Note: Descriptions are shown in the official language in which they were submitted.
CA 02392979 2002-05-29
WO 01/40190 PCT/IB00/01650
-1-
4-CARBOXYAMINO-2-ETHYL-1,2,3,4-TETRAHYDROQUINOLINE CRYSTAL AS CETP INHIBITOR
Background Of The Invention
This invention relates to cholesteryl ester transfer protein (CETP)
inhibitors,
pharmaceutical compositions containing such inhibitors and the use of such
inhibitors
to elevate certain plasma lipid levels, including high density lipoprotein
(HDL)-
cholesterol and to lower certain other plasma lipid levels, such as low
density
lipoprotein (LDL)-cholesterol and triglycerides and accordingly to treat
diseases which
are affected by low levels of HDL cholesterol and/or high levels of LDL-
cholesterol
and triglycerides, such as atherosclerosis and cardiovascular diseases in
certain
mammals (i.e., those which have CETP in their plasma), including humans.
More particularly, this invention relates to CETP inhibitor crystals,
pharmaceutical compositions comprising these crystals, a process for preparing
these crystals and to methods of treating atherosclerosis, obesity, and
related
diseases and/or conditions with the crystals.
Atherosclerosis and its associated coronary artery disease (CAD) is the
leading cause of mortality in the industrialized world. Despite attempts to
modify
secondary risk factors (smoking, obesity, lack of exercise) and treatment of
dyslipidemia with dietary modification and drug therapy, coronary heart
disease
(CHD) remains the most common cause of death in the U.S., where cardiovascular
disease accounts for 44% of all deaths, with 53% of these associated with
atherosclerotic coronary heart disease.
Risk for development of this condition has been shown to be strongly
correlated with certain plasma lipid levels. While elevated LDL-cholesterol
may be
the most recognized form of dyslipidemia, it is by no means the only
significant lipid
associated contributor to CHD. Low HDL-cholesterol is also a known risk factor
for
CHD (Gordon, D.J., et al.,: "High-density Lipoprotein Cholesterol and
Cardiovascular
Disease", Circulation, (1989), 79: 8-15).
High LDL-cholesterol and triglyceride levels are positively correlated, while
high levels of HDL-cholesterol are negatively correlated with the risk for
developing
cardiovascular diseases. Thus, dyslipidemia is not a unitary risk profile for
CHD but
may be comprised of one or more lipid aberrations.
Among the many factors controlling plasma levels of these disease
dependent principles, cholesteryl ester transfer protein (CETP) activity
affects all
CA 02392979 2002-05-29
WO 01/40190 PCT/IB00/01650
-2-
three. The role of this 70,000 dalton plasma glycoprotein found in a number of
animal
species, including humans, is to transfer cholesteryl ester and triglyceride
between
lipoprotein particles, including high density lipoproteins (HDL), low density
lipoproteins
(LDL), very low density lipoproteins (VLDL), and chylomicrons. The net result
of
CETP activity is a lowering of HDL cholesterol and an increase in LDL
cholesterol.
This effect on lipoprotein profile is believed to be pro-atherogenic,
especially in
subjects whose lipid profile constitutes an increased risk for CHD.
No wholly satisfactory HDL-elevating therapies exist. Niacin can significantly
increase HDL, but has serious toleration issues which reduce compliance.
Fibrates
and the HMG CoA reductase inhibitors raise HDL-C only modestly (--10-12%). As
a
result, there is a significant unmet medical need for a well-tolerated agent
which can
significantly elevate plasma HDL levels, thereby reversing or slowing the
progression
of atherosclerosis.
Commonly assigned U.S. application ser. No. 09/391,152 filed September 7,
1999 entitled 4-CARBOXYAMINO-2-SUBSTITUTED-1,2,3,4-
TETRAHYDROQUINOLINES, the disclosure of which is hereby incorporated by
reference, is directed to compounds of the following general formula:
O
R3 ~
\N- _ R
Rs R I O a
4~
6 ~~ 3
2
1~
N- _R
R z
R8 R~
Specifically, the compound [2R,4S] 4-[(3,5-bis-trifluoromethyl-benzyl)-
methoxycarbonyl-amino]-2-ethyl-6-trifluoromethyl-3,4-dihydro-2H-quinoline-1-
carboxylic acid ethyl ester is described.
Thus, although there are a variety of anti-atherosclerosis therapies, there is
a
continuing need and a continuing search in this field of art for alternative
therapies.
CA 02392979 2002-05-29
WO 01/40190 PCT/IB00/01650
-3-
Summary Of The Invention
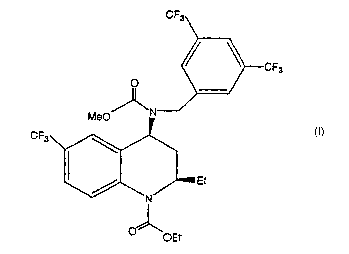
This invention is directed to a Formula I crystal
r~
CF3
O
~N
CF3
Et
Alternatively, a crystal of the above Formula I is named as [2R,4S] 4-
[(3,5-bis-trifluoromethyl-benzyl)-methoxycarbonyl-amino]-2-ethyl-6-
trifluoromethyl-3,4-dihydro-2H-quinoline-1-carboxylic acid ethyl ester.
Another aspect of this invention is directed to an anhydrous crystal of
Formula
Another aspect of this invention is directed to the corresponding anhydrous
crystal having the X-ray powder diffraction pattern as shown in Figure 1.
Another aspect of this invention is directed to an ethanolate crystal of
Formula
Another aspect of this invention is directed to the corresponding ethanolate
crystal having the X-ray powder diffraction pattern as shown in Figure 2.
A preferred dosage is about 0.01 to 100 mg/kg/day of a Formula I crystal. An
especially preferred dosage is about 0.1 to 10 mg/kg/day of a Formula I
crystal.
In the text herein including the following methods, pharmaceutical
compositions, combinations and kits reference is made to a crystal of Formula
I.
While it is understood that if the crystal is in solution, the crystal form is
not present
(in contrast to e.g., a dry tablet formulation), the following methods
pharmaceutical
compositions combinations and kits are intended to include a method or
formulation
IV
O' \
OEt
CA 02392979 2002-05-29
WO 01/40190 PCT/IB00/01650
-4-
resulting from a use of such crystal (e.g., administering a gelatin capsule
including an
oil formulation solution of the crystal).
Yet another aspect of this invention is directed to methods for treating
atherosclerosis, peripheral vascular disease, dyslipidemia,
hyperbetalipoproteinemia,
hypoalphalipoproteinemia, hypercholesterolemia, hypertriglyceridemia, familial-
hypercholesterolemia, cardiovascular disorders, angina, ischemia, cardiac
ischemia,
stroke, myocardial infarction, reperfusion injury, angioplastic restenosis,
hypertension,
vascular complications of diabetes, obesity or endotoxemia in a mammal
(including a
human being either male or female) by administering to a mammal in need of
such
treatment an atherosclerosis, peripheral vascular disease, dyslipidemia,
hyperbetalipoproteinemia, hypoalphalipoproteinemia, hypercholesterolemia,
hypertriglyceridemia, familial-hypercholesterolemia, cardiovascular disorders,
angina,
ischemia, cardiac ischemia, stroke, myocardial infarction, reperfusion injury,
angioplastic restenosis, hypertension, vascular complications of diabetes,
obesity or
endotoxemia treating amount of a Formula I crystal.
Yet another aspect of this invention is directed to a method for treating
atherosclerosis in a mammal (including a human being) by administering to a
mammal in need of such treatment an atherosclerosis treating amount of a
Formula I
crystal.
Yet another aspect of this invention is directed to a method for treating
peripheral vascular disease in a mammal (including a human being) by
administering
to a mammal in need of such treatment a peripheral vascular disease treating
amount of a Formula I crystal.
Yet another aspect of this invention is directed to a method for treating
dyslipidemia in a mammal (including a human being) by administering to a
mammal
in need of such treatment a dyslipidemia treating amount of a Formula I
crystal.
Yet another aspect of this invention is directed to a method for treating
hyperbetalipoproteinemia in a mammal (including a human being) by
administering to
a mammal in need of such treatment a hyperbetalipoproteinemia treating amount
of a
Formula I crystal.
Yet another aspect of this invention is directed to a method for treating
hypoalphalipoproteinemia in a mammal (including a human being) by
administering to
a mammal in need of such treatment a hypoalphalipoproteinemia treating amount
of
a Formula I crystal.
CA 02392979 2002-05-29
WO 01/40190 PCT/IB00/01650
-5-
Yet another aspect of this invention is directed to a method for treating
hypercholesterolemia in a mammal (including a human being) by administering to
a
mammal in need of such treatment a hypercholesterolemia treating amount of a
Formula I crystal.
Yet another aspect of this invention is directed to a method for treating
hypertriglyceridemia in a mammal (including a human being) by administering to
a
mammal in need of such treatment a hypertriglyceridemia treating amount of a
Formula I crystal.
Yet another aspect of this invention is directed to a method for treating
familial-hypercholesterolemia in a mammal (including a human being) by
administering to a mammal in need of such treatment a familial-
hypercholesterolemia treating amount of a Formula I crystal.
Yet another aspect of this invention is directed to a method for treating
cardiovascular disorders in a mammal (including a human being) by
administering to
a mammal in need of such treatment a cardiovascular disorder treating amount
of a
Formula I crystal.
Yet another aspect of this invention is directed to a method for treating
angina
in a mammal (including a human being) by administering to a mammal in need of
such treatment an angina treating amount of a Formula I crystal.
Yet another aspect of this invention is directed to a method for treating
ischemia in a mammal (including a human being) by administering to a mammal in
need of such treatment an ischemic disease treating amount of a Formula I
crystal.
Yet another aspect of this invention is directed to a method for treating
cardiac ischemia in a mammal (including a human being) by administering to a
mammal in need of such treatment a cardiac ischemic treating amount of a
Formula I
crystal.
Yet another aspect of this invention is directed to a method for treating
stroke
in a mammal (including a human being) by administering to a mammal in need of
such treatment a stroke treating amount of a Formula I crystal.
Yet another aspect of this invention is directed to a method for treating a
myocardial infarction in a mammal (including a human being) by administering
to a
mammal in need of such treatment a myocardial infarction treating amount of a
Formula I crystal.
CA 02392979 2002-05-29
WO 01/40190 PCT/IB00/01650
-6-
Yet another aspect of this invention is directed to a method for treating
reperfusion injury in a mammal (including a human being) by administering to a
mammal in need of such treatment a reperfusion injury treating amount of a
Formula
I crystal.
Yet another aspect of this invention is directed to a method for treating
angioplastic restenosis in a mammal (including a human being) by administering
to a
mammal in need of such treatment an angioplastic restenosis treating amount of
a
Formula I crystal.
Yet another aspect of this invention is directed to a method for treating
hypertension in a mammal (including a human being) by administering to a
mammal
in need of such treatment a hypertension treating amount of a Formula I
crystal.
Yet another aspect of this invention is directed to a method for treating the
vascular complications of diabetes in a mammal (including a human being) by
administering to a mammal in need of such treatment a vascular complications
of
diabetes treating amount of a Formula I crystal.
Yet another aspect of this invention is directed to a method for treating
obesity
in a mammal (including a human being) by administering to a mammal in need of
such treatment an obesity treating amount of a Formula I crystal.
Yet another aspect of this invention is directed to a method for treating
endotoxemia in a mammal (including a human being) by administering to a mammal
in need of such treatment an endotoxemia treating amount of a Formula I
crystal.
This invention is also directed to pharmaceutical compositions which comprise
a therapeutically effective amount of a crystal of Formula I and a
pharmaceutically
acceptable carrier, vehicle or diluent.
This invention is also directed to pharmaceutical compositions for the
treatment of atherosclerosis, peripheral vascular disease, dyslipidemia,
hyperbetalipoproteinemia, hypoalphalipoproteinemia, hypercholesterolemia,
hypertriglyceridemia, familial-hypercholesterolemia, cardiovascular disorders,
angina,
ischemia, cardiac ischemia, stroke, myocardial infarction, reperfusion injury,
angioplastic restenosis, hypertension, vascular complications of diabetes,
obesity or
endotoxemia in a mammal (including a human being) which comprise a
therapeutically effective amount of a crystal of Formula I and a
pharmaceutically
acceptable carrier, vehicle or diluent.
CA 02392979 2002-05-29
WO 01/40190 PCT/IB00/01650
This invention is also directed to pharmaceutical compositions for the
treatment of atherosclerosis in a mammal (including a human being) which
comprise
an atherosclerosis treating amount of a crystal of Formula I and a
pharmaceutically
acceptable carrier, vehicle or diluent.
This invention is also directed to pharmaceutical compositions for the
treatment of peripheral vascular disease in a mammal (including a human being)
which comprise a peripheral vascular disease treating amount of a crystal of
Formula
I and a pharmaceutically acceptable carrier, vehicle or diluent.
This invention is also directed to pharmaceutical compositions for the
treatment of dyslipidemia in a mammal (including a human being) which comprise
a
dyslipidemia treating amount of a crystal of Formula I and a pharmaceutically
acceptable carrier, vehicle or diluent.
This invention is also directed to pharmaceutical compositions for the
treatment of hyperbetalipoproteinemia in a mammal (including a human being)
which
comprise a hyperbetalipoproteinemia treating amount of a crystal of Formula I
and a
pharmaceutically acceptable carrier, vehicle or diluent.
This invention is also directed to pharmaceutical compositions for the
treatment of hypoalphalipoproteinemia in a mammal (including a human being)
which
comprise a hypoalphalipoproteinemia treating amount of a crystal of Formula I
and a
pharmaceutically acceptable carrier, vehicle or diluent.
This invention is also directed to pharmaceutical compositions for the
treatment of hypercholesterolemia in a mammal (including a human being) which
comprise a hypercholesterolemia treating amount of a crystal of Formula I and
a
pharmaceutically acceptable carrier, vehicle or diluent.
This invention is also directed to pharmaceutical compositions for the
treatment of hypertriglyceridemia in a mammal (including a human being) which
comprise a hypertriglyceridemia treating amount of a crystal of Formula I and
a
pharmaceutically acceptable carrier, vehicle or diluent.
This invention is also directed to pharmaceutical compositions for the
treatment of familial-hypercholesterolemia in a mammal (including a human
being)
which comprise a familial-hypercholesterolemia treating amount of a crystal of
Formula I and a pharmaceutically acceptable carrier, vehicle or diluent.
This invention is also directed to pharmaceutical compositions for the
treatment of angina in a mammal (including a human being) which comprise an
CA 02392979 2002-05-29
WO 01/40190 PCT/IB00/01650
_g_
angina treating amount of a crystal of Formula I and a pharmaceutically
acceptable
carrier, vehicle or diluent.
This invention is also directed to pharmaceutical compositions for the
treatment of ischemia in a mammal (including a human being) which comprise an
ischemic treating amount of a crystal of Formula I and a pharmaceutically
acceptable
carrier, vehicle or diluent.
This invention is also directed to pharmaceutical compositions for the
treatment of cardiac ischemia in a mammal (including a human being) which
comprise a cardiac ischemic treating amount of a crystal of Formula 1 and a
pharmaceutically acceptable carrier, vehicle or diluent.
This invention is also directed to pharmaceutical compositions for the
treatment of stroke in a mammal (including a human being) which comprise a
stroke
treating amount of a crystal of Formula I and a pharmaceutically acceptable
carrier,
vehicle or diluent.
This invention is also directed to pharmaceutical compositions for the
treatment of a myocardial infarction in a mammal (including a human being)
which
comprise a myocardial infarction treating amount of a crystal of Formula I and
a
pharmaceutically acceptable carrier, vehicle or diluent.
This invention is also directed to pharmaceutical compositions for the
treatment of reperfusion injury in a mammal (including a human being) which
comprise a reperfusion injury treating amount of a crystal of Formula I and a
pharmaceutically acceptable carrier, vehicle or diluent..
This invention is also directed to pharmaceutical compositions for the
treatment of angioplastic restenosis in a mammal (including a human being)
which
comprise an angioplastic restenosis treating amount of a crystal of Formula I
and a
pharmaceutically acceptable carrier, vehicle or diluent.
This invention is also directed to pharmaceutical compositions for the
treatment of hypertension in a mammal (including a human being) which comprise
a
hypertension treating amount of a crystal of Formula I and a pharmaceutically
acceptable carrier, vehicle or diluent.
This invention is also directed to pharmaceutical compositions for the
treatment of the vascular complications of diabetes in a mammal (including a
human
being) which comprise a vascular complications of diabetes treating amount of
a
crystal of Formula I and a pharmaceutically acceptable carrier, vehicle or
diluent.
CA 02392979 2002-05-29
WO 01/40190 PCT/IB00/01650
_g_
This invention is also directed to pharmaceutical compositions for the
treatment of obesity in a mammal (including a human being) which comprise an
obesity treating amount of a crystal of Formula I and a pharmaceutically
acceptable
carrier, vehicle or diluent.
This invention is also directed to pharmaceutical compositions for the
treatment of endotoxemia in a mammal (including a human being) which comprise
an
endotoxemia treating amount of a crystal of Formula I and a pharmaceutically
acceptable carrier, vehicle or diluent.
This invention is also directed to a pharmaceutical combination composition
comprising: a therapeutically effective amount of a composition comprising
a first compound, said first compound being a Formula I crystal;
a second compound, said second compound being an HMG-CoA reductase
inhibitor, an microsomal triglyceride transfer protein (MTP)/Apo B secretion
inhibitor,
a PPAR activator, a bile acid reuptake inhibitor, a cholesterol absorption
inhibitor, a
cholesterol synthesis inhibitor, a fibrate, niacin, an ion-exchange resin, an
antioxidant, an ACAT inhibitor or a bile acid sequestrant; and/or optionally
a pharmaceutical carrier, vehicle or diluent.
Preferred among the second compounds are an HMG-CoA reductase
inhibitor and a MTP/Apo B secretion inhibitor.
A particularly preferred HMG-CoA reductase inhibitor is lovastatin,
simvastatin, pravastatin, fluvastatin, atorvastatin or rivastatin.
Another aspect of this invention is a method for treating atherosclerosis in a
mammal comprising administering to a mammal suffering from atherosclerosis
a first compound, said first compound being a Formula I crystal; and
a second compound, said second compound being an HMG-CoA reductase
inhibitor, an MTP/Apo B secretion inhibitor, a cholesterol absorption
inhibitor, a
cholesterol synthesis inhibitor, a fibrate, niacin, an ion-exchange resin, an
antioxidant, an ACAT inhibitor or a bile acid sequestrant wherein the amounts
of the
first and second compounds result in a therapeutic effect.
A preferred aspect of the above method is wherein the second compound is
an HMG-CoA reductase inhibitor or an MTP/Apo B secretion inhibitor.
A particularly preferred aspect of the above method is wherein the HMG-CoA
reductase inhibitor is lovastatin, simvastatin, pravastatin, fluvastatin,
atorvastatin or
rivastatin.
CA 02392979 2002-05-29
WO 01/40190 PCT/IB00/01650
-10-
Yet another aspect of this invention is a kit comprising:
a. a first compound, said first compound being a Formula I crystal, and a
pharmaceutically acceptable carrier in a first in a unit dosage form;
b. a second compound, said second compound being an HMG CoA
reductase inhibitor, an MTP/Apo B secretion inhibitor, a cholesterol
absorption
inhibitor, a cholesterol synthesis inhibitor, a fibrate, niacin, an ion-
exchange resin, an
antioxidant, an ACAT inhibitor or a bile acid sequestrant and a
pharmaceutically
acceptable carrier in a second unit dosage form; and
c. means for containing said first and second dosage forms wherein the
amounts of the first and second compounds result in a therapeutic effect.
A preferred second compound is an HMG-CoA reductase inhibitor or an
MTP/Apo B secretion inhibitor.
A particularly preferred HMG-CoA reductase inhibitor is lovastatin,
simvastatin, pravastatin, fluvastatin, atorvastatin or rivastatin.
The present invention is also directed to processes for preparing crystalline
anhydrous [2R,4S] 4-[(3,5-bis-trifluoromethyl-benzyl)-methoxycarbonyl-amino]-2-
ethyl-6-trifluoromethyl-3,4-dihydro-2H-quinoline-1-carboxylic acid ethyl ester
by
dissolving or mixing [2R,4S] 4-[(3,5-bis-trifluoromethyl-benzyl)-
methoxycarbonyl-
amino]-2-ethyl-6-trifluoromethyl-3,4-dihydro-2H-quinoline-1-carboxylic acid
ethyl ester
in the presence of a suitable organic solvent, preferably hexanes.
Another aspect of this invention is directed to a process for preparing
crystalline ethanolate [2R,4S] 4-[(3,5-bis-trifluoromethyl-benzyl)-
methoxycarbonyl-
amino]-2-ethyl-6-trifluoromethyl-3,4-dihydro-2H-quinoline-1-carboxylic acid
ethyl ester
by dissolving or mixing [2R,4S] 4-[(3,5-bis-trifluoromethyl-benzyl)-
methoxycarbonyl-
amino]-2-ethyl-6-trifluoromethyl-3,4-dihydro-2H-quinoline-1-carboxylic acid
ethyl ester
in ethanol/water at ambient temperature for about 0.5 to about 18 hours.
Preferably ethanol is used without water.
This invention is also directed to a process for preparing crystalline
anhydrous
[2R,4S] 4-[(3,5-bis-trifluoromethyl-benzyl)-methoxycarbonyl-amino]-2-ethyl-6-
trifluoromethyl-3,4-dihydro-2H-quinoline-1-carboxylic acid ethyl ester
comprising
dissolving or mixing [2R,4S] 4-[3,5-bis-trifluoromethyl-benzyl)-
methoxycarbonyl-
amino]-2-ethyl-6-trifluoromethyl-3,4-dihydro-2H-quinoline-1-carboxylic acid
ethyl ester
in ethanol at ambient temperature for about 2 to about 24 hours.
CA 02392979 2002-05-29
WO 01/40190 PCT/IB00/01650
-11-
It is noted that as the anhydrous and ethanolate crystals are of different
energy levels seeding with either anhydrous or ethanolate may determine the
resulting isolated crystalline form. As is known in the art the presence of
seed
crystals in the air in a lab may be sufficient "seeding." In one embodiment
anhydrous
crystals may be obtained using hexanes and the resulting anhydrous crystals
may be
used to seed the production of further anhydrous crystals from ethanol.
As used herein the term mammals is meant to refer to all mammals which
contain CETP in their plasma, for example, rabbits and primates such as
monkeys
and humans. Certain other mammals e.g., dogs, cats, cattle, goats, sheep and
horses do not contain CETP in their plasma and so are not included herein.
The term ethanolate refers to an ethanol of solvation.
The term "treating", "treat" or "treatment" as used herein includes
preventative
(e.g., prophylactic) and palliative treatment.
By "pharmaceutically acceptable" it is meant the carrier, vehicle, diluent,
excipients, and/or salt must be compatible with the other ingredients of the
formulation, and not deleterious to the recipient thereof.
As used herein, the expressions "reaction-inert solvent" and "inert solvent"
refers to a solvent or mixture of solvents which does not interact with
starting
materials, reagents, intermediates or products in a manner which adversely
affects
the yield of the desired product.
It will be recognized that the compound of this invention can exist in
radiolabelled form, i.e., said compound may contain one or more atoms
containing an
atomic mass or mass number different from the atomic mass or mass number
usually
found in nature. Radioisotopes of hydrogen, carbon, phosphorous, fluorine and
chlorine include 3H,'4C, 32P, 355,'8F and 36C1, respectively. The compound of
this
invention which contains those radioisotopes and/or other radioisotopes of
other
atoms is within the scope of this invention. Tritiated, i.e., 3H, and carbon-
14, i.e., '4C,
radioisotopes are particularly preferred for their ease of preparation and
detectability.
A radiolabelled compound of this invention can generally be prepared by
methods
well known to those skilled in the art. Conveniently, such radiolabelled
compounds
can be prepared by carrying out the procedures disclosed in the Examples below
by
substituting a readily available radiolabelled reagent for a non-radiolabelled
reagent.
Other features and advantages will be apparent from the specification and
claims which describe the invention.
CA 02392979 2002-05-29
WO 01/40190 PCT/IB00/01650
-12
Brief Description of the Drawings
FIG. 1 is a characteristic x-ray powder diffraction pattern showing that
anhydrous [2R,4S] 4-[(3,5-bis-trifluoromethyl-benzyl)-methoxycarbonyl-amino]-2-
ethyl-6-trifluoromethyl-3,4-dihydro-2H-quinoline-1-carboxylic acid ethyl ester
is
crystalline. (Vertical Axis: Intensity (CPS); Horizontal Axis: Two theta
(degrees))
FIG. 2 is the characteristic x-ray powder diffraction pattern of the
ethanolate
[2R,4S] 4-[(3,5-bis-trifluoromethyl-benzyl)-methoxycarbonyl-amino]-2-ethyl-6-
trifluoromethyl-3,4-dihydro-2H-quinoline-1-carboxylic acid ethyl ester as
crystalline
Vertical Axis: Intensity (CPS); Horizontal Axis: Two theta (degrees))
Detailed Description Of The Invention
In general the compound of this invention can be made by processes which
include analogous processes known in the chemical arts, particularly in light
of the
description contained herein. Certain processes for the manufacture of the
compound
of this invention are provided as further features of the invention and are
described
below including in the Examples.
The amorphous form of the compound of this invention [2R,4S] 4-[(3,5-bis-
trifluoromethyl-benzyl)-methoxycarbonyl-amino]-2-ethyl-6-trifluoromethyl-3,4-
dihydro-
2H-quinoline-1-carboxylic acid ethyl ester is prepared as disclosed below (see
Example 1 ).
An anhydrous crystalline form of the above compound may be prepared from
the amorphous compound by recrystallization from hexanes (solvent comprised of
hexane isomers (e.g., n-hexane, cyclohexane, methyl pentane, etc.)) at a
temperature of about 40°C to about 80°C, preferably 60°
followed typically by
granulating, for about 2 to about 24 hours, then filtering the material and
subsequent
air drying.
Alternatively, the anhydrous crystal may be prepared from the ethanolate
crystalline form (described below) utilizing analogous procedures to the
immediately
preceding procedure. In addition, the yield in this procedure may be enhanced
by
azeotroping the ethanol from the hexanes.
An ethanolate crystalline form of the above compound may be prepared from
the amorphous compound by recrystallization from ethanol/water at a
temperature of
about 20°C to about 25°C, preferably ambient temperature for
about 0.5 hour to
about 18 hours. Typically the range is about 3% to about 10% ethanol and about
CA 02392979 2002-05-29
WO 01/40190 PCT/IB00/01650
-13
90% to about 97% water. Preferably the ratio is about 10% to about 90%
ethanol/water.
Alternatively, the ethanolate crystalline form may be prepared utilizing
procedures analogous to those described above but using ethanol alone. The
filtered
materials are typically granulated for about 2 hours to about 24 hours
followed by air
drying.
The following Table 1 details important properties for three forms of [2R,4S]
4-[(3,5-bis-trifluoromethyl-benzyl)-methoxycarbonyl-amino]-2-ethyl-6-
trifluoromethyl-
3,4-dihydro-2H-quinoline-1-carboxylic acid ethyl ester: the amorphous (A); and
the
two crystalline forms ethanolate (B) and crystalline anhydrous (C).
CA 02392979 2002-05-29
WO 01/40190 PCT/IB00/01650
-14
TABLE 1
Thermal
Stability Crvstallinitv Solubility Stability
Amorphous M.P. 21 C Non- Most solublehygrocopic
'
A crystallinein aqueous
'EthanolateMelt onset CrystallineHigher non-hygroscopic
B 45C solubility C~ 90% relative
in
(Fig. 2) aqueous thanhumidity
over 24
Anhydrous hours
C
Anhydrous M.P. 89-90C CrystallineLeast solublenon-hygroscopic
C in water at 80% &
100%
(Fig. 1 relative
) humidity
over 3 da
s.
'Loses thanol at
some e closed bottle
ambient
conditions
but remains
crystalline
The compound of the instant invention is orally administrable and is
accordingly
used in combination with a pharmaceutically acceptable vehicle, carrier or
diluent
suitable to oral dosage forms. Suitable pharmaceutically-acceptable carriers
include
inert solid fillers or diluents and sterile aqueous or organic solutions. The
active
compound will be present in such pharmaceutical compositions in amounts
sufficient
to provide the desired dosage amount in the range described below. Thus, for
oral
administration the compound may be combined with a suitable solid or liquid
carrier
or diluent to form capsules, tablets, powders, syrups, solutions, suspensions
and the
like. The pharmaceutical compositions may, if desired, contain additional
components such as flavorants, sweeteners, excipients and the like.
The tablets, pills, capsules, and the like may also contain a binder such as
gum
tragacanth, acacia, corn starch or gelatin; excipients such as dicalcium
phosphate; a
disintegrating agent such as corn starch, potato starch, alginic acid; a
lubricant such
as magnesium stearate; and a sweetening agent such as sucrose, lactose or
saccharin. When a dosage unit form is a capsule, for example a gel capsule, it
may
contain, in addition to or instead of materials of the above type, a liquid
carrier such
as a fatty glyceride or mixtures of fatty glycerides, such as olive oil, or
MigIyoITM or
CapmuITM glycerides. Dosage forms may also include orral suspensions.
Various other materials may be present as coatings or to modify the physical
form of the dosage unit. For instance, tablets may be coated with shellac,
sugar or
both. A syrup or elixir may contain, in addition to the active ingredient,
sucrose as a
CA 02392979 2002-05-29
WO 01/40190 PCT/IB00/01650
-15
sweetening agent, methyl and propylparabens as preservatives, a dye and a
flavoring
such as cherry or orange flavor.
The compound of the instant invention may also be administered parenterally.
For parenteral administration the compound may be combined with sterile
aqueous or
organic media to form injectable solutions or suspensions. The injectable
solutions
prepared in this manner can then be administered intravenously,
intraperitoneally,
subcutaneously, or intramuscularly.
The pharmaceutical forms suitable for injectable use include sterile solutions
or
dispersions and sterile powders for the extemporaneous preparation of sterile
injectable solutions or dispersions. In all cases, the form must be sterile
and must be
fluid to the extent that easy syringability exists. It must be stable under
the conditions
of manufacture and storage and must be preserved against the contaminating
action
of microorganisms such as bacteria and fungi. They may be sterilized, for
example,
by filtration through a bacteria-retaining filter, by incorporating
sterilizing agents into
the compositions, or by irradiating or heating the compositions where such
irradiating
or heating is both appropriate and compatible with the drug formulation.
Additional pharmaceutical formulations may include, inter alia, suppositories,
sublingual tablets, topical dosage forms and the like and these may be
prepared
according to methods which are commonly accepted in the art.
Controlled release, sustained release, and delayed release oral or parenteral
compositions may be used.
The dosage of the compound of the instant invention which is administered will
generally be varied according to principles well known in the art taking into
account
the severity of the condition being treated and the route of administration.
In general,
the compound will be administered to a warm blooded animal (such as a human,
livestock or pet) so that an effective dose, usually a daily dose administered
in unitary
or divided portions, is received, for example a dose in the range of about
0.01 to
about 100 mg/kg/day body weight, preferably about 0.1 to about 10 mg/kg/day
body
weight. The above dosages are exemplary of the average case; there can, of
course, be individual instances where higher or lower dosage ranges are
merited,
and such deviations are within the scope of this invention.
EXAMPLES
Melting points were determined with a Thomas Hoover melting point
apparatus or a DSC apparatus. Unless otherwise stated, CD3C13 was used for NMR
CA 02392979 2002-05-29
WO 01/40190 PCT/IB00/01650
-16
spectra. Microanalysis was performed by Schwarzkopf Microanalytical
Laboratory.
All reagents and solvents were obtained commercially and used without
purification.
Example 1
cis-4-((3 5-Bis-trifluoromethVl-benzyl)-methoxycarbonyl-aminol-2-ethyl-6-
trifluoromethyl-3 4-dihydro-2H-auinoline-1-carboxylic acid ethyl ester:
A solution of cis-4-(3,5-bis-trifluoromethyl-benzylamino)-2-ethyl-6-
trifluoromethyl-3,4-
dihydro-2H-quinoline-1-carboxylic acid ethyl ester (2.0 g, 3.7 mmol) and
pyridine
(0.58 g, 7.4 mmol) in 100 mL of dichloromethane was cooled in an ice/water
bath as
methyl chloroformate (0.87 g, 9.2 mmol) was added slowly. After stirring
overnight at
room temperature, the reaction mixture was washed twice with a 2N hydrochloric
acid
solution, dried over magnesium sulfate, filtered and concentrated in vacuo to
afford
the crude product, which was purified by silica gel chromatography using 5-10%
ethyl
acetate/hexanes as eluent to afford 1.8 g of the title product. MS mfz 601 (M+
+ 1 );
'H NMR (coalescing mixture of conformers, CDC13) 8 0.6-0.8 (bm, 3H), 1.2-1.3
(bm,
3H), 1.3-1.5 (bm, 2H), 1.6-1.75 (bm, 1 H), 2.1-2.3 (bm, 1 H), 3.7-3.9 (bs,
3H), 4.0-4.4
(bm, 4H), 5.0-5.6 (bm, 2H), 7.1 (s, 1 H), 7.4-7.6 (bm, 2H), 7.6-7.8 (bm, 3H).
[2R,4S]4-[(3,5-bis-trifluoromethyl-benzyl)-methoxycarbonyl-amino]-2-ethyl-6-
trifluoromethyl-3,4-dihydro-2H-quinoline-1-carboxylic acid ethyl ester was
prepared in
optically enriched form by resolution of the corresponding racemate, or an
intermediate in its synthesis, using standard methods.
Example 2
(1-Benzotriazol-1-yl-propel)-(4-trifluoromethyl-phenyl)-amine
A two liter, four neck flask under nitrogen atmosphere was charged with
benzotriazole (36.96 g, 310 mmol, 1.0 equiv) and dry toluene (400 mL). A room
temperature solution of 4-(trifluoromethyl)aniline (39.1 mL, 310 mmol, 1.0
equiv) and
50 mL toluene was added over one minute. A room temperature solution of
propionaldehyde (24.6 mL, 341 mmol, 1.1 equiv) and 50 mL toluene was then
added
over 20 minutes. There was an exotherm from 23°C to 30°C during
this addition.
After stirring 24 h, n-heptane (500 mL) was added, and the slurry stirred an
additional
1 h. The suspension was filtered, the solids were washed with n-heptane (1 x
100
mL, then 1 x 200 mL, and dried. (1-Benzotriazol-1-yl-propyl)-(4-
trifluoromethyl-
phenyl)-amine was isolated as shiny white needles (81.3 g, 82%). After 24 h, a
second crop was isolated from the filtrate (8.7 g, 9%). mp 130-132 °C;
'H NMR
CA 02392979 2002-05-29
WO 01/40190 PCT/IB00/01650
-17_
(DMSO-d6, 400 MHz) 8 0.82 (t, 3H, J=7.5 Hz), 2.25 (m, 2H), 6.49 (m, 1 H), 6.80
(d,
2H, J=8.7 Hz), 7.35 (m, 3H), 7.50 (m, 1 H), 7.88 (d, 1 H, J=8.3 Hz), 7.99 (m,
1 H), 8.09
(d, 1H, J=8.5 Hz);'3C NMR (DMSO-d6, 100 MHz) 8 149.32, 146.19, 131.46, 127.73,
126.8, 125.33 (q, J=270 Hz), 124.44, 119.88, 118.27 (q, J=31.7 Hz), 112.91,
111.56,
71.03, 28.08, 10.29; DEPT spectrum: quaternary carbons 8 149.32, 146.19,
131.46,
125.33, 118.27; CH carbons 8 127.73, 126.8, 124.44, 119.88, 112.91, 111.56,
71.03;
CH2 carbon 8 28.08; CH3 carbon 8 10.29; IR (drifts) 3292 (s), 3038 (m), 2975
(m),
1621 (s), 1331 (s), 1320 (s), 1114 (vs); Anal. Calcd for C,6H,SN4F3: C, 59.99;
H, 4.72;
N, 17.49. Found (first crop): C, 60.16; H, 4.74; N, 17.86. Found (second
crop): C,
59.97; H, 4.66; N, 17.63.
Example 3
cis-(2-Ethyl-6-trifluoromethyl-1 2 3 4-tetrahydro-quinolin-4-yl)-carbamic acid
benzyl
ester
A one liter, four neck flask under nitrogen atmosphere was charged with N-
vinyl-
carbamic acid benzyl ester (27.66 g, 156 mmol, 1.0 equiv) and dry toluene (500
mL).
(1-Benzotriazol-1-yl-propyl)-(4-trifluoromethyl-phenyl)-amine (50.0 g, 156
mmol, 1.0
equiv) and p-toluenesulfonic acid monohydrate (297 mg, 1.56 mmol, 0.01 equiv)
were
added, and the mixture heated to 70°C. After 2 h, the mixture was
cooled to room
temperature and transfered to a separatory funnel. Ethyl acetate (500 mL) was
added. The mixture was washed 1 x 200 mL 1 N NaOH, 1 x 200 mL H20, 1 x 200 mL
brine, and dried (MgS04). The mixture was filtered and the solids washed 1 x
50 mL
ethyl acetate: The filtrate was concentrated to approximately 250 mL. 500 mL
toluene were added, and the mixture concentrated to approximately 500 mL. 500
mL
n-heptane were added, the slurry was stirred 1 h, filtered through a Buchner
funnel,
and dried. cis-(2-Ethyl-6-trifluoromethyl-1,2,3,4-tetrahydro-quinolin-4-yl)-
carbamic
acid benzyl ester was isolated as a white powder (45.04 g, 76%): mp 155-157
°C; ' H
NMR (DMSO-d6, 400 MHz) b 0.92 (t, 3H, J=7.5 Hz), 1.5 (m, 3H), 2.00 (m, 1 H),
3.35
(m, 1 H), 4.77 (m, 1 H), 5.07 (d, 1 H, J=12.5 Hz), 5.15 (d, 1 H, J=12.5 Hz),
6.35 (s, 1 H),
6.61 (d, 1 H, J=8.5 Hz), 7.12 (s, 1 H), 7.18 (dd, 1 H, J=1.9, 8.5 Hz), 7.4 (m,
5H), 7.70
(d, 1 H, J=9.1 Hz);'3C NMR (DMSO-d6, 100 MHz) cS 157.03, 149.02, 137.79,
128.82,
128.23, 128.03, 125.9 (q, J=270 Hz), 125.06, 123.50, 121.73, 115.2 (q, J=31.7
Hz),
113.33, 65.85, 52.09, 47.83, 34.02, 28.68, 9.93; DEPT spectrum: quaternary
carbons 8 157.03, 149.02, 137.79, 125.9, 121.73, 115.2; CH carbons 8 128.82,
CA 02392979 2002-05-29
WO 01/40190 PCT/IB00/01650
_18_
128.23, 128.03, 125.06, 123.50, 113.33, 52.09, 47.83; CH2 carbons b 65.85,
34.02,
28.68; CH3 carbon 8 9.93; IR (drifts) 3430 (m), 3303 (s), 2951 (m), 1686 (vs),
1542
(vs), 1088 (vs); MS (APCI+) m/z (rel. intensity) 379 (M+H+, 53), 228 (100);
Anal.
Calcd for C2oH2,N202F3: C, 63.48; H, 5.59; N, 7.40; Found: C, 63.69; H, 6.06,
N,
7.36.
Example 4
cis-4-Benzyloxycarbonylamino-2-ethyl-6-trifluoromethyl-3,4-dihydro-2H-
quinoline-1-
carboxylic acid ethyl ester
A three liter, four neck flask under nitrogen atmosphere was charged with cis-
(2-
ethyl-6-trifluoromethyl-1,2,3,4-tetrahydro-quinolin-4-yl)-carbamic acid benzyl
ester
(96.0 g, 254 mmol, 1.0 equiv), dry dichloromethane (720 mL), and dry pyridine
(103
mL, 1.27 mol, 5.0 equiv). A solution of ethyl chloroformate (121 mL, 1.27 mol,
5.0
equiv), in dry dichloromethane (240 mL), was added slowly over 4 h. The
addition
was exothermic and required a reflux condenser. Once the chloroformate
addition
was complete, the reaction was cooled in an ice bath and 1350 mL 1 N NaOH were
added. The mixture was stirred 15 min, then transferred to a separatory
funnel. The
layers were separated and the aqueous extracted 1 x 1 L dichloromethane. The
combined dichloromethane layers were washed 1 x 1350 mL 1 N HCI, 1 x 1 L
saturated aq. NaHC03, 1 x 1 L brine, and dried (Na2SOa). The mixture was
filtered,
and the filtrate concentrated to an orange oil. 570 mL abs. ethanol were
added, and
the solution was concentrated. The solids were dissolved in 1370 mL abs.
ethanol.
570 mL H20 were added dropwise over 45 min. The resultant thick slurry was
stirred
18 h and filtered. The solids were washed with cold 7:3 abs. ethanol/water (1
x 250
mL, then 1 x 100 mL) and dried (vac oven, 45°C) to give cis-4-
benzyloxycarbonylamino-2-ethyl-6-trifluoromethyl-3,4-dihydro-2H-quinoline-1-
carboxylic acid ethyl ester as a white, crystalline solid (94.54 g, 83%): mp
92-96°C;
'H NMR (CDC13, 400 MHz) 8 0.84 (t, 3H, J=7.4 Hz), 1.28 (t, 3H, J=7.0 Hz), 1.4
(m,
2H), 1.62 (m, 1 H), 2.53 (m, 1 H), 4.23 (m, 2H), 4.47 (m, 1 H), 4.79 (m, 1 H),
5.01 (d,
1 H, J=9.2 Hz), 5.18 (m, 2H), 7.4 (m, 5H), 7.5 (m, 2H), 7.57 (m, 1 H);'3C NMR
(CDCI3,
100 MHz) 8 155.97, 154.43, 139.44, 136.21, 134.33, 128.61, 128.33, 128.22,
126.32
(q, J=31.7 Hz), 126.18, 124.22, 124.19, 124.12 (q, J=273 Hz), 120.74, 120.70,
67.22,
62.24, 53.47, 46.79, 37.75, 28.25, 14.38, 9.78; DEPT spectrum: quaternary
carbons
8 155.97, 154.43, 139.44, 136.21, 134.33, 126.32, 124.12; CH carbons 8 128.61,
CA 02392979 2002-05-29
WO 01/40190 PCT/IB00/01650
_19_
128.33, 128.22, 126.18, 124.22, 124.19, 120.74, 120.70, 53.47, 46.79; CH2
carbons ~
67.22, 62.24, 37.75, 28.25; CH3 carbons 8 14.38, 9.78; IR (drifts) 3304 (s),
3067 (m),
3033 (m), 2982 (m), 2932 (m), 1723 (s), 1693 (s), 1545 (s); MS (APCI+) m/z
(rel.
intensity) 451 (M+H+, 2), 300 (100); Anal. Calcd for C23H2sN20aF3: C, 61.33;
H, 5.60;
N, 6.22. Found: C, 61.07; H, 5.69; N, 6.22.
Example 5
cis-4-Amino-2-ethyl-6-trifluoromethyl-3 4-dihydro-2H-quinoline-1-carboxylic
acid ethyl
ester
A one liter, four neck flask under nitrogen atmosphere was charged with cis-4-
benzyloxycarbonylamino-2-ethyl-6-trifluoromethyl-3,4-dihydro-2H-quinoline-1-
carboxylic acid ethyl ester (40.1 g, 89 mmol, 1.0 equiv), methanol (400 mL),
and
ammonium formate (14.0 g, 223 mmol, 2.5 equiv). 10% Pd/C, 50% water wet (4.0
g)
was added, and the slurry heated to 40° C over 1 h. After 1.5 h, the
mixture was
cooled to room temperature and filtered through Celite~. The cake was washed 2
x
100 mL methanol. The filtrate was concentrated to approximately 75 mL,
transferred
to a separatory funnel, and diluted with 400 mL ethyl acetate. The mixture was
washed 1 x 125 mL saturated aq. NaHC03, 1 x 100 mL brine, and dried (Na2S04).
The mixture was filtered and the filtrate concentrated to a clear oil. The oil
was
crystallized from 100 mL n-heptane to give cis-4-amino-2-ethyl-6-
trifluoromethyl-3,4-
dihydro-2H-quinoline-1-carboxylic acid ethyl ester as a white crystalline
solid (26.05 g,
93%): mp 61.5-63.5° C;'H NMR (CDC13, 400 MHz) 8 0.79 (t, 3H, J=7.5 Hz),
1.24 (m,
4H), 1.42 (m, 1 H), 1.51 (br s, 2H), 1.62 (m, 1 H), 2.46 (m, 1 H), 3.73 (m, 1
H), 4.17 (m,
2H), 4.36 (m, 1 H), 7.44 (m, 2H), 7.66 (m, 1 H); '3C NMR (CDC13, 100 MHz) 8
154.6,
139.3, 138.9, 126.3 (q, J=32 Hz), 125.7, 124.3 (q, J=271 Hz), 123.5, 119.8,
61.96,
54.16, 46.91, 41.50, 28.85, 14.38, 9.60; DEPT spectrum: quaternary carbons 8
154.6, 139.3, 138.9, 126.3, 124.3; CH carbons b 125.7, 123.5, 119.8, 54.16,
46.91;
CHz carbons 8 61.96, 41.50, 28.85; CH3 carbons 8 14.38, 9.60; IR (drifts) 3350
(s),
3293 (m), 2972 (s), 1697 (vs); MS (ES+) m/z (rel. intensity) 358 (M+H+CH3CN+,
55),
317 (M+H+, 7), 300 (100); Anal. Calcd for C,SH,gN202F3: C, 56.96; H, 6.06; N,
8.86.
Found: C, 56.86; H, 6.28; N, 8.82.
Example 6
(-) (2R 4S)-4-Amino-2-ethyl-6-trifluoromethyl-3,4-dihydro-2H-quinoline-1-
carboxylic
acid ethyl ester hemi-(-)-dibenzoyl-L-tartrate salt
CA 02392979 2002-05-29
WO 01/40190 PCT/IB00/01650
-20-
A one liter flask under nitrogen atmosphere was charged with cis-4-
benzyloxycarbonylamino-2-ethyl-6-trifluoromethyl-3,4-dihydro-2H-quinoline-1-
carboxylic acid ethyl ester (24.0 g, 75.9 mmol, 1.0 equiv) and (-) dibenzoyl-L-
tartaric
acid (anhydrous) (27.19 g, 75.9 mmol, 1.0 equiv). 300 mL of approximately 97%
ethanol (prepared by adding 10.5 mL H20 to 500 mL absolute ethanol, mixing,
and
measuring out 300 mL) was added. The mixture was stirred at room temperature
for
18 h, then filtered. The solids were washed 1 x 48 mL approximately 97%
ethanol,
and dried to give (-) (2R, 4S)-4-amino-2-ethyl-6-trifluoromethyl-3,4-dihydro-
2H-
quinoline-1-carboxylic acid ethyl ester hemi-(-)-dibenzoyl-L-tartrate salt as
a white
crystalline solid (14.77 g, 39%): mp 189.5-191.5 °C (dec);'H NMR (DMSO-
d6, 400
MHz) 8 0.62 (t, 3H, J=7.3 Hz), 1.16 (t, 3H, J=7.1 Hz), 1.3 (m, 3H), 2.5 (m, 1
H), 4.1 (m,
4H), 5.63 (s, 1 H, methine proton in DBTA), 7.47 (m, 2H, DBTA aromatic H's),
7.6 (m,
3H, DBTA aromatic H's), 7.68 (s, 1 H), 7.95 (m, 2H), 8.2 (br s, NH3+, did not
integrate);'3C NMR (DMSO-d6, 100 MHz) 8 169.85, 165.53, 154.10, 140.14,
134.59,
133.51, 130.74, 129.69, 128.98, 126.74, 124.82 (q, J=31.7 Hz), 124.69 (q,
J=271
Hz), 124.50, 120.90, 74.49, 62.14, 53.51, 45.94, 38.81, 28.23, 14.63, 9.58;
DEPT
spectrum: quaternary carbons 8 169.85, 165.53, 154.10, 140.14, 134.59, 130.74,
124.82, 124.69; CH carbons 8 133.51, 129.69, 128.98, 126.74, 124.50, 120.90,
74.49, 53.51, 45.94; CH2 carbons 8 62.14, 38.81, 28.23; CH3 carbons 8 14.63,
9.58;
IR (drifts) 3278 (m), 2400-3100 (broad), 1703 (vs); MS (ES+) m/z (rel.
intensity) 358
(M+H+CH3CN+, 55), 317 (M+H+, 7), 300 (100); Anal. Calcd for
C,5H,9N202F3.C9H,04:
C, 58.18; H, 5.29; N, 5.65. Found: C, 57.99; H, 5.15; N, 5.64; Chiral HPLC:
mobile
phase 950:50:2 n-hexane:2-propanoI:HOAc, flow rate 1.50 mUmin, column temp
40°C, chiralpakTM AD 4.6 x 250 mm, sample concentration approximately
0.5 mg/mL
in approximately 1:1 n-hexane:2-propanol. Authentic racemate shows retention
times
of 7.5 min and 10.0 min. (-) (2R, 4S)-4-Amino-2-ethyl-6-trifluoromethyl-3,4-
dihydro-
2H-quinoline-1-carboxylic acid ethyl ester hemi-(-)-dibenzoyl-L-tartrate salt:
10.0 min,
88.9%, 7.5 min «1 %, 2.0 min (solvent front) 11.1 %; [a]p = -153 (c=1.07,
CH30H).
Example 7
(-)-(2R, 4S)-4-(3,5-Bis-trifluoromethyl-benzylamino)-2-ethyl-6-trifluoromethyl-
3,4-
dihydro-2H-guinoline-1-carboxylic acid ethyl ester tosylate salt
(-) (2R, 4S)-4-Amino-2-ethyl-6-trifluoromethyl-3,4-dihydro-2H-quinoline-1-
carboxylic
acid ethyl ester hemi-(-)-dibenzoyl-L-tartrate salt (13.0 g, 26.2 mmol, 1.0
equiv) was
CA 02392979 2002-05-29
WO 01/40190 PCT/IB00/01650
-21
suspended in 1,2-dichloroethane (260 mL) in a 500 mL separatory funnel. The
mixture was washed 1 x 65 mL 1 N NaOH, 1 x 65 mL brine, and dried (MgS04). The
mixture was filtered, concentrated to approximately approximately 80 mL, and
transferred to a 250 mL three neck flask. 3,5-Bis(trifluoromethyl)benzaldehyde
(4.53
mL, 27.5 mmol, 1.05 equiv) was added, and the mixture stirred 1 h at room
temperature under nitrogen atmosphere. Sodium triacetoxyborohydride (11.1 g,
52.4
mmol, 2.0 equiv) was added in one portion, and the white slurry was stirred 18
h. 50
mL 1,2-dichloroethane and 50 mL 2N NaOH were added, and the aqueous layer
extracted 2 x 50 mL 1,2-dichloroethane. The combined organic extracts were
washed 1 x 31 mL 1 N HCI, 1 x 50 mL saturated aq. NaHC03, 1 x 50 mL brine, and
dried (Na2S04). The mixture was filtered and concentrated to a clear oil. The
oil was
dissolved in methanol (71 mL). p-Toluenesulfonic acid monohydrate (5.23 g,
27.5
mmol, 1.05 equiv) was added. After 5 min, 284 mL isopropyl ether was added.
The
solution was concentrtated to approximately 35mL, transferred to a 500 mL
three
neck flask (mech. stirrer), and diluted with 284 mL isopropyl ether. A thick
white
slurry formed in 10 minutes. After stirring 3 h, the slurry was filtered and
the cake
washed 2 x 70 mL isopropyl ether. After drying, (-)-(2R, 4S)-4-(3,5-bis-
trifluoromethyl-benzylamino)-2-ethyl-6-trifluoromethyl-3,4-dihydro-2H-
quinoline-1-
carboxylic acid ethyl ester tosylate salt was isolated as a white powder
(16.18 g, 86%
overall): mp 191-192°C; ' H NMR (DMSO-d6, 400 MHz) 8 0.78 (t, 3H, J=7.5
Hz), 1.21
(t, 3H, J=7.0 Hz), 1.5 (m, 3H), 2.24 (s, 3H), 3.08 (m, 1 H), 4.17 (m, 2H),
4.41 (m, 1 H),
4.50 (m, 2H), 4.79 (m, 1 H), 7.04 (d, 2H, J=7.9 Hz), 7.42 (d, 2H, J=7.9 Hz),
7.7 (m,
2H), 7.81 (s, 1 H), 8.21 (s, 1 H), 8.35 (s, 2H), 9.58 (br s, 1 H), 9.83 (br s,
1 H);'3C NMR
(DMSO-d6, 100 MHz) 8 154.00, 145.46, 140.21, 138.39, 135.33, 132.51, 131.62,
130.79 (q, J=33.2 Hz), 128.49, 127.40, 125.82, 125.36, 124.99 (q, J=31.7 Hz),
124.59 (q, J=271 Hz), 123.69 (q, J=273 Hz), 123.44, 120.33, 62.32, 53.99,
53.79,
47.98, 33.30, 28.61, 21.13, 14.63, 9.58; DEPT spectrum: quaternary carbons b
154.00, 145.46, 140.21, 138.39, 135.33, 130.79, 124.99, 124.59, 123.69; CH
carbons
8 132.51, 131.62, 128.49, 127.40, 125.82, 125.36, 123.44, 120.33, 53.99,
53.79; CH2
carbons 8 62.32, 47.98, 33.30. 28.61; CH3 carbons S 21.13, 14.63, 9.58: IR
(drifts)
2300-3100 (broad), 2974 (m), 2731 (m), 2620 (m), 2455 (m), 1714 (s), 1621 (m),
1283 (vs), 1169 (vs), 1126 (vs); MS (ES+) m/z (rel. intensity) 584
(M+H+CH3CN+,
100), 543 (M+H+, 80); Anal. Calcd for C24H23N202F9.C~Ha03S: C, 52.11; H, 4.37;
N,
CA 02392979 2002-05-29
WO 01/40190 PCT/IB00/01650
-22
3.92. Found: C, 52.15; H, 4.22; N, 3.69; [a]o = -77.9 (c = 1.05, CH30H).
Example 8
(-)-(2R 4S)-4-f(3 5-Bis-trifluoromethyl-benzyl)-methoxycarbonyl-aminol-2-ethyl-
6-
trifluoromethyl-3 4-dihydro-2H-quinoline-1-carboxylic acid ethyl ester mono
ethanolate
Na2C03 (s) (6.75 g, 63.7 mmol, 3.5 equiv) was added to a room temperature
solution
of (-)-(2R, 4S)-4-(3,5-bis-trifluoromethyl-benzylamino)-2-ethyl-6-
trifluoromethyl-3,4-
dihydro-2H-quinoline-1-carboxylic acid ethyl ester tosylate salt (13.0 g, 18.2
mmol,
1.0 equiv) in dry THF (130 mL). Methyl chloroformate (3.51 mL, 45.5 mmol, 2.5
equiv) was added neat, dropwise over 2 min. After 24 h, the mixture was
concentrated to 65 mL, diluted with 260 mL ethyl acetate, and transferred to a
separatory funnel. The mixture was washed 1 x 90 mL 1 N HCI (C02 evolution), 1
x
90 mL saturated aq. NaHC03, 1 x 90 mL brine, and dried (MgS04). Filtration and
concentration of filtrate afforded a clear oil, which was costripped 3 x 33 mL
2B
ethanol. The oil was dissolved in 33 mL 2B ethanol and seeded with a few
milligrams
of (-)-(2R, 4S)-4-[(3,5-bis-trifluoromethyl-benzyl)-methoxycarbonyl-amino]-2-
ethyl-6-
trifluoromethyl-3,4-dihydro-2H-quinoline-1-carboxylic acid ethyl ester mono
ethanolate. After stirring 18 h at room temperature, the slurry was filtered
and dried
to give (-)-(2R, 4S)-4-[(3,5-bis-trifluoromethyl-benzyl)-methoxycarbonyl-
amino]-2-
ethyl-6-trifluoromethyl-3,4-dihydro-2H-quinoline-1-carboxylic acid ethyl ester
mono
ethanolate as a white crystalline powder (8.66 g, 74%): mp 54-58 °C;'H
NMR
(CDCI3, 400 MHz, 55°C) 8 0.73 (t, 3H, J=7.0 Hz), 1.20 (t, EtOH), 1.27
(t, 3H, J=7.1
Hz), 1.42 (m, 2H), 1.66 (m, 1 H), 2.25 (br s, 1 H), 3.67 (q, EtOH), 3.79 (s,
3H), 4.2 (m,
3H), 4.33 (m, 1 H), 5.2 (br s, 2H), 7.12 (s, 1 H), 7.49 (d, 1 H, J=8.3 Hz),
7.57 (d, 1 H,
J=8.5 Hz), 7.73 (s, 2H), 7.78 (s, 1 H);'3C NMR (CDC13, 400 MHz) S 157.74,
154.37,
141.73, 140.05, 133.83, 132.14 (q, J=33 Hz), 126.94, 124.49, 123.96 (q, J=273
Hz),
123.13 (q, J=273 Hz), 121.31, 119.17, 62.29, 58.28, 54.42, 53.71, 53.08,
46.67,
37.01, 29.02, 18.29, 14.32, 9.22, (note: the fourth quartet appears to be
buried under
the 8 126.94 peak, with J approximately 32 Hz); DEPT spectrum: quaternary
carbons
8 157.74, 154.37, 141.73, 140.05, 133.83, 132.14, 123.96, 123.13; CH carbons 8
126.94, 124.49, 121.31, 119.17, 54.42, 53.08; CH2 carbons 8 62.29, 58.28,
46.67,
37.01, 29.02; CH3 carbons 8 53.71, 18.29, 14.32, 9.22; IR (drifts) 3489 (s),
2974 (s),
2884 (m), 1701 (vs), 1280 (vs), 1131 (vs); MS (ES+) m/z (rel. intensity) 601
(M+H+,
100); Anal. Calcd for C26H2sN20aFs.C2H60: C, 52.01; H, 4.83; N, 4.33. Found:
C,
CA 02392979 2002-05-29
WO 01/40190 PCT/IB00/01650
-23
51.84; H, 4.54; N, 4.33; chiral HPLC: mobile phase 950:50:2 n-hexane:2-
propanoI:HOAc, flow rate 1.0 mUmin, 254 nm, chiralpak AD 4.6 x 250 mm, column
temp 40°C, sample concentration approximately 0.5 mg/mL in 90:10 n-
hexane:2-
propanol, authentic racemate retention times 3.6 and 4.6 min. (-)-(2R, 4S)-4-
[(3,5-Bis-
trifluoromethyl-benzyl)-methoxycarbonyl-amino]-2-ethyl-6-trifluoromethyl-3,4-
dihydro
2H-quinoline-1-carboxylic acid ethyl ester mono ethanolate shows 4.6 min, 99.1
and 3.6 min, not detected; [a]p = -93.3 (c = 1.08, CH30H).
Example 9
Anhydrous (-)-(2R 4S)-4-f(3 5-Bis-trifluoromethyl-benzyl)-methoxycarbonyl-
aminol-2-
ethyl-6-trifluoromethyl-3 4-dihydro-2H-auinoline-1-carboxylic acid ethyl
ester.
A 2.6g portion of 4(S)-[(3,5-bis-trifluoromethyl-benzyl)-methoxycarbonyl-
amino]-2(R)-
ethyl-6-trifluoromethyl-3,4-dihydro-2H-quinoline-1-carboxylic acid ethyl ester
(a
mixture of predominantly amorphous material with traces of ethanolate
crystalline
form; the title compound was also prepared in an analogous manner starting
from
pure amorphous or pure ethanolate material) was charged to 13 milliliters of
hexanes
and heated to effect a solution at about 60°C. The heat was removed and
the
reaction was allowed to cool to ambient over a one hour period. The reaction
was
seeded with anhydrous (-)-(2R,4S)-4-[(3,5-bis-trifluoromethyl-benzyl)-
methoxycarbonyl-amino]-2-ethyl-6-trifluoromethyl-3,4-dihydro-2H-quinoline-1-
carboxylic acid ethyl ester and granulated for eighteen hours under ambient
conditions. Alternately, the anhydrous crystals may be prepared from hexanes
without seeding. The product was collected by filtration and air dried. The
isolated
product X-ray pattern matched the calculated powder pattern.
Density: 1.406
Crystal System: Trigonal
Microscopy: Well formed rods and equant (fractured rods) crystals
demonstrating
high birefringence when viewed across the C axis. Being in the Trigonal
crystal
system the crystals do not demonstrate birefringence when viewed down the C
axis.
The crystals demonstrate a cleavage plane perpendicular to the C axis.
Fusion Microsocopy: In Type A oil--------dissolution at 50°C.
Dry-------------------clear melt at 86°C.
NMR: No trace of ethanolate
Degree of crystallinity: Highly crystalline
CA 02392979 2002-05-29
WO 01/40190 PCT/IB00/01650
-24-
Hygroscopicity: Non-hygroscopic at 100% relative humidity over 48 hours.
Appearance: Free flowing white powder.
The X-Ray diffraction d-spacing is provided in Table 2.
CA 02392979 2002-05-29
WO 01/40190 PCT/IB00/01650
-25-
TABLE 2
Anode: CU - Wavelength 1: 1.54056 Wavelength 2: 1.54439 (Rel Intensity: 0.500)
Range #1 - Coupled: 3.000 to 40.000 StepSize: 0.040 StepTime: 1.00
Smoothing Width: 0.300 Threshold: 1.0
d A 1 rel d A I rel d A I rel
11.21659 34.8 5.52958 60.0 4.04469 36.6
10.50618 12.0 5.39152 75.7 3.89345 39.6
9.66890 11.0 5.24818 80.5 3.72038 80.7
8.88669 4.1 4.84992 13.2 3.64330 15.0
7.31083 3.7 4.44170 100.0 3.49463 5.9
6.34185 56.4 4.32558 16.8 3.44831 7.2
6.09484 5.9 4.25150 31.0 3.33_631 14.7
5.92806 38.4 4.08413 42.7 3.22157 6.7
~
d A I rel d A I rel
3.16983 8.3 2.57207 8.5
3.11970 14.0 2.49503 3.6
2.96985 16.3 2.44562
2.87051 8.7 2.42250
2.81002 6.8 2.38844
2.75539 6.8 2.36135
2.70226 3.6 2.32612
2.64524 8.9
Example 10
Monoethanolate, (-)-(2R,4S)-4-f(3,5-Bis-trifluoromethyl-benzyl)-
methoxycarbonyl-
amino-2-ethyl-6-trifluoromethyl-3,4-dihydro-2H-auinoline-1-carboxylic acid
ethyl
ester.
4.0 grams of (-)-(2R,4S)-4-[(3,5-bis-trifluoromethyl-benzyl)-methoxycarbonyl-
amino]-
2-ethyl-6-trifluoromethyl-3,4-dihydro-2H-quinoline-1-carboxylic acid ethyl
ester-were
dissolved in 3.5 ml ethanol and sonicated for two minutes to complete
dissolution. A
white solid formed to which 10 ml ethanol was added and stirred at ambient
temperature overnight. A white powder was filtered and collected on 0.22 ~m LS
filter paper followed by washing with about 15 ml. ethanol. The isolated
product X-ray
pattern matched the calculated powder pattern.
Density: 1.402
Crystal System: orthorhombic
Microscopy: crystalline needles with moderate birefringence.
Fusion Microsocopy: In Type A oil-----melt and dissolution at 43°C with
loss of water
Dry----------------clear melt at 43°C
CA 02392979 2002-05-29
WO 01/40190 PCT/IB00/01650
-26
NMR: shows ethanol of solvation
Degree of crystallinity: highly crystalline
Hygroscopicity: non-hygroscopic
Appearance: free-flowing white power
The X-Ray diffraction d-spacing is provided in Table 3.
TABLE 3
Anode' CU - Wavelength 1: 1.54056 Wavelength 2: 1.54439 (Rel Intensity: 0.500)
Range #1 - Coupled: 3.000 to 40.000 StepSize: 0.040 StepTime: 1.00
Smoothing Width: 0.300 Threshold: 1.0
d A 1 rel d A I rel d A I rel
22.15759 37.6 5.69284 6.9 4.18443 23.3
8.61222 15.1 5.45839 5.8 4.03073 30.9
8.15185 9.5 5.19975 19.0 3.96396 33.9
7.83462 47.0 4.90695 53.6 3.83314 35.0
7.47295 100.0 4.68527 42.1 3.77447 40.8
7.00403 9.6 4.80453 18.9 3.72125 33.1
6.46476 17.2 4.38780 16.3 3.62106 26.6
6.23035 14.8 4.30354 19.7 3.52462 17.1
5.90921 7.9
d A I rel d A I rel
3.44170 12.6 2.77147 5.0
3.35282 6.7 2.70399 7.5
3.25110 11.7 2.63859 4.6
3.12884 5.7 2.53872 6.4
3.03164 4.4 2.49493 5.3
2.94892 5.8 2.47186 5.0
2.86853 4.2 2.34837 4.7
4.3 2.26951 4.1
2.79318
Example 11
Anhydrous (-)-(2R 4S)-4-((3 5-bis-trifluromethylbenzyl)-methoxycarbonyl-aminol-
2
ethvl-6-trifluoromethvl-3,4-dihvdro-2H-quinoline-1-carboxylic acid ethyl
ester.
A crude solution of approximately 42 g of (-)-(2R,4S)-4-[(3,5-bis-
trifluromethylbenzyl)-
methoxycarbonyl-amino]-2-ethyl-6-trifluoromethyl-3,4-dihydro-2H-quinoline-1-
carboxylic acid ethyl ester in 500 ml of ethyl acetate (obtained via the
process
described in Example 8) was concentrated under vacuum to a volume of 100-135
ml.
The remaining ethyl acetate was displaced with 3 X 220 ml 2B EtOH to a final
volume
CA 02392979 2002-05-29
WO 01/40190 PCT/IB00/01650
-27
of 100-135 ml. This solution was seeded with a crystal of anhydrous (-)-
(2R,4S)-4-
[(3,5-bis-trifluromethylbenzyl)-methoxycarbonyl-amino]-2-ethyl-6-
trifluoromethyl-3,4-
dihydro-2H-quinoline-1-carboxylic acid ethyl ester. After stirring 18 hr at
room
temperature the slurry was filtered and vacuum dried to give 19.81 g of
anhydrous (-)-
(2R,4S)-4-[(3,5-bis-trifluromethylbenzyl)-methoxycarbonyl-amino]-2-ethyl-6-
trifluoromethyl-3,4-dihydro-2H-quinoline-1-carboxylic acid ethyl ester. The
melting
point behaviour was the same as the material prepared via Example 9 confirming
the
anhydrous nature of the material.