Note: Descriptions are shown in the official language in which they were submitted.
CA 02385029 2002-03-13
WO 01/21310 PCT/US00/25851
-1-
Device for Rapid DNA Sample Processing with Integrated Liquid Handling,
Thermoeycling, and Purification
Reference to Related Applications
This application claims priority to U.S. Provisional Application No.
60/155,299, filed September 21, 1999. The aforementioned application, and the
references cited therein, are incorporated herein by reference.
Field of the Invention
The invention relates to devices and methods for high speed, low volume
automated sample handling of biological samples, which are useful in the field
of
genomics for a variety of processes, including DNA sequencing, genetic
analysis, and
gene expression analysis. The invention further relates to devices and methods
for
setting up and executing assays for high throughput compound screening for
pharmaceutical applications.
Background of the Invention
Laboratory automation has played a key role in the advancement of
genomics and drug discovery over the past decade. Early work in genomics
focused on
the automation of fingerprinting and STS mapping procedures through the
adaptation of
pipetting robots and image acquisition systems (Garcia et al., 1995, Kwok et
al., 1992,
Lamerdin and Carrano, 1993, MacMurray etal.,1991, Nizetic et al., 1994, Sloan
et
a1.,1993). An interesting example was the "Genomatron" for STS/EST mapping,
jointly developed by Intelligent Automation Systems and the Whitehead
Institute
Genome Center in Cambridge, MA (Dietrich et al., 1995, Hudson et al., 1995).
This
system performed all the necessary steps for high-speed PCR setup,
thermocycling,
sample processing for transfer of the reaction products onto nylon membranes,
and
hybridization with biotinylated probes for CCD based optical signal detection.
However, the machine was large, expensive to operate, and could not be easily
adapted
to performing other tasks. From 1990 onward, a large number and variety of
laboratory
CA 02385029 2002-03-13
WO 01/21310 PCT/US00/25851
-2-
automation devices became available from an ever expanding set of
instrumentation
companies. Automated systems are now used in high-throughput sample
preparation for
DNA sequencing at many of the large sequencing centers.
The degree of automation of the various functions performed in
sequencing centers varies widely, ranging from manually fed systems to fully
integrated
processes. Each configuration has demonstrated positive attributes that
contribute to its
successful implementation in the sequencing laboratory. However, with a manual
setup,
there are significant problems with human error resulting in misidentification
of
sequencing reads, while the fully integrated process is prone to system
failure should
one of the modules break.
The current approach in the automation community is to move away from
large fully integrated systems to smaller workstations that fulfill specific
independent
functions in the sequencing process. This means that malfunction of one
workstation
does not result in a total system breakdown. In addition, this paradigm allows
flexibility, which can accommodate changes in requirements as sequencing
processes
change and improve over time. Specifically, as it takes time to build an
automation unit,
having flexibility allows one to alter and modify different components as
improvements
become available. In high throughput sequencing facilities there are several
functions
which are tedious, inefficient and error prone. Examples of these are colony
picking,
template preparation, sequencing reaction setup, clone retrieval and gel
loading.
Modern laboratories employ partially automated procedures for handling
samples. In these procedures, reagents and templates are combined by manually
feeding
96-channel pipettors with thermocycling plates. Other laboratories utilize
pipetting
robots, such as the Tecan Genesis (Ahmadi, 1997) to accomplish the same task.
Integrated systems that utilize a variety of pipetting robots, and plate-to-
plate liquid
transfers, plate sealing, and plate-based thermocycling with magnetic bead or
filtration
based purification procedures have been constructed. However, these systems
are
complicated, expensive to build, and suffer from sample evaporation problems
and
volume constraints.
Several groups have proposed using glass capillaries to handle large
numbers of DNA sequencing samples. For example, the first protocols for
chemical
sequencing developed by Maxam and Gilbert ( 1977) utilized sealed glass
capillaries to
handle the samples. In one case, the capillaries are filled, mixed and handled
individually as they are moved through several functional "stations" on a
conveyor belt
type of arrangement (Friedman and Meldrum, 1998). In another developmental
project,
CA 02385029 2002-03-13
WO 01/21310 PCT/US00/25851
-3-
96 capillaries are attached to a Hydra dispenser (Robbins Scientific) so that
the samples
can be moved up and down past heating elements to perform PCR (Hunicke-Smith,
1997). In a revision of this device, copper heating elements were moved up and
down
with respect to the position of the samples (Stanford Technology Lab, 1998).
A significant drawback in standard 5-10 ~.l sequencing reactions is that at
least 50% of the sample is wasted, never being loaded on the gel.
Summary of the Invention
The present invention addresses the foregoing by providing a capillary
cassette
having a frame defining an interior chamber, a plurality of capillaries, each
having a first
and second end, wherein at least one of the first end and the second end is
mounted to
the frame, such that each of the capillaries is fluidly coupled to an external
surface of the
frame.
According to another aspect of the invention, the present invention provides a
sample handling cassette including a frame having a first end and a second
end, with a
passage through the frame extending from the first end to the second end, a
first flat
membrane layer disposed along opposed sides of the frame and defining, along
with the
frame a sample handling chamber.
According to another aspect of the invention, a docking port is provided
including a guide cap having a concave end and, optionally, a fluid-tight
seal.
According to another aspect of the invention, a method of performing dialysis
on
a biological sample is provided involving the use of a capillary cassette.
According to another aspect of the invention, a method of performing
temperature processing on a biological sample involving the use of a capillary
cassette is
provided.
According to another aspect of the invention, a biological sample handling
system for use with a liquid handling machine is provided involving the use of
a
capillary cassette and a needle bed.
According to another aspect of the invention, a hotel for processing multiple
biological samples is provided including a housing and a fluid management
system.
Optionally, the hotel of the present invention can process a plurality of
capillary
cassettes.
CA 02385029 2002-03-13
WO 01/21310 PCT/US00/25851
-4-
According to another aspect of the invention, a template preparation module is
provided involving an apparatus management device and a capillary cassette.
Brief Description of the Drawings
The foregoing and other objects, features and advantages of the
invention will be apparent from the following description and apparent from
the
accompanying drawings, in which like reference characters refer to the same
parts
throughout the different views. The drawings illustrate principles of the
invention
and, although not to scale, show relative dimensions.
Figure 1 is a perspective view of a capillary cassette according to a
variation of a first embodiment of the invention;
Figure 2 is a perspective view of a frame according to the first
embodiment of the invention;
Figure 3 is a close-up perspective cross-sectional view of a docking port
according to the first embodiment of the invention;
Figure 4 is a cross-sectional view of a docking port according to the first
embodiment of the invention;
Figure 5 is a cross-sectional view of a portion of a sample handling
cassette according to a second embodiment of the invention;
Figures 6 and 7 are perspective views of a capillary cassette used in
conjunction with a liquid handling machine according to a third embodiment of
the
invention.
Figure 8 is a perspective cross-sectional view of the third embodiment of
the invention;
Figures 9 and 10 are a cross-sectional perspective view of a sample
handling cassette according to the second embodiment of the invention used
with a
needle bed and microtiter plate according to the third embodiment of the
invention;
Figure 11 is a perspective view of a template preparation module in
accordance with a fourth embodiment of the invention;
CA 02385029 2002-03-13
WO 01/21310 PCT/US00/25851
-5-
Figures 12 and 13 are close-up perspective views of the template
preparation module according to the fourth embodiment of the invention;
Figure 14 is a perspective view of a hotel according to a fifth
embodiment of the invention;
Figure 15 is a perspective view of a hotel according to a sixth
embodiment of the invention; and
Figure 16 is a close-up perspective view of a capillary cassette and hotel
according to the sixth embodiment of the invention.
Detailed Description of the Invention
The present invention addresses a need in the art for small-volume DNA
purification methods, preferably with re-usable components, that can be easily
integrated with capillary-based sample handling, and that eliminate the need
for
centrifugation. The invention also performs capillary-based clean-up devices
and
sample handling alternatives to air-driven thermocycling, and methods for
efficiently
handling sample amounts that are just sufficient for each separation, in order
to achieve
significant cost savings.
Before further description of the invention, certain terms employed in the
specification, examples and appended claims are, for convenience, collected
here.
The term "biological sample" refers to a sample comprising one or more
cellular or extracellular components of a biological organism. Such components
include, but are not limited, to nucleotides (e.g., DNA, RNA, fragments
thereof and
plasmids), peptides (e.g., structural proteins and fragments thereof, enzymes,
etc.),
carbohydrates, etc. The biological samples described herein may also include
transport
media, biological buffers and other reagents well know in the art for carrying
out the
processes described above. Although the methods of the invention can be
carried out
with a biological sample of just about any volume, biological samples in
accordance
with the invention preferably have microliter (p,L) volumes and therefore can
be referred
to as microsamples, e.g., biological microsamples.
The term "cassette" refers to a structure or "module" capable of handling
a plurality of samples, for example, 96 or more samples.
CA 02385029 2002-03-13
WO 01/21310 PCT/US00/25851
-6-
The term "dialysis" is art-recognized and is understood to refer to the
separation of substances in solution by means of their unequal diffusion
through a
membrane. As used herein, "equilibrium dialysis" refers to dialysis which
occurs
without exchange or flow of dialysate. "Flow dialysis" refers to dialysis
which occurs
with a flow (or counterflow) of dialysate. "Exchange dialysis" refers to
dialysis which
includes at least one change of the dialysate surrounding the membrane.
The term "frame" refers to any suitable structure for providing
mechanical support to a capillary.
The term "hotel" refers to a unit for housing one or more cassettes and
provides a platform for sample processing. In one embodiment, the hotel is
adapted for
sample thermocycling by the inclusion of means for circulating a fluid, for
example,
water or air, to provide temperature control. The hotel also provides a
platform for
sample purification. For example, in one embodiment, the hotel is adapted for
dialysis
of the sample by the inclusion of means for circulating a dialysis fluid. In
yet another
embodiment, the hotel advantageously provides a washing platform by the
inclusion of
means for circulating a liquid (e.g., water or a chemical cleaning solution)
such that
cassettes can be washed to prevent sample carryover, or can be regenerated.
The terms "filter" and "membrane element" refer to a material which
may used to separate substances in solution by means of unequal diffusion, for
example., by size exclusion. Exemplary membrane elements and filters are
semipermeable; i.e., the membrane elements or filters are capable of
permitting dialysis
to take place.
The term "purification" is intended to encompass, in its various
grammatical forms and synonyms (e.g., purification, purifying, clean up, etc.)
any
operation whereby an undesired components) is/are separated from a desired
component(s). Such operations include, but are not limited to, filtration,
ultrafiltration,
dialysis/equilibrium dialysis, chromatography, etc. In certain embodiments,
purification
is achieved by molecular size discrimination among the components of the
biological
sample. Purification by molecular size discrimination can be achieved using
any
number of materials of varying porosity well known in the art including, but
not limited
to, filters, membranes, and semipermeable ultrafiltration fiber materials.
The terms "temperature processing," "temperature treating," and
"thermal processing" are used interchangeably herein to refer to the
application of a
variety of temperature conditions to the sample, depending on the particular
process
3~ underway and include, but are not limited to, continuous and discontinuous
heating
CA 02385029 2002-03-13
WO 01/21310 PCT/US00/25851
regimens, e.g., denaturation, annealing, incubation, precipitation, etc. For
example, the
terms broadly encompass thermocycling associated with PCR and similar
processes.
The term "ultrafiltration" refers to any method of dialysis wherein the
sample is under positive pressure relative to the dialysate.
According to the invention, purification of a biological sample may be
achieved by a variety of methods, including dialysis, filtration,
ultrafiltration and
chromatography. The invention further provides various configurations to
achieve
purification, depending on the method of purification selected. For example,
when
equilibrium dialysis is the method of purification, the apparatus of the
invention
provides at least one capillary comprising a membrane element in operative
contact with
a dialysate, for example, water. When exchange dialysis is the method of
purification
method, the capillary may be exposed successively to at least two dialysates.
When
flow dialysis is employed as the method of purification, the capillary
cassette 10 may
further include one or more ports for inflow and/or outflow of dialysate.
The invention further includes microdialysis-based sample clean up and
plasmid clean up.
As set forth herein, the present invention includes dialysis techniques,
which may be used effectively to "clean up" polymerase chain reaction (PCR)
and cycle
sequencing reactions. Until now, one of the problems with conventional
dialysis
techniques has been one of scale. Typically, dialysis is carried out on
relatively large
sample volumes of at least 1 mL or more. The typical PCR or sequencing
reaction, on
the other hand, generally utilizes sample volumes of approximately 10 ~L or
less,
significantly smaller than the sample volumes in conventional dialysis
techniques.
The present invention addresses this disparity by optionally using a
membrane element, such as one or more microfibers inserted within one of the
capillaries. The microfiber performs the same separation functions as the much
larger
dialysis operations, but with much smaller sample volumes and without the use
of
centrifugation. The microfibers can be generated or manufactured by removing
one or
more hollow fibers from commercially available filtration cartridges. Typical
cartridges
contain many hundreds of fibers, since the cartridge is solely designed to
perform
dialysis on large sample volumes, e.g., 1 mL or more. Many types and sizes of
hollow
fiber filtration cartridges are available through such suppliers as Millipore
Corp.
Bedford, MA or Spectrum Labs Laguna Hills, CA. Typically these cartridges are
used
as ultrafiltration devices, where the dialysis membrane acts as a filter,
excluding the
CA 02385029 2002-03-13
WO 01/21310 PCT/US00/25851
_g_
desired products while allowing the undesired components to pass through when
pressure or vacuum is applied to the system. The present invention achieves
proper
filtration or separation of components from small volumes of a biological
sample by
employing one fiber for each biological sample. In this way, dialysis on
sample
volumes of 10 to 0.05 ~L volumes is achieved.
According to one mode of operation, the present invention achieves
appropriate purification of a sample by first performing a standard Big Dye
Terminator
Cycle Sequencing Ready Reaction Kit, part # 4303154 PE Applied Biosystems
Foster
City CA, on a reaction sample size of between 0.05-10 ~1. The sample volume is
drawn
up into a hollow fiber filter which has been cut out of a Spectrum cartridge
cat # 132229
Spectrum Labs Laguna Hills, CA using a 10 ~.l syringe from Hamilton, Reno,
Nevada
(see FIG. 2). Purif cation is then achieved according to any of the various
methods
described herein.
The technique of dialysis, although well established, has heretofore been
difficult to perform on small sample volumes without suffering loss of the
sample.
Semi-permeable microfiber ultrafiltration materials are available in a variety
of
porosities, which allow small components to freely pass through while larger
components are selectively retained. Although these are commonly used for
ultrafiltration of proteins, only some of the materials are suitable for
capillary-based
dialysis. Because all of the reaction components to be removed from PCR and
DNA
sequencing reactions are much smaller than the desirable products, the process
of the
present invention is an optimal method to "clean up" these reactions.
The invention also relates to purifying and cleaning methods that remove
contaminants quickly and efficiently from a DNA reaction mix. Current
sequencing
machines use electrophoresis through a gel to separate and detect different
lengths of
DNA that have been appropriately labeled. To make these machines provide
results
faster and more accurately, the shapes of the gel separation media have gone
from thick
gels to a gel captured by thin capillaries. A major drawback is the
contaminants in the
DNA being sequenced tend to physically plug the capillary and interfere with
the
accurate detection of the different DNA lengths. One major source of
contaminants in
the DNA sample is the result of by-products of the thermocycling reaction that
generates
the DNA sample. Both regular and dye-labeled nucleotides that are not
incorporated
into the DNA strings during the reaction become contaminants that degrade the
DNA
sequencer. Additionally, ionic components of the reaction reagents remaining
in the
reaction (e.g., salts) also degrade the machines.
CA 02385029 2002-03-13
WO 01/21310 PCT/US00/25851
-9-
In mode of operation, the present invention provides for effective
removal of contaminants from a thermocycling reaction. Once the reaction
mixture is
thermocycled, purification may be achieved by placing the mixture into a
hollow
membrane element, which is in contact with a solution having a lower
concentration of
ionic components. The difference in osmotic pressure across the membrane
forces
contaminants in the product to migrate across the membrane into the aqueous
solution,
effectively removing them from the product.
In another mode of operation, the invention provides an apparatus and
method for purifying DNA molecules produced in host cells.
The invention involves processes including, but is not limited to,
template purification, polymerase chain reaction (PCR), DNA sequencing,
polynucleotide ligation, cloning, ligase chain reaction (LCR), single
nucleotide
extension reaction, exonuclease treatment, and oligonucleotide hybridization
reactions.
Process steps associated with these processes include, for example, the
aspiration,
mixing, incubation, purification, temperature treating, such as heating or
cooling, and
delivery of the biological sample alone or in a biologically compatible
carrier fluid in a
selected manner.
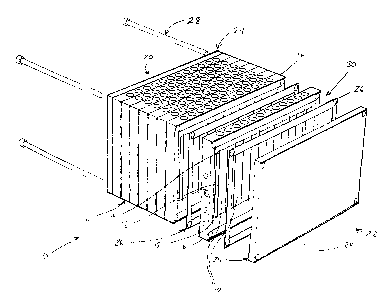
As shown in Figure l, a first embodiment of the present invention
involves a capillary cassette 10 based on a standard microtiter plate
configuration, such
as an 8 x 12 array of elements on 9 mm centers, to allow immediate integration
with
existing laboratory automation devices and capillary electrophoresis (CE)
sequencing
instruments. Optionally, the footprint of this embodiment will be of the same
dimensions as a 96-well plate, but the height may be increased to accommodate
an
appropriate length of capillary tubing. This embodiment enables the optional
use of an
existing 96 channel pipetting device, such as the Robbins Hydra, to perform
the liquid
handling aspects of the process. The capillary cassette 10 of the present
invention may
optionally be formed with any number of channel pipetting devices. Other
devices may
include 384 channels or more.
The capillary cassette 10 of the present invention includes a plurality of
capillaries 12. Each capillary 12 has a first end 14 and a second end 15, each
of which is
securely mounted within a frame 16. Frame 16 may be formed so as to
accommodate 96
capillaries 12, or may optionally be formed to accommodate a subset of the
capillaries
12 within the capillary cassette 10, as shown in Figure 1. Open space 18 is
preferably
CA 02385029 2002-03-13
WO 01/21310 PCT/US00/25851
-10-
provided between capillaries 12, so as to allow fluids, such as liquids or
gasses, to pass
within or through an interior of capillary cassette 10.
The assembled capillary cassette 10 thus defines an interior chamber 30,
with capillaries located therein, and through which air or water can be
introduced and
circulated over the capillaries to achieve thermocycling or dialysis.
According to a preferred practice, the capillaries preferably have internal
volumes that accommodate fluid sizes of less than about 1 microliter. The
methods of
the invention are advantageously practiced with biological samples having
volumes
ranging down to approximately 0.05 ~L, preferably 0.1 ~L to 3 ~.L.
An advantage of employing the novel submicroliter capillaries is that
minimal amounts of expensive sequencing reagents and relatively small volumes
of
biological samples may be used in an automated sample handling format. The
invention
can be used, for example, to perform purification procedures on polymerase
chain
reaction (PCR) products, preparing sequencing ladders, and injecting the
sequencing
ladders into appropriate microtiter plates, or aspirating the biological
products.
The capillary cassette 10 of the present invention is formed with a first
and second end 20, 22. Both the first and second end 20, 22 of the capillary
cassette 10
may be open or closed by an optional end plate 24. The end plate 24 may
further
optionally be provided with ports to facilitate the entry and exit of fluids,
such as gasses
or liquids, passed within an interior chamber 30 of capillary cassette 10. A
sealing
gasket 26 is preferably optionally provided between frame 16 and the optional
end plate
24. An optional sealing gasket 26 is also provided between frames 16, if more
than one
frame 16 is used, as shown in Figure 1.
In a variation of the present invention involving a plurality of frames 16
forming a capillary cassette 10, holes are formed in the frames to accommodate
one or
more fasteners, such as pins 28, that are optionally provided to mount the
plurality of
frames 16 to one another. The pins 28 may also secure optional end plates 24
to the
frames 16. Preferably, four pins 28, each having threads and associated nuts
are used,
spaced along a perimeter of frames 16, as shown in Figure 1. Optionally,
screws, rivets
or other compressive fasteners may be used in combination with or in place of
the pins
28. The pins 28, or their alternative, are preferably formed of stainless
steel.
Use of frames 16 accommodating subsets of the total number of
capillaries 12 within capillary cassette 10 provides the ability to replace a
portion of the
capillaries 12 within the capillary cassette 10 in the event of a capillary
failure.
CA 02385029 2002-03-13
WO 01/21310 PCT/US00/25851
-11-
Therefore, all capillaries 12 within capillary cassette 10 need not be
discarded. Only the
capillaries 12 sharing a frame 16 with the failed capillary are discarded.
Figure 2 illustrates a frame 16 according to a variation of the first
embodiment of the invention. Optionally, a docking port 40 may be provided
according
to the first embodiment of the invention. The docking port 40 may optionally
be
mounted to frames 16 so as to be fluidly connected to the first end 14 and the
second
end 15 of capillary 12. Optionally, a docking port 40 may only be provided to
a single
end of capillary 12, or may be omitted entirely.
As shown in Figures 3 and 4, the docking port 40 includes a guide cap
42, optionally configured with a concave surface facing away from the
capillary 12 so as
to guide a needle 44 to be co-axially aligned with the capillary 12.
Preferably, the
needle 44 will be a blunt syringe needle. The guide cap 42 is optionally
securely
mounted to the frame 16. The guide cap 42 may be press fit within a portion of
the
frame 16. However, an adhesive is preferably used for mounting the guide cap
42 to the
frame 16 within a portion of the frame 16. Optionally, a sealing element 46 is
provided
within docking port 40. The sealing element 46 is preferably a rubber o-ring
gland seal
from Apple Rubber Corp., press fit within a cavity 47 of guide cap 42. The
dimensions
of the sealing element 46 and cavity 47 are formed so as to allow a blunt
needle 44 to be
inserted through the sealing element 46 without damaging the sealing element
46, while
simultaneously providing a fluid-tight seal to the corresponding end of the
capillary 12.
The use of a blunt needle 44 aids in reducing damage to the sealing element
46, and
provides for extensive repetitive use of the docking port 40.
Different solutions (for instance, DNA and Big Dye Terminator Cycle
Sequencing Ready Reaction Mix (DT-mix)) can be aspirated in separate "slugs"
with a
small air gap in between (with minimal cross-contamination). In such a case,
preferably, a docking ports 40 is mounted on each end of each capillary 12.
In preferred embodiments, the capillaries are made of glass, fused silica,
polyimide coated fused silica, or TEFLON~. The frames and end plates are
preferably
fabricated as injection molded parts.
Assembly of individual capillaries in the apparatus of the present
invention may be achieved in a variety of ways. In one embodiment, capillaries
may be
cut to size, assembled into cast grooves, and then secured in place.
Capillaries may be
secured using a waterproof and temperature resistant glue.
A significant advantage of employing multiple capillaries is that the
sample volumes provided by each capillary tube allows the processing of
significantly
CA 02385029 2002-03-13
WO 01/21310 PCT/US00/25851
-12-
smaller sample portions, since relatively small volumes of the overall carrier
fluid
disposed within the capillaries are subject to evaporation. This sample
conservation
advantage significantly reduces the sample volumes necessary to achieve
selected
processing of the sample, while concomitantly affording sample outputs that
have
sequencing ladders with improved signal strength and resolution. By way of
example,
the amount of fluorescently labeled DNA that can be detected on current
sequencing
machines is much lower than the amounts that are typically processed; 0.5-1 ~l
samples
are sufficient.
Figure 5 illustrates a second embodiment of the invention involving a
variation of the construction of the sample handling chamber. The second
embodiment
of the invention uses a flat membrane 61 in place of capillaries. A flat
membrane 61 is
provided along both sides of a frame 62. In Figure 5, a sample handling
chamber 64 is
formed by a cutout of the frame 62 and two flat membranes 61 layers. A first
flat
membrane layer 66 is provided along a forward surface of the frame 62 as shown
in
Figure 5. A second flat membrane layer 68 is mounted along a back side of the
frame
62 as illustrated in Figure 5.
Optionally, a hollow fiber 70 may be mounted within the first or second
embodiment of the invention.
The second embodiment of the invention, as described above, provides
additional durability and economy. By omitting capillaries, there is no glass
to break
during rough handling of the frame 62. Furthermore, by the use of the flat
membrane 61
to form a plurality of sample handling chambers 64, each frame can be quickly
and
easily formed.
According to a third embodiment of the invention, the capillary cassette
10 is used in conjunction with a liquid handling machine 80. Hydra liquid
handling
machines, manufactured by Robbins Scientific, U.S.A., are convenient because
of the
ability to simultaneously, accurately, and coherently aspirate and deliver
selected
volumes from parallel channels. These systems offer ease of integration with
physical
plate-handling systems and PC-based programming systems through an RS232 port.
The Robbins Hydra is also preferred because of the Teflon seals on the syringe
plungers,
availability of 384-channel models, and lower overall cost.
According to the third embodiment of the invention, the liquid handling
machine 80 is provided with needles 44 and optionally a needle alignment frame
82.
The needle alignment frame 82 is preferably configured so as to align the
needles 44
axially with the capillaries 12 of the capillary cassette 10. Optionally,
docking ports 40
CA 02385029 2002-03-13
WO 01/21310 PCT/US00/25851
-13-
are provided at a first end 14 of the capillaries 12 to assist in alignment of
the needles 44
with the capillaries 12.
Optionally, a needle bed 90 is provided below the capillary cassette 10
and is provided with a plurality of needles 92. Each needle 92 has a first end
94 oriented
toward the capillary cassette 10 and a second end 96 oriented away from the
capillary
cassette 10. As shown in Figure 8, the first end 94 of needles 92 is axially
aligned with
the second end 15 of the capillaries 12 so as to be able to be inserted
through docking
port 40 located in fluid contact with the second end 15 of capillary 12. A
second end 96
of the needles 96 may optionally be configured so as to be inserted within a
well 102 of
microtiter plate 100.
The liquid handling machine 80 is preferably configured so as to provide
relative vertical movement between the needles 44, the needle alignment frame
82, the
capillary cassette 10, the needle bed 90 and the microtiter plate 100.
Preferably the
liquid handling machine 80 is provided with a platen surface 84 to support the
microtiter
plate 100.
Figures 9 provides a cross-sectional perspective view of the non-capillary
configuration of the second embodiment of the invention, used with a needle
bed 90 and
microtiter plate 100. Figure 10 provides a further perspective view showing an
exemplary sample handling chamber 64 provided with a hollow fiber 70 and
provided
with guide caps 40. As described above, a first flat membrane layer 66 is
provided
along a forward side of frame 62 and a second flat membrane layer 68 is
provided along
a rear surface of frame 62.
For temperature control and thermocycling, a two-temperature fluid
circulation system with appropriately placed valves may be used to enable a
wide range
of fluid temperatures to be quickly attained. For example, the heating source
40 can be
employed to heat a sample disposed in the capillary 12. Various flow patterns
can be
created, such as front to back, side to side, and general circulation.
Optionally, the thermocycler may optionally use a combination of hot
and cold fluid to change sample temperature. Simple blowers or fluid pumps or
blowing
ambient air and air heated by resistance heaters over the capillaries are
another
alternative to change the temperature. The temperature may be measured and
controlled
by standard Proportional Integral Differential (PID) controllers. The heating
rate may
be increased as desired by using, for example, superheated fluid for the first
part of the
heating cycle, then cooler fluid to avoid excessive overshoot of the
temperature of the
capillaries.
CA 02385029 2002-03-13
WO 01/21310 PCT/US00/25851
-14-
Optical sensors may optionally be employed in connection with the
invention to detect liquid levels at one or more points, and provide open loop
or
feedback control to adjust, if necessary, the sample or fluid level volumes.
The present invention is capable of processing many samples in parallel,
if desired, using standard micro-titer plates as reagent sources. The use of
capillaries in
connection with the present invention is beneficial in that only a small
fraction of the
liquid volume is exposed to the atmosphere, so that evaporation is minimized.
This
promotes the processing of the sample, while concomitantly eliminating or
reducing
sample loss. The capillaries of the system can be used to retrieve, mix and
dispense
fluids by integration with air or liquid-filled volumetric devices, such as
piezoelectric
elements, movable pistons or syringe-type plungers.
Typically, DNA sequencing products are purified to remove excess salt,
nucleotides, primers, and templates from the biological sample. The
illustrated
microfiber 70 can be employed to perform the filtration process upon the DNA,
to
exclude the desired products, while concomitantly allowing undesired
components to
pass therethrough when the processing assembly is exposed to a pressure or
vacuum
condition at a proximal end. The DNA sample is cycled through the microfiber
by the
pressure formed within the system, thereby resulting in relatively small
components
being filtered out of the hollow fibers and hence the sample. The use of a
capillary tube
with one or more microfibers 70 disposed therein, provides for the ability to
perform
equilibrium dialysis upon very small volumes of between about 10 to 0.05
microliters.
A well plate, such as a microtiter plate, can be positioned, so that the
pipettes attached to the top of the cassette can draw the samples from the
microtiter plate
into the capillaries for processing. The needles may then be withdrawn from
the top and
the bottom of the cassette, allowing the gland seals to close, thereby
effectively sealing
the capillaries in the cassette. The cassettes may be thermocycled or dialyzed
in place,
or moved to separate hotels for these operations, as described below.
In another embodiment of the invention, cassettes are hard-mounted to 96
channel pipettors, and a needle bed is hard mounted to the bottom of the
cassette.
During processing of the samples, the top of the capillaries are sealed by
syringes, and
the bottom of the capillaries are sealed by driving the lower syringe tips
against gasket
material.
One variation of the invention involves a capillary cassette with ports to
allow inflow and outflow of dialysate (e.g., water). Preferably the ports are
located at
opposite ends of the cassette.
CA 02385029 2002-03-13
WO 01/21310 PCT/US00/25851
-15-
Figure 14 illustrates one embodiment of a hotel. A hotel may be used
while thermocycling and/or dialysis occurs, to provide a washing function, to
prevent
sample cross-contamination, and/or regenerate chemically cassettes that fail
to perform
adequately. In this embodiment, the system may also recirculate water, or some
other
solution (acid, base, or detergent), through the lumen of the thermocycling or
dialysis
capillaries. In another embodiment, the water or other solution is heated.
The invention provides "hotels" that accept a plurality of cassettes for
parallel processing to process large numbers of samples efficiently during the
dialysis
and thermocycling steps. In one embodiment, a hotel can process up to 15-20
cassettes.
Each housing of the hotel accepts and processes a cassette independently of
the others.
The hotel includes a fixture that accepts an individual cassette, and makes
the
appropriate fluid connections between the cassette and the dialysis,
thermocycling or
wash media. In a preferred embodiment, the end plates of the cassettes
incorporate
specialized fluid connectors that mate with similar fittings in the hotel. The
cassette is
optionally seated on a tray that includes means for grasping the cassette and
holding it
firmly against the housing of the hotel during processing.
In one embodiment, the dialysis hotel recirculates dialysis solution to the
cassettes, and contains a reservoir and pump to perform this function. The
pump
constantly recirculates the solution through all the stations of the hotel.
When a cassette
is mounted, a valve opens to allow a stream of this recirculating dialysate to
pass
through the cassette and back to the reservoir. Figure 15 depicts one
embodiment of a
dialysis hotel according to the invention. A needle bed 390 may optionally be
used to
provide the dialysis solution.
In another embodiment, the thermocycling hotel recirculates hot and cold
fluid, such as air or liquid, through a set of closed conduits. The hot air
source is
maintained at approximately 100 °C, for example, by electric resistance
elements, and
the cool air source may be ambient air. In other embodiments, the cool air
source is a
refrigeration unit. At each hotel housing, there is a proportional mixing
valve that
controls the temperature of the air circulating through the cassette by
selectively mixing
the hot and cold air sources. When a cassette is mounted, a valve may open to
allow the
air mixture to pass through the cassette. The invention also optionally
provides a
temperature sensor in the air-flow stream entering (or leaving) the cassette
to provide
positive feedback to a controller that operates the mixing valve to control
the
temperature and time profile during thermocycling. A control unit in each
station of the
CA 02385029 2002-03-13
WO 01/21310 PCT/US00/25851
- 16-
hotel causes the preset thermocycling protocol to initiate, and to preferably
proceed until
finished. In another embodiment, liquid, such as water, may be used instead of
air as a
heat transfer medium to achieve a uniform temperature profile across all of
the
capillaries within the cassette.
In yet another embodiment, a washing hotel serves to rinse out the inner
surfaces of the capillaries after each use to minimize and/or eliminate cross
contamination of samples, and also to retain the microporosity characteristics
of the
membrane element. In a preferred embodiment, the washing hotel circulates a
wash
fluid, followed by a water rinse whenever a cassette is introduced to a
particular station.
The cassettes may be mounted differently in the washing station than in the
thermocycling hotels to allow the circulating solutions to be directed to the
insides of the
capillaries rather than the outside. The circulation pumps are preferably
capable of
developing pressures in the range of about 15 psi to dislodge deposits from
the capillary
walls. Chemical regeneration may also be performed, by the use of appropriate
regeneration chemicals.
As shown in Figures 11-13, one embodiment of the invention pertains to
a system to prepare cleared lysates for plasmid template preparation or
"template
preparation module." The system is mounted on a Hydra, and includes heating
and
filtration functions to process cells from deep-well plates. The unit is based
on the
transfer Hydra design, but includes a specialized filtration manifold that
utilizes a roll of
filter material instead of mufti-well filter plates. According to a preferred
embodiment,
a deep-well plate containing resuspended cells in lysis buffer is placed on
the system
and the samples are aspirated into a large-volume (100 ~L) thermocycling
cassette. The
samples are heated at 95-100 °C for 1 minute, and the deep-well plate
is removed. The
filtration manifold is brought into place under the cassette, the filter
material firmly
clasped in place by the manifold (effectively sealing off each individual
well), and the
samples are drawn through the filter material into a receiving plate below the
manifold
by vacuum (about 3 min). Alternatively, pressure is applied to the samples to
effect
filtration. The filter material is then disengaged from the manifold,
advanced, and the
manifold and cassette is washed to prepare for the next set of samples (about
1 min).
The filtration apparatus moves laterally with respect to the cassette and
needle bed, and
the roll of filter material moves in and out to permit washing of the
cassette, needles and
manifold. Figure 16 depicts one embodiment of a template preparation module.
CA 02385029 2002-03-13
WO 01/21310 PCT/US00/25851
-17-
An apparatus management device 300 is preferably used to manipulate
the above-noted components during processing.
According to the invention, automated processing may be provided by
commercially available hardware and software. Custom automated systems to
facilitate
key aspects of the sequencing and finishing process have been developed and
are known
to the skilled artisan. Such systems preferably include: 1 ) a robust platform
for silica-
bead based template preparation, quantification, and sample reconfiguration
utilizing a
CRS systems robot, two TECAN liquid handling stations, a fluorometer, a Sagian
96
channel pipettor, and several plate stackers and sealers, 2) a custom platform
for
automated fabrication and spotting of "paper combs" using a Seiko D-Tran robot
and
plate handling system designed for microarray spotting and built for GTC by
Intelligent
Automation Systems, Inc., and 3) a finishing automation system built around a
sophisticated storage and retrieval system (Gira), a Tecan liquid handling
station, a 96
channel Quadra pipettor, two plate stackers and a plate sealer. In addition,
the skilled
artisan may use known databases and software tools to automate the apparatuses
and
methods of the invention.
In a preferred embodiment, the invention provides a method of plasmid
isolation ("Automated Template Preparation" or "ATP") which is based on
standard
alkaline lysis chemistry coupled with reversible capture on silica beads
(Engelstein,
1998). This method meets the design criteria for a filtration-based process
that can
produce templates yielding data of comparable or better quality than Qiagen-
generated
preparations, and the samples are stable upon storage at 4° C for 6
months. The ATP
hardware preferably includes a Tecan Genesis 200 with Robotic Manipulator
(RoMa), a
CRS T475, a Sagian Multipette 96-channel pipettor, Tecan shakers, Scitec
vacuum
manifolds and a drying station.
The automated microplate heat sealer (Marsh BioMedical, NY) has
RS232 capabilities and can function with plates manufactured from several
different
plastics. The material used to seal the plates is aluminum foil backed with a
thin plastic
film, which differs depending on the composition of the microplates to be
sealed. The
sealing process actually welds the seal to each well rim, with temperature and
thickness
of the plastic film controlling the strength of the seal. This permits both
permanent and
removable seals to be used in the process. Alternatively, thinner foils are
also available
which allow the seal to be pierced ("easy pierce seals") by the pipetting
robot to gain
access to samples.
CA 02385029 2002-03-13
WO 01/21310 PCT/US00/25851
-18-
The sample handling device may also be constructed as a series of
independent modules that can be stacked together, or independently attached
temporarily to a liquid handling device to effect the liquid handling steps.
An advantage
of this approach is that a single liquid handling device, which is the most
expensive part
of the system to build, could be more optimally utilized to process samples in
a large
number of dialysis and thermocycling modules. The latter modules can then be
handled
in a similar fashion as plates are handled on a typical integrated automation
system, such
that the independent units would be advantageously sealed automatically when
detached
from the liquid handling device, and that plate-to-plate transfers would be
replaced by
capillary-to-capillary transfers (avoiding contact with the atmosphere).
Visual examination (using a microscope) of 100 nanoliter samples being
heated inside a capillary revealed rapid sample dispersal unless both ends of
the
capillary were sealed (presumably due to rapid outgassing or localized
boiling). In
experiments with larger sample volumes where only one end of the tubing was
effectively sealed by a syringe, the sample slug was observed to rapidly
migrate back
and forth in the tubing during thermocycling. This was assumed to be a result
of the
changing vapor pressure in the closed air space within the capillary tubing.
The
application of slight pressure to the open end of the tubing eliminated the
movement and
allowed successful thermocycling to be achieved in long TEFLON~ capillaries.
Therefore, sealing means, valves, or a pressure control system are a desirable
part of a
robust flow-through capillary thermocycling system.
The invention relates to an integrated, capillary-based sample handling
system for capillary-based aspiration, incubation, purification and delivery
of a
biological sample that is capable of processing many samples in parallel. The
invention
further provides integration of pipetting, mixing, temperature treatment, and
sample
purification that is easy to operate and can be re-used many times.
In another embodiment, the invention provides a capillary cassette
system which allows integration with existing laboratory automation devices
and
sequencing instruments. The cassette includes open frames comprising a
plurality of
openings on the top and the bottom portions of the frame. In a preferred
embodiment,
there are twelve openings on the top and bottom portions of the frame. At each
corner
of the frame, slots are provided for means, e.g., pins for connecting the
frames. In one
embodiment, the frames are separated by sealing gaskets.
In another embodiment of the invention, the capillaries are designed to
prevent leakage, e.g., by sealing, but at the same time to allow penetration,
e.g., by a
CA 02385029 2002-03-13
WO 01/21310 PCT/US00/25851
-19-
syringe. This arrangement allows for the application of positive or negative
pressure.
For example, this arrangement permits aspiration of samples from microtiter
plates into
the capillaries, and subsequent dispensing back into plates for further
processing.
In a further embodiment of the invention, multiple samples are
thermocycled in a single capillary at the same time by the use of air gaps
and,
preferably, sealing elements at each end of the capillary.
In another embodiment of the invention, DNA and sequencing reagents
are metered and mixed in a single capillary.
In yet another embodiment, the invention provides a guide cap located
above the seals at the top and bottom of each capillary tube. This arrangement
serves to
guide a syringe needle into the opening of the capillary.
In still another embodiment of the invention, the cassette includes open
frames to allow for air or water flow through the capillaries for
thermocycling and
dialysis.
In an another embodiment of the invention, cassettes are processed in a
hotel, e.g., an air or water temperature controlled station, for thermocycling
or dialysis,
or as a washing station to regenerate the cassettes.
Although not explicitly discussed herein, it will be appreciated that one
or more fluid regulating elements could be positioned along the capillaries
discussed
herein.
References
Maxam AM, et al. (1977) A new method for sequencing DNA. Proc Natl Acad Sci U
S
A. 74: 560-4.
Bashkin, J., Roach, D., Leong, J., Bartosiewicz, M., Barker, D., Johnston,
R.G. J. (1996)
Capillary Electrophor. 3: 61-68.
Boddy, AV; Idle, JR. (1993) Cancer Surv., 17 (Pharmacokinetics and Cancer
Chemotherapy), 79-104.
Boffa L.C., Carpaneto E.M. & Allfrey V.G., (1995).Proc. Natl. Acad. Sci. USA
92, 1901
Caporaso, N.; Landi, M.T. (1995) Med. Lav., 86, 199-206.
Carrilho, E; Ruiz-Martinez, M., Berka, J., Smirnov, L., Goetzinger, W.,
Miller, A.w.,
CA 02385029 2002-03-13
WO 01/21310 PCT/US00/25851
-20-
Brady, D., Karger, B.L. (1996) Anal. Chem. 68: 3305-3313
Dubiley S., Kirillov E., Lysov Y. & Mirzabekov A., (1997).Nucleic Acid Res.
25, 2259
Carrilho, E., Miller, A., Ruiz Martinez, M.C., Kotler, L., Keisilman, j.,
Karger, B.L.
(1997) B. L. Proc. SPIE 2985A, 4-18.
Egholm M., Buchard O., Christensen L., Behrens C., Freier S.M., Driver D.A.,
Berg R.,
Kim S.K., Norden B., & Nielsen P.E., (1993). Nature 365, 566
Evensen, H. T.; Meldrum, D. R.; Cunningham, D. L. (1998) Rev. Sci. Instrum,
519-526.
Figeys D., Ahmadzedeh H., Arriaga E. & Dovichi N.J., (1996) J. Chromatogr. A
698,
375.
Gingeras, TA.; Mack, D; Chee, MS.; Berno, AJ.; Stryer, L; Ghandour, G; Wang,
C. PCT
Int. Appl., 132 pp. WO 9729212 A1 970814.
Haff L., Atwood J.G., . DiCesareJ, Katz E., Picozza E., Williams J.F. &
Woudenberg T.,
BioTechniques 10, 102 (1991).
Meldrum D., et al., (1998) http://isdl.ee.washington.edu/GNI~/acapella/
Hacia, JG.; Makalowski, W; Edgemon, K; Erdos, M R.; Robbins, CM.; Fodor, SPA.;
Brody, LC.; Collins, FS. (1998) Nat. Genet., 18, 155-158.
Hacia, JG.; Brody, LC.; Chee, MS.; Fodor, SPA.; Collins, FS. (1996) Nat.
Genet., 14,
441-447.
Hunicke-Smith, S.P. PCT Int. Appl., 42 pp. Application: WO 97-US10365 970616.
Hunicke-Smith, S.P. (1997) Ph.D. Dissertation, Stanford Univ., Stanford, CA,
USA.
Witter C.T. & Garling D.J., BioTechniques 10, 76 (1991).
Linder, M W.; Prough, R A.; Valdes, R, Jr. (1997) Clin. Chem., 43, 254-266.
Masson, E; Zamboni, WC. Can. (1997) Clin. Pharmacokinet., 32, 324-343.
Nickerson, D.A., Tobe, V.O., Taylor S.L. (1997) Nucleic Acids Res. 25: 2745-
2751.
Nielsen P.E., Egholm M., Berg R.H., Buchard O., ( 1991 ).Science 254, 1497
Parinov S., Barsky V., Yershov G., Kirillov E., Timofeev E., Belgovskiy A. &
Mirzabekov A., (1996).Nucleic Acid Res. 24, 2998
PerSeptive Biosystem manual.
CA 02385029 2002-03-13
WO 01/21310 PCT/US00/25851
-21 -
Rose D.J., Anal. Chem 65, 3545 (1993).
Ruiz-Martinez, M.C., Salas-Solano O., Carrilho, E., Kotler, L., Karger, B.L.
(1998)
Anal. Chem. 70.
Salas-Solano O., Ruiz-Martinez, M.C., Carrilho, E., Kotler, L., Karger, B.L.
(1998)
Anal. Chem. 70.
Seeger C., Batz H.G. & Orum H., (1997). BioTechniques 23, 512
Smith, D.R., Doucette-Stamm, L.A., Deloughery, C., et al., (1997) J.Bact.179,
7135-55.
Stanford Technology Lab, ( 1998) http://sequence-
www.stanford.edu/group/techdev/therm.htm
Swerdlow, H., Jones, B., Witter, C.T. (1997) Anal. Chem. 69: 848-855
Tan, H., Yeung, E.S. (1997) Analytical Chem. 69: 664-674.
'Tan S.S. & Weis J.H., (1992) PCR Methods and Applications: CSH Laboratory
Press,
137
Wang J., J.Amer. Chem. Soc. 118, 7667 ( 1996).
Wang, K. Gan, L., Boysen C. & Hood, L. (1995).Anal. Biochem. 226, 85
Wang R., Cao W., Cerniglia C.E. & Jonson M.G., The rapid Cyclist, 12 (1995)
Weiler J., Gausepohl H., Hauser N., Jensen O.N., & Hoheisel J.D., (
1997).Nucleic Acid
Res. 25, 2792
incorporation By Reference
All patents, published patent applications and other references disclosed
herein
are hereby expressly incorporated herein in their entireties by reference.
Equivalents
Those skilled in the art will recognize, or will be able to ascertain using no
more
than routine experimentation, many equivalents to the specific embodiments of
the
CA 02385029 2002-03-13
WO 01/21310 PCT/US00/25851
-22-
invention described herein. Such equivalents are intended to be encompassed by
the
following claims.