Note: Descriptions are shown in the official language in which they were submitted.
CA 02392400 2002-05-22
WO 01/38496 PCT/US00/31968
A HUMAN NORMAL COUNTERPART OF CD4+CD56+
CUTANEOUS NEOPLASM
FIELD OF THE INVENTION
The invention is directed to purified and isolated novel CD56+ plasmacytoid
monocytes, the healthy counterpart of CD4+CD56+ cutaneous neoplasm, and uses
thereof.
BACKGROUND OF THE INVENTION
Various glycoproteins, such as CD4, CD56, CD68, and CD123, are expressed on
cells and serve as markers for a variety of diseases. Expression of these
markers has
allowed the scientific community to better understand the associated diseases
and how
they are caused. Understanding the markers' expression may further allow the
scientific
community to develop new treatments.
CD4 is a 55-kd glycoprotein expressed on a subset of peripheral T cells,
thymocytes, and monocytes/macrophages. In hematopoietic neoplasms, CD4 has
been
shown to be a marker for T-cell acute lymphocytic leukemias, T-cell lymphomas,
and
acute myeloid leukemias (AMLs). (Kameoka et al., Am J Clin Pathol 110:478-488
(1998).)
CD56 is a 175- to 185-kd surface glycoprotein normally expressed on all human
peripheral natural killer (NK) cells and on a subset of peripheral CD8+ T
cells and a
subset of peripheral monocytes. In hematopoietic neoplasms, CD56 has been
shown to
be a marker for NK-like T-cell lymphomas, plasma cell myelomas, and de novo
AMLs
with cytogenetic abnormalities such as t(8:21) and t(8:16). (Id.) Further,
CD56 molecules
may be involved in the recognition of target cells by human natural killer
cells. (Suzuki
et al. J. Ex. Med. Vol 173, 1451 (1991).)
Recently, cases of lymphomas expressing both CD4+ and CD56+ have been
published. (Kameoka et al. (1988); Petrella et al., Am J Surg Path 23(2):137-
146 (1999).)
The CD4+ CD56+ cutaneous neoplasm described in these publications is
characterized by
cutaneous nodules or papules and has cells that exhibit a CD4+ CD56+ CD45+
CD43+
HLA-DR+ phenotype. The lymphoma cells also lack the characteristics of both T
cells
and NK cells. (Petrella et al. at 137.) At initial diagnosis, CD4+CD56+
cutaneous
CA 02392400 2002-05-22
WO 01/38496 PCT/US00/31968
neoplasm is usually misclassified as either T-cell lymphoma, NK cell lymphoma,
or
myeloid sarcoma, closely related diseases to CD4+ CD56+ cutaneous neoplasm.
Initially
the patient responds favorably to treatment for those diseases but relapses
relatively
quickly. (Id.) Presently, there is no known cure for CD4+ CD56+ cutaneous
neoplasm
and patients usually die within a two-year period from the discovery of the
disease.
Plasmacytoid monocytes (also called plasmacytoids) are cell types that were
originally described as being in human lymph nodes based on their plasma cell-
like
morphology, the expression of CD4, CD31, CD36, CD68 and cutaneous lymphoctye-
associated antigen, and the lack of other cell lineage markers. (Cells et al.,
Nature
Medicine 5(8):919-923 (1999).) CD4+ CD56+cells have been identified in T-cell
lines
and in kidney allografts. (Lamer et al., J. Immunol. 138:2019-2023 (1987);
Vergelli et al.,
J. Immunol. 157:679-688 (1996); Bachetoni et al., Clin Transpl. 9:433-437
(1995).)
These cells appear to belong to either T cells or NK cells. (Id.) CD4+
CD56+cells that
are neither T cells nor NK cells have not been detected in the human
peripheral blood.
Identification of such cells, the healthy counterparts to CD4+ CD56+cutaneous
neoplasm,
could be of great interest and usefulness in developing treatment for CD4+
CD56+
cutaneous neoplasm.
Thus there is a need in the art for a method of treating CD4+ CD56+ cutaneous
neoplasm. Further, there is a need in the art for tools and methods which may
further the
discovery of such treatment methods.
SUMMARY OF THE INVENTION
The instant invention answers these needs by providing the isolated
plasmacytoid
monocyte counterpart to CD4+ CD56+ cutaneous neoplasm cells. This plasmacytoid
monocyte counterpart has the identical phenotype as the cutaneous neoplasm
cells and
may be used in the development of treatments for CD4+ CD56+ cutaneous
neoplasm.
The instant invention further provides for antibodies directed against the
plasmacytoid monocyte and the use of these antibodies to treat the CD4+ CD56+
cutaneous neoplasm.
The instant invention further provides for a more efficient method of
generating
plasmacytoid monocytes with the same phenotype as CD4+ CD56+ cutaneous
neoplasm
cells and the plasmacytoid monocytes produced by this method.
2
CA 02392400 2002-05-22
WO 01/38496 PCT/US00/31968
BRIEF DESCRIPTION OF THE FIGURES
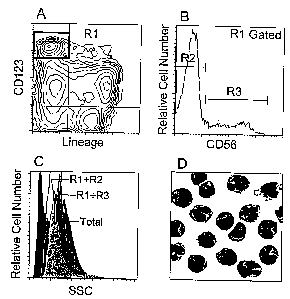
Figure 1A discloses that Peripheral Blood mononuclear cells (PBMCs), obtained
from blood of healthy volunteers treated for ten consecutive days with Flt-3L,
were
labeled with Fluorescein-conjugated monoclonal antibodies against CD3, CD14,
CD15,
CD16, CD19, CD20 and CD34 (lineage cocktail) as well as Allophycocyanin-
conjugated
antibody against CD123. The plasmacytoid cells appear as CD123"' Lineage-
cells (R1
gate. black box).
Figure 1B shows the pattern of expression of CD56(NCAM) on the plasmacytoid
cells as identified in figure 1A. In brief, CD123"' Lineage- PBMCs were
further labeled
with phycoerythrin-conjugated antibody against CD56. The CD56+plasmacytoid
monocytes (PM) are shown in gate R3. The CD56~PMs are shown in R2.
Figure 1C compares the granulosity of CD56-PM (transparent histogram) with
that of the CD56+PM (gray histogram) and that of total PBMCs (Black
histogram). Light
Side scatter parameters are shown.
Figure 1D shows the morphology of CD56+PM. In practice, R3 gated cells (see
figure 1A, B) were purified by flow cytometry (FACS vantage, Becton &
Dickinson).
The isolated cells were then submitted to a standard GIEMSA staining and
observed by
optical microscope at x400 magnification.
DETAILED DESCRIPTION OF THE INVENTION
Plasmacytoid monocytes are difficult to detect in human peripheral blood,
making
identification of markers on the plasmacytoid monocytes even more difficult.
The level
of plasmacytoid monocytes produced, however, may be increased by applying the
pre-
enrichment steps of the instant invention. A higher volume of plasmacytoid
monocytes
increases the opportunity to isolate healthy counterparts to CD4+ CD56+
cutaneous
neoplasm.
The instant invention identifies CD56+ plasmacytoid monocytes as the healthy
counterpart of CD4+ CD56+ cutaneous neoplasm cells. The CD56+ plasmacytoid
monocyte ("CD56+ PM") expresses the markers CD56, CD68, CXCR3 and CD4. The
CD56+PMs also exhibit very bright levels of CD123 and low levels of CDllc.
CD56+
PMs are CD3~ and do not uniformly have the plasma cell morphology typical of
plasmacytoid monocytes.
3
CA 02392400 2002-05-22
WO 01/38496 PCT/US00/31968
The plasmacytoid monocytes of the instant invention may be "isolated" or
"purified". Isolated plasmacytoids are not in an environment identical to the
environment
in which they can be found in nature. A purified plasmacytoid is essentially
free of
association with other plasmacytoids.
"Fragments" of CD56+ plasmacytoid monocytes encompass truncated components
of the plasmacytoid monocytes that retain the biological ability to express
the markers
CD4, CD56, CD123 and are also provided for by the present invention.
Flt-3 ligand is a hematopoietic growth factor that induces the proliferation
and
survival of primitive progenitor and stem cells. Administering Flt-3 ligand to
humans can
expand the volume of plasmacytoid cells greater than 5 fold. A screening assay
can then
be performed on the increased volume of plasmacytoids to determine the number
of
plasmacytoids present that express the markers CD4, CD56, CD68, and CD123.
These
plasmacytoid monocytes may then be isolated. The increased volume of
plasmacytoid
monocytes available enables the screening process to produce a sufficiently
useful
number of CD56+ PMs.
Thus, in one embodiment of the instant invention, pre-enrichment steps are
conducted in which CD56+ PMs are detected by administering Flt-3 ligand (as
described
in Examples 1 and 2) to produce increased levels of plasmacytoids. The
plasmacytoids
are screened to determine the number of which express CD4 and CD56 (as
described in
Example 3) and then isolated. In general, this method involves administering
Flt-3 ligand
for 10 consecutive days; incubating the peripheral blood monocyte cells (PBMC)
taken
after the administration of Flt-3 ligand with directly conjugated antibodies
including CD4,
CD56 and CD123; and then performing flow cytometry to isolate the plasmacytoid
monocytes expressing the desired markers.
Alternatively, the plasmacytoid monocytes may be isolated, for example, by the
use of a version of the Magnetic Cell Sorting (MACS) (Miltenyi Biotech)
technique. In
this method, an enrichment step is performed using anti-CD123 antibody (Olweus
et al.,
Proc Natl Acad Sci USA (1997)). The selected cells are then labeled with anti-
CD56
coated magnetic beads (e.g., Dynabeads (Dynal)). By applying a low intensity
magnetic
field, the CD56+CD123h' cells may be specifically retained on the magnet. The
CD56
CD123h' cells would not be attached to the beads and would therefore be
discarded.
Other methods of isolation are known in the art and may also be used. Further,
CD56+
PMs that are identified by any of these methods are included in the instant
invention.
4
CA 02392400 2002-05-22
WO 01/38496 PCT/US00/31968
The present invention further provides for antibodies that are immunoreactive
with the CD56+PMs of the invention. Such antibodies specifically bind to the
plasmacytoid monocytes via the antigen-binding sites of the antibody (as
opposed to non-
specific binding). Thus, the plasmacytoids monocytes and fragments, as well as
variants,
fusion proteins, etc., may be employed as "immunogens" in producing antibodies
immunoreactive therewith. More specifically, the plasmacytoid monocytes,
fragment,
variants, fusion proteins, etc. contain antigenic determinants or epitopes
that elicit the
formation of antibodies.
These antigenic determinants or epitopes can be either linear or
conformational
(discontinuous). Linear epitopes are composed of a single section of amino
acids of
plasmacytoid monocytes, while conformational or discontinuous epitopes are
composed
of amino acids sections from different regions of the polypeptide chain that
are brought
into close proximity upon protein folding (C. A. Janeway, Jr. and P. Travers,
Immuno
Biology 3:9 (Garland Publishing Inc., 2nd ed. 1996)). Because folded proteins
have
complex surfaces, the number of epitopes available is quite numerous; however,
due to
the conformation of the protein and steric hinderances, the number of
antibodies that
actually bind to the epitopes is less than the number of available epitopes
(C. A. Janeway,
Jr. and P. Travers, Immuno Biology 2:14 (Garland Publishing Inc., 2nd ed.
1996)).
Epitopes may be identified by any of the methods known in the art.
Thus, one aspect of the present invention relates to the antigenic epitopes of
the
CD56+PMs of the invention. Such epitopes are useful for raising antibodies, in
particular
monoclonal antibodies, as described in more detail below. Additionally,
epitopes from
the plasmacytoid monocytes of the invention can be used as research reagents,
in assays,
and to purify specific binding antibodies from substances such as polyclonal
sera or
supernatants from cultured hybridomas. Such epitopes or variants thereof can
be
produced using techniques well known in the art such as solid-phase synthesis,
chemical
or enzymatic cleavage of a polypeptide, or using recombinant DNA technology.
As to the antibodies that can be elicited by the epitopes of the CD56+PMs of
the
invention, whether the epitopes have been isolated or remain part of the
plasmacytoid
monocytes, both polyclonal and monoclonal antibodies may be prepared by
conventional
techniques. See, for example, Monoclonal Antibodies, Hybridomas: A New
Dimension in
Biological Analyses, Kennet et al. (eds.), Plenum Press, New York (1980); and
Antibodies: A Laboratory Manual, Harlow and Land (eds.), Cold Spring Harbor
CA 02392400 2002-05-22
WO 01/38496 PCT/US00/31968
Laboratory Press, Cold Spring Harbor, NY, (1988). One method of producing
antibodies
is by phage display. (de Kruif et al., Immunol Today 17(10):453-5 (1996).)
Hybridoma cell lines that produce monoclonal antibodies specific for the CD56+
PMs of the invention are also contemplated herein. Such hybridomas may be
produced
and identified by conventional techniques. One method for producing such a
hybridoma
cell line comprises immunizing an animal with a polypeptide; harvesting spleen
cells
from the immunized animal; fusing said spleen cells to a myeloma cell line,
thereby
generating hybridoma cells; and identifying a hybridoma cell line that
produces a
monoclonal antibody that binds the polypeptide. The monoclonal antibodies may
be
recovered by conventional techniques.
The monoclonal antibodies of the present invention include chimeric
antibodies,
e.g., humanized versions of murine monoclonal antibodies. Such humanized
antibodies
may be prepared by known techniques and offer the advantage of reduced
immunogenicity when the antibodies are administered to humans. In one
embodiment, a
humanized monoclonal antibody comprises the variable region of a murine
antibody (or
just the antigen-binding site thereof) and a constant region derived from a
human
antibody. Alternatively, a humanized antibody fragment may comprise the
antigen-
binding site of a murine monoclonal antibody and a variable region fragment
(lacking the
antigen-binding site) derived from a human antibody. Procedures for the
production of
chimeric and further engineered monoclonal antibodies include those described
in
Riechmann et al. (Nature 332:323, 1988), Liu et al. (PNAS 84:3439, 1987),
Larnck et al.
(Bio/fechnology 7:934, 1989), and Winter and Hams (TIPS 14:139, May, 1993).
Procedures that have been developed for generating human antibodies in
non-human animals may also be employed in producing antibodies of the instant
invention. The antibodies may be partially human or preferably completely
human. For
example, transgenic mice into which genetic material encoding one or more
human
immunoglobulin chains has been introduced may be employed. Such mice may be
genetically altered in a variety of ways. The genetic manipulation may result
in human
immunoglobulin polypeptide chains replacing endogenous immunoglobulin chains
in at
least some, and preferably virtually all, antibodies produced by the animal
upon
immumzarion.
Mice in which one or more endogenous immunoglobulin genes have been
inactivated by various means have been prepared. Human immunoglobulin genes
have
6
CA 02392400 2002-05-22
WO 01/38496 PCT/US00/31968
been introduced into the mice to replace the inactivated mouse genes.
Antibodies
produced in the animals incorporate human immunoglobulin polypeptide chains
encoded
by the human genetic material introduced into the animal.
Examples of techniques for the production and use of such transgenic animals
are
described in U.S. Patent Nos. 5,814,318, 5,569,825, and 5,545,806 and Waldman,
T.
Science Vol. 252, 1657-62 (1991).
Antigen-binding fragments of the antibodies, which may be produced by
conventional techniques, are also encompassed by the present invention.
Examples of
such fragments include, but are not limited to, Fab and F(ab~2 fragments.
Antibody
fragments and derivatives produced by genetic engineering techniques are also
provided.
In one embodiment, the antibodies are specific for the CD56+PMs of the instant
invention and do not cross-react with other proteins. Screening procedures by
which such
antibodies may be identified are well known, and may involve immunoaffinity
chromatography, for example.
The plasmacytoid monocytes, fragments thereof or antibodies may be used in the
developing treatments for any disorder mediated (directly or indirectly) by
defective, or
overly sufficient or insufficient amounts of the genes corresponding to the
plasmacytoid
monocytes of the instant invention. The plasmacytoid monocytes, fragments
thereof or
antibodies further can be used in the treatment of CD4+ CD56+ cutaneous
neoplasm.
Antibodies may naturally exert an agonist or antagonist activity that would be
beneficial in the treatment of tumors. For instance, antibodies may transduce
a signal to
cells that would mediate apoptosis. Alternatively, antibodies could fix
Complement or
mediate killer activity through Fc receptor-bearing natural killer cells.
Antibodies of the instant invention may also find use as Garners for
delivering
agents attached thereto to tumor cells bearing the cell surface antigen. The
antibody
binds to the target cells, thus allowing detection thereof (in the case of
diagnostic agents)
or treatment thereof (with therapeutic agents).
Diagnostic and therapeutic agents that may be attached to an antibody include,
but
are not limited to, drugs, toxins, radionuclides, chromophores, enzymes that
catalyze a
colorimetric or fluorometric reaction, cytokines or chemokines, active
fragments of
cytokines or chemokines, and the like, with the particular agent being chosen
according to
the intended application. Cytokines that may be used in the instant invention
include, but
are not limited to, IL-3, IL-2, CD40-L, GM-CSF and PIXY (Immunex). Chemokines
7
CA 02392400 2002-05-22
WO 01/38496 PCT/US00/31968
include, but are not limited to, CXCR3-ligands (ITAC, MIG, and IP-10). Active
fragments of cytokines or chemokines are any fragments that maintain the
biological
activity of the cytokine or chemokine. Among the toxins are ricin, abrin,
saporin toxin,
diptheria toxin, Pseudomonas aeruginosa exotoxin A, ribosomal inactivating
proteins,
mycotoxins such as trichothecenes, and derivatives and fragments (e.g., single
chains)
thereof. Radionuclides suitable for diagnostic use include, but are not
limited to, 1231,
131h 99Tc, 111In, S~Cr, 3H, 14C, 2' MTh and 76Br. Radionuclides suitable for
therapeutic use
include, but are not limited to, 1311, 77gr, 32P, 212Bi, 64Cu, and 67Cu.
Such agents may be attached to the antibodies of the instant invention by any
suitable conventional procedure. Antibodies, being proteins, comprise
functional groups
on amino acid side chains that can be reacted with functional groups on a
desired agent to
form covalent bonds, for example. The agent may be covalently linked to the
antibody
via an amide bond, hindered disulfide bond, acid-cleavable linkage, and the
like, which
are among the conventional linkages chosen according to such factors as the
structure of
the desired agent. Alternatively, the antibody or the agent to be linked
thereto may be
derivatized to generate or attach a desired reactive functional group. The
derivatization
may involve attachment of one of the bifunctional coupling reagents available
for linking
various molecules to proteins (Pierce Chemical Company, Rockford, Illinois). A
number
of techniques for radiolabeling proteins are known. Radionuclide metals may be
attached
to the antibody by using a suitable bifunctional chelating agent, examples of
which are
described in U.S. patents 4,897,255 and 4,965,392.
Conjugates comprising the antibodies of the instant invention and a suitable
diagnostic or therapeutic agent (preferably covalently linked) are thus
prepared. The
conjugates are administered or otherwise employed in an amount appropriate for
the
particular application. The conjugates find use in in vitro or in vivo
procedures.
Among the use of antibodies of the instant invention is the use in assays to
detect
the presence of CD56+ PMs and CD4+ CD56+ cutaneous neoplasm cells either in
vitro or
in vivo. The antibodies may be employed in purifying CD56+ PMs or CD4+ CD56+
cutaneous neoplasm cells by immunoaffinity chromatography. Further, these
antibodies
may be used to inhibit the growth or induce differentiation of either CD56+
PMs or CD4+
CD56+ cutaneous neoplasm cells. Such antibodies may be identified using any
suitable
assay procedure, such as by testing antibodies for the ability to inhibit
growth of CD56+
8
CA 02392400 2002-05-22
WO 01/38496 PCT/US00/31968
PMs or CD4+ CD56+ cutaneous neoplasm, induce apoptotic death of these cells or
induce
differentiation into dendritic cells.
Alternatively, blocking antibodies may be identified in assays for the ability
to
inhibit a biological effect that results from the binding of CD56+ PM or CD4+
CD56+
cutaneous neoplasm cells to partner cells, such as endothelial cells or T-
lymphocytes.
One therapeutic method involves in vivo administration of a blocking antibody
to a
mammal in an amount effective in inhibiting CD56+ PM-mediated or CD4+ CD56+
cutaneous neoplasm cell-mediated biological activity. Disorders caused or
exacerbated
by CD56+ PM or CD4+ CD56+ cutaneous neoplasm cells directly or indirectly, are
thus
treated. Monoclonal antibodies are generally preferred for use in such
therapeutic
methods. In one embodiment, an antigen-binding antibody fragment is employed.
Further, antibodies raised against CD56+ PM or CD4+ CD56+ cutaneous neoplasm
cells may be screened for agonistic properties. Such antibodies, upon binding
to CD56+
PM or CD4+ CD56+ cutaneous neoplasm, induce biological effects (e.g.,
transduction of
biological signals), leading to differentiation into dendritic cells or cells
with natural killer
activity. Agonistic antibodies may also be used to induce apoptosis of certain
cancer
cells, such as CD4+ CD56+ cutaneous neoplasm.
Plasmacytoid monocytes have been shown to produce large amounts of type-1
interferon in response to virus infection. Thus, agonistic antibodies against
CD56+PM
may be used to increase type-1 interferon production and exacerbate the anti-
viral activity
of CD56+ PM. Conventional techniques may be employed to confirm the
susceptibility
of various cancer cell types and virally infected cells to cell death induced
by agonistic
antibodies of the instant invention.
Viral infections and associated conditions include, but are not limited to
cytomegalovirus, encephalomyocarditis, influenza, Newcastle disease virus,
vesicular
stomatitus virus, herpes simplex virus, hepatitis, adenovirus-2, bovine viral
diarrhea virus,
HIV, and Epstein-Ban virus. Agonistic antibodies of the present invention.may
be
administered alone or in combination with other agents useful for combating a
particular
virus. Agonistic antibodies may also be employed in conjunction with other
agents)
useful in treating cancer. Examples of such agents include both proteinaceous
and non-
proteinaceous drugs and radiation therapy.
The present invention also provides pharmaceutical compositions comprising an
antibody against CD56+ PM or CD4+ CD56+ cutaneous neoplasm cells and a
suitable
9
CA 02392400 2002-05-22
WO 01/38496 PCT/US00/31968
diluent, excipient, or carrier. Such Garners will be nontoxic to patients at
the dosages and
concentrations employed. Ordinarily, the preparation of such compositions
entails
combining a mammalian antibody or derivative thereof with buffers,
antioxidants such as
ascorbic acid, low molecular weight (less than about 10 residues) peptides,
proteins,
amino acids, carbohydrates including glucose, sucrose, or dextrans, chelating
agents such
as EDTA, glutathione, or other stabilizers and excipients. Neutral buffered
saline is one
appropriate diluent.
For therapeutic use, the compositions are administered in a manner and dosage
appropriate to the indication and the patient. As will be understood by one
skilled in the
pertinent field, a therapeutically effective dosage will vary according to
such factors as
the nature and severity of the disorder to be treated and the age, condition,
and size of the
patient. Administration may be by any suitable route, including but not
limited to
intravenous injection, continuous infusion, local infusion during surgery, or
sustained
release from implants (gels, membranes, and the like).
The compositions of the present invention may contain an antibody in any form
described above, including variants, derivatives, biologically active
fragments, and
oligomeric forms thereof. Antibodies derived from the same mammalian species
as the
patient is generally preferred for use in pharmaceutical compositions. In one
embodiment
of the invention, the composition comprises a soluble human antibody. In
another
embodiment of the invention, the pharmaceutical composition comprises an
antibody
having a diagnostic or therapeutic agent attached thereto. Such compositions
may be
administered to diagnose or treat conditions characterized by CD56+ PM cells,
e.g., CD4+
CD56+ cutaneous neoplasm, as discussed above. The foregoing compositions may
additionally contain, or be co-administered with, additional agents effective
in treating
malignancies characterized by CD56+ PM cells.
It is known that Interleukin 3 (IL-3) can produce the differentiation of
plasmacytoid monocyte cells into dendritic cells. These dendritic cells
present the
antigens the cells are expressing. As discussed above, CD4+CD56+ cutaneous
neoplasm
express very high levels of CD123, the IL-3 receptor alpha chain. Thus, in one
embodiment of the invention, differentiation of CD4+CD56+ cutaneous neoplasm
is
induced by administering IL-3 or an IL-3 agonist. The dendritic cells formed
by this
differentiation would present antigens from the CD4+CD56+ cutaneous neoplasm
to
effector cells, such as helper, cytotoxic T-lymphocytes or natural killer
cells. This
CA 02392400 2002-05-22
WO 01/38496 PCT/US00/31968
immune response could be used in the treatment of such tumors. Since CD4+
CD56+
cutaneous neoplasm express high levels of CD123 or CXCR-3, the toxins
described
above could be coupled with antibodies against CD123 or CXCR-3 to deliver
therapeutic
agents, preferentially to the tumor cells. Alternatively, a cytokine, such as
IL,-3, or a
chemokine, such as IP-10, ITAC or MIG, could be coupled to a toxin described.
Upon
delivery, the cytokine-toxin conjugate or chemokine-toxin conjugate would
target the
CD4+ CD56+ cutaneous neoplasm cells.
In another embodiment of the instant invention, CD56+ PMs are used to create a
cDNA library. The cDNA library may be created by any known means. In one
embodiment, a PCR-select cDNA subtraction kit (ClonTech) may be used to
generate a
library of plasmacytoid specific cDNAs. Peripheral blood leukocytes from
plasmacytoid
monocytes, from which the plasmacytoid cells have been removed, may be used to
generate the driver cDNA of CD56+ PMs. The peripheral blood leukocytes may be
excluded by flow cytometry, by use of magnetic beads as discussed above, or by
any
method known in the art. Plasmacytoid monocyte cell cDNA may then be generated
by
the subtraction kit and used as the target DNA for the subtraction procedure.
The
subtracted plasmacytoid monocyte cDNA library may be used to probe arrays of
human
cDNAs and sequence clones, directly sequenced, or used as a cDNA source of
cDNA
expressing cloning of CD56+PM specific genes. The clones or sequences of CD56+
PMs
may then be examined to determine whether they encode genes that influence the
survival, activation or death of CD56+ PMs. The proteins encoded by these
genes may be
administered to treat CD4+CD56+ cutaneous neoplasm.
Isolated or purified CD56+PMs, fragments or antibodies specific to CD56+ PM or
CD4+ CD56+ cutaneous neoplasm cell thereof can be administered through well-
known
means, including parenterally (subcutaneous, intramuscular, intravenous,
intradermal, etc.
injection) and with a suitable carrier. Formulations suitable for parenteral
administration
include aqueous and non-aqueous sterile injection solutions which may contain
anti-
oxidants, buffers, bacteriostats and solutes which render the formulation
instonic with the
blood of the recipient; and aqueous and non-aqueous sterile suspensions which
may
include suspending agents or thickening agents. The dosage will depend on the
specific
activity of the vaccine and can be readily determined by routine
experimentation.
The following examples are offered by way of illustration, and not by way of
limitation. Those skilled in the art will recognize that variations of the
invention
11
CA 02392400 2002-05-22
WO 01/38496 PCT/US00/31968
embodied in the example can be made, especially in light of the teachings of
the various
references cited herein, the disclosures of which are incorporated by
reference in their
enrirety.
Example 1
Peripheral blood samples were obtained from a randomized, placebo controlled,
phase 1 dose escalation study performed in healthy human volunteers. Flt-3
ligand was
produced by well-known recombinant DNA technology in a Chinese hamster ovary
(CHO) cell line. Flt-3 ligand was supplied as a sterile lyophilized
preparation of 5 mg of
Flt-3 ligand, with 40 mg mannitol, 10 mg sucrose, and 35 mM of tromethamine
(TRIS)
per vial. Prior to administration, the Flt-3 ligand was reconstituted in
bacteriostatic water
for injection.
Volunteers were subcutanously injected once daily with 10, 25, 50, 75 or 100
~g/kg/day of human Flt-3 ligand for 10 consecutive days.
Example 2
The peripheral blood mononuclear cells (PBMC) from the subjects of Example 1
were isolated after centrifugation over a discontinuous density gradient using
Ficoll
(1.077g/ml Accu-Prep, Accurate Chemicals, Westbury NY), and washed in PBS
containing 5% fetal bovine serum (FBS) (Intergen, New York, NY). Cells were
then
incubated with directly conjugated antibodies which included CD2, CD4, CD56,
and
CDwl23 (IL-3Ra) for 30 minutes at 4°C in PBS containing 0.01% NaN3
supplemented
with 10% goat serum and 10% rabbit serum to block Fc-receptors. Propidium
iodide (PI)
(lp,g/ml) was included in the final wash to allow exclusion of dead cells.
Example 3
The PBMC cells, obtained as described in Examples 1 and 2, cells were then
cryogenically preserved in approximately 2 to 3 x 107 cells/ml in 12%DMSO
(Dimethyl
Sulfoxide; ICN, Aurora, OH)/p,H FBS (Characterized Fetal Bovine Serum;
HyClone,
Logan, UT). Cryovials were then stored in a liquid nitrogen dewar.
Cells were thawed in 10% pH FBS/10% Versene (Life Technologies, Bethesda,
MD); 20 Kunitz Units/ml of DNasel(Sigma, St.Louis, MO) and Super McCoys
medium.
Thawed cells were resuspended at approximately 2 x 107 cells/ml in 10% pH
human
12
CA 02392400 2002-05-22
WO 01/38496 PCT/US00/31968
serum (Cellect Human Pooled Serum; ICN, Aurora, OH) and Phosphate Buffered
Saline
(PBS).
Typically stained cells were distributed 1 to 2 x 106 cells per well in a
polystyrene
V-bottom 96-well plate (Costar, Corning, NY). The wells were diluted to a
ratio of 1/50
using APC-conjugated mouse anti-human CDw123 (anti-IL-3Ra; non-blocking; clone
9F5 at approximately 164~g/ml; Pharmingen, San Diego, CA) and PE-conjugated
mouse
anti-human CD56 (clone B 159; at approximately 200 ~g/ml; Pharmingen, San
Diego,
CA). Mouse antibody isotype controls (i.e., APC-conjugated IgGI and PE-
conjugated
IgGI) were also included in the media. The media was incubated for ten minutes
at
approximately 4°C.
The cells were washed with PBS and resuspended in 300p,1 of FACS buffer.
Several hours after the completion of staining, flow cytometry was performed.
Example 4
This example illustrates a method for preparing monoclonal antibodies that
bind
CD56+ PM cells. Suitable immunogens that may be employed in generating such
antibodies include, but are not limited to, purified CD56+ PM cells.
Purified CD56+ PM cells can be used to generate monoclonal antibodies
immunoreactive therewith, using conventional techniques such as those
described in U.S.
Patent 4,411,993. Briefly, mice are immunized with CD56+ PM immunogen
emulsified
in complete Freund's adjuvant, and injected in amounts ranging from 10-100:g
subcutaneously or intraperitoneally. Ten to twelve days later, the immunized
animals are
boosted with additional CD56+ PM emulsified in incomplete Freund's adjuvant.
Mice are
periodically boosted thereafter on a weekly to bi-weekly immunization
schedule. Serum
samples are periodically taken by retro-orbital bleeding or tail-tip excision
to test for
antibodies against CD56+ PM by dot blot assay, ELISA (Enzyme-Linked
Immunosorbent
Assay) or flow cytometry.
Following detection of an appropriate antibody titer, positive animals are
provided
one last intravenous injection of CD56+ PM cells in saline. Three to four days
later, the
animals are sacrificed, spleen cells harvested, and spleen cells are fused to
a murine
myeloma cell line, e.g., NS1 or preferably P3x63Ag8.653 (ATCC CRL 1580).
Fusions
generate hybridoma cells, which are plated in multiple microtiter plates in a
HAT
13
CA 02392400 2002-05-22
WO 01/38496 PCT/US00/31968
(hypoxanthine, aminopterin and thymidine) selective medium to inhibit
proliferation of
non-fused cells, myeloma hybrids, and spleen cell hybrids.
The hybridoma cells are screened by ELISA for reactivity against purified
CD56+
PM cells by adaptations of the techniques disclosed in Engvall et al.,
Immunochem.
8:871, 1971 and in U.S. Patent 4,703,004. A preferred screening technique is
the
antibody capture technique described in Beckmann et al., (J. Immunol.
144:4212, 1990)
Positive hybridoma cells can be injected intraperitoneally into syngeneic
BALB/c mice to
produce ascites containing high concentrations of anti- CD56+ PM monoclonal
antibodies. Alternatively, hybridoma cells can be grown in vitro in flasks or
roller bottles
by various techniques. Monoclonal antibodies produced in mouse ascites can be
purified
by ammonium sulfate precipitation, followed by gel exclusion chromatography.
Alternatively, affinity chromatography based upon binding of antibody to
Protein A or
Protein G can also be used, as can affinity chromatography based upon binding
to CD56+
PM cells.
14