Note: Descriptions are shown in the official language in which they were submitted.
CA 02395298 2002-06-18
WO 01/55085 PCT/DKO1/00058
NEW COMPOUNDS, THEIR PREPARATION AND USE
FIELD OF INVENTION
The present invention relates to novel compounds, pharmaceutical compositions
containing
them, methods for preparing the compounds and their use as medicaments. More
specifi-
cally, compounds of the invention can be utilised in the treatment and/or
prevention of condi-
tions mediated by nuclear receptors, in particular the Peroxisome Proliferator-
Activated Re-
ceptors (PPAR).
BACKGROUND OF THE INVENTION
Coronary artery disease (CAD) is the major cause of death in Type 2 diabetic
and metabolic
syndrome patients (i.e. patients that fall within the 'deadly quartet'
category of impaired glu-
cose tolerance, insulin resistance, hypertriglyceridaemia and/or obesity).
The hypolipidaemic fibrates and antidiabetic thiazolidinediones separately
display moderately
effective triglyceride-lowering activities although they are neither potent
nor efficacious
enough to be a single therapy of choice for the dyslipidaemia often observed
in Type 2 dia-
betic or metabolic syndrome patients. The thiazolidinediones also potently
lower circulating
glucose levels of Type 2 diabetic animal models and humans. However, the
fibrate class of
compounds are without beneficial effects on glycaemia. Studies on the
molecular actions of
these compounds indicate that thiazolidinediones and fibrates exert their
action by activating
distinct transcription factors of the peroxisome proliferator activated
receptor (PPAR) family,
resulting in increased and decreased expression of specific enzymes and
apolipoproteins
respectively, both key-players in regulation of plasma triglyceride content.
Fibrates, on the
one hand, are PPARa activators, acting primarily in the liver.
Thiazolidinediones, on the other
hand, are high affinity ligands for PPARy acting primarily on adipose tissue.
Adipose tissue plays a central role in lipid homeostasis and the maintenance
of energy
balance in vertebrates. Adipocytes store energy in the form of triglycerides
during periods of
nutritional affluence and release it in the form of free fatty acids at times
of nutritional
deprivation. The development of white adipose tissue is the result of a
continuous
CA 02395298 2002-06-18
WO 01/55085 PCT/DKO1/00058
2
differentiation process throughout life. Much evidence points to the central
role of PPARy
activation in initiating and regulating this cell differentiation. Several
highly specialised
proteins are induced during adipocyte differentiation, most of them being
involved in lipid
storage and metabolism. The exact link from activation of PPARy to changes in
glucose
metabolism, most notably a decrease in insulin resistance in muscle, has not
yet been
clarified. A possible link is via free fatty acids such that activation of
PPARy induces v
Lipoprotein Lipase (LPL), Fatty Acid Transport Protein (FATP) and Acyl-CoA
Synthetase
(ACS) in adipose tissue but not in muscle tissue. This, in turn, reduces the
concentration of
free fatty acids in plasma dramatically, and due to substrate competition at
the cellular level,
skeletal muscle and other tissues with high metabolic rates eventually switch
from fatty acid.
oxidation to glucose oxidation with decreased insulin resistance as a
consequence.
PPARa is involved in stimulating (3-oxidation of fatty acids. In rodents, a
PPARa-mediated
change in the expression of genes involved in fatty acid metabolism lies at
the basis of the
phenomenon of peroxisome proliferation, a pleiotropic cellular response,
mainly limited to .
liver and kidney and which can lead to hepatocarcinogenesis in rodents. The
phenomenon
of peroxisome proliferation is not seen in man. In addition to its role in
peroxisome
proliferation in rodents, PPARa is also involved in the control of HDL
cholesterol levels in
rodents and humans. This effect is, at least partially, based on a PPARa-
mediated transcrip-
tional regulation of the major HDL apolipoproteins, apo A-t and apo A-li. The
hypotriglyceridemic action of fibrates and fatty acids also involves PPARa and
can be
summarised as follows: (I) an increased lipolysis and clearance of remnant
particles, due to
changes in lipoprotein lipase and apo, C-III levels, (II) a stimulation of
cellular fatty acid
uptake and their subsequent conversion to acyl-CoA derivatives by the
induction of fatty acid
binding protein and acyl-CoA synthase, (III) an induction of fatty acid a-
oxidation pathways,
(IV) a reduction in fatty acid and triglyceride synthesis, and finally (V) a
decrease in VLDL
production. Hence, both enhanced catabolism of triglyceride-rich particles as
well as reduced
secretion of VLDL particles constitutes mechanisms that contribute to the
hypolipidemic
effect of fibrates.
A number of compounds have been reported to be useful in the treatment of
hyperglycemia,
hyperlipidemia and hypercholesterolemia (U.S. Pat. 5,306,726, PCT Publications
nos.
CA 02395298 2002-06-18
WO 01/55085 PCT/DKO1/00058
3
V1I091/19702, WO 95/03038, WO 96/04260, WO 94/13650, WO 94/01420, WO 97/36579,
WO 97/25042, WO 95/17394, WO 99/08501, WO 99/19313 and WO 99/16758).
SUMMARY OF THE INVENTION
Glucose lowering as a single approach does not overcome the macrovascular
complications
associated with Type 2 diabetes and metabolic syndrome. Novel treatments of
Type 2 dia-
betes and metabolic syndrome must therefore aim at lowering both the overt
hypertriglyceri-
daemia associated with these syndromes as well as alleviation of
hyperglycaemia.
The clinical activity of fibrates and thiazolidinediones indicates that
research for compounds
displaying combined PPARa and PPARy activation should lead to the discovery of
effica
cious glucose and triglyceride lowering drugs that have great potential i,n
the treatment of
Type 2 diabetes and the metabolic syndrome (i.e. impaired glucose tolerance,
insulin resis
15, tance, hypertriglyceridaemia and/or obesity).
DETAILED DESCRIPTION OF THE INVENTION
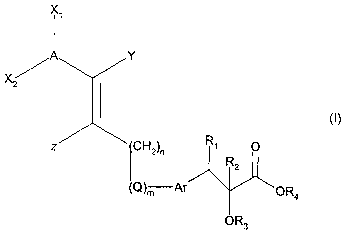
Accordingly, the present invention relates to compounds of the general formula
(I):
XA
Y
X,
CH2)n R~ O
R2
(Q)m Ar ~/ ~OR4
OR3
CA 02395298 2002-06-18
WO 01/55085 PCT/DKO1/00058
4
wherein A is aryl or heteroaryl and wherein A is optionally substituted with
one or more sub-
stituents. selected from hydroxy, halogen, perhalomethyl, perhalomethoxy,
acyl, cyano,
amino, C~_s-alkylamino, C~_s-dialkylamino, methylenedioxy, aralkenyl,
aralkynyl, heteroary-
loxy, heteroaralkoxy, aralkyl, heteroaralkyl, arylamino, or
A is optionally substituted with C~~-alkyl, Cz~-alkenyl or C2~-alkynyl each of
which is option-
ally substituted with one or more substituents selected from C~_s-
alkoxycarbonyl or carboxy,
or
A is optionally substituted with C~~-alkoxy, C~_s-alkylthio or C2_s-alkenyloxy
each of which is
optionally substituted with one or more halogens, or
A is optionally substituted with aryloxy, arylthio or aralkoxy each of which
is optionally substi-
tuted with one or more substituents selected from C~_s-alkoxy, nitro, carboxy
or C~_s-
alkoxycarbonyl; and
X~ and X2 independently are
hydrogen,
aryl or heteroaryl each of which is optionally substituted with one or more
substituents se-
lected from hydroxy, aryloxy, arylthio, aralkoxy, heteroaryloxy, aralkoxy,
C~_s-alkoxy, C~_s-
alkylthio, halogen, perhalomethyl, perhalomethoxy, acyl, aryl, heteroaryl,
aralkyl, heteroaral-
kyl, cyano, amino, C~~-alkylamino, C~_s-dialkylamino, arylamino or
methylenedioxy, or
aryl or heteroaryl each of which is optionally substituted with one or more
substituents se-
lected from C~_s-alkyl, C2~-alkenyl or C2_s-alkynyl each of which is
optionally substituted with
hydroxy; or
A is selected from the ring systems consisting of
CA 02395298 2002-06-18
WO 01/55085 PCT/DKO1/00058
\ I I \ \ I I ~N / I I ~N / I I \
N N N~ \
Rs Rs Rs
I \
\ \ \ ~ \ \ \ \ /
/ / / / / ~ / /
\ \ \ ~ \ N \ ~ \ S \ \ C \
I
/ N / N / N / N
R5 R5
Figure 1
wherein the attachment point of A to the remaining part of the structure of
formula (I) is as
indicated on the chemical structures in Figure 1, and wherein A is optionally,
substituted with
one or more substituents selected from C~_s-alkyl, C2_s-alkenyl, CZ_s-alkynyl,
hydroxy, aryloxy,
arylthio, aralkoxy, C~_s-alkoxy, C~_s-alkylthio, heteroaryloxy,
heteroaralkoxy, halogen, perha-
lomethyl, perhalomethoxy, acyl, aralkyl, heteroaralkyl, cyano, amino, C~.s-
alkylamino, C~_s-
dialkylamino, arylamino or methylenedioxy; and
wherein X~ and X2 are hydrogen; and
R5 is hydrogen or C~_s-alkyl; and
Y is hydrogen, or
Y is C~_~2-alkyl, C2_~2-alkenyl, C2_~2-alkynyl, C4_~2-alkenynyl, aralkyl or
heteroaralkyl each of
which is optionally substituted with one or more substituents selected from
halogen, C~_s-
alkyl, perhalomethyl, hydroxy, aryl, heteroaryl, carboxy or amino; and
Z is hydrogen, halogen, hydroxy, or
Z is C~_s-alkyl or C~~-alkoxy each of which is optionally substituted with one
or more substitu-
ents selected from halogen, hydroxy, carboxy, amino, cyano or C~_s-alkoxy; and
CA 02395298 2002-06-18
WO 01/55085 PCT/DKO1/00058
6
Q is O, S or NR6, wherein R6 is hydrogen, C~~-alkyl, C2~-alkenyl, CZ_s-
alkynyl, C4_6-alkenynyl,
aralkyl, heteroaralkyl and wherein Rs is optionally substituted with one or
more substituents
selected from halogen, hydroxy, C~_6-alkoxy, amino or carboxy; and
Ar is arylene, heteroarylene or a divalent heterocyclic group each of which
can be optionally
substituted with one or more substituents selected from C~_s-alkyl, aryl or
C~_6-alkoxy each of
which can be optionally substituted with halogen, hydroxy, carboxy, cyano or
heterocyclyl;
and
R~ is hydrogen, hydroxy or halogen; or R~ forms a bond together with RZ; and
R2 is hydrogen or C~_B-alkyl; or R2 forms a bond together with R~; and
R3 is hydrogen, or
R3 is C~_6-alkyl, CZ_6-alkenyl, C2_6-alkynyl, C~6-alkenynyl, aryl, aralkyl,
C~~-alkoxyC~.~alkyl,
acyl, heterocyclyl, heteroaryl or heteroaralkyl each of which is optionally
substituted with one
or more substituents selected from halogen, perhalomethyl, hydroxy or cyano;
and
R4 is hydrogen, C~~-alkyl, C2_6-alkenyl, C2~-alkynyl, C4_6-alkenynyl or aryl;
and
n is an integer ranging from 0 to 3; and
m is an integer ranging from 0 to 1;
or a pharmaceutically acceptable salt thereof, or a pharmaceutically
acceptable solvate
thereof, or any tautomeric forms, stereoisomers, mixture of stereoisomers
including a race-
mic mixture, or polymorphs.
In a preferred embodiment, the present invention is concerned with compounds
of formula (I)
CA 02395298 2002-06-18
WO 01/55085 PCT/DKO1/00058
7
X~
/A Y
X/2
CH2)n R~ O
R2
(Q)m Ar ~OR4
R3
wherein A is aryl or heteroaryl and wherein A is optionally substituted with
one or more sub-
stituents selected from hydroxy, halogen, perhalomethyl, perhalomethoxy, acyl,
cyano,
amino, C~~-alkylamino, C~_s-dialkylamino, methylenedioxy, aralkenyl,
aralkynyl, heteroary-
loxy, heteroaralkoxy, aralkyl, heteroaralkyl, arylamino, or
A is optionally substituted with C,_s-alkyl, C2_s-alkenyl or C2.~-alkynyl each
of which is option-
ally substituted with one or more substituents selected from C~~-
alkoxycarbonyl or carboxy,
or
A is optionally substituted with C~_s-alkoxy, C~_s-alkylthio or C2_s-
alkenyloxy each of which is
optionally substituted with one or more halogens, or
A is optionally substituted with aryloxy, arylthio or aralkoxy each of which
is optionally substi-
tuted with one or more substituents selected from C~_s-alkoxy, nitro, carboxy
or C~_s-
alkoxycarbonyl; and
X~ and X2 independently are
hydrogen,
aryl or heteroaryl each of which is optionally substituted with one or more
substituents se-
lected from hydroxy, aryloxy, arylthio, aralkoxy, heteroaryloxy, aralkoxy,
C,_s-alkoxy, C~~-
alkylthio, halogen, perhalomethyl, perhalomethoxy, acyl, aryl, heteroaryl,
aralkyl, heteroaral-
2o kyl, cyano, amino, C~_s-alkylamino, C~_s-dialkylamino, arylamino or
methylenedioxy, or
aryl or heteroaryl each of which is optionally substituted with one or more
substituents se-
lected from C~_s-alkyl, CZ_s-alkenyl or C2_s-alkynyl each of which is
optionally substituted with
hydroxy; or
CA 02395298 2002-06-18
WO 01/55085 PCT/DKO1/00058
A is selected from the ring systems consisting of
~ I I \ \ I I ~N / I I N ~ I I ~
N N'~~ N
Rs R5 ~ Rs
\ \ \ \ \ \ \ I /
I / / / I / / I / /
I \ \ \ ~ \ N \ I \ S \ \ ~ \
/ N / N / N I / N
Rs Rs
Figure 1
wherein the attachment point of A to the remaining part of the structure of
formula (I) is as
indicated on the chemical structures in Figure 1, and wherein A is optionally
substituted with
one or more substituents selected from C~_6-alkyl, C2_6-alkenyl, C2_6-alkynyl,
hydroxy, aryloxy,
arylthio, aralkoxy, C~_s-alkoxy, C~~-alkylthio, heteroaryloxy, heteroaralkoxy,
halogen, perha-
lomethyl, perhalomethoxy, acyl, aralkyl, heteroaralkyl, cyano, amino, C~_6-
alkylamino, 0_6-
dialkylamino, arylamino or methylenedioxy; and
wherein X, and X2 are hydrogen; and
R5 is hydrogen or C~_6-alkyl; and
Y is hydrogen, or
Y is C~_~2-alkyl, C2_~2-alkenyl, CZ_~2-alkynyl, C4_~2-alkenynyl, aralkyl or
heteroaralkyl each of
which is optionally substituted with one or more substituents selected from
halogen, C~.~-
alkyl, perhalomethyl, hydroxy, aryl, heteroaryl, carboxy or amino; and
CA 02395298 2002-06-18
WO 01/55085 PCT/DKO1/00058
9
Z is hydrogen, halogen, hydroxy, or
Z is C~_6-alkyl or C~_s-alkoxy each. of which is optionally substituted with
one or more. substitu-
ents selected from halogen, hydroxy, carboxy, amino, cyano or C~_6-alkoxy; and
Q is O, S or NR6, wherein R6 is hydrogen, C~_s-alkyl, C2~-alkenyl, C2_6-
alkynyl, C4_6-alkenynyl,
aralkyl, heteroaralkyl and wherein R6 is optionally substituted with one or
more substituents
selected from halogen, hydroxy, C~_s-alkoxy, amino or carboxy; and
Ar is arylene, heteroarylene or a divalent heterocyclic group each of which
can be optionally
substituted with one or more substituents selected from C~_6-alkyl, aryl or
C~_6-alkoxy each of
which can be optionally substituted with halogen, hydroxy, carboxy, cyano or
heterocyclyl;
and
R~ is hydrogen, hydroxy or halogen; or R~ forms a bond together with R2; and
R2 is hydrogen or C~~-alkyl; or R2 forms a bond together with R~; and
R3 is hydrogen, or
R3 is C~_6-alkyl, C2~-alkenyl, CZ~-alkynyl, C~6-alkenynyl, aryl, aralkyl, C~_6-
alkoxyC~~alkyl,
acyl, heterocyclyl, heteroaryl or heteroaralkyl each of which is optionally
substituted with one
or more substituents selected from halogen, perhalomethyl, hydroxy or cyano;
and
R4 is hydrogen, C~_6-alkyl, CZ_6-alkenyl, C2~-alkynyl, C~s-alkenynyl or aryl;
and
n is an integer ranging from 1 to 3; and
m is 1;
or a pharmaceutically acceptable salt thereof, or a pharmaceutically
acceptable solvate
thereof, or any tautomeric forms, stereoisomers, mixture of stereoisomers
including a race-
mic mixture, or polymorphs.
CA 02395298 2002-06-18
WO 01/55085 PCT/DKO1/00058
In another preferred embodiment, the present invention is concerned with
compounds of
formula (I)
X1
/P Y
X/z
CH2)n R1 O
Rz
(Q)m Ar ~/ ~OR4
R3
5
wherein A is. aryl or heteroaryl and wherein A is optionally substituted with
one or more sub-
stituents selected from C~_6-alkyl, C2~-alkenyl, C2~-alkynyl, hydroxy,
aryloxy, arylthio,
aralkoxy, C~_6-alkoxy, C,~-alkylthio, heteroaryloxy, heteroaralkoxy, halogen;
perhalomethyl,
perhalomethoxy, acyl, aralkyl, heteroaralkyl, cyano, amino, C~_6-alkylamino,
C~~-
dialkylamino, arylamino or methylenedioxy, and
X~ and X2 independently are hydrogen, aryl or heteroaryl optionally
substituted with one or
more substituents selected from C~_6-alkyl, C2_6-alkenyl, CZ_6-alkynyl,
hydroxy, aryloxy, aryl-
thio, aralkoxy, heteroaryloxy, aralkoxy, C~~-alkoxy, C~_6-alkylthio, halogen,
perhalomethyl,
perhalomethoxy, acyl, aryl, heteroaryl, aralkyl, heteroaralkyl, cyano, amino,
C~~-alkylamino,
C~_6-dialkylamino, arylamino or methylenedioxy;
or
wherein A is selected from the ring systems consisting of
CA 02395298 2002-06-18
WO 01/55085 PCT/DKO1/00058
11
i ( ~ \ \ ~ ~ \
N
I
R5
\ \ I \ \ \ \ N \
/ / / N ~ /
N
\ S \ \ O \
(/
N ~ ~N
Rs R5
Figure 1
wherein the attachment point of A to the remaining part of the structure of
formula (I) is as
indicated on the chemical structures in Figure 1, and
wherein A is optionally substituted with one or more substituents selected
from C~_6-alkyl, C2_
6-alkenyl, C2_6-alkynyl, hydroxy, aryloxy, arylthio, aralkoxy, C~_6-alkoxy,
C~_6-alkylthio, het-
eroaryloxy, heteroaralkoxy, halogen, perhalomethyl, perhalomethoxy, acyl,
aralkyl, het-
eroaralkyl, cyano, amino, C~~-alkylamino, C~.~-dialkylamino, arylamino or
methylenedioxy,
and wherein X~ and X2 are hydrogen; and
R5 is hydrogen or C~~-alkyl; and
Y is hydrogen, C~_~2-alkyl, C2_~2-alkenyl, C2_~2-alkynyl, C~~2-alkenynyl,
aralkyl or heteroaralkyl
optionally substituted with one or more substituents selected from halogen,
C~_6-alkyl, perha-
lomethyl, hydroxy, aryl, heteroaryl, carboxy or amino; and
Z is hydrogen, halogen, hydroxy, C'_6-alkyl or C~_6-alkoxy optionally
substituted with one or
more substituents selected from halogen, hydroxy, carboxy, amino, cyano or
C~_6-alkoxy; and
CA 02395298 2002-06-18
WO 01/55085 PCT/DKO1/00058
12
Q is O, S or NRs, wherein Rs is hydrogen, C~_s-alkyl, C2_s-alkenyl, C2_s-
alkynyl, C4~-alkenynyl,
aralkyl, heteroaralkyl and wherein Rs is optionally substituted with one or
more substituents
selected from halogen, hydroxy, C~_s-alkoxy, amino or carboxy; and
Ar is arylene, heteroarylene or a divalent heterocyclic group each of which
can be optionally
substituted with one or more substituents selected from C~~-alkyl, aryl or
C~_s-alkoxy each of
which can be optionally substituted with halogen, hydroxy, carboxy, cyano or
heterocyclyl;
and
R, is hydrogen, hydroxy or halogen; or R~ forms a bond together with R2; and
R2 is hydrogen or C~_s-alkyl; or R2 forms a bond together with R~; and
R3 is hydrogen, C~_s-alkyl, CZ_s-alkenyl, CZ~-alkynyl, C4_s-alkenynyl, aryl,
aralkyl, C~_s-
alkoxyC~_salkyl, acyl, heterocyclyl, heteroaryl or heteroaralkyl groups
optionally substituted
15, with one or more substituents selected from halogen, perhalomethyl,
hydroxy or cyano; and
R4 is hydrogen, C~_s-alkyl, CZ_s-alkenyl, C~_s-alkynyl, C4_s-alkenynyl or
aryl; and
n is an integer ranging from 0 to 3; and
m is an integer ranging from 0 to 1.
In another preferred embodiment, the present invention is concerned with
compounds of
formula (I)
CA 02395298 2002-06-18
WO 01/55085 PCT/DKO1/00058
13
X.
Y
X,
CH2)n R~ O
R2
(Q)m Ar ~/ ~OR4
R3
wherein A is aryl or heteroaryl and wherein A is optionally substituted with
one or more sub-
stituents selected from C~_s=alkyl, CZ_s-alkenyl, C2_s-alkynyl, hydroxy,
aryloxy, arylthio,
aralkoxy, C~~-alkoxy, C~_s-alkylthio, heteroaryloxy, heteroaralkoxy, halogen,
perhalomethyl,
perhalomethoxy, acyl, aralkyl, heteroaralkyl, cyano, amino, C~_s-alkylamino,
C~_s-
dialkylamino, arylamino or methylenedioxy; or
provided X~ and X2 is hydrogen, A is selected from the ring systems consisting
of
CA 02395298 2002-06-18
WO 01/55085 PCT/DKO1/00058
14
/ ~ ~ \ \ ~ ~ \
N
I
R5
\ \ \ ~ \ \ \ \ N \
/ / / N ( /
N
\ S \ \ O \
N ~ ~N
Rs Rs
wherein A is optionally substituted with one or more substituents selected
from C~_s-alkyl, CZ_
6-alkenyl, C~_6-alkynyl, hydroxy, aryloxy, arylthio, aralkoxy, C~_6-alkoxy,
C~_6-alkylthio; heteroa-
ryloxy, heteroaralkoxy, halogen, perhalomethyl, perhalomethoxy, acyl, aralkyl,
hei:eroaralkyl,
cyano, amino, C~_6-alkylamino, C~_6-dialkylamino, arylamino or methylenedioxy;
and
R5 is hydrogen or C~_6-alkyl; and
X~ and XZ independently are hydrogen, aryl or heteroaryl optionally
substituted with one or
more substituents selected from C~_6-alkyl, CZ_s-alkenyl, C2_s-alkynyl,
hydroxy, aryloxy, aryl-
thio, aralkoxy, heteroaryloxy, aralkoxy, C~_6-alkoxy, C~~-alkylthio, halogen,
perhalomethyl,
perhalomethoxy, acyl, aryl, heteroaryl, aralkyl, heteroaralkyl, cyano, amino,
C~~-alkylamino,
C~_s-dialkylamino, arylamino or methylenedioxy; and
Y is hydrogen, C~_~2-alkyl, C2_~Z-alkenyl, C2_~2-alkynyl, C~~2-alkenynyl,
aralkyl or heteroaralkyl
optionally substituted with one or more substituents selected from halogen,
C~_6-alkyl, perha-
lomethyl, hydroxy, aryl, heteroaryl, carboxy or amino; and
Z is hydrogen, halogen, hydroxy, C~_6-alkyl or C~_6-alkoxy optionally
substituted with one or
more substituents selected from halogen, hydroxy, carboxy, amino, cyano or
C,_6-alkoxy; and
CA 02395298 2002-06-18
WO 01/55085 PCT/DKO1/00058
Q is O, S or NR6, wherein R6 is hydrogen, C~_6-alkyl, C~~-alkenyl, C2_6-
alkynyl, C~6-alkenynyl,
aralkyl, heteroaralkyl and wherein Rs is optionally substituted with one or
more substituents
selected from halogen, hydroxy, C~_6-alkoxy, amino or carboxy; and
5 Ar is arylene, heteroarylene or a divalent heterocyclic group each of which
can be optionally
substituted with one or more substituents selected from C~_6-alkyl, aryl or
C~_s-alkoxy each of
which can be optionally substituted with halogen, hydroxy, carboxy, cyano or
heterocyclyl;
and
R~ is hydrogen, hydroxy or halogen; or R~ forms a bond together with R2; and
R2 is hydrogen or C~_6-alkyl; or R~ forms a bond together with R~; and
R3 is hydrogen, C~_6-alkyl, C2_s-alkenyl, CZ_6-alkynyl, C4_6-alkenynyl, aryl,
aralkyl, C~_6-
alkoxyC~_salkyl, acyl, heterocyclyl, heteroaryl or heteroaralkyl groups
optionally substituted
with one or more substituents selected from halogen, perhalomethyl! hydroxy or
cyano; and
R4 is hydrogen, C~~-alkyl, C2_6-alkenyl, C2~-alkynyl, C4~-alkenynyl or aryl;
and
n is an integer ranging from 0 to 3; and
m is an integer ranging from 0 to 1.
In another preferred embodiment, the present invention is concerned with
compounds of for-
mula I wherein A is aryl or heteroaryl optionally substituted with one or more
substituents
selected from from C~_s-alkyl, CZ_6-alkenyl each of which is optionally
substituted with one or
more substituents selected from C~_6-alkoxycarbonyl or carboxy, or
A is optionally substituted with aryloxy optionally substituted with one or
more C~_6-alkoxy, or
A is optionally substituted with aralkoxy optionally substituted with one or
more substituents
selected from C~_6-alkoxy, nitro, carboxy or C~~-alkoxycarbonyl, or
A is optionally substituted with C~_6-alkoxy optionally substituted with one
or more halogens,
or
A is optionally substituted with aralkenyl, C2_6-alkenyloxy, aralkynyl,
halogen, perhalomethyl,
perhalomethoxy, acyl, aralkyl or methylenedioxy.
CA 02395298 2002-06-18
WO 01/55085 PCT/DKO1/00058
16
In another preferred embodiment, the present invention is concerned with
compounds of for-
mula I wherein A is aryl, heteroaryl or
optionally substituted with one or more substituents selected from aryloxy,
arylthio, aralkoxy,
C~~-alkoxy, C~_6-alkylthio, halogen, perhalomethyl, aralkyl, heteroaralkyl,
heteroaryloxy or
heteroaralkoxy.
In another preferred embodiment, the present invention is concerned with
compounds of for-
mula I wherein A is aryl optionally substituted with one or more substituents
selected from
aryloxy, arylthio, aralkoxy, C~_6-alkoxy, C~~-alkylthio, halogen,
perhalomethyl, aralkyl, het-
eroaralkyl, heteroaryloxy or heteroaralkoxy.
In another preferred embodiment, the present invention is concerned with
compounds of for-
mula I wherein A is aryl optionally substituted with one or more substituents
selected from
from C~_6-alkyl, C2_6-alkenyl each of which is optionally substituted. with
one or more substitu-
ents selected from C~_6-alkoxycarbonyl or carboxy, or
A is optionally substituted with aryloxy optionally substituted with one or
more C~_6-alkoxy, or
A is optionally substituted with aralkoxy optionally substituted with one or
more.substituents
selected from C~_6-alkoxy, nitro, carboxy or C~_6-alkoxycarbonyl, or
A is optionally substituted with C~_s-alkoxy optionally substituted with one
or more halogens,
or
A is optionally substituted with aralkenyl, C2_6-alkenyloxy, aralkynyl,
halogen, perhalomethyl,
perhalomethoxy, acyl, aralkyl or methylenedioxy.
In another preferred embodiment, the present invention is concerned with
compounds of for-
mula I wherein. A is aryl optionally substituted with one or more substituents
selected from
from C~_6-alkyl, or
A is optionally substituted with aryloxy optionally substituted with one or
more C~~-alkoxy,
CA 02395298 2002-06-18
WO 01/55085 PCT/DKO1/00058
17
A is optionally substituted with aralkoxy optionally substituted with one or
more substituents
selected from C~_6-alkoxy, or
A is optionally substituted with C~~-alkoxy optionally substituted with one or
more halogens,
or
A is optionally substituted with aralkenyl, aralkynyl, halogen, perhalomethyl,
perhalomethoxy
or aralkyl.
In another preferred embodiment, the present invention is concerned with
compounds of
formula I wherein A is heteroaryl optionally substituted with one or more
substituents se-
lected from aryloxy, arylthio, aralkoxy, C~_6-alkoxy, C~~-alkylthio, halogen,
perhalomethyl,
aralkyl, heteroaralkyl, heteroaryloxy or heteroaralkoxy.
In another preferred embodiment, the present invention is concerned with
compounds of for-
mula I wherein A is heteroaryl.
In another preferred embodiment, the present invention is concerned with
compounds of formula I wherein A is
v
optionally substituted with one or more substituents selected from aryloxy,
arylthio, aralkoxy,
C~_6-alkoxy, C~_6-alkylthio, halogen, perhalomethyl, aralkyl, heteroaralkyl,
heteroaryloxy or
heteroaralkoxy.
In another preferred embodiment, the present invention is concerned with
compounds of formula I wherein A is
N
I
R5
optionally substituted with one or more substituents selected from C~_6-alkyl,
and wherein R5
is hydrogen or C~_6-alkyl.
CA 02395298 2002-06-18
WO 01/55085 PCT/DKO1/00058
18
In another preferred embodiment, the present invention is concerned with
compounds of
formula I wherein X~ and Xz independently are hydrogen, aryl or heteroaryl
optionally substi-
tuted with one or more substituents selected from aryloxy, arylthio, aralkoxy,
halogen, perha-
lomethyl, aryl, heteroaryl, aralkyl, heteroaralkyl, heteroaryloxy or
heteroaralkoxy.
In another preferred embodiment, the present invention is concerned with
compounds of
formula I wherein X~ and Xz independently are
hydrogen,
aryl or heteroaryl optionally substituted with one or more substituents
selected from halogen,
acyl, aryl, or
aryl or heteroaryl optionally substituted with one or more substituents
selected from C~_6-
alkyl, C2_6-alkynyl each of which is optionally substituted with hydroxy.
In another preferred embodiment, the present invention is concerned with
compounds.of
formula I wherein X~ and X~ independently are
hydrogen, or
aryl or heteroaryl optionally substituted with one or more substituents
selected from halogen.
In another preferred embodiment, the present invention is concerned with
compounds of for-
mula I wherein X~ and XZ independently are hydrogen or aryl optionally
substituted with one
or more substituents selected from aryloxy, arylthio, aralkoxy, halogen,
perhalomethyi, aryl, .
heteroaryl, aralkyl, heteroaralkyl, heteroaryloxy or heteroaralkoxy.
In another preferred embodiment, the present invention is concerned with
compounds of
formula I wherein X~ and X2 independently are
hydrogen,
aryl optionally substituted with one or more substituents selected from
halogen, acyl, aryl, or
aryl optionally substituted with one or more substituents selected from C~_6-
alkyl, C2~-alkynyl
each of which is optionally substituted with hydroxy.
In another preferred embodiment, the present invention is concerned with
compounds of
formula I wherein X~ and XZ independently are
hydrogen, or
CA 02395298 2002-06-18
WO 01/55085 PCT/DKO1/00058
19
phenyl optionally substituted with one or more substituents selected from
halogen.
In another preferred embodiment, the present invention is concerned with
compounds of for-
mula I wherein X~ and X2 independently are hydrogen or heteroaryl optionally
substituted
with one or more substituents selected from aryloxy, arylthio, aralkoxy,
halogen, perha-
lomethyl, aryl, heteroaryl, aralkyl, heteroaralkyl, heteroaryloxy or
heteroaralkoxy.
In another preferred embodiment, the present invention is concerned with
compounds of for-
mula I wherein X~ and X2 independently are hydrogen or heteroaryl.
In another preferred embodiment, the present invention is concerned with
compounds of formula I wherein X~ and X2 is hydrogen.
In another preferred embodiment, the present invention is concerned with
compounds of for-
mula I wherein Y is hydrogen or C~_~2-alkyl.
In another preferred embodiment, the present invention is concerned with
.compounds of for-
mula I wherein Y is hydrogen or methyl.
In another preferred embodiment, the. present invention is concerned
with.compounds of for-
mula I wherein Z is hydrogen or C~_6-a(koxy.
In another preferred embodiment, the present invention is concerned with,
compounds of for-
mula I wherein Z is hydrogen or C~_s-alkyl.
In another preferred embodiment, the present invention is concerned with
compounds of for-
mula I wherein Z is hydrogen.
In another preferred embodiment, the present invention is concerned with
compounds of for-
mula I wherein Q is O.
In another preferred embodiment, the present invention is concerned with
compounds of for-
mula I wherein Ar is arylene optionally substituted with one or more
substituents. selected
from C,_6-alkyl or C~~-alkoxy each of which can be optionally substituted with
carboxy.
CA 02395298 2002-06-18
WO 01/55085 PCT/DKO1/00058
In another preferred embodiment, the present invention is concerned with
compounds of for-
mula I wherein Ar is arylene.
5 In another preferred embodiment, the. present invention is concerned with.
compounds of for-
mula 1 wherein R~ is hydrogen or R~ forms a bond together with R2.
In another preferred embodiment, the present invention is concerned with
compounds of for-
mula I wherein R~ is hydrogen.
In another preferred embodiment, the present invention is concerned with
compounds of for-
mula I wherein R2 is hydrogen or R2 forms a bond together with R~.
In another preferred embodiment, the present invention is concerned with
compounds of for-
mina I wherein R2 is hydrogen.
In another preferred embodiment, the present invention is concerned with
compounds of for-
mula I wherein R3 is C~_6-alkyl or aralkyl.
In another preferred embodiment,.the present invention is concerned with
compounds of for-
mula I wherein R3 is C~_6-alkyl.
In another preferred embodiment, the present invention is concerned with
compounds of for-
mula I. wherein R4 is hydrogen, C~_3-alkyl.
In another preferred embodiment, the present invention is concerned with
compounds of for-
mula I wherein R4 is hydrogen.
In another preferred embodiment, the present invention is concerned with
compounds of for-
mula I wherein n is 1.
In another preferred embodiment, the present invention is concerned with
compounds of for-
mula I wherein m is 1.
CA 02395298 2002-06-18
WO 01/55085 PCT/DKO1/00058
21
In another preferred embodiment, the present invention is concerned with
compounds of
formula I wherein alkyl is methyl, ethyl, n-propyl, iso-propyl, butyl, tert-
butyl, pentyl, hexyl,
cyclopropyl or cyclopentyl.
In another preferred embodiment, the present invention is concerned with
compounds of
formula I wherein alkenyl is vinyl or 1-propenyl.
In another preferred embodiment, the present invention is concerned with
compounds of
formula I wherein alkynyl is ethynyl, 1-propynyl and 2-propynyl.
In another preferred embodiment, the present invention is concerned with
compounds of
formula I wherein alkoxy is methoxy, ethoxy, propoxy, butoxy, pentoxy, hexoxy,
isopropoxy or
cyclopentyloxy.
In another preferred embodiment, the present invention is concerned with
compounds of
formula I wherein alkylthio is methylthio, ethylthio, propylthio or
cyclopentylthio.
In another preferred embodiment, the present invention is concerned with
compounds of
formula I wherein aryl is phenyl optionally substituted with halogen.
In another preferred embodiment, the present invention is concerned with
compounds of
formula I wherein arylene is phenylene optionally substituted with halogen.
In another preferred embodiment, the present invention is concerned with
.compounds of
formula I wherein halogen is fluorine or chlorine.
In another preferred embodiment, the present invention is concerned with
compounds of
formula I wherein perhalomethyl is trifluoromethyl.
In another preferred embodiment, the present invention is concerned with
compounds of
formula I wherein acyl is acetyl.
CA 02395298 2002-06-18
WO 01/55085 PCT/DKO1/00058
22
In another preferred embodiment, the present invention is concerned with
compounds of
formula I wherein heteroaryl is furan, thiophene, pyrrole, imidazole,
pyrazole, pyridine,
quinoline, isoquinoline, quinazoline, quinoxaline, indole, benzimidazole or
benzofuran.
In another preferred embodiment, the present invention is concerned with
compounds of
formula I wherein heteroaryl is furan, pyrrole, indole or benzofuran.
In another preferred embodiment, the present invention is concerned with
compounds of
formula I wherein heteroarylene is furan, thiophene, pyrrole, imidazole,
pyrazole, triazole,
pyridine, pyrazine, pyrimidine or pyridazine.
In another preferred embodiment, the present invention is concerned with
compounds of
formula I wherein aralkyl is benzyl or phenethyl.
In another preferred embodiment, the present invention is concerned with
compounds of
formula I wherein aryloxy is phenoxy.
In another preferred embodiment, the present invention is concerned with
compounds of
formula I wherein aralkoxy is benzyloxy.
In another preferred embodiment, the present invention is concerned with
compounds of
formula I wherein n is an integer ranging from 1 to 3 and m is 1.
In another preferred embodiment, the present invention is concerned with
compounds of
formula I wherein the substituents Z and Y are arranged in a trans-
configuration.
In another preferred embodiment, the present invention is concerned with
compounds of
formula l wherein the substituents Z and Y are arranged in a cis-
configuration.
Preferred compounds of the invention are:
(E)-(S)-Ethyl 3-[4-(3-biphenyl-4-yl-but-2-enyloxy)-phenyl]-2-ethoxy-
propionate,
(E)-(S)-3-[4-(3-Biphenyl-4-yl-but-2-enyloxy)-phenyl]-2-ethoxy-propionic acid,
(E)-(S)-3-{4-[3-(4'-Bromo-biphenyl-4-yl)-but-2-enyloxy]-phenyl}-2-ethoxy-
propionic acid ethyl
ester,
(E)-(S)-3-{4-[3-(4'-Bromo-biphenyl-4-yl)-but-2-enyloxy]-phenyl}-2-ethoxy-
propionic acid,
CA 02395298 2002-06-18
WO 01/55085 PCT/DKO1/00058
23
(E)-(S)-2-Ethoxy-3-{4-[3-(4-phenoxy-phenyl)-but-2-enyloxy]-phenyl}-propionic
acid ethyl es-
ter,
(E)-(S)-2-Ethoxy-3-{4-[3-(4-phenoxy-phenyl)-but-2-enyloxy]-phenyl}-propionic
acid,
(E)-(S)-2-Ethoxy-3-(4-{3-[4-(4-methoxy-phenoxy)-phenyl]-but-2-enyloxy}-phenyl)-
propionic
acid ethyl ester,
(E)-(S)-2-Ethoxy-3-(4-{3-[4-(4-methoxy-phenoxy)-phenyl]-but-2-enyloxy}-phenyl)-
propionic
acid,
(E)-(S)-2-Ethoxy-3-{4-[3-(9H-fluoren-2-yl)-but-2-enyloxy]-phenyl}-propionic
acid ethyl ester,
(E)-(S)-2-Ethoxy-3-{4-[3-(9H-fluoren-2-yl)-but-2-enyloxy]-phenyl}-propionic
acid,
(E)-(S)-3-{4-[3-(3,4-Dimethoxy-phenyl)-but-2-enyloxy]-phenyl}-2-ethoxy-
propionic acid ethyl
ester,
(E)-(S)-3-{4-[3-(3,4-Dimethoxy-phenyl)-but-2-enyloxy]-phenyl}-2-ethoxy-
propionic acid,
(E)-(S)-3-{4-[3-(3,5-Bis-trifluoromethyl-phenyl)-but-2-enyloxy]-phenyl}-2-
ethoxy-propionic acid
ethyl ester,
(E)-(S)-3-{4-[3-(3,5-Bis-trifluoromethyl-phenyl)-but-2-enyloxy]-phenyl}-2-
ethoxy-propionic
acid,
(E)-(S)-3-[4-(3-Biphenyl-4-yl-allyloxy)-phenyl]-2-ethoxy-propionic acid ethyl
ester,
(E)-(S)-3-[4-(3-Biphenyl-4-yl-allyloxy)-phenyl]-2-ethoxy-propionic acid,
(E)-(S)-2-Ethoxy-3-[4-(3-naphthalen-2-yl-but-2-enyloxy)-phenyl]-propionic acid
ethyl ester,
(E)-(S)-2-Ethoxy-3-[4-(3-naphthalen-2-yl-but-2-enyloxy)-phenyl]-propionic
acid,
(E)-(S)-2-Ethoxy-3-[4-(3-pyridin-2-yl-but-2-enyloxy)-phenyl]-propionic acid
ethyl ester,
(E)-(S)-2-Ethoxy-3-[4-(3-pyridin-2-yl-but-2-enyloxy)-phenyl]-propionic acid,
(E)-(S)-3-{4-[3-(3,5-Bis-benzyloxy-phenyl)-but-2-enyloxy]-phenyl}-2-ethoxy-
propionic acid
ethyl ester,
(E)-(S)-3-{4-[3-(3,5-Bis-benzyloxy-phenyl)-but-2-enyloxy]-phenyl}-2-ethoxy-
propionic acid,
(E)-(S)-2-Ethoxy-3-[4-(3-naphthalen-2-yl-allyloxy)-phenyl]-propionic acid
ethyl ester,
(E)-(S)-2-Ethoxy-3-[4-(3-naphthalen-2-yl-allyloxy)-phenyl]-propionic acid,
(E)-(S)-2-Ethoxy-3-{4-[3-(3-phenoxy-phenyl)-allyloxy]-phenyl}-propionic acid
ethyl ester,
(E)-(S)-2-Ethoxy-3-{4-[3-(3-phenoxy-phenyl)-allyloxy]-phenyl}-propionic acid,
(S)-3-[4-(2-Benzofuran-3-yl-allyloxy)-phenyl]-2-ethoxy-propionic acid ethyl
ester,
(S)-3-[4-(2-Benzofuran-3-yl-allyloxy)-phenyl]-2-ethoxy-propionic acid;
or a salt thereof with a pharmaceutically acceptable acid or base, or any
optical isomer or
mixture of optical isomers, including a racemic mixture, or any tautomeric
forms.
CA 02395298 2002-06-18
WO 01/55085 PCT/DKO1/00058
24
Also preferred compounds of the invention are:
(E7-(S)-3-{4-[3-(4-Benzyloxy-phenyl)-allyloxy]-phenyl}-2-ethoxy-propionic acid
ethyl ester,
(~-(S)-3-{4-[3-(4-Benzyloxy-phenyl)-allyloxy]-phenyl}-2-ethoxy-propionic acid,
(~-(S)-3-[4-(3-Benzo[1,3]dioxol-5-yl-allyloxy)-phenyl]-2-ethoxy-propionic acid
ethyl ester,
(E~-(S)-3-[4-(3-Benzo[1,3]dioxol-5-yl-allyloxy)-phenyl]-2-ethoxy-propionic
acid,
(~-(S)-3-{4-[3-(4-Allyloxy-phenyl)-allyloxy]-phenyl}-2-ethoxy-propionic acid
ethyl ester,
(~-(S)-3-{4-[3-(4-Allyloxy-phenyl)-allyloxy]-phenyl}-2-ethoxy-propionic acid,
(~-(S)-3-[4-(3-Benzofuran-7-yl-allyloxy)-phenyl]-2-ethoxy-propionic acid ethyl
ester, .
(E)-(S)-3-[4-(3-Benzofuran-7-yl-ailyloxy)-phenyl]-2-ethoxy-propionic acid,
(S)-3-[4-(3-Benzo[1,3]dioxol-4-yl-allyloxy)-phenyl]-2-ethoxy-propionic acid
ethyl ester,
(S)-3-[4-(3-Benzo[1,3]dioxol-4-yl-allyloxy)-phenyl]-2-ethoxy-propionic acid;
(~-(S)-2-Ethoxy-3-(4-[3-(9H-fluoren-2-yl)-allyloxy]-phenyl)-propionic
acid.ethyl ester,.
(~-(S)-2-Ethoxy-3-(4-[3-(9H-fluoren-2-yl)-allyloxy]-phenyl)-propionic acid,
(S)-2-Ethoxy-3-[4-(3-quinolin-2-yl-allyloxy)-phenyl]-propionic acid ethyl
ester,
(S)-2-Ethoxy-3-[4-(3-quinolin-2-yi-allyloxy)-phenylj-propionic acid,
(~-(S)-3-{4-[3-(3,5-Bis-benzyloxy-phenyl)-allyloxy]-phenyl}-2-ethoxy-propionic
acid ethyl es-
ter,
(~-(S)-3-{4-[3-(3,5-Bis-benzyloxy-phenyl)-allyloxy]-phenyl}-2-ethoxy-propionic
acid,
(~-(S)-3-{4-[3-(3!5-Dimethoxy-phenyl)-allyloxy]-phenyl}-2-ethoxy-propionic
acid ethyl ester,
(~-(S)-3-{4-[3-(3,5-Dimethoxy-phenyl)-allyloxy]-phenyl}-2-ethoxy-propionic
acid,
(~-(S)-2-Ethoxy-3-[4-(3-phenanthren-9-yl-allyloxy)-phenyl]-propionic acid
ethyl ester!
(~-(S)-2-Ethoxy-3-{4-[3-(2-methoxy-naphthalen-1-yl)-allyloxy]-phenyl}-
propionic acid ethyl
ester,
(~-(S)-2-Ethoxy-3-{4-[3-(2-methoxy-naphthalen-1-yl)-allyloxy]-phenyl}-
propionic acid,
(~-(S)-Ethyl 3-{4-[3-(4-Bromophenyl)-but-2-enyloxy]-phenyl}-2-ethoxy-
propionate,
(E~-(S)-3-{4-[3-(4-Bromophenyl)-but-2-enyloxy]-phenyl}-2-ethoxy-propionic
acid,
(E)-(S)-Ethyl 3-{4-[3-(4'-Chloro-biphenyl-4-yl)-but-2-enyioxy]-phenyl}-2-
ethoxy-propionate,
(~-(S)-3-{4-[3-(4'-Chloro-biphenyl-4-yl)-but-2-enyloxy]-phenyl}-2-ethoxy-
propionic acid,
(E~-(S)-Ethyl 2-Ethoxy-3-{4-[3-(5'-isopropyl-2'-methoxy-biphenyl-4-yl)-but-2-
enyloxy]-phenyl}-
propionate,
(E'-(S)-2-Ethoxy-3-{4-[3-(5'-isopropyl-2'-methoxy-biphenyl-4-yl)-but-2-
enyloxy)-phenyl}-
propionic acid,
CA 02395298 2002-06-18
WO 01/55085 PCT/DKO1/00058
(E~-(S)-Ethyl 2-Ethoxy-3-{4-[3-(5'-chloro-2'-methoxy-biphenyl-4-yl)-but-2-
enyloxy]-phenyl}-
propionate,
(E7-(S)-3-{4-[3-(5'-Chloro-2'-methoxy-biphenyl-4-yl)-but-2-enyloxy]-phenyl}-2-
ethoxy-
propionic acid,
5 (E~-(S)-Ethyl3-{4-[3-(2',3'-Dichloro-biphenyl-4-yl)-but-2-enyloxy]-phenyl}-2-
ethoxy-
propionate,
(E~-(S)-3-{4-[3-(2',3'-Dichloro-biphenyl-4-yl)-but-2-enyloxy]-phenyl}-2-ethoxy-
propionic acid,
(E7-(S)-Ethyl 3-{4-[3-(2',6'-Dimethoxy-biphenyl-4-yl)-but-2-enyloxy]-phenyl}-2-
ethoxy-
propionate,
10 (E)-(S)-3-{4-[3-(2',6'-Dimethoxy-biphenyl-4-yl)-but-2-enyloxy]-phenyl}-2-
ethoxy-propionic
acid,
(E)-(S)-Ethyl 3-{4-[3-(4-Bromophenyl)-2-methyl-but-2-enyloxy]-phenyl}-2-ethoxy-
propionate,
'. (E)-(S)-3-{4-[3-(4-Bromophenyl)-2-methyl-but-2-enyloxy]-phenyl}-2-ethoxy-
propionic acid,
(Z)-(S)-Ethyl 3-{4-[3-(4-Bromophenyl)-2-methyl-but-2-enyloxy]-phenyl}-2-ethoxy-
propionate,
15 (Z)-(S)-3-{4-[3-(4-Bromophenyl)-2-methyl-but-2-enyloxy]-phenyl}-2-ethoxy-
propionic acid,
(E)-(S)-Ethyl 2-Ethoxy-3-[4-(3-[1,1';3',1 "]terphenyl-4"-yl-but-2-enyloxy)-
phenyl]-propionate,
(E7-(S)-2-Ethoxy-3-[4-(3-[1,1';3',1 "]terphenyl-4"-yi-but-2-enyloxy)-phenyl]-
propionic acid,
(E7-(S)-Ethyl 2-Ethoxy-3-{4-[3-(3'-methyl-biphenyl-4-yl)-but-2-enyloxy]-
phenyl}-propionate, .
(E)-(S)-2-Ethoxy-3-{4-[3-(3'-methyl-biphenyl-4-yl)-but-2-enyloxy]-phenyl}-
propionic acid,
20 (E)-(S)-3-{4-[3-(3'-Acetyl-biphenyl-4-yl)-but-2-enyloxy]-phenyl}-2-ethoxy-
propionate,
(E7-(S)-2-Ethoxy-3-{4-[3-(5'-isopropyl-2'-methoxy-biphenyl-4-yl)-allyloxy]-
phenyl}-propionic
acid ethyl ester,
(E)-(S)-2-Ethoxy-3-{4-[3-(5'-isopropyl-2'-methoxy-biphenyl-4-yl)-allyloxy]-
phenyl}-propionic
acid,
25 (E)-(S)-3-{4-[3-(3,5-Distyryl-phenyl)-allyloxy]-phenyl}-2-ethoxy-propionic
acid ethyl ester,
(E7-(S)-3-{4-[3-(3,5-Distyryl-phenyl)-allyloxy]-phenyl}-2-ethoxy-propionic
acid;
or a salt thereof with a pharmaceutically acceptable acid or base, or any
optical isomer or
mixture of optical isomers, including a racemic mixture, or any tautomeric
forms.
Also preferred compounds of the invention are:
(E)-(S)-3-{4-[3-(3,5-Diisopropoxy-phenyl)-allyloxy]-phenyl}-2-ethoxy-propionic
acid ethyl es-
ter,
(E)-(S)-3-{4-[3-(3,5-Diisopropoxy-phenyl)-allyloxy]-phenyl}-2-ethoxy-propionic
acid,
CA 02395298 2002-06-18
WO 01/55085 PCT/DKO1/00058
26
(S)-3-{4-[3-(3-Bromo-5-styryl-phenyl)-allyloxy]-phenyl}-2-ethoxy-propionic
acid ethyl ester,
(S)-3-{4-[3-(3-Bromo-5-styryl-phenyl)-allyloxy]-phenyl}-2-ethoxy-propionic
acid,
(E)-(S)-2-Ethoxy-3-[4-(3-phenyl-allyloxy)-phenyl]-propionic acid ethyl ester,
(E)-(S)-2-Ethoxy-3-[4-(3-phenyl-allyloxy)-phenyl]-propionic acid,
10 (E)-(S)-3-{4-[3-(2',3'-Dichloro-biphenyl-4-yl)-allyloxy]-phenyl}-2-ethoxy-
propionic acid ethyl
esfier,
(E)-(S)-3-{4-[3-(2',3'-Dichloro-biphenyl-4-yl)-allyloxy]-phenyl}-2-ethoxy-
propionic acid,
(E)-(S)-3-{4-[3-(3,5-Bis-phenylethynyl-phenyl)-allyloxy]-phenyl}-2-ethoxy-
propionic acid ethyl
ester,
(E)-(S)-3-{4-[3-(3,5-Bis-phenylethynyl-phenyl)-allyloxy]-phenyl}-2-ethoxy-
propionic acid,
(E)-(S)-3-{4-[3-(3,5-Diphenethyl-phenyl)-allyloxy]-phenyl}-2-ethoxy-propionic
acid ethyl ester,
(E)-(S)-3-{4-[3-(3,5-Diphenethyl-phenyl)-allyloxy]-phenyl}-2-ethoxy-propionic
acid,
3-{4-[3-(3,5-Bis-cyclopentyloxy-phenyl)-allyloxy]-phenyl}-2-ethoxy-propionic
acid ethyl ester,
(E)-(S)-3-{4-[3-(3,5-Bis-cyclopentyloxy-phenyl)-allyloxy]-phenyl}-2-ethoxy-
propionic acid,
(E)-(S)-3-(4-{3-[3,5-Bis-(2,2,2-trifluoro-ethoxy)-phenyl]-allyloxy}-phenyl)-2-
ethoxy-propionic
acid ethyl ester,
(E)-(S)-3-(4-{3-[3,5-Bis-(2,2,2-trifluoro-ethoxy)-phenyl]-allyloxy}-phenyl)-2-
ethoxy-propionic
acid,
(~-(S)-Ethyl 2-Ethoxy-3-{4-[3-(4-furan-2-yl-phenyl)-but-2-enyloxy]-phenyl}-
propionate,
(E7-(S)-Ethyl 2-Ethoxy-3-{4-[3-(2'-methyl-biphenyl-4-yl)-but-2-enyloxy]-
phenyl}-propionate,
(E7-(S)-2-Ethoxy-3-{4-[3-(2'-methyl-biphenyl-4-yl)-but-2-enyloxy]-phenyl}-
propionic acid,
(E7-(S)-Ethyl3-{4-[3-(2',5'-Dimethoxy-biphenyl-4-yl)-but-2-enyloxy]-phenyl}-2-
ethoxy-
propionate,
(~-(S)-3-{4-[3-(2',5'-Dimethoxy-biphenyl-4-yl)-but-2-enyloxy]-phenyl}-2-ethoxy-
propionic
acid,
(E)-(S)-Ethyl 3-{4-[3-(4-Bromophenyl)-2-ethyl-but-2-enyloxyJ-phenyl}-2-ethoxy-
propionate,
(E~-(S)-3-{4-[3-(4-Bromophenyl)-2-ethyl-but-2-enyloxy]-phenyl}-2-ethoxy-
propionic acid,
(L7-(S)-Ethyl3-{4-[3-(4-Bromophenyl)-2-ethyl-but-2-enyloxy]-phenyl}-2-ethoxy-
propionate,
CA 02395298 2002-06-18
WO 01/55085 PCT/DKO1/00058
27
15
(~-(S)-3-{4-[3-(4-Bromophenyl)-2-ethyl-but-2-enyloxy]-phenyl}-2-ethoxy-
propionic acid,
(E)-(S)-Ethyl 3-{4-[3-(4'-tert Butyl-biphenyl-4-yl)-but-2-enyloxy]-phenyl}-2-
ethoxy-propionate,
(E)-(S)-Ethyl 3-{4-[3-(3',5'-Bis-trifluoromethyl-biphenyl-4-yl)-but-2-enyloxy]-
phenyl}-2-ethoxy-
propionate,
(E)-(S)-3-{4-[3-(3',5'-Bis-trifluoromethyl-biphenyl-4-yl)-but-2-enyloxy]-
phenyl}-2-ethoxy-
propionic acid,
(E)-(S)-Ethyl 2-Ethoxy-3-{4-[3-(4'-isopropyl-biphenyl-4-yl)-but-2-enyloxy]-
phenyl}-propionate,
(E)-(S)-2-Ethoxy-3-{4-[3-(4'-isopropyl-biphenyl-4-yl)-but-2-enyloxy]-phenyl}-
propionic acid,
(E)-(S)- 3-{4-[3-(3,5-Dimethoxy-phenyl)-but-2-enyloxy]-phenyl}-2-ethoxy-
propionic acid ethyl
ester,
(E)-(S)-3-{4-[3-(3'-Acetyl-biphenyl-4-yl)-but-2-enyloxy]-phenyl}-2-ethoxy-
propionic acid,
(E)-(S)-Ethyl 3-{4-[3-(4'-Acetyl-biphenyl-4-yl)-but-2-enyloxy]-phenyl}-2-
ethoxy-propionate,
(E)-(S)-3-{4-[3-(4'-Acetyl-biphenyl-4-yl)-but-2-enyloxy]-phenyl}-2-ethoxy-
propionic acid,
(E)-(S)-Ethyl 2-Ethoxy-3-[4-(3-[1,1';3',1 "]terphenyl-5'-yl-allyloxy)-phenyl]-
propionate,
(E)-(S)-2-Ethoxy-3-[4-(3-[1,1';3',1"]terphenyl-5'-yl-allyloxy)-phenyl]-
propionic acid,
(E,E)-(S)-Ethyl 3-(4'-{3-[4-(2-Ethoxy-2-ethoxycarbonyl-ethyl)-phenoxy]-1-
methyl-propenyl}-
biphenyl-3-yl)-but-2-enoate,
(E,E)-(S)- 3-(4'-{3-[4-(2-Carboxy-2-ethoxy-ethyl)-phenoxy]-1-methyl-propenyl}-
biphenyl-3-yl)-
but-2-enoic acid,
(~-(S)-Ethyl2-Ethoxy-3-{4-[3-(3'-methoxy-biphenyl-4-yl)-but-2-enyfoxy]-phenyl}-
propionate,
(E)-(S)-2-Ethoxy-3-{4-[3-(3'-methoxy-biphenyl-4-yl)-but-2-enyloxy]-phenyl}-
propionic acid,
(E)-(S, S/R)-Ethyl 2-Ethoxy-3-(4-{3-[3'-(1-hydroxy-ethyl)-biphenyl-4-yl]-but-2-
enyloxy}-phenyl)-
propionate,
(E)-(S, S/R)-2-Ethoxy-3-(4-{3-[3'-(1-hydroxy-ethyl)-biphenyl-4-yl]-but-2-
enyloxy}-phenyl)-
propionic acid,
(E)-(S)-Ethyl3-{4-[3-(3,5-Dibromophenyl)-but-2-enyloxy]-phenyl}-2-ethoxy-
propionate,
(E)-(S)-3-{4-[3-(3,5-Dibromophenyl)-but-2-enyloxy]-phenyl}-2-ethoxy-propionic
acid,
(E)-(S)-Ethyl 3-{4-[3-(3,5-Dibromophenyl)-allyloxy]-phenyl}-2-ethoxy-
propionate,
(E)-(S)-3-{4-[3-(3,5-Dibromophenyl)-allyloxy]-phenyl}-2-ethoxy-propionic acid,
CA 02395298 2002-06-18
WO 01/55085 PCT/DKO1/00058
28
(E~-(S)-Ethyl 3-{4-[3-(4,4"-Di-tart-butyl-[1,1';3',1 "]terphenyl-5'-yl)-
allyloxy]-phenyl}-2-ethoxy-
propionate,
(E)-(S)-3-{4-[3-(4,4"-Di-tart butyl-[1,1';3',1 "]terphenyl-5'-yl)-allyloxy]-
phenyl}-2-ethoxy-
propionic acid,
(~-(S)-Ethyl 3-{4-[3-(3',5'-Dibromo-biphenyl-4-yl)-but-2-enyloxy]-phenyl}-2-
ethoxy-
propionate,
15
(~-(S)-3-{4-[3-(3',5'-Dibromo-biphenyl-4-yl)-but-2-enyloxy]-phenyl}-2-ethoxy-
propionic acid,
(~-(S)-Ethyl 3-{4-[3-(3',5'-Dichloro-biphenyl-4-yl)-but-2-enyloxy]-phenyl}-2-
ethoxy-
propionate,
(~-(S)-3-{4-[3-(3',5'-Dichloro-biphenyl-4-yl)-but-2-enyloxy]-phenyl}-2-ethoxy-
propionic acid,
(r~-(S)-Ethyl 3-{4-[3-(3',5'-Dichloro-biphenyl-4-yl)-allyloxy]-phenyl}-2-
ethoxy-propionate,
(~-(S)-3-{4-[3-(3',5'-Dichloro-biphenyl-4-yl)-allyloxy]-phenyl}-2-ethoxy-
propionic acid,
(~-(S)-Ethyl 3-{4-[3-(3',5'-di-tart butyl-biphenyl-4-yl)-allyloxy]-phenyl}-2-
ethoxy-propionate,
(~-(S)-3-{4-[3-(3',5'-Di-tart-butyl-biphenyl-4-yl)-allyloxy]-phenyl}-2-ethoxy-
propionic acid,
(~-(S)-Ethyl 3-{4-[3-(3',5'-Di-tart butyl-biphenyl-4-yl)-but-2-enyloxy]-
phenyl}-2-ethoxy-
propionate,
(~-(S)-3-{4-[3-(3',5'-Di-tart-butyl-biphenyl-4-yl)-but-2-enyloxy]-phenyl}-2-
ethoxy-propionic
acid,
(~-(S/R)-Ethyl 3-[4-(3-Biphenyl-4-yl-but-2-enyloxy)-phenyl]-2-isopropoxy-
propionate,
(~-(S/R)-3-[4-(3-Biphenyl-4-y!-but-2-enyloxy)-phenyl]-2-isopropoxy-propionic
acid,
(E~-(S/R)-Ethyl 3-[4-(3-Biphenyl-4-yl-but-2-enyloxy)-phenyl]-2-butoxy-
propionate,
(~-(S/R)-3-[4-(3-Biphenyl-4-yl-but-2-enyloxy)-phenyl]-2-butoxy-propionic acid,
(~-(S/R)-Ethyl3-[4-(3-Biphenyl-4-yl-but-2-enyloxy)-phenyl]-2-hexyloxy-
propionate,
(t7-(S/R)-3-[4-(3-Biphenyl-4-yl-but-2-enyloxy)-phenyl]-2-hexyloxy-propionic
acid,
(~-(S/R)-Ethyl 3-[4-(3-Biphenyl-4-yl-but-2-enyloxy)-phenyl]-2-(3-phenyl-
propoxy)-propionate,
(~-(S/R)-3-[4-(3-Biphenyl-4-yl-but-2-enyloxy)-phenyl]-2-(3-phenyl-propoxy)-
propionic acid,
(~-(S/R)-Ethyl 3-[4-(3-Biphenyl-4-yl-but-2-enyloxy)-phenyl]-2-(4-phenyl-
butoxy)-propionate,
(~-(S/R)-3-[4-(3-Biphenyl-4-yl-but-2-enyloxy)-phenyl]-2-(4-phenyl-butoxy)-
propionic acid,
CA 02395298 2002-06-18
WO 01/55085 PCT/DKO1/00058
29
(E)-(S/R)-Propyl 3-[4-(3-Biphenyl-4-yl-but-2-enyloxy)-phenyl]-2-propoxy-
propionate,
(E'-(S/R)-3-[4-(3-Biphenyl-4-yl-but-2-enyloxy)-phenyl]-2-propoxy-propionic
acid,
(E~-(S)-3-{4-[3-(3,5-Diethoxyoxy-phenyl)-allyloxy]-phenyl}-2-ethoxy-propionic
acid ethyl ester,
(E)-(S)-3-{4-[3-(3,5-Diethoxyoxy-phenyl)-allyloxy]-phenyl}-2-ethoxy-propionic
acid,
(E)-(S)-3-{4-[3-(3,5-Bis-trifluoromethyl-phenyl)-allyloxy]-phenyl}-2-ethoxy-
propionic acid ethyl
ester,
(E~-(S)-3-{4-[3-(3,5-Bis-trifluoromethyl-phenyl)-allyloxy]-phenyl}-2-ethoxy-
propionic acid,
(E)-(R,S)-3-[4-(3-Biphenyl-4-yl-allyloxy)-phenyl]-2-ethoxy-propionic acid
ethyl ester,
(L~-(R,S)-3-[4-(3-Biphenyl-4-yl-allyloxy)-phenyl]-2-ethoxy-propionic acid,
(E)-(S)- 4-(3-{3-[4-(2-Ethoxy-2-ethoxycarbonyl-ethyl)-phenoxy]-propenyl}-
phenoxymethyl)-
benzoic acid methyl ester,
(E'-(S)- 4-(3-{3-[4-(2-Carboxy-2-ethoxy-ethyl)-phenoxy]-propenyl}-
phenoxymethyl)-benzoic
acid;
or a salt thereof with a pharmaceutically acceptable acid or base, or any
optical isomer or
mixture of optical isomers, including a racemic mixture, or any tautomeric
forms.
Also preferred compounds of the invention are:
(E)-(S)-Ethyl 2-Ethoxy-3-{4-[3-(4'-fluoro-biphenyl-4-yl)-but-2-enyloxy]-
phenyl}~propionate,
(E)-(S)-2-Ethoxy-3-{4-[3-(4'-fluoro-biphenyl-4-yl)-but-2-enyloxy]-phenyl}-
propionic acid,
(E)-(S)-Ethyl 2-Ethoxy-3-{4-[3-(4-lodophenyl)-but-2-enyloxy]-phenyl}-
propionate;
or a salt thereof with a pharmaceutically acceptable acid or base, or any
optical isomer or
mixture of optical isomers, including a racemic mixture, or any tautomeric
forms.
In the above structural formulas and throughout the present specification, the
following terms
have the indicated meaning:
The term "C~_~2-alkyl" as used herein, alone or in combination is intended to
include those alkyl
groups of the designated length in either a linear or branched or cyclic
configuration represents
e.g. cyclopropyl, cyclobutyl, cyclopentyl, cyclohexyl, cycloheptyl and
cyclooctyl and the like.
Typical C~_~2-alkyl groups include, but are not limited to, methyl, ethyl, n-
propyl, iso-propyl,
CA 02395298 2002-06-18
WO 01/55085 PCT/DKO1/00058
butyl, iso-butyl, sec-butyl, tert-butyl, pentyl, iso-pentyl, hexyl, iso-hexyl,
cyclopropyl, cyclob-
utyl, cyclopentyl, cyclohexyl, cycloheptyl and cyclooctyl and the like,
especially preferred is
methyl, ethyl, n-propyl, iso-propyl, butyl, tert-butyl, pentyl, hexyl,
cyclopropyl and cyclopentyl.
5 The term "Cz_~z-alkenyl" as used herein, represents an olefinically
unsaturated branched or
straight group having from 2 to the specified number of carbon atoms and at
least one dou-
ble bond. Examples of such groups include, but are not limited to, vinyl, 1-
propenyl, 2-
propenyl, allyl, iso-propenyl, 1,3-butadienyl, 1-butenyl, hexenyl, pentenyl
and the like, espe-
cially preferred is vinyl and 1-propenyl.
The term "Cz_~z-alkynyl", as used herein, represent an unsaturated branched or
straight
group having from 2 to the specified number of carbon atoms and at least;one
triple bond.
Examples of such groups include, but are not limited to ethynyl, 1-propynyl, 2-
propynyl, 1-
butynyl, 2-butynyl, 1-pentynyl, 2-pentynyl and the like, especially preferred
is ethynyl, 1-
propynyl and 2-propynyl.
The term "C4_~z-alkenynyl" as used herein, represent an unsaturated branched
or straight hy-
drocarbon group having from 4 to the specified number of carbon atoms and both
at least
one double bond and at least one triple bond. Examples of such groups include,
but are not. .
limited to, 1-penten-4-yne, 3-penten-1-yne, 1,3-hexadiene-5-yne and the like.
The term "C~~-alkoxy" as used herein, alone or in combination is intended to
include those C~.~-
alkyl groups of the designated length in either a linear or branched or cyclic
configuration linked
thorugh an ether oxygen having its free valence bond from the ether oxygen.
Examples of linear
alkoxy groups are methoxy, ethoxy, propoxy, butoxy, pentoxy, hexoxy and the
like, especially
preferred is methoxy, ethoxy, propoxy, butoxy, pentoxy, hexoxy. Examples of
branched alkoxy
are isopropoxy, sec-butoxy, tert-butoxy, isopentoxy, isohexoxy and the like,
especially preferred
is isopropoxy. Examples of cyclic alkoxy are cyclopropyloxy, cyclobutyloxy,
cyclopentyloxy,
cyclohexyloxy and the like, especially preferred is cyclopentyloxy.
The term "C~_6-alkylthio" as used herein, alone or in combination, refers to a
straight or
branched or cyclic monovalent substituent comprising a C~_6-alkyl group linked
through a
divalent sulfur atom having its free valence bond from the sulfur atom and
having.1 to 6
carbon atoms e.g. methylthio, ethylthio, propylthio, butylthio, pentylthio and
the like,
CA 02395298 2002-06-18
WO 01/55085 PCT/DKO1/00058
31
especially preferred is methylthio, ethylthio and propylthio. Examples of
cyclic alkylthio are
cyclopropylthio, cyclobutylthio, cyclopentylthio, cyclohexylthio and the like,
especially preferred
is cyclopentylthio.
The term "C~~-alkylamino" as used herein, alone or in combination, refers to a
straight or
branched or cyclic monovalent substituent comprising a C~_6-alkyl group linked
through amino
having a free valence bond from the nitrogen atom e.g. methylamino,
ethylamino,
propylamino, butylamino, pentylamino and the like. Examples of cyclic
alkylamino are
cyclopropylamino, cyclobutylamino, cyclopentylamino, cyclohexylamino and the
like.
The term "arylamino" as used herein, alone or in combination, refers to an
aryl as defined
herein linked through amino having a free valence bond from the nitrogen atom
e.g.
phenylamino, naphthylamino and the like.
The term "C~_6-alkoxyC~_6-alkyl" as used herein, alone or in combination,
refers to a C~_6-alkyl
as defined herein whereto is attached a C~_6-alkoxy as defined herein, e.g.
methoxymethyl,
ethoxymethyl, methoxyethyl, ethoxyethyl and the. like.
The term "aryl" is intended to include a bicyclic aromatic ring, such as
carbocyclic aromatic rings
selected from the group consisting of phenyl and naphthyl, (1-naphthyl or 2-
naphthyl), optionally
substituted with halogen, amino, hydroxy, C~~-alkyl, C~~-alkoxy, carboxy or
C~~-alkylester and
the like, especially preferred is halogen.
The term "arylene" is intended to include divalent aromatic rings, such as
carbocyclic aromatic
rings selected from the group consisting of phenylene, naphthylene and the
like optionally
substituted with halogen, amino, hydroxy, C~.~-alkyl, C~.~-alkoxy, carboxy or
C~~-alkylester and
the like.
The term "halogen" means fluorine, chlorine, bromine or iodine, especially
preferred is
fluorine and chlorine.
The term "perhalomethyl" means trifluoromethyl, trichloromethyl,
tribromomethyl or
triiodomethyl, especially preferred is trifluoromethyl.
CA 02395298 2002-06-18
WO 01/55085 PCT/DKO1/00058
32
The term "C~_6-dialkylamino" as used herein refers to an amino group wherein
the two
hydrogen atoms independently are substituted with a straight or branched,
saturated.
hydrocarbon chain having the indicated number of carbon atoms; such as
dimethylamino, N-
ethyl-N-methylamino, diethylamino, dipropylamino, N-(n-butyl)-N-methylamino,
di(n-
pentyl)amino and the like.
The term "acyl" as used herein refers to a monovalent substituent comprising a
C~_6-alkyl
group linked through a carbonyl group; such as e.g. acetyl, propionyl,
butyryl, isobutyryl,
pivaloyl, valeryl and the like, especially preferred is acetyl.
The term "heteroaryl" as used herein, alone or in combination, refers to a
monovalent
substituent comprising a 5-6 membered monocyclic aromatic system or a 9-10
membered
bicyclic aromatic system containing one or more heteroatoms selected from
nitrogen, oxygen
and sulfur, e.g. furan, thiophene, pyrrole, imidazole, pyrazole, triazole,
pyridine, pyrazine,
pyrimidine, pyridazine, isothiazole, isoxazole, oxazole, oxadiazole,
thiadiazole, quinoline,
isoquinoline, quinazoline, quinoxaline, indole, benzimidazole, benzofuran,
pteridine and
purine and the like, preferred is furan, thiophene, pyrrole, imidazole,
pyrazole, pyridine,
quinoline, isoquinoline, quinazoline~ quinoxaline, indole, benzimidazole,
benzofuran,
especially preferred is furan,: pyrrole, indole and benzofuran.
The term "heteroarylene" as used herein, alone or in combination, refers to a
divalent group
comprising a 5-6 membered monocyclic aromatic system or a 9-10 membered
bicyclic
aromatic system containing one or more heteroatoms selected from nitrogen,
oxygen and
sulfur, e.g. furan, thiophene, pyrrole, imidazole, pyrazole, triazole,
pyridine, pyrazine,
pyrimidine, pyridazine, isothiazole, isoxazole, oxazole, oxadiazole,
thiadiazole, quinoline,
isoquinoline, quinazoline, quinoxaline, indole, benzimidazole, benzofuran,
pteridine and
purine and the like, especially preferred is furan, thiophene, pyrrole,
imidazole, pyrazole,
triazole, pyridine, pyrazine, pyrimidine, pyridazine.
The. term "heteroaryloxy" as used herein, alone or in combination, refers to a
heteroaryl as
defined herein linked to an oxygen atom having its free valence bond from the
oxygen atom
e.g. pyrrole, imidazole, pyrazole, triazole, pyridine, pyrazine, pyrimidine,
pyridazine, isothi-
azole, isoxazole, oxazole, oxadiazole, thiadiazole, quinoline, isoquinoline,
quinazoline,
CA 02395298 2002-06-18
WO 01/55085 PCT/DKO1/00058
33
quinoxaline, indole, benzimidazole, benzofuran, pteridine and purine linked to
oxygen, and
the like.
The term "aralkyl" as used herein refers to a straight or branched saturated
carbon chain
containing from 1 to 6 carbons substituted with an aromatic carbohydride; such
as benzyl,
phenethyl, 3-phenylpropyl, 1-naphthylmethyl,.2-(1-naphthyl)ethyl and the like,
especially
preferred is benzyl and phenethyl.
The term "aryloxy" as used herein refers to phenoxy, 1-naphthyloxy, 2-
naphthyloxy and the
like, especially preferred is phenoxy.
The term "aralkoxy" as used herein refers to a C~_6-alkoxy group substituted.
with an aromatic
carbohydride, such as benzyloxy, phenethoxy, 3-phenylpropoxy, 1-
naphthylmethoxy, 2-(1-
naphthyl)ethoxy and the like, especially preferred is benzyloxy.
The term "heteroaralkyl" as used herein refers to a straight or branched
saturated carbon
chain containing from 1 to 6 carbons substituted with a heteroaryl group; such
as.(2-
furyl)methyl;.(3-furyl)methyl, (2-thienyl)methyl, (3-thienyl)methyl, (2-
pyridyl)methyl, 1-methyl-
1-(2-pyrimidyl)ethyl and the like.
The term "heteroaralkoxy" as used herein refers to a heteroaralkyl as defined
herein linked to
an oxygen atom having its free valence bond from the oxygen atom, e.g. (2-
furyl)methyl, (3-
furyl)methyl, (2-thienyl)methyl, (3-thienyl)methyl, (2-pyridyl)methyl, 1-
methyl-1-(2-
pyrimidyl)ethyl linked to oxygen, and the like.
The term "arylthio" as used herein, alone or in combination, refers to an aryl
group linked
through a divalent sulfur atom having its free valence bond from the sulfur
atom, the aryl group
optionally being mono- or polysubstituted with C~~-alkyl, halogen, hydroxy or
C~.~-alkoxy; e.g.
phenylthio, (4-methylphenyl)-thio, (2-chlorophenyl)thio and the like.
As used herein, the phrase "heterocyclyl" means a monovalent saturated or
unsaturated non
aromatic group being monocyclic and containing one or more, such. as from one
to four car-
bon atom(s), and from one to four N, O or S atoms) or a combination thereof.
The phrase
"heterocyclyl" includes, but is not limited to, 5-membered heterocycles having
one hetero
CA 02395298 2002-06-18
WO 01/55085 PCT/DKO1/00058
34
atom (e.g. pyrrolidine, pyrroline and the like); 5-membered heterocycles
having two heteroa-
toms in 1,2 or 1,3 positions (e.g. pyrazoline, pyrazolidine, 1,2-oxathiolane,
imidazolidine, imi-
dazoline, 4-oxazolone and the like); 5-membered heterocycles having three
heteroatoms
(e.g. tetrahydrofurazan and the like); 5-membered heterocycles having four
heteroatoms; 6-
membered heterocycles with one heteroatom (e.g. piperidine and the like); 6-
membered het-
erocycles with two heteroatoms (e.g. piperazine, morpholine and the like); 6-
membered het-
erocycles with three heteroatoms; and 6-membered heterocycles with four
heteroatoms, and
the like.
As used herein, the phrase "a divalent heterocyclic group" means a divalent
saturated or un-
saturated system being monocyclic and containing one or more, such as from one
to four
carbon atom(s), and one to four N, O or S atoms) or a combination thereof. The
phrase a
divalent heterocyclic group includes, but is not limited to, 5-membered
heterocycles. having
one hetero atom (e.g. pyrrolidine, pyrroline and the like); 5-membered
heterocycles having
two heteroatoms in 1,2 or 1,3 positions (e.g. pyrazoline, pyrazolidine, 1,2-
oxathiolane, imida-
zolidine, imidazoline, 4-oxazolone and the like); 5-membered heterocycles
having three het-
eroatoms (e.g. tetrahydrofurazan and the like); 5-membered heterocycles having
four het-
eroatoms; 6-membered heterocycles with one heteroatom (e.g. piperidine and
the' like); 6-
membered heterocycles with two heteroatoms (e.g. piperazine, morpholine and
the like); 6-
2o membered heterocycles with three heteroatoms; and 6-membered heterocycles
with four
heteroatoms, and the like.
As used herein the term "treatment" includes treatment, prevention and
management of such
condition.
Certain of the above defined terms may occur more than once in the above
formula (I), and
upon such occurrence each term shall be defined independently of the other.
The present invention also encompasses pharmaceutically acceptable salts of
the present
compounds. Such salts include pharmaceutically acceptable acid addition salts,
pharmaceu-
tically acceptable base addition salts, pharmaceutically acceptable metal
salts, ammonium
and alkylated ammonium salts. Acid addition salts include salts of inorganic
acids as well as
organic acids. Representative examples of suitable inorganic acids include
hydrochloric, hy
drobromic, hydroiodic, phosphoric, sulfuric, nitric acids and the like.
Representative exam
CA 02395298 2002-06-18
WO 01/55085 PCT/DKO1/00058
pies of suitable organic acids include formic, acetic, trichloroacetic,
trifluoroacetic, propionic,
benzoic, cinnamic, citric, fumaric, glycolic, lactic, malefic, malic, malonic,
mandelic, oxalic,
picric, pyruvic, salicylic, succinic, methanesulfonic, ethanesulfonic,
tartaric, ascorbic, pamoic,
bismethylene salicylic, ethanedisulfonic, gluconic, citraconic, aspartic,
stearic, palmitic,
5 EDTA, glycolic, p-aminobenzoic, glutamic, benzenesulfonic, p-toluenesulfonic
acids, sul-
phates, nitrates, phosphates, perchlorates, borates, acetates, benzoates,
hydroxynaphtho-
ates, glycerophosphates, ketoglutarates and the like. Further examples of
pharmaceutically
acceptable inorganic or organic acid addition salts include the
pharmaceutically acceptable
salts listed in J. Pharm. Sci. 1977, 66, 2, which is incorporated herein by
reference. Exam-
10 pies of metal salts include lithium, sodium, potassium, magnesium salts and
the like. Exam-
ples of ammonium and alkylated ammonium salts include ammonium,
methylammonium, di-
methylammonium, trimethylammonium, ethylammonium, hydroxyethylammonium,
diethyl-
ammonium, butylammonium, tetramethylammonium salts and the like. Examples of
organic
bases include lysine, arginine, guanidine, diethanolamine, choline and the
like.
The pharmaceutically acceptable salts are prepared by reacting the compound
~of formula I
with 1 to 4 equivalents of a base such as sodium hydroxide, sodium methoxide,
sodium hy-
dride; potassium t-butoxide, calcium hydroxide, magnesium hydroxide and the
like, in sol-
vents like ether, THF, methanol, t-butanol, dioxane, isopropanol, ethanol etc.
Mixture of sol-
vents may be used. Organic bases like lysine, arginine, diethanolamine,
choline, guandine
and their derivatives etc. may also be used. Alternatively, acid addition
salts wherever appli-
cable are prepared by treatment with acids such as hydrochloric acid,
hydrobromic acid, ni-
tric acid, ~immer0e~ acid, phosphoric acid, p-toluenesulphonic acid,
methanesulfonic acid,
acetic acid, citric acid, malefic acid salicylic acid, hydroxynaphthoic acid,
ascorbic acid, pal-
mitic acid, succinic acid, benzoic acid, benzenesulfonic acid, tartaric acid
and the like in sol-
vents like ethyl acetate, ether, alcohols, acetone, THF, dioxane etc. Mixture
of solvents may
also be used.
The stereoisomers of the compounds forming part of this invention may be
prepared by using
reactants in their single enantiomeric form in the process wherever possible
or by conducting
the reaction in the presence of reagents or catalysts in their single
enantiomer form or by re-
solving the mixture of stereoisomers by conventional methods. Some of the
preferred meth-
ods include use of microbial resolution, enzymatic resolution, resolving the
diastereomeric
salts formed with chiral acids such as mandelic acid, camphorsulfonic acid,
tartaric acid, lac-
CA 02395298 2002-06-18
WO 01/55085 PCT/DKO1/00058
36
tic acid, and the like wherever applicable or chiral bases such as brucine,
(R)- or (S)-
phenylethylamine, cinchona alkaloids and their derivatives and the like.
Commonly used
methods are compiled by Jaques et al in "Enantiomers, Racemates and
Resolution" (Wiley
Interscience, 1981 ). More specifically the compound of formula I may be
converted to a 1:1
mixture of diastereomeric amides by treating with chiral amines, aminoacids,
aminoalcohols
derived from aminoacids; conventional reaction conditions may be employed to
convert acid
into an amide; the dia-stereomers may be separated either by fractional
crystallization or
chromatography and the stereoisomers of compound of formula I may be prepared
by hydro- ,
lysing the pure diastereomeric amide.
Various polymorphs of compound of general formula I forming part of this
invention may be
prepared by crystallization of compound of formula I under different
conditions. For example,
using different solvents commonly used or their mixtures for
recrystallization; crystallizations
at different temperatures; various modes of cooling, ranging from very fast to
very slow cool-
ing during crystallizations. Polymorphs may also be obtained by heating or
melting the com- ,
pound followed by gradual or fast cooling. The presence of polymorphs may be
determined
by solid probe nmr spectroscopy, it spectroscopy, differential scanning
calorimetry, powder
X-ray diffraction or such other techniques.
The invention also encompasses prodrugs of the present compounds, which on
administra-
tion undergo chemical conversion by metabolic processes before becoming active
pharma-
cological substances. In general, such prodrugs will be functional derivatives
of the present
compounds, which are readily convertible in vivo into the required compound of
the formula
(I). Conventional procedures for the selection and preparation of suitable
prodrug derivatives
are described, for example, in "Design of Prodrugs", ed. H. Bundgaard,
Elsevier, 1985.
The invention also encompasses active metabolites of the present compounds.
Furthermore, the present compounds of formula I can be utilised in the
treatment and/or pre-
vention of conditions mediated by nuclear receptors, in particular the
Peroxisome Prolifera-
tor-Activated Receptors (PPAR).
CA 02395298 2002-06-18
WO 01/55085 PCT/DKO1/00058
37
In a further aspect, the present invention relates to a method of treating
and/or preventing Type I
or Type II diabetes.
In a still further aspect, the present invention relates to the use of one or
more compounds of the
general formula I or pharmaceutically acceptable salts thereof for the
preparation of a
medicament for the treatment and/or prevention of Type I or Type II diabetes.
In a still further aspecfi, the. present compounds are useful for the
treatment and/or prevention
of IGT.
In a still further aspect, the. present compounds are useful for the treatment
and/or prevention
of Type 2 diabetes.
In a still further aspect, the present compounds are useful for the delaying
or prevention of
the progression from IGT to Type 2 diabetes.
In a still further aspect, the present compounds are useful for the delaying
or prevention of
the progression from non-insulin requiring Type 2 diabetes to insulin
requiring Type 2 diabe-
tes.
In another aspect, the present compounds reduce blood glucose and triglyceride
levels and
are accordingly useful for the treatment and/or prevention of ailments and
disorders such as
diabetes and/or obesity.
In still another aspect, the present compounds are useful for the treatment
and/or prophylaxis
of insulin resistance (Type 2 diabetes), impaired glucose tolerance,
dyslipidemia, disorders
related to Syndrome X such as hypertension, obesity, insulin resistance,
hyperglycaemia,
atherosclerosis, hyperlipidemia, coronary artery disease, myocardial ischemia
and other car-
diovascular disorders.
In still another aspect, the present compounds are effective in decreasing
apoptosis in mam-
malian cells such as beta cells of Islets of Langerhans.
CA 02395298 2002-06-18
WO 01/55085 PCT/DKO1/00058
38
In still another aspect, the present compounds are useful for the treatment of
certain renal
diseases including glomerulonephritis, glomerulosclerosis, nephrotic syndrome,
hypertensive
nephrosclerosis.
In still another aspect, the present compounds may also be useful for
improving cognitive
functions in dementia, treating diabetic complications, psoriasis, polycystic
ovarian syndrome
(PCOS) and prevention and treatment of bone loss, e.g. osteoporosis.
The invention also relates to pharmaceutical compositions comprising, as an
active ingredi-
ent, at least one compound of the formula I or any optical or geometric isomer
or tautomeric
form thereof including mixtures of these or a pharmaceutically acceptable salt
thereof to-
gether with one or more pharmaceutically acceptable carriers or diluents.
Furthermore, the invention relates to the use of compounds of the general
formula I or their
tautomeric forms, their stereoisomers, their polymorphs, their
pharmaceutically acceptable
salts or pharmaceutically acceptable solvates thereof for the preparation of a
pharmaceutical
composition for the treatment and/or prevention of conditions mediated by
nuclear receptors,
in particularthe Peroxisome Proliferator-Activated Receptors (PPAR) such as
the conditions
mentioned above.
The present invention also relates to a process for the preparation of the
above said novel
compounds, their derivatives, their analogs, their tautomeric forms, their
stereoisomers, their
polymorphs, their pharmaceutically acceptable salts or pharmaceutically
acceptable solvates.
The method comprises:
a)
Reacting a compound of formula II
X~
~/A~Y
~O
(II)
CA 02395298 2002-06-18
WO 01/55085 PCT/DKO1/00058
39
wherein A, X~, X~ and Y are defined as above, through a Wittig process with
e.g.
(EtO)ZPO(CHZ)(CH2)tCOOR6 (wherein R6 is an alkyl group), in the presence of a
base such
as sodium hydride, EtONa and the like to give a compound of formula III.
X1
/A Y
X2
Z ~ CH2)t
O O
Rs
(III)
wherein A, X~, XZ, Y, Z and Rs are defined as above, and wherein t is 0-2, and
b)
reducing a compound of formula III, wherein A, X~, X2, Y, Z, R6 and t are
defined as above
with a suitable reagent such a diisobutylaluminum hydride, to give a compound
of formula IV.
X1
/A Y
X
z ~ CH2)t
OH
(IV)
CA 02395298 2002-06-18
WO 01/55085 PCT/DKO1/00058
wherein A, X~, X2, Y, Z and t are defined as above, and
5 C)
reacting a compound of IV, wherein A, X~, X2, Y, Z and t are defined as above,
with a com-
pound of formula V
R~ O
R2
H_~Q)m Ar WOR4
OR3
(V)
wherein Q, Ar, R~, R2; R3, R4 and m are defined as above, under Mitsunobu
conditions, using
a reagent such as triphenylphosphine/diethylazodicarboxylate and the like to
obtain a com-
pound of formula I, wherein A, X~, X~, Y, Z, Q, Ar, R~, R2, R3, R4, n and m
are defined as
above, except that R4 is not H, n and m are not 0, and
d)
converting the -OH functionality in a compound of formula IV wherein A, X~,
X2, Y, Z and t are
defined as above to an appropriate leaving group (L) such as p-
toluenesulfonate, methane-
sulfonate, halogen (in examples by methods according to: Houben-Weyl, Methoden
der or-
ganischen Chemie, Alkohole III, 6/1 b, Thieme Verlag 1984, 4th Ed., pp. 927-
939; Compre-
hensive Organic Transformations. A guide to functional group preparations, VCH
Publishers
1989, 1St Ed., pp. 353-363), triflate and the like, to give a compound of
formula VI
CA 02395298 2002-06-18
WO 01/55085 PCT/DKO1/00058
41
X1
/A Y
X
z ~ CH2)t
L
(VI)
e)
reacting a compound of formula VI
X1
/A Y
X2
z ~ CH2)t
L
(VI)
wherein L is a leaving group such as p-toluenesulfonate, methanesulfonate,
halogen , triflate
and the like and wherein A, X1, X2, Y, Z and t are defined as above with a
compound of for-
mula V
R1 O
R2
H-(Q)mAr ~OR4
OR3
CA 02395298 2002-06-18
WO 01/55085 PCT/DKO1/00058
42
(V)
wherein Q, Ar,. R~, RZ, R3, R4 and m are defined as above, to give a compound
of formula I
wherein A, X~, X2, Y, Z, Q, Ar, R~, R2, R3, R4, n and m are defined as above
except that R4 is
not H, n and m are not 0, or
e)
reacting a compound of formula VII
X~
/A~H
X2
(VII)
wherein A, X~ and X2 are defined as above, through a Friedel-Crafts
acylation..with in exam-
ple CIOCCHZ(CHZ)~R~ (wherein n and Z is defined as above and R~ are halogen or
OH), in
the presence of a Friedel-Crafts catalysts such as aluminium trichloride and
the like, to give a
compound of formula VIII
X~
/A O
X2
z ~~CH2)n
IRS
(VIII)
CA 02395298 2002-06-18
WO 01/55085 PCT/DKO1/00058
43
wherein A, X~, X2, Z, R~ and n are defined as above, and
f)
reacting a compound of formula VIII, wherein A, X~, X2, Z and R~ are defined
as above with
a Grignard reagents such a MgBrY or a lithium reagent such as LiY or
organozinc reagent
such as ZnY, wherein Y is defined as above, followed by a acidic workup to
give a compound
of formula IX
X1
/A Y
X2
z ~~CHZ)~
1o R~
(IX)
wherein A,~X~, X2, Z, Y, R~ and n are defined as above, and
g)
reacting a compound of IX, wherein A, X~, X2, Z, Y, R7 and n are defined as
above, with a
compound of formula V
R1 O
R2
H-(Q)m Ar ~OR4
OR3
(V)
CA 02395298 2002-06-18
WO 01/55085 PCT/DKO1/00058
44
wherein Q, Ar, R~, R2, R3, R4 and m are defined as above except that m is not
0, under either
basic condition e.g. potassium carbonate/acetone (if R~ is halogen) or
Mitsunobu conditions
( if R~ is OH) using a reagent such as
triphenylphosphine/diethylazodicarboxylate and the
like to obtain a compound. of formula I, wherein A, X~, X2, Y, Z, Q, Ar, R,,
Rz, R3, R4, n and m
are defined as above, except that R4 is not H, n and m arenot 0, or
h)
by chemical or enzymatic saponification of a compound of formula I
X1
/A Y
X2
R1 R O
z CH2)~ 2
(Q}~4r wOR4
OR3
wherein A, X~, X2, Y, Z, Q, Ar, R~, R2, R3, R4, n and m are defined as above,
except that R4
is not H, to obtain a compound of formula I, wherein A, X~, X2, Y, Z, Q, Ar,
R~, RZ, R3, R4, n
and m are defined as above, except that R4 is H.
Trans-cis or cis-trans isomerization of compounds I, III, IV, VI, and IX (Arai
et al., Chem.
Rev., 93, pp 23-39, 1993; J. March, Advanced Organic Chemistry, 4t" Ed., J.
Wiley & Sons,
New York 1992, pp. 218, 245, 745).
PHARMACOLOGICAL METHODS
CA 02395298 2002-06-18
WO 01/55085 PCT/DKO1/00058
In vitro PPAR alpha and PPAR gamma activation activity.
Principle.
5
The PPAR gene transcription activation assays were based on transient
transfection into
human HEK293 cells of two plasmids encoding a chimeric test protein and a
reporter protein
respectively. The chimeric test protein was a fusion of the DNA binding domain
(DBD) from
the yeast GAL4 transcription factor to the ligand binding domain (LBD) of the
human PPAR
10 proteins. The PPAR LBD harbored in addition to the ligand binding pocket
also the native
activation domain (activating function 2 = AF2) allowing the fusion protein to
function as a
PPAR ligand dependent transcription factor. The GAL4 DBD will force the fusion
protein to
bind only to Gal4 enhancers (of which none existed in HEK293 cells). The
reporter plasmid
contained a Gal4 enhancer driving the expression of the firefly luciferase
protein. After trans-
15 fection, HEK293 cells expressed the GAL4-DBD-PPAR-LBD fusion protein. The
fusion pro-
tein will in turn bind to the Gal4 enhancer controlling the luciferase
expression, and do noth-
ing in the absence of ligand. Upon addition to the cells of a PPAR ligand,
luciferase protein
will be produced in amounts corresponding to the activation of the PPAR
protein. The
amount of luciferase protein is measured by light emission after addition of
the appropriate
20 substrate.
Methods
25 In vitro transactivation assays.
Cell culture and transfection: HEK293 cells were grown in DMEM + 10% FCS.
Cells
were seeded in 96-well plates the day before transfection to give a confluency
of 50-80 % at
transfection. A total of 0,8 p,g DNA containing 0,64 ~,g pM1a/yLBD, 0,1 ~g
pCMV(3Gal, 0,08
30 ~g pGL2Ga14DBD and 0,02 p.g pADVANTAGE was transfected per well using
FuGene trans-
fection reagent according to the manufacturers instructions (Roche). Cells
were allowed to
express protein for 48 h followed by addition of compound.
CA 02395298 2002-06-18
WO 01/55085 PCT/DKO1/00058
46
Plasmids: Human PPAR a and y was obtained by PCR amplification using cDNA
synthesized
by reverse transcription of mRNA from liver and adipose tissue. respectively.
Amplified
cDNAs were cloned into pCR2.1 and sequenced. The ligand binding domain (LBD)
of each
PPAR isoform was generated by PCR (PPARa: as 167 - C-terminus; PPARy: as 165 -
C-
terminus) and fused to the DNA binding domain (DBD) of the yeast transcription
factor GAL4
by subcloning fragments in frame into the vector pM1 generating the plasmids
pM1aLBD and
pM1yLBD. Ensuing fusions were verified by sequencing. The reporter was
constructed by
inserting an oligonucleotide encoding five repeats of the GAL4 recognition
sequence (5 x
CGGAGTACTGTCCTCCG(AG)) into the vector pGL2 promotor (Promega) generating the
plasmid pGL2(GAL4)5. pCMV[3Gal was purchased from Clontech and pADVANTAGE was
purchased from Promega.
Luciferase assay: Medium including test compound was aspirated and 100 ~,I PBS
incl. 1 mM
Mg++ and Ca++ was added to each well. The luciferase assay was performed using
the Lu-
cLite kit according to the manufacturers instructions (Packard Instruments).
Light emission
was quantified by counting SPC mode on a Packard Instruments top-counter.
To.measure (3-
galactosidase activity 25 p1 'supernatant from each transfection lysate was
transferred to a
new microplate. ~i-galactosidase assays were performed in the microwell
plates, using a kit
from Promega and read in a microplate reader. The (3-galactosidase data were
used to nor-
malize. (transfection efficiency, cell growth etc.) the luciferase data.
Compounds: All compounds were dissolved in DMSO and diluted 1:1000 upon
addition to
the cells. Compounds were tested in quadruple in five concentrations ranging
form 0.01 to 30
,uM. Cells were treated with compound for 24 h followed by luciferase assay.
Each compound
was tested in three separate experiments. EC5o values were calculated via non-
linear regres-
sion. using GraphPad PRISM 3.02 (GraphPad Software, San Diego, CA).The results
were
expressed as means.
CA 02395298 2002-06-18
WO 01/55085 PCT/DKO1/00058
47
Table 1.
In vitro PPAR alpha and PPAR gamma activation of examples according to the
present in-
vention.
In vitro
activation
PPAR a PPAR y
Example EC5o, p.M % maxa ECSO, ~M % max
no
4 3.1 212 0.72 156
9 0.038 234 0.35 125
27 0.10 185 0.11 99
57 0.38 178 0.70 110
124 . 0.35 102 0.30 83
134 2.90 122 0.89 155
Compounds were tested in at least three separate experiments in five
concentrations ranging
from 0.01 to 30 wM. ECSO's were not calculated for compounds producing
transactivation lo-
wer than 25% at 30 p,M.. aFold activation relative to maximum activation
obtained with
Wy14643 (approx. 20 fold corresponded to 100%) and with brosiglitazone
(approx. 120 fold
corresponded to 100%).
PHARMACEUTICAL COMPOSITIONS
In another aspect, the present invention includes within its scope
pharmaceutical compositions
comprising, as an active ingredient, at least one of the compounds of the
general formula I or a
pharmaceutically acceptable salt thereof together with a pharmaceutically
acceptable carrier or
diluent.
The present compounds may also be administered in combination with one or more
further
pharmacologically active substances eg., selected from antiobesity agents,
antidiabetics, an-
tihypertensive agents, agents for the treatment and/or prevention of
complications resulting
from or associated with diabetes and agents for the treatment and/or
prevention of complica-
tions and disorders resulting from or associated with obesity.
CA 02395298 2002-06-18
WO 01/55085 PCT/DKO1/00058
48
Thus, in a further aspect of the invention the present compounds may be
administered in
combination with one or more antiobesity agents or appetite regulating agents.
Such agents may be selected from the group consisting of CART (cocaine
amphetamine
regulated transcript) agonists, NPY (neuropeptide Y) antagonists, MC4
(melanocortin 4)
agonists, orexin antagonists, TNF (tumor necrosis factor) agonists, CRF
(corticotropin releas-
ing factor) agonists, CRF BP (corticotropin releasing factor binding protein)
antagonists, uro-
cortin agonists, (33 agonists, MSH (melanocyte-stimulating hormone) agonists,
MCH
(melanocyte-concentrating hormone) antagonists, CCK (cholecystokinin)
agonists, serotonin
re-uptake inhibitors, serotonin and noradrenaline re-uptake inhibitors, mixed
serotonin and
noradrenergic compounds, 5HT (serotonin) agonists, bombesin agonists, galanin
antago-
nists, growth hormone, growth hormone releasing compounds, TRH (thyreotropin
releasing
hormone) agonists, UCP 2 or 3 (uncoupling protein 2 or 3) modulators, leptin
agonists, DA
agonists (bromocriptin, doprexin), lipase/amylase inhibitors, RXR (retinoid X
receptor)
modulators or TR [3 agonists.
In one embodiment of the invention the antiobesity agent is leptin.
In another embodiment the antiobesity agent is dexamphetamine or amphetamine.
In another embodiment the. antiobesity agent is fenfluramine or
dexfenfluramine.
In still another embodiment the antiobesity agent is sibutramine.
In a further embodiment the antiobesity agent is orlistat.
In another embodiment the antiobesity agent is mazindol or phentermine.
Suitable antidiabetics comprise insulin, GLP-1 (glucagons like peptide-1 )
derivatives such as
those disclosed in WO 98/08871 to Novo Nordisk A/S, which is incorporated
herein by refer-
ence as well as orally active hypoglycaemic agents.
CA 02395298 2002-06-18
WO 01/55085 PCT/DKO1/00058
49
The orally active hypoglycaemic agents preferably comprise sulphonylureas,
biguanides,
meglitinides, glucosidase inhibitors, glucagon antagonists such as those
disclosed in WO
99/01423 to Novo Nordisk A/S and Agouron Pharmaceuticals, Inc., GLP-1
agonists, potas-
sium channel openers such as those disclosed in WO 97/26265 and WO 99/03861 to
Novo
Nordisk A/S which are incorporated herein by reference, DPP-IV (dipeptidyl
peptidase-IV)
inhibitors, inhibitors of hepatic enzymes involved in stimulation of
gluconeogenesis andlor
glycogenolysis, glucose uptake modulators, compounds modifying the lipid
metabolism such
as antihyperlipidemic agents and antilipidemic agents as HMG CoA inhibitors
(statins), com-
pounds lowering food intake, RXR agonists and agents acting on the ATP-
dependent potas-
sium channel of the (3-cells.
In one embodiment of the invention the present compounds are administered in
combination
with insulin:
In a further embodiment the present compounds are administered in combination
with a sul-
phonylurea eg. tolbutamide, glibenclamide, glipizide or glicazide.
In another embodiment the present compounds are administered in combination
with a bi-
guanide eg. metformin.
2o
In yet another embodiment the present compounds are administered in.
combination with a
meglitinide eg. repaglinide or senaglinide.
In a further embodiment the present compounds are administered in combination
with an
a-glucosidase inhibitor eg. miglitol or acarbose.
In another embodiment the present compounds are administered in combination
with an
agent acting on the ATP-dependent potassium channel of the (3-cells eg.
tolbutamide, gliben-
clamide, glipizide, glicazide or repaglinide.
Furthermore, the present compounds may be administered in combination with
nateglinide.
CA 02395298 2002-06-18
WO 01/55085 PCT/DKO1/00058
In still another embodiment the present compounds are administered in
combination with an
antihyperlipidemic agent or antilipidemic agent eg. cholestyramine,
colestipol, clofibrate,
gemfibrozil, lovastatin, pravastatin, simvastatin, probucol or
dextrothyroxine.
5 In a further embodiment the present compounds are administered in
combination with more
than one of the above-mentioned compounds eg. In combination with a
sulphonylurea and
metformin, a sulphonylurea and acarbose, repaglinide and metformin, insulin
and a
sulphonylurea, insulin and metformin, insulin,. insulin and lovastatin, etc.
10 Furthermore, the present compounds may be administered in combination with
one or more
antihypertensive agents. Examples of antihypertensive agents are (3-blockers
such as alpre-
nolol, atenolol, timolol, pindolol, propranolol and metoprolol, ACE
(angiotensin converting en-
zyme) inhibitors such as benazepril, captopril, enalapril, fosinopril,
lisinopril, quinapril and
ramipril, calcium channel blockers such as nifedipine, felodipine,
nicardipine, isradipine, ni-
15 modipine, diltiazem and verapamil, and a-blockers such as doxazosin,
urapidil, prazosin and
terazosin. Further reference can be made to Remington: The Science and
Practice of Phar-
macy, 19t" Edition, Gennaro, Ed., Mack Publishing Co., Easton, PA, 1995.
It should be understood that any suitable combination of the compounds
according to the in-
20 vention with one or more. of the above-mentioned compounds and optionally
one or more~fur-
ther pharmacologically active substances are considered to be within the scope
of the pre-
sent invention.
Pharmaceutical compositions containing a compound of the present invention may
be prepared
25 by conventional techniques, e.g. as described in Remington: The Science and
Practise of
Pharmacy, 19t" Ed., 1995. The compositions may appear in conventional forms,
for example
capsules, tablets, aerosols, solutions, suspensions or topical applications.
Typical compositions include a compound of formula I or a pharmaceutically
acceptable acid
30 addition salt thereof, associated with a pharmaceutically acceptable
excipient which may be
a carrier or a diluent or be diluted by a carrier, or enclosed within a
carrier which can be in
the form of a capsule, sachet, paper or other container. In making the
compositions,
conventional techniques for the preparation of pharmaceutical compositions may
be used.
For example, the active compound will usually be mixed with a carrier, or
diluted by a carrier,
CA 02395298 2002-06-18
WO 01/55085 PCT/DKO1/00058
51
or enclosed within a carrier which may be in the form of a ampoule, capsule,
sachet, paper,
or other container. When the carrier serves as a diluent, it may be solid,
semi-solid, or liquid
material which acts as a vehicle, excipient, or medium for the active
compound. The active
compound can be adsorbed on a granular solid container for example in a
sachet. Some
examples of suitable carriers are water, salt solutions, alcohols,
polyethylene glycols,
polyhydroxyethoxylated castor oil, peanut oil, olive oil, gelatine, lactose,
terra alba, sucrose,
cyclodextrin, amylose, magnesium stearate, talc, gelatin, agar, pectin,
acacia, stearic acid or
lower alkyl ethers of cellulose, silicic acid, fatty acids, fatty acid amines,
fatty acid
monoglycerides and diglycerides, pentaerythritol fatty acid esters,
polyoxyethylene,
hydroxymethylcellulose and polyvinylpyrrolidone. Similarly, the carrier or
diluent may include
any sustained release material known in the art, such as glyceryl monostearate
or glyceryl
distearate, alone or mixed with a wax. The formulations may also include
wetting agents,
emulsifying and suspending agents, preserving agents, sweetening agents or
flavouring
agents. The formulations of the invention may be formulated so as to provide
quick;
sustained, or delayed release of the active ingredient after administration to
the patient by
employing procedures well known in the art.
The pharmaceutical compositions can be sterilized and mixed, if desired, with
auxiliary
agents, emulsifiers, salt for influencing osmotic pressure, buffers andlor
colouring sub-
stances and the like, which do not deleteriously react with the active
compounds.
The route of administration may be any route, which effectively transports.
the active com-
pound to the appropriate or desired site of action, such as oral, nasal,
pulmonary,. tra~sdermal
orp~ar~enteral e.g. rectal, depot, subcutaneous, intravenous, intraurethral,
intramuscular, in-
tranasal,.ophthalmic solution or an ointment, the oral route being preferred.
If a solid carrier is used for oral administration, the preparation may be
tabletted, placed in a
hard gelatin capsule in powder or pellet form or it can be in the form of a
troche or lozenge. If a
liquid carrier is used, the preparation may be in the form of a syrup,
emulsion, soft gelatin
capsule or sterile injectable liquid such as an aqueous or non-aqueous liquid
suspension or
solution.
For nasal administration, the preparation may contain a compound of formula I
dissolved or
suspended in a liquid carrier, in particular an aqueous carrier, for aerosol
application. The carrier
CA 02395298 2002-06-18
WO 01/55085 PCT/DKO1/00058
52
may contain additives such as solubilizing agents, e.g. propylene glycol,
surfactants, absorption
enhancers such as lecithin (phosphatidylcholine) or cyclodextrin, or
preservatives such as
parabenes.
For parenteral application, particularly suitable are injectable solutions or
suspensions, pref-
erably aqueous solutions with the active compound dissolved in
polyhydroxylated castor oil.
Tablets, dragees, or capsules having talc and/or a carbohydrate carrier or
binder or the like
are particularly suitable for oral application. Preferable carriers for
tablets, dragees, or cap-
sules. include lactose, corn starch, and/or potato starch. A syrup or elixir
can be used in
cases where a sweetened vehicle can be employed.
A typical tablet which may be prepared by conventional tabletting techniques
may contain:
Core:
Active compound (as free compound or salt thereof) 5 mg
Colloidal silicon dioxide (Aerosil) 1.5 mg
Cellulose, microcryst. (Avicel) 70 mg
Modified cellulose gum (Ac-Di-Sol) 7.5 mg
Magnesium stearate Ad.
Coating:
HPMC approx. 9 mg
*Mywacett 9-40 T approx. 0.9 mg
*Acylated monoglyceride used as plasticizer for film coating.
The compounds of the invention may be administered to a mammal, especially a
human in
need of such treatment, prevention, elimination, alleviation or amelioration
of diseases
related to the regulation of blood sugar.
Such mammals include also animals, both domestic animals, e.g. household pets,
and non-
domestic animals such as wildlife.
CA 02395298 2002-06-18
WO 01/55085 PCT/DKO1/00058
53
The compounds of the invention are effective over a wide dosage range. For
example, in the
treatment of adult humans, dosages from about 0.05 to about 100 mg, preferably
from about
0.1 to about 100 mg, per day may be used. A most preferable dosage is about
0.1 mg to
about 70 mg per day. In choosing a regimen for patients it may frequently be
necessary to
begin with a dosage of from about 2 to about 70 mg per day and when the
condition is under
control to reduce the dosage as low as from about 0.1 to about 10 mg per day.
The exact
dosage will depend upon the mode of administration, on the therapy desired,
form in which
administered, the subject to be treated and the body weight of the subject to
be treated, and
the preference and experience of the physician or veterinarian in charge.
Generally, the compounds of the present invention are dispensed in unit dosage
form
comprising from about 0.1 to about 100 mg of active ingredient together with a
pharmaceutically
acceptable carrier per unit dosage.
Usually, dosage forms suitable for oral, nasal, pulmonary or transdermal
administration
comprise from about 0.001 mg to about 100 mg, preferably from about 0.01 mg to
about 50-mg
of the compounds of formula I admixed with a pharmaceutically acceptable
carrier or diluent.
Any novel feature or combination of features described herein is considered
essential to this
invention.
EXAMPLES
The process for preparing compounds of formula I, and preparations containing
them, is
further illustrated in the following examples, which however, are not to
be,construed as
limiting.
The structures of the compounds are confirmed by either elemental analysis
(MA) nuclear
magnetic resonance (NMR) or mass spectrometry (MS). NMR shifts (s) are given
in parts per
million (ppm) and only selected peaks are given. Mp is melting point and is
given in °C. Col
umn chromatography was carried out using the technique described by W.C. Still
et al, J.
Org. Chem. 1978, 43, 2923-2925 on Merck silica gel 60 (Art 9385). Compounds
used as
starting materials are either known compounds or compounds which can readily
be prepared
by methods known per se.
CA 02395298 2002-06-18
WO 01/55085 PCT/DKO1/00058
54
Abbreviations:
THF: tetrahydrofuran
DIBAL-H: diisobutylaluminum
hydride
Na2S04: sodium sulfate
MgS04: magnesium sulfate
DMSO: dimethylsulfoxide
CDCI3: deuterated chloroform
DMF: N,N-dimethylformamide
HCI: hydrochloric acid
1o DME: 1,2-dimethoxyethane
min: minutes
h: hours
EXAMPLE 1
\ / ~ ~ \ ~o 0
o ~ ~ o
(E)-(S)-Ethyl 3-[4-(3-biphenyl-4-yl-but-2-enyloxy)-phenyl]-2-ethoxy-propionate
a)
Sodium (1.75 g, 73.4 mmol) was added to ethanol (45 ml) at 20°C and the
mixture stirred
until the metal had fully reacted. Triethyl phosphonoacetate (14.69 g, 73.4
mmol) was added,
the mixture stirred for 5 min., then 4-acetylbiphenyl (12.00. g, 61.1 mmol)
was added to the
stirred solution. The mixture was stirred at room temperature for 24h, the
resulting suspen-
sion filtered, and the filter-cake collected and recrystallised from ethanol
to give (E)-3-
biphenyl-4-yl-but-2-enoic acid ethyl ester as white crystals; 5.73 g (36%)
'H NMR (300 MHz, CDCI3) 8: 1.32 (3H, t), 2.62 (3H, d), 4.21 (2H, q), 6.2 (1H,
d), 7.31-7.65
(9H, m). MS: 267 (M+), 266(100%), 221, 194, 178.
3o Microanalysis Calculated % C: 81.00, H: 7Ø Found C: 80.86, H: 6.90.
CA 02395298 2002-06-18
WO 01/55085 PCT/DKO1/00058
b)
A 1 M solution of DIBAL-H in toluene (40 ml, 40 mmol) was added dropwise at -
70°C over 20
min. to a stirred solution of 3-biphenyl-4-yl-but-2-enoic acid ethyl ester
(2.66 g, 10Ø mmol) in
dry THF (100 ml) and the mixture stirred for 30 min. Methanol (2 ml) was
added, followed by
5 saturated aqueous Rochelle's salt (100 ml), and the resulting mixture
extracted with ethyl
acetate (200 ml), separated and the organic phase washed with brine, dried
(Na2S04),
evaporated and dried in vacuo yielding (E)-3-biphenyl-4-yl-but-2-en-1-of as
colorless crystals:
1.94 g (86%)
'H NMR (300 MHz, CDCI3) 8: 1.40. (1 H, br s), 2.12 (3H, d), 4.45 (2H, dd),
6.05 (1 H, dt), 7.35-
10 7.7 (9H, m). MS: 225 (M+), 224(100%), 209, 181,165.
Microanalysis Calculated % C: 86.00, H: 7.00. Found C: 85.67, H: 7.29
c)
Diethyl azodicarboxylate (0.346 ml, 2.2 mmol) was added at 0°C to a
stirred solution of
15 triphenyl- phosphine (0.656 g, 2.2 mmol) and (E)-3-biphenyl-4-yl-but-2-en-1-
of (0.270 g, 1.2
mmol) in dry THF (20 ml) and the mixture stirred for 5 min. A solution of (S)-
ethyl 2-ethoxjr-3-
(4-hydroxy-phenyl)-propionate (0.238 g, 1.0 mmol) in dry THF (10 ml) was
added, the mix-
ture allowed to warm to room temperature, and stirring continued for 48 h. The
resulting mix-.
ture was evaporated in vacuo and the residue purified by column chromatography
on silica
20 gel (20% ethyl acetate in n-heptane) to give (E)-(S)-ethyl 3-[4-(3-biphenyl-
4-yl-but-2-
enyloxy)-phenyl]-2-ethoxy-propionate as an oil; 0.288 g (65%).
'H NMR (300 MHz, CDCI3) b: 1.13-1.25 (6H, m), 2.13 (3H, d), 2.94 (2H, d), 3.29-
3.37 (1 H,
m), 3.54-3.61 (1 H, m), 3.97 (1 H, t), 4.1 (2H, q), 4.70 (2H, d), 6.11 (1 H,
dt), 6.86 (2H, d), 7.16
(2H, d), 7.25-7.63 (9H, m).
EXAMPLE 2
\ / / \ \ ~° off
-,
o \ / o
(E)-(S)-3-[4-(3-Biphenyl-4-yl-but-2-enyloxy)-phenyl]-2-ethoxy-propionic acid
CA 02395298 2002-06-18
WO 01/55085 PCT/DKO1/00058
56
Sodium hydroxide (1 M, 0.45 ml, 0.45 mmol) was added to a solution of (E)-(S)-
ethyl 3-[4-(3-
biphenyl-4-yl-but-2-enyloxy)-phenyl]-2-ethoxy-propionate (example 1 ) (0.100
g, 0.225 mmol)
in ethanol (20 ml) and the mixture stirred at 70°C for 2.5 h. After
cooling to room temperature
the resulting mixture was partitioned between water (50 ml) and ethyl acetate
and the aque-
ous phase collected. The aqueous phase was acidified with 1 N hydrochloric
acid (5 ml) and
extracted with ethyl acetate (100 ml), and the organic phase collected, washed
with brine,
dried (Na2S04) and evaporated to give (E)-(S)-3-[4-(3-biphenyl-4-yl-but-2-
enyloxy)-phenyl]-2-
ethoxy-propionic acid as a white solid; 0.014 g (15%).
'H NMR (300 MHz, CDCI3) 8: 1.19 (3H, t), 2.63 (3H, d), 2.93 (1 H, dd), 3.1 (1
H, dd), 3.4-3.65
(2H, m), 4.1 (2H, q), 4.72 (2H, d), 6.1 (1 H, dt), 6.9 (2H, d), 7.2 (2H, d),
7.35-7.60 (9H, m).
EXAMPLE 3
Br ~ ~ ~ ~ ~ ~o o-
o ~ ~ o
(E)-(S)-3-{4-[3-(4'-Bromo-biphenyl-4-yl)-but-2-enyloxy]-phenyl}-2-ethoxy-
propionic acid ethyl
ester
a)
(E)-3-(4'-Bromo-biphenyl-4-yl)-but-2-enoic acid ethyl ester was prepared from
4-(4-
bromophenyl)acetophenone (12.0 g, 0.044 mol), sodium (1.25 g, 0.052 mol) and
triethyl
phosphonoacetate (11.73 g, 0.052 mol) by a procedure analogous to that
described in ex-
ample 1 a yielding 11.97 g (80%).
'H NMR (300 MHz, CDCI3) 8: 1.32 (3H, t), 2.61 (3H, d),°4.23 (2H, q),
6.19 (1 H, d), 7.40-7.58
(8H, m).
b)
CA 02395298 2002-06-18
WO 01/55085 PCT/DKO1/00058
57
(E)-3-(4'-bromo-biphenyl-4-yl)-but-2-en-1-of was prepared from (E)-3-(4'-bromo-
biphenyl-4-
yl)-but-2-enoic acid ethyl ester (3.45 g, 10.0 mmol) and DIBAL-H (1 M in
toluene, 40 ml, 40
mmol) by a procedure analogous to that described in example 1 b, yielding
1.68. g (55%).
'H NMR (300MHz, CDCI3) 8: 2.14 (3H, d), 4.4 (2H, t), 6.05 (1H, dt), 7.45-7.55
(8H, m),
C)
The title compound was prepared from (E)-3-(4'-bromo-biphenyl-4-yl)-but-2-en-1-
of (0.364 g,
1.2 mmol), triphenylphosphine (0.328 g, 1.3 mmol), diethyl azodicarboxylate
(0.173 ml, 1.1
mmol) and (S)-ethyl 2-ethoxy-3-(4-hydroxy-phenyl)-propionate (0.238 g, 1.0
mmol) by a pro-
cedure analogous to that described in example 1 c, yielding 0.180 g (34%) of
(E)-(S)-3-{4-[3-
(4'-bromo-biphenyl-4-yl)-but-2-enyloxy]-phenyl}-2-ethoxy-propionic acid ethyl
ester.
'H NMR (300 MHz, CDCI3) b: 1.15-1.25 (6H, m), 2.15 (3H, d), 2.95 (2H, d) 3.29-
3.4 (1H, m),
3.5-3.65 (1 H, m), 3.96 (1 H, t), 4.15 (2H, q), 4.75 (2H, dd), 6.11 (1 H, dt),
6.85 (2H, d), 7.14
(2H, d), 7.4-7.55 (8H, m).
EXAMPLE 4
Br ~ ~ ~ ~ ~ '-O OH
O \ / O
(E)-(S)-3-{4-[3-(4'-Bromo-biphenyl-4-yl)-but-2-enyloxy]-phenyl}-2-ethoxy-
propionic acid
The title compound was prepared from (E)-(S)-3-{4-[3-(4'-bromo-biphenyl-4-yl)-
but-2-
enyloxy]-phenyl}-2-ethoxy-propionic acid ethyl ester (example 3) (0.150 g,
0.29 mmol) and
sodium hydroxide (1 M, 0.45 ml, 0.45 mmol) by a procedure analogous to that
described in
example 2 yielding 0.180 g (34%) of (E)-(S)-3-{4-[3-(4'-bromo-biphenyl-4-yl)-
but-2-enyloxy]-
phenyl}-2-ethoxy-propionic acid ethyl ester.
'H NMR (300 MHz, CDCI3) 8: 1.14 (3H, t), 2.13 (3H, d), 2.86-3.10 (2H, m), 3.37-
3.45 (1 H, m),
3.55-3.65 (1 H, m), 4.05 (2H, q), 4.70 (2H, dd), 6.12 (1 H, dt), 6.9 (2H, d),
7.18 (2H, d), 7.4-
7.60 (8H, m).
CA 02395298 2002-06-18
WO 01/55085 PCT/DKO1/00058
58
EXAMPLE 5
o~
i ~ ~ o~ o o~
0
(E)-(S)-2-Ethoxy-3-{4-[3-(4-phenoxy-phenyl)-but-2-enyloxy]-phenyl}-propionic
acid ethyl ester
The title compound was prepared from 4-phenoxyacetophenone (12.0 g, 0.056 mol)
by a se-
quence analogous to that described in example 3, yielding 0.190 g (41 %) of
(E)-(S)-2-ethoxy-
3-{4-[3-(4-phenoxy-phenyl)-but-2-enyloxy]-phenyl}-propionic acid ethyl ester.
'H NMR (300 MHz, CDCI3) s: 1.2 (6H, m), 2.12 (3H, s), 2.97 (2H, d), 3.30-3.42
(1H, m), 3.59-
3.70 (1 H, m), 3.98 (1 H, t), 4.15 (2H, q), 4.73 (2H, dd), 6.05 (1 H, dt),
6.85-7.45 (13H, m).
EXAMPLE 6
o~
~ ~ O- v O OH
O
(E)-(S)-2-Ethoxy-3-{4-[3-(4-phenoxy-phenyl)-but-2-enyloxy]-phenyl}-propionic
acid
The title compound was prepared from (E)-(S)-2-ethoxy-3-{4-[3-(4-phenoxy-
phenyl)-but-2-
enyloxy]-phenyl}-propionic acid ethyl ester (example 5) (0.170 g, 0.37 mmol)
and sodium hy-
droxide (1 M, 0.74 ml, 0.74 mmol) by a procedure analogous to that described
in example 2
yielding 0.136 g (85%) of (E)-(S)-2-ethoxy-3-{4-[3-(4-phenoxy-phenyl)-but-2-
enyloxy]-
phenyl}-propionic acid.
'H NMR (300 MHz, CDCI3) 8: 1.14 (3H, t), 2.13 (3H, d), 2.86-3.10 (2H, m), 3.38-
3.45 (1H, m),
3.55-3.65(1 H, m), 4.05 (2H, q), 4.70 (2H, dd), 6.12 (1 H, dt), 6.9 (2H, d),
7.18 (2H, d), 7.4-
7.60 (8H, m).
CA 02395298 2002-06-18
WO 01/55085 PCT/DKO1/00058
59
EXAMPLE 7
° ~ i ~ ~ \ ° \ / °
° ~° o~
(E)-(S)-2-Ethoxy-3-(4-{3-[4-(4-methoxy-phenoxy)-phenyl]-but-2-enyloxy}-phenyl)-
propionic
acid ethyl ester
The title compound was prepared from 4-(4-methoxyphenoxy)acetophenone (2.63 g,
0.01.1
mol) by a sequence analogous to that described in example 3 yielding 0.200 g
(41 %) of (E)-
(S)-2-ethoxy-3-(4-~3-[4-(4-methoxy-phenoxy)-phenyl]-but-2-enyloxy}-phenyl)-
propionic acid
ethyl ester.
'H NMR (300 MHz, CDCI3) 8: 1.15-1.23 (6H, m), 2.12 (3H, s), 2.97 (2H, d), 3.30-
3.40 (1H,
m), 3.57-3.65 (1 H, m), 3.80 (3H, s), 3.98 (1 H, t), 4.18 (2H, q), 4.63 (2H,
dd), 5.97-6.05 (1 H,
m), 6.85-6.96 (8H, m), 7.15 (2H, d), 7.35 (2H, d).
MS 490 (M~), 417, 359 (100%), 269.
EXAMPLE 8
I _
° ~ i ~ ~ \ ° \ / °
° ~O OH
(E)-(S)-2-Ethoxy-3-(4-{3-j4-(4-methoxy-phenoxy)-phenyl]-but-2-enyloxy}-phenyl)-
propionic
acid
CA 02395298 2002-06-18
WO 01/55085 PCT/DKO1/00058
The title compound was prepared from (E)-(S)-2-ethoxy-3-(4-~3-[4-(4-methoxy-
phenoxy)-
phenyl]-but-2-enyloxy}-phenyl)-propionic acid ethyl ester (example 7) (0.176
g, 0.36 mmol)
and sodium hydroxide (1 M, 0.74 ml, 0.74 mmol) by a procedure analogous to
that described
5 in example 2 yielding 0.140 g (84%) of (E)-(S)-2-ethoxy-3-(4-{3-[4-(4-
methoxy-phenoxy)-
phenyl]-but-2-enyloxy}-phenyl)-propionic acid.
'H NMR (300 MHz, CDCI3) 8: 1.15 (3H, t), 2.1 (3H, s), 2.9-3.1 (2H, m), 3.36-
3.43 (1 H, m),
3.55-3.64 (1 H, m), 3.78 (3H, s), 4.00 (1 H, dd), 4.70 (2H, dd), 6.0 (1 H,
dt), 6.8-6.9 (8H, m),
7.19 (2H, d), 7.35 (2H, d).
10 MS 462 (M+)(100%), 436, 359, 252.
EXAMPLE 9
(E)-(S)-2-Ethoxy-3-{4-[3-(9H-fluoren-2-yl)-but-2-enyloxy]-phenyl}-propionic
acid ethyl ester
The title compound was prepared from 2-acetylfluorene (12.0 g, 0.058 mmol) by
a sequence
analogous to that described in example 3 yielding 0.200. g (41 %) of (E)-(S)-2-
ethoxy-3-f4-[3
(9H-fluoren-2-yl)-but-2-enyloxy]-phenyl}-propionic acid ethyl ester.
'H NMR (300 MHz, CDCl3) 8: 1.16-1.22 (6H, m), 2.2 (3H, s), 2.96 (2H, d), 3.30-
3.40 (1 H, m),
3.51-3.65 (1 H, m), 3.9 (2H, s), 3.98 (1 H, t), 4.15 (2H, q), 4.75 (2H, d),
6.04-6.13 (1 H, m), 6.88
(2H, d), 7.17 (2H, d), 7.3-7.8 (7H, m).
MS 456 (M+), 410, 325 (100%), 238.
Microanalysis Calculated % C: 78.92, H: 7.06. Found C: 78.72, H: 7.30.
CA 02395298 2002-06-18
WO 01/55085 PCT/DKO1/00058
61
EXAMPLE 10
i i
0
Ho
0
(E)-(S)-2-Ethoxy-3-{4-[3-(9H-fluoren-2-yl)-but-2-enyloxyJ-phenyl}-propionic
acid
The title compound was prepared from (E)-(S)-2-ethoxy-3-{4-[3-(9H-fluoren-2-
yl)-but-2-
enyloxy]-phenyl}-propionic acid ethyl ester (example 9) (0.230 g, 0.504 mol)
and sodium hy-
droxide (1 M, 1.008 ml, 1.008 mmol) by a procedure analogous to that described
in example
2 yielding 0.140 g (84%) of (E)-(S)-2-ethoxy-3-{4-[3-(9H-fluoren-2-yl)-but-2-
enyloxy]-phenyl}-
propionic acid.
'H NMR (300 MHz, CDCI3) 8: 1.20 (3H, t), 2.18 (3H, s), 2.9-3.15 (2H, m), 3.4-
3.6 (2H, m),
3.87 (2H, s), 4.05 (1 H, dd), 4.75 (2H, d), 6.11 (1 H, dt), 6.88 (2H, d), 7.17
(2H, d), 7.3-7.8 (7H,
m).
EXAMPLE 11
O
O
l ~ i
0
CA 02395298 2002-06-18
WO 01/55085 PCT/DKO1/00058
62
(~-(S)-3-{4-[3-(3,4-Dimethoxy-phenyl)-but-2-enyloxy]-phenyl}-2-ethoxy-
propionic acid ethyl
ester
The title compound was prepared from 3,4-dimethoxyacetophenone (10.00 g, 0.055
mol) by
a sequence analogous to that described in example 3 yielding 0.160 g (31 %) of
(E7-(S)-3-{4
[3-(3,4-dimethoxy-phenyl)-but-2-enyloxy]-phenyl}-2-ethoxy-propionic acid ethyl
ester.
'H NMR (300 MHz, CDCI3) 8: 1.1-1.19 (6H, m), 2.17 (3H, s), 2.98 (2H, d), 3.37-
3.45 (1H, m),
3.58-3.65 (1 H, m), 3.9 (6H, ds), 4.02 (1 H, t), 4.15 (2H, q), 4.7 (2H, d),
6.0 (1 H, dt), 6.81-6.86
(3H, m), 7.0 (2H, d), 7.15 (2H, d).
MS 428 (M+), 382, 355,297 (100%), 207.
EXAMPLE 12
I
0
I \
~o
~ / O
~O OH
O
(~-(S)-3-{4-[3-(3,4-Dimethoxy-phenyl)-but-2-enyloxy]-phenyl}-2-ethoxy-
propionic acid
The title compound was prepared from (~-(S)-3-{4-[3-(3,4-dimethoxy-phenyl)-but-
2-enyloxy]-
phenyl}-2-ethoxy-propionic acid ethyl ester (example 11 ) (0.150 g, 0.350
mmol) and sodium
hydroxide (1 M, 1.05 ml, 1.05 mmol) by a procedure analogous to that described
in example 2
yielding 0.120 g (86%) of (~-(S)-3-{4-[3-(3,4-dimethoxy-phenyl)-but-2-enyloxy]-
phenyl}-2-
ethoxy-propionic acid.
'H NMR (300 MHz, CDCI3) 8: 1.15 (3H, t), 2.15 (3H, s), 2.9-3.15 (2H, m), 3.40-
3.48 (1H, m),
3.56-3.63 (1 H, m), 3.9 (6H, ds), 4.08 (1 H, dd), 475 (2H, d), 6.01 (1 H, dt),
6.80-6.91 (3H, m),
7.0 (2H, d), 7.15 (2H, d).
CA 02395298 2002-06-18
WO 01/55085 PCT/DKO1/00058
63
EXAMPLE 13
F
F F
O
F ~ ~ ~ \ ~ o~/
F ~° o
(E)-(S)-3-{4-[3-(3,5-Bis-trifluoromethyl-phenyl)-but-2-enyloxy]-phenyl}-2-
ethoxy-propionic acid
ethyl ester
The title compound was prepared from 3,5-bis(trifluoromethyl)acetophenone
(5.12 g, 0.02
mol) by a sequence analogous to that described in example 3 yielding 0.370 g
(73%) of (~-
(S)-3-{4-[3-(3,5-bis-trifluoromethyl-phenyl)-but-2-enyloxy]-phenyl)-2-ethoxy-
propionic acid
ethyl ester.
'H NMR (300 MHz, CDCI3) S: 1.1-1.25 (6H, m), 2.20 (3H, s), 2.97 (2H, d), 3.3-
3.4 (1H, m),
3.62-3.7 (1 H, m), 4.0 (1 H, t), 4.15 (2H, q), 4.75 (2H, d), 6.2 (1 H, dt),
6.85 (2H, d), 7.2 (2H, d), .
7.78 (1 H, br s), 7.87 ( 2H, br s).
MS 504 (M+), 458, 431 (100%), 373 , 267, 192
EXAMPLE 14
F
F F
O
F \ ~ ~ \ / OH
F ~° O
(E~-(S)-3-{4-[3-(3,5-Bis-trifluoromethyl-phenyl)-but-2-enyloxy]-phenyl}-2-
ethoxy-propionic acid
CA 02395298 2002-06-18
WO 01/55085 PCT/DKO1/00058
64
The title compound was prepared from (E)-(S)-3-{4-[3-(3,5-bis-trifluoromethyl-
phenyl)-but-2-
enyloxy]-phenyl}-2-ethoxy-propionic acid ethyl ester (example 13) (0.200 g,
0.396 mmol) and
sodium hydroxide (1 M, 0.792 ml, 0.792 mmol) by a procedure analogous to that
described in
example 2 yielding 0.150 g (79%) of (E)-(S)-3-{4-[3-(3,5-bis-trifluoromethyl-
phenyl)-but-2-
enyloxy]-phenyl}-2-ethoxy-propionic acid.
'H NMR (300. MHz, CDCI3) 8: 1.12 (3H, t), 2.18 (3H, s), 2.9 (1 H, dd), 3.1 (1
H, dd), 3.34-3.42
(1 H, m), 3.5-3.65 (1 H, m), 4.0 (1 H, dd), 4.7 (2H, d), 6.11 (1 H, dt), 6.83
(2H, d), 7.19 (2H, d)
7.72 (1 H, br s), 7.83 (2H, br s).
EXAMPLE 15
~ / o
~o o~
(E)-(S)-3-[4-(3-Biphenyl-4-yl-allyloxy)-phenyl]-2-ethoxy-propionic acid ethyl
ester
The title compound was prepared from 3-biphenyl-4-yl-acrylic acid ethyl ester
(2.5 g, 0.01
mol) by a sequence analogous to that described in example 3b-c yielding 0.370
g (73%) of
(E)-(S)-3-[4-(3-biphenyl-4-yl-allyloxy)-phenyl]-2-ethoxy-propionic acid ethyl
ester.
'H NMR (200 MHz, CDCI3) 8: 1.1-1.25 (6H, m), 2.97 (2H, d), 3.3-3.4 (1H, m),
3.52-3.7 (1H,
m), 4.0 (1 H, t), 4.15 (2H, q), 4.75 (2H, dd), 6.35-6.5 (1 H, dt), 6.75 (1 H,
d), 6.87 (2H, d), 7.15
(2H, d), 7.4-7.65 (9H, m).
CA 02395298 2002-06-18
WO 01/55085 PCT/DKO1/00058
EXAMPLE 16
o \ / o
/ \ /-o off
/ \
5 (E7-(S)-3-[4-(3-Biphenyl-4-yl-allyloxy)-phenyl]-2-ethoxy-propionic acid
The title compound was prepared from (E)-(S)-3-[4-(3-biphenyl-4-yl-allyloxy)-
phenyl)-2-
ethoxy-propionic acid ethyl ester (example 15) (0.200 g, 0.464 mmol) and
sodium hydroxide
(1 M, 0.928 ml, 0.928 mmol) by a procedure analogous to that described in
example 2 yield-
10 ing 0.043 g (23%) of (E'7-(S)-3-[4-(3-biphenyl-4-yl-allyloxy)-phenyl]-2-
ethoxy-propionic acid.
H NMR (300 MHz, CDCI3) 8: 1.15 (3H, t), 2.9 (1 H, dd), 3.12 (1 H, dd) 3.45-
3.55 (2H, m),
3.84-3.96 (2H, m), 4.1 (1 H, dd), 4.7 (2H, d), 6.35-6.5 (1 H, dt), 6.78 (1 H,
d), 6.88 (2H, d), 7.15
(2H, d) 7.4-7.6 (9H, m).
EXAMPLE 17
(E7-(S)-2-Ethoxy-3-[4-(3-naphthalen-2-yl-but-2-enyloxy)-phenyl]-propionic acid
ethyl ester
The title compound was prepared from 2-acetonaphthone (10.0 g, 0.06. mol) by a
sequence
analogous to that described in example 3 yielding 0.190 g (38%) of (E~-(S)-2-
ethoxy-3-[4-(3-
naphthalen-2-yl-but-2-enyloxy)-phenyl]-propionic acid ethyl ester.
CA 02395298 2002-06-18
WO 01/55085 PCT/DKO1/00058
66
'H NMR (200 MHz, CDC13) 8: 1.1-1.2 (6H, m), 2.20 (3H, s), 2.95 (2H, d), 3.3-
3.4 (1H, m),
3.52-3.65 (1 H, m), 3.95 (1 H, t), 4.15 (2H, q), 4.76 (2H, d), 6.2 (1 H, t),
6.85 (2H, d), 7.15 (2H,
d), 7.35-7.42 (2H, m), 7.6 (1 H, dd), 7.75-7.85 (4H, m).
EXAMPLE 18
(E7-(S)-2-Ethoxy-3-[4-(3-naphthalen-2-yl-but-2-enyloxy)-phenyl]-propionic acid
The title compound was prepared from (E7-(S)-2-ethoxy-3-[4-(3-naphthalen-2-yl-
but-2-
enyloxy)-phenyl]-propionic acid ethyl ester (example 17) (0.165 g, 0.394 mmol)
and sodium
hydroxide (1 M, 0.789 ml, 0.789 mmol) by a procedure analogous to that
described in exam-
ple 2 yielding 0.030 g (19%) of (E7-(S)-2-ethoxy-3-[4-(3-naphthalen-2-yl-but-2-
enyloxy)-,
phenyl]-propionic acid.
'H NMR (400 MHz, CDCI3) 8: 1.13 (3H, t), 2.18 (3H, s), 2.95 (1 H, dd), 3.05 (1
H, dd), 3.3-3.45
(1 H, m), 3.65-3.63 (1 H, m), 3.95 (1 H, dd), 4.72 (2H, d), 6.15 (1 H, t),
6.84 (2H, d), 7.14 (2H,
d), 7.35-7.45 (2H, m), 7.6 (1 H, d), 7.7-7.8 (4H, m).
EXAMPLE 19
o ~ ~ o-~
~0 0
N
(E7-(S)-2-Ethoxy-3-[4-(3-pyridin-2-yl-but-2-enyloxy)-phenyl]-propionic acid
ethyl ester
CA 02395298 2002-06-18
WO 01/55085 PCT/DKO1/00058
67
The title compound was prepared from 2-acetylpyridine (9.6 g, 0.08 mol) by a
sequence
analogous to that described in example 3. yielding 0.230 g (23%) of (~-(S)-2-
ethoxy-3-[4-(3-
pyridin-2-yl-but-2-enyloxy)-phenyl]-propionic acid ethyl ester.
'H NMR (400 MHz, CDCI3) 8: 1.1-2.5 (6H, m), 2.21 (3H, s), 2.97 (2H, d), 3.3-
3.4 (1H, m),
3.58-3.64 (1 H, m), 3.97 (1 H, t), 4.15 (2H, q), 4.78 (2H, d), 6.65 (1 H, t),
6.85 (2H, d), 7.05-
7.15 (3H, m), 7.42 (2H, d), 7.6 (1 H, dd), 8,52 (1 H, d).
EXAMPLE 20
o ~ ~ off
~--0 0
N
(~-(S)-2-Ethoxy-3-[4-(3-pyridin-2-yl-but-2-enyloxy)-phenyl]-propionic acid
The title compound was prepared from (~-(S)-2-ethoxy-3-[4-(3-pyridin-2-yl-but-
2-enyloxy)-
phenyl]-propionic acid ethyl ester (example 19) (0.220 g, 0.595 mmol) and
sodium hydroxide
(1 M, 1.19 ml, 1.19 mmol) by a procedure analogous to that described in
example 2 yielding
0.200 g (98%) of (E~-(S)-2-ethoxy-3-[4-(3-pyridin-2-yl-but-2-enyloxy)-phenyl]-
propionic acid.
'H NMR (300 MHz, CDCI3) 8: 1.2 (3H, t), 2.1 (3H, s), 2.7-2.85 (1 H, m), 3.0-
3:25 (2H, m), 3.5-
3.6 (1 H, m), 3.8-3.92 (1 H, m), 4.6 (2H, d), 6.5 (1 H, t), 6.75 (2H, d), 7.1-
7.2 (3H, m), 7.35 (1 H,
d), 7.6 (1 H, t), 8,5 (1 H, d).
CA 02395298 2002-06-18
WO 01/55085 PCT/DKO1/00058
68
EXAMPLE 21
o ~ o
i I Ii
i
y
0
i
0
~o
(E)-(S)-3-~4-[3-(3,5-Bis-benzyloxy-phenyl)-but-2-enyloxy]-phenyl}-2-ethoxy-
propionic acid
ethyl ester
The title compound was prepared from 3,5-dibenzyloxyacetophenone (6.64 g, 0.02
mol) by a
sequence analogous to that described. in example. 3 yielding 0.460 g (53%) of
(E7-(S)-3-{4-[3
(3,5-bis-benzyloxy-phenyl)-but-2-enyloxy]-phenyl}-2-ethoxy-propionic acid
ethyl ester.
'H NMR (300 MHz, CDCI3) 8: 1.1-1.21 (6H, m), 2.14 (3H, s), 2.95 (2H, d) 3.28-
3.41 (1H, m),
3.51-3.65 (1 H, m), 3.94 (1 H, t), 4.12 (2H, q), 4.7 (2H, d), 5.05 (4H, s),
6.05 (1 H, t), 6.53-6.57
(1 H, m), 6.67 (2H, d), 6.85 (2H, d), 7.12 (2H, d), 7.3-7.45 (10H, m).
EXAMPLE 22
o ~ o
I
i
y
0
1
i
0
OH
CA 02395298 2002-06-18
WO 01/55085 PCT/DKO1/00058
69
(~-(S)-3-{4-[3-(3,5-Bis-benzyloxy-phenyl)-but-2-enyloxy]-phenyl}-2-ethoxy-
propionic acid
The title compound was prepared from (E7-(S)-3-{4-[3-(3,5-bis-benzyloxy-
phenyl)-but-2-
enyloxy]-phenyl}-2-ethoxy-propionic acid ethyl ester (example 21 ) (0.430 g,
0.741 mmol) and
sodium hydroxide (1 M, 1.5 ml, 1.5 mmol) by a procedure analogous to that
described in ex-
ample 2 yielding 0.300 g (73%) of (E'7-(S)-3-{4-[3-(3,5-bis-benzyloxy-phenyl)-
but-2-enyloxy]-
phenyl}-2-ethoxy-propionic acid.
H NMR (300 MHz, CDCI3) 8: 1.15 (3H, t), 2.1 (3H, s), 2.95 (1 H, dd), 3.05 (1
H, dd), 3.36-3.44
(1 H, m), 3.57-3.65 (1 H, m), 4.05 (1 H, dd), 4.68 (2H, d), 5.05 (4H, s), 6.05
(1 H, t), 6.52 (1 H,
m), 6.65 (2H, d), 6.85 (2H, d), 7.15 (2H, d), 7.3-7.45 (10H, m).
EXAMPLE 23
O~
(~-(S)-2-Ethoxy-3-[4-(3-naphthalen-2-yl-allyloxy)-phenyl]-propionic acid ethyl
ester
a)
Triethyl phosphonoacetate (8.9 g, 40.0 mmol) was added at 0°C over a
period of 10 min. to a
stirred suspension of sodium hydride (60% in oil, 1.44 g, 36.0 mmol) in dry
THF (145 mL).
After stirring at 0°C for 15 min. a solution of 2-naphthaldehyde (3.12
g, 20.0 mmol) in dry
THF (15 mL) was added, the mixture slowly warmed to room temperature, and
stirring con-
tinued for 16h. The reaction mixture was quenched with water (100 mL) and
acidified to pH 6
with 1 N hydrochloric acid. Additional water (200 mL) was added, the organic
phase sepa-
rated, and the aqueous phase further extracted with ethyl acetate (300 mL).
The combined
organic phases were washed with water (200 mL x 3), dried (MgS04), filtered
and concen-
trated in vacuo to give 6.5 g of crude (~-3-naphthalen-2-yl-acrylic acid ethyl
ester.
CA 02395298 2002-06-18
WO 01/55085 PCT/DKO1/00058
b)
Crude (E'-3-naphthalen-2-yl-acrylic acid ethyl ester (4.5 g, 20.0 mmol) was
reduced by a
procedure analogous to that described in example 1 b. The product was purified
by flash col-
5 umn chromatography to give 3.1 g (86%) of (E'-3-naphthalen-2-yl-prop-2-en-1-
ol.
c)
Under an atmosphere of nitrogen, (~-3-naphthalen-2-yl-prop-2-en-1-of (190 mg,
0.8 mmol),
tributylphosphine (323 mg, 1.6 mmol) and (S)-2-ethoxy-3-(4-hydroxy-phenyl)-
propionic acid
10 ethyl ester (184 mg, 1.0 mmol) were successively dissolved in dry benzene
(20 mL). Solid
1,1'-(azodicarbonyl) dipiperidine (403 mg, 1.6 mmol) was added at 0°C
with stirring. After 10
min. the reaction was warmed to room temperature and the stirring continued
for 1 h. The
reaction mixture was concentrated in vacuo and the product purified by flash
column chroma-
tography, eluting with heptane/ethyl acetate (3:2), to give 180 mg (55%) of
the title com-
15 pound.
~H NMR (CDCI3, 300 MHz) 8: 1.15 (t, 3H), 1,22 (t, 3H), 2.95 (d, 2H), 3.28-3.40
(m, 1H), 3.55-
3.65 (m, 1 H), 3.96 (t, 1 H), 4.15 (q, 2H), 4.72 (dd, 2H), 6.53 (dt, 1 H),
6.83-6.93 (m, 3H), 7.18
(d, 2H), 7.40-7.50 (m, 2H), 7.13 (dd, 1 H), 7.72-7.85 (m, 4H).
EXAMPLE 24
(~-(S)-2-Ethoxy-3-[4-(3-naphthalen-2-yl-allyloxy)-phenyl]-propionic acid
(E~-(S)-2-Ethoxy-3-[4-(3-naphthalen-2-yl-allyloxy)-phenyl]-propionic acid
ethyl ester (example
23) (170 mg, 0.42 mmol) was dissolved in ethanol (20 mL) at 35°C and
sodium hydroxide
(1 N, 2.1 mL, 2.1 mmol) added. The mixture was stirred at 35°C for 1 h,
the ethanol evapo-
CA 02395298 2002-06-18
WO 01/55085 PCT/DKO1/00058
71
rated in vacuo and the mixture acidified to pH 1 with 1 N hydrochloric acid.
The product was
isolated by extraction with ethyl acetate (30 mL x 2). The combined organic
phases were
dried (MgS04), filtered and evaporated to give 155 mg (98%) of the title
compound as crys-
tals.
'H NMR (CDC13, 300 MHz) 8: 1.18 (t, 3H), 2.90-3.12 (m, 2H), 3.35-3.48 (m, 1H),
3.55-3.68
(m, 1 H), 4.03 (q, 1 H), 4.70 (dd, 2H), 6.52 (dt, 1 H), 6.80-6.95 (m, 3H),
7.18 (d, 2H), 7.40-7.48
(m, 2H), 7.60 (dd, 1 H), 7.70-7.80 (m, 4H).
EXAMPLE 25
\ I \ I . H
I
H
O \ O O~
O
(E~-(S)-2-Ethoxy-3-{4-(3-(3-phenoxy-phenyl)-allyloxy]-phenyl}-propionic
acid,ethyl ester
The title compound was prepared from 3-phenoxybenzaldehyde (4.0 g, 20.0 mmol)
by a se-
quence analogous to that described in example 23.
'H NMR (CDCI3, 300 MHz) 8: 1.15 (t, 3H), 1,22 (t, 3H), 2.95 (d, 2H), 3.30-3.40
(m, 1H), 3.55-
3.68 (m, 1 H), 3.98 (t, 1 H), 4.15 (q, 2H), 4.72 (dd, 2H), 6.38 (dt, 1 H),
6.67 (d, 1 H), 6.83-6.93
(m, 3H), 6.97-7.20 (m, 7H), 7.22-7.38 (m, 3H).
CA 02395298 2002-06-18
WO 01/55085 PCT/DKO1/00058
72
EXAMPLE 26
\ ~ \ ~ H
O
H
O \ O O
O
H
(E7-(S)-2-Ethoxy-3-{4-[3-(3-phenoxy-phenyl)-allyloxy]-phenyl}-propionic acid
(E7-(S)-2-Ethoxy-3-{4-[3-(3-phenoxy-phenyl)-allyloxy]-phenyl}-propionic acid
ethyl ester (ex-
ample 25) (150 mg, 0.34 mmol) was dissolved in ethanol (7 mL) and sodium
hydroxide. (1 N,
4.4 mL, 4.4 mmol) added. The mixture was heated slightly to obtain a clear
solution and then
stirred at room temperature for 1.5 h. The ethanol was evaporated in vacuo and
the mixture
acidified to pH 1 with 1 N hydrochloric acid. The product was isolated by
extraction with ethyl
acetate (40 mL x 2). The combined organic phases were dried (MgSOø), filtered
and evapo-
rated to give 130 mg (91 %) of the title compound as an oil.
' H NMR (CDCI3, 300 MHz) 8: 1.18 (t, 3H), 2.95 (dd, 1 H), 3.08 (dd, 1 H), 3.38-
3.50 (m, 1 H),
7 5 3.55-3-65 (m, 1 H), 4.05 (q, 1 H), 4.65 (dd, 1 H), 6.35 (dt, 1 H), 6.66
(d, 1 H), 6.85-6.92 (m, 3H),
6.98-7.20 (m,. 7H), 7.25-7.40 (m, 3H).
EXAMPLE 27
(S)-3-[4-(2-Benzofuran-3-yl-allyloxy)-phenyl]-2-ethoxy-propionic acid ethyl
ester
CA 02395298 2002-06-18
WO 01/55085 PCT/DKO1/00058
73
The title compound was prepared from benzo[b]furan-2-carboxaldehyde (9.8 g,
0.07 mol) by
a sequence analogous to that described in. example 23.
'H NMR (CDCI3, 300 MHz) 8: 1.15 (t, 3H), 1.22 (t, 3H), 2.95 (d, 2H), 3.30-3.42
(m, 1H), 3.55
3.65 (m, 1 H), 3.98 (t, 1 H), 4.15 (q, 2H), 4.73 (d, 2H), 6.65-6.70 (m, 3H),
6.88 (d, 2H), 7.15 (d,
2H), 7.20-7-30 (m, 2H), 7.45 (d, 1 H), 7.53. (d, 1 H).
EXAMPLE 28
OH
O~
(S)-3-[4-(2-Benzofuran-3-yl-allyloxy)-phenyl]-2-ethoxy-propionic acid
The title compound was prepared from (S)-3-[4-(2-benzofuran-3-yl-allyloxy)-
phenyl]-2-
ethoxy-propionic acid ethyl ester (example 27) (127 mg, 0.3 mmol) by a
procedure analo-
gous to that described in example 26.
'H NMR (CDCI3, 300 MHz) 8: 1.15 (t, 3H), 3.30 (dd, 1 H), 3.08 (dd, 1 H), 2.38-
3.50 (m, 1 H),
3.55-3.65 (m, 1 H), 4.05 (q, 1 H), 4.72 (d, 2H), 6.55-6.68 (m, 3H), 6.90 (d, 1
H), 7-13-7.30 (m,
5H), 7.42 (d, 1 H), 7.50 (d, 1 H).
CA 02395298 2002-06-18
WO 01/55085 PCT/DKO1/00058
74
EXAMPLE 29
i
0
0
o
0
(E)-(S)-3-{4-[3-(4-Benzyloxy-phenyl)-allyloxy]-phenyl}-2-ethoxy-propionic acid
ethyl ester
The title compound was prepared from 4-benzyloxybenzaldehyde (21.2 g, 0.1 mol)
by a se-
quence analogous to that described in example 23. The title compound was
purified on -
HPLC, using ethyl acetate/heptane (20:80) as eluent.
'H NMR (CDCI3, 300 MHz) 8: 1.15 (t, 3H), 1.22 (t, 3H), 2.95 (d, 2H), 3.35 (m,
1H), 3.6 (m,
1 H), 3.98 (t, 1 H), 4.15 (q, 2H), 4.65 (dd, 2H), 5.05 (s, 2H), 2.28 (dt, 1
H), 6.65 (d, 1 H), 6.85 (d,
2H), 6.93 (d, 2H), 7.15 (d! 2H), 7.30-7.48 (m, 7H).
EXAMPLE 30
(E)-(S)-3-{4-[3-(4-Benzyloxy-phenyl)-allyloxy]-phenyl}-2-ethoxy-propionic acid
CA 02395298 2002-06-18
WO 01/55085 PCT/DKO1/00058
The title compound was prepared from (~-(S)-3-~4-[3-(4-Benzyloxy-phenyl)-
allyloxy]-
phenyl}-2-ethoxy-propionic acid ethyl ester (example 29) (80 mg, 0.17 mmol) by
a procedure
analogous to that described in example 26.
5 'H NMR (CDCI3, 300 MHz): 1.18 (t, 3H), 2.95 (dd, 1 H), 3.12 (dd, 1 H), 3.45-
3.60 (m, 2H), 4.15
(dd, 1 H), 4.65 (dd, 2H), 5.06 (s, 2H), 6.25 (dt, 1 H), 6.65 (d, 1 H), 6.90
(d, 2H), 6.93 (d, 2H),
7.15 (d, 2H), 7.30-7.45 (m, 7H).
10 EXAMPLE 31
0
(~-(S)-3-[4-(3-Benzo[1,3]dioxol-5-yl-allyloxy)-phenyl]-2-ethoxy-propionic acid
ethyl ester
The title compound was prepared from piperonal (3.0 g, 20 mmol) by a sequence
analogous
to that described in example 23. The title compound was purified on HPLC,
using ethyl ace-
tate/heptane (10:90) as eluent.
'H NMR (CDCI3, 300 MHz) 8: 1.15 (t, 3H), 1.22 (t, 3H), 2.96 (d, 2H), 3.30-3.42
(m, 1H), 3.55-
3.65 (m, 1 H), 3.97 (t, 1 H), 4.15 (q, 2H), 4.63 (dd, 2H), 5.96 (s, 2H), 6.25
(dt, 1 H), 6.63 (d,
1 H), 6,75 (d, 1 H), 6.80-6.90 (m, 3H), 6.95 (d, 1 H), 7.15 (d, 2H).
CA 02395298 2002-06-18
WO 01/55085 PCT/DKO1/00058
76
EXAMPLE 32
OH
(E7-(S)-3-[4-(3-Benzo[1,3]dioxol-5-yl-allyloxy)-phenyl]-2-ethoxy-propionic
acid
The title compound was prepared from (E'7-(S)-3-[4-(3-benzo[1,3]dioxol-5-yl-
allyloxy)-phenyl]-
2-ethoxy-propionic acid ethyl ester (example 31) (100 mg, 0.25 mmol) by a
procedure analo-
gous to that described in example 26.
'H NMR (CDCI3, 300 MHz): 1.18 (t, 3Hj, 2.95 (dd, 1 H), 3.08 (dd, 1 H), 3.38-
3.50 (m, 1 H),
3.55- 3.68 (m, 1 H), 4.05 (dd, 1 H), 4.65 (dd, 2H), 5.95 (s, 2H), 6.25 (dt, 1
H), 6.63 (d, 1 H), 6.75
(d, 1 H), 6.83 (dd, 1 H), 6.88 (d, 2H), 6.95 (d, 1 H), 7.17 (d, 2H).
EXAMPLE 33
0
(E)-(S)-3-~4-[3-(4-Allyloxy-phenyl)-allyloxy]-phenyl}-2-ethoxy-propionic acid
ethyl ester
CA 02395298 2002-06-18
WO 01/55085 PCT/DKO1/00058
77
The title compound was prepared from 4-allyloxybenzaldehyde (3.24 g, 20 mmol)
by a se-
quence analogous to that described in example 23. The title compound was
purified on
HPLC, using ethyl acetate/heptane (10:90) as eluent.
'H NMR (CDCI3, 300 MHz) 8: 1.18 (t, 3H), 1.22 (t, 3H), 2.95 (d, 2H), 3.30-3.42
(m, 1 H), 3.55-
3.68 (m, 1 H), 3.98 (t, 1 H), 4.17 (q, 2H), 4.53 (d, 2H), 4.65 (dd, 2H), 5.29
(dd, 1 H), 5.40 (dd,
1 H), 5.97-6.13 (m, 1 H), 6.28 (dt, 1 H), 6.65 (d, 1 H), 6.88 (d, 4H), 7.15
(d, 2H), 7.35 (d, 2H).
EXAMPLE 34
0
H
(~-(S)-3-{4-[3-(4-Allyloxy-phenyl)-allyloxy]-phenyl}-2-ethoxy-propionic acid
The title compound was prepared from (~-(S)-3-{4-[3-(4-allyloxy-phenyl)-
allyloxy]-phenyl}-2-
ethoxy-propionic acid ethyl ester (example 33) (40 mg, 0.1 mmol) by a
procedure analogous
to that described in example 26.
'H NMR (CDCI3, 300 MHz): 1.18 (t, 3H), 2.95 (dd, 1 H), 3.10 (dd, 1 H), 3.39-
3.50 (m, 1 H),
3.53-3.65 (m, 1 H), 4.05 (dd, 1 H), 4.53 (d, 2H), 4.65 (d, 2H), 5.29 (dd, 1
H), 5.40 (dd, 1 H),
5.98-6.14 (m, 1 H), 6.28 (dt, 1 H), 6.65 (d, 1 H), 6.85-6.95 (m, 4H), 7.15 (d,
2H), 7.35 (d, 2H).
CA 02395298 2002-06-18
WO 01/55085 PCT/DKO1/00058
78
EXAMPLE 35
(E'7-(S)-3-[4-(3-Benzofuran-7-yl-allyloxy)-phenyl]-2-ethoxy-propionic acid
ethyl ester
The title compound was prepared from benzofuran-7-carboxaldehyde (1.46 g, 10
mmol) by a
sequence analogous to that described in example 23.
'H NMR (CDCI3, 300 MHz) 8: 1.15 (t, 3H), 1.22 (t, 3H), 2.95 (d, 2H), 3.30-
3.42, (m, 1H), 3.55
3.65 (m, 1 H), 3.98 (t, 1 H), 4.15 (q, 2H), 4.75 (dd, 2H), 6,79 (d, 1 H), 6.87-
7.00 (m, 4H), 7.13
7.30 (m, 4H), 7.50 (dd, 1 H), 7.65 (d, 1 H).
EXAMPLE 36
O ~ ~O
off
0
(E7-(S)-3-[4-(3-Benzofuran-7-yl-allyloxy)-phenyl]-2-ethoxy-propionic acid
The title compound was prepared from (E'7-(S)-3-[4-(3-benzofuran-7-yl-
allyloxy)-phenyl]-2-
ethoxy-propionic acid ethyl ester (example 35) (100 mg, 0.25 mmol) by a
procedure analo-
gous to that described in example 26.
CA 02395298 2002-06-18
WO 01/55085 PCT/DKO1/00058
79
' H NMR (CDC13, 300 MHz) 8: 1.15 (t, 3H), 2.95 (dd, 1 H), 3.08 (dd, 1 H), 3.35-
3.48 (m, 1 H)
3.55-3.68 (m, 1 H), 4.03 (dd, 1 H), 4.75 (dd, 2H), 6.78 (d, 1 H), 6.90-7.00
(m, 4H), 7.13-7.32
(m, 4H), 7.50 (dd, 1 H), 7.65 (d, 1 H), 10.1 (bs, 1 H).
EXAMPLE 37
i
0
~-o
o ~ ~o
~ i o
0
(S)-3-[4-(3-Benzo[1,3]dioxol-4-yl-allyloxy)-phenyl]-2-ethoxy-propionic acid
ethyl ester
The title compound was prepared from 2,3-methylenedioxybenzaldehyde (1.5. g,
10 mmol) by
a sequence analogous to that described in example 23.
'H NMR (CDCI3,.300 MHz) 8: 1.18 (t, 3H), 1.22 (t, 3H), 2.95 (d, 2H), 3.30-3.42
(m, 1H), 3.55-
3.65 (m, 1 H), 3.97 (t, 1 H), 4.15 (q, 2H), 4.65 (d, 2H), 6.00 (s, 2H), 6.55-
6.92 (m, 7H), 7.15 (d,
2H).
EXAMPLE 38
(S)-3-[4-(3-Benzo[1,3]dioxol-4-yl-allyloxy)-phenyl]-2-ethoxy-propionic acid
CA 02395298 2002-06-18
WO 01/55085 PCT/DKO1/00058
The title compound was prepared from (S)-3-[4-(3-benzo[1,3]dioxol-4-yl-
allyloxy)-phenyl]-2-
ethoxy-propionic acid ethyl ester (example 37) (100 mg, 0.24 mmol) by a
procedure analo-
gous to that described in example 26.
5 'H NMR (CDCI3, 300 MHz) 8: 1.17 (t, 3H), 2.95 (dd, 1 H), 3.05 (dd, 1 H),
3.35-3.48 (m, 1 H),
3.55-3.68 (m, 1 H), 4.03 (dd, 1 H), 4.65 (d, 2H), 6.00 (s, 2H), 6.55-6.95 (m,
7H), 7.19 (d, 2H).
EXAMPLE 39
O w
O
O
(E7-(S)-2-Ethoxy-3-(4-[3-(9H-fluoren-2-yl)-allyloxy]-phenyl)-propionic acid
ethyl ester
The title compound was prepared from fluorene-2-carboxaldehyde (9.7 g, 50
mmol) by a se-
quence analogous to that described in example 23.
'H NMR (CDCI3, 300 MHz) 8: 1.18 (t, 3H), 1.22 (t, 3H), 2.97 (d, 2H), 3.32-3.42
(m, 1H), 3.55-
3.67 (m, 1 H), 3.90 (s, 2H), 3.98 (t, 1 H), 4.16 (q, 2H), 4.70 (dd, 2H), 6.45
(dt, 1 H), 6.80 (d,
1 H), 6.90 (d, 2H), 7.1 (d, 2H), 7.24-7.46 (m, 3H), 7.55 (d, 1 H), 7.62 (s, 1
H), 7.72-7.80 (m,
2H).
CA 02395298 2002-06-18
WO 01/55085 PCT/DKO1/00058
81
EXAMPLE 40
(E~-(S)-2-Ethoxy-3-(4-(3-(9H-fluoren-2-yl)-allyloxy]-phenyl)-propionic acid
The title compound was prepared from (E)-(S)-2-ethoxy-3-(4-[3-(9H-fluoren-2-
yl)-allyloxy]-
phenyl)-propionic acid ethyl ester (example 39) (275 mg, 0.6 mmol) by a
procedure analo-
gous to that described in example 26.
'H NMR (CDCI3, 300 MHz) 8: 1.20 (t, 3H), 3.46 (dd, 1 H), 3.12 (dd, 1 H), 3.43-
3.65 (m, 2H),
3.90 (s, 2H), 4.05 (dd, 1 H), 4.70 (dd, 2H), 6.46 (dt, 1 H), 6.80 (d, 1 H),
6.92 (d, 2H), 7.17 (d,
2H), 7.23-7.46 (m, 3H), 7.53 (d, 1 H), 7.60 (s, 1 H), 7.70-7.80 (m, 2H).
EXAMPLE 41
I~
i
N
O
I~ o
O
(S)-2-Ethoxy-3-[4-(3-quinolin-2-yl-allyloxy)-phenyl]-propionic acid ethyl
ester
The title compound was prepared from 2-quinoline-carboxaldehyde (5.12 g, 32.5
mmol) by a
sequence analogous to that described in example 23.
CA 02395298 2002-06-18
WO 01/55085 PCT/DKO1/00058
82
'H NMR (CDC13, 300 MHz) 8: 1.18 (t, 3H), 1.22 (t, 3H), 2.98 (d, 2H), 3.32-3.42
(m, 1H), 3.55-
3.66 (m, 1 H), 3.98 (t, 1 H), 4.17 (q, 2H), 4.80 (d, 2H), 6.92 (d, 2H), 7.02
(m, 2H), 7.18 (d, 2H),
7.47-7.60 (m, 2H), 7.70 (dt, 1 H), 7.78 (d, 1 H), 8.05 (d, 1 H), 8.13 (d, 1
H).
EXAMPLE 42
(S)-2-Ethoxy-3-[4-(3-quinolin-2-yl-allyloxy)-phenyl]-propionic acid
(S)-2-Ethoxy-3-[4-(3-quinolin-2-yl-allyloxy)-phenyl]-propionic acid ethyl
ester (example 41 ) .
(150 mg, 0.37 mmol) was dissolved in ethanol (2 mL) and sodium hydroxide (1 N,
2.0 mL, 2.0
mmol) added. The mixture was stirred at room temperature for 16 h. The
reaction mixture
was concentrated in vacuo; added 2-propanol (2 mL) and diethyl ether (2 mL).
The title com-
pound was isolated by filtration.
'H. NMR (CDCI~/MeOD, 300 MHz) 8: 1.12 (t, 3H), 2.83 (dd, 1 H), 3.02 (dd, 1 H),
3.32 (m, 1 H),
3.56 (dd, 1 H), 3.84 (dd, 1 H), 4.85 (d, 2H), 6.90-7.10 (m, 4H), 7.25 (m, 2H),
7.5-7.6 (m, 1 H),
7.68-7.75 (m, 2H), 7.85 (d, 1 H), 8.03 (d, 1 H), 8.23 (d, 1 H).
CA 02395298 2002-06-18
WO 01/55085 PCT/DKO1/00058
83
EXAMPLE 43
i
0
0
0 '
i
0
0
(~-(S)-3-~4-[3-(3,5-Bis-benzyloxy-phenyl)-allyloxy]-phenyl}-2-ethoxy-propionic
acid ethyl es-
ter
The title compound was prepared from 3,5-dibenzyloxybenzaldehyde (3.1 g, 9.7
mmol) by a
sequence analogous to that described' in example 23.
'H NMR (CDCI3, 300 MHz) 8: 1.16 (t, 3H), 1.22 (t, 3H), 2.96 (d, 2H), 3.30-3.42
(m, 1 H), 3.54-
3.65 (m, 1 H), 3.98 (t, 1 H), 4.17 (q, 2H), 4.65 (d, 2H), 5.02 (s, 4H), 6.38
(dt, 1 H), 6.55 (s, 1 H),
6.58-6.70 (m, 3H), 6.88 (d, 2H), 7.15 (d, 2H), 7.30-7.45 (m, 10H).
CA 02395298 2002-06-18
WO 01/55085 PCT/DKO1/00058
84
EXAMPLE 44
i
0
(E~-(S)-3-~4-[3-(3,5-Bis-benzyloxy-phenyl)-allyloxy]-phenyl}-2-ethoxy-
propionic acid
The title compound was prepared from (E)-(S)-3-{4-[3-(3,5-bis-benzyloxy-
phenyl)-allyloxy]-
phenyl}-2-ethoxy-propionic acid ethyl ester (example 43) (587 mg, 1.1 mmol) by
a procedure
analogous to that described in example 26..
' H NMR (CDCI3, 300 MHz) b: 1.15 (t, 3H), 2.95 (dd, 1 H), 3.08 (dd, 1 H), 3.38-
3.48 (m, 1 H),
3.54-3.65 (m 1 H), 4.03 (dd, 1 H), 4.65 (d, 2H), 5.03 (s, 4H), 6.35. (dt, 1
H), 6.54 (t, 1 H), 6:60-
6.70 (m, 3H), 6.88 (d, 2H); 7.16 (d, 2H), 7.30-7:45 (m, 10 H).
EXAMPLE 45
0
CA 02395298 2002-06-18
WO 01/55085 PCT/DKO1/00058
(E7-(S)-3-{4-[3-(3,5-Dimethoxy-phenyl)-allyloxy]-phenyl}-2-ethoxy-propionic
acid ethyl ester
The title compound was prepared from 3,5 dimethoxybenzaldehyde (5.5 g, 33.1
mmol) by a
sequence analogous to that described in example 23.
5 'H NMR (CDCI3, 300 MHz) 8: 1.18 (t, 3H), 1.22 (t, 3H), 2.95 (d, 2H), 3.30-
3.40 (m, 1H), 3.53-
3.65 (m, 1 H), 3.78 (s, 6H), 3.97 (t, 1 H), 4.15 (q, 2H), 4.65 (dd, 1 H), 6.33-
6.43 (m, 2H), 6.55
(d, 2H), 6.88 (d, 2H), 7.15 (d, 2H).
10 EXAMPLE 46
OH
(E'7-(S)-3-{4-[3-(3,5-Dimethoxy-phenyl)-allyloxy]-phenyl)-2-ethoxy-propionic
acid
The title compound was prepared from (E7-(S)-3-~4-[3-(3,5-dimethoxy-phenyl)-
allyloxy]-
phenyl}-2-ethoxy-propionic acid ethyl ester (example 45) (300 mg, 0.7 mmol) by
a procedure
analogous to that described in example 26.
' H NMR (CDCI3, 300 MHz) 8: 1.18 (t, 3H), 2.95 (dd, 1 H), 3.07 (dd, 1 H), 3.37-
3.48 (m, 1 H),
3.55-3.67 (m, 1 H), 3.80 (s, 6H), 4.05 (dd, 1 H), 4.67 (d, 2H), 6.33-6.45 (m,
1 H), 6.55 (d, 2H),
6.65 (d, 1 H), 6.88 (d, 2H), 7.18 (d, 2H).
CA 02395298 2002-06-18
WO 01/55085 PCT/DKO1/00058
86
EXAMPLE 47
(E7-(S)-2-Ethoxy-3-[4-(3-phenanthren-9-yl-allyloxy)-phenyl]-propionic acid,
ethyl ester
The title compound was prepared from phenanthrene-9-carboxaldehyde (4.1 g,
20.0 mmol)
by a sequence analogous to that described in example 23.
'H NMR (CDCI3, 300 MHz) 8: 1.16 (t, 3H), 1.22 (t, 3H), 2.95 (d, 2H), 3.30-3.42
(m, 1H), 3.53-
3.65 (m,1 H), 3.98 (t, 1 H), 4.15 (q, 2H), 4.47 (d, 2H), 6.47 (dt, 1 H), 6.74
(d, 2H), 7.08 (d, 2H),
7.38 (d, 1 H), 7.53-7.70 (m, 4H), 7.82 (s, 1 H),7.85 (d, 1 H), 8.15 (d, 1 H),
8.65 (d, 1 H), 8.72 (d,
1 H).
EXAMPLE 48
0
(E'7-(S)-2-Ethoxy-3-{4-[3-(2-methoxy-naphthalen-1-yl)-allyloxy]-phenyl}-
propionic acid ethyl
ester
CA 02395298 2002-06-18
WO 01/55085 PCT/DKO1/00058
87
The title compound was prepared from 2-methoxy-1-naphthaldehyde (4.1 g, 22.1
mmol) by a
sequence analogous to that described in example 23.
'H NMR (CDCI3, 300 MHz) 8: 1.16 (t, 3H), 1.22 (t, 3H), 2.97 (d, 2H), 3.30-3.42
(m, 1H), 3.55-
3.65 (m, 1 H), 3.93 (s, 3H), 3.97 (t, 1 H), 4.15 (q, 2H), 4.85 (d, 2H), 6.48
(dt, 1 H), 6.95 (d, 2H),
7.10-7.35 (m, 5H), 7.45 (dt, 1 H), 7.75-7.78 (m, 2H), 8.12 (d, 1 H).
EXAMPLE 49
~ o~
i
o ~ ~o
I , off
0
(E7-(S)-2-Ethoxy-3-{4-[3-(2-methoxy-naphthalen-1-yl)-allyloxy]-phenyl}-
propionic acid
The title compound was prepared from (E7-(S)-2-ethoxy-3-{4-[3-(2-methoxy-
naphthalen-1-yl)-
allyloxy]-phenyl}-propionic acid ethyl ester (example 48) (327 mg, 0.75 mmol)
by a procedure
analogous to that described in example 26.
'H NMR (CDCI3, 300 MHz) 8: 1.16 (t, 3H), 2.95 (dd, 1 H), 3.08 (dd, 1 H), 3.35-
3.48 (m, 1 H),
3.53-3.65 (m, 1 H), 3.93 (s, 3H), 4.05 (dd, 1 H), 4.82 (dd, 2H), 6.49 (dt, 1
H), 6.95 (d, 2H), 7.13
(d, 1 H), 7.20 (d, 2H), 7.23-7.35 (m, 2H), 7.44 (dt, 1 H), 7.74 (d, 2H), 8.12
(d, 1 H).
FXA~API F 5n
Et0 OEt
\O
Br
CA 02395298 2002-06-18
WO 01/55085 PCT/DKO1/00058
88
(~-(S)-Ethyl 3-~4-[3-(4-Bromophenyl)-but-2-enyloxy]-phenyl}-2-ethoxy-
propionate
a)
Sodium (5.52 g, 0.24 mol) was added to ethanol (250 ml) at 20°C and the
mixture stirred until
the metal had fully reacted. Triethyl phosphonoacetate (62.72 g, 0.28 mol) was
added,. the
mixture stirred for 20 min, then a solution of 4-bromoacetophenone (39.81 g,
0.20 mol) in
ethanol (250 ml) was added and the reaction mixture heated to 80°C
under reflux for 17h.
The solution was cooled, the ethanol evaporated and the resulting orange
residue partitioned
between 1 N HCI (200 ml) and ethyl acetate (200 ml). The aqueous layer was
collected and
further extracted with ethyl acetate (2 x 200 ml). The organic layers were
combined, washed
with brine, dried (MgS04) and evaporated to an orange gum. This was
purified.by column
chromatography on silica gel (3% diethyl ether in n-heptane eluent) to give,
the product, (~-
ethyl 3-(4-bromophenyl)-but-2-enoate, as a colourless oil; 44.08 g (82%)
'H NMR (300 MHz, CDCI3) 8: 1.31 (3H, t), 2.54 (3H, s), 4.21 (2H, q), 6.11 (1
H, s), .7.34 (2H,
dm), 7.48 (2H, dm). MS: 268/270 (M+), 240/242, 239/241, 196/198, 116, 115
(100%).
Microanalysis Calculated % C: 53.55, H: 4.87. Found % C: 53.86, H: 4.90.
b)
A 1 M solution of DIBAL-H in toluene (42 ml, 42 mmol) was added dropwise,.at -
70°C over
min, to a stirred solution of (~-ethyl 3-(4-bromophenyl)-but-2-enoate (4.55 g,
16.92 mmol)
in dry THF (100 ml), and the mixture stirred for 1 h. Methanol (5 ml) was
carefully added fol-
lowed by 1 N HCI (300 ml) and the resulting mixture extracted with ethyl
acetate (3 x 200 ml).
The combined organic extracts were washed with brine, dried (NaZS04), and
evaporated to
25 give the crude product as an off white solid, which was purified by
recrystallisation from hot
1:4 ether/n-heptane (250 ml) to give the product (E)-3-(4-bromophenyl)-but-2-
en-1-of as col-
ourless needles: 3.10 g (81 %)
Mpt. 58-59.5°C. ' H NMR (300 MHz, CDCI3) s: 1.41 (1 H, br s), 2.05 (3H,
d), 4.36 (2H, d), 5.96
(1H, tq), 7.27 (2H, dm), 7.44 (2H, dm). MS: 226/228 (M+), 211/213, 193/195,
183/185, 147
30 (100%), 132, 129, 115. Microanalysis Calculated % C: 52.89, H: 4.88, Br:
35.18. Found C:
53.24, H: 4.86, Br: 35.08.
CA 02395298 2002-06-18
WO 01/55085 PCT/DKO1/00058
89
C)
Azodicarboxylic dipiperidide (0.756 g, 3.0 mmol) was added at 0-5°C to
a stirred solution of
tributylphosphine (0.74 ml, 0.61 g, 3.0 mmol), (S)-ethyl 2-ethoxy-3-(4-
hydroxyphenyl)-
propionate (0.500 g, 2.10 mmol) and (E)-3-(4-bromophenyl)-but-2-en-1-of (0.454
g, 2.0
mmol) in dry benzene (20 ml), the mixture warmed to room temperature, and
stirred for 2.5
days. The resulting mixture was diluted with water and ethyl acetate (50 ml
each), the aque-
ous layer collected and further extracted with ethyl acetate (50 ml). The
organic layers were
combined, washed with brine, dried (MgS04) and evaporated. The crude product
was then
purified by column chromatography on silica gel (20% ethyl acetate in n-
heptane eluent) to
give (E)-(S)-ethyl 3-{4-[3-(4-bromophenyl)-but-2-enyloxy]-phenyl-2-ethoxy-
propionate as an
oil; 0.780 g (87%).
'H NMR (300 MHz, CDCI3) 8: 1.17 (3H, t), 1.22 (3H, t), 2.10 (3H, s), 2.96 (2H,
d), 3.30-3.45
(1 H, m), 3.55-3.70 (1 H, m), 3.98 (1 H, t), 4.17 (2H, q), 4.70 (2H, d), 6.04
(1 H, t), 6.86 (2H, m),
7.16 (2H, m), 7.29 (2H, m), 7.44 (2H, m).
EXAMPLE 51
Et0 OH
O ~ \ \O
Br
(E)-(S)-3-{4-[3-(4-Bromophenyl)-but-2-enyloxy]-phenyl}-2-ethoxy-propionic acid
Sodium hydroxide (1 M, 1.10 ml, 1.10 mmol) was added to a solution of (E)-(S)-
ethyl 3-{4-[3-
(4-bromophenyl)-but-2-enyloxy]-phenyl-2-ethoxy-propionate (example 50) (0.245
g, 0.548
mmol) in ethanol (10 ml) and the mixture stirred at room temperature for 18 h.
The resulting
mixture was partitioned between water (50 ml) and ethyl acetate (50 ml) and
the aqueous
layer acidified to pH1 by addition of 1 N HCI. The aqueous layer was separated
and further
extracted with ethyl acetate (2 x 50 ml). The combined organic layers were
washed with
CA 02395298 2002-06-18
WO 01/55085 PCT/DKO1/00058
brine, dried (MgS04), evaporated and vacuum dried at 40°C for 18 h, to
give (~-(S)-3-{4-[3-
(4-bromophenyl)-but-2-enyloxy]-phenyl}-2-ethoxy-propionic acid as a colourless
gum which
contained 0.1 molar equivalents of ethyl acetate; 0.22 g (96%).
'H NMR (300 MHz, CDCI3) 8: 1.18 (3H, t), 1.26 (ethyl acetate impurity, 0.3H,
t), 2.04 (ethyl
5 acetate impurity, 0.2H, s), 2.11 (3H, s), 2.96 (1 H, dd), 3.08 (1 H, dd),
3.40-3.55 (1 H, m), 3.55-
3-68 (1 H, m), 4.06 (1 H, dd), 4.15 (ethyl acetate impurity, 0.2H, q), 4.70
(2H, d), 6.04 (1 H, t),
6.88 (2H, m), 7.17 (2H, m), 7.29 (2H, m), 7.44 (2H, m), carboxylic acid proton
not observed.
LCMS: 441/443 (M+Na), 209/211 (100%).
EXAMPLE 52
ci ~ o
OEt
OEt
~/ a
(~-(S)-Ethyl3-{4-[3-(4'-Chloro-biphenyl-4-yl)-but-2-enyloxy]-phenyl}-2-ethoxy-
propionate
a)
Tetrakis(triphenylphoshine)palladium(0) (0.26 g, 0.22 mmol, 4 mol%) was added,
under ni-
trogen, to a stirred solution of (~-ethyl 3-(4-bromophenyl)-but-2-enoate (1.5
g, 5.57 mmol)
{prepared as detailed in example 50 a} in DME (70 ml), and the resulting
orange coloured
solution stirred at room temperature for 10 min. Aqueous 2M sodium carbonate.
(16.7 ml,
33.4 mmol) was then added, the mixture stirred for 10 min, then 4-chlorophenyl
boronic acid
(1.3 g, 8.36 mmol) was added, and the reaction mixture heated to 80°C
for 18 h, under re-
flux. The reaction mixture was diluted with 1 N HCI (100. ml) and the products
extracted into
ethyl acetate (2 x 100 ml). The combined organic extracts were washed with
brine, dried
(MgS04), and evaporated to give the crude product, which was purified by
column chroma-
tography on silica gel (20% ethyl acetate in n-heptane eluent) to give the
product, (E~-ethyl-3-
(4'-chloro-biphenyl-4-yl)-but-2-enoate as a colourless solid; 1.17 g (70%).
'H NMR (300 MHz, CDCI3) 8: 1.33 (3H, t), 2.60 (3H, s), 4.23 (2H, q), 6.20 (1H,
s), 7.41 (2H,
m), 7.52 (2H, m). MS: 300/302 (100%, M+), 271/273, 255/257, 228/230, 165.
CA 02395298 2002-06-18
WO 01/55085 PCT/DKO1/00058
91
b)
A 1 M solution of DIBAL-H in toluene (10 ml, 10 mmol) was added dropwise, at -
70°C over
min, to a stirred solution of (~-ethyl-3-(4'-chloro-biphenyl-4-yl)-but-2-
enoate (1.0 g,. 3.32
5 mmol) in dry THF (25 ml), and the mixture warmed to room temperature over 4
h. Methanol
(1 ml) was carefully added, followed by 1 N HCI (50 ml) and the resulting
mixture extracted
with ethyl acetate (2 x 50 ml). The combined organic extracts were washed with
brine, dried
(MgS04), and evaporated to give the product, (~-3-(4'-chloro-biphenyl-4-yl)-
but-2-en-1-of as
a colourless solid: 0.86 g (100%).
10 Mpt. 137-142°C. ' H NMR (300 MHz, CDCI3) 8: 1.79 (1 H, br s), 2.11
(3H, d), 4.40 (2H, d),
6.05 (1 H, tq), 7.41 (2H, dm), 7.45-7.60 (6H, m).
c)
Azodicarboxylic dipiperidide (0.731 g, 2.9 mmol) was added at 0-5°C to
a stirred solution of
tributylphosphine (0.71 ml, 0.58 g, 2.9 mmol), (S)-ethyl 2-ethoxy-3-(4-
hydroxyphenyl)-
propionate (0.483 g, 2.03 mmol) and (~-3-(4'-chloro-biphenyl-4-yl)-but-2-en-1-
of (0.500 g,
1.93 mmol) in dry benzene (15 ml), the mixture warmed to room temperature, and
stirred for
3 h. The resulting mixture was diluted with water and ethyl acetate (30 ml
each), the aqueous
layer collected and further extracted with ethyl acetate (30 ml). The organic
layers were com-
bined, washed with brine, dried (MgS04) and evaporated. The crude product was
then puri-
fied by column chromatography on silica (20% ethyl acetate in n-heptane
eluent) to give (~-
(S)-ethyl 3-~4-[3-(4'-chloro-biphenyl-4-yl)-but-2-enyloxy]-phenyl}-2-ethoxy-
propionate as a
gum; 0.69 g (75%).
'H NMR (300 MHz, CDCI3) 8: 1.17 (3H, t), 1.23 (3H, t), 2.16 (3H, s), 2.96 (2H,
d), 3.30-3.45
(1 H, m), 3.55-3.68 (1 H, m), 3.98 (1 H, t), 4.17 (2H, q), 4.74 (2H, d), 6.12
(1 H, t), 6.88 (2H, m),
7.18 (2H, m), 7.40 (2H, m), 7.45-7.60 (6H, m).
EXAMPLE 53
CI ~ O
i i I off
oEt
CA 02395298 2002-06-18
WO 01/55085 PCT/DKO1/00058
92
(~-(S)-3-{4-[3-(4'-Chloro-biphenyl-4-yl)-but-2-enyloxy]-phenyl}-2-ethoxy-
propionic acid
Sodium hydroxide (1 M, 2.3 ml, 2.3 mmol) was added to a solution of (E~-(S)-
ethyl 3-{4-[3-(4'-
chloro-biphenyl-4-yl)-but-2-enyloxy]-phenyl}-2-ethoxy-propionate (example 52)
(0.600 g, 1.25
mmol) in ethanol (10 ml) and the mixture stirred at room temperature for 18h,
then heated to
80°C for 2 h. The resulting mixture was partitioned between water (50
ml) and ethyl acetate
(50 ml) and the aqueous layer acidified to pH1 by addition of 1 N HCI. The
aqueous layer was
separated and further extracted with ethyl acetate (2 x 50 ml). The combined
organic layers
were washed with brine, dried (MgS04), evaporated, and the product and vacuum
dried at
40°C for 72 h, to give (~-(S)-3-~4-[3-(4'-chloro-biphenyl-4-yl)-but-2-
enyloxy]-phenyl}-2-
ethoxy-propionic acid as a colourless solid; 0.53 g (94%).
'H NMR (300 MHz, CDCI3) s: 1.18 (3H, t), 2.16 (3H, s), 2.97 (1 H, dd), 3.08 (1
H, dd), 3.40-.
3.53 (1 H, m), 3.55-3-68 (1 H, m), 4.07 (1 H, dd), 4.74 (2H, d), 6.11 (1 H,
t), 6.90 ,(2H, m), 7.17
015 (2H, m), 7.39 (2H, m), 7.45-7.60 (6H, m), carboxylic acid proton not
observed.
EXAMPLE 54
0
/ / ~ ~ ,'' ~OEt
OMe ~ ~ / O ~ OEt
(~-(S)-Ethyl 2-Ethoxy-3-~4-[3-(5'-isopropyl-2'-methoxy-biphenyl-4-yl)-but-2-
enyloxy]-phenyl}-
propionate
a)
Tetrakis(triphenylphoshine)palladium(0) (0.20 g, 0.18 mmol, 4 mol%) was added,
under ni-
trogen, to a stirred solution of (~-3-(4-bromophenyl)-but-2-en-1-of (1.0 g,
4.40 mmol) {pre-
pared as detailed in example 50 b} in DME (55 ml), and the resulting orange
coloured solu-
tion stirred at room temperature for 10 min. Aqueous 2M sodium carbonate (13.2
ml, 26.4
CA 02395298 2002-06-18
WO 01/55085 PCT/DKO1/00058
93
mmol) was then added, the mixture stirred for 10 min, then 5-isopropyl-2-
methoxyphenylboronic acid (1.28 g, 6.60 mmol) was added, and the reaction
mixture heated
to 80°C for 18 h, under reflux. The reaction mixture was diluted with 1
N HCI (100 ml) and the
products extracted into ethyl acetate (2 x 100 ml). The combined organic
extracts were
washed with brine, dried (MgS04), and evaporated to give the crude product,
which was puri-
fied by column chromatography on silica gel (1 % methanol in dichloromethane
eluent) to give
the product, 3-(5'-isopropyl-2'-methoxy-biphenyl-4-yl)-but-2-en-1-of as a
colourless oil; 1.15 g
(88%).
H NMR (300 MHz, CDCI3) b: 1.26 (6H, d), 1.33 (1 H, br t), 2.12 (3H, s), 2.91
(1 H, septet),
3.80 (3H, s), 4.39 (2H, br t), 6.04 (1 H, 7), 6.92 (1 H, d), 7.15-7.20 (2H,
m), 7.42-7.55 (4H, m).
MS: 296 (100%, M+), 281, 263, 253.
b)
Azodicarboxylic dipiperidide (0.756 g, 3.0 mmol) was added at 0-5°C to
a stirred solution of
tributylphosphine (0.74 ml, 0.61 g, 3.0 mmol), (S)-ethyl 2-ethoxy-3-(4-
hydroxyphenyl)-
propionate (0.50 g, 2.10 mmol) and 3-(5'-isopropyl-2'-methoxy-biphenyl-4-yl)-
but-2-en-1-of
(0.593 g, 2.0 mmol) in dry benzene (15 ml), the mixture warmed to room
temperature, and
stirred for 4 h. The resulting mixture was diluted with water (100 ml) and
ethyl acetate (50
ml), the aqueous layer collected and further extracted with ethyl acetate
(50;m1). The organic
layers were combined, washed with brine, dried (MgS04) and evaporated. The
crude product
was then purified by column chromatography on silica (10% ethyl acetate in n-
heptane elu-
ent) to give (~-(S)-ethyl 2-ethoxy-3-{4-[3-(5'-isopropyl-2'-methoxy-biphenyl-4-
yl)-but-2-
enyloxy]-phenyl}-propionate as a colourless oil; 0.67 g (65%).
'H NMR (300 MHz, CDCI3) 8: 1.17 (3H, t), 1.22 (3H, t), 1.26 (6H, d), 2.16 (3H,
s), 2.91 (1 H,
septet), 2.96 (2H, d), 3.30-3.45 (1 H, m), 3.54-3.66 (1 H, m), 3.79 (3H, s),
3.98 (1 H, t), 4.17
(2H, q), 4.74 (2H, d), 6.10 (1 H, t), 6.84-6.95 (3H, m), 7.12-7.20 (4H, m),
7.42-7.57 (4H, m).
CA 02395298 2002-06-18
WO 01/55085 PCT/DKO1/00058
94
EXAMPLE 55
0
/ / ~ ~ ~OH
OEt
OMe ~ ~ / O
(E7-(S)-2-Ethoxy-3-{4-[3-(5'-isopropyl-2'-methoxy-biphenyl-4-yl)-but-2-
enyloxy]-phenyl}-
propionic acid
The title compound was prepared from (E~-(S)-ethyl 2-ethoxy-3-{4-[3-(5'-
isopropyl-2'-
methoxy-biphenyl-4-yl)-but-2-enyloxy]-phenyl}-propionate (example 54) (0.50 g,
0.968 mmol)
and sodium hydroxide (1 M, 1.93 ml, 1.93 mmol) by a procedure analogous to
that described
in example 51, yielding (E7-(S)-2-ethoxy-3-{4-[3-(5'-isopropyl-2'-methoxy-
biphenyl-4-yl)-but-2- .
enyloxy]-phenyl}-propionic acid as a colourless gum, which contained 0.44 mol
equivalents
of ethyl acetate; 0.48 g (94%).
'H NMR (300 MHz, CDCI3) 8: 1.18 (3H, t), 1.26 (6H, d), 1.26 (ethyl acetate
impurity, 1.32H,
t), 2.04 (ethyl acetate impurity, 0.88H, s), 2.16 (3H, s), 2.82-3.02 (2H-,
m),:3.08 (1 H, dd), 3.40-
3.52 (1 H, m), 3.52-3.68 (1 H,. m), 3.79 (3H, s), 4.06 (1 H, dd), 4.15 (ethyl
acetate impurity,
0.88H, q), 4.75 (2H, d), 6.09 (1 H, t), 6.88-6.95 (3H, m), 7.12-7.20 (4H, m),
7.42-7.57 (4H, m),
carboxylic acid proton not observed.
EXAMPLE 56
OEt
CA 02395298 2002-06-18
WO 01/55085 PCT/DKO1/00058
(E7-(S)-Ethyl 2-Ethoxy-3-{4-[3-(5'-chloro-2'-methoxy-biphenyl-4-yl)-but-2-
enyloxy]-phenyl}-
propionate
a)
5 (E~-Ethyl 3-(5'-chloro-2'-methoxy-biphenyl-4-yl)-but-2-enoate (1.07 g, 91%
yield) was pre-
pared from 5-chloro-2-methoxyphenylboronic acid (1.0 g, 5.36 mmol) and (E7-
ethyl 3-(4-
bromophenyl)-but-2-enoate (0.96 g, 3.57 mmol) by a procedure analogous to
thatdescribed
in example 52 a.
'H NMR (300 MHz, CDCI3) 8: 1.33 (3H, t), 2.60 (3H, s), 3.81 (3H, s), 4.23 (2H,
q), 6.20 (1H,
10 s), 6.91 (1 H, d), 7.25-7.33 (2H, m), 7.47-7.57 (4H, m). MS: 330/332 (100%,
M+).
b)
(E)-Ethyl 3-(5'-chloro-2'-methoxy-biphenyl-4-yl)-but-2-enoate (0.90 g, 2.72
mmol) was re-
duced with DIBAL-H by a procedure analogous to that described in example 52 b
to give (E7-
15 3-(5'-chloro-2'-methoxy-biphenyl-4-yl)-but-2-en-1-of as a colourless oil;
0.785 g (100%).
'H NMR (300 MHz, CDCI3) 8: 1.49 (1 H, br s), 2.11 (3H, s), 3.80 (3H, s), 4.39:
(2H, d), 6.04
(1 H, t), 6.90 (1 H, d), 7.22-7.32 (2H, m), 7.47-7.57 (4H, m). MS: 288/290
(100%, M~),
270/272, 255/257, 245/247.
20 c)
The title compound (0.54 g, 61 % yield) was prepared from (E)-3-(5'-chloro-2'-
methoxy-
biphenyl-4-yl)-but-2-en-1-of (0.50 g, 1.73 mmol) and (S)-ethyl 2-ethoxy-3-(4-
hydroxyphenyl)-
propionate (0.433 g, 1.82 mmol) by a procedure analogous to that described in
example 52
c.
25 'H NMR (300 MHz, CDCI3) 8: 1.17 (3H, t), 1.23 (3H, t), 2.16 (3H, s), 2.96
(2H, d), 3.30-3.43
(1 H, m), 3.55-3.65 (1 H, m), 3.79 (3H, s),. 3.98 (1 H, t), 4.17 (2H, q), 4.74
(2H, d), 6.10 (1 H, t),
6.84-6.92 (3H, m), 7.12-7.20 (2H, m), 7.22-7.32 (2H, m), 7.45-7.50 (4H, m).
LCMS: 331/333 (M+Na).
CA 02395298 2002-06-18
WO 01/55085 PCT/DKO1/00058
96
EXAMPLE 57
ci
0
/ / ~ ~ ~OH
OEt
OMe ~ ~ / O
(E)-(S)-3-{4-[3-(5'-Chloro-2'-methoxy-biphenyl-4-yl)-but-2-enyloxy]-phenyl}-2-
ethoxy-
propionic acid
The title compound was prepared from (E)-(S)-ethyl 2-ethoxy-3-{4-[3-(5'-chloro-
2'-methoxy-
biphenyl-4-yl)-but-2-enyloxy]-phenyl}-propionate (example 56) (0.47 g, 0.92
mmol) and so-
dium hydroxide (1 M, 1.8 ml, 1.8 mmol) by a procedure analogous to that
described in exam-
ple 51, yielding (~-(S)-3-{4-[3-(5'-chloro-2'-methoxy-biphenyl-4-yl)-but-2-
enyloxy]-phenyl}-2-
ethoxy-propionic acid as a colourless gum, which contained 0.2 mol equivalents
of ethyl ace-
tate; 0.43 g (98%).
'H NMR (300 MHz, CDCI3) 8: 1.19 (3H, t), 1.26 (ethyl acetate impurity, 0.6H,
t), 2.04 (ethyl
acetate impurity, 0.4H, s), 2.16 (3H, s), 2.96 (1 H, dd), 3.10 (1 H, dd), 3.42-
3.52 (1 H, m), 3.53-
3.68 (1 H, m), 3.80 (3H, s), 4.07 (1 H, dd), 4.12 (ethyl acetate impurity,
0.4H), 4.74 (2H, d),
6.10 (1 H, t), 6.85-6.95 (3H, m), 7.12-7.20 (2H, m), 7.21-7.32 (2H, m), 7.45-
7.50 (4H, m), car-
boxylic acid proton not observed.
LCMS: 503/505 (M+Na).
EXAMPLE 58
ci
c~ o
/ I oEt
t o w oEt
(E~-(S)-Ethyl3-{4-[3-(2',3'-Dichloro-biphenyl-4-yl)-but-2-enyloxy]-phenyl)-2-
ethoxy-propionate
CA 02395298 2002-06-18
WO 01/55085 PCT/DKO1/00058
97
a)
(E7-Ethyl 3-(2',3'-Dichloro-biphenyl-4-yl)-but-2-enoate (1.07 g, 73% yield)
was prepared from
2,3-dichlorophenylboronic acid (1.26 g, 6.60 mmol) and (E'-ethyl 3-(4-
bromophenyl)-but-2-
enoate (1.0 g, 4.40 mmol) by a procedure analogous to that described in
example 52 a.
Mpt. 64-66°C. ' H NMR (300 MHz, CDCI3) 8: 1.33 (3H, t), 2.62 (3H, d),
4.23 (2H, q), 6.21 (1 H,
m), 7.20-7.30 (2H, m), 7.40-7.50 (3H, m), 7.50-7.60 (2H, m). MS: 334/336/338
(100%, M+),
305/307/309, 289/291/293, 262/264/266, 189/191.
b)
(E~-Ethyl 3-(2',3'-dichloro-biphenyl-4-yl)-but-2-enoate (1.07 g, 3.19 mmol)
was reduced with
DIBAL-H by a procedure analogous to that described in example 52 b to give (E)-
3-(2',3'-
dichloro-biphenyl-4-yl)-but-2-en-1-of as a colourless solid; 0.74 g (79%).
Mpt. 95-100°C.'H NMR (300 MHz, CDCI3) 8: 1.45 (1H, br s), 2.13 (3H, s),
4.40 (2H, d), 6.07
(1 H, t), 7.20-7.28 (2H, m), 7.35-7.42 (2H, m), 7.42-7.53 (3H, m).
C)
The title compound (0.41 g, 80% yield) was prepared from (E7-3-(2',3'-dichloro-
biphenyl-4-
yl)-but-2-en-1-of (0.293 g, 1.0 mmol) and (S)-ethyl 2-ethoxy-3-(4-
hydroxyphenyl)-propionate
(0.25-g, 1.05 mmol) by a procedure analogous to that described in example 52
c.
'H NMR (300 MHz, CDCI3) 8: 1.17 (3H, t), 1.23 (3H, t), 2.18 (3H, s), 2.96 (2H,
d), 3.30-3.43
(1 H, m), 3.55-3.65 (1 H, m), 3.98 (1 H, t), 4.17 (2H, q), 4.75 (2H, d), 6.13
(1 H, t), 6.84-6.92
(2H, m), 7.12-7.20 (2H, m), 7.21-7.32 (2H, m), 7.35-7.42 (2H, m), 7.43-7.53
(3H, m).
EXAMPLE 59
ci
ci o
i I off
i
o w oEt
(E7-(S)-3-{4-[3-(2',3'-Dichloro-biphenyl-4-yl)-but-2-enyloxy]-phenyl}-2-ethoxy-
propionic acid
CA 02395298 2002-06-18
WO 01/55085 PCT/DKO1/00058
98
The title compound was prepared from (E7-(S)-ethyl 3-{4-[3-(2',3'-dichloro-
biphenyl-4-yl)-but-
2-enyloxy]-phenyl}-2-ethoxy-propionate (example 58) (0.325 g, 0.63 mmol) and
sodium hy-
droxide (1 M, 1.27 ml, 1.27 mmol) by a procedure analogous to that described
in example 51,
giving (E~-(S)-3-{4-[3-(2',3'-dichloro-biphenyl-4-yl)-but-2-enyloxy]-phenyl-2-
ethoxy-propionic
acid as a gum, which contained 0.28 mol equivalents of ethyl acetate; 0.24 g
(80%).
'H NMR (300 MHz, CDCI3) 8: 1.19 (3H, t), 1.26 (ethyl acetate impurity, 0.84H,
t), 2.05 (ethyl
acetate impurity,. 0.56H, s), 2.18 (3H, m), 2.98 (1 H, dd), 3.08 (1 H, dd),
3.42-3.52 (1 H, m),
3.53-3.68 (1 H, m), 3.80 (3H, s), 4.07 (1 H, dd), 4.12 (ethyl acetate
impurity, 0.56H), 4.75 (2H,
d), 6.13 (1 H, tm), 6.85-6.95 (2H, m), 7.14-7.20 (2H, m), 7.21-7.30 (2H, m),
7.35-7.42 (2H, m),
7.42-7.53 (3H, m), carboxylic acid proton not observed.
EXAMPLE 60
OMe O
/ / ~ OEt
/ I
OMe ~ ~ / O ~ OEt
(E7-(S)-Ethyl 3-{4-[3-(2',6'-Dimethoxy-biphenyl-4-yl)-but-2-enyloxy]-phenyl}-2-
ethoxy-
propionate
a)
(E7-Ethyl 3-(2',6'-dimethoxy-biphenyl-4-yl)-but-2-enoate (1.02 g, 73% yield)
was prepared
from 2,6-dimethoxyphenylboronic acid (1.20 g, 6.60 mmol) and (E7-ethyl 3-(4-
bromophenyl)-
but-2-enoate (1.0 g, 4.40 mmol) by a procedure analogous to that described in
example 52 a.
Mpt. 120-123.5°C.'H NMR (300 MHz, CDCI3) 8: 1.32 (3H, t), 2.62 (3H, d),
3.75 (6H, s), 4.23
(2H, q), 6.22 (1 H, m), 6.67 (2H, d), 7.29 (1 H, t), 7.38 (2H, dm), 7.53 (2H,
dm). MS: 326
(100%, M+), 297, 281.
b)
CA 02395298 2002-06-18
WO 01/55085 PCT/DKO1/00058
99
(~-Ethyl 3-(2',6'-dimethoxy-biphenyl-4-yl)-but-2-enoate (0.90 g, 2.76 mmol)
was reduced
with DIBAL-H by a procedure analogous to that described in example 52 b to
give (~-3-
(2',6'-dimethoxy-biphenyl-4-yl)-but-2-en-1-of as a colourless solid; 0.82 g
(100%).
Mpt. 70-75°C.'H NMR (300 MHz, CDCI3) 8: 1.44 (1H, br s), 2.12 (3H, d),
3.74 (6H, s), 4.38
(2H, d), 6.06 (1 H, tm), 6.66 (2H, d), 7.13-7.37 (3H, m), 7.42-7.50 (2H, m).
MS: 284 (100%,
M+), 266, 251, 241.
c)
The title compound (0.41 g, 80% yield) was prepared from (~-3-(2',6'-dimethoxy-
biphenyl-4-
yl)-but-2-en-1-of (0.50 g, 1.76 mmol) and (S)-ethyl 2-ethoxy-3-(4-
hydroxyphenyl)-propionate
(0.44 g, 1.85 mmol) by a procedure analogous to that described in example 52
c.
'H NMR (300 MHz, CDCI3) 8: 1.17 (3H, t), 1.22 (3H, t), 2.16 (3H, m), 2.96 (2H,
d), 3.30-3.43
(1 H, m), 3.53-3.65 (1 H, m), 3.73 (6H, s), 3.97 (1 H, t), 4.17 (2H, q), 4.75
(2H, d), 6.10 (1 H,
tm), 6.66 (2H, d), 6.84-6.90 (2H, m), 7.13-7.20 (2H, m), 7.27 (1 H, t), 7.30-
7.38 (2H, m), 7.45-
7.52 (2H, m). LCMS: 527 (M+Na), 267 (100%).
EXAMPLE 61
OMe O
/ ~ ~ OH
OEt
OMe ~ ~ / O ~
(E)-(S)-3-{4-[3-(2',6'-Dimethoxy-biphenyl-4-yl)-but-2-enyloxy]-phenyl}-2-
ethoxy-propionic acid
The title compound was prepared from (~-(S)-ethyl 3-{4-[3-(2',6'-dimethoxy-
biphenyl-4-yl)-
but-2-enyloxy]-phenyl}-2-ethoxy-propionate (example 60) (0.565 g, 1.12 mmol)
and sodium
hydroxide (1 M, 2.20 ml, 2.20 mmol) by a procedure analogous to that described
in example
51; giving (~-(S)-3-{4-[3-(2',6'-dimethoxy-biphenyl-4-yl)-but-2-enyloxy]-
phenyl}-2-ethoxy-
propionic acid as a gum; 0.49 g (92%).
'H NMR (300 MHz, CDCl3) 8: 1.18 (3H, t), 2.16 (3H, m), 2.98 (1 H, dd), 3.08 (1
H, dd), 3.42-
3.52 (1 H, m), 3.53-3.68. (1 H, m), 3.73 (6H,. s), 4.07 (1 H, dd), 4.75 (2H,
d), 6.10 (1 H, tm), 6.66
CA 02395298 2002-06-18
WO 01/55085 PCT/DKO1/00058
100
(2H, d), 6.86-6.92 (2H, m), 7.13-7.20 (2H, m), 7.27 (1 H, t), 7.28-7.35 (2H,
m), 7.45-7.50 (2H,
m), carboxylic acid proton not observed. LCMS: 499 (M+), 267 (100%).
EXAMPLE 62
0
Br / ~ ~ ~ ~OEt
O ~ OEt
(E7-(S)-Ethyf 3-~4-[3-(4-Bromophenyl)-2-methyl-but-2-enyloxy]-phenyl}-2-ethoxy-
propionate
a)
Sodium (1.37 g, 59.6 mmol) was added to ethanol (50 ml) at 20°C and the
mixture stirred
until the metal had fully reacted. Triethyl phosphonoacetate (17.78 g, 74.62
mmol) was
added, the mixture stirred for 20 min, then a solution of 4-bromoacetophenone
(9.90 g! 49.74
mmol) in ethanol (50 ml) was added and the reaction mixture heated to
80°C under reflux for
17h. The solution was cooled, the ethanol evaporated and the residue
partitioned between
1 N HCI (100 ml) and ethyl acetate (100 ml). The aqueous layer was collected
and further ex-
tracted with ethyl acetate (2 x 200 ml). The organic layers were combined,
washed with
brine, dried (MgS04) and evaporated, to an orange gum. This was purified by
column chro-
2o matography on silica gel (2% diethyl ether in n-heptane eluent) to give the
two double-bond
isomer products as colourless oils.
(~-Ethyl 3-(4-bromophenyl)-2-methyl-but-2-enoate; 5.38 g (38%).
'H NMR (300 MHz, CDCI3) 8: 1.34 (3H, t), 1.75 (3H, m), 2.22 (3H, m), 4.26 (2H,
q), 7.04 (2H,
dm), 7.49 (2H, dm). MS: 282/284 (M+), 253/255, 237/239, 208/210, 175, 157,
130, 129
(100%), 115.
And
(~-Ethyl 3-(4-bromophenyl)-2-methyl-but-2-enoate; 3.15 g (22%).
'H NMR (300 MHz, CDCI3) b: 0.90 (3H, t), 2.01 (3H, s), 2.06 (3H, s), 3.88 (2H,
q), 7.00 (2H,
dm), 7.41 (2H, dm). MS: 282/284 (M+), 253/255, 237/239, 208/210, 157, 130, 129
(100%),
115.
CA 02395298 2002-06-18
WO 01/55085 PCT/DKO1/00058
101
b)
(E7-Ethyl 3-(4-bromophenyl)-2-methyl-but-2-enoate (2.83 g, 9.99 mmol) was
reduced with
DIBAL-H by a procedure analogous to that described in example 52b to give (E7-
3-(4-
bromophenyl)-2-methyl-but-2-en-1-of as a colourless oil; 1.82 g (75%).
'H NMR (300 MHz, CDCI3) 8: 1.60 (1 H, br s), 1.66 (3H, m), 2.00 (3H, m), 4.29
(2H, s)~ 7.01
(2H, dm), 7.44 (2H, dm). MS: 240/242 (M+), 225/227, 183/185 (100%), 161, 146,
143, 128,
115.
1 o c)
The title compound (0.83 g, 87% yield) was prepared from (~-3-(4-bromophenyl)-
2-methyl-
but-2-en-1-of (0.50 g, 2.07 mmol) and (S)-ethyl 2-ethoxy-3-(4-hydroxyphenyl)-
propionate
(0.519 g, 2.18 mmol) by a procedure analogous to that described in example
52c.
'H NMR (300 MHz, CDCI3) ~: 1.18 (3H, t), 1.23 (3H, t), 1.68 (3H, m), 2.04 (3H,
m), 2.97 (2H,
d), 3.30-3.43 (1 H, m), 3.53-3.68 (1 H, m), 3.98 (1 H, t), 4.18 (2H, q), 4.61
(2H, s), 6.88 (2H,
dm), 7.04 (2H, dm), 7.17 (2H, dm), 7.45 (2H, dm).
EXAMPLE 63
0
Br / ~ ~ ~ ~OH
O ~ OEt
(E7-(S)-3-{4-[3-(4-Bromophenyl)-2-methyl-but-2-enyloxy]-phenyl}-2-ethoxy-
propionic acid
The title compound was prepared from (E'-(S)-ethyl 3-{4-[3-(4-bromophenyl)-2-
methyl-but-2
enyloxy]-phenyl}-2-ethoxy-propionate (example 62) (0.710 g, 1.54 mmol) and
sodium hydrox
ide (1 M, 3.10 ml, 3.10 mmol) by a procedure analogous to that described in
example 51; giv
ing (~-(S)-3-{4-[3-(4-bromophenyl)-2-methyl-but-2-enyloxy]-phenyl}-2-ethoxy-
propionic acid
as a colourless solid, which contained approximately 13 mol% of ethyl acetate
impurity; 0.67
g (98%).
CA 02395298 2002-06-18
WO 01/55085 PCT/DKO1/00058
102
'H NMR (300 MHz, CDC13) 8: 1.19 (3H, t), 1.68 (3H, m), 2.04 (3H, m), 2.98 (1H,
dd), 3.08
(1 H, dd), 3.42-3.54 (1 H, m), 3.54-3.68 (1 H, m), 4.07 (1 H, dd), 4.61 (2H,
s), 6.90 (2H, dm),
7.04 (2H, dm), 7.17 (2H, dm), 7.45 (2H, dm), carboxylic acid proton not
observed.
EXAMPLE 64
(Z)-(S)-Ethyl3-{4-[3-(4-Bromophenyl)-2-methyl-but-2-enyloxy]-phenyl}-2-ethoxy-
propionate
a)
(Z)-Ethyl 3-(4-bromophenyl)-2-methyl-but-2-enoate (1.42 g, 5.01 mmol), which
was prepared
as described in example 62 a, was reduced with DIBAL-H by a procedure
analogous to that
described in example 52 b. to give (Z)-3-(4-bromophenyl)-2-methyl-but-2-en-1-
of as a colour-
less oil; 1.19 g (98%).
'H NMR (300 MHz, CDCI3) 8: 1.38 (1 H, br s), 1.89 (3H, s), 1.97 (3H, s), 3.92
(2H, s), 7.01
(2H, dm), 7.42 (2H, dm). MS: 240/242 (M+), 225/227, 183/185 (100%), 161, 146,
143, 128,
115.
C)
The title compound (0.91 g, 95% yield) was prepared from (Z)-3-(4-bromophenyl)-
2-methyl-
but-2-en-1-of (0.50 g, 2.07 mmol) and (S)-ethyl 2-ethoxy-3-(4-hydroxyphenyl)-
propionate
(0.519 g, 2.18 mmol) by a procedure analogous to that described in example 52
c.
'H NMR (300 MHz, CDCI3) 8: 1.16 (3H, t), 1.21 (3H, t), 1.93 (3H, s), 2.02 (3H,
s), 2.93 (2H,
d), 3.28-3.42 (1 H, m), 3.53-3.68 (1 H, m), 3.95. (1 H, t), 4.16 (2H, q), 4.25
(2H, s), 6.69 (2H,
dm), 7.04 (2H, dm), 7.09 (2H, dm), 7.41 (2H, dm).
CA 02395298 2002-06-18
WO 01/55085 PCT/DKO1/00058
103
EXAMPLE 65
/ I \~
0
\ OH
O
(27-(S)-3-f4-[3-(4-Bromophenyl)-2-methyl-but-2-enyloxy]-phenyl}-2-ethoxy-
propionic acid
The title compound was prepared from (~-(S)-ethyl 3-{4-[3-(4-bromophenyl)-2-
methyl-but-2-
enyloxy]-phenyl)-2-ethoxy-propionate (example 64) (0.82 g, 1.78 mmol) and
sodium hydrox-
ide (1 M, 3.60 ml, 3.60 mmol) by a procedure analogous to that described in
example 51; giv-
ing (27-(S)-3-{4-[3-(4-bromophenyl)-2-methyl-but-2-enyloxy]-phenyl}-2-ethoxy-
propionic acid
as a colourless solid, which contained approximately 15 mol% of ethyl acetate
impurity;
0.766 g (100%).
' H NMR (300 MHz, CDCI~) S: 1.17 (3H,. t), 1.93 (3H, s), 2.02.(3H, s), 2.93 (1
H, dd)~ 3.04 (1 H,
dd), 3.40-3.52 (1 H, m), 3.52-3.65 (1 H, m), 4.03 (1 H! dd), 4.26 (2H, s),
6.71 (2H, dm), 7.04.
(2H, dm), 7.09 (2H, dm), 7.41 (2H, dm), carboxylic acid proton not observed.
EXAMPLE 66
0
\
~OEt
\ / /
/ \ ~ / O \ OEt
(~-(S)-Ethyl 2-Ethoxy-3-[4-(3-[1,1';3',1 "]terphenyl-4"-yl-but-2-enyloxy)-
phenyl]-propionate
a)
CA 02395298 2002-06-18
WO 01/55085 PCT/DKO1/00058
104
(~-Ethyl 3-[1,1';3',1 "]terphenyl-4"-yl-but-2-enoate (1.02 g, 68% yield) was
prepared from 3-
biphenylboronic acid (1.31 g, 6.60 mmol) and (~-ethyl 3-(4-bromophenyl)-but-2-
enoate (1.0
g, 4.40. mmol) by a procedure analogous to that described in example 52 a.
'H NMR (300 MHz, CDCI3) s: 1.33 (3H, t), 2.62 (3H, d), 4.23 (2H, q), 6.21 (1H,
s), 7.30-7.70
(12H, m), 7.82 (1 H, m). LCMS: 343 (100%, M+), 297.
b)
(~-Ethyl 3-[1,1';3',1"]terphenyl-4"-yl-but-2-enoate (0.95 g, 2.77 mmol) was
reduced with DI-
BAL-H by a procedure analogous to that described in example 52 b to give (,E)-
3-
[1,1';3',1"]terphenyl-4"-yl-but-2-en-1-of as a colourless solid; 0.81 g (97%).
'H NMR (300 MHz, CDCI3) 8: 1.37 (1 H, br s), 2.13 (3H, s), 4.40 (2H, d), 6.06:
(1 H, tm), 7.30-
7.70 (12H, m), 7.81 (1 H, m). LCMS: 283 (100%, M+H-H20).
Microanalysis Calculated % C: 87.96, H: 6.71. Found % C: 87.85, H: 6.74.
c)
The title compound (0.41 g, 80% yield) was prepared from (~-3-
[1,1';3',1"]terphenyl-4"-y[-
but-2-en-1-of (0.30 g, 1.0 mmol) and (S)-ethyl 2-ethoxy-3-(4-hydroxyphenyl)-
propionate (0:25
g, 1.05 mmol) by a procedure analogous to that described in example 52 c.
'H NMR (300 MHz, CDCI3) 8: 1.17 (3H, t)"1.22 (3H, t), 2.18 (3H, s), 2.96 (2H,
d), 3.30-3.43
(1 H, m), 3.55-3.68 (1 H, m), 3.98 (1 H, t), 4.17 (2H, q), 4.75 (2H, d), 6.13
(1 H, t), 6.89 (2H,
dm), 7.17 (2H, dm), 7.30-7.70 (12H, m), 7.81 (1 H, m).
Microanalysis Calculated % C: 80.74, H: 6.97. Found % C: 80.84, H: 7.28.
EXAMPLE 67
0
~ ' i I ~ ~oH
O ~ OEt
(~-(S)-2-Ethoxy-3-[4-(3-[1,1';3',1"]terphenyl-4"-yl-but-2-enyloxy)-phenyl]-
propionic acid
CA 02395298 2002-06-18
WO 01/55085 PCT/DKO1/00058
105
The title compound was prepared from (~-(S)-ethyl 2-ethoxy-3-[4-(3-[1,1';3',1
"]terphenyl-4"-
yl-but-2-enyloxy)-phenyl]-propionate (example 66) (0.185 g, 0.36 mmol) and
sodium hydrox-
ide (1 M, 0.71 ml, 0.71 mmol) by a procedure analogous to that described in
example 51; giv-
ing. (~-(S)-2-ethoxy-3-[4-(3-[1,1';3',1 "]terphenyl-4"-yl-but-2-enyloxy)-
phenyl]-propionic acid
as a gum; 0.145 g (83%).
' H NMR (300 MHz, CDCI3) 8: 1.19 (3H, t), 2.18 (3H, m), 2.99 (1 H, dd), 3.09
(1 H, dd), 3.40-
3.53 (1 H, m), 3.53-3.68 (1 H, m), 4.07 (1 H, dd), 4.75 (2H, d), 6.13 (1 H,
tm), 6.90 (2H, dm),
7.17 (2H, dm), 7.30-7.70 (12H, m), 7.81 (1 H, m), carboxylic acid proton not
observed.
EXAMPLE 68
0
/ / ~ ~ ~OEt
a /
OEt
(~-(S)-Ethyl2-Ethoxy-3-{4-[3-(3'-methyl-biphenyl-4-yl)-but-2-enyloxy]-phenyl)-
propionate
a)
(~-3-(3'-Methyl-biphenyl-4-yl)-but-2-enoate (0.795 g, 65% yield) was prepared
from 3-
tolylboronic acid (0.90 g, 6.60 mmol) and (~-ethyl 3-(4-bromophenyl)-but-2-
enoate (1.0 g,
4.40 mmol) by a procedure analogous to that described in example..52 a.
'H NMR (300 MHz, CDCI3) 8: 1.33 (3H, t), 2.43 (3H, s), 2.61 (3H, s), 4.23 (2H,
q), 6.20 (1 H,
s), 7.18 (1 H, dm), 7.34 (1 H, tm), 7.41 (2H, dm), 7.52-7.63 (4H, m). LCMS:
281 (M+H), 235
(100%).
b)
(~-3-(3'-Methyl-biphenyl-4-yl)-but-2-enoate (0.74 g, 2.64 mmol) was reduced
with DIBAL-H
by a procedure analogous to that described in example 52 b to give (~-3-(3'-
methyl-
biphenyl-4-yl)-but-2-en-1-of as a colourless solid; 0.63 g (85%).
CA 02395298 2002-06-18
WO 01/55085 PCT/DKO1/00058
106
' H NMR (300 MHz, CDC13) 8: 1.36 (1 H, br s), 2.12 (3H, s), 2.42 (3H, s), 4.39
(2H, d), 6.05
(1 H,. tm), 7.16 (1 H, dm), 7.33 (1 H, tm), 7.40 (2H, dm), 7.48 (2H, dm), 7.56
(2H, dm). LCMS:
221 (100%, M+H-HZO). '
C)
The title compound (0.365 g, 78% yield) was prepared from (E~-3-(3'-methyl-
biphenyl-4-yl)-
but-2-en-1-of (0.238 g, 1.0 mmol) and (S)-ethyl 2-ethoxy-3-(4-hydroxyphenyl)-
propionate
(0.25 g, 1.05 mmol) by a procedure analogous to that described in example 52
c.
'H NMR (300 MHz, CDCI3) 8: 1.17 (3H, t), 1.22 (3H, t), 2.17 (3H, s), 2.42 (3H,
s), 2.96 (2H,
d), 3.30-3.43 (1 H, m), 3.55-3.68 (1 H, m), 3.98 (1 H, t), 4.17 (2H, q), 4.74
(2H, d), 6.11 (1 H, t),
6.90 (2H, dm), 7.13-7.23 (3H, m), 7.33 (1 H, t), 7.36-7.44 (2H, m), 7.45-7.60
(4H, m).
Microanalysis Calculated % C: 78.57, H: 7.47. Found % C: 78.90, H: 7.70.
EXAMPLE 69
0
~ ~ ~OH
U
OEt
(~-(S)-2-Ethoxy-3-{4-[3-(3'-methyl-biphenyl-4-yl)-but-2-enyloxy]-phenyl}-
propionic acid
The title compound was prepared from (~-(S)-2-ethoxy-3-f4-[3-(3'-methyl-
biphenyl-4-yl)-but-
2-enyloxy]-phenyl}-propionate (example 68) (0.225 g, 0.49 mmol) and sodium
hydroxide (1 M,
0.98 ml, 0.98 mmol) by a procedure analogous to that described in example 51;
giving (E~-
(S)-2-ethoxy-3-{4-[3-(3'-methyl-biphenyl-4-yl)-but-2-enyloxy]-phenyl}-
propionic acid as a
gum; 0.20 g (95%).
'H NMR (300 MHz, CDCI3) 8: 1.18 (3H, t), 2.17 (3H, m), 2.42 (3H, s), 2.97 (1
H, dd), 3.09 (1 H,
dd), 3.42-3.54 (1 H, m), 3.55-3.68 (1 H, m), 4.07 (1 H, dd), 4.75 (2H, d),
6.11 (1 H, tm), 6.90
(2H, dm), 7.10-7.23 (3H, m), 7.35 (1 H, t), 7.37-7.44 (2H, m), 7.45-7.60 (4H,
m), carboxylic
acid proton not observed.
CA 02395298 2002-06-18
WO 01/55085 PCT/DKO1/00058
107
EXAMPLE 70
0
/ / I Y ~OEt
a /
O \ I / O
OEt
(~-(S)-Ethyl 3-{4-[3-(3'-Acetyl-biphenyl-4-yl)-but-2-enyloxy]-phenyl}-2-ethoxy-
propionate
a)
3-Acetylphenylboronic acid (7.10 g, 43.3 mmol) was coupled with (~-3-(4-
bromophenyl)-but-
2-en-1-of (5.76 g, 25.0 mmol) by a procedure analogous to that described in
example 54 a to
give (~-3-(3'-acetyl-biphenyl-4-yl)-but-2-en-1-of as an off-white solid; 5.33
g (79%). This
solid was recrystallised from aqueous ethanol to give a first crop of very
pure .(E)-3-(3'-acetyl-
biphenyl-4-yl)-but-2-en-1-of as colourless platelets; 2.78 g (41%) and a
second crop of (E~-3-
(3'-acetyl-biphenyl-4-yl)-but-2-en-1-of as an amorphous off-white solid; 2.53
g (37%).
Mpt. 85-86°C.'H NMR (300 MHz, CDCI3) 8: 1.46 (1H, brt), 2.13 (3H, d),
2.66 (3H, s), 4.41
(2H, br t),, 6.07 (1 H, tm), 7.50-7.62 (5H, m), 7.80 (1 H, dm), 7.92 (1 H,
dm), 8.19 (1 H, .m). MS:
266 (M+), 251, (M-Me), 248 (M-H20), 223 (100%). Microanalysis Calculated %.C:
81.17, H:
6.81. Found % C: 81.22, H: 6.83.
b)
The title compound (0.16 g, 65% yield) was prepared from (~-3-(3'-acetyl-
biphenyl-4-yl)-but-
2-en-1-of (0.133 g, 0.50 mmol) and (S)-ethyl 2-ethoxy-3-(4-hydroxyphenyl)-
propionate (0.125
g, 0.525 mmol) by a procedure analogous to that described in example 52 c.
'H NMR (300 MHz, CDCI3) b: 1.17 (3H, t), 1.23 (3H, t), 2.18 (3H, s), 2.66 (3H,
s), 2.92 (2H,
d), 3.30-3.43 (1 H, m), 3.55-3.68 (1 H, m), 3.98 (1 H, t), 4.17 (2H, q), 4.75
(2H, d), 6.13 (1 H, t),
6.89 (2H, dm), 7.17 (2H, dm), 7.50-7.64 (5H, m), 7.80 (1 H, dm), 7.92 (1 H,
dm), 8.19 (1 H, m).
CA 02395298 2002-06-18
WO 01/55085 PCT/DKO1/00058
108
EXAMPLE 71
o~
(E7-(S)-2-Ethoxjr-3-{4-[3-(5'-isopropyl-2'-methoxy-biphenyl-4-yl)-allyloxy]-
phenyl}-propionic
acid ethyl ester
a)
(E)-3-(4-Bromo-phenyl)-acrylic acid ethyl ester was prepared from 4-
bromobenzaldehyde
(20.0 g,. 0.11 mol) by a procedure analogous to that described in example 23
a.
b)
(E'7-3-(4-Bromo-phenyl)-acrylic acid ethyl ester (450 mg, 2.0 mmol) was
reacted with 5-
isopropyl-2-methoxy-benzene boronic acid (776 mg, 4.0 mmol) by a procedure
described in
example 52 a, to give (E)-3-(5'-Isopropyl-2'-methoxy-biphenyl-4-yl)-acrylic
acid. ethyl ester.
c)
(E)-3-(5'-Isopropyl-2'-methoxy-biphenyl-4-yl)-acrylic acid ethyl ester was
reduced by DIBAL-
H by a procedure analogous to that described in example 52 b to give (E)-3-(5'-
isopropyl-2'-
methoxy-biphenyl-4-yl)-prop-2-en-1-ol.
CA 02395298 2002-06-18
WO 01/55085 PCT/DKO1/00058
109
d)
The title compound was prepared from (E)-3-(5'-isopropyl-2'-methoxy-biphenyl-4-
yl)-prop-2-
en-1-of by a procedure analogous to that described in 52 c.
'H NMR (300 MHz, CDCI3) 8: 1.13-1.30 (m, 12H), 2.85-3.0 (m, 3H), 3.30-3.42 (m,
1 H), 3.53-
3.67 (m, 1 H), 2.78 (s, 3H), 3.98. (t, 1 H), 4.15 (q, 2H), 4.70 (dd, 2H), 6.43
(dt, 1 H), 6.75 (d,
1 H), 6.85-6.95 (m, 3H), 7.15 (d, 4H), 7.44 (d, 2H), 7.52 (d, 2H).
GYA~ADI G 7~
OH
(E'7-(S)-2-Ethoxy-3-{4-[3-(5'-isopropyl-2'-methoxy-biphenyl-4-yl)-allyloxy]-
phenyl}-propionic
acid
The title compound was prepared from (E'7-(S)-2-ethoxy-3-{4-[3-(5'-isopropyl-
2'-methoxy-
biphenyl-4-yl)-allyloxy]-phenyl}-propionic acid ethyl ester (example 71 ) (370
mg, 0.78 mmol)
by a procedure analogous to that described in example 26.
~H NMR (CDCI3, 300 MHz) 8: 1.18 (t, 3H), 1.26 (d, 6H), 2.85-3.03 (m, 2H), 3.08
(dd, 1 H),
3.35-3.48 (m, 1 H), 3.55-3.68 (m, 1 H), 3.75 (s, 3H), 4.03 (dd, 1 H), 4.67 (d,
2H), 6.43 (dt, 1 H),
6.75 (d. 1 H), 6.87-6.95 (m, 3H), 7.13-7.23 (m, 4H), 7.43 (d, 2H), 7.53 (d,
2H).
CA 02395298 2002-06-18
WO 01/55085 PCT/DKO1/00058
110
EXAMPLE 73
o~
(~-(S)-3-{4-[3-(3,5-Distyryl-phenyl)-allyloxy]-phenyl}-2-ethoxy-propionic acid
ethyl ester
a)
Bu4NBr (2.0 g, 6.3 mmol), K2C03 (7.8 g, 56.7 mmol), Pd(Oac)2 (250 mg, 1.1
mmol) and sty-
rene (20 mL, 175 mmol) were stirred for 5 min under nitrogen. To the mixture
was added 3,5-
dibromobenzaldehyde (5.0 g, 18.9 mmol) in dry DMF (5.0 mL), and the mixture
was.stirred at
65°C for 16h. The reaction mixture. was diluted with ethyl acetate (20
mL) and the solution
filtered. The filtrate was diluted with water and extracted with ethyl acetate
(3 x. 50 mL). The.
organic layers were combined, dried over MgS04, and concentrated under
vacuum.. To the
residue was added a mixture of toluene/petroleum ether (1:1 ) (50 mL) and 3,5-
distyryl-
benzaldehyde (4.95 g, 85%) was isolated by filtration.
b)
The title compound was prepared from 3,5-distyryl-benzaldehyde ( 3.8 g, 10.0
mmol) by a
sequence analogous to that described in example 23 b-c.
'H NMR (CDCI3, 300 MHz) 8: 1.15 (t, 3H), 1.22 (t, 3H), 2.98 (d, 2H), 3.32-3.42
(m, 1H), 3.55-
3.68 (m, 1 H), 3.98 (t, 1 H), 4.12 (t, 1 H), 4.18 (q, 2H), 4.72 (dd, 2H), 6.50
(dt, 1 H), 6.78 (d, 1 H),
6.90 (d, 1 H), 7.08-7.32 (m, 8H), 7.39 (t, 4H), 7.45 (s, 2H), 7.53 (d, 5H).
CA 02395298 2002-06-18
WO 01/55085 PCT/DKO1/00058
111
EXAMPLE 74
OH
(E7-(S)-3-{4-[3-(3,5-Distyryl-phenyl)-allyloxy]-phenyl-2-ethoxy-propionic acid
(E'7-(S)-3-{4-[3-(3,5-Distyryl-phenyl)-allyloxy]-phenyl}-2-ethoxy-propionic
acid ethyl ester (ex-
ample 73) (335 mg, 0.6 mmol) was dissolved in warm ethanol (20 mL) and sodium
hydroxide
(1N, 0.9 mL, 0.9 mmol) added. The mixture was stirred at room
temperature~for~l6h. The title
compound as a sodium salt was isolated by filtration and washed with
ethanol/water (10:1 ),
yielding 190 mg (57°t°).
'H NMR (CDCI3, 300 MHz) 8: 0.98 (t, 3H), 2.63 (dd, 1 H), 2.85 (dd, 1 H), 3.05-
3:15 (m, 1 H),
3.50-3.64 (m, 2H), 4.75 (d, 2H), 6.68 (dt, 1 H), 6.80 (d, 1 H), 6.90 (d, 2H),
7.15 (d, 2H), 7.25-
7.48 (m, 1 OH), 7.60-7.70 (m, 6H), 7.75 (s, 1 H).
EXAMPLE 75
Y
1
CA 02395298 2002-06-18
WO 01/55085 PCT/DKO1/00058
112
(E)-(S)-3-{4-[3-(3,5-Diisopropoxy-phenyl)-allyloxy]-phenyl}-2-ethoxy-propionic
acid ethyl ester
a)
To a solution of 3,5-dihydroxybenzaldehyde (3.0 g, 22.0 mmol) in DMF (17 mL)
was added
potassium carbonate (12.1 g, 87.0 mmol) and 2-bromopropane (28.5 g, 232 mmol).
The re-
action mixture was heated at 100°C for 3 h. The mixture was filtered
and washed with ethyl
acetate. The filtrate was added water and the organic phase isolated. The
aqueous phase
was extracted once more with ethyl acetated. The combined organic phases were
dried
(MgS04), filtered and concentrated in vacuo. The residue was purified by flash
chromatogra-
phy eluting with toluene to give 3.8 g (79%) of 3,5-diisopropoxy-benzaldehyde
as a yellow oil.
'H NMR (CDCI3, 300 MHz) 8: 1.35 (d, 12H), 4.60 (heptet, 2H), 6.68 (t, 1H),
6.97 (d, 2H).
b)
The title compound was prepared from 3,5-diisopropoxy-benzaldehyde by a
sequence
analogous to that described in example 23.
'H NMR (CDCI3, 300 MHz) 8: 1.15 (t, 3H), 1.22 (t, 3H), 1.32 (d, 12H), 2.96 (d,
2H), 3:32-3.42
(m, 1 H), 3.55-3.65 (m,1 H), 3.98 (t, 1 H), 4.16 (q, 2H), 4.53 (heptet, 2H),
4.65 (dd, 2H), 6.30-
6.40 (m, 2H), 6.52 (d, 2H), 6.62 (d, 1 H), 6.88 (d, 2H), 7.15 (d, 2H).
EXAMPLE 76
0
\ _
\ / o \ / o
O ~O OH
(E)-(S)-3-{4-[3-(3,5-Diisopropoxy-phenyl)-allyloxy]-phenyl}-2-ethoxy-propionic
acid
The title compound was prepared from (E)-(S)-3-{4-[3-(3,5-diisopropoxy-phenyl)-
allyloxy]-
phenyl}-2-ethoxy-propionic acid ethyl ester (example 75) (800 mg, 1.7 mmol) by
a procedure
analogous to that described in example 26.
CA 02395298 2002-06-18
WO 01/55085 PCT/DKO1/00058
113
'H NMR (CDC13, 300 MHz) 8: 1.18 (t, 3H), 1.32 (d, 12H), 2.95 (dd, 1 H), 3.10
(dd, 1 H), 3.40-
3.52 (m,. 1 H), 3.55-3.65 (m, 1 H), 4.05 (dd, 1 H), 4.53 (heptet, 2H), 6.30-
6.40 (m, 2H), 6.52 (d,
2H), 6.62 (d, 1 H), 6.88 (d, 2H), 7.15 (d, 2H).
EXAMPLE 77
Rr
(S)-3-{4-[3-(3-Bromo-5-styryl-phenyl)-allyloxy]-phenyl}-2-ethoxy-propionic
acid ethyl ester
a)
A mixture of styrene. (2.0 g, 18.9.~mmol), potassium carbonate (7.8 g, 56.7
mmol); tetra-N-
butylammonium bromide (2.0 g, 6.3 mmol) and palladium(II) acetate (250. mg,
1.11 mmol),
under nitrogen, was stirred for 10 min. A solution of 3,5-dibromobenzaldehyde
(5.0 g, 18.9
mmol) in dry DMF (10 mL) was added and the mixture heated at 65°C for
16 h: The reaction
mixture was concentrated in vacuo, and the product purified by flash
chromatography (hep-
tane/ethyl acetate 1:4) to give 1.7 g of 3-bromo-5-styryl-benzaldehyde.
b)
The title compound was prepared from 3-bromo-5-styryl-benzaldehyde by a
sequence
analogous to that described in example 23.
'H NMR (CDCI3, 300 MHz) 8: 1.18 (t, 3H), 1.23 (t, 3H), 2.96 (d, 2H), 3.30-3.42
(m, 1H), 3.55
3.65 (m, 1 H), 3.98 (t, 1 H), 4.15 (q, 2H), 4.68 (d, 2H), 6.43 (dt, 1 H), 6.66
(d, 1 H), 6.88 (d, 2H),
6.93-7.56 (m, 12H).
CA 02395298 2002-06-18
WO 01/55085 PCT/DKO1/00058
114
EXAMPLE 78
Br
\ _
\ ~ O O
~O OH
(S)-3-{4-[3-(3-Bromo-5-styryl-phenyl)-allyloxy]-phenyl}-2-ethoxy-propionic
acid
The title compound was prepared from (S)-3-{4-[3-(3-bromo-5-styryl-phenyl)-
allyloxy]- ,
phenyl-2-ethoxy-propionic acid ethyl ester (example 77) (800 mg, 1.7 mmol) by
a procedure
analogous to that described in example 26.
'H NMR (CDCI3, 300 MHz) 8: 1.18 (t, 3H), 2.98 (dd, 1 H), 3.10 (dd, 1 H), 3.40-
3.53 (m, 1 H),
3.54-3.68 (m, 1 H), 4.05 (dd, 1 H), 4.68 (dd, 2H), 6.43 (dt, 1 H), 6.68 (s, 1
H), 6.88 (d, 2H), 6.94-
7.56 (m, 12H).
EXAMPLE 79
(E)-(S)-2-Ethoxy-3-[4-(3-phenyl-allyloxy)-phenyl]-propionic acid ethyl ester
The title compound was prepared from 3-phenyl-prop-2-en-1-of (270 mg, 2.0
mmol) by a se-
quence analogous to that described in example 23c.
'H NMR (CDCI3, 300 MHz) 8: 1.18 (t, 3H), 1.22 (t, 3H), 2.95 (d, 2H), 3.30-3.42
(m, 1H), 3.53-
3.65 (m, 1 H), 3.98 (t, 1 H), 4.15 (q, 2H), 4.68 (dd, 2H), 6.41 (dt, 1 H),
6.73 (dt, 1 H), 6.88 (d,
2H), 7.15 (d, 2H), 7.21-7.38 (m, 3H), 7.38-7.43 (m, 2H).
CA 02395298 2002-06-18
WO 01/55085 PCT/DKO1/00058
115
EXAMPLE 80
\ _
0 0
\ /
~O OH
/5
(E)-(S)-2-Ethoxy-3-[4-(3-phenyl-allyloxy)-phenyl]-propionic acid
The title compound was prepared from (E)-(S)-2-ethoxy-3-[4-(3-phenyl-allyloxy)-
phenyl]-
propionic acid ethyl ester (example 79) (700 mg, 2.0 mmol) by a procedure
analogous to
that described in example 26.
' H NMR (CDCI3, 300 MHz) 8: 1.18 (t, 3H), 2.95 (dd,.1 H), 3.10 (dd, 1 H), 4.42-
3.53 (m, 1 H),
3.53-3.64 (m, 1 H), 4.05 (dd, 1 H), 4.68 (dd, 2H), 6.42 (dt, 1 H), 6.72 (d, 1
H), 6.89 (d, 2H), 7.15
(d, 1 H), 7.22-7.37 (m, 3H), 7.40 (d, 2H).
EXAMPLE 81
(E)-(S)-3-~4-[3-(2',3'-Dichloro-biphenyl-4-yl)-allyloxy]-phenyl}-2-ethoxy-
propionic acidethyl
ester
a)
(E)-3-(4-Bromo-phenyl)-acrylic acid ethyl ester was prepared from 3-
bromobenzaldehyde
(20.0 g, 0.11 mol) by a sequence analogous to that described in example 23a.
CA 02395298 2002-06-18
WO 01/55085 PCT/DKO1/00058
116
b)
The title compound was prepared from (E)-3-(4-bromo-phenyl)-acrylic acid ethyl
ester and
2,3-dichlorobenzene boronic acid by a sequence analogous to that described in
example
52a-c.
'H NMR (CDCI3, 300 MHz) 8: 1.18 (t, 3H), 1.23 (t, 3H), 2.95 (d, 2H), 3.30-3.42
(m, 1H), 3.53-
3.65 (m, 1 H), 3.98 (t, 1 H), 4.15 (q, 2H), 4.70 (dd, 2H), 6.47 (dt, 1 H), 6.7
(d, 1 H), 6.88 (d, 2H),
7.15 (d, 2H), 7.20-7.28 (m, 2H), 7.35 (d, 2H), 7.43-7.52 (m, 3H).
EXAMPLE 82
\ _
/ \ \ / 0 0
\ /
~O OH
C /I
(E)-(S)-3-{4-[3-(2',3'-Dichloro-biphenyl-4-yl)-allyloxy]-phenyl}-2-ethoxy-
propionic acid
The title compound was prepared from (E)-(S)-3-{4-[3-(2',3'-dichloro-biphenyl-
4-yl)-allyloxy]-
phenyl}-2-ethoxy-propionic acid ethyl ester (example 81 ) by a procedure
analogous to that
described in example 26.
'H NMR (MeOD, 300 MHz) 8: 1.12 (t, 3H), 2.88 (dd, 1 H), 3.0 (dd, 1 H), 3.30-
3.42 (m, 1 H),
3.3-3.65 (m, 1 H), 4.0 (dd, 1 H), 4.70 (dd, 2H), 6.52 (dt, 1 H), 6.80 (d, 1
H), 6.90 (d, 2H), 7.18
(d, 2H), 7.25-7.40 (m, 4H), 7.48-7.55 (m, 3H).
CA 02395298 2002-06-18
WO 01/55085 PCT/DKO1/00058
117
EXAMPLE 83
0
(E)-(S)-3-~4-[3-(3,5-Bis-phenylethynyl-phenyl)-allyloxy]-phenyl}-2-ethoxy-
propionic acid ethyl
ester
a)
A mixture of potassium carbonate (2.1 g, 15.2 mmol), tetra-N-butylammonium
bromide (0.75
~ g, 2.4 mmol) and palladium(II) acetate (75 mg, 0.33 mmol) in dry DMF (8 ML),
under nitro-
gen, was stirred for 10 min. 3-(3,5-Dibromophenyl)-acrylic acid ethyl ester
(1.2. g, 3.6 mmol)
was added and the mixture cooled on ice. Pfienylacetylene (4.0 mL, 36.0 mmol)
was added
and the mixture. stirred at room temperature for 7 days. The reaction mixture
was added wa-
ter and the product extracted with ethyl acetate (x 3). The combined organic
phases were
dried and concentrated in vacuo to give crude 3-(3,5-bis-phenylethynyl-phenyl)-
acrylic acid
ethyl ester.
b)
The title compound was prepared from 3-(3,5-bis-phenylethynyl-phenyl)-acrylic
acid ethyl
ester by a sequence analogous to that described in example 23b-c:
'H NMR (CDCI3, 300 MHz) 8: 1.18 (t, 3H), 1.23 (t, 3H), 2.96 (d, 2H), 3.30-3.42
(m, 1H), 3.55-
3.67 (rri, 1 H), 3.98 (t, 1 H), 4.15 (q, 2H), 4.70 (d, 2H), 6.46 (dt, 1 H),
6.68 (d, 1 H), 6.88 (d, 2H),
7.15 (d, 2H), 7.30-7.38 (m, 6H), 7.48-7.58 (m, 6H), 7.60 (s, 1 H).
CA 02395298 2002-06-18
WO 01/55085 PCT/DKO1/00058
118
EXAMPLE 84
~H
(E)-(S)-3-{4-[3-(3,5-Bis-phenylethynyl-phenyl)-allyloxy]-phenyl}-2-ethoxy-
propionic acid.
The title compound was prepared from (E)-(S)-3-{4-[3-(3,5-bis-phenylethynyl-
phenyl)-
allyloxy]-phenyl}-2-ethoxy-propionic acid ethyl ester (example 83) (130 mg,
0.24 mmol) by a
procedure analogous to that described in example 26.
H NMR (CDCI3, 300 MHz) 8: 1.18 (t, 3H), 2.96 (dd, 1 H), 3.98 (dd, 1,H), 3.37-
3.48 (m; 1 H),
3.53-3.67 (m, 1 H), 4.03 (dd, 1 H), 4.68 (d, 2H), 6.47 (dt, 1 H), 6.68 (d, 1
H), 6.88 (d, 2H), 7.18
(d, 2H), 7.30-7.42 (m, 6H), 7.48-7.58 (m, 6H), 7.60 (s, 1 H).
EXAMPLE 85
\ _
0 0
\ /
~o
\ /
(E)-(S)-3-{4-[3-(3,5-Diphenethyl-phenyl)-allyloxy]-phenyl}-2-ethoxy-propionic
acid ethyl ester
CA 02395298 2002-06-18
WO 01/55085 PCT/DKO1/00058
119
a)
A solution of 3,5-distyryl-benzaldehyde (2.0 g, 6.44 mmol) (prepared as
described in exam-
ple 79) in ethyl acetate (150 mL) was hydrogenated at 3 atm for 16 h using 5%
Pd-C (2 g) as
catalyst. The catalyst was removed by filtration and the solvent evaporated to
give (3,5-
diphenethyl-phenyl)-methanol (2.0 g) as an oil.
b)
To a solution of (3,5-diphenethyl-phenyl)-methanol (2.0 g, 6.4 mmol) in dry
dichloromethane
(30 mL) was added pyridinium chlorochromate (1.4 g, 6.4 mmol) and the mixture
was stirred
at room temperature for 16 h. The product was purified by flash chromatography
using di-
chloromethane as solvent to give 1.3 g 3,5-diphenethyl-benzaldehyde.
c)
The title compound was prepared from 3,5-diphenethyl-benzaldehyde by a
sequence analo-
gnus to that described in example 23.
EXAMPLE 86
H
(E)-(S)-3-{4-[3-(3,5-Diphenethyl-phenyl)-allyloxy]-phenyl}-2-ethoxy-propionic
acid
The title compound was prepared from (E)-(S)-3-{4-[3-(3,5-Diphenethyl-phenyl)-
allyloxy]-
phenyl}-2-ethoxy-propionic acid ethyl ester (example 85) (449 mg, 0.80 mmol)
by a proce-
dure analogous to that described in example 26.
CA 02395298 2002-06-18
WO 01/55085 PCT/DKO1/00058
120
'H NMR (CDC13, 300 MHz) 8: 1.18 (t, 3H), 2.96 (dd, 1 H), 3.08 (dd, 1 H), 3.35-
3.48 (m, 1 H),
3.55-3.67 (m, 1 H), 4.03 (dd, 1 H), 4.65 (dd, 1 H), 6.35 (dt, 1 H), 6.68 (d, 1
H), 6.82-6.92 (m,
3H), 7.04 (d, 2H), 7.12-7.32 (m, 12H).
EXAMPLE 87
Q
0
3-{4-[3-(3,5-Bis-cyclopentyloxy-phenyl)-allyloxy]-phenyl}-2-ethoxy-propionic
acid ethyl ester
The title compound was prepared from dihydroxybenzaldehyde (1.0 g, 7.2 mmol)
and
cyclopentylbromide (4.0 g, 29.0 mmol) by a sequence analogous to that
described in exam-
ple 75.
'H NMR (CDCI3, 300 MHz) 8: 1.18 (t, 3H), 1.21 (t, 3H), 1.50-1.68 (m, 4H), 1.68-
1.97 (m,
12H), 2.95 (d, 2H), 3.28-3.42 (m, 1 H), 3.54-3.65 (m, 1 H), 3.97 (t, 1 H),
4.15 (q, 2H), 4.67 (dd,
2H), 4.67-4.77 (m, 2H), 6.28-6.40 (m, 2H), 6.50 (d, 2H), 6.60 (d, 1 H), 6.87
(d, 2H), 7.15 (d,
2H).
CA 02395298 2002-06-18
WO 01/55085 PCT/DKO1/00058
121
EXAMPLE 88
0
\ _
\~ 0 0
\ /
~o
1~/ ~O OH
(E)-(S)-3-{4-[3-(3,5-Bis-cyclopentyloxy-phenyl)-allyloxy]-phenyl-2-ethoxy-
propionic acid
The title compound was prepared from (E)-(S)-3-{4-[3-(3,5-bis-cyclopentyloxy-
phenyl)-
allyloxy]-phenyl}-2-ethoxy-propionic acid ethyl ester (example 87) (220 mg,
0.42 mmol) by a
procedure analogous to that described in example 26.
'H NMR (CDCI3, 300 MHz) 8: 1.18 (t, 3H), 1.52-1.70 (m, 4H), 1.70-1.98 (m,
12H), 2.95 (dd,
1 H), 3.07 (dd, 1 H), 3.37-3.48 (m, 1 H), 3.55-3.65 (m, 1 H), 4.03 (dd, 1 H),
4:65 (dd, 2H), 4.70-
4.78 (m, 2H), 6.29-6.40 (m, 2H), 6.50 (d, 2H), 6.60 (d, 1 H), 6.88 (d, 2H),
7.15 (d, 2H).
EXAMPLE 89
F
~F
F
O
(E)-(S)-3-(4-{3-[3,5-Bis-(2,2,2-trifluoro-ethoxy)-phenyl]-allyloxy}-phenyl)-2-
ethoxy-propionic
acid ethyl ester
CA 02395298 2002-06-18
WO 01/55085 PCT/DKO1/00058
122
a)
To a solution of 3,5-dihydroxybenzaldehyde (2.0 g, 14.5 mmol) in DMF (35 mL)
was added
potassium carbonate (11.0 g, 80.0 mmol) and 1,1,1-trifluoro-2-iodoethane (33.3
g, 160
mmol). The reaction mixture was heated I a sealed reactor at 50°C for 7
days. The mixture
was filtered and washed with ethyl acetate. The filtrate was added water and
the organic
phase isolated. The aqueous phase was extracted once more with ethyl acetated.
The com-
bined organic phases were dried (MgS04), filtered and concentrated in vacuo.
The residue
was purified by flash chromatography eluting with toluene to give 906 mg (18%)
of 3,5-bis-
(2,2,2-trifluoro-ethoxy)-benzaldehyde .'H NMR (CDCI3, 300 MHz) 8: 4.43 (q,
4H), 6.85 (t,
1 H), 7.15 (d, 2H), 9.95 (s, 1 H).
b)
The title compound was prepared from 3,5-bis-(2,2,2-trifluoro-ethoxy)-
benzaldehyde by a se-
quence analogous to that described in example 23.
'H NMR (CDCI3, 300 MHz) 8: 1.15 (t, 3H), 1.22 (t, 3H), 2.95 (d, 2H), 3.30-3.40
(m,1H), 3.55-
3.67 (m, 1 H), 3.97 (t, 1 H), 4.15 (q, 2H), 4.33 (q, 4H), 4.65 (d, 2H), 6.32-
6.48 (m, 2H), 6.55-
6.70 (m, 3H), 6.85 (d, 2H), 7.15 (d, 2H).
EXAMPLE 90
F
~F
F
O
(E)-(S)-3-(4-{3-[3,5-Bis-(2,2,2-trifluoro-ethoxy)-phenyl]-allyloxy}-phenyl)-2-
ethoxy-propionic
acid
CA 02395298 2002-06-18
WO 01/55085 PCT/DKO1/00058
123
The title compound was prepared from (E)-(S)-3-(4-{3-[3,5-bis-(2,2,2-trifluoro-
ethoxy)-phenyl]-
allyloxy)-phenyl)-2-ethoxy-propionic acid ethyl ester (example 89) (200 mg,
0.36 mmol) by a pro-
cedure analogous to that described in example 26.
H NMR (CDCI3, 300 MHz) 8: 1.20 (t, 3H), 2.97 (dd, 1 H), 3.10 (dd, 1 H), 3.41-
3.53 (m, 1 H),
3.55-3.68 (m, 1 H), 4.05 (dd, 1 H), 4.35 (q, 4H), 4.67 (d, 2H), 6.35-6.48 (m,
2H), 6.60-6.70 (m,
3H), 6.87 (d, 2H), 7.15 (d, 2H).
EXAMPLE 91
Et0 OEt
O
/ O ~ ~ O
(E'7-(S)-Ethyl 2-Ethoxy-3-{4-[3-(4-furan-2-yl-phenyl)-but-2-enyloxy]-phenyl}-
propionate
a)
Sodium (5.52 g, 0.24 mol) was added to ethanol (250 ml) at 20°C and the
mixture stirred until
the metal had fully reacted.. Triethyl phosphonoacetate (62.72 g, 0.28 mol)
was added as an
ethanol (50 ml) solution, the mixture stirred for 20 min, then a solution of 4-
iodoacetophenone (49.21 g, 0.20 mol) in ethanol (300 ml) was added and the
reaction mix-
ture heated to 80°C under reflux for 17h. The solution was cooled, the
ethanol evaporated.
and the resulting orange residue partitioned between 1 N HCI (200 ml) and
ethyl acetate (200
ml). The aqueous layer was collected and further extracted with ethyl acetate
(3 x 200 ml).
The organic layers were combined, washed with brine, dried (MgS04) and
evaporated to an
orange/yellow oil, which was purified by column chromatography on silica gel
(2% diethyl
ether in n-heptane eluent) to give the product, (E'7-ethyl 3-(4-iodophenyl)-
but-2-enoate, as a
pale yellow oil; 54.83 g (87%)
'H NMR (300 MHz, CDCI3) 8: 1.31 (3H, t), 2.53 (3H, s), 4.21 (2H, q), 6.11 (1H,
s), 7.20 (2H,
dm), 7.69 (2H, dm).'3CNMR (75MHz, CDCI3) 8: 13.0 (q), 16.4 (q), 58.6 (t), 93.7
(s), 116.2
(d), 126.7 (d), 136.3 (d), 140.3 (s), 152.8 (s), 165.2 (s). MS: 316 (M+), 287,
271, 244, 144,
115 (100%). Microanalysis Calculated % C: 45.59, H: 4.14. Found % C: 45.72, H:
4.20.
CA 02395298 2002-06-18
WO 01/55085 PCT/DKO1/00058
124
b)
Tetrakis(triphenylphoshine)palladium(0) (0.69 g, 0.60 mmol, 6 mol%) was added,
under ni-
trogen, to a stirred solution of (~-ethyl 3-(4-iodophenyl)-but-2-enoate (3.16
g, 10.0 mmol) in
DME (100 ml), and the resulting orange coloured solution stirred at room
temperature for 10
min. Aqueous 2M sodium carbonate (30.0 ml, 60.0 mmol) was then added, the
mixture
stirred for 10 min, then furan-2-boronic acid (2.25 g, 20.11 mmol) was added,
and the reac-
tion mixture heated to 80°C for 20 h, under reflux. The reaction
mixture was cooled, diluted
with water (100 ml) and the products extracted into ethyl acetate (3 x 100
ml). The combined
organic extracts were washed with brine, dried (MgS04), and evaporated to give
the crude
product, which was purified by column chromatography on silica gel (3% diethyl
ether in n-
heptane eluent) to give the product, (E~-ethyl 3-(4-furan-2-yl-phenyl)-but-2-
enoate as an off-
white solid; 2.46 g (96%).
Mpt. 85.5-56.5°C.'H NMR (300 MHz, CDCI3) s: 1.32 (3H, t), 2.59 (3H, d),
4.22 (2H, q), 6.18
(1 H, m), 6.49 (1 H, dd), 6.70 (1 H, d), 7.46-7.56 (3H, m), 7.66 (2H, dm). MS:
256 (100%,. M+),
227, 211, 184, 153, 115. Microanalysis Calculated % C: 74.98, H: 6.29. Found %
C: 74.99,
H: 6.39.
c)
(~-Ethyl 3-(4-furan-2-yl-phenyl)-but-2-enoate was reduced with DIBAL-H by a
procedure
analogous to that described in example 50b, to give the colourless solid (~-3-
(4-furan-2-yl-
phenyl)-but-2-en-1-ol.
d)
The title compound (678 mg, 77%) was prepared from (~-3-(4-furan-2-yl-phenyl)-
but-2-en-1-
0l (430 mg, 2.0 mmol) and (S)-ethyl 2-ethoxy-3-(4-hydroxyphenyl)-propionate
(526 mg, 2.21
mmol) by a procedure analogous to that described in example 52c.
'H NMR (300 MHz, CDCI3) 8: 1.17 (3H, t), 1.22 (3H, t), 2.14 (3H, d), 2.96 (2H,
d), 3.31-3.41
(1 H, m), 3.55-3.66 (1 H, m), 3.98 (1 H, t), 4.16 (2H, q), 4.73 (2H, d), 6.10
(1 H, tm), 6.47 (1 H,
dd), 6.64 (1 H, d), 6.88 (2H, dm), 7.17 (2H, dm), 7.43-7.48 (3H, m), 7.62 (2H,
dm). LCMS:
457 (M+Na), 452 (M+NH4), 197 (100%).
CA 02395298 2002-06-18
WO 01/55085 PCT/DKO1/00058
125
EXAMPLE 92
i
\ ~ / Et0 OEt
/ O ~ ~ O
(E7-(S)-Ethyl2-Ethoxy-3-{4-[3-(2'-methyl-biphenyl-4-yl)-but-2-enyloxy]-phenyl}-
propionate
a) .
The colourless solid, (E7-ethyl 3-(2'-methyl-biphenyl-4-yl)-but-2-enoate was
prepared from
(E7-ethyl 3-(4-iodophenyl)-but-2-enoate (example 91 a) and or~ho-tolyl boronic
acid by a pro-
cedure analogous to that described in example 91 b.
b)
The colourless oil (E7-3-(2'-methyl-biphenyl-4-yl)-but-2-en-1-of was prepared
by DIBAL-H re-
duction of (E'7-ethyl 3-(2'-methyl-biphenyl-4-yl)-but-2-enoate by a procedure
analogous to that
described in example 50b.
c)
The title compound (1.80 g, 78%) was prepared as a colourless oil from (E7-3-
(2'-methyl-
biphenyl-4-yl)-but-2-en-1-of (1.19 g, 4.99 mmol) and (S)-ethyl 2-ethoxy-3-(4-
hydroxyphenyl)-
propionate (1.31 g, 6.48 mmol) by a procedure analogous to that described in
example 52c.
'H NMR (300 MHz, CDCI3) 8: 1.17 (3H, t), 1.22 (3H, t), 2.17 (3H, d), 2.28 (3H,
s), 2.96 (2H,
d), 3.30-3.41 (1 H, m), 3.54-3.66 (1 H, m), 3.98 (1 H, t), 4.16 (2H, q), 4.74
(2H, d), 6.11 (1 H,
tm), 6.88 (2H, dm), 7.16. (2H, dm), 7.20-7.32 (6H, m), 7.47 (2H, dm). LCMS:
679 (M+221 ),
633 (679-EtOH), 481 (M+Na), 476 (M+NH4), 221 (100%).
CA 02395298 2002-06-18
WO 01/55085 PCT/DKO1/00058
126
EXAMPLE 93
/
\ I / Et0 OH
\ ~ / O / \ O
(E7-(S)-2-Ethoxy-3-{4-[3-(2'-methyl-biphenyl-4-yl)-but-2-enyloxy]-phenyl}-
propionic acid
The title compound was prepared by hydrolysis of (E)-(S)-ethyl 2-ethoxy-3-{4-
[3-(2'-methyl-
biphenyl-4-yl)-but-2-enyloxy]-phenyl}-propionate (example 92) (918 mg, 2.0
mmol) with so-
dium hydroxide by a procedure analogous to that described in example. 51,
yielding (E~-(S)-
2-ethoxy-3-{4-[3-(2'-methyl-biphenyl-4-yl)-but-2-enyloxy]-phenyl}-propionic
acid as a colour-
less gum, which contained 0.25 mol equivalents of ethyl acetate; 586 mg (64%).
'H NMR (300 MHz, CDCI3) 8: 1.17 (3H, t), 1.27 (0.75H, t, AcOEt), 2.04 (0.75H,
s, AcOEt),
2.19 (3H, d), 2.29 (3H, s), 2.96 (1 H, dd), 3.09 (1 H,. dd), 3.41-3.53 (1 H,
m), 3.53-3.65; (1 H, m),
' 4.06 (1 H, dd), 4.12 (0.5H, q, AcOEt), 4.75 (2H, d), 6.12 (1 H, tm), 6.89
(2H, dm), 7.16 (2H,
dm), 7.20-7.34 (6H, m), 7.48 (2H, dm), carboxylic acid proton not observed.
LCMS: 651
(M+221 ), 453 (M+Na), 221 (100%).
EXAMPLE 94
OMe
O
/ / ~ ~ ~OEt
OEt
OMe \ ~ / O \
(E7-(S)-Ethyl 3-{4-[3-(2',5'-Dimethoxy-biphenyl-4-yl)-but-2-enyloxy]-phenyl}-2-
ethoxy-
propionate
a)
CA 02395298 2002-06-18
WO 01/55085 PCT/DKO1/00058
127
The colourless oil, (E7-ethyl 3-(2',5'-dimethoxy-biphenyl-4-yl)-but-2-enoate
was prepared
from (E7-ethyl 3-(4-bromophenyl)-but-2-enoate (example 50a) and 2,5-
dimethoxyphenyl bo-
ronic acid by a procedure analogous to that described in example 52a.
b)
The colourless gum (~-3-(2',5'-dimethoxy-biphenyl-4-yl)-but-2-en-1-of was
prepared by DI-
BAL-H reduction of (E7-ethyl 3-(2',5'-dimethoxy-biphenyl-4-yl)-but-2-enoate by
a procedure
analogous to that described in example 52b.
1 o c)
The title compound (0.765 g, 61 %) was prepared as a colourless gum from (~-3-
(2',5'-
dimethoxy-biphenyl-4-yl)-but-2-en-1-of (0.711 g, 2.50 mmol) and (S)-ethyl 2-
ethoxy-3-(4-
hydroxyphenyl)-propionate (0.655 g, 2.75 mmol) by a procedure analogous to
that described
in example 52c.
'H NMR (300 MHz, CDCI3) 8: 1.17 (3H, t), 1.22 (3H, t), 2.16 (3H, d), 2.96 (2H;
d), 3:31-3.41
(1 H, m), 3.55-3.65 (1 H, m), 3.76 (3H, s), 3.81 (3H, s), 3.98 (1 H, t), 4.17
(2H, q), 4.74 (2H, d),
6.10 (1 H, tm), 6.81-6.95 (5H, m), 7.16 (2H, dm), 7.45-7.53 (4H, m). LCMS: 771
(M+267), 527
(M+Na), 422 (M+NH4), 267 (100%).
EXAMPLE 95
OMe
O
/ / ~ Y ~OH
OEt
OMe \ ~ / O \
(~-(S)-3-{4-[3-(2',5'-Dimethoxy-biphenyl-4-yl)-but-2-enyloxy]-phenyl}-2-ethoxy-
propionic acid
The title compound was prepared from (E7-(S)-ethyl 3-~4-[3-(2',5'-dimethoxy-
biphenyl-4-yl)-
but-2-enyloxy]-phenyl}-2-ethoxy-propionate (Example 94) (0.62 g, 1.23 mmol)
and sodium
hydroxide (1 M, 2.0 ml, 2.0 mmol) by a procedure analogous to that described
in example 51,
CA 02395298 2002-06-18
WO 01/55085 PCT/DKO1/00058
128
yielding (E~7-(S)-ethyl 3-~4-[3-(2',5'-dimethoxy-biphenyl-4-yl)-but-2-enyloxy]-
phenyl}-2-ethoxy-
propionic acid as a colourless glass; 0.485 g (83%).
' H NMR (300 MHz, CDCI3) 8: 1.18 (3H, t), 2.16 (3H, d), 2.96 (1 H, dd), 3.10
(1 H, dd), 3.42-
3.51 (1 H, m), 3.51-3.65 (1 H, m), 3.75 (3H, s), 3.80 (3H, s), 4.05 (1 H, dd),
4.74 (2H, d), 6.10
(1 H, tm), 6.81-6.94 (5H, m), 7.17 (2H, dm), 7.43-7.53 (4H, m), carboxylic
acid proton not ob-
served. LCMS: 743 (M+267), 499 (M+Na), 494 (M+NH4), 267 (100%).
EXAMPLE 96
O
Br / I ~ ~OEt
OEt
O \
(E7-(S)-Ethyl 3-{4-[3-(4-Bromophenyl)-2-ethyl-but-2-enyloxy]-phenyl}-2-ethoxy-
propionate
a)
A solution of triethyl 2-phosphonobutyrate (17.7 g, 70.0 mmol) in dry THF (30
ml) was added
dropwise, at 0°C, to a stirred suspension of sodium hydride (50%
dispersion in mineral oil,
2.90 g, 60.4 mmol) in dry THF (30 ml) and the mixture stirred for 30 min. A
solution of 4-
bromoacetophenone (7.96 g, 39.99 mmol) in THF (80 ml) was added over 20 min.,
the re-
suiting mixture warmed to room temperature and stirring continued overnight.
Second por-
tions of triethyl 2-phosphonobutyrate (10.11 g, 40.1 mmol) and sodium hydride
(2.90 g, 60.4
mmol) were then added at room temperature, and stirring continued for a
further 24 h; TLC at
this stage showed that a substantial amount of unreacted 4-bromo acetophenone
starting
material was still present. The reaction was worked up by adding 1 N HCI (200
ml) and ethyl
acetate (100m1), the organic layer collected and the aqueous layer extracted
with ethyl ace-
tate (3 x 100m1). The combined organic layers were washed with brine, dried
(MgS04) and
concentrated to give an orange gum, which was purified by column
chromatography on silica
gel (2% diethyl ether in n-heptane eluent) to give the orange oil, (E/Z)-ethyl
3-(4-
bromophenyl)-2-ethyl-but-2-enoate (3.47 g, 29%) as a mixture of double-bond
isomers.
b)
CA 02395298 2002-06-18
WO 01/55085 PCT/DKO1/00058
129
A toluene solution of DIBAL-H (1 M, 29.0 ml, 29.0 mmol) was added dropwise at -
70°C to a
stirred THF (100 ml) solution of (E/Z)-ethyl 3-(4-bromophenyl)-2-ethyl-but-2-
enoate (3.45 g,
11.6 mmol), and the solution stirred for 40 min. Methanol (1 ml) was carefully
added, fol-
lowed by 1 N HCI (300 ml) and ethyl acetate (200 ml). The aqueous layer was
separated and
further extracted with ethyl acetate (2 x 150 ml). The combined organic layers
were washed.
with brine, dried (MgS04), and concentrated to give an orange gum, which was
separated
into its two major constituents by column chromatography on silica gel (15%
ethyl acetate in
n-heptane eluent). The two products, in order of elution, were (Z)-3-(4-bromo-
phenyl)-2-ethyl-
but-2-en-1-of (0.365 g, 12%) and (~-3-(4-bromophenyl)-2-ethyl-but-2-en-1-of
(0.89 g,. 30%).
C)
The title compound (843 mg, 89%) was prepared from (E7-3-(4-bromophenyl)-2-
ethyl-but-2-
en-1-of (510 mg, 2.0 mmol) and (S)-ethyl 2-ethoxy-3-(4-hydroxyphenyl)-
propionate (500 mg,
2.10 mmol) by a procedure analogous to that described in example 52c.
'H NMR (300 MHz, CDCI3) 8: 0.94 (3H, t), 1.20 (3H, t), 1.23 (3H, t), 2.01 (3H,
s), 2.05 (2H, q),
2.97 .(2H, d), 3.31-3.43 (1 H, m), 3.54-3.68 (1 H,. m), 3.99 (1 H, t), 4.17
(2H, q), 4.61 (2H, s),
6.89 (2H, dm), 7.04 (2H, dm), 7.18 (2H, dm), 7.45 (2H, dm).
EXAMPLE 97
0
gr / ~ ~ 'OH
OEt
\ I / O \
(E7-(S)-3-{4-[3-(4-Bromophenyl)-2-ethyl-but-2-enyloxy]-phenyl}-2-ethoxy-
propionic acid
The title compound was prepared from (~-(S)-ethyl. 3-{4-[3-(4-bromophenyl)-2-
ethyl-but-2-
enyloxy]-phenyl}-2-ethoxy-propionate (Example 96) (0.78 g, 1.64 mmol) and
sodium hydrox-
ide (1M, 3.3 ml, 3.3 mmol) by a procedure analogous to that described in
example 51, yield-
ing (E7-(S)-3-{4-[3-(4-bromophenyl)-2-ethyl-but-2-enyloxy]-phenyl}-2-ethoxy-
propionic acid
CA 02395298 2002-06-18
WO 01/55085 PCT/DKO1/00058
130
(0.703 g, 96%) as a pale yellow oil, which contained a small amount of
dichloromethane;
0.703 g (96%).
'H NMR (300 MHz, CDCI3) 8: 1.19 (3H, t), 1.26 (ethyl acetate impurity, 0.6H,
t), 2.04 (ethyl
acetate impurity, 0.4H, s), 2.16 (3H, s), 2.96 (1 H, dd), 3.10 (1 H, dd), 3.42-
3.52 (1 H, m), 3.53-
3.68 (1 H, m), 3.80 (3H, s), 4.07 (1 H, dd), 4.12 (ethyl acetate impurity,
0.4H), 4.74 (2H, d),
5.30 (CHZCIz, trace), 6.10 (1 H, t), 6.85-6.95 (3H, m), 7.12-7.20 (2H, m),
7.21-7.32 (2H, m),
7.45-7.50 (4H, m), carboxylic acid proton not observed.
EXAMPLE 98
0
OEt
Br ~ I O ~ ~ OEt
(Z)-(S)-Ethyl 3-{4-[3-(4-Bromophenyl)-2-ethyl-but-2-enyloxy]-phenyl}-2-ethoxy-
propionate
The title compound (535 mg, 81 %) was prepared from (Z)-3-(4-bromophenyl)-2-
ethyl-but-2-
en-1-of (prepared as described in example 96b) (355 mg, 1.39 mmol) and (S)-
ethyl 2-ethoxy-
3-(4-hydroxyphenyl)-propionate (348 mg, 1.46 mmol) by a procedure analogous to
that de-
scribed in example 52c.
'H. NMR (300 MHz, CDCI3) 8: 1.11 (3H, t), 1.16 (3H, t), 1.21 (3H, t), 2.04
(3H, s), 2.37 (2H, q),
2.93 (2H, d), 3.29-3.40 (1 H, m), 3.53-3.65 (1 H, m), 3.95 (1 H, t), 4.16 (2H,
q), 4.25 (2H, s),
6.70 (2H, dm), 7.03-7.12 (4H, m), 7.40 (2H, dm). Microanalysis Calculated % C:
63.16, H:
6.57. Found % C: 63.34, H: 6.66.
EXAMPLE 99
0
OH
Br ~ ~ p
CA 02395298 2002-06-18
WO 01/55085 PCT/DKO1/00058
131
(Z)-(S)-3-{4-[3-(4-Bromophenyl)-2-ethyl-but-2-enyloxy]-phenyl}-2-ethoxy-
propionic acid
The title compound was prepared from (Z)-(S)-ethyl 3-{4-[3-(4-bromophenyl)-2-
ethyl-but-2-
enyloxy]-phenyl}-2-ethoxy-propionate (Example 98) (475 mg, 1.0 mmol) and
sodium hydrox-
ide (1 M, 2.0 ml, 2.0 mmol) by a procedure analogous to that described in
example 51, yield-
ing (Z)-(S)-3-{4-[3-(4-bromophenyl)-2-ethyl-but-2-enyloxy]-phenyl}-2-ethoxy-
propionic acid
(0.424 g, 95%) as a pale yellow oil.
'H NMR (300 MHz, CDCI3) 8: 1.11 (3H, t), 1.18 (3H, t), 2.04 (3H, s), 2.37 (2H,
q), 2.94 (1 H,
dd), 3.04 (1 H, dd), 3.40-3.53 (1 H, m), 3.53-3.64 (1 H, m), 4.03 (1 H, dd),
4.25 (2H, s), 6.71
(2H, dm), 7.02-7.14 (4H, m), 7.40 (2H, dm), carboxylic acid proton not
observed.
EXAMPLE 100
0
/ ~ I ~ ~OEt
O ~ OEt
~~ v
(E7-(S)-Ethyl 3-{4-[3-(4'-tart Butyl-biphenyl-4-yl)-but-2-enyloxy]-phenyl}-2-
ethoxy-propionate
a)
The colourless oil, (E7-ethyl 3-(4'-tart butyl-biphenyl-4-yl)-but-2-enoate was
prepared from
(E'7-ethyl 3-(4-bromophenyl)-but-2-enoate (example 50a) and 4-tart
butylphenylboronic acid
by a procedure analogous to that described in example 52a
b)
The colourless gum (E7-3-(4'-tart butyl-biphenyl-4-yl)-but-2-en-1-of was
prepared by DIBAL-H
reduction of (E7-ethyl 3-(4'-tart butyl-biphenyl-4-yl)-but-2-enoate by a
procedure analogous to
that described in example 52b.
c)
CA 02395298 2002-06-18
WO 01/55085 PCT/DKO1/00058
132
The title compound (0.375 g, 75%) was prepared as a colourless gum from (E)-3-
(4'-tert-
butyl-biphenyl-4-yl)-but-2-en-1-of (0.280 g, 1.00 mmol) and (S)-ethyl 2-ethoxy-
3-(4-
hydroxyphenyl)-propionate (0.250 g, 1.05 mmol) by a procedure analogous to
that described
in example 52c.
'H NMR (300 MHz, CDCI3) 8: 1.17 (3H, t), 1.22 (3H, t), 1.37 (9H, s), 2.17 (3H,
d), 2.97 (2H,
d), 3.30-3.43 (1 H, m), 3.53-3.67 (1 H, m), 3.98 (1 H, t), 4.17 (2H, q), 4.73
(2H, d), 6.11 (1 H,
tm), 6.88 (2H, dm), 7.17 (2H, dm), 7.43-7.60 (8H, m). LCMS: 763 (M+263), 523
(M+Na), 263
(100%).
EXAMPLE 101
CF3
O
/ ~ I ~ ~OEt
C
O ~ OEt
(E)-(S)-Ethyl3-{4-[3-(3',5'-Bis-trifluoromethyl-biphenyl-4-yl)-but-2-enyloxy)-
phenyl}-2-ethoxy-
propionate
a)
The colourless solid, (E7-ethyl 3-(3',5'-bis-trifluoromethyl-biphenyl-4-yl)-
but-2-enoate was .-
prepared from (E~-ethyl 3-(4-iodophenyl)-but-2-enoate (example 91 a) and 3,5-
bis-
(trifluoromethyl)phenyl boronic acid by a procedure analogous to that
described in example
91 b.
b)
The colourless solid (E)-3-(3',5'-bis-trifluoromethyl-biphenyl-4-yl)-but-2-en-
1-of was prepared
by DIBAL-H reduction of (E)-ethyl 3-(3',5'-bis-trifluoromethyl-biphenyl-4-yl)-
but-2-enoate by a
procedure analogous to that described in example 50b.
c)
CA 02395298 2002-06-18
WO 01/55085 PCT/DKO1/00058
133
The title compound (656 mg, 81 %) was prepared as a colourless oil from (E7-3-
(3',5'-bis-
trifluoromethyl-biphenyl-4-yl)-but-2-en-1-of (500 mg, 1.39 mmol) and (S)-ethyl
2-ethoxy-3-(4-
hydroxyphenyl)-propionate (348 mg, 1.46 mmol) by a procedure analogous to that
described
in example 52c.
'H NMR (300 MHz, CDCI3) 8: 1.17 (3H, t), 1.23 (3H, t), 2.18 (3H, d), 2.97 (2H,
d), 3.30-3.43
(1 H, m), 3.56-3.69 (1 H, m), 3.98 (1 H, t), 4.17 (2H, q), 4.75 (2H, d), 6.15
(1 H, tm), 6.89 (2H,
dm), 7.18 (2H, dm), 7.52-7.62 (4H, m), 7.85 (1 H, s), 8.01 (2H, s). LCMS: 603
(100%, M+Na),
598 (M+NH4), 343.
EXAMPLE 102
CF3
O
(\
/ / ~ ~ ~OH
F3C ~ /
O \ OEt
~/ v
(E~-(S)-3-~4-[3-(3',5'-Bis-trifluoromethyl-biphenyl-4-yl)-but-2-enyloxy]-
phenyl}-2-ethoxy-
propionic acid
The title compound was prepared from (E)-(S)-ethyl 3-{4-[3-(3',5'-bis-
trifluoromethyl-biphenyl-
4-yl)-but-2-enyloxy]-phenyl}-2-ethoxy-propionate (Example 101 ) (625 mg, 1.08
mmol) and
sodium hydroxide (1 M, 4.3 ml, 4.3 mmol) by a procedure analogous to that
described in ex-
ample 51, yielding (E)-(S)-3-{4-[3-(3',5'-bis-trifluoromethyl-biphenyl-4-yl)-
but-2-enyloxy]-
phenyl}-2-ethoxy-propionic acid (535 mg, 90%) as a colourless gum.
'H NMR (300 MHz, CDCI3) 8: 1.20 (3H, t), 2.18 (3H, d), 2.98 (1 H, dd), 3.10 (1
H, dd), 3.43-
3.53 (1 H, m), 3.53-3.66 (1 H, m), 4.07 (1 H, dd), 4.76 (2H, d), 6.15 (1 H,
tm), 6.90 (2H, dm),
7.19 (2H, dm), 7.50-7.62 (4H, m), 7.85 (1 H, s), 8.01 (2H, s), carboxylic acid
proton not ob-
served.
CA 02395298 2002-06-18
WO 01/55085 PCT/DKO1/00058
134
EXAMPLE 103
0
~ ( ~ ~OEt
O ~ OEt
(E')-(S)-Ethyl2-Ethoxy-3-f4-[3-(4'-isopropyl-biphenyl-4-yl)-but-2-enyloxy]-
phenyl}-propionate
a)
The colourless solid, (E7-ethyl 3-(4'-isopropyl-biphenyl-4-yl)-but-2-enoate
was prepared from
(E)-ethyl 3-(4-bromophenyl)-but-2-enoate (example 50a) and 4-isopropylphenyl
boronic acid
by a procedure analogous to that described in example 52a.
Mpt. 96.5-97.5°C.'H NMR (300 MHz, CDCI3) 8: 1.29 (6H, d), 1.32 (3H, t),
2.61 (3H, d), 2.97
(1 H, septet), 4.22 (2H, q), 6.20 (1 H, m), 7.32 (2H, dm), 7.50-7.65 (6H, m).
MS: 308 (100%,
M+), 293, 178. Microanalysis Calculated % C: 81.78, H: 7.84. Found % C: 81.96,
H: 8.22.
b)
The colourless solid (E7-3-(4'-isopropyl-biphenyl-4-yl)-but-2-en-1-of was
prepared by DIBAL-
H reduction of (~-ethyl 3-(4'-isopropyl-biphenyl-4-yl)-but-2-enoate by a
procedure analogous
to that described in example 50b.
Mpt. 110.5-112.5°C.'H NMR (300 MHz, CDCI3) 8: 1.29 (6H, d), 2.10 (3H,
s), 2.94 (1H, sep
tet), 4.37 (2H, d), 6.03 (1 H, t), 7.29 (2H, dm), 7.40-7.60 (6H, m). MS: 266
(M+), 251 (M-Me),
223 (100%, M-i-Pr). Microanalysis Calculated % C: 85.67, H: 8.32. Found % C:
85.55, H:
8.55.
c)
The title compound (410 mg, 84%) was prepared as a colourless solid from (~-3-
(4'-
isopropyl-biphenyl-4-yl)-but-2-en-1-of (266 mg, 1.00 mmol) and (S)-ethyl 2-
ethoxy-3-(4-
hydroxyphenyl)-propionate (250 mg, 1.05 mmol) by a procedure analogous to that
described
in example 52c.
Mpt. 70-73°C.'H NMR (300 MHz, CDCI3) 8: 1.17 (3H, t), 1.22 (3H, t),
1.29 (6H, d), 2.17 (3H,
3o d), 2.89-3.01 (3H, m), 3.30-3.41 (1 H, m), 3.55-3.66 (1 H, m), 3.98 (1 H,
t), 4.17 (2H, q), 4.74
CA 02395298 2002-06-18
WO 01/55085 PCT/DKO1/00058
135
(2H, d), 6.11 (1 H, tm), 6.88 (2H, dm), 7.17 (2H, dm), 7.30 (2H, dm), 7.46-
7.57 (6H, m).
LCMS: 735 (M+249), 509 (M+Na), 249 (100%).
EXAMPLE 104
0
/ / I ~ ~OH
O ~ OEt
~/ v
(E)-(S)-2-Ethoxy-3-{4-[3-(4'-isopropyl-biphenyl-4-yl)-but-2-enyloxy]-phenyl}-
propionic acid
The title compound was prepared from (~-(S)-ethyl 2-ethoxy-3-(4-[3-(4'-
isopropyl-biphenyl-
4-yl)-but-2-enyloxy]-phenyl}-propionate (Example 103) (400 mg, 0.822 mmol) and
sodium
hydroxide (1M, 3.29 ml, 3.29 mmol) by a procedure analogous to that
described.in example
51, yielding (E~-(S)-2-ethoxy-3-{4-[3-(4'-isopropyl-biphenyl-4-yl)-but-2-
enyloxy]-phenyl}-
propionic acid (380 mg, 100%) as a beige coloured solid.
'H NMR (300 MHz, CDCI3) 8: 1.19 (3H, t), 1.29 (6H, d), 2.17 (3H, d), 2.89-3.01
(2H; m), 3.10
(1 H, dd), 3.42-3.64 (2H, m), 4.06 (1 H, dd), 4.74 (2H, d), 6.11 (1 H, tm),
6.90 (2H, dm), 7.16
(2H, dm), 7.30 (2H, dm), 7.46-7.57 (6H, m), carboxylic acid proton not
observed. LCMS: 707
(M+249), 481 (M+Na), 249 (100%).
CA 02395298 2002-06-18
WO 01/55085 PCT/DKO1/00058
136
EXAMPLE 105
, (E)-(S)- 3-{4-[3-(3,5-Dimethoxy-phenyl)-but-2-enyloxy]-phenyl}-2-ethoxy-
propionic acid ethyl
ester
The title compound was prepared from 3',5'-dimethoxyacetophenone (7.0 g,
0.0388 mol) by
a sequence analogous to that described in example 3, yielding 0.165 g (35%) of
(E'-(S)- 3-
, {4-[3-(3,5-Dimethoxy-phenyl)-but-2-enyloxy]-phenyl}-2-ethoxy-propionic acid
ethyl ester.
'H NMR (200 MHz, CDCI3) b: 1.1-1.27 (6H, m), 2.97 (2H, d), 3.3-3.4 (1 H, m),
3.52-3.7 (1 H,
m), 4.0 (1 H, t), 4.15 (2H, q), 4.7 (2H, d), ), 6.39 (1 H, dd), 6.57 (2H, dd),
6.88 (2H, d), 7.17
(2H, d)..
EXAMPLE 106
0
\
/ / ~ ~ ~OH
a /
OEt
O \ ~ / O \
(~-(S)-3-{4-[3-(3'-Acetyl-biphenyl-4-yl)-but-2-enyloxy]-phenyl}-2-ethoxy-
propionic acid
CA 02395298 2002-06-18
WO 01/55085 PCT/DKO1/00058
137
The title compound was prepared from (~-(S)-ethyl 3-{4-[3-(3'-acetyl-biphenyl-
4-yl)-but-2-
enyloxy]-phenyl}-2-ethoxy-propionate (Example 105) (140 mg, 0.288 mmol) and
sodium hy-
droxide (1 M, 0.58 ml, 0.58 mmol) by a procedure analogous to that described
in example 51,
yielding (~-(S)-3-{4-[3-(3'-acetyl-biphenyl-4-yl)-but-2-enyloxy]-phenyl}-2-
ethoxy-propionic
acid (33 mg, 25%) as a yellow coloured solid.
' H NMR (300 MHz, CDCI3) 8: 1.19 (3H, t), 2.18 (3H, d), 2.66 (3H, s), 2.97 (1
H, dd), 3.10 (1 H,
dd), 3.41-3.65 (2H, m), 4.07 (1 H, dd), 4.75 (2H, d), 6.13 (1 H, tm), 6.90
(2H, dm), 7.17 (2H,
dm), 7.49-7.63 (5H, m), 7.80 (1 H, dm), 7.93 (1 H, dm), 8.19 (1 H, m),
carboxylic acid proton
not observed. LCMS: 707 (M+249), 481 (M+Na), 249 (100%).
EXAMPLE 107
(~-(S)-Ethyl 3-{4-[3-(4'-Acetyl-biphenyl-4-yl)-but-2-enyloxy]-phenyl}-2-ethoxy-
propionate
a)
(~-3-(4-lodophenyl)-but-2-en-1-of was prepared by DIBAL-H reduction of (~-
ethyl 3-(4-
iodophenyl)-but-2-enoate (example 91a) by a procedure analogous to that
described in ex-
ample 50b.
'H NMR (300 MHz, CDCI3) 8: 1.36 (1 H, br s), 2.04 (3H, d), 2.66 (3H, s), 4.36
(2H, br d), 5.96
(1 H, tm), 7.15 (2H, dm), 7.65 (2H, dm).
,b~
The pale yellow solid, (E)-1-[4'-(3-hydroxy-1-methyl-propenyl)-biphenyl-4-yl]-
ethanone was
prepared from 4-acetylphenyl boronic acid and (~-3-(4-iodophenyl)-but-2-en-1-
of by a pro-
cedure analogous to that described in example 54a.
'H NMR (300 MHz, CDCI3) 8: 1.55 (1 H, br s), 2.12 (3H, d), 2.64 (3H, s), 4.41
(2H, d), 6.07
(1 H, tm), 7.52 (2H, dm), 7.61 (2H, dm), 7.70 (2H, dm), 8.03 (2H, dm).
CA 02395298 2002-06-18
WO 01/55085 PCT/DKO1/00058
138
C)
The title compound (275 mg, 75%) was prepared from (~-1-[4'-(3-hydroxy-1-
methyl-
propenyl)-biphenyl-4-yl]-ethanone (200 mg, 0.75 mmol) and (S)-ethyl 2-ethoxy-3-
(4-
hydroxyphenyl)-propionate (188 mg, 0.79 mmol) by a procedure analogous to that
described
in example 52c.
'H NMR (300 MHz, CDCI3) 8: 1.17 (3H, t), 1.23 (3H, t), 2.18 (3H, d), 2.64 (3H,
s), 2.97 (2H,
d), 3.30-3.42 (1 H, m), 3.55-3.67 (1 H, m), 3.98 (1 H, t), 4.17 (2H, q), 4.75
(2H, d), 6.14 (1 H,
tm), 6.89 (2H, dm), 7.17 (2H, dm), 7.54 (2H, dm), 7.61 (2H, dm), 7.70 (2H,
dm), 8.03 (2H,
dm).
EXAMPLE 108
0
0
I\
/ / ~ ~ ~OH
O \ ( OEt
(~-(S)-3-{4-[3-(4'-Acetyl-biphenyl-4-yl)-but-2-enyloxy]-phenyl}-2-ethoxy-
propionic acid
The title compound was prepared from (~-(S)-ethyl 3-{4-[3-(4'-acetyl-biphenyl-
4-yl)-but-2-
enyloxy]-phenyl}-2-ethoxy-propionate (Example 107) (200 mg, 0.411 mmol) and
sodium hy-
droxide (1 M, 1.64 ml, 1.64 mmol) by a procedure analogous to that described
in example 51,
yielding (~-(S)-3-{4-[3-(4'-acetyl-biphenyl-4-yl)-but-2-enyloxy]-phenyl}-2-
ethoxy-propionic
acid (70 mg, 37%) as a yellow coloured solid.
'H NMR (300 MHz, CDCI3) 8: 1.19 (3H, t), 2.18 (3H, d), 2.64 (3H, s), 2.97 (1
H, dd), 3.11 (1 H,
dd), 3.45-3.65 (2H, m), 4.08 (1 H, dd), 4.75 (2H, d), 6.14 (1 H, tm), 6.90
(2H, dm), 7.17 (2H,
dm), 7.54 (2H, dm), 7.61 (2H, dm), 7.70 (2H, dm), 8.03 (2H, dm), carboxylic
acid proton not
observed. LCMS: 707 (M+249), 459 (M+H), 249 (100%).
CA 02395298 2002-06-18
WO 01/55085 PCT/DKO1/00058
139
EXAMPLE 109
/ o
/, / ~ ~ ~OEt
/ O ~ OEt
a a a
(E)-(S)-Ethyl2-Ethoxy-3-[4-(3-[1,1';3',1"]terphenyl-5'-yl-allyloxy)-phenyl]-
propionate
a)
Sodium (0.90 g, 39.1 mmol) was added to ethanol (50 ml) at 20°C and the
mixture stirred
until the metal had fully reacted. Triethyl phosphonoacetate (10.1 g, 45 mmol)
was added,
the mixture stirred for 10 min, then a solution of 3,5-dibromobenzaldehyde
(7.92 g, 30 mmol) ,
in ethanol (50 ml) was added and the reaction mixture heated to 80°C
under reflux~for 72h.
The solution was cooled, the ethanol evaporated and the resulting yellow
residue partitioned
between 1 N HCI (100 ml) and ethyl acetate (100 ml). The aqueous layer was
collected and
further extracted. with ethyl acetate (3 x 100 ml). The organic layers were
combined, washed
with brine, dried (MgS04) and evaporated to a yellow solid, which was purified
by column
chromatography on silica gel (2% diethyl ether in n-heptane eluent) to give
the product, (E)-
ethyl 3-(3,5-dibromophenyl)-acrylate, as a colourless solid; 4.54 g (45%).
Mpt. 80-82°C. ' H NMR (300 MHz, CDCI3) s: 1.33 (3H, t), 4.27 (2H, q),
6.42 (1 H, d), 7.51 (1 H,
d), 7.58 (2H, d), 7.66 (1H, t). MS: 336/334/332 (M+), 308/306/304, 291/289/287
(100%, M-
Oet), 180/182.
b)
The colourless solid, (~-ethyl 3-[1,1';3',1"]terphenyl-5'-yl-acrylate was
prepared from (-
ethyl 3-(3,5-dibromophenyl)-acrylate and phenylboronic acid by a procedure
analogous to
that described in example 52a.
Mpt. 78.5-81.5°C.'H NMR (300 MHz, CDCI3) 8: 1.36 (3H, t), 4.29 (2H, q),
6.58 (1H, d), 7.33-
7.54 (6H, m), 7.60-7.68 (4H, m), 7.72 (2H, d), 7.81 (1 H, t), 7.82 (1 H, d).
MS: 328 (100%, M+),
283, 256, 252, 241, 239.
CA 02395298 2002-06-18
WO 01/55085 PCT/DKO1/00058
140
C)
The colourless solid (~-3-[1,1';3',1"]terphenyl-5'-yl-prop-2-en-1-of was
prepared by DIBAL-H
reduction of (~-ethyl 3-[1,1';3',1"]terphenyl-5'-yl-acrylate by a procedure
analogous to that
described for example 52b.
Mpt. 140-141.5°C.'H NMR (300 MHz, CDCI3) 8: 1.54 (1 H, br s), 4.38 (2H,
d), 6.50 (1 H, dt),
6.75 (1 H, d), 7.30-7.52 (6H, m), 7.53-7.73 (7H, m). MS: 286 (M+), 258 (100%),
243, 230, 165,
91, 77.
d)
The title compound (426 mg, 80%) was prepared from (~-3-[1,1';3',1"]terphenyl-
5'-yl-prop-2
en-1-of (300 mg, 1.05 mmol) and (S)-ethyl 2-ethoxy-3-(4-hydroxyphenyl)-
propionate-(262
mg, 1.10 mmol) by a procedure analogous to that described in example 52c.
'H NMR (300 MHz, CDCI3) b: 1.16 (3H, t), 1.22 (3H, t), 2.96 (2H, d), 3.29-3.41
(1 H, m), 3.54-
3.66 (1 H, m), 3.98 (1 H, t), 4.16 (2H, q), 4.73 (2H, d), 6.54 (1 H, dt), 6.85
(1 H, d), 6.90 (2H,
dm), 7.17 (2H, dm), 7.30-7.50 (6H, m), 7.55-7.71 (7H, m). LCMS: 775 (M+269),
729 (100%,
M+269-EtOH), 461 (M+H-EtOH), 269.
EXAMPLE 110
/ O
/ / I ~ ~OH
/ O ~ OEt
(~-(S)-2-Ethoxy-3-[4-(3-[1,1';3',1 "]terphenyl-5'-yl-allyloxy)-phenyl]-
propionic acid
The title compound was prepared from (E~-(S)-ethyl 2-ethoxy-3-[4-(3-
[1,1';3',1"]terphenyl-5'
yl-allyloxy)-phenyl]-propionate (Example 109) (405 mg, 0.8 mmol) and sodium
hydroxide
(1 M, 1.6 ml, 1.6 mmol) by a procedure analogous. to that described in example
51, yielding
(~-(S)-2-ethoxy-3-[4-(3-[1,1';3',1 "]terphenyl-5'-yl-allyloxy)-phenyl]-
propionic acid (352 mg,
92%) as a colourless glass.
CA 02395298 2002-06-18
WO 01/55085 PCT/DKO1/00058
141
' H NMR (300 MHz, CDC13) b: 1.18 (3H, t), 2.97 (1 H, dd), 3.09 (1 H, dd), 3.41-
3.53 (1 H, m),
3.53-3.65 (1 H, m), 4.07 (1 H, dd), 4.73 (2H, dd), 6.54 (1 H, dt), 6.85 (1 H,
dm), 6.92 (2H, dm),
7.17 (2H, dm), 7.32-7.50 (6H, m), 7.55-7.71 (7H, m), carboxylic acid proton
not observed.
LCMS: 747 (M+269), 501 (M+Na), 496 (M+NH4), 269 (100%).
EXAMPLE 111
/ o
OEt
Et0 ~ ~ ~ / O ~ OEt
O
(E,E7-(S)-Ethyl 3-(4'-{3-[4-(2-Ethoxy-2-ethoxycarbonyl-ethyl)-phenoxy]-1-
methyl-propenyl}-
biphenyl-3-yl)-but-2-enoate
a)
The yellow oil (E,E7-ethyl 3-[4'-(3-hydroxy-1-methyl-propenyl)-biphenyl-3-yl]-
but-2-enoate
was prepared from (E7-1-[4'-(3-hydroxy-1-methyl-propenyl)-biphenyl-3-yl]-
ethanone (example
105a) and triethyl phosphonoacetate by a reaction analogous to that described
for example
50a.
'H NMR (300 MHz, CDCI3) s: 1.33 (3H, t), 1.37 (1 H, br t), 2.13 (3H, d), 2.62
(3H, d), 4.23
(2H, q), 4.41 (2H, br t), 6.06 (1 H, tm), 6.19 (1 H, m), 7.40-7.62 (7H, m),
7.68 (1 H, m). MS: 336
(M+), 334, 308, 293, 43 (100%).
b)
The title compound (230 mg, 58%) was prepared from (E,E7-ethyl 3-[4'-(3-
hydroxy-1-methyl-
propenyl)-biphenyl-3-yl]-but-2-enoate (235 mg, 0.70 mmol) and (S)-ethyl 2-
ethoxy-3-(4-
hydroxyphenyl)-propionate (175 mg, 0.73 mmol) by a procedure analogous to that
described
in example 52c.
'H NMR (300 MHz, CDCI3) 8: 1.17 (3H, t), 1.23 (3H, t), 1.33 (3H, t), 2.17 (3H,
d), 2.62 (3H,
d), 2.96 (2H, d), 3.30-3.42 (1 H, m), 3.55-3.67 (1 H, m), 3.98 (1 H, t), 4.17.
(2H, q), 4.23 (2H,. q),
CA 02395298 2002-06-18
WO 01/55085 PCT/DKO1/00058
142
4.75 (2H, d), 6.13 (1 H, tm), 6.20 (1 H, m), 6.89 (2H, dm), 7.17 (2H, dm),
7.42-7.62 (7H, m),
7.67 (1 H, m). MS: 556 (M+), 319 (100%).
EXAMPLE 112
o
off
i
HO I ~ I / O ~ OEt
i
O
(E,E)-(S)- 3-(4'-{3-[4-(2-Carboxy-2-ethoxy-ethyl)-phenoxy]-1-methyl-propenyl}-
biphenyl-3-yl)-
but-2-enoic acid
The title compound was prepared from (E,E)-(S)-ethyl 3-(4'-~3-[4-(2-ethoxy-2-
ethoxycarbonyl-ethyl)-phenoxy]-1-methyl-propenyl}-biphenyl-3-yl)-but-2-enoate
(example
111 ) (190 mg, 0.34 mmol). and sodium hydroxide (1 M, 1.4 ml, 1.4 mmol) by a
procedure
analogous to that described in example 51, yielding (E,E)-(S)- 3-(4'-{3-[4-(2-
carboxy-2-
ethoxy-ethyl)-phenoxy]-1-methyl-propenyl}-biphenyl-3-yl)-but-2-enoic acid (135
mg~.79%) as
a colourless solid.
'H NMR (300 MHz, CDCI3) 8: 1.19 (3H, t), 2.18 (3H, m), 2.64 (3H, s), 3.01 (1
H, dd), 3.08 (1 H,
dd), 3.40-3.70 (2H, m), 4.07 (1 H, dd), 4.75 (2H, dd), 6.12 (1 H, br m), 6.23
(1 H, s), 6.89 (2H,
dm), 7.18 (2H, dm), 7.40-7.70 (8H, m), carboxylic acid protons not observed.
EXAMPLE 113
O
.i ' oEt
o w oEt
~/ v
CA 02395298 2002-06-18
WO 01/55085 PCT/DKO1/00058
143
(E7-(S)-Ethyl 2-Ethoxy-3-{4-[3-(3'-methoxy-biphenyl-4-yl)-but-2-enyloxy]-
phenyl}-propionate
a)
The colourless oil (E7-ethyl 3-(3'-methoxy-biphenyl-4-yl)-but-2-enoate was
prepared from (E'7
ethyl 3-(4-bromophenyl)-but-2-enoate (example 50a) and 3-methoxyphenyl boronic
acid by a
procedure analogous to that described in example 52a.
'H NMR (300 MHz, CDCI3) 8: 1.33 (3H, t), 2.61 (3H, d), 3.87 (3H, s), 4.23 (2H,
q), 6.20 (1H,
m), 6.91 (1 H, ddd), 7.13 (1 H, dd), 7.19 (1 H, ddd), 7.37 (1 H, dd), 7.51-
7.62 (4H, m). MS: 296
(100%, M+), 281, 267, 251, 224. Microanalysis Calculated % C: 77.00, H: 6.80.
Found % C:
77.02, H: 6.93.
b).
The colourless solid (E7-3-(3'-methoxy-biphenyl-4-yl)-but-2-en-1-of was
prepared by DIBAL-H
reduction of (E~-ethyl 3-(3'-methoxy-biphenyl-4-yl)-but-2-enoate as described
for example
52b.
Mpt. 62-68°C.'H NMR (300 MHz, CDCI3) s: 1.40 (1H, br s), 2.12 (3H, d),
3.87 (3H, s), 4.39
(2H, d), 6.05 (1 H, tm), 6.89 (1 H, ddd), 7.13 (1 H, dd), 7.19 (1 H, ddd),
7.35 (1 H, dd), 7.49 (2H,
dm), 7.56 (2H, dm). MS: 254 (M+), 239, 211 (100%).
2o c)
The title compound (280 mg, 59%) was prepared as a colourless solid from (E)-3-
(3'-
methoxy-biphenyl-4-yl)-but-2-en-1-of (254 mg, 1.00 mmol) and (S)-ethyl 2-
ethoxy-3-(4-
hydroxyphenyl)-propionate (250 mg, 1.05 mmol) by a procedure analogous to that
described
in example 52c.
'H NMR (300 MHz, CDCI3) 8: 1.17 (3H, t), 1.22 (3H, t), 2.17 (3H, d), 2.96 (2H,
d), 3.30-3.42
(1 H, m), 3.55-3.67 (1 H, m), 3.87 (3H, s), 3.98 (1 H, t), 4.17 (2H, q), 4.74
(2H, d), 6.12 (1 H,
tm), 6.85-6.92 (3H, m), 7.11-7.22 (4H, m), 7.35 (1 H, dd), 7.47-7.59 (4H, m).
MS: 474 (M+),
237 (100%). Microanalysis Calculated % C: 75.92,. H: 7.22. Found % C: 76.04,
H: 7.39.
CA 02395298 2002-06-18
WO 01/55085 PCT/DKO1/00058
144
EXAMPLE 114
0
off
i
O ~ OEt
~/ a
(E~-(S)-2-Ethoxy-3-{4-[3-(3'-methoxy-biphenyl-4-yl)-but-2-enyloxy]-phenyl}-
propionic acid
The title compound was prepared from (~-(S)-ethyl 2-ethoxy-3-{4-[3-(3'-methoxy-
biphenyl-4- -
yl)-but-2-enyloxy]-phenyl)-propionate (example 113) (230 mg, 0.49 mmol) and
sodium hy-
droxide (1M, 0.97 ml, 0.97 mmol) by a procedure analogous to that described in
example 51,.
yielding (~-(S)-2-ethoxy-3-{4-[3-(3'-methoxy-biphenyl-4-yl)-but-2-enyloxy]-
phenyl}-propionic
acid (183 mg, 85%) as a colourless solid.
' H NMR (300 MHz, CDCI3) 8: 1.18 (3H, t), 2.17 (3H, d), 2.97 (1 H, dd), 3.10
(1 H, dd); 3.41-
3.53 (1 H, m), 3.53-3.65 (1 H, m), 3.87 (3H, s), 4.07 (1 H, dd), 4.74 (2H,
dd), 6.11 (1 H, tm),
6.86-6.93 (3H, m), 7.11-7.22 (4H, m), 7.35 (1 H, dd), 7:47-7.59 (4H, m),
carboxylic acid pro-
ton not observed. LCMS: 683 (M+237), 469 (M+Na), 237 (100%).
EXAMPLE 115
o
OEt
/ I
OEt
OH ~ ~ / O
(E)-(S, S/R)-Ethyl 2-Ethoxy-3-(4-{3-[3'-(1-hydroxy-ethyl)-biphenyl-4-yl]-but-2-
enyloxy}-phenyl)-
propionate
CA 02395298 2002-06-18
WO 01/55085 PCT/DKO1/00058
145
a)
The colourless solid (E)-(S/R)-3-[3'-(1-hydroxy-ethyl)-biphenyl-4-yl]-but-2-en-
1-of was pre-
pared by DIBAL-H reduction of (~-1-[4'-(3-hydroxy-1-methyl-propenyl)-biphenyl-
3-yl]-
ethanone (example 105a) by a procedure analogous to that described in example
52b.
Mpt. 94-100°C.'H NMR (300 MHz, DMSO-ds) 8: 1.37 (3H, d), 2.02 (3H, d),
4.18 (2H, br dd),
4.75 (1 H, br t, OH), 4.78 (1 H, dq), 5.21 (1 H, d, OH), 5.98 (1 H, tm), 7.33
(1 H, dm), 7.40 (1 H,
dd), 7.48-7.54 (3H, m), 7.60-7.66 (3H, m). MS: 268 (100%, M+), 253, 235, 225.
Microanalysis
Calculated % C: 80.56, H: 7.51. Found % C: 80.21, H: 7.78.
b)
The title compound (490 mg, 57%) was prepared as a colourless oil from (~-
(S/R)-3-[3'-(1-
hydroxy-ethyl)-biphenyl-4-yl]-but-2-en-1-of (500 mg, 1.86 mmol) and (S)-ethyl
2-ethoxy-3-(4-
hydroxyphenyl)-propionate (422.mg, 1.77 mmol) by a procedure analogous to that
described
in example 52c.
'H NMR (300 MHz, CDCI3) 8: 1.17 (3H, t), 1.22 (3H, t), 1.56 (3H, d), 1.89 (1H,
d, OH), 2.18
(3H, d), 2.97 (2H, d), 3.30-3.42 (1 H, m), 3.54-3.66 (1 H, m), 3.98 (1 H, t),
4.17 (2H, q), 4.75
(2H, d), 4.99 (1 H, dq), 6.12 (1 H, tm), 6.85 (2H, dm), 7.18 (2H, dm), 7.32-
7.48 (2H, m), 7.48-
7.67 (6H, m).
EXAMPLE 116
0
off
i
OEt
OH ~ ~ / O
(~-(S,S/R)-2-Ethoxy-3-(4-{3-[3'-(1-hydroxy-ethyl)-biphenyl-4-yl]-but-2-
enyloxy}-phenyl)-
propionic acid
The title compound was prepared from (~-(S,S/R)-ethyl 2-ethoxy-3-(4-~3-[3'-(1-
hydroxy-
ethyl)-biphenyl-4-yl]-but-2-enyloxy}-phenyl)-propionate (example 115) (460 mg,
0.94 mmol)
and sodium hydroxide (1 M, 1.9 ml, 1.9 mmol) by a procedure analogous to that
described in
CA 02395298 2002-06-18
WO 01/55085 PCT/DKO1/00058
146
example 51, yielding (~-(S,S/R)-ethyl 2-ethoxy-3-(4-{3-[3'-(1-hydroxy-ethyl)-
biphenyl-4-yl]-
but-2-enyloxy}-phenyl)-propionic acid (434 mg, 100%) as a colourless gum.
'H NMR (300 MHz, CDCI3) 8: 1.18 (3H, t), 1.55 (3H, d), 2.17 (3H, d), 2.96 (1
H, dd), 3.09 (1 H,
dd), 3.41-3.53 (1 H, m), 3.54-3.66 (1 H, m), 4.06 (1 H, dd), 4.75 (2H, d),
4.98 (1 H, q), 6.12 (1 H,
tm), 6.90 (2H, dm), 7.17 (2H, dm), 7.32-7.46 (2H, m), 7.47-7.63 (6H, m),
carboxylic acid pro-
ton not observed.
EXAMPLE 117
O
Br
/ / ~ ~ 'OEt
OEt
Br \ / O
(~-(S)-Ethyl 3-{4-[3-(3,5-Dibromophenyl)-but-2-enyloxy]-phenyl}-2-ethoxy-
propionate
a)
Sodium (0.49 g, 21.3 mmol) was added to ethanol (50 ml) at room temperature
and the mix-
ture stirred. until the metal had fully reacted. Triethyl phosphonoacetate
(5.49. g, 24.5 mmol)
was added, the solution stirred for 15 min, then an ethanol (100 ml) solution
of 3,5-
dibromoacetophenone (4.60 g, 16.6 mol) was added and the reaction mixture
heated to 80°C
under reflux for 72 h. The solution was cooled, the ethanol evaporated and the
resulting or-
ange residue partitioned between 1 N HCI (150 ml) and ethyl acetate (150 ml).
The aqueous .
layer was collected and further extracted with ethyl acetate (2 x 100 ml). The
organic layers
were combined, washed with brine, dried (MgS04) and evaporated to an
orange/yellow gum,
which was purified by column chromatography on silica gel (3% diethyl ether in
n-heptane
eluent) to give the product, (E7-ethyl 3-(3,5-dibromophenyl)-but-2-enoate, as
a colourless
wax; 4.06 g (70%).
'H NMR (300 MHz, CDCI3) 8: 1.32 (3H, t), 2.51 (3H, d), 4.22 (2H, q), 6.09 (1
H, m), 7.52 (2H,
d), 7.64 (1H, t). MS: 350/348/346 (M+), 304/302/300 (M-EtOH), 115 (100%).
Microanalysis
Calculated % C: 41.41, H: 3.48, Br: 45.92. Found % C: 41.75, H: 3.52, Br:
45.62.
CA 02395298 2002-06-18
WO 01/55085 PCT/DKO1/00058
147
b)
(E~-Ethyl 3-(3,5-dibromophenyl)-but-2-enoate was reduced with DIBAL-H by a
procedure
analogous to that described in example 50b, to give the colourless oil (~-3-
(3,5-dibromo-
phenyl)-but-2-en-1-ol.
~H NMR (300 MHz, CDCI3) S: 1.64 (1 H, br s), 2.01 (3H, d), 4.36 (2H, d), 5.96
(1 H, tm), 7.46
(2H, d), 7.54 (1H, t). MS: 308/306/304 (M+), 293/291/289 (M-Me), 266/264/262,
227/225/223,
131, 128, 115 (100%), 102.
c)
The title compound (851 mg, 81 %) was prepared from (E7-3-(3,5-dibromo-phenyl)-
but-2-en-
1-0l (612 mg, 2.0 mmol) and (S)-ethyl 2-ethoxy-3-(4-hydroxyphenyl)-propionate
(500 mg,
2.10. mmol) by a procedure analogous to that described in example 52c.
'H NMR (300 MHz, CDCI3) 8: 1.17 (3H, t), 1.23 (3H, t), 2.09 (3H, d), 2.96 (2H,
d), 3.30-3.42
(1 H, m), 3.54-3.66 (1 H, m), 3.98 (1 H, t), 4.17 (2H, q), 4.69 (2H, d), 6.04
(1 H, tm), 6.85 (2H,
dm), 7.16 (2H, dm), 7.48 (2H, d), 7.57 (1 H, t). LCMS: 551/549/547 (100%,
M+Na),
546/544/542 (M+NH4), 483/481/479 (M+H-EtOH).
EXAMPLE 118
O
Br
~OH
O ~ OEt
Br v
(E'7-(S)-3-{4-[3-(3,5-Dibromophenyl)-but-2-enyloxy]-phenyl}-2-ethoxy-propionic
acid
The title compound was prepared from (E7-(S)-ethyl 3-{4-[3-(3,5-dibromophenyl)-
but-2-
enyloxy]-phenyl}-2-ethoxy-propionate (example 117) (840 mg, 1.60 mmol) and
sodium hy-
droxide (1 M, 16 ml, 16 mmol) by a procedure analogous to that described in
example 51,
yielding (E7-(S)-3-{4-[3-(3,5-dibromophenyl)-but-2-enyloxy]-phenyl)-2-ethoxy-
propionic acid
(781 mg, 98%) as a colourless gum.
CA 02395298 2002-06-18
WO 01/55085 PCT/DKO1/00058
148
'H NMR (300 MHz, CDC13) b: 1.19 (3H, t), 2.08 (3H, d), 2.96 (1H, dd), 3.09
(1H, dd), 3.41-
3.53 (1 H, m), 3.55-3.67 (1 H, m), 4.07 (1 H, dd), 4.70 (2H, d), 6.04 (1 H,
tm), 6.87 (2H, dm),
7.17 (2H, dm), 7.48 (2H, d), 7.58 (1 H, t), carboxylic acid proton not
observed. LCMS:
523/521/519 (100%, M+Na), 518/516/514 (M+NH4), 455/453/451 (M+H-EtOH),
291/289/287.
EXAMPLE 119.
0
Br
OEt
OEt
Br \ I / O
(E7-(S)-Ethyl 3-{4-[3-(3,5-Dibromophenyl)-allyloxy]-phenyl}-2-ethoxy-
propionate
a)
(E~-Ethyl 3-(3,5-dibromophenyl)-acrylate (example109a) was reduced with D1BAL-
H by a
procedure analogous to that described in example 50b; to give the colourless
.solid (E7-3
(3,5-dibromophenyl)-prop-2-en-1-ol.
' H NMR (300 MHz, CDCI3) 8: 1.52 (1 H, t, OH), 4.35 (2H, ddd), 6.36 (1 H, dt),
6.50 (1 H, dm),
7.44 (2H, d), 7.53 (1 H, t). LCMS: 277/275/273 (100%, M+H-H20), 196/194 (M+H-
H20-Br),
100.
b)
The title compound (780 mg, 78%) was prepared from (E'7-3-(3,5-dibromophenyl)-
prop-2-en-
1-0l (584 mg, 2.0 mmol) and (S)-ethyl 2-ethoxy-3-(4-hydroxyphenyl)-propionate
(500 mg,
2.10 mmol) by a procedure analogous to that described in example 52c.
'H NMR (300 MHz, CDCI3) 8: 1.17 (3H, t), 1.22 (3H, t), 2.96 (2H, d), 3.30-3.42
(1 H, m), 3.55-
3.67 (1 H, m), 3.97 (1 H, t), 4.17 (2H, q), 4.68 (2H, dd), 6.41 (1 H, dt),
6.59 (1 H, dm), 6.86 (2H,
dm), 7.17. (2H, dm), 7.46 (2H, d), 7,53 (1 H, t).
CA 02395298 2002-06-18
WO 01/55085 PCT/DKO1/00058
149
EXAMPLE 120
0
Br
~OH
OEt
Br \ ~ / O \
(E)-(S)-3-{4-[3-(3,5-Dibromophenyl)-allyloxy]-phenyl}-2-ethoxy-propionic acid
The title compound was prepared from (~-(S)-ethyl 3-{4-[3-(3,5-dibromophenyl)-
allyloxy]-
phenyl)-2-ethoxy-propionate (example 119) (512 mg, 1Ø mmol) and sodium
hydroxide (1 M,
ml, 10 mmol) by a procedure analogous to that described in example 51,
yielding (~-(S)-
10 3-{4-[3-(3,5-dibromophenyl)-allyloxy]-phenyl}-2-ethoxy-propionic acid (96
mg! 20%)'as a col-
ourless gum.
'H NMR (300 MHz, CDCI3) 8: 1.19 (3H, t), 2.97 (1 H, dd), 3.09 (1 H, dd), 3.40-
3.66 (2H, m),
4.06 (1 H, dd), 4.68 (2H, dd), 6.42 (1 H, dt), 6.59 (1 H, dm), 6.88 (2H, dm),
7.18 (2H, dm), 7.46
(2H, d), 7.58 (1 H, t), carboxylic acid proton not observed. LCMS: 509/507/505
(M+Na),
504/502/500 (100%, M+NH4), 441/439/437 (M+H-EtOH), 277/275/273.
EXAMPLE 121
\
/
/ Et0 OEt
\ \ ~ / O ~ ~ O
_ _
(~-(S)-Ethyl 3-{4-[3-(4,4"-Di-tern butyl-[1,1';3',1"jterphenyl-5'-yl)-
allyloxyj-phenyl}-2-ethoxy-
propionate
CA 02395298 2002-06-18
WO 01/55085 PCT/DKO1/00058
150
The colourless glass, (~-ethyl 3-(4,4"-di-tert-butyl-[1,1';3',1"]terphenyl-5'-
yl)-acrylate was
prepared from (~-ethyl 3-(3,5-dibromophenyl)-acrylate (example 109a) and 4-
tert
butylphenylboronic acid by a procedure analogous to. that described in example
52a.
'H NMR (300 MHz, CDCI3) 8: 1.36 (3H, t), 1.38 (18H, s), 4.29 (2H, q), 6.55
(1H, d), 7.43-7.52
(4H, m), 7.52-7.61 (4H, m), 7.70 (2H, d), 7.81 (1 H, t), 7.82 (1 H, d).. MS:
440 (M+), 425 (100%,
M-Me), 205.
b)
The colourless gum (~-3-(4,4"-di-tert butyl-[1,1';3',1"]terphenyl-5'-yl)-prop-
2-en-1-ol,was
prepared by DIBAL-H reduction of (~-ethyl 3-(4,4"-di-tert butyl-
[1,1';3',1"]terphenyl-5'-yl)-
acrylate by a procedure analogous to that described for example 52b.
'H NMR (300 MHz, CDCI3) 8: 1.37 (18H, s), 1.48 (1 H, br t), 4.37 (2H, m),
6.49(1 H, dt), 6.75
(1 H, dm), 7.45-7.52 (4H, m), 7.54-7.61 (6H, m), 7.68 (1 H, t). LCMS: 779
(M+381 ), 761 (779-
H20), 437, 421 (M+Na), 399 (M+H, 381 (100%, M+H-H20).
c)
The title compound (368 mg, 79%) was prepared from (E~-3-(4,4"-di-tert butyl-
[1,1';3',1"]terphenyl-5'-yl)-prop-2-en-1-of (300 mg, 0.75 mmol) and (S)-ethyl
2-ethoxy-3-(4-
hydroxyphenyl)-propionate (188 mg, 0.79 mmol) by a procedure analogous to that
described
in example 52c.
'H NMR (300 MHz, CDCI3) 8: 1.17 (3H, t), 1.22 (3H, t), 1.37 (18H, s), 2.96.
(2H, d), 3.30-3.43
(1 H, m), 3.54-3.67 (1 H, m), 3.98 (1 H, t), 4.16 (2H, q), 4.72 (2H, d), 6.52
(1 H, dt), 6.85 (1 H, d),
6.90 (2H, dm), 7.18 (2H, dm), 7.44-7.52 (4H, m), 7.54-7.63 (6H, m), 7.69 (1 H,
m). LCMS:
641 (100%, M+Na), 636 (M+NH4), 381.
CA 02395298 2002-06-18
WO 01/55085 PCT/DKO1/00058
151
EXAMPLE 122
/
/ Et0 OH
\ \ ~ / O ~ ~ O
_ _
(~-(S)-3-~4-[3-(4,4"-Di-tert butyl-[1,1';3',1"]terphenyl-5'-yl)-allyloxy]-
phenyl}-2-ethoxy-
propionic acid
The title compound was prepared from (~-(S)-ethyl 3-{4-[3-(4,4"-di-tert butyl-
[1,1';3',1"]terphenyl-5'-yl)-allyloxy]-phenyl-2-ethoxy-propionate (example
121) (345 mg, 0.56
mmol) and sodium hydroxide (1 M, 1.1 ml, 1.1 mmol) by a procedure analogous to
that de-
,, scribed in example 51, yielding (~-(S)-3-~4-[3-(4,4"-di-tert-butyl-
[1,1';3',1"]terphenyl-5'-yl)-
allyloxy]-phenyl}-2-ethoxy-propionic acid (284 mg, 86%) as a colourless foam..
'H NMR (300 MHz, CDCI3) 8: 1.18 (3H, t), 1.38 (18H, s), 2.97 (1 H, dd), 3.10
(1 H, dd), 3.41-
3.65 (2H, m), 4.07 (1 H, dd), 4.72 (2H, dm), 6.52 (1 H, dt), 6.85 (1 H, d),
6.92 (2H, dm), 7.17
(2H, dm), 7.44-7.52 (4H, m), 7.55-7.62 (6H, m), 7.69 (1 H, m), carboxylic acid
proton not ob-
served.
EXAMPLE 123
Br
/ O
\ I / ~ OEt
Br \ ~ O \ OEt
~/ a
(~-(S)-Ethyl 3-~4-[3-(3',5'-Dibromo-biphenyl-4-yl)-but-2-enyloxy]-phenyl}-2-
ethoxy-propionate
CA 02395298 2002-06-18
WO 01/55085 PCT/DKO1/00058
152
a)
The colourless gum (E~-ethyl 3-(3',5'-dibromo-biphenyl-4-yl)-but-2-enoate was
prepared from
(E~-ethyl 3-(4-iodophenyl)-but-2-enoate (example 91 a) and 3,5-dibromobenzene
boronic acid
by a procedure analogous to that described in example 52a.
'H NMR (300 MHz, CDCI3) 8: 1.33 (3H, t), 2.61 (3H, d), 4.22 (2H, q), 6.20 (1H,
m), 7.44-7.80
(7H, m). LCMS: 427/425/423 (100%, M+H), 381/379/377 (M+H-EtOH).
b)
The colourless gum (E)-3-(3',5'-dibromo-biphenyl-4-yl)-but-2-en-1-of was
prepared by DI-
BAL-H reduction of (E)-ethyl 3-(3',5'-dibromo-biphenyl-4-yl)-but-2-enoate as
described fo,r
example 52b, with the purification of the product being carried out by
preparative HPLC.
'H NMR (300 MHz, CDCI3) 8: 1.45 (1 H, br s), 2.12 (3H, d), 4.41 (2H, d), 6.05
(1 H, tm), 7.45-
7.54 (4H, m), 7.62 (1 H, t), 7.66 (2H, d). LCMS: 367/365/363 (100%, M+H-H20),
286/284.
C)
The 'title compound (177 mg, 83%) was prepared as a colourless gum from (~-3-
(3',5'-
dibromo-biphenyl-4-yl)-but-2-en-1-of (135 mg, 0.35 mmol) and (S)-ethyl 2-
ethoxy-3-(4-
hydroxyphenyl)-propionate (90 mg, 0.38 mmol) by a procedure analogous to that
described
in example 52c.
'H NMR (300 MHz, CDCI3) s: 1.17 (3H, t), 1.23 (3H, t), 2.16 (3H, d), 2.96 (2H,
d), 3.31-3.43
(1 H, m), 3.55-3.67 (1 H, m), 3.98 (1 H, t), 4.17 (2H, q), 4.74 (2H, d), 6.13
(1 H, tm), 6.88 (2H,
dm), 7.17 (2H, dm), 7.45-7.56 (4H, m), 7.63 (1 H, t), 7.66 (2H, d). LCMS:
627/625/623 (100%,
M+Na), 365.
EXAMPLE 124
Br
/ O
/ I OH
Br \ ~ O ~ OEt
~/ v
CA 02395298 2002-06-18
WO 01/55085 PCT/DKO1/00058
153
(~-(S)-3-f4-[3-(3',5'-Dibromo-biphenyl-4-yl)-but-2-enyloxy]-phenyl}-2-ethoxy-
propionic acid
The title compound was prepared from (E)-(S)-ethyl 3-{4-[3-(3',5'-dibromo-
biphenyl-4-yl)-but-
2-enyloxy]-phenyl-2-ethoxy-propionate (example 123) (110 mg, 0.18 mmol) and
sodium hy-
droxide (1 M, 1.0 ml, 1.0 mmol) by a procedure analogous to that described. in
example 51,
yielding (,E~-(S)-3-{4-[3-(3',5'-dibromo-biphenyl-4-yl)-but-2-enyloxy]-phenyl}-
2-ethoxy
propionic acid (90 mg, 86%) as a colourless gum.
'H NMR (300 MHz, CDCI3) 8: 1.20 (3H, t), 2.18 (3H, d), 2.99 (1 H, dd), 3.12 (1
H, dd), 3.43
3.68 (2H, m), 4.08 (1 H, dd), 4.75 (2H, d), 6.13 (1 H, tm), 6.91 (2H, dm),
7.19 (2H, dm), 7.45
7.60 (4H, m), 7.60-7.74 (3H, m), carboxylic acid proton not observed. LCMS:
599/597/595
(100%, M+Na), 365.
EXAMPLE 125
CI
O
OEt
v
CI \ ~ O ~ OEt
a
(~-(S)-Ethyl 3-{4-[3-(3',5'-Dichloro-biphenyl-4-yl)-but-2-enyloxy]-phenyl}-2-
ethoxy-propionate
a)
The colourless solid (~-ethyl 3-(3',5'-dichloro-biphenyl-4-yl)-but-2-enoate
was prepared from
(~-ethyl 3-(4-iodophenyl)-but-2-enoate (example 91a) and 3,5-dichlorobenzene
boronic acid
by a procedure analogous to that described in example 52a.
Mpt. 96.3-97.3°C.'H NMR (300 MHz, CDCI3) 8: 1.33 (3H, t), 2.61 (3H, d),
4.23 (2H, q), 6.19
(1 H, m), 7.35 (1 H, t), 7.47 (2H, d), 7.51-7.61 (4H, m). LCMS: 335/337/339
(100%, M+H),
289/291/293 (M+H-EtOH). Microanalysis Calculated % C: 64.49, H: 4.81, CI:
21.15; found C:
64.41, H: 4.80, CI: 20.80.
b)
CA 02395298 2002-06-18
WO 01/55085 PCT/DKO1/00058
154
The colourless oil (~-3-(3',5'-dichloro-biphenyl-4-yl)-but-2-en-1-of was
prepared by DIBAL-H
reduction of (~-ethyl 3-(3',5'-dichloro-biphenyl-4-yl)-but-2-enoate as
described for example
52b.
' H NMR (300 MHz, CDCI3) 5: 1.39 (1 H, br s), 2.12 (3H, d), 4.40 (2H, d), 6.05
(1 H, tm), 7.33
(1 H, t), 7.46 (2H, d), 7.45-7.65 (4H, m). MS: 296/294/292 (100%, M+),
281/279/277 (M-Me),
278/276/274 (M-H20), 253/251/249.
c)
The title compound (794 mg, 77%) was prepared as a colourless gum from (E)-3-
(3',5'-
dichloro-biphenyl-4-yl)-but-2-en-1-ol. (586 mg, 2.0 mmol) and (S)-ethyl 2-
ethoxy-3-(4-
hydroxyphenyl)-propionate (500 mg, 2.10 mmol) by a procedure analogous to that
described
in example 52c.
'H NMR (300 MHz, CDCI3) 8: 1.17 (3H, t), 1.23 (3H, t), 2.17 (3H, d), 2.96 (2H,
d), 3.30-3.42
(1 H, m), 3.55-3.67 (1 H, m), 3.98 (1 H, t), 4.17 (2H, q), 4.74 (2H, d), 6.13
(1 H, tm), 6.88 (2H,
dm), 7.18 (2H, dm), 7.33 (1 H, t), 7.46 (2H, d), 7.48-7.54 (4H, m). LCMS:
539/537/535 (100%,
M+Na), 534/532/530 (M+NH4), 279/277/275.
EXAMPLE 126
CI
O
i I off
c1 v i I
o w opt
(t~-(S)-3-{4-[3-(3',5'-Dichloro-biphenyl-4-yl)-but-2-enyloxy]-phenyl}-2-ethoxy-
propionic acid
The title compound was prepared. from (~-(S)-ethyl 3-{4-[3-(3',5'-dichloro-
biphenyl-4-yl)-but-
2-enyloxy]-phenyl}-2-ethoxy-propionate (example 125) (513 mg, 1.0 mmol) and
sodium hy-
droxide (1 M, 5.0 ml, 5.0 mmol) by a procedure analogous to that described in
example 51,
yielding (t~-(S)-3-{4-[3-(3',5'-dichloro-biphenyl-4-yl)-but-2-enyloxy]-phenyl}-
2-ethoxy-
propionic acid (422 mg, 87%) as a colourless glass.
CA 02395298 2002-06-18
WO 01/55085 PCT/DKO1/00058
155
'H NMR (300 MHz, CDC13) 8: 1.19 (3H, t), 2.16 (3H, d), 2.97 (1 H, dd), 3.09 (1
H, dd), 3.40-
3.54 (H, m), 3.54-3.67 (1 H, m), 4.07 (1 H, dd), 4.74 (2H, d), 6.12 (1 H, tm),
6.90 (2H, dm), 7.18
(2H, dm), 7.32 (1 H, t), 7.46 (2H, d), 7.48-7.55 (4H, m), carboxylic acid
proton not observed.
LCMS: 511/509/507 (100%, M+Na), 279/277/275.
EXAMPLE 127
ci
0
i I oEt
i
oEt
(E7-(S)-Ethyl 3-{4-[3-(3',5'-Dichloro-biphenyl-4-yl)-allyloxy]-phenyl}-2-
ethoxy-propionate
a)
The colourless solid (E7-ethyl 3-(3',5'-dichloro-biphenyl-4-yl)-acrylate was
prepared:from (E7
ethyl 3-(4-bromo-phenyl)-acrylate (example 71 a) and 3,5-dichlorobenzene
boronic acid by a
procedure analogous to that described in example 52a.
Mpt. 70-78°C. 'H NMR (300 MHz, CDCI3) 8: 1.35 (3H, t), 4.29 (2H, q),
6.49 (1 H, d), 7.36 (1 H,
t), 7.47 (2H, d), 7.53-7.64 (4H, m), 7.71 (1 H, d). LCMS: 325/323/321 (100,%,
M+H). Micro-
analysis Calculated % C: 63.57, H: 4.39; found C: 63.37, H: 4.43.
b)
The colourless gum (E)-3-(3',5'-dichloro-biphenyl-4-yl)-prop-2-en-1-of was
prepared by DI-
BAL-H reduction of (~-ethyl 3-(3',5'-dichloro-biphenyl-4-yl)-acrylate as
described for exam-
ple 52b.
'H NMR (300 MHz, CDCI3) 8: 1.47 (1 H, br t), 4.36 (2H, ddd), 6.43 (1 H, dt),
6.65 (1 H, dm),
7.33 (1 H, t), 7.37-7.55 (6H, m). MS: 282/280/278 (100%, M+), 2391237/235,
226/224/222.
c)
The title compound (732 mg, 70%) was prepared as a yellow gum, (containing
0.25 molar
equivalents of ethyl acetate) from (E7-3-(3',5'-dichloro-biphenyl-4-yl)-prop-2-
en-1-of (559 mg,
CA 02395298 2002-06-18
WO 01/55085 PCT/DKO1/00058
156
2.0 mmol) and (S)-ethyl 2-ethoxy-3-(4-hydroxyphenyl)-propionate (500 mg, 2.10
mmol) by a
procedure analogous to that described in example 52c.
'H NMR (300 MHz, CDCI3) 8: 1.17 (3H, t), 1.22 (3H, t), 1.25 (0.75H, t, AcOEt),
2.04 (0.75H,
s, AcOEt), 2.97 (2H, d), 3.30-3.41 (1 H, m), 3.53-3.67 (1 H, m), 3.98 (1 H,
t), 4.12 (0.5H, q,
AcOEt), 4.17 (2H, q), 4.71 (2H, dd), 6.47 (1 H, dt), 6.76 (1 H, dm), 6.89 (2H,
dm), 7.17 (2H,
dm), 7.33 (1 H, t), 7.43-7.55 (6H, m). LCMS: 525/523/521 (100%, M+Na),
265/263/261.
EXAMPLE 128
ci
0
ci ~ I i ~ ~ off
OEt
(~-(S)-3-{4-[3-(3',5'-Dichloro-biphenyl-4-yl)-allyloxy]-phenyl}-2-ethoxy-
propionic acid
The title compound was prepared from (~-(S)-ethyl 3-{4-[3-(3',5'-dichloro-
biphenyl-4-yl)-
allyloxy]-phenyl}-2-ethoxy-propionate (example 127) (522 mg, 1.0 mmol) and
sodium hydrox-
ide (1M, 10.0 ml, 10.0 mmol) by a procedure analogous to that described in
example 51,
yielding (E7-(S)-3-~4-[3-(3',5'-dichloro-biphenyl-4-yl)-allyloxy]-phenyl}-2-
ethoxy-propionic acid
(325 mg, 67%) as a colourless wax, which contained 0.167 molar equivalents of
AcOEt..
'H NMR (300 MHz, CDCI3) 8: 1.19 (3H, t), 1.26 (0.5H, t, AcOEt), 2.04 (0.5H, sr
AcOEt), 2:97
(1 H, dd), 3.09 (1 H, dd), 3.41-3.53 (1 H, m), 3.53-3.68 (1 H, m), 4.07 (1 H,
dd), 4.12 (0.33H, q,
AcOEt), 4.71 (2H, dd), 6.48 (1 H, dt), 6.76 (1 H, dm), 6.91 (2H, dm), 7.18
(2H, dm), 7.33 (1 H,
t), 7.40-7.60 (6H, m), carboxylic acid proton not observed. LCMS:
497/495/493(100%,
M+Na), 492/490/488 (M+NH4), 265/263/261.
CA 02395298 2002-06-18
WO 01/55085 PCT/DKO1/00058
157
EXAMPLE 129
(~-(S)-Ethyl 3-{4-[3-(3',5'-di-tert butyl-biphenyl-4-yl)-allyloxy]-phenyl}-2-
ethoxy-propionate
a)
The colourless solid (~-3-(4-bromo-phenyl)-prop-2-en-1-of was prepared by
DIBAL-H reduc-
tion of (~-ethyl 3-(4-bromo-phenyl)-acrylate (example 71 a) as described for
example 52b.
Mpt. 65.5-67.5°C.'H NMR (300 MHz, CDCI3) s: 1.50 (1H, br s), 4.33 (2H,
d), 6.35 (1H, dt),
6.55 (1 H, d), 7.23 (2H, dm), 7.43 (2H, dm). MS: 214/212 (M+), 171/169,
158/156, 133 (M-Br,
100%), 115, 91, 77.
b)
tert Butyl chlorodimethylsilane (1.33 g, 19.5,mmol) was added to a stirred
solution of (~-3-
(4-bromo-phenyl)-prop-2-en-1-of (3.20 g, 15.0 mmol), and imidazole (2.72 g,
18.0 mmol) in
dry dichloromethane (75 ml) and the resulting mixture stirred at room
temperature for 18 h, a
colourless precipitate being formed. The mixture was diluted with
dichloromethane (100 ml)
and 1 N hydrochloric acid (100 ml). The aqueous layer was separated, further
extracted with
dichloromethane (2 x 100m1) and the combined organic layers washed with brine,
dried
(MgS04) and evaporated. The product was purified by column chromatography on
silica gel
(1 % diethyl ether in n-heptane eluent) to give the colourless solid, (~-[3-(4-
bromo-phenyl)-
allyloxy]-tert butyldimethylsilane (4.39 g, 89%).
Mpt. 46.5-48°C.'H NMR (300 MHz, CDCI3) 8: 0.11 (6H, s), 0.94 (9H, s),
4.34 (2H, dd), 6.27
(1 H, dt), 6.54 (1 H, dt), 7.24 (2H, dm), 7.42 (2H, dm), 7.71 (1 H, d).
Microanalysis Calculated
%. C: 55.04, H: 7.08, Br: 24.41; found C: 54.81, H: 7.22, Br: 24.51.
c)tert Butyllithium (1.7M in pentane, 3.5 ml, 6.0 mmol) was added dropwise, at
-78°C to a
stirred THF (10 ml) solution of (E)-[3-(4-bromo-phenyl)-allyloxy]-tern
butyldimethylsilane (982
3o mg, 3.0 mmol) and the resulting solution stirred for 45 min.
Trimethylborate (0.51 ml, 4.50
CA 02395298 2002-06-18
WO 01/55085 PCT/DKO1/00058
158
mmol) was added, the solution allowed to warm to room temperature over 2h, and
the sol-
vents evaporated to give the crude boronate ester as a yellow gum, which was
dissolved in
DME (10 ml). Tetraleis(triphenylphosphine)palladium(0) (69 mg, 0.06 mmol), was
added to a
stirred DME (20 ml) solution of 1-bromo-3,5-di-tert butylbenzene (538 mg, 2.0
mmol), the so-
y lution stirred for 10 min, aqueous sodium carbonate (2M, 9 ml, 18.0 mmol)
added, and stir-
ring continued for a further 10 min. The boronate ester solution was added and
the mixture
heated to 80°C, under reflux, for 24h. The resulting mixture was
diluted with 1 N HCI (50 ml),
the products extracted into ethyl acetate (3 x 50 ml), and the combined
extracts washed with
brine, dried (MgS04), and evaporated. The resulting yellow gum was dissolved
in dry THF
(20 ml), tetra-n-butyl ammonium fluoride (1.26 g, 4.0 mmol) added and the
solution stirred at
room temperature for 18h. The resulting mixture was diluted with 1 N HCI (50
ml), and the
products extracted into ethyl acetate (2 x 50 ml). The combined organic phases
were washed
with brine, dried (MgSO~), and evaporated to give the colourless glass, (E)-3-
(3',5'-di-tert
butyl=biphenyl-4-yl)-prop-2-en-1-of (127 mg, 20%).
'H NMR (300 MHz, CDCI3) 8: 1.38. (18H, s), 1.45 (1 H, br t), 4.35 (2H, br t),
6.41 (1 H, dt), 6.66
(1 H, br dm), 7.40-7.49 (5H, m), 7.53-7.58 (2H, m). MS: 322 (M+), 307 (100%, M-
Me), 57.
d)
°The title compound (110 mg, 51 %) was prepared as a colourless gum
from (E)-3-(3'!5'-di-
tent butyl-biphenyl-4-yl)-prop-2-en-1-of (127 mg, 0.39 mmol) and (S)-ethyl 2-
ethoxy-3-(4-
hydroxyphenyl)-propionate (99 mg, 0.41 mmol) by a procedure analogous to that
described
in example 52c.
'H .NMR (300 MHz, CDCI3) s: 1.17 (3H, t), 1.23 (3H, t), 1.38 (18H, s), 2.97
(2H~ d), 3.29-3:42
(1 H, m), 3.53-3.67 (1 H, m), 3.98 (1 H, t), 4.17 (2H, q), 4.70 (2H, dd), 6.45
(1 H, dt), 6.77 (1 H,
dm), 6.90 (2H, dm), 7.18 (2H, dm), 7.40-7.60 (7H, m).
EXAMPLE 130
o
off
i
o w opt
CA 02395298 2002-06-18
WO 01/55085 PCT/DKO1/00058
159
(~-(S)-3-{4-[3-(3',5'-Di-tert butyl-biphenyl-4-yl)-allyloxy]-phenyl}-2-ethoxy-
propionic acid
The title compound was prepared from (~-(S)-ethyl 3-{4-[3-(3',5'-di-tert butyl-
biphenyl-4-yl)-
allyloxy]-phenyl}-2-ethoxy-propionate (example 129) (110 mg, 0.20 mmol) and.
sodium hy-
droxide (1 M, 0.8 ml, 0.8 mmol) by a procedure analogous to that described in
example 51,
yielding ((,E~-(S)-3-~4-[3-(3',5'-di-tert butyl-biphenyl-4-yl)-allyloxy]-
phenyl}-2-ethoxy-propionic
acid (92 mg, 88%) as a colourless solid.
'H NMR (300 MHz, CDCI3) 8: 1.20 (3H, t), 1.38 (18H, s), 2.97 (1 H, dd), 3.10
(1 H, dd), 3.40-
3.65 (2H, m), 4.07 (1 H, dd), 4.70 (2H, dd), 6.45 (1 H, dt), 6.77 (1 H, dm),
6.90 (2H, dm), 7.18
(2H, dm), 7.38-7.60 (7H, m), carboxylic acid proton not observed.
EXAMPLE 131
O
i I oEt
O ~ OEt
(E)-(S)-Ethyl 3-{4-[3-(3',5'-Di-tert-butyl-biphenyl-4-yl)-but-2-enyloxy]-
phenyl}-2-ethoxy-
propionate
a)
The colourless oil, (~-[3-(4-bromophenyl)-but-2-enyloxy]-tert butyl-
dimethylsilane. was pre-
pared from (~-3-(4-bromophenyl)-but-2-en-1-of (example 50b), imidazole and
tert butyl
chlorodimethylsilane by a procedure analogous to that described in example
129b.
'H NMR (300 MHz, CDCI3) 8: 0.10 (6H, s), 0.92 (9H, s), 4.37 (2H, d), 5.88 (1H,
tm), 7.25 (2H,
dm), 7.42 (2H, dm). MS: 342/340 (M+), 327/325 (M-Me), 285/283 (M-Bu), 130, 75
(100%).
b)
CA 02395298 2002-06-18
WO 01/55085 PCT/DKO1/00058
160
The colourless wax, (~-3-(3',5'-di-tert-butyl-biphenyl-4-yl)-but-2-en-1-of was
prepared via a
metallation, boronation, cross coupling and deprotection sequence analogous to
that de-
scribed for example 129c.
'H NMR (300 MHz, CDCI3) 8: 1.26 (1H, br m), 1.38 (18H, s), 2.12 (3H, d), 4.40
(2H, br t),
6.05 (1 H, dt), 7.40-7.42 (3H, m), 7.43-7.59 (4H, m). LCMS: 331 (M+H), 319
(100%, M+H-
H20).
c)
The title compound (429 mg, 74%) was prepared as a colourless gum from (EJ-3-
(3',5'-di-
tert butyl-biphenyl-4-yl)-but-2-en-1-of (350 mg, 1.04 mmol) and (S)-ethyl 2-
ethoxy-3-(4-
hydroxyphenyl)-propionate (260 mg, 1.09 mmol) by a procedure analogous to that
described
in example 52c.
'H NMR (300 MHz, CDCI3) s: 1.17 (3H, t), 1.22 (3H, t), 1.38 (18H, s), 2.17
(3H, d), 2.96 (2H,
d), 3.30-3.43 (1 H, m), 3.55-3.68 (1 H, m), 3.98 (1 H, t), 4.17 (2H, q), 4.75
(2H, d), 6.11 (1 H,
tm), 6.89 (2H, dm), 7.17 (2H, dm), 7.40-7.45 (3H, m), 7.46-7.60 (4H, m). LCMS:
579 (100%,
M+Na), 574 (M+NH4), 511 (M+H-EtOH).
EXAMPLE 132
O
/ I ~ 'OH
a /
O ~ OEt
~/ a
(E7-(S)-3- f4-[3-(3',5'-Di-tert-butyl-biphenyl-4-yl)-but-2-enyloxyj-phenyl}-2-
ethoxy-propionic
acid
The title compound was prepared from (~-(S)-ethyl 3-{4-[3-(3',5'-di-tert butyl-
biphenyl-4-yl)-
but-2-enyloxy]-phenyl}-2-ethoxy-propionate (example 131 ) (400 mg, 0.72 mmol)
and sodium
hydroxide (1M, 2.9 ml, 2.9 mmol) by a procedure analogous to that described in
example 51,
CA 02395298 2002-06-18
WO 01/55085 PCT/DKO1/00058
161
yielding (E7-(S)-3-~4-[3-(3',5'-di-tert-butyl-biphenyl-4-yl)-but-2-enyloxy]-
phenyl}-2-ethoxy-
propionic acid (315 mg, 83%) as a colourless gum.
'H NMR (300 MHz, CDCI3) 8: 1.19 (3H, t), 1.39 (18H, s), 2.18 (3H, d), 2.97
(1H, dd), 3.10
(1 H, dd), 3.40-3.67 (2H, m), 4.07 (1 H, dd), 4.70 (2H, d), 6.11 (1 H, tm),
6.90 (2H, dm), 7.18
(2H, dm), 7.40-7.45 (3H, m), 7.46-7.60 (4H, m), carboxylic acid proton not
observed. LCMS:
847 (M+319), 551 (M+Na), 319 (100%).
EXAMPLE 133
o
i I oet
0
i
(E7-(S/R)-Ethyl 3-[4-(3-Biphenyl-4-yl-but-2-enyloxy)-phenyl]-2-isopropoxy-
propionate
a)
(S/R)-Ethyl 2-(diethoxyphosphoryl)-2-isopropoxy-acetate was prepared as a pale
green oil by
the rhodium(II) acetate dimer catalysed reaction of ethyl diazo-
(diethoxyphosphoryl)-acetate
with isopropyl alcohol, according to the method described by C.J. Moody et al
(Tetrahedron,
1992, 48, 3991-4004).
'H NMR (300 MHz, CDCI3) s: 1.21 (3H, d), 1.23 (3H, d), 1.28-1.39 (9H, m), 3.74
(1H, septet),
4.15-4.35 (6H, m), 4.39 (1 H, d, JHP = 19.9 Hz). LCMS: 283 (M+H), 241 (100%),
213.
b)
A THF (20 ml) solution of (S/R)-ethyl 2-(diethoxyphosphoryl)-2-isopropoxy-
acetate (6.40 g,
22.7 mmol) was added dropwise, at 0°C, to a stirred suspension of
sodium hydride (60% dis-
persion in mineral oil, 0.92 g, 23.0 mmol) in THF (20 ml), and the resulting
mixture stirred for
min. A THF (20 ml) solution of 4-benzyloxybenzaldehyde (3.21 g, 15.1 mmol) was
added,
the resulting solution allowed to warm to room temperature, and stirring
continued for 72 h.
The mixture was carefully diluted with.1 N HCI (150 ml), the products
extracted into ethyl ace-
30 tate (3 x 100 ml), and the combined organic phases washed with brine, dried
(MgS04) and
CA 02395298 2002-06-18
WO 01/55085 PCT/DKO1/00058
162
(MgS04) and evaporated to give a yellow gum, which was purified by column
chromatogra-
phy on silica gel (10% ethyl acetate in n-heptane eluent) to give the
intermediate, (E/~-ethyl
3-(4-benzyloxyphenyl)-2-isopropoxy-acrylate as a colourless gum.
The (E/Z)-ethyl 3-(4-benzyloxyphenyl)-2-isopropoxy-acrylate was dissolved in
ethanol (100
ml), palladium on activated charcoal (10 wt. %, 0.80 g, 0.75 mmol) added and
the mixture
hydrogenated at 30 Ib/in2 H2 pressure for 18 h. The catalyst was removed by
filtration through
celite and the solvent evaporated to give (S/R)-ethyl 3-(4-hydroxyphenyl)-2-
isopropoxy-
propionate (3.44 g, 90%) as a pale orange gum.
'H NMR (300 MHz, CDCI3) 8: 0.98 (3H, d), 1.15 (3H, d), 1.24 (3H, t), 2.82-2.98
(2H, m), 3.51
(1 H, septet), 4.02 (1 H, dd), 4.17 (2H, q), 5.49 (1 H, br s), 6.75 (2H, dm),
7.09. (2H, dm).
LCMS: 275 (M+Na), 253 (M+H), 235, 211, 193, 151, 137 (100%).
c)
The title compound (324 mg, 70%) was prepared as a colourless solid from (S/R)-
ethyl 3-(4-
hydroxyphenyl)-2-isopropoxy-propionate (280 mg, 1.11 mmol) and (E7-3-biphenyl-
4-yl-but-2-
. en-1-of (225 mg, 1.0 mmol) by a procedure analogous to that described in
example 52c.
Mpt. 79-81°C.'H NMR (300 MHz, CDCI3) 8: 0.97 (3H, d), 1.16 (3H, d),
1.24 (3H, t), 2.17 (3H,
d), 2.85-3.02 (2H, m), 3.51 (1 H, septet), 4.01 (1 H, dd), 4.08-4.25 (2H,. m),
4.75 (2H; d), 6:11
(1 H, tm), 6,.88 (2H, dm), 7.17 (2H, dm), 7.30-7.38 (1 H, m), 7.40-7.65 (8H,
m). LCMS: 665
(M+207), 481 (M+Na), 476 (M+NH4), 207 (100%).
EXAMPLE 134
o
I i I off
i
w o~
I i o
(~-(S/R)-3-[4-(3-Biphenyl-4-yl-but-2-enyloxy)-phenyl]-2-isopropoxy-propionic
acid
The title compound was prepared from (E)-(S/R)-ethyl 3-[4-(3-biphenyl-4-yl-but-
2-enyloxy)-
phenyl]-2-isopropoxy-propionate (example 133) (230 mg, 0.50 mmol) and sodium
hydroxide
CA 02395298 2002-06-18
WO 01/55085 PCT/DKO1/00058
163
(1M, 1.5 ml, 1.5 mmol) by a procedure analogous to that described in example
51, yielding
(E7-(S/R)-3-[4-(3-biphenyl-4-yl-but-2-enyloxy)-phenyl]-2-isopropoxy-propionic
acid (190 mg,
88%) as a colourless solid.
Mpt. 125-127.5°C.'H NMR (300 MHz, CDCI3) s: 1.03 (3H, d), 1.16 (3H, d),
2.17 (3H, d), 2.90
(1 H, dd), 3.08 (1 H, dd), 3.55 (1 H, septet), 4.10 (1 H, dd), 4.75 (2H, d),
6.12 (1 H, tm), 6.90
(2H, dm), 7.17 (2H, dm), 7.30-7.38 (1 H, m), 7,40-7.65 (8H, m), carboxylic
acid proton not ob-
served. LCMS: 637 (M+207), 453 (M+Na), 448. (M+NH4), 207 (100%)
EXAMPLE 135
o
opt
0
(E)-(S/R)-Ethyl 3-[4-(3-Biphenyl-4-yl-but-2-enyloxy)-phenyl]-2-butoxy-
propionate
a)
Butyl di-butoxy acetate (23.75 g, 91.2 mmol) was mixed with acetyl chloride
(15.5 ml, 218
mmol) and iodine (0.2 g, 0.79 mmol) and the resulting brown solution heated to
60°C, under
reflux, for 6 h. The product was then fractionally distilled, under reduced
pressure, yielding
(S/R)-butyl 2-butoxy-2-chloro-acetate (17.58 g, 79%) as an orange coloured
oil.
Bpt. 130-135°C/approx. 15 mmHg.'H NMR (300 MHz, CDCI3) 8: 0.89-0.99
(6H, m), 1.41
(4H, sextet), 1.60-1.75 (4H, m), 3.60 (1 H, dt), 3.98 (1 H, dt), 4.25 (2H, t),
5.81 (1 H, s).
b)
A mixture of triethylphosphite (13.05 ml, 75.0 mmol) and (S/R)-butyl 2-butoxy-
2-chloro-
acetate (16.70 g, 75.0 mmol) was heated to 140°C, under reflux, for 6h.
The resulting oil was
fractionally distilled under reduced pressure to give the product, (S/R)-butyl
2-butoxy-2-
(diethoxyphosphoryl)-acetate (20.42 g, 84%) as a colourless oil.
CA 02395298 2002-06-18
WO 01/55085 PCT/DKO1/00058
164
Bpt. 170-175°C/1-5 mmHg.'H NMR (300 MHz, CDC13) s: 0.87-0.99 (6H, m),
1.30-1.49 (10H,
m), 1.57-1.73 (4H, m), 3.52 (1 H, dt), 3.66 (1 H, dt), 4.15-4.30 (6H, m), 4.30
(1 H, d, JHP = 19
Hz). LCMS: 325 (100%, M+H), 269, 167.
C)
A THF (40 ml) solution of (S/R)-butyl 2-butoxy-2-(diethoxyphosphoryl)-acetate
(14.60 g, 45.0
mmol) was added dropwise, at 0°C, to a stirred suspension of sodium
hydride.(55% disper-
sion in mineral oil, 2.61 g, 59.8 mmol) in THF (50 ml), and the resulting
mixture stirred for 30
min. A THF (50 ml) solution of 4-benzyloxybenzaldehyde (6.37 g, 30.0 mmol) was
added, the
resulting solution allowed to warm to room temperature, and stirring
continued~for 48 h. The
mixture was carefully diluted with 1 N HCI (200 ml), the products extracted
into ethyl acetate
(4 x 100 ml), and the combined organic phases. washed with brine, dried
(MgS04) and
evaporated to give a yellow gum, which was purified by column chromatography
on silica gel
(10% ethyl acetate in n-heptane eluent) to give the intermediate, (E/~-butyl 3-
(4-
benzyloxyphenyl)-2-butoxy-acrylate as a colourless gum.
The (E/Z)-butyl 3-(4-benzyloxyphenyl)-2-butoxy-acrylate was dissolved in
ethanol (200 ml),
palladium on activated. charcoal (10 wt. %, 1.60 g, 1.5 mmol) added and the
mixture hydro-
genated at 30 Ib/in2 H2 pressure for 18 h. The catalyst was removed by
filtration through
celite and the solvent evaporated to give an orange gum, which contained both
(S/R)-butyl 3-
(4-hydroxyphenyl)-2-butoxy-propionate and the trans-esterification product,
(S/R)-ethyl 3-(4-
hydroxyphenyl)-2-butoxy-propionate. These were separated by column
chromatography on
silica gel (15% ethyl acetate in n-heptane eluent) to give, in respective
order of elution, (S/R)-
butyl 3-(4-hydroxyphenyl)-2-butoxy-propionate (6.74 g, 76%) and (S/R)-ethyl 3-
(4-
hydroxyphenyl)-2-butoxy-propionate (0.40 g, 5%) as colourless oils.
(S/R)-butyl 3-(4-hydroxyphenyl)-2-butoxy-propionate:'H NMR (300 MHz, CDC13) 8:
0.85 (3H,
t), 0.91 (3H, t), 1.22-1.43 (4H, m), 1.43-1.65 (4H, m), 2.89-2.98 (2H, m),
3.28 (1 H, dt), 3.54
(1 H, dt), 3.97 (1 H, dd), 4.11 (2H, t), 5.56 (1 H, br s), 6.74 (2H, dm), 7.08
(2H, dm). LCMS: 317
(M+Na), 295 (M+H), 221 (100%, M+H-BuOH), 193, 179, 165, 137.
(S/R)-ethyl 3-(4-hydroxyphenyl)-2-butoxy-propionate:'H NMR (300 MHz, CDC13) 8:
0.85 (3H,
t), 1.23 (3H, t), 1.21-1.39 (2H, m), 1.43-1.60 (2H, m), 2.89-2.99 (2H, m),
3.28 (1H, dt), 3.55
(1 H, dt), 3.97 (1 H, dd), 4.17 (2H, q), 5.63 (1 H, br s), 6.74 (2H, dm), 7.08
(2H, dm). LCMS:
289 (M+Na), 267 (M+H), 193 (100%, M+H-BuOH), 151, 137.
CA 02395298 2002-06-18
WO 01/55085 PCT/DKO1/00058
165
d)
The title compound (420 mg, 71 %) was prepared as a colourless solid from
(S/R)-ethyl 3-(4-
hydroxyphenyl)-2-butoxy-propionate (385 mg, 1.45 mmol) and (~-3-biphenyl-4-yl-
but-2-en-
1-0l (280 mg, 1.25 mmol) by a procedure analogous to that described in example
52c.
Mpt. 62-63.5°C.'H NMR (300 MHz, CDCI3) 8: 0.85 (3H, t), 1.23 (3H, t),
1.20-1.40 (2H, m),
2.17 (3H, d), 2.90-3.00 (2H, m), 3.27 (1 H, dt), 3.55 (1 H, dt), 3.95 (1 H,
dd), 4.10-4.23 (2H, m),
4.74 (2H, d), 6.11 (1 H, tm), 6.88 (2H, dm), 7.17 (2H, dm), 7.30-7.38 (1 H,
m), 7.40-7.63 (8H,
m). LCMS: 679 (M+207), 495 (M+Na), 490 (M+NH4), 207 (100%).
EXAMPLE 136
o
~ I i I off
w I o w o~
(~-(S/R)-3=[4-(3-Biphenyl-4-yl-but-2-enyloxy)-phenyl]-2-butoxy-propionic acid
.
The title compound was prepared from (~-(S/R)-ethyl 3-[4-(3-biphenyl-4-yl-but-
2-enyloxy)
phenyl]-2-butoxy-propionate (example 135) (331 mg, 0.70 mmol) and sodium
hydroxide (1 M,
2.1 ml, 2.1 mmol) by a procedure analogous to that described in example 51,
yielding (~=
(S/R)-3-j4-(3-biphenyl-4-yl-but-2-enyloxy)-phenyl]-2-butoxy-propionic acid (42
mg, 13%) as a
colourless solid.
'H NMR (300 MHz, CDCI3) 8: 0.87 (3H, t), 1.21-1.38 (2H, m), 1.47-1.60 (2H, m),
2.17 (3H, br
s), 2.96 (1 H, dd), 3.09 (1 H, dd), 3.33-3.44 (1 H, m), 3.47-3.60 (1 H, m),
4.04 (1 H, dd), 4.75
(2H, d), 6.12 (1 H, br t), 6.90 (2H, dm), 7.17 (2H, dm), 7.30-7.38 (1 H, m),
7.38-7.65 (8H, m),
carboxylic acid proton not observed. LCMS: 651 (M+207), 467 (100%, M+Na), 207.
CA 02395298 2002-06-18
WO 01/55085 PCT/DKO1/00058
166
EXAMPLE 137
o
i I oEt
i
0
i o
(L~-(S/R)-Ethyl3-[4-(3-Biphenyl-4-yl-but-2-enyloxy)-phenyl]-2-hexyloxy-
propionate
a)
(S/R)-Ethyl 2-(diethoxyphosphoryl)-2-hexyloxy-acetate was prepared as a pale
green oil by
the rhodium(II) acetate dimer catalysed reaction of ethyl diazo-
(diethoxyphosphoryl)-acetate
with 1-hexanol, by a method analogous to that described for example 133a.
'H NMR (300 MHz, CDCI3) 8: 0.88 (3H, t), 1.23-1.44 (15H, m), 1.57-1.69 (2H,
m), 3.51 (1H,
dt), 3.65 (1 H, dt), 4.15-4.38 (7H, m). LCMS: 671 (2M+Na), 649 (2M+H), 325
(100%, M+H),
297, 241.
b)
Sodium hydride (60% dispersion in mineral oil, 1.0 g, 25.0 mmol) was added at
0°C, in small
portions, to a stirred THF (50 ml) solution of (S/R)-ethyl 2-
(diethoxyphosphoryl)-2-hexyloxy-
acetate (8.12 g, 25.0 mmol), and the resulting suspension stirred for 30 min.
A THF (50 ml)
solution of 4-benzyloxybenzaldehyde (4.25. g, 20.0 mmol) was added, the
resulting solution
allowed to warm to room temperature, and stirring continued for 4 h. The
mixture was care-
fully diluted with 0.5N HCI (150 ml), the products extracted into ethyl
acetate (4. x 75 ml), and
the combined organic phases washed with brine, dried (MgS04) and evaporated to
give an
orange coloured gum, which was purified by column chromatography on silica gel
(15% ethyl
acetate in n-heptane eluent) to give the intermediate, (E/L7-ethyl 3-(4-
benzyloxyphenyl)-2-
hexyloxy-acrylate as a pale yellow oil.
The (E/L~-ethyl 3-(4-benzyloxyphenyl)-2-hexyloxy-acrylate was dissolved in
ethanol (150 ml),
palladium on activated charcoal (10 wt. %, 1.40 g, 1.32 mmol) added and the
mixture hydro-
genated at 30 Ib/in? H2 pressure for 18 h. The catalyst was removed by
filtration through
celite and the solvent evaporated to give a colourless gum, which was purified
by column
chromatography on silica gel (10% ethyl acetate in n-heptane eluent) to give
(S/R)-ethyl 3-(4-
hydroxyphenyl)-2-hexyloxy-propionate (9.83 g, 30%) as a colourless gum.
CA 02395298 2002-06-18
WO 01/55085 PCT/DKO1/00058
167
'H NMR (300 MHz, CDC13) 8: 0.85 (3H, t), 1.14-1.32 (9H, m), 1.45-1.60 (2H, m),
2.94 (2H, d),
3.28 (1 H, dt), 3.54 (1 H, dt), 3.97 (1 H, dd), 4.17 (2H, q), 5.95 (1 H, br
s), 6.74 (2H, dm), 7.07
(2H, dm). MS: 294 (M+), 221 (M-COOEt), 192 (M-hexanol), 137, 107 (100%).
C)
The title compound (248 mg, 81 %) was prepared as a waxy solid from (S/R)-
ethyl 3-(4-
hydroxyphenyl)-2-hexyloxy-propionate (215 mg, 0.72 mmol) and (E7-3-biphenyl-4-
yl-but-2-
en-1-of (137 mg, 0.61 mmol) by a procedure analogous to that described in
example 52c:
'H NMR (300 MHz, CDCI3) 8: 0.86 (3H, t), 1.13-1.35 (9H, m), 1.46-1.60 (2H, m),
2.17 (3H, br
s), 2.88-3.00 (2H, m), 3.26 (1 H, dt), 3.55 (1 H, dt), 3.95 (1 H, dd), 4.10-
4.23 (2H, m), 4.74 (2H,
d), 6.12 (1 H, tm), 6.88 (2H, dm), 7.17 (2H, dm), 7.30-7.37 (1 H, m), 7.40-
7.63 (8H, m).
EXAMPLE 138
/ O
off
o ~ o
(E~-(S/R)-3-[4-(3-Biphenyl-4-yl-but-2-enyloxy)-phenyl]-2-hexyloxy-propionic
acid
The title compound was prepared from (E7-(S/R)-ethyl 3-[4-(3-biphenyl-4-yl-but-
2-enyloxy)-
phenyl]-2-hexyloxy-propionate (example 137) (172 mg, 0.34 mmol) and sodium
hydroxide
(1 M, 1.0 ml, 1.0 mmol) by a procedure analogous to that described in example
51, yielding
(E7-(S/R)-3-[4-(3-biphenyl-4-yl-but-2-enyloxy)-phenyl]-2-hexyloxy-propionic
acid (160 mg,
99%) as a colourless solid.
Mpt. 117-119°C.'H NMR (300 MHz, CDCI3) 8: 0.87 (3H, t), 1.13-1.38 (6H,
m), 1.45-1.60 (2H,
m), 2.17 (3H, d), 2.96 (1 H, dd), 3.10 (1 H, dd), 3.40 (1 H, dt), 3.53 (1 H,
dt), 4.05 (1 H, dd), 4.75.
(2H, d), 6.12 (1 H, tm), 6.90 (2H, dm), 7.16 (2H, dm), 7.30-7.38 (1 H, m),
7.40-7.63 (8H, m),
carboxylic acid proton not observed.
CA 02395298 2002-06-18
WO 01/55085 PCT/DKO1/00058
168 .,
EXAMPLE 139
/ o
\ I / I OEt /
\ I O~ O \
~/ a
(E7-(S/R)-Ethyl3-[4-(3-Biphenyl-4-yl-but-2-enyloxy)-phenyl]-2-(3-phenyl-
propoxy)-propionate
a)
(S/R)-Ethyl 2-(diethoxyphosphoryl)-2-(3-phenyl-propoxy)-acetate was prepared
as a pale
green oil by the rhodium(II) acetate Dimmer catalysed reaction of 3-phenyl-1-
propanol with
ethyl diazo-(diethoxyphosphoryl)-acetate, by a method analogous to that
described for ex-
ample 133a.
'H NMR (300 MHz, CDCI3) 8: 1.23-1.40 (9H, m), 1.98 (2H, quintet), 2.72 (2H, ),
3.52 (1H;
dt), 3.67 (1 H, dt), 4.15-4.35 (7H, m), 7.12-7.22 (3H, m), 7.22-7.32 (2H, m).
b)
A THF (50 ml) solution of (S/R)-ethyl 2-(diethoxyphosphoryl)-2-(3-phenyl-
propoxy)-acetate
(14.2 g, 39.6 mmol) was added dropwise, at 0°C, to a stirred mixture of
sodium hydride (60%
dispersion in mineral oil, 2.35 g, 58.8 mmol) and 4-benzyloxybenzaldehyde
(4.20 g, 19.8 ,
mmol) in THF (50 ml), and the resulting mixture allowed to warm slowly to room
temperature
over 18 h. The mixture was carefully diluted with water (150 ml), the products
extracted into
ethyl acetate (2 x 150 ml), and the combined organic phases washed. with
brine, dried
(MgS04) and evaporated to give (E/Z)-ethyl 3-(4-benzyloxyphenyl)-2-(3-phenyl-
propoxy)-
acrylate as a yellow oil.
The (E/~-ethyl 3-(4-benzyloxyphenyl)-2-(3-phenyl-propoxy)-acrylate was
dissolved in etha-
nol (50 ml), palladium on activated charcoal (10 wt. %, 1.0 g, 0.94 mmol)
added and the mix-
ture hydrogenated at 30 Ib/in2 H2 pressure for 18 h. The catalyst was removed
by filtration
through celite and the solvent evaporated to give a colourless oil.
'H NMR (300 MHz, CDC13) 8: 1.23 (3H, t), 1.72-1.97 (2H, m), 2.52-2.64 (2H, m),
2.87-3.02
(2H, m), 3.17-3.27 (1 H, m), 3.53-3.63 (1 H, m), 3.94 (1 H, dd), 4.17 (2H, q),
4.93 (1 H, s), 6.76
(2H, dm), 7.02-7.29 (7H, m).
CA 02395298 2002-06-18
WO 01/55085 PCT/DKO1/00058
169
C)
The title compound (150 mg, 56%) was prepared as a waxy solid from (S/R)-ethyl
3-(4-
hydroxyphenyl)-2-(3-phenyl-propoxy)-propionate (172 mg, 0.53 mmol) and (~-3-
biphenyl-4-
yl-but-2-en-1-o! (112 mg, 0.50 mmo!) by a procedure analogous to that
described in example
52c.
'H NMR (300 MHz, CDCI3) s: 1.23 (3H, t), 1.72-1.97 (2H, m), 2.16 (3H, br s),
2.51-2.65 (2H,
m), 2.89-3.05 (2H, m), 3.17-3.27 (1 H, m), 3.59 (1 H, dt), 3.95 (1 H, dd),
4.18 (2H, q), 4.74 (2H,
d), 6.11 (1H, tm), 6.90 (2H, dm), 7.01-7.63 (16H, m). LCMS: 741 (M+207), 557
(100%,.
M+Na), 552 (M+NH4), 207.
EXAMPLE 140
0
i I off
i
o~ o
(~-(S/R)-3-[4-(3-Biphenyl-4-yl-but-2-enyloxy)-phenyl]-2-(3-phenyl-propoxy)-
propionic acid
The title compound was prepared from (E)-(S/R)-ethyl 3-[4-(3-biphenyl-4-yl-but-
2-enyloxy)-
phenyl]-2-(3-phenyl-propoxy)-propionate (example 139) (130 mg, 0.24 mmol) and
sodium
hydroxide (1 M, 0.73 ml, 0.73 mmol) by a procedure analogous to that described
in example
51, yielding (E'-(S/R)-3-[4-(3-biphenyl-4-yl-but-2-enyloxy)-phenyl]-2-(3-
phenyl-propoxy)-
propionic acid (90 mg, 73%) as a colourless solid.
Mpt. 115-117°C.'H NMR (300 MHz, CDCI3) 8: 1.75-1.98 (2H, m), 2.16 (3H,
d), 2.52-2.68
(2H, m), 2.96 (1 H, dd), 3.10 (1 H, dd), 3.36 (1 H, dt), 3.57 (1 H, dt), 4.03
(1 H, dd), 4.74 (2H, d),
6.11 (1 H, tm), 6.91 (2H, dm), 7.06 (2H, dm), 7.12-7.63 (14H, m), carboxylic
acid proton not
observed. LCMS: 529 (M+Na), 525 (M+NH4), 207 (100%).
CA 02395298 2002-06-18
WO 01/55085 PCT/DKO1/00058
170
EXAMPLE 141
/ o
\ ~ / ~ OEt
\ ~ O \ O /
(E7-(S/R)-Ethyl3-[4-(3-Biphenyl-4-yl-but-2-enyloxy)-phenyl]-2-(4-phenyl-
butoxy)-propionate
a)
(S/R)-Ethyl 2-(diethoxyphosphoryl)-2-(4-phenyl-butoxy)-acetate was prepared as
a pale
green oil by the rhodium(II) acetate Dimmer catalysed reaction of 4-phenyl-1-
butanol with
ethyl diazo-(diethoxyphosphoryl)-acetate, by a method analogous to that
described for ex-
ample 133a.
'H NMR (300 MHz, CDCI3) b: 1.25-1.40 (9H, m), 1.60-1.78 (4H, m), 2.63 (2H, t),
3.47-3.56.
(1 H, m), 3.61-3.70 (1 H, m), 4.12-4.35 (7H, m), 7.12-7.21 (3H, m), 7.21-7.31
(2H, m). LCMS:
745 (2M+H), 373 (100%, M+H), 241.
b)
A THF (30 ml) solution of (S/R)-ethyl 2-(diethoxyphosphoryl)-2-(4-phenyl-
butoxy)-acetate
(15.64 g, 42.0 mmol) was added dropwise, at 0°C, to a stirred
suspension of sodium hydride
(60% dispersion in mineral oil, 2.52 g, 63.0 mmol) in THF (30 ml), and the
resulting mixture
stirred for 20 min. A THF (50 ml) solution of 4-benzyloxybenzaldehyde (4.46 g,
21.0 mmol)
was added, and the mixture warmed, resulting in a vigorous reaction. The
mixture was
cooled, carefully diluted with 0.5N HCI (150 ml), the products extracted into
ethyl acetate (2 x
150 ml), and the combined organic phases washed with brine, dried (MgS04) and
evapo-
rated to give (E/L7-ethyl 3-(4-benzyloxyphenyl)-2-(4-phenyl-butoxy)-acrylate
as a yellow oil.
The (E/Z)-ethyl 3-(4-benzyloxyphenyl)-2-(4-phenyl-butoxy)-acrylate was
dissolved in ethanol
(175 ml), palladium on activated charcoal (10 wt. %, 0.50 g, 0.47 mmol) added
and the mix-
ture hydrogenated at 30 Ib/in2 H2 pressure for 18 h. The catalyst was removed
by filtration
through celite and the solvent evaporated to give a colourless gum, which was
purified by
column chromatography on silica gel to give (S/R)-ethyl 3-(4-hydroxyphenyl)-2-
(4-phenyl-
butoxy)-propionate (1.73 g, 24%) as a colourless oil.
CA 02395298 2002-06-18
WO 01/55085 PCT/DKO1/00058
171
'H NMR (300 MHz, CDC13) 8: 1.22 (3H, t), 1.50-1.67 (4H, m), 2.50-2.60 (2H, m),
2.85-3.0
(2H, m), 3.21-3.31 (1 H, m), 3.53-3.63 (1 H, m), 3.94 (1 H, dd), 4.16 (2H, q),
6.72 (2H, dm),
7.06-7.31 (7H, m), phenol proton not observed.
C)
The title compound (245 mg, 80%) was prepared as a yellow, waxy solid from
(S/R)-ethyl 3-
(4-hydroxyphenyl)-2-(4-phenyl-butoxy)-propionate (200 mg, 0.58 mmol) and (~-3-
biphenyl-
4-yl-but-2-en-1-of (125 mg, 0.56 mmol) by a procedure analogous to that
described in exam-
ple 52c.
'H NMR (300 MHz, CDCI3) 8: 1.22 (3H, t), 1.50-1.68 (4H, m), 2.16 (3H, d), 2.50-
2.60 (2H, m),
2.88-3.02 (2H, m), 3.21-3.33 (1 H, m), 3.52-3.64 (1 H, m), 3.95 (1 H, dd),
4.17 (2H, q), 4.72
(2H, d), 6.11 (1 H, tm), 6.87 (2H, dm), 7.06-7.63 (16H, m).
EXAMPLE 142
0
I ~I o
0
i
i
(E)-(S/R)-3-[4-(3-Biphenyl-4-yl-but-2-enyloxy)-phenyl]-2-(4-phenyl-butoxy)-
propionic acid
The title compound was prepared from (E~-(S/R)-ethyl 3-[4-(3-biphenyl-4-y!-but-
2-enyloxy)-
phenyl]-2-(4-phenyl-butoxy)-propionate (example 141 ) (225 mg, 0.41 mmol) and
sodium hy-
droxide (1M, 1.64 ml, 1.64 mmol) by a procedure analogous to that described in
example 51,
yielding (E)-(S/R)-3-[4-(3-biphenyl-4-yl-but-2-enyloxy)-phenyl]-2-(4-phenyl-
butoxy)-propionic
acid (210 mg, 99%) as a pale yellow solid.
'H NMR (300 MHz, CDCI3) 8: 1.50-1.70 (4H, m), 2.17 (3H, d), 2.53-2.61 (2H, m),
2.95 (1 H,
dd), 3.09 (1 H, dd), 3.34-3.44 (1 H, m), 3.50-3.60 (1 H, m), 4.04 (1 H, dd),
4.72 (2H, d), 6.11
(1 H, tm), 6.88 (2H, dm), 7.06-7.63 (16H, m), carboxylic acid proton not
observed.
CA 02395298 2002-06-18
WO 01/55085 PCT/DKO1/00058
172
EXAMPLE 143
o
off/
o ~ ~
(~-(S/R)-Propyl3-[4-(3-Biphenyl-4-yl-but-2-enyloxy)-phenyl]-2-propoxy-
propionate
a)
Morpholinium dimorpholinoacetate was prepared according to the method
described by ,
Bourguignon and Wermuth (Bourguignon, J.J.; Wermuth, C.G. J. Org. Chem. 1981,
46,
4889-4894): an ethanol (100 ml) solution of morpholine (310 ml, 3.55 mol) was
added, at
0°C, to a stirred ethanol (500 ml) solution of glyoxylic acid
monohydrate (92.06.g, 1.0 mol)
and the resulting mixture refrigerated for 60 h, a colourless precipitate
being formed. The
solid was collected by filtration, washed with diethyl ether (2 x 300 ml) and
vacuum dried at
30°C to give morphilinium dimorhpholinoacetate (298 g, 94%) as a
colourless solid, which
contained a small amount of water.
Mpt. 139-139.5°C. 'H NMR (300 MHz, CDCI3) 8: 2.83 (12H, br m), 3.26 (1
H, s); 3.78. (12H, br
m), 7.78 (2H, br s). Microanalysis Calculated % C: 52.93, H: 8.58, N: 13.24,
water: 0.1 %;
found C: 52.84, H: 8.84, N: 13.15, water: 0.1 %.
2o b)
Using a method based on that described by Kerfanto and Jegou, (Kerfanto, M.;
Jegou, D.
Compt. Rendus. 1965, 267 (77), 2232-2233) morphilinium dimorpholinoacetate
(127 g, 0.40
mol) was added to a stirred solution of hydrochloric acid (94.3 g, 6.5 mol) in
1-propanol (600
ml) and the resulting mixture heated to 80°C, under reflux, for 2 h.
The resulting colourless
suspension was filtered to remove morphiline hydrochloride, and the filtrate
fractionally dis-
tilled, under reduced pressure, to give excess 1-propanol and the colourless
oil, propyl 2,2-
dipropoxyacetate (57.14 g, 65%).
'H NMR (300. MHz, CDCI3) 8: 0.86-1.05 (9H, m), 1.55-1.78 (6H, m), 3.47-3.65
(4H, m), 4.15
(2H, t), 4.89 (1 H, s).
CA 02395298 2002-06-18
WO 01/55085 PCT/DKO1/00058
173
C)
A mixture of propyl 2,2-dipropoxyacetate (43.66 g, 0.20 mol), acetyl chloride
(28 ml, 0.394
mol) and iodine (0.25 g, 1.0 mmol) was heated to 55°C, under reflux,
for 16 h. Since GC
analysis showed that some propyl 2,2-dipropoxyacetate starting material was
still present,
second portions of acetyl chloride (14 ml, 0.197 mol) and iodine (0.25 g, 1.0
mmol) were
added, and heating continued for a further 6 h. The product was then purified
by fractional
distillation under reduced pressure to give (S/R)-propyl 2-chloro-2-
propoxyacetate (32.67 g,
84%) as a pale orange oil (trace of iodine present).
Bpt. 116-119.5°C/approx. 10 mmHg.'H NMR (300 MHz, CDCI3) 8: 0.97 (3H,
t), 0.98 (3H, t),
1.63-1.82 (4H, m), 3.58 (1 H, dt), 3.93 (1 H, dt), 4.13-4.26 (2H, m), 5.83 (1
H, s).
d)
Triethylphosphite (27 ml, 0.155 mol) was added to (S/R)-propyl 2-chloro-2-
propoxyacetate
(29.21 g, 0.15 mol), resulting in an immediate decolourisation of the pale
orange acetate, and
the resulting mixture heated,to 140°C, under reflux, for 6 h, a
colourless gas being evolved.
,The mixture was then fractionally distilled under reduced pressure to give
the product, (S/R)-
propyl 2-(diethoxyphosphoryl)-2-propoxyacetate (35.78 g, 80%) as a colourless
oil.
Bpt. 155-160°C/approx. 3 mmHg.'H NMR (300 MHz, CDCI3) 8: 0.95 (3H, t),
0.98 (3H, t),
1.31-1.39 (6H, m), 1.60-1.76 (4H, m), 3.49 (1 H, dt), 3.62 (1 H, dt), 4.12-
4.30 (6H, m), 4.31.
(1 H, d, JHP = 19 Hz).
e)
A THF (50 ml) solution of (S/R)-propyl 2-(diethoxyphosphoryl)-2-propoxyacetate
(18.52 g,
62.5 mmol) was added dropwise, at 0°C, to a stirred suspension of
sodium hydride (60% dis-
persion in mineral oil, 2.50 g, 62.5 mmol) in THF (50 ml), and the resulting
mixture stirred for
min. A THF (100 ml) solution of 4-benzyloxybenzaldehyde (10.62 g, 50.0 mmol)
was
added, the resulting solution allowed to warm to room temperature, and
stirring continued for
24 h. TLC showed a considerable amount of unreacted 4-benzyloxybenzaldehyde
was still
present so a further portion of sodium hydride (60% dispersion in mineral oil,
1.0 g, 25.0
30 mmol) was added and stirring continued for a further 18 h. The mixture was
carefully diluted
with 0.5N HCI (400 ml), the products extracted into ethyl acetate (3 x 200
ml), and the com-
bined organic phases washed with brine, dried (MgS04) and evaporated to give a
yellow
gum, which was purified by column chromatography on silica gel (10% ethyl
acetate in n-
CA 02395298 2002-06-18
WO 01/55085 PCT/DKO1/00058
174
heptane eluent) to give the intermediate, (E/~-propyl 3-(4-benzyloxyphenyl)-2-
propoxy-
acrylate as a colourless gum.
The (E/L7-propyl 3-(4-benzyloxyphenyl)-2-propoxy-acrylate was dissolved in
ethanol (200,
ml), palladium on activated charcoal (10 wt. %, 2.18 g, 2.05 mmol) added and
the mixture
hydrogenated at 30 Ib/in2 HZ pressure for 20 h. The. catalyst was removed by
filtration through
celite and the solvent evaporated to give an orange gum, which contained both
the propyl:
and the ethyl (formed by trans-esterification) esters of (S/R)-3-(4-
hydroxyphenyl)-2-propoxy-
propionic acid. These were separated by column chromatography on silica gel
(15% ethyl
acetate in n-heptane eluent) to give, in respective order of elution, (S/R)-
propyl 3-(4-
hydroxyphenyl)-2-propoxy-propionate (4.52 g, 41 %) and. a mixture of both
(S/R)-propyl and
(S/R)-ethyl 3-(4-hydroxyphenyl)-2-propoxy-propionates (4.98 g, approx. 45%) as
colourless
oils.
(S/R)-propyl 3-(4-hydroxyphenyl)-2-propoxy-propionate:'H NMR (300 MHz, CDCI3)
8: 0.84
(3H, t), 0.90 (3H, t), 1.48-7 .69 (4H, m), 2.95 (2H, d), 3.25 (1 H, dt), 3.52
(1 H, dt), 4.00 (1 H, t),
4.07 (2H, t), 6.43 (1 H, br s), 6.74 (2H, dm), 7.07 (2H, dm). '3C NMR (75 MHz,
CDCI3) 8: 10.2
(q), 10.3 (q), 21.8 (t), 22.7 (t), 38.4 (t), 66.6 (t), 72.5 (t), 80.6 (d),
115.2 (d), 128.5 (s), 130.4
(d), 154.7 (s), 173.2 (s). MS: 266 (M+), 206 (M-PrOH), 179, 164, 137, 107.
(100%).
f)
The title compound (350 mg, 74%) was prepared as a colourless gum from (S/R)-
propyl 3-(4-
hydroxyphenyl)-2-propoxy-propionate (280 mg, 1.05 mmol) and (~-3-biphenyl-4-yl-
but-2-en-
1-0l (224 mg, 1.0 mmol) by a procedure analogous to that described in example
52c.
'H NMR (300 MHz, CDCI3) 8: 0.85 (3H, t), 0.90 (3H, t), 1.49-1.69 (4H, m), 2.17
(3H, d), 3.91-
3.02 (2H, m), 3.23 (1 H, dt), 3.52 (1 H, dt), 3.97 (1 H, dd), 4.07 (2H, t),
4.74 (2H; d), 6.11 (1 H,
tm), 6.88 (2H, dm), 7.17 (2H, dm), 7.30-7.38 (1 H, m), 7.40-7.63 (8H, m).
LCMS: 679
(M+207), 495 (100%, M+Na), 490 (M+NH4), 207.
CA 02395298 2002-06-18
WO 01/55085 PCT/DKO1/00058
175
EXAMPLE 144
o
I i I off
w I o w o~
(E~-(S/R)-3-[4-(3-Biphenyl-4-yl-but-2-enyloxy)-phenyl]-2-propoxy-propionic
acid
The title compound was prepared from (E~-(S/R)-propyl 3-[4-(3-biphenyl-4-yl-
but-2-enyloxy)-
phenyl]-2-propoxy-propionate (example 143) (330 mg, 0.70 mmol) and sodium
hydroxide
(1 M, 1.4 ml, 1.4 mmol) by a procedure analogous to that described in example
51, yielding
(~-(S/R)-3-[4-(3-biphenyl-4-yl-but-2-enyloxy)-phenyl]-2-propoxy-propionic acid
(300 mg,
100%) as a colourless gum.
'H NMR (300 MHz, CDCI3) 8: 0.88 (3H, t), 1.58 (2H, sextet), 2.17 (3H, s),
2.96,(1 H, dd), 3.10
(1 H, dd), 3.37 (1 H, dt), 3.50 (1 H, dt), 4.06 (1 H, dd), 4.75 (2H, d), 6.11
(1 H, t), 6.90 (2H, dm),
7.17 (2H, dm), 7.30-7.38 (1 H, m), 7.40-7.63 (8H, m), carboxylic acid proton
not observed: .
LCMS: 637 (M+207), 453 (100%, M+Na), 207.
EXAMPLE 145
0
\~ 0 0
\ /
~o
~o
(~-(S)-3-{4-[3-(3,5-Diethoxyoxy-phenyl)-allyloxy]-phenyl}-2-ethoxy-propionic
acid ethyl ester
The title compound was prepared from 3,5-dihydroxybenzaldehyde (3.0 g, 22.0
mmol) and
ethyl iodide (17.2 g, 110 mmol) by a sequence analogous to that described in
example 75.
CA 02395298 2002-06-18
WO 01/55085 PCT/DKO1/00058
176
'H NMR (300 MHz, CDC13) 8: 1.15 (t, 3H), 1.20 (t, 3H), 1.38 (t, 6H), 2.95 (d,
2H), 3.30-3.40
(m, 1 H), 3.53-3.65 (m, 1 H), 3.98 (q, 4H), 4.15 (q, 2H), 4.63 (d, 2H), 6.28-
6.40 (m, 2H), 6.53
(d, 2H), 6.60 (d, 1 H), 6.87 (d, 2H), 7.15 (d, 2H).
EXAMPLE 146
0
'' \ _
\ I o \ / o
~O OH
(E7-(S)-3-{4-[3-(3,5-Diethoxyoxy-phenyl)-allyloxy]-phenyl}-2-ethoxy-propionic
acid
The title compound was prepared from (E)-(S)-3-f4-[3-(3,5-diethoxyoxy-phenyl)-
allyloxy]- .
phenyl}-2-ethoxy-propionic acid ethyl ester (730 mg, 1.6 mmol) by a procedure
analogous to
that described in example 26.
'H NMR (300 MHz, CDCI3) 8: 1.15 (t, 3H), 1.22 (t, 3H), 1.38 (t, 6H), 2.95 (d,
2H), 3.28-3.38
(m, 1 H), 3.53-3.65 (m, 1 H), 3.98 (q, 4H), 4.15 (q, 2H), 4.63 (d, 2H), 6.28-
6.40 (m, 2H), 6.53
(d, 2H), 6.60 (d, 1 H), 6.85 (d, 2H), 7.15 (d, 2H).
EXAMPLE 147
F F
F
i \
\ ~ ~ O
\ /
F F F ~O
CA 02395298 2002-06-18
WO 01/55085 PCT/DKO1/00058
177
(~-(S)-3-{4-[3-(3,5-Bis-trifiuoromethyl-phenyl)-allyloxy]-phenyl}-2-ethoxy-
propionic acid ethyl
ester
The title compound was prepared from 3,5-bis(trifluoromethyl)benzaldehyde (5.0
g, 20.7
mmol) by a sequence analogous to that described in example 23. The title
compound was
purified on HPLC, using ethyl acetate/heptane (20:80) as eluent.
'H NMR (300 MHz, CDCI3) 8: 1.15 (t, 3H), 1.22 (t, 3H), 2.97 (d, 2H),. 3.30-
3.42-(m, 1H), 3.55-
3.67 (m, 1 H), 3.98 (t, 1 H), 4.18 (q, 2H), 4.72 (d, 2H), 6.55 (dt, 1 H), 6.80
(d, 1 H), 6.89 (d, 2H),
7.18 (d, 2H), 7.75 (bs, 1 H), 7.82 (bs, 2H).
EXAMPLE 148
i
~ \ /. o
~O OH
(E~-(S)-3-{4-[3-(3,5-Bis-trifluoromethyl-phenyl)-allyloxy]-phenyl}-2-ethoxy-
propionic acid
The title compound was prepared from (~-(S)-3-{4-[3-(3,5-bis-trifluoromethyl-
phenyl)
allyloxy]-phenyl}-2-ethoxy-propionic acid ethyl ester (0.58 g, 1.2 mmol) by a
procedure
20 analogous to that described in example 26.
'H NMR (300 MHz, CDCI3) 8: 1.18 (t, 3H), 2.98 (dd, 1 H), 3.08 (dd, 1 H), 3.36-
3.48 (m, 1 H);
3.58-3.71 (m, 1 H), 4.05 (dd, 1 H), 4.72 (d, 2H), 6.55 (dt, 1 H), 6.80 (d, 1
H), 6.89 (d, 2H), 7.20
(d, 2H), 7.75 (bs, 1 H), 7.82 (bs, 2H).
CA 02395298 2002-06-18
WO 01/55085 PCT/DKO1/00058
178
EXAMPLE 149
(E)-(R,S)-3-[4-(3-Biphenyl-4-yl-allyloxy)-phenyl]-2-ethoxy-propionic acid
ethyl ester
The title compound was prepared from 3-biphenyl-4-yl- prop-2-en-1-of (0.25 g,
0.001 mol) by
a procedure analogous to that described in example 3c yielding 0.050 g of (E'7-
(R,S)-3-[4-(3-
biphenyl-4-yl-allyloxy)-phenyl]-2-ethoxy-propionic acid ethyl ester.
'H NMR (200 MHz, CDCI3) 8: 1.1-1.26 (6H, m), 2.97 (2H, d), 3.3-3.4 (1 H, m),
3.52-3.7 (1 H,
m), 4.0 (1 H, t), 4.15 (2H, q), 4.75 (2H, dd), 6.35-6.5 (1 H, dt), 6.75 (1 H,
d), 6.87 (2H, d), 7:15
(2H, d), 7.4-7.7 (9H, m).
EXAMPLE 150
(~-(R,S)-3-[4-(3-Biphenyl-4-yl-allyioxy)-phenyl]-2-ethoxy-propionic acid
The title compound was prepared from (~-(S)-3-[4-(3-biphenyl-4-yl-aliyloxy)-
phenyl]-2-
ethoxy-propionic acid ethyl ester (example 149) (0.040 g) by a procedure
analogous to that
described in example 2 yielding. 0.0045 g of (E)-(R,S)-3-[4-(3-biphenyl-4-yl-
allyloxy)-phenyl]-
2-ethoxy-propionic acid.
CA 02395298 2002-06-18
WO 01/55085 PCT/DKO1/00058
179
~ H NMR (300 MHz, CDC13) 8: 1.14 (3H, t), 2.85 (1 H, dd), 3.1 (1 H, dd) 3.42-
3.57 (2H, m),
3.84-3.96 (2H, m), 4.1 (1 H, dd), 4.7 (2H, d), 6.3-6.5 (1 H, dt), 6.78 (1 H,
d), 6.88 (2H, d), 7.15
(2H, d) 7.4-7.6 (9H, m).
EXAMPLE 151
o \ / o
/ \ ~o o~
0
/ \
0
o \
(~-(S)- 4-(3-{3-[4-(2-Ethoxy-2-ethoxycarbonyl-ethyl)-phenoxy]-propenyl}-
phenoxymethyl)-
benzoic acid methyl ester
a)
(~-3-(3-Hydroxy-progeny!)-phenol was prepared from 3-hydroxybenzaldehyde. (6.0
g, 0.049
mol) by a procedure analogous to that described in example 1 a-b yielding 1.5
g
~H NMR (300 MHz, CDCI3) 8: 1.4 (1 H, t), 4.27 (2H, m), 4.88 (1 H, s), 6.35 (1
H, dt), 6.57 (1 H,
d), 6.68 (1 H, dd), 6.87 (1 H, s), 6.96 (1 H, d), 7.19 (1 H, dd).
b)
A mixture of (~-3-(3-Hydroxy-propenyl)-phenol (0.5 g, 3.33 mmol), methyl 4-
(bromomethyl)
benzoate (763 mg, 3.33 mmol) and potassium carbonate (1.8 g, 13.3 mmol) in
acetone (40
ml) was stirred at roomtemperature over night. The reaction mixtyre was added
water (30m1)
and acidified with 1 N HCI and extracted with ethyl acetate (90 ml). The
organic phase was
washed with water, brine and dried with sodium sulphate and evaporated and
dried in vacuo
CA 02395298 2002-06-18
WO 01/55085 PCT/DKO1/00058
180
yielding 954 mg (96%) (~- 4-[3-(3-Hydroxy-propenyl)-phenoxymethyl]-benzoic
acid methyl
ester.
' H NMR (300MHz, CDCI3) 8: 3.8 (3H, s), 4.24 (2H, d), 5.15 (2H, s), 6.3 (1 H,
dt), 6.57 (1 H, d),
7.0 (2H, d), 7.2 (1 H, d),. 7.51 (2H, d), 8.08 (2H, d).
C)
The title compound was prepared from (~- 4-[3-(3-Hydroxy-propenyl)-
phenoxymethyl]-
benzoic acid methyl ester (0.298 g, 1.0 mmol) by a procedure analogous to that
described in
example 3c yielding 0.184 g (35%) of (E7-(S)- 4-(3-{3-[4-(2-Ethoxy-2-
ethoxycarbonyl-ethyl)-
phenoxy]-propenyl}-phenoxymethyl)-benzoic acid methyl ester.
'H NMR (300 MHz, CDCI3) 8: 1.15-1.35 (6H, m), 2.9 (2H, d) 3.3-3.45 (1H, m),
3.53-3.68 (,1H,
m), 3.89 (3H, s), 3.97 (1 H, t), 4.13 (2H, q), 4.68 (2H, dd), 5.15 (2H, s),
6.35 (1 H, dt), 6.62
(1 H, d), 6.87 (3H, d), 7.05 (2H, d), 7.13-7.3 (3H, m), 7.5 (2H, d), 8.10 (2H,
d).
EXAMPLE 152
o ~ ~ o
/ \ ~o off
0
/ \
OH
O
(E~-(S)- 4-(3-{3-[4-(2-Carboxy-2-ethoxy-ethyl)-phenoxy]-propenyl}-
phenoxymethyl)-benzoic
acid
The title compound was prepared from (E7-(S)- 4-(3-(3-[4-(2-ethoxy-2-
ethoxycarbonyl-ethyl)-
phenoxy]-propenyl}-phenoxymethyl)-benzoic acid methyl ester (example 151 )
(0.220 g) by a
procedure analogous to that described in example 2 yielding 0.160 g (77%) (~-
(S)-4-(3-(3-
[4-(2-carboxy-2-ethoxy-ethyl)-phenoxy]-propenyl)-phenoxymethyl)-benzoic acid.
CA 02395298 2002-06-18
WO 01/55085 PCT/DKO1/00058
181
'H NMR (300 MHz, CDC13) 8: 8: 1.17 (3H, t), 2.9-3.15 (2H, m) 3.3-3.68 (2H, m),
4.1 (2H, q),
4.67 (2H, d), 5.17 (2H, s), 6.35 (1 H, dt), 6.68 (1 H, d), 6.86 (3H, d), 7.05
(2H, d), 7.12-7.32
(3H, m), 7.52 (2H, d), 8.12 (2H, d).
EXAMPLE 153
0
/ I ~ -oEt
\ \ O ~ OEt
\ ~ /
/
F
(~-(S)-Ethyl 2-Ethoxy-3-{4-[3-(4'-fluoro-biphenyl-4-yl)-but-2-enyloxy]-phenyl}-
propionate
a)
The colourless solid (~-ethyl 3-(4'-fluoro-biphenyl-4-yl)-but-2-enoate was
prepared from (~-
ethyl 3-(4-iodophenyl)-but-2-enoate (example 91a) and 4-fluorobenzene boronic
acid by a
procedure analogous to that described in example 52a.
Mpt. 63.5-64.5°C.'H NMR (300 MHz, CDCI3) 8: 1.33 (3H, t), 2.61 (3H, d),
4.23 (2H, q), 6.20
(1 H, m), 7.14 (2H, dd), 7.52-7.62 (6H, m). MS: 284 (100%, M+), 255, 239, 212,
196. Micro-
analysis Calculated % C: 76.04, H: 6.03; found C: 76.10, H; 6.17.
b)
The colourless solid (~-3-(4'-fluoro-biphenyl-4-yl)-but-2-en-1-of was prepared
by DIBAL-H
reduction of (~-ethyl 3-(4'-fluoro-biphenyl-4-yl)-but-2-enoate as described
for example 52b.
Mpt. 120.5-122°C (n-heptane).'H NMR (300 MHz, CDCI3) 8: 1.39 (1 H, br
s), 2.12 (3H, d),
4.40 (2H, d), 6.05 (1 H, tm), 7.12 (2H, dd), 7.42-7.60 (6H, m). MS: 242 (100%,
M+), 227 (M-
Me), 224 (M-H20), 203, 199. Microanalysis Calculated % C: 79.32, H: 6.24;
found C: 79.34,
H: 6.37.
c)
CA 02395298 2002-06-18
WO 01/55085 PCT/DKO1/00058
182
The title compound (849mg, 89%) was prepared as a colourless gum from (E)-3-
(4'-fluoro-
biphenyl-4-yl)-but-2-en-1-of (500 mg, 2.06 mmol) and (S)-ethyl 2-ethoxy-3-(4-
hydroxyphenyl)-propionate (516 mg, 2.17 mmol) by a procedure analogous to that
described
in example 52c.
'H NMR (200 MHz, CDCI3) 8: 1.17 (3H, t), 1.23 (3H, t), 2.17 (3H, d), 2.97 (2H,
d), 3.27-3.44
(1 H, m), 3.52-3.69 (1 H, m), 3.98 (1 H, t), 4.17 (2H, q), 4.75 (2H, d), 6.12
(1 H, tm), 6.88 (2H,
dm), 7.05-7.22 (4H, m), 7.44-7.62 (6H, m). LCMS: 687 (M+225), 641 (687-EtOH),
485
(M+Na), 480 (M+NH4), 225 (100%).
EXAMPLE 154
OH
(E7-(S)-2-Ethoxy-3-(4-[3-(4'-fluoro-biphenyl-4-yl)-but-2-enyloxy]-phenyl}-
propionic acid
The title compound was prepared from (E7-(S)-ethyl 2-ethoxy-3-{4-[3-(4'-fluoro-
biphenyl-4-yl)-
but-2-enyloxy]-phenyl}-propionate (example 153) (463 mg, 1.0 mmol) and sodium
hydroxide
(1 M, 1.5 ml, 1.5 mmol) by a procedure analogous to that described in example
51, yielding
(~-(S)-2-ethoxy-3-f4-[3-(4'-fluoro-biphenyl-4-yl)-but-2-enyloxy]-phenyl}-
propionic acid (229
mg, 53%) as a colourless solid containing a trace of water.
'H NMR (300 MHz, CDCI3) 8: 1.19 (3H, t), 2.16 (3H, d), 2.97 (1 H, dd), 3.09 (1
H, dd), 3.42-
3.65 (2H, m), 4.07 (1 H, dd), 4.75 (2H, d), 6.11 (1 H, tm), 6.90 (2H, dm),
7.07-7.20 (4H, m),
7.45-7.60 (6H, m), carboxylic acid proton not observed. LCMS: 457 (M+Na), 225
(100%). Mi-
croanalysis for CZ~H27F04~0.05H20 Calculated % C: 74.48, H: 6.27, HZO: 0.21;
found C:
74.25, H: 6.39, H2O: 0.21.
CA 02395298 2002-06-18
WO 01/55085 PCT/DKO1/00058
183
EXAMPLE 155
0
~OEt
O ~ OEt
/
I
(E'7-(S)-Ethyl2-Ethoxy-3-{4-[3-(4-lodophenyl)-but-2-enyloxy]-phenyl}-
propionate
The title compound (398 mg, 80%) was prepared as a colourless gum, from (E~-3-
(4-
iodophenyl)-but-2-en-1-of (example 107a) (275 mg, 1.0 mmol) and (S)-ethyl 2-
ethoxy-3-(4-
hydroxyphenyl)-propionate (256 mg, 1.07 mmol) by a procedure analogous to that
described
in example 52c.
'H NMR (300 MHz, CDCI3) 8: 1.17 (3H, t), 2.10 (3H, d), 2.96 (2H, d), 3.30-3.40
(1 H, m), 3.55-
3.65 (1 H, m), 3.97 (1 H, t), 4.96 (2H, q), 4.70 (2H, d), 6.04 (1 H, tm), 6.86
(2H, dm), 7.13-7.20
(4H, m), 7.64 (2H, dm). LCMS: 751 (M+257), 705 (751-EtOH), 517 (100%, M+Na),
512
(M+NH4), 449 (M+H-EtOH), 257, 130.