Note: Descriptions are shown in the official language in which they were submitted.
CA 02391710 2002-01-29
= ,
-1-
METHOD AND DEVICE FOR CATALYTICALLY TREATING
EXHAUST GAS CONTAINING DUST AND OXYGEN
Description
In chemical processes or during the combustion of fossil or secondary fuels -
such
as garbage or processed garbage - exhaust gases are produced, which apart from
other pollutants also contain sulfur oxides and nitrogen oxides. Among
experts, the
sulfur oxides (SO2 and S03) are referred to as SOX, and the nitrogen oxides
(NO,
N02 and N20) are referred to as NOX. Sulfur oxides and nitrogen oxides are
gase-
ous pollutants which act as toxins to the environment and must therefore be re-
moved from the exhaust gases, before the same get into the atmosphere. In the
preceding years, considerable efforts were made to reduce the emissions of
sulfur
oxides and nitrogen oxides. In connection with the denitrification of exhaust
gases
several processes are being employed. The process most frequently used at pre-
sent is the SCR process (SCR = Selective Catalytic Reduction). In this
process,
ammonia or ammonium-containing compounds are introduced into the catalyst-
containing reaction chamber, and the nitrogen oxides in the flue gas are
reacted to
obtain nitrogen and steam. In connection with the SCR process it is reported
that
in the case of S02-containing exhaust gases sulfuric acid and ammonium hydro-
gen sulfate are formed. The formation of sulfuric acid and ammonium hydrogen
sulfate is undesired, as in those parts of the plant which are disposed behind
the
reactor they lead to considerable corrosion problems. In the case of S02-
containing exhaust gases, separate desulfurization plants are therefore
generally
provided before the SCR process, which desulfurization plants operate
according
to the principle of the dry or wet flue gas desulfurization plant (REA). In
the case of
wet processes, the exhaust gas is cooled and reheated for the subsequent SCR
process, which is the case in most power plants and garbage incineration
plants.
Such processes involve high costs, and the formation of CaS03 corresponding to
the reaction CaO + SO2 > CaSO3 cannot be avoided. The presence of CaS03
in landfill materials is a hazard to the environment.
CA 02391710 2005-04-21
2
EP-A-0 671 201 describes a process for separating sulfur trioxide and for
denitrifi-
cation - in particular in garbage incineration plarits - ammonia or ammonium-
containing compounds being introduced into the flue gas stream before a heat
ex-
changer package, preferably before the last heat exchanger package, or before
the flue gas cleaning, so that the catalytic denitrification of the dedusted
flue gases
is then effected in the low-temperature range, in particular between 100 C and
280 C. The object is to reduce the SO3 concentration before the SCR reactor by
forming ammonium sulfate. The disadvantage of this process consists in that
not
only ammonium sulfate aerosols are formed, but also ammonium hydrogen sul-
fate, which later on is precipitated on the catalysts. The ammonium sulfate
aero-
sols can hardly be dedusted in succeeding filter means, so that they represent
a
considerable burden to the environment. Moreover, a separate gas washer is nec-
essary for the removal of SO2. The flue gas must be reheated behind the gas
washer, which is not achieved by heat exchange alone. Thus, an additional
firing
means, e.g. a surface burner with natural gas, is reiquired. Disadvantages
include
high investment and operating costs.
It is the object underlying the invention to develop a process for the
simultaneous
desulfurization and denitrification without the formation of ammonium sulfate
or
ammonium hydrogen sulfate, wherein NOx is decomposed to obtain N2 and N20.
This object is solved in that the treatment of the extiaust gases containing
sulfur
oxides and nitrogen oxides is performed by a process, at temperatures in the
range from 200 C to 500 C by means of reducing agents in a reactor which is
equipped with solid catalyst with flow passages, in which the free opening
surface of the catalyst is more than 50% and in which the passages of the
catalyst have a hydraulic diameter of more than 2mrn, wherein:
- the treatment is performed in the piresence of and/or with the
addition of one or more substances selected from the group including free
oxides, carbonates, hydroxides of calcium, magnesium, sodium and potassium,
- said substances being present in said exhaust gases prior to
contact of said exhaust gases with said catalyst; and
CA 02391710 2005-04-21
2a
- that during the treatment the operating conditions of the gas flow in
the free reaction space corresponding to the Froude numbers lie in the range
of
7
I :53 / 4 = p = 9 < 100 with 2 Fr'
g=dk Pk Pg g=dk
in which:
p = the relative gas speed in m/s
Fr = the Froude number
CA 02391710 2002-01-29
-3-
p9 = the density of the gas in kg/m3
Pk = the density of the solid particle in kg/m3
dk = the diameter of the spherical dust particle in m
g = the gravitational constant in m/s2
Surprisingly, it was noted that despite the approximately stoichiometric
operation
of the NH3/NOX ratios, a degree of denitrification of 95 % to 98 % and a
degree of
desulfurization of 80 % to 90 % can be achieved with the inventive process,
the
formation of ammonium sulfate, ammonium hydrogen sulfate and sulfuric acid be-
ing avoided. This advantage is based on the fact that in the catalytic
treatment not
only NOX is converted to nitrogen and steam, but also SO2 is converted to SO3
and
incorporated in the presence of free oxides, carbonates, hydroxides of
calcium,
magnesium, sodium and potassium. The formation of ammonium sulfates, ammo-
nium bisulfates and sulfuric acid is suppressed. These incorporated sulfates
of
calcium, magnesium, sodium and potassium can very easily be separated and
utilized in a succeeding filter plant, e.g. a bag collector or electrostatic
precipitator.
A preferred aspect of the invention is the use of honeycomb or plate
catalysts,
which apart from titanium dioxide and tungsten contain more than 0.5 wt-% vana-
dium pentoxide. The catalytic conversion is increased. In accordance with a
par-
ticularly preferred aspect of the invention, the catalysts preferably contain
2 % to 8
% vanadium pentoxide. With this operation, degrees of denitrification and
desulfu-
rization of more than 95 % are achieved.
Another preferred aspect is the treatment in the presence of and/or with the
addi-
tion of one or more substances selected from the group including free oxides,
car-
bonates, hydroxides of calcium, magnesium, sodium and potassium, with an aver-
age particle size d50 between 5 pm and 100 pm. The removal of the sulfur
oxides
is effected very quickly with little consumption of additives.
Furthermore, costs are preferably minimized by the treatment in the presence
of
and/or with the addition of one or more substances selected from the group in-
cluding free oxides, carbonates, hydroxides of calcium, as calcium compounds
are
more economic as compared to alkali compounds.
CA 02391710 2002-01-29
-4-
As reducing agent, NH3-releasing compounds such as (NH4)2SO4, (NHa)2CO3,
(NH4)HCO3, (COONH3)2H20, HCOONH4, NH3, NH4OH, H20-CO-NH2, NH2CN,
Ca(CN)2, NaOCN, C2H4N4, C3H6N6 and NH3-containing waste waters from photo-
chemical plants, singly or several of them, are introduced into the flue gas
stream
at several points in the gaseous, liquid or solid condition at temperatures in
the
range between 20000 and 1000 C before entering the catalytic reactor.
A preferred aspect consists in that as reducing agent NH3-releasing compounds
in
the form of dilute aqueous solutions are introduced into the flue gas stream,
pref-
erably at temperatures in the range between 300 C and 550 C. The partial steam
pressure in the reaction space is increased and thus the improvement of the in-
corporation of sulfur is achieved.
A particularly preferred aspect of the invention is the presence or addition
of one
or more substances selected from the group including free oxides, carbonates,
hydroxides of calcium, magnesium, sodium and potassium to the flue gas stream
before the addition of NH3-releasing compounds. The formation of ammmonium
hydrogen sulfate, ammonium sulfate and sulfuric acid is suppressed completely.
Flow to the reactor can be effected from above or from below. A particularly
pre-
ferred aspect of the invention consists in that the flow to the reactor
equipped with
the catalysts can alternately be effected from above and from below. By means
of
this alternate flow, the reactor can easily be kept clean of dust-laden
exhaust
gases, and blockage of the passages by dust can be avoided. Furthermore, the
service life of the catalysts can be increased by alternating the flow to the
reactor.
Another preferred aspect of the invention consists in that beside the
breakdown of
sulfur oxide and nitrogen oxide, the reactor equipped with catalyst can at the
same
time be used for the breakdown of halogen compounds, halogenated organic
compounds, hydrocarbons and CO.
A preferred aspect of the invention consists in that the reactor equipped with
cata-
lyst can be used for the breakdown of sulfur oxides and nitrogen oxides in
dust-
laden exhaust gases in the chemical and metallurgical industries as well as in
the
cement and lime industries, in power plants and in garbage incineration plants
in
CA 02391710 2005-04-21
the process flow at temperatures in the range between 200 C and 500 C without
additional preheating of the exhaust gas.
The object of the invention is also solved by means of an apparatus for the
treatment of dust- and oxygen-containing exhaust gases of a cement factory,
which exhaust gases in an exhaust gas strearrr contain sulfur oxides and
nitrogen oxides, comprising:
- a reactor (19) equipped with a catalyst (20), said reactor (19) being
disposed in the exhaust gas stream behind a cyclone heat exchanger
(13) (and before a raw material grinder (21) and before a by-pass I to
an evaporative cooler (22),
- the treatment being performed in the presence of one or more
substances selected from the group consisting of free oxides,
carbonates, hydroxides of calcium, magnesium, sodium and
potassium, said substances being preserit in said exhaust gases prior
to contact of said exhaust gases with said catalyst.
The drawings represent examples of the apparatus for performing above
mentioned the process, which are explained in deta'il below. In the drawings:
Fig. 1 schematically shows an arrangement of the apparatus in the cement
industry;
Fig. 2 schematically shows an apparatus for the cement industry;
Fig. 3 schematically shows an arrangement of the apparatus for power
plants;
Fig. 4 schematically shows an apparatus for power plants.
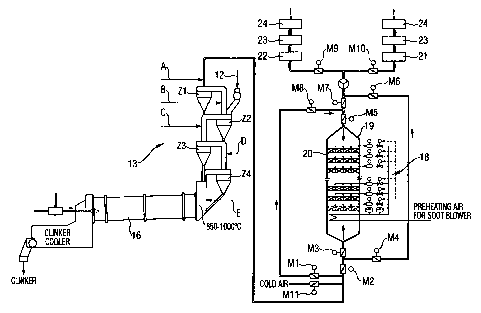
Fig. 1 shows the arrangement of the inventive apparatus in a cement factory
with
rotary kiln 16 for the production of clinker. The SCR reactor 19 with catalyst
mod-
ules 20 and dust blowers 18 is arranged in flow direction behind the
suspension-
type cyclone heat exchanger 13 comprising the cyclones Zl to Z4 which are con-
CA 02391710 2005-04-21
5a
nected with each other. For metering Ni-l3-releaslrig uompounds, a plurality
of,
points A, B, C, D and E are provided at temperatures in the range from 300 C
to
1000 C. For metering ammonia, ammonia solution or urea solution, the metering
points A, B and C are preferred. For metering NH3-containing waste water from
phototechnical plants and other compounds of 'NH3, the metering points D and E
are preferably used. The calcium-containing raw meal 12 is charged between the
cyclones Z1 and Z2. After the treatment in the SCR reactor 19, the exhaust gas
is
either supplied to the chimney 24 via the raw material grinder 21 and the
dedust-
ing means 23 in the case of a combined operation, or is supplied to the
chimney
24 via the evaporative cooler 22 and the dedusting means 23 in the case of a
di-
rect operation.
Fig. 2 shows an apparatus with gas conduit from the bottom to the top, from
the
top to the bottom and alternately from below and from above.
CA 02391710 2002-01-29
-6-
For an alternate gas conduit from below and from above in operation, some addi-
tional lines and flaps are provided, which are shown in Fig. 2. When
alternately
switching the exhaust gases from the bottom to the top, calcium-containing com-
pounds and NH3-containing exhaust gas are introduced into the reactor 19 from
below via line F and are withdrawn via line G. The flaps M1, M4, M6 and M8 re-
main closed, and the flaps M2, M3, M5 and M7 remain open. In the case of a
combined operation, the exhaust gas is then passed via the WT blower 25 and
line
H to the raw material grinder 21 and the dedusting means 23 to the chimney 24,
or
in the case of a direct operation via line I to the evaporative cooler 22 and
the de-
dusting means 23 to the chimney 24. The flaps M9 and M10 mutually act to block
the combined operation or the direct operation. When alternately switching the
gas
conduit from above, calcium compounds and NH3-containing exhaust gas are in-
troduced into the SCR reactor 19 from above via lines J and G behind the
cyclone
heat exchanger 13, and are discharged from below via lines F and K to the WT
blower 25. The flaps Ml, M8, M5, M3, M4 and M6 remain open, and the flaps M2
and M7 remain closed. In the case of a combined operation, the exhaust gas is
then passed through the WT blower 25 via line H to the raw material grinder 21
and the dedusting means 23 to the chimney 24, or in the case of a direct
operation
via line I to the evaporative cooler 22 and the dedusting means 23 to the
chimney
24.
In the case of an accident or shut-down of the SCR reactor 19, the addition of
NH3-releasing compounds is stopped and discharged via a bypass, i.e. via line
K
through the WT blower 25 either to the raw material grinder 21 or to the
evapora-
tive cooler 22. The flaps M2, M4, M6 remain open and the flaps M3, Ml, M8, M7
and M5 remain closed. The cold-air flap M11 is provided to control the exhaust
gas temperature before the SCR reactor 19.
In the case of a design with gas conduit only from below, line J and the flaps
Ml,
M8 and M7 are superfluous and thus the apparatus is only provided with an SCR
reactor 19, bypass line K and the flaps M3, M4, M5, M6. In connection with
space
and cost savings, two individual flaps may be equipped with a switching flap.
In
addition, the WT blower 25 may be installed shortly behind the cyclone heat ex-
changer 13, depending on space requirements and design.
CA 02391710 2002-01-29
-7-
For instance, the SCR reactor 19 is provided with five catalyst layers with
modules
for the breakdown of SOX and NO,, and one catalyst layer with modules for the
breakdown of hydrocarbons and carbon monoxide. Depending on the content of
SO,, NOX, hydrocarbons and carbon monoxide, the number of catalyst layers may
be changed. On the gas side, the catalyst elements or catalyst modules 20 are
provided with a protection against wear or with antiwear grids made of hard
metal
or ceramics against the erosion of dust-laden exhaust gases. In the case of an
alternate gas conduit from above and from below, a protection against wear of
about 5-20 mm is mounted on both sides.
For cleaning the catalyst surface, dust blowers 18 are furthermore provided
for
each catalyst layer on the gas side. In the case of an alternate gas conduit
in op-
eration from above and from below, the dust blowers 18 are provided on both
sides. Before entering the reactor 19, the air for the dust blowers 18 is
heated to
about 250 C.
Fig. 3 shows the arrangement of the inventive apparatus for power plants
between
boiler 27 and air preheater 26. Additives 28, e.g. Ca(OH)2, are added behind
the
boiler 27 and before the NHOH dosage 29.
Fig. 4 shows the gas conduit from below or from above or in an alternate
operation
from below and from above analogous to the description given in Fig. 2 for
cement
factories. In power plants, as compared to cement factories, the exhaust gas
is
supplied behind the SCR reactor 19 via the air preheater 26 and the dedusting
means 23 to the chimney 24.
The process in accordance with the invention will be explained below with
refer-
ence to embodiments.
In a cement factory with an exhaust gas volume of 100000 m3N.tr/h a system is
in-
stalled as it is shown in Fig. 2. Experiments are made with partial gas
streams of
3000-10000 m3N.tr. Before being introduced into the reactor, the raw gas has
the
following composition:
NOX content (calculated as NO2) = 1500 mg/m3N,tr
SOz content = 500 mg/m3N.tr
CA 02391710 2002-01-29
-8-
dust content = 8000 mg/m3N.t,
O2 content = 3.2 vol-%
Temperature in the reactor = 320 C
The density of the gas is calculated with reference to the gas composition.
The
dust content before entrance into the reactor (chiefly CaO and Ca(OH)2) is
8000
mg/m3N.tr. The determined particle density of the dust is about 3.1 kg/m3.
With ref-
erence to these data and operating conditions, a gas speed of 6.5 m/s is deter-
mined corresponding to the Froude numbers.
In the experiments, there were used honeycomb catalysts with different
contents
of active components and with the following specifications:
free opening surface = 85 %
pitch = 11 mm
clear width of the passages = 10 mm
wall thickness = 1 mm.
The content of active component (e.g. V205) in the catalysts is 0.1 %, 0.3 %,
1%,
3 % and 5 %. As reducing agent, gaseous NH3 with a stoichiometry, i.e. a molar
ratio NH3/NO, of 0.85 is added before entry in the reactor.
For the experiments, a steel grid made of stainless steel is mounted on the
module
of the catalyst as a protection against wear before entry of the dust-laden
exhaust
gas.
The gas components NOX, SOX, NH3, CO, CO2 and H20 are measured continu-
ously before and behind the reactor by means of a multicomponent analyzer MCS-
100.
The breakdown of the most important components in dependence on the content
of active component V205 is represented in the following Table:
CA 02391710 2002-01-29
-9-
Active component of Breakdown of Breakdown of Breakdown of
the catalyst NOX SO, hydrocarbons
V205 content 0.1 % 34 3 10
V205 content 0.3 % 42 5 15
V2O5 content 1.0 % 56 22 30
V2O5 content 3.0 % 75 70 55
V2O5 content 5.0 % 95 90 70
The results show that with the inventive process NOX and SOX are decomposed
when suitable operating conditions - gas flow and selection of the active
compo-
nents - are adjusted.
In the experiments with 5 % V205, the NH3 content of the exhaust gas is about
< 1
mg/m3N,tr. The analyses of the dust behind the catalyst exhibit no formation
of am-
monium sulfate, ammonium hydrogen sulfate or CaSO3. The SOx content is bound
as CaSO4. Moreover, these experiments exhibit not dust deposits in the reactor
or
in the catalyst passages.
In another series of experiments, the operating conditions of the gas flow in
the
free reaction space are changed outside the inventive Froude numbers with the
same gas composition, the same dust content and the same catalysts. It is
noted
that at gas speeds below 4 m/s the breakdown of NOX decreased considerably
and the differential pressure at the reactor 19 increased. The result is a
complete
blockage of the catalyst passages with dust.