Note: Descriptions are shown in the official language in which they were submitted.
CA 02384474 2002-05-O1
CBIt 3.8-Od7
CaMFO~x'Tr4NS ANb lulETtiaDS POR PROTECnON AO~'ST
Sl~Xt7ALLY TRANSM~D DxSEA.SE9
BACKf3Rt)UND OF ?I~ 1N'~ENTION
I o 0 0 i1 'I~e caaageonan idly of polymers ors palyaacaharidnc obtained $om
the red
algae commonly lmowa as seaweed. Caaagaenana have been uaad extensively in the
food,
pbarmaccutical and cosmetics industries as luhrica~sts, ernttlsifiexs, and
crabilizirtg and
dispersing agears. Extensive pharmacological and toxicological studies hare
been conduatad,
Cattageenan ltas bast found to be na~rt-tea by anal, deal, anti fnhalatioa
tauter of
administrations even at extremely high doses, The cartageenans wrra therefore
elaaei~ed as
''ge~ally recommended as safe" (GRAS by the F1~A. in I97Z'. Furtber extensive
anal
pnacolanetic studies conducted in pigs, rats, twice, gerbils, guinea pigs,
ferrets, bam:tezs,
dvga, aid monkeys ~" showed that the breakdown of chc carrageenans in the
gasC~iatestittal
tract taste minimal at best and that absorption was virtually non-existent
SUMMARY Ql? TldE SON
~ 0 0 0 21 Ot~ aspect of thr present iavtation is directed to s mia~fiu~es of
kappa and lambda
~eoa~.ln~" ~ ulat3o:as aontai~ning thtc mixtures and nsethoda of use. 'f he
iui.ctuxa
of tiaras apeaifie carrain the n~Iative amounts provides uz~acpectodly
superior
atttimicrobial acti~iry compared to other carregeenans, ?he caxxageenan
mixtures of ~
present invention xnay ba easily formulaxd for admiaixtration to a human or
snitaah
(preferably via the vaginal route) using sa aqueaua solution. In'a preferxtd
embodiment,the
mixture is present in tbt foo~lation in an amount of about 3°l°
~by ~veEght. The formulations
ors particularly useful is protection agair~at contraction of sexually
tra~nitted diaeases
I o 0 0 3 7 Anotlxar aspect of the present invention is directed to a raetitod
of processing,
ze$tua~ or stabilixiaE a sulfatod- polysacel~ide such as ca~ageerlari, and
more plef~bly
the caarag~nan ~ixtura of the sent ir~~ention (herminafcer "a IrJ7t cawageenan
miJ.-ture").
The ~sthod ails mixing the sulfated-polysaccharide in anhydrous yr powdery
form with at
least one dry buffer salt, followed by hydration of tl~e canrageonan e.g., by
the aciditivn of
orator or another aqueous solution. The meB~od overcomes several disadvantage
associated
with current techniques for processing catrugeeaszts from seaweed, and
facilitates finer
processing into ~oncaulations suibblc for phairaaceutical applications.
IaESCRIPTION OF THI; bfiA'WINGS
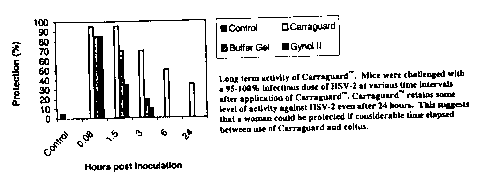
Iaooal Fig.t is a graph showing long term activity ofa ICI7~. carrngecnan
mixture, Mica
1
CA 02384474 2002-05-O1
were cb~alleaged. with a 95-100°Y° iut~actaons dose of SSV-2 at
various time intervals afEGr
application oftha KI74 canagoenan niixtttte. Tba Kl7v. carrageanate rxtixtutc
rr!ta;na some ravel
of activity against HSV 2 avers a#ter 2~4 hopes. The data suggest that a
woxne~a could be
protected even if oo>osideeablc time elagscd bohxreen u.sa of the IGlA.
rarrageenaa mixture and
CBitttB.
~ooo~l Pig 2 is a graph of Soutlurrn Blot byrbxidizstion of ItT pC~~uoducta
from RNA
extracted from the spleens of rhice. Lang 2 s=td 3 are positive ceratrols.
x,anGS 4 to 8 are from
mica that w~erc pretreated with a I~17~. carrag~an mixtctre S minutes befbxe
viral challenge,
Lanes 9 to 14~ are frog mica in4culatsd vaginally nvitir 1~1V.
DBTAtLBD DESCRXP1IDN OF I'FI8 fiTV~NT~DI~Ir
100101 ~azzageanaa is the structural compattsnt of;saweari and is extracted as
three
main types, ~aatnsty iota, kappa and lambda. xhe mixture corltaitLS about 83
to about 99%
lrratbda-carrageeaarr sad about 1 to about 15 °~ kappa-cazr&geanan,
preferably ft~om aboac 1
to about 10°.~a, and mare preferably aboett 3°!e kappa
Ca'trageexlan. In Othe7C pzefeired
embodia, the ~mi~cture ig substantially or entirely ~ree of dextrose, an
iagredieu:t
oornenonty found in carra~roenaos used ins the food industry. 'the nnixatre o~
these specific
carragaenans in the ~lativa amQUnts provide uacxlrectedly superior
axt~mi~ro6ial activity
compared to ocher catragcattans. Although carcageenan has ~t cQatinuutn of
rrrolocuiar
weights, tree mixtures of the pnesertt invemiort have an average molecular
weight of about Z x
105 daltaets with lines than about 1% of molecules having an average
moleon'tar weight of 2 x
lOs daltot~ (as dctarinined by gel pertxteation chromatography and light
scntterln~. Tints
tenique physical propariy imparts nonabsorbability to the final formulation
wltiah in 'rum
provides prolonged aati~microbial aetiv~ity.
IoOZiI 'I'he oarragccnan mixtures of the present ~nrantion may br easily
formulated
fez vaginal (and even rectal) administration using an aqueov~s solution.
Vaginal
ad~Oistratioa is the preferred mode of administration, In ~neral, tba amonat
of the
canragccnan znixmre wih vary from about 2 w about ~4 °~b by weight Tn a
prcftrrad
ranboditnent, the mixtxue is present in the formulation in set amount Qf about
3% by weight
The fomduiatioa may iusther carttain other active te,g., vaginally
administrable) actxv~e agtmts
andlor ic~trt ints, dependi~zg upon the iarend~ed nat. The ~bsrnulatians are
parDoulaxty
useful is prateadon ag~air~st sexually txansxnattcd diseases a.g., by
inhibiting infection by ~V,
HPY, $5"il-2 and rJe~ssaria goxorrltaeae.
2
CA 02384474 2002-05-O1
ZI The sanxatg~nan mixtuxo of the present irxvention remains stable if exposed
to
fiteezittg, ambient, ox boiling crmpaxatinvs, is compatible with the hua~m
traginal
enviroametxt, and is sat Systemically absorbed not degraded td any absoibablt
by~ptoductg
det~mental to htunans.
t00131 The kappa and laatbda c~z'ageenatts are commercially avaiailabla. They
caa be
taixad and fotnaulated inn aocardattce with standard techniques. A prefcrzed
matbod for
fomnulatfng the ~ cazrageenan mixtures of the present invention ivvolvas the
uss of dry
buffer salts. T'he mekhod oxtails mixing s Solid buffer salt and the IfJ7~
ca~geenan mixture,
in a weight ratio of from about 1:1 ~ about 10:1, The mixture of solid buffer
salt at~d the
c~c~cagoeaan mixture is then satubilixed in watax or in, an aqueous soluti~,
oo make the
sulfatadgolysaccharide formulation, the p13 of dte sulfated pvlysacchaxide
farmu~latioct is
rhea adjusted to be fibm about 6.0 tp about $.0, This is typically achieved by
t'he addition of
en acid, such as FiCI or a base, such as NaOH. ha general, the viscority of
the a~:Ifated
polysaccharide founulutlon is from about 20,000 to about 100,000 ~S,
prefbsably &oxn
about 30,000 to about 35,000 CPS.
~oo~~tl Solid buffer salts itrcludc solid alkaline metal salh of acetic acid,
citric acid
and phosphoric acid, nrherei~a the solid alicalirte foetal phoapltatc buffer
includes solid mixture
of tri-basic attd dl basic al1'ali Salts of phosphate, preferably in aphydxous
form, wherein
alkaline metal includes, but is not limited to potassitun and sadiarn. Any
physiologically
acceptable buffet can ho used
i0oi5! To enhance the stability of ttro sulfated polysacaharids formulation,
at least
one preservatirne can be added to the fatmutaticm. datives iztclude aTlc~r1
esters of para-
hydrouybenzoie acid, such as methyl parao~drbenzoatey pxopyl pataoxybeazante,
hydaratoin
derivatives. parabens, such as ranthyl parahe~a, propioniate salts, triclosah
tricarbapilide, tea
use oil, alcohals, fstmtsol, ~tesol acetate, hexachlorophene and quaternary
aanuonium
Salts, streh as benzolconjure, zinc and aluminum salts, sodium benzoate,
benxyl aloQkrol,
bcnzal3tonzum ch~orlds and chlombntanol.
=00161 The preservative can be pxtsent iti the propofions indicated i:a the
various
pharmacopoeias, and is particular is a weight ratio to sulfated polysaccharide
of from about
80:1 to about 1,0:1, p~a~rably from X0;1 to about 15:1.
too171 The mmthocl may also be u$ed to fomtulate orre~na other negatively
c>~argad
sulfated polysaccharides provided that thby exhibit anti~miembial propartios
and arc nan-
3
CA 02384474 2002-05-O1
absorbable to vtro. Without #ending to be.bout~d by any paxaoular theoxY of
operation, it is
'beticved that Sulfated-poiysacchasides such as oarrageenans including the
iCl7~ carragocnan
r~xturaa, azs dry povyders that arc cattcemely bY~'~~pio whatw eycposed tA the
atmosphere.
The uptalct of atmosphexie xnaisture rata flue dry ittgrediem causes eluting
of the aueterial.
The probtern compounded wl~au the maroexfal is then introduced into ~e aqueous
base
solution, such that complete incorpoxatiaa of the sulfated polyeaccltar~ds
into the aqueous
Solution cantrot be obtained. It is also believed that by mixing sulfated-
pol~rsaccha~cide and at
least one solid bni~r salt together, the solid buyer satt absorbs the
atmospheric znoiatwe shat
the sulfated-polysaccharide would have absotbcd when eatposed to the
atmoaphgre, and
prevents cluzctping of the sul~ted-polyascoharidt. It is rather believed that
the process
Sesves to increaso the solubility of sulfasad~olya*ccharida is water, arid
acbiawes
stabiliution of tbei p8.
La Q l81 The follawht$ axarrZples are inbetuied to fxu~Qter illustrabs cemin
~e~Onced
embodzmtentss of the invention and are riot fnttndtd en limit the itttrention
in any way.
Exempla I . P'RpDUCTxDN Op 50Q LIx'15RS ofthe KID. cantageenat~ mixture
~40i91 In preparing the 1Cla. caa~a~geenan mtxturt~, (1) the :formulation
ingrc~dimts
should be weighed individually in a clean, dry weighing vessel;: (2) the
ingr~dierst's "aeraaf.
weight, oat protyocvl wdght; should be reeoxded in the rttanufactuilng
production Ing
regardless of even slight va~iatian haturcen the rivo; (3J ony bulls
ingredient container
containing an artifacts) or r~oata should not ha used at~d the container
should be
rinsed, sealed, marked "~ON~'AMINATED" and rcmorrrd fra~u production arca~ (4)
in
process prodnct<ori batch ahpuld not be txanafezred ~ro:a one vessel to
another before
maauficharing is completed end formuletiou has passed quality control testing;
and (3J
pmd~ac~tion vessel should remain closod during raanufactuair~ to avoid loss of
avatar due to
evaporation, especially dut'sng any steps that require heating.
ID 0 ~ O1 Additionally, aarrageenan has proven to be stable in tba solid.
state and the
productiat~ state under a rraxiety of adverse canditians, including Erecting
ox autoclaving, for
24 months.
300~~,1 In the courSa of preparing the xC/7,. coon mixture from 100 mL
labaraaory size bat~ehas on to scalo-up of x5 and 30 liter Iabaxatary batches
to Snalizing the
maanfacsuting procedure of 50Q litx batches, it bccxxna difficult so al~tam
batxhto-batch
coneisten:ry of the desired formulation. ?hr present method surprisingly
overcutrc these
4
CA 02384474 2002-05-O1
dif~autties and produced fans of the ~ carragecttan mires having comds~nr
batch to-batch guality,
EQLT>pMENT:
i00Z~7 Producfiion Vessel - IKA, IrMA 9I500AI'lF'f'~" is a water jacket
prodaction
vessel that allows fot rapid heating and cooling of solution Boring
production.
1~C#~'1'S:
10023 theK/A.CatfBgCCnenmixture;
Phosphate buffer ss;lirie (PB5) [ca~inung: NaCI - 1x0 mmollL, KCl -- 2.'~
mmollL,
Phosp>:ata buffer (potstaaium phosphate monobasia aad sodium phosphate
dibasic) - 10
mmolJY, - (Sigcua Aldrich, Saint Louis MO);
p-Hydroxybenzoie methyl ester (Methyl parabcn)- (N'ipa Laboratos~es,
Poptypridd, L1K);
~yd~oric Acid (1d4~ - Merck, Dnaaatadt, (3crmany;
Purified rwator- Clean Chemical Sweddn, 8orlange, Sweden.
(1). Weigh ingredients iu the following qnatnitias:
ING 1. -..~-"'..~~.,.,QUAD.
'
Purred watcQ (3 484.Q
Fates .
~e ~ ~~~ ~- l i.0 ka
Pho atst i~uffer 4.8 ~
saline B9
o.s ~
drc~chloric acid .5 kg
109/r
(2). Carefully end thoroughly mix the dry ingiodieats~ the KI~ catraagemttaa
mLxture and
Phospkuate buf9fer ale (pBS) togcthtr~
(3). Inspect production vasstI to ensure Q~t mixing chamber is clean, dry and
flea of
artif~, and that the botitnn tralue is closed;
(4). Fill the pcoductlan vessel v~rith 100,01:. (Part ~ of purified water at~d
begin stircir~g:
turbizi 500 rpm, emd anchor 20 rpm. water is added in 3 parts, 'fhc first part
is enough
to dissol~ the mttlzyl pxteben. The secoad part aids irt reducing the
temptratuce,
sufficiently dilutes the HGl ao acidio hydrolyses of canrageenan does not
occur ~ahila
maintaining low enough Salutien levtl so when adding the caaageenan/F8S
mixture
the dalivezy siove caps be lowered into the mixing vessel such that it does
not come
into aot~taet with the base solution and is lower than the rr~sal access
hotels so ~e
excessive 'dusthtg' of tht mi~-ture is sot lost. The third part completes the
final
CA 02384474 2002-05-O1
Couaeutratxam,
(5). Continua stirring :ad add 0.51cg of mothyl parabea attd 0.5 kg of HCI.
Clare vessel
access hatch aa,d ixcat vvatx 75~ to 85°G. Once mmpemturo is reached
continue stirring
for a mi~rir~um of 10 minutos to dissolve rnettryl paraben:
(f). Discolttinue heating and add 250.0 kg (.part 1I] of purified water. Cool
solution to 25°
to 30~G. The addition of the ovaber will expedite tlta cooling process. 1"he
solution
needs to be cooled so that it is not produciung steam whets the next sddiboa
of
ingredients is made, Besides prevtotinyecraber loss whop the vessel is Qpen
for ~e
next addition, steam causes the caaage~arnll~HS mixture to clump end stick to
the
sievd that is uaed in the addition;
(7). Open crease hatch and begin the addition of cur:agsovan. I PBS muxiure
slowly
through a sieve with gentle shakier. lAddition takes approxir~rely 20
mirnytes.
Cainaide the addition of the mixture with increasing the atlxrtng speed to s
maati:mrm
speed of turbo 1200 rpm end anchor z0 rpm. T'he viscoairy of't~ solution,
increases
expona~ia~y with the addition of cazragsan3n. If the stirring speed is not
sigaiRcaat,
the camgeenan forms 'hydra-sealed' clumps, which never become dissolved And
iacorporatad into the solution, thereby tendering the bstdh ux~acceptsble.
('Plydro-
sealed' clumps a:C pockets of day ra~ageenan, which ere surtou~ed with an
outside
casting of aeatf hydrated caarago~an, which becomes impenetrable to caster due
to
aarrageenan'a ext~mtly latge molecular weight and ffexi'hIe structure.);
(8). Close aace9s hatoh and continue stirring at maximuta~ spend, turbin 1200
rpm and
anctaox ~0 rpzn. Add 13.0 kg ptuifled water (Part Ill) and disvannevt the
waterlfae,
close value. Teat aolutiou rrr ~5" to 80°C by applying 52~/ heat; and
(9). G'Iteck that all the values are alased and apply the vacuum to the vessel
at a00 mbar,
Stir selution at slightly reduced speed, turbin 1100 rpm.and anchor ZO ipm,
under
vacuum fox 1.5 hr at 75° m 80~C. The constant stinting of the solution,
which is
necessary for even distn'bittion and complete ineoxpotatian of ingredients,
causes
txressive air entrapment 'rhe vacuum pulls dais air vut of the solution;
(10). Turn heating OFF. stirring OPF, and vacuum Ok~. liemave Testing Sample
Pram
~psodt~tion vessel and tsst for Control Test #1 Complete incorporation and
overt
distribution;
Control Test #I: Complete ittcarpoiation and event distxt-butian
b
CA 02384474 2002-05-O1
toaZ41 Remove approximately 90 ~L o~ the inrproress mixtw~e (use a Large
orlfllce
Z00 E~. pipette tfp to aid in removing the carrageenan solutioon) and min: is
10 NI. of a 0.1s/.
methyl blue ~'S (I:1, isopropyl alcohol: d~-12U) is a 500 ~.L Fppertdorfftube.
The mixture in
the tube should nppeat as an even blue color. This indicates that,the 1GI~,
carraEeenaa mixture
is avartly distributed wxthia the solution. Prepare a mxcmacope elide v~nith a
10 uL, of this
m3xt~ue; cover with a conrar slip and view under lrnv magni$catloa (10~. The
iCl~.
carragoanatr miaoture atlouId appear as !ar$e purple atcauds. This indicates
tI~ the IC/?~,
carzagaaaan mixn~xa x8 complotely incorporated snd the solution is "PASS". If
the strands are
blue or large blue cluaaps era viseble, tftea the KI7v, carrageenan mixture is
not campletcly
incorporated and stilndoa is "FAIL", Continue processing the solution under
the conditionQ
of step ~9. Itecjreck aalutiozr at O.S hour intervals until solution is
"PASS".
(11 ). Whim the solution is "PASS" far Cool Tact #1, test for fontrol Test #2,
pii;
Corttxol Test #2: ply
Ioozsl Tlxe tasdng samgle should be cooled to ?SQCt 2 (a range o~23aG to
Z?°C) tbr
nesting. The pl:I should ba 7.0 t 0.1 (a range of b.9 to 7.1). This irzdicatcs
that the solutloaf's
ply is umfornt and the sohxtion is "PASS". If the soluko~a is not ar'sthin the
accgptabls pH
range (b.9 to 7.1 ) the solution is "FAIL". If the solution is "FAIL", the
solution needs to be
adjusted, as needed with either 10°Io HCl (to decrease the p~ or 1N
NaOI:YT (to uxrease tlae
pH) is 25 m~, iacr~~raeat$ until the solation is "PASS". With each iuerementsl
addition o~
either acid or base, thorough atim~g (atirriag and vacuum condiriora step #9,
ac added. heat) is
seeded to amture even distdbutian throughout batch before re-testing the pFl.
kachacl:
solution altar stixringlvacuurn far 0.5 hoax. Continua in this manor until
aaluaan it "'PASS".
(1~), Wren the sciatica is "PASS" for Control Test #~, begin cooling the
msaesare to 25aC
t 2~ (f3oC to 2'ToC). The stirring sped, which should be OFF st dais point,
will need to be
increased as the solution thiclrent upon eooling_ 'At start; turbix~ C~ and
anchor zQrpm,
ina~se turban 20 xgm / 15 mm and increase anchor 10 rpm / 30 mm, ending twith
fi~in
1000 rpm and anchor d0 xlrm, It is preferred not to increase stiirirtg to
rapidly; athacwise, air
entrapz<xent may result, rf this should bsppen, apply the vacuumv 400 mbar
until adlutioa is
free of sir babbles;
(13). Remove Final Teatiag Sa~uple from the production vessel and rarest for
Control Test #2
pH an8 for Control Teat #3, 'Viscosity.
Control Tast i~3; Viscosity
CA 02384474 2002-05-O1
IO o~ 61 The testing aunpte should be heated to 35aC t2a (a rye of 33~ to
37°C).
Te optimize pcrformanee, the visa~ity chonld be about 34,000 bo about 44,440
CP. Viscosity
meas~u:aa~eats indicate that the solution's viscosity is urtifatm with tf~e FC
Rafe~rance sample
ind GCS pxoductiaat batches utd the solution is 'PASS". if the solution is
"FAIL" obtain
testing samples from tha top sad the bottom of pcoduation vessel and conduct
Coaaol Test
#Z, pH and ~annol Test #3, Viscosity on each sample. If the solution is still
'FAIL", ccpcac
step #9 and step #12 end ictest the solution fox Co~rol 'Test 3#, Yiscnsity_
Tf solution is
'~A1L" sa Out of Speoi~cadaas Study shall be undertaken to detcxmine tha
source of our of
sptcificatto~x production.
t d 0 ~ ~ ] It area di$covered that sdjustiu~g viscosity rovith t]te addition
of water ykklS an
usdcaovirn percentlctrat~on to the final production batch reoaeriag the
production batch
naacceptsble, '
(14). 'VVhe~t the solution is "'PA9S° for Goatral Tests #1, #2,and #3
it is an acceptable
prpduetion' batch which cart be prxessed far the iiuas control testing. Comet
the ccaasfex
tube ca~ntainin~ a alter bad to the bottom value of the production vessel end
transfer the
formuiaxion into storage containers. Retain a ?est Sample fbr Micmbiological
Tes#ng~ before
filling applicators.
L o d ~ s~ The fuel fbanulation prepares. in the proaesa discecssed , above
hes the
following cor~npoacnt~
Weight/Peroent: 500 L~tars offarmulation
oncnt ~ocnt
pttri~ed Waccr 4 9ti.8
_0
k
lvtatlr. $W 0.
I
ben
pBS: -
NaGl 120
zruool/I,
ICCI ~_7
mmollL
F11 1
llHt~e d
S3ltt ~QI~L
10~C1 $
the 15 3.
cairra~eenan kg
nnixtelre
[0D2 ton bas a
9 p of about
] 7.0 whir
Tho , waS adlua
Vital by Addis
fotaxula
~IG1
solution
and
1:
t
ratio
of
K~Pb,
and
xTa~HHi~tS~~.
ExampIt 2. F~'ea of Carrageenaa on ,Fftv Infections ih vitro
It7o301 GactatgCCnus hoe been shown to block HfV'.and other enveloped virnses
by
savexal Iaboratorxes including the laboratory of the Fh"S. several different
t~~pes of forget
8
CA 02384474 2002-05-O1
calls surd strains of FIIV have been araployed in these studies. Generally,
509~o b~iooldng is
observed at a fevc~ micro~nsin~L This is sitru'lar to other sulfated
poIysacclrarides such as
and dextrixr snlfats.
'l~an~rle 3. Iatrs vaginal veal iafecdon studaes - HSV-2lMouse
I0031I Besides being the first and the priz~capal in v~vo system to evaluate
fin
potential of candidate xni~crabicides in bloaki~ag viral infection, the HSY
2lsnouae (BslWC~
aysteut is widely ntili~d by most investigatory groups engaged is the
dev~elopmrnt of a
micxobioide. An important difference between the system established by
Phillips m~° and
other ~ysurns is the utt~lization of viral dose rarAge comparison. The
staadaxd viral challenge
dose, Z00°r6 infection dose ox 10' gfu, usod by others for cwaluation
of a micmbicide is rate
limiting. The large m~jorny of the microbieides under development, as well as
marry of dse
4T'C spermicidea will sl~vv a si~nificattt rate of protection against USV 2
iofeotion at this
viral ahallBnge doses. However, i~hillips has utilized a vii c~os~tetitratirnn
mothod that wilt
enable evahtatfan at viral clrallcnge doses of IQ°,10°, acrd
1,000 x 140%inf~4on dose.
Iao321 Using this viral cballenga doss System, a comparison study was
conducted to
evaluate the eorapaxiaoa protection rates of a number of rnVcrobicides under
deveiopmtrnt,
4TC sptamiaides .aid lubricants, and possible forurulatiotrs fox ~tso as a
placd~o in the clinical
trials to cval>rate efficacy of a microbicide. In addition to the KI7v,
cat5tagmixture,
comparative test formulations wtna: micmbicidea under develo~rmant such es
Buffe:Gel'~
and No Fam'1, OTC spermicides: K 'Y Flus° ~ytrol III, and. ~dvsntsge
5~, OTC yaaipal
lubriea:r~: Rtplensa and Kr'Y' relly'~'; and possibly placebo foanulations:
.1.SR~6 Carbopol' and
2.5% methyl odlIuiose.
100331 Test formulations fell into three categozies with respect to officuoy
izr
pmroecting mice flrona vaginal HSV z inferevn. At the vinI ahallc~e dose of
10' pfti, with
the exception of K-7~ JeIIy, Carbopol sad methyl cellutose, art Fannulatioa$
prawidad s
significant tevet of piotec#an against in#hctioa from T~SV-2, Hovvevrt, at thB
~~iral challenge
dose of 1 Q ; with tire cxceptioh of tt:e ~. caxrageenaa r~aixt~u~re, art
formulations only
pQOVided a minimum level of protection. Tire l~, carregeottatr mix4m twas~ the
only
folmulativn still affording a level of protactian against vial infection at
tire viral challenge
dose of l d' pfu ~°.
9
CA 02384474 2002-05-O1
too3W Thtreftita, it can be concluded that the i~sV Zlmouse system csa bs
etnployrd
as a tneaus by rwbich candidate microbieides can be evaluated assd compared
under the~aarnr
reefing conditions to identify potential effective microbinides,
fixsmple 4._ Duration of actirrity - HSV-/Mouse
L 0 0 ~ 5 ~ Came of tea criteria set ~oith by Uh?,AIDS (World Health
Orgaai~stioa, ADDS
branch) for an ideal micr4bicide states 'it arontld be activ~a upon insertion
and for a long
padod of tithe, gii, iag a woman male flexibility in product use. ~dditio~lly,
tho time course
for iafectioa by sell-flee or r"e11,-assxista3 ~'V' en occur may hat be
immediate. Th: I:ISV-.
?,/atous8 system can be employed to evaluate the duration of time drat a
microbicide ~ronld
retain activity. This is done by iutra-vagin.'tl applioati4tt of a te$t
founulation, waiting a set
period of tithe, and then claalleaging mine with a lotown doss of vin~.a.
"Datation of activity"
tosting was conducted uaittg Oynol T.Te (a 2°r6 N-9 containing OTG
sgcrmicide), BuffetGtI° (a
law pH mierobicida tuuler devclapment) and the alt, carrag~nnn mixtvro, at
five minutes
and 1.5, 3, and 5 hours follow formulation application. Hy the 1'lrhour time
point, Gynoi
A° no loatger a~'orded any pmtectiaxr against infection and Hu~fezGel'
had dropped. td being
only 30% ef$ctivc. &uffexGel's efEcacy continued to drop aver tune and ha
longss afforded
attar protection by ~6 hours. In rnsrictd coxatraat, the ~/a. carrageetian
mioturo repnaitted 90-
t0o~~o effecsive in protecting against HSV 2 infection up tp LS htar and in
:ame cxpazimaats
up to ~ hr3. The I:CfA. caaageanatt mL~ture continued to retain so3nc level of
activity for up to
24 hours. Sre fxg. 1.
Example 5. iatta rectal viral infection studies - HSV-2JMouse
too36~ Ideallyr, a atierabioide fat was effective in protecting against
izifectiorl by
filV could be used rectaily as well as vaginally. Using an ir~t~n- xec~tai
viral challenge
moditkatian of the HS'lt-lmouae systara an avalaation of the efficacy and
safely of a
mi4robioida was explored.
tooay~ Pre.-tre$tment of me rectum with the L~J~ catragcenan m3xhua
significantly
reduct8 the auaabec of anialals that became infected fdllovving rectal
ahalleage with IiSV 2,
cotttpared to pre~atmant with P89 or taethylcellulose {an inert placebo)".
~tatt~pla 6. Erect ofthe Itlh. carrageenan mixture on vaginal flora
to ca el It is important that the use of a microbicide dons not disrupt the
batance of the
natural vaginal flara_ In vitro studies indicated that carrageenan, did not
enhance or inhibit the
grQwr6 rate of .Lacto6acil)us aeidophdlus. the mosx common bacteria present in
the vaginal
IO
CA 02384474 2002-05-O1
ffoxa. A study canduotad fn 35 wamGn pazticipatittg in a Pha3e I cliuicsi
trial tbr tho vaginat
safety of the ICIJ~. carrageenaa mixture showed no significaa~t change in
vag~aal flora, as
aLeasurad by the presence at abssncc o~bactezial vaginvsie~'
8xampla 7. I~VIMonat viral tranapoxt systzrn
100391 ~4lthough mica oenttot ba infected with HTV, its has been ihavvn that
v~
active at inattivatad vlNa is in~tilied unto t'6e vagina of mice, virus can be
subsequently
defeated is the lymph lodes by the use of reverse transccriptase polymexase
chain reartian
(RT-pCR}a fividonaa has been pxeaented rbac dandritia cells played a mie in
the upr,~ka of
virus end subsequent traagpork to the ly~ztpb nodes. 'l7ais c4nchrsian is in
agreement mith
studies impiicatiu$ detulZitic cells in the initial stage of saxuai
transmission of HTV~.
i0o~01 Results indic~xe that the KIa. ceaageenan mixture is efifcacions in
preveatlng
HIV from reaahin,g the lymph node, preaumsbiy by blocking HIV transport from
the ~ragina_
t O o ~i 11 HIV transport using a mouse system and Aldritl~ol~ 2 israotivatod
virets were
nsed_ Thris is a standard method for iaxctivating IiIV that dOCS nut alter the
rriral enrrelnpe.
The spleen cad the lymph nodes ware assayed fox the deter#an of ~ in order to
establish
the spleen as as alternate r~osfto~ty xite for HIV. The splaen(as opposed to
the lynrtph nodes)
allows for obtaining ralativety iargc~r amounts of RNA for pcxfaami~ag 12T-PGR
far thr
detectiaa of ItIIV_ Txr add'stivn, rxtracdon of spleens is lass time consuming
t3ian xemaval of
the Iyrnph modes thoteby lmasening the probability of RNA tlagrs~ation.
too~~1 To detesxnine the cfficaa}' of the ItI~, carrageenan mi~cairo in
pxovonting HIV
trora crosain$ the cerv~icaiwaginaI battier, mice vrrermandomized into three
groups: 1) aon-
traatad PAS eontrnl mine; Z) mica pro-treated with methyl cellulose (inert
placebo); and 3)
mica pretxeatod wide tht Y!?~, carxagaanan mixture. Results are shown can the
Southern Blot in
Fig. 2 and the table below.
'rreauuent .~_Itotalosier a ge
lvt~G~iy1
CelluIosa
-tne ~a Y~o
~ppollatnb
raxrageenan
'
m
a
to04~1 Dsia from saline aaa methylcetiulose (control) treated and mice treated
with
11
CA 02384474 2002-05-O1
the ~,/~. 03T12g~TJ3xl t111Xtt1Te. ZitC (IHIa ~h,OW ~tt tltC ~. ~gee~ ~
$1~1~f~, C~M~y
reduces five number ofpositiva ~.e., irifectedj animals. Mathylccllulasa Karl
no effect.
IOD44I 1"1t8 data indicates that the ~J7i. carrageenan mi~cturc is affeativc
is prevesiting
HxY frame leavistg the vaginal vault.
Example $. Cell ~'taf~cldng/Mottse System
o g 5 7 It has pre~niously been suggested that sexual a~ansmission of HIV
could be
mediated by ~Y-fnfoeted lymphocytes or macrophages in semen thst cross the
genital tract
epithelium"~~, In ordex to test the hypatha~is t'bat moraonuclaar blood calls
ttaffio from the
vsginal vault thron~"h intact epithelia, donble<-vitally-sGtained activated
mononuclear blood
cells (mouse) were placed in the vagina of mice. pour hours later, animals
were sacrificed
and tissues were p~rapa~tcd for examination by fluorescence microscopy.
Numesnus double-
staiaad ceps ~rrare of~aarvtd is d1e con~neetiva tissue beneath the vaginal
epithelium and is the
iliac lymph nncios~. To evaluate the effect that the K/7~, carrageenan
nai~ctura may have on
blocking this procosS, at~ir~ala ~nvwra pre.oreatcd vwith the test formulation
priox to instillation
of labeled cello.
a t " a
~
aimala ned r.aIls
i
4 FteC2e tll~9V~d
IltaprCphBge 0 's
control
macro a 40f4
~
~~
~
~
g
cot with
~c~tm
p
t
trit kacppallalmbda
earn muctu~
lOoa61 Number of double stained cells is the iliac Iymgh nodes e~ mica.
Appauximately 1_S million cells were examined in each animal, Tbc data show
thatthe K/7~
caaageanan mixture sigt~if carttIy mdaced the ,umber of Galls that ware
observed in the -
Iymph nodes.
>rxample 9 lvlicrobicide affect on papillousaviius
~ao~9] 'Ihe YGI7,, aan~agea~an mixture is also effective on blocking bovitle
papillomavirus (BPV~ foal formation in vitro (data oat shown). The xC/~
carrageertan mixtvze
is efficacious in preventing papillomavirus ifrorn trausfozmir~ human vaginal
explants in a
xenogxsph system, x'hc STAID mouse xmograph sysnm tmploys cxplants of human
vaginal
1z
CA 02384474 2002-05-O1
tissue rolled into ayli~Cical tubes that are gxafted subcutaneously on
Irli~DISCID
(i~mmuaodo~cient) mice. The grafts are aliawad to heal fbr two areelrs, at
which time o~,e
end of the tuba is opened and a tsar ac~ound it instilled followed by HPV
chslla'xge. In
experimonLS evaluating the KIJI. csrtagrenan mixture, In 14 out of 14 saline
treated coritral
axptsusts were transformed. xn contrast, anly 3 Attt of 17 ratplat~ts 2raated
veith the ICIa.
cairogc4nau miytuze was trat~sfbzmed (darts not shanvn).
All patent arts non patent pablicstidns cited in this apecif cataon are
indicative
of the level of skill of those skilled in the arc to which Ibis invention
pertains. All these
publications are hcrain iacorporat~ed by reference to the snn~e axteat as if
each individual
publication or patent application was speoifically at~d individually indieatad
as being
incorporated hexeia by refatance.
10097 ?hoe skilled in tha art will rcco~niza, or be able to ascertain, using
na snore
than routine expernneratatlon, antnaoua equivalents to tha specific 3ubstnaces
and pmaedures
described herein. Such equivaleixts are eot~sl,daned to be within the scrape
of this i~a~~entian.
I3
CA 02384474 2002-05-O1
tc.~c~cr,~laa..1
I. Fo~ovd and Drug Adminigtratian. G12AS (Generally recognized as safe} tbad
iagredients:
Caxrageet~an. IAA Publications PB~?.2I 206, (1970.
Z. $enitz, R.F,, Abraham. ri" Cxolberg, L. & Goulstost, F. ~C.auagaanan: un
ulctro~caic
agent. Tox~ftol. Appi. PJtarr~racol. 22, 282 (I972).
3. Fox, M.R.B. & Jaaobs, R.M. MetuI.Xotis in $iologiaal Systems., pp. 214-
7'.48 (Maieel
Ticker, Inc.,I9$~.
4.LuscQmbe, D.K. 8t Nichalls, P.J. Acute and subpcutC oral toxicity of AI~t
24388, a
pnri~ed sodium Ii~sulphanate in rats.Fd Cos~d. TaxfcoZ lT, 229 237 (1973).
5. Naesa; H. T'b~c effect of mic~cabial and animal prQteinases on peptide= and
protein
Hgnoseilphonic acid complexes in agar gal. Actn VaG Scarad. I2, 592-6Da, x971.
Ref
'I~pe: Aba~act
b. Sa:nman, S. 6c ltoberts, 13.C.K. The erect of xinc sulrplements on plaama
zinc and
copper lavels snd floe reported symptoms is ltealtliy volunteers, Jklc~l J.
A~rtralia rd6,
~4b-249 (1987.
7. L_S.~A. 8aatt~r Effects Assessmelox~ of for Zinc (and Cbmpounds). B3~.AJ540-
i-96-048.
1984. Wa$hiutgtoa, D~.C., tJS EnvixanmentmJ Prdtc~ction ~.gency, dfFce
o~Researoh at~d
lhwalopment. Ref Typt: Data File
8. Walden, 1,T. & Derttth, 17. FDA Nevv Release 12155. ,FIaA PuSI'akiv~ns
y2lSS,
(1972).
9. ~Valkar, A.F. et al. Test guidelines fo: the assossment of skin, talaaace
of potantially
i~at cosmetic ingredicxtts In man. Fd Client. Toxic. 3S~ I099..1 lOb (1997).
lO,Weiner, M.L. Intestinal #sansport of some taacromolecules in food. Fd
~heFn.
Tasic.26, I0~ 867-$80 (19$8j.
1 x. Phillips, D.'I~. & ~'au, X. Mechanism of trophobtast infection by
HIV.~tX,D$~t~,s Xsetn
Red ~ovirusts 9, ld9T-1705 (1992).
12. baba, M. er al. pentogan polysulfaate, a sulfated aligosaecharide, is a
potent and seleetxve
anti-HTV age»t in vitro. An~viral Res 9, 335-343 (1988).
13. $aba, M. Qt al. Mechanism o~ inbibitoty effect of dextran sulfate and
heparin an
replicaaan of bsunau iazm~.snodeficiency virus in ~~itro. Proc Narl Aran! Sri
85, b132-G 126
(1988).
y
14
CA 02384474 2002-05-O1
14. Pe~rce-Prstt, R 8t Phillips, D.M. S~die: of adhesion of lyrmphaoytic
acllg; Iatglicatioms
lox se7u~a1 t~nission of httmaa imravunodeficicncy virus. .8iol lteprad 48,
431.1.5
(1993).
15. Pearve-Platt, 1~.. & Phollips, D.M. SnIfated palyaaccha~rides itrlnbit
lymphoaytQto-
epifhelisl traxrsmission of HIV=I. .~i01 Reprod .54,173-182 (~99~.
16. Maguire, R.;4,., Zxcharopoulo:, V.R, &c Phillips. p.M. Carragsenati N9
Spatmicides for
Prevexntng Prcgnn~ecy and. Sexually ?ransmitted xn~ectiams. Sir Tra>asm DDS
25, 4~-
500 (x998).
I7. Phillzps, D.M Perspectives in Dtug X?iscovery anr3 l~asi~. Fantini, r. &
Sabatiec, d.M.
(eds.), pp. 213 223 (19~.
18. Zacharopoulos, V.IZ 8t 1'I~illips, D.M. Vaginal i;oxmulations of
carrageenan protoect
mice i~om herspes simplex virus infection. Chn. bia,~ T"ab, xmmrotol. 4 465-
468 (199.
19. p'hillips, D.M. & Zacharopoulaa, Y.R Nonaxynol-9 Ezrhanees Rectal
Infection by ~Icipes
Simplex Yirus in Mice. Gorciracepon» .f7, 341-348 (199$).
20. );line, C.J. et aL C4lpOSCapic Evaluation o~ a Vaginal Gel 1?ormulatiOn of
iota-
Cauagerpaa. Contraceptfm 36, 387-389 (199'x.
21. Hlasuncr, C, et aL Dendritia aella ronta Human rmmunodcfciency Yutus to
lymph
apde3 alter vaginal ax intraveaaus administration ~co mice_ J l~,rd72, 7822-
78.29 (1998).
22. htasuter, C. dl aL l~endritic Cells in Fuud,tmental end Clinical
Trnrauaoiogy.
lticcciaadi-Castsg~noli (ed.), pp~.~4I1-414 (Plenum Piesa, New YorIc,199~).
23. An~d~erson, DJ. Meclyanisnns of FH'V'-I transmission vie somen;. J: NIH
Res. ~, 104-111
(1992).
24. Lavy, r.A. Tl~e txsnsaziasion of AIDS: tha case of the infected
cell..L4ltT~ ,259, 3037-
3038 (1988).
25, 2,acharopaulos, Y.R, ~'erot'ti, M.E. & P'Itillips, b.M. A rote for call
migration in the
scxuel transmission of HIV? Gusrcnf Bfo~. 7, 53~-537 (1997).
25. Howetk M.K.,Kr.W.C.~.D. I~nnaan xaaografts. A model systeza for hutuan
papillomavieus infactior~. TntcrvrY. 31,109-115 (1990).
.,