Note: Descriptions are shown in the official language in which they were submitted.
CA 02389669 2009-02-04
TRANSCRANIAL ULTRASOUND THROMBOLYSIS SYSTEM AND
METHOD OF TREATING A STROKE
TECHNICAL FIELD OF THE INVENTION
The present invention is directed generally to a transcranial ultrasound
tFiromliotysis sy.stem and method for transcranial ultrasound thrombolysis
and,
more -specifcally, to a system and method of using ultrasonic energy in
combination with a thrombolytic agent to assistin dissolving intracranial
thrombi
and to trnhance the efficacy of a thrombolytic agent. :
CA 02389669 2002-04-30
WO 01/32258 PCT/US00/30104
BACKGROUND OF THE INVENTION
Seven hundred thousand strokes occur each year in the United States
alone and many result in death. Ischemic strokes are generally caused by an
occlusion or blockage (either partial or complete) resulting from a blood clot
in
one of the blood vessels in the head. Successful treatment of stroke patients
depends on early recognition of the stroke, and almost immediate treatment,
such as within three to four hours of the onset of the stroke.
Currently, one treatment for acute ischemic stroke patients is the use of
a specific dose of the thrombolytic agent recombinant tissue plasminogen
activator, commonly known as rt-PA, administered intravascularly. However,
this
treatment is not commonly administered due to a variety of factors. The
treatment may not be administered because of a delay in recognizing and
diagnosing stroke symptoms and transporting stroke patients to an appropriate
medical facility. In addition, physicians are often reluctant to administer rt-
PA
due to the increased risk of an intracerebral hemorrhage. Accordingly,
hospitals
are less likely to use rt-PA on an acute stroke patient if they do not have a
specialized stroke neurologist present to diagnose correctly the need for rt-
PA
and address any subsequent complications.
As can be seen, current treatments have a number of shortcomings that
can greatly reduce the availability of treatments for acute stroke patients.
The
current medical treatment is generally not used by front-line medical
personnel.
Such treatments can also have adverse side effects, and can have limited use
2
WO 01/32258 CA 02389669 2002-04-30 PCT/US00/30104
and application. A need exists for a system and treatment method for providing
quicker and/or easier treatment for acute ischemic stroke patients and/or for
improving the efficacy of thrombolytic medicines, such as rt-PA, and reducing
undesirable side effects.
SUMMARY OF THE INVENTION
It is an object of the present invention to provide a system and method for
the treatment of strokes that addresses and overcomes the above-mentioned
shortcomings and problems.
Another object of the present invention is to provide a system and method
for the treatment of strokes that can be administered as soon as possible at
the
onset of stroke without a need for radiologic or imaging guidance to determine
the specific vascular location of a clot.
Still another object of the present invention is to provide a system and
method for the treatment of strokes that can be administered by front line
medical personnel.
Still another object of the present invention is to provide a system and
method for the treatment of strokes that avoid and/or reduce undesirable
bioeffects, either cavitational, mechanical or thermal in nature.
To achieve the foregoing and other objects, and in accordance with the
purpose herein, one embodiment of the present invention comprises a method
of intracranial thrombolysis comprising the steps of providing a predetermined
level of ultrasonic energy substantially throughout a primary treatment zone
3
CA 02389669 2009-08-28
encompassing at least a substantial portion of the Ml branch and the M2
branches of the middle cerebral artery in one hemisphere of a brain of an
individual and further administering a thrombolytic agent to the individual.
To achieve further objects and in accordance with the purposes herein,
another embodiment of the invention is directed to a thrombolytic device
comprising a transducer adapted to provide a predetermined level of ultrasonic
energy substantially throughout a primary treatment zone encompassing at least
a
substantial portion of the Ml branch and the M2 branches of the middle
cerebral artery in one hemisphere of a brain.
The method and system are advantageous in providing for relatively quick
treatment of stroke without requiring radiologic or imaging guidance to
determine
a specific vascular location of a clot. Still other advantages and objects of
the
present invention will become apparent to those skilled in the art from the
following description wherein there are shown and described alternative
exemplary embodiments of this invention. As will be realized, the invention is
capable of other different, obvious aspects, objects and embodiments, all
without
departing from the scope of the invention. Accordingly, the drawings, objects
and descriptions should be regarded as illustrative and exemplary in nature
only,
and not as restrictive.
In accordance with an aspect of the present invention, there is provided
the use of non-invasive predetermined levels of ultrasonic energy of an
ultrasonic frequency of from about 100 kHz to 1 MHz and a thrombolytic agent
for the treatment of transcranial ultrasound thrombolysis in a patient,
wherein
said energy is used substantially throughout a primary treatment zone
4
CA 02389669 2009-08-28
emcompassing at least a substantial portion of the Ml branch and M2 branches
of the middle cerebral artery in one hemisphere of a brain of said patient.
In accordance with another aspect of the present invention, there is
provided a transcranial ultrasound thrombolysis system comprising: a
transducer adapted to be placed adjacent to an exterior surface of a cranium;
and an ultrasonic driver adapted to generate energy that can be converted at
the transducer to ultrasonic energy suitable for penetrating the cranium and
transmitting through cranial tissue without generating undesirable thermal,
mechanical or cavitational effects, wherein the system is adapted to non-
invasively provide a predetermined level of ultrasonic energy substantially
throughout a primary treatment zone encompassing at least a substantial
portion of the Ml branch and the M2 branches of the middle cerebral artery in
one hemisphere of a brain, and further wherein the transducer provides an
ultrasonic frequency of from about 100 kHz to about 1 MHz.
BRIEF DESCRIPTION OF THE DRAWINGS
While the specification concludes with claims particularly pointing out and
distinctly claiming the present invention, it is believed the same will be
better
4a
WO 01/32258 CA 02389669 2002-04-30 pCT/US00/30104
understood from the following description taken in conjunction with the
accompanied drawings in which:
FIG. 1 is a schematic diagram of a transcranial ultrasonic thrombolysis
system in accordance with the teachings of the present invention;
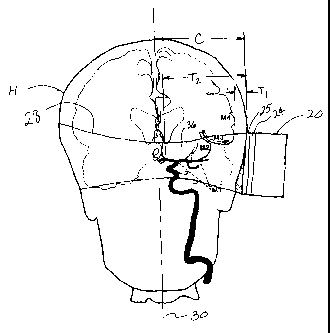
FIG. 2 is a vertical cross-sectional view of a transducer employed in a
system of the present invention placed adjacent the head;
FIG. 3 is a front elevational view of a transducer employed in a system in
accordance with the present invention;
FIG. 4 is a front elevational view of a transducer in the form of a 2-
dimensional array employed in a system in accordance with another embodiment
of the present invention;
FIG. 5 is an elevational view of an exemplary transducer employed in a
system in accordance with the present invention, with a corresponding beam
profile; and
FIG. 6 is an axial beam profile of the normalized intensity versus distance
from the transducer of an ultrasound beam in a system in accordance with the
present invention.
DETAILED DESCRIPTION OF THE PREFERRED EMBODIMENTS
Referring now to the drawings in detail, wherein like numerals indicate the
same elements throughout the figures, FIG. 1 exemplifies a system 10 for
assisting in dissolving intracranial thrombi and for enhancing the
thrombolytic
action of a thrombolytic agent. The system 10 comprises a transducer 20 which
is described in further detail below. As shown in Fig. 1, the system 10 may
5
WO 01/32258 CA 02389669 2002-04-30 pCT/US00/30104
further include an ultrasound system 11 and may be used in combination with
a medicine delivery system 40. The ultrasound system 11 used with the present
invention can include an ultrasonic driver 14 for generating electrical energy
that
can be converted to ultrasound waves or energy at transducer 20. Driver 14
may be of a conventional design with an adjustable frequency generator 16
and/or an adjustable power amplifier 18. The driver 14 should be such that the
ultrasound waves or energy are suitable and can be selected to penetrate the
temporal bone of the head (H), and to be transmitted through cranial tissue
without generating undesirable thermal, mechanical or cavitational effects. A
frequency generator 16 used with the present invention should have an
adjustable frequency range preferably from about 100 kHz to about 1 MHZ. The
power amplifier 18 used with the present invention should also have an
adjustable range up to about 150 W, and/or provide up to about 60 dB of gain.
Also, the driver 14 should have an adjustable duty cycle from about 10% to
100% so that the wave operation can be pulsating, continuous, or both, as
desired.
Transducer 20 is preferably electrically connected to the ultrasonic driver
14 by an electrical cord 26. Transducer 20 may be configured for converting
electricity from an electrical source (e.g., ultrasonic driver 14) into
ultrasound
waves or energy, and for radiating or directing such ultrasonic waves or
energy
into the head (H). Use of the transducer 20, along with the medicine delivery
system 40, should assist in dissolving or removing the blockage or occlusion
in
the cerebral blood vessel, and/or enhancing the efficacy of the dose of
medicine
6
CA 02389669 2002-04-30
WO 01/32258 PCT/USOO/30104
(e.g., thrombolytic agent) being used. The size and configuration of
transducer
20 used with the present invention should be selected so that ultrasound waves
or energy, and preferably low energy ultrasound waves, can penetrate the
temporal bone. Furthermore, the transducer 20 should be configured such that
undesirable heat energy and cavitation effects are not created. Undesirable
attenuating and heating of tissue may result with relatively high ultrasound
frequencies. Frequencies that are too low penetrate the tissue but may cause
cavitation and tissue damage. Suitable frequency ranges emitted by the
transducer 20 can be from about 100 kHz to about 1 MHZ. In one example, the
transducer 20 can emit frequencies from about 100 kHz to about 250 kHz. In
another particular example, the frequericy range of the transducer is about
120
kHz. Accordingly, it is desirable to select frequencies sufficiently low to
minimize
or prevent attenuation and heating of tissue while allowing sufficient
penetration
of tissue including the temporal bone. In addition, the selected frequency
should
not be low enough to cause cavitation and tissue damage at similar amplitudes.
Also, the transducer 20 should be sized and configured so that it can deliver
an
intensity range from about 0.5 W/cm2 to about 10 W/cm2. In another
embodiment, the transducer 20 can deliver an intensity range up to about
2 W/cmZ.
A tip including a quarter-wave matching layer and/or a lens 24 may
optionally be provided in a front portion of the transducer 20, such as the
end
portion of the transducer 20, for efficiently coupling ultrasound waves or
energy
into the head and/or focusing, concentrating or specifically directing
ultrasound
7
CA 02389669 2002-04-30
WO 01/32258 PCT/US00/30104
waves or energy to a desired area or volume in the body. In one embodiment,
a predetermined level of ultrasonic energy is provided substantially
throughout
a primary treatment zone 36 encompassing at least a substantial portion of the
Ml branch and M2 branches of the middle cerebral artery as shown in FIGS. 2
and 5. In other embodiments, the primary treatment zone 36 includes at least
a substantial portion of the M3 branch other extravascular thrombi, or other
intracranial vascular thrombi. Tip 24 should be sized and configured to
optimize
the transmission of ultrasound energy or waves through the temporal bone. As
shown in FIG. 3, the transducer 20 may have a diameter or aperture greaterthan
about 2 cm. In one embodiment, the transducer 20 may also have a diameter
or aperture greater than about 5 cm. In still another embodiment, the
transducer
may have a diameter or aperture of about 6 cm.
A beam width from about 3 cm to about 4 cm may be provided to allow
for variations and the differences in human anatomy and in the position of a
15 blockage or occlusion. In one particular embodiment, a beam width of about
3
centimeters is provided. Accordingly, by providing a sufficiently large beam
width, the system may provide a secondary treatment zone 34 that is effective
to encompass the primary treatment zone 36, and therefore treat the majority
of
strokes without the need for imaging or other techniques to determine the
20 specific vascular location of a thrombi.
One example of a suitable transducer 20 to assist in concentrating or
specifically directing ultrasound waves or energy to the area or location of
the
middle cerebral artery area is a transducer having a diameter of about 6 cm
and
8
WO 01/32258 CA 02389669 2002-04-30 pCT/US00/30104
a pillbox shaped configuration made of a piezoelectric ceramic. In one
example,
the transducer 20 may be sized and configured to have a Rayleigh distance (R)
as shown in FIGS. 5 and 6, which is generally the distance between the front
of
the transducer (e.g., located adjacent the skin above the temporal bone) and
the
location of the natural focus having the highest intensity from the ultrasonic
waves.
FIG. 5 shows one example of a transducer 20 emitting a beam 28 of
ultrasonic energy or waves. As illustrated in FIG. 5, the beam 28 of
ultrasonic
energy has a natural focus commonly known as the Rayleigh distance (R) from
the transducer to the distance at which the intensity of the beam reaches its
maximum. The Rayleigh distance (R) is determined by the operating frequency
of the transducer 20, the dimensions of the transducer and the speed of sound
through the medium in which the ultrasonic sound waves are traveling. With a
transducer with circular cross-section (e.g., as illustrated in FIGS. 2 and 3)
the
following relationship exists:
R=(f/a)(D/2)2
wherein:
R = Rayleigh distance
f = Operating frequency of the transducer
D Diameter of the transducer
a speed of sound through the cranial tissue.
As shown in FIG. 6, an exemplary axial profile of the beam 28 is
displayed. It is understood that the exact profile may vary depending on the
medium in which the ultrasonic waves are traveling. For instance, the axial
profile would show a decrease in intensity if the energy is attenuated when
9
CA 02389669 2002-04-30
WO 01/32258 PCT/US00/30104
traveling through the medium (e.g., cranial tissues). The effective secondary
treatment zone 34 is defined between the (X,) and (XZ) positions. For example,
FIG. 6 shows one embodiment where (X,) and (XZ) are located at 50% of the
maximum intensity. In this instance, a therapeutic effect may be achieved by
exposing the thrombi to an intensity of at least 50% of the maximum intensity.
Accordingly, a predetermined intensity level of ultrasonic energy may be
provided to expose all of the secondary treatment zone to at least the
predetermined level of ultrasonic energy. In one embodiment, as shown in
FIGS. 5 and 6, the predetermined level is at least 50% of the maximum
intensity.
In other embodiments, the predetermined level is at least 75% of the maximum
intensity level. In still other embodiments, the predetermined level is at
least
90% or at least 95% of the maximum intensity level. It will be appreciated
that
intensity levels of less then 50% of the maximum level could be used.
As illustrated in FIG. 6, the relationship of intensity as a function of
distance results in the distance between (XZ) and (R) being greater than the
distance between (X,) and (R). Accordingly, when locating the secondary
treatment zone, the ultrasound system may be designed such that the Rayleigh
distance (R) of the ultrasound beam is positioned at least substantially at
the
center of the primary treatment zone 36 as shown in FIGS. 5 and 6. Locating
the Rayleigh distance (R) at the center maximizes the intensity of the
ultrasonic
energy at the center of the primary treatment zone 36. Alternatively, the beam
28 can be oriented such that the Rayleigh distance (R) is offset from the
center
of the primary treatment zone 36 and positioned closer to the transducer such
CA 02389669 2002-04-30
WO 01/32258 PCT/USOO/30104
that the intensity of the sound waves at (T,) and (T2) are approximately
equal.
This location of the beam would be useful to maximize the intensity of the
sound
waves at each location in the primary treatment zone 36. In still another
embodiment, the beam can be positioned so that the center of the primary
treatment zone 36 is located at the middle of the secondary treatment zone,
i.e.,
midway between X, and X2. This location of the beam would maximize the
additional coverage beyond the normal primary treatment zone on each side to
cover additional possible thrombi locations.
The concepts of the present invention may treat both sides of the brain
at once. However, the invention is also useful to treat one side of the brain.
It
is understood that treating one side of the brain may also result in
incidental
treatment of the other side of the brain as well. Symptoms of the patient will
indicate which side of the brain contains the thrombi. For example, paralysis
or
weakness on the right side of the body indicates the thrombi is located on the
left
side of the brain. The center line 30 (see FIG. 2) of an adult brain will
typically
be located a distance (C) of about 6 to 71/2 centimeters from the transducer
20.
In one embodiment, the primary treatment zone begins at a distance T, of about
2 centimeters from the transducer 20 and continues to a distance Tz of about 7
centimeters from the transducer 20. In addition, the primary treatment zone
width (W) throughout the primary treatment zone is from about 3 centimeters to
about 4 centimeters. There is a very high probability that any intracranial
thrombi
will be located within this primary treatment zone.
In orderto locate the secondary treatment zone 34 such that (X,) and (X2)
11
CA 02389669 2002-04-30
WO 01/32258 PCT/USOO/30104
encompass t he primary treatment zone 36, the Rayleigh distance (R) should be
from about 3 centimeters to about 6 centimeters. In another example, the
Rayleigh distance (R) is about 6.2 centimeters.
In one embodiment, the beam width (W) of the beam between (X,) and
(X2) is about 3 centimeters to about 4 centimeters. The beam width can be
controlled by changing the diameter (D) or aperture of the transducer 20 while
keeping the frequency fixed for example. The beam width (W) at the Rayleigh
distance (R), otherwise known as the 3-dB beam width, is about half the
diameter (D) of the transducer 20. Thus, a transducer 20 having a circular
aperture with a diameter of about 6 centimeters will produce a beam having a 3-
dB beam width of about 3 centimeters at the natural focus of the transducer.
In
embodiments wherein the treatment zone is exposed to ultrasonic energy at
least half of the maximum ultrasonic energy, the half-intensity beam width
will be
between about 3 centimeters and 4 centimeters.
As shown in FIG. 6, one or more pre-focus high intensity spots 32 may
exist in the beam profile. In certain embodiments, it might be desirable to
reduce
or eliminate these spots 32. For example, a conformal array transducer around
part of the head (H) may be arranged to eliminate or reduce the spot 32.
To enhance and optimize insonification into the head (H), the transducer
20 may have a quarter wave matching layer. An integral gel pad 25 may be
present for assisting in coupling an ultrasound energy or waves (US) to heads
of different geometries.
The transducer 20 used with the present invention can be a transducer
12
WO 01/32258 CA 02389669 2002-04-30 pCT/US00/30104
configured for transcranial use to minimize the invasiveness of the treatment
as
exemplified in FIG. 2.
A conventional cooling system may optionally be present in the transducer
20 employed in the method and system of the present invention to assist in
preventing the surrounding body tissue from becoming burned or overheated
due to the transfer and transmission of ultrasound waves or energy. A
thermocouple may be mounted on the edge of the transducer 20 to permit
temperature monitoring during use. Also, a cooling medium may be directed to
the transducer 20 from a source away from the transducer 20, and the cooling
medium may be either air or liquid.
One or more transducers 20 may be used with the present invention, and
each may be selectively adjusted to account for variations in head geometry.
As
shown in FIG. 4, the transducer 120 may comprise an array of transducers, such
as a 2-dimensional conformal array. Individual elements of the array may be
square, hexagonal, segmented rings, or any other pattern which fills the
emitting
area of the transducer and can be controlled by a suitably designed driver
system. The beam can be characterized, with a focus for example, by the
cumulative ultrasound emissions from each of the individual transducers in the
array.
The system of the present invention may also include a holding device to
assist in appropriately positioning the transducer 20 on the head (H) to
enhance
its effect on an intracranial circulatory system, and in particular, the
middle
cerebral artery area. Furthermore, the holding device should be configured for
13
WO 01/32258 CA 02389669 2002-04-30 pCT/US00/30104
maintaining the transducer's 20 desired position during use and treatment to
enhance the effect and/or efficacy of the ultrasound waves or energy. Suitable
examples of such devices may include a head harness, straps, frames, helmets,
and the like. The transducer 20 may be releasably detached, or permanently
affixed to the holding device.
The medicine delivery system 40 can include any conventional
intravascular IV delivery system for the delivery of fluids into the
circulatory
system of the body B. The thrombolytic agent or solution 48 is generally
housed
in a container 42, such as an IV bag or bottle, and is in fluid communication
to
the body B via a catheter 44. Solution 48 is preferably injected and delivered
intravascular into the body B with a needle 46 having an appropriate gauge,
such as an 18-22 gauge needle.
The solution orthrombolytic agent 48 used with the present invention can
be any solution or medicine used to assist in the removal cerebral vessel
blockages or obstructions, such as a blood clot, or to enhance the
thrombolytic
action in a blocked cerebral vessels. A suitable example of a solution or
thrombolytic agent 48 used with the present invention may include an
appropriate solution or suitable dose of a thrombolytic drug.
Any thrombolytic agent or anti-platelet drug can be used with the present
invention. Illustrative examples of suitable agents for use in alleviating
cerebral
blood clots, or other blockages or occlusions which might be used with the
present invention include recombinanttissue plasminogen activator, forexample
rt-PA. In another example, abciximab or other antiplatelet agents are used. A
14
WO 01/32258 CA 02389669 2002-04-30 PCT/US00/30104
suitable dosage or concentration of rt-PA may be about 0.9 mg per kg of body
weight. About 10% of the dosage is preferably given as a bolus at the onset of
treatment, and the remaining portion is preferably given over the period of
about
an hour. Alternatively, a suitable dosage or concentration of rt-PA used with
the
present invention may be less than 0.9 mg per kg of body weight.
In an alternative embodiment, the thrombolytic agent (e.g., t-PA) may be
encapsulated or otherwise contained in a medium that is sufficiently
protective
so that the thrombolytic agent can be delivered to the body (6) and
transmitted
through the circulatory system without effecting nontargeted areas. The
protective medium is capable of being ruptured or otherwise exposing the
thrombolytic agent by the ultrasound waves or energy generated by the
ultrasound device 11 used with the present invention. This arrangement will
target the exposure of the thrombolytic agent to the affected area, thereby
minimizing adverse affects in other parts of the body. Suitable examples of
such
encapsulating materials include microballoons made of a cross-linked albumin,
a lipid vehicle and a targeting moiety, or other protein compatible with blood
products. The size of the encapsulation should be optimized to allow
circulation
of the encapsulated drug throughout the body (e.g., including the lungs) and
yet
be readily destroyed by the application of external ultrasound. In still
another
embodiment, the present invention may use targeted gas-filled echocontrast
agents to act as cavitation nuclei at the site of the clot.
In use, the present invention can be used to treat acute stroke patients.
Once a decision is made to administer a course of treatment, the medicine
WO 01/32258 CA 02389669 2002-04-30 PCT/US00/30104
delivery system 40 is intravenously connected to the body (B) of a patient.
More
specifically, the needle 46 can be inserted through the skin and is inserted
into
the circulatory system. Preferably, the needle 46 is inserted into a suitable
artery
or vein so that the solution 48 is quickly and efficiently directed to the
site of the
obstruction or clot. Exemplary vessels include the radial vein (e.g., see FIG.
1),
antecubital vessels, subclavian vein, femoral vein, or femoral artery. Once
the
needle 46 is appropriately inserted and securely positioned, a valve 50 may be
switched to the open position so that the solution 48 can flow from the
container
42, through a catheter 44, through the needle 46, and into the body B.
As exemplified in FIG. 2, the transducer 20 may be placed near or
adjacent the head (H) of the body (B), and preferably, near or adjacent the
temple. In particular, the transducer 20 is selected, positioned, and
activated
with a natural focus having a Rayleigh distance (R) from about 3 centimeters
to
about 6 centimeters such that the secondary treatment zone 34 will encompass
a zone 36 that has a high probability of containing a thrombus as shown in
FIGS.
5 and 6.
The driver 14 can be connected to an electrical source, activated, or
turned on, and an electrical current is transmitted through the cord 26 to the
transducer 20. The electrical energy is converted or transformed to ultrasound
waves or energy at the transducer 20. The resulting ultrasound energy or waves
are emitted, provided or directed into the body B, preferably through the
temporal bone and toward the blockage or occlusion (e.g., blood clot), such as
within the middle cerebral artery. The transducer 20 can radiate, direct, emit
or
16
WO 01/32258 CA 02389669 2002-04-30 pCT/US00/30104
provide ultrasound waves or energy (US) at a frequency range from about 100
kHz to about 1 MHZ, such as from about 100 kHz to about 250 kHz. In one
particular embodiment, the frequency of the transducer is about 120 kHz. The
amplitude, or intensity, of the sound waves are from about 0.5 W/cm2 to about
10 W/cm2. In one embodiment, the amplitude or intensity might be up to 2
W/cm2, as desired. The duty cycle of the ultrasound waves or energy may be
adjustable, as desired, and can be set at a range from about 10% to 100% (or
continuous wave). The ultrasound waves or energy are radiated, directed,
emitted and directed during the period that the solution 48 is being
administered
intravenously. The ultrasound waves or energy may be radiated, directed,
emitted and directed for about an hour, although larger or smaller time
periods
may be employed.
In a preferred embodiment, a transducer 20 may also be fixed in the
desired position near or adjacent the temple of the head using a strap, or
other
affixation device.
It will be appreciated that the system 10 and methods described herein
are useful in the lysis of intracranial thrombi. The application of ultrasonic
energy
to a primary treatment zone allows increased efficacy of thrombolytic agents.
Accordingly, in some embodiments of the present invention, the techniques
described herein will result in a reduced dosage of thrombolytic agent,
thereby
reducing possible occurrences of undesirable side effects such as hemorrhage
complications. In addition, the device and methods described herein provide an
ultrasonic zone that targets the primary zone. Accordingly, therapy may be
17
WO 01/32258 CA 02389669 2002-04-30 PCT/US00/30104
initiated sooner since there is no need for radiologic or imaging guidance to
determine the exact vascular location of the thrombi.
The portability and ease of use of the device may even allow treatment
to begin before arrival at the hospital. For instance, emergency technicians
may
start therapy on site and/or may administer therapy on an ambulance for
example.
Having shown and described the preferred embodiments to the present
invention, further adaptations of the present invention as described herein
can
be accomplished by appropriate modifications by one of ordinary skill in the
art
without departing from the scope of the present invention. For example, other
thrombolytics may be used with the present invention. In addition, while
certain
transducers shown and described throughout this application have circular
section, it is understood that they could be formed with other shapes
including
polygons (e.g., triangle, square, or other polygon with four or more sides),
elliptical, or other geometric shapes. In addition, other methods of providing
a
secondary treatment zone can involve focusing the beam with a spherical
segment for example, attaching or forming the transducer with a lens, a
conformal 2-dimensional array, and/or forming a helmet to receive the
transducers to place over the head. Several such potential modifications have
been discussed and others will be apparent to those skilled in the art.
Accordingly, the scope of the present invention should be considered in terms
of the following claims and is understood not to be limited in the details,
structure
and operation shown and described in its specification and drawings.
18