Note: Descriptions are shown in the official language in which they were submitted.
CA 02389273 2002-04-24
WO 01/38102 PCT/US00/08156
METAL ION FUNCTIONALIZED SILICA SURFACES FOR INK RECEPTORS
This invention relates to ink receptor media and, more particularly, to silica-
filled
microporous substrates having a coating that improves drying times of the ink,
improves
abrasion resistance of the image after drying, improves waterfastness of the
printed image,
and provides high quality images that are resistant to smudging and
feathering.
Pigment fixation in a porous substrate is different than that in a non-porous
substrate. In such microporous substrates as polypropylene membranes prepared
using
thermally induced phase separation techniques (that is, TIPS film) according
to the
disclosures of U.S. Patent Nos. 4,539,256 and 5,120,594, and EVAL (ethylene
vinyl
alcohol) film, available from Minnesota Mining and Manufacturing Company, St.
Paul,
MN, pigments are fixed at the sub-surface and sometimes within the wall of the
capillaries
in the bulk of the substrates. The fixation is usually carried out by metal
ion flocculation
of the pigmented ink at certain sheltered locations dictated by the flow and
position of a
surfactant or a combination of surfactants, migration inhibitor, and ink
drying agent(s).
This, in turn, is controlled by the type and nature of the surfactants) and
other additives,
as well as the pore size, size-distribution of the porous substrates)
concerned.
In the case of TIPS film, for example, pigment flocculation is caused by a
metal
ion and flocculated or agglomerated pigmented ink is located at such a
sheltered position,
that is, in the bulk of the film where the surfactant and other additives are
positioned,
which is dictated by their flow. The chemical entanglement of the pigment is
caused by
the metal ion with the pigment dispersant but there is sufficient mechanical
entanglement
being provided by the pores due to pore-sizes. The entire process of pigment
anchoring in
porous films such as TIPS film is. in part, chemical. However, there are
additional
physical phenomena such as physical adsorption (physisorption) along the pores
inside the
pore-interconnected bulk of the film. The optical density of the imaged film
usually is
somewhat lower than in the case where all the pigmented ink could be laid down
on top of
the surface.
Substrates with very limited pore sizes may allow only water percolation
retaining
the pigment onto the surface level. This is the situation with the surface of
TESLIN brand
-1-
CA 02389273 2002-04-24
WO 01/38102 PCT/US00/08156
film, available from PPG Industries, Pittsburgh, PA. TESLIN film is a silica-
filled high
density polyethylene substrate having very small pore sizes (typically 0.02-
0.5
micrometers). When TESLIN film is imaged with pigmented ink, the pigment
remains on
the surface and does not penetrate below the surface into the pores for
sheltering at the
sub-surface level. Such a substrate provides very high optical density and
high quality
images, but such images are not water-fast.
In one embodiment, the invention provides an ink receptor medium comprising a
silica-filled microporous substrate having a silica surface and an
organometallic
multivalent metal salt on the silica surface of the silica-filled microporous
substrate. The
ink receptor media of the invention may also further comprise a surfactant, a
binder, or a
combination of surfactant and binder on the surface of the silica-filled
microporous
substrate. The surfactant is preferably hydrophilic. The surface of the silica-
filled
microporous substrate may be partially or substantially fully covered with the
organometallic multivalent salt.
The novel ink receiving media when imaged using an inkjet printer provide
durable, high color intensity and high quality images which are tack-free and
rapidly dry
to the touch.
In another embodiment, the invention provides an imaged ink receptor medium
comprising a silica-filled microporous substrate having a silica surface and
an
organometallic multivalent metal salt on the silica surface of the silica-
filled microporous
substrate and ink on the surface of silica-filled microporous substrate and in
contact with
the organometallic multivalent metal salt. In a preferred embodiment, the ink
colorant is a
pigment dispersion having a dispersant bound to the pigment that will
destabilize,
flocculate, agglomerate, or coagulate on contact with the ink receiving
medium.
Preferably, the image is made using an ink jet printer head.
In another embodiment, the invention provides an ink receptor medium
intermediate comprising an aqueous composition comprising an organometallic
salt on a
silica surface of a silica-filled microporous substrate.
The present invention also provides an ink receiving medium/ink set comprising
a
silica-filled microporous substrate having a silica surface and an
organometallic
multivalent metal salt on the silica surface of the silica-filled microporous
substrate, and
an ink that contains pigment colorants.
-2-
CA 02389273 2002-04-24
WO 01/38102 PCT/US00/08156
The ink receptor media of the invention provides silica-filled microporous
substrates that are water and abrasion resistant (that is, substantially wash-
fast) by
chemically modifying the silica surface of the silica-filled substrate.
Additionally, the ink
receptor media of the invention provide higher color density and sharper
images than those
of the non-porous silica-filled substrates.
An advantage of ink receiving media of the present invention is that a
laminated
protective cover layer is not necessary to achieve water resistant images.
Other features of ink receiving media of the invention include that they: work
with
pigmented inks, have high resolution, have high color density, provide wide
color gamut,
are waterfast, are smudge resistant, and provide rapid drying.
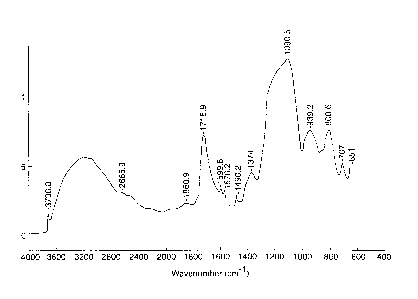
Figure 1 is an infrared spectrum of silica particles modified by
multifunctional
organic acid.
The microporous substrates useful in the invention are so called "silica-
filled"
microporous substrates. "Silica-filled" substrates are substrates that are
filled with silica
particles. These substrates are typically made by impregnation of silica
particles into the
polymer matrix. The process involves co-extrusion of both the polymer and the
silica at
appropriate processing temperature and pressure as well as axial orientation
to induce
pores into the resulting film. Typically, these substrates have very small
pore sizes on the
order of 0.02 to 0.5 micrometers and may be prepared according to methods
described in
one or more of U.S. Patent Nos. 4,833,172; 4,861,644; 4,877,679; and
4,892,779.
The silica present on the surface of the microporous substrates is amorphous
silica.
Amorphous silica usually consists of a silicon dioxide wherein the silicon
atom is
tetrahedrally bound to the oxygen atoms bridged between the silicon atoms. In
an aqueous
environment, a certain percentage of silanol groups exist on the silica
surface. Under
acidic conditions, this percentage is increased. In the present invention, for
example, the
Al+3 ion and the -COON functionality are believed to react with the Si-OH
group to give
-Al-O-Si- and -C(=O)-O-Si- linkages which are surface-bound. Evidence for the
surface
interactions has been shown by infrared spectroscopy. Infrared spectroscopy
for
aluminosilicates (for example, Kaolinite, Kaolin, feldspar, etc.) show
characteristic
absorption bands at 680-1100 cm-1. (See Sadder Research Lab, Division of Bio-
Rad Lab,
Spectrum Nos. 354, 355). The IR spectrum of silica particles modified by
multifunctional
organic acids (such as sulfocarboxylic acid) according to the present
invention has a
-3-
CA 02389273 2002-04-24
WO 01/38102 PCT/US00/08156
similar absorption band accompanied by a carbonyl absorption band at 1715 cm-1
as
shown in Figure 1.
While wishing not to be bound by theory, it is believed that pigmented inks
are
flocculated, that is, the pigment is removed from the dispersion, by binding
to surface
bound M+(3-n) ions.
Pigment usually rests on the dispersions) used in the preparation and
composition
of the pigmented based ink(s). For the anionic dispersant(s), the anions, for
example, a
carboxylate anion, instantaneously react with the surface-bound aluminum canon
leading
to coagulation/flocculation of the ink. The ink, thus, is also surface-bound
and is not
prone to hydrolysis (no wash-away). The product materials on the silica
surface,
aluminum carboxylates, and aluminum silicates are usually insoluble end-
products.
Preferred silica-filled substrates are commercially available from PPG
Industries,
Pittsburgh, PA, having the tradename TESLIN, and from Texwipe, Saddle River,
NJ,
under the tradename TEXWRITE (for example TEXWRITE MP10).
Useful organometallic salts are those which have a multivalent metal ion and a
multifunctional organic acid anion or counterion. Examples of the multivalent
metal ions
include, but are not limited, Al, Ga, Ti, Zr, Hf, Zn, Mg, Ca, Nb, Ta, Fe, Cu,
Sn, Co, and
the like. Examples of the organic acid include, but are not limited to,
aromatic
dicarboxylic, tri-, tetra- and penta-carboxylic, sulfocarboxylic, di-, tri-,
tetra
sulfocarboxylic, and any combination thereof, hydroxycarboxylic,
hydroxysulfonic,
hydroxysulfocarboxylic, and any combination in number thereof in any aromatic
system.
Specific examples of useful organometallic salts include metal sulfocarbolates
having the formula:
M O3s O OH
y
wherein
when M is Cu, Mg, Zn, Ca, or Co, the ratio of x:y is 1:2; and when M is Al,
Ga, B, then
the ratio of x:y is 1:3 or 2:3;
metal hydroquinonesulfonates having the formula:
-4-
CA 02389273 2002-04-24
WO 01/38102 PCT/US00/08156
OH
M X 03S
Y
OH
wherein
when M is Cu, Mg, Zn, Ca, or Co, the ratio of x:y is 1:2 or 2:2; and when M is
Al, Ga, B,
Ti, Zr, or Hf, the ratio of x:y is 1:3, 2:3 or 2:2;
metal dihydroxybenzenedisulfonates having the formula:
HO
~3
M 03S
Y
OH
wherein
when M is Cu, Mg, Zn, Ca, or Co, the ratio of x:y is 1:2 or 2:2; and when M is
Al, Ga, B,
Ti, Zr, or Hf, the ratio of x:y is 1:1, 1:3, 2:2, or 4:3;
metal sulfosalicylates having the formula:
R
Mx 03S O OH
Y
wherein
when M is Cu, Mg, Zn, Ca, or Co, the ratio of x:y is 1:2 or 1:1; when M is Al,
Ga, B, Ti,
Zr, or Hf, the ratio of x:y is 1:3, 2:3, or 3:3; and R is -COOH (Li, Na, or
K);
metal sulfophthalates having the formula:
R1
MX 03S
R2 y
-5-
CA 02389273 2002-04-24
WO 01/38102 PCT/US00/08156
wherein
when M is Cu, Mg, Zn, Ca, or Co, the ratio of x:y is 3:2, 2:2 or 1:1; when M
is Al, Ga, B,
Ti, Zr, or Hf, the ratio of x:y is 1:3, 2:2, or 2:3; R1 is H or-COON (Li, Na,
or K); and R2
is -COOH (Li, Na, or K); and
metal carboxylates having the formula:
1
MX -00C
Y
wherein
when M is Cu, Mg, Zn, Ca or Co, then the ratio of x:y is l :l, 2:2, or 3:2;
when M is Al,
Ga, B, Ti, Zr, or Hf, then the ratio of x:y is 1:3, 2:2, or 2:3; R1 is H or
COOH (Li, Na, or
K) and R2 is COOH ((Li, Na, or K).
Specific examples of preferred organometallic multivalent metal salts include
magnesium sulfophthalate, copper sulfophthalate, zirconium sulfophthalate,
zirconium
phthalate, aluminum sulfophthalate, aluminum sulfoisophthalate, and
combinations
thereof.
The surfaces of the silica-filled microporous substrates of the invention may
also
further optionally comprise surfactant, binder, or a combination thereof.
Sometimes, the
chemical and physical properties (for example, surface energy) of the
microporous surface
requires assistance from surfactants to aid in the management of ink fluids.
Therefore, at
least one surfactant may be advantageously impregnated into the pore volume of
the
microporous substrate. Application of the surfactant may be performed as a
separate and
distinct step, or combined with the organometallic salt and coated onto the
substrate in a
single step, followed by removal of any water and/or organic solvent or
solvents, to
provide particularly suitable surfaces for the particular fluid components of
the pigmented
inkjet inks.
Surfactants can be cationic, anionic, nonionic, or zwitterionic. Many of each
type
of surfactant are widely available to one skilled in the art. Accordingly, any
surfactant or
-6-
CA 02389273 2002-04-24
WO 01/38102 PCT/US00/08156
combination of surfactants or less preferably, polymers) that will render said
substrate
hydrophilic, could be employed.
These may include but are not limited to fluorochemical, silicone, and
hydrocarbon-based surfactants wherein the said surfactants may be anionic or
non-ionic.
Furthermore, the non-ionic surfactant may be used either as it is or in
combination with
another anionic surfactant in water and/or organic solvent or solvents, said
organic solvent
being selected from the group consisting of alcohols, ethers, amides, ketones,
and the like.
Various types of non-ionic surfactants can be used, including but not limited
to:
ZONYL fluorocarbons, for example, ZONYL FSO, available from E.I. du Pont de
Nemours and Co., Wilmington, DE; FLUORAD FC- 170 or 171 surfactants, available
from Minnesota Mining and Manufacturing Company; PLURONIC block copolymers of
ethylene and propylene oxide to an ethylene glycol base, available from BASF
Corp.
Chemicals Division, Mount Olive, NJ; TWEEN polyoxyethylene sorbitan fatty acid
esters,
available from ICI Americas, Inc., Wilmington, DE; TRITON X series
octylphenoxy
polyethoxy ethanol, available from Rohm and Haas Co., Philadelphia, PA;
SURFYNOL
tetramethyl decynediol, available from Air Products and Chemicals, Inc.,
Allentown, PA;
and SILWET L-7614 and L-7607 silicon surfactants, available from Union Carbide
Corp.,
Danbury, CT, and the like known to those skilled in the art.
Useful anionic surfactants include, but are not limited to, alkali metal and
(alkyl)ammonium salts of: 1 ) alkyl sulfates and sulfonates such as sodium
dodecyl sulfate
and potassium dodecanesulfonate; 2) sulfates of polyethoxylated derivatives of
straight or
branched chain aliphatic alcohols and carboxylic acids; 3) alkylbenzene or
alkylnaphthalene sulfonates and sulfates such as sodium laurylbenzene-
sulfonate; 4)
ethoxylated and polyethoxylated alkyl and aralkyl alcohol carboxylates; 5)
glycinates such
as alkyl sarcosinates and alkyl glycinates; 6) sulfosuccinates including
dialkyl
sulfosuccinates; 7) isethionate derivatives; 8) N-acyltaurine derivatives such
as sodium N-
methyl-N-oleyltaurate); 9) amphoteric alkyl carboxylates such as amphoteric
propionates
and alkyl and aryl betaines, optionally substituted with oxygen, nitrogen
and/or sulfur
atoms; and 10) alkyl phosphate mono or di-esters such as ethoxylated dodecyl
alcohol
phosphate ester, sodium salt.
Useful cationic surfactants include alkylammonium salts having the formula
CnH2n+1 N(CH3)3X, where X is OH, Cl, Br, HS04 or a combination of OH and Cl,
and
_7_
CA 02389273 2002-04-24
WO 01/38102 PCT/US00/08156
where n is an integer from 8 to 22, and the formula CnH2n+1N(C2H5)3X. where n
is an
integer from 12 to 18; gemini surfactants, for example those having the
formula:
~C 16H33N(CH3)2CmH2m+1 ~X~ wherein m is an integer from 2 to 12 and X is as
defined
above; aralkylammonium salts such as, for example, benzalkonium salts; and
cetylethylpiperidinium salts, for example, C16H33N(C2H5)(CSH10)X~ wherein X is
as
defined above.
The amount of organometallic multivalent salt that can be used in the
intermediate
for imbibing in the microporous substrate of the present invention can range
from 0.1
weight percent to 50 weight percent, and preferably from 0.5 weight percent to
20 weight
percent.
The amount of surfactant that can be used in the intermediate for imbibing in
the
microporous substrate of the present invention can range from 0.01 weight
percent to 10
weight percent, and preferably from 0.1 weight percent to 5 weight percent.
Optionally, organic binders can be used in the ink receiving media of the
invention.
Preferably, the organic binders are soluble or dispersible in water so that
they may be
easily incorporated into the intermediate compositions used to coat
microporous substrates
in forming the ink receiving media of the invention. Non-limiting examples of
such
organic binders include acrylic emulsions, styrene-acrylic emulsions,
polyvinyl alcohol,
polyvinyl alcohol/acrylic acid combinations, and combinations thereof, and the
like. Such
organic binders can be present in the coating or intermediate solution from
0.1 to 50
weight percent, preferably 1 to 30 weight percent based on total weight of the
coating or
intermediate solution, including surfactants and metal salts, with the
remainder being
water and/or organic solvent.
Optionally, opacifying pigments can be used in ink receiving media of the
present
invention. Non-limiting examples of such opacifying pigments include titanium
dioxide
pigments, barium sulfate pigments, and the like. Such opacifying pigments can
be present
in the coating solution and can range from 0.01 weight percent to 50 weight
percent.
Preferably, the opacifying pigment is present in an amount from 1 to 30 weight
percent.
Optionally, heat or ultraviolet light stabilizers can be used in ink receptors
of the
present invention. Non-limiting examples of such additives include TINUVIN 123
or
622LD, or CHIMASSORB 944 (hindered amine light stabilizers), available from
Ciba
Specialty Chemicals Corp., Tarrytown, NY); and UVINUL 3008, available from
BASF
_g_
CA 02389273 2002-04-24
WO 01/38102 PCT/US00/08156
Corporation Chemicals Division. Such stabilizers can be present in a coating
or
intermediate solution to be impregnated into the microporous substrate in the
range from
0.2 weight percent to 20 weight percent. Preferably, the stabilizer is present
in an amount
from 0.1 to 10 weight percent, more preferably in an amount of from 0.~ to 5
weight
percent.
Optionally, ultraviolet light absorbers can be used in ink receiving media of
the
present invention. Non-limiting examples of such absorbers include TINUVIN II
30 or
326, available from Ciba Specialty Chemicals Corp.; UVINUL 40501 l, available
from
BASF Corporation, and SANDUVOR VSU or 3035, available from Sandoz Chemicals,
Charlotte, NC. Such absorbers can be present in the coating or intermediate
solution and
can range from 0.01 weight percent to 20 weight percent. Preferably, the
absorber is
present in an amount from 1 to 10 weight percent.
Optionally, anti-oxidants can be used in ink receiving media of the present
invention. Non-limiting examples of such anti-oxidants include IRGANOX 1010 or
1076,
available from Ciba Specialty Chemicals Corp.; and UVINUL 2003 AD, available
from
BASF Corporation Chemicals Division.
Such anti-oxidants can be present in the coating or intermediate solution and
can
range from 0.2 weight percent to 20 weight percent. Preferably, the anti-
oxidant is present
in an amount from 0.4 to 10 weight percent, and more preferably in an amount
from 0.5 to
5 weight percent.
An ink receiving medium of the present invention has two major opposing
surfaces
and can be employed for printing (for example, by inkjet methods) on both
surfaces.
Optionally, one of the major surfaces can be dedicated for the purpose of
adhering the
finished image graphic to a supporting surface such as a wall, a floor, or a
ceiling of a
building, a sidewall of a truck, a billboard, or any other location where an
excellent quality
image graphic can be displayed for education, entertainment, or information.
Minnesota Mining and Manufacturing Company offers a variety of image graphic
receptor media and has developed an array of pressure-sensitive adhesive
formulations
that can be employed on the major surface opposing the surface intended for
imaging.
Among these adhesives are those disclosed in U.S. Patent Nos. 5,141,790
(Calhoun et al.);
5,229,207 (Paquette et al.); 5,800,919 (Peacock et al.); 5,296,277 (Wilson et
al.);
5,362,516 (Wilson et al.); EPO Patent Publication EP 0 570 515 B 1 (Steelman
et al.), and
-9-
CA 02389273 2002-04-24
WO 01/38102 PCT/US00/08156
PCT Patent Application Nos. WO 97/31076 (Peloquin et al.) and WO 98/29516
(Sher et
al.).
Any of these adhesive surfaces should be protected by a release or storage
liner
such as those commercially available from Rexam Release, Bedford Park, IL.
Alternatively to adhesives, mechanical fasteners can be used if laminated in
some known
manner to that opposing major surface of the receptor of the present
invention. Non-
limiting examples of mechanical fasteners include hook and loop, VelcroTM,
ScotchmateTM, and Dual LockTr'' fastening systems, as disclosed in published
PCT Patent
Application No. WO 98/39759 (Loncar).
The invention in its preferred mode is made by impregnation of the microporous
substrate with organometallic salt and with a suitable surfactant as required
followed by
drying at a temperature of 100 to 120 °C. After the receptor is dried,
it can be imaged
using conventional inkjet imaging techniques embodied in commercially
available
printers.
Impregnation of the organometallic multivalent salt may be accomplished by
dissolving or mixing the salt or salt and surfactant in de-ionized water or a
mixture of an
alcohol and de-ionized water. Impregnation of the solution may be done using
conventional equipment and techniques such as slot fed knife, rotogravure
devices,
padding operations, dipping, spraying, and the like. It is preferred that the
organometallic
multivalent metal salt fills the pores of the substrate without leaving
substantial quantities
on the surface. Excessive amounts of solids could plug the pores and in turn
causes
smearing and slow dry times during imaging. Coating weights depend on
porosity,
thickness, and chemical nature of the substrate, but may be readily determined
by routine
optimization. Typical wet coating weights are from 1 up to 500 grams per
square meter,
preferably from 10 up to 50 grams per square meter, more preferably from 15 to
30 grams
per square meter. Optional additives may be added before, during, or after
impregnation
of the ink receptor intermediate.
The printing industry has previously employed dye-based inks, although pigment-
based inks are becoming more prevalent. Use of pigment colorants is preferred
over dye
colorants because of durability and ultraviolet light stability in outdoor
applications.
Further, reference to ink with respect to this invention concerns aqueous-
based
inks, not solvent-based inks. Aqueous-based inks are currently preferred in
the printing
- 10-
CA 02389273 2002-04-24
WO 01/38102 PCT/US00/08156
industry for environmental and health reasons, among other reasons.
Minnesota Mining and Manufacturing Company produces a number of excellent
pigmented inkjet inks for thermal inkjet printers. Among these products are
Series 8551,
8552, 8553, and 8554 pigmented inkjet inks. The use of four principal colors:
cyan,
magenta, yellow, and black permit the formation of as many as 256 colors or
more in the
digital image. Further, pigmented inkjet inks, and components for them, are
also produced
by others, including Hewlett-Packard Corp., Palo Alto, CA and E.I. du Pont de
Nemours
and Co., and a number of other companies that can be located at many
commercial trade
shows dedicated to the imaging and signage industries.
The metal-ion functionalized silica surfaces of the present invention are
capable of
capturing and flocculating or coagulating pigmented inks or colorants and thus
fixing the
colorants onto the silica surfaces. This property makes the metal-ion
functionalized silica
surfaces of the invention particularly suitable for use as an ink receptor.
Examples
In the following examples, the term "parts" means parts by weight, unless
otherwise specified.
The HP Designjet 2500cp thermal inkjet printer, available from Hewlett-Packard
Corp, was used with manufacturer's recommended inks (Part Nos. C 1806A, C
1807A,
C 1808A, C 1809A). It is believed that the above black, yellow, magenta, and
cyan inks are
pigment based.
The exact nature of the image printed in the examples below is not critical in
order
to be able to reproduce the result obtained therein. The image employed was
standard test
patterns (for example CCL, circle tests, photographic images, etc.)
Wash-fastness Test
Printed films were evaluated for wash-fastness by placing them under a fully
open
running utility sink water faucet (25-30 °C). The wet imaged film was
wiped with a paper
towel until dry. If no discernible smearing or reduction of color density
occurred, then the
wash-fastness test was passed. Percentage color density loss was measured
using a
GRETAG M50 REFLECTANCE SPECTROPHOTOMETER, available from Gretag-
Macbeth, Gastonia, NC, unless otherwise noted.
-11-
CA 02389273 2002-04-24
WO 01/38102 PCT/L1S00/08156
Example 1
Composition-I was flood-coated onto the surface of a silica-filled film
(CHANGEABLE OPAGUE IMAGING MEDIA 8522CP), available from Minnesota
Mining and Manufacturing Company, using a #4 Meyer Rod, available from RD
Specialties, Webster, NY, to produce a nominal wet coating thickness of 0.0091
mm. The
coated substrate was dried at 120-130 ~C for 1-2 minutes to provide an ink
receptor
medium. When imaged using HP Designjet 2500cp thermal inkjet printers, a very
high
density, high quality, instantaneously dry image was obtained. The image was
resistant to
smudging with fingers, had good edge definition (without noticeable
feathering), and
visually passed the wash-fastness test.
Composition-I
Ingredients Parts
Aluminum(III) Sulfophthalate (Organometallic Salt)10
Dihexylsulfosuccinate-Na Salt (Surfactant) 6
Poly vinylpyrrolidone/Acrylic Acid) (PVP/AA) (75:25)2
(Binder)
Isopropyl Alcohol 25
De-ionized Water 57
Example 2
A 24 inch x 16 inch (61 x 41 cm) TESLIN film substrate was flood coated with
Composition-I over one-half of its surface (as described in Example 1) and the
remaining
half of the surface was left uncoated. The coated substrate was dried as
described above to
form an ink receptor medium. The dry substrate was imaged using a HP Designjet-
2500cp
thermal inkjet printer to obtain a very high color density image as described
above. The
image on the coated portion of the image provided a higher density image than
did the un-
coated surface. The imaged substrate was laminated onto an aluminum
board/backing
(poster board) and subjected to the wash-fastness test. The image on the un-
coated
TESLIN film substrate washed away. The image fading was very pronounced and
easily
observed by the naked eye. The image on the coated TESLIN film surface (the
present
invention) passed the wash-fastness test with insignificant loss (about 1
percent) of color.
Example 3
The procedure of Example 2 was repeated except that the dihexylsulfosuccinate-
Na salt of Composition-I was eliminated and replaced with an equivalent amount
of
- 12-
CA 02389273 2002-04-24
WO 01/38102 PCT/L1S00/08156
deionized water. The image on the coated substrate was a very high density
image that
was smudge-free and free of feathering, except about 5-10 percent of the
colors black and
green were lost during the wash-fastness test.
Example 4
The procedure of Example 2 was repeated except that the PVP/AA binder was
eliminated from Composition-I and replaced with an equivalent amount of
deionized
water. The image on the coated substrate was a very high density image that
was smudge-
free and free of feathering, except that the color black appeared to be more
on top of the
surface and about 5-10 percent of the colors black and green was lost in the
wash-fastness
test.
Example 5
The procedure of Example 2 was repeated except that both the PVP/AA binder and
dihexylsulfosuccinate-Na salt were eliminated from Composition-I and replaced
with an
equivalent amount of deionized water. The image on the coated substrate was a
very high
density image that was smudge-free and free of feathering, except that the
color black
appeared to be more on top of the surface and there was some pigment beading
and
coalescence together with some minor banding. In the wash-fastness test, about
5-10
percent of the colors black and green were lost.
Example 6
The procedure of Example 2 was repeated except that bis-aluminum (III)
sulfophthalate was substituted for aluminum(III)sulfophthalate in Composition-
I. The
effect was an increase in the amount of aluminum ions in the composition. The
image on
the coated substrate was a very high density image that was smudge-free and
free of
feathering. However, the image was slightly less dry after printing than the
image of
Example 2 and in the wash-fastness test, about 5-10 percent of the colors red
and green
color was lost.
Example 7
The procedure of Example 2 was repeated except that tris-aluminum (III)
sulfophthalate was substituted for aluminum(III) sulfophthalate in Composition-
I. The
effect was an increase in the amount of aluminum ions in the composition. The
image on
the coated substrate showed ink beading, coalescence, feathering, smudginess
and the
image was not dry after printing. In the wash-fastness test, about 30-40
percent of the
colors red and green were lost.
-13-
CA 02389273 2002-04-24
WO 01/38102 PCT/US00/08156
Example 8
Composition-II was flood-coated onto a surface of a silica-filled substrate as
described in Example 1 to provide a nominal wet coating thickness of about
0.0091 mm.
The coated film was dried as in Example 1 to form an ink receptor medium. When
imaged using an HP Designjet 2500cp thermal inkjet printer, the ink receptor
medium
provided a very high density, high quality, instantaneously dry image. The
image was
smudge-free, feathering-free, and substantially passed the wash-fastness test.
In Examples 1-8, the image on the uncoated part of the substrate washed away
in
the wash-fastness test (failed).
Composition-II
In reg diem Parts
Zirconium Tetrakis(Sulfophthalate) (Organometallic 9
Salt)
Dihexylsulfosuccinate-Na Salt (Surfactant) 6
Isopropyl Alcohol 25
De-ionized Water 58
Example 9
A 24 inch x 16 inch (61 x 41 cm) piece TESLIN film was flood coated with
Composition-II over one-half of its surface (as described in Example 1 ) and
the remaining
half of the surface was left uncoated. The coated substrate was dried as
described above to
form an ink receptor medium. The dry substrate was imaged using an HP
Designjet
thermal inkjet to obtain a very high density image as described above. The
image on the
coated portion of the image provided a higher density image than did the un-
coated
surface. The imaged substrate was laminated onto an aluminum board/backing
(poster
board). The image was then subjected to the wash-fastness test. Ninety-percent
of the
image on the un-coated surface was washed away. The image on the coated
portion of the
image (the invention) survived with insignificant loss (about 3-5 percent) of
color.
Example 10
The procedure of Example 9 was repeated except that titanium
tetrakis(sulfophthalate) was substituted for zirconium
tetrakis(sulfophthalate). The printed
image had similar image quality to that of the image of Example 9. The image
on the
uncoated part of the substrate washed away and about 10-20 percent of the
image on the
coated part of the substrate was washed away after the wash-fastness test.
- 14-
CA 02389273 2002-04-24
WO 01/38102 PCT/US00/08156
Example 11
The procedure of Example 9 was repeated except that copper(II) sulfosalicylate
was substituted for zirconium tetrakis(sulfophthalate). The printed image had
similar
image quality to that of the image of Example 9. The image on the uncoated
part of the
substrate washed away and about 5-10 percent of the image on the coated part
of the
substrate was washed away after the wash-fastness test.
Example 12
Composition-III was flood-coated onto a CHANGEABLE OPAQUE IMAGING
MEDIA 8522CP film to form a nominal wet coating thickness of 0.0091 mm and
then
dried as described in Example 1 to form an ink receptor medium. When the ink
receptor
medium was imaged using an HP Designjet 2500cp thermal inkjet printer, the ink
receptor
medium provided a very high density, high quality, instantaneously dry image
that was
also smudge-free, feathering-free, and passed the wash-fastness test.
Composition-III
Ing'edients Parts
Magnesium Sulfophthalate (Organometallic 10
Salt)
Dihexylsulfosuccinate-Na Salt (Surfactant) 6
PVP/AA (75:25) (Binder) 2
Isopropyl Alcohol 25
De-ionized Water
Example 13
A 24 inch x 16 inch (61 x 41 cm) piece of TESLIN film was flood coated with
Composition-III over one-half of its surface (as described in Example 1 ) and
the remaining
half of the surface was left uncoated. The coated substrate was dried as
described above to
form an ink receptor medium. The dry substrate was imaged using an HP
Designjet
2500cp thermal inkjet printer to obtain a very high density image as described
above. The
image on the coated portion of the image had a higher density image than did
the un-
coated surface. The imaged substrate was laminated onto an aluminum
board/backing
(poster board). The image was then subjected to the wash-fastness test. Ninety-
percent of
the image on the un-coated surface was washed away. The image on the coated
portion of
the surface (the invention) survived with a small loss (about 10-15 percent)
of color, and
substantially passed the wash-fastness test.
-15-
CA 02389273 2002-04-24
WO 01/38102 PCT/US00/08156
Example 14
Composition-IV was flood-coated onto a TESLIN film to form a nominal wet
coating thickness of 0.0091 mm and then dried, as described in Example 1 to
form an ink
receptor medium. When the ink receptor medium was imaged using an HP Designjet
2500cp thermal inkjet printer, the ink receptor medium provided a very high
density, high
quality, instantaneously dry image having a significant amount of ink beading
and
coalescence.
Composition-IV
In reg diem Parts
Aluminum Sulfate, 14 H20 (Inorganic Salt) 3
Dihexylsulfosuccinate-Na Salt (Surfactant) 6
PVP/AA (75:25) (Binder) 2
Isopropyl Alcohol 25
De-ionized Water 64
Example 15 (Comparative)
A 24 inch x 16 inch (61 x 41 cm) piece of TESLIN film was flood coated with
Composition-IV over one-half of its surface (as described in Example 1) and
the
remaining half of the surface was left uncoated. The coated substrate was
dried as
described above to form an ink receptor medium. The dry substrate was imaged
using an
HP Designjet 2500cp thermal inkjet printer to obtain a very high density image
as
described above. The image on the coated portion of the image provided a
higher density
image than did the un-coated surface. The imaged substrate was laminated onto
an
aluminum board/backing (poster board). The image was then subjected to the
wash-
fastness test. Both images were washed away to an extent of 75-85 percent.
Example 16
The Procedure of Example 15 was repeated except that sulfophthalic acid (9.6
parts) was added to 93.4 parts Composition-IV. The printed image had a similar
image
quality as that of the image of Example 15. However, only about 20-30 percent
of the
image color was lost in the wash-fastness test. This marked improvement
illustrates the
advantages of using organometallic salts according to the invention.
- 16-
CA 02389273 2002-04-24
WO 01/38102 PCT/CTS00/08156
Example 17
The procedure of Example 15 was repeated except that phthalic acid (4.5 parts,
at
aluminum-sulfate:phthalic acid molar ratio of 1:3) was added to 95.5 parts
Composition-
IV. The printed image had a similar image quality as that of the image of
Example 15.
However, only about 10-20 percent of the image color was lost in the wash-
fastness test.
This marked improvement illustrates the advantages of using organometallic
salts
according to the invention.
Example 18
The procedure of Example 15 was repeated except that 1,2,4-
benzenetricarboxylic
acid (5.7 percent, at aluminum sulfatearicarboxylic acid molar ratio of 1:3)
was added to
94.3 parts of Composition-IV. Following the wash-fastness test, only about 20-
30 percent
of the image color was lost and some feathering occurred. This improvement
illustrates
the advantages of using organometallic salts according to the present
invention.
Example 19
Composition-I was flood coated onto a TEXWRTTE MP-10 silica filled substrate
(silica-filled HDPE film from Texwipe Co.) and then dried as described in
Example 1 to
form an ink receptor medium. The substrate was imaged as described in Example
1,
providing an image having similar image quality as the image of Example 1.
About 5-7
percent of the image was washed away in the wash-fastness test test.
Example 20
The procedure of Example 1 was repeated except a piece of TESLIN film was used
as the substrate. The image quality and wash-fastness of the sample was the
same as that
of Example 1.
Exam 1p a 21
The procedure of Example 8 was repeated except that a piece of TESLIN film was
used as the substrate. The image quality and wash-fastness of the sample was
the same as
that of Example 8.
The invention is not limited to the above embodiments.
-17-