR
medium and allow the latent heat to liquefy the HTR medium. As the pressure of
HTR is
increased, the melting point of the HTR medium will increase and the HTR
medium will
transfer its latent heat at a higher temperature out of the HTR and back to
the HCM
medium. The capacity of the heat elevation of the HTR depends on the speed of
the HTR
1 S pressure alternation between high-pressure and low-pressure. The
alternation between
high-pressure and low-pressure of HTR is dependent on the rate of the heat of
HCM
medium transferring into HTR medium or the rate of heat of HTR medium
transferring
back out to HCM medium.
The heat transfer resistance in condensing HCM-1 vapor is low and the heat
transfer
resistance through the conduit wall is low. The major heat transfer resistance
is in the heat
transfer through the HTR medium itself.
The condensing rate of HCM medium vapor is very fast, and the heat transfer
rate
through the wall of the conduits is very fast, but the heat transfer rate of
the HTR medium
inside the metallic tube is very slow. Therefore, the rate of the alternation
of HTR between
high-pressure and low-pressure depends on the heat transfer rate of the medium
inside the
HTR. Hence, in order to increase the rate of the heat transfer resistance of
the HTR
medium in the conduits, it is necessary to install a set of fins inside the
conduits of the HTR.
There are many types of fins such as longitudinal radial fins and longitudinal
radial fins
with holes on the fins. There are many methods and materials can be used to
form these
heat conductive fins. For example, a piece of thin metal may be folded in a
zigzag shape
and then formed into a circular shape as shown in figure 2B to become a
longitudinal radial
fin. One skilled in the art can readily select a fin design from many well-
known designs.
SUBSTITUTE SHEET (RULE 26)
CA 02383489 2002-02-12
WO 01/13050 PCT/US99/18344
16
The fins will greatly increase the heat transfer rate of the medium inside the
HTR because
the radial fms transfer heat in the radial direction of the tube. The
installation of the fms
will greatly reduced the heat transfer resistance of the HTR and allow
increase in the speed
of the pressure alternation of the HTR, and thereby increase the heat raising
capacity of the
HTR.
The Heat Temperature Raiser (HTR) is preferably a stationary device only
acting as
an elevator to elevate the temperature of the latent heat of the HTR medium.
It does not by
itself have the ability to transfer heat from the low temperature heat source
to the higher
temperature heat sink. Therefore, one or two Heat Carrying Mediums (HCM
mediums) are
needed to assist in transferring heat from low temperature heat source to a
higher
temperature heat sink. Any compound that has proper vapor pressure at the
desired
operating temperature can be used as the Heat Carrying Medium.
HCM-1 medium in the Zone with heat source either enters in direct contact or
through a heat exchanger absorbs heat from the heat source and vaporizes
itself to become
HCM-1 vapor. The HCM-1 medium vapor flows to the Zone where HTR is located and
condenses onto the surface of the HTR. As HCM-1 medium vapor condenses on the
surface of the HTR, HTR medium solid melts and the heat absorbed by the HTR
medium is
stored as the latent heat of the HTR medium. After the HTR medium is melted, a
high
pressure is applied onto the HTR medium to elevate the melting point of the
HTR medium
to a high temperature. At the same time, the applied high pressure will cause
the latent heat
of melting to be elevated to a higher temperature as well. Then, the HTR
medium releases
heat to the HCM-2 medium and the HCM-2 medium vaporizes at a higher
temperature.
The HCM-2 medium vapor enters into the higher temperature heat sink Zone where
HCM-
2 vapor releases heat to the higher temperature heat sink through direct or
indirect heat
exchange. The whole process of this invention comprises subjecting the HTR
medium
inside of the HTR unit to batch processing of elevating the heat temperature;
and subjecting
the HCM mediums to vaporization, condensation, absorbing, and releasing of the
heat. The
HCM mediums perform the functions of transferring heat from a low temperature
heat
source to a high temperature heat sink. Therefore, a Heat Temperature Raising
System
comprises a Heat Temperature Raiser, a Heat Temperature Raising Medium, and
one or
two Heat Carrying Mediums.
SUBSTITUTE SHEET (RULE 26)
CA 02383489 2002-02-12
WO 01/13050 PCT/US99/18344
17
Since the process for elevating heat temperature by the HTR is a batch
process, the
amount of latent heat of HTR medium elevated from each batch is limited. Thus,
when the
amount of the latent heat produced by one process is not enough to cover the
sensible heat
loss of the outer walls of the conduits, multiple sets of HTR systems can be
used to elevate
the process stepwise to the desired temperature.
Under different types of heat source, there are different operation methods.
The
methods are as follows:
When the heat source and the HCM medium cannot be allowed to make direct
contact, a heat exchanger can be used for the heat transfer. For example, in
an air
conditioning operation, water is used as HCM-1 and room air serves as the heat
source and
an indirect heat exchange takes place.
When the heat source and the HCM medium may make direct contact, HCM
medium absorbs heat directly from the low temperature heat source and HCM-2
medium
condenses and releases the heat to the high temperature heat sink. For
example, one may
1 S use a water insoluble substance as HCM medium to remove heat from an
aqueous solution.
The material processed may provide a HCM medium and also serves as the heat
source. For example, in flash vaporizing an aqueous solution, a part of the
water becomes
HCM-1 and the remaining part serves as heat source.
Exotherrnal chemical reaction produces heat for vaporizing Heat Carrying
Medium
to produce HCM medium vapor.
A unit of HTR is shown in Figure 2, whereby the Heat Temperature Raising Unit
1
has the multitude of heat conductive pressure sustaining conduit 2, and a heat
temperature
raising medium 3 filled inside of the conduit 2 and a header 4 and a
pressurizing device 5.
In order to increase heat transfer rate one may install a longitudinal radial
fin 6
inside of each of the heat conductive pressure sustain conduits 2 as shown in
Figure 2A.
The system illustrated by Figures 3A, 4A, and SA illustrates the cross section
taken
at section AA of Figure 2 and Figures 3B, 4B, and SB illustrates the cross
section taken at
section AA of Figure 2A.
Figure 3A illustrates a cross section view of a multiple connected conduits
containing HTR medium 7 and heat conductive conduit with pressure sustaining
wall 8 and
connecting walls between neighboring conduits 9. Figure 3B illustrates a cross
section
view of a multiple connected conduits containing HTR medium 7 and heat
conductive
SUBSTITUTE SHEET (RULE 26)
CA 02383489 2002-02-12
WO 01/13050 PCT/US99/18344
18
conduit with pressure sustaining wall 8 and connecting walls between
neighboring conduits
9 and a heat conductive fin 10 installed inside of the conduit. Figure 4A
illustrates a cross
section view of a multiple tube assembly containing HTR medium 7 and heat
conductive
and pressure sustaining wall 8 enclosing each conduit. It shows that there are
no connecting
walls between two neighboring conduits. Figure 4B illustrates a cross section
view of a
multiple tube assembly containing HTR medium 7 and heat conductive and
pressure
sustaining wall 8 enclosing each tube and it shows there are no connecting
wall between
two neighboring tube and a heat conductive fin 10 installed inside of the
tube. Figure SA
illustrates a cross section view of multivoid heat conductive block containing
multitude of
conduits 11 which containing HTR medium 12. Figure SB illustrates a cross
section view
of mufti void heat conductive block containing multitude of conduits 11 which
containing
HTR medium 12 and a heat conductive fin 13 installed inside of the conduit.
The system illustrated by Figures 6A and 6B is a vapor pressure raising
system. It
comprises a vapor pressure raising Zone Z-1 15, a low pressure first vapor
generation Zone,
1 S Z-2 16, and high pressure vapor condensing Zone Z-3 17, and a valve 18
connecting from
Zone Z-2 to Zone Z-1 and a valve 19 connecting from Zone Z-1 to Zone Z-3.
Figure 6A
illustrates that the first step of generating first vapor, HCM-1 vapor, in
Zone 2. Adjusting
the pressure of the HTR medium at the first pressure, where the melting
temperature of the
HTR medium is lower than the condensing temperature of the first vapor, HCM-1
vapor,
thereby condensing the first vapor, HCM-1 vapor, and melting the HTR medium
inside of
the HTR conduits. Upon actuating a pressure variation device, one can control
the
transformation temperature such that the first vapor, HCM vapor, generating in
Zone 2
enters through a self actuating valve (made of a grating and flaps made of
thin films
attached on to the grating) 18 transfers heat from the first vapor, HCM vapor,
to HTR
medium thereby condenses the HCM-1 vapor into the solid or liquid form and
melts the
HTR medium.
Figure 6B illustrates that upon applying the pressure to the medium by
actuating the
HTR pressurizing device, and applying a HCM-2 liquid outside of the HTR
conduits, heat
transfers from the HTR medium to the HCM-2 liquid thereby solidifies the HTR
medium
and vaporizes the HCM-2 liquid thereby forming a high pressure vapor, HCM-2
vapor.
The HCM-2 vapor flows from Zone 1 to Zone 3 through another self actuating
valve 19 to
thereby condense inside of Zone 3.
SUBSTITUTE SHEET (RULE 26)
CA 02383489 2002-02-12
WO 01/13050 PCT/US99/18344
19
The system illustrated by Figures 7A and 7B are similar of those of Figures 6A
and
6B with two added Zones; a low temperature heat source Zone and a high
temperature heat
sink Zone. Figure 7A and 7B illustrate a system for providing air conditioning
or producing
chill water. It comprises vapor pressure raising Zone Z-l and first vapor
generating Zone
Z-2A and second vapor condensing Zone Z-3A and low temperature heat source
Zone Z-2B
containing low temperature heat exchanger coil 26 and high temperature heat
sink Zone Z-
3B containing high temperature heat exchanger coil 27. Air or water is
introduced into
Zone Z-2B to exchange heat with the process liquid HCM medium thereby forming
a first
HCM1 vapor. The first HCM1 vapor enters into Zone Z-1 heat exchange with HTR
medium condenses therein and melts the HTR medium. Referring to Figure 7B the
pressure
is adjusted to raise the solidification temperature of HTR medium and applying
process
liquid on the outside of the heat transfer conduits. Thereby, heat is
transferred from the
HTR medium to the process liquid and solidifying the HTR medium and generating
a
second HCM-2 vapor. Second HCM-2 vapor enters into Zone Z-3A and the air is
circulated in Zone Z-3B. The second HCM-2 vapor is heat exchanged with air or
water in
Zone Z-3B to thereby condenses and heat is removed by the outside air or cool
water.
The system illustrated by Figures 8A and 8B are similar to that of Figures 6A
and
6B. In this system, simultaneous vaporization and freezing operations take
place to produce
HCM-1 vapor and a mass of solid of the process substance. Figures 8A and 8B
illustrate a
system for providing pure water. It comprises vapor pressure raising Zone Z-1
and first
vapor generating Zone Z-2 and second vapor condensing Zone Z-3 and a crystal
washing
Zone Z-4. The process substance 32 is feed into the Zone Z-2 to generate first
HCM1 vapor
and solid simultaneously. The solid generated in Zone Z-2 along with mother
liquid is sent
to the Zone Z-4 for crystal washing. The first HCM 1 vapor generated in Zone Z-
2 enters
into Zone Z-1 heat exchange with HTR medium condensed therein and melts the
HTR
medium. Figure 8B illustrates the pressure is adjusted to raise the
solidification temperature
of HTR medium and applying process liquid on the outside of the heat transfer
conduits.
Thereby, heat is transferred from the HTR medium to the process liquid and
solidifies the
HTR medium, generating second HCM-2 vapor. The washed crystals 33 from Zone Z-
4 are
then sent to Zone Z-3 to allow the second HCM-2 vapor to condense and thereby
melt and
generate pure water 39.
SUBSTITUTE SHEET (RULE 26)
CA 02383489 2002-02-12
WO 01/13050 PCT/US99/18344
The system illustrated in Figures 9A and 9B is similar to that of Figures 8A
and 8B
and operations conducted in this system are also similar. In this system the
HCM-2 vapor is
brought into an indirect contact heat exchange with the purified crystals in
Zone 3.
The system illustrated by Figures 10A and lOB is useful in vacuum
crystallization
5 of aqueous solution and non-aqueous mixtures. In this system the HCM-2 vapor
formed is
condensed by a cooling medium in Zone 3, and the crystal formed is not melted
by the
HCM-2 vapor. This system is particularly usefizl in making ice blocks whereby
the small
pieces of ice made in Zone 2 can be compressed to form an ice block. This
system is also
very useful in conducting the distillative freezing process invented by Chen-
Yen Cheng and
10 Sing-Wang Cheng and described in U.S. Patent Nos.: 4218893, 4433558,
4451273 and
4578093.
Figures 11A and 11B illustrate a multi-effect evaporating system that
comprises a
first multiple effect evaporator, Z-lA, a second multiple effect evaporator, Z-
1B, in the
major processing Zone Z-1, a first HTR unit 61 in Z-2A and a second HTR unit
62 in Z-2B
15 at the first end of the system, a third HTR unit 63 in Z-3A and a fourth
HTR unit 64 in Z-3B
at the second end of the system. The HTR units are operated cyclically and the
mufti-effect
evaporators are operated nearly continuously.
The first multiple effect evaporator Z-lA comprises, as an example, nine
evaporators ZE-1 through ZE-9, 69 through 77 connected in series, with
operating pressures
20 decreasing successively in the direction from the left end ZE-1 toward the
right end ZE-9.
The second multiple effect evaporator Z-1B, comprises nine evaporators ZE'-1
through
ZE'-9, 78 through 86 connected in series, with operating pressures decreasing
successively
in the direction from the right end ZE'-1 toward the left end ZE'-9.
Each of the four HTR units is operated cyclically and alternately serves as an
evaporator and as a condenser. The four HTR units are operated in a
coordinated manner.
While one of the two HTR units at each end serves as an evaporator, the other
unit serves as
a condenser. As illustrated in Figure 11A, the HTR units 61 in Z-2A and Z-3B
64 serves as
two vapor generators and the HTR units in Z-2B 62 and Z-3A 63 serve as two
condensers.
The vapor streams generated are used as steam supplies to the two ZE-1 Zones
and
ZE'-1 Zones and thereby initiates the multiple effect evaporator operations.
The vapors
leaving the last effect ZE-9 and ZE'-9 are condensed in the two HTR-units
serving as
condensers. Figure 11B illustrates the same system in the other half of the
cycle, in which
SUBSTITUTE SHEET (RULE 26)
CA 02383489 2002-02-12
WO 01/13050 PCT/US99/18344
21
the HTR units in Z-2B and Z-3A become the vapor generators and the HTR units
in Z-2A
and Z-3B become the condensers.
Figures 12A and 12B illustrate a multiple effect evaporator system similar to
those
illustrated by Figures 11A and 11B. In this system, corrugated metal walls are
used to form
falling film evaporators. The operations of this system are similar to those
described in
connection with figures 11A and 11B.
Figure 13 illustrates a self actuating valuing system that provides vapor
passages
from one chamber to another without any mechanical device or any electrical
switch. The
valuing system is made of a mesh 105 for structural support as well as a
divider for the two
chambers with flaps 106 (made of thin film) attached onto the divider. These
dividers with
attached thin films 106 will be the dividers between two chambers. The thin
film flaps 106
are pressure sensitive wherein when one chamber's pressure is higher than the
other, the
flap will automatically opens allowing the vapor to flow from the first
chamber with higher
pressure to the second chamber with low pressure. It automatically closes when
the
1 S pressure of the second chamber becomes higher than the pressure in the
first chamber.
Figure 13A illustrates a single vent made of thin film 106 attached onto a
holder 107
on the top of the vent.
The concept of the present invention has a wide range of usage, such as air
condition, water purification, distillative freezing, ice making, waste water
treatment,
desalinization, distillation operation under ambient temperature or high
temperature, or
organic chemical purification and separation, and other areas which may
require the use of
raising heat temperature from low temperature heat source to a high
temperature heat sink.
SUBSTITUTE SHEET (RULE 26)