Note: Descriptions are shown in the official language in which they were submitted.
CA 02386614 2002-04-05
WO 01/23626 PCT/AUOO/01203
-1-
TITLE
"THE ELUTION OF GOLD FROM ANION EXCHANGE RESINS"
FIELD OF THE INVENTION
This invention relates to an improved process for the elution of gold from
anion
exchange resins.
More particularly, the improved process of the present invention may be used
in
the recovery of gold from thiosulphate leach pulps and solutions.
BACKGROUND ART
Precious metals, including gold, have traditionally been leached from ore
using
cyanide-containing solutions. The precious metal is then recovered from that
solution or pulp. Importantly however, several factors have made cyanide
unsuitable for precious metal recovery. The most important of these are the
increasing environmental concerns with the use of cyanide and the increasing
proportion of so-called refractory gold ores which do not respond favourably
to
cyanidation. For these reasons, alternative lixiviants such as thiosulphate
have
been proposed.
The conventional method for the recovery of gold from cyanide leach pulps is
based on the use of activated carbon granules. However, activated carbon does
not adsorb gold from thiosulphate solutions or pulps. For this reason, ion
exchange resins have been suggested as alternatives to activate carbon. If
looking to recover gold from a thiosulphate leach pulp using an anion exchange
resin where that leach pulp also contains copper which is used as a catalyst
in the
leaching process, a number of particular problems arise. For example, known
procedures for the elution of gold thiosulphate from resins involve the use of
300
g/L ammonium thiosulphate or 200 g/L potassium thiocyanate. Copper has been
shown to be selectively eluted using initially 200 g/L ammonium thiosulphate
solution followed by elution of the gold using 200 g/L potassium thiocyanate.
PCT/AU00/01203
CA 02386614 2002-04-05 Received 27 July 2001
-2-
These eluants are relatively costly. Eluant concentrations at this level are
relatively high and may prove uneconomical in a commercial plant setting.
A major problem with thiosulphate leaching is the high concentrations of
thiosulphate needed and the high rate of lixiviant losses during a leach. The
thiosulphate can be lost from solution through oxidation to tetrathionate,
complexation with other noble metal ions or solution losses to the tails,
which
adds to the cost of the process.
The improved process for the recovery of gold from anion exchange resins of
the
present invention has as one object thereof to overcome the above-mentioned
problems of the prior art.
The preceding discussion of the background art is intended to facilitate an
understanding of the present invention only. It should be appreciated that the
discussion is not an acknowledgement or admission that any of the material
referred to was part of the common general knowledge in Australia as at the
priority date of the application.
Throughout the specification, unless the context requires otherwise, the word
"comprise" or variations such as "comprises" or "comprising", will be
understood to
imply the inclusion of a stated integer or group of integers but not the
exclusion of
any other integer or group of integers.
DISCLOSURE OF THE INVENTION
In accordance with the present invention there is provided a process for the
recovery of gold from thiosulphate leach solution or pulp, the method
comprising
the method steps of:
a) Loading gold from a thiosulphate leach solution on to an anion exchange
resin;
b) Eluting with a nitrate salt to displace gold from the resin; and
AMlEEoED SHEET
rP+E~u
CA 02386614 2008-11-26
~
-3-
c) Recovering gold from the eluate.
Preferably, the nitrate salt is provided in the form of ammonium nitrate. The
ammonium nitrate is further preferably provided at a concentration of less
than or about 2M (160 g/L).
The gold may be recovered in step c) by way of electrowinning or
precipitation, or any other similar process.
The process of the present invention may comprise the additional method
step of recovering thiosulphate from the leach solution by absorbing same
onto a resin and subsequently eluting it therefrom.
Preferably, the process further comprises the method step of recycling the
recovered thiosulphate to a leaching process from which the thiosulphate
leach solution was obtained. The elution of thiosulphate from the resin may
be performed with either a nitrate or sulphate solution.
Where the method of the present invention is conducted in the presence of
copper, before eluting with a nitrate salt the method comprises the additional
step of exposing the resin to excess oxygenated ammonia which displaces
the copper, loaded on the resin as copper (I) thiosulphate, as copper (li)
ammine.
According to an aspect of the present invention, there is provided a process
for the recovery of gold from thiosulphate leach solution or pulp, the method
comprising the method steps of:
a) loading gold from a thiosulphate leach solution on to an anion
exchange resin;
b) eluting with a nitrate salt to displace gold from the resin to yield
an eluate; and
CA 02386614 2008-11-26
-3a-
c) recovering gold from the eluate.
Preferably, a buffer is provided to maintain pH at about 9.2. The buffer
may preferably be ammonium sulphate.
BRIEF DESCRIPTION OF THE DRAWINGS
The improved process of the present invention will now be described,
by way of example only, with reference to one embodiment thereof and
the accompanying drawings, in which:
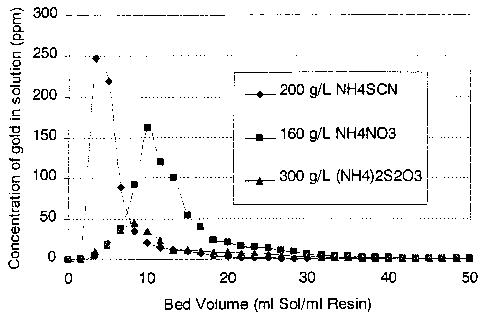
Figure 1 is a graphical representation of the elution of gold from
a commercial strong base anionic exchange resin by ammonium
nitrate, ammonium thiocyanate and ammonium thiosulphate;
CA 02386614 2002-04-05 PCT/AUOO/01203
Received 27 July 2001
-4-
Figure 2 is a graphical representation of the elution of gold from the same
resin by two concentrations of ammonium nitrate in accordance with the
present invention;
Figure 3 is a graphical representation of gold elution from the same resin by
different nitrate salts in accordance with the present invention;
Figure 4 is a graphical representation of the total recovery and separation of
copper, then gold, for a commercial gel type resin (A) and a commercial
macroporous resin (B);
Figure 5 is a graphical representation of the elution of thiosulphate from
resin
by either a nitrate or sulphate solution in accordance with a further
embodiment of the present invention;
Figure 6 is a graphical representation of the equilibrium loading of gold onto
the resins (A) and (B) in the presence of varying ammonium nitrate
concentrations;
Figure 7 is a graphical representation of consecutive equilibrium loadings
onto resin with a simulated leach solution containing gold, silver and copper;
Figure 8 is a graphical representation of the rate at which gold is eluted
from
resins (A) and (B); and
Figure 9 is a graphical representation of the elution of copper and gold from
a loaded resin from a small scale resin-in-pulp operation by ammonium
sulphate and ammonium nitrate solutions respectively.
BEST MODE(S) FOR CARRYING OUT THE INVENTION
The improved process for the recovery of gold from anion exchange resins of
the
present invention will now be described with reference to a preferred
embodiment.
However, it is to be appreciated that the following description of a preferred
form
of the invention is not to limit the above generality of the invention.
AMS, D:';-, St'iE=-T
IP-~, A F 1
CA 02386614 2008-11-26
-5-
The strong base anion exchange resins used in the embodiment include a gel
type resin (A), being Vitrokele 911, and a macroporous resin (B), being
Amberjet 4200. The resins used were of a particular size fraction, being
0.85<x<0.6 mm in diameter. The inventors have found that gold loading rates
onto each of these resins is similar and followed a simple first-order rate
equation for absorption to equilibrium. The kinetics of loading of gold onto
these resins is largely unaffected by the presence of thiosulphate.
After trials of methods of the prior art and several other known eluants,
nitrate
salts have been found to be the preferred eluant for gold. A concentration of
2M (160 g/L) ammonium nitrate was determined to be the most efficient at
eluting the gold. Figure 1 shows results for tests of the gold elution
characteristics of ammonium nitrate, ammonium thiocyanate and ammonium
thiosulphate. A result of 99% recovery of gold was achieved. 1 M(80 g/L)
ammonium nitrate was also found to account for 99% of the gold. However,
the elution curve was broad compared to that of the 2M ammonium nitrate.
Figure 2 shows the results for the elution of gold from the gel resin (A) by
the
two concentrations of ammonium nitrate.
CA 02386614 2002-04-05
WO 01/23626 PCT/AUOO/01203
-6-
To confirm that the active species is the nitrate ion, a number of nitrate
solutions
of the same concentration, but from different salts were used, as shown in
Figure
3. It is apparent that it is the nitrate ions in solution which are eluting
the gold
from the resin and the cations of the salt play no part in the elution
process.
The presence of copper in the system was determined to be problematic as
copper simultaneously loads onto the resin and does not selectively elute off
the
resin. For example, it was found that treatment with a lower concentration of
ammonium nitrate (0.1 M) did not elute any of the copper or gold off the
resin.
Further, treatment with 0.5M ammonium nitrate eluted 70% of the copper and
15% of the gold. Whilst this demonstrates some selectivity, gold was lost in
the
initial strip (with 0.5M ammonium nitrate) and there was carry over of copper
into
the final strip for the gold using the 2M ammonium nitrate. The inventors have
consequently observed that the copper thiosulphate complex behaves on the
resin in a similar manner to the gold thiosulphate complex.
A clue to the solution for the above problem may be found in the chemistry of
the
leaching of gold with thiosulphate in the presence of copper and ammonia. It
is to
be noted that the exact mechanism of this process is presently uncertain.
However, it is known that there is a cyclic system that reverts from copper
(II) to
copper (I) and which promotes the dissolution of gold.
The copper (II) ammine drives the cathodic reaction for the dissolution of
gold. As
such, it should be desirable to have as high a concentration of copper (II)
ammine
present within a leach solution. However, a high copper (II) ammine
concentration would result in increased degradation of thiosulphate.
The most likely cycle of copper during the dissolution of gold in a
thiosulphate
leach solution is copper (II) ammine to copper (I) thiosulphate. Copper (II)
ammine is the more stable complex in aerated solutions. As such, the copper
(I)
thiosulphate that loads onto the resin should be displaced off the resin by
converting back to the copper (II) ammine complex with the addition of excess
oxygenated ammonia solution. Figure 4 shows the separation of copper and gold
for both resins under this process.
CA 02386614 2002-04-05
WO 01/23626 PCT/AUOO/01203
-7-
A buffer of ammonium sulphate is desirable when ammonia is added to the
system so as to maintain the desired pH at 9.2.
Accordingly, once gold and copper have loaded onto the resin, the process of
the
present invention is able to selectively elute these species by initially
displacing
the copper as the copper (II) ammine with ammonia, then finally eluting with
ammonium nitrate for the displacement of the gold.
A means to minimise the loss of thiosulphate during thiosulphate leaching may
be
to recover as much of the thiosulphate from the leach solution before it
leaves to
the tail, and then to recycle it back into the leaching process. Resin could
be used
to absorb the anionic species (which would include all the poly-thionate
compounds) from leach solutions.
The elution of thiosulphate from the resin could be performed with either a
nitrate
or sulphate solution, see Figure 5. Since the eluted thiosulphate would be
reused
in the leaching process, ammonium sulphate would be the prefered eluant since
nitrate ions may prevent gold loading onto resin. Another good reason for
using
ammonium sulphate is that it would not elute any gold so any absorbed anions
other than gold would be eluted prior to using nitrate solution for gold.
To obtain a thermodynamic understanding of the elution process, equilibrium
loadings of gold in the presence of ammonium nitrate were performed on the two
resins (A) and (B). Both resins gave similar gold loadings for a particular
ammonium nitrate concentration and the data in Figure 6 contains the results
from
both resins. The results show that nitrate ions are very good at preventing
the
gold from loading onto the resin. The results also show that as the ammonium
nitrate concentration decreases the gold loading increases greatly, indicating
that
nitrate ions absorbed to the resin after elution could have minimal effect on
the
gold loading onto the resin if reintroduced to the absorption process.
Therefore
there is no need for regeneration of the resin from the nitrate form.
CA 02386614 2002-04-05
WO 01/23626 PCT/AUOO/01203
-8-
To examine the effect of consecutive loading/elution cycles on equilibrium
loadings a simulated leach solution was prepared consisting of 0.01 M
trithionate,
0.05 M thiosulphate, 0.2 M ammonia at pH 9.5. Trithionate was added to the
solution to mimic a real pulp solution. To this solution 10 ppm Cu, 10 ppm Ag
or
ppm Au was added to investigate the effect of copper and silver on the loading
of gold. Although it is known that gold, silver and copper will load onto the
resin it
was considered desirable to investigate the capacity of the resin after many
cycles
in order to assess how practical a resin-in-pulp (RIP) process would be for
the
recovery of gold from thiosulphate leach solutions.
In the elution cycle the resin was initially eluted with 0.5 M ammonia
buffered with
1 M ammonium sulphate, then further eluted with 2 M ammonium nitrate. The
ammonia solution was buffered to prevent any precipitation of metal complexes
onto the resin during elution and to recover the loaded thiosulphate. The
copper
that was loaded as the copper(l) thiosulphate [Cu(S203)z]3" was eluted by
converting it to the copper(II) tetrammine [Cu(NH3) 4]2+ with the addition of
excess
oxygenated ammonia solution. The gold was then eluted with a nitrate solution.
The resin was then dried and the loading and elution cycles repeated.
Evaluation
of the elution data is presented in Table 1. Although the conditions were not
optimised for the silver and gold elution, the low overall recovery for silver
and
gold would suggest some precipitation of these metal ions onto the resin
surface
over time. It can also be noted that there will be some carry over of gold and
silver back to the leaching circuit in the ammonia eluate.
Table 1. Averaged metal recovery over eight cycles.
Metal ion Ammonia elution Nitrate elution Overall
recovery
Copper 98 8 110
Gold 2 91 93
Silver 12 65 77
CA 02386614 2002-04-05
WO 01/23626 PCT/AUOO/01203
-9-
The equilibrium loadings from eight cycles showed very little difference in
the
equilibrium loading for any metal ion, see Figure 7. Although there was no
effect
of elution on the equilibrium loading, the resin darkened when cycled. At the
conclusion of these experiments, some of the resin was washed in nitrate
solution
and some in thiosulphate solution (2M), both were then fire assayed to
determine
what was precipitating onto the resin, see Table 2. The fire assay results
showed
there was about 7000 ppm silver and about 3000 ppm gold. There was very little
copper precipitated on the resin. The thiosulphate removed 99 % of the silver,
70
% of the copper and 30 % of the gold. Therefore, it is apparent that the resin
may
be successfully cleaned with thiosulphate when needed.
Table 2. Fire Assay of resin for gold, silver or copper.
Experiment Metal ion Concentration Concentration Average
(ppm) (ppm)
Concentration
Nitrate Copper 111 111 111
Elution Gold 2760 2980 2870
Silver 6640 6710 6675
Thiosulphate Copper 38 38 38
Elution Gold 1790 1820 1805
Silver 38 50 44
To improve the understanding of the rate at which the gold is eluted from the
resin, a loaded resin was added to a solution of ammonium nitrate and samples
taken over time. Both resins, the macroporus resin (A) and the gel resin (B)
gave
similar elution profiles. There was an initial fast elution of the gold from
the resin
followed by a slower elution, see Figure 8. This slower elution is expected as
the
nitrate solution now needs to diffuse into the pores of the resin before
releasing
the gold into solution. The rate of elution was very fast for 1.0 and 2.0 M
ammonium nitrate with both solutions reaching the expected equilibrium loading
calculated from the thermodynamic study. The macroporus resin (B) eluted
faster
since having greater rate of prenetration of the eluant into the pores of the
resin.
CA 02386614 2002-04-05
WO 01/23626 PCT/AU00/01203
-10-
A fixed-bed elution of some of the resin from the counter-current adsorption
run
showed that there is fast and effective elution of copper and gold when using
the
elution cycle of 1 M ammonium sulphate and 2 M ammonium nitrate solutions
respectively, see Figure 9. The volume of solution required by either eluant
was
low with only 5 bed volumes necessary to elute 95 % copper and 98 % gold from
the resin.
It is envisaged that the process of the present invention may be used to
ultimately
provide a cost effective and viable process for the recovery of gold from
thiosulphate leach pulp.
Modifications and variations such as would be apparent to the skilled
addressee
are considered to fall within the scope of the present invention.