Note: Descriptions are shown in the official language in which they were submitted.
i
' CA 02390879 2002-05-09
COMMUNICATION SYSTEM FOR THE INTERACTION BETWEEN MEDICAL
LABORATORIES AND MEDICAL TREATMENT CENTERS
Description
The invention relates to a communication system for the interaction between
medical laboratories and medical treatment centers.
Due to the high cost for material and personnel, a medical laboratory is
typically
operated centrally. For physicians in an individual practice, such laboratory
is
frequently set up as a shared laboratory, whereas university hospitals and
other
hospitals tend to operate a central laboratory with several specialized
departments.
Many laboratories have started to format the test results in such a way that
they
can be recalled from the commissioning hospital ward or medical practice via
data transmission. Although this expedites the transmission of the completed
test
data, accepting and processing orders is still handled in a conventional
manner.
A laboratory is therefore unable to fully plan its workload ahead of time,
since a
decision about the equipment and materials, etc., can only be made after an
order and the corresponding sample materials have been received. Frequently,
orders have to transferred to a second, more highly specialized laboratory, or
~i
CA 02390879 2002-05-09
tests cannot be started due to a high workload, which can delay order
processing, often to the detriment of the patient.
It is therefore an object of the invention to provide a communication system
for
the aforementioned purpose which can optimize and accelerate order processing
and balance the workload.
The object is solved by the invention with the characterizing features of
claim 1 in
conjunction with the features recited in the preamble. Advantageous
embodiments of the invention are recited in the dependent claims.
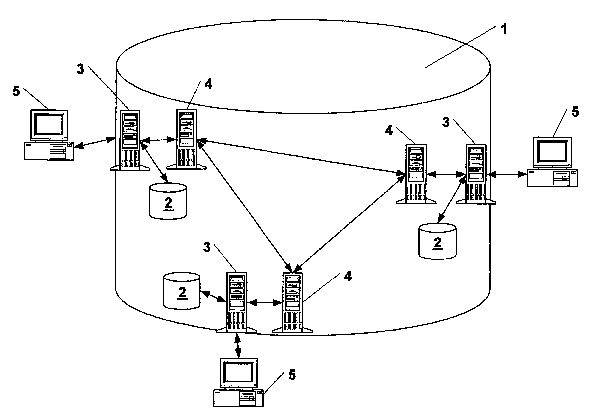
Accordingly, all laboratories participating in the communication system and
their
specialized laboratory departments, on one hand, and medical treatment
facilities
on the other hand are networked with an integrated central laboratory database
1
via data transmission systems 6. The integrated central laboratory database 1
is
used for the following purposes:
- the required patient and laboratory data are acquired in an acquisition
module
of a data input device located at the commissioning treatment facility and
forwarded to the respective laboratory or laboratory department via data
output
devices located at that laboratory or laboratory department under coordination
by
the integrated central laboratory database 1,
si
CA 02390879 2002-05-09
- samples to be tested are labeled andlor encoded in the treatment facilities
by
acquisition modules according to predetermined criteria and sent to the
respective laboratory or laboratory department,
- any already preordered tests are activated when the samples arrive at the
laboratory or laboratory department, and
- the test results are stored after completion of the tests in the integrated
central
laboratory database 1 and transmitted via data transmission devices 6 to the
commissioning treatment facility.
The invention will be described hereinafter with reference to embodiments that
are at least in part illustrated in the drawings.
It is shown in:
Fig. 1 a schematic diagram of a communication system,
Fig. 2 a network environment of a local laboratory site.
The client requests that the laboratory determines certain parameters. To
determine these parameters, the laboratory has to examine the received test
materials. The test samples are distributed from the material receiving
station to
the appropriate laboratory site, where they are logged in. The test result is
then
immediately transmitted via data transmission.
3
~i
~ ' ~ ' CA 02390879 2002-05-09
All the data generated during this process - from the initial inquiry, through
ordering process, gathering the test results, to obtaining information about
treatment methods andlor previous illnesses - are stored for each patient in
an
integrated central laboratory database 1. This database can be located at a
separate database server 3. Advantageously, however, the integrated central
laboratory database can also exist in form of several identical copies 2 which
are
stored on different servers 3 connected to a data transmission network 6. Each
of
these copies 2 provides complete functionality. All information concerning
changes made on one copy 2 are transmitted to the other copies via the data
transmission network 6, which guarantees that all copies have identical
information and data. For this reason, reference is made herein to only a
single
virtual integrated central laboratory database 1, even if copies are
distributed
across several servers.
An exemplary detailed process flow is as follows:
Each client 5, i.e., each commissioning hospital ward and/or each
commissioning
physician's office has a data input module that is networked with the
integrated
central laboratory database 1 in the laboratory. When an order is initiated,
the
required patient data and order data are entered in the data input module.
These
include: requester, patient, all parameters to be determined on the sample,
optionally also the diagnosis, and the tests and equipment, on which the tests
are
to be performed and which are required to determine the parameters. This can
4
~i
CA 02390879 2002-05-09
be accomplished by using direct ISDN connections, WAN connections via ISDN,
Internet connections or email. Labels required for the samples are also
printed.
Annotations related to pre-analysis, completion date and the like can also be
added. Pre-analysis refers to the treatment of the samples before testing and
can
relate to merely storing the samples or to their preparation for the
subsequent
tests.
After the suspected diagnoses or the diagnoses according to the ICD 9 and ICD
classification (International Classification of Diseases) have been entered,
the
requisition module identifies stored laboratory diagnostic guidelines and
proposes a standardized palette of laboratory tests reflecting the major and
minor
diagnoses. The generated process can be reviewed and has a defined quality
standard for the prevention as well as the diagnosis and treatment of
diseases.
Any tests that are not indicated for the selected diseases are eliminated.
Test
that are absolutely necessary for the prevention, diagnosis and therapy cannot
be missed. The patient identification system also checks any laboratory test
results that remain unchanged during a predefined interval. The client is
informed
about the results that are already confirmed, even if he did not request the
tests.
Diagnoses are thereby available online to each participating medical facility
to
facilitate cooperative treatment.
The data input module is advantageously executed on an IBM-compatible PC.
For example, any documents accompanying the order can be scanned in.
i
CA 02390879 2002-05-09
After the data are entered, the order can be immediately confirmed or set
aside.
Orders that have been set aside are collected in an order wait queue and can
be
confirmed at a later date. Optionally, another laboratory can be selected to
accept the order. The labels are preferably printed after the order is
confirmed,
so that all laboratory data can be considered.
With this method, work locations can be concentrated at a single laboratory
site;
however, the work locations can also be spread over several laboratory sites.
The system is adapted to provide laboratory test results to regional health
facilities (a hospital, a physicians' network, rehab clinics, nursing homes, a
physician's office). It is an important feature that the database 1 associates
all
diagnoses with the patient. Every partner in the health organization who is
part
of the supply network can view this electronic patient folder, if the patient
grants
permission. This approach eliminates duplication of procedures, accelerates
the
course of the treatment andlor time for a diagnosis, and enhances quality. A
complex system of this type will advantageously employ the aforedescribed
integrated central laboratory database that is distributed across several
servers.
This takes advantage of the particular feature of the invention, since the
integrated central laboratory database 1 optimizes the process flow in this
complex system. According to the invention, when an order is placed, the
laboratories are selected based on the compatibility of their equipment with
the
~i
CA 02390879 2002-05-09
requested tests and their actual workload at that time, whereby transportation
and traffic routes can be optimized for economy and convenient scheduling.
Appropriate nearby laboratories are selected and new orders are assigned based
on the most favorable shipping routes. In special situations and emergencies,
the
most suited facilities can be identified, since the associated facilities are
listed in
hierarchical order.
When the material arrives, the laboratory activates the previously placed
order.
Samples that are intended, for example, for a clinical or hematology
laboratory
can hence be routed directly to the corresponding laboratory site.
A sample can be logged in at the laboratory by placing the sample in front of
a
barcode reader, with the data associated with the sample being displayed, for
example, on a monitor.
The received patient data are transmitted to a patient identification module.
This
module can return several results:
- If the patient is uniquely identified, the order is processed immediately.
- If no patient was identified, then the patient data included with the order
are
checked for completeness. If the data are sufficient, then a new patient data
set is generated and the order is processed immediately. If the data are
insufficient, then a message is generated that individual data will first have
to
be collected in the laboratory, for example, by a telephone inquiry.
si
CA 02390879 2002-05-09
After the tests are completed, the results are transmitted to the
communication
system and subjected to a delta-check. In a delta-check, the measured value is
compared to a previously determined parameter for the patient, to a standard
parameter range and/or to a fixed technical limit. Any detected errors are
immediately processed at designated work places. Optionally, a follow-up test
can be requested during the delta-check. Conversely, all results falling in a
"release" range are released. In addition, some results may need to be
validated
by a medical specialist.
The communication system also allows the laboratory physician to access the
medical record of the patient, because the medical history of the patient
provides
important information about a possible diagnosis and treatment strategy. At a
minimum, data relating to diagnoses, drugs and any chronic illnesses of the
patient should be accessible.
A patient is frequently treated at different facilities. The facilities
connected to the
communication system (hospitals, physicians' offices, laboratories, nursing
homes, ...) can hereby advantageously view the electronic patient folder,
since
the integrated central laboratory database 1 stores all information about the
entire course of the treatment relating to the patient. The result is a
cumulative
summary of all ambulatory and in-patient services. This transparency of events
i
' ' ' CA 02390879 2002-05-09
makes it easier to arrive at a diagnosis and can accelerate the therapy and
make
it more successful.
The test results advantageously include an editable section where the
laboratory
physician can insert annotations and comments.
For some parameters, a decision is made based on the results from a single
test
to optionally perform additional tests, which can be requested automatically.
Some diseases have to be reported by law. If a laboratory detects such a
disease in a patient, then this event is reported to the health department and
the
attending physician. A similar procedure applies to highly infectious
bacteria. If
these are detected, the finding is reported to the hospital ward and/or the
physician so as to immediately initiate preventive measures.
The data can be transmitted from the integrated central laboratory database 1
via
email, network printer, fax or LDT. Transmission via LDT should be feasible as
an email attachment or directly via ISDN. Several parallel transmission paths
can
also be provided.
Advantageously, all transmissions of the test results are logged with date and
time, laboratory department, type of diagnosis and the like.
9
CA 02390879 2002-05-09
A custom result layout can be designed for each hospital ward andlor
physician's
office, wherein the parameters obtained in the tests can be presented in a
certain
order andlor arrangement.
The fundamental process flow can be summarized as follows, regardless if the
communication system is used by an individual laboratory, a group of
laboratories or an entire health service region:
A client (a physician in a hospital or in private practice) initiates a
laboratory
order. The database books the order and prints at the client's site
corresponding
labels for the materials. When the client enters the patient's diagnosis
according
to the international diagnostic code ICD or DRG, certain tests that should be
ordered are proposed to the client. The client is also informed about
available
possibilities for receiving and shipping the samples.
After the order is submitted, the order is activated in the system and the
database plans, based on a predetermined hierarchical structure, at which work
site (laboratory A, B, C, ...) the tests should be performed. The condition of
the
test equipment (load, quality) plays hereby a significant role. The workflow
through the laboratory is optimized through specific order disposition,
allowing
the results to be returned to the client in less time than would otherwise be
possible.
The communication system has the advantage that the requester as well as the
laboratory always have an overview over the current status of arriving,
activated
io
CA 02390879 2002-05-09
and completed requests for testing, allowing them to react promptly. When
problems with materials are encountered, actions can be taken in close
cooperation with the patient.
The database automatically reacts to critical test results after their
technical
release, contacts immediately the requester who ordered the laboratory tests
and
optionally orders additional tests. A complex diagnosis can be generated at a
display monitor across several sites by consulting with another colleague
working
at a clinic, optionally directly at the patient's bed.
Repeat examinations are avoided. The system also helps minimizing the amount
of information and the cost associated with managing the information. All the
data are stored centrally and are available - not only to the original
requester - for
performing follow-up tests and displaying the patient's history.
Integrated with the system is an inventory administration module which Nlogs
out"
consumables and reagents for each test and administers supplies for the
workplace by minimizing the inventory for each workplace. The order volume and
quantity discounts can be optimally managed across the networked laboratories.
In addition, the module coordinates the shipments of blood-drawing equipment
and other accessories to the party that requested the laboratory tests. The
hospital wards check in the material received from each supplier. In this way,
n
i
CA 02390879 2002-05-09
transaction data an be generated automatically and the inventory at the
requester's site can be determined.
A local laboratory site advantageously includes in addition to the integrated
central laboratory database 1 andlor a local copy of the database 2, the
database
server 3 and the replication server 4, also laboratory workstations 9 which
are
connected via device drivers 12 to additional devices, such as card readers 18
or
online devices 19. The laboratory database 1 can be accessed externally, for
example, from a hospital ward 20, a physician's office 21 or, for a remote
diagnosis 22, via a web access server 8. A communication server 7 controls the
data exchange between fax machine 14, printer 15, the workstations 17 located
at the different stations as well as other devices for file transfer. The
basic
system advantageously includes a diagnosis server 11, as well as minicomputers
connected to the laboratory database 1 via a dedicated server 10.
The invention is not limited to the illustrated embodiments. It is feasible to
arrive
at different embodiments by combining the aforedescribed means and features,
without deviating form the scope of the invention.
m
~i
~ - CA 02390879 2002-05-09
List of reference numerals
1. (virtual) integrated central laboratory database
2. local database
3. database server
4. replication server
5. client
6. data transmission device
7. communication server
8. web access server
9. laboratory workstation
10.dedicated server
11.diagnosis server
12.device driver
13.minicomputer
14.fax machine, fax software
15.printer
16.file transfer
17.hospital ward workstation
18.card reader
19.online device
20.hospital ward
21.physicians' office
22.remote diagnosis
13