Note: Descriptions are shown in the official language in which they were submitted.
CA 02386520 2002-O1-30
WO 01/19178 PCT/DE00/02997
Method for marking an animal and products originating
therefrom
The invention relates to a method for marking an animal
and products originating therefrom, to a transponder
and a kit.
According to the prior art, livestock, such as cattle
and pigs, for recording purposes and for identification
of origin, are usually marked, such as using an ear
tag, a tattoo or a transponder.
Transponders, as are known, for example, from
US 5,697,384, are injected under the skin or into the
muscle tissue at various points in the animal's body.
The transponder is identified on the basis of the
following principle: a reader unit emits an
electromagnetic field into the surroundings via an
antenna. If the transponder passes into the region of
the field, this field produces a current in the
transponder due to inductive coupling. The current is
utilized to transmit a signal via an antenna. The
signal is detected and decoded by the reader unit.
Operation of such passive transponders does not require
any further energy supply, for example in the form of a
battery.
A disadvantage found with these conventional
transponders is the low integration distance, which is
generally 30-50 cm, so that even with a slight shift of
the transponder in the animal, errors on reading can
occur. In order to counteract shifting, DE 37 45 053
discloses coating the transponder with a roughened
plastic layer.
A further disadvantage of the abovementioned markings
is that after separating the animal from the marking,
for example after slaughtering the animal and cutting
CA 02386520 2002-O1-30
WO 01/19178 PCT/DE00/02997
- 2 -
the carcass, it is no longer possible to assign
individual parts of the animal to the respective
slaughter animal.
WO 98/18003 and WO 99/36775 disclose biological marking
for slaughter animals. In this method at least one
immunogen which is harmless to the animal and the
consumers is administered to the animal. Detection of
the induced specific antibodies makes possible forgery-
proof identification of origin of the living animal and
products originating therefrom, such as meat and meat
products. The disadvantage of this method is that a
sample must be taken to identify the origin of the
animal.
It is an object of the invention to eliminate the
disadvantages of the prior art. In particular, a
reliable, rapid and forgery-proof marking of an animal
is to be made possible. The marking is to be
identifiable as far as possible without a sample
needing to be taken from the animal. In addition, a
reliable and forgery-proof identification is also to be
made possible by which the origin of products
originating from the animal can be unambiguously
established after slaughter.
This object is achieved by the features of claims 1, 14
and 18. Expedient embodiments of the invention arise
from features 2 to 13, 15 to 17 and 19 to 21.
To achieve the object, there are provided a method for
marking an animal and products originating therefrom in
which a transponder is introduced into the body of the
animal and at least one substance triggering an immune
reaction is administered to the animal which does not
pass into the animal's body as vaccine, or via the food
chains, or via the environment during usual animal
husbandry, so that, for a predetermined period,
CA 02386520 2002-O1-30
- i~10 01/19178 PCT/DE00/02997
- 3 -
biological and physical marking are achieved
simultaneously.
The immune reaction initiated by the substance does not
usually occur in the animals, because these do not come
into contact with these substances triggering the
immune reaction. In selection of the substance, care
must be taken to ensure that it does not pass into the
body of the animal either as vaccine, or via the food
chain, or via the environment during conventional
animal husbandry. Furthermore, the substance should be
harmless both for the animal and for the consumer.
The inventive method has the advantage that at least
for a predetermined period the immune reaction creates
a biological marking which is inseparably connected to
the animal. The biological marking cannot be exchanged
or forged; it cannot be removed by removing the
transponder which is prescribed during slaughter and
carcass cutting. The biological marking can be detected
both in parts of the animal and in products originating
therefrom. Detection is possible, for example, in
blood, in milk or in other body secretions of the
animal or in the drip or press fluid from the meat. At
the same time, the transponder gives the possibility of
identifying the animal reliably, rapidly and in a
contact-free manner using physical methods. The
security against forgery is achieved in particular by a
double marking, physical and biological.
The immune reaction can take place in a time-delayed
manner after administration of the substance. During
this time, the marked animal can already be identified
using the transponder. In a predetermined period, for
example at least 6 months before slaughter, the animal
is marked biologically and physically. The
predetermined period can obviously also be longer than
6 months. It preferably starts a few days after
CA 02386520 2002-O1-30
_ WO 01/19178 PCT/DE00/02997
- 4 -
administration of the substance and extends up to
slaughter of the animal.
In addition, in the inventive method, it is
advantageous that the transponder and the substance can
be administered in one and the same working step. Thus
it is possible, for example, to inject together with
the transponder the substance which is present in
solution or as suspension. A suitably prepared
transponder can also be administered.
It is particularly advantageous in the inventive method
that the double marking of the animal ensures markedly
increased security. Thus in the case of an animal in
which the transponder has been lost or damaged, the
origin of the animal can nevertheless be detected by
the biological marking. Vice versa, the transponder
makes an unambiguous identification of an animal
possible where, for some reason or other, no immune
reaction, or no sufficient immune reaction, against the
substance has occurred.
The substance can be coupled to a carrier and can be
administered together with auxiliaries, for example
additional adjuvants, in solution, as suspension or as
an implant. Administration of a mixture of substances
is also provided.
In a preferred embodiment of the inventive method a
housing of the transponder is coated with the
substance. The substance can be bound to the material
by adsorbent chemicals, for example biopolymers. It is
advantageous here that, due to the adsorption, an
adjuvant effect can be achieved which leads to an
enhanced immune response. The substance, however, can
also be bound to the housing via an intermediate layer.
The binding can be performed, for example, via binding
agents, for example biotin. The intermediate layer can
CA 02386520 2002-O1-30
- WO 01/19178 PCT/DE00/02997
- 5 -
consist, for example, of polystyrene or polycarbonate.
By means of the binding an adjuvant effect can be
achieved. In addition, this can cause the substance to
be released slowly in the body of the animal. This
causes intense and lasting stimulation of the immune
response.
In a further preferred embodiment of the inventive
method, the substance is enclosed by, and/or embedded
in, a composition delaying accessibility in the
animal's body. As a result of this composition, the
substance is protected against the attack of the immune
system for a predetermined time. This gives the
advantage that implantation can be performed even in
the perinatal period, for example together with other
treatments customarily carried out. Antibody formation
is delayed and not induced until after, for example, a
few weeks.
A further possibility is to provide a plurality of
layers having intermediate delaying compositions. As a
result, repeated release of the substance is possible
over a relatively long period, so that the repeated
stimulation of the immune reaction achieves high
antibody titer lasting for a long period. A further
advantage of a multilayer structure is that various
substances can be used. By means of successive release
of these substances taking place over the lifetime of
the animal, biological marking can also be performed
via which the age of the animal can be established both
on the live animal and on individual products
originating from the animal, such as meat and meat
products. The compositions delaying availability which
can be used are, for example, polyglycolic acid,
polydioxanone, polyglycolides, polylactides, homopoly-
mers and/or copolymers of glycolides and d/1-lactides
and other biodegradable constituents, as described in
US 3,887,699, US 3,991,766, US 4,045,418 and
CA 02386520 2002-O1-30
WO 01/19178 PCT/DE00/02997
- 6 -
US 4,137,921. The disclosure content of said US patents
is hereby incorporated by reference.
In a further preferred embodiment of the method, a
specific digital code is transmitted by the transponder
on appropriate excitation. This is possible, in
particular, using a transponder which contains a
digital circuit. This permits error-free detection of
the transponder signal, with simultaneously lower
transmission power. The code can be read even from
relatively large distances. The digital code can
contain information on the biological marking of the
animal. This further increases the security of the
method against forging. A transponder cannot be
transplanted unnoticed into the body of another animal.
The substance can contain peptides and/or nucleic
acids. They can cause an immune reaction either
directly or indirectly. Substances which trigger an
immune reaction directly are, for example, proteins or
peptides or fragments thereof, such as: keyhole limpet
hemocyanines, green fluorescent protein, from Aequoria
victoria, inactivated snake venoms, virus proteins,
hirudine, pheromonotropine, renalexine and artificial
proteins and peptides whose amino acid sequence does
not correspond to any previously known substance, but
which are harmless to animals and consumers. Suitable
peptides are, in particular, those having at least one
nonphysiological amino acid, a modified amino acid, a
D-amino acid and/or a derivative of such an amino acid.
Modified amino acids and derivatives can contain, for
example, selenium, alkyl, phenyl or other radicals or
carbohydrates.
An advantage of the peptides containing at least one
nonphysiological amino acid is that they can be used to
prepare substances with which the animals to be marked
have not previously come into contact. Furthermore,
CA 02386520 2002-O1-30
_ WO 01/19178 PCT/DE00/02997
_ 7 _
these amino acids offer the advantage that by combining
them a great number of different peptides can be
provided. Individual marking of individual animals is
possible. In addition, it is advantageous that by using
the amino acids mentioned, peptides can be provided
which are more stable in the body of the animal than
are peptides having naturally occurring amino acids. A
particularly persistent immune stimulation and improved
antibody titer can be achieved. Specially modified
vaccines, that is to say vaccines suitable for marking
purposes or appropriately produced vaccines, are also
considered in this context to be a substance suitable
for marking.
Reference is also made in this context to WO 98/18003
and WO 99/36775, the disclosure content of which is
hereby incorporated by reference.
The nucleic acids can be present in an expression
2 0 vector . They can code for a defined immunogen . Such an
immunogen can be, for example, one of the above
specified proteins or peptides. An immune reaction can
be triggered indirectly by administering the nucleic
acids.
Furthermore, the substance can alternatively be a
chemical agent or a chemical group, such as the
dinitrophenyl group. Frequently, chemical agents or
chemical groups must be coupled to relatively large
molecules in order to be immunological. The
dinitrophenyl group can be coupled, for example, to a
protein, such as boroserum albumin, and as a result
becomes immunogenic.
Conveniently, a code from the transponder can be read
by an external transmitter and detector device. In this
manner it is possible, for example, to supply farm
animals in an enterprise with feed and defined
CA 02386520 2002-O1-30
- WO 01/19178 PCT/DE00/02997
_ g _
additives individually at an automated feeder or
rapidly to record the origin of individual animals as
they are being driven through. The method also makes it
possible to record mechanically the origin of animals
which have already been slaughtered and still contain
the transponder, so that as a result, for example, in a
carcass cutting operation, a processing route dependent
on the origin can be controlled automatically.
The invention further relates to a method for
identifying the origin of an animal marked by an
inventive method, the antibodies and/or T cells and/or
their receptors specifically formed by the substance
being identified from a body fluid of the animal, from
milk or from the drip fluid of meat originating
therefrom.
A method for identifying antibodies has the advantage
that it is always simple and rapid to carry out.
In a preferred embodiment of the inventive method, at
least one of the following methods is used: Enzyme
Linked Immunosorbent Assay (= ELISA), Enzyme Immuno
Assay (= EIA), Immunoblot (= western blot), Immunodif-
fusion, Immunofluorescence, Radio Immuno Assay (= RIA) ,
identification using a test stick and identification
using a test chip. Identification is particularly
simple using test sticks, which are dipped, for
example, into the drip fluid of the meat of the
slaughtered animal, or into milk. The origin of
products obtained from livestock can thus be tested and
demonstrated by anyone.
The invention further relates to a transponder whose
housing is coated with a substance triggering an immune
reaction in the animal that does not pass into the
animal's body as vaccine, or via the food chain, or via
the environment during usual animal husbandry. The
CA 02386520 2002-O1-30
WO 01/19178 PCT/DE00/02997
- 9 -
transponder advantageously has a digital circuit for
transmitting a digital code. Such transponders have a
low power consumption. They can be read from a
relatively great distance. The invention further
relates to a kit having a transponder and at least one
substance triggering an immune reaction in an animal
that does not pass into the animal's body as vaccine,
or via the food chain, or via the environment during
usual animal husbandry. Because of the advantageous
embodiments, reference is made in each case to the
preceding descriptions.
Obviously, the features mentioned above and still to be
explained hereinafter can be used not only in the
combinations specified in each case, but also in other
combinations or alone, without departing from the
context of the present invention.
Other advantages result from the exemplary embodiments
together with the drawings. In the drawings:
fig. 1 shows a diagrammatic cross section of a first
exemplary embodiment and
fig. 2 shows a diagrammatic cross section of a second
exemplary embodiment.
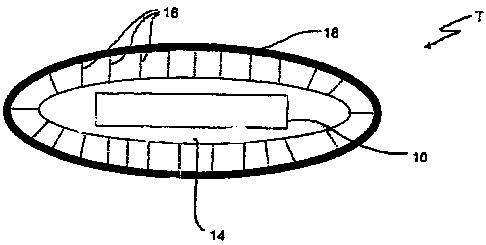
In the figures a general transponder apparatus denoted
by T exhibits a transponder 10 having a housing 14
which is expediently produced from a dielectric and
biologically inert material. It can be glass,
polyethylene, polystyrene or polycarbonate. The housing
14 is coated with a substance 16. The substance 16 is
enclosed by a composition 18 which delays or retards
the accessibility in the animal's body. This
composition can be, for example, polyglycolic acid.
CA 02386520 2002-O1-30
- WO 01/19178 PCT/DE00/02997
- 10 -
In the case of the transponder apparatus T shown in
fig. 2, the substance 16 is bound to the housing 14 via
an intermediate layer 15. The intermediate layer can be
formed, for example, of polystyrene or polycarbonate.
In the inventive method, the transponder apparatus T is
introduced into an animal to be marked by, for example,
injecting it intramuscularly. After some weeks, the
retardant composition 18 consisting, for example, of
polyglycolic acid, dissolves. The substance 16, which
can be, for example, keyhole limpet hemocyanine, then
becomes accessible to the animal's immune system and
causes an immune reaction in the animal directly or
indirectly. The substance 16 can be released slowly,
that is to say over the entire life of the animal, so
that there is a high antibody titer in the animal over
a long period. The substance 16 can be bound to the
surface of the housing 14 directly or via adsorbent
chemicals. The transponder 10 can contain a digital
circuit which generates a specific digital code and
thus permits a greater reading distance than do
conventional transponders. Provided that the animal
body marked in this way contains the transponder 10, it
can be recorded via a corresponding transmitter and
detector device. After slaughter and cutting up a
carcass, the antibodies formed against the substance 16
can be identified, for example, in the meat drip fluid
from the slaughtered animal. This can be performed, for
example, by ELISA, in which the substance 16 has been
immobilized to a microtiter plate. The antibodies from
the meat drip fluid which bind to the microtiter plate
can then be identified by means of the binding of
enzyme-labeled secondary antibodies directed toward
antibodies of the animal, and a corresponding enzyme
reaction.