Note: Descriptions are shown in the official language in which they were submitted.
CA 02387549 2002-05-06
WO 01/09282 PCT/EP00/06153
Device for culturing and/or treating cells
The invention relates to a device for culturing and/or
treating cells of the type defined in detail in the
precharacterizing clause of claim 1. The invention also
relates to a method for culturing and treating cells.
DE 42 06 585 C2 describes a device for mass culture of
cells, especially hepatocytes, on plate-like cell
culture carriers, where at least part of the surfaces
of the cell culture carriers is gas permeable. Oxygen
can be passed into the interior of the cell culture
carriers, and a collagen layer covering the cells is
applied to the cell culture carrier. The next cell
culture carrier is arranged directly or a small
distance above the collagen layer. Culture medium can
be passed into the collagen layer or into the space
between the collagen layer and the next cell culture
carrier. The device is constructed like a sandwich. A
disadvantage of this device is that cell chambers which
are precisely defined and have particular volumes are
always present. In addition it is difficult to observe
the cells in the cell chambers in order to ascertain
any changes or developmental states.
DE 42 22 345 A1 describes a method for culturing a cell
type in a coculture method with liver cells, where
liver cells are cultured on a support in a sandwich
method. A first matrix layer is arranged between the
liver cells and the support to anchor the liver cells,
and a second matrix layer is located above the liver
cells.
WO 95/17526 describes a method for culturing skin by a
so-called air lift method. This entails gas or sterile
air being brought into a cell growth chamber. The cells
in this case are located on a semipermeable membrane.
Basal cells lying on the semipermeable membrane are
supplied with nutrients through the medium chamber by
CA 02387549 2002-05-06
- 2 -
diffusion. The basal cells show multilayer growth
toward the air phase. However, the disadvantage in this
case is that with the previously disclosed method the
skin structure is "upside down". This means that other
cell types such as, for example, connective tissue
cells (fibroblasts) either must be initially seeded and
time allowed to elapse, or the skin must be
mechanically raised. However, this may lead to damage
to the sensitive structures.
DE 197 19 751 A1 relates to a device for culturing
and/or treating cells, where a cell chamber is arranged
on a gas-permeable carrier. The cell chamber is formed
by a gas-permeable, non liquid-permeable film which is
laid on the carrier, and by a second microporous film,
which is arranged over the first film or is integral
with the latter. At least one of the two films is
elastic such that the final volume in the cell chamber
can be many times its initial volume. The cell chamber
is provided with at least one supply and/or discharge
line. This previously disclosed device is particularly
suitable for a mass culture system. In addition, it can
be adapted to the particular requirements since it is
possible to begin with a very small volume and a low
number of cells which are being treated or cultured in
the cell chamber, and then to increase the starting
volume many times during the treatment. It is also
possible for one or more units to be placed, one above
the other in the form of a sandwich, in a conventional
incubator.
The present invention is based on the object of improv
ing a device as disclosed in DE 197 19 751 A1 further,
in particular a further simplification and an even
greater variability of the device.
This is achieved according to the invention by the
features mentioned in claim 1.
CA 02387549 2002-05-06
- 3 -
A method of the invention is indicated in claim 37.
One of the essential advantages of the device of the
invention and of the method of the invention is that a
very shallow cell culture chamber with minimal layer
thickness is obtained in this way, with the outer walls
of the cell culture chamber being at least partly gas
permeable. A large variability of the device together
with a simple design is achieved through the
moldability of the cell culture chamber. A further
advantage is that moldability of the cell culture
chamber in virtually any way desired is possible owing
to the geometry of the device.
The frames and stability structures indicated in the
dependent claims for forming the cell culture chamber
and the cell cultures make even further moldability
possible, in particular both before and during the
treatment of the cells.
A further improvement of the invention may comprise the
frames and/or the stability structures themselves also
being moldable, in particular both before and during
the culturing method.
. . 25
A further considerable advantage of the achievement of
the invention is that the complete unit with cells can
be frozen and stored in a defined shape in a controlled
manner. This makes it possible to use the device of the
invention also as transport system.
As is evident, one of the considerable differences
compared with the prior art, where only flexible cell
culture chambers or films are present, is now the
achievement not only of the possibility of flexibility
but also moldability or modelability of the cell
culture chamber and its geometry. It is possible in
this way to produce virtually any shapes and designs of
the cell culture chamber and thus practice-oriented
CA 02387549 2002-05-06
- 4 -
results.
If the cell culture chamber is in the form of a thin
layer structure in a design of the invention, then very
small oxygen diffusion pathways are present, especially
at the start of the culturing.
Advantageous designs and further developments are
evident from the dependent claims and from the
exemplary embodiments which are described in principle
hereinafter on the basis of the drawing.
This shows in
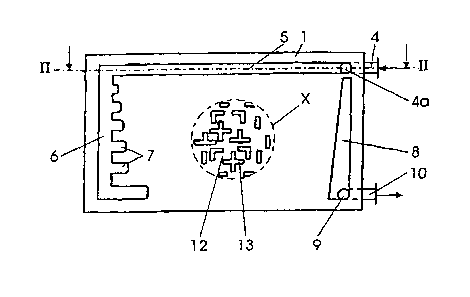
Fig. 1 a diagrammatic representation of a top view of
a carrier plate of the device of the invention;
Fig. 2 a section along the line II-II in Fig. 1 with a
flexible film and a cover plate;
Fig. 3 an arrangement of the device shown in Fig. 2
(without cover plate) rotated by 180°;
Fig. 4 a perspective view of a frame-like carrier
plate;
Fig. 5 a cross section along line V-V in Fig. 4 in
enlarged representation;
Fig. 6 an outline representation of a bioreactor;
Fig. 7 a perspective representation of a carrier plate
which is curved to form a circle;
Fig. 8 a perspective representation of a carrier plate
which is curved to form a spiral;
Fig. 9 a top view of a structure as heart valve;
CA 02387549 2002-05-06
- 5 -
Fig. 10 a structure as heart valve in lengthwise
section; and
Fig. 11 a structure for forming a heart valve in a
container.
In a basic design, the device has a moldable carrier
plate, which is principally composed of plastic, as
carrier 1. A gas-permeable and flexible plastic film 2
is applied to the carrier plate 1 and is tightly
connected to the edges of the carrier plate 1.
The carrier plate 1 is provided on one end face in the
edge region with an inflow connector 4 through which is
fed, for example, nutrient medium which opens from an
internal bore 9 which is connected to the inflow
connector 4 into an inflow channel 5 which runs along a
side wall of the carrier plate 1. The nutrient medium
flows at the side in the inflow channel 5 which is
inlet into the surface of the carrier plate 1 in the
direction of the end face opposite to the inflow side.
The inflow channel 5 opens on this end face into a
transverse channel 6 which extends at least approxi-
mately over the entire width of the carrier plate 1.
Projections 7 which have an inclined upward course lead
from the transverse channel 6 to the upper side of the
carrier plate 1.
As is evident from Fig. 1, the length and, where
appropriate, also the width of the projections 7
increases as the distance from the inflow channel 5
increases, so that the nutrient medium is uniformly
distributed over the surface of the carrier plate 1
and, in this way, a uniform flow is moved toward an
outflow channel 8, which likewise has a transverse
course. In order to generate a uniform flow here too,
and a correspondingly uniform outflow, the outflow
channel 8 can likewise be provided with projections 7
which lie as mirror images thereto. An alternative
CA 02387549 2002-05-06
possibility is also - as represented in Fig. 1 - to
provide a transverse channel 8 which continuously
widens toward an outflow bore 9. The outflow bore 9 is
connected via a bore in the interior of the carrier
plate 1 through an outflow connector 10 which is placed
on the carrier plate 1. The shape of the two transverse
channels 6 and 8 and the course of the projections 7 is
indicated only by way of example. The only essential
point is that a uniform and continuous flow of the
10. nutrient medium results over the entire width of the
carrier plate 1.
The flexible film 2 can be connected to the carrier
plate 1 at the edges in any suitable manner, for
example by clamping, bonding, welding or bolting. A
cell culture 11 can be placed on the carrier plate 1
together with the nutrient medium or else with another
carrier liquid in a cell culture chamber 11a which is
formed by the carrier film 3 and the cell culture film
2 as thin-layer structure.
It is, of course, also possible to distribute the cell
culture 11 on the carrier plate 1 beforehand, and this
can likewise be introduced via the inflow connector 4.
The cell culture 11 settles directly on the surface of
the carrier plate 1 in accordance with the embodiment
depicted in Fig. 2. To avoid shear stress and flow
stress, the surface of the carrier plate 1 can be
structured in such a way that the cells become lodged
in small depressions 12 in the surface. For this
purpose, the surface of the carrier plate 1 can have a
checkerboard structure, for example, with corresponding
depressions 12 and side walls 13 raised as far as the
surface of the carrier plate 1. This structure is
evident in the enlargement depicted in the broken-line
circle X in Fig. 1. In order to create an appropriate
connection between the individual depressions 12 and
thus the cells, the walls 13 can also be provided with
perforations resulting in a type of labyrinth. It is
CA 02387549 2002-05-06
also possible for the films per se to be profiled, for
example, in checkerboard fashion, or contain glass
fibers or another material which define a particular
structure which are possibly altered appropriately by
fusion processes.
It is evident from Fig. 4 that the carrier plate 1 may
also consist only of a frame la to which the flexible
film 2 is connected. Below the flexible film 2 and
likewise connected to the frame-like carrier plate 1a,
the space between the frame can be filled by a carrier
film 3 which forms the outer film. The carrier film 3
may, but need not necessarily, be flexible and/or gas
permeable. Oxygen supply to the cell culture 11 takes
place both in the example shown in Fig. 2 and in that
shown in Fig. 4 via the elastic film 2.
The frame 1a can be provided with a multiplicity of
knobs 27 which serve to clamp an applied flexible film
or fasten it. For this purpose, the frame 1a can also
be designed - similar to two halves of a projector
slide frame - in which case flexible films 2 are placed
and clamped between two frames 1a.
The frame 1a may also be designed as a type of clamping
frame or as clamping spring. A clamping frame results
in the films 2 connected to the frames being clamped by
the latter, thus avoiding loop formation or pouch
formation. Because of the elasticity of the films 2,
the cell culture chamber 11a grows appropriately during
the culture. The design of a correspondingly open
clamping frame is indicated in Fig. 4 by a slit 28 on
one side. The frame la is designed in this case so that
forces acting in the direction of the arrow arise to
clamp the films 2 lying in between. In place of the
rectangular design, in this case an open round clamping
frame will also be used where appropriate, through
which all-round clamping of the films 2 is achieved
more easily.
CA 02387549 2002-05-06
-
The knobs 27 may also be designed as holes into which,
for clamping, the films 2 are lowered and appropriately
clamped.
Fig. 5 shows a cross section along the line V-V in
Fig. 4 in enlarged representation (especially of the
films). As can be seen, in this case the frame 1a or an
edge strip has a slope downward toward the interior.
This sloping avoids dead spaces in the cell culture
chamber 11a when the films 2 and, where appropriate,
the film 3 are placed on appropriately. In this case, a
cover plate 17 which is depicted in Fig. 2 can be put
on top of the film 2 for accurate guidance and loca-
tion, the cover plate 17 having a shape appropriately
complementary to the sloping of the frame 1a. The
object of the cover plate 17 and its shape will be
described in more detail below.
Fig. 3 shows an inverted arrangement for culturing or
treating a cell culture 11 such that, in this case, the
carrier plate 1 is at the top and the elastic film 2
hangs underneath. Since the cells in the cell culture
11 sediment in the direction of gravity, in this case
they lie on the inside of the elastic film 2. It is
possible in this case if necessary for the elastic film
2 likewise to be structured like the surface design of
the carrier plate 1 in Fig. 1. Owing to the surface
structuring, the nutrient medium flows over the cells
present in the depressions 12 in the form of a fine
film. The elastic film in the arrangement shown in
Fig. 3 may be appropriately etched for example to form
a honeycomb-like structure. Connection of the two films
2 and 3 with the frame-like carrier plate 1a can take
place, for example, by bolting or clamping, preferably
with an appropriate seal as intermediate layer.
It is also evident from the broken-line depiction in
Fig. 3 that a unit can also be reflected on a carrier
CA 02387549 2002-05-06
- 9 -
plate 1, making it possible to use a single carrier
plate 1 to form two cell culture chambers 11a. It is
possible in this case for the two units to be operated
separately from one another, or they can communicate
with one another through one or more perforations 26.
It is possible in this case where appropriate for a
common inflow and a common outflow to be provided. The
cells may in this case be placed both on the inside of
the cell culture film 2 and on the carrier plate 1. A
marked saving in material and costs is achieved in this
way.
It is, of course, also possible in a simple way for
nutrient medium to flow over the carrier plate 1
directly from the inflow side with inflow connector 4
to the opposite side and then be discharged there. In
this case, the inflow channel 5 would be dispensed
with, and the transverse channel 8 would merely instead
of forming the outflow then be designed as inflow
channel and, on the other side, an appropriate outflow
channel would be inserted in the carrier plate 1.
However, for practical reasons, the direction of flow
chosen in Fig. 1 will be provided because, in this
case, all inlet and outlet lines, which are generally
provided in the form of tubing, will take place from
one side and thus be completely accessible from one
side. This design will be provided in particular when -
as depicted in Fig. 6 - a plurality of devices are
treated in a type of bioreactor 14, for example in the
form of a vertical rack. For this purpose, the
bioreactor 14 has a plurality of slide-in compartments
15 which are arranged appropriately one on top of
another and into which sliding of the devices shown in
Figures 1 to 4 is possible. All the inflows and
outflows are then in this case on one side. The
individual modules can also be directly connected with
one another.
It is also possible - as can be seen - for a plurality
CA 02387549 2002-05-06
- 10 -
of rows of slide-in compartments 15 to be arranged side
by side in the bioreactor 14. The bioreactor l4 will
generally also be provided with wheels 16 for ease of
transport.
The cover plate 17 is illustrated by means of Fig. 2 as
a further possibility for treating a cell culture 11.
The cover plate 17 is placed on the film 2. The volume
can be minimized by the cover plate 17. The spacings
can also be adjusted flexibly. This is a particular
advantage in the preparation phase, for example in the
first 48 hours, when it is wished to leave the cell
culture 11 undisturbed. This results in the dead space
over the system not being too large and, at the same
time, the diffusion exchange is increased too. As can
be seen, the cover plate 17 is also provided with
channels 18 or a honeycomb structure which is open at
the lateral edges so that air or oxygen is supplied
through the channels 18 and from there through the air-
permeable film 2 to the cell culture 11. Connection of
the cover plate 17 to the carrier plate 1 and a tight
connection of the film 2 and - where appropriate of the
carrier film 3 - to the edges can be effected by clips
which are not depicted or an appropriate screw
connection.
The cover plate 17 shown in Fig. 2 can,also be designed
so that the channels 18 are connected on a supply side
to an oxygen carrier (not depicted) and are closed on
the other edges. It is possible in this way to produce
an individual oxygen supply for the cells. The advan-
tage of such a self-contained device is that air can
then be supplied with a particular temperature and/or
humidity and, in this way, an individual "biosphere"
can be produced. A further advantage of this design is
that, if necessary, air can also be fed in under
pressure, making it possible to increase the mass
transfer and the partial pressure. It would, of course,
also be possible in principle to increase the oxygen
CA 02387549 2002-05-06
- 11 -
concentration correspondingly, but certain cell
cultures react unfavorably to an oxygen content which
is too high. A treatment under an elevated pressure
may, however, have beneficial effects - depending on
the cell cultures to be treated.
A further design may comprise a network structure being
present in the cell chamber 11a. As is well known, bone
marrow grows in bone, and bone-like structures or,
correspondingly, a type of network structure or an
extracellular matrix can be placed in the interior in
order to improve the result, thus providing the cells
with a type of microenvironment coming close to the
normal environment. Such a network structure may be
placed freely in the cell chamber space 11a, or it may
be incorporated in the film 2 or the carrier film 3. It
would thus become possible to put, for example,
calcareous structures (tricalcium phosphate) into the
cell chamber 11a.
The elastic film 2 and, if used, the carrier film 3 can
be appropriately strengthened in the edge region so
that the films do not tear. Inflow and/or outflow
connectors can also be sealed in this strengthening and
then replace the inflow connector 4 and the return flow
connector 10 of the carrier plate 1. tnlhen a frame-like
carrier plate 1a as depicted in Fig. 4 is used, the
carrier film 3 and the elastic film 2 result in cell
culture chambers 11a. Just like the design shown in
Figures 1 to 3, it is possible for frame-like carrier
plates 1a with films 2 and 3 fastened thereto to be
stacked one on top of the other as desired, in which
case the inflow and outflow connectors will be sealed
in films 2 and 3 - as mentioned above.
The individual devices with their carrier plates 1 can
also be connected together in the form of a fit-
together system to give units of any length. In this
case, appropriate bores and pins are used for the
CA 02387549 2002-05-06
- 12 -
connection. It is then merely necessary for this
purpose to connect the individual inflow connectors 3
and the outflow connectors 10 appropriately.
Instead of a carrier plate 1a in the form of a frame as
depicted in Fig. 4, it is also possible for a frame-
like carrier plate 1a to be replaced by a clamping
frame or a clamping wire in any shape, for example in
rectangular, square or circular shape, with which the
two films 2 and 3 are connected together. In other
words: the frame construction shown in Fig. 4 can be
curved to form a circle as depicted in principle in
Fig. 7, or else in a spiral shape as can be seen in
Fig. 8, in which case the material can be moldable or
elastic for this purpose. The designs in Figures 7 and
8, in particular Fig. 7, result in a flexible system in
the form of a tube with an [sic] in each case one
carrier plate or one carrier ring 1b and films 2 and 3
in between, in whose interior the cell culture 11a is
present. If the starting material chosen in this case
is a frame-like carrier plate 1a as shown in Fig. 4, on
which the two films 2 and 3 are fastened, stiffening
rings are present only on the two end faces of the tube
and are connected together along the surface line by
' 25 two connecting strips lying side by side. Otherwise,
the peripheral wall of the tube is flexible. It is
possible, where appropriate, for the two connecting
strips also to be omitted. In this case there are only
two stiffening rings on the two end faces.
The structure in the shape of a ring depicted in Fig. 7
represents only a design in principle and can
advantageously be modified from this basic shape. One
possible area of use is, for example, a coronary vessel
bypass, it having been necessary to date to remove this
bypass from a vein of the patient's leg. However, the
disadvantage of this is that veins frequently close
again after several years because they do not withstand
the higher pressure in the region of the arterial flows
CA 02387549 2002-05-06
- 13 -
on the heart.
With an embodiment of the invention as shown in Fig. 7
it is possible in this way to culture a vessel with the
patient's own cells, in which case connective tissue
cells are injected together with a collagen mixture
between the films 2 and 3 in the cell culture chamber
11a. The cells are then allowed to grow for a
particular time until used.
Another possible use is for neuronal cells. These cells
can also be put into the tubes produced in this way,
but in this case it is necessary to avoid them adhering
=-,-;x
in certain phases of culturing. The tubes in this case
serve merely for multiplication of the neuronal cells
so that they can subsequently be removed again and used
for the neurological patient.
Fig. 9 shows an outline representation in a top view of
a tube with three "flaps" 19 drawn inward. This design
produces a stability structure which can serve as heart
valve.
Fig. 10 depicts in lengthwise section a stability
structure which can form a heart valve. As can be seen,
the tube has inward protrusions 20. Depending on the
purpose of use of such a tube, the inward protrusions
may also go so far inward that they make contact or
even form a transverse connection in the annular cell
culture chamber 11a.
In the manufacture of this form it is possible, for
example, for stabilizing rings 21 to be arranged above
and below the inward protrusions 20. Clamps 22 can also
be placed on the inside above the inward protrusions 20
for accurate determination of the shape (represented by
broken lines). The carrier film 3 as carrier on the
outside and also the carrier film 3 on the inside are
permeable to air and moldable. Nutrient medium flows in
CA 02387549 2002-05-06
- 14 -
each case between the outer films 3 and the elastic
films 2 which are arranged at a small distance
therefrom. The elastic films 2 are in this case
additionally microporous so that the nutrients or the
nutrient medium can diffuse into the annular cell
culture chamber 11 located on the inside and having the
cells 11 disposed therein.
The cell culture chamber 11a in which the cells 11
appropriate for the particular use are introduced can
also be formed by a structure with microporous cells in
the manner of an "injection molding process". In this
case, the structure in practice represents the carrier
portion for the cells 11 and corresponds in its shape
to the later shape for use, for example a heart valve
or else a bladder as replacement for the tumor
patient's own bladder.
In the latter case, the cells can be cultured in the
particular shape of a type of double-air balloon which
has an inlet and outlet and which is elastic. The outer
film 3 in each case is moreover gas permeable, and is
elastic where appropriate, and the microporous film 2
is present inside.
The structure may, for example, comprise a housing
which is either biodegradable and thus decomposes, or
it consists of a plastic which is removed again later.
The housing ought to be flexible and correspond in its
shape to the shape of the organ to be replaced or
formed. In this way, the cells are provided in practice
with a mold into which a connective tissue matrix or
collagen matrix with cells is then injected. The whole
system becomes solid and can form networks, also in
three-dimensional growth, and absorb the matrix during
this.
As a further advantageous use, the microporous film 2
can also be produced from a material which itself
CA 02387549 2002-05-06
- 15 -
dissolves after a certain time. This applies, for
example, to particular polymers such as, for example,
polylactide, alkanoates or butyrates which dissolve,
for example, after several weeks. If, for example,
connective tissue cells have formed the framework in a
matrix structure, then the "inner lining" of many
organs must be attached, which is done with endothelial
cells. These endothelial cells can be applied, for
example, when the microporous film 2 has dissolved. In
this case, separate flow into the cell culture chamber
11a is still always possible. In general, the cells 11
will be introduced together with a matrix, for example
collagen, into the cell culture chamber 11a, where they
can multiply appropriately. In the case of replacement
for a heart valve, the endothelial cells are applied on
the inside and the connective tissue cells with muscle
are applied on the outside. This means that the
endothelial cells are applied on the side of the film 2
which is directed toward the interior of the tube. It
is likewise possible for the film 2 to be designed to
be dissolvable, or removable.
The fact that the films are elastic produces a
substantially natural environment for the cells 11 to
be cultivated, since a heart pumps, resulting corres-
pondingly in pressure waves which act correspondingly
on the cells 11.
When the culturing is finished, the rings 21 and, where
appropriate, the clamps 22 can be removed, since the
unit is then stable on its own and it can be inserted
at the desired site.
Fig. 11 shows another possibility for producing a heart
valve or else another structure. In this case, the tube
with the inward protrusions 20 is present in a
container 23 in which nutrient medium is present. The
container, which is provided with inflow orifice and
outflow orifice which are not depicted, can be provided
CA 02387549 2002-05-06
- 16 -
with a lid 24, with the structure "floating" in the
nutrient medium or being suspended therein. In this
case, the two outer films 3 can be omitted and only the
microporous elastic films 2 are present, between which
the annular cell culture chamber 11a with the inward
protrusions 20 is located. Oxygen or air is also
introduced into the container 23, it being possible for
a stirring element 25, which is not depicted in detail,
to be disposed in the containers 23 for uniform
agitation or distribution.
In a simplification of the structure depicted in
Fig. 11 and of the method resulting therefrom, the
outer film 3 can also be designed to be only air
permeable and acts as carrier film. The supply of
nutrient medium from the container takes place in this
case only from the inside through the microporous inner
film 2.
The cell culture and cell treatment method can in
principle take place with all the exemplary embodiments
mentioned above in the cell culture chamber 11a in the
following way:
In a first step, the matrix or the collagen is prepared
externally. The matrix or the collagen is then injected
into the cell culture chamber 11a. The structures or
the cell culture chamber 11a can be either already
coated with cells, or the cells 11 are injected
directly together with the collagen or with the matrix.
In a next step, the modeling phase or
the cell
multiplication place, with the cells 11
takes
transforming the matrix It is then
correspondingly.
possible - necessary - for further cell types to
if be
added, for example if a film has dissolved
appropriately or it has been removed.
Another very great advantage of the invention is that
CA 02387549 2002-05-06
- 17 -
the finished structure can subsequently be frozen. This
is possible with the device of the invention and the
method of the invention, in contradistinction to other
reactor systems, since virtually a thin-layer system is
present. Heat is released in the cells when the cells
are frozen and the associated crystal formation takes
place. This release of heat must be countered by
cooling. It is therefore impossible with the known
reactor systems to freeze the cells 11 in a coordinated
manner. In the present case, however, because of the
thin-layer system it is possible to freeze all the
cells more or less uniformly, it being possible to
counteract the production of heat during crystal
formation in a specific and uniform manner. It is
possible in this way for the cells 11 to freeze in a
coordinated manner, it being possible to counteract in
practice the production of heat by a simple
introduction of gas, for example of nitrogen.
Structures frozen in this way can thus be kept as long
as desired for later use and/or else be transported,
appropriately cooled, into a clinic and there implanted
shortly after thawing and, where appropriate, after a
further rinsing. This means that the device of the
invention and the method is not just a cultivation
system but also a transport system, with the sterile
chain being uninterrupted.
When used for liver cells, an extracorporeal connection
or use is also possible.
It is also possible in a very advantageous manner to
produce and multiply skin cells with the invention. In
this case, connective tissue cells of the skin are
introduced, for example with collagen, into the cell
culture 11a - as depicted in Fig. 3. In a second phase,
the keratinocytes, i.e. keratinizing cells which
comprise the epidermis, are applied, so that the whole
system grows inward in a plurality of layers. After
CA 02387549 2002-05-06
- 18 -
completion of the culture method, the film can be cut
up or pulled off. To ensure sterility, the structure
can also be put in a housing or form a small closed
box. Controlled culturing of skin is possible in this
way.
In contradistinction to the prior art, the skin in this
case is built up in the way in which it must later be
applied. This means that the keratinization takes place
on the outside and not on the inside, and in this case
there is not initial production of connective tissue
but first the epidermal cells are applied directly to
the film and then the collagen is injected.
With the device of the invention it is also possible to
use embryonic cells. Embryonic cells are cells which
have not yet developed their own tissue expression
pattern. The microenvironment and the matrix can
provide the possibility of these cells being converted
into tissue expression which corresponds to terminal
differentiation, i.e. definitive differentiation. A
coating with peptides is possible.
Films which can be used are PTFE, silicone,
polylactide, polyhydroxyalkanoate and polyhydroxide-
butyrates. The essential point is that they belong very
generally to the group of biodegradable polymers, i.e.
structures which can be broken down either by
hydrolysis or enzymatically, which makes them
implantable.
An essential feature of all carrier structures and of
the various designs of carrier 1 is that they can be
moldable or modelable. Besides changes in the volume of
the system and of the cell culture chamber, it is
possible in this way to change the geometry of the
bioreactor or of the device. The system can also be
reversibly changed in shape during operation through
alterations in the perfusion pressure because of the
CA 02387549 2002-05-06
- 19 -
elastic properties of the materials used (memory
effect) .
Cells 11 which can be modeled by the cell culture
chamber 11a can be introduced with matrix into the cell
culture chamber 11a.