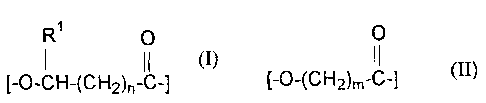Note: Descriptions are shown in the official language in which they were submitted.
CA 02387075 2002-04-10
WO 01/30893 PCT/USOO/29885
METHODS FOR PREPARING SOFT AND ELASTIC BIODEGRADABLE
POLYHYDROXYALKANOATE COPOLYMER COMPOSITIONS AND
POLYMER PRODUCTS COMPRISING SUCH COMPOSITIONS
FIELD OF THE INVENTION
The present invention is directed to polvmer products, including, but not
limited to,
films, fibers, nonwovens, and sheets, obtained by stretching a composition
comprising a
biodegradable polyhydroxyalkanoate copolymer. The products exhibit a desirable
combination of softness and elasticity while maintaining strength. The
products are useful
for various biodegradable articles including diaper topsheets, diaper
backsheets, garbage
bags, food wrap, disposable garments and the like.
BACKGROUND OF THE INVENTION
Biodegradable polymers and products formed from biodegradable polymers are
becoming increasingly important in view of the desire to reduce the volume of
solid waste
materials generated by consumers each year.
In the past, the biodegradability and physical properties of a variety of
polyhydroxyalkanoates have been studied. Polyhydroxyalkanoates are polyester
compounds produced by a variety of microorganisms, such as bacteria and algae.
While
polyhydroxyalkanoates have been of general interest because of their
biodegradable nature,
their actual use as a plastic material has been hampered by their thermal
instability. For
example, poly-3-hydroxybutyrate (PHB) is a natural energy-storage product of
bacterial
and algae, and is present in discrete granules within the cell cytoplasm. PHB
is
thermoplastic and has a high degree of crystallinity and a well-defined melt
temperature of
about 180 C. Unfortunately, PHB becomes unstable and degrades at elevated
temperatures near its melt temperature. Due to this thermal instability,
commercial
applications of PHB have been extremely limited.
Other polyhydroxyalkanoates, such as poly(3-hydroxybutyrate-co-3-
hydroxyvalerate) (PHBV), have also been investigated. Examples of PHB
homopolymer
and PHBV copolymers are described in the Holmes et al. U.S. Patents Nos.
4,393,167 and
4,880,59, and PHBV copolymers are commercially available from Monsanto under
the
1
CA 02387075 2005-08-10
trade nameBIOPOL .Unfortunately, polyhydroxyalkanoates such as PHB and PHBV
are
difficult to process into films for use in various applications. As previously
discussed, the
thermal instability of PHB makes such processing nearly impossible.
Furthermore, the
slow crystallization rates and flow properties of PHB and PHBV make film
processing
difficult. PHBV copolymers are typically produced with valerate contents
ranging from
about 5 to about 24 mol %. Increasing valerate content decreases the melt
temperature of
the polymer. However, owing to the relatively small changes in crystallinity,
PHBV films
often remain stiff and brittle for many applications.
Improved biodegradable copolymers are disclosed by Noda, for example in U.S.
Patents Nos. 5,498,692, 5,536,564, 5,602,227 and 5,685,756. The biodegradable
copolymers of Noda comprise at least two randomly repeating monomer units
(RRMUs)
wherein the first RRMU has the structure [-O-CH(R')-(CH2)n-C(O)-] wherein R'
is H or
C 1 or C2 alkyl, and n is I or 2, and the second RRMU has the structure [-O-
CH(R2)-CH2-
C(O)-] wherein R2 is a C4-C 19 alkyl or alkenyl, and wherein at least 50% of
the RRMUs
have the structure of the first RRMU. These copolymers are advantageous in
that they are
biodegradable and exhibit a good combination of physical properties which
allow their
processing into films, sheets, fibers, foams, molded articles, nonwoven
fabrics and the like
to provide a variety of useful articles. However, these copolymers are not
soft and elastic
while maintaining strength when they are in their original unstretched state.
Polyhydroxyalkanoate (PHA) copolymers consisting essentially of the repeat
units
having relatively long alkyl pendant groups of three to nine carbons, such as
polyhdroxyoctanoate, are known to exhibit soft and rubber-like elasticity with
some level
of strength. (See for example, K. D. Gagnon, R. W. Lenz, R. J. Farris, and R.
C. Fuller,
Macromolecules, vol. 25, pp.3723-3728, 1992.) The utility of soft and elastic
products
made of such PHA copolymers, however, is severely limited by the
disappointingly low
melt temperature around 60 C. The dimensional stability of the product is
compromised
even at a temperature of a warehouse in summer which can reach above 80 C.
Thus, a
biodegradable soft and elastic product made of polymers having a higher melt
temperature
range is desired.
2
CA 02387075 2002-04-10
WO 01/30893 PCT/US00/29885
It is often desirable to stretch thermoplastic polymers in order to alter
their physical
properties. Unfortunately, PHB and PHBV form brittle products that typically
break even
when drawn to only a very small extent. Various methods have been attempted to
improve the stretching processes and the resulting properties of stretched or
drawn PHB
and PHBV products, for example as disclosed in the Holmes U.S. Patent No.
4,537,738
and the Barham et al U.S. Patent No. 4,427,614. Additional methods are
disclosed in the
Safta European Reference EP 736,563 Al, the Institute of Physical and Chemical
Research European Reference EP 849,311 A2, the Waldock WO reference 97/22459,
Kusaka et al Pure Appl. Chem., 834(2):319-335 (1998) and Yamamoto et al,
Intern.
Polymer Processing XII, (1997) 1:29-37. However, such methods have not been
particularly successful in providing means for easily forming stretched
products having a
certain combination of desired physical properties. In particular, the prior
art does not
provide polymer products having softness and elasticity, while maintaining
strength.
Accordingly, it would be advantageous to obtain polymer products which are
biodegradable and which have a desirable combination of soft and elastic
properties
allowing use of the products in a wide range of applications.
SUMMARY OF THE INVENTION
Accordingly, it is an object of the present invention to provide new products
and
methods which overcome disadvantages of the prior art. It is a related object
of the
present invention to provide polymer products formed of compositions
comprising a
biodegradable polymer. It is a further object of the invention to provide
polymer products
which exhibit advantageous combinations of physical properties. It is a more
specific
object of the invention to provide biodegradable polymer products which
exhibit softness
and elasticity while maintaining strength. It is another object of the
invention to provide
methods for easily forming such products. It is yet a further object of the
invention to
provide articles comprising such polymer products.
These and additional objects and advantages are provided by the products and
methods of the present invention. In one embodiment, the invention is directed
to polymer
products which are obtained by stretching a composition comprising a
biodegradable
polyhydroxyalkanoate copolymer. The biodegradable polyhydroxyalkanoate
copolymer
3
CA 02387075 2002-04-10
WO 01/30893 PCT/US00/29885
comprises at least two randomly repeating monomer units (RRMUs). The first
RRIVIU has
the structure (I):
R 0
1 11
[-O-CH-(CH2)n-C-] (I)
wherein R' is H, or C1 or C2 alkyl and n is 1 or 2. The second RRMU is
different from
the first RRMU and has the structure (II):
0
11
[-O-(CH2)m-C-] (II)
wherein m is from 2 to about 9. At least about 70 mole % of the copolymer
comprises
RRMUs having the structure of the first RRMU of formula (I). Suitable polymer
products
include, but are not limited to, films, sheets, fibers, nonwovens, and
products formed by
bonding a plurality of fibers, for example nonwoven sheets and the like. The
polymer
products of the invention are advantageous in that they exhibit a combination
of softness
and elasticity while maintaining strength.
In another embodiment, the invention is directed to methods of forming
improved
biodegradable polymer products. The methods comprise stretching a composition
comprising a biodegradable polyhydroxyalkanoate copolymer at a temperature
above the
glass transition temperature T. of the composition and below the melting
temperature Tm
of the composition. The biodegradable polyhydroxyalkanoate copolymer comprises
at
least two RRMUs, wherein the first RRMU has the structure of formula (I) and
the second
RRMU is different from the first RRMU and has the structure of formula (II),
all as
defined above, with at least about 70 mole % of the copolymer comprising RRMUs
having
the structure of the first RRIVIU of formula (I). Conveniently, conventional
solid state
stretching may be employed. Thus, the methods of the invention comprise
relatively easy
steps as compared with many of the cumbersome methods of the prior art for
producing
stretched polymer products, and provide stretched polymer products having an
advantageous combination of physical properties.
These and additional objects and advantages will be more fully understood in
view
of the following detailed description.
4
CA 02387075 2002-04-10
WO 01/30893 PCT/USOO/29885
DETAILED DESCRIPTION
While not intending to be bound by theory, it is believed that the stretching
process, to some degree, orients the copolymer chains of the products
according to the
invention. The stretched polymer products of the invention unexpectedly
exhibit an
advantageous combination of physical properties, and particularly exhibit
softness and
elasticity while maintaining strength. Particularly, the stretched polymer
products of the
invention exhibit both a higher strength, for example as measured by a higher
tensile stress
at break, and a higher softness, for example as measured by a lower tensile
modulus, as
compared with an unstretched product of the same composition. This combination
of
properties is a highly unexpected outcome of a stretching process. Typically,
a stretching
process results in products that are both stronger and stiffer (less soft),
not stronger and
softer. For example, for fibers, M.S.M. Mark describes in Polymer Science
Dictionary,
Elsevier Applied Science, New York (1989), page 295, that "drawn or spun
fibres are
deliberately oriented along their length to enhance strength and stiffness in
this direction
due to uniaxial orientation", and L.E. Nielson and R.F. Landel describe in
Mechanical
Properties of Polymers and Composites, 2"d Edition, Marcel Dekker, Inc., New
York
(1994), page 116 that "many highly oriented fibers have Young's moduli about
an order of
magnitude greater than that of the unoriented polymers". For films, Nielson
and Landel
(page 116) also describe that "Biaxially oriented films, made by stretching in
two mutually
perpendicular directions, have reduced creep and stress relaxation compared to
unoriented
materials. Part of the effect is due to the increased modulus."
The products exhibit good elasticity in that they are able to recover quickly
when a
deforming force or pressure is removed. In preferred embodiments, the
elasticity exhibited
by the present products is springy in that the products easily respond to an
applied stress
and quickly return to their original shape after release of the deforming
stress. This
springy behavior which is exhibited by preferred products according to the
invention may
be similar to the elasticity of vulcanized rubber and certain synthetic
thermoplastic
elastomers. However, the present products also exhibit high strength and
resistance to
creep, whereby the products resist premature deformation at low stress and
resist sagging
when used. The products of the invention also exhibit good softness in that
they can be
CA 02387075 2002-04-10
WO 01/30893 PCT/USOO/29885
bent, twisted or folded without breaking. In preferred embodiments, the
softness is
accompanied by a supple quality which allows the materials to be readily bent,
twisted or
folded without any sign of injury. Thus, the present products are able to
exhibit high
strength at large deformations that is generally exceeded only by highly
oriented materials,
while exhibiting supple and springy characteristics at small deformations
which provide
enhanced pliability or drape, i.e., the products more easily conform to and
fit more snugly
around objects. Additionally, the products do not exhibit tackiness which is
associated
with many conventional elastomers without the use of powders or other
antiblock agents
that may affect performance or interact negatively in a desired application.
Importantly,
the present products are biodegradable.
The stretched polymer products are formed from a composition comprising a
biodegradable polyhydroxyalkanoate copolymer comprising at least two RRMUs.
The
first RRMU has the structure (I):
R~ 0
1 11
[-O-CH-(CH2)n-C-] (I)
wherein R' is H, or Cl or C2 alkyl, and n is 1 or 2. In a preferred
embodiment, R' is a
methyl group (CH3), whereby the first RRMU has the structure:
CH3 0
1 11
[-O-CH-(C H2)n-C-J
wherein n is 1 or 2. In a further preferred embodiment of the first RRMU, R'
is methyl
and n is 1, whereby the polyhydroxyalkanoate copolymer comprises 3-
hydroxybutyrate
units.
The second RRMU included in the biodegradable polyhydroxyalkanoate copolymer
is different from the first RRMU and has the structure (II):
0
11
[-0-(CH2)m-C-] (II)
wherein m is from 2 to about 9. Generally, in the RRMU of formula (II), the
length of
(CH2)m will, to some extent, influence the reduction in overall crystallinity
of the
6
CA 02387075 2005-08-10
copolymer. In a preferred embodiment, m is from 2 to about 9, and more
preferably is
from about 2 to about 5. In a further preferred embodiment, m is about 3.
In order to obtain the advantageous combination of physical properties
exhibited
by the stretched polymer products of the present invention while maintaining
the
biodegradability of the polyhydroxyalkanoate copolymer, at least about 70 mole
% of the
copolymer comprises RRMUs having the structure of the first RRMU of formula
(I).
Suitably, the molar ratio of the first RRMUs to the second RRMUs in the
copolymer is in
the range of from about 70:30 to about 98:2. More preferably, the molar ratio
is in the
range of from about 75:25 to about 95:5, and even more preferred, the mole
ratio is in the
range of from about 80:20 to about 90:10. As a result, the
polyhydroxyalkanoate
copolymer suitably has a number average molecular weight of greater than about
150,000
g/mole. While not intending to be bound by theory, it is believed that the
combination of
the second RRMU chain lengths and the indicated molar amounts sufficiently
decrease the
crystallinity of the first RRMU to form the copolymer with desired physical
properties.
In further embodiments of the polyhydroxyalkanoate copolymer employed in the
compositions, one or more additional RRMUs may be included. Suitably, the
additional
RRMUs may have the structure (III):
R3 0
1 11
[-O-CH-(CH2)P-C-] (III)
wherein R3 is H, or a C 1-C 19 alkyl or alkenyl group and p is 1 or 2, with
the provision that
the additional RRMUs are not the same as the first or second RRMUs.
The biodegradable polyhydroxyalkanoate copolymers can be synthesized by
chemical or biological based methods as disclosed, for example, by Noda in
U.S. Patent
No. 5,618,855, and Noda et al. in U.S. Patent No. 5,942,597.
The compositions preferably comprise greater than about 50 weight percent of
the
biodegradable polyhydroxyalkanoate copolymer, and it is preferred that the
copolymer is
present as a continuous phase in the composition. In one embodiment, the
composition
may comprise the polyhydroxyalkanoate copolymer as the only polymeric
component,
7
CA 02387075 2002-04-10
WO 01/30893 PCT/US00/29885
while in yet other embodiments, one or more additional polymers or copolymers
may be
included in combination with the polyhydroxyalkanoate copolymer. For example,
the
compositions may include a combination of two or more of such biodegradable
polyhydroxyalkanoate copolymers, a combination of the biodegradable
polyhydroxyalkanoate copolymer as defined herein, and other
polyhydroxyalkanoate
copolymers, and/or additional polymeric components, for example additional
polyester
components or the like. In such embodiments, the biodegradable
polyhydroxyalkanoate
copolymer preferably comprises at least about 50 weight percent, more
preferably at least
about 60 weight percent, and even more preferably at least about 75 weight
percent, of the
composition.
The compositions may further include various nonpolymeric components
including,
among others, nucleating agents, antiblock agents, antistatic agents, slip
agents, pro-heat
stabilizers, antioxidants, pro-oxidant additives, pigments, fillers and the
like. These
additives may be employed in conventional amounts although, typically, such
additives are
not required in the composition in order to obtain the advantageous
combination of
softness, elasticity, and strength. Additionally, one or more plasticizers may
be employed
in the compositions in conventional amounts although again, plasticizer are
typically not
required in order to obtain the advantageous combination of properties
discussed above.
The upper limit of the use temperature of biodegradable polymer products,
which
exhibit a desirable combination of softness and elasticity, while maintaining
strength, may
be substantially higher than room temperature, because of the relatively high
melt
temperature of the polymer used to fabricate such products. Preferably the
upper limit of
the use temperature of the products can exceed above 80 C without melting or
becoming
excessively soft, more preferably above 100 C, even more preferably above 120
C.
The polymer products may be in any physical form and typically will comprise a
stretched film, sheet, fiber, or nonwoven or a product from a stretched film,
sheet, fiber, or
nonwoven. For example, a stretched nonwoven product can be produced by
stretching a
nonwoven structure that has been made by conventional means including
spunbonding,
melt blowing, air-laying, carding, hydroentangling, or combinations of the
forementioned
and the like, and in which the nonwoven can be bonded by any means known in
the art,
8
CA 02387075 2002-04-10
WO 01/30893 PCT/US00/29885
including but not limited to thermal, mechanical, chemical, or adhesive
bonding.
Alternatively, a plurality of stretched fibers can be bonded to form a
nonwoven web which
can exhibit similar softness, elastic and strength properties to the previous
approach.
Additionally, the stretched polymer product need not be limited to single
component
structures, for example, monolayer film or monofilament fibers. The stretched
polymer
products can also include various multiconstituent products, including but not
limited to
(1) fiber or nonwovens having side-side, sheath-core, multiple-segment,
islands-in-the-sea,
and matrix-fibril morphologies, (2) coextruded films or sheets consisting or
two or more
layers, (3) film/fiber, sheet/fiber, film/nonwoven, or sheet/nonwoven
composites, or (4)
combinations of 1-3, as long as the PHA copolymer(s) comprise at least 50
weight percent
of the composition, more preferably at least about 60 weight percent, and even
more
preferably at least about 75 weight percent. Further, one skilled in the art
will appreciate
that the level of elasticity, strength, and softness exhibited by a stretched
multiconstituent
polymer product will be influenced by the particular morphology and
configuration, such
as described in part in Polymer Blends and Alloys, M.J. Folkes and P.S. Hope
(Editors),
Chapman & Hall, New York (1993), and in Plastics Films, 2"d Edition, J.H.
Briston,
Longman Inc., New York (1983).
The stretched polymer products may be conveniently formed by conventional
solid
state stretching techniques wherein the composition is stretched at a
temperature above the
glass transition temperature Tg of the composition and below the melting
temperature T.
of the composition. Preferably, the product is obtained by solid state
stretching the
composition at a temperature at least 20 C above the glass transition
temperature (Tg +
20 C) and at least 20 C below the melting temperature (Tm - 20 C) of the
composition.
The glass transition temperature of the polyhydroxyalkanoate copolymers
employed in the
compositions of the invention are generally below room temperature, i.e., less
than about
25 C. Generally, the melting temperatures of the copolymers are greater than
about
100 C.
One skilled in the art will appreciate that the level of elasticity, including
the
springy characteristics, strength and softness exhibited by the stretched
product will be
influenced by not only the stretching temperature, but also by the rate and
extent of
9
CA 02387075 2002-04-10
WO 01/30893 PCT/US00/29885
stretching, whether the stretching is carried out at a constant or variable
rate of
displacement, strain or stress, the type of stretching and, for films, sheets,
or nonwovens,
whether the stretching is uniaxial or biaxial and, if biaxial, whether the
stretching steps are
performed sequentially, simultaneously, or some combination thereof. The
stretching may
be conducted at a constant or variable rate of displacement, strain or stress
in accordance
with techniques known in the art, for example, by a tenter framing process for
films,
sheets, and nonwovens, such as described by J.H. Briston in Plastics Films, 2d
Edition,
Longman Inc., New York (1983), pages 83-85, or for fiber products by a
spinning
operation with Godet rolls or filament winding, such as described by J.E.
McIntyre and
M.J. Denton in Concise Encyclopedia of Polymer Science and Engineeriniz, John
Wiley &
Sons, New York (1990) pages 390, 391, and 395. Additionally, for films,
sheets, and
nonwovens, the stretching may be performed uniformly across the form, for
example as
achieved in a tenter framing process, or incrementally across the form, for
example as in a
ring-rolling operation such as described in US Patents 4,116,892 and 5,296,184
where
alternating parallel regions that are stretched coexist with with regions that
remain virtually
unstretched. Additionally, for blown films and sheets, the stretching can be
performed by
the double or tubular bubble process, such as described by J.H. Briston in
Plastics Films,
2 a Edition, Longman Inc., New York (1983), page 85. Further, depending upon
the end
use, the stretching of films, sheets, and nonwovens may be performed
uniaxially or
biaxially, and, if biaxially, the stretching steps may be performed
sequentially,
simultaneously or any combination thereof, in accordance with techniques known
in the
art. All of the above described methods of stretching films, sheets, and
nonwovens,
including uniform as well as incremental stretching processes, can be used and
are within
the scope of the present invention.
The extent of stretching must exceed the yield or neck point in at least one
direction of stretch while remaining below the failure point in all stretch
directions.
Preferably, the product is obtained by stretching the composition in at least
one direction
to an extent greater than about 50% strain from its initial unstretched state,
and more
preferably in at least one direction greater than about 100% strain from its
initial
unstretched state. In further preferred embodiments, the product is obtained
by stretching
CA 02387075 2002-04-10
WO 01/30893 PCT/US00/29885
the composition in at least one direction to an extent in the range from about
200% to
about 1500% strain from its initial unstretched state, and more preferably in
at least one
direction in the range from about 300% to about 1000% strain from its initial
unstretched
state. Additionally, one skilled in the art will further appreciate that the
effective strain in
each stretch direction will depend on the deformation process and geometry.
For example,
in a uniform stretching process like fiber spinning the effective strain is
determined by the
drawdown ratio, which is the ratio of the velocity of the fiber exiting the
Godet or
stretching rolls divided by the velocity of the fiber entering the Godet or
stretching rolls,
and as such is proportional to the overall change in sample length. By
contrast, for
example, in an incremental stretching process like ring rolling the effective
strain is
determined by the draw ratio within each stretch or gauge section such as
disclosed in US
Patent 4,116,892 referenced above, and as such is proportional to localized
changes in
length within each stretch region and not generally to the overall change in
sample length.
Generally, once the stretching has been completed, the stretched polymer
product
may be cooled to below its glass transition temperature or may be subjected to
a heat-
setting step where the stretched form is annealed under strain at a
temperature above the
glass transition temperature of the composition but below the melting
temperature of the
composition and typically in the range of from about T, + 20 C to about T. -
20 C.
The solid state stretching may be conducted using any suitable apparatus known
in
the art, such as discussed above. The examples set forth below describe the
use of an
Instron universal testing machine, but one of ordinary skill will appreciate
other apparatus
which may be employed. The Instron or other stretching apparatus which is
employed is
preferably equipped with an environmental chamber to provide a thermally
controlled
stretching process. One skilled in the art will appreciate that the stretching
conditions and
the annealing conditions, if employed, can be determined for a given
composition as
described herein depending on the desired end use application.
The stretched products of the present invention are advantageous in exhibiting
a
good combination of softness and elasticity while maintaining strength. More
specifically,
the stretched polymer products of the invention exhibit (1) higher strength,
for example as
measured by a higher tensile stress at break, (2) higher softness, for example
as measured
11
CA 02387075 2002-04-10
WO 01/30893 PCT/US00/29885
by a lower Young's modulus, and (3) higher elasticity, for example as measured
by a
higher percent recovery after release of the deforming stress, as compared
with an
unstretched product of the same composition. In preferred embodiments, the
stretched
polymer products have a tensile strength of greater than about 15 MPa, as
measured for
example according to ASTM D882-97 for films, and preferably greater than about
20
MPa. Additionally, in preferred embodiments, the stretched polymer products
have a
Young's modulus of less than about 400 MPa, as measured for example according
to
ASTM D882-97 for films, more preferably less than about 300 MPa, and even more
preferably less than about 200 MPa.
The elasticity of the stretched products, and particularly the springiness of
the
products, allows the products to substantially recover when the stretched
products are
elongated, for example during use. Thus, in a preferred embodiment, the
stretched
polymer products exhibit elastic behavior that results in greater than about
65% recovery
of the product in less than about 15 seconds when the product is elongated up
to about
50%, as measured for example according to ASTM D5459-95 for films. More
preferably
greater than about 75% recovery, and even more preferably greater than about
85%
recovery.
The stretched products are useful for comprising various biodegradable
articles
including disposable, environmentally benign packaging, overwrap, absorbent
articles
including diaper/catemenial/feminine hygiene topsheets, nonwoven cores, and
backsheets,
stretch films including food and pallet wrap, agricultural films, mulch film,
balloons, skin
packaging, stretch packaging, bags including food and garbage bags,
contraceptives
including condoms and diaphraghms, shrink packaging, synthetic paper,
carpeting, fishing
line, hospital gowns, gloves, band-aids, wound dressings, disposable garments
including
shirts and socks, disposable surgical drapes, sutures, mailing envelopes, and
agricultural
uses including row covers, bed covers, turf covers, and weed barriers.
The products and methods of the present invention are further exemplified in
the
following examples. In the examples and throughout the present specification,
parts and
percentages are by weight unless otherwise specified.
EXAMPLE 1
12
CA 02387075 2002-04-10
WO 01/30893 PCTIUSOO/29885
This example demonstrates uniaxial stretching of a melt extrusion cast film
according to
the invention. Specifically, a copolymer of 3 -hydroxybutyrate and 11.1 mole
percent 6-
hydroxyhexanoate (hereafter a PHBHH copolymer) is melt extruded into cast
films of
varying thicknesses ranging from about 0.004 to about 0.005 inches.
Rectangular strips of
about 1 x 4 inches are cut from the film with the long dimension parallel to
the machine
direction of fabrication. Individual strips are placed in an Instron universal
testing machine
(Model 1122, Canton, MA) such that the long dimension is in the pull
direction, with a test
gage length of one inch. The test machine is equipped with a Sintech ReNewTM
1122/R
upgrade package, TestWorksTM V3.02 software for test control and analysis, and
200 lbf
high/low temperature pneumatic grips (model S512.01), all from MTS Systems
Corp.,
Research Triangle Park, NC, as well as an environmental chamber, Series 3710,
from MTS
Direct, Eden Prairie, MN, to provide thermally controlled uniaxial stretching.
For tests in
which the stretching temperature is different from ambient, the test strips
are allowed to
equilibrate for about 2-3 minutes before starting the stretching process.
Using a stretching
temperature of about 60 C and an initial strain rate of about 2 in/in-min,
film strips of the
PHBHH cast film can be extended beyond 600% elongation before failure. In
addition,
the stretched strips exhibit elastic behavior, i.e., when clamped between the
thumb and
forefinger on each hand of a technician and then pulled apart, the film is
easily extended
and quickly returns to its original length after release. This behavior
demonstrates that the
PHBHH film composition is highly ductile and the stretched PHBHH film
composition is
springy.
EXAMPLE 2
This Example demonstrates uniaxial stretching of a PHBHH copolymer melt spun
fiber
according to the invention. The PHBHH copolymer from Example 1 is melt spun
into
fibers having a diameter of about 4 mm. Test strands about 3 inches long are
cut from the
PHBHH fiber. Following the stretching procedure described in Example 1, using
a
stretching temperature of about 60 C and an initial strain rate of about 2
in/in-min, strands
of the PHBHH fiber can be elongated in excess of 400% before failure. In
addition, the
stretched fiber strands exhibit an elastic behavior, i.e., when clamped
between the thumb
and forefinger on each hand of a technician and then pulled apart, the fiber
is easily
13
CA 02387075 2002-04-10
WO 01/30893 PCT/USOO/29885
extended and quickly returns to its original length after release. This
behavior
demonstrates that the PHBHH fiber composition is highly ductile and the
stretched
PHBHH fiber is springy.
EXAMPLE 3
This Example demonstrates biaxial stretching of a PHBHH melt extrusion cast
film
according to the invention. A film strip of the PHBHH copolymer cast film from
Example
1 is first stretched about 150% according to the procedure described in
Example 1 at a
temperature of about 60 C and an initial strain rate of about 4 in/in-min.
This stretched
sample is then rotated ninety degrees within the test machine such that the
first stretch
direction is perpendicular to the pull direction. A second stretch of about
150%
elongation is carried out a temperature of about 60 C and an initial strain
rate of about 4
in/in-min. The springy nature of the resulting biaxially oriented film is
readily discerned by
simply stretching the film by hand, as the film quickly returns to its
original length after
release of the deforming strain.
EXAMPLE 4
This Example compares tensile properties of stretched and unstretched PHBHH
melt
extrusion cast films. Specifically, the tensile properties of stretched and
unstretched
PHBHH melt extruded cast film strips from Example l are determined by a method
outlined in ASTM D882-97 using an Instron universal testing machine such as
described in
Example 1. The stretched samples are produced at a stretch temperature of
about 60 C
and an initial strain rate of about 4.0 in/in-min. The stretched films are
stronger, as
evidenced by a higher stress at break, softer, as evidenced by a lower Young's
modulus,
but less extensible, as evidenced by a lower strain at break, than the
unstretched
counterparts.
EXAMPLE 5
This Example demonstrates the elastic recovery of a stretched PHBHH melt
extrusion cast
film according to the invention. The elastic properties of a stretched PHBHIi
cast film
from Example 1 are measured by determining the dimensional recovery exhibited
by a film
when it is stretched in an Instron universal testing machine, such as
described in Example
1. The stretched films are extended at ambient temperature to a predetermined
extension
14
CA 02387075 2002-04-10
WO 01/30893 PCT/US00/29885
at an initial strain rate of 1.0 in/in-min, the applied stress removed, and
the decrease in
strain measured after about 10-15 seconds relaxation time. The stretched PHBHH
film
strips show a short-term recovery of greater than about 85% from up to about
100%
extension. This behavior demonstrates the long-range mechanical elasticity of
the
products according to the invention.
EXAMPLE 6
This Example demonstrates an elastic, pliable band-aid formed from stretched
PHBHH
cast film according to the invention. A stretched film is prepared from a
PHBHH cast film
of Example 1, where the stretching process is carried out at a temperature of
about 60 C
and an initial strain rate of about 4.0 in/in-min. A 0.75 x 3 inch film strip
is cut from the
stretched PHBHH film. An absorbent pad 0.75 x 1.0 inch is glued lengthwise to
the center
of the strip, and self-adhering Velcro pieces are attached to the ends to form
a band-aid.
Use of the band-aid on an index finger shows that the film easily flexes, and
follows both
the back-and-forth and bending motions of the index finger without sagging.
EXAMPLE 7
This Example demonstrates the fabrication of a springy, soft nonwoven sheet
from
stretched PHBHH fiber according to the present invention. A nonwoven sheet is
prepared
from stretched PHBHH fibers. Melt spun PHBHH fibers are stretched as described
in
Example 2 at a temperature of about 60 C and an initial strain rate of about
3.0 in/in-min.
Several of the stretched strands are cut to 3 inch lengths and placed randomly
between two
mm think, 6 x 6 inch sheets of polytetrafluoroethylene (Teflon ), the whole
being
placed between the platens of a Carver hydraulic laboratory press. The upper
platen is
preheated to about 15 C above the calorimetrically determined melting point of
the
PHBHH, and has a equally spaced spot bonding pattern of 25 1.0 mm diameter
bonds per
square inch. Sufficient pressure is applied so as to cause the bond spots to
soften and fuse.
The pressure is released and the nonwoven sheet is allowed to cool to room
temperature
before removing the outer polytetrafluoroethylene sheets. The springy nature
of the
nonwoven sheet is readily discerned by simply stretching the sheet by hand, as
the sheet
quickly returns to its original dimensions after release of the applied
strain.
EXAMPLE 8
CA 02387075 2005-08-10
The Example demonstrates uniaxial stretching of conventional melt extrusion
cast films
and tensile properties thereof. Melt extruded cast films are made from several
biodegradable polymers including polycaprolactone (Tone P787, Union
Carbide,),
Bionolle 1001 and 3001 (Showa Highpolymer Co., LTD., Tokyo, JP), Eastar 14766
(Eastman Chemical Company, Kingsport, TN), and BAK 1095 (Bayer Corporation,
Pittsburgh, PA), as well as from several nondegradable polymers including
polypropylene
(type 7300KF, Millennium Petrochemicals, Cincinnati, OH), high density
polyethylene
(HDPE) (type LTPR059, Millennium Petrochemicals, Cincinnati, OH), and a 50:50
by
weight low density polyethylene (LDPE):linear low density polyethylene (LLDPE)
blend
(type NA940000 and GA5010110, respectively, Millennium Petrochemicals,
Cincinnati,
OH). Following the sample preparation and stretching procedure described in
Example 1,
using a stretching temperature of about 25 C and an initial strain rate of
about 1.0 in/in-
min, film strips of various cast films can be extended beyond 300% elongation
before
failure. This behavior is consistent with film compositions that are ductile.
The tensile properties of the stretched melt extruded cast film strips are
determined
by the method outlined in ASTM 882-97 using an Instron universal testing
machine such
as described in Example 1. Typical of stretching processes, the various
stretched films are
stronger, as evidenced by higher stress at break, but stiffer, as evidenced by
higher
Young's modulus, and less extensible, as evidenced by lower stress at break,
than the
unstretched counterparts.
Comparing the tensile properties of the stretched PHBHH films from Example 4
with the tensile properties of the stretched films from this Example shows
that the PHBHH
films are softer than the stretched films formed from the conventional
compositions. In
fact, the stretching process enhances the softness of the PHBHH films, as
evidenced by a
decrease in the Young's modulus, whereas the conventional compositions all
show an
increase in stiffness as evidenced by an increase in Young's modulus. In all
cases, the
stretched films show some level of recovery from small extensional
deformations;
however, it becomes much harder to extend the stretched films formed from the
conventional compositions beyond relatively low elongations, compared with a
stretched
film according to the invention. For example, a 10 lb stretching force, or
equivalently a 35
16
CA 02387075 2002-04-10
WO 01/30893 PCT/US00/29885
MPA stress for a one inch wide film strip 0.002 inches thick, results in an
immediate 45%
extension for a stretched PHBHH film, whereas, the stretched films formed from
the
conventional compositions at best show an immediate 8% extension.
EXAMPLE 9
This example demonstrates the fabrication of a nonwoven sheet using a melt
blown
process. Specifically, the PHBHH copolymer from Example 1 is fed into an
extruder
which gradually melts the polymer as it feeds the melt blowing die. The die
meters the
polymer into a balancing channel that is oriented linearly in the cross
machine direction and
that narrows to a spinneret of several holes per linear inch. At the point of
exit the
polymer strands are attentuated by heated, high velocity air. The fibers that
are formed are
continuous and extremely fine, and are blown onto a moving collector screen to
form the
nonwoven web structure. The web is thermally bonded by passing the web through
a 2-
roll stainless steel stack roll on which one roll there is a spot bonding
pattern of about 25
1.0 mm diameter bonds per square inch. The stack rolls are preheated to about
15 C
above the calorimetrically determined melting point of the PHBHH composition,
and
sufficient pressure is applied to the web as it passes through the stack roll
so as to cause
the spot bonds to soften and fuse. The lack of elasticity is readily discerned
by simply
stretching the nonwoven web by hand, as the web does not easily elongate and
does not
return to its original dimensions after release of the applied strain.
EXAMPLE 10
This example demonstrates the fabrication of a springy nonwoven sheet from a
melt blown
nonwoven web. Rectangular strips of about 1 x 4 inches are cut from the bonded
PHBHH
web described in Example 9, with the long dimension parallel to the machine
direction of
fabrication. Following the stretching procedure described in Example 1, using
a stretching
temperature of about 60 C and an initial strain rate of about 1.0 in/in-min,
elongated strips
of the PHBHH nonwoven web can be produced. The springy nature of the stretched
nonwoven sheet is readily discerned by simply stretching the sheet by hand, as
the sheet is
easily elongated and quickly returns to it original dimensions after release
of the applied
strain. Comparing this behavior with that of Example 7 shows that similar
springy
17
CA 02387075 2002-04-10
WO 01/30893 PCT/US00/29885
nonwoven products can be produced by either stretching a nonwoven sheet made
by
conventional means or by fabricating a nonwoven sheet from stretched fibers.
EXAMPLE 11
This example demonstrates the fabrication of a springy film product by
incrementally
stretching a PHBHH film in a ring rolling operation according to U.S. Patent
4,116,892.
The melt extruded PHBHH cast film from Example I is introduced in the machine
direction of manufacture through a pair of grooved rolls that are preheated to
a
temperature of about 60 C. The grooves are perpendicular to the machine
direction of the
film, have an approximate sinusoidal shape 3 mm deep and 3 mm apart, and
produce a
draw ratio of about 2. When the film is stretched to conform with the shape of
the
grooves, 8 groove tips simultaneously engage the film. The film is introduced
into the nip
of the intermeshing grooved rolls rotating at about 2 RPM to produce a feed
velocity of
approximately 2 feet per minute, and wound at about 4 feet per minute. The
film has
relatively transparent lines at 3 mm intervals corresponding to the contact
points, or
stretched areas, with undrawn opaque sections in between. The springy nature
of the ring-
rolled PHBHH film is readily discerned in directions parallel to the machine
direction by
simply stretching the sheet by hand, as the sheet is easily elongated and
quickly returns to
it original dimensions after release of the applied strain. By contrast,
stretching the film
product in directions perpendicular to the machine direction indicates no
apparent
springiness or elasticity, as the sheet does not easily elongate and does not
return to its
original dimensions after release of the applied strain. This example
illustrates that a ring
rolling operation can impart a uniaxial or directional elastic behavior to a
preferred film
product. This approach is also applicable for preferred sheet and nonwoven
products.
EXAMPLE 12
This example demonstrates the fabrication of a stretched film product by
incrementally
stretching a film of Bionolle 3001 (Showa Highpolymer Co., LTD., Tokyo, JP) in
a ring
rolling operation. Specifically, a melt extruded cast film of Bionolle 3001
with a thickness
of about 0.002 inches is ring rolled according to the procedure described in
Example 11,
using a grooved roll temperature of about 25 C. The lack of springiness is
readily
discerned by simply stretching the ring rolled film product by hand in
directions parallel
18
CA 02387075 2002-04-10
WO 01/30893 PCT/US00/29885
and perpendicular to the machine direction of manufacture, as the film does
not easily
elongate and does not return to its original dimensions after release of the
applied strain.
In fact, the ring rolling operation permanently deforms the Bionolle 3001 film
in the
machine direction.
EXAMPLE 13
This example demonstrates the fabrication of a contractive film product by
incrementally
stretching a multilayer film in a ring rolling operation. Specifically, the
PHBHH copolymer
from Example 1 is coextruded with the Bionolle 3001 resin from Example 12 into
a two-
layer cast film product where the thickness of the PHBHH layer is about 0.002
inches and
the Bionolle 3001 layer is about 0.001 inches. This PHBHH/Bionolle film is
then ring
rolled according to the procedure described in Example 11, using a grooved
roll
temperature of about 60 C. The result of the ring rolling operation is a
contractive film
product, in which the PHBHH layer is springy as described in Example 11 and
the Bionolle
3001 layer is nonspringy and permanently deformed as described in Example 12,
and in
which the Bionolle layer forms gathers or pleats as the PHBHH layer contracts
upon
release of an applied strain. Additionally, the Bionolle layer limits the
extent to which the
product is rendered elastically extensible, at least up to the point of
initial stretching.
EXAMPLE 14
This example demonstrates the fabrication of a disposable baby diaper, where
the
dimensions listed are intended for use with a child in the 6-10 kilogram size
range. These
dimensions can be modified proportionally for different size children, or for
adult
incontinence briefs, according to standard practice.
1. Backsheet: 0.020-.038 mm film consisting of the PHBHH copolymer from
Example 1; width at top and bottom 33 cm; notched inwardly on both sides to a
width-at-
center of 28.5 cm; length 50.2 cm.
2. Topsheet: carded and thermally bonded staple-length polyproplyene fibers
(Hercules type 151 polypropylene); width at top and bottom 33 cm; nothched
inwardly on
both sides to a width-at-center of 28.5 cm; length 50.2 cm.
3. Absorbent core: 28.6 g of cellulose wood pulp and 4.9 g of absorbent
gelling
material particles (commercial polyacrylate from Nippon Shokubai); 8.4 mm
thick,
19
CA 02387075 2002-04-10
WO 01/30893 PCT/USOO/29885
calendered; width at top and bottom 28.6 cm; notched inwardly at both sides to
a width-
at-center of 10.2 cm; length 44.5 cm.
4. Elastic leg bands: four individual rubber strips (2 per side); width 4.77
cm;
length 37 cm; thickness 0.178 mm (all the foregoing dimensions being in the
relaxed state).
The diaper is prepared in standard fashion by positioning the core material
covered
with the topsheet on the backsheet and gluing.
The elastic bands (designated "inner" and "outer", corresponding to the bands
closest to, and farthest from, the core, respectively) are stretched to ca.
50.2 cm and
positioned between the topsheet/backsheet along each longitudinal side (2
bands per side)
of the core. The inner bands along each side are positioned ca. 55 mm from the
narrowest
width of the core (measured from the inner edge of the elastic bank). This
provides a
spacing element along each side of the diaper comprising the flexible
topsheet/backsheet
material between the inner elastic and the curved edge of the core. The inner
bands are
glued down along their length in the stretched state. The outer bands are
positioned ca. 13
mm from the inner bands, and are glued down along their length in the
stretched state.
The topsheet/backsheet assembly is flexible, and the glued-down bands contract
to
elasticize the sides of the diaper.
EXAMPLE 15
The diaper of Example 14 is modified by replacing the elastic leg bands with
the springy
PHBHH film product described in Example 1.
EXAMPLE 16
The diaper of Example 14 is modified by replacing the elastic leg bands with
the
contractive film product described in Example 13.
The specific embodiments and examples set forth above are provided for
illustrative
purposes only and are not intended to limit the scope of the following claims.
Additional
embodiments of the invention and advantages provided thereby will be apparent
to one of
ordinary skill in the art and are within the scope of the claims.