Note: Descriptions are shown in the official language in which they were submitted.
CA 02388782 2002-04-18
WO 01/28367 PCT/US99/24386
METHOD AND SYSTEM FOR ASSAY AND REMOVAL OF HARMFUL TOXINS DURING PROCESSING OF
TOBACCO PRODUCTS
BACKGROUND OF THE INVENTION
The present invention relates to an improved process and apparatus for
detecting and removing harmful toxins, such as mycotoxins and benzpyrene
(BZP),
found in tobacco and tobacco products to ensure that the products are safe for
human
association and/or consumption. More specifically, the invention relates to a
novel
process and apparatus for continuously detecting, monitoring and removing
harmful
mycotoxins, in particular, but not limited to, aflatoxins, and benzpyrene and
its
precursors, during processing of tobacco for human association, consumption
and use.
Moreover, the novel process and apparatus provides for inhibiting production
of
harmful toxins in tobacco and tobacco products, and for continuous monitoring
and
removal of such toxins from solvent and gaseous effluent streams arising from
processing tobacco. ..
Since at least as early as the 1980's, an increasing concern about public
safety
has led tobacco processors and refiners to attempt to reduce the tar content
of
cigarettes. ~It was this concern about consumer safety that resulted in
research in the
field of tobacco treatments for manufacturing reformulated tobacco with lower
tar.
U.S. Patent No. 4,944,316 to Stuhl et al., entitled "Process for Treating
Tobacco and
Similar Organic Materials."
It is believed that safety initiatives by the tobacco companies, however, have
only
recently addressed some of the most potent carcinogens: mycotoxins. A class of
mycotoxins, commonly known as aflatoxins, is one of the most potent
carcinogens
-1-
CA 02388782 2002-04-18
WO 01/28367 PCT/US99/24386
known to man. Eaton, David L., and John D. Groopman, The Toxicology of
Aflatoxins,
Academic Press, New York, 1994. Aflatoxins are estimated to be 200 times more
carcinogenic than benzpyrene, the most regularly acknowledged carcinogen in
tobacco
smoke. Moreover, benzpyrene pre-treatment of some species has been associated
with
an increase in bioactivity of aflatoxins. Ma, Xinfang, Jacqueline A. Gibbons
and John
G. Babish, "Benzo[e]pyrene Pretreatment of Immature, Female C57BL/6J Mice
Results
in Increased Bioactivation of Aflatoxin Bl in Vitro." Toxicology Letters,
1991; 59: 51-
58.
Additionally, aflatoxins have been shown to be profound immunosuppressants.
Denning, D.W., "Aflatoxin and Outcome from Acute Lower Respiratory Infection
in
Children in the Philippines." Annals of Tropical Paediatrics, 1995; 15: 209-
216. A
400% increase in the titers of human immunodeficiency virus (HIV) occurs when
exposed to aflatoxin. Yao, Yan, Amy Hoffer, Ching-yi Chang, and Alvaro Puga,
"Dioxin Activates HIV-1 Gene Expression by an Oxidative Stress Pathway
Requiring a
Functional Cytochrome P450 CYP1A1 Enzyme." Environmental Health Perspectives,
March,1995; 103: 366-371.
The potency of aflatoxins is further illustrated by its presence as one of the
chemical agents in Iraq's arsenal of chemical weapons. See a study by Anthony
H.
Cordesman, co-director of the Middle East Program at the Center for Strategic
and
International Studies, entitled "Weapons of Mass Destruction in Iraq"
(November 14,
1996). ,
-2-
CA 02388782 2002-04-18
WO 01/28367 PCT/US99/24386
It has been observed that many tumor types found in experimental animals that
are exposed to aflatoxins are the same as the tumor types found in cigarette
smokers.
As is well known, tobacco use has been associated with an increased incidence
of many
cancers, typically cancer of the lung, esophagus, mouth, throat, stomach,
colon, kidney,
bladder, and breast, among others. The presence of mycotoxins, such as
aflatoxins, on
tobacco may be a cause of the high incidence of cancer associated directly and
indirectly
with cigarette smoking. Dvorackova, Ivana, M.D. "Aflatoxin Inhalation and
Alveolar
Cell Carcinoma." British Medical Journal, March 20, 1976; 691. El-Maghraby,
O.M.
and M.A. Abdel-Sater, "Mycoflora and Natural Occurrence of Mycotoxins from
Cigarettes in Egypt." Zentralblatt fur Mikrobiologie, 1993; 148(4): 253-264.
In addition to danger to a cigarette smoker by the presence of aflatoxins in
primary cigarette smoke, aflatoxins may be a special hazard in secondhand
smoke.
Both aflatoxins, which are dihydrobenzofurofurans, and benzpyrene, are
aromatic
heterocyclics, which means they are relatively stable. Therefore, although
some
aflatoxins present in tobacco may be combusted at the combustion temperatures
that
are produced when a cigarette is burnt by inhaling at one end, aflatoxins have
been
shown under some smoking conditions, especially idling of a burning cigarette,
to
survive the combustion process. Lofroth, Goran and Yngve Zebuhr,
"Polychlorinated
Dibenzo-p-dioxins (PCDDs) and Dibenzofurans (PCDFs) in Mainstream and
Sidestream Cigarette Smoke." Bulletin of Environmental Contamination
Toxicology,
1992; 48: 789-794. As secondhand smoke is often combusted at lower
temperatures
than primary smoke, a larger proportion of aflatoxins may remain undestroyed
in
secondhand smoke, posing an environmental danger to others. In at least one
study,
-3-
CA 02388782 2002-04-18
WO 01/28367 PCT/US99/24386
passive or secondary smoke has been linked to repeated occurrences of acute
otitis
media among pre-school children. Collet, J.P., et al., "Parental Smoking and
Risk of
Otitis Media in Pre-school Children." Canadian Journal of Public Health, July-
August,
1995; 86(4): 269-273.
Inhalation of primary or secondhand smoke contaminated with aflatoxins may
be inadvertently increasing titers of HIV in individuals thus exposed; for
example,
pregnant women with HIV, thus increasing the chances of infecting their
offspring.
Yao, Yan, supra; and Vlahov, David, Ph.D., et al., "Prognostic Indicators for
AIDS and
Infectious Disease Death in HIV-Infected Injection Drug Users: Plasma Viral
Load and
CD4+ Cell Count." JAMA, January 7, 1998; 279 (1): 35-40.
These potent health hazards are produced by the Aspergillus and Penicillium
fungi, among others, and were known to be present in tobacco and tobacco
products
since at least the 1960's. Pattee, Harold E., "Production of Aflatoxins by
Aspergillus
flavus Cultured on Flue-Cured Tobacco." Applied Microbiology, November, 1969;
18:
952-953; Welty, R.E., G.B. Lucas, J.T. Fletcher, and H. Yang, "Fungi Isolated
from
Tobacco Leaves and Brown-Spot Lesions Before and After Flue-Curing." Applied
Microbiology, September, 1968; 16: 1309-1313; Hamilton, P.B., G.B. Lucas and
R.E.
Welty, "Mouse Toxicity of Fungi of Tobacco." Applied Microbiology, October,
1968; 18:
570-574; and Welty, R.E. and G.B. Lucas, "Fungi Isolated from Flue-Cured
Tobacco at
Time of Sale and After Storage." Applied Microbiology, March, 1969; 17: 360-
365.
However, the significance and potential health hazard of allatoxins were not
considered
by the tobacco industry until now. In a 1997 United States Patent to Subbiah,
entitled
-4-
CA 02388782 2002-04-18
WO 01/28367 PCT/US99/24386
"Method of Inhibiting Mycotoxin Production," No. 5,698,599, and assigned on
its face to
the R.J. Reynolds Tobacco Company, a method is disclosed for inhibiting
mycotoxin
production in tobacco.
Mycotoxins in general, and aflatoxins in particular, are monitored and
controlled in agricultural feed and foodstuffs to minimize their impact.
Current Food
and.Drug Administration (FDA) regulations ban use of aflatoxin-contaminated
corn
and grain when aflatoxin levels exceed 20 parts per billion (ppb). Similar
regulations
apply for other mycotoxins. Yet, due to lack of FDA authority, no regulations
presently
exist to mandate permissible levels of these toxins. on tobacco products, for
both chewing
and smoking. Presently there is no regulatory oversight to ensure that tobacco
and
tobacco products consumed by the public are adequately screened and treated
for
mycotoxins, such as aflatoxins, and benzpyrene. Furthermore, there is no
publicly
available information which reveals that adequate measures are being taken by
the
tobacco industry to monitor, treat and remove these potent toxins from tobacco
and
tobacco products.
Treatment of tobacco to reduce such harmful toxins is of critical importance.
Monitoring the production process to ensure continuous diminution is of equal
importance. A failure to adequately monitor, treat and remove these harmful
toxins
could result in their continued presence in tobacco and tobacco products with
attendant
negative public health consequences.
-5-
CA 02388782 2002-04-18
WO 01/28367 PCT/US99/24386
Prior art tobacco treatment processes do not fully acknowledge or address the
implications of mycotoxins (such as aflatoxins) on tobacco leaves, and
therefore, the
prior art processes do not adequately monitor or treat the toxins.
Reformulation and
reconstitution processes currently used in cigarette manufacturing appear to
mimic
many of the known processes for removing mycotoxins, especially aflatoxins,
from
agricultural products. U.S. Patent No. 5,082,679 to Chapman, entitled "Method
for
Detoxifying Foodstuffs"; U.S. Patent No. 4,962,774 to Thomasson et al.,
entitled
"Tobacco Reconstitution Process"; U.S. Patent No. 4,531,529 to White et al.,
entitled
"Process for Increasing Filling Capacity of Tobacco"; and U.S. Patent No.
4,055,674 to
Yano et al., entitled "Method for the Removal of Aflatoxin from Cereals, Oil
Seeds and
Feedstuffs." However, these processes do not disclose continuously assaying
and
treating in-process tobacco to ensure adequate removal and continuous
diminution of
harmful toxins, such as mycotoxins and benzpyrene, from tobacco and tobacco
end
products.
OBJECTS AND BRIEF SUMMARY OF THE INVENTION
It is therefore a general object of the invention to provide a novel process
and
system, which will minimize a potent toxin in tobacco, a toxin with negative
public-
health consequences.
It is another general object of the invention to provide a novel process and
system that inhibits production of and greatly reduces levels of harmful
toxins in
tobacco products.
-6-
CA 02388782 2002-04-18
WO 01/28367 PCT/US99/24386
It is another general object of the invention to provide a novel process and
system for continuous analysis and treatment of harmful toxins during
processing of
tobacco products.
It is another general object of the invention to provide a novel process and
system for continuous monitoring of a wide array of harmful toxins during
processing
of tobacco to detect and eliminate in-process product having unacceptably high
levels of
toxins.
It is another general object of the invention to provide a novel process and
system, which can be utilized for a wide range of tobacco products with
respect to which
microbial toxin detection and removal is desirable or necessary.
It is a specific object of the invention to provide for continuous assay and
analysis and removal of harmful toxins, such as mycotoxins and benzpyrene,
from
tobacco during processing for human and animal consumption and use.
It is another specific object of the invention to provide a novel process and
system for continuous assay and analysis and removal of harmful toxins from
solvent
and gaseous extraction streams and other processing steps.
It is another specific object of the invention to provide a novel process and
system for treating tobacco prior to processing to inhibit production of
harmful toxins
CA 02388782 2002-04-18
WO 01/28367 PCT/US99/24386
and to monitor and ensure the absence of harmful levels of the toxins in final
end
products.
It is another specific object of the invention to provide a novel process and
system for removing harmful toxins from tobacco processing solvent or gaseous
effluent
streams so that the toxin-free solvents or gases are safe for reuse or
disposal.
It is another specific object of the invention to provide a novel process and
system for making tobacco inert with respect to production and reformation of
harmful
toxins.
BRIEF SUMMARY OF PREFERRED EMBODIMENTS
OF THE INVENTION
Preferred embodiments of the invention that are intended to accomplish at
least
some of the foregoing objects comprise a process and system for storage,
handling, and
processing of tobacco in a cigarette manufacturing facility. Production of
harmful
toxins is inhibited, and harmful toxins that are present are continuously
monitored,
detected, and eliminated. The invention provides a process and system for
continuous
assay and treatment of toxins in an in-process product by contacting the
product with a
solvent. The solvent is extracted and assayed for toxin content. The in-
process product
is again contacted with a solvent if the assayed toxin content exceeds a
predetermined
level of toxin. The solvent contacting, extracting and assaying steps are
repeated until
the assayed toxin content does not exceed a predetermined level of harmful
toxin.
_g_
CA 02388782 2002-04-18
WO 01/28367 PCT/US99/24386
In one preferred embodiment of the invention, the in-process product is
intended
for human and animal consumption and use, such as tobacco. The toxin is a
mycotoxin,
and in particular an aflatoxin, or benzpyrene and its precursors. The process
and
system further comprises remediating the extracted solvent to remove harmful
toxin
and reusing the remediated solvent. Advantageously, the assaying is done by
chromatography, including high-pressure liquid chromatography (HPLC), reversed-
phase liquid chromatography, thin-layer chromatography, adsorption
chromatography,
immunoaffinity chromatography, gas chromatography; enzyme-linked
immunoadsorbent assay (ELISA), fluorescent immunoassay, radioimmunoassay;
spectroscopy, including mass spectroscopy, infrared spectroscopy, raman
spectroscopy,
packed-cell fluorescent spectroscopy; polymerase chain reaction (PCR),
supercritical
fluid extraction, bio-luminescence, chemical luminescence, and combinations
thereof.
Fluorescent immunoassay is a presently preferred best mode for assaying for
aflatoxin
on tobacco.
The process and system provides for monitoring toxin content to less than 300
parts per billion (ppb), in particular, less than 20 parts per billion (ppb),
and more
particularly, less than 0.5 parts per billion (ppb). The process and system
also provides
for treating in-process product to inhibit production and reformation of
toxin.
Advantageously, in-process product is treated prior to processing with
irradiation to
sterilize the product; with an inert gas environment;.or with non-toxigenic
fungal spores
to inhibit toxin production.
-9-
CA 02388782 2002-04-18
WO 01/28367 PCT/US99/24386
In another embodiment, the process includes heating in-process product, and
collecting and analyzing vapors emitted from the heated product to determine
toxin
content in the product. Product that has toxin content greater than 300 parts
per billion
(ppb) is separated from product that has toxin content less than 300 parts per
billion
(ppb) to eliminate grossly contaminated product.
The process and system provides for detecting toxin contamination in an in-
process product and separating contaminated product. Conveying means is used
for
conveying in-process product to means for retaining in-process product for
illumination
by ultraviolet light. Detector means detects fluorescence emitted from in-
process
product illuminated by the ultraviolet light indicative of toxin content.
Preferably,
computer means may be used for controlling the retaining means to retain
product for
further processing when no fluorescence is detected and to discharge product
when
fluorescence indicative of toxin is detected.
DRAWINGS
Other objects and advantages of the present invention will become apparent
from the following detailed description of preferred embodiments thereof taken
in
conjunction with the accompanying drawings, wherein:
FIGURE 1 is a schematic diagram of process steps representative of one
embodiment of the present invention;
FIGURE 2 is a schematic diagram of a representative apparatus for performing
the process of the subject invention;
-10-
CA 02388782 2002-04-18
WO 01/28367 PCT/US99/24386
FIGURE 3 is a schematic diagram of another representative apparatus for
performing the process of this invention;
FIGURE 4 is a schematic diagram of yet another representative apparatus for
performing the subject process;
FIGURE 5 is a schematic diagram of yet another representative apparatus for
performing the process in accordance with the invention;
FIGURE 6 shows an embodiment of a continuous assay device for performing
the process of the invention; and
FIGURE 7 shows another embodiment of a continuous assay device for
performing the process of the invention.
DETAILED DESCRIPTION
The process and system of the invention provides a product that contains
minimal amounts of harmful toxins, such as mycotoxins and benzpyrene, in the
final
end products, such as tobacco products. Tobacco leaves are particularly suited
for the
process and system of the invention. Tobacco strips, shredded tobacco, diced
tobacco,
tobacco rag, tobacco plant extracts, tobacco nicotine extracts, or any other
tobacco-
based product-all are considered within the scope of the invention.
As used herein, the terms "tobacco" and "tobacco products" mean all tobacco
and nicotine-based products intended for human and animal consumption,
association
and/or use, which may be contaminated with toxigenic microbial contaminants,
and in
particular, immunosuppressive and carcinogenic toxins. The term "in-process
-11-
CA 02388782 2002-04-18
WO 01/28367 PCT/US99/24386
product" means any product or commodity that is to be or is being processed
for
human and animal consumption or use. The term "grossly contaminated product"
means any product that is found to be contaminated, based upon visual
examination,
irradiation with ultraviolet light, measurement of moisture content, or any
other
general examination, such that the contamination cannot be removed or treated
as a
practical matter. The terms "toxins" and "harmful toxins" include mycotoxins,
such as
aflatoxins, ochratoxins, which are produced by Aspergillus ochraeus and are
both
nephrotoxins and promoters of lung tumors, zearealone, an estrogenic
carcinogen,
produced by the fungi species fusarium, which is known in particular to
contaminate
tobacco, and other mycotoxins that are known to be produced by a variety of
fungi that
regularly inhabit tobacco depending on the microenvironment; the at least 40
other
carcinogens known to exist in tobacco, the prototypical being benzpyrene; and
other
compounds such as tobacco-specific nitrosamines, which may be detected by
optical
fluoroscopy in solvent streams, and as such are amenable to a treatment
process to
remove them.
In its broadest aspect, the present invention is directed to reducing
contamination in tobacco and tobacco products by inhibiting production of
harmful
microbes, and in particular phytopathogenic fungi, and continuously monitoring
and
removing contamination from products, such as tobacco, which are prone to
contamination by phytopathogenic fungi that produce toxic metabolites known as
mycotoxins, and other harmful toxins. Contamination is reduced at each stage
of a
production process including storage, pre-processing, and during actual
processing into
end product. Of importance are mycotoxins such as aflatoxin, tricothecene
mycotoxins,
-12-
CA 02388782 2002-04-18
WO 01/28367 PCT/US99/24386
ochratoxins, rubratoxins, patulin, stachybotrys, T2 toxins, sterigmatocystin,
fusarium-
based toxins; benzpyrene and its precursors; and other toxins and contaminants
typically found in tobacco and tobacco products.
In-process product determined to be grossly contaminated is continuously
eliminated from further processing. The products are treated to prevent
production of
harmful microbes and are continuously monitored during processing into
products for
human and animal consumption and/or use to detect and remove known harmful
toxins. Pre- and post-production treatments of the products provide added
protection
against microbial growth and remediation of solvents and other agents used in
processing permits safe reuse or disposal of the solvents/agents.
In particular, the process and system of the invention is directed to
detecting,
monitoring and removing one of the most dangerous of the mycotoxins known to
man: a
class of toxins commonly referred to as aflatoxins. The process includes
continuously
assaying or testing effluent streams derived from processing the commodity to
monitor
levels of aflatoxins in the effluent streams. This continuous assaying ensures
a minimal
presence of harmful toxins in final end products. The subject invention is
particularly
applicable to tobacco and products such as cigarettes because it provides a
continuous
monitoring and treating process and system for application in tobacco
processing and
manufacturing facilities.
-13-
CA 02388782 2002-04-18
WO 01/28367 PCT/US99/24386
The invention is also directed to detecting, monitoring and removing
benzpyrene
and its precursors. With respect to benzpyrene, see United States Patent No.
3,863,645
to Tso, entitled "Process for Treating Tobacco."
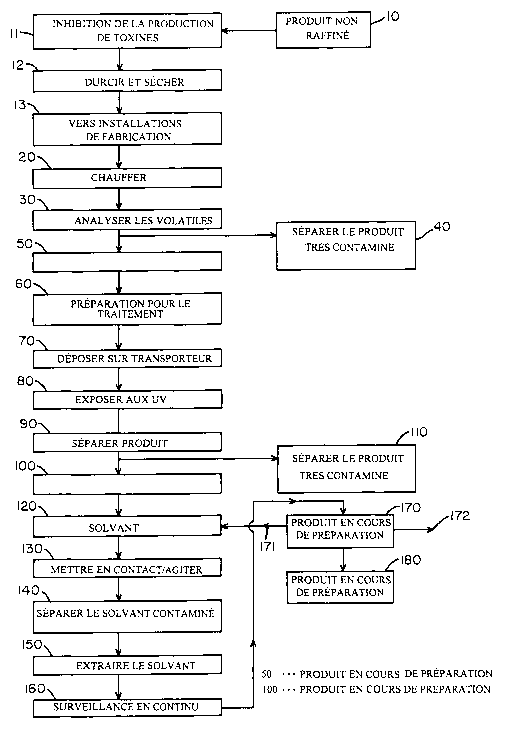
Refer now to the drawings and particularly to Figure 1, which shows that a
commodity 10, such as unrefined tobacco material, is treated prior to
processing to
inhibit toxin production 11. The unrefined tobacco is placed in a storage
facility 12, to
cure and dry. This step of curing and drying typically occurs prior to
transport to a
manufacturing facility, such as a cigarette manufacturing facility, for
processing into an
end product. The tobacco product 10 may be washed post-harvesting with a
detergent
or other suitable cleansing solution to remove debris, pesticides, fungicides,
etc., and
placed on a conveying device for irradiation with gamma, x-ray or electron-
beam
radiation in a dose sufficient to sterilize most microbial contaminants.
Typically,
electron-beam irradiation in the range of about 1.5 kilograys (Kgys) or less
is used
within an energy range of about 0.5 to about 2.0 Mev to penetrate thin
material less
than 1 cm. thick. A 1997 United States Patent to McFarland entitled
"Irradiation
Method and Apparatus," No. 5,603,972, discloses an irradiation method and
apparatus.
The disclosure of that patent and all other references cited and/or discussed
herein is
hereby incorporated herein by reference as though set forth at length.
Alternatively, gamma radiation in the range of 20 to 30 Kgys is used for
thicker
products. A 1994 United States Patent to Kent entitled "Method for Sterilizing
Products with Gamma Radiation," No. 5,362,442, discloses a method for
sterilizing
products with gamma irradiation and the disclosure of that patent is also
incorporated
-14-
CA 02388782 2002-04-18
WO 01/28367 PCT/US99/24386
herein by reference. Since fungal spores are more resistant to radiation, a
dose of 50-75
Kgys should be effective. As an alternative to irradiation, the product 10 may
be
treated with a suitable sporicidal composition. A United States Patent to
Allen entitled
"Method for Killing or Inhibiting the Growth of Sporulating Microorganisms
with
Haloperoxidase-Containing Compositions," No. 5,510,104, discloses one method
for
such treatment and its disclosure is incorporated herein by reference as
though set forth
at length.
A step to separate grossly contaminated product at this stage involves
removing
a known volume of product 10 and weighing it before further processing. If
certain
threshold weights are exceeded, moisture in the product could be excessive and
a
likelihood of fungal content is increased. Generally, aflatoxin formation is
found to
occur only when relative humidity exceeds about 85%, or when moisture content
of the
commodity exceeds about 18%. Pattee, Harold E., "Production of Aflatoxins by
Aspergillus Flavus Cultured on Flue-Cured Tobacco." Applied Microbiology,
November, 1969; 18: 952-953. Hence, such grossly contaminated product may be
rejected at the outset. The weighing process is preferably done on a
continuous
conveyor.
Figure 2 shows in diagrammatic form an apparatus for treating product 10 to
inhibit production of harmful microbes. (Step 11 in Figure 1.) The product 10
is placed
in a suitable treatment chamber 200, such as a curing barn. The product 10 may
previously have been sterilized or otherwise treated as discussed above. A
prepackaged
cartridge 210 is provided to inject non-toxigenic benevolent fungal spores 211
into the
-15-
CA 02388782 2002-04-18
WO 01/28367 PCT/US99/24386
chamber 200. The purpose of the benevolent fungal spores 211 is to crowd out
harmful
toxigenic microbes with a harmless species. The treatment is generally done in
an
enclosed semi-airtight chamber 200, but a curing barn may suffice. United
States
Patent to Cotty, entitled "Method for the Control or Prevention of Aflatoxin
Contamination Using a Non-Toxigenic Strain of Aspergillus Flavus," No.
5,294,442, and
United States Patent to Miller et al., entitled "Packaged Fungal Culture
Stable to Long-
Term Storage," No. 5,679,362, disclose a benevolent spore production device
and their
disclosure is hereby incorporated by reference as though set forth at length.
In Figure 2, a moisture source 220, such as moistened sponges on rotating
cylinders, is provided to aerosolize the spores 211 ejected from the fungal
spore
cartridge 210 and a blower device 230 is provided for blowing the fungal
spores 211
from cartridge 210 through the chamber 200 so that benevolent fungal spores
211 are
fully dispersed through out the chamber 200 and the product 10. A water bath
240 is
provided for spore production and a heating device 250 for heating the water
bath 240.
If liquid distribution of aerosolized spores is desirable or necessary, a mist-
generating
apparatus (not shown) may be provided to provide a benevolent fungal spore
bearing
mist for distribution through out the chamber 200. A conveying device 260 is
provided
for conveying the product 10 from the chamber 200 for further processing.
As an alternative to the benevolent fungal spores discussed above, the chamber
200 may have a nitrogen generator (not shown) to provide an inert atmosphere
in the
chamber. One example of a nitrogen generator has a semi-permeable membrane
that
separates out nitrogen from air. Other nitrogen generators are known in the
art and
-16-
CA 02388782 2002-04-18
WO 01/28367 PCT/US99/24386
therefore are not discussed in detail here. A United States Patent to Ward
entitled
"Nitrogen Generator Process for the Production of Low Volumes of High Purity
Nitrogen from Compressed Air," No. 4,572,723, discloses one example of a
preferred
nitrogen generator. In the instance of use of nitrogen, or other suitable
inert gas, the
chamber is airtight, purged of air, and the air is replaced with pure nitrogen
from the
nitrogen generator, or with another suitable inert gas. An inert atmosphere
inhibits
and/or prevents production of harmful fungal toxins. Pattee, Harold E.,
"Production of
Aflatoxins by Aspergillus Flavus Cultured on Flue-Cured Tobacco." Applied
Microbiology 1969; 18: 952-953. Preferably, the product .10 is stored in an
airtight
storage container that contains a minimal, but optimally, substantially zero
amount of
oxygen so as to prevent formation of toxins. Preferably, the product 10 is
surrounded
by an inert gas, generally, but not limited to nitrogen, to inhibit and
prevent further
toxin production.
As another alternative for inhibiting toxin production, the product 10 can be
treated to inhibit production of microbes, as disclosed in U.S. Patent No.
5,698,599,
supra, the disclosure of which is hereby incorporated herein by reference in
its entirety.
Refer again to Figure 1, which shows that the product 10 is transported to a
manufacturing facility 13, such as a cigarette manufacturing plant, and is
preferably
heated 20, for example, by steam, infrared irradiation, or microwave
irradiation. U.S.
Patent to Lasch et al., entitled "Method of and Apparatus for Manipulating
Bales of
Condensed Tobacco Particles," No. 5,139,035, discloses methods of heating
tobacco and
its disclosure is hereby incorporated herein by reference as though set forth
at length.
-17-
CA 02388782 2002-04-18
WO 01/28367 PCT/US99/24386
Continuous monitoring 30 of the heated tobacco is performed to analyze vapors
emitted
from the product 10 for toxin content. In this, gas chromatography or
gas/solvent
immunoantibody fluorescence or any other suitable analysis technique may be
used to
analyze the vapors. United States Patent to Stahr entitled "Method of
Detecting Mold
Toxin Infected Grains," No. 4,314,027, discloses one suitable method to
analyze vapors
and its disclosure is hereby incorporated herein by reference in its entirety.
There are presently no guidelines for aflatoxin contamination with respect to
tobacco products, but given the increasing incidence of all cancers associated
with
smoking, and the potency of aflatoxins, the process and system of the present
invention
provides for a substantially reduced concentration of this carcinogen, i.e.,
the mycotoxin
is substantially eliminated from in-process product. Grossly contaminated
product, i.e.,
product contaminated to such an extent that removal of contamination is
impossible as
a practical matter, is separated 40 and removed from further processing to be
discarded
or otherwise handled as appropriate. U.S. Patent No. 4,991,598 to Henderson et
al.,
entitled "Method of and Apparatus for Automatically Analyzing the Degradation
of
Processed Leaf Tobacco." In-process product 50, which can be treated and
processed
effectively, is retained for further processing and treatment.
Although, as discussed above, there are presently no guidelines with respect
to
mycotoxin contamination in tobacco, some guidance may be obtained from
mycotoxin-
contamination guidelines with respect to other agricultural products. For
example,
some state regulations ban foodstuffs and animal feed when aflatoxin
contamination
exceeds 200 to 300 parts per billion (ppb), the United States Food and Drug
-18-
CA 02388782 2002-04-18
WO 01/28367 PCT/US99/24386
Administration (FDA) currently bans sale of foodstuffs when aflatoxin
contamination
exceeds 20 ppb, and milk is banned for human consumption when levels of
aflatoxins
exceed 0.5 ppb. However, it will be appreciated that in the main, experience
will dictate
to a skilled practitioner when threshold levels of mycotoxins in general, and
in
particular aflatoxins, are above critical levels, at which they cannot be
practically
removed from the product. In other words, the skilled practitioner knows when
the
commodity is grossly contaminated.
Once the in-process product 50 is acceptable for further processing, it may be
prepared for processing 60 by treatments designed to volatilize, vaporize,
heat, freeze
dry, irradiate, wet, solubilize or provide other desired treatment before
further
processing begins. The nature and extent of such preparation 60 depends upon
the
product 50 and the treatments that are considered desirable or necessary for
the
product 50 before further processing. As a part of the preparation for
processing 60,
preferably individual sheets of product 50, i.e., tobacco leaves, are
deposited 70 on a
conveyor device such that a maximum amount of surface area of the product 50
is
exposed. The preparation 60 may include slicing the product 50 with a sharp
knife
device, cutting it with a reciprocating or band saw, burning of sections with
high-energy
laser, etc., so as to expose a maximum surface area of the in-process product.
After the product 50 is deposited on a conveying device 70, the product 50 is
exposed 80 to ultraviolet radiation to separate 90 uncontaminated in-process
product
100 from contaminated product 110, as discussed in detail below. U.S. Patent
No.
4,866,283 to Hill, Jr., entitled "Optical Inspection of Food Products."
-19-
CA 02388782 2002-04-18
WO 01/28367 PCT/US99/24386
Generally, ultraviolet radiation in the range of, but not limited to, about
362 to
about 363 manometers is used for aflatoxin detection. Exposure of aflatoxins
in
particular to ultraviolet radiation results in optical fluorescence at about
425 to about
450 manometers, which can be seen by the naked eye in a darkened environment.
Similarly, other mycotoxins may be detected using ultraviolet radiation having
frequencies specific to the particular mycotoxins. As is generally known,
different
species of mycotoxins have associated excitation-emission frequencies.
Detection of such
mycotoxins using their associated excitation-emission frequencies is within
the scope of
the present invention. Moreover, various other types of harmful carcinogenic
compounds present in tobacco and tobacco products may also be removed using
their
excitation-emission frequencies as shown in Table 1.
Table 1: Excitation-Emission
Maximums for Various Polynuclear
Aromatic H drocarbons
Pol nuclear Aromatic H drocarbonsExcitation Emission
P rene 331 384
Phenanthrene 248 365
Fluoranthrene 284 454
Anthracene 248 ~ 395
Chr sene 262 377
Benzo(a) rene 378 400
Benzo(a)anthracene 282 385
Benzo c henanthrene 275 390
Benzo b fluoranthrene 295 426
Benzo ' fluoranthrene 313 498
Benzo ,h,i er lene 295 415 .
Meth lcholanthrene 291 414
Dibenz(a,h)anthracene 280 380
The optical fluorescence emitted may be detected by devices such as electronic-
image intensifiers, enhancers coupled with charged coupled devices, etc.
Preferably, the
-20-
CA 02388782 2002-04-18
WO 01/28367 PCT/US99/24386
devices for detecting optical fluorescence are connected to a computer
programmed for
controlling other devices that separate 90 uncontaminated in-process product
100 from
contaminated product 110. In this, devices that can be controlled by a
computer for
separating contaminated product include, but are not limited to, a swinging or
extending sweeping-arm device, which sweeps contaminated product into a waste
bin or
onto a second conveyor running at any desired angle and in any direction
relative to a
first conveying device. In addition, a blast of air may be used for separation
of
contaminated product to another conveyor and a vacuum device may be used for
sucking up the contaminated product.
Figure 3 shows in block-diagram form a preferred embodiment of a system 300
for exposing product 50 to ultraviolet light and separating contaminated
product 110
(steps 80 and 90 in Figure 1). A loading device 310 conveys in-process product
50 to a
conveying device 320 having means 330 for applying negative pneumatic
pressure, i.e., a
suction device. The product 50 is retained on the conveying device 320 and is
carried
thereon while being exposed to ultraviolet radiation from an ultraviolet light
source 340
of a specific frequency. The ultraviolet light source may be a laser operable
for
producing ultraviolet light. In one embodiment, the laser may be a laser
diode.
Preferably, the conveying device 320 is made of a material that is optically
transparent
to ultraviolet light so that the product 50 may be exposed to ultraviolet
light 340 from
top and bottom. A computer 350 controls pneumatic device 330 so that when
toxin
contamination is detected by fluorescence detector 360 the pneumatic pressure
of the
pneumatic device 330 is reversed, i.e., a blower device, and contaminated
product 110 is
blown off the conveying device 320 into a reject bin 370. The fluorescence
detector 360
-21-
CA 02388782 2002-04-18
WO 01/28367 PCT/US99/24386
is connected with the computer 350, which controls separation of contaminated
material
from uncontaminated commodity. Uncontaminated in-process product 100 is
retained
on the conveying device 320 and carried away for further processing into an
end
product, such as cigarettes. The contaminated product 110, however, is carried
from
the bin 370 by conveying means 380 for appropriate disposal. In a preferred
embodiment of the system 300, the conveying device 320 is a conveyor belt or
screw
conveyor, or any other suitable conveyor apparatus, and is made of a clear
material that
is optically transparent to ultraviolet light. Optical radiation is easily
transmitted
through the device 320 to allow detection of contamination on both upper and
lower
surfaces of the product 50, thereby increasing efficiency and accuracy in
selecting and
separating contaminated product from the production line. A similar result may
also be
obtained by blowing the in-process product 50 over a glass plate irradiated
with a
desired optical radiation.
Tobacco is prone to aflatoxin contamination when stored in the open and wet by
rain. In this, the system 300 may advantageously be used to continuously
expose
tobacco carried by optically transparent screw conveyors, augers, or belts to
ultraviolet
radiation of a specific frequency.
Figure 4 shows an apparatus 400 for sorting tobacco (steps 80 and 90 in Figure
1) having a clear, transparent chamber 410 and a conveying device 401 for
conveying
in-process tobacco to the chamber 410. A number of channels 420 in the chamber
410
have openings 421 at the bottom for gravity separation of contaminated product
from
uncontaminated product. Preferably, the channels 420 in the optically
transparent
-22-
CA 02388782 2002-04-18
WO 01/28367 PCT/US99/24386
chamber 410 are parallel to each other and are separated by a minimal amount
of
distance so that in-process product in the channels 420 passes through the
channels 420
with each surface being exposed to UV radiation from an ultraviolet light
source 430 of
specific frequency. This arrangement enhances detection of toxin contamination
and
ensures that contaminated tobacco does not pass through undetected.
Preferably, a
plurality of fiber-optic illumination and receiving fibers 440 are placed in
the
transparent panels of the chamber 410, making the unit 400 compact and
eliminating
need for bulky UV light sources between the clear panels. A computer 450
connected
with the fiber- optic strands 440 controls the bottom openings 421 of the
channels 420 to
eject contaminated product into a reject bin 460. A conveying device 470
carries
uncontaminated product out of the chamber 410, preferably to another treatment
chamber 480 for treating the uncontaminated product with a suitable treatment
gas,
such as, for example, ammonia (NH3), to remove or treat any toxin
contamination
remaining undetected in the preceding chamber 410 and to inhibit production or
reformation of harmful toxins. Another conveying device 490 carries treated
product
out of treatment chamber 480 for further processing, if desirable or
necessary.
Refer again to Figure 1, which shows that a solvent 120 for removing toxins is
contacted and agitated 130 with in-process product 100. Solvents considered
particularly suitable for use in the subject invention include aqueous
solutions having
adjunct solvents added to facilitate separation of toxins such as acids,
bases, oils,
detergents, fatty acids, esters, emulsifiers; organic-based solvents,
including ethers,
ethanol, methanol, chloroform, dichloromethane; other alcohols, ammonia,
bleaches,
hydrogen peroxide, polyethyleneglycol, amines, methylamines, hydroxides of
salts,
-23-
CA 02388782 2002-04-18
WO 01/28367 PCT/US99/24386
formalin, ozone; or other solvents or solutions. Reagents that cause the
toxins to
separate as precipitates, as well as solvents that solubilize the toxins, are
considered
within the scope of the invention. Tobacco-processing solvents and solvents
used to
extract mycotoxins, such as aflatoxins, during processing regimens are
numerous and,
in many respects, the same. For example, alcohols may be used, in particular
methanol
and ethanol. Halogenated hydrocarbons, ethers, and other wetting agents may
also be
used. Liquid carbon dioxide may also be used as a solvent.
Mycotoxins, and in particular aflatoxins, are removed by contacting and
agitating 130 the in-process product with a suitable solvent 120, separating
contaminants. 140 from the product, and then removing the toxins as a
suspension in
extracted solvent 150. Typically, product 100 is contacted with a suitable
solvent or
solvents, and the mixture is then physically agitated by stirring, shaking,
subjecting to
ultrasonic cavitation, or any other similar agitation process, to physically
separate any
contaminants from the in-process product. Preferably, the solvent-product
mixture is
tested for toxin level prior to treatment and then is subjected to
intermittent or
continuous ultrasonic cavitation (U.S. Patent No. 5,498,431 to Lindner,
entitled
"Decontamination and Detoxification of Cereals Contaminated with Mycotoxins")
and
ultraviolet illumination (U.S. Patent No. 5,194,161 to Heller et al., entitled
"Materials
and Methods for Enhanced Photocatalyzation of Organic Compounds with
Palladium")
until such time that the extracted solvent 150 no longer contains a
significant level of
toxins, as discussed in further detail below. Solvent treatment of in-process
product and
continuous monitoring of extracted solvent streams ensures that even minute
quantities
of contaminants, which would otherwise escape detection, are eliminated from
in-
-24-
CA 02388782 2002-04-18
WO 01/28367 PCT/US99/24386
process product, thereby ensuring that end products, such as cigarettes, are
free of
harmful toxins.
Toxin levels in extracted solvent streams 150 are continuously monitored 160
to
detect levels of toxins present prior to treatment and remaining in the
product 100. For
example, solvent streams extracted from the solvent-product slurry mixture are
filtered,
clarified, or otherwise rendered relatively optically transparent, such that
the solvent
streams can then be subjected to ultraviolet radiation, in particular to
monitor for
aflatoxins. U.S. Patent No. 4,285,698 to Otto et al., entitled "Analysis of
Aflatoxins in
Peanuts by High Pressure Liquid Chromatograph." Preferably, the solvent
streams are
passed through an immunoaflinity column, or clay-type filtration column, to
clean up
other contaminants in the solvent streams so that aflatoxins in the solvent
streams may
be better detected. Stubblefield, R.D., J.I. Green O.L. Shotwell, and A.M.
Aikens,
"Rapid Immunochemical Screening Method for Aflatoxin Bl in Humans and Animal
Urine" JOAC 1991; 74: 530.
A number of alternative assaying techniques may be used to continuously or
intermittently monitor levels of toxins. These assaying techniques include,
but are not
limited to, high-pressure liquid chromatography (HPLC), reversed-phase liquid
chromatography, thin-layer chromatography, radioimmunoassay (RIA), antibody-
linked RIA, ELISA, spectrophotometry, mass spectrophotometry, infrared
spectroscopy, raman spectroscopy, lyophilized ligand-receptor complexes for
assays and
sensors, packed-flow cell fluorescence liquid chromatography (PFCFLC),
antibody-
-25-
CA 02388782 2002-04-18
WO 01/28367 PCT/US99/24386
linked immunoassay, adsorption chromatography, immunoaffinity chromatography,
supercritical fluid extraction, bio-luminescence, chemical luminescence, and
others.
Preferably, the extracted solvent streams 150 may be irradiated with optical
radiation of a desired wavelength delivered and/or sensed through a fiber-
optic device.
In this, continuous assay of effluent streams involves use of fiber-optic
fibers or strands
to carry and receive optical radiation used in the toxin identification
process. The
illumination apparatus may be located at a considerable distance from the
point of
solvent stream toxin identification. Advantageously, use of fiber optics
allows a
plurality of wavelengths of light in close proximity to each other to be used
for multiple
toxin identification, and for a plurality of receiving fiber strands to be
placed adjacent
to each other, if necessary or desirable. The fiber optics may advantageously
be mated
to an electro-optical processing unit such that incident optical radiation is
converted
into an electrical analog or digital data stream, and the data is then
transmitted
electrically to a computer processing unit. In this, a liquid or gaseous
effluent-solvent
stream is illuminated at various specific frequencies and the reflected
fluorescing
radiation is transmitted back to a central computer. A program or algorithm
designed
to signal the presence of predetermined levels of toxins or other undesirable
chemicals is
preferably used to monitor levels of toxins in the effluent streams at, or in
excess of,
preset levels. Once the alert to excessive levels of toxin contamination is
given, further
treatment steps can be effected, possibly resulting in total rejection of an
entire batch if
treatment and removal of toxins cannot be reliably achieved.
-26-
CA 02388782 2002-04-18
WO 01/28367 PCT/US99/24386
Refer again to Figure 1, which shows that remediation 170 of contaminants in
extracted solvents 150, used to extract, treat, or remove toxins from in-
process product
100, is provided. Such treatments include, but are not limited to,
acidification,
oxidation, reduction, peroxidation, ammoniation, addition of a base, dilution,
microwave irradiation, nuclear irradiation, ozonation, ultraviolet
irradiation, heating,
cooling, saponification, precipitation, condensation, chemical alteration or
ultrasonic
cavitation.
Tobacco processing currently used in manufacturing reformulated tobacco
product involves a series of steps designed to separate tobacco leaves from
certain
pharmacologically active substrates, especially nicotine. The tobacco
processing steps
then continue, and certain components of tobacco such as flavorings and likely
carcinogens are extracted out. The tobacco product is then treated further,
and at some
point, nicotine and/or other extracts may be added back into the nearly
finished
product. Preferably, any extracts added back into the in-process tobacco are
tested for
toxins and treated, if necessary.
As aflatoxins are deadly poisons, it is not adequate to merely treat to remove
them without knowing the level of contamination before treatment and what
remains
after treatment. The process and system of the present invention treats toxins
in
effluent solvent streams, and other potential additives, as a quality control
measure,
especially to prevent reintroduction of contaminants back into the in-process
tobacco.
The remediated solvent, or tested and treated additives, may be reused 171, or
at least
safely disposed of 172, when the level of contamination is known. In this, the
process
-27-
CA 02388782 2002-04-18
WO 01/28367 PCT/US99/24386
and system of the invention quantifies the levels of toxins in the effluent
streams, thus
indirectly revealing the level of toxins remaining on the tobacco, especially
when the
solvents separate the toxins from the tobacco with great avidity. For
remediation of a
solvent stream identified as toxin-tainted, a computer can be programmed to
institute
5- appropriate treatment regimens, which are effected as quickly and
economically as
possible. The process and system continues treatment until such time as the
solvent
stream is deemed as safe as possible.
The in-process product is treated 180 near the end of its
treatment/manufacturing process, but not necessarily as a last step, to
prevent
reformation of toxins. Ammoniation of smoking compositions has been shown to
decrease biological activity. U.S. Patent No. 3,631,865 to Michelson, entitled
"Smoking
Composition of Reduced Toxicity and Method of Making Same." The reformation
typically occurs when the pH of the processed product changes at the end of
the process.
For example, addition of gaseous or liquid ammonia (NH3) may be used to
protect
decontaminated tobacco from recontamination by reformation of aflatoxins.
Additionally, processed product could advantageously be packaged within an
airtight
container that contains an inert gas, such as nitrogen, which prevents growth
of toxins,
or ammonia (NH3), which prevents reformation of toxins on the finished
product.
Figure 5 shows in diagrammatic form an apparatus 500 for continuous assay of
effluent solvent streams and remediation of solvent streams to decontaminate
harmful
toxins separated from in-process product 100. (Steps 160 and 170 of Figure 1.)
Effluent
-28-
CA 02388782 2002-04-18
WO 01/28367 PCT/US99/24386
solvent derived from treatment of product 100 is delivered to a solvent
assaying device
501.
Figure 6 shows one preferred embodiment of a solvent-assaying device according
to the present invention having a continuously moving transparent strip 600
comprising
a substrate 601 with slots 602 therein arranged longitudinally along the strip
600, for
example, similar to a 35 mm photography film. U.S. Patent No. 4,071,315 to
Chateau
entitled "Analytical Tape Medium." A plurality of mycotoxin-specific
antibodies 610
are provided on the substrate 601 extending longitudinally along the strip
600. U.S.
Patent No. 4,168,146 to Grubb et al., entitled "Immunoassay with Test Strip
Having
Antibodies Bound Thereto." When the strip 600 is exposed to a continuous
effluent
solvent stream, toxins present in the effluent solvent adhere to the
antibodies 610 on the
strip 600 specific to the toxins in the solvent. The strip 600 may be
contacted with
effluent solvent, for example, by dripping the solvent, brushing it, or
otherwise
contacting the effluent solvent with the strip 600.
The strip 600, after exposure to effluent solvent, preferably has fluorescent
probes 620 attached chemically to the toxin-antibody complex forming toxin-
specific
antibody fluorescent probe complexes 630. U.S. Patent No. 4,036,946 to
Kleinerman,
entitled "Immunofluorometric Method for Measuring Minute Quantities of
Antigens,
Antibodies and Other Substances." The strip 600 with the complexes 630 is
exposed to
ultraviolet light 640 having a wavelength that is specific to the fluorescent
complex to be
identified. The radiation emitted is measured and quantified by a detector
650,
advantageously connected to a computer, to yield a continuous assay of toxins
in the
-29-
CA 02388782 2002-04-18
WO 01/28367 PCT/US99/24386
effluent solvent, and therefore, the toxins present in the in-process product
100. The
product 100 is retreated with solvent till such time that it meets acceptable
standards
for toxin content. In this, by use of the strip 600 an in-process product can
be tested
simultaneously and continuously for 5 to 10 different toxins. Advantageously,
a control
or pilot antibody strip (not shown) specific to a control chemical in the
effluent stream is
provided on the strip 600 to verify that the strip 600 is properly exposed to
the effluent
stream. U.S. Patent No. 4,772,551 to Hart et al., entitled "Method and Test
Kit for
Detecting A Trichothecene Using Novel Monoclonal Antibodies"; and U.S. Patent
No.
4,835,100 to Dixon et al., entitled "Method and Test Kit for Detecting an
Aflatoxin Bl
and Gl Using Novel Monoclonal Antibodies."
Figure 7 shows another preferred embodiment of a solvent assaying device
according to the present invention having a continuous solvent testing device
700 with
automated, continuously moving transparent cuvettes 710; for example, cuvettes
or cells
continuously unwound from a roll using suitable means. Inlet means 711 are
provided
for delivering effluent solvent and other assaying agents described below into
the
cuvettes 710. U.S. Patent No. 3,763,374 to Tiffany et al., entitled "Dynamic
Multistation
Photometer-Fluorometer." Laser produced ultraviolet light 720 is transmitted
by fiber-
optic cable 730 (U.S. Patent No. 3,992,631 to Harte, entitled ~ "Fluorometric
System,
Method And Test Article") to illuminate, for example, aflatoxin- specific
antibody-
coated beads 740 in the cuvettes 710. The toxin-specific antibody-coated beads
740 may
be coated with antibodies specific to any toxin that is to be detected. The
beads 740 are
contacted with effluent solvent, which is introduced in the cuvettes 710 via
inlets 711.
The antibody bead-cuvettes 710 preferably use fluorescent probes, which, when
-30-
CA 02388782 2002-04-18
WO 01/28367 PCT/US99/24386
combined with antibodies for specific toxins, will fluoresce even if the toxin
in question
does not fluoresce well or at all. An accelerator reagent may be used, if
desirable or
necessary, and the cuvettes 710 may be agitated, heated or otherwise treated
to enhance
assay sensitivity, as with addition of a cyclodextrin. Cepeda, A., et al.,
"Postcolumn
Excitation of Aflatoxins Using Cyclodextrins in Liquid Chromatography for Food
Analysis." Journal of Chromatography,1996; 721: 69-74.
The fiber-optic apparatus 730 transmits fluorescent radiation, detected by
detecting means 750, such as, for example, an image enhancer or intensifier
device, to
an electro-optical computer or evaluation unit 760. If a light source that
consists of a
plurality of wavelengths is used, a filter may also be used to screen out or
eliminate
undesired optical radiation from being transmitted to the optical-radiation
computer.
Preferably, optically transparent cuvettes are used to hold the testing
complex, and the
cuvettes may move sequentially in an automated-testing regimen. The automated
testing regimen may advantageously use a circular spinning tray to hold
samples, or
may consist of a continuous strip of test wells that move into the testing
zone. Test
cuvettes may be preloaded with toxin-specific antibody fluorescent probe
complexes
(TSAFPC) (Haugland, Richard P., Handbook of Fluorescent Probes and Research
Chemicals, Sixth Ed. Molecular Probes, Inc., Eugene, Oregon, 1996) with
presealed
needle-penetrable rubber stoppers, or test cuvettes may be loaded with toxin-
specific
antibody fluorescent probe complexes (TSAFPC) through inlet means 711 just
prior to
testing a solvent sample. An advantage of point of testing loading (POTL) of
cuvettes is
that the desired test may be chosen by a suitably programmed computer
depending on
the anticipated or suspected toxin and quantities present.
-31-
CA 02388782 2002-04-18
WO 01/28367 PCT/US99/24386
The toxin-specific antibody fluorescent probe complexes (TSAFPC) may be
coated onto transparent microspheres of various sizes to obtain optimal
fluorescence,
thereby enhancing detection sensitivity. The TSAFPC-coated microspheres may be
used in test cuvettes, as described above. Alternatively, the TSAFPC-coated
microspheres may be affixed to a suitable substrate and used in the manner
described
above in connection with Figure 6. Moreover, as another embodiment, the TSAFPC-
coated microspheres may be introduced into a flowing solvent stream, captured
by a
restrictive or screen-type device, and illuminated with suitable optical
radiation, and
thereby, assayed. U.S. Patent No. 4,181,853 to Abu-Shumays et al., entitled
"Liquid
Chromatography System With Packed Flow Cell For Improved Fluorescence
Detection"; and U.S. Patent No. 5,322,799 to Miller, Robert J. and James D.
Ingle,
entitled "Observation Cell And Mixing Chamber."
In addition, mirrors and filters may be used, if desirable or necessary, to
optimize detection of the optical radiation by the fiber-optic detection unit.
Another
feature of the process and system relates to alignment of the solvent-
containing tubes or
cuvettes 710 and the fiber-optic illumination cables 730 and/or receiving
detector 750 so
that optical radiation is optimized.
Refer again to Figure 5, which shows that after continuous assay of effluent
solvent and quantification of levels of toxins in the solvent, the solvent is
remediated in
treatment chamber 502 of apparatus 500. Treatment gas/solvent is provided via
input
means 510, and preferably, an ultrasonic transducer/cavitator device 520 is
used to
-32-
CA 02388782 2002-04-18
WO 01/28367 PCT/US99/24386
promote remediation of the effluent solvent. Preferably, neutrally buoyant
palladium,
or other suitable catalyst-coated spheres 530 are provided in the treatment
chamber 502
to enhance cavitation treatment. In this, palladium-catalyst coating is in the
range of
about 0.001 to about 3.0 percent by weight. Advantageously, the spheres 530
have
diameters in the range of about 30 to about 100 manometers. Advantageously, an
ultraviolet light source 540 is provided for biocidal treatment of the
effluent solvent. In
this, ultraviolet light in excess of about 10 watts/meterz is an effective
biocide and
enhances catalytic degradation of toxins. United States Patent No. 5,194,161
to Heller et
al., supra, discloses materials and methods for enhanced photocatylization of
organic
compounds with palladium and said disclosure is hereby incorporated herein by
reference in its entirety. Typically, aflatoxin contaminants of agricultural
commodities
are remediated by contacting the products with an ammonia-based solution or
gas. U.S.
Patent No. 5,082,679 to Chapman, supra. After remediation, the remediated
effluent
solvent is once again assayed using another assaying device 503, which
preferably is one
of the assaying devices described above. Outlet means 550 removes remediated
solvent
for further remediation, if desirable or necessary, or for reuse or safe
disposal, as
desired.
In addition to mycotoxins, tobacco contains at least some 40 other
carcinogens,
the prototypical being benzpyrene and its precursors and its congeners, which
have
their own specific excitation-emission frequencies and are thus subject to
detection and
remediation. Fungi known in particular to contaminate tobacco are the species
fusarium, which produce zearealone, an estrogenic carcinogen. Aspergillus
ochraeus
can produce a mycotoxin known as ochratoxin, which is both a nephrotoxin and
-33-
CA 02388782 2002-04-18
WO 01/28367 PCT/US99/24386
promoter of lung tumors. A variety of fungi regularly inhabit tobacco,
depending on
the microenvironment, and many are known to produce mycotoxins. Other
compounds, such as tobacco-specific nitrosamines, may be detected by optical
fluoroscopy in solvent streams, and as such are amenable to a treatment
process to
remove them.
SUMMARY OF MAJOR ADVANTAGES OF THE INVENTION
After reading and understanding the foregoing detailed description of a
process
and system for continuous assay and elimination of toxins, in accordance with
preferred
embodiments of the invention, several distinct advantages of the subject
process and
system are obtained.
The present invention provides a novel process and apparatus for detecting and
removing harmful toxins found in tobacco and tobacco products by continuously
detecting, monitoring and removing harmful mycotoxins, such as aflatoxins, and
benzpyrene and its precursors during processing of tobacco for human and
animal
association, consumption and use. The novel process and apparatus provides for
inhibiting production of mycotoxins in and on tobacco and tobacco products,
and for
continuous monitoring and removal of harmful toxins from solvent and gaseous-
effluent
streams arising from processing tobacco. This continuous assaying and
monitoring is
necessary to ensure adequate removal and continuous diminution of harmful
toxins
from tobacco and tobacco products and to ensure that the products are safe for
human
consumption.
-34-
CA 02388782 2002-04-18
WO 01/28367 PCT/US99/24386
Treatment of tobacco to eliminate immunosuppressive carcinogens is of critical
importance. Monitoring the tobacco production process to ensure continuous
diminution is of equal importance. A failure to adequately monitor, treat and
remove
these harmful toxins could result in their continued presence in tobacco and
tobacco
products, with attendant negative public- health consequences. In contrast
with prior
art processes, the process and system of the present invention continuously
assays and
treats in-process tobacco to ensure adequate removal and continuous diminution
of
harmful toxins from tobacco and tobacco end products.
The process and system of the invention assays and verifies multiple toxins in
tobacco, and in processing-extraction streams, for treatment and removal, to
ensure
that processed end products, and in particular cigarettes, and effluent
streams, do not
contain dangerous levels of the toxins. The invention provides for optical
fluorescence
of in-process tobacco solvent- streams, in combination with other confirmatory
qualitative or quantitative tests, to correlate the optical fluorescence with
tests that are
traditionally more definitive, thereby increasing the accuracy and sensitivity
of
detection and assaying of harmful toxins. For example, if a fast-flowing
solvent stream
is fluorescing markedly for a particular toxin, minimal amounts of solvent are
withdrawn or extracted from the solvent stream for further testing by
techniques such
as high-pressure liquid chromatography (HPLC), reversed- phase liquid
chromatography, thin-layer chromatography, adsorption chromatography,
immunoaffinity chromatography, ELISA, fluorescent immunoassay, gas
chromatography, mass spectroscopy, infrared spectroscopy, raman spectroscopy,
-35-
CA 02388782 2002-04-18
WO 01/28367 PCT/US99/24386
packed-cell fluorescent spectroscopy, radioimmunoassay, ~polymerase chain
reaction
(PCR), supercritical fluid extraction, bio-luminescence, chemical
luminescence, or any
combination thereof.
In the flexibility and mde range of alternatmes promded for detection of
multiple
toxins, the advantages of such a feature are numerous. This continuous
monitoring and
assaying of contaminants provides an ongoing quality control of the
decontamination
process, ensuring that harmful toxins and other contaminants in end products
do not
rise above generally acceptable levels. Effluent solvents derived from
processing
tobacco are also analyzed for toxin content, and are treated before reuse or
disposal to
reduce, minimize, or eliminate the toxins.
Inherent flexibility and adaptability of the process and apparatus provide for
continuous or intermittent assay of mycotoxins, and in particular aflatoxins,
benzpyrene
and its precursors, and other contaminants, such as pesticides, biotoxins or
any other
undesirable toxins or agents that may threaten human or animal health. Of
particular
concern are smokers and individuals who inhale secondhand or environmental
tobacco
smoke. Tobacco is treated while being processed to remove such contaminants.
Levels
of contaminants in solvents, gases, and other process agents used to process
tobacco,
and in tobacco additives, are continuously monitored and controlled to provide
a
comprehensive, dependable solution to a grave problem that these dangerous
contaminants pose to human and animal safety. As a part of this comprehensive
approach to the problem, a tobacco product near the end of its treatment
process, but
-36-
CA 02388782 2002-04-18
WO 01/28367 PCT/US99/24386
not necessarily at the last step, is treated to prevent reformation of toxins
on or in the
tobacco.
Without attempting to set forth all of the desirable features of the instant
process
and system for continuous assay and elimination of toxins, at least some of
the major
advantages include the following: After removal of any tobacco that is
excessively
contaminated, the in-process tobacco is treated by a suitable process to
remove toxins,
including but not limited to solvent immersion, aqueous immersion,
gasification, heating
and cooling by any means, etc. These initial steps eliminate gross
contamination, if any,
and are followed by continuous analysis of extracted gases, solvents, liquids,
vapors,
and/or solids for toxins, to provide in-process quality control. Toxin levels
are
continuously and accurately monitored as the tobacco is treated, and harmful
toxins
present on or in the tobacco are removed, neutralized, or otherwise taken out
of the end
products. In a novel embodiment of the instant invention, this simultaneous
quality-
control monitoring system ensures that if a particular processing step ~is not
sufficient to
remove toxins, the step can be repeated or the product in question can be
discarded,
retreated, reformulated or otherwise modified so that it meets required
standards
insuring a safe end product.
In a comprehensive and global solution to the contamination problem, solvents,
gases and vapors eluted in various treatment steps are further treated to
remove
dangerous toxins from the elution stream so that the solvents, gases and
vapors can
safely be reused without recontaminating the product, or if desired, safely
disposed of
without placing harmful toxins in the wastewater stream. Such decontamination
-37-
CA 02388782 2002-04-18
WO 01/28367 PCT/US99/24386
processes include, but are not limited to, acidification, ammoniation,
saponification,
irradiation, proteolysis, ozonation, cavitation, sonoluminescence,
precipitation,
alkalization, chemical neutralization by any means, not excluding heating,
cooling,
freezing or high temperature pyrolisis, among others.
The instant process and system provides for analyzing and treating re-
additives
to in-process tobacco so that they do not inadvertently reintroduce harmful
toxins back
into the cleaned and reformulated product. Current tobacco reformulation
technology
involves removal of extracts, flavorings, nicotine, etc., in early processing
steps and
returning them back into the tobacco near the end of the processing scheme as
re-
additives. These additives, like the solvents used to clean and extract
toxins, are
subjected to the same continuous or intermittent sampling for toxins and are
cleansed of
toxin contamination by means similar, but not limited, to those listed above.
In describing the invention, reference has been made to preferred embodiments
and illustrative advantages of the invention. Those skilled in the art,
however, and
familiar with the instant disclosure of the subject invention, may recognize
additions,
deletions, modifications, substitutions and other changes that fall within the
purview of
the subject invention.
-38-