Note: Claims are shown in the official language in which they were submitted.
57
CLAIMS
1. A pharmaceutical composition which comprises an
insulin sensitizer in combination with at least one member
selected from the group consisting of
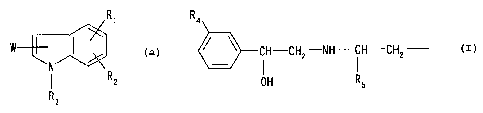
1) a compound of the formula [I] :
<IMG>
wherein R1 represents a lower alkyl group optionally
substituted by hydroxyl group, phenylsulfonylamino group,
a lower alkylsulfonylamino group, a mono- or di-lower
alkylaminosulfonyl group, or a group selected from the
following (a) to (d), or combines with R2 to form
methylenedioxy group, said methylenedioxy group being
optionally substituted by carboxyl group or a lower
alkoxycarbonyl group,
(a) a group of the formula : -Xa-Ra
wherein Xa is O, S or NH, Ra is hydrogen atom or a lower
alkyl group, provided that Ra is a lower alkyl group when
Xa is S;
(b) a group of the formula : -[O(CH2)p-CH(Rb)]q Rbb
wherein Rb is hydrogen atom, a lower alkyl group, a lower
alkoxycarbonyl group or carboxyl group, Rbb is a lower
alkoxycarbonyl group or carboxyl group, p is an integer of
0 to 3, q is 0 or 1,
(c) a group of the formula : -O(CH2)r-Rc
wherein Rc is a lower alkanoyl group, hydroxyl group, cyano
group, phenyl group, a mono- or di-lower alkylaminocarbonyl
group or a group of the formula : -P(=O)(OR A)(OR A)
wherein R A is hydrogen atom or a lower alkyl group, r is
an integer of 1 to 4,
(d) a group of the formula : -Ya- (CH2)s-Rd
wherein Ya is NH or S, Rd is carboxyl group or a lower
alkoxycarbonyl group, s is an integer of 1 to 4,
58
R2 represents hydrogen atom, a halogen atom, a lower alkyl
group optionally substituted by hydroxyl group, hydroxyl
group, a lower alkoxy group, or the same group as the above
(b) or (c), or combines with R1 to form the above
methylenedioxy group, said methylenedioxy group being
optionally substituted by carboxyl group or a lower
alkoxycarbonyl group,
R3 represents hydrogen atom or a lower alkyl group,
W represents a group of the formula which bonds to the 2-
or 3-position of the indole ring in the formula [I]:
<IMG>
wherein R4 represents a halogen atom or a halogeno lower
alkyl group, R5 represents a lower alkyl group, or a salt
thereof;
2) a compound of the formula [II] :
<IMG>
wherein R6 represents a halogen atom or a halogeno lower
alkyl group, R7 represents hydrogen atom, a lower alkyl
group or a halogeno lower alkyl group, R8 represents
hydrogen atom, a halogen atom, a halogeno lower alkyl group,
nitro group or cyano group, or a salt thereof;
3) a compound of the formula [III] :
<IMG>
wherein j represents an integer of 0 to 7,
k represents 0 or 1,
59
t represents an integer of 0 to ~3,
ring A' represents benzene ring; naphthalene ring; a 5- or
6-membered heterocyclic ring containing 1 to 4 hetero atoms
selected from O, S and N; benzene ring condensed with C3-8
cycloalkyl ring; benzene ring condensed with a 5- or 6-
membered heterocyclic ring containing 1 to 3 hetero atoms
selected from O, S and N; or a 5- or 6-membered heterocyclic
ring containing 1 to 3 hetero atoms selected from O, S and
N which is condensed with a 5- or 6-membered heterocyclic
ring containing 1 to 3 hetero atoms selected from O, S and
N;
R11 represents hydroxy, oxo, halogen, cyano, nitro, NR18R18,
SR18, a halogeno lower alkyl, C1-6 alkyl, C1-6 alkoxy, C3-8
cycloalkyl, phenyl, SO2R19, NR18COR19, COR19, NR18SO2R19,
NR18CO2R18; or a C1-6 alkyl group substituted by hydroxy, nitro,
halogen, cyano, NR18R18, SR18, a halogeno lower alkyl, C1-6
alkoxy, C3-8 cycloalkyl, phenyl, NR18COR19, COR19, SO2R19,
NR18SO2R19 or NR18CO2R18;
or R11 represents a 5- or 6-membered heterocyclic group
containing 1 to 3 hetero atoms selected from O, S and N;
R12 and R13 represent independently hydrogen atom, C1-6 alkyl,
or C1-6 alkyl substituted by hydroxy, C1-6 alkoxy or halogen;
X' represents -CH2-, -CH2-CH2-, -CH=CH- or -CH2O- ;
R14 and R15 represent independently hydrogen atom, C1-6 alkyl,
halogen, NHR18, OR18, SO2R19 or NHSO2R19;
R16 represents hydrogen atom or C1-6 alkyl;
R17 represents C1-6 alkyl, C3-8 cycloalkyl or -B' -(R11)j
wherein R11 and j have the same meanings as above;
ring B' represents benzene ring; naphthalene ring; a 5- or
6-membered heterocyclic ring containing 1 to 4 hetero atoms
selected from O, S and N; benzene ring condensed with C3-8
cycloalkyl ring; benzene ring condensed with a 5- or 6-
membered heterocyclic ring containing 1 to 3 hetero atoms
selected from O, S and N; or a 5- or 6-membered heterocyclic
ring containing 1 to 3 hetero atoms selected from O, S and
N which is condensed with a 5- or 6-membered heterocyclic
60
ring containing 1 to 3 hetero atoms selected from O, S and
N;
R18 represents hydrogen atom; C1-10 alkyl; C3-8 cycloalkyl;
phenyl substituted by halogen, C1-6 alkyl or C1-6 alkoxy; C1-10
alkyl substituted by hydroxy, halogen, CO2H, C1-6
alkoxy-carbonyl, C3-8 cycloalkyl, C1-6 alkoxy, or phenyl
substituted by halogen, C1-6 alkyl or C1-6 alkoxy;
R19 represents R18, NHR18 or NR18 wherein R18 has the same
meaning as above, or a salt thereof;
4) a compound of the formula [V] :
<IMG>
wherein R20 represents hydrogen atom or methyl group,
R21 represents hydrogen atom, halogen atom, hydroxy group,
benzyloxy group, amino group or hydroxymethyl group,
R22 represents hydrogen atom, hydroxymethyl group, NHR23,
SO2NR24R24' or nitro group,
R23 represents hydrogen atom, methyl group, SO2NR25, formyl
group or CONHR26',
R25 represents a lower alkyl group, benzyl group or NR24R24',
R24 and R24' are the same or different, and represent hydrogen
atom, a lower alkyl group or benzyl group,
R26' represents hydrogen atom or a lower alkyl group,
R26 represents hydrogen atom or a lower alkyl group,
na is 1 or 2,
Xb represents secondary nitrogen atom, O or S,
one of R27 or R28 is hydrogen atom, and the other is hydrogen
atom, amino group, acetylamino group or hydroxy group, when
na is 1,
R28 is hydrogen atom, and R27 is hydrogen atom, amino group,
acetylamino group or hydroxy group, when na is 2,
61
*1 represents an asymmetric carbon atom,
*2 and *3 represent an asymmetric carbon atom when R26 and
R28 are respectively not hydrogen atom, or a salt thereof;
and
5) a compound of the formula [VI] :
<IMG>
wherein R30 represents hydrogen atom or methyl group,
R21 represents hydrogen atom, a halogen atom, hydroxy group,
benzyloxy group, amino group or hydroxymethyl group,
R32 represents hydrogen atom, hydroxymethyl group, NHR33,
SO2NR34R34' or nitro group,
R33 represents hydrogen atom, methyl group, SO2NR35, formyl
group or CONHR36',
R35 represents a lower alkyl group, benzyl group or NR34R34',
R34 and R34' are the same or different, and represent hydrogen
atom, a lower alkyl group or benzyl group,
R36' represents hydrogen atom or a lower alkyl group,
R36 represents hydrogen atom or a lower alkyl group,
Xc represents secondary nitrogen atom, O, S or methylene
group,
R39 is hydrogen atom, one of R37 or R38 is hydrogen atom, and
the other is hydrogen atom, amino group, acetylamino group
or hydroxy group, when Xc is secondary nitrogen atom, O or
S,
R37 and R38 are both hydrogen atom, and R39 is hydrogen atom,
amino group, acetylamino group or hydroxy group, when Xc
is methylene group,
*4 represents an asymmetric carbon atom,
*5 represents an asymmetric carbon atom when R36 is a lower
alkyl group, or a salt thereof.
62
2. A pharmaceutical composition according to claim 1,
wherein the insulin sensitizer is a compound of the formula
<IMG>
wherein R represents a hydrocarbon group or a heterocyclic
group, each of which may be substituted; Y represents a
group of the formula : -CO-, -CH(OH)- or -NR3- wherein R3
represents an alkyl group that may be substituted; m is 0
or 1; n is 0, 1 or 2; X represents CH or N; A represents
a chemical bond or a bivalent aliphatic hydrocarbon group
having 1 to 7 carbon atoms; Q represents O or S; R1 represents
hydrogen atom or an alkyl group; ring E may have further
1 to 4 substituents, which may form a ring in combination
with R1; L and M respectively represent hydrogen atom or
may be combined with each other to form a chemical bond,
or a salt thereof.
3. A pharmaceutical composition according to claim 2,
wherein the compound of the formula [IV] or a salt thereof
is pioglitazone or its salt.
4. A pharmaceutical composition according to claim 2,
wherein the compound of the formula [IV] or a salt thereof
is rosiglitazone or its salt.
5. A pharmaceutical composition according to claim 1,
which comprises pioglitazone or its hydrochloride in
combination with 2-[3-(7-carboxymethoxyindol-3-yl)-
(2R)-2-propylamino]-(1R)-1-(3-chlorophenyl)ethanol.
63
6. A pharmaceutical composition according to claim 1,
which comprises rosiglitazone or its maleate in combination
with 2-[3-(7-carboxymethoxyindol-3-yl)-(2R)-2-
propylamino]-(1R)-1-(3-chlorophenyl)ethanol.
7. A pharmaceutical composition according to claim 1,
which is for preventing or treating diabetes.
8. A pharmaceutical composition according to claim 7,
wherein the diabetes is noninsulin-dependent diabetes
mellitus.
9. A pharmaceutical composition according to claim 1,
which is for preventing or treating impaired glucose
tolerance, hyperlipidemia, hyperinsulinemia, obesity,
hyperphagia, hypertension, cardiovascular diseases,
polycystic ovary syndrome, gestational diabetes,
pancreatitis, glomerulonephritis, glomerular sclerosis,
hypertensive nephrosclerosis, inflammatory bowel diseases,
syndrome X, visceral fat obesity syndrome or diabetic
complications.
10. A pharmaceutical composition according to claim 9,
wherein the diabetic complications are retinopathy,
nephropathy, neuropathy, macroangiopahty, diabetic
hyperosmolar coma, infectious disease, diabetic
osteoporosis, diabetic gangrene, xerostomia, lowered sense
of hearing, myocardial infarction, angina pectoris,
cerebrovascular disease or peripheral circulatory
disturbance.
11. A pharmaceutical composition for inhibiting body
weight increase after stopping a smoking habit, which
comprises at least one member selected from the group
consisting of
1) a compound of the formula [I] :
64
<IMG>
wherein R1 represents a lower alkyl group optionally
substituted by hydroxyl group, phenylsulfonylamino group,
a lower alkylsulfonylamino group, a mono- or di-lower
alkylaminosulfonyl group, or a group selected from the
following (a) to (d), or combines with R2 to form
methylenedioxy group, said methylenedioxy group being
optionally substituted by carboxyl group or a lower
alkoxycarbonyl group,
(a) a group of the formula : -Xa-Ra
wherein Xa is O, S or NH, Ra is hydrogen atom or a lower
alkyl group , provided that Ra is a lower alkyl group when
Xa is S;
(b) a group of the formula : -[O(CH2)p-CH(Rb))q Rbb
wherein Rb is hydrogen atom, a lower alkyl group, a lower
alkoxycarbonyl group or carboxyl group, Rbb is a lower
alkoxycarbonyl group or carboxyl group, p is an integer of
0 to 3, q is 0 or 1,
(c) a group of the formula : -O(CH2)r-Rc
wherein Rc is a lower alkanoyl group, hydroxyl group, cyano
group, phenyl group, a mono- or di-lower alkylaminocarbonyl
group or a group of the formula : -P(=O)(OR A)(OR A)
wherein R A is hydrogen atom or a lower alkyl group, r is
an integer of 1 to 4,
(d) a group of the formula : -Ya-(CH2)s-Rd
wherein Ya is NH or S, Rd is carboxyl group or a lower
alkoxycarbonyl group, s is an integer of 1 to 4,
R2 represents hydrogen atom, a halogen atom, a lower alkyl
group optionally substituted by hydroxyl group, hydroxyl
group, a lower alkoxy group, or the same group as the above
(b) or (c), or combines with R1 to form the above
methylenedioxy group, said methylenedioxy group being
65
optionally substituted by carboxyl group or a lower
alkoxycarbonyl group,
R3 represents hydrogen atom or a lower alkyl group,
W represents a group of the formula which bonds to the 2-
or 3-position of the indole ring in the formula [I]:
<IMG>
wherein R4 represents a halogen atom or a halogeno lower
alkyl group, R5 represents a lower alkyl group, or a salt
thereof;
2) a compound of the formula [II] :
<IMG>
wherein R6 represents a halogen atom or a halogeno lower
alkyl group, R7 represents hydrogen atom, a lower alkyl
group or a halogeno lower alkyl group, R8 represents
hydrogen atom, a halogen atom, a halogeno lower alkyl group,
nitro group or cyano group, or a salt thereof;
3) a compound of the formula [III] :
<IMG>
wherein j represents an integer of 0 to 7,
k represents 0 or 1,
t represents an integer of 0 to 3,
ring A' represents benzene ring; naphthalene ring; a 5- or
6-membered heterocyclic ring containing 1 to 4 hetero atoms
selected from O, S and N; benzene ring condensed with C3-8
cycloalkyl ring; benzene ring condensed with a 5- or 6-
66
membered heterocyclic ring containing 1 to 3 hetero atoms
selected from O, S and N; or a 5- or 6-membered heterocyclic
ring containing 1 to 3 hetero atoms selected from O, S and
N which is condensed with a 5- or 6-membered heterocyclic
ring containing 1 to 3 hetero atoms selected from O, S and
N;
R11 represents hydroxy, oxo, halogen, cyano, nitro, NR18R18,
SR18, a halogeno lower alkyl, C1-6 alkyl, C1-6 alkoxy, C3-8
cycloalkyl, phenyl, SO2R19, NR18COR19, COR19 , NR18SO2R19,
NR18CO2R18; or a C1-6 alkyl group substituted by hydroxy, nitro,
halogen, cyano, NR18R18, SR18, a halogeno lower alkyl, C1-6
alkoxy, C3-8 cycloalkyl, phenyl, NR18COR19, COR19, SO2R19,
NR18SO2R19 or NR18CO2R18;
or R11 represents a 5- or 6-membered heterocyclic group
containing 1 to 3 hetero atoms selected from O, S and N;
R12 and R13 represent independently hydrogen atom, C1-6 alkyl,
or C1-6 alkyl substituted by hydroxy, C1-6 alkoxy or halogen;
X' represents -CH2-, -CH2-CH2-, -CH=CH- or -CH2O-;
R14 and R15 represent independently hydrogen atom, C1-6 alkyl,
halogen, NHR18, OR18, SO2R19 or NHSO2R19;
R16 represents hydrogen atom or C1-6 alkyl;
R17 represents C1-6 alkyl, C3-8 cycloalkyl or -B'-(R11)j
wherein R11 and j have the same meanings as above;
ring B' represents benzene ring; naphthalene ring; a 5- or
6-membered heterocyclic ring containing 1 to 4 hetero atoms
selected from O, S and N; benzene ring condensed with, C3-8
cycloalkyl ring; benzene ring condensed with a 5- or 6-
membered heterocyclic ring containing 1 to 3 hetero atoms
selected from O, S and N; or a 5- or 6-membered heterocyclic
ring containing 1 to 3 hetero atoms selected from O, S and
N which is condensed with a 5- or 6-membered heterocyclic
ring containing 1 to 3 hetero atoms selected from O, S and
N;
R18 represents hydrogen atom; C1-10 alkyl; C3-8 cycloalkyl;
phenyl substituted by halogen, C1-6 alkyl or C1-6 alkoxy; C1-10
alkyl substituted by hydroxy, halogen, CO2H, C1-6
67
alkoxy-carbonyl, C3-8 cycloalkyl, C1-6 alkoxy, or phenyl
substituted by halogen, C1-6 alkyl or C1-6 alkoxy;
R19 represents R18, NHR18 or NR18 wherein R18 has the same
meaning as above, or a salt thereof;
4) a compound of the formula [V] :
<IMG>
wherein R20 represents hydrogen atom or methyl group,
R21 represents hydrogen atom, halogen atom, hydroxy group,
benzyloxy group, amino group or hydroxymethyl group,
R22 represents hydrogen atom, hydroxymethyl group, NHR23,
SO2NR24R24' or nitro group,
R23 represents hydrogen atom, methyl group, SO2NR25, formyl
group or CONHR26',
R25 represents a lower alkyl group, benzyl group or NR24R24',
R24 and R24' are the same or different, and represent hydrogen
atom, a lower alkyl group or benzyl group,
R26' represents hydrogen atom or a lower alkyl group,
R26 represents hydrogen atom or a lower alkyl group,
na is 1 or 2,
Xb represents secondary nitrogen atom, O or S,
one of R27 or R28 is hydrogen atom, and the other is hydrogen
atom, amino group, acetylamino group or hydroxy group, when
na is 1,
R28 is hydrogen atom, and R27 is hydrogen atom, amino group,
acetylamino group or hydroxy group, when na is 2,
*1 represents an asymmetric carbon atom,
*2 and *3 represent an asymmetric carbon atom when R26 and
R28 are respectively not hydrogen atom, or a salt thereof;
and
5) a compound of the formula [VI] :
68
<IMG>
wherein R30 represents hydrogen atom or methyl group,
R21 represents hydrogen atom, a halogen atom, hydroxy group,
benzyloxy group, amino group or hydroxymethyl group,
R32 represents hydrogen atom, hydroxymethyl group, NHR33,
SO2NR34R34' or nitro group,
R33 represents hydrogen atom, methyl group, SO2NR35, formyl
group or CONHR33',
R35 represents a lower alkyl group, benzyl group or NR34R34',
R34 and R34' are the same or different, and represent hydrogen
atom, a lower alkyl group or benzyl group,
R36' represents hydrogen atom or a lower alkyl group,
R36 represents hydrogen atom or a lower alkyl group,
Xc represents secondary nitrogen atom, O, S or methylene
group,
R39 is hydrogen atom, one of R37 or R38 is hydrogen atom, and
the other is hydrogen atom, amino group, acetylamino group
or hydroxy group, when Xc is secondary nitrogen atom, O or
S,
R37 and R38 are both hydrogen atom, and R39 is hydrogen atom,
amino group, acetylamino group or hydroxy group, when Xc
is methylene group,
*4 represents an asymmetric carbon atom,
*5 represents an asymmetric carbon atom when R36 is a lower
alkyl group, or a salt thereof.
12. A pharmaceutical composition for inhibiting body
weight increase after stopping alimentary therapy, which
comprises at least one member selected from the group
consisting of
69
1) a compound of the formula [I] :
<IMG>
wherein R1 represents a lower alkyl group optionally
substituted by hydroxyl group, phenylsulfonylamino group,
a lower alkylsulfonylamino group, a mono- or di-lower
alkylaminosulfonyl group, or a group selected from the
following (a) to (d), or combines with R2 to form
methylenedioxy group, said methylenedioxy group being
optionally substituted by carboxyl group or a lower
alkoxycarbonyl group,
(a) a group of the formula : -Xa-Ra
wherein Xa is O, S or NH, Ra is hydrogen atom or a lower
alkyl group, provided that Ra is a lower alkyl group when
Xa is S;
(b) a group of the formula : -[O(CH2)p-CH(Rb)]q Rbb
wherein Rb is hydrogen atom, a lower alkyl group, a lower
alkoxycarbonyl group or carboxyl group, Rbb is a lower
alkoxycarbonyl group or carboxyl group, p is an integer of
0 to 3, q is 0 or 1,
(c) a group of the formula : -O(CH2)r-Rc
wherein Rc is a lower alkanoyl group, hydroxyl group, cyano
group, phenyl group, a mono-or di-lower alkylaminocarbonyl
group or a group of the formula : -P(=O)(OR A)(OR A)
wherein R A is hydrogen atom or a lower alkyl group, r is
an integer of 1 to 4,
(d) a group of the formula : -Ya-(CH2)s-Rd
wherein Ya is NH or S, Rd is carboxyl group or a lower
alkoxycarbonyl group, s is an integer of 1 to 4,
R2 represents hydrogen atom, a halogen atom, a lower alkyl
group optionally substituted by hydroxyl group, hydroxyl
group, a lower alkoxy group, or the same group as the above
(b) or (c), or combines with R1 to form the above
70
methylenedioxy group, said methylenedioxy group being
optionally substituted by carboxyl group or a lower
alkoxycarbonyl group,
R3 represents hydrogen atom or a lower alkyl group,
W represents a group of the formula which bonds to the 2-
or 3-position of the indole ring in the formula [I]:
<IMG>
wherein R4 represents a halogen atom or a halogeno lower
alkyl group, R5 represents a lower alkyl group, or a salt
thereof;
2) a compound of the formula [II] :
<IMG>
wherein R6 represents a halogen atom or a halogeno lower
alkyl group, R7 represents hydrogen atom, a lower alkyl
group or a halogeno lower alkyl group, R8 represents
hydrogen atom, a halogen atom, a halogeno lower alkyl group,
nitro group or cyano group, or a salt thereof;
3) a compound of the formula [III] :
<IMG>
wherein j represents an integer of 0 to 7,
k represents 0 or 1,
t represents an integer of 0 to 3,
ring A' represents benzene ring; naphthalene ring; a 5- or
6-membered heterocyclic ring containing 1 to 4 hetero atoms
selected from O, S and N; benzene ring condensed with C3-8
71
cycloalkyl ring; benzene ring condensed with a 5- or 6-
membered heterocyclic ring containing 1 to 3 hetero atoms
selected from O, S and N; or a 5- or 6-membered heterocyclic
ring containing 1 to 3 hetero atoms selected from O, S and
N which is condensed with a 5- or 6-membered heterocyclic
ring containing 1 to 3 hetero atoms selected from O, S and
N;
R11 represents hydroxy, oxo, halogen, cyano, nitro, NR18R18,
SR18, a halogeno lower alkyl, C1-6 alkyl, C1-6 alkoxy, C3-8
cycloalkyl, phenyl, SO2R19, NR18COR19, COR19, NR18SO2R19,
NR18CO2R18; or a C1-6 alkyl group substituted by hydroxy, nitro,
halogen, cyano, NR18R18, SR18, a halogeno lower alkyl, C1-6
alkoxy, C3-8 cycloalkyl, phenyl, NR18COR19, COR19, SO2R19,
NR18SO2R19 or NR18CO2R18;
or R11 represents a 5- or 6-membered heterocyclic group
containing 1 to 3 hetero atoms selected from O, S and N;
R12 and R13 represent independently hydrogen atom, C1-6 alkyl,
or C1-6 alkyl substituted by hydroxy, C1-6 alkoxy or halogen;
X' represents -CH2-, -CH2-CH2-, -CH=CH- or -CH2O-;
R14 and R15 represent independently hydrogen atom, C1-6 alkyl,
halogen, NHR18, OR18, SO2R19 or NHSO2R19;
R16 represents hydrogen atom or C1-6 alkyl;
R17 represents C1-6 alkyl, C3-8 cycloalkyl or -B'-(R11)j
wherein R11 and j have the same meanings as above;
ring B' represents benzene ring; naphthalene ring; a 5- or
6-membered heterocyclic ring containing 1 to 4 hetero atoms
selected from O, S and N; benzene ring condensed with C3-8
cycloalkyl ring; benzene ring condensed with a 5- or 6-
membered heterocyclic ring containing 1 to 3 hetero atoms
selected from O, S and N; or a 5- or 6-membered heterocyclic
ring containing 1 to 3 hetero atoms selected from O, S and
N which is condensed with a 5- or 6-membered heterocyclic
ring containing 1 to 3 hetero atoms selected from O, S and
N;
R18 represents hydrogen atom; C1-10 alkyl; C3-8 cycloalkyl;
phenyl substituted by halogen, C1-6 alkyl or C1-6 alkoxy; C1-10
72
alkyl substituted by hydroxy, halogen, CO2H, C1-6
alkoxy-carbonyl , C3-8 cycloalkyl , C1-6 alkoxy , or phenyl
substituted by halogen, C1-6 alkyl or C1-6 alkoxy;
R19 represents R18, NHR18 or NR18 wherein R18 has the same
meaning as above, or a salt thereof;
4) a compound of the formula [V]:
<IMG>
wherein R20 represents hydrogen atom or methyl group,
R21 represents hydrogen atom, halogen atom, hydroxy group,
benzyloxy group, amino group or hydroxymethyl group,
R22 represents hydrogen atom, hydroxymethyl group, NHR23,
SO2NR24R24 or nitro group,
R23 represents hydrogen atom, methyl group, SO2NR25, formyl
group or CONHR26',
R25 represents a lower alkyl group, benzyl group or NR24R24',
R24 and R24' are the same or different, and represent hydrogen
atom, a lower alkyl group or benzyl group,
R26' represents hydrogen atom or a lower alkyl group,
R26 represents hydrogen atom or a lower alkyl group,
na is 1 or 2,
Xb represents secondary nitrogen atom, O or S,
one of R27 or R28 is hydrogen atom, and the other is hydrogen
atom, amino group, acetylamino group or hydroxy group, when
na is 1,
R28 is hydrogen atom, and R27 is hydrogen atom, amino group,
acetylamino group or hydroxy group, when na is 2,
*1 represents an asymmetric carbon atom,
*2 and *3 represent an asymmetric carbon atom when R26 and
R28 are respectively not hydrogen atom, or a salt thereof;
and
73
5) a compound of the formula [VI]:
<IMG>
wherein R30 represents hydrogen atom or methyl group,
R21 represents hydrogen atom, a halogen atom, hydroxy group,
benzyloxy group, amino group or hydroxymethyl group,
R32 represents hydrogen atom, hydroxymethyl group, NHR33,
SO2NR34R34' or nitro group,
R33 represents hydrogen atom, methyl group, SO2NR35, formyl
group or CONHR36',
R35 represents a lower alkyl group, benzyl group or NR34R34',
R34 and R34' are the same or different, and represent hydrogen
atom, a lower alkyl group or benzyl group,
R36' represents hydrogen atom or a lower alkyl group,
R36 represents hydrogen atom or a lower alkyl group,
Xc represents secondary nitrogen atom, O, S or methylene
group,
R39 is hydrogen atom, one of R37 or R38 is hydrogen atom, and
the other is hydrogen atom, amino group, acetylamino group
or hydroxy group, when Xc is secondary nitrogen atom, O or
S,
R37 and R38 are both hydrogen atom, and R39 is hydrogen atom,
amino group, acetylamino group or hydroxy group, when Xc
is methylene group,
*4 represents an asymmetric carbon atom,
*5 represents an asymmetric carbon atom when R36 is a lower
alkyl group, or a salt thereof.
13. A method for preventing or treating diabetes in a
mammal in need thereof, which comprises administering to
said mammal an effective amount of an insulin sensitizer
74
in combination with at least one member selected from the
group consisting of
1) a compound of the formula [I]:
<IMG>
wherein R1 represents a lower alkyl group optionally
substituted by hydroxyl group, phenylsulfonylamino group,
a lower alkylsulfonylamino group, a mono- or di-lower
alkylaminosulfonyl group, or a group selected from the
following (a) to (d), or combines with R2 to form
methylenedioxy group, said methylenedioxy group being
optionally substituted by carboxyl group or a lower
alkoxycarbonyl group,
(a) a group of the formula . -Xa-Ra
wherein Xa is O, S or NH, Ra is hydrogen atom or a lower
alkyl group , provided that Ra is a lower alkyl group when
Xa is S;
(b) a group of the formula : -[O(CH2)p-CH(Rb)]q Rbb
wherein Rb is hydrogen atom, a lower alkyl group, a lower
alkoxycarbonyl group or carboxyl group, Rbb is a lower
alkoxycarbonyl group or carboxyl group, p is an integer of
0 to 3 , q is 0 or 1,
(c) a group of the formula : -O(CH2)r-Rc
wherein Rc is a lower alkanoyl group, hydroxyl group, cyano
group, phenyl group, a mono- or di-lower alkylaminocarbonyl
group or a group of the formula : -P(=O)(OR A)(OR A)
wherein R A is hydrogen atom or a lower alkyl group, r is
an integer of 1 to 4,
(d) a group of the formula : -Ya-(CH2)s-Rd
wherein Ya is NH or S, Rd is carboxyl group or a lower
alkoxycarbonyl group, s is an integer-of 1 to 4,
R2 represents hydrogen atom, a halogen atom, a lower alkyl
group optionally substituted by hydroxyl group, hydroxyl
75
group, a lower alkoxy group, or the same group as the above
(b) or (c), or combines with R1 to form the above
methylenedioxy group, said methylenedioxy group being
optionally substituted by carboxyl group or a lower
alkoxycarbonyl group,
R3 represents hydrogen atom or a lower alkyl group,
W represents a group of the formula which bonds to the 2-
or 3-position of the indole ring in the formula [I]:
<IMG>
wherein R4 represents a halogen atom or a halogeno lower
alkyl group, R5 represents a lower alkyl group, or a salt
thereof;
2) a compound of the formula [II]:
<IMG>
wherein R6 represents a halogen atom or a halogeno lower
alkyl group, R7 represents hydrogen atom, a lower alkyl
group or a halogeno lower alkyl group, R8 represents
hydrogen atom, a halogen atom, a halogeno lower alkyl group,
nitro group or cyano group, or a salt thereof;
3) a compound of the formula [III]:
<IMG>
wherein j represents an integer of 0 to 7,
k represents 0 or 1,
t represents an integer of 0 to 3,
ring A' represents benzene ring; naphthalene ring; a 5- or
76
6-membered heterocyclic ring containing 1 to 4 hetero atoms
selected from O, S and N; benzene ring condensed with C3-8
cycloalkyl ring; benzene ring condensed with a 5- or 6-
membered heterocyclic ring containing 1 to 3 hetero atoms
selected from O, S and N; or a 5- or 6-membered heterocyclic
ring containing 1 to 3 hetero atoms selected from O, S and
N which is condensed with a 5- or 6-membered heterocyclic
ring containing 1 to 3 hetero atoms selected from O, S and
N;
R11 represents hydroxy, oxo , halogen , cyano , nitro , NR18R18 ,
SR18, a halogeno lower alkyl, C1-6 alkyl, C1-6 alkoxy, C3-8
cycloalkyl, phenyl, SO2R19, NR18COR19, COR19, NR18SO2R19,
NR18CO2R18; or a C1-6 alkyl group substituted by hydroxy, nitro,
halogen, cyano, NR18R18, SR18, a halogeno lower alkyl, C1-6
alkoxy, C38 cycloalkyl, phenyl, NR18COR19, COR19, SO2R19,
NR18SO2R19 or NR18CO2R18;
or R11 represents a 5- or 6-membered heterocyclic group
containing 1 to 3 hetero atoms selected from O, S and N;
R12 and R13 represent independently hydrogen atom, C1-6 alkyl,
or C1-6 alkyl substituted by hydroxy, C1-6 alkoxy or halogen;
X' represents -CH2-, -CH2-CH2-, -CH=CH- or -CH2O-;
R14 and R15 represent independently hydrogen atom, C1-6 alkyl,
halogen, NHR18, OR18, SO2R19 or NHSO2R19;
R16 represents hydrogen atom or C1-6 alkyl;
R17 represents C1-6 alkyl, C3-8 cycloalkyl or -B' -(R11)j
wherein R11 and j have the same meanings as above;
ring B' represents benzene ring; naphthalene ring; a 5- or
6-membered heterocyclic ring containing 1 to 4 hetero atoms
selected from O, S and N; benzene ring condensed with C3-8
cycloalkyl ring; benzene ring condensed with a 5- or 6-
membered heterocyclic ring containing 1 to 3 hetero atoms
selected from O, S and N; or a 5- or 6-membered heterocyclic
ring containing 1 to 3 hetero atoms selected from O, S and
N which is condensed with a 5- or 6-membered heterocyclic
ring containing 1 to 3 hetero atoms selected from O, S and
N;
77
R18 represents hydrogen atom; C1-10 alkyl; C3-8 cycloalkyl;
phenyl substituted by halogen, C1-6 alkyl or C1-6 alkoxy; C1-10
alkyl substituted by hydroxy, halogen, CO2H, C1-6
alkoxy-carbonyl, C3-8 cycloalkyl, C1-6 alkoxy, or phenyl
substituted by halogen, C1-6 alkyl or C1-6 alkoxy;
R19 represents R18, NHR18 or NR18 wherein R18 has the same
meaning as above, or a salt thereof;
4) a compound of the formula [V]:
<IMG>
wherein R20 represents hydrogen atom or methyl group,
R21 represents hydrogen atom, halogen atom, hydroxy group,
benzyloxy group, amino group or hydroxymethyl group,
R22 represents hydrogen atom, hydroxymethyl group, NHR23,
SO2NR24R24' or nitro group,
R23 represents hydrogen atom, methyl group, SO2NR25, formyl
group or CONHR26',
R25 represents a lower alkyl group, benzyl group or NR24R24',
R24 and R24' are the same or different, and represent hydrogen
atom, a lower alkyl group or benzyl group,
R26' represents hydrogen atom or a lower alkyl group,
R26 represents hydrogen atom or a lower alkyl group,
na is 1 or 2,
Xb represents secondary nitrogen atom, O or S,
one of R27 or R28 is hydrogen atom, and the other is hydrogen
atom, amino group, acetylamino group or hydroxy group, when
na is 1,
R28 is hydrogen atom, and R27 is hydrogen atom, amino group,
acetylamino group or hydroxy group, when na is 2,
*1 represents an asymmetric carbon atom,
*2 and *3 represent an asymmetric carbon atom when R26 and
78
R28 are respectively not hydrogen atom, or a salt thereof ;
and
5) a compound of the formula [VI] :
<IMG>
wherein R30 represents hydrogen atom or methyl group,
R21 represents hydrogen atom, a halogen atom, hydroxy group,
benzyloxy group, amino group or hydroxymethyl group,
R32 represents hydrogen atom, hydroxymethyl group, NHR33,
SO2NR34R34' or nitro group,
R33 represents hydrogen atom, methyl group, SO2NR35, formyl
group or CONHR36',
R35 represents a lower alkyl group, benzyl group or NR34R34',
R34 and R34' are the same or different , and represent hydrogen
atom, a lower alkyl group or benzyl group,
R36' represents hydrogen atom or a lower alkyl group,
R36 represents hydrogen atom or a lower alkyl group,
Xc represents secondary nitrogen atom, O, S or methylene
group,
R39 is hydrogen atom, one of R37 or R38 is hydrogen atom, and
the other is hydrogen atom, amino group, acetylamino group
or hydroxy group, when Xc is secondary nitrogen atom, O or
S,
R37 and R38 are both hydrogen atom, and R39 is hydrogen atom,
amino group, acetylamino group or hydroxy group, when Xc
is methylene group,
*4 represents an asymmetric carbon atom,
*5 represents an asymmetric carbon atom when R36 is a lower
alkyl group, or a salt thereof.
14. A method for preventing or treating impaired glucose
79
tolerance, hyperlipidemia, hyperinsulinemia, obesity,
hyperphagia, hypertension, cardiovascular diseases,
polycystic ovary syndrome, gestational diabetes,
pancreatitis, glomerulonephritis, glomerular sclerosis,
hypertensive nephrosclerosis, inflammatory bowel diseases,
syndrome X, visceral fat obesity syndrome or diabetic
complications, in a mammal in need thereof, which comprises
administering to said mammal an effective amount of an
insulin sensitizer in combination with at least one member
selected from the group consisting of
1) a compound of the formula [I] :
<IMG>
wherein R1 represents a lower alkyl group optionally
substituted by hydroxyl group, phenylsulfonylamino group,
a lower alkylsulfonylamino group, a mono- or di-lower
alkylaminosulfonyl group, or a group selected from the
following (a) to (d), or combines with R2 to form
methylenedioxy group, said methylenedioxy group being
optionally substituted by carboxyl group or a lower
alkoxycarbonyl group,
(a) a group of the formula : -Xa-Ra
wherein Xa is O, S or NH, Ra is hydrogen atom or a lower
alkyl group, provided that Ra is a lower alkyl group when
Xa is S;
(b) a group of the formula : -[O(CH2)p -CH(Rb)]q Rbb
wherein Rb is hydrogen atom, a lower alkyl group, a lower
alkoxycarbonyl group or carboxyl group, Rbb is a lower
alkoxycarbonyl group or carboxyl group, p is an integer of
0 to 3, q is 0 or 1,
(c) a group of the formula : -O(CH2)r -Rc
wherein Rc is a lower alkanoyl group , hydroxyl group , cyano
group, phenyl group, a mono- or di-lower alkylaminocarbonyl
80
group or a group of the formula : -P(=O)(OR A)(OR A)
wherein R A is hydrogen atom or a lower alkyl group, r is
an integer of 1 to 4,
(d) a group of the formula : -Ya-(CH2)s -Rd
wherein Ya is NH or S, Rd is carboxyl group or a lower
alkoxycarbonyl group, s is an integer of 1 to 4,
R2 represents hydrogen atom, a halogen atom, a lower alkyl
group optionally substituted by hydroxyl group, hydroxyl
group, a lower alkoxy group, or the same group as the above
(b) or (c), or combines with R1 to form the above
methylenedioxy group, said methylenedioxy group being
optionally substituted by carboxyl group or a lower
alkoxycarbonyl group,
R3 represents hydrogen atom or a lower alkyl group,
W represents a group of the formula which bonds to the 2-
or 3-position of the indole ring in the formula [I]:
<IMG>
wherein R4 represents a halogen atom or a halogeno lower
alkyl group, R5 represents a lower alkyl group, or a salt
thereof ;
2) a compound of the formula [II] :
<IMG>
wherein R6 represents a halogen atom or a halogeno lower
alkyl group, R7 represents hydrogen atom, a lower alkyl
group or a halogeno lower alkyl group, R8 represents
hydrogen atom, a halogen atom, a halogeno lower alkyl group,
nitro group or cyano group, or a salt thereof;
3) a compound of the formula [III] :
81
<IMG>
wherein j represents an integer of 0 to 7,
k represents 0 or 1,
t represents an integer of 0 to 3,
ring A' represents benzene ring; naphthalene ring; a 5- or
6-membered heterocyclic ring containing 1 to 4 hetero atoms
selected from O, S and N: benzene ring condensed with C3-8
cycloalkyl ring; benzene ring condensed with a 5- or 6-
membered heterocyclic ring containing 1 to 3 hetero atoms
selected from O, S and N; or a 5- or 6-membered heterocyclic
ring containing 1 to 3 hetero atoms selected from O, S and
N which is condensed with a 5- or 6-membered heterocyclic
ring containing 1 to 3 hetero atoms selected from O, S and
N;
R11 represents hydroxy, oxo, halogen, cyano, nitro, NR18R18,
SR18, a halogeno lower alkyl, C1-6 alkyl, C1-6 alkoxy, C3-8
cycloalkyl, phenyl, SO2R19, NR18COR19, COR19, NR18SO2R19,
NR18CO2R18; or a C1-6 alkyl group substituted by hydroxy, nitro,
halogen, cyano, NR18R18, SR18, a halogeno lower alkyl, C1-6
alkoxy, C3-8 cycloalkyl, phenyl, NR18COR19, COR19, SO2R19,
NR18SO2R19 or NR18CO2R18;
or R11 represents a 5- or 6-membered heterocyclic group
containing 1 to 3 hetero atoms selected from O, S and N;
R12 and R13 represent independently hydrogen atom, C1-6 alkyl,
or C1-6 alkyl substituted by hydroxy, C1-6 alkoxy or halogen;
X' represents -CH2-, -CH2-CH2-, -CH=CH- or -CH2O-;
R14 and R15 represent independently hydrogen atom, C1-6 alkyl,
halogen, NHR18, OR18, SO2R19 or NHSO2R19;
R16 represents hydrogen atom or C1-6 alkyl;
R17 represents C1-6 alkyl, C3-8 cycloalkyl or -B'-(R11)j
wherein R11 and j have the same meanings as above;
ring B' represents benzene ring; naphthalene ring; a 5- or
82
6-membered heterocyclic ring containing 1 to 4 hetero atoms
selected from O, S and N; benzene ring condensed with C3-8
cycloalkyl ring; benzene ring condensed with a 5- or 6-
membered heterocyclic ring containing 1 to 3 hetero atoms
selected from O, S and N; or a 5- or 6-membered heterocyclic
ring containing 1 to 3 hetero atoms selected from O, S and
N which is condensed with a 5- or 6-membered heterocyclic
ring containing 1 to 3 hetero atoms selected from O, S and
N;
R18 represents hydrogen atom; C1-10 alkyl; C3-8 cycloalkyl;
phenyl substituted by halogen, C1-6 alkyl or C1-6 alkoxy; C1-10
alkyl substituted by hydroxy, halogen, CO2H, C1-6
alkoxy-carbonyl, C3-8 cycloalkyl, C1-6 alkoxy, or phenyl
substituted by halogen, C1-6 alkyl or C1-6 alkoxy;
R19 represents R18, NHR18 or NR18 wherein R18 has the same
meaning as above, or a salt thereof;
4) a compound of the formula [V] :
<IMG>
wherein R20 represents hydrogen atom or methyl group,
R21 represents hydrogen atom, halogen atom, hydroxy group,
benzyloxy group, amino group or hydroxymethyl group,
R22 represents hydrogen atom, hydroxymethyl group, NHR23,
SO2NR24R24' or nitro group,
R23 represents hydrogen atom, methyl group, SO2NR25, formyl
group or CONHR26',
R25 represents a lower alkyl group, benzyl group or NR24R24',
R24 and R24' are the same or different, and represent hydrogen
atom, a lower alkyl group or benzyl group,
R26' represents hydrogen atom or a lower alkyl group,
R26 represents hydrogen atom or a lower alkyl group,
83
na is 1 or 2,
Xb represents secondary nitrogen atom, O or S,
one of R27 or R28 is hydrogen atom, and the other is hydrogen
atom, amino group, acetylamino group or hydroxy group, when
na is 1,
R28 is hydrogen atom, and R27 is hydrogen atom, amino group,
acetylamino group or hydroxy group, when na is 2,
*1 represents an asymmetric carbon atom,
*2 and *3 represent an asymmetric carbon atom when R26 and
R28 are respectively not hydrogen atom, or a salt thereof ;
and
5) a compound of the formula [VI] :
<IMG>
wherein R30 represents hydrogen atom or methyl group,
R21 represents hydrogen atom, a halogen atom, hydroxy group,
benzyloxy group, amino group or hydroxymethyl group,
R32 represents hydrogen atom, hydroxymethyl group, NHR33,
SO2NR34R34' or nitro group,
R33 represents hydrogen atom, methyl group, SO2NR35, formyl
group or CONHR36',
R35 represents a lower alkyl group, benzyl group or NR34R34',
R34 and R34' are the same or different, and represent hydrogen
atom, a lower alkyl group or benzyl group,
R36' represents hydrogen atom or a lower alkyl group,
R36 represents hydrogen atom or a lower alkyl group,
Xc represents secondary nitrogen atom, O, S or methylene
group,
R39 is hydrogen atom, one of R37 or R38 is hydrogen atom, and
the other is hydrogen atom, amino group, acetylamino group
or hydroxy group, when Xc is secondary nitrogen atom, O or
84
S,
R37 and R38 are both hydrogen atom, and R39 is hydrogen atom,
amino group, acetylamino group or hydroxy group, when Xc
is methylene group,
*4 represents an asymmetric carbon atom,
*5 represents an asymmetric carbon atom when R36 is a lower
alkyl group, or a salt thereof.
15. A method according to claim 14, wherein the diabetic
complications are retinopathy, nephropathy, neuropathy,
macroangiopahty, diabetic hyperosmolar coma, infectious
disease, diabetic osteoporosis, diabetic gangrene,
xerostomia, lowered sense of hearing, myocardial
infarction, angina pectoris, cerebrovascular disease or
peripheral circulatory disturbance.
16. A method for inhibiting body weight increase after
stopping a smoking habit in human in need thereof, which
comprises administering to said human an effective amount
of at least one member selected from the group consisting
of
1) a compound of the formula [I] :
<IMG>
wherein R1 represents a lower alkyl group optionally
substituted by hydroxyl group, phenylsulfonylamino group,
a lower alkylsulfonylamino group, a mono- or di-lower
alkylaminosulfonyl group, or a group selected from the
following (a) to (d), or combines with R2 to form
methylenedioxy group, said methylenedioxy group being
optionally substituted by carboxyl group or a lower
alkoxycarbonyl group,
(a) a group of the formula : -Xa-Ra
85
wherein Xa is O, S or NH, Ra is hydrogen atom or a lower
alkyl group, provided that Ra is a lower alkyl group when
Xa is S;
(b) a group of the formula : -[O(CH2)p -CH(Rb)]q Rbb
wherein Rb is hydrogen atom, a lower alkyl group, a lower
alkoxycarbonyl group or carboxyl group, Rbb is a lower
alkoxycarbonyl group or carboxyl group, p is an integer of
0 to 3, q is 0 or 1,
(c) a group of the formula : -O(CH2)r -Rc
wherein Rc is a lower alkanoyl group, hydroxyl group, cyano
group, phenyl group, a mono- or di-lower alkylaminocarbonyl
group or a group of the formula : -P(=O)(OR A)(OR A)
wherein R A is hydrogen atom or a lower alkyl group, r is
an integer of 1 to 4,
(d) a group of the formula : -Ya-(CH2)s -Rd
wherein Ya is NH or S, Rd is carboxyl group or a lower
alkoxycarbonyl group, s is an integer of 1 to 4,
R2 represents hydrogen atom, a halogen atom, a lower alkyl
group optionally substituted by hydroxyl group, hydroxyl
group, a lower alkoxy group, or the same group as the above
(b) or (c), or combines with R1 to form the above
methylenedioxy group, said methylenedioxy group being
optionally substituted by carboxyl group or a lower
alkoxycarbonyl group,
R3 represents hydrogen atom or a lower alkyl group,
W represents a group of the formula which bonds to the 2-
or 3-position of the indole ring in the formula [I]:
<IMG>
wherein R4 represents a halogen atom or a halogeno lower
alkyl group, R5 represents a lower alkyl group, or a salt
thereof;
2) a compound of the formula [II] :
86
<IMG>
wherein R6 represents a halogen atom or a halogeno lower
alkyl group, R7 represents hydrogen atom, a lower alkyl
group or a halogeno lower alkyl group, R8 represents
hydrogen atom, a halogen atom, a halogeno lower alkyl group,
nitro group or cyano group, or a salt thereof;
3) a compound of the formula [III] :
<IMG>
wherein j represents an integer of 0 to 7,
k represents 0 or 1,
t represents an integer of 0 to 3,
ring A' represents benzene ring; naphthalene ring; a 5- or
6-membered heterocyclic ring containing 1 to 4 hetero atoms
selected from O, S and N; benzene ring condensed with C3-8
cycloalkyl ring; benzene ring condensed with a 5- or 6-
membered heterocyclic ring containing 1 to 3 hetero atoms
selected from O, S and N; or a 5- or 6-membered heterocyclic
ring containing 1 to 3 hetero atoms selected from O, S and
N which is condensed with a 5- or 6-membered heterocyclic
ring containing 1 to 3 hetero atoms selected from O, S and
N;
R11 represents hydroxy, oxo, halogen, cyano, nitro, NR18R18,
SR18, a halogeno lower alkyl, C1-6 alkyl, C1-6 alkoxy , C3-8
cycloalkyl, phenyl, SO2R19, NR18COR19, COR19, NR18SO2R19,
NR18CO2R18; or a C1-6 alkyl group substituted by hydroxy, nitro,
halogen, cyano, NR18R18, SR18, a halogeno lower alkyl , C1-6
alkoxy, C3-8 cycloalkyl, phenyl, NR18COR19, COR19, SO2R19,
NR18SO2R19 or NR18CO2R18;
87
or R11 represents a 5- or 6-membered heterocyclic group
containing 1 to 3 hetero atoms selected from O, S and N;
R12 and R13 represent independently hydrogen atom, C1-6 alkyl,
or C1-6 alkyl substituted by hydroxy, C1-6 alkoxy or halogen;
X' represents -CH2-, -CH2-CH2-, -CH=CH- or -CH2O-;
R14 and R15 represent independently hydrogen atom, C1-6 alkyl,
halogen, NHR18, OR18, SO2R19 or NHSO2R19;
R16 represents hydrogen atom or C1-6 alkyl;
R16 represents C1-6 alkyl, C3-8 cycloalkyl or -B'-(R11)j
wherein R11 and j have the same meanings as above;
ring B' represents benzene ring; naphthalene ring; a 5- or
6-membered heterocyclic ring containing 1 to 4 hetero atoms
selected from O, S and N; benzene ring condensed with C3-8
cycloalkyl ring; benzene ring condensed with a 5- or 6-
membered heterocyclic ring containing 1 to 3 hetero atoms
selected from O, S and N; or a 5- or 6-membered heterocyclic
ring containing 1 to 3 hetero atoms selected from O, S and
N which is condensed with a 5- or 6-membered heterocyclic
ring containing 1 to 3 hetero atoms selected from O, S and
N;
R18 represents hydrogen atom; C1-10 alkyl; C3-8 cycloalkyl;
phenyl substituted by halogen, C1-6 alkyl or C1-6 alkoxy; C1-10
alkyl substituted by hydroxy, halogen, CO2H, C1-6
alkoxy-carbonyl, C3-8 cycloalkyl, C1-6 alkoxy, or phenyl
substituted by halogen, C1-6 alkyl or C1-6 alkoxy;
R19 represents R18, NHR18 or NR18 wherein R18 has the same
meaning as above, or a salt thereof;
4) a compound of the formula [V] :
<IMG>
wherein R20 represents hydrogen atom or methyl group,
88
R21 represents hydrogen atom, halogen atom, hydroxy group,
benzyloxy group, amino group or hydroxymethyl group,
R22 represents hydrogen atom, hydroxymethyl group, NHR23,
SO2NR24R24' or nitro group,
R23 represents hydrogen atom, methyl group, SO2NR25, formyl
group or CONHR26',
R25 represents a lower alkyl group, benzyl group or NR24R24',
R24 and R24' are the same or different, and represent hydrogen
atom, a lower alkyl group or benzyl group,
R26' represents hydrogen atom or a lower alkyl group,
R26 represents hydrogen atom or a lower alkyl group,
na is 1 or 2,
Xb represents secondary nitrogen atom, O or S,
one of R27 or R28 is hydrogen atom, and the other is hydrogen
atom, amino group, acetylamino group or hydroxy group, when
na is 1,
R28 is hydrogen atom, and R27 is hydrogen atom, amino group,
acetylamino group or hydroxy group, when na is 2,
*1 represents an asymmetric carbon atom,
*2 and *3 represent an asymmetric carbon atom when R26 and
R28 are respectively not hydrogen atom, or a salt thereof;
and
5) a compound of the formula (VI] :
<IMG>
wherein R30 represents hydrogen atom or methyl group,
R21 represents hydrogen atom, a halogen atom, hydroxy group,
benzyloxy group, amino group or hydroxymethyl group,
R32 represents hydrogen atom, hydroxymethyl group, NHR33,
SO2NR34R34' or nitro group,
R33 represents hydrogen atom, methyl group, SO2NR35, formyl
89
group or CONHR36',
R35 represents a lower alkyl group, benzyl group or NR34R34',
R34 and R34' are the same or different, and represent hydrogen
atom, a lower alkyl group or benzyl group,
R36' represents hydrogen atom or a lower alkyl group,
R36 represents hydrogen atom or a lower alkyl group,
Xc represents secondary nitrogen atom, O, S or methylene
group,
R39 is hydrogen atom, one of R37 or R38 is hydrogen atom, and
the other is hydrogen atom, amino group, acetylamino group
or hydroxy group, when Xc is secondary nitrogen atom, O or
S,
R37 and R38 are both hydrogen atom, and R39 is hydrogen atom,
amino group, acetylamino group or hydroxy group, when Xc
is methylene group,
*4 represents an asymmetric carbon atom,
*5 represents an asymmetric carbon atom when R36 is a lower
alkyl group, or a salt thereof.
17. A method for inhibiting body weight increase after
stopping alimentary therapy in human in need thereof, which
comprises administering to said human an effective amount
of at least one member selected from the group consisting
of
1) a compound of the formula [I] :
<IMG>
wherein R1 represents a lower alkyl group optionally
substituted by hydroxyl group, phenylsulfonylamino group,
a lower alkylsulfonylamino group, a mono- or di-lower
alkylaminosulfonyl group, or a group selected from the
following (a) to (d), or combines with R2 to form
methylenedioxy group, said methylenedioxy group being
90
optionally substituted by carboxyl group or a lower
alkoxycarbonyl group,
(a) a group of the formula : -Xa-Ra
wherein Xa is O, S or NH, Ra is hydrogen atom or a lower
alkyl group, provided that Ra is a lower alkyl group when
Xa is S;
(b) a group of the formula : -[O(CH2)p -CH(Rb)]q Rbb
wherein Rb is hydrogen atom, a lower alkyl group, a lower
alkoxycarbonyl group or carboxyl group, Rbb is a lower
alkoxycarbonyl group or carboxyl group, p is an integer of
0 to 3, q is 0 or 1,
(c) a group of the formula : -O(CH2)r -Rc
wherein Rc is a lower alkanoyl group, hydroxyl group, cyano
group, phenyl group, a mono-or di-lower alkylaminocarbonyl
group or a group of the formula : -P(=O)(OR A)(OR A)
wherein R A is hydrogen atom or a lower alkyl group, r is
an integer of 1 to 4,
(d) a group of the formula : -Ya-(CH2)s -Rd
wherein Ya is NH or S, Rd is carboxyl group or a lower
alkoxycarbonyl group, s is an integer of 1 to 4,
R2 represents hydrogen atom, a halogen atom, a lower alkyl
group optionally substituted by hydroxyl group, hydroxyl
group, a lower alkoxy group, or the same group as the above
(b) or (c), or combines with R1 to form the above
methylenedioxy group, said methylenedioxy group being
optionally substituted by carboxyl group or a lower
alkoxycarbonyl group,
R3 represents hydrogen atom or a lower alkyl group,
W represents a group of the formula which bonds to the 2-
or 3-position of the indole ring in the formula [I]:
<IMG>
wherein R4 represents a halogen atom or a halogeno lower
alkyl group, R5 represents a lower alkyl group, or a salt
91
thereof ;
2) a compound of the formula [II] :
<IMG>
wherein R6 represents a halogen atom or a halogeno lower
alkyl group, R7 represents hydrogen atom, a lower alkyl
group or a halogeno lower alkyl group, R8 represents
hydrogen atom, a halogen atom, a halogeno lower alkyl group,
nitro group or cyano group, or a salt thereof;
3) a compound of the formula [III] :
<IMG>
wherein j represents an integer of 0 to 7,
k represents 0 or 1,
t represents an integer of 0 to 3,
ring A' represents benzene ring; naphthalene ring; a 5- or
6-membered heterocyclic ring containing 1 to 4 hetero atoms
selected from O, S and N; benzene ring condensed with C3-8
cycloalkyl ring; benzene ring condensed with a 5- or 6-
membered heterocyclic ring containing 1 to 3 hetero atoms
selected from O, S and N; or a 5- or 6-membered heterocyclic
ring containing 1 to 3 hetero atoms selected from O, S and
N which is condensed with a 5- or 6-membered heterocyclic
ring containing 1 to 3 hetero atoms selected from O, S and
N;
R11 represents hydroxy, oxo, halogen, cyano, nitro, NR18R18,
SR18, a halogeno lower alkyl, C1-6 alkyl, C1-6 alkoxy, C3-8
cycloalkyl, phenyl, SO2R19, NR18COR19, COR19, NR18SO2R19,
NR18CO2R18; or a C1-6 alkyl group substituted by hydroxy, nitro,
halogen, cyano, NR18R18, SR18, a halogeno lower alkyl, C1-6
92
alkoxy, C3-8 cycloalkyl, phenyl, NR18COR19, COR19, SO2R19,
NR18SO2R19 or NR18CO2R18;
or R11 represents a 5- or 6-membered heterocyclic group
containing 1 to 3 hetero atoms selected from O, S and N;
R12 and R13 represent independently hydrogen atom, C1-6 alkyl,
or C1-6 alkyl substituted by hydroxy, C1-6 alkoxy or halogen;
X' represents -CH2-, -CH2-CH2-, -CH=CH- or -CH2O-;
R14 and R15 represent independently hydrogen atom, C1-6 alkyl,
halogen , NHR18, OR18, SO2R19 or NHSO2R19;
R16 represents hydrogen atom or C1-6 alkyl;
R17 represents C1-6 alkyl, C3-8 cycloalkyl or -B' -(R11)j
wherein R11 and j have the same meanings as above;
ring B' represents benzene ring; naphthalene ring; a 5- or
6-membered heterocyclic ring containing 1 to 4 hetero atoms
selected from O, S and N; benzene ring condensed with C3-8
cycloalkyl ring; benzene ring condensed with a 5- or 6-
membered heterocyclic ring containing 1 to 3 hetero atoms
selected from O, S and N; or a 5- or 6-membered heterocyclic
ring containing 1 to 3 hetero atoms selected from O, S and
N which is condensed with a 5- or 6-membered heterocyclic
ring containing 1 to 3 hetero atoms selected from O, S and
N;
R18 represents hydrogen atom; C1-10 alkyl; C3-8 cycloalkyl;
phenyl substituted by halogen, C1-6 alkyl or C1-6 alkoxy; C1-10
alkyl substituted by hydroxy, halogen, CO2H, C1-6
alkoxy-carbonyl, C3-8 cycloalkyl, C1-6 alkoxy, or phenyl
substituted by halogen, C1-6 alkyl or C1-6 alkoxy;
R19 represents R18, NHR18 or NR18 wherein R18 has the same
meaning as above, or a salt thereof;
4) a compound of the formula [V] :
93
<IMG>
wherein R20 represents hydrogen atom or methyl group,
R21 represents hydrogen atom, halogen atom, hydroxy group,
benzyloxy group, amino group or hydroxymethyl group,
R22 represents hydrogen atom, hydroxymethyl group, NHR23,
SO2NR24R24' or nitro group.
R23 represents hydrogen atom, methyl group, SO2NR25, formyl
group or CONHR26',
R25 represents a lower alkyl group, benzyl group or NR24R24',
R24 and R24' are the same or different, and represent hydrogen
atom, a lower alkyl group or benzyl group,
R26' represents hydrogen atom or a lower alkyl group,
R26 represents hydrogen atom or a lower alkyl group,
na is 1 or 2,
Xb represents secondary nitrogen atom, O or S,
one of R27 or R28 is hydrogen atom, and the other is hydrogen
atom, amino group, acetylamino group or hydroxy group, when
na is 1,
R28 is hydrogen atom, and R27 is hydrogen atom, amino group,
acetylamino group or hydroxy group, when na is 2,
*1 represents an asymmetric carbon atom,
*2 and *3 represent an asymmetric carbon atom when R26 and
R28 are respectively not hydrogen atom, or a salt thereof;
and
5) a compound of the formula [VI] :
94
<IMG>
wherein R30 represents hydrogen atom or methyl group,
R21 represents hydrogen atom, a halogen atom, hydroxy group,
benzyloxy group, amino group or hydroxymethyl group,
R32 represents hydrogen atom, hydroxymethyl group, NHR33,
SO2NR34R34' or nitro group,
R33 represents hydrogen atom, methyl group, SO2NR35, formyl
group or CONHR36',
R35 represents a lower alkyl group, benzyl group or NR34R34',
R34 and R34' are the same or different, and represent hydrogen
atom, a lower alkyl group or benzyl group,
R36' represents hydrogen atom or a lower alkyl group,
R36 represents hydrogen atom or a lower alkyl group,
Xc represents secondary nitrogen atom, O, S or methylene
group,
R39 is hydrogen atom, one of R37 or R38 is hydrogen atom, and
the other is hydrogen atom, amino group, acetylamino group
or hydroxy group, when Xc is secondary nitrogen atom, O or
S,
R37 and R38 are both hydrogen atom, and R39 is hydrogen atom,
amino group, acetylamino group or hydroxy group, when Xc
is methylene group,
*4 represents an asymmetric carbon atom,
*5 represents an asymmetric carbon atom when R36 is a lower
alkyl group, or a salt thereof.
18. Use of an insulin sensitizer for the manufacture of
a pharmaceutical preparation for treating diabetes, which
is used in combination with at least one member selected
from the group consisting of
95
1) a compound of the formula [I] :
<IMG>
wherein R1 represents a lower alkyl group optionally
substituted by hydroxyl group, phenylsulfonylamino group,
a lower alkylsulfonylamino group, a mono- or di-lower
alkylaminosulfonyl group, or a group selected from the
following (a) to (d), or combines with R2 to form
methylenedioxy group, said methylenedioxy group being
optionally substituted by carboxyl group or a lower
alkoxycarbonyl group,
(a) a group of the formula : -Xa-Ra
wherein Xa is O, S or NH, Ra is hydrogen atom or a lower
alkyl group , provided that Ra is a lower alkyl group when
Xa is S;
(b) a group of the formula : -[O(CH2)p -CH(Rb)]q Rbb
wherein Rb is hydrogen atom, a lower alkyl group, a lower
alkoxycarbonyl group or carboxyl group, Rbb is a lower
alkoxycarbonyl group or carboxyl group, p is an integer of
0 to 3, q is 0 or 1,
(c) a group of the formula : -O(CH2)r -Rc
wherein Rc is a lower alkanoyl group, hydroxyl group, cyano
group, phenyl group, a mono- or di-lower alkylaminocarbonyl
group or a group of the formula : -P(=O)(OR A)(OR A)
wherein R A is hydrogen atom or a lower alkyl group, r is
an integer of 1 to 4,
(d) a group of the formula : -Ya-(CH2)s -Rd
wherein Ya is NH or S, Rd is carboxyl group or a lower
alkoxycarbonyl group, s is an integer of 1 to 4,
R2 represents hydrogen atom, a halogen atom, a lower alkyl
group optionally substituted by hydroxyl group, hydroxyl
group, a lower alkoxy group, or the same group as the above
(b) or (c), or combines with R1 to form the above
96
methylenedioxy group, said methylenedioxy group being
optionally substituted by carboxyl group or a lower
alkoxycarbonyl group,
R3 represents hydrogen atom or a lower alkyl group,
W represents a group of the formula which bonds to the 2-
or 3-position of the indole ring in the formula [I]:
<IMG>
wherein R4 represents a halogen atom or a halogeno lower
alkyl group, R5 represents a lower alkyl group, or a salt
thereof;
2) a compound of the formula [II] :
<IMG>
wherein R6 represents a halogen atom or a halogeno lower
alkyl group, R7 represents hydrogen atom, a lower alkyl
group or a halogeno lower alkyl group, R8 represents
hydrogen atom, a halogen atom, a halogeno lower alkyl group,
nitro group or cyano group, or a salt thereof;
3) a compound of the formula [III] :
<IMG>
wherein j represents an integer of 0 to 7,
k represents 0 or 1,
t represents an integer of 0 to 3,
ring A' represents benzene ring; naphthalene ring; a 5- or
6-membered heterocyclic ring containing 1 to 4 hetero atoms
selected from O, S and N; benzene ring condensed with C3-8
97
cycloalkyl ring; benzene ring condensed with a 5- or 6-
membered heterocyclic ring containing 1 to 3 hetero atoms
selected from O, S and N; or a 5- or 6-membered heterocyclic
ring containing 1 to 3 hetero atoms selected from O, S and
N which is condensed with a 5- or 6-membered heterocyclic
ring containing 1 to 3 hetero atoms selected from O, S and
N;
R11 represents hydroxy, oxo, halogen, cyano, nitro, NR18R18,
SR18, a halogeno lower alkyl, C1-6 alkyl, C1-6 alkoxy, C3-8
cycloalkyl, phenyl, SO2R19, NR18COR19, COR19, NR18SO2R19,
NR18CO2R18; or a C1-6 alkyl group substituted by hydroxy, nitro,
halogen, cyano, NR18R18, SR18, a halogeno lower alkyl, C1-6
alkoxy, C3-8 cycloalkyl, phenyl, NR18COR19, COR19, SO2R19,
NR18SO2R19 or NR18CO2R18;
or R11 represents a 5- or 6-membered heterocyclic group
containing 1 to 3 hetero atoms selected from O, S and N;
R12 and R13 represent independently hydrogen atom, C1-6 alkyl,
or C1-6 alkyl substituted by hydroxy, C1-6 alkoxy or halogen;
X' represents -CH2-, -CH2-CH2-, -CH=CH- or -CH2O-;
R14 and R15 represent independently hydrogen atom, C1-6 alkyl,
halogen, NHR18, OR18, SO2R19 or NHSO2R19;
R16 represents hydrogen atom or C1-6 alkyl;
R17 represents C1-6 alkyl, C3-8 cycloalkyl or -B'-(R11)j
wherein R11 and j have the same meanings as above;
ring B' represents benzene ring; naphthalene ring; a 5- or
6-membered heterocyclic ring containing 1 to 4 hetero atoms
selected from O, S and N; benzene ring condensed with C3-8
cycloalkyl ring; benzene ring condensed with a 5- or 6-
membered heterocyclic ring containing 1 to 3 hetero atoms
selected from O, S and N; or a 5- or 6-membered heterocyclic
ring containing 1 to 3 hetero atoms selected from O, S and
N which is condensed with a 5- or 6-membered heterocyclic
ring containing 1 to 3 hetero atoms selected from O, S and
N;
R18 represents hydrogen atom; C1-10 alkyl; C3-8 cycloalkyl;
phenyl substituted by halogen, C1-6 alkyl or C1-6 alkoxy; C1-10
98
alkyl substituted by hydroxy, halogen, CO2H, C1-6
alkoxy-carbonyl, C3-8 cycloalkyl, C1-6 alkoxy, or phenyl
substituted by halogen, C1-6 alkyl or C1-6 alkoxy;
R19 represents R18, NHR18 or NR18 wherein R18 has the same
meaning as above, or a salt thereof;
4) a compound of the formula [V] :
<IMG>
wherein R20 represents hydrogen atom or methyl group,
R21 represents hydrogen atom, halogen atom, hydroxy group,
benzyloxy group, amino group or hydroxymethyl group,
R22 represents hydrogen atom, hydroxymethyl group, NHR23,
SO2NR24R24' or nitro group,
R23 represents hydrogen atom, methyl group, SO2NR25, formyl
group or CONHR26',
R25 represents a lower alkyl group, benzyl group or NR24R24',
R24 and R24' are the same or different, and represent hydrogen
atom, a lower alkyl group or benzyl group,
R26' represents hydrogen atom or a lower alkyl group,
R26 represents hydrogen atom or a lower alkyl group,
na is 1 or 2,
Xb represents secondary nitrogen atom, O or S,
one of R27 or R28 is hydrogen atom, and the other is hydrogen
atom, amino group, acetylamino group or hydroxy group, when
na is 1,
R28 is hydrogen atom, and R27 is hydrogen atom, amino group,
acetylamino group or hydroxy group, when na is 2,
*1 represents an asymmetric carbon atom,
*2 and *3 represent an asymmetric carbon atom when R26 and
R28 are respectively not hydrogen atom, or a salt thereof;
and
99
5) a compound of the formula [VI] :
<IMG>
wherein R30 represents hydrogen atom or methyl group,
R21 represents hydrogen atom, a halogen atom, hydroxy group,
benzyloxy group, amino group or hydroxymethyl group,
R32 represents hydrogen atom, hydroxymethyl group, NHR33,
SO2NR34R34' or nitro group,
R33 represents hydrogen atom, methyl group, SO2NR35, formyl
group or CONHR36',
R35 represents a lower alkyl group, benzyl group or NR34R34',
R34 and R34' are the same or different, and represent hydrogen
atom, a lower alkyl group or benzyl group,
R36' represents hydrogen atom or a lower alkyl group,
R36 represents hydrogen atom or a lower alkyl group,
Xc represents secondary nitrogen atom, O, S or methylene
group,
R39 is hydrogen atom, one of R37 or R38 is hydrogen atom, and
the other is hydrogen atom, amino group, acetylamino group
or hydroxy group, when Xc is secondary nitrogen atom, O or
S,
R37 and R38 are both hydrogen atom, and R39 is hydrogen atom,
amino group, acetylamino group or hydroxy group, when Xc
is methylene group,
*4 represents an asymmetric carbon atom,
*5 represents an asymmetric carbon atom when R36 is a lower
alkyl group, or a salt thereof.
19. Use of at least one member selected from the group
consisting of
1) a compound of the formula [I] :
100
<IMG>
wherein R1 represents a lower alkyl group optionally
substituted by hydroxyl group, phenylsulfonylamino group,
a lower alkylsulfonylamino group, a mono- or di-lower
alkylaminosulfonyl group, or a group selected from the
following (a) to (d), or combines with R2 to form
methylenedioxy group, said methylenedioxy group being
optionally substituted by carboxyl group or a lower
alkoxycarbonyl group,
(a) a group of the formula : -Xa-Ra
wherein Xa is O, S or NH, Ra is hydrogen atom or a lower
alkyl group, provided that Ra is a lower alkyl group when
Xa is S;
(b) a group of the formula : -[O(CH2)p -CH(Rb)]q Rbb
wherein Rb is hydrogen atom, a lower alkyl group, a lower
alkoxycarbonyl group or carboxyl group, Rbb is a lower
alkoxycarbonyl group or carboxyl group, p is an integer of
0 to 3, q is 0 or 1,
(c) a group of the formula : -O(CH2)r -Rc
wherein Rc is a lower alkanoyl group, hydroxyl group, cyano
group, phenyl group, a mono- or di-lower alkylaminocarbonyl
group or a group of the formula : -P(=O)(OR A)(OR A)
wherein R A is hydrogen atom or a lower alkyl group, r is
an integer of 1 to 4,
(d) a group of the formula : -Ya-(CH2)s -Rd
wherein Ya is NH or S, Rd is carboxyl group or a lower
alkoxycarbonyl group, s is an integer of 1 to 4,
R2 represents hydrogen atom, a halogen atom, a lower alkyl
group optionally substituted by hydroxyl group, hydroxyl
group, a lower alkoxy group, or the same group as the above
(b) or (c), or combines with R1 to form the above
methylenedioxy group, said methylenedioxy group being
101
optionally substituted by carboxyl group or a lower
alkoxycarbonyl group,
R3 represents hydrogen atom or a lower alkyl group,
W represents a group of the formula which bonds to the 2-
or 3-position of the indole ring in the formula [I]:
<IMG>
wherein R4 represents a halogen atom or a halogeno lower
alkyl group, R5 represents a lower alkyl group, or a salt
thereof;
2) a compound of the formula [II] :
<IMG>
wherein R6 represents a halogen atom or a halogeno lower
alkyl group, R7 represents hydrogen atom, a lower alkyl
group or a halogeno lower alkyl group, R8 represents
hydrogen atom, a halogen atom, a halogeno lower alkyl group,
nitro group or cyano group, or a salt thereof;
3) a compound of the formula [III] :
<IMG>
wherein j represents an integer of 0 to 7,
k represents 0 or 1,
t represents an integer of 0 to 3,
ring A' represents benzene ring; naphthalene ring; a 5- or
6-membered heterocyclic ring containing 1 to 4 hetero atoms
selected from O, S and N; benzene ring condensed with C3-8
cycloalkyl ring; benzene ring condensed with a 5- or 6-
102
membered heterocyclic ring containing 1 to 3 hetero atoms
selected from O, S and N; or a 5- or 6-membered heterocyclic
ring containing 1 to 3 hetero atoms selected from O, S and
N which is condensed with a 5- or 6-membered heterocyclic
ring containing 1 to 3 hetero atoms selected from O, S and
N;
R11 represents hydroxy, oxo, halogen, cyano, nitro, NR18R18,
SR18, a halogeno lower alkyl, C1-6 alkyl, C1-6 alkoxy, C3-8
cycloalkyl, phenyl, SO2R19, NR18COR19, COR19, NR18SO2R19,
NR18CO2R18; or a C1-6 alkyl group substituted by hydroxy, nitro,
halogen, cyano, NR18R18, SR18, a halogeno lower alkyl, C1-6
alkoxy, C3-8 cycloalkyl, phenyl, NR18COR19, COR19, SO2R19,
NR18SO2R19 or NR18CO2R18;
or R11 represents a 5- or 6-membered heterocyclic group
containing 1 to 3 hetero atoms selected from O, S and N;
R12 and R13 represent independently hydrogen atom, C1-6 alkyl,
or C1-6 alkyl substituted by hydroxy, C1-6 alkoxy or halogen;
X' represents -CH2-, -CH2-CH2-, -CH=CH- or -CH2O-;
R14 and R15 represent independently hydrogen atom, C1-6 alkyl,
halogen, NHR18, OR18, SO2R19 or NHSO2R19;
R16 represents hydrogen atom or C1-6 alkyl;
R17 represents C1-6 alkyl, C3-8 cycloalkyl or -B'-(R11)j
wherein R11 and j have the same meanings as above;
ring B' represents benzene ring; naphthalene ring; a 5- or
6-membered heterocyclic ring containing 1 to 4 hetero atoms
selected from O, S and N; benzene ring condensed with C3-8
cycloalkyl ring; benzene ring condensed with a 5- or 6-
membered heterocyclic ring containing 1 to 3 hetero atoms
selected from O, S and N; or a 5- or 6-membered heterocyclic
ring containing 1 to 3 hetero atoms selected from O, S and
N which is condensed with a 5- or 6-membered heterocyclic
ring containing 1 to 3 hetero atoms selected from O, S and
N;
R18 represents hydrogen atom; C1-10 alkyl; C3-8 cycloalkyl;
phenyl substituted by halogen, C1-6 alkyl or C1-6 alkoxy; C1-10
alkyl substituted by hydroxy, halogen, CO2H, C1-6
103
alkoxy-carbonyl, C3-8 cycloalkyl, C1-6 alkoxy, or phenyl
substituted by halogen, C1-6 alkyl or C1-6 alkoxy;
R19 represents R18, NHR18 or NR18 wherein R18 has the same
meaning as above, or a salt thereof;
4) a compound of the formula [V] :
<IMG>
wherein R20 represents hydrogen atom or methyl group,
R21 represents hydrogen atom, halogen atom, hydroxy group,
benzyloxy group, amino group or hydroxymethyl group,
R22 represents hydrogen atom, hydroxymethyl group, NHR23,
SO2NR24R24' or nitro group,
R23 represents hydrogen atom, methyl group, SO2NR25, formyl
group or CONHR26',
R25 represents a lower alkyl group, benzyl group or NR24R24',
R24 and R24' are the same or different, and represent hydrogen
atom, a lower alkyl group or benzyl group,
R26' represents hydrogen atom or a lower alkyl group,
R26 represents hydrogen atom or a lower alkyl group,
na is 1 or 2,
Xb represents secondary nitrogen atom, O or S,
one of R27 or R28 is hydrogen atom, and the other is hydrogen
atom, amino group , acetylamino group or hydroxy group, when
na is 1,
R28 is hydrogen atom, and R27 is hydrogen atom, amino group,
acetylamino group or hydroxy group, when na is 2,
*1 represents an asymmetric carbon atom,
*2 and *3 represent an asymmetric carbon atom when R26 and
R28 are respectively not hydrogen atom, or a salt thereof;
and
5) a compound of the formula [VI] :
104
<IMG>
wherein R30 represents hydrogen atom or methyl group,
R21 represents hydrogen atom, a halogen atom, hydroxy group,
benzyloxy group, amino group or hydroxymethyl group,
R32 represents hydrogen atom, hydroxymethyl group, NHR33,
SO2NR34R34' or nitro group,
R33 represents hydrogen atom, methyl group, SO2NR35, formyl
group or CONHR36',
R35 represents a lower alkyl group, benzyl group or NR34R34',
R34 and R34' are the same or different, and represent hydrogen
atom, a lower alkyl group or benzyl group,
R36' represents hydrogen atom or a lower alkyl group,
R36 represents hydrogen atom or a lower alkyl group,
Xc represents secondary nitrogen atom, O, S or methylene
group,
R39 is hydrogen atom, one of R37 or R38 is hydrogen atom, and
the other is hydrogen atom, amino group, acetylamino group
or hydroxy group, when Xc is secondary nitrogen atom, O or
S,
R37 and R38 are both hydrogen atom, and R39 is hydrogen atom,
amino group, acetylamino group or hydroxy group, when Xc
is methylene group,
*4 represents an asymmetric carbon atom,
*5 represents an asymmetric carbon atom when R36 is a lower
alkyl group, or a salt thereof,
for the manufacture of a pharmaceutical preparation for
treating diabetes, which is used in combination with an
insulin sensitizer.
20. Use of an insulin sensitizer for the manufacture of
105
a pharmaceutical preparation for treating impaired glucose
tolerance, hyperlipidemia, hyperinsulinemia, obesity,
hyperphagia, hypertension, cardiovascular diseases,
polycystic ovary syndrome, gestational diabetes,
pancreatitis, glomerulonephritis, glomerular sclerosis,
hypertensive nephrosclerosis, inflammatory bowel diseases,
syndrome X, visceral fat obesity syndrome or diabetic
complications, which is used in combination with at least
one member selected from the group consisting of
1) a compound of the formula [I] :
<IMG>
wherein R1 represents a lower alkyl group optionally
substituted by hydroxyl group, phenylsulfonylamino group,
a lower alkylsulfonylamino group, a mono- or di-lower
alkylaminosulfonyl group, or a group selected from the
following (a) to (d), or combines with R2 to form
methylenedioxy group, said methylenedioxy group being
optionally substituted by carboxyl group or a lower
alkoxycarbonyl group,
(a) a group of the formula : -Xa-Ra
wherein Xa is O, S or NH, Ra is hydrogen atom or a lower
alkyl group, provided that Ra is a lower alkyl group when
Xa is S;
(b) a group of the formula : -[O(CH2)p-CH(Rb)]q Rbb
wherein Rb is hydrogen atom, a lower alkyl group, a lower
alkoxycarbonyl group or carboxyl group, Rbb is a lower
alkoxycarbonyl group or carboxyl group, p is an integer of
0 to 3, q is 0 or 1,
(c) a group of the formula : -O(CH2)r-Rc
wherein Rc is a lower alkanoyl group, hydroxyl group, cyano
group, phenyl group, a mono- or di-lower alkylaminocarbonyl
group or a group of the formula : -P(=O)(OR A)(OR A)
106
wherein R A is hydrogen atom or a lower alkyl group, r is
an integer of 1 to 4,
(d) a group of the formula : -Ya-(CH2)s-Rd
wherein Ya is NH or S, Rd is carboxyl group or a lower
alkoxycarbonyl group, s is an integer of 1 to 4,
R2 represents hydrogen atom, a halogen atom, a lower alkyl
group optionally substituted by hydroxyl group, hydroxyl
group, a lower alkoxy group, or the same group as the above
(b) or (c), or combines with R1 to form the above
methylenedioxy group, said methylenedioxy group being
optionally substituted by carboxyl group or a lower
alkoxycarbonyl group,
R3 represents hydrogen atom or a lower alkyl group,
W represents a group of the formula which bonds to the 2-
or 3-position of the indole ring in the formula [I]:
<IMG>
wherein R4 represents a halogen atom or a halogeno lower
alkyl group, R5 represents a lower alkyl group, or a salt
thereof;
2) a compound of the formula [II] :
<IMG>
wherein R6 represents a halogen atom or a halogeno lower
alkyl group, R7 represents hydrogen atom, a lower alkyl
group or a halogeno lower alkyl group, R8 represents
hydrogen atom, a halogen atom, a halogeno lower alkyl group,
nitro group or cyano group, or a salt thereof;
3) a compound of the formula [III] :
107
<IMG>
wherein j represents an integer of 0 to 7,
k represents 0 or 1,
t represents an integer of 0 to 3,
ring A' represents benzene ring; naphthalene ring; a 5- or
6-membered heterocyclic ring containing 1 to 4 hetero atoms
selected from O, S and N; benzene ring condensed with C3-8
cycloalkyl ring; benzene ring condensed with a 5- or 6-
membered heterocyclic ring containing 1 to 3 hetero atoms
selected from O, S and N; or a 5- or 6-membered heterocyclic
ring containing 1 to 3 hetero atoms selected from O, S and
N which is condensed with a 5- or 6-membered heterocyclic
ring containing 1 to 3 hetero atoms selected from O, S and
N;
R11 represents hydroxy, oxo, halogen, cyano, nitro, NR18R18,
SR18, a halogeno lower alkyl, C1-6 alkyl, C1-6 alkoxy, C3-8
cycloalkyl, phenyl, SO2R19, NR18COR19, COR19, NR18SO2R19,
NR18CO2R18; or a C1-6 alkyl group substituted by hydroxy, nitro,
halogen, cyano, NR18R18, SR18, a halogeno lower alkyl, C1-6
alkoxy, C3-8 cycloalkyl, phenyl, NR18COR19, COR19, SO2R19,
NR18SO2R19 or NR18CO2R18;
or R11 represents a 5- or 6-membered heterocyclic group
containing 1 to 3 hetero atoms selected from O, S and N;
R12 and R13 represent independently hydrogen atom, C1-6 alkyl,
or C1-6 alkyl substituted by hydroxy, C1-6 alkoxy or halogen;
X' represents -CH2-, -CH2-CH2-, -CH=CH- or -CH2O-;
R14 and R15 represent independently hydrogen atom, C1-6 alkyl,
halogen, NHR18, OR18, SO2R19 or NHSO2R19;
R16 represents hydrogen atom or C1-6 alkyl;
R17 represents C1-6 alkyl, C3-8 cycloalkyl or -B'-(R11)j
wherein R11 and j have the same meanings as above;
ring B' represents benzene ring; naphthalene ring; a 5- or
108
6-membered heterocyclic ring containing 1 to 4 hetero atoms
selected from O, S and N; benzene ring condensed with C3-8
cycloalkyl ring; benzene ring condensed with a 5- or 6-
membered heterocyclic ring containing 1 to 3 hetero atoms
selected from O, S and N; or a 5- or 6-mertibered heterocyclic
ring containing 1 to 3 hetero atoms selected from O, S and
N which is condensed with a 5- or 6-membered heterocyclic
ring containing 1 to 3 hetero atoms selected from O, S and
N;
R18 represents hydrogen atom; C1-10 alkyl; C3-8 cycloalkyl;
phenyl substituted by halogen, C1-6 alkyl or C1-6 alkoxy; C1-10
alkyl substituted by hydroxy, halogen, CO2H, C1-6
alkoxy-carbonyl, C3-8 cycloalkyl, C1-6 alkoxy, or phenyl
substituted by halogen, C1-6 alkyl or C1-6 alkoxy;
R19 represents R18, NHR18 or NR18 wherein R18 has the same
meaning as above, or a salt thereof;
4) a compound of the formula [V]:
<IMG>
wherein R20 represents hydrogen atom or methyl group,
R21 represents hydrogen atom, halogen atom, hydroxy group,
benzyloxy group, amino group or hydroxymethyl group,
R22 represents hydrogen atom, hydroxymethyl group, NHR23,
SO2NR24R24 or vitro group,
R21 represents hydrogen atom, methyl group, SO2NR25, formyl
group or CONHR26',
R25 represents a lower alkyl group, benzyl group or NR24R24,
R24 and R25 are the same or different, and represent hydrogen
atom, a lower alkyl group or benzyl group,
R26' represents hydrogen atom or a lower alkyl group,
R26 represents hydrogen atom or a lower alkyl group,
109
na is 1 or 2,
Xb represents secondary nitrogen atom, O or S,
one of R27 or R28 is hydrogen atom, and the other is hydrogen
atom, amino group, acetylamino group or hydroxy group, when
na is 1,
R28 is hydrogen atom, and R27 is hydrogen atom, amino group,
acetylamino group or hydroxy group, when na is 2,
*1 represents an asymmetric carbon atom,
*2 and *3 represent an asymmetric carbon atom when R26 and
R26 are respectively not hydrogen atom, or a salt thereof;
and
5) a compound of the formula [VI]:
<IMG>
wherein R30 represents hydrogen atom or methyl group,
R21 represents hydrogen atom, a halogen atom, hydroxy group,
benzyloxy group, amino group or hydroxymethyl group,
R32 represents hydrogen atom, hydroxymethyl group, NHR33,
SO2NR34R34' or nitro group,
R33 represents hydrogen atom, methyl group, SO2NR35, formyl
group or CONHR36',
R35 represents a lower alkyl group, benzyl group or NR34R34',
R36 and R3 are the same or different, and represent hydrogen
atom, a lower alkyl group or benzyl group,
R36' represents hydrogen atom or a lower alkyl group,
R36 represents hydrogen atom or a lower alkyl group,
Xc represents secondary nitrogen atom, O, S or methylene
group,
R39 is hydrogen atom, one of R37 or R38 is hydrogen atom, and
the other is hydrogen atom, amino group, acetylamino group
or hydroxy group, when Xc is secondary nitrogen atom, O or
110
S,
R37 and R38 are both hydrogen atom, and R39 is hydrogen atom,
amino group, acetylamino group or hydroxy group, when Xc
is methylene group,
*4 represents an asymmetric carbon atom,
*5 represents an asymmetric carbon atom when R36 is a lower
alkyl group, or a salt thereof.
21. Use of at least one member selected from the group
consisting of
1) a compound of the formula [I].
<IMG>
wherein R1 represents a lower alkyl group optionally
substituted by hydroxyl group, phenylsulfonylamino group,
a lower alkylsulfonylamino group, a mono- or di-lower
alkylaminosulfonyl group, or a group selected from the
following (a) to (d), or combines with R2 to form
methylenedioxy group, said methylenedioxy group being
optionally substituted by carboxyl group or a lower
alkoxycarbonyl group,
(a) a group of the formula : -Xa-Ra
wherein Xa is O, S or NH, Ra is hydrogen atom or a lower
alkyl group, provided that Ra is a lower alkyl group when
Xa is S;
(b) a group of the formula : - [O(CH2)p-CH(Rb)]pRbb
wherein Rb is hydrogen atom, a lower alkyl group, a lower
alkoxycarbonyl group or carboxyl group, Rbb is a lower
alkoxycarbonyl group or carboxyl group, p is an integer of
0 to 3 , q is 0 or 1,
(c) a group of the formula: -O(CH2)r-Rc
wherein Rc is a lower alkanoyl group, hydroxyl group, cyano
group, phenyl group, a mono- or di-lower alkylaminocarbonyl
111
group or a group of the formula : -P(=O)(ORA)(ORA)
wherein R A is hydrogen atom or a lower alkyl group, r is
an integer of 1 to 4,
(d) a group of the formula : -Ya- (CH2)s-Rd
wherein Ya is NH or S, Rd is carboxyl group or a lower
alkoxycarbonyl group, s is an integer of 1 to 4,
R2 represents hydrogen atom, a halogen atom, a lower alkyl
group optionally substituted by hydroxyl group, hydroxyl
group, a lower alkoxy group, or the same group as the above
(b) or (c), or combines with R1 to form the above
methylenedioxy group, said methylenedioxy group being
optionally substituted by carboxyl group or a lower
alkoxycarbonyl group,
R3 represents hydrogen atom or a lower alkyl group,
W represents a group of the formula which bonds to the 2-
or 3-position of the indole ring in the formula [I]:
<IMG>
wherein R4 represents a halogen atom or a halogeno lower
alkyl group, R5 represents a lower alkyl group, or a salt
thereof;
2) a compound of the formula [II]
<IMG>
wherein R6 represents a halogen atom or a halogeno lower
alkyl group, R7 represents hydrogen atom, a lower alkyl
group or a halogeno lower alkyl group, R8 represents
hydrogen atom, a halogen atom, a halogeno lower alkyl group,
nitro group or cyano group, or a salt thereof;
3) a compound of the formula [III] :
112
<IMG>
wherein j represents an integer of 0 to 7,
k represents 0 or 1,
t represents an integer of 0 to 3,
ring A' represents benzene ring; naphthalene ring; a 5- or
6-membered heterocyclic ring containing 1 to 4 hetero atoms
selected from O, S and N; benzene ring condensed with C3-8
cycloalkyl ring; benzene ring condensed with a 5- or 6-
membered heterocyclic ring containing 1 to 3 hetero atoms
selected from O, S and N; or a 5- or 6-membered heterocyclic
ring containing 1 to 3 hetero atoms selected from O, S and
N which is condensed with a 5- or 6-membered heterocyclic
ring containing 1 to 3 hetero atoms selected from O, S and
N;
R11 represents hydroxy, oxo, halogen, cyano, nitro, NR18R18,
SR18, a halogeno lower alkyl, C1-6 alkyl, C1-6 alkoxy , C3-8
cycloalkyl, phenyl, SO2R19, NR1BCOR19, COR19, NR18SO2R19,
NR18CO2R18; or a C1-6 alkyl group substituted by hydroxy, nitro,
halogen, cyano, NR18R, SR18, a halogeno lower alkyl, C1-6
alkoxy, C3-8 cycloalkyl, phenyl, NR18COR19, COR19, SO2R19,
NR18SO2R19 or NR18CO2R18;
or R11 represents a 5- or 6-membered heterocyclic group
containing 1 to 3 hetero atoms selected from O, S and N;
R12 and R13 represent independently hydrogen atom, C1-6 alkyl,
or C1-6 alkyl substituted by hydroxy, C1-6 alkoxy or halogen;
X' represents -CH2-, -CH2-CH2-, -CH=CH- or -CH2O-;
R14 and R15 represent independently hydrogen atom, C1-6 alkyl,
halogen, NHR18, OR15, SO2R19 or NHSO2R19;
R16 represents hydrogen atom or C1-6 alkyl;
R16 represents C1-6 alkyl, C3-8 cycloalkyl or -B' -(R11)j
wherein R11 and j have the same meanings as above;
ring B' represents benzene ring; naphthalene ring; a 5- or
113
6-membered heterocyclic ring containing 1 to 4 hetero atoms
selected from O, S and N; benzene ring condensed with C3-8
cycloalkyl ring; benzene ring condensed with a 5- or 6-
membered heterocyclic ring containing 1 to 3 hetero atoms
selected from O, S and N; or a 5- or 6-membered heterocyclic
ring containing 1 to 3 hetero atoms selected from O, S and
N which is condensed with a 5- or 6-membered heterocyclic
ring containing 1 to 3 hetero atoms selected from O, S and
N;
R18 represents hydrogen atom; C1-10 alkyl; C3-e cycloalkyl;
phenyl substituted by halogen, C1-6 alkyl or C1-6 alkoxy; C1-10
alkyl substituted by hydroxy, halogen, CO2H, C1-6
alkoxy-carbonyl, C3-8 cycloalkyl, C1-6 alkoxy, or phenyl
substituted by halogen, C1-6 alkyl or C1-6 alkoxy;
R19 represents R18, NHR18 or NR18 wherein R18 has the same
meaning as above, or a salt thereof;
4) a compound of the formula [V]:
<IMG>
wherein R20 represents hydrogen atom or methyl group,
R21 represents hydrogen atom, halogen atom, hydroxy group,
benzyloxy group, amino group or hydroxymethyl group,
R22 represents hydrogen atom, hydroxymethyl group, NHR23,
SO2NR24R24' or nitro group,
R23 represents hydrogen atom, methyl group, SO2NR25, formyl
group or CONHR26',
R25 represents a lower alkyl group, benzyl group or NR24R24',
R24 and R24 are the same or different, and represent hydrogen
atom, a lower alkyl group or benzyl group,
R26' represents hydrogen atom or a lower alkyl group,
R26 represents hydrogen atom or a lower alkyl group,
114
na is 1 or 2,
Xb represents secondary nitrogen atom, O or S,
one of R27 or R28 is hydrogen atom, and the other is hydrogen
atom, amino group, acetylamino group or hydroxy group, when
na is 1,
R28 is hydrogen atom, and R27 is hydrogen atom, amino group,
acetylamino group or hydroxy group, when na is 2,
*1 represents an asymmetric carbon atom,
*2 and *3 represent an asymmetric carbon atom when R26 and
R28 are respectively not hydrogen atom, or a salt thereof;
and
5) a compound of the formula [VI].
<IMG>
wherein R30 represents hydrogen atom or methyl group,
R21 represents hydrogen atom, a halogen atom, hydroxy group,
benzyloxy group, amino group or hydroxymethyl group,
R32 represents hydrogen atom, hydroxymethyl group, NHR33,
SO2NR33R34 or nitro group,
R33 represents hydrogen atom, methyl group, SO2NR35, formyl
group or CONHR36',
R35 represents a lower alkyl group, benzyl group or NR34R34,
R34 and R34' are the same or different, and represent hydrogen
atom, a lower alkyl group or benzyl group,
R36' represents hydrogen atom or a lower alkyl group,
R36 represents hydrogen atom or a lower alkyl group,
Xc represents secondary nitrogen atom, O, S or methylene
group,
R39 is hydrogen atom, one of R37 or R38 is hydrogen atom, and
the other is hydrogen atom, amino group, acetylamino group
or hydroxy group, when Xc is secondary nitrogen atom, O or
115
S,
R37 and R38 are both hydrogen atom, and R39 is hydrogen atom,
amino group, acetylamino group or hydroxy group, when Xc
is methylene group,
*4 represents an asymmetric carbon atom,
*5 represents an asymmetric carbon atom when R36 is a lower
alkyl group, or a salt thereof,
for the manufacture of a pharmaceutical preparation for
treating impaired glucose tolerance, hyperlipidemia,
hyperinsulinemia, obesity, hyperphagia, hypertension,
cardiovascular diseases, polycystic ovary syndrome,
gestational diabetes, pancreatitis, glomerulonephritis,
glomerular sclerosis, hypertensive nephrosclerosis,
inflammatory bowel diseases, syndrome X, visceral fat
obesity syndrome or diabetic complications;
which is used in combination with an insulin sensitizer.
22. Use according to claim 20 or 21, wherein the diabetic
complications are retinopathy, nephropathy, neuropathy,
macroangiopahty, diabetic hyperosmolar coma, infectious
disease, diabetic osteoporosis, diabetic gangrene,
xerostomia, lowered sense of hearing, myocardial
infarction, angina pectoris, cerebrovascular disease or
peripheral circulatory disturbance.
23. Use of at least one member selected from the group
consisting of
1) a compound of the formula [I].
<IMG>
wherein R1 represents a lower alkyl group optionally
substituted by hydroxyl group, phenylsulfonylamino group,
a lower alkylsulfonylamino group, a mono- or di-lower
116
alkylaminosulfonyl group, or a group selected from the
following (a) to (d), or combines with R2 to form
methylenedioxy group, said methylenedioxy group being
optionally substituted by carboxyl group or a lower
alkoxycarbonyl group,
(a) a group of the formula : -Xa-Ra
wherein Xa is O, S or NH, Ra is hydrogen atom or a lower
alkyl group, provided that Ra is a lower alkyl group when
Xa is S;
(b) a group of the formula : -(O(CH2)p-CH(Rb)]q Rbb
wherein Rb is hydrogen atom, a lower alkyl group, a lower
alkoxycarbonyl group or carboxyl group, Rbb is a lower
alkoxycarbonyl group or carboxyl group, p is an integer of
0 to 3, q is 0 or 1,
(c) a group of the formula : -O(CH2)r-Rc
wherein Rc is a lower alkanoyl group, hydroxyl group, cyano
group, phenyl group, a mono- or di-lower alkylaminocarbonyl
group or a group of the formula : -P(=O)(ORA)(ORA)
wherein R A is hydrogen atom or a lower alkyl group, r is
an integer of 1 to 4,
(d) a group of the formula : -Ya-(CH2)s-R d
wherein Ya is NH or S, Rd is carboxyl group or a lower
alkoxycarbonyl group, s is an integer of 1 to 4,
R2 represents hydrogen atom, a halogen atom, a lower alkyl
group optionally substituted by hydroxyl group, hydroxyl
group, a lower alkoxy group, or the same group as the above
(b) or (c), or combines with R1 to form the above
methylenedioxy group, said methylenedioxy group being
optionally substituted by carboxyl group or a lower
alkoxycarbonyl group,
R3 represents hydrogen atom or a lower alkyl group,
W represents a group of the formula which bonds to the 2-
or 3-position of the indole ring in the formula [I]:
117
<IMG>
wherein R4 represents a halogen atom or a halogeno lower
alkyl group, R5 represents a lower alkyl group, or a salt
thereof;
2) a compound of the formula [II] :
<IMG>
wherein R6 represents a halogen atom or a halogeno lower
alkyl group, R7 represents hydrogen atom, a lower alkyl
group or a halogeno lower alkyl group, R8 represents
hydrogen atom, a halogen atom, a halogeno lower alkyl group,
nitro group or cyano group, or a salt thereof;
3) a compound of the formula [III] :
<IMG>
wherein j represents an integer of 0 to 7,
k represents 0 or 1,
t represents an integer of 0 to 3,
ring A' represents benzene ring; naphthalene ring; a 5- or
6-membered heterocyclic ring containing 1 to 4 hetero atoms
selected from O, S and N; benzene ring condensed with C3-8
cycloalkyl ring; benzene ring condensed with a 5- or 6-
membered heterocyclic ring containing 1 to 3 hetero atoms
selected from O, S and N; or a 5- or 6-membered heterocyclic
ring containing 1 to 3 hetero atoms selected from O, S and
N which is condensed with a 5- or 6-membered heterocyclic
ring containing 1 to 3 hetero atoms selected from 0, S and
118
N;
R11 represents hydroxy, oxo, halogen, cyano, nitro, NR18R18,
SR18, a halogeno lower alkyl, C1-6 alkyl, C1-6 alkoxy, C3-8
cycloalkyl, phenyl, SO2R19, NR18COR19, COR19, NR18SO2R19,
NR18CO2R18; or a C1-6 alkyl group substituted by hydroxy, nitro,
halogen, cyano, NR18R18, SR18, a halogeno lower alkyl, C1-6
alkoxy, C3-8 cycloalkyl, phenyl, NR18COR19, COR19, SO2R19,
NR18SO2R19 or NR18CO2R18;
or R11 represents a 5- or 6-membered heterocyclic group
containing 1 to 3 hetero atoms selected from O, S and N;
R12 and R13 represent independently hydrogen atom, C1-6 alkyl,
or C1-6 alkyl substituted by hydroxy, C1-6 alkoxy or halogen;
X' represents -CH2-, -CH2-CH2-, -CH=CH- or -CH2O- ;
R14 and R15 represent independently hydrogen atom, C1-6 alkyl,
halogen, NHR18, OR18, SO2R19 or NHSO2R19;
R16 represents hydrogen atom or C1-6 alkyl;
R11 represents C1-6 alkyl, C3-8 cycloalkyl or -B'-(R11)j
wherein R11 and j have the same meanings as above;
ring B' represents benzene ring; naphthalene ring; a 5- or
6-membered heterocyclic ring containing 1 to 4 hetero atoms
selected from O, S and N; benzene ring condensed with C3-8
cycloalkyl ring; benzene ring condensed with a 5- or 6-
membered heterocyclic ring containing 1 to 3 hetero atoms
selected from O, S and N; or a 5- or 6-membered heterocyclic
ring containing 1 to 3 hetero atoms selected from O, S and
N which is condensed with a 5- or 6-membered heterocyclic
ring containing 1 to 3 hetero atoms selected from O, S and
N;
R18 represents hydrogen atom; C1-10 alkyl; C3-8 cycloalkyl;
phenyl substituted by halogen, C1-6 alkyl or C1-6 alkoxy; C1-10
alkyl substituted by hydroxy, halogen, CO2H, C1-6
alkoxy-carbonyl, C3-8 cycloalkyl, C1-6 alkoxy, or phenyl
substituted by halogen, C1-6 alkyl or C1-6 alkoxy;
R19 represents R18, NHR18 or NR18 wherein R18 has the same
meaning as above, or a salt thereof;
4) a compound of the formula [V] :
119
<IMG>
wherein R20 represents hydrogen atom or methyl group,
R21 represents hydrogen atom, halogen atom, hydroxy group,
benzyloxy group, amino group or hydroxymethyl group,
R22 represents hydrogen atom, hydroxymethyl group, NHR23,
SO2NR24R24' or nitro group,
R23 represents hydrogen atom, methyl group, SO2NR25, formyl
group or CONHR26',
R25 represents a lower alkyl group, benzyl group or NR24R24',
R24 and R24' are the same or different, and represent hydrogen
atom, a lower alkyl group or benzyl group,
R26' represents hydrogen atom or a lower alkyl group,
R26 represents hydrogen atom or a lower alkyl group,
na is 1 or 2,
Xb represents secondary nitrogen atom, O or S,
one of R27 or R28 is hydrogen atom, and the other is hydrogen
atom, amino group, acetylamino group or hydroxy group, when
na is 1,
R28 is hydrogen atom, and R27 is hydrogen atom, amino group,
acetylamino group or hydroxy group, when na is 2,
*1 represents an asymmetric carbon atom,
*2 and *3 represent an asymmetric carbon atom when R26 and
R28 are respectively not hydrogen atom, or a salt thereof;
and
5) a compound of the formula [VI] :
120
<IMG>
wherein R30 represents hydrogen atom or methyl group,
R21 represents hydrogen atom, a halogen atom, hydroxy group,
benzyloxy group, amino group or hydroxymethyl group,
R32 represents hydrogen atom, hydroxymethyl group, NHR33,
SO2NR34R34' or nitro group,
R33 represents hydrogen atom, methyl group, SO2NR35, formyl
group or CONHR36',
R35 represents a lower alkyl group, benzyl group or NR34R34',
R34 and R34' are the same or different, and represent hydrogen
atom, a lower alkyl group or benzyl group,
R36' represents hydrogen atom or a lower alkyl group,
R36 represents hydrogen atom or a lower alkyl group,
Xc represents secondary nitrogen atom, O, S or methylene
group,
R39 is hydrogen atom, one of R37 or R38 is hydrogen atom, and
the other is hydrogen atom, amino group, acetylamino group
or hydroxy group, when Xc is secondary nitrogen atom, O or
S,
R37 and R38 are both hydrogen atom, and R39 is hydrogen atom,
amino group, acetylamino group or hydroxy group, when Xc
is methylene group,
*4 represents an asymmetric carbon atom,
*5 represents an asymmetric carbon atom when R36 is a lower
alkyl group, or a salt thereof,
for the manufacture of a pharmaceutical preparation for
inhibiting body weight increase after stopping a smoking
habit.
24. Use of at least one member selected from the group
121
consisting of
1) a compound of the formula [I] :
<IMG>
wherein R1 represents a lower alkyl group optionally
substituted by hydroxyl group, phenylsulfonylamino group,
a lower alkylsulfonylamino group, a mono- or di-lower
alkylaminosulfonyl group, or a group selected from the
following (a) to (d), or combines with R2 to form
methylenedioxy group, said methylenedioxy group being
optionally substituted by carboxyl group or a lower
alkoxycarbonyl group,
(a) a group of the formula : -Xa-Ra
wherein Xa is O, S or NH, Ra is hydrogen atom or a lower
alkyl group, provided that Ra is a lower alkyl group when
Xa is S;
(b) a group of the formula : -[O(CH2)p-CH(Rb)]q Rbb
wherein Rb is hydrogen atom, a lower alkyl group, a lower
alkoxycarbonyl group or carboxyl group, Rbb is a lower
alkoxycarbonyl group or carboxyl group, p is an integer of
0 to 3 , q is 0 or 1,
(c) a group of the formula : -O(CH2)r-Rc
wherein Rc is a lower alkanoyl group, hydroxyl group, cyano
group, phenyl group, a mono- or di-lower alkylaminocarbonyl
group or a group of the formula : -P(=O)(OR A)(OR A)
wherein R A is hydrogen atom or a lower alkyl group, r is
an integer of 1 to 4,
(d) a group of the formula : -Ya-(CH2)s-Rd
wherein Ya is NH or S, Rd is carboxyl group or a lower
alkoxycarbonyl group, s is an integer of 1 to 4,
R2 represents hydrogen atom, a halogen atom, a lower alkyl
group optionally substituted by hydroxyl group, hydroxyl
group, a lower alkoxy group, or the same group as the above
122
(b) or (c), or combines with R1 to form the above
methylenedioxy group, said methylenedioxy group being
optionally substituted by carboxyl group or a lower
alkoxycarbonyl group,
R3 represents hydrogen atom or a lower alkyl group,
W represents a group of the formula which bonds to the 2-
or 3-position of the indole ring in the formula [I]:
<IMG>
wherein R4 represents a halogen atom or a halogeno lower
alkyl group, R5 represents a lower alkyl group, or a salt
thereof;
2) a compound of the formula [II] :
<IMG>
wherein R6 represents a halogen atom or a halogeno lower
alkyl group, R7 represents hydrogen atom, a lower alkyl
group or a halogeno lower alkyl group, R8 represents
hydrogen atom, a halogen atom, a halogeno lower alkyl group,
nitro group or cyano group, or a salt thereof;
3) a compound of the formula [III] :
<IMG>
wherein j represents an integer of 0 to 7,
k represents 0 or 1,
t represents an integer of 0 to 3,
ring A' represents benzene ring; naphthalene ring; a 5- or
6-membered heterocyclic ring containing 1 to 4 hetero atoms
123
selected from O, S and N; benzene ring condensed with C3-8
cycloalkyl ring; benzene ring condensed with a 5- or 6-
membered heterocyclic ring containing 1 to 3 hetero atoms
selected from O, S and N; or a 5- or 6-membered heterocyclic
ring containing 1 to 3 hetero atoms selected from O, S and
N which is condensed with a 5- or 6-membered heterocyclic
ring containing 1 to 3 hetero atoms selected from O, S and
N;
R11 represents hydroxy, oxo, halogen, cyano, nitro, NR18R18,
SR18, a halogeno lower alkyl, C1-6 alkyl, C1-6 alkoxy, C3-8
cycloalkyl, phenyl, SO2R19, NR18COR19, COR19, NR18SO2R19,
NR18CO2R18; or a C1-6 alkyl group substituted by hydroxy, nitro,
halogen, cyano, NR18R18, SR18, a halogeno lower alkyl, C1-6
alkoxy, C3-8 cycloalkyl, phenyl, NR18COR19, COR19, SO2R19,
NR18SO2R19 or NR18CO2R18;
or R11 represents a 5- or 6-membered heterocyclic group
containing 1 to 3 hetero atoms selected from O, S and N;
R12 and R13 represent independently hydrogen atom, C1-6 alkyl,
or C1-6 alkyl substituted by hydroxy, C1-6 alkoxy or halogen;
X' represents -CH2-, -CH2-CH2-, -CH=CH- or -CH2O-;
R14 and R15 represent independently hydrogen atom, C1-6 alkyl,
halogen, NHR18, OR18, SO2R19 or NHSO2R19;
R16 represents hydrogen atom or C1-6 alkyl;
R17 represents C1-6 alkyl, C3-8 cycloalkyl or -B' -(R11)j
wherein R11 and j have the same meanings as above;
ring B' represents benzene ring; naphthalene ring; a 5- or
6-membered heterocyclic ring containing 1 to 4 hetero atoms
selected from O, S and N; benzene ring condensed with C3-8
cycloalkyl ring; benzene ring condensed with a 5- or 6-
membered heterocyclic ring containing 1 to 3 hetero atoms
selected from O, S and N; or a 5- or 6-membered heterocyclic
ring containing 1 to 3 hetero atoms selected from O, S and
N which is condensed with a 5- or 6-membered heterocyclic
ring containing 1 to 3 hetero atoms selected from O, S and
N;
R18 represents hydrogen atom; C1-10 alkyl; C3-8 cycloalkyl;
124
phenyl substituted by halogen, C1-6 alkyl or C1-6 alkoxy; C1-10
alkyl substituted by hydroxy, halogen, CO2H, C1-6
alkoxy-carbonyl , C3-8 cycloalkyl , C1-6 alkoxy, or phenyl
substituted by halogen, C1-6 alkyl or C1-6 alkoxy;
R19 represents R18, NHR18 or NR18 wherein R18 has the same
meaning as above, or a salt thereof;
4) a compound of the formula [V]:
<IMG>
wherein R20 represents hydrogen atom or methyl group,
R21 represents hydrogen atom, halogen atom, hydroxy group,
benzyloxy group, amino group or hydroxymethyl group,
R22 represents hydrogen atom, hydroxymethyl group, NHR23,
SO2NR24R24 or nitro group,
R23 represents hydrogen atom, methyl group, SO2NR25, formyl
group or CONHR26',
R25 represents a lower alkyl group, benzyl group or NR24R24-,
R24 and R24' are the same or different, and represent hydrogen
atom, a lower alkyl group or benzyl group,
R26' represents hydrogen atom or a lower alkyl group,
R26 represents hydrogen atom or a lower alkyl group,
na is 1 or 2 ,
Xb represents secondary nitrogen atom, O or S,
one of R27 or R28 is hydrogen atom, and the other is hydrogen
atom, amino group, acetylamino group or hydroxy group, when
na is 1,
R28 is hydrogen atom, and R27 is hydrogen atom, amino group,
acetylamino group or hydroxy group, when na is 2,
*1 represents an asymmetric carbon atom,
*2 and *3 represent an asymmetric carbon atom when R26 and
R28 are respectively not hydrogen atom, or a salt thereof;
125
and
5) a compound of the formula [VI]:
<IMG>
wherein R30 represents hydrogen atom or methyl group,
R21 represents hydrogen atom, a halogen atom, hydroxy group,
benzyloxy group, amino group or hydroxymethyl group,
R32 represents hydrogen atom, hydroxymethyl group, NHR33,
SO2NR34R34' or nitro group,
R33 represents hydrogen atom, methyl group, SO2NR35, formyl
group or CONHR36',
R35 represents a lower alkyl group, benzyl group or NR34R34',
R34 and R34' are the same or different, and represent hydrogen
atom, a lower alkyl group or benzyl group,
R36' represents hydrogen atom or a lower alkyl group,
R36 represents hydrogen atom or a lower alkyl group,
Xc represents secondary nitrogen atom, O, S or methylene
group,
R39 is hydrogen atom, one of R37 or R38 is hydrogen atom, and
the other is hydrogen atom, amino group, acetylamino group
or hydroxy group, when Xc is secondary nitrogen atom, O or
S,
R37 and R38 are both hydrogen atom, and R39 is hydrogen atom,
amino group, acetylamino group or hydroxy group, when Xc
is methylene group,
*4 represents an asymmetric carbon atom,
*5 represents an asymmetric carbon atom when R36 is a lower
alkyl group, or a salt thereof,
for the manufacture of a pharmaceutical preparation for
inhibiting body weight increase after stopping alimentary
therapy.