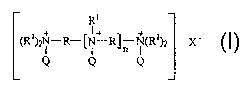Note: Descriptions are shown in the official language in which they were submitted.
CA 02389768 2002-04-08
WO 01/34748 PCT/US00/30645
LAUNDRY DETERGENT COMPOSITIONS COMPRISING
ZWITTERIONIC POLYAMINES
FIELD OF THE INVENTION
The present invention relates to laundry detergent compositions comprising one
or more
hydrophobically modified polyamines which provide enhanced hydrophilic soil,
inter alia, clay,
removal benefits. The present invention also relates to methods for removing
hydrophilic soil
form wearing apparel.
BACKGROUND OF THE INVENTION
Fabric, especially clothing, can become soiled with a variety of foreign
substances
ranging from hydrophobic stains (grease, oil) to hydrophilic stains (clay).
The level of cleaning
which is necessary to remove said foreign substances depends to a large degree
upon the amount
of stain present and the degree to which the foreign substance has contacted
the fabric fibers.
Grass stains usually involve direct abrasive contact with vegetative matter
thereby producing
highly penetrating stains. Clay soil stains, although in some instances
contacting the fabric fibers
with less force, nevertheless provide a different type of soil removal problem
due to the high
degree of charge associated with the clay itself. This high surface charge
density may act to repel
some laundry adjunct ingredients, inter alia, clay dispersants, thereby
resisting any appreciable
peptization and dispersal of the clay into the laundry liquor.
A surfactant per se is not all that is necessary to remove unwanted clay soils
and stains.
In fact, most surfactants by themselves in water are surprisingly poor at
removing clay soils from
fabric. not all surfactants work equally well on all types of stains. In
addition to surfactants,
polyamine-based hydrophilic soil dispersants are added to laundry detergent
compositions to
"carry away" clay soils from the fabric surface and to stabilize the removed
particles in solution
sufficiently to minimize the possibility that the clay soil will be re-
deposited upon the fabric.
However, unless the clay can be initially removed from the soiled fabric,
especially in the case of
hydrophilic fibers, inter alia, cotton, there will be nothing in solution for
the dispersants to bind to
and keep suspended.
There is a long felt need in the art for laundry detergent compositions which
can
effectively break up and remove embedded clay and other hydrophilic soils from
fabric. In
addition, as the concentration of hydrophilic soil increases in the laundry
liquor, there is a need
for a surfactant system which will be able to handle this increased soil load.
Also there is a long
CA 02389768 2002-04-08
WO 01134748 PCT/US00/30645
felt need for a clay soil active adjunct ingredient which can be optimized to
fit the particular
laundry detergent embodiment, inter alia, granular, liquid, and which can be
therefore tailored to
match the surfactant system. There has further been a long felt need for a
method for cleaning
hydrophilic soils from fabric wherein the hydrophilic soils are effectively
peptized, dispersed, and
suspended in the laundry liquor.
SUMMARY OF THE INVENTION
It has now been surprisingly discovered that laundry detergent compositions
comprising
fully quaternized polyethoxylated polyamines wherein said polyethoxy units are
capped with
anionic units and wherein the polyamine backbone is comprised of relatively
hydrophobic
backbone spacer units, said polyamines can be hydrophobically modified by the
selection of
certain quaternizing units to provide enhanced removal of soils from clothing.
The laundry
detergent compositions of the present invention are especially effective in
removal of clay and
other hydrophilic soils from fabric. When used together with a suitable
surfactant system, the
hydrophobically modified polyamines of the present invention provides for
removal of stains
which were once believed ruinous to fabric, especially cellulose comprising
fabric.
The first aspect of the present invention relates to laundry detergent
compositions
comprising:
A) from about 0.01 %, preferably from about 0.1 %, more preferably from about
1 %,
most preferably from about 3% to about 50%, preferably to about 20%, more
preferably to about 10%, most preferably to about 7% by weight, of a
hydrophobically modified polyamine having the formula:
R~
(R1)zN-R-~N~ R~p N(R~)z X-
Q Q Q
wherein R is C5-CZ° linear or branched alkylene, and mixtures thereof;
R' is an
alkyleneoxy unit having the formula:
_(Rz0)X Rs
wherein Rz is CZ-C4 linear or branched alkylene, and mixtures thereof; R3 is
an
anionic unit, and mixtures thereof; x is from about 15 to about 30; Q is a
hydrophobic quaternizing unit selected from the group consisting of C8-
C3° linear
or branched alkyl, C~-C3° cycloalkyl, C,-C3° substituted or
unsubstituted
2
CA 02389768 2002-04-08
WO 01/34748 PCT/US00/30645
alkylenearyl, and mixtures thereof; X is an anion present in sufficient amount
to
provide electronic neutrality; n is from 0 to 4;
B) from about 0.01% by weight, of a surfactant system comprising one or more
surfactants selected from:
i) from 0% to 100% by weight, of one or more anionic surfactants;
ii) from 0% to 100% by weight, of one or more nonionic surfactants;
iii) optionally from 0.1 % to about 80% by weight, of one or more cationic
surfactants;
iv) optionally from 0.1% to about 80% by weight, of one or more
zwitterionic surfactants;
v) optionally from 0.1 % to about 80% by weight, of one or more
ampholytic surfactants; or
vi) mixtures thereof;
C) the balance carriers and adjunct ingredients.
The present invention also relates to a method for cleaning fabric, said
method
comprising the step of contacting an article of manufacture comprising fabric,
preferably clothing,
with an aqueous solution of a laundry detergent composition comprising a
hydrophobically
modified polyamine of the present invention.
These and other objects, features and advantages will become apparent to those
of
ordinary skill in the art from a reading of the following detailed description
and the appended
claims. All percentages, ratios and proportions herein are by weight, unless
otherwise specified.
All temperatures are in degrees Celsius (° C) unless otherwise
specified. All documents cited are
in relevant part, incorporated herein by reference.
DETAILED DESCRIPTION OF THE INVENTION
The present invention relates to hydrophobically modified quaternized
zwitterionic
polyamines which are suitable for use in laundry detergent compositions. The
hydrophobically
modified zwitterionic polyamines of the present invention provide enhanced
body soil and
perspiration soil removal benefits.
It has been surprisingly discovered that hydrophobically modified quaternized
zwitterionic polyamines have increased effectiveness when treating fabric
which is soiled with
human body oils, perspiration, etc. Without wishing to be limited by theory,
the hydrophobically
modified quaternary zwitterionic polyamines of the present invention have an
unexpected balance
of properties which makes the compounds amenable to penetrating fabric to
solublize greasy, oily
3
CA 02389768 2002-04-08
WO 01/34748 PCT/US00/30645
stains, while maintaining water solubility, and preserving the particulate
soil suspension
properties needed to direct the dirt away from the fabric thereby avoiding re-
deposition. In
addition, the hydrophobically modified zwitterionic polyamines of the present
invention reinforce
the cleaning actions of high suds and high phosphate cleaning systems.
When present in laundry detergent compositions, the zwitterionic polyamines
are
effective in an amount from about 0.01 %, preferably from about 0.1 %, more
preferably from
about 1%, most preferably from about 3% to about 50%, preferably to about 20%,
more
preferably to about 10%, most preferably to about 7% by weight, of said
laundry detergent
composition.
Hydrophobicallv Modified Quaternized Zwitterionic Polyamines
For the purposes of the present invention the term "hydrophobically modified"
is defined
herein as the "reaction of a linear polyamine comprising from 2 to 5 nitrogens
wherein each
nitrogen has its backbone hydrogens replaced by an anionic unit-capped
polyalkyleneoxy unit
comprising at least about 15 alkyleneoxy units, with at least one equivalent
per nitrogen of a
quaternizing agent, said quaternizing agents comprising a linear alkyl moiety
having at least 8
carbon atoms, a cyclic alkyl moiety having at least 6 carbon atoms, an
alkylenearyl unit, inter
alia, benzyl, having at least 7 carbon atoms, or mixtures thereof '.
A "polyamine" for the purposes of the present invention is "an amine having
less than 6
backbone nitrogen atoms and no branching", whereas for the purposes of the
present invention,
amines comprising more than 5 nitrogens are defined as "oligomeric amines"
(oligoamines) or
"polymeric amines" (polyalkyleneamines or polyalkyleneimines).
The hydrophobically modified zwitterionic polyamines of the present invention
have the
formula:
R~
(R1)?N-R-~N~ R~ n N(Rl)2 X
Q Q Q
wherein R is C~ CZ~ linear or branched alkylene, and mixtures thereof; in one
embodiment R is C~_
-C,o linear alkylene, in another embodiment R is C~,-C8 linear alkylene, in
yet another embodiment
each of the R units are hexylene units, typically linear hexylene units.
R' is an anionic unit-capped polyalkyleneoxy unit having the formula:
(R20)X R3
wherein Rz is Cz-C4 linear or branched alkylene, and mixtures thereof. The
term linear or
branched as it refers to R' include any linear or branched alkylene unit
comprising a total of from
4
CA 02389768 2002-04-08
WO 01/34748 PCT/US00/30645
2 to 4 carbon atoms. In one embodiment RZ comprises ethylene, 1,2-propylene,
and mixtures
thereof; in still another embodiment each RZ unit is an ethylene unit. One
embodiment of the
present invention which provides advantages in a bleach comprising composition
relates to
hydrophobically modified zwitterionic polyamines comprising the first 1-6,
preferably the first 1-
3 of alkyleneoxy units as 1,2-propyleneoxy units followed by the balance
ethyleneoxy units.
R' is an anionic capping unit, and mixtures thereof. What is meant herein as
"an anionic
capping unit, and mixtures thereof ' is the R3 unit may comprise a single
anionic unit or each R;
may be a different anionic capping unit, or R3 may comprise any mixture of
anionic units." Non-
limiting examples of anionic units which comprise one embodiment of the
present invention are
anionic units selected from the group consisting of:
a) -(CHz),COzM;
b) -C(O)(CHZ),COzM;
c) -(CHZ)rP03M;
d) -(CHz),OP03M;
e) -(CHZ),S03M;
f) -CHZ(CHS03M)(CHz),SO,M;
g) -CHZ(CHSOZM)(CH~),,S03M;
h) -C(O)CHzCH(S03M)COzM;
i) -C(O)CHzCH(COzM)NHCH(COzM)CHZCOzM;
j) and mixtures thereof;
wherein M is hydrogen or a canon which provides charge neutrality. For the
purposes of the
present invention, all M units, whether associated with a hydrophobically
modified zwitterionic
polyamine, surfactant, or adjunct ingredient, can either be a hydrogen atom or
a canon depending
upon the form isolated by the artisan or the relative pH of the system wherein
the compound is
used. Non-limiting examples of preferred canons include sodium, potassium,
ammonium, and
mixtures thereof. The index f is from 0 to about I 0, one embodiment of the
present invention
fixes the range of the index f from 0 to 2.
The index x which describes the average number of alkyleneoxy units attached
to the
backbone nitrogen is from about I 5 to about 30,preferably from I S to 25,
more preferably from
18 to 23, most preferred average value of alkyleneoxy units is 20. The
formulator will recognize
that when ethoxylating a zwitterionic polyamine, only an average number or
statistical
distribution of alkyleneoxy units will be know. Therefore, depending upon how
"tightly" or how
"exactly" a zwitterionic polyamine is alkoxylated, the average value may vary
from embodiment
to embodiment.
5
CA 02389768 2002-04-08
WO 01/34748 PCT/US00/30645
Q is a hydrophobic quaternizing unit and each Q is independently C8-C3o linear
or
branched alkyl, C~-C3o cycloalkyl, C,-C3o substituted or unsubstituted
alkylenearyl, and mixtures
thereof; in one embodiment of the present invention Q is a hydrophobic
quaternizing unit selected
from the group consisting of C,-C3o substituted or unsubstituted alkylenearyl,
and mixtures
thereof; more preferably benzyl, substituted benzyl, naphthyl, substituted
naphthyl, and mixtures
thereof. For the purposes of the present invention the formulae:
-(CHZ)W
-(CH2)W
or
stands for the term "naphthyl" depending upon whether said unit comprises a-
substitution or (3-
substitution. The index w has the value from 0 to 20. Other alkylene aryl
units include besides
benzyl, alkylenearyl units having the formula:
-(CHZ)L
wherein the index z is from 1 to 24.
For the purposes of the present invention the term "substituted" as it applies
to
alkylenearyl units suitable as Q units, are one or more C,-C,2 linear or
branch alkyl moieties,
provided the total number of carbon atoms including the aromatic ring does not
exceed 30 carbon
atoms.
A non-limiting example of a substitued alkylenearyl unit according to the
present
invention has the formula:
-(CHz)
which is a 3,5-di-tert-butyl benzyl moiety.
The index n represents the number of secondary nitrogens in the backbone. The
index n
has the value from 0 to 4, preferably from 0 to 2.
X is an anion present in sufficient amount to provide electronic neutrality.
Non- limiting
examples of anions are chlorine, bromine, iodine, methylsulfate, and mixtures
thereof.
6
CA 02389768 2002-04-08
WO 01/34748 PCT/US00/30645
An example of a preferred hydrophobically modified zwitterionic polyamine
according to
the present invention has the formula:
X _ +~HzCHZO)zoSO~ _ +(~HZCH20)zoS03M
(CHZCH20)zS03M X
M03S(OCHZCHz)zo-N N-(CHZCH20)zoS03M
_ +N
X
\ ~ / ~ \
wherein X is a water soluble anion selected from the group consisting of
chlorine, bromine,
iodine, methylsulfate, and mixtures thereof.
SURFACTANTSYSTEM
The laundry detergent compositions of the present invention comprise from
about 0.01 %,
preferably from about 1 %, more preferably from about 5%, most preferably from
10% to about
80%, preferably to about 50%, more preferably to about 30%, by weight of a
surfactant system,
said surfactant system comprising one or more surfactants selected from:
i) from 0% to 100% by weight, of one or more anionic surfactants;
ii) from 0% to 100% by weight, of one or more nonionic surfactants;
iii) optionally from 0.1 % to about 80% by weight, of one or more cationic
1 S surfactants;
iv) optionally from 0.1 % to about 80% by weight, of one or more zwitterionic
surfactants;
v) optionally from 0.1 % to about 80% by weight, of one or more ampholytic
surfactants; or
vi) mixtures thereof.
A preferred surfactant system according to the present invention comprises
from about 0.01 % by weight, of one or more surfactants selected from:
i) from 1 % to about 100% by weight, of an anionic surfactant selected from:
a) linear alkyl benzene sulfonates;
b) mid-chain branched aryl sulfonate surfactants having the formula:
A R2
S03M'
7
CA 02389768 2002-04-08
WO 01/34748 PCT/US00/30645
wherein A is a mid-chain branched alkyl unit having the formula:
R R1
CH3(CHZ),~CH(CHZ)yCH(CHZ)Z
wherein R and R' are each independently hydrogen, C,-C3 alkyl, and
$ mixtures thereof, provided the total number of carbon atoms in said alkyl
unit is from 6 to 18 and at least one of R and R' is not hydrogen; x is an
integer from 0 to 13; y is an integer from 0 to 13; z is 0 or l; RZ is
hydrogen, C,-C3 alkyl, and mixtures thereof; M' is a water soluble canon
with sufficient charge to provide neutrality;
c) branched alkyl sulfate surfactants having the formula:
CH3CH2(CHZ)r"CHZOS03M.
or the formula:
CH3CHz(CHZ)",CHZ(OCH2CH2)yOS03M~
d) mid-chain branched alkyl sulfate surfactants having the formula:
R R~ RZ
CH3CHz(CHZ)WCH(CHZ),~CH(CHZ)yCH(CH2)ZOS03M~
or the formula:
R R~ R2
CH3CH2(CHZ)w,CH(CHZ)XCH(CHZ)yCH(CHZ)z(OR3)n,OS03M
wherein R, R', and R~ are each independently hydrogen, C,-C, alkyl, and
mixtures thereof, provided the total number of carbon atoms in said
surfactant is from 14 to 20 and at least one of R, R', and Rz is not
hydrogen; the index w is an integer from 0 to 13; x is an integer from 0 to
13; y is an integer from 0 to 13; z is an integer of at least 1; provided w +
x + y + z is from 8 to 14 and the total number of carbon atoms in a
surfactant is from 14 to 20; R3 is ethylene, 1,2-propylene, 1,3-propylene,
1,2-butylene, 1,4-butylene, and mixtures thereof; the average value of the
index m is at least about 0.01; M is a water soluble canon of sufficient
charge to provide electronic neutrality;
ii) from 0% to 100% by weight, of one or more nonionic surfactants;
iii) optionally from 0.1 % to about 80% by weight, of one or more cationic
surfactants;
8
CA 02389768 2002-04-08
WO 01/34748 PCT/US00/30645
iv) optionally from 0.1 % to about 80% by weight, of one or more zwitterionic
surfactants;
v) optionally from 0.1% to about 80% by weight, of one or more ampholytic
surfactants; or
S vi) mixtures thereof.
Depending upon the embodiment of the present invention one or more categories
of
surfactants may be chosen by the formulator, however, at least one anionic or
at least one
nonionic surfactant must be present. Within each category of surfactant, more
than one type of
surfactant can be selected.
Nonlimiting examples of surfactants useful herein include:
a) C"-C,8 alkyl benzene sulfonates (LAS);
b) C,~ C~o primary, branched-chain and random alkyl sulfates (AS);
c) C",-C,8 secondary (2,3) alkyl sulfates having the formula:
OS03- M+ OS03- M+
CH3(CH2)X(CH)CH3 or CH3(CHZ)y(CH)CH2CH3
wherein x and (y + 1 ) are integers of at least about 7, preferably at least
about 9; said
surfactants disclosed in U.S. 3,234,258 Morris, issued February 8, 1966; U.S.
5,075,041
Lutz, issued December 24, 1991; U.S. 5,349,101 Lutz et al., issued September
20, 1994;
and U.S. 5,389,277 Prieto, issued February 14, 1995 each incorporated herein
by
reference;
d) C,~ C,g alkyl alkoxy sulfates (AEXS) wherein preferably x is from 1-7;
e) C,~-C,8 alkyl alkoxy carboxylates preferably comprising 1-5 ethoxy units;
f) C,,-C,g alkyl ethoxylates, C~ C,z alkyl phenol alkoxylates wherein the
alkoxylate units
are a mixture of ethyleneoxy and propyleneoxy units, C,2-C,g alcohol and C~-
C,Z alkyl
phenol condensates with ethylene oxide/propylene oxide block polymers inter
alia
Pluronic~ ex BASF which are disclosed in U.S. 3,929,678 Laughlin et al.,
issued
December 30, 1975, incorporated herein by reference;
g) Alkylpolysaccharides as disclosed in U.S. 4,565,647 Llenado, issued January
26, 1986,
incorporated herein by reference;
h) Polyhydroxy fatty acid amides having the formula:
9
CA 02389768 2002-04-08
WO 01/34748 PCT/US00/30645
O
R-C-N-[(RIO)x(R20)yR3]m
(R4)n
wherein R is C,-C2, linear alkyl, C~-C2, branched alkyl, C,-CZ, linear
alkenyl, C,-C2, branched
alkenyl, and mixtures thereof.
R' is ethylene; Rz is C,-C4 linear alkyl, C3-C4 branched alkyl, and mixtures
thereof;
preferably RZ is 1,2-propylene. Nonionic surfactants which comprise a mixture
of R' and Rz units
preferably comprise from about 4 to about 12 ethylene units in combination
with from about 1 to
about 4 1,2-propylene units. The units may be alternating, or grouped together
in any
combination suitable to the formulator. Preferably the ratio of R' units to Rz
units is from about 4
1 to about 8 : 1. Preferably an R' units (i.e. 1,2-propylene) is attached to
the nitrogen atom
followed by the balance of the chain comprising from 4 to 8 ethylene units.
R' is hydrogen, C,-C4 linear alkyl, C3-C4 branched alkyl, and mixtures
thereof; preferably
hydrogen or methyl, more preferably hydrogen.
R4 is hydrogen, C,-C4 linear alkyl, C;-C,, branched alkyl, and mixtures
thereof; preferably
hydrogen. When the index m is equal to 2 the index n must be equal to 0 and
the R'' unit is absent
and is instead replaced by a -[( R'O)x(R20)yR3] unit.
The index m is 1 or 2, the index n is 0 or 1, provided that when m is equal to
1, n is equal
to 1; and when m is 2 n is 0; preferably m is equal to 1 and n is equal to
one, resulting in one -
[(R'O)~(RZO)YR3] unit and R4 being present on the nitrogen. The index x is
from 0 to about S0,
preferably from about 3 to about 25, more preferably from about 3 to about 10.
The index y is
from 0 to about 10, preferably 0, however when the index y is not equal to 0,
y is from I to about
4. Preferably all of the alkyleneoxy units are ethyleneoxy units. Those
skilled in the art of
ethoxylated polyoxyalkylene alkyl amide surface active agents will recognized
that the values for
the indices x and y are average values and the true values may range over
several values
depending upon the process used to alkoxylate the amides.
Suitable means for preparing the polyoxyalkylene alkylamide surface active
agents of the
present invention can be found in "Surfactant Science Series", Editor Martin
Schick, Volume I,
Chapter 8 ( I 967) and Volume XIX, Chapter 1 ( 1987) included herein by
reference.
Mid-chain Branched Alk~ Sulfates
The surfactant systems of the present invention may comprise a mid-chain
branched alkyl
sulfate surfactant and/or a mid-chain branched alkyl alkoxy sulfate
surfactant. Because mid-chain
branched alkyl sulfate or alkyl alkoxy sulfate surfactants are not required
when mid-chain
branched aryl sulfonate surfactants are present, the surfactant system
comprises from 0%, when
CA 02389768 2002-04-08
WO 01/34748 PCT/US00/30645
present from 0.01 %, preferably from about 0.1 % more preferably from about 1
% to about 100%,
preferably to about 80% by weight, preferably to about 60%, most preferably to
about 30% by
weight, of the surfactant system. When the mid-chain branched alkyl sulfate
surfactants or mid-
chain branched alkyl alkoxy sulfate surfactants comprise 100% of the
surfactant system said
surfactants will comprise up to 60% by weight of the final laundry detergent
composition.
The mid-chain branched alkyl sulfate surfactants of the present invention have
the
formula:
R R1 RZ
CH3CH2(CHZ)~,,CH(CHZ),iCH(CHZ)yCH(CHZ)ZOS03M
the alkyl alkoxy sulfates have the formula:
R R1 R2
CH3CH2(CHZ)WCH(CHZ),~CH(CH2)yCH(CHZ)~(OR3)~,OS03M
wherein R, R', and Rz are each independently hydrogen, C,-C3 alkyl, and
mixtures thereof;
provided at least one of R, R', and RZ is not hydrogen; preferably R, R', and
Rz are methyl;
preferably one of R, R', and R' is methyl and the other units are hydrogen.
The total number of
carbon atoms in the mid-chain branched alkyl sulfate and alkyl alkoxy sulfate
surfactants is from
14 to 20; the index w is an integer from 0 to 13; x is an integer from 0 to
13; y is an integer from 0
to 13; z is an integer of at least 1; provided w + x + y + z is from 8 to 14
and the total number of
carbon atoms in a surfactant is from 14 to 20; R3 is C,-C4 linear or branched
alkylene, preferably
ethylene, 1,2-propylene, 1,3-propylene, 1,2-butylene, 1,4-butylene, and
mixtures thereof.
However, a preferred embodiment of the present invention comprises from 1 to 3
units wherein
R' is 1,2-propylene, 1,3-propylene, or mixtures thereof followed by the
balance of the R3 units
comprising ethylene units. Another preferred embodiment comprises R3 units
which are
randomly ethylene and 1,2-propylene units. The average value of the index m is
at least about
0.01. When the index m has low values, the surfactant system comprises mostly
alkyl sulfates
with a small amount of alkyl alkoxy sulfate surfactant. Some tertiary carbon
atoms may be
present in the alkyl chain, however, this embodiment is not desired.
M denotes a cation, preferably hydrogen, a water soluble canon, and mixtures
thereof.
Non-limiting examples of water soluble cations include sodium, potassium,
lithium, ammonium,
alkyl ammonium, and mixtures thereof.
11
CA 02389768 2002-04-08
WO 01/34748 PCT/US00/30645
The preferred mid-chain branched alkyl sulfate and alkyl alkoxy sulfate
surfactants of the
present invention are "substantially linear" surfactants. The term
"substantially linear" is defined
for the purposes of the present invention as "alkyl units which comprise one
branching unit or the
chemical reaction products which comprise mixtures of linear (non-branched)
alkyl units and
alkyl units which comprise one branching unit". The term "chemical reaction
products" refers to
the admixture obtained by a process wherein substantially linear alkyl units
are the desired
product but nevertheless some non-branched alkyl units are formed. When this
definition is taken
together with preferably one of R, R', and RZ is methyl and the other units
are hydrogen, the
preferred mid-chain branched alkyl sulfate and alkyl alkoxy sulfate
surfactants comprise one
methyl branch, preferably said methyl branch is not on the a,, (3, or the
second to the last carbon
atom. Typically the branched chains are a mixture of isomers.
The following illustrate preferred examples of mid-chain branched alkyl
sulfate and
alkoxy alkyl sulfate surfactants.
8-Methylundecyl sulfate:
~S03M
3-Methylundecyl sulfate:
~ ~S03M
3-Methyltridecyl sulfate:
S03M
8-Methyltridecyl sulfate:
S03M
Mid-chain Branched Aryl Sulphonates
The surfactant systems of the present invention may comprise a mid-chain
branched aryl
sulphonate surfactant. Because mid-chain branched aryl sulfonate surfactants
may not be present
when mid-chain branched alkyl sulfate and/or alkyl alkoxy surfactants are
present, the surfactant
system comprises from 0%, when present from 0.01 %, preferably from about 0.1
% more
12
CA 02389768 2002-04-08
WO 01/34748 PCT/US00/30645
preferably from about 1 % to about 100%, preferably to about 80% by weight,
preferably to about
60%, most preferably to about 30% by weight, of the surfactant system. When
the mid-chain
branched aryl sulphonate surfactants comprise 100% of the surfactant system
said mid-chain
branched aryl sulphonate surfactants will comprise up to 60% by weight of the
final laundry
detergent composition.
The mid-chain branched aryl sulphonates of the present invention have the
formula:
A R2
S03M'
wherein A is a mid-chain branched alkyl unit having the formula:
R R'
CH3(CH2),~CH(CHZ)yCH(CHZ)Z
wherein R and R' are each independently hydrogen, C,-C; alkyl, and mixtures
thereof, provided at
least one of R and R' is not hydrogen; preferably at least one R or R' is
methyl; wherein the total
number of carbon atoms in said alkyl unit is from 6 to 18. Some tertiary
carbon atoms may be
present in the alkyl chain, however, this embodiment is not desired.
The integer x is from 0 to 13. The integer y is from 0 to 13. The integer z is
either 0 or 1,
preferably 0.
R2 is hydrogen, C,-C3 alkyl, and mixtures thereof. Preferably R'' is hydrogen.
M' denotes a water soluble cation with sufficient charge to provide
neutrality, preferably
hydrogen, a water soluble canon, and mixtures thereof. Non-limiting examples
of water soluble
canons include sodium, potassium, lithium, ammonium, alkyl ammonium, and
mixtures thereof.
In one embodiment of the present invention mid-chain branched aryl sulphonate
surfactants are "substantially linear aryl" surfactants. The term
"substantially linear aryl" is
defined for the purposes of the present invention as "an alkyl unit which is
taken together with an
aryl unit wherein said alkyl unit preferably comprises one branching unit,
however, a non-
branched linear alkyl unit having an aryl unit bonded to the 2-carbon position
as part of an
admixture is included as a substantially linear aryl surfactant". The
preferred alkyl units do not
have a methyl branch on the second to the last carbon atom. Typically the
branched chains are a
mixture of isomers. However, in the case of the mid-chained branched aryl
sulphonates of the
13
CA 02389768 2002-04-08
WO 01/34748 PCT/US00/30645
present invention, the relative position of the aryl moiety is key to the
functionality of the
surfactant. Preferably the aryl moiety is attached to the second carbon atom
in the branched chain
as illustrated herein below.
In one or more embodiments mid-chain branched aryl sulphonates of the present
invention will comprise a mixture of branched chains. Preferably R' is methyl,
the index z is
equal to 0, and the sulphate moiety is para ( 1,4) to the branched alkyl
substituent thereby
resulting in a "2-phenyl aryl sulphonate" defined herein by the general
formula:
R I H3
CH3(CHZ),~CH(CH2)y-CH
S03M
Typically 2-phenyl aryl sulphonates are formed as a mixture together with "3-
phenyl aryl
sulphonates" defined herein by the general formula:
R i HZCH3
CH3(CHZ),~CH(CHZ)y-CH
S03M
The surfactant properties of the mid-chain branched aryl sulphonates of the
present
invention can be modified by varying the ratio of 2-phenyl to 3-phenyl isomers
in the final
surfactant mixture. A convenient means for describing the relative amounts of
isomers present is
the "2/3 phenyl index" defined herein as "100 times the quotient of the amount
of 2-phenyl
isomer present divided by the amount of the 3-phenyl isomer which is present".
Any convenient
means, NMR, inter alia, can be used to determine the relative amounts of
isomers present. A
preferred 2/3 phenyl index is at least about 275 which corresponds to at least
2.75 times more 2-
phenyl isomer present than the 3-phenyl isomer in the surfactant mixture. The
preferred 2/3-
phenyl index according to the present invention is from about 275, more
preferably from about
14
CA 02389768 2002-04-08
WO 01/34748 PCT/US00/30645
350, most preferably from about 500 to about 10,000, preferably to about 1200,
more preferably
to about 700.
Those of ordinary skill in the art will recognize that the mid-chain branched
surfactants of
the present invention will be a mixture of isomers and the composition of the
mixture will vary
depending upon the process which is selected by the formulator to make the
surfactants. For
example, the following admixture is considered to comprise a substantially
linear mid-chain
branched aryl sulphonate admixture according to the present invention. Sodium
para-(7-
methylnonan-2-yl)benzenesulphonate, sodium para-(6-methylnonan-2-
yl)benzenesulphonate,
sodium para-(7-methylnonan-3-yl)benzene-sulphonate, sodium para-(7-methyldecan-
2-
yl)benzenesulphonate, sodium para-(7-methylnonanyl)benzenesulphonate.
FORMULATIONS
The compositions of the present invention may be in any form, inter alia,
liquid, granular,
paste. Depending upon the specific form of the laundry composition, as well
as, the expected use
thereof, the formulator may will use different surfactant and adjunct
ingredient combinations.
The Heavy Duty Granular compositions according to the present invention
comprise:
a) from about 0.01 %, preferably from about 0.1 %, more preferably from 1 %,
most
preferably from 3% to about 20%, preferably to about 10%, more preferably to
about 7% by weight, of a hydrophobically modified polyamine; and
b) from about 0.01 % by weight, preferably from about 0.1 % more preferably
from
about 1 % to about 60%, preferably to about 30% by weight, of said
composition,
of a surfactant system, said surfactant system comprising:
i) from 0.01 %, preferably from about 0.1 % more preferably from about 1
to about 100%, preferably to about 80% by weight, preferably to about
60%, most preferably to about 30% by weight, of a surfactant selected
from the group consisting of alkyl sulfate surfactants, alkoxy sulfate
surfactants, mid-chain branched alkyl sulfate surfactants, mid-chain
branched alkoxy sulfate surfactants, mid-chain branched aryl sulfonate
surfactants, and mixtures thereof;
ii) optionally and preferably, from 0.01 %, preferably from about 0.1 % more
preferably from about 1 % to about 100%, preferably to about 80% by
weight, preferably to about 60%, most preferably to about 30% by
weight, of one or more anionic surfactants;
CA 02389768 2002-04-08
WO 01/34748 PCT/US00/30645
iii) optionally, from 0.01 %, preferably from about 0.1 % more preferably
from about 1 % to about 100%, preferably to about 80% by weight,
preferably to about 60%, most preferably to about 30% by weight, of one
or more nonionic surfactants.
HDG laundry detergent compositions will typically comprise more of anionic
detersive
surfactants. Therefore, the formulator will employ a zwitterionic polyamine
having a greater
number of anionic units than the number of backbone cationic units. This net
charge balance will
ameliorate the negative interaction of the surfactant molecules with the
hydrophilic soil active
zwitterionic polymers.
By contrast, the Heavy Duty Liquid (HDL) compositions according to the present
invention comprise:
a) from about 0.01 %, preferably from about 0.1 %, more preferably from 1 %,
most
preferably from 3% to about 20%, preferably to about 10%, more preferably to
about 5% by weight, of a zwitterionic polyamine wherein said polyamine
comprises less than or equal number of anionic substituents than the number of
backbone quaternary nitrogen units; and
b) from about 0.01 % by weight, preferably from about 0.1 % more preferably
from
about 1 % to about 60%, preferably to about 30% by weight, of said
composition,
of a surfactant system, said surfactant system comprising:
i) from 0.01 %, preferably from about 0.1 % more preferably from about 1
to about 100%, preferably to about 80% by weight, preferably to about
60%, most preferably to about 30% by weight, of a surfactant selected
from the group consisting of mid-chain branched alkyl sulfate
surfactants, mid-chain branched alkoxy sulfate surfactants, mid-chain
branched aryl sulfonate surfactants, and mixtures thereof;
ii) preferably, from 0.01 %, preferably from about 0.1 % more preferably
from about 1 % to about 100%, preferably to about 80% by weight,
preferably to about 60%, most preferably to about 30% by weight, of one
or more nonionic surfactants, said nonionic surfactants selected form the
group consisting of alcohols, alcohol ethoxylates, polyoxyalkylene
alkylamides, and mixtures thereof;
iii) optionally, from 0.01 %, preferably from about 0.1 % more preferably
from about 1 % to about 100%, preferably to about 80% by weight,
16
CA 02389768 2002-04-08
WO 01/34748 PCT/US00/30645
preferably to about 60%, most preferably to about 30% by weight, of one
or more nonionic surfactants.
HDL laundry detergent compositions will typically comprise more of a lesser
amount of
an anionic detersive surfactant and more nonionic surfactants. Therefore, the
formulator will
employ a zwitterionic polyamine having an equal number of anionic units as the
number of
cationic units or a greater number of cationic backbone units than the number
of anionic units.
BLEACHING SYSTEM
The hydrophobically modified polyamine-comprising laundry detergent
compositions of
the present invention may optionally comprise a bleaching system. Bleaching
systems typically
comprise a "bleaching agent" (source of hydrogen peroxide) and an "initiator"
or "catalyst".
Preferred laundry detergent compositions of the present invention which
comprise a
bleaching system, comprise:
a) from about 0.01% by weight, of a hydrophobically modified polyamine
according to the present invention;
b) from about 0.01 % by weight, of a surfactant system comprising:
i) from 0% to 100% by weight, of the surfactant system one or more
anionic surfactants;
ii) from 0% to 100% by weight, of the surfactant system one or more
nonionic surfactants;
iii) optionally from 0.1 % to about 80% by weight, of the surfactant system
one or more cationic surfactants;
iv) optionally from 0.1 % to about 80% by weight, of the surfactant system
one or more zwitterionic surfactants;
v) optionally from 0.1 % to about 80% by weight, of the surfactant system
one or more ampholytic surfactants; or
vi) mixtures thereof;
c) from about 1%, preferably from about 5% to about 80%, preferably to about
50%
by weight, of the laundry detergent composition, a peroxygen bleaching system
comprising:
i) from about 40%, preferably from about 50%, more preferably from about
60% to about 100%, preferably to about 95%, more preferably to about
80% by weight, of the bleaching system, a source of hydrogen peroxide;
17
CA 02389768 2002-04-08
WO 01/34748 PCT/US00/30645
ii) optionally from about 0. I %, preferably from about 0.5% to about 60%,
preferably to about 40% by weight, of the beaching system, a beach
activator;
iii) optionally from about 1 ppb (0.0000001%), more preferably from about
100 ppb (0.00001 %), yet more preferably from about 500 ppb
(0.00005%), still more preferably from about 1 ppm (0.0001 %) to about
99.9%, more preferably to about 50%, yet more preferably to about 5%,
still more preferably to about 500 ppm (0.05%) by weight of the
composition, of a transition-metal bleach catalyst;
iv) optionally from about 0.1 % by weight, of a pre-formed peroxygen
bleaching agent; and
d) the balance carriers and other adjunct ingredients.
Bleaching Agents - Hydrogen peroxide sources are described in detail in the
herein
incorporated Kirk Othmer's Encyclopedia of Chemical Technology, 4th Ed (1992,
John Wiley &
Sons), Vol. 4, pp. 271-300 "Bleaching Agents (Survey)", and include the
various forms of sodium
perborate and sodium percarbonate, including various coated and modified
forms.
Sources of hydrogen peroxide which are suitable for use in the compositions of
the
present invention include, but are not limited to, perborates, percarbonates,
perphosphates,
persulfates, and mixtures thereof. Preferred sources of hydrogen peroxide are
sodium perborate
monohydrate, sodium perborate tetrahydrate, sodium percarbonate and sodium
persulfate, more
preferably are sodium perborate monohydrate, sodium perborate tetrahydrate,
and sodium
percarbonate. When present the source of hydrogen peroxide is present at a
level of from about
40%, preferably from about 50%, more preferably from about 60% to about 100%,
preferably to
about 95%, more preferably to about 80% by weight, of the bleaching system.
Embodiments
which are bleach comprising pre-soak compositions may comprise from 5% to 99%
of the source
of hydrogen peroxide.
A preferred percarbonate bleach comprises dry particles having an average
particle size in
the range from about 500 micrometers to about 1,000 micrometers, not more than
about 10% by
weight of said particles being smaller than about 200 micrometers and not more
than about 10%
by weight of said particles being larger than about 1,250 micrometers.
Optionally, the
percarbonate can be coated with a silicate, borate or water-soluble
surfactants.
Bleach Activators
Preferably, the source of hydrogen peroxide (peroxygen bleach component) in
the
composition is formulated with an activator (peracid precursor). The activator
is present at levels
18
CA 02389768 2002-04-08
WO 01/34748 PCT/US00/30645
of from about 0.01%, preferably from about 0.5%, more preferably from about 1%
to about 15%,
preferably to about 10%, more preferably to about 8%, by weight of the
composition. Also,
bleach activators will comprise from about 0.1 % to about 60% by weight, of
the beaching system.
When the herein described bleaching system comprises 60% by weight, of an
activator (the
maximal amount) and said composition (bleaching composition, laundry
detergent, or otherwise)
comprises 15% by weight of said activator (the maximal amount by weight), said
composition
will comprise 25% by weight of a bleaching system (60% of which is bleach
activator, 40% a
source of hydrogen peroxide). However, this is not meant to restrict the
formulator to a 60:40
ratio of activator to hydrogen peroxide source.
Preferably the mole ratio of peroxygen bleaching compound (as Av0) to bleach
activator
in the present invention generally ranges from at least 1:1, preferably from
about 20:1, more
preferably from about 10:1 to about I :1, preferably to about 3:1.
Preferred activators are selected from the group consisting of tetraacetyl
ethylene diamine
(TAED), benzoylcaprolactam (BzCL), 4-nitrobenzoylcaprolactam, 3-
chlorobenzoylcaprolactam,
1 S benzoyloxybenzenesulphonate (BOBS), nonanoyloxybenzenesulphonate (NOBS),
phenyl
benzoate (PhBz), decanoyloxybenzenesulphonate (C,°-OBS),
benzoylvalerolactam (BZVL),
octanoyloxybenzenesulphonate (C8-OBS), perhydrolyzable esters and mixtures
thereof, most
preferably benzoylcaprolactam and benzoylvalerolactam. Particularly preferred
bleach activators
in the pH range from about 8 to about 9.5 are those selected having an OBS or
VL leaving group.
Preferred hydrophobic bleach activators include, but are not limited to,
nonanoyloxybenzenesulphonate (NOBS), 4-(N-(nonaoyl) amino hexanoyloxy]-benzene
sulfonate
sodium salt (NACA-OBS) an example of which is described in U.S. Patent No.
5,523,434,
dodecanoyloxybenzenesulphonate (LOBS or C,2 -OBS), 10-
undecenoyloxybenzenesulfonate
(UDOBS or C"-OBS with unsaturation in the 10 position), and decanoyloxybenzoic
acid
(DOBA).
Preferred bleach activators are those described in U.S. 5,698,504 Christie et
al., issued
December 16, 1997; U.S. 5,695,679 Christie et al. issued December 9, 1997;
U.S. 5,686,401
Willey et al., issued November 11, 1997; U.S. 5,686,014 Hartshorn et al.,
issued November 11,
1997; U.S. 5,405,412 Willey et al., issued April 11, 1995; U.S. 5,405,413
Willey et al., issued
April 11, 1995; U.S. 5,130,045 Mitchel et al., issued July 14, 1992; and U.S.
4,412,934 Chung et
al., issued November 1, 1983, and copending patent applications U. S. Serial
Nos. 08/709,072,
08/064,564; acyl lactam activators, as described in U.S. 5,698,504, U.S.
5,695,679 and U.S.
5,686,014, each of which is cited herein above, are very useful herein,
especially the acyl
19
WO 01/34748 CA 02389768 2002-04-08 pCT/US00/30645
caprolactams (see for example WO 94-28102 A) and acyl valerolactams, U.S.
5,503,639 Willey et
al., issued April 2, 1996 all of which are incorporated herein by reference.
Quaternary substituted bleach activators may also be included. The present
cleaning
compositions preferably comprise a quaternary substituted bleach activator
(QSBA) or a
quaternary substituted peracid (QSP); more preferably, the former. Preferred
QSBA structures
are further described in U.S. 5,686,015 Willey et al., issued November I 1,
1997; U.S. 5,654,421
Taylor et al., issued August 5, 1997; U.S. 5,460,747 Gosselink et al., issued
October 24, 1995;
U.S. 5,584,888 Miracle et al., issued December 17, 1996; and U.S. 5,578,136
Taylor et al., issued
November 26, I 996; all of which are incorporated herein by reference.
Highly preferred bleach activators useful herein are amide-substituted as
described in
U.S. 5,698,504, U.S. 5,695,679, and U.S. 5,686,014 each of which are cited
herein above.
Preferred examples of such bleach activators include: (6-octanamidocaproyl)
oxybenzenesulfonate, (6-nonanamidocaproyl)oxybenzenesulfonate,
(6-decanamidocaproyl)oxybenzenesulfonate and mixtures thereof.
Other useful activators, disclosed in U.S. 5,698,504, U.S. 5,695,679, U.S.
5,686,014
each of which is cited herein above and U.S. 4,966,723Hodge et al., issued
October 30, 1990,
include benzoxazin-type activators, such as a C~HQ ring to which is fused in
the 1,2-positions a
moiety --C(O)OC(R')=N-.
Depending on the activator and precise application, good bleaching results can
be
obtained from bleaching systems having with in-use pH of from about 6 to about
13, preferably
from about 9.0 to about 10.5. Typically, for example, activators with electron-
withdrawing
moieties are used for near-neutral or sub-neutral pH ranges. Alkalis and
buffering agents can be
used to secure such pH.
Transition Metal Bleach Catalyst
The laundry detergent compositions of the present invention optionally
comprises a
bleaching system which contains one or more bleach catalysts. Selected bleach
catalysts inter
alia 5,12-dimethyl-1,5,8,12-tertaaza-bicyclo[6.6.2]hexadecane manganese (II)
chloride may be
formulated into bleaching systems which do not require a source of hydrogen
peroxide or
peroxygen bleach. The compositions comprise from about 1 ppb (0.0000001%),
more preferably
from about 100 ppb (0.00001%), yet more preferably from about 500 ppb
(0.00005%), still more
preferably from about 1 ppm (0.0001 %) to about 99.9%, more preferably to
about 50%, yet more
preferably to about 5%, still more preferably to about 500 ppm (0.05%) by
weight of the
composition, of a transition-metal bleach catalyst
CA 02389768 2002-04-08
WO 01/34748 PCT/US00/30645
Non-limiting examples of suitable manganese-based catalysts are disclosed in
U.S.
5,576,282 Miracle et al., issued November 19, 1996; U.S. 5,246,621 Favre et
al., issued
September 21, 1993; U.S. 5,244,594 Favre et al., issued September 14, 1993;
U.S. 5,194,416
Jureller et al., issued March 16, 1993; U.S. 5,114,606 van Vliet et al.,
issued May 19, 1992; U.S.
4,430,243 Bragg, issued February 7, 1984; U.S. 5,114,611 van Kralingen, issued
May 19, 1992;
U.S. 4,728,455 Rerek, issued March 1, 1988; U.S. 5,284,944 Madison, issued
February 8, 1994;
U.S. 5,246,612 van Dijk et al., issued September 21, 1993; U.S. 5,256,779
Kerschner et al., issued
October 26, 2993; U.S. 5,280,117 Kerschner et al., issued January 18, 1994;
U.S. 5,274,147
Kerschner et al., issued December 28, 1993; U.S. 5,153,161 Kerschner et al.,
issued October 6,
1992; and U.S. 5,227,084 Martens et al., issued July 13, 1993; and European
Pat. App. Pub. Nos.
549,271 A 1, 549,272 A 1, 544,440 A2, and 544,490 A 1.
Non-limiting examples of suitable cobalt-based catalysts are disclosed in U.S.
5,597,936
Perkins et al., issued January 28, 1997; U.S. 5,595,967 Miracle et al., issued
January 21, 1997;
U.S. 5,703,030 Perkins et al., issued December 30, 1997; U.S. Patent 4,810,410
Diakun et al,
issued March 7,1989; M. L. Tobe, "Base Hydrolysis of Transition-Metal
Complexes", Adv. Inorg.
Bioino~g. Mech., (1983), 2, pages I-94; J. Chem. Ed. (1989), 66 (12), 1043-45;
The Synthesis and
Characterization of Inorganic Compounds, W.L. Jolly (Prentice-Hall; 1970), pp.
461-3; Inorg.
Chem., 18, 1497-1502 (1979); Inorg. Chem., 21, 2881-2885 (1982); Inorg.
Chern., 18, 2023-2025
( 1979); Inorg. Synthesis, 173-176 ( I 960); and Journal of Physical
Chemistry, 56, 22-25 ( 1952).
Further examples of preferred macrocyclic ligand comprising bleach catalysts
are
described in WO 98/39406 A1 published September 11, 1998 and included herein
by reference.
Suitable examples of these bleach catalysts include:
Dichloro-5,12-dimethyl-1,5,8,12-tetraazabicyclo[6.6.2]hexadecane manganese(II)
Diaquo-5,12-dimethyl-1,5,8,12-tetraazabicyclo[6.6.2]hexadecane manganese(II)
hexafluorophosphate
Aquo-hydroxy-5,12-dimethyl-1,5,8,12-tetraazabicyclo[6.6.2]hexadecane
manganese(III)
hexafluorophosphate
Diaquo-5,12-dimethyl-1,5,8,12-tetraazabicyclo[6.6.2]hexadecane manganese(II)
tetrafluoroborate
Dichloro-5,12-dimethyl-1,5,8,12-tetraazabicyclo[6.6.2]hexadecane
manganese(III)
hexafluorophosphate
Dichloro-5,12-di-n-butyl-1,5,8,12-tetraaza bicyclo[6.6.2]hexadecane
manganese(II)
Dichloro-5,12-dibenzyl-1,5,8,12-tetraazabicyclo[6.6.2]hexadecane manganese(II)
Dichloro-5-n-butyl-12-methyl-1,5,8,12-tetraaza- bicyclo[6.6.2]hexadecane
manganese(II)
Dichloro-5-n-octyl-12-methyl-1,5,8,12-tetraaza- bicyclo[6.6.2]hexadecane
manganese(II)
Dichloro-5-n-butyl-12-methyl-1,5,8,12-tetraaza- bicyclo[6.6.2]hexadecane
manganese(II).
21
CA 02389768 2002-04-08
WO 01/34748 PCT/US00/30645
Pre-formed Bleaching Agents
The bleaching systems of the present invention may optionally further comprise
from
0.1 %, preferably from 1 %, more preferably from 5% to about 10%, preferably
to about 7% by
weight, of one or more pre-formed bleaching agents. Pre-formed bleaching
materials typically
have the general formula:
O
HO-O- C- R- Y
wherein R is a C,-Cz2 alkylene, C,-CZ, substituted alkylene, phenylene, C~ C22
substituted
phenylene, and mixtures thereof, Y is hydrogen, halogen, alkyl, aryl, -C(O)OH,
-C(O)OOH, and
mixtures thereof.
The organic percarboxylic acids usable in the present invention can contain
either one or
two peroxy groups and can be either aliphatic or aromatic. When the organic
percarboxylic acid is
aliphatic, the unsubstituted acid has the general formula:
O
HO-O-C-(CHZ)~ Y
wherein Y can be hydrogen, methyl, methyl chloride, carboxylate,
percarboxylate; and n is an
integer having the value from 1 to 20.
When the organic percarboxylic acid is aromatic, the unsubstituted acid has
the general
formula:
O
HO-O-C / \ Y
wherein Y can be hydrogen, alkyl, haloalkyl, carboxylate, percarboxylate, and
mixtures thereof.
Typical monoperoxy percarboxylic acids useful herein include alkyl
percarboxylic acids
and aryl percarboxylic acids such as:
i) peroxybenzoic acid and ring-substituted peroxybenzoic acids, e.g., peroxy-o-
naphthoic acid;
ii) aliphatic, substituted aliphatic and arylalkyl monoperoxy acids, e.g.
peroxylauric
acid, peroxystearic acid, and N,N-phthaloylaminoperoxycaproic acid (PAP).
Typical diperoxy percarboxylic acids useful herein include alkyl diperoxy
acids and aryldiperoxy acids, such as:
iii) 1,12-diperoxydodecanedioic acid;
22
CA 02389768 2002-04-08
WO 01/34748 PCT/US00/30645
iv) 1,9-diperoxyazelaic acid;
v) diperoxybrassylic acid; diperoxysebacic acid and diperoxyisophthalic acid;
vi) 2-decyldiperoxybutane-1,4-dioic acid;
vii) 4,4'-sulfonybisperoxybenzoic acid.
A non-limiting example of a highly preferred pre-formed bleach includes 6-
nonylamino-6-
oxoperoxycaproic acid (NAPAA) as described in U.S. Pat. No. 4,634,551 Burns et
al., issued Jan. 6,
1987 included herein by reference.
As well as the herein described peroxygen bleaching compositions, the
compositions of
the present invention may also comprise as the bleaching agent a chlorine-type
bleaching
material. Such agents are well known in the art, and include for example
sodium
dichloroisocyanurate ("NaDCC"). However, chlorine-type bleaches are less
preferred for
compositions which comprise enzymes.
ADJUNCT INGREDIENTS
The following are non-limiting examples of adjunct ingredients useful in the
laundry
compositions of the present invention, said adjunct ingredients include
builders, optical
brighteners, soil release polymers, dye transfer agents, dispersents, enzymes,
suds suppressers,
dyes, perfumes, colorants, filler salts, hydrotropes, photoactivators,
fluorescers, fabric
conditioners, hydrolyzable surfactants, preservatives, anti-oxidants,
chelants, stabilizers, anti-
shrinkage agents, anti-wrinkle agents, germicides, fungicides, anti corrosion
agents, and mixtures
thereof.
Enzymes
The term "enzyme" or "detersive enzyme", as used herein, means any enzyme
having a
cleaning, stain removing or otherwise beneficial effect in a laundry, hard
surface cleaning, or
other cleaning formulation or composition as described herein. In general,
enzymes are present in
the compositions of the present invention at a level of from 0.0001 %, more
preferably from
0.0005%, most preferably from 0.001 % to 2%, preferably to 0.1 %, more
preferably to 0.02% by
weight, of pure enzyme. Preferred enzymes are hydrolases such as proteases,
amylases and
lipases. Preferred enzymes for liquid laundry purposes include, but are not
limited to, inter alia
proteases, cellulases, lipases and peroxidases.
Protease Enzymes
The preferred liquid laundry detergent compositions according to the present
invention
further comprise at least 0.001 % by weight, of a protease enzyme. However, an
effective amount
of protease enzyme is sufficient for use in the liquid laundry detergent
compositions described
23
CA 02389768 2002-04-08
WO 01/34748 PCT/US00/30645
herein. The term "an effective amount" refers to any amount capable of
producing a cleaning,
stain removal, soil removal, whitening, deodorizing, or freshness improving
effect on substrates
such as fabrics. In practical terms for current commercial preparations,
typical amounts are up to
about 5 mg by weight, more typically 0.01 mg to 3 mg, of active enzyme per
gram of the
detergent composition. Stated otherwise, the compositions herein will
typically comprise from
0.001% to 5%, preferably 0.01%-1% by weight of a commercial enzyme
preparation. The
protease enzymes of the present invention are usually present in such
commercial preparations at
levels sufficient to provide from 0.005 to 0.1 Anson units (AU) of activity
per gram of
composition.
Preferred liquid laundry detergent compositions of the present invention
comprise
modified protease enzymes derived from Bacillus arnyloliquefaciens or Bacillus
lentus. For the
purposes of the present invention, protease enzymes derived from B.
anzyloliguefacierZS are further
referred to as "subtilisin BPN"' also referred to as "Protease A" and protease
enzymes derived
from B. Lentus are further referred to as "subtilisin 309". For the purposes
of the present
invention, the numbering of Bacillus amyloliguefaciens subtilisin, as
described in the patent
applications of A. Baeck, et al, entitled "Protease-Containing Cleaning
Compositions" having US
Serial No. 08/322,676, serves as the amino acid sequence numbering system for
both subtilisin
BPN' and subtilisin 309.
Derivatives of Bacillus amylol~uefaciens subtilisin -BPN' enzymes
A preferred protease enzyme for use in the present invention is a variant of
Protease A
(BPN') which is a non-naturally occurring carbonyl hydrolase variant having a
different
proteolytic activity, stability, substrate specificity, pH profile and/or
perforn~ance characteristic as
compared to the precursor carbonyl hydrolase from which the amino acid
sequence of the variant
is derived. This variant of BPN' is disclosed in EP 130,756 A, January 9,
1985.
Protease B
A preferred protease enzyme for use in the present invention is Protease B.
Protease B is
a non-naturally occurring carbonyl hydrolase variant having a different
proteolytic activity,
stability, substrate specificity, pH profile and/or performance characteristic
as compared to the
precursor carbonyl hydrolase from which the amino acid sequence of the variant
is derived.
Protease B is a variant of BPN' in which tyrosine is replaced with leucine at
position +217 and as
further disclosed in EP 303,761 A, April 28, 1987 and EP 130,756 A, January 9,
1985.
Protease C
A preferred protease enzyme for use in the compositions of the present
invention Protease
C. Protease C is a variant of an alkaline serine protease from Bacillus in
which lysine replaced
24
CA 02389768 2002-04-08
WO 01/34748 PCT/US00/30645
arginine at position 27, tyrosine replaced valine at position 104, serine
replaced asparagine at
position 123, and alanine replaced threonine at position 274. Protease C is
described in EP
90915958:4, corresponding to WO 91/06637, Published May 16, 1991. Genetically
modified
variants, particularly of Protease C, are also included herein.
Protease D
A preferred protease enzyme for use in the present invention is Protease D.
Protease D is a carbonyl hydrolase variant derived from Bacillus lentus
subtilisin as described in
WO 95/10615 published April 20, 1995 by Genencor International.
A further preferred protease enzyme for use in combination with the modified
polyamines
of the present invention is ALCALASE~ from Novo. Another suitable protease is
obtained from
a strain of Bacillus, having maximum activity throughout the pH range of 8-12,
developed and
sold as ESPERASE~ by Novo Industries A/S of Denmark, hereinafter "Novo". The
preparation
of this enzyme and analogous enzymes is described in GB 1,243,784 to Novo.
Other suitable
proteases include SAVINASE~ from Novo and MAXATASE~ from International Bio-
Synthetics, Inc., The Netherlands. See also a high pH protease from Bacillus
sp. NCIMB 40338
described in WO 9318140 A to Novo. Enzymatic detergents comprising protease,
one or more
other enzymes, and a reversible protease inhibitor are described in WO 9203529
A to Novo.
Other preferred proteases include those of WO 9510591 A to Procter & Gamble .
When desired, a
protease having decreased adsorption and increased hydrolysis is available as
described in WO
9507791 to Procter & Gamble. A recombinant trypsin-like protease for
detergents suitable herein
is described in WO 9425583 to Novo.
Other particularly useful proteases are described in PCT Application Nos.
PCT/US98/22588, PCT/US98/22482 and PCT/US98/22486 all filed on October 23,
1998 from
The Procter & Gamble Company.
Also suitable for the present invention are proteases described in patent
applications EP
251 446 and WO 91/06637, protease BLAP~ described in W091/02792 and their
variants
described in WO 95/23221.
See also a high pH protease from Bacillus sp. NCIMB 40338 described in WO
93/18140 A to Novo. Enzymatic detergents comprising protease, one or more
other enzymes, and
a reversible protease inhibitor are described in WO 92/03529 A to Novo. When
desired, a
protease having decreased adsorption and increased hydrolysis is available as
described in WO
95/07791 to Procter & Gamble. A recombinant trypsin-like protease for
detergents suitable
herein is described in WO 94/25583 to Novo. Other suitable proteases are
described in EP 516
200 by Unilever.
CA 02389768 2002-04-08
WO 01/34748 PCT/US00/30645
Commercially available proteases useful in the present invention are known as
ESPERASE~, ALCALASE~, DURAZYM~, SAVINASE~, EVERLASE~ and KANNASE~
all from Novo Nordisk A/S of Denmark, and as MAXATASE~, MAXACAL~, PROPERASE~
and MAXAPEM~ all from Genencor International (formerly Gist-Brocades of The
Netherlands).
A preferred protease for use in the present invention is the protease enzyme
as described in
W099/20771 published April 29, 1999.
In addition to the above-described protease enzymes, other enzymes suitable
for use in
the liquid laundry detergent compositions of the present invention are further
described herein
below.
Other Enzymes
Enzymes in addition to the protease enzyme can be included in the present
detergent
compositions for a variety of purposes, including removal of protein-based,
carbohydrate-based,
or triglyceride-based stains from surfaces such as textiles, for the
prevention of refugee dye
transfer, for example in laundering, and for fabric restoration. Suitable
enzymes include
amylases, lipases, cellulases, peroxidases, and mixtures thereof of any
suitable origin, such as
vegetable, animal, bacterial, fungal and yeast origin. Preferred selections
are influenced by
factors such as pH-activity and/or stability optima, thermostability, and
stability to active
detergents, builders and the like. In this respect bacterial or fungal enzymes
are preferred, such as
bacterial amylases and proteases, and fungal cellulases.
Amylases suitable herein include, for example, a-amylases described in GB
1,296,839 to
Novo; RAPIDASE~, International Bio-Synthetics, lnc. and TERMAMYL~, Novo.
FUNGAMYL~ from Novo is especially useful. Engineering of enzymes for improved
stability,
e.g., oxidative stability, is known. See, for example J. Biological Chem.,
Vol. 260, No. I 1, June
1985, pp 6518-6521 and WO 9402597 to Novo, Feb. 3, 1994, and WO 9509909 A to
Novo.
Cellulases usable herein include both bacterial and fungal types, preferably
having a pH
optimum between 5 and 9.5. U.S. 4,435,307, Barbesgoard et al, March 6, 1984,
discloses
suitable fungal cellulases from Humicola insolens or Humicola strain DSM 1800
or a cellulase
212-producing fungus belonging to the genus Aeromonas, and cellulase extracted
from the
hepatopancreas of a marine mollusk, Dolabella Auricula Solander. Suitable
cellulases are also
disclosed in GB-A-2.075.028; GB-A-2.095.275 and DE-OS-2.247.832. CAREZYME~
(Novo)
is especially useful. See also WO 9117243 to Novo.
Suitable lipase enzymes for detergent usage include those produced by
microorganisms of
the Pseudomonas group, such as Pseudomonas stutzeri ATCC 19.154, as disclosed
in GB
1,372,034. See also lipases in Japanese Patent Application 53,20487, laid open
Feb. 24, 1978.
26
CA 02389768 2002-04-08
WO 01/34748 PCT/US00/30645
This lipase is available from Amano Pharmaceutical Co. Ltd., Nagoya, Japan,
under the trade
name Lipase P "Amano," or "Amano-P." Other suitable commercial lipases include
Amano-CES,
lipases ex Chromobacter viscosum, e.g. Chromobacter viscosum var. lipolyticum
NRRLB 3673
from Toyo Jozo Co., Tagata, Japan; Chromobacter viscosum lipases from U.S.
Biochemical
Corp., U.S.A. and Disoynth Co., The Netherlands, and lipases ex Pseudomonas
gladioli.
LIPOLASE~ enzyme derived from Humicola lanuginosa and commercially available
from
Novo, see also EP 341,947, is a preferred lipase for use herein. Lipase and
amylase variants
stabilized against peroxidase enzymes are described in WO 9414951 A to Novo.
See also WO
9205249 and RD 94359044.
Cutinase enzymes suitable for use herein are described in WO 8809367 A to
Genencor.
Peroxidase enzymes may be used in combination with oxygen sources, e.g.,
percarbonate,
perborate, hydrogen peroxide, etc., for "solution bleaching" or prevention of
transfer of dyes or
pigments removed from substrates during the wash to other substrates present
in the wash
solution. Known peroxidases include horseradish peroxidase, ligninase, and
haloperoxidases such
as chloro- or bromo-peroxidase. Peroxidase-containing detergent compositions
are disclosed in
WO 89099813 A, October 19, 1989 to Novo and WO 8909813 A to Novo.
A range of enzyme materials and means for their incorporation into synthetic
detergent
compositions is also disclosed in WO 9307263 A and WO 9307260 A to Genencor
International,
WO 8908694 A to Novo, and U.S. 3,553,139 McCarty et al., issued January 5,
1971. Enzymes
are further disclosed in U.S. 4,101,457 Place et al, issued July 18, 1978, and
U.S. 4,507,219
Hughes, issued March 26, 1985. Enzyme materials useful for liquid detergent
formulations, and
their incorporation into such formulations, are disclosed in U.S. 4,261,868
Hora et al., issued
April 14, 1981. Enzymes for use herein can be stabilized by various
techniques. Enzyme
stabilization techniques are disclosed and exemplified in U.S. 3,600,319 Gedge
et al., issued
August 17, 1971; EP 199,405 and EP 200,586, October 29, 1986, Venegas. Enzyme
stabilization
systems are also described, for example, in U.S. 3,519,570. A useful Bacillus,
sp. AC13 giving
proteases, xylanases and cellulases, is described in WO 9401532 A to Novo.
Enzyme Stabilizing System
Enzyme-containing, including but not limited to, liquid compositions, herein
may
comprise from about 0.001%, preferably from about 0.005%, more preferably from
about 0.01%
to about 10%, preferably to about 8%, more preferably to about 6% by weight,
of an enzyme
stabilizing system. The enzyme stabilizing system can be any stabilizing
system which is
compatible with the detersive enzyme. Such a system may be inherently provided
by other
formulation actives, or be added separately, e.g., by the formulator or by a
manufacturer of
27
CA 02389768 2002-04-08
WO 01/34748 PCT/US00/30645
detergent-ready enzymes. Such stabilizing systems can, for example, comprise
calcium ion, boric
acid, propylene glycol, short chain carboxylic acids, boronic acids, and
mixtures thereof, and are
designed to address different stabilization problems depending on the type and
physical form of
the detergent composition.
One stabilizing approach is the use of water-soluble sources of calcium and/or
magnesium ions in the finished compositions which provide such ions to the
enzymes. Calcium
ions are generally more effective than magnesium ions and are preferred herein
if only one type of
canon is being used. Typical detergent compositions, especially liquids, will
comprise from
about 1 to about 30, preferably from about 2 to about 20, more preferably from
about 8 to about
12 millimoles of calcium ion per liter of finished detergent composition,
though variation is
possible depending on factors including the multiplicity, type and levels of
enzymes incorporated.
Preferably water-soluble calcium or magnesium salts are employed, including
for example
calcium chloride, calcium hydroxide, calcium formate, calcium malate, calcium
maleate, calcium
hydroxide and calcium acetate; more generally, calcium sulfate or magnesium
salts corresponding
to the exemplified calcium salts may be used. Further increased levels of
Calcium and/or
Magnesium may of course be useful, for example for promoting the grease-
cutting action of
certain types of surfactant.
Another stabilizing approach is by use of borate species disclosed in U.S.
4,537,706
Severson, issued August 27, 1985. Borate stabilizers, when used, may be at
levels of up to 10%
or more of the composition though more typically, levels of up to about 3% by
weight of boric
acid or other borate compounds such as borax or orthoborate are suitable for
liquid detergent use.
Substituted boric acids such as phenylboronic acid, butaneboronic acid, p-
bromophenylboronic
acid or the like can be used in place of boric acid and reduced levels of
total boron in detergent
compositions may be possible though the use of such substituted boron
derivatives.
Stabilizing systems of certain cleaning compositions may further comprise from
0,
preferably from about 0.01 % to about 10%, preferably to about 6% by weight,
of chlorine bleach
scavengers, added to prevent chlorine bleach species present in many water
supplies from
attacking and inactivating the enzymes, especially under alkaline conditions.
While chlorine
levels in water may be small, typically in the range from about 0.5 ppm to
about 1.75 ppm, the
available chlorine in the total volume of water that comes in contact with the
enzyme, for
example during fabric-washing, can be relatively large; accordingly, enzyme
stability to chlorine
in-use is sometimes problematic. Since perborate or percarbonate, which have
the ability to react
with chlorine bleach, may present in certain of the instant compositions in
amounts accounted for
separately from the stabilizing system, the use of additional stabilizers
against chlorine, may,
28
CA 02389768 2002-04-08
WO 01/34748 PCT/US00/30645
most generally, not be essential, though improved results may be obtainable
from their use.
Suitable chlorine scavenger anions are widely known and readily available,
and, if used, can be
salts containing ammonium cations with sulfite, bisulfate, thiosulfite,
thiosulfate, iodide, etc.
Antioxidants such as carbamate, ascorbate, etc., organic amines such as
ethylenediaminetetraacetic acid (EDTA) or alkali metal salt thereof,
monoethanolamine (MEA),
and mixtures thereof can likewise be used. Likewise, special enzyme inhibition
systems can be
incorporated such that different enzymes have maximum compatibility. Other
conventional
scavengers such as bisulfate, nitrate, chloride, sources of hydrogen peroxide
such as sodium
perborate tetrahydrate, sodium perborate monohydrate and sodium perearbonate,
as well as
phosphate, condensed phosphate, acetate, benzoate, citrate, formate, lactate,
malate, tartrate,
salicylate, etc., and mixtures thereof can be used if desired. In general,
since the chlorine
scavenger function can be performed by ingredients separately listed under
better recognized
functions, (e.g., hydrogen peroxide sources), there is no absolute requirement
to add a separate
chlorine scavenger unless a compound performing that function to the desired
extent is absent
from an enzyme-containing embodiment of the invention; even then, the
scavenger is added only
for optimum results. Moreover, the formulator will exercise a chemist's normal
skill in avoiding
the use of any enzyme scavenger or stabilizer which is majorly incompatible,
as formulated, with
other reactive ingredients, if used. In relation to the use of ammonium salts,
such salts can be
simply admixed with the detergent composition but are prone to adsorb water
and/or liberate
ammonia during storage. Accordingly, such materials, if present, are desirably
protected in a
particle such as that described in US 4,652,392 Baginski et al., issued March
24, 1987.
Builders - The laundry detergent compositions of the present invention
preferably
comprise one or more detergent builders or builder systems. When present, the
compositions will
typically comprise at least about 1% builder, preferably from about 5%, more
preferably from
about 10% to about 80%, preferably to about 50%, more preferably to about 30%
by weight, of
detergent builder.
The level of builder can vary widely depending upon the end use of the
composition and
its desired physical form. When present, the compositions will typically
comprise at least about
1% builder. Formulations typically comprise from about S% to about 50%, more
typically about
5% to about 30%, by weight, of detergent builder. Granular formulations
typically comprise
from about 10% to about 80%, more typically from about 15% to about 50% by
weight, of the
detergent builder. Lower or higher levels of builder, however, are not meant
to be excluded.
Inorganic or P-containing detergent builders include, but are not limited to,
the alkali
metal, ammonium and alkanolammonium salts of polyphosphates (exemplified by
the
29
WO 01/34748 CA 02389768 2002-04-08 pCT~S00/30645
tripolyphosphates, pyrophosphates, and glassy polymeric meta-phosphates),
phosphonates, phytic
acid, silicates, carbonates (including bicarbonates and sesquicarbonates),
sulphates, and
aluminosilicates. However, non-phosphate builders are required in some
locales. Importantly,
the compositions herein function surprisingly well even in the presence of the
so-called "weak"
builders (as compared with phosphates) such as citrate, or in the so-called
"underbuilt" situation
that may occur with zeolite or layered silicate builders.
Examples of silicate builders are the alkali metal silicates, particularly
those having a
Si02:Na20 ratio in the range 1.6:1 to 3.2: I and layered silicates, such as
the layered sodium
silicates described in U.S. 4,664,839 Rieck, issued May 12, 1987. NaSKS-6 is
the trademark for
a crystalline layered silicate marketed by Hoechst (commonly abbreviated
herein as "SKS-6").
Unlike zeolite builders, the Na SKS-6 silicate builder does not contain
aluminum. NaSKS-6 has
the delta-Na2Si05 morphology form of layered silicate. It can be prepared by
methods such as
those described in German DE-A-3,417,649 and DE-A-3,742,043. SKS-6 is a highly
preferred
layered silicate for use herein, but other such layered silicates, such as
those having the general
formula NaMSix02x+1 ~yH20 wherein M is sodium or hydrogen, x is a number from
1.9 to 4,
preferably 2, and y is a number from 0 to 20, preferably 0 can be used herein.
Various other
layered silicates from Hoechst include NaSKS-5, NaSKS-7 and NaSKS-11, as the
alpha, beta and
gamma forms. As noted above, the delta-Na2Si05 (NaSKS-6 form) is most
preferred for use
herein. Other silicates may also be useful such as for example magnesium
silicate, which can
serve as a crispening agent in granular formulations, as a stabilizing agent
for oxygen bleaches,
and as a component of suds control systems.
Examples of carbonate builders are the alkaline earth and alkali metal
carbonates as
disclosed in German Patent Application No. 2,321,001 published on November 15,
1973.
Aluminosilicate builders are useful in the present invention. Aluminosilicate
builders are
of great importance in most currently marketed heavy duty granular detergent
compositions, and
can also be a significant builder ingredient in liquid detergent formulations.
Aluminosilicate
builders include those having the empirical formula:
[Mz(zA102)y]~xH20
wherein z and y are integers of at least 6, the molar ratio of z to y is in
the range from 1.0 to about
0.5, and x is an integer from about 15 to about 264.
Useful aluminosilicate ion exchange materials are commercially available.
These
aluminosilicates can be crystalline or amorphous in structure and can be
naturally-occurring
aluminosilicates or synthetically derived. A method for producing
aluminosilicate ion exchange
materials is disclosed in U.S. 3,985,669, Krummel et al, issued October 12,
1976. Preferred
WO 01/34748 CA 02389768 2002-04-08 pCT~S00/30645
synthetic crystalline aluminosilicate ion exchange materials useful herein are
available under the
designations Zeolite A, Zeolite P (B), Zeolite MAP and Zeolite X. In an
especially preferred
embodiment, the crystalline aluminosilicate ion exchange material has the
formula:
Nal2[(A102)12(Si02)12~~xH20
wherein x is from about 20 to about 30, especially about 27. This material is
known as Zeolite A.
Dehydrated zeolites (x = 0 - 10) may also be used herein. Preferably, the
aluminosilicate has a
particle size of about 0.1-10 microns in diameter.
Organic detergent builders suitable for the purposes of the present invention
include, but
are not restricted to, a wide variety of polycarboxylate compounds. As used
herein, "poly-
carboxylate" refers to compounds having a plurality of carboxylate groups,
preferably at least 3
carboxylates. Polycarboxylate builder can generally be added to the
composition in acid form,
but can also be added in the form of a neutralized salt. When utilized in salt
form, alkali metals,
such as sodium, potassium, and lithium, or alkanolammonium salts are
preferred.
Included among the polycarboxylate builders are a variety of categories of
useful mate-
rials. One important category of polycarboxylate builders encompasses the
ether polycarboxy-
lates, including oxydisuccinate, as disclosed in U.S. 3,128,287 Berg, issued
April 7, 1964, and
U.S. 3,635,830 Lamberti et al., issued January 18, 1972. See also "TMS/TDS"
builders of U.S.
4,663,071 Bush et al., issued May 5, 1987. Suitable ether polycarboxylates
also include cyclic
compounds, particularly alicyclic compounds, such as those described in U.S.
3,923,679 Rapko,
issued December 2, 1975; U.S. 4,158,635 Crutchfield et al., issued June 19,
1979; U.S. 4,120,874
Crutchfield et al., issued October 17, 1978; and U.S. 4,102,903 Crutchfield et
al., issued July 25,
1978.
Other useful detergency builders include the ether hydroxypolycarboxylates,
copolymers
of malefic anhydride with ethylene or vinyl methyl ether, 1, 3, 5-trihydroxy
benzene-2, 4, 6-
trisulphonic acid, and carboxymethyloxysuccinic acid, the various alkali
metal, ammonium and
substituted ammonium salts of polyacetic acids such as ethylenediamine
tetraacetic acid and
nitrilotriacetic acid, as well as polycarboxylates such as mellitic acid,
succinic acid, oxydisuccinic
acid, polymaleic acid, benzene 1,3,5-tricarboxylic acid,
carboxymethyloxysuccinic acid, and
soluble salts thereof.
Citrate builders, e.g., citric acid and soluble salts thereof (particularly
sodium salt), are
polycarboxylate builders of particular importance for heavy duty liquid
detergent formulations
due to their availability from renewable resources and their biodegradability.
Citrates can also be
used in granular compositions, especially in combination with zeolite and/or
layered silicate
builders. Oxydisuccinates are also especially useful in such compositions and
combinations.
31
CA 02389768 2002-04-08
WO 01/34748 PCT/US00/30645
Also suitable in the detergent compositions of the present invention are the
3,3-dicar-
boxy-4-oxa-1,6-hexanedioates and the related compounds disclosed in U.S.
4,566,984, Bush,
issued January 28, 1986. Useful succinic acid builders include the C5-C20
alkyl and alkenyl
succinic acids and salts thereof. A particularly preferred compound of this
type is do-
decenylsuccinic acid. Specific examples of succinate builders include:
laurylsuccinate,
myristylsuccinate, palmitylsuccinate, 2-dodecenylsuccinate (preferred), 2-
pentadecenylsuccinate,
and the like. Laurylsuccinates are the preferred builders of this group, and
are described in
European Patent Application 86200690.5/0,200,263, published November 5, 1986.
Other suitable polycarboxylates are disclosed in U.S. 4,144,226, Crutchfield
et al., issued
March 13, 1979 and in U.S. 3,308,067, Diehl, issued March 7, 1967. See also
Diehl U.S. Patent
3,723,322.
Fatty acids, e.g., C 12-C 1 g monocarboxylic acids, can also be incorporated
into the
compositions alone, or in combination with the aforesaid builders, especially
citrate and/or the
succinate builders, to provide additional builder activity. Such use of fatty
acids will generally
result in a diminution of sudsing, which should be taken into account by the
formulator.
In situations where phosphorus-based builders can be used, and especially in
the for-
mutation of bars used for hand-laundering operations, the various alkali metal
phosphates such as
the well-known sodium tripolyphosphates, sodium pyrophosphate and sodium
orthophosphate
can be used. Phosphonate builders such as ethane-1-hydroxy-1,1-diphosphonate
and other
known phosphonates (see, for example, U.S. Patents 3,159,581; 3,213,030;
3,422,021; 3,400,148
and 3,422,137) can also be used.
Dispersants
A description of other suitable polyalkyleneimine dispersants which may be
optionally
combined with the bleach stable dispersants of the present invention can be
found in U.S.
4,597,898 Vander Meer, issued July 1, 1986; European Patent Application
111,965 Oh and
Gosselink, published June 27, 1984; European Patent Application 1 I 1,984
Gosselink, published
June 27, 1984; European Patent Application 112,592 Gosselink, published July
4, 1984; U.S.
4,548,744 Connor, issued October 22, 1985; and U.S. 5,565,145 Watson et al.,
issued October 15,
1996; all of which are included herein by reference. However, any suitable
clay/soil dispersant or
anti-redepostion agent can be used in the laundry compositions of the present
invention.
In addition, polymeric dispersing agents which include polymeric
polycarboxylates and
polyethylene glycols, are suitable for use in the present invention. Polymeric
polycarboxylate
materials can be prepared by polymerizing or copolymerizing suitable
unsaturated monomers,
preferably in their acid form. Unsaturated monomeric acids that can be
polymerized to form
32
CA 02389768 2002-04-08
WO 01/34748 PCT/US00/30645
suitable polymeric polycarboxylates include acrylic acid, malefic acid (or
malefic anhydride),
fumaric acid, itaconic acid, aconitic acid, mesaconic acid, citraconic acid
and methylenemalonic
acid. The presence in the polymeric polycarboxylates herein or monomeric
segments, containing
no carboxylate radicals such as vinylmethyl ether, styrene, ethylene, etc. is
suitable provided that
such segments do not constitute more than about 40% by weight.
Particularly suitable polymeric polycarboxylates can be derived from acrylic
acid. Such
acrylic acid-based polymers which are useful herein are the water-soluble
salts of polymerized
acrylic acid. The average molecular weight of such polymers in the acid form
preferably ranges
from about 2,000 to 10,000, more preferably from about 4,000 to 7,000 and most
preferably from
about 4,000 to 5,000. Water-soluble salts of such acrylic acid polymers can
include, for example,
the alkali metal, ammonium and substituted ammonium salts. Soluble polymers of
this type are
known materials. Use of polyacrylates of this type in detergent compositions
has been disclosed,
for example, in U.S. 3,308,067 Diehl, issued March 7, 1967.
Acrylic/maleic-based copolymers may also be used as a preferred component of
the
1 S dispersing/anti-redeposition agent. Such materials include the water-
soluble salts of copolymers
of acrylic acid and malefic acid. The average molecular weight of such
copolymers in the acid
form preferably ranges from about 2,000, preferably from about 5,000, more
preferably from
about 7,000 to 100,000, more preferably to 75,000, most preferably to 65,000.
The ratio of
acrylate to maleate segments in such copolymers will generally range from
about 30:1 to about
1:1, more preferably from about 10:1 to 2:1. Water-soluble salts of such
acrylic acid/maleic acid
copolymers can include, for example, the alkali metal, ammonium and
substituted ammonium
salts. Soluble acrylate/maleate copolymers of this type are known materials
which are described
in European Patent Application No. 66915, published December 15, 1982, as well
as in EP
193,360, published September 3, 1986, which also describes such polymers
comprising
hydroxypropylacrylate. Still other useful dispersing agents include the
maleic/acrylic/vinyl
alcohol terpolymers. Such materials are also disclosed in EP 193,360,
including, for example, the
45/45/10 terpolymer of acrylic/maleic/vinyl alcohol.
Another polymeric material which can be included is polyethylene glycol (PEG).
PEG
can exhibit dispersing agent performance as well as act as a clay soil removal-
antiredeposition
agent. Typical molecular weight ranges for these purposes range from about 500
to about
100,000, preferably from about 1,000 to about 50,000, more preferably from
about 1,500 to about
10,000.
33
CA 02389768 2002-04-08
WO 01/34748 PCT/US00/30645
Polyaspartate and polyglutamate dispersing agents may also be used, especially
in
conjunction with zeolite builders. Dispersing agents such as polyaspartate
preferably have a
molecular weight (avg.) of about 10,000.
Soil Release A ents
S The compositions according to the present invention may optionally comprise
one or
more soil release agents. If utilized, soil release agents will generally
comprise from about
0.01 %, preferably from about 0. I %, more preferably from about 0.2% to about
10%, preferably to
about 5%, more preferably to about 3% by weight, of the composition. Polymeric
soil release
agents are characterized by having both hydrophilic segments, to hydrophilize
the surface of
hydrophobic fibers, such as polyester and nylon, and hydrophobic segments, to
deposit upon
hydrophobic fibers and remain adhered thereto through completion of the
laundry cycle and, thus,
serve as an anchor for the hydrophilic segments. This can enable stains
occuring subsequent to
treatment with the soil release agent to be more easily cleaned in later
washing procedures.
The following, all included herein by reference, describe soil release
polymers suitable
for use in the present invention. U.S. 5,843,878 Gosselink et al., issued
December 1, 1998; U.S.
5,834,412 Rohrbaugh et al., issued November 10, 1998; U.S. 5,728,671 Rohrbaugh
et al., issued
March 17, 1998; U.S. 5,691,298 Gosselink et al., issued November 25, 1997;
U.S. 5,599,782 Pan
et al., issued February 4, 1997; U.S. 5,415,807 Gosselink et al., issued May
16, 1995; U.S.
5,182,043 Morrall et al., issued January 26, 1993; U.S. 4,956,447 Gosselink et
al., issued
September 11, 1990; U.S. 4,976,879 Maldonado et al. issued December 11, 1990;
U.S. 4,968,451
Scheibel et al., issued November 6, 1990; U.S. 4,925,577 Borcher, Sr. et al.,
issued May 15,
1990; U.S. 4,861,512 Gosselink, issued August 29, 1989; U.S. 4,877,896
Maldonado et al., issued
October 31, 1989; U.S. 4,771,730 Gosselink et al., issued October 27, 1987;
U.S. 711,730
Gosselink et al., issued December 8, 1987; U.S. 4,721,580 Gosselink issued
January 26, 1988;
U.S. 4,000,093 Nicol et al., issued December 28, 1976; U.S. 3,959,230 Hayes,
issued May 25,
1976; U.S. 3,893,929 Basadur, issued July 8, 1975; and European Patent
Application 0 219 048,
published April 22, 1987 by Kud et al.
Further suitable soil release agents are described in U.S. 4,201,824 Voilland
et al.; U.S.
4,240,918 Lagasse et al.; U.S. 4,525,524 Tung et al.; U.S. 4,579,681 Ruppert
et al.; U.S.
4,220,918; U.S. 4,787,989; EP 279,134 A, 1988 to Rhone-Poulenc Chemie; EP
457,205 A to
BASF (1991); and DE 2,335,044 to Unilever N.V., 1974; all incorporated herein
by reference.
METHOD OF USE
The present invention further relates to a method for removing hydrophobic
soils, inter
alia, body oils, perspiration and other human body soils form fabric,
preferably clothing, said
34
WO 01/34748 CA 02389768 2002-04-08 pCT~S00/30645
method comprising the step of contacting fabric in need of cleaning with an
aqueous solution
containing at least 0.01 % by weight, of a laundry detergent composition
comprising:
A) from about 0.01% by weight of a hydrophobically modified polyamine having
the formula:
R1
(R~)2N- R-~N~ R~ n N(R~)2 X
Q Q Q
wherein R is CS-CZ° linear or branched alkylene, and mixtures thereof;
R' is an
alkyleneoxy unit having the formula:
(Rz0)X R3
wherein RZ is C,-CQ linear or branched alkylene, and mixtures thereof; R' is
an
anionic unit, and mixtures thereof; x is from about 15 to about 30; Q is a
hydrophobic quaternizing unit selected from the group consisting of C8-C",
linear
or branched alkyl, C~,-C3° cycloalkyl, C,-C3° substituted or
unsubstituted
alkylenearyl, and mixtures thereof; X is an anion present in sufficient amount
to
provide electronic neutrality; n is from 0 to 4;
B) from about 0.01 % by weight, of a surfactant system comprising one or more
surfactants selected from:
i) from 0% to 100% by weight, of one or more anionic surfactants;
ii) from 0% to 100% by weight, of one or more nonionic surfactants;
iii) optionally from 0.1 % to about 80% by weight, of one or more cationic
surfactants;
iv) optionally from 0.1 % to about 80% by weight, of one or more
zwitterionic surfactants;
v) optionally from 0.1 % to about 80% by weight, of one or more
ampholytic surfactants; or
vi) mixtures thereof;
C) the balance carriers and adjunct ingredients
Preferably the aqueous solution comprises at least about 0.01 % ( 100 ppm),
preferably at
least about 1 % ( 1000 ppm)by weight, of said laundry detergent composition.
The compositions of the present invention can be suitably prepared by any
process chosen
by the formulator, non-limiting examples of which are described in U.S.
5,691,297 Nassano et al.,
issued November 11, 1997; U.S. 5,574,005 Welch et al., issued November 12,
1996; U.S.
CA 02389768 2002-04-08
WO 01/34748 PCT/US00/30645
5,569,645 Dinniwell et al., issued October 29, 1996; U.S. 5,565,422 Del Greco
et al., issued
October 15, 1996; U.S. 5,516,448 Capeci et al., issued May 14, 1996; U.S.
5,489,392 Capeci et
al., issued February 6, 1996; U.S. 5,486,303 Capeci et al., issued January 23,
1996 all of which
are incorporated herein by reference.
EXAMPLE 1
Synthesis of ethoxylated (E20) bis(hexamethylene)triamine
tribenzyl quaternary ammonium bromide
Ethoxylation of Bis(hexamethylene)triamine to Average E20 per NH - The
ethoxylation
is conducted in a 2 gallon stirred stainless steel autoclave equipped for
temperature measurement
and control, pressure measurement, vacuum and inert gas purging, sampling, and
for introduction
of ethylene oxide as a liquid. A ~20 1b. net cylinder of ethylene oxide (ARC)
is set up to deliver
ethylene oxide as a liquid by a pump to the autoclave with the cylinder placed
on a scale so that
the weight change of the cylinder could be monitored.
A 362 g portion of Bis(hexamethylene)triamine (BHMT) (m.w. 215, (Aldrich),
1.68
moles, 5.04 moles nitrogen, 8.4 moles ethoxylatable (NH) sites, is added to
the autoclave. The
autoclave is then sealed and purged of air (by applying vacuum to minus 28" Hg
followed by
pressurization with nitrogen to 250 psia, then venting to atmospheric
pressure). The autoclave
contents are heated to 80 °C while applying vacuum. After about one
hour, the autoclave is
charged with nitrogen to about 250 psia while cooling the autoclave to about
105 °C. Ethylene
oxide is then added to the autoclave incrementally over time while closely
monitoring the
autoclave pressure, temperature, and ethylene oxide flow rate. The ethylene
oxide pump is turned
off and cooling is applied to limit any temperature increase resulting from
any reaction exotherm.
The temperature is maintained between 100 and 110 °C while the total
pressure is allowed to
gradually increase during the course of the reaction. After a total of 370
grams of ethylene oxide
(8.4 moles) has been charged to the autoclave, the temperature is increased to
110 °C and the
autoclave is allowed to stir for an additional 2 hours. At this point, vacuum
is applied to remove
any residual unreacted ethylene oxide.
Next, vacuum is continuously applied while the autoclave is cooled to about 50
°C while
introducing 181.5 g of a 25% sodium methoxide in methanol solution (0.84
moles, to achieve a
10% catalyst loading based upon ethoxylatable sites functions). The methoxide
solution is
removed from the autoclave under vacuum and then the autoclave temperature
controller setpoint
is increased to 100 °C. A device is used to monitor the power consumed
by the agitator. The
36
CA 02389768 2002-04-08
WO 01/34748 PCT/US00/30645
agitator power is monitored along with the temperature and pressure. Agitator
power and
temperature values gradually increase as methanol is removed from the
autoclave and the
viscosity of the mixture increases and stabilizes in about 1.5 hours
indicating that most of the
methanol has been removed. The mixture is further heated and agitated under
vacuum for an
additional 30 minutes.
Vacuum is removed and the autoclave is cooled to 105 °C while it is
being charged with
nitrogen to 250 psia and then vented to ambient pressure. The autoclave is
charged to 200 Asia
with nitrogen. Ethylene oxide is again added to the autoclave incrementally as
before while
closely monitoring the autoclave pressure, temperature, and ethylene oxide
flow rate while
maintaining the temperature between 100 and 110 °C and limiting any
temperature increases due
to reaction exotherm. After the addition of 4180 g of ethylene oxide ( 95 mol,
resulting in a total
of 20 moles of ethylene oxide per mole of ethoxylatable sites on BHMT), the
temperature is
increased to 110 °C and the mixture stirred for an additional 2 hours.
The reaction mixture is then collected into a 22 L three neck round bottomed
flask purged
with nitrogen. The strong alkali catalyst is neutralized by slow addition of
80.7 g methanesulfonic
acid (0.84 moles) with heating (100 °C) and mechanical stirring. The
reaction mixture is then
removed of residual ethylene oxide and deodorized by sparging an inert gas
(argon or nitrogen)
into the mixture through a gas dispersion frit while agitating and heating the
mixture to 120 °C for
1 hour. The final reaction product is cooled slightly and stored in a glass
container purged with
nitrogen.
Quaternization of BHMT E20 to 90 mol% (3 mol N per mol polymer) - Into a
weighed,
1000m1, 3 neck round bottom flask fitted with argon inlet, condenser, addition
funnel,
thermometer, mechanical stirring and argon outlet (connected to a bubbler) is
added BHMT E020
(522.8g, 0.333 mol N, 98% active, m.w.-4615) under argon. The material is
heated to 80°C with
stirring until melted. Next, benzyl bromide (61.6g, 0.36mo1, Aldrich, m.w.-
171.04) is slowly
added to the melted BHMT E020 using an addition funnel over a period of 10
minutes. The
reaction complete after stirring at 80°C for 6 hours. The reaction
mixture is dissolved in SOOg
water and adjusted to pH>7 using 1N NaOH followed by transfer to a plastic
container for
storage.
Sulfation of BHMT E20 to 90% - Under argon, the reaction mixture from the
quaternization step is cooled to 5°C using an ice bath (BHMT E20,
90+mol% quat, 0.59 mol
OH). Chlorosulfonic acid (72g, 0.61 mol, 99%, mw-I 16.52) is slowly added
using an addition
37
CA 02389768 2002-04-08
WO 01/34748 PCT/US00/30645
funnel. The temperature of the reaction mixture is not allowed to rise above
10°C. The ice bath
is removed and the reaction is allowed to rise to room temperature. After 6
hrs. the reaction is
complete. The reaction is again cooled to 5°C and sodium methoxide
(264g, 1.22 mol, Aldrich,
25% in methanol, m.w.-54.02) is slowly added to the rapidly stirred mixture.
The temperature of
the reaction mixture is not allowed to rise above 10°C. The reaction
mixture is transferred to a
single neck round bottom flask. Purified water (1300m1) is added to the
reaction mixture and the
methylene chloride, methanol and some water is stripped off on a rotary
evaporator at 50°C. The
clear, light yellow solution is transferred to a bottle for storage. The final
product pH is checked
and adjusted to ~9 using 1 N NaOH or 1 N HCl as needed.
The following are non-limiting examples of the compositions according to the
present
mvenrion.
TABLEI
weight
Ingredients 2 3 4 5
C,4 C,5 alkyl E1.0 sulfate22.5 22.5 22.5 22.5
Linear alkyl benzene 3.0 3.0 3.0 3.0
sulfonate
C, amidopropyl DMA 1.5 1.5 1.5 1.5
C,2-C,4 alkyl E7.0 3.0 3.0 3.0 3.0
Citric Acid 2.5 2.5 2.5 2.5
C,z-C,8 alkyl fatty acid3.5 3.5 3.5 3.5
Rapeseed fatty acid 5.0 5.0 5.0 5.0
protease 0.8 1.57 1.57 1.57
amylase 0.055 0.088 0.088 0.088
cellulase 0.188 0.055 0.055 0.055
lipolase 0.06 -- -- --
mannanase 0.007 0.0033 0.0033 0.0033
Sodium metaborate 2.0 2.5 2.5 2.5
Ca formate/CaClz 0.02 0.10 0.10 0.10
Modified polyamine '
Bleach catalyst z 0.035 0.034 0.034 0.034
Hydrophobic dispersant' 0.65 0.76 0.76 0.76
Soil release agent 4 0.147 -- -- --
38
CA 02389768 2002-04-08
WO 01/34748 PCT/US00/30645
Soil release agent -- 0.10 0.10 0.10
Suds suppresser 0.60 0.60 0.60 0.60
Water and minors balance balance balance balance
1. Hydrophobically modified polyamine according to Example 1.
2. 1,5-bis(hydroxymethylene)-3,7-dimethyl-2,4-bis(2-pyridyl)-3,7-
diazabicyclo[3.3.1 ]-nonan-9-
ol manganese(II) dichloride 1/2H20.
3. PEI 189 E15-18 according to U.S. Patent 4,597,898 Vander Meer, issued July
1, 1986.
S 4. Soil release agent according to U.S. Patent 4,702,857 Gosselink, issued
October 27, 1987.
5. Soil release agent according to U.S. Patent 4,968,451, Scheibel et al.,
issued November 6,
1990.
The following examples include compositions which comprise an adjunct
bleaching
agent.
TABLE II
weight
Ingredients 6 7 8 9
Sodium CI 1-C13 alkylbenzene-sulfonate13.3 13.7 10.4 11.1
Sodium C 14-C 15 alcohol sulfate3.9 4.0 4.5 11.2
Sodium C14-C15 alcohol ethoxylate2.0 2.0 -- --
(0.5)
sulfate
Sodium C14-C15 alcohol ethoxylate0.5 0.5 0.5 1.0
(6.5)
Tallow fatty acid -- -- -- 1.1
Sodium tripolyphosphate -- 41.0 -- --
Zeolite A, hydrate (0.1-10 26.3 -- 21.3 28.0
micron size)
Sodium carbonate 23.9 12.4 25.2 16.1
Sodium Polyacrylate (45%) 3.4 -- 2.7 3.4
Sodium silicate (1:6 ratio 2.4 6.4 2.1 2.6
Na0/Si02)(46%)
Sodium sulfate 10.5 10.9 8.2 15.0
Sodium perborate 1.0 1.0 5.0 --
Poly(ethyleneglycol), MW 4000 1.7 0.4 1.0 1.1
(50%)
Citric acid -- -- 3.0 --
Bleach catalyst' 0.035 0.030 0.034 0.028
Bleach activator Z -- -- 5.9 --
39 .
WO 01/34748 CA 02389768 2002-04-08 pCT~S00/30645
Soil release agent 3 -- 0.10 0.10 0.10
Polyamine 4
Suds suppresser 0.60 0.60 0.60 0.60
Water and minors 5 balance balancebalancebalance
1. 1,5-bis(hydroxymethylene)-3,7-dimethyl-2,4-bis(2-pyridyl)-3,7-
diazabicyclo[3.3.1 ]-nonan-9-
ol manganese(II) dichloride 1/2H20.
2. Nonyl ester of sodium p-hydroxybenzene-sulfonate.
3. Soil release agent according to U.S. 5,415,807 Gosselink et al., issued May
16, 1995.
4. Hydrophobically modified polyamine according to Example 1.
5. Balance to 100% can, for example, include minors like optical brightener,
perfume, soil
dispersant, chelating agents, dye transfer inhibiting agents, additional
water, and fillers,
including CaC03, talc, silicates, etc.
The following is a non-limiting example of the bleaching system of the present
invention
I O in the absence of a source of hydrogen peroxide.
TABLE III
weight
Ingredients 10 11 12 13
Sodium C1 I-C13 alkylbenzene-sulfonate13.3 13.7 10.4 1 I.1
Sodium C 14-C I 5 alcohol 3.9 4.0 4.5 11.2
sulfate
Sodium C 14-C I 5 alcohol 2.0 2.0 -- --
ethoxylate (0.5)
sulfate
Sodium C14-C15 alcohol ethoxylate0.5 0.5 0.5 1.0
(6.5)
Tallow fatty acid -- -- -- 1.1
Sodium tripolyphosphate -- 41.0 -- --
Zeolite A, hydrate (0.1-10 26.3 -- 21.3 28.0
micron size)
Sodium carbonate 23.9 12.4 25.2 16.1
Sodium Polyacrylate (45%) 3.4 -- 2.7 3.4
Sodium silicate (I :6 ratio 2.4 6.4 2.1 2.6
Na0/Si02)(46%)
Sodium sulfate 10.5 10.9 8.2 15.0
Poly(ethyleneglycol), MW 40001.7 0.4 I .0 I .1
(50%)
Citric acid -- -- 3.0 --
Bleach catalyst' 0.10 0.07 0.035 0.028
Hydrophobically modified polyamine
Z
CA 02389768 2002-04-08
WO 01/34748 PCT/US00/30645
Hydrophobic dispersant 5 0.65 0.76 0.76 0.76
Soil release agent G 0.147 0.10 0.10 0.10
Suds suppresser 0.60 0.60 0.60 0.60
Water and minors' balancebalance balancebalance
1. 1,5-bis(hydroxymethylene)-3,7-dimethyl-2,4-bis(2-pyridyl)-3,7-
diazabicyclo[3.3.1 ]-nonan-9-
ol manganese(II) dichloride 1/2Hz0.
2. Hydrophobically modified polyamine according to Example 1.
3. Potassium sulfite.
4. PEI 189 E15-18 according to U.S. Patent 4,597,898 Vander Meer, issued July
1, 1986.
6. Soil release agent according to U.S. 5,415,807 Gosselink et al., issued May
16, 1995.
7. Balance to 100% can, for example, include minors like optical brightener,
perfume, soil
dispersant, chelating agents, dye transfer inhibiting agents, additional
water, and fillers,
including CaC03, talc, silicates, etc.
The compositions of the present invention can be suitably prepared by any
process chosen
by the formulator, non-limiting examples of which are described in U.S.
5,691,297 Nassano et al.,
issued November 11, 1997; U.S. 5,574,005 Welch et al., issued November 12,
1996; U.S.
5,569,645 Dinniwell et al., issued October 29, 1996; U.S. 5,565,422 Del Greco
et al., issued
October 15, 1996; U.S. 5,516,448 Capeci et al., issued May 14, 1996; U.S.
5,489,392 Capeci et
al., issued February 6, 1996; U.S. 5,486,303 Capeci et al., issued January 23,
1996 all of which
are incorporated herein by reference.
41