Note: Descriptions are shown in the official language in which they were submitted.
0~5~~510(5 CA 02396583 2002-07-05
1
4-Aryl-1-difluoromethoxyimidazole
The present invention relates to
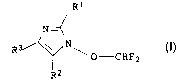
4-aryl-1-difluoromethoxyimidazoles of the formula I
R1
N
R3i \ N~ I r
O-CHFz
R2
in which the variables R1, R2 and R3 are as defined be:Low:
R1 is hydrogen, C1-C4-alkyl or C1-C4-haloalkyl;
RZ is hydrogen, halogen, cyano, C1-C4-alkyl or C1-C4-haloalkyl;
R3 is a 5- or 6-membered aromatic radical which may have one,
two or three heteroatoms selected from oxygen, nitrogen and
sulfur, which is unsubstituted or substituted and/or may have
one further fused-on 5- or 6-membered carbocyclic or
heterocyclic ring having 1 to 3 heteroatoms selected from
nitrogen, oxygen and sulfur atoms, where the fused-on ring is
partially or fully unsaturated, may be unsubstituted or may
for its part carry one, two or three substituents andlor may
contain one or two nonadjacent carbonyl, thiocarbonyl or
sulfonyl ring members,
and the agriculturally useful salts of I.
WO 91/13065, WO 97105115, WO 96/33994, WO 94/17059 and WO
99/05125 disclose herbicidally active compounds having a
phenyl-substituted heterocycle. Suitable heterocycles are, inter
olio, substituted imidazoles.
EP 590 843 describes 4-phenylimidazoles which may have a
C1-Clo-alkyl, C1-C6-haloalkyl, C3-C5-alkenyl or C3-C5-alkynyl group
at the imidazole nitrogen, and their use as herbicides.
Since the herbicidal properties of the prior art arylimidazoles
are, with respect to the harmful plants, nvt always entirely
satisfactory, it is an object of the present invention to provide
novel arylimidazoles which allow better targeted control of
X050/51065 CA 02396583 2002-07-05
2
undesirable plants than hitherto. This object also extends to
providing novel compounds having desiccant/defoliant action.
We have found that this object is achieved by
4-aryl-1-difluoromethoxyimidazoles which carry a difluoromethoxy
group in the 1 position of the imidazole ring.
Accordingly, the present invention relates to the
4-aryl-1-difluoromethoxyimidazoles of the formula I defined at
the outset and their agriculturually useful salts.
Moreover, the invention relates to
- the use of compounds I and their salts as herbicides and/or
for the desiccation and/or defoliation of plants,
- herbicidal compositions and compositions for the desiccation
and/or defoliation of plants, which compositions comprise the
compounds I and/or their salts as active substances,
- processes for preparing the compounds I and herbicidal
compositions and compositions for the desiccation and/or
defoliation of plants using the compounds I and/or ther
salts, and
- methods for controlling undesirable vegetation (harmful
plants) and for the desiccation and/or defoliation of plants
using the compounds I and/or their salts.
In the substituents, the compounds of the formula I may have one
or more chiral centers, in which case they are present as
enantiomer or diastereomer mixtures. The invention provides both
the pure enantiomers or diastereomers and mixtures thereof.
Suitable agriculturally useful salts are, in particular, the
salts of those cations or the acid addition salts of those acids
whose cations or anions, respectively, do not negatively affect
the herbicidal action of the compounds I. Thus, suitable cations
are, in particular, the ions of the alkali metals, preferably
sodium and potassium, of the alkaline earth metals, preferably
calcium, magnesium and barium, and of the transition metals,
preferably manganese, copper, zinc and iron, and the ammonium ion
which, if desired, may carry one to four C1-C4-alkyl substituents
and/or one phenyl or benzyl substituent, preferably
diisopropylammmonium, tetramethylammonium, tetrabutylammonium,
trimethylbenzylammonium, furthermore phosphonium ions, sulfonium
ions, preferably tri(C1-C4-alkyl)sulfonium, and sulfoxonium ions,
preferably tri(C1-C4-alkyl)sulfoxonium.
0050/51065 CA 02396583 2002-07-05
3
Anions of useful acid addition salts are primarily chloride,
bromide, fluoride, hydrogen sulfate, sulfate, dihydrogen
phosphate, hydrogen phosphate, phosphate, nitrate, hydrogen
carbonate, carbonate, hexafluorosilicate, hexafluorophosphate,
benzoate, and also the anions of C1-C4-alkanoic acids, preferably
formate, acetate, propionate and butyrate. They can be formed by
reaction of I with an acid of the corresponding anion, preferably
hydrochloric acid, hydrobromic acid, sulfuric acid, phosphoric
acid or nitric acid.
The organic molecule moieties mentioned in the definition of the
substituents R1, R2, R5, R8 to R19 or as radicals on cycloalkyl,
phenyl or heterocyclic rings or on X, Y and Z are - :like the term
halogen - collective terms for individual enumerations of the
individual group members. All carbon chains, i.e. al:L alkyl,
haloalkyl, phenylalkyl, cycloalkylalkyl, alkoxy, haloalkoxy,
alkylthio, haloalkylthio, alkylsulfinyl, haloalkylsu:Lfinyl,
alkylsulfonyl, haloalkylsulfonyl, alkenyl, haloalkenyl, alkynyl
and haloalkynyl groups and corresponding group moieties in larger
groups such as alkoxycarbonyl, phenylalkyl, cycloalkylalkyl,
alkoxycarbonylalkyl, etc., can be straight-chain or branched, the
prefix C"-Cm in each case denoting the possible number of carbon
atoms in the group. Halogenated substituents preferably carry
one, two, three, four or five identical or different halogen
atoms. In each case, the term halogen denotes fluorine, chlorine,
bromine or iodine.
Other examples of meanings are:
- C1-C4-alkyl: CH3, CZHS, n-propyl, CH(CH3)2, n-butyl,
CH(CH3)-CZHS, CHZ-CH(CH3)2 and C(CH3)3;
- C1-C4-haloalkyl: a C1-C4-alkyl radical as mentioned above
which is partially or fully substituted by fluorine,
chlorine, bromine and/or iodine, i.e. for example CH2F, CHF2,
CF3, CHzCl, dichloromethyl, trichloromethyl,
chlorofluoromethyl, dichlorofluoromethyl, chlorodifluoro-
methyl, 2-fluoroethyl, 2-chloroethyl, 2-bromoethyl,
2-iodoethyl, 2,2-difluoroethyl, 2,2,2-trifluoroethyl,
2-chloro-2-fluoroethyl, 2-chloro-2,2-difluoroethyl,
2,2-dichloro-2-fluoroethyl, 2,2,2-trichloroethyl, C2F5,
2-fluoropropyl, 3-fluoropropyl, 2,2-difluoropropyl,
2,3-difluoropropyl, 2-chloropropyl, 3-chloropropyl,
2,3-dichloropropyl, 2-bromopropyl, 3-bromopropyl,
3,3,3-trifluoropropyl, 3,3,3-trichloropropyl,
2,2,3,3,3-pentafluoropropyl, heptafluoropropyl,
1-fluoromethyl-2-fluoroethyl, 1-chloromethyl-2-chloroethyl,
~05~/51065 CA 02396583 2002-07-05
4
1-bromomethyl-2-bromoethyl, 4-fluorobutyl, 4-chlorobutyl,
4-bromobutyl or nonafluorobutyl;
- Cl-C6-alkyl: C1-C4-alkyl as mentioned above, and also, for
example, n-pentyl, 1-methylbutyl, 2-methylbutyl,
3-methylbutyl, 2,2-dimethylpropyl, 1-ethylpropyl, n-hexyl,
1,1-dimethylpropyl, 1,2-dimethylpropyl, 1-methylpentyl,
2-methylpentyl, 3-methylpentyl, 4-methylpentyl,
1,1-dimethylbutyl, 1,2-dimethylbutyl, 1,3-dimethylbutyl,
2,2-dimethylbutyl, 2,3-dimethylbutyl, 3,3-dimethylbutyl,
1-ethylbutyl, 2-ethylbutyl, 1,1,2-trimethylpropyl,
1,2,2-trimethylpropyl, 1-ethyl-1-methylpropyl or
1-ethyl-2-methylpropyl, preferably methyl, ethyl, n-propyl,
1-methylethyl, n-butyl, 1,1-dimethylethyl, n-pentyl or
n-hexyl;
- C1-C6-haloalkyl: a C1-C6-alkyl radical as mentioned above
which is partially or fully substituted by fluorine,
chlorine, bromine and/or iodine, i.e. for example one of
the radicals mentioned under C1-C4-haloalkyl, and also
5-fluoro-1-pentyl, 5-chloro-1-pentyl, 5-bromo-1-pentyl,
5-iodo-1-pentyl, 5,5,5-trichloro-1-pentyl, undecafluoro-
pentyl, 6-fluoro-1-hexyl, 6-chloro-1-hexyl, 6-bromo-1-hexyl,
6-iodo-1-hexyl, 6,6,6-trichloro-1-hexyl or dodecafluorohexyl;
- phenyl-C1-C4-alkyl: benzyl, 1-phenylethyl, 2-phenylethyl,
1-phenylprop-1-yl, 2-phenylprop-1-yl, 3-phenylprop-1-yl,
1-phenylbut-1-yl, 2-phenylbut-1-yl, 3-phenylbut-1-yl,
4-phenylbut-1-yl, 1-phenylbut-2-yl, 2-phenylbut-2-yl,
3-phenylbut-2-yl, 4-phenylbut-2-yl, 1-phenylmethyleth-1-yl,
1-phenylmethyl-1-methyleth-1-yl or 1-phenylmethy:Lprop-1-yl,
preferably benzyl or 2-phenylethyl;
- heterocyclyl-C1-C4-alkyl: heterocyclylmethyl,
1-heterocyclylethyl, 2-heterocyclylethyl,
1-heterocyclylprop-1-yl, 2-heterocyclylprop-1-yl,
3-heterocyclylprop-1-yl, 1-heterocyclylbut-1-yl,
2-heterocyclylbut-1-yl, 3-heterocyclylbut-1-yl,
4-heterocyclylbut-1-yl, 1-heterocyclylbut-2-yl,
2-heterocyclylbut-2-yl, 3-hetero-cyclylbut-2-yl,
4-heterocyclylbut-2-yl, 1-heterocyclylmethyleth-:l-yl,
1-heterocyclylmethyl-1-methyleth-1-yl or
1-heterocyclylmethylprop-1-yl, preferably heterocyclylmethyl
or 2-heterocyclylethyl;
0050/51065
CA 02396583 2002-07-05
- C1-C4-alkoxy: OCH3, OC2H5, n-propoxy, OCH(CH3)z, n-butoxy,
OCH(CH3)-C2H5, OCHZ-CH(CH3)z Or OC(CH3)3, preferably OCH3, OC2H5
or OCH(CH3)z;
5 - C1-C4-haloalkoxy: a C1-C4-alkoxy radical as mentioned above
which is partially or fully substituted by fluorine,
chlorine, bromine and/or iodine, i.e. for example OCHZF,
OCHFZ, OCF3, OCHzCl, OCH(C1)z, OC(Cl)3, chlorofluoromethoxy,
dichlorofluoromethoxy, chlorodifluoromethoxy, 2-fluoroethoxy,
2-chloroethoxy, 2-bromoethoxy, 2-iodoethoxy,
2,2-difluoroethoxy, 2,2,2-trifluoroethoxy,
2-chloro-2-fluoroethoxy, 2-chloro-2,2-difluoroethoxy,
2,2-dichloro-2-fluoroethoxy, 2,2,2-trichloroethoxy, OC2F5,
2-fluoropropoxy, 3-fluoropropoxy, 2,2-difluoropropoxy,
2,3-difluoropropoxy, 2-chloropropoxy, 3-chloropropoxy,
2,3-dichloropropoxy, 2-bromopropoxy, 3-bromopropoxy,
3,3,3-trifluoropropoxy, 3,3,3-trichloropropoxy,
2,2,3,3,3-pentafluoropropoxy, OCFZ-C2F5, 1-(CH2F)-2-fluoro-
ethoxy, 1-(CH2C1)-2-chloroethoxy, 1-(CHZBr)-2-bramoethoxy,
4-fluorobutoxy, 4-chlorobutoxy, 4-bromobutoxy or
nonafluorobutoxy, preferably OCHFZ, OCF3,
dichlorofluoromethoxy, chlorodifluoromethoxy or
2,2,2-trifluoroethoxy;
- C1-C6-alkylthio: SCH3, SC2H5, n-propylthio, SCH(CH3)z.
n-butylthio, SCH(CH3)-CZHS, SCHZ-CH(CH3)z or SC(CH3)3.
preferably SCH3 or SC2H5;
- C1-C4-haloalkylthio: a C1-C4-alkylthio radical as mentioned
above which is partially or fully substituted by fluorine,
chlorine, bromine and/or iodine, i.e. for example SCH2F,
SCHFZ, SCH2C1, SCH(C1)z, SC(C1)3, SCF3,
chlorofluoromethylthio, dichlorofluoro-
methylthio, chlorodifluoromethylthio, 2-fluoroethylthio,
2-chloroethylthio, 2-bromoethylthio, 2-iodoethylthio,
2,2-difluoroethylthio, 2,2,2-trifluoroethylthio, 2-chloro-
2-fluoroethylthio, 2-chloro-2,2-difluoroethylthio,
2,2-dichloro-2-fluoroethylthio, 2,2,2-trichloroethylthio,
SC2F5, 2-fluoropropylthio, 3-fluoropropylthio, 2,2-difluoro-
propylthio, 2,3-difluoropropylthio, 2-chloropropylthio,
3-chloropropylthio, 2,3-dichloropropylthio, 2-bromopropyl-
thio, 3-bromopropylthio, 3,3,3-trifluoropropylthio,
3,3,3-trichloropropylthio, SCHZ-C2F5, SCFz-C2F5,
1-(CHzF)-2-fluoroethylthio, 1-(CH2C1)-2-chloro-
ethylthio, 1-(CHZBr)-2-bromoethylthio, 4-fluorobutylthio,
4-chlorobutylthio, 4-bromobutylthio or SCFZ-CFZ-C2F5,
0050/51065
CA 02396583 2002-07-05
6
preferably SCHFZ, SCF3, dichlorofluoromethyl-
thio, chlorodifluoromethylthio or 2,2,2-trifluoroethylthio;
- C1-C4-alkoxy-C1-C4-alkyl: C1-C4-alkyl which is substituted by
C1-C4-alkoxy as mentioned above, i.e. for example CH2-OCH3,
CHz-OCZHS, n-propoxymethyl, CHZ-OCH(CH3)2, n-butoxymethyl,
(1-methylpropoxy)methyl, (2-methylpropoxy)methyl,
CHZ-OC(CH3)3, 2-(methoxy)ethyl, 2-(ethoxy)ethyl,
2-(n-propoxy)ethyl, 2-(1-methylethoxy)ethyl,
2-(n-butoxy}ethyl, 2-(1-methylpropoxy}ethyl, 2-(2-methyl-
propoxy)ethyl, 2-(1,1-dimethylethoxy)ethyl, 2-(methoxy)-
propyl, 2-(ethoxy)propyl, 2-(n-propoxy)propyl, 2-(1-methyl-
ethoxy)propyl, 2-(n-butoxy)propyl, 2-(1-methylpropoxy)propyl,
2-(2-methylpropoxy)propyl, 2-(1,1-dimethylethoxy)propyl,
3-(methoxy)propyl, 3-(ethoxy)propyl, 3-(n-propox;y)propyl,
3-(1-methylethoxy)propyl, 3-(n-butoxy)propyl, 3-(1-methyl-
propoxy)propyl, 3-(2-methylpropoxy)propyl, 3-(1,1-dimethyl-
ethoxy)propyl, 2-(methoxy)butyl, 2-(ethoxy)butyl,
2-(n-propoxy)butyl, 2-(1-methylethoxy)butyl, 2-(n-butoxy}
butyl, 2-(1-methylpropoxy)butyl, 2-(2-methylpropoxy)butyl,
2-(1,1-dimethylethoxy)butyl, 3-(methoxy)butyl, 3-(ethoxy)
butyl, 3-(n-propoxy)butyl, 3-(1-methylethoxy)butyl,
3-(n-butoxy}butyl, 3-(1-methylpropoxy)butyl, 3-(2-methyl-
propoxy)butyl, 3-(1,1-dimethylethoxy)butyl, 4-(methoxy)butyl,
4-(ethoxy)butyl, 4-(n-propoxy)butyl, 4-(1-methylethoxy)butyl,
4-(n-butoxy)butyl, 4-(1-methylpropoxy)butyl, 4-(2-methyl-
propoxy)butyl or 4-(1,1-dimethylethoxy)butyl, preferably
CHZ-OCH3, CHZ-OCZHS, 2-methoxyethyl or 2-ethoxyethyl;
- C1-C4-alkylthio-C1-C4-alkyl: C1-C4-alkyl which is substituted
by C1-C4-alkylthio as mentioned above, i.e. for example
CHZ-SCH3, CHZ-SC2H5, n-propylthiomethyl, CH2-SCH(CH3)2.
n-butylthiomethyl, (1-methylpropylthio)methyl,
(2-methylpropylthio)methyl, CHZ-SC(CH3)3, 2-(methylthio}ethyl,
2-(ethylthio)ethyl, 2-(n-propylthio)ethyl,
2-(1-methylethylthio}ethyl, 2-(n-butylthio)ethyl,
2-(1-methylpropylthio)ethyl, 2-(2-methylpropylthio)ethyl,
2-(l,l-dimethylethylthio)ethyl, 2-(methylthio)propyl,
2-(ethylthio)propyl, 2-(n-propylthio)-
propyl, 2-(1-methylethylthio)propyl, 2-(n-butylthio)propyl,
2-(1-methylpropylthio)propyl, 2-(2-methylpropylthio)propyl,
2-(1,1-dimethylethylthio)propyl, 3-(methylthio)propyl,
3-(ethylthio)propyl, 3-(n-propylthio)propyl, 3-(1-methyl-
ethylthio)propyl, 3-(n-butylthio)propyl, 3-(1-methylpropyl-
thio)propyl, 3-(2-methylpropylthio)propyl, 3-(1,1-dimethyl-
ethylthio)propyl, 2-(methylthio)butyl, 2-(ethylthio)butyl,
2-(n-propylthio)butyl, 2-(1-methylethylthio)butyl,
0050/51065 CA 02396583 2002-07-05
7
2-(n-butylthio)butyl, 2-(1-methylpropylthio)butyl,
2-(2-methylpropylthio)butyl, 2-(1,1-dimethylethylthio)butyl,
3-(methylthio)butyl, 3-(ethylthio)butyl, 3-(n-propylthio)-
butyl, 3-(1-methylethylthio)butyl, 3-(n-butylthio)butyl,
3-(1-methylpropylthio)butyl, 3-(2-methylpropylthio)butyl,
3-(l,l-dimethylethylthio)butyl, 4-(methylthio)butyl,
4-(ethylthio)butyl, 4-(n-propylthio)butyl, 4-(1-methyl-
ethylthio)butyl, 4-(n-butylthio)butyl, 4-(1-methylpropyl-
thio)butyl, 4-(2-rnethylpropylthio)butyl or 4-(1,1-dimethyl-
ethylthio)butyl, preferably CHZ-SCH3, CHz-SCzHS.
2-methylthioethyl or 2-ethylthioethyl;
- (C1-C4-alkyl)carbonyl: CO-CH3, CO-CzHS, CO-CH2-CZH5,
CO-CH(CH3)2, n-butylcarbonyl, CO-CH(CH3)-CZHS, CO-CHZ-CH(CH3)Z
or CO-C(CH3)3, preferably CO-CH3 or CO-CzHS;
(C1-C4-haloalkyl)carbonyl: a (C1-C4-alkyl)carbonyl radical as
mentioned above which is partially or fully substituted by
fluorine, chlorine, bromine and/or iodine, i.e. for example
CO-CHZF, CO-CHF2, CO-CF3, CO-CHZC1, CO-CH(Cl)2, CO-C(C1)3,
chlorofluoromethylcarbonyl, dichlorofluoromethylcarbonvl,
0050/51065 CA 02396583 2002-07-05
O-CO-CH2F, O-CO-CHF2, O-CO-CFg, O-CO-CH2C1, O-CO-CH(C1)2,
O-CO-C(C1)3, chlorofluoromethylcarbonyloxy,
dichlorofluoromethylcarbonyloxy,
chlorodifluoromethylcarbonyloxy, 2-fluoroethylcarbonyloxy,
2-chloroethylcarbonyloxy, 2-bromoethylcarbonyloxy,
2-iodoethylcarbonyloxy, 2,2-difluoroethylcarbonyloxy,
2,2,2-trifluoroethylcarbonyloxy, 2-chloro-2-fluoroethyl-
carbonyloxy, 2-chloro-2,2-difluoroethylcarbonylo:xy,
2,2-dichloro-2-fluoroethylcarbonyloxy, 2,2,2-trichloroethyl-
carbonyloxy, 0-CO-C2F5, 2-fluoropropylcarbonyloxy,
3-fluoropropylcarbonyloxy, 2,2-difluoropropylcarbonyloxy,
2,3-difluoropropylcarbonyloxy, 2-chloropropylcarbonyloxy,
3-chloropropylcarbonyloxy, 2,3-dichloropropylcarbonyloxy,
2-bromopropylcarbonyloxy, 3-bromopropylcarbonyloxy,
3,3,3-trifluoropropylcarbonyloxy, 3,3,3-trichloropropyl-
carbonyloxy, 2,2,3,3,3-pentafluoropropylcarbonyloxy,
heptafluoropropylcarbonyloxy, 1-(CHZF)-2-fluoroethyl-
carbonyloxy, 1-(CH2C1)-2-chloroethylcarbonyloxy,
1-(CH2Br)-2-bromoethylcarbonyloxy, 4-fluorobutylcarbonyloxy,
4-chlorobutylcarbonyloxy, 4-bromobutylcarbonyloxy or
nonafluorobutylcarbonyloxv, preferably O-CO-CFA. O-CO-CH~Cl.
CA 02396583 2002-07-05
0050/51065
9
3-(n-propoxycarbonyl)propyl, 3-(1-methylethoxycarbonyl)-
propyl, 3-(n-butoxycarbonyl)propyl, 3-(1-methylp:ropoxy-
carbonyl)propyl, 3-(2-methylpropoxycarbonyl)propyl,
3-(1,1-dimethylethoxycarbonyl)propyl, 2-(methoxycarbonyl)
butyl, 2-(ethoxycarbonyl)butyl, 2-(n-propoxycarbonyl)butyl,
2-(1-methylethoxycarbonyl)butyl, 2-(n-butoxycarbonyl)butyl,
2-(1-methylpropoxycarbonyl)butyl, 2-(2-methylpropoxy
carbonyl)butyl, 2-(1,1-dimethylethoxycarbonyl)butyl,
3-(methoxycarbonyl)butyl, 3-(ethoxycarbonyl)buty:l,
3-(n-propoxycarbonyl)butyl, 3-(1-methylethoxycarbonyl)butyl,
3-(n-butoxycarbonyl)butyl, 3-(1-methylpropoxycarbonyl)butyl,
3-(2-methylpropoxycarbonyl)butyl, 3-(1,1-dimethy:lethoxy-
carbonyl)butyl, 4-(methoxycarbonyl)butyl, 4-(ethoxy-
carbonyl)butyl, 4-(n-propoxycarbonyl)butyl, 4-(1-methyl-
ethoxycarbonyl)butyl, 4-(n-butoxycarbonyl)butyl, 4-(1-methyl-
propoxycarbonyl)butyl, 4-(2-methylpropoxycarbonyl)butyl or
4-(1,1-dimethylethoxycarbonyl)butyl, preferably
methoxycarbonylmethyl, ethoxycarbonylmethyl,
1-(methoxycarbonyl)ethyl or 1-(ethoxycarbonyl)ethyl;
- (C1-C4-alkoxy)carbonyl-C1-C4-alkoxy: C1-C4-alkoxy which is
005/51065 CA 02396583 2002-07-05
3-(n-butoxycarbonyl)propoxy,
3-(1-methylpropoxycarbonyl)propoxy,
3-(2-methylpropoxycarbonyl)propoxy,
3-(1,1-dimethylethoxycarbonyl)propoxy,
5 2-(methoxycarbonyl)butoxy, 2-(ethoxycarbonyl)butoxy,
2-(n-propoxycarbonyl)butoxy,
2-(1-methylethoxycarbonyl)butoxy, 2-(n-butoxycarbonyl)butoxy,
2-(1-methylpropoxycarbonyl)butoxy,
2-(2-methylpropoxycarbonyl)butoxy,
10 2-(1,1-dimethylethoxycarbonyl)butoxy,
3-(methoxycarbonyl)butoxy, 3-(ethoxycarbonyl)butoxy,
3-(n-propoxycarbonyl)butoxy,
3-(1-methylethoxycarbonyl)butoxy, 3-(n-butoxycarbonyl)butoxy,
3-(1-methylpropoxycarbonyl)butoxy,
3-(2-methylpropoxycarbonyl)butoxy,
3-(1,1-dimethylethoxycarbonyl)butoxy,
4-(methoxycarbonyl)butoxy, 4-(ethoxycarbonyl)butoxy,
4-(n-propoxycarbonyl)butoxy,
4-(1-methylethoxycarbonyl)butoxy, 4-(n-butoxycarbonyl)butoxy,
4-(1-methylpropoxycarbonyl)butoxy,
4-(2-methylpropoxycarbonyl)butyl or
4-(1,1-dimethylethoxycarbonyl)butoxy, preferably
methoxycarbonylmethoxy, ethoxycarbonylmethoxy,
1-(methoxycarbonyl)ethoxy or 1-(ethoxycarbonyl)ethoxy;
- (C1-C4-alkoxy)carbonyl-C1-C4-alkylthio: C1-C4-alkylthio which
is substituted by (C1-C4-Alkoxy)carbonyl as mentioned above,
i.e., for example, methoxycarbonylmethylthio,
ethoxycarbonylmethylthio, n-propoxycarbonylmethylthio,
(1-methylethoxycarbonyl)methylthio,
n-butoxycarbonylmethylthio,
(1-methylpropoxycarbonyl)methylthio,
(2-methylpropoxycarbonyl)methylthio,
(1,1-di.methylethoxycarbonyl)methylthio,
1-(methoxycarbonyl)ethylthio, 1-(ethoxycarbonyl)ethylthio,
1-(n-propoxycarbonyl)ethylthio,
1-(1-methylethoxycarbonyl)ethylthio,
1-(n-butoxycarbonyl)ethylthio, Z-(methoxycarbonyl)ethylthio,
2-(ethoxycarbonyl)ethylthio, 2-(n-propoxycarbony:l)ethylthio,
2-(1-methylethoxycarbonyl)ethylthio,
2-(n-butoxycarbonyl)ethylthio,
2-(1-methylpropoxycarbonyl)ethylthio,
2-(2-methylpropoxycarbonyl)ethylthio,
2-(1,1-dimethylethoxycarbonyl)ethylthio,
2-(methoxycarbonyl)propylthio, 2-(ethoxycarbonyl)propylthio,
2-(n-propoxycarbonyl)propylthio,
2-(1-methylethoxycarbonyl)propylthio,
CA 02396583 2002-07-05
0050/51065
11
2-(n-butoxycarbonyl)propylthio,
2-(1-methylpropoxycarbonyl)propylthio,
2-(2-methylpropoxycarbonyl)propylthio,
2-(1,1-dimethylethoxycarbonyl)propylthio,
3-(methoxycarbonyl)propylthio, 3-(ethoxycarbonyl)propylthio,
3-(n-propoxycarbonyl)propylthio,
3-(1-methylethoxycarbonyl)propylthio,
3-(n-butoxycarbonyl)propylthio,
3-(1-methylpropoxycarbonyl)propylthio,
3-(2-methylpropoxycarbonyl)propylthio,
3-(1,1-dimethylethoxycarbonyl)propylthio,
2-(methoxycarbonyl)butylthio, 2-(ethoxycarbonyl)butylthio,
2-(n-propoxycarbonyl)butylthio,
2-(1-methylethoxycarbonyl)butylthio,
2-(n-butoxycarbonyl)butylthio,
2-(1-methylpropoxycarbonyl)butylthio,
2-(2-methylpropoxycarbonyl)butylthio,
2-(1,1-dimethylethoxycarbonyl)butylthio,
3-(methoxycarbonyl)butylthio, 3-(ethoxycarbonyl)butylthio,
3-(n-propoxycarbonyl)butylthio,
3-(1-methylethoxycarbonyl)butylthio,
3-(n-butoxycarbonyl)butylthio,
3-(1-methylpropoxycarbonyl)butylthio,
3-(2-methylpropoxycarbonyl)butylthio,
3-(1,1-dimethylethoxycarbonyl)butylthio,
4-(methoxycarbonyl)butylthio, 4-(ethoxycarbonyl)butylthio,
4-(n-propoxycarbonyl)butylthio,
4-(1-methylethoxycarbonyl)butylthio,
4-(n-butoxycarbonyl)butylthio,
4-(1-methylpropoxycarbonyl)butylthio,
4-(2-methylpropoxycarbonyl)butyl or
4-(1,1-dimethylethoxycarbonyl)butylthio, preferably
methoxycarbonylmethylthio, ethoxycarbonylmethylthio,
1-(methoxycarbonyl)ethylthio or 1-(ethoxycarbonyl)ethylthio;
- C1-Cq-alkylsulfinyl: SO-CH3, SO-CZHg, SO-CHZ-C2H5, SO-CH(CH3)2,
n-butylsulfinyl, SO-CH(CH3)-CZHS, SO-CH2-CH(CH3)z or
SO-C(CH3)3, preferably SO-CH3 or SO-C2H5;
- C1-C4-haloalkylsulfinyl: a C1-C4-alkylsulfinyl radical as
mentioned above which is partially or fully substituted by
fluorine, chlorine, bromine and/or iodine, i.e. for example
SO-CHZF, SO-CHF2, SO-CF3, SO-CHZCl, SO-CH(Cl)y, SO-C(C1)3,
chlorofluoromethylsulfinyl, dichlorofluoromethylsulfinyl,
chlorodifluoromethylsulfinyl, 2-fluoroethylsulfinyl,
2-chloroethylsulfinyl, 2-bromoethyl-
sulfinyl, 2-iodoethylsulfinyl, 2,2-difluoroethylsulfinyl,
0050J51065
CA 02396583 2002-07-05
12
2,2,2-trifluoroethylsulfinyl, 2-chloro-2-fluoroethylsulfinyl,
2-chloro-2,2-difluoroethylsulfinyl, 2,2-dichloro-2-fluoro-
ethylsulfinyl, 2,2,2-trichloroethylsulfinyl, SO-C2F5,
2-fluoropropylsulfinyl, 3-fluoropropylsulfinyl, 2,2-difluoro-
propylsulfinyl, 2,3-difluoropropylsulfinyl, 2-ch:loropropyl-
sulfinyl, 3-chloropropylsulfinyl, 2,3-dichloropropylsulfinyl,
2-bromopropylsulfinyl, 3-bromopropylsulfinyl,
3,3,3-trifluoropropylsulfinyl, 3,3,3-trichloropropylsulfinyl,
SO-CHz-CZFS, SO-CFz-C2F5, 1-(fluoromethyl)-2-
fluoroethylsulfinyl, 1-(chloromethyl)-2-chloroethylsulfinyl,
1-(bromomethyl)-2-bromoethylsulfinyl, 4-fluorobutylsulfinyl,
4-chlorobutylsulfinyl, 4-bromobutylsulfinyl or
nonafluorobutylsulfinyl, preferably SO-CF3, SO-CHzCl or
2,2,2-trifluoroethylsulfinyl;
- C1-C4-alkylsulfonyl: SOZ-CH3, SOZ-C2H5, SOz-CHZ-CZHS,
SOz-CH(CH3)z. n-butylsulfonyl, SOz-CH(CH3)-CzHSr
50z-CHZ-CH(CH3)z or SOz-C(CH3)3, preferably SOz-CHI or SOz-C2H5;
- C1-C4-haloalkylsulfonyl: a C1-C4-alkylsulfonyl radical as
mentioned above which is partially or fully substituted by
0050/51065 CA 02396583 2002-07-05
13
N-methyl-N-(1-methylethyl)amino, N-butyl-N-methylamino,
N-methyl-N-(1-methylpropyl)amino,
N-methyl-N-(2-methylpropyl)amino, N-(1,1-dimethylethyl)-
N-methylamino, N-ethyl-N-propylamino, N-ethyl-N-(1-methyl-
ethyl)amino, N-butyl-N-ethylamino, N-ethyl-N-(1-methyl-
propyl)amino, N-ethyl-N-(2-methylpropyl)amino, N-ethyl-
N-(1,1-dimethylethyl)amino, N-(1-methylethyl)-N-propylamino,
N-butyl-N-propylamino, N-(1-methylpropyl)-N-propylamino,
N-(2-methylpropyl)-N-propylamino, N-(1,1-dimethy.lethyl)-
N-propylamino, N-butyl-N-(1-methylethyl)amino, N~-(1-methyl-
ethyl)-N-(1-methylpropyl)amino, N-{1-methylethyl)-
N-(2-methylpropyl)amino, N-(1,1-dimethylethyl)-
N-(1-methylethyl)amino, N-butyl-N-(1-methylpropy:l)amino,
N-butyl-N-(2-methylpropyl)amino, N-butyl-N-(1,1-dimethyl-
ethyl)amino, N-(1-methylpropyl)-N-(2-methylpropy:l)amino,
N-(1,1-dimethylethyl)-N-(1-methylpropyl)amino or
N-(1,1-dimethylethyl)-N-(2-methylpropyl)amino, preferably
N{CH3)2 or N(C2H5);
- Di(C1-C4-alkyl)aminocarbonyl: for example
N,N-dimethylaminocarbonyl, N,N-diethylaminocarbanyl,
N,N-di-(1-methylethyl)aminocarbonyl,
N,N-dipropylaminocarbonyl, N,N-dibutylaminocarbonyl,
N,N-di-(1-methylpropyl)aminocarbonyl,
N,N-di-(2-methylpropyl)aminocarbonyl,
N,N-di-(1,1-dimethylethyl)aminocarbonyl,
N-ethyl-N-methylaminocarbonyl,
N-methyl-N-propylaminocarbonyl,
N-methyl-N-(1-methylethyl)aminocarbonyl,
N-butyl-N-methylaminocarbonyl,
N-methyl-N-(1-methylpropyl)aminocarbonyl,
N-methyl-N-(2-methylpropyl)aminocarbonyl,
N-(1,1-dimethylethyl)-N-methylaminocarbonyl,
N-ethyl-N-propylaminocarbonyl,
N-ethyl-N-(1-methylethyl)aminocarbonyl,
N-butyl-N-ethylaminocarbonyl,
N-ethyl-N-(1-methylpropyl)aminocarbonyl,
N-ethyl-N-(2-methylpropyl)aminocarbonyl,
N-ethyl-N-(1,1-dimethylethyl)aminocarbonyl,
N-(1-methylethyl)-N-propylaminocarbonyl,
N-butyl-N-propylaminocarbonyl,
N-(1-methylpropyl)-N-propylaminocarbonyl,
N-(2-methylpropyl)-N-propylaminocarbonyl,
N-(1,1-dimethylethyl)-N-propylaminocarbonyl,
N-butyl-N-(1-methylethyl)aminocarbonyl,
N-(1-methylethyl)-N-(1-methylpropyl)aminocarbonyl,
N-(1-methylethyl)-N-(2-methylpropyl)aminocarbonyl,
0050/51065
CA 02396583 2002-07-05
14
N-(1,1-dimethylethyi)-N-(1-methylethyl}aminocarbonyl,
N-butyl-N-(1-methylpropyl)aminocarbonyl,
N-butyl-N-(2-methylpropyl}aminocarbonyl,
N-butyl-N-(1,1-dimethylethyl)aminocarbonyl,
N-(1-methylpropyl)-N-(2-methylpropyl)aminocarbonyl,
N-(1,1-dimethylethyl)-N-(1-methylpropyl)aminocarbonyl or
N-(1,1-dimethylethyl)-N-(2-methylpropyl)aminocarbonyl;
- Di(C1-Cq-alkyl)aminocarbonyl-C1-C4-alkyl: C1-C4-alkyl which is
monosubstituted by di(C1-C4-alkyl)aminocarbonyl, for example
di(C1-C4-alkyl)aminocarbonylmethyl, 1- or
2-di(C1-C4-alkyl)aminocarbonylethyl, 1-, 2- or
3-di(C1-C4-alkyl)aminocarbonylpropyl;
- Di(C1-C4-alkyl)aminocarbonyl-C1-C4-alkoxy: C1-C4-alkoxy which
is monosubstituted by di(C1-C4-alkyl)aminocarbonyl, for
example di(C1-C4-alkyl)aminocarbonylmethoxy, 1- or
2-di(C1-C4-alkyl)aminocarbonylethoxy, 1-, 2- or
3-di(C1-C4-alkyl)aminocarbonylpropoxy;
- Di(C1-C4-alkyl)aminocarbonyl-C1-C4-alkylthio: C1-C4-alkylthio
which is monosubstituted by di(C1-C4-alkyl)aminocarbonyl, for
example di(C1-C4-alkyl)aminocarbonylmethylthio, 7.- or
2-di(C1-C4-alkyl)aminocarbonylethylthio, 1-, 2- or
3-di(C1-C4-alkyl)aminocarbonylpropylthio;
C2-C6-alkenyl: vinyl, prop-1-en-1-yl, allyl, 1-methylethenyl,
1-buten-1-yl, 1-buten-2-yl, 1-buten-3-yl, 2-buten-1-yl,
1-methylprop-1-en-1-yl, 2-methylprop-1-en-1-yl, :1-methyl-
prop-2-en-1-y1, 2-methylprop-2-en-1-yl, n-penten-1-yl,
n-penten-2-yl, n-penten-3-yl, n-penten-4-yl, 1-methyl-
but-1-en-1-yl, 2-methylbut-1-en-1-yl, 3-methylbut-1-en-1-yl,
1-methylbut-2-en-1-yl, 2-methylbut-2-en-1-yl, 3-methyl-
but-2-en-1-yl, 1-methylbut-3-en-1-yl, 2-methylbut-3-en-1-yl,
3-methylbut-3-en-1-yl, 1,1-dimethylprop-2-en-1-yl,
1,2-dimethylprop-1-en-1-yl, 1,2-dimethylprop-2-en-1-yl,
1-ethylprop-1-en-2-yl, 1-ethylprop-2-en-1-yl, n-hex-1-en-
1-yl, n-hex-2-en-1-yl, n-hex-3-en-1-yl, n-hex-4-en-1-yl,
n-hex-5-en-1-yl, 1-methylpent-1-en-1-yl, 2-methylpent-1-en-
1-yl, 3-methylpent-1-en-1-yl, 4-methylpent-1-en-1-yl,
1-methylpent-2-en-1-yl, 2-methylpent-2-en-1-yl, 3-methyl-
pent-2-en-1-yl, 4-methylpent-2-en-1-yl, 1-methylpent-3-en-
1-yl, 2-methylpent-3-en-1-yl, 3-methylpent-3-en-1-yl,
4-methylpent-3-en-1-yl, 1-methylpent-4-en-1-yl, 2-methyl-
pent-4-en-1-yl, 3-methylpent-4-en-1-yl, 4-methylpent-4-en-
1-yl, 1,1-dimethylbut-2-en-1-yl, 1,1-dimethylbut-3-en-1-yl,
1,2-dimethylbut-1-en-1-yl, 1,2-dimethylbut-2-en-1-yl,
005/51065 CA 02396583 2002-07-05
1,2-dimethylbut-3-en-1-yl, 1,3-dimethylbut-1-en-1-yl,
1,3-dimethylbut-2-en-1-yl, 1,3-dimethylbut-3-en-1-yl,
2,2-dimethylbut-3-en-1-yl, 2,3-dimethylbut-1-en-1-yl,
2,3-dimethylbut-2-en-1-yl, 2,3-dimethylbut-3-en-1-yl,
5 3,3-dimethylbut-1-en-1-yl, 3,3-dimethylbut-2-en-1-yl,
1-ethylbut-1-en-1-yl, 1-ethylbut-2-en-1-yl, 1-ethyl-
but-3-en-1-yl, 2-ethylbut-1-en-1-yl, 2-ethylbut-2-en-1-yl,
2-ethylbut-3-en-1-yl, 1,1,2-trimethylprop-2-en-1-yl,
1-ethyl-1-methylprop-2-en-1-yl, 1-ethyl-2-methylprop-1-en-
10 1-yl or 1-ethyl-2-methylprop-2-en-1-yl;
- CZ-C6-haloalkenyl: C2-C6-alkenyl as mentioned above which is
partially or fully substituted by fluorine, chlorine and/or
bromine, i.e. for example 2-chlorovinyl, 2-chloroallyl,
15 3-chloroallyl, 2,3-dichloroallyl, 3,3-dichloroallyl,
2,3,3-trichloroallyl, 2,3-dichlorobut-2-enyl, 2-bromoallyl,
3-bromoallyl, 2,3-dibromoallyl, 3,3-dibromoallyl,
2,3,3-tribromoallyl and 2,3-dibromobut-2-enyl, preferably C3-
or C4-haloalkenyl;
- C2-C6-alkynyl: ethynyl and C3-C6-alkynyl, such as
prop-1-yn-1-yl, prop-2-yn-1-yl, n-but-1-yn-1-yl,
n-but-1-yn-3-yl, n-but-1-yn-4-yl, n-but-2-yn-1-yl,
n-pent-1-yn-1-yl, n-pent-1-yn-3-yl, n-pent-1-yn-4-yl,
n-pent-1-yn-5-yl, n-pent-2-yn-1-yl, n-pent-2-yn-4-yl,
n-pent-2-yn-5-yl, 3-methylbut-1-yn-3-yl,
3-methylbut-1-yn-4-yl, n-hex-1-yn-1-yl, n-hex-1-yn-3-yl,
n-hex-1-yn-4-yl, n-hex-1-yn-5-yl, n-hex-1-yn-6-yl,
n-hex-2-yn-1-yl, n-hex-2-yn-4-yl, n-hex-2-yn-5-yl,
n-hex-2-yn-6-yl, n-hex-3-yn-1-yl, n-hex-3-yn-2-yl,
3-methylpent-1-yn-1-yl, 3-methylpent-1-yn-3-yl,
3-methylpent-1-yn-4-yl, 3-methylpent-1-yn-5-yl,
4-methylpent-1-yn-1-yl, 4-methylpent-2-yn-4-yl or
4-methylpent-2-yn-5-yl, preferably prop-2-yn-1-yl;
- CZ-C6-haloalkynyl: C2-C6-alkynyl as mentioned above which is
partially or fully substituted by fluorine, chlorine and/or
bromine, i.e. for example 1,1-difluoroprop-2-yn-1-yl,
1,1-difluorobut-2-yn-1-yl, 4-fluorobut-2-yn-1-yl,
4-chlorobut-2-yn-1-yl, 5-fluoropent-3-yn-1-yl or
6-fluorohex-4-yn-1-yl, preferably C3- or C4-haloalkynyl;
- C3-C8-cycloalkyl: cyclopropyl, cyclobutyl, cyclopentyl,
cyclohexyl, cycloheptyl or cyclooctyl;
~05~/51065 CA 02396583 2002-07-05
16
- C3-Ce-cycloalkyl containing a carbonyl or thiocarbonyl ring
member: for example cyclobutanon-2-yl, cyclobutanon-3-yl,
cyclopentanon-2-yl, cyclopentanon-3-yl, cyclohexanon-2-yl,
cyclohexanon-4-yl, cycloheptanon-2-yl, cyclooctanon-2-yl,
cyclobutanethion-2-yl, cyclobutanethion-3-yl,
cyclopentanethion-2-yl, cyclopentanethion-3-yl,
cyclohexanethion-2-yl, cyclohexanethion-4-yl,
cycloheptanethion-2-yl or cyclooctanethion-2-yl, preferably
cyclopentanon-2-yl or cyclohexanon-2-yl;
- C3-C$-cycloalkyl-C1-C4-alkyl: cyclopropylmethyl,
1-cyclopropylethyl, 2-cyclopropylethyl,
1-cyclopropylprop-1-yl, 2-cyclopropylprop-1-yl,
3-cyclopropylprop-1-yl, 1-cyclopropylbut-1-yl,
2-cyclopropylbut-1-yl, 3-cyclopropylbut-1-yl,
4-cyclopropylbut-1-yl, 1-cyclopropylbut-2-yl,
2-cyclopropylbut-2-yl, 3-cyclopropylbut-2-yl,
4-cyclopropylbut-2-yl, 1-(cyclopropylmethyl)eth-:1-yl,
1-(cyclopropylmethyl)-1-(methyl)eth-1-yl,
1-(cyclopropylmethyl)prop-1-yl, cyclobutylmethyl,
1-cyclobutylethyl, 2-cyclobutylethyl, 1-cyclobutylprop-1-yl,
2-cyclobutylprop-1-yl, 3-cyclobutylprop-1-yl,
1-cyclobutylbut-1-yl, 2-cyclobutylbut-1-yl,
3-cyclobutylbut-1-yl, 4-cyclobutylbut-1-yl,
1-cyclobutylbut-2-yl, 2-cyclobutylbut-2-yl,
3-cyclobutylbut-2-yl, 4-cyclobutylbut-2-yl,
1-(cyclobutylmethyl)eth-1-yl,
1-(cyclobutylmethyl)-1-(methyl)eth-1-yl,
1-(cyclobutylmethyl)prop-1-yl, cyclopentylmethyl,
1-cyclopentylethyl, 2-cyclopentylethyl,
1-cyclopentylprop-1-yl, 2-cyclopentylprop-1-yl,
3-cyclopentylprop-1-yl, 1-cyclopentylbut-1-yl,
2-cyclopentylbut-1-yl, 3-cyclopentylbut-1-yl,
4-cyclopentylbut-1-yl, 1-cyclopentylbut-2-yl,
2-cyclopentylbut-2-yl, 3-cyclopentylbut-2-yl,
4-cyclopentylbut-2-yl, 1-(cyclopentylmethyl)eth-I-yl,
1-(cyclopentylmethyl)1-(methyl)eth-1-yl,
1-(cyclopentylmethyl)prop-1-yl, cyclohexylmethyl,
1-cyclohexylethyl, 2-cyclohexylethyl, 1-cyclohexylprop-1-yl,
2-cyclohexylprop-1-yl, 3-cyclohexylprop-1-yl,
1-cyclohexylbut-1-yl, 2-cyclohexylbut-1-yl,
3-cyclohexylbut-1-yl, 4-cyclohexylbut-1-yl,
1-cyclohexylbut-2-yl, 2-cyclohexylbut-2-yl,
3-cyclohexylbut-2-yl, 4-cyclohexylbut-2-yl,
1-(cyclohexylmethyl)eth-1-yl,
1-(cyclohexylmethyl)-1-(methyl)eth-1-yl,
1-(cyclohexylmethyl)prop-1-yl, cycloheptylmethyl,
0050/51065 CA 02396583 2002-07-05
17
1-cycloheptylethyl, 2-cycloheptylethyl,
1-cycloheptylprop-1-yl, 2-cycloheptylprop-1-yl,
3-cycloheptylprop-1-yl, 1-cycloheptylbut-1-yl,
2-cycloheptylbut-1-yl, 3-cycloheptylbut-1-yl,
4-cyclvheptylbut-1-yl, 1-cycloheptylbut-2-yl,
2-cycloheptylbut-2-yl, 3-cycloheptylbut-2-yl,
4-cycloheptylbut-2-yl, 1-(cycloheptylmethyl)eth-1-yl,
1-(cycloheptylmethyl)-1-(methyl)eth-1-yl,
1-(cycloheptylmethyl)prop-1-yl, cyclooctylmethyl,
1-cyclooctylethyl, 2-cyclooctylethyl, 1-cyclooctylprop-1-yl,
2-cyclooctylprop-1-yl, 3-cyclooctylprop-1-yl,
1-cyclooctylbut-1-yl, 2-cyclooctylbut-1-yl,
3-cyclooctylbut-1-yl, 4-cyclooctylbut-1-yl,
1-cyclooctylbut-2-yl, 2-cyclooctylbut-2-yl,
3-cyclooctylbut-2-yl, 4-cyclooctylbut-2-yl,
1-(cyclooctylmethyl)eth-1-yl,
1-(cyclooctylmethyl)-1-(methyl)eth-1-yl or
1-(cyclooctylmethyl)prop-1-yl, preferably cyclopropylmethyl,
cyclobutylmethyl, cyclopentylmethyl or cyclohexylmethyl;
- C3-Cg-cycloalkyl-C1-C4-alkyl containing a carbonyl or
thiocarbonyl ring member: for example cyclobutanon-2-yl-
methyl, cyclobutanon-3-ylmethyl, cyclopentanon-2-ylmethyl,
cyclopentanon-3-ylmethyl, cyclohexanon-2-ylmethyl,
cyclohexanon-4-ylmethyl, cycloheptanon-2-ylmethyl,
cyclooctanon-2-ylmethyl, cyclobutanethion-2-ylmethyl,
cyclobutanethion-3-ylmethyl, cyclopentanethion-2-ylmethyl,
cyclopentanethion-3-ylmethyl, cyclohexanethion-2-ylmethyl,
cyclohexanethion-4-ylmethyl, cycloheptanethion-2-ylmethyl,
cyclooctanethion-2-ylmethyl, 1-(cyclobutanon-2-yl)ethyl,
1-(cyclobutanon-3-yl)ethyl, 1-(cyclopentanon-2-yl)ethyl,
1-(cyclopentanon-3-yl)ethyl, 1-(cyclohexanon-2-yl)ethyl,
1-(cyclohexanon-4-yl)ethyl, 1-(cycloheptanon-2-yl)ethyl,
1-(cyclooctanon-2-yl)ethyl, 1-(cyclobutanethion-2-yl)ethyl,
1-(cyclobutanethion-3-yl)ethyl, 1-(cyclopentanethion-2-yl)-
ethyl, 1-(cyclopentanethion-3-yl)ethyl, 1-(cyclohexane-
thion-2-yl)ethyl, 1-(cyclohexanethion-4-yl)ethyl, 1-(cyclo-
heptanethion-2-yl)ethyl, 1-(cyclooctanethion-2-yl)ethyl,
2-(cyclobutanon-2-yl)ethyl, 2-(cyclobutanon-3-yl)ethyl,
2-(cyclopentanon-2-yl)ethyl, 2-(cyclopentanon-3-yl)ethyl,
2-(cyclohexanon-2-yl)ethyl, 2-(cyclohexanon-4-yl)ethyl,
2-(cycloheptanon-2-yl)ethyl, 2-(cyclooctanon-2-yl)ethyl,
2-(cyclobutanethion-2-yl)ethyl, 2-(cyclobutanethian-
3-yl)ethyl, 2-(cyclopentanethion-2-yl)ethyl,
2-(cyclopentanethion-3-yl)-ethyl, 2-(cyclohexanethion-
2-yl)ethyl, 2-(cyclohexanethion-4-yl)ethyl,
2-(cycloheptanethion-2-yl)ethyl, 2-(cyclooctanethion-2-
0050/51065
CA 02396583 2002-07-05
18
yl)ethyl, 3-(cyclobutanon-2-yl)propyl, 3-(cyclobutanon-3-
yl)propyl, 3-(cyclopentanon-2-yl)propyl, 3-(cyclopentanon-~3-
yl)propyl, 3-(cyclohexanon-2-yl)propyl, 3-(cyclohexanon-4-
yl)propyl, 3-(cycloheptanon-2-yl)propyl, 3-(cyclooctanon-
2-yl)propyl, 3-(cyclobutanethion-2-yl)propyl,
3-(cyclobutanethion-3-yl)propyl, 3-(cyclopentanethion-2-yl)-
propyl, 3-(cyclopentanethion-3-yl)propyl, 3-(cyclohexane-
thion-2-yl)propyl, 3-(cyclohexanethion-4-yl)propyl, 3-(cyclo-
heptanethion-2-yl)propyl, 3-(cyclooctanethion-2-yl)propyl,
4-(cyclobutanon-2-yl)butyl, 4-(cyclobutanon-3-yl)butyl,
4-(cyclopentanon-2-yl)butyl, 4-(cyclopentanon-3-yl)butyl,
4-(cyclohexanon-2-yl)butyl, 4-(cyclohexanon-4-yl)butyl,
4-(cycloheptanon-2-yl)butyl, 4-(cyclooctanon-2-y.l)butyl,
4-(cyclobutanethion-2-yl)butyl, 4-(cyclobutanethion-3-yl)-
butyl, 4-(cyclopentanethion-2-yl)butyl, 4-(cyclopentane-
thion-3-yl)butyl, 4-(cyclohexanethion-2-yl)butyl, 4-(cyclo-
hexanethion-4-yl)butyl, 4-(cycloheptanethion-2-yl)butyl or
4-(cyclooctanethion-2-yl)butyl, preferably cyclopenta-
non-2-ylmethyl, cyclohexanon-2-ylmethyl, 2-(cyclopenta-
non-2-yl)ethyl or 2-(cyclohexanon-2-yl)ethyl.
3- to 7-membered heterocyclyl is a saturated, partially or fully
unsaturated or aromatic heterocycle having one to three
heteroatoms selected from a group consisting of nitrogen atoms,
oxygen atoms and sulfur atoms.
Examples of saturated heterocycles containing a carbonyl or
thiocarbonyl ring member are:
oxiranyl, thiiranyl, aziridin-1-yl, aziridin-2-yl,
diaziridin-1-yl, diaziridin-3-yl, oxetan-2-yl, oxetan-3-yl,
thietan-2-yl, thietan-3-yl, azetidin-1-yl, azetidin-2-yl,
azetidin-3-yl, tetrahydrofuran-2-yl, tetrahydrofuran-3-yl,
tetrahydrothiophen-2-yl, tetrahydrothiophen-3-yl, pyrrolidin-
1-yl, pyrrolidin-2-yl, pyrrolidin-3-yl, 1,3-dioxolan-2-yl,
1,3-dioxolan-4-yl, 1,3-oxathiolan-2-yl, 1,3-oxathiolan-4-yl,
1,3-oxathiolan-5-yl, 1,3-oxazolidin-2-yl, 1,3-oxazolidin-3-yl,
1,3-oxazolidin-4-yl, 1,3-oxazolidin-5-yl, 1,2-oxazolidin-2-yl,
1,2-oxazolidin-3-yl, 1,2-oxazolidin-4-yl, 1,2-oxazolidin-5-yl,
1,3-dithiolan-2-yl, 1,3-dithiolan-4-yl, pyrrolidin-1-yl,
pyrrolidin-2-yl, pyrrolidin-5-yl, tetrahydropyrazol-1-yl,
tetrahydropyrazol-3-yl, tetrahydropyrazol-4-yl, tetrahydro-
pyran-2-yl, tetrahydropyran-3-yl, tetrahydropyran-4-yl, tetra-
hydrothiopyran-2-yl, tetrahydrothiopyran-3-yl, tetrahydro-
pyran-4-yl, piperidin-1-yl, piperidin-2-yl, piperidin-3-yl,
piperidin-4-yl, 1,3-dioxan-2-yl, 1,3-dioxan-4-yl, 1,3-dioxan-
5-yl, 1,4-dioxan-2-yl, 1,3-oxathian-2-yl, 1,3-oxathian-4-yl,
1,3-oxathian-5-yl, 1,3-oxathian-6-yl, 1,4-oxathian-2-yl,
005/51065 CA 02396583 2002-07-05
19
1,4-oxathian-3-yl, morpholin-2-yl, morpholin-3-yl, morpholin-
4-yl, hexahydropyridazin-1-yl, hexahydropyridazin-3-yl,
hexahydropyridazin-4-yl, hexahydropyrimidin-1-yl,
hexahydropyrimidin-2-yl, hexahydropyrimidin-4-yl,
hexahydropyrimidin-5-yl, piperazin-1-yl, piperazin-2-yl,
piperazin-3-yl, hexahydro-1,3,5-triazin-1-yl, hexahydro-
1,3,5-triazin-2-yl, oxepan-2-yl, oxepan-3-yl, oxepan-4-yl,
thiepan-2-yl, thiepan-3-yl, thiepan-4-yl, 1,3-dioxepan-2-yl,
1,3-dioxepan-4-yl, 1,3-dioxepan-5-yl, 1,3-dioxepan-6-yl,
1,3-dithiepan-2-yl, 1,4-dioxepan-2-yl, 1,4-dioxepan-7-yl,
hexahydroazepin-1-yl, hexahydroazepin-2-yl, hexahydraazepin-3-yl,
hexahydroazepin-4-yl, hexahydro-1,3-diazepin-1-yl, hexahydro-
1,3-diazepin-2-yl, hexahydro-1,3-diazepin-4-yl, hexahydro-
1,4-diazepin-1-yl and hexahydro-1,4-diazepin-2-yl.
Examples of unsaturated heterocycles containing a carbonyl or
thiocarbonyl ring member are:
dihydrofuran-2-yl, 1,2-oxazolin-3-yl, 1,2-oxazolin-5-yl,
1,3-oxazolin-2-yl.
Examples of aromatic heterocyclyl are the 5- and 6-membered
aromatic heterocyclic radicals, for example furyl, such as
2-furyl and 3-furyl, thienyl, such as 2-thienyl and 3-thienyl,
pyrrolyl, such as 2-pyrrolyl and 3-pyrrolyl, isoxazolyl, such as
3-isoxazolyl, 4-isoxazolyl and 5-isoxazolyl, isothiazolyl, such
as 3-isothiazolyl, 4-isothiazolyl and 5-isothiazolyl, pyrazolyl,
such as 3-pyrazolyl, 4-pyrazolyl and 5-pyrazolyl, oxazolyl, such
as 2-oxazolyl, 4-oxazolyl and 5-oxazolyl, thiazolyl, such as
2-thiazolyl, 4-thiazolyl and 5-thiazolyl, imidazolyl, such as
2-imidazolyl and 4-imidazolyl, oxadiazolyl, such as
1,2,4-oxadiazol-3-yl, 1,2,4-oxadiazol-5-yl and
1,3,4-oxadiazol-2-yl, thiadiazolyl, such as 1,2,4-thiadiazol-
3-yl, 1,2,4-thiadiazol-5-yl and 1,3,4-thiadiazol-2-yl, triazolyl,
such as 1,2,4-triazol-1-yl, 1,2,4-triazol-3-yl and 1,2,4-triazol-
4-yl, pyridinyl, such as 2-pyridinyl, 3-pyridinyl and
4-pyridinyl, pyridazinyl, such as 3-pyridazinyl and
4-pyridazinyl, pyrimidinyl, such as 2-pyrimidinyl, 4-pyrimidinyl
and 5-pyrimidinyl, and furthermore 2-pyrazinyl, 1,3,5-triazin-
2-yl and 1,2,4-triazin-3-yl, in particular pyridyl, pyrimidyl,
furanyl and thienyl.
In the context of the present invention, 5- or 6-membered
aromatic radicals are phenyl and the abovementioned 5- or
6-membered aromatic heterocyclyl radicals, in particular phenyl
or pyridyl, for example 2-pyridyl. According to the invention,
these radicals can be substituted and/or have a fused-on, 5- or
6-membered carbocyclic or heterocyclic ring with 1 to 3
0050/51065 CA 02396583 2002-07-05
heteroatoms selected from the group consisting of nitrogen,
oxygen and sulfur atoms, where the fused-on ring is partially or
fully unsaturated and may be unsubstituted or may for its part
carry one, two or three substituents or else contain one or two
5 nonadjacent carbonyl, thiocarbonyl or sulfonyl ring members.
Examples of suitable substituents on the aromatic radical are the
meanings given below for R4, R5 and R6.
Examples of fused-on rings are, in addition to phenyl, the
10 abovementioned heteroaromatic groups, in particular pyridine,
pyrazine, pyridazine, pyrimidine, furan, dihydrofuran, thiophene,
dihydrothiophene, pyrrole, dihydropyrrole, 1,3-dioxolane,
1,3-dioxolan-2-one, isoxazole, oxazole, oxazolinone, isothiazole,
thiazole, pyrazole, pyrazoline, imidazole, imidazolinone,
15 dihydroimidazole, 1,2,3-triazole, 1,1-dioxodihydroisothiazole,
dihydro-1,4-dioxine, pyridone, dihydro-1,4-oxazine,
dihydro-1,4-oxazin-2-one, dihydro-1,4-oxazin-3-one,
dihydro-1,3-oxazine, dihydro-1,3-thiazin-2-one,
dihydro-1,4-thiazine, dihydro-1,4-thiazin-2-one,
20 dihydro-1,4-thiazin-3-one, dihydro-1,3-thiazine and
dihydro-1,3-thiazin-2-one which for their part may have one, two
or three substituents. Examples of suitable substituents on the
fused-on ring are the meanings given below for R16, R17, R1$ and
R19.
30
With a view to the use of the 4-aryl-1-difluoromethoxyimidazoles
I as herbicides or desiccants/defoliants, preference is given to
those compounds I where the variables are as defined below, in
each case on their own or in combination:
R1 is hydrogen, methyl, ethyl or C1-C2-haloalkyl;
R2 is hydrogen, halogen, preferably chlorine or bromine, cyano,
methyl or trifluoromethyl, in particular hydrogen or halogen;
R3 is a radical of the formula II
R4
R5-~ ~~- (II).
-Q
X-R6
in which the variables X, Q, R4, R5 and R6 are as defined
below:
CA 02396583 2002-07-05
0050!51065
21
R4 is hydrogen or halogen;
R5 is hydrogen, cyano, vitro, halogen, Ci-C4-alkyl, OH, SH,
NH2, Ci-C4-haloalkyl, Ci-C4-alkoxy or Ci-C4-haloalkoxy;
X is a chemical bond or a methylene, ethylene,
propane-1,3-diyl or ethene-1,2-diyl chain or an
oxymethylene or thiamethylene chain which is attached to
the phenyl ring via the heteroatom, where all chains may
be unsubstituted or may carry one or two substituents. in
each case selected from the group consisting of cyano,
carboxyl, halogen, Ci-C4-alkyl, Ci-C4-haloalkyl,
Ci-C4-alkoxy, (Ci-C4-alkoxy)carbonyl, di(Ci-C4-alkyl)amino
and phenyl;
Q is nitrogen or a group C-R7 in which R7 is hydrogen, NH2,
OH or SH;
R6 is hydrogen, vitro, cyano, halogen, halosulfonyl,
-O-Y-R8, -O-CO-Y-R8, -N(Y-R8)(Z-R9), -N(Y-R8)-S02-Z-R9,
-N(S02-Y-Re)(S02-Z-R9), -N(Y-R$)-CO-Z-R9,
-N(Y-RB)(0-Z-R9), -S-Y-R8, -SO-Z-Rg, -S02-Y-R$,
-S02-O-Y-Rg, -S02-N(Y-R$)(Z-R9), -CO-Y-R8, -C(=NORi~)-Y-R8,
-C(=NOR1~)-0-Y-R8, -CO-O-Y-Re, -CO-S-Y-Re.
-CO-N(Y-R8)(Z-R9), -CO-N(Y-Re)(0-Z-R9) or -PO(0-Y-Re)2; in
which the variables Y, Z, R8, R9 and R1~ are as defined
below:
Y, Z are, independently of one another:
a chemical bond, a methylene or ethylene group which
may be unsubstituted or may carry one or two
substituents, in each case selected from the group
consisting of carboxyl, Ci-C4-alkyl, Ci-C4-haloalkyl,
(Ci-C4-alkoxy)carbonyl and phenyl;
Re, R9 are, independently of one another:
hydrogen, Ci-C6-haloalkyl, C2-C6-alkenyl,
C2-C6-haloalkenyl, C2-C6-alkynyl, C2-C6-haloalkynyl,
_CH(R11) (R12) ~ _C(Rll) (R12)_Np2~ _G(R11) (R12)_CN~
_C(Rii)(R12)_halogen, -C(Rii)(R1z)_ORi3~
_C(R11) (R12)_j~(R13)R14~ _C(R11) (R12)_N(R13)_pRl4~
_C ( Rl l ) ( R12 ) _SR13 ~ _C ( R11 ) ( R12 ) _gp_R13 ~
_C ( Rll ) ( R12 ) _S02_R13 ~ _C ( R11 ) ( R12 ) _S02_OR13 ~
_C ( R11 ) ( R12 ) _Sp2_N ( R13 ) Ri4 ~ _C ( Rll ) ( R12 ) _Cp_R13 ~
_C ( R11 ) ( R12 ) _C ( =NOR15 ) -R13 r _C ( R11 ) ( R12 ) _CO_OR13 ,
_C(Rll)(R12)_Cp_SR13, -C(Ril)(R12)_Cp_N(R13)R14~
_C(R11)(R12)_Cp_N(R13)_pRl4~ _C(Ril)(R12)_pp(OR13)2.
0050/51065
CA 02396583 2002-07-05
22
is C3-C8-cycloalkyl which may contain a carbonyl or
thiocarbonyl ring member,
is phenyl or 3- to 7-membered heterocyclyl which may
contain a carbonyl or thiocarbonyl ring member, where
each cycloalkyl, the phenyl and each heterocyclyl
ring may be unsubstituted or may carry one to four
substituents, in each case selected from the group
consisting of cyano, nitro, amino, hydroxyl,
carboxyl, halogen, C1-C4-alkyl, C1-C4-haloalkyl,
C1-C4-alkoxy, C1-C4-haloalkoxy, C1-C~-alkylthio,
C1-C4-haloalkylthiv, C1-C4-alkylsulfonyl,
C~-C4-haloalkylsulfonyl, (C1-C4-alkyl)carbonyl,
(C1-C4-haloalkyl)carbonyl, (C1-C4-alkyl)carbonyloxy,
(C1-C4-haloalkyl)carbonyloxy, (C1-C4-alkoxy)carbonyl
and di-(C1-C4-alkyl)amino;
R1~ is hydrogen, C1-C6-alkyl, C1-C6-haloalkyl,
C2-C6-alkenyl, Cz-C6-haloalkenyl, C2-C6-a:Lkynyl,
Cy-C6-haloalkynyl, C3-C8-cycloalkyl, phenyl or
phenyl-C1-C4-alkyl;
' 005o~51os~
CA 02396583 2002-07-05
23
Ci-C4-haloalkylthio, Ci-C4-alkylsulfonyl,
Ci-C4-haloalkylsulf.onyl, (Ci-C4-alkyl)carbonyl,
(Ci-C4-haloalkyl)carbonyl, (Ci-C4-alkyl)carbonyloxy,
(Ci-C4-haloalkyl)carbonyloxy, (Ci-C4-alkoxy)carbonyl
and di-(Ci-C4-alkyl)amino;
R15 is hydrogen, Ci-C6-alkyl, Ci-C6-haloalkyl,
Cz-C6-alkenyl, Cz-C6-haloalkenyl, Cz-C6-alkynyl,
Cz-C6-haloalkynyl, C3-Cg-cycloalkyl, phenyl or
phenyl-Ci-C4-alkyl.
Examples of combinations of the variables X-R6 are given in Table
1. In the formula II, the variables Q, R4, R5, X and R6 preferably
have the following meanings, in each case on their own or in
combination:
Q is CH;
ao
R4 is hydrogen, fluorine or chlorine;
R5 is halogen, in particular chlorine;
X is a chemical bond, methylene or ethane-1,2-diyl,
ethene-1,2-diyl, 2-chloroethane-1,2-diyl and
2-chloroethene-1,2-diyl;
R6 is hydrogen, -O-Y-Re, -0-CO-Y-Re, -N(Y-Re)-SOz-Z-R9,
-N(SOz-Y-R~)(SOz-Z-R9), -S-Y-R8, -SOz-N(Y-R$)(Z-R9),
-C(=NOR1~)-O-Y-R8, -CO-0-Y-Ra, -CO-N(Y-RS)(Z-R9) or
-PO(0-Y-R8)z.
in particular hydrogen, -0-Y-RB, -N(Y-R$)-SOz-Z-R9, -S-Y-R8 or
-CO-0-Y-R8,
particularly preferably hydrogen or -0-Y-R8.
The variables R8, R9, Rio, Y and Z mentioned in the definition of
the variable R6 preferably have the following meanings:
Y, Z are, independently of one another, a chemical bond or
methylene;
R8, R9 are, independently of one another,
hydrogen, Ci-C6-haloalkyl, Cz-C6-alkenyl, Cz-C6-haloalkenyl,
Cz-C6-alkynyl, -CH(Rii)(R1z), _C(R11)(R12)_Npz~ -C(Rii)(R12)_CN~
_C ( Ri 1 ) ( R12 ) _halogen, -C ( Ri 1 ) ( R12 ) -pRi3 ~ _C ( Ri 1 ) ( Ri z )
_N ( R13 ) R14 ~
-C ( Ri 1 ) ( Ri2 ) _N ( R13 ) _pgi4 ~ _C ( Ri l ) ( Ri2 ) _SR13 ~ _C ( Ri 1 )
( R12 ) _gp_R13 ~
_C(R11)(Rlz)_gpz_R13~ _C(Ril)(R12)_gpz_pRl3~
-C(R11)(R12)_gp2_N(R13)R14, _C(R11)(R12)_Cp_R13~
0050/51065 CA 02396583 2002-07-05
a4
_C ( R11 ) ( R12 ) _C ( -NOR15 ) -R13 ~ _C ( Rll ) ( R12 ) _Cp_OR13 ,
_C ( R11 ) ( R12 ) _Cp_ SRi3 , -C ( R11 ) ( R12 ) _pp_N ( R13 ) R14 ~
_C ( R1l ) ( R12 ) _Cp_N ( Ri3 ) _pRl4 ~
C3-C8-cycloalkyl which may contain a carbonyl or thiocarbonyl
ring member, phenyl or 3- to 7-membered heterocyclyl having
one or two nitrogen and/or one oxygen or sulfur atom as
heteroatom and, if desired, a carbonyl or thiocarbonyl ring
member,
where each cycloalkyl, the phenyl and each heteracyclyl ring
may be unsubstituted or may carry one or two substituents, in
each case selected from the group consisting of cyano, nitro,
halogen, Ci-C4-alkyl, Ci-C4-alkoxy, Ci-C4-alkylsul.fonyl,
(Ci-C4-alkyl)carbonyl, (Ci-C4-alkyl)carbonyloxy and
(Ci-C4-alkoxy)carbonyl;
in particular hydrogen, Ci-C6-haloalkyl, C2-C6-alkenyl,
C2-C6-alkynyl, -CH(Rii)(R12), _C(Rii)(R12)_CO_OR13,
-C(Rii)(R12)_CO-N(R13)R14 or. C3-C8-cycloalkyl, particularly
preferably hydrogen, Ci-C6-alkyl, C2-C6-alkenyl,
C2-C6-alkynyl, -C(Rii)(R12)_CO-OR13 or C3-C8-cycloalkyl.
0050/51065
CA 02396583 2002-07-05
Among these, preference is given to compounds I in which R5
together with X-R6 in formula II is a chain of the formulae:
_p_G(R16~R17)_Cp_N(Rie)_~ _g_G(R16~R17)_Gp_N(Ri8)_~ _N=C(Ris)_p_ or
-N=C(Ri9)-S- (compounds IC) in which the variables Rib to R19 are
5 as defined below:
R16, R17 are, independently of one another,
hydrogen, Ci-C6-alkyl, Ci-C6-haloalkyl, C2-C6-alkenyl,
C2-C6-haloalkenyl, C2-C6-alkynyl, CZ-C6-haloalkynyl,
10 C3-C8-cycloalkyl, phenyl or phenyl-Ci-C4-alkyl;
Ri8 is hydrogen, hydroxyl, Ci-C6-alkyl, Ci-C6-haloalkyl,
CZ-C6-alkenyl, C2-C6-haloalkenyl, Cz-C6-alkynyl, Ci-C4-alkoxy,
Ci-C4-haloalkoxy, C3-C6-alkenyloxy, C3-C6-alkynyloxy,
15 Ci-C4-alkylsulfonyl, Ci-C4-haloalkylsulfonyl,
Ci-C4-alkylcarbonyl, Ci-C4-haloalkylcarbonyl,
Ci-C4-alkoxycarbonyl, Ci-C4-alkoxycarbonyl-Ci-C4-alkyl,
Ci-C4-alkoxycarbonyl-Ci-C4-alkoxy,
di(Ci-C4-alkyl)aminocarbonyl,
20 di(Ci-C4-alkyl)aminocarbonyl-Ci-C4-alkyl,
di(Ci-C4-alkyl)aminocarbonyl-Ci-C4-alkoxy, phenyl or
phenyl-Ci-C4-alkyl;
Ri9 is hydrogen, halogen, cyano, amino, Ci-C6-alkyl,
25 Ci-C6-haloalkyl, CZ-C6-alkenyl, CZ-C6-haloalkenyl,
C2-C6-alkynyl, Ci-G4-alkoxy, Ci-C4-haloalkoxy,
C3-C6-alkenyloxy, C3-C6-alkynyloxy, Gi-C4-alkylamino,
di(Ci-C4-alkyl)amino, Ci-C4-haloalkoxy, Ci-C4-alkylthio,
Ci-C4-haloalkylthio, Ci-C4-alkylsulfinyl,
Ci-C4-haloalkylsulfinyl, Ci-C4-alkylsulfonyl,
Ci-C4-haloalkylsulfonyl, Ci-C4-alkylcarbonyl,
Ci-C4-haloalkylcarbonyl, Ci-C4-alkoxycarbonyl,
Ci-C4-alkoxycarbonyl-Ci-C4-alkyl,
Ci-C4-alkoxycarbonyl-Ci-C4-alkoxy,
Ci-C4-alkoxycarbonyl-Ci-C4-alkylthio,
di(Ci-C4-alkyl)aminocarbonyl,
di(Ci-C4-alkyl)aminocarbonyl-Ci-C4-alkyl,
di(Ci-C4-alkyl)aminocarbonyl-Ci-C4-alkoxy,
di(Ci-C4-alkyl)aminocarbonyl-Ci-C4-alkylthio, phenyl or
phenyl-Ci-C4-alkyl.
The variables R16 to R19 are preferably as defined below:
R16, R17 are, independently of one another,
hydrogen or methyl;
0050/51065
CA 02396583 2002-07-05
26
R18 is hydrogen, hydroxyl, C1-C6-alkyl, C1-C6-haloalkyl,
C2-C6-alkenyl, C2-C6-alkynyl, Ci-C4-alkoxy, C1-C~-haloalkoxy,
C3-C6-alkenyloxy, C3-C6-alkynyloxy,
C1-C4-alkoxycarbonyl-C1-C4-alkyl,
C1-C4-alkoxycarbonyl-C1-C4-alkoxy or phenyl-C1-C4-alkyl;
R19 is hydrogen, halogen, amino, C1-C6-alkyl, C1-C6-haloalkyl,
C1-C4-alkoxy, C3-C6-alkenyloxy, C3-C6-alkynyloxy,
C1-C4-alkylamino, di(C1-C4-alkyl)amino, C1-C~-alkylthio,
C1-C4-alkoxycarbonyl-C1-C4-alkyl,
C1-C4-alkoxycarbonyl-C1-C4-alkoxy,
C1-C4-alkoxycarbonyl-C1-C4-alkylthio, phenyl or
phenyl-C1-C4-alkyl.
In these compounds, Q and RQ are as defined above, and Q is in
particular CH and R4 has in particular the meanings given above as
being preferred.
Among the compounds IC, particular preference is given to those
compounds in which RS together with X-R6 is a chain of the formula
-O-CH(R16)-CO-N(R18)-, -S-CH(R16)-CO-N(R18)-. R16 and R1s in
particular have the meanings given as being preferred. Among
these, very particular preference is given to compounds IC in
which the nitrogen atom of the chain -0-CH(R16)-CO-N(R18)-,
-S-CH(R16)-CO-N(R18)- is attached to the carbon atom of the phenyl
ring in formula II which is adjacent to the group Q (meta
position with respect to the imidazolyl group).
Among the compounds of the formula I in which R3 is a radical of
the formula II, preference is furthermore given to compounds I in
which Q is a group C-R7 and R7 together with X-R6 in the formula
II is a chain of the formulae: -0-C(R16,R17)-CO-N(Rle)-,
-S-C(R16,R17)-CO-N(R18)-, -N=C(R19)-O- or -N=C(R19)-S-, in which
the variables R16 - Ris have the meanings given above, in
particular the meanings given as being preferred. Hereinbelow,
such compounds are referred to as compounds ID. Amongst these,
preference is given to those compounds in which R7 together with
X-R6 is a chain of the formula -N=C(R19)-0- or -N=C(R19)-S-. In
these compounds, R4 and R~ have the meanings mentioned above, in
particular the meanings mentioned as being preferred.
0050/51065
CA 02396583 2002-07-05
27
Particular preference is giver. to the compounds of the formula
IAa (compounds IA where Q = CH, R1 = H and R5 = C1) in which the
variables Rz, R4 and X-R6 together have the meanings given in each
case in one row of Table 1 (compounds IAa.l-IAa.936).
C 1- N ( IAa )
~ O-CHF2
X_R6 R2
Table 1
No . R2 R4 -X-R6
1 H H H
2 H F H
3 H C1 H .
4 C1 H H
5 Cl F H
_.
.
6 C1 C1 -
_
H
7 Br H H
8 Br F H
9 Br C1 H
10 CH3 H H
11 CH3 F H
12 CH3 C1 H
13 H H CH3
14 H F CH3
15 H C1 CH3
16 C1 H CH3
1~ C1 F CH3
18 Cl C1 CH3
19 Br H CH3
20 Br F CH3
21 Br C1 CH3
22 CH3 H CH3
23 CH3 F CH3
24 CH3 Cl CH3
25 H H NOZ
26 H F NOp
27 H Cl NO2
2g C1 H NOz
29 C1 F NOZ
30 C1 C1 NOZ
31 Br H N02
32 Br F N02
33 Br C1 NOZ
34 CH3 H N02
35 CH3 F NO~
36 CH3 C1 NO~
R4
N=~
0050!51065
CA 02396583 2002-07-05
28
No . ~ R R -X-R .~__ _
37 H H CN
38 H F CN
39 H C1 CN
40 C1 H CN
41 C1 F CN
42 C1 C1 CN
43 Br H CN
44 Br F CN
45 Br Cl CN
46 CH3 H CN _ _
47 - CH3 F CN _
48 CH3 C1 CN
49 H H F
50 H F F
51 H Cl F
52 C1 H F
53 C1 F F
54 C1 C1 F
55 Br H F
5 6 Br F F _
57 Br C1 F -_. _ _
58 CH3 H F
59 CH3 F F
60 CH3 C1 F
61 H H Cl
62 H F C1
63 H C1 C1
64 C1 H C1
65 C1 F C1
66 C1 C1 C1
67 Br H C1
6g Br F C1
69 Br C1 C1
70 CH3 H Cl
71 CH3 F C1
72 CH3 C1 C1
73 H H Br
74 H F Br
75 H C1 Br
76 Cl H Br
77 Cl F Br
78 C1 C1 Br
7g Br H Br
80 Br F Br
81 Br C1 Br
82 CH3 H Br
83 CHg F Br
84 CH3 C1 Br
85 H H OH
86 H F OH
87 H C1 OH
0050/51065 CA 02396583 2002-07-05
29
No. R R4 -X-
88 C1 H OH
89 C1 F OH
90 C1 C1 OH
91 Br H OH
92 Br F OH
93 Br C1 OH
94 CH3 H OH
95 CH3 F OH -
96 CH3 C1 OH
97 H H OCH3
98 H F OCH3
99 H C1 OCH3
100 C1 H OCH3
101 C1 F OCH3
102 C1 C1 OCH3
103 Br H OCH3
104 Br F OCH3
105 Br C1 OCH3
106 CH3 H OCH3
107 CH3 F OCH3
108 CH3 C1 OCH3
109 H H OCH(CH3)z
110 H F OCH(CH3)z
111 H Cl OCH(GH3)z
112 C1 H - OCH(CH3)2
113 Cl F OCH(CH3)z
114 Cl C1 OCH(CH3)z
115 Br H OCH(CH3)z
116 Br F OCH(CH3)2
117 Br C1 OCH(CH3)z
118 CH3 H OCH(CH3)2
119 CH3 F OCH(CH3)2
120 CH3 C1 OCH(CH3)z
121 H H OCHz-CH=CHz
122 H F OCHz-CH=CHz
123 H C1 OCHZ-CH=CHz
124 C1 H OCHz-CH=CHz
125 C1 F OCHz-CH=CHz
126 C1 C1 OCHz-CH=CHz
127 Br H OCHZ-CH=CHz
128 Br F OCHz-CH=CHz
129 Br Cl OCHz-CH=CHz
130 CH3 H OCHZ-CH=CHz
131 CH3 F OCHz-CH=CHz
132 CH3 C1 OCHZ-CH=CHz
133 H H OCHz-CCH
134 H F OCHZ-C~CH
135 H C1 OCHz-C~CH
136 C1 H OCHZ-C~CH
137 C1 F OCHZ-C$CH
138 C1 C1 OCHZ-C~CH
0050/51065
CA 02396583 2002-07-05
No . R2 R4 -X-R
139 Br H OCHZ-CgCH
140 Br F OCHZ-C~CH
141 Br C1 OCHZ-C~CH
5 142 CH3 H OCH2-C~CH
143 CH3 F OCH2-C~CH
144 CH3 C1 OCHZ-C~CH
145 H H OCHz-COOH
146 H F OCH2-COOH
147 H C1 OCH2-COON
10148 C1 H OCHZ-COON
149 C1 F OCHZ-COOH
150 C1 G1 OCH2-COOH
151 Br H OCHy-COOH
152 Br F OCH2-COOH
15153 Br C1 OCHZ-COOH
154 CH3 H OCH2-COOH
155 CH3 F OCHZ-COOH
156 CH3 C1 OCH2-COOH
157 H H OCH2-COOCH3
158 H F OCH2-COOCH3
20159 H Cl OCHp-COOCH3
160 C1 H OCH2-COOCH3
161 C1 F OCH2-COOCH3
162 C1 C1 OCHZ-COOCH3
163 Br H OCHy-COOCH3
25164 Br F OCH2-COOCH3
165 Br C1 OCH2-COOCH3
166 CH3 H OCH2-COOCH3
167 CH3 F OCHz-COOCH3
168 CH3 C1 OCH2-COOCH3
169 H H OCHZ-COOC2H5
30170 H F OCHZ-COOCzHS
171 H C1 OCHZ-COOCzHS
172 C1 H OCH2-COOC2H5
173 C1 F OCHZ-COOCZHS
174 C1 C1 OCHZ-COOC2H5
35175 Br H OCHz-COOCZHS
176 Br F OCH2-COOC2H5
177 Br C1 OCH2-COOC2H5
178 CH3 H OCH2-COOC2H5
179 CH3 F OCH2-COOCZHS
180 CH3 C1 OCH2-COOCZHS
40181 H H OGH2-CO-N(CH3)2
182 H F OCH2-CO-N(CH3)Z
183 H C1 OCH2-CO-N(CH3)2
184 C1 H OCH2-CO-N(CH3)2
185 C1 F OCHZ-CO-N(CH3)y
45186 C1 C1 OCHZ-CO-N(CH3)Z
187 Br H OCH2-CO-N(CH3)2
188 Br F OCH2-CO-N(CH3)2
189 Br C1 OCH2-CO-N(CH3)Z
0050/51065
CA 02396583 2002-07-05
31
No . R R~ -X-R
190 CH3 H OCH2-CO-N(CH3)_2
191 CH3 F OCH2-CO-N(CH3)2
192 CH3 C1 OCH2-CO-N(CH3)2
193 H H OCH(CH3)-COOH
194 H F OCH(CH3)-COOH
195 H C1 OCH(CH3)-COOH
196 C1 H OCH(CH3)-COOH
197 C1 F OCH(CH3)-COOH
198 C1 C1 OCH(CH3)-COON
10199 Br H OCH(CH3)-COON _-
200 Br F OCH(CH3)-COOH
201 Br C1 OCH(CH3)-COOH- _. _
202 CH3 H - ~CH(CH3)-COOH _
203 CH3 F OCH(CH3)-COOH
15204 CH3 C1 OCH(CH3)-COOH
205 H H OCH(CH3)-COOCH3
206 H F OCH(CH3)-COOCH3
207 H C1 OCH(CH3)-COOCH3
208 C1 H OCH(CH3)-COOCH3
209 C1 F OCH(CH3)-COOCH3
20210 Cl C1 OCH(CH3)-COOCH3
211 Br H OCH(CH3)-COOCH3
212 Br F OCH(CH3)-COOCH3
213 Br C1 OGH(CH3)-COOCH3
214 CH3 H OCH(CH3)-COOCH3
25215 CH3 F OCH(CH3)-CUOCH3
216 CHg C1 OCH(CH3)-COOCH3
217 H H OCH(CH3)-COOC2H5
218 H F OCH(CH3)-COOC2H5
219 H C1 OCH(CHa)-COOC2H5
220 C1 H OCH(CH3)-COOC2H5
30221 C1 F OCH(CH3)-COOC2H5
222 C1 Cl OCH(CH3)-COOC2H5
223 Br H OCH(CH3)-COOC2H5
224 Br F OCH(CH3)-COOC2H5
225 Br C1 OCH(CH3)-COOC2H5
35226 CH3 H OCH(CH3)-COOC2H5
227 CH3 F OCH(CH3)-COOC2H5
228 CH3 C1 OCH(CH3)-COOC2H5
229 H H OCH(CH3)-CO-N(CH3)2
230 H F OCH(CH3)-CO-N(CH3)2
231 H C1 OCH(CH3)-CO-N(CH3)2
40232 C1 H OCH(CH3)-CO-N(CH3)2
233 C1 F OCH(CH3)-CO-N(CH3)2
234 C1 C1 OCH(CH3)-CO-N(CH3)2
235 Br H OCH(CH3)-CO-N(CH3)2
236 Br F OCH(CH3)-CO-N(CH3)2
45237 Br C1 OCH(CH3)-CO-N(CH3)2
238 CH3 H OCH(CH3)-CO-N(CH3)2
239 CH3 F UCH(CH3)-CO-N(CH3)2
240 CH3 C1 OCH(CH3)-CO-N(CH3)2
0050/51065
CA 02396583 2002-07-05
32
No. R R -X-R~
241 Ii H O-cyclopentyl
242 H F 0-cyclopen~yl
243 H C1 O-cyclopentyl
244 Cl H 0-cyclopentyl
245 C1 F 0-cyclopentyl
246 C1 C1 0-cyclopentyl
247 Br H O-cyclopentyl
248 Br F 0-cyclopentyl
249 Br C1 O-cyclopentyl
250 CH3 H - O-cyclopentyl
251 CH3 ~.__I O=~yclopentyl- _ _ _ -
252 CH3 C1 0-cyclopentyl
253 H H 0-CH2-phenyl
254 H F 0-CH2-phenyl
255 H C1 O-CHz-phenyl
256 C1 H 0-CH2-phenyl
257 C1 F O-CH2-phenyl
258 C1 C1 O-CHZ-phenyl
259 Br H O-CHZ-phenyl
260 Br F O-CHz-phenyl
261 Br C1 0-CH2-phenyl
262 CH3 H. O-~H2_phenyl _
_
_
263 CH3 F o-CH2_phenyl -..
264 CH3 C1 O-CHZ-phenyl - _. -.. -.
2 6 5 H- H _ NH2 _ _
266 H F NH2 _. _
267 H C1 NHy
268 C1 H NH2
269 C1 F NH2
270 Cl C1 NH2
2 71 Br H NH2
272 Br F NHZ
273 Br C1 NH2
274 CH3 H NHz
275 CH3 F NHZ
276 CH3 Cl NH2
277 H H N(CH3)-CHZ-C~CH
278 H F N(CH3)-CH2-C~CH
279 H C1 N(CH3)-CH2-C~CH
280 C1 H N(CH3)-CH2-C~CH
281 C1 F N(CH3)-CHZ-C~CH
282 C1 C1 N(CH3)-CH2-C=-CH
283 Br H N(CH3j-CH2-C~CH
284 Br F N(CH3)-CH2-C~CH
285 Br C1 N(CH3)-CHZ-C~CH
286 CH3 H N(CH3)-CH2-C~CH
287 CH3 F N(CH3)-CHy-C~CH
288 CH3 C1 N(CH3)-CHZ-C~CH
289 H H N(S02-CH3)Z
290 H F N(S02-CH3)2
291 H C1 N(SOZ-CH3)z
0050/51065
CA 02396583 2002-07-05
33
No . R2 R -X-R
292 C1 H N(S02-_CH3)2 __
~
293 C1 F N(
S02-CH3)2
294 Cl C1 N(S02-CH3)2
295 Br H N(S02-CH3)2
296 Br F N(S02-CH3)2
297 Br C1 N(S02-CH3)2
298 CH3 H N(S02-CH3)z
299 CH3 F N(S02-CH3)2
300 CH3 Cl N(S02-CH3)2
10301 H _ H NH-(S02-CHg)
302 H F NH-(SOZ-CH3)
303 H C1 NH-(SOZ-CH3)
304 C1 H NH-(SOZ-CH3)
305 C1 F NH-(SOz-CH3)
15306 C1 C1 NH-(SOZ-GH3)
307 Br H NH-(S02-CH3)
308 Br F NH-(SOz-CH3)
309 Br C1 NH-(SOZ-CH3)
310 CH3 H NH-(SOZ-CH3)
311 CH3 F NH-(S02-CH3)
20312 CHg-- C1 NH-(S02-CH3). -..
313 H H - N (( CH3 ) _ ( 502_CH3 )
314 H F N(CH3)-(S02-CH3)
315 H C1 N(CH3)-(SOZ-CH3)
316 C1 H- N(CH3)-(SOZ-CH3)
25317 C1 F N(CH3)-(SOy-CH3)
318 C1 C1 N(CH3)-(S02-CH3)
319 Br H N(CH3)-(SOZ-CH3)
320 Br F N(CH3)-(S02-GH3)
321 Br C1 N(CH3)-(SOZ-CH3)
322 CH3 H N(CH3)-(SOz-CH3)
30323 CH3 F N(CH3)-(SOZ-CH3)
324 CH3 C1 N(CH3)-(SOz-CH3)
325 H H SH
326 H F SH
327 H Cl SH
35328 Cl H SH
329 C1 F SH
330 Cl C1 SH
331 Br H SH
332 Br F SH
333 Br C1 SH
40334 CH3 H SH
335 CH3 F SH
336 CH3 C1 SH
337 H H SCH3
338 H F SCH3
45339 H C1 SCH3
340 C1 H SCH3
341 C1 F SCH3
342 C1 C1 SCH3
0050/51065
CA 02396583 2002-07-05
34
No. R R -X-R6
343 Br H SCH3
344 Br F SCH3
345 Br C1 SCH3-_ _ _ _. _.-
346 CH3 H SCH3
347 CH3 F SCH3
348 CH3 C1 SCH3
349 H H SCH(CH3)2
350 H F SCH(CH3)z
351 H C1 SCH(CH3)2
352 C1 H SCH(CH3)z
353 C1 F SCH(CH3)2
354 C1 C1 SCH(CIi3)2
355 Br H SCH(CH3)z
356 Br F SCH(CH3)~
357 Br Cl SCH(CH3)2
358 CH3 H SCH(CH3)2
359 CH3 F SCH(CH3)2
360 CH3 C1 SCH(CH3)2
361 H H SCH2-CH=CHZ
362 H F SCH2-CH=CH2
363 H C1 SCH2-CH=CH2
364 C1 H SCH2-CH=CH2
365 C1 F SCHZ-CH=CH2
366 C1 C1 SCH2-CH=CH2
367 Br H SCH2-CH=CH2
368 Br F SCH2-CH=CHz
369 Br C1 SCHz-CH=CHZ
370 CH3 H SCHZ-CH=CH2
371 CH3 F SCHZ-CH=CHZ
372 CH3 C1 SCH2-CH=CHy
373 H H SCH2-C~CH
374 H F SCH2-C~CH
375 H C1 SCHZ-C~CH
376 C1 H SCHZ-C~CH
377 C1 F SCH2-C-=CH
378 C1 Cl SCHz-C~CH
379 Br H SCHZ-CsCH
380 Br F SCH2-C~CH
381 Br Cl SCH2-C~CH
382 CHg H SCH2-C~CH
383 CH3 F SCH2-C~CH
384 CH3 C1 SCHZ-C~CH
385 H H SCHZ-COON
386 H F SCHz-COOH
387 H Cl SCHZ-COOH
388 Cl H SCH2-COOH
389 C1 F SCHZ-COOH
3g0 C1 C1 SCHZ-COON
391 Br H SCHZ-COOH
392 Br F SCH~-COON
393 Br C1 SCHz-COON
0050151065
CA 02396583 2002-07-05
No. R2 Fad X-R
394 CH3 H ~ HZ~COOH
395 CH3 F SCHz-COOH
396 CH3 C1 SCH2-COOH
397 H H SCH2-COOCH3
398 H F SCHZ-COOCH3
399 H C1 SCH2-COOCH3
400 C1 H SCH2-COOCH3
401 C1 F SCH2-COOCH3
402 C1 C1 SCHZ-COOCH3
10 403 - Br H SCH2-COOCH3
-
404 Br F SCH2-COOCH3
405 Br C1 SCHy-COOCH3
406 CH3 H SCH2-COOCH3
407 CH3 F SCH2-COOCH3
15 408 CH3 C1 SCH2-COOCH3
409 H H SCHZ-COOC2H5
410 H F SCH2-COOCzHS
411 H C1 SCH2-COOC2H5
412 C1 H SCH2-COOC2H5
413 C1 F SCH2-COOC2H5
20 414 C1 G1 SCH2-COOC2H5
415 Br H SCH2-COOC2H5
416 Br F SCH2-COOC2H5
417 Br C1 SCH2-COOCZHS
418 CH3 H SCHz-COOCZHS
25 419 CH3 F SCH2-COOC2H5
420 CH3 C1 SCH2-COOCZHg
421 H H SCHZ-CO-N(CH3)2
422 H F SCHZ-CO-N(CH3)2
423 H C1 SCHZ-CO-N(CH3)2
424 Cl H SCHZ-CO-N(CH3)2
30 425 C1 F SCH2-CO-N(CH3)2
426 C1 C1 SCHz-CO-N(CH3)2
427 Br H SCH2-CO-N(CH3)2
428 Br F SCH2-CO-N(CH3)2
429 Br C1 SCHZ-CO-N(CH3)2
35 430 CH3 H SCHz-CO-N(CH3)z
431 CH3 F SCH2-CO-N(CH3)2
432 CH3 G1 SCHa-CO-N(CH3)z
433 H H SCH(CH3)-COOH
434 H F SCH(CH3)-COOH
435 H Cl SCH(CH3)-COOH
436 C1 H SCH(CH3)-COOH
437 C1 F SCH(CH3)-COOH
438 C1 C1 SCH(CH3)-COOH
439 Br H SCH(CH3)-COOH
440 Br F SCH(CH3)-COOH
441 Br C1 SCH(CH3)-COON
442 CH3 H SCH(CH3)-COOH _
443 CH3 F SCH(CH3)-COON
444 CH3 C1 SCH(CH3)-COOH
0050/51065
CA 02396583 2002-07-05
36
No.~ R R -X-R
' .--,_
~
445 H H SCH
(_CHg)-COOCH3
446 H F SCH(CH3)-COOCH3
447 H Gi SCH(CH3)-COOCH3
448 C1 H -. SCH(CH3)-COOCH3 _..
449 C1 F SCH(CH3)-COOCH3
450 C1 C1 SCH(CH3)-COOCH3
451 Br H SCH(CH3)-COOCH3
452 Br F SCH(CH3)-COOCH3
453 Br C1 SCH(CH3)-COOCH3
10454 CH3 H SCH(CH3)-COOCH3
455 CH3 F SCH(CH3)-COOCH3
456 CH3 C1 SCH(CH3)-COOCH3
457 H H SCH(CH3)-COOC2H5
458 H F SCH(CH3)-COOCzHS
15459 H C1 SCH(CH3)-COOCyHS
460 C1 H SCH(CH3)-COOC2H5
461 C1 F SCH(CH3)-COOCzHS
462 C1 C1 SCH(CH3)-COOC2H5
463 Br H SCH(CH3)-COOCZHS
464 Br F SCH(CH3)-COOG2H5
20465 Br C1 SCH(CH3)-COOCZHS
466 CH3 H - -SCH(CH3)-COOCzH5. -.
467 CH3 F SCH(CH3)-COOCZH5
468 CH3 C1 SCH(CH3)-COOC2H5
469 H H SCH(CH3)-CO-N(CH3)2
25470 H F SCH(CH3)-GO-N(CH3)2
471 H C1 SCH(CH3)-CO-N(CH3)2
472 C1 H SCH(CH3)-CO-N(CH3)2
473 C1 F SCH(CH3)-CO-N(CH3)2
474 Cl Cl SCH(CH3)-CO-N(CH3)z
475 Br H SCH(CH3)-CO-N(CH3)2
304'76 Br F SCH(CH3)-CO-N(CH3)Z
477 Br C1 SCH(CH3)-CO-N(CHg)Z
478 CH3 H SCH(CH3)-CO-N(CH3)2
479 CH3 F SCH(CH3)-CO-N(CH3)2
480 CH3 C1 SCH(CH3)-CO-N(CH3)2
35481 H H S-cyclopentyl
482 H F S-cyclopentyl
483 H C1 S-cyclopentyl
484 C1 H S-cyclopentyl
485 C1 F S-cyclopentyl
486 C1 C1 S-cyclopentyl
40487 Br H S-cyclopentyl
488 Br F S-cyclopentyl
489 Br C1 S-cyclopentyl
490 CH3 H S-cyclopentyl
491 CHg F S-cyclopentyl
45492 CH3 C1 S-cycl.opentyl
493 H H S-CHZ-phenyl
494 H F S-CHz-phznyl
495 H C1 S-CH2-phenyl
0050/51065
CA 02396583 2002-07-05
37
No. R2 R -X-R6
496 C1 H _S-CHz-phenyl
497 C1 F S-CHZ-phenyl
498 Cl C1 S-CEiz-phenyl -. _ _ _ _- _-
499 Br H S-CHz-phenyl
500 Br F S-CHz-phenyl
501 Br C1 S-CHz-phenyl
502 CH3 H S-CHz-phenyl
503 CH3 F S-CHz-phenyl
504 CH3 Cl S-CHz-phenyl
505 H _ H _.- SOz~Ci - _ _.
506 H F SOz-C1
507 H C1 SOz-C1
508 Cl H SOz-C1
509 C1 F SOz-C1
510 C1 C1 SOz-C1
511 Br H SOz-C1
512 Br F SOz-C1
513 Br C1 SOz-C1
514 CH3 H SOz-C1
515 CH3 F SOz-Cl
516 CH3 C1 SOz-Cl
517 H H SOz-NHz
518 H F SOz-NHz
519 H C1 SOz-NHz
520 Cl H SOZ-NHz
521 C1 F SOz-NHz
522 C1 C1 SOz-NHz
523 Br H SOz-NHz
524 Br F SOz-NHz
525 Br C1 SOz-NHz
526 CH3 H SOz-NHz
527 CH3 F SOz-NHz
528 CH3 Cl SOz-NHz
529 H H SOZ-N(CH3)z
530 H F SOz-N(CH3)z
531 H C1 SOz-N(CH3)z
532 C1 H SOz-N(CH3)z
533 C1 F SOz-N(CH3)z
534 C1 C1 SOz-N(CH3)2
535 Br H SOz-N(CH3)z
536 Br F SOz-N(CH3)2
537 Br C1 SOz-N(CH3)z
538 CH3 H SOz-N(CH3)z
539 CH3 F SOz-N(CH3)z
540 CH3 C1 SOz-N(CH3)z
541 H H CHO
542 H F CHO
543 H Cl CHO
544 C1 H CHO
545 C1 F CHO
546 C1 C1 CHO
0050/51065
CA 02396583 2002-07-05
38
No .~ R R -X-R
~~~
547 Br H ___ CHO
:i48 Br F CHO
549 Br Cl CHO
550 CH3 H CHO
551 CH3 F CHO
552 CH3 C1 CHO
553 H H CH=N-OH
554 H F CH=N-OH
555 H C1 CH=N-OH
556 - ~1--__H CH=N-OH _ _. -
557 C1 F CH=N-OH
558 C1 Cl CH=N-OH
559 Br H CH=N-OH
560 Br F CH=N-0H
561 Br C1 CH=N-OH
562 CH3 H CH=N-OH
563 CH3 F CH=N-OH
564 CH3 C1 CH=N-OH
565 H H CH=N-OCH3
566 H F CH=N-OCH3
567 H Cl CH=N-OCH3
_
_
568 C1 H _ -
CH=N-OCH3
569 Cl F CH=N-OCH3
570 C1 C1 CH=N-OCH3
571 Br H CH=N-OCH3
572 Br F CH=N-OCH3
573 Br Cl CH=N-OCH3
574 CH3 H CH=N-OCH3
575 CH3 F CH=N-OCH3
576 CH3 C1 CH=N-OCH3
577 H H CH=N-OC2H5
57g H F CH=N-OCZHS
579 H C1 CH=N-OCzHS
580 C1 H CH=N-OC2H5
581 C1 F CH=N-OCzHS
582 C1 Cl CH=N-OCyHS
583 Br H CH=N-OC2H5
584 Br F CH=N-OCZHS
585 Br C1 CH=N-OC2H5
586 CH3 H CH=N-OCZHS
587 CH3 F CH=N-OCZHS
588 CH3 C1 CH=N-OC2H5
589 H H COOH
590 H F COON
591 H C1 COOH
592 C1 H GOOH
593 Cl F COOH
594 Cl C1 COOH
595 Br H COOH
596 Br F COOH
597 Br C1 COON
0050/51065
CA 02396583 2002-07-05
39
No. R2 R4 _X_R -_
598 ~ H COOH
599 CH3 F COOH
CH3
600 CH3 C1 COOH
6 O 1 H H COOCH3
602 H F COOCH3
603 H CL COOCH3
604 CL H COOCH3
605 C1 F COOCH3
606 C1 C1 COOCH3
607 Br H COOCH3
608 Br F COOCH3
609 Br C1 COOCH3
610 CH3 H COOCK3
611 CH3 F COOCH3
612 CH3 C1 COOCH3
613 H H COOC2Hs
614 H F COOC2H5
615 H C1 COOC2Hs
616 C1 H COOCzHs
617 C1 F COOCZHs
618 C1 Cl COOCzHs
619 Br H COOCZHs
620 Br F COOC2Hs
621 Br Cl COOCzHs
622 CH3 H COOC2Hs
623 CH3 F COOC2Hs
624 CH3 C1 COOC2Hs
625 H H COOCH(CH3)z
626 H F COOCH(CH3)z
627 H C1 COOCH(CH3)z
628 C1 H COOCH(CH3)2
629 C1 F COOCH(CH3)z
630 C1 C1 COOCH(CH3)z
631 Br H COOCH(CH3)z
632 Br F COOCH(CH3)z
633 Br C1 COOCH(CH3)2
634 CH3 H COOCH(CH3)z
635 CH3 F COOCH(CH3)z
636 CH3 C1 COOCH(CH3)z
637 H H COO-CHz-COOCH3
638 H F COO-CHz-COOCH3
639 H C1 COO-CHz-COOCH3
640 Cl H C00-CHz-COOCH3
641 C1 F COO-CHZ-COOCH3
642 C1 C1 COO-CHz-COOCH3
643 Br H COO-CHz-COOCH3
644 Br F COO-CHz-COOCH3
645 Br C1 COO-CHz-COOCH3
646 CH3 H C00-CHz-COOCH3
647 CH3 F COO-CHz-COOCH3
648 CH3 C1 C00-CHZ-COOCH3
CA 02396583 2002-07-05
0050/51065
No,. R, R4 -~-R~ - -
649 H H COO-CH(CH3)-COOCH3
650 H F COO-CH(CH3)-COOCH3
651 H C1 COO-CH(CH3)-COOCH3
5 652 C1 H C00-CH(CH3)-COOCH3
653 C1 F COO-CH(CH3)-COOCH3
654 C1 C1 C00-CH(CH3)-COOCH3
655 Br H C00-CH(CH3)-COOCH3
656 Br F COO-CH(CH3)-COOCH3
657 Br C1 COO-CH(CH3)-COOCH3
10 658 CH3 H C00-CH(CH3)-COOCH3
659 CH3 F COO-CH(CH3)-COOCH3
660 CH3 C1 C00-CH(CH3)-COOCH3
661 H H COO-C(CH3)2-COO-CHZ-CH=CH2
662 H F COO-C(CH3)2-COO-CH2-CH=CH2
15 663 H C1 COO-C(GH3)y-COO-CH2-CH=CH2
664 C1 H C00-C(CH3)p-COO-CH2-CH=CHZ
665 C1 F COO-C(CH3)2-C00-CH2-CH=CHZ
666 C1 C1 COO-C(CH3)z-COO-CH2-CH=CH2
667 Br H COO-C(CH3)z-COO-GH2-CH=CHz
668 Br F C00-C(CH3)Z-COO-CHZ-CH=CH2
20 669 Br Cl COO-C(CH3)z-COO-CHz-CH=CH2
670 CH3 H COO-C(CH3)2-COO-CH2-CH=CH2
671 CH3 F COO-C(CH3)2-C00-CHZ-CH=CHZ
672 CH3 C1 COO-C(CH3)2-C00-CH2-CH=CHz
673 H H CH2-CH(C1)-COOH
25 674 H F CH2-CH(C1)-COON
675 H Cl CH2-CH(C1)-COOH
676 C1 H CH2-CH(C1)-COOH
677 C1 F CHy-CH(C1)-COON
678 C1 C1 CHZ-CH(C1)-COON
679 Br H CH2-CH(C1)-COOH
30 680 Br F CHZ-CH(C1)-COON
681 Br C1 CHZ-CH(C1)-COOH
682 CH3 H CHZ-CH(C1)-COOH
683 CH3 F CH2-CH(Cl)-COOH
684 CH3 C1 CH2-CH(C1)-COOH
35 685 H H CH2-CH(C1)-COOCH3
686 H F CH2-CH(C1)-COOCH3
687 H C1 CHZ-CH(C1)-COOCH3
688 C1 H CH2-CH(Cl)-COOCH3
689 C1 F CHZ-CH(Cl)-COOCH3
690 C1 C1 CH2-CH(C1)-COOCH3
40 691 Br H CH2-CH(C1)-COOCH3
692 Br F CHZ-CH(C1)-COOCH3
693 Br C1 CHZ-CH(C1)-COOCH3
694 CH3 H CHZ-CH(C1)-COOCH3
69S CH3 F CHy-CH(C1)-COOCH3
696 CH3 C1 CH2-CH(C1)-COOCH3
697 H H CH2-CH(C1)-COOCZHS
698 H F CHy-CH(C1)-COOCyHS
699 H C1 CH2-CH(C1)-COOCzHS
0050/51065 CA 02396583 2002-07-05
41
T~To R R -X-R
.
- _
700 C1 H CH2-CH(C1)-COOC2H5
701 Cl F CH2-CH(C1)-COOC2H5
702 Cl C1 CH2-CH(C1)-COOC2H5
703 Br H CH2-CH(Cl)-COOC2H5
704 Br F CH2-CH(C1)-COOC2H5
705 Br C1 CH2-CH(C1)-COOC2H5
706 CH3 H CH2-CH(Cl)-COOC2H5
707 CH3 F CH2-CH(C1)-COOC2H5
708 CH3 C1 CH2-CH(Cl)-COOC2H5
7pg H H CH2-CH(Cl)-CO-N(CH3)2
710 H F CH2-CH(Cl)-CO-N(CH3)2
711 H C1 CH2-CH(Cl)-CO-N(CH3)2 I
712 C1 H CH2-CH(C1)-CO-N(CH3)2
713 C1 F CH2-CH(C1)-CO-N(CH3)2
714 C1 C1 CH2-CH(Cl)-CO-N(CH3)2
715 Br H CH2-CH(C1)-CO-N(CH3)2
716 Br F CH2-CH(C1)-CO-N(CH3)2
717 Br C1 CH2-CH(Cl)-CO-N(CH3)2
718 CH3 H CH2-CH(C1)-CO-N(CH3)2
719 CH3 F CH2-CH(C1)-CO-N(CH3)2
720 GH3 C1 CH2-CH(C1)-CO-N(CH3)2
721 H H CH=C(C1)-COOH
722 H F CH=C(C1)-COOH
723 H C1 CH=C(C1)-COOH
724 C1 H CH=C(Cl)-COOH
725 Cl F CH=C(C1)-COOH
726 C1 Cl CH=C(Cl)-COOH
727 Br H CH=C(C1)-COOH
728 Br F CH=C(Cl)-COOH
729 Br C1 CH=C(C1)-COON
730 CH3 H CH=C(C1)-COOH
731 CH3 F CH=C(C1)-COON
732 CH3 Cl CH=C(C1)-COOH
733 H H CH=C(C1)-COOCH3
734 H F CH=C(C1)-COOCH3
735 H C1 CH=C(C1)-COOCH3
736 C1 H CH=C(C1)-COOCH3
737 C1 F CH=C(C1)-COOCH3
738 C1 C1 CH=C(C1)-COOCH3
739 Br H CH=C(C1)-COOCH3
740 Br F CH=C(Cl)-COOGH3
741 Br C1 CH=C(Cl)-COOCH3
742 CH3 H CH=C ( Cl ) -COOCH3
743 CH3 F CH=C(C1)-COOCH3
744 CH3 C1 CH=C(Cl)-COOCH3
745 H H CH=C(C1)-COOC2H5
746 H F CH=C(C1)-COOC2H5
747 H C1 CH=C(Cl)-COOC2H5
748 C1 H CH=C(C1)-COOC2H5
749 C1 F CH=C(C1)-COOC2H5
750 C1 C1 CH=C(C1)-COOC2H5
0050/51065
CA 02396583 2002-07-05
42
No. R R -X-R
751 Br H CH=c(C1)-COOCzHS
752 Br F CH=C(C1)-COOC2H5
753 Br C1 CH=C(C1)-COOCZHS
754 CH3 H CH=C(C1)-COOCZHS
755 CH3 F CH=C(C1)-COOCZHS
756 CH3 C1 CH=C(C1)-COOC2H5
757 H H CH=C(Cl)-CO-N(CH3)z
758 H F CH=C(C1)-CO-N(CH3)2
759 H C1 CH=C(C1)-CO-N(CH3)z
10760 C1 H CH=C(CI)-CO-N(CH3)2 ___
761 C1 F CH=C(G1)-CO-N(CH3)2
762 C1 Cl CH=C(C1)-CO-N(CH3)z
763 Br H CH=C(C1)-CO-N(CH3)z
?64 Br F CH=C(C1)-CO-N(CH3)2
15765 Br Cl CH=C(C1)-CO-N(CH3)z
766 CH3 H CH=C(C1)-CO-N(CH3)z
767 CH3 F CH=C(C1)-CO-N(CH3)z
768 CH3 Cl CH=C(C1)-CO-N(CH3)z
769 H H CO-NHz
770 H F CO-NHz
20771 H Cl CO-NHz
772 C1 ~ CO-NHz
773 C1 F- CO-NHZ
-
774 C1 Cl CO-NHZ __.. _..
775 Br H CO-NHz
25776 Hr F CO-NHz
777 Br C1 CO-NHz
778 CH3 H CO-NHz
779 CH3 F CO-NHz
780 CH3 C1 CO-NHz
781 H H CO-NH-CHg
30782 H F CO-NH-CH3
783 H C1 CO-NH-CH3
784 C1 H CO-NH-CH3
785 C1 F CO-NH-CH3
786 C1 Cl CO-NH-CH3
35787 Br H CO-NH-CH3
788 Br F CO-NH-CH3
789 Hr C1 CO-NH-CH3
790 CH3 H CO-NH-CH3
791 CH3 F CO-NH-CH3
792 CH3 C1 CO-NH-CH3
40793 H H CO-N(CH3)z
794 H F CO-N(CH3)2
795 H C1 CO-N(CH3)z
796 C1 H CO-N(CH3)z
797 C1 F CO-N(CH3)z
45798 C1 C1 CO-N(CH3)2
799 Br H CO-N(CH3)z
800 Br F CO-N(CH3)z
801 Br C1 CO-N(CH3)z
0050/51065
CA 02396583 2002-07-05
43
No. R R -X-R~
802 CH3 F3 CO--N ( CH3 ) z
803 CH3 F CO-N(CH3)z
804 CH3 C1 CO-N(CH3)z
805 H H CO-NH-OCH3
806 H F CO-NH-OCH3
807 H C1_ C~-NH-OCHg _ _ -
808 C1 H CO-NH-OCH3 ___ _ _ - _
809 C1 F CO-NH-OCH3
810 C1 C1 CO-NH-OCH3
811 Br H CO_NH-OCH3 _ - _
812 Br F CO-NH-OCH3
813 Br Cl CO-NH-OCH3
814 CH3 H CO-NH-OCH3
815 CH3 F CO-NH-OCH3
816 CH3 C1 CO-NH-OCH3
$17 H H C(N=OCH3)-OCH3
818 H F C(N=OCH3)-OCH3
819 H C1 C(N=OCH3)-OCH3
820 C1 H C(N=OCH3)-OCH3
821 Cl F C(N=OCH3)-OCH3
g22 C1 C1 C(N=OCH3)-OCH3
823 Br H C(N=OCH3)-OCH3
824 Br F C(N=OCH3)-OCH3
825 Br C1 C(N=OCH3)-OCH3
826 CH3 H C(N=OCH3)-OCH3
827 CH3 F C(N=OCH3)-OCH3
828 CH3 Cl C(N=OCH3)-OCH3
829 H H C(N=OCH3)-OCHz-COOCH3
830 H F C(N=OCH3)-OCHz-COOCH3
831 H C1 C(N=OCH3)-OCHz-COOCH3
832 C1 H C(N=OCH3)-OCHZ-COOCH3
833 C1 F C(N=OCH3)-OCHz-COOCH3
834 C1 C1 C(N=OCH3)-OCHZ-COOCH3
835 Br H C(N=OCH3)-OCHz-COOCH3
836 Br F C(N=OCH3)-OCHZ-COOCH3
837 Br C1 C(N=OCH3)-OCHz-COOCH3
838 CH3 H C(N=OCH3)-OCHZ-COOCH3
839 CH3 F C(N=OCH3)-OCHz-COOCH3
840 CH3 C1 C(N=OCHg)-OCHz-COOCH3
841 H H PO(OCH3)z
842 H F PO(OCH3)z
843 H C1 PO(OCH3)2
$44 C1 H PO(OCH3)z
845 C1 F PO(OCH3)2
846 C1 Cl PO(OCH3)z
847 Br H PO(OCH3)z
848 Br F-- PO(OCH3)z
849 Br C1 P0(OCH3)2
850 CH3 H PO(OCH3)2 _
851 CH3 F PO(OCH3)z
B52 CH3 C1 PO(OCH3)z
0050/51065
CA 02396583 2002-07-05
44
No . R2 R4 -X-R
853 H H PO(OCzHS)z
854 H F PO(OC2H5)z
855 H -.- G1 P~~OCyHS)z
856 Cl H PO(OCaHS)z
857 C1 F PO(OC2H5)z
858 C1 C1 PO(OCZHS)2
859 Br H PO(OCyHS)z
860 Br F PO(OCaHS)z
861 Br C1 PO(OC2H5)2
862 CH3 H pO~OC2H5)2
863 CH3 F PO(OC2H5)z
864 CH3 C1 PO(OC2H5)z
865 H H CHz-PO(OCH3)z
866 H F CHZ-PO(OCH3)z
867 H C1 CHz-PO(OCH3)z
868 C1 H CHZ-PO(OCH3)z
869 C1 F CHz-PO(OCH3)z
870 C1 C1 CHz-PO(OCH3)z
871 Br H CHz-PO(OCH3)z
872 Br F CHz-PO(OCH3)2
873 Br.- C1 CHZ-PC1(OCH3)z
874 CH3 H CHz_p~(pCH3)z
875 CH3 F CHZ-PO(OCH3)z
876 CH3 C1 CHZ-PO(OCH3)2
877 H H CHZ-PO(OCZHg)z
878 H F CHZ-PO(OCZHS)z
879 H C1 CHZ-PO(OC2H5)z
880 C1 H CHZ-PO(OCZHS)z
881 C1 F CHz-PO(OCzHS)z
882 C1 C1 CHz-PO(OCzHS)z
883 Br H CHz-PO(OCZHS)z
gg4 Br F CHz-PO(OCZHg)z
885 Br C1 CHZ-PO(OC2H5)z
886 CH3 H CHZ-PO(OCzHS)z
887 CH3 F CHZ-PO(OCZHS)z
888 CH3 C1 CHz-PO(OC2H5)2
889 H H CHZ-CH(C1)-PO(OCH3)z
890 H F CHZ-CH(C1)-PO(OCH3)z
891 H C1 CHZ-CH(C1)-PO(OCH3)z
892 C1 H CHz-CH(Cl)-PO(OCH3)2
B93 C1 F CHz-CH(C1)-PO(OCH3)z
894 C1, C1 CHz-CH(C1)-PO(OCH3)z
895 Br H CHz-CH(C1)-PO(OCH3)2
896 Br F CHZ-GH(C1)-PO(OCH3)2
897 Br C1 CHz-CH(C1)-PO(OCH3)z
898 CH3 H CHz-CH(C1)-PO(OCH3)z
899 CH3 F CHz-CH(C1)-PO(OCH3)z
900 GH3 C1 CHz-CH(C1)-PO(OCH3)z
901 H H CHz-CH(G1)-PO(OC2H5)z
902 H F CHz-CLi(C1)-PO(OCzHS)z
903 H C1 CHZ-CH(C1)-PO(OC2H5)z I
0050/51065
CA 02396583 2002-07-05
No. R2 R4 -X-R
904 Cl H Cud-CH(Cl)-PO(0CyH5)2
905 C1 F CHy-CH(Cl)-PO(OCZHS)2
906 C1 C1 CH2-CH(C1)-PO(OCzH5)y
5 907 Br H CHZ-CH(C1)-PO(OCzHS)z
908 Br F CH2-CH(C1)-PO(OCZHS)z
909 Br Cl CHz-CH(C1)-PO(OC2H5)z
910 CH3 H CHZ-CH(Cl)-PO(OC2H5)Z
911 CH3 F CH2-CH(C1)-PO(OC2H5)2
912 CH3 Cl CHZ-CH(C1)-PO(_OCZHS)2
10913 H H CH=C(C1)-PO(OCH3)a
914 H F CH=C(C1)-PO(OCH3)2
915 H C1 CH=C(G1)-PO(OCH3)2
916 C1 H CH=C(C1)-PO(OCH3)z
917 C1 F CH=C(C1)-PO(OCH3)2
15918 C1 C1 CH=C(Cl)-PO(OCH3)2
919 Br H CH=C(Cl)-PO(OCH3)z
920 Br F CH=C(C1)-PO(OCH3)2
921 Br C1 CH=C(Cl)-PO(OCH3)2
922 CH3 H CH=C(C1)-PO(OCH3)2
923 CH3 F CH=C(Cl)-PO(OCH3)2
20924 CH3 C1 CH=C(C1)-PO(OCH3)2
925 H H CH=C(C1)-PO(OC~HS)2
926 H F CH=C(C1)-PO(OC2H5)Z
927 H C1 CH=C(C1)-PO(OCyHS)2
928 C1 H CH=C(C1)-PO(OCyHS)z
25929 C1 F CH=C(C1)-PO(OC2H5)2
930 C1 C1 CH=C(C1)-PO(OC2H5)2
931 Br H CH=C(C1)-PO(OC2H5)z
932 Br F CH=C(C1)-PO(OC2H5)z
933 Br C1 CH=C(C1)-PO(OCzHS)z
934 CH3 H CH=C(C1)-PO(OCaHS)z
30935 CH3 F CH=C(Cl)-PO(OC2H5)2
936 CH3 C1 CH=C(C1)-PO(OCZHS)z
Particular preference is also given to the compounds of the
formula IAb (compounds IA where Q = CH, R1 = CH3 and R5 = C1) in
35 which the variables Rz, R4 and X-R6 together have the meanings
given in each case in one row of Table 1 (compounds
IAb.l-IAb.936).
R4 CH3
- ~ ~ N- \
40 Gl ~ N (IAb)
~ 0-CHF2
X-R6
R
0050/51065 CA 02396583 2002-07-05
46
Particular preference is also given to the compounds of the
formula IAc (compounds IA where Q = CH, R1 = C2H5 and R5 = C1) in
which the variables Rz, R4 and X-R6 together have the meanings
given in each case in one row of Table 1 (compounds
IAc.l-IAc.936).
R4 CH2CH3
N=
C1- ~ ~ \ N\ ( IAc )
O-CHFz
X-R6 ~2
Particular preference is also given to the compounds of the
formula IAd (compounds IA where Q = CH, R1 = CH(CH3)z and R5 = C1)
in which the variables Rz, R4 and X-R6 together have the meanings
given in each case in one row of Table 1 (compounds
IAd.l-IAd.936).
R4 CH(CH3)z
- ~ ~ N
C1 ~ N (IAd)
~' O-CHFz
X_R6 R2
Particular preference is also given to the compounds of the
formula IAe (compounds IA where Q = CH, R1 = C(CH3)3 and R5 = Cl)
in which the variables Rz, R4 and X-R6 together have the meanings
given in each case in one row of Table 1 (compounds
IAe.l-IAe.936).
R4 C(CH3)3
N
C1- ~ ~ ~ N ( IAe )
~ O-CHFz
X-R6 Rz
Preference is furthermore given to the compounds of the formula
IBa (compounds IB where Q = N, R1 = H and RS = C1) in which the
variables Rz, R4 and X-R6 together have the meanings given in each
case in one row of Table 1 (compounds IBa.l-IBa.936).
R4
N=~
- N
C1- N ( IBa )
\ O-CHFz
X-R6 Rz
CA 02396583 2002-07-05
0050/51065
47
Preference is also given to the compounds of the formula IBb
(compounds IB where Q = N, R1 = CHI and RS = C1) in which the
variables R2, R4 and X-R6 together have the meanings given in each
case in one row of Table 1 (compounds IBb.l-IBb.936).
R4 CH3
N="~
C1-~ ~) ~ N (IBb)
'- N ~ 0-CHF2
X-R6 R2
Preference is also given to the compounds of the formula IBc
(compounds IB where Q = N, R1 = CZHS and R5 = Cl) in which the
variables Rz, R4 and X-R6 together have the meanings given in each
case in one row of Table 1 (compounds IBc.l-IBc.936).
R4 CHZCH3
N ='~
C1- ~ ~ ~ N ( IBc )
N ~ ~ 0-CHF2
X-R6 R2
Preference is also given to the compounds of the formula IBd
(compounds IB where Q = N, R1 = CH(CH3)2 and R5 = C1) :in which the
variables Rz, R4 and X-R6 together have the meanings given in each
case in one row of Table 1 (compounds IBd.l-IBd.936).
R4 CH(CH3)2
N =
Cl- f \~ ~ N~ ( IBd)
N O-CHF2
X-R6 R2
Preference is also given to the compounds of the formula IBe
(compounds IB where Q = N, R1 = C(CH3)3 and R5 = C1) in which the
variables R2, R4 and X-R6 together have the meanings given in each
case in one row of Table 1 (compounds IBe.I-IBe.936).
R~ C(CH3)3
- ~ ~ N
Cl ~ N (IBe)
' N ~ ~ O-CHFZ
X-R6 R2
0050f51065
CA 02396583 2002-07-05
48
Particular preference is furthermore given to the compounds of
the formula ICa (compounds IC where Q = CH, R1 = H and where R5
and X-R6 form a chain -OCH(R16)-C(O)-NR18-) in which the variables
R2, R4 and R16 and R1$ together have the meanings given in each
case in one row of Table 2 (compounds ICa.l-ICa.696).
Rls N~ ( ICa )
O-CHF2
0 ~R18
Table 2:
No. R R R R
1 H H H H
2 H F H H
203 H G1 H H
4 Cl H H H
5 C1 F H H
6 C1 C1 H H
7 Br H H H
8 Br F H H
25g Br Cl H H
10 CH3 H H H
11 CH3 F H H
12 CH3 Cl H H
13 H H CH3 H
3014 H F CH3 H
15 H C1 CH3 H
16 C1 H CH3 H
17 C1 F CH3 H
18 C1 C1 CH3 H
19 Br H CH3 H
3520 Br F CH3 H
21 Br Cl CH3 H
22 CH3 H CH3 H
23 CH3 F CH3 H
24 CH3 C1 CH3 H
402 5 H H H OH
26 H F H OH
27 H C1 H OH
28 C1 H H OH
29 C1 F H OH
30 C1 C1 H OH
4531 Br H H OH
32 Br F H OH
33 Br C1 H OH
R4
p-~ ~ N
-N R2
0050!51065
CA 02396583 2002-07-05
49
No . R R Rl R18
34 CH3 H H OH __-
35 CH3 F H OH
36 CH3 C1 H OH
3 7 H H CH3 OH
38 H F CH3 OH
39 H C1 CH3 OH
40 C1 H CH3 OH
41 C1 F CHg OH
42 C1 C1 CH3 OH
1043 Br H CH3 OH _ -
44 Br F CH3 OH
45 Br CZ CH3 OH
46 CH3 H CH3 OH
47 CH3 F CH3 OH
1548 CH3 C1 CH3 OH
49 H H H CH3
50 H F H CH3
51 H C1 H GH3
52 C1 H H CH3
53 C1 F H CH3
2054 CZ -Ci H CH3 _
55 Br H _ H _ CH3 _ _ __ _ _
__
..
_
56 Br F H -
-
CH3
57 Br C1 H CH3
58 CH3 H H CH3
2559 CH3 F H CH3
60 CH3 C1 H CH3
61 H H CH3 CH3
62 H F CH3 CH3
63 H C1 CH3 CH3
64 C1 H CH3 CH3
3065 C1 F CH3 CH3
66 C1 C1 CH3 CH3
67 Br H CH3 CH3
68 Br F CH3 CH3
69 Br C1 CH3 CH3
3570 CH3 H CH3 CH3
71 CH3 F CH3 CH3
72 CH3 C1 CH3 CH3
73 H H H C2H5
74 H F H C2H5
75 H C1 H C2H5
4076 C1 H H C2H5
77 C1 F H CZHS
78 C1 C1 H CZHS
79 Br H H CZHS
80 Br F H CzHS
4581 Br C1 H CzHS -
82 CH3 H H C2H5
$3 CH3 F H CZHS
84 CH3 C1 H C2H5
0050/51065
CA 02396583 2002-07-05
No . R A A1 R18 .-
85 H H CH3 C2H
86 H F CH3 C2H5
87 H C1 CH3 CZHS
5 88 C1 H CH3 C2H5
89 C1 F CH3 C2H5
90 C1 C1 CH3 C2H5
91 Br H CH3 C2H5
92 Br F CH3 C2H5
93 Br Cl CH3 C2H5
10 g4 _.. CH3.. H CH3 C2H5
-
95 CH3 F CH3 C2H5
96 CH3 C1 CH3 C2H5
97 H H H n-C3H7
98 H F H n-C3H7
15 99 H C1 H n-C3H7
100 Cl H H n-C3H7
101 C1 F H n-C3H7
102 C1 C1 H n-C3H7
103 Br H H n-C3H7
104 Br F H n-C3Hq
20 105 Br C1 H n-C3H7
106 CH3 H H n-C3H7
107 CH3 F H n-C3H~
108 CH3 C1 H n-C3H7
109 H H CHg n-C3H7
25 110 H F CH3 n-C3H7
111 H C1 CH3 n-C3H7
112 Cl H CH3 n-C3H7
113 C1 F CH3 n-C3H7
114 C1 C1 CH3 n-C3H7
115 Br H CH3 n-C3H7
30 116 Br F CH3 n-C3H7
117 Br C1 CH3 n-C3H~
118 CH3 H CH3 n-C3H7
119 CH3 F CH3 n-C3H7
120 CH3 C1 CH3 n-C3H7
35 121 H H H CH(CH3)y
122 H F H CH(CH3)2
123 H Cl H CH(CH3)2
124 C1 H H CH(CH3)2
125 C1 F H CH(CH3)2
126 C1 C1 H CH(CH3)2
40 127 Br H H CH(CH3)y
128 Br F H CH(CH3)2
129 Br C1 H CH(CH3)2
130 CH3 H H CH(CH3)2
131 CH3 F H CH(CH3)2
45 132 CH3 C1 H CH(CH3)2
133 H H CH3 CH(CH3)2
134 H F CH3 CH(CH3)2
135 H C1 CH3 CH(CH3)2
0050/51065
CA 02396583 2002-07-05
51
No. R~ R Rl R1$ _.
136 C1 H CH3 C_H(CH3)p _
~
137 C1 F CH3 CH(CH3)z
138 C1 C1 CHg CH(CH3)a
139 Br H CH3 CH(CH3)2
140 Br F CH3 CH(CH3)a
141 Br Cl CH3 CH(CH3)a
142 CH3 H CH3 CH(CH3)a
143 CH3 F CH3 CH(CH3)2
144 CH3 C1 CH3 CH(CH3)a
I45 H H H n_~4H9 _ _. __.
146 H F H n_~4H9 -.- _-
147 H C1 H - _n~C~H9 _ _
-.
148 C1 H H n-C4Hg
149 C1 F H n-C4Hg
150 Cl C1 H n-C4Hg
151 Br H H n-C4Hg
152 Br F H n-C4H9
153 Br C1 H n-C4H9
154 CH3 H H n-C4Hg
155 CH3 F H n-C4Hg
156 CH3 Cl H n-C4Hg
157 H H CH3 n-C4Hg
158 H F CH3 n-CqHg
159 H C1 CH3 n-C4Hg
160 CI H CHg n-C4Hg
161 C1 F CH3 n-C4Hg
162 C1 Cl CH3 n-C4Hg
163 Br H CH3 ri-C4Hg
164 Br F CH3 n-C4Hg
165 Br C1 CH3 n-C4Hg
166 CH3 H CH3 n-CgHg
167 CH3 F CH3 n-C4Hg
168 CH3 C1 CH3 n-C4Hg
169 H H H CH(CH3)-C2H5
170 H F H CH(CH3)-C2H5
171 H C1 H CH(CH3)-C2H5
172 C1 H H CH(CH3)-C2H5
173 C1 F H CH(CH3)-CaHS
174 C1 C1 H CH(CH3)-C2H5
175 Br H H CH(CH3)-C2H5
176 Br F H CH(CH3)-C2H5
177 Br C1 H CH(CH3)-CZHS
178 CH3 H H CH(CH3)-CZHS
179 CH3 F H CH(CH3)-CZH5
180 CH3 C1 H CH(CH3)-CZHS
181 H H CH3 CH(CH3)-CaHS
182 H F CH3 CH(CH3)-C2H5
183 H C1 CH3 CH(CH3)-CZHS
184 Cl H CH3 CH(CH3)-CZH5
185 C1 F CH3 CH(CH3)-CzHS _ _
186 C1 C1 CH3 CH(CH3)-CzHS
0050/51065
CA 02396583 2002-07-05
52
No. R2 R4 R Rig _- __ _
187 Br H CH3 CH(CH3)-CzHS
188 Br F CH3 CH(CH3)-CzHS
189 Br ci CH3- CH~CH3~~~ZHS _.
190 CH3 H CH3 CH ~ CH3 ) -C2g5-- - _-
- _
191 CH3 F CH3 CH(CH3)-CzHS
192 CH3 Cl CH3 CH(CH3)-CZHS
193 H H H CHz-CH(CH3)z
194 H F H CHz-CH(CH3)z
195 H C1 H CHz-CH(CH3)z
10196 C1 H H CHz-CH(CH3)2
197 Cl F H CHZ-CH(CH3)z
198 Cl G1 H CHZ-CH(CH3)2
199 Br H H CHz-CH(CH3)z
200 Br F H CHz-CH(CH3)z
15201 Br C1 H CHz-CH(CH3)z
202 CH3 H H CHz-CH(CH3)z
203 CH3 F H CHz-CH(CH3)2
204 CH3 C1 H CHZ-CH(CH3)2
205 H H CH3 CHz-CH(CH3)z
206 H F CH3 CHz-CH(CH3)z
20207 H C1 CH3 CHz-CH(CH3)z _
208 C1 H CH3 CHz-CH(CH3)2
209 C1 F CH3 CHz-CH(CH3)2
210 C1 C1 CH3 CHz-CH(CH3)z
211 Br H CH3 CHz-CH(CH3)z
25212 Br F CH3 CHz-CH(CH3)z
213 Br C1 CH3 CHz-CH(CH3)z
214 CH3 H CH3 CHz-CH ( CH3 ) 2
215 CH3 F CH3 CHz-CH(CH3)z
216 CH3 C1 CH3 CHz-CH(CH3)2
217 H H H CHz-CF3
30218 H F H CHz-CF3
219 H C1 H CHZ-CF3
220 C1 H H CHz-CF3
221 C1 F H CHz-GF3
222 C1 C1 H CHz-GF3
35223 Br H H CHz-CF3
224 Br F H CHz-CF3
225 Br C1 H CHZ-CFg
226 CH3 H H CHz-CF3
227 CH3 F H CHz-CF3
228 CH3 C1 H CHz-CF3
40229 H H CH3 CHz-CF3
230 H F CH3 CHz-CF3
231 H C1 CH3 CHZ-CF3
232 C1 H CH3 CHz-CF3
233 C1 F CH3 CHz-CF3
45234 C1 C1 CH3 CHz-CF3
_
235 Br H CH3 CHz-CF3
236 Br F CH3 CHz-CF3
237 Br C1 CH3 CHZ-CFg
0050/51065
CA 02396583 2002-07-05
53
No. R2 R4 ~1~ ~1& _
238 CH3 H CH3 CHp-CFg
239 CH3 F GH3 CH2-CF3
240 CH3 C1 CH3 CH2-CF3
241 H H H CHZ-CH=CHy
242 H F H CHz-CH=CHz
243 H C1 H CH2-CH=GH2
244 Cl H H CH2-CH=CHz
245 C1 F H CHZ-CH=CH2
246 C1 C1 H CHz-CH=CH2
10247 Br H H _ GH2_~H-CH2_. __-
248 Br F H CHZ-CH=CHZ
249 Br C1 H CHZ-CH=CHZ
250 CH3 H H CHp-CH=CHI
251 CH3 F H CH2-CH=CH2
15252 CH3 C1 H CHz-CH=CHy
253 H H CH3 CHZ-CH=CH2
254 H F CH3 CHp-CH=CHp
255 H Cl CH3 CHZ-CH=CHy
256 C1 H CH3 CHy-CH=CH2
257 C1 F CH3 CHz-CH=CH2
20258 C1 C1 CH3 CHZ-CH=GH2
259 Br H CH3 CH2-CH=CHy
260 Br F CH3 CHZ-CH=CHZ
261 Br C1 CH3 CHZ-CH=CHZ
262 CH3 H CH3 CH2-CH=CH2
25263 CH3 F CH3 CH2-CH=CHz
264 CH3 C1 CH3 CHZ-CH=CH2
265 H H H CHZ-C~CH
266 H F H CHZ-C~CH
267 H C1 H CH2-C~CH
268 Cl H H CHZ-C~CH
30269 C1 F H CH2-C--=CH
270 C1 C1 H CH2-C~CH
271 Br H H CH2-C-=CH
272 Br F H CH2-C~CH
273 Br C1 H CH2-C~CH
35274 CH3 H H CHy-C~CH
275 CH3 F H CH2-C~CH
276 CH3 C1 H CHy-C~CH
277 H H CH3 CHZ-C~CH
278 H F CH3 CHz-C=CH
279 H C1 CH3 CHy-C~CH
40280 C1 H CH3 CH2-C~CH
281 C1 F CH3 CHZ-C~CH
282 Cl C1 CH3 CHZ-C~CH
283 Br H CH3 CHz-C$CH
284 Br F CH3 CH2-C~CH
45285 Br C1 CH3 CH2-C~CH
286 CH3 H CH3 CH2-C~CH
287 CH3 F CH3 CH2-C~CH
288 CH3 C1 CH3 CHz-C~CH
0050/51065
CA 02396583 2002-07-05
54
N~o . R2 R4 R1 R
289 H H H OCH3
290 H F H OCH3
291 H C1 H OCH3
292 C1 H H OCH3
293 C1 F H OCH3
294 C1 Cl H OCH3
295 Br H H OCH3
296 Br F H OCH3
297 Br C1 H OGH3
298 CH3 H H OCH3
299 CH3 F H OCH3
300 CH3 C1 H OCH3
301 H H CH3 OCH3
302 H F CH3 OCH3
303 H C1 CH3 OCH3
304 C1 H CH3 OCH3
305 Cl F CH3 OCH3
306 C1 C1 CH3 OCH3
307 Br H CH3 OCH3
308 Br F CH3 OCH3
309 Br C1 CH3 OCH3
310 CH3 H CH3 OCH3
311 CH3 F CH3 OCH3
312 CH3 C1 CH3 OCH3
313 H H H OCZHS
314 H F H OCzHS
315 H C1 H OC2H5
316 C1 H H OC2H5
317 C1 F H OCZHS
318 C1 C1 H OC2H5
319 Br H H OCZHS
320 Br F H OC2H5
321 Br Cl H OC2H5
322 CH3 H H OC2H5
323 CH3 F H OCyHS
324 CH3 C1 H OCZHS
325 H H CH3 OCzHS
326 H F CH3 OC2H5
327 H C1 CH3 OC2H5
328 C1 H CH3 OC2H5
329 C1 F CH3 OC2H5
330 C1 C1 CH3 OC2H5
331 Br H CH3 OC2HS
332 Br F CH3 OC2H5
333 Br Cl CH3 OC2H5
334 CH3 H CHg OC2H5
335 CH3 F CH3 OC2H5
336 CH3 C1 CH3 OC2H5
337 H H H O-n-C3H7
338 H F H 0-n-C3H7
339 H C1 H 0-n-C3H7
0050/51065
CA 02396583 2002-07-05
No. R R4 R1 Ri
340 C1 H H 0-n-C3H7
341 C1 F H 0-n-C3H7
342 C1 C1 H O-n-C3H7
343 Br H H 0-n-C3H7
344 Br F H 0-n-C3H7
345 Br C1 H 0-n-C3H7
346 CH3 H H O-n-C3H7
347 CH3 F H 0-n-C3H7
348 CH3 C1 H 0-n-C3H7
10349 H - H_. CH3 0-n-C3H~ -.
350 H F CH3 O-n-C3H7
351 H C1 CH3 0-n-C3H7
352 C1 H CH3 0-n-C3H7
353 C1 F CH3 0-n-C3H7
15354 Cl C1 CH3 0-n-C3H7
355 Br H CH3 0-n-C3H7
356 Br F CH3 O-n-C3H7
357 Br C1 CH3 0-n-C3H7
358 CH3 H CH3 O-n-C3H7
359 CH3 F CH3 O-n-C3H7
20360 CH3 C1 CH3 O-n-C3H7
361 H H H OCH(CH3)2
362 H F H OCH(CH3)2
363 H C1 H OCH(CH3)2
364 Cl H H OCH(CH3)2
25365 C1 F H OCH(CH3)2
366 C1 C1 H OCH(CH3)2
367 Br H H OCH(CH3)2
368 Br F H OCH(CH3)2
369 Br C1 H OCH(CH3)z
370 CHg H H OCH(CH3)2
30371 CH3 F H OCH(CH3)y
372 CH3 C1 H OCH(CH3)2
373 H H CH3 OCH(CH3)2
374 H F CH3 OCH(CH3)2
375 H C1 CH3 OCH(CH3)2
35376 C1 H CH3 OCH(CH3)z
377 C1 F CH3 OCH(CH3)2
378 C1 C1 CH3 OCH(CH3)2
379 Br H CH3 OCH(CH3)2
380 Br F CH3 OCH(CH3)2
381 Br C1 CH3 OCH(CHg)2
40382 CH3 H CH3 OCH(CH3)2
383 CH3 F CH3 OCH(CH3)2
384 CH3 C1 CH3 OCH(CH3)2
385 H H H O-n-C4Hg
386 H F H 0-n-CgH9
45387 H C1 H O-n-C4Hg
388 C1 H H 0-n-CQHg
389 C1 F H O-n-C4H9
390 C1 C1 H O-n-CqHg
0050/51065
CA 02396583 2002-07-05
56
No . R R4 .R1 R 8
391 Br H___ H 0-n-C4Hg
~
392 Br F H 0-n-C4Hg
393 Br C1 H O-n-C4Hg
394 CH3 H H 0-n-C4Hg
395 CH3 F H 0-n-C4Hg
396 CH3 C1 H 0-n-C4Hg
397 H H CH3 O-n-C4Hg
398 H F CH3 0-n-C4Hg
399 H C1 CH3 O-n-CqHg
400 C1 H CH3 0-n-C4Hg
401 C1 F CH3 O-n-C4Hg
402 C1 C1 CH3 0-n-C4Hg
403 Br H CH3 0-n-C4Hg
404 Br F CH3 0-n-C4H9
405 Br Cl CH3 0-n-CqHg
406 CH3 H CH3 0-n-CqHg
407 CH3 F CH3 p_n_C4Hg -
408 CH3 C1 CH3 O-n-CqHg
409 H H H OCH(CH3)-C2H5
410 H F H OCH(CH3)-C2H5
411 H C1 H OCH(CH3)-C2H5
412 C1 H H OCH(CH3)-CZH5
413 Cl F H OCH(CH3)-C2H5
414 C1 Cl H OCH(CH3)-C2H5
415 Br H H OCH(CH3)-CZHS
416 Br F H OCH(CH3)-CyHs
417 Br Cl H OCH(CH3)-C2H5
418 CH3 H H OCH(CH3)-C2H5
419 CH3 F H OCH(CH3)-CZHS
420 CH3 Cl H OCH(CH3)-C2H5
421 H H CH3 OCH(CH3)-C2H5
422 H F CH3 OCH(CH3)-CZHS
423 H C1 CH3 OCH(CH3)-C2H5
424 Cl H CH3 OCH(CH3)-C2H5
425 Cl F CH3 OCH(CH3)-CZHS
426 C1 C1 CH3 OCH(CH3)-CZHS
427 Br H CH3 OCH(CH3)-C2H5
428 Br F CH3 OCH(CH3)-CzHS
429 Br C1 CH3 OCH(CH3)-CzHS
430 CH3 H CH3 OCH(CH3)-CZHS
431 CH3 F CH3 OCH(CH3)-CZHS
432 CH3 C1 CH3 OCH(CH3)-CpHS
433 H H H OCHZ-CH(CH3)2
434 H F H OCHZ-CH(CH3)2
435 H Cl H OCHz-CH(CH3)2
436 C1 H H OCHZ-CH(CH3)2
437 C1 F H OCHZ-CH(CH3)2
438 C1 C1 H OCHz-CH(CH3)y
439 Br H H OCHZ-CH(CH3)2
440 Br F H OCHZ-CH(CH3)2
441 Br C1 H OCHZ-CH(CH3)2
0050/51065
CA 02396583 2002-07-05
57
No. R R4 Rl R
-~~
442 CH3 H H OCHZ-CH(CH3)2
443 CH3 F H OCH2-CH(CH3)2
444 CH3 C1 H OCH2-CH(CH3)2
445 H H CH3 OCHZ-CH(CH3)y
446 H F CH3 OCHZ-CH(CH3)2
447 H C1 CH3 OCH2-CH(CH3)2
448 Cl H CH3 OCH2-CH(CH3)2
449 C1 F CH3 ~CH2-CH(CH3)Z -
-
-
450 Cl C1 CH3 OCH2-CH(CH3)2
4 51 Br H- CH3 ~CH2-CH ( CH3 ) 2
452 Br F -. CH3 OCH2-CH(CH3)z-
453 Br C1 CH3 OCH2-CH(CH3)z
454 CH3 H CH3 OCHy-CH(CH3)Z
455 CH3 F CH3 OCHZ-CH(CH3)2
456 GH3 C1 CH3 OCH2-CH(CH3)Z
457 H H H OCH2-CH=CH2
458 H F H OCH2-CH=CH2
459 H C1 H OCH2-CH=CHZ
460 C1 H H OCHy-CH=CH2
461 C1 F H OCH2-CH=CH2
462 C1 Cl H OCH2-CH=CHz
463 Br H H OCH2-CH=CHZ
464 Br F H OCH2-CH=CHz
465 Br C1 H OCH2-CH=CHZ
466 CH3 H H OCH2-CH=CH2
467 CH3 F H OCH2-CH=CHZ
468 CH3 C1 H OCH2-CH=CH2
469 H H CH3 OCH2-CH=CH2
470 H F CH3 OCH2-CH=CH2
471 H C1 CH3 OCH2-CH=CH2
472 C1 H CH3 OCHz-CH=CH2
473 C1 F CH3 OCH2-CH=CHz
474 C1 Cl CH3 OCH2-CH=CH2
475 Br H CH3 OCHZ-CH=CHZ
476 Br F CHg OCH2-CH=CHZ
477 Br C1 CH3 OCHy-CH=CHZ
478 CH3 H CH3 OCH2-CH=CHZ
479 CH3 F CH3 OCH2-CH=CH2
480 CHg C1 CH3 OCH2-CH=CH2
481 H H H OCH2-C~CH
482 H F H OCH2-C~CH
483 H C1 H OCH2-C~CH
484 C1 H H OCH2-C~CH
485 C1 F H OCHy-C~CH
486 C1 C1 H OCH2-C~CH
487 Br H H OCH2-C=CH
488 Br F H OCHz-CCH
489 Br C1 H OCH2-C~CH
490 CH3 H H OCHZ-C~CH
491 CH3 F H OCHZ-C--=CH
492 CH3 C1 H OCHZ-C~CH
0050/51065
CA 02396583 2002-07-05
58
No. R R R1 R18
493 H H CH3 OCHZ-C=CH
i
494 H F CH3 OCHy-C~CH
495 H C1 CH3 OCHZ-C=CH
496 C1 H CH3 OCH2-CCH
497 C1 F CH3 OCH2-C~CH
498 C1 C1 CH3 OCH2-C~CH
499 Br H CH3 OCHZ-C~CH
500 Br F CH3 OCHZ-C$CH
501 Br C1 CH3 OCHz-C~CH
502 CH3 H CH3 OCH~-C~CH
503 CH3 F CH3 OCHZ-C~CH
504 CH3 C1 CH3 OCHZ-C~CH
505 H H H CHZ-COOCH3
506 H F H CH2-COOCH3
507 H C1 H CH2-COOCH3
508 C1 H H CHZ-COOCH3
509 C1 F H CH2-GOOCH3
510 C1 C1 H CHz-COOCH3
511 Br H H CHZ-COOCH3
512 Br F H CHZ-COOCH3
513 Br Cl H CH2-COOCH3
514 CH3 H H CHZ-COOCH3
515 CH3 F H CHZ-COOCH3
516 CH3 C1 H CHZ-COOCH3
517 H H CH3 CHZ-COOCH3
518 H F CH3 CHZ-COOCH3
519 H C1 CHg CHZ-COOCH3
520 C1 H CH3 CHZ-GOOCH3
521 C1 F CH3 CH2-COOCH3
522 C1 Cl CH3 CHZ-COOCH3
523 Br H CH3 CH2-COOCH3
524 Br F CH3 CH2-COOCH3
525 Br C1 CH3 CHy-COOCH3
526 CH3 H CH3 CH2-COOCH3
527 CH3 F CH3 CH2-COOCH3
528 CH3 C1 CH3 CHz-COOCH3
529 H H H CHy-COOC2H5
530 H F H CH2-COOC2H5
531 H Cl H CHz-COOCZHS
532 Cl H H CHZ-COOCzHS
533 C1 F H CH2-COOC2H5
534 C1 C1 H CHZ-COOCZHS
535 Br H H CHZ-COOCzHS
536 Br F H CH2-COOG2H5
537 Br Cl H CHz-COOC2H5
538 CH3 H H CHy-COOC2H5
539 CH3 F H CH2-COOC2H5
540 CH3 C1 H CH2-COOCZHS
541 H H CH3 CHZ-COOC2H5
542 H F CH3 CH2-COOCzHS
543 H C1 CH3 ~CH2-COOCzHS j
0050/51065
CA 02396583 2002-07-05
59
No . RZ R4 R16 R $
~
544 C1 H CH3 2-COOC2H5
~ CH
545 C1 F CH3 CH2-COOC2H5
546 C1 C1 CH3 CHy-COOC2H5
547 Br H CHg CH2-COOC2H5
548 Br F GH3 CHZ-COOC2H5
549 Br C1 CH3 CH2-COOC2H5
550 CH3 H CHg CH2-COOC2Hg
551 CH3 F CH3 CHy-COOCyHS
552 CH3 C1 CH3 CHZ-COOCZHS
10553 H H H CH(CH3)-COOCH3
554 H F H CH(CH3)-COOCH3
555 H Cl H CH(CH3)-COOCH3
556 C1 H H CH(CH3)-COOCH3
557 C1 F H CH(CH3)-COOCH3
15558 C1 C1 H CH(CH3)-COOCH3
559 Br H H CH(CH3)-COOCH3
560 Br F H CH(CH3)-COOCH3
561 Br Cl H CH(CH3)-COOCH3
562 CH3 H H CH(CH3)-COOCH3
563 CH3 F H CH(CH3)-COOCH3
20564 CH3 Cl H CH(CH3)-COOCH3
565 H H CH3 CH(CH3)-COOCH3
566 H F CH3 CH(CH3)-COOCH3
567 H Cl CH3 CH(CH3)-COOCH3
568 C1 H CH3 CH(CH3)-COOCH3
25569 C1 F CH3 CH(CH3)-COOCH3
570 C1 C1 CH3 CH(CH3)-COOCH3
571 Br H CH3 CH(CH3)-COOCH3
572 Br F GH3 CH(CH3)-COOCH3
573 Br C1 CH3 CH(CH3)-COOCH3
574 CH3 H CH3 CH(CH3)-COOCH3
30575 CH3 F CH3 CH(CH3)-COOCH3
576 CH3 C1 CH3 CH(CH3)-COOCH3
577 H H H CH(CH3)-COOC2H5
578 H F H CH(CH3)-COOC2H5
579 H C1 H CH(CH3)-COOCyHS
35580 C1 H H CH(CH3)-COOC2H5
581 C1 F H CH(CH3)-COOCZHS
582 C1 C1 H CH(CH3)-COOC2H5
583 Br H H CH(CH3)-COOC2H5
584 Br F H CH(CH3)-COOC2H5
585 Br Cl H CH(CH3)-COOC2H5
40586 CH3 H H CH(CH3)-COOC2H5
587 CH3 F H CH(CH3)-COOC2H5
588 CH3 C1 H CH(CH3)-COOC2H5
589 H H CH3 CH(CH3)-COOC2H5
590 H F CH3 CH(CH3)-COOC2H5
45591 H C1 CH3 CH(CH3)-COOC2H5
592 C1 H CH3 CH(CH3)-COOC2H5
593 C1 F CH3 CH(CH3)-COOC2H5
594 C1 C1 CH3 CH(CH3)-COOC2H5
0050/51065
CA 02396583 2002-07-05
No. R R R R $
595 Br H CH3 CH(CHz)-G00_CZHS
596 Br F CH3 CH(CH3j-COOC2H5
597 Br C1 CH3 CH(CH3)-COOCZHS
598 CH3 H CH3 CH(CH3)-COOCZHS
599 CH3 F CH3 CH(CH3)-COOC2H5
600 CH3 C1 CH3 CH(CH3)-COOC2H5
601 H H H OCH2-COOCH3
602 H F H OCH2-COOCH3
603 H C1 H OCH2-COOCH3
10604 Cl H H OCH2-COOGH3
605 C1 F H OCHz-COOCH3
606 C1 C1 H OCH2-COOCH3
607 Br H H OCHz-COOCH3
608 Br F H OCH2-COOCH3
15609 Br C1 H OCH2-COOCH3
610 CH3 H H OCH2-COOCH3
611 CH3 F H OCH2-COOCH3
612 GH3 C1 H OCHZ-COOCH3
613 H H CH3 OCHZ-COOCH3
614 H F CH3 OCHZ-COOCH3
20615 H C1 CH3 OCHZ-COOCH3
616 C1 H CH3 OCH2-COOCH3
617 C1 F CH3 OCHZ-COOCH3
618 Cl G1 CH3 OCH2-COOCH3
619 Br H CH3 OCHZ-COOCH3
25620 Br F CH3 OCH2-GOOCH3
621 Br C1 CH3 OCHz-COOCH3
622 CH3 H CH3 OCHZ-COOCH3
623 CH3 F CH3 OCH2-COOCH3
624 CH3 C1 CH3 OCH2-COOCH3
625 H H H OCH2-COOCZH5
30626 H F H OCH2-COOCZHS
627 H C1 H OCH2-COOCzHS
628 Cl H H OCH2-COOCZHS
629 C1 F H OCH2-COOC2H5
630 Cl C1 H OCH2-COOC2H5
35631 Br H H OCH2-COOCZHS
632 Br F H OCHz-COOC2H5
633 Br Cl H OCH2-COOC2H5
634 CH3 H H OGH2-COOC2H5
635 CH3 F H OCHy-COOC2H5
636 CH3 Cl H OCHp-COOC2H5
4063? H H CH3 OCH2-COOCyHS
638 H F CH3 OCH2-COOCZHS
639 H G1 CH3 OCH2-COOCzH5
640 C1 H CH3 OCHZ-COOC2H5
641 C1 F CH3 OCH2-COOC2H5
45642 C1 c1 CH3 OCHZ-COOCZHS
643 Br H CH3 OCHZ-COOCZHS
644 Br F CH3 !OCH2-CUOCZHS
645 Br Cl CH3 OCH2-COOC2H5
0050/51065
CA 02396583 2002-07-05
61
No . RZ R R~~ R18
646 GH3 H ~ O
CHI, CHI-COOC2H5
t
647 CH3 F CH3 OCH2-COOCZHS
648 CH3 C1 CH3 OCH2-COOCZHS
649 H H H OCH(CH3)-COOCH3
650 H F H OCH(CHg)-COOCH3
651 H C1 H OCH(CH3)-COOCH3
652 C1 H H OCH(CH3)-COOCH3
653 C1 F H OCH(CH3)-COOCHg
654 C1 C1 H OCH(CH3)-COOCH3
655 Br-_ H H OCH(CH3)-COOCHg _ _
656 Br F H OCH(CH3}-COOCH3 _. _-_ -_
657 Br C1 H OCH(CH3)-COOCH3
658 CH3 H H OCH(CH3)-COOCH3
659 CH3 F H OCH(CH3)-COOCH3
660 CH3 C1 H OCH(CH3)-COOCH3
661 H H CH3 OCH(CH3)-COOGH3
662 H F CH3 OCH(CH3)-COOCH3
663 H C1 CHg OCH(CH3)-COOCH3
664 C1 H CH3 OCH(CH3)-COOCHg
665 C1 F CH3 OCH(CH3)-COOCH3
666 C1 C1 CH3 OCH(CH3)-COOCH3
667 Br H CH3 OCH(CH3)-COOCH3
668 Br F CH3 OCH(CH3)-COOCH3
669 Br C1 CH3 OCH(CH3)-COOCH3
670 CH3 H CH3 OCH(CH3)-COOCH3
671 CH3 F CH3 OCH(CH3)-COOCH3
672 CH3 C1 CH3 OCH(CH3)-COOCH3
673 H H H OCH(CH3)-COOCzHS
674 H F H OCH(CH3)-COOC2H5
675 H C1 H OCH(CH3)-GOOCZHS
676 C1 H H OCH(CH3)-COOC2H5
6'77 C1 F H OCH(CH3)-COOC2H5
678 C1 Cl H OCH(CH3)-COOCzHS
679 Br H H OCH(CH3)-COOCZHS
680 Br F H OCH(CH3)-GOOCZHS
681 Br C1 H OCH(CH3)-GOOCZHS
682 CH3 H H OCH(CH3)-COOCyHS
683 CH3 F H OCH(CH3)-COOC2H5
684 CH3 Cl H OCH(CH3)-COOC2H5
685 H H CH3 OCH(CH3)-COOG2H5
686 H F GHg OCH(CH3)-GOOC2H5
687 H C1 CH3 OCH(CH3)-COOC2H5
Egg C1 H CH3 OCH(CH3)-COOCzH5
689 C1 F CH3 OCH(CH3)-GOOC2H5
690 C1 C1 CH3 OCH(CH3)-COOCzHS
691 Br H CH3 OCH(CH3)-COOCZHS
692 Br F CH3 OCH(CH3)-COOC2H5
693 Br C1 CH3 OCH(CH3)-COOC2H5
694 CH3 H CH3 OCH(CH3)-COOC2H5
695 CH3 F CH3 OCH(CH3)-COOCzHS
696 CH3 C1 CH3 OCH(CH3)-COOC2H5
0050/51065
CA 02396583 2002-07-05
62
Particular preference is furthermore given to the compounds of
the formula ICb (compounds IC where Q = CH, R1 = CH3 and in which
R5 and X-R6 form a chain -OCH(R16)-C(O)-NR18-) in which the
variables R2, R4 and Rlb and R18 together have the meanings given
in each case in one row of Table 2 (compounds ICb.l-ICb.696).
R4 CH3
0- ~ \ N'-\
R16 ~ N~ ( ICb
O-CHF2
N R2
0 ~R18
Particular preference is furthermore given to the compounds of
the formula ICc (compounds IC where Q = CH, R1 = CHZCH3 and in
which R5 and X-R6 form a chain -OCH(R16)-C(O)-NR18-) in which the
variables R2, R4 and R16 and R18 together have the meanings given
in each case in one row of Table 2 (compounds ICe.l-ICc.696).
R4 CH2CH3
p ._ ~ \ N
Rls ~ N~ ( ICc
0 -CHFZ
N R2
0 ~R18
Particular preference is furthermore given to the compounds of
the formula ICd (compounds IC where Q = CH, R1 = CH(CH3)2 and in
which RS and X-R6 form a chain -OCH(R16)-C(0)-NR18-) in which the
variables Rz, R4 and R16 and R18 together have the meanings given
in each case in one row of Table 2 (compounds ICd.l-ICd.696).
R~ CH(CH3)2
\ N-\
N (ICd)
R16 ~ p-CHFy
N R2
O ~ R18
Particular preference is furthermore given to the compounds of
the formula ICe (compounds IC where Q = CH, R1 = C(CH3)3 and in
which R5 and X-R6 form a chain -OCH(R16)-C(0)-NR18-) in which the
variables Rz, R4 and R16 and R18 together have the meanings given
in each case in one row of Table 2 (compounds ICe.l-ICe.696).
CA 02396583 2002-07-05
0050/51065
63
R4 C(CH3)3
0- / \ N"\
R16 ~ N~ ( ICe )
O-CHFz
N R2
O ~R18
Particular preference is furthermore given to the compounds of
the formula ICf (compounds IC where Q = CH, R1 = H and in which RS
and X-R6 form a chain -SCH(R16)-C(O)-NR18-) in which the variables
Rz, R4 and R16 and R18 together have the meanings given in each
case in one row of Table 2 (compounds ICf.l-ICf.696).
N (ICf)
R \' O-CHFz
0 ~ R18
Particular preference is furthermore given to the compounds of
the formula IGg (compounds IC where Q = CH, R1 = CH3 and in which
R5 and X-R6 form a chain -SCH(R16)-C(0)-NR18-) in which the
variables Rz, R4 and R16 and R18 together have the meanings given
in each case in one row of Table 2 (compounds ICg.l-ICg.696).
R4 CH3
S - / \ N'- \
R16 ~ N~ ( ICg )
0-CHFz
N Rz
0 ~R18
Particular preference is furthermore given to the compounds of
the formula ICh (compounds IC where Q = CH, R1 = CH2CH3 and in
which R5 and X-R6 form a chain -SCH(R16)-C(O)-NR18-) in which the
variables Rz, R4 and R16 and R18 together have the meanings given
in each case in one row of Table 2 (compounds ICh.l-ICh.696).
R4 CHZCH3
S-I \ N
R16 ~ N~ ( ICh )
0-CHFz
N Rz
O ~ R18
R4
S-. / \ N
16
N Rz
CA 02396583 2002-07-05
0050/51065
64
Particular preference is furthermore given to the compounds of
the formula ICi (compounds IC where Q = CH, R1 = CH(CH3)2 and in
which R5 and X-R6 form a chain -SCH(R16)-C(0)-NR18-) in which the
variables R2, R4 and R16 and R18 together have the meanings given
in each case in one row of Table 2 (compounds ICi.l-ICi.696).
R4 CH(CH3)2
S- ~ ~ N-\
R16 ~ N~ ( ICi )
0 -CHFy
N R2
O ~ R18
Particular preference is furthermore given to the compounds of
the formula ICk (compounds IC where Q = H, R1 = C(CH3,13 and in
which R5 and X-R6 form a chain -SCH(R16)-C(O)-NR18-) in which the
variables R2, R4 and R16 and R18 together have the meanings given
in each case in one row of Table 2 (compounds ICk.l-ICk.696).
R4 C(CH3)3
~
S .- ~ ' N- \
R16 ~ N~ ( ICk )
0-CHF2
N R2
0 ~R18
35
45
0050/51065
CA 02396583 2002-07-05
Particular preference is furthermore given to the compounds of
the formula IDa (compounds ID where Q = C-R~, R1 = H and RS = C1
and in which R7 and X-R6 form a chain -0-C(R19)=N-) in. which the
5 variables Rz, R4 and R19 together have the meanings given in each
case in one row of Table 3 (compounds IDa.l-IDa.444).
C1- N ( IDa )
10 ~ 0-CHFz
N ~ 0 Rz
' 19
R
Table 3
No . R2 R4 R19
._ ~
1 H H H
_
2 H F H
3 H Cl H
4 C1 H H
5 C1 F H
6 C1 C1 H
7 Br H H
8 Br F H
9 Br Cl H
10 CH3 H H
11 CH3 F H
12 CH3 C1 H
13 H H CH3
14 H F CH3
15 H C1 CH3
16 C1 H CH3
17 Cl F CH3
18 Cl C1 CH3
19 Br H CH3
20 Br F CH3
21 Br Cl CH3
22 CH3 H CH3
23 CH3 F CH3
24 CH3 C1 CH3
25 H H CzHS
26 H F C2H5
27 H C1 CpHS
28 C1 H CZH5
29 C1 F C2H5
30 C1 C1 CpHS
31 Br H CZHS
32 Br F CzHS
R4
N=~
0050/51065
CA 02396583 2002-07-05
66
No . R2 R4 R -
33 Br C1 CzHs
_~-' --.. _
34 CH3 H CzHS
35 CH3 F C2H5
36 CH3 C1 C2H5
37 H H n-C3H7
38 H F n-C3H7
39 H C1 n-C3H7
40 Cl H n-C3H~
41 C1 F n-C3H7
42 C1 C~ n-C3H7 _. _ -_. _. -_ -
- _-
43 Br H n-CgH7
44 Br F n-C3H7
45 Br C1 n-C3H7
46 CH3 H n-C3H7
47 CH3 F n-C3H7
48 CH3 Cl n-C3H7
49 H H F
50 H F F
51 H Cl F
52 C1 H F
53 C1 F F
54 Cl C1 F
55 Br H F
56 Br F F
57 Br C1 F
58 CH3 H F
59 CH3 F F
60 CH3 C1 F
61 H H C1
62 H F C1
63 H C1 C1
64 C1 H C1
65 Cl F C1
66 C1 C1 C1
67 Hr H C1
68 Hr F C1
69 Br C1 C1
70 CH3 H C1
71 CH3 F C1
72 CH3 C1 C1
73 H H Br
74 H F Br
75 H C1 Br
76 C1 H Br
77 Cl F Br
78 C1 C1 Br
7 9 Br H Br _.
80 Br F Br _ _
81 Br C1 Br
82 CH3 H Br
83 CH3 F Br
0050/51065
CA 02396583 2002-07-05
67
No. RZ R~ Rl
84 CH3 C1 Br
85 H H CH(CH3)2
86 H F CH(CH3)2
87 H C1 CH(CH3)2
88 C1 H CH(CH3)2
89 C1 F CH(CH3)2
90 Cl C1 CH(CH3)2
91 Br H CH(CH3)2
92 Br F CH(CH3)2
1093 Br ~1 CH ~ CH3 ) 2 -
94 CH3 H CH(CH3)z -._
95 CH3 F CH(CH3)2
96 CH3 C1 CH ( GHg ~ 2-- _ __ _ _ -
97 H H OCH3
1598 H F OCH3
99 H C1 OCH3
100 C1 H OCH3
101 C1 F OCH3
102 C1 C1 OCH3
103 Br H OCH3
20104 Br F OCH3
105 Br C1 OCH3
106 CH3 H OCH3
107 CH3 F OCH3
108 CH3 C1 OCH3
25109 H H OCH(CH3)2
110 H F OCH(CH3)2
111 H C1 OCH(CH3)2
112 Cl H OCH(CH3)2
113 C1 F OCH(CH3)2
114 Cl C1 OCH(CH3)Z
30115 Br H OCH(CH3)2
116 Br F OCH(CH3)2
117 Br C1 OCH(CH3)z
118 CH3 H OCH(CH3)2
119 CH3 F OCH(CH3)2
35120 CH3 C1 OCH(CH3)Z
121 H H OCHz-CH=CHZ
122 H F OCHZ-CH=CH2
123 H C1 OCHZ-CH=CH2
124 C1 H OCH2-CH=CH2
125 C1 F OCHZ-CH=CHZ
40126 C1 C1 OCHz-CH=CH2
127 Br H OCH2-CH=CH2
128 Br F OCH2-CH=CH2
129 Br Cl OCH2-CH=CHz
130 CH3 H OCHZ-CH=CHz
45131 CH3 F OCH2-CH=CH2
132 CH3 C1 OCHZ-CH=CH2
133 H H OCHZ-CgCH
134 H F OCHZ-C~CH
0050!51065
CA 02396583 2002-07-05
68
No . R R4 R
135 H C1 OCH2-C~CH
136 C1 H OCHZ-C$CH
137 C1 F OCH2-C~CH
138 Cl Cl OCH2-C~CH
139 Br H OCH2-C~CH
140 Br F OCH2-C~CH
141 Br C1 OCHZ-C~CH
142 CH3 H OCH2-C=CH
143 CH3 F OCH2-C~CH
10144 CH3- C1 OCH2-C~CH
145 H H n-C4Hg
146 H F n-C4Hg
147 H C1 n-C4Hg
148 C1 H n-C4Hg
15149 C1 F n-C4Hg
150 C1 Cl n-C4Hg
151 Br H n-C4Hg
152 Br F n-C4Hg
153 Br Cl n-C4Hg
154 CH3 H n-C4Hg
20155 CH3 F n_C4Hg
_
156 CH3 C1 ~_C4Hg
157 H H OCHZ-COOCH3
158 H F OCHZ-COOCH3
159 H C1 OCH2-COOCH3
25160 Cl H OCHa-COOCH3
161 C1 F OCH2-COOCH3
162 C1 C1 OCH2-COOCH3
163 Br H OCH2-COOCH3
164 Br F OCHZ-COOCH3
165 Br C1 OCHZ-COOCH3
30166 CH3 H OCH2-COOCH3
167 CH3 F OCH2-COOCH3
168 CH3 C1 OCH2-COOCH3
169 H H OCH2-COOC2H5
170 H F OCHz-COOCZHS
35171 H C1 OCHZ-COOC2H5
172 C1 H OCHZ-COOC2H5
173 Cl F OCHZ-COOCZHS
174 C1 C1 OCH2-COOC2H5
175 Br H OCH2-COOCzH5
176 Br F OCHZ-COOC2H5
40177 Br Cl OCHZ-COOCzHS
178 CH3 H OCH2-COOC2H5
179 CH3 F OCH2-COOCzHS
180 CH3 C1 OCH2-COOC2H5
181 H H CH(CH3)-C2H5
45182 H F CH(CH3)-CyHS
183 H C1 CH(CH3)-C2H5
184 Cl H CH(CH3)-C2H5
185 Cl F CH(CH3)-CzHS
0050/51065
CA 02396583 2002-07-05
69
No. R R4 Rl~'
186 C1 C1 CH(CH3)-CzH5
18 7 Br H CH ( CH3 ) -C2H5
188 Br F CH(CH3)-C2H5
189 Br Cl CH(CH3)-CZH5
190 CH3 H CH(CH3)-C2H5
191 CH3 F CH(CH3)-C2H5
192 CH3 C1 CH(CH3)-C2H5
193 H H CHZ-CH(CH3)z
194 H F CHz-CH(CH3)2
195 H C1 CHz-CH(CH3)z-_...
196 Cl H CHZ-CH(CH3)2
197 C1 F CHz-CH(CH3)z
198 Cl C1 CHz-CH(CH3)z
199 Br H CHZ-CH ( CH3 ) z--_
200 Br F CHZ-CH(CH3)2
201 Br C1 CHZ-CH(CH3)z
202 CH3 H CHz-CH(CH3)z
203 CH3 F CHz-CH(CH3)z
204 CH3 C1 CHz-CH(CH3)z
205 H H OCH(CH3)-COOCH3
206 H F OCH(CH3)-COOCH3
207 H C1 pCH(CH3)-COOCH3 _.
208 C1 H OCH(CH3)-COOCH3
209 C1 F OCH(CH3)-COOCH3
210 Cl C1 OCH(CH3)-COOCH3
2~ 211 Br H OCH(CH3)-COOCH3
212 Br F OCH(CH3)-COOCH3
213 Br C1 OCH(CH3)-COOCH3
214 CH3 H OCH(CH3)-COOCH3
215 CH3 F OCH(CH3)-COOCH3
2I6 CH3 C1 OCH(CHg)-COOCH3
217 H H OCH(CH3)-COOC2H5
218 H F OCH(CH3)-COOC2H5
219 H C1 OCH(CH3)-COOCZH5
220 C1 H OCH(CH3)-COOCZHS
221 Cl F OCH(CH3)-COOC2H5
222 Cl C1 OCH(CH3)-COOCZHS
223 Br H OCH(CH3)-COOCZHS
224 Br F OCH(CH3)-COOC2H5
225 Br C1 OCH(CH3)-COOC2H5
226 CH3 H OCH(CH3)-COOC2H5
227 CH3 F OCH(CH3)-COOC2H5
228 CH3 C1 OCH(CH3)-COOCZHS
229 H H cyclopropyl
230 H F cyclopropyl
231 H C1 cyclopropyl
232 C1 H cyclopropyl
4~ 233 C1 F cyclopropyl
234 C1 Cl cyclopropyl
235 Br H cyclopropyl
236 Br F cyclopropyl
0050/51065
CA 02396583 2002-07-05
No.
237 Br C1 cyclopropyl
238 CH3 H cyclopropyl
239 CH3 F cyclopropyl
5 240 GH3 C1 cyclopropyl
241 H H cyclopentyl
242 H F cyclopentyl
243 H C1 cyclopentyl
244 C1 H cyclopentyl
245 C1 F cyclopentyl
10246 Cl Cl cyclopentyl
247 Br H cyclopentyl
248 Br F cyclopentyl
249 Br C1 cyclopentyl
250 CH3 H cyclvpentyl
15251 CH3 F cyclopentyl
252 GH3 C1 cyclopentyl
253 H H CH2-COOCH3
254 H F CH2-COOCH3
255 H C1 CH2-COOCH3
256 C1 H CH2-COOCH3
20257 C1 F CH2-COOCH3
258 C1 ~i _CH2-COOCH3
_-
259 Br H CH2-COOCH3
260 Br F CH2-COOCH3
261 Br C1 CH2-COOCH3
25262 CH3 H CH2-COOCH3
263 CH3 F CH2-COOCH3
264 CH3 Cl CH2-COOCH3
265 H H NH2
266 H F NH2
267 H C1 NH2
30268 C1 H NH2
269 C1 F NH2
270 C1 C1 NH2
271 Br H NH2
272 Br F NH2
35273 Br C1 NH2
274 CHg H NH2
275 CH3 F NH2
276 CH3 C1 NH2
277 H H N(CH3)2
27$ H F N(CH3)2
40279 H C1 N(CH3)2
280 C1 H N(CH3)2
281 G1 F N(CH3)2
282 C1 C1 N(CH3)2
283 Br H N(CH3)2
45284 Br F N(CH3)2
285 Br C1 N(CH3)2
286 CH3 H N(CH3)2
287 CH3 F N(CH3)2
0050/51065
CA 02396583 2002-07-05
71
No. R R R19
288 CHI C1 N(CH3)z
289 H H CHZ-COOCyHS
290 H F CHZ-COOC2H5
291 H C1 CHZ-COOCzHS
292 C1 H CHz-COOC2H5
293 C1 F CHy-COOCZHS
294 C1 C1 CH2-COOCyHS
295 Br H CHa-COOC2H5
296 Br F CHZ-COOCyHS
10297 Br C1 CH2-COOCzHS
298 CHg H CHZ-COOC2H5
299 CH3 F CH2-COOC2H5
30o CH3 C1 CHy-COOCzHS
301 H H GHz-CH2-COOCH3
15302 H F CH2-CHZ-COOCH3
303 H C1 GH2-CH2-COOCH3
304 C1 H CH2-CHZ-COOCH3
305 C1 F CH2-CHz-COOCH3
306 Cl C1 CHZ-CH2-COOCH3
307 Br H CHZ-CH2-COOCH3
203O8 Br F __ CHZ_CH2-COOCH3 _. __ _
-..
309 Br C1 GH2-=CHz-COOCH3. _
_
310 CH3 H CH2-CH2-COOCH3
311 CH3 F CHZ-CH2-COOCH3
312 CHg C1 CH2-CH2-COOCH3
25313 H H CH2-CH2-COOC2H5
314 H F CHp-CHZ-COOCZHS
315 H C1 CH2-CHZ-COOCyHS
316 C1 H CH2-CH2-COOC2H5
317 C1 F CH2-CH2-COOC2H5
318 C1 C1 CH2-CH2-COOC2H5
30319 Br H CHZ-CH2-COOCzHS
320 Br F CHZ--CH2-COOC2H5 _
321 Br C1 CH2-CHZ-COOCzHS
322 CH3. H. CH2_CH2-COOC2H5
323 CH3 F CHZ-CHz-COOC2H5
35324 CH3 Cl CH2-CHZ-COOC2H5
325 H H SCH3
326 H F SCH3
327 H C1 SCH3
328 C1 H SCH3
329 C1 F SCH3
40330 C1 C1 SCH3
331 Br H SCH3
332 Br F SCH3
333 Br C1 SCH3
334 CH3 H SCH3
45335 CH3 F SCH3
336 CH3 C1 SCH3
337 H H SCH(CH3)2 _
338 H F SCH(CH3)z
0050/51065
CA 02396583 2002-07-05
72
No. R R Rlg
339 H C1 SCH(CH3)~
340 Gl H SCH(CH3)2
341 C1 F SCH(CH3)2
342 C1 C1 SCH(CH3)z
343 Br H SCH(CH3)2
344 Br F SCH(CH3)2
345 Br C1 SCH(CH3)2
346 CH3 H SCH(CH3)2
347 CH3 F SCH(CH3)z
348 CH3 C1 SCH(CH3)2
349 H H SCH2-CH=CHZ
350 H F SCH2-CH=CHz
351 H C1 SCHZ-CH=CH2
352 C1 H SCHZ-CH=CH2
353 C1 F SCHy-CH=CH2
354 Cl Cl SCHy-CH=CH2
355 Br H SCHZ-CH=CHz
356 Br F SCHZ-CH=CHZ
357 Br C1 SCHy-CH=CH2
358 CH3 H SCHZ-CH=CH2
359 CH3 F SCHZ-CH=CHZ
360 CH3 C1 SCHZ-CH=CHZ
361 H H SCH2-C~CH
362 H F SCHZ-C~CH
363 H C1 SCHZ-C~CH
364 C1 H SCH2-CCH
365 C1 F SCH2-C~CH
366 C1 C1 SCH2-C~CH
367 Br H SCH2-C~CH
368 Br F SCH2-C~CH
369 Br C1 SCHZ-C--=CH
370 CH3 H SCHZ-C~CH
371 GH3 F SCHZ-C~CH
372 CH3 C1 SCH2-C~CH
373 H H SCH2-COOCH3
374 H F SCHZ-COOCH3
375 H C1 SCHz-COOCH3
376 C1 H SCHZ-COOCH3
377 C1 F SCHZ-COOCH3
378 C1 Cl SCHZ-COOCH3
379 Br H SCH2-COOCH3
380 Br F SCH2-COOCH3
381 Br C1 SCHZ-COOCH3
382 CH3 H SCHZ-COOCH3
383 CH3 F SCH2-COOCH3
384 CH3 C1 SCHy-COOCH3
385 H H SCHZ-COOC2H5
386 H F SCHz-COOC2H5
387 H Cl SCH2-COOCZHS
388 C1 H SCH2-COOCZHS
389 C1 F SCHZ-COOCZHS
0050/51065
CA 02396583 2002-07-05
73
No . R R4 R19
390 C1 C1 SCH2-COOC2H5
391 Br H SCHZ-COOC2H5
392 Br F SCH2-COOCZH5
393 Br C1 SCH2-COOC2H5-- _.
394 CH3 H SCH2-COOC2H5
395 CH3 F SCH2-COOCyHS
396 CH3 C1 SCHZ-COOC2H5
397 H H SCH(GH3)-COOCH3
398 H F SCH(CH3)-COOCHg
399 H C1 SCH(CH3)-COOCH3
400 C1 H SCH(CH3)-COOCH3
401 C1 F SCH(CH3)-COOCH3
402 C1 C1 SCH(CH3)-COOCH3
403 Br H SCH(CH3)-COOCH3
404 Br F SCH(CH3)-COOCH3
405 Br C1 SCH(CH3)-COOCH3
406 CH3 H SCH(CH3)-COOCH3
407 CH3 F SCH(CH3)-COOCH3
408 CH3 C1 SCH(CH3)-COOCH3
409 H H SCH(CH3)-COOCZHS
410 H F SCH(CH3)-COOC2H5
411 H C1 SGH(CH3)-COOC2H5
412 C1 H SCH(CH3)-COOC2H5
413 C1 F SCH(CH3)-COOCzHS
414 C1 C1 SCH(CH3)-COOCZH5
415 Br H SCH(CH3)-COOC2H5
416 Br F SCH(CH3)-COOC2H5
417 Br C1 SCH(CH3)-COOC2H5
418 CH3 H SCH(CH3)-COOC2H5
419 CH3 F SCH(CH3)-COOC2H5
420 CH3 G1 SCH(CH3)-COOC2H5
421 H H COOCH3
422 H F COOCH3
423 H Cl COOCH3
424 C1 H COOCH3
425 C1 F COOCH3
426 C1 C1 COOCH3
427 Br H COOCH3
428 Br F COOCH3
429 Br C1 COOCH3
430 CH3 H COOCH3
431 CH3 F COOCH3
432 CH3 C1 GOOCH3
433 H H COOC2H5
434 H F COOCzHS
435 H C1 COOCZHS
436 C1 H COOCZHS
437 C1 F COOC2H5
438 C1 Cl COOC2H5
439 Br H COOC2H5
440 Br F COOC2H5
0050/51065
CA 02396583 2002-07-05
74
N'o. R2 R R19
441 Br C1 COOCyHS
442 CH3 H COOCZHS
443 CH3 F COOC2H5
444 CH3 C1 COOC2H5
Particular preference is furthermore given to the compounds of
the formula IDb (compounds ID where Q = C-R~, R1 = CH3 and R5 = C1
and in which R7 and X-R6 form a chain -0-C(R19)=N-) in which the
variables R2, Rø and R19 together have the meanings given in each
case in one row of Table 3 (compounds IDb.l-IDb.444).
R4 CH3
C 1- ~ ~ N
N (IDb)
~ ~ 0-CHFZ
N~O R2
~R19
Particular preference is furthermore given to the compounds of
the formula IDc (compounds ID where Q = C-R7, R1 = CHZCH3 and R5 =
C1 and in which R7 and X-R6 form a chain -0-C(R~9)=N-) in which
the variables R2, R~ and R19 together have the meanings given in
each case in one row of Table 3 (compounds IDc.l-IDc.444).
R4 CHZCH3
C 1- ~ ~ N
N (IDc)
~ O-CHF2
N ~ O R2
19
R
Particular preference is furthermore given to the compounds of
the formula IDd (compounds ID where Q = C-R7, R1 = CH(CH3)Z and R5
- C1 and in which R7 and X-R6 form a chain -0-C(R19)=N-) in which
the variables R2, R4 and R~9 together have the meanings given in
each case in one row of Table 3 (compounds IDd.2-IDd.444).
R4 CH(CH3)2
C 1- ~ ~ N
N (IDd)
~ 0-CHFZ
N \ 'O R2
R19
CA 02396583 2002-07-05
0050151065
Particular preference is furthermore given to the compounds of
the formula IDe (compounds ID where Q = C-R~, R1 = C(CH3)3 and R'
C1 and in which R7 and X-R6 form a chain -0-G(R19)=N-) in which
the variables R2, R4 and R19 together have the meanings given in
5 each case in one row of Table 3 (compounds IDe.l-IDe.444).
R4 C(CH3)3
c1- / \ N
N (IDe)
10 ~ O--CHF2
N\ /O R2
SIR 19
15 Particular preference is furthermore given to the compounds of
the formula IDf (compounds ID where Q = C-R~, R1 = H and R5 = C1
and in which R~ and X-R6 form a chain -S-C(R19)=N-) in which the
variables R2, R~ and R19 together have the meanings given in each
case in one row of Table 3 (compounds IDf.l-IDf.444).
20 R4
C 1-- / \ N ~
N (IDf)
~ O-CHF~
25 N \ S R2
' 19
R
Particular preference is furthermore given to the compounds of
30 the formula IDg (compounds ID where Q = C-R7, R1 = CH3 and R5 = C1
and in which R7 and X-R6 form a chain -S-C(Rlg)=N-) in which the
variables R2, R4 and R19 together have the meanings given in each
case in one row of Table 3 (compounds IDg.l-IDg.444).
R4 CH3
N='~
C1- / \ ~ N~ ( IDg )
O-CHF2
N\ /S R2
R19
Particular preference is furthermore given to the compounds of
the formula IDh (compounds ID where Q = C-R7, R1 = CH2CH3 and R5 =
C1 and in which R7 and X-R6 form a chain -S-C(R19)=N-) in which
the variables R2, R4 and R19 together have the meanings given in
each case in one row of Table 3 (compounds IDh.l-IDh.444).
CA 02396583 2002-07-05
0050/51065
76
R4 CHzCH3
N=
C1-- ' \ ~ N ( IDh )
~" O-CHFz
N\ 'S Rz
YIR19
Particular preference is furthermore given to the compounds of
the formula IDi (compounds ID where Q = C-R7, R1 = CH(CH3)z and R5
- C1 and in which R7 and X-R6 farm a chain -S-C(R19)=N-) in which
the variables Rz, R~ and R19 together have the meanings given in
each case in one row of Table 3 (compounds IDi.l-IDi.444).
R4 CH(CH3)z
c1- I \ N=
N (IDi)
~ O-CHFz
N \/ S R2
R
Particular preference is furthermore given to the compounds of
the formula IDk (compounds ID where Q = C-R7, R1 = C(CH3)3 and R5 =
Cl and in which R7 and X-R6 form a chain -S-C(R19)=N-) in which
the variables Rz, R4 and R19 together have the meanings given in
each case in one row of Table 3 (compounds IDk.l-IDk.444).
R4 C(CH3)3
G 1--- ~ \ N
N (IDk)
~ O-CHFz
N ~ S R2
~
19
R
Particular preference is furthermore given to the compounds of
the formula IDl (compounds ID where Q = C-R7, R1 = H and R5 = Cl
and in which R7 and X-R6 form a chain -N=C(R19)-O-) in which the
variables Rz, R~ and R19 together have the meanings given in each
case in one row of Table 3 (compounds IDl.l-ID1.444).
CA 02396583 2002-07-05
0050/51065
77
C1- N ( ID1 )
~ O-CHFz
O / N R2
' 19
R
Particular preference is furthermore given to the compounds of
the formula IDm (compounds ID where Q = C-R7, R1 = CH-; and R5 = Cl
and in which R7 and X-R6 form a chain -N=C(R19)-0-) in which the
variables Rz, R4 and R19 together have the meanings given in each
case in one row of Table 3 (compounds IDm.l-IDm.444).
R4 CH3
C 1- / \ N
\ N (IDm)
~ O-CHFz
O~N Rz
YIR19
Particular preference is furthermore given to the compounds of
the formula IDn (compounds ID where Q = C-R7, R1 = CHZCH3 and R5 =
Cl and in which R7 and X-R6 form a chain -N=C(R19)-O-) in which
the variables Rz, R4 and R19 together have the meanings given in
each case in one row of Table 3 (compounds IDn.l-IDn.444).
R4 CHZCH3
C 1- / \ N
\ N (IDn)
~' O-CHFz
O / N Rz
~
19
R
Particular preference is furthermore given to the compounds of
the formula IDo (compounds ID where Q = C-R7, R1 = CH(CH3)z and
R5 = C1 and in which R7 and X-R6 form a chain -N=C(R19)-O-) in
which the variables Rz, R4 and R19 together have the meanings
given in each case in one row of Table 3 (compounds
IDo.l-IDo.444).
R4
N=~
0050/51065
CA 02396583 2002-07-05
7$
R4 CH(CH3)2
c 1- / \ N
~ N (IDo)
0-CHF2
0' /'N R2
SIR 19
Particular preference is furthermore given to the compounds of
the formula IDp (compounds ID where Q = C-R7, R1 = C(CH3)3 and R5 =
C1 and in which R7 and X-R6 form a chain -N=C(Ri9)-0-) in which
the variables R2, R4 and R19 together have the meanings given in
each case in one row of Table 3 (compounds IDp.l-IDp.444).
R4 C(CH3)3
c1- / \ N=-
\ N.~ ( IDp
O-CHFy
0 / N R2
' 19
R
Particular preference is furthermore given to the compounds of
the formula IDq (compounds ID where Q = C-R7, R1 = H and R5 = Cl
and in which R7 and X-R6 form a chain -N=C(R19)-S-) in which the
variables R2, R4 and R19 together have the meanings given in each
case in one row of Table 3 (compounds IDq.l-IDq.444).
C1- N ( IDq)
~ O-CHFz
S ~ N R2
R19
Particular preference is furthermore given to the compounds of
the formula IDr (compounds ID where Q = C-R7, R1 = CH3 and R5 = Cl
and in which R7 and X-RS form a chain -N=C(R19)-S-) in which the
variables R2, R4 and R19 together have the meanings given in each
case in one row of Table 3 (compounds IDr.l-IDr.444).
R4
f \ N=\
CA 02396583 2002-07-05
0050/51065
79
R4 CH3
C 1-- / \
N (IDr)
~ 0-CHFy
S / N R2
19
R
Particular preference is furthermore given to the compounds of
the formula IDs (compounds ID where Q = C-R7, R1 = CHJCH3 and R5 =
C1 and in which R7 and X-R6 form a chain -N=C(R19)-S-) in which
the variables R2, R4 and R19 together have the meanings given in
each case in one row of Table 3 (compounds IDs.l-IDs.444).
R4 CH2CH3
C 1-- / \ N
N (IDs)
~ O-CHF2
S I N R2
R19
Particular preference is furthermore given to the compounds of
the formula IDt (compounds ID where Q = C-R7, R1 = CH(CH3)2 and R5
- C1 and in which R7 and X-R6 form a chain -N=C(R19)-S-) in which
the variables Rz, R4 and R19 together have the meanings given in
each case in one row of Table 3 (compounds IDt.l-IDt.444).
R4 CH(CH3)Z
C 1- / \ N
\ N.~ ( IDt )
O-CHF2
S / N R2
19
R
Particular preference is furthermore given to the compounds of
the formula IDu (compounds ID where Q = C-R7, R1 = C(CH3)3 and R5 =
C1 and in which R7 and X-Rs form a chain -N=C(R19)-S-) in which
the variables R2, R4 and R19 together have the meanings given in
each case in one row of Table 3 (compounds IDu.l-IDu.444).
R C(CH3)3
C 1- / \ N
N (IDu)
~ O-CHFZ
S ~ N R2
R19
CA 02396583 2002-07-05
0050/51065
$0
The 4-aryl-1-difluoromethoxyimidazoles of the formula I according
to the invention can be prepared by the synthesis routes
described below:
A) An important route to the 4-aryl-1-difluoromethoxyimidazoles
of the formula I according to the invention is the
difluoromethylation of 4-aryl-1-hydroxyimidazoles of the
formula III
R1
N='~
R~ ~ N~ ( I I I )
OH
R2
in which the variables R1 - R3 are as defined in claim 1.
To this end, the 4-aryl-1-hydroxyimidazole of the formula III
is reacted with chlorodifluoromethane, preferably in an
organic solvent. This reaction is preferably carried out in
the presence of a base. Examples of suitable bases are alkali
metal hydroxides, such as sodium hydroxide or potassium
hydroxide, alkali metal carbonates and bicarbonates, such as
potassium carbonate or bicarbonate or sodium carbonate or
bicarbonate, or an organic base, for example an alkoxide,
such as sodium methoxide or ethoxide or potassium methoxide
or ethoxide, in particular tertiary amines, such as
triethylamine or pyridine.
The gaseous chlorodifluoromethane is preferably introduced
slowly into the reaction mixture comprising the compound III,
preferably dissolved or suspended in a solvent, and, if
appropriate, a base and/or further catalysts. If the reaction
is carried out under atmospheric pressure, excess
chlorodifluoromethane gas is preferably trapped using a
low-temperature condenser. However, the reaction can also be
carried out under elevated chlorodifluoromethane pressure in
a closed apparatus (autoclave), at pressures between about
0.1 and 100 bar.
The reaction temperature is usually between the melting point
and the boiling point of the reaction mixture, preferably in
the range from 50 to 150~C.
0050/51065
CA 02396583 2002-07-05
81
To obtain a high yield, it may be advantageous to employ an
excess of chlorodifluoromethane (based on III). The excess
can, for example, be up to five times the molar amount of the
1-hydroxyimidazole IIT used.
Suitable solvents are inert organic solvents, fo.r example
hydrocarbons, such as toluene or hexane, ethers, such as
diethyl ether, dimethoxyethane, methyl t-butyl ether, dioxane
or tetrahydrofuran (THF), amides, such as dimethylformamide
(DMF), N,N-dimethylacetamide (DMA) or N-methylpyrrolidone
(NMP), C1-C6-alkanols, such as methanol or ethanol, or else
mixtures of such solvents with one another or with water.
To improve the conversion or to increase the reaction rate,
it is frequently advantageous to add catalytic amounts
(0.01-20 mold, based on III) of a phase-transfer catalyst,
for example a tetraalkylammonium salt, such as
tetrabutylammonium chloride, or a crown ether, such as
18-crown-6 or 15-crown-5.
The 1-hydroxyimidazoles of the formula III are in principle
known from the literature (for example A. Katritzky, C. Rees,
Ed., Comprehensive Heterocyclic Chemistry, Vol. 5, p. 474 et
seq.), or they can be prepared analogously to compounds known
from the literature. In particular, they can be prepared by
the two processes mentioned below:
1. Reaction of a styrene derivative of the formula IV with a
nitroso compound of the formula V and a nitr:Lle of the
formula VI to give the hydroxyimidazoles of the formula
IIIa (compounds III where R2 = H) according to the scheme
below:
R1
N-\
R3-CH=CHy + NOXa + R1-CAN ---'1'
R
~~~ N~ OH
(IV) (V) (VI)
(IIIa) ~RZ = H}
In the formulae IV, VI and IIIa, R1 and R3 are as defined
above. NOXa is a customary nitrosating agent, Xa being,
for example, an inorganic anion, far example halogen, in
particular chlorine, hydrogen sulfate or
tetrafluoroborate, or an alkoxide radical, such as
tert-butoxide.
CA 02396583 2002-07-05
0050/51065
82
Such reactions are known in principle from the literature
(for example M. Scheinbaum, M. Dines, Tetrahedron Lett.
24 (1971), 2205; B. Lipshutz, B. Huff, W. Vaccaro,
Tetrahedron Lett. 27 (1986), 4241; J. Beger, J. prakt.
Chem. 311 (1969), 746). Suitable nitroso compounds are,
in addition to nitrosonium tetrafluoroborate, which is
customarily used, also other nitrosating agents, such as,
for example, nitrosyl chloride, nitrosyl sulfuric acid,
alkyl nitrites, such as, for example, t-butyl nitrite, or
salts of nitrous acid, such as, for example, sodium
nitrite.
2. Reaction of the oxime VII of an a-dicarbonyl compound
with ammonia and an aldehyde of the formula VIII
according to the scheme below:
0 R1
l RZ.
R3/ 1 N=
+ R -C HO -----~. R ~~ N~ ( I I I b )
N-OH OH
(VII) (VIII) R2'
In this scheme, R1 and R3 are as defined above. In the
formulae VII and IIIb, R2' is hydrogen, CN, C1-C4-alkyl or
C1-C4-haloalkyl. '
These reactions, too, are known in principle
("Diels-Reaktion"; see, for example, H. Lettau, Z. Chem.
10 (1970), 338 and literature quoted therein).
B) Moreover, 4-aryl-1-difluoromethoxyimidazoles I can be
prepared by functionalization, for example by halogenation of
4-aryl-1-difluoromethoxyimidazoles I in which R2 is hydrogen:
halogenation
I {R2 = H} ~ I {R2 = ha:Logen}
Suitable halogenating agents are, for example, fluorine, DAST
(diethylaminosulfur trifluoride), chlorine,
N-chlorosuccinimide, sulfuryl chloride, thionyl chloride,
phosgene, phosphorus trichloride, phosphorus oxychloride,
bromine, N-bromosuccinimide, phosphorus tribromide and
phosphorus oxybromide.
The reaction is usually carried out in an inert
solvent/diluent, for example in a hydrocarbon, such as
n-hexane and toluene, a halogenated hydrocarbon, such as
~ CA 02396583 2002-07-05
0050/51065
83
dichloromethane, tetrachloromethane and chloroform, an ether,
such as methyl tert-butyl ether, an alcohol, such as methanol
and ethanol, a carboxylic acid, such as acetic acid, or in a
polar aprotic solvent, such as acetonitrile.
The reaction temperature is usually between the melting point
and the boiling point of the reaction mixture, preferably
from 0 to 100~C.
To obtain as high a yield of the product of value as
possible, the halogenating agent is employed in approximately
equimolar amounts or in an excess of up to five times the
molar amount, based on the amount of starting material.
C) Compounds I in which R3 is a radical of the formula II where
Q = CH (compounds IA or IC) can be converted into other
compounds IA by functionalization of the phenyl ring in the
aromatic radical R3. Examples of this are:
C.1 Nitration of 4-aryl-1-difluoromethoxyimidazoles IA in which
XR6 is hydrogen, and conversion of the products of the
process into further compounds of the formula IA:
R~
R1 R4
R4 N=
N=
nitration ' ~ I \ N~~ O-CHFZ
N~ O-CHFy R5 \
R5 \ R2
NOz
IA ~XR6 = H} IA ~XR6 = NOy}
Suitable nitrating agents are, for example, nitric acid in
various concentrations, including concentrated and fuming
nitric acid, mixtures of sulfuric acid and nitric. acid,
acetyl nitrates and alkyl nitrates.
The reaction can be carried out either without using a
solvent in an excess of the nitrating agent, or in an inert
solvent or diluent, suitable solvents or diluents being, for
example, water, mineral acids, organic acids, halogenated
hydrocarbons, such as rnethylene chloride, anhydrides, such as
acetic anhydride, and mixtures of these.
Starting material IA ~XR6 = H} and nitrating agent are
advantageously employed in about equimolar amounta; however,
to optimize the conversion of the starting material, it may
CA 02396583 2002-07-05
0050/51065
84
be advantageous to use an excess of nitrating agent, up to
about 10 times the molar amount based on IA. When the
reaction is carried out without a solvent in the nitrating
agent, the latter is present in an even greater excess.
The reaction temperature is usually from -100~C to 200~C,
preferably from -30 to 50~C.
The compounds IA where XR6 = N02 can then be reduced to give
compounds IA where X-R6 = NH2 or -NHOH:
reduction
IA {XR6 = NOZ} IA {XR6 = NH2, NHOH}
The reduction is generally carried out by reacting the nitro
compound with a metal, such as iron, zinc or tin, under
acidic reaction conditions, or using a complex hydride, such
as lithium aluminum hydride and sodium borohydride, the
reaction being carried out without dilution or in a solvent
or diluent. Suitable solvents are - depending on the reducing
agent chosen - for example water, alcohols, such as methanol,
ethanol and isopropanol, or ethers, such as diethyl ether,
methyl tert-butyl ether, dioxane, tetrahydrofuran and
ethylene glycol dimethyl ether.
If the reduction is carried out using a metal, the reaction
is preferably carried out without a solvent in an inorganic
acid, in particular in concentrated or dilute hydrochloric
acid, or in a liquid organic acid, such as acetic; acid or
propionic acid. However, it is also possible to dilute the
acid with an inert solvent, for example one of those
mentioned above. The reductian with complex hydrides is
preferably carried out in a solvent, for example an ether or
an alcohol.
The nitro compound IA {X-R6 = NOZ} and the reducing agent are
frequently employed in approximately equimolar amounts; to
optimize the reaction it may be advantageous to use an excess
of one of the two components, up to about 10 times the molar
amount.
The amount of acid is not critical. To ensure as complete a
reduction of the starting material as possible, i.t is
advantageous to use at least an equivalent amount. of acid.
Frequently, an excess of acid, based on IA {X-R6 = N02}, is
employed.
0050/51065
CA 02396583 2002-07-05
The reaction temperature is usually in the range from -30~C
to 200~C, preferably in the range from O~C to 80«C.
For work-up, the reaction mixture is usually diluted with
5 water, and the product is isolated by filtration,
crystallization or extraction with a substantially
water-immiscible solvent, for example ethyl acetate, diethyl
ether or methylene chloride. If desired, the product can then
be purified in a conventional manner.
The nitro group of the compounds IA {X-R6 = NOz} can also be
hydrogenated catalytically using hydrogen. Catalysts suitable
for this purpose are, for example, Raney nickel, palladium on
activated carbon, palladium oxide, platinum and ;platinum
oxide, an amount of catalyst of from 0.05 to 10.0 mold, based
on the compound to be reduced, generally being sufficient.
The reaction is carried out either without a solvent or in an
inert solvent or diluent, for example an acetic acid, a
mixture of acetic acid and water, ethyl acetate, ethanol or
in toluene.
After removal of the catalyst, the reaction solution can be
worked up in a conventional manner to afford the product.
The hydrogenation can be carried out under atmospheric
hydrogen pressure or under elevated hydrogen pressure.
The amino group in IA {X-R6 = NH2} can then be diazotized in a
conventional manner. The diazonium salts then give access to
the compounds I where:
- X-R6 = cyano or halogen {for example by the Sandmeyer
reaction: cf., for example, Houben-Weyl, Met:hoden der
Organischen Chemie, Georg Thieme Verlag Stuttgart,
Vol. 5/4, 4th Edition 1960, p. 438 et seq.},
- X-R6 = hydroxyl {for example by generating phenols by
heating diazonium salts: cf. for example Org. Synth.
Coll. Vol. 3 (1955), p. 130},
- X-R6 = mercapto or C1-C6-alkylthio {cf., for example,
Houben-Weyl, Methoden der Organischen Chemie, Georg
Thieme Verlag Stuttgart, Vol. E11 1984, p. 4:3 and 176},
- X-R6 = halosulfonyl {cf., for example, Houben-Weyl,
Methoden der Organischen Chemie, Georg Thieme: Verlag
Stuttgart, Vol. E11 1984, p. 1069 et seq.},
0050/51065
CA 02396583 2002-07-05
86
- X-R6 = for example -CHZ-CH(halogen)-CO-0-Y-RH,
-CH=C(halogen)-CO-O-Y-R8~ -CH2-CH(halogen)-PO-(O-Y-Re)2..
-CH=C(halogen)-CO-(0-Y-R$)2 {these are generally products
of a Meerwein arylation; cf., for example, C.S.
Rondestredt, Org. React. 1~ (1960), 189 and H.P. Doyle et
al., J. Org. Chem. ~ (1977), 2431}.
The diazonium salt in question of IA {X-R6 = Na+} is generally
prepared in a manner known per se by reacting IA {X-R6 = NHZ}
in an aqueous solution of acid, for example in hydrochloric
acid, hydrobromic acid or sulfuric acid, with a nitrite, such
as sodium nitrite and potassium nitrite.
For preparing the diazonium salt IA {X-R6 = NZ+}, the amino
compound IA {X-R6 = NH2} can be reacted in the absence of
water, for example in glacial acetic acid containing hydrogen
chloride, in absolute alcohol, in dioxane or tetrahydrafuran,
in acetonitrile or in acetone, with a nitrite, such as
tert-butyl nitrite and isopentyl nitrite.
The conversion of the resulting diazonium salt into the
corresponding compound IA where X-R6 = cyano, chlorine,
bromine or iodine is particularly preferably carried out by
treatment with a solution or suspension of a copper(I) salt,
such as copper(I) cyanide, chloride, bromide or iodide, or
with a solution of an alkali metal salt.
The conversion of the resulting diazonium salt into the
corresponding hydroxyl compound IA {X-R6 = hydroxyl} is
advantageously carried out by treatment of the diazonium salt
IA with an aqueous acid, preferably sulfuric acid. The
addition of a copper(II) salt, such as copper(II) sulfate,
can have a positive effect on the course of the reaction. In
general, this reaction is carried out at from 0 to 100~C,
preferably at the boiling point of the reaction mixture.
Compounds IA where X-R6 = mercapto, C1-C6-alkylthi.o or
halosulfonyl are obtained, for example, by reacting the
diazonium salt in question of IA with hydrogen sulfide, an
alkali metal sulfide, a dialkyl disulfide, such as dimethyl
disulfide, or with sulfur dioxide.
The Meerwein arylation usually entails reacting the diazonium
salts with alkenes or alkynes. The alkene or alkyne is
preferably employed in an excess of up to 3000 mold, based on
the amount of the diazonium salt.
0050/51065
CA 02396583 2002-07-05
87
The reactions described above of the diazonium salt IA
~X-R6 = N2+} can be carried out, for example, in water, in
aqueous hydrochloric acid or hydrobromic acid, i:n a ketone,
such as acetone, diethyl ketone and methyl ethyl ketone, in a
nitrile, such as acetonitriie, in an ether, such as dioxane
and tetrahydrofuran, or in an alcohol, such as methanol and
ethanol.
Unless stated otherwise for the individual reactions, the
reaction temperatures are usually from -30~C to .°iO~C.
All reaction partners are preferably employed in
approximately stoichiometric amounts; however, an excess of
one or the other component of up to about 3000 mall may be
advantageous.
The mercapto compounds IA ~X-R6 = SH} can also be obtained by
reducing the compounds IA described below where X-R6 =
halosulfonyl. Suitable reducing agents are, for example,
transition metals, such as iron, zinc and tin (cf., for
example, "The Chemistry of the Thiol Group", John Wiley,
1974, p. 216).
C.2 Halosulfonation of 4-aryl-1-difluoromethoxyimidazoles IA in
which XR6 is hydrogen:
IA ~XR6 = H} IA ~XR6 = -S02~-halogen}
The halosulfonation can be carried out in the absence of a
solvent in an excess of sulfonating agent, or in an inert
solvent/diluent, for example in a halogenated hydrocarbon, an
ether, an alkylnitrile or a mineral acid.
Chlorosulfonic acid is the preferred agent as wel_1 as the
preferred solvent.
The amount of sulfonating agent used is usually slightly less
(up to about 95 mol$) or an excess of 1 to 5 times the molar
amount of the starting material IA (where X-R6 = H). In the
absence of an inert solvent, it may be advantageous to employ
an even larger excess.
The reaction temperature is usually from O~C to the boiling
point of the reaction mixture.
0050/51065
CA 02396583 2002-07-05
88
For work-up, the reaction mixture is mixed, for example, with
water, whereupon the product can be isolated as usual.
C.3 Side-chain halogenation of 4-aryl-1-difluoromethoxyimidazoles
IA in which X-R6 is methyl, and conversion of the products
into further compounds of the formula IA:
i
R4 R R4 N ~ R.1
N=
\ N
/ I \ U
I N~ 0-CHFy .--~". ' I 2 O-CHFZ
R5 \ R2 R5 ~ R
/CHZ
CH3 halogen
IA {XR6 = CH3? IA {XR6 = CH2-halogen}
or
R1
R4
N=
\ N
I ~ ~ O-CHFZ
RS R2
C~
halogens I halogen
H
IA {XR6 = CH(halogen)Z~
Examples of suitable solvents include organic acids,
inorganic acids, aliphatic or aromatic hydrocarbons, which
may be halogenated, and also ethers, sulfides, sulfoxides and
sulfones.
Suitable halogenating agents are, for example, chlorine,
bromine, N-bromosuccinimide, N-chlorosuccinimide or sulfuryl
chloride. Depending on the starting material and the
halogenating agent used, the addition of a free-radical
initiator, for example an organic peroxide, such as dibenzoyl
peroxide, or an azo compound, such as azobisisobutyronitrile,
or irradiation with light, may have an advantageous effect on
the course of the reaction.
The amount of halogenating agent is not critical. Both
substoichiometric amounts and large excesses of halogenating
agent, based on the compound IA to be halogenated (where X-R6
- methyl), are possible.
0050/51065
CA 02396583 2002-07-05
89
When using a free-radical initiator, a catalytic amount is
usually sufficient.
The reaction temperature is usually from -100~C to 200~C,
mainly from 10 to 100~C or the boiling point of the reaction
mixture.
By nucleophilic substitution, those halogenated ;products IA
where X-R6 = CHZ-halogen can be converted according to the
scheme below into their corresponding ethers, thioethers,
esters, amines or hydroxylamines:
R1 R1
4
R4 Nc \ R N-\
/ ~ N~ , ~ N
0-CHF2 ~ \ ~ ~ ~ O-CHFZ
R5 ~ R2 R5 ~ Rz
CH2-halogen CH2-R6
IA {X = CHZ; R6 = -O-Y-R8,
IA {XR6 = CHZ-halogen} -O-CO-gY-R8,-N 9-R8)(Z-R8),
-N(Y R )(-O-Z-R ), -S-Y R }
The nucleophile used is either a suitable alcohol, thiol,
carboxylic acid or amine, the reaction in this case being
preferably carried out in the presence of a base (for example
an alkali metal hydroxide or an alkaline earth metal
hydroxide or an alkali metal carbonate or alkaline earth
metal carbonate), or the alkali metal salts of these
compounds obtained by reaction of the alcohol, thiol,
carboxylic acid or amine with a base (for example an alkali
metal hydride).
Particularly suitable solvents are aprotic organic solvents,
for example tetrahydrofuran, dimethylformamide, dimethyl
sulfoxide, or hydrocarbons, such as toluene and n-hexane.
The reaction is carried out at a temperature from the melting
point to the boiling point of the reaction mixture,
preferably at from 0 to 100~C.
Those halogenation products IA where X-R6 = CH(halogen)Z can
be hydrolyzed to the corresponding aldehydes (IA where X-R6 =
CHO). The latter can in turn be oxidized analogously to known
processes to give the carboxylic acids IA {X-R6 =- COON}:
0050/51065
CA 02396583 2002-07-05
R1 R1
4
R4
5 ~ ~ ~ \ O-CHF2 -i I ~ ~ O-CHF2
R2 ~ R2
R5 hydrolysis R5
C
CHO
halogens I \ halogen
H IA ~XR6 = CHO}
10 IA fXR6 = CH(halogen)2} ~ oxidation
I ~ XR6 = COOH }
The hydrolysis of the compounds IA where X-R6 = dihalomethyl
is preferably carried out under acidic conditions, in
15 particular without a solvent in hydrochloric acid, acetic
acid, formic acid or sulfuric acid, or in an aqueous solution
of one of the acids mentioned, for example in a mixture of
acetic acid and water (for example 3:1).
20 The reaction temperature is usually at from 0 to 120~C.
The oxidation of the hydrolysis products IA where XR6 =
formyl to the corresponding carboxylic acids can be carried
out in the manner known per se, for example according to
25 Kornblum (cf. in particular pages 179 to 181 of the volume
"Methods for the Oxidation of Organic Compounds" by A.H.
Haines, Academic Press 1988, in the series "Best Synthetic
Methods"). A suitable solvent is, for example, dimethyl
sulfoxide.
35
The aldehydes IA ~X-R6 = CHO} can also be converted in a
manner known per se into olefinic compounds IA where X =
unsubstituted or substituted ethene-1,2-diyl:
IA {XR6 = CHO} olefination IA .{X = (un)substituted
ethene-1,2-diyl}
The olefination is preferably carried out by the method of
Wittig or one of its modifications, suitable reacaion
partners being phosphorus ylides, phosphonium sa7.ts and
phosphonates, or by aldol condensation.
If a phosphonium salt or a phosphonate is used, it is
advantageous to carry out the reaction in the presence of a
base, particularly suitable bases being alkali metal alkyls,
such as n-butyllithium, alkali metal hydrides and alkoxides,
0050/51065
CA 02396583 2002-07-05
91
such as sodium hydride, sodium ethoxide and potassium
tert-butoxide, and alkali metal hydroxides and alkaline earth
metal hydroxides, such as calcium hydroxide.
For a complete conversion, all reaction partners are employed
in a ratio which is about stoichiometric; however, preference
is given to using an excess of phosphorus compound and/or
base of up to about 10 mold, based on the starting material
(IA where X-R6 = CHO).
The reaction temperature is generally from -40 to 150~C.
The 4-aryl-1-difluoromethoxyimidazoles IA where X-R6 = formyl
can be converted in a manner known per se into the compounds
IA where X-R6 = -CO-Y-R8, for example by reaction with a
suitable organometal compound Me-Y-R8 - where Me is
preferably lithium or magnesium - and subsequent oxidation of
the alcohols obtained in this reaction (cf., for example, J.
March, Advanced Organic Chemistry, 3rd Ed., John Wiley, New
York 1985, p. 816 et seq. and 1057 et seq.).
The compounds IA where X-R6 = -CO-Y-RB can in turn be reacted
further in a Wittig reaction. The phosphonium salts,
phosphonates or phosphorus ylides required as reaction
partner which are not already known can be prepared in a
manner known per se (cf., for example, Houben-Weyl, Methoden
der Organischen Chemie, Vol. E1, p. 636 et seq. and Vol. E2,
p. 345 et seq., Georg Thieme Verlag Stuttgart 1982; Chem.
Ber. ~ (1962), 3993}.
Further possible ways of preparing other
4-aryl-1-difluoromethoxyimidazoles IA from compounds IA where
X-R6 = formyl include the aldol condensation known per se,
and condensation reactions according to Knoevenagel or
Perkin. Suitable conditions for these processes are
described, for example, in Nielson, Org. React. ~~ (1968),
1 et seq. {aldol condensation}, Org. React. 15 (1967), 204 et
seq. {Knoevenagel condensation} and Johnson, Org. React. _1
(1942), 210 et seq. {Perkin condensation}.
The compounds IA where X-R6 = -CO-Y-R8 can also be converted
in a manner known per se into their corresponding oximes
{cf., for example, Houben-Weyl, Methoden der Organischen
Chemie, Georg Thieme Verlag Stuttgart, Vol. 10/4, 4th Edition
1968, p. 55 et seq. and p. 73 et seq.}:
0050/51065
CA 02396583 2002-07-05
92
R4 R1 R4 R1
N=
N-
/ \
v
N\ \ i N
~ I 0-CHFa '~ ' ~ \ O-CHFa
R5 R2 HaN---~,,. Rs '.. R:~
R8-y ~CO
R8-Y \ NOR:tO
I {XR6 _- _CO_y-R8} I {XR6 = -C(=NOR1~)-Y-Re}
C.4 Synthesis of ethers, thioethers, amines, esters, amides,
sulfonamides, thioesters, hydroximic esters, hydroxylamines,
sulfanic acid derivatives, oximes or carboxylic acid
derivatives:
4-Aryl-1-difluoromethoxyimidazoles IA where R6 is hydroxyl,
amino, -NH-Y-Re, hydroxylamino, -N(Y-R8)-OH, -NH-O-Y-R8,
mercapto, halosulfonyl, -C(=NOH)-Y-Re, carboxyl or
-CO-NH-O-Z-R9 can be converted in a manner known per se by
alkylation, acylation, sulfonylation, esterification or
amidation into the corresponding ethers {IA where R6 =
-O-Y-R8}, esters {IA where R6 = -O-CO-Y-R8}, amines {IA where
R6 = -N(X-R8)(Z-R9)}, amides {IA where R6 = -N(Y-R8)-CO-Z-R9},
sulfonamides {IA where R6 = -N(Y-R8)-SOa-Z-Rg or
-N(SOa-Y-R8)(SOa-Z-R9)}, hydroxylamines {IA where R6 =
-N(Y-R8)(O-Z-R9)}, thioethers {IA where R6 = -S-Y~-Re},
sulfonic acid derivatives {IA where R6 = -SOa-Y-R.B,
-SOa-O-Y-R8 or -SOa-N(Y-R8)(Z-R9)}, oximes {IA where R6 =
-C(=NORi~)-Y-R$}, carboxylic acid derivatives {IA where R6 =
-CO-0-Y-Re, -CO-S-Y-R8, -CO-N(Y-R8)(Z-R9),
-CO-N(Y-R8)(O-Z-R9)} or hydroximic esters {IA where
R6 =_ _ C ( =NOR1 ~ ) -0-Y-Re } .
Such conversions are described, for example, in Houben-Weyl,
Methoden der Organischen Chemie, Georg Thieme Verlag
Stuttgart (Vol. El6d, p. 1241 et seq.; Vol. 6/1a, 4th Edition
1980, p. 262 et seq.; Vol. 8, 4th Edition 1952, p. 471 et
seq., 516 et seq., 655 et seq. and p. 686 et seq.; Vol. 6/3,
4th Edition 1965, p. 10 et seq.; Vol. 9, 4th Edition 1955, p.
103 et seq., 227 et seq., 343 et seq., 530 et sect., 659 et
seq., 745 et seq. and p. 753 et seq.; Vol. E5, p. 934 et
seq., 941 et seq. and p. 1148 et seq.).
D) Preparation of compounds of the formula I in which R3 is a
radical of the formula II where Q is nitrogen (compounds IB).
0050/51065
CA 02396583 2002-07-05
93
In addition to the processes already mentioned in sections A,
B and C above, the processes D.1 and D.2 below are
particularly suitable:
D.1 Halogenation of the pyridine ring of compounds IB where X-R6
- H: to this end, a pyridyl imidazole of the formula IB (X-R6
- H) is preferably initially converted into the corresponding
pyridine N-oxide of the formula IX. In the formula IX, R1, R2,
R4 and R5 are as defined above.
i
R4 R1 R4 ~R
N='~ N ~.
N oxidation ~ N
~. N ~ \ O-CHFz ~. \ ~N ~ ~ O-CHFZ
R5 R2 R5 R
IB {X-R6 = H) (IX)
Suitable oxidizing agents for this reaction are, for example,
hydrogen peroxide or organic peracids, for example performic
acid, peracetic acid, trifluoroperacetic acid or
m-chloroperbenzoic acid.
Suitable solvents are organic solvents which are inert to
oxidation, such as, for example, hydrocarbons, such as
toluene or hexane, ethers, such as diethyl ether,
dimethoxyethane, methyl t-butyl ether, dioxane or THF,
alcohols, such as methanol or ethanol, or else m:i.xtures of
such solvents with one another or with water. If the
oxidation is carried out using an organic peracid, the
preferred solvent is the parent organic acid, i.e., for
example, formic, acetic or trifluoroacetic acid, if
appropriate in a mixture with one or more of the
abovementioned solvents.
The reaction temperature is usually from the melting point to
the boiling point of the reaction mixture, preferably
0-150°C.
To obtain a high yield, it is frequently advantageous to
employ the oxidizing agent in a molar excess of up to about
5 times, based on the IB (where X-R6 = H) used.
The pyridine N-oxide IX is then converted into IB (X-R6 =
halogen) by reaction with a halogenating agent.
0050/51065
CA 02396583 2002-07-05
9~
IB {-X-R6 = H} IB {-X-R6 = halogen}
Suitable halogenating agents are phosphoryl halides, such as
POC13 or POBr3, phosphorus halides, such as PC15, PBrS, PC13
or PBr3, phosgene or organic or inorganic acid halides, such
as, for example, trifluoromethanesulfonyl chloride, acetyl
chloride, bromoacetyl bromide, acetyl bromide, benzoyl
chloride, benzoyl bromide, phthaloyl dichloride,
toluenesulfonyl chloride, thionyl chloride or sulfuryl
chloride. If appropriate, it may be advantageous to carry out
the reaction in the presence of a base, such as, for example,
trimethylamine or triethylamine or hexamethyldisilazane.
Suitable solvents are inert organic solvents, such as, for
example, hydrocarbons, such as toluene or hexane, ethers,
such as diethyl ether, dimethoxyethane, methyl t-butyl ether,
dioxane or THF, amides, such as DMF, DMA or NMP, or mixtures
thereof. If the reaction is carried out using a liquid
halogenating agent, this can preferably also be used as
solvent, if appropriate in a mixture with one of the
abovementioned solvents.
The reaction temperature is usually from the melting point to
the boiling point of the reaction mixture, preferably at
50-150~C.
To obtain a high yield, it may be advantageous to employ an
excess of halogenating agent or base of up to about five
times, based on the IX used.
D.2 Nucleop~.~lic substitution on halopyridines of the formula IB
(X-R6 = halogen). In the scheme below, examples of the
classes of compounds obtainable by this route are shown.
nucleophile
IB {X-R6 = halogen} -~. IB {X-R6 = -0-Y-RB}
IB {X-R6 = _p_CO_y_RB}
Ig {X_R6 = _p(y_Rs)(Z_R9)}
IB {X-R6 =- -N(y_Re)(0_Z_R9)}
IB {X-R6 =- -S-Y-Ra}
Suitable nucleophiles are alcohols, thiols, amines,
carboxylic acids or CH-acidic compounds, for example
nitroalkanes, such as nitromethane, malonic acid derivatives,
~ CA 02396583 2002-07-05
0050/51065
such as diethyl malonate, or cyanoacetic acid derivatives,
such as methyl cyanoacetate. To carry out the reaction, what
has been said under C.3 applies.
5 E) Preparation of compounds of the formula I in which R3 is a
radical of the formula II, where R5 together with X-R6 or R7
together with X-R6 is one of the chains N=C(R19)-S- (compounds
IC-1 or compounds ID-1) or N=C(R19)-0- (compounds IC-2 and
compounds ID-2).
For the preparation of the compounds IC and ID, it is
likewise possible to use the processes mentioned in sections
A and B, or to use these processes for preparing suitable
starting materials.
Furthermore, the compounds IC-1, IC-2, ID-1 and ID-2 can be
synthesized analogously to known processes by ring closure
reactions from the corresponding ortho-aminophenols or
ortho-mercaptoanilines of the formulae IA-1, IA-:?, IA-3 or
IA-4; numerous methods for this purpose are known from the
literature (see, for example, Houben-Weyl, Methoden der
Organischen Chemie, Vol. EBa, p. 1028 et seq.,
Georg-Thieme-Verlag, Stuttgart 1993 and Vol. E8b, p. 881 et
seq., Georg-Thieme-Verlag, Stuttgart 1994). In the formulae
IA-1 to IA-4, the variables Ri, R2, R4 and RS are as defined
above. The variables X1, Xz, X3 and X4 are, independently of
one another, OH or SH.
R1 R1
4
I 4 N ' R N
~ N\ , ~ N
O-CHFZ ~ ~ \ 0-CHF2
X1 ~ RZ R5 ~ \ R2
X2
NHz NHy
(IA-1) (IA-2)
R1 Rn
4
R4 N~ R N-\
i ~ N\ i I ~ Nw
O-CHFy i ~O-CHF2
NH2 R2 R5 RZ
X3 X4 NH2
(IA-3) (IA-4)
CA 02396583 2002-07-05
'tJ050/51065
96
E.1 Compounds IC-1 or ID-1 in which R5 together with X-R6 or R7
together with X-R6 form one of the chains -N=C(R~9)-S- can
also be prepared, in particular, by the process chown below:
This process entails the reaction of an aminophenylimidazole
of the formula IA-5, IA-6, IA-7 or IA-8 with halogen and
ammonium thiocyanate or with an alkali metal or alkaline
earth metal thiocyanate. This gives compounds of the formulae
IC-la, IC-lb or ID-la or ID-lb (compounds IC-1 o:r ID-1 in
which R19 is NH2).
R1 R4 R1
R4 ~ /
N N ~,
N S- ~ ~ N
~ I I/ ~ O_CHF2 -fir- HZ~\ ~ ~ O_CHF2
R2 N R2
NH2
(IA-5) (IC-la)
R1 R4 R1
R4 N=~ N ~~
\ N N-I ~ ~ .N
/ ~ ~ \ O-CHF2 ~' H2N~ \ O-CHFZ
NH2 R2 S R2
(IA-6) (IC-lb)
R1 R4 R:~
R4
N=~ N=
\ N\ R5- ~ ~ ~ Ny
0-CHFy ~ O-CHFy
R5 R2 N \ S RZ
NHp
( IA-~ ) NHz
(ID-la)
R1 R4 CH3
R4~ ~
N-\ N-\
R5- ~ ~ ~ N
N~ 0-CHFZ --~ \ 0-CHFZ
R5 RZ S / N R2
NHZ
(IA-8) NHZ (ID-lb)
CA 02396583 2002-07-05
0050/51065
97
By subsequent reactions at the amino group, these compounds
can be converted into other compounds IC-1 or ID-1.
Preferred halogen is chlorine or bromine; among the alkali
metal/alkaline earth metal thiocyanates, preference is given
to sodium thiocyanate.
In general, the reaction is carried out in an inert
solvent/diluent, for example in a hydrocarbon, such as
toluene and hexane, in a halogenated hydrocarbon,. such as
dichloromethane, in an ether, such as tetrahydrofuran, in an
alcohol, such as ethanol, in a carboxylic acid, such as
acetic acid, or in a polar aprotic solvent/diluent, such as
dimethylformamide, acetonitrile and dimethyl sulfoxide.
The reaction temperature is usually from the melting point to
the boiling point of the reaction mixture, preferably at from
0 to 150°C .
To obtain a high yield of the product of value, the halogen
and ammonium thiocyanate or alkali metal/alkaline earth metal
thiocyanate are preferably employed in an about equimolar
amount or in an excess of up to about 5 times the molar
amount, based on the amount of IA-5, IA-6, IA-7 or IA-8.
A variant of the process comprises converting the NHZ group
of the aminophenylimidazoles IA-5, IA-6, IA-7 or IA-8
initially with ammonium thiocyanate or an alkali metal or
alkaline earth metal thiocyanate into a thiourea group
(NH-C(S)-NH2 group), which is then converted by treatment
with a halogen into the benzothiazoles (compounds IC-1 or
ID-1 where R19 = NH2).
Finally, it is possible to carry out reactions analogous to
those which have already been described in section C.1) at
the amino group of the chain -N=C(NH2)-S-.
E.2 Compounds of the formulae IC and ID in which R5 together with
X-R6 or R7 together with X-R6 form one of the chains
-N=C(R19)-O- can be prepared by conversion of the NHZ group
into the aminophenylimidazoles of the formula IA-5, IA-6,
IA-7 or IA-8 into an azide group (N3 group) and subsequent
cyclization of the resulting azidophenylimidazoles with a
carboxylic acid to give compounds of the formula IC-2a,
IC-2b, ID-2a or ID-2b.
. CA 02396583 2002-07-05
0050/51065
98
R1 R4 R1
Rq 1. azide formation
N ~ 2. R19-COOH N
N 0- ~ ~ N
\ ~ ~ ~ O-CHFz Rl9--~\ '~ ~ O-CHFz
Rz N R2
NHz
(IA-5)
(IC-2a)
R1 R4 R1
R4 1. azide formation
2. R19-COOH N=~~
N N- / \ N
~' 0-CHFZ R19 ~ ~ ~ O-CHFz
NHZ Rz O Rz
(IA-6) (IC-2b)
R1 R4 R7.
R4 1. azide formation
2. R19-COOH
\ N R5- ~ ~ \ N\
\ ~ ~ ~ O-CHFZ ~ 0-CHFz
R5 ~ RZ N \ 0 R2
NH2
(IA-7) R19 (ID-2a)
R1 R4 CH3
R4 ~ 1. azide formation
N- 2. R19-COOH N=
~ N R5- ~ ~ ~ N
~ O-CHFz \ O-CHF2
R5 Rz O / N R2
NHz
(IA-8) R19 (ID-2b)
The conversion of the amino group in the
aminophenylimidazoles of the formula IA-5, IA-6, IA-7 or IA-8
into an azide group is generally carried out in two steps,
i.e. by diazotization of the amino group and subsequent
treatment of the resulting diazonium salt with an azide. To
carry out the diazotization, what has been stated in process
C.1) applies. The conversion into the aryl azides is
preferably carried out by reacting diazonium salts with an
CA 02396583 2002-07-05
0050/51065
99
alkali. metal or alkaline earth metal azide, such as sodium
azide, or by reaction with trimethylsilyl azide.
The reaction of the azide compounds IA (X-R6 = N3) with the
carboxylic acid R19-COOH is carried out either in. an inert
organic solvent, for example in hydrocarbons, such as toluene
or hexane, in halogenated hydrocarbons, such as
dichloromethane or chloroform, in ethers, such as diethyl
ether, dimethoxy ethane, methyl t-butyl ether, dioxane or
THF, in amides, such as DMF, DMA or NMP, in acetonitrile or,
preferably, in the absence of solvent in an excess of
carboxylic acid R19COOH. In the latter case, it may be helpful
to add a mineral acid, such as phosphoric acid, or a
silylating agent, such as a mixture of phosphorus pentoxide
and hexamethyldisiloxane.
The reaction is preferably carried out at elevated
temperature, for example at the boiling point of the mixture.
F) Compounds of the formula I in which R3 is a radical of the
formula II where X-R6 together with R5 or R7 forms one of the
chains -O-C(R16,R17)-CO-N(R18)- or -S-C(R16,R17)_C0-N(RlB)- can
be prepared by the processes mentioned in sections A and B.
Moreover, they can be prepared in principle from the
corresponding aminophenols or mercaptoanilines IA-1, IA-2,
IA-3 or IA-4 by known processes, for example by the process
described in US 4,798,620. With a view to this reaction, the
disclosure of this publication is expressly incorporated
herein by way of reference.
In particular, those compounds of the formula I i.n which X-R6
together with R5 or together with R7 in the formula II form a
chain -0-C(R16,R17)-CO-N(R1$)- can also be prepared from the
nitrophenoxyacetic acid derivatives of the formulae IA-9,
IA-10, IA-11 and IA-12. The conversion is carried out by
reducing the nitro groups in IA-9, IA-10, IA-11 or IA-12,
where generally simultaneously with the reduction ring
closure takes place, to give the compounds of the formula
IC-3a, IC-3b, ID-3a or ID-3b.
45
0050/51065 CA 02396583 2002-07-05
100
R1 R4 Ri
R4
N- N=('
/ \ N R17 O-~ ~ \ N
~ ( ~ ~ 0-CHF2 ~"' ~ \ 0-CHFZ
0 R2 R 6// N 2
R
I Ri6 N02 O
Ra00C~i7
(IA-9j (IC-3a)
R1 R4 R1
R4 ~ R1B~ /
N ~ - ~ ~ N _--~'
/ \ N~ N \ N
~ ~ ~ 0-CHF2 "~'' O ~ \ O-CHFZ
R2 R1 O
02 ~ 0 R2
R16
R16
Ra00C~gi7 ( IA-10 ) ( IC-3b)
R1 R4 R1
R4
N- / \ N=(,
/ \ N~ \ N~
~ ~ 0-CHF2 ~ ~ O-CHF2
R Rie N p 2
O R
N02J R16 ~'~-R16
Ra00C ~17 ( IA-11 ) O Ri7 ( ID-3a
)
R1 R4 Ri
N R5 ~ ~ N-
\ N~ \ N
O-CHF2 ~' ~ \ 0-CHF2
R R2 0 N-Ri8'
R2
0 N02
Ri6 R16~0
Ra00C~g17 ( IA-12 ) ( ID-3b )
In the formulae IA-9, IA-10, IA-11, IA-12, IC-3a, IC-3b,
ID-3a or ID-3b, Ri, R2, R4, R5, Ri6 and Ri7 are as defined
above. Ri8' is H or OH. Ra is a nucleophilically displaceable
leaving group, for example a Ci-C4-alkyl radical, such as
methyl or ethyl.
~ CA 02396583 2002-07-05
0050/51065
101
These reductions can be carried out under the conditions
mentioned in section C.1) for the reduction of aromatic nitro
groups.
If desired, the reaction products can be converted by
alkylation into further compounds of the formula IC-3 or
ID-3. For carrying out these reactions, what has been said in
section C.4 applies correspondingly.
If not stated otherwise, all the processes described above are
advantageously carried out under atmospheric pressure or under
the autogenous vapor pressure of the reaction mixture in
question.
The work-up of the reaction mixtures is usually carried out in a
manner known per se. If not stated otherwise in the processes
described above, the products of value are obtained, for example,
after the dilution of the reaction solution with water by
filtration, crystallization or solvent extraction, ox- by removing
the solvent, partitioning the residue in a mixture of water and a
suitable organic solvent and work-up of the organic phase to
afford the product.
The 4-aryl-1-difluoromethoxyimidazoles of the formula I can be
obtained as isomer mixtures in the preparation; however, if
desired, these can be separated into largely pure isamers using
customary methods such as crystallization or chromatography,
including chromatography over an optically active adsorbate. Pure
optically active isomers can be prepared advantageously from
suitable optically active starting materials.
Agriculturally useful salts of the compounds I can be formed by
reaction with a base of the corresponding ration, preferably an
alkali metal hydroxide or hydride, or by reaction with an acid of
the corresponding anion, preferably of hydrochloric acid,
hydrobromic acid, sulfuric acid, phosphoric acid or nitric acid.
Salts of I where the metal ion is not an alkali metal. ion can be
prepared by ration exchange of the corresponding alkali metal
salt in a conventional manner, similarly ammonium, phosphonium,
sulfonium and sulfoxonium salts by means of ammonia, phosphonium,
sulfonium or sulfoxonium hydroxides.
The compounds I and their agriculturally useful salts are
suitable, both in the form of isomer mixtures and in the form of
the pure isomers, as herbicides. The herbicidal compositions
comprising I control vegetation on non-crop areas very
CA 02396583 2002-07-05
0050/51065
102
efficiently, especially at high rates of application. They act
against broad-leaved weeds and grass weeds in crops such as
wheat, rice, maize, Soya and cotton without causing any
significant damage to the crop plants. This effect is mainly
observed at low rates of application.
15
25
35
45
CA 02396583 2002-07-05
0050/51065
103
Depending on the application method in question, the compounds I,
or herbicidal compositions comprising them, can additionally be
employed in a further number of crop plants for eliminating
undesirable plants. Examples of suitable crops are the following:
Allium cepa, Ananas comosus, Arachis hypogaea, Asparagus
officinalis, Beta vulgaris spec. altissima, Beta vulgaris spec.
rapa, Brassica napus var. napus, Brassica napus var.
napobrassica, Brassica rapa var. silvestris, Camellia sinensis,
Carthamus tinctorius, Carya illinoinensis, Citrus limon, Citrus
sinensis, Coffea arabica (Coffea canephora, Coffea liberica),
Cucumis sativus, Cynodon dactylon, Daucus carota, Elaeis
guineensis, Fragaria vesca, Glycine max, Gossypium hirsutum,
(Gossypium arboreum, Gossypium herbaceum, Gossypium vitifolium),
Helianthus annuus, Hevea brasiliensis, Hordeum vulgare, Humulus
lupulus, Ipomoea batatas, Juglans regia, Lens culinaris, Linum
usitatissimum, Lycopersicon lycopersicum, Malus spec., Manihot
esculenta, Medicago sativa, Musa spec., Nicotiana tabacum
(N.rustica), Olea europaea, Oryza sativa , Phaseolus lunatus,
Phaseolus vulgaris, Picea abies, Pinus spec., Pisum sativum,
Prunus avium, Prunus persica, Pyrus communis, Ribes sylvestre,
Ricinus communis, Saccharum officinarum, Secale cereale, Solanum
tuberosum, Sorghum bicolor (s. vulgare), Theobroma cacao,
Trifolium pratense, Triticum aestivum, Triticum durum, Vicia
faba, Vitis vinifera and Zea mays.
In addition, the compounds I may also be used in crops which
tolerate the action of herbicides owing to breeding, including
genetic engineering methods.
In addition, the 4-aryl-1-difluormethoxyimidazoles and their
agriculturally utilizable salts are also suitable for the
desiccation and/or defoliation of plants.
As desiccants, they are suitable, in particular, for drying out
the above-ground parts of crop plants such as potatoes, rapeseed,
sunflower and soybeans. Completely mechanical harvesting of these
important crop plants is made possible in this way.
Also of economic interest is
- the time-controlled fall of fruit or the reductian in their
firmness of attachment to the plant, for example in the case
of citrus fruits, olives and other types of pomes, drupes and
0050/51065 CA 02396583 2002-07-05
104
indehiscent fruit, since by this means the harvesting of this
fruit is facilitated.
- Controlled defoliation in the case of useful plants,
especially cotton (defoliation).
The fall is based on the formation of abscission tissue between
the fruit or leaf and sprout part of the plants and is promoted
by the 4-aryl-1-difluormethoxyimidazoles according to the
invention and their agriculturally utilizable salts. Controlled
defoliation of cotton is of particular economic interest, since
by this means the harvesting is facilitated. By means of the
shortening of the time interval in which the individual cotton
plants become ripe, increased quality of the harvested fiber
material is achieved.
The active compounds I or the herbicidal compositions can be
applied pre- or post-emergence. If the active ingredients are
less well tolerated by certain crop plants, application
techniques may be used in which the herbicidal compositions are
sprayed, with the aid of the spraying equipment, in such a way
that they come into as little contact as possible, if_ any, with
the leaves of the sensitive crop plants, while the active
ingredients reach the leaves of undesirable plants growing
underneath, or the bare soil surface (post-directed, lay-by).
The compounds I, or the herbicidal compositions comprising them,
can be used for example in the form of ready-to-spray aqueous
solutions, powders, suspensions, also highly-concentrated
aqueous, oily or other suspensions or dispersions, emulsions, oil
dispersions, pastes, dusts, materials for spreading, or granules,
by means of spraying, atomizing, dusting, spreading or pouring.
The use forms depend on the intended aims; in any case, they
should ensure the finest possible distribution of the active
ingredients according to the invention.
Suitable inert additives are essentially: mineral oil fractions
of medium to high boiling point, such as kerosene and diesel oil,
furthermore coal tar oils and oils of vegetable or animal origin,
aliphatic, cyclic and aromatic hydrocarbons, e.g. paraffins,
tetrahydronaphthalene, alkylated naphthalenes and their
derivatives, alkylated benzenes and their derivatives, alcohols
such as methanol, ethanol, propanol, butanol and cyclohexanol,
ketones such as cyclohexanone, and strongly polar solvents, e.g.
amides such as N-methylpyrrolidone, and water.
~ CA 02396583 2002-07-05
~ 0050/51065
105
Aqueous use forms can be prepared from emulsion concentrates,
suspensions, pastes, wettable powders or water-dispersible
granules by adding water. To prepare emulsions, pastes or oil
dispersions, the 4-aryl-1-difluoromethoxyimidazoles I, either as
such or dissolved in an oil or solvent, can be homogenized in
water by means of a wetting agent, tackifier, dispersant or
emulsifier. Alternatively, it is possible to prepare concentrates
comprising active ingredient, wetting agent, tackifier,
dispersant or emulsifier and, if desired, solvent or oil, which
are suitable for dilution with water.
Suitable surfactants are the alkali metal salts, alkaline earth
metal salts and ammonium salts of aromatic sulfonic acids, e.g.
ligno-, phenol-, naphthalene- and dibutyl-
naphthalenesulfonic acid, and of fatty acids, alkyl- and
alkylarylsulfonates, alkyl sulfates, lauryl ether sulfates and
fatty alcohol sulfates, and salts of sulfated hexa-, hepta- and
octadecanols, and also of fatty alcohol glycol ethers,
condensates of sulfonated naphthalene and its derivatives with
formaldehyde, condensates of naphthalene, or of the
naphthalenesulfonic acids with phenol and formaldehyde,
polyoxyethylene octylphenol ether, ethoxylated isooctyl-, octyl-
or nonylphenol, alkylphenyl or tributyl-
phenyl polyglycol ether, alkylaryl polyether alcohols,
isotridecyl alcohol, fatty alcohol/ethylene oxide condensates,
ethoxylated castor oil, polyoxyethylene alkyl ethers or
polyoxypropylene alkyl ethers, lauryl alcohol polyglycol ether
acetate, sorbitol esters, lignin-sulfite waste liquors or
methylcellulose.
Powders, materials for spreading and dusts can be prepared by
mixing or grinding the active ingredients together with a solid
carrier.
Granules, e.g. coated granules, impregnated granules and
homogeneous granules, can be prepared by binding the active
ingredients to solid carriers. Solid carriers are mineral earths,
such as silicas, silica gels, silicates, talc, kaolin, limestone,
lime, chalk, bole, loess, clay, dolomite, diatomaceous earth,
calcium sulfate, magnesium sulfate, magnesium oxide, ground
synthetic materials, fertilizers such as ammonium sulfate,
ammonium phosphate, ammonium nitrate, ureas, and products of
vegetable origin, such as cereal meal, tree bark meal, wood meal
and nutshell meal, cellulose powders, or other solid carriers.
The concentrations of the active compounds I in the ready-to-use
preparations can be varied within wide ranges. In general, the
0050/51065
CA 02396583 2002-07-05
106
formulations comprise approximately from 0.001 to 98'~ by weight,
preferably 0.01 to 95~ by weight, of at least one active
compound. The active compounds are employed in a purity of from
90~ to 100, preferably 95~ to 100 (according to NMR spectrum).
To widen the spectrum of action and to achieve synergistic
effects, the 4-aryl-1-difluoromethoxyimidazoles I may be mixed
with a large number of representatives of other groups of
herbicidal or growth-regulating active ingredients and then
applied concomitantly. Suitable components for mixtures are, for
example, 1,2,4-thiadiazoles, 1,3,4-thiadiazoles, amides,
aminophosphoric acid and its derivatives, aminotriazoles,
anilides, (het)aryloxyalkanoic acids and their derivatives,
benzoic acid and its derivatives, benzothiadiazinones, 2-aroyl-
1,3-cyclohexanediones, hetaryl aryl ketones, benzyl-
isoxazolidinones, meta-CF3-phenyl derivatives, carbamates,
quinolinecarboxylic acid and its derivatives, chloroacetanilides,
cyclohexane-1,3-dione derivatives, diazines, dichlorapropionic
acid and its derivatives, dihydrobenzofurans, dihydrofuran-
3-ones, dinitroanilines, dinitrophenols, diphenyl ethers,
dipyridyls, halocarboxylic acids and their derivatives, ureas,
3-phenyluracils, imidazoles, imidazolinones, N-phenyl-
3,4,5,6-tetrahydrophthalimides, oxadiazoles, oxiranes, phenols,
aryloxy- or hetaryloxyphenoxypropionic esters, phenylacetic acid
and its derivatives, phenylpropionic acid and its derivatives,
pyrazoles, phenylpyrazoles, pyridazines, pyridinecarboxylic acid
and its derivatives, pyrimidyl ethers, sulfonamides,
sulfonylureas, triazines, triazinones, triazolinones,
triazolecarboxamides and uracils.
It may furthermore be advantageous also to apply the compounds I,
alone or in combination with other herbicides, in the' form of a
mixture with other crop protection agents, for example together
with agents for controlling pests or phytopathogenic fungi or
bacteria. Also of interest is the miscibility with mineral salt
solutions, which are employed for treating nutritional and trace
element deficiencies. Nonphytotoxic oils and oil concentrates may
also be added.
The rates of application of active ingredient are from 0.001 to
3.0, preferably 0.01 to 1.0, kg/ha of active substance (a.s.),
depending on the control target, the season, the target plants
and the growth stage.
The examples given hereinbelow are meant to illustrate the
invention without limiting it.
~
~ 0050/51065 CA 02396583 2002-07-05
107
I Preparation Examples:
Example 1:
4-(4-Chlorophenyl)-1-difluoromethoxy-2-methyl-1H-imidazole
(compound No. IAb.l; see Table 1)
1.1: 4-(4-Chlorophenyl)-1-hydroxy-2-methyl-1H-imidazole:
5 g (43 mmol) of nitrosonium tetrafluoroborate were dissolved
in 40 ml of aeetonitrile. Over the course of 8 h, the
solution was admixed dropwise with a solution of 5.9 g
(43 mmol) of 4-chlorostyrene in 50 ml of acetonitrile. The
mixture was then allowed to react at room temperature for
another 3 days, the pH was then adjusted to about 7-8 using
dil. sodium bicarbonate solution and the precipitated product
of value was filtered off and dried. Yield 2.8 g. MS (m/e):
208 [M]+.
1.2: 2.6 g (13 mmol) of 4-(4-chlorophenyl)-1-hyd:roxy-2-
methyl-1H-imidazole were dissolved in 100 ml of :DMF and
admixed with 1.85 g (13 mmol) of potassium carbonate, and the
mixture was heated to 90~C. Gaseous chlorodifluoromethane was
then introduced until the reaction had gone to completion.
The resulting solution was admixed with water and the aqueous
phase was extracted with ethyl acetate. The organic phase was
washed with water, dried over magnesium sulfate, filtered and
concentrated. The residue was purified by silica gel
chromatography. This gave 0.9 g of the title compound (mobile
phase cyclohexane/ethyl acetate 4:1).
1H-NMR (400 MHz, in CDC13): 8 = 2.42 (s, 3 H), 6.59 (t, 1 H),
7.30 (m, 3 H), 7.64 (d, 2 H).
Example 2:
5-Chloro-4-(4-chlorophenyl)-1-difluoromethoxy-2-methyl-1H-
imidazole (compound No. IAb.4)
0.55 g (2.1 mmol) of 4-(4-chlorophenyl)-1-difluoromethoxy-2-
methyl-1H-imidazole from Example 1 was dissolved in 30 ml of
carbon tetrachloride and admixed with 0.32 g (2.3 mmol) of
sulfuryl chloride. After 4 h, a dilute aqueous sodium
bicarbonate solution was added and the organic phase was
separated off, washed with water, dried over magnesium
sulfate, filtered and concentrated. The residue was purified
by silica gel chromatography. This gave 0.3 g of the title
compound (mobile phase cyclohexane/ethyl acetate 4:1).
MS (m/e): 293 [M+H]+.
' , 0050/51065 CA 02396583 2002-07-05
108
Example 3:
4-(2,4-Dichlorophenyl)-1-difluoromethoxy-2-methyl-1H-
imidazole (compound No. IAb.3)
3.1: 4-(2,4-Dichlorophenyl)-1-hydroxy-2-methyl-1H-imidazole
36 g (0.2 mol) of 2,4-dichlorostyrene were reacted by the
procedure of Example 1.1. This gave 16 g of the
hydroxyimidazole.
1H-NMR (270 MHz, in DMSO-d6): b = 2.29 (s, 3 H), 7.41 (dd,
1 H), 7.59 (d, 1 H}, 7.81 (s, 1 H), 8.10 (d, 1 H).
3.2: 16 g (66 mmol) of the 4-(2,4-dichlorophenyl)-1-hydroxy-
2-methyl-1H-imidazole obtained according to 3.1 were reacted
by the procedure of Example 1.2. This gave 4 g of the title
compound.
1H-NMR (400 MHz, in CDC13): b = 2.44 (s, 3 H), 6.61 (t, 1 H),
7.29 (dd, 1 H), 7.41 (d, 1 H), 7.79 (s, 1 H}, 8.08 (d, 1 H).
Example 4:
5-Chloro-4-(2,4-dichlorophenyl)-1-difluoromethoxy-2-methyl-
1H-imidazole (compound No. IAb.6)
4 g (13 mmol) of 4-(2,4-dichlorophenyl)-1-difluoromethoxy-
2-methyl-1H-imidazole were reacted according to the procedure
of Example 2. Yield 2.4 g.
1H-NMR (400 MHz, in CDC13): b = 2.48 (s, 3 H), 6.67 (t, 1 H),
7.31 (dd, 1 H), 7.40 (d, 1 H), 7.48 (d, 1 H).
Example 5:
5-Chloro-4-(2,4-dichloro-5-nitrophenyl)-1-difluoromethoxy-2-
methyl-1H-imidazole (compound No. IAb.30)
At -40°C, 2.1 g (34 mmol) of nitric acid were admixed with
2.0 g (20 mmol) of sulfuric acid. A solution of 2.1 g
(6.7 mmol) of 5-chloro-4-(2,4-dichlorophenyl)-1-
difluoromethoxy-2-methyl-1H-imidazole in 50 ml of methylene
chloride was added dropwise. The mixture was allowed to warm
to 0°C, and this temperature was maintained for 1 h. The
reaction mixture was then poured into ice water and extracted
with dichloromethane, and the combined organic phases were
washed with dilute aqueous sodium bicarbonate solution and
water. The organic phase was subsequently dried over
magnesium sulfate, filtered and concentrated. Yield 2 g.
1H-NMR (400 MHz, in CDC13): b = 2.50 (s, 3 H), 6.71 (t, 1 H),
7.68 (s, 1 H), 8.09 (s, 1 H).
0050/51065 CA 02396583 2002-07-05
109
Example 6:
2,4-Dichloro-5-(5-chloro-1-difluoromethoxy-2-methyl-IH-
imidazol-4-yl)aniline (compound No. IAb.270)
A solution of 20 ml of acetic acid in 30 ml of ethanol was
admixed successively with 1.5 g (27 mmol) of iron powder and
2 g (5.4 mmol) of 5-chloro-4-(2,4-dichloro-5-nitrophenyl)-
1-difluoromethoxy-2-methyl-1H-imidazole (prepared, for
example, according to Example 5) and heated to 70pC. After
2 h at this temperature, the mixture was diluted with ethyl
acetate to five times its original volume. The solids were
then filtered off through a bed of diatomaceous earth and the
filtrate was concentrated and admixed with dichloromethane.
The organic phase was washed with dilute aqueous sodium
bicarbonate solution and water, dried over magnesium sulfate,
filtered and concentrated. The resulting residue was purified
by silica gel chromatography (mobile phase cyclo:hexane/ethyl
acetate 4:1). This gave 0.9 g of the title compound.
1H-NMR (270 MHz, in CDC13): b = 2.48 (s, 3 H), 4.10 (s, 2 H),
6.67 (t, 1 H), 6.85 (s, 1 H), 7.36 (s, 1 H).
Example 7:
5-Chloro-4-(2,4-dichloro-5-(di(methylsulfonyl)am.ino)phenyl)-
1-difluoromethoxy-2-methyl-1H-imidazole (compound No.
IAb.294)
0.9 g (2.7 mmol) of 2,4-dichloro-5-(5-chloro-1-
difluoromethoxy-2-methyl-1H-imidazol-4-yl)aniline (prepared,
for example, according to Example 6) was dissolved in 50 ml
of THF and admixed with 0.6 g (5.3 mmol) of methanesulfonyl
chloride and 0.79 g (7.9 mmol) of triethylamine. After 8 h,
the mixture was concentrated, and ethyl acetate was added.
The organic phase was washed with water, dried over magnesium
sulfate, filtered and concentrated. Yield 1.2 g.
1H-NMR (270 MHz, in CDC13): b = 2.49 (s, 3 H), 3.50 (s, 6 H),
6.68 (t, 1 H), 7.54 (s, 1 H), 7.67 (s, 1 H).
Example 8:
Methane sulfonic acid (2,4-dichloro-5-(5-chloro-
1-difluoromethoxy-2-methyl-1H-imidazol-4-yl)anil:ide)
(compound No. IAb.306)
0.65 g (1.3 mmol) of 5-chloro-4-(2,4-dichloro-5-
(di(methylsulfonyl)amino)phenyl)-1-difluoromethoxy-2-methyl-
1H-imidazole (prepared, for example, according to Example 7)
were dissolved in 100 ml of methanol and admixed with 0.17 g
(2.6 mmol) of potassium hydroxide. After 4 h, the mixture was
0050/51065 CA 02396583 2002-07-05
110
concentrated and the residue was admixed with water and ethyl
acetate. The organic phase was washed with water, dried over
magnesium sulfate, filtered and concentrated. The resulting
crude product was purified by silica gel chromatography
(mobile phase cyclohexane/ethyl acetate 4:1). This gave 0.2 g
of the title compound.
MS (m/e): 419 [MJ+.
Example 9:
4-(4-Chloro-2-fluoro-5-isopropoxyphenyl)-1-difluoromethoxy-2-
methyl-1H-imidazole (compound No. IAb.110)
9.1: 4-Chloro-2-fluoro-5-isopropoxystyrene
5.7 g (16 mmol) of methyltriphenylphosphonium bromide were
suspended in 50 ml of THF and admixed with 6.8 g (16 mmol) of
a 15~ strength solution of butyllithium in hexane. The yellow
suspension was cooled to -78~C. A solution of 2.3 g (10 mmol)
of 4-chloro-2-fluoro-5-isopropoxybenzaldehyde in 20 ml of THF
was added and the mixture was warmed to room temperature.
After 2 h at room temperature, the solution was concentrated
and the residue was chromatographed over silica gel (mobile
phase hexane/ethyl acetate 2:1). Yield 2 g.
1H-NMR (270 MHz, in CDC13): b = 1.37 (d, 6 H), 4.48 (sept.,
1 H), 5.39 (d, 1 H), 5.77 (d, 1 H), 6.79 (dd, 1 H), 7.03 (d,
1 H), 7.09 (d, 1 H).
9.2: 4-(4-Chloro-2-fluoro-5-isopropoxyphenyl)-1-hydroxy-
2-methyl-1H-imidazole
2 g (9.3 mmol) of 4-chloro-2-fluoro-5-isopropoxystyrene were
reacted by the procedure of Example 1.1. Yield 1.5 g.
MS (m/e): [M+HJ+.
9.3: 1.5 g (5.2 mmol) of 4-(4-chloro-2-fluoro-5-
isopropoxyphenyl)-1-hydroxy-2-methyl-1H-imidazole were
reacted by the procedure of Example 1.2. Yield 0.2 g.
1H-NMR (360 MHz, in CDC13): b = 1.39 (d, 6 H), 2.44 (s, 3 H),
4.64 (sept., 1 H), 6.60 (t, 1 H), 7.12 (d, 1 H), 7.50
(d, 1 H), 7.70 (d, 1 H).
Example 10:
5-Chloro-4-(4-chloro-2-fluoro-5-isopropoxyphenyl)-1-
difluoromethoxy-2-methyl-1H-imidazole (compound No. IAb.113)
0.2 g (0.5 mmol) of 4-(4-chloro-2-fluoro-5-
isopropoxyphenyl)-1-difluoromethoxy-2-methyl-1H-imidazole was
reacted by the procedure of Example 2. Yield 0.07 g.
0050/51065
CA 02396583 2002-07-05
111
1H-NMR (360 MHz, in CDC13): 8 = 1.38 (d, 6 H), 2.49 (s, 3 Fi),
4.55 (sept., 1 H), 6.66 (t, 1 H), 7.19 (m, 3 H).
Example 11:
4-(2,4-Dichlorophenyl)-1-difluoromethoxy-2-isopropyl-1H-
imidazole (compound No. IAd.3)
11.1: 4-(2,4-Dichlorophenyl)-1-hydroxy-2-isopropyl-1H-
imidazole: 20 g (0.17 mol) of nitrosonium tetrafluoroborate
were dissolved in 200 ml of isobutyronitrile and, over a
period of 5 h, admixed dropwise with a solution of 30 g (0.17
mol) of 2,4-dichlorostyrene in 100 ml of isobutyronitrile.
The mixture was allowed to react at room temperature for
another 16 h, the pH was adjusted to about 7-8 using dil.
sodium bicarbonate solution and the precipitated product of
value was filtered off and dried. Yield 20 g.
11.2: 20 g (74 mmol) of 4-(2,4-dichlorophenyl)-1-hydroxy-
2-isopropyl-1H-imidazole were reacted by the procedure of
Example 1.2. This gave the title compound in a yield of 6 g.
1H-NMR (270 MHz, in CDC13): b = 1.37 (d, 6 H), 3.14 (sept.,
1 H), 6.61 (t, 1 H), 7.29 (dd, 1 H), 7.41 (d, 1 H), 7.77 (s,
1 H), 8.16 (d, 1 H).
Example 12:
5-Chloro-4-(2,4-dichlorophenyl)-1-difluoromethoxy-2-
isopropyl-1H-imidazole (compound No. IAd.6)
6 g (19 mmol) of 4-(2,4-dichlorophenyl)-1-difluoromethoxy-
2-isopropyl-1H-imidazole were reacted by the procedure of
Example 2. Yield 4.3 g.
1H-NMR (400 MHz, in CDC13): S = 1.37 (d, 6 H), 3.20 (sept.,
1 H), 6.66 (t, 1 H), 7.30 (dd, 1 H), 7.42 (d, 1 H), 7.46 (d,
1 H).
Example 13:
2-t-Butyl-4-(2,4-dichlorophenyl)-1-difluoromethoxy-1H-
imidazole (compound No. IAe.3)
13.1: 2-t-Butyl-4-(2,4-dichlorophenyl)-1-hydroxy-1H-imidazole
20 g (0.17 mol) of nitrosonium tetrafluoroborate were
dissolved in 200 ml of pivalonitrile and, over a period of
5 h, admixed dropwise with a solution of 30 g (0.17 mol) of
2,4-dichlorostyrene in 50 ml of pivalonitrile. The mixture
was allowed to react at room temperature for another 16 h,
the pH was adjusted to about 7-8 using dil. sodium
bicarbonate solution and the precipitated product of value
0~5~/51065 CA 02396583 2002-07-05
112
was filtered off and dried. Yield 19 g.
13.2: 19 g (67 mmol) of 2-t-butyl-4-(2,4-dichlorophenyl)-
1-hydroxy-1H-imidazole were reacted analogously to the
procedure of Example 1.2. Yield 4 g.
1H-NMR (270 MHz, in CDC13): b = 1.45 (s, 9 H), 6.57 (t, 1 H),
7.29 (dd, 1 H), 7.40 (d, 1 H), 7.83 (s, 1 H), 8.17 (d, 1 H).
Example 14:
2-t-Butyl-5-chloro-4-(2,4-dichlorophenyl)-1-difluoromethoxy-
1H-imidazole (compound No. IAe.6)
4 g (12 mmol) of 2-t-butyl-4-(2,4-dichlorophenyl)-
1-difluoromethoxy-1H-imidazole were reacted by the procedure
of Example 2. Yield 1.7 g.
1H-NMR (400 MHz, in CDC13): b = 1.45 (s, 9 H), 6.62 (t, 1 H),
7.30 (dd, 1 H), 7.41 (d, 1 H), 7.47 (d, 1 H).
The compounds prepared in the examples above are represented by
the formula below in which the variables R1, R2, R4 and R6' are as
defined in Table 4.
R4 R1
N
Cl ~ N
\' 0-CHF2
R6' R2
35
45
0050/51065 CA 02396583 2002-07-05
113
Table 4
Example (No. ) R ~ R2 R4 R ' (= X-R6)
1 (IAb.l) CH3 H H H
_ _
2 ( IAb. 4 ) CH3 - H H ._
C1
3 (IAb.3) CH3 H C1 H
4 (IAb.6) CH3 C1 C1 H
5 (IAb.30) CH3 C1 C1 N02
6 (IAb.270) CHg C1 C1 NHZ
7 (IAb.294) CH3 C1 Gl N(S02CH3)2
108 (IAb.306) CH3 C1 C1 NH(SOZCH3)
9 (IAb.110) CH3 H F OCH(CH3)2
(IAb.113) CH3 C1 F OCH(CH3)2
11 (IAd.3) CH(CH3)2 H C1 H
12 (IAd.6) CH(CH3)2 C1 C1 H
1513 (IAe.3) C(CH3)3 H C1 H
14 (IAe.6) C(CH3)3 C1 C1 H
II Formulation examples and examination of the herbicidal
activity
The compounds I according to the invention can be formulated,
for example, as follows:
I. 20 parts by weight of the compound example 1 are
dissolved in a mixture composed of 80 parts by weight of
alkylated benzene, 10 parts by weight of the adduct of 8 to
10 mol of ethylene oxide to 1 mol of oleic acid
N-monoethanolamide, 5 parts by weight of calcium
dodecylbenzenesulfonate and 5 parts by weight of the adduct
of 40 mol of ethylene oxide to 1 mol of castor oil. Pouring
the solution into 100,000 parts by weight of water and
finely distributing it therein gives an aqueous dispersion
which comprises 0.02$ by weight of the active :ingredient.
II. 20 parts by weight of the compound example 2 are
dissolved in a mixture composed of 40 parts by weight of
cyclohexanone, 30 parts by weight of isobutano:L, 20 parts
by weight of the adduct of 7 mol of ethylene oxide to 1 mol
of isooctylphenol and 10 parts by weight of the adduct of
mol of ethylene oxide to 1 mol of castor oi:L. Pouring
40 the solution into 100,000 parts by weight of water and
finely distributing it therein gives an aqueous dispersion
which comprises 0.02 by weight of the active ingredient.
III. 20 parts by weight of the active ingredient example 4
are dissolved in a mixture composed of 25 parts by weight
of cyclohexanone, 65 parts by weight of a mineral oil
fraction of boiling point 210 to 280~C and 10 parts by
~~~J~/51065 CA 02396583 2002-07-05
114
weight of the adduct of 40 mol of ethylene oxide to 1 mol
of castor oil. Pouring the solution into 100,000 parts by
weight of water and finely distributing it therein gives an
aqueous dispersion which comprises 0.02 by weight of the
active ingredient.
IV. 20 parts by weight of the active ingredient example
are mixed thoroughly with 3 parts by weight of sodium
diisobutylnaphthalenesulfonate, 17 parts by weight of the
10 sodium salt of a lignosulfonic acid from a sulfite waste
liquor and 60 parts by weight of pulverulent silica gel,
and the mixture is ground in a hammer mill. Finely
distributing the mixture in 20,000 parts by weight of water
gives a spray mixture which comprises 0.1~ by weight of the
active ingredient.
V. 3 parts by weight of the active ingredient example 7
are mixed with 97 parts by weight of finely divided kaolin.
This gives a dust which comprises 3~ by weight of active
ingredient.
VI. 20 parts by weight of the active ingredient example 8
are mixed intimately with 2 parts by weight of calcium
dodecylbenzenesulfonate, 8 parts by weight of :fatty alcohol
polyglycol ether, 2 parts by weight of the sodium salt of a
phenol/urea/formaldehyde condensate and 68 parts by weight
of a paraffinic mineral oil. This gives a stab:Le oily
dispersion.
VII. 1 part by weight of the compound example 12 is
dissolved in a mixture composed of 70 parts by weight of
cyclohexanone, 20 parts by weight of ethoxylated
isooctylphenol and 10 parts by weight of ethoxylated castor
oil. This gives a stable emulsion concentrate.
VIII. 1 part by weight of the compound example 14 is
dissolved in a mixture composed of 80 parts by weight of
cyclohexanone and 20 parts by weight of Wettol~'~ EM 31
(nonionic emulsifier based on ethoxylated castor oil). This
gives a stable emulsion concentrate.
The herbicidal activity of the 4-aryl-1-difluoromethoxyimidazoles
of the formula I was demonstrated by the following greenhouse
experiments:
0050/51065 CA 02396583 2002-07-05
115
The culture containers used were plastic pots containing loamy
sand with approximately 3.0~ of humus as the substrate. The seeds
of the test plants were sown separately for each species.
For the pre-emergence treatment, the active ingredients, which
had been suspended or emulsified in water, were applied directly
after sowing by means of finely distributing nozzles. The
containers were irrigated gently to promote germination and
growth and subsequently covered with transparent plastic hoods
until the plants had rooted. This cover causes uniform
germination of the test plants, unless this has been adversely
affected by the active ingredients.
For the post-emergence treatment, the plants were first grown to
a height of 3 to 15 cm, depending on the plant habit, and only
then treated with the active ingredients which had been suspended
or emulsified in water. The test plants for this purpose were
either sown directly and grown in the same containers, or they
were first grown separately as seedlings and transplanted into
the test containers a few days prior to the treatment. The rate
of application for the post-emergence treatment was 0.125 and
0.063 kg of a.s./ha.
Depending on the species, the plants were kept at 10-25~C or
20-35~C. The test period extended over 2 to 4 weeks. During this
time, the plants were tended, and their response to the
individual treatments was evaluated.
Evaluation was carried out using a scale from 0 to 100. 100 means
no emergence of the plants, or complete destruction of at least
the above-ground parts, and 0 means no damage, or normal course
of growth.
The plants used in the greenhouse experiments belong to the
following species:
Bayer code Common Name
SETFA giant foxtail
ABUTH velvetleaf
AMARE redroot pigweed
POLPE lady's thumb
Herbicidal activity when used post-emergence in a greenhouse
(Example 4: 5-chloro-4-(2,4-dichloro-phenyl)-1-difluaromethoxy-2-
methyl-1H-imidazole (Compound No. IAb.6)
0050/51065 CA 02396583 2002-07-05
116
At application rates of 0.125 kg/ha a.S, and 0.063 kg/ha s.a.
compound Nr. IAb.6 from example 4 shows a good herbicidal action
against SETFA, ABUTH, AMARE and POLPE, when applied by the
post-emergence method.
10
20
30
40