Note: Descriptions are shown in the official language in which they were submitted.
CA 02396972 2002-08-07
REFERENCE TO CO-PENDING APPLICATION
The subject matter of each of the following applications is incorporated
herein by
reference:
- U.S. Patent Application 09/275,932, filed on March 24, 1999 entitled METHODS
OF
PURIFYING COBALT;
- U.S. Patent Application 09/306,311, filed on May 6, 1999 entitled BASE METAL
RECOVERY; and
- PCT Patent Application PCT/CA00/00352, filed April 5, 2000 entitled
PURIFICATION OF ZINC MATERIALS
BACKGROUND OF THE INVENTION
1. FIELD OF THE INVENTION
The present invention relates to techniques for the processing of nickel
bearing solutions.
2. DESCRIPTION OF THE RELATED ART
Typical nickel solutions include cobalt and impurities such as iron and
manganese. There
are several ways in which nickel has been recovered from these solutions.
These are widely
known and generally involve removal of iron, followed by:
a) solvent extraction of cobalt, manganese and zinc using the well known
CYANEX 272
(a trademark) solvent, followed by a nickel solvent extraction using VERSATIC
10 (a
trademark), then cobalt sulphide precipitation from the CYANEX 272 raffinate,
followed
-1-
CA 02396972 2002-08-07
by a cobalt sulphide pressure leach, purification and cobalt recovery.
b) precipitation of cobalt and nickel using Mg0 or CaO, with the cobalt and
nickel being
re-leached in NH3 for separation and recovery.
c) precipitation of cobalt and nickel sulphides which are later subjected to a
pressure
leach, followed by a cobalt solvent extraction and a nickel precipitation
using Hz.
The processes b) and c) therefore remove iron in the first step, then cobalt
and nickel are
precipitated from the rest of the impurities, in particular manganese, and
then cobalt is separated
from nickel. Therefore, to recover nickel, which is generally the most
valuable element, nickel
and cobalt must usually be separated from manganese and then the nickel
recovered from the
cobalt.
It is an object of the present invention to provide a novel method for
processing nickel
bearing solutions.
SUMMARY OF THE INVENTION
Briefly stated, the invention involves a process for recovering a nickel
constituent from a
solution containing iron, cobalt and manganese, comprising the steps of:
a) removing the iron from the solution using an oxidation and partial
neutralization step;
b) removing cobalt and manganese together from the solution using an oxidation
step at a
pH ranging from about 1 to about 4, to leave substantially all of the nickel
constituent in
solution; and
c) recovering the nickel constituent from solution.
-2-
CA 02396972 2002-08-07
In one embodiment, the solution is reacted, in step (b), with hypochlorite to
precipitate
both the cobalt and manganese, preferably at a pH from about 3.0 to about 3.5.
In another embodiment, the solution, in step (b), is reacted with a mixture of
sulphur
dioxide and oxygen to precipitate both the cobalt and manganese, again at a pH
from about 3.0 to
about 3.5.
In another aspect, the present invention provides a method of processing a
nickel bearing
solution containing iron, cobalt and manganese constituents, comprising the
steps of:
a) removing the iron constituent from the solution using an oxidation and
partial
neutralization step; and
b) removing cobalt and manganese constituents together from the solution using
an
oxidation step at a pH ranging from about 1 to about 4, to leave substantially
all of the
nickel constituent in solution.
In one embodiment, the method further comprises the step of:
c) recovering the nickel constituent from solution.
In still another of its aspects, the present invention provides a method of
processing a
laterite leach solution, comprising the steps of
a) providing a laterite leach solution including 1 to 10 g/L nickel, 10 to
1000 mg/L cobalt,
and 50 to 1500 mg/L manganese;
b) removing cobalt and manganese together from the solution using an oxidation
step at a
pH ranging from about 1 to about 4, to leave substantially all of the nickel
in solution.
-3-
CA 02396972 2002-08-07
In one embodiment, the solution of step a) includes 0 to 2000 mg/L iron, the
method
further comprising, before step b), the step of:
c) removing the iron from the solution using an oxidation and partial
neutralization step.
In another embodiment, the method includes the step of:
d) recovering the nickel from solution.
In still another embodiment, the method further comprises the steps of:
e) re-leaching the cobalt and manganese from step b) into an intermediate
leach solution;
and
f) recovering the cobalt from the intermediate leach solution.
In still another embodiment, the method may further comprise the steps of:
e) re-leaching the cobalt and manganese from step b) into an intermediate
leach solution;
and
f) recovering the manganese from the intermediate leach solution.
In one embodiment, the solution includes at least one of the following
impurities: 0 to 50
g/L chloride, 2 to 15 g/L magnesium and 300 to 600 mg/L calcium, further
comprising the step
of:
d) recovering the nickel from solution, while leaving the impurities in
solution.
-4-
CA 02396972 2002-08-07
In yet another of its aspects, there is provided a method of processing a
laterite leach
solution, comprising the steps of
a) providing a laterite leach solution including 1 to 10 g/L nickel, 10 to
1000 mg/L cobalt,
50 to 1500 mg/L manganese, and at least one of the following impurities: 0 to
50 g/L
chloride, 2 to 15 g/L magnesium and 300 to 600 mg/L calcium;
b) removing cobalt and manganese together from the solution using an oxidation
step at a
pH ranging from about 1 to about 4, to leave substantially all of the nickel
and the
impurities in the solution.
In one embodiment, the solution of step a) includes 0 to 2000 mg/L iron, the
method
further comprising, before step b), the step of:
c) removing the iron from the solution using an oxidation and partial
neutralization step.
It will be understood by those skilled in the art that the steps of a
hydrometallurgy process
to 'remove' or 'recover' an impurity or a metal of value will often not be
absolute. Rather, trace
elements will sometimes remain, the amount of which will vary depending on the
selected
process parameters, such as pH, temperature and the like. Therefore, terms
used herein such as
'remove' or 'recover' are intended to take this into account.
Thus, in one embodiment, the present invention provides a method of recovering
either
nickel or cobalt, or both, from a leach solution containing impurities such as
iron, manganese and
others.
-5-
CA 02396972 2002-08-07
DESCRIPTION OF THE PREFERRED EMBODIMENTS
An embodiment of the present invention is illustrated in figure 1, this case
employing a
laterite leach solution. It will, of course, be understood that the present
process may be used on
any other leach solutions.
As shown, at step 10, the leach solution is first subjected to an oxidation
and partial
neutralization step to remove iron (as is known to those skilled in the art),
which leaves as a solid
phase residue as shown in the separation step 12. The solution is then exposed
to a suitable
oxidant at step 14, (at a pH ranging from about 1 to about 4, more preferably
from about 3 to
about 3.5, for example at 3.5 as illustrated in the example herein below) to
oxidize cobalt and
manganese together. The cobalt and manganese oxides are then removed in a
later separation
step 16, while the nickel bearing solution is directed to a nickel recovery
step, not shown (but
which is known to those skilled in the art).
The cobalt and manganese oxides are re-leached in a re-leach step 18 and the
resulting
leach solution is directed to a cobalt manganese separation step as shown in
figure 2.
As figure 2 illustrates, some trace amounts of nickel will, in some cases, be
present in the
cobalt manganese leach solution. However, the presence of nickel is only an
economic issue and
should not otherwise impair the removal of cobalt and manganese. The former is
removed by
subjecting the leach solution, for example by solvent extraction or
precipitation, the latter using
complexants such as NaSH or others (as is known to those skilled in the art),
at step 20, to form a
cobalt sulphide, which is then recovered in a separation step 22, with the
manganese bearing
leach solution advancing to a later recovery step (as is known to those
skilled in the art) not
shown.
The present process is advantageous in that cobalt, manganese and nickel may
be
recovered from the solution using well known process steps.
-6-
CA 02396972 2002-08-07
Feed solutions, for example, may include laterite leach solutions having the
following:
- 1 to 10 g/L, more preferably 1 to 5 g/L Ni
- 10 to 1000 mg/L Co,
- 50 to 1500 mg/L, more preferably 60 to 1000 mg/L Mn
-Oto50g/LCl
- 2 to 15 g/L Mg;
- 0 to 200 mg/L Fe;
- 300 to 600 mg/L Ca;
- Cu, Cr, Zn or other trace elements, each not usually exceeding 100 mg/L.
The oxidation steps may involve hypochlorite or other oxidants such as
mixtures of SOZ
and OZ (and others as known to those skilled in the art) at temperatures
ranging, for example,
from 25 to 80 degrees Celsius, more preferably 50 to 70 degrees Celsius.
Typical reactions
involve an excess of oxidant ranging from 1 to 20 percent of the
stoichiometric amount.
IS
Thus, the nickel constituent can be removed by using the VERSATIC 10 solvent.
The
cobalt constituent can be upgraded by precipitation and recovery in a side
stream. The present
process thus enables the selective precipitation of Co and Mn by oxidation to
Co 3+, Mn 4+
without oxidizing Ni z'.
The oxidation steps herein may include, for example, SOZ/OZ at lower pH's or
NaClO.
Other oxidants include C12, Os, and Caro's acid. Preferably, the Co-Mn re-
leach step is carried
out using reductive leaching (for example using metabisulphite or SOZ).
Co Mn separation can also be carried out in several ways, such as by a
sulphide
precipitation of CoS leaving Mn behind, and a re-leach CoS in an autoclave, or
selective
oxidation of Mn and selective precipitation of MnOx, using SOZ/OZ , then
recovering the cobalt
as metal (by solvent extraction or electrowinning ), or by recovering the
cobalt in other forms
such as a carbonate or a hydroxide.
_7_
CA 02396972 2002-08-07
If desired, the iron constituent may, in some cases, be removed together with
the cobalt
and manganese from the nickel bearing leach solution, for example, by reacting
the solution with
an appropriate oxidant such as hypochlorite. However, this may complicate, to
some extent, the
subsequent recovery of cobalt from the precipitate.
Embodiments of the present invention will be described with reference to the
following
examples which are presented for illustrative purposes only and are not
intended to limit the
scope of the invention.
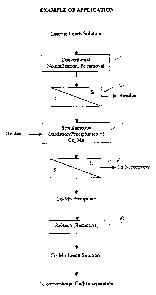
EXAMPLE:
1. Feed Solution
An example of the present process was conducted on a laterite feed solution,
having the
following composition:
Ni: 1.9 g/L
Co: 60 mg/L
Mn: 410 mg/L
Cl: 28 g/L
Mg: 12 g/L
Fe: <5 mg/L
+ Cu, Cr, Zn: < 100 mg/L each
2. Oxidation-precipitation of Co + Mn
Following a conventional oxidation of Fe, the oxidation and precipitation of
Co and Mn
proceeded under the following conditions:
_g_
CA 02396972 2002-08-07
2.1. Conditions
Temperature: 68°C
pH = 3.5
Duration: 120 min.
Oxidant: sodium hypochlorite
2.2. Results
The results are shown in the table below indicating that the process was very
selective for
the recovery/removal of Co (95.4%) and Mn (99.9%) while removing a relatively
small amount of
Ni (2%).
Stream Ni Co Mn
PLS (mg/L) 1930 62 414
Final Solution (mg/L)1800 2.9 0.3
Co/Mn precip (%) 3.45 5.75 33.5
Recovery in Precipitate2.0 95.4 99.9
3. Re-dissolution of Co + Mn precipitate
The Co Mn precipitate was re-dissolved under the following conditions:
3.1. Conditions
Reductive leach: sodium metabisulphite
pH = 1.5
Temperature = 50°C
Time = 15 minutes
-9-
CA 02396972 2002-08-07
3.2. Results:
The sample was completely re-dissolved with a 100 percent recovery of Co, Ni
and Mn, resulting
in the leach solution having the following composition:
Leach solution:
2.9 g/L Ni
3.0 g/L Co
21.2 g/L Mn
-10-