Note: Descriptions are shown in the official language in which they were submitted.
CA 02388847 2002-04-23
Process for the direct reduction of
iron-oxide-containing material
The invention relates to a process for the direct
reduction of iron-oxide-containing material by means of
a CO- and H2-containing reducing gas in at least one
fluidized-bed reduction zone, COz-containing, used
reducing gas which emerges from the at least one
fluidized-bed reduction zone being recirculated and
fresh reducing gas being produced by COz reforming of
the used reducing gas and of a methane-containing gas,
in particular natural gas, and to an installation for
carrying out the process.
Processes in which CO- and HZ-containing reducing gas
is produced by what is known as steam reforming of
methane-containing gas and steam, the steam reforming
being carried out at high pressures and high
temperatures and hydrocarbons and steam being converted
into CO and HZ by means of nickel catalysts in
accordance with the following reaction:
Steam reforming reaction: CH4 + HZO -~ CO + 3 Hz
are known from the prior art, for example from
US-A-5,082,251.
In a CO shift reaction which follows the steam
reforming, the CO which is formed during the reforming
is then converted into C02 and Hz in accordance with the
following equation:
CO shift reaction: CO + H20 -~ COz + Hz
The C02 usually then has to be removed from the
reformed gas, and the gas from which the COz has been
removed has to be heated.
By contrast, in the case of COZ reforming, which is
CA 02388847 2002-04-23
- 2 -
known, for example, from DE-A 196 37 180 and
DE-A-195 17 766, not only steam is converted, but also
C02, in accordance with the following equation:
COz reforming reaction: CH4 + C02 ~ 2 CO + 2 HZ
The advantage of the C02 reforming is that there is no
need for any removal of COZ or for any subsequent
heating of the reducing gas to the desired reduction
temperature.
DE-A-196 37 180 has disclosed a process in which fine
iron oxide particles are reduced by means of a CO- and
HZ-containing reducing gas in a spouted bed and a
bubbling bed which is connected downstream of the
spouted bed, the reducing gas being produced from the
used CO-, COZ- and H20-containing reducing gas by means
of COZ reforming. The reforming and the direct
reduction take place at low pressures of from 1.6 to
2.4 bar.
DE-A-195 17 766 has. disclosed a process in which fine
iron oxide particles are reduced in a plurality of
circulating fluidized beds, which are connected in
series, likewise by means of a CO- and H2-containing
reducing gas, fresh reducing gas likewise, as in
DE-A-196 37 180, being produced from the used CO-, COz-
and H20-containing reducing gas by COZ reforming.
US-A-4,348,226 has disclosed a process in which off-gas
from a reducing shaft furnace is mixed with natural
gas, and the gas mixture is reformed in a heated
reformer, and in which further natural gas is admixed
with the reformed gas, and the gas mixture which is
then formed is subjected, in an unheated reactor, to an
endothermic reforming reaction, fresh reducing gas
being formed for the reduction shaft furnace. The
sensible heat of the gas which has been reformed in the
heated reformer is utilized in the second, endothermic
CA 02388847 2002-04-23
- 3 -
reforming reaction, and the desired reducing-gas
temperature is established.
It is known that COZ reforming takes place more
successfully at lower pressures and that the reformer
tubes can be designed to be thinner and therefore less
expensive at low pressures.
The invention is based on the object of providing a
process for the direct reduction of
iron-oxide-containing material, in which CO- and HZ-
containing reducing gas can be produced by COZ
reforming of a methane-containing gas, in particular
natural gas, and used reducing gas, in which, however,
the drawbacks of the known processes, which use a COz
reformer, such as the formation of carbon, deposits,
large reactor diameters, etc., are to be avoided. The
overall size of a reactor which accommodates the
reduction zone is to be kept small, but at the same
time a quantity of reducing gas which satisfies the
metallurgical requirements is to pass through the
reduction zone.
According to the invention, this object is achieved by
the fact that the C02 reforming and the direct
reduction are carried out at high pressure, preferably
at a pressure of at least 4 bar superatmospheric
pressure (5 bar absolute), in particular at a pressure
of approximately 7 bar superatmospheric pressure. The
pressure range which is appropriate in a technical
context in a process of this type is 6 to 8 bar
superatmospheric pressure; the upper pressure limit is
15 bar superatmospheric pressure.
Surprisingly, it has been found that, in this way, many
factors which have a disruptive effect on the reduction
process, such as the formation of carbon and deposits,
can be avoided in the fluidized-bed reduction zone.
CA 02388847 2002-04-23
- 4 -
Furthermore, a sufficiently high supply of gas per unit
volume of the reduction reactor to satisfy the
metallurgical requirements is provided for the
reduction, so that the reactors which accommodate the
' 5 fluidized-bed reduction zones can be of smaller
dimensions. Nevertheless, a sufficient gas throughput
is still ensured. Moreover, the reduction potential of
the reducing gas is higher.
Furthermore, iron sponge which is produced during the
direct reduction of iron-oxide-containing material can
advantageously be fed by pneumatic conveying by means
of the reducing gas to be briquetted, so that a
briquetting device which is used for the briquetting
can be arranged next to a direct reduction device which
is used for the direct reduction, with the result that
the overall size of the entire installation for
carrying out the process according to the invention can
be kept small.
The advantage of the process according to the invention
is that the C02 which is present in the used reducing
gas does not have to be removed, but rather is used
directly for the production of fresh reducing gas.
Compared to known direct reduction processes, for
example that described in US-A-5,082,251, which was
mentioned in the introduction, in which the reducing
gas is produced by steam reforming, without the steam
reformer being connected into the reducing-gas circuit,
connecting the C02 reformer into the reducing-gas
circuit means that a lower specific flow of reducing
gas is required for the direct reduction; the specific
flow of reducing gas is understood to mean the flow
rate of freshly supplied reducing gas based on the
material which is to be reduced.
It is preferable for the used reducing gas to be
subjected to a CO shift reaction at least in part prior
to the reforming. In this way, the CO is converted into
CA 02388847 2002-04-23
- 5 -
C02 and H2 by means of steam in accordance with the
following equation:
CO shift reaction: CO + H20 -> COZ + H2.
' 5
The CO content of the gas supplied to the reformer is
advantageously minimized in the process, and the CO/COZ
ratio is set.
On account of a high CO content in the reducing gas, in
particular if the gas which is to be reformed already
contains CO, problems caused by metal dusting, which is
understood as meaning destruction of the metallic parts
of the installation by CO, may occur in metallic parts
of the installation. If the gas which is to be
reformed, should it contain CO, is subjected to a CO
shift reaction, metal dusting can be substantially
avoided.
If the H20 content of the COz- and CO-containing gas is
not high enough for a CO shift reaction, steam is
advantageously added to the CO shift reaction.
On account of the once-through operation, which is
understood as meaning the fact that the reformer is
connected directly into the reducing-gas circuit,
without any devices which have a significant influence
on the temperature and composition of the reducing gas
being provided between the reformer and a reduction
reactor which accommodates the fluidized-bed reduction
zone, there are fewer possible ways of adjusting the
reducing-gas quality than if the reformer is connected
outside the reducing-gas circuit. According to
WO-A-96 00304, which, like US-A-5,082,251, has
disclosed a direct reduction process using a steam
reformer connected outside the reducing-gas circuit,
there are, for example, possible ways of setting the
reducing-gas quality by changing the way in which the
reformer operates, by changing the extent to which COz
CA 02388847 2002-04-23
- 6 -
is scrubbed out of the reformed gas and/or used
reducing gas, etc.
With the aid of the CO shift reaction which is provided
according to a preferred variant of the process
according to the invention, it is possible even when
using once-through operation for the gas ratios
required for the reforming and the direct reduction to
be set as required, i.e. for the CO/HZ ratio to be
varied or the CO content to be reduced according to the
specific requirements.
According to a further preferred embodiment, the used
reducing gas is compressed prior to the reforming,
preferably to a pressure of approximately 8 bar
superatmospheric pressure.
It is preferable for the waste heat of the reforming to
be used to preheat air, H20, natural gas, etc.
The used reducing gas is advantageously compressed
prior to the CO shift reaction, preferably to a
pressure of approximately 8 bar superatmospheric
pressure.
The used reducing gas is expediently heated prior to
the reforming and prior to the optional CO shift
reaction.
The present invention also relates to an installation
for carrying out the process according to the
invention, having at least one fluidized-bed reactor,
which accommodates a fluidized-bed reduction zone, a
feed line for feeding a CO- and H2-containing reducing
gas to the fluidized-bed reactor and a gas discharge
line for discharging used reducing gas, which leads
from the fluidized-bed reactor to a C02 reformer in
order to produce the CO- and HZ-containing reducing gas
from a methane-containing gas, in particular natural
CA 02388847 2002-04-23
- 7 _
gas, and the used reducing gas, the C02 reformer being
line-connected to the fluidized-bed reactor via the
feed line.
According to the invention, this installation is
characterized in that there is a compression device for
compressing the gas which is supplied to the
fluidized-bed reactor to a high pressure, preferably to
a pressure of at least 5 bar superatmospheric pressure,
in particular to a pressure of approximately 8 bar
superatmospheric pressure, upstream of the COZ
reformer.
It is preferable for a CO shift reactor to be provided
upstream of the C02 reformer for used reducing gas. The
feed line for steam may in this case open out upstream
of the CO shift reactor into a feed line for the COz-
and, if appropriate, CO-containing gas and/or into the
CO shift reactor itself.
According to an even more preferred embodiment, the
compression device for compressing the used reducing
gas is provided upstream of the CO shift reactor.
In the installation according to the invention, it is
preferable for at least three, and in particular
preferably four, fluidized-bed reactors which are
connected in series to be provided.
To accurately set the chemical composition of the
reducing gas for optimum efficiency of the COZ
reformer, the CO shift reactor can expediently be
bypassed by means of a bypass line for the used
reducing gas.
It is advantageous for a line which supplies a CH4-
containing gas, in particular natural gas, to open out
into the gas line which supplies used reducing gas to
the COz reformer.
CA 02388847 2002-04-23
The installation according to the invention is
expediently characterized by a heating device for the
cleaned and compressed used reducing gas.
' 5
The invention is explained in more detail below with
reference to the drawing, in which Figures 1 and 2 in
each case illustrate a preferred embodiment of the
invention, identical components in each case being
provided with identical reference symbols.
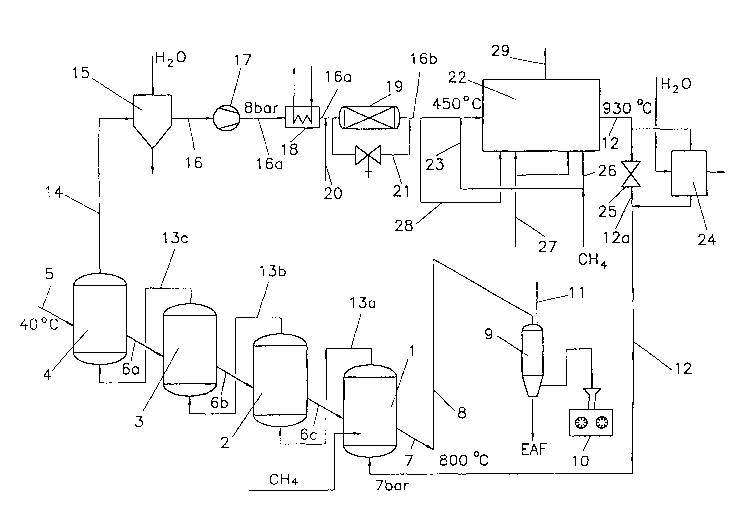
Figure 1 shows four fluidized-bed reactors 1 to 4 which
are connected in series and each accommodate a
steady-state fluidized bed, iron-oxide-containing
material, such as fine ore, being supplied via an ore
feed line 5 to the uppermost fluidized-bed reactor 4,
in which heating to reduction temperature and, if
appropriate, preliminary reduction take place, and then
being passed from fluidized-bed reactor 4 to
fluidized-bed reactors 3, 2 and 1 via delivery lines 6a
to 6c. The fully reduced material (iron sponge) is fed,
via a discharge line 7 and a riser 8, which is
understood as meaning a substantially vertical section
of pipe which has a refractory lining and is used to
convey the iron sponge pneumatically upwards by means
of the reducing gas, to a storage hopper 9 and, from
there, to a briquetting device 10, in which the iron
sponge is hot-briquetted. If appropriate, the reduced
material is protected from reoxidation during the
briquetting by an inert-gas system (not shown) or is
fed to an electric arc furnace situated below.
The reducing gas which is used to convey the iron
sponge through the riser 8 is extracted and expanded
via a line 11 and is then fed for further use, for
example for heating purposes (not illustrated). The use
of a riser 8 has the advantage that the briquetting
device 10 can be arranged next to the reduction device
formed from the fluidized-bed reactors 1 to 4, with the
CA 02388847 2002-04-23
- 9 -
result that the overall height of the entire
installation can be lowered. A further possibility (not
illustrated) of conveying the iron sponge into the
storage hopper 9 without using a riser 8 consists in
the lowermost fluidized-bed reactor 1 being arranged at
a height which is such that the iron sponge can flow
into the storage hopper 9, which is arranged at a lower
level, by means of the force of gravity; in this case,
however, the drawback of a greater overall height of
the entire installation has to be accepted.
Before the iron-oxide-containing material is introduced
into the first fluidized-bed reactor 4, as seen in the
direction of flow of the material, it is subjected to a
preparation treatment, such as a drying treatment (not
illustrated in more detail).
Reducing gas is fed to the lowermost fluidized-bed
reactor 1 via a feed line 12, is carried from
fluidized-bed reactor 1 to fluidized-bed reactors 2, 3
and 4 via lines 13a to 13c in countercurrent to the
flow of the material which is to be reduced and is
extracted via a gas discharge line 14 as used reducing
gas. By way of example, the reducing gas flows into the
lowermost fluidized-bed reactor 1 at a temperature of
approximately 800°C and a pressure of approximately 8
bar absolute and leaves the uppermost fluidized-bed
reactor 4 as used reducing gas at a temperature of
approximately 550°C and a pressure of approximately 6
bar absolute.
The used reducing gas is cooled and scrubbed in a
cooler/cleaner 15, where dust and steam are removed.
The cooled and cleaned gas, which according to the
embodiments illustrated is passed through a circuit, is
then fed to a compressor 17 via a line 16. In the
compressor 17, the used reducing gas is compressed, for
example to a pressure of approximately 8 bar. Following
the compressor 17 there is a heating device 18, which
CA 02388847 2002-04-23
- 10 -
is used to heat the used reducing gas, which has been
greatly cooled during the cleaning by the
' cooler/cleaner 15, back up to a temperature which it
needs for a CO shift reaction. The used reducing gas
which has been heated in this way is then fed via the
line 16a to a CO shift reactor 19, in which the CO
which is present in the used reducing gas is partly
converted, by means of steam, to CO2 and H2. In the
exemplary embodiment illustrated in Fig. 1, steam is
fed via a feed line 20 into the line 16a by means of
which the used reducing gas is carried to the CO shift
reactor 19. However, the steam may also, by way of
example, be fed directly into the CO shift reactor 19.
In the CO shift reactor 19, the CO which is present in
the used reducing gas is (partially) converted into COZ
and Hz by means of steam.
The provision of the CO shift reactor 19 on the one
hand advantageously increases the COZ content of the
gas which is fed to the COZ reformer, which promotes
the reformer reaction, and, on the other hand, reduces
the CO content, with the result that metal dusting,
i.e. the destruction of metallic parts of the
installation by CO, is substantially avoided. In
addition, the CO shift reactor 19 results in more
possible ways of setting the desired reducing-gas
quality. The gas ratios required for the reforming and
the direct reduction can be set according to the
particular requirements, i.e. the CO/HZ ratio can be
varied and/or the CO content can be reduced according
to requirements.
The CO shift reactor 19 can be bypassed by means of a
bypass line 21, resulting in a wide range of
possibilities for setting the desired reducing-gas
quality, for example as a result of a partial quantity
of the used reducing gas being fed directly to the COz
reformer 22 without being passed through the CO shift
reactor 19.
CA 02388847 2002-04-23
- 11 -
In the C02 reformer 22, the gas which is supplied via
the line 16b, if appropriate prior to heating, is
reacted together with methane-containing gas, in the
example illustrated natural gas, which is supplied via
a line 23, so that CO and H2 are formed.
The reformed gas leaves the COz reformer for example at
a temperature of approximately 930°C. To allow it to
be used as fresh reducing gas, the reformed gas still
has to be heated to the desired reducing-gas
temperature. In the exemplary embodiment illustrated,
the reformed gas which is extracted from the C02
reformer 22 via a line 12 is in part guided via a
cooler 24 and the remaining part is guided via a line
12a which bypasses the cooler and has a valve 25,
during which process a reducing-gas temperature of
approximately 800°C is established.
The COZ reformer 22 is heated by burning natural gas,
which is supplied via a line 26, with an
oxygen-containing gas, such as air, which gas is
supplied via a line 27. Part of the used, heated
reducing gas can be branched off via a line 28 and can
likewise be burned with an oxygen-containing gas, such
as air, in order to heat the CO2 reformer 22. The
combustion off-gases which are formed in the process
are extracted from the COZ reformer 22 via a line 29.
The high pressure in the reducing-gas circuit, for
example approximately 7 to 8 bar absolute upstream of
the COz reformer 22 and approximately 6 to 7 bar before
the gas is introduced into the lowermost fluidized-bed
reactor 1, allows all the internal fittings (lines,
fluidized-bed reactors) to be of correspondingly small
dimensions. Furthermore, the formation of carbon and
deposits is substantially avoided in all components.
Finally, a riser 8 may advantageously be used to convey
CA 02388847 2002-04-23
, - 12 -
the reduced material to the briquetting device 10, as
has already been explained in more detail above.
According to the embodiment illustrated in Fig. 2, the
used reducing gas, after it has been heated in the
heating device 18, is fed directly to the COZ reformer
22, with the result that the installation is
simplified, but there is not such a wide range of
possibilities for influencing the composition of the
reducing gas leaving the COz reformer as there are in
the embodiment illustrated in Fig. 1.
Chemical compositions of the gases, temperatures and
pressures in accordance~with the exemplary embodiment
illustrated in Fig. 1 are explained in more detail in
the example which follows (pressure details are in bar
absolute).
A) Flow of ore
Ore introduced into the fluidized-bed reactor 4 via the
ore feed line 5:
Temperature: approx. 50°C, ore weight based on the
product approx. 1.44.
Composition: hematite (Fe203) with a pure iron content
of approx. 67%, grain size up to at most
12.5 mm.
Ore discharged from the fluidized-bed reactor 1 via the
discharge line 7:
Temperature: approx. 800°C, reduced ore
Composition: total iron content approx. 93% (Fe),
metallization 92%
C = 1.5 - 2.5%
Grain size: up to at most 6.3 mm
The reduced ore is conveyed for briquetting 10 via the
riser 8.
CA 02388847 2002-04-23
- 13 -
B) Gas flow
Gas introduced into the fluidized-bed reactor 1 via the
line 13:
Pressure: approx. 7 bar superatmospheric pressure
Temperature: approx. 800°C
Reducing-gas composition: CO: 21.7%
CO2: 3.2%
H2: 57.2%
H20 : 5 . 6 %
CH4: 6.2%
N2: 6.1%
Gas discharge of the used reducing gas from the
fluidized-bed reactor 4 via the gas discharge line 14:
Pressure: approx. 5 bar superatmospheric pressure
Temperature: approx. 550°C
Gas composition: CO: 15.4%
C02: 8.8%
H2: 46.5%
CH4: 4.4%
H20: 18.3%
N2: 6.5%
Dust content in the gas: approx. 27 kg/t of product,
with 9.5 g/m3n.
Deposition of the dust through reducing-gas scrubber 15
(also referred to as cooler/cleaner):
Used reducing gas after scrubber 15:
Pressure: approx. 4 bar superatmospheric pressure
Temperature: approx. 40°C
Dust content: 27.3 g/t of product with
approx. 10 mg/m3n.
Used reducing gas after the compressor 16:
Pressure increase to approx. 8 bar superatmospheric
pressure
' CA 02388847 2002-04-23
- 14 -
Temperature: approx. 100°C
' Used reducing gas after the heating device 18:
Pressure: approx. 7.8 bar superatmospheric pressure
Temperature: approx. 350°C
Input into the CO shift reactor 19:
Pressure: approx. 7.8 bar superatmospheric pressure
Temperature: approx. 350°C
Gas composition: C0: 14.0%
CO2 : 8 . 0
%
H2: 42.4%
H20: 26.6%
CH4: 4.0%
N2: 5.2%
Used reducing gas after the CO shift reactor 19:
Pressure: approx. 7.5 bar superatmospheric pressure
Temperature: approx. 450°C
Entry of the used reducing gas into the COz reformer 22
(after CH4 has been admixed):
Pressure: approx. 7.5 bar superatmospheric pressure
Temperature: approx. 450°C
Gas composition: CO: 4.4%
COz: 13.6%
H2: 43.9%
Hz0 : 14 . 9
CH4: 17.5%
N2: 5.8%
Reducing-gas discharge from COZ reformer 22 via the
line 12:
Pressure: approx. 7 bar superatmospheric pressure
Temperature: approx. 930°C
Gas composition: CO: 22.6%
CO2: 3.3%
H2: 59.5%
<IMG>