Note: Descriptions are shown in the official language in which they were submitted.
CA 02384029 2002-03-04
WO 01/18240 PCT/CA00/01043
DIAGNOSTIC AND THERAPEUTIC METHODS IN
AUTOIMMUNE DISEASE
FIELD OF THE INVENTION
The invention is in the field of pharamacogenomics, particularly the
utilization of
genetic alleles as diagnostic markers.
BACKGROUND OF THE INVENTION
Rheumatoid arthritis is a chronic polyarticular inflammatory disease with a
variable
course and outcome (Combe et al., 1995, Br. J. Rheumatol. 34: 529). Clinical
expression
ranges from a mild, non-deforming arthropathy with little long-term disability
to severe,
incapacitating, erosive articular destruction which may be refractory to
conventional disease
modifying agents (Schiff, 1997, Am. J. Med. 102 (suppl 1A): 11 S-15S).
Prediction of disease
progression is imprecise, and is often based on a combination of demographic,
clinical and
laboratory factors, including low socioeconomic status and educational levels,
severe initial
disease activity, systemic manifestations and extra-articular features, the
early appearance of
joint erosions, an elevated erythrocyte sedimentation rate, C-reactive protein
and the presence
of rheumatoid factor. There is epidemiological evidence that there is a
genetic relationship
between loci in the major histocompatibility complex (MHC) class II region and
disease
susceptibility and severity (Reveille, 1998, Curr. Op. Rheumatol. 10: 187;
Nepom et al.,
1996, J.Rheumatol. 23 (suppl 44): S; Weyand and Goronzy, 1995, Curr. Op.
Rheumatol. 7:
206).
Interferon gamma is a homodimeric 34 Kd peptide. IFN gamma may be secreted by
T-lymphocytes under certain conditions of activation and by NK cells (Boehm,
U., et al.
1997). IFN gamma binds to cell surface receptors on cellular targets including
mononuclear
phagocytes, endothelial cells and NK cells, and is thought to play an
important role in the
coordinated regulation and expression of the immune response through the
stimulation or
repression of genes (Farrar, M.A. et al., 1993; Revel, M. et al., 1986). IFN
gamma appears to
be produced by T-cells infiltrating the inflamed synovium and may be secreted
into the joint
space, but the role of this peptide in the progression of the articular injury
in arthritis remains
controversial (Feldemann, M. et al. 1996).
-1-
CA 02384029 2002-03-04
WO 01/18240 PCT/CA00/01043
IFN gamma is encoded by a gene which in humans is mapped to 12q24 on
chromosome 12. The known sequence of the gene consists of 4 exons with 3
intervening
regions. A variable length dinucleotide repeat polymorphism has been described
in humans
and lower primates within the first intron of this gene, between positions
1349 and 1373. The
number of alleles reported at this microsatellite region appears to vary
according to the
detection methodology employed to characterize it (Ruiz-Linares A, 1993;
Awata, T. et al.
1994; Pravica, V. et al., 1998).
The human leukocyte antigens (HLA) are a family of polymorphic cell-surface
proteins that are involved in intercellular interactions in the immune system.
The HLA genes
are part of the major histocompatibiiity complex (MHC). The HLA proteins are
designated
HLA-A, -B, -DR, -DQ and -DP. The HLA-A, -B and -C proteins are described as
Class I
HLA proteins, while the HLA-DR, -DQ and -DP proteins are described as Class II
proteins,
and are composed of two polypeptide chains, an alpha chain and a highly
polymorphic beta
chain.
The Class II HLA proteins are expressed on the cell surface of macrophages, B-
cells
and activated T-cells, where they are thought to be involved in binding and
presenting
antigens to helper T-lymphocytes (see Giles and Capra, 1985, Adv. Immunol.
37:1). The
Class II DP, DQ and DR genes are located in separate regions of the MHC
(Trowsdale et al.,
1985, Imunol. Rev. 85:5). In the DR region, the DRA locus encodes the alpha
chain and five
different DRB loci encode the beta chain: DRBl, DRB2 (now known as DRB6),
DRB3,
DRB4, and DRBS.
The Class II protein genes have been segregated into a number of known
haplotypes
(the specific allele combination at multiple loci on the same chromosome, see
for example
Dupont, 1989, Hum: Immunol. 26:3), such as the DR4 haplotype which may be
associated
with rheumatoid arthritis. Some efforts have been made to determine which
locus within the
DR4 haplotype is most tightly associated with predisposition to rheumatoid
arthritis (Zanelli
et al., 1998, Immunogenetics 48:394-401). A complex interrelationship of loci
appears to be
involved in various aspect of rheumatoid arthritis, including a 'shared
epitope' Q(K/R)RAA at
amino acids 70-74 of the DRBI encoded peptide. This shared epitope has been
associated
-2-
CA 02384029 2002-03-04
WO 01/18240 PCT/CA00/01043
with severe rheumatoid arthritis (Gregerson et al., 1987, Arthritis Rheum
30:1205-1213;
Williams et al., 1993, DNA and Cell Biology 12(5):425-434), although its
frequency in the
normal population has been suggested to preclude its use as a positive
indicator of disease
prognosis (Khani-Hanjani et al., 1998, Abstract 293, Poster Session B,
American College of
Rheumatorolgy 62nd National Meeting). The role of specific residues within the
shared
epitope of DRBI has been investigated (Zanelli et al., 1997, J. Immunol
158:3545-3551;
Wucherpfennig et al., 1995, Proc. Natl. Acad. Sci. 92:11935-11939).
SUMMARY OF THE INVENTION
In one aspect, the invention provides a method of diagnosis. The diagnostic
method
may include steps of identifying a patient at risk of an arthritis, the
patient having an
interferon gamma gene. The patient may be tested to characterize a
polymorphism in a first
intron of the interferon gamma gene. The polymorphism may comprise a variable
length
dinucleotide repeat region within the first intron, and the dinucleotide
repeat region may be
1 S located at least partly between nucleotides 1349 and 1373 in the
interferon gamma gene. The
method may be carried out so as to be capable of identifying alleles such as
the 126 by allele
and the 122 by allele, as further described herein. The polymorphisms may be
distinguished
based on a difference in the number of CA repeats in a portion of the first
intron of the
interferon gamma gene. To characterize the polymorphism, a region of the first
intron may be
amplified, such as a region comprising a variable length dinucleotide repeat.
The use of an allele of an interferon, gamma gene as described herein may
provide
prognostic information with respect to the likelihood of particular clinical
outcomes for the
patient, and as a result may be utilized to modify treatment regimens. In
particular, the
presence of alleles associated with relatively severe disease, such as the 126
by allele, may be
taken as an indication that aggressive therapy should be pursued relatively
early in the
progression of the disease.
In one aspect, the invention involves the identification of high and low risk
interferon
gamma alleles. In another aspect, the invention involves a further refinement
of patient
differentiation involving the use of an HLA locus in conjunction with the IFN
locus.
-3-
CA 02384029 2002-03-04
WO 01/18240 PCT/CA00/01043
BRIEF DESCRIPTION OF THE DRAWINGS
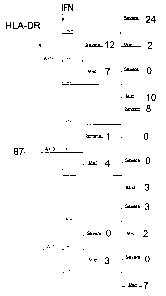
Figure 1 is a risk analysis tree showing the association between the severity
of RA
(severe or mild disease) and the IFN gamma (shown by the arrow labelled "IFN")
and HLA-
DRBI (shown by the arrow labelled "HLA") genotype of the patient.
Figure 2 shows the HLA-DRB 1 amino acid sequences 70-74 for both alleles for
patients with severe RA.
Figure 3 shows the HLA-DRB1 amino acid sequences 70-74 for both alleles for
patients with mild RA.
DETAILED DESCRIPTION OF THE INVENTION
In one aspect, the invention discloses a correlation between two alleles,
designated
126 by and 122 bp, and the occurrence of particular disease states in
arthritis. There is a
positive correlation between the occurrence of the 126 by allele and severe
rheumatoid
arthritis. There is a negative correlation between the occurrence of the 122
by allele and
severe rheumatoid arthritis. For each allele, there is a corresponding and
reverse correlation
with relatively mild rheumatoid arthritis. One aspect of the present invention
therefore
provides pharmacogenomic methods to assist in diagnosis of arthritis disease,
including
prediction of disease severity and selection of therapy regimens in particular
patients.
In another aspect, the invention discloses embodiments of partial DNA
sequences
corresponding to the 122 bp, 124 by and 126 by alleles. Alleles which share a
particular PCR
fragment length, such as the 122 by alleles, need not be identical in all
other respects. In
effect, there may be a'family' of alleles characterized by a particular PCR
amplification
fragment size. Sequencing of individual embodiments of the alleles of interest
produced the
following sequence information (in which "N" indicates that it was not
possible to
unambiguously identify the base at the relevant position):
A 122 by fragment partial sequence:
AACCACAAATNCAATNCTCACACACACACACACACACACACACCC
NNANATTTTGGAAACNANCTTTAAANCCNCNNAAAAAAANNCCCN
ANAGNANGGT
-4-
CA 02384029 2002-03-04
WO 01/18240 PCT/CA00/01043
A 124 by fragment partial sequence:
AAAACCACAAAATTCAAATNCNCACACACACACACACACACACAC
ACNCCCANATTTTTGNNAANANNCTTTTAAACCCCTNAAAAAAAAN
CCCCANANGNGAGNGGGGAT
A 126 by fragment partial sequence:
AAANCCACAAATTCAAATNCACACACACACACACACACACACACA
CACCCACANATTTTTGGAAACNANCTTTAAANCCCCNNAAAAAAA
ACCCCCAANAGGGGANGGGGATN
The various alleles have been found to have different numbers of CA repeats
according to their fragment size, as follows:
120 by allele: 11 CA repeats
122 by allele: 12 CA repeats
124 by allele: 13 CA repeats
126 by allele: 14 CA repeats
128 by allele: 15 CA repeats
130 by allele: 16 CA repeats
Hutchinson et al., 1999, Transplant Proc., 31 (1-2): 734, indicate that the
allele
containing the 122 by fragment, is associated with high levels of IFN-gamma
production,
while other alleles, including the 126 by allele, are associated with low
levels of IFN-gamma
production. Accordingly, in one aspect, the invention provides for a
genotyping assay to
identify IFN-gamma alleles that are associated with low levels of IFN-gamma
production, to
provide an indication that the patient having the allele is likely to suffer
from severe
rheumatoid arthritis. Conversely, the invention provides assays for
identifying IFN-gamma
alleles associated with high levels of IFN-gamma production to provide an
indication that the
patient having such an allele is less likely to suffer from severe rheumatoid
arthritis.
The invention may be utilized in patients identified as at risk of an
arthritis, such as
patients diagnosed by a medical practitioner as suffering from RA. Patients
may for example
be identified as at risk of an arthritis on the basis epidemiological criteria
such as sex, age,
socioeconomic factors or family history, on the basis of which an assessment
may be made
that the patient is more likely than other persons to suffer from an
arthritis. Physicians
typically diagnose RA based on the overall pattern of symptoms, medical
history, physical
-5-
CA 02384029 2002-03-04
WO 01/18240 PCT/CA00/01043
exam, X-rays and tests for rheumatoid factor or established genetic markers
such as HLA-
DR4. Typical symptoms of patients at risk of RA may include: general fatigue,
soreness,
stiffness and aching, with pain and swelling Typically occurring in the same
joints on both
sides of the body and starting in the hands or feet, particularly the wrist
and many of the hand
joints. Other diagnostic symptoms may include rheumatoid nodules. The
diagnosis of the
patient as being at risk of an arthritis may also comprise identifying
symptoms such as the
following: joint erosions, elevated erythrocyte sedimentation rate, C-reactive
protein,
polyarticular disease, joint deformities, radiological evidence of subchondral
erosions, extra-
articular arthritis or the presence of rheumatoid factor. Patients may be
identified as at risk by
virtue of an inadequate response to one or more arthritis therapies, such as
an inadequate
response to DMARDs (disease-modifying antirheumatic drugs) or other
medicaments for
treating the arthritis.
In one aspect, the invention provides a method of treating a patient having an
interferon gamma gene, comprising testing the patient to characterize a
polymorphism in the
interferon gamma gene; and, treating the patient for an arthritis if the
polymorphism indicates
that the patient is at risk of an arthritis. The polymorphism in the
interferon gamma gene may
be in the length of the dinucleotide repeat region within the first intron.
The presence of the
high risk allele of the present invention may for example be taken as
indicative of
susceptibility to arthritis or to a more severe form of arthritis.
In accordance with various aspects of the invention, a patient may be treated
for an
arthritis. For example, treating a patient for RA may comprise administering
to the patient an
effective amount of a medicament. An effective amount of a medicament may be a
therapeutically effective amount or a prophylactically effective amount. A
"therapeutically
effective amount" refers to an amount effective, at dosages and for periods of
time necessary,
to achieve the desired therapeutic result, such as reducing signs and symptoms
of RA and
delaying structural damage of RA . A therapeutically effective amount of a
therapeutic may
vary according to factors such as the disease state, age, sex, and weight of
the individual, and
the ability of the therapeutic to elicit a desired response in the individual.
Dosage regimens
may be adjusted to provide the optimum therapeutic response. A therapeutically
effective
amount is also typically one in which any toxic or detrimental effects of the
therapeutic are
-6-
CA 02384029 2002-03-04
WO 01/18240 PCT/CA00/01043
outweighed by the therapeutically beneficial effects. A "prophylactically
effective amount"
refers to an amount effective, at dosages and for periods of time necessary,
to achieve the
desired prophylactic result, such as reducing signs and symptoms of RA and
delaying
structural damage of RA . A prophylactic dose may be used in subjects prior to
or at an
earlier stage of disease, and a prophylactically effective amount may be more
or less than a
therapeutically effective amount in some cases.
Medicaments for treating an arthritis may for example include drugs approved
by the
FDA for treating patients with moderately to severely active rheumatoid
arthritis, such as
drugs that reduce signs and symptoms of RA and delay structural damage of RA
in patients.
Such drugs may for example include: nonsteroidal anti-inflammatory drugs
(NSAIDs), COX-
2 inhibitors such as celecoxib (Celebrex) or rofecoxib (Vioxx ), salicylates,
glucocorticoids,
TNF inhibitors such as infliximab (Remicade ), DMARDs such as leflunomide
(Arava),
cyclosporine, mycophenolate mofetil (Cellcept), anti-TNF antibodies (as
described in US
Patnent No. 5,698,195), methotrexate, or soluble versions of the TNF receptor
(such as
ENBREL(TM), available from Immunex Corp. of Seattle, Washington, USA) or the
IL-1
receptor.
In one aspect, the invention relates to the use in gene therapy of an IFN
gamma
nucleic acid. The IFN gamma nucleic acid may be delivered by a therapeutically
acceptable
gene therapy vector to modify a patient's IFN gamma allele profile. Gene
therapy may for
example be used to replace a high risk IFN gamma allele with a low risk IFN
gamma allele.
Gene therapy vectors may for example be an adeno-associated vector (AAV). Such
a
vector may comprise for example: a 5' inverted terminal repeat (ITR); a
promoter, such as a
CMV enhancer-promoter with a muscle specific enhancer; an intron; a 3'-
untranslated region
(3'-UTR); a polyadenylation signal, such as an SV40 polyadenylation signal;
and a 3'-ITR.
For gene therapy vectors, the dosage to be administered may depend to a large
extent on the
condition and size of the subject being treated as well as the therapeutic
formulation,
frequency of treatment and the route of administration. Regimens for
continuing therapy,
including dose, formulation, and frequency may be guided by the initial
response and clinical
judgment. The parenteral route of injection into the interstitial space of
tissue may be
preferred, although other parenteral routes, such as inhalation of an aerosol
formulation, may
CA 02384029 2002-03-04
WO 01/18240 PCT/CA00/01043
be required in specific administration. In some protocols, a formulation
comprising the gene
and gene delivery system in an aqueous carrier is injected into tissue in
appropriate amounts.
The tissue target may be specific, for example the muscle or liver tissue, or
it may be a
combination of several tissues, for example the muscle and liver tissues.
Exemplary tissue
targets may include liver, skeletal muscle, heart muscle, adipose deposits,
kidney, lung,
vascular endothelium, epithelial and/or hematopoietic cells. A nucleic acid of
the invention
may be delivered to cells in vivo using methods such as direct injection of
DNA, receptor-
mediated DNA uptake, viral-mediated transfection or non-viral transfection and
lipid based
transfection, all of which may involve the use of gene therapy vectors. Direct
injection has
been used to introduce naked DNA into cells in vivo (see e.g., Acsadi et al.
(1991) Nature
332:815-818; Wolff et al. (1990) Science 247:1465-1468). A delivery apparatus
(e.g., a "gene
gun") for injecting DNA into cells in vivo may be used. Such an apparatus may
be
commercially available (e.g., from BioRad). Naked DNA may also be introduced
into cells
by complexing the DNA to a canon, such as polylysine, which is coupled to a
ligand for a
cell-surface receptor (see for example Wu, G. and Wu, C. H. (1988) J. Biol.
Chem.
263:14621; Wilson e1 al. (1992) J. Biol. Chem. 267:963-967; and U.S. Pat. No.
5,166,320).
Binding of the DNA-ligand complex to the receptor may facilitate uptake of the
DNA by
receptor-mediated endocytosis. A DNA-ligand complex linked to adenovirus
capsids which
disrupt endosomes, thereby releasing material into the cytoplasm, may be used
to avoid
degradation of the complex by intracellular lysosomes (see for example Curiel
e1 al. (1991)
Proc. Natl. Acad. Sci. USA 88:8850; Cristiano et al. (1993) Proc. Natl. Acad.
Sci. USA
90:2122-2126). Defective retroviruses are well characterized for use as gene
therapy vectors
(for a review see Miller, A. D. (1990) Blood 76:271). Protocols for producing
recombinant
retroviruses and for infecting cells in vitro or in vivo with such viruses can
be found in
Current Protocols in Molecular Biology, Ausubel, F. M. et al. (eds.) Greene
Publishing
Associates, (1989), Sections 9.10-9.14 and other standard laboratory manuals.
Examples of
suitable retroviruses include pLJ, pZIP, pWE and pEM which are well known to
those skilled
in the art. Examples of suitable packaging virus lines include .pyri.Crip,
.pWi.Cre, .pyri.2 and
.pyi.Am. Retroviruses have been used to introduce a variety of genes into many
different cell
types, including epithelial cells, endothelial cells, lymphocytes, myoblasts,
hepatocytes, bone
marrow cells, in vitro and/or in vivo (see for example Eglitis, et al. (1985)
Science 230:1395-
1398; Danos and Mulligan (1988) Proc. Natl. Acad. Sci. USA 85:6460-6464;
Wilson et al.
_g_
CA 02384029 2002-03-04
WO 01/18240 PCT/CA00/01043
(1988) Proc. Natl. Acad. Sci. USA 85:3014-3018; Armentano et al. (1990) Proc.
Natl. Acad.
Sci. USA 87:6141-6145; Huber et al. (1991) Proc. Natl. Acad. Sci. USA 88:8039-
8043; Ferry
et al. (1991) Proc. Natl. Acad. Sci. USA 88:8377-8381; Chowdhury et al. (1991)
Science
254:1802-1805; van Beusechem et al. (1992) Proc. Natl. Acad. Sci. USA 89:7640-
7644; Kay
et al. (1992) Human Gene Therapy 3:641-647; Dai et al. (1992) Proc. Natl.
Acad. Sci. USA
89:10892-10895; Hwu et al. (1993) J. Immunol. 150:4104-4115; U.S. Pat. No.
4,868,116;
U.S. Pat. No. 4,980,286; PCT Application WO 89/07136; PCT Application WO
89/02468;
PCT Application WO 89/05345; and PCT Application WO 92/07573).
For use as a gene therapy vector, the genome of an adenovirus may be
manipulated so
that it includes an IFN gamma nucleic acid, but is inactivated in terms of its
ability to
replicate in a normal lytic viral life cycle. See for example Berkner et al.
(1988)
BioTechniques 6:616; Rosenfeld et al. (1991) Science 252:431-434; and
Rosenfeld et al.
(1992) Cell 68:143-155. Suitable adenoviral vectors derived from the
adenovirus strain Ad
type 5 d1324 or other strains of adenovirus (e.g., Ad2, Ad3, Ad7 etc.) are
well known to those
skilled in the art. Recombinant adenoviruses are advantageous in that they do
not require
dividing cells to be effective gene delivery vehicles and can be used to
infect a wide variety
of cell types, including airway epithelium (Rosenfeld et al. (1992) cited
supra), endothelial
cells (Lemarchand et al. (1992) Proc. Natl. Acad. Sci. USA 89:6482-6486),
hepatocytes
(Herz and Gerard (1993) Proc. Natl. Acad. Sci. USA 90:2812-2816) and muscle
cells
(Quantin e1 al. (1992) Proc. Natl. Acad. Sci. USA 89:2581-2584).
Adeno-associated virus (AAV) may be used as a gene therapy vector for delivery
of
DNA for gene therapy purposes. AAV is a naturally occurring defective virus
that requires
another virus, such as an adenovirus or a herpes virus, as a helper virus for
efficient
replication and a productive life cycle (Muzyczka et al. Curr. Topics in
Micro. and Immunol.
(1992) 158:97-129). AAV may be used to integrate DNA into non-dividing cells
(see for
example Flotte et al. (1992) Am. J. Respir. Cell. Mol. Biol. 7:349-356;
Samulski et al. (1989)
J. Virol. 63:3822-3828; and McLaughlin et al. (1989) J. Virol. 62:1963-1973).
An AAV
vector such as that described in Tratschin et al. (1985) Mol. Cell. Biol.
5:3251-3260 may be
used to introduce DNA into cells (see for example Hermonat et al. (1984) Proc.
Natl. Acad.
Sci. USA 81:6466-6470; Tratschin et al. (1985) Mol. Cell. Biol. 4:2072-2081;
Wondisford et
-9-
CA 02384029 2002-03-04
WO 01/18240 PCT/CA00/01043
al. (1988) Mol. Endocrinol. 2:32-39; Tratschin et al. (1984) J. Virol. 51:611-
619; and Flotte
et al. (1993) J. Biol. Chem. 268:3781-3790). Lentiviral gene therapy vectors
may also be
adapted for use in the invention.
S General methods for gene therapy are known in the art. See for example, U.S.
Pat.
No. 5,399,346 by Anderson et al. (incorporated herein by reference). A
biocompatible
capsule for delivering genetic material is described in PCT Publication WO
95/05452 by
Baetge et al. Methods of gene transfer into hematopoietic cells have also
previously been
reported (see Clapp, D. W., et al., Blood 78: 1132-1139 (1991); Anderson,
Science 288:627-9
(2000); and , Cavazzana-Calvo et al., Science 288:669-72 (2000), all of which
are
incorporated herein by reference).
EXAMPLE 1
48 adult Caucasian patients with severe rheumatoid arthritis and 39 patients
with mild
rheumatoid arthritis were selected sequentially from a hospital patient
population. 50 patients
that did not present with symptoms of arthritic disease were selected as a
control comparator
group. Patients with severe rheumatoid arthritis were aged 58+ 12 years and
were
predominantly female and had a mean disease duration of 19+ 12 years. All such
patients
had clinically severe polyarticular disease with joint deformities and
radiological evidence of
subchondral erosions. 75% of such patients had extra-articular manifestations
of disease
other than the sicca syndrome. 87% of patients with severe rheumatoid
arthritis were
rheumatoid factor positive. All such patients had not responded favourably to
therapy with
conventional disease-modifying anti-rheumatic drugs (DMR.DS), and had been
maintained on
cyclosporine treatment for a mean period of 26 months.
Patients with mild disease were aged,61+ 13 years, predominantly female, and
had a
mean disease duration of 12+ 7 years. All such patients had clinically mild
disease, which
had been controlled for a mean period of 90 months by antimalarials alone
without prior or
current use of DMRDS. Only 26% of such patients had joint deformities, and 36%
had extra-
articular disease manifestations other than sicca syndrome. 34% of such
patients were
rheumatoid factor positive.
-10-
CA 02384029 2002-03-04
WO 01/18240 PCT/CA00/01043
Peripheral blood was obtained from patients and control subjects, and genomic
DNA
extracted by proteinase K digestion and by salting out. Molecular typing at
the IFN gamma
(12q24.12) microsatellite polymorphism was performed by PCR followed by use of
a DNA
sequencer and gene analysis software. Locus or group-specific amplification
was performed
using 5' and 3' oligonucleotide amplification primers as follows:
5'6 FAMAG ACA TTC ACA ATT GAT TTT ATT CTT AC 3'
5' CCT TCC TGT AGG GTA TTA TTA TAC G3'
The primers were designed with a high annealing temperature to enhance
specificity.
Both primers were obtained from Perkin-Elmer-ABI-PRISM and the forward primer
was
fluorescently labelled at the 5' end.
Genomic DNA (100 ng) was amplified using 50 pmoles each of the oligonucleotide
primers, 100 uM, each of dNTP, 1.5 mM MgCl2 and 0.8u of TAQ polymerase in a
Perkin-
Elmer PCR cycler. Cycling conditions included a 5-minute hot start at
95° C. followed by 32
cycles of 95° C for 45 seconds (denaturation) and 62° C for one
minute (annealing and
extension) with a final extension of 5 minutes at 62° C in the last
cycle. The amplified
product was run on a 1.5% agarose gel for detection of positive amplification
and then on a
long-range gel on a 377 DNA sequencer ABI-PRISM) data were collected using 377
collection software and size analysis was performed using Genescan 2Ø2
software and
Genescan 2Ø2 software and Genescan-500 ROX as a size standard (ABI-PRISM).
A total of six alleles were documented in the patients and controls, ranging
in length
from 120 by to 130 bp, as shown in Table 1.
-11-
CA 02384029 2002-03-04
WO 01/18240 PCT/CA00/01043
TABLE 1: Proportion of subjects expressing individual alleles in the first
intron of
the IFNy gene. Controls (n=50), patients with severe RA (n=48), patients with
mild
RA (n=39).
IFNy AllelesControlsSevere Mild
RA RA
(Size) % % % ORb
ORa ORa pb
Pa p
A2 (130 0 6 7.77 NS 0 - - 6.08 NS
bp)
A3 (128 16 15 0.90 NS 15 0.95 NS 0.94 NS
bp)
A4 (126 12 73 19.74p<0.000121 1.89 NS 10.43p<0.0001
bp)
A5 (124 68 77 1.58 NS 67 0.94 NS 1.68 NS
bp)
A6 (122 68 6 0.019p<0.000164 0.50 NS 0.037p<0.0001
bp)
A7 ( 120 0 0 - - 3 3.93 NS 0.27 NS
bp)
severe or mild RA compared with controls
b severe compared with mild RA
NS: not statistically significant
OR: odds ratio
The frequency of the polymorphisms in normal subjects ranged from 0% for the
130
by and 120 by alleles, to 68% for the 122 by allele. The genotype frequencies
did not deviate
from the expected value by Hardy-Weinberg equilibrium. The alleles identified
herein
appear to correspond closely in length to those initially reported by Ruiz-
Linares, which
ranged from 122-134 by (Ruiz-Linares, 1994 Hum. Mol. Genet. 2(9):1508). Such
alleles
may vary, for example, depending upon the ethnic origin of the subjects and
the methodology
of characterization (see for example Awata, T. et al. 1994 Diabetologia
37:1159). In some
populations, 6-8 alleles encompassing at least 11-15 CA repeats may exist at
this site,
including the intermediate polymorphism of 128 bp.
As shown in this example, patients with severe rheumatoid arthritis differ
significantly in the frequency of 2 alleles. The 126 by allele was present in
73% of patients
with severe rheumatoid arthritis compared with 21% of patients with mild
rheumatoid
arthritis (OR: 10.43, p < 0.0001) and 12% of normal subjects (OR: 19.74, p <
0.0001). In
contrast, the 122 by allele was detected in only 6% of patients with severe
rheumatoid
arthritis compared with 64% of patients with mild disease (OR: 0.037, p <
0.0001) and 68%
of normal subjects (OR: 0.019, p < 0.0001). There was no significant
difference in the
-12-
CA 02384029 2002-03-04
WO 01/18240 PCT/CA00/01043
frequencies of the other microsatellite polymorphisms between the three groups
of
individuals.
Table 2 shows a grouping of the subjects into one of four categories,
depending upon
their expression of the 126 by allele and the 122 by allele. Almost three
quarters (73%) of
patients with severe rheumatoid arthritis expressed the 126 by allele without
the 122 by
allele, compared with 10% of patients with mild rheumatoid arthritis (OR:
23.56, p < 0.0001)
and 4% of normal subjects (OR: 60.62, p < 0.0001). In contrast, only 6% of
patients with
severe rheumatoid arthritis expressed the 122 by allele without the 126 by
allele compared
with 54% of patients with mild rheumatoid arthritis (OR: 0.057, p < 0.0001)
and 70% of
normal subjects (OR: 0.029, p < 0.0001). The 126 by and 122 by alleles were
not conjointly
expressed by any patients with severe disease compared with conjoint
expression in 10% of
patients with mild disease (OR: 0.081, p = NS) and conjoint expression in 8%
of normal
subjects (OR: 0.1 l, p = NS).
Table 2: Proportion of subjects expressing the 126 by allele, 122 by allele,
both or
neither in the first intron of the IFNy gene. Controls (n=50), patients with
severe RA
(n=48) patients with mild RA (n=39).
ControlsSevere Mild
RA RA
Categories% % % ORb
ORa ORa pb
pa pa
126 by 4 73 60.62<0.000110 2.74NS 23.56<0.0001
allele
122 by 70 6 0.029<0.000154 0.50NS 0.057<0.0001
allele
Both 8 0 0.11 NS 10 1.31NS 0.081NS
Neither 18 21 1.20 NS 26 1.57NS 0.76 NS
compared with controls
b severe compared with mild
NS: not statistically significant
OR: odds ratio
-13-
CA 02384029 2002-03-04
WO 01/18240 PCT/CA00/01043
Table 3: Results of logistic regression for the effects of HLA-DR, IFN-g, and
clinical
measures on the odds of severe disease. Odds ratios and chi-square statistics
are
marginal (i.e. have been adjusted for all other factors). Odds ratios here
reflect the
distribution of patients observed rather than underlying prevalence of mild
and severe
RA. Controls (n=50), patients with severe RA (n=48) patients with mild RA
(n=39).
Severe vs. Severe vs. Severe vs.
Control Mild Mild
actor .f. x~ x2
HLA-DR 3 11.23* 13.04** 10.66*
IFN-y 3 65.88** 43.91*** 28.09***
2 _- _- 9.33**
Age 1 -- -- 0.46
Duration 1 -- -- 6.57*
Gender 1 -- -- 3.84**
Factor Effect O.R. 0.R. 0.R.
HLA-DR H vs. 23.95* 14.55 48.27
L
B vs. 7.23 0.43 0.63
L
N vs. 2.10 2.15 2.52
L
IFN-Y H vs. 327.09*** 107.57*** 278.02**
L
B vs. 0.03 0.00 0.00
L
N vs. 19.26*** 5.13* 9.95
L
RF NA vs. 2.10
neg
pos vs. 25.07*
neg
Age 10 years 0.77
Duration 10 years 4.71
Gender m vs. 11.16
f
*p<0.05; **p<0.01 level; ***p<0.001 level.
HLA-DR: H = QKRRA/QRRAA, L = DERAA, B = Both, N = Neither
IFN-g: H = 126 by allele; L = 122 by allele; B = Both; N = Neither
These striking results were confirmed in a subsequent and independent group of
12
patients with severe RA who were selected according to the same clinical and
laboratory
criteria. Seventy-five percent of these patients expressed the 126 by allele
and 8% the 122 by
-14-
CA 02384029 2002-03-04
WO 01/18240 PCT/CA00/01043
allele; when all subjects with severe RA were combined (n=60) the patient
frequencies were
unchanged from those reported in tables l and 2.
Logistic regression was used to examine the influences of IFN-y polymorphism,
HLA
DR-B 1 genotype (Wayland and Goronzy, 1997, J. Mol. Med. 75:772) and other
prognostic
factors. The results are shown in table 3. Inheritance of the INF-y 126 by
allele is strongly
associated with the presence of severe RA even after accounting for HLA-DRB 1
polymorphism, while possession of the IFNy 122 by polymorphism is highly
negatively
associated with the presence of severe disease. The association of these IFNy
alleles with
severe RA is considerably greater than that noted for the most tightly
associated MHC class
II alleles or other clinical predictors including gender, age at onset,
duration of disease, or
rheumatoid factor positivity.
In accordance with one aspect of the present invention, the diagnostic test
for the
presence of IFN gamma alleles may be carried out on asymptomatic individuals
to assess the
individual's susceptibility to rheumatoid arthritis. In individuals presenting
with arthritic
symptoms, the test may be utilized to assess the likelihood of progression to
the severe form
of the disease. In accordance with these aspects of the invention, the
presence of the 126 by
allele may be taken as an indication of increased susceptibility to rheumatoid
arthritis,
including increased susceptibility to progression of the arthritis to the
severe form of the
disease, as set out in Table 3.
The diagnostic test may in some embodiments be utilized to determine whether
the
IFN gamma alleles are homozygous or heterozygous for example, in the exemplary
embodiment, 10% (6/60) of patients with severe rheumatoid arthritis were
homozygous for
the 126 by allele, compared with 3% (1/39) of those with mild disease and 0%
(0/50) of
normal controls. In contrast, none of the 60 patients with severe rheumatoid
arthritis were
homozygous for the 122 by allele, compared with 8% (3/39) of those with mild
disease and
14% (7/50) of normal controls.
-15-
CA 02384029 2002-03-04
WO 01/18240 PCT/CA00/01043
EXAMPLE 2
As shown in Figures 1, 2 and 3, 87 patients were segregated into three groups
on the basis of their HLA-DRBI alleles, on the basis of the charge on the
amino acid at
position 71, being positive ("+", i.e. lysine (K), arginine (R) or histidine
(H)), negative ("-",
i.e. glutamate (E) or aspartate (D)) or neutral ("0", the remaining amino
acids). Figure 2
shows the HLA-DRB 1 amino acid sequences 70-74 for both alleles for patients
with severe
RA. Figure 3 shows the HLA-DRB 1 amino acid sequences 70-74 for both alleles
for patients
with mild RA. Individuals are segregated in the chart of Figure 1 into three
groups based on
their HLA-DRB 1 sequence:
i) A+/+ individuals are homozygous for a positive amino acid at position 71;
ii) A+/0 individuals are heterozygous, having a positive amino acid allele and
a
neutral amino acid allele;
iii) A-/* individuals have at least one copy of an allele with a negative
amino acid
at position 71.
With respect to the IFN-gamma alleles in the chart of Figure 1 "+" indicates a
high
risk allele, while "-" indicates a low risk allele. For example, individuals
who are
homozygous for the high risk allele are grouped on the branch of the tree
denoted by "+/+",
The risk analysis chart indicates, for example, that patients homozygous a
positive
amino acid at position 71 of HLA-DRB l and homozygous for the high risk IFN
allele suffer
from severe RA in 24 out of 26 cases, while patients having at least one HLA-
DRB1 allele
with a negative amino acid (shown as "-/*) who are also homozygous for the low
risk IFN
allele ("-/-) present with mild RA in 7 out of 7 cases.
Although various embodiments of the invention are disclosed herein, many
adaptations and modifications may be made within the scope of the invention in
accordance
with the common general knowledge of those skilled in this art. Such
modifications include
the substitution of known equivalents for any aspect of the invention in order
to achieve the
same result in substantially the same way. Numeric ranges are inclusive of the
numbers
defining the range. In the claims, the word "comprising" is used as an open-
ended term,
substantially equivalent to the phrase "including, but not limited to".
-16-