Note: Descriptions are shown in the official language in which they were submitted.
CA 02383523 2002-02-27
WO 01/17377 PCT/EP00/08646
Crosslinked composition for producing a long-lasting
satiation effect and process for its preparation
The present invention relates to a composition for
producing a satiation effect.
Numerous attempts have been made by medical means to
break down excessive accumulations of fat in the human
body, or to prevent them developing. There are, for
example, appetite suppressants which attempt by
biochemical means to induce in the body a disinclination
to take food. These compositions have in some cases
considerable harmful side effects.
Besides the numerous dietary products which have been
proposed, there are also mechanical and
electromechanical means intended specifically to break
down fat and build up muscle. However, the effect of
such means is very doubtful.
German Patent DE 4025912 discloses a composition for
oral intake which consists of a container which is
soluble in the stomach and releases the contents. This
container is filled with a substance whose volume
increases after it is released in the stomach, and thus
it induces a feeling of satiation in the body.
From the prior art a number of elastic materials are
already known that can be compressed on passage through
the esophagus and which can, after leaving the
esophagus, be decompressed in water and/or gastro-
intestinal fluid. Such sponge-like structures are taken
to mean foams which consist of gas-filled
spherical/polyhedral cells which are limited by highly
viscous or solid cell walls. It is possible to employ
according to the invention both naturally occurring
sponges and synthetically prepared sponge-like
structures.
CA 02383523 2002-02-27
- - 2 -
' Natural materials which are already used are collagen
and cellulose. However, these abovementioned materials
are relatively expensive raw materials. Both materials
require complex isolation or work-up processes which,
in addition, are very environmentally polluting. The
latter applies especially to cellulose, the isolation
of which means that large amounts of acids have to be
employed.
Soluble collagen is isolated from animal hides, for
example, preferably young cattle or pigs, since the
soluble collagen content in the animal becomes ever
smaller with increasing age. This is also only possible
with complex isolation and work-up processes.
Not least since the discovery of a number of diseases
in pigs and cattle, which are suspected to be
transmissible to humans, in particular the cattle
disease BSE, and a possible risk of infection for
humans, the acceptance of such collagen-containing
products by the end consumer has fallen drastically.
It is an object of the present invention, therefore, to
provide a material for preparing a composition for
producing a long-lasting satiation effect, which
material does not have the abovementioned
disadvantages.
This is achieved according to the invention by a
composition for oral intake comprising uronic-acid-
containing polysaccharides stably crosslinked to one
another in the form of a sponge-like structure which is
characterized in that it is slightly soluble or of low
absorbability in water and/or gastrointestinal fluids.
According to the invention the uronic-acid-containing
polysaccharides are crosslinked to one another by ionic
bonds and in addition are stably crosslinked to one
another by covalent bonds. Particularly preferred
polyuronic acid-containing-polysaccharides are alginic
acids and their salts (alginates). However, low degree
CA 02383523 2002-02-27
- 3 -
' of esterification pectins, xanthan, tragacanth,
chondroitin sulfate and all other uronic-acid-
containing compounds can also be used according to the
invention.
Alginic acid is a linear polyuronic acid of alternating
parts of D-mannuronic acid and L-guluronic acid which
are linked to one another by (3-glycosidic bonds, the
carboxyl groups not being esterified. One molecule of
alginic acid can be composed of about 150-1 050 uronic
acid units, where the mean molecular weight can range
from 30-200 kDa.
The polysaccharide alginic acid is a constituent of
cell walls of brown algae. The alginic acid content can
make up to 40% of the dry matter of the algae in this
case. The alginic acid is produced by alkaline
extraction with methods known per se according to the
prior art. The resultant pulverulent alginic acid is
thus purely of plant origin and has high
biocompatibility. It can absorb 300 times its own
weight of water, forming highly viscous solutions. In
the presence of polyvalent cations, alginic acid forms
gels. The formation of alginate gels in the presence of
divalent cations, such as calcium or barium, is
described in Shapiro I., et al. (Biomaterials, 1997,
18: 583-90). The latter is not suitable for use in
biomedicine, however, on account of its toxicity. In
addition to calcium chloride, calcium gluconate also
provides suitable divalent cations. In general, all
physiologically safe polycations can be used, in
particular divalent cations.
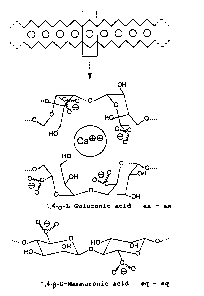
The unbranched concertina-like alginate chains are
fixed by ionic bonds via the free bonding positions of
the cations, preferably calcium ions (Fig. 1). This
produces a three-dimensional network in which the
divalent cations are situated like "eggs in an eggbox"
as in the "egg-box model" presented in Smidsrod et al.
(Trends in Biotechnology, 1990, 8: 71).
CA 02383523 2002-02-27
- 4 -
The sponge-like or sponge-shaped structures are
produced by methods known per se from the prior art.
Depending on the starting material employed, in the
simplest case, a foam can be obtained by blowing, by
beating, shaking, spraying or stirring in the relevant
gas atmosphere. In the case of polymers, the foam
structure is produced by chemical reactions. Thus, for
example, polyurethanes are foamed by adding blowing
agents which decompose at a defined temperature during
processing, with gas formation, or by addition of
liquid solvents during the polymerization. The foaming
takes place either on leaving the extrusion die, that
is to say following the extrusion or injection molding,
or in open molds. The curing takes place under the
conditions characteristic of the respective chemical
compound of the material.
An indispensable prerequisite for the usability of the
material is that it can be compressed without breaking
the cell walls. This is because in order to be able to
use the inventive material for oral intake, the foam-
like or foamy material must be directly compressible on
passage through the esophagus. In particular, no
trouble must occur on passage through the esophagus.
A particular advantage of the present invention is that
the alginates crosslinked according to the invention
are more flexible and softer, and as a result have very
much more favorable mechanical properties for
gastrointestinal application than the materials
previously available on the market. For the user this
is accompanied by the advantage of improved tolerance,
so that even in the case of patients having mucosal
lesions, neither a feeling of pressure nor mucosal
irritation is caused.
For the selection of the material and the type of foam
formation, it is furthermore essential that the
material remains swellable without destroying the cell
CA 02383523 2002-02-27
- 5 -
walls. After passage through the esophagus, the sponge
like structure is to resume at least the size which it
had before entry into the esophagus. If appropriate,
the material may also swell to a size which goes beyond
the original volume.
The sponge-like structure can have any desired shape
and size in the compressed and decompressed states.
However, preference is given to cuboid or rectangular
or round embodiments.
Preferably, the material is designed so that the
sponge-like structure can be compressed to 1/2 to
1/100, preferably 1/4 to 1/50, particularly preferably
1/10 to 1/20, of its volume or of its size. Under
physiological conditions, the compressed material,
after passage through the esophagus, is to be able to
expand, preferably to two to twenty times, particularly
preferably to four to fifty times, and very
particularly preferably to ten to twenty times, its
volume.
As material for the sponge-like structure, according to
the invention natural, semisynthetic or synthetic
polymers can be used, which, in addition, can be
crosslinked by stable crosslinks.
Various processes are known from the prior art for
crosslinking polymers. Thus, for example, the free-
radical polymerization of lactose-O-(p-vinylbenzyl)-
oxime for forming hydrogels is described in Zhou, W-Z,
et al. (Macromolecules, 1997, 30: 7063-7068) and a
polymerization of N-vinylpyrrolidone by electron-beam
irradiation is described in Rosiak, J.M. (J Contr Rel.,
1994, 31: 9-19). In addition, for example, crosslinked
polymers of saccharide acrylates or poly(2-
hydroxyethylmethacrylate)gelatin and also collagen or
chitosan are known (Martin, B.D., et al. (Biomaterials,
1998, 19: 69-76; Santin, M., et al. (Biomaterials,
_ CA 02383523 2002-02-27
- 6 -
1996, 17: 1459-1467); Weadock, K.S., et al. (J Biomed
Mater Res, 1995, 29: 1371-1379); Groboillot, A., et al.
(Biotech Bioeng, 1993, 42: 1157-1163)).
Examples of starting materials particularly suitable
according to the invention are uronic-acid-containing
polysaccharides which still have free reactive groups,
preferably carboxyl groups and/or hydroxyl groups, for
forming stable crosslinks, for example ester bonds.
Very high preference is given here to alginic acids,
low degree of esterification pectins, xanthan,
tragacanth, chondroitin sulfate and all uronic-acid-
containing compounds and their salts.
Crosslinking alginates by polyvalent cations is
described in Shapiro L. et al., Biomaterials, 1997,
18:583-590. However, these compounds are unstable in
water or a surrounding medium having a calcium
concentration less than 3 mmolar, since the calcium is
extracted from the chain cluster and/or may be
displaced by other (monovalent) ions. This leads to a
dissolution of the crosslinking between the concertina-
like polyuronic-acid-containing polysaccharide chains.
It is a disadvantage here that the alginates which are
only crosslinked by ionic bonds dissolve relatively
rapidly in water and/or gastrointestinal fluids and are
thus not suitable for producing a satiation effect. A
particular advantage of the inventive composition is
stable crosslinking by covalent bonds, in particular
ester bonds, the formation of which is catalyzed by
mineral acids. Covalently linked alginate molecules
have also already been described in Moe et al. (Food
Hydrocolloids, 1991, 119). However, the preparation
process requires relatively long reaction times. In
addition, resultant products, owing to the chemicals
used for their production, are toxic and are thus not
suitable for the fields of application according to the
invention.
CA 02383523 2002-02-27
The inventive composition can comprise, inter alia,
pharmaceutically active substances, foodstuffs or food
supplements, for example vitamins, dietary fiber,
proteins, minerals and other food constituents, taste
and stimulant substances or flavorings.
In addition to said substances, it is also possible to
add other ancillary substances to the carrier material.
Inter alia, release-slowing substances may additionally
be suitable in the case where pharmaceutically active
substances are used.
In addition, the compositions according to the present
invention can additionally contain fillers, dis-
integrants, binders and lubricants and also excipients.
Active compounds can also be introduced into the
sponge-like structure.
For the purposes of the invention, active compounds are
all substances having a pharmaceutical or biological
action. Examples are betamethasone, thioctic acid,
sotalol, salbutamol, norfenefrine, silymarin, dihydro-
ergotamine, buflomedil, etofibrate, indomethacin,
oxazepam, beta-acetyldigoxin, piroxicam, haloperidol,
ISMN, amitriptyline, diclofenac, nifedipine, verapamil,
pyritinol, nitrendipine, doxycycline, bromhexine,
methylprednisolone, clonidine, fenofibrate, allo-
purinol, pirenzepine, levothyroxine, tamoxifen, metil-
digoxin, o-(beta-hydroxyethyl)rutoside, propicillin,
aciclovir mononitrate, paracetamol, naftidrofuryl,
pentoxyfylline, propafenone, acebutolol, L-thyroxine,
tramadol, bromocriptine, loperamide, ketotifen,
fenoterol, Ca dobesilate, propranolol, minocycline,
nicergoline, ambroxol, metoprolol, beta-sitosterol,
enalapril hydrogen maleate, bezafibrate, ISDN,
gallopamil, xanthinol nicotinate, digitoxin, fluni-
trazepam, bencyclane, dexapanthenol, pindolol, lor-
azepam, diltiazem, piracetam, phenoxymethylpenicillin,
CA 02383523 2002-02-27
furosemide, bromazepam, flunarizine, erythromycin,
metoclopramide, acemetacin, ranitidine, biperiden;
metamizole, doxepin, dipotassium chlorazepate,
tetrazepam, estramustine phosphate, terbutaline, capt-
opril, maprotiline, prazosin, atenolol, glibenclamide,
cefaclor, etilefrine, cimetidine, theophylline, hydro-
morphone, ibuprofen, primidone, clobazam, oxaceprol,
medroxyprogesterone, flecainide, Mg pyridoxal 5-phos-
phate glutamate, hymecromone, etofylline clofibrate,
vincamine, cinnarizine, diazepam, ketoprofen, flupen-
tixol, molsidomine, glibornuride, dimetindene, mel-
perone, soquinolol, dihydrocodeine, clomethiazole,
clemastine, glisoxepide, kallidinogenase, oxyfedrine,
baclofen, carboxymethylcysteine, thioridazine, beta-
histine, L-tryptophan, myrtol, bromelains, prenylamine,
salazosulfapyridine, astemizole, sulpiride, benser-
azide, dibenzepin, acetylsalicylic acid, miconazole,
nystatin, ketoconazole, Na picosulfate, colestyramine,
gemfibrozil, rifampicin, fluorocortolone, mexiletine,
amoxicillin, terfenadine, mucopolysaccharide poly-
sulfates, triazolam, mianserin, tiaprofenic acid,
amezinium methyl sulfate, mefloquine, probucol, quin-
idine, carbamazepine, Mg L-aspartate, penbutolol,
piretanide, amitriptyline, cyproterone, Na valproate,
mebeverine, bisacodyl, 5-aminosalicylic acid, dihydral
azine, magaldrate, phenprocoumon, amantadine, naproxen,
carteolol, famotidine, methyldopa, auranofin, estriol,
nadolol, levomepromazine, doxorubicin, meclofenoxate,
azathioprine, flutamide, norfloxacin, fendiline,
prajmalium bitartrate, escin.
Further examples are the following active substances:
acetaminophen (= paracetamol), acetohexamide, acetyl-
digoxin, acetylsalicylic acid, acromycin, anipamil,
benzocaine, beta-carotene, chloramphenicol, chlor-
diazepoxide, chlormadinone acetate, chlorthiazide,
cinnarizine, clonazepam, codeine, dexamethasone,
diazepam, dicumarol, digitoxin, digoxin, dihydro-
ergotamine, drotaverine, flunitrazepam, furosemide,
CA 02383523 2002-02-27
_ g _
gramicidin, griseofulvin, hexobarbital, hydrochloro-
thiazide, hydrocortisone, hydroflumethazide, indo-
methacin, ketoprofen, lonetil, medazepam, mefruside,
methandrostenolone, methylprednisolone, methylsulfa-
diazine (= sulfaperin), nalidixic acid, nifedipine,
nitrazepam, nitrofurantoin, nystatin, estradiol,
papaverine, phenacetin, phenobarbital, phenylbutazone,
phenytoin, prednisone, reserpine, spironolactone,
streptomycin, sulfadimidine (= sulfamethazine), sulfa-
methizole, sulfamethoxazole, (= sulfameter), sulfa-
perin, sulfathiazole, sulfisoxazole, testosterone,
tolazamide, tolbutamide, trimethoprim, tyrothricin,
vitamins, minerals.
Active compounds which also come into consideration are
those having prophylactic action, for example in the
field of tumor therapy.
In addition to said active compounds, it is also
possible to add other ancillary substances to the
carrier material. Inter alia, release-slowing
substances can additionally come into consideration.
Release-slowing ancillary substances which can be used
are essentially water-insoluble ancillary substances or
mixtures thereof, such as lipids, inter alia fatty
alcohols, for example cetyl alcohol, stearyl alcohol
and cetostearyl alcohol; glycerides, for example
glycerol monostearate or mixtures of mono-, di- and
triglycerides of vegetable oils; hydrogenated oils,
such as hydrogenated castorr oil or hydrogenated
cottonseed oil; waxes, for example beeswax or carnauba
wax; solid hydrocarbons, for example paraffin or earth
wax; fatty acids, for example stearic acid; certain
cellulose derivatives, for example ethyl cellulose or
acetyl cellulose; polymers or copolymers, such as
polyalkylenes, for example polyethylene, polyvinyl
compounds, for example polyvinylchloride or
polyvinylacetate, and also vinylchloride-vinylacetate
CA 02383523 2002-02-27
- 10 -
copolymers and copolymers with crotonic acid, or
polymers and copolymers of acrylates and methacrylates,
for example copolymers of acrylates and methyl
methacrylate.
The resultant material which is slightly soluble or has
low absorbability in water and/or gastrointestinal
fluids can then be compressed. This can be achieved by
pressing, rolling or comparable methods. In addition,
the material can be compressed by chewing movements
during the oral intake of the material.
Before, during or after the preparation of the sponge-
like structure, the material can be loaded, for
example, with the abovmentioned active substances. All
conventional methods are suitable for this purpose. In
the simplest case, this can take place during the
preparation phase of the sponge material by mixing
carrier material and active substance. Also, these
substances can be applied to the surface.
The sponge-like structure thus prepared can, in a
preferred embodiment of the invention, be encased with
the abovementioned substances . That is to say either a
container, for example a capsule, is produced from the
substance and the sponge-like structure is introduced
into this, or the substance is applied directly onto
the structure, for instance by immersion, spraying,
spreading or similar methods. In another embodiment of
the invention, the sponge-like structure is introduced
into the substance. This can be achieved, for example,
by impregnation.
The purpose of the inventive process is to obtain a
composition which is sufficiently compressed on passage
through the esophagus and is not decompressed until in
the stomach. This purpose is achieved by said process
steps.
CA 02383523 2002-02-27
- 11 -
In contrast to other food/food supplement/dietary or
drug products which are rapidly decomposed in the
stomach or pass into it already in a comminuted state,
the sponge or foam body which is prepared in the
described manner and consists of natural, semisynthetic
or synthetic polymers retains its original shape for
several hours due to particular crosslinking points, in
particular covalent bonds. Owing to the decompression
of the inventive composition in the stomach, the
stretch receptors of the stomach are excited, which
triggers a feeling of satiation. The inventive sponge
is dissolved only slightly or absorbed only to a
limited extent in the stomach in the course of this.
In addition, the present invention relates to a process
for preparing compositions for producing a long-lasting
satiation effect. In the process, polyuronic-acid-
containing polysaccharides are crosslinked via ionic
bonds, frozen, freeze-dried, stably crosslinked via
covalent bonds, then dried and, if appropriate,
pressed. Particularly preferably here the unbranched
polyuronic-acid-containing polysaccharides used are
alginic acids and their salts. In addition, pectins,
xanthan, tragacanth, chondroitin sulfate and all other
uronic-acid-containing compounds or their salts are
also conceivable.
According to the invention, alginic acids or their
salts are used in concentrations of 0.3 to 10% by
weight, preferably 0.5 to 5o by weight, particularly
preferably at concentrations of 1 to 3% by weight.
In addition, it is essential to the invention that by
immersing the sponge-like structure in mineral acids,
preferably hydrochloric acid, after the freeze-drying,
additional stable crosslinking points are introduced
into the sponge material by forming covalent ester
bonds (fig. 2). In this case, according to the
discretion of those skilled in the art, at least
CA 02383523 2002-02-27
- 12 -
catalytic amounts of mineral acids are used, but at
most an amount such that the material is not broken
down into its constituents by acid hydrolysis.
Particular preference is given to a concentration of
0.1 mol/1 of mineral acid, in particular hydrochloric
acid. The stable crosslinking due to mineral acids
causes solubility of the sponge body in water and/or
gastrointestinal fluids to be only slight for a long
time. This slight solubility is a prerequisite for long
residence of the sponge in the stomach and the long-
lasting satiation effect caused as a result.
The invention is not restricted to the described
process, but also applies to all other processes in
which sponges or sponge-like structures are prepared
which are to, or can, achieve a long-lasting satiation
effect due to only slight solubility in water and/or
gastrointestinal fluids and the resultant long
residence time in the stomach.
The inventive composition is taken orally. The solid
sponge or solid foam body passes through the mouth,
throat and esophagus by the addition of beverage and
gentle chewing or swallowing movements, and swells
again in the stomach, preferably to its original
volume, owing to the gastric fluid. If appropriate, the
volume may alternatively be greater than or less than
the original volume.
The oral intake of the inventive composition means that
the solid sponge or solid foam body, owing to the only
slight solubility in the stomach, resides for several
hours in the stomach. As a result, a long-lasting
feeling of satiation or repletion can be achieved,
which results in a reduced food intake. However, the
composition can also be used in the fields of pharmacy
and/or health, preferably (dietetic) nutrition or food
supplementation. For this purpose the composition
CA 02383523 2002-02-27
- i3 -
comprises the above-described active compounds or
foodstuffs.
Depending on the degree of satiation desired, a
different number of sponge bodies can be taken daily at
differing time intervals. The ~~stretch receptors"
triggered by the sponge volume situated in the stomach
generate via the diencephalon a satiation effect which
decreases again only when the stomach is emptied. It is
thus possible to control the period of satiation by the
length of residence of the bulk sponges.
In addition, the present invention relates to the use
of the inventive compositions for preparing
compositions to produce a satiation effect and for
preparing drugs which can be administered orally,
foodstuffs, food supplements or dietetic foods loaded
with active compounds.
In addition, the inventive compositions can also
develop their action after passage through the stomach,
that is to say in the intestine. Here the composition
acts by exciting the stretch receptors in the
intestinal wall, in particular stimulating intestinal
activity.
In a particular embodiment of the invention, the
composition can also be designed such that the
decompression does not take place until it is in the
intestine. That is to say the composition in this case
does not develop its action in the stomach, but only in
the intestine. For this purpose, preferably, it is
envisaged to provide the polymers with a compound which
does not dissolve in the stomach, but only in the
intestine, so that the compressed sponge-like structure
is also not able to decompress until it reaches there.
The dissolution of the compound is affected in this
case by various parameters, in part also prevailing
simultaneously in the intestine, for example pH,
CA 02383523 2002-02-27
- 14 -
pressure, redox potential and enzymatic dissolution via
the intestinal flora. In addition, the residence time
of the composition in the intestine also affects the
rate at which the compound dissolves.
For preference, the compound dissolves at a pH between
5 and 10, preferably between 7 and 9, particularly
preferably between 5.5 and 8.5. Dissolution in the pH
environment of the intestine at a pH between 6.4 ~ 0.6
and 7.0 ~ 0.7 is most preferred. In particular, those
compounds are suitable which dissolve depending on the
redox potential, enzymatic activities and pressure.
The compound is applied to the sponge-like structure
according to the invention preferably in the form of a
coating which, if appropriate, can also be made up of a
plurality of layers. The minimum layer thickness here
can vary considerably and is dependent on the film-
former used and its composition. Osterwald H. et al.
(Acta Pharm Technol, 1980, 26: 201-209) describes, for
example, a minimum layer thickness of 46 um for the
preparation of a film-former in organic solvents,
preparation with an ammonium salt solution requires a
layer thickness of 161 N,m, as an emulsion 46 ~m and as
a latex dispersion 52 ~.m. According to the invention,
the layer thickness is from 10 ~m to several
millimeters, preferably from 15 ~,m to 3 mm.
However, instead of a coating applied directly to the
structure, the sponge-like structure can be introduced
into a container which dissolves under the above-
described conditions. That is to say the container is
stable in the stomach, but dissolves in the intestine.
In another variant of the invention, the compound can
be introduced into the sponge-like structure. This may
be achieved, for example, by impregnation in a solution
of the compound or by adding the compound during
preparation of the sponge-like structure. Obviously, a
CA 02383523 2002-02-27
- 15 -
structure impregnated, for example, in such a manner
can additionally be provided with a coating of the
compound. In addition, the impregnated structure can
also be introduced into the above-described container.
In addition, the structure can be introduced into a
container which itself is coated or impregnated with
the compound or into which the compound is introduced.
The time and location of the dissolution of the
compound may be influenced by the selection and
combination of the compounds, which achieves targeted
release of the sponge-like structure in the intestine
and, in particular, in the various intestinal sections,
such as the jejunum, ileum and colon. The solubility of
the compounds can depend on one or more factors, for
example pH, time of exposure, redox potential of the
intestine, enzymatic activities of the intestinal
flora, or pressure which is produced by intestinal
peristalsis. The various possibilities for controlling
the release of active compounds are described
extensively. The pH-dependent solubility is described,
for example, in Marvola et al., Eur J Pharm Sci, 1999,
7:259-267 and Khan Z1 et al., J Controlled Release,
1999, 58:215-222. Pozzi F. et al., J Controlled
Release, 1994, 31:99-108; Wilding IR et al., Pharmacol
Ther, 1994, 62:97-124; Niwa K. et a1, J Drug Target,
1995, 3:83-89 and US-4871549 disclose systems which
release the active compounds as a function of time.
Examples of systems having a combined pH and time
dependency are described in Rodriguez M. et al.,
J Controlled Release, 1998, 55:67-77 and Gazzinga A.
et al., STP Pharm Sci, 1995, 5:83-88. The dissolution
of compounds due to changed redox potential in the
intestine is dealt with by Bronsted H. et al., Pharm
Res 1992, 9:1540-1545; Yeh PY et al., J Controlled
Release, 1995, 36:109-124; Shanta KL et al.,
Biomaterials, 1995, 16:1313-1318 and Kimura Y et a1<,
Polymer, 1992, 33:5294-5299. Examples of systems which
are released by the enzymes of the intestinal flora are
CA 02383523 2002-02-27
- 16 -
described in Ashford M et al., J Controlled Release,
1994, 30:225-232; Fernandez-Hervas MJ et al., Int J
Pharm, 1998, 169:115-119; EP-0460921 US-4432966 and
Milojevic S et a1. , J Controlled Release, 1996, 38 : 75-
84. The dissolution of systems due to the pressure of
intestinal peristalsis is covered in Muraoka M et al.,
J Controlled Release, 1998, 52:119-129.
Preference is given according to the invention to the
following compounds and their combinations which are,
however, in no way limiting for the present invention:
hydroxypropyl methyl cellulose phthalate (HPMCP 55),
hydroxypropyl methyl cellulose acetate succinate (Aqoat
AS-MF, Aqoat AS-HF), 1:1 copolymer of methacrylic acid
and ethyl acrylate (Eudragit~L), copolymer of vinyl
acetate and crotonic acid (Coating CE 5142), cellulose
acetate phthalate (CAP, Aquateric), methacrylate
copolymers (Eudragit~S), shellac, Time Clock System~,
carnauba wax, hydroxypropyl methyl cellulose (TC-5),
Pulsincap~, polyethylene glycol, crosslinked poly-
ethylene glycol, ethyl cellulose, ethyl cellulose/
ethanol mixture, hydroxypropyl cellulose, hydroxypropyl
methyl cellulose, glycerol monostearate, Eudragit~E. In
addition, hydrogels from azo compounds are possible,
for example N-substituted methacrylamide, N-tert-
butylacrylamide, acrylic acid in the presence of
4,4'-bis(methacryloylamino)azobenzenes, 4,4'-bis(N-
methacryloyl-6-aminohexanoylamino)azobenzene or 3,3',5,
5'-tetrabromo-4,4,4'4'-tetra(methacryloylamino)azobenz-
ene. Examples of other compounds are unbranched polymer
precursors, for example containing N,N-dimethyl-
acrylamide, N-tertbutylacrylamide, acrylic acid,
N-methacryloylglycylglycine p-nitrophenyl ester, cross-
linked by suitable crosslinkers, for example
N,N'-(t~-aminocaproyl)-4,4'-diaminoazobenzene and
polymers containing azo compounds, for example
2-hydroxyethyl methacrylate, 4-(methacryloyloxy)azo-
benzene, N-(2-hydroxypropyl)methacrylamide copolymers,
copolymers containing styrene and 2-hydroxy-
CA 02383523 2002-02-27
- 1'7 -
ethylmethacrylate crosslinked by, for example, 4,4'-
divinylazobenzene or N, N' -bis ((3-sterylsulfonyl ) -4, 4' -
diaminoazobenzene. Also, poly(ether-ester)azo polymers
can also be used according to the invention, for
example copolymers containing 4-[4-[(6-hydroxyhexyl)-
oxy]phenyl]azobenzoic acid and 16-hydroxyhexadecanoic
acid, copolymers containing 4-[2-[2-(2-hyrdoxy-
ethoxy) ethoxy] ethoxy] benzoic acid, 4- [4- [2- [2- (2-
hydroxyethoxy)ethoxy]ethoxy]phenyl]azobenzoic acid and
16-hydroxyhexadecanoic acid or 12-hydroxydodecanoic
acid and segmented polyurethanes containing m-xylene
diisocyanate, 3,3'-dihydroxyazobenzene, polyethylene
glycol or 1,2-propanediol. In addition, usable
compounds are azo-compound-containing polyamides or
copolymers of 4-[4-(chlorocarbonyl)phenyl)]azobenzoyl
chloride and a,cu-bis(aminopropyl)poly(tetramethylene
oxide) and copolymers of 4-[4-chlorocarbonyl)-
phenyl]azobenzoyl chloride and Jeffamine ED-600.
In addition, pectins are used, which can be
additionally coated or embedded in a matrix, for
example, methoxy pectin, amidated pectin, calcium
pectate, pectin in combination with ethyl cellulose
(Aquacoat, Surelease), acrylic ester polymers
(Eudragit RS30D, Eudragit NE30D). In addition,
combinations of pectins with other dietary fibers are
used. Examples of dietary fibers are guar
(galactomannan) or chitosan, the dietary fibers
themselves in turn being able to be coated or a
constituent of a matrix. In this case the following
substances are used as film-formers: polymethacrylate
solutions, copolymers containing polyurethane and di-,
oligo- or polysaccharides (galactomannans) and ethyl-
galactomannans or acetylgalactomannans. In addition,
cyanoacrylate, inulin, inulin suspensions containing
Eudragit-RS, methacrylated inulin, chondroitin sulfate,
chondroitin polymers containing 1,12-diaminododecane
and dicyclohexylcarbodiimide, amorphous amylose or
amorphous amylose together with other film-forming
polymers are used as film-former. In addition, dextrans
CA 02383523 2002-02-27
- 18 -
can be used which can be crosslinked in various ways,
for example with diisocyanates, fatty acid esters, for
example lauric acid, glutaraldehyde. conjugates of
biphenylacetic acid and (3-cyclodextrin, films of
cyclodextrins with methacrylic acid copolymers or
acrylic acid polymers with disaccharide side groups are
also used according to the invention.
The choice of compounds and their many possible
combinations make targeted release of the sponge-like
structure in the large intestine possible.
The invention is described in more detail below with
reference to the following example:
Preparation of alginate sponges:
Into each of the recesses of a microtiter plate
(diameter 16 mm, height 20 mm) are pipetted 0.5 ml of a
1% strength sodium alginate solution (w/v) and 0.5 ml
of distilled water and, with intensive stirring, a 0.20
strength calcium gluconate solution (w/v) are added to
each. The hydrogels thus produced are frozen overnight
at -20°C and are then freeze-dried at 0.007 mm Hg
(column mercury) and -60°C.
The freeze-dried small sponges are removed from the
microtiter plate and immersed for 30 seconds in 0.1
molar hydrochloric acid. The hydrochloric acid is
removed by rinsing with distilled water. The small
sponges are dried in a drying cabinet at 30°C and are
then pressed.