Note: Descriptions are shown in the official language in which they were submitted.
CA 02389228 2002-04-25
WO 01/32828 PCT/US00/29543
BIOLOGICAL FLUID PROCESSING
This application claims the benefit of U. S. Provisional Application Nos.
60/162,234, filed October 29, 1999; 60/178,746, filed January 28, 2000; and
60/216,467,
filed July 6, 2000, each of which is incorporated by reference.
TECHNICAL FIELD
This invention relates to analyzing a biological fluid such as blood and blood
components, e.g., to determine the presence of material, preferably
microorganisms such
1 o as bacteria, in a portion of the fluid.
BACKGROUND OF THE INVENTION
Blood is conventionally processed, e.g., separated into components, to provide
a
variety of valuable products such as transfusion products. Blood components or
products such as huffy coat and platelets may be pooled during processing,
e.g., 4-6 units
of platelet concentrate can be pooled before administration as a transfusion
product.
Additionally, blood components processed in a closed system (e.g., without
exposing the
components to the outside environment) can be stored before administration.
For
example, red blood cells can be stored for several weeks, and platelets can be
stored for
2 o several days (e.g., 5 days according to current U.S. practice).
Stored and/or non-stored components typically include undesirable material
such
as bacteria. Bacteria can contaminate the blood or blood component during
blood
collection (including blood sampling) and/or storage. One source of bacterial
contamination may be the blood donor's skin, which may contain one or more
varieties
2 5 of bacteria, e.g., gram positive bacteria such as Stanhvl~c~ccns enid~ and
~taflh~rlc, .~~ccn.s _anr .~, and/or gram negative bacteria such as ~erratia
marce~cens and
~erratia liauefacien~. Other bacterial contaminants include, for example,
.~g1,1
n .egat~iv~ styl~encci, and YerSinia entPr~ .nliti . .
Since swabbing the donor's skin (e.g., with alcohol) prior to venipuncture may
be
3 o inadequate to assure sterility, the bacteria may pass into the blood
collection container,
WO 01/32828 CA 02389228 2002-04-25 PCT/LTS00/29543
2
and the bacteria may reproduce while the blood or blood component is stored.
Additionally, phlebotomy needles may cut a disc of skin when the phlebotomy
needle is
inserted into the donor, allowing the bacteria-containing skin plug to pass
with the blood
into the blood collection container.
s Other sources of contamination include the donor's blood, the environment
(including the air, and the equipment in the environment), and the
phlebotomist.
Contamination can occur while the unit of blood is being donated and/or while
samples
of blood are being obtained.
Since some blood components (e.g., platelets) are typically stored at ambient
1 o temperatures, the problem of contamination may be magnified, as most
bacteria
reproduce more rapidly at ambient temperatures.
Contaminated blood products, especially bacterially contaminated blood
products, pose a potential health risk to those who come into contact with, or
receive,
these products. For example, the administration of transfusion products with
bacterial
1 s contamination can have adverse affects on the recipient, and the
administration of
platelets with massive levels of bacterial contamination is implicated in
about 150 cases
of severe morbidity or death each year in the U.S.
Some bacterial detection techniques include opening the container of collected
blood, obtaining a sample of the blood, transferring the sample to a container
including a
2 o growth medium, and incubating the bacteria. The bacteria are subsequently
detected,
e.g., by changes in pH.
However, these techniques are labor- and time-intensive and may require
expensive equipment. Some of the techniques may provide inaccurate results.
Additionally, the techniques may introduce contamination from the environment
into the
2 5 samples.
The present invention provides for ameliorating at least some of the
disadvantages of the prior art. These and other advantages of the present
invention will
be apparent from the description as set forth below.
CA 02389228 2002-04-25
WO 01/32828 PCT/LTS00/29543
3
SUMMARY OF THE INVENTION
In accordance with an embodiment of the invention, a system is provided
comprising a first container suitable for containing a biological fluid, and a
processing
arrangement comprising a biological fluid analysis chamber suitable for
receiving a
microbe- and plasma-containing portion of biological fluid from the first
container and a
filter interposed between the first container and the analysis chamber,
wherein the filter
allows a microbe- and plasma-containing portion of biological fluid to pass
from the first
container to the analysis chamber, while reducing the passage of at least one
of platelets,
red blood cells and white blood cells therethrough, and the system is disposed
to allow
1 o the detection of microbes in the analysis chamber. In an embodiment, the
first container
is suitable for holding a plurality of units of biological fluid (e.g., pooled
platelet
concentrate). In some embodiments, the analysis chamber can be detached from
other
components of the system before the microbes are detected.
The system is disposed to allow microbes in the biological fluid passing
through
the filter to be detected, preferably by detecting an indicator of microbe
metabolism in
the analysis chamber. In a preferred embodiment, the system is disposed to
allow the
early detection of clinically significant levels of bacteria in the analysis
chamber.
A method according to an embodiment of the instant invention provides a
filtered, microbe-containing sample of biological fluid in a biological fluid
analysis
2 o chamber, wherein microbes in the filtered sample are subsequently
detected. In a
preferred embodiment, the filtered, microbe-containing sample comprises a
plasma-rich,
bacteria-containing, platelet-reduced fluid. In some embodiments, the filtered
sample
comprises a red blood cell and/or white blood cell-depleted plasma-rich fluid.
Systems and methods according to the present invention are especially suitable
2 5 for use by transfusion services, blood centers and/or blood bank
personnel.
BRIEF DESCRIPTION OF THE DRAWINGS
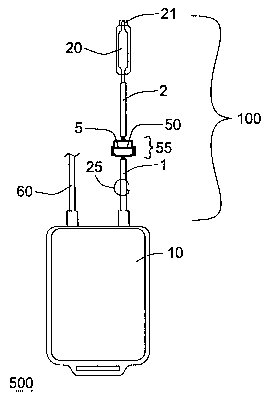
Figure 1 is an embodiment of a system according to the present invention,
including a container, and a processing arrangement including a filter and an
analysis
3 0 chamber, wherein the analysis chamber includes a port (e.g., allowing
communication
WO ~l/32828 CA 02389228 2002-04-25 PCT/[JS00/29543
4
with a probe device).
Figure 2 is another embodiment of a system according to the present invention,
including a container, and a processing arrangement including a filter and an
analysis
chamber.
Figure 3 is another embodiment of a system according to the present invention,
including a plurality of containers, and a processing arrangement including a
filter and
an analysis chamber.
SPECIFIC DESCRIPTION OF THE INVENTION
1 o In accordance with an embodiment of the invention, a biological fluid
processing
system comprises a first container suitable for holding a biological fluid,
the container
having an internal volume for receiving the biological fluid, and a processing
arrangement comprising an analysis chamber, suitable for receiving a plasma-
containing
portion of biological fluid passed from the internal volume of the container
and a filter
15 interposed between, and in fluid communication with, the first container
and the analysis
chamber, the filter comprising at least one filter element including at least
one porous
medium, wherein the filter allows the passage of microbes and plasma from the
first
container to the analysis chamber, and reduces the passage of platelets from
the first
container to the analysis chamber.
2 o A biological fluid processing system provided by another embodiment of the
invention comprises a first flexible container suitable for holding a
biological fluid, the
container having an internal volume for receiving the biological fluid, and a
processing
arrangement comprising a biological fluid analysis chamber, adapted for
receiving a
microbe- and plasma-containing portion of biological fluid passed from the
internal
2 5 volume of the first flexible container and a filter interposed between,
and in fluid
communication with, the first flexible container and the biological fluid
analysis
chamber, the filter comprising at least one filter element including at least
one porous
medium, wherein the filter allows the passage of microbes and plasma from the
first
flexible container to the analysis chamber, and reduces the passage of white
blood cells
3 o from the first flexible container to the analysis chamber, wherein the
system is arranged
CA 02389228 2002-04-25
WO 01/32828 PCT/US00/29543
to allow microbes in the biological fluid passing through the filter to be
detected, e.g., in
the analysis chamber.
In accordance with yet another embodiment, a microbe detection system is
provided comprising a first container suitable for holding a biological fluid
to be
s analyzed for the presence of microbes, the container having an internal
volume for
receiving the biological fluid; a microbe detection chamber, adapted for
receiving a
microbe-containing portion of the biological fluid passed from the internal
volume of the
first flexible container; a filter interposed between, and in fluid
communication with, the
first flexible container and the microbe detection chamber, the filter
allowing a portion
of the microbes in the biological fluid to pass from the first flexible
container into the
detection chamber, wherein the detection system is arranged to allow microbes
to be
detected in the microbe detection chamber.
Another embodiment of a biological fluid processing system according to the
invention comprises a first container suitable for holding a biological fluid,
the container
having an internal volume far receiving the biological fluid, and a filter in
fluid
communication with the first container, the filter comprising at least one
filter element
including at least one porous medium, the filter including an analysis
chamber, suitable
for receiving a plasma-containing portion of biological fluid passed from the
internal
volume of the container, wherein the filter allows the passage of microbes and
plasma
2 o from the first container to the analysis chamber, and reduces the passage
of platelets
from the first container to the analysis chamber.
In some embodiments of the system, the filter reduces the passage of at least
one
of platelets, white blood cells and red blood cells (more preferably, reducing
the passage
of platelets and white blood cells), from the first container to the analysis
chamber.
2 5 In one embodiment of the system, the first container is suitable for
holding two or
more units of blood components, e.g., pooled or combined platelets, for
example,
multiple units of buffy coat or platelet concentrate. Illustratively, the
first container can
be suitable for holding four or more combined units of platelet concentrate
(PC).
Embodiments of the system, that can comprise a microbe detection system, can
3 o include a detachable analysis chamber. For example, a conduit allowing
fluid
CA 02389228 2002-04-25
WO 01/32828 PCT/CTS00/29543
6
communication between the first container and the analysis chamber can be cut
(preferably by heat-sealing to maintain the sterility of the contents of the
analysis
chamber and the first container) after the fluid has been passed therethrough,
and the
microbes can be subsequently detected. Alternatively, or additionally, the
system can
include an analysis system that is connected (e.g., to the first container) by
a tether,
preferably a flexible tether such as a plastic cord or cable. Illustratively,
the conduit
described above can be cut and sealed, and the separate tether keeps the
analysis
chamber associated with the first container, e.g., until the microbe analysis
is completed.
The present invention also provides a biological fluid analysis device,
wherein
one embodiment of the device comprises a filter comprising a filter element
that allows
the passage of plasma- and bacteria-containing biological fluid therethrough,
while
preventing the passage of blood cells therethrough; and a biological fluid
analysis
chamber downstream of the filter, wherein the chamber is disposed to receive
the fluid
passing through the filter, and all fluid passing into the chamber first
passes through the
filter, the chamber comprising a flexible container.
In accordance with the invention, methods for processing a biological fluid
are
also provided. For example, in accordance with one embodiment of a method, a
biological fluid is passed from a first container through a filter to provide
a filtered,
microbe-containing portion of biological fluid in an analysis chamber, and
microbes
2 o present in the filtered portion are subsequently detected. In one
preferred embodiment,
the method includes passing a biological fluid through a filter to provide a
plasma-rich,
bacteria-containing, platelet-depleted fluid in the analysis chamber.
Another embodiment of the invention provides a method for processing a
platelet-containing biological fluid comprising obtaining a platelet- and
2 5 plasma-containing biological fluid in a first container (wherein the
biological fluid
possibly includes microbes), passing a portion of the fluid from the first
container into a
processing arrangement comprising a filter including at least one filter
element including
at least one porous medium, wherein the filter allows the passage of microbe-
and
plasma-containing fluid therethrough and reduces the passage of platelets
therethrough,
3 o the processing arrangement also including an analysis chamber downstream
of the filter
CA 02389228 2002-04-25
WO 01/32828 PCT/US00/29543
7
element; receiving a filtered plasma-containing fluid in the analysis chamber;
and
determining whether microbes are present in the filtered plasma-containing
fluid.
In some embodiments of a method according to the invention, two or more units
of a biological fluid (e.g., from the same donor, or from different donors) to
be analyzed
for the presence of microorganisms are pooled or combined, and the biological
fluid
passed through the filter according to the embodiments described above
includes a
pooled or combined fluid. For example, four or more units of platelet-
containing fluid,
e.g., platelet concentrate (PC), can be combined, and a portion of the
combined PC is
passed through the filter to provide a filtered plasma-containing fluid in the
analysis
1 o chamber, preferably to provide a plasma-rich, bacteria-containing,
platelet- and white
blood cell-depleted fluid in the analysis chamber. A variety of techniques,
protocols,
devices and systems for pooling or combining units are suitable for carrying
out the
invention and known in the art.
Since microorganisms (especially bacteria) can be detected in accordance with
the invention, embodiments of the present invention can be suitable for
providing blood
components that can be stored for longer periods than are currently allowed by
the
regulations in various countries. For example, due, at least in part, to fears
that platelet
concentrate (PC) can be contaminated with bacteria, current U.S. practice
requires that
individual units of PC be utilized within 5 days, and pooled PC be utilized
within 8
2 o hours of pooling. However, since embodiments of the invention allow the
detection of
contaminated PC, pooled and unpooled PC can be monitored, and if determined to
be
uncontaminated, can be used after the 5 day/8 hour limits that are currently
required.
Illustratively, individual units of PC or pooled PC can be transfused after,
for example, 7
days of storage.
2 5 As used herein, a biological fluid includes any treated or untreated fluid
associated with living organisms, including, but not limited to saliva, lymph,
cerebrospinal
fluid, ascites fluid, and urine, particularly blood, including whole blood,
warm or cold
blood, and stored or fresh blood; treated blood, such as blood diluted with at
least one
physiological solution, including but not limited to saline, nutrient, and/or
anticoagulant
3 o solutions; blood components, such as platelet concentrate (PC), platelet-
rich plasma
WU U1/32828 CA 02389228 2002-04-25 pCT/US00/29543
8
(PRP), platelet-poor plasma (PPP), platelet-free plasma, plasma, components
obtained
from plasma, packed red cells (PRC), transition zone material or huffy coat
(BC); blood
products derived from blood or a blood component or derived from bone marrow;
red
cells separated from plasma and resuspended in a physiological fluid or a
cryoprotective
fluid; and platelets separated from plasma and resuspended in a physiological
fluid or a
cryoprotective fluid.
A "unit" is the quantity of biological fluid from a donor or derived from one
unit
of whole blood. It may also refer to the quantity drawn during a single
donation.
Typically, the volume of a unit varies, the amount differing from patient to
patient and
from donation to donation. Multiple units of some blood components,
particularly
platelets and huffy coat, may be pooled or combined, typically by combining
four or
more units.
Each of the components of the invention will now be described in more detail
below, wherein like components have like reference numbers.
. Figures 1 and 2 illustrate embodiments of a system 500 according to the
invention, including at least one container 10, in fluid communication with a
processing
arrangement 100 comprising a filter 50 (comprising at least one filter element
5
comprising at least one porous medium) and an analysis chamber 20, wherein the
filter
50 is interposed between the analysis chamber 20 and the container 10. In the
illustrated
2 o embodiments, the filter 50 is disposed in a housing to provide a filter
device 55, and
processing arrangement 100 includes at least two conduits 1, 2, and at least
one flow
control device 25. The embodiment illustrated in Figure 1 also includes a port
21, e.g.,
allowing access to the interior of analysis chamber 20.
Figure 3 illustrates another embodiment of a system according to the
invention,
2 5 including a multiple bag set 1000 (such as a multiple blood bag set)
comprising
containers 11, 12, and 13 in communication with the system 500 comprising a
container
10 and a processing arrangement 100 including a filter 50 and an analysis
chamber 20.
The illustrated set also includes a plurality of filter devices 200 and 201,
preferably
leukocyte depletion filter devices, a plurality of conduits 60-66, and a
connector 40.
3 o In accordance with the invention, the filter 50 comprises at least one
filter
CA 02389228 2002-04-25
WO 01/32828 PCT/CTS00/29543
9
element 5 comprising at least one porous medium, wherein the filter allows the
passage
therethrough of a microbe-containing portion of a biological fluid (if
microbes are
present in the biological fluid in container 10), while being capable of
reducing the
passage therethrough of white blood cells. Preferably, the filter 50 reduces
the passage
therethrough of white blood cells and platelets. In some embodiments, the
filter reduces
the passage therethrough of red blood cells.
The filter can allow a variety of microbes (microorganisms) to pass
therethrough
(the terms "microbes" and "microorganisms" are used interchangeably). In a
preferred
embodiment, the filter allows bacteria to pass therethrough. The 'filter can
allow
1 o gram-positive and gram-negative bacteria to pass therethrough.
Illustratively, the filter
can allow the passage of one or more of the following bacteria:
~t~nh~rlc,cc,ccu~
~pidermidis, ~t~anh~~rlc~cnccm anreus, ~erratia marcescen~, Serratia lianPf .i
.n~, Yersinia
entPr~c~litica, KlehSiella nnenmcni , Kl .h~i .11 cx~rt~, RSCherichia cnli,
RntPrchacter
cl.~..a~e., RntPrchactPr aern,genec, P~eucl~mcnas .rm in~isa, ~almcnella ~nn_,
Ba .illm
~p~., such as Racillm cereLS, Group B streptococcus, and ~.nagulas~ neg tiv .
StTVIc .~~cci,
The filter allows sufficient microbes to pass through the filter and to be
detected
within a suitable amount of time. Typically, as the microbe-containing fluid
is passed
through the filter, the filter allows at least about 25%, in some embodiments,
at least
2 o about 50%, or a higher percentage (in some embodiments, about 60% or
more), of the
microbes) of interest to pass therethrough. However, the filter does not
necessarily
allow the passage of substantially all of the microbes through the filter. For
example, the
filter can prevent the passage of some of the microbes and/or prevent the
passage of
some amount of the microbes. Illustratively, as a microbe- and plasma-
containing
2 5 biological fluid is passed from the container 10 to the analysis chamber
20, the filter can
remove a level of the microbes from the portion of the biological fluid.
Nevertheless,
while the level of microbes can be reduced during filtration, sufficient
microbes pass
through the filter to be detected, more typically, to be detected after a
period allowing for
microbe growth. '
3 o In some embodiments, at least one filter element 5 comprises a membrane,
that
CA 02389228 2002-04-25
WO 01/32828 PCT/US00/29543
can be supported or unsupported. Typically, at least one filter element 5
comprises a
fibrous porous medium, preferably a non-woven medium, more preferably a
fibrous
leukocyte depletion medium, even more preferably a fibrous synthetic polymeric
leukocyte depletion medium comprising melt-blown fibers.
A variety of materials can be used, including synthetic polymeric materials,
to
produce the porous media of the filter elements according to the invention.
Suitable
synthetic polymeric materials include, for example, polybutylene terephthalate
(PBT),
polyethylene, polyethylene terephthalate (PET), polypropylene,
polymethylpentene,
polyvinylidene fluoride, polysulfone, polyethersulfone, nylon 6, nylon 66,
nylon 6T,
10 nylon 612, nylon 11, and nylon 6 copolymers.
Suitable media prepared from melt-blown fibers include, but are not limited
to,
those prepared as disclosed in, for example, U.S. Patent Nos. 4,880,548;
4,925,572,
5,152,905, 5,443,743, 5,472,621, 5,582,907, and 5,670,060, as well as
International
Publication Nos. WO 91/04088 and WO 93/04763. The filter (and the filter
element),
which can comprise a preform, can include a plurality of layers and/or media.
In some embodiments, the filter includes a plurality of filter elements andlor
a
composite filter element, e.g., the filter can include at least two fibrous
media, at least
one fibrous medium and at least one membrane, or at least two membranes.
The filter elements) 5 can be treated for increased efficiency in processing a
2 o biological fluid. For example, the element may be modified, e.g., surface
modified, to
affect the critical wetting surface tension (CWST). Typically, an element
according to
embodiments of the invention, that can comprise, for example, a leukocyte
depletion
medium, has a CWST of greater than about 53 dynes/cm (about 0.53 erg/mm2),
more
typically greater than about 58 dynes/cm (about 0.58 erg/mmz), and can have a
CWST of
2 5 about 66 dynes/cm (about 0.66 erg/mmz) or more. In some embodiments, the
CWST is
75 dynes (about 0.75 erg/mm2) or more. In some embodiments, the element may
have a
CWST in the range from about 62 dynes/cm to about 115 dynes/cm (about 0.62
erg/mm2
to about 1.62 erg/mm2), e.g., in the range of about 80 to about 100 dynes/cm
(about 0.80
to about 1.00 erg/mm2). In some embodiments, the element has a CWST of about
85
3 o dynes/cm (0.85 erg/mm2), or greater, e.g., in the range from about 90 to
about 105
CA 02389228 2002-04-25
WO 01/32828 PCT/US00/29543
11
dynes/cm (about .90 to about 1.05 erg/mm2), or in the range from about 85
dynes/cm to
about 98 dynes/cm (about .85 to .98 erg/mmz).
Surface characteristics of the element can be modified (e.g., to affect the
CWST,
to provide a low affinity for amide-group containing materials, to include a
surface
charge, e.g., a positive or negative charge, and/or to alter the polarity or
hydrophilicity of
the surface) by chemical reaction including, for example, wet or dry
oxidation, by coa-
ting or depositing a polymer on the surface, or by a grafting reaction.
Modifications
include, e.g., irradiation, a polar or charged monomer, coating and/or curing
the surface
with a charged polymer, and carrying out chemical modification to attach
functional
1 o groups on the surface. Grafting reactions may be activated by exposure to
an energy
source such as gas plasma, heat, a Van der Graff generator, ultraviolet light,
electron
beam, or to various other forms of radiation, or by surface etching or
deposition using a
plasma treatment. In some embodiments, the elements) can be modified as
described in,
for example, the U.S. patents listed above.
Typically, the filter 50 has a pore structure, e.g., a pore size (for example,
as
evidenced by bubble point, or by KL as described in, for example, U.S. Patent
No.
4,340,479), a pore rating, or a pore diameter (e.g., when characterized using
the modified
OSU F2 test as described in, for example, U.S. Patent No. 4,925,572), that
reduces the
passage therethrough of white and/or red blood cells. For example, the filter
can have a
2 0 pore diameter of about 10 micrometers (gym) or less. In one embodiment,
the f lter has a
pore diameter in the range of from about 8 micrometers to about 5 micrometers.
In
another embodiment, the filter and/or at least one filter element 5 has a pore
diameter
about 5 micrometers or less. In another embodiment, the filter and/or at least
one filter
element has a pore diameter of about 3 micrometers, or less.
2 5 The filter can include a plurality of filter elements having different
pore structures
and/or at least one element can have a varied pore structure.
In some embodiments of the invention, the filter and/or at least one filter
element
has a voids volume of about 75% or more, or about 80% or more, e.g., in the
range of
85% to about 96%. In one embodiment, at least one filter element has a voids
volume of
3 o at least about 90%.
CA 02389228 2002-04-25
WO 01/32828 PCT/LTS00/29543
12
In some embodiments wherein the filter includes at least one filter element
comprising a fibrous medium, e.g., a polymeric non-woven medium, including
some
embodiments wherein the biological fluid comprises blood or a blood component,
the
filter has a nominal effective fiber surface area of at least about .2 M2. In
another
illustrative embodiment, the filter has a nominal effective fiber surface area
of at least
about .3 MZ, and can have a nominal effective fiber surface area of at least
about .35 MZ.
In accordance with some embodiments of the invention, wherein the filter
comprises at least one filter element comprising a fibrous medium, the filter
and/or the at
least one filter element has a density of about 4000 g/ft3 (about 0.14 g/cm3)
or less (in
l0 some embodiments about 3800 g/ft3 (about 0.13 g/cm3) or less), wherein the
density is
calculated according to the following equation, at a given average fiber
diameter and
voids volume:
Density (g/ft3) _
ha~i~ weight of the fiber (g~~~ X m~mhPr ~f 1 yer~ in the filter element X l1
inch ~/1 ftl
thickness of the element (inches).
For example, a fibrous filter element according to some embodiments of the
invention has a density in the range of from about 2550 g/ft3 to about 4000
g/ft3 (about
2 0 0.09 to about 0.14 g/cm3). In other illustrative embodiments, a fibrous
filter element has
a density in the range of from about 2550 g/ft3 to about 3200 g/ft3 (about
0.09 to about
0.11 g/cm3), or a density in the range of from about 3220 g/ft3 to about 4000
g/ft3 (about
0.11 to about 0.14 g/cm3).
Typically, the filter removes at least some level of the white blood cells
(and
2 5 possibly other biological fluid components) by sieving. In some
embodiments, the filter
also removes at least some level of white blood cells (and possibly other
biological fluid
components such as platelets) by adsorption.
In a typical embodiment, the filter reduces the level of platelets and white
blood
cells in the biological fluid passing therethrough by a factor of at least
about 1 log for
3 o each component, preferably, at least about 2 logs. In some embodiments,
the filter
reduces the level of platelets by a level of at least one log (e.g., reducing
the level from
about 1 x 109/mL to about 1 x 108/mL), and reduces the level of white blood
cells by a
WO 01/32828 CA 02389228 2002-04-25 pCT/jJS00/29543
13
level of at least three logs.
Without being limited to any particular mechanism, it is believed the
reduction or
elimination of other components of the biological fluid as the fluid passes
through the
filter reduces the potential for "noise" (particularly background noise) in
the analysis
chamber. Since the background noise is reduced, the markers or indicators of
microbe
metabolism (such as, for example, p02 and glucose) can be more accurately
detected.
For example, in those embodiments wherein oxygen (e.g., p02) is detected in
the
analysis chamber, the reduced level of platelets present minimizes the change
in the level
of p02 that could be attributed to the metabolism of the platelets: In other
words, since
1 o p02 can be consumed by platelets and microbes such as bacteria, the
reduced presence of
the non-microbe components improves the capability of detecting "microbe
consumed"
p02.
In another illustration, in those embodiments wherein lactate and/or glucose
is
detected in the analysis chamber, the reduced level of white blood cells and
red blood
cells present minimizes the change in the level of lactate and/or glucose that
could be
attributed to the metabolism of these components. Since glucose can be
consumed by
red and white blood cells and microbes such as bacteria, and lactate can be
formed as the
blood cells and microbes metabolize, the reduced presence of the non-microbe
components improves the capability of detecting "microbe consumed" glucose
and/or
2 o "microbe formed" lactate.
Typically, using the illustrated embodiments for reference, the microbes are
detected in the analysis chamber 20. However, if desired, the filtered fluid,
or a portion
thereof, can be transferred from at least one chamber or container to another,
e.g., passed
to at least one additional chamber, container or device (not shown), before
detection of
2 5 the microbes. Accordingly, the analysis chamber can comprise the
additional
chamber(s), container(s), or device(s). For example, the filtered fluid can be
passed to
an additional container, and at least a portion of the filtered fluid can be
passed to one or
more analysis chambers for analysis. Alternatively, or additionally, at least
a portion of
the filtered fluid can be passed to an additional container for analysis. If
desired, the
3 o additional chambers) or containers) can have different configurations
and/or be made
CA 02389228 2002-04-25
WO 01/32828 PCT/US00/29543
14
from different materials) than that of analysis chamber 20. Illustratively,
the analysis
chamber 20 can have a "bag-like" configuration and be made from a plasticized
flexible
material, and the additional chamber can have a "tube-like" configuration and
be made
from, for example, glass or plastic. The chamber 20 and the additional
containers) can
also differ with respect to, for example, the reagents) contained therein.
Alternatively, or additionally, in some embodiments (not shown), the filter
device
includes the analysis chamber, and the microbes are detected therein. For
example, in
one embodiment wherein the filter comprises a filter device, e.g., comprising
a filter
housing having an inlet and an outlet and defining a fluid flow path between
the inlet and
the outlet, with the filter element disposed across the fluid flow path, the
"downstream"
portion of the filter includes the analysis chamber (e.g., a portion of the
downstream
section of the housing can allow the filtered sample to be separated and/or
isolated from
the filter element) where microbes can be detected.
In accordance with the invention, microbes can be detected directly (e.g.,
using
reagents that bind to the microbe), or, more preferably, indirectly (e.g., by
detecting
indicators and/or markers of the microbes' metabolism, e.g., nutrients
consumed by the
microbes, metabolic products and/or byproducts produced by the microbes).
Illustrative
indicators and/or markers include at least one of ATP, pH, glucose, lactate
and lactic
acid (e.g., as reflected by changes in pH), p02 and pC02. In some preferred
2 o embodiments, the detected indicators and/or markers include at least one
of pOz and
pC02.
The indicators and/or markers can be detected in liquid and/or in gas. For
example, a probe can be placed in an analysis chamber wherein the chamber is
partially
filled with liquid containing microorganisms, and the probe can be placed in
the liquid,
2 5 or in the gas or "head space" above the liquid. Accordingly,
microorganisms can be
detected upon detecting the indicators and/or markers in the liquid or in the
gas above
the liquid. In some embodiments, the liquid and/or gas can be withdrawn from
the
chamber and analyzed elsewhere, e.g., in an additional chamber, container, or
device. In
accordance with some embodiments of the invention, e.g., wherein microorganism
3 o metabolism is monitored over time, the withdrawn liquid and/or gas can be
returned to
WO 01/32828 CA 02389228 2002-04-25 pCT/US00/29543
the analysis chamber after each analysis. If desired, particularly for those
embodiments
wherein gas is withdrawn and it is desired to maintain the initial volume of
gas in the
chamber, the returned gas can be supplemented, e.8., with a controlled volume
of sterile
air.
5 A variety of equipment, devices and/or protocols are suitable for detecting
microbes. Illustratively, one or more probes, sensors, instruments, reagents
andlor
reagent strips can be utilized, e.8., placed in or on the analysis chamber 20.
In some
embodiments, the devices, e.8., probes and/or sensors, are self contained and
suitable for
one-time use. Embodiments of systems according to the invention can include
these
1 o items pre-assembled and/or pre-attached, e.8., before biological fluid is
passed into the
container 10 and/or analysis chamber 20. Alternatively, or additionally, one
or more of
these items can be assembled and/or attached during or after the passage of
fluid into
container 10 or analysis chamber 20.
In a variation of the embodiment illustrated in Figure 1, conduit 2 can
include a
15 sheathed connector (e.8., a needle) or a dockable portion, allowing
subsequent
connection to analysis chamber 20 when desired. Such configurations are
especially
suitable for those embodiments wherein different sterilization protocols are
utilized for
different elements of the system.
Suitable equipment and devices include, but are not limited to, at least one
of
2 o probe devices (e.8., gas probes); gas chromatographs; blood gas analyzers;
head space
analyzers, including head space analyzers for oxygen, carbon dioxide, and
combined
oxygen/carbon dioxide analyzers (such as, for example, the CHECKMATE~ system,
Topac Inc., Hingham, MA); biosensors, e.8., enzymatic systems, including
enzymatic
systems wherein the measured voltage reflects the presence and/or level of the
2 5 indicators) present; membrane interfaces; reagent (e.8., dye) detection
systems
(including fluorescent reagent detection systems, particularly for detecting
fluorescent
compounds that are quenched in the presence of oxygen); the BioProbe~
Luminorneter
(Pall Corporation), e.8., for detecting ATP; as well as the BACTECT~ MGITTM
960
System (Becton Dickinson Microbiology Systems, Sparks, Maryland), e.8.,
utilizing
3 o fluorescent compounds sensitive to the presence of oxygen.
w0 ~l/32828 CA 02389228 2002-04-25 pCT/US00/29543
16
Typically, using the exemplary multiple bag set 1000 illustrated in Figure 3
for
reference, the containers 10-13, and the conduits 1, 2, and 60-66, are made
from
commercially available materials used in biological fluid (e.g., blood)
processing
systems. More typically, they are made from plasticized materials, e.g.,
plasticized
polyvinyl chloride (PVC). Exemplary plasticized PVC materials include, but are
not
limited to, PVC plasticized with dioctylphthalate (DOP),
diethylhelxylphthalate (DEHP),
or trioctyltrimelliate (TOTM), e.g., triethylhexyl trimellitate.
In some embodiments, the analysis chamber 20 (sometimes referred to below as
the detection chamber) is made from the same materials as the containers and
conduits.
1 o In other embodiments, the chamber can be made from a different material,
e.g., glass, or
a thermoplastic material (for example, as used for the housing for filter
device 55).
In accordance with the invention, including some embodiments wherein pOz
andlor pCOz is detected in the- analysis chamber, at least a portion of the
analysis
chamber, for example, at least about 50% of the surface area of at least one
side wall of
the chamber, is capable of allowing gas transmission therethrough. However, in
other
embodiments, the chamber can be substantially impermeable to gas.
If desired, the analysis chamber 20 can be substantially flexible, e.g., it
can
collapse or deform when the reservoir is empty and is unsupported by external
means.
For example, the chamber 20 shown in Figures 1-3 can be substantially
flexible.
2 o Alternatively, the analysis chamber 20 can have sufficient rigidity that
it does not
collapse or deform when the reservoir is empty and is unsupported by external
means.
In some embodiments, at least a portion of the analysis chamber 20, (e.g., a
side
and/or bottom wall) is resilient. For example, the side walls of the chamber
20 as shown
in the Figures can be resilient, and the chamber can have sufficient rigidity
that it does
2 5 not collapse when empty. As used herein, the term "resilient" refers to
the property of
springing back, e.g., to regain, either fully, or approximately, an original
position or
shape after having been deformed, e.g., bent, stretched, or compressed.
Illustratively, at
least one wall (or a portion thereof) "springs back" to its previous position
or shape after
compression. Typically, during use, the process of the wall springing back to
its
3 o previous position creates a negative differential pressure in the chamber,
and this causes
WO 01/32828 CA 02389228 2002-04-25 pCT/US00/29543
17
fluid to enter the chamber.
In yet another embodiment, the analysis chamber 20 comprises an evacuated
container, e.8., a stoppered tube. For example, in a variation of the
embodiment
illustrated in Figure 1, the conduit 2 includes a sheathed connector such as a
needle that
can be unsheathed and used to puncture the stopper of the tube. Since the tube
can be
evacuated before use, the negative pressure can cause fluid to be passed from
the
container 10 and through the filter 50 into the chamber 20.
The containers 10-13, as well as the analysis chamber 20, can be of any
suitable
size and shape, and can include other structure, e.8., any suitable number of
ports,
1 o transfer leg closures, connectors and/or attached conduits.
Typically, the analysis chamber 20 is suitable for containing at least about 2
ml,
more typically at least about 3 ml of biological fluid, e.8., in the range of
from about 5
ml to about 50 ml, or more. In some embodiments, containers 10-13 are
commercially
available flexible blood bags.
The system can include at least one connector, and typically includes a
plurality
of connectors. In the embodiment illustrated in Figure 3, the system 1000
includes at
least one connector 40, that has at least three branches, e.8., the connectors
can be in the
form of Y- or T-connectors. Suitable connectors are known in the art.
The system can include one or more flow control devices such as a clamp, seal,
2 o valve, transfer leg closure, or the like. Typically, the system includes a
plurality of flow
control devices, and they can be located within or on the conduits and/or the
containers.
For example, the Figures illustrate embodiments having a flow control device
25
associated with conduit 1. Typically, using the illustrative set illustrated
in Figure 3 for
reference, flow control devices are associated with one or more of the
conduits
2 5 interposed between containers 10-13.
Embodiments of the system can include additional elements such as at least one
of a vent (including a gas inlet and/or a gas outlet), and a gas collection
and displacement
loop. Additionally, or alternatively, the system can include, for example, one
or more
additional conduits, containers, and/or connectors.
3 o As noted above, in a typical embodiment the filter device 55 comprises a
housing
WO 01/32828 CA 02389228 2002-04-25 pCT~S00/29543
18
and a filter 50 comprising a filter element 5. Illustratively, the filter
device comprises at
least one inlet and at least one outlet, and defining a fluid flow path
between the inlet and
the outlet, and at least one filter comprising a filter element across the
fluid flow path.
Any housing of suitable shape to provide an inlet and an outlet may be
employed. In
those embodiments having a rigid housing, the housing may be fabricated from
any
suitably rigid, impervious material, including any impervious thermoplastic
material,
which is compatible with the fluid being processed. In an embodiment, the
housing is
fabricated by injection molding from a polymer, more preferably a transparent
or
translucent polymer, such as an acrylic, polypropylene, polystyrene, or a
polycarbonate
l0 resin. Not only is such a housing easily and economically fabricated, but
also it allows
observation of the passage of the liquid through the housing.
The housing may include an arrangement of one or more channels, grooves,
conduits, passages, ribs, or the like, which may be serpentine, parallel,
curved, circular,
or a variety of other configurations.
Preferably, the filter 50 (e.g., the filter device 55) is sterilizable, as is
the
sampling arrangement 100. Systems according to the invention (including
systems
further comprising the analysis and/or detection equipment such as probes
and/or
sensors) are preferably closed systems. A variety of suitable sterilization
protocols are
known in the art.
2 0 The present invention is suitable for use with a variety of biological
fluids, and a
plurality of processing arrangements can be included in a system or set. For
example, a
multiple blood bag set can include multiple processing arrangements, e.g., for
detecting
microorganisms in a plurality of separated blood components, blood products
and/or in
reagents utilized during the processing of blood components and products:
Illustratively,
2 5 processing arrangements can be placed in fluid communication with
containers for
containing packed red cells, platelet-rich-plasma, platelet concentrate and/or
plasma, and
portions of each of these fluids can be passed into the processing
arrangements) to allow
microbes to be detected.
In some embodiments, microbes can be detected at a concentration of about 106
3 0 (or less) colony forming units (CFU)/mL in the portion of biological fluid
in the analysis
WO 01/32828 CA 02389228 2002-04-25 pCT/US00/29543
19
chamber. Illustratively, microbes can be detected at a concentration in the
range of
about 103 to about 10' CFU/mL of fluid in the chamber. In one embodiment of
the
invention, microbes can be detected at a concentration of at least about 105
CFU/mL of
fluid.
In some embodiments, the system (for example, the analysis or detection
chamber) can include at least one reagent, solution, additive, growth medium
and/or
culture medium, e.g., to prevent a lag in growth of the microbes and/or to
improve the
growth rate. In some embodiments, minimizing the lag in growth and/or
improving the
growth rate allows the microbes to be detected more quickly. A variety of
reagents,
1 o solutions, additives, growth media and/or culture media are suitable.
These additional
materials can be in dry form (e.g., a powder or a "tablet") or liquid form. If
desired, e.g.,
wherein the materials are in dry form, for example, tablet form, further
components,
ingredients and/or additives, that can be inert materials such as at least one
of maltose
and mannitol, can be included, e.g., to provide bulk. In one embodiment, the
analysis
chamber includes sodium polyanethol sulfonate (SPS) in dry or liquid form. As
noted
above, additional materials can be included in the chamber with the SPS.
Alternatively, or additionally, some components, ingredients and/or additives
can
provide for interacting with, neutralizing and/or inhibiting other components,
ingredients, additives, products and/or byproducts present in and/or produced
in the
2 o analysis chamber. Illustratively, in an embodiment wherein the presence of
citrate, or
the presence of a high level of citrate in the analysis chamber is
undesirable,
embodiments of the invention include providing calcium, e.g., to inhibit
and/or
neutralize the citrate. In some embodiments of the invention, a complement
activation
inhibitor is provided in the analysis chamber.
2 5 In one embodiment of a method according to the invention, and using the
exemplary system illustrated in Figure 3 for reference, a unit of blood is
obtained from a
source such as a donor, and passed along conduit 64 into container 11 (such as
collection
bag, that typically includes anticoagulant). Typically, the blood is
centrifuged, to form a
sediment layer comprising red blood cells and a supernatant layer comprising
3 o platelet-rich-plasma (or to form a sediment layer comprising red blood
cells, an
WO U1/32828 CA 02389228 2002-04-25 pCT/US00/29543
intermediate buffy coat layer, and a supernatant layer comprising platelet-
poor-plasma).
If desired, one or more layers can be passed through a filter device, such as
a
leukocyte depletion filter device. For example, packed red blood cells can be
passed
from container 11 along conduit 65 and through leukocyte depletion filter
device 201
5 and conduit 66 into container 13. Additionally, (typically before the red
blood cells are
passed from container 11) platelet-rich-plasma (PRP) can be passed from
container 11
along conduit 63 and through leukocyte depletion filter device 200, conduit
62,
connector 40 and conduit 60 into container 10 of system 500.
Subsequently, the PRP (typically leukocyte-reduced PRP) is centrifuged, and
l0 plasma can be passed from container 10 into container 12 via conduit 60,
connector 40,
and conduit 61, leaving platelet concentrate in container 10.
If desired, two or more units of platelet concentrate can be combined (e.g.,
pooled) in a container (e.g., container 10 as shown in Figures 1 and 2) before
passing a
portion of platelet concentrate into the processing arrangement.
15 A portion of the platelet concentrate (e.g., single donor apheresis
platelets or
pooled platelets) is passed into the processing arrangement 100, such that
fluid passes
along conduit 25 and through the filter element 5 of filter 50. The filtered
fluid, that may
contain microbes such as bacteria, passes into analysis chamber 20. The filter
depletes
the fluid of biological fluid components by reducing the level of components
such as at
2 0 least one of platelets, white blood cells and red blood cells, thus
reducing the potential
for noise in the analysis chamber.
In a preferred embodiment, microbes, if present, are detected in the analysis
chamber 20. If desired, the microbes can be allowed to grow in the chamber 20
(e.g., for
about 8 hours or more, in some embodiments, for at least about 24 hours or
more) before
2 5 detection. Alternatively, or additionally, the presence of microbes can be
monitored
continuously or intermittently over time, and, if desired, a plurality of
analysis chambers
can be utilized.
If desired, particularly in some of those embodiments wherein the analysis
chamber is detached from the source container, e.g., the source container is
no longer in
3 o fluid communication with the analysis chamber, and a tether allows the
source container
CA 02389228 2002-04-25
WO 01/32828 PCT/US00/29543
21
to be associated with the analysis chamber, the source container can be
processed
differently than the analysis chamber. For example, after a conduit (e.g.,
conduit 1
and/or 2 in Figures 1 and 2) interposed between the source container and
analysis
chamber is sealed and cut, the analysis chamber can be processed in conditions
more
conducive to rapid microorganism growth (e.g., stored at a higher than ambient
temperature, e.g., a temperature of about 35-37° C), and the source
container can be
processed in a more conventional manner (e.g., stored at an ambient
temperature of
about 22° C).
Microbes can be detected (directly or indirectly) using a variety of
indicators,
1 o markers, equipment, devices and/or protocols as described earlier. In some
embodiments, microbes can be detected without adding a microbe growth medium
to the
biological fluid.
If desired, embodiments of the invention can include automated tracking and/or
automated detection protocols and equipment. For example, one or more
containers and
analysis chambers can include indicia (e.g., bar coding labels) with
information such as
the sources) of the biological fluid, blood type, additives) utilized, an
indication
whether a level of indicator and/or marker (e.g., pOz or COZ) was reached, and
this
information can be tracked, combined with the detection results, and provided
in
whatever format is suitable, e.g., indicated (in machine readable form if
desired) on at
2 0 least one of the analysis chamber and the storage container and/or as a
print-out.
EXAMPLE 1
Two units of leukocyte-reduced platelet-rich-plasma (each unit is
approximately
250 ml) are obtained, and each unit is separated into 50 ml portions that are
placed in
2 5 individual plastic satellite bags, wherein the bags are suitable for
storing platelets.
Two groups of filter devices are provided, as described in more detail below.
One group of filter devices is used to filter "spiked" platelet-rich-plasma,
and the other
group is used as a control, i.e., to filter "non-spiked" platelet-rich-plasma.
Two groups of systems according to an embodiment of the invention are provided
3 o wherein, for each system, the bag suitable for storing platelets is placed
in fluid
CA 02389228 2002-04-25
WO 01/32828 PCT/US00/29543
22
communication with an analysis chamber via flexible plastic tubing, with a
filter device
interposed between the bag and the chamber, as generally shown in Figure 1.
The
analysis chamber includes a port (as shown in Figure 1 ), and the system also
includes an
adapter for use with a p02 probe. The adapter is a length of tubing including
a
duckbill-type check valve.
The two groups of systems are essentially identical.
Each filter device includes a 25 mm diameter housing having an inlet and an
outlet defining a fluid flow path between the inlet and the outlet with a
filter across the
fluid flow path. The filter, that has a face area of .849 inz (.000542 m2) and
a nominal
l0 effective fiber surface area of .399 m2, is a filter element made from 10
layers of
melt-blown polybutylene terephthalate fibers. Each layer, comprising fibers
having an
average fiber diameter of about 3 micrometers, has a voids volume of 92% and a
thickness of 0.020 inches. The filter element is gas plasma treated as
disclosed in
International Publication No. WO 93/04763.
The analysis chamber in each system is a 3 cm x 5 cm pouch manufactured from
PVC film plasticized with diethylhelxylphthalate (DEHP), wherein the walls of
the
pouch are permeable to gas. The plasticized PVC film, having a wall thickness
of 0.015
inches, has an oxygen transfer rate of 5 micromoles per hour for a bag having
a surface
area of 350 cm2 (corresponding to an ASTM oxygen transfer rate of about 470
2 o mL/mz/day).
Five of the 50 ml portions of platelet-rich-plasma (PRP) are inoculated with
F~cherichia c~li obtained from the American Type Culture Collection (ATCC) at
a
suspension level to provide a target inoculum of about 1 CFU/mL. The other
five
portions of PRP are not inoculated.
2 5 About 24 hours after preparing the portions of PRP, about 5 mL of fluid
from
each container is passed from the container, through a filter device, into the
analysis
chamber, and the clamp between the filter device and the container is closed.
After an
additional 24 hours, a fiber optic oxygen sensor (Ocean Optics, Inc.), is
inserted into the
adapter connected to each analysis chamber and operated according to the
manufacturer's
3 0 instructions.
WO ~l/32828 CA 02389228 2002-04-25 pCT~S00/29543
23
The p02 in each analysis chamber containing unspiked fluid has reached an
equilibrium oxygen tension level of at least 100 mm Hg. The p02 is each
chamber
containing inoculated fluid is reduced to a level well below 100 mm Hg.
This example shows that systems according to an embodiment of the invention
provide for the passage of plasma and a level of E~ali through a filter, and
allow the E.
~nli to be detected.
EXAMPLE 2
Multiple units of leukocyte-reduced platelet concentrate (PC) from human
donors
are obtained. Each unit of PC is prepared in a closed system from a unit of
whole blood,
and is stored overnight (on a platelet shaker) in a commercially available
platelet storage
bag. As will be described in more detail below, 4 units of PC are placed into
various
groups (each group contains 4 individual units to be processed and analyzed)
to provide
"spiked/filtered" and "unspiked/filtered" units.
The "spiked" units are spiked with either gram positive or gram negative
bacteria.
The bacteria are: ~taThvlncnccn~ ameus (gram positive), Kleh~iella_ nneum~niae
(gram
negative), Fnt .roha .ter .l~ .a . (gram negative), and (~'Tr~un R
Strent~c~ccuS (gram
positive). Each unit in a given group is spiked with a single type of bacteria
at a level of
200-500 cfu/ml and filtered shortly after spiking.
2 o For each group, an equal number of unspiked/filtered units are studied in
parallel,
as negative controls.
Each filter device includes 25 mm diameter housing, and a filter comprising a
single fibrous filter element having a face area of .000542 m2, a nominal
effective fiber
surface area of .399 m2, and 10 layers of melt-blown polybutylene
terephthalate fibers.
2 5 The basis weight of the fibers is 5.2 g/ft2. Each layer, including fibers
having an average
fiber diameter of about 3 micrometers, has a voids volume of 92% and a
thickness of
0.020 inches. The filter element is surface modified as disclosed in U.S.
Patent No.
4,925,572. The filter has a critical wetting surface tension of about 66
dynes/cm. The
filter has a pore diameter of 8 micrometers as determined by the modified OSU
F-2 test
3 o as generally described in U.S. Patent No. 4,925,572.
CA 02389228 2002-04-25
WO 01/32828 PCT/LTS00/29543
24
The density of the filter element, wherein the density is calculated as [the
basis
weight of the fibers (5.2 g/ft2) x the number of layers (10) x (12 inches/1
ft)]/the
thickness of the filter (0.2 inches) = 3120 g/ft3 (= 0.110 g/cm3).
The platelet storage bag is sterile-docked to a filter device leading to a
sterile
flexible pouch. As will be explained in more detail below, a first sample of
the PC is
passed through the filter into the pouch. Subsequently, a first portion of the
first sample
is passed from the first pouch into a tube containing a fluorescent indicator,
and a second
portion of the first sample is passed from the first pouch into another tube
containing the
fluorescent indicator, wherein the tube also contains media (broth).
After the first filter device/flexible pouch is disconnected from the platelet
storage bag, a second filter device/flexible pouch is sterile-docked to the
bag. The
second flexible pouch has sodium polyanethol sulfonate (SPS), therein. A
second
sample is filtered, and passed into the second pouch. The concentration of the
SPS in the
second sample is 0.05%. First and second portions of the second sample (each
portion
containing filtered fluid and SPS) are passed from the second pouch into
separate tubes
containing fluorescent indicators, one tube with media, and one tube without
media.
Unspiked PC is also filtered to provide first and second samples, and the
samples
are divided into portions and passed into separate tubes in the same manner as
described
above.
2 o Accordingly, for each unit of PC, a total of four different Indicator
Tubes are
utilized for the samples: Media/SPS, Media/no SPS, no MedialSPS, no Medialno
SPS.
The tubes are commercially available BBLTM MGITTM Mycobacteria Growth
Indicator Tubes (Becton Dickinson) including a fluorescent indicator
containing
ruthenium chloride petahydrate. One set of Indicator Tubes also includes
media, i.e., a
2 5 broth base conventionally used for mycobacteria growth, the other set
lacks the broth.
The fluorescent indicator in each Indicator Tube is sensitive to the presence
of
oxygen, and as bacteria in the sample consume the oxygen, the fluorescence is
detected.
The fluorescence is monitored using a BACTECTM MGITTM 960 System (Becton
Dickinson).
3 o On the average, at least about 25% of each type of bacteria passes through
the
WO 01/32828 CA 02389228 2002-04-25 pCT/US00/29543
filter, with the exception of B streptococci, wherein about 12% passes
through. The
level of platelets and white blood cells in the filtered samples is below the
detection
limit.
The spiked/filtered samples show a change in fluorescence. The
unspiked/filtered
5 samples show essentially no change in fluorescence.
On the average, the bacteria in the spiked samples are positively detected in
less
than 24 hours in both sets of tubes. However, the Kleh~iell nne ~mc,nia in the
Indicator
Tube without SPS is positively detected in about 50 hours.
This example shows bacteria are detected if platelets and white blood cells,
that
1 o are metabolically active, are removed.
EXAMPLE 3
Three units of leukocyte-reduced platelet concentrate (PC) are obtained. Each
unit of PC, that is approximately 55 ml, is prepared in a closed system from a
unit of
15 whole blood, and is stored overnight in a commercially available platelet
storage bag.
Three units of PC are spiked with F.~ali, at 100-500 cfu/ml.
The filter device is as described in Example 2. The system is arranged as
generally shown in Figure 2, and the analysis chamber 20 is a 3 cm x 5 cm
pouch
manufactured from PVC film plasticized with diethylhelxylphthalate (DEHP),
wherein
2 o the walls of the pouch are permeable to gas. The plasticized PVC film,
having a wall
thickness of 0.015 inches, has an oxygen transfer rate of 5 micromoles per
hour for a bag
having a surface area of 350 cm2 (corresponding to an ASTM oxygen transfer
rate of
about 470 mL/m2/day).
Four 6 ml filter samples are taken from each unit of PC, wherein a new filter
2 5 device and analysis chamber is sterile docked to the PC bag for each
filtration. Two of
the four analysis chambers for the samples from a unit of PC contain a
detergent, sodium
polyanethol sulfonate (SPS), to provide a concentration of .0S% SPS in the
sample.
The concentration of the platelets in the PC is about 1.3 x 109 platelets/ml.
The
concentration of the platelet in the filtered samples is about 1.1 x 10'
platelets/ml.
3 o The platelet bags and pouches are stored on a platelet shaker for 24 hours
at 22°
W~ 01/32828 CA 02389228 2002-04-25 pCT~S00/29543
26
C. Portions of each sample are passed into a blood gas analyzer (model Stat
Profile 3,
Nova Biomedical) that is operated in accordance with the manufacturer's
instructions to
determine the P02.
Each of the spiked samples exhibits P02 levels well below 100 mrn/Hg. The
samples including SPS exhibit POz levels well below 100 mm/Hg in less than 24
hours.
The samples without SPS exhibit POz levels well below 100 mrn/Hg in 30 to 48
hours.
This example shows that systems according to an embodiment of the invention
provide for the passage of plasma and a level of E.~nli through a filter,
while depleting the
platelet concentration by about 2 logs, and allow the E.~oli to be detected.
l0
EXAMPLE 4
Units of leukocyte-reduced platelet concentrate are obtained, spiked with
E~.ali at
100-500 cfu, and samples are passed from platelet storage bags through filter
devices into
flexible pouches (analysis chambers) having SPS therein as generally described
in Example
3. Analysis of the influents and effluents shows the concentrations of the
platelets are
decreased by about 2 logs upon passing through the filter devices.
At 0, 24, 30 and 48 hours, samples are taken from the platelet storage bags
and
analysis chambers to determine the bacteria counts, and the oxygen
concentration is
determined in the storage bags and analysis chambers using a head space
analyzer for
2 0 oxygen (CHECKMATE~ oxygen analyzer, Topac, Inc.).
At 24 hours, the p02 concentrations in the storage bags are well below 100
mm/Hg,
and the pOz concentrations in the analysis chambers are above 100 mrri/Hg. The
bacteria
counts in the bags and chambers show little growth has occurred.
At 30 hours, the pOz concentrations in the storage bags and the analysis
chambers
2 5 are well below 100 mm/Hg. The bacteria counts in the bags and chambers
show the bacteria
counts have increased by several logs.
This example shows systems according to embodiments of the invention improve
the
capability of detecting bacteria-consumed p02 by reducing the presence of
platelets (that
also consume p02).
W~ 01/32828 CA 02389228 2002-04-25 pCT/LTS00/29543
27
All of the references cited herein, including publications, patents, and
patent
applications, are hereby incorporated in their entireties by reference.
While the invention has been described in some detail by way of illustration
and
example, it should be understood that the invention is susceptible to various
modifications and alternative forms, and is not restricted to the specific
embodiments set
forth. It should be understood that these specific embodiments are not
intended to limit
the invention but, on the contrary, the intention is to cover all
modifications, equivalents,
and alternatives falling within the spirit and scope of the invention.