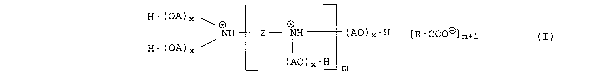Note: Descriptions are shown in the official language in which they were submitted.
Use of fatty acid salts of alkoxylated oligoamines as lubricity
enhancers for mineral oil products
The present invention relates to the use of fatty acid salts of
alkoxylated oligoamines as lubricity enhancers for mineral oil
products, in particular for gasoline fuels and middle
distillates, especially diesel fuels, and additive concentrates
for such mineral oil products and such mineral oil products
themselves which contain these fatty acid salts of alkoxylated
oligoamines.
Carburettors and intake systems of gasoline engines, but also
injection systems for fuel metering, are increasingly becoming
contaminated by impurities due to dust particles from the air,
uncombusted hydrocarbon residues from the combustion chamber and
the crank case vent gases passed into the carburretor.
These residues shift the air/fuel ratio during idling and in the
lower part-load range in such a way that the mixture becomes
leaner, the combustion more incomplete and in turn the amounts of
uncombusted or partially combusted hydrocarbons in the exhaust
gas become larger and the gasoline consumption increases.
It is known that these disadvantages can be avoided by using fuel
additives for keeping valves and carburretors or injection
systems for gasoline engines clean (cf. for example:
M. Rossenbeck in Katalysatoren, Tenside, Mineraloladditive,
Editors J. Falbe, U. Hasserodt, page 223, G. Thieme Verlag,
Stuttgart 1978).
Such fuel additives (detergents) which maintain cleanliness and
can derive from a large number of classes of chemical substances,
such as polyalkeneamines, polyetheramines, polybutene Mannich
bases or polybutenylsuccinimides, are used in general in
combination with carrier oils and in some cases further additive
components, such as corrosion inhibitors and demulsifiers.
However, gasoline fuels with and without such additive components
generally exhibit inadequate behavior in need of improvement,
with regard to their lubricity and antiwear properties in
gasoline engines.
The low-sulfur diesel fuel increasingly used nowadays also gives
rise to lubricity problems which result, for example, in greater
wear in the injection pumps. The reduction of the sulfur content
in diesel fuels is required for reducing or avoiding
CA 02388065 2002-04-18
2
sulfur-dioxide and particle emissions. To achieve lower sulfur
contents, the diesel fuels must be hydrogenated. As a result,
polar and polynuclear aromatic components in the fuel, which are
responsible for the natural lubricating effect of the diesel
fuel, are also destroyed. Here too, as in the case of the
gasoline fuels, there is therefore a need for additives which
sufficiently increase the lubricity in the fuel (also referred to
as lubricity additive or friction modifier).
Lubricity-enhancing additives known to date for gasoline fuel
are, for example, fatty acids, as described, for example in WO
98!111'75, alkenylsuccinic esters, bis(hydroxyalkyl)-fatty amines
and hydroxyacetamides. For diesel fuels, in particular fatty
acids and fatty acid derivatives, for example esters of glycerol
with unsaturated fatty acids, or castor oil, as described in EP-B
605 857, are used as lubricity-enhancing additives.
However, since said prior art materials are unsatisfactory in
their property profile, in particular with regard to their
lubricating and antiwear effect, it is an object of the present
invention to provide more efficient lubricity enhancers for
mineral oil products, in particular for gasoline fuels and middle
distillates.
We have found that this object is achieved by the use of fatty
acid salts of alkoxylated oligoamines of the formula I
H-(OA)x ~
NH Z - NH
( AO ) x-H [ R-COOD) m+1 ( I )
H_(OA)x /
(AO)x-H m
where
A is an alkylene group of 2 to 8 carbon atoms,
R is C~- to C23-alkyl or mono- or polyunsaturated C~- to
C23-alkenyl, which may additionally carry hydroxyl groups,
Z is a C1- to C~-alkylene group, C3- to C8-cycloalkylene group
or Cs- to C12-arylene or arylalkylene group,
m is 0 or an integer from 1 to 5 and
CA 02388065 2002-04-18
3
the sum of all variables x has a value of from 50 to 300% of
(m+3), as lubricity enhancers for mineral oil products.
Said compounds of the formula I are already known as such.
US 4 131 583 describes salts of, in particular, unsaturated Cli-
to CZO=carboxylic acids with
N,N,N',N'-tetrahydroxyethyl-C2-C4-alkylenediamines, these are
recommended exclusively as corrosion inhibitors in aqueous
coating materials for metal surfaces.
JP-A 11/050076 discloses that N,N,N',N'-tetrakis(2-hydroxypropyl)
ethylenediamine in the presence of fatty acids, such as stearic
acid or oleic acid, in aqueous lubricating oil compositions, such
as cutting oil or milling oil, prevents rust formation and fungal
attack and stabilizes the composition. An improvement of the
lubricating effect is not mentioned therein.
JP-A 11/209773 discloses aqueous lubricants for conveyor belts in
the filling of bottles with beverages, which lubricants contain
fatty acid salts of alkanolamines, such as
N,N,N',N'-tetrakis(2-hydroxypropyl)ethylenediamine. For example,
palmitic acid, tetradecanoic acid, oleic acid and lauric acid are
mentioned as fatty acids.
Mineral oil products are to be understood here as meaning power
fuels, operating materials, combustion fuels and lubricating
oils, which however are based not only on mineral oil but also
partially or completely on synthetic and/or naturally occurring
raw materials. Examples of such raw materials are natural gas,
methanol, ethanol, coal liquefaction products or rapeseed oil,
which are processed to give fuels or incorporated in fuels based
on mineral oil. The designated mineral oil products are as a rule
virtually anhydrous or contain water at least only in minor
amounts. Examples of water-containing mineral oil products are
fuel emulsions, such as diesel/water emulsions, which may usually
contain up to about 35% by weight of water. Mineral oil products
preferred for the present invention are on the one hand gasoline
fuels and on the other hand middle distillates, in particular
diesel fuels.
The alkylene group A is preferably derived from corresponding
alkylene oxides, such as ethylene oxide, 1,2-propylene oxide,
1,2-butylene oxide and cis- or trans-2,3-butylene oxide. However,
they may also be 1,3-propylene, 1,4-butylene, 1,6-hexylene or
1,8-octylene. A may likewise be a mixture of different groups
CA 02388065 2002-04-18
4
from among said groups. A is particularly preferably an ethylene,
1,2-propylene or 1,2-butylene group.
The relatively long-chain radical R occurring in the carboxylate
anion is, for example, branched or preferably linear C~- to C23-
alkyl, preferably C11- to C21-alkyl, especially C15- to C19-alkyl,
which may additionally carry hydroxyl groups. Examples of parent
carboxylic acids are octanoic acid, 2-ethylhexanoic acid,
nonanoic acid, decanoic acid, undecanoic acid, dodecanoic acid
(lauric acid), tridecanoic acid, isotridecanoic acid,
tetradecanoic acid (myristic acid), hexadecanoic acid (palmitic
acid), octadecanoic acid (stearic acid) and eicosanoic acid. Said
acids may be of natural or synthetic origin. The carboxylate
anions may also be based on mixtures of said acids.
The relatively long-chain radical R occurring in the carboxylate
anion is however preferably a mono- or polyunsaturated C~- to
C23-radical, in particular mono- or polyunsaturated C11- to
C21-alkenyl, especially C15- to Clg-alkenyl, which may additionally
carry hydroxyl groups. These unsaturated radicals are preferably
linear. In the case of polyunsaturated alkenyl groups, they
preferably contain two or three double bonds. Examples of parent
carboxylic acids are elaidic acid, ricinoleic acid, linoleic acid
and linolenic acid. Particularly good results are obtained with
oleic acid. The carboxylate anions may also be based on mixtures
of such unsaturated carboxylic acids with one another and also
with the abovementioned saturated carboxylic acids. Such mixtures
are, for example, tall oil, tall oil fatty acid and rapeseed oil
fatty acid. Said unsaturated carboxylic acids and said mixtures
are as a rule of natural origin.
Z is in particular a C1- to C4-alkylene group, such as methylene,
1,2-propylene, 1,2-butylene, 1,3-butylene or 2,3-butylene, C5- to
C6-cycloalkylene group, such as 1,3-cyclopentylidene or 1,3- or
1,4-cyclohexylidene or C6- to C8-arylene or arylalkylene group,
such as 1,3- or 1,4-phenylene, 2-methyl-I,4-phenylene or 1,3- or
1,4-bismethylenephenylene.
Z is however preferably a polymethylene group of the formula
-(CH2)n- where n is from 2 to 8, in particular from 2 to 6, i.e.
in particular 1,2-ethylene, 1,3-propylene, 1,4-butylene,
1,5-pentylene and 1,6-hexylene, but also 1,7-heptylene and
1,8-octylene.
m is 0, the fatty acid salts used according to the invention are
as a rule, dependent on the sum (~) of,all variables x, based on
mixtures of mono-, di- and/or trialkanolamines or pure
CA 02388065 2002-04-18
5
trialkanolamines as cationic components. Examples of such
alkanolamines are monoethanolamine, diethanolamine,
triethanolamine, monoisopropanolamine, diisopropanolamine,
triisopropanolamine and the associated mixtures. In this group,
the oleic acid salt of triethanolamine (fix = 3) is of particular
interest.
m is however preferably 1 or 2. For m = 1, completely andlor
partially alkoxylated alkylenediamines such as
1,2-ethylenediamine, 1,3-propylenediamine or 1,4-butylenediamine,
form the basis. For m = 2, in general completely and/or partially
alkoxylated dialkylenetriamines such as di(1,2-ethylene)triamine,
di(1,3-propylene)triamine or di(1,4-butylene)triamine, form the
basis. In this group, the bisoleic acid salts of
N,N,N',N'-tetrakis(2'-hydroxyethyl)-1,2-ethylenediamine (Fx = 4)
and N,N,N',N'-tetrakis(2'-hydroxypropyl)-1,2-ethylenediamine (fix
- 4) and the trisoleic acid salts of di(1,2-ethylene)triamine
reacted with from 4 to 5 mol of ethylene oxide or~l,2-propylene
oxide are of particular interest.
However, it is also possible to use higher homologues of said
alkylenediamines and dialkylenetriamines, for example
tetraethylenetetramine (m = 3), tetraethylenepentamine (m = 4) or
pentaethylenehexamine (m = 5), as parent amine components for the
fatty acid salts used according to the invention.
The number of alkylene oxide units (OA) introduced per amine
molecule may correspond to the number of N-H bonds in the parent
amine (fix = m+3). However, it is also possible to incorporate
more or less OA units. In the case of superstoichiometric
incorporation, triple alkoxylation per N-H bond [300% of (m+3)]
is an upper limit with respect to the properties of the resulting
fatty acid salts. In the case of substoichiometric incorporation,
50% alkoxylation on average [50% of (m+3)j is a corresponding
lower limit; here, mixtures of species having different degrees
of alkoxylation are then generally present.
In a preferred embodiment, the sum (E) of all variables x has a
value of from 75 to 125% of (m+3).
The fatty acid salts used according to the invention and of the
formula I can usually be easily prepared by alkoxylation of the
parent amines by conventional methods and subsequent
neutralization with the fatty acids of the formula R-COON.
CA 02388065 2002-04-18
6
When C2- to C4-alkylene oxides are used, the alkoxylation is
expediently carried out in the presence of small amounts of water
(at most from 0.5 to 5% by weight, based on the amount of amine
used), without a catalyst, at from 80 to 140°C, for the
introduction of the first alkylene oxide unit into the N-H bond,
and the absence of water, in the presence of basic catalysts,
such as alkali metal hydroxides, e.g. sodium hydroxide or
potassium hydroxide, at from 100 to 150°C, for the introduction of
further alkylene oxide units.
The neutralization is carried out as a rule by heating the
alkoxylated amines obtained with the corresponding stoichiometric
or slightly substoichiometric amount (i.e. from 90 to 100%, in
particular from 95 to 100%, of theory) of fatty acid to
temperatures of from 30 to 100°C, in particular from 40 to 80°C,
for from 15 minutes to 10 hours, in particular from 30 minutes to
5 hours. The neutralization reaction should be carried out in
such a way that no carboxylic ester fractions result in the
product. In-many-cases, both the alkoxylated amine and the fatty
acid can be used in the form of liquids, which makes the reaction
to the corresponding fatty acid salt particularly simple. The
order in which alkoxylated amine and fatty acid are combined is
not critical, either the alkoxylated amine can be initially taken
and the fatty acid added or the fatty acid can be initially taken
and the alkoxylated amine added.
However, it is in principle also possible to add the alkoxylated
amine and the fatty acid as individual components to the additive
concentrates or the mineral oil products and to allow the salt
formation to take place there.
The preparation of the fatty acid salts used according to the
invention and of the formula I generally requires substantially
less effort and energy than in the case of conventional lubricity
enhancers of the prior art, in particular those based on amides
or esters, in the preparation of which higher temperatures,
longer reaction times and expensive working-up procedures
precipitating off undesired byproducts, which occur in particular
in the case of condensation reactions, are as a rule required.
The described fatty acid salts of the formula I are very suitable
as lubricity enhancers (lubricity additives, lubricity modifiers)
in mineral oil products, in particular in gasoline fuels and in
middle distillates, especially diesel fuels. The fatty acid salts
I are in general highly efficient and hence widely usable. Where
fatty acid salts I are used, the tendency for wear to occur in
CA 02388065 2002-04-18
the parts of the machines and units operated with the mineral oil
products is substantially reduced.
The fatty acid salts used according to the invention in gasoline
fuels and of the formula I can be advantageously used in
combination with, in principle, all conventional gasoline fuel
additives.
Examples of conventional gasoline fuel additives having a
detergent effect are:
(a) polyisobuteneamines which are obtainable according to
EP-A 244 616 by hydroformylation of highly reactive
polyisobutene having a number average molecular weight of
from 300 to 5000 and subsequent reductive amination with
ammonia, monoamines or polyamines, such as
dimethyleneaminopropylamine, ethylenediamine,
diethylenetriamine, triethylenetetramine or
tetraethylenepentamine;
(b) poly(iso)buteneamines which are obtainable by chlorination of
polybutenes or polyisobutenes having double bonds
predominantly in the ~- and y-position and subsequent
amination with ammonia, monoamines or the polyamines
mentioned above under (a);
(c) poly(iso)buteneamines which are obtainable by oxidation of
double bonds in poly(iso)butenes with air or ozone to give
carbonyl or carboxyl compounds and subsequent amination under
reducing (hydrogenating) conditions;
(d) polyisobuteneamine which are obtainable according to
DE-A 196 20 262 from polyisobutene epoxides by reaction with
amines and subsequent dehydration and reduction of the amino
alcohols;
(e) polyisobuteneamines which may contain a hydroxyl group and
which are obtainable according to WO-A 97/03946 by reaction
of polyisobutenes having an average degree of polymerization
P of from 5 to 100 with oxides of nitrogen or mixtures of
oxides of nitrogen and oxygen and subsequent hydrogenation of
these reaction products;
CA 02388065 2002-04-18
8
(f) hydroxyl-containing polyisobuteneamines which are obtainable
according to EP-A 476 485 by reaction of polyisobutene
epoxides with ammonia, monoamines or the abovementioned
polyamines;
(g) polyetheramines which are obtainable by reaction of C2- to
C3o-alkanols, C6- to C3o-alkanediols, mono-, di- or tri-C2- to
C3o-alkylamines, C1- to C3o-alkylcyclohexanols or C1- to
C3o-alkylphenols with from 1 to 30 mol of ethylene oxide
and/or propylene oxide andlor butylene oxide per hydroxyl or
amino group and subsequent reductive amination with ammonia,
monoamines or the abovementioned polyamines; such products
also have carrier oil properties;
(h) polyisobutene Mannich bases which are obtainable in
particular according to EP-A 831 141 or according to German
Patent Applications 199 48 111.3 and 199 48 114.8 by reaction
of polyisobutene-substituted phenols with aldehydes and
monoamines of the abovementioned polyamines;
(i) polypropyleneamines according to WO 94/24231, which are
obtainable by metallocene-catalyzed propene oligomerization
and, subsequently, the hydroformylation and reductive
amination steps stated above under (a);
(j) additives containing carboxylic ester groups, preferably
based on esters of mono-, di- or tricarboxylic acids with
long-chain alcohols or polyols, in particular those having a
minimum viscosity of 2 mm2ls at 100°C, as described in
DE-A 38 38 918; adipates, phthalates, isophthalates,
terephthalates and trimellitates of isooctanol, of
isononanol, of isodecanol and of isotridecanol may be
mentioned as examples here; such products also have carrier
oil properties;
(k) imides, amides, esters and ammonium and alkali metal salts of
polyisobutenylsuccinic anhydrides which are obtainable from
conventional or highly reactive polyisobutene and malefic
anhydride by a thermal method or via chlorinated
polyisobutene and can be used in particular in the form of
the derivatives with aliphatic polyamines; such gasoline fuel
additives are described in particular in US-A 4 849 572.
Examples of conventional gasoline fuel additives which inhibit
valve seat wear are:
CA 02388065 2002-04-18
9
(1) additives containing carboxyl groups or their alkali metal or
alkaline earth metal salts, in particular the copolymers of
CZ-C4o-olefins with malefic anhydride, which are described in
EP-A 307 815 and have a total molar mass of from 500 to
20,000 and some or all of whose carboxyl groups have been
reacted to give the alkali metal or alkaline earth metal
salts and a remainder of the carboxyl groups have been
reacted with alcohols or amines;
(m) additives containing sulfo groups or their alkali metal or
alkaline earth metal salts, in particular the alkali metal or
alkaline earth metal salts of alkyl sulfosuccinates, which
salts are described in EP-A 639 632.
In addition to and together with said gasoline fuel additives (a)
to (m), further conventional carrier oils, additive components
and assistants may be combined with the fatty acid salts of the
formula I for use in gasoline fuels.
Examples of conventional carrier oils for gasoline fuel additives
are mineral carrier oils (base oils), in particular those of
viscosity class "Solvent Neutral (SN) 500 to 2000n, synthetic
carrier oils based on olefin polymers having MN = 400 to 1800,
especially based on polybutene or polyisobutene (hydrogenated or
unhydrogenated), and on poly-alphaolefins or
poly-internal-olefins, and synthetic carrier oils based on
alkoxylated long-chain alcohols or phenols. Mixtures of said
carrier oils can of course also be used.
Fractions obtained in mineral oil processing such as kerosine,
naphtha or brightstock, are also suitable as mineral carrier oils
and/or diluents or solvents for gasoline fuel additives. Aromatic
hydrocarbons, paraffinic (aliphatic) hydrocarbons and
alkoxyalkanols are furthermore suitable for this purpose.
Of particular importance here as carrier oils for gasoline fuel
additives are polyetherols which are obtainable by reaction of C2-
to C3o-alkanols, C6- to C3o-alkanediols, mono-, di- or tri-C2- to
C3o-alkylamines, C1- to C3a-alkylcyclohexanols or C1- to
C3p-alkylphenols with from 1 to 30 mol of ethylene oxide andlor
propylene oxide andlor butylene oxide per hydroxyl or amino
group. Particular examples here are alcohol-initiated
polyetherols having from about 10 to 35, in particular from 15 to
30, propylene oxide and/or butylene oxide units; particularly
suitable initiator alcohols here are linear or branched C6- to
C15-alkanols.
CA 02388065 2002-04-18
10
Further conventional additive components and assistants for
gasoline fuel are corrosion inhibitors, for example based on
ammonium salts of organic carboxylic acids, which salts tend to
form films, or heterocyclic aromatics in the case of corrosion
protection of nonferrous metals, antioxidants or stabilizers, for
example based on amines such as p-phenylenediamine,
dicyclohexylamine or derivatives thereof or on phenols, such as
2,4-di-tert-butylphenol or 3,5-di-tert-butyl-4-hydroxyphenyl-
propionic acid, demulsifiers, antistatic agents, metallocenes,
such as ferrocene or methylcyclopentadienylmanganesetricarbonyl,
and markers. Medium-chain linear or branched alkanols (having,
for example, from 6 to 12 carbon atoms), e.g. 2-ethylhexanol, are
often used as solubilizers. Amines are sometimes also added for
lowering the pH of the fuel.
The fatty acid salts used according to the invention in gasoline
fuels and of the formula I can also be employed together with
other lubricity enhancers customary for this purpose, such as
specific fatty acids, alkenylsuccinic esters,
bis(hydroxyalkyl)-fatty-amines or hydroxyacetamides.
The carboxylic acids or fatty acids used as corrosion inhibitors
or lubricity enhancers may be present as monomeric or oligomeric,
in particular dimeric species. Mixtures of monomeric and dimeric
and, if required, higher oligomeric species may also be present.
Combinations of the fatty acid salts used according to the
invention in gasoline fuels and of the formula I with the
detergents of the abovementioned groups (a), (d) and (g), in
particular with polyisobuteneamines of group (a), are preferred.
Particularly suitable polyisobuteneamines (a) here are those
which are prepared by hydroformylation of highly reactive
polyisobutenes having a number average molecular weight of from
500 to 2300, in particular from 800 to 1500, especially from 900
to 1200, and subsequent reductive amination with ammonia. The
polyisobuteneamines (a) are preferably used together with carrier
oils, for example polyetherols or aliphatic or aromatic
hydrocarbons, and, if required, with the abovementioned corrosion
inhibitors, antioxidants or stabilizers, demulsifiers, antistatic
agents, metallocenes and/or markers. A typical example of such a
polyisobuteneamine (a) is the product sold under the tradename
Rerocom~ PIBA by BASF Aktiengesellschaft.
Suitable gasoline fuels are all commercial gasoline fuel
compositions. A typical example here is the commercial Eurosuper
base fuel according to EN 228. The gasoline fuel composition
CA 02388065 2002-04-18
11
described in German Patent Application 199 05 211.5 is also of
interest here.
The fatty acid salts used according to the invention in middle
distillates and of the formula I can advantageously be used in
all conventional middle distillates.
These middle distillates, of which diesel fuels constitute the
most important group, include refined mineral oil products which
usually have a boiling range from 100 to 400°C. These are
generally distillates having a 95% point up to 360°C or higher.
However, they may also be ultra low sulfur diesel or city diesel,
characterized by a 95% point of, for example, not more than 345°C
and a sulfur content of not more than 0.005% by weight or by a
95% point of, for example, 285°C and a sulfur content of not more
than 0.001% by weight. Moreover, these middle distillates may
also be heating oils having a sulfur content of not more than
0.20, in particular, not more than 0.10, % by weight, and
aviation fuels.
Said distillates are usually composed of components which are
obtained from the distillation of mineral oil under atmospheric
or reduced pressure or are obtained from conversion processes,
for example cracker, coker or visbreaker gas oil.
Said middle distillates, especially diesel fuels, are
distinguished by a low sulfur content, as a rule not more than
0.05, in particular not more than 0.02, especially not more than
0.005, very particularly preferably not more than 0.001, % by
weight.
The fatty acid salts used according to the invention in middle
distillates, especially diesel fuels, and of the formula I can be
incorporated herein as pure liquid substances or as liquid
concentrates in a solvent or diluent. In principle, all
substances stated above as such compositions for use with
gasoline fuel additives can be used here as solvents or diluents.
Mineral oil fractions such as naphtha, kerosine, diesel fuel and
aromatic hydrocarbons, such as heavy solvent naphtha, Solvesso~
or Shellsol~ are particularly suitable. These concentrates may be
solutions or dispersions, clear solutions being preferred.
Mixtures of said solvents or diluents may also be used.
By using said solvents or diluents, in particular in a weight
ratio to the fatty acid salts I of from 1:10 to 10:1, in
particular from 1:4 to 4:1, especially,from 1:2 to 2:1, the
solubility of the fatty acid salts used according to the
CA 02388065 2002-04-18
12
invention and of the formula I can be improved. Such solutions or
dilutions are advantageous when the middle distillate already
contains further additives, when the mixing-in temperatures are
low, when the metering means is not tailored to low doses or when
mixtures with other middle distillates are to be prepared.
The fatty acid salts used according to the invention in middle
distillates and of the formula I can advantageously be employed
in combination with in principle all conventional middle
distillate or diesel fuel additives.
Conventional middle distillate or diesel fuel additives in this
context are in particular detergents, corrosion inhibitors,
dehazers, demulsifiers, antifoams, antioxidants, metal
deactivators, multifunctional stabilizers, cetane number
improvers, combustion improvers, dyes, markers, solubilizers,
antistatic agents, other conventional lubricity additives and the
additives which improve low temperature properties, such as flow
improvers (MDFIj, paraffin dispersants (WASA) and the combination
thereof (WAFI).
The present application also relates to a process for improving
the lubricity of mineral oil products, in particular gasoline
fuels and middle distillates, wherein effective amounts of fatty
acid salts of alkoxylated oligoamines of the formula I 1 to 6
[sic] are added to the mineral oil products.
The present invention furthermore relates to additive
concentrates for mineral oil products, in particular for gasoline
fuels and middle distillates, which contain the fatty acid salts
of alkoxylated oligoamines of the formula I in amounts of from
0.05 to 50, in particular from 0.1 to 30, % by weight, based on
the total amount of the concentrates. These concentrates usually
also contain the abovementioned further additives, carrier oils,
solvents or diluents and/or assistants. In the case of additive
concentrates for gasoline fuels, these are in particular
detergents and/or compositions inhibiting valve seat wear,
especially the abovementioned additives (a) to (m), and further
components and assistants customary for this purpose, in
particular carrier oils, corrosion inhibitors, antioxidants or
stabilizers, demulsifiers, antistatic agents, metallocenes and
markers.
The present invention furthermore relates to mineral oil
products, in particular gasoline fuel and middle distillate
compositions, which contain the fatty acid salts of alkoxylated
oligoamines of the formula I in effective amounts. Effective
CA 02388065 2002-04-18
13
amounts are as a rule to be understood, both in the case of
gasoline compositions and in the case of diesel fuel
compositions, as meaning from 1 to 1000, in particular from 5 to
500, especially from 10 to 250, particularly preferably from 20
to 100, ppm by weight, based in each case on the total amount of
the composition. These mineral oil products, in particular the
gasoline fuel and middle distillate compositions, usually contain
the abovementioned additives, additive components and assistants
in addition to the fatty acid salts I used according to the
invention in them.
The fatty acid salts of alkoxylated oligoamines of the formula I
are highly effective as lubricity enhancers even in low
concentrations in the mineral oil products and effectively
protect wear in those parts of the machines and assemblies
operated with the mineral oil products, for example in fuel
intake systems or injection pumps.
Furthermore, the fatty acid salts of alkoxylated oligoamines of
the formula I have good compatibility with lubricating oil, which
is important in particular when used in diesel fuels. As a result
of the interaction of acidic lubricity enhancers (such as dimeric
fatty acids), used to date, with basic components of lubricating
oil, which are known to be in contact with the fuel in gasoline
and diesel engines, corresponding salts may be deposited in the
fuel and on undesired areas of the engine or of the injection
system and may give rise to faults. This disadvantage is avoided
by the novel use of the fatty acid salts of alkoxylated
oligoamines of the formula I.
35
Furthermore, the fatty acid salts of alkoxylated oligoamines of
the formula I, which salts are used according to the invention,
have virtually no tendency to undesired emulsion formation in the
mineral oil products and are sufficiently stable to hydrolysis.
The examples which follow illustrate the invention without
restricting it.
Preparation Examples
Example 1: Preparation of the bisoleic acid salt of
N,N,N',N'-tetrakis(2'-hydroxypropyl)-1,2-ethylenediamine
58.4 g (0.2 mol) of N,N,N',N'-tetrakis(2'-hydroxypropyl)
1,2-ethylenediamine (prepared by conventional reaction of
1,2-ethylenediamine with 4 mol of propylene oxide in the presence
of about 3% by weight, based on the amount of the amine used, of
CA 02388065 2002-04-18
.. ,
14
water, at from 100 to 110°C) were heated to 60-80°C and mixed
with
110.4 g (0.4 mol) of oleic acid in the course of 2 hours while
stirring. During this procedure, the pH did not fall below 7.
Stirring was then carried out for a further 2 hours in the stated
temperature interval. The product obtained had an N titer of
2.39 mmol/g (calculated 2.37 mmol/g).
Example 2: Preparation of the bisoleic acid salt of
N,N,N',N'-tetrakis(2'-hydroxyethyl)-1,2-ethylenediamine
The title compound was prepared analogously to Example 1 using
the analogous amounts of N,N,N',N'-tetrakis(2'-hydroxy-
ethyl)-1,2-ethylenediamine.
Use Examples
Example 3: Determination of the fretting values in gasoline fuel
To test the- lubricity or the wear in gasoline fuels, a high
frequency reciprocating rig (HFRR) apparatus from PCS
Instruments, London was used. The measuring conditions were
adapted to the use of gasoline fuels. The applicability of this
test method to gasoline fuel is demonstrated by the publications
D. Margaroni, Industrial Lubrication and Tribology, Vol. 50, No.
3, May/June 1998, pages 108-118, and W. D. Ping, S. Korcek,
H. Spikes, SAE Techn. Paper 962010, pages 51-59 (1996).
The gasoline fuel (GF) used here (typically gasoline fuel
according to EN 228) were evaporated down to 50% by volume by
distillation under mild conditions for the measurements. This 50%
residue was used in the test in the wear measuring apparatus for
determining the blank value. Further additives were added to this
residue according to the examples shown below, and the fretting
values was [sic] determined according to the abovementioned
method. The resulting fretting values (F) are stated in
micrometers (Eun); the lower this value, the lower is the
resulting wear.
The 50% by volume residue of a Eurosuper gasoline fuel GF1 gave a
blank value of F = 873 Eun in the HFRR test, while a 50% by volume
residue of a comparable Eurosuper gasoline fuel GF2 gave a blank
value of F = ?54 dun in the HFRR test. The addition of 500 mg/kg
of a commercial gasoline fuel additive package P1 (based on a
polyisobuteneamine detergent, a synthetic carrier oil and a
conventional corrosion inhibitor) or a commercial gasoline fuel
additive package P2 (analogous to P1 but with another synthetic
carrier oil) to the said residue in each case led to fretting
CA 02388065 2002-04-18
15
values of the same order of magnitude. The addition of in each
case 50 mg/kg of the novel lubricity enhancers from Examples 1
and 2 or of the lubricity enhancers known from the prior art
resulted in correspondingly lower values, the novel products
being in general substantially superior to those of the prior
art. The values obtained are shown below in table 1.
Table 1: Fretting values F in gasoline fuel
Additive introduced GF R [gym]
None GF1 873
500 mg/kg of package P1 GF1 853
500 mg/kg of package P1 + 50 mg/kg
of product from Example 2 GF1 686
none GF2 754
540 mglkg of package P1 GF2 717
500 mg/kg of package P1 + 50 mg/kg
of product from Example 1 GF2 593
500 mg/kg of package P1 + 50 mg/kg
of product from Example 2 GF2 634
500 mg/kg of package P1 + 50 mg/kg
of comparative product GF2 659
500 mg/kg of package P2 GF2 775
500 mg/kg of package P2 + 50 mg/kg
of product from Example 1 GF2 680
500 mg/kg of package P2 + 50 mg/kg
of product from Example 2 GF2 633
500 mg/kg of package P2 + 50 mg/kg
of comparative product GF2 684
In each case a commercial lubricity enhancer based on tall oil
fatty acid and according to WO 98!11175 was used as the
comparative product.
CA 02388065 2002-04-18
16
Example 4: Determination of the fretting values in diesel fuels
Standard lubricity tests were carried out using an HFRR apparatus
from PCS Instruments, London, in which a steel ball rubs against
a steel plate in the test fuel. ISO 12156-1 describes this method
and has been introduced into the diesel standard EN 590-1999. The
limit specified here is an abrasion on the steel ball of not more
than 460 Eun. Low-sulfur diesel fuels without additives may have
fretting values F of, typically, from 400 to 700 Eun.
The test diesel fuels (DF1 to DF4) stated in Table 2 below had
the stated characteristics.
Table 2: Characteristics of the test diesel fuel
DF 1 DF 2 DF 3 DF 4
Quality [mg/kg] EN 590 EN 590 EN 590 C-DK MK
1
Sulfur content[kg/m3] 180 700 430 6
Density [C] 841.3 835.6 830.6 810.6
CP [C] -6 -6 -7 -31
CFPP [C] -9 -10 -10 -32
Initial boil- [C] 170 176 175 184
ing point
5
20% by volume (C] 235 214 218 219
95% by volume {C] 363 352 343 280
Final boiling [C] 371 370 356 289
point
The sulfur content was determined according to EN ISO 14 596.
The following diesel fuel additives were used:
Additive A:
product from Example 2, used according to the invention
(undiluted)
Additive B:
product from Example 1, used according to the invention
(undiluted)
Additive C:
as a comparison, commercial lubricity enhancer based on a mixture
of a sterically hindered alkylphenol and a long-chain carboxylic
acid
CA 02388065 2002-04-18
,, , ,
17
Additive D:
as a comparison, commercial lubricity enhancer based on a mixture
of a carboximide and a natural fatty ester
Additive E:
as a comparison, commercial lubricity enhancer based on a mixture
of glyceryl monooleate and glyceryl monolinolate
Additive F:
as a comparison, commercial lubricity enhancer based on a mixture
of long-chain carboxylic acids
Table 3 below shows the results of the determination of the
fretting values F in the test diesel fuels. It is clear that the
effect of the products A and B used according to the invention is
superior to that of the commercial products C to F.
Table 3:
Fretting values F (Eun) in diesel fuel (WS 1,4 values according to
HFRR according to ISO 12 156-1 at 60°C)
Additive DFl DF2 DF3 DF4
introduced
None 532 (0) 624 (0) 615 (0) 564 (0)
Additive 389 (50) 375 (50) 399 (75) 358 (200)
A
363 (100)
Additive 355 (50) 471 (50) 401 (75) 388 (200)
B
359 (100)
Additive 436 (50) 455 (50) 435 (200)
C
330 (100) 453 (250)
Additive 517 (50) 474 (150) 518 (150)
D
252 (200) 387 (200)
Additiv E 471 (50) 473 (100) 578 (250)
282 (50) 350 (350)
35Additive 421 (75)
F
The values stated in brackets indicate the respective dose in ppm
by volume.
Example 5: Compatibility with lubricating oil
The tendency of an additive to form insoluble precipitates with
lubricating oil can be checked by various standardized laboratory
tests. Here, the test prescribed by DGMK (Deutsche
Wissenschaftliche Gesellschaft fur Erdol, Erdgas and Kohle e.V.,
Research Board 531 ~~Aufstellung eines Rriterienkataloges zur
CA 02388065 2002-04-18
.. .
., , , ,,
18
Testung von Lubricity Additiven in Dieselkraftstoffen fur den
Raffinerieeinsatz", Hamburg 1998) was used. Here, 10 ml of engine
oil (CEC RL 189; SAE 15W40) and 10 ml of additive are homogenized
in a 500 ml flask. After 72 hours at 90°C, the mixture is cooled
and visually assessed. This mixture is made up to 500 ml with
diesel fuel and filtered over a 0.8 Eun filter (SEDAB
specification). The occurrence of gel or sediment and exceeding a
filtration time of 300 seconds leads to "fail". "Pass" on the
other hand means that the test requirements have been fulfilled.
15
Table 4 below, containing the results of this test, illustrates
the fact that the lubricity enhancers A and B used according to
the invention are likely to have no adverse interactions with
lubricating oil.
Table 4: Lubricating oil compatibility tests according to DGMK FB
531
Additive after 72 h at 90~CFiltration Assessment
time [sec]
None - 112 -
Additive A clear 86 Pass
Additive B clear 89 Pass
Additive D cloudy > 300 Fail
( for
comparison)
Additive G cloudy, inhomoge- > 1800 Fail
(for neous, gel-like
comparison) particles
A commercial dimeric fatty acid was used as further comparative
additive G.
40
CA 02388065 2002-04-18