Note: Descriptions are shown in the official language in which they were submitted.
CA 02385859 2002-03-27
WO 01/25189 . PCT/EPOO/09151
Description
2'-Substituted 1,1'-biphenyl-2-carboxamides, processes for their
preparation, their use as medicament, and pharmaceutical preparations
comprising them
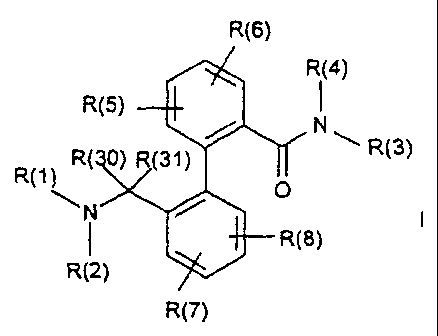
The invention relates to compounds of the formula I,
R(6)
R(4)
R(5) 1
/
R( 0) R(31) ~ R(3)
R(1) O
i /
R(8)
R(2)
R(7)
in which R(1), R(2), R(3), R(4), R(5), R(6), R(7), R(8), R(30) and R(31)
have the meanings indicated below, their preparation and their use, in
particular in pharmaceuticals.
The compounds of the formula I according to the invention were hitherto
unknown. They act on the so-called Kv1.5 potassium channel and inhibit a
potassium current described as "ultra-rapidly activating delayed rectifier" in
the human atrium. The compounds are therefore very particularly suitable
as novel antiarrhythmic active compounds, in particular for the treatment
and prophylaxis of atrial arrhythmias, e.g. atrial fibrillation (AF) or atrial
flutter.
Atrial fibrillation (AF) and atrial flutter are the most frequent persistent
cardiac arrhythmias. The occurrence increases with increasing age and
frequently leads to fatal sequelae, such as, for example, cerebral apoplexy.
AF affects about 1 million Americans annually and leads to more than
80,000 strokes each year in the USA. The presently customary
antiarrhythmics of classes I and III, reduce the reoccurrence rate of AF, but
because of their potential proarrhythmic side effects only have restricted
use. There is therefore a great medical need for the development of better
medicaments for the treatment of atrial arrhythmias (S. Nattel, Am. Heart J.
. CA 02385859 2002-03-27
2
130, 1995, 1094-1106; "Newer developments in the management of atrial
fibrillation").
It was shown that most supraventricular arrhythmias are subject to
so-called "reentry" excitatory waves. Such reentries occur when the cardiac
tissue has a slow conductivity and at the same time very short refractory
periods. The increasing of the myocardial refractory time by prolongation of
the action potential is a recognized mechanism for ending arrhythmias or
preventing their formation (T.J. Colatsky et al., Drug Dev. Res. 19, 1990,
129-140; "Potassium channels as targets for antiarrhythmic drug action").
The length of the action potential is essentially determined by the extent of
repolarizing K+ currents which flow out of the cell via various K+ channels.
Particularly great importance is ascribed here to the so-called "delayed
rectifier" IK, which consists of 3 different components: lKr, IKs and IKur.
Most known class III antiarrhythmics (e.g. dofetilide, E4031 and d-sotalol)
mainly or exclusively block the rapidly activating potassium channel lKr,
which can be detected both in cells of the human ventricle and in the
atrium. However, it has been shown that at low or normal heart rates these
compounds have an increased proarrhythmic risk, arrhythmias which are
described as "Torsades de pointes" being observed in particular (D. M.
Roden, Am. J. Cardiol. 72, 1993, 44B-49B; "Current status of class III
antiarrhythmic drug therapy"). In addition to this high, in some cases fatal,
risk at low frequency, a decrease in the efficacy under the conditions of
tachycardia, in which the action is especially needed, has been found for
the lKr blockers ("negative use-dependence").
While some of these disadvantages can possibly be overcome by blockers
of the slowly activating components (IKs), their efficacy has hitherto not
been confirmed, as no clinical investigations with IKS channel blockers are
known.
The "particularly rapidly" activating and very slowly inactivating component
of the delayed rectifier IKur (= ultra-rapidly activating delayed rectifier),
which corresponds to the Kv1.5 channel, plays a particularly large role in
the repolarization period in the human atrium. In comparison to the
inhibition of lKr or IKS, inhibition of the IKur potassium outward current is
thus a particularly effective method for the prolongation of the atrial action
CA 02385859 2002-03-27
3
potential and thus for the ending or prevention of atrial arrhythmias.
Mathematical models of the human action potential suggest that the
positive effect of a blockade of the lKur, especially under the pathological
conditions of chronic atrial fibrillation, should be particularly pronounced
(M. Courtemanche, R. J. Ramirez, S. Nattei, Cardiovascular Research
1999, 42, 477-489: "Ionic targets for drug therapy and atrial fibrillation-
induced electrical remodeling: insights from a mathematical model").
In contrast to IKr and IKs, which also occur in the human ventricle, the 1Kur
admittedly plays an important role in the human atrium, but not in the
ventricle. For this reason, on inhibition of the IKur current in contrast to
the
blockade of lKr or IKs, the risk of a proarrhythmic action on the ventricle is
excluded from the start (Z. Wang et al., Circ. Res. 73, 1993, 1061-1076:
"Sustained Depolarisation-induced Outward Current in Human Atrial
Myocytes"; G.-R. Li et al, Circ. Res. 78, 1996, 689 - 696: "Evidence for Two
Components of Delayed Rectifier K+ Current in Human Ventricular
Myocytes"; G. J. Amos et al., J. Physiol. 491, 1996, 31-50: "Differences
between outward currents of human atrial and subepicardial ventricular
myocytes").
Antiarrhythmics which act via a selective blockade of the IKur current or
Kv1.5 channel were previously not available, however, on the market. For
numerous pharmaceutical active compounds (e.g. tedisamil, bupivacaine or
sertindole), a blocking action on the Kv1.5 channel was admittedly
described, but the Kv1.5 blockade here in each case represents only a side
effect next to other principal actions of the substances.
WO 98 04 521 claims aminoindans as potassium channel blockers which
block the Kv1.5 channel. The applications WO 98 18 475 and
WO 98 18 476 claim the use of various pyridazinones and phosphine
oxides as antiarrhythmics, which should act via a blockade of the 1Kur=
However, the same compounds were originally also described as immuno-
suppressants (WO 96 25 936). The compounds described in these
mentioned applications are structurally completely different to the
compounds according to the invention of this application.
It has now surprisingly been found that the 2'-substituted 1,1'-biphenyl-
2-carboxamides described here are potent blockers of the human Kv1.5
= CA 02385859 2002-03-27
4
channel. They can therefore be used as novel antiarrhythmics having a
particularly advantageous safety profile. In particular, the compounds are
suitable for the treatment of supraventricular arrhythmias, e.g. atrial
fibrillation or atrial flutter.
The compounds can be employed for the termination of existing atrial
fibrillation or flutters for re-establishing the sinus rhythm (cardioversion).
Moreover, the substances reduce the susceptibility to the formation of new
flutter events (retention of the sinus rhythm, prophylaxis).
The compounds according to the invention were previously unknown.
Some structurally related compounds are described in Helv. Chim. Acta
1994 (70) 70 and references cited there. For the peptide compounds
described there (e.g. compound A), however, no potassium channel-
blocking activity is known. Moreover, compounds of this type should have
too low a metabolic stability for use as antiarrhythmics on account of the
numerous peptide bonds.
o 0
C11< / N-1-1- N N1'1j~ 0
o-~' N / O
I
compound A
A further similar compound (compound B) is mentioned in European Patent
Application EP 0620216. The compound B and all other compounds of this
application carry, in the position of R(3), a specific substituent (e.g.
benzoyl-1,2,3,4-tetrahydroisoquinoline), which is not included in the
compounds according to the invention of this application. The compounds
mentioned in EP 0 620 216 act as vasopressin antagonists and thus have a
completely different biological activity to the blockers of the Kv1.5 channel
described here.
~ ~ N \
compound B
The present invention relates to compounds of the formula I
CA 02385859 2002-03-27
. 5
R(6)
(4)
R(5)
/ R(3)
R(1) R( 0 R(31)
O
N ~
R(8)
R(2)
R(7)
in which:
R(1) is C(O)OR(9), SO2R(10), COR(11), C(O)NR(12)R(13) or
C(S)NR(12)R(13);
R(9) is CXH2x-R(14);
x is 0, 1, 2, 3 or 4,
where x cannot be 0 if R(14) is OR(15) or SO2Me;
R(14) is alkyl having 1, 2, 3, 4, 5 or 6 carbon atoms,
cycloalkyl having 3, 4, 5, 6, 7, 8, 9, 10 or 11 carbon
atoms, CF3, C2F5, C3F7, CH2F, CHF2, OR(15),
SO2Me, phenyl, naphthyl, biphenylyl, furyl, thienyl
or an N-containing heteroaromatic having 1, 2, 3,
4, 5, 6, 7, 8 or 9 carbon atoms,
where phenyl, naphthyl, biphenylyl, furyl,
thienyl and the N-containing hetero-
aromatic are unsbustituted or substituted
by 1, 2 or 3 substituents selected from the
group consisting of F, Cl, Br, I, CF3, OCF3,
NO2, CN, COOMe, CONH2, COMe, NH2,
OH, alkyl having 1, 2, 3 or 4 carbon atoms,
alkoxy having 1, 2, 3= or 4 carbon atoms,
dimethylamino, sulfamoyl, methylsulfonyl
and methylsulfonylamino;
R(15) is alkyl having 1, 2, 3, 4 or 5 carbon
atoms, cycloalkyl having 3, 4, 5 or 6
carbon atoms, CF3 or phenyl which is
unsubstituted or substituted by 1, 2 or 3
substituents selected from the group
= CA 02385859 2002-03-27
= 6
consisting of F, Cl, Br, I, CF3, NO2, CN,
COOMe, CONH2, COMe, NH2, OH, alkyl
having 1, 2, 3 or 4 carbon atoms, alkoxy
having 1, 2, 3 or 4 carbon atoms,
dimethylamino, sulfamoyl, methylsulfonyl
and methylsulfonylamino;
R(10), R(11) and R(12)
independently of one another are defined as R(9);
R(13) is hydrogen, alkyl having 1, 2, 3 or 4 carbon atoms or CF3;
R(2) is hydrogen, alkyl having 1, 2, 3 or 4 carbon atoms or CF3;
R(3) is CyH2y-R(16);
y is 0, 1, 2, 3 or 4,
where y cannot be 0 if R(16) is OR(17) or SO2Me;
R(16) is alkyl having 1, 2, 3, 4, 5 or 6 carbon atoms, cycloalkyl
having 3, 4, 5, 6, 7, 8, 9, 10 or 11 carbon atoms, CF3,
C2F5, C3F7, CH2F, CHF2, OR(17), SO2Me, phenyl,
naphthyl, furyl, thienyl or an N-containing heteroaromatic
having 1, 2, 3, 4, 5, 6, 7, 8 or 9 carbon atoms,
where phenyl, naphthyl, furyl, thienyl and the
N-containing heteroaromatic are unsubstituted or
substituted by 1, 2 or 3 substituents selected from
the group consisting of F, Cl, Br, I, CF3, OCF3,
NO2, CN, COOMe, CONH2, COMe, NH2, OH, alkyl
having 1, 2, 3 or 4 carbon atoms, alkoxy having 1,
2, 3 or 4 carbon atoms, dimethylamino, sulfamoyl,
methylsulfonyl and methylsulfonylamino;
R(17) is hydrogen, alkyl having 1, 2, 3, 4 or 5 carbon
atoms, cycloalkyl having 3, 4, 5 or 6 carbon atoms,
CF3, phenyl or 2-, 3- or 4-pyridyl,
where phenyl or 2-, 3- or 4-pyridyl are
unsubstituted or substituted by 1, 2 or 3
substituents selected from the group
consisting of F, Cl, Br, I, CF3, OCF3, NO2,
CN, COOMe, CONH2, COMe, NH2, OH,
alkyl having 1, 2, 3 or 4 carbon atoms,
alkoxy having 1, 2, 3 or 4 carbon atoms,
dimethylamino, sulfamoyl, methylsulfonyl
and methylsulfonylamino;
= CA 02385859 2002-03-27
7
or
R(3) is CHR(18)R(19);
R(18) is hydrogen or CzH2z-R(16), where R(16) is defined as
indicated above;
z is 0, 1, 2 or 3;
R(19) is COOH, CONH2, CONR(20)R(21), COOR(22), CH2OH;
R(20) is hydrogen, alkyl having 1, 2, 3, 4 or 5 carbon
atoms, CvH2õ-CF3 or CWH2w-phenyl,
where the phenyl ring is unsubstituted or
substituted by 1, 2 or 3 substitutents
selected from the group consisting of F, Cl,
Br, I, CF3, OCF3, NO2, CN, COOMe,
CONH2, COMe, NH2, OH, alkyl having 1,
2, 3 or 4 carbon atoms, alkoxy having 1, 2,
3 or 4 carbon atoms, dimethylamino,
sulfamoyl, methylsulfonyl and
methylsulfonylamino;
v is0, 1,2or3;
w is0, 1,2or3;
R(21) is hydrogen or alkyl having 1, 2, 3, 4 or 5 carbon
atoms;
R(22) is alkyl having 1, 2, 3, 4 or 5 carbon atoms;
R(4) is hydrogen, alkyl having 1, 2, 3, 4, 5 or 6 carbon atoms or CF3;
or
R(3) and R(4)
together are a chain of 4 or 5 methylene groups, of which one
methylene group can be replaced by -0-, -S-, -NH-, -N(methyl)- or
-N(benzyl)-;
R(5), R(6), R(7) and R(8)
independently of one another are hydrogen, F, Cl, Br, I, CF3, N02,
CN, COOMe, CONH2, COMe, NH2, OH, alkyl having 1, 2, 3 or 4
carbon atoms, alkoxy having 1, 2, 3 or 4 carbon atoms, dimethyl-
amino, sulfamoyl, methylsulfonyl or methylsulfonylamino;
R(30) and R(31)
independently of one another are hydrogen or alkyl having 1, 2 or
3 carbon atoms;
or
R(30) and R(31)
= CA 02385859 2002-03-27
8
together are a chain of 2 methylene groups;
and their pharmaceutically acceptable salts.
Preferred compounds of the formula I are those in which:
R(1) is C(O)OR(9), SO2R(10), COR(11) or C(O)NR(12)R(13);
R(9) is CXH2x-R(14);
x is 0, 1, 2, 3 or 4,
where x cannot be 0 if R(14) is OR(15);
R(14) is alkyl having 1, 2, 3 or 4 carbon atoms, cycloalkyl
having 3, 4, 5, 6, 7, 8 or 9 carbon atoms, CF3,
C2F5, OCF3, OR(15), phenyl, furyl, thienyl or an
N-containing heteroaromatic having 1, 2, 3, 4, 5, 6,
7, 8 or 9 carbon atoms,
where phenyl, furyl, thienyl 'and the
N-containing heteroaromatic are
unsubstituted or substituted by 1, 2 or 3
substituents selected from the group
consisting of F, Cl, Br, CF3, NO2, CN,
COOMe, CONH2, COMe, NH2, OH, alkyl
having 1, 2, 3 or 4 carbon atoms, alkoxy
having 1, 2, 3 or 4 carbon atoms, dimethyl-
amino, sulfamoyl, methylsulfonyl and
methylsulfonylamino;
R(15) is alkyl having 1, 2, 3, 4 or 5 carbon atoms,
cycloalkyl having 3, 4, 5 or 6 carbon
atoms, CF3 or phenyl,
which is unsubstituted or substitu-
ted by 1, 2 or 3 substituents
selected from the group consisting
of F, Cl, Br, CF3, NO2, CN,
COOMe, CONH2, COMe, OH,
alkyl having 1, 2, 3 or 4 carbon
atoms, alkoxy having 1, 2, 3 or 4
carbon atoms, dimethylamino,
sulfamoyl, methylsulfonyl and
methylsulfonylamino;
R(10), R(11) and R(12)
independently of one another are defined as R(9);
, CA 02385859 2002-03-27
. 9
R(13) is hydrogen, alkyl having 1, 2, 3 or 4 carbon atoms or CF3;
R(2) is hydrogen, alkyl having 1, 2, 3 or 4 carbon atoms or CF3;
R(3) is CyH2y-R(16);
y is0, 1,2,3or4,
where y cannot be 0 if R(16) is OR(17);
R(16) is alkyl having 1, 2, 3 or 4 carbon atoms, cycloalkyl having
3, 4, 5, 6, 7, 8 or 9 carbon atoms, CF3, C2F5, OR(17),
phenyl, furyl, thienyl or an N-containing heteroaromatic
having 1, 2, 3, 4, 5, 6, 7, 8 or 9 carbon atoms,
where phenyl, furyl, thienyl and the N-containing
heteroaromatic are unsubstituted or substituted by
1, 2 or 3 substituents selected from the group
consisting of F, CI, Br, CF3, OCF3, NO2, CN,
COOMe, CONH2, COMe, NH2, OH, alkyl having 1,
2, 3 or 4 carbon atoms, alkoxy having 1, 2, 3 or 4
carbon atoms, dimethylamino, sulfamoyl,
methylsulfonyl and methylsulfonylamino;
R(17) is alkyl having 1, 2, 3, 4 or 5 carbon atoms,
cycloalkyl having 3, 4, 5 or 6 carbon atoms, CF3,
phenyl or 2-, 3-, or 4-pyridyl,
where phenyl or 2-, 3-, or 4-pyridyl are
unsubstituted or substituted by 1, 2 or 3
substituents selected from the group
consisting of F, Cl, Br, CF3, OCF3, NO2,
CN, COOMe, CONH2, COMe, OH, alkyl
having 1, 2, 3 or 4 carbon atoms, alkoxy
having 1, 2, 3 or 4 carbon atoms,
dimethylamino, sulfamoyl, methylsulfonyl
and methylsulfonylamino;
or
R(3) is CHR(18)R(19);
R(18) is hydrogen or CZH2z-R(16), where R(16) is defined as
indicated above;
z is 0, 1, 2 or 3;
R(19) is CONH2, CONR(20)R(21), COOR(22), CH2OH;
R(20) is hydrogen, alkyl having 1, 2, 3, 4 or 5 carbon
atoms, CvH2v-CF3 or CWH2w-phenyl,
CA 02385859 2002-03-27
where the phenyl ring is unsubstituted or
substituted by 1, 2 or 3 substituents
selected from the group consisting of F, Cl,
Br, CF3, OCF3, N02, CN, COOMe,
5 CONH2, COMe, NH2, OH, alkyl having 1,
2, 3 or 4 carbon atoms, alkoxy having 1, 2,
3 or 4 carbon atoms, dimethylamino,
sulfamoyl, methylsulfonyl and
methylsulfonylamino;
10 v is 0, 1, 2 or 3;
w is 0, 1, 2 or 3;
R(21) is hydrogen or alkyl having 1, 2, 3, 4 or 5 carbon
atoms;
R(22) is alkyl having 1, 2, 3, 4 or 5 carbon atoms;
R(4) is hydrogen, alkyl having 1, 2, 3, 4, 5 or 6 carbon atoms or CF3;
R(5), R(6), R(7) and R(8)
independently of one another are hydrogen, F, Cl, Br, CF3, NO2,
CN, COOMe, CONH2, COMe, NH2, OH, alkyl having 1, 2, 3 or 4
carbon atoms, alkoxy having 1, 2, 3 or 4 carbon atoms, dimethyl-
amino, sulfamoyl, methylsulfonyl or methylsulfonylamino;
R(30) and R(31)
independently of one another are hydrogen or alkyl having 1,2 or 3
carbon atoms;
or
R(30) and R(31)
together are a chain of 2 methylene groups;
and their pharmaceutically acceptable salts.
Particularly preferred compounds of the formula I are those in which:
R(1) is C(O)OR(9), SO2R(10), COR(11) or C(O)NR(12)R(13);
R(9) is CXH2x-R(14);
x is 0, 1, 2, 3 or 4,
where x cannot be 0 if R(14) is OR(15);
R(14) is cycloalkyl having 3, 4, 5, 6, 7, 8 or 9 carbon
atoms, CF3, OR(15), phenyl, furyl, thienyl or an
N-containing heteroaromatic having 3, 4 or 5
carbon atoms,
CA 02385859 2002-03-27
- 11
where phenyl, furyl, thienyl and the
N-containing heteroaromatic are
unsubstituted or substituted by 1 or 2
substituents selected from the group
consisting of F, Cl, Br, CF3, OCF3, CN,
COOMe, CONH2, COMe, OH, alkyl having
1, 2 or 3 carbon atoms, alkoxy having 1 or
2 carbon atoms, dimethylamino, sulfamoyl,
methylsulfonyl and methylsulfonylamino;
R(15) is alkyl having 1 or 2 carbon atoms, cyclo-
alkyl having 3, 4, 5 or 6 carbon atoms, CF3
or phenyl,
which is unsubstituted or substitu-
ted by 1 or 2 substituents selected
from the group consisting of F, Cl,
Br, CF3, CN, COOMe, CONH2,
COMe, OH, alkyl having 1, 2 or 3
carbon atoms, alkoxy having 1 or 2
carbon atoms, dimethylamino,
sulfamoyl, methylsulfonyl and
methylsulfonylamino;
R(10), R(11) and R(12)
independently of one another are defined as R(9);
R(13) is hydrogen;
R(2) is hydrogen or alkyl having 1, 2 or 3 carbon atoms;
R(3) is CHR(18)R(19);
R(18) is hydrogen or CZH2z-R(16);
z is0, 1,2or3;
R(19) is CONH2, CONR(20)R(21), COOR(22) or CH2OH;
R(20) is hydrogen, alkyl having 1, 2, 3, 4 or 5 carbon
atoms, CvH2i-CF3 or CwH2w-phenyl,
where the phenyl ring is unsubstituted or
substituted by 1, 2 or 3 substituents selec-
ted from the group consisting of F, Cl, Br,
CF3, OCF3, CN, COOMe, CONH2, COMe,
OH, alkyl having 1, 2 or 3 carbon atoms,
alkoxy having 1 or 2 carbon atoms,
. CA 02385859 2002-03-27
12
dimethylamino, sulfamoyl, methylsulfonyl
and methylsulfonylamino;
v is 0, 1, 2 or 3;
w is 0, 1, 2 or 3;
R(21) is hydrogen or alkyl having 1, 2, 3, 4 or 5 carbon
atoms;
R(22) is alkyl having 1, 2, 3, 4 or 5 carbon atoms;
R(16) is alkyl having 1, 2 or 3 carbon atoms, cycloalkyl
having 3, 4, 5, 6, 7, 8 or 9 carbon atoms, CF3,
OR(17), phenyl, furyl, thienyl or an N-containing
heteroaromatic having 3, 4 or 5 carbon atoms,
where phenyl, furyl, thienyl and the
N-containing heteroaromatic are
unsubstituted or substituted by 1 or 2
substitutents selected from the group
consisting of F, Cl, Br, CF3, OCF3, CN,
COOMe, CONH2, COMe, NH2, OH, alkyl
having 1, 2 or 3 carbon atoms, alkoxy -
having 1 or 2 carbon atoms,
dimethylamino, sulfamoyl, methylsulfonyl
and methylsulfonylamino;
R(17) is alkyl having 1, 2, 3 or 4 carbon atoms,
cycloalkyl having 3, -4, 5 or 6 carbon atoms, CF3,
phenyl or 2-, 3- or 4-pyridyl,
where phenyl or 2-, 3- or 4-pyridyl are
unsubstituted or substituted by 1, 2 or 3
substituents selected from the group
consisting of F, Cl, Br, CF3, OCF3, CN,
COOMe, CONH2, COMe, OH, alkyl having
1, 2, 3 or 4 carbon atoms, alkoxy having 1,
2, 3 or 4 carbon atpms, dimethylamino,
sulfamoyl, methylsulfonyl and methyl-
sulfonylamino;
R(4) is hydrogen or alkyl having 1 or 2 carbon atoms;
R(5), R(6), R(7) and R(8)
independently of one another are hydrogen, F, Cl, Br, CF3, CN,
COOMe, CONH2, COMe, NH2, OH, alkyl having 1, 2 or 3 carbon
' CA 02385859 2002-03-27
' 13
atoms, alkoxy having 1 or 2 carbon atoms, dimethylamino,
sulfamoyl, methylsulfonyl or methylsulfonylamino;
R(30) and R(31)
independently of one another are hydrogen or methyl
or
R(30) and R(31)
together are a chain of 2 methylene groups;
and their pharmaceutically acceptable salts.
Particularly preferred compounds of the formula I are also those in which:
R(1) is C(O)OR(9), SO2R(10), COR(11) or C(O)NR(12)R(13);
R(9) is CxH2x-R(14);
x is 0, 1, 2, 3 or 4,
where x cannot be 0 if R(14) is OR(15);
R(14) is alkyl having 1, 2, 3 or 4 carbon atoms, cycloalkyl
having 3, 4, 5, 6, 7, 8 or 9 carbon atoms, CF3,
OR(15), phenyl, furyl, thienyl or an N-containing
heteroaromatic having 3, 4 or 5 carbon atoms,
where phenyl, furyl, thienyl and the
N-containing heteroaromatic are
unsubstituted or substituted by 1 or 2
substituents selected from the group
consisting of F, Cl, Br, CF3, OCF3, CN,
COOMe, CONH2, COMe, OH, alkyl having
1, 2 or 3 carbon atoms, alkoxy having 1 or
2 carbon atoms, dimethylamino, sulfamoyl,
methylsulfonyl and methylsulfonylamino;
R(15) is alkyl having 1 or 2 carbon atoms,
cycloalkyl having 3, 4, 5 or 6 carbon atoms,
CF3 or phenyl,
which is unsubstituted or substitu-
ted by 1 or 2 substituents selected
from the group consisting of F, Cl,
Br, CF3, CN, COOMe, CONH2,
COMe, OH, alkyl having 1, 2 or 3
carbon atoms, alkoxy having 1 or 2
carbon atoms, dimethylamino,
CA 02385859 2002-03-27
14
sulfamoyl, methylsulfonyl and
methylsulfonylamino;
R(10), R(11) and R(12)
independently of one another are defined as R(9);
R(13) is hydrogen;
R(2) is hydrogen or alkyl having 1, 2 or 3 carbon atoms;
R(3) is CyH2y-R(16);
y is 0, 1, 2, 3 or 4,
where y cannot be 0 if R(16) is OR(17);
R(16) is alkyl having 1, 2 or 3 carbon atoms, cycloalkyl having 3,
4, 5, 6, 7, 8 or 9 carbon atoms, CF3, OR(17), phenyl, furyl,
thienyl or an N-containing heteroaromatic having 3, 4 or 5
carbon atoms,
where phenyl, furyl, thienyl and the N-containing
heteroaromatic are unsubstituted or substituted by
1 or 2 substituents selected from the group
consisting of F, CI, Br, CF3, OCF3, CN, COOMe,
CONH2, COMe, NH2, OH, alkyl having 1, 2 or 3
carbon atoms, alkoxy having 1 or 2 carbon atoms,
dimethylamino, sulfamoyl, methylsulfonyl and
methylsulfonylamino;
R(17) is alkyl having 1, 2, 3, 4 or 5 carbon atoms,
cycloalkyl having 3, 4, 5 or 6 carbon atoms, CF3,
phenyl or 2-, 3- or 4-pyridyl,
where phenyl or 2-, 3- or 4-pyridyl are
unsubstituted or substituted by 1, 2 or 3
substituents selected from the group
consisting of F, CI, Br, CF3, OCF3, NO2,
CN, COOMe, CONH2, COMe, OH, alkyl
having 1, 2, 3 or 4 carbon atoms, alkoxy
having 1, 2, 3 or 4 carbon atoms,
dimethylamino, sulfamoyl, methylsulfonyl
and methylsulfonyiamino;
R(4) is hydrogen or alkyl having 1 or 2 carbon atoms;
R(5), R(6), R(7) and R(8)
independently of one another are hydrogen, F, CI, Br, CF3, CN,
COOMe, CONH2, COMe, NH2, OH, alkyl having 1, 2 or 3 carbon
CA 02385859 2002-03-27
atoms, alkoxy having 1 or 2 carbon atoms, dimethylamino,
sulfamoyl, methylsulfonyl or methylsulfonylamino;
R(30) and R(31)
independently of one another are hydrogen or methyl
5 or
R(30) and R(31)
together are a chain of 2 methylene groups;
and their pharmaceutically acceptable salts.
10 Very particularly preferred compounds of the formula I are those in which:
R(1) is C(O)OR(9), SO2R(10), COR(11) orC(O)NR(12)R(13);
R(9) is CXH2x-R(14);
x is 0, 1, 2 or 3;
R(14) is alkyl having 1, 2, 3 or 4 carbon atoms, cycloalkyl
15 having 3, 4, 5, 6, 7, 8 or 9 carbon atoms, CF3,
phenyl or pyridyl,
where phenyl and pyridyl are unsubstituted
or substituted by 1 or 2 substituents selected from the group consisting of F,
Cl,
CF3, OCF3, OH, alkyl having 1, 2 or 3
carbon atoms and alkoxy having 1 or 2
carbon atoms;
R(10), R(11) and R(12)
independently of one another are defined as R(9);
R(13) is hydrogen;
R(2) is hydrogen;
R(3) is CyH2y-R(16);
y is 0, 1or 2;
R(16) is alkyl having 1, 2 or 3 carbon atoms, cycloalkyl having 5
or 6 carbon atoms, CF3, phenyl or pyridyl,
where phenyl and pyridyl are unsubstituted or
substituted by 1 or 2 substituents selected from the
group consisting of F, Cl, CF3, OCF3, OH, alkyl
having 1, 2 or 3 carbon atoms and alkoxy having 1
or 2 carbon atoms;
R(4) is hydrogen;
R(5), R(6), R(7) and R(8)
= CA 02385859 2002-03-27
- 16
independently of one another are hydrogen, F, CF3, CN, COOMe,
CONH2, NH2, OH, alkyl having 1, 2 or 3 carbon atoms or alkoxy
having 1 or 2 carbon atoms;
R(30) and R(31)
independently of one another are hydrogen or methyl
or
R(30) and R(31)
together are a chain of 2 methylene groups;
and their pharmaceutically acceptable salts.
Especially preferred compounds of the formula I are those in which:
R(1) is C(O)OR(9) or COR(11);
R(9) is CxH2x-R(14);
x is 0, 1, 2 or 3;
R(14) is cycloalkyl having 5 or 6 carbon atoms or phenyl,
where phenyl is unsubstituted or
substituted by 1 or 2 substituents selected
from the group consisting of F, Cl, CF3,
OCF3, alkyl having 1, 2 or 3 carbon atoms
and alkoxy having 1 or 2 carbon atoms;
R(11) is defined as R(9);
R(2) is hydrogen;
R(3) is CyH2y-R(16);
y is 0, 1 or 2;
R(16) is alkyl having 1, 2 or 3 carbon atoms, cycloalkyl having 5
or 6 carbon atoms, CF3, phenyl or pyridyl
where, phenyl and pyridyl are unsubstituted or
substituted by 1 or 2 substituents selected from the
group consisting of F, Cl, CF3, OCF3, alkyl having
1, 2 or 3 carbon atoms and alkoxy having 1 or 2
carbon atoms;
R(4) is hydrogen;
R(5), R(6), R(7) and R(8)
independently of one another are hydrogen, F, CF3, alkyl having 1,
2 or 3 carbon atoms or alkoxy having 1 or 2 carbon atoms;
R(30) and R(31)
are hydrogen;
and their pharmaceutically acceptable salts.
CA 02385859 2002-03-27
17
Alkyl radicals and alkylene radicals can be straight-chain or branched. This
also applies to the alkylene radicals of the formulae CXH2X, CyH2y, CzH2z,
CvH2v and CWH2w. Alkyl radicals and alkylene radicals can also be
straight-chain or branched if they are substituted or are contained in other
radicals, e.g. in an alkoxy radical or in a fluorinated alkyl radical.
Examples
of alkyl radicals are methyl, ethyl, n-propyl, isopropyl, n-butyl, isobutyl,
sec-butyl, tert-butyl, n-pentyl, isopentyl, neopentyl, n-hexyl, 3,3-dimethyl-
butyl, heptyl, octyl, nonyl, decyl, undecyl, dodecyl, tridecyl, tetradecyl,
pentadecyl, hexadecyl, heptadecyl, octadecyl, nonadecyl, eicosyl. The
divalent radicals derived from these radicals, e.g. methylene, 1,1-ethylene,
1,2-ethylene, 1,1-propylene, .1,2-propylene, 2,2-propylene, 1,3-propylene,
1,1-butylene, 1,4-butylene, 1,5-pentylene, 2,2-dimethyl-1,3-propylene,
1,6-hexylene, etc., are examples of alkylene radicals.
Cycloalkyl radicals can likewise be branched. Examples of cycloalkyl
radicals having 3 to 11 carbon atoms are cyclopropyl, cyclobutyl, 1-methyl-
cyclopropyl, 2-methylcyclopropyl, cyclopentyl, 2-methylcyclobutyl, 3-methyl-
cyclobutyl, cyclopentyl, cyclohexyl, 2-methylcyclohexyl, 3-methylcyclohexyl,
4-methylcyclohexyl, menthyl, cycloheptyl, cyclooctyl etc.
N-containing heteroaromatics having 1, 2, 3, 4, 5, 6, 7, 8 or 9 carbon atoms
are considered in particular as 1-, 2- or 3-pyrrolyl, 1-, 2-, 4- or 5-
imidazolyl,
1-, 3-, 4- or 5-pyrazolyl, 1,2,3-triazol-l-, -4- or -5-yl, 1,2,4-triazol-l-, -
3- or
-5-yl, 1- or 5-tetrazolyl, 2-, 4- or 5-oxazolyl, 3-, 4- or 5-isoxazolyl,
1,2,3-oxadiazol-4- or -5-yl, 1,2,4-oxadiazol-3- or -5-y1, 1,3,4-oxadiazol-2-yl
or -5-yi, 2-, 4- or 5-thiazolyl, 3-, 4- or 5-isothiazolyl, 1,3,4-thiadiazol-2-
or
-5-yl, 1,2,4-thiadiazol-3- or -5-yl, 1,2,3-thiadiazol-4- or -5-y1, 2-, 3- or
4-pyridyl, 2-, 4-, 5- or 6-pyrimidinyl, 3- or 4-pyridazinyl, pyrazinyl, 1-, 2-
, 3-,
4-, 5-, 6- or 7-indolyl, 1-, 2-, 4- or 5-benzimidazolyl, 1-, 3-, 4-, 5-, 6- or
7-indazolyl, 2-, 3-, 4-, 5-, 6-, 7- or 8-quinolyl, 1-, 3-, 4-, 5-, 6-, 7- or
8-isoquinolyl, 2-, 4-, 5-, 6-, 7- or 8-quinazolinyl, 3-, 4-, 5-, 6-, 7- or
8-cinnolinyl, 2-, 3-, 5-, 6-, 7- or 8-quinoxalinyl, 1-, 4-, 5-, 6-, 7- or
8-phthalazinyl. The corresponding N-oxides of these compounds are
furthermore included, i.e., for example, 1-oxy-2-, -3- or -4-pyridyl.
The N-containing heterocycles pyrrolyl, imidazolyl, quinolyl, pyrazolyl,
pyridyl, pyrazinyl, pyrimidinyl and pyridazinyl are particularly preferred.
CA 02385859 2002-03-27
18
Pyridyl is either 2-, 3- or 4-pyridyl. Thienyl is either 2- or 3-thienyl.
Furyl is
either 2- or 3-furyl.
Monosubstituted phenyl radicals can be substituted in the 2, 3 or 4 position,
disubstituted in the 2,3, 2,4, 2,5, 2,6, 3,4 or 3,5 position, or
trisubstituted in
the 2,3,4, 2,3,5, 2,3,6, 2,4,5, 2,4,6 or 3,4,5 position. The same
correspondingly also applies analogously to the N-containing hetero-
aromatics, the thiophene or the furyl radical.
If a radical is di- or trisubstituted, the substituents can be identical or
different.
If R(3) and R(4) are together a chain of 4 or 5 methylene groups, of which
one methylene group can be replaced by -0-, -S-, -NH- etc., then these
radicals together with the nitrogen atom of the formula I form a 5- or
6-membered nitrogen heterocycle, such as, for example, pyrrolidine,
piperidine, morpholine, thiomorpholine etc.
If the compounds of the formula I contain one or more.acidic or basic
groups or one or more basic heterocycles, the invention also includes the
corresponding physiologically or toxicologically tolerable salts, in
particular
the pharmaceutically utilizable salts. Thus the compounds of the formula I
which carry acidic groups, e.g. one or more COOH groups, for example as
alkali metal salts, preferably sodium or potassium salts, or as alkaline earth
metal salts, e.g. calcium or magnesium salts, or as ammonium salts, e.g.
as salts with ammonia or organic amines or amino acids, can be used.
Compounds of the formula I which carry one or more basic, i.e.
protonatable, groups or contain one or more basic heterocyclic rings can
also be used in the form of their physiologically tolerable acid addition
salts
with inorganic or organic acids, for example as hydrochlorides, phosphates,
sulfates, methanesulfonates, acetates, lactates, maleates, fumarates,
malates, gluconates etc. If the compounds of the formula I simultaneously
contain acidic and basic groups in the molecule, the invention also includes
internal salts, so-called betaines, in addition to the salt forms described.
Salts can be obtained from the compounds of the formula I according to
customary processes, for example by combination with an acid or base in a
solvent or dispersant or alternatively from other salts by anion exchange.
- CA 02385859 2002-03-27
19
If they are appropriately substituted, the compounds of the formula I can be
present in stereoisomeric forms. If the compounds of the formufa I contain
one or more asymmetric centers, these independently of one another can
have the S configuration or the R configuration. The invention includes all
possible stereoisomers, e.g. enantiomers or diastereomers, and mixtures of
two or more stereoisomeric forms, e.g. enantiomers and/or diastereomers,
in any desired ratios. The invention thus includes enantiomers, for
example, in enantiomerically pure form, both as levo- and as dextrorotatory
antipodes, and also in the form of mixtures of the two enantiomers in
different ratios or in the form of racemates. If desired, the individual
stereoisomers can be prepared by resolution of a mixture according to
customary methods or, for example, by stereoselective synthesis. If mobile
hydrogen atoms are present, the present invention also comprises all
tautomeric forms of the compounds of the formula I.
The compounds of the formula I can be prepared by different chemical
processes, which are likewise included in the present invention. Some
typical routes are outlined in the reaction sequences designated below as
schemes 1, 2, 3 and 4. The radicals R(1) to R(8) used here are in each
case defined as indicated above, if not stated otherwise below.
Thus a compound of the formula I according to scheme 1 is obtained, for
example, starting from diphenic anhydride derivatives of the formula II as
precursors which are commercially obtainable or known from the literature.
Reduction of the compounds II using sodium borohydride followed by
reaction with potassium phthalimide as described in Tetrahedron 45 (1989)
1365-1376 yields the biphenylcarboxylic acids of the formula IV. By
coupling with amines of the formula HNR(3)R(4) followed by hydrazinolysis
of the phthalimide, the aminomethyl compounds of the formula VI are
obtained, from which, by reaction with suitable derivatives of the formula
R(1)-X, the compounds of the formula I according to the invention are
obtained in which R(2) is hydrogen and R(1), R(3), R(4), R(5), R(6), R(7)
and R(8) have the meanings indicated above. Subsequent alkylation using
suitable alkylating agents of the formula R(2)Y, in which Y is a nucleofugic
leaving group, e.g. Cl, Br or I, yields the corresponding compounds of the
formula I in which R(2) is alkyl having 1 to 4 carbon atoms.
Alternatively, the biphenylcarboxylic acids of the formula IV can also be
CA 02385859 2002-03-27
720
converted by hydrazinolysis to the aminocarboxylic acids of the formula VII
which are then converted by reaction of the amino group with compounds
of the formulae R(1)-X and R(2)-Y followed by amidation of the carboxylic
acids with amines of the formula HNR(3)R(4) to give compounds of the
formula I according to the invention (scheme 2).
In some cases, it can be useful to first prepare compounds of the formula Ia
(scheme 3) in which R(9) is an easily removable radical, such as, for
example, tert-butyl or benzyl, by one of the previously mentioned methods.
After removal of the corresponding protective group, e.g. with trifluoroacetic
acid for the Boc group or by catalytic hydrogenation for the benzyioxy-
carbonyl radical, the compounds of the formula IX are obtained, which can
then in turn be converted into other compounds of the formula I according
to the invention by reaction with compounds of the formula R(1)-X.
Another possibility for the preparation of compounds according to the
invention consists in the palladium-catalyzed coupling of a phenyl bromide
or iodide of the formula X with a phenylboronic acid of the formula XI
(Suzuki coupling; scheme 4), which can be carried out, for example, in the
presence of Pd[(PPh)3]4 as a catalyst, sodium carbonate as a base and
1,2-dimethoxyethane as a solvent. If R(9) is an easily removable radical,
such as, for example, tert-butyl or benzyl, the compounds of the formula lb
can then be converted into other compounds of the formula I according to
the invention as described above and in scheme 3.
The required boronic acids of the formula XI can be obtained from the
compounds of the formula XII, in which Z is hydrogen, bromine or iodine,
by ortholithiation or metal-halogen exchange followed by reaction with
trimethyl borate.
The abovementioned reactions of the compounds of the formulae VI, VII
and IX with compounds of the formula R(1)-X correspond to the known
conversion of an amine to a carboxamide, sulfonamide, carbamate, urea or
thiourea derivative. The radical X here is a suitable nucleofugic leaving
group, such as, for example, F, Cl, Br, imidazole, 0-succinimide etc.
For the preparation of compounds of the formula I or VIII in which R(1) is
C(O)OR(9), i.e. carbamates, compounds of the formula R(1)-X, for
CA 02385859 2002-03-27
21
example, are used in which X is chlorine or 0-succinimide, i.e.
chloroformates or succinimidocarbonates.
For the preparation of compounds of the formula I or VIII in which R(1) is
SO2R(10), i.e. sulfonamides, as a rule compounds of the formula R(1)-X
are used in which X is chlorine, i.e. sulfonyl chlorides.
For the preparation of compounds of the formula I or VIII in which R(1) is
COR(11), i.e. carboxamides, compounds of the formula R(1)-X, for
example, are used in which X is chlorine, imidazole or acetoxy, i.e.
carbonyl chlorides, carboxylic acid imidazolides or mixed anhydrides.
However, the free acids of the formula R(1)-OH can also be used in the
presence of suitable condensing agents such as carbodiimides or uronium
salts such as TOTU.
For the preparation of compounds of the formula I or VIII in which R(1) is
CONR(12)R(13) or C(S)NR(12)R(13), i.e. ureas or thioureas, instead of the
compounds of the formula R(1)-X it is also possible to use compounds of
the formula R(12)N(=C=O), or R(12)N(=C=S), i.e. isocyanates or
isothiocyanates.
The abovementioned reactions of the compounds of the formulae IV or VIII
with amines of the formula HNR(3)R(4) correspond to the known
conversion of a carboxylic acid to a carboxamide. Numerous methods have
been described in the literature for carrying out these reactions. They can
be carried out particularly advantageously by activation of the carboxylic
acid, e.g. with dicyclohexylcarbodiimide (DCC), if appropriate with addition
of hydroxybenzotriazole (HOBT) or dimethylaminopyridine (DMAP), or with
O-[(cyano(ethoxycarbonyl)methylene)amino]-1,1,3,3-tetramethyluronium
tetrafluoroborate (TOTU). However, reactive acid derivatives can also be
synthesized first according to known methods, e.g. acid chlorides by
reaction of the carboxylic acids of the formula IV or VIII with inorganic acid
halides, such as, for example, SOCI2, or acid imidazolides by reaction with
carbonyidiimidazole, which are then reacted with the amines of the formula
HNR(3)R(4), if appropriate with the addition of an auxiliary base.
In all procedures, it may be appropriate to temporarily protect functional
groups in the molecule in certain reaction steps. Such protective group
CA 02385859 2002-03-27
22
techniques are familiar to the person skilled in the art. The choice of a
protective group for groups under consideration and the processes for their
introduction and removal are described in the literature and can if
necessary be adapted to the individual case without difficulties.
= CA 02385859 2002-03-27
23
Scheme 1:
R(6) R(6)
R(5) R(5) /
O NaBH4 O
/
O
tR(8) O
R(8)
)
R(7) II R(7) III
R(6)
0 R(5)
OH
N-K 0
0 N IV
/ \ R(a)
0
R(7)
R(6)
R(4)
R(5)
R(3)
HNR(3)R(4)
R(g) v
O
R(7) (6)
I(4)
R(5)
R(3)
N2H4 0
-- - - H2N
R(8) VI
R(7)
R(6)
I (4)
R(5)
R(3)
1. R(1)-X R(l) 0
2. R(2)-Y R($)
R(2) I
R(7)
CA 02385859 2002-03-27
24
Scheme 2:
R(6) R(6)
R(5) OH R(5) OH
0
N O IV N2H4 H2N O VII 311- R(a) R(8)
O
R(7) R(7)
R(6)
OH
R(1)-X R(5)
R(N Vill
R(2)-Y R(8)
R(2)
R(7)
R(6)
R(4)
R(5)
HNR(3)R(4) R(1) 0 R(3)
R($)
R(2)
R(7)
CA 02385859 2002-03-27
25 -.
Scheme 3:
R(6) R(6)
R(4) R(4)
N
O R(5 R 3 R(5 I
R(9) O ( ) \ R(3)
O i R(g) H i O
R(8)
R(2) R(7) la R(2) IX
R(7)
R(6)
R(4)
R(5 N
1-~ R(3)
R(1~
R(1)-X N O
--~ I R(8).
R(2) I
R(7)
Scheme 4:
R(6)
R(4)
R(5) I R(6)
/ N. R(3) R(4)
R(s) I
[Br,i] O ~
x 0 ~ 31) N R(3)
Pd (0) R(9)~O~N O
O R(30) +
(31) B(OH)2
Base ~ R(s)
R(9j~0 N R(2)
I R(8) R(7)
R(2)
R(m
XI lb
1. tert. BuLi
2. B(OMe)3
0 R(30) (31) Z
R(9)~
O N
I R(8)
R(2)
Rm xil
The compounds of the formula I according to the invention and their
physiologically tolerable salts can thus be used in animals, preferably in
mammals, and in particular in humans, as pharmaceuticals on their own, in
mixtures with one another or in the form of pharmaceutical preparations.
The present invention also relates to the compounds of the formula I and
- CA 02385859 2002-03-27
26
their physiologically tolerable salts for use as pharmaceuticals, their use in
the therapy and prophylaxis of the syndromes mentioned and their use for
the production of medicaments therefor and of medicaments having K+
channel-blocking action. The present inverition furthermore relates to
pharmaceutical preparations which, as active constituent, contain an
efficacious dose of at least one compound of the formula I and/or of a
physiologically tolerable salt thereof in addition to customary,
pharmaceutically innocuous vehicles and excipients. The pharmaceutical
preparations normally contain 0.1 to 90% by weight of the compounds of
the formula I and/or their physiologically tolerable salts. The pharmaceutical
preparations can be prepared in a manner known per se. To this end, the
compounds of the formula I and/or their physiologically tolerable salts are
brought, together with one or more solid or liquid pharmaceutical vehicles
and/or excipients and, if desired, in combination with other pharmaceutical
active compounds, into a suitable administration form or dose form, which
can then be used as a pharmaceutical in human medicine or veterinary
medicine.
Pharmaceuticals which contain compounds of the formula I according to
the invention and/or their physiologically tolerable salts can be
administered orally, parenterally, e.g. intravenously, rectally, by inhalation
or topically, the preferred administration being dependent on the individual
case, e.g. the particular form of the disease to be treated.
The person skilled in the art is familiar on the basis of his/her expert
knowledge with which excipients are suitable for the desired pharma-
ceutical formulation. In addition to solvents, gel-forming agents, suppository
bases, tablet excipients and other active compound carriers, it is possible
to use, for example, antioxidants, dispersants, emulsifiers, antifoams, flavor
corrigents, preservatives, solubilizers, agents for achieving a depot effect,
buffer substances or colorants.
For the obtainment of an advantageous therapeutic action, the compounds
of the formula I can also be combined with other pharmaceutical active
compounds. Thus in the treatment of cardiovascular diseases advanta-
geous combinations with substances having cardiovascular activity are
possible. Possible combination partners of this type which are advanta-
geous for cardiovascular disorders are, for example, other antiarrhythmics,
CA 02385859 2002-03-27
27
i.e. class I, class II or class III antiarrhythmics, such as, for example, IKS
or
lKr channel blockers, e.g. dofetilide, or furthermore hypotensive substances
such as ACE inhibitors (for example enalapril, captopril, ramipril),
angiotensin antagonists, K+ channel activators, and also alpha- and beta-
receptor blockers, but also sympathomimetic compounds and compounds
having adrenergic activity, as well as Na+/H+ exchange inhibitors, calcium
channel antagonists, phosphodiesterase inhibitors and other substances
having a positive inotropic action, such as, for example, digitalis
glycosides,
or diuretics.
For an oral administration form, the active compounds are mixed with the
additives suitable therefor, such as vehicles, stabilizers or inert diluents,
and brought by the customary methods into the suitable administration
forms, such as tablets, coated tablets, hard gelatin capsules, aqueous,
alcoholic or oily solutions. Inert carriers which can be used are, for
example, gum arabic, magnesia, magnesium carbonate, potassium
phosphate, lactose, glucose or starch, in particular cornstarch. In this case,
preparation can be carried out both as dry and as moist granules. Suitable
oily vehicles or solvents are, for example, vegetable or animal oils, such as
sunflower oil or cod-liver oil. Suitable solvents for aqueous or alcoholic
solutions are, for example, water, ethanol or sugar solutions or mixtures
thereof. Further excipients, also for other administration forms, are, for
example, polyethylene glycols and polypropylene glycols.
For subcutaneous or intravenous administration, the active compounds, if
desired with the substances customary therefor such as solubilizers,
emulsifiers or further excipients, are brought into solution, suspension or
emulsion. The compounds of the formula I and their physiologically
tolerable salts can also be Iyophilized and the lyophilizates obtained used,
for example, for the production of injection or infusion preparations.
Suitable solvents are, for example, water, physiological saline solution or
alcohols, e.g. ethanol, propanol, glycerol, and in addition also sugar
solutions such as glucose or mannitol solutions, or alternatively mixtures of
the various solvents mentioned.
Suitable pharmaceutical formulations for administration in the form of
aerosols or sprays are, for example, solutions, suspensions or emulsions of
the active compounds of the formula I or their physiologically tolerable salts
CA 02385859 2002-03-27
28
in a pharmaceutically acceptable solvent, such as, in particular, ethanol or
water, or a mixture of such solvents. If required, the formulation can also
contain other pharmaceutical excipients such as surfactants, emulsifiers
and stabilizers, and a propellant. Such a preparation contains the active
compound customarily in a concentration of approximately 0.1 to 10, in
particular of approximately 0.3 to 3, percent by weight.
The dose of the active compound of the formula I or of the physiologically
tolerable salts thereof to be administered depends on the individual case
and is to be adjusted to the conditions of the individual case as customary
for an optimum action. Thus it depends, of course, on the frequency of
administration and on the potency and duration of action of the compounds
in each case employed for therapy or prophylaxis, but also on the nature
and severity of the illness to be treated and on sex, age, weight and
individual responsiveness of the human or animal to be treated and on
whether treatment is acute or prophylactic. Customarily, the daily dose of a
compound of the formula I on administration to a patient weighing
approximately 75 kg is 0.001 mg/kg of body weight to 100 mg/kg of body
weight, preferably 0.01 mg/kg of body weight to 20 mg/kg of body weight.
The dose can be administered in the form of an individual dose or can be
divided into two or more, e.g. 2, 3 or 4, individual doses. In particular in
the
treatment of acute cases of cardiac arrhythmias, for example in an
intensive care unit, parenteral administration by injection or infusion, e.g.
by
an intravenous continuous infusion, can also be advantageous.
Experimental section
List of abbreviations
CDI Carbonyldiimidazole
DIC Diisopropylcarbodiimide
DMAP 4-Dimethylaminopyridine
DMF N,N-Dimethylformamide
EDAC N-Ethyl-N'-(3-dimethylaminopropyl)carbodiimide hydrochloride
EA Ethyl acetate
m.p. Melting point (if not stated otherwise the melting points of the
unpurified crude products are stated; the melting points of the
respective pure substances can definitely be markedly higher)
= CA 02385859 2002-03-27
29
HOBT 1-Hydroxy-1 H-benzotriazole
in vac. In vacuo
S Solvent
Me Methyl
RT Room temperature
THF Tetrahydrofuran
TOTU O-[(Cyano(ethoxycarbonyl)methylene)amino]-1,1,3,3-tetramethyl-
uronium tetrafluoroborate
Precursor 1: 7H-Dibenzo[c,e]oxepin-5-one
9.0 g (0.24 moI) of sodium borohydride were added in portions at 5 C in
the course of 10 min to a suspension of 50.0 g (0.22 mol) of diphenic
anhydride in 220 ml of DMF. After stirring at RT for 1 h, the reaction
mixture was poured onto 220 ml of 6 M hydrochloric acid, diluted with
750 ml of water and stirred for 2 h. The deposited precipitate was filtered
off with suction and 35.0 g of 7H-dibenzo[c,e]oxepin-5-one were obtained;
m.p. 131 C.
Precursor 2: 2'-Phthaloimidomethylbiphenyl-2-carboxylic acid
A mixture of 35 g(0.17 moI) of 7H-dibenzo[c,e]oxepin-5-one and 30.8 g
(0.17 moI) of potassium phthalimide in 330 ml of DMF was heated at
170 C for 18 h. After cooling, the' deposited precipitate was filtered off
with
suction and introduced into 160 ml of glacial acetic acid. After stirring for
1 h, the mixture was diluted with 650 ml of ice water and the deposited
product was filtered off with suction and dried in vacuo. 44.8 g of
2'-phthalimidomethylbiphenyl-2-carboxylic acid were obtained; m.p.
198 C.
Precursor 3: 2'-Aminomethylbiphenyl-2-carboxylic acid
A suspension of 10.0 g (28 mmol) of 2'-phthaloimidomethylbiphenyl-
2-carboxylic acid in 450 ml of methanol was treated with 20 ml of
hydrazine hydrate and heated at 40 C for 1.5 h. The reaction mixture was
concentrated and the residue was taken up in 250 ml of methylene
chloride. After filtering off undissolved 2,3-dihydrophthalazine-1,4-dione,
the mother liquor was concentrated and 4.8 g of 2'-aminomethylbiphenyl-
2-carboxylic acid were obtained.
General procedure for the synthesis of mixed succinimidocarbonates from
CA 02385859 2002-03-27
'. 30
alcohols (precursors 4 a - 4 k)
5.0 g (19.5 mmol) of disuccinimidocarbonate are added in portions at 0 C
to a solution of 19.5 mmol of the appropriate alcohol and 1.2 g (9.8 mmol)
of DMAP in 30 ml of methylene chloride and 30 ml of acetonitrile. After
stirring at RT for 2.5 to 10 h, 25 ml of water are added and the organic
phase is washed a further 2 times with water. After drying and
concentration, the corresponding succinimidocarbonates are obtained,
usually as crystalline solids.
Precursor 4 a:
According to the general procedure, 3.2 g of 4-fluorobenzyl N-succinimido-
carbonate were obtained; m. p. 89 C (ether).
Precursor 4 b:
From 11.7 mmol of 4-trifluoromethylbenzyl alcohol, corresponding to the
general procedure 2.3 g of 4-trifluoromethylbenzyl N-succinimido-
carbonate were obtained; m.p. 102 C (ether).
Precursor 4 c:
From 10.5 mmol of a-methyl-4-(trifluoromethyl)benzyl alcohol, correspond-
ing to the general procedure 1.6 g of a-methyl-4-(trifluoromethyl)benzyl
N-succinimidocarbonate were obtained; m.p. 115 C (ether).
Precursor 4 d:
From 19.5 mmol of 4,4,4-trifluorobutanol, corresponding to the general
procedure 4.0 g of 4,4,4-trifluorobutyl N-succinimidocarbonate were
obtained; m.p. 72 C (ether).
Precursor 4 e:
From 26.3 mmol of a-methyl-3-(trifluoromethyl)benzyl alcohol, correspond-
ing to the general procedure 5.1 g of a-methyl-3-(trifluoromethyl)benzyi
N-succinimidocarbonate were obtained; m.p. 77 C (ether).
Precursor 4 f:
From 31.6 mmol of a-methyl-2,6-difluorobenzyl alcohol, corresponding to
the general procedure 1.6 g of a-methyl-2,6-difluorobenzyl N-succinimido-
carbonate were obtained; m.p. 108 C (ether).
CA 02385859 2002-03-27
31
Precursor 4 g:
From 25 mmol of a-methyl-2-(trifluoromethyl)benzyl alcohol, correspond-
ing to the general procedure 3.5 g of a-methyl-2-(trifluoromethyl)benzyl
N-succinimidocarbonate were obtained.
Precursor 4 h:
From 25 mmol of (S)-1-phenylethanol, corresponding to the general
procedure 3.5 g of (S)-a-methylbenzyl N-succinimidocarbonate were
obtained.
Precursor 4 i:
From 25 mmol of (R)-1-phenylethanol, corresponding to the general
procedure 3.5 g of (R)-a-methylbenzyl N-succinimidocarbonate were
obtained.
Precursor 4 j:
From 25 mmol of a-methyl-4-fluorobenzyl alcohol, corresponding to the
general procedure 4.3 g of a-methyl-4-fluorobenzyl N-succinimido-
carbonate were obtained.
Precursor 4 k:
From 9.8 mmol of (S)-1-phenyl-1-butanol, corresponding to the general
procedure 1.7 g of (S)-a-propylbenzyl N-succinimidocarbonate were
obtained.
Precursor 5 a: 2'-Aminomethylbiphenyl-2-carboxylic acid phenethylamide
From 2'-phthaloimidomethylbiphenyl-2-carboxylic acid (precursor 2), after
activation with CDI and reaction with phenethylamine, 2'-phthaloimido-
methylbiphenyl-2-carboxylic acid phenethylamide was obtained; m.p.
156 C.
5.0 g (10.9 mmol) of the product were dissolved in 200 ml of methanol and
treated with 5 ml of hydrazine hydrate. After stirring at 40 C for 1 h, the
reaction mixture was concentrated and the residue was taken up in
methylene chloride. After filtering off the 2,3-dihydrophthalazine-1,4-dione
formed, the mother liquor was concentrated and the residue was purified
by flash chromatography using methylene chloride/methanol 20:1. 3 g of
2'-aminomethylbiphenyl-2-carboxylic acid phenethylamide were obtained.
CA 02385859 2002-03-27
= 32
Precursor 5 b: 2'-Aminomethylbiphenyl-2-carboxylic acid benzylamide
From 2'-phthaloimidomethylbiphenyl-2-carboxylic acid (precursor 2), after
conversion into the acid chloride using thionyl chloride and reaction with
benzylamine, 2'-phthaloimidomethylbiphenyl-2-carboxylicacid benzylamide
was obtained. 1.2 g (2.7 mmol) of the product were dissolved in 55 ml of
methanol and treated with 1.35 ml of hydrazine hydrate. After stirring at
40 C for 1 h, the reaction mixture was concentrated and the residue was
taken up in methylene chloride. After filtering off the 2,3-dihydro-
phthalazine-1,4-dione formed, the mother liquor was concentrated and the
residue was purified by flash chromatography using methylene
chloride/methanol 30:1. 0.49 g of 2'-aminomethylbiphenyl-2-carboxylic
acid benzylamide was obtained.
Precursor 5 c: 2'-Aminomethylbiphenyl-2-carboxylic acid isopentylamide
From 3 g(8.4 mmol) of 2'-phthaloimidomethylbiphenyl-2-carboxylic acid
(precursor 2), by reaction with isopentylamine in the presence of HOBT
and DIC, 3.2 g of 2'-phthaloimidomethylbiphenyl-2-carboxylic acid
isopentylamide were obtained; m.p. 169 C. The product was dissolved in
100 ml of methanol and treated with 5 ml of hydrazine hydrate. After
stirring at 40 C for 1 h, the cooled reaction mixture was filtered. The
filtrate
was concentrated and the residue was taken up in methylene chloride.
After washing with water, drying and concentrating, 1.8 g of 2'-amino-
methylbiphenyl-2-carboxylic acid isopentylamide were obtained.
Precursor 5 d: 2'-Aminomethylbiphenyl-2-carboxylic acid 2-(2-pyridyl)ethyl-
amide
From 10 g (28 mmol) of 2'-phthaloimidomethylbiphenyl-2-carboxylic acid
(precursor 2), by reaction with 2-(2-pyridyl)ethylamine in the presence of
HOBT and DIC, 13 g of 2'-phthaloimidomethylbiphenyl-2-carboxyiic acid
2-(2-pyridyl)ethylamide were obtained; m.p. 155 C. The product was
suspended in 300 ml of methanol and treated with, 20 ml of hydrazine
hydrate. After stirring at 40 C for 1 h, the cooled reaction mixture was
filtered. The filtrate was concentrated and the residue was taken up in EA.
The product was extracted into the aqueous phase 2 times using 2 M
hydrochloric acid. The aqueous phase was then rendered alkaline with
potassium carbonate and extracted 2 times with EA. After washing with
water, drying and concentrating, 7.3 g of 2'-aminomethylbiphenyl-
2-carboxylic acid 2-(2-pyridyl)ethylamide were obtained.
= CA 02385859 2002-03-27
33
Precursor 6: 2'-(Benzyloxycarbonylaminomethyl)biphenyl-2-carboxylic acid
500 mg (2 mmol) of benzyl N-succinimidocarbonate dissolved in 2.5 ml of
dioxane were added dropwise at 0 C to a solution of 455 mg (2 mmol) of
2'-aminomethylbiphenyl-2-carboxylic acid (precursor 3) and 336 mg
(4 mmol) of sodium hydrogencarbonate in 5 ml of dioxane and 5 ml of
water. After stirring at RT for 4 h, the mixture was concentrated in vacuo,
diluted with water, acidified and extracted with ethyl acetate. 590 mg of
2'-(benzyloxycarbonylaminomethyl)biphenyl-2-carboxylic acid were
obtained.
Precursor 7: 2'-(tert-Butoxycarbonylaminomethyl)biphenyl-2-carboxylic
acid
65 ml of 1 M sodium hydroxide solution were added to a solution of 12.0 g
(53 mmol) of 2'-aminomethylbiphenyl-2-carboxylic acid (precursor 3) in
130 ml of 1,4-dioxane and 65 ml of water and, after complete dissolution,
12.6 g (58 mmol) of di-tert-butyl dicarbonate were added. After. stirring at
RT for 2 h, the mixture was concentrated in vacuo, diluted with-water and
extracted 2 times with methylene chloride. The aqueous phase was
acidified with 1 M potassium hydrogensulfate solution and. extracted with
ethyl acetate. After extensive concentration, addition of n-heptane and
allowing to stand overnight, the product precipitated and 7.6 g of 2'-(tert-
butoxycarbonylaminomethyl)biphenyl-2-carboxylic acid were obtained;
m.p. 136 C.
General procedure for the removal of the Boc protective group:
The n-Boc-protected aminomethylbiphenyl derivative (1 g to 10 ml of
solution) was added to a solution of trifluoroacetic acid in dichloromethane
(30% strength). The mixture was stirred at room temperature for
minutes and the solvent was then removed in vacuo on a rotary
30 evaporator. The residue was taken up in ethyl acetate and washed with
saturated sodium hydrogencarbonate solution. The organic phase was
dried over magnesium sulfate, the solvent was removed in vacuo and the
corresponding 2'-aminomethylbiphenyl-2-carboxamides were obtained.
Precursor 8 a: 2'-Aminomethylbiphenyl-2-carboxylic acid (2,4-difluoro-
benzyl)amide
The compound was obtained from the Boc-protected compound
(Example 8 c) according to the general procedure. Alternatively, the
CA 02385859 2002-03-27
34
compound can also be isolated directly as the trifluoroacetate and reacted
further.
Further precursors 8:
The corresponding amines were analogously liberated from the
Boc-protected compounds of Examples 8 d - 8 o and 10 a - 10 o.
General procedure for the reaction of aminomethylbiphenyis with
succinimidocarbonates to give carbamates (Examples 1 a to 1 u)
0.45 mmol of the respective succinimidocarbonate dissolved in 2 ml of
dioxane is slowly added dropwise to a solution of 0.45 mmol of the
respective 2'-aminomethylbiphenyl and 38 mg (0.45 mmol) of sodium
hydrogencarbonate in 2 ml of dioxane and 2 ml of water. The mixture is
stirred at RT for 2 to 12 h, concentrated, diluted with water and extracted
with EA, and the organic phase is washed with water. After drying and
concentration, the corresponding carbamates are obtained.
Example 1 a: 2'-(4-Trifluoromethylbenzyloxycarbonylaminomethyl)bi-
phenyl-2-carboxylic acid phenethylamide
O / N \
I-z O 'k N O
F3C I / \ I .
From 0.45 mmol of 2'-aminomethylbiphenyl-2-carboxylic acid phenethyl-
amide and 4-trifluoromethylbenzyl N succinimidocarbonate (precursor 4b),
according to the general working procedure 226 mg of 2'-(4-trifluoro-
methylbenzyloxycarbonylaminomethyl)biphenyl-2-carboxylic acid phen-
ethylamide were obtained. MS (ES+): m/e = 533 (M+1).
Example 1 b: 2'-(Benzyloxycarbonylaminomethyl)biphenyl-2-carboxylic
acid phenethylamide
O / N \
)~ N O
O
\
CA 02385859 2002-03-27
From 0.3 mmol of 2'-aminomethylbiphenyl-2-carboxylic acid phenethyl-
amide and benzyl N-succinimidocarbonate, according to the general
working procedure 66 mg of 2'-(benzyloxycarbonylaminomethyl)biphenyl-
2-carboxylic acid phenethylamide were obtained as an oil. MS (ES+):
5 m/e = 456 (M+1).
Example 1 c: 2'-(Methylsulfonylethyloxycarbonylaminomethyl)biphenyl-
2-carboxylic acid phenethylamide
O N \
O II
~
/
S~~~OJ~N O
o
From 0.45 mmol of 2'-aminomethylbiphenyl-2-carboxylic acid phenethyl-
amide (precursor 5 a) and methylsulfonylethyl N-succinimidocarbonate,
according to the general working procedure 164 mg of 2'-(methyl-
sulfonylethyloxycarbonylaminomethyl)biphenyl-2-carboxylic acid phen-
ethylamide were obtained as an oil. MS (ES+): m/e = 481 (M+1).
Example 1 d: 2'-(4-Trifluoromethylbenzyloxycarbonylaminomethyl)biphenyl-
2-carboxylic acid 2-(2-pyridyl)ethylamide
I\
O / N N
\ ~ N O
O
~
FC /
3
From 0.3 mmol of 2'-aminomethylbiphenyl-2-carboxylic acid 2-(2-pyridyl)-
ethylamide (precursor 5 d) and 4-trifluoromethylbenzyl N-succinimido-
carbonate (precursor 4 b), according to the general working procedure
170 mg of 2'-(4-trifluoromethylbenzyloxycarbonylaminomethyl)biphenyl-
2-carboxylic acid 2-(2-pyridyl)ethylamide were obtained. MS (ES+):
m/e = 534 (M+1).
Example 1 e: 2'-(4-Fluorobenzyloxycarbonylaminomethyl)biphenyl-2-
carboxylic acid 2-(2-pyridyl)ethylamide
CA 02385859 2002-03-27
36
O N N
~ O I /
O N
F i / \ I
From 0.3 mmol of 2'-aminomethylbiphenyl-2-carboxylic acid 2-(2-pyridyl)-
ethylamide (precursor 5 d) and 4-fluorobenzyl N-succinimidocarbonate
(precursor 4 a), according to the general working procedure 150 mg of
2'-(4-fluorobenzyloxycarbonylaminomethyl)biphenyl-2-carboxylic acid
2-(2-pyridyl)ethylamide were obtained. MS (ES+): m/e = 484 (M+1).
Example 1 f: ( )-2'-(a-Methyl-4-(trifluoromethyl)benzyloxycarbonylamino-
methyl)biphenyl-2-carboxylic acid 2-(2-pyridyl)ethylamide
O / N N
O
I \ O N
F3C / \
From 0.3 mmol of 2'-aminomethylbiphenyl-2-carboxylic acid 2-(2-pyridyl)-
ethylamide (precursor 5 d) and a-methyl-4-(trifluoromethyl)benzyl
N-succinimidocarbonate (precursor 4 c), according to the general working
procedure 170 mg of 2'-(a-methyl-4-(trifluoromethyl)benzyloxycarbonyl-
aminomethyl)biphenyl-2-carboxylic acid 2-(2-pyridyl)ethylamide were
obtained as a racemate. MS (ES+): m/e = 548 (M+1).
Example 1 g: (S)-2'-(a-Methyl-4-(trifluoromethyl)benzyloxycarbonylamino-
methyl)biphenyl-2-carboxylic acid 2-(2-pyridyl)ethylamide
I \ .
O N N
~ O
JOI,~ O N F3C
The S enantiomer was obtained from the corresponding racemate
(Example 1 f) by preparative HPLC on a Chiralpak AD 250x4.6 column
= CA 02385859 2002-03-27
= 37
using n-hexane/ethanoVisopropanol (10:1:1, 0.3% each of trifluoroacetic
acid/diethylamine) as solvent.
Example 1 h: (R)-2'-(a-Methyl-4-(trifluoromethyl)benzyloxycarbonylamino-
methyl)biphenyl-2-carboxylic acid 2-(2-pyridyl)ethylamide
\
O N N
~ O
\ O N
F3C I ~
The R enantiomer was obtained from the corresponding racemate
(Example 1 f) by preparative HPLC on a Chiralpak AD 250x4.6 column
using n-hexane/ethanoVisopropanol (10:1:1, 0.3% each of trifluoroacetic
acid/diethylamine) as solvent.
Example 1 i: 2'-(4,4,4-Trifluorobutyloxycarbonylaminomethyl)biphenyl-2-
carboxylic acid 2-(2-pyridyl)ethylamide
O N N
~~ O
F3C~ O~ N (
\
From 0.3 mmol of 2'-aminomethylbiphenyl-2-carboxylic acid 2-(2-pyridyl)-
ethylamide (precursor 5 d) and 4,4,4-trifluorobutyl N-succinimidocarbonate
(precursor 4 d), according to the general working procedure 140 mg of 2'
(4,4,4-trifluorobutyloxycarbonylaminomethyl)biphenyl-2-carboxylic acid
2-(2-pyridyl)ethylamide were obtained. MS (ES+): m/e = 486 (M+1).
Example 1 j: (S)-2'-(a-Methylbenzyloxycarbonylaminomethyl)biphenyi-2-
carboxylic acid 2-(2-pyridyl)-ethylamide
\
O I N N
O
~
O N
~ / \
= CA 02385859 2002-03-27
38
From 0.3 mmol of 2'-aminomethylbiphenyl-2-carboxylic acid 2-(2-pyridyl)-
ethylamide (precursor 5 d) and (S)-a-methylbenzyl N-succinimido-
carbonate (precursor 4 h), according to the general working procedure
60 mg of (S)-2'-((x-methylbenzyloxycarbonylaminomethyl)biphenyl-2-
carboxylic acid 2-(2-pyridyl)ethylamide were obtained. MS (ES+): m/e =
480 (M+1).
Example 1 k: (R)-2'-(a-Methyibenzyloxycarbonylaminomethyl)biphenyl-
2-carboxylic acid 2-(2-pyridyl)ethylamide
N N
O ~ o y i
N
From 0.3 mmol of 2'-aminomethylbiphenyl-2-carboxylic acid 2-(2-pyridyl)-
ethylamide (precursor 5 d) and (R)-a-methylbenzyl N-succinimido-
carbonate (precursor 4 i), according to the general working procedure
60 mg of (R)-2'-(a-methylbenzyloxycarbonylaminomethyl)biphenyl-
2-carboxylic acid 2-(2-pyridyl)ethylamide were obtained. MS (ES+): m/e =
480 (M+1).
Examples 1 I-1 u
The following compounds were obtained from the respective precursors
according to the general working procedure and analogously to
Examples 1 a-1 k:
Example Pre- Structure MS (ES+):
No. cursors m/e =
1 1 5 d+ 548
4 g
&O1N0 \ ~
CA 02385859 2002-03-27
= 39
1 m 5 d+ 548
4 e o N N
F3c ~ O
1 \ O N
1 n 5 d+ 516
4 f F 0 N N
o'it- N 0 F
lo 8 a + I\ F/ I F501
4i 0 N
0'k N
I / \ I
1p 8a+ F F 569
4 c
0 "
( \ O~" / ~ O
F3C / \
1 q 8 a+ `I F F ~ F 529
"~'~/
4 k 0
p'k N / 0
~ / \ I
1r 5d+ 508
4k J o `/ N N~
0N
1s 8a+ 505
4 a 0 (m.p.
oxrv
F \ ~ \ 104 C)
1 t 8 a+ F F 507
4 d F q N (m.p.
F-~OJ~N 111 C)
CA 02385859 2002-03-27
1 u \ I N I~ 452
N
0 R N
General procedure for the reaction of aminomethylbiphenyls with
chioroformic acid esters to give carbamates (Examples 2 a to 2 m):
0.32 mmol of the respective chloroformic acid ester dissolved in 1 ml of
5 methylene chloride is slowly added dropwise at 5 C to a solution of
0.3 mmol of the respective 2'-aminomethylbiphenyl and 37 mg
(0.36 mmol) of triethylamine in 6 ml of methylene chloride. The mixture is
stirred at RT overnight, poured onto water and the organic phase is
washed once more with water. After concentration, the residue is purified
10 by flash chromatography.
Example 2 a: 2'-(Butoxycarbonylaminomethyl)biphenyl-2-carboxylic acid
2-(2-pyridyl)ethylamide
N N
0
Jl N 0 15
From 0.3 mmol of 2'-aminomethylbiphenyl-2-carboxylic acid 2-(2-pyridyl)-
ethylamide (precursor 5 d) and butyl chloroformate, according to the
general working procedure 69 mg of 2'-(butoxycarbonylaminomethyl)-
20 biphenyl-2-carboxylic acid 2-(2-pyridyl)ethylamide were obtained as an oil.
MS (ES+): m/e = 432 (M+1).
Example 2 b: 2'-(Benzyloxycarbonylam i nom ethyl) b ip henyl-2-carboxyl ic
acid (3-methylbutyl)amide
I N
/
C7o O
~ {
From 0.27 mmol of 2'-aminomethylbiphenyl-2-carboxylic acid (3-methyl-
CA 02385859 2002-03-27
= 41
butyl)amide (precursor 5 c) and benzyl chloroformate, according to the
general working procedure 44 mg of 2'-(benzyloxycarbonylaminomethyl)-
biphenyl-2-carboxylic acid (3-methylbutyl)amide were obtained;
m.p. 112 C. MS (ES+): m/e = 431 (M+1).
Example 2 c: 2'-(Benzyloxycarbonylaminomethyl)biphenyl-2-carboxylic
acid 2-(2-pyridyl)ethylamide
N N
I*-~
O
O'k N O
From 0.24 mmol of 2'-aminomethylbiphenyl-2-carboxylic acid
2-(2-pyridyl)-ethylamide (precursor 5 d) and benzyl chloroformate,
according to the general working procedure 59 mg of
2'-(benzyloxycarbonylaminomethyl)-biphenyl-2-carboxylic acid
2-(2-pyridyl)ethylamide were obtained; m.p.140 C (heptane/EA). MS
(ES+): m/e = 466 (M+1).
Example 2 d: 2'-(Butoxycarbonylaminomethyl)biphenyl-2-carboxylic acid
(3-methylbutyl)amide
O
IN /
zz~-, 1
From 0.34 mmol of 2'-aminomethylbiphenyl-2-carboxylic acid (3-methyl-
but)4)amide (precursor 5 c) and butyl chloroformate, according to the
general working procedure 66 mg of 2'-(butoxyca'rbonylaminomethyl)-
biphenyi-2-carboxyfic acid (3-methylbutyl)amide were obtained as a resin.
MS (ES+): m/e = 397 (M+1).
Example 2 e: 2'-(2-Chlorobenzyloxycarbonylaminomethyl)biphenyl-
2-carboxylic acid (3-methylbutyl)amide
CA 02385859 2002-03-27
42
cl O
O/\N O
From 0.34 mmol of 2'-aminomethylbiphenyl-2-carboxylic acid
(3-methylbutyl)amide (precursor 5 c) and 2-chlorobenzyl chioroformate,
according to the general working procedure 75 mg of 2'-(2-chlorobenzyl-
oxycarbonylaminomethyl)biphenyl-2-carboxylic acid (3-methylbutyl)amide
were obtained as a resin. MS (ES+): m/e = 465 (M+1).
Example 2 f: 2'-(Methoxycarbonylaminomethyl)biphenyl-2-carboxylic acid
(3-methylbutyl)amide
O
/ O +
O N ~
From 0.34 mmol of 2'-aminomethylbiphenyl-2-carboxylic acid (3-methyl-
butyl)amide (precursor 5 c) and methyl chloroformate, according to the
general working procedure followed by extraction with EA and purification
by flash chromatography 29 mg of 2'-(methoxycarbonylaminomethyl)-
biphenyl-2-carboxylic acid (3-methylbutyl)amide were obtained as a resin.
MS (ES+): m/e = 355 (M+1).
Example 2 g: 2'-(Phenoxycarbonylaminomethyl)biphenyl-2-carboxylic acid
(3-methylbutyl)amide
~ \ .
~
O O
N I
From 0.34 mmol of 2'-aminomethylbiphenyl-2-carboxylic acid (3-methyl-
butyl)amide (precursor 5 c) and phenyl chioroformate, according to the
general working procedure followed by extraction with EA and purification
- - - - --------- ----- - ---------
= CA 02385859 2002-03-27
=, 43
by flash chromatography 55 mg of 2'-(phenoxycarbonylaminomethyl)-
biphenyl-2-carboxylic acid (3-methylbutyl)amide were obtained as a resin.
MS (ES+): m/e = 417 (M+1).
Example 2 h: 2'-(4-Carbomethoxyphenoxycarbonylaminomethyl)biphenyl-
2-carboxylic acid 3-methylbutyl)amide
o ~
~ /
I 0
O / N
)LNo /
\
From 0.34 mmol of 2'-aminomethylbiphenyl-2-carboxylic acid (3-methyl-
butyl)amide (precursor 5 c) and (4-carbomethoxy)-phenyl chloroformate,
according to the general working procedure followed by extraction with EA
and purification by flash chromatography 77 mg of 2'-(4-carbomethoxy-
phenoxycarbonylaminomethyl)biphenyl-2-carboxylic acid (3-methylbutyl)-
amide were obtained as a resin. MS (ES+): m/e = 475 (M+1).
Example 2 i: 2'-(2,2-Dimethylpropoxycarbonyiaminomethyl)biphenyl-
2-carboxylic acid phenethylamide
N
O
O', N O
From 0.45 mmol of 2'-aminomethylbiphenyl-2-carboxylic acid phen-
ethylamide (precursor 5 a) and neopentyl chloroformate, according to the
general working procedure followed by extraction with EA and purification
by flash chromatography 156 mg of 2'-(2,2-dimethylpropoxycarbonyl-
aminomethyl)biphenyl-2-carboxylic acid phenethylamide were obtained.
MS (ES+): m/e = 445 (M+1).
CA 02385859 2002-03-27
= 44
Examples 2 j-2 m
The following compounds were obtained analogously to Examples 2 a-2 i:
Example Structure MS (ES+): M.p.
No. m/e =
F F
N
F
2j 0 491
O N I ~
F
DO
O N 2 k ~ ~ Olul N O 473 107
F F
~ao~ N 21 0 503 123
N
/ I
a O N \N
2 m ol~,N 482
General procedure for the reaction of aminomethylbiphenyls with sulfonyl
chlorides to give sulfonamides (Examples 3 a to 3 t):
0.66 mmol of the respective sulfonyl chloride is slowly added dropwise at
0 C to a solution of 0.61 mmol of the respective 2'-aminomethylbiphenyl
and 74 mg (0.73 mmol) of triethylamine in 5 ml of methylene chloride.
After stirring at RT for 12 h, the reaction mixture is concentrated in vacuo,
the residue is stirred with 25 ml of water for 2 h and the crystallized
product is filtered off with suction.
= CA 02385859 2002-03-27
Example 3 a: 2'-(3-Trifluoromethylphenylsulfonylaminomethyl)biphenyl-
2-carboxyfic acid phenethylamide
~
~
F3C \ ZN O
O
5
From 0.61 mmol of 2'-aminomethylbiphenyl-2-carboxylic acid phen-
ethylamide (precursor 5 a) and 3-trifluoromethylphenyisulfonyl chloride,
according to the general working procedure 272 mg of 2'-(3-trifluoromethyl-
phenyisulfonylaminomethyl)biphenyl-2-carboxylic acid phenethylamide
10 were obtained; m.p. 145 C. MS (ES+): m/e = 539 (M+1).
Example 3 b: 2'-(4-Acetylphenylsulfonylaminomethyl)biphenyl-2-carboxylic
acid phenethylamide
N
O
11
OS, N O
From 0.61 mmol of 2'-aminomethylbiphenyl-2-carboxylic acid phen-
ethylamide (precursor 5 a) and 4-acetyiphenylsulfonyl chloride, according
to the general working procedure 258 mg of 2'-(4-acety4phenylsulfonyl-
aminomethyl)biphenyl-2-carboxylic acid phenethylamide were obtained;
m.p. 145 C. MS (ES+): m/e = 513 (M+1).
Example 3 c: 2'-(3-Nitrophenylsulfonylaminomethyl)biphenyl-2-carboxylic
acid phenethylamide
OO% O, From 0.61 mmol of 2'-aminomethylbiphenyl-2-carboxylic acid phenethyl-
CA 02385859 2002-03-27
= 46
amide (precursor 5 a) and 3-nitrophenylsulfonyl chloride, according to the
general working procedure 272 mg of 2'-(3-nitrophenylsulfonyl-
aminomethyl)biphenyl-2-carboxylic acid phenethylamide were obtained; m.
p. 145 C. MS (ES+): m/e = 516 (M+1).
Example 3 d: 2'-(Phenylsulfonylaminomethyl)biphenyl-2-carboxylic acid
phenethylamide
I\
~ O \
~SNN / O
O
From 0.61 mmol of 2'-aminomethylbiphenyl-2-carboxylic acid phen-
ethylamide (precursor 5 a) and phenyisuffonyl chloride according to the
general working procedure 224 mg of 2'-(phenyisulfonylaminornethyl)-
biphenyf-2-carboxyfic acid phenethylamide were obtained; m. p. 154 C. MS
(ES+): m/e = 471 (M+1).
Example 3 e: 2'-(3-Fluorophenylsuifonylaminomethyl)biphenyl-2-carboxylic
acid phenethylamide
I O \
1`N O
O
From 0.61 mmol of 2'-aminomethylbiphenyl-2-carboxylic acid phen-
ethylamide (precursor 5 a) and 3-fluorophenylsulfonyl chloride, according to
the general working procedure 221 mg of 2'-(3-fluorophenylsulfonyl-
aminomethyl)biphenyl-2-carboxylic acid phenethylamide were obtained;
m.p. 153 C. MS (ES+): m/e = 489 (M+1).
Example 3 f: 2'-(4-Ethylphenylsulfonylaminomethyl)biphenyl-2-carboxylic
acid phenethylamide
CA 02385859 2002-03-27
47
~ o \
N O
O I
\
From 0.61 mmol of 2'-aminomethylbiphenyl-2-carboxylic acid phen-
ethylamide (precursor 5 a) and 4-ethylphenylsulfonyl chloride, according to
the general working procedure 250 mg of 2'-(4-ethylphenylsulfonyl-
aminomethyl)biphenyl-2-carboxylic acid phenethylamide were obtained;
m.p. 163 C. MS (ES+): m/e = 499 (M+1).
Example 3 g: 2'-(3-Trifluoromethylphenylsulfonylaminornethyl)biphenyl-
2-carboxylic acid benzylamide
~ N \ I
FC ~ ~
3 O
/-N
O
From 0.28 mmol of 2'-aminomethylbiphenyl-2-carboxylic acid benzylamide
(precursor 5 b) and 3-trifluoromethylphenylsulfonyl chloride, according to
the general working procedure 131 mg of 2'-(3-trifluoromethylphenyl-
sulfonylaminomethyl)biphenyl-2-carboxylic acid benzylamide were
obtained; m.p. 126 C. MS (ES+): m/e = 525 (M+1).
Example 3 h: 2'-(3-Acetylphenylsulfonylaminomethyl)biphenyl-2-carboxylic
acid benzylamide
/ ~ / \ (
N,
~ ~ O
~s~N O
O 0
From 0.28 mmol of 2'-aminomethylbiphenyl-2-carboxylic acid benzylamide
(precursor 5 b) and 3-acetylphenyisulfonyl chloride, according to the
general working procedure 110 mg of 2'-(3-acetylphenylsulfonyl-
aminomethyl)biphenyl-2-carboxylic acid benzylamide were obtained;
CA 02385859 2002-03-27
48
m.p. 182 C. MS (ES+): m/e = 499 (M+1).
Example 3 i: 2'-(3-Nitrophenylsulfonylaminomethyl)biphenyl-2-carboxylic
acid benzylamide
o N
N O
O N 5~;;'
O I
\
From 0.28 mmol of 2'-aminomethylbiphenyl-2-carboxylic acid benzylamide
(precursor 5 b) and 3-nitrophenylsulfonyl chloride, according to the general
working procedure 115 mg of 2'-(3-nitrophenylsulfonylaminomethyl)-
biphenyl-2-carboxylic acid benzylamide were obtained; m.p. 175 C. MS
(ES+): m/e = 502 (M+1).
Example 3 j: 2'-(3-Phenylsulfonylaminomethyl)biphenyl-2-carboxylic acid
benzylamide
I\ ~I
c(IO
.S-N / O
O I
\
From 0.28 mmol of 2'-aminomethylbiphenyl-2-carboxylic acid benzylamide
(precursor 5 b) and phenylsulfonyl chloride, according to the general
working procedure 95 mg of 2'-(phenylsulfonylaminomethyl)biphenyt-
2-carboxylic acid benzylamide were obtained; m.p. 162 C. MS (ES+):
m/e = 457 (M+1).
Example 3 k: 2'-(3-Fluorophenylsulfonylaminomethyl)biphenyl-2-carboxylic
acid benzylamide
N
O
S~N / I
O
O
= CA 02385859 2002-03-27
= 49
From 0.28 mmol of 2'-aminomethylbiphenyl-2-carboxylic acid benzylamide
(precursor 5 b) and 3-fluorophenylsulfonyl chloride, according to the
general working procedure 112 mg of 2'-(3-fluorophenylsulfonyl-
aminomethyl)biphenyl-2-carboxylic acid benzylamide were obtained;
m.p. 147 C. MS (ES+): m/e = 475 (M+1).
Example 3 I: 2'-(Phenylsulfonylaminomethyl)biphenyl-2-carboxylic acid
(3-methylbutyl)amide
I\ .
O
.S~N O N
OI
From 0.34 mmol of 2'-aminomethylbiphenyl-2-carboxylic acid (3-methyl-
butyl)amide (precursor 5 c) and phenylsulfonyl chloride, according to the
general working procedure 100 mg of 2'-(phenylsulfonylaminomethyl)-
biphenyl-2-carboxylic acid (3-methylbutyl)amide were obtained;
m.p. 127 C. MS (ES+): m/e = 437 (M+1).
Example 3 m: 2'-(4-Fluorophenylsulfonylaminomethyl)biphenyl-
2-carboxylic acid (3-methylbutyl)amide
F I \
O
s~N / I O
O
\
From 0.34 mmol of 2'-aminomethylbiphenyl-2-carboxylic acid (3-methyl-
butyl)amide (precursor 5 c) and 4-fluorophenylsulfonyl chloride, according
to the general working procedure 122 mg of 2'-(4-fluorophenylsulfonyl-
aminomethyl)biphenyl-2-carboxylic acid (3-methylbutyl)amide were
obtained; m.p. 149 C. MS (ES+): m/e = 455 (M+1).
------ - --------
CA 02385859 2002-03-27
'= 50
Example 3 n: 2'-(3-Fluorophenylsulfonylaminomethyl)biphenyl-2-carboxylic
acid (3-methylbutyl)amide
\ I O ~
N
F 'N / O
OI
\
From 0.34 mmol of 2'-aminomethylbiphenyl-2-carboxylic acid (3-methyl-
butyl)amide (precursor 5 c) and 3-fluorophenylsulfonyl chloride, according
to the general working procedure 118 mg of 2'-(3-fluorophenylsulfonyl-
aminomethyl)biphenyl-2-carboxylic acid (3-methylbutyl)amide were
obtained; m.p. 141 C. MS (ES+): m/e = 455 (M+1).
Example 3 o: 2'-(Isopropylsulfonylaminomethyl)biphenyl-2-carboxylic acid
(3-methylbutyl)amide
~ \ .
N
~SO O
O`N
From 0.34 mmol of 2'-aminomethylbiphenyl-2-carboxylic acid (3-methyl-
butyl)amide (precursor 5 c) and isopropylsulfonyl chloride, according to the
general working procedure followed by purification by flash chromato-
graphy 16 mg of 2'-(isopropylsulfonylaminomethyl)biphenyl-2-carboxylic
acid (3-methylbutyl)amide were obtained as an oil. MS (ES+): m/e = 403
(M+1).
Example 3 p: 2'-(Phenylsulfonylaminomethyl)biphenyl-2-carboxylic acid
2-(2-pyridyl)ethylamide
I\
N N
`N O I /
O
CA 02385859 2002-03-27
51
From 0.3 mmol of 2'-aminomethylbiphenyl-2-carboxylic acid 2-(2-pyridyl)-
ethylamide (precursor 5 d) and phenylsulfonyl chloride, according to the
general working procedure 117 mg of 2'-(phenylsulfonylaminomethyl)-
biphenyl-2-carboxylic acid 2-(2-pyridyl)ethylamide were obtained;
m.p. 131 C. MS (ES+): m/e = 472 (M+1).
Example 3 q: 2'-(4-Fluorophenylsulfonylaminomethyl)biphenyl-2-carboxylic
acid 2-(2-pyridyl)ethylamide
F I
N N
.SN / 0
O I
\
From 0.3 mmol of 2'-aminomethylbiphenyl-2-carboxylic acid 2-(2-pyridyl)-
ethylamide (precursor 5 d) and 4-fluorophenylsulfonyl chloride, according
to the general working procedure 106 mg of 2'-(4-fluorophenyisulfonyl-
aminomethyl)biphenyl-2-carboxylic acid 2-(2-pyridyl)ethylamide were
obtained; m.p. 130 C. MS (ES+): m/e = 490 (M+1).
Example 3 r: 2'-(3-Fluorophenylsulfonylaminomethyl)biphenyl-2-carboxylic
acid 2-(2-pyridyl)ethylamide
\
a N
F ~s O
O .N 0
From 0.3 mmol of 2'-aminomethylbiphenyl-2-carboxylic acid 2-(2-pyridyl)-
ethylamide (precursor 5 d) and 3-fluorophenylsulfonyl chloride, according
to the general working procedure 102 mg of 2'-(3-fluorophenylsulfonyl-
aminomethyl)biphenyl-2-carboxylic acid 2-(2-pyridyl)ethylamide were
obtained; m.p. 123 C. MS (ES+): m/e = 490 (M+1).
Example 3 s: 2'-(Isopropylsulfonylaminomethyl)biphenyl-2-carboxylic acid
2-(2-pyridyl)ethylamide
CA 02385859 2002-03-27
52
N \
~
O
O N
From 0.3 mmol of 2'-aminomethylbiphenyl-2-carboxylic acid 2-(2-pyridyl)-
ethylamide (precursor 5 d) and isopropyisulfonyl chloride, according to the
general working procedure and subsequent extraction with EA 40 mg of
2'-(isopropylsulfonylaminomethyl)biphenyl-2-carboxylic acid 2-(2-pyridyl)-
ethylamide were obtained as an oil. MS (ES+): m/e = 438 (M+1).
The following compound was obtained analogously to Examples 3 a-3 s:
Example Structure MS (ES+):
No. m/e =
Example Structure MS (ES+):
No. m/e =
/ ~ I N \
3 t 1p, CF3 539
O N
General procedure for the reaction of aminomethylbiphenyls with carbonyl
chlorides to give carboxamides (Examples 4 a to 4 I):
0.36 mmol of the respective sulfonyl chloride is slowly added dropwise at
0 C to a solution of '0.34 mmol of the respective 2'-aminomethylbiphenyl
and 41 mg (0.41 mmol) of triethylamine in 5 mi of methylene chloride.
After stirring at RT for 3 h, the reaction mixture is concentrated in vacuo,
the residue is stirred with 25 ml of water and the precipitated product is
filtered off with suction or isolated by extraction with EA.
Example 4 a: 2'-(Benzoylaminomethyl)biphenyl-2-carboxylic acid
(3-methylbutyl)amide
CA 02385859 2002-03-27
53
\
I / N
1
O N / O
\
From 0.34 mmol of 2'-aminomethylbiphenyl-2-carboxylic acid (3-methyl-
butyl)amide (precursor 5 c) and benzoyl chloride, according to the general
working procedure 75 mg of 2'-(benzoylaminomethyl)biphenyl-2-carboxylic
acid (3-methylbutyl)amide were obtained; m.p. 147 C. MS (ES+):
m/e = 401 (M+1).
Example 4 b: 2'-(Benzoylaminomethyl)biphenyl=2-carboxylic acid
2-(2-pyridyl)ethylamide
\
I / N
O
O N / I
\
From 0.3 mmol of 2'-aminomethylbiphenyl-2-carboxylic acid 2-(2-pyridyl)-
ethylamide (precursor 5 d) and benzoyl chloride, according to the general
working procedure 98 mg of 2'-(benzoylaminomethyl)biphenyl-2-carboxylic
acid 2-(2-pyridyl)ethylamide were obtained; m.p. 135 C. MS (ES+):
m/e = 436 (M+1).
Example 4 c: 2'-{[2-(4-Methoxyphenyl)acetylamino]methyl}biphenyl-
2-carboxylic acid 2,4-difluorobenzylamide
F F
i0 N
~ N / =
From 0.5 mmol of 2'-aminomethylbiphenyl-2-carboxylic acid (2,4-di-
fluorobenzyl)amide (precursor 8 a) and 4-methoxyphenylacetyl chloride,
according to the general working procedure 160 mg of 2'-{[2-(4-methoxy-
phenyl)acetylamino]methyl}biphenyl-2-carboxylic acid 2,4-difluoro-
benzylamide were obtained; m.p. 138 C. MS (ES+): m/e = 501 (M+1).
CA 02385859 2002-03-27
= 54
The following compounds were obtained analogously to Examples 4 a-4 c:
Example Structure ME (ES+)
M.P.
m/e = p
0 O N N
4 d ~ 480
N I \
/
~ ~
0 / N \ N
4 e 0 466
N
O
4 f 466
N
O
4g 481
N \ O
0 N
4 h 0 465
N
4 i o N 480 116 C
N \ \
O j N
41 iv 480
N
O \ I N N
4 k 508
0
CA 02385859 2002-03-27
=. 55
i
i N N
41 \ N o 478
I ~ I ~.
General procedure for the reaction of aminomethylbiphenyls with
isocyanates to give ureas (Examples 5 a-5 e):
0.36 mmol of the respective isocyanate dissolved in 0.5 ml of methylene
chloride is slowly added dropwise at 0 C to a solution of 0.34 mmol of the
respective 2'-aminomethylbiphenyl and 41 mg (0.41 mmol) of triethylamine
in 5 ml of methylene chloride. After stirring at RT for 3 h, the reaction
mixture is concentrated in vacuo, the residue is stirred with 25 ml of water
and the precipitated product is filtered off with suction or isolated by
extraction with EA.
Example 5 a: 2'-[(3-Phenylureido)methyl]biphenyl-2-carboxylic acid
(3-methylbutyl)amide
I\
O / o N
~ ,
N N
\
From 0.34 mmol of 2'-aminomethylbiphenyl-2-carboxylic acid (3-methyl-
butyl)amide (precursor 5 c) and phenyl isocyanate, according to the
general working procedure 85 mg of 2'-[(3-phenylureido)methyl]biphenyl-
2-carboxylic acid (3-methylbutyl)amide were obtained; m.p. 194 C. MS
(ES+): m/e = 416 (M+1).
Example 5 b: 2'-[(3-Phenylureido)methyl]biphenyl-2-carboxylic acid
2-(2-pyridyl)ethylamide
~ \
O / N N
Jk ~
N N ~
\
From 0.3 mmol of 2'-aminomethylbiphenyl-2-carboxylic acid 2-(2-pyridyl)-
CA 02385859 2002-03-27
56
ethylamide (precursor 5 d) and phenyl isocyanate, according to the
general working procedure 101 mg of 2'-[(3-phenylureido)methyl]biphenyl-
2-carboxylic acid 2-(2-pyridyl)ethylamide were obtained; m.p. 99 C. MS
(ES+): m/e = 451 (M+1).
Examples 5 c-5 e
The following compounds were obtained analogously from 2'-amino-
methylbiphenyl-2-carboxylic acid 2-(2-pyridyl)ethylamide (precursor 5 d)
and the corresponding isocyanates:
Example Structure MS (ES+):
No. m/e =
0 N N
5 c '' 465
"I, N N
Chiral
N N
5 d NJLN p 479
Chiral
O I N ~N
5 e NN O 479
General procedure for the reaction of biphenylcarboxylic acids with amines
to give amides (Examples 6 a-6 h):
0.3 mmol of the respective amine is added dropwise at 0 C to a solution of
0.28 mmol of the appropriate biphenylcarboxylic acid, 0.3 mmol of HOBT
and 0.3 mmol of DIC in 5 ml of THF and it is stirred at RT for 12 [lacuna].
The reaction mixture is diluted with EA and washed with dilute
hydrochloric acid and sodium bicarbonate solution. After drying over
magnesium sulfate and concentrating in vacuo, the corresponding amide
is obtained.
CA 02385859 2002-03-27
57
Example 6 a: 2'-(Benzyloxycarbonylaminomethyl)biphenyl-2-carboxylic
acid benzylmethylamide
\ I~
0 N
Olk N O
/ \
From 0.28 mmol of 2'-(benzyloxycarbonylaminomethyl)biphenyl-
2-carboxylic acid (precursor. 6) and benzylmethylamine, according to the
general working procedure 89 mg of 2'-(benzyloxycarbonylaminomethyl)-
biphenyl-2-carboxylic acid benzylmethylamide were obtained. MS (ES+):
m/e = 465 (M+1).
. ~
Example 6 b: 2'-(Benzyloxycarbonylaminomethyl)biphenyl-2-carboxylic
acid cyclohexylamide
O N_0
O~N O
\ .
Frori 0.28 mmol of 2'-(benzyloxycarbonylaminomethyl)biphenyl-
2-carboxylic acid (precursor 6) and cyclohexylamine, according to the
general working procedure 99 mg of 2'-(benzyloxycarbonylaminomethyl)-
biphenyl-2-carboxylic acid cyclohexylamide were obtained. MS (Et+):
m/e = ¾43 (M+1).
Example 6 c: 2'-(Benzyloxycarbonylaminomethyl)biphenyl-2-carboxylic
acid phenylamide
O N \
~ O I /
O N
From 0.28 mmol of 2'-(benzyloxycarbonylaminomethyl)biphenyl-
CA 02385859 2002-03-27
58
2-carboxylic acid (precursor 6) and aniline, according to the general
working procedure 66 mg of 2'-(benzyloxycarbonylaminomethyl)biphenyl-
2-carboxylic acid phenylamide were obtained. MS (ES+): m/e = 437 (M+1).
Example 6 d: 2'-(Benzyloxycarbonylaminomethyl)biphenyl-2-carboxylic
acid {N-methyl-N-[2-(2-pyridyl)ethyl]}amide
O / N
D~N / I ~ JN10 From 0.28 mmol of 2'-(benzyloxycarbonylaminomethyl)biphenyl-
2-carboxylic acid (precursor 6) and 2-[2-(methylaminoethyl)]pyridine,
according to the general working procedure and subsequent purification by
flash chromatography 54 mg of . 2'-(benzyloxycarbonylaminomethyl)-
biphenyl-2-carboxylic acid {N-methyl-N-[2-(2-pyridyl)ethyl]}amide were
obtained. MS (ES+): m/e = 480 (M+1).
Example 6 e: 2'-(Benzyloxycarbonylaminomethyl)biphenyl-2-carboxylic
acid dibutylamide
0 N
0N 0
I / \ I
0
From 0.28 mmol of 2'-(benzyloxycarbonylaminomethyl)biphenyl-
2-carboxylic acid (precursor 6) and dibutylamine, according to the general
working procedure and subsequent purification by flash chromatography
82 mg of 2'-(benzyloxycarbonylaminomethyl)biphenyl-2-carboxylic acid
dibutylamide were obtained. MS (ES+): m/e = 473 (M+1).
= CA 02385859 2002-03-27
= 59
Example 6 f: 2'-(Benzyloxycarbonylaminomethyl)biphenyl-
2-carboxylic acid 2-(2-pyridyl)ethylamide
O \
'k O
O N
From 0.28 mmol of 2'-(benzyloxycarbonylaminomethyl)biphenyl-
2-carboxylic acid (precursor 6) and 2-(2-pyridyl)ethylamine, according to
the general working procedure and subsequent purification by flash
chromatography 85 mg of 2'-(benzyloxycarbonylaminomethyl)biphenyl-2-
carboxylic acid 2-(2-pyridyl)ethylamide were obtained. M.p. 140 C
(heptane/EA); MS (ES+): m/e = 466 (M+1).
Example 6 g: 2'-(Benzyloxycarbonylaminomethyl)biphenyl-2-carboxylic
acid (2,4-difluorobenzyl)amide
F
DaZ--,
O N 0-1k N O
~ \
From 0.28 mmol of 2'-(benzyloxycarbonylaminomethyl)biphenyl-
2-carboxylic acid (precursor 6) and 2,4-difluorobenzylamine, according to
the general working procedure and subsequent purification by flash
chromatography 99 mg of 2'-(benzyloxycarbonylaminomethyl)biphenyl-2-
carboxylic acid (2,4-difluorobenzyl)amide were obtained. MS (ES+): m/e =
487 (M+1).
Example 6 h: 2'-(Benzyloxycarbonylaminomethyl)biphenyl-2-carboxylic
acid (2,2,2-trifluoroethyl)amide
\
0 N --"ICF3
OA, N O
\% \
CA 02385859 2002-03-27
From 0.28 mmol of 2'-(benzyloxycarbonylaminomethyl)biphenyl-
2-carboxylic acid (precursor 6) and 2,2,2-trifluoroethylamine, according to
the general working procedure and subsequent purification by flash
chromatography 19 mg of 2'-(benzyloxycarbonylaminomethyl)biphenyl-2-
5 carboxylic acid (2,2,2-trifluoroethyl)amide were obtained. MS (ES+): m/e =
443 (M+1).
Example 7 a: 2'-(Benzyloxycarbonylaminomethyl)biphenyl-2-carboxylic
acid 2-[2-(1-oxypyridyl)]ethylamide
o
O N N
O N
~ 00
47 mg of m-chloroperbenzoic acid dissolved in 2 ml of methylene chloride
were added dropwise at 0 C to a solution of 85 mg (0.18 mmol) of
2'-(benzyloxycarbonylaminomethyl)biphenyl-2-carboxylic acid 2-(2-pyridyl)-
ethylamide (Example 6 f) in 13 ml of methylene chloride and the reaction
mixture was stirred at RT for 12 h. The organic phase was washed 2 times
with sodium bicarbonate solution, dried over magnesium sulfate and
concentrated in vacuo. 79 mg of 2'-(benzyloxycarbonylaminomethyl)-
biphenyl-2-carboxylic acid 2-[2-(1-oxypyridyl)]ethylamide were obtained.
MS (ES+): m/e = 482 (M+1).
The following compounds were obtained from the corresponding pyridines
analogously to Example 7 a:
Example No. Structure MS (ES+):
m/e =
~~ .
7 b N \\ \ O_ 482
Example No. Structure MS (ES+):
m/e
N Q
7 c N ~ \ N
496
CA 02385859 2002-03-27
61
General procedure for the reaction of biphenylcarboxylic acids with amines
to give amides (Examples 8 a-8 c):
0.44 mmol of the respective amine is added dropwise at 0 C to a solution
of 0.42 mmol of the appropriate biphenylcarboxylic acid, 0.44 mmol of
HOBT and 0.44 mmol of EDAC in 5 ml of THF and it is stirred at RT for 4
to 12 h. The reaction mixture is diluted with EA and washed with dilute
hydrochloric acid and sodium bicarbonate solution. After drying over
magnesium sulfate and concentrating in vacuo, the corresponding amide
is obtained.
Example 8 a: Benzyl [2'-(1-carbamoyl-3-methylbutylcarbamoyl)biphenyl-
2-ylmethyl]carbamate
1 \ o
0 N ''' N
0O)LN O
From 0.28 mmol of 2'-(benzyloxycarbonylaminomethyl)biphenyl-
2-carboxylic acid (precursor 6) and L-leucinamide hydrochloride/
triethylamine, according to the general working procedure 180 mg of benzyl
[2'-(1-carbamoyl-3-methylbutylcarbamoyl)biphenyl-2-ylmethyl]carbamate
were obtained. MS (ES+): m/e = 474 (M+1).
Example 8 b: methyl 2-{[2'-(benzyloxycarbonylaminomethyl)biphenyl-
2-carbonyl]amino}-3-phenylpropionate
I \ o
o "1 N ''' o
o
~N
~ I \ I ~ /
)",
From 0.28 mmol of 2'-(benzyloxycarbonylaminomethyl)biphenyl-
2-carboxylic acid (precursor 6) and L-phenylaianine methyl. ester
hydrochloride/triethylamine, according to the general working procedure
230 mg of methyl 2-{[2'-(benzyloxycarbonylaminomethyl)biphenyl-
2-carbonyl]amino}-3-phenylpropionate were obtained. MS (ES+): m/e = 523
CA 02385859 2002-03-27
62
(M+1).
Example 8 c: 2'-(tert-Butoxycarbonylaminomethyl)biphenyl-2-carboxylic
acid (2,4-difluorobenzyl)amide
F F
1
O N
0 'k N O
From 10 mmol of 2'-(tert-butoxycarbonylaminomethyl)biphenyl-
2-carboxylic acid (precursor 7) and 2,4-difluorobenzylamine, according to
the general working procedure 3.8 g of 2'-(tert-butoxycarbonylamino-
methyl)biphenyl-2-carboxylic acid (2,4-difluorobenzyl)amide were
obtained. MS (ES+): m/e = 453 (M+1).
Examples 8 d-8 p:
The following products were obtained from 2'-(tert-butoxy-
carbonylaminomethyl)biphenyl-2-carboxylic acid (precursor 7) and the
corresponding amines analogously to Examples 8 a-8 c:
Example Structure MS (ES+): M.p.
No. m/e =
N
8 d 40 ~ N N 418 139
\
QNQ
O 8 e 1 O~N O 418 171
\`
O N
8 f O 432 161
N
CA 02385859 2002-03-27
= 63
p N
8 g O~N \ O 432 59
O \ I N `~
8i 431 171
ON p
p N
8 j p ~~ 433 165
Q
f
p N
8 k N p 421 199
I
N
81 p 397
O N
~ Chiral
I \ ; \ ~
~ -\-O
N
8 m 4~ p 461
O N
p \ N
8 n /` ~ \ O N 490
o N o p
CA 02385859 2002-03-27
64
O N
8o O N / 432
O N
Examples 8 p-8 ac
The following products were obtained from 2'-(benzyloxycarbonylamino-
methyl)biphenyl-2-carboxylic acid (precursor 6) and the corresponding
amines analogously to Examples 8 a-8 c:
Example Structure MS (ES+): M.p.
No. m/e =
O
O N .'' N
I O 460 119 C
cr O
O N 480
OA, N O
O
O N Oi
8 r cr OO 489 F
O N`"I'F
8s OxN I\ O F 471
~ ~
-= CA 02385859 2002-03-27
N
8 x O 494
O-U-N i 0
O ~) C
8 y p~N N N O 460
~
O N
8 Z OA \N O O O N 524
/ ( / (
\ I N N
8 aa 0 N 0 466
/I
\ N \
8 ab \0 N
O N 466 132 C
O
O N N
8 ac 0 474
I \ O N / (
General procedure for the reaction of aminomethylbiphenyls with isothio-
cyanates to give thioureas (Examples 9 a-9 i):
0.36 mmol of the respective isothiocyanate dissolved in 0.5 ml of
5 methylene chloride is slowly added dropwise at 0 C to a solution of
0.34 mmol of the respective 2'-aminomethylbiphenyl and 41 mg
CA 02385859 2002-03-27
66
(0.41 mmol) of triethylamine in 5 ml of methylene chloride. After stirring at
RT for 3 h, the reaction mixture is concentrated in vacuo, the residue is
stirred with 25 ml of water and the precipitated product is filtered off with
suction or purified by preparative HPLC.
The following products, inter alia, were obtained in this way:
Example Structure MS (ES+):
No. m/e =
S N
9 a 483
,
O
~
S
9 c 466
1 1 /
1 ~ O N
F
F
F
S4z:< N
9 d N 609
o-.
p N O
CA 02385859 2002-03-27
67
ci
ci
N
9 e 534
N N
O N
~
N
S
9 f 480
1 N,
9 g 480
N
~
/ O N /
F
N
9 h SZ*( 537
N F
\
/ N I FF
O
/
CA 02385859 2002-03-27
68
qF
N
9 i S~ ci 551
N Ci
o N
Examples 10 a-10 o
The following products, inter alia, were obtained by coupling 2'-(tert-
butyloxycarbonylaminomethyl)biphenyl-2-carboxylic acid (precursor 7) with
corresponding amines analogously to the methods described for
Example 6 or 8:
Example Structure MS (ES+):
No. m/e =
O
a O NH 384
O--~ N O
H
O
O
10 b >~O NH 506
O~N ~ O
H ,~ I
CA 02385859 2002-03-27
69
O
c NH 460
O
O-1--N / O
H \ (
CI
CI
10 d >~ 498
O NH
O-~- N
H
>~ H
O N
10 e O-~- N O 484
H F
F
F
N
10f >~O NH 431
O-~- N
H
CA 02385859 2002-03-27
{ \
O ~
10 g >~O NH 446
O;~ N
H
N
10 h NH 417
O--~ N O
H
O,S,NH2
10 i
~ 509
O NH
OJ, N 0
H ~
\
10j NH 422
~
O N O
H
~ H
N
O
10 k ~ O 417
0 H \ I \ (
=' CA 02385859 2002-03-27
71
NH
101 O 406
o ^~H
\
N,
ClN
m >~O NH 434
O-~- N / O
H
H
>~O N
10 n O-~-N O 466
H
OO
10 o O 412
H
Examples 11 a-11 r:
General procedure for the conversion of the Boc derivatives of
Examples 10 to ureas:
5 For the removal of the Boc protective group, 1 g of the appropriate
compound of Example 10 were added to 10 ml of a 30% solution of TFA in
dichloromethane. The mixture was stirred at RT for 30 min and the solvent
was removed in vacuo on a rotary evaporator. The residue was taken up
in ethyl acetate and washed with saturated sodium hydrogencarbonate
10 solution. The organic phase was dried over magnesium sulfate and
CA 02385859 2002-03-27
= 72
filtered, and the solvent was removed in vacuo. The 2'-aminomethyl-
biphenyl-2-carboxamides obtained were then reacted with isocyanates to
give the corresponding ureas according to the procedure for Examples 5.
The following products, inter alia, were obtained in this way:
Example Structure MS (ES+):
No. m/e =
0
/
11 a o---~' 480
N N
O N
F
F
N
11 b 04:*( 486
N N
1 /
~ N
-" O
11 c o~ N 466
N
N
0
CA 02385859 2002-03-27
== 73
F
F
F
~
11 d N 504
N
~
O N N ~
F F
F
11 e 521
N
N.-,/~ ~^y
~ o
F
0-F
11 f O--~ 439
N
O N
CI
0
11 g O----~ 437
N
O--
O
CA 02385859 2002-03-27
74
F
O~-F
N
11 h ~ ~ 561
O,
1
'
O N O
III-q
O
=
F
F
F
11 i o N 547
N
O N ~O
F
F
F
11 j o-t~ N cI 585
N CI `
O N
F
F
N
11 k 0 Ci 553
N CI
80 N
CA 02385859 2002-03-27
== 75
CI
11 1 O~N 484
N NbR O
N
F
F F
11 m Ozz~ N 519
N N1
1 / O N
F
0-F
11 n Q~~ 473
N
O N N
F F
~ F
~ / .
11 o O:--~ N 519
N N N
O
CA 02385859 2002-03-27
76
F
0-F
N
11 p O~ 502
N
/
` O ~
N
~
1
/ O
O N
llq ~ 446
~
,~../ / 1
1 / o N ''
F
F F
11 r 0 N 0~'O 597
N
~
1 / o N
General working procedures for the preparation of compounds according
to the invention by means of soiid-phase synthesis:
The quantitative data in the procedures in each case always relate to the
resin loading, which were determined by UV photometry after removal of
the Fmoc protective group (see for example"The Combinatorial Chemistry
Catalog", Novabiochem).
CA 02385859 2002-03-27
77
General procedure for the coupling of a-Fmoc-amino acids to Rink amide
resin
A solution of 1.5 equivalents each of HOBT, TOTU, DIPEA and the
a-Fmoc amino acid in DMF (5 ml/g of resin) was added to Rink amide
polystyrene resin (loading 1.2 mmol/g) and the mixture was shaken at
room temperature for 12 h. The resin was filtered off and washed 3 times
with 10 ml each of DMF, once with 10 ml of toluene, once with 10 ml of
methanol and 3 times with 10 ml of dichloromethane. Determination of the
loading according to the Fmoc method showed a loading of 0.9 mmoVg of
carrier.
Removal of the Fmoc protective group
For the removal of the Fmoc protective group, the resin was preswollen in
DMF at room temperature for 5 min. After addition of a solution of
DMF/piperidine (4 ml/g of resin, 1:1), the mixture was shaken at room
temperature for 20 min. The solution was filtered off with suction and the
process was repeated. The removal of an analytical sample showed
complete reaction according to HPLC/MS investigation. After complete
reaction, the resin was washed three times with dichloromethane and
employed directly in the coupling.
General working procedure for the coupling of the resin-bonded amino
acids with the 2'-phthalimidomethylbiphenyl-2-carboxylic acid (precursor 2)
A solution of 12.2 mg (0.09 mmol) of HOBT, 29.5 mg (0.09 mmol) of
TOTU, 16,ul (0.09 mmol) of DIPEA and 0.09 mmol of 2'-phthalimido-
methylbiphenyl-2-carboxylic acid (precursor 2) in 5 ml of DMF was added
to 100 mg of resin loaded with the amino acid (0.6-0.8 mmol/g) and the
mixture was shaken at room temperature for 12 h. The resin was filtered
off and washed 3 times with 10 ml each of DMF, once with with 10 ml of
toluene, once with 10 ml of methanol and 3 times with 10 ml of
dichloromethane.
General procedure for the removal of the phthalimido protective group on
the carrier
== CA 02385859 2002-03-27
78
ml of a 10% strength solution of hydrazine in DMF were added to 1 g of
resin loaded with the Fmoc-protected amino compound and the mixture
was shaken at room temperature for 2 h. The resin was filtered off with
suction. The resin was then washed 3 times each with 10 ml each of DMF
5 and dichloromethane. The removal of an analytical sample showed
complete reaction according to HPLC/MS investigation.
General procedure for coupling with sulfonyl chlorides
A solution of 0.16 ml (0.027 mmol) of DIPEA and 0.027 mmol of the
sulfonyl chloride in 5 ml of DMF was added to 100 mg of resin loaded with
the functionalized 2'-aminomethylbiphenyl-2-carboxylic acid and the
mixture was shaken at room temperature for 12 h. The resin was filtered
off and washed 3 times with 10 ml each of DMF, once with 10 ml of
toluene, once with 10 ml of methanol and 3 times with 10 ml of
dichloromethane.
General working procedure for removal from the resin
For removal, the resin was suspended in dichloromethane/trifluoroacetic
acid (3 ml/0.1 g of resin) and shaken for 1 h. The resin was filtered and
washed with 1 ml of dichloromethane. The combined removal solution was
concentrated in a rotary concentrator. The residue was taken up in
dichloromethane and chromatographed on silica gel using
dichloromethane and ethyl acetate or purified by preparative HPLC.
The following products, inter alia, were obtained in this way:
Example Structure MS (ES+):
No. m/e =
Chiral
O
N~
I O N
12 a S",N p 542
~ ~ ~
CA 02385859 2002-03-27
79
=
O Chiral
Nl~N
O
p ~ // p 480
12 b ~N O
Chiral
N
~ p = 516
12c S'/ O
p N
Chiral
N O
O1- '0 ~.\
N p 534
12 d F
F F
Chiral
N".AN
CI a~/j p 506
N
12 e ~ S~,p
Chiral
O
N
O
p\S\ 0 528
12f N
Chiral
O
N
564
12 g ~~O p
N
p
CA 02385859 2002-03-27
=~ 80
0 Chiral
O
O\SO N
12 h N / I I\ 582
f \ /
F
F F
0 Chiral
CI ~ ( N N
12 i g S~ O = 554
O/ \N
O Chiral
O I / N~! N
O~//
N O O
12j S\ / I 522
~
F
F F
O Chiral
O I/ N Y N
0 //
12 k N 0 530
O\
CI ~
0 Chiral
O I / Nv N
121 565
uQ
CA 02385859 2002-03-27
81
p Chiral
0 N
p p
12 m S~
O~ N 556
\ I I /
Example 13 a: 2-{[2'-(Benzyloxycarbonylaminomethyl)biphenyl-
2-carbonyl]amino}-4-methylpentanoic acid
1 O
O N .'' O
p'k N 0
The compound was obtained from the methyl ester of Example 8 r by
hydrolysis with potassium hydroxide in methanol/water at 60 C.
Examples 13 b-13 e
The following compounds were obtained by coupling of the carboxylic acid
of Example 13 a with the appropriate amines according to the general
method indicated in Example 8:
Example Structure MS (ES+):
No. m/e =
Chiral
O ~
13 b ON O N 550
Chiral
0 N
13 c N ~ O O"N 564
)-0'
CA 02385859 2002-03-27
82
Chiral
13 d OxN O ON 556
~F
FF
Chiral
~
13 e O~N O O N 544
The following compounds were obtained by hydrogenolytic removal of the
Z protective group of the compound of Example 13 c and subsequent
reaction with the appropriate acid chlorides:
Example Structure MS (ES+):
No. m/e =
O Chiral
13 f NxY 556
O N I~ O N O
F FF
Chiral
13 g -~ ~ N~ 554
O N N'~O
F j>17F
F
O Chiral
13 h O~ N592
SN O N O
I F FF
Starting from the compound of Example 8 z, the following compound was
== CA 02385859 2002-03-27
=- 83
obtained by hydrolysis and reaction with isopropylamine analogously to
Examples 13 a-13 e:
Example Structure MS (ES+):
No. m/e =
O N
13 i Cro ~ N O N N551 5 General procedure for the coupling of 2'-
aminomethylbiphenyl-2-carboxylic
acid (2,4-difluorobenzyl)amide with carboxylic acids to give carboxamides
(Examples 14 a-14 f):
0.27 mmol of the appropriate carboxylic acid was stirred at RT for 30 min
with 0.27 mmol of HOBT and 0.27 mmol of EDAC in 1 ml of THF.
0.26 mmol of 2'-aminomethylbiphenyl-2-carboxylic acid (2,4-difluoro-
benzyl)amide trifluoroacetate dissolved in 1 ml of THF was then added
and the mixture was stirred at RT overnight. The reaction mixture was
diluted with EA and washed with sodium bicarbonate solution and water.
After concentrating the organic phase, the products were purified by
means of preparative HPLC.
The following compounds were prepared in this way:
Example Structure MS (ES+):
No. m/e =
14a O F 499
N
O N F
~
14 b N F 503
1
F
14 c N F 499
il
-= CA 02385859 2002-03-27
== 84
F
14 d ~ HO cj--~N , 0 F 501 O F
0
14 e 515
~yF
O
14f ;~ H 1 0 F 521
F
F
General procedure for the synthesis of biphenyls by Suzuki coupling
(Examples 15 a-15 b)
58 mg (0.05 mmol) of tetrakistriphenylphosphine palladium and 1 mmol of
the appropriate bromide were added to dimethoxyethane (10 ml) gassed
with argon. After 10 min, 1.5 mmol of the appropriate boronic acid were
added and finally 1 ml of a 2 molar sodium carbonate solution (2 mmol).
The mixture was heated to reflux under argon for 18 h, cooled and diluted
with 30 ml of methylene chloride. The mixture was washed with water and
saturated sodium chloride solution, dried over sodium sulfate, concentrated
and purified by chromatography on silica gel.
Example 15 a: 2'-(tert-Butoxycarbonylaminomethyl)-4-nitrobiphenyl-
2-carboxylic acid (3-methylbutyl)amide
NOZ
0 N
ON
H
According to the general procedure, 350 mg (79% yield) of the nitro-
substituted compound were obtained as a yellow solid.
CA 02385859 2002-03-27
Example 15 b: 2'-(t-Butoxycarbonylaminomethyl)-4-methoxybiphenyl-
2-carboxylic acid (3-methylbutyl)amide
OMe
O \ N
O
0N
H
5
According to the general procedure, 170 mg (41% yield) of the methoxy-
substituted compound were obtained as a viscous pale oil.
Example 16 a: 2'-(tert-Butoxycarbonylaminomethyl)-4-aminobiphenyl-2-
10 carboxylic acid (3-methylbutyl)amide
N H2
O \ N
'k
H
330 mg (0.75 mmol) of the nitro-substituted compound of Example 15 a
15 were dissolved in ethyl acetate and hydrogenated under a hydrogen
atmosphere (1 bar) using a spatula tipful of 10% palladium on carbon. After
2 h, the mixture was filtered through Celite and the clear solution was
concentrated. Yield: 260 mg (84%).
20 Example 16 b: 2'-(Benzyioxycarbonylaminomethyl)-4-hydroxybiphenyl-
2-carboxylic acid (3-methylbutyl)amide
OH
/
IN /
H
\ I
=' CA 02385859 2002-03-27
=- 86
150 mg (0.35 mmol) of the methoxy-substituted compound of Example 15 b
were dissolved in 5 ml of anhydrous methylene chloride and siowly treated
at -70 C with 1.4 ml (1.4 mmol) of a 1 moiar solution of boron tribromide in
n-hexane. After 10 min, the reaction solution was slowly warmed to 0 C.
After 2 h at this temperature, it was neutralized with saturated sodium
hydrogencarbonate solution, extracted with a total of 40 ml of methylene
chloride, dried over sodium sulfate and concentrated. Of the crude product
(88 mg) of 2'-aminomethyl-4-hydroxybiphenyl-2-carboxylic acid (3-methyl-
butyl)amide obtained, 30 mg (0.1 mmol) were dissolved in 3 ml of
methylene chloride and treated with 11 mg (0.11 mmol) of triethylamine
and 27 mg (0.11 mmol) of benzyloxycarbonyloxysuccinimide. After 3 h, the
mixture was diluted with methylene chloride, washed with water, and the
organic phase was dried over sodium sulfate and purified by RP-HPLC.
8 mg of 2'-(benzyloxycarbonylaminomethyl)-4-hydroxybiphenyl-2-carboxylic
acid (3-methylbutyl)amide were obtained as a dark oil.
Example 17a: tert-Butyl {1-[2'-(3-methylbutylcarbamoyl)biphenyl-2-yl]ethyl}-
carbamate
N
O
O
O
2.2 g (10 mmol) of N-Boc-(R)-phenylethylamine were dissolved in 50 ml of
anhydrous THF, cooled to -78 C and treated dropwise with 14 ml (1.5 M
solution in pentane, 21 mmol) of tert-butyllithium. The mixture was warmed
to -20 C in the course of 2 h, then 4.5 ml (40 mmol) of trimethyl borate
were added and it was warmed to RT. The solution was cooled to 0 C,
acidified to pH 6 using 10% HCI, the aqueous phase was extracted with
dichloromethane, and the combined organic phases were washed with
saturated NaCI solution, dried and concentrated. The boronic acid was
obtained as a pale yellow solid foam which was used without further
purification.
CA 02385859 2002-03-27
87
The Suzuki coupling was carried out according to the general working
procedure (see example 15) using 1 mmol of 2-bromo-N-(3-methylbutyl)-
benzamide and, after chromatographic purification, afforded 85 mg
(0.2 mmol) of the biphenyl.
Examples 17 b - 17 e:
The enantiomer 17 b was obtained analogously to example 17 a. By
removal of the Boc group and conversion into the corresponding
carbamates, the compounds 17a and 17b were converted into the
compounds of examples 17 c - 17e.
MS (ES+):
=
Example No. Structure miz
17 b 0 411
40''N o
N` ^f
17 c O 445 17 d I 445
0
p N
i
I~ .
Ti7e o
0 " 'I( 459
ON
The following compounds were prepared analogously to the procedures
described in examples 1 to 17:
'= CA 02385859 2002-03-27
88
MS (ES+):
Example No. Structure m/Z
18 a 0 0 379
N \ I
0
18b 0 427
i N
18 c ~ 431
O N
18 d 0 443
N 0
CA 02385859 2002-03-27
89
F F
I / N \ I
18 e o 451
N
N
18 f 0 0 464
\ ~ N \ ~
O N
18 g N O 416
18 h N 478
\ I N \ I
181 0 478
\ ( . N \ I N
~l
O N
18j 523
18k F\ 4 \ o N ~ j 457
N ~ \ F
CA 02385859 2002-03-27
CI
181 o )<~O)~N o F 487 18 m 0 0 395
o
0
18 n 0 433
O N
O ~ N
18o OAIM 395
~ \f
F
18 p ^N 1 465
N,
\ / ~
N
18 q ox" 466
CA 02385859 2002-03-27
91
\
N N
18 r 0 p ~ 494
0 N
18 s 0 1 \
p 480
F F
18t O N yj p 487
~./`"
N
F F
;p,
18 u 489
0
-I
~. /
O I / N N 18v o 515
ip,
, F
I
/ N \ I
18w 487
0
oj
N / f
0
18 x a 473
CA 02385859 2002-03-27
92
18 y ~~ ~ o N~'o 475
N
O \ \ ( N
18 z O O,,.o 443
N
N
18 aa 0N Q~~0~ 433
O ~ \ N
18 ab / 0 0 459
N
\V~~
O N
18 ac 0 432
18 ad ~^ 478
OY-Y N p
N M
N
O:S_Q
/ F
18 ae o N ~~ 566
OxN O F
~ , ,
=^ CA 02385859 2002-03-27
93
\ N ~ ~
18 af N 505
N
1
/ I \
18 ag o N \ 543
o
N
18 ah 0 11)'-
~ N N 491
OON o
~ N
N ~. ~ 494
18ai o
cr O
Pharmacological investigations
Kv1.5 channels from humans were expressed in Xenopus oocytes. For
this, oocytes from Xenopus laevis were first isolated and defolliculated.
RNA encoding Kv1.5 synthesized in vitro was then injected into these
oocytes. After Kv1.5 protein expression for 1-7 days, Kv1.5 currents were
measured on the oocytes using the two-microelectrode voltage clamp
technique. The Kv1.5 channels were in this case as a rule activated using
voltage jumps to 0 mV and 40 mV lasting 500 ms. The bath was rinsed
with a solution of the following composition: NaCl 96 mM, KCI 2 mM,
CaCI2 1.8 mM, MgC12 1 mM, HEPES 5 mM (titrated with NaOH to
pH 7.4). These experiments were carried out at room temperature. The
following were employed for data acquisition and analysis: Geneclamp
amplifier (Axon Instruments, Foster City, USA) and MacLab D/A converter
and software (ADlnstruments, Castle Hill, Australia). The substances
according to the invention were tested by adding them in different
concentrations to the bath solution. The effects of the substances were
'CA 02385859 2002-03-27
94
calculated as the percentage inhibition of the Kv1.5 control current which
was obtained when no substance was added to the solution. The data
were then extrapolated using the Hill equation in order to determine the
inhibitory concentration IC50 for the respective substances.
The following IC50 values were determined in this way for the compounds
listed below:
Example IC50 Example IC50 Example IC50 Example IC50
No. M No. M No. M No. M
1 a 6.1 2 a 2.6 4 a 4.1 6 h 3.0
1 b 3.3 2 b 0.8 4 c 1.4 7 a -6.0
1 d 1.0 2 c 0.7 4 d 1.8 8 a 0.3
1 e 0.5 2 d 1.7 4 g 3.4 8 b 0.9
1 f 0:4 2 e 3.4 4 h 1.8 8 d 6.4
1 g 0.4 2f 7.1 4i 4.7 8j 4.5
1 h 4.3 2 g 3.3 4 j 7.1 8 k 3.1
1 i 1.7 2 h 2.5 4 k 2.2 81 3.5
1 j 0.2 2 i 3.3 41 0.8 8 m 5.2
1 k 2.4 2 j 2.5 5 a 4.5 8 n 3.7
1 1 1.4 2 k 3.8 5 c 7.8 8 o 8.4
1 m 0.7 2 m 2.6 5 d 1.9 8 p 1.4
1 n 1.4 3 d 1.7 5 e 7.2 8 q 7.3
1 o 4.4 3 k 2.4 6 a 4.4 8 r 1.0
1 r 0.8 31 2.6 6 b 1.8 8s 1.0
Example IC50 Example IC50 Example IC50 Example IC50
No. M No. M No. M No. M
1 s 1.7 3 p 1.9 6 c 2.5 8 x 3.3
it 1.3 3 r 1.5 6 d 3.1 8 y 2.8
1 u 0.8 3 3.0 6 e 3.6 8 z 1.6
8aa 0.8 8ab 1.2 8ac 1.1 9b 3.0
9 c 2.0 9 f 2.2 9 g 2.2 11 a 2.3
11 b 7.3 11 d 3.3 11 g 7.8 11 h 5.8
111 2.7 11 m 3.3 11 n 5.9 11o 4.4
11 p 7.3 12c 11.2 12f 11.3 12g 9.1
12 h 4.8 121 10.3 12 m 7.7 13 b -3.0
13 c 1.4 13 d 0.5 13 e 2.8 13f 3.4
13g 1.1 13h 1.4 13i 1.2 14a 3.6
= CA 02385859 2002-03-27
14 b 2.7 14 d 2.0 14 e 0.8 14f 2.5
15 b 3.1 16 b 5.2 18a 7.2 18b 0.4
18c 4.2 18d 0.4 18e 1.7 18f 1.3
18 g 3.9 18 h 0.8 18i 0.4 18j 0.7
18k 3.0 18m 2.1 18n 0.4 180 3.6
18 p 4.7 18 q 3.2 18 r 0.7 18s 0.9
18u 1.1 18v 0.4 18w 5.4 18x 4.6
17d 1.3 17e 1.8 17c 2.1 18y 1.9
18 z 1.2 18aa 0.4 18 ab 1.1 18 ac 10
18ad 0.3 18af 5.8 18ah 2.1 18ai 6.6