Note: Descriptions are shown in the official language in which they were submitted.
WO 01/44294 CA 02394167 2002-06-11 PCT/US00/34039
1
COMPOSITIONS AND METHODS FOR 1NHIBITING
ENDOTHELIAL CELL PROLIFERATION
TECHNICAL FIELD
This application relates to novel uses for kringle domain-containing
proteins and peptides such as hepatocyte growth factor (HGF), also known as
scatter
factor, and macrophage stimulating protein (MSP), as regulators of
angiogenesis
useful for treating angiogenesis-related diseases including angiogenesis-
dependent
cancer. More specifically, the present invention relates to kringle domain
containing
proteins and peptides including active fragments of HGF and/or MSP, capable of
regulating, and preferably inhibiting angiogenesis. The invention further
relates to
novel HGF and/or MSP fragment compositions and methods for curing angiogenesis-
dependent disorders such as cancer, arthritis, blindness, diabetic
retinopathy, macular
degeneration, psoriasis and artherosclerosis. In addition, the present
invention relates
to kringle domain containing proteins and peptides antibodies that, for
example, block
the interaction of HGF (and fragments thereof) to the c-met receptor, the
molecular
probes for monitoring biosynthesis to antibodies that are specific for kringle
domain
containing proteins and peptides, to the development of peptide agonists and
antagonists to kringle domain containing proteins and peptides, and to
cytotoxic
agents linked to receptors peptides.
BACKGROUND OF THE INVENTION
Kringle domain containing proteins and peptides ark unique in that
they are characterized by triple disulfide loops structures. Examples of such
proteins
comprise hepatocyte growth factor, macrophage stimulating protein, tissue
plasminogen activator, apolipoprotein (a), prothrombin, urokinase, and
ANGIOSTATIN proteins.
Hepatocyte growth factor (HGF) is a mesenchyme derived
glycoprotein and is named for its ability to induce kidney epithelial cells in
a collagen
matrix to form branching networks of tubules. (Grant et al. PNAS 1993; 90:1937-
1941 ) As characterized in the art, HGF is considered to be a potent
angiogenic
molecule that primarily acts on endothelial cells promoting cell motility,
WO 01/44294 CA 02394167 2002-06-11 PCT/US00/34039
2
proliferation, protease production, invasion and organization into capillary-
like
tubules. (Rosen et al. Adv. Cancer Res. 1995; 67:257-79)
HGF contains 29% identity with plasminogen and within the first four
kringle domains HGF contains 44% similarity with plasminogen. HGF contains
approximately 44% identity with macrophage stimulating protein (MSP). In
addition, all of the cystenes and most of the aromatic amino acids such as
trytophanes
and prolines are conserved between HGF and plasminogen. It is produced as a
preproprecursor of 728 amino acids that is cleaved to a large heterodimeric
molecule
made up of an a-chain (69kDa) and a [3-chain (34kDa). (Lokker et al. Prot.
Engin.,
1994; 7:895-903) HGF is a basic heparin-binding glycoprotein consisting of a
heavy
(58kDa) and a light (3lkDa) subunit. (Grant et al.) The biological effects of
HGF
are triggered by the interaction of HGF with its high-affinity receptor c-Met.
The c-
Met is a receptor-type tyrosine kinase containing a 145kDa (3-chain that
transverses
the membrane once and an extracellular SOkDa a-chain. HGF-Met signaling has
been implicated in supporting significant roles in the pathogenesis and
biology of
human cancers. Specifically it is thought that by autocrine or paracrine
mechanisms
HGF-Met signaling promotes tumor cell growth, invasion and angiogenesis.
As mentioned above, the structure of HGF is similar to that of
plasminogen. The a-chain is distinguished by the presence of an N-terminal
hairpin
loop followed by four kringle domains, and the (3-chain by a non-functional
serine
protease-like domain. Structural-functional studies have demonstrated that the
hairpin loop and kringle domains are important in the binding of HGF to its
receptors
and proteoglycans. (To et al. Oncl. Rep., 1998; 5:1013-1024)
Macrophage Stimulating Protein
Macrophage stimulating protein (MSP) is a 78 KD plasma protein
(711 aa) that is secreted by the liver into the circulation as single-chain,
biologically
inactive pro-MSP. After the proteolytic cleavage at a single site MSP becomes
biologically active disulfide-linked alpha beta-chain heterodimeric molecule.
MSP is
a growth and motility factor which interacts to its transmembrane tyrosine
kinase
called RON to induce activation of signal transduction pathway that mediates
its
biological effects.
Both MSP and HGF are plasminogen-related growth and motility
factors that interact with cell-surface protein tyrosine kinase receptors.
Each one is a
heterodimeric protein comprising a disulfide-linked alpha chain and a serine
protease-like beta chain. Despite structural similarities between MSP and HGF,
the
WO 01/44294 CA 02394167 2002-06-11 PCT/US00/34039
3
primary receptor binding site is located on the alpha chain of HGF but on the
beta
chain of MSP.
Evaluation studies indicated that HGF and HGFI/MSP evolved along
with plasminogen and other members of the kringle-serine proteinase
superfamily
from an ancestral gene that contained a single copy of the kringle domain, a
serine
protease domain and an activation peptide connecting the two domains. So the
kringle domains from plasminogen, HGF and HGFI/MSP may still possess similar
biological functions such as anti-angiogenesis.
Angiogenesis and Cancer
The hypothesis that tumor growth is angiogenesis-dependent was first
proposed in 1971 by Judah Folkman (N. Engl. Jour. Med. 285:1182 1186, 1971 ).
In
its simplest terms the hypothesis proposes that expansion of tumor volume
beyond a
certain phase requires the induction of new capillary blood vessels. For
example,
pulmonary micrometastases in the early prevascular phase in mice would be
undetectable except by high power microscopy on histological sections. Further
indirect evidence supporting the concept that tumor growth is angiogenesis
dependent is found in U.S. Patent Nos. 5,639,725, 5,629,327, 5,792,845,
5,733,876,
and 5,854,205, all of which are incorporated herein by reference.
To stimulate angiogenesis, tumors upregulate their production of a
variety of angiogenic factors, including the fibroblast growth factors (aFGF
and
bFGF) (Kandel et al., 1991 ) and vascular endothelial cell growth
factor/vascular
permeability factor (VEGF/VPF) and HGF. However, many malignant tumors also
generate inhibitors of angiogenesis, including ANGIOSTATIN protein and
thrombospondin. (Chen et al., 1995; Good et al., 1990; O'Reilly et al., 1994).
It is
postulated that the angiogenic phenotype is the result of a net balance
between these
positive and negative regulators of neovascularization. (Good et al., 1990;
O'Reilly
et al., 1994; Parangi et al., 1996; Rastinejad et al., 1989). Several other
endogenous
inhibitors of angiogenesis have been identified, although not all are
associated with
the presence of a tumor. These include, platelet factor 4 (Gupta et al., 1995;
Maione
et al., 1990), interferon-alpha, interferon-inducible protein 10 (Angiolillo
et al., 1995;
Strieter et al., 1995), which is induced by interleukin-12 and/or interferon-
gamma
(Voest et al., 1995), gro-beta (Cao et al., 1995), and the 16 kDa N-terminal
fragment
of prolactin (Clapp et al., 1993).
One example of an angiogenesis inhibitor that specifically inhibits
endothelial cell proliferation is ANGIOSTATIN protein. (O'Reilly et al.,
1994).
WO 01/44294 CA 02394167 2002-06-11 PCT/US00/34039
4
ANGIOSTATIN protein is a.n approximately 38 kiloDalton (kDa) specific
inhibitor
of endothelial cell proliferation. ANGIOSTATIN protein is an internal fragment
of
plasminogen containing at least three of the five kringles of plasminogen.
ANGIOSTATIN protein has been shown to reduce tumor weight and to inhibit
metastasis in certain tumor models. (O'Reilly et al., 1994). Another
angiogenesis
inhibitor is ENDOSTATIN protein, which is a carboxy fragment of collagen or
XVIII. (O'Reilly et al., 1997).
What is needed is the discovery and development of additional anti
angiogenic agents that may be used alone or in combination with known
angiogenic
agents in order to treat cancer and hyperproliferative disorders.
SUMMARY OF THE INVENTION
The present invention comprises novel uses for kringle domain
containing proteins and peptides such as hepatocyte growth factor (HGF), also
known as scatter factor, and macrophage stimulating protein (MSP), as
regulators of
angiogenesis useful for treating angiogenesis-related diseases including
angiogenesis-
dependent cancer. More specifically, the present invention relates to kringle
domain
containing proteins and peptides including active fragments of HGF and/or MSP,
capable of regulating, and preferably inhibiting angiogenesis. The invention
further
relates to novel HGF and/or MSP fragment compositions and methods for curing
angiogenesis-dependent disorders such as cancer, arthritis, blindness,
diabetic
retinopathy, macular degeneration, psoriasis and artherosclerosis. In
addition, the
present invention relates to kringle domain containing proteins and peptides
antibodies that, for example, block the interaction of HGF (and fragments
thereof) to
the c-met receptor, the molecular probes for monitoring biosynthesis to
antibodies
that are specific for kringle domain containing proteins and peptides, to the
development of peptide agonists and antagonists to kringle domain containing
proteins and peptides, and to cytotoxic agents linked to receptors
peptides.The
present invention generally relates to kringle domain containing proteins and
peptides
and active fragments thereof, as angiogenesis inhibitors and methods of use
thereof.
Examples of such proteins include hepatocyte growth factor (HGF), macrophage
stimulating protein (MSP), apolipoprotein, prothrombin urokinase and tissue
plasminogen activator. Whole HGF is a potent and specific regulator of
endothelial
cell function and angiogenesis, however, as demonstrated herein, HGF fragments
comprising kringle domains serve the reverse effects of whole HGF. Systemic
WO 01/44294 CA 02394167 2002-06-11 PCT/US00/34039
therapy with active HGF fragments, causes suppression of tumor-induced
angiogenesis, and exhibits strong antitumor activity.
HGF, also known as scatter factor, has a molecular weight of
approximately 87 kiloDaltons as determined by amino acid composition. As
5 described herein, novel HGF fragments are capable of inhibiting endothelial
cell
function in cultured endothelial cells, tumor cells, smooth muscle cells, and
other
variety of cells.
The present invention provides methods and compositions for treating
diseases and processes mediated by undesired and uncontrolled angiogenesis by
administering to a human or animal with the undesired angiogenesis a
composition
comprising kringle domain containing proteins and peptides such as novel HGF
and/or MSP active fragments of the present invention, or derivatives thereof,
in a
dosage sufficient to regulate, and preferably inhibit, angiogenesis. More
specifically,
the present invention is directed kringles 1-3, and/or kringles 2-3 of HGF
and/or MSP.
The present invention is particularly useful for treating or for repressing
the growth of
tumors. Administration of the presently identified novel HGF and/or MSP
fragments
to a human or animal with metastasized tumors prevents the growth or expansion
of
those tumors. The invention further provides methods and compositions for
regulating endothelial cell function in vivo as well as ira vitro.
The present invention also includes kringle domain-containing proteins
and peptides such as HGF peptide fragments that can be labeled isotopically or
with
other molecules or proteins for use in the detection and visualization of HGF
binding
sites with state of the art techniques, including, but not limited to,
positron emission
tomography, autoradiography, flow cytometry, radioreceptor binding assays, and
immunohistochemistry.
The present invention also includes kringle domain containing proteins
and peptides , kringle domain containing proteins and peptides fragments, or
kringle
domain containing proteins and peptides receptor agonists and antagonists
linked to
cytotoxic agents for therapeutic and research applications.
The present invention also includes HGF, HGF fragments, or HGF
receptor agonists and antagonists linked to cytotoxic agents for therapeutic
and
research applications.
The present invention also includes MSP, MSP fragments, or MSP
receptor agonists and antagonists linked to cytotoxic agents for therapeutic
and
research applications.
WO 01/44294 CA 02394167 2002-06-11 PCT/US00/34039
6
In addition, kringle domain containing proteins and peptides may act as
agonists and antagonists of kringle domain containing protein receptors,
thereby
enhancing or blocking the biological activity of such proteins. Such proteins
and
peptides are used in the isolation of the receptors such as the HGF receptor.
S A surprising discovery is that various active fragments of HGF, can
serve as sustained release anti-angiogenesis compounds when administered to a
tumor-bearing animal.
The present invention also relates to methods of using kringle domain
containing proteins and peptides and fragments thereof, corresponding nucleic
acid
sequences, and antibodies that bind specifically to the inhibitor and its
peptides, to
diagnose endothelial cell-related diseases and disorders.
The invention further encompasses a method for identifying receptors
specific HGF fragments, and the receptor molecules identified and isolated
thereby.
An important medical method is a new form of birth control, wherein
an effective amount of a kringle domain from a kringle domain containing
protein
such as HGF or MSP is administered to a female such that uterine endometrial
vascularization is inhibited and embryo implantation cannot occur or be
sustained.
A particularly important aspect of the present invention is the discovery
of a novel and effective method for treating angiogenesis-related diseases,
particularly
angiogenesis-dependent cancer, in patients, and for curing angiogenesis-
dependent
cancer in patients. The method unexpectedly provides the medically important
result
of inhibition of tumor growth and reduction of tumor mass. The method relates
to the
co-administration of an active HGF (or MSP) fragment of the present invention
and
another anti-angiogenesis compound, such as ANGIOSTATIN protein (EntreMed,
Inc. Rockville, MD) or ENDOSTATIN protein (EntreMed, Inc. Rockville, MD).
Accordingly, the present invention also includes formulations containing HGF
kringle
fragments, MSP kringle fragments, ANGIOSTATIN protein, and/or
ENDOSTATIN protein, which are effective for treating or curing angiogenesis-
dependent diseases.
Accordingly, it is an object of the present invention to provide
compositions and methods comprising kringle domain containing proteins and
peptides such as HGF, including active HGF fragments, useful for the treatment
of
angiogenic disorders.
Another object of the present invention is to provide compositions and
methods comprising kringle domain containing proteins and peptides, or kringle
domain containing proteins and peptides fragments in combination with other
WO 01/44294 CA 02394167 2002-06-11 PCT/US00/34039
7
antiangiogenic compounds such as ANGIOSTATIN protein or ENDOSTATINT"''
protein, useful for the treatment of angiogenic disorders.
Another object of the present invention is to provide compositions and
methods comprising HGF, or HGF kringle domain containing fragments in
combination with other antiangiogenic compounds such as ANGIOSTATIN protein
or ENDOSTATINT"'' protein, useful for the treatment of angiogenic disorders.
Another object of the present invention is to provide compositions and
methods comprising MSP, or MSP kringle domain containing fragments, in
combination with other antiangiogenic compounds such as ANGIOSTATIN protein
or ENDOSTATIN protein, useful for the treatment of angiogenic disorders.
It is another object of the present invention to provide compositions and
methods of treating diseases and processes that are mediated by angiogenesis.
It is yet another object of the present invention to provide compositions
and methods for treating diseases and processes that are mediated by
angiogenesis
including, but not limited to, hemangioma, solid tumors, leukemia, metastasis,
telangiectasia psoriasis scleroderma, pyogenic granuloma, myocardial
angiogenesis,
plaque neovascularization, coronary collaterals, cerebral collaterals,
arteriovenous
malformations, ischemic limb angiogenesis, corneal diseases, rubeosis,
neovascular
glaucoma, diabetic retinopathy, retrolental fibroplasia, arthritis, diabetic
neovascularization, macular degeneration, wound healing, surgical adhesions,
peptic
ulcer, fractures, keloids, vasculogenesis, hematopoiesis, ovulation,
menstruation, and
placentation.
It is another object of the present invention to provide compositions and
methods for treating or repressing the growth of a cancer.
Still another object of the present invention is to provide compositions
and methods comprising antibodies to kringle domain containing proteins and
peptides, or kringle domain containing proteins and peptides fragments, that
are
selective for specific regions of the kringle domain containing proteins and
peptides
molecule.
Another object of the present invention is to provide compositions and
methods comprising antibodies to HGF, or HGF fragments, that are selective for
specific regions of the HGF molecule.
Still another object of the present invention is to provide compositions
and methods comprising antibodies to MSP, or MSP fragments, that are selective
for
specific regions of the MSP molecule.
WO 01/44294 CA 02394167 2002-06-11 PCT/US00/34039
g
It is another object of the present invention to provide compositions and
methods for the detection or prognosis of anti-angiogenesis activity.
It is yet another object of the present invention to provide a therapy for
cancer that has minimal side effects.
S Still another object of the present invention is to provide compositions
comprising kringle domain containing proteins and peptides such as HGF or HGF
peptide fragments, linked to a cytotoxic agent for treating or repressing the
growth of
a cancer.
These and other objects, features and advantages of the present
invention will become apparent after a review of the following detailed
description of
the disclosed embodiments and the appended claims.
BRIEF DESCRIPTION OF THE FIGURES
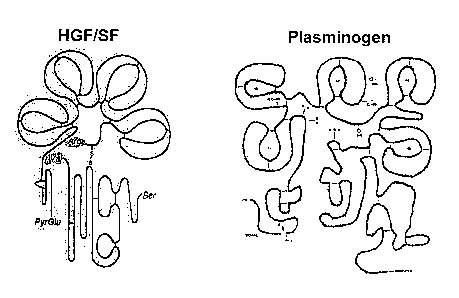
Figure 1 shows a structural comparison of HGF and plasminogen.
Mature HGF is a heterodimeric molecule which is composed of an alpha-chain,
containing the N-terminal hairpin domain and four kringle domains, and a beta-
chain
containing the serine protease-like domain. The alpha-chain has been
identified as the
receptor binding domain. HGF/NK1, HGF/NK2, HGF/NK3 and HGF/NK4 are
defined as N-terminal hairpin domain plus the first kringle (NK 1 ), or N-
terminal
hairpin domain plus the first 2 kringles (NK2) and so on HGF K2-3 comprises
the
second and the third kringles of HGF. Plasminogen is composed of kringle
domains
and serine protease domain. Angiostatin protein comprises the first 3 kringles
of
plasminogen (K1-3).
Figure 2 shows protein sequence comparison between kringles 1-4 of
HGF and plasminogen. Primary protein sequence comparison revealed that
plasminogen kringle domain and HGF kringle domain has over 49% sequence
similarity and all the cysteines are highly conserved.
Figure 3 provides the amino acid sequence for HGF (SEQ ID NO: l ).
Figure 4 provides the amino acid sequence for HGF fragments (SEQ
ID NOS:2 and 3).
Figure 5 provides the amino acid sequence for MSP (SEQ ID NO: 4).
Figure 6 is a SDS-PAGE gel of recombinant HGF K2-3 expressed in
Pichia pastori.
Figures 7(a) and 7(b) are graphs showing the HGF K2-3 inhibits
VEGF as well as HGF stimulated HUVEC migration.
WO 01/44294 CA 02394167 2002-06-11 PCT/US00/34039
9
Figure 8 is a graph showing HGF K2-3 inhibits HUVEC tube
formation.
Figure 9 is a graph showing that Angiostatin protein inhibits HGF
stimulated HUVEC migration.
Figure 10 shows that Angiostatin protein does not inhibit HGF
stimulated c-met phosphorylation.
Figure 11 shows Western blots probed with anti-phosphotyrosine
antibody (4G 10) or anti-c-met antibodies.
DETAILED DESCRIPTION OF THE INVENTION
The present invention may be understood more readily by reference to
the following detailed description of specific embodiments included herein.
Although the present invention has been described with reference to specific
details
of certain embodiments thereof, it is not intended that such details should be
regarded
as limitations upon the scope of the invention. The entire text of the
references
mentioned herein are hereby incorporated in their entireties by reference.
Applicants have discovered a novel property for a class of protein
molecules. These protein molecules are characterized in that they typically
include
kringle domains, and/or protease domains, and have the surprising ability to
regulate
angiogenic function when added to proliferating endothelial cells. "Hepatocyte
Growth Factor" (HGF) and macrophage stimulating protein (MSP) along with
tissue
plasminogen activator, apolipoprotein (a), prothrombin and urokinase, are
examples
of such proteins. HGF and MSP belong to the plasminogen-related kringle domain
family, and as used herein, it is to be understood that the term HGF includes
HGF
analogs, homologs, HGF kringle domain fragments and active peptides thereof,
and
that the term MSP includes MSP analogs, homologs, and active peptides thereof.
The term "Hepatocyte Growth Factor" (HGF) refers generally to a
protein that is approximately 87 kiloDaltons in size as determined by amino
acid
composition, more specifically to a protein that is approximately 43
kiloDaltons, and
more preferably to a protein that is approximately 31 kiloDaltons. HGF shares
both
structural (Figure 1 ) and sequence homology (Figure 2) with plasminogen. The
amino acid sequence of a human HGF is provided in SEQ ID. NO: I as shown in
Figure 3. The term HGF also includes precursor forms of the prepropeptide and
propeptide as well as modified proteins and peptides that have a substantially
similar
amino acid sequence, and which are capable of inhibiting proliferation of
endothelial
cells. For example, silent substitutions of amino acids, wherein the
replacement of an
WO 01/44294 CA 02394167 2002-06-11 PCT/US00/34039
amino acid with a structurally or chemically similar amino acid does not
significantly
alter the structure, conformation or activity of the protein, are well known
in the art.
Such silent substitutions, additions and deletions, are intended to fall
within the scope
of the appended claims.
5 The term "Macrophage Stimulating Protein" (MSP) refers generally to
a protein that is approximately 78 kilodaltons in size as determined by amino
acid
composition, and shares both structural and sequence homology with
plasminogen.
The amino acid sequence of a human HGF is provided in SEQ ID. NO: 4 as shown
in
Figure 5. The term MSP also includes precursor forms of the prepropeptide and
10 propeptide as well as modified proteins and peptides that have a
substantially similar
amino acid sequence, and which are capable of inhibiting proliferation of
endothelial
cells. For example, silent substitutions of amino acids, wherein the
replacement of an
amino acid with a structurally or chemically similar amino acid does not
significantly
alter the structure, conformation or activity of the protein, are well known
in the art.
Such silent substitutions, additions and deletions, are intended to fall
within the scope
of the appended claims.
It will be appreciated that the terms "HGF" and "MSP" include
shortened proteins or peptides wherein one or more amino acid is removed from
either or both ends of the protein, or from an internal region of the protein,
yet the
resulting molecule retains angiogenic regulating activity. HGF and MSP also
include
lengthened proteins or peptides wherein one or more amino acid is added to
either or
both ends of the protein, or to an internal location in the protein, yet the
resulting
molecule retains angiogenic regulating activity. Such molecules, for example
with
tyrosine added in the first position, are useful for labeling such as
radioiodination
with 125lodine, for use in assays. Labeling with other radioisotopes may be
useful in
providing a molecular tool for isolating and identifying the target cell
containing
HGF of MSP receptors. Other labeling, with molecules such as ricin, may
provide a
mechanism for destroying cells with HGF or MSP receptors. The invention also
contemplates that active peptides of HGF may be used alone or combined with
other
peptides and proteins to form chimeric proteins containing active HGF or MSP
peptides. Active HGF fragments of particular interest include kringles 1-3 or
HGF as
set forth in SEQ ID N0:2 (269 amino acids, 31 kiloDaltons) and kringles 1-4 of
HGF
as set forth in SEQID N0:3 (368 amino acids, 43 kiloDaltons). Both SEQ ID NOS:
2 and 3 were obtained from the Pichia production clone.
"Substantial sequence homology" means at least approximately 70%
homology between amino acid residue sequence in the kringle domain containing
WO 01/44294 CA 02394167 2002-06-11 PCT/US00/34039
protein analog, homolog or derivative sequence and that of kringle domain
containing protein, preferably at least approximately 80% homology, and more
preferably at least approximately 90% homology.
Kringle domain containing proteins and peptides such as HGF can be
isolated from normal, hyperplastic, primary and metatstatic tissue from a
variety of
species including humans. HGF and MSP can also be isolated from body fluids
including, but not limited to, semen, serum, urine and ascites, or synthesized
by
chemical or biological methods (e.g. peptide synthesis and in vitro enzymatic
catalysis of precursor molecules to yield active HGF). Kringle domain
containing
proteins and peptides may be produced from recombinant sources, from
genetically
altered cells implanted into animals, from tumors, and from cell cultures as
well as
other sources. Recombinant techniques include gene amplification from DNA
sources using the polymerase chain reaction (PCR), and gene amplification from
RNA sources using reverse transcriptase/PCR.
Though not wishing to be bound by the following theory, fragments of
glycoproteins in the family of kringle domain containing proteins and peptides
such
as HFG or MSP regulate angiogenic activity by specifically, and most likely
reversibly, inhibiting endothelial cell proliferation. The inhibitor protein
molecules
of the present invention are useful as birth control drugs, and for treating
angiogenesis-related diseases, particularly angiogenesis-dependent cancers and
tumors. The protein molecules are also useful for curing angiogenesis-
dependent
cancers and tumors. The unexpected and surprising ability of these novel
compounds
to treat and cure angiogenesis-dependent cancers and tumors answers a long-
felt,
unfulfilled need in the medical arts, and provides an important benefit to
mankind.
Important terms that are used herein are defined as follows. "Cancer"
means angiogenesis-dependent cancers and tumors, i.e. tumors that require for
their
growth (expansion in volume and/or mass) an increase in the number and density
of
the blood vessels supplying them with blood. "Regression" refers to the
reduction of
tumor mass and size.
As used herein, the term "angiogenesis" and related terms such as
"angiogenic" refer to activities associated with blood vessel growth and
development,
including, but not limited to, endothelial cell proliferation, endothelial
cell migration
and capillary tube formation.
As used herein, the term "antiangiogenic" refers to compositions and
the like that are capable of inhibiting the formation of blood vessels,
including but
WO 01/44294 CA 02394167 2002-06-11 PCT/US00/34039
12
not limited to inhibiting endothelial cell proliferation, endothelial cell
migration and
capillary tube formation.
The process of angiogenesis is complex and involves a number of
orchestrated steps that can be separately studied in vitro, such as FGF-2-
and/or
VEGF-stimulated endothelial cell proliferation and migration. For example,
ANGIOSTATIN protein and ENDOSTATIN protein inhibit these processes (see
U.S. Pat. No. 5,639,725 and U.S. Pat. No. 5,854,205). The inventors of the
present
invention have suprisingly discovered antiangiogenic properties of proteins
belonging to the family of kringle domain containing proteins and peptides by
demonstrating and systematically evaluating the effects of such proteins, for
example
HGF, on endothelial cell proliferation, migration, and invasion.
Contrary to the teachings of the prior art wherein HGF is characterized
as "potent angiogenic molecule, primarily act[ing] on endothelial cells
inducing cell
motility, proliferation, protease production, invasion, and organization into
capillary-
like tubes" (Rosen et al. Adv. Cancer Res. 1995; 67:257-79), the inventors of
the
present invention demonstrate the opposite effect of novel HGF fragments as an
anti-
angiogenic molecule. Until the discovery of the novel HGF fragments as
described
herein, HGF was considered to be only related to the induction of
angiogenesis:
"HGF/SF has been shown to stimulate endothelial cell proliferation and
migration,
and induce angiogenesis in vivo. HGF/SF may also potentiate new blood vessel
formation by up-regulating the expression of vascular endothelial cell growth
factor
(VEGF) in vascular smooth muscle cells." (To et al. 1998) Accordingly the
prior art
in fact teaches away from the study of HGF related proteins or HGF fragments
as
exhibiting any angiogenic activity.
The effects of the novel HGF fragments of the present invention on
angiogenic activity are demonstrated in Human Umbilical Vein Endothelial Cells
(HUVEC). Purified human HGF fragments demonstrate a potent and dose related
inhibitory activity on FGF-2-stimulated proliferation of HUVEC cells. To
determine
if HGF fragments inhibit a variety of endothelial cells or simply display
specificity
for HUVECs, the ability of HGF to inhibit bovine adrenal cortex endothelial
cell
(BCE) and human microvascular dermal cell (HMVEC-d) proliferation is also
demonstrated. The effects of HGF on FGF-2-stimulated endothelial cell
proliferation
are also conducted.
In order to demonstrate that HGF fragments exert antiangiogenic
effects as opposed to general inhibition of cell proliferation, the inventors
WO 01/44294 CA 02394167 2002-06-11 PCT/US00/34039
13
demonstrate experiments to show direct stimulatory or inhibitory effect on the
proliferation of cancer cells.
The effects of HGF fragments on endothelial cell migration are
demonstrated by the inventors to further confirm the antiangiogenic effects of
such
fragments. In order to evaluate the in vitro effects of HGF fragments on
endothelial
cell migration in response to FGF-2 or VEGF, confluent monolayers of HUVEC are
scraped to remove a section of monolayer and cultured with FGF-2 or VEGF in
the
presence or absence of purified human HGF fragments.
The inventors further demonstrate antiangiogenic properties of HGF
fragments by demonstrating effects on endothelial cell invasion. These
experiments
demonstrate that inhibition is dose dependent and not the result of toxicity
as the
endothelial cells appear viable and no junctions are made by the cells. These
findings
further support the inhibitory effects of HGF fragments on endothelial cell
invasion
and further confirm HGF fragment antiangiogenic activity.
Further studies are conducted to determine whether HGF fragments of
the present invention function similarly to other kringle domain containing
proteins
such as ANGIOSTATIN protein. For examples the inventors are conducting
experiments to determine whether ANGIOSTATIN protein binds the HGF receptor
c-met and acts as an antagonist to compete with HGF binding, thus inhibiting
tumorgenesis. The inventors are using a tumor cell line with expresses c-met
receptor to determine whether ANGIOSTATIN protein blocks downstream effects
(cell migration, proliferation, morphology, cytokine production level,
phosphorylations, etc.) of HGF.
Corresponding experiments described above for HGF fragments, are
also conducted for MSP.
Protocols and methods for conducting the above-described
experiments are well-known to those skilled in the art and are described in
further
detail in the Examples below, and in United States Patent Application Serial
No.
09/316,802.
Though not wishing to be bound by the following theory, it is believed
that the antiangiogenic properties of the present HGF fragments are related to
the
kringle activity of the protein. More specifically it is believed that the
antiangiogenic
activity is most likely located within kringles 1-3 (SEQ ID N0:2) or within
kringles
1-4 (SEQ ID N0:3). It is also believed that the antiangiogenic properties of
the MSP
and fragments thereof are related to the kringle activity of the protein and
that such
antiangiogenic activity is located within kringles I-3 or within kringles 1-4
of MSP.
WO 01/44294 CA 02394167 2002-06-11 PCT/US00/34039
14
In conducting the above-described experiments, the inventors of the
present invention suprisingly demonstrate for the first time that certain
novel HGF
fragments are endothelial cell-specific inhibitors of angiogenesis that
exhibit potent
anti-proliferative and anti-migratory activity on a variety of cultured
endothelial cells.
Furthermore, these novel HGF fragments inhibit the endothelial-cell specific
angiogenesis process of capillary tube formation in matrigel.
Based on the novel findings of the inventors, the present invention is
directed to methods and compositions comprising the administration of proteins
belonging to the kringle domain containing protein family for the regulation
of
antiangiogenic processes. More particularly, the methods and compositions of
the
present invention comprise the administration of novel HGF or MSP fragments
for
inhibiting angiogenesis and for reducing related cancer or tumor growth.
The novel antiangiogenic HGF or MSP fragments of the present
invention can be made by automated protein synthesis methodologies well-known
to
one skilled in the art. Alternatively, HGF or MSP and peptide fragments
thereof,
may be isolated from larger known prepropeptides that share a common or
similar
amino acid sequence.
Proteins and peptides derived from these and other sources, including
manual or automated protein synthesis, may be quickly and easily tested for
antiangiogenic activity using a biological activity assay such as the human
umbilical
vein endothelial cell proliferation assay (HUVEC) and the bovine capillary
endothelial cell proliferation assay (BCE). Such assays are described in U.S.
Patent
No. 5,639,725 which is incorporated herein by reference. Other bioassays for
inhibiting activity include the chick CAM assay, the mouse corneal assay, and
the
effect of administering isolated or synthesized proteins on implanted tumors.
The
chick CAM assay is described by O'Reilly, et al. in "Angiogenic Regulation of
Metastatic Growth" Cell, vol. 79 (2), October 21, 1994, pp. 315-328, which is
hereby
incorporated by reference in its entirety. Applicants' invention also
encompasses nucleic acid sequences that correspond to, and code for
antiangiogenic
kringle domain containing proteins and peptides, and to monoclonal and
polyclonal
antibodies that bind specifically to such protein molecules. The biologically
active
protein molecules, nucleic acid sequences corresponding to the proteins, and
antibodies that bind specifically to the proteins of the present invention are
useful for
modulating angiogenic processes in vivo, and for diagnosing and treating
endothelial
cell-related diseases, for example by gene therapy.
WO 01/44294 CA 02394167 2002-06-11 PCT/US00/34039
Nucleic acid sequences that correspond to, and code for, kringle
domain containing proteins and peptides such as HGF fragments and HGF fragment
analogs, can be prepared based upon the knowledge of the amino acid sequence,
and
the art recognized correspondence between codons (sequences of three nucleic
acid
5 bases), and amino acids. Because of the degeneracy of the genetic code,
wherein the
third base in a codon may vary yet still code for the same amino acid, many
different
possible coding nucleic acid sequences are derivable for any particular
protein or
peptide fragment.
Nucleic acid sequences are synthesized using automated systems well
10 known in the art. Either the entire sequence may be synthesized or a series
of smaller
oligonucleotides are made and subsequently ligated together to yield the full
length
sequence. Alternatively, the nucleic acid sequence may be derived from a gene
bank
using oligonucleotides probes designed based on the N-terminal amino acid
sequence
and well known techniques for cloning genetic material.
15 The present invention also encompasses gene therapy whereby genes
encoding kringle domain containing proteins and peptides such as HGF or MSP
fragments, are regulated in a patient. Various methods of transferring or
delivering
DNA to cells for expression of the gene product protein, otherwise referred to
as
gene therapy, are disclosed in Gene Transfer into Mammalian Somatic Cells in
vivo,
N. Yang, Crit. Rev. Biotechn. 12(4): 335-356 ( I 992). Gene therapy
encompasses
incorporation of DNA sequences into somatic cells or germ line cells for use
in either
ex vivo or in vivo therapy. Gene therapy functions to replace genes, augment
normal
or abnormal gene function, and to combat infectious diseases and other
pathologies.
Strategies for treating these medical problems with gene therapy
include therapeutic strategies such as identifying the defective gene and then
adding a
functional gene to either replace the function of the defective gene or to
augment a
slightly functional gene; or prophylactic strategies, such as adding a gene
for the
product protein that will treat the condition or that will make the tissue or
organ more
susceptible to a treatment regimen. As an example of a prophylactic strategy,
a gene
such as that for a desired HGF fragment may be placed in a patient and thus
prevent
occurrence of angiogenesis; or a gene that makes tumor cells more susceptible
to
radiation could be inserted and then radiation of the tumor would cause
increased
killing of the tumor cells.
Many protocols for transfer of kringle domain containing protein
DNA, or corresponding regulatory sequences are envisioned in this invention.
Transfection of promoter sequences, other than ones normally found
specifically
WO 01/44294 CA 02394167 2002-06-11 PCT/US00/34039
16
associated with such proteins or other sequences which would increase
production of
these proteins are also envisioned as methods of gene therapy. An example of
this
technology is found in Transkaryotic Therapies, Inc., of Cambridge,
Massachusetts,
using homologous recombination to insert a "genetic switch" that turns on an
erythropoietin gene in cells. See Genetic Engineering News, April 15, 1994.
Such
"genetic switches" could be used to activate HGF (or HGF receptors) in cells
not
normally expressing HGF (or the HGF receptor).
Gene transfer methods for gene theranv fall ;r,t~ thrPP hrnar~
categories: physical (e.g., electroporation, direct gene transfer and particle
bombardment), chemical (lipid-based carriers, or other non-viral vectors) and
biological (virus-derived vector and receptor uptake). For example, non-viral
vectors
may be used which include liposomes coated with DNA. Such liposome/DNA
complexes may be directly injected intravenously into the patient. It is
believed that
the liposome/DNA complexes are concentrated in the liver where they deliver
the
DNA to macrophages and Kupffer cells. These cells are long lived and thus
provide
long term expression of the delivered DNA. Additionally, vectors or the
"naked"
DNA of the gene may be directly injected into the desired organ, tissue or
tumor for
targeted delivery of the therapeutic DNA.
Gene therapy methodologies can also be described by delivery site.
Fundamental ways to deliver genes include ex vivo gene transfer. zr2 Vlvo gene
transfer, and irz vitro gene transfer. In ex vivo gene transfer, cells are
taken from the
patient and grown in cell culture. The DNA is transfected into the cells, the
transfected cells are expanded in number and then reimplanted in the patient.
In in
vitro gene transfer, the transformed cells are cells growing in culture, such
as tissue
culture cells, and not particular cells from a particular patient. These
"laboratory
cells" are transfected, the transfected cells are selected and expanded for
either
implantation into a patient or for other uses.
In vivo gene transfer involves introducing the DNA into the cells of the
patient when the cells are within the patient. Methods include using virally
mediated
gene transfer using a noninfectious virus to deliver the gene in the patient
or injecting
naked DNA into a site in the patient and the DNA is taken up by a percentage
of cells
in which the gene product protein is expressed. Additionally, the other
methods
described herein, such as use of a "gene gun," may be used for in vitro
insertion of
DNA encoding HGF kringle domains or HGF regulatory sequences.
Chemical methods of gene therapy may involve a lipid based
compound, not necessarily a liposome, to ferry the DNA across the cell
membrane.
WO 01/44294 CA 02394167 2002-06-11 PCT/US00/34039
17
Lipofectins or cytofectins, lipid-based positive ions that bind to negatively
charged
DNA, make a complex that can cross the cell membrane and provide the DNA into
the interior of the cell. Another chemical method uses receptor-based
endocytosis,
which involves binding a specific ligand to a cell surface receptor and
enveloping
and transporting it across the cell membrane. The ligand binds to the DNA and
the
whole complex is transported into the cell. The ligand gene complex is
injected into
the blood stream and then target cells that have the receptor will
specifically bind the
ligand and transport the ligand-DNA complex into the cell.
Many gene therapy methodologies employ viral vectors to insert genes
into cells. For example, altered retrovirus vectors have been used in ex vivo
methods
to introduce genes into peripheral and tumor-infiltrating lymphocytes,
hepatocytes,
epidermal cells, myocytes, or other somatic cells. These altered cells are
then
introduced into the patient to provide the gene product from the inserted DNA.
Viral vectors have also been used to insert genes into cells using in
vivo protocols. To direct tissue-specific expression of foreign genes, cis-
acting
regulatory elements or promoters that are known to be tissue specific can be
used.
Alternatively, this can be achieved using in situ delivery of DNA or viral
vectors to
specific anatomical sites in vivo. For example, gene transfer to blood vessels
in vivo
was achieved by implanting iiz vitro transduced endothelial cells in chosen
sites on
arterial walls. The virus infected surrounding cells which also expressed the
gene
product. A viral vector can be delivered directly to the in vivo site, by a
catheter for
example, thus allowing only certain areas to be infected by the virus, and
providing
long-term, site specific gene expression. 1u vivo gene transfer using
retrovirus
vectors has also been demonstrated in mammary tissue and hepatic tissue by
injection
of the altered virus into blood vessels leading to the organs.
Viral vectors that have been used for gene therapy protocols include
but are not limited to, retroviruses, other RNA viruses such as poliovirus or
Sindbis
virus, adenovirus, adeno-associated virus, herpes viruses, SV 40, vaccinia and
other
DNA viruses. Replication-defective murine retroviral vectors are the most
widely
utilized gene transfer vectors. Murine leukemia retroviruses are composed of a
single strand RNA complexed with a nuclear core protein and polymerase (pol)
enzymes, encased by a protein core (gag) and surrounded by a glycoprotein
envelope
(env) that determines host range. The genomic structure of retroviruses
include the
gag, pol, and env genes enclosed at by the 5' and 3' long terminal repeats
(LTR).
Retroviral vector systems exploit the fact that a minimal vector containing
the 5' and
3' LTRs and the packaging signal are sufficient to allow vector packaging,
infection
WO 01/44294 CA 02394167 2002-06-11 PCT/US00/34039
18
and integration into target cells providing that the viral structural proteins
are
supplied in traps in the packaging cell line. Fundamental advantages of
retroviral
vectors for gene transfer include efficient infection and gene expression in
most cell
types, precise single copy vector integration into target cell chromosomal
DNA, and
ease of manipulation of the retroviral genome.
The adenovirus is composed of linear, double stranded DNA
complexed with core proteins and surrounded with capsid proteins. Advances in
molecular virology have led to the ability to exploit the biology of these
organisms to
create vectors capable of transducing novel genetic sequences into target
cells in
vivo. Adenoviral-based vectors will express gene product peptides at high
levels.
Adenoviral vectors have high efficiencies of infectivity, even with low titers
of virus.
Additionally, the virus is fully infective as a cell free virion so injection
of producer
cell lines are not necessary. Another potential advantage to adenoviral
vectors is the
ability to achieve long term expression of heterologous genes in vivo.
Mechanical methods of DNA delivery include fusogenic lipid vesicles
such as liposomes or other vesicles for membrane fusion, lipid particles of
DNA
incorporating cationic lipid such as lipofectin, polylysine-mediated transfer
of DNA,
direct injection of DNA, such as microinjection of DNA into germ or somatic
cells,
pneumatically delivered DNA-coated particles, such as the gold particles used
in a
"gene gun," and inorganic chemical approaches such as calcium phosphate
transfection. Another method, ligand-mediated gene therapy, involves
complexing
the DNA with specific ligands to form ligand-DNA conjugates, to direct the DNA
to
a specific cell or tissue.
It has been found that injecting plasmid DNA into muscle cells yields
high percentage of the cells which are transfected and have sustained
expression of
marker genes. The DNA of the plasmid may or may not integrate into the genome
of
the cells. Non-integration of the transfected DNA would allow the transfection
and
expression of gene product proteins in terminally differentiated, non-
proliferative
tissues for a prolonged period of time without fear of mutational insertions,
deletions,
or alterations in the cellular or mitochondria) genome. Long-term, but not
necessarily permanent, transfer of therapeutic genes into specific cells may
provide
treatments for genetic diseases or for prophylactic use. The DNA could be
reinjected
periodically to maintain the gene product level without mutations occurring in
the
genomes of the recipient cells. Non-integration of exogenous DNAs may allow
for
the presence of several different exogenous DNA constructs within one cell
with all
of the constructs expressing various gene products.
WO 01/44294 CA 02394167 2002-06-11 PCT/US00/34039
19
Particle-mediated gene transfer methods were first used in
transforming plant tissue. With a particle bombardment device, or "gene gun,"
a
motive force is generated to accelerate DNA-coated high density particles
(such as
gold or tungsten) to a high velocity that allows penetration of the target
organs,
S tissues or cells. Particle bombardment can be used in in vitro systems, or
with ex
vivo or in vivo techniques to introduce DNA into cells, tissues or organs.
Electroporation for gene transfer uses an electrical current to make
cells or tissues susceptible to electroporation-mediated gene transfer. A
brief electric
impulse with a given field strength is used to increase the permeability of a
membrane in such a way that DNA molecules can penetrate into the cells. This
technique can be used in in vitro systems, or with ex vivo or in vivo
techniques to
introduce DNA into cells, tissues or organs.
Carrier mediated gene transfer in vivo can be used to transfect foreign
DNA into cells. The carrier-DNA complex can be conveniently introduced into
body
fluids or the bloodstream and then site specifically directed to the target
organ or
tissue in the body. Both liposomes and polycations, such as polylysine,
lipofectins or
cytofectins, can be used. Liposomes can be developed which are cell specific
or
organ specific and thus the foreign DNA carried by the liposome will be taken
up by
target cells. Injection of immunoliposomes that are targeted to a specific
receptor on
certain cells can be used as a convenient method of inserting the DNA into the
cells
bearing the receptor. Another carrier system that has been used is the
asialoglycoportein/polylysine conjugate system for carrying DNA to hepatocytes
for
in vivo gene transfer.
The transfected DNA may also be complexed with other kinds of
carriers so that the DNA is carried to the recipient cell and then resides in
the
cytoplasm or in the nucleoplasm. DNA can be coupled to carrier nuclear
proteins in
specifically engineered vesicle complexes and carried directly into the
nucleus.
Gene regulation of kringle domain containing proteins and peptides
such as HGF kringle fragments may be accomplished by administering compounds
that for example bind to HGF genes, or control regions associated with the HGF
genes, or corresponding RNA transcript to modify the rate of transcription or
translation. Additionally, cells transfected with a DNA sequence encoding HGF
may
be administered to a patient to provide an in vivo source of HGF fragments.
For
example, cells may be transfected with a vector containing a nucleic acid
sequence
encoding HGF. The term "vector" as used herein means a carrier that can
contain or
associate with specific nucleic acid sequences, which functions to transport
the
WO 01/44294 CA 02394167 2002-06-11 PCT/US00/34039
specific nucleic acid sequenc~a into a cell. Examples of vectors include
plasmids and
infective microorganisms such as viruses, or non-viral vectors such as ligand-
DNA
conjugates, liposomes, lipid-DNA complexes. It may be desirable that a
recombinant
DNA molecule comprising HGF DNA sequence is operatively linked to an
S expression control sequence to form an expression vector capable of
expressing
HGF. The transfected cells may be cells derived from the patient's normal
tissue, the
patient's diseased tissue, or may be non-patient cells.
For example, tumor cells removed from a patient can be transfected
with a vector capable of expressing HGF protein of the present invention, and
re-
10 introduced into the patient. The transfected tumor cells produce HGF levels
in the
patient that inhibit the growth of the tumor. Patients may be human or non-
human
animals. Cells may also be transfected by non-vector, or physical or chemical
methods known in the art such as electroporation, ionoporation, or via a "gene
gun."
Additionally, HGF DNA may be directly injected, without the aid of a carrier,
into a
15 patient. In particular, HGF DNA may be injected into skin, muscle or blood.
The gene therapy protocol for transfecting a kringle domain containing
protein such as HGF into a patient may either be through integration of HGF
DNA
into the genome of the cells, into minichromosomes or as a separate
replicating or
non-replicating DNA construct in the cytoplasm or nucleoplasm of the cell. HGF
20 expression may continue for a long-period of time or may be reinjected
periodically
to maintain a desired level of HGF protein in the cell, the tissue or organ or
a
determined blood level.
The present invention includes methods of treating or preventing
angiogenic diseases and processes including, but not limited to, arthritis and
tumors
by stimulating the production of kringle domain containing proteins and
peptides
such as HGF fragments, and/or by administering substantially purified HGF
fragments, or HGF fragment agonists or antagonists, and/or HGF fragment
antisera
to a patient. Additional treatment methods include administration of HGF
fragments,
HGF antisera, or HGF receptor agonists and antagonists linked to cytotoxic
agents.
It is to be understood that HGF can be animal or human in origin. HGF can also
be
produced synthetically by chemical reaction or by recombinant techniques in
conjunction with expression systems. HGF, and fragments thereof, can also be
produced by enzymatically cleaving different molecules, including HGF
precursors,
containing sequence homology or identity with segments of HGF to generate
peptides having anti-angiogenesis activity.
WO 01/44294 CA 02394167 2002-06-11 PCT/US00/34039
21
The present invention further includes methods of treating or
preventing angiogenic diseases and processes including, but not limited to,
arthritis
and tumors by stimulating the production of kringle domain containing proteins
and
peptides such as MSP fragments, and/or by administering substantially purified
MSP
fragments, or MSP fragment agonists or antagonists, and/or HGF fragment
antisera
to a patient. Additional treatment methods include administration of HGF, MSP
fragments, MSP antisera, or MSP receptor agonists and antagonists linked to
cytotoxic agents. It is to be understood that MSP can be animal or human in
origin.
MSP can also be produced synthetically by chemical reaction or by recombinant
techniques in conjunction with expression systems. MSP, and fragments thereof,
can
also be produced by enzymatically cleaving different molecules, including MSP
precursors, containing sequence homology or identity with segments of MSP to
generate peptides having anti-angiogenesis activity.
Antibodies that specifically bind kringle domain containing proteins
and peptides such as HGF fragments can be employed to modulate endothelial
dependent processes such as reproduction, development, and wound healing and
tissue repair. In addition, antisera directed to the Fab regions of HGF
antibodies for
example can be administered to block the ability of endogenous HGF antisera to
bind
HGF fragments.
Antibodies specific kringle domain containing proteins and peptides
such as HGF fragments, and HGF fragment analogs, are made according to
techniques and protocols well known in the art. The antibodies may be either
polyclonal or monoclonal. The antibodies are utilized in well know immunoassay
formats, such as competitive and non-competitive immunoassays, including
ELISA,
sandwich immunoassays and radioimmunoassays (RIAs), to determine the presence
or absence of the endothelial proliferation inhibitors of the present
invention in body
fluids. Examples of body fluids include but are not limited to semen, blood,
serum,
peritoneal fluid, pleural fluid, cerebrospinal fluid, uterine fluid, saliva,
and mucus.
The proteins, nucleic acid sequences and antibodies of the present
invention are useful for diagnosing and treating endothelial cell-related
diseases and
disorders. A particularly important endothelial cell process is angiogenesis,
the
formation of blood vessels. Angiogenesis-related diseases may be diagnosed and
treated using the endothelial cell proliferation inhibiting proteins of the
present
invention. Angiogenesis-related diseases include, but are not limited to,
angiogenesis-dependent cancer, including, for example, solid tumors, blood
born
tumors such as leukemias, and tumor metastases; benign tumors, for example
WO 01/44294 CA 02394167 2002-06-11 PCT/US00134039
22
hemangiomas, acoustic neuromas, neurofibromas, trachomas, and pyogenic
granulomas; rheumatoid arthritis; psoriasis; ocular angiogenic diseases, for
example,
diabetic retinopathy, retinopathy of prematurity, macular degeneration,
corneal graft
rejection, neovascular glaucoma, retrolental fibroplasia, rubeosis; Osler-
Webber
Syndrome; myocardial angiogenesis blindness; plaque neovascularization;
telangiectasia; hemophiliac joints; angiofibroma; and wound granulation. The
endothelial cell proliferation inhibiting proteins of the present invention
are useful in
the treatment of disease of excessive or abnormal stimulation of endothelial
cells.
These diseases include, but are not limited to, intestinal adhesions,
atherosclerosis,
scleroderma, and hypertrophic scars, i.e., keloids. They are also useful in
the
treatment of diseases that have angiogenesis as a pathologic consequence such
as cat
scratch disease (Rochele minalia guintosa) and ulcers (Helicobacter pylorii).
The angiogenic regulating proteins of the present invention can be
used as a birth control agent by reducing or preventing uterine
vascularization
required for embryo implantation. Thus, the present invention provides an
effective
birth control method when an amount of a kringle domain containing protein
composition comprising for example inhibitory HGF fra~rnents sufficient to
prevent
embryo implantation is administered to a female. In on;; aspect of the birth
control
method, an amount of the inhibiting protein sufficient to block embryo
implantation
is administered before or after intercourse and fertilization have occurred,
thus
providing an effective method of birth control, possibly a "morning after"
method.
While not wanting to be bound by this statement, it is believed that
inhibition of
vascularization of the uterine endometrium interferes with implantation of the
blastocyst. Similar inhibition of vascularization of the mucosa of the uterine
tube
interferes with implantation of the blastocyst, preventing occurrence of a
tubal
pregnancy. Administration methods may include, but are not limited to, pills,
injections (intravenous, subcutaneous, intramuscular), suppositories, vaginal
sponges,
vaginal tampons, and intrauterine devices. It is also believed that
administration of
the anti-angiogenic compositions of the present invention will interfere with
normal
enhanced vascularization of the placenta, and also with the development of
vessels
within a successfully implanted blastocyst and developing embryo and fetus.
Conversely, blockade of kringle domain containing protein receptors
with corresponding analogs which act as receptor antagonists, may promote
angiogenic activity such as endothelialization and vascularization. Such
effects may
be desirable in situations of inadequate vascularization of the uterine
endometrium
and associated infertility, wound repair, healing of cuts and incisions,
treatment of
WO 01/44294 CA 02394167 2002-06-11 PCTNS00/34039
23
vascular problems in diabetics, especially retinal and peripheral vessels,
promotion of
vascularization in transplanted tissue including muscle and skin, promotion of
vascularization of cardiac muscle especially following transplantation of a
heart or
heart tissue and after bypass surgery, promotion of vascularization of solid
and
relatively avascular tumors for enhanced cytotoxin delivery, and enhancement
of
blood flow to the nervous system, including but not limited to the cerebral
cortex and
spinal cord.
The present invention also relates to methods of using angiogenic
peptide fragments of kringle domain containing proteins and peptides such as
HGF,
nucleic acid sequences corresponding to HGF fragments, and antibodies that
bind
specifically to HGF fragments and related peptides, to diagnose endothelial
cell-
related diseases and disorders.
The invention further encompasses a method for identifying kringle
domain containing protein-specific receptors, and the receptor molecules
identified
and isolated thereby. The present invention also provides a method for
quantitation
of such receptors.
A particularly important aspect of the present invention is
administration of HGF fragments either alone or in combination with one or
more
anti-angiogenic agents, such as ENDOSTATIN protein, ANGIOS'FATIN protein, or
METASTATINTM protein (Entremed, Inc., Rockville, MD), in an amount sufficient
to inhibit tumor growth and cause sustainable regression of tumor mass to
microscopic size. Accordingly, the present invention also includes
formulations
effective for treating or curing angiogenesis-dependent cancers and tumors.
More particularly, recombinant HGF fragments, from insect cells or E.
coli, for example, can potently inhibit angiogenesis and the growth of
metastases. It
is contemplated as part of the present invention that HGF fragments can be
isolated
from a body fluid such as semen, blood or urine of patients, or that HGF
fragments
can be produced by recombinant DNA methods or synthetic peptide chemical
methods that are well known to those of ordinary skill in the art. Protein
purification
methods are well known in the art.
One example of a method of producing a desired kringle domain
containing protein such as HGF fragments using recombinant DNA techniques
entails the steps of (1) identifying an HGF fragment as discussed above, and
as more
fully described below, (2) synthetically generating a DNA oligonucleotide
probe that
corresponds to the protein sequence, (3) conducting PCR from human liver cDNA
(4)
inserting the gene into an appropriate vector such as an expression vector,
(5)
WO 01/44294 CA 02394167 2002-06-11 PCT/US00/34039
24
inserting the gene-containing vector into a microorganism or other expression
system
capable of expressing the inhibitor gene, and (6) isolating the recombinantly
produced inhibitor. The above techniques are more fully described in
laboratory
manuals such as "Molecular Cloning: A Laboratory Manual" Latest Edition by
Sambrook et al., Cold Spring Harbor Press, 1989.
Yet another method of producing desired proteins of the present
invention such as HGF fragments is by peptide synthesis. For example, once a
biologically active fragment of HGF is found, it can be sequenced, for example
by
automated peptide sequencing methods. Alternatively, once the gene or DNA
sequence which codes for HGF fragment is isolated, for example by the methods
described above, the DNA sequence can be determined, which in turn provides
information regarding the amino acid sequence. Thus, if the biologically
active
fragment is generated by specific methods, such as tryptic digests, or if the
fragment
is N-terminal sequenced, the remaining amino acid sequence can be determined
from
the corresponding DNA sequence.
Once the amino acid sequence of the peptide is known, for example the
N-terminal 20 amino acids, the fragment can be synthesized by techniques well
known in the art, as exemplified by "Solid Phase Peptide Synthesis: A
Practical
Approach" E. Atherton and R.C. Sheppard, IRL Press, Oxford England. Similarly,
multiple fragments can be synthesized which are subsequently linked together
to
form larger fragments. These synthetic peptide fragments can also be made with
amino acid substitutions at specific locations in order to test for agonistic
and
antagonistic activity in vitro and in vivo.
The synthetic peptide fragments of kringle domain containing proteins
and peptides such as HGF have a variety of uses. The peptide that binds to the
HGF
receptor with high specificity and avidity is radiolabeled and employed for
visualization and quantitation of binding sites using autoradiographic and
membrane
binding techniques. Knowledge of the binding properties of the HGF receptor
facilitates investigation of the transduction mechanisms linked to the
receptor.
Different peptide fragments of the intact HGF molecule can be
synthesized for use in several applications including, but not limited to the
following;
as antigens for the development of specific antisera, as agonists and
antagonists
active at HGF binding sites, as peptides to be linked to cytotoxic agents for
targeted
killing of cells that bind HGF. The amino acid sequences that comprise these
peptides
are selected on the basis of their position on the exterior regions of the
molecule and
are accessible for binding to antisera. Peptides can be synthesized in a
standard
WO 01/44294 CA 02394167 2002-06-11 PCT/US00/34039
microchemical facility and purity checked with HPLC and mass
spectrophotometry.
Methods of peptide synthesis, HPLC purification and mass spectrophotometry are
commonly known to those skilled in these arts.
HGF kringle containing fragments or peptides can also be produced in
5 recombinant E. coli, or in insect or yeast expression systems, mammalian
cell
expression systems and transgenic expression systems and purified with column
chromatography.
HGF peptides can be chemically coupled to isotopes, enzymes, carrier
proteins, cytotoxic agents, fluorescent molecules and other compounds for a
variety
10 of applications. The efficiency of the coupling reaction is determined
using different
techniques appropriate for the specific reaction.
Systematic substitution of amino acids within the synthesized peptides
yields high affinity peptide agonists and antagonists to HGF receptors that
enhance or
diminish HGF binding to its receptor. Such agonists are used to suppress the
growth
15 of primary and metastatic tumors, thereby limiting the spread of cancer.
Antagonists
to HGF are applied in situations of inadequate vascularization, to block the
inhibitory
effects of HGF and possibly promote angiogenesis. This treatment may have
therapeutic effects to promote wound healing in diabetics.
HGF peptides are employed to develop affinity columns for isolation
20 of the HGF receptor from cultured cells. Isolation and purification of the
HGf
receptor is followed by amino acid sequencing. Next, nucleotide probes are
developed for insertion into vectors for expression of the receptor. These
techniques
are well known to those skilled in the art. These techniques can be helpful in
defining minimal structures of HGF for receptor engagement.
25 Cytotoxic agents, such as ricin, are linked to the kringle domain
containing proteins and peptides of the present invention such as HGF kringle
fragments and high affinity HGF peptide fragments, thereby providing a tool
for
destruction of cells that bind HGF. These cells may be found in many
locations,
including but not limited to, metastases and primary tumors. Peptides linked
to
cytotoxic agents are infused in a manner designed to maximize delivery to the
desired
location. For example, ricin-linked high affinity HGF fragments are delivered
through a cannula into vessels supplying the target site or directly into the
target.
Such agents are also delivered in a controlled manner through osmotic pumps
coupled to infusion cannulae. A combination of HGF antagonists may be co-
applied
with stimulators of angiogenesis to increase vascularization of tissue.
WO 01/44294 CA 02394167 2002-06-11 PCT/US00/34039
26
Antiserum against kringle domain containing proteins and peptides
such as HGF fragments can be generated. After peptide synthesis and
purification,
both monoclonal and polyclonal antisera are raised using established
techniques
known to those skilled in the art. For example, polyclonal antisera may be
raised in
rabbits, sheep, goats or other animals. HGF peptides conjugated to a carrier
molecule
such as bovine serum albumin, are combined with an adjuvant mixture,
emulsified
and injected subcutaneously at multiple sites on the back, neck, flanks, and
sometimes in the footpads. Booster injections are made at regular intervals,
such as
every 2 to 4 weeks. Blood samples are obtained by venipuncture, for example
using
the marginal ear veins after dilation, approximately 7 to 10 days after each
injection.
The blood samples are allowed to clot overnight at 4°C and are
centrifuged at
approximately 2400 X g at 4°C for about 30 minutes.
All serum samples from generation of polyclonal antisera or media
samples from production of monoclonal antisera are analyzed for determination
of
titer. Titer is established through several means, for example, using dot
blots and
density analysis, and also with precipitation of radiolabeled peptide-
z~ntibod~y
complexes using protein A, secondary antisera, cold ethanol or charcoal-
dextran
followed by activity measurement with a gamma counter. The highest titer
antisera
are also purified on affinity columns which are commercially available. HGF
peptides are coupled to the gel in the affinity column. Antiserum samples are
passed
through the column and anti-HGF fragment antibodies remain bound to the
column.
These antibodies are subsequently eluted, collected and evaluated for
determination
of titer and specificity.
The highest titer HGF fragment antisera is tested to establish the
following; a) optimal antiserum dilution for highest specific binding of the
antigen
and lowest non-specific binding, b) the ability to bind increasing amounts of
HGF
peptide in a standard displacement curve, c) potential cross-reactivity with
related
peptides and proteins, including HGF related species, d) ability to detect HGF
peptides in extracts of, semen, plasma, urine, tissues, and in cell culture
media.
According to the present invention, kringle domain containing protein
compositions such as HGF fragment compositions, may be used in combination
with
other compositions and procedures for the treatment of diseases. For example,
a
tumor may be treated conventionally with surgery, radiation or chemotherapy
combined with or without HGF fragment compositions and then such compositions
may be subsequently administered to the patient to extend the dormancy of
micrometastases and to stabilize any residual primary tumor.
WO 01/44294 CA 02394167 2002-06-11 PCT/US00/34039
27
It is to be understood that the present invention is contemplated to
include any derivatives of kringle domain containing proteins and peptides
that have
angiogenic activity. The present invention includes the entire HGF protein
including
the kringle domains, derivatives of the HGF protein and biologically-active
fragments of the HGF protein. These include proteins with HGF activity that
have
amino acid substitutions or have sugars or other molecules attached to amino
acid
functional groups. The present invention also includes genes that code for HGF
and
HGF receptors, HGF kringle domains, and to proteins that are expressed by
those
genes.
The present invention includes the entire MSP protein, derivatives of
the MSP protein, MSP kringle fragments, and biologically-active fragments of
the
MSP protein. These include proteins with MSP activity that have amino acid
substitutions or have sugars or other molecules attached to amino acid
functional
groups. The present invention also includes genes that code for MSP and MSP
receptors as well as MSP kringle domain fragments, and to proteins that are
expressed by those genes.
The kringle domain containing protein having antiangiogenic activity
described above can be provided as isolated and substantially purified
proteins and
protein fragments in phaz-maceutically acceptable formulations using
formulation
methods known to those of ordinary skill in the art. These formulations can be
administered by standard routes. In general, the combinations may be
administered
by the topical, transdermal, intraperitoneal, intracranial,
intracerebroventricular,
intracerebral, intravaginal, intrauterine, oral, rectal or parenteral (e.g.,
intravenous,
intraspinal, subcutaneous or intramuscular) route. In addition, the proteins
may be
incorporated into biodegradable polymers allowing for sustained release of the
compound, the polymers being implanted in the vicinity of where drug delivery
is
desired, for example, at the site of a tumor or implanted so that the HGF is
slowly
released systemically. Osmotic minipumps may also be used to provide
controlled
delivery of high concentrations of HGF kringle fragments through cannulae to
the
site of interest, such as directly into a metastatic growth or into the
vascular supply to
that tumor. The biodegradable polymers and their use are described, for
example, in
detail in Brem et al., J. Neurosurg. 74:441-446 ( 1991 ).
The formulations of the present invention include those suitable for
oral, rectal, ophthalmic (including intravitreal or intracameral), nasal,
topical
(including buccal and sublingual), intrauterine, vaginal or parenteral
(including
subcutaneous, intraperitoneal, intramuscular, intravenous, intradermal,
intracranial,
WO 01/44294 CA 02394167 2002-06-11 PCT/US00/34039
28
intratracheal, and epidural) ;administration. The formulations may
conveniently be
presented in unit dosage form an~~i may be prepared by conventional
pharmaceutical
techniques. Such techniques include the step of bringing into association the
active
ingredient and the pharmaceutical carriers) or excipient(s). In general, the
formulations are prepared by uniformly and intimately bringing into
association the
active ingredient with liquid carriers or finely divided solid carriers or
both, and then,
if necessary, shaping the product.
Formulations suitable for parenteral administration include aqueous
and non-aqueous sterile injection solutions which may contain anti-oxidants,
buffers,
bacteriostats and solutes which render the formulation isotonic with the blood
of the
intended recipient; and aqueous and non-aqueous sterile suspensions which may
include suspending agents and thickening agents. The formulations may be
presented
in unit-dose or multi-dose containers, for example, sealed ampules and vials,
and may
be stored in a freeze-dried (lyophilized) condition requiring only the
addition of the
sterile liquid carrier, for example, water for injections, immediately prior
to use.
Extemporaneous injection solutions and suspensions may be prepared from
sterile
powders, granules and tablets of the kind previously described.
The dosage of the compositions of the present invention will depend
on the disease state or condition being treated and other clinical factors
such as
weight and condition of the human or animal and the route of administration of
the
compound. For example, for treating humans or animals, between approximately
0.5
to 500 mg/kilogram is typical broad range for administering a HGF protein, or
a
composition comprising kringle domain fragments of HGF. Depending upon the
half life of the protein in the particular animal or human, the protein can be
administered between several times per day to once a week. It is to be
understood
that the present invention has application for both human and veterinary use.
The
methods of the present invention contemplate single as well as multiple
administrations, given either simultaneously or over an extended period of
time.
Preferred unit dosage formulations are those containing a daily dose or
unit, daily sub-dose, as herein above recited, or an appropriate fraction
thereof, of the
administered ingredient. It should be understood that in addition to the
ingredients,
particularly mentioned above, the formulations of the present invention may
include
other agents conventional in the art having regard to the type of formulation
in
question.
This invention is further illustrated by the following examples, which
are not to be construed in any way as imposing limitations upon the scope
thereof. On
WO 01/44294 CA 02394167 2002-06-11 PCT/US00/34039
29
the contrary, it is to be clearly understood that resort may be had to various
other
embodiments, modifications, and equivalents thereof which, after reading the
description herein, may suggest themselves to those skilled in the art without
departing from the spirit of the present invention and/or the scope of the
appended
claims.
EXAMPLE 1
Purified human recombinant HGFK2-3 expressed in Pichia pastori (Figure 6)
The HGF K2-3 gene was cloned into pPICZaA and expressed in X33 P.
pastoris strain. The fermentation supernatant was purified through ion
exchange and
hydrophobic interaction chromatography. 11 ~g of purified HGF K2-3 was
analyzed
on the denaturing and non-reducing SDS-PAGE gel stained with Coomassie blue.
EXAMPLE 2
HGF K2-3 inhibits VEGF as well as HGF stimulated HUVEC migration.
(Figut°es 7a
and 7b)
Micro Chemotaxis Assay
Apparatus:
Chamber: Neuro Probe Standard 48 Well Chemotaxis Chamber Cat #
AP48 (301-229-8598)
Filter Membrane: Poretics Membrane Polycarbonate PVP Free 8
micron 25 X 80 mm (Osmonics Inc.800-444-8212 Cat #10474)
Forceps: VWR Scientific 800- 234-9300 Cat# 25681-269. For ease of
handling membranes use these forceps.
Preparation of Filter Membrane:
Coat membranes with Rat tail Collagen Type 1 (BD Collaborative Res
Cat # 40236) at 100 mg/ml in 29 ml of .2N Acetic Acid. For .2N Acetic Acid add
345
ml of Glacial Acetic Acid (99%) in 29.66 sterile H20 (BRL).
1. Dilute collagen (100 mg/ml) in .2N Acetic Acid in 50 ml conical
tube. Mix well.
2. Place collagen solution in either a petri dish or small plastic staining
box. Submerge membranes individually ( 10 membranes).
3. Agitate slowly on rocker at room temperature for 48 hours.
WO 01/44294 CA 02394167 2002-06-11 PCT/US00/34039
4. Air dry filters in laminar flow hood by laying out filters on open
petri dishes.
5. Store dry filters in covered container at room temperature (2 weeks).
5 Assay:
Assay Media: Medium -200 supplemented with 1 % L-glutamine
(BioWhittaker) and 0.1 % BSA (Sigma Cat# A8412)
1. Harvest HUVEC p2-p7 from flasks by trypsinization. Neutralize
trypsin with growth medium. Remove trypsin/versene/media by centrifugation at
10 1000 rpm for 5 minutes. Resuspend in 10 ml of assay media. Do not over
trypsinize
cells or over centrifuge cells.
2. Count cells by dilution with Trypan Blue solution. Determine
viability. Resuspend cells at 2 X 105/m1 in assay media.
3. Before adding cells to chambers, preincubate cells with test proteins
15 in 17X100 mm (14m1) polypropylene round bottom tube (Falcon 35-2059) for 30
minutes at 37°C with 5% C02. Do not snap top of tube. Incubate control
cells with
assay media.
4. Approximately 5 minutes before addition of cells prepare chamber.
To the first three columns of the bottom chamber add approximately 28 ml of
assay
20 media alone. This volume varies with individual chambers from 25-30 ml.
Adjust
volume so that a slight positive meniscus is formed over well. Fill rest of
wells with
chemoattractant (e.g., VEGF165 R&D Cat #293-VE) 2-10 ng/ml. Reconstitute
VEGF in assay media. Optimum concentration depends on cells. The last three
columns are stimulated controls.
25 5. All manipulations with membranes are done with forceps. Cut off
small corner of membrane and orient cut off corner with NP trademark. Place
membrane on top of lower chamber shiny side up. Do not adjust membrane as this
will contaminate wells.
6. Gently place silicone gasket on top of membrane. Then place the top
30 portion of the chamber oriented with the trademark. With firm even pressure
screw
on the thumb nuts tightly. Note: Cells will leak if this is not done
correctly.
7. Add 50 ml of either control cells or treated cells to upper chambers.
To avoid bubbles lift pipette tip while adding cells. Be careful not to
puncture
membrane.
8. Place chamber in 1 SOmm petri dish with moist paper towel. Incubate
chamber or 6 h at 37°C with 5% C02.
WO 01/44294 CA 02394167 2002-06-11 PCT/US00/34039
31
9. After 6 hours, gently remove thumb nuts and upper chamber. Peel
off membrane which is stuck to the gasket with forceps.
10. Fix and stain filter membrane with Diff Quik (bade Int. Cat#
84132-10) Fix for 2 minutes; Solution I for 2 minutes; Solution II for 3
minutes.
11. Rinse membrane 2X in distilled H20. Place membrane on top of 3
X 2 glass microscope slide (VWR Cat # 28351-100). With a wet Kim-Wipe remove
non-migrating cells while holding onto membrane to prevent movement. After
removal of cells allow membrane to dry. Place 4 small drops of super-glue (use
pipette tip on each corner of the slide. Place additional slide on top. View
under
microscope to determine total number of migrated cells.
The migration of HUVECs was evaluated with the micro chemotaxis
chamber (modified Boyden chamber, Neuro Probe, Inc., Gaithersburg, MD)
described
above which allows for the measurement of cell movement and directionality.
HUVECs pretreated with HGF K2-3 or basal media (M-200 containing 0.1 % BSA)
for 30' at 37°C were placed into the upper chamber. The lower chamber
contained
basal media with (a) VEGF (5 ng/ml) or (b) HGF (50 ng,~mL) or without. Cells
were
allowed to migrate through an 8 mm polycarbonate PVP free f lter coated with
100
~g/ml of rat tail collagen type 1 (Collaborative Biomedical Products, Bedford,
MA).
The chamber was then incubated as above for 6 h. The non-migrated cells were
removed and the filter was fixed and stained with Diff Quik (Dale Diagnostics,
Aquado, Puerto Rico). The numbers of migrated cells were determined using the
Image-Pro Plus analysis system (Media Cybernetics, Silver Spring, MD). BSA at
100
~g/ml showed no inhibition effect on the growth factor stimulated HUVEC
migration
(data not shown).
EXAMPLE 3
HGF K2-3 inhibits HUVEC tube formation. (Figure 8)
HUVECs were trypsinized and resuspend at 1 X 10 5 cells/ml in the
assay media [medium -200 supplemented with LSGS and 5% heat inactivated FBS
(Hyclone)]. 100 ml of HGFK2-3 dilution in assay and 100 u1 of HUVECs were
plated
with onto a Matrigel substratum(Collaborative Biomedical Products, Bedford,
MA)
for 16 h at 37°C. After incubation, endothelial cells were examined
microscopically
and evaluated for tube formation by counting the number of junctions. BSA at
200
~g/ml showed no inhibition effect (data not shown).
WO 01/44294 CA 02394167 2002-06-11 PCT/US00/34039
32
EXAMPLE 4
Angiostatira protein inhibits t~GF stimulated HUVEC migration. (Figure 9)
The migration of HUVECs was evaluated with a micro chemotaxis
chamber (modified Boyden chamber, Neuro Probe, Inc., Gaithersburg, MD).
HUVECs pretreated with Angiostatin protein or basal media (M-200 containing
0.1
BSA) for 30' at 37°C were placed into the upper chamber. The lower
chamber
contained basal media with HGF (50 ng/ml) or without. Cells were allowed to
migrate
through an 8 mm polycarbonate PVP free filter coated with 100 mg/ml of rat
tail
collagen type 1 (Collaborative Biomedical Products, Bedford, MA). The chamber
was
then incubated as above for 6 h. The non-migrated cells were removed and the
filter
was fixed and stained with Diff Quik (Dade Diagnostics, Aquado, Puerto Rico).
The
numbers of migrated cells were determined using the Image-Pro Plus analysis
system
(Media Cybernetics, Silver Spring, MD). BSA at 100 ug/ml showed no inhibition
effect on the growth factor stimulated HUVEC migration (data not shown).
EXAMPLE 5
Angiostatin protein does not induce c-rnet phosphorylation. (Figure 1 ~)
A549 cells were starved in serum free media plus 0.1 % BSA for 6 hours
and then treated with Angiostatin protein or HGF for 10 min. at 37 o C. The
cells were
lysed and the supernatant was then immunoprecipated with 15 u1 of rabbit anti-
c-met
antibodies and analyzed with Western blots probed with anti-phosphotyrosine
antibody (4610) or anti-c-met antibodies.
EXAMPLE 6
Angiostatin protein does not inhibit HGF stimulated c-rnet phosphorylation.
(Figure
11)
The HUVECs were starved in serum free media plus 0.1 % BSA for 6
hours and then treated with Angiostatin protein at 4° C for 30 min. The
cold media
was replaced with fresh media (37° C) with the same concentration of
Angiotstatin
and then induced with 20 ng/ml HGF for 10 min. at 37° C. The cells were
lysed and
the supernatant was then immunoprecipated with 15 u1 of rabbit anti-c-met anti-
bodies
and analyzed with Western blots probed with anti-phosphotyrosine antibody (4G
10)
or anti-c-met antibodies.